Note: Descriptions are shown in the official language in which they were submitted.
= CA 02688507 2012-01-17
"Alloys with High Corrosion Resistance for Engine Valve Applications"
Field of the Invention
[0001] This invention deals with an Ni-Fe-Cr precipitation hardened
superalloy for application in internal combustion engine valves, having as
chief
characteristics a high hot resistance through the precipitation of
Ni3(AI,Ti,Nb) and
a higher oxidation resistance as compared to the state-of-the-art alloys. The
alloy
project, based on its microstructure aspects, also allows for the alloy hereof
to be
provided with properties equal to or higher than those of the high nickel
alloys used
in internal combustion engine valves, associated with the alloy's significant
cost
reduction because of the lower nickel content.
[0002] The alloy hereof is intended for valves' manufacture, where
the alloy
is required to have several properties, among them the following: high
temperature
oxidation resistance and heat resistance, because of the high temperatures
used
in the application.
Background of the Invention
[0003] Conventionally, the materials used for exhaust valves in
diesel and
gasoline engines were JIS SUH 35 or JIS SUH 38 with STELLITE (trademark of
Delore Stellite Co.) coating (cobalt base alloy) on the valve's hardfacing.
With the
historic increased application temperatures of the new engines' valves, higher
performance materials started to be used in some applications, as it occurs
with
nickel base superalloys.
[0004] Currently, the reduction in the production costs of high
performance
materials is a tendency of the industry, as it occurs with exhaust valves,
which are
parts that are exposed to the highest temperatures and highest mechanical
stress
in an internal combustion engine. Such extreme stresses in terms of mechanical
i
. CA 02688507 2012-01-17
2
resistance and corrosion resistance at high temperatures require the use of
costly
nickel-based superalloys.
[0005] One example of an excellent performance alloy in these
applications
is the UNS N07751 alloy, which is very costly because of its high nickel
content,
above 70%. In this sense, alloys with lower nickel contents with high
temperature
resistance, corrosion resistance and long term microstructural stability at
high
temperature have been developed. Examples are the state-of-the-art alloys,
NCF3015 (JIS3015D - U.S. Pat. No. 5,660,938) and the alloy of U.S. Pat. No.
5,951,789.
[0006] Through the use of the lead-free gasoline since the 70's,
the
requirement in terms of corrosion resistance of the exhaust valve material has
been
reduced, so that the lead oxide corrosion by the lead oxide is no longer a
primary
concern. The high temperature oxidation resistance is a property to be
reviewed in
terms of corrosion, having the good performance of the UNS N07751 alloy.
[0007] Accordingly, the need is evident to develop new superalloy
compositions resistant to high temperature mechanical stress in connection
with
high temperatures, corrosion resistant, with high hot workability, and meeting
the
different stresses under the conditions of using intake or exhaust valves,
able to
meet the need of a lower cost, which is related to the lower content of costly
alloy
elements. Alloy UNS N07751 is the most important material to be replaced.
[0008] The alloys hereof are intended to meet all such needs.
Summary of the Invention
[0009] The properties of the Ni-Fe-Cr alloys used in exhaust valves
are
closely related to the presence of intermetallic phases in their
microstructures. The
intermetallic phases are very important for high temperature resistance. As
regards
the solid solution elements in the alloy, a composition providing the material
with
1
CA 02688507 2009-12-16
3
corrosion resistance required in the use environment is very important. The
performance of the alloy elements to form these phases has been carefully
reviewed
and modified as regards the conventional concept. In this sense, this
invention
employs the niobium in relatively high quantities (higher than the state-of-
the-art
alloys) as an alloy element, mainly in the form of fine intermetallic
precipitate.
[0010] Other elements used by this invention in higher quantities than the
state-of-the-art alloys are aluminum and chromium, which have prevailing
functions
in the alloys' corrosion resistance. Chromium is responsible for the formation
of a
passivating film of chromium oxide on the material's surface, which prevents
the
corrosive process progression. Aluminum is also a builder of a coherent
intermetallic
containing niobium, Ni3(AI,Nb), thus improving the material's heat resistance.
