Note: Descriptions are shown in the official language in which they were submitted.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
1
METHOD OF PRODUCING METALS AND ALLOYS BY CARBOTHERMAL
REDUCTION OF METAL OXIDES
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Patent
Application Serial No.
60/954328, filed on 07 August 2007, under 35 U.S.C. 119(e). U.S. Provisional
Patent
Application Serial No. 60/954328 is hereby incorporated by reference.
FIELD OF THE INVENTION
[0002] The present invention relates to a method of producing metals and
alloys by
carbothermal reduction of metal oxides and more particularly to a method
comprising heating
raw materials comprising at least one metal oxide, and agglomerates comprising
a
carbonaceous reducing agent and a cured binder to effect reduction of the
metal oxide to the
metal, wherein each agglomerate has at least one molded open channel, and an
apparent
density not greater than 99% of the apparent density of an identical
agglomerate except
without the channel.
BACKGROUND OF THE INVENTION
[0003] The large scale production of various metals and alloys by the
carbothermal
reduction of metal oxides remains an important process in extractive
metallurgy. For
example, metals and alloys such as silicon, ferrosilicon, aluminum, iron,
steel, and tungsten
are widely produced by reduction of the corresponding metal oxides with a
carbonaceous
reducing agent in an electric arc furnace.
[0004] In carbothermal reductions, the carbonaceous reducing agent is
typically introduced
into the furnace in the form of agglomerates, such as pellets, lumps, or
briquettes. Moreover,
agglomerates having low apparent density and low bulk density are highly
desired for
optimal operation of the furnace and control of the process. The advantages of
using
agglomerates having low apparent and bulk densities typically include
relatively high yield,
high metal production rate, and low energy consumption. Furthermore,
agglomerates should
have sufficient strength to withstand conventional handling operations and the
harsh
environment of a metallurgical furnace, without being crushed.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
2
[0005] Although numerous processes for the carbothermal reduction of metal
oxides are
reported in the art, a need persists for a process employing a carbonaceous
reducing agent
having substantially lower bulk and apparent densities, while still meeting
the requirement
for adequate strength.
SUMMARY OF THE INVENTION
[0006] The present invention is directed to a method of producing a metal, the
method
comprising heating raw materials comprising at least one metal oxide, and
agglomerates
comprising a carbonaceous reducing agent and a cured binder to effect
reduction of the metal
oxide to the metal, wherein each agglomerate has at least one molded open
channel, and an
apparent density not greater than 99% of the apparent density of an identical
agglomerate
except without the channel.
[0007] The process of the instant invention employs agglomerates comprising a
carbonaceous reducing agent and a cured binder, wherein each agglomerate has
at least one
molded open channel. Importantly, the agglomerates have low apparent density
and low bulk
density. In particular, the channels increase the porosity of the furnace
charge, thus
increasing the circulation of the reacting gases and facilitating the removal
of CO gas. As a
result, the process exhibits relatively high yield, high metal production
rate, and low energy
consumption. Further, the process can be carried out using conventional
equipment and
techniques. Still further, the method is scaleable to a high throughput
manufacturing process.
[0008] The method of the present invention can be used to produce various
metals and
alloys from the corresponding metal oxides. In particular, the method can be
used to produce
metallurgical grade and solar grade silicon from silicon dioxide. Moreover,
metals and alloys
produced according to the present method have widespread utility in chemical,
electrical, and
mechanical products and processes.
BRIEF DESCRIPTION OF THE DRAWINGS
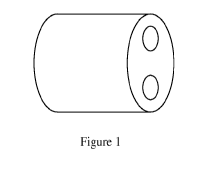
[0009] Figure 1 shows a perspective view of a first embodiment of an
agglomerate
according to the present method.
[0010] Figure 2 shows a perspective view of a second embodiment of an
agglomerate
according to the present method.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
3
[0011] Figure 3 shows a cross-sectional view of a third embodiment of an
agglomerate
according to the present method.
