Note: Descriptions are shown in the official language in which they were submitted.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
HYDROGEL POLYMERIC COMPOSITIONS AND METHODS
TECHNICAL FIELD
The technical field, in general, relates to synthetic polymeric resins that
are
hydrogel compositions, as applied to certain medical conditions.
BACKGROUND
Age-related macular degeneration (AMD), diabetic retinopathy, diabetic macular
edema (DME) posterior uveitis, chororidal neovascularization (CNV) and cystoid
macular
edema (CME) are sight-threatening back-of-the-eye diseases. Age related
macular
degeneration and diabetic retinopathy are significant causes of visual
impairment in the
United States and elsewhere; these conditions are generally caused by
angiogenesis
(unwanted blood-vessel growth in the eye) that damages the retina and
ultimately can
cause blindness. Posterior uveitis is a chronic inflammatory condition that
causes about
ten percent of the blindness in the United States.
SUMMARY
One invention disclosed herein is a crosslinked hydrogel formed in-situ that
releases a therapeutic agent that can be used to treat back-of-the eye
diseases. In this
embodiment, aqueous polymeric precursors are combined ex vivo in flowable
concentrations/viscosities with a drug and injected intravitreally or via
subconjunctival
routes through a small gauge needle into the eye, where the precursors form a
crosslinked
hydrogel that releases the drug over time. The hydrogel may be formulated to
adhere to a
tissue in or around the eye to enhance drug release effects and stability, to
degrade to
biocompatible components without causing inflammation, and to crosslink in
place. A
shape-stable hydrogel thus formed can effectively deliver the drug and
advantageously
have a well-controlled size, shape, and surface area. A small gauge needle or
blunt tip
cannula for sub-Tenon's injections may be used to inject the materials since
soluble or
flowable precursors may be used instead of an already-formed material.
A biocompatible material may be created for eye treatments, one that causes
minimal inflammation. The hydrogels are made using biocompatible precursors,
contain
high proportions of water, and make biocompatible degradation products. The
materials
may thus be soft, hydrophilic, and conforming to space where they are made,
without hard
edges, corners, or sharp surfaces.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
2
Biodegradable materials can also be made that are effectively self-removing
or, if
removed, leave only portions that are self-removing. Some embodiments are
implants
made with a soft, flexible biomaterials crosslinked for strength so the
implants they can be
pulled out or otherwise evacuated though a small opening in case their
retrieval is needed.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1 depicts anatomical features of an eye from a frontal view;
Figure 2 is a partially cut-away perspective view of an eye;
Figure 3 is a cross-sectional view of an eye; and
Figure 4 is an enlarged view of the cross-sectional view of Figure 3.
Figure 5 depicts various delivery alternatives for the implants;
Figure 6A depicts introduction of an implant into the eye, with a small
opening
being made;
Figure 6B depicts the method of Figure 6A, with a cannula introduced through
the
opening;
Figure 6C depicts the method of Figure 6B, with closure of the opening by
cauterization
Figure 7A depicts delivery of implants in the intravitreal space, with an
opening
being made on the eyes surface;
Figure 7B depicts the method of Figure 7A with a cannula for delivery of an
implant to one or more locations internal to the eye;
Figure 8 depicts delivery of a bolus of a material into an eye;
Figure 9 depicts data gathered as per Example 2;
Figure 10 depicts data gathered as per Example 3;
Figure 11 depicts data gathered as per Example 4;
Figure 12 depicts data gathered as per Example 5;
Figure 13 depicts data gathered as per Example 6;
Figure 14 depicts data gathered as per Example 7;
Figure 15 depicts data gathered as per Example 8; and
Figure 16 depicts data gathered as per Example 9.
DETAILED DESCRIPTION
Locally formed hydrogels made in-situ from precursors in aqueous solution can
serve as depots of drugs or other therapeutic agents for ocular drug delivery.
These depots
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
3
can be formed as needed, e.g., topically on the surface of the eye, trans-
scleral in and/or
between the conjunctival and scleral tissues, injected intraocularly, or
formed periocularly.
There are a variety of serious eye diseases that need treatment with a drug
regimen.
Described herein are hydrogels that can be formed in situ on a tissue to
deliver drugs. In
situ refers to forming a material at its intended site of use. Thus a hydrogel
may be formed
in situ in a patient at the site wherein the hydrogel is intended to be used,
e.g., as a drug
depot for controlled release.
The hydrogel is, in one embodiment, formed from precursors having functional
groups that form covalent crosslinks to crosslink the hydrogels and thereby
form the
hydrogel. The hydrogel delivers drugs to the eye. Some embodiments use highly
flowable precursors that gel slowly enough to be forced through a very small
bore cannula
or needle to essentially cross-link only after injection, but nonetheless gel
quickly enough
so that they do not migrate back through the track of the incision. This gel
then swells
minimally after crosslinking. The gel degrades in the physiological fluid in
or around the
eye without causing inflammation by degrading into parts that are
biocompatible and not
acidic. The hydrogel also has enough mechanical strength so that it can be
recovered by
means of either manual or mechanical irrigation/aspiration techniques if
necessary.
Moreover, in some embodiments the gel adheres to the tissue.
In general, precursors may be combined as described herein at a site in or
near an
eye to make a covalently-crosslinked hydrogel that comprises a therapeutic
agent that is
released into the eye to treat an opthalmic disease over a suitable period of
time. The
hydrogel may be low-swelling, as measurable by the hydrogel having a weight
increasing
no more than about 10% or about 50% upon exposure to a physiological solution
for
twenty-four hours relative to a weight of the hydrogel at the time of
formation; artisans
will immediately appreciate that all the ranges and values within the
explicitly stated
ranges are contemplated. The hydrogel also may be water-degradable, as
measurable by
the hydrogel being dissolvable in vitro in an excess of water by degradation
of water-
degradable groups in the hydrogel. A composition with the precursors mixed
therein can
be introduced through a.small-gauge needle provided that the composition has a
suitable
viscosity, which in turn depends on precursor properties, concentrations, and
chemistry.
Further, the hydrogels' mechanical strengths and reaction time are adjusted
though control
of the precursors and functional groups. The precursors and hydrogels may have
various
features that can be mixed-and-matched as guided by the considerations for
making an
effective device; the following sections describe some of these features.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
4
Precursor materials
The precursors can be triggered to react to form a crosslinked hydrogel. In
general, the precursors are polymerizable and include crosslinkers that are
often, but not
always, polymerizable precursors. Polymerizable precursors are thus precursors
that have
functional groups that react with each other to form polymers made of
repeating units.
Some precursors thus react by chain-growth polymerization, also referred to as
addition polymerization, and involve the linking together of monomers
incorporating
double or triple chemical bonds. These unsaturated monomers have extra
internal bonds
which are able to break and link up with other monomers to form the repeating
chain.
Monomers are polymerizable molecules with at least one group that reacts with
other
groups to form a polymer. A macromonomer is a polymer or oligomer that has at
least one
reactive group, often at the end, which enables it to act as a monomer; each
macromonomer molecule is attached to the polymer by reaction the reactive
group. Thus
macromonomers with two or more monomers or other functional groups tend to
form
covalent crosslinks. Addition polymerization is involved in the manufacture
of, e.g.,
polypropylene or polyvinyl chloride. One type of addition polymerization is
living
polymerization.
Some precursors thus react by condensation polymerization that occurs when
monomers bond together through condensation reactions. Typically these
reactions can be
achieved through reacting molecules incorporating alcohol, amine or carboxylic
acid (or
other carboxyl derivative) functional groups. When an amine reacts with a
carboxylic acid
an amide or peptide bond is formed, with the release of water. Some
condensation
reactions follow a nucleophilic acyl substitution, e.g., as in U.S. Pat. No.
6,958,212, which
is hereby incorporated by reference to the extent it does not contradict what
is explicitly
disclosed herein.
Some precursors react by a chain growth-step system. Chain growth polymers are
defined as polymers formed by the reaction of monomers or macromonomers with a
reactive center. A reactive center is a particular location within a chemical
compound that
is the center of a reaction in which the chemical is involved. In chain-growth
polymer
chemistry, this is also the point of propagation for a growing chain. The
reactive center is
commonly radical, anionic, or cationic in nature, but can also take other
forms. Chain
growth-step systems include free radical polymerization, which involves a
process of
initiation, propagation and termination. Initiation is the creation of free
radicals necessary
for propagation, as created from radical initiators, e.g., organic peroxide
molecules.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
Termination occurs when a radical reacts in a way that prevents further
propagation. The
most common method of termination is by coupling where two radical species
react with
each other forming a single molecule.
Some precursors react by a step growth mechanism, and are polymers formed by
5 the stepwise reaction between functional groups of monomers. Most step
growth polymers
are also classified as condensation polymers, but not all step growth polymers
release
condensates.
Monomers may be polymers or small molecules. A polymer is an organic
molecule formed by combining many smaller molecules (monomers) in a regular
pattern,
and includes those formed from at least two monomers and also oligomers, which
is a
term herein referring to polymers having less than about 20 monomeric repeat
units. A
small molecule generally refers to a molecule that is less than about 2000
Daltons.
The precursors must thus be small molecules, such as acrylic acid or vinyl
caprolactam, larger molecules containing polymerizable groups, such as
acrylate-capped
polyethylene glycol (PEG-diacrylate), or other polymers containing
ethylenically-
unsaturated groups, such as those of U.S. Pat. No. 4,938,763 to Dunn et al,
U.S. Pat. Nos.
5,100,992 and 4,826,945 to Cohn et al, or U.S. Pat. Nos. 4,741,872 and
5,160,745 to
DeLuca et al., each of which are hereby incorporated by reference to the
extent they do not
contradict what is explicitly disclosed herein.
To form covalently crosslinked hydrogels, the precursors must be crosslinked
together. In general, polymeric precursors will form polymers that will be
joined to other
polymeric precursors at two or more points, with each point being a linkage to
the same or
different polymers. Precursors with at least two monomers can serve as
crosslinkers since
each monomer can participate in the formation of a different growing polymer
chain. In
the case of monomers with a reactive center, each monomer effectively has one
functional
group for reacting with other precursors. In the case of functional groups
without a
reactive center, among others, crosslinking requires three or more such
functional groups
on a precursor. For instance, many electrophilic-nucleophilic reactions
consume the
electrophilic and nucleophilic functional groups so that a third functional
group is needed
for the precursor to form a crosslink. Such precursors thus may have three or
more
functional groups and may be crosslinked by precursors with two or more
functional
groups. Thus some precursors have functional groups for participating in
polymer and/or
crosslink formation but are free of polymerizable reactive centers or are free
of radical
and/or anionic and/or cationic reactive centers, or have only some combination
of the
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
6
same. A crosslinked molecule may be crosslinked via an ionic or covalent bond,
a
physical force, or other attraction. A covalent crosslink, however, will
typically offer
stability and predictability in reactant product architecture.
In some embodiments, each precursor is multifunctional, meaning that it
comprises
two or more electrophilic or nucleophilic functional groups, such that a
nucleophilic
functional group on one precursor may react with an electrophilic functional
group on
another precursor to form a covalent bond. At least one of the precursors
comprises more
than two functional groups, so that, as a result of electrophilic-nucleophilic
reactions, the
precursors combine to form crosslinked polymeric products.
The precursors may have biologically inert and water soluble portions, e.g., a
core.
A core refers to a contiguous portion of a molecule that is generally at least
about 80% of
the molecule by weight, sometimes with arms that extend from the core, with
the arms
having a functional group, which is often at the terminus of the branch. A
water soluble
portion is a water soluble molecule or polymer that is joined to a hydrophobic
polymer.
