Note: Descriptions are shown in the official language in which they were submitted.
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 NANO-SIZED METAL AND METAL OXIDE PARTICLES FOR MORE COMPLETE FUEL
2 COMBUSTION
3
4 TECHNICAL FIELD
Provided are nano-sized metal particles and metal oxide particles to
facilitate fuel
6 combustion and methods of improving fuel combustion.
7
8 BACKGROUND
9 Engine manufacturers continue to seek improved fuel economy through engine
design.
Alternative approaches in improving fuel economy include formulating new fuels
and engine
11 oils. Combustion engines such as automobile engines typically require high
octane gasoline for
12 efficient operation. In the past, lead was added to gasoline to increase
the octane number. Due
13 to health and environmental concerns, however, lead was removed from
gasoline. Lead can
14 also poison a catalytic converter dramatically reducing its lifetime.
Oxygenates, such as methyl-
t-butyl ether (MTBE) and ethanol, may be added to gasoline to increase the
octane number.
16 While generally less toxic than lead, some suggest MTBE can be linked to
ground water
17 contamination. There is also a desire by some to reduce some of the high
octane components
18 normally present in gasoline, such as benzene, aromatics, and olefins.
19
SUMMARY
21 The following presents a simplified summary of the invention in order to
provide a basic
22 understanding of some aspects of the invention. This summary is not an
extensive overview of
23 the invention. It is intended to neither identify key or critical elements
of the invention nor
24 delineate the scope of the invention. Rather, the sole purpose of this
summary is to present
some concepts of the invention in a simplified form as a prelude to the more
detailed description
26 that is presented hereinafter.
27 The subject invention provides nano-sized metal particles and nano-sized
metal oxide
28 particles that can be used to improve combustion, decrease harmful exhaust
emissions, and
29 increase catalytic chemical oxidation of fuel.
One aspect of the invention relates to a fuel composition containing a liquid
fuel and at
31 least one of nano-sized metal particles, or nano-sized metal oxide
particles, or combination
32 thereof. Another aspect of the invention relates to a fuel additive
composition containing a
33 carrier/organic solvent and at least one of nano-sized metal particles, or
nano-sized metal oxide
34 particles or combination thereof. Other aspects of the invention include
methods of making fuel
21951351.1 1
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 compositions, methods of improving combustion, and methods of increasing
catalytic chemical
2 oxidation of a fuel composition.
3 To the accomplishment of the foregoing and related ends, the invention
comprises the
4 features hereinafter fully described and particularly pointed out in the
claims. The following
description and the annexed drawings set forth in detail certain illustrative
aspects and
6 implementations of the invention. These are indicative, however, of but a
few of the various
7 ways in which the principles of the invention may be employed. Other
objects, advantages and
8 novel features of the invention will become apparent from the following
detailed description of
9 the invention when considered in conjunction with the drawings.
11 BRIEF SUMMARY OF THE DRAWINGS
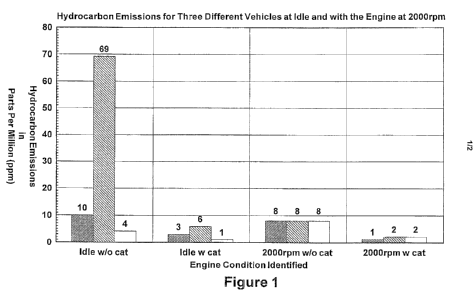
12 Figure 1 illustrates a bar graph demonstrating the hydrocarbon emissions
from various
13 fuels from various engines.
14 Figure 2 illustrates a bar graph demonstrating the octane ratings of
various fuel
compositions.
16
17 DETAILED DESCRIPTION
18 Nano-sized metal particles and/or nano-sized metal oxide particles are
combined with
19 fuel to improve fuel combustion. The nano-sized metal particles may be
present in a fuel
additive composition which is combined (that is, either suspended or
dispersed) with fuel to
21 make a fuel composition, or present in a fuel composition.
22 While not wishing to be bound by any theory, when nano-sized metal
particles are
23 present in a liquid fuel composition which is oxidized in the combustion
process, an added
24 energy source is provided. The nano-sized metal particles may increase the
catalytic chemical
oxidation or combustion of hydrocarbon based fuels. Consequently, an increase
in engine
26 power is achieved. Still not wishing to be bound by any theory, it is
believed that nano-sized
27 metal or metal oxide particles or combinations thereof present in a liquid
fuel composition
28 provide a catalytic surface capable of supplying oxygen to the combustion
process during
29 transient reducing atmospheric episodes generated by the combustion
process. Since the
combustion process is more complete, an environmentally friendly internal
combustion engine
31 fuel is provided.