[0012] Figures 4A and 4B are photographs showing perspective and cross-
sectional views,
respectively, of a honeycomb briquette prepared as described in the Examples
Section,
below.
[0013] Figure 5 shows a cross-sectional view of a multi-hole die used to
prepare the
honeycomb briquettes in the Examples below.
DETAILED DESCRIPTION OF THE INVENTION
[0014] As used herein, the term "metal" refers to a single metal or a mixture
comprising
two or more different metals, i.e., an alloy. Moreover, the term "metal"
includes semi-metals
(metalloids) such as silicon and boron. As used herein, the term "median
particle size," also
known as the "mass median diameter," is defined as the particle diameter at
which fifty
percent by mass of the particles have a larger diameter and fifty percent of
the particles by
mass have a smaller diameter. Graphically, the median particle size
corresponds to the
diameter at which the cumulative arithmetic curve (plot of percentage by
weight versus
particle diameter) intersects the 50% line.
[0015] A method of producing a metal according to the present invention,
comprises
heating raw materials comprising at least one metal oxide, and agglomerates
comprising a
carbonaceous reducing agent and a cured binder to effect reduction of the
metal oxide to the
metal, wherein each agglomerate has at least one molded open channel, and an
apparent
density not greater than 99% of the apparent density of an identical
agglomerate except
without the channel.
[0016] The metal oxide can be any metal oxide capable of undergoing
carbothermal
reduction to produce the corresponding metal. The metal oxide may be an
isolated (i.e.,
substantially pure) compound or a metal oxide-containing ore. The metal oxide
can have a
variety of physical forms including, but not limited to, lumps, granules,
flakes, powder, sand,
and gravel. Moreover the metal oxide, when compounded with a binder, may have
the form
of agglomerates, such as briquettes and pellets.
[0017] Examples of metal oxides include, but are not limited to, compounds
such as silicon
dioxide, iron oxide, aluminum oxide, molybdenum oxide, chromium oxide, boric
oxide,
tungsten oxide, magnesium oxide, cobalt oxide, nickel oxide, copper oxide,
calcium oxide,
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
4
lead oxide, calcium phosphate, manganese dioxide, beryllium oxide, zirconium
oxide, tin
oxide, zinc oxide, titanium oxide, and vanadium oxide; and ores containing any
of the
aforementioned metal oxides.
[0018] In one embodiment of the present invention, the metal oxide is silica
(i.e., silicon
dioxide). Examples of silica include, but are not limited to, crystalline
silica such as quartz;
and noncrystalline (amorphous) silica such as fused quartz, fumed silica,
silica gel, and
precipitated silica.
[0019] The metal oxide can be a single metal oxide or a mixture comprising two
or more
metal oxides of different metals or the same metal in different oxidation
states. Methods of
preparing metal oxides are well known in the art; many metal oxides, including
metal oxide-
containing ores, are commercially available.
[0020] The agglomerates comprise a carbonaceous reducing agent and a cured
binder,
wherein each agglomerate has at least one molded open channel, and an apparent
density not
greater than 99% of the apparent density of an identical agglomerate except
without the
channel.
[0021] As used herein, the term "molded open channel" refers to a macroscopic
cavity
present in the agglomerate by design, where the cavity is continuous with the
external surface
of the agglomerate. The channel may be open at one end (dead-end channels) or
open at two
ends (through channels). The channel may have any regular or irregular
geometric shape,
including cylindrical, slit-shaped, and conical.
[0022] The width of the channel is typically at least 1 mm, alternatively at
least 3 mm,
where the width is defined as the minimum distance between two opposite walls
of the
channel. For example, the width of a cylindrical channel is the diameter of
the cylinder,
whereas the width of a slit-shaped channel is the width of the slit. The width
of the channel is
typically from 1 to 50 mm, alternatively from 1 to 30 mm, alternatively from 2
to 20 mm.
The length of a channel is typically at least 2 times the width of the
channel.
