Note: Descriptions are shown in the official language in which they were submitted.
CA 02688647 2012-01-17
Wear Resistant Alloy for High Temperature Applications
Field of the Invention
[0001] This invention deals with an Ni-Fe-Cr precipitation hardened
superalloy
for application in internal combustion engine valves, having as chief
characteristics
the precipitation of Ni3(AI,Ti,Nb) and niobium and titanium carbides in its
microstructure. The alloy project, based on its microstructure aspects, allows
for the
alloy hereof to be provided with properties equal to or higher than those of
the alloys
used in internal combustion engine valves, associated with the alloy's
significant cost
reduction because of the lower nickel content.
[0002] The alloy hereof is intended for valves' manufacture, where the
alloy
is required to have several properties, among them the following: oxidation
resistance, wear resistence and heat resistance, because of the high
temperatures
used in the application.
Background of the Invention
[0003] Conventionally, the materials used for exhaust valves in diesel
and
gasoline engines were JIS SUH 35 or JIS SUH 38 with STELLITE (trademark of
Delore Stellite Co.) coating (cobalt base alloy) on the valve's hardfacing.
With the
historic increased application temperatures of the new engines' valves, higher
performance materials started to be used in some applications, as it occurs
with
nickel base superalloys.
[0004] Currently, the reduction in the production costs of high
performance
materials is a tendency of the industry, as it occurs with exhaust valves,
which are
parts that are exposed to the highest temperatures and highest mechanical
stress
in an internal combustion engine. Such extreme stresses in terms of mechanical
resistance and corrosion resistance at high temperatures require the use of
costly
CA 02688647 2012-01-17
=
2
nickel-based superalloys. Another point to be reviewed is the abrasion
resistance.
Many alloys are coated with STELLITE (cobalt base alloy) on the valve's
hardfacing, which also increases the final cost of the valve. Accordingly,
high
performance materials have been increasingly sought in terms of abrasion, so
as to
eliminate the need to coat the valve's face.
[0005] One example of an excellent performance alloy in these
applications
is the UNS N07751 alloy, which is very costly because of its high nickel
content,
above 70%. In this sense, alloys with lower nickel contents with high
temperature
resistance, corrosion resistance and long term microstructural stability at
high
temperature and abrasion resistance have been developed. Examples are the
state-of-the-art alloys, NCF3015 (JIS3015D - U.S. Pat. No. 5,660,938) and HI
461
alloy.
[0006] Through the use of the lead-free gasoline since the 70's,
the
requirement in terms of corrosion resistance of the exhaust valve material has
been
reduced, so that the lead oxide corrosion by the lead oxide is no longer a
primary
concern. The high temperature oxidation resistance is a property to be
reviewed in
terms of corrosion, having the good performance of the UNS N07751 alloy.
[0007] Accordingly, the need is evident to develop new superalloy
compositions resistant to high temperature mechanical stress in connection
with high
temperatures, corrosion resistant, abrasion resistant with high hot
workability, and
meeting the different stresses under the conditions of using intake or exhaust
valves,
able to meet the need of a lower cost, which is related to the lower content
of costly
alloy elements. Alloy UNS N07751 is the most important material to be
replaced.
[0008] The alloys hereof are intended to meet all such needs.
CA 02688647 2009-12-16
3
Summary of the Invention
[0009] The properties of the Ni-Fe-Cr alloys used in exhaust valves are
closely
related to the presence of intermetallic phases, alloy elements and carbides
in their
microstructures. The intermetallic phases are very important for high
temperature
resistance. As regards the solid solution elements in the alloy, a composition
providing the material with corrosion resistance required in the use
environment is
very important. Additionally, the presence of carbides is important for wear
resistance as a result of abrasion to the material. The performance of the
alloy
elements to form these phases has been carefully reviewed and modified as
regards
the conventional concept. In this sense, this invention employs the niobium in
relatively high quantities (higher than the state-of-the-art alloys) as an
alloy element,
not only as a carbide builder, but mainly in the form of fine intermetallic
precipitate.
[0010] Another element used by this invention in higher quantities than
the
state-of-the-art alloys is aluminum, which has a prevailing function of
coherent
intermetallic containing niobium, Ni3(AI,Nb), thus improving the material's
heat
resistance. Additionally, aluminum improves the alloy hot oxidation
resistance.