The water soluble precursor or precursor portion preferably has a solubility
of at least 1
g/100 mL in an aqueous solution. A water soluble portion may be, for instance,
a
polyether, for example, polyalkylene oxides such as polyethylene glycol (PEG),
polyethylene oxide (PEO), polyethylene oxide-co-polypropylene oxide (PPO), co-
polyethylene oxide block or random copolymers, and polyvinyl alcohol (PVA),
poly
(vinyl pyrrolidinone) (PVP), poly (amino acids, dextran, or a protein. The
precursors may
have a polyalkylene glycol portion and may be polyethylene glycol based, with
at least
about 80% or 90% by weight of the polymer comprising polyethylene oxide
repeats. The
polyethers and more particularly poly (oxyalkylenes) or poly (ethylene glycol)
or
polyethylene glycol are generally hydrophilic.
A precursor may also be a macromolecule, which is a molecule having a
molecular
weight in the range of a few thousand to many millions. In some embodiments,
however,
at least one of the precursors is a small molecule of about 1000 Da or less.
The
macromolecule, when reacted in combination with a small molecule of about 1000
Da or
less, is preferably at least five to fifty times greater in molecular weight
than the small
molecule and is preferably less than about 60,000 Da; artisans will
immediately appreciate
that all the ranges and values within the explicitly stated ranges are
contemplated. A more
preferred range is a macromolecule that is about seven to about thirty times
greater in
molecular weight than the crosslinker and a most preferred range is about ten
to twenty
times difference in weight. Further, a macromolecular molecular weight of
5,000 to
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
7
50,000 is useful, as is a molecular weight of 7,000 to 40,000 or a molecular
weight of
10,000 to 20,000.
Certain macromeric precursors are the crosslinkable, biodegradable, water-
soluble
macromers described in U.S. Pat. No. 5,410,016 to Hubbell et al, which is
hereby
incorporated herein by reference to the extent it does not contradict what is
explicitly
disclosed. These monomers are characterized by having at least two
polymerizable groups,
separated by at least one degradable region.
Synthetic precursors may be used. Synthetic refers to a molecule not found in
nature or not normally found in a human. Some synthetic polymers are free of
amino
acids or free of amino acid sequences that occur in nature. Some synthetic
molecules are
polypeptides that are not found in nature or are not normally found in a human
body, e.g.,
di-, tri-, or tetra-lysine. Some synthetic molecules have amino acid residues
but only have
one, two, or three that are contiguous, with the amino acids or clusters
thereof being
separated by non-natural polymers or groups.
Alternatively, natural proteins or polysaccharides may be adapted for use with
these methods, e.g., collagens, fibrin(ogen)s, albumins, alginates, hyaluronic
acid, and
heparins. These natural molecules may further include chemical derivitization,
e.g.,
synthetic polymer decorations. The natural molecule may be crosslinked via its
native
nucleophiles or after it is derivatized with functional groups, e.g., as in
U.S. Pat. Nos.
5,304,595, 5,324,775, 6,371,975, and 7,129,210, each of which is hereby
incorporated by
reference to the extent it does not contradict what is explicitly disclosed
herein. Natural
refers to a molecule found in nature. Natural polymers, for example proteins
or
glycosaminoglycans, e.g., collagen, fibrinogen, albumin, and fibrin, may be
crosslinked
using reactive precursor species with electrophilic functional groups. Natural
polymers
normally found in the body are proteolytically degraded by proteases present
in the body.
Such polymers may be reacted via functional groups such as amines, thiols, or
carboxyls
on their amino acids or derivatized to have activatable functional groups.
While natural
polymers may be used in hydrogels, their time to gelation and ultimate
mechanical
properties must be controlled by appropriate introduction of additional
functional groups
and selection of suitable reaction conditions, e.g., pH. In contrast, fibrin
glues, which rely
on polymerization of fibrinogen to form fibrin, have a limited range of
mechanical
properties, a limited range of degradability, and are not generally suited to
many of the
ophthalmic therapeutic applications that are available when hydrogels as
described herein
are formulated.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
8
Precursors may be made with a hydrophobic portion. In some cases, the
precursor
is nonetheless soluble in water because it also has a hydrophilic portion. In
other cases,
the precursor makes dispersion in the water (a suspension) but is nonetheless
reactable to
from a crosslinked material. Some hydrophobic portions may include a plurality
of alkyls,
polypropylenes, alkyl chains, or other groups. Some precursors with
hydrophobic portions
are sold under the trade names PLURONIC F68, JEFFAMINE, or TECTRONIC. A
hydrophobic portion is one that is sufficiently hydrophobic to cause the
macromer or
copolymer to aggregate to form micelles in an aqueous continuous phase or one
that, when
tested by itself, is sufficiently hydrophobic to precipitate from, or
otherwise change phase
while within, an aqueous solution of water at pH from about 7 to about 7.5 at
temperatures
from about 30 to about 50 degrees Centigrade.
Precursors may have, e.g., 2-100 arms, with each arm having a terminus,
bearing in
mind that some precursors may be dendrimers or other highly branched
materials. Thus
hydrogels can be made, e.g., from a multi-armed precursor with a first set of
functional
groups and a low molecular-weight precursor having a second set of functional
groups.
For example, a six-armed or eight-armed precursor may have hydrophilic arms,
e.g.,
polyethylene glycol, terminated with primary amines, with the molecular weight
of the
arms being about 1,000 to about 40,000; artisans will immediately appreciate
that all
ranges and values within the explicitly stated bounds are contemplated. Such
precursors
may be mixed with relatively smaller precursors, for example, molecules with a
molecular
weight of between about 100 and about 5000, or no more than about 800, 1000,
2000, or
5000 having at least about three functional groups, or between about 3 to
about 16
functional groups; ordinary artisans will appreciate that all ranges and
values between
these explicitly articulated values are contemplated. Such small molecules may
be
polymers or non-polymers and natural or synthetic.
Some embodiments include a precursor that consists essentially of an
oligopeptide
sequence of no more than five residues, e.g., amino acids comprising at least
one amine,
thiol, carboxyl, or hydroxyl side chain. A residue is an amino acid, either as
occurring in
nature or derivatized thereof. The backbone of such an oligopeptide may be
natural or
synthetic. In some embodiments, peptides of two or more amino acids are
combined with
a synthetic backbone to make a precursor; certain embodiments of such
precursors have a
molecular weight in the range of about 100 to about 10,000 or about 300 to
about 500
Artisans will immediately appreciate that all ranges and values between these
explicitly
articulated bounds are contemplated.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
9
Precursors may be prepared to be free of amino acid sequences cleavable by
enzymes present at the site of introduction, including free of
metalloproteinases and/or
collagenases. Further, precursors may be made to be free of all amino acids,
or free of
amino acid sequences of more than about 50, 30, 20, 10, 9, 8, 7, 6, 5, 4, 3,
2, or 1 amino
acids. Precursors may be non-proteins, meaning that they are not a naturally
occurring
protein and can not be made by cleaving a naturally occurring protein and can
not be made
by adding synthetic materials to a protein. Precursors may be non-collagen,
non-fibrin
(ogen), non-hyaluronic acid, and non-albumin, meaning that they are not one of
these
proteins and are not chemical derivatives of one of these proteins. The use of
non-protein
precursors and limited use of amino acid sequences can be helpful for avoiding
immune
reactions, avoiding unwanted cell recognition, and avoiding the hazards
associated with
using proteins derived from natural sources.
Peptides may be used as precursors. In general, peptides with less than about
10
residues are preferred, although larger sequences (e.g., proteins) may be
used. Artisans
will immediately appreciate that every range and value within these explicit
bounds is
included, e.g. 1-10, 2-9. 3-10, 1, 2, 3, 4, 5, 6, or 7. Some amino acids have
nucleophilic
groups (e.g., primary amines or thiols) or groups that can be derivatized as
needed to
incorporate nucleophilic groups or electrophilic groups (e.g., carboxyls or
hydroxyls).
Polyamino acid polymers generated synthetically are normally considered to be
synthetic
if they are not found in nature and are engineered not to be identical to
naturally occurring
biomolecules.
Some hydrogels are made with a polyethylene glycol-containing precursor.
Polyethylene glycol (PEG, also referred to as polyethylene oxide) refers to a
polymer with
a repeat group (CHZCH2O) n, with n being at least 3. A polymeric precursor
having a
polyethylene glycol thus has at least three of these repeat groups connected
to each other
in a linear series. The polyethylene glycol content of a polymer or arm is
calculated by
adding up all of the polyethylene glycol groups on the polymer or arm, even if
they are
interrupted by other groups. Thus, an arm having at least 1000 MW polyethylene
glycol
has enough CHZCH2O groups to total at least 1000 MW. As is customary
terminology in
these arts, a polyethylene glycol polymer does not necessarily terminate in a
hydroxyl
group.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
Initiating Systems
Some precursors react using initiators. An initiator group is a chemical group
capable of initiating a free radical polymerization reaction. For instance, it
may be present
as a separate component, or as a pendent group on a precursor. Initiator
groups include
5 thermal initiators, photoactivatable initiators, and oxidation-reduction
(redox) systems.
Long wave UV and visible light photoactivatable initiators include, for
example, ethyl
eosin groups, 2, 2-dimethoxy-2-phenyl acetophenone groups, other acetophenone
derivatives, thioxanthone groups, benzophenone groups, and camphorquinone
groups.
Examples of thermally reactive initiators include 4, 4' azobis (4-
cyanopentanoic acid)
10 groups, and analogs of benzoyl peroxide groups. Several commercially
available low
temperature free radical initiators, such as V-044, available from Wako
Chemicals USA,
Inc., Richmond, Va., may be used to initiate free radical crosslinking
reactions at body
temperatures to form hydrogel coatings with the aforementioned monomers.
Metal ions may be used either as an oxidizer or a reductant in redox
initiating
systems. For example, ferrous ions may be used in combination with a peroxide
or
hydroperoxide to initiate polymerization, or as parts of a polymerization
system. In this
case, the ferrous ions would serve as a reductant. Alternatively, metal ions
may serve as
an oxidant. For example, the ceric ion (4+ valence state of cerium) interacts
with various
organic groups, including carboxylic acids and urethanes, to remove an
electron to the
metal ion, and leave an initiating radical behind on the organic group. In
such a system,
the metal ion acts as an oxidizer. Potentially suitable metal ions for either
role are any of
the transition metal ions, lanthanides and actinides, which have at least two
readily
accessible oxidation states. Particularly useful metal ions have at least two
states
separated by only one difference in charge. Of these, the most commonly used
are
ferric/ferrous; cupric/cuprous; ceric/cerous; cobaltic/cobaltous; vanadate V
vs. IV;
permanganate; and manganic/manganous. Peroxygen containing compounds, such as
peroxides and hydroperoxides, including hydrogen peroxide, t-butyl
hydroperoxide, t-
butyl peroxide, benzoyl peroxide, cumyl peroxide may be used.
An example of an initiating system is the combination of a peroxygen compound
in
one solution, and a reactive ion, such as a transition metal, in another. In
this case, no
external initiators of polymerization are needed and polymerization proceeds
spontaneously and without application of external energy or use of an external
energy
source when two complementary reactive functional groups containing moieties
interact at
the application site.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
11
Functional Groups
The precursors have functional groups that react with each other to form the
material in situ. The functional groups generally have reactive centers for
polymerization
or react with each other in electrophile-nucleophile reactions or are
configured to
participate in other polymerization reactions. Various aspects of
polymerization reactions
are discussed in the precursors section herein.
Thus in some embodiments, precursors have a polymerizable group that is
activated by photoinitiation or redox systems as used in the polymerization
arts, e.g., or
electrophilic functional groups that are carbodiimidazole, sulfonyl chloride,
chlorocarbonates, n-hydroxysuccinimidyl ester, succinimidyl ester or
sulfasuccinimidyl
esters, or as in U.S. Pat. Nos. 5,410,016, or 6,149,931, each of which are
hereby
incorporated by reference to the extent they do not contradict what is
explicitly disclosed
herein. The nucleophilic functional groups may be, for example, amine,
hydroxyl,
carboxyl, and thiol. Another class of electrophiles are acyls, e.g., as in
U.S. Pat. No.
