Note: Descriptions are shown in the official language in which they were submitted.
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
TITLE
BIOMASS TREATMENT APPARATUS
STATEMENT OF GOVERNMENT RIGHTS
This invention was made with United States Government support
under Contract Nos. 04-03-CA-70224 and DE-FC36-03G013146 awarded
by the Department of Energy. The government has certain rights in this
invention.
FIELD OF THE INVENTION
An apparatus for treatment of biomass is provided. The apparatus
is one through which biomass is moved while maintaining a non-
compacted state. Also provided is a method for biomass treatment where
reactants are added to the biomass in the apparatus.
BACKGROUND
Cellulosic and lignocellulosic feedstocks and wastes, such as
agricultural residues, wood, forestry wastes, sludge from paper
manufacture, and municipal and industrial solid wastes, provide a
potentially large renewable feedstock for the production of valuable
products such as fuels and other chemicals. Cellulosic and lignocellulosic
feedstocks and wastes, composed of carbohydrate polymers comprising
cellulose, hemicellulose, glucans and lignin are generally treated by a
variety of chemical, mechanical and enzymatic means to release primarily
hexose and pentose sugars, which can then be fermented to useful
products.
First, biomass feedstocks are treated to make the carbohydrate
polymers of cellulosic and lignocellulosic materials more readily available
to saccharification enzymes, which is often called pretreatment. The
pretreated biomass is then further hydrolyzed in the presence of
saccharification enzymes to release oligosaccharides and/or
monosaccharides in a hydrolysate. Saccharification enzymes used to
produce fermentable sugars from pretreated biomass typically include one
or more glycosidases, such as cellulose-hydrolyzing glycosidases,
hemicellulose-hydrolyzing glycosidases, and starch-hydrolyzing
1
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
glycosidases, as well as peptidases, lipases, ligninases and/or feruloyl
esterases. Saccharification enzymes and methods for biomass treatment
are reviewed in Lynd, L. R., et al. (Microbiol. Mol. Biol. Rev. (2002) 66:506-
577).
It is desirable to have a system and/or method for treating biomass
that is effective and economical for use on a large scale. Treatment of
biomass as a concentrated, high dry weight material is needed to produce
the high concentrations of fermentable sugars needed for fermentation to
products economically. Thus movement of material including a high dry
weight fraction of biomass through a reactor while maintaining the ability of
treatment reactants to penetrate and optimally prepare the biomass for
saccharification, in addition to using minimal chemical and energy inputs,
is a challenge for biomass treatment processes. Also a system or method
that includes low capital cost equipment is desired. Systems or methods
including reactors with no requirement for stirring or reactor rotation may
provide lower capital cost for equipment and lower energy input.
Systems not requiring stirring or reactor rotation and specifying
means for moving biomass through a reactor have been described.
US4186658 discloses an apparatus for conveying particulate material,
such as wood chips, straw, bagasse and other fibrous material, which
compacts the material into a solid "plug" state. A screw conveyor pre-
compacts the material, with further compaction by a reciprocating piston.
The compact plug is so dense that it is capable of effectively preventing
blow-back within the system. The plug may then be fed to a means for
processing the material. A dense plug of biomass material would not be
optimally accessible by pretreatment reactants.
Similarly, US4136207 discloses a process for preparing cellulosic
material with enhanced digestibility by ruminants that begins with
mechanically compacting the material. It is then subjected to high steam
pressure in the absence of chemical reagents, and is further compacted to
form a solid plug of biomass which prevents escape of steam through the
inlet. Small portions of the material are then discharged, subjecting it to
2
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
rapid reduction in pressure. The compacting of biomass into a plug would
not allow optimal accessibility by reactants used in pretreatment.
US6176176 discloses an apparatus for treating cellulosic materials
that uses a rotatable screw mounted in a barrel of an extruder. Liquid
ammonia under pressure is fed into the barrel and mixed with
lignocellulosic material in the barrel, then the lignocellulosic material
containing the ammonia is expanded explosively by change of liquid
ammonia to a gas as it exits the barrel through a heated die. Use of an
extruder in a large scale commercial process would be very costly and
therefore not provide an economical process.
There remains a need for an economical reactor for treating
biomass that moves high dry weight of biomass through the low-cost
reactor while allowing for maximal accessibility by reactants, to prepare
the biomass for saccharification.
SUMMARY OF THE INVENTION
The present invention provides an apparatus for treating biomass
prior to saccharification and methods for treating biomass using said
apparatus. In addition, the present invention provides treated biomass
produced by the present methods in the present apparatus, as well as
hydrolysate containing fermentable sugars produced by subsequent
saccharification of the pretreated biomass. The apparatus comprises:
a) a cylindrical barrel having a first end fitted with a piston and a
second end fitted with a discharge valve;
b) optionally, an offset attached at one offset end to the cylindrical
barrel near the cylindrical barrel first end, and said offset having a
sealable
valve at the unattached offset end;
c) at least 2 sealable ports in the cylindrical barrel or in the offset;
d) optionally, a valve in the cylindrical barrel dividing the barrel into
separate first and second chambers, said first chamber having the barrel
first end fitted with said piston, and said second chamber having the barrel
second end with the discharge valve,; and
e) a flash tank attached to the discharge valve at the second end of
the barrel,
3
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
wherein said apparatus provides for a sealable impermeable barrel
and non-compacting flow of treated biomass.
In another aspect a method for treating biomass in the apparatus
comprises:
a) loading biomass using a non-compacting feeder into the cylindrical
barrel through the open first end or the offset;
b) fitting the piston;
c) optionally applying vacuum via at least one port in the cylindrical
barrel;
d) adding at least one reactant other than steam via at least one port
in the cylindrical barrel or offset;
e) adding steam via at least one port in the cylindrical barrel to reach
a suitable temperature inside the barrel;
f) sealing the ports of c), d), and e) and all valves to provide an
impermeable chamber;
g) allowing said biomass and at least one reactant to react for a time
that is between about 30 seconds and about 4 hours; and
h) opening the discharge valve and moving the reacted biomass
product from step (g) from the impermeable chamber by displacement
with the piston into the flash tank;
wherein steps a) and b) may be in either order, wherein d) and e) may be
in any order or concurrent, and whereby non-compacted treated biomass
is produced.
Yet additional aspects of the present invention are directed to the
treated biomass that has been prepared in the present apparatus,
according to the present treatment methods, and the hydrolysate
containing fermentable sugars produced by saccharification of biomass
that has been treated in the present apparatus by the present methods.
Biomass refers to any cellulosic and/or lignocellulosic material
which may include bioenergy crops, agricultural residues, municipal solid
waste, industrial solid waste, yard waste, wood, forestry waste or
combinations thereof. Energy may be applied to the biomass before (a), in
order to reduce the size, increase the exposed surface area, and/or
4
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
increase the accessibility of cellulose, hemicellulose and/or
oligosaccharides present in the biomass.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic drawing of one embodiment of an
apparatus of the present invention.
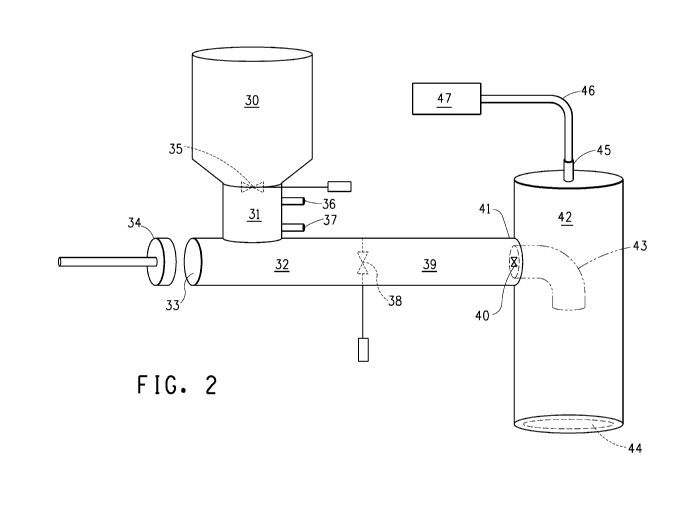
Figure 2 is a schematic drawing of a second embodiment of an
apparatus of the present invention.
Figure 3 is a schematic drawing of one embodiment of a gradual
expansion venturi used as a discharge valve, with the valve closed.
Figure 4 is a schematic drawing of the gradual expansion venturi
embodiment of Figure 3, with the valve open.
Figure 5 is a schematic drawing of an embodiment of a V-port valve
gradual expansion venturi.
Figure 6 is a schematic drawing of an embodiment of a swingcheck
valve gradual expansion venturi, with the valve closed in A and open in B.
DETAILED DESCRIPTION
Applicants specifically incorporate the entire contents of all cited
references in this disclosure. Further, when an amount, concentration, or
other value or parameter is given as either a range, preferred range, or a
list of upper preferable values and lower preferable values, this is to be
understood as specifically disclosing all ranges formed from any pair of
any upper range limit or preferred value and any lower range limit or
preferred value, regardless of whether ranges are separately disclosed.
Where a range of numerical values is recited herein, unless otherwise
stated, the range is intended to include the endpoints thereof, and all
integers and fractions within the range. It is not intended that the scope of
the invention be limited to the specific values recited when defining a
range.
The present invention provides an apparatus for the treatment of
biomass to prepare it for undergoing saccharification to produce
fermentable sugars. The sugars may be fermented to produce valuable
products such as fuels and other chemicals. Through the pretreatment,
saccharification and fermentation steps, renewable biomass, including
5
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
waste biomass, may be used to produce valuable chemicals which may
decrease the need for oil.
Definitions:
In this disclosure, a number of terms are used. The following
definitions are provided:
"Biomass" refers to any cellulosic or lignocellulosic material and
includes materials comprising cellulose, and optionally further comprising
hemicellulose, lignin, starch, oligosaccharides and/or monosaccharides.
Biomass may also comprise additional components, such as protein
and/or lipid. According to the invention, biomass may be derived from a
single source, or biomass can comprise a mixture derived from more than
one source; for example, biomass could comprise a mixture of corn cobs
and corn stover or fiber, or a mixture of grass and leaves. Biomass
includes, but is not limited to, bioenergy crops, agricultural residues,
municipal solid waste, industrial solid waste, sludge from paper
manufacture, yard waste, wood and forestry waste. Examples of biomass
include, but are not limited to, corn grain, corn cobs, crop residues such as
corn husks, corn stover, corn fiber, grasses, wheat, wheat straw, hay, rice
straw, switchgrass, waste paper, sugar cane bagasse, sorghum stalks,
soy hulls or stalks, components obtained from milling of grains, trees,
branches, roots, leaves, wood chips, sawdust, shrubs and bushes,
vegetables, fruits, flowers and ruminant animal manure. In one
embodiment, biomass that is useful for the invention includes biomass that
has a relatively high carbohydrate value, is relatively dense, and/or is
relatively easy to collect, transport, store and/or handle. In one
embodiment of the invention, biomass that is useful includes corn cobs,
corn stover, corn fiber, and sugar cane bagasse.