32 The nano-sized metal or metal oxide particles or combination thereof may
also be
33 involved in other reactions that improve the combustion. For example, the
nano-sized metal
34 oxide particles can sequester low levels of water which otherwise can
contaminate fuels,
21951351.1 2
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 especially those fuels containing oxygenates such as alcohol. It is believed
that this
2 sequestration with the presence of ethanol provides an added benefit by
decreasing the
3 sensitivity or difference between the RON and the MON levels for ethanol.
The decrease in
4 sensitivity increases the fuels performance when the engine is under load
and can give rise to
an increased octane rating for the fuel. The nano-sized metal or metal oxide
particles may
6 function to form a coating on metal parts within the internal combustion
engine, thereby not only
7 adding lubricity but also preventing carbon deposition on the internal
engine parts. This reduces
8 engine maintenance.
9 Nano-sized metal or metal oxide particles or combination thereof are added
to
hydrocarbon based fuels to increase power output during combustion. Combustion
processes
11 (oxidation of hydrocarbon fuels) can occur an order of magnitude faster by
a substantially
12 heterogeneous reaction on solid catalytic surfaces (provided by the nano-
sized metal and metal
13 oxide particles) than do the same oxidation processes in homogeneous gas
phase reactions
14 without the metal and metal oxide particles. The invention thus provides
nano sized solid
catalyst having a significantly increased surface area needed for more
complete combustion.
16 The nano-sized metal particles and metal oxide particles have a size
suitable to catalyze
17 the combustion reaction of fuels, yet have 1) an ability to pass through
fuel filters and 2) at least
18 substantially combust themselves, or sublime, or otherwise be consumed so
that particulate
19 emissions are minimized and/or eliminated. In one embodiment, the nano-
sized metal particles
and metal oxide particles have a size where at least about 90% by weight of
the particles have a
21 size from about 1 nm to about 990 nm. In this connection, size refers to
average cross-section
22 of a particle, such as diameter. In another embodiment, the nano-sized
metal particles and
23 metal oxide particles have a size where at least about 90% by weight of the
particles have a
24 size from about 1 nm to about 75 nm. In yet embodiment, the nano-sized
metal particles and
metal oxide particles have a size where at least about 90% by weight of the
particles have a
26 size from about 1.5 nm to about 40 nm. In still yet embodiment, the nano-
sized metal particles
27 and metal oxide particles have a size where at least about 90% by weight of
the particles have a
28 size from about 2 nm to about 20 nm. In still yet embodiment, the nano-
sized metal particles
29 and metal oxide particles have a size where at least about 90% by weight of
the particles have a
size from about 1 nm to about 10 nm. In another embodiment, about 100% by
weight of the
31 particles have any of the sizes described above, including a size of less
than about 20 nm.
32 The nano-sized metal particles and metal oxide particles have a surface
area suitable to
33 catalyze the combustion reaction of fuels and to increase the rate of
combustion compared to
34 using the same amount of catalyst in bulk form. Increased surface area is
often better achieved
21951351.1 3
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 via small sized particles rather than particles with high porosity. In one
embodiment, the nano-
2 sized metal particles and metal oxide particles have a surface area from
about 50 m2/g to about
3 1,000 mz/g. In another embodiment, the nano-sized metal particles and metal
oxide particles
4 have a surface area from about 100 mZ/g to about 750 mz/g. In yet another
embodiment, the
nano-sized metal particles and metal oxide particles have a surface area from
about 150 m2/g to
6 about 600 m2/g.
7 The nano-sized metal particles and metal oxide particles have a morphology
suitable to
8 catalyze the combustion reaction of fuels, increase the rate of combustion
compared to using
9 the same amount of catalyst in bulk form, yet have an ability to pass
through fuel filters.
Examples of the one or more morphologies the nano-sized metal particles and
metal oxide
11 particles may have include, spherical, substantially spherical, oval,
popcorn-like, plate-like,
12 cubic, pyramidal, cylindrical, and the like. The nano-sized metal particles
and metal oxide
13 particles may be crystalline, partially crystalline, or amorphous.
