Note: Descriptions are shown in the official language in which they were submitted.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
8-Beta-Substituted Estratrienes as Selectively Active Estrogens
Field of the Invention
This invention relates to new 80-substituted estra-1,3,5(10)-triene
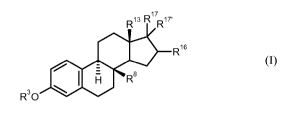
derivatives of general formula
R13 17 R17'
R16
H Rs
R30
(I)
in which radicals R3, Rg, R13 R16 as well as Ri' and Ri'', independently of
one another, have the
following meaning:
3
R means a hydrogen atom or a group Rig, in which
Rig means a straight-chain or branched-chain, saturated or unsaturated
Ci-C6-alkyl radical, a trifluoromethyl group,
an aryl, heteroaryl or aralkyl radical optionally substituted with at least
one
radical independently chosen from a methyl, ethyl, trifluoromethyl,
pentafluoroethyl, trifluoromethylthio, methoxy, ethoxy, nitro, cyano, halo-,
hydroxy, amino,
mono(Ci-Cs-alkyl) or di(Ci-Cs-alkyl)amino whereby both alkyl groups are
identical or different, di(aralkyl)amino whereby both aralkyl groups are
identical
or different, carboxyl, carboxyalkoxy, Ci-Czo-acyl or Ci-Czo-acyloxy as
substituents;
an acyl radical -C(=O)R'9, in which R19 is a straight-chain or branched-chain
of a
Ci-Cio -alkyl radical that is saturated or unsaturated in up to three places
and is
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
2
partially or completely halogenated, or
Rig means a group R20S02, in which
20 2122 21 22
N group, whereby R and R
R is an R R , independently of one another,
23
mean a hydrogen atom, a Ci-CS -alkyl radical, a group -C(=0)R , in
23
which R means a unsubstituted or substituted, straight-chain or
branched-chain Ci-Cio -alkyl radical with that is saturated or unsaturated
in up to three places and is partially or completely halogenated, a
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl group, a
C4-Cis-cycloalkylalkyl radical with 3 to 7 carbon atoms in the cycloalkyl
portion and with an alkyl portion of up to 8 carbon atoms or an aryl,
heteroaryl or aralkyl radical, optionally substituted, with at least
one radical independently chosen from a methyl, ethyl, trifluoromethyl,
pentafluroethyl, trifluoromethylthio, methoxy, ethoxy, nitro, cyano, halo-,
hydroxy, amino, mono(Ci-Cg-alkyl) or di(Ci-Cg-alkyl) amino, whereby
both alkyl groups are identical or different, di(aralkyl)amino, whereby
both aralkyl groups are identical or different, carboxyl, carboxyalkoxy,
Ci-C2o-acyl or Ci -C20-acyloxy as substituents; or,
together with the N atom, a polymethylenimino radical with 4 to 6 carbon
atoms or a morpholino radical,
8
R is a straight-chain or branched-chain alkenyl or alkinyl radical with 2 to 6
carbon atoms,
which optionally can be partially or completely fluorinated,
R13 is a methyl group or an ethyl group,
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
3
16
R is a fluorine atom,
Ri' and Ri'' , in each case independently of one another, are a hydrogen atom
and a hydroxy
group; or
a hydrogen atom and a group RigO-, R20S02- or OC(O)R23 with Rig, R20 and R23
in each
3
case in the meaning that is indicated under R
This invention further relates to the use of the new 80-substituted estra-
1,3,5(10)-triene
as pharmaceutical active ingredients, which have in vitro a higher affinity to
estrogen receptor
preparations from rat prostates than to estrogen receptor preparations from
rat uteri and in vivo a
preferential action in the ovary in comparison to the uterus, their
production, their therapeutic
use and pharmaceutical dispensing forms that contain the new compounds.
The 80-substituted estra-1,3,5(10)-triene derivatives of the present
inventionare new,
steroidal, estrogen receptor subtype -selective estratriene with improved
potency and metabolic
stability.
Background of the Invention
The efficiency of estrogens in the treatment of hormone-deficiency-induced
symptoms
such as hot flashes, atrophy of estrogen target organs and incontinence, as
well as the successful
use of estrogen therapies for prevention of bone mass loss in peri- and
postmenopausal women,
is well documented and generally accepted (Grady et al. 1992, Ann Intern Med
117: 1016-
1037). It is also well documented that estrogen replacement therapy in
postmenopausal women
or in women with ovarian dysfunction that is caused in some other way reduces
the risk of
cardiovascular diseases compared to women who are not treated with estrogen
(Grady et al., loc.
cit.).
In conventional estrogen or hormone replacement therapy (= HRT), natural
estrogens,
such as estradiol, and conjugated estrogens that consist of equine urine are
used either by
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
4
themselves or in combination with a gestagen. Instead of the natural
estrogens, derivatives that
are obtained by esterification, such as, e.g., 17(3-estradiol-valerate, can
also be used.
Because of the stimulating action of the estrogens that are used on the
endometrium,
which results in an increase of the risk of endometrial carcinoma (Harlap, S.
1992, Am J Obstet
Gynecol 166: 1986-1992), estrogen/gestagen combination preparations are
preferably used in
hormone replacement therapy. The gestagenic component in the estrogen/gestagen
combination
avoids hypertrophy of the endometrium, but the occurrence of undesirable
intracyclic menstrual
bleeding is also linked to the gestagen-containing combination.
Selective estrogens represent a more recent alternative to the
estrogen/gestagen
combination preparations. Up until now, selective estrogens have been defined
as those
compounds that have an estrogen-like effect on the brain, bones and vascular
system, owing to
their antiuterotropic (i.e., antiestrogenic) partial action, but they do not
have a proliferative effect
on the endometrium.
A class of substances that partially meet the desired profile of a selective
estrogen is the
so-called "Selective Estrogen Receptor Modulators" (SERM) (R. F. Kauffman, H.
U. Bryant
1995, DNAP 8 (9): 531-539). In this case, these are partial agonists of
estrogen receptor
subtype "ERa." This substance type is ineffective, however, with respect to
the therapy of acute
postmenopausal symptoms, such as, e.g., hot flashes. As an example of a SERM,
the raloxifene
that was recently introduced for the indication of osteoporosis can be
mentioned.
For the treatment of fertility disorders of women, frequently caused by
ovarian
dysfunction that is caused by surgery, medication, etc., new possible
therapies are also opened
up by the use of new selective estrogens. The in-vitro fertility treatment is
a process that has
been established for more than 20 years. Numerous methods for treating ovarian-
induced
infertility with exogenic gonadotropins are known. By administration of
gonadotropins such as
FSH (FSH = follicle-stimulating hormone), a stimulation of the ovaries, which
is to make
possible a healthy follicular maturation, is to be produced.
The follicle is the functional unit of the ovary and has two purposes: it
accommodates
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
the oocytes and provides for the latter the possibility for growth and for
maturation.