Additionally, aluminum improves the alloy hot resistance oxidation.
[0011] As the carbon atoms are stabilized by the presence of titanium and
niobium (strong carbide formers) in great quantities in the alloy, the
chromium atoms
are "free" to react with the outer oxygen on the alloy's surface and build a
thin
chromium oxide layer thereon. This is a protective layer as it is impermeable
to
oxygen and other gases and fluids of the outer environment, so as not to allow
for a
progressive corrosion process.
[0012] It is extremely important that a small distortion exists between the
network parameters of the phases y and 7', which leads to a low interface
energy (y/
y"). The main coarsening driving force of these intermetallic precipitates is
the
minimization of the total interface energy, so that one coherent or semi-
coherent low
energy interface leads to a more stable microstructure. Metallurgical
stability is a
highly recommendable property for high temperature applications.
[0013] The morphology of these precipitates is determined by the surface
energy of the y / f interface, and the elastic energy generated by phases y e
y"
CA 02688507 2009-12-16
4
lattices mismatch, being primarily determined by the lattice distortion. If it
is a small
distortion, the morphology that will minimize the surface energy and the
distortion
energy per volume will be the spherical one. However, in case the lattice
distortion is
considerably big, the morphology of the precipitates will not be spherical,
but rather
cubic. Whenever the lattice mismatch is up to 0.02 %, the 7' precipitates will
be
spherical; in case of mismatches between 0.5 and 1.0 %, these precipitates
will be
cubic; above 1.25 %, they are plate-shaped.
[0014] Niobium shows an ordered phase Ni3Nb precipitation kinetics lower
than when compared to elements such as titanium and aluminum in phases
Ni3(Ti,AI). In the Ni-Cr-Fe system superalloys, high niobium contents lead to
the
ordered phase 7" (Ni3Nb) precipitation, similar to phase 7'. Whenever added to
lower
content alloy, niobium only increases the gamma prime precipitate volume and
the
solution temperature of this phase, leading its hardening effect to even
higher
temperatures.
[0015] With a view to meet the above referred conditions, the alloys hereof
are
provided with alloy element compositions, which, in bulk percentage, consist
of:
= 15.0 to 25.0 chromium, preferably 16.0 to 24.0 chromium, typically 18.6
chromium.
= 3.0 to 10.0 for the (Nb + 2 Ti) ratio, preferably (Nb + 2Ti) between 3.0
and 7.0, typically (Nb + 2Ti) equal to 4.2; in this equation, titanium and
niobium can take any value within the limits; however, a minimum
niobium content shall be maintained, equal to 1.20%, preferably higher
than 1.8%.
= 0.02 to 0.07 carbon, preferably 0.03 to 0.06 carbon, typically 0.05%
carbon.
= 0.1 to 3.0 aluminum, preferably 0.5 to 2.5 aluminum, typically 1.85%
CA 02688507 2009-12-16
aluminum.
= Maximum 1.0 copper, preferably maximum 0.5 copper, typically
maximum 0.1 copper.
[0016] = Iron balance and inevitable metallic and non-metallic impurities
to the
melting shop process, where said non-metallic impurities include, without
limitation,
the following elements, in bulk percentage:
= Maximum 5.0 for the manganese, molybdenum and tungsten elements,
preferably maximum 2.0, typically maximum 0.50.
= Maximum 0.20 for phosphorus and sulfur, preferably maximum 0.05,
typically maximum 0.005.
[0016] Find below the reasons for the specification of the new material
composition, describing the effect of each alloy element. The indicated
percentages
relate to bulk percentages.