[0023] The number, width, and depth of the channels are such that the
agglomerate has an
apparent density not greater than 99%, alternatively not greater than 80%,
alternatively not
greater than 75%, of the apparent density of an identical agglomerate except
without the
channel(s). For example, the agglomerate typically has an apparent density of
from 10 to
99%, alternatively from 40 to 90%, alternatively from 60 to 80%, of the
apparent density of
an identical agglomerate except without the channel(s).
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
[0024] The "apparent density" of an agglomerate is defined as the ratio of the
mass of the
agglomerate to the total volume of the agglomerate, where the total volume of
the
agglomerate is the sum of the volumes of the solid material, pore(s), and
molded open
channel(s). Also, the term "solid material" refers to an agglomerated mixture
comprising
carbonaceous reducing agent, a cured binder and, optionally, other
ingredients, described
below. Further, the term "pore" refers to any void present in the agglomerate
not by design,
where the void may or may not be continuous with the external surface of the
agglomerate.
Also, pores typically have a width not greater than 1 m, where the width is
defined as the
minimum distance between two opposite walls of the pore.
[0025] The apparent density of an agglomerate can be calculated by measuring
the mass
and total volume of the agglomerate. Mass of an agglomerate can be determined
using a
balance. Total volume of an agglomerate having a simple three-dimensional
shape (e.g.,
spherical and cylindrical) can be calculated from the external dimensions of
the agglomerate.
Total volume of an agglomerate having a complex shape can be determined by
measuring the
volume of mercury displaced by an identical agglomerate, except without the
channel(s). In
the later case, the identical agglomerate without the channel(s) is submerged
in mercury at
room temperature (-23 2 C). The change in volume of the mercury is equal to
the total
volume of the corresponding agglomerate having at least one molded open
channel. The
identical agglomerate without the channel(s) has the same external shape and
solid
composition as the corresponding agglomerate with the channel(s). The
agglomerate without
the channel(s) can be prepared using the same materials and methods as used
for the
corresponding agglomerate with the channel(s), described below, except the
mixture of
carbonaceous reducing agent, curable binder, and any optional ingredients, are
compressed to
form an agglomerate having the same external shape, but without the
channel(s).
[0026] The agglomerates have sufficient strength to withstand handling
operations and the
harsh environment of a metallurgical furnace, without being crushed. For
example, the
agglomerates typically have a compression strength of from 5 to 1501bf,
alternatively from 5
to 701bf, alternatively from 10 to 401bf.
[0027] The agglomerates can have a variety of shapes and sizes, depending on
the method
used to produce them, described below. For example, the agglomerates can be
briquettes or
pellets. Moreover, the agglomerates can have a variety of regular or irregular
three-
dimensional external shapes including, but not limited to, cylindrical,
cuboidal, cubical,
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
6
conical, octahedral, spherical, hemispherical, and pyramidal. In one
embodiment, the
agglomerates have parallel channels open at both ends arranged in a honeycomb
pattern.
[0028] Examples of agglomerates useful in the method of the present invention
include, but
are not limited to, cylindrical briquettes (Figure 1), cuboidal briquettes
(Figure 2), and star-
shaped briquettes (Figure 3).
[0029] The agglomerates can be identical or a mixture comprising two or more
agglomerates that differ in at least one of the following properties: apparent
density, shape,
number of channels, dimensions of channels, mass, concentration of
carbonaceous reducing
agent and/or cured binder, and composition of carbonaceous reducing agent
and/or cured
binder.
[0030] The carbonaceous reducing agent can be any solid particulate containing
primarily
elemental carbon effective in the carbothermal reduction of metal oxides or
ores to the
corresponding metals and alloys. The carbonaceous reducing agent typically has
a median
particle size of from 0.01 to 3000 m, alternatively from 0.1 to 1000 m,
alternatively from
to 500 m.
[0031] Examples of carbonaceous reducing agents include, but are not limited
to, carbon
black, activated carbon, coal, coke, and charcoal. Moreover, the carbonaceous
reducing
agent can be a single reducing agent or a mixture comprising two or more
different agents,
each as described above.
