Note: Descriptions are shown in the official language in which they were submitted.
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
IMPROVED CATHETER
RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application Serial No.
60/946,807, filed June 28, 2007, entitled "ULTRASOUND CATHETER", the entirety
of
which is hereby incorporated by reference, and U.S. Patent Application Serial
No.
12/163,325, filed June 27, 2008, entitled "IMPROVED CATHETER", the entirety of
which is hereby incorporated by reference.
FIELD OF THE INVENTION
The invention relates to improved catheters, and is particularly apt to
catheters
for imaging and interventional device delivery (e.g. ultrasound catheters with
diagnostic
or therapeutic device, agent or energy delivery capabilities) that can be used
to obtain
targeted images of interventional devices positioned at desired locations in
the body of a
patient and/or delivery target locations.
BACKGROUND OF THE INVENTION
Catheters are tubular medical devices that may be inserted into a body vessel,
cavity or duct, and manipulated utilizing a portion that extends out of the
body.
Typically, catheters are relatively thin and flexible to facilitate
advancement/retraction
along non-linear paths. Catheters may be employed for a wide variety of
purposes,
including the internal bodily positioning of diagnostic and/or therapeutic
devices. For
example, catheters may be employed to position internal imaging devices,
deploy
implantable devices (e.g., stents, stent grafts, vena cava filters), and/or
deliver energy
(e.g., ablation catheters).
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
In this regard, use of ultrasonic imaging techniques to obtain visible images
of
structures is increasingly common, particularly in medical applications.
Broadly stated,
an ultrasonic transducer, typically comprising a number of individually
actuated
piezoelectric elements, is provided with suitable drive signals such that a
pulse of
ultrasonic energy travels into the body of the patient. The ultrasonic energy
is reflected
at interfaces between structures of varying acoustic impedance. The same or a
different transducer detects the receipt of the return energy and provides a
corresponding output signal. This signal can be processed in a known manner to
yield
an image, visible on a display screen, of the interfaces between the
structures and
hence of the structures themselves.
Numerous prior art patents discuss the use of ultrasonic imaging in
combination
with specialized surgical equipment in order to perform very precise surgical
procedures. For example, a number of patents show use of ultrasonic techniques
for
guiding a "biopsy gun", i.e., an instrument for taking a tissue sample from a
particular
area for pathological examination, for example, to determine whether a
particular
structure is a malignant tumor or the like. Similarly, other prior art patents
discuss use of
ultrasonic imaging techniques to assist in other delicate operations, e.g.,
removal of
viable eggs for in vitro fertilization, and for related purposes.
As internal diagnostic and therapeutic procedures continue to evolve, the
desirability of enhanced procedure imaging via compact and maneuverable
catheters
has been recognized. More particularly, the present inventors have recognized
the
desirability of providing catheter features that facilitate selective
positioning and control
of componentry located at a distal end of a catheter, while maintaining a
relatively small
profile, thereby yielding enhanced functionality for various clinical
applications.
2
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
SUMMARY OF THE INVENTION
The present invention relates to improved catheter designs. For purposes
hereof, a catheter is defined as a device which is capable of being inserted
into a body
vessel, cavity or duct, wherein at least a portion of the catheter extends out
of the body
and the catheter is capable of being manipulated and/or removed from the body
by
manipulating/pulling on the portion of the catheter extending out of the body.
In the
various designs the catheter comprises an outer tubular body having a wall, a
proximal
end and a distal end. The catheter may further include a deflectable member
located at
the distal end of the outer tubular body. The deflectable member may include
one or
more therapeutic and/or diagnostic devices. For example, the deflectable
member may
include an imaging device such as an ultrasound transducer array. The
deflectable
member may be selectively deflectable relative to the outer tubular body to
facilitate
operation of componentry comprising the deflectable member.
In an additional aspect, at least a portion of the deflectable member may be
permanently located outside of the outer tubular body. In this regard, the
deflectable
member may be selectively deflectable away from a center axis of the outer
tubular
body. In certain embodiments, such deflectability may be at least partially or
entirely
distal to the distal end of the outer tubular body.
In one aspect, the catheter may also include a lumen for delivering an
interventional device extending through the outer tubular body from the
proximal end of
the outer tubular body to a point distal thereto. For purposes hereof,
"interventional
device" includes without limitation diagnostic devices (e.g. pressure
transducers,
conductivity measurement devices, temperature measurement devices, flow
measurement devices, electro- and neuro-physiology mapping devices, material
detection devices, imaging devices, central venous pressure (CVP) monitoring
devices,
3
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
intracardiac echocardiography (ICE) catheters, balloon sizing catheters,
needles, biopsy
tools), therapeutic devices (e.g. ablation catheters (e.g., radio-frequency,
ultrasonic,
optical), patent foramen ovale (PFO) closure devices, cryotherapy catheters,
vena cava
filters, stents, stent-grafts, septostomy tools), and agent delivery devices
(e.g., needles,
cannulae, catheters, elongated members). For purposes hereof, "agent" includes
without limitation therapeutic agents, pharmaceuticals, chemical compounds,
biologic
compounds, genetic materials, dyes, saline, and contrast agents. The agent may
be
liquid, gel, solid, or any other appropriate form. Furthermore, the lumen may
be used to
delivery agents therethrough without the use of an interventional device. The
combinative inclusion of a deflectable member and lumen for interventional
device
delivery therethrough facilitates multi-functionality of the catheter. This is
advantageous
because it reduces the number of catheters and access sites required during
the
procedure, provides the potential to limit the interventional procedure time,
and
enhances ease of use.
In this regard, in certain embodiments the lumen may be defined by an inside
surface of the wall of the outer tubular body. In other embodiments, the lumen
may be
defined by an inside surface of an inner tubular body located within the outer
tubular
body and extending from the proximal end to the distal end thereof.
In another aspect, a deflectable member may be selectively deflectable through
an arc of at least 45 degrees, and in various implementations at least 90
degrees. For
example, the deflectable member may be deflectable in a pivot-like manner
about a
pivot, or hinge, axis through an arc of at least 90 degrees. Further, the
deflectable
member may be selectively deflectable and maintainable at a plurality of
positions
across a range of different angled positions. Such embodiments are
particularly apt for
implementing a deflectable member comprising an imaging device.
4
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
In certain embodiments, a deflectable imaging device may be selectively
deflectable from an exposed (e.g., where at least a portion of the aperture of
the
deflectable imaging device is free from interference from the outer tubular
body) side-
looking first position to an exposed forward-looking, second position. "Side-
looking" as
used herein is defined as the position of the deflectable imaging device where
the field
of view of the deflectable imaging device is oriented substantially
perpendicular to the
distal end of the outer tubular body. "Forward-looking" includes where the
imaging field
of view of the deflectable imaging device is at least partially deflected to
enable imaging
of a volume distal to the distal end of the catheter. For example, a
deflectable imaging
device (e.g., an ultrasound transducer array) may be aligned with (e.g.,
disposed
parallel to or coaxially with) a center axis of the outer tubular body in a
first position.
Such an approach accommodates imaging of anatomical landmarks during catheter
positioning (e.g. during insertion and advancement of the catheter into a
vascular
passageway or bodily cavity), wherein anatomical landmark images may be
employed
to precisely position an exit port of a lumen comprising the catheter. In
turn, the
ultrasound transducer array may be deflected from the side-looking, first
position to a
forward-looking, second position (e.g., angled at least 45 degrees, or in some
applications at least 90 degrees) relative to a center axis of the catheter.
An
interventional device may then be selectively advanced through a lumen of the
catheter
and into a work area located adjacent to a lumen exit port and within an
imaging field of
view of the ultrasound transducer array, wherein imaged internal procedures
may be
completed utilizing the interventional device with imaging from the ultrasound
transducer
array alone or in combination with other imaging modalities (e.g.,
fluoroscopy). The
deflectable imaging device may be deflected such that no part of the
deflectable imaging
device occupies a volume with the same cross section as the exit port and
extending
5
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
distally from the exit port. As such, the imaging field of view of the
deflectable imaging
device may be maintained in a fixed registration relative to the outer tubular
body while
the interventional device is being advanced through the outer tubular body,
through the
exit port, and into the imaging field of view of the deflectable imaging
device.
In a related aspect, a deflectable member may comprise an ultrasound
transducer array having an aperture length at least as large as a maximum
cross-
dimension of the outer tubular body. Correspondingly, the deflectable
ultrasound
transducer array may be provided for selective deflection from a first
position that
accommodates advancement of the catheter through a vascular passageway to a
second position that is angled relative to the first position. Again, in
certain
embodiments the second position may be selectively established by a user.
In a related aspect, deflectable member may be deflectable from a first
position
aligned with the center axis of the catheter (e.g. parallel thereto) to a
second position
angled relative to the center axis, wherein when in the second position the
deflectable
member is disposed outside of a working area located adjacent to a lumen exit
port. As
such, an interventional device may be advanceable through the exit port free
from
interference with the deflectable member.
In certain embodiments, the deflectable member may be provided so that the
cross-sectional configuration thereof generally coincides with the cross-
sectional
configuration of the outer tubular body at the distal end thereof. For
example, when a
cylindrically-shaped outer tubular body is employed, a deflectable member may
be
located beyond the distal end of the outer tubular body and configured to
coincide with
(e.g., slightly exceed, occupy, or fit within) an imaginary cylindrical volume
defined by
and adjacent to such distal end, wherein the deflectable member is selectively
6
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
deflectable out of such volume. Such an approach facilitates initial
advancement and
positioning of the catheter through vascular passageways.
In certain embodiments, a deflectable member may be provided to deflect along
an arc path that extends away from a center axis of the outer tubular body. By
way of
example, in various implementations the deflectable member may be disposed to
deflect
from a first position that is located distal to a lumen exit port, to a second
position that is
lateral to the outer tubular body (e.g. to one side of the outer tubular
body).
In another aspect, a deflectable member may be provided to deflect from a
longitudinal axis of the catheter, wherein upon deflection a displacement arc
is defined.
In a catheter with a tip fixed relative to the outer tubular body, the
displacement arc is
the minimum curvature of the catheter. In a catheter with a deflectable member
movable relative to the outer tubular body, the displacement arc is the
minimum arc that
is tangent to a face of the deflectable member and tangent to the center axis
of the
catheter. In the present aspect, a deflectable member may be provided wherein
a ratio
of a maximum cross-dimension of the distal end of the outer tubular body to
the
displacement arc radius is at least about 1. By way of example, for a
cylindrical outer
tubular body, the ratio may be defined by the outer diameter of the distal end
of the
outer tubular body over the displacement arc radius, wherein such ratio may be
advantageously established to be at least about 1.
