Note: Descriptions are shown in the official language in which they were submitted.
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
METHODS AND DEVICES FOR CHARGED MOLECULE MANIPULATION
FIELD OF THE INVENTION
The present invention relates to the micromanipulation of charged molecules.
Accordingly, this invention involves the fields of biotechnology, chemistry,
and
micromanipulation.
BACKGROUND OF THE INVENTION
Microinjection of foreign materials is often problematic, particularly if such
1Q microinjection is being performed on a biological structure such as a
living cell. Various
transfection techniques include the microinjection of foreign genetic material
such as
DNA into the nucleus of a cell to facilitate the expression of foreign DNA.
For example,
when a fertilized oocyte (egg) is transfected, cells arising from that oocyte
will carry the
foreign genetic material. Thus in one application organisms can be produced
that exhibit
additional, enhanced, or repressed genetic traits. As one example, researchers
have used
microinjections to create strains of mice that carry a foreign genetic
construct causing
macrophages to auto-fluoresce and undergo cell death when exposed to a certain
drugs.
Such transgenic mice have since played roles in investigations of macrophage
activity
during immune responses and macrophage activity during tumor growth.
Prior art microinjectors function in a similar manner to macro-scale syringes:
a
pressure differential forces a liquid through a needle and into the cell. In
some cases a
glass needle that has been fire drawn from a capillary tube can be used to
pierce the
cellular and nuclear membranes of an oocyte. Precise pumps then cause the
expulsion of
minute amounts of genetic material from the needle and into the nucleus.
Recently, researchers have produced fine microinjection needles from silicon
nitride and silica glass that are smaller than fire drawn capillaries. These
finer needles,
however, still employ macro-scale pumps similar to those used in traditional
microinj ectors.
SUMMARY OF THE INVENTION
Accordingly, the present invention provides systems and methods for
manipulating molecular material. In one aspect, for example, a method for
manipulating
-1-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
molecular material may include positioning an uncharged needle structure in
electrical
proximity with a charged molecular material at a first locus in a liquid
environment,
charging the needle structure such that at least a portion of the charged
molecular material
becomes associated with the needle structure, moving the needle structure and
the first
locus relative to one another, and discharging the needle structure to
disassociate the
charged molecular material at a second locus. It should be noted that
discharging the
needle structure can occur in a variety of ways. For example, in one aspect
the needle
structure can be discharged to a degree sufficient to release the associated
charged
molecular material. In another aspect, discharging the needle structure can
include
reversing the polarity of the needle structure charge to drive the charged
molecular
material away from the needle structure.
A variety of charged molecular materials are contemplated that can be
manipulated according to aspects of the present invention. Non-limiting
examples can
include DNA, RNA, peptides, polymers, organic molecules, inorganic molecules,
ions,
and combinations thereof. In one specific aspect of the present invention, the
charged
molecular material can include DNA, RNA, peptides, and combinations thereof.
In
another specific aspect, the charged molecular material can include DNA.
Furthermore, numerous first and second loci are contemplated, and the nature
and
extent of manipulation of molecular material can vary depending on the
environment and
intended use of the techniques of the present invention. In one aspect, for
example, the
second locus can be located within a single cell. In another aspect, the
second locus can
be located within a cell nucleus of the single cell. In yet another aspect,
the first locus can
be located outside of the single cell.
Additionally, the movement of the needle structure and the first locus
relative to
one another can vary depending on the relative locations of the first and
second loci and
the nature and configuration of the movement apparatus. In one aspect, for
example,
moving the needle structure and the first locus relative to one another
comprises moving
the needle structure from the first locus to the second locus. In another
aspect, the needle
structure includes an elongate axis and a loci axis is defined between the
first locus and
the second locus. As the needle structure and the first locus are moved
relative to one
another, an angular relationship between the elongate axis and the loci axis
remains
constant.
-2-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
The present invention also provides systems for manipulating molecular
material.
In one aspect, for example, a system for manipulating molecular material can
include a
needle structure, a charging system electrically coupled to the needle
structure, the
charging system being operable to charge and discharge the needle structure,
and a
movement system operable to move the needle structure from a first locus to a
second
locus. In one specific aspect, the system can further include a charged
molecular material
sample associated with the needle structure. In another specific aspect, the
system can
further include a single cell positioned to receive the needle structure upon
operation of
the movement system. In one aspect the single cell can be an oocyte.
In another aspect, a system for manipulating molecular material can include a
moveable support frame and a needle structure associated with the moveable
support
frame, where the needle structure is operable to carry molecular material
therewith.