Folliculogenesis comprises the development of an ovarian follicle from a
primordial stage to a
continuously increasing antral follicle, which represents the last stage
before ovulation. Only an
optimally developed antral follicle can release a mature oocyte by ovulation.
Patients with ovarian-induced infertility (PCOS = syndrome of polycystic
ovaries) suffer
from a disrupted follicular maturation, which is associated both with hormonal
and ovulatory
disruptions and with inadequately matured oocytes. The number of primary and
secondary
follicles is approximately twice as high here as in the normal ovary
(Hughesden et al., Obstet.
Gynecol. Survey 37, 1982, pp. 59-77).
There are indications that the early development stages of folliculogenesis
(which relates
to the development of primordial follicles to antral follicles) are
gonadotropin-independent. It is
not clearly explained how great the influence of known paracrine and autocrine
factors is on
early folliculogenesis (Elvin et al., Mol. Cell Endocrinol. 13, 1999, pp. 1035-
1048; McNatty et
al., J. Reprod. Fertil. Suppl. 54, 1999, pp. 3-16).
Gonadotropins such as FSH are mainly involved in the last development stages
of
folliculogenesis in follicular maturation, i.e., in the development of the
early antral follicle to a
mature follicle that can undergo ovulation.
The in-vivo and in-vitro infertility is preferably treated with gonadotropins
(FSH and
antiestrogens) (White et al., J. Clin. Endocrinol. Metab. 81, 1996, pp. 3821-
3824). In in-vitro
fertilization treatment, oocytes are removed from preovulatory antral
follicles to be able to
mature in vitro into an oocyte that can be fertilized. After fertilization and
early embryonic
development, one to three embryos are implanted in the uterus of the woman.
In many respects, the treatment with exogenic gonadotropins is accompanied by
numerous risks and side effects. The greatest risk consists in an
overstimulation of the ovaries,
which in severe cases can represent a serious danger to life (OHSS = Ovarian
Hyperstimulation
Syndrome). Other side effects are the high costs of the in-vitro fertility
treatment that must be
paid by the couples. Negative side effects such as weight gain, bloatedness,
nausea, vomiting
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
6
and an as yet unknown long-term risk of developing cancer are attributed to
the gonadotropin
treatment.
One method to avoid the above-mentioned drawbacks and risks is to ensure the
maturation and stimulation in vivo of follicular growth in the case of ovarian-
induced infertility
with a suitable active ingredient before treatment with exogenic gonadotropins
begins.
Estrogen Receptor Beta (ER(3)
Several years ago, estrogen receptor (3 (ER(3) was discovered as a second
subtype of the
estrogen receptor (Kuiper et al. (1996), Proc. Natl. Acad. Sci. 93: 5925-5930;
Mosselman,
Dijkema (1996) Febs Letters 392: 49-53; Tremblay et al. (1997), Molecular
Endocrinology 11:
353-365). The expression pattern of ER(3 differs from that of the ERa (Kuiper
et al. (1996),
Endocrinology 138: 863-870). ER(3 thus predominates over ERa in the rat
prostate, while ERa
predominates over ER(3 in the rat uterus. The highest concentrations of ER(3
and mRNA were
found in the ovaries (Couse et al. Endocrinology 138, 1997, pp. 4612-4613).
Other organ systems with comparatively higher ER(3-expression comprise the
bones
(Onoe, Y. et al., 1997, Endocrinology 138: 4509-4512), the vascular system
(Register, T. C.,
Adams, M. R. 1998, J. Steroid Molec Bio164: 187-191), the urogenital tract
(Kuiper, G. J. M. et
al. 1997, Endocrinology 138: 863-870), the gastrointestinal tract (Campbell-
Thopson 1997,
BBRC 240: 478-483), as well as the testis (Mosselmann, S. et al. 1996 FEBS
Lett. 392, 49-53)
including the spermatides (Shugrue et al. 1998, Steroids 63: 498-504). The
tissue distribution
suggests that estrogens regulation of organ functions via ER(3 is highly
relevant. The fact that
ER(3 is functional in this respect also follows by studies in ERa- (ERKO) or
ER(3-((3ERKO)-
knockout mice: ovariectomy produces bone mass loss in ERKO-mice, which can be
eliminated
by estrogen substitution (Kimbro et al. 1998, Abstract OR7-4, Endocrine
Society Meeting, New
Orleans). Estradiol in the blood vessels of female ERKO mice also inhibits
vascular media and
smooth muscle cell proliferation (lafrati, M. D. et al. 1997, Nature Medicine
3: 545-548). These
protective actions of estradiol are carried out in the ERKO mouse presumably
via ER(3.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
7
The fact that ERa and ER(3 have a functionally different action was confirmed
after
successful production of ERKO and (3ERKO mice. ERa consequently plays an
important role
in the adult uterus, in mammary gland tissue, in the negative regulation of
the gonadotropin
activity, while ER(3 is mainly bonded in the processes of ovarian physiology,
especially that of
folliculogenesis and ovulation (Couse et al., Endocrine Reviews 20, 1999, pp.
358-417).
Observations of (3ERKO mice provide an indication on a function of ER(3 in the
prostate
and bladder: in the case of older male mice, symptoms of prostate and bladder
hyperplasia occur
(Krege, J. H. et al. 1998, Proc Natl Acad Sci 95: 15677-15682). In addition,
female ERKO mice
(Lubahn, D. B. et al. 1993, Proc Natl Acad Sci 90: 11162-11166) and male ERKO
mice (Hess,
R. A. et al. 1997, Nature 390: 509-512) as well as female (3ERKO mice (Krege,
J. H., 1998,
Proc Natl Acad Sci 95: 15677-15682) have fertility disorders. Consequently,
the important
function of estrogens with respect to maintaining testis and ovary functions
as well as fertility is
confirmed.
It is possible to achieve a selective estrogenic action on specific target
organs by subtype-
specific ligands based on the different tissue or organ distribution of the
two subtypes of the
ERs. Substances with a preference for ER(3 compared to ERa in the in-vitro
receptor binding
test were described by Kuiper et al. (Kuiper et al. (1996), Endocrinology 138:
863-870). A
selective action of subtype-specific ligands of the estrogen receptor on
estrogen-sensitive
parameters in vivo was not previously shown.
The patent application WO 01/77139 Al describes 8(3-substituted estratrienes
wherein
R 8 means a straight-chain or branched-chain, optionally partially or
completely halogenated
alkyl or alkenyl radical with up to 5 carbon atoms, an ethinyl- or prop-l-inyl
radical, as
pharmaceutical active ingredients that have in vitro a higher affinity to
estrogen receptor
preparations of rat prostates than to estrogen receptor preparations of rat
uteri, their production,
their therapeutic use and pharmaceutical dispensing forms that contain the
said compounds. The
compound 3-methoxy-8-vinyl-estra-1,3,5(10)-trien-17-one (Example 6) is also
described in
WO01/77139.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
8
Compounds with potent estrogen activity and a higher affinity to estrogen
receptor preparations
of rat prostates than to estrogen receptor preparations of rat uteri for the
production of
medicaments are highly needed in the present technical field.