In another aspect, a deflectable member may be interconnected to the catheter
body wall at the distal end of the outer tubular body. As will be further
described, such
interconnection may provide support functionality and/or selective deflection
functionality. In the latter regard, the deflectable member may be deflectable
about a
deflection axis that is offset from a center axis of the outer tubular body.
For example,
the deflection axis may lie in a plane that extends transverse to the center
axis of an
7
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
outer tubular body and/or in a plane that extends parallel to the center axis.
In the
former regard, in one embodiment the deflection axis may lie in a plane that
extends
orthogonal to the center axis. In certain implementations, the deflection axis
may lie in a
plane that extends tangent to an exit port of a lumen that extends through the
outer
tubular body of the catheter.
In yet another aspect, the catheter may comprise a lumen for delivering an
interventional device extending from the proximal end to an exit port located
at the distal
end of the outer tubular body, wherein the exit port has a center axis
coaxially aligned
with a center axis of the outer tubular body. Such an arrangement facilitates
the
realization of relatively small catheter cross-dimensions, thereby enhancing
catheter
positioning (e.g. within small and/or tortuous vascular passageways). The
deflectable
member may also be disposed for deflection away from the coaxial center axes,
thereby
facilitating angled lateral positioning away from the initial catheter
introduction (e.g., 0
degree) position of the deflectable member. In certain embodiments, the
deflectable
member may be deflectable through an arc of at least 90 degrees.
In a further aspect, the catheter may include an actuation device, extending
from
the proximal end to the distal end of the outer tubular body, wherein the
actuation device
may be interconnected to the deflectable member. The actuation device and
outer
tubular body may be disposed for relative movement such that the deflectable
member
is deflectable through an arc of at least 45 degrees in response to 0.5 cm or
less relative
movement between the actuation device and the outer tubular body. By way of
example, in certain embodiments the deflectable member may be deflectable
through
an arc of at least 90 degrees in response to 1.0 cm or less relative movement
of the
actuation device and outer tubular body.
8
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
In a further aspect, the deflectable member may be interconnected to the outer
tubular body. In one approach, the deflectable member may be supportably
interconnected to the outer tubular body at the distal end thereof. In turn,
an actuation
device comprising one or more elongate members (e.g. of wire-like
construction) may
be disposed along the outer tubular body and interconnected at a distal end to
the
deflectable member, wherein upon applying a tensile force (e.g. a pull force)
to a
proximal end of the elongate member(s) the distal end of the elongate
member(s) may
cause the deflectable member to deflect. In this approach, the outer tubular
body may
define a lumen therethrough for delivering an interventional device extending
from the
proximal end of the outer tubular body to an exit port located distal to the
proximal end.
In another approach, a deflectable member may be supportably interconnected
to one of the outer tubular body and an actuation device, and restrainably
interconnected by a restraining member (e.g. a ligature) to the other one of
the outer
tubular body and actuation device, wherein upon relative movement of the outer
tubular
body and actuation device the restraining member restrains movement of the
deflectable member to affect deflection thereof.
For example, the deflectable member may be supportably interconnected to an
actuation device and restrainably interconnected to the outer tubular body at
the distal
end thereof. In this approach, the actuation device may comprise an inner
tubular body
defining a lumen therethrough for delivering an interventional device
extending from the
proximal end of the catheter body to an exit port located distal to the
proximal end.
More particularly, and in a further aspect, the catheter may comprise an inner
tubular body, disposed within the outer tubular body for relative movement
therebetween (e.g., relative slidable movement). A deflectable member located
at the
distal end may be supportably interconnected to the inner tubular body. In
certain
9
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
embodiments, the deflectable member may be disposed so that upon selective
relative
movement of the outer tubular body and inner tubular body the deflectable
member is
selectively deflectable and maintainable in a desired angular orientation.
For example, in one implementation an inner tubular body may be slidably
advanced and retracted relative to an outer tubular body, wherein engagement
between
surfaces of the two components provides a mechanism interface sufficient to
maintain a
selected relative position of the two components and corresponding deflected
position of
the deflectable member. A proximal handle may also be provided to facilitate
the
maintenance of selected relative positioning of the two components.
In an additional aspect, the catheter may include an actuation device,
extending
from a proximal end to a distal end of the outer tubular body and moveable
relative to
the outer tubular body to apply a deflection force to the deflectable member.
In this
regard, the actuation device may be provided so that deflection force is
communicated
by the actuation device from the proximal end to the distal end in a balanced
and
distributed manner about a center axis of the outer tubular body. As may be
appreciated, such balanced and distributed force communication facilitates the
realization of a non-biased catheter yielding enhanced control and positioning
attributes.
In conjunction with one or more of the above-noted aspects, the catheter may
include a hinge that is supportably interconnected to the outer tubular body
or, in certain
embodiments, to an included actuation device (e.g. an inner tubular body). The
hinge
may be structurally separate from and fixedly interconnected to the catheter
body (e.g.,
the outer tubular body or the inner tubular body). The hinge may be further
fixedly
interconnected to the deflectable member, wherein the deflectable member is
deflectable in a pivot-like manner. The hinge member may be at least partially
elastically deformable to deform from a first configuration to a second
configuration
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
upon the application of a predetermined actuation force, and to at least
partially return
from the second configuration to the first configuration upon removal of the
predetermined actuation force. Such functionality facilitates the provision of
a
deflectable member that may be selectively actuated via an actuation device to
move
from an initial first position to a desired second position upon the
application of a
predetermined actuation force (e.g. a tensile or pulling force, or a
compressive pushing
force applied thereto), wherein upon selective release of the actuation force
the
deflectable member may automatically at least partially retract to its initial
first position.
In turn, successive deflectable positioning/retraction of the deflectable
member may be
realized during a given procedure, thereby yielding enhanced functionality in
various
clinical applications.
In certain embodiments, the hinge member may be provided to have a column
strength sufficient to reduce unintended deflection of the deflectable member
during
positioning of the catheter (e.g. due to mechanical resistance associated with
advancement of the catheter). By way of example, the hinge member may exhibit
a
column strength at least equivalent to that of the outer tubular body.
In certain implementations the hinge may be a portion of a one-piece,
integrally
defined member. For example, the hinge may comprise a shape memory material
(e.g.,
Nitinol). In one approach, the hinge member may include a curved first portion
and a
second portion interconnected thereto, wherein the second portion is
deflectable about a
deflection axis defined by the curved first portion. By way of example, the
curved first
portion may comprise a cylindrically-shaped surface. In one embodiment, the
curved
first portion may include two cylindrically-shaped surfaces having
corresponding center
axes that extend in a common plane and intersect at an angle, wherein a
shallow,
saddle-like configuration is defined by the two cylindrically-shaped surfaces.
11
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
In yet a further aspect, the outer tubular body may be constructed to
facilitate the
inclusion of electrical componentry at the distal end thereof. More
particularly, the outer
tubular body may comprise a plurality of interconnected electrical conductors
extending
from the proximal end to the distal end. For example, in certain embodiments
the
electrical conductors may be interconnected in a ribbon-shaped member that is
helically
disposed about and along all or at least a portion of a catheter center axis,
thereby
yielding enhanced structurally qualities to the wall of the outer tubular body
and avoiding
excessive strain on the electrical conductors during flexure of the outer
tubular body.
For example, in certain embodiments the electrical conductors may be braided
along at
least a portion of the catheter center axis, thereby yielding enhanced
structurally
qualities to the wall of the outer tubular body. The outer tubular body may
further
include a first layer disposed inside of the first plurality of electrical
conductors and
extending from the proximal end to the distal end, and a second layer disposed
on the
outside of the first plurality of electrical conductors, extending from the
proximal end to
the distal end. The first tubular layer and second tubular layer may each be
provided to
have a dielectric constant of about 2.1 or less, wherein capacitive coupling
may be
advantageously reduced between the plurality of electrical conductors and
bodily fluids
present outside of the catheter and within a lumen extending through the outer
tubular
body.
In another aspect, the outer tubular body may comprise a plurality of
electrical
conductors extending from a proximal end to the distal end and a set of
tubular layers
inside and/or outside of the first plurality of electrical conductors. The set
of tubular
layers may comprise a low dielectric constant layer (e.g., located closest to
the electrical
conductors), and a high withstand voltage layer. In this regard, the low
dielectric
constant layer may have a dielectric constant of 2.1 or less, and the high
withstand
12
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
voltage layer may be provided to yield a withstand voltage of at least about
2500 volts
AC. In certain embodiments, a set of low dielectric and high withstand voltage
layers
may be provided both inside and outside of the plurality of electrical
conductors along
the length of the outer tubular body.
In certain embodiments tie layers may be interposed between the electrical
conductors and one or more inner and/or outer layers. By way of example, such
tie
layers may comprise a film material that may have a melt temperature that is
lower than
other components of the outer tubular body, wherein the noted layers of
components
may be assembled and the tie layers selectively melted to yield an
interconnected
structure. Such selectively melted tie layers may prevent other layers of the
outer
tubular body from migrating relative to each other during manipulation of the
outer
tubular body (e.g., during insertion into a patient).
For some arrangements, the outer tubular body may further include a shielding
layer disposed outside of the electrical conductors. By way example, the
shielding layer
may be provided to reduce electromagnetic interference (EMI) emissions from
the
catheter as well as shield the catheter from external EMI.
In certain embodiments, lubricious inside and outside layers and/or coatings
may
also be included. That is, an inner layer may be disposed within the first
tubular layer
and an outer layer may be disposed outside of the second tubular layer.
In yet a further aspect, the catheter may be provided to comprise a first
electrical
conductor portion extending from a proximal end to a distal end of the
catheter, and a
second electrical conductor portion electrically interconnected to the first
electrical
conductive portion at the distal end. The first electrical conductor portion
may comprise
a plurality of interconnected electrical conductors arranged side-by-side with
electrically
non-conductive material therebetween. In certain implementations, the first
electrical
13
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
conductor portion may be helically disposed about a catheter center axis from
the
proximal end to the distal end thereof. In conjunction with such
implementations, the
second electrical conductor portion may comprise a plurality of electrical
conductors
interconnected to the plurality of interconnected electrical conductors of the
first
electrical conductor portion, and extending parallel to a center axis of the
outer tubular
body at the distal end. In certain embodiments, the first electrical conductor
portion may
be defined by a ribbon-shaped member included within the wall of the outer
tubular
body, thereby contributing to the structural integrity thereof.
In conjunction with the noted aspect, the first electrical conductor portion
may
define a first width across the interconnected plurality of electrical
conductors, and the
second electrical conductor portion may define a second width across the
corresponding
plurality of electrical conductors. In this regard, the second electrical
conductor portion
may be defined by electrically conductive traces disposed on a substrate. By
way of
example, the substrate may extend between the end of the first electrical
conductor
portion and electrical componentry provided at the distal end of a catheter,
including for
example an ultrasound transducer array.
