Note: Descriptions are shown in the official language in which they were submitted.
CA 02688610 2009-12-11
System and Process for Converting Non-Fresh Water to Fresh
Water or Steam
FIELD OF THE INVENTION
[001] The present invention relates to the conversion of non-fresh water to
fresh water or
steam.
BACKGROUND
[002] Water is one of the most vital natural resources for all life on Earth.
The availability
and quality of water has always played an important part in determining not
only where
people can live, but also their quality of life. Domestic use includes water
that is used in the
home every day such as for drinking, food preparation, bathing, washing
clothes and dishes,
flushing toilets, and watering lawns and gardens. Commercial water use
includes fresh
water for motels, hotels, restaurants, office buildings, other commercial
facilities, and civilian
and military institutions. Industrial water use is a valuable resource to a
nation's industries
for such purposes as processing, cleaning, transportation, dilution, and
cooling in
manufacturing facilities. Major water-using industries include steel,
chemical, paper, and
petroleum refining. Water is used in the production of electricity in
thermoelectric power
plants that are fueled by fossil fuels, nuclear fission, or geothermal.
Irrigation water use is
water artificially applied to farm, orchard, pasture, and horticultural crops,
as well as water
used to irrigate pastures, for frost and freeze protection, chemical
application, crop cooling,
and harvesting. Livestock water use includes water for stock animals, feed
lots, dairies, fish
farms, and other nonfarm needs. Water is needed for the production of red
meat, poultry,
eggs, milk, and wool, and for horses, rabbits, and pets.
1
CA 02688610 2009-12-11
[003] The planet's water reserves are estimated at 1,304,100 teratons (1
teraton is 1012
tons) of which freshwater reserves only account for 2.82% of this figure.
Agriculture
consumes 70% of the world's freshwater, industry 20% and households 10%.
Between
1900 and 1995, drinking water demand grew twice as fast as the world
population. By
2025, this demand should grow another 40%. In fifty years, the Canadian Agency
for
International Development has predicted that some forty countries could lack
adequate
drinking water. This will inevitably lead to conflict, even wars, as local
areas, provinces and
countries will go to any length to defend their fresh water resources.
[004] Almost all conventional power plants, including coal, oil, natural gas,
and nuclear
facilities, employ water cycles in the generation of electricity. Recently
available data from
the U.S. Geologic Survey shows that thermoelectric power plants, in the
U.S.A., use more
than 195 billion gallons of water per day. Such immense water needs produce
equally
immense concerns given the likelihood of future droughts and shortages,
especially during
the summer months. The addition of new conventional power plants therefore,
has inherent
water-related risks that may result in electric utilities no longer able to
construct them.
[005] In Canada, there are vast oil sand resources estimate at 1.7 trillion
barrels
(270X109 m) of bitumen. Water is required to convert bitumen into synthetic
crude oil. A
recent report by the Pembina Institute shows that it requires about 2-4.5 m3
of water to
produce one cubic metre (m) of synthetic crude. The need for industrial water
use will
increase with population growth and global warming as the demand for fuel and
electricity
increases.
2
CA 02688610 2009-12-11
[006] According to recent numbers by UNICEF and the World Health Organization,
there
are an estimated 884 million people without adequate drinking water, and a
correlating 2.5
billion without adequate water for sanitation (e.g. wastewater disposal).
Also, cross-
contamination of drinking water by untreated sewage is the chief adverse
outcome of
inadequate safe water supply. Consequently, disease and significant deaths
arise from
people using contaminated water supplies; these effects are particularly
pronounced for
children in underdeveloped countries, where 3900 children per day die of
diarrhea alone.
The greatest irony is that 97% of the water exists as seawater which is unfit
for human
consumption. Consequently, as the world population grows it is increasingly
important to
find ways to produce fresh water such as by converting non-fresh water and in
particular
seawater, waste water, brackish water and polluted waters to fresh water.
"Fresh water" as
used herein is potable water.
[007] Seawater contains about 3% salts and minerals, with 97% of the seawater
being
water. Brackish water contains more than 500 ppm of salts but less than sea
water, which
has between 34,000 to 36,000 ppm of salt. Desalination refers to any of
several processes
that convert seawater to fresh water. Sometimes the process produces table
salt as a by-
product. It is also used on many seagoing ships and submarines.
3
CA 02688610 2009-12-11
DESCRIPTION OF PRIOR ART
[008] . The two most popular desalination technologies are Multi Stage Flash
Distillation
(MSF) and Reverse Osmosis (RO), or some variations of them, which account for
about
90% of the technologies that desalinate seawater across the globe. Most
desalination plants
convert only about 30% - 60% of the seawater to fresh water.
[009] Multi-stage flash distillation ("MSF") is a desalination process that
distills sea water by
flashing a portion of the water into steam in multiple stages of what are
essentially
regenerative heat exchangers. Seawater is first heated in a container known as
a brine
heater. This is usually achieved by condensing steam on a bank of tubes
carrying sea water
through the brine heater. Heated water is passed to another container known as
a "stage",
where the surrounding pressure is lower than that in the brine heater. It is
the sudden
introduction of this water into a lower pressure "stage" that causes it to
boil so rapidly as to
flash into steam. As a rule, only a small percentage of this water is
converted into steam.
Consequently, it is normally the case that the remaining water will be sent
through a series
of additional stages, each possessing a lower ambient pressure than the
previous "stage."
As steam is generated, it is condensed on tubes of heat exchangers that run
through each
stage. MSF distillation plants, especially large ones, are paired with power
plants in a
cogeneration configuration where the waste heat from the power plant is used
to heat the
seawater rather than generate electricity or be used in an industrial/chemical
process. The
power plants consume large amounts of fossil fuels thereby contributing
significantly to
global warming. The world's largest MSF desalination plant is the Jebel Ali
Desalination
Plant located in the United Arab Emirates and is capable of producing 820,000
cubic meters
(215 million gallons/day) of fresh water per day.
4
CA 02688610 2009-12-11
[010] Reverse Osmosis ("RO") is a filtration process typically used for water.
It works by
using pressure to force a solution through a membrane, retaining the solute on
one side and
allowing the pure solvent to pass to the other side. This is the reverse of
the normal
osmosis process, which is the natural movement of solvent from an area of low
solute
concentration, through a membrane, to an area of high solute concentration
when no
external pressure is applied. The largest Sea Water Reverse Osmosis (SWRO)
installation
is built in Ashkelon, Israel capable of producing 320,000 cubic meters of
fresh water per
day. The Ashkelon plant has a dedicated 80 MW gas turbine to supply the
required
electrical need. The Tampa Bay plant (the largest in North America) takes 44
million gallons
of seawater and converts it to 25 million gallons (95,000 cubic meters) of
fresh water every
day (a 56.8% conversion rate).
[011] Electrolysis of water is the decomposition of water (H2O) into oxygen
(02) gas and
hydrogen (H2) gas due to an electric current being passed through the water.
An electrical
power source is connected to two electrodes, or two plates, (typically made
from some inert
metal such as platinum or stainless steel) which are placed in the water.
Hydrogen will
appear at the cathode (the negatively charged electrode, where electrons are
pumped into
the water), and oxygen will appear at the anode (the positively charged
electrode). The
generated amount of hydrogen is twice the amount of oxygen, and both are
proportional to
the total electrical charge that was sent through the water. Electrolysis of
pure water is very
slow, and can only occur due to the self-ionization of water. Pure water has
an electrical
conductivity about one millionth that of seawater. It is sped up dramatically
by adding an
electrolyte (such as a salt, an acid or a base). Electrolysis at normal
conditions (25 C and 1
atm) is completely impractical for electrolyzing water for anything but a
small lab experiment.