The term "fermentable sugar" or "sugars" refers to oligosaccharides
and monosaccharides that can be readily fermented to target chemicals.
The term "lignocellulosic" refers to material comprising both lignin
and cellulose. Lignocellulosic material may also comprise hemicellulose.
The term "cellulosic" refers to material comprising cellulose.
6
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
The term "saccharification" refers to the production of fermentable
sugars from polysaccharides.
By "dry weight" of biomass is meant the weight of the biomass
having all or essentially all water removed. Dry weight is typically
measured according to American Society for Testing and Materials
(ASTM) Standard E1756-01 (Standard Test Method for Determination of
Total Solids in Biomass) or Technical Association of the Pulp and Paper
Industry, Inc. (TAPPI) Standard T-412 om-02 (Moisture in Pulp, Paper and
Paperboard).
An "aqueous solution comprising ammonia" refers to the use of
ammonia gas (NH3), compounds comprising ammonium ions (NH4+) such
as ammonium hydroxide or ammonium sulfate, compounds that release
ammonia upon degradation such as urea, and combinations thereof in an
aqueous medium.
The term "treatment" refers to a process of a reactant acting on a
material wherein the physical and/or chemical properties of the material
are altered.
The term "reactant" refers to a composition that is able to alter the
physical and/or chemical properties of a target material under conditions
used in a treatment process.
An "enzyme consortium" for saccharification is a combination of
enzymes that are able to act on a biomass mixture to produce fermentable
sugars. Typically, a saccharification enzyme consortium may comprise
one or more glycosidases; the glycosidases may be selected from the
group consisting of cellulose-hydrolyzing glycosidases, hemicellulose-
hydrolyzing glycosidases and starch-hydrolyzing glycosidases. Other
enzymes in the saccharification enzyme consortium may include
peptidases, lipases, ligninases and feruloyl esterases.
The terms "treat" and "pretreat" with respect to biomass are related
in the following manner. Biomass is treated with reactant to form a treated
biomass product, which may also be referred to as treating to form
pretreated biomass or pretreating to form pretreated biomass. The use of
7
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
"pre" distinguishes the treating of biomass that is prior to saccharification
of biomass.
Biomass Treatment Apparatus
The apparatus of the present invention is designed to handle the
process of treatment of high concentration biomass with reactants to
prepare the biomass for saccharification. The treatment of high
concentration biomass is successful in the present apparatus due to the
avoidance of compacting the biomass at any stage, and thereby allowing
for improved access of treatment reactants to the biomass over that which
occurs in a process that includes biomass compaction. In an apparatus
where biomass is compacted, the biomass can be de-compacted for
improved reaction with treatment reactants, but this requires high energy
input and thereby raises the cost of operating the apparatus. When using
the apparatus of the present invention, no de-compaction function is
needed. Additional economy in the present apparatus is provided by the
stationary aspect of the reaction chamber. There is no rotation of the
reaction chamber, or of paddles or other mixers within the chamber. Thus
the present apparatus surprisingly provides effective penetration of
treatment reactants into high concentration biomass for creating effectively
pretreated biomass in a low-cost manner.
In the present apparatus, biomass is added to the stationary
apparatus without compacting, and is moved through the apparatus
without compacting. By maintaining the biomass in a non-compacted
state, the natural pores and channels of the biomass material are not
crushed. The treatment reactants added to the biomass in the reaction
chamber are able to penetrate through the non-compacted natural
biomass pores and channels providing rapid and thorough effects on the
cellulosic or lignocellulosic material of the biomass. This apparatus is
highly effective in producing pretreated biomass that undergoes effective
saccharification to produce fermentable sugars, in that it leads to a high
conversion of biomass carbohydrates to de-polymerized sugars per
enzyme dosage and reaction time.
8
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
The present biomass treatment apparatus may best be understood
by making reference to the schematic drawings in Figures 1 and 2, which
show two embodiments of a piston/barrel-type apparatus, and the
following description of use of the apparatus in treatment systems and/or
methods. These drawings are simplified for clarity of illustration, where
some elements are omitted such as the flanges shown in Figures 3 and 4.
The apparatus in Figure 1 is a test scale reactor. It comprises a horizontal
cylindrical barrel chamber (10) with an open first end for adding biomass
(11) that is then sealed following biomass loading by inserting a moveable
plug (12), which is used as a type of piston. The cylindrical chamber has a
first sealable port (13) and a second sealable port (14) for adding
treatment reactants to the biomass in the cylindrical chamber, and a third
sealable port (15) for applying a vacuum. An insulation jacket (16) covers
the cylindrical chamber.
Following loading biomass and application of vacuum, typically a
treatment reactant and steam are added through ports, then all ports are
sealed and a desired temperature is maintained. Following a period of
time, a previously closed discharge valve (17) is opened in the cylindrical
barrel second end (18) by moving the valve shaft (19). The valve shaft
extends through a hole in a downward directed internal separating elbow
(20) in the adjacent flash tank (21) and through a packing gland (22) on
the far side of the flash tank to an actuator (23). The biomass and reactant
mixture is pushed through the discharge valve (17) by moving the plug in
the cylindrical barrel first end towards the second end. The biomass
mixture passes through the discharge valve and into the flash tank (21)
through the elbow (20). A cover (24) over an opening in the bottom of the
flash tank allows access to pretreated biomass. A port (25) in the top of
the flash tank allows exit of vapors, and is connected through a tubing (26)
to a condenser (27).
Further description of an embodiment of the apparatus of Figure 1
and its use in Examples herein is as follows. This large barrel piston
reactor consisted of a 5.1 cm x 68.6 cm stainless steel barrel equipped
with a piston, oriented horizontally. The piston was sealed to the barrel
9
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
with four 0-rings and was pressurized with nitrogen (up to about 5600
kPa) on the backside of the piston during the discharge stroke. The 68.6
cm barrel was equipped with eight multiple use ports, 4 each along the top
and bottom surfaces, allowing application of vacuum, injection of
reactants, and insertion of thermocouples for measurement of temperature
inside the barrel. The reactor barrel was equipped with a steam jacket for
even heating of the barrel. The reactor barrel was directly attached to a
15.2 cm x 61 cm stainless steel flash tank, oriented vertically. The barrel
was isolated from the flash tank by a conical nozzle and seat end shearing
valve arrangement. The diameter of the end valve shearing die was 3.5
cm. The backpressure on the conical nozzle and seat was adjustable,
with most tests performed using -138 kPa (gauge pressure) of
backpressure into a 10.2 cm diameter air cylinder connected to the cone of
the end shear valve. The cone of the end shearing valve could move back
up to 1.6 cm to allow discharge of particles in the flash tank. An elbow at
the outlet of the end shear valve directed the treated solids down into the
bottom of the flash tank where the solids were easily removed by unbolting
a domed end flange in the bottom of the tank. An upper domed flange to
the flash tank incorporated a special outlet fitting with slots machined at
right angles to the axis of the flash tank, which caused released vapors to
travel around a corner path to an exit fitting, helping to prevent carry-over
of entrained biomass particles and water droplets into a vent condenser.
Three electrical band heaters (set at 60 C) and insulation were added
along the flash tank to allow hot treated solids to flash into a heated
vessel, better simulating a commercial scale process.
In another embodiment a small barrel piston reactor was built as
described above, except having a 45.7 cm barrel, no steam jacket, three
electrical band heaters, a 2.5 cm thick fiberglass mat covered with a
silicone impregnated fiberglass jacket as insulation, and three multiple use
ports. Other features including the flash tank, shearing valve, and elbow
were as described for the large barrel piston reactor.
The apparatus of the invention embodied in Figure 2 is a
commercial scale reactor design. It comprises a horizontal cylindrical
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
barrel fitted with a piston (34) at the first end (33) and a discharge valve
(40) at the second end (41). The barrel is insulated and has impermeable
walls. An offset (31) is attached near the first end and a valve (35), that is
an infeed valve, is located at the unattached end of the offset. A hopper
(30) is attached to the valve end of the offset. Biomass is added through
the hopper. There may be non-compacting flow-inducing means to control
biomass addition from the hopper (30) to the offset (31). The offset has a
first sealable port (36) and a second sealable port (37) for adding
treatment reactants to the biomass in the offset as it moves into the
cylindrical barrel. A second valve (38) separates the barrel into a first
cylindrical chamber (32) and a second cylindrical chamber (39). Biomass
and reactant pass through the offset into the first chamber where a desired
temperature and pressure is reached. Movement of the piston through the
impermeable barrel pushes the biomass and reactant mixture from the first
chamber into the second chamber, through the opened second valve (38),
and displacing contents in the second chamber (39) through the opened
discharge valve (40) into a flash tank (42). Contents of the second
chamber are biomass and reactant(s) that were previously moved into this
chamber and held for as long as necessary for the treatment reaction
under the conditions used. The second valve (38) is then closed and the
piston (34) is retracted so as to prepare the first cylindrical chamber (32)
to
be reloaded and the process cycle repeated. In the flash tank (42), the
biomass moves through a downward directed elbow (43). A cover (44)
over an opening in the bottom of the flash tank allows access to pretreated
biomass. A port (45) in the top of the flash tank allows exit of vapors, and
is connected through a tubing (46) to a condenser (47).
The embodiments shown in Figures 1 and 2 function similarly in
that biomass is added to and moved through the reactors without
compaction. The embodiment of Figure 1, with one chamber, is a batch
system for processing one sample of biomass at a time. The embodiment
of Figure 2, with two chambers that are separated by a valve, allows a
semi-continuous or fed-batch operation wherein multiple loadings of
biomass are processed concurrently. In this second embodiment, each
11
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
piston displacement cycle, where each successive loading of biomass
enters the second chamber, is accompanied by the discharge of
corresponding volume through the discharge orifice once the second
chamber is fully loaded. The number of piston displacement cycles in the
second chamber at one time, and therefore the size of the second
chamber, is related to the residence time required for each biomass
sample. Residence time is discussed further below in relation to
temperature and time for treatments in the present methods using the
present apparatus. Thus, the size of chamber two with respect to the size
of chamber one may vary depending on the specific conditions required for
the specific treatment being used, as is readily determined by one skilled
in the art.
The present apparatus and treatment methods are particularly
suited to treatment of biomass at a high dry weight of biomass relative to
the weight of biomass, reactant and steam mixture of the treatment
reaction. It is desirable to treat biomass in a high dry weight concentration
to provide biomass that will produce a high sugars concentration
hydrolysate following saccharification. The features of the present
apparatus that provide that the biomass is not compacted allow effective
treatment of high dry weight concentration of biomass. The initial dry
weight of biomass used in the present apparatus and treatment methods is
at least about 15% of the total weight of the biomass, reactant and steam
mixture of the treatment reaction. More typically, the dry weight of biomass
is at least about 20% and may be at least about 30%, 45%, 50%, or more.
