Note: Descriptions are shown in the official language in which they were submitted.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
1
METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER, TUMORS, AND
TUMOR-RELATED DISORDERS
CROSS-REFERENCE
[0001] This application claims the benefit of U. S. Provisional Application
No. 60/949,483, filed July
12, 2007; U.S. Provisional Application No. 60/990,900, filed November 28,
2007; and U.S.
Provisional Application No. 61/044,425, filed April 11, 2008, the contents of
which are incorporated
herein by reference in their entirety.
FIELD
[0002] The present invention relates to combination compositions and the use
of such combinations
for the treatment of cancer, tumors, and tumor-related disorders.
BACKGROUND
[0003] Cancer, tumors, tumor-related disorders, and neoplastic disease states
are serious and often
times life-threatening conditions. These diseases and disorders, which are
characterized by rapidly-
proliferating cell growth, continue to be the subject of research efforts
directed toward the
identification of therapeutic agents which are effective in the treatment
thereof. Such agents prolong
the survival of the patient, inhibit the rapidly-proliferating cell growth
associated with the neoplasm,
or effect a regression of the neoplasm.
[0004] Generally, surgery and radiation therapy are the first modalities
considered for the treatment
of cancer that is considered locally confmed, and offer the best prognosis.
Chemotherapy treatment of
certain cancers typically results in disappointing survival rates but still
offer a survival benefit. For
example, in patients with non-small cell lung cancer, platinum-based
chemotherapy regimens, such as
the use of either cisplatin or carboplatin plus one of either paclitaxel,
docetaxel, gemcitabine,
vinorelbine, irinotecan, etoposide, vinblastine, or bevacizumab is employed.
If patients cannot tolerate
this therapy, a single agent, such as N-(3-ethynylphenyl)-6,7-bis(2-
methoxyethoxy)quinazolin-4-
amine, commonly known as erlotinib (Tarceva ), can be used. Erlotinib targets
the epidermal growth
factor receptor tyrosine kinase which is highly expressed and occasionally
mutated in various forms of
cancer. If patients fail to respond to an erlotinib treatment, additional
conventional treatment offers
limited benefit.
[0005] Despite erlotinib's approval for the treatment of stage IIIB and IV non-
small cell lung cancer,
as with most therapeutic agents, side-effects result from its use. For
example, common side effects,
occurring in greater than 30% of patients taking erlotinib, include, rash,
diarrhea, poor appetite,
fatigue, shortness of breath, cough, nausea and vomiting. Additionally, less
common side effects
include infection, mouth sores, itching, dry skin, eye irritation, pulmonary
fibrosis, and abdominal
pain. Of greater concern, is the growing view that, while utilization of
erlotinib for the treatment of
tumors may initially shrink the size of the tumor, the tumor may eventually
enlarge in size, indicating,
among other things, the development of resistance. Erlotinib may be
representative of the types of
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
2
therapeutic agents being used for cancer treatment; in that its use has an
effect on cancer, but because
of other factors, which are not entirely known, the tumor develops resistance
and progresses.
[0006] What is needed, therefore, are compositions and/or methods of treatment
for cancer which
take advantage of the synergy found in a therapeutic combination that could
increase the effectiveness
of the agents and reduce and/or eliminate the side effects typically
associated with conventional
treatments.
SUMMARY OF THE INVENTION
[0007] The present invention is based at least in part on a synergistic effect
obtained in treating
conditions such as cancer by a combination comprising a COX-2 selective
inhibitor and an EGFR
inhibitor. Some of the possible favorable outcomes for synergism include 1)
increasing the efficacy of
the therapeutic effect, 2) decreasing the dosage but increasing or maintaining
the same efficacy to
avoid toxicity, 3) minimizing or slowing down the development of drug
resistance, and 4) providing
selective synergism against target (or efficacy synergism) versus host (or
toxicity antagonism).
[0008] While some embodiments of the invention are illustrated through a fixed
dose combination
comprising a COX-2 inhibitor and an EGFR inhibitor, the embodiments of the
invention cover
compositions and methods wherein the COX-2 inhibitor and the EGFR inhibitor
are provided in
separate dosage forms.
[0009] The present invention is based, at least in part on the discovery that
combinations of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor provide numerous advantages
in the treatment of
cancer, tumors, and tumor-related disorders. These combinations may allow for
lesser amounts of
each particular agent to be delivered and may produce the same or better
effect as a greater amount of
each particular agent. For example, a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor (provided in one fixed dose or in two separate dosage forms) will
increase therapeutic
efficacy, reduce side effects, reduce resistance, and when a fixed dose is
used reduce the pill burden,
enhance patient compliance, and thus, support a more favorable treatment
setting and outcome. Such
combinations can further increase the benefit of each particular agent which
otherwise would be only
marginally effective. As such, this synergistic or enhancement effect can be
employed in the treatment
of cancer, tumors, and tumor-related disorders, and may overcome resistance of
tumors to drugs at
conventional doses.
Methods of Use
[0010] The present invention provides a method for treating cancer, tumors,
and tumor-related
disorders. For example, the method comprises administering to a subject in a
combination therapy an
amount of a COX-2 selective inhibitor and an EGFR inhibitor, wherein the COX-2
selective inhibitor
and the EGFR inhibitor combine to give a therapy suitable for treating a
cancer, tumor, and tumor-
related disorders.
[0011] The invention further provides combinations of COX-2 selective
inhibitors and EGFR
inhibitors for antineoplastic, anti-tumor, anti-cancer combination therapy. In
one embodiment the
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
3
invention provides compositions comprising a combination of a COX-2 selective
inhibitor and an
EGFR inhibitor. The invention also provides for the use of combinations of 1,2-
diphenylpyrrole
derivatives and an EGFR inhibitor and/or their pharmaceutically acceptable
salt, solvate, or prodrug,
as antineoplastic, anti-tumor, or anti-cancer combination therapy.
[0012] In one embodiment, the invention provides a method for treating a
subject having cancer,
comprising administering to the subject, a therapeutically effective amount of
a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor or their
respective
pharmaceutically acceptable salt, solvate or prodrug.
[0013] In one embodiment, the 1,2-diphenylpyrrole derivative of the
combination composition has
the following formula:
R4
R3
R2 N
% R
SO2R1
wherein:
R is a hydrogen atom, a halogen atom or an alkyl group having from 1 to 6
carbon atoms;
R' is an alkyl group having from 1 to 6 carbon atoms or an amino group;
R2 is a phenyl group which is unsubstituted or is substituted by at least one
substituent
selected from the group consisting of substituents a and substituents (3;
R3 is a hydrogen atom, a halogen atom or an alkyl group which has from 1 to 6
carbon atoms
and which is unsubstituted or is substituted by at least one substituent
selected from the group
consisting of a hydroxy group, a halogen atom, an alkoxy group having from 1
to 6 carbon atoms and
an alkylthio group having from 1 to 6 carbon atoms;
R4 is a hydrogen atom; an alkyl group which has from 1 to 6 carbon atoms and
which is
unsubstituted or is substituted by at least one substituent selected from the
group consisting of a
hydroxy group, a halogen atom, an alkoxy group having from 1 to 6 carbon atoms
and an alkylthio
group having from 1 to 6 carbon atoms; a cycloalkyl group having from 3 to 8
carbon atoms, an aryl
group; or an aralkyl group; said aryl group having from 6 to 14 ring carbon
atoms in a carbocyclic ring
and are unsubstituted or are substituted by at least one substituent selected
from the group consisting
of substituents a and substituents (3;
said aralkyl group are an alkyl group having from 1 to 6 carbon atoms and
which are
substituted by at least one aryl group as defined above;
said substituents a are selected from the group consisting of a hydroxy group,
a halogen atom,
an alkoxy group having from 1 to 6 carbon atoms and an alkylthio group having
from 1 to 6 carbon
atoms;
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
4
said substituents 0 are selected from the group consisting of an alkyl group
which has from 1 to 6
carbon atoms and which is unsubstituted or are substituted by at least one
substituent selected from the
group consisting of a hydroxy group, a halogen atom, an alkoxy group having
from 1 to 6 carbon
atoms and an alkylthio group having from 1 to 6 carbon atoms; an alkanoyloxy
group having from 1 to
6 carbon atoms; a mercapto group; an alkanoylthio group having from 1 to 6
carbon atoms; an
alkylsulfinyl group having from 1 to 6 carbon atoms; a cycloalkloxy group
having from 3 to 8 carbon
atoms; a haloalkoxy group having from 1 to 6 carbon atoms; and an
alkylenedioxy group having from
1 to 6 carbon atoms; or a pharmaceutically acceptable salt, solvate, or
prodrug.
[0014] In another embodiment, a 1,2-diphenylpyrrole derivative of the
combination composition has
the following formula:
3 R4
R2 N
R
S02R1
R is a hydrogen atom, a halogen atom or an alkyl group having from 1 to 4
carbon atoms;
R' is a methyl group or an amino group;
RZ is an unsubstituted phenyl group or a phenyl group which is substituted by
at least one
substituent selected from the group consisting of a halogen atom; an alkoxy
group having from 1 to 4
carbon atoms; an alkylthio group having from 1 to 4 carbon atoms; an
unsubstituted alkyl group
having from 1 to 4 carbon atoms; an alkyl group having from 1 to 4 carbon
atoms and which is
substituted by at least one substituent selected from the group consisting of
a halogen atom, an alkoxy
group having from 1 to 4 carbon atoms and an alkylthio group having from 1 to
4 carbon atoms; a
haloalkoxy group having from 1 to 4 carbon atoms; and an alkylenedioxy group
having from 1 to 4
carbon atoms;
R3 is a hydrogen atom, a halogen atom, an unsubstituted alkyl group having
from 1 to 4
carbon atoms or a substituted alkyl group having from 1 to 4 carbon atoms and
substituted by at least
one substituent selected from the group consisting of a halogen atom, an
alkoxy group having from 1
to 4 carbon atoms and an alkylthio group having from 1 to 4 carbon atoms;
R4 is a hydrogen atom; an unsubstituted alkyl group having from 1 to 4 carbon
atoms; a
substituted alkyl group having from 1 to 4 carbon atoms and substituted by at
least one substituent
selected from the group consisting of a hydroxy group, a halogen atom, an
alkoxy group having from
1 to 4 carbon atoms and an alkylthio group having from 1 to carbon atoms; a
cycloalkyl group having
from 3 to 6 carbon atoms; an aryl group which has from 6 to 10 ring carbon
atoms and which is
unsubstituted or is substituted by at least one substituent selected from the
group consisting of a
halogen atom; an alkoxy group having from 1 to 4 carbon atoms; an alkylthio
group having from 1 to
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
4 carbon atoms; an unsubstituted alkyl group having from 1 to 4 carbon atoms;
an alkyl group having
from 1 to 4 carbon atoms and substituted by at least one substituent selected
from the group consisting
of a hydroxy group, a halogen atom, an alkoxy group having from 1 to 4 carbon
atoms and an
alkylthio group having from 1 to 4 carbon atoms; and a cycloalkyloxy group
having from 3 to 7
5 carbon atoms; an aralkyl group having from 1 to 4 carbon atoms in the alkyl
part and containing at
least one said aryl group; or a pharmaceutically acceptable salt, solvate, or
prodrug.
[0015] In a further embodiment, the invention provides a 1,2-diphenylpyrrole
derivative of the
combination composition wherein:
R is a hydrogen atom;
R' is an amino group;
RZ is an unsubstituted phenyl group or a phenyl group which is substituted by
at least one
substituent selected from the group consisting of a halogen atom, an alkoxy
group having from 1 to 4
carbon atoms, an alkylthio group having from 1 to 4 carbon atoms, an alkyl
group having from 1 to 4
carbon atoms, a haloalkyl group having from 1 to 4 carbon atoms, a haloalkoxy
group having from 1
to 4 carbon atoms and a alkylenedioxy group having from 1 to 4 carbon atoms;
R3 is a hydrogen atom, a halogen atom, an alkyl group having from 1 to 4
carbon atoms or a
haloalkyl group having from 1 to 4 carbon atoms;
R4 is a hydrogen atom; an unsubstituted alkyl group having from 1 to 4 carbon
atoms; a
substituted alkyl group having from 1 to 4 carbon atoms and substituted by at
least one substituent
selected from the group consisting of a hydroxy group and an alkoxy group
having from 1 to 4 carbon
atoms; a cycloalkyl group having from 3 to 6 carbon atoms; an aryl group which
has from 6 to 10 ring
carbon atoms and which is unsubstituted or is substituted by at least one
substituent selected from the
group consisting of a hydroxy group; a halogen atom; an alkoxy group having
from 1 to 4 carbon
atoms; an unsubstituted alkyl group having from 1 to 4 carbon atoms; an alkyl
group having from 1 to
4 carbon atoms and which is unsubstituted or substituted by at least one
halogen atom; and a
cycloalkyloxy group having from 3 to 7 carbon atoms; and an aralkyl group
having from 1 to 4 carbon
atoms in the alkyl part and containing at least one said aryl group; or a
pharmaceutically acceptable
salt, solvate, or prodrug.
[00161 In yet a further embodiment, the 1,2-diphenylpyrrole derivative is
selected from the group
consisting of: 4-methyl-2-(4-methylphenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-
methoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)pyrrole; 2-(4-chlorophenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 4-
methyl-2-(4-methylthiophenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-(4-methoxy-3-methylphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-
(3-fluoro-4-methoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)pyrrole; 2-(3,4-
dimethylphenyl)-4-
methyl-l-(4-sulfamoylphenyl)pyrrole; 4-methyl-l-(4-methylthiophenyl)-2-(4-
sulfamoylphenyl)pyrrole; 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(4-
methoxyphenyl)pyrrole;
and 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(3,4-dimethylphenyl)pyrrole.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
6
[0017] In one embodiment the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole.
[0018] In another embodiment the EGFR inhibitor is erlotinib.
[0019] In a further embodiment, the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-
1-(4-sulfamoylphenyl)-pyrrole2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and the
EGFR inhibitor is erlotinib.
[0020] In yet a further embodiment the 1,2-diphenylpyrrole derivative and the
EGFR inhibitor are
administered sequentially in either order or simultaneously.
100211 In one embodiment the 1,2-diphenylpyrrole derivative is administered
first.
[0022] In another embodiment the EGFR inhibitor is administered first.
[0023] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders, comprising administering the combination by a mode of
administration comprising
oral, parenteral, buccal, intranasal, epidural, sublingual, pulmonary, local,
rectal, or transdermal
administration.
[0024] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders wherein the combination is orally administered as a single
dosage form.
[0025] In another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single dosage form enhances patient
compliance and/or reduces
pill burden.
[0026] In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single dosage form is a single capsule or
a single tablet.
[0027] In yet a further embodiment, the invention provides a method for
treating cancer, tumors, and
tumor-related disorders wherein the composition is provided as a single
tablet.
[0028] In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders, comprising administering the combination by a mode of
parenteral
administration selected from intravenous, subcutaneous, intrathecal, and
intramuscular administration.
Dosages
[0029] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders comprising administering the combination in a single tablet
wherein the single tablet
comprises from about 1 mg to about 1200 mg of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and from about 25 mg to about 450 mg of erlotinib.
[0030] In another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 25 mg of
erlotinib.
[0031] In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl- 1 -(4-sulfamoylphenyl)-pyrrole and about 100 mg of
erlotinib.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
7
[0032] In yet a further embodiment, the invention provides a method for
treating cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 150 mg of
erlotinib.
[0033] In another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 200 mg of
erlotinib.
[0034] In yet another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and about 300 mg of
erlotinib.
[0035] In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises from about 1 mg to
about 1200 mg 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and about 450 mg of
erlotinib.
[0036] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders wherein the single tablet comprises about 1 mg, about 5 mg,
about 10 mg, about 25
mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400 mg, about
600 mg, about
800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and about 25 mg of erlotinib.
[0037] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders wherein the single tablet comprises about 1 mg, about 5 mg,
about 10 mg, about 25
mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400 mg, about
600 mg, about
800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and about 100 mg of erlotinib.
[0038] In another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises about 1 mg, about
5 mg, about 10 mg,
about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400
mg, about 600 mg,
about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 150 mg of erlotinib.
[0039] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders wherein the single tablet comprises about 1 mg, about 5 mg,
about 10 mg, about 25
mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400 mg, about
600 mg, about
800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and about 200 mg of erlotinib.
[0040] In another embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises about 1 mg, about
5 mg, about 10 mg,
about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400
mg, about 600 mg,
about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 300 mg of erlotinib.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
8
100411 In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders wherein the single tablet comprises about 1 mg, about
5 mg, about 10 mg,
about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300 mg, about 400
mg, about 600 mg,
about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 450 mg of erlotinib.
[00421 In one embodiment, the invention provides a method of treating cancer,
tumors, and tumor-
related disorders comprising administering a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the composition is
suitable for once-daily
administration.
[0043] In a further embodiment, the invention provides a method wherein
administering the
combination enhances treatment of the subject compared to administering one
component of the
combination alone.
Types of Cancer
[0044] In yet a further embodiment, the invention provides a method wherein
administering the
combination reduces the side effects of treatment for a cancer, tumor, and
tumor-related disorders.
[0045] In one embodiment the cancer is selected from the group consisting o
oral cancer, prostate
cancer, rectal cancer, non-small cell lung cancer, lip and oral cavity cancer,
liver cancer, lung cancer,
anal cancer, kidney cancer, vulvar cancer, breast cancer, oropharyngeal
cancer, nasal cavity and
paranasal sinus cancer, nasopharyngeal cancer, urethra cancer, small intestine
cancer, bile duct cancer,
bladder cancer, ovarian cancer, laryngeal cancer, hypopharyngeal cancer,
gallbladder cancer, colon
cancer, colorectal cancer, head and neck cancer, glioma; parathyroid cancer,
penile cancer, vaginal
cancer, thyroid cancer, pancreatic cancer, esophageal cancer, Hodgkin's
lymphoma, leukemia-related
disorders, mycosis fungoides, and myelodysplastic syndrome.
[0046] In another embodiment the cancer is non-small cell lung cancer,
pancreatic cancer, breast
cancer, ovarian cancer, colorectal cancer, or head and neck cancer.
[0047] In yet another embodiment the cancer is a carcinoma, a tumor, a
neoplasm, a lymphoma, a
melanoma, a glioma, a sarcoma, or a blastoma.
[0048] In one embodiment the carcinoma is selected from the group consisting
of: carcinoma,
adenocarcinoma, adenoid cystic carcinoma, adenosquamous carcinoma,
adrenocortical carcinoma,
well differentiated carcinoma, squamous cell carcinoma, serous carcinoma,
small cell carcinoma,
invasive squamous cell carcinoma, large cell carcinoma, islet cell carcinoma,
oat cell carcinoma,
squamous carcinoma, undifferentiatied carcinoma, verrucous carcinoma, renal
cell carcinoma,
papillary serous adenocarcinoma, merkel cell carcinoma, hepatocellular
carcinoma, soft tissue
carcinomas, bronchial gland carcinomas, capillary carcinoma, bartholin gland
carcinoma, basal cell
carcinoma, carcinosarcoma, papilloma/carcinoma, clear cell carcinoma,
endometrioid
adenocarcinoma, mesothelial, metastatic carcinoma, mucoepidermoid carcinoma,
cholangiocarcinoma,
actinic keratoses, cystadenoma, and hepatic adenomatosis.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
9
[0049] In another embodiment the tumor is selected from the group consisting
of: astrocytic tumors,
malignant mesothelial tumors, ovarian germ cell tumors, supratentorial
primitive neuroectodermal
tumors, Wilm's tumors, pituitary tumors, extragonadal germ cell tumors,
gastrinoma, germ cell tumors,
gestational trophoblastic tumors, brain tumors, pineal and supratentorial
primitive neuroectodermal
tumors, pituitary tumors, somatostatin-secreting tumors, endodermal sinus
tumors, carcinoids, central
cerebral astrocytoma, glucagonoma, hepatic adenoma, insulinoma,
medulloepithelioma,
plasmacytoma, vipoma, and pheochromocytoma.
[0050] In yet another embodiment the neoplasm is selected from the group
consisting o
intraepithelial neoplasia, multiple myeloma/plasma cell neoplasm, plasma cell
neoplasm,
interepithelial squamous cell neoplasia, endometrial hyperplasia, focal
nodular hyperplasia,
hemangioendothelioma, and malignant thymoma.
[0051] In a further embodiment the lymphoma is selected from the group
consisting of: nervous
system lymphoma, AIDS-related lymphoma, cutaneous T-cell lymphoma, non-
Hodgkin's lymphoma,
lymphoma, and Waldenstrom's macroglobulinemia.
[0052] In another embodiment the melanoma is selected from the group
consisting o acral
lentiginous melanoma, superficial spreading melanoma, uveal melanoma, lentigo
maligna melanomas,
melanoma, intraocular melanoma, adenocarcinoma nodular melanoma, and
hemangioma.
[0053] In yet another embodiment the sarcoma is selected from the group
consisting of: adenomas,
adenosarcoma, chondosarcoma, endometrial stromal sarcoma, Ewing's sarcoma,
Kaposi's sarcoma,
leiomyosarcoma, rhabdomyosarcoma, sarcoma, uterine sarcoma, osteosarcoma, and
pseudosarcoma.
[0054] In one embodiment the glioma is selected from the group consisting of:
glioma, brain stem
glioma, and hypothalamic and visual pathway glioma.
[0055] In another embodiment the blastoma is selected from the group
consisting of: pulmonary
blastoma, pleuropulmonary blastoma, retinoblastoma, neuroblastoma,
medulloblastoma, glioblastoma,
and hemangiblastomas.
EGFR Inhibitors
[0056] In a further embodiment, the invention provides a method wherein the
EGFR inhibitor is a
small molecule compound or an antibody.
[0057] In one embodiment, the invention provides a method wherein the small
molecule compound
is selected from the group consisting of: ZM-254530, BIBX-1382, reveromycin A,
gefitinib, CGP-
57148, CGP-59326, 4-(3-chloro)-5,6-dimethyl-7H-pyrrolo[2,3-d]pyrimidine,
tyrphostin, PKI-166, PD
153035, EKB-569, and 4-(phenylamino)quinazolines, or their pharmaceutically
acceptable salts,
solvates, or prodrugs.
[0058] In another embodiment the invention provides a method wherein the
antibody is selected from
the group consisting of: EGF receptor antibody, MR1scFvPE38KDEL MDX-447, MDX-
210, MD-
72000, MDX-260, wayne anti-EGFR Mabs, anti-EGFr Mab, anti-EGFr MAb, Genen anti-
EGFR Mab,
MAb DC-101, trastuzumab, anti-VEGF monoclonal, anti-EGFR-DM1 Ab, MAb 4D5, BAB-
447,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
EMD-55900, EMD-6200, -82633, anti-EGFR Mab, MAb 4D5, cetuximab, anti-EGFr MAb,
anti-flk-1,
CCX, CCZ, anti-flk-1, AG-514, AG-568, nti-EGFR-DMl Ab, MDX-447, TgDCC-ElA and
C225,
matuzumab, panitumumab, DWP-408, and RC-394011.
[0059] In another embodiment the EGFR inhibitor is selected from the group
consisting of:
5 muellerian-inhibiting hormone, TNP-470, tecogalan sodium, EGF receptor
antisense, PI-88,
oligonucleotide, bromelain molecules, amphiregulin, EGF fusion toxin, EGF
fusion protein,
Amphiregulin hbEGF-toxin, hbEGF-toxin, and EGF fusion protein.