Furthermore, the moveable support frame is operable to moving the needle
structure from
an initial position to an extended position, where an elongate axis of the
needle structure
is maintained in a substantially constant orientation as the moveable support
frame moves
the needle structure from the initial position to the extended position. In
one specific
aspect, the vertical elevation of the needle structure in the extended
position is different
from a vertical elevation of the needle structure in the initial position. In
another specific
aspect, the system can include a charging system electrically coupled to the
needle
structure, where the charging system is operable to charge and discharge the
needle
structure. In yet another aspect, the system can include a support structure
positioned
adjacent to the moveable support frame, where the support structure is
operable to secure
a single cell in a position to receive the needle structure when in the
extended position.
In another aspect, a system for manipulating molecular material can include a
moveable support frame, and a needle structure associated with the moveable
support
frame, where the needle structure is chargeable between a first charged state
and a second
charged state. Furthermore, the moveable support frame is operable to moving
the needle
structure from an initial position to an extended position, wherein an
elongate axis of the
needle structure is maintained in a substantially constant orientation as the
moveable
support frame moves the needle structure from the initial position to the
extended
position. In one specific aspect, the vertical elevation of the needle
structure in the
-3-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
extended position is different from a vertical elevation of the needle
structure in the initial
position.
DEFINITIONS OF KEY TERMS
In describing and claiming the present invention, the following terminology
will
be used in accordance with the definitions set forth below.
The singular forms "a," "an," and, "the" include plural referents unless the
context
clearly dictates otherwise. Thus, for example, reference to "a support"
includes reference
to one or more of such supports, and reference to "an oocyte" includes
reference to one or
more of such oocytes.
As used herein, the term "charged molecular material" may be used to refer to
any
molecular material that is capable of 6eing attracted to or associated with an
electrically
charged structure. Accordingly, charged molecular material may be used to
refer to those
molecules having a net charge, as well as those molecules that have a net
neutral charge
but possess a charge distribution that allows attraction to the structure.
As used herein, the term "peptide" may be used to refer to a natural or
synthetic
molecule comprising two or more amino acids linked by the carboxyl group of
one amino
acid to the alpha amino group of another. A peptide of the present invention
is not
limited by length, and thus "peptide" can include polypeptides and proteins.
As used herein, the term "uncharged" when used in reference to a needle
structure
may be used to refer to the relative level of charge in the needle structure
as compared to
a charged molecular material. In other words, a needle structure may be
considered to be
"uncharged" as long as the amount of charge on the needle structure is
insufficient to
attract a useable portion of the charged molecular material. Naturally what is
a useable
portion may vary depending on the intended use of the molecular material, and
it should
be understood that one of ordinary skill in the art would be aware of what a
useable
portion is given such an intended use. Additionally it should be noted that a
needle
structure with no measurable charge would be considered "uncharged" according
to the
present definition.
As used herein, the term "sample" when used in reference to a sample of a
molecular material may be used to refer to a portion of molecular material
that has been
purposefully attracted to or associated with the needle structure. For
example, a sample
of a molecular material such as DNA that is described as being associated with
a needle
-4-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
structure would include DNA that has been purposefully attracted thereto, but
would not
include DNA that is attracted thereto through the mere exposure of the needle
structure to
the environment. One example of DNA that would not be considered to be a
"sample"
includes airborne DNA fragments that may associate with the needle structure
following
exposure to the air.
As used herein, "adjacent" refers to near or close sufficient to achieve a
desired
effect.
As used herein, "associate" is used to describe molecular material that is in
electrostatic contact with a structure due to attraction of opposite charges.
For example,
DNA that has been attracted to a structure by a positive charge is said to be
associated
with the structure.
As used herein, the term "substantially" refers to the complete or nearly
complete extent or degree of an action, characteristic, property, state,
structure, item, or
result. For example, an object that is "substantially" enclosed would mean
that the object
is either completely enclosed or nearly completely enclosed. The exact
allowable degree
of deviation from absolute completeness may in some cases depend on the
specific
context. However, generally speaking the nearness of completion will be so as
to have
the same overall result as if absolute and total completion were obtained. The
use
of "substantially" is equally applicable when used in a negative connotation
to refer to
the complete or near complete lack of an action, characteristic, property,
state, structure,
item, or result. For example, a composition that is "substantially free of"
particles would
either completely lack particles, or so nearly completely lack particles that
the effect
would be the same as if it completely lacked particles. In other words, a
composition that
is "substantially free of' an ingredient or element may still actually contain
such item as
long as there is no measurable effect thereof
As used herein, the term "about" is used to provide flexibility to a numerical
range
endpoint by providing that a given value may be "a little above" or "a little
below" the
endpoint without affecting the desired result.
As used herein, a plurality of items, structural elements, compositional
elements,
and/or materials may be presented in a common list for convenience. However,
these
lists should be construed as though each member of the list is individually
identified as a
separate and unique member. Thus, no individual member of such list should be
-5-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
construed as a de facto equivalent of any other member of the same list solely
based on
their presentation in a common group without indications to the contrary.
