Note: Descriptions are shown in the official language in which they were submitted.
CA 02690651 2010-01-21
PRESERVATION OF FETAL NUCLEIC ACIDS IN MATERNAL PLASMA
FIELD OF THE INVENTION
[0001] This invention relates to prenatal diagnosis of fetal abnormalities and
more particularly to the preservation of fetal nucleic acids within a maternal
blood
sample.
BACKGROUND OF THE INVENTION
[0002] The demonstration by Leon et al. Cancer Res 37 (1977) 646-650
that cell-free plasma DNA is elevated in cancer patients paved the way for the
present day interest in cell-free plasma nucleic acid in disease diagnosis.
Relatively recently, Lo et al. Lancet 350 (1997) 485-487 have identified the
existence of circulating cell-free fetal nucleic acids in maternal plasma.
Since this
work, a number of studies have demonstrated that cell-free fetal nucleic acids
present in maternal plasma can be used in non-invasive prenatal diagnosis.
[0003] The analysis of nucleic acids can serve as a predictor of patient
vulnerabilities by identifying chromosomes and corresponding genes that
represent possible disease-related issues for a patient or a patient's
offspring.
Research has provided the chromosomal locations of many hereditary diseases
and also the genotype or chromosomal mutation that corresponds with the
disease. As the genetic markers for these hereditary diseases are ascertained,
there is a parallel interest identifying patients that carry these genetic
traits,
especially when such diseases may only manifest in a patient's offspring.
Further, hereditary diseases may only affect a child if both parents carry a
necessary allele. In the interest of identifying the offspring that may be
stricken
with a fatal or debilitating hereditary condition, prenatal testing has become
a
much more routine practice. However, the difficulty in obtaining the genetic
material of a fetus has presented a number of barriers to testing for the many
known genetic markers for hereditary disease.
[0004] The most thorough and accurate prenatal screening procedures for
fetal abnormalities generally involve invasive techniques such as
amniocentesis
and chorionic villus sampling. While providing reliable results, these
procedures
1
CA 02690651 2010-01-21
are often regarded as carrying a substantial potential risk of pregnancy
complications due to their invasive nature. In recent years, the
identification of
fetal nucleic acids within maternal blood has led to extensive research with a
focus on isolating such fetal DNA and RNA to test for any number of fetal
abnormalities. Such testing desirably is performed using only a maternal blood
sample thereby eliminating the need for the invasive testing procedures.
Unfortunately, it has proved challenging to isolate fetal nucleic acids from
maternal nucleic acids.
[0005] More specifically, in order to obtain consistent and reliable results
from the testing of fetal nucleic acids within maternal blood, it is important
to both
distinguish the fetal nucleic acids from the maternal nucleic acids and to
preserve
the structural integrity of the fetal nucleic acids. Traditionally, the first
step of
isolating cell-free nucleic acid from blood is obtaining either serum or
plasma and
then isolating the cell-free nucleic acids within the serum or plasma.
However,
serum is generally not suitable for cell-free nucleic acid isolation since
blood
clotting processes release cellular nucleic acids which contaminate cell-free
plasma DNA (see Figure 1) as well as other deleterious substances that may
destabilize the nucleated blood cells. Therefore efforts have been directed
also at
plasma as preferred starting material for cell-free nucleic acid isolation.
Under
such an approach, efforts at plasma separation from blood have been carried
out
to obtain a cell-free plasma sample. Unfortunately, this is frequently a
tedious
and time consuming multi-step process as it is important to use carefully
controlled conditions to prevent cell breakage during centrifugation which
will
contaminate the cell-free nucleic acids with cellular nucleic acids released
during
breakage. Another important consideration is that cellular nucleic acid
releases
into plasma due to cell breakage during ex vivo incubation, typically within a
relatively short period of time from a blood draw event. Once maternal cell
lysis
begins, the lysed maternal cells release additional nucleic acids which become
mixed with the cell-free fetal nucleic acids and it becomes increasingly
difficult to
recover the fetal nucleic acids for testing. Further, the amount and
recoverability
of available cell-free DNA will decrease substantially over a relatively short
period
2
CA 02690651 2010-01-21
of time due to degradation (e.g., from deoxyribonuclease (DNase) or
ribonuclease (RNase) activity) of fetal cell-free DNA (which reduces the
already
finite supply of fetal DNA that can be recovered for analysis). For example,
after
a period of about 36 hours, an untreated sample is expected to be sufficiently
corrupt that it would not lead to reliable or conclusive analysis. Thus, cell-
free
nucleic acids desirably are isolated as soon as plasma is separated or the
plasma may be frozen at -80 C until the nucleic acids can be isolated. This
too
imposes practical constraints upon processing. It would therefore be of great
benefit to develop sample processing techniques that would increase the amount
of fetal nucleic acids (DNA and/or RNA) recoverable from maternal plasma,
making the isolation and testing of the fetal nucleic acids more reliable and
consequently improving the diagnostic capabilities of the fetal nucleic acids.
[0006] The problems generally associated with the isolation of cell-free
nucleic acids include the time consuming and tedious nature of traditional
isolation protocols and the requirement that blood samples be processed
immediately in an effort to avoid maternal cell lysis. Often, maternal blood
samples are immediately treated to remove all maternal cells and the resulting
plasma is frozen. However, this process is lengthy and often cell lysis begins
before cells are removed. Further, any protocols for removing the maternal
cells,
including centrifuging the maternal cells out of the sample and plasma
freezing
may have deleterious effects on the fetal nucleic acids.
[0007] In an effort to counter these problems and avoid cell degradation,
blood samples have been subjected to a protocol which includes contacting the
samples with formaldehyde. Formaldehyde is often used to stabilize cell
membranes and its use could therefore reduce maternal cell lysis. Formaldehyde
has also been thought to inhibit DNase and RNase thereby increasing the
preservation and stability of the cell-free fetal nucleic acids. Studies by
Dhallan et
al. JAMA 291 (2004) 1114-1119 have demonstrated a decrease in cell lysis and
a substantial increase in the amount of recoverable cell-free fetal nucleic
acids.
However, other studies have countered this data indicating that the
formaldehyde
does not have the desired effect. Most recently, Zhang et al., Clinical
Chimica
3
CA 02690651 2010-01-21
Acta 397 (2008) 60-64, determined that the effect of formaldehyde on the
percentage of fetal DNA in maternal plasma depends on processing time,
wherein formaldehyde has little to no effect on samples processed at 6 hours,
but
has substantial preservation effect on samples processed at 36 hours. More
particularly, samples contacted with formaldehyde and processed at 36 hours
were found have reduced cell lysis and increased inhibition of plasma DNase
activity. The use of formaldehyde for such purposes is discussed in U.S.
Patent
Nos. 7,332,277 and 7,442,506.
[0008] The potential for unreliability and toxicity considerations attendant
with formaldehyde processing make its use for maternal plasma preservation
undesirable. Given the immense discrepancies regarding the use of
formaldehyde for fetal DNA sample preservation, there remains a need for a
processing protocol that will consistently reduce one or any combination of
maternal cell lysis and DNase and/or RNase activity within maternal plasma
samples. It is further desired that such protocol allow for increased sample
storage time, so that samples can be taken from a pregnant patient and
subsequently stored or sent to a remote location for testing without fear of
reduced integrity of the fetal nucleic acids.