CA 02688610 2009-12-11
[012] High-temperature electrolysis ("HTE"), also known as steam electrolysis,
is the same
concept as electrolysis except that it occurs at high temperatures. High
temperature
electrolysis is more efficient economically than traditional room-temperature
electrolysis
because some of the energy is supplied as heat, which commercially is
generally less
expensive to supply than electricity, and because the electrolysis reaction is
more efficient at
higher temperatures.
[013] As we go to higher temperatures, the energy necessary for electrolysis
comes from
heat (thermal energy) rather than electricity. It is known that at around 1000
C, about 70%
of the energy requirement comes from electricity and about 30% can come from
heat. This
increases the efficiency and reduces the cost significantly.
[014] Thermal decomposition, also called thermolysis, is defined as a chemical
reaction
when a chemical substance breaks up into at least two chemical substances when
heated.
The reaction is usually endothermic as heat is required to break chemical
bonds in the
compound undergoing decomposition. The decomposition temperature of a
substance is
the temperature at which the substance decomposes into smaller substances or
into its
constituent atoms. As explained previously, water will decompose to its
elements at
temperatures around 3200 C at 1 atm. In this case the entire required energy
for hydrogen
and oxygen production is completely provided by heat and no electricity is
necessary.
[015] As discussed above, fresh water scarcity is a growing problem in many
parts of the
world. However, in parts of the world where fresh water is more abundant, the
fresh water
supply can also be threatened, not by scarcity, but rather by contamination.
For example,
an investigation by the Associated Press has revealed that the drinking water
of at least 41
6
CA 02688610 2009-12-11
million people in the United States is contaminated with pharmaceutical drugs.
It has long
been known that drugs are not wholly absorbed or broken down by the human
body.
Significant amounts of any medication taken eventually pass out of the body,
primarily
through the urine. While sewage is treated before being released back into the
environment
and water from reservoirs or rivers is also treated before being funneled back
into the
drinking water supply, none of these treatments are able to remove all traces
of
medications.
[016] Medications for animals are also contaminating the water supply. Drugs
given to
animals are also entering the water supply. One study found that 10 percent of
the steroids
given to cattle pass directly through their bodies. Another study found that
steroid
concentrations in the water downstream of a Nebraska feedlot were four times
as high as
the water upstream. Male fish downstream of the feedlot were found to have
depressed
levels of testosterone and smaller than normal heads, most likely due to the
pharmaceutical
contamination in their water.
[017] In most modern cities, rivers and lakes, within their vicinity have
become the focal
point of business, resulting in heavy development and commercialization of
these primary
natural resources. The Seine River in Paris, the Singapore River in the Lion
City, the Chao
Phraya in Bangkok and the Thames in London, to name just a few famous ones,
have all
been turned into tourist destinations with massive commercial development
around them. In
all these cities, businesses flourish along their river corridors and the
aesthetic values the
rivers offer to the city denizens such as scenic beauty, solitude, natural
environment cannot
be described with words but need to be experienced. But, there is a heavy
price to pay for
the massive economic development and the booming commercial activities along
these
7
CA 02688610 2009-12-11
rivers and within their vicinity. These rivers are slowly being killed by the
unrestrained
development which is often accompanied by massive pollution and other
ecological
damage.
[018] Conventional desalination methods (most notably Multi-Stage Flashing and
Reverse
Osmosis) can help to close the gap between the supply and demand of fresh
water.
However, these desalination methods require a lot of capital expenditures and
consume an
enormous amount of fossil fuels. The sad reality is that the countries that
need the fresh
water most are the developing countries (and in many cases the poorest
countries) who do
not have the required capital and can not afford to purchase the enormous
annual amount
of fossil fuel that is required to operate these plants.
[019] In the last decade, there has been much discussion about using nuclear
energy to
provide the required energy for the desalination plants. While nuclear plants
may offer
some solutions, they also create many other problems. Nuclear plants require
significant
capital, take a long time to be put in place (permitting, construction etc.)
and require the
availability of highly trained staff to run the plants. Unfortunately, this
option will not be
available to most developing countries and in particular the poorest
countries. In the world
of instability, the last thing that the world need is the proliferation of
nuclear plants that may
lead to a nuclear race in many unstable regions of the world. Moreover, it is
impractical to
have a nuclear plant in every province much less in every village where fresh
water is often
needed most.
[020] Produced water is a term used in the oil and gas industry to describe
water that is
produced along with oil and gas obtained from a well. To achieve increased oil
recovery
8
CA 02688610 2009-12-11
additional water is often injected into the reservoirs to help force the oil
to the surface. Both
the formation water and the injected water are eventually produced along with
the oil and
therefore as the field becomes depleted the produced water content of the oil
increases.
Produced water is often used as an injection fluid. This reduces the potential
of causing
formation damage due to incompatible fluids, although the risk of scaling or
corrosion in
injection flowlines or tubing remains. Also, the produced water, being
contaminated with
hydrocarbons and solids, must be disposed of in some manner, and disposal to
sea or river
requires a certain level of clean-up of the water stream first. However, the
processing
required to render produced water fit for reinjection may be equally costly.
As the volumes
of water being produced are never sufficient to replace all the production
volumes (oil & gas,
in addition to water), additional "make-up" water must be provided. Mixing
waters from
different sources exacerbates the risk of scaling. Consequently, the
acquisition of fresh
water and the disposal of produced water are significant cost in oil and gas
production.
[021] The technique of hydraulic fracturing is used to increase or restore the
rate at which
fluids, such as oil, gas or water, can be produced from the desired formation.
The method
is informally called fracking or hydro-fracking. By creating fractures, the
reservoir surface
area exposed to the borehole is increased. The fracture fluid can be any
number of fluids,
ranging from water to gels, foams, nitrogen, carbon dioxide or even air in
some cases. The
fracture, which is kept open using a proppant such as sand or ceramic beads,
provides a
conductive path connecting a larger area of the reservoir to the well, thereby
increasing the
area from which fluids can be produced from the desired formation. The
produced water
(called flowback water) is contaminated and must be treated prior to disposal.
In many
instances flowback water is trucked away to be treated elsewhere.
Consequently, the
9
CA 02688610 2009-12-11
acquisition of fresh water and the disposal of the flowback water are
significant cost of
production.
[022] Bituminous sands (tar sands) are a major source of unconventional oil.
The extra-
heavy oil and bitumen flow very slowly, if at all, toward producing wells
under normal
reservoir conditions. The sands must be extracted by strip mining or the oil
made to flow
into wells by in situ techniques which reduce the viscosity by injecting
steam, solvents,
and/or hot air into the sands. These processes use vast amounts of fresh water
and require
larger amounts of energy to produce the vast amounts of steam that is used in
the extraction
operation. Between 2 to 4.5 volume units of water are used to produce each
volume unit of
synthetic crude oil. Despite recycling, almost all of the water used in the
extraction ends up
in tailings ponds. Consequently, the acquisition of fresh water and the
disposal of produced
water are a significant cost of production.
[023] Industrial water pollution occurs across all industries. To illustrate
these sources of
water pollution consider the following few examples. The production of iron
from ore
involves powerful reduction reactions in blast furnaces. Cooling waters are
inevitably
contaminated with products especially ammonia and cyanide. Production of coke
from coal
in coking plants also requires water cooling and the use of water in by-
products separation.
Contamination of waste streams includes gasification products such as benzene,
naphthalene, cyanide, ammonia, phenols, cresols and other chemicals. The
conversion of
iron or steel into sheet, wire or rods requires hot and cold mechanical
transformation stages
frequently employing water as a lubricant and coolant. Contaminants include
hydraulic oils,
tallow and particulate solids. Final treatment of iron and steel products
before onward sale
into manufacturing includes pickling in strong mineral acid to remove rust and
prepare the
CA 02688610 2009-12-11
surface for tin or chromium plating or for other surface treatments such as
galvanizations or
painting. The two acids commonly used are hydrochloric acid and sulfuric acid.