Concentrations, amounts, and other numerical data may be expressed or
presented
herein in a range format. It is to be understood that such a range format is
used merely
for convenience and brevity and thus should be interpreted flexibly to include
not only the
numerical values explicitly recited as the limits of the range, but also to
include all the
individual numerical values or sub-ranges encompassed within that range as if
each
numerical value and sub-range is explicitly recited. As an illustration, a
numerical range
of "about 1 to about 5" should be interpreted to include not only the
explicitly recited
values of about 1 to about 5, but also include individual values and sub-
ranges within the
indicated range. Thus, included in this numerical range are individual values
such as 2, 3,
and 4 and sub-ranges such as from 1-3, from 2-4, and from 3-5, etc., as well
as 1, 2, 3, 4,
and 5, individually.
This same principle applies to ranges reciting only one numerical value as a
minimum or a maximum. Furthermore, such an interpretation should apply
regardless of
the breadth of the range or the characteristics being described.
BRIEF DESCRIPTION OF THE DRAWINGS
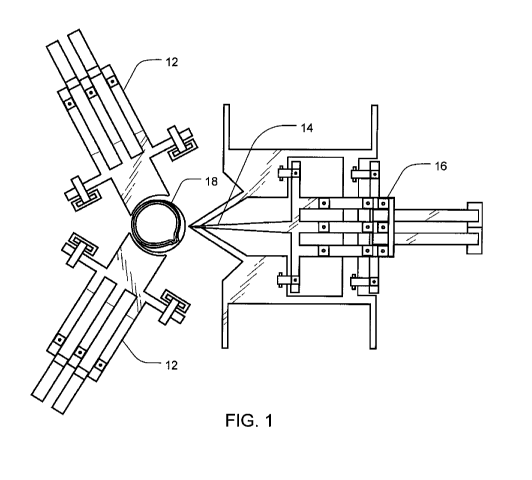
FIG. 1 is a view of a molecular material manipulation system in accordance
with
one embodiment of the present invention.
FIG. 2 is a view of a molecular material manipulation system in accordance
with
another embodiment of the present invention.
FIG. 3 is a view of a molecular material manipulation system in accordance
with
yet another embodiment of the present invention.
FIG. 4 is a view of a molecular material manipulation system in accordance
with a
further embodiment of the present invention.
FIG. 5 is graphical plot of DNA concentration to pixel intensity in accordance
with yet a further embodiment of the present invention.
DETAILED DESCRIPTION
Aspects of the present invention provide methods and systems for manipulating
molecular material. Such methods and systems can include the actual
manipulation of the
-6-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
molecular material as well as the movement and positioning of a device or
devices used
for the manipulation.
It has thus now been discovered that molecular material may be manipulated
through the use of a needle structure without the use of a pump mechanism.
Such a
"pump-less" needle structure utilizes electrical charge to associate and
release charged
molecular material therefrom. For example, in one aspect a method for
manipulating
molecular material can include positioning an uncharged needle structure in
electrical
proximity with a charged molecular material at a first locus in a liquid
environment,
charging the needle structure such that at least a portion of the charged
molecular material
becomes associated with the needle structure, moving the needle structure and
the first
locus relative to one another, and discharging the needle structure to
disassociate the
charged molecular material at a second locus. Such a method utilizes the
charge of a
molecular material to facilitate the transfer from the first locus to the
second locus. In the
case of DNA, for example, such a transfer is made feasible by the unequal
charge
distributions within DNA molecules. More specifically, the phosphate backbone
of DNA
has a net charge of one electron per phosphate, giving a total of two
electrons per base
pair. This net negative charge on the outer backbone of the DNA molecule makes
it
possible to move DNA from the first locus to the second locus using a charged
needle
structure.
As further description, an electrical charge is introduced into the needle
structure
to attract the charged molecular material onto its outer surface. The needle
structure can
then be moved from the first locus to the second locus along with the
associated
molecular material. It should be noted that such movement may include moving
the
needle structure to the second locus, moving the second locus to the needle
structure, or a
combination of both. Moving to the second locus may also include moving from
the
outside of a cell to the inside of the cell, moving from one portion of a
liquid to another
portion of the sarne liquid, moving from one liquid to another liquid, and the
like.
Following arrival at the second locus, the molecular material can then be
released from
the surface of the needle structure. In one aspect, such release may be
accomplished by
releasing the charge of the needle structure and allowing the molecular
material to diffuse
away from the structure. In another aspect, the polarity of the charge of the
needle may
-7-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
be reversed to electrostatically repel the molecular material from the
needle's outer
surface at the second locus.