14 The nano-sized metal particles and metal oxide particles contain any
material suitable to
catalyze the combustion reaction of fuels. General examples of materials of
metal particles
16 and/or metal oxide particles include one or more of the following
(referring to Groups of the
17 Periodic Table of Elements): Group Ila metals, Group Ila metal oxides,
Group Illa metals, Group
18 Illa metal oxides, Group IVa metals, Group lVa metal oxides, Group VIII
metals, Group VIII
19 metal oxides, Group lb metals, Group lb metal oxides, Group IIb metals,
Group llb metal oxides,
Group -IIb metals, and Group lllb metal oxides. More specific examples of
materials of metal
21 particles and/or metal oxide particles include one or more of the
following: magnesium, calcium,
22 strontium, barium, cerium, titanium, zirconium, iron, ruthenium, osmium,
cobalt, rhodium,
23 iridium, nickel, palladium, platinum, copper, silver, gold, tin, zinc,
aluminum, mixed metal
24 particles, alloy metal particles, calcium oxides, strontium oxides, barium
oxides, cerium oxides,
titanium oxides, zirconium oxides, iron oxides, ruthenium oxides, osmium
oxides, cobalt oxides,
26 rhodium oxides, iridium oxides, nickel oxides, palladium oxides, platinum
oxides, copper oxides,
27 silver oxides, gold oxides, tin oxides, zinc oxides, aluminum oxides, mixed
metal oxide particles,
28 and mixed metal-metal oxide particles.
29 In one embodiment, the nano-sized metal particles and/or metal oxide
particles do not
contain health hazardous and environmentally non-friendly (by current or
future standards)
31 metals and metal oxides. For example, in one embodiment, the nano-sized
metal particles
32 and/or metal oxide particles do not contain lead and/or lead oxide.
33 In one embodiment, the nano-sized metal particles contains mixed metal
particles and/or
34 mixed metal oxide particles containing at least two metals/metal oxides, at
least three
21951351.1 4
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 metals/metal oxides, or at least four metals/ metal oxides. Examples of
mixed metal particles
2 and mixed metal oxide particles include one or more of the following:
aluminum-magnesium,
3 aluminum-iron, aluminum-zinc, zinc-magnesium, zinc-magnesium-iron, calcium-
magnesium,
4 calcium-magnesium-zinc, calcium-magnesium-iron, nickel-magnesium, aluminum-
nickel, nickel-
magnesium-aluminum, aluminum-cerium, aluminum-magnesium oxides, aluminum-iron
oxides,
6 aluminum-zinc oxides, zinc-magnesium oxides, zinc-magnesium-iron oxides,
calcium-
7 magnesium oxides, calcium-magnesium-zinc oxides, calcium-magnesium-iron
oxides, nickel-
8 magnesium oxides, aluminum-nickel oxides, nickel-magnesium-aluminum oxides,
aluminum-
9 cerium oxides, and the like.
Many of the nano-sized metal particles and/or metal oxide particles are
commercially
11 available from a number of sources including Sigma-Aldrich Inc.
Alternatively, metal oxides can
12 be made by converting a metal salt to its corresponding metal or metal
oxide by methods known
13 in the art. The conversion can take place in an inert atmosphere or in air
via heating, such as
14 calcining in an inert or atmospheric environment or heating in solution.
In one embodiment, a metal salt is dissolved in a liquid and subjected to
ultrasound
16 irradiation followed by its conversion to metal or metal oxide. Metal salts
include metal
17 carboxylates, metal halides, and metal acetylacetonates. That is, metal
carboxylates, metal
18 halides, and metal acetylacetonates may be used to make metal oxides. Metal
carboxylates
19 include metal acetates, metal ethylhexanoates, metal gluconates, metal
oxalates, metal
propionates, metal pantothenates, metal cyclohexanebutyrates, metal
bis(ammonium
21 lacto)dihydroxides, metal citrates, and metal methacrylates. Specific
examples of metal
22 carboxylates include aluminum lactate, calcium acetate, calcium
ethylhexanoate, calcium
23 gluconate, calcium oxalate, calcium propionate, calcium pantothenate,
calcium
24 cyclohexanebutyrate, cerium acetate, cerium oxalate, cesium acetate, cesium
formate, iron
acetate, iron citrate, iron oxalate, magnesium acetate, magnesium
methylcarbonate,
26 magnesium gluconate, nickel acetate, nickel ethylhexanoate, nickel
octanoate, tin acetate, tin
27 oxalate, titanium bis(ammonium lacto)dihydroxide, zinc acetate, zinc
methacrylate, zinc
28 stearate, zinc cyclohexanebutyrate, zirconium acetate, zirconium citrate.
29 Two or more metal salts may be used to form mixed metal oxides. Mixed metal
oxides
contain at least two different metal oxides. Mixed metal oxides contain at
least three different
31 metal oxides. Alternatively, mixed metal oxides contain at least four
different metal oxides.
32 Any suitable liquid can be used to convert a metal salt such as a metal
carboxylate to a
33 metal oxide. Examples of liquids include water and organic solvents such as
alcohols, ethers,
34 esters, ketones, alkanes, aromatics, and the like. When using an absolute
alcohol such as
21951351.1 5
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 absolute ethanol as the liquid, the alcohol complexes with water that may be
liberated during the
2 conversion process.
