Note: Descriptions are shown in the official language in which they were submitted.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
NEW PHARMACEUTICAL FORMULATION COMPRISING CANNABIDIOL AND
TETRAHYDROCANNABIDIVARIN
FIELD OF THE INVENTION
The present invention relates to a novel pharmaceutical
formulation comprising a ratioed mix of: (i) one or more
compounds that acts as an inverse agonist of the CB1 and /
or CB2 receptor; and (ii) one or more compounds that acts
as a neutral antagonist of the CB1 and / or CB2 receptor.
Preferably both the inverse agonist of the CB1 and / or
CB2 receptor and the neutral antagonist of the CB1 and /
or CB2 receptor are cannabinoids. Preferably the
cannabinoids are tetrahydrocannabidivarin (THCV) and
cannabidiol (CBD).
BACKGROUND DESCRIPTION
Cannabinoids are a group of chemicals known to activate
cannabinoid receptors in cells. These chemicals, which
are found in cannabis plants, are also produced
endogenously in humans and other animals, and are termed
endocannabinoids. Synthetic cannabinoids are manmade
chemicals with the same structure as plant cannabinoids
or endocannabinoids.
Cannabinoids are generally known to be cannabinoid
receptor agonists. When a cannabinoid receptor agonist
binds to a cannabinoid receptor a response is triggered.
This response is known as a signalling pathway.
Compounds which are known to bind to the CB1 cannabinoid
receptor include delta-9-tetrahydrocannabinol (THC), R-
(+)-WIN55212 and anandamide. These compounds are as such
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
described as CB1 agonists as when they bind to the CB1
receptor a specific response is produced.
Agonism at a receptor will often lead to an active
response by the cell. Many disease states result from the
overactive or overabundant effects of agonists at their
receptors.
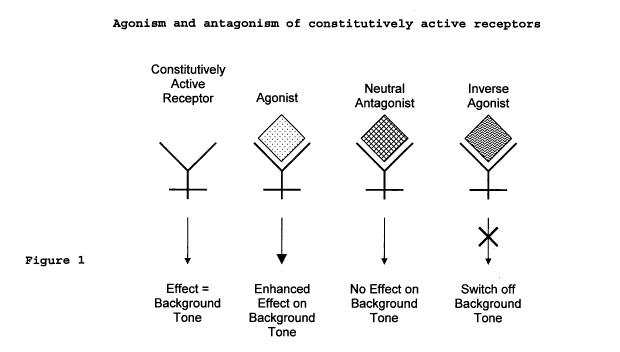
Cannabinoid receptors are known to be constitutively
active. This means that the receptors undergo some degree
of coupling to their signalling pathways even in the
absence of an agonist. As such they exhibit a background
tone.
In the presence of an agonist this background tone is
increased. This can cause an intensification of a disease
state that has resulted from the active response of the
cell.
Research into compounds that are able to oppose the
ability of such agonists has led to the discovery of
compounds that act as cannabinoid receptor antagonists.
A neutral antagonist is a compound that will bind to the
receptor but will lack any efficacy as a receptor
agonist. Such a neutral antagonist will compete with
agonists for its receptor and once bound will not result
in any active response. In constitutively active
receptors the background tone remains unaffected.
An inverse agonist will also bind to its receptor and
will lack any efficacy as a receptor agonist. Once an
inverse agonist is bound to a receptor it is able to
produce an opposite effect of the active response.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Therefore in constitutively active receptors an inverse
agonist is able to either partially or completely switch
off the background tone.
The way in which constitutively active receptors work in
the presence of agonists and different types of receptor
antagonists is shown in Figure 1.
The ability of a compound to have antagonistic properties
at a constitutively active receptor may be extremely
beneficial in the treatment of diseases where a change in
the background tone of a cell is the cause of the disease
state.
Examples of diseases and conditions that are the result
of the background tone of constitutively active
cannabinoid receptors include but are not limited to
obesity, schizophrenia, epilepsy, cognitive disorders
such as Alzheimer's disease, bone disorders such as
osteoporosis, bulimia, obesity associated with type II
diabetes (non-insulin dependant diabetes), the treatment
of drug, alcohol and nicotine abuse or dependency and
inflammatory disorders (Pertwee, R.G., 2000).
There is evidence that the endogenous CB1 agonist,
anandamide, is released in the brain to mediate processes
such as feeding and appetite (Di Marzo et al., 2001).
This raises the possibility that a CB1 receptor antagonist
could be effective in the clinic as an appetite
suppressant.
One such cannabinoid receptor antagonist is SR141716A.
The use of this compound in the regulation of appetite
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
has been described by Maruani and Soubrie in US 6,444,474
and EP0969835.
The compound SR141716A is a synthetic compound and as
such its long-term effects cannot be completely
quantified by clinical trials. It is not known how a
synthetic compound such as this will interfere with the
cannabinoid receptors on a very long-term basis (it is
likely from data accumulated in a clinical study with
SR141716A that appetite suppressant treatments will have
to be chronic). The clinical study showed a significant
increase in depression in at least some of the patients
enrolled in the trials. Also a recent article in the
journal Multiple Sclerosis describes a patient whose
previously subclinical case of multiple sclerosis became
active when treatment with SR141716A was started.