[0017] When defining this alloy, a crucial point was the chromium content
variation. Chromium is used to provide the alloy with high temperature
corrosion and
oxidation resistance; accordingly, its content shall be higher than 10% in
case of
exhaust valve superalloys. Contents above 25% threaten the microstructure
stability
since they tend to form phases such as sigma and alpha prime phases (a and
a'),
which deteriorate ductility. On the other hand, contents above 16% bulk show a
positive answer as regards the improvement of high temperature oxidation
resistance. Accordingly, one concludes that the alloy chromium content would
be
between such limits, preferably between 16.0% and 22.0%, typically 18.6%.
[0018] Titanium and niobium are carbide formers. Whenever they are added
to the alloy, they firstly combine with carbon, because of the high chemical
affinity
between these elements. The resulting carbides contribute to the abrasive wear
resistance. The titanium and niobium content that is non-combined with carbon
will
CA 02688507 2009-12-16
6
combine with nickel to form the y e intermetallic phases. For these two
effects, the
titanium and niobium contents shall be added to the alloy hereof according to
the Nb
+ 2 Ti ratio, which accounts for the atomic mass difference of both elements.
Thus,
in order to obtain the desired effect in the hot resistance properties, the Nb
+ 2Ti
ratio must be higher than 3.0%, typically equal to 4.2%.
[0019] Another determining fact to define this alloy was the titanium and
niobium content variation in order to define an optimum composition, within
the ratio
in question. It could be noticed that the niobium introduction in amounts
above 1.2%
causes beneficial effects as regards niobium residual content (non-combined in
the
form of carbides), and such content is crucial to improve the alloy hot
properties.
What is desired when introducing Nb in higher quantities is to cause the y
intermetallic phase precipitation (Ni3Nb) and the phase y modification through
the
introduction of a greater niobium content in its structure. A wide range for
the Nb
element is between 0.9 up to 4.0% (bulk), with an intermediate range of 1.2 up
to
3.5% (bulk) of Nb and a narrow interval of 1.5 up to 3.0% (bulk) of Nb, or
even
narrower, of 1.8 up to 2.5%.
[0020] In addition to the improved heat resistance, Nb also improves the
weldability of hardened superalloys by phase 7" precipitation; additionally,
it
improves corrosion in sulfurous environments, such as diesel engines.
[0021] Nb can be partially replaced with tantalum (Ta) on equiatomic bases.
Like Nb, Ta is also a builder of the intermetallic phase with nickel and
strongly
stabilizes primary carbides, being equally beneficial for hot hardness and
abrasion
resistance.
[0022] The increase in the niobium amount has shown effects in hot
resistance properties. Although the mechanism is not completely defined, in
the
alloys hereof the niobium content that is not combined with carbon must build
CA 02688507 2009-12-16
7
different intermetallics as compared to the titanium intermetallics, probably
the two-
line gamma type (r), which are very stable to coalescence and, accordingly,
effective in improving the high temperature resistance properties.
[0023] For a same content of (Nb + 2 Ti) ratio, niobium addition causes a
reduction in the alloy's total titanium percentage. The studies hereof showed
that
such reduction is also beneficial to improve the high temperature oxidation
resistance ¨ an also essential property in high temperature working valves.
[0024] The reduced total titanium percentage in the alloy by the niobium
addition in quantities higher than 1.2% improves its hot workability, since
the alloy's
hot ductility is threatened to values above 4.0% for the sum of titanium and
aluminum contents; desired (Ti + Al) < 4.0%.
[0025] For all such effects ¨ hot resistance and oxidation resistance ¨ the
(Nb
+ 2 Ti) ratio must show a minimum 1.2% niobium content, preferably niobium
above
1.5%, with an optimum niobium content equal to or higher than 1.8%.
[0026] In spite of niobium and titanium beneficial aspects, the content of
such
elements cannot be excessively high, since it would cause the formation of
coarse
intermetallics, thus jeopardizing the mechanical properties of the alloy in
terms of
mechanical resistance and ductility, in addition to increasing the alloy cost.