In various embodiments, the second electrical conductor portion may be
interconnected to a deflectable member and may be of a bendable construction,
wherein at least a portion of the second electrical conductor portion is
bendable with and
in response to deflection of the deflectable member. More particularly, the
second
electrical conductor portion may be defined by electrically conductive traces
on a
substrate that is bendable in tandem with a deflectable member through an arc
of at
least 90 degrees.
In a further aspect, the catheter may comprise a deflectable member that
includes an ultrasound transducer array, wherein at least a portion of the
deflectable
14
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
ultrasound transducer array may be located within the outer tubular body wall
at the
distal end. Further, the catheter may include a lumen for delivering an
interventional
device extending from the proximal end to a point distal thereto.
In a still further aspect, the catheter may comprise a steerable or pre-curved
catheter segment located near the distal end of the outer tubular body and the
deflectable member may comprise an ultrasound transducer array. Further, the
catheter may include a lumen for delivering an interventional device extending
from the
proximal end to a point distal thereto.
In another aspect, the catheter may comprise an outer tubular body having a
wall, a proximal end and a distal end. The catheter may further include a
lumen for
delivering an interventional device extending through the outer tubular body
from the
proximal end to an exit port located distal to the proximal end. The catheter
may further
include a first electrical conductor portion comprising a plurality of
interconnected
electrical conductors arranged side-by-side with electrically non-conductive
material
therebetween. The first electrical conductor portion may extend from the
proximal end
to the distal end. The catheter may further include a second electrical
conductor portion
electrically interconnected to the first electrical conductor portion at the
distal end. The
second electrical conductor portion may comprise a plurality of electrical
conductors.
The catheter may further include a deflectable member located at the distal
end. The
second electrical conductor portion may be electrically interconnected to the
deflectable
member and may be bendable in response to deflection of the deflectable
member.
In another aspect, the catheter may comprise an outer tubular body having a
wall, a proximal end and a distal end. The catheter may further include a
lumen for
delivering an interventional device or agent delivery device extending through
the outer
tubular body from the proximal end to an exit port located distal to the
proximal end.
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
The catheter may further include a deflectable member, at least a portion of
which is
permanently located outside of the outer tubular body at the distal end,
selectively
deflectable relative to the outer tubular body and distal to the exit port. In
an
embodiment, the catheter may further include a hinge located at the distal end
where
the deflectable member may be supportably interconnected to the hinge. In such
an
embodiment, the deflectable member may be selectively deflectable relative to
the outer
tubular body about a hinge axis defined by the hinge.
Numerous aspects described hereinabove comprising a selectively deflectable
imaging device disposed at a distal end of an outer tubular body of a
catheter.
Additional aspects of the present invention may include deflectable members in
place of
such deflectable imaging devices. Such deflectable members may include imaging
devices, diagnostic devices, therapeutic devices, or any combination thereof.
In another aspect, a method is provided for operating a catheter having a
deflectable imaging device located at a distal end thereof. The method may
include
moving the distal end of the catheter from an initial position to a desired
position and
obtaining image data from the deflectable imaging device during at least a
portion of the
moving step. The deflectable imaging device may be located in a first position
during
the moving step. The method may further include utilizing the image data to
determine
when the catheter is located at the desired position, deflecting the
deflectable imaging
device from the first position to a second position after the moving step; and
advancing
an interventional device through an exit port at the distal end of the
catheter and into an
imaging field of view of the deflectable imaging device in the second
position.
In an arrangement, the deflecting step may further include translating a
proximal
end of at least one of an outer tubular body of the catheter and actuation
device of the
16
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
catheter relative to a proximal end of the other one of the outer tubular body
and
actuation device.
A deflection force may be applied to a hinge in response to the translating
step.
The deflectable imaging device may be supportably interconnected by the hinge
to one
of the outer tubular body and the actuation device. The deflection force may
be initiated
in response to the translating step. The deflection force may be communicated
in a
balanced and distributed manner about a center axis of the outer tubular body.
In an arrangement, the position of the deflectable imaging device may be
maintained relative to the distal end of the catheter during the moving and
obtaining
steps. In an embodiment, the deflectable imaging device may be side-looking in
the first
position and forward-looking in the second position. In an embodiment, the
imaging
field of view may be maintained in substantially fixed registration to the
distal end of the
catheter during the advancing step.
The various features discussed above in relation to each aforementioned aspect
may be utilized by any of the aforementioned aspects. Additional aspects and
corresponding advantages will be apparent to those skilled in the art upon
consideration
of the further description that follows.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a catheter embodiment having a deflectable ultrasound
transducer array located at an end of the catheter.
Figure 2A shows a cross-sectional view of the catheter embodiment of Figure 1.
Figure 2B shows a catheter embodiment having a deflectable ultrasound
transducer array located at a distal end of the catheter.
17
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Figures 2C and 2D show the catheter embodiment of Figures 2A and 2B, wherein
the catheter further includes an optional steerable segment.
Figures 3A and 3B show a further catheter embodiment having a deflectable
ultrasound transducer array located at a distal end of the catheter.
Figure 4 shows a catheter embodiment having electrically conductive wires
attached to an ultrasound transducer array located near the distal end of the
catheter,
wherein the electrically conductive wires helically extend to the proximal end
of the
catheter and are embedded in the catheter wall.
Figure 4A shows an exemplary conductive wire assembly.
Figure 5A shows an embodiment of a catheter that includes a deflectable
member.
Figures 5B through 5E show an embodiment of a catheter that includes a
deflectable member wherein the deflectable member is deflectable by moving an
inner
tubular body relative to an outer tubular body.
Figures 5F shows an embodiment of an electrical interconnection between a
helically disposed electrical interconnection member and a flexible electrical
member.
Figures 6A through 6D show an embodiment of a catheter that includes a
deflectable member wherein the deflectable member is deflectable by moving an
elongate member relative to a catheter body.
Figures 7A and 7B show a further aspect wherein an ultrasound transducer array
is located near the distal end of the catheter. The array can be manipulated
between
side-looking and forward-looking by utilizing an actuation device attached to
the array
and extending to the proximal end of the catheter.
Figures 8A through 8D show various exemplary variations of the catheter of
Figures 7A and 7B.
18
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Figures 9, 9A and 9B demonstrate further embodiments wherein an ultrasound
array is deflectable.
Figures 10A and 10B demonstrate further alternative embodiments.
Figures 11, 11A and 11 B demonstrate further embodiments.
Figure 12 demonstrates a still further embodiment.
Figure 13 is a flow chart for an embodiment of a method of operating a
catheter.
DETAILED DESCRIPTION OF THE DRAWINGS
The detailed description that follows is directed to various catheter
embodiments
that include a deflectable member that comprises an ultrasound transducer
array, and a
lumen for delivering an interventional device. Such embodiments are for
exemplarily
purposes and are not intended to limit the scope of the present invention. In
that
regard, the deflectable member may comprise componentry other than or in
addition to
an ultrasound transducer array. Further, additional embodiments may utilize
inventive
features described herein that do not necessitate the inclusion of a lumen.
An ultrasound transducer array built into a catheter presents unique design
challenges. Two critical points include, for example, the resolution in the
image plane
and the ability to align that image plane with an interventional device.
The resolution in the imaging plane of an ultrasound array can be approximated
by the following equation:
Lateral resolution = Constant * wavelength * Image Depth / Aperture Length
For catheters being described here, the wavelength is typically in the range
of 0.2 mm
(at 7.5 MHz). The constant is in the range of 2Ø The ratio of (Image
Depth/Aperture
Length) is a critical parameter. For ultrasound imaging in the range of 5 - 10
MHz for
19
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
catheters presented here, acceptable resolution in the imaging plane can be
achieved
when this ratio is in the range of 10 or less.
For imaging with a catheter in the major vessels and the heart, it is
desirable to
image at depths of 70 to 100 mm. Catheters used in the heart and major vessels
are
typically 3 to 4 mm in diameter or smaller. Thus while conceptually a
transducer array
can be made of arbitrary size and placed at any position within the catheter
body, this
model shows that transducer arrays that readily fit within the catheter
structure do not
have sufficient width for acceptable imaging.
The ultrasound image plane produced by the array placed on the catheter
typically has a narrow width typically called the out of plane image width.
For objects to
be seen in the ultrasound image, it is important that they be in this image
plane. When
a flexible/bendable catheter is placed in a major vessel or heart, the image
plane can be
aligned to some degree. It is desirable to guide a second device placed in the
body with
the ultrasound image, but doing so requires placing that second device in the
plane of
the ultrasound image. If the imaging array and the interventional device are
both on
flexible/bendable catheters that are inserted into the body, it is extremely
difficult to
orient one interventional device into the ultrasound image plane of the
imaging catheter.
Certain embodiments of the present invention utilize an ultrasound image to
guide an interventional device. To accomplish this, a large enough aperture is
needed
to produce an image of acceptable resolution while being able to place the
device in a
known position that is stable relative to the imaging array and/or to be able
to align
and/or register the interventional device to the ultrasound image plane.
In certain implementations, the aperture length of the ultrasound array may be
larger than the maximum cross dimension of the catheter. In certain
implementations,
the aperture length of the ultrasound array may be much larger (2 to 3 times
larger) than
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
the diameter of the catheter. This large transducer, however, may fit within
the 3 to
4 mm maximum diameter of the catheter to be inserted into the body. Once in
the body,
the imaging array is deployed out of the catheter body leaving space to pass
an
interventional device through that same catheter that will then be located in
a known
position relative to the imaging array. In certain arrangements, the imaging
array may
be deployed in a way so that the interventional device can be readily kept
within the
ultrasound image plane.
The catheter may be configured for delivery through a skin puncture at a
remote
vascular access site (e.g., vessel in the leg). Through this vascular access
site, the
catheter may be introduced into regions of the cardiovascular system such as
the
inferior vena cava, heart chambers, abdominal aorta, and thoracic aorta.
Positioning the catheter in these anatomic locations provides a conduit for
delivery of devices or therapy to specific target tissues or structures. One
example of
this includes bedside delivery of inferior vena cava filters in patients for
whom transport
to the catheterization laboratory is either high risk or otherwise
undesirable. The
catheter with the ultrasound transducer array allows the clinician to not only
identify the
correct anatomical location for placement of the inferior vena cava filter,
but also
provides a lumen through which the vena cava filter can be delivered under
direct
ultrasound visualization. Both location identification and delivery of a
device can occur
without withdrawal or exchange of the catheter and/or imaging device. In
addition, post-
delivery visualization of the device allows the clinician to verify placement
location and
function(s) prior to removal of the catheter.