Wastewaters
include acidic rinse waters together with waste acid. Although many plants
operate acid
recovery plants, (particularly those using Hydrochloric acid), where the
mineral acid is boiled
away from the iron salts, there remains a large volume of highly acid ferrous
sulfate or
ferrous chloride to be disposed of. The principal water pollution associated
with mines and
quarries are slurries of rock particles in water. These arise from rainfall
washing exposed
surfaces and haul roads and also from rock washing and grading processes.
Volumes of
water can be very high, especially rainfall related arisings on large sites.
Some specialized
separation operations, such as coal washing to separate coal from native rock
using density
gradients, can produce wastewater contaminated by fine particulate hematite
and
surfactants. Oils and hydraulic oils are also common contaminants. Polluted
water from
metal mines and ore recovery plants are inevitably contaminated by the
minerals present in
the native rock formations. Following crushing and extraction of the desirable
materials,
undesirable materials may become contaminated in the wastewater. For metal
mines, this
can include unwanted metals such as zinc and other materials such as arsenic.
Extraction of
high value metals such as gold and silver may generate slimes containing very
fine particles
in where physical removal of contaminants becomes particularly difficult.
Consequently, the
acquisition of fresh water and the disposal of polluted water are a
significant cost of
production.
[024] A range of industries manufacture or use complex organic chemicals.
These include
pesticides, pharmaceuticals, paints and dyes, petro-chemicals, detergents,
plastics, paper
pollution, etc. Waste waters can be contaminated by feed-stock materials, by-
products,
11
CA 02688610 2009-12-11
product material in soluble or particulate form, washing and cleaning agents,
solvents and
added value products such as plasticizers. Consequently, the acquisition of
fresh water and
the disposal of produced water are significant cost of production across all
industries.
[025] Oil wastes that enter the ocean come from many sources, some being
accidental
spills or leaks, and some being the results of chronic and careless habits in
the use of oil
and oil products. Most waste oil in the ocean consists of oily stormwater
drainage from cities
and farms, untreated waste disposal from factories and industrial facilities,
and unregulated
recreational boating. It is estimated that approximately 706 million gallons
of waste oil enter
the ocean every year, with over half coming from land drainage and waste
disposal; for
example, from the improper disposal of used motor oil. Offshore drilling and
production
operations and spills or leaks from ships or tankers typically contribute less
than 8 percent of
the total. The remainder comes from routine maintenance of ships (nearly 20
percent),
hydrocarbon particles from onshore air pollution (about 13 percent), and
natural seepage
from the seafloor (over 8 percent). When oil is spilled in the ocean, it
initially spreads in the
water (primarily on the surface), depending on its relative density and
composition. The oil
slick formed may remain cohesive, or may break up in the case of rough seas.
Waves,
water currents, and wind force the oil slick to drift over large areas,
impacting the open
ocean, coastal areas, and marine and terrestrial habitats in the path of the
drift. The largest
accidental oil spill on record (Persian Gulf, 1991) put 240 million gallons of
oil into the ocean
near Kuwait and Saudi Arabia when several tankers, port facilities, and
storage tanks were
destroyed during war operations. The blowout of the Ixtoc I exploratory well
offshore Mexico
in 1979, the second largest accidental oil spill, gushed 140 million gallons
of oil into the Gulf
of Mexico. By comparison, the wreck of the Exxon Valdez tanker in 1989 spilled
11 million
12
CA 02688610 2009-12-11
gallons of oil into Prince William Sound offshore Alaska, and ranks fifty-
third on the list of oil
spills involving more than 10 million gallons. Oil spills present the
potential for enormous
harm to deep ocean and coastal fishing and fisheries. The immediate effects of
toxic and
smothering oil waste may be mass mortality and contamination of fish and other
food
species, but long-term ecological effects may be worse. Oil waste poisons the
sensitive
marine and coastal organic substrate, interrupting the food chain on which
fish and sea
creatures depend, and on which their reproductive success is based. Commercial
fishing
enterprises may be affected permanently. The techniques used to clean up an
oil spill
depend on oil characteristics and the type of environment involved; for
example, open
ocean, coastal, or wetland. Pollution-control measures include containment and
removal of
the oil (either by skimming, filtering, or in situ combustion), dispersing it
into smaller droplets
to limit immediate superficial and wildlife damage, biodegradation (either
natural or
assisted), and normal weathering processes. Individuals of large-sized
wildlife species are
sometimes rescued and cleaned, but micro-sized species are usually ignored.
The costs of
an oil spill are both quantitative and qualitative. Quantitative costs include
loss of the oil,
repair of physical facilities, payment for cleaning up the spill and
remediating the
environment, penalties assessed by regulatory agencies, and money paid in
insurance and
legal claims. Qualitative costs of an oil spill include the loss of pristine
habitat and
communities, as well as unknown wildlife and human health effects from
exposure to water
and soil pollution.
SUMMARY OF THE INVENTION
[026] In one aspect, the present invention relates to a method comprising the
steps of (a)
subjecting steam to high temperature electrolysis whereby hydrogen gas and
oxygen gas
13
CA 02688610 2009-12-11
are produced and separated; (b) combusting the hydrogen gas and the oxygen gas
to
produce superheated steam; and; c) collecting superheated steam produced by
the
combustion in step (b). The steam can also include other gases besides water
vapour.
[027] .In another aspect, the present invention relates to a system comprising
a high
temperature electrolysis unit for producing and separating hydrogen and oxygen
gas from
steam and/or steam containing other gases besides water vapour; a combustor
combusting
hydrogen and oxygen to produce superheated steam; a collector connected to the
combustor for collecting superheated steam produced by the combustor.
[028] In one aspect, the present invention relates to using high temperature
electrolysis to
dissociate steam to hydrogen and oxygen and to separate the non-water
material, and then
combusting the generated hydrogen and oxygen at elevated pressure to form high
pressure
high temperature superheated steam wherein a closed loop heat recovery system
is utilized
to recycle the heat generated by the combustion process to the high
temperature
electrolysis unit for the dissociation of steam. The extraction of heat from
superheated
steam by a heat recovery system can be used to condense the superheated steam
to
produce fresh water. Methods embodying the principles of the present invention
have been
given the name of "The Rosenbaum-Weisz Process" by the inventor. The reference
to
Rosenbaum and Weisz is in honour of the inventor's parents.
[029] In another aspect, the present invention relates to the Rosenbaum-Weisz
Process
which utilizes high temperature electrolysis of non-fresh water to produce
fresh water. The
required heat for high temperature electrolysis is obtained by capturing and
utilizing heat
that is generated by the combustion of hydrogen and oxygen. When hydrogen and
oxygen
14
CA 02688610 2009-12-11
are combusted, the resulting product is heat and superheated steam. The
combustion
temperature is around 3200 C at 1 atm (same as thermolysis). The heat
generated by the
combustion of hydrogen and oxygen is extracted by a heat exchanger system and
recycled
to be used in the high temperature electrolysis process. The extraction of the
heat by the
heat exchanger system condenses the superheated steam into fresh water. The
overall
process includes the following steps: non-fresh water treatment; evaporation
of the treated
non-fresh water, high temperature electrolysis; hydrogen and oxygen
production; hydrogen
and oxygen storage; combustion of hydrogen and oxygen; heat exchanger recovery
system;
and the condensing of the superheated steam into fresh water.