Object of the Invention
The object of this invention is therefore to prepare compounds that have in
vitro a high
dissociation with respect to the binding to estrogen receptor preparations
from rat prostates and
rat uteri. The compounds are to show in vitro a higher affinity to estrogen
receptor preparations
from rat prostates than to estrogen receptor preparations from rat uteri.
The compounds according to the present invention have a high potent estrogen
activity and
efficacy, namely
a higher binding affinity to rat prostate estrogen receptor and a better
dissociation regarding
binding to rat prostate versus rat uterus estrogen receptor with respect to
the known compounds.
The compounds according to the invention are to produce enhanced fertility in
the ovary while at
the same time affecting the uterus very little in cases of ovarian-associated
infertility.
The advantageous profile of the compounds according to the invention was
achieved by
the specific combination of the substituents R8, R13 R16 Ri' and Ri''.
According to the invention, the object above is achieved by the provision of
80-substituted estra-1,3,5(10)-triene derivatives of general formula I
R13 17 R17'
R16
H Rs
R30
(I)
in which radicals R3, Rg, R'3, R16 as well as Ri' and Ri'~, independently of
one another,
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
9
have the following meaning:
R3 means a hydrogen atom or a group Rig, in which
Rig means a straight-chain or branched-chain, saturated or unsaturated
Ci-C6-alkyl radical, a trifluoromethyl group,
an aryl, heteroaryl or aralkyl radical optionally substituted with at least
one radical independently chosen from a methyl, ethyl, trifluoromethyl,
pentafluoroethyl, trifluoromethylthio, methoxy, ethoxy, nitro, cyano, halo-
, hydroxy, amino, mono(Ci-Cg-alkyl) or di(Ci-Cg-alkyl)amino whereby
both alkyl groups are identical or different, di(aralkyl) amino whereby both
aralkyl groups are identical or different, carboxyl, carboxyalkoxy, Ci-C2o-
acyl or Ci-C2o-acyloxy as substituents;
an acyl radical -C(=O)R'9, in which R19 is a straight-chain or branched-
chain of a Ci-Cio -alkyl radical that is saturated or unsaturated in up to
three places and is partially or completely halogenated, or
Rig means a group R20S02, in which
20 2122 21 22
N group, whereby R and R
R is an R R , independently of one
another, mean a hydrogen atom, a Ci-CS -alkyl radical, a group
23 23
-C(=O)R , in which R means a unsubstituted or substituted,
straight-chain or branched-chain Ci-Cio -alkyl radical that is
saturated or unsaturated in up to three places and is partially or
completely halogenated, a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl or cycloheptyl group, a C4-Cis-cycloalkylalkyl radical
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
with 3 to 7 carbon atoms in the cycloalkyl portion and with an alkyl
portion of up to 8 carbon atoms or an aryl, heteroaryl or aralkyl
radical, optionally substituted with at least
one radical independently chosen from a methyl, ethyl,
trifluoromethyl, pentafluroethyl, trifluoromethylthio, methoxy,
ethoxy, nitro, cyano, halo- , hydroxy, amino, mono(Ci-Cg-alkyl) or
di(Ci-Cg-alkyl) amino, whereby both alkyl groups are identical or
different, di(aralkyl)amino, whereby both aralkyl groups are
identical or different, carboxyl, carboxyalkoxy,
Ci -C20-acyl or Ci -Cz -acyloxy groups as substituents; or,
together with the N atom, a polymethylenimino radical with 4 to 6
carbon atoms or a morpholino radical,
8
R is a straight-chain or branched-chain alkenyl or alkinyl radical with 2 to 6
carbon
atoms, which optionally can be partially or completely fluorinated,
R13 is a methyl group or an ethyl group,
16
R is a fluorine atom,
Ri' and Ri'' , in each case independently of one another, are a hydrogen atom
and a
hydroxy group; or
a hydrogen atom and a group Rig0-, R20S02- or OC(O)R23 with Rig, R20 and R~3
3
in each case in the meaning that is indicated under R
A particular embodiment of the present invention are the compounds of general
formula
I, in which R3 is a hydrogen atom.
According to a further embodiment of the invention, compounds of general
formula I
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
11
comprise R 8 being a vinyl, ethinyl or prop-l-inyl group.
Other possible forms of embodiment of the present invention are defined by the
depending claims.
Compounds of general formula I in which Ri7 and Ri7' are a hydrogen atom and a
hydroxy group atom are further preferred.
Compounds of general formula I in which R16 is in a-position or compounds of
general
formula I in which R16 is in 0-position are equally preferred forms of
embodiment of the
invention.
Furthermore, a particular embodiments of the present invention are the
compounds of
general formula I in which R 8 is a vinyl, ethinyl or prop-l-inyl group, R16
is a fluorine atom, Ri7
and Ri'' are independently from one another a hydrogen atom and a hydroxy
group atom.
Compounds of general formula I in which Ri7 and/or Ri7' are a hydrogen atom
and a
group Rig-O- or R19S02-O- with Rig and R'9 in each case in the meaning that is
indicated under
R3 are further particular form of embodiment of the present invention.
17
Another variant of the invention in particular calls for compounds in which R
stands for
a group R1g0- or R20 SOz-O- with R1g and R20
in each case in the meaning that is indicated under
3
R.
Compounds according to the present invention are:
8(3-vinyl-l6a-fluoro-estra-1,3,5(10)-triene-3,17a-diol
8(3-vinyl-l6a-fluoro-estra-1,3,5(10)-triene-3,17(3-diol
8(3-vinyl-16(3-fluoro-estra-1,3,5(10)-triene-3,17(3-diol
As halo- or halogen, a fluorine, chlorine, bromine or iodine atom is intended
according to the
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
12
present invention
As alkyl radical is generally intended a(Ci-C6)alkyl radical, as far as it is
not differently
specified, said alkyl radical being a straight or branched chain, saturated or
unsaturated.
Representative groups for an alkyl radical according to the present invention
are methyl, ethyl,
n- propyl, 1-methylethyl (iso-propyl), n-butyl, n-pentyl, 1, 1 -dimethylethyl
(t-butyl) and n-hexyl.
The Ci-C6-alkyl radical can be partially or completely substituted by halogen
atoms, hydroxy
groups or Ci-C6-alkoxy groups.
is
According to the above definition R is, for example, a methyl, ethyl, propyl,
isopropyl,
butyl, isobutyl, tert.-butyl, pentyl, isopentyl, neopentyl, or hexyl radical.
18
Alkoxy groups OR in the compounds of general formula I in each case can
contain an
alkyl radical according to the definition given above, whereby methoxy,
ethoxy, propoxy,
isopropoxy and t-butyloxy groups are preferred alkoxy radical.