Another application of such a catheter is as a conduit through which ablation
catheters can be delivered within the atria of the heart. Although ultrasound
imaging
catheters are utilized today in many of these cardiac ablation procedures, it
is very
21
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
difficult to achieve proper orientation of the ablation catheters and
ultrasound catheter
so as to attain adequate visualization of the ablation site. The catheter
described herein
provides a lumen through which the ablation catheter can be directed and the
position of
the ablation catheter tip monitored under direct ultrasound visualization. As
described,
the coaxial registration of this catheter and other interventional devices and
therapy
delivery systems provides the means by which direct visualization and control
can be
achieved.
Turning now to the figures, Figure 1 shows a catheter embodiment having an
ultrasound transducer array 7 located on a deflectable distal end of the
catheter 1.
Specifically, catheter 1 comprises a proximal end 3 and a distal end 2.
Located on the
distal end 2 is the ultrasound transducer array 7. Attached to ultrasound
transducer
array 7 is at least one electrically conductive wire 4 (such as a
microminiature flat cable)
that extends from the array 7 to the proximal end 3 of catheter 1. The at
least one
electrically conductive wire 4 exits the catheter proximal end 3 through a
port or other
opening in the catheter wall and is connected to transducer driver; image
processor 5
which provides a visual image via device 6.
Figure 2A is a cross-section of Figure 1 taken along lines A-A. As can be seen
in
Figure 2A, the catheter 1 includes a catheter wall portion 12 that extends at
least the
length of proximal end 3 and further defines lumen 10 that extends at least
the length of
proximal end 3. Catheter wall 12 can be any suitable material or materials,
such as
extruded polymers, and can comprise one or more layers of materials. Further
shown is
the at least one electrically conductive wire 4 located at the bottom portion
of wall 12.
Operation of the catheter 1 can be understood with reference to Figures 1 and
2B. Specifically, the catheter distal end 2 can be introduced into the desired
body lumen
and advanced to a desired treatment site with ultrasound transducer array 7 in
a "side-
22
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
looking" configuration (as shown in Figure 1). Once the target area is
reached,
interventional device 11 can be advanced through the lumen 10 of the catheter
1 and
out the distal port 13 and advanced in a distal direction. As can be seen, the
catheter 1
can be configured such that advancing interventional device 11 in a distal
direction out
distal port 13 can deflect distal end 2 and thus result in ultrasound
transducer array 7
being converted from "side-looking" to "forward-looking". Thus, the physician
can
advance interventional device 11 into the field of view of ultrasound
transducer array 7.
"Deflectable" is defined as the ability to move the ultrasound transducer
array, or
a portion of the catheter body containing the ultrasound transducer array,
away from the
longitudinal axis of the catheter body, such that 1) the transducer face is
fully or partially
forward facing, and 2) the distal exit port of the delivery lumen and the
catheter body
can be opened. Deflectable can include 1) "actively deflectable" meaning that
the array
or catheter portion containing the array can be moved by remote application of
force
(e.g., electrical (e.g., wired or wireless), mechanical, hydraulic, pneumatic,
magnetic,
etc.), transmission of that force by various means including pull wires,
hydraulic lines, air
lines, or electrical conductors; and 2) "passively deflectable" meaning that
the array or
catheter portion containing the array when in the resting, unstrained
condition, tends to
be in alignment with the catheter longitudinal axis and may be moved by local
forces
imparted by the introduction of interventional device 11.
In certain embodiments, the ultrasound transducer array may be deflected up to
90 degrees from the longitudinal axis of the catheter, as shown in Figure 2B.
Moreover,
the deflectable ultrasound transducer array 7 can be attached to the catheter
by a hinge
9 as shown in Figure 2C. In an embodiment, hinge 9 can be a spring-loaded
hinged
device. Such a spring-loaded hinge can be actuated from the proximal end of
the
23
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
catheter by any suitable means. In an embodiment, the spring-loaded hinge is a
shape
memory alloy actuated by withdrawal of an outer sheath.
With reference to Figures 2C and 2D, the catheter 1 can further comprise a
steerable segment 8. "Steerable" is defined as the ability to direct the
orientation of the
portions of the catheter 1 and lumen 10 distal to the steerable segment at an
angle with
respect to the catheter proximal to the steerable segment. Figure 2D shows the
steerable segment 8 deflected at an angle with respect to the catheter
proximal to the
steerable segment.
In a further embodiment, Figures 3A and 3B demonstrate a catheter 1 including
an ultrasound transducer array 7 on a deflectable distal end 17 of the
catheter 1. The
catheter 1 comprises a proximal end (not shown) and a deflectable distal end
17.
Ultrasound transducer array 7 is located at the deflectable distal end 17.
Conductive
wires 4 are attached to the ultrasound transducer array 7 and extend in a
proximal
direction to the proximal end of catheter 1. The catheter 1 also includes a
generally
centrally located lumen 10 that extends from the proximal end to the distal
tip of the
catheter. At distal end 17, the generally centrally located lumen 10 is
essentially
blocked or closed off by ultrasound transducer array 7. Finally, the catheter
1 also
includes at least one longitudinally extending slit 18 that extends through a
region
proximal to the ultrasound transducer array 7.
As can be seen in Figure 3B, once interventional device 11 is advanced
distally
through lumen 10, the interventional device 11 deflects deflectable distal end
17 and
ultrasound transducer array 7 in a downward motion, thus opening lumen 10 so
that
interventional device 11 may be advanced distally past the ultrasound
transducer array
7.
24
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
In various embodiments described herein, catheters may be provided having an
ultrasound transducer array located near the distal end thereof. The catheter
body may
comprise a tube having a proximal end and a distal end. Moreover, the catheter
may
have at least one lumen extending from the proximal end to at least near the
ultrasound
transducer array. The catheter may comprise electrically conductive wires
(e.g., a
microminiature flat cable) attached to the ultrasound transducer array and
being
imbedded in the catheter wall and helically extending from the ultrasound
transducer
array to the proximal end of the catheter.
Such a catheter is depicted, for example, in Figures 4 and 4A. Specifically,
Figures 4 and 4A demonstrate catheter 20 having a proximal end (not shown) and
a
distal end 22 with ultrasound transducer array 27 located at the distal end 22
of catheter
20. As can be seen, lumen 28 is defined by the inner surface of polymer tube
26, which
can be formed from a suitable lubricious polymer (such as, for example, PEBAX
72D,
PEBAX 63D, PEBAX 55D, high density polyethylene, polytetrafluoroethylene,
and
expanded polytetrafluoroethylene, and combinations thereof) and extends from
the
proximal end to the distal end 22 near the ultrasound transducer array 27. The
electrically conductive wires (e.g., microminiature flat cable) 24 are
helically wrapped
about polymer tube 26 and extend from near the ultrasound transducer array 27
proximally to the proximal end. An example of a suitable microminiature flat
cable is
shown in Figure 4A where microminiature flat cable 24 includes electrically
conductive
wires 21 and suitable ground, such as copper 23. A conductive circuit element
43 (such
as a flexboard) is attached to ultrasound transducer array 27 and to the
electrically
conductive wires 24. A suitable polymer film layer 40 (such as a lubricious
polymer and
or shrink wrap polymer) can be located over electrically conductive wires 24
to act as an
insulating layer between the electrically conductive wires 24 and a shielding
layer 41.
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Shielding layer 41 may comprise any suitable conductor that can be helically
wrapped
over polymer film 40, for example, in the opposing direction of the
electrically conductive
wires 21. Finally, outerjacket 42 can be provided over shielding layer 41 and
can be of
any suitable material, such as a lubricious polymer. Suitable polymers
include, for
example, PEBAX 70D, PEBAX 55D, PEBAX 40D, and PEBAX film 23D. The
catheter depicted in Figures 4 and 4A can include the deflectable distal end
and
steerable segments discussed above.
The above catheter provides a means to electrically interface with an
ultrasound
probe at the distal end of a catheter while providing a working lumen to
facilitate delivery
of interventional devices to the imaged area. The construction of the catheter
utilizes
the conductors both to power the array as well as to provide mechanical
properties that
enhance kink resistance and torqueability. The novel construction presented
provides a
means to package the conductors and necessary shielding in a thin wall, thus
providing
a sheath profile that is suited for interventional procedures, with an OD
targeted at or
below 14 French (Fr) and an ID targeted at above 8 Fr, thus facilitating
delivery of
typical ablation catheters, filter delivery systems, needles, and other common
interventional devices designed for vascular and other procedures.
Figure 5A shows an embodiment of a catheter 50 that includes a deflectable
member 52 and a catheter body 54. The catheter body 54 may be flexible and
capable
of bending to follow the contours of a body vessel into which it is being
inserted. The
deflectable member 52 may be disposed at a distal end 53 of the catheter 50.
The
catheter 50 includes a handle 56 that may be disposed at a proximal end 55 of
the
catheter 50. During a procedure where the deflectable member 52 is inserted
into the
body of a patient, the handle 56 and a portion of the catheter body 54 remain
outside of
the body. The user (e.g., physician, technician, interventionalist) of the
catheter 50 may
26
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
control the position and various functions of the catheter 50. For example,
the user may
hold the handle 56 and manipulate a slide 58 to control a deflection of the
deflectable
member 52. In this regard, the deflectable member 52 may be selectively
deflectable.
The handle 56 and slide 58 may be configured such that the position of the
slide 58
relative to the handle 56 may be maintained, thereby maintaining the selected
deflection
of the deflectable member 52. Such maintenance of position may at least
partially be
achieved by, for example, friction (e.g., friction between the slide 58 and a
stationary
portion of the handle 56), detents, and/or any other appropriate means. The
catheter 50
may be removed from the body by pulling (e.g., pulling the handle56).
Furthermore, the user may insert an interventional device (e.g., a diagnostic
device and/or therapeutic device) through an interventional device inlet 62.
The user
may then feed the interventional device through the catheter 50 to move the
interventional device to the distal end 53 of the catheter 50. Electrical
interconnections
between an image processor and the deflectable member may be routed through an
electronics port 60 and through the catheter body 54 as described below.
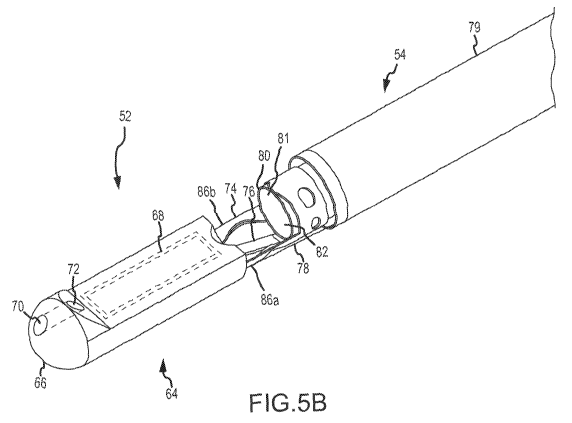
Figures 5B through 5E show an embodiment of a catheter that includes a
deflectable member 52 wherein the deflectable member 52 is deflectable by
moving an
inner tubular body 80 relative to an outer tubular body 79 of the catheter
body 54. As
shown in Figure 513, the illustrated deflectable member 52 includes a tip 64.