6,958,212, which describes, among other things, Michaels addition schemes for
reacting
polymers.
Certain functional groups, such as alcohols or carboxylic acids, do not
normally
react with other functional groups, such as amines, under physiological
conditions (e.g.,
pH 7.2-11.0, 37 C). However, such functional groups can be made more reactive
by using
an activating group such as N-hydroxysuccinimide. Certain activating groups
include
carbonyldiimidazole, sulfonyl chloride, aryl halides, sulfosuccinimidyl
esters, N-
hydroxysuccinimidyl ester, succinimidyl ester, epoxide, aldehyde, maleimides,
imidoesters and the like. The N-hydroxysuccinimide esters or N-
hydroxysulfosuccinimide
(NHS) groups are useful groups for crosslinking of proteins or amine-
containing
polymers, e.g., amino terminated polyethylene glycol. An advantage of an NHS-
amine
reaction is that the reaction kinetics are favorable, but the gelation rate
may be adjusted
through pH or concentration. The NHS-amine crosslinking reaction leads to
formation of
N-hydroxysuccinimide as a side product. Sulfonated or ethoxylated forms of N-
hydroxysuccinimide have a relatively increased solubility in water and hence
their rapid
clearance from the body. The sulfonic acid salt on the succinimide ring does
not alter the
reactivity of NHS group with the primary amines. An NHS-amine crosslinking
reaction
may be carried out in aqueous solutions and in the presence of buffers, e.g.,
phosphate
buffer (pH 5.0-7.5), triethanolamine buffer (pH 7.5-9.0), or borate buffer (pH
9.0-12), or
sodium bicarbonate buffer (pH 9.0-10.0). Aqueous solutions of NHS based
crosslinkers
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
12
and functional polymers preferably are made just before the crosslinking
reaction due to
reaction of NHS groups with water. The reaction rate of these groups may be
delayed by
keeping these solutions at lower pH (pH 4-7).
In some embodiments, each precursor comprises only nucleophilic or only
electrophilic functional groups, so long as both nucleophilic and
electrophilic precursors
are used in the crosslinking reaction. Thus, for example, if a crosslinker has
nucleophilic
functional groups such as amines, the functional polymer may have
electrophilic
functional groups such as N-hydroxysuccinimides. On the other hand, if a
crosslinker has
electrophilic functional groups such as sulfosuccinimides, then the functional
polymer
may have nucleophilic functional groups such as amines or thiols. Thus,
functional
polymers such as proteins, poly(allyl amine), or amine-terminated di-or
multifunctional
poly(ethylene glycol) can be used.
An arm on a hydrogel precursor refers to a linear chain of chemical groups
that
connect a crosslinkable functional group to polymer a core. Some embodiments
are
precursors with between 3 and 300 arms; artisans will immediately appreciate
that all the
ranges and values within the explicitly stated ranges are contemplated, e.g.,
4 to 16, 8 to
100, or at least 6 arms. Precursors may be dendrimers, e.g., as in Patent
Application Pub.
Nos. US20040086479, US20040131582, W007005249, W007001926, W006031358, or
the U.S. counterparts thereof; dendrimers may also be useful as
multifunctional
precursors, e.g., as in U.S. Pat. Pub. Nos. US20040131582, US20040086479 and
PCT
Applications No. W006031388 and W006031388; all of which US and PCT
applications
are hereby incorporated by reference to the extent they do not contradict what
is explicitly
disclosed herein. Dendrimers are highly ordered possess high surface area to
volume
ratios, and exhibit numerous end groups for potential functionalization. Some
dendrimers
are regularly ordered, meaning that each arm has an identical structure. Some
dendrimers
have arms with a plurality of serial branches meaning that a polymer branches
into at least
two arms that each branch into at least two more arms. Consequently,
dendrimers tend to
display low, polydispersity indexes, low viscosities, and high solubility and
miscibility.
Some embodiments are directed to dendrimers with a relatively high molecular
weight
used with a relatively lower molecular weight multifunctional precursor, with
suitable
functional groups on the precursors. Other embodiments are directed to using
dendrimers
functionalized with electrophiles and/or nucleophiles. In some embodiments,
the
dendrimers serve as precursors with a relatively lower molecular weight (e.g.,
less than
about half, less than about one-third) than another crosslinking precursor,
e.g., with a
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
13
dendrimer being between about 600 and about 3000 Da and a multifunctional
precursor
being between about 2000 to about 5000 Da. In some embodiments, the precursor
is a
hydrophilic dendrimer, e.g., comprising PEG. In some embodiments, each
dendrimer arm,
or at least half of the arms, terminates in a functional group for reaction
with functional
groups on other precursors. In some embodiments, dendrimer precursors of at
least about
10,000 molecular weight are reacted with small precursors that crosslink the
dendrimers,
with the small precursors having a molecular weight of less than about 1000.
In some
embodiments, at least about 90% by number of the arms of the dendrimers are
reacted to
form links to the hydrogel; in other embodiments, less than about 25% by
number of the
arms are reacted so as to increase the mobility of the free arms.
One embodiment has reactive precursor species with 3 to 16 nucleophilic
functional groups each and reactive precursor species with 2 to 12
electrophilic functional
groups each; artisans will immediately appreciate that all the ranges and
values within the
explicitly stated ranges are contemplated.
Hydrogel Formation
In general, precursors may be combined in a flowable composition with a
delayed
crosslinking chemistry to make a covalently-crosslinked material in situ that
comprises a
therapeutic agent that is released over a suitable period of time. The
crosslinking reactions
generally occur in aqueous solution under physiological conditions. The
crosslinking
reactions preferably do not release heat of polymerization or require
exogenous energy
sources for initiation or to trigger polymerization. Photochemical initiation,
for instance,
is generally to be avoided in the eye so as to avoid damage to the eye. In the
case of
injected materials, the viscosity may be controlled so that the material is
introduced
through a small diameter catheter or needle. In the case of materials applied
around an
eye, which are optionally delivered through such a catheter/needle, viscosity
may further
be controlled to keep precursors in place until they form a gel so that the
precursors do not
run-off the intended site of use.
The hydrogel is generally low-swelling, as measurable by the hydrogel having a
weight increasing no more than about 0% to about 10% or to about 50% upon
exposure to
a physiological solution for twenty-four hours relative to a weight of the
hydrogel at the
time of formation. One embodiment for reducing swelling is to increase the
number of
crosslinks, bearing in mind, however, that crosslinks can increase rigidity or
brittleness.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
14
Another embodiment is to reduce the average chain distance between crosslinks.
Another
embodiment is to use precursors with many arms, as explained below.
Another embodiment to reduce swelling is to control the degree of
hydrophilicity,
with less hydrophilic materials tending to swell less; for instance, highly
hydrophilic
materials such as PEOs can be combined with less hydrophilic materials such as
PPO or
even hydrophobic groups such as alkyls.
Another embodiment to reduce swelling is to choose precursors that have a high
degree of solvation at the time of crosslinking but subsequently become less
solvated and
having a radius of solvation that effectively shrinks; in other words, the
precursor is
spread-out in solution when crosslinked but later contracts. Changes to pH,
temperature,
solids concentration, and solvent environment can cause such changes;
moreover, an
increase in the number of branches (with other factors being held effectively
constant) will
tend to also have this effect. The number of arms are believed to stericly
hinder each other
so that they spread-out before crosslinking, but these steric effects are
offset by other
factors after polymerization. In some embodiments, precursors have a plurality
of similar
charges so as to achieve these effects, e.g., a plurality of functional groups
having a
negative charge, or a plurality of arms each having a positive charge, or each
arm having a
functional group of similar charges before crosslinking or other reaction.
Hydrogels described herein can include hydrogels that swell minimally after
deposition. Such medical low-swellable hydrogels may have a weight upon
polymerization that increases no more than, e.g., about 50%, about 10%, about
5%, about
0% by weight upon exposure to a physiological solution, or that shrink
(decrease in weight
and volume), e.g., by at least about 5%, at least about 10%, or more. Artisans
will
immediately appreciate that all ranges and values within or otherwise relating
to these
explicitly articulated limits are disclosed herein. Unless otherwise
indicated, swelling of a
hydrogel relates to its change in volume (or weight) between the time of its
formation
when crosslinking is effectively complete and the time after being placed in
vitro a
physiological solution in an unconstrained state for twenty-four hours, at
which point it
may be reasonably assumed to have achieved its equilibrium swelling state. For
most
embodiments, crosslinking is effectively complete within no more than about
fifteen
minutes such that the initial weight can generally be noted at about 15
minutes after
formation as Weight at initial formation. Accordingly, this formula is used: %
swelling =
[(Weight at 24 hours - Weight at initial formation)/ Weight at initial
formation] * 100. n
the case of hydrogels that have substantial degradation over twenty-four
hours, the
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
maximum weight may be used instead of a 24-hour weight, e.g., as measured by
taking
successive measurements. The weight of the hydrogel includes the weight of the
solution
in the hydrogel. A hydrogel formed in a location wherein it is constrained is
not
necessarily a low-swelling hydrogel. For instance, a swellable hydrogel
created in a body
5 may be constrained from swelling by its surroundings but nonetheless may be
a highly
swellable hydrogel as evidenced by measurements of its swelling when
unconstrained
andlor the forces against a constraint.
Reaction kinetics are generally controlled in light of the particular
functional
groups unless an external initiator or chain transfer agent is required, in
which case
10 triggering the initiator or manipulating the transfer agent can be a
controlling step. In
some embodiments, the molecular weights of the precursors are used to affect
reaction
times. Precursors with lower molecular weights tend to speed the reaction, so
that some
embodiments have at least one precursor with a molecular weight of at least
5,000 to
50,000 or 150,000 Daltons. Preferably the crosslinking reaction leading to
gelation occurs
15 within about 2 to about 10 or to about 30 minutes; artisans will
immediately appreciate
that all the ranges and values within the explicitly stated ranges are
contemplated, e.g., at
least 120 seconds, or between 180 to 600 seconds. Gelation time is measured by
applying
the precursors to a flat surface and determining the time at which there is
substantially no
flow down the surface when it is titled at an angle of about 60 degrees (i.e.,
a steep angle,
close to perpendicular).
The crosslinking density of the resultant biocompatible crosslinked polymer is
controlled by the overall molecular weight of the crosslinker and functional
polymer and
the number of functional groups available per molecule. A lower molecular
weight
between crosslinks such as 500 will give much higher crosslinking density as
compared to
a higher molecular weight such as 10,000. The crosslinking density also may be
controlled by the overall percent solids of the crosslinker and functional
polymer
solutions. Increasing the percent solids increases the probability that an
electrophilic
functional group will combine with a nucleophilic functional group prior to
inactivation by
hydrolysis. Yet another method to control crosslink density is by adjusting
the
stoichiometry of nucleophilic functional groups to electrophilic functional
groups. A one
to one ratio leads to the highest crosslink density. Precursors with longer
distances
between crosslinks are generally softer, more compliant, and more elastic.
Thus an
increased length of a water-soluble segment, such as a polyethylene glycol,
tends to
enhance elasticity to produce desirable physical properties. Thus certain
embodiments are
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
16
directed to precursors with water soluble segments having molecular weights in
the range
of 3,000 to 100,000 or, e.g., 10,000 to 35,000.
The solids content of the hydrogel can affect its mechanical properties and
biocompatibility and reflects a balance between competing requirements. In
general, a
relatively low solids content tends to be most useful, e.g., between about
2.5% to about
25%, including all ranges and values there between, e.g., about 2.5% to about
10%, about
5% to about 15%, or less than about 15%.