[030] The heat for the high temperature electrolysis can come from different
sources. One
way to create on-site heat is by burning fossil fuels such as natural gas to
produce the
required heat. Another way is to capture waste heat from a nearby cogeneration
plant. The
typical temperature of the waste heat from a cogeneration plant is between 800
C and
1000 C. Yet another way is to locate a HTE facility near a nuclear plant
thereby utilizing the
heat from the nuclear plant. For HTE occurring at around 1500 C, the energy
contribution
can be approximately 50% from the electrical input and 50% from the heat and
at around
2000 C, the energy contribution can be approximately 25% from the electrical
input and
75% from the heat. At even higher temperatures, thermal decomposition occurs.
It will be
understood by persons of ordinary skill in the art that the ratio of
electricity to thermal energy
used as input energy for the HTE process can be varied according to the
conditions under
which the HTE operates. In general, if more heat energy is used, less
electricity is required
and vice versa.
CA 02688610 2009-12-11
[031] If seawater is to be converted to fresh-water, the seawater is
preferably pretreated to
remove organics, algae, and fine particles if brackish water is used.
Conventional
processes can be used for the pretreatment.
[032] If waste water or polluted water is to be converted to fresh water,
pretreatment to
remove waste material is preferred and conventional processes can be used for
such
pretreatment. The treated non-fresh water is then subjected to high
temperature
electrolysis.
[033] An HTE system according to aspect of the present invention can operate
at
temperatures ranging from about 100 C to about 850 C, a typical known range
for HTE. At
higher temperatures, more of the energy is derived from the heat thus
requiring less
electricity for the electrolysis. An HTE system according to another aspect of
the present
invention can operate at temperatures ranging from 850 C to just below the
thermolysis
temperature (thermolysis temperature is about 3200 C at 1 atm). An HTE system
according
to another aspect of the present invention can operate at temperature ranging
from 1000 C
to just below the thermolysis temperature. An HTE system according to a still
further aspect
of the present invention can operate at temperature ranging from 100 C to just
below the
thermolysis temperature.
[034] Operating the HTE system at just below the thermolysis temperature, the
energy
required for hydrogen and oxygen production comes mainly (can be almost 100%)
from heat
generated by the combustion of hydrogen and oxygen (in a later stage of the
process) and
the remaining negligible amount from electricity. In this way, the hydrogen
and oxygen
16
CA 02688610 2009-12-11
production is mostly through heat, and electricity is used primarily to
separate produced
hydrogen and oxygen and avoid their recombination.
[035] In one aspect, the present invention relates to converting almost all of
the input
seawater to fresh water where the Rosenbaum-Weisz Process has the potential of
converting 97% seawater and 3% salts/mineral into 97% fresh water and 3%
salts/minerals
thereby providing fresh water for humans, industries, livestock and
agriculture.
[036] In another aspect, the present invention relates to a desalination
system where the
high temperature electrolysis units are operated at pressures greater than 1
atm. Such
higher or elevated pressure reduces the volume required for the HTE and thus
the volume
of the electrolysis units and in turn the number of high temperature
electrolysis units
needed.
[037] In a further aspect, the present invention provides to a system and
method where the
energy required for the HTE process is provided by harnessing the heat that is
generated by
the combustion of the hydrogen and oxygen (a green and renewable energy
process) rather
than burning fossil fuels, which are known to cause global warming.
[038] In a still further aspect, the present invention relates to a system and
method where
fresh drinking water is provided from polluted waters by increasing water
temperature
thereby rejuvenating polluted rivers and stream, eliminating drugs and other
deadly bacteria
in waste treatment plants. The standard requirement for eliminating hazardous
material in
typical incineration process is by keeping the material at 2000 C for 2
seconds. The present
system in one embodiment provides such conditions for polluted and waste
water.
17
CA 02688610 2009-12-11
[039] In other embodiments of the present invention, a system using the
Rosenbaum-
Weisz Process can be installed in existing MSF desalination plants as well as
SWRO
desalination plants. Thus, the extensive non-renewable energy that contributes
significantly
to global warming, that is currently being consumed can be replaced by the
implementation
of the Rosenbaum-Weisz Process. In the case of the MSF desalination process,
the waste
heat from the adjacent cogeneration plants can be used to produce electricity
or be used in
an industrial/chemical process, since they will not be closed down.
[040] In another embodiment of the present invention, a new plant using the
Rosenbaum-
Weisz Process does not require massive investments in the construction of an
adjacent
cogeneration power plant. Consequently, plants employing the Rosenbaum-Weisz
Process
can be located anywhere in the world since they are dependant on having a
cogeneration
power plant beside them to supply the required energy. Plants employing the
Rosenbaum-
Weisz Process can be located in a small village in Africa that has a small
plant to convert
seawater, brackish or polluted water to fresh water or in a large metropolitan
city that has
large plant converting, seawater, brackish or polluted water to fresh water
since they are not
depended on being located near a cogeneration power plant.
[041] In a further embodiment of the present invention, plants employing the
Rosenbaum-
Weisz Process can be set up to provide vast amounts of fresh water that are
required for
industrial use and for power plants.
[042] In a further embodiment of the present invention, plants employing the
Rosenbaum-
Weisz Process can be set up at or near the oil and/or gas field to process the
produced
water and flowback water produced in the oil and gas field thus providing the
vast amounts
18
CA 02688610 2009-12-11
of fresh water that are required for oil and gas production, oil and gas
fracking and in
bituminous sands (tar sands) operations thereby significantly reducing the
water supply
costs and water disposal costs.
[043] In a further embodiment of the present invention the Rosenbaum-Weisz
Process can
be set up at or near industrial/chemical/processing plants, mines, foundries
etc. to process
the polluted water there from thereby significantly reducing the water supply
costs and water
disposal costs.
[044] In a further embodiment of the present invention, portable units
employing the
Rosenbaum-Weisz Process can be set up near the oil spill to process the
polluted water
thereby reducing the oil spillage cost.
[045] In a still further embodiment, methods and systems according to the
present
invention can be used to electrolyze steam from an industrial process and
recover heat
energy either for reuse in the process or for use in another process.
[046] In still further embodiment of the present invention, the Rosenbaum-
Weisz Process
can provide fresh water from many non-fresh water sources and does not require
the
consumption of large amounts of non-renewable fossil fuels. Consequently, the
Rosenbaum-Weisz Process can be a major contributor to the slowing down of the
consumption of non-renewable fossil fuel and thus significantly contributing
to the slowing
down of global warming and thereby extending the life of non-renewable fossil
fuel reserves.
[047] The Rosenbaum-Weisz Process can be utilized by both rich and poor
nations across
the world since it requires very little purchase of external energy to
operate.
19
CA 02688610 2009-12-11
BRIEF DESCRIPTION OF THE DRAWINGS
[048] FIG. 1 illustrates processes in high temperature electrolysis of non-
fresh water
producing fresh water according to certain embodiments of the invention.
[049] FIG. 2 illustrates a high temperature electrolysis unit according to one
embodiment of
the present invention.
[050] FIG. 3 illustrates a hydrogen and oxygen combustor according to one
embodiment of
the present invention.
[051] FIG. 4 illustrates one embodiment of a heat exchanger used for
extracting heat from
a combustion of hydrogen and oxygen to produce superheated steam according to
one
embodiment of the present invention.
[052] FIG. 5 illustrates one embodiment of the present process that is
utilizing part of
hydrogen and oxygen produced for external use and sale according to one
embodiment of
the present invention.
[053] FIG. 6 illustrates one embodiment of the present process that is
utilizing part of the
heat extracted from superheated steam to generate electricity according to one
embodiment
of the present invention.