Representatives of the Ci-CS-alkyl radicals R21 and R22 are methyl, ethyl,
propyl,
isopropyl, butyl, isobutyl, tert-butyl, pentyl, isopentyl and neopentyl.
As representatives of straight-chain or branched-chain Ci-Cio -alkyl radicals
R23, for
example, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl,
pentyl, isopentyl, neopentyl,
heptyl, hexyl, and decyl can be mentioned; methyl, ethyl, propyl and isopropyl
are preferred.
As a C3-C7-cycloalkyl group, a cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl or
cycloheptyl group can be mentioned.
A C4-Cis-cycloalkylalkyl radical has 3 to 7 carbon atoms in the cycloalkyl
portion;
typical representatives are the cycloalkyl groups that are mentioned directly
above. The alkyl
portion has up to 8 carbon atoms.
As examples of a C4-Cis-cycloalkylalkyl radical, the cyclopropylmethyl,
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
13
cyclopropylethyl, cyclopentylmethyl, cyclopentylpropyl groups, etc., can be
mentioned.
In terms of this invention, an aryl radical is a phenyl, 1- or 2-naphthyl
radical; the phenyl
radical is preferred.
Examples of a heteroaryl radical according to the present invention are the 2-
, 3- or 4-
pyridinyl, the 2- or 3-furyl, the 2- or 3-thienyl, the 2-or 3-pyrrolyl, the 2-
, 4- or 5-imidazolyl, the
pyrazinyl, the 2-, 4- or 5-pyrimidinyl or 3- or 4-pyridazinyl radical.
As substituents that can be present on an aryl or heteroaryl radical, for
example, a
methyl-, ethyl-, trifluoromethyl-, pentafluoroethyl-, trifluoromethylthio-,
methoxy-, ethoxy-,
nitro-, cyano-, halogen- (fluorine, chlorine, bromine, iodine), hydroxy-,
amino-,
mono(Ci-Cg-alkyl) or di(Ci-Cg-alkyl)amino, whereby both alkyl groups are
identical or different,
di(aralkyl)amino, whereby both aralkyl groups are identical or different,
carboxyl,
carboxyalkoxy, Ci-Czo-acyl or Ci-Czo-acyloxy groups can be mentioned.
An aralkyl radical is a radical that contains in the ring up to 14, preferably
6 to 10 C
atoms, and in the alkyl chain 1 to 8, preferably 1 to 4, C atoms. Thus, as
aralkyl radicals, for
example, benzyl, phenylethyl, naphthylmethyl, naphthylethyl, furylmethyl,
thienylethyl, and
pyridylpropyl are suitable.
The alkyl groups and radicals can be partially or completely substituted by 1-
5 halogen
atoms, hydroxy groups or Ci-C4-alkoxy groups.
A vinyl or allyl radical is primarily defined with a C2-C6-alkenyl radical.
A C2-C6-alkinyl radical is preferably defined as an ethinyl radical or a prop-
l-inyl
radical.Ci_io-Acyl radicals mean, for example, acetyl, propionyl, butyryl,
valeroyl, isovaleroyl,
pivaloyl, hexanoyl, octyl, nonyl, or decanoyl.
One or two hydroxyl groups at C atoms 3 and 16 can be esterified with an
aliphatic,
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
14
straight-chain or branched-chain, saturated or unsaturated C1-C14-mono- or
polycarboxylic acid
or an aromatic carboxylic acid.
Suitable as such carboxylic acids for esterification are, for example:
- Monocarboxylic acids: formic acid, acetic acid, propionic acid, butyric
acid,
isobutyric acid, valeric acid, isovaleric acid, pivalic acid, lauric acid,
myristic acid,
acrylic acid, propionic acid, methacrylic acid, crotonic acid, isocrotonic
acid, oleic
acid, and elaidic acid.
Esterification with acetic acid, valeric acid or pivalic acid is preferred.
- Dicarboxylic acids: oxalic acid, malonic acid, succinic acid, glutaric acid,
adipic
acid, pimelic acid, suberic acid, azelaic acid, sebacic acid, maleic acid,
fumaric
acid, muconic acid, citraconic acid, and mesaconic acid.
- Aromatic carboxylic acids: benzoic acid, phthalic acid, isophthalic acid,
terephthalic acid, naphthoic acid, o-, m- and p-toluic acid, hydratropic acid,
atropic acid, cinnamic acid, nicotinic acid, and isonicotinic acid.
Esterification with benzoic acid is preferred.
As prodrugs, the esters of the 8(3-substituted estratrienes according to the
invention have
advantages compared to the unesterified active ingredients with respect to
their method of
administration, their type of action, strength and duration of action.
Especially the sulfamates of 8(3-substituted estratrienes according to the
invention have
pharmacokinetic and pharmacodynamic advantages. Related effects were already
described in
other steroid-sulfamates (J. Steroid Biochem. Molec. Biol, 55, 395-403 (1995);
Exp. Opinion
Invest. Drugs 7, 575-589 (1998)).
In this patent application, steroids based on a 8(3-substituted estra-
1,3,5(10)-triene
skeleton are described for the treatment of estrogen receptor (3-mediated
disorders and conditions
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
as selective estrogens, which have in-vitro dissociation with respect to their
binding to estrogen
receptor preparations from rat prostates and rat uteri and which have in vivo
preferably a
dissociation with respect to ovary action in comparison to uterus action.
The ER(3-specific compounds according to the present invention are to mediate
in vivo a
profertility action in the ovary. At the same time, the compounds are to
exhibit a dissociation
with respect to ovary action in comparison to uterus action.
It was found that the 80-substituted estra-1,3,5(10)-trienes according to
general formula I
are suitable as selective estrogens for the treatment of various conditions
and disorders that are
characterized by a higher content of estrogen receptor (3 than estrogen
receptor a in the
corresponding target tissue or target organ. Said compounds have improved
potency and
metabolic stability.
The invention also relates to pharmaceutical preparations that contain at
least one
compound of general formula I (or physiologically compatible addition salts
with organic or
inorganic acids thereof) and the use of the compounds of general formula I for
the production of
pharmaceutical agents, especially for the indications mentioned below.
The new selective estrogens that are described here can be used as individual
components
in pharmaceutical preparations or in combination especially with gestagens.
Part of the present
invention is the combination of selective estrogens with ERa-selective
antiestrogens that are
peripherally-selectively active, i.e., that do not pass through the blood-
brain barriers, as well as
with selective estrogen receptor modulators (SERM).
The ER(3-selective compounds according to the invention can be used in
particular for
the production of pharmaceutical agents for treating fertility disorders, for
prevention and
therapy of prostate hyperplasia, for prevention and treatment of hormone-
deficiency-induced
mood swings in women and men and for use in hormone replacement therapy (HRT)
in men and
women.
Furthermore, the compounds according to the invention have antiproliferative
effects in
models of colon and intestinal hyperplasia and can therefore be administered
for the prevention
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
16
and treatment of diseases associated with colon and intestinal epithelium
proliferation like for
the treatment and the prevention of cancer.