[0009] A number of patent documents address such processes for the
stabilizing, identification and testing of fetal cells and/or nucleic acids
located
within blood. See, generally, U.S. Patent Nos. 5,447,842; 5,457,024;
5,861,253;
6,258,540; 6,617,170; 6,821,789; 7,332,277; 7,442,506 and U.S. Patent
Publication Nos. 2007/0111233; 2007/0134658; 2007/0202525; 2008/0020390;
and 2008/0108071. Further, a substantial amount of academic research has
been published in regard to fetal cell-free DNA and associated topics.
[0010] Notwithstanding the above, there remains a need for fetal nucleic
acid isolation and preservation methods that are simplified and less time
consuming. It is further desirable that these methods increase the amount of
recovered fetal DNA and RNA from maternal plasma (e.g., as compared with
methods that do not employ the teachings herein) while maintaining the
integrity
of the DNA and RNA and producing reliable diagnostic results. Efforts to
increase
4
CA 02690651 2010-01-21
the reliability and consistency of fetal nucleic acid analysis include
treating a
maternal blood sample so that the amount of viable fetal DNA and/or RNA
recovered is increased. The concentration of cell-free fetal DNA found within
samples of maternal plasma at the time of blood draw generally ranges from
3.4% to 6.2% of the total amount of the cell-free DNA that is present in the
plasma, depending on duration of gestation.
[0011] The present invention addresses the need for an efficient and
consistent method of preserving and testing fetal nucleic acids from within
maternal plasma. By providing an improved method for the reduction of maternal
cell lysis and nuclease activity, the present invention includes a protocol
that
increases the amount of recoverable fetal nucleic acids thereby improving the
diagnostic reliability of the fetal nucleic acids. The present invention helps
prevent contamination of plasma cell-free nucleic acids with cellular nucleic
acids
that are released from damaged cells. The present invention further helps to
inhibit nuclease activity to protect the integrity of the cell-free plasma
nucleic
acid. The stabilizing of the nucleated blood cells within a blood sample makes
it
no longer necessary to separate plasma immediately after blood draw. The
present invention may further allow for blood samples to be stored at room
temperature for up to about 14 days without compromising the integrity of the
cell-free nucleic acids present in the plasma and without contaminating the
sample with cellular nucleic acids originating from lysed cells. The present
invention may also make it possible to avoid any freezing of the plasma and/or
contact with any formaldehyde.
[0012] One advantage of the present invention is the possibility for
essentially simultaneous stabilizing of both the nucleated blood cells and
cell-free
nucleic acids. This helps to prevent cellular genomic nucleic acids (e.g.,
maternal
cellular genomic nucleic acids) from being released into plasma, and further
diluting the fetal nucleic acids (and associated biomarkers) of interest,
while also
maintaining the structural integrity of the fetal nucleic acids. An additional
possible advantage of the present invention lies in its ability to maintain
relative
amounts of fetal nucleic acids. In vivo there is constant replenishment of the
fetal
CA 02690651 2010-01-21
nucleic acids to maintain a consistent amount of fetal nucleic acids but upon
blood draw the fetal nucleic acid amounts will deteriorate without
replenishment.
The teachings of the present invention also contemplate the possibility to
arrest
the degradation of the fetal nucleic acids post-blood draw.
SUMMARY OF THE INVENTION
[0013] In a first aspect, the present invention contemplates a non-invasive
prenatal screening method for the identification of fetal characteristics. The
method includes the steps of: contacting a drawn maternal blood sample that
includes a plurality of blood cells with a nucleic acid protective agent in an
amount and time sufficient so that the blood cells are substantially prevented
from (i) releasing genomic nucleic acids into the blood sample and from (ii)
experiencing nuclease activity that degrades fetal nucleic acid; isolating
fetal
nucleic acids from the maternal blood sample; and analyzing the isolated fetal
nucleic acids to identify a fetal characteristic.
[0014] The nucleic acid protective agent may include a formaldehyde
releaser preservative agent such as one selected from the group consisting of:
diazolidinyl urea, imidazolidinyl urea, dimethoylol-5,5dimethylhydantoin,
dimethylol urea, 2-bromo-2.-nitropropane-1,3-diol, oxazolidines, sodium
hydroxymethyl glycinate, 5-hydroxymethoxymethyl-1-1 aza-3,7-dioxabicyclo
[3.3.0]octane, 5-hydroxymethyl-1 -1 aza-3,7dioxabicyclo[3.3.0]octane, 5-
hydroxypoly[methyleneoxy]methyl-1-1 aza-3, 7dioxabicyclo[3.3.0]octane,
quaternary adamantine and any combination thereof. The concentration of the
preservative agent prior to the contacting step may be between about 0.1 g/ml
and about 3 g/ml. The concentration of the preservative agent prior to the
contacting step may be between about 0.4 g/ml and about 0.8 g/ml. The
concentration of the preservative agent prior to the contacting step may be a
concentration at which cross-linking of nucleic acids and proteins is
observed, as
indicated by agarose gel electrophoresis. The amount of the preservative agent
in a treated sample may be less than about 20 mg/ ml of the blood sample.
[0015] The isolating step may include isolating nucleic acid from maternal
6
CA 02690651 2010-01-21
plasma and isolating the fetal nucleic acid in the absence of any cell. Either
or
both of the isolating or analyzing steps may occur at least 2 hours, 7 days,
or
even 14 days after the blood sample is drawn. Either or both of the isolating
or
analyzing steps may occur without and/or prior to any freezing the blood
sample
or any of its constituents (e.g. to a temperature colder than about -30 C
(more
preferably colder than about -70 C)). In one embodiment the isolating step
occurs without prior freezing of the blood sample. In another embodiment, the
analyzing steps occurs without prior freezing of the fetal nucleic acids
isolated
therefrom, In another embodiment both steps can occur without prior freezing
of
the respective material.
[0016] The fetal nucleic acid may be DNA, RNA or both. The analyzing step,
the isolating step or both may include a step of contacting the fetal nucleic
acid
with an enzyme, an amplifier or both. The contacting step may take place in a
blood collection tube into which the blood sample is drawn (e.g., while the
blood
sample is entering a blood collection tube). The contacting step may take
place
as the blood sample is drawn. The contacting step may be sufficient so that
after
a period of at least 7 days (or even 14 days) from the time the blood sample
is
drawn, the amount of fetal nucleic acid is at least about 90% of the amount of
fetal nucleic acid at the time the blood sample is drawn. The contacting step
may
be sufficient so that after a period of at least 7 days from the time the
blood
sample is drawn, the amount of fetal nucleic acid present in the sample is
about
100% of the amount of fetal nucleic acid present in the sample at the time the
blood sample is drawn. The contacting step may be sufficient so that after a
period of at least about 14 days from the time the blood sample is drawn, the
concentration of fetal nucleic acid relative to the total nucleic acid in the
blood
sample that is present is at least about 10 to at least about 50 times the
amount
of fetal nucleic acid that would be present in the absence of the contacting
step.
[0017] The protective agent may include a nuclease inhibitor selected from
the group consisting of: diethyl pyrocarbonate, ethanol, aurintricarboxylic
acid
(ATA), formamide, vanadyl-ribonucleoside complexes, macaloid,
ethylenediamine tetraacetic acid (EDTA), proteinase K, heparin, hydroxylamine-
7
CA 02690651 2010-01-21
oxygen-cupric ion, bentonite, ammonium sulfate, dithiothreitol (DTT), beta-
mercaptoethanol, cysteine, dithioerythritol, tris (2-carboxyethyl) phosphene
hydrochloride, a divalent cation such as Mg", Mn+2, Zn+2, Fe+2, Ca+2, Cu+2 and
any combination thereof. The protective agent may include an anticoagulant
selected from the group consisting of heparin, ethylenediamine tetraacetic
acid,
citrate, oxalate, and any combination thereof. The protective agent may
include a
preservative agent and an anticoagulant. The protective agent may include
imidazolidinyl urea and ethylenediamine tetraacetic acid.