[054] FIG. 7 illustrates one embodiment of the present process that is
utilizing part of
hydrogen and oxygen produced for external use and sale and utilizing part of
heat extracted
from superheated steam to generate electricity according to one embodiment of
the present
invention.
[055] FIG. 8 illustrates one embodiment of the present process where hydrogen
and
oxygen are provided from other source(s) and/or process(es), in addition to
hydrogen and
oxygen generated by the high temperature electrolysis. The combined generated
and
provided hydrogen and oxygen are combusted to produce superheated steam and
heat.
The heat extracted from the superheated steam can be used to compensate for
the heat
losses in the system, to generate electricity and/or be used in an
industrial/chemical process
according to one embodiment of the present invention.
CA 02688610 2009-12-11
[056) FIG 9 illustrates the impact of temperature on the contribution of heat
and electricity
according to one embodiment of the present invention.
[057] FIG. 10 illustrates a system according to one embodiment of the present
invention
where an evaporator and an electrolysis unit are separate.
[058] FIG. 11 illustrates a system according to one embodiment of the present
invention
which includes a mixing station to reduce scaling.
[059] FIG. 12 illustrates a system according to one embodiment of the present
invention
which includes utilizing heat from cooling and compression of hydrogen and
oxygen gases.
[060] FIG. 13 illustrates a system according to one embodiment of the present
invention
where a high temperature electrolysis unit also includes a combustor and a
water pipe. This
embodiment does not require the use of a high temperature heat exchanger
system.
[061] FIG. 14 illustrates a system according to one embodiment of the present
invention
that details a high temperature electrolysis unit that includes a combustor
and a water pipe.
[062] FIG. 15 illustrates a system according to one embodiment of the present
invention
that details a high temperature electrolysis unit that includes a combustor
and a multiple
water pipes.
[063] FIG. 16 illustrates a system according to one embodiment of the present
invention in
which a temperature reducing agent injected into the combustor and/or water
pipe so as to
reduce the steam temperature.
[064] FIG. 17 illustrates a system according to one embodiment of the present
invention in
which a temperature reducing agent is injected into the combustor and/or steam
mixing
chamber so as to reduce the steam temperature.
[065] FIG. 18 illustrates a system according to one embodiment of the present
invention in
which the high temperature electrolysis of non-fresh water produces steam
rather than fresh
water.
21
CA 02688610 2009-12-11
[066] FIG. 19 illustrates a system according to one embodiment of the present
invention in
which hydrogen is injected into the high temperature electrolysis unit.
[067] FIG. 20 illustrates a system according to one embodiment of the present
invention in
which hydrogen and steam are produced at the cathode; and
[068] FIG. 21 illustrates a system according to one embodiment of the present
invention in
which a methanation process is combined with the Rosenbaum-Weisz process.
22
CA 02688610 2009-12-11
DESCRIPTION OF THE PREFERRED EMBODIMENT
[069] Referring initially to FIG. 1, in one embodiment, of the present
invention all of the
hydrogen and oxygen that is generated by the high temperature electrolysis
process is
combusted at elevated pressure to produce high pressure high temperature
superheated
steam. The heat generated through the combustion of hydrogen and oxygen is
then
extracted by the heat exchanger system and is recycled to be used in the high
temperature
electrolysis process. The extraction of the heat by the heat exchanger system
condenses
the superheated steam to produce fresh water.
[070] The process can be summarized as follows:
HTE
H2O + HEAT => H2+1/202
Non-Fresh
Water
Combustion
H2+1/202=> H2O + HEAT (2)
Fresh
Water
[071] As shown in equation (1), non-fresh water is heated to create
supersaturated steam
and using the high temperature electrolysis process the supersaturated steam
is separated
into hydrogen and oxygen. The generated hydrogen and oxygen is then combusted
to
create supersaturated steam and heat as shown in equation (2). The heat
generated by the
process of combustion of hydrogen and oxygen is then recovered to be used for
the
required heat in the high temperature electrolysis process.
23
CA 02688610 2009-12-11
[072] Non-fresh water 1 is first taken to a treatment station 2. Non-fresh
water is treated to
remove organics, algae and particulate such as sand. Fine particles are
removed if brackish
water is used as the input water. Waste material is removed if waste water is
used as input
water. Polluted water from any source (including but not limited to: water
from oil and gas
production, flowback water from fracking, water production from tar sands,
water from
chemical/industrial/processing plants, water from mining/foundries operations
and oil
spillage etc.) can be used as the input water. Conventional processes can be
used for such
removal of non-water materials (such as gas, oil residues, minerals, metals,
salts etc.) from
the non-fresh water will be understood by those of ordinary skill in the art.
[073] Scaling can be an issue, as in the case of seawater where salts and
minerals can
cause scaling issues, in the conversion of non-fresh water to fresh water.
Using seawater
as an example, the scaling issue becomes more acute as the treated seawater is
evaporated thereby increasing the relative concentration of salts and minerals
in the
remaining seawater. In another embodiment of the present invention as shown in
FIG. 11 in
order to minimize the scaling caused by the evaporation of the treated
seawater, the relative
concentration of the salts and minerals in the treated seawater is diluted by
mixing the
treated seawater with fresh water, as provided by loop 4, in the mixing
station 2A prior to the
high temperature electrolysis ("HTE") unit 5. The amount of fresh water that
is used to
dilution can be substantially greater than the amount of original treated
seawater. The
resulting increased combined treated seawater is then directed to the HTE
unit. The
resulting increased quantity of hydrogen and oxygen that is produced by the
HTE process
will generate increased quantities of heat and fresh water by the combustion
of hydrogen
and oxygen in the combustion chamber. A portion of the fresh water that is
produced at the
24
CA 02688610 2009-12-11
end of the water pipe is diverted back to mixing station 2A by loop 4 while
the remaining part
will be the net output of fresh water produced by the Rosenbaum-Weisz Process.
It should
be noted that the mixing station 2A and this looping back process is not
necessary where
scaling is not an issue. It will be understood by those skilled in the art
that any number of
suitable types of collection vessels (referred to generally as a "collector")
can be used in
place of a water pipe for condensing steam and the present invention is not
limited to the
use of a water pipe.
[074] The next step in the process is the high temperature electrolysis
process 5. In this
stage, the treated non-fresh water is electrolyzed into hydrogen and oxygen.
The
electrolysis process is through high temperature electrolysis, in which the
treated non-fresh
water is heated to extreme temperature operation just below the thermolysis
temperature.
Electrolysis at a temperature of 3150 C can be used for example. Consequently,
only a
relatively small amount of electricity is required to cause the hydrogen and
oxygen to
separate and flow in different channels after decomposition. The required heat
for the high
temperature electrolysis is provided from the combustion of hydrogen and
oxygen at
elevated pressure in a later stage of the process. The required electricity
for the electrolysis
process, whose only purpose is to separate hydrogen and oxygen, can be
purchased from
an outside source or may even be produced by utilizing the excess heat
produced at various
stages of the present method. Alternatively, the excess heat can be used as an
energy
input for an electricity generator such as a steam turbine and the energy
produced can be
sold. High temperature electrolysis process is an established process and
consequently,
the selection of electrodes and the construction of HTE unit are within the
knowledge of a
person of ordinary skill in the art. There are several methods of constructing
high
CA 02688610 2009-12-11
temperature electrolysis systems. One method is described by Jensen, Larsen
and
Mogensen, the details of which are incorporated herein by reference
(International Journal
of Hydrogen Energy, 32 (2007) 3253-3257.