[0011] It is extremely important that a small distortion exists between
the
network parameters of the phases y and y', which leads to a low interface
energy (y /
y"). The main coarsening driving force of these intermetallic precipitates is
the
minimization of the total interface energy, so that one coherent or semi-
coherent low
energy interface leads to a more stable microstructure. Metallurgical
stability is a
highly recommendable property for high temperature applications.
[0012] The morphology of these precipitates is determined by the surface
energy of the y / y" interface, and the elastic energy generated by phases y e
lattices mismatch, being primarily determined by the lattice distortion. If it
is a small
distortion, the morphology that will minimize the surface energy and the
distortion
CA 02688647 2009-12-16
4
energy per volume will be the spherical one. However, in case the lattice
distortion is
considerably big, the morphology of the precipitates will not be spherical,
but rather
cubic. Whenever the lattice mismatch is up to 0.02 %, the y" precipitates will
be
spherical; in case of mismatches between 0.5 and 1.0 %, these precipitates
will be
cubic; above 1.25 %, they are plate-shaped.
[0013] Niobium shows an ordered phase N13Nb precipitation kinetics lower
than when compared to elements such as titanium and aluminum in phases
Ni3(Ti,AI). In the Ni-Cr-Fe system superalloys, high niobium contents lead to
the
ordered phase y" (Ni3Nb) precipitation, similar to phase y". Whenever added to
lower
content alloy, niobium only increases the gamma prime precipitate volume and
the
solution temperature of this phase, leading its hardening effect to even
higher
temperatures.
[0014] With a view to meet the above referred conditions, the alloys hereof
are
provided with alloy element compositions, which, in bulk percentage, consist
of:
= 12.0 to 25.0 chromium, preferably 14.0 to 24.0 chromium, typically 18.0
chromi urn.
= 4.0 to 15.0 for the (Nb + 2 Ti) ratio, preferably (Nb + 2Ti) between 5.0
and 11.0, typically (Nb + 2T1) equal to 8.0; in this equation, titanium and
niobium can take any value within the limits; however, a minimum
niobium content shall be maintained, equal to 3.1%, preferably higher
than 3.7%.
= 0.05 to 1.0 carbon, preferably 0.20 to 0.40 carbon, typically 0.27%
carbon.
= 0.1 to 4.0 aluminum, preferably 1.0 to 3.0 aluminum, typically 2.0%
aluminum.
[0015] Iron balance and inevitable metallic and non-metallic impurities to
the
CA 02688647 2009-12-16
steel mill process, where said non-metallic impurities include, without
limitation, the
following elements, in bulk percentage:
= Maximum 5.0 for the manganese, copper, molybdenum and tungsten
elements, preferably maximum 2.0, typically maximum 0.50.
= Maximum 0.20 for phosphorus and sulfur, preferably maximum 0.05,
typically maximum 0.005.
[0016] Find below the reasons for the specification of the new material
composition, describing the effect of each alloy element. The indicated
percentages
relate to bulk percentages.
[0017] Chromium is used to provide the alloy with high temperature
corrosion
and oxidation resistance; accordingly, its content shall be higher than 10% in
case of
exhaust valve superalloys. Contents above 25% threaten the microstructure
stability
since they tend to form phases such as sigma and alpha prime phases (ci and
a'),
which deteriorate ductility. Accordingly, one concludes that the alloy
chromium
content would be between such limits, preferably between 14.0% and 22.0%,
typically 18.0%.
[0018] Titanium and niobium are carbide formers. Whenever they are added
to the alloy, they firstly combine with carbon, because of the high chemical
affinity
between these elements. The resulting carbides contribute to the abrasive wear
resistance. The titanium and niobium content that is non-combined with carbon
will
combine with nickel to form the y e intermetallic phases. For these two
effects, the
titanium and niobium contents shall be added to the alloy hereof according to
the Nb
+ 2 Ti ratio, which accounts for the atomic mass difference of both elements.
Thus,
in order to obtain the desired effect in both wear and hot resistance
properties, the
Nb + 2Ti ratio must be higher than 4.0%, preferably higher than 5.0%,
typically equal
to 8.0%.