BRIEF DESCRIPTION OF THE DRAWINGS
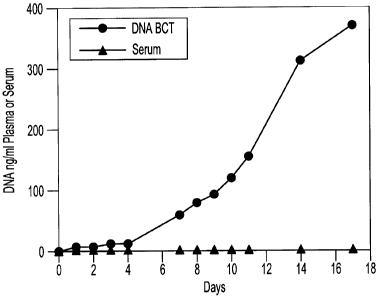
[0018] FIG. 1 is an illustrative graphic representation showing the relative
amounts of cellular DNA present as a result of cell leakage within two blood
samples stored at room temperature over time; there is seen a plot of "DNA
BCT"
data that extends substantially entirely along the x-axis at a y-axis (0 DNA)
value
of zero (0).
[0019] FIG. 2 is an illustrative graphic representation showing the relative
amounts of Y-chromosomal DNA present as a result of male white blood cell
leakage within two female blood samples over time; again there is seen a plot
of
"cell-free DNA BCT" that extends substantially entirely along the x-axis at a
y-
axis value of zero (0) DNA.
[0020] FIG. 3 is an illustrative graphic representation showing the relative
amounts of cell-free DNA present within blood samples over time using lambda
DNA as a marker.
[0021] FIG. 4 is an illustrative graphic representation showing the relative
amounts of cell-free fetal DNA present within two blood samples over time
using
the RASSF1A promoter region as a marker.
[0022] FIG. 5 is an illustrative graphic representation showing the relative
amounts of plasma DNA over time in a blood sample drawn into standard
K3EDTA tubes. In each box-plot, the total amount of cell-free plasma DNA is
represented as genome equivalents per milliliter of plasma (GE/ ml). The line
inside of the box indicates the median value. The limits of the box represent
the
8
CA 02690651 2010-01-21
75t" and 25t" percentiles. The upper and lower error bars indicate the 10th
and
90th percentiles, respectively. The uppermost and lowermost dots indicate the
maximum and minimum values. The y-axis is in logarithmic scale.
[0023] FIG. 6 is an illustrative graphic representation showing the relative
amounts of plasma DNA over time in a blood sample drawn into a device of the
present teachings. In each box-plot, the total amount of cell-free plasma DNA
is
represented as genome equivalents per milliliter of plasma (GE/ ml). The line
inside of the box indicates the median value. The limits of the box represent
the
75th and 25th percentiles. The upper and lower error bars indicate the 10th
and
90th percentiles, respectively. The uppermost and lowermost dots indicate the
maximum and minimum values. The y-axis is in logarithmic scale.
[0024] FIG. 7 is an illustrative graphic representation showing the relative
amounts of fetal cell-free DNA over time in a blood sample drawn into standard
K3EDTA tubes. In each box plot, the percentage of cell-free plasma DNA is
represented as genome equivalents per milliliter of plasma (GE/ ml). The line
inside of the box indicates the median value. The limits of the box represent
the
75t" and 25th percentiles. The upper and lower error bars indicate the 10th
and
90th percentiles, respectively. The upper most and lower most dots indicate
the
maximum and minimum values. The y-axis is in logarithmic scale. Over time, a
statistically significant decrease in the percentage of fetal cell-free DNA is
seen
only in K3EDTA tubes (*P< 0.05, **P:5 0.01 by paired Student's t test).
[0025] FIG. 8 is an illustrative graphic representation showing the relative
amounts of fetal cell-free DNA over time in a blood sample drawn into a device
of
the present teachings. In each box plot, the percentage of cell-free plasma
DNA
is represented as genome equivalents per milliliter of plasma (GE/ ml). The
line
inside of the box indicates the median value. The limits of the box represent
the
75th and 25t" percentiles. The upper and lower error bars indicate the 10th
and
90th percentiles, respectively. The upper most and lower most dots indicate
the
maximum and minimum values. The y-axis is in logarithmic scale. Over time, a
statistically significant decrease in the percentage of fetal cell-free DNA is
seen
only in K3EDTA tubes (*P< 0.05, **P:5 0.01 by paired Student's t test).
9
CA 02690651 2010-01-21
[0026] FIG. 9 is an illustrative graphic representation showing amplification
of fetal cell-free DNA from maternal plasma by whole genome amplification
(WGA). One aliquot (without amplification) is used directly to quantify (by
real-
time PCR) the Y chromosomal SRY sequence from maternal plasma (an
indicator of fetal DNA in maternal plasma). The other aliquot (with
amplification)
is subjected to WGA and then SRY sequence quantification by real-time PCR is
performed. Enrichment in fetal cell-free DNA from maternal plasma by eighty
fold
is observed with WGA. A plasmid DNA construct containing a single copy of the
Y chromosomal SRY sequence is used to plot the standard curve for the
quantification.
DETAILED DESCRIPTION
[0027] In general, the invention herein contemplates a method of prenatal
screening which includes the isolation and preservation of fetal nucleic acids
located within maternal blood. A unique preservation step acts to increase the
amount of recoverable fetal nucleic acids thereby improving the diagnostic
capabilities of the fetal DNA and RNA.
[0028] More particularly, the present invention provides a method for the
isolation of fetal nucleic acids including a preservation step that includes
contacting a maternal blood sample with a protective agent. The nucleic acid
may be DNA or RNA or any combination thereof. The fetal nucleic acid may be
cell-free DNA or RNA. The samples from which the nucleic acids may be isolated
include any maternal blood sample. The fetal nucleic acids may be located in
maternal plasma. The method disclosed herein allows for the efficient
isolation
and preservation of fetal nucleic acids while avoiding confusion with maternal
nucleic acids that enter a blood sample due to maternal cell lysis after blood
draw.
[0029] The process for improved fetal nucleic acid isolation from a maternal
blood sample begins by contacting a blood sample with a protective agent
containing an active ingredient to maintain the integrity of the components
within
the sample. Ingredients that may be used include, but are not limited to,
CA 02690651 2010-01-21
diazolidinyl urea, imidazolidinyl urea, dimethoylol-5,5dimethylhydantoin,
dimethylol urea, 2-bromo-2.-nitropropane-1,3-diol, oxazolidines, sodium
hydroxymethyl glycinate, 5-hydroxymethoxymethyl-1-1 aza-3, 7-
d ioxabicyclo[3.3.0]octane, 5-hydroxymethyl-1-1 aza-
3,7dioxabicyclo[3.3.0]octane,
5-hydroxypoly[methyleneoxy]methyl-1 -1 aza-3, 7dioxabicyclo [3.3.0]octane,
quaternary adamantine, 2-aminoacetic acid or any combination thereof.
Preferred ingredients are selected from the group consisting of diazolidinyl
urea
(DU), imidazolidinyl urea (IDU), and any combination thereof.
[0030] The protective agent may consist essentially of the active ingredient.
It may be at least about 10%, 50%, or even 80% by volume of the protective
agent. For instance, the amount of active ingredient within the protective
agent
used may be generally about 100 to about 800 grams per liter. The amount of
active ingredient within the protective agent may be at least about 25 grams
per
liter or even 50 grams per liter. The amount of active ingredient within the
protective agent may be less than about 1500 grams per liter or even 1200
grams per liter. For example, the protective agent may comprise about 0.05 to
about 0.4 grams of a formaldehyde releaser preservation agent (e.g., IDU) per
0.2 ml of the total protective agent.