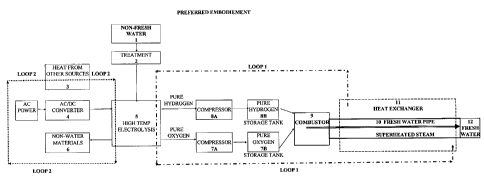
[075] FIG 1 illustrates, heat from combustion, the addition of heat 3 (if
required), and
electricity 4 are provided to the high temperature electrolysis unit 5. The
high temperature
electrolysis unit contains two sections, the evaporation chamber and the high
temperature
electrolysis section. Additional heat from outside sources may be required so
as to
compensate for any heat losses in the system such as heat exchanger
inefficiencies.
Electricity, whose sole purpose will be to separate the hydrogen and oxygen,
will be
negligible and may be purchased from outside sources or generated by capturing
the lost
heat at various stages in the plant. External sources, such as energy from
wind, solar, fossil
fuel, nuclear and geothermal sources can be used to compensate for the heat
losses and/or
supply the minimal electrical need to separate the hydrogen and oxygen.
[076] The treated non-fresh water is taken into the evaporation chamber
section where the
treated non-fresh water is turned into steam by the addition of some of the
recycled heat
(carried by suitable piping) from the combustion of hydrogen and oxygen at
elevated
pressure in a later stage of the process. The purpose of the separate
evaporation chamber
section is to pre-heat the treated non-fresh water thereby separating the
water portion of the
non-fresh water from the non-water materials (such as gas, oil residues,
minerals, metals,
etc.) by evaporating the water component of the treated non-fresh water into
steam and then
subjecting the steam to extreme temperatures, just below the thermolysis
temperature in the
high temperature electrolysis section.
26
CA 02688610 2009-12-11
[077] Consequently, the steam in the evaporation chamber section will be
substantially
pure and will not contain non-water materials. As a result of thermal
expansion the steam
then flows into the high temperature electrolysis section where additional
heat, the balance
of the recycled heat from the combustion of hydrogen and oxygen at elevated
pressure, is
added. Non-water materials at the bottom 6 of the HTE unit are removed,
preferably
continuously. Conventional processes can be used for such removal of non-water
materials
and will be understood by those of ordinary skill in the art.
[078] As shown in FIG. 2, treated non-fresh water enters the evaporation
chamber section
of the HTE unit at 51. Some heat is diverted from the recycled combustion heat
at 52 and it
heats up the treated non-fresh water to create steam. The remaining non-water
materials
are removed, preferably continuously from the evaporation chamber at 53. The
recovered
non-water materials (in the case of seawater recovered salts and minerals) can
be sold
thereby providing an additional source of revenue. As a result of thermal
expansion, the
steam in the evaporator chamber section will then flow into the high
temperature electrolysis
section of the HTE unit 5 where additional heat from the recycled combustion
heat is added
to the steam through a heat exchanging system 55 and 54. Most of the heat
needed for this
process is generated internally 54 through loop 1 that recycles the heat that
is provided by
the combustion of the hydrogen and oxygen at elevated pressure in a later
stage of the
process. Any additional heat, if needed, comes from external sources 55
through loop 2.
Two electrodes, cathode 56 and anode 57 located inside the HTE unit 5 act to
separate the
oxygen 58 and hydrogen 59. The minimal amount of electricity that is required
for the high
temperature electrolysis process is supplied to the electrodes by the AC/DC
converter unit
4. In cases where the non-fresh water contain gas, oil residues or other
gases, the heat
27
CA 02688610 2009-12-11
generated by the electrolysis process may release addition gases other than
hydrogen or
oxygen at some stage of the process. These other gases 60 will be recovered
and can be
sold thereby providing an additional source of revenue.
[079] To demonstrate the ability of this method to minimize the electricity
usage for
hydrogen and oxygen production two sample cases have been considered. FIG. 9
(taken
from an article published in the International Journal of Hydrogen Energy 32
(2007) 3253-
3257 by Soren H. Jensen, Peter H. Larsen, Mogens Mogensen of the Riso National
Laboratory) illustrates the relationship between the contribution of heat and
electricity as a
function of temperature. The temperature range is consistent with the typical
temperature of
the waste heat from a cogeneration plant. Extrapolating the relationship, for
electrolysis
occurring at 1500 C, it is estimated that 50% of the required energy will come
from heat and
50% from electricity (Case A). If the electrolysis occurs at 2000 C then it is
estimated that
75% of the required energy comes from heat and 25% from electricity (Case B).
It should
be noted that energy provided by the heat is almost 100% if the electrolysis
is at around
thermolysis.
[080] The above cases clearly demonstrate that electricity purchases are
significantly
reduced even in the cases where only 75% of the energy requirement comes from
heat. For
the proposed invention almost 100% of the energy will be provided from the
heat generated
by the combustion of hydrogen and oxygen. It can be easily predicted that
electricity
purchase, whose sole purpose will be to separate the hydrogen and oxygen, will
be
negligible.
28
CA 02688610 2009-12-11
[081] In an alternate embodiment of the present invention as shown in FIG. 10,
the
evaporation chamber section and the high temperature electrolysis section can
be two
separate equipment units rather than two sections within the same unit.
[082] In an alternate embodiment of the present invention, the evaporation
chamber
section in the HTE unit may not be employed. In this situation all of the
heating and the
removal of the salts and minerals occur in the high temperature electrolysis
section.
[083] Preferably, the evaporator section (whether part of the HTE unit or
separated) and
the high temperature electrolysis section of the HTE unit 5, the combustor 9
and the high
temperature heat exchanger 11 are insulated so as to minimize heat loss and
maximize
their efficiencies. The selection of insulating materials is within the
knowledge of a person of
ordinary skill in the art.
[084] Preferably, the evaporator section (whether part of the HTE unit or
separated) and
the high temperature electrolysis section of the HTE unit 5 and the mixing
station 2A are
made of material suitable to withstand the presence of the salts and minerals
so that to
minimize corrosion. The selection of the appropriate material is within the
knowledge of a
person of ordinary skill in the art.
[085] Once hydrogen and oxygen are generated and separated by the HTE unit 5,
they are
compressed and stored in different storage tanks under pressure. Elevated
pressure is
used so as to minimize the amount of the required storage. A compressor 7A is
used to
compress and move the oxygen into a storage tank 7B, and a compressor 8A is
used to
compress and move hydrogen into a storage tank 8B. The hydrogen and oxygen
gases
leaving the HTE unit will be at elevated temperature. Heat may be extracted
from the
29
CA 02688610 2009-12-11
hydrogen and oxygen gases so as to reduce their volatility and/or to reduce
the required
storage space. The hydrogen and oxygen gases will then be compressed by their
respective compressor operating at elevated pressure (i.e. greater than 1
atmosphere). A
compression pressure of 2 atmospheres can be used for example.
[086] Steam in the high temperature electrolysis unit reacts with the cathode
electrode
causing corrosion. One way to reduce corrosion is to inject a gas, for example
hydrogen,
into the steam that is being electrolyzed in the HTE unit. In another
embodiment of the
present invention as shown in FIG. 19, hydrogen is separated from the main
hydrogen
stream going to the compressor 8A and then is injected into the HTE unit via
loop 8. The
injected hydrogen along with the hydrogen that is produced by the electrolysis
of the steam
exits the HTE unit at the cathode. Thus, the total volume of hydrogen will
exceed the
amount of hydrogen that is required for combustion. The excess amount of
hydrogen will
be the amount that was injected. Thus the hydrogen in loop 8 is a self
sustaining
continuous loop.