The ER(3-specific compounds according to the present invention can be
advantageously
used for the selective stimulation of hair growth.
A therapeutic product that contains an estrogen and a pure antiestrogen for
simultaneous,
sequential or separate use for the selective estrogen therapy of
perimenopausal or
postmenopausal conditions is already described in EP-A 0 346 014.
Because of their dissociation of action in the ovary in comparison to the
action of the
uterus, the substances and the pharmaceutical agents that contain them are
especially suitable for
the treatment in the case of ovarian dysfunction that is caused by surgery,
medication, etc., such
as female infertility for stimulation of folliculogenesis for treatment by
itself in terms of
enhanced fertility, for supporting in-vitro fertility treatment (IVF) in
connection with an in-vivo
treatment and for treatment of ovarian-induced disorders in later age ("late
fertility") as well as
for treatment of hormone-deficiency-induced symptoms.
The compounds of this invention are also suitable for therapy of ovarian
diseases such as
polycystic ovarian syndrome, POF (premature ovarian failure) syndrome, and
ovulation
disorders.
Finally, the compounds of general formula I can be used in connection with
selective
estrogen receptor modulators (SERM) or raloxifene, specifically in particular
for use in hormone
replacement therapy (HRT) and for treatment of gynecological disorders.
The 8(3-substituted estratrienes according to the invention are also suitable
as individual
components for the treatment of perimenopausal and postmenopausal symptoms, in
particular
hot flashes, sleep disturbances, irritability, mood swings, incontinence,
vaginal atrophy and
hormone-deficiency-induced mental disorders. The above 8(3-substituted
estratrienes are also
suitable for hormone substitution and for the therapy of hormone-deficiency-
induced symptoms
in ovarian dysfunction that is caused by surgery, medication, etc.
In addition, the 8(3-substituted estratrienes according to the invention can
also be used to
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
17
prevent cardiovascular diseases, in particular vascular diseases such as
arteriosclerosis, high
blood pressure, hypertensive heart disease and to prevent hormone-deficiency-
induced
neurodegenerative diseases, such as Alzheimer's disease, as well as hormone-
deficiency-induced
impairment of memory and learning capacity.
In addition, the compounds according to the present invention can be used as
active
ingredients in preparations for treating inflammatory diseases and diseases of
the immune
system, in particular autoimmune diseases, such as, e.g., rheumatoid
arthritis, multiple sclerosis,
lupus, Crohn's disease and other inflammatory intestinal diseases,
inflammatory diseases of the
skin, such as psoriasis, as well as for treating endometriosis. Building on
the evidence from
preclinical models of human inflammatory diseases, the ER(3-specific compounds
can therfore be
used for the prevention and treatment of the diseases mentioned above
(Heather, H.A.; Mol
Endocrinol. 2007 Jan;21(1):1-13. Epub 2006 Mar 23. Review)..
In addition, the compounds are effective against inflammatory diseases of the
respiratory
system, the lungs and bronchial tubes, such as, e.g., asthma.
The medication is suitable for therapy and prophylaxis of estrogen-deficiency-
induced
diseases both in women and in men.
The present compounds are also suitable for prevention and therapy of prostate
hyperplasia.
The compounds can be further used for prophylaxis and therapy of humans age-
related
dysfunctions or diseases. In particular, they can be used for prevention and
treatment of an age-
related drop of androgens, such as testosterone and DHEA, as well as of the
growth hormone.
The amount of a compound of general formula I that is to be administered
fluctuates
within a wide range and can cover any effective amount. On the basis of the
condition that is to
be treated and the type of administration, the amount of the compound that is
administered can
be 0.01 g/kg - 100 mg/kg of body weight, preferably 0.04 g/kg - 1 mg/kg of
body weight, per
day.
In humans, this corresponds to a dose of 0.8 g to 8 g, preferably 3.2 g to
80 mg, daily.
According to the invention, a dosage unit contains 1.6 g to 2000 mg of one or
more
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
18
compounds of general formula I.
The compounds according to the invention and the acid addition salts are
suitable for the
production of pharmaceutical compositions and preparations. The pharmaceutical
compositions
or pharmaceutical agents contain as active ingredients one or more of the
compounds according
to the invention or their acid addition salts, optionally mixed with other
pharmacologically or
pharmaceutically active substances. The production of the pharmaceutical
agents is carried out
in a known way, whereby the known and commonly used pharmaceutical adjuvants
as well as
other commonly used vehicles and diluents can be used.
As such vehicles and adjuvants, for example, those are suitable that are
recommended or
indicated in the following bibliographic references as adjuvants for
pharmaceutics, cosmetics
and related fields: Ullmans Encyklopadie der technischen Chemie [Ullman's
Encyclopedia of
Technical Chemistry], Volume 4 (1953), pages 1 to 39; Journal of
Pharmaceutical Sciences,
Volume 52 (1963), page 918 ff., issued by Czetsch-Lindenwald, Hilfsstoffe fur
Pharmazie und
angrenzende Gebiete [Adjuvants for Pharmaceutics and Related Fields]; Pharm.
Ind., Issue 2,
1961, p. 72 and ff.: Dr. H. P. Fiedler, Lexikon der Hilfsstoffe fiSr
Pharmazie, Kosmetik und
angrenzende Gebiete [Dictionary of Adjuvants for Pharmaceutics, Cosmetics and
Related
Fields], Cantor KG, Aulendorf in Wurttemberg 1971.
The compounds can be administered orally or parenterally, for example
intraperitoneally,
intramuscularly, subcutaneously or percutaneously. The compounds can also be
implanted in the
tissue.
For oral administration, capsules, pills, tablets, coated tablets, etc., are
suitable. In
addition to the active ingredient, the dosage units can contain a
pharmaceutically compatible
vehicle, such as, for example, starch, sugar, sorbitol, gelatin, lubricant,
silicic acid, talc, etc.
For parenteral administration, the active ingredients can be dissolved or
suspended in a
physiologically compatible diluent. As diluents, very often oils with or
without the addition of a
solubilizer, a surfactant, a suspending agent or an emulsifying agent are
used. Examples of oils
that are used are olive oil, peanut oil, cottonseed oil, soybean oil, castor
oil and sesame oil.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
19
The compounds can also be used in the form of a depot injection or an implant
preparation, which can be formulated so that a delayed release of active
ingredient is made
possible.
As inert materials, implants can contain, for example, biodegradable polymers,
or
synthetic silicones such as, for example, silicone rubber. In addition, for
percutaneous
administration, the active ingredients can be added to, for example, a patch.
For the production of intravaginal systems (e.g., vaginal rings) or
intrauterine systems
(e.g., pessaries, coils, IUDs, Mirena(R) ) that are loaded with active
compounds of general formula
I for local administration, various polymers are suitable, such as, for
example, silicone polymers,
ethylene vinyl acetate, polyethylene or polypropylene.