CA 02688647 2009-12-16
6
[0019] When defining this alloy, a crucial point was the titanium and
niobium
content variation in order to define an optimum composition, within the ratio
in
question. It could be noticed that the niobium introduction in amounts above
3.0%
causes beneficial effects, both as regards the carbides that were formed and
the
residual niobium content (non-combined in the form of carbides), and such
content is
crucial to improve the alloy hot properties. What is desired when introducing
Nb in
higher quantities is to cause the IP intermetallic phase precipitation (Ni3Nb)
and the
phase I modification through the introduction of a greater niobium content in
its
structure. Additionally, a high niobium amount causes the precipitation of
primary
NbC-type carbides. These MC-type niobium carbides are more effective as
regards
abrasion resistance that titanium carbides, because of their greater hot
hardness.
The niobium content shall be carefully balanced to the carbide content. Since
niobium has a greater chemical affinity to carbon, the available niobium to
form the
intermetallic phase with nickel will be the quantity of this element as
dissolved in the
alloy matrix after reaction with carbon to form the primary carbides.
Accordingly, the
Nb:C ratio shall be higher than 7.4 : 1 (bulk), so that dissolved Nb in the
austenitic
matrix still exists, which will precipitate as Ni3Nb. A wide range for the Nb
element is
between 2.0 up to 8.0% (bulk), with an intermediate range of 3.0 up to 8.0%
(bulk) of
Nb and a narrow interval of 3.1 up to 8.0% (bulk) of Nb, or even narrower, of
3.5 up
to 8.0%.
[0020] In addition to the improved heat and abrasion resistance, Nb also
improves the weldability of hardened superalloys by phase 7" precipitation;
additionally, it improves corrosion in sulfurous environments, such as diesel
engines.
[0021] Nb can be partially replaced with tantalum (Ta) on equiatomic
bases.
Like Nb, Ta is also a builder of the intermetallic phase with nickel and
strongly
stabilizes primary carbides, being equally beneficial for hot hardness and
abrasion
CA 02688647 2009-12-16
7
resistance.
[0022] The increase in the niobium amount has shown effects in hot
resistance properties. Although the mechanism is not completely defined, in
the
alloys hereof the niobium content that is not combined with carbon must build
different intermetallics as compared to the titanium intermetallics, probably
the two-
line gamma type (r), which are very stable to coalescence and, accordingly,
effective in improving the high temperature resistance properties. As regards
carbides, a greater volumetric fraction of large-sized carbides was noticed,
with
increased niobium content and reduction of the titanium content, thus
resulting in
greater wear resistance.
[0023] For a same content of (Nb + 2 Ti) ratio, niobium addition causes a
reduction in the alloy's total titanium percentage. The studies hereof showed
that
such reduction is also beneficial to improve the high temperature oxidation
resistance ¨ an also essential property in high temperature working valves.
[0024] The reduced total titanium percentage in the alloy by the niobium
addition in quantities higher than 3.5% improves its hot workability, since
the alloy's
hot ductility is threatened to values above 4.0% for the sum of titanium and
aluminum contents (Ti + Al).
[0025] For all such effects ¨ hot resistance, oxidation resistance and wear
resistance ¨ the (Nb + 2 Ti) ratio must show a minimum 2.0% niobium content,
preferably niobium above 3.5%, with an optimum niobium content equal to or
higher
than 3.7%.
[0026] In spite of niobium and titanium beneficial aspects, the content of
such
elements cannot be excessively high, since it would cause the formation of
coarse
intermetallics, thus jeopardizing the mechanical properties of the alloy in
terms of
mechanical resistance and ductility, in addition to increasing the alloy cost.
CA 02688647 2009-12-16
8
Accordingly, the value of the (Nb + 2 Ti) ratio must be below 15.0%,
preferably below
13.0%.
[0027] Carbon is added with the intent of combining titanium and niobium in
order to form hard carbide particles and provide abrasion resistance. For that
function, the carbide content shall be at least 0.05%, preferably above 0.1%.
However, the percentage of hard particles shall be below 5% in volume, so as
not to
deteriorate the toughness and hot workability properties, the latter being
essential for
hot forged valves. Such particles' volume is determined by carbon, since, when
forming NbC or TiC, the alloy is provided with excessive Ti and Nb.
Accordingly, the
carbon content is used as controlling element of the volume of particles that
was
formed, being below 1.0%, preferably below 0.40%.