Other compounds which have been identified as CB1 and / or
CB2 cannabinoid receptor antagonists include the
following: SR144528; 0-2654; 0-2050; NESS0327; AM281;
AM251; LY320135; and AM630.
Naturally occurring CB1 and CB2 receptor antagonists which
are produced by the cannabis plant are likely to have a
less complex pharmacology than those of an inverse
agonist which has been chemically synthesised to bind
with the cannabinoid receptor. This is because the human
body has been in contact with such substances for
millennia and as such the body's pharmacological systems
have developed in the presence of plant cannabinoids and
if there were any untoward side effects these would be
known already. However, until recently none of the
cannabinoids produced by the cannabis plant have been
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
found to possess inverse agonism properties of the
cannabinoid receptor.
The applicant's co-pending patent application GB2434312
describes a THC extract which comprises a small amount of
THCV, which is less than 2% (w/w). When the amount of
THCV is lower than the amount of THC in the extract then
it is impossible to determine what the effects of the
THCV would be.
In the applicant's co-pending International patent
application WO 2005/120478, the application describes
that THCV could be used in place of THC. It has been
subsequently found that this is not the case. THCV has
been discovered to work as a CB1 receptor antagonist,
which is completely opposite from THC which acts as a CB1
agonist.
The applicants have described in their co-pending
application PCT/GB2005/004388 the cannabinoid receptor
antagonist properties of the cannabinoid
tetrahydrocannabidivarin (THCV). Here it is shown that
the cannabinoid THCV acts as a neutral antagonist of the
CB1 and CB2 cannabinoid receptors
More recently the applicants have described in their co-
pending application PCT/GB2007/002008 the cannabinoid
receptor antagonist properties of the cannabinoid
cannabidiol (CBD). The cannabinoid CBD acts as an inverse
agonist of the CB1 and CB2 cannabinoid receptors.
The applicants therefore believe that the combination of
the cannabinoids tetrahydrocannabidivarin (THCV) and
cannabidiol (CBD) will exhibit benefits as a
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
pharmaceutical formulation as compared the use of each of
the cannabinoids alone.
The cannabinoid THCV is a classical plant cannabinoid,
which is structurally related to THC, in that instead of
the 3-pentyl side chain of THC, the THCV molecule has a
3-propyl side chain. The cannabinoid CBD is again another
classical plant cannabinoid, which is known to be non-
psychoactive. CBD has previously been shown to be useful
in the treatment of inflammation, nausea and anxiety. The
structures of the two cannabinoids are shown in Figure 2.
The two cannabinoids THCV and CBD can work together to
provide a beneficial formulation, and this is of
particular value. The diseases and conditions that the
formulation with a combination of THCV and CBD will be
useful in the treatment of are diseases and conditions
that benefit from antagonism of the CB1 and / or CB2
cannabinoid receptors. It is thought that the
combinations described herein provide a better treatment
option due to the difference in the ways the two
cannabinoids have an affect.
THCV is thought to act directly on the cannabinoid
receptors and bind to cause a neutral antagonist effect.
This means that the receptor itself is blocked to binding
with an agonist such as an endocannabinoid; however the
background tone of the receptor remains unaffected. When
THCV is provided as a pharmaceutical formulation alone
the unaffected background tone means that some of the
diseases and conditions that antagonism is useful to
treat may not be fully alleviated as the background tone
may still cause an effect on the body.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Conversely, CBD is thought to act as an inverse agonist,
which means that the background tone of the receptor is
switched off. However, CBD is thought to bind at a site
distinct from the cannabinoid receptors themselves and as
such may allow an agonist to bind with the receptor.
A combination of the two cannabinoid receptor antagonists
may therefore prove to be a very useful treatment option
in diseases and conditions that benefit from antagonism
of the CB1 and / or CBZ cannabinoid receptors.
SUMMARY OF THE INVENTION
According to the first aspect of the present invention
there is provided a pharmaceutical formulation comprising
a ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB1 and / or CB2 receptor; and
(ii) one or more compounds that acts as a neutral
antagonist of the CB1 and / or CB2 receptor.
The above ratioed mix will include the alternatives as
follows:
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB1 receptor; and (ii) one or
more compounds that acts as a neutral antagonist of the
CB1 receptor;
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB1 receptor; and (ii) one or
more compounds that acts as a neutral antagonist of the
CB2 receptor;
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB2 receptor; and (ii) one or
more compounds that acts as a neutral antagonist of the
CB1 receptor;
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CBZ receptor; and (ii) one or
more compounds that acts as a neutral antagonist of the
CB2 receptor;
A ratioed mix of.: (i) one or more compounds that acts as
an inverse agonist of both the CB1 and the CB2 receptors;
and (ii) one or more compounds that acts as a neutral
antagonist of both the CB1 and the CB2 receptors;
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of both the CB1 and the CBZ receptors;
and (ii) one or more compounds that acts as a neutral
antagonist of the CB1 receptor;
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of both the CB1 and the CB2 receptors;
and (ii) one or more compounds that acts as a neutral
antagonist of the CBZ receptor;
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB1 receptor; and (ii) one or
more compounds that acts as a neutral antagonist of both
the CB1 and the CB2 receptors; and
A ratioed mix of: (i) one or more compounds that acts as
an inverse agonist of the CB2 receptor; and (ii) one or
more compounds that acts as a neutral antagonist of both
the CB1 and the CB2 receptors.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Preferably the pharmaceutical formulation comprises a
cannabinoid which acts as inverse agonist of the CB1 and /
or CB2 receptor.