Anatomy of the eye
The structure of the mammalian eye can be divided into three main layers or
tunics: the fibrous tunic, the vascular tunic, and the nervous tunic. The
fibrous tunic, also
known as the tunica fibrosa oculi, is the outer layer of the eyeball
consisting of the cornea
and sclera. The sclera is the supporting wall of the eye and gives the eye
most of its white
color. It is extends from the cornea (the clear front section of the eye) to
the optic nerve at
the back of the eye. The sclera is a fibrous, elastic and protective tissue,
composed of
tightly packed collagen fibrils, containing about 70% water.
Overlaying the fibrous tunic is the conjunctiva. The conjunctiva is a membrane
that covers the sclera (white part of the eye) and lines the inside of the
eyelids. It helps
lubricate the eye by producing mucus and tears, although a smaller volume of
tears than
the lacrimal gland. The conjunctiva is typically divided into three parts: (a)
Palpebral or
tarsal conjunctivam which is the conjunctiva lining the eyelids; the palpebral
conjunctiva
is reflected at the superior fornix and the inferior fornix to become the
bulbar conjunctiva.
(b) Fornix conjunctiva: the conjunctiva where the inner part of the eyelids
and the eyeball
meet. (c) Bulbar or ocular conjunctiva: The conjunctiva covering the eyeball,
over the
sclera. This region of the conjunctiva is bound tightly and moves with the
eyeball
movements.
The conjunctiva effectively surrounds, covers, and adheres to the sclera. It
is has
cellular and connective tissue, is somewhat elastic, and can be removed,
teased away, or
otherwise taken down to expose a surface area of the sclera. As explained
below, it can be
removed or used in conjunction with transcleral drug delivery schemes.
The vascular tunic, also known as the tunica vasculosa oculi, is the middle
vascularized layer which includes the iris, ciliary body, and choroid. The
choroid contains
blood vessels that supply the retinal cells with oxygen and remove the waste
products of
respiration.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
17
The nervous tunic, also known as the tunica nervosa oculi, is the inner
sensory
which includes the retina. The retina contains the photosensitive rod and cone
cells and
associated neurons. The retina is a relatively smooth (but curved) layer. It
does have two
points at which it is different; the fovea and optic disc. The fovea is a dip
in the retina
directly opposite the lens, which is densely packed with cone cells. The fovea
is part of
the macula. The fovea is largely responsible for color vision in humans, and
enables high
acuity, which is necessary in reading. The optic disc is a point on the retina
where the
optic nerve pierces the retina to connect to the nerve cells on its inside.
The mammalian eye can also be divided into two main segments: the anterior
segment and the posterior segment. The anterior segment consists of an
anterior and
posterior chamber. The anterior chamber is located in front of the iris and
posterior to the
corneal endothelium and includes the pupil, iris, ciliary body and aqueous
fluid. The
posterior chamber is located posterior to the iris and anterior to the
vitreous face where the
crystalline lens and zonules fibers are positioned between an anterior and
posterior capsule
in an aqueous environment.
The cornea and lens help to converge light rays to focus onto the retina. The
lens,
behind the iris, is a convex, springy disk which focuses light, through the
second humour,
onto the retina. It is attached to the ciliary body via a ring of suspensory
ligaments known
as the Zonule of Zinn. The ciliary muscle is relaxed to focus on an object far
away, which
stretches the fibers connecting it with the lens, thus flattening the lens.
When the ciliary
muscle contracts, the tension of the fibers decreases, which brings the lens
back to a more
convex and round shape. The iris, between the lens and the first humour, is a
pigmented
ring of fibrovascular tissue and muscle fibers. Light must first pass though
the center of
the iris, the pupil. The size of the pupil is actively adjusted by the
circular and radial
muscles to maintain a relatively constant level of light entering the eye.
Light enters the eye, passes through the cornea, and into the first of two
humors,
the aqueous humour. Approximately two-thirds of the total eyes refractive
power comes
from the cornea which has a fixed curvature. The aqueous humor is a clear mass
which
connects the cornea with the lens of the eye, helps maintain the convex shape
of the cornea
(necessary to the convergence of light at the lens) and provides the corneal
endothelium
with nutrients.
The posterior segrnent is located posterior to the crystalline lens and in
front of the
retina. It represents approximately two-thirds of the eye that includes the
anterior hyaloid
membrane and all structures behind it: the vitreous humor, retina, c, and
optic nerve. On
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
18
the other side of the lens is the second humour, the vitreous humour, which is
bounded on
all sides: by the lens, ciliary body, suspensory ligaments and by the retina.
It lets light
through without refraction, helps maintain the shape of the eye and suspends
the delicate
lens.
Figure 1 depicts eye 10 having sclera 12, iris 14, pupil 16, and eyelid 18.
Figure 2
depicts a perspective view of eye 10 with a partial cross-section that depicts
lens 20,
inferior oblique muscle 21, inferior rectus muscle 23, and optic nerve 25.
Figure 3 is a
cross-section of eye 10 and depicts cornea 22 that is optically clear and
allows light to pass
iris 14 and penetrate lens 20. Anterior chamber 24 underlies cornea 22 and
posterior
chamber 26 lies between iris 14 and lens 20. Ciliary body 28 is connected to
lens 20.
Figure 3 depicts a portion of the conjunctiva 30, which overlies the sclera
12. The
vitreous body 32 comprises the jelly-like vitreous humor, with hyaloid canal
34 being in
the same. Fovea 36 is in the macula and retina 38 overlies choroid 37. Zonular
spaces 42
are depicted. Figure 4 shows eye 10 in partial view, and shows portions of
conjunctiva 30
on sclera 12, including tendon of the superior rectus muscle 44 emerging from
the same.
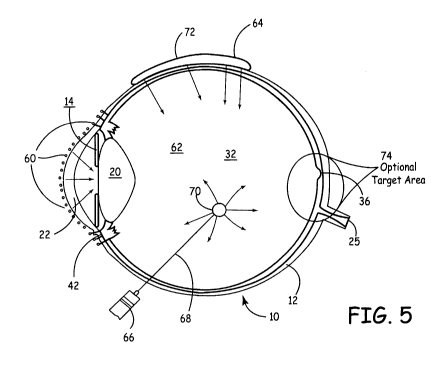
Figure 5 shows certain points of delivery at or near eye 10. One area is
topically at
60, with area 60 being indicated by dots on surface of eye 10. Another area is
intravitreally as indicated by numera162, or trans-sclerally, as indicated by
numeral 64. In
use, for example a syringe 66, catheter (not shown) or other device is used to
deliver
hydrogel or a hydrogel precursors, optionally through needle 68, into the eye,
either
intravitrealy, as at 70 or peri-ocularly, as at 72. Drugs or other therapeutic
agents are
released to the intra-ocular space. In the case of back-of-the-eye diseases,
drugs may be
targeted via the peri-ocular or intravitreal route to target approximate area
74, where they
interact with biological features to achieve a therapy.
Eye Disease States
The materials described herein may be used to deliver drugs or other
therapeutic
agents (e.g., imaging agents or markers) to eyes or tissues nearby. Some of
the disease
states are back-of-the-eye diseases. The term back-of-the eye disease is
recognized by
artisans in these fields of endeavor and generally refers to any ocular
disease of the
posterior segment that affects the vasculature and integrity of the retina,
macula or choroid
leading to visual acuity disturbances, loss of sight or blindness. Disease
states of the
posterior segment may result from age, trauma, surgical interventions, and
hereditary
factors. Some back-of-the-eye disease are; age-related macular degeneration
(AMD)
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
19
cystoid macular edema (CME), diabetic macular edema (DME), posterior uveitis,
and
diabetic retinopathy. Some back-of-the-eye diseases result from unwanted
angiogenesis or
vascular proliferation, such as macular degeneration or diabetic retinopathy.
Drug
treatment options for these and other conditions are further discussed
elsewhere herein.
Application of Precursors to form hydrogels in situ
One mode of application is to apply a mixture of precursors and other
materials
(e.g., therapeutic agent, viscosifying agent, accelerator, initiator) through
a needle,
cannula, catheter, or hollow wire to a site in or near an eye. The mixture may
be
delivered, for instance, using a manually controlled syringe or mechanically
controlled
syringe, e.g., a syringe pump. Alternatively, a dual syringe or multiple-
barreled syringe or
multi-lumen system may be used to mix the precursors at or near the site.
One system that has been tested involved mixing a drug into a diluent, and
drawing
200 microliters of the drug/diluent into a 1 ml syringe. About 66 mg of a
precursor
powder consisting of trilysine was placed into a separate 1 ml syringe. The
two syringes
were attached via a female-female LUER connector, the solution was moved back
and
forth between the syringes until the dry precursor was completely dissolved. A
solution of
multi-armed electrophilic precursor in 200 l of water was drawn into a third
1-ml
syringe. Using another female-female LUER connector, the user mixed the
reconstituted
PEG / drug solution with the electrophilic precursor. The solutions were
rapidly inject
back and forth at least about ten times to ensure good mixing. The solutions
were drawn
into 1 syringe and were then available for further use.
Sites where drug delivery depots may be formed include the anterior chamber,
the
vitreous, episcleral, in the posterior subtenon's space (Inferior fornix),
subconjunctival, on
the surface of the cornea or the conjunctiva, among others.
Back of the eye diseases can be treated with drugs utilizing, e.g., topical,
systemic,
intraocular and subconjunctival delivery routes. Systemic and topical drug
delivery
modalities fall short in delivering therapeutic drug levels to treat posterior
segment
diseases. These methods of drug delivery encounter diffusion and drug dilution
issues due
to the inherent anatomical barriers of the intraocular and systemic systems,
causing
significant patient side effects (due to multiple daily dosing), poor
bioavailability and
compliance issues. Pericular drug delivery of an ophthalmic hydrogel implant
using
subconjunctival, retrobulbar or sub-Tenon's placement has the potential to
offer a safer
and enhanced drug delivery system to the retina compared to topical and
systemic routes.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
The delivery site for placement of an intraocular drug delivery implant is
generally
dependent upon the disease that needs to be treated and the type of drug
therapy. For
example; steroids like dexamethasone and triamicinolone acetonide may be mixed
with
the hydrogel precursor to form a sustained-release drug implant. The liquid
hydrogel
5 could then be injected in-situ into the sub-Tenon's capsule where it could
deliver a
constant or tunable release profile of the drug over a over a three to four
month time
period. The minimally invasive procedure could be performed in a doctor's
office, or after
a cataract operation under topical anesthesia, to treat chronic back of the
eye diseases.
In some embodiments, a retractor 80 is used to hold back eyelids 82, and the
user
10 would create a small buttonhole 84 (Figure 6A) in the conjunctiva about 5-6
mm from the
inferior/nasal limbus and dissect the conjunctiva down through Tenon's
capsule; to the
bare sclera. Next, a 23-gauge blunt cannula 86 (e.g., 15 mm in length) is
inserted through
the opening and the liquid drug implant is injected onto the scleral surface
(Figure 6B).
The cannula is then removed and the conjunctive is closed with a cauterization
device 88
15 (Figure 6C).
One advantage of an implant having three dimensional integrity is that it will
tend
to resist cellular infiltration and be able to prevent the locally
administered drug from
being phagocytosed and cleared prematurely from the site. Instead, it stays in
place until
delivered. By way of contrast a microparticle, liposome, or pegylated protein
tends to be
20 rapidly cleared from the body by the reticuloendothelial system before
being bioeffective.
Intravitreal Drug Delivery Implants
The delivery of therapeutic amounts of a drug to the retina in posterior
segment
eye diseases remains a challenge. Although intravitreal injections into the
vitreous cavity
of anti-VEG F agents have shown promise to arrest and in some cases reverse
chronic age-
related diseases like macular degeneration, these techniques and procedures
are not
without risks and side effects. Intravitreal administration of therapeutic
agents into the
vitreous cavity can cause cataracts, endophthalmitis and retinal detachments.