[0028] Aluminum is very important for the gamma line (y) phase
precipitation,
and therefore for high temperature resistance. Another extremely important
function
of aluminum in the alloy is to increase high temperature oxidation resistance
by
increasing formation of A1203 upon heating. Nevertheless, aluminum contents
must
be restricted, as very high quantities thereof can lead to deterioration of
high
temperature resistance and hot workability because of the formation of
nitrites and
such phases as n and 8 for long heating periods. Therefore, the aluminum
content
shall be between 0.5% and 4.0%, preferably between 1.0% and 3.0%, typically
equal
to 2.0%.
[0029] Residuals: Other elements such as manganese, tungsten,
molybdenum, copper, sulphur, phosphorus and those usually obtained as regular
residual elements in the preparation process of steel or liquid nickel alloys,
shall be
understood as impurities in connection with the melting shop deoxidization
processes or inherent to the manufacturing processes. Therefore, the
manganese,
copper, tungsten and molybdenum content is reduced to 5%, preferably below
2.0%,
CA 02688647 2012-01-17
9
because of the ratio destabilization between the austenite and ferrite phases,
and
because of any effects in the intermetallic phases present in the alloy.
Phosphorus
and sulphur segregate in grain contours and other interfaces, and therefore
they
shall be below 0.20%, preferably below 0.05%, preferably maximum 0.005%.
[0029a] In
accordance with one aspect then, there is provided a wear resistant
alloy for internal combustion engine valves, having a chemical composition of
elements comprising C, Mn, Si, Cr, Ni, Mo, W, V, Cu, Al, Ti, Nb, B, Zr, Co, Fe
and
impurities, and wherein the chemical composition of the elements comprises, in
percentage by mass:
0.15 to 0.50% C,
0.01 to 1.5% Mn,
0.01 to 1.0% Si,
12.0 to 21.0% Cr,
25.0 to 39.0% Ni,
0.01 to 2.5% Mo,
0.01 to 0.50% W,
0.01 to 0.50% V,
0.01 to 0.50% Cu,
1.0 to 3.0% Al,
1.0 to 4.5% Ti,
3.3 to 8.0% Nb,
0.001 to 0.02% B,
0.001 to 0.10% Zr,
0.01 to 2.0% Co,
the balance including Fe and impurities, where the sum of Ni + Co is
between 25.0% and 39.0% by mass; the proportion of percentages by mass of Nb:C
is in the range from 14:1 to 54:1 and the ratio of percentages by mass of
Ti/AI is
between 0.5 and 2Ø
CA 02688647 2012-01-17
9a
[0030] The described alloy can be made by conventional or special
processes
such as melting in electric arc or vacuum furnaces, followed by re-melting
processes
or not. Casting can be made in ingots by means of conventional or continuous
casting, or even by other manufacturing processes involving disaggregation of
the
liquid metal and further aggregation, such as power metallurgy and the spray
forming
or continuous casting process. The end products can be obtained after hot or
cold
forming, and end products are produced in the form of wire rods, blocks, bars,
wires,
sheets, strips, or can be even products in the as cast state.
Brief Description of the Drawings
[0031] Figure 1 shows the microstructure observed in an optical
microscope
of alloys ET1 and PI1 through P14.
Figure 2 shows the result of the image computer analysis to quantify
the carbides observed in the alloys studied with different Ti, Nb and Al
contents;
Figure 3 shows the results of the creep testing of the alloys hereof as
compared to ET1 and ET2 alloys;
Figure 4 compares hot resistance of the alloys hereof to ET1 and ET2
alloys, as of the flow stress for several temperatures;
Figures 5 and 6 show the result of the abrasive wear test carried out
with ET1, ET2 alloys and PI1 through PI7 alloys;
Figure 7 and 8 show the aging response after heat treatment at 750 C
and 690 C respectively; and
= CA 02688647 2012-01-17
Figure 9 and 10 show the properties of resistance to temperature and
resistance to hot oxidation can be examined in accordance with ratios (Nb/C)
and
(Ti/AI), respectively.
DETAILED DESCRIPTION OF THE INVENTION
[0032]
Figure 1 shows the microstructure observed in an optical microscope
of alloys ET1 and PI1 through P14, after polishing and attack with Gliceregia
reagent
for 15 seconds and 120 times magnification.
[0033]
Figure 2 shows the result of the image computer analysis to quantify
the carbides observed in the alloys studied with different Ti, Nb and Al
contents.