The per cent dry weight of biomass may vary and the optimal per cent may
be different for different types of biomass. For example, biomass of at
least about 24% is desired when using corn cob, to provide pretreated
biomass that is saccharified to produce fermentable sugars concentrated
sufficiently for cost-effective fermentation to ethanol. More suitable is corn
cob biomass that is at least about 30%. The preferred per cent dry weight
of a particular type of biomass for use in the present methods for
producing a high sugars hydrolysate is readily determined by one skilled in
the art.
12
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
The biomass may be used directly as obtained from a source, or
energy may be applied to the biomass to reduce the size, increase the
exposed surface area and/or increase the availability of cellulose,
hemicellulose, and/or oligosaccharides present in the biomass. Energy
means useful for this purpose include those that do not crush or compact
the biomass, such that the ultrastructure of the biomass is not destroyed.
For example, biomass may be shredded, chopped, or chipped. A jaw
crusher may also be used when used in a manner that shears the biomass
without crushing the ultrastructure. A tooth disk refiner is also useful for
reducing the biomass size prior to treatment in the present methods.
In the present apparatus and treatment methods biomass is moved
into a cylindrical barrel using a non-compacting feeder. If there are two
chambers in the barrel, loading is into the first chamber. In the simplest
case a non-compacting feeder refers to loading biomass by hand. This
method is described in examples herein using an apparatus as set forth in
Figure 1. The non-compacting feeder exemplified in the apparatus of
Figure 2 is a hopper. The hopper may be self-dumping, and/or it may be
equipped with a flow-inducing device that does not provide compacting
force. For example various types of live-bottom bin flow inducers followed
by flow metering conveyors such as various types of drag chains, bucket
elevators, or rotating helixes (such as Acrison devices) may be used.
The amount of biomass loaded in the first cylindrical chamber is limited so
that room is allowed for biomass expansion, which may occur upon
addition of treatment reactant.
Vacuum may be applied to the cylindrical barrel containing
biomass. If there are two chambers in the barrel, vacuum is applied to the
first chamber containing biomass. Typically if applied, the vacuum reduces
the pressure to less than about 20 kPa. At least one reactant is added
(more than one may be added) through one or more ports in the cylindrical
barrel, or the offset. If there are two chambers in the barrel, reactant is
added to the first chamber containing biomass. The present apparatus
may be used in treatment processes that make use of various types of
reactants, as known to one skilled in the art. For example, reactants may
13
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
be acids, bases, organosolvents, oxidizing agents, steam, or various
combinations of these. More suitably, steam may be used alone, or in
combination with an acid, acid and oxidizing agent, base, base and
oxidizing agent, or organosolvent in a reatment process in the present
apparatus.
An example of high concentration biomass treatment with low
ammonia and steam as reactants is described in co-owned and co-
pending US NA 11/402757. Use of the present apparatus in a method of
treatment of high biomass with low ammonia and steam is described in co-
owned and co-pending US patent application CL3950, as follows. An
aqueous solution comprising ammonia is added through one or more ports
in the first cylindrical chamber, or its offset, in an amount so that ammonia
is less than about 12 weight percent relative to dry weight of biomass in
the chamber. More than one port, with ports being distributed, may be
used so that ammonia solution contact is substantially evenly distributed to
the biomass. Also ammonia may be added in an amount that is between
about 4% and about 6% relative to dry weight of biomass in the chamber.
The ammonia solution may be preheated, which will contribute to raising
the temperature of the biomass. In an alternative embodiment, the
aqueous ammonia solution is premixed with the biomass prior to loading
into the first cylindrical chamber. Biomass and aqueous ammonia may be
premixed in a vessel that feeds into the first cylindrical chamber. For
example, aqueous ammonia may be pumped through an inline heater and
into a paddle mixer containing biomass. The biomass and aqueous
ammonia mixture is then fed into the first cylindrical chamber, where
steam is injected after closing off the chamber. Steam is injected to reach
a desired temperature that is between about 85 C and about 180 C. The
reaction time may be between a few minutes and about four hours,
depending on the temperature and the specific biomass used. The
required time decreases as the temperature increases. For low ammonia
treatment, typically suitable conditions for biomass comprising corn cob
include treatment at between about 120 C and about 160 C for between
about 60 minutes to about 5 minutes. Particularly suitable conditions are
14
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
between about 140 C and about 150 C for between about 30 minutes
and about 10 minutes.
A treatment with a high concentration of ammonia is described by
Teymouri et al. (Bioresource Technology, 96:2014-2018 (2005)) where
biomass is treated with anhydrous ammonia at moderate temperatures for
short times. This treatment may be carried out in the present apparatus in
the following manner, as an example. Biomass is loaded without
compacting into a premixer. Water is added to the biomass to bring the
moisture content to about 60%. Liquid ammonia is then added in an
amount equal to the dry weight of biomass. This mixture is transferred to
the first cylindrical chamber of the present single chamber apparatus,
which is then sealed. Steam is injected to bring the chamber temperature
to 90 C. The mixture is held at this temperature for 5 minutes and then
moved by displacement of the piston without compacting through the
discharge orifice to the flash tank. The two-chamber apparatus may be
used as well, with multiple loadings of biomass and non-compacting
movement by piston displacement between the first and second cylindrical
chambers.
An example of an acid treatment process is described in Aden et al.
(National Renewable Energy Laboratory report TP-510-32438 (2002)). In
this process dilute H2SO4 is added to biomass and the mixture is heated
by direct steam injection to the desired temperature. The process may be
carried out as a continuous or batch process. Acid treatment may be used
in the present apparatus in the following manner, as an example. Biomass
is loaded into the first cylindrical chamber of the present single chamber
reactor by non-crushing means, then dilute sulfuric acid (about 1 % or less
w/w) is added through at least one port to achieve a dry biomass
concentration of about 30% in the total mixture. Steam is injected through
a port to bring the chamber temperature to about 190 C. The biomass-
acid mixture is held in the chamber for about 5 minutes, then moved by
displacement with the piston without compacting through the discharge
valve into the flash tank. The two-chamber apparatus may be used as
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
well, with multiple loadings of biomass and non-compacting movement by
piston displacement between the first and second cylindrical chambers.
An example of steam only biomass treatment is described by Lloyd
and Wyman (Appl. Biochem. and Biotechnol. 105: 53-57 (2003) and
Bioresource Technol. 96:1967-1977 (2005)) where corn stover at 50% dry
biomass concentration was loaded into a steam gun reactor, and steam
was injected to bring the mixture to a temperature of 150-210 C. The
mixture was held at temperature for 2-107 minutes, and then explosively
discharged. This type of treatment may be used in the present apparatus
in the following manner, as an example. Biomass in water at about 50%
(w/w) dry biomass concentration is loaded without compacting into the first
cylindrical chamber of the present single chamber apparatus. Steam is
injected through a port into the chamber to bring the chamber temperature
to 210 C. The mixture is held at temperature for 10 min, and then moved
by displacement with the piston without compacting through the discharge
valve into the flash tank. The two-chamber apparatus may be used as
well, with multiple loadings of biomass and non-compacting movement by
piston displacement between the first and second cylindrical chambers.
An example of organosolvent biomass treatment is described in US
4409032 where cellulosic material is treated in a pressure vessel with a
water solvent mixture containing a small amount of mineral acid. After the
treatment, the mixture is rapidly cooled to prevent degradation of sugars to
non-sugars. This type of treatment may be used in the present apparatus
in the following manner, as an example. Biomass is loaded into the first
cylindrical chamber of the present single chamber apparatus and a 60:40
volume ratio of acetone:water containing 0.25% H2SO4 is added through
at least one port. Steam is injected through a port into the chamber to
bring the temperature to about 200 C. The mixture is held at temperature
for 10 min and then moved by displacement with the piston without
compacting through the discharge orifice to the flash tank. Cooling of the
pretreated biomass occurs in the flash tank. The two-chamber apparatus
may be used as well, with multiple loadings of biomass and non-
16
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
compacting movement by piston displacement between the first and
second cylindrical chambers.
An example of alkaline peroxide treatment of lignocellulosic
substrates is described in US 4859283, where a first period of treatment is
with alkaline solution, followed by addition of magnesium ions and
peroxide. The entire treatment occurs between 25 C and 100 C. This
type of treatment may be used in the present apparatus in the following
manner, as an example. Biomass is loaded without compacting into a
premixer and aqueous NaOH is added to a concentration of 10% NaOH
based on dry weight of biomass. Magnesium sulfate is added to reach a
concentration of 0.2% magnesium ion based on initial biomass dry weight,
followed by addition of H202 to reach a concentration of 5% H202 based
on initial biomass dry weight. The well-blended biomass mixture is loaded
without compacting into the first cylindrical chamber of the present single
chamber apparatus which is then sealed. Steam is injected through a port
into the chamber to bring the temperature to about 95 C. The mixed
solids are maintained at 95 C for 1 hour and then moved by displacement
with the piston without compacting through the discharge orifice to the
flash tank. The two-chamber apparatus may be used as well, with multiple
loadings of biomass and non-compacting movement by piston
displacement between the first and second cylindrical chambers.
In all of these treatment methods, multiple ports may be used for
introducing reactant and/or steam to the biomass. Multiple reactants may
be added through one or multiple ports. Reactant may be preheated prior
to its introduction, so that it contributes to raising the temperature of the
biomass. Reactant and biomass may be premixed prior to loading into the
first cylindrical chamber, such as in a vessel that feeds into the first
cylindrical chamber.
For any type of treatment, the present apparatus is constructed of
materials that are compatible with the particular reactant and conditions
used in the treatment. In most cases, the apparatus may be constructed
using carbon steel or stainless steel. However when using acid as a
reactant, materials that withstand the corrosive nature of the acid are
17
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
employed. For example, the reactor chamber may be made using a high
performance alloy such as a Hastelloy . Materials compatibility is well
known to one skilled in the art.
In the present apparatus, the cylindrical barrel may be horizontal as
depicted in Figures 1 and 2, or it may be vertical. With a vertical barrel the
offset and hopper as shown in Figure 2 would be reconfigured to allow
loading of biomass into the barrel chamber, such as at a less than 90
degree angle. One skilled in the art would be able to readily configure the
apparatus with a vertical barrel. For example, the vertical barrel may be
located above the flash tank and be connected without an elbow directing
flow downward, since flow through the discharge valve would already be
directed downward. It is also within the ability of one skilled in the art to
orient the flash tank in a vertical or horizontal manner. The orientation
desired would be one that best fits the specific treatment being used in the
apparatus. For example, a vertical tank is more suitable with ammonia
treatment to facilitate removal and capture of ammonia gas released in the
flash tank.