Further Methods of Use
[0060] In one embodiment, the invention provides a method of inducing
differentiation of tumor
10 cells, the method comprising contacting the cells with an effective amount
of a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor whereby the
combination induces
differentiation of tumor cells.
[0061] In one embodiment, the invention provides a method of inhibiting
proliferation of cancer cells,
the method comprising contacting a cancer cell with a combination comprising a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor whereby the combination inhibits
proliferation of cancer cells.
[0062] In another embodiment, the invention provides a method for reducing
proliferation of cancer
cells, the method comprising delivering to the cells a combination comprising
a 1,2-diphenylpyrrole
derivative and an EGFR inhibitor, whereby the reduction of cell proliferation
is greater than a
reduction caused by either a 1,2-diphenylpyrrole derivative alone or an EGFR
inhibitor alone.
[0063] In one embodiment, the invention provides a method of modulating
autophosphorylation with
a molecule of ATP, the method comprising delivering to a cancer cell an
effective amount of a
combination comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor
wherein the
combination inhibits autophosphorylation with a molecule of ATP.
[0064] In a further embodiment, the invention provides a method of inhibiting
metastases of tumor
cells, the method comprising administering an effective amount of a
combination comprising a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor such that the combination
inhibits metastatic
activity of tumor cells.
[0065] In one embodiment, the invention provides a method for inducing
apoptosis in cancer cells,
the method comprising contacting the cancer cells with a combination
comprising a 1,2-
diphenylpyrrole derivative and an EGFR kinase sufficient to induce apoptosis.
[0066] In another embodiment, the invention provides a method for sensitizing
EGFR-inhibitor
resistant cancer cells to an EGFR inhibitor, the method comprising
administering a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the
combination
sensitizes the cancer cells to the EGFR inhibitor.
[0067] In one embodiment, the invention provides a method of treating EGFR
resistance in a cancer
cell, the method comprising, administering a combination comprising a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
11
[0068] In a further embodiment, the invention provides a method of modulating
prostaglandin
synthesis in a cancer cell, the method comprising contacting the cell with a
combination comprising a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the combination
inhibits prostaglandin
synthesis in a cancer cell.
[0069] In one embodiment, the invention provides a method of modulating
cyclooxygenase
expression in a cancer cell, the method comprising delivering to the cell a
combination comprising a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the combination
inhibits
cyclooxygenase expression in a cancer cell.
[0070] In one embodiment, the invention provides a method of modulating
angiogenesis in a cancer
cell, the method comprising contacting the cell with a combination comprising
a 1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the combination inhibits angiogenesis
in a cancer cell.
[0071] In another embodiment, the invention provides a method of reducing the
dosage in
conventional treatment for neoplasia and/or neoplasia related disorders in a
subject, the method
comprising administering to a subject a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor wherein the combination reduces the dosage in conventional treatment
for neoplasia and/or
neoplasia-related disorders.
[0072] In one embodiment, the invention provides a method of treating
neoplasia and/or neoplasia
related disorders, the method comprising administering a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor
[0073] In one embodiment, the invention provides a composition for treating
cancer comprising, a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor or their
respective
pharmaceutically acceptable salt, solvate or prodrug.
[0074] In one embodiment, the invention provides a method of treatment
comprising administering a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl- 1 -
(4-sulfamoylphenyl)-
pyrrole.
[0075] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor, wherein the EGFR inhibitor is erlotinib.
[0076] In yet another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib.
[0077] In one embodiment, the invention provides a method of treatment
comprising administering a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the composition is a single dosage form.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
12
[0078] In a further embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein a single dosage form enhances patient compliance and/or
reduces pill burden.
[0079] In one embodiment, the invention provides a method for treating cancer,
tumors, and tumor-
related disorders, comprising administering a combination according to the
invention by a mode of
administration comprising oral, parenteral, buccal, intranasal, epidural,
sublingual, pulmonary, local,
rectal, or transdermal administration.
[0080] In a further embodiment, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders, comprising a combination according to the invention
by parenteral
administration selected from intravenous, subcutaneous, intrathecal, and
intramuscular administration.
[0081] In one embodiment, the invention provides a method comprising
administering the
combination in a single dosage form.
[0082] In yet a further embodiment, the invention provides a method of
treatment comprising
administering a composition comprising a single tablet combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein a single dosage form is a single
capsule or a single tablet.
[0083] In one embodiment, the invention provides a method of treatment
comprising administering a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the composition is in the form of a single tablet.
[0084] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and from about 25 mg to
about 450 mg of
erlotinib.
[0085] In yet another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 25 mg of
erlotinib.
[0086] In one embodiment, the invention provides a method of treatment
comprising administering a
single tablet composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor wherein the single tablet comprises from about 1 mg to about 1200 mg
2-(4-ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and about 100 mg of erlotinib.
[0087] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 150 mg of
erlotinib.
[0088] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
13
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 200 mg of
erlotinib.
[0089] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 3 00 mg of
erlotinib.
[0090] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the single tablet comprises from about 1 mg to about
1200 mg 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and about 450 mg of
erlotinib.
[0091] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 25 mg of erlotinib.
[0092] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 100 mg of erlotinib.
[0093] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 150 mg of erlotinib.
[0094] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 200 mg of erlotinib.
[0095] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
14
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-1-(4-
sulfamoylphenyl)-pyrrole and about 300 mg of erlotinib.
[0096] In a further embodiment, the invention provides a method of treatment
comprising
administering a single tablet composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the single tablet comprises from
about 1 mg, about 5 mg,
about 10 mg, about 25 mg, about 50 mg, about 100 mg, about 200 mg, about 300
mg, about 400 mg,
about 600 mg, about 800 mg, about 1000 mg, or about 1200 mg 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and about 450 mg of erlotinib.
[0097] In one embodiment, the invention provides a method of treatment
comprising administering a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the composition is suitable for once-daily administration.
[0098] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the EGFR inhibitor is a small molecule compound or an
antibody.
[0099] In a further embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and a small
molecule wherein the small molecule compound is selected from the group
consisting of: ZM-
254530, BIBX-1382, reveromycin A, gefitinib, CGP-57148, CGP-59326, 4-(3-
chloro)-5,6-dimethyl-
7H-pyrrolo[2,3-d]pyrimidine, tyrphostin, PKI-166, PD 153035, EKB-569, and 4-
(phenylamino)quinazolines, or their pharmaceutically acceptable salts,
solvates, or prodrugs.
[00100] In another embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the EGFR inhibitor is an antibody selected from the
group consisting of: EGF
receptor antibody, MR1scFvPE38KDEL MDX-447, MDX-210, MD-72000, MDX-260, wayne
anti-
EGFR Mabs, anti-EGFr Mab, anti-EGFr MAb, Genen anti-EGFR Mab, MAb DC-101,
trastuzumab,
anti-VEGF monoclonal, anti-EGFR-DM1 Ab, MAb 4D5, BAB-447, EMD-55900, EMD-6200,
-
82633, anti-EGFR Mab, MAb 4D5, cetuximab, anti-EGFr MAb, anti-flk-1, CCX, CCZ,
anti-flk-1,
AG-514, AG-568, nti-EGFR-DMI Ab, MDX-447, TgDCC-E1A, C225, matuzumab,
panitumumab,
DWP-408, and RC-394011.
[00101] In yet another embodiment, the invention provides a method of
treatment comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the composition contains a lower dose of an EGFR
inhibitor than a
conventional treatment for cancer.
[00102] In a further embodiment, the invention provides a method of treatment
comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the composition reduces the side effects of cancer
treatment.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
[00103] In yet a further embodiment, the invention provides a method of
treatment comprising
administering a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an
EGFR inhibitor wherein the composition enhances treatment of cancer.
Pharmaceutical Compositions
5 [00104] In one embodiment the invention provides a pharmaceutical
composition for treating cancer
comprising, a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor, and a
pharmaceutically acceptable excipient or carrier.
[00105] In one embodiment, the invention provides a pharmaceutical composition
for treating cancer
wherein the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
10 pyrrole.
[00106] In another embodiment, the invention provides a pharmaceutical
composition for treating
cancer wherein the EGFR inhibitor is erlotinib.
[00107] In yet another embodiment, the invention provides a pharmaceutical
composition for treating
cancer wherein the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-
methyl-l-(4-
15 sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib.
Kits/Articles of Manufacture
[00108] In one embodiment the invention provides a kit for treating cancer
comprising a single dosage
form comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor and
instructions on administration.
[00109] In one embodiment the invention provides a kit for treating cancer
having a composition
comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib.
INCORPORATION BY REFERENCE
[00110] All publications, patents, and patent applications described in this
specification are herein
incorporated by reference to the same extent as if each individual
publication, patent, or patent
application was specifically and individually indicated to be incorporated by
reference.
BRIEF DESCRIPTION OF THE DRAWINGS
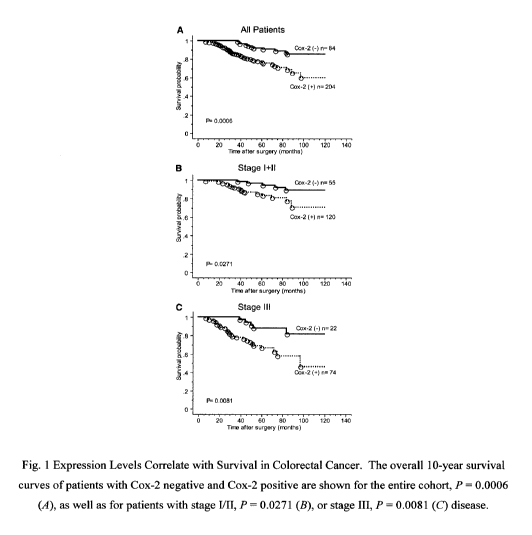
[00111] Figure 1 provides graphs illustrating COX-2 expression levels in
colorectal cancer.
[00112] Figure 2 provides an illustration of the interactions between the EGFR
and COX pathways.
1001131 Figure 3 provides a graph illustrating the results of a A43 1 squamous
vulvar carcinoma
xenograft study.
DETAILED DESCRIPTION
[00114] To facilitate understanding of the disclosure set forth herein, a
number of terms are defined
below.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
16
Definitions
[00115] As used herein, "abnormal cell growth," refers to cell growth that is
independent of normal
regulatory mechanisms (e.g., loss of contact inhibition), including the
abnormal growth of normal cells
and the growth of abnormal cells. This includes, but is not limited to, the
abnormal growth of: (1)
tumor cells (tumors), both benign and malignant, expressing an activated Ras
oncogene; (2) tumor
cells, both benign and malignant, in which the Ras protein is activated as a
result of oncogenic
mutation in another gene; (3) benign and malignant cells of other
proliferative diseases in which
aberrant Ras activation occurs. Examples of such benign proliferative diseases
are psoriasis, benign
prostatic hypertrophy, human papilloma virus (HPV), and restenosis. Abnormal
cell growth, also
refers to and includes the abnormal growth of cells, both benign and
malignant, resulting from activity
of the enzymes farnesyl protein transferase, protein kinases, protein
phosphatases, lipid kinases, lipid
phosphatases, or activity of transcription factors, or intracellular or cell
surface receptor proteins.
[00116] "Neoplasia" as described herein, is an abnormal, unregulated and
disorganized proliferation
of cells that is distinguished from normal cells by autonomous growth and
somatic mutations. An
accumulation of neoplastic cells is also known as a neoplasm, or tumor. As
neoplastic cells grow and
divide they pass on their genetic mutations and proliferative characteristics
to progeny cells. In some
embodiments, the neoplasm can be benign or malignant.
[00117] "Metastasis," as used herein, refers to the dissemination of tumor
cells via lymphatics or blood
vessels. Metastasis also refers to the migration of tumor cells by direct
extension through serous
cavities, or subarachnoid or other spaces. Through the process of metastasis,
tumor cell migration to
other areas of the body establishes neoplasms in areas away from the site of
initial appearance.
[00118] As discussed herein, "angiogenesis" is prominent in tumor formation
and metastasis.
Angiogenic factors have been found associated with several solid tumors such
as rhabdomyosarcomas,
retinoblastoma, Ewing sarcoma, neuroblastoma, and osteosarcoma. A tumor cannot
expand without a
blood supply to provide nutrients and remove cellular wastes. Tumors in which
angiogenesis is
important include solid tumors such as renal cell carcinoma, hepatocellular
carcinoma, and benign
tumors such as acoustic neuroma, and neurofibroma, trachoma and pyogenic
granulomas.
Angiogenesis has been associated with blood-born tumors such as leukemias. It
is believed that
angiogenesis plays a role in the abnormalities in the bone marrow that give
rise to leukemia.
Prevention of angiogenesis could halt the growth of cancerous tumors and the
resultant damage to the
subject due to the presence of the tumor.
[00119] The term "subject" refers to an animal, including, but not limited to,
a primate (e.g., human),
cow, sheep, goat, horse, dog, cat, rabbit, rat, or mouse. The terms "subject"
and "patient" are used
interchangeably herein in reference, for example, to a mammalian subject, such
as a human subject.
[00120] The terms "treat," "treating," and "treatment" are meant to include
alleviating or abrogating a
disorder, disease, or condition; or one or more of the symptoms associated
with the disorder, disease,
or condition; or alleviating or eradicating the cause(s) of the disorder,
disease, or condition itself.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
17
[00121] The term "therapeutically effective amount" refers to the amount of a
compound that, when
administered, is sufficient to prevent development of, or alleviate to some
extent, one or more of the
symptoms of the disorder, disease, or condition being treated. The term
"therapeutically effective
amount" also refers to the amount of a compound that is sufficient to elicit
the biological or medical
response of a cell, tissue, system, animal, or human that is being sought by a
researcher, veterinarian,
medical doctor, or clinician.
[00122] The term "pharmaceutically acceptable carrier," "pharmaceutically
acceptable excipient,"
"physiologically acceptable carrier," or "physiologically acceptable
excipient" refers to a
pharmaceutically-acceptable material, composition, or vehicle, such as a
liquid or solid filler, diluent,
excipient, solvent, or encapsulating material. Each component must be
"pharmaceutically acceptable"
in the sense of being compatible with the other ingredients of a
pharmaceutical formulation. It must
also be suitable for use in contact with the tissue or organ of humans and
animals without excessive
toxicity, irritation, allergic response, inmmunogenicity, or other problems or
complications,
commensurate with a reasonable benefit/risk ratio. See, Remington: The Science
and Practice of
Pharmacy, 21st Edition; Lippincott Williams & Wilkins: Philadelphia, PA, 2005;
Handbook of
Pharmaceutical Excipients, 5th Edition; Rowe et al., Eds., The Pharmaceutical
Press and the
American Pharmaceutical Association: 2005; and Handbook of Pharmaceutical
Additives, 3rd Edition;
Ash and Ash Eds., Gower Publishing Company: 2007; Pharmaceutical
Preformulation and
Formulation, Gibson Ed., CRC Press LLC: Boca Raton, FL, 2004).
[00123] The term "pharmaceutical composition" refers to a mixture of a
compound disclosed herein
with other chemical components, such as diluents or carriers. The
pharmaceutical composition
facilitates administration of the compound to an organism. Multiple techniques
of administering a
compound exist in the art including, but not limited to, oral, injection,
aerosol, parenteral, and topical
administration. Pharmaceutical compositions can also be obtained by reacting
compounds with
inorganic or organic acids such as hydrochloric acid, hydrobromic acid,
sulfuric acid, nitric acid,
phosphoric acid, methanesulfonic acid, ethanesulfonic acid, p-toluenesulfonic
acid, salicylic acid and
the like.
Cyclooxygenase
1001241 Cyclooxygenase (COX) is an enzyme that is responsible for the
formation of important
biological mediators called prostanoids, including prostaglandins,
prostacyclin and thromboxane.
COX converts arachidonic acid, an co-6 essential fatty acid, to prostaglandin
H2 (PGH2), the precursor
of the series-2 prostanoids. The enzyme contains two active sites: a heme with
peroxidase activity,
responsible for the reduction of PGG2 to PGH2, and a cyclooxygenase site,
where arachidonic acid is
converted into the hydroperoxy endoperoxide prostaglandin G2 (PGG2). The
reaction proceeds
through a hydrogen atom abstraction from arachidonic acid by a tyrosine
radical generated by the
peroxidase active site, then two oxygen molecules react with the arachidonic
acid radical, giving
PGGz.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
18
1001251 COX-1 is a constitutive enzyme responsible for biosynthesis of
prostaglandins in the gastric
mucosa and in the kidney among other sites. COX-2 is an enzyme that is
produced by an inducible
gene that is responsible for biosynthesis of prostaglandins in inflammatory
cells. Inflammation causes
induction of COX-2, leading to release of prostanoids (prostaglandin E2),
which sensitize peripheral
nociceptor terminals and produce localized pain hypersensitivity, inflammation
and edema.
[00126] Historically, physicians have treated inflammation-related disorders
with a regimen of
NSAIDs such as, for example, aspirin and ibuprofen. Undesirably, however, some
NSAIDs are
known to cause gastrointestinal (GI) bleeding or ulcers in patients undergoing
consistent long term
regimens of NSAID therapy. Henry et al., Lancet, 1991, 337, 730. A reduction
of unwanted side
effects of common NSAIDs was made possible by the discovery that the two
cyclooxygenases
involved in the transformation of arachidonic acid as the first step in the
prostaglandin synthesis
pathway were cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2).
[00127]Many common NSAIDs are now known to be inhibitors of both COX-1 and COX-
2.
Accordingly, when administered in sufficiently high levels, these NSAIDs not
only alleviate the
inflammatory consequences of COX-2 activity, but also inhibit the beneficial
gastric maintenance
activities of COX-1. Research into the area of arachidonic acid metabolism has
resulted in the
discovery of compounds that selectively inhibit the COX-2 enzyme to a greater
extent than they
inhibit COX-1. These COX-2 selective inhibitors are believed to offer
advantages that include the
capacity to prevent or reduce inflammation while avoiding harmful side effects
associated with the
inhibition of COX- 1. Thus, COX-2 selective inhibitors have shown great
promise for use in therapies,
especially in therapies that require maintenance administration, such as for
pain and inflammation
control.
[00128] As described herein, the terms "cyclooxygenase-2 inhibitor" and "Cox-2
inhibitor", which can
be used interchangeably herein, denote compounds which inhibit the
cyclooxygenase-2 enzyme
(COX-2) regardless of the degree of inhibition of the cyclooxygenase-1 enzyme
(COX-1), and include
pharmaceutically acceptable racemates, enantiomers, tautomers, salts, esters
and prodrugs of those
compounds. Thus, for purposes of the present disclosure, a compound is
considered a COX-2
inhibitor although the compound inhibits COX-2 to an equal, greater, or lesser
degree than it inhibits
COX-l. COX-2 inhibitors herein therefore encompass many traditional non-
selective NSAIDs.
COX-2 Selective Inhibitors
[00129] COX-2 inhibitors useful according to embodiments of the present
disclosure are agents and
compounds that selectively or preferentially inhibit COX-2 to a greater degree
than they inhibit COX-
1. Such agents and compounds are termed "COX-2 selective inhibitors" herein.
[00130] In practice, in a test for selectivity of a COX-2 selective inhibitor,
the observed selectivity
varies depending upon the conditions under which the test is performed and on
the compound being
tested. For example, selectivity of a COX-2 inhibitor can be measured as a
ratio of the in vitro or in
vivo IC50 value for inhibition of COX-1, divided by the corresponding IC50
value for inhibition of
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
19
COX-2 (COX-1 IC50/COX-2 ICso). A COX-2 selective inhibitor herein is thus any
inhibitor for which
COX-1 IC50/COX-2 IC50 is greater than 1. In various embodiments, this ratio is
greater than about 2,
greater than about 5, greater than about 10, greater than about 50, or greater
than about 100.
1001311 In one embodiment, the invention provides a composition, comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the ratio of
selectivity of COX-2 over
COX-1 inhibition is greater than about 1. In another embodiment, the invention
provides a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the ratio of selectivity of COX-2 over COX-1 inhibition is greater
than about 2. In a further
embodiment, the invention provides a composition comprising combination of a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the ratio of selectivity of COX-2
over COX-1 inhibition is
greater than about 5. In another embodiment, the invention provides a
composition comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the ratio of selectivity
of COX-2 over COX-1 inhibition is greater than about 7.8. In yet a further
embodiment, the invention
provides a composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor wherein the ratio of selectivity of COX-2 over COX-1 inhibition is
greater than about 10. In
another embodiment, the invention provides a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the ratio of
selectivity of COX-2 over
COX-1 inhibition is greater than about 20. In a further embodiment, the
invention provides a
composition comprising a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the ratio of selectivity of COX-2 over COX-1 inhibition is greater
than about 50. In yet a
further embodiment, the invention provides a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the ratio of
selectivity of COX-2 over
COX-1 inhibition is greater than about 100. In one embodiment, the invention
provides a composition
comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein 1-2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole. In another
embodiment, the invention provides a composition comprising a combination of a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the EGFR inhibitor is erlotinib. In a
further embodiment,
the invention provides a composition comprising a combination of a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor wherein the 1-2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib and wherein
the ratio of selectivity of
COX-2 over COX-1 inhibition is greater than about 1, about 2, about 5, about
7.8, about 10, about 20,
about 50, and about 100.
1001321 In one embodiment, the invention provides a method for treating a
tumor and/or tumor related
disease comprising administering a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor wherein the ratio of selectivity of COX-2 over COX-1 inhibition is
greater than about 1. In
one embodiment, the invention provides a method for treating a tumor and/or
tumor related disease
comprising administering a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
wherein the ratio of selectivity of COX-2 over COX- 1 inhibition is greater
than about 2. In a further
embodiment, the invention provides a method for treating a tumor and/or tumor
related disease
comprising administering a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the ratio of selectivity of COX-2 over COX-1 inhibition is greater
than about 5. In yet a
5 further embodiment, the invention provides a method for treating a tumor
and/or tumor related disease
comprising administering a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
wherein the ratio of selectivity of COX-2 over COX-1 inhibition is greater
than about 10. In some
embodiments, the invention provides methods for treating a tumor and/or tumor
related disease
comprising administering a combination of a 1,2-diphenylpyrrole derivative and
an EGFR inhibitor
10 wherein the ratio of selectivity of COX-2 over COX-1 inhibition is greater
than about 20, than about
50, and about 100. In other embodiments, the invention provides methods for
treating a tumor and/or
tumor related disease comprising administering a combination of a 1,2-
diphenylpyrrole derivative and
an EGFR inhibitor wherein the 1-2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib wherein the ratio
of selectivity of COX-
15 2 over COX-1 inhibition is greater than about 1, about 2, about 5, about
7.8, about 10, about 20, about
50, and about 100.