3 Methods of making metal particles and metal oxide particles are known in the
art and
4 described in U.S. Patent 5,039,509; U.S. Patent 5,106,608; U.S. Patent
5,654,456; U.S. Patent
6,179,897 (combining metal with graphite, heating to form an intermediate
metal carbide,
6 applying apply more heat to decompose the metal carbide and release the
metal as a vapor,
7 then oxidizing to form a pure metal oxide powder); PCT Publication Number
WO/2007/000014;
8 all of which are hereby incorporated by reference.
9 The nano-sized metal particles and/or metal oxide particles (or the fuel
compositions or
fuel additive compositions) may contain or have coated thereon one or more
surfactants.
11 Surfactants can facilitate one or more of suspending the particles within
the fuel composition,
12 preventing agglomeration, promoting compatibility between the particles and
liquid fuel, and the
13 like. Any suitable surfactant can be employed including ionic surfactants,
anionic surfactants,
14 cationic surfactants, amphoteric surfactants, and nonionic surfactants.
Surfactants are known in
the art, and many of these surfactants are described in McCutcheon's "Volume
I: Emulsifiers
16 and Detergents", 1995, North American Edition, published by McCutcheon's
Division MCP
17 Publishing Corp., Glen Rock, N.J., and in particular, pp. 1-232 which
describes a number of
18 anionic, cationic, nonionic and amphoteric surfactants and is hereby
incorporated by reference
19 for the disclosure in this regard.
Examples of anionic (typically based on sulfate, sulfonate or carboxylate
anions)
21 surfactants include sodium dodecyl sulfate (SDS), ammonium lauryl sulfate,
and other alkyl
22 sulfate salts, sodium laureth sulfate, also known as sodium lauryl ether
sulfate (SLES), alkyl
23 benzene sulfonate, soaps, or fatty acid salts (see acid salts).
24 Examples of cationic (typically based on quaternary ammonium cations)
surfactants
include cetyl trimethylammonium bromide (CTAB) a.k.a. hexadecyl trimethyl
ammonium
26 bromide, and other alkyltrimethylammonium salts, cetylpyridinium chloride
(CPC),
27 polyethoxylated tallow amine (POEA), benzalkonium chloride (BAC), and
benzethonium
28 chloride (BZT).
29 Examples of zwitterionic surfactants or amphoteric surfactants include
dodecyl betaine,
dodecyl dimethylamine oxide, cocamidopropyl betaine, and coco ampho glycinate.
31 Examples of nonionic surfactants include alkyl poly(ethylene oxide); alkyl
32 polyglucosides, such as octyl glucoside, and decyl maltoside; fatty
alcohols such as cetyl
33 alcohol and oleyl alcohol; cocamide MEA, cocamide DEA, and cocamide TEA.
34 In one embodiment, the fuel composition contains from about 0.001% to about
1% by
21951351.1 6
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 weight of one or more surfactants. In another embodiment, the fuel
composition contains from
2 about 0.01 % to about 0.1 % by weight of one or more surfactants.
3 The nano-sized metal particles and metal oxide particles can be at least
partially
4 suspended, but typically suspended, in a liquid fuel composition in any
suitable manner. The
relatively small size of the nano-size particles contributes to the inherent
ability to remain
6 suspended over a longer period of time compared to relatively larger
particles (larger than a
7 micron), even though the density and/or specific gravity of the nano-size
particles may be
8 several times greater than the corresponding density and/or specific gravity
of the liquid fuel.
9 The longer suspension times mean that the liquid fuel containing the nano-
size particles
entering the engine over time contains a more uniform and/or consistent
dispersion of the nano-
11 size particles.
12 A suspension contains the nano-sized metal particles and/or metal oxide
particles and a
13 carrier fluid that is compatible with the fuel. For example, when the
nanoparticles are made in
14 the alcohol solution, or when toluene or xylenes are used as a carrier
fluid, the resulting
suspension can be added directly to pump gasoline. Analogously, for diesel
fuels, another
16 carrier fluid which is more of a cetane enhancer can be employed. The use
of one or more
17 suitable surfactants with a carrier fluid that is compatible with the fuel
can enhance the
18 suspension of the nanoparticles.
19 The nano-sized metal particles and metal oxide particles can be in dry
powder form.
The powdered form may be prepared by spray drying a suspension of the nano-
sized metal
21 particles and metal oxide particles. An inert gas such as nitrogen can be
used to spray dry the
22 particles. The coated powder can then be added to fuel or an engine as a
powder or made into
23 a fuel compatible paste. The powder can be directly added into the air
intake of an engine
24 instead of adding the powder to the fuel.