[0032] The concentration of the carbonaceous reducing agent in each
agglomerate is
typically from 60 to 98 parts by weight, alternatively from 70 to 90 parts by
weight,
alternatively from 75 to 85 parts by weight, wherein the total amount of the
carbonaceous
reducing agent and the cured binder is 100 parts by weight.
[0033] The cured binder can be any cured solid binder that provides the
agglomerates
sufficient compression strength to withstand handling operations and the harsh
environment
of a metallurgical furnace, without being crushed. As used herein, the term
"cured binder"
refers to a cross-linked binder having a continuous three-dimensional network
structure
throughout the agglomerate. The cured binder is typically a thermoset polymer,
a cross-
linked thermoplastic polymer, or a cross-linked product of thermally
polymerizable
monomers.
[0034] Examples of cured binders include, but are not limited to, cured
silicone resins,
cured polybutadienes, cured polyesters, cured products of carbohydrates and
dicarboxylic
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
7
acids or anhydrides, cured epoxy resins, cured polyvinyl alcohols, cured amino
resins, cured
polyurethanes, cured polyimides, cured phenolic resins, cured cyanate ester
resins, cured
furan resins, cured bismaleimide resins, and cured acrylic resins. In one
embodiment of the
agglomerates, the cured binder is a cured product of sucrose (sugar) and
adipic acid.
[0035] The cured binder can be a single cured binder or a mixture comprising
two or more
different cured binders, each as described above. Moreover, the concentration
of the cured
binder is typically from 2 to 40 parts by weight, alternatively from 10 to 30
parts by weight,
alternatively from 15 to 25 parts by weight, wherein the total amount of the
carbonaceous
reducing agent and the cured binder is 100 parts by weight.
[0036] In addition to the carbonaceous reducing agent and the cured binder,
the
agglomerates can further comprise at least one additional ingredient, provided
the ingredient
does not inhibit reduction of the metal oxide to the corresponding metal, as
described below.
Examples of additional ingredients include, but are not limited to, fibers
such as polyethylene
fibers and paper fibers; and metal oxides. In particular, the agglomerates can
comprise at
least a portion (i.e., part or all) of the metal oxide, described above, in
the raw materials.
When present in the agglomerates, the metal oxide typically has the form of
particles having
a median particle size of from 0.01 to 3000 m, alternatively from 0.1 to 1000
m,
alternatively from 10 to 500 m.
[0037] An agglomerate according to the present invention can be prepared by
(i) forming a
mixture comprising a carbonaceous reducing agent and a curable binder into a
green
agglomerate having at least one molded open channel; and (ii) curing the
curable binder of
the green agglomerate to produce an agglomerate having an apparent density not
greater than
99% of the apparent density of an identical agglomerate except without the
channel.
[0038] In step (i) of the method of preparing an agglomerate, a mixture
comprising a
carbonaceous reducing agent and a curable binder is formed into a green
agglomerate having
at least one molded open channel.
[0039] The carbonaceous reducing agent is as described and exemplified above
for the
agglomerate of the present invention. The concentration of the carbonaceous
reducing agent
in the mixture is typically from 60 to 98 parts by weight, alternatively from
70 to 90 parts by
weight, alternatively from 75 to 85 parts by weight, wherein the total amount
of the
carbonaceous reducing agent and the curable binder is 100 parts by weight.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
8
[0040] The curable binder can be any binder that can be cured (i.e., cross-
linked) upon
application of heat. The curable binder is typically a thermosetting polymer,
a mixture of a
thermoplastic polymer and a cross-linking agent, or a mixture of thermally
polymerizable
monomers. When the curable binder is a solid at ambient temperature and
pressure, the
curable binder typically has the form of particles having a median particle
size of from 0.01
to 3000 m, alternatively from 0.1 to 1000 m, alternatively from 10 to 500
m.