This form of
therapy requires many patients to receive monthly intraocular injections of an
anti-VEGF
drug over a 12 month time period thus increasing the risk of infection,
vitreous wicks and
retinal detachments. Embodiments directed to an in situ hydrogel biodegradable
drug
implant will provide an effective alternative treatment for back of the eye
diseases, and are
expected to reduce the common side-effects associated with repeated
intravitreal
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
21
injections. Embodiments of an intravitreal biodegradable drug delivery implant
system
are summarized below.
In Figure 7A, a hydrogel implant is injected intravitrealy about 2.5 mm
posterior to
the limbus through a pars plana incision 90 using a sub-retinal cannula 92, as
shown by
depiction of magnifying glass 94 held so as to visualization incision 90 on
eye 10, which
may be made following dissecting-away or otherwise clearing the conjunctiva,
as needed.
A 25, 27 or 30 gauge sub-retinal cannula 94 (or other appropriate cannulas) is
then
inserted through incision 90 and positioned intraocularly to the desired
target site, e.g., at
least one of sites 96, 98, 100 (Figure 7B) where the flowable precursors are
introduced to
form a hydrogel in-situ. The precursors then forms into an absorbable gel 102,
104, and/or
106, adhering to the desired target site.
As described in more detail in other sections, a drug depot of the in-situ
hydrogel
drug delivery implant may be designed for controlled, long term drug release
ranging
from, e.g., about one to about three months; and may optionally be directed to
treatment of
diseases of the posterior segment including, for example, age-related macular
degeneration, diabetic retinopathy, diabetic macular edema, and the cystoid
macular. The
device can carry a drug payload of various types of therapeutic agents for
various
conditions, of which some include, for example, steroids, antibiotics, NSAIDS
and/or
antiangiogenic agents, or combinations thereof.
The in-situ implant embodiments can improve the efficacy and pharmacokinetics
of potent therapeutic agents in the treatment of chronic back of the eye
diseases and
minimize patient side effects in several ways. First, the implant can be
placed in the
vitreous cavity at a specific disease site, bypassing the topical or systemic
routes and
thereby increasing drug bioavailability. Secondly, the implant maintains local
therapeutic
concentrations at the specific target tissue site over an extended period of
time. Thirdly,
the number of intravitreal injections would be substantially reduced over a 12
month
therapy regimen, thereby reducing patient risk of infection, retinal
detachment and
transient visual acuity disturbances (white specks floating in the vitreous)
that can occur
until the drug in the vitreous migrates down toward the inferior wall of the
eye and away
from the portion of the central vitreous or macula. As shown in Figure 8, a
bolus 120 of
conventionally-injected drugs forms in the vitreous body and displaces the
vitreous humor
until dispersed. Dispersion typically takes a significant amount of time since
the vitreous
humor is quite viscous. The bolus thus interferes with vision, particularly
when it is
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
22
moved around the eye in response to sudden accelerations, e.g., as the patient
stands up or
quickly turns the head.
Trans-scleral Drug Delivery
The hydrogels may be formed on scleral tissue either with or without the
presence
of the conjunctiva. The hydrogel may be adhesive to the sclera or other tissue
near the
sclera to promote drug diffusion through the intended tissue or to provide a
stable depot to
direct the therapeutic agents as required. In some embodiments, the
conjunctiva of the eye
may be removed, macerated, dissected away, or teased-free so that the tissue
can be lifted
away from the sclera to access a specific region of the sclera for
implantation or injection
of the hydrogel. A hydrogel is formed in situ that makes a layer on, and
adheres, to the
surface area. The conjunctiva may be allowed to contact the tissue if it is
still present or
retains adequate mechanical integrity to do so. In some embodiments the
hydrogel is
comprised of at least 50%, 75%, 80%, 90%, or 99% w/w water-soluble precursors
(calculated by measuring the weight of the hydrophilic precursors and dividing
by the
weight of all precursors, so that the weight of water or solvents or non-
hydrogel
components is ignored) to enhance the non-adhesive properties of the hydrogel.
In some
embodiments, such hydrophilic precursors substantially comprise PEOs. In some
embodiments, drugs to reduce tissue adherence mediated by biological
mechanisms
including cell mitosis, cell migration, or macrophage migration or activation,
are included,
e.g., anti-inflammatories, anti-mitotics, antibiotics, PACLITAXEL, MITOMYCIN,
or
taxols.
In other embodiments, the sclera is not substantially cleared of the
conjunctiva.
The conjunctiva is a significant tissue mass that overlays much or all of the
sclera. The
conjunctiva may be punctured or penetrated with a needle or catheter or trocar
and
precursors introduced into a space between the sclera and conjunctiva. In some
cases the
conjunctiva may be punctured to access a natural potential space between the
tissues that
is filled by the precursors. In other cases, a potential or actual space is
created
mechanically with a trocar, spreader, or the like, that breaks the adherence
between the
sclera and conjunctiva so that precursors may be introduced. The conjunctiva
has enough
elasticity to allow useful amounts of precursors to be introduced or forced
into such
natural or created spaces. Similarly, in the case of intravitreal hydrogel
formation,
relatively large columns may also be used. Accordingly, in some cases, the
amount is
between about 0.25 to about 10 ml; artisans will immediately appreciate that
all the ranges
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
23
and values within the explicitly stated ranges are contemplated, e.g., about 1
ml or from
0.5 ml to about 1.5 ml.
Moreover, removal of a hydrogel, whether present intraocularly or
periocularly, is
also readily achieved using either a vitrectomy cutter if the implant is
located in the
vitreous cavity or a manual I/A syringe and cannula if the implant is located
on the scleral
surface or irrigation/aspiration handpiece. This contrasts with major surgical
procedures
needed for the removal of some conventional non-absorbable implants.
In some aspects, in-situ formation of the hydrogel lets the hydrogel gel or
crosslink
in place, so that it does not flow back out through the tract of the needle
and diffuse
extraocularly through the incision site upon the removal of the needle or
cannula. A
shape-stable hydrogel thus formed can effectively deliver the drug and
advantageously can
have well-controlled size, shape, and surface area. A small needle may be used
to inject
the materials since soluble or flowable precursors may be used instead of an
already-
formed material. By way of contrast, alternative materials that do not cross-
link quickly
and firmly upon introduction tend to flow back out of the incision. And
materials that do
not covalently cross-link are subject to creep or weeping as the material
continually
reorganizes and some or all of the material flows out.
Delivery across the sclera is an important advance in these arts that is made
possible by the hydrogels and other materials disclosed herein. Transcleral
drug delivery
would conventionally not be considered since the diffusion of the drug across
the scleral
tissue is an unknown. Not only is the actual diffusion of the drug an issue,
but the rate of
that potential diffusion had to be balanced against the competing tendency of
the drug to
diffuse away to other relatively more permeable tissues, especially in
response to tear or
other fluid production. Moreover, fluid production in response to irritation
is also a
potential factor, e.g., as by flow of tears, lymph, edema, or a foreign body
response. But
the biocompatible materials and various available features, e.g., softness,
biocompatible
degradation products, conformability to surrounding tissues, adherence to the
sclera,
applicability over, in, or under the conjunctiva, crosslinking, non-irritating
shape and
deposition techniques, can be used to make suitable materials.
Adherence
Adhesivity can play an important role for in situ hydrogel-based therapies.
For
instance, a hydrogel that is adhesive to a scleral tissue can have good
surface-area contact
with the sclera to promote diffusion of drugs or other agents into the sclera.
By way of
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
24
contrast, a failure to adhere will create a diffusion barrier or allow entry
of fluids between
the drug depot and eye so that the drugs are washed away. On the other hand,
if a peri-
ocular hydrogel adheres to the tissues around it, or allows tissues to grow
and adhere to it,
the delivery of the drug may be compromised. Thus a hydrogel depot that
adheres
tenaciously to the sclera (the hydrogel's anterior surface) but does not
adhere to tissues on
its opposing surface (the posterior surface for a coating) or surfaces (for
more complex
geometries) would be useful. The in-situ made materials can reconcile these
opposing
needs by allowing forming the material in situ on the sclera with its other
surfaces being
free or substantially free of tissue contact during the time of gelation
and/or crosslinking.
As already explained, some embodiments relate to providing hydrogels that
adhere to
specific sites, e.g., the sclera and/or conjunctiva.
A test of adherence of a hydrogel to a tissue is, unless otherwise indicated,
to apply
it to a rabbit cornea and show that it is immobilized and is not displaced
when placed on
an uninjured rabbit cornea, despite unrestricted blinking by the rabbit. By
way of contrast,
a nonadherent material will be pushed out of, or to the side of the eye.
Some embodiments of forming a hydrogel involve mixing precursors that
substantially crosslink after application to a surface, e.g., on a tissue of a
patient to form a
biodegradable hydrogel depot. Without limiting the invention to a particular
theory of
operation, it is believed that reactive precursor species that crosslink after
contacting a
tissue surface will form a three dimensional structure that is mechanically
interlocked with
the coated tissue. This interlocking contributes to adherence, intimate
contact, and
essentially continuous coverage of the coated region of the tissue. Moreover,
formulations
with strongly electrophilic functional groups may tend to react with
nucleophilic groups
on the tissue to form covalent crosslinks, provided that the electrophiles are
present in
suitable concentrations and the nucleophiles are at a suitable pH.
By way of contrast, conventional materials tend to be non-adhesive to an
ocular
surface. Lenticels made of hydrogels, for instance, are not adherent. Fibrin
glue, for
instance, is generally not adherent as that term is used herein, although the
fact that it may
stick somewhat to an ocular tissue is acknowledged. Moreover, for many
materials, it is
generally unknown whether or not they will be adherent to an ocular tissue, or
to a
particular ocular tissue.
Another aspect of adherence is that the implant is prevented from moving from
the
site of its intended use. This tends to increase patient comfort, reduce
irritation, and
reduce tearing or fluid-flowing reactions that affect the therapeutic agent in
the implant.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
Also, the implant may be placed with precision, e.g., between certain tissues
or on a tissue,
with confidence that it will continue to affect the intended site.
Adherence can be useful for drug delivery. In some embodiments, specific zones
are targeted for adherence, e.g., as in Figure 5. For instance a material with
the drug can
5 be made to adhere to the sclera and/or conjunctiva. Or the material can be
made to adhere
to a surface inside the eye, a surface in the anterior portion of the eye. In
some cases, the
material is targeted to adhere to a surface inside the eye and within 1-10 mm
of the
macula; artisans will immediately appreciate that all the ranges and values
within the
explicitly stated ranges are contemplated, e.g., less than 10, 9, 8, 7, 6, 5,
4 mm or at least
10 1-10 mm or 2 mm up to about 25 mm distant; such targeting may be performed
to avoid
the macula itself, or not, as needed. Alternatively, or example, such
targeting may be used
to place the material is a position to an interior side of the eye where it
does not intrude
into the light path through the eye to the retina.
15 Drugs or other therapeutic agents for delivery
The hydrogel may be used to deliver classes of drugs including steroids, Non-
steroidal anti-inflammatory drugs (NSAIDS), intraocular pressure lowering
drugs,
antibiotics, or others. The hydrogel may be used to deliver drugs and
therapeutic agents,
e.g., an anti-inflammatory (e.g., Diclofenac), a pain reliever (e.g.,
Bupivacaine), a Calcium
20 channel blocker (e.g., Nifedipine), an Antibiotic (e.g., Ciprofloxacin), a
Cell cycle
inhibitor (e.g., Simvastatin), a protein (e.g., Insulin). The rate of release
from the hydrogel
will depend on the properties of the drug and the hydrogel, with factors
including drug
sizes, relative hydrophobicities, hydrogel density, hydrogel solids content,
and the
presence of other drug delivery motifs, e.g., microparticles.