Accordingly, the value of the (Nb + 2 Ti) ratio must be below 8.0%, preferably
below
7.0%.
[0027] Carbon is added with the intent of combining titanium and niobium in
order to form carbides, which precipitate in the grain contours and improve
the
alloy's creep resistance, since they hinder the deformation mechanism by
"grain
contour sliding". For that function, the carbide content shall be at least
0.03%,
preferably above 0.03% and below 0.06%, preferably 0.05%.
[0028] Aluminum is very important for the gamma line (y) phase
precipitation,
CA 02688507 2009-12-16
8
and therefore for high temperature resistance. Another extremely important
function
of aluminum in the alloy is to increase high temperature oxidation resistance
by
increasing the formation of A1203 during the heating phase. Nevertheless,
aluminum
contents must be restricted, as very high quantities thereof can lead to
deterioration
of high temperature resistance and hot workability because of nitrite
formation and
such phases as T1 and 8 for long heating periods. Therefore, the aluminum
content
shall be between 0.5% and 4.0%, preferably between 1.0% and 3.0%, typically
equal
to 2.0%.
[0029] Copper must be controlled in low contents, as this element is
harmful
for some properties, mainly the high temperature oxidation, which is the great
improvement obtained by the alloys hereof. Additionally, no nickel alloy or
superalloy
uses copper and, once such element is added, it cannot be removed by the
melting
shop processes. This causes the intemal scrap, namely, the returns of the
production process, not to be used in other alloys' process, thus considerably
increasing the production cost or even rendering unfeasible the alloy
production.
Accordingly, in the alloys hereof the copper content must be below 1.0%,
preferably
below 0.5% and typically below 0.1%. As shown in Table 1 of the Example, US
patent 5,951,789 has a high copper content, and this is its main disadvantage
as
compared to the alloys hereof, because of such reasons of hot oxidation and
scrap
contamination.
[0030] Residual: Other elements such as manganese, tungsten, molybdenum,
sulfur, phosphorus and those usually obtained as regular residual elements in
the
preparation process of steel or liquid nickel alloys, shall be understood as
impurities
in connection with the melting shop deoxidization processes or inherent to the
manufacturing processes. Therefore, the manganese, tungsten and molybdenum
content is reduced to 5%, preferably below 2.0%, because of the ratio
destablization
CA 02688507 2013-09-09
9
between the austenite and ferrite phases, and because of any effects in the
intermetallic phases present in the alloy. Phosphorus and sulphur segregate in
grain
boundaries and other interfaces, and therefore they shall be below 0.20%,
preferably below 0.05%, preferably maximum 0.005%.
[0030a] In
accordance with one aspect then, there is provided an alloy for
internal combustion engine valves, components, tools, and structural, static
or
dynamic parts, for use in applications which demand resistance at high
temperatures, resistance to creep, and resistance to abrasion; presenting a
chemical composition of elements comprising, C, Mn, Si, Cr, Ni, Mo, W, V, Al,
Ti,
Nb, B, Zr, Cu, Co, Fe and impurities, wherein the ratio M = (Nb+2Ti) is
greater than
3.0% and lower than 11.0% by mass, presenting the chemical composition of
elements comprising, in percentage by mass,
0.01 to 0.10% C,
0.05 to 1.0% Mn,
0.05 to 1.0% Si,
18.5 to 25.0% Cr,
25.0 to 39.0% Ni,
0.01 to 1.0% Mo,
0.01 to 1.0% W,
0.01 to 1.0% V,
1.7 to 3.0% Al,
0.5 to 2.5% Ti,
2.1 to 3.2% Nb,
0.001 to 0.02% B,
0.001 to 0.1% Zr,
0.01 to 0.5% Cu,
0.01 to 2.0% Co,
the balance including Fe and impurities, wherein the minimum Fe
content is 30%,
where the sum of Co and Ni mass percent is between 25.0% and
39.0%, and the ratio of percentages in mass Ti:Al is between 0.50 and 1.3.