[087] In certain embodiments of the present invention not all of the steam is
electrolyzed to
produce hydrogen and oxygen. Some examples that cause this are: small cell
surface
area, the ratio between the current and the steam flow rate, smaller
difference between
applied voltage and the open circuit voltage. Consequently, steam and hydrogen
will be
produced at the cathode. In some situation it may be desirable to inject the
steam and
hydrogen mixture directly into the combustor. In other situation it may be
desirable to
separate the steam from the hydrogen. One way to separate steam and hydrogen
is to cool
the gases thereby condensing the steam into fresh water. In another embodiment
of the
present invention as shown in FIG. 20, steam is separated from the hydrogen
and
CA 02688610 2009-12-11
condensed to produce fresh water. Since not all of the steam is converted into
hydrogen
and oxygen then less will be available for combustion and thus less heat will
be available to
the HTE unit. Consequently, more external energy will be required for
electrolysis and that
will be supplied by loop 2. The extracted heat can be used in the evaporation
chamber
and/or the evaporator unit, generate electricity, or in other parts of the
process. If the
extracted heat is used to generate electricity then the generated electricity
can be used for
internal use (thereby reducing the plant's external electrical purchase) or be
sold to an
external source resulting in a revenue stream. The fresh water produced by the
condensing of the steam can be sold as fresh water or could be used in other
part of the
process.
[088] Another embodiment of the present invention as shown in FIG. 12, the
heat from the
heated hydrogen and oxygen, and other gases if produced during the HTE
process, exiting
the HTE unit is extracted by way of one or more heat exchangers 18 and by the
compression of the gases. The extracted heat can be used in the evaporation
chamber
and/or the evaporator unit, generate electricity, or in other parts of the
process for example,
the drying of the salts/minerals/metals if extracted. If the extracted heat is
used to generate
electricity then the generated electricity can be used for internal use
(thereby reducing the
plant's external electrical purchase) or be sold to an external source
resulting in a revenue
stream.
[089] As shown in FIG. 3, hydrogen at elevated pressure 91 and oxygen at
elevated
pressure 92 are then injected into a combustor 9 to generate superheated steam
93. The
pressurized hydrogen and oxygen ensures that the combustion will occur under
high
pressure thus preventing air from entering the combustor thereby preventing
the creation of
31
CA 02688610 2009-12-11
nitrous oxide ("NOX"). The combustion pressure will exceed 1 atmosphere so as
to
exclude the air from entering the combustor. A combustion pressure of 2
atmospheres can
be used for example. The combustion chamber is designed to withstand high
combustion
temperatures without significant heat loss. The combustion chamber is
preferably
constructed of refractory materials or has high temperature ceramic surface
coatings 94.
Another means for carrying out high temperature combustion is described in
U.S. Patent No.
7,128,005, details of which are incorporated herein by reference. The
combustion process
produces superheated steam at high pressure and high temperature. The heat
from the
superheated steam is extracted through a high temperature heat exchanger
system 11. The
material in the system is chosen from material that is suitable for high
temperature
operation. Current technology has the capacity to deal with heat in excess of
3200 C. For
example, there are ceramics that can withstand the heat and thus could line
the surface of
the combustor, the appropriate selection of which is within the knowledge of a
person of
ordinary skill in the art.
[090] As shown in FIG 4, the superheated steam 101 so produced is at a
combustion
temperature of about 3200 C at 1 atm. The actual combustion temperature will
be higher
since the combustion will occur at elevated pressure. The higher the pressure
the higher
the combustion temperature (for example, the combustion temperature is about
3353 C at
the pressure of 2 atms). This high temperature superheated steam then flows
through a
water pipe 10, transferring heat to a high temperature heat exchanger system
11. The
returned heat exchanger fluid enters the heat exchanger system at 102. The
heat energy
extracted by the heat exchanger system from the high pressure high temperature
superheated steam is then returned to the high temperature electrolysis unit
103 to heat the
32
CA 02688610 2009-12-11
treated non-fresh water through loop 1. The superheated steam produced by the
high
pressure combustion process is cooled by the extraction of the heat by the
high temperature
heat exchanger system to produce fresh water stored in a fresh water tank 12.
The water
pipe 104 serves the purpose of containing the superheated steam isolated so
that no
impurities are introduced into the process of fresh water creation. The water
pipe and the
combustor are hermetically sealed thereby ensuring that no air or contaminants
will enter
the process. The superheated steam exiting from the combustor to the water
pipe is also
under high pressure thus ensuring that no air will enter the water pipe.
[091] The wall thickness of the water pipe can be tapered as the temperature
gradient
reduces along the water pipe due to heat extraction. The tapered wall reduces
the cost of
the water pipe. Heat is extracted from the water pipe by way of suitable high
temperature
heat exchanger system. The combustor and the water pipe containing high
pressure high
temperature superheated steam and are made of material that can stand high
pressure and
high temperatures. The heat exchanger fluid is not in direct contact with the
super saturated
steam which is contained in the water pipe. Many known industries such as
nuclear plants,
foundries, rockets etc. operate at very high temperatures and consequently,
the selection of
appropriate heat exchanger and heat exchanger fluids suitable for the
Rosenbaum-Weisz
Process is within the knowledge of a person of ordinary skill in the art.
[092] In another embodiment of the present invention as illustrated in FIG.
13, where the
HTE unit also contains, the combustor and the water pipe. This configuration
does not
require the high temperature heat exchanger system thereby reducing the
capital cost and
significantly reducing the system heat loss. Unlike previous embodiments, in
this
embodiment the water pipe is in direct contact with the HTE unit.
33
CA 02688610 2009-12-11
[093] In another embodiment of the present invention as illustrated in FIG. 14
illustrates the
details of the HTE unit that also has the combustor and the water pipe. The
wall that the
water pipe and combustor share in common is covered by ceramic tiles so as to
prevent
heat transfer between them so as to eliminate heat losses. Conversely, the
wall that the
water pipe and the HTE unit share in common is not covered by ceramic tile so
that there is
maximum heat transfer from the water pipe to the high temperature electrolysis
section.
The higher the amount of heat transfer to the high temperature electrolysis
section the lower
the amount of electricity that is required for electrolysis. This embodiment
may be furthered
refined by excluding the evaporation section from the HTE unit. The selection
of the
ceramics that can withstand the heat and thus could line the surface of the
combustor and
the water pipe is within the knowledge of a person of ordinary skill in the
art. The selection
of appropriate materials suitable for the water pipe is within the knowledge
of a person of
ordinary skill in the art. This is the only situation in which part of the
surface of the water
pipe is covered by ceramic tiles so as to prevent heat transfer. In all other
embodiments
the contain heat exchanger system none of the water pipe surface is covered by
ceramic so
as to maximize the heat transfer from the water pipe to the heat exchanger
system This
embodiment may be further refined, as illustrated in FIG. 15, by having the
high temperature
steam split into multiple streams and going to multiple water pipes where some
of the water
pipes are imbedded in the HTE unit thereby improving the distribution of heat
in the HTE
unit. In this situation, the walls of the water pipes imbedded in the HTE unit
will not be
covered by ceramic materials.
[094] In another embodiment of the present invention as illustrated in FIG. 5,
some of the
hydrogen and oxygen is sold rather than be used to generate heat. Some of the
oxygen
34
CA 02688610 2009-12-11
and hydrogen are extracted from the storage tanks 7B and 8B for external use.
Thus, this
process can be used to generate hydrogen for the hydrogen economy. The selling
of some
of the hydrogen and oxygen implies that less hydrogen and oxygen is combusted
in the
combustor. The extraction of hydrogen and oxygen results in reducing the
amount of heat
available to the HTE process from the combustion of hydrogen and oxygen. Thus,
the
reduction of the heat from the combustion can be made up by increasing the
amount of heat
and or electricity that would be required to be purchased from outside
sources. This is an
arbitrage situation. The amount of hydrogen that can be sold is a function of
the difference
in the sum of the cost of purchasing heat and/or electricity and the reduction
of fresh water
revenue versus the revenue that could be generated by the sale of hydrogen and
oxygen.