Such analysis was performed in a total surface area of 65,990,417 1Jm2 of the
sample, in 50 random fields with 500 times magnification.
[0034]
Figure 3 shows the results of the creep testing of the alloys hereof as
compared to ET1 and ET2 alloys, by assessing the creep rupture time for an 800
C
temperature and 3 tensile stress levels. Figure 4 compares hot resistance of
the
alloys hereof to ET1 and ET2 alloys, as of the flow stress for several
temperatures.
[0035]
Figures 5 and 6 show the result of the abrasive wear test carried out
with ET1, ET2 alloys and P11 through PI7 alloys. The test was made by pin
against
sandpaper; the test specimens pins were provided after aging heat treatment
and
using alumina abrasive paper grit #120. The average contact speed between the
abrasive paper and the pins was 100 m/min.
[0036]
Figures 7 and 8 show the aging response after heat treatment at
750 C and 690 C respectively. The hardness is always higher for the alloys of
the
present invention (PI5, P16) when compared with the alloys of the state of the
art for
CA 02688647 2012-01-17
11
the same time of treatment. In Figure 8, Alloys PI5 and PI6 also have a better
response to the aging heat treatment at 690 C than the alloy of the state of
the art
ET3, by reaching hardness higher than the minimum value required for
application
after one hour of treatment.
[0037] Figures 9 and 10 show the properties of resistance to temperature
and
resistance to hot oxidation can be examined in accordance with ratios (Nb/C)
and
(Ti/AI), respectively. In Figure 9, shows the optimum range of the ratio Nb/C
for the
optimization of the heat resistance property, represented by the time of creep
disruption at 800 C under 100 Mpa stress. Figure 10 shows that the alloys of
the
present invention are at the optimum range of the ratio Ti/AI to optimize the
property
of hot oxidation resistance, represented by a reversal of the gain in mass (in
mg/cm2)
after 400 hours at 800 C in atmosphere (air).
[0038] In order to define the compositions of the alloys hereof, several
alloys
were made and compared to the state-of-the-art alloys. The chemical
compositions
are shown in Table 1. The alloys hereof are hereinafter called PI, and the
state-of-the-art alloys are hereinafter called ET. ET1 alloy corresponds to HI
461
alloy, ET2 alloy corresponds to UNS N07751 alloy, and ET3 alloy corresponds to
NCF 3015 alloy (of US patent 5,660,938). The following ratios are also
quantified:
(Nb + 2 Ti); (Nb/C) and (Ti/AI) in Table 1.
[0039] In Table 1 one can notice a significant reduction in the nickel
content
of the alloy in the compositions hereof as regards ET2 alloy, thus resulting
in
considerably lower cost. ET2 and ET3 alloys are not either provided with
significant
carbon contents, causing no formation of carbides or the high wear resistance
shown
by the other alloys.
CA 02688647 2009-12-16
12
[0040] Table 1 also shows the addition of different niobium contents to the
alloys hereof, unlike the state-of-the-art alloy (ET1), which shows titanium
only. The
review of the (Nb + 2 Ti) ratio is also interesting, since it normalizes the
atomic mass
difference and is then related to the atomic content.
[0041] This is approximately consistent between the ones in the present
invention (P11 to P16) and in alloy ET1. Thus, the atoms of Ti in the alloys
of the
present invention are gradually replaced with niobium, until titanium is fully
replaced
with niobium in alloy P14. Despite having similar chemical nature, titanium
and
niobium have different effects in the alloys studied, so that such replacement
made
was of great benefit to end properties, as described below. In this sense, the
quantification and the differentiation of the alloys under study through the
content of
niobium not combined in form of carbides becomes very interesting. This
quantification can be evaluated through the ratio (Nb/C).
[0042] The differences between the titanium and aluminum contents in the
different alloys can be evaluated through the ratio (Ti/AI), which is very
important for
the hot oxidation resistance and the hot workability properties. Such ratio
(Ti/AI) is
displayed in Table 1 as well.
[0043] The ingots were cast by means of a close procedure for such ten
alloys
(ET1, ET2, ET3, P11, P12, P13, P14, P15, P16, P17), in a vacuum induction
furnace.
The casting was made into cast iron moulds, producing an ingot of about 55 kg.