The tip 64
may encase various components and members.
The tip 64 may have a cross section that corresponds to the cross section of
the
outer tubular body 79. For example, and as illustrated in Figure 513, the tip
64 may have
a rounded distal end 66 that corresponds to the outer surface of the outer
tubular body
79. The portion of the tip 64 that houses the ultrasound transducer array 68
may be
shaped to at least partially correspond (e.g., along the lower outer surface
of the tip 64
27
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
as viewed in Figure 5B) to the outer surface of the outer tubular body 79. At
least a
portion of the tip 64 may be shaped to promote transport through internal
structures of
the patient such as the vasculature. In this regard, the rounded distal end 66
that may
aid in moving the deflectable member 52 through the vasculature. Other
appropriate
end shapes may be used for the shape of the distal end 66 of the tip 64.
In an embodiment, such as the one illustrated in Figures 5B through 5D, the
tip
64 may hold an ultrasound transducer array 68. As will be appreciated, as
illustrated in
Figure 5B, the ultrasound transducer array 68 may be side-looking when the
deflectable
member 52 is aligned with the outer tubular body 79. The field of view of the
ultrasound
transducer array 68 may be located perpendicular to the flat upper face (as
oriented in
Figure 513) of the ultrasound transducer array 68. As illustrated in Figure
513, the field of
view of the ultrasound transducer array 68 may be unobstructed by the outer
tubular
body 79 when the ultrasound transducer array 68 is side-looking. In this
regard, the
ultrasound transducer array 68 may be operable to image during catheter body
54
positioning, thereby enabling imaging of anatomical landmarks to aid in
positioning the
distal end of a lumen 82. The ultrasound transducer array 68 may have an
aperture
length. The aperture length may be greater than a maximum cross dimension of
the
outer tubular body 79. At least a portion of the deflectable member 52 may be
permanently positioned distal to the distal end of the outer tubular body 79.
In an
embodiment, the entirety of the deflectable member 52 may be permanently
positioned
distal to the distal end of the outer tubular body 79. In such an embodiment,
the
deflectable member may be incapable of being positioned within the outer
tubular body
79.
The tip 64 may further include a feature to enable the catheter to follow a
guide
wire. For example, as illustrated in Figure 513, the tip 64 may include a
distal guide wire
28
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
aperture 70 functionally connected to a proximal guide wire aperture 72. In
this regard,
the catheter may be operable to travel along the length of a guide wire
threaded through
the distal 70 and proximal 72 guide wire apertures.
As noted, the deflectable member 52 may be deflectable relative to the outer
tubular body 79. In this regard, the deflectable member 52 may be
interconnected to
one or more members to control the motion of the deflectable member 52 as it
is being
deflected. A tether 78 may interconnect the deflectable member 52 to the
catheter body
54. The tether 78 may be anchored to the deflectable member 52 on one end and
to
the catheter body 54 on the other end. The tether 78 may be configured as a
tensile
member operable to prevent the anchor points from moving a distance away from
each
other greater than the length of the tether 78. In this regard, through the
tether 78, the
deflectable member 52 may be restrainably interconnected to the outer tubular
body 79.
An inner tubular body 80 may be disposed within the outer tubular body 79. The
inner tubular body 80 may include the lumen 82 passing through the length of
the inner
tubular body 80. The inner tubular body 80 may be movable relative to the
outer tubular
body 79. This movement may be actuated by movement of the slide 58 of Figure
5A. A
support 74 may interconnect the deflectable member 52 to the inner tubular
body 80.
The support 74 may be structurally separate from the inner tubular body 80 and
the
outer tubular body 79. A flexboard 76 may contain electrical interconnections
operable
to electrically connect the ultrasound transducer array 68 to an electrical
interconnection
member 104 (shown in Figure 5E) disposed within the outer tubular body 79. The
exposed portion of flexboard 76 between the tip 64 and the outer tubular body
79 may
be encapsulated to isolate it from possible contact with fluids (e.g., blood)
when the
deflectable member 52 is disposed within a patient. In this regard, the
flexboard 76 may
be encapsulated with an adhesive, a film wrap, or any appropriate component
operable
29
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
to isolate the electrical conductors of the flexboard 76 from the surrounding
environment. In an embodiment, the tether 78 may be wrapped around the portion
of
the flexboard 76 between the tip 64 and the outer tubular body 79.
Deflection of the deflectable member 52 will now be discussed with reference
to
Figures 5C and 5D. Figures 5C and 5D illustrate the deflectable member 52 with
the
portion of the tip 64 surrounding the ultrasound image array 68 and support 74
removed. As illustrated in Figure 5C, the support 74 may include a tubular
body
interface portion 84 operable to fix the support 74 to the inner tubular body
80. The
tubular body interface portion 84 may be fixed to the inner tubular body 80 in
any
appropriate manner. For example, the tubular body interface portion 84 may be
secured to the inner tubular body 80 with an external shrink wrap. In such a
configuration, the tubular body interface portion 84 may be placed over the
inner tubular
body 80 and then a shrink-wrap member may be placed over the tubular body
interface
portion 84. Heat may then be applied causing the shrink wrap material to
shrink and fix
the tubular body interface portion 84 to the inner tubular body 80. An
additional wrap
may then be applied over the shrink wrap to further fix the tubular body
interface portion
84 to the inner tubular body 80. In another example, the tubular body
interface portion
84 may be secured to the inner tubular body 80 with an adhesive, a weld,
fasteners, or
any combination thereof.
The support 74 may comprise, for example, a shape memory material (e.g., a
shape memory alloy such as Nitinol). The support 74 may further include a
hinge
portion 86. The hinge portion 86 may comprise one or more members
interconnecting
the tubular body interface portion 84 with a cradle portion 88. The hinge
portion 86, as
illustrated in Figures 5B through 5C, may comprise two members. The cradle
portion 88
may support the ultrasound transducer array 68. The support 74, including the
hinge
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
portion 86, may possess a column strength adequate to keep the deflectable
member
52 substantially aligned with the outer tubular body 79 in the absence of any
advancement of the inner tubular body 80 relative to the outer tubular body
79. In this
regard, the deflectable member 52 may be operable to remain substantially
aligned with
the outer tubular body 79 when the outer tubular body 79 is being inserted
into and
guided through the patient.
The hinge portion 86 may be shaped such that upon application of an actuation
force, the hinge portion 86 elastically deforms along a predetermined path
about a
deflection axis 92. The predetermined path may be such that the tip 64 and the
hinge
portion 86 each are moved to a position where they do not interfere with an
interventional device emerging from the distal end of the lumen 82. An imaging
field of
view of the ultrasound transducer array 68 may be substantially maintained in
a position
relative to the outer tubular body 79 when the interventional device is
advanced through
the exit port 81 at the distal end of the lumen 82 and into the field of view.
As illustrated
in Figures 5B through 5D, the hinge portion may comprise two generally
parallel
sections 86a and 86b, where the ends of each of the generally parallel
sections 86a and
86b (e.g., where the hinge portion 86 meets the cradle portion 88 and where
the hinge
portion 86 meets the tubular body interface portion 84) may be generally
shaped to
coincide with a cylinder oriented along a center axis 91 of the inner tubular
body 80. A
central portion of each of the generally parallel sections 86a and 86b may be
twisted
toward the center axis 91 of the outer tubular body 79 such that the central
portions are
generally aligned with the deflection axis 92. The hinge portion 86 is
disposed such that
it is disposed about less than the entirety of the circumference of the inner
tubular body
80.
31
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
To deflect the deflectable member 52 relative to the outer tubular body 79,
the
inner tubular body 80 may be moved relative to the outer tubular body 79. Such
relative
movement is illustrated in Figure 5D. As shown in Figure 5D, movement of the
inner
tubular body 80 in an actuation direction 90 (e.g., in the direction of the
ultrasound
transducer array 68 when the deflectable member 52 is aligned with the outer
tubular
body 79) may impart a force on the support 74 in the actuation direction 90.
However,
since the cradle portion 88 is restrainably connected to the outer tubular
body 79 by the
tether 78, the cradle portion 88 is prevented from moving substantially in the
actuation
direction 90. In this regard, the movement of the inner tubular body 80 in the
actuation
direction 90 may result in the cradle portion 88 pivoting about its interface
with the tether
78 and also in the hinge portion 86 bending as illustrated in Figure 5D. Thus
the
movement of the inner tubular body 80 in the actuation direction 90 may result
in the
cradle portion 88 (and the ultrasound transducer array 68 attached to the
cradle portion
80) rotating 90 degrees as illustrated in Figure 5D. Accordingly, movement of
the inner
tubular body 80 may cause a controlled deflection of the deflectable member
52. As
illustrated, the deflectable member 52 may be selectively deflectable away
from the
center axis 91 of the outer tubular body 79.
In an exemplary embodiment, a movement of the inner tubular body 80 of about
0.1 cm may result in the deflectable member 52 deflecting through an arc of
about 9
degrees. In this regard, movement of the inner tubular body 80 of about 1 cm
may
result in the deflectable member 52 deflecting about 90 degrees. Thusly, the
deflectable
member 52 may be selectively deflected from a side-looking position to a
forward-
looking position. Intermediate positions of the deflectable member 52 may be
achieved
by moving the inner tubular body 80 a predeterminable distance. For example,
in the
current exemplary embodiment, the deflectable member 52 may be deflected 45
32
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
degrees from the side-looking position by moving the inner tubular body 80
about 0.5
cm relative to the outer tubular body 79 in the actuation direction 90. Other
appropriate
member geometries may be incorporated to produce other relationships between
inner
tubular body 80 and deflectable member 52 deflection. Moreover, deflections of
greater
than 90 degrees may be obtained. Moreover, an embodiment of the catheter 50
may be
configured such that a predeterminable maximum deflection of the deflectable
member
52 may be achieved. For example, the handle 56 may be configured to limit the
movement of the slide 58 such that the full range of movement of the slide 58
corresponds to a 45 degree deflection (or any other appropriate deflection) of
the
deflectable member 52.
The slide 58 and handle 56 may be configured such that substantially any
relative
motion of the slide 58 to the handle 56 results in a deflection of the
deflectable member
52. In this regard, there may be substantially no dead zone of the slide 58
where slide
58 movement does not result in deflection of the deflectable member 52.
Furthermore,
the relationship between movement of the slide 58 (e.g., relative to the
handle 56) and
the amount of corresponding deflection of the deflectable member 52 may be
substantially linear.