1001331 As used herein, the term "IC50" with respect to a COX-1 or COX-2
inhibitor refers to the
concentration of a compound that is required to produce 50% inhibition of
activity of COX-1 or COX-
2. In one embodiment, 1,2-diphenylpyrrole derivatives useful in the present
disclosure can have a
20 COX-2 IC50 of less than about 3 M. In another embodiment, the 1,2-
diphenylpyrrole derivative has a
COX-2 IC50 of less than about 2.8 M. In yet another embodiment, the 1,2-
diphenylpyrrole derivative
has a COX-2 IC50 of less than about 2 M. In some embodiments, 1,2-
diphenylpyrrole derivative
useful in the present disclosure can have a COX-2 IC50 of less than about 1
gM, less than about 0.5
gM, or less than about 0.2 M. 1,2-diphenylpyrrole derivatives useful in the
present disclosure can
have a COX-1 IC50 of greater than about 1 M, for example greater than about
20 M. In one
embodiment, the invention provides a composition comprising a combination of a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the IC50 is less than about I M. In
another embodiment,
the invention provides a composition comprising a combination of a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor wherein the IC50 is less than about 0.5 M. In a further
embodiment, the
invention provides a composition comprising a combination of a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor wherein the IC50 is less than about 0.2 M. In one embodiment
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole. In another
embodiment the EGFR inhibitor is erlotinib. In a further embodiment the 1,2-
diphenylpyrrole
derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
the EGFR inhibitor is
erlotinib wherein the IC50 is less than about 3 M, about 2.8 M, about 2 M,
about 1 M, about 0.5
M, and about 0.2 M.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
21
[00134] COX-2 inhibitors exhibiting a high degree of selectivity for COX-2
over COX-1 inhibition
can indicate ability to reduce incidence of common NSAID-induced side effects.
In one embodiment,
the invention provides a composition comprising a combination of a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor wherein the NSAID-induced side effects are substantially
diminished. For
example, NSAID-induced side effects include, but are not limited to, nausea,
vomiting, diarrhea,
constipation, decreased appetite, rash, dizziness, headache, drowsiness, fluid
retention, edema, kidney
failure, liver failure, ulcers and prolonged bleeding after surgery. In
another embodiment, the
invention provides a composition comprising a combination of a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib and wherein the
NSAID-induced side
effects are substantially diminished.
[001351 A COX-2 selective inhibitor can be used in a form of a prodrug
thereof. As described herein,
a "COX-2 prodrug" is a compound that can be converted into an active COX-2
selective inhibitor by
metabolic or simple chemical processes within the body of the subject. One
example of a prodrug for
a COX-2 selective inhibitor is parecoxib, for example in a form of a salt such
as parecoxib sodium,
which is a therapeutically effective prodrug of the tricyclic COX-2 selective
inhibitor valdecoxib.
Overexpression of COX-2 and Cancer
{00136] The overexpression of COX-2 and also the upstream and downstream
enzymes of the
prostaglandin synthesis pathway has been demonstrated in multiple cancer types
and some pre-
neoplastic lesions. Direct interactions of prostaglandins with their receptors
through autocrine or
paracrine pathways to enhance cellular survival or stimulate angiogenesis have
been proposed as
molecular mechanisms underlying the pro-carcinogenic functions of COX enzymes.
In this respect,
preclinical studies suggest that COX-2 may be involved in the molecular
pathogenesis of some types
of lung cancer. Most of the studies point to its involvement in non-small cell
lung cancer. Survival of
patients with non-small cell lung cancer expressing high levels of COX-2 is
markedly reduced.
Treatment of humans with the selective COX-2 inhibitor celecoxib augments the
antitumor effects of
chemotherapy in patients with non-small cell lung cancer.
Studies indicate that prostaglandins synthesized by cyclooxygenase play a
critical role in the initiation
and promotion of cancer. Aberrant COX-2 expression was reported in colorectal
carcinomas and
adenomas, and has been detected in various human cancers, including those of
the breast. Moreover,
COX-2 is overexpressed in neoplastic lesions of the colon, breast, lung,
prostate, esophagus, pancreas,
intestine, cervix, ovaries, urinary bladder and head and neck (see Table 1
below).
Tumor Type % Tissue expressing COX-2
Colorectal Cancer 70-95
Non-small Cell Lung Cancer 70-90
Gastric Cancer 45-75
Pancreatic Cancer 40-80
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
22
Glioblastoma Multiforme 40-70
Bladder Cancer 50-60
Esophageal Cancer 50-60
Breast Cancer 40-50
Ovarian Cancer 40-60
Prostate Cancer 40-60
Table 1. COX-2 Expression in Tumors
[00137] COX-2 overexpression in murine mammary glands is sufficient to cause
tumor formation. In
several in vitro and animal models, COX-2 inhibitors have inhibited tumor
growth and metastasis.
[00138] In addition to cancers per se, COX-2 is also expressed in the
angiogenic vasculature within
and adjacent to hyperplastic and neoplastic lesions indicating that COX-2
plays a role in angiogenesis.
In both the mouse and rat, COX-2 inhibitors markedly inhibited bFGF-induces
neovascularization.
The utility of COX-2 inhibitors as chemopreventive, antiangiogenic and
chemotherapeutic agents is
described in the literature. Koki et al., Exp. Opin., Invest. Drugs, 1999,
8(10) 1623-38.
[00139] COX-2 has been shown to regulate some embodiments of tumor-associated
angiogenesis.
Angiogenesis is an attractive therapeutic target because it is a multi-step
process that occurs in a
specific sequence, thus providing several possible targets for drug action.
Angiogenesis is important
in two stages of tumor metastasis. The first stage where angiogenesis
stimulation is important is in the
vascularization of the tumor which allows the tumor cells to enter the blood
stream and to circulate
throughout the body. After the tumor cells have left the primary site and have
settled into the
secondary, metastasis site, angiogenesis must occur before the new tumor can
grow and expand.
Therefore, prevention of angiogenesis could lead to the prevention of
metastasis of tumors and
possibly contain the neoplastic growth at the primary site. Examples of agents
that interfere with
several of these steps include, angiostatin, endostatin, interferon alpha and
COX-2 selective inhibitors
that prevent the growth of cells that form new blood vessels; and protein-
based compounds that
simultaneously interfere with several of these targets.
[00140] Additionally, several studies have suggested that COX-2 expression is
associated with
parameters of aggressive breast cancer, including large tumor size, positive
axillary lymph node
metastases, and HER2-positive tumor status. Studies of mammary tumors in mice
and rats have
indicated that moderate to high COX-2 expression is related to the genesis of
mammary tumors that
are sensitive to treatment with nonspecific and specific COX-2 inhibitors.
Further studies have shown
that increased amounts of prostaglandins and COX-2 are commonly found in a
wide range of
premalignant tissues and malignant tumors including cervical dysplasia and
cancer. Elevated
prostaglandin and COX-2 levels substantially contribute to carcinogenesis by
inhibiting apoptosis and
stimulating angiogenesis. Tsujii and DuBois, Cell, 1995, 83, 493-501.
[00141] Further, COX-2 is also highly expressed in prostate cancer,
particularly in the epithelial cell of
high-grade prostatic intraepithelial neoplasia and cancer. It was shown that
treatment of human
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
23
prostate cancer cell lines with a selective COX-2 inhibitor induces apoptosis
both in vitro and in vivo.
The in vivo results also indicate that the COX-2 inhibitor decreases tumor
microvessel density and
angiogenesis. COX-2 inhibitors can prevent the hypnoxic upregulation of a
potent angiogenic factor,
vascular endothelial growth factor.
[00142] Overexpression of COX-2 has been documented in several premalignant
and malignant
tissues. Subbaramaiah et al., Trends Pharmacol. Sci., 2003, 24, 96-102.
Without wishing to be bound
by any particular theory, this increase in expression is thought to be a
product of stimulation of PKC
signaling, which stimulates activity of MAPK, enhancing transcription of COX-2
by nuclear factors.
Additionally, enhanced stability of COX-2 mRNA transcripts in cancer cells due
to augmented
binding of the RNA-binding protein HuR, as well as activation of extracellular
signal related kinase 1
and 2 (ERK 1 and 2) and p38, is thought to contribute to increased expression
of COX-2.
[00143] These results indicate that COX-2 inhibitors may serve as effective
chemopreventive and
therapeutic agents in cancer of the prostate.
COX-2 Selective Inhibitors
[00144] It has recently been found that the use of nonsteroidal anti-
inflammatory drugs (NSAIDs) has
been associated with the prevention and treatment of several types of cancer.
Thun et al., J. National
Cancer Inst., 2002, 94(4), 252-66. COX-2 inhibitors have been utilized for the
treatment of cancer
and for the treatment of tumors. For example, celecoxib, a COX-2 selective
inhibitor, exerted a potent
inhibition of fibroblast growth factor-induced corneal angiogenesis in rats.
Masferrer et al., Proc. Am.
Assoc. Cancer Research, 1999, 40, 396. Other COX-2 inhibitors have been
described for the
treatment of cancer, tumors and neoplasia. FR 27 71 005 describes compositions
containing a COX-2
inhibitor and N-methyl-d-aspartate (NMDA) antagonist used to treat cancer and
other diseases.
Further, WO 99/18960 describes a combination comprising a COX-2 inhibitor that
can be used to treat
colorectal and breast cancer. Additionally, WO 97/36497 describes a
combination comprising a COX-
2 inhibitor and a 5-lipoxygenase inhibitor useful in treating cancer.
1,2-Diphenylpyrrole Derivatives
[00145] 1,2-Diphenylpyrrole derivatives and pharmaceutically acceptable salts,
solvates, or prodrugs
are known to have analgesic and antiphlogistic properties. Further, they have
been shown to act as
COX-2 selective inhibitors and are thus effective for the prophylaxis and
therapy of diseases mediated
by COX-2 and/or inflammatory cytokines. In addition, 1,2-diphenylpyrrole
derivatives have been
shown to treat diseases involving or resulting from the resorption of bone,
such as osteoporosis,
rheumatoid arthritis and osteoarthritis.
[00146] These types of analgesics, anti-inflammatory agents and/or
antipyretics exhibit effects not
only on inflammatory diseases, such as pain, pyrexia, and edema, but also on
chronic inflammatory
diseases, such as chronic rheumatoid arthritis and osteoarthritis, allergic
inflammatory diseases,
asthma, sepsis, psoriasis, various autoimmune diseases, systemic lupus
erythematosus, juvenile onset
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
24
diabetes, autoimmune intestinal diseases (such as ulcerative colitis, Crohn's
disease), viral infection,
and glomerulonephritis.
[00147] 1,2-Diphenylpyrrole derivatives described herein have the general
formula:
R4
R3
)[~
R2 N
R
SO2R1
wherein:
R is a hydrogen atom, a halogen atom or an alkyl group having from 1 to 6
carbon atoms;
R' is an alkyl group having from 1 to 6 carbon atoms or an amino group;
R2 is a phenyl group which is unsubstituted or is substituted by at least one
substituent
selected from the group consisting of substituents a and substituents f3;
R3 is a hydrogen atom, a halogen atom or an alkyl group which has from 1 to 6
carbon atoms
and which is unsubstituted or is substituted by at least one substituent
selected from the group
consisting of a hydroxy group, a halogen atom, an alkoxy group having from 1
to 6 carbon atoms and
an alkylthio group having from 1 to 6 carbon atoms;
R4 is a hydrogen atom; an alkyl group which has from 1 to 6 carbon atoms and
which is
unsubstituted or is substituted by at least one substituent selected from the
group consisting of a
hydroxy group, a halogen atom, an alkoxy group having from 1 to 6 carbon atoms
and an alkylthio
group having from 1 to 6 carbon atoms; a cycloalkyl group having from 3 to 8
carbon atoms, an aryl
group; or an aralkyl group; said aryl group having from 6 to 14 ring carbon
atoms in a carbocyclic ring
and are unsubstituted or are substituted by at least one substituent selected
from the group consisting
of substituents a and substituents [3; said aralkyl group are an alkyl group
having from 1 to 6 carbon
atoms and which are substituted by at least one aryl group as defined above;
said substituents a are selected from the group consisting of a hydroxy group,
a halogen atom,
an alkoxy group having from 1 to 6 carbon atoms and an alkylthio group having
from 1 to 6 carbon
atoms;
said substituents 0 are selected from the group consisting of an alkyl group
which has from 1
to 6 carbon atoms and which is unsubstituted or are substituted by at least
one substituent selected
from the group consisting of a hydroxy group, a halogen atom, an alkoxy group
having from 1 to 6
carbon atoms and an alkylthio group having from 1 to 6 carbon atoms; an
alkanoyloxy group having
from 1 to 6 carbon atoms; a mercapto group; an alkanoylthio group having from
1 to 6 carbon atoms;
an alkylsulfinyl group having from 1 to 6 carbon atoms; a cycloalkloxy group
having from 3 to 8
carbon atoms; a haloalkoxy group having from 1 to 6 carbon atoms; and an
alkylenedioxy group
having from 1 to 6 carbon atoms; or a pharmaceutically acceptable salt,
solvate, or prodrug.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
[00148] In one embodiment, the invention provides a 1,2-diphenylpyrrole
derivative having the
formula:
R4
R3
Rz N
R
SOzRI
wherein:
5 R is a hydrogen atom, a halogen atom or an alkyl group having from 1 to 4
carbon atoms;
Rl is a methyl group or an amino group;
RZ is an unsubstituted phenyl group or a phenyl group which is substituted by
at least one
substituent selected from the group consisting of a halogen atom; an alkoxy
group having from 1 to 4
carbon atoms; an alkylthio group having from 1 to 4 carbon atoms; an
unsubstituted alkyl group
10 having from 1 to 4 carbon atoms; an alkyl group having from 1 to 4 carbon
atoms and which is
substituted by at least one substituent selected from the group consisting of
a halogen atom, an alkoxy
group having from 1 to 4 carbon atoms and an alkylthio group having from 1 to
4 carbon atoms; a
haloalkoxy group having from 1 to 4 carbon atoms; and an alkylenedioxy group
having from 1 to 4
carbon atoms;
15 R3 is a hydrogen atom, a halogen atom, an unsubstituted alkyl group having
from 1 to 4
carbon atoms or a substituted alkyl group having from 1 to 4 carbon atoms and
substituted by at least
one substituent selected from the group consisting of a halogen atom, an
alkoxy group having from 1
to 4 carbon atoms and an alkylthio group having from 1 to 4 carbon atoms;
R4 is a hydrogen atom; an unsubstituted alkyl group having from 1 to 4 carbon
atoms; a
20 substituted alkyl group having from 1 to 4 carbon atoms and substituted by
at least one substituent
selected from the group consisting of a hydroxy group, a halogen atom, an
alkoxy group having from
1 to 4 carbon atoms and an alkylthio group having from 1 to carbon atoms; a
cycloalkyl group having
from 3 to 6 carbon atoms; an aryl group which has from 6 to 10 ring carbon
atoms and which is
unsubstituted or is substituted by at least one substituent selected from the
group consisting of a
25 halogen atom; an alkoxy group having from 1 to 4 carbon atoms; an alkylthio
group having from 1 to
4 carbon atoms; an unsubstituted alkyl group having from 1 to 4 carbon atoms;
an alkyl group having
from I to 4 carbon atoms and substituted by at least one substituent selected
from the group consisting
of a hydroxy group, a halogen atom, an alkoxy group having from 1 to 4 carbon
atoms and an
alkylthio group having from 1 to 4 carbon atoms; and a cycloalkyloxy group
having from 3 to 7
carbon atoms; an aralkyl group having from I to 4 carbon atoms in the alkyl
part and containing at
least one said aryl group; or a pharmaceutically acceptable salt, solvate, or
prodrug.
[00149] In one embodiment, the invention provides a 1,2-diphenylpyrrole
derivative wherein:
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
26
R is a hydrogen atom;
R' is an amino group;
R2 is an unsubstituted phenyl group or a phenyl group which is substituted by
at least one
substituent selected from the group consisting of a halogen atom, an alkoxy
group having from 1 to 4
carbon atoms, an alkylthio group having from 1 to 4 carbon atoms, an alkyl
group having from 1 to 4
carbon atoms, a haloalkyl group having from 1 to 4 carbon atoms, a haloalkoxy
group having from 1
to 4 carbon atoms and a alkylenedioxy group having from 1 to 4 carbon atoms;
R3 is a hydrogen atom, a halogen atom, an alkyl group having from 1 to 4
carbon atoms or a
haloalkyl group having from 1 to 4 carbon atoms;
R4 is a hydrogen atom; an unsubstituted alkyl group having from 1 to 4 carbon
atoms; a
substituted alkyl group having from 1 to 4 carbon atoms and substituted by at
least one substituent
selected from the group consisting of a hydroxy group and an alkoxy group
having from 1 to 4 carbon
atoms; a cycloalkyl group having from 3 to 6 carbon atoms; an aryl group which
has from 6 to 10 ring
carbon atoms and which is unsubstituted or is substituted by at least one
substituent selected from the
group consisting of a hydroxy group; a halogen atom; an alkoxy group having
from 1 to 4 carbon
atoms; an unsubstituted alkyl group having from I to 4 carbon atoms; an alkyl
group having from 1 to
4 carbon atoms and which is unsubstituted or substituted by at least one
halogen atom; and a
cycloalkyloxy group having from 3 to 7 carbon atoms; and an aralkyl group
having from 1 to 4 carbon
atoms in the alkyl part and containing at least one said aryl group; or a
pharmaceutically acceptable
salt, solvate, or prodrug.
[00150] In one embodiment, R is a hydrogen atom. In another embodiment, R is a
fluorine atom. In a
further embodiment, R is a chlorine atom. In yet a further embodiment, R is a
methyl group.
[00151] In one embodiment, Rl is a methyl group. In another embodiment, Rl is
an amino group.
[00152] In one embodiment, RZ is a phenyl group.
[00153] In one embodiment, R3 is a hydrogen atom. In another embodiment, R3 is
a halogen atom.
[00154] In one embodiment, R4 is a hydrogen atom.
[00155] The term "aryl" refers to a carbocyclic aromatic hydrocarbon group
having from 6 to 14
carbon atoms in one or more aromatic rings or such a group which is fused to a
cycloalkyl group
having from 3 to 10 carbon atoms, and the group is unsubstituted or it is
substituted by at least one
substituent selected from the group consisting of hydroxy groups, halogen
atoms, lower alkoxy
groups, lower alkylthio groups, lower alkyl groups, alkanoyloxy groups,
mercapto groups, alknoylthio
groups, lower alkylsulfinyl groups, lower alkyl groups having at least one
substituent selected from the
group consisting of cycloalkloxy groups, lower haloalkoxy groups, and lower
alkylenedioxy groups.
[00156] In some embodiments, the 1,2-diphenylpyrrole derivative is selected
from the group consisting
of compounds 2-1 - 2-213 of Table 2 as disclosed in U.S. 6,887,893, which is
herein incorporated in
its entirety by reference.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
27
[00157] In one embodiment, the 1,2-diphenylpyrrole derivative is selected from
the group consisting
of: 4-methyl-2-(4-methylphenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-
methoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-(4-chlorophenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 4-methyl-2-(4-
methylthiophenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-ethoxyphenyl)-4-methyl-l-
(4-
sulfamoylphenyl)pyrrole; 2-(4-methoxy-3-methylphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-
(3-fluoro-4-methoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)pyrrole; 2-(3,4-
dimethylphenyl)-4-
methyl-l-(4-sulfamoylphenyl)pyrrole; 4-methyl-l-(4-methylthiophenyl)-2-(4-
sulfamoylphenyl)pyrrole; 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(4-
methoxyphenyl)pyrrole;
and 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(3,4-dimethylphenyl)pyrrole. In
another
embodiment, the invention provides a method wherein the 1,2-diphenylpyrrole
derivative is 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole.
[00158] In another embodiment, the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-pyrrole.
[00159] The methods for synthesizing 1,2-diphenylpyrrole derivatives,
illustrated herein, are described
in the Examples section and in U.S. RE39,420, which is incorporated herein by
reference in its
entirety.
Me
N
EtO C
SO2NH2
2-(4-ethoxyphenyl)-4-methyl- 1 -(4-sulfamoylphenyl)-pyrrole
[00160]2-(4-Ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole has
alternative chemical names,
including 4-[2-(4-ethoxyphenyl)-4-methyl-lH-pyrrol-1-yl benzenesulfonamide, 4-
[2-(4-
ethoxyphenyl)-4-methyl-lH-pyrrol-1-yl]benzenesulfonamide, TGO1, CS-706 and
apricoxib. It has a
molecular formula of C19H2ON203S, a molecular weight of 356.4 and the CAS
registry number is
197904-84-0.
Receptor Tyrosine Kinases
[00161] Protein tyrosine kinases are a class of enzymes that catalyze the
transfer of a phosphate group
from ATP or GTP to the tyrosine residue located on protein substrates. Protein
tyrosine kinases
clearly play a role in normal cell growth. Many of the growth factor receptor
proteins function as
tyrosine kinases and it is by this process that they effect signaling. The
interaction of growth factors
with these receptors is a necessary event in normal regulation of cell growth.
Under certain
conditions, however, as a result of either mutation or overexpression, these
receptors can become
deregulated; the result of which is uncontrolled cell proliferation which can
lead to tumor growth and
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
28
ultimately to the disease known as cancer. Wilks, Adv. Cancer Res., 1993, 60,
43. Among the growth
factor receptor kinases and their proto-oncogenes that have been identified
and which are targets of the
combinations presented herein are the epidermal growth factor receptor kinase
(EGFR kinase, the
protein product of the erbB oncogene), and the product produced by the erbB-2
(also referred to as the
neu or HER2) oncogene. Since the phosphorylation event is a necessary signal
for cell division to
occur and since overexpressed or mutated kinases have been associated with
cancer, an inhibitor of
this event, a protein tyrosine inhibitor, will have therapeutic value for the
treatment of cancer and other
diseases characterized by uncontrolled or abnormal cell growth. For example,
overexpression of the
receptor kinase product of the erbB-2 oncogene has been associated with human
breast and ovarian
cancers. Slamon et. al., Science, 1989, 244, 707. Deregulation of EGFR kinase
has been associated
with epidermoid tumors and tumors involving other major organs. Because of the
importance of the
role played by deregulated receptor kinases in the pathogenesis of cancer,
many recent studies have
dealt with the development of specific PTK inhibitors as potential anti-cancer
therapeutic agents.
[00162] Receptor tyrosine kinases span the cell membrane and possess an
extracellular binding domain
for growth factors such as epidermal growth factor (EGF), a transmembrane
domain, and an
intracellular portion which functions as a kinase to phosphorylate specific
tyrosine kinase residues in
proteins and hence to influence cell proliferation. The EGF receptor tyrosine
kinase family has four
members: EGFR (HER1, erbB 1); HER2 (c-erbB2, erbB2, neu); HER3 (erbB3); and
HER4 (erbB4).
The ErbB receptors generally transduce signals through two pathways. It is
known that such kinases
are frequently and aberrantly expressed in common human cancers such as breast
cancer,
gastrointestinal cancer of colon, rectum or stomach, leukemia, and ovarian,
bronchial or pancreatic
cancer. As discussed previously, epidermal growth factor receptor (EGFR), is
mutated and/or
overexpressed in many human cancers such as brain, lung, squamous cell,
bladder, gastric, breast,
head and neck, oesophageal, gynecological and thyroid tumors.
Epidermal Growth Factor Receptors
[00163] Control of cell growth is regulated by the interaction of soluble
growth factors and cell
membrane receptors. The first step in the mitogenic stimulation of epidermal
cells is the specific
binding of epidermal growth factor (EGF) to a membrane glycoprotein known as
the epidermal growth
factor receptor (EGFR). The EGFR is composed of 1,186 amino acids which are
divided into an
extracellular portion of 621 residues and a cytoplasmic portion of 542
residues connected by a single
hydrophobic transmembrane segment of 23 residues. Ullrich et al., Nature,
1986, vol. 309, 418-25.