Numerous types of charged molecular material are contemplated for use
according
to aspects of the present invention, all of which would be considered to be
within the
present scope. Non-limiting examples include DNA, RNA, peptides, polymers,
organic
molecules, inorganic molecules, ions, and combinations thereof. In one
specific aspect,
DNA may be any form of natural or synthetic DNA, including genomic DNA, cDNA,
plasmid DNA, and the like. In another specific aspect, RNA may be any form of
RNA,
including RNAi, siRNA, shRNA, mRNA, tRNA, rRNA, microRNA, and hybrid
sequences or synthetic or semi-synthetic sequences thereof.
The manipulation of molecular material according to aspects of the present
invention may be useful in a variety of situations and environments. For
example, the
transfer of such material may be utilized to transfer molecular material into
a single cell.
Although any single cell would be considered to be within the present scope,
in one
specific aspect the single cell may be an oocyte. Other non-limiting examples
of single
cells include neuronal cells, fibroblasts, cancer cells, and the like.
Additionally, it is also
contemplated that molecular material may be transferred to particular regions
or
biological structures within a single cell. In one specific example, molecular
material
may be transferred into the nucleus of a single cell.
A variety of motions are contemplated to move the needle structure and the
associated molecular material from the first locus to the second locus. In one
aspect, for
example, the needle structure can be moved along a linear or substantially
linear path
from the first locus to the second locus. One example of such a motion would
include
situations where the first locus and the second locus are substantially
aligned with an
elongate axis of the needle structure, with the first locus at or near the tip
of the needle
structure. By moving the needle structure forward along the elongate axis, the
tip of the
needle will move along a linear path from the first locus to the second locus.
Alternatively, in other aspects the needle structure may also be moved in an
additional
direction that is out of the linear path of the needle structure. In such
cases, however, it
may be beneficial to maintain the orientation of the needle structure,
particularly in those
aspects where molecular material is being transferred into a cell. If such an
orientation is
not maintained, there may be a risk of damage to the cell being injected. More
-8-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
specifically, if a loci axis is defined between the first locus and the second
locus, the
angular relationship between the elongate axis of the needle structure and the
loci axis
should remain constant as the needle structure and the first locus are moved
relative to
one another. As such, in situations where the needle structure is moved in a
direction
away from the loci axis, the orientation of the needle structure would still
be maintained
parallel to the elongate axis established prior to movement. In another
aspect, the angular
relationship between the loci axis and the elongate axis need not remain
constant,
provided the needle structure be positioned so as the second loci is
approached in a
direction that is along the elongate axis. In other words, the needle
structure may be
moved to an intermediate locus, and movement of the needle structure from the
intermediate locus to the second locus can be in a direction along the
elongate axis of the
needle structure at the intermediate locus.
Upon arrival of the needle structure at the second locus, the molecular
material
can be released. This can be accomplished in a variety of ways, and any method
that
releases the molecular material should be considered to be within the scope of
the present
invention. In one aspect, the charge of the needle structure can simply be
released, thus
allowing the molecular material to diffuse away at the second locus. It should
be noted
that the needle structure may not necessarily be completely discharged, but in
some cases
could be discharged to a degree sufficient to substantially release the
charged molecular
xnaterial associated with the needle stnxcture. In another aspect, discharging
the needle
structure can include reversing the polarity of the needle structure charge.
By utilizing
this method, the molecular material may be actively driven from the needle
structure, thus
minimizing the amount of time the needle structure is present at the second
locus.
The present invention additionally provides systems for manipulating molecular
material. In one aspect for example, a system for manipulating molecular
material can
include a needle structure, a charging system electrically coupled to the
needle structure,
the charging system being operable to charge and discharge the needle
structure, and a
movement system operable to move the needle structure from a first locus to a
second
locus. In one specific aspect, the system may further include a charged
molecular
material sample associated with the needle structure. As has previously been
described,
such molecular material may include any molecular material sample that is
purposefully
attracted to or associated with the needle structure. Non-limiting examples of
such
-9-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
samples include DNA, RNA, peptides, polymers, organic molecules, inorganic
molecules, ions, and combinations thereof. A more specific list of non-
limiting examples
may include DNA, RNA, peptides, and combinations thereof.
In another specific aspect, the system can further include a single cell
positioned
to receive the needle structure upon operation of the movement system.
AIthough a
variety of single cells are contemplated, in one aspect the single cell is an
oocyte.
Numerous needle structure configurations and materials are contemplated, and
it
should be noted that any material and configuration that allows the
manipulation of
molecular material through electrical charge and discharge should be
considered to be
within the present scope. In one aspect, for example, the material of the
needle structure
may include a metal or metal alloy, a conductive glass, a polymeric material,
a
semiconductor material, and combinations thereof. Non-limiting examples of
metals for
use in the needle structure include indium, gold, platinum, silver, copper,
associated
alloys, palladium, tungsten, aluminum, titanium, and combinations thereof.