CA 02688507 2012-01-17
9a
[0031] The described alloy can be made by conventional or special
processes such as melting in electric or vacuum furnaces, followed by re-
melting
processes or not. Casting can be made in ingots by means of conventional or
continuous casting, or even by other manufacturing processes involving
disaggregation of the liquid metal and further aggregation, such as power
metallurgy
and the spray forming or continuous casting process. The end products can be
obtained after hot or cold forming, and end products are produced in the form
of
wire rods, blocks, bars, wires, sheets, strips, or can be even products in the
as cast
state.
Brief Description of the Drawings
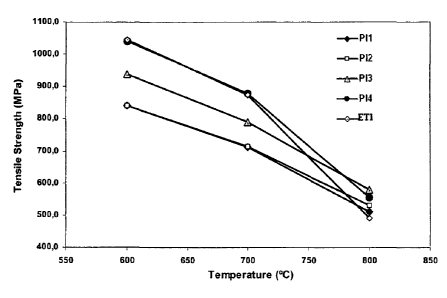
[0032] Figure 1 compares the heat resistance of the alloys hereof (PI) to
the
state-of-the-art alloys ET1 and ET2 as of the resistance limit thereof for
several
temperatures;
Figure 2 shows the test oxidation resistance results; and
Figure 3 shows the oxidation resistance results as related to the Ti/AI
ratio.
Detailed Description of the Invention
[0033] Figure 1 depicts the heat resistance of the alloys compared to
other
known alloys. Figure 2 shows the test oxidation resistance results. In such
test,
cylindrical test specimens (diameter=12 mm, height=14 mm), as solubilized and
aged, were duly weighed and kept at an 800 C temperature for 100 hours. After
being removed from the furnace, the test specimen is air cooled and weighed
again,
and the mass variation is measured. The bigger the mass gain, the lower the
oxidation resistance. Such process is repeated until the total test time is
completed.
Ceramic alumina crucibles were used as sample holders during the test. The
oxidation process at 800 C was reviewed for 400 hours, when it was possible to
= CA 02688507 2012-01-17
notice a stabilization of the corrosion process. The test was carried out so
that all
samples of all alloys involved had identical sizes, for them also to have an
identical
contact surface. Figure 3 shows the oxidation resistance results as related to
the
Ti/AI ratio. The oxidation resistance calculation is made by inverting the
mass
variation after 200 hours at 800 C (g-1).
[0034] In order to define the compositions of the alloys hereof,
several alloys
were made and compared to the state-of-the-art alloys. The chemical
compositions
are shown in Table 1. The alloys hereof are hereinafter called PI, and the
state-of-the-art alloys are hereinafter called ET. ET1 alloy corresponds to
UNS N07751, and ET2 alloy corresponds to NCF 3015 alloy (of U.S. Pat. No.
5,660,938). The following ratios are also quantified: (Nb + 2 Ti) and (Ti/AI)
in
Table 1.
[0035] In Table 1 one can notice a significant reduction in the
nickel content
of the alloy in the compositions hereof as regards ET1 alloy, thus resulting
in
considerably lower cost. Table 1 also shows the addition of different niobium
contents to the alloys hereof, and aluminum and titanium contents.
[0036] Table-1: Chemical compositions of two alloys of the state of
the art
(ET1 and ET2) and the alloys of the present invention (P11 to P14). Percentage
in
mass and balance in iron.