[0031] As used throughout the present teachings, the protective agent
composition preferably is substantially non-toxic. For example, the methods
herein (and compositions used herein) may be free of separately adding and/or
handling of any materially significant concentration (e.g., less than about 1%
by
weight, more preferably less than about 2000 parts per million, more
preferably
less than about 1000 parts per million, and still more preferably less than
about
500 parts per million) of formaldehyde and/or paraformaldehyde prior to any
contact with a blood product sample.
[0032] The protective agent may include a nuclease inhibitor in a suitable
amount to prevent DNase and RNase activity from further decreasing (e.g. by at
least about 10% by weight, and more preferably at least about 50% by weight)
the quality and amount of fetal nucleic acids recoverable from the blood
sample
as compared with a sample that does not include a nuclease inhibitor. Nuclease
11
CA 02690651 2010-01-21
inhibitors that may be used include, but are not limited to diethyl
pyrocarbonate,
ethanol, au rintricarboxylic acid (ATA), formamide, vanadyl-ribonucleoside
complexes, macaloid, ethylenediamine tetraacetic acid (EDTA), proteinase K,
heparin, hydroxylamine-oxygen-cupric ion, bentonite, ammonium sulfate,
dithiothreitol (DTT), beta-mercaptoethanol, cysteine, dithioerythritol, tris
(2-
carboxyethyl) phosphene hydrochloride, or a divalent cation such as Mg+2,
Mn+2,
Zn+2, Fe+2, Ca+2, Cu+2 or any combination thereof. Further, the protective
agent
may be substantially free of guanidinium salts, sodium dodecyl sulfate (SDS),
or
any combination thereof.
[0033] The initial contacting of the blood sample may be for a time sufficient
to inhibit one or both of maternal cell lysis, nuclease activity, or any
combination
thereof. Contacting may occur for at least about 10 seconds, more preferably
at
least about 1 minute, still more preferably at least about 2 minutes.
Contacting
can occur for longer periods of time. For example, contacting may be
commenced substantially contemporaneously from the time of blood draw (e.g.,
within less than about 10 minutes of the blood draw) and it may last until
nucleic
acids are isolated, screened, and/or tested. The contacting step may also be
employed to provide a sample with a longer shelf life. Thus, it is possible
that a
lapse of time of at least about 2 hours, more preferably at least about 6
hours, at
least about 24 hours, at least about 7 days or even at least about 14 days can
elapse between the time of blood draw (which may be substantially
contemporaneous with the contacting step), and the time of any testing or
screening of the sample, and/or isolation of the nucleic acids.
[0034] The protective agent may comprise an active agent in solution.
Suitable solvents include water, saline, dimethylsulfoxide, alcohol and
mixtures
thereof. The protective agent may comprise diazolidinyl urea (DU) and/or
imidazolidinyl urea (IDU) in a buffered salt solution. The protective agent
may
further comprise EDTA and 2-aminoacetic acid. Alternatively, the protective
agent may contain only a fixative (e.g., an active ingredient) and may be free
of
any additional additives.
[0035] The amount of any active ingredient within the protective agent may
12
CA 02690651 2010-01-21
generally be about 10% to about 90% by weight. The active ingredient or
fixative
may comprise about 70% to about 90% by weight of the protective agent. The
protective agent may further contain an anticoagulant such as about 5% to
about
20% by weight EDTA. The protective agent may contain about 10% by weight
EDTA. The protective agent may include from about 1 % to about 40% by weight
of nuclease inhibitor.
[0036] The amount of active ingredient or fixative (e.g. the formaldehyde
releaser) relative to the amount of EDTA may be about 1 to about 10 parts
(more
preferably about 2 to about 8 parts) by weight of fixative to about 1 part by
weight
EDTA. The amount of protective agent within a tube prior to blood draw may be
about 0.05 to about 1.0 ml and more preferably about 0.1 to about 0.3 ml.
[0037] The combination of an active ingredient or fixative (e.g. the
formaldehyde releaser) and anticoagulant within the protective agent results
in
improved ability to maintain the amount and quality of fetal DNA within a
maternal blood sample. These results are believed unexpected and superior to
results obtained by the use of only the fixative or only the anticoagulant.
Therefore it is believed that a synergistic effect may occur when both a
fixative
and anticoagulant are combined. The compositions disclosed herein specifically
envision the possibility to include the combination of a formaldehyde releaser
and
an anticoagulant.
[0038] The protective agent may be located within a specialized device,
wherein the protective agent is already present in the device prior to
addition of
the blood sample, such as that disclosed in U.S. Patent Publication No.
2004/0137417. The device may be an evacuated collection container, usually a
tube. The tube may be made of a transparent material that will also resist
adherence of the cells within a given sample. The interior wall of the tube
may be
coated or otherwise treated to modify its surface characteristics, such as to
render it more hydrophobic and/or more hydrophilic, over all or a portion of
its
surface. The tube may have an interior wall flame sprayed, subjected to corona
discharge, plasma treated, coated or otherwise treated. The tube may be
treated
by contacting an interior wall with a substance so that the nucleic acids of
interest
13
CA 02690651 2010-01-21
will resist adhering to the tube walls. The surface of the tube may be
modified to
provide a dual functionality that simultaneously provides an appropriate
balance
of desired hydrophilicity and hydrophobicity, to allow collection of blood,
dispersion of the protective agent disclosed herein, while resisting adhesion
of
nucleic acids to the inner wall of the blood collection tube.
[0039] It is possible that any coating may be a functionalized polymeric
coating that includes a first polymer and one or more second monomeric and/or
polymeric functionalities that are different from (e.g., chemically different
from)
the first polymer. The coating may include one or more co-polymers (e.g.,
block
copolymer, graft copolymer, or otherwise). For example, it may include a
copolymer that includes a first hydrophobic polymeric portion, and a second
hydrophilic polymeric portion. The coating may be a water based coating. The
coating may optionally include an adhesion promoter. The coating may be
applied in any suitable manner, it may be sprayed, dipped, swabbed, or
otherwise applied onto some or all of the interior of the blood collection
tube. The
coating may also be applied in the presence of heat. Preferably any coating
applied to the inner wall of a blood collection tube will form a sufficiently
tenacious bond with the glass (e.g., borosilicate glass) or other material
(e.g.,
polymeric material) of the tube so that it will not erode or otherwise get
removed
from the inner wall. Examples of suitable polymeric coatings may include
silicon
containing polymers (e.g., silanes, siloxanes, or otherwise); polyolefins such
as
polyethylene or polypropylene; polyethylene terephthalate; fluorinated
polymers
(e.g., polytetrafluoroethylene); polyvinyl chloride, polystyrene or any
combination
thereof. Examples of teachings that may be employed to coat an interior of a
blood collection tube may be found in U.S. Patent Nos. 6,551,267; 6,077,235;
5,257,633; and 5,213,765.
[0040] The tube as described above may preferably include an
anticoagulant agent and an active ingredient such as a fixative agent
including
but not limited to those active ingredients disclosed herein. The tube may
also
may further include a nuclease inhibitor. Preferably, the compounds included
in
the tube are in an amount sufficient to preserve maternal cell morphology and
14
CA 02690651 2010-01-21
prevent cell degradation while also preventing deleterious DNase and RNase
activity within the fetal cell-free nucleic acids. However, the amount of
protective
agent may also be sufficiently small so that any consequential dilution of the
sample is substantially avoided, and cell-free nucleic acids in the sample are
not
materially diluted. A blood sample may be fixed simultaneously as it is drawn
into
the specialized tube. The tube may also be coated over an exterior wall with a
protective coating (e.g., a containment barrier that helps control glass shard
fragmentation) such as that disclosed in U.S Patent No. 7,419,832.