When the deflectable member 52 is deflected from the position illustrated in
Figure 5C so that no part of the tip 64 occupies a cylinder the same diameter
as and
extending distally from the exit port 81, an interventional device may be
advanced
through the exit port 81 without contacting the tip 64. As such, the imaging
field of view
of the ultrasound transducer array 68 may be maintained in a fixed
registration relative
to the catheter body 54 while the interventional device is being advanced into
the
catheter body 54, through the exit port 81, and into the imaging field of view
of the
ultrasound transducer array 68.
33
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
When in a forward-looking position, the field of view of the ultrasound
transducer
array 68 may encompass an area in which an interventional device may be
inserted
through the lumen 82. In this regard, the ultrasound transducer array 68 may
be
operable to aid in the positioning and operation of the interventional device.
The deflectable member 52 may deflect about the deflection axis 92 (deflection
axis 92 is aligned with the view of Figure 5D and therefore is represented by
a point).
The deflection axis 92 may be defined as a point fixed relative to the tubular
body
interface portion 84 about which the cradle portion 88 rotates. As illustrated
in Figure
5D, the deflection axis 92 may be offset from the center axis 91 of the outer
tubular
body 79. For any given deflection of the deflectable member 52, a displacement
arc 93
may be defined as the minimum arc that is tangent to a face of the deflectable
member
52 and tangent the center axis 91 of the catheter. In an embodiment of the
catheter 50,
the ratio of a maximum cross-dimension of the distal end of the outer tubular
body 79 to
the radius of the displacement arc 93 may be at least about 1.
The deflectable member 52 may deflect about the deflection axis 92 such that
the
ultrasound transducer array 68 is positioned proximate to the exit port 81.
Such
positioning, in conjunction with a small displacement arc 93, reduces the
distance an
interventional device must travel between emerging from the exit port 81 and
entering
the field of view of the ultrasound transducer array 68. For example, upon
deflection of
90 degrees as shown in Figure 5D, the ultrasound transducer array 68 may be
positioned such that the acoustical face of the ultrasound transducer array 68
is a
distance from the exit port 81 (as measured along the central axis 91) that is
less than
the maximum cross dimension of the distal end of the outer tubular body 79.
34
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
As illustrated in Figures 5C and 5D, the flexboard 76 may remain
interconnected
to the catheter body 54 and the deflectable member 52 independent of the
deflection of
the deflectable member 52.
Figure 5E illustrates an embodiment of the catheter body 54. The catheter body
54 as illustrated comprises the inner tubular body 80 and the outer tubular
body 79. In
the illustrated embodiment, the outer tubular body 79 comprises all of the
components
illustrated in Figure 5E except for the inner tubular body 80. For the
illustration of Figure
5E, portions of various layers have been removed to reveal the construction of
the
catheter body 54. The outer tubular body 79 may include an outer covering 94.
The
outer covering 94 may, for example, be a high voltage breakdown material. In
an
exemplary configuration the outer covering 94 may comprise a substantially non-
porous
composite film including expanded polytetrafluoroethylene (ePTFE) with a
thermal
adhesive layer of ethylene fluoroethylene perfluoride on one side. The
exemplary
configuration may have a width of about 25 mm, a thickness of about 0.0025 mm,
an
isopropyl alcohol bubble point of greater than about 0.6 MPa, and a tensile
strength of
about 309 MPa in the length direction (e.g., the strongest direction). The
outer covering
94 may be lubricious to aid in the passage of the outer tubular body 79
through the
patient. The outer covering 94 may provide a high voltage breakdown. Within
the outer
covering 94 may be disposed an outer low-dielectric constant layer 96. The
outer low-
dielectric constant layer 96 may reduce capacitance between the electrical
interconnection member 104 and materials (e.g., blood) outside of the outer
covering
94. The outer low-dielectric constant layer 96 may have a dielectric constant
of less
than about 2.2. In an embodiment, the outer low-dielectric constant layer 96
may be
about 0.07-0.15 mm thick. In an embodiment, the outer low-dielectric constant
layer 96
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
may comprise a porous material, such as ePTFE. The voids in the porous
material may
be filled with a low-dielectric material such as air.
Moving toward the center of the outer tubular body 79, the next layer may be
first
tie layer 97. The first tie layer 97 may comprise a film material that may
have a melt
temperature that is lower then other components of the outer tubular body 79.
During
fabrication of the outer tubular body 79, the first tie layer 100 may be
selectively melted
to yield an interconnected structure. For example, selectively melting the
first tie layer
97 may serve to secure the outer low-dielectric constant layer 96, the first
tie layer 97,
and a shield layer 98 (discussed below) to each other.
Moving toward the center of the outer tubular body 79, the next layer may be
the
shield layer 98. The shield layer 98 may be used to reduce electrical
emissions from the
outer tubular body 79. The shield layer 98 may be used to shield components
internal
to the shield layer 98 (e.g., the electrical interconnection member 104) from
external
electrical noise. The shield layer 98 may be in the form of a double served
wire shield
or braid. In an exemplary embodiment, the shield layer 98 may be about 0.05-
0.08 mm
thick. Moving toward the center of the outer tubular body 79, the next layer
may be a
second tie layer 100. The second tie layer 100 may comprise a film material
that may
have a melt temperature that is lower then other components of the outer
tubular body
79. During fabrication of the outer tubular body 79, the second tie layer 100
may be
selectively melted to yield an interconnected structure.
Interior to the second tie layer 100 may be the electrical interconnection
member
104. The electrical interconnection member 104 may comprise a plurality of
conductors
arranged in a side-by-side fashion with an insulative (e.g., non-conductive)
material
between the conductors. The electrical interconnection member 104 may comprise
one
or more microminiature flat cables. The electrical interconnection member 104
may
36
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
contain any appropriate number of conductors arranged in a side-by-side
fashion. By
way of example, the electrical interconnection member 104 may contain 32 or 64
conductors arranged in a side-by-side fashion. The electrical interconnection
member
104 may be helically disposed within the outer tubular body 79. In this
regard, the
electrical interconnection member 104 may be helically disposed within the
wall of the
outer tubular body 79. The electrical interconnection member 104 may be
helically
disposed such that no part of the electrical interconnection member 104
overlies itself.
The electrical interconnection member 104 may extend from the proximal end 55
of the
catheter 50 to the distal end 53 of the outer tubular body 79. In an
embodiment, the
electrical interconnection member 104 may be disposed parallel to and along
the center
axis of the outer tubular body 79.
As illustrated in Figure 5E, there may be a gap of width Y between the coils
of the
helically wound electrical interconnection member 104. In addition, the
electrical
interconnection member 104 may have a width of X as illustrated in Figure 5E.
The
electrical interconnection member 104 may be helically disposed such that the
ratio of
the width X to the width Y is greater than 1. In such an arrangement, the
helically
disposed electrical interconnection member 104 may provide significant
mechanical
strength to the outer tubular body 79. This may, in certain embodiments,
obviate or
reduce the need for a separate reinforcing layer within the outer tubular body
79.
Moreover, the gap Y may vary along the length of the outer tubular body 79
(e.g.,
continuously or in one or more discrete steps). For example, it may be
beneficial to
have a greater stiffness to the outer tubular body 79 toward the proximal end
of the
outer tubular body 79. Accordingly, the gap Y may be made smaller toward the
proximal end of the outer tubular body 79.
37
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
An inner tie layer 102 may be disposed interior to the electrical
interconnection
member 104. The inner tie layer 102 may be configured similar to and serve a
similar
function as the second tie layer 100. The inner tie layer 102 may have a
melting point
of, for example, 160 degrees Celsius. Moving toward the center of the outer
tubular
body 79, the next layer may be an inner low-dielectric constant layer 106. The
inner
low-dielectric constant layer 106 may be configured similar to and serve a
similar
function as the outer low-dielectric constant layer 96. The inner low-
dielectric constant
layer 106 may be operable to reduce capacitance between the electrical
interconnection
member 104 and materials (e.g., blood, interventional device) within the outer
tubular
body 79. Moving toward the center of the outer tubular body 79, the next layer
may be
an inner covering 108. The inner covering 108 may be configured similar to and
serve a
similar function as the outer covering 94.
The tie layers (first tie layer 97, second tie layer 100, and inner tie layer
102) may
each have substantially the same melting point. In this regard, during
construction, the
catheter body 54 may be subjected to an elevated temperature that may melt
each of
the tie layers simultaneously and fix various layers of the catheter body 54
relative to
each other. Alternatively, the tie layers may have different melting points
allowing
selective melting of one or two of the tie layers while leaving the other tie
layer or tie
layers unmelted. Accordingly, embodiments of catheter bodies 54 may comprise
zero,
one, two, three, or more tie layers that have been melted to secure various
layers of the
catheter body 54 to other layers of the catheter body 54.
The aforementioned layers (from the outer covering 94 through the inner
covering 108) may each be fixed relative to each other. Together these layers
may form
the outer tubular body 79. Interior to these layers and movable relative to
these layers
may be the inner tubular body 80. The inner tubular body 80 may be disposed
such that
38
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
there is an amount of clearance between the outside surface of the inner
tubular body
80 and the interior surface of the inner covering 108. The inner tubular body
80 may be
a braid reinforced polyether block amide (e.g., the polyether block amide may
comprise
a PEBAX material available from Arkema Inc. Philadelphia, PA) tube. The inner
tubular body 80 may be reinforced with a braided or coiled reinforcing member.
The
inner tubular body 80 may possess a column strength adequate that it may be
capable
of translating a lateral motion of the slide 58 along the length of the inner
tubular body
80 such that the deflectable member 52 may be actuated by the relative
movement of
the inner tubular body 80 where its interfaces with the support 74. The inner
tubular
body 80 may also be operable to maintain the shape of the lumen 82 passing
through
the length of the inner tubular body 80 during deflection of the deflectable
member 52.
Accordingly, a user of the catheter 50 may be capable of selecting and
controlling the
amount of deflection of the deflectable member 52 through manipulation of the
handle
56. The lumen 82 may have a center axis aligned with the center axis 91 of the
outer
tubular body 79.
In a variation of the embodiment illustrated in Figure 5E, the inner tubular
body
80 may be replaced with an external tubular body that is disposed outside of
the outer
covering 94. In such an embodiment, the components of the outer tubular body
79
(from the outer covering 94 to the inner covering 108) may remain
substantially
unchanged from as illustrated in Figure 5E (the diameters of the components
may be
reduced slightly to maintain similar overall inner and outer diameters of the
catheter
body 54). The external tubular body may be fitted outside of the outer
covering 94 and
may be movable relative to the outer covering 94. Such relative movement may
facilitate deflection of the deflectable member 52 in a manner similar to as
described
with reference to Figures 5A through 5D. In such an embodiment, the electrical
39
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
interconnection member 104 would be a part of the outer tubular body 79 that
would be
located inside of the external tubular body. The external tubular body may be
constructed similarly to the inner tubular body 80 described above.