= CA 02688507 2012-01-17
11
C Si Mn Cr Ni Al Ti Nb
Cu Nb + 2Ti/AI
Ti
ET1
(UNS NO7751) 0.05 0.03 0.05 15.5 70 1.2 2.45 0.9
0.01 5.5 1.92
ET2
(NCF 3015) 0.04 0.03 0.05 16.0 32 1.4 2.50
0.65 0.01 5.65 1.79
ET3
(US 5,951,789) 0.03 0.21 0.21 16.0 32.3 1.43
2.53 0.82 2.05 3.35 1.77
P11
0.05 0.1 0.16 18.2 35.2 1.87 2.1 0.89 0.01 5.09 1.12
P12
0.05 0.1 0.15 21.5 35.9 1.85 2.1 0.90 0.01 5.1 1.14
P13
0.05 0.1 0.15 18.6 35.6 1.83 1.14 1.92 0.01 4.2 0.62
P14
0.06 0.1 0.15 19.1 36.8 1.82 2.08 1.93 0.01 6.09 1.14
[0037]
This new alloy project showed to be of great benefit to the end
properties, as discussed below, when comparing the alloys of the present
invention
with alloys ET1 and ET2. As for alloy ET3, the alloys of the present invention
have
an important advantage--they reaching higher mechanical and corrosion
properties,
without the need of high contents of copper. As discussed above, copper is an
important contaminant of nickel alloy scrap, because copper cannot be removed
through the process of scrap remelting and, thus, returns of materials with a
high
content of copper cannot be used to prepare conventional alloys, which have
limits
to the maximum content of copper. Besides, copper tends to be damaging to
corrosion properties at high temperature.
[0038]
The differences between the titanium and aluminum contents in the
different alloys can be evaluated through the ratio (Ti/AI), which is very
important as
for the alloy properties of resistance to hot oxidation and workability. Such
ratio
(Ti/AI) is displayed in Table 1 as well.
[0039]
The ingots were cast by means of a close procedure for such six
alloys (ET1, ET2, P11, P12, PI3 and P14), in a vacuum induction furnace. The
casting
was made into cast iron moulds, producing an ingot of about 55 kg. After the
solidification, the ingots were forged and rolled for round gauges with
diameter of
18 mm. In addition to these alloys, the typical composition of U.S. Pat. No.
5,951,789 is also displayed for comparison purposes (called ET3).
i
. CA 02688507 2012-01-17
12
[0040] Table 2 displays the hardness of alloys ETI , ET2, P11, P12,
P13, and
PI4 after solution at 1050 C and aging at 750 C for 1 hour and, also after
solution
at 1050 C and aging for 4 hours. These data show equivalent values as for the
hardness of aged alloys, except for alloy ET3, which has lower hardness. The
alloys
with niobium have lower hardness in the solution state, what is interesting to
machine the material in this condition.
[0041] Table 2: Response to the heat treatment of the alloys of the
state of
the art (ETI , and ET2), and the alloys of the present invention (P11, P12,
P13, and
P14). Results of hardness in HB after solution at 1050 C and aging at 750 C
for 1
hour and 4 hours.
Solution Aging Aging
(750 C - 1h) (750 C - 4h)
ETI 254 330 330
ET2 250 335 335
P11 307 333 357
PI2 323 343 359
PI3 276 327 346
P14 319 352 374
[0042] Another important parameter for these alloys are the
mechanical
properties at high temperature, as displayed in Figure 1. The alloys of the
present
invention, despite having nickel content substantially lower than alloy ETI ,
are more
resistant at high temperature than alloy ETI . The values of the limit of
traction
resistance (FIG. 1), for instance, show that all the alloys of the present
invention,
P11, P12, P13, and P14, are more resistant than alloy ETI for 800 C
temperature.
Alloy PI4 is highlighted, seen as equivalent to or even more resistant than
alloy
UNS N07751 (ET1) in all temperatures tested.