Polymeric
materials that can be used to construct the needle structure can include any
conductive
polymer, non-limiting examples of which include polypyrrole doped with dodecyl
benzene sulfonate ions, SU-8 polymer with embedded metallic particles, and
combinations thereof. Non-limiting examples of useful semiconductor materials
can
include monocrystalline silicon, polycrystalline silicon, germanium, gallium
arsenide,
indium-tin oxide, and combinations thereof. It should additionally be noted
that in some
aspects the conductive material may be a conductive layer that is coated on a
second
material, where the second material provides the physical structure of the
needle
structure. Additionally, the needle structure may be comprised of a hollow,
non-
conductive material, such as a glass, where the hollow material is filled with
a conductive
material such as a conductive liquid. The needle structure may be of a variety
of sizes
depending on the intended use of the device. In one aspect, however, the tip
of the needle
structure may be less than about 5 microns across. In another aspect, the tip
of the needle
stracture may be less than about 1 microns across. In yet another aspect, the
tip of the
needle structure may be less than about 100 nanometers across.
The charging system operatively coupled to the needle structure may include
any
system capable of electrically charging, maintaining the charge, and
subsequently
discharging the device. Non-limiting examples can include batteries, DC power
supplies,
-10-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
photovoltaic cells, static electricity generators, capacitors, and the like.
The charging
system can include a switch for activation and deactivation, and in some
aspects can also
include a polarity switch to reverse polarity of the charge on the needle
structure. In one
aspect the system may additionally include multiple charging systems, one
system for
charging the needle structure with a charge, and another charging system for
charging the
needle structure with an opposite polarity charge. In one example scenario, an
initially
uncharged needle structure is brought into contact with a sample of a charged
molecular
material. The molecular material may be in water, saline, or any other liquid
useful for
molecular material manipulation. A charge opposite in polarity to the charge
of the
charged molecular material is applied to the needle structure, thus attracting
a portion of
the sample of molecular material thereto. The needle structure is then moved
from the
first locus to the second locus while the molecular material is held in place
by the charge.
Once at the second locus, the polarity of the charge at the needle structure
can be
reversed, thus releasing the molecular material from the needle structure, and
in some
cases actively driving the molecular material into the region surrounding the
second
locus. The necdle structure can subsequently be withdrawn from the second
locus.
A variety of movement systems are contemplated to move the needle structure
from the first locus to the second locus. Any technique capable of moving the
needle
structure with sufficient precision to allow the manipulation of molecular
material is
considered to be within the present scope. Non-limiting examples of movement
systems
include, mechanical systems, magnetic systems, piezoelectric systems,
electrostatic
systems, thermo-mechanical systems, pneumatic systems, hydraulic systems, and
the like.
The needle structure may also be moved manually by a user. For example, a user
may
push the needle structure along a track from the first locus to the second
locus.
Additional movement systems are described more fully below.
In another aspect of the present invention, a system for manipulating
molecular
material is provided including a moveable support frame, a needle structure
associated
with the moveable support frame, the needle structure operable to carry
molecular
material therewith, where the moveable support frame is operable to move the
needle
structure from an initial position to an extended position, and wherein an
elongate axis of
the needle structure is maintained in a substantially constant orientation as
the moveable
support frame moves the needle structure from the initial position to the
extended
-11-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
position. In a more specific aspect, a vertical elevation of the needle
structure in the
extended position is different from a vertical elevation of the needle
structure in the initial
position. In such a case, the elongate axis of the needle structure in the
extended position
is parallel to or substantially parallel to the elongate axis of the needle
structure in the
initial position. Furthermore, the system can include a charging system as
described
above. It should be noted that the term "vertical" as has been used herein
refers to
movement relative to the elongate axis of the needle structure in the initial
position.
Additional components of the system are contemplated, depending on the nature
of the molecular material manipulation. For example, a system that is utilized
to transfer
a molecular material such as DNA to the nucleus of a single cell can include a
support
structure positioned adjacent to the moveable support frame, where the support
structure
is operable to secure a single cell in a position to receive the needle
structure when in the
extended position. One example of a system having such a support structure for
holding
an oocyte is shown in FIG. 1. A pair of oocyte supports 12 are used to hold
the oocyte 18
in a position to receive the needle structure 14 when moved into the extended
position by
the moveable support frame 16. In this aspect the center of the oocyte 18 is
located such
that the nucleus of the oocyte is aligned with the needle structure when it is
brought to the
extended position. In another aspect, an oocyte may be placed in a recess in
the substrate
holding the moveable support frame such that the approximate center of the
oocyte is
aligned with the elongate axis of the needle structure when in the initial
position (not
shown). The extended position in this configuration can thus be achieved by
moving the
needle structure along the elongate axis toward the oocyte. Additional
configurations for
support structures would be readily apparent to one of ordinary skill in the
art once in
possession of the present disclosure, and such configurations are considered
to be within
the present scope.