[00411 Additionally, the protective agent may be in a highly viscous or
substantially solid state, such that (for example) it can be used effectively
as a
substantially solid state coating. Examples of such substantially solid state
preservatives can be found in commonly owned co-pending U.S. Application
Serial No. 12/646,204, filed December 23, 2009. Liquid removal techniques can
be performed on the protective agent in order to obtain a substantially solid
state
protective agent. Liquid removal conditions may be such that they result in
removal of at least about 50% by weight, at least about 75% by weight, or at
least about 85% by weight of the original amount of the dispensed liquid
protective agent. Liquid removal conditions may be such that they result in
removal of sufficient liquid so that the resulting composition is in the form
of a
film, gel or other substantially solid or highly viscous layer. For example it
may
result in a substantially immobile coating (preferably a coating that can be
re-
dissolved or otherwise dispersed upon contact with a blood product sample). It
is
possible that lyophilization or other techniques may be employed for realizing
a
substantially solid form of the protective agent (e.g., in the form of one or
more
pellet). Thus, liquid removal conditions may be such that they result in a
material
that upon contact with the sample under consideration (e.g., a maternal blood
sample) the protective agent will disperse in the sample, and substantially
preserve components (e.g., cell-free nucleic acids) in the sample. Liquid
removal
conditions may be such that they result in a remaining composition that is
substantially free of crystallinity; has a viscosity that is sufficiently high
that the
remaining composition is substantially immobile at ambient temperature (e.g.,
it
CA 02690651 2010-01-21
does not exhibit any visibly detectable (as seen by the naked eye) flow when
held in a storage device at room temperature on an incline of at least about
450
for at least one hour); or both. A colorant may also be employed.
[0042] As discussed herein, contacting a maternal blood or plasma sample
with the protective agent allows the sample to be stored for a period of time
prior
to isolating and testing the fetal nucleic acids. More preferably, a maternal
blood
or plasma sample may be drawn at one location (e.g., a health care facility),
contacted with the protective agent, and later transported to a different
remote
location (e.g., a laboratory, such as one that is separately housed at a
distance of
at least about 1 km, 2 km, 3 km, or further away from the draw site) for the
nucleic acid isolation and testing process. Fetal nucleic acids may be
isolated
from the maternal blood or plasma sample and tested for various fetal
characteristics (including but not limited to chromosomal abnormalities) at
the
remote location and the resulting diagnostic information may then be reported
to
the site of the original blood draw. The fetal nucleic acid isolation process
may be
performed at one remote location and the resulting information can be analyzed
to identify fetal characteristics including chromosomal abnormalities at a
third
location. Moreover, the results of the fetal nucleic acid isolation process
may be
sent back to the site of the initial blood draw and analyzed there. The
resulting
diagnostic information may then be sent to a third location or back to the
remote
location or the site of the initial blood draw.
[0043] At any time after the initial contact of the maternal blood or plasma
sample with the protective agent, the sample can be treated to isolate the
cell-
free fetal nucleic acids located within the maternal blood. The nucleic acids
may
be isolated using any isolation method including those methods disclosed in
commonly owned United States Application Publication No. 2009/0081687
Preferably, the maternal blood cells will stay generally intact, so that
maternal
nucleic acids are not released into the sample from broken blood cells, making
isolation of the fetal nucleic acids more difficult. The fixative acts to
prevent cell
lysis so that the maternal cells remain intact and substantially all maternal
nucleic
acids remain intra-cellular to avoid unwanted contamination of the cell-free
fetal
16
CA 02690651 2010-01-21
nucleic acids.
[0044] After the fetal nucleic acids have been isolated, they can be tested to
identify various fetal characteristics including but not limited to sex of the
fetus,
preeclampsia in the mother, rhesus status of the fetus and the presence of any
chromosomal abnormalities including but not limited to any chromosomal
inversions, translocations, aneuploidies, other mutations, or any combination
thereof. The methods herein thus further contemplate a step of nucleic acid
testing. Testing of the fetal nucleic acids can be performed using any nucleic
acid
testing method including, but not limited to polymerase chain reaction (PCR),
reverse transcription polymerase chain reaction (RT-PCR), quantitative real
time
polymerase chain reaction (Q-PCR), gel electrophoresis, capillary
electrophoresis, mass spectrometry, fluorescence detection, ultraviolet
spectrometry, DNA hybridization, allele specific polymerase chain reaction,
polymerase cycling assembly (PCA), asymmetric polymerase chain reaction,
linear after the exponential polymerase chain reaction (LATE-PCR), helicase-
dependent amplification (HDA), hot-start polymerase chain reaction,
intersequence-specific polymerase chain reaction (ISSR), inverse polymerase
chain reaction, ligation mediated polymerase chain reaction, methylation
specific
polymerase chain reaction (MSP), multiplex polymerase chain reaction, nested
polymerase chain reaction, solid phase polymerase chain reaction, or any
combination thereof.
[0045] One aspect of the teachings herein contemplates a method for
isolating and testing cell-free fetal DNA from maternal plasma. The method may
be performed on a single sample or on a multitude of samples (e.g., in a multi-
well plate). The method may include contacting the maternal plasma sample with
a protective agent. The protective agent may include a fixative as previously
discussed so that the maternal cells remain intact throughout the blood draw
and
DNA isolation process. The protective agent may further include a DNase
inhibitor to maintain the structural integrity of the fetal DNA. After
contacting the
maternal plasma sample with the protective agent, the sample may be
centrifuged to separate the plasma and the supernatant is discarded. By
17
CA 02690651 2010-01-21
contacting a maternal blood sample with the protective agent, the blood sample
does not necessarily require immediate processing and may be stored for a
prolonged period, such as up to about 14 days or longer at room temperature.
Thus the inventions herein contemplate one or more steps of storing and/or
otherwise waiting a relatively lengthy period from the time of blood draw
and/or
contacting until the time of screening, testing or other analysis.
[0046] Once, the sample is processed, an appropriate concentration of an
agent for inducing precipitation (e.g., a composition of salt and/or alcohol)
may
be added to precipitate the fetal DNA containing material. An organic or other
compound such as a phenol derivative or the like may be added to remove any
remaining protein contaminants. Any protein contaminants that still remain may
be removed by adding additional amounts of an organic or other compound such
as a phenol derivative or the like. After centrifugation, ethanol may be added
and
the sample centrifuged again. Any remaining liquid may be removed from the
sample so only the fetal DNA will remain. The finished product of isolated
fetal
DNA may then be contacted with a buffer.
[0047] One or more steps of incubation may be performed. Incubation may
occur on ice or at any temperature between -30 C and 70 C. For example, a
sample may be incubated at about -20 C. A sample may also be stored at room
temperature and thus substantially free of freezing upon blood draw.