Since biomass is not compacted in the present apparatus and
methods, it cannot block the passage of steam as occurs in systems with
compacted biomass. Therefore the chamber to which steam is added is
closed off prior to steam injection. Ports, other than the one or more
through which steam is being added, are sealed. The barrel first end
piston, or plug serving as a piston, is put in place and valves are closed.
Valves used may be any opening and closing type of valve, such as
poppet-valves or rotating knife-gate valves.
Steam is added through one or more ports in the first cylindrical
barrel, or the offset, in an amount that is needed to raise the temperature
of the biomass and reactant mixture to the desired point. It is more suitable
to use more than one port, with ports being spaced so that steam contact
is distributed over the biomass. Steam is added to raise the temperature of
the biomass and reactant mixture to between about 85 C and about 300
C. More typical temperatures are between about 85 C and about 275 C,
and depend on the specific treatment method being used in the apparatus.
18
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Some examples of treatments with temperatures used were described
above, and additional treatments are well known to one skilled in the art.
Additional steam may be added through a port in the second cylindrical
chamber when present, if needed to maintain the desired temperature.
The present apparatus may include a heating jacket, steam jacket, band
heaters, or insulation jacket to contribute to raising and/or maintaining the
temperature. Heating or steam jackets are particularly suited to small scale
reactors while insulation jackets are suited to large scale reactors.
Heating may occur at different stages, including preheating the barrel prior
to treating or pretreating.
Different treatments are carried out at different temperatures and
times, as exemplified in descriptions above for specific reactants. The type
of biomass being treated also can affect the optimum time and
temperature for treatment in the present apparatus and methods, as can
readily be assessed by one skilled in the art. A wide range of times and
temperatures may be used in the present apparatus. Function of the batch
feeding cycle as used in the apparatus of Figure 2 requires adequate time
for multiple loadings. It is therefore desirable to choose a time and
temperature combination for a particular treatment that has limited time,
which is long enough for function of the apparatus embodiment used, yet a
moderate temperature to provide an economical process. A benefit of
using moderate temperatures in the present apparatus is that lower
pressure steam, which has lower cost, may be used.
The time that biomass is held at the desired temperature within a
reactor chamber is the residence time. When using an apparatus with only
a first chamber, the residence time takes place in the first chamber. When
using an apparatus with a first chamber and a second chamber, time in the
first chamber may be that which is long enough to combine biomass with
reactant prior to moving the mixture to the second chamber, with the bulk
of residence time occurring in the second chamber. In this case time in the
first chamber may be as little as about 30 seconds, and time in the second
chamber may be between about 2 minutes and 4 hours.
19
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Bringing the biomass to the described temperatures using steam in
the present apparatus results in pressures within the barrel chamber that
are between about 60 kPA and about 8600 kPa. More typical pressures
used are between about 300 kPA and about 4700 kPA, or between about
300 kPA and about 2200 kPA, depending on the treatment reactants used.
In the present apparatus and methods biomass is moved through
the first chamber and, if present, the second chamber, without compaction.
This may be achieved using a piston and impermeable cylinder chamber.
For purposes of the present disclosure, a piston may include any article
that may be used as a piston such as a plug that is pushed into the
chamber, as well as any type of standard piston. The plug of a type of
apparatus as exemplified in Figure 1 may be pushed into the chamber
using any method that applies adequate pressure to move the biomass. A
particularly suitable method is to provide a static closure at the end of the
chamber after inserting the plug, such as a bolted cylinder head, then to
introduce nitrogen between the closure and the plug to build up pressure
and move the plug. The plug may be moved by other means, such as
using a pushrod connected to a hydraulic, pneumatic, or electric actuator.
The barrel of the apparatus is impermeable (with all ports and
valves closed) in that there are no unsealed wall penetrations, so liquid
does not leave the barrel. Retention of liquid allows the piston to move the
biomass without compacting it. Liquid in treatment methods used in the
apparatus is limited, and what is there may serve to lubricate the chamber
walls to enable non-compacting flow in response to piston pressure. In
fact, piston pressure may temporarily slightly squeeze the biomass, as
with a sponge, without being squeezed enough that the biomass pores
and channels are collapsed. Upon removing the piston pressure, the
biomass may reabsorb the liquid into the pores and channels that have not
been crushed. To aid in biomass flow, a lubricating liquid such as
vegetable oil soap may be introduced into the chamber. Flow may be
enhanced by rifling of the internal chamber wall, wherein adding
discontinuities such as angled grooves may reduce friction, thereby
reducing yield stress and improving biomass flow. Movement of biomass
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
without compaction maintains the swollen liquid-filled pores generated by
treatment, which enhances subsequent saccharification.
In the present apparatus, following treatment for the desired time at
the desired temperature, the biomass and reactant mixture is moved
through a discharge valve at the end of the cylindrical barrel into a flash
tank. The discharge valve is closed during biomass reaction with reactant
at the desired temperature, then opened for passage of the biomass. In a
two chamber reactor, as exemplified in Figure 2, the discharge valve
opens in synchronism with the opening of the valve between the first and
second chambers, after the piston has built up pressure in the first
chamber in order to displace the entire contents of the second chamber by
the volume of the first chamber's contents.
Discharge valves which may be used are exemplified by rotary V-
port valves, swingcheck valves, and poppet discharge valves. Particularly
useful in a smaller scale reactor, as exemplified in Figure 1, is a piston-
operated poppet-type discharge valve, where the hardfaced upstream side
of the valve seat is the discharge orifice, and the softer downstream side
of the valve seat seals against a hardfaced valve plunger, with the flow
area increasing continually beyond the valve seat when the valve plunger
is retracted to open.
Most suitably the poppet-type discharge valve would incorporate a
gradual expansion venturi. One embodiment of a gradual expansion
venturi poppet valve, that is suitable for a small scale reactor as
exemplified in Figure 1, is diagrammed in Figure 3. This valve incorporates
a conical nozzle and a seat end shearing valve arrangement. To avoid
plugging, the gradual expansion venturi as exemplified in Figure 3 (closed
position) and Figure 4 (open position) was designed to accelerate solids
through a steadily enlarging gap between the stationary outside cone of
the venturi (50) and the moveable inside cone of the venturi (51) that is
mounted on the end of a valve shaft (52). The venturi outside cone is a
generally-toroidal venturi-shape clamped between a flange (53) at the
reactor chamber (54; equivalent to 10 in Figure 1) exit and a flash tank
inlet flange (55). The venturi inside cone (51) is the nose on the end of the
21
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
reactor exit valve shaft (52). The venturi inside cone and valve shaft are
within the discharge elbow (56; equivalent to 20 in Figure 1) that is within
the flash tank (57; equivalent to 21 in Figure 1). The valve shaft is attached
to an actuator (58) for control of movement. The actuator may be any
device that is able to move the valve shaft back and forth in a horizontal
motion, such as an electric, pneumatic or hydraulic motor, pneumatic valve
actuator, or hydraulic piston. When the valve shaft is in its farthest
leftward
position the outer edge of the inside cone seats against the inner edge of
the outside cone to seal the discharge end of the reactor during treatment.
When it is time to discharge the chamber contents, the valve shaft is
moved to the right to provide the size of opening that is desired for the
flash venturi.
This design provides a flash zone of some length which expands
smoothly in the direction of flow. In this design, biomass solids are
accelerated down the axis of the gradually-opening annular cone, which
avoids allowing sudden radial expansion leading to plugging.
Another embodiment of a gradual expansion venturi, that is suitable
as a discharge valve, particularly in a larger scale apparatus as
exemplified in Figure 2, is diagrammed in Figure 5. This is an embodiment
of a V-port plugcock where the flash venturi expansion is machined into
the valve body. Within the flash venturi stationary body (70) there is a
narrowing (71) from the exit end of the reaction chamber (72) and an
expansion (73) to the entrance to the flash tank (74). In the rotary core
(75) of the plugcock is an angled opening (76) that aligns with the reactor
chamber narrowing (71) and the expansion to the flash tank (73) when in
the open position. The rotary core (75) is turned in a half of a full rotation
to block alignment of the plugcock which closes the valve.
A further embodiment of a gradual expansion venturi, that is
suitable as a discharge valve, particularly in a larger scale reactor as
exemplified in Figure 2, is diagrammed in Figure 6. This is an embodiment
of a swingcheck valve that has a cone (80) which fits into the narrowed
junction (81) between the reactor chamber (72) and the entrance to the
flash tank (74) (Figure 6A). The cone is on an arm (82) that is attached to
22
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
a shaft (83) that extends through a packing gland to a rotary valve
actuator. The shaft is rotated in the direction of the dotted arrow to move
the arm counterclockwise to open the junction, forming a gradual
expansion venturi (Figure 6B). In another embodiment of a swingcheck
valve used for a gradual expansion venturi, the cone may be several feet
in diameter, with the distance moved counterclockwise to open the valve
being only a few inches, which is less that 8 cm.
Biomass and reactant moving through the discharge valve enters a
flash tank, which is able to hold a vacuum. When using a treatment
including ammonia, the ammonia is released from the treated biomass and
the biomass is cooled in the flash tank, in preparation for saccharification.
Any typical flash tank may be used, with one having a tangential or volute
entrance that provides the function of a separating elbow most suitable. It
is particularly suitable to impose flashing several times in sequence at
different pressures to release ammonia from the treated biomass. For
example, a first flash to a pressure near atmospheric typically removes
most of the free ammonia and cools material to about 100 C. A second
flash to a pressure less than about 20 kPa removes the remaining free
ammonia and cools material to a temperature of about 50 C, which is
desired for saccharification.
Ammonia vapor, released in the flash tank from the biomass and
ammonia mixture passed through the discharge valve, may be recovered
from the flash tank, and may be recycled. Vapor from lower-pressure
flashes may be recycled using a standard vapor recompression apparatus
(such as a turbine or a steam jet pump) without intercooling. Thus
ammonia vapor may be recycled directly to treatment without
condensation, or it may be condensed prior to re-use. In the latter case,
collected vapor is fed to a condenser as in Figure 1. Reducing the
ammonia in the treated biomass will lower the pH and reduce the amount
of acid needed to reach a pH that is satisfactory for activity of
saccharification enzymes. This is desirable since the extensive addition of
acid may result in the formation of salts at concentrations that are
inhibitory to saccharification enzymes or to microbial growth. On the other
23
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
hand, ammonia left in the biomass may serve as a nitrogen source to
support growth of microorganisms during fermentation. Thus remaining
ammonia may reduce or eliminate the need to supplement the growth
medium used during fermentation with a nitrogen source. Typically, at
least a portion of the ammonia is removed, which reduces the pH but
leaves some nitrogen that provides this nutrient for use in subsequent
fermentation.