In yet another aspect of the present invention, a system for manipulating
molecular material can include a moveable support frame, a needle structure
associated
with the moveable support frame, the needle structure being chargeable between
a first
charged state and a second charged state, the moveable support frame being
operable to
move the needle structure from an initial position to an extended position,
wherein an
elongate axis of the needle structure is maintained in a substantially
constant orientation
as the moveable support frame moves the needle structure from the initial
position to the
-12-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
extended position. Furthermore, in one specific aspect a vertical elevation of
the needle
structure in the extended position is different from a vertical elevation of
the needle
structure in the initial position.
As has been described, a variety of configurations are contemplated for
manipulating molecular material according to aspects of the present invention.
In one
specific example, a pumpless microelectromechanical system (MEMS) device is
provided
for the introduction of foreign molecular material into a single cell. Due to
the small size
of such devices, it may be beneficial to create a system that is at least
substantially self-
contained. The use of the molecular material manipulation techniques of the
present
invention eliminates the need for precise injection pumps, thus facilitating
such a self-
contained system.
In order to inject foreign molecular material into a single cell such as an
oocyte,
the system should effectively constrain the cell and introduce foreign
molecular material
into the cell's nucleus. As was shown in FIG. 1, the system may utilize three
polysilicon
arms arranged around the oocyte. Two of these arms (support structures 12) are
arranged
and configured to constrain the cell, while the third (moveable support frame
16) includes
a needle structure for introducing molecular material into the cell. In one
aspect, the three
arms will cause the oocyte to align in a proper position as they come into
contact. As has
also been described, any of the three arms may be actuated manually by the
user, or they
may be actuated by motors or other movement systems.
To hold an oocyte in place, the two support structure arms rise up out of
plane and
move toward the cell. The arms are lamina-emergent, change point, parallel-
guiding, six-
bar mechanisms, kinematically grounded to the chip substrate, and receive a
linear input
from a slider. The constraining arms are designed to contact the cell wall
above the cell's
midline to prevent both lateral and vertical displacements during transfer of
molecular
materiaI. For oocytes such as mouse cells (80 - 100 gm in diameter), the
constraining
arms are designed to have a total vertical displacement of 68.2 m and a total
horizontal
displacement of 84.6 m from its fabricated position. Given such dimensions,
one of
ordinary skill in the art could readily modify the present system to be
adaptable to other
cell sizes. The constraining arm's large horizontal displacement thus allows
for a larger
area into which the cell can be initially placed. The needle arm, similar to
the
constraining arms, can be made of a lamina-emergent, change-point, parallel-
guiding, six-
-13-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
bar mechanism receiving a linear input from a slider. However, the needle
structure
mechanism is attached to a moveable support frame. The moveable support frame
is a
fully compliant, parallel guiding mechanism with, for mouse oocytes, 50 m of
in-plane
travel. In one aspect, the moveable support frame is sufficiently stiff so
that as the slider
moves forward, the needle rises up out of the plane with minimal horizontal
displacement
in the translating stage. Further forward movement causes the moveable support
frame
to deflect and move the needle structure horizontally in its extended position
toward the
second locus, thus maintaining a parallel relationship between the elongate
axis of the
needle structure in the initial position with the elongate axis in the
extended position. The
previously described system is shown more fully in FIGs. 2 and 3. FIG. 2 shows
the
system in the initial position, including the moveable support frame 22, the
needle
structure, 24, and a compliant folded beam suspension 26 to facilitate the
horizontal
movement of the needle structure 24 once in the extended position. The aspect
shown in
FIG. 2 additionally shows a manual slider 28 to allow a user to manually
actuate the
device from the initial position to the extended position. The system can
additionally
includes electrical contacts to charge the needle structure (not shown).
FIG. 3 shows the system in the extended position. Note that as the moveable
support frame 32 is moved from the initial position shown in FIG. 2, the
needle structure
34 is maintained in an orientation that is parallel to the needle structure in
the initial
position.
The following description relates to DNA, however the present scope should not
be limited to such. Rather, DNA is used to describe various embodiments for
convenience. Though it has a net charge of zero, DNA has an unequal
distribution of
internal charges resulting in a negative character equal to two electrons for
every base
pair. Exploiting the electrical characteristics of DNA, the needle structure
may be
designed to attract, associate, and release molecular material using static
electric charges.