More preferably the cannabinoid which is an inverse
agonist of the CB1 and / or CB2 receptor is cannabidiol
(CBD).
Preferably the pharmaceutical formulation comprises a
cannabinoid which acts as a neutral antagonist of the CB1
and / or CB2 receptor.
More preferably the cannabinoid which is a neutral
antagonist of the CB1 and / or CB2 receptor is
tetrahydrocannabidivarin (THCV).
More preferably still, the ratioed mix of (i) and (ii) is
a ratioed mix of THCV and CBD.
Such a pharmaceutical formulation may used in the
manufacture of a medicament for the treatment of diseases
such as obesity, schizophrenia, epilepsy or cognitive
disorders such as Alzheimer's, bone disorders, bulimia,
obesity associated with type II diabetes (non-insulin
dependant diabetes) and in the treatment of drug, alcohol
or nicotine abuse or dependency. These diseases may be
caused by agonism of the CB1 receptor and therefore can be
treated with different ratioed mixtures of the inverse
agonist and neutral antagonist of the CB1 receptor.
Inflammatory diseases may be caused by agonism of the CB2
receptor can also be treated with different ratioed
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
mixtures of the inverse agonist and neutral antagonist of
the CB2 receptor.
Such formulations may be of particular value in the
treatment of diseases with multiple symptoms as the
combined mixture of inverse agonist of the CB1 and / or
CB2 receptor and neutral antagonist of the CB1 and / or
CB2 receptor will provide a dual benefit.
The rationale behind producing a formulation which has
the properties of both neutral antagonism and inverse
agonism of the CB1 or CB2 receptors is to enable diseases
which would normally be treated by either a neutral
antagonist or an inverse agonist to have an enhanced
treatment option.
For example, as has already been described by the
applicants in their co-pending application
(PCT/GB05/004388), THCV is useful in producing beneficial
weight loss in obese mammals. This appears to be due to
an increase in the energy expenditure and food conversion
efficiency. It is thought that THCV achieves such
properties by antagonism of the CB1 receptor.
Unfortunately there are associated problems with the
treatment of diseases such as obesity with THCV due to
the ongoing background tone in the cells of mammals
suffering from obesity. A treatment option that combines
THCV with an inverse CB1 agonist which is able to switch
off the background tone of the cells provides a valuable
solution.
The combination of a neutral antagonist and an inverse
agonist enables the treatment of obese animals. The
combination results in a lowered blood triglyceride level
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
and in consequence an increase in HDL-cholesterol (which
is often referred to as `good cholesterol').
The combination of a neutral antagonist and an inverse
agonist also enables the treatment of diabetic animals.
The combination results in a reduction in plasma insulin
levels and improved glucose tolerance.
References to THCV and CBD, THCV- and CBD-type compounds
or derivatives thereof, particularly with regard to
therapeutic use, will be understood to also encompass
pharmaceutically acceptable salts of such compounds. The
term "pharmaceutically acceptable salts" refers to salts
or esters prepared from pharmaceutically acceptable non-
toxic bases or acids, including inorganic bases or acids
and organic bases or acids, as would be well known to
persons skilled in the art. Many suitable inorganic and
organic bases are known in the art.
The scope of the invention also extends to derivatives of
THCV or CBD that retain the desired activity of neutral
antagonism or inverse agonism of the CB1 and / or CB2
receptor. Derivatives that retain substantially the same
activity as the starting material, or more preferably
exhibit improved activity, may be produced according to
standard principles of medicinal chemistry, which are
well known in the art. Such derivatives may exhibit a
lesser degree of activity than the starting material, so
long as they retain sufficient activity to be
therapeutically effective. Derivatives may exhibit
improvements in other properties that are desirable in
pharmaceutically active agents such as, for example,
improved solubility, reduced toxicity, enhanced uptake.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Preferably the THCV and CBD are in the form of a
cannabinoid-containing plant extract from at least one
cannabis plant.
More preferably the cannabinoid-containing plant extract
from at least one cannabis plant is a botanical drug
substance.
In one embodiment the cannabinoid-containing plant
extract from at least one cannabis plant is produced by
extraction with supercritical or subcritical CO2.
Alternatively the cannabinoid-containing plant extract
from at least one cannabis plant is produced by
contacting plant material with a heated gas at a
temperature which is greater than 100 C, sufficient to
volatilise one or more of the cannabinoids in the plant
material to form a vapour, and condensing the vapour to
form an extract.
Preferably the cannabinoid-containing plant extract from
at least one cannabis plant comprises all the naturally
occurring cannabinoids in the plant.
Alternatively the THCV and / or CBD are in a
substantially pure or isolated form.