[0048] Centrifugation may be performed at a suitable rate. For example,
centrifugation may be done at about 500 to about 20,000 rpm. Centrifugation
may occur at about 1,000 to 16,000 rpm. Centrifugation may be performed at
about room temperature or cooler. For example, it may be performed at about 1-
20 C, or still more specifically at about 4-9 C.
[0049] The following illustrates how a blood collection device in accordance
with the present teachings can preserve fetal cell-free DNA and help minimize
the cell-free DNA background in maternal plasma at ambient temperature. As
will
be seen, blood samples are drawn from healthy pregnant donors into (i)
standard
K3EDTA (sold under the name BD Vacutainer by Becton Dickinson of Franklin
Lakes, New Jersey) blood collection tubes and (ii) blood collection tubes
18
CA 02690651 2010-01-21
containing the protective agent taught herein ("the protective agent of the
present
teachings"), and kept at ambient temperature. For example, the protective
agent
of the present teachings may include about 500 g/L IDU, about 81 g/L
Tripotassium EDTA, and about 47 g/I glycine. The protective agent of the
present
teachings may be placed within a tube so that the tube contains about 0.20 ml
of
the protective agent. The tube containing the protective agent may receive
about
ml of patient blood. The patient blood may be drawn directly into the tube
containing the protective agent. It is believed that results shown will vary
by
about 25% of that described across a range of about 300 to about 700 g/L IDU
(with similar results expected for other formaldehyde releasers described
herein)
and from about 60 to about 100 g/L Tripotassium EDTA, and about 20 to about
60 g/L glycine. The protective agent may include roughly about 6 parts by
weight
IDU per about 1 part by weight EDTA, and roughly about 10 parts by weight IDU
per about 1 part glycine. The protective agent may include about 80% by volume
of IDU, 12.8% by volume Tripotassium EDTA, and 7.25 by volume glycine. An
example of a commercially available tube in accordance with the present
teachings is sold under the name Cell-Free DNA BCT by Streck, Inc., Omaha,
Nebraska.
[0050] For comparison purposes, a blood sample that is not treated with the
compositions disclosed herein is centrifuged to cause plasma separation and
cell-free DNA is extracted. Cell-free DNA from plasma is quantified by
quantitative real-time PCR. These maternal blood samples (drawn into standard
K3EDTA tubes) show a steady reduction in the amount of fetal cell-free DNA
during an extended time period (e.g., 36 hours, 7 days, 2 weeks etc.) at
ambient
temperature. Conversely, blood drawn into a device containing the protective
agent of the present teachings shows no change in the amount of fetal cell-
free
DNA over the same time period.
[0051] Using maternal plasma stored in a device containing the protective
agent of the present teachings for an extended period, fetal cell-free DNA may
be
amplified at least 10-fold (e.g., 80-fold) using whole genome amplification at
the
end of the extended period, and there is sufficient quantity of DNA available
for
19
CA 02690651 2010-01-21
meaningful analysis. Thus, use of the protective agent of the present
teachings
makes it possible to preserve fetal cell-free DNA for extended times as well
as
minimize any post-sampling maternal cell-free DNA background. Preserved in
this way, fetal cell-free DNA can be amplified by whole genome amplification
technology for producing sufficient amounts of fetal nucleic acids as a
starting
material for nucleic acid-based prenatal diagnostic tests.
[0052] For the discussion that follows, there is envisioned a protocol that
employs some or all of the following steps, following direct draw of a blood
sample into an evacuated blood collection tube. In accordance with the present
invention, the blood sample may be contacted by a protective agent such as
those protective agents described herein. The processing of nucleic acids for
analysis may include a step of purifying the nucleic acids and amplifying the
nucleic acids.
[0053] Samples of the treated blood (e.g., one and one half ml aliquots of
blood) may be removed from each tube periodically; cell-free plasma DNA may
be purified; primers and probes for the real-time PCR quantification of one or
more antibody or protein sequences (e.g., (3-actin, SRY, RASSFIA and/or other
markers for fetal DNA) may be prepared; real-time PCR quantification of one or
more antibody or protein sequences (e.g., 13-actin, SRY, RASSFIA and/or other
markers for fetal DNA) may be carried out; re-suspended plasma DNA may be
treated with a restriction enzyme; a promoter region sequence (such as those
associated with the fetal DNA markers discussed herein) may be used as a
universal marker for fetal DNA; a suitable amplifier may be used to amplify
fetal
cell-free plasma DNA obtained from maternal blood stored in the device of the
present teachings; or statistical analysis may be carried out.
[0054] Primers and probes for the real-time PCR quantification of certain
antibody or protein sequences discussed herein (e.g., f3-actin, RASSFIA and/or
other markers for fetal DNA) may be prepared in accordance with art-disclosed
teachings, such as described by Chan et al. Clinical Chemistry 52:2211-2218
(2006). Primers for the Y-chromosomal sex determining region (SRY) may be
prepared in accordance with art-disclosed teachings, such as Lee et al., Blood
CA 02690651 2010-01-21
93:3127-3139. An example probe that may be used for the quantification of SRY
sequence is 6FAM-ATG GCT CTA GAG AAT CCC AGA ATG CGA AAC TCA
GAG A-TAMRA. Commercially available primers, probes and PCR master mix,
(e.g., TagMan Universal PCR master mix) may be purchased from Applied
Biosystems, Foster City, CA. Plasmid DNA constructs may be prepared so that
each contains a single copy of the antibody or protein sequences discussed
herein ((3-actin, RASSFIA, SRY, and/or other fetal DNA markers). These plasmid
constructs may be used to plot the standard curves.
[0055] After re-suspension of the plasma DNA, the plasma may be treated
with a restriction enzyme (e.g., 25 U of BstUl restriction enzyme, available
from
New England Biolabs, Ipswich, MA) in accordance with art-disclosed teachings,
such as described by Chan et al. Clinical Chemistry 52:2211-2218 (2006).
[0056] Following re-suspension, a suitable amplifier (e.g., a QIAGEN REPLI-
g UltraFast Mini whole genome amplification kit available from QIAGEN, Inc.,
Valencia, California) may be used for the step of amplifying the fetal cell-
free
plasma DNA obtained from maternal blood stored in the device of the present
teachings. Purified cell-free DNA is prepared from a volume of plasma (e.g.,
at
least about 100 pl, or less than about 800 pl) as described above, but is re-
suspended in a small volume (e.g., at least about 0.05 pl, or less than about
10
pl) and amplified using the kit according to the manufacturer's instructions.
After
amplification, the sample may be diluted (e.g., by about 25-fold) prior to PCR
analysis.
[0057] In verifying the protective capabilities of the compositions disclosed
herein, standard K3EDTA blood collection tubes are thus compared against tubes
containing the protective agent of the present teachings, which thus contains
a
composition that stabilizes nucleated blood cells and inhibits plasma
nucleases.
In the examples and results discussed below, statistical analysis is carried
out
using software available at the Tools for Science website of the Physics
Department, College of Saint Benedict Saint John's University, St. Joseph,
Minnesota (http://www.physics.csbsiu.edu/). Paired Student's t test is used
and
P < 0.05 is considered statistically significant.