The uniformity of dispersion and/or duration of suspension can also be
established or
26 facilitated by the use of one or more suitable surfactants. Examples of
such surfactants include
27 amphoteric surfactants, ionic surfactants, and non-ionic surfactants. In
one embodiment,
28 however, the surfactant does not contain sulfur atoms. In another
embodiment, the surfactant
29 does not contain halide atoms. If employed, the surfactant can be added to
the liquid fuel
composition before, during, or after the nano-size particles are combined with
the fuel.
31 Alternatively, the nano-size particles may be contacted or coated with the
surfactant before
32 addition to the fuel. The powdered form can be prepared by spray drying a
suspension of the
33 nano-sized metal particles or metal oxide particles containing one or more
suitable surfactants.
34 Alternatively, oven drying or vacuum drying may be employed to form the
surfactant coated
21951351.1 7
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 particles. To be safe during spray drying, an inert gas such as nitrogen can
be used to spray
2 dry the particles with surfactant. The powder coated with surfactant can
then be added to fuel.
3 The uniformity of dispersion and/or duration of suspension can also be
established or
4 facilitated by mixing, stirring, blending, shaking, sonicating, or otherwise
agitating the liquid fuel
composition containing the nano-size particles.
6 The liquid fuel composition contains a suitable amount of at least partially
suspended
7 nano-si2ed metal particles and/or metal oxide particles to catalyze the
combustion reaction of
8 fuels. In one embodiment, the liquid fuel composition contains a liquid fuel
and from about 0.01
9 ppm to about 500 ppm of suspended nano-sized metal particles and/or metal
oxide particles. In
another embodiment, the liquid fuel composition contains a liquid fuel and
from about 0.05 ppm
11 to about 250 ppm of suspended nano-sized metal particles and/or metal oxide
particles. In yet
12 another embodiment, the liquid fuel composition contains a liquid fuel and
from about 0.1 ppm
13 to about 100 ppm of suspended nano-sized metal particles and/or metal oxide
particles. In still
14 yet another embodiment, the liquid fuel composition contains a liquid fuel
and from about 1 ppm
to about 75 ppm of suspended nano-sized metal particles and/or metal oxide
particles.
16 A fuel additive composition provides an efficient means to store and
transport the nano-
17 sized metal particles and/or metal oxide particles prior to the addition
with a liquid fuel. In one
18 embodiment, the fuel additive composition is simply a dry powder coated
with one or more
19 suitable surfactants. Or in another embodiment, no surfactant is used. In
another embodiment,
the fuel additive composition is a paste containing from about 10% by weight
to about 95% by
21 weight of the nano-sized metal particles and/or metal oxide particles and
from about 5% by
22 weight to about 90% by weight of a fuel compatible organic solvent and from
about 5% by
23 weight to about 10% by weight of one or more suitable surfactants. In yet
embodiment, the fuel
24 additive composition is a combination of a carrier liquid and the nano-
sized metal particles
and/or metal oxide particles and one or more suitable surfactants.
26 The fuel composition or fuel additive composition may optionally contain a
bicyclic
27 aromatic compound. Examples of bicyclic aromatic compounds include
naphthalene,
28 substituted naphthalenes, biphenyl compounds, biphenyl compound
derivatives, and mixtures
29 thereof. In one embodiment, the fuel composition contains from about 0.01
ppm to about 1000
ppm while the fuel additive composition contains from about 0.1 % by weight to
about 10% by
31 weight of one or more bicyclic aromatic compounds. In another embodiment,
the fuel
32 composition contains from about 0.1 ppm to about 500 ppm while the fuel
additive composition
33 contains from about 0.5% by weight to about 5% by weight of one or more
bicyclic aromatic
34 compounds.
21951351.1 8
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 The nano-sized metal and/or metal oxide particles and the optional bicyclic
aromatic
2 compound in the fuel additive composition can be dispersed in a carrier
liquid to form a fuel
3 additive composition. A carrier liquid has a flash point of at least 100 F
and an auto-ignition
4 temperature of at least 400 F or is a Cl -C3 alcohol. Examples of carrier
liquids include one or
more of toluene, xylenes, kerosene and Cl -C3 monohydric, dihydric or
polyhydric aliphatic
6 alcohols. Examples of aliphatic alcohols include methanol, ethanol, n-
propanol, isopropyl
7 alcohol, ethylene glycol, propylene glycol, and the like. In one embodiment,
the fuel additive
8 composition contains at least 90% by weight of a carrier liquid and no more
than 10% by weight
9 of the nano-sized metal and/or metal oxide particles.