When using other reactants, the flash vapor will include water and
any volatile components that are present. Pretreated biomass is cooled in
the flash tank. As the pretreated biomass accumulates at the bottom of the
flash tank, it may be stirred by a paddle mixer that may be attached at the
bottom of the flash tank. Pretreated biomass is removed from the flash
tank, typically by opening a cover in the bottom of the tank. A live-bottom
mechanical means for extracting the pretreated biomass continuously is
particularly suitable. For processing of multiple batches of biomass in the
present apparatus, one batch of biomass and reactant may be in the barrel
chamber, while another batch is in the flash tank. In the two chamber
apparatus, batches may concurrently be in both chambers and in the flash
tank. In addition, multiple batches of pretreated biomass may be collected
in the flash tank prior to removal.
Following treatment, the product typically comprises a mixture of
reactant, partially degraded biomass and some fermentable sugars. The
entire pretreated biomass comprising both soluble and insoluble fractions
may be removed from the flash tank and utilized in a saccharification
reaction. Alternatively, some liquid may be drained from the pretreated
biomass mixture prior to saccharification so that the dry weight of biomass
remains high in the saccharification reaction. Excess liquid may be present
following treatment, particularly when large amounts of steam are required
to raise and maintain the temperature of the biomass for treatment. With
some treatments, excess liquid may contain sugars that may be isolated
for use in fermentation, or the liquid containing sugars may be used during
fermentation.
24
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
In another alternative, biomass solids may be recycled through
treatment in the present apparatus and methods.
Saccharification
Pretreated biomass from the present apparatus is further hydrolyzed in
the presence of saccharification enzymes, which may be referred to as a
saccharification enzyme consortium, to release oligosaccharides and/or
monosaccharides in a hydrolyzate. Saccharification enzymes and
methods for biomass treatment are reviewed in Lynd, L. R., et al.
(Microbiol. Mol. Biol. Rev. (2002) 66:506-577).
Prior to saccharification, the pretreated biomass may be treated to
alter the pH, composition or temperature such that the enzymes of the
saccharification enzyme consortium will be active. The pH may be altered
through the addition of acids or bases in solid or liquid form. Alternatively,
carbon dioxide (C02), which may be recovered from fermentation, may be
utilized to lower the pH. For example, C02 may be collected from a
fermenter and fed into the pretreatment product headspace in the flash
tank or bubbled through the pretreated biomass if adequate liquid is
present while monitoring the pH, until the desired pH is achieved. The
temperature may be brought to a temperature that is compatible with
saccharification enzyme activity, as noted below. Any cofactors required
for activity of enzymes used in saccharification may be added.
The saccharification enzyme consortium comprises one or more
enzymes selected primarily, but not exclusively, from the group
"glycosidases" which hydrolyze the ether linkages of di-, oligo-, and
polysaccharides and are found in the enzyme classification EC 3.2.1.x
(Enzyme Nomenclature 1992, Academic Press, San Diego, CA with
Supplement 1(1993), Supplement 2 (1994), Supplement 3 (1995,
Supplement 4 (1997) and Supplement 5[in Eur. J. Biochem. (1994) 223:1-
5, Eur. J. Biochem. (1995) 232:1-6, Eur. J. Biochem. (1996) 237:1-5, Eur.
J. Biochem. (1997) 250:1-6, and Eur. J. Biochem. (1999) 264:610-650,
respectively]) of the general group "hydrolases" (EC 3.). Glycosidases
useful in the present method can be categorized by the biomass
component that they hydrolyze. Glycosidases useful for the present
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
method include cellulose-hydrolyzing glycosidases (for example,
cellulases, endoglucanases, exoglucanases, cellobiohydrolases, R-
glucosidases), hemicellulose-hydrolyzing glycosidases (for example,
xylanases, endoxylanases, exoxylanases, P-xylosidases,
arabinoxylanases, mannases, galactases, pectinases, glucuronidases),
and starch-hydrolyzing glycosidases (for example, amylases, a-amylases,
P-amylases, glucoamylases, a- glucosidases, isoamylases). In addition, it
may be useful to add other activities to the saccharification enzyme
consortium such as peptidases (EC 3.4.x.y), lipases (EC 3.1.1.x and
3.1.4.x), ligninases (EC 1.11.1.x), and feruloyl esterases (EC 3.1.1.73) to
help release polysaccharides from other components of the biomass. It is
well known in the art that microorganisms that produce polysaccharide-
hydrolyzing enzymes often exhibit an activity, such as cellulose
degradation, that is catalyzed by several enzymes or a group of enzymes
having different substrate specificities. Thus, a "cellulase" from a
microorganism may comprise a group of enzymes, all of which may
contribute to the cellulose-degrading activity. Commercial or non-
commercial enzyme preparations, such as cellulase, may comprise
numerous enzymes depending on the purification scheme utilized to
obtain the enzyme. Thus, the saccharification enzyme consortium of the
present method may comprise enzyme activity, such as "cellulase",
however it is recognized that this activity may be catalyzed by more than
one enzyme.
Saccharification enzymes may be obtained commercially, such as
Spezyme CP cellulase (Genencor International, Rochester, NY) and
Multifect xylanase (Genencor). In addition, saccharification enzymes may
be produced biologically, including using recombinant microorganisms.
One skilled in the art would know how to determine the effective
amount of enzymes to use in the consortium and adjust conditions for
optimal enzyme activity. One skilled in the art would also know how to
optimize the classes of enzyme activities required within the consortium to
obtain optimal saccharification of a given pretreatment product under the
selected conditions.
26
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Preferably the saccharification reaction is performed at or near the
temperature and pH optima for the saccharification enzymes. The
temperature optimum used with the saccharification enzyme consortium in
the present method ranges from about 15 C to about 100 C. In another
embodiment, the temperature optimum ranges from about 20 C to about
80 C. The pH optimum can range from about 2 to about 11. In another
embodiment, the pH optimum used with the saccharification enzyme
consortium in the present method ranges from about 4 to about 10.
The saccharification can be performed for a time of about several
minutes to about 120 hr, and preferably from about several minutes to
about 48 hr. The time for the reaction will depend on enzyme
concentration and specific activity, as well as the substrate used and the
environmental conditions, such as temperature and pH. One skilled in the
art can readily determine optimal conditions of temperature, pH and time
to be used with a particular substrate and saccharification enzyme(s)
consortium.
The saccharification can be performed batch-wise or as a
continuous process. The saccharification can also be performed in one
step, or in a number of steps. For example, different enzymes required for
saccharification may exhibit different pH or temperature optima. A primary
treatment can be performed with enzyme(s) at one temperature and pH,
followed by secondary or tertiary (or more) treatments with different
enzyme(s) at different temperatures and/or pH. In addition, treatment with
different enzymes in sequential steps may be at the same pH and/or
temperature, or different pHs and temperatures, such as using
hemicellulases stable and more active at higher pHs and temperatures
followed by cellulases that are active at lower pHs and temperatures.
The degree of solubilization of sugars from biomass following
saccharification can be monitored by measuring the release of
monosaccharides and oligosaccharides. Methods to measure
monosaccharides and oligosaccharides are well known in the art. For
example, the concentration of reducing sugars can be determined using
the 1,3-dinitrosalicylic (DNS) acid assay (Miller, G. L., Anal. Chem. (1959)
27
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
31:426-428). Alternatively, sugars can be measured by HPLC using an
appropriate column as described herein in the General Methods section.
Fermentation
Fermentable sugars released from biomass can be used by suitable
microorganisms to produce target chemicals. Following saccharification,
but prior to fermentation, the saccharification mixture may be concentrated
by evaporation, for example, to increase the concentration of fermentable
sugars. Optionally, liquid in the saccharification product may be separated
from solids in a batch or continuous method. Optionally, the liquid or the
entire saccharification product may be sterilized prior to fermentation.
Depending on the microorganism(s) used during fermentation and the pH
used during saccharification, the pH may be adjusted to that suitable for
fermentation. In addition, the saccharification mixture may be
supplemented with additional nutrients required for microbial growth.
Supplements may include, for example, yeast extract, specific amino
acids, phosphate, nitrogen sources, salts, and trace elements.
Components required for production of a specific product made by a
specific biocatalyst may also be included, such as an antibiotic to maintain
a plasmid or a cofactor required in an enzyme catalyzed reaction. Also
additional sugars may be included to increase the total sugar
concentration. The saccharification mixture may be used as a component
of a fermentation broth, for example, making up between about 100% and
about 10% of the final medium
Temperature and/or headspace gas may also be adjusted,
depending on conditions useful for the fermentation microorganism(s).
Fermentation may be aerobic or anaerobic. Fermentation may occur
subsequent to saccharification, or may occur concurrently with
saccharification by simultaneous saccharification and fermentation (SSF).
SSF can keep the sugar levels produced by saccharification low, thereby
reducing potential product inhibition of the saccharification enzymes,
reducing sugar availability for contaminating microorganisms, and
improving the conversion of pretreated biomass to monosaccharides
and/or ol igosaccharides.
28
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Target chemicals that may be produced by fermentation include, for
example, acids, alcohols, alkanes, alkenes, aromatics, aldehydes,
ketones, biopolymers, proteins, peptides, amino acids, vitamins,
antibiotics, and pharmaceuticals. Alcohols include, but are not limited to
methanol, ethanol, propanol, isopropanol, butanol, ethylene glycol,
propanediol, butanediol, glycerol, erythritol, xylitol, and sorbitol. Acids
include acetic acid, lactic acid, propionic acid, 3-hydroxypropionic, butyric
acid, gluconic acid, itaconic acid, citric acid, succinic acid and levulinic
acid. Amino acids include glutamic acid, aspartic acid, methionine, lysine,
glycine, arginine, threonine, phenylalanine and tyrosine. Additional target
chemicals include methane, ethylene, acetone and industrial enzymes.
The fermentation of sugars to target chemicals may be carried out
by one or more appropriate biocatalysts in single or multistep
fermentations. Biocatalysts may be microorganisms selected from
bacteria, filamentous fungi and yeast. Biocatalysts may be wild type
microorganisms or recombinant microorganisms, and include Escherichia,
Zymomonas, Saccharomyces, Candida, Pichia, Streptomyces, Bacillus,
Lactobacillus, and Clostridium. In another embodiment, biocatalysts may
be selected from the group consisting of recombinant Escherichia coli,
Zymomonas mobilis, Bacillus stearothermophilus, Saccharomyces
cerevisiae, Clostridia thermocellum, Thermoanaerobacterium
saccharolyticum, and Pichia stipitis
Many biocatalysts used in fermentation to produce target chemicals
have been described and others may be discovered, produced through
mutation, or engineered through recombinant means. Any biocatalyst that
uses fermentable sugars produced from saccharification of pretreated
biomass using the present apparatus and methods may be used to make
the target chemical(s) that it is known to produce by fermentation.