In an exemplary embodiment, the catheter body 54 may have a capacitance of
less than 2,000 picofarads. In an embodiment, the catheter body 54 may have a
capacitance of about 1,600 picofarads. In the above-described embodiment of
Figure
5E, the outer covering 94 and outer low-dielectric constant layer 96 may, in
combination,
have a withstand voltage of at least about 2,500 volts AC. Similarly, the
outer covering
108 and inner low-dielectric constant layer 106 may, in combination, have a
withstand
voltage of at least about 2,500 volts AC. Other embodiments may achieve
different
withstand voltages by, for example, varying the thicknesses of the covering
and/or low-
dielectric constant layers. In an exemplary embodiment, the outer diameter of
the outer
tubular body 79 may, for example, be about 12.25 Fr. The inner diameter of the
inner
tubular body may, for example, be about 8.4 Fr.
The catheter body 54 may have a kink diameter (the diameter of bend in the
catheter body 54 below which the catheter body 54 will kink) that is less than
ten times
the diameter of the catheter body 54. Such a configuration is appropriate for
anatomical
placement of the catheter body 54.
As used herein, the term "outer tubular body" refers to the outermost layer of
a
catheter body and all layers of that catheter body disposed to move with the
outermost
layer. For example, in the catheter body 54 as illustrated in Figure 5E, the
outer tubular
body 79 includes all illustrated layers of the catheter body 54 except the
inner tubular
body 80. Generally, in embodiments where there is no inner tubular body
present, the
outer tubular body may coincide with the catheter body.
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Figure 5F shows an embodiment of an electrical interconnection between the
helically disposed electrical interconnection member 104 and the flexboard 76
(a
flexible/bendable electrical member). For explanatory purposes, all the parts
of the
catheter body 54 except the electrical interconnection member 104 and the
flexboard 76
are not illustrated in Figure 5F. The flexboard 76 may have a curved section
109. The
curved section 109 may be curved to correspond with the curvature of the outer
tubular
body 79. The curved section 109 of the flexboard 76 may be disposed within the
outer
tubular body 79 at the end of the outer tubular body 79 proximate to the
deflectable
member 52 in the same position with respect to the layers of the outer tubular
body 79
as the electrical interconnection member 104. Accordingly, the curved section
109 of
the flexboard 76 may come into contact with the electrical interconnection
member 104.
In this regard, the distal end of the electrical interconnection member 104
may
interconnect to the flexboard 76 in an interconnect region 110.
Within the interconnect region 110, the electrically conductive portions
(e.g.,
wires) of the electrical interconnection member 104 may be interconnected to
electrically
conductive portions (e.g., traces, conductive paths) of the flexboard 76. This
electrical
interconnection may be achieved by peeling back or removing some of the
insulative
material of the electrical interconnection member 104 and contacting the
exposed
electrically conductive portions to corresponding exposed electrically
conductive
portions on the flexboard 76. The end of the electrical interconnection member
104 and
the exposed conductive portions of the electrical interconnection member 104
may be
disposed at an angle relative to the width of the electrical interconnection
member 104.
In this regard, the pitch (e.g., the distance between exposed electrically
conductive
portions) between the exposed electrically conductive portions of the
flexboard 76 may
be greater than the pitch (as measured across the width) of the electrical
41
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
interconnection member 104, while maintaining an electrical interconnection
between
each conductor of both the electrical interconnection member 104 and the
flexboard 76.
As illustrated in Figure 5F, the flexboard 76 may comprise a flexing or
bending
region 112 that has a width narrower than the width of the electrical
interconnection
member 104. As will be appreciated, the width of each individual electrically
conductive
path through the flexing region 112 may be smaller than the width of each
electrically
conductive memberwithin the electrical interconnection member 104. Furthermore
the
pitch between each electrically conductive member within the flexing region
112 may be
smaller than the pitch of the electrical interconnection member 104.
The flexing region 112 may be interconnected to an array interface region 114
of
the flexboard 76 through which the electrically conductive paths of the
electrical
interconnection member 104 and the flexboard 76 may be electrically
interconnected to
individual transducers of the ultrasound transducer array 68.
As illustrated in Figures 5C and 5D, the flexing region 112 of the flexboard
76
may be operable to flex during deflection of the deflectable member 52. In
this regard,
the flexing region 112 may be bendable in response to deflection of the
deflectable
member 52. The individual conductors of the electrical interconnection member
104
may remain in electrical communication with the individual transducers of the
ultrasound
transducer array 68 during deflection of the deflectable member 52.
In an embodiment, the electrical interconnection member 104 may comprises two
or more separate sets of conductors (e.g., two or more microminiature flat
cables). In
such an embodiment, each of the separate sets of conductors may be
interconnected to
the flexboard 76 in a manner similar to as illustrated in Figure 5F.
Furthermore, the
electrical interconnection member 104 (either a unitary electrical
interconnection
member 104 as illustrated in Figure 5F or an electrical interconnection member
104
42
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
comprising a plurality of generally parallel distinct cables) may comprise
members that
extend from the distal end 53 to the proximal end 55 of the catheter body 54
or the
electrical interconnection member 104 may comprise a plurality of discrete,
serially
interconnected members that together extend from the distal end 53 to the
proximal end
55 of the catheter body 54. In an embodiment, the flexboard 76 may include the
electrical interconnection member 104. In such an embodiment, the flexboard 76
may
have a helically wrapped portion extending from the distal end 53 to the
proximal end 55
of the catheter body 54. In such an embodiment, no electrical conductor
interconnections (e.g., between the flexboard 76 and a microminiature flat
cable)) may
be required between the array interface region 114 and the proximal end of the
catheter
body 54.
Figures 6A through 6D show an embodiment of a catheter that includes a
deflectable member 116 wherein the deflectable member 116 is deflectable by
moving
an elongate member relative to an outer tubular body 118. It will be
appreciated that the
embodiment illustrated in Figures 6A through 6D does not include an inner
tubular body
and the outer tubular body 118 may also be characterized as a catheter body.
The deflectable member 116 may be selectively deflectable. As shown in Figure
6A, the illustrated deflectable member 116 includes a tip 120. The tip 120 may
include
the ultrasound transducer array 68 and may include a rounded distal end 66 and
guide
wire aperture 70 similar to the tip 64 described with reference to Figure 5B.
As with the
tip 64 of Figure 513, the ultrasound transducer array 68 may be side-looking
when the
deflectable member 116 is aligned with the outer tubular body 118. In this
regard, the
ultrasound transducer array 68 may be operable to image anatomical landmarks
during
catheter insertion to aid in guiding and/or positioning the outer tubular body
118.
43
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
The outer tubular body 118 may include a lumen 128 operable to allow an
interventional device to pass therethrough. At least a portion of the
deflectable member
116 may be permanently positioned distal to the distal end of with the outer
tubular body
118. In an embodiment, the entirety of the deflectable member 116 may be
permanently positioned distal to the distal end of the outer tubular body 118.
The deflectable member 116 may be deflectable relative to the outer tubular
body
118. In this regard, the deflectable member 116 may be interconnected to one
or more
elongate members to control the motion of the deflectable member 116 as it is
being
deflected. The elongate member may take the form of a pull wire 130. The pull
wire 130
may be a round wire. Alternatively, for example, the pull wire 130 may be
rectangular in
cross-section. For example, the pull wire may be rectangular in cross-section
with a
width-to-thickness ratio of about 5 to 1.
As with the catheter embodiment illustrated in Figures 5B through 5E, the
catheter of Figures 6A through 6D may include a support 126 that supports the
ultrasound transducer array 68. The support 126 may interconnect the
deflectable
member 116 to the outer tubular body 118. A flexboard 122 may contain
electrical
interconnections operable to electrically connect the ultrasound transducer
array 68 to
an electrical interconnection member 104 (shown in Figure 6D) disposed within
the
outer tubular body 118. The exposed portion of flexboard 122 may be
encapsulated
similarly to the flexboard 76 discussed above.
The outer tubular body 118 may include a distal portion 124. The distal
portion
124 may comprise a plurality of wrapped layers disposed about a securement
portion
133 (shown in Figures 6B and 6C) of the support 126. The wrapped layers may
serve
to secure the securement portion 133 to an inner portion of the outer tubular
body 118
as discussed below with reference to Figure 6D.
44
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Deflection of the deflectable member 116 will now be discussed with reference
to
Figures 6B and 6C. Figures 6B and 6C illustrate the deflectable member 116
with the
portion of the tip 120 surrounding the ultrasound image array 68 and support
126
removed. Also, the distal portion 124 of the outer tubular body 118 wrapped
around the
securement portion 133 has been removed. The support 126 may be configured
similarly to the support 74 discussed above. The support 126 may further
include a
hinge portion 131 similar to the hinge portion 86.
To deflect the deflectable member 116 relative to the outer tubular body 118,
the
pull wire 130 may be moved relative to the outer tubular body 118. As shown in
Figure
6C, pulling the pull wire 130 (e.g., toward the handle 56) may impart a force
on the
support 126 at a pull wire anchor point 132 directed along the pull wire 130
toward a pull
wire outlet 134. The pull wire outlet 134 is the point where the pull wire 130
emerges
from a pull wire housing 136. The pull wire housing 136 may be fixed to the
outer
tubular body 118. Such a force may result in the deflectable member 116
bending
toward the pull wire outlet 134. As in the embodiment illustrated in Figures
5C and 5D,
the deflection of the deflectable member will be constrained by the hinge
portion 131 of
the support 126. As illustrated in Figure 6C, the resultant deflection of the
deflectable
member 116 may result in the ultrasound transducer array 68 being pivoted to a
forward-looking position. It will be appreciated that varying amounts of
deflection of the
deflectable member 116 may be achieved through controlled movement of the pull
wire
130. In this regard, any deflection angle between 0 degrees and 90 degrees may
be
achievable by displacing the pull wire 130 a lesser amount than as illustrated
in Figure
6C. Furthermore, deflections of greater than 90 degrees may be obtainable by
displacing the pull wire 130 a greater amount than as illustrated in Figure
6C.. As
illustrated in Figures 6B and 6C, the flexboard 122 may remain interconnected
to the
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
outer tubular body 118 and the deflectable member 116 independent of the
deflection of
the deflectable member 116.