[0041] Examples of curable binders include, but are not limited to, curable
silicone resins,
polybutadienes, unsaturated polyesters, mixtures of carbohydrates and
dicarboxylic acids or
anhydrides, epoxy resins, amino resins, polyurethanes, polyimides, phenolic
resins, cyanate
ester resins, furan resins, bismaleimide resins, and acrylic resins. In one
embodiment of the
agglomerate, the curable binder is a mixture of sucrose and adipic acid. The
mole ratio of
adipic acid to sucrose is typically from 0.2 to 1.2, alternatively from 0.4 to
1.0, alternatively
from 0.5 to 0.7.
[0042] The curable binder can be a single curable binder or a mixture
comprising two or
more different curable binders, each as described above. Moreover, methods of
preparing
curable binders are well known in the art; many curable binder compositions
are
commercially available.
[0043] The concentration of the curable binder in the mixture is typically
from 2 to 40 parts
by weight, alternatively from 10 to 30 parts by weight, alternatively from 15
to 25 parts by
weight, wherein the total amount of the carbonaceous reducing agent and the
curable binder
is 100 parts by weight.
[0044] The mixture comprising the carbonaceous reducing agent and curable
binder can
further comprise additional ingredients, provided the ingredient does not
prevent the mixture
from forming a green agglomerate having at least one molded open channel,
inhibit cure of
the binder, or inhibit reduction of the metal oxide to the corresponding metal
in the process of
the present invention. Examples of additional ingredients include, but are not
limited to,
fibers such as polyethylene fibers and paper fibers; metal oxides; and
solvents. In particular,
the mixture can further comprise at least a portion (i.e., part or all) of the
metal oxide,
described above, in the raw materials.
[0045] The mixture can further comprise at least one solvent. The solvent may
be used to
aid dispersal of the ingredients (i.e., carbonaceous reducing agent, curable
binder, etc.) in the
mixture, adjust the consistency of the mixture prior to forming the green
agglomerate, or
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
9
improve cohesion of the ingredients in the green agglomerate formed from the
mixture. The
solvent can be any nonpolar or polar (protic, aprotic, or dipolar aprotic)
solvent that does not
react with the carbonaceous reducing agent, curable binder, or other
ingredients in the
mixture in step (i) or step (ii) of the method of preparing the agglomerate.
The solvent
typically has a normal boiling point below the cure temperature of the curable
binder. The
solvent may be miscible or immiscible with the curable binder. For example,
the solvent may
form a suspension or an emulsion with the curable binder.
[0046] Examples of solvents include, but are not limited to, saturated
aliphatic
hydrocarbons such as n-pentane, hexane, n-heptane, isooctane and dodecane;
cycloaliphatic
hydrocarbons such as cyclopentane and cyclohexane; aromatic hydrocarbons such
as
benzene, toluene, xylene and mesitylene; cyclic ethers such as tetrahydrofuran
(THF) and
dioxane; ketones such as methyl isobutyl ketone (MIBK); halogenated alkanes
such as
trichloroethane; halogenated aromatic hydrocarbons such as bromobenzene and
chlorobenzene; alcohols such as methanol, ethanol, 1-propanol, 2-propanol, 1-
butanol, 2-
butanol, 2-methyl-l-butanol, 1,1-dimethyl-l-ethanol, pentanol, hexanol,
cyclohexanol,
hepatanol, and octanol; and water. Moreover, the solvent can be a single
solvent or a mixture
comprising two or more different solvents, each as described and exemplified
above.
[0047] The concentration of the solvent is typically from 0 to 70% (w/w),
alternatively
from 20 to 60% (w/w), alternatively from 40 to 50% (w/w), based on the total
weight of the
mixture.
[0048] The mixture can be prepared by combining the carbonaceous reducing
agent, curable
binder, and any optional ingredients, in any order and thoroughly mixing the
ingredients.