To achieve better bio-availability of the active ingredient, the compounds can
also be
formulated as cyclodextrin clathrates. For this purpose, the compounds are
reacted with a-, (3-,
or y-cyclodextrin or derivatives of the latter (PCT/EP95/02656).
According to the invention, the compounds of general formula I can also be
encapsulated
with liposomes.
Methods
Estrogen Receptor Binding Studies:
The binding affinity of the new selective estrogens was tested in competitive
experiments
with use of 3H-estradiol as a ligand to estrogen receptor preparations from
rat prostates and rat
uteri. The preparation of prostate cytosol and the estrogen receptor test with
prostate cytosol
was carried out as described by Testas et al. (1981) (Testas, J. et al., 1981,
Endocrinology 109:
1287-1289).
The preparation of rat uterus cytosol as well as the receptor test with the ER-
containing
cytosol were basically performed as described by Stack and Gorski, (1985)
(Stack, Gorski 1985,
Endocrinology 117, 2024-2032) with some modifications as described in Fuhrmann
et al. (1995)
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
(Fuhrmann, U. et al. 1995, Contraception 51: 45-52).
The substances that are described here have higher binding affinity to the
estrogen
receptor of rat prostates than to estrogen receptors of rat uteri. In this
case, it is assumed that
ER(3 predominates in the rat prostates over ERa, and ERa predominates in rat
uteri over ER(3.
Table 1 shows that the ratio of the binding to prostate and uterus receptors
qualitatively
coincides with the quotient of relative binding affinity (RBA) to human ER(3
and ERa of rats
(according to Kuiper et al. (1996), Endocrinology 138: 863-870) (Table 1).
Table 1
Estrogen Structure hERa hER(3 ER(3/ Rat uterus Rat prost. prost.
RBA* RBA* ERa ER(RBA) ER(RBA) ER/uterus
ER
Estradiol 100 100 1 100 100 1
Estrone o~`h,~~ 60 37 0.6 3 2 0.8
17a-Estra- 58 11 0.2 2.4 1.3 0.5
diol o -
Estriol Ho lhll 14 21 1.5 4 20 5
~~ o~
5-Andro- 6 17 3 0.1 5 50
stene-diol Genisteine 5 36 7 0.1 10 100
o o
Coumes- ~~ 94 185 2 1.3 24 18
trol
*Cited from: Kuiper et al. (1996), Endocrinology 138: 863-870
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
21
Table 2 shows the results for one of the 80 -vinyl-estra-1,3,5(10)-triene-3,17-
diol
derivatives (compounds 1) according to the invention. Compound 2(8(3-vinyl-
estra-1,3,5(10)-
triene-3,17-diol is shown as a reference.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
22
Table 2
Com ound Rat uterus Rat prost.
p prost. ER/uterus ER
ER(RBA) ER(RBA)
80-Vinyl-estra- 1.7 167 98
1,3,5(10)-16a-fluoro-
3,170-diol (1)
80-Vinyl-estra- 0.7 63 90
1,3,5 10 -3,17 -diol (2)
Compounds 1 according to the invention as well as compound 2 show a higher
binding
affinity to the estrogen receptor of rat prostates than to the estrogen
receptor of rat uteri.
Compound 1 is superior to compound 2 by a higher binding affinity to rat
prostate estrogen
receptor and by a better dissociation regarding binding to rat prostate versus
rat uterus estrogen
receptor.
In addition, the predictability of the prostate-ER versus the uterus-ER test
system is
confirmed with respect to tissue-selective action by in-vivo studies.
Substances with a
preference for prostate-ER are dissociated in vivo preferably with respect to
ovary and uterus
action as well as pituitary gland action in favor of action on the ovary.
Studies for Dissociation of Action of the Uterus and Pituitary Gland
The studies with respect to the action on uterus growth and ovulation
(indirect effect by
influencing the secretion of pituitary gland hormones) are performed on adult
female rats (body
weight of 220-250 g). The substances are subcutaneously administered four
times on four
consecutive days. The first administration is carried out in the metestrus.
One day after the last
administration, the autopsy is carried out. The number of oocytes in the
Fallopian tube (effect on
the ovulation) as well as the uterus weight are determined.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
23
While estradiol produces a dose-dependent ovulation inhibition and an increase
in uterus
weight with an ED50 of 0.004 mg/kg of body weight, the substance according to
the invention
does not exert any effect on pituitary gland and uterus weight.
Ovary Studies:
The compounds are tested in vivo on hypophysectomized juvenile rats. In a
modification
of this operative method, a GnRH antagonist (Cetrorelix) is administered to
the animals. It is
examined whether the substance stimulates follicular proliferation
(maturation) in the ovary.
The ovary weight is the measurement parameter.
In each case, five animals (body weight 40-50 g) are assigned randomly to the
treatment
groups. The animals are fed at libitum with a standard diet (altromin) in
Makrolon cages in air-
conditioned rooms with a lighting program (12 hours of darkness, 12 hours of
light) and are
given acidified tap water to drink. For the s.c administration, the test
substance as well as the
control substance (estradiol E2) are dissolved in benzylbenzoate/castor oil
(1+4 v/v).
Juvenile female rats are either hypophysectomized on day 0 and subcutaneously
treated
(administration 1 x daily) from day 1 to day 4 with estradiol, the compound
according to the
invention, or subcutaneously treated (administration 1 x daily) with a vehicle
(castor oil/benzyl
benzoate). In the modified version of the method, 0.5 mg/animal/day of
Cetrorelix is
administered to the animals simultaneously with the compound according to the
invention or the
vehicle and the control substance estradiol over four days of treatment. In
both cases, the
animals are sacrificed 24 hours after the last administration, and ovary
weight as well as follicle
stages are determined.
The compounds according to the invention thus shows a clear dissociation of
action in
the ovary in comparison to the uterus action and the pituitary gland action
and is excellently
suited for the treatment of female infertility, because of its follicle-
stimulating action.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
24
Production of the Compounds According to the Invention
The compounds of general formula I according to the invention are produced as
described in the examples. Additional compounds of general formula I can be
obtained by an
analogous procedure using reagents that are homologous to the reagents that
are described in the
examples.
Etherification and/or esterification of free hydroxy groups is carried out
according to
methods that are common to one skilled in the art.
The compounds according to the invention can be present in carbon atoms 16 and
17 as
a,(3-stereoisomers. In the production of compounds according to the described
processes, the
compounds in most cases accumulate as mixtures of the corresponding a,(3-
isomers. The
mixtures can be separated by, for example, chromatographic processes.
According to general formula I, possible substituents can already be present
in final form
or in the form of a precursor even in the starting product, a substituted
estrone already
corresponding to the desired end product.
17-Substituents are also introduced according to known processes by
nucleophilic
addition of the desired substituent or a reactive precursor thereof and are
optionally further built
up.