Thus, the needle requires no pumps, no capillaries, and consists only of an
easily
fabricated, solid, pointed body. The needle and the bottommost monosilicon
layer of a
MEMS chip may form a capacitor. Voltage is applied to the needle structure
using a
compliant beam attached to either side the moveable support frame. The
compliant beam
may be highly folded and attach to the needle structure about halfway between
the two
vertical legs to prevent the generation of moments that might cause the needle
structure to
-14-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
rotate about a horizontal axis. The compliant beams are electrically coupled
to fixed
electrical bonding pads near the device through which charge can be applied.
In this case,
a positive charge would be applied to attract the negatively charged DNA
molecules. The
negative terminal of the voltage supply can be attached to a bonding pad
connected to the
underlying monosilicon substrate.
The MEMS construction can be achieved by a variety of methods. The MEMS
methods themselves are well known, and will not be discussed in detail. One
exemplary
process, however, is polyMUMPs (Polysilicon MEMS Multi-User Processes), a
fabrication process for surface micromachined polysilicon structures (MEMSCAP
USA).
Accordingly, the MEMS system described can be operated as follows: DNA is
introduced into the solution containing the needle structure. A positive
charge is applied
to the needle structure to attract the DNA sample. A single cell such as an
oocyte is
positioned at a point to receive the needle structure and the associated DNA.
As is shown
in FIG. 4, constraining structures 42 are raised to align and constrain the
oocyte. In FIG.
4, the oocyte would be located on top of the pad shown at 44. The moveable
support
frame 46 is raised to the extended position, and the needle structure 48 is
extended
horizontally forward into the oocyte until the nucleus has been punctured by
the needle.
The polarity of the electrical charge can then be reversed to release the DNA
into the
oocyte nucleus, and the needle structure can be retracted from the cell.
In one aspect, to be useful a practical, self-contained pumpless MEMS
injection
device should satisfy three principle constraints. First, to be practically
useful, the MEMS
device should must concentrate measurable amounts of DNA on its surface in a
reasonably short amount of time. Second, to increase the likelihood of cell
survival, the
MEMS needle structure should remain inside the cell for as little time as
possible; e.g. the
MEMS needle structure should be capable of repelling DNA concentrated on its
surface
in a matter of seconds. Third, to prevent damage to the device or the cell,
and to prevent
unwanted bubble formation, the MEMS needle structure should not cause
electrolysis of
the surrounding water. In some cases, not causing electrolysis of the water
surrounding
the MEMS needle structure, may be particularly limiting. In initial
feasibility testing,
electrolysis occurred at approximately 1.8 V on gold bond pads submerged in an
aqueous
solution of 0.9% NaCI (i.e. saline solution). In initial testing, when
electrolysis was
allowed to occur, delamination of gold-on-polysilicon bond pads, and near
complete
-15-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
removal of gold from gold on polysilicon bond pads were observed. Thus, it
appears that
for some applications the operating voltage for the MEMS needle structure
should be less
than 1.8 V.
Examples
The following examples are provided to promote a more clear understanding of
certain embodiments of the present invention, and are in no way meant as a
limitation
thereon.
Example I:DNA Visualization and Ima ing Methods
4',6-diamidino-2-phenylindole dihydrochloride (DAPI) is used to visualize the
DNA in the following example. DAPI exhibits low toxicity and its strong
fluorescence
under ultra-violet illumination. When dissolved in water and not bound to DNA,
DAPI
has an excitation maximum of 355 nm (ultra-violet light) and an emission
maximum of
453 nm (blue light). When DAPI is bound to DNA its excitation maximum changes
to
388 nm and its emission maximum shifts to 454 nm, and the intensity of the
emitted light
increases roughly twenty-fold compared to free DAPI. The increase in emission
intensity
when DAPI binds with DNA makes it possible to distinguish between unbound DAPI
and
DAPI-stained DNA.
DAPI-stained DNA is visualized using a Zeiss Axioskop Fluorescence
Microscope with UV illumination and a purpose-built blue light filter for
imaging DAPI
stained samples. Because the DAPI-DNA complex fluoresces in the blue portion
of the
visible spectrum, the blue color channel is isolated from raw RGB images to
simplify
image analysis. To provide quantitative estimates of the concentration of DNA
on or near
the needle structure, a regression model is made of blue pixel intensity (I)
as a function of
DNA concentration (C). DAPI stained DNA samples of known and uniform
concentration are imaged using the aforementioned parameters, and the mean
blue
channel pixel intensities of the images are calculated using a MATLAB script.
The
relationship between concentration in ng1 L and blue pixel intensity is linear
as shown in
FIG. 5. A linear fit to the data gives the relationship shown in Equation I
C = (I- 83.07)136:788 1
-16-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
The blue channel intensity measurements are shown with 95% error bounds, and
the
linear
model of the data given in Equation I is represented by the solid line.