A "substantially pure" preparation of cannabinoid is
defined as a preparation having a chromatographic purity
(of the desired cannabinoid) of greater than 90%, more
preferably greater than 95%, more preferably greater than
96%, more preferably greater than 97%, more preferably
greater than 98%, more preferably greater than 99% and
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
most preferably greater than 99.5%, as determined by area
normalisation of an HPLC profile.
Preferably the substantially pure cannabinoid used in the
invention is substantially free of any other naturally
occurring or synthetic cannabinoids, including
cannabinoids which occur naturally in cannabis plants. In
this context "substantially free" can be taken to mean
that no cannabinoids other than the target cannabinoid
are detectable by HPLC.
Particularly in the case of THCV, it is known that the
cannabinoid THCV is produced together with THC in the
cannabis plant. The psychoactive side effects of THC are
not wanted especially when producing a pharmaceutical
formulation and as such the plant extracts used in the
formulations of the invention can be selectively treated
to remove other cannabinoids such as THC.
In another aspect of the present invention the
cannabinoids are in a synthetic form.
Preferably the pharmaceutical formulation further
comprises one or more pharmaceutically acceptable
carriers, excipients or diluents.
The invention also encompasses pharmaceutical
formulations, formulated into pharmaceutical dosage
forms, together with suitable pharmaceutically acceptable
carriers, such as diluents, fillers, salts, buffers,
stabilizers, solubilizers, etc. The dosage form may
contain other pharmaceutically acceptable excipients for
modifying conditions such as pH, osmolarity, taste,
viscosity, sterility, lipophilicity, solubility etc. The
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
choice of diluents, carriers or excipients will depend on
the desired dosage form, which may in turn be dependent
on the intended route of administration to a patient.
Suitable dosage forms include, but are not limited to,
solid dosage forms, for example tablets, capsules,
powders, dispersible granules, cachets and suppositories,
including sustained release and delayed release
formulations. Powders and tablets will generally comprise
from about 5% to about 70% active ingredient. Suitable
solid carriers and excipients are generally known in the
art and include, e.g. magnesium carbonate, magnesium
stearate, talc, sugar, lactose, etc. Tablets, powders,
cachets and capsules are all suitable dosage forms for
oral administration.
Liquid dosage forms include solutions, suspensions and
emulsions. Liquid form preparations may be administered
by intravenous, intracerebral, intraperitoneal,
parenteral or intramuscular injection or infusion.
Sterile injectable formulations may comprise a sterile
solution or suspension of the active agent in a non-
toxic, pharmaceutically acceptable diluent or solvent.
Liquid dosage forms also include solutions or sprays for
intranasal, buccal or sublingual administration. Aerosol
preparations suitable for inhalation may include
solutions and solids in powder form, which may be
combined with a pharmaceutically acceptable carrier, such
as an inert compressed gas.
Also encompassed are dosage forms for transdermal
administration, including creams, lotions, aerosols
and/or emulsions. These dosage forms may be included in
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
transdermal patches of the matrix or reservoir type,
which are generally known in the art.
Pharmaceutical preparations may be conveniently prepared
in unit dosage form, according to standard procedures of
pharmaceutical formulation. The quantity of active
compound per unit dose may be varied according to the
nature of the active compound and the intended dosage
regime. Generally this will be within the range of from
0.1mg to 1000mg.
It may be preferable depending on the disease or
condition which is to be treated to have a high dose of
the inverse agonist the CB1 and / or CB2 receptor and a
low dose of the neutral antagonist of the CB1 and / or CB2
receptor, or vice versa. For example a high dose of CBD
of 1000mg may be combined with a low dose of THCV of 10
mg. Alternatively the dose of each inverse agonist or
neutral antagonist may be approximately the same.
Preferably the ratio of (i):(ii) in the pharmaceutical
formulation is from 99:1 to 1:99.
Preferably the ratio of THCV and CBD in the
pharmaceutical formulation are in a ratio of from 99:1
and 1:99 THCV:CBD (w/w).
More preferably the ratio of THCV:CBD is from 85:15 to
15:85 THCV:CBD (w/w).
More preferably the ratio of THCV:CBD is from 75:25 to
25:75 THCV:CBD (w/w).
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
More preferably the ratio of THCV:CBD is from 65:35 to
35:65 THCV:CBD (w/w).
More preferably the ratio of THCV:CBD is from 55:45 to
45:55 THCV:CBD (w/w).
More preferably the ratio of THCV:CBD is approximately
50:50 THCV:CBD (w/w).
.Certain aspects of this invention are further described,
by way of example only, with reference to the
accompanying drawings in which:
Figure 1 shows the agonism and antagonism of
constitutively active receptors; and
Figure 2 shows the 2-dimensional structure of the
cannabinoid tetrahydrocannabidivarin (THCV) and
cannabidiol (CBD).
SPECIFIC DESCRIPTION
The examples described below relate to the preparation of
a dosage form containing a mixture of extracts of
cannabis. The extracts are referred to as cannabis-based
medicinal extracts (CBME) for ease of reference.