Particularly of interest are biocatalysts that produce biofuels
including ethanol and butanol. For example, fermentation of carbohydrates
to acetone, butanol, and ethanol (ABE fermentation) by solventogenic
Clostridia is well known (Jones and Woods (1986) Microbiol. Rev. 50:484-
524). A fermentation process for producing high levels of butanol, also
29
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
producing acetone and ethanol, using a mutant strain of Clostridium
acetobutylicum is described in US 5192673. The use of a mutant strain of
Clostridium beijerinckii to produce high levels of butanol, also producing
acetone and ethanol, is described in US 6358717. Co-owned and co-
pending patent applications WO 2007/041269 and WO 2007/050671,
disclose the production of 1-butanol and isobutanol, respectively, in
genetically engineered microbial hosts. Co-owned and co-pending US
patent applications #11/741892 and #1 1 /741 91 6 disclose the production of
2-butanol in genetically engineered microbial hosts. Isobutanol, 1-butanol
or 2-butanol may be produced from fermentation of hydrolysate produced
using the present apparatus and methods by a microbial host following the
disclosed methods.
Genetically modified strains of E. coli have also been used as
biocatalysts for ethanol production (Underwood et al., (2002) Appl.
Environ. Microbiol.68:6263-6272). A genetically modified strain of
Zymomonas mobilis that has improved production of ethanol is described
in US 2003/0162271 Al. A further engineered ethanol-producing strain of
Zymomonas mobilis and its use for ethanol production are described in co-
owned and co-pending US patent applications 60/847813 and 60/847856,
respectively. Ethanol may be produced from fermentation of hydrolysate
produced using the present apparatus and methods by Zymomonas
mobilis following the disclosed methods.
Lactic acid has been produced in fermentations by recombinant
strains of E. coli (Zhou et al., (2003) Appl. Environ. Microbiol. 69:399-407),
natural strains of Bacillus (US20050250192), and Rhizopus oryzae (Tay
and Yang (2002) Biotechnol. Bioeng. 80:1-12). Recombinant strains of E.
coli have been used as biocatalysts in fermentation to produce 1,3
propanediol (US 6013494, US 6514733), and adipic acid (Niu et al., (2002)
Biotechnol. Prog. 18:201-211). Acetic acid has been made by fermentation
using recombinant Clostridia (Cheryan et al., (1997) Adv. Appl. Microbiol.
43:1-33), and newly identified yeast strains (Freer (2002) World J.
Microbiol. Biotechnol. 18:271-275). Production of succinic acid by
recombinant E. coli and other bacteria is disclosed in US 6159738, and by
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
mutant recombinant E. coli in Lin et al., (2005) Metab. Eng. 7:116-127).
Pyruvic acid has been produced by mutant Torulopsis glabrata yeast (Li et
al., (2001) Appl. Microbiol. Technol. 55:680-685) and by mutant E. coli
(Yokota et al., (1994) Biosci. Biotech. Biochem. 58:2164-2167).
Recombinant strains of E. coli have been used as biocatalysts for
production of para-hydroxycinnamic acid (US20030170834) and quinic
acid (US20060003429).
A mutant of Propionibacterium acidipropionici has been used in
fermentation to produce propionic acid (Suwannakham and Yang (2005)
Biotechnol. Bioeng. 91:325-337), and butyric acid has been made by
Clostridium tyrobutyricum (Wu and Yang (2003) Biotechnol. Bioeng.
82:93-102). Propionate and propanol have been made by fermentation
from threonine by Clostridium sp. strain 17cr1 (Janssen (2004) Arch.
Microbiol. 182:482-486). A yeast-like Aureobasidium pullulans has been
used to make gluconic acid (Anantassiadis et al., (2005) Biotechnol.
Bioeng. 91:494-501), by a mutant of Aspergillis niger (Singh et al., (2001)
Indian J. Exp. Biol. 39:1136-43). 5-keto-D-gluconic acid was made by a
mutant of Gluconobacter oxydans (Elfari et al., (2005) Appl Microbiol.
Biotech. 66:668-674), itaconic acid was produced by mutants of
Aspergillus terreus (Reddy and Singh (2002) Bioresour. Technol. 85:69-
71), citric acid was produced by a mutant Aspergillus niger strain (Ikram-
Ul-Haq et al., (2005) Bioresour. Technol. 96:645-648), and xylitol was
produced by Candida guilliermondii FTI 20037 (Mussatto and Roberto
(2003) J. Appl. Microbiol. 95:331-337). 4-hydroxyvalerate-containing
biopolyesters, also containing significant amounts of 3-hydroxybutyric acid
3-hydroxyvaleric acid, were produced by recombinant Pseudomonas
putida and Ralstonia eutropha (Gorenflo et al., (2001) Biomacromolecules
2:45-57). L-2,3-butanediol was made by recombinant E. coli (Ui et al.,
(2004) Lett. Appl. Microbiol. 39:533-537).
Production of amino acids by fermentation has been accomplished
using auxotrophic strains and amino acid analog-resistant strains of
Corynebacterium, Brevibacterium, and Serratia. For example, production
of histidine using a strain resistant to a histidine analog is described in
31
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Japanese Patent Publication No. 8596/81 and using a recombinant strain
is described in EP 136359. Production of tryptophan using a strain
resistant to a tryptophan analog is described in Japanese Patent
Publication Nos. 4505/72 and 1937/76. Production of isoleucine using a
strain resistant to an isoleucine analog is described in Japanese Patent
Publication Nos. 38995/72, 6237/76, 32070/79. Production of
phenylalanine using a strain resistant to a phenylalanine analog is
described in Japanese Patent Publication No. 10035/81. Production of
tyrosine using a strain requiring phenylalanine for growth, resistant to
tyrosine (Agr. Chem. Soc. Japan 50 (1) R79-R87 (1976), or a recombinant
strain (EP263515, EP332234), and production of arginine using a strain
resistant to an L-arginine analog (Agr. Biol. Chem. (1972) 36:1675-1684,
Japanese Patent Publication Nos. 37235/79 and 150381/82) have been
described. Phenylalanine was also produced by fermentation in Eschericia
coli strains ATCC 31882, 31883, and 31884. Production of glutamic acid in
a recombinant coryneform bacterium is described in US 6962805.
Production of threonine by a mutant strain of E. coli is described in
Okamoto and Ikeda (2000) J. Biosci Bioeng. 89:87-79. Methionine was
produced by a mutant strain of Corynebacterium lilium (Kumar et al,
(2005) Bioresour. Technol. 96: 287-294).
Useful peptides, enzymes, and other proteins have also been made
by biocatalysts (for example, in US6861237, US6777207, US6228630).
The pretreatment and saccharification of biomass to fermentable
sugars, followed by fermentation of the sugars to a target chemical is
exemplified in Example 5 herein for the production of ethanol from
pretreated corn cobs using Z. mobilis as the biocatalyst for the
fermentation of sugars to ethanol. The method of the present invention
can also be used for the production of 1,3-propanediol from biomass.
Biomass treated using the present apparatus and methods may be
saccharified; following saccharification, E. coli is used to produce 1,3-
propanediol as described in Example 10 of co-owned and co-pending US
application #11/403087.
32
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Target chemicals produced in fermentation by biocatalysts may be
recovered using various methods known in the art. Products may be
separated from other fermentation components by centrifugation, filtration,
microfiltration, and nanofiltration. Products may be extracted by ion
exchange, solvent extraction, or electrodialysis. Flocculating agents may
be used to aid in product separation. As a specific example, bioproduced
1-butanol may be isolated from the fermentation medium using methods
known in the art for ABE fermentations (see for example, Durre, Appl.
Microbiol. Biotechnol. 49:639-648 (1998), Groot et al., Process. Biochem.
27:61-75 (1992), and references therein). For example, solids may be
removed from the fermentation medium by centrifugation, filtration,
decantation, or the like. Then, the 1 -butanol may be isolated from the
fermentation medium using methods such as distillation, azeotropic
distillation, liquid-liquid extraction, adsorption, gas stripping, membrane
evaporation, or pervaporation. Purification of 1,3-propanediol from
fermentation media may be accomplished, for example, by subjecting the
reaction mixture to extraction with an organic solvent, distillation, and
column chromatography (U.S. 5,356,812). A particularly good organic
solvent for this process is cyclohexane (U.S. 5,008,473). Amino acids may
be collected from fermentation medium by methods such as ion-exchange
resin adsorption and/or crystallization.
EXAMPLES
GENERAL METHODS AND MATERIALS
The following abbreviations are used:
"HPLC" is High Performance Liquid Chromatography, "C" is
Centigrade, "kPa" is kiloPascal, "m" is meter, "mm" is millimeter, "kW" is
kilowatt, " m" is micrometer, " L" is microliter, "mL" is milliliter, "L" is
liter,
"min" is minute, "mM" is millimolar, "cm" is centimeter, "g" is gram, "kg" is
kilogram, "wt" is weight, "hr" is hour, "temp" or "T" is temperature,
"theoret"
is theoretical, "pretreat" is pretreatment, "DWB" is dry weight of biomass,
"ASME" is the American Society of Mechanical Engineers, "s.s." is
stainless steel, in" or is inch.
33
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Sulfuric acid, ammonium hydroxide, acetic acid, acetamide, yeast extract,
glucose, xylose, sorbitol, MgSO4.7H2O, phosphoric acid and citric acid
were obtained from Sigma-Aldrich (St. Louis, MO).
Treatment is referred to as pretreatment in the Examples.
Small Barrel Piston Reactor
A small barrel piston reactor (piston/barrel reactor) was constructed
that consisted of a 5.1 cm x 45.7 cm stainless steel barrel equipped with a
piston, oriented horizontally. The piston was sealed to the barrel with four
0-rings and was pressurized with nitrogen on the backside of the piston
during the discharge stroke. The 45.7 cm barrel was equipped with three
multiple use ports allowing application of vacuum, injection of aqueous
ammonia, injection of steam, and insertion of thermocouples for
measurement of temperature inside the barrel. To avoid excess steam
condensation upon steam injection, the outside of the barrel was heated
with three band heaters and insulated with a 2.5 cm thick fiberglass mat
covered with a silicone impregnated fiberglass jacket.
The reactor barrel was directly attached to a 15.2 cm x 61 cm
stainless steel flash tank, oriented vertically. The barrel was isolated from
the flash tank by a conical nozzle and seat end shearing valve
arrangement. The diameter of the end shearing valve die was 3.5 cm.