After
the solidification, the ingots were forged and rolled for round gauges with
diameter of
18 mm. The bars were examined in an optical microscope after the solution
treatment, and the result is shown in Figure 1. Such images display the
increase in
the size of the carbides due to the replacement of titanium with niobium. Such
fact is
confirmed through the quantitative analyses of the images displayed in Figure
2.
CA 02688647 2012-01-17
13
[0044] Table-1: Chemical compositions of three alloys of the state of the
art
(ET1, ET2, and ET3) and the alloys of the present invention (P11 to P17).
Percentage in mass and balance in iron.
C Si Mn Cr Ni Al Ti Nb Nb + 2 Ti N b/C
Ti/A1
ET1
(HI 461) 0.27 0.10 0.15 18.0 46.0 1.20 4.00 - 8.00 - 3.3
ET2
(UNS NO7751) 0.05 0.03 0.05 15.5 70.0 1.20 2.45 0.90 5.50 18 1.9
ET3
(NCF 3015) 0.04 0.03 0.05 16.0 32.0 1.40 2.50 0.65 5.65 16.3 1.8
P11 0.27 0.10 0.15 18.0 46.0 1.20
3.00 1.90 7.90 7 2.5
P12 0.27 0.10 0.15 18.0 46.0 1.20
2.00 3.85 7.85 14.3 1.7
P13 0.27 0.10 0.15 18.0 46.0 1.20
1.00 5.80 7.85 21.5 0.8
P14 0.27 0.10 0.15 18.0 46.0 1.20 - 7.70 7.70 28.5 -
P15 0.27 0.10 0.15 18.0 46.0 1.90
2.00 3.95 7.95 14.6 1.1
P16 0.25 0.10 0.15 15.2 32.1 1.92
2.10 3.90 7.92 15.6 1.1
P17 0.25 0.10 0.15 18.8 36.0 1.30
1.71 3.38 6.80 13.5 1.3
[0045] Table 2 displays the hardness of alloys ET1, ET2, ET3, P11 P12,
P13,
P14, P15, PI6 and PI7 after solution at 1050 C and aging at 750 C for 1 hour
and,
also after solution at 1050 C and aging for 4 hours. These data show
equivalent
values as for the hardness of aged alloys, except for alloy ET3, which has
lower
hardness. The alloys with niobium have lower hardness in the solution state,
what
is interesting to machine the material in this condition.
[0046] Table 2: Response to the heat treatment of the alloys of the state
of
the art (ET1, ET2 and ET3), and the alloys of the present invention (P11, P12,
P13,
P14, P15, PI6 and P17). Results of hardness in HB after solution at 1050 C and
aging
at 750 C for 1 hour and 4 hours.
CA 02688647 2012-01-17
14
Solution Aging Aging
(750 C - 1h) (750 C - 4h)
ET1 254 330 330
ET2 250 335 335
ET3 238 260 300
PI1 192 334 340
PI2 177 326 345
PI3 185 316 335
PI4 207 331 340
PI5 178 333 348
PI6 172 331 350
PI7 171 315 330
[0047] Another important parameter for these alloys are the mechanical
properties at high temperature; such results are displayed in Figures 3 and 4.
The
alloys of the present invention are significantly more resistant concerning
creep than
alloy ET1. Alloys P12, P13, PI5 and PI6 are either equivalent to or better
than alloy
ET2 (UNS N07751), despite having nickel content substantially lower than this
alloy.
As for resistance at high temperature, measured by the yield stress (Figure
4), the
same behavior is seen. Alloys P12, P13, P15, and especially P16, are more
resistant
than alloys ET1 and ET2. Due to a higher concentration of coarse phases, alloy
P14
has reduction in the hot resistance in terms of creep resistence.
[0048] In terms of oxidation resistance, the alloys of the present
invention are
also superior to alloys ET1 and ET2, as shown in Table 3; we see that the
lower the
content of titanium, the higher the resistance to the alloy oxidation. This is
the best
resistance seen for titanium-free alloy P14. This occurs because titanium
destabilizes
the oxide layer formed on the surface of the alloys in the nickel-iron system
and,
thus, it reduces oxidation resistance. Another interesting effect to be seen
is that,
among the alloys with the lowest titanium content (PI2, P13, P14, P15, PI6 and
P17),
those with the highest aluminum content (PI5, P16, and P17) have higher
resistance
to hot oxidation under test conditions. The test was carried out so that all
samples
of all alloys involved had identical sizes, so that their contact surface was
identical
as
CA 02688647 2009-12-16
well. Solution and aged cylindrical samples (diameter = 12 mm and height = 14
mm)
were duly weighed and maintained at 800 C for 100 hours. After being removed
from
the furnace, the sample is cooled by air and weighed again, by measuring mass
variation. This process is repeated until the full test time is completed.