Some fuels and fuel additives contain relatively large or small quantities of
ketones, such
11 as acetone, or ethers, such MTBE. A relatively large or small quantity of a
ketone or ether is not
12 necessary in the fuel compositions and fuel additive compositions. In one
embodiment, a
13 relatively large quantity (more than 5% by volume) of a ketone or ether is
not present in the fuel
14 compositions and/or fuel additive compositions because ketones and ethers
may decrease the
solubility of the nano-sized metal and/or metal oxide particles and
undesirably reduce the flash
16 point of the resultant fuel composition.
17 Fuel compositions are made by combining the nano-sized metal and/or metal
oxide
18 particles and a liquid fuel. Examples of liquid fuels include hydrocarbon
fuels such as gasoline,
19 reformulated gasoline, diesel, jet fuel, marine fuel, kerosene, biofuels
such as biodiesel,
bioalcohols such as bioethanol, and the like. Gasoline contains one or more of
the following
21 components that may, by themselves, constitute liquid fuel: straight-run
products, reformate,
22 cracked gasoline, high octant stock, isomerate, polymerization stock,
alkylate stock,
23 hydrotreated feedstocks, desulfurization feedstocks, alcohol, and the like.
24 In one embodiment, the fuel additive composition or the nano-sized metal
and/or metal
oxide particles coated with or without one or more suitable surfactants is/are
added to the liquid
26 fuel in an amount sufficient to provide decrease of at least about 10% in
hydrocarbon and/or
27 carbon monoxide emissions from the exhaust system as compared to the
corresponding
28 emissions from use of the liquid fuel without inclusion of the nano-sized
metal and/or metal
29 oxide particles. In another embodiment, the fuel additive composition or
the nano-sized metal
and/or metal oxide particles coated with or without one or more suitable
surfactants is/are added
31 to the liquid fuel in an amount sufficient to provide decrease of at least
about 25% in
32 hydrocarbon and/or carbon monoxide and/or nitrogen oxides emissions from
the exhaust
33 system as compared to the corresponding emissions from use of the liquid
fuel without inclusion
34 of the nano-sized metal and/or metal oxide particles.
21951351.1 9
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 In one embodiment, the fuel additive composition or the nano-sized metal
and/or metal
2 oxide particles coated with or without one or more suitable surfactants
is/are added to the liquid
3 fuel in an amount sufficient to provide a decrease of at least 5% in the
amount of the liquid fuel
4 consumed by the internal combustion engine when compared with the
corresponding amount of
liquid fuel consumed by the engine when the nano-sized metal and/or metal
oxide particles are
6 not included. In another embodiment, the fuel additive composition or the
nano-sized metal
7 and/or metal oxide particles is/are added to the liquid fuel in an amount
sufficient to provide a
8 decrease of at least 10% in the amount of the liquid fuel consumed by the
internal combustion
9 engine when compared with the corresponding amount of liquid fuel consumed
by the engine
when the nano-sized metal and/or metal oxide particles are not included.
11 The quality of a fuel such as gasoline can be determined by octane. Octane
is
12 measured relative to a mixture of isooctane (2,2,4-trimethylpentane, an
isomer of octane) and n-
13 heptane. For example, an 87-octane gasoline has the same octane rating as a
mixture of 87
14 vol-% isooctane and 13 vol-% n-heptane. A low octane rating is undesirable
in a gasoline
engine. The most common type of octane rating worldwide is the Research Octane
Number
16 (RON). RON is determined by running the fuel through a specific test engine
with a variable
17 compression ratio under controlled conditions, and comparing these results
with those for
18 mixtures of isooctane and n-heptane. In this connection, RON can be
determined using the
19 procedure set forth in ASTM D 2699, which is hereby incorporated by
reference in its entirety.
Another type of octane rating, called Motor Octane Number (MON), which is in
some instances
21 a better measure of how the fuel behaves when under load. MON testing uses
a similar test
22 engine to that used in RON testing, but with a preheated fuel mixture, a
higher engine speed,
23 and variable ignition timing to further stress the fuel's knock resistance.
24 Cetane number or CN a measure of the combustion quality of diesel fuel
under compression,
one measure of fuel quality. CN is actually a measure of a diesel fuel's
ignition delay; the time
26 period between the start of injection and start of combustion (ignition) of
the fuel.
27 In one embodiment, a fuel composition containing a liquid fuel and the nano-
sized metal
28 and/or metal oxide particles has a higher RON, MON, and/or CN than a RON,
MON, and/or CN
29 for a fuel composition with the same ingredients except without the nano-
sized metal and/or
metal oxide particles. In another embodiment, a fuel composition containing a
liquid fuel and
31 the nano-sized metal and/or metal oxide particles can have less than about
5% higher RON,
32 MON, and/or CN than a RON, MON, and/or CN for a fuel composition with the
same ingredients
33 except without the nano-sized metal and/or metal oxide particles. In yet
another embodiment, a
34 fuel composition containing a liquid fuel and the nano-sized metal and/or
metal oxide particles
21951351.1 10
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 has can have less than 10% higher RON, MON, and/or CN than a RON, MON,
and/or CN for a
2 fuel composition with the same ingredients except without the nano-sized
metal and/or metal
3 oxide particles.