An extract from a chemovar of cannabis producing
cannabidiol (CBD) as a main cannabinoid and an extract
from a chemovar producing tetrahydrocannabidivarin (THCV)
as a main cannabinoid have been used in many of the
examples below. These cannabinoids were used to produce
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
formulations as the binding properties of these
cannabinoids have been explored; the data from these
experiments is detailed in Example 1.
The remainder of the examples describe different types of
pharmaceutical formulations that may be useful for
administration of a neutral antagonist of the CB1 and / or
CB2 receptor combined with an inverse agonist of the CB1
and / or CB2 receptor.
The formulas described in these examples can be varied to
accommodate CBME with a greater or lesser amount of
cannabinoid in order to achieve the desired ratio of THCV
to CBD or other cannabinoids or active agents. Different
ratios of neutral antagonists of the CB1 and / or CB2
receptor and inverse agonists of the CB1 and / or CB2
receptor will be useful in the treatment of specific
therapeutic conditions.
Example 1:
Experiments were performed with membranes prepared from
healthy brain tissue, which is densely populated with CB1
but not CB2 receptors. Further experiments were undertaken
with Chinese hamster ovary (CHO) cells transfected with
hCB2 receptors. These membranes were used to investigate
the ability of the test compound to displace [3H]CP55940
CB2 binding sites
These experiments were used to determine whether the test
compounds behaved as a CB1 and / or a CBZ receptor agonist
or antagonist. For these experiments the test compounds
used were THCV (cannabinoid-containing plant extract) and
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
CBD (cannabinoid-containing plant extract), both singly
and as a mixture.
Methods:
Radioligand displacement assay
The assays were carried out with [3H]CP55940, 1 mg ml-1
bovine serum albumin (BSA) and 50mM Tris buffer, total assay
volume 500pl.
Binding was initiated by the addition of either the brain
membranes (33pg protein per tube) or the transfected hCB2
cells (25pg protein per tube).
All assays were performed at 37 C for 60 min before
termination by addition of ice-cold wash buffer (50mM Tris
buffer, 1 mg ml-1 bovine serum albumin, pH 7.4) and vacuum
filtration using a 24-well sampling manifold and GF/B
filters that had been soaked in wash buffer at 4 C for at
least 24 h.
Each reaction tube was washed six times with a 1.2 ml
aliquot of wash buffer. The filters were oven-dried for 60
min and then placed in 5ml of scintillation fluid.
Radioactivity was quantified by liquid scintillation
spectrometry.
Specific binding was defined as the difference between the
binding that occurred in the presence and absence of luM
unlabelled CP55940. The THCV and CBD were stored as a stock
solution of 10mM in DMSO, the vehicle concentration in all
assay tubes being 0.1% DMSO.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
The binding parameters for [3H]CP55940, were 2336 fmol mg-1
protein (Bmax) and 2.31 nM (Kd) in mouse brain membranes, and
72570 fmol/mg protein (B,,x) and 1.043 nM (Kd) in hCB2
transfected cells.
[35S]GTPyS binding assay
The assays were carried out with GTPyS binding buffer
(50mM Tris-HC1; 50mM Tris-Base; 5mM MgC12; 1mM EDTA; 100mM
NaCl; imM DTT; 0.1% BSA) in the presence of [35S]GTPyS and
GDP, in a final volume of 500ul. Binding was initiated by
the addition of [35S]GTPyS to the tubes. Nonspecific
binding was measured in the presence of 30pM GTPyS.
The drugs were incubated in the assay for 60 min at 30 C.
The reaction was terminated by a rapid vacuum filtration
method using Tris buffer (50mM Tris-HC1; 50mM Tris-Base;
0.1% BSA), and the radioactivity was quantified by liquid
scintillation spectrometry.
The concentrations of [35S ]GTPyS and GDP present in the
assay varied depending on whether the assay was conducted
with mouse brain or transfected cell membranes. When the
assay was conducted with mouse brain membranes, 0.1nM
[35S ]GTPyS and 30uM GDP were present, whereas the
corresponding concentrations present when the assay was
conducted with transfected cell membranes were 1nM and
320pM respectively.
Additionally, mouse brain membranes were preincubated for
30 minutes at 30 C with 0.5 U ml-1 adenosine deaminase to
remove endogenous adenosine. Agonists and antagonists
were stored as a stock solution of 1 or 10mM in DMSO, the
vehicle concentration in all assay tubes being 0.11%
DMSO.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Analysis of data
Values are expressed as means and variability as s.e.mean or
as 95% confidence limits. The concentration of THCV that
produced a 50% displacement of radioligand from specific
binding.
Net agonist-stimulated [35S]GTPyS binding values were
calculated by subtracting basal binding values (obtained in
the absence of agonist) from agonist-stimulated values
(obtained in the presence of agonist) as detailed elsewhere
(Ross et al., 1999a).
Inhibition of the electrically-evoked twitch response of the
vas deferens has been expressed in percentage terms and this
has been calculated by comparing the amplitude of the twitch
response after each addition of a twitch inhibitor with its
amplitude immediately before the first addition of the
inhibitor. Contractile responses to phenylephrine and (3,y-
methylene-ATP have been expressed as increases in tension
(g).