The external portion of the EGFR can be subdivided into four domains.
Recently, it has been
demonstrated that domain III, residues 333 to 460 which is flanked by two
cysteine domains is likely
to contain the EGFR binding site of the receptor. Lax et al., Mol. And Cell
Biol., 1988, vol. 8, 1831-
34. The binding of EGF to domain III leads to the initiation of pleiotropic
responses leading to DNA
synthesis and cell proliferation.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
29
[00164] EGFR has also been found in various types of human tumor cells and
that those cells
overexpress EGFR. For example, the cancerous cells of bladder tumors have been
shown to have a
relatively large population of EGF receptors. Neal et al., Lancet, 1985, vol.
1, 366-67. Breast cancer
cells exhibit a positive correlation between EGFR density and tumor size and a
negative correlation
with the extent of differentiation. Sainsbury et al., Lancet, 1985, vol. 1,
364-66. The tumorigenicity
of a series of human vulval epidermoid carcinoma (A43 1) clonal variants
implanted into athymic mice
having different levels of EGFR was found to correlate directly with the level
of expression of the
EGF receptor. Santon et al., Cancer Res., 1986, vol. 46, 4700-01. Thus, it has
been proposed that
overexpression of EGFRs play a role in the origin or tumorigenesis of cancer
cells.
EGFR and COX Pathways
[00165] The relationship between the EGFR and COX pathways, to date, has not
yet been fully
elucidated. It has been submitted, however, that induction of COX-2 results in
the production of
increased levels of prostaglandins which then stimulate angiogenesis, cell
proliferation and cell
differentiation in an autocrine and/or paracrine manner. As described above,
NSAIDs inhibit this
process. Prostaglandins promote angiogenesis, along with cellular differention
and proliferation.
They also activate signaling through the EGFR. EGFR activation leads to
phosphorylation on tyrosine
residues by the receptor's tyrosine kinase domain, initiating a signaling
pathway that includes the
molecules Grb-2, SOS, the small G protein Raf. Raf activates Mitogen-Activated
Protein Kinase
Kinase (MAPKK) and group of nuclear transcription factors (c-myc, c-fos, c-
jun); (see Figure 2).
These factors initiate transcription of genes involved in the regulation of
cell proliferation and
differentiation. Additionally, these factors induce transcription of the COX-
2. These effects may
significantly amplify the original EGFR mediated signal and lead to pro-
neoplastic effects. Inhibiting
both signaling pathways could lead to a significant anti-neoplastic effect.
[00166] Studies have provided support that EGFR induces COX-2 in intestinal
epithelial cells and
anti-HER2 antibodies can inhibit COX-2 expression in colorectal cancer cells.
In addition,
overexpression of COX-1 or COX-2 in colon carcinoma cells has been shown to
increase EGFR
expression. Thus, COX and EGFR levels appear to be linked in a positive
feedback cycle during
colon cancer development. While the exact mechanism by which dysregulated EGF
signaling
promotes colon carcinogenesis is not clearly understood, it has been submitted
that EGFR activation in
a variety of cell types results in stimulation of cell proliferation and
alterations in cell motility and/or
adhesion to extra-cellular matrix. Studies have suggested that COX can
positively influence tumor
growth by promoting tumor associated angiogenesis.
[00167] It has also recently been proposed that the activation and
overexpression of COX-2 in
adenomatous polyps is due to activation of the EGFR. EGFR stimulation by one
of its ligands,
amphiregulin (AR), induces the nuclear targeting of COX-2, release of
prostaglandins and subsequent
mitogenesis, in polarized colonic epithelial cells. COX-2 inhibitors have been
shown to prevent this
series of events.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
EGFR Inhibitors
[00168] Accordingly, it has been recognized that inhibitors of receptor
tyrosine kinases are useful as
selective inhibitors of the growth of mammalian cancer cells. For example,
erbstatin, a tyrosine kinase
inhibitor, selectively attenuates the growth in athymic nude mice of a
transplanted human mammary
5 carcinoma which expresses EGFR but is without effect on the growth of
another carcinoma which
does not express the EGFR.
[00169] Various other compounds, such as styrene derivatives, have also been
shown to possess
tyrosine kinase inhibitory properties. Others have disclosed that certain
quinazoline derivatives
possess anti-cancer properties which result from their tyrosine inhibitory
properties.
10 [00170] As described herein, an "EGFR inhibitor" is a molecule which
inhibits the kinase domain of
the epidermal growth factor receptor. Compounds which are EGFR inhibitors can
readily be
identified by one skilled in the art using methods such as, for example,
standard pharmacological test
procedures which measure the inhibition of the phosphorylation of the tyrosine
residue of a peptide
substrate catalyzed by EGFR. Briefly, the peptide substrate (RR-SRC) has the
sequence arg-arg-leu-
15 ile-glu-asp-ala-glu-tyr-ala-ala-arg-gly. The enzyme is obtained as a
membrane extract of A431 cells
(American Type Culture Collection, Rockvilie, Md.). A431 cells are grown in
T175 flasks to 80%
confluency. The cells are washed twice with phosphate buffered saline (PBS)
without Ca2+. Flasks
are rotated for 1.5 hours in 20 ml PBS with 1.0 mM ethylenediaminetetraacetic
acid (EDTA) at room
temperature and centrifuged at 600 g for 10 minutes. The cells are solubilized
in 1 ml per 5x106 cells
20 of cold lysis buffer { 10 mM 4-(2-hydroxyethyl)- 1 -
piperazineethanesulfonic acid (HEPES), pH 7.6, 10
mM NaCl, 2 mM EDTA, 1 mM phenylmethylsulfonyl-fluoride (PMSF), 10 mg/ml
aprotinin, 10
mg/ml leupeptin, 0.1 mM sodium orthovanadate} in a Dounce homogenizer with 10
strokes on ice.
The lysate is centrifuged at 600 g for 10 minutes first to clear cell debris
and the supernatant further
centrifuged at 100,000 g for 30 min at 4° C. The membrane pellet is
suspended in 1.5 ml HNG
25 buffer (50 mM HEPES, pH 7.6, 125 mM NaCI, 10% glycerol). The membrane
extract is divided into
aliquots, immediately frozen in liquid nitrogen and stored at -70 C.
[00171] Compositions to be evaluated are made into 10 mg/mi stock solutions in
100%
dimethylsulfoxide (DMSO). Stock solutions are diluted to 500 mM with buffer
(30 mM Hepes pH
7.4) and then serially diluted to the desired concentration. An aliquot of the
A431 membrane extract
30 (10 mg/ml) is diluted in 30 mM HEPES (pH 7.4) to give a protein
concentration of 50 g/ml. To 4 l
of enzyme preparation, EGF (1 l at 12 g/ml ) is added and incubated for 10
min on ice followed by
4 1 of the test compound or buffer; this mix is incubated on ice for 30 min.
To this is added the 33P-
ATP (10 mCi/ml) diluted 1:10 in assay buffer along with the substrate peptide
at a concentration of 0.5
mM (control reactions get no test compound) and the reaction is allowed to
proceed for 30 min at
30 C. The reaction is stopped with 10% TCA and left on ice for at least 10 min
after which tubes are
microcentrifuged at full speed for 15 min. A portion of the supernatants are
then spotted on P81
phosphocellulose discs and washed twice in 1% acetic acid then water for 5 min
each followed by
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
31
scintillation counting. The results obtained can be expressed as an IC50. The
IC50 is the concentration
of test compound needed to reduce the total amount of phosphorylated substrate
by 50%. The %
inhibition of the test compound is determined for at least three different
concentrations and the IC50
value is evaluated from the dose response curve. The % inhibition is evaluated
with the following
formula:
% inhibition = 100-[CPM(drug)/CPM(control)]x100
where CPM(drug) is in units of counts per minute and is a number expressing
the amount of
radiolabeled ATP (g-33 P) incorporated onto the RR-SRC peptide substrate by
the enzyme after 30
minutes at 30 C in the presence of test compound as measured by liquid
scintillation counting.
CPM(control) is in units of counts per minute and is a number expressing the
amount of radiolabeled
ATP (g-33 P) incorporated into the RR-SRC peptide substrate by the enzyme
after 30 minutes at 30 C.
in the absence of test compound as measured by liquid scintillation counting.
The CPM values are
corrected for the background counts produced by ATP in the absence of the
enzymatic reaction.
Compounds having an IC50 of about 200 nM or less are considered to be
significantly active EGFR
inhibitors.
[00172] The identification of EGFR as an oncogene has led to the development
of anticancer
therapeutics directed against EGFR, including gefitinib and cetuximab for
colon cancer. Cetuximab is
an example of a monoclonal antibody inhibitor, while gefitinib is a small
molecule inhibitor.
Monoclonal Antibodies
[00173) The monoclonal antibodies block the extracellular ligand binding
domain. With the binding
site blocked, signal molecules can no longer attach there and activate the
tyrosine kinase.
1001741 Cetuximab is a chimeric monoclonal antibody generated from fusion of
the variable region of
the murine anti-EGFR monoclonal antibody M225 and the human IgGI constant
region. The resulting
antibody retains high affinity and specificity to EGFR and reduces
immunogenicity. Preclinical
studies have demonstrated that cetuximab effectively inhibits the
proliferation of a variety of EGFR-
expressing cancer cells in vitro and that it inhibits tumor growth in
xenograft models. In an orthotopic
pancreatic cancer model, cetuximab significantly suppressed the growth of
orthotopically implanted
pancreatic tumors, and this effect was enhanced by the addition of
gemcitabine. Histologic analysis of
tumor specimens revealed that cetuximab induced apoptosis and suppressed
proliferation of tumor
cells. Interestingly, cetuximab also induced apoptosis of endothelial cells,
which are not believed to
be direct targets of EGFR inhibition. Moreover, an antiangiogenic effect,
characterized by decreased
microvascular densities associated with reduced expression of tumor-related
VEGF and interleukin-8,
was observed. These data suggest that, in addition to direct antiproliferative
activity, antiangiogenic
activity contributes significantly to the antitumor effect of EGFR inhibitors.
1001751 Panitumumab (ABX-EGF) is a fully human monoclonal antibody specific to
EGFR and is
produced by immunization of transgenic mice, that are able to produce human
immunoglobulin light
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
32
and heavy chains. Following immunization, a specific clone of B cells that
produced the antibody
against EGFR are selected and immortalized for the generation of the antibody.
[00176] Effective anti-EGFR monoclonal antibodies compete with endogenous
ligands, primarily EGF
and transforming growth factor a for receptor ligand-binding sites. Binding to
EGFR blocks critical
signaling pathways and interferes with the growth of tumors expressing EGFR.
Anti-EGFR
monoclonal antibodies that are currently under study include EMD 55900 and ICR
62.
Small Molecules
[00177] Another method is using small molecules to inhibit the EGFR tyrosine
kinase, which is on the
cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to
activate itself, which is a
prerequisite for binding of downstream adaptor proteins. Ostensibly by halting
the signaling cascade
in cells that rely on this pathway for growth, tumor proliferation and
migration is diminished.
[00178] Gefitinib is currently only indicated for the treatment of locally
advanced or metastatic non-
small cell lung cancer (NSCLC) in patients who have previously received
chemotherapy. While
gefitinib has yet to be proven to be effective in other cancers, there is
potential for its use in the
treatment of other cancers where EGFR overexpression is involved.
Erlotinib
[00179]N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine, also
known as erlotinib
(Tarceva ), is currently being used to help treat advanced non-small cell lung
cancer, and in
combination with gemcitabine in the treatment for advanced metastatic
pancreatic cancer. U.S.
5,747,498 describes the preparation of erlotinib and other chemically-related
compounds.
Additionally, U.S. 6,900,221 describes the use of a stable polymorph of
erlotinib as an inhibitor of the
erbB family of oncogenic and protoncogenic protein kinases such as EGFR. Also,
the patent
illustrates methods for the treatment of non-small cell lung cancer, pediatric
malignancies, cervical and
other tumors caused or promoted by human papilloma virus (HPV), melanoma,
Barrett's esophagus
(pre-malignant syndrome), adrenal and skin cancers and auto immune, neoplastic
cutaneous diseases
and atherosclerosis.
0
OO / N
O \ I ~ N
HN
N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine
Gemcitabine
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
33
NH2
N
HO N O
O
OH F Gemcitabine
1001801 Gemcitabine is a nucleoside analog that exhibits antitumor activity.
The chemical name for
gemcitabine is (3-2'-deoxy-2',2'-difluorocytidine and is administered as the
hydrochloride salt. The
cytotoxic effect of gemcitabine is attributed to two actions. First,
gemcitabine diphosphate inhibits
ribonucleotide reductase causing a reduction in deoxynucleotides available for
DNA synthesis.
Second, gemcitabine triphosphate competes with dCTP for incorporation into
DNA. The two actions
working in combination lead to high levels of gemcitabine nuceotide
incorportion into growing DNA
chains. This leads to inhibition of further DNA synthesis and chain
termination.
[00181] Gemcitabine is indicated for use in the treatment of ovarian, breast,
NSCL and pancreatic
cancer. Gemcitabine hydrochloride is administered as an intravenous infusion
of a 1000 mg/m2 dose
over 30 minutes once a week for up to 7 weeks. This is followed by one week of
rest and then
subsequent cycles of weekly infusions for 3 weeks of a 4 week cycle. Complete
details are provided
in the prescribing instructions for gemcitabine, which is included by
reference in its entirety.
[00182] In one embodiment the invention provides a composition comprising a
combination of a
COX-2 selective inhibitor and an EGFR inhibitor disclosed herein for the
treatment and prevention of
cancer, tumors, and tumor-related disorders, and neoplastic disease states. In
one embodiment, the
EGFR inhibitor is a small molecule compound or an antibody.
[00183] In one embodiment, the EGFR inhibitor is selected from gefitinib,
cetuximab, and erlotinib.
In another embodiment, the EGFR inhibitor is erlotinib.
[00184) In another embodiment the small molecule compound is selected from the
group consisting of:
ZM-254530, BIBX-1382, reveromycin A, gefitinib, CGP-57148, CGP-59326, 4-(m-
chloro)-5,6-
dimethyl-7H-pyrrolo[2,3-d]pyrimidine, tyrphostin, PKI-166, PD 153035, EKB-569,
and 4-
(phenylamino)quinazolines, or their pharmaceutically acceptable salts,
solvates, or prodrugs. In
another embodiment, the small molecule is selected from the compounds
disclosed in
PCT/USO4/027574 which is herein incorporated by reference in its entirety.
[00185] In another embodiment, the antibody is selected from the group
consisting of: EGF receptor
antibody, MR1 scFvPE3 8KDEL MDX-447, MDX-2 10, MD-72000, MDX-260, wayne anti-
EGFR
Mabs, anti-EGFr Mab, anti-EGFr MAb, Genen anti-EGFR Mab, MAb DC-101,
trastuzumab, anti-
VEGF monoclonal, anti-EGFR-DMl Ab, MAb 4D5, BAB-447, EMD-55900, EMD-6200, -
82633,
anti-EGFR Mab, MAb 4D5, cetuximab, anti-EGFr MAb, anti-flk-1, CCX, CCZ, anti-
flk-l, AG-514,
AG-568, nti-EGFR-DM1 Ab, MDX-447, TgDCC-EIA, of: muellerian-inhibiting
hormone, TNP-470,
tecogalan sodium, C C225, matuzumab, panitumumab, DWP-408, and RC-394011. In
another
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
34
embodiment are provided compositions wherein the EGFR inhibitor is selected
from the inhibitors
disclosed in PCT/USO4/027574 which is herein incorporated by reference in its
entirety.
[00186] In a further embodiment the EGFR inhibitor is selected from the group
consisting EGF
receptor antisense, PI-88, oligonucleotide, bromelain molecules, amphiregulin,
EGF fusion toxin, EGF
fusion protein, Amphiregulin hbEGF-toxin, hbEGF-toxin, and EGF fusion protein.
[00187] As described herein "a selective EGFR inhibitory effect" is meant that
the composition
comprising a combination of a 1,2-diphenylpyrrole derivative with erlotinib
displays selective
inhibition against EGFR than other kinases. In some embodiments, combinations
presently disclosed
display selective inhibition against EGFR kinase than against other tyrosine
kinases such as other
erbBRs such as erbB2. For example in one embodiment, the invention provides a
composition
comprising a combination of a 1,2-diphenylpyrrole derivative with erlotinib is
at least 5 times or at
least 10 times selective against EGFR than it is against erbB2, as determined
from the relative ICs0
values in suitable assays (for example by comparing the IC50 value from the KB
cell assay with the
IC50 value from the Clone 24 phospho-erbB2 cell assay for a given composition
as described above).
In another embodiment, the invention provides a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is
erlotinib wherein
the combination is at least 5 times or at least 10 times selective against
EGFR than it is against erbB2.
[00188] The compositions provided herein may be enantiomerically pure, such as
a single enantiomer
or a single diastereomer, or be stereoisomeric mixtures, such as a mixture of
enantiomers, a racemic
mixture, or a diastereomeric mixture, or a polymorph of the active agent, for
example for erlotinib ,
polymorphs, including but not limited to polymorphs A, B, and E, and amorphous
forms or solid
amorphous dispersions as disclosed in US20060154941. As such, one of skill in
the art will recognize
that administration of a compound in its (R) form is equivalent, for compounds
that undergo
epimerization in vivo, to administration of the compound in its (S) form.
Conventional techniques for
the preparation/isolation of individual enantiomers include chiral synthesis
from a suitable optically
pure precursor or resolution of the racemate using, for example, chiral
chromatography,
recrystallization, resolution, diastereomeric salt formation, or
derivatization into diastereomeric
adducts followed by separation.
[00189] When the composition described herein contains an acidic or basic
moiety, it may also be
provided as a pharmaceutically acceptable salt (See, Berge et al., J. Pharm.
Sci. 1977, 66, 1-19; and
"Handbook of Pharmaceutical Salts, Properties, and Use," Stah and Wermuth,
Ed.; Wiley-VCH and
VHCA, Zurich, 2002).
[00190] Suitable acids for use in the preparation of pharmaceutically
acceptable salts include, but are
not limited to, acetic acid, 2,2-dichloroacetic acid, acylated amino acids,
adipic acid, alginic acid,
ascorbic acid, L-aspartic acid, benzenesulfonic acid, benzoic acid, 4-
acetamidobenzoic acid, boric
acid, (+)-camphoric acid, camphorsulfonic acid, (+)-(1S)-camphor-10-sulfonic
acid, capric acid,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
caproic acid, caprylic acid, cinnamic acid, citric acid, cyclamic acid,
cyclohexanesulfamic acid,
dodecylsulfuric acid, ethane-1,2-disulfonic acid, ethanesulfonic acid, 2-
hydroxy-ethanesulfonic acid,
formic acid, fumaric acid, galactaric acid, gentisic acid, glucoheptonic acid,
D-gluconic acid, D-
glucuronic acid, L-glutamic acid, a-oxo-glutaric acid, glycolic acid, hippuric
acid, hydrobromic acid,
5 hydrochloric acid, hydroiodic acid, (+)-L-lactic acid, ( )-DL-lactic acid,
lactobionic acid, lauric acid,
maleic acid, (-)-L-malic acid, malonic acid, (=i:)-DL-mandelic acid,
methanesulfonic acid, naphthalene-
2-sulfonic acid, naphthalene-1,5-disulfonic acid, 1-hydroxy-2-naphthoic acid,
nicotinic acid, nitric
acid, oleic acid, orotic acid, oxalic acid, palmitic acid, pamoic acid,
perchloric acid, phosphoric acid,
L-pyroglutamic acid, saccharic acid, salicylic acid, 4-amino-salicylic acid,
sebacic acid, stearic acid,
10 succinic acid, sulfuric acid, tannic acid, (+)-L-tartaric acid, thiocyanic
acid, p-toluenesulfonic acid,
undecylenic acid, and valeric acid.
[00191] Suitable bases for use in the preparation of pharmaceutically
acceptable salts, including, but
not limited to, inorganic bases, such as magnesium hydroxide, calcium
hydroxide, potassium
hydroxide, zinc hydroxide, or sodium hydroxide; and organic bases, such as
primary, secondary,
15 tertiary, and quaternary, aliphatic and aromatic amines, including L-
arginine, benethamine,
benzathine, choline, deanol, diethanolamine, diethylamine, dimethylamine,
dipropylamine,
diisopropylamine, 2-(diethylamino)-ethanol, ethanolamine, ethylamine,
ethylenediamine,
isopropylamine, N-methyl-glucamine, hydrabamine, lH-imidazole, L-lysine,
morpholine, 4-(2-
hydroxyethyl)-morpholine, methylamine, piperidine, piperazine, propylamine,
pyrrolidine, 1-(2-
20 hydroxyethyl)-pyrrolidine, pyridine, quinuclidine, quinoline, isoquinoline,
secondary amines,
triethanolamine, trimethylamine, triethylamine, N-methyl-D-glucamine, 2-amino-
2-(hydroxymethyl)-
1,3-propanediol, and tromethamine.
1001921 The composition described herein may also be provided as a prodrug,
which is a functional
derivative of the 1,2-diphenylpyrrole derivative and/or the EGFR inhibitor and
is readily convertible
25 into the parent compound in vivo. Prodrugs are often useful because, in
some situations, they may be
easier to administer than the parent compound. They may, for instance, be
bioavailable by oral
administration whereas the parent compound is not. The prodrug may also have
enhanced solubility in
pharmaceutical compositions over the parent compound. A prodrug may be
converted into the parent
drug by various mechanisms, including enzymatic processes and metabolic
hydrolysis. See Harper,
30 Progress in Drug Research 1962, 4, 221-294; Morozowich et al. in "Design of
Biopharmaceutical
Properties through Prodrugs and Analogs," Roche Ed., APHA Acad. Pharm. Sci.
1977; "Bioreversible
Carriers in Drug in Drug Design, Theory and Application," Roche Ed., APHA
Acad. Pharm. Sci.
1987; "Design of Prodrugs," Bundgaard, Elsevier, 1985; Wang et al., Curr.
Pharm. Design 1999, 5,
265-287; Pauletti et al., Adv. Drug. Delivery Rev. 1997, 27, 235-256; Mizen et
al., Pharm. Biotech.
35 1998, 11, 345-365; Gaignault et al., Pract. Med. Chem. 1996, 671-696;
Asgharnejad in "Transport
Processes in Pharmaceutical Systems," Amidon et al., Ed., Marcell Dekker, 185-
218, 2000; Balant et
al., Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 143-53; Balimane and Sinko,
Adv. Drug Delivery
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
36
Rev. 1999, 39, 183-209; Browne, Clin. Neuropharmacol. 1997, 20, 1-12;
Bundgaard, Arch. Pharm.
Chem. 1979, 86, 1-39; Bundgaard, Controlled Drug Delivery 1987, 17, 179-96;
Bundgaard, Adv. Drug
Delivery Rev. 1992, 8, 1-38; Fleisher et al., Adv. Drug Delivery Rev. 1996,
19, 115-130; Fleisher et al.,
Methods Enzymol. 1985, 112, 360-381; Farquhar et al., J. Pharm. Sci. 1983, 72,
324-325; Freeman et
al., J. Chem. Soc., Chem. Commun. 1991, 875-877; Friis and Bundgaard, Eur. J.