Mixing can be accomplished using techniques known in the art such as milling,
blending, and
stirring, either in a batch or continuous process. Mixing is typically
continued until the
ingredients are uniformly distributed throughout the mixture. The mixture can
also be
prepared by combining the ingredients in a screw feeder, which provides the
advantage of
precompacting and deaerating the incoming materials. A screw feeder can also
crush infeed
particles to achieve a more favorable size consistency for extrusion or
molding. In some
cases, heat generated by the screw feeding process may be beneficial, for
example, by
softening the binder prior to agglomeration. Furthermore, a twin screw
extruder can be used
to sequentially mix the ingredients and extrude the mixture into a green
agglomerate of
desired shape.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
[0049] The mixture can be formed into a green agglomerate having at least one
molded
open channel using conventional methods, such as compression molding,
injection molding,
and extrusion. As used herein, the term "green agglomerate" refers to an
agglomerate
comprising a curable, but uncured, binder. For example, the mixture can be
formed into a
green agglomerate of a desired shape by extruding the mixture through a die
containing
apertures corresponding to molded open channels in the resulting green
agglomerate.
[0050] In step (ii) of the method of preparing the agglomerate of the present
invention, the
curable binder of the green agglomerate is cured. The curable binder can be
cured by heating
the green agglomerate at a temperature and for a time sufficient to cure the
binder. The green
agglomerate is typically heated at a temperature below the decomposition
temperatures of
both the curable binder and the cured binder produced there from. The cure
conditions for a
particular curable binder are known in the art. The green agglomerate can be
heated in air or
an inert atmosphere (e.g., nitrogen or argon), depending on the stability of
the curable binder
in air.
[0051] The green agglomerate can be heated in a conventional oven, conveyor
oven, or
furnace. The green agglomerate can also be heated in a furnace with a metal
oxide in the
carbothermal reduction process of the present invention to effect cure of the
curable binder
prior to production of the metal.
[0052] The method of preparing the agglomerate can further comprise, before
curing the
binder of the green agglomerate, heating the green agglomerate at a
temperature and for a
time sufficient to at least partially remove solvent and/or partially cure the
curable resin. The
resulting green agglomerate remains flexible, but has the advantages of being
less tacky and
more durable than the green agglomerate before heat treatment.
[0053] According to the present method of producing a metal, the ratio of the
number of
moles of total carbon to the number of moles of the metal oxide in the raw
materials is
typically a stoichiometric amount, plus or minus 20%, based on the balanced
chemical
equation for the carbothermal reduction of the particular metal oxide. As used
herein, "moles
of total carbon" refers to the sum of the number of moles of carbon from the
carbonaceous
reducing agent, binder, and any optional carbon-containing ingredients, e.g.,
wood chips, in
the raw materials. For example, the mole ratio of the total carbon to silicon
dioxide used in
the carbothermal reduction of silicon dioxide is typically 2 0.4, based on
the following
chemical equation: Si02 + 2C ---> Si + 2C0.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
11
[0054] The raw materials may comprise at least one additional ingredient,
provided the
ingredient does not inhibit reduction of the metal oxide to the corresponding
metal, as
described below. Examples of additional ingredients include, but are not
limited to, wood
chips and limestone.
[0055] The raw materials can be heated in any furnace commonly used in
smelting
operations, particularly the carbothermal reduction of metal oxides or ores
containing metal
oxides. Suitable furnaces are typically equipped with a tap for recovering the
metal in a
molten state at regular intervals during the process. Examples of furnaces
include, but are not
limited to, blast furnaces; and electric arc furnaces such as plasma-arc
furnaces, DC-arc
furnaces, submerged-arc furnaces, and arc-resistance furnaces. Furnace designs
suitable for
the carbothermal production of various metals from their corresponding oxides
are well
known in the art. For example, submerged-arc furnaces are typically used for
the production
of silicon and ferrosilicon.
[0056] The raw materials are heated at a sufficient temperature and for a
sufficient time to
effect reduction of the metal oxide to the metal. The temperature and time of
heating will
depend on the particular metal oxide, the furnace design, and quantities of
raw materials.