Values for EC50, for maximal effect (Emax) and for the
s.e.mean or 95% confidence limits of these values have been
calculated by nonlinear regression analysis using the
equation for a sigmoid concentration-response curve
(GraphPad Prism).
The apparent dissociation constant (KB) values for
antagonism of agonists by THCV in the vas deferens or
[35S]GTPyS binding assay have been calculated by Schild
analysis from the concentration ratio, defined as the
concentration of an agonist that elicits a response of a
particular size in the presence of a competitive reversible
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
antagonist at a concentration, B, divided by the
concentration of the same agonist that produces an identical
response in the absence of the antagonist.
The methods used to determine concentration ratio and
apparent KB values and to establish whether log
concentration-response plots deviated significantly from
parallelism are detailed elsewhere (Pertwee et al., 2002).
Mean values have been compared using Student's two-tailed t-
test for unpaired data or one-way analysis of variance
(ANOVA) followed by Dunnett's test (GraphPad Prism). A P-
value <0.05 was considered to be significant.
Results:
THCV
THCV displaced [3H]CP55940 from specific binding sites in
mouse brain and CHO-hCB2 cell membranes in a manner that
fitted significantly-better to a one-site than a two-site
competition curve (P<0.05; GraphPad Prism 4). Its mean Ki
values were 75.4nM and 62.8nM respectively.
THCV also displaced [3H]R-(+)-WIN55212 and [3H]SR141716A
from specific binding sites in mouse brain membranes, its
mean EC50 values with 95% confidence limits shown in
brackets being 61.3nM (48.6 and 77.3nM; n=4 to 7) and 86.8nM
(63.8 and 188.lnM; n=4 to 6) respectively. The corresponding
EC50 value of THCV for displacement of [3H]CP55940 is 98.2nM
(69.6 and 138.6nM; n=4 to 8).
The ability of CP55940 to enhance [35S]GTPyS binding to
mouse brain and CHO-hCB2 membranes was attenuated by THCV,
which at l M produced significant dextral shifts in the log
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
concentration response curves of this cannabinoid receptor
agonist that did not deviate significantly from parallelism.
The mean apparent KB values for this antagonism are shown in
Table 1, as are mean apparent KB values of SR141716A for
antagonism of CP55940 in mouse brain membranes and of
SR144528 for antagonism of CP55940 in the CHO-hCB2 cell
membranes. At 1pM, THCV also produced a significant parallel
dextral shift in the log concentration response curve of R-
(+)-WIN55212 for enhancement of GTPyS binding to mouse brain
membranes.
Table 1:
Antagonist Agonist Membrane Mean 95% n
prepara- apparent confiden
tion KB ce
(nM) limits
(nM)
THCV CP55940 Brain 93.1 66.5, 6
(1000 nM) 130.6
THCV R- (+) -WIN55212 Brain 85.4 29.3, 5
(1000 nM) 270.5
SR141716A CP55940 Brain 0.09 0.021, 4
(10 nM) 0.41
THCV CP55940 CHO-hCB2 10.1 5.0, 6
(1000 nM) 20.5
SR144528 CP55940 CHO-hCB2 0.49 0.26, 6
(100 nM) 0.85
CBD
Table 2 describes the data produced by CBD and the known CB1
receptor inverse agonist'SR141716A at the CB1 receptor.
The table describes the KB-values for the CP55940 induced
activation of [35S]GTPyS binding to the cell membrane in the
presence of the known CB1 receptor inverse agonist and CBD.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
The Ki-value for the displacement of the [3H]CP55940 from
the membranes is also shown.
Table 2:
Test Article KB-value for Ki-value for
Binding Displacement
SR141716A 0.09 nM 2.2 nM
(10 nM)
CBD 78.8 nM 4.9 pM
(luM)
Both SR141617A and CBD were able to produce a rightward
shift in the log-concentration response curve of the
established CB1/CB2 receptor agonist CP55940 in the mouse
brain membranes when the measured response was stimulation
of [35S]GTPyS binding. These data show that both compounds
were able to inhibit the response caused by the activation
of the CB1 receptor by CP55940.
The KB-value of SR141716A was 0.09nM which is only slightly
less than its CB1 Ki-value of 2.2nM for the displacement of
[3H]CP55940 from the mouse brain membranes. This infers that
this compound is able to produce an inverse response in the
cell at a similar concentration to that at which it competes
and binds to the receptor.
However the KB-value of CBD was 78.8 nM this was well below
its CB1 Ki-value of 4.9 pM for the displacement of
[3H]CP55940 from the mouse brain membranes. These data show
that CBD is able to act as an inverse agonist at the CB1
receptor. They also show that CBD is able to act as an
inverse agonist at concentrations much below that at which
it will compete with the agonist for the binding site.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
This property may be of significant value as it infers that
CBD will form a less strong interaction with the cannabinoid
receptor in vivo and as such is likely to produce fewer side
effects in use than the compound SR141716A.
Further experiments were undertaken at different
concentrations of the test compounds. At concentrations of 1
and 10 pM CBD produced a significant inhibition of
[35S]GTPyS binding to the mouse brain membrane. The
inhibitory effect of CBD at 1 pM was.similar to that of
SR141716A at 1 pM, whereas the inhibitory effect of CBD at
10 pM greatly exceeded that of SR141716A at the same
concentration. At the higher concentration CBD is a more
potent inverse agonist of the CB1 receptor than SR141716A.