The 8(3-substituted estratriene-carboxylic acid esters according to the
invention are
produced from the corresponding hydroxy steroids analogously to processes that
are also known
(see, e.g., Pharmazeutische Wirkstoffe, Synthesen, Patente, Anwendungen
[Pharmaceutical
Active Ingredients, Syntheses, Patents, Applications]; A. Kleemann, J. Engel',
Georg Thieme
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
Verlag Stuttgart 1978, Arzneimittel, Fortschritte [Pharmaceutical Agents,
Improvements] 1972
to 1985; A. Kleemann, E. Lindner, J. Engel (Editors), VCH 1987, pp. 773-814).
The estratriene-sulfamates according to the invention are available in a way
that is known
in the art from the corresponding hydroxy steroids by esterification with
sulfamoyl chlorides in
the presence of a base (Z. Chem. 15, 270-272 (1975); Steroids 61, 710-717
(1996)).
Subsequent acylation of the sulfamide group results in the (N-acyl)sulfamates
according
to the invention, for which pharmacokinetic advantages were already detected
in the case of the
absence of an 8-substituent (cf. DE 195 40 233 Al).
The regioselective esterification of polyhydroxylated steroids with N-
substituted and N-
unsubstituted sulfamoyl chlorides is carried out according to partial
protection of those hydroxyl
groups that are to remain unesterified. Silyl ethers have turned out to be
protective groups with
selective reactivity that is suitable for this purpose, since these silyl
ethers are stable under the
conditions of sulfamate formation, and the sulfamate group remains intact when
the silyl ethers
are cleaved again for regeneration of the residual hydroxyl group(s) still
contained in the
molecule (Steroids 61, 710-717 (1996)). The production of the sulfamates
according to the
invention with one or more additional hydroxyl groups in the molecule is also
possible in that the
starting material is suitable hydroxy-steroid ketones. First, depending on the
goal, one or more
hydroxyl groups that are present are subjected to sulfamoylation. Then, the
sulfamate groups
optionally can be converted with a desired acyl chloride in the presence of a
base into the (N-
acyl)sulfamates in question. The now present oxosulfamates or oxo-(N-
acyl)sulfamates are
converted by reduction into the corresponding hydroxysulfamates or hydroxy-(N-
acyl)sulfamates (Steroids 61, 710-717 (1996)). Sodium borohydride and the
borane-dimethyl
sulfide complex are suitable as suitable reducing agents.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
26
The introduction of variable substituents in rings D of the estratriene
skeleton,
particularly the halogen atom (for example a fluorine atom) at C-atom 16, can
basically be
carried out according to the chemical teaching that is known to one skilled in
the art, with which
the corresponding estratriene derivatives that are not substituted in 8-
position are produced (see,
i.a.: Steroide [Steroids], L. F. Fieser, M. Fieser, Verlag Chemie,
Weinheim/Bergstr., 1961;
Organic Reactions in Steroid Chemistry, J. Fried, J. A. Edwards, Van Nostrand
Reinhold
Company, New York, Cincinnati, Toronto, London, Melbourne, 1972; Medicinal
Chemistry of
Steroids, F. J. Zeelen, Elsevier, Amsterdam, Oxford, New York, Tokyo, 1990).
Substituents according to general formula I can also be introduced in the
stage of
estratrienes that are already substituted in 8-position, according to known
methods in the art.
This can be useful or necessary especially in the case of multiple
substitutions of the desired
final compound.
Characteristic, but not limiting synthesis processes, which are useful for
providing
representative substitution patterns on the estrone skeleton, also in
combination with several
substituents, are found in, for example: C(1) J. Chem. Soc. (C) 1968, 2915;
C(7) Steroids 54,
1989, 71; C(8a) Tetrahedron Letters 1991, 743; C(8(3) Tetrahedron Letters
1964, 1763; J. Org.
Chem. 1970, 35, 468; C(11) J. Steroid Biochem. 31, 1988, 549; Tetrahedron 33,
1977, 609 and J.
Org. Chem. 60, 1995, 5316; C(9) DE-OS 2035879; J. Chem. Soc. Perk. 1 1973,
2095; C(15) J.
Chem. Soc. Perk. 1 1996, 1269.); C(13a) Mendeleev Commun. 1994, 187; C(14(3)
Z. Chem. 23,
1983, 410.
The examples below are used for a more detailed explanation of the invention.
Analogously to the degradation of the 8(3-vinyl grouping, other compounds of
general
formula I can be obtained with use of reagents that are homologous to the
reagents that are
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
27
described in the examples.
Etherification and/or esterification of free hydroxy groups is carried out
according to the
methods that are common to one skilled in the art.
Example 1
80-Vinyl-16a-Fluoro-estra-1,3,5(10)-triene-3,17-diol
3-Methoxy-16a-f1uor-8-vinyl-estra-1,3,5(10)-trien-17-one
7,3 g (23,5 mmol) of 3-methoxy-8-vinyl-estra-1,3,5(10)-trien-l7-one in 65 ml
THF are added
dropwise under argon to 30 ml of a 2M solution of lithium-diisopropylamide
(60,0 mmol) in
THF / Heptan / Ethylbenzol, cooled down at -78 C, and then 16 ml (115,4 mmol)
Triethylamin
and 7,6 ml (59,9 mmol) chlortrimethylsilane are added one after the other. The
reaction mixture
is subsequently warmed up to room temperature in 1,5 h, washed with sodium
bicarbonate
solution, and extracted with n-Hexane. The collected organic phases are washed
with water,
drayed over sodium sulphate and concentrated by evaporation in a vacuum.
The obtained (3-methoxy-8-vinyl-estra- 1,3,5(10),16-trien- 17-yloxy)-
trimethylsilane crude
product (12 g of a yellowish liquid residue) is used without any further
purification in the next
step.
Rf= 0,54 (Cyclohexane / Ethyl Acetate = 8 / 2)
6,8 g of the (3-Methoxy-8-vinyl-estra- 1,3,5(10),16-tetraen- 17-yloxy)-
trimethylsilane crude
product are dissolved in 50 ml methylenchloride, combined with 5g (15,9mmo1) N-
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
28
fluordibenzensulfonimide and mixed for 16 hours at room temperature in the
absence of light.
25 m12N hydrochloric acid are added, the organic phase is separated and
extracted several times
with methylchloride. The collected organic phases are washed with a sodium
bicarbonate
solution, with water and with a saturated sodium chloride solution one after
the other, drayed
over magnesium sulphate and concentrated in vacuo. The obtained crude product
(8.43
yellowish-brown oil) is purified by chromatography on silica gel (19/1
cyclohexane/ethyl
acetate). In this way 1.65g (38%, colourless foam) of 3-methoxy-l6a-fluor-8-
vinyl-estra-
1,3,5(10)-trien-l7-one and 2,08g (51% colourless foam) of 3-methoxy-8-vinyl-
estra-1,3,5(10)-
trien-17-one are obtained.