Although certain metal oxides can be reduced at temperatures lower than the
temperature
required to melt the metal, typically the raw materials are heated at
temperatures high enough
to melt the resulting metal, so the metal can be promptly removed from the
furnace.
Conditions for carrying out the carbothermal reduction of particular metal
oxides are known
in the art.
[0057] The method of the present invention can further comprise recovering the
metal from
the furnace. For example, the metal can be recovered from the furnace by
tapping the molten
metal at regular intervals during the production cycle. Moreover, the method
can further
comprise refining (i.e., purifying) the metal product. Methods of refining
metals are well
known in the art, and are exemplified by physical methods such as liquation,
zone melting,
distillation, degassing, vacuum melting, and filtration; and chemical methods
such as
oxidation, deoxidation, precipitation of intermetallic compounds, and electro
slag refining.
[0058] Examples of metals produced by the present method include, but are not
limited to,
silicon, iron, aluminum, molybdenum, chromium, boron, tungsten, magnesium,
cobalt, nickel,
copper, calcium, lead, phosphorus, manganese, beryllium, zirconium, tin, zinc,
titanium, and
vanadium. Examples of alloys include, but are not limited to, nonferrous
alloys such as
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
12
copper-beryllium alloys, nickel-beryllium alloys, aluminum-beryllium alloys;
and ferroalloys
such as ferrosilicon, ferromolybdenum, ferromanganese, ferrochromium,
ferrophosphorus,
ferrotitanium, ferroboron, ferrotungsten, and ferrovanadium.
[0059] The process of the instant invention employs agglomerates comprising a
carbonaceous reducing agent and a binder, wherein each agglomerate has at
least one molded
open channel. Importantly, the agglomerates have low apparent density and low
bulk density.
In particular, the channels increase the porosity of the furnace charge, thus
increasing the
circulation of the reacting gases and facilitating the removal of CO gas. As a
result, the
process exhibits relatively high yield, high metal production rate, and low
energy
consumption. Also, the agglomerates have sufficient strength to withstand
handling
operations and the harsh environment of a metallurgical furnace, without being
crushed.
Further, the process can be carried out using conventional equipment and
techniques. Still
further, the method is scaleable to a high throughput manufacturing process.
[0060] The method of the present invention can be used to produce various
metals and
alloys from the corresponding metal oxides. In particular, the method can be
used to produce
metallurgical grade and solar grade silicon from silicon dioxide. Moreover,
metals and alloys
produced according to the present method have widespread utility in chemical,
electrical, and
mechanical products and processes.
EXAMPLES
[0061] The following examples are presented to better illustrate the method of
the present
invention, but are not to be considered as limiting the invention, which is
delineated in the
appended claims. Unless otherwise noted, all parts and percentages reported in
the examples
are by weight. The following methods and materials were employed in the
examples:
[0062] The yield of silicon metal was calculated using the following equation:
Yield Si (%) = actual yield of Si (kg)/theoretical yield Si (kg) x 100.
The theoretical yield of silicon was calculated from the total weight of
silicon dioxide in the
raw materials using the following chemical equation: Si02 + 2C ---> Si + 2C0.
[0063] Wood Chips: Douglas fir woodchips obtained from Weyerhaeuser (Albany,
OR)
and sieved to obtain a mesh size greater than 0.5 in.
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
13
[0064] Quartz: Lumps of quartz having a silica content of 99.2% and a mesh
size of from
0.5 in. to 2.5 in.
[0065] Carbon Black: Pellets of carbon black, sold under the trademark Corax
N 660
(Degussa Corporation), having a sieve residue (325 Mesh) of 300 ppm max, a
CTAB surface
area of 38 m2/g, a DBP (dibutyl phthalate) absorption of 90 mL/100g, an ash
content of 0.5%
max, and an individual pellet hardness (1.4-1.7 mm) of 30 g.
[0066] Sugar: Granulated sugar, sold under the trademark Great Value (Walmart
Corporation).
[0067] Adipic Acid (99%) was obtained from Alfa Aesar (Ward Hill, MA).