Table 3 describes the data produced by CBD and the known CB2
receptor inverse agonist SR144528 at the CB2 receptor.
The table describes the KB-values for the CP55940 induced
activation of [35S]GTPyS binding to the cell membrane in the
presence of the known CB1 receptor inverse agonist and CBD.
The Ki-value for displacement of the [3H]CP55940 from the
membranes is also shown.
Table 3:
Test Article KB-value for Ri-value for
binding displacement
SR144528 0.49 nM 7.5 nM
(100 nM)
CBD 65.1 nM 4.2 pM
(1}~M)
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Both SR144528 and CBD were able to produce a downward and
rightward shift in the log-concentration response curve of
the established CB1/CB2 receptor agonist CP55940 in the CHO
cell membranes when the measured response was stimulation of
[35S]GTPyS binding. These data show that both compounds were
able to inhibit the response caused by the activation of the
CB2 receptor by CP55940.
The KB-value of SR144528 was 0.49 nM which was 15 times less
than its CB1 Ki-value of 7.5 nM for the displacement of
[3H]CP55940 from the CHO cell membranes.
The KB-value of CBD was 65.1 nM which was 65 times less than
its CB1 Ki-value of 4.2 pM for the displacement of
[3H]CP55940 from the CHO cell membranes.
Conclusions:
18 9-tetrahydrocannabivarin (THCV) displaced [3H]CP55940 from
specific binding sites on brain and CHO-hCB2 cell membranes
(K1 = 75.4 and 62.8nM respectively), indicating that THCV is
both a CB1 and CB2 receptor antagonist.
THCV (1pM) also antagonized CP55940-induced enhancement of
[35S]GTPyS binding to these membranes (apparent KB = 93.1 and
10.1nM respectively), indicating that it is a reasonably
potent competitive antagonist. The KB values indicate that
THCV is more potent as a CB2 than a CB1 receptor antagonist.
THCV produced its antagonism of cannabinoids at
concentrations that by themselves did not affect the
amplitude of the electrically-evoked contractions, or the
ability of [35S]GTPyS to bind to mouse brain membranes or
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
CHO-hCB2 cell membranes, suggesting that THCV is a neutral
cannabinoid receptor antagonist.
CBD is able to act as an inverse agonist at the CB1 and CB2
receptors. CBD acts as inverse agonist at concentrations
below that at which it competes with the agonist for the
binding site. However CBD was shown to compete at a far
lower concentration than SR144528.
In summary the data produced in this example indicates that
CBD is an inverse agonist at both the CB1 and CB2 receptors.
It is also shown that CBD will only displace agonists from
their cannabinoid receptor binding sites at far higher
concentrations than that at which it is able to produce the
inverse agonism in the cell.
Example 2:
A mixture is prepared by melting together the following
ingredients:
Glycerol mono-oleate 10 parts
Soy lecithin 5 parts
CBME - to give CBD 1 part
CBME - to give THCV 2 parts
Alpha-tocopherol 0.1 part
Ascorbyl palmitate BP 0.1 part
Glycogelatin to produce 100 parts
The components are mixed together over a gentle heat and
poured into moulds whilst hot. The product in moulds is
formed into a rigid gel and sealed in an inert
atmosphere. The relatively large size of this dosage form
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
(1-2g) allows a large amount of active ingredient to be
incorporated into the dosage form. Each dose unit may be
administered by allowing to dissolve in the mouth,
sublingually, buccally or swallowed whole or in smaller
units.
Example 3:
A smaller unit dosage form may be prepared using the
following example, whereby a smaller amount of active can
be incorporated. The following example is particularly
suitable for an oral dosage form such as a tablet.
Glycerol monosterate (self emulsifying grade) 5 parts
Polysorbate 80 0.5 parts
Lactose (direct compression grade) 79.3 parts
Soluble starch 10 parts
CBME - to give CBD 2.5 parts
CBME - to give THCV 2.5 parts
Ascorbyl palmitate 0.1 part
Alpha-tocopherol 0.1 part
Ethanol (dehydrated) BP 10 parts
The glycerol monosterate, polysorbate, alpha-tocopherol
and CBMEs are dispersed and dissolved in the ethanol.
This solution is then sprayed onto the dry poweder
ingredients which have been thoroughly mixed. The ethanol
is allowed to evaporate and the granules are dusted with
1% talc and compressed to the target tablet weight of
101mg in a conventional tablet press. Biconvex punches
with a diameter of 7-9mm are used to produce tablets with
a high surface to weight ratio. These are able to absorb
water when placed under the sublingual or buccal mucosae
and disperse in a period'of 30 seconds to 5 minutes.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Alternatively the tablets may be swallowed whole as an
oral dosage form.