Pharm. Sci. 1996, 4,
49-59; Gangwar et al., Des. Biopharm. Prop. Prodrugs Analogs, 1977, 409-42 1;
Nathwani and Wood,
Drugs 1993, 45, 866-94; Sinhababu and Thakker, Adv. Drug Delivery Rev. 1996,
19, 241-273; Stella
et al., Drugs 1985, 29, 455-73; Tan et al., Adv. Drug Delivery Rev. 1999, 39,
117-151; Taylor, Adv.
Drug Delivery Rev. 1996, 19, 131-148; Valentino and Borchardt, Drug Discovery
Today 1997, 2, 148-
155; Wiebe and Knaus, Adv. Drug Delivery Rev. 1999, 39, 63-80; Waller et al.,
Br. J. Clin. Pharmac.
1989, 28, 497-507.
Methods of Use
[00193] The invention provides a method for treating a subject having tumors,
tumor-related disorders,
and/or cancer, comprising administering to the subject, a therapeutically
effective amount of a
combination comprising 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and erlotinib.
[00194] In one embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the 1,2-diphenylpyrroie derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole.
[00195] In another embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the EGFR inhibitor is erlotinib.
[00196] In yet another embodiment, the invention provides a method for
treating a subject having a
tumors, tumor-related disorders, and/or cancer, comprising administering to
the subject, a
therapeutically effective amount of a combination comprising a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor or their respective pharmaceutically acceptable salt, solvate
or prodrug.
[00197] In one embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the 1,2-diphenylpyrrole derivative is selected from the group
consisting of: 4-methyl-2-(4-
methylphenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-methoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-(4-chlorophenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 4-methyl-2-(4-
methylthiophenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-ethoxyphenyl)-4-methyl-l-
(4-
sulfamoylphenyl)pyrrole; 2-(4-methoxy-3 -methylphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-
(3-fluoro-4-methoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)pyrrole; 2-(3,4-
dimethylphenyl)-4-
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
37
methyl-l-(4-sulfamoylphenyl)pyrrole; 4-methyl-l-(4-methylthiophenyl)-2-(4-
sulfamoylphenyl)pyrrole; 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(4-
methoxyphenyl)pyrrole;
and 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(3,4-dimethylphenyl)pyrrole.
[001981 In yet another embodiment, the invention provides a method for
treating a subject having
tumors, tumor-related disorders, and/or cancer, comprising administering to
the subject, a
therapeutically effective amount of a combination comprising a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative and the EGFR
inhibitor are administered
sequentially in either order or simultaneously. In a further embodiment, the
invention provides a
method for treating a subject having tumors, tumor-related disorders, and/or
cancer, comprising
administering to the subject, a therapeutically effective amount of a
combination comprising a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is
administered first. In one embodiment, the invention provides a method for
treating a subject having
tumors, tumor-related disorders, and/or cancer, comprising administering to
the subject, a
therapeutically effective amount of a combination comprising a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor wherein the EGFR inhibitor is administered first. In another
embodiment, the
invention provides a method for treating a subject having tumors, tumor-
related disorders, and/or
cancer, comprising administering to the subject, a therapeutically effective
amount of a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
administering the
combination enhances treatment of the subject in comparison to a treatment of
either a 1,2-
diphenylpyrrole derivative or an EGFR inhibitor alone. In yet another
embodiment, the invention
provides a method for treating a subject having tumors, tumor-related
disorders, and/or cancer,
comprising administering to the subject, a therapeutically effective amount of
a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
administering the
combination reduces the side effects of the treatment of tumors, tumor-related
disorders, and/or
cancer.
[001991 In one embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the cancer is selected from the group consisting of: oral cancer,
prostate cancer, rectal cancer,
non-small cell lung cancer, lip and oral cavity cancer, liver cancer, lung
cancer, anal cancer, kidney
cancer, vulvar cancer, breast cancer, oropharyngeal cancer, nasal cavity and
paranasal sinus cancer,
nasopharyngeal cancer, urethra cancer, small intestine cancer, bile duct
cancer, bladder cancer, ovarian
cancer, laryngeal cancer, hypopharyngeal cancer, gallbladder cancer, colon
cancer, colorectal cancer,
head and neck cancer, parathyroid cancer, penile cancer, vaginal cancer,
thyroid cancer, pancreatic
cancer, esophageal cancer, Hodgkin's lymphoma, leukemia-related disorders,
mycosis fungoides, and
myelodysplastic syndrome.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
38
[00200] In another embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the cancer is non-small cell lung cancer, pancreatic cancer, breast
cancer, ovarian cancer,
colorectal cancer, and head and neck cancer.
[00201] In one embodiment, the invention provides a method for treating a
subject having tumors,
tumor-related disorders, and/or cancer, comprising administering to the
subject, a therapeutically
effective amount of a combination comprising a 1,2-diphenylpyrrole derivative
and an EGFR inhibitor
wherein the cancer is a carcinoma, a tumor, a neoplasm, a lymphoma, a
melanoma, a glioma, a
sarcoma, and a blastoma. In another embodiment, the invention provides a
method for treating a
subject having a carcinoma, comprising administering to the subject, a
therapeutically effective
amount of a combination comprising a 1,2-diphenylpyrrole derivative and an
EGFR inhibitor wherein
the carcinoma is selected from the group consisting of: carcinoma,
adenocarcinoma, adenoid cystic
carcinoma, adenosquamous carcinoma, adrenocortical carcinoma, well
differentiated carcinoma,
squamous cell carcinoma, serous carcinoma, small cell carcinoma, invasive
squamous cell carcinoma,
large cell carcinoma, islet cell carcinoma, oat cell carcinoma, squamous
carcinoma, undifferentiatied
carcinoma, verrucous carcinoma, renal cell carcinoma, papillary serous
adenocarcinoma, merkel cell
carcinoma, hepatocellular carcinoma, soft tissue carcinomas, bronchial gland
carcinomas, capillary
carcinoma, bartholin gland carcinoma, basal cell carcinoma, carcinosarcoma,
papilloma/carcinoma,
clear cell carcinoma, endometrioid adenocarcinoma, mesothelial, metastatic
carcinoma,
mucoepidermoid carcinoma, cholangiocarcinoma, actinic keratoses, cystadenoma,
and hepatic
adenomatosis. In a further embodiment the tumor is selected from the group
consisting of: astrocytic
tumors, malignant mesothelial tumors, ovarian germ cell tumor, supratentorial
primitive
neuroectodermal tumors, Wilm's tumor, pituitary tumors, extragonadal germ cell
tumor, gastrinoma,
germ cell tumors, gestational trophoblastic tumor, brain tumors, pineal and
supratentorial primitive
neuroectodermal tumors, pituitary tumor, somatostatin-secreting tumor,
endodermal sinus tumor,
carcinoids, central cerebral astrocytoma, glucagonoma, hepatic adenoma,
insulinoma,
medulloepithelioma, plasmacytoma, vipoma, and pheochromocytoma. In yet another
embodiment, the
invention provides a method for treating a subject having a neoplasm,
comprising administering to the
subject, a therapeutically effective amount of a combination comprising a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the neoplasm is selected from the
group consisting of
intaepithelial neoplasia, multiple myeloma/plasma cell neoplasm, plasma cell
neoplasm, interepithelial
squamous cell neoplasia, endometrial hyperplasia, focal nodular hyperplasia,
hemangioendothelioma,
and malignant thymoma. In one embodiment the lymphoma is selected from the
group consisting of:
nervous system lymphoma, AIDS-related lymphoma, cutaneous T-cell lymphoma, non-
Hodgkin's
lymphoma, lymphoma, and Waldenstrom's macroglobulinemia. In another embodiment
the melanoma
is selected from the group consisting of: acral lentiginous melanoma,
superficial spreading melanoma,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
39
uveal melanoma, lentigo maligna melanomas, melanoma, intraocular melanoma,
adenocarcinoma
nodular melanoma, and hemangioma.
[00202] In a further embodiment, the invention provides a method for treating
a subject having a
sarcoma, comprising administering to the subject, a therapeutically effective
amount of a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the
sarcoma is selected
from the group consisting of: adenomas, adenosarcoma, chondosarcoma,
endometrial stromal
sarcoma, Ewing's sarcoma, Kaposi's sarcoma, leiomyosarcoma, ,
rhabdomyosarcoma, sarcoma, uterine
sarcoma, osteosarcoma, and pseudosarcoma. In one embodiment, the glioma is
selected from the
group consisting of: glioma, brain stem glioma, and hypothalamic and visual
pathway glioma. In
another embodiment, the invention provides a method for treating a subject
having a blastoma,
comprising administering to the subject, a therapeutically effective amount of
a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the
blastoma is selected
from the group consisting of: pulmonary blastoma, pleuropulmonary blastoma,
retinoblastoma,
neuroblastoma, medulloblastoma, glioblastoma, and hemangiblastomas.
[00203] In one embodiment the EGFR inhibitor is a small molecule compound or
an antibody. In
another embodiment the EGFR inhibitor is a small molecule compound is selected
from the group
consisting of: ZM-254530, BIBX-1382, reveromycin A, gefitinib, CGP-57148, CGP-
59326, 4-(m-
chloro)-5,6-dimethyl-7H-pyrrolo[2,3-d]pyrimidine, tyrphostin, PKI-166, PD
153035, EKB-569, and 4-
(phenylamino)quinazolines, or their pharmaceutically acceptable salts,
solvates, or prodrugs. In a
further embodiment, the EGFR inhibitor is an antibody is selected from the
group consisting of: EGF
receptor antibody, MR1scFvPE38KDEL MDX-447, MDX-210, MD-72000, MDX-260, wayne
anti-
EGFR Mabs, anti-EGFr Mab, anti-EGFr MAb, Genen anti-EGFR Mab, MAb DC-101,
trastuzumab,
anti-VEGF monoclonal, anti-EGFR-DMl Ab, MAb 4D5, BAB-447, EMD-55900, EMD-6200,
-
82633, anti-EGFR Mab, MAb 4D5, cetuximab, anti-EGFr MAb, anti-flk-1, CCX, CCZ,
anti-flk-1,
AG-514, AG-568, nti-EGFR-DM1 Ab, MDX-447, TgDCC-E1A, C225, matuzumab,
panitumumab,
DWP-408, and RC-3940II. In yet a further embodiment the EGFR inhibitor is
selected from the group
consisting of: muellerian-inhibiting hormone, TNP-470, tecogalan sodium, EGF
receptor antisense,
PI-88, oligonucleotide, bromelain molecules, amphiregulin, EGF fusion toxin,
EGF fusion protein,
Amphiregulin hbEGF-toxin, hbEGF-toxin, and EGF fusion protein.
[00204] In one embodiment the invention provides a method of inducing
differentiation of tumor cells,
the method comprising contacting the cells with an effective amount of a
combination comprising a
1,2-diphenylpyrrole derivative and an EGFR inhibitor whereby the combination
induces
differentiation of tumor cells. In one embodiment, the invention provides a
method of inducing
differentiation of tumor cells, the method comprising contacting the cells
with an effective amount of a
combination comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor
wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
[00205] In one embodiment the invention provides a method of inhibiting
proliferation of cancer cells,
the method comprising contacting a cancer cell with a combination comprising a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor whereby the combination inhibits
proliferation of cancer cells. In
one embodiment, the invention provides a method of inhibiting proliferation of
cancer cells, the
5 method comprising contacting a cancer cell with a combination comprising a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is
2-(4-ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib.
[00206] In another embodiment the invention provides a method for reducing
proliferation of cancer
cells, the method comprising delivering to the cells a combination comprising
a 1,2-diphenylpyrrole
10 derivative and an EGFR inhibitor, whereby the reduction of cell
proliferation is greater than a
reduction caused by either a 1,2-diphenylpyrrole derivative alone or an EGFR
inhibitor alone. In one
embodiment, the invention provides a method for reducing proliferation of
cancer cells, the method
comprising delivering to the cells a combination comprising a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-1-(4-
15 sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib.
[00207] In one embodiment the invention provides a method of modulating
autophosphorylation with
a molecule of ATP, the method comprising delivering to a cancer cell an
effective amount of a
combination comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor
wherein the
combination inhibits autophosphorylation with a molecule of ATP. In one
embodiment, the invention
20 provides a method of modulating autophosphorylation with a molecule of ATP,
the method
comprising delivering to a cancer cell an effective amount of a combination
comprising a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is
erlotinib.
[00208] In a further embodiment the invention provides a method of inhibiting
metastases of tumor
25 cells, the method comprising administering an effective amount of a
combination comprising a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor such that the combination
inhibits metastatic
activity of tumor cells. In one embodiment, the invention provides a method of
inhibiting metastases
of tumor cells, the method comprising administering an effective amount of a
combination comprising
a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is
30 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR
inhibitor is erlotinib.
[00209] In one embodiment the invention provides a method for inducing
apoptosis in cancer cells, the
method comprising contacting the cancer cells with a combination comprising a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor sufficient to induce apoptosis. In one
embodiment, the invention
provides a method for inducing apoptosis in cancer cells, the method
comprising contacting the cancer
35 cells with a combination comprising a 1,2-diphenylpyrrole derivative and an
EGFR inhibitor wherein
the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and
the EGFR inhibitor is erlotinib.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
41
[00210] In another embodiment the invention provides a method for sensitizing
EGFR inhibitor
resistant cancer cells to an EGFR inhibitor, the method comprising
administering a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the
combination
sensitizes the cancer cells to the EGFR inhibitor. In one embodiment, the
invention provides a method
for sensitizing EGFR inhibitor resistant cancer cells to an EGFR inhibitor,
the method comprising
administering a combination comprising a 1,2-diphenylpyrrole derivative and an
EGFR inhibitor
wherein the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-
pyrrole and the EGFR inhibitor is erlotinib.
[00211] In a further embodiment the invention provides a method of modulating
prostaglandin
synthesis in a cancer cell, the method comprising contacting the cell with a
combination comprising a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the combination
inhibits prostaglandin
synthesis in a cancer cell. In one embodiment, the invention provides a method
of modulating
prostaglandin synthesis in a cancer cell, the method comprising contacting the
cell with a combination
comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the
1,2-diphenylpyrrole
derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
the EGFR inhibitor is
erlotinib.
1002121 In one embodiment the invention provides a method of modulating
cyclooxygenase expression
in a cancer cell, the method comprising delivering to the cell a combination
comprising a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the combination
inhibits cyclooxygenase
expression in a cancer cell. In one embodiment, the invention provides a
method of modulating
cyclooxygenase expression in a cancer cell, the method comprising delivering
to the cell a
combination comprising a 1,2-diphenylpyrrole derivative and an EGFR inhibitor
wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-i-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib.
[00213] In one embodiment the invention provides a method of modulating
angiogenesis in a cancer
cell, the method comprising contacting the cell with a combination comprising
a 1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the combination inhibits angiogenesis
in a cancer cell. In
one embodiment the invention provides a method of modulating angiogenesis in a
cancer cell, the
method comprising contacting the cell with a combination comprising a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib. In another
embodiment the
invention provides a method of reducing the dosage in conventional treatment
for neoplasia and/or
neoplasia related disorders in a subject, the method comprising administering
to a subject a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the combination
reduces the dosage in conventional treatment for neoplasia and/or neoplasia-
related disorders. In one
embodiment, the invention provides a method of reducing the dosage in
conventional treatment for
neoplasia and/or neoplasia related disorders in a subject, the method
comprising administering to a
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
42
subject a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib.
[00214] In one embodiment the invention provides a method of treating
neoplasia and/or neoplasia
related disorders, the method comprising administering a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor. In one embodiment, embodiment the invention
provides a method
of treating neoplasia and/or neoplasia related disorders, the method
comprising administering a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl- 1-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib.
[00215] The combinations presently described herein may also be useful in the
treatment of additional
disorders in which aberrant expression ligand/receptor interactions or
activation or signalling events
related to various protein tyrosine kinases are involved. Such disorders may
include those of neuronal,
glial, astrocytal, hypothalamic, glandular, macrophagal, epithelial, stromal,
or blastocoelic nature in
which aberrant function, expression, activation or signalling of the erbB
tyrosine kinases are involved.
In addition, the combinations presented herein may have therapeutic utility in
inflammatory,
angiogenic and immunologic disorders involving both identified and as yet
unidentified tyrosine
kinases that are inhibited by the combinations presented herein.
Combination Therapy
[00216] In some embodiments, the composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor described herein, has an effect that is
additive of the effects of the
1,2-diphenylpyrrole derivative alone and the effects of the EGFR inhibitor
alone. In another
embodiment, the invention provides a composition comprising, a combination of
a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is
erlotinib, wherein
the combination has an effect that is additive of the effects of the 2-(4-
ethoxyphenyl)-4-methyl- 1 -(4-
sulfamoylphenyl)-pyrrole alone and the effects of erlotinib alone.
[00217] In some other embodiments, the composition comprising a combination of
a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor described herein, has an
effect that is greater than
the additive effects of the 1,2-diphenylpyrrole derivative alone and the
effects of the EGFR inhibitor
alone. In another embodiment, the invention provides a composition comprising,
a combination of a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-
(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor
is erlotinib,
wherein the combination has an effect that is greater than the additive
effects of the 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole alone and the effects of
erlotinib alone.
1002181 In some embodiments, the composition comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor described herein, has an effect that is
greater than the effects of the
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
43
1,2-diphenylpyrrole derivative alone (e.g., cyclooxygenase-2 inhibition
alone). In another
embodiment, the invention provides a composition comprising, a combination of
a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is
erlotinib, wherein
the combination has an effect that is greater than the effects of the 2-(4-
ethoxyphenyl)-4-methyl- 1 -(4-
sulfamoylphenyl)-pyrrole alone.
[00219] In other embodiments, the composition comprising a combination of a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor described herein, has an effect that is
greater than the effects of the
EGFR inhibitor alone (epidermal growth factor receptor kinase inhibition
alone). In another
embodiment, the invention provides a composition comprising, a combination of
a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is
erlotinib, wherein
the combination has an effect that is greater than the effects of erlotinib
alone.
[00220] In other embodiments, the invention provides a method for treating
cancer, tumors, and tumor-
related disorders comprising administering a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor described herein, wherein the
combination has an
effect that is additive of the effects of the 1,2-diphenylpyrrole derivative
alone and the effects of the
EGFR inhibitor alone. In further embodiments, the invention provides a method
for treating cancer,
tumors, and tumor-related disorders comprising administering a composition
comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib, wherein the combination has an effect that is
additive of the effects of 2-
(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole alone and the effects
of erlotinib alone.
1002211 In some other embodiments, the invention provides a method for
treating cancer, tumors, and
tumor-related disorders, comprising administering a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor described herein, wherein the
combination has an
effect that is greater than the additive effects of the effects of the 1,2-
diphenylpyrrole derivative alone
and the effects of the EGFR inhibitor alone. In other embodiments, the
invention provides method for
treating cancer, tumors, and tumor-related disorders, comprising administering
a composition
comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib, wherein the combination has an effect that is
greater than the additive
effects of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole alone and
the effects of
erlotinib alone.
1002221 In some embodiments, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders comprising administering a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor described herein, wherein the
combination has an
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
44
effect that is greater than the effects of the 1,2-diphenylpyrrole derivative
alone (e.g., cyclooxygenase-
2 inhibition alone). In other embodiments, the invention provides a method for
treating cancer,
tumors, and tumor-related disorders comprising administering a composition
comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib, wherein the combination has an effect that is
greater than the effects of is
2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole alone.
[00223] In further embodiments, the invention provides a method for treating
cancer, tumors, and
tumor-related disorders comprising administering a composition comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor described herein, wherein the
combination has an
effect that is greater than the effects of the EGFR inhibitor alone (epidermal
growth factor receptor
kinase inhibition alone). In other embodiments, the invention provides a
method for treating cancer,
tumors, and tumor-related disorders comprising administering a composition
comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib, wherein the combination has an effect that is
greater than the effects of
erlotinib alone.
1002241 Synergism of the composition comprising a combination of a 1,2-
diphenylpyrrole derivative
and an EGFR inhibitor, may be used to obtain the desired effect at doses to
which side effects are
minimal. For example, a patient may be treated for a disease, disorder, or
condition which benefits
from EGFR inhibition, such as tumors, tumor-related diseases, cancer,
neoplasia, while concomitantly
being treated for a side effect of the EGFR inhibition, such as inflammation,
through the benefit of the
1,2-diphenylpyrrole derivative inhibitor. In one embodiment, the invention
provides a combination of
2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib which
may be used to
obtain the desired effect at doses to which side effects are minimal.
[00225] The composition comprising a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor, may be applied as a sole therapy or may involve one or more other
materials and treatment
agents such as but not limited to a combination of inhibitors of MMP (matrix-
metallo-proteinase),
other tyrosine kinases including VEGFR (vascular endothelial growth factor
receptor), farnesyl
transferase, CTLA4 (cytotoxic T-lymphocyte antigen 4) and erbB2, as well as
MAb to VEGFr, and
other cancer-related antibodies including rhuMAb-VEGF, the erbB2 MAb, or avb3.
[00226] Thus, the composition comprising a combination of a 1,2-
diphenylpyrrole derivative and an
EGFR inhibitor, may be applied with one or more other anti-tumor substances,
for example, those
selected from, mitotic inhibitors, for example vinblastine; alkylating agents,
for example, cis-platin,
carboplatin, and cyclophosphamide; anti-metabolites, for example 5-
fluorouracil, cytosine arabinoside
and hydroxyurea, or, for example, anti-metabolites such as N-(5-[N-(3,4-
dihydro-2-methyl-4-
oxoquinazolin-6-ylmethyl)-N-methylamino]-2 -thenoyl)-L-glutamic acid; growth
factor inhibitors; cell
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
cycle inhibitors; intercalating antibiotics, for example adriamycin and
bleomycin; enzymes, for
example interferon; and anti-hormones, for example anti-estrogens such as
Nolvadex (tamoxifen) or,
for example anti-androgens such as Casodex (4'-cyano-3-(4-fluorophenyl
sulphonyl)-2-hydroxy-2-
methyl-3'-(trifluoromethyl)propionanilide). In one embodiment, the invention
provides a combination
5 a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is
2-(4-ethoxyphenyl)-4-methyl-i-(4-sulfamoylphenyl)-pyrrole and the EGFR
inhibitor is erlotinib, may
be applied with one or more other anti-tumor substances, for example, those
selected from, mitotic
inhibitors, for example vinblastine; alkylating agents, for example, cis-
platin, carboplatin, and
cyclophosphamide; anti-metabolites, for example 5-fluorouracil, cytosine
arabinoside and
10 hydroxyurea, or, for example, anti-metabolites such as N-(5-[N-(3,4-dihydro-
2-methyl-4-
oxoquinazolin-6-ylmethyl)-N-methylamino]-2 -thenoyl)-L-glutamic acid; growth
factor inhibitors; cell
cycle inhibitors; intercalating antibiotics, for example adriamycin and
bleomycin; enzymes, for
example interferon; and anti-hormones, for example anti-estrogens such as
Nolvadex (tamoxifen) or,
for example anti-androgens such as Casodex (4'-cyano-3-(4-fluorophenyl
sulphonyl)-2-hydroxy-2-
15 methyl-3'-(trifluoromethyl)propionanilide).