Figure 6D illustrates an embodiment of the outer tubular body 118. For the
illustration of Figure 6D, portions of various layers have been removed to
reveal the
construction of the outer tubular body 118. Layers similar to those of the
embodiment of
Figure 5E are labeled with the same reference numbers as in Figure 5E and will
not be
discussed at length here. The pull wire housing 136 housing the pull wire 130
may be
disposed proximate to the outer covering 94. An external wrap 138 may then be
disposed over the outer covering 94 and pull wire housing 136 to secure the
pull wire
housing 136 to the outer covering 94. Alternatively, the pull wire housing 136
and pull
wire 130 may, for example, be disposed between the outer covering 94 and the
outer
low-dielectric constant layer 96. In such an embodiment, the outer wrap 138
may not be
needed. Other appropriate locations for the pull wire housing 136 and pull
wire 130 may
be utilized.
Disposed interior to the outer low-dielectric constant layer 96 may be the
shield
layer 98. A first tie layer (not shown in Figure 6D), similar to first tie
layer 97, may be
disposed between the outer low-dielectric constant layer 96 and the shield
layer 98.
Disposed interior to the shield layer may be the second tie layer 100.
Disposed interior
to the second tie layer 100 may be the electrical interconnection member 104.
Disposed interior to the electrical interconnection member 104 may be an inner
low-
dielectric constant layer 142. In this regard, the electrical interconnection
member 104
may be helically disposed within the wall of the outer tubular body 118.
Moving toward the center of the outer tubular body 118, the next layer may be
a
coiled reinforcement layer 144. The coiled reinforcement layer 144 may, for
example,
comprise a stainless steel coil. In an exemplary embodiment, the coiled
reinforcement
46
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
layer 144 may be about 0.05-0.08 mm thick. Moving toward the center of the
outer
tubular body 118, the next layer may be an inner covering 146. The inner
covering 146
may be configured similar to and serve a similar function as the outer
covering 94. The
lumen 128 may have a center axis aligned with the center axis of the outer
tubular body
118.
As noted above, the wrapped layers of the distal portion 124 of the outer
tubular
body 118 may serve to secure the securement portion 133 of the support 126 to
an
inner portion of the outer tubular body 118. For example, each layer outboard
of the
electrical interconnection member 104 may be removed in the distal portion
124.
Furthermore, the electrical interconnection member 104 may be electrically
interconnected to the flexboard 122 proximal to the distal portion 124 in a
manner
similar to as described with reference to Figure 5F. Accordingly, the
securement portion
133 of the support 126 may be positioned over the remaining inner layers
(e.g., the
inner low-dielectric constant layer 142, the coiled reinforcement layer 144
and the inner
covering 146) and a plurality of layers of material may be wrapped about the
distal
portion 124 to secure the securement portion 133 to the outer tubular body
118.
The outer diameter of the outer tubular body 118 may, for example, be about
12.25 Fr. The inner diameter of the outer tubular body 118 may, for example,
be about
8.4 Fr.
Figures 7A and 7B demonstrate further embodiments. As shown, the catheter 30
comprises a deflectable distal end 32. Located at deflectable distal end 32 is
ultrasound
transducer array 37. The catheter also includes wire 33 attached to the
ultrasound
transducer array 37 and extending to the proximal end of catheter 30 where it
exits
through a port or other opening at the proximal end of catheter 30. As shown
in Figure
7A, ultrasound transducer array 37 is in a "side-looking" configuration. The
catheter can
47
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
be delivered to the treatment site with the ultrasound transducer array 37 in
the "side-
looking" configuration, as shown in Figure 7A. Once the treatment site is
reached, wire
33 can be pulled in a proximal direction to deflect deflectable distal end 32
to result in
ultrasound transducer array 37 being moved to a "forward-looking"
configuration, as
shown in Figure 7B. As shown in Figure 7B, once ultrasound transducer array 37
is
positioned in the "forward-looking" position and deflectable distal end 32 is
deflected as
shown, generally centrally located lumen 38 is then available for delivery of
a suitable
interventional device to a point distal to the catheter distal end 32.
Alternatively, a tube
containing lumen 38 and movable relative to the outer surface of the catheter
30 may be
used to deflect the deflectable distal end 32 to the "forward-looking"
configuration.
Figure 8A is a front view of a single lobe configuration of the device shown
in
Figures 7A and 7B. Figure 8B shows a dual-lobe configuration of the catheter
shown in
Figures 7A and 7B. Figure 8C shows a tri-lobe configuration and Figure 8D
shows a
quad-lobe configuration. As will be understood, any suitable number of lobes
can be
constructed as desired. Moreover, in multiple-lobe configurations, ultrasound
transducer arrays 37 may be disposed on one or more of the lobes.
Further embodiments are shown in Figures 9, 9A and 9B. Figure 9 shows
catheter 1 having an ultrasound transducer array 7 near the distal end
thereof. The
ultrasound transducer array 7 is attached to catheter 1 by hinge 9.
Electrically
conductive wires 4 are connected to ultrasound transducer array 7 and extend
proximally to the proximal end of the catheter 1. The catheter 1 includes
distal exit port
13. The hinge 9 can be located at the distal end of ultrasound transducer
array 7, as
shown in Figure 9A, or at the proximal end of ultrasound transducer array 7,
as shown in
Figure 9B. In any event, the ultrasound transducer array 7 can be either
passively or
actively deflectable, as discussed above. Ultrasound transducer array 7 can be
48
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
deflected up to the forward-looking configuration (as shown in Figures 9A and
9B) and
an interventional device can be advanced at least partially out of distal exit
port 13, such
that at least a portion of the interventional device will be in the field of
view of the
ultrasound transducer array 7.
Figures 10A and 10B demonstrate a further embodiment where the catheter
includes ultrasound transducer array 7 near the catheter distal end 2 of the
catheter.
The catheter further includes steerable segment 8 and lumen 10. Lumen 10 can
be
sized to accept a suitable interventional device that can be inserted at the
proximal end
of the catheter and advanced through lumen 10 and out port 13. The catheter
can
further include guidewire receiving lumen 16. Guidewire receiving lumen 16 can
include
proximal port 15 and distal port 14, thus allowing for the well known "rapid
exchange" of
suitable guidewires.
As further demonstrated in Figures 11 and 11A and 11 B, the catheter steerable
segment 8 can be bent in any suitable direction. For example, as shown in
Figure 11A
the steerable segment is bent away from port 13 and as shown in Figure 11 B
the
steerable segment is bent toward port 13.
Figure 12 demonstrates yet another embodiment. Specifically, catheter 1 can
include ultrasound transducer array 7 located at the distal end 2 of the
catheter 1.
Electrically conductive wires 4 are attached to the ultrasound transducer
array 7 and
extend to the proximal end of the catheter 1. Lumen 19 is located proximal to
the
ultrasound transducer array 7 and includes proximal port 46 and distal port
45. The
lumen 19 can be sized to accept a suitable guidewire and/or interventional
device.
Lumen 19 can be constructed of a suitable polymer tube material, such as
ePTFE. The
electrically conductive wires 4 can be located at or near the center of the
catheter 1.
49
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
Figure 13 is a flow chart for an embodiment of a method of operating a
catheter
having a deflectable imaging device located at a distal end thereof. The first
step 150 in
the method may be to move the distal end of the catheter from an initial
position to a
desired position, wherein the deflectable imaging device is located in a first
position
during the moving step. The deflectable imaging device may be side-looking
when in
the first position. The moving step may include introducing the catheter into
a body
through an entry site that is smaller than the aperture of the deflectable
imaging device.
The moving step may include rotating the catheter relative to its
surroundings.
The next step 152 may be to obtain image data from the deflectable imaging
device during at least a portion of the moving step. The obtaining step may be
performed with the deflectable imaging device located in the first position.
During the
moving and obtaining steps, a position of the deflectable imaging device
relative to the
distal end of the catheter may be maintained. Thus the deflectable imaging
device may
be moved and images may be obtained without moving the deflectable imaging
device
relative to the distal end of the catheter. During the moving step, the
catheter, and
therefore the deflectable imaging device, may be rotated relative to its
surroundings.
Such rotation may allow the deflectable imaging device to obtain images in a
plurality of
different directions transverse to the path traveled by the catheter during
the moving
step.
The next step 154 may be to utilize the image data to determine when the
catheter is located at the desired position. For example, the image data may
indicate
the position of the deflectable imaging device, and therefore the distal end
of the
catheter, relative to a landmark (e.g., an anatomical landmark).
The next step 156 may be to deflect the deflectable imaging device from the
first
position to a second position. The deflecting step may follow the moving step.
The
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
deflectable imaging device may be forward-looking in the second position. The
deflectable imaging device may be angled at least 45 degrees relative to a
center axis of
the catheter when in the second position. Optionally, after the deflecting
step, the
deflectable imaging device may be returned to the first position and the
catheter
repositioned (e.g., repeating the moving step 150, the obtaining step 152, and
the
utilizing step 154). Once repositioned, the deflecting step 156 may be
repeated and the
method may be continued.
In an embodiment, the catheter may comprise an outer tubular body and an
activation device, each extending from a proximal end to the distal end of the
catheter.
In such an embodiment, the deflecting step may include translating a proximal
end of at
least one of the outer tubular body and actuation device relative to a
proximal end of the
other one of the outer tubular body and actuation device. The deflectable
imaging
device may be supportably interconnected by a hinge to one of the outer
tubular body
and the actuation device, and the deflecting step may further comprise
applying a
deflection force to the hinge in response to the translating step.
Furthermore, the
deflecting step may further include initiating the application of the
deflection force to the
hinge in response to the translating step. The deflection force may be applied
and then
maintained by manipulating a handle interconnected to the proximal end of the
catheter.
Moreover, the applying step may comprise communicating the deflection force by
the
actuation device from the proximal end to the distal end of the catheter in a
balanced
and distributed manner about a center axis of the outer tubular body.
The next step 158 may be to advance an interventional device through an exit
port at the distal end of the catheter and into an imaging field of view of
the deflectable
imaging device in the second position. The imaging field of view may be
maintained in
51
CA 02691449 2009-12-18
WO 2009/006335 PCT/US2008/068643
substantially fixed registration to the distal end of the catheter during the
advancing
step.
After advancing and using the interventional device (e.g., to perform a
procedure,
to install or retrieve a device, to make a measurement), the interventional
device may be
withdrawn through the exit port. The deflectable imaging device may then be
returned
to the first position. The return to the first position may be facilitated by
an elastic
deformation quality of the hinge. For example, the hinge may be biased toward
positioning the deflectable imaging device in the first position. As such,
when the
deflectable imaging device is in the second position and the deflection force
is removed,
the deflectable imaging device may return to the first position. After
withdrawal of the
interventional device through the exit port (and optionally from the entire
catheter) and
return of the deflectable imaging device to the first position, the catheter
may then be
repositioned and/or removed.
Additional modifications and extensions to the embodiments described above
will
be apparent to those skilled in the art. Such modifications and extensions are
intended
to be within the scope of the present invention as defined by the claims that
follow.
52