4 The fuel composition can be effectively used in both fuel-injected and non
fuel-injected
engines. The fuel composition can be effectively used in two-stroke engines,
four-stroke
6 engines, and vehicle engines such as automobile engines, motorcycle engines,
jet engines,
7 marine engines, truck/bus engines, and the like. The fuel composition can be
effectively used in
8 any type of internal combustion engine including an Otto-cycle engine, a
diesel engine, a rotary
9 engine, and a gas turbine engine. The fuel composition can be effectively
used in an
intermittent internal combustion engine or a continuous internal combustion
engine.
11 The fuel composition can supply to the fuel chamber the liquid fuel and the
nano-sized
12 metal and/or metal oxide particles as a mixture, or the liquid fuel and the
nano-sized metal
13 and/or metal oxide particles can be supplied to the fuel chamber
separately.
14 The fuel compositions are tailored to reduce the percentages of
hydrocarbons, carbon
monoxide, nitrogen oxides, and molecular oxygen in motor vehicle exhaust
emissions. Use of
16 the fuel compositions may also result in a desirable increase in the
percentage of carbon
17 dioxide in combustion exhaust emissions. Thus, the fuel compositions, when
used to fuel
18 internal combustion engines, lead to efficient operation and the resultant
emissions meet or
19 exceed E.P.A. standards. The fuel compositions are also tailored to have
more effective
combustion thereby reducing little or less deposition of carbon residue in the
21 internal chamber of the combustion engine.
22 The following examples illustrate the subject invention. Unless otherwise
indicated in
23 the following examples and elsewhere in the specification and claims, all
parts and percentages
24 are by weight, all temperatures are in degrees Centigrade, and pressure is
at or near
atmospheric pressure.
26 Table 1 reports hydrocarbon emissions in parts per million (ppm) from three
different
27 engines at idle and at 2000 rpm using a fuel without the nano-sized metal
and/or metal oxide
28 particles and a fuel with the nano-sized metal and/or metal oxide
particles. The base fuel is
29 regular unleaded gasoline having an octane rating of 87. The nano-sized
metal and/or metal
oxide particles are present at a level of about 50 ppm and are zinc oxide
particles having a size
31 from 1 nm to 20 nm. Engine 1 is a year 2002 Ford F-150 pick-up V-8; engine
2 is a year 2000
32 Dodge Ram pick-up V-8; and engine 3 is a 1999 Audi A8 V-8. Hydrocarbon
emissions are
33 measured using a five gas analyzer with a tailpipe probe (Model 5002
Exhaust Gas Analyzer
34 made by Emission Systems Inc.).
21951351.1 11
CA 02691890 2009-12-29
Agent Ref: 75974/00002
2 TABLE 1
3 Engine idle w/o cat idle w cat 2000rpm w/o cat 2000rpm w cat
4 1 10 3 8 1
2 69 6 8 2
6 3 4 1 8 2
7
8 Figure 1 is a bar graph for hydrocarbon readings to facilitate visual
comparisons of
9 emissions reported in Table 1. On the bar graph of Figure 1, the first set
of bars (idle w/o cat)
shows the hydrocarbon emissions from three engines at idle using a fuel
without the nano-sized
11 metal and/or metal oxide particles. The second set of bars (idle w cat)
shows hydrocarbon
12 emissions from the same three engines at idle using a fuel with the nano-
sized metal and/or
13 metal oxide particles. The final two sets of bars (2000rpm w/o cat and
2000rpm w cat) shows
14 the hydrocarbon emissions either without or with the nano-sized metal
and/or metal oxide
particles from the same three engines, but with the engine turning at 2000 rpm
(a typical turn
16 rate for highway travel). For both idle and cruising engine turning rates,
the reduction in
17 hydrocarbon emissions is substantial.
18 Table 2 reports nitrogen oxide (NOx) emissions in parts per million (ppm)
from two
19 different engines at idle and at 2000 rpm using a fuel without the nano-
sized metal and/or metal
oxide particles and a fuel with the nano-sized metal and/or metal oxide
particles. For both idle
21 and cruising engine turning rates, the reduction in nitrogen oxide
emissions is substantial. The
22 base fuel is regular unleaded gasoline having an octane rating of 87. The
nano-sized metal
23 and/or metal oxide particles are present at a level of about 50 ppm and are
zinc oxide particles
24 having a size from 1 nm to 20 nm. Engine 1 is a year 2002 Ford F-150 pick-
up V-8 and engine
3 is a 1999 Audi A8 V-8. Nitrogen oxide emissions are measured using a five
gas analyzer with
26 a tailpipe probe (Model 5002 Exhaust Gas Analyzer made by Emission Systems
Inc.).