21
CA 02690651 2010-01-21
Example 1
[0058] Blood samples are taken from a female donor and a male donor. The
female blood sample is transferred into two tubes, tube A containing about 500
g/L IDU, about 80 g/L Tripotassium EDTA, and about 50 g/L glycine and tube B
containing only the Tripotassium EDTA. Both tubes are stored at room
temperature. White blood cells from the male blood sample are isolated and
spiked into both tube A and tube B. 3m1 of blood are taken from each tube on
day
0, day 1, day 2, day 3, day 4, day 7 and day 11. Each sample is centrifuged at
room temperature at 800 g for 10 minutes and the upper plasma layer is
transferred to a new tube and further centrifuged at 1500 g at room
temperature
for 10 minutes. The free circulating DNA in each tube is then purified using
the
NucleoSpin Plasma XS kit available from Macherey-Nagel Inc., Bethlehem,
Pennsylvania. The samples are then amplified by Real Time PCR amplification of
a fragment of the Y-chromosome (using iQ SYBR Green Supermix reagents
available from BIO-RAD Laboratories (Hercules, California)). Any rupture of
the
male white blood cells during sample processing will cause Y-chromosomal DNA
to be detectable within the female blood sample. Tube A shows no Y-
chromosomal DNA presence within the plasma sample, while the amount of Y-
chromosomal DNA identified in tube B increases at each measurement,
indicating male white blood cell rupture in tube B. The expected results of
this
example are shown in graphic format at Figure 2, and supports that use of the
compositions disclosed herein are capable of substantially preventing lysis of
the
blood cells spiked into the samples.
Example 2
[0059] Blood samples from the same donor are drawn into two different
types of blood collection tubes. One tube contains 500 g/L IDU, 81 g/L
Tripotassium EDTA and 47 g/L glycine. The other tube contains only Heparin.
All
samples are centrifuged at 2100 g for 30 minutes at room temperature to
separate the plasma. The plasma is then transferred to new tubes and non-
human (lambda) DNA is then spiked into the plasma tubes. The spiked samples
are then stored at room temperature for 0, 1, 2, 3, 4, 7, 11, and 14 days.
Free
22
CA 02690651 2010-01-21
circulating DNA is purified using the QlAamp DNA Blood Mini Kit available
from
QIAGEN Inc. (Valencia, CA). DNA is extracted from each plasma sample. The
samples are then amplified by Quantitative PCR (using iQ SYBR Green
Supermix reagents available from BIO-RAD Laboratories (Hercules, California))
to identify the amount of lambda DNA present. Results show a consistent
relative
percentage of lambda DNA presence at each measurement, indicating little if
any
decline in the percentage of cell-free DNA in the plasma samples contacted by
both IDU and Tripotassium EDTA. The amount of lambda DNA decreases at
every consecutive measurement in those samples contacted with only Heparin,
indicating a gradual decline in the relative percentage of cell-free DNA. The
expected results of this example are shown in graphic format at Figure 3. This
example confirms that the compositions of the present invention are able to
maintain the integrity and amount of DNA present in a blood sample.
Example 3
[0060] Two maternal blood samples from the same donor are drawn into two
separate blood collection tubes. One tube contains about 500 g/L IDU, about 80
g/L Tripotassium EDTA, and about 50 g/L glycine. The other tube contains only
the Tripotassium EDTA. Both tubes are stored at room temperature and 1ml
aliquots of blood are removed from each tube on day 0, day 7, and day 14 and
plasma is separated. All samples are centrifuged at 800 g for 10 minutes at
room
temperature to separate the plasma. The plasma is then transferred into new
tubes and centrifuged at 1500 g for 10 minutes at room temperature. Free
circulating DNA is purified using the NucleoSpin Plasma XS kit available from
Macherey-Nagel Inc., Bethlehem, Pennsylvania. DNA is extracted from each
plasma sample and eluted in 60p1 of elution buffer. An amount of 32p1 of
eluted
DNA is digested with 40 U of BstU I enzyme at 60 for 6 hours. The samples are
then amplified by Real Time PCR (using TagMan RT PCR reagents available
from Applied Biosystems, Foster City, California) using primers for RASSFIA
promoter region. Results show a consistent relative percentage of RASSFIA
presence at each measurement, indicating little if any decline in the
percentage
of fetal cell-free DNA in the maternal plasma samples contacted by both IDU
and
23
CA 02690651 2010-01-21
Tripotassium EDTA. The amount of RASSFIA decreases at every consecutive
measurement in those samples contacted with only the Tripotassium EDTA,
indicating a gradual decline in the relative percentage of fetal cell-free
DNA. The
results of this example are shown in graphic format at Figure 4.
[0061] Figure 5 shows the expected result of ex-vivo incubation of maternal
blood when drawn into standard K3EDTA tubes on the cell-free DNA
concentration in plasma. Initially (3 hrs), the median cell-free DNA
concentration
is found to be 762 genome equivalents per ml of plasma (GE/ ml) which
increased markedly over time. Compared to the initial 3 hrs value,
statistically
significant increases are observed in the cell-free DNA concentration at 24
hrs (P
< 0.05), 48 hrs (P < 0.05), 72 hrs (P < 0.05), 7 days (P < 0.05) and 14 days
(P:5
0.001). This steady increase may reflect the lysis of nucleated blood cells
and the
subsequent release of cellular genomic DNA into the plasma that continued for
14 days.
[0062] Figure 6 illustrates the expected effect of ex-vivo incubation of
maternal blood drawn into tubes containing the protective agent of the present
teachings on the plasma cell-free DNA concentration. Here, an initial median
cell-
free DNA concentration of 672 GE/ ml does not increase significantly
throughout
the entire 14 day experimental period, indicating that an enhanced integrity
of
nucleated blood cells is observed. After 3 hrs of incubation, a comparison of
the
plasma cell-free DNA concentration in K3EDTA tubes and tubes containing the
protective agent of the present teachings showed a statistically significant
difference (P < 0.05). The mean cell-free DNA concentration in K3EDTA tubes is
6341 GE/ ml whereas it is 680 GE/ ml in those tubes containing the protective
agent of the present teachings. The higher cell-free plasma DNA concentration
in
the K3EDTA tube compared to those tubes containing the protective agent of the
present teachings indicates that cellular DNA is released into plasma by
nucleated blood cell lysis.
[0063] Figure 7 shows the expected effect of ex vivo incubation on the fetal
cell-free DNA in maternal plasma in K3EDTA blood collection tubes. A
statistically
significant decrease in the percentage of fetal cell-free DNA is observed.
This
24
CA 02690651 2010-01-21
downward trend in the median values of the percentage of fetal cell-free DNA;
4.05, 1.33, 0.45, 0.11, 0.10 and 0.023 (at 3 hrs, 24 hrs, 48 hrs, 72 hrs, 7
days
and 14 days, respectively), demonstrates that K3EDTA tubes are not capable of
maintaining the fetal cell-free DNA percentage in maternal plasma at a
constant
level.
[0064] Figure 8 shows the expected effect of ex-vivo incubation on the fetal
cell-free DNA in maternal plasma when contacted by the protective agent of the
present teachings. Here, the percentage of fetal cell-free DNA does not change
significantly in the trend of median values; 6.8, 5.5, 6.2, 6.1, 6.8 and 6.5
at 3 hrs,
24 hrs, 48 hrs, 72 hrs, 7 days and 14 days, respectively.
[0065] In further testing, donor plasma that had tested positive for the Y
chromosome (data not shown) was used for whole genome amplification (WGA).
By real-time PCR, 398 SRY DNA copies are detected without WGA, whereas
32,300 SRY DNA copies are detected following WGA. This represents
enrichment in fetal cell-free DNA by at least about 10 fold, 20 fold, 40 fold
or
even 80 fold from maternal plasma that had been stored in a device containing
the protective agent of the present teachings at ambient temperature for the
same period (e.g., 2 weeks) (Figure 9).