Example 4:
The generation of an emulsion from a self-emulsifying
formulation is not limited to solid dosage forms. In the
following example three liquid formulations suitable for
sublingual application are exemplified. A solution is
produced by melting together, at a temperature not
exceeding 50 C, the following ingredients:
A B C D E
Glycerol mono-oleate 2 2 2 2 2
(self-emulsifying)
Medium chain triglyceride 5 - - - -
Cremophor RH40 30 26.5 - - -
CBME - to give CBD 5 1 9 7.5 2.5
CBME - to give THCV 5 9 1 2.5 7.5
Alpha-tocopherol 0.1 0.1 0.1 0.1 0.1
Ascorbyl palmitate 0.1 0.1 0.1 0.1 0.1
Propylene glycol - - 44 - -
Ethanol (to give) 100 100 100 100 100
The products formed by mixing these ingredients are
dispersed in lOml quantities into a glass vial ad closed
with a pump action break-up button. Each actuation of the
pump delivers a fine spray which can be directed to an
area of the buccal or sublingual mucosae or can be simply
sprayed into the mouth and swallowed.
Solutions based on ethanol alone are generally not
suitable to be used as a mouth spray. The addition of a
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
self-emulsifying agent allows this problem to be
overcome.
Example 5:
The solid dosage form may be a soft gelatine capsule
which can be crushed to release the medicament to give an
emulsion or swallowed orally. The soft gelatine capsule
described below provides an emulsified form of medicament
which can be absorbed from any part of the GI tract.
Glycerol monosterate (self emulsifying grade) 5 parts
Polysorbate 80 1 part
Beeswax 5 parts
CBME - to give CBD 10 parts
CBME - to give THCV 10 parts
Ascorbyl palmitate 0.1 part
Alpha-tocopherol 0.1 part
Hemp oil (to produce) 100 parts
Example 6:
A dosage form as described above which uses vegetable
rather than animal gelling agents may be made as.follows:
Sorbitol 35 parts
Gum acacia 20 parts
Glycerol mono-oleate 10 parts
Egg lecithin 10 parts
CBME - to give CBD 2.5 parts
CBME - to give THCV 2.5 parts
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Ascorbyl palmitate 0.1 part
Alpha-tocopherol 0.1 part
Ethanol (dehydrated) BP 10 parts
Vanillin 0.1 parts
BHT 0.01 parts
Glycerol 5 parts
Water (to give) 100 parts
The fat soluble ingredients are melted together at a
temperature of 70 C. Sorbitol is mixed with the acacia
gum, dispersed in glycerol, and added to the other solid
ingredients. Water is added and the mass heated on a
boiling water bath until evenly dispersed. While still at
a temperature of 60 C the mass can be distributed into
moulds.
Example 7:
A product providing a fast release of one constituent and
a slower release of another constituent can be produced
by making a combination dose unit. Using the formulation
described in example 5 a quantity of heated mass is
filled into a mould or cast into a film, and allowed to
set. A layer of material as described in example 2 is
then cast onto the surface of the gel. Variations of the
proportions and active content in the two layers provides
opportunities for the treatment of different diseases and
conditions where the administration of either a neutral
antagonist of the CB1 and / or CBz receptor is useful
either before or after the administration of an inverse
agonist of the CB1 and / or CB2 receptor.
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Example 8:
The example described below details the features of
formulations intended for spray application to the buccal
mucosae.
A solution is produced by dissolving the following
ingredients at a temperature not exceeding 50 C.
A B C D E
Glycerol monostearate 2 - 2 - 2
(self-emulsifying)
Glycerol mono-oleate - 2 - 2 2
Cremophor RH40 20 30 30 20 30
CBME - to give CBD 5 2.5 5 1.5 3.5
CBME - to give THCV 5 5 2.5 3.5 1.5
Alpha-tocopherol 0.1 0.1 0.1 0.1 0.1
Ascorbyl palmitate 0.1 0.1 0.1 0.1 0.1
Ethanol (to give) 100 100 100 100 100
The product formed by mixing together these ingredients
is dispensed into glass vials and closed with a pump
action or aerosol spray.
Example 9:
The example described below details the features of
formulations which can be dispensed from a pump action
spray device. The product can be dispensed to produce a
ribbon of gel which can either be swallowed or can be
applied to the buccal or other mucosae.
Carboxymethylcellulose sodium 2 parts
Glycerol monosterate (self emulsifying grade) 10 parts
CA 02692539 2010-01-04
WO 2009/007697 PCT/GB2008/002315
Glycerol 10 parts
CBME - to give CBD 10 parts
CBME - to give THCV 10 parts
Ascorbic acid 0.1 part
Alpha-tocopherol 0.1 part
Water (to produce) 100 parts
The non-aqueous ingredients are melted together at a
temperature of not more than 50 C until evenly suspended.
Water is then added to form a creamy gel. The product is
dispensed into containers whilst still warm and sealed
with a pump dispenser head.
Example 10:
The example described below details the features of
formulations produced with less than 5% water. The
presence of water can sometimes cause precipitation of
the active ingredients. The product can be dispensed from
a pump action spray device. The product can be dispensed
to produce a spray which can either be swallowed or can
be applied to the buccal or other mucosae.
Propylene glycol 50 parts
CBME - to give CBD 2.5 parts
CBME - to give THCV 2.5 parts
Peppermint oil 0.005 part
Ethanol (to produce) 100 parts