Because of the long
exposure used (six seconds), the intercept value (blue pixel intensity at 0
ng/ L) is highly
susceptible to changes in ambient lighting conditions. In cases where the
ambient
lighting conditions cause the intercept to deviate from the value of 83.07
shown in
Equation I, a reference image of a MEMS die submerged in the appropriate media
(either
distilled water or 0.9% NaCI) with no DNA present can be used to calculate the
value of
the intercept under those lighting conditions.
Example 2: DNA Attraction Experiment
The DNA attraction and repulsion experiments are performed both in distilled
water and in 0.9% saline solution. In both cases, the experiments follow
identical
protocols, with
the exception of the media into which the device is submerged. A MEMS device
as has
been described herein is covered in approximately 2 mm of either distilled
water or 0.9%
saline solution. A 1-2 L drop of 306 ng/ L DAPI stained DNA is placed in the
solution
near the device using a calibrated pipette. The needle structure of the MEMS
device is
connected to the positive terminal of a voltage source providing 1.5 V DC. The
substrate
of the MEMS device is connected to the negative terminal of the voltage
source. Images
can be taken to verify the DAPI-stained DNA on the surface of the needle
structure.
Approximate concentrations of the DNA can be calculated using the linear model
of
Equation I.
Example 3: DNA Repulsion Experiment
DNA is attracted to the tip of a MEMS needle structure as is described in
Example
2, by connecting the needle structure to the positive terminal of a 1.5 V DC
voltage
source and connecting the MEMS device substrate to the negative terminal of
the voltage
source. Following attraction of DNA, the polarity of the electrical charge is
then reversed
so that the positive terminal is connected to the MEMS device substrate and
the negative
terminal is connected to the needle structure. Images can be taken from the
time the
polarity is reversed to verify DNA release and repulsion. Additionally, the
time between
-17-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
connecting the MEMS needle structure to the negative terminal and when DNA is
clearly
repelled from the needle structure can be calculated, and approximate
concentrations can
be calculated using the linear model given in Equation I.
Example 4: Distilled Water Experiment
A MEMS device as has been described herein is covered in approximately 2 mm
of distilled water. A 1-2 L drop of 306 ng/pL DAPI stained DNA is placed in
the
distilled water near the device using a calibrated pipette. The needle
structure of the
MEMS device is connected to the positive terminal of a voltage source
providing 1.5 V
DC. The substrate of the MEMS device is connected to the negative terminal of
the
voltage source. Images can be taken to verify the DAPI-stained DNA on the
surface of
the needle structure. After 1 hour 17 minutes of incubation, approximately 2.4-
2.5 ng/ L
is attracted to the tip of the charged needle structure. The approximations
are derived
from the linear model of Equation I.
Following attraction of DNA, the polarity of the electrical charge is then
reversed
so that the positive terminal is connected to the MEMS device substrate and
the negative
terminal is connected to the needle structure. The negatively charged needle
structure
repels measurable amounts of DNA from its tip within six seconds of the
polarity change.
Example 5;0.9% Saline Solution Experiment
A MEMS device as has been described herein is covered in approximately 2 mm
of 0.9% saline solution, A 1-2 L drop of 306 ng/gL DAPI stained DNA is placed
in the
saline solution near the device using a calibrated pipette. The needle
structure of the
MEMS device is connected to the positive terminal of a voltage source
providing 1.5 V
DC. The substrate of the MEMS device is connected to the negative terminal of
the
voltage source. When connected in this manner and submerged in 0.9% NaCI
solution,
the MEMS needle structure has a capacitance of approximately 230 pf. Images
can be
taken to verify the DAPI-stained DNA on the surface of the needle structure.
After 5
minutes 46 seconds of incubation, approximately 2.2-2.4 ng/ L is attracted to
the tip of
the charged needle structure. The approximations are derived from the linear
model of
Equation I.
-18-
CA 02692742 2010-01-05
WO 2009/009610 PCT/US2008/069550
Following attraction of DNA, the polarity of the electrical charge is then
reversed
so that the positive terminal is connected to the MEMS device substrate and
the negative
terminal is connected to the needle structure. The negatively charged needle
structure
repels measurable amounts of DNA from its tip within six seconds of the
polarity change.
It is to be understood that the above-described compositions and modes of
application are only illustrative of preferred embodiments of the present
invention.
Numerous modifications and alternative arrangements may be devised by those
skilled in
the art without departing from the spirit and scope of the present invention
and the
appended claims are intended to cover such modifications and arrangements.
Thus, while
the present invention has been described above with particularity and detail
in connection
with what is presently deemed to be the most practical and preferred
embodiments of the
invention, it will be apparent to those of ordinary skill in the art that
numerous
modifications, including, but not limited to, variations in size, materials,
shape, form,
function and manner of operation, assembly and use may be made without
departing from
the principles and concepts set forth herein.
-19-