Example 1
[0068] This example demonstrates preparation of carbon black honeycomb
briquettes.
Carbon black (45.4 kg), 13.0 kg of refined sugar, and 6.5 kg of adipic acid
were combined in
a 55-gallon plastic-lined steel drum. The contents of the drum were mixed for
7 h using a
drum roller. Deionized water (47 kg) was added to the mixture, and mixing was
continued
for an additional 15 h to give a viscous paste.
[0069] The mixture was then extruded into cylindrical green honeycomb
briquettes at a rate
of 24 kg/h using a Bonnot 3" Twin-Packer Extruder (480 V, 7 hp), equipped with
a multi-
hole die (see Figure 5). The briquettes were placed on a conveyor belt (steel
mesh) in a
single layer and passed at a rate of 1.1 m/min. through a hot air oven at 260
C for a dwell
time of 4 min. The temperature of the briquettes upon exiting the oven was
generally not
greater than 80 C. The partially dried briquettes were cooled to room
temperature with the
aid of a fan positioned by the exit port of the oven.
[0070] The briquettes were placed in an oven (Blue M, 120 V, 30 A) on aluminum
trays
and heated to 170 C at a rate of 2 C/min. in a nitrogen atmosphere. After 5 h
at 170 C, the
oven was turned off and the briquettes were allowed to cool to room
temperature. A
representative briquette is shown in Figures 4A and 4B.
Example 2
[0071] A120-KVA electric arc furnace equipped with a 24-in. diameter graphite
hearth and
a 6-in. diameter graphite electrode was charged with quartz lumps (6.00 kg),
2.78 kg of wood
chips, and 2.48 kg of the honeycomb briquettes of Example 1. About 8 h after
striking the
CA 02692541 2010-01-04
WO 2009/020890 PCT/US2008/072050
14
arc, the furnace tap hole was opened to remove the silicon. Thereafter, the
furnace was
tapped about every two hours during a total period of 60 h. After each tap,
the furnace was
manually stoked and charged with raw materials, i.e., briquettes, quartz, and
wood chips in
the weight ratio of the initial charge, to maintain a constant charge depth.
During the 60-h
run, 468 kg of quartz, 171.1 kg of honeycomb briquettes, and 130.3 kg of wood
chips were
fed into the furnace to produce a total of 124 kg of silicon with an energy
consumption of
4249 kwh. During the fina124 h of the run, 192 kg of quartz, 77.4 kg of
honeycomb
briquettes, and 101.8 kg of woodchips were fed into the furnace to produce
72.2 kg (80.5%)
of silicon with a specific energy consumption of 26.6 kwh/kg Si. The silicon
had average
boron and phosphorous contents of 4.2 ppmw and 47.9 ppmw, respectively, as
determined by
glow discharge mass spectrometry.
Example 3
[0072] A 120-KVA electric arc furnace equipped with a 24-in. diameter graphite
hearth
and a 6-in. diameter graphite electrode was charged with 6.00 kg of quartz
lumps and 3.02
kg of the honeycomb briquettes of Example 1. About 9 h after striking the arc,
the furnace
tap hole was opened to remove the silicon. Thereafter, the furnace was tapped
about every
two hours during a total period of 55 h. After each tap, the furnace was
manually stoked and
charged with raw materials, i.e., briquettes and quartz in the weight ratio of
the initial charge,
to maintain a constant charge depth. During the 55-h run, 474 kg of quartz and
232.2 kg of
honeycomb briquettes were fed into the furnace to produce a total of 131 kg of
silicon with
an energy consumption of 4073.1 kwh. During the fina124 h of the run, 282 kg
of quartz and
142 kg of honeycomb briquettes were fed into the furnace to produce 102.1 kg
(77.6%) of
silicon with a specific energy consumption of 25.3 kwh/kg Si. The silicon had
average boron
and phosphorous contents of 2.8 ppmw and 16.7 ppmw, respectively, as
determined by glow
discharge mass spectrometry.