I
CA 02688507 2009-12-16
13
[0043] In terms of oxidation resistance, the alloys of the present
invention are
also superior to alloy ET1, as shown in Table 3 and in Figure 2; we see that
the
higher the content of chromium and aluminum, the lower the content of
titanium, and
the higher the resistance to the oxidation. This is the best resistance seen
for alloy
P13. This occurs for two reasons. Firstly, because a higher content of
chromium and
aluminum provides a larger and quicker formation of the passive film made of
chromium oxide or aluminum oxide on the surface of the material. Secondly, the
effect caused by titanium, i.e., the destabilization of the oxide layer formed
on the
surface of the alloys in the iron-nickel-chromium system and, thus, the
decrease in
the oxidation resistance of alloys with a higher content of such element. For
instance, it is interesting to notice that, among the alloys with high content
of
chromium (P11, P12, P13, and P14), the one with the lowest titanium content
(PI3) has
the highest hot oxidation resistance in the tested conditions.
[0044]Table 3: Gain in mass (in mg/cm2) after 100, 300 and 400 hours in
atmosphere air (800 C). The lower the gain in mass, the higher the resistance
to the
oxidation of the material.
100 hours 300 hours 400 hours
ET1 (Ti = 2.5%; Cr = 15.5%) 0.40 0.54 0.54
PI1 (Ti = 3.0%; Cr = 18.2%) 0.00 0.49 0.49
PI2 (Ti = 2.0%; Cr = 21.5%) 0.00 0.44 0.44
PI3 (Ti = 1.1%; Cr = 18.6%) 0.00 0.30 0.30
PI4 (Ti = 2.1%; Cr = 19.1%) 0.00 0.49 0.49
[0045] The property of resistance to hot =oxidation can be examined in
accordance with ratio (Ti/AI) as well. Figure 3 shows this analysis concerning
the
alloys of the present invention (P11 to P14) and of the state of the art
(ET1). In these
results, we can see clearly that the alloys of the present invention are in
the optimum
range of the ratio Ti/AI for the optimization of the property of hot oxidation
resistance,
represented by a reversal of the gain in mass (in mg/cm2) after 400 hours at
800 C
CA 02688507 2009-12-16
14
in atmosphere (air).
[0046] Therefore, the comparison between the alloys of the state of the art
and the alloys of the present invention showed that the introduction of higher
contents of niobium and aluminum, together with the contents of titanium,
cause
improvements in the hot resistance properties, creep resistance, and
resistance to
oxidation. A summary of such effects is displayed in Table 4. Alloys P12, P13,
and
PI4 are always superior to the alloys of the state of the art, in terms of all
properties
examined. Alloys PI3 and PI4 are highlighted, since they have the best
results.
[0047] In summary, we can state that the results discussed herein show that
the alloys of the present invention, in addition to the economic advantage of
working
with a lower content of nickel, have better properties as well. As for the
alloys of the
state of the art, the alloys of the present invention have higher levels of
properties at
high temperature. So, they are material improvements for industrial
application in
combustion engine valves or even other components used at high temperatures
and
corrosive sites. Besides, they can be produced with no scrap contamination
problems, since they use a low content of copper.
[0048]Table 4: Comparison of Properties among all alloys studied, in absolute
figures and relative figures (the reference is alloy ET1 = 10V/0).
CA 02688507 2009-12-16
ET1 ET2 PI1 PI2 PI3 PI4
Hardness After Aging Treatment (HB) 335 300 357 359 346 '374
Rupture Strength at 800 C (MPa) 490 490 512 530 5791 555
Oxidation Resistance (Inverse of weight 1.9 _ 2.1 2.3 2.1 3:3
variation) after 200 hours at 800 C (g-1)
In relation to ET1 (ET1 = 100)
ET1 ET2 PI1 P12i P13 PI4
Hardness After Aging Treatment 100 90 106 1075 10311 112
Rupture Strength at 800 C 100 100 104 108, 118: 113
Oxidation Resistance (Inverse of Weight 100 - 111 121 111 174
Variation) after 200 hours at 800 C
[0049] While the invention has been described with reference to preferred
embodiments, variations and modifications would be apparent to one of ordinary
skill
in the art. The invention encompasses such variations and modifications.