25 The hydrogel precursor may be used to deliver classes of drugs including
steroids,
NSAIDS (See Table 1), intraocular pressure lowering drugs, antibiotics, pain
relievers,
inhibitors or vascular endothelial growth factor (VEGF), chemotherapeutics,
anti viral
drugs etc. The drugs themselves may be small molecules, proteins, RNA
fragments,
proteins, glycosaminoglycans, carbohydrates, nucleic acid, inorganic and
organic
biologically active compounds where specific biologically active agents
include but are
not limited to: enzymes, antibiotics, antineoplastic agents, local
anesthetics, hormones,
angiogenic agents, anti-angiogenic agents, growth factors, antibodies,
neurotransmitters,
psychoactive drugs, anticancer drugs, chemotherapeutic drugs, drugs affecting
reproductive organs, genes, and oligonucleotides, or other configurations. The
drugs that
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
26
have low water solubility may be incorporated, e.g., as particulates or as a
suspension.
Higher water solubility drugs may be loaded within microparticles or
liposomes.
Microparticles can be formed from, e.g., PLGA or fatty acids.
Table 1: NSAIDS that may be delivered.
Item Drug Structure Solubility
1 Ibuprofen l Omg/ml @ pH 7
1I \ COOH
/
2 Meclofenamate COO If~ CI < 50 g/ mL @ pH 7.2
sodium I\ N 50mg/mL @ pH 9.0
~ CI ~
3 Mefanamic COO II 40 g/ml @ pH 7.1
Acid N
4 Salsalate COOH
/
O \ I
0 OH
5 Sulindac Practically insoluble
\ \ ~
CooH below pH 4.5: Very
IN, S soluble >pH 6
O
F
6 Tolmetin 0 Freely soluble in water
\
sodium N
COONa
7 Ketoprofen O Not less than 0.25
COOH mg/ml @pH 7.35
8 Diflunisal COOH 3.43mg/ml @ pH 7
F Q-6 OH
F
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
27 -
9 Piroxicam O O 0.03mg/ml
N N
S:NH H
O O
Naproxen Freely soluble at pH 8
I ~ COOH
MeO Ito
11 Etodolac Insoluble in water
O
N
H
OOC
12 Flurbiprofen 0.9 mg/mL
F
COOH
13 Fenoprofen 0 0 Slightly soluble in
Calcium O'CaiO water
s o
14 Indomethacin COO O @ pH 7
N I~ Form I: 0.54mg/ml
~ CI
Form II: 0.80mg/ml
MeO
Celecoxib 5 g/ml
CF3
N-N
0
H2NO2S
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
28
16 Ketorolac 10.5mg/ml in IPB;
25mg/ml
o N a as tromethamine salt.
o
17 Nepafenac 0 NH2 <lmg/ml
NH2 (The drug is available
as 0.1 % suspension)
In some embodiments, the therapeutic agent is mixed with the precursors prior
to
making the aqueous solution or during the aseptic manufacturing of the
functional
polymer. This mixture then is mixed with the precursor to produce a
crosslinked material
in which the biologically active substance is entrapped. Functional polymers
made from
inert polymers like PLURONIC, TETRONICS or TWEEN surfactants are preferred in
releasing small molecule hydrophobic drugs.
In some embodiments, the therapeutic agent or agents are present in a separate
phase when crosslinker and crosslinkable polymers are reacted to produce a
crosslinked
polymer network or gel. This phase separation prevents participation of
bioactive
substance in the chemical crosslinking reaction such as reaction between NHS
ester and
amine group. The separate phase also helps to modulate the release kinetics of
active
agent from the crosslinked material or gel, where `separate phase' could be
oil (oil-in
water emulsion), biodegradable vehicle, and the like. Biodegradable vehicles
in which the
active agent may be present include: encapsulation vehicles, such as
microparticles,
microspheres, microbeads, micropellets, and the like, where the active agent
is
encapsulated in a bioerodable or biodegradable polymers such as polymers and
copolymers of: poly(anhydride), poly(hydroxy acid)s, poly(lactone)s,
poly(trimethylene
carbonate), poly(glycolic acid), poly(lactic acid), poly(glycolic acid)-co-
poly(glycolic
acid), poly(orthocarbonate), poly(caprolactone), crosslinked biodegradable
hydrogel
networks like fibrin glue or fibrin sealant, caging and entrapping molecules,
like
cyclodextrin, molecular sieves and the like. Microspheres made from polymers
and
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
29
copolymers of poly (lactone) s and poly (hydroxy acid) are particularly
preferred as
biodegradable encapsulation vehicles.
In using crosslinked materials which are described herein as drug delivery
vehicles, the active agent or encapsulated active agent may be present in
solution or
suspended form in crosslinker component or functional polymer solution
component. The
nucleophilic component, whether it be in the crosslinker or the functional
polymer is the
preferred vehicle due to absence of reactive groups. The functional polymer
along with
bioactive agent, with or without encapsulating vehicle, is administered to the
host along
with equivalent amount of crosslinker and aqueous buffers. The chemical
reaction
between crosslinker and the functional polymer solution readily takes place to
form a
crosslinked gel and acts as a depot for release of the active agent to the
host. Such
methods of drug delivery find use in both systemic and local administration of
an active
agent.
A variety of drugs or other therapeutic agents may be delivered using these
systems. A list of agents or families of drugs and examples of indications for
the agents
are provided. The agents may also be used as part of a method of treating the
indicated
condition or making a composition for treating the indicated condition. For
example,
AZOPT (a brinzolamide opthalmic suspension) may be used for treatment of
elevated
intraocular pressure in patients with ocular hypertension or open-angle
glaucoma.
BETADINE in a Povidone-iodine ophthalmic solution may be used for prepping of
the
periocular region and irrigation of the ocular surface. BETOPTIC (betaxolol
HCI) may be
used to lower intraocular pressure, or for chronic open-angle glaucoma and/or
ocular
hypertension. CILOXAN (Ciprofloxacin HCI opthalmic solution) may be used to
treat
infections caused by susceptible strains of microorganisms. NATACYN (Natamycin
opthalmic suspension) may be used for treatment of fungal blepharitis,
conjunctivitis, and
keratitis. NEVANAC (Nepanfenac opthalmic suspension) may be used for treatment
of
pain and inflammation associated with cataract surgery. TRAVATAN (Travoprost
ophthalmic solution) may be used for reduction of elevated intraocular
pressure - open-
angle glaucoma or ocular hypertension. FML FORTE (Fluorometholone ophthalmic
suspension) may be used for treatment of corticosteroid-responsive
inflammation of the
palperbral and bulbar conjunctiva, cornea and anterior segment of the globe.
LUMIGAN
(Bimatoprost ophthalmic solution) may be used for reduction of elevated
intraocular
pressure - open-angle glaucoma or ocular hypertension. PRED FORTE
(Prednisolone
acetate) may be used for treatment of steroid-responsive inflammation of the
palpebral and
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
bulbar conjunctiva, cornea and anterior segment of the globe. PROPINE
(Dipivefrin
hydrochloride) may be used for control of intraocular pressure in chronic open-
angle
glaucoma. RESTASIS (Cyclosporine ophthalmic emulsion) may be used to increases
tear
production in patients, e.g., those with ocular inflammation associated with
5 keratoconjunctivitis sicca. ALREX (Loteprednol etabonate ophthalmic
suspension) may
be used for temporary relief of seasonal allergic conjunctivitis. LOTEMAX
(Loteprednol
etabonate ophthalmic suspension) may be used for treatment of steroid-
responsive
inflammation of the palpebral and bulbar conjunctiva, cornea and anterior
segment of the
globe. MACUGEN (Pegaptanib sodium injection) may be used for Treatment of
10 neovascular (wet) age-related macular degeneration. OPTIVAR (Azelastine
hydrochloride) may be used for treatment of itching of the eye associated with
allergic
conjunctivitis. XALATAN (Latanoprost ophthalmic solution) may be used to
reduce
elevated intraocular pressure in patients, e.g., with open-angle glaucoma or
ocular
hypertension. BETIMOL (Timolol opthalmic solution) may be used for treatment
of
15 elevated intraocular pressure in patients with ocular hypertension or open-
angle glaucoma.
In using the crosslinked composition for drug delivery as mentioned above, the
amount of crosslinkable polymer, crosslinker and the dosage agent introduced
in the host
will necessarily depend upon the particular drug and the condition to be
treated.
Administration may be by any convenient means such as syringe, cannula,
trocar, catheter
20 and the like.
Certain embodiments of the invention are accomplished by providing
compositions
and methods to control the release of relatively low molecular weight
therapeutic species
using hydrogels. A therapeutic agent first is dispersed or dissolved within
one or more
relatively hydrophobic rate modifying agents to form a mixture. The mixture
may be
25 formed into particles or microparticles, which are then entrapped within a
bioabsorbable
hydrogel matrix so as to release the water soluble therapeutic agents in a
controlled
fashion. Alternatively, the microparticles may be formed in situ during
crosslinking of the
hydrogel.
In one method, hydrogel microspheres are formed from polymerizable macromers
30 or monomers by dispersion of a polymerizable phase in a second immiscible
phase,
wherein the polymerizable phase contains at least one component required to
initiate
polymerization that leads to crosslinking and the immiscible bulk phase
contains another
component required to initiate crosslinking, along with a phase transfer
agent. Pre-formed
microparticles containing the water soluble therapeutic agent may be dispersed
in the
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
31
polymerizable phase, or formed in situ, to form an emulsion. Polymerization
and
crosslinking of the emulsion and the immiscible phase is initiated in a
controlled fashion
after dispersal of the polymerizable phase into appropriately sized
microspheres, thus
entrapping the microparticles in the hydrogel microspheres. Visualization
agents may be
included, for instance, in the microspheres, microparticles, and/or
microdroplets.
Embodiments of the invention include compositions and methods for forming
composite hydrogel-based matrices and microspheres having entrapped
therapeutic
compounds. In one embodiment, a bioactive agent is entrapped in microparticles
having a
hydrophobic nature (also termed hydrophobic microdomains), to retard leakage
of the
entrapped agent. In some cases, the composite materials that have two phase
dispersions,
where both phases are absorbable, but are not miscible. For example, the
continuous
phase may be a hydrophilic network (such as a hydrogel, which may or may not
be
crosslinked) while the dispersed phase may be hydrophobic (such as an oil,
fat, fatty acid,
wax, fluorocarbon, or other synthetic or natural water immiscible phase,
generically
referred to herein as an "oil" or "hydrophobic" phase).
The oil phase entraps the drug and provides a barrier to release by slow
partitioning of the drug into the hydrogel. The hydrogel phase in turn
protects the oil from
digestion by enzymes, such as lipases, and from dissolution by naturally
occurring lipids
and surfactants. The latter are expected to have only limited penetration into
the hydrogel,
for example, due to hydrophobicity, molecular weight, conformation, diffusion
resistance,
etc. In the case of a hydrophobic drug which has limited solubility in the
hydrogel matrix,
the particulate form of the drug may also serve as the release rate modifying
agent.
Hydrophobic microdomains, by themselves, may be degraded or quickly cleared
when administered in vivo, making it difficult to achieve prolonged release
directly using
microdroplets or microparticles containing the entrapped agent in vivo. In
accordance
with the present invention, however, the hydrophobic microdomains are
sequestered in a
gel matrix. The gel matrix protects the hydrophobic microdomains from rapid
clearance,
but does not impair the ability of the microdroplets or microparticles to
release their
contents slowly. Visualization agents may be included, for instance, in the
gel matrix or
the microdomains.