[095] Another embodiment of the present invention is illustrated in FIG. 6,
where some of
the heat that is generated by the combustion of hydrogen and oxygen can be
diverted to a
steam generator to be converted by a steam turbine into electricity. All of
the hydrogen and
oxygen are used for combustion. There is no sale of hydrogen or oxygen. Part
of the
combustion heat is captured through another heat exchanger 12 and carried
through loop 3
to a steam generator 14. The generated steam is then taken to a steam turbine
15 to
generate electricity 16. The extraction of the heat to generate electricity
will result in
reducing the amount of heat available to the HTE process from the combustion
of hydrogen
and oxygen. Thus, the reduction of the heat from the combustion can be made up
by
increasing the amount of heat and/or electricity that would be required to be
purchased from
outside sources. One reason that one would do this is because some of the
generated
electricity may be classified as "green electricity" thereby enabling the
plant to get a high
premium price for the generated electricity. This is an arbitrage situation.
Typically,
CA 02688610 2009-12-11
however, the capital cost required for the generation of electricity would
make it
uneconomical to generate and sell electricity unless there was a premium paid
for the
generated electricity.
[096] Another embodiment of the present invention as shown in FIG. 7 is a
combination of
extraction of hydrogen and oxygen as well as producing electricity.
[097] Another embodiment of the present invention as shown in FIG. 8
illustrates a process
where hydrogen and oxygen are provided from other source(s) and/or process(es)
and the
hydrogen and oxygen that is produced by the high temperature electrolysis are
combined to
be combusted at elevated pressure to produce superheated steam at high
pressure and
high temperature. The heat extracted from the superheated steam can be used to
compensate for the heat losses in the system, generate electricity and/or be
used in an
industrial/chemical process. This may be done where the cost of the additional
hydrogen
and oxygen is less than the purchase of heat from other sources to compensate
for the heat
losses in the system. Another reason for doing this is if the revenue from
electricity
produced exceeds the cost of the additional hydrogen and oxygen.
[098] Referring to FIG. 16, in another embodiment of the present invention a
temperature
reducing agent is introduced, such as by injection, into the combustor 9
and/or the water
pipe 10. The temperature reducing agent can comprise a suitable liquid, a gas,
a gel or a
foam, or combinations thereof and must not contaminate the quality of the
produced water
or alternatively must be capable of removal from the produced water prior to
human
consumption. The purpose of introducing a temperature reducing agent is to
reduce the
steam temperature thereby reducing the material costs of the combustor and/or
water pipe.
36
CA 02688610 2009-12-11
The temperature reducing agent should therefore be at a lower temperature when
introduced than the steam temperature. The temperature reducing agent can then
be
separated from the fresh water 13 and recycled by a closed Loop 5. Heat is
extracted from
the water pipe 10 by a heat exchanger to generate electricity. The generated
electricity can
be used internally (thereby reducing the plant's external electrical purchase)
or be sold to an
external source resulting in a revenue stream.
[099] Referring to FIG. 17, in another embodiment of the present invention, a
temperature
reducing agent which can comprise a suitable liquid, a gas, a gel or a foam,
or combinations
thereof is introduced into the combustor 9 and/or steam mixing chamber 20. The
temperature reducing agent must not contaminate the quality of the produced
water or
alternatively must be capable of removal from the produced water prior to
human
consumption. The temperature reducing agent and/or a portion of the steam from
the
combustor and/or mixing chamber are then separated from the steam in the
combustor
and/or steam mixing chamber and recycled by a closed Loop 6. Heat is extracted
from the
separated temperature reducing agent and/or steam by a heat exchanger in a
closed Loop
7 to generate electricity. The generated electricity can be used internally
(thereby reducing
the plant's external electrical purchase) or be sold to an external source
resulting in a
revenue stream.
[0100] In other embodiments, other methods for reducing the temperature of
steam in the
combustor, steam mixing chamber and water pipe (collectively sometimes
referred to herein
as the "chambers") are contemplated. For example, instead of introducing an
temperature
reducing agent into one or more of the chambers, one or more of the chambers
can be
cooled directly such as by the use of heat exchangers to circulate cooling
fluid such as
37
CA 02688610 2009-12-11
within the various chambers or in the chamber walls or in the environment
exterior to the
chambers. Heat recovered by these methods can be used to generate electricity.
[0101] Referring to FIG. 18, in another embodiment of the present invention,
many industrial
operations require steam in their processes. For example, steam is used in
production of
heavy oil/tar sand such as Steam Assisted Gravity Drainage (SAG-D) process
where steam
is pumped into the heavy oil reservoir so as to reduce the oil viscosity.
Steam is also used
in the petrochemical/pharmaceutical/mining operations. As FIG 18 illustrates,
by removing
less heat from the water pipe 10 than is required to condense the steam in the
water pipe,
the process yields pure steam at the end of the water pipe 12. Removing less
heat from the
water pipe, so as to produce steam versus water at the end of the water pipe,
will require
more energy to be supplied from other sources (electrical and or by loop 2) to
the high
temperature electrolysis unit. The steam can then be used in the required
industrial
operation. Steam pressure can be adjusted by compressing the hydrogen gas 8A
and
oxygen gas 7A to the required pressure.
[0102] The unfortunate side effect of oil sands upgrading processing is the
production of
carbon dioxide (CO2). The CO2 gas is then emitted to the atmosphere thereby
contributing
to global warming. One way to reduce the emission of CO2 to the atmosphere is
by a
known process call methanation. The methanation process is described by the
following
chemical reaction:
CO2 + 4H2 = CH4 + 2H20 + HEAT
38
CA 02688610 2009-12-11
[0103] Referring to FIG. 21, in a further embodiment, CO2 from the oil sand
upgrading
process is combined with hydrogen in a methanation process to produce methane,
steam
and heat. The steam created by the methanation process is the subjected to the
Rosenbaum-Weisz process described above. Heat produced by the methanation
process
could be used in the Rosenbaum-Weisz process or any other part of the oil sand
process.
The methane produced by the methanation process could be used in the Rosenbaum-
Weisz
process, any other part of the oil sand process or be sold thereby generating
additional
revenue.
[0104] In an alternate embodiment, the system and process of the present
invention with
appropriate modification can be used with a sewage treatment plant to
eliminate impurities
and hazardous materials in the non-fresh water being processed. Current
process to
elimination hazardous material requires the incineration of such materials at
2000 C for 2
seconds which is very expensive. Using the Rosenbaum-Weisz Process results in
an
electrolysis temperature in excess of 3000 C thereby eliminating all of the
hazardous
material as part of the process.
[0105] It will be understood by those skilled in the art that the process of
the present
invention can be used on a variety of scales such as from a small plant that
purifies water in
a small village to large desalination plant providing fresh water to a major
metropolitan city.
[0106] It will be further understood by those skilled in the art that the
system of the present
invention can be configured in a number of ways. For example, in certain
embodiments,
multiple units can be used such as, but not limited to, two HTE units, three
combustors, and
four heat exchangers. The mixing station, steam mixing station, heat
exchangers and other
39
CA 02688610 2009-12-11
embodiments described above can likewise be optionally included in systems
according to
the invention as needed.
[0107] It will be further understood that methods and systems embodying the
principles of
the present invention can be used for purposes other than converting non-fresh
water to
fresh water. For example, steam from any number of sources and/or processes
can be
used as an input such as steam from a methanation process. The steam may also
contain
other gases.
[0108] While preferred processes are described, various modifications,
alterations, and
changes may be made without departing from the spirit and scope of the process
according
to the present invention as defined in the appended claims. Many other
configurations of the
described processes may be useable by one skilled in the art.