[00227] For the combination therapies including combination therapies having
pharmaceutical
compositions described herein, the effective amounts of the compound presently
described herein
useful for inhibiting abnormal cell growth (e.g., other antiproliferative
agent, anti-angiogenic, signal
transduction inhibitor or immune-system enhancer) can be determined by those
of ordinary skill in the
20 art, based on the effective amounts for the compound described herein and
those known or described
for the chemotherapeutic or other agent. The formulations and routes of
administration for such
therapies and compositions can be based on the information described herein
for compositions and
therapies comprising the combinations presented herein as the sole active
agent and on information
provided for the chemotherapeutic or other agent in combination therewith.
25 [00228] In one embodiment, the invention provides a method for inhibiting
abnormal cell growth in a
subject comprising administering to the subject an effective amount of a
composition comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor, or
their pharmaceutically
acceptable salt, solvate or prodrug thereof, in combination with radiation
therapy effective in
inhibiting abnormal cell growth in the subject. Techniques for administering
radiation therapy are
30 known to a person of skill in the art and these techniques can be used in
the combination therapy
described herein.
Additional Therapy
[00229] Available additional treatments for cancer that may be advantageously
employed in
combination with the therapies and compositions disclosed herein include,
without limitation, surgery,
35 radiation therapy, chemotherapy, high dose chemotherapy with stem cell
transplant; hormone therapy,
and monoclonal antibody therapy.
[00230] Surgical procedure are often used in the treatment of cancer.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
46
[00231] Radiation therapy is a cancer treatment that uses high-energy x-rays
or other types of radiation
to kill cancer cells or keep them from growing.
[00232] Chemotherapy is a cancer treatment that uses drugs to stop the growth
of cancer cells, either
by killing the cells or by stopping them from dividing. When chemotherapy is
taken by mouth or
injected into a vein or muscle, the drugs enter the bloodstream and can reach
cancer cells throughout
the body (systemic chemotherapy). When chemotherapy is placed directly into
the spinal column, an
organ, or a body cavity such as the abdomen, the drugs mainly affect cancer
cells in those areas
(regional chemotherapy). The way the chemotherapy is given depends on the type
and stage of the
cancer being treated.
[00233] Different chemotherapeutic agents are known in the art for treating
cancer. Cytoxic agents
used for treating cancer include doxorubicin, cyclophosphamide, methotrexate,
5-fluorouracil,
mitomycin C, mitoxantrone, paclitaxel, taxane formulations such as by way of
example only,
Abraxane (ABI-007), Paclitaxel-Cremophor EL, Paclitaxel poliglumex, and
Paclitaxel injectable
emulsion (PIE), gemcitabine, docetaxel, capecitabine and epirubicin.
100234] Other chemotherapy against cancer includes treatment with one or more
of bendamustine,
carboplatin (for example, Paraplatin ), carmustine (for example, BCNU ),
chlorambucil (for
example, Leukeran(g), cisplatin (for example, Platinol ), cyclophosphamide
injection (for example,
Cytoxan ), oral cyclophosphamide (for example, Cytoxan ), dacarbazine (for
example, DTIC ),
ifosfamide (for example, ifex ), lomustine (for example, CCNU ),
mechlorethamine (for example,
nitrogen mustard, Mustargen(T), melphalan (for example, Alkeran ),
procarbazine (for example,
Matulane ), bleomycin (for example, Blenoxane ), doxorubicin (for example,
Adriamycin ,
Rubex(X), epirubicin, Idarubicin (for example, Idamycin ), mitoxantrone (for
example,
Novantrone ), gemcitabine (for example, Gemzar(t), oral mercaptopurine (for
example,
Purinethol ). methotrexate, pentostatin IV (for example, Nipent(t), oral
thioguanine (for example,
Lanvis ), oral etoposide (for example, VP-16, VePesid , Etopophos) - etoposide
N(for example,
VP- 16, VePesid , Etopophos), vinblastine (for example, Velban ), vincristine
(for example,
Oncovin ), vinorelbine (for example, Navelbine(g), dexamethasone (for example,
Decadron ),
methylprednisolone (for example, Medrol(t), and prednisone (for example,
Deltasone(t). Erlotinib in
combination with gemcitabine is indicated for the treatment of advanced
pancreatic cancer.
[00235] Monoclonal antibody therapy is a cancer treatment that uses antibodies
made in the laboratory,
from a single type of immune system cell. These antibodies can identify
substances on cancer cells or
normal substances that may help cancer cells grow. The antibodies attach to
the substances and kill the
cancer cells, block their growth, or keep them from spreading. Monoclonal
antibodies are given by
infusion. They may be used alone or to carry drugs, toxins, or radioactive
material directly to cancer
cells. Monoclonal antibodies are also used in combination with chemotherapy as
adjuvant therapy.
[00236] Trastuzumab (Herceptin ) is a monoclonal antibody that blocks the
effects of the growth
factor protein HER2, which transmits growth signals to breast cancer cells.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
47
[00237] Trastuzumab leads to clinical responses as a single agent and improves
survival when added to
chemotherapy for advanced HER2-positive breast cancer. However, some patients
do not respond to
trastuzumab, and most eventually develop clinical resistance. Mechanisms of
intrinsic and acquired
trastuzumab resistance are poorly understood. One study which utilized a cell
line-based approach to
delineate genetic and protein alterations associated with resistance has been
reported. (D. Tripathy et al
Journal of Clinical Oncology, 2005 Vol 23, No 16S, 3121. These researchers
studied two HER2-
positive breast cancer cell lines (BT474 and SKBR3) that were serially
passaged in the presence of
trastuzumab until in vitro resistance was documented. Resistant cell lines
emerged after 12 months
and exhibited a 3-fold more rapid growth rate in the absence of trastuzumab.
Following trastuzumab
exposure, Go/Gl arrest was observed in sensitive compared to resistant cells
(84 vs. 68%), with fewer
cells in S-phase (3 vs. 14%). Resistant cell lines exhibited fewer changes in
gene expression with
trastuzumab as well as upregulation of the chemokine receptor CXCR4 and
mitotic checkpoint
regulators, and downregulation of PTEN compared to sensitive cells.
[00238] Additional, illustrative, treatments that may be advantageously
combined with the
compositions and therapies disclosed herein may include, without limitation,
administration of agents
including, but not limited to capecitabine, docetaxel, epirubicin, epothilone
A, B or D, goserelin
acetate, paclitaxel, pamidronate, bevacizumab, and trastuzumab.
EGFR Resistance
[00239] Studies have shown that after a period of about 8-12 months of EGFR-
directed treatments, the
cancer cells become resistant to the treatment, most commonly by 1) recruiting
a mutated IGF-1
receptor to act as one of the EGFR partners in the homodimer, thus, forming a
heterodimer (this
allows the signal to be transmitted even in the presence of an EGFR
inhibitor); 2) the presence of
redundant tyrosine kinase receptors; 3) increased angiogenesis; 4) the
constitutive activation of
downstream mediators; and 5) the existence of specific EGFR mutations.
Understanding the
molecular mechanisms of resistance and sensitivity may lead to improvements in
therapies that target
EGFR.
[00240] IGF-1R activates many of the same downstream pathways as EGFR and can
lead to
tumorigenesis, increased proliferation, angiogenesis, and metastasis. PI-
3K/Akt signaling is a critical
component of the downstream mediation of EGFR and also plays a functional role
in IGF-1R
signaling. Without wishing to be bound by any particular theory, this
redundancy may explain how
the receptors can mimic the function of one another. Chakravarti et al.,
identified two glioblastoma
cell lines that each overexpressed EGFR but exhibited very different responses
to EGFR inhibitors.
The resistant cell line significantly overexpressed IGF-IR and showed further
increases in IGF-1R
expression in response to EGFR inhibition by AG1478, an EGFR TKI. PI-3K/Akt
signaling persisted
in these resistant cell lines in response to AG1478 treatment, and these cells
also maintained their
invasive and antiapoptotic characteristics. These studies support the concept
of redundant signaling
through IGF-1R that maintains activation of critical pathways for survival in
the presence of EGFR
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
48
inhibition. Inhibiting both IGF-IR and EGFR may significantly reduce the
growth and invasiveness of
cells that are resistantto EGFR inhibitors alone. Further evidence that IGF-1R
activation may bypass
inhibition of other tyrosine kinase receptors comes from a study by Lu et al.,
who showed that the
degree of overexpression of IGF-1Rwas inversely related to the response of
breast cancer cells to
trastuzumab, an antibody directed against ErbB2. SKBR3 human breast cancer
cells, which normally
overexpress HER2/neu and minimally express IGF- 1R, showed a 42% decrease in
proliferation in
response to trastuzumab. Unlike the parental cell line
[00241 ], SKBR3 cells that were engineered to overexpress IGF-1 R showed no
response to
trastuzumab. When the IGF-1R was inhibitedby IGF-binding protein-3 in the
engineered cell lines,
the response to trastuzumab returned to normal. These studies clearly indicate
that activation of
alternative tyrosine kinase receptors in tumor cells may override the effect
of EGFR family inhibitors.
These examples suggest that a combination may be able to overcome resistance
to a single receptor
inhibitor and thus more effectively inhibit pathways leading to cancer growth
and survival. Recently,
an enhanced response was shown in breast cancer cell lines treated with either
recombinant bispecific
antibodies to both EGFR andIGF-1R or a combination of single receptor
antibodies comparedwith
either antibody alone. Signaling pathways downstream of the receptors were
also more effectively
inhibited by the combination therapy. By combining therapies that attack
multiple cell surface and
intracellular signaling pathways, redundant receptor signaling might be
blocked and greater clinical
benefit achieved. Thus, identifying key downstream signaling molecules in
which growth factor
receptor signals converge may be important in the development of therapeutic
agents that block signals
from multiple activated growth factor receptors. Similarly, Jung et al.
discloses the use of a
combination of mAbs to EGFR and mAbs to VEGFR-2 to treat gastric cancer grown
in nude mice.
Both mAbs were modestly effective at inhibiting tumor growth, but the
combination achieved
significantly greater tumor growth inhibition that was also associated with
decreased tumor vascularity
and increased tumor cell apoptosis.
[00242] In one embodiment the invention provides a method for treating a
subject having an EGFR
inhibitor resistant cancer cell comprising administering to the subject a
therapeutically effective
amount of a composition comprising a 1,2-diphenylpyrrole derivative in
combination with an EGFR
inhibitor. In one embodiment, the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-pyrrole. In another embodiment the 1,2-diphenylpyrrole
derivative is 2-(4-
ethoxyphenyl)-4-methyl- I -(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor
is erlotinib.
[00243] In one embodiment the invention provides a method for sensitizing EGFR
inhibitor resistant
cancer cells to an EGFR inhibitor, the method comprising administering a
combination comprising a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the combination
sensitizes the cancer
cells to the EGFR inhibitor. In one embodiment, the invention provides a
method for sensitizing
EGFR inhibitor resistant cancer cells to erlotinib, the method comprising
administering a combination
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
49
comprising a 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
erlotinib wherein the
combination sensitizes the cancer cells eriotinib.
Lung Cancer
[00244] In many countries including Japan, Europe and America, the number of
patients with lung
cancer is fairly large and continues to increase year after year and is the
most frequent cause of cancer
death in both men and women. Although there are many potential causes for lung
cancer, tobacco use,
and particularly cigarette smoking, is the most important. Additionally,
etiologic factors such as
exposure to asbestos, especially in smokers, or radon are contributory
factors. Also occupational
hazards such as exposure to uranium have been identified as an important
factor. Finally, genetic
factors have also been identified as another factor that increases the risk of
cancer.
[00245] Lung cancers can be histologically classified into non-small cell lung
cancers (e.g. squamous
cell carcinoma (epidermoid), adenocarcinoma, large cell carcinoma (large cell
anaplastic), etc.) and
small cell lung cancer (oat cell). Non-small cell lung cancer (NSCLC) has
different biological
properties and responses to chemotherapeutics from those of small cell lung
cancer (SCLC). Thus,
chemotherapeutic formulas and radiation therapy are different between these
two types of lung cancer.
Non-Small Cell Lung Cancer
[00246] Where the location of the non-small cell lung cancer tumor can be
easily excised (stage I and
II disease) surgery is the first option of therapy and offers a relatively
good chance for a cure. In more
advanced disease (stage IIIa and greater), however, where the tumor has
extended to tissue beyond the
bronchopulmonary lymph nodes, surgery may not lead to complete excision of the
tumor. In such
cases, the patient's chance for a cure by surgery alone is greatly diminished.
Where surgery will not
provide complete removal of the NSCLC tumor, other types of therapies must be
utilized. Today,
chemoradiation therapy, is the standard treatment to control unresectable or
inoperable NSCLC.
Improved results have been seen when radiation therapy has been combined with
chemotherapy, but
gains have been modest and the search continues for improved methods of
combining modalities.
[00247] Radiation therapy is based on the principle that high-dose radiation
delivered to a target area
will result in the death of reproductive cells in both tumor and normal
tissues. The radiation dosage
regimen is generally defined in terms of radiation absorbed dose (rad), time
and fractionation, and
must be carefully defined by the oncologist. The amount of radiation a patient
receives will depend on
various consideration but the two most important considerations are the
location of the tumor in
relation to other critical structures or organs of the body, and the extent to
which the tumor has spread.
One course of treatment for a patient undergoing radiation therapy for NSCLC
will be a treatment
schedule over a 5 to 6 week period, with a total dose of 50 to 60 Gy
administered to the patient in a
single daily fraction of 1.8 to 2.0 Gy, 5 days a week. A Gy is an abbreviation
for Gray and refers to
100 rad of dose.
[00248] As NSCLC is a systemic disease, however, and radiation therapy is a
local modality, radiation
therapy as a single mode of therapy is unlikely to provide a cure for NSCLC,
at least for those tumors
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
that have metastasized distantly outside the zone of treatment. Thus, the use
of radiation therapy with
other modality regimens has important beneficial effects for the treatment of
NSCLC.
[00249] Generally, radiation therapy has been combined temporally with
chemotherapy to improve the
outcome of treatment. There are various terms to describe the temporal
relationship of administering
5 radiation therapy in combination with COX-2 inhibitors and chemotherapy, and
the following
examples are the treatment regimens and are provided for illustration only and
are not intended to limit
the use of other combinations. "Sequential" therapy refers to the
administration of a composition
comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and
erlotinib and/or radiation therapy separately in time in order to allow the
separate administration of the
10 composition, and/or radiation therapy. "Concomitant" therapy refers to the
administration of the
composition, and/or radiation therapy on the same day.
[00250] Finally, "alternating" therapy refers to the administration of
radiation therapy on the days in
which the composition would not have been administered if it was given alone.
It is reported that
advanced non-small cell lung cancers do not respond favorably to single-agent
chemotherapy and
15 useful therapies for advanced inoperable cancers have been limited.
(Journal of Clinical Oncology,
vol. 10, pp. 829-83 8 (1992)).
[00251] Japanese Patent Kokai 5-163293 refers to some specified antibiotics of
16-membered-ring
macrolides as a drug delivery carrier capable of transporting anthracycline-
type anticancer drugs into
the lungs for the treatment of lung cancers. Thus, the use of a composition
comprising a combination
20 of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib
and a macrolide as a drug
delivery carrier is contemplated herein for the treatment of non-small cell
lung cancer.
[00252] Several chemotherapeutic agents have been shown to be efficacious
against NSCLC.
Chemotherapeutic agents that can be used in the present disclosure against
NSCLC include cisplatin,
carboplatin, paclitaxel, docetaxel, taxane formulations such as by way of
example only, Abraxane
25 (ABI-007), Paclitaxel-Cremophor EL, Paclitaxel poliglumex, and Paclitaxel
injectable emulsion (PIE),
gemcitabine, navelbine, pemetrexate, etoposide, methotrexate, 5-Fluorouracil,
epirubicin, doxorubicin,
vinblastine, cyclophosphamide, ifosfamide, mitomycin C, epirubicin, vindesine,
camptothecins,
fotemustine, and edatrexate.
[00253] In one embodiment, the invention provides a method of the treatment of
NSCLC which
30 utilizes a therapeutically effective amounts of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib. Further,
therapy for the
treatment of NSCLC utilizes a therapeutically effective amounts of a
composition comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole with
erlotinib, and one of
the following antineoplastic agents: bevacizumab, docetaxel, gefitinib,
gemcitabine, cisplatin,
35 carboplatin, etoposide, paclitaxel, pemetrexate, vinorelbine, or radiation
therapy.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
51
Small Cell Lung Cancer
1002541 Approximately 15 to 20 percent of all cases of lung cancer reported
worldwide are small cell
lung cancer (SCLC). Ihde DC: Cancer 54:2722, 1984. Currently, treatment of
SCLC incorporates
multi-modal therapy, including chemotherapy and radiation therapy. Response
rates of localized or
disseminated SCLC remain high to systemic chemotherapy, however, persistence
of the primary tumor
and persistence of the tumor in the associated lymph nodes has led to the
integration of several
therapeutic modalities in the treatment of SCLC.
[00255] Therapy for the treatment of lung cancer according to one embodiment
of the invention
utilizes a combination of therapeutically effective amount of a composition
comprising a combination
of 2-(4-ethoxyphenyl)-4-methyl- 1 -(4-sulfamoylphenyl)-pyrrole and erlotinib.
In another embodiment,
therapy for the treatment of lung cancer according to the invention utilizes a
combination of
therapeutically effective amount of a composition comprising a combination of
2-(4-ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib and one of the following
antineoplastic agents:
vincristine, docetaxel, camptothecin, topotecan cisplatin, carboplatin,
cyclophosphamide, epirubicin
(high dose), etoposide (VP-16) I.V., etoposide (VP-16) oral, isofamide,
teniposide (VM-26),
doxorubicin, and amrubicin. Other preferred single-agents chemotherapeutic
agents that may be used
in the present disclosure include BCNU (carmustine), vindesine,
hexamethylmelamine (altretamine),
methotrexate, nitrogen mustard, and CCNU (lomustine). Other chemotherapeutic
agents under
investigation that have shown activity against SCLC include iroplatin,
gemcitabine, lonidamine, and
taxol.
Glioma
[00256] A glioma is a type of primary central nervous system (CNS) tumor that
arises from glial cells.
The most common site of involvement of gliomas is the brain, but they can also
affect the spinal cord
or any other part of the CNS, such as the optic nerves. Treatment for brain
gliomas depends on the
location and the grade. Often, treatment is a combined approach, using
surgery, radiation therapy, and
chemotherapy. The radiation therapy is in the form of external beam radiation
or the stereotactic
approach using radiosurgery. Spinal cord tumors can be treated by surgery and
radiation.
Temozolomide is a chemotherapeutic drug that is able to cross the blood-brain
barrier effectively and
is being used in therapy.
[00257] EGFR is frequently amplified, overexpressed, or mutated in
glioblastomas, but gnereally only
10 to 20 % of patients have a response to EGFR inhibitors, It has been shown
that glioblastoma that
overexpresses the EGFRvIII oncogene and are PTEN tumor suppressor wildtype are
sensitive to
erlotinib treatment.
[00258] Therapy for the treatment of glioma according to one embodiment of the
invention utilizes a
combination of therapeutically effective amount of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and erlotinib. In another
embodiment,
therapy for the treatment of glioma according to the invention utilizes a
combination of therapeutically
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
52
effective amount of a composition comprising a combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and erlotinib and temozolomide.
Head and Neck Cancer
[00259] Head and neck cancer includes cancers of the mouth, nose, sinuses,
salivary glands, throat and
lymph nodes in the neck. Most begin in the moist tissues that line the mouth,
nose and throat.
Symptoms include a lump or sore that does not heal, a sore throat that does
not go away, trouble
swallowing and a change or hoarseness in the voice. Using tobacco or alcohol
increases cancer risk.
Treatments of head and neck cancer may include surgery, radiation therapy,
chemotherapy or a
combination. Treatments can affect eating, speaking or even breathing, so
patients may need
rehabilitation.
[00260] Head and neck cancer is often complex, with many different sites and
staging systems.
However, current therapy offers several alternatives, including surgery,
radiation, and chemotherapy,
either alone or in combination.
[00261] Combined modality therapy is becoming the principal method of treating
patients with locally
advanced head and neck cancers. Meanwhile, researchers are actively
investigating new treatments
such as gene therapy. Newer chemotherapy agents (e.g., paclitaxel [Taxol ],
docetaxel [Taxotere ],
gemcitabine [Gemzar ], doxorubicin [Doxil ]) may be combined with established
chemotherapeutic
agents (e.g., methotrexate [Trexall , Methotrex ]) to improve results.
[00262] In March 2006, the Food and Drug Administration (FDA) approved
cetuximab (Erbitux(9), in
combination with radiation, for patients with squamous cell carcinoma of the
head and neck that
cannot be treated surgically. Cetuximab also may be used alone (called
monotherapy) in patients with
head and neck cancer that has spread (metastasized) following standard
chemotherapy.
[00263] Therapy for the treatment of head and neck cancer according to one
embodiment of the
invention utilizes a combination of therapeutically effective amount of a
composition comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
erlotinib. In another
embodiment,therapy for the treatment of head and neck cancer according to the
invention utilizes a
combination of therapeutically effective amount of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl- 1 -(4-sulfamoylphenyl)-pyrrole and erlotinib and one
or more chemotherapy
agents (e.g., paclitaxel [Taxol ], docetaxel [Taxotere ], gemcitabine [Gemzar
], doxorubicin
[Doxil ]) which may be further combined with established chemotherapeutic
agents (e.g.,
methotrexate [Trexall , Methotrex ]). In another embodiment, therapy for the
treatment of head and
neck cancer according to the invention utilizes a combination of
therapeutically effective amount of a
composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and erlotinib and Erbitux .
Colorectal Cancer
[00264] COX-2 expression levels correlate with survival rates in colorectal
cancer (see Figure 1.)
Survival from colorectal cancer depends on the stage and grade of the tumor,
for example precursor
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
53
adenomas to metastatic adenocarcinoma. Generally, colorectal cancer can be
treated by surgically
removing the tumor, but overall survival rates remain between 45 and 60
percent. Colonic excision
morbidity rates are fairly low and are generally associated with the
anastomosis and not the extent of
the removal of the tumor and local tissue. In patients with a high risk of
reoccurrence, however,
chemotherapy has been incorporated into the treatment regimen in order to
improve survival rates.
[00265] Tumor metastasis prior to surgery is generally believed to be the
cause of surgical intervention
failure and up to one year of chemotherapy is required to kill the non-excised
tumor cells. As severe
toxicity is associated with the chemotherapeutic agents, only patients at high
risk of recurrence are
placed on chemotherapy following surgery. Thus, the incorporation of an
antiangiogenesis inhibitor
into the management of colorectal cancer plays an important role in the
treatment of colorectal cancer
and lead to overall improved survival rates for patients diagnosed with
colorectal cancer.
[00266] One embodiment of the invention provides a combination therapy for the
treatment of
colorectal cancer including surgery, followed by a regimen of a composition
comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
erlotinib. Further,
another embodiment of the invention provides a combination therapy for the
treatment of colorectal
cancer including surgery, followed by a regimen of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib, and one or
more antiangiogenic
agents including an MMP inhibitor, or an integrin antagonist, cycled over a
one year time period. A
further embodiment of the invention provides a combination therapy for the
treatment of colorectal
cancer including a regimen of a composition comprising a combination of 2-(4-
ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib, followed by surgical
removal of the tumor from
the colon or rectum and then followed be a regimen of a composition comprising
a combination of 2-
(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib cycled
over a one year time
period. Another therapy for the treatment of colon cancer comprises
administering a combination of
therapeutically effective amounts of a composition comprising a combination of
2-(4-ethoxyphenyl)-
4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib.