The backpressure on the conical nozzle and seat was adjusted to about
138 kPa (gauge pressure) of backpressure into a 10.2 cm diameter air
cylinder connected to the cone of the end shear valve. The cone of the
end shearing valve could move back up to 1.6 cm to allow discharge of
particles in the flash tank. An elbow at the outlet of the end shear valve
directed the pretreated solids down into the bottom of the flash tank where
the solids were easily removed by unbolting a domed end flange in the
bottom of the tank. An upper domed flange to the flash tank incorporated
a special outlet fitting with slots machined at right angles to the axis of
the
flash tank which caused released vapors to travel around a corner path to
an exit fitting, helping to prevent carry-over of entrained biomass particles
and water droplets into a vent condenser.
34
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Large Barrel Piston Reactor
A second barrel for the piston reactor (ASME code stamped) was
fabricated with the same 5.1 cm diameter, but a longer 68.6 cm length to
hold additional biomass volume. The piston was sealed to the barrel with
four 0-rings and was pressurized with nitrogen on the backside of the
piston during the discharge stroke. The 68.6 cm barrel was equipped with
eight multiple use ports, 4 each along the top and bottom surfaces,
allowing application of vacuum, injection of aqueous ammonia, injection of
steam, and insertion of thermocouples for measurement of temperature
inside the barrel. The reactor barrel was equipped with a steam jacket for
even heating of the barrel. The reactor barrel was directly attached to a
15.2 cm x 61 cm stainless steel flash tank, oriented vertically. The barrel
was isolated from the flash tank by a conical nozzle and seat end shearing
valve arrangement. The diameter of the end valve shearing die was 3.5
cm. The backpressure on the conical nozzle and seat was adjustable,
with most tests performed using -138 kPa (gauge pressure) of
backpressure into a 10.2 cm diameter air cylinder connected to the cone of
the end shear valve. The cone of the end shearing valve could move back
up to 1.6 cm to allow discharge of particles in the flash tank. An elbow at
the outlet of the end shear valve directed the pretreated solids down into
the bottom of the flash tank where the solids were easily removed by
unbolting a domed end flange in the bottom of the tank. An upper domed
flange to the flash tank incorporated a special outlet fitting with slots
machined at right angles to the axis of the flash tank, which caused
released vapors to travel around a corner path to an exit fitting, helping to
prevent carry-over of entrained biomass particles and water droplets into a
vent condenser. Three electrical band heaters (set at 60 C) and insulation
were added along the flash tank to allow hot pretreated solids to flash into
a heated vessel, better simulating a commercial scale process.
Steam Gun Reactor batch digestion system
The 4-liter steam gun reactor (Autoclave Engineers, Erie, PA) is a
steam-jacketed reactor consisting of a length of 102 mm schedule 80
Hastelloy pipe closed by two ball valves. Additional electrical heaters
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
were placed on all exposed, non-jacketed surfaces of the reactor and
controlled to the pretreatment set point temperature. Direct steam
injection was also used to rapidly bring the biomass up to pretreatment
temperature. Steam pressure was adjusted and controlled to maintain the
desired pretreatment temperature. The bottom of the reactor was necked
down to 51 mm. All pretreated material exited through a replaceable die at
the bottom of the reactor and was collected in a nylon (Hotfill ) 0.21 m3
bag supported within a heavy walled, jacketed, and cooled flash tank.
Pretreatment and Enzymatic Hydrolysis Reactor (PEHReactor)
The 9-L PEHReactor (constructed at NREL, Golden, CO; see co-
pending US patent application 11/402464) has an approximately 15 cm x
51 cm stainless steel reaction vessel and the 3.2-L PEHReactor has a 15
cm x 18 cm stainless steel reaction vessel. Each vessel has an injection
lance extending through the longitudinal center of the reaction vessel for
introduction of processing reactants. The injection lance is connected
using a rotary joint to a port in a cover on one end of the vessel, which has
an additional port for vessel access. Four baffles run the length of the
vessel wall, and are attached perpendicularly to the wall. The baffles and
ceramic attrition media cylinders of 3.2 cm X 3.2 cm (E.R. Advanced
Ceramics, East Palestine, OH), free floating in the vessel, apply
mechanical mixing of biomass and reactant as the vessel is rotated,
promoting assimilation of reactant into the biomass. Seven cylinders are
used in the small reactor and twenty-two in the large reactor. The
PEHReactor is placed on a Bellco Cell-Production Roller Apparatus
(Bellco Technology, Vineland, NJ) which provides a mechanism for
rotation, and the reactor with roller apparatus is housed in a temperature
controlled chamber which provides heat. Vacuum and pressure may be
applied to the reaction vessel by attaching external sources to the lance-
connected port in the cover.
Fed-batch saccharification reactor
The fed-batch saccharification reactor is a 15-L fermentor (B. Braun
Biotech International, Allentown, PA) controlled by a BioStat ED data
control unit and associated control module containing a circulating pump,
36
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
acid and base pumps, solenoid valves, heat exchangers for temperature
control, steam supply, process water, air supply control valves and
filtration, and back pressure control valves and exhaust filters. The
fermentor was equipped with two 11.4 cm diameter three-blade high
efficiency Ligntnin A-310 impellers. The bottom impeller was located 7.6
cm from the reactor bottom (it could not be located any closer due to the
presence of a large seal arrangement near the bottom of the shaft for the
bottom-drive shaft penetration) and the upper impeller was located 22.9
cm from the reactor bottom. The fermentor vessel has a diameter of 19.0
cm and a maximum height of 55.9 cm. Four removable baffles were
installed, each of which has a width of 1.6 cm and a length of 48.3 cm and
extended from the vessel bottom to within - 7.6 cm of the top. Plumbed
into the top and bottom ports on the fermenter system was a pump-around
loop consisting of an APV lobe pump (model M1/028/06), 1-1/2-in (3.81
cm) flexible hoses and a Teflon sight flow indicator. The pump around
loop was isolated from the fermentation vessel with 1-1/2-in (3.81 cm)
Valmicro and SVF full port ball valves with CF8M bodies, 316 s.s. balls,
and PTFE seats. Additionally, a V-port shear valve (Triac Controls) was
located downstream of the lobe pump, prior to the ball valve isolating the
pump from the top port of the fermentor. During the recirculation cycles,
this valve was gradually closed to up to 60 to provide greater shearing of
the recirculating pretreated solids.
Analytical methods
Cellulose quantitation
The amount of cellulose in each starting biomass sample was
determined using methods well known in the art, such as ASTM E1758-01
"Standard method for the determination of carbohydrates by HPLC".
Measurement of sugar, acetamide, lactic acid and acetic acid
content
Soluble sugars (glucose, cellobiose, xylose, galactose, arabinose
and mannose), acetic acid and ethanol in saccharification liquor or
fermentation broth were measured by HPLC (Agilent Model 1100, Agilent
37
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Technologies, Palo Alto, CA) using Bio-Rad HPX-87P and Bio-Rad HPX-
87H columns (Bio-Rad Laboratories, Hercules, CA) with appropriate guard
columns. The sample pH was measured and adjusted to 5-6 with sulfuric
acid if necessary. The sample was then passed through a 0.2 m syringe
filter directly into an HPLC vial. The HPLC run conditions were as follows:
HPX-87P (for carbohydrates):
Injection volume: 10 - 50 L, dependent on concentration and
detector limits
Mobile phase: HPLC grade water, 0.2 m filtered and degassed
Flow rate: 0.6 mL / minute
Column temperature: 80 - 85 C, guard column temperature <60 C
Detector temperature: as close to main column temperature as
possible
Detector: refractive index
Run time: 35 minute data collection plus 15 minute post run (with
possible adjustment for later eluting compounds)
Biorad Aminex HPX-87H (for carbohydrates, acetic acid and
ethanol)
Injection volume: 5-10 L, dependent on concentration and detector
limits
Mobile phase: 0.01 N Sulfuric acid, 0.2 m filtered and degassed
Flow rate: 0.6 mL / minute
Column temperature: 55 C
Detector temperature: as close to column temperature as possible
Detector: refractive index
Run time: 25 - 75 minute data collection
After the run, concentrations in the sample were determined from standard
curves for each of the compounds.
Examgle 1
Pretreatment of cob in the small barrel giston reactor
Whole corn cobs were processed with a jaw crusher (2.2 kW motor)
with a jaw spacing of approximately 0.95 cm, followed by a delumper (1.5
38
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
kW motor, Franklin Miller Inc., Livingston, NJ), followed by screening with
a Sweco screen equipped with a 1.9 cm U.S. Standard screen to fracture
the whole cobs into smaller pieces. The small barrel piston reactor
(described in General Methods) was charged with 115 g (dry weight basis)
fractured cobs, by hand placing of cobs into the end of the reactor with the
piston removed. The piston was replaced to plug the end. A vacuum was
applied to the reactor vessel to bring the reactor pressure to < 10 kPa (0.1
bar), and dilute ammonium hydroxide solution was injected to give an
ammonia concentration of either 4 g or 6 g per 100 g dry weight of
biomass (as given in Table 1) and a dry weight of biomass concentration
of 50 g per 100 g total biomass-aqueous ammonia mixture. After the
ammonia solution was charged, steam was injected to bring the
temperature to 145 C inside the reactor. The biomass was held at
temperature for 20 minutes and then discharged into the flash tank by
activating the piston. During the 20 minute pretreatment, temperature was
monitored and steam was added as necessary to maintain temperature.
Pretreated cobs were harvested through the bottom of the flash tank.
Excess free liquid was removed and remaining solids were used in
saccharification.
For saccharification, about 470 g of pretreated biomass was added
to the 3.2-L PEHR reactor described in General Methods. The pH of the
contents was adjusted to approximately 5.5 by injecting 1 M citric acid
buffer at pH 4.8 plus adding citric acid monohydrate. Once the desired pH
was reached, 12.9 mg/g cellulose or 25.8 mg/g cellulose of Spezyme CP
cellulase (Genencor International, Rochester, NY) and 4.2 mg active
protein /g cellulose or 8.4 mg active protein /g cellulose of hemicellulase
enzyme consortium (Diversa; San Diego, CA) consisting of P-glucosidase,
xylanase, P-xylosidase and arabinofuranosidase were loaded into the
reactor. Buffer, enzymes and water were added such that the final mixture
in the reactor consisted of 23 g dry biomass/1 00 g pretreated biomass-
saccharification enzyme consortium mixture. The reactor remained in an
incubator at 50 C rolling at 19 rpm for 72 hr. Yields given in Table 1 below
are the release as percent of theoretical yield.
39
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Table 1: Yields following saccharification of cob pretreated in the small
barrel piston reactor.