In one embodiment, a microemulsion of a hydrophobic phase and an aqueous
solution of a water soluble molecular compound, such as a protein, peptide or
other water
soluble chemical is prepared. The emulsion is of the "water-in-oil" type (with
oil as the
continuous phase) as opposed to an "oil-in-water" system (where water is the
continuous
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
32 -
phase). Other aspects of drug delivery are found in commonly assigned U.S.
patents
6,632,457; 6,379,373; and 6,514,534, each of which are hereby incorporated by
reference.
Moreover, drug delivery schemes as described in commonly owned Compositions
And
Methods For Controlled Drug Delivery From Biodegradable Hydrogels, now
60/899,898
filed 02-06-07, which is hereby incorporated by reference herein, may also be
used with
the hydrogels herein.
Controlled rates of drug delivery also may be obtained with the system
disclosed
herein by degradable, covalent attachment of the bioactive molecules to the
crosslinked
hydrogel network. The nature of the covalent attachment can be controlled to
enable
control of the release rate from hours to weeks or longer. By using a
composite made from
linkages with a range of hydrolysis times, a controlled release profile may be
extended for
longer durations.
Biodegradation
The hydrogel is, in general, water-degradable, as measurable by the hydrogel
being
dissolvable in vitro in an excess of water by degradation of water-degradable
groups. This
test is predictive of hydrolytically-driven dissolution in vivo, a process
that is in contrast to
cell or protease-driven degradation. The hydrogels can be selected to be
absorbable over
days, weeks, or months, depending on the drug selected, disease being treated,
the
duration for release that is needed, and the release profile of the specific
drug selected.
Some embodiments, however, are specifically directed to 30 to 120 days since
longer
periods of time allow for less user-control of the dosing regimen, a factor
that may be
important if the drug does not exert its intended effect.
The biodegradable linkage may be water-degradable or enzymatically degradable.
Illustrative water-degradable biodegradable linkages include polymers,
copolymers and
oligomers of glycolide, dl-lactide, 1-lactide, dioxanone, esters, carbonates,
and
trimethylene carbonate. Illustrative enzymatically biodegradable linkages
include peptidic
linkages cleavable by metalloproteinases and collagenases. Examples of
biodegradable
linkages include polymers and copolymers of poly(hydroxy acid)s,
poly(orthocarbonate)s,
poly(anhydride)s, poly(lactone)s, poly(aminoacid)s, poly(carbonate)s, and
poly(phosphonate)s.
Significantly, however, polyanhydrides or other conventionally-used degradable
materials that degrade to acidic components tend to cause inflammation in the
eye. The
hydrogels, however, may exclude such materials, and may be free of
polyanhydrides,
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
33
anhydride bonds, or precursors that degrade into acid or diacids. Instead, for
example, SG
(succinimidyl glutarate), SS (succinimidyl succinate), SC (succinimidyl
carbonate),
carboxymethyl hydroxybutyric acid (CM-HBA) may be used and have esteric
linkages
that are hydrolytically labile.
If it is desired that the biocompatible crosslinked polymer be biodegradable
or
absorbable, one or more precursors having biodegradable linkages present in
between the
functional groups may be used. The biodegradable linkage optionally also may
serve as
the water soluble core of one or more of the precursors. For each approach,
biodegradable
linkages may be chosen such that the resulting biodegradable biocompatible
crosslinked
polymer will degrade or be absorbed in a desired period of time.
The crosslinked hydrogel degradation will generally proceed by the water-
driven
hydrolysis of the biodegradable segment when water-degradable materials are
used. If
polyglycolate is used as the biodegradable segment, for instance, the
crosslinked polymer
could be made to degrade in about 1 to about 30 days depending on the
crosslinking
density of the network. Similarly, a polycaprolactone based crosslinked
network can be
made to tend to degrade in about 1 to about 8 months. The degradation time
generally
varies according to the type of degradable segment used, in the following
order:
polyglycolate < polylactate < polytrimethylene carbonate < polycaprolactone.
Thus it is
possible to construct a hydrogel with a desired degradation profile, from a
few days to
many months, using a degradable segment.
Visualization agents
A visualization agent may be used with the hydrogel; it reflects or emits
light at a
wavelength detectable to a human eye so that a user applying the hydrogel can
observe
the gel.
Preferred biocompatible visualization agents are FD&C BLUE #1, FD&C BLUE
#2, and methylene blue. These agents are preferably present in the final
electrophilic-
nucleophilic reactive precursor species mix at a concentration of more than
0.05 mg/ml
and preferably in a concentration range of at least 0.1 to about 12 mg/ml, and
more
preferably in the range of 0.1 to 4.0 mg/ml, although greater concentrations
may
potentially be used, up to the limit of solubility of the visualization agent.
These
concentration ranges can give a color to the hydrogel without interfering with
crosslinking
times (as measured by the time for the reactive precursor species to gel).
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
34 -
Visualization agents may be selected from among any of the various non-toxic
colored substances suitable for use in medical implantable medical devices,
such as FD&C
BLUE dyes 3 and 6, eosin, methylene blue, indocyanine green, or colored dyes
normally
found in synthetic surgical sutures. The visualization agent may be present
with either
reactive precursor species, e.g., a crosslinker or functional polymer
solution. The
preferred colored substance may or may not become chemically bound to the
hydrogel.
The visualization agent may generally be used in small quantities, preferably
less than 1%
weight/volume, more preferably less that 0.01% weight/volume and most
preferably less
than 0.001 % weight/volume concentration.
Additional machine-aided imaging agents may be used, such as fluorescent
compounds, x-ray contrast agents (e.g., iodinated compounds) for imaging under
x-ray
imaging equipment, ultrasonic contrast agents, or MRI contrast agents (e.g.,
Gadolinium
containing compounds).
Viscosity
A composition with the precursors mixed therein can be made with viscosity
suitable for introduction through a small gauge needle using manual force. A
small gauge
needle has a diameter less than the diameter of a needle with a gauge of 27,
e.g., 28, 29,
30, 31, 32, or 33 gauge, with the gauge being specific for inner and/or outer
diameters.
Moreover, hollow-tube wires, as used in the intravascular arts, may be used to
deliver the
materials, including those with inn and/or outer diameters equivalent to the
small gauge
needles, or smaller. Thus a viscosity of between about 1 to about 100,000
centipoise may
be used; artisans will immediately appreciate that all the ranges and values
within the
explicitly stated ranges are contemplated e.g., about 10 to about 10,000
centipoise, less
than about 5 to about 10,000 centipoise, less than about 100 or about 500
centipoise, or
between about 1 and about 100 centipoise. The viscosity may be controlled,
e.g., by
choosing appropriate precursors, adjusting solids concentrations, and reaction
kinetics. In
general, lower concentrations of precursors, increased hydrophilicity, lower
molecular
weights favor a lower viscosity.
Viscosity enhancers may be used in conjunction with precursors. In general,
the
viscosity enhancers do not react with the precursors to form covalent bonds.
While it is
appreciated that precursors that are generally free of such bonding may
sometimes
participate in unwanted side reactions, these have little effect on the
hydrogel so that the
precursors are "free" of such reactions. For instance, if the precursors react
by
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
electrophile-nucleophile reactions, the viscosity enhancers may be free of
electrophiles or
nucleophiles that can form covalent bonds with functional groups of the
precursors, even
if there is some low level of unwanted side reactions. Viscosity enhancers
are, in general,
hydrophilic polymers with a molecular weight of at least 20,000, or from about
10,000 to
5 about 500,000 Daltons; artisans will immediately appreciate that all values
and ranges
between these explicitly stated values are described, e.g., at least about
100,000 or
200,000. A concentration of about 5% to about 25% w/w may be used, for
instance. PEG
(e.g., M.W. 100,000 to 250,000) is useful, for example. Viscosity enhancers
may be free
of electrophiles and/or nucleophiles. Viscosity enhancers may be fee of one or
more
10 functional groups such as hydroxyl, carboxyl, amine, or thiol. Viscosity
enhancers may
include one or more biodegradable links as described herein for precursors.
Viscosity
enhancers can be useful to prevent precursors from running-off a tissue site
before the
precursor's crosslink to form a gel.
15 Overview
Certain polymerizable hydrogels made using synthetic precursors are known in
the medical arts, e.g., as used in products such as FOCALSEAL (Genzyme, Inc.),
COSEAL (Angiotech Pharmaceuticals), and DURASEAL (Confluent Surgical, Inc), as
in,
for example, U.S. Patent Nos. 6,656,200; 5,874,500; 5,543,441; 5,514,379;
5,410,016;
20 5,162,430; 5,324,775; 5,752,974; and 5,550,187; each of which are hereby
incorporated by
reference to the extent they do not contradict what is explicitly disclosed
herein. None of
these materials seem to be suited to use inside the eye or around the eye. One
reason is
that they polymerize too quickly to be injected in a controlled fashion. Also,
COSEAL
and DURASEAL have a very high pH, which can be detrimental to ocular tissues
(above
25 pH 9). Another reason is that they apparently swell too much. The swelling
of COSEAL
and DURASEAL has been measured using an in vitro model in comparison to fibrin
sealant (Campbell et al., Evaluation of Absorbable Surgical Sealants: In vitro
Testing,
2005). Over a three day test, COSEAL swelled an average of about 558% by
weight,
DURASEAL increased an average of about 98% by weight, and fibrin sealant
swelled
30 about 3%. Assuming uniform expansion along all axes, the percent increase
in a single
axis was calculated to be 87%, 26%, and 1% for COSEAL, DURASEAL, and fibrin
sealant respectively. FOCALSEAL is known to swell over 300%. And also needs an
external light to be activated, so is not well suited as an injectable drug
delivery depot,
especially in or around the eye, which is sensitive to such radiation. Fibrin
sealant is a
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
36
proteinaceous glue that has adhesive, sealing, and mechanical properties that
are inferior
to COSEAL, DURASEAL, and other hydrogels disclosed herein. Further, it is
typically
derived from biological sources that are potentially contaminated, is cleared
from the body
by mechanisms distinct from water-degradation, and typically requires
refrigeration while
stored.
Some gel systems exist that relate to healing a wound or providing a lens on
the
cornea, e.g., as in U.S. Pat. No. 5,874,500, 6,458,889, 6,624,245 or PCT WO
2006/031358
or WO 2006/096586; other gels or systems for drug delivery are set forth in
U.S. Pat. No.
6,777,000, 7,060,297, US2006/0182771, US2006/0258698, US2006/0100288, or
US2006/0002963; each of which are hereby incorporated by reference to the
extent they
do not contradict what is explicitly disclosed herein.
Some systems to deliver drugs to the eye rely on topical eye drops. For
example,
after cataract and vitreoretinal surgery, antibiotics may need to be
administered every few
hours for several days. In addition, other drugs such as non-steroidal anti
inflammatory
drugs (NSAIDS) may also need to be given frequently. Often some of these eye
drops, for
example RESTASIS (Allergan) also has a stinging and burning sensation
associated with
its administration. RESTASIS is indicated for dry eye and has to be used by
the patient
several times a day. Similarly treatments for other ophthalmic diseases such
as cystoid
macular edema, diabetic macular edema (DME), and diabetic retinopathy also
need
administration of steroidal or NSAID drugs. Several vascular proliferative
diseases such
as macular degeneration are treated using intravitreal injections of VEGF
inhibitors.
These include drugs such as LUCENTIS and AVASTIN (Genentech) and MACUGEN
(OSI). Such drugs may be delivered using the hydrogel systems herein, with the
steps of
repeated dosings being avoided; e.g., not making new applications of the drug
daily,
weekly, or monthly, or not using topical eye drops to administer the drug.