27
28 TABLE 2
29 Engine idle w/o cat idle w cat 2000rpm w/o cat 2000rpm w cat
1 10 1 207 31
31 3 3 0 37 2
32
33 Table 3 reports carbon dioxide emissions in parts per million (ppm) from
three different
34 engines at idle and at 2000 rpm using a fuel without the nano-sized metal
and/or metal oxide
21951351.1 12
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 particles and a fuel with the nano-sized metal and/or metal oxide particles.
The base fuel is
2 regular unleaded gasoline having an octane rating of 87. The nano-sized
metal and/or metal
3 oxide particles are present at a level of about 50 ppm and are zinc oxide
particles having a size
4 from 1 nm to 20 nm. Engine 1 is a year 2002 Ford F-150 pick-up V-8 and
engine 2 is a year
2000 Dodge Ram pick-up V-8. Carbon dioxide emissions are measured using a five
gas
6 analyzer with a tailpipe probe (Model 5002 Exhaust Gas Analyzer made by
Emission Systems
7 Inc.).
8
9 TABLE 3
Engine idle w/o cat idle w cat 2000rpm w/o cat 2000rpm w cat
11 1 13.8 13.7 17.7 15
12 2 14.3 14.7 14.9 14.8
13
14 Table 4 reports octane ratings from five different fuel compositions; one
without the
nano-sized metal and/or metal oxide particles additive and four with varying
amounts of the
16 nano-sized metal and/or metal oxide particles additive. Each of the five
different fuel
17 compositions contains Murphy's USA regular unleaded fuel having an octane
rating of 87 with
18 or without an additive. The additive is a different amount of 1 nm to 20 nm
zinc oxide particles.
19 The octane number is measured using an IR scanner (Model ZX-101XL portable
octane and
fuel analyzer made by Zeltex Inc.).
21
22 TABLE 4
23 Fuel Octane Reading
24 without additive 87.1
with 50 ppm additive 87.8
26 with 100 ppm additive 88.2
27 with 150 ppm additive 88.6
28 with 200 ppm additive 88.8
29
Figure 2 is a bar graph for octane readings to facilitate visual comparisons
of the fuel
31 compositions reported in Table 4. On the bar graph of Figure 2, the first
bar shows the octane
32 reading from a fuel composition without the nano-sized metal and/or metal
oxide particles while
33 the second to fifth bars show fuel compositions with varying amounts of the
nano-sized metal
34 and/or metal oxide particles. All of the fuel compositions with varying
amounts of the nano-
21951351.1 13
CA 02691890 2009-12-29
Agent Ref: 75974/00002
1 sized metal and/or metal oxide particles have higher octane readings than
the fuel composition
2 without the nano-sized metal and/or metal oxide particles.
3 Table 5 illustrates that NOx emissions from diesel fuel with catalyst were
reduced from
4 125 ppm level to 58 ppm level: approximately a 53% reduction. Each of the
two different diesel
fuel compositions contains Phillips's USA diesel fuel with or without an
additive. The additive is
6 1 nm to 20 nm zinc oxide particles. Nitrogen oxide emissions are measured
using a five gas
7 analyzer with a tailpipe probe (Model 5002 Exhaust Gas Analyzer made by
Emission Systems
8 Inc.).
9
TABLE 5 NOx Reduction Test Results using Diesel Fuel with/without catalyst
11 NOx (ppm)
12 Engine Speed: Idle 2,000rpm
13 1) Diesel w/o catalyst 264 125
14 2) Diesel w/catalyst 257 58
16 The data were calculated from averaged where multiple readings were taken
at two
17 engine speeds: 1) Idle and 2) 2,000 rpm. As shown in Table 5, a two
different fuel compositions
18 were used; 1) diesel fuel only and 2) diesel with catalyst. These two fuels
were run sequentially
19 with an initial pump diesel base line followed by testing with
diesel/catalyst.
With respect to any figure or numerical range for a given characteristic, a
figure or a
21 parameter from one range may be combined with another figure or a parameter
from a different
22 range for the same characteristic to generate a numerical range.
23 While the invention has been explained in relation to certain embodiments,
it is to be
24 understood that various modifications thereof will become apparent to those
skilled in the art
upon reading the specification. Therefore, it is to be understood that the
invention disclosed
26 herein is intended to cover such modifications as fall within the scope of
the appended claims.
27
21951351.1 14