Ceramic
crucibles of alumina were used as sample holders during the test. The progress
of
the oxidation process at 800 C was evaluated for 400 hours, when it was
possible to
see stabilization in the corrosion process.
[0049]Table 3: Mass variation (mg/cm2) after 100, 200 and 400 hours at 800 C
in
atmosphere (air). The lower the gain in mass, the higher the oxidation
resistance to
the oxidation of the material.
100 hours 200 hours 400 hours
ET1 (Ti = 4.0%; Al = 1.2cro) 0.40 0.66 0.66
ET2 (Ti = 2.5%; Nb = 0.9%; Al = 1.2%) 0.41 0.54 0.54
PI1 (Ti = 3.0%; Nb = 1.9%; Al = 1.2%) 0.40 0.54 0.54
PI2 (Ti = 2.0%; Nb= 3.85 %; Al = 1.2%) 0.14 0.27 0.27
PI3 (Ti = 1.0%; Nb= 5.8%; Al = 1.2%) 0.14 0.27 0.27
PI4 (Nb= 7.7 %; Al = 1.2%) 0.14 0.14 0.14
PI5 (Ti = 2.0%; Nb= 3.9%; Al = 1.9%) 0 0.25 0.25
PI6 (Ti = 2.0%; Nb= 3.9%; Al = 1.9%) 0 0.25 0.25
PI7 (Ti = 1.7%; Nb= 3.4%; Al = 1.3%) 0 0.25 0.25
[0050] The resistance to abrasive wear, compared in Figures 5 and 6, and
quantified in Table 4, follows the same tendency of oxidation resistance, for
different
reasons, though. Alloys ET1 and PI1 to PI9 have wear resistance significantly
higher
than alloy ET2, due to the presence of hard particles in their microstructures
(as
shown in Figure 1). However, we also see that the higher the content of
niobium, the
lower the rate of wear and, therefore, the higher the resistance to abrasive
wear.
This occurs because of the larger size of the carbides existing in the
microstructure
CA 02688647 2009-12-16
16
of alloys with the highest niobium content, as displayed in Figure 1 and
quantified in
Figure 2.
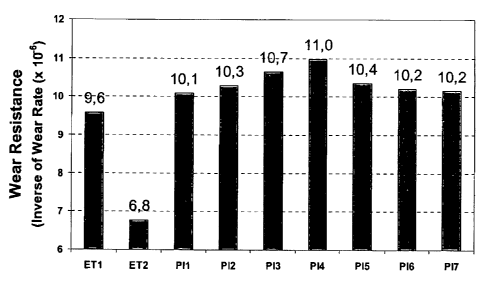
[0051]Table 4: Wear rate in the alloys studied, calculated from the division
of the
slopes in the curves of Figure 5 by the area of the sample (Wear rate =
(1/area) * a
AV / a AL). The lower the wear rate, the higher the resistance to the wear of
the
material, since the loss of material for wear is lower. Thus, the material
with the
highest value toward the reverse of the wear rate is more resistant to wear.
i.e.:
1/Rate = Wear Resistance.
Wear Resistance
Alloy
Absolute Value Concerning ET1
ET1 (Ti = 4.0%) 9.6 100%
ET2 (Ti = 2.5%; Nb = 0.9%) 6.8 71%
PI1 (Ti = 3.0%; Nb= 1.9%) 10.1 105%
P12 (Ti = 2.0%; Nb= 3.85 %) 10.3 107%
PI3 (Ti = 1.0%; Nb= 5.8%) 10.7 111%
PI4 (Nb= 7.7 %) 11.0 115%
P15 (Ti = 2.0%; Nb= 3.9%) 10.4 108%
P16 (Ti = 2.0%; Nb= 3.9%) 10.2 106%
P17 (Ti = 1.7%; Nb= 3.4%) 10.2 106%
[0052] The industrial application of these alloys includes a phase of aging
heat
treatment after the final formation of the piece. The alloys of the present
invention
are easier to obtain the minimum hardness required for application purposes
(about
330 HB ¨ Brinell hardness scale), that is, the achievement of hardness over
330 HB
is seen after only 20 minutes of treatment at 750 C. The hardness is always
higher
for the alloys of the present invention (PI5, P16) when compared with the
alloys of
the state of the art for the same time of treatment, as seen in Figure 7.