Several alternative drug delivery systems are known. These other systems
generally include intravitreal implant reservoir type systems, biodegradable
depot systems,
or implants that need to be removed (non-erodeable). The state of the art in
this regard has
been delineated in texts such as "Intraocular Drug Delivery" (Jaffe et al.,
Taylor & Francis
pub., 2006. However, most of these implants either need to be removed at term,
can
detach from their target site, may cause visual disturbances in the back of
the eye or can be
inflammatory themselves because of the liberation of a substantial amount of
acidic
degradation products. These implants are thus made to be very small with a
very high
drug concentration. Even though they are small, they still need to be deployed
with
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
37
needles over 25G (25 gauge) in size, or a surgical approach delivery system
for
implantation or removal as needed. In general, these are localized injections
of drug
solutions into the vitreous humor or intravitreal implants that use a
biodegradable-
approach or a removable-reservoir approach.
For instance, localized injections delivered into the vitreous humor include
anti-
VEGF agents LUCENTIS or AVASTIN. POSURDEX (Allergan) is a biodegradable
implant with indications for use being diabetic macular edema (DME) or retinal
vein
occlusions, with a 22 g delivery system used for delivery into the vitreous
cavity; these are
powerful drugs in a short drug delivery duration setting. The therapeutic
agent is in
dexamethasone with polylactic/polyglycolic polymer matrix. Phase III trials
with
POSURDEX for diabetic retinopathy are in progress.
And for instance, a Medidure implant (PSIVIDA) is used for DME indications.
This implant is about 3 mm in diameter, cylindrical in shape, and non-
erodeable. It is
placed with a 25 gauge injector delivery system, the therapeutic agent is
fluocinolone
acetonide, and has a nominal delivery life of 18 months or 36 months (two
versions).
Phase III trials in progress.
Surmodics has a product that is an intravitreal, removable implant. It is
placed
surgically, with a therapeutic agent being triamcinolone acetonide. Its
nominal delivery
life is about two years. Its indication is for DME. It is presently in about
Phase I trials.
In contrast to these conventional systems, hydrogels can be made that are
biocompatible for the eye, which is an environment that is distinctly
different from other
environments. The use of minimally inflammatory materials avoids angiogenesis,
which
is harmful in the eye in many situations. Biocompatible ocular materials thus
avoid
unintended angiogenesis; in some aspects, avoiding acidic degradation products
achieves
this goal. Further, by using hydrogels and hydrophilic materials (components
having a
solubility in water of at least one gram per liter, e.g., polyethylene
glycols/oxides), the
influx of inflammatory cells is also minimized; this process is in contrast to
conventional
use of non-hydrogel or rigid, reservoir-based ocular implants. Moreover,
certain proteins
may be avoided to enhance biocompatibility; collagen or fibrin glues, for
instance, tend to
promote inflammation or unwanted cellular reactions since these releases
signals as they
are degraded that promote biological activity. Instead, synthetic materials
are used, or
peptidic sequences not normally found in nature. Moreover, the hydrogels may
be made
without external energy and/or without photoactivation so as to avoid heating
or
degradation of tissues, bearing in mind that the eye is a sensitive tissue.
Additionally,
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
38 _
biodegradable materials may be used so as to avoid a chronic foreign body
reaction, e.g.,
as with thermally-formed gels that do not degrade. Further, soft materials or
materials
made in-situ to conform the shape of the surrounding tissues can minimize
ocular
distortion, and low-swelling materials may be used to eliminate vision-
distortion caused
by swelling. High pH materials may be avoided, both in the formation,
introduction, or
degradation phases.
Kits or Systems
Kits or systems for making hydrogels may be prepared. The kits are
manufactured
using medically acceptable conditions and contain precursors that have
sterility, purity and
preparation that is pharmaceutically acceptable. The kit may contain an
applicator as
appropriate, as well as instructions. A therapeutic agent may be included pre-
mixed or
available for mixing. Solvents/solutions may be provided in the kit or
separately, or the
components may be pre-mixed with the solvent. The kit may include syringes
and/or
needles for mixing and/or delivery.
In some embodiments, the kit has at least one precursor and an applicator. In
some
embodiments, a biodegradable, polymeric, synthetic hydrogel is formed by the
reaction of
an 8 armed 15,000 MW polyethylene glycol (PEG) having NHS-esters on each
terminus
of each arm with trilysine (which has primary amine nucleophiles) in phosphate
or other
buffer solutions. Visualization agents (e.g., FD&C Blue #1) may be
incorporated into the
sealant material.
In some embodiments the kit's applicator includes (or consists essentially of)
syringes for syringe-to-syringe mixing. The delivery device is one of the
syringes, and has
a small bore tube with a LUER-lock on at least one end. After reconstitution
of the
product, an applicator tube is attached to the delivery syringe and the
hydrogel is applied
to the target tissue.
In some embodiments, kits having precursors and other materials as needed to
form a hydrogel in situ with a therapeutic agent may be provided, with the
component
parts including those described herein. In some aspects, features of the
hydrogels can thus
be chosen to make hydrogels that are minimally swelling, delivered through a
small
needle, can be put into an aqueous low viscosity preparation to gel after
placement. The
hydrogel is not inflammatory or angiogenic, relies on biocompatible
precursors, and is
soft, hydrophilic, and conforming to the space wherein it is placed. The
hydrogel may be
easily removable or self-removing, and can be biodegradable or suited to
delivery to easily
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
39 -
accessible areas without dispersal. It can be made so it is easy to mix and
use, with an
option to combine all the precursors in a single container. The hydrogel may
be made
with safe, all-synthetic materials. The hydrogel formulations may be made to
be adhesive
to tissues. The degradation and/or delivery rate may be controlled to fit the
time periods
described. Since the hydrogel is cross-linked, it will not come out of the
needle tract or
other hole created for its delivery because it is shape-stable as deposited.
The hydrogel
depots have advantages relative to eye drops. Over 97% of topically
administered eye
drops are cleared via the tear ducts and do not end up penetrating the eye.
Patient
compliance may be enhanced by avoiding repeated dosing.
The use of fluent aqueous precursors to form a biodegradable drug depot allows
for
administration through small (e.g., 30 gauge) needles. Also, since the
hydrogel can be
made to not break down s into acidic by products, the drug depots are well
tolerated by
sensitive tissues, such as the eye. Due to this, the implants can be made
rather large in size
(e.g., 1 ml capacity) relative to implants that are made from conventional
biodegradable
polymers, which are conventionally much smaller. Accordingly, some embodiments
are
hydrogels with volumes between about 0.5 to about 5 ml; artisans will
immediately
appreciate that all the ranges and values within the explicitly stated ranges
are
contemplated, e.g., 0.5 ml to about 1 ml. This makes such hydrogels eminently
suited for
periocular (episclereal or posterior subtenon injections (PST)) drug depots.
While some of the agent in one of the hydrogels or other crosslinked materials
may
be lost to the systemic circulation through a periocular route, a
significantly larger implant
size has the capability to retain therapeutic agent concentrations and
accommodate larger
implants to enable adequate transscleral diffusion of drugs across the sclera
and into the
back of the eye, bearing in mind that the human sclera surface area is about
17 cm2. The
hydrogels also help in localizing the drug; by way of contrast, if a drug
suspension or
microparticles are injected within the vitreous, they can migrate into the
visual field and
interfere with vision.
Example 1, Drug incorporation into hydrogel
Two precursors and a diluent were prepared. The first precursor was an 8-armed
polyethylene glycol with a succinimidyl glutarate on the terminus of each arm,
having a
molecular weight of about 15,000. It was provided as a powder and blended with
a dye
(FD&A Blue) at a concentration of 0.11 % w/w. The second precursor was
trilysine in an
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
0.2 M sodium phosphate buffer at pH 8. A diluent for the first precursor was
prepared to
be 0.01 M sodium phosphate, pH 4.8.
A drug (as indicated in Examples below) was mixed into drug into diluent, and
about 200 l of the drug/diluent was drawn into a 1 ml syringe. 66 mg of the
first
5 precursor powder was placed into a separate 1 ml syringe. The two syringes
were attached
via a female-female luer connector, and the solution was injected back-and-
forth until the
powder was completely dissolved. The second precursor in its solution was
drawn (200
gl) into a third syringe. With another female-female luer connector, the first
and second
precursors were thoroughly mixed. The mixed solution was drawn into one of the
10 syringes and attached to a 4 inch length of silicone tubing that received
the contents. After
allowing a suitable reaction time, the tubing was cut into desired lengths,
and the gel
inside pushed out with a mandrel. Resulting hydrogel plugs were, in general,
0.125 inch
in diameter and about 6.4 mm thick.
Unless otherwise indicated, the analysis of drug release profiles was
ascertained
15 using high-pressure liquid chromatography (HPLC). The disks were kept in a
solution and
the solution was periodically sampled and tested by HPLC to measure the
concentration of
drug in the solution. Total drug loading was determined by dissolving the
disks in
aqueous solution or in the presence of an alcohol such as octanol at high pH
and
measuring the drug content in the disks. Drug loading was 5% (weight of drug /
total
20 weight of hydrogel including contents) unless otherwise indicated.
Example 2: Release of Diclofenac Sodium
Diclofenac Sodium has a water solubility of about 1113 mg/L. It is an anti-
inflammatory drug. It was loaded into a hydrogel as per Example 1 and was
released as
25 indicated in Figure 9. Essentially complete release of the drug was
observed in about 8
hours.
Example 3: Release of Bupivacaine
Bupivacaine has a water solubility of about 86 mg/L. It is a pain reliever
that was
30 converted from an HCl-salt to a free base to decrease water solubility. It
was loaded into a
hydrogel as per Example 1 and was released as indicated in Figure 10.
Sustained zero
order release was observed over six days.
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
41
Example 4: Release of Nifedipine
Nifedipine has a water solubility of less than 1 mg/L. It is a calcium channel
blocker
and Anti-hypertensive that relieves angina by increasing blood flow to the
heart. It was
loaded into a hydrogel as per Example 1 and was released as indicated in
Figure 11.
Sustained zero order release over 45 days.
Example 5: Release of Ciprofloxacin
Ciprofloxacin has a water solubility of 160 mg/L. It is an antibiotic. It was
loaded
into a hydrogel as per Example 1 and was released as indicated in Figure 12,
which shows
testes at ph 9.0 (squares) or pH 7.4 (circles). 50 % of drug released by day
three.
Example 6: Release of Mefenamic Acid
Mefenamic Acid is an NSAID for treating pain. It was loaded into a hydrogel as
per Example 1 and was released as indicated in Figure 13, which shows tests at
ph 9.0
(squares) or pH 7.4 (circles). It was released over about 15 days although
further
degradation to its component parts at later times would release additional
amounts of the
drug.
Example 7: Release of Indomethacin
Indomethacin is another NSAID for treating pain. It was loaded into a hydrogel
as
per Example 1 and was released as indicated in Figure 14, which shows tests at
ph 9.0
(squares) or pH 7.4 (circles). The Figure shows the release profile over about
six days;
further release was observed but not quantified.
Example 8: Release of Triamcinolone
Triamcinolone has very little solubility in water and is a synthetic
corticosteroid
conventionally given orally, by injection, inhalation, or as a topical cream.
It was loaded
into a hydrogel as per Example 1 except that the loading was about 4% instead
of 5%, and
was released as indicated in Figure 15, which shows tests up to about a week.
Example 9: Release of dexamethasone
Dexamethasone is a glucocorticoid-type steroid hormone. It acts as an anti-
inflammatory and immunosuppressant. It was loaded into a hydrogel as per
Example 1
CA 02692545 2010-01-04
WO 2009/008946 PCT/US2008/006114
42
and was released as indicated in Figure 16. The Figure shows the release
profile over
about six days; further release was observed but not quantified.
* * * * * *
Many embodiments have been set forth herein. In general, components of the
embodiments may be mixed-and-matched with each other as guided for the need to
make
functional embodiments.