Enzyme Monomer Total Monomer Total
Spezyme
Ammonia CP consortium Glucose Glucose Xylose Xylose
(g/100 g (Diversa) Release Release Release Release
(mg/g
DWB) cellulose) (mg/g (% (% (% (%
cellulose) theoret) theoret) theoret) theoret)
4 25.8 8.4 78 90 50 80
6 12.9 4.2 65 77 48 72
Example 2
Pretreatment in the large barrel piston reactor at different times
Steam was added to the jacket of the barrel to preheat the barrel of
the large barrel piston reactor (described in General Methods) to -130 C.
The flash receiver was preheated to -60 C with band heaters. Fractured
cobs were prepared as described in Example 1. These cobs (175 g, dry
weight basis) were loaded into the large barrel reactor, by hand placing of
cobs into the end of the reactor with the piston removed. The piston was
replaced to plug the end. A vacuum was applied to the reactor vessel and
to the flash receiver to bring the pressure down < 10 kPa, and dilute
ammonium hydroxide solution was injected into the reactor to give an
ammonia concentration of 6 g/100 g dry weight of biomass and a dry
weight of biomass concentration of 45 g/100 g total biomass-aqueous
ammonia mixture. Once the ammonia was charged, steam was injected
into the reactor to bring the temperature to 145 C. The mixture was held
at this temperature for 10 or 20 minutes by monitoring the temperature and
adding steam as necessary and then discharged into the preheated flash
tank by activating the piston. Vacuum was pulled on the flash tank until
the flash receiver reached - 59 C. Three 10 minute pretreatments and six
20 minute pretreatments were carried out, with all material pretreated for
the same length of time pooled at the end. Upon harvest from the flash
receiver, free liquid was separated from the pretreated solids and not
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
added back for saccharification. A sample of the pretreated cob was
subsequently saccharified as described in Example 1 in the small
PEHReactor. All saccharifications were done with 12.9 mg/g cellulose of
Spezyme CP cellulase and 4.2 mg active protein/g cellulose of
hemicellulase enzyme consortium (Diversa) containing xylanase, R-
xylosidase, arabinofuranosidase and P-glucosidase at 50 C and pH 5.5 for
72 hr. Yields given in Table 2 below are the release as percent of
theoretical yield.
Table 2: Yields following saccharification of cob pretreated in the large
barrel piston reactor.
Monomer Total Monomer Total Xylose
Pretreatment Glucose Glucose Xylose Release Release
time (min) Release Release (% theoret) (% theoret)
(% theoret) (% theoret)
10 68.2 79.5 32.1 77.0
68.0 83.2 39.1 84.3
Examgle 3
Pretreatment in large barrel piston reactor compared to steam gun
15 Size-reduced cobs were prepared as described in Example 1.
Pretreatment in the large barrel piston reactor was carried out as
described in Example 2. For pretreatment in the steam gun, cobs were
first loaded into a 9-L PEHReactor. The reactor was cooled to 4 C by
rotation in contact with ice on the outer surface. A vacuum was applied to
20 the vessel, and dilute ammonium hydroxide solution that was pre-cooled in
a cold room at 4 C and passed through tubing immersed in an ice-water
bath was injected to give an ammonia concentration of 6 g/100 g dry
weight of biomass and a dry weight of biomass concentration of 45 g/100
g total biomass-aqueous ammonia mixture. The PEHReactor charged
with ammonia and cob was cooled to 4 C by applying ice to the surface of
the rotating reactor vessel and rotated at 4 C for 30 min. At this time the
contents were transferred to the steam gun reactor that is described in
41
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
General Methods. Once the steam gun reactor was charged with the
ammonia-cob mixture, the temperature was increased to 145 C by direct
injection of steam. The cob-ammonia mixture was held at this
temperature for 20 min, and then the mixture was discharged into a flash
tank.
Samples of pretreated cob were taken from both the large barrel
piston reactor and steam gun reactor, and saccharified as described in
Example 1. Saccharifications were carried out with 12.9 mg/g cellulose
Spezyme CP cellulase (Genencor) and 4.2 mg active protein /g cellulose
of hemicellulase enzyme consortium (Diversa) consisting of P-glucosidase,
xylanase, P-xylosidase and arabinofuranosidase. The reactor remained in
the incubator at 50 C and 19 rpm for 72 hr. Resulting glucose yields for
pretreatment in each reactor are shown in Table 3 below.
Table 3: Yields following saccharification of cobs pretreated in either the
large barrel piston reactor or steam gun.
Monomer Total Monomer Total
Pretreat Pretreat
Pretreat DWB ment ment Glucose Glucose Xylose Xylose
conc in Release Release Release Release
ment time tempera
reactor reactor (min) ture ( C) (% (% (% (%
theoret) theoret) theoret) theoret)
piston
50% 20 145 68.0 83.2 39.1 84.3
reactor
Steam
60% 40 150 65 77 48 82
gun
Example 4
Pretreatment of corn cob and fiber blends in large barrel piston reactor
Fractured corn cobs were prepared as described in Example 1.
Fractured cobs alone and fractured cobs blended with Cargill Bran 80
(Cargill, Minnetonka, MN) were pretreated in the large barrel piston
reactor. Fractured cobs and Cargill Bran 80 corn fiber were combined such
that the fiber was approximately 33% of the total dry biomass of the mixed
42
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
sample. In each case 175 g (dry weight basis) feedstock was added to the
reactor. Pretreatment was carried out essentially as described in Example
2. However, in these experiments, after addition of ammonia solution, the
reactor contents were held for 10 min before injecting steam to bring the
temperature to 145 C. After steam injection, temperature was held for 10
min at 145 C by adding steam when necessary. Following the
pretreatment, the sample was discharged into a flash tank with activation
of the piston.
Samples of the pretreated cob and cob-fiber blend were taken from
the flash tank of the large barrel piston reactor and saccharified in small
PEHReactors as described in Example 1. Biomass was added such that
20% of the reactor volume was filled. Saccharifications were carried out
with 12.9 mg/g cellulose of Spezyme CP cellulase (Genencor) and 15 mg
/g cellulose of Multifect xylanase (Genencor). The PEHReactors remained
in the incubator at 50 C and 19 rpm for 72 hr. Resulting glucose and
xylose yields for pretreatment are shown in Table 4 below.
Table 4: Yields following saccharification of cob and cob/bran samples
pretreated in the large barrel piston reactor.
Monomer Total Monomer Total
Feedstock DWB conc Glucose Glucose Xylose Xylose
in reactor Release Release Release Release
(% theoret) (% theoret) (% theoret) (% theoret)
Cob only 45% 40.2 67.2 29.4 83.9
Cob + bran
45% 37.0 65.4 21.6 77.2
20 Example 5
Production of ethanol from corn cobs pretreated in the large barrel piston
reactor
Pretreatment of corn cobs was carried out for 10 minutes as
described in Example 2. A total of 17 such pretreatments were carried
25 out. Pretreated cobs from 4 pretreatments were pooled for
saccharification to provide initial hydrolysate for the fed-batch
43
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
saccharification. Pretreated cobs from the remaining 13 runs were pooled
for use in the fed-batch saccharification.
To start the fed-batch saccharification, the fed-batch
saccharification reactor described in General Methods was first loaded
with hydrolysate to fill the reactor volume up to the bottom of the first
impeller. This hydrolyzate was prepared by saccharifying pretreated cobs
in 2.8-L shake flasks. These shake flasks were loaded with 465 g
pretreated solids, 1000 ml DI water, and enzymes at 28.4 mg Spezyme
CP /g cellulose and 4.2 mg active protein /g cellulose hemicellulase
enzyme consortium (Diversa) comprising P-glucosidase, xylanase, P-
xylosidase and arabinofuranosidase. Prior to enzyme addition, pH was
adjusted to 5 with 8.5% H3PO4. The shake flasks were maintained at
50 C and 150 rpm in a rotary shaker for 48 hr, at which time the
hydrolysate was loaded into the fed-batch reactor.
Once the initial hydrolysate was loaded, the first aliquot of the
pretreated biomass-ammonia mixture (- 700 g) was added to the reactor.
The pH was maintained at a setpoint of 5.5 by addition of 8.5% H3PO4.
Once the pH readjusted to the setpoint, 28.4 mg of Spezyme CP /g
cellulose and 4.2 mg active protein /g cellulose of hemicellulase enzyme
consortium (Diversa) comprising P-glucosidase, xylanase, P-xylosidase
and arabinofuranosidase were added. Additional aliquots of the pretreated
biomass-ammonia mixture, Spezyme CP cellulase and hemicellulase
enzyme consortium were added at t = 4, 8, 12, 22, 26, 30 and 34 hr. The
pump around loop was generally started about 1 hr after enzyme addition
and was run for about 1 hr up through the 22 hr solids addition. After the
26 hr and 30 hr additions, the pump was started about 50 min after
enzyme addition and run for 30 minutes. After the 34 hr addition, the
pump was started -3 hr after enzyme addition and run for 30 minutes.
The pump was also run for 30 minutes at t = 29, 33, 47 and 49 hr. Total
saccharification time was 120 hr. At this time, hydrolysate contained - 60
g/L monomer glucose, 25 g/L monomer xylose and 10 g/L acetic acid.
This hydrolyzate was used for fermentation of Zymomonas mobilis
strains ZW800 or ZW658 (ATCC # PTA-7858). ZW658 is a strain of
44
CA 02692717 2010-01-05
WO 2009/045652 PCT/US2008/073416
Zymomonas mobilis that has been engineered for xylose fermentation to
ethanol and is described in co-owned and co-pending US Patent
Application 60/847813. ZW658 was constructed by integrating two
operons, PgapxylAB and Pgaptaltkt, containing four xylose-utilizing genes
encoding xylose isomerase, xylulokinase, transaldolase and transketolase,
into the genome of ZW1 (ATCC #31821) via sequential transposition
events, and followed by adaptation on selective media containing xylose.
ZW800 is the ZW658 strain with the gene encoding glucose-fructose
oxidoreductase inactivated, which is also described in co-owned and co-
pending US Patent Application 60/847813.
Fermentations were carried out in sterilized 1-liter fermentors
(BIOSTATO B-DCU system, Sartorius BBI System Inc., Bethlehem,
Pennsylvania, USA) with 500 ml initial working volume. Inoculum was
added to the fermentor at a level of 10% (v/v) such that the OD600 - 1 in
the broth after addition. Hydrolysate was present at 80% or 40% (v/v),
with the balance as water. Additional glucose and xylose were added to
bring final concentrations in the broth to 92 g/L and 82 g/L, respectively.
Broth was also supplemented with 10 mM sorbitol and 1 g/L MgSO4.7H2O.
Fermentation was carried out for 72 hr at 33 C, pH 5.8 with 150 rpm
agitation. Final ethanol titers for the ZW800 strain were 8 g/L in the 40%
hydrolysate and 7 g/L in the 80% hydrolysate. For ZW658, the final
ethanol titers were 8 g/L in 40% hydrolyzate and 6.5 g/L in 80%
hydrolyzate.