Alloys PI5
CA 02688647 2009-12-16
17
and PI6 also have a better response to the aging heat treatment at 690 C than
the
alloy of the state of the art ET3, by reaching hardness higher than the
minimum
value required for application after one hour of treatment. This can be seen
in Figure
8. Reducing the temperature and the time of the aging treatment is of the
utmost
importance to reduce costs and enhance yield when processing the material.
[0053] The properties of resistance to temperature and resistance to hot
oxidation can be examined in accordance with ratios (Nb/C) and (Ti/AI),
respectively.
Figures 9 and 10 show this analysis concerning the alloys of the present
invention
(P11 to P17) and of the state of the art (Eli and ET2). In Figure 9, we can
see clearly
that the alloys of the present invention are in the optimum range of the ratio
Nb/C for
the optimization of the heat resistance property, represented by the time of
creep
disruption at 800 C under 100 Mpa stress. Figure 10 shows that the alloys of
the
present invention are at the optimum range of the ratio Ti/AI to optimize the
property
of hot oxidation resistance, represented by a reversal of the gain in mass (in
mg/cm2)
after 400 hours at 800 C in atmosphere (air).
[0054] Therefore, the comparison between the alloys of the state of the
art
and the alloys of the present invention showed that the introduction of higher
contents of niobium and aluminum, together with the contents of titanium,
cause
improvements in the properties of resistance to hot, creep, resistance to
oxidation
and wear. A summary of such effects is displayed in Table 5. Alloys P12, P13,
P15,
PI6 and PI7 are always superior to the alloys of the state of the art, in
terms of all
properties examined. However, alloy PI4 has better result in situations where
resistance to wear and oxidation should prevail.
[0055] In summary, we can state that the results discussed herein shown
that
the alloys of the present invention, in addition to the economic advantage of
working
with a lower content of nickel, have better properties as well. As for the
alloys of the
CA 02688647 2009-12-16
18
state of the art, the alloys of the present invention have higher levels of
properties at
high temperature and resistance to wear. So, they are material improvements
for
industrial application in combustion engine valves or even other components
used at
high temperatures and corrosive sites.
[0056]Table 5: Comparison of Properties among all alloys studied, in absolute
figures and relative figures (the reference is alloy ET1 = 100%).
CA 02688647 2012-10-26
19
FIGURES
ET1 ET2 ET3 PI1 PI2 PI3 PI4 PI5 PI6 PI7
Hardness After Aging
330 335 300 334 330 316 331 350 340 330
Treatment (HB)
Yield Strength at
538 484 525 550 552 520 431 564 560 600
800 C (MPa)
Rupture Time at 800 C
302 900 - 312 906 1188 301 906 1060 578
and 100 MPa (hours)
Carbides Fraction over
1.25 <0.1 <0.1 1.28 1.29 1.65 2.22 1.29 1.29 1.29
8 microns (% Volume)
Wear Resistance
9.6 6.8 - 10.1 10.3 10.7 11 10.4 10.2 10.2
(Inverse of Wear Rate)
Oxidation Resistance
(Inverse of Weight 1.5 1.9 - 1.9 3.7 3.7 7.1 4.0 4.0
4.0
Variation) after 200
hours at 800 C (g4)
PERCENTAGE ON THE ALLOY ET1 (%)
ET1 ET2 ET3 PI1 PI2 PI3 PI4 PI5 PI6 PI7
Hardness After Aging
100 102 91 101 100 96 100 106 103 100
Treatment
Yield Strength at
100 90 98 102 103 97 80 103 104 93
800 C
Rupture Time at 800 C
100 298 - 103 300 393 100 300 351 191
and 100 MPa
Wear Resistance 100 71 - 106 107 111
115 108 106 103
(Inverse of Wear Rate)
Oxidation Resistance
(Inverse of Weight
100 122 - 122 244 244 471 264 264 106
Variation) after 200
hours at 800 C
[0057] While the invention has been described with reference to preferred
embodiments, variations and modifications would be apparent to one of ordinary
skill
in the art.