[0066] As discussed in reference to Figure 5, maternal blood collected into
standard K3EDTA tubes shows a 7-fold and 98-fold increase in total cell-free
DNA in maternal plasma at 24 hrs and 14 days as compared to 3 hrs,
respectively. However, total cell-free DNA concentration is constant at
ambient
temperature for up to 14 days in maternal blood contacted by the protective
agent of the present teachings (Figure 6). Without intending to be bound by
theory, this indicates that the chemicals present in the protective agent of
the
present teachings are able to fix the nucleated blood cells thereby preventing
apoptosis, cell death and cell lysis associated cellular genomic DNA release
into
plasma. A comparison of total cell-free DNA concentrations in K3EDTA and the
device of the present teachings at initial time point (3 hrs) show a
statistically
significant difference. The mean total cell-free DNA concentration in K3EDTA
tube at 3 hrs is 6341 GE/ ml, whereas in a tube containing the protective
agent of
CA 02690651 2010-01-21
the present teachings, it is only 680 GE/ ml. This multi-fold (e.g., 9-fold)
increase
in total cell-free DNA concentration in the samples contacted by only K3EDTA
as
compared to samples contacted by the protective agent of the present teachings
may result from increased cellular genomic DNA release from nucleated blood
cell apoptosis, death and lysis during post blood draw ex vivo incubation and
sample processing, and may be substantially avoided by use of the teachings
herein.
[0067] Figure 7 shows the expected effect of ex vivo incubation (of blood
drawn into K3EDTA tube) on fetal cell-free DNA percentage in maternal blood
plasma. There is a statistically significant decrease in fetal cell-free DNA
percentage over time. The major contributor to this steady decrease in fetal
cell-
free DNA percentage may be the increased background maternal cell-free DNA,
as fetal cell-free DNA degradation may occur due to nuclease action. In
contrast,
as shown in Figure 8, fetal cell-free DNA percentage of maternal blood
contacted
by the protective agent of the present teachings may be substantially constant
over time at ambient temperature. It is believed that this protective effect
may be
the result of the chemicals present in the protective agent of the present
teachings that stabilize blood cells preventing cellular DNA release as well
as
nuclease inhibitory activity that protect fetal cell-free DNA from
degradation.
Thus, the teachings herein contemplate treating a sample in a matter such that
limits the deleterious effects of DNase and RNase on the fetal nucleic acids
present in the plasma.
[0068] As evidenced by the examples and testing results disclosed herein,
fetal cell-free DNA found in maternal blood plasma is a valuable source for
noninvasive prenatal diagnosis. However, a major factor that limits the
effective
use of fetal cell-free DNA in nucleic acid-based prenatal testing is that the
total
DNA concentration present in maternal plasma, comes largely from the mother
herself. Thus, samples may be free of cell-free DNA attributable to apoptosis,
cell
death and lysis of nucleated maternal blood cells. This may be the case after
about 4 hours from blood draw, 6 hours from blood draw, or even 24 hours from
blood draw.
26
CA 02690651 2010-01-21
[0069] When the integrity of a DNA target is compromised, the targeted
DNA sequence fails to be amplified. Since most of the DNA-based prenatal
diagnostic tests depend on subsequent DNA amplification, it is important to
protect the integrity of rare DNA targets such as fetal cell-free DNA during
all pre-
analytical procedures. Here, fetal cell-free DNA percentage is determined by
real-time quantitative PCR. Figure 8 shows that fetal cell-free DNA percentage
stays substantially constant for up to 14 days and provides evidence that the
protective agent of the present teachings can protect the integrity of fetal
cell-free
DNA at ambient temperature for up to 14 days.
[0070] One of the factors that limit the use of fetal cell-free DNA in
maternal
plasma in noninvasive prenatal diagnosis is its low relative level in maternal
plasma. Therefore, we amplify fetal cell-free DNA in maternal plasma by whole
genome amplification (WGA). A first trimester pregnant donor with a male fetus
is
identified by amplifying Y chromosomal SRY region sequence. Amplification of
cell-free plasma DNA obtained from this donor by WGA and subsequent
detection of Y chromosomal SRY region sequence by real-time PCR shows a
multi-fold (e.g., at least 10, 20, 40 or -80-fold) increase in fetal cell-free
DNA
concentration (Figure 9). When cell-free DNA for WGA isolated from blood
contacted by the protective agent of the present teachings and stored at
ambient
temperature for 14 days, results are expected to provide strong evidence that
the
protective agent of the present teachings is able to preserve the integrity of
long
fetal cell-free DNA molecules that are required for WGA.
[0071] This new methodology can be used to circumvent many existing pre-
analytical issues that can affect the detection of fetal cell-free DNA in
maternal
blood. Since the protective agent of the present teachings stabilizes
nucleated
blood cells and inhibits plasma nucleases, it is possible to store maternal
blood
samples in a device containing the protective, agent of the present teachings
at
ambient temperature for up to 14 days without any increase in background
maternal cell-free DNA concentration and without any alteration in cell-free
DNA
integrity. Methods herein contemplate such storing. The methods herein also
contemplate using the device of the present teachings to draw maternal blood
for
27
CA 02690651 2010-01-21
noninvasive prenatal diagnosis when blood drawing and nucleic acid testing are
not done at the same location. The methods herein thus may be free of any step
of immediate separation of plasma after blood draw, freezing of the plasma
(e.g.,
at -800C) or both, for shipping. The methods herein may also be free of any
use
of magnetic beads or particles of any kind. The methods herein may be free of
any addition of formaldehyde to the blood sample immediately following the
blood draw.
[0072] The examples and testing results discussed above demonstrate an
unexpected synergistic effect occurring only in blood samples contacted by
both
a fixative and an anticoagulant, or more specifically, by IDU, EDTA and
glycine.
Maternal blood samples contacted by only a fixative or only an anticoagulant
do
not demonstrate the ability to maintain the integrity of the maternal blood
cells or
the integrity of the fetal nucleic acids. The combined effect of the IDU, EDTA
and
glycine far exceeds any expectations based on the effect, or lack thereof, of
the
IDU or EDTA or glycine used alone.
[0073] It will be appreciated that concentrates or dilutions of the amounts
recited herein may be employed. In general, the relative proportions of the
ingredients recited will remain the same. Thus, by way of example, if the
teachings call for 30 parts by weight of a Component A, and 10 parts by weight
of
a Component B, the skilled artisan will recognize that such teachings also
constitute a teaching of the use of Component A and Component B in a relative
ratio of 3:1. Teachings of concentrations in the examples may be varied within
about 25% (or higher) of the stated values and similar results are expected.
Moreover, such compositions of the examples may be employed successfully in
the present methods to isolate fetal nucleic acids (e.g., cell-free fetal
DNA).
[0074] It will be appreciated that the above is by way of illustration only.
Other ingredients may be employed in any of the compositions disclosed herein,
as desired, to achieve the desired resulting characteristics. Examples of
other
ingredients that may be employed include antibiotics, anesthetics,
antihistamines, preservatives, surfactants, antioxidants, unconjugated bile
acids,
mold inhibitors, nucleic acids, pH adjusters, osmolarity adjusters, or any
28
CA 02690651 2010-01-21
combination thereof.
[0075] The explanations and illustrations presented herein are intended to
acquaint others skilled in the art with the invention, its principles, and its
practical
application. Those skilled in the art may adapt and apply the invention in its
numerous forms, as may be best suited to the requirements of a particular use.
Accordingly, the specific embodiments of the present invention as set forth
are
not intended as being exhaustive or limiting of the invention. The scope of
the
invention should, therefore, be determined not with reference to the above
description, but should instead be determined with reference to the appended
claims, along with the full scope of equivalents to which such claims are
entitled.
Other combinations are also possible as will be gleaned from the following
claims.
29