[00267] A further therapy for the treatment of colon cancer is a combination
of a treatment with a
composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-
pyrrole and erlotinib in combination with the following antineoplastic agents:
fluorouracil,
Levamisole, camptothecin, oxaliplatin, bevacizumab, cetuximab, panitumumab,
irinotecan,
leucovorin, and capecitabine.
Breast Cancer
[00268] Today, among women in the United States, breast cancer remains the
most frequent diagnosed
cancer. One in 8 women in the United States is at risk of developing breast
cancer in their lifetime.
Age, family history, diet, and genetic factors have been identified as risk
factors for breast cancer.
Breast cancer is the second leading cause of death among women.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
54
[00269] Different chemotherapeutic agents are known in art for treating breast
cancer. Cytoxic agents
used for treating breast cancer include doxorubicin, cyclophosphamide,
methotrexate, 5-fluorouracil,
mitomycin C, mitoxantrone, paclitaxel, taxane formulations such as by way of
example only,
Abraxane (ABI-007), Paclitaxel-Cremophor EL, Paclitaxel poliglumex, and
Paclitaxel injectable
emulsion (PIE), gemcitabine, docetaxel, capecitabine, lapatanib, trastuzumab,
anastrozole, letrozole,
exemestane, and epirubicin. In the treatment of locally advanced
noninflammatory breast cancer, a
composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and erlotinib can be used to treat the disease. Additionally, in
combination with surgery,
radiation therapy or with chemotherapeutic or other antiangiogenic agents.
Other combinations of
chemotherapeutic agents, that can be used in combination with the present
disclosure include, but are
not limited to anastrozole, capecitabine, docetaxel, epirubicin, exemestane,
fulvestrant, epothilone A,
B or D, goserelin acetate, letrozole, bevacizumab, paclitaxel, pamidronate,
tamoxifen, toremifene, and
trastuzumab.
Hormone Positive
[00270] Many breast cancers require the hormone oestrogen to grow. In women
who have had their
menopause, the main source of oestrogen is through the conversion of androgens
into oestrogens.
This process is carried out by the aromatase enzyme. In the treatment of
hormone positive breast
cancer a composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-
1-(4-
sulfamoylphenyl)-pyrrole and erlotinib can be used to treat the disease in
combination with other
agents, such as, aromatase inhibitors, for e.g., exemestane, letrozole, and
anastrozole.
HER2/neu Positive Breast Cancer
[00271] In the treatment of HER2/neu positive breast cancer, compositions
comprising a combination
of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib can
be used to treat the
disease in combination with other antiangiogenic agents, or in combination
with surgery, radiation
therapy or with chemotherapeutic agents. Other chemotherapeutic agents
include, for example,
trastuzumab, lapatinib, and CL-387785.
Triple Negative Breast Cancer
In the treatment of triple negative breast cancer wherein the cancer is
estrogen receptor-negative,
progesterone receptor-negative and HER2-negative, compositions comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-1-(4-sulfamoylphenyl)-pyrrole and erlotinib can be used
to treat the disease
in combination with other therapeutic agents. Such agents include, by way of
example only,
cetuximab, paclitaxel, docetaxel, taxane formulations, for example, Abraxane
(ABI-007), Paclitaxel-
Cremophor EL, Paclitaxel poliglumex, and Paclitaxel injectable emulsion (PIE).
Ovarian Cancer
[00272] Celomic epithelial carcinoma accounts for approximately 90% of ovarian
cancer cases. One
therapy for the treatment of ovary cancer is a combination of therapeutically
effective amounts of a
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and erlotinib.
[00273] Other agents that can be used in combination with a composition
comprising a combination 2-
(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib include,
but are not limited to:
5 alkylating agents, ifosfamide, cisplatin, carboplatin, paclitaxel,
docetaxel, PEGylated liposomal
doxorubicin, gemcitabine, doxorubicin, epothilone A, B, or D, topotecan,
liposomal doxorubicin 5-
fluorouracil, methotrexate, mitomycin, hexamethylmelamine, progestins,
antiestrogens,
prednimustine, dihydroxybusulfan, galactitol, interferon alpha, and interferon
gama.
[00274] Other combinations for the treatment of celomic epithelial carcinoma
is a combination of
10 therapeutically effective amounts of a composition comprising a combination
of 2-(4-ethoxyphenyl)-
4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib and one of the following
combinations of
antineoplastic agents: 1) cisplatin, doxorubicin, cyclophosphamide; 2)
hexamthylmelamine,
cyclosphamide, doxorubicin, cisplatin; 3) cyclophosphamide,
hexamehtylmelamine, 5-flurouracil,
cisplatin; 4) melphalan, hexamethylmelamine, cyclophosphamide; 5) melphalan,
doxorubicin,
15 cyclophosphamide; 6) cyclophosphamide, cisplatin, carboplatin; 7)
cyclophosphamide, doxorubicin,
hexamethylmelamine, cisplatin; 8) cyclophosphamide, doxorubicin,
hexamethylmelamine,
carboplatin; 9) cyclophosphamide, cisplatin; 10) hexamethylmelamine,
doxorubicin, carboplatin; 11)
cyclophosphamide, hexamethimelamine, doxorubicin, cisplatin; 12) carboplatin,
cyclophosphamide;
13) cisplatin, cyclophosphamide.
20 [00275] Cancer of the fallopian tube accounts for approximately 400 new
cancer cases per year in the
United States. One therapy according to the invention is for the treatment of
fallopian tube cancer
which includes administering a combination of therapeutically effective amount
of a composition
comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and
erlotinib. Other agents that can be used in combination with a composition
comprising a combination
25 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib
include, but are not limited
to: alkylating agents, ifosfamide, cisplatin, carboplatin, paclitaxel,
docetaxel, PEGylated liposomal
doxorubicin, gemcitabine, doxorubicin, epothilone A, B, or D, topotecan,
liposomal doxorubicin, 5-
fluorouracil, methotrexate, mitomycin, hexamethylmelamine, progestins,
antiestrogens,
prednimustine, dihydroxybusulfan, galactitol, interferon a, and interferon y.
30 [00276] Germ cell ovarian cancer accounts for approximately 5% of ovarian
cancer cases. Germ cell
ovarian carcinomas are classified into two main groups: dysgerminoma, and
nondysgerminoma.
Nondysgerminoma is further classified into teratoma, endodermal sinus tumor,
embryonal carcinoma,
chloricarcinoma, polyembryoma, and mixed cell tumors. One therapy for the
treatment of germ cell
carcinoma is a combination of therapeutically effective amount of a
composition comprising a
35 combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole
and erlotinib.
[00277] One therapy according to the invention is for the treatment of germ
cell carcinoma which
comprises administering a combination of a therapeutically effective amount of
a composition
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
56
comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and
erlotinib and the following combinations of antineoplastic agents: 1)
vincristine, actinomycin D,
cyclophosphamide; 2) bleomycin, etoposide, cisplatin; 3) vinblastine,
bleomycin, cisplatin.
Pancreatic Cancer
[002781 Approximately 2% of new cancer cases diagnosed in the United States
are pancreatic cancer.
Pancreatic cancer is generally classified into two clinical types: 1)
adenocarcinoma (metastatic and
non-metastatic), and 2) cystic neoplasms (serous cystadenomas, mucinous cystic
neoplasms, papillary
cystic neoplasms, acinar cell systadenocarcinoma, cystic choriocarcinoma,
cystic teratomas,
angiomatous neoplasms). Erlotinib in combination with gemcitabine is indicated
for treatment of
patients with locally advanced, unresectable or metastatic pancreatic cancer.
[00279] Combinations of therapy for the treatment of non-metastatic
adenocarcinoma that may be used
in the present disclosure include the use of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and erlotinib along with
preoperative bilary
tract decompression (patients presenting with obstructive jaundice); surgical
resection, including
standard resection, extended or radial resection and distal pancreatectomy
(tumors of body and tail);
adjuvant radiation; antiangiogenic therapy; and chemotherapy.
[00280] For the treatment of metastatic adenocarcinoma, a combination therapy
consists of a
composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole and erlotinib of the present disclosure in combination with continuous
treatment of 5-
fluorouracil, followed by weekly cisplatin therapy.
[00281] Another combination therapy for the treatment of cystic neoplasms is
the use of a composition
comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and
erlotinib in combination with gemcitabine.
Pharmaceutical Compositions
[00282] Provided herein is a pharmaceutical composition for treating cancer
comprising a combination
of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor; and a
pharmaceutically acceptable
excipient or carrier.
[00283] In one embodiment, the invention provides a pharmaceutical composition
comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and
erlotinib as an active
ingredient, or a pharmaceutically acceptable salt, solvate, or prodrug
thereof, in a pharmaceutically
acceptable vehicle, carrier, diluent, or excipient, or a mixture thereof; and
one or more
pharmaceutically acceptable excipients or carriers.
[00284] In another embodiment, the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-pyrrole and a pharmaceutically acceptable excipient or
carrier. In another
embodiment, the EGFR inhibitor is erlotinib and a pharmaceutically acceptable
excipient or carrier. In
a further embodiment the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-
4-methyl-l-(4-
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
57
sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib and a
pharmaceutically acceptable
excipient or carrier.
[00285] In one embodiment, the invention provides a pharmaceutical composition
for treating cancer
comprising a combination of a 1,2-diphenylpyrrole derivative selected from the
group consisting of: 4-
methyl-2-(4-methylphenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-methoxyphenyl)-4-
methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-(4-chlorophenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 4-methyl-2-(4-
methylthiophenyl)-1-(4-sulfamoylphenyl)pyrrole; 2-(4-ethoxyphenyl)-4-methyl-l-
(4-
sulfamoylphenyl)pyrrole; 2-(4-methoxy-3-methylphenyl)-4-methyl-l-(4-
sulfamoylphenyl)pyrrole; 2-
(3-fluoro-4-methoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)pyrrole; 2-(3,4-
dimethylphenyl)-4-
methyl-l-(4-sulfamoylphenyl)pyrrole; 4-methyl-l-(4-methylthiophenyl)-2-(4-
sulfamoylphenyl)pyrrole; 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(4-
methoxyphenyl)pyrrole;
and 1-(4-acetylaminosulfonylphenyl)-4-methyl-2-(3,4-dimethylphenyl)pyrrole. In
another
embodiment, the invention provides a method wherein the 1,2-diphenylpyrrole
derivative is 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and an EGFR inhibitor;
and a
pharmaceutically acceptable excipient or carrier.
[00286] Provided herein are pharmaceutical compositions comprising a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor as an active ingredient, or a
pharmaceutically
acceptable salt, solvate, or prodrug thereof, in a pharmaceutically acceptable
vehicle, carrier, diluent,
or excipient, or a mixture thereof; and one or more pharmaceutically
acceptable excipients or carriers.
Also provided herein are pharmaceutical compositions comprising a combination
of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor as an active ingredient, or a
pharmaceutically
acceptable salt, solvate, or prodrug thereof, in a pharmaceutically acceptable
vehicle, carrier, diluent,
or excipient, or a mixture thereof; and one or more release controlling
excipients as described herein.
Provided herein are pharmaceutical compositions comprising a combination of a
1,2-diphenylpyrrole
derivative and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is
2-(4-ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib as an
active ingredient, or a
pharmaceutically acceptable salt, solvate, or prodrug thereof, in a
pharmaceutically acceptable vehicle,
carrier, diluent, or excipient, or a mixture thereof; and one or more release
controlling excipients as
described herein. Suitable modified release dosage vehicles include, but are
not limited to,
hydrophilic or hydrophobic matrix devices, water-soluble separating layer
coatings, enteric coatings,
osmotic devices, multi-particulate devices, and combinations thereof. The
pharmaceutical
compositions may also comprise non-release controlling excipients.
[00287] Provided herein are pharmaceutical compositions in film-coated dosage
forms, which
comprise a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor as an active
ingredient, or a pharmaceutically acceptable salt, solvate, or prodrug
thereof; and one or more
tabletting excipients to form a tablet core using conventional tabletting
processes and subsequently
coating the core. The tablet cores can be produced using conventional
granulation methods, for
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
58
example wet or dry granulation, with optional comminution of the granules and
with subsequent
compression and coating. Granulation methods are described, for example, in
Voigt, pages 156-69
(Voigt, R. (1984): Lehrbuch der pharmazeutischen Technologie (Textbook of
Pharmaceutical
Technology); Verlag Chemie Weinheim-Beerfield Beach/Florida-Basle).
[00288] Suitable excipients for the production of granules are, for example
pulverulent fillers
optionally having flow-conditioning properties, for example talcum, silicon
dioxide; for example
synthetic amorphous anhydrous silicic acid of the Syloid type (Grace), for
example SYLOID 244
FP, microcrystalline cellulose, for example of the Avicel type (FMC Corp.),
for example of the types
AVICEL PH101, 102, 105, RC581 or RC 591, Emcocel type (Mendell Corp.) or
Elcema type
(Degussa); carbohydrates, such as sugars, sugar alcohols, starches or starch
derivatives, for example
lactose, dextrose, saccharose, glucose, sorbitol, mannitol, xylitol, potato
starch, maize starch, rice
starch, wheat starch or amylopectin, tricalcium phosphate, calcium hydrogen
phosphate or magnesium
trisilicate; binders, such as gelatin, tragacanth, agar, alginic acid,
cellulose ethers, for example
methylcellulose, carboxymethylcellulose or hydroxypropylmethylcellulose,
polyethylene glycols or
ethylene oxide homopolymers, especially having a degree of polymerization of
approximately from
2.Ox103 to 1.Ox105 and an approximate molecular weight of about from 1.Ox105
to 5.Ox106, for
example excipients known by the name Polyox (Union Carbide),
polyvinylpyrrolidone or povidones,
especially having a mean molecular weight of approximately 1000 and a degree
of polymerization of
approximately from about 500 to about 2500, and also agar or gelatin; surface-
active substances, for
example anionic surfactants of the alkyl sulfate type, for example sodium,
potassium or magnesium n-
dodecyl sulfate, n-tetradecyl sulfate, n-hexadecyl sulfate or n-octadecyl
sulfate, of the alkyl ether
sulfate type, for example sodium, potassium or magnesium n-dodecyloxyethyl
sulfate, n-
tetradecyloxyethyl sulfate, n-hexadecyloxyethyl sulfate or n-octadecyloxyethyl
sulfate, or of the
alkanesulfonate type, for example sodium, potassium or magnesium n-
dodecanesulfonate, n-
tetradecanesulfonate, n-hexadecanesulfonate or n-octadecanesulfonate, or non-
ionic surfactants of the
fatty acid polyhydroxy alcohol ester type, such as sorbitan monolaurate,
monooleate, monostearate or
monopalmitate, sorbitan tristearate or trioleate, polyoxyethylene adducts of
fatty acid polyhydroxy
alcohol esters, such as polyoxyethylene sorbitan monolaurate, monooleate,
monostearate,
monopalmitate, tristearate or trioleate, polyethylene glycol fatty acid
esters, such as polyoxyethyl
stearate, polyethylene glycol 400 stearate, polyethylene glycol 2000 stearate,
especially ethylene
oxide/propylene oxide block polymers of the Pluronics (BWC) or Synperonic
(ICI) type
[00289] Further provided herein are pharmaceutical compositions in enteric
coated dosage forms,
which comprise a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor as an active
ingredient, or a pharmaceutically acceptable salt, solvate, or prodrug
thereof; and one or more release
controlling excipients for use in an enteric coated dosage form. Provided
herein are pharmaceutical
compositions in enteric coated dosage forms comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is
2-(4-ethoxyphenyl)-4-
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
59
methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib as an
active ingredient, or a
pharmaceutically acceptable salt, solvate, or prodrug thereof, in a
pharmaceutically acceptable vehicle,
carrier, diluent, or excipient, or a mixture thereof; and one or more release
controlling excipients for
use in an enteric coated dosage form. The pharmaceutical compositions may also
comprise non-
release controlling excipients.
[00290] Further provided herein are pharmaceutical compositions in
effervescent dosage forms, which
comprise a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor as an active
ingredient, or a pharmaceutically acceptable salt, solvate, or prodrug
thereof; and one or more release
controlling excipients for use in effervescent dosage forms. Also provided
herein are pharmaceutical
compositions in effervescent dosage forms comprising a combination of a 1,2-
diphenylpyrrole
derivative and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is
2-(4-ethoxyphenyl)-4-
methyl-1-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib as an
active ingredient, or a
pharmaceutically acceptable salt, solvate, or prodrug thereof, in a
pharmaceutically acceptable vehicle,
carrier, diluent, or excipient, or a mixture thereof; and one or more release
controlling excipients for
use in an effervescent dosage forms. The pharmaceutical compositions may also
comprise non-release
controlling excipients.
[00291] Additionally provided are pharmaceutical compositions in a dosage form
that has an instant
releasing component and at least one delayed releasing component, and is
capable of giving a
discontinuous release of the compound in the form of at least two consecutive
pulses separated in time
from 0.1 up to 24 hours. The pharmaceutical compositions comprise a
combination of a 1,2-
diphenylpyrrole derivative and an EGFR inhibitor as an active ingredient, or a
pharmaceutically
acceptable salt, solvate, or prodrug thereof; and one or more release
controlling and non-release
controlling excipients, such as those excipients suitable for a disruptable
semi-permeable membrane
and as swellable substances. Additionally, the invention provides
pharmaceutical compositions
comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl- 1 -(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib as an active ingredient, or a pharmaceutically
acceptable salt, solvate, or
prodrug thereof; and one or more release controlling and non-release
controlling excipients, such as
those excipients suitable for a disruptable semi-permeable membrane and as
swellable substances.
[00292] Provided herein also are pharmaceutical compositions in a dosage form
for oral administration
to a subject, which comprises a combination of a 1,2-diphenylpyrrole
derivative and an EGFR
inhibitor as an active ingredient, or a pharmaceutically acceptable salt,
solvate, or prodrug thereof; and
one or more pharmaceutically acceptable excipients or carriers, enclosed in an
intermediate reactive
layer comprising a gastric juice-resistant polymeric layered material
partially neutralized with alkali
and having cation exchange capacity and a gastric juice-resistant outer layer.
Additionally, the
invention provides pharmaceutical compositions in a dosage form for oral
administration to a subject
comprising a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein the 1,2-
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib enclosed in an intermediate reactive layer
comprising a gastric juice-
resistant polymeric layered material partially neutralized with alkali and
having cation exchange
capacity and a gastric juice-resistant outer layer.
5 [00293] Provided herein are pharmaceutical compositions that comprise about
0.1 to about 1200 mg,
about 1 to about 500 mg, about 2 to about 100 mg, about 1 mg, about 2 mg,
about 3 mg, about 5 mg,
about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 100 mg,
about 200 mg,
about 300 mg, about 400 mg, about 500 mg, about 600mg, about 800 mg, about
1200mg of a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor as an
active ingredient, or a
10 pharmaceutically acceptable salt, solvate, or prodrug thereof, in the form
of enteric-coated granules, as
delayed-release capsules for oral administration. Also, the invention provides
for pharmaceutical
compositions that comprise about 0.1 to about 1000 mg, about 1 to about 500
mg, about 2 to about 100
mg, about 1 mg, about 2 mg, about 3 mg, about 5 mg, about 10 mg, about 20 mg,
about 30 mg, about
40 mg, about 50 mg, about 100 mg, about 500 mg of a combination of a 1,2-
diphenylpyrrole
15 derivative and an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative
is 2-(4-ethoxyphenyl)-4-
methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib as an
active ingredient, or a
pharmaceutically acceptable salt, solvate, or prodrug thereof, in the form of
enteric-coated granules, as
delayed-release capsules for oral administration.
[00294] Provided herein are pharmaceutical compositions that comprise about
0.1 to about 1200 mg,
20 about 1 to about 500 mg, about 2 to about 100 mg, about 1 mg, about 2 mg,
about 3 mg, about 5 mg,
about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 100 mg,
about 500 mg,
about 600 mg, about 800 mg, about 1200 mg of a combination of a 1,2-
diphenylpyrrole derivative and
an EGFR inhibitor as an active ingredient, or a pharniaceutically acceptable
salt, solvate, or prodrug
thereof in the form of enteric-coated pellets, as delayed-release capsules for
oral administration. Also,
25 the invention provides for pharmaceutical compositions that comprise about
0.1 to about 1200 mg,
about 1 to about 500 mg, about 2 to about 100 mg, about 1 mg, about 2 mg,
about 3 mg, about 5 mg,
about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 100 mg,
about 500 mg,
about 600 mg, about 800 mg, about 1200 mg of a combination of a 1,2-
diphenylpyrrole derivative and
an EGFR inhibitor wherein the 1,2-diphenylpyrrole derivative is 2-(4-
ethoxyphenyl)-4-methyl- 1 -(4-
30 sulfamoylphenyl)-pyrrole and the EGFR inhibitor is erlotinib as an active
ingredient, or a
pharmaceutically acceptable salt, solvate, or prodrug thereof in the form of
enteric-coated pellets, as
delayed-release capsules for oral administration. The pharmaceutical
compositions further comprise
glyceryl monostearate 40-50, hydroxypropyl cellulose, hypromellose, magnesium
stearate,
methacrylic acid copolymer type C, polysorbate 80, sugar spheres, talc, and
triethyl citrate.
35 [00295] Provided herein are pharmaceutical compositions that comprise about
0.1 to about 1200 mg,
about 1 to about 500 mg, about 2 to about 100 mg, about 1 mg, about 2 mg,
about 3 mg, about 5 mg,
about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 100 mg,
about 500 mg,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
61
about 600 mg, about 800 mg, about 1200 mg of a combination of a 1,2-
diphenylpyrrole derivative and
an EGFR inhibitor as an active ingredient, or a pharmaceutically acceptable
salt, solvate, or prodrug
thereof, as enteric-coated delayed-release tablets for oral administration.
Also, the pharmaceutical
compositions that comprise about 0.1 to about 1200 mg, about 1 to about 500
mg, about 2 to about 100
mg, about I mg, about 2 mg, about 3 mg, about 5 mg, about 10 mg, about 20 mg,
about 30 mg, about
40 mg, about 50 mg, about 100 mg, about 500 mg, about 600 mg, about 800 mg,
about 1200 mg of a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein
the 1,2-
diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and the
EGFR inhibitor is erlotinib as an active ingredient, or a pharmaceutically
acceptable salt, solvate, or
prodrug thereof, as enteric-coated delayed-release tablets for oral
administration. The pharmaceutical
compositions further comprise carnauba wax, crospovidone, diacetylated
monoglycerides,
ethylcellulose, hydroxypropyl cellulose, hypromellose phthalate, magnesium
stearate, mannitol,
sodium hydroxide, sodium stearyl fumarate, talc, titanium dioxide, and yellow
ferric oxide.
[00296] Provided herein are pharmaceutical compositions that comprise about
0.1 to about 1200 mg,
about 1 to about 500 mg, about 2 to about 100 mg, about 1 mg, about 2 mg,
about 3 mg, about 5 mg,
about 10 mg, about 20 mg, about 30 mg, about 40 mg, about 50 mg, about 100 mg,
about 500 mg,
about 600 mg, about 800 mg, about 1200 mg of a combination of a 1,2-
diphenylpyrrole derivative and
an EGFR inhibitor as an active ingredient, or a pharmaceutically acceptable
salt, solvate, or prodrug
thereof, as enteric-coated delayed-release tablets for oral administration.