3-Methoxy-l6a-fluor-8-vinyl-estra-1,3,5(10)-trien-17-one 1H-NMR (CDC13): 6 =
0,96 (s, 3H;
18-CH3), 3,76 (s, 3H; OMe), 5,01 (d, 1H; CH=CH2), 5,05 / 5,18 (d, 1H; 16-H),
5,13 (d, 1H;
CH=CH2), 5,54 (dd, 1H; CH=CH2), 6,59 (d, 1H; 4-H), 6,69 (dd, 1H; 1-H, 2-H),
7,15 (d, 1H;
1-H, 1-H).
3-Methoxy-16a-f1uor-8-vinyl-estra-1,3,5(10)-trien-17a-o1 und 3-methoxy-16a-
fluor-8-vinyl-
estra-1,3,5(10)-trien-17(3-o1
1,65 g (5,0 mmol) of 3-Methoxy-l6a-fluor-8-vinyl-estra-1,3,5(10)-trien-17-one
are dissolved in
a mixture of 150 ml of THF and 150 ml of Methanol, cooled at 5 C and combined
to 1,9 g (50,2
mmol) of sodiumborohydride. During the warming up to room temperature in 1,5h,
the reaction
mixture is stirred. 10 ml of acetic acid are then added and the solution
finally dried in vacuo.
The residue is redissolved in water and extracted several times with ethyl
acetate. The organic
phases are collected, dried with magnesium sulfate and concentrated in vacuo.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
29
The obtained crude product is separated by chromatography on silica gel
(Cyclohexane / Methyl-
tert.-Buthylether = 13 / 1). According to this procedure 0,67 g (40%) of 3-
methoxy-l6a-fluor-8-
vinyl-estra-1,3,5(10)-trien-17a-ol and 1,00 g (60%) 3-of inethoxy-l6a-fluor-8-
vinyl-estra-
1,3,5(10)-trien-17(3-ol are obtained in the form of a colorless foam.
3-Methoxy-l6a-fluor-8-vinyl-estra-1,3,5(10)-trien-l7a-o11H-NMR (CDC13): 6 =
0,72 (s, 3H;
18-CH3), 3,72 (d, 1H, 17-H), 3,75 (s, 3H; OMe), 4,90 (d, 1H; CH=CH2), 5,04 (d,
1H;
CH=CH2), 5,14 / 5,28 (dd, 1H; 16-H), 5,51 (dd, 1H; CH=CH2), 6,57 (d, 1H; 4-H),
6,67 (dd, 1H;
1-H, 2-H), 7,16 (d, 1H; 1-H, 1-H).
3-Methoxy-l6a-fluor-8-vinyl-estra-1,3,5 (10)-trien-17(3-ol
1H-NMR (CDC13): 6 = 0,79 (s, 3H; 18-CH3), 3,75 (s, 3H; OMe), 3,81 (d, 1H, 17-
H), 4,82 / 4,95
(dd, 1H; 16-H), 4,89 (d, 1H; CH=CH2), 5,06 (d, 1H; CH=CH2), 5,51 (dd, 1H;
CH=CH2), 6,57
(d, 1H; 4-H), 6,68 (dd, 1H; 1-H, 2-H), 7,15 (d, 1H; 1-H, 1-H).
16a-F1uor-8-vinyl-estra-1,3,5 (10)-trien-3,17 (3-diol
690 mg (1,87 mmol) of tetrabutylammoniumiodide are added to a solution of 70
mg (0,21
mmol) 3-methoxy-16a-fluor-8-vinyl-estra-1,3,5(10)-trien-17(3-ol in 9 ml of
methylenchloride
under argon. The reaction mixture is cooled at -78 C and then combined with
1,9 ml (1,9 mmol)
of a 1M solution of borotrichloride in methylenchloride. The mixture is
stirred for 0,5 h at -78 C,
finally warmed up at room temperature and quenched with water. The organic
phase is
separated, the water phase is extracted several times with methylenchloride,
and the organic
phases are collected, washed with a saturated sodium chloride solution, dried
with magnesium
sulfate and concentrated in vacuo.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
The obtained crude product is separated by chromatography on silica gel
(Cyclohexane / Ethyl
Acetate = 9 / 1). 26 mg (39%) of 16a-Fluor-8-vinyl-estra-1,3,5(10)-trien-
3,17(3-diol are obtained
according to this procedure.
16a-Fluor-8-vinyl-estra-1,3,5(10)-trien-3,17(3-diol 1H-NMR (DMSO[D6]): 6 =
0,64 (s, 3H; 18-
CH3), 3,49 / 3,57 (t, 1H, 17-H), 4,70 / 4,84 (dd, 1H; 16-H), 4,85 (d, 1H;
CH=CH2), 4,98 (d, 1H;
CH=CH2), 5,19 (d, 1H, 17-OH), 5,46 (dd, 1H; CH=CH2), 6,37 (d, 1H; 4-H), 6,48
(dd, 1H; 1-H,
2-H), 6,99 (d, 1H; 1-H, 1-H), 8,93 (s, 1H, 3-OH).
Example 2
16a-F1uor-8-vinyl-estra-1,3,5 (10)-trien-3,17a-diol
295 mg (0,80 mmol) of tetrabutylammoniumiodide are added to a solution of 30
mg (0,09
mmol) of 3-methoxy-16a-fluor-8-vinyl-estra-1,3,5(10)-trien-17a-ol in 4 ml
methylenchloride
under argon. The reaction mixture is cooled at -78 C and then combined with
0,8 ml (0,8 mmol)
of a 1M solution of borotrichloride in methylenchloride. The mixture is
stirred for 0,5 h at -78 C,
finally warmed up at room temperature and quenched with water. The organic
phase is
separated, the water phase is extracted several times with methylenchloride,
and the organic
phases are collected, washed with a saturated Sodium Chloride solution, dried
with magnesium
sulfate and concentrated in vacuo.
The obtained crude product is separated by chromatography on silica gel
(Cyclohexane / Ethyl
Acetate = 7 / 3). According to this procedure 27 mg (94%) 16a-Fluor-8-vinyl-
estra-1,3,5(10)-
trien-3,17a-diol are obtained.
CA 02692727 2010-01-06
WO 2009/007454 PCT/EP2008/059115
31
16a-Fluor-8-vinyl-estra-1,3,5(10)-trien-3,17a-diol 1H-NMR (DMSO[D6]): 6 = 0,63
(s, 3H; 18-
CH3), 3,51 (t, 1H, 17-H), 4,82 (d, 1H, 17-OH), 4,85 (d, 1H; CH=CH2), 4,97 (d,
1H; CH=CH2),
5,05 / 5,18 (dd, 1H; 16-H), 5,47 (dd, 1H; CH=CH2), 6,37 (d, 1H; 4-H), 6,48
(dd, 1H; 1-H, 2-H),
6,99 (d, 1H; 1-H, 1-H), 8,97 (s, 1H, 3-OH).