Also provided herein are
pharmaceutical compositions that comprise about 0.1 to about 1200 mg, about 1
to about 500 mg,
about 2 to about 100 mg, about 1 mg, about 2 mg, about 3 mg, about 5 mg, about
10 mg, about 20 mg,
about 30 mg, about 40 mg, about 50 mg, about 100 mg, about 500 mg, about 600
mg, about 800 mg,
about 1200 mg of a combination of a 1,2-diphenylpyrrole derivative and an EGFR
inhibitor wherein
the 1,2-diphenylpyrrole derivative is 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole and
the EGFR inhibitor is erlotinib as an active ingredient, or a pharmaceutically
acceptable salt, solvate,
or prodrug thereof, as enteric-coated delayed-release tablets for oral
administration. The
pharmaceutical compositions further comprise calcium stearate, crospovidone,
hydroxypropyl
methylcellulose, iron oxide, mannitol, methacrylic acid copolymer, polysorbate
80, povidone,
propylene glycol, sodium carbonate, sodium lauryl sulfate, titanium dioxide,
and triethyl citrate.
[002971 The pharmaceutical compositions provided herein may be provided in
unit-dosage forms or
multiple-dosage forms. Unit-dosage forms, as used herein, refer to physically
discrete units suitable
for administration to human and animal subjects and packaged individually as
is known in the art.
Each unit-dose contains a predetermined quantity of the active ingredient(s)
sufficient to produce the
desired therapeutic effect, in association with the required pharmaceutical
carriers or excipients.
Examples of unit-dosage forms include ampules, syringes, and individually
packaged tablets and
capsules. Unit-dosage forms may be administered in fractions or multiples
thereof. A multiple-
dosage form is a plurality of identical unit-dosage forms packaged in a single
container to be
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
62
administered in segregated unit-dosage form. Examples of multiple-dosage forms
include vials,
bottles of tablets or capsules, or bottles of pints or gallons.
[00298] The compositions provided herein may be administered alone, or in
combination with one or
more other compounds provided herein, one or more other active ingredients.
The pharmaceutical
compositions that comprise a compound provided herein may be formulated in
various dosage forms
for oral, parenteral, buccal, intranasal, epidural, sublingual, pulmonary,
local, rectal, transdermal, or
topical administration. The pharmaceutical compositions may also be formulated
as a modified
release dosage form, including delayed-, extended-, prolonged-, sustained-,
pulsatile-, controlled-,
accelerated- and fast-, targeted-, programmed-release, and gastric retention
dosage forms. These
dosage forms can be prepared according to conventional methods and techniques
known to those
skilled in the art (see, Remington: The Science and Practice of Pharmacy,
supra; Modified-Release
Drug Deliver Technology, Rathbone et al., Eds., Drugs and the Pharmaceutical
Science, Marcel
Dekker, Inc.: New York, NY, 2002; Vol. 126).
[00299] The pharmaceutical compositions provided herein may be administered at
once, or multiple
times at intervals of time. It is understood that the precise dosage and
duration of treatment may vary
with the age, weight, and condition of the patient being treated, and may be
determined empirically
using known testing protocols or by extrapolation from in vivo or in vitro
test or diagnostic data. It is
further understood that for any particular individual, specific dosage
regimens should be adjusted over
time according to the individual need and the professional judgment of the
person administering or
supervising the administration of the formulations.
[00300] In the case wherein the patient's condition does not improve, upon the
doctor's discretion the
administration of the combinations may be administered chronically, that is,
for an extended period of
time, including throughout the duration of the patient's life in order to
ameliorate or otherwise control
or limit the symptoms of the patient's disease or condition.
[00301] In the case wherein the patient's status does improve, upon the
doctor's discretion the
administration of the combinations may be given continuously or temporarily
suspended for a certain
length of time (i.e., a "drug holiday").
[00302] Once improvement of the patient's conditions has occurred, a
maintenance dose is
administered if necessary. Subsequently, the dosage or the frequency of
administration, or both, can be
reduced, as a function of the symptoms, to a level at which the improved
disease, disorder or condition
is retained. Patients can, however, require intermittent treatment on a long-
term basis upon any
recurrence of symptoms.
[00303] As described herein, the compositions and methods for using the
composition comprising a
combination of a 1,2-diphenylpyrrole derivative and an EGFR inhibitor, may be
formulated without
carriers or excipients or may be combined with one or more pharmaceutically
acceptable carriers for
administration. For example, solvents, diluents and the like, and may be
administered orally in such
forms as tablets, capsules, dispersible powders, granules, or suspensions
containing, for example, from
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
63
about 0.05 to about 5% of suspending agent, syrups containing, for example,
from about 10 to about
50% of sugar, and elixirs containing, for example, from about 20 to about 50%
ethanol, and the like.
Such pharmaceutical preparations may contain, for example, from about 0.05 up
to about 90% of the
active ingredient in combination with the carrier, more usually between about
5% and about 60% by
weight. Also, the compositions and methods for using the composition
comprising a combination of a
1,2-diphenylpyrrole derivative and an EGFR inhibitor wherein the 1,2-
diphenylpyrrole derivative is 2-
(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and the EGFR inhibitor
is erlotinib, may be
formulated without carriers or excipients or may be combined with one or more
pharmaceutically
acceptable carriers for administration.
[00304] The effective dosage of each active ingredient employed may vary
depending on the particular
compound employed, the mode of administration and the severity of the
condition being treated. The
projected daily dosage of the EGFR inhibitor will depend on many factors.
Numerous methods for
evaluating and comparing 1,2-diphenylpyrrole derivative inhibitor potency are
known to one of skill in
the art. In one embodiment, an oral daily dosage of the 1,2-diphenylpyrrole
derivative inhibitor is in
the range of about 0.01 to about 30 mg/kg, and the projected daily dosage of
the EGFR inhibitor is in
the range of about 0.3 to about 8 mg/kg. This dosage regimen may be adjusted
to provide the optimal
therapeutic response. For example, several divided doses may be administered
daily or the dose may
be proportionally reduced as indicated by the exigencies of the therapeutic
situation. The 1,2-
diphenylpyrrole derivative inhibitor and the EGFR inhibitor may also be
administered as a combined
dosage unit, or as separate components. When administered as separate
components, each component
may be administered at the same time, or at different times during the
treatment period.
[00305] It is understood, however, that a specific dose level for any
particular patient will depend upon
a variety of factors such as, for example, decreases in the liver and kidney
function.
[00306] Treatment dosages generally may be titrated to optimize safety and
efficacy. Typically,
dosage-effect relationships from in vitro studies initially can provide useful
guidance on the proper
doses for patient administration. Studies in animal models also generally may
be used for guidance
regarding effective dosages for treatment of cancers in accordance with the
present disclosure. In
terms of treatment protocols, it should be appreciated that the dosage to be
administered will depend
on several factors, including the particular agent that is administered, the
route administered, the
condition of the particular patient, etc. Determination of these parameters
are well within the skill of
the art. These considerations, as well as effective formulations and
administration procedures are well
known in the art and are described in standard textbooks.
Oral Formulations
[00307] Oral formulations containing the active combinations described herein
may comprise any
conventionally used oral forms, including: tablets, capsules, pills, troches,
lozenges, pastilles, cachets,
pellets, medicated chewing gum, granules, bulk powders, effervescent or non-
effervescent powders or
granules, solutions, emulsions, suspensions, solutions, wafers, sprinkles,
elixirs, syrups, buccal forms,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
64
and oral liquids. Capsules may contain mixtures of the active compound(s) with
inert fillers and/or
diluents such as the pharmaceutically acceptable starches (e.g. corn, potato
or tapioca starch), sugars,
artificial sweetening agents, powdered celluloses, such as crystalline and
microcrystalline celluloses,
flours, gelatins, gums, etc. Useful tablet formulations may be made by
conventional compression, wet
granulation or dry granulation methods and utilize pharmaceutically acceptable
diluents, binding
agents, lubricants, disintegrants, surface modifying agents (including
surfactants), suspending or
stabilizing agents, including, but not limited to, magnesium stearate, stearic
acid, talc, sodium lauryl
sulfate, microcrystalline cellulose, carboxymethylcellulose calcium,
polyvinylpyrrolidone, gelatin,
alginic acid, acacia gum, xanthan gum, sodium citrate, complex silicates,
calcium carbonate, glycine,
dextrin, sucrose, sorbitol, dicalcium phosphate, calcium sulfate, lactose,
kaolin, mannitol, sodium
chloride, talc, dry starches and powdered sugar. In some embodiments are
surface modifying agents
which include nonionic and anionic surface modifying agents. For example,
surface modifying agents
include, but are not limited to, poloxamer 188, benzalkonium chloride, calcium
stearate, cetostearyl
alcohol, cetomacrogol emulsifying wax, sorbitan esters, colloidal silicon
dioxide, phosphates, sodium
dodecylsulfate, magnesium aluminum silicate, and triethanolamine. Oral
formulations herein may
utilize standard delay or time release formulations to alter the absorption of
the active compound(s).
The oral formulation may also consist of administering the active ingredient
in water or a fruit juice,
containing appropriate solubilizers or emulsifiers as needed.
Oral Administration
[00308] As described herein, the combination regimen can be given
simultaneously or can be given in
a staggered regimen, with a 1,2-diphenylpyrrole derivative being given at a
different time during the
course of chemotherapy than an EGFR inhibitor. This time differential may
range from several
minutes, hours, days, weeks, or longer between administration of the two
compounds. Therefore, the
term combination does not necessarily mean administered at the same time or as
a unitary dose, but
that each of the components are administered during a desired treatment
period. The agents may also
be administered by different routes. As is typical for chemotherapeutic
regimens, a course of
chemotherapy may be repeated several weeks later, and may follow the same
timeframe for
administration of the two compounds, or may be modified based on patient
response.
[00309] In other embodiments, the pharmaceutical compositions provided herein
may be provided in
solid, semisolid, or liquid dosage forms for oral administration. As used
herein, oral administration
also include buccal, lingual, and sublingual administration. Suitable oral
dosage forms include, but are
not limited to, tablets, capsules, pills, troches, lozenges, pastilles,
cachets, pellets, medicated chewing
gum, granules, bulk powders, effervescent or non-effervescent powders or
granules, solutions,
emulsions, suspensions, solutions, wafers, sprinkles, elixirs, and syrups. In
addition to the active
ingredient(s), the pharmaceutical compositions may contain one or more
pharmaceutically acceptable
carriers or excipients, including, but not limited to, binders, fillers,
diluents, disintegrants, wetting
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
agents, lubricants, glidants, coloring agents, dye-migration inhibitors,
sweetening agents, and flavoring
agents.
[00310] Binders or granulators impart cohesiveness to a tablet to ensure the
tablet remaining intact
after compression. Suitable binders or granulators include, but are not
limited to, starches, such as
5 corn starch, potato starch, and pre-gelatinized starch (e.g., STARCH 1500);
gelatin; sugars, such as
sucrose, glucose, dextrose, molasses, and lactose; natural and synthetic gums,
such as acacia, alginic
acid, alginates, extract of Irish moss, Panwar gum, ghatti gum, mucilage of
isabgol husks,
carboxymethylcellulose, methylcellulose, polyvinylpyrrolidone (PVP), Veegum,
larch arabogalactan,
powdered tragacanth, and guar gum; celluloses, such as ethyl cellulose,
cellulose acetate,
10 carboxymethyl cellulose calcium, sodium carboxymethyl cellulose, methyl
cellulose,
hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxypropyl
methyl cellulose
(HPMC); microcrystalline celluloses, such as AVICEL-PH- 10 1, AVICEL-PH- 103,
AVICEL RC-581,
AVICEL-PH-105 (FMC Corp., Marcus Hook, PA); and mixtures thereof. Suitable
fillers include, but
are not limited to, talc, calcium carbonate, microcrystalline cellulose,
powdered cellulose, dextrates,
15 kaolin, mannitol, silicic acid, sorbitol, starch, pre-gelatinized starch,
and mixtures thereof. The binder
or filler may be present from about 50 to about 99% by weight in the
pharmaceutical compositions
provided herein.
[00311] Suitable diluents include, but are not limited to, dicalcium
phosphate, calcium sulfate, lactose,
sorbitol, sucrose, inositol, cellulose, kaolin, mannitol, sodium chloride, dry
starch, and powdered
20 sugar. Certain diluents, such as mannitol, lactose, sorbitol, sucrose, and
inositol, when present in
sufficient quantity, can impart properties to some compressed tablets that
permit disintegration in the
mouth by chewing. Such compressed tablets can be used as chewable tablets.
[00312] Suitable disintegrants include, but are not limited to, agar;
bentonite; celluloses, such as
methylcellulose and carboxymethylcellulose; wood products; natural sponge;
cation-exchange resins;
25 alginic acid; gums, such as guar gum and Veegum HV; citrus pulp; cross-
linked celluloses, such as
croscarmellose; cross-linked polymers, such as crospovidone; cross-linked
starches; calcium
carbonate; microcrystalline cellulose, such as sodium starch glycolate;
polacrilin potassium; starches,
such as corn starch, potato starch, tapioca starch, and pre-gelatinized
starch; clays; aligns; and
mixtures thereof. The amount of disintegrant in the pharmaceutical
compositions provided herein
30 varies upon the type of formulation, and is readily discernible to those of
ordinary skill in the art. The
pharmaceutical compositions provided herein may contain from about 0.5 to
about 15% or from about
I to about 5% by weight of a disintegrant.
[00313] Suitable lubricants include, but are not limited to, calcium stearate;
magnesium stearate;
mineral oil; light mineral oil; glycerin; sorbitol; mannitol; glycols, such as
glycerol behenate and
35 polyethylene glycol (PEG); stearic acid; sodium lauryl sulfate; talc;
hydrogenated vegetable oil,
including peanut oil, cottonseed oil, sunflower oil, sesame oil, olive oil,
corn oil, and soybean oil; zinc
stearate=, ethyl oleate; ethyl laureate; agar; starch; lycopodium; silica or
silica gels, such as AEROSIL
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
66
200 (W.R. Grace Co., Baltimore, MD) and CAB-O-SIL (Cabot Co. of Boston, MA);
and mixtures
thereof. The pharmaceutical compositions provided herein may contain about 0.1
to about 5% by
weight of a lubricant.
[00314] Suitable glidants include colloidal silicon dioxide, CAB-O-SIL (Cabot
Co. of Boston, MA),
and asbestos-free talc. Coloring agents include any of the approved,
certified, water soluble FD&C
dyes, and water insoluble FD&C dyes suspended on alumina hydrate, and color
lakes and mixtures
thereof. A color lake is the combination by adsorption of a water-soluble dye
to a hydrous oxide of a
heavy metal, resulting in an insoluble form of the dye. Flavoring agents
include natural flavors
extracted from plants, such as fruits, and synthetic blends of compounds which
produce a pleasant
taste sensation, such as peppermint and methyl salicylate. Sweetening agents
include sucrose, lactose,
mannitol, syrups, glycerin, and artificial sweeteners, such as saccharin and
aspartame. Suitable
emulsifying agents include gelatin, acacia, tragacanth, bentonite, and
surfactants, such as
polyoxyethylene sorbitan monooleate (TWEEN 20), polyoxyethylene sorbitan
monooleate 80
(TWEEN 80), and triethanolamine oleate. Suspending and dispersing agents
include sodium
carboxymethylcellulose, pectin, tragacanth, Veegum, acacia, sodium
carbomethylcellulose,
hydroxypropyl methylcellulose, and polyvinylpyrolidone. Preservatives include
glycerin, methyl and
propylparaben, benzoic add, sodium benzoate and alcohol. Wetting agents
include propylene glycol
monostearate, sorbitan monooleate, diethylene glycol monolaurate, and
polyoxyethylene lauryl ether.
Solvents include glycerin, sorbitol, ethyl alcohol, and syrup. Examples of non-
aqueous liquids utilized
in emulsions include mineral oil and cottonseed oil. Organic acids include
citric and tartaric acid.
Sources of carbon dioxide include sodium bicarbonate and sodium carbonate.
[00315] It should be understood that many carriers and excipients may serve
several functions, even
within the same formulation.
[00316] In further embodiments, the pharmaceutical compositions provided
herein may be provided as
compressed tablets, tablet triturates, chewable lozenges, rapidly dissolving
tablets, multiple
compressed tablets, or enteric-coating tablets, sugar-coated, or film-coated
tablets. Enteric-coated
tablets are compressed tablets coated with substances that resist the action
of stomach acid but
dissolve or disintegrate in the intestine, thus protecting the active
ingredients from the acidic
environment of the stomach. Enteric-coatings include, but are not limited to,
fatty acids, fats,
phenylsalicylate, waxes, shellac, ammoniated shellac, and cellulose acetate
phthalates. Sugar-coated
tablets are compressed tablets surrounded by a sugar coating, which may be
beneficial in covering up
objectionable tastes or odors and in protecting the tablets from oxidation.
Film-coated tablets are
compressed tablets that are covered with a thin layer or film of a water-
soluble material. Film coatings
include, but are not limited to, hydroxyethylcellulose, sodium
carboxymethylcellulose, polyethylene
glycol 4000, and cellulose acetate phthalate. Film coating imparts the same
general characteristics as
sugar coating. Multiple compressed tablets are compressed tablets made by more
than one
compression cycle, including layered tablets, and press-coated or dry-coated
tablets.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
67
[00317] The tablet dosage forms may be prepared from the active ingredient in
powdered, crystalline,
or granular forms, alone or in combination with one or more carriers or
excipients described herein,
including binders, disintegrants, controlled-release polymers, lubricants,
diluents, and/or colorants.
Flavoring and sweetening agents are especially useful in the formation of
chewable tablets and
lozenges.
[00318] The pharmaceutical compositions provided herein may be provided as
soft or hard capsules,
which can be made from gelatin, methylcellulose, starch, or calcium alginate.
The hard gelatin
capsule, also known as the dry-filled capsule (DFC), consists of two sections,
one slipping over the
other, thus completely enclosing the active ingredient. The soft elastic
capsule (SEC) is a soft,
globular shell, such as a gelatin shell, which is plasticized by the addition
of glycerin, sorbitol, or a
similar polyol. The soft gelatin shells may contain a preservative to prevent
the growth of
microorganisms. Suitable preservatives are those as described herein,
including methyl- and propyl-
parabens, and sorbic acid. The liquid, semisolid, and solid dosage forms
provided herein may be
encapsulated in a capsule. Suitable liquid and semisolid dosage forms include
solutions and
suspensions in propylene carbonate, vegetable oils, or triglycerides. Capsules
containing such
solutions can be prepared as described in U.S. Pat. Nos. 4,328,245; 4,409,239;
and 4,410,545. The
capsules may also be coated as known by those of skill in the art in order to
modify or sustain
dissolution of the active ingredient.
[00319] In other embodiments, the pharmaceutical compositions provided herein
may be provided in
liquid and semisolid dosage forms, including emulsions, solutions,
suspensions, elixirs, and syrups.
An emulsion is a two-phase system, in which one liquid is dispersed in the
form of small globules
throughout another liquid, which can be oil-in-water or water-in-oil.
Emulsions may include a
pharmaceutically acceptable non-aqueous liquids or solvent, emulsifying agent,
and preservative.
Suspensions may include a pharmaceutically acceptable suspending agent and
preservative. Aqueous
alcoholic solutions may include a pharmaceutically acceptable acetal, such as
a di(lower alkyl) acetal
of a lower alkyl aldehyde (the term "lower" means an alkyl having between 1
and 6 carbon atoms),
e.g., acetaldehyde diethyl acetal; and a water-miscible solvent having one or
more hydroxyl groups,
such as propylene glycol and ethanol. Elixirs are clear, sweetened, and
hydroalcoholic solutions.
Syrups are concentrated aqueous solutions of a sugar, for example, sucrose,
and may also contain a
preservative. For a liquid dosage form, for example, a solution in a
polyethylene glycol may be
diluted with a sufficient quantity of a pharmaceutically acceptable liquid
carrier, e.g., water, to be
measured conveniently for administration.
[00320] Other useful liquid and semisolid dosage forms include, but are not
limited to, those
containing the active ingredient(s) provided herein, and a dialkylated mono-
or poly-alkylene glycol,
including, 1,2-dimethoxymethane, diglyme, triglyme, tetraglyme, polyethylene
glycol-350-dimethyl
ether, polyethylene glycol-550-dimethyl ether, polyethylene glycol-750-
dimethyl ether, wherein 350,
550, and 750 refer to the approximate average molecular weight of the
polyethylene glycol. These
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
68
formulations may further comprise one or more antioxidants, such as butylated
hydroxytoluene
(BHT), butylated hydroxyanisole (BHA), propyl gallate, vitamin E,
hydroquinone, hydroxycoumarins,
ethanolamine, lecithin, cephalin, ascorbic acid, malic acid, sorbitol,
phosphoric acid, bisulfite, sodium
metabisulfite, thiodipropionic acid and its esters, and dithiocarbamates.
[00321] The pharmaceutical compositions provided herein for oral
administration may be also
provided in the forms of liposomes, micelles, microspheres, or nanosystems.
Miccellar dosage forms
can be prepared as described in U.S. Pat. No. 6,350,458.
[00322] In other embodiments, the pharmaceutical compositions provided herein
may be provided as
non- effervescent or effervescent, granules and powders, to be reconstituted
into a liquid dosage form.
Pharmaceutically acceptable carriers and excipients used in the non-
effervescent granules or powders
may include diluents, sweeteners, and wetting agents. Pharmaceutically
acceptable carriers and
excipients used in the effervescent granules or powders may include organic
acids and a source of
carbon dioxide.
[00323] Coloring and flavoring agents can be used in all of the above dosage
forms.
[00324] The pharmaceutical compositions provided herein may be formulated as
immediate or
modified release dosage forms, including delayed-, sustained, pulsed-,
controlled, targeted-, and
programmed-release forms.
[00325] In further embodiments, the pharmaceutical compositions provided
herein may be co-
formulated with other active ingredients which do not impair the desired
therapeutic action, or with
substances that supplement the desired action, such as other cholinergic
agents, other serotoninergic
agents, alpha adrenergic agents, CCKA antagonists, 5-HT3 antagonists, NMDA
receptor antagonists,
opioids, prokinetics, tachykinins, antalarmin, and Z-338.
Parenteral Administration
[00326] In some embodiments, the pharmaceutical compositions provided herein
may be administered
parenterally by injection, infusion, or implantation, for local or systemic
administration. Parenteral
administration, as used herein, include intravenous, intraarterial,
intraperitoneal, intrathecal,
intraventricular, intraurethral, intrasternal, intracranial, intramuscular,
intrasynovial, and subcutaneous
administration.
[00327] In other embodiments, the pharmaceutical compositions provided herein
may be formulated in
any dosage forms that are suitable for parenteral administration, including
solutions, suspensions,
emulsions, micelles, liposomes, microspheres, nanosystems, and solid forms
suitable for solutions or
suspensions in liquid prior to injection. Such dosage forms can be prepared
according to conventional
methods known to those skilled in the art of pharmaceutical science (see,
Remington: The Science and
Practice of Pharmacy, supra).
[00328] The pharmaceutical compositions intended for parenteral administration
may include one or
more pharmaceutically acceptable carriers and excipients, including, but not
limited to, aqueous
vehicles, water-miscible vehicles, non-aqueous vehicles, antimicrobial agents
or preservatives against
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
69
the growth of microorganisms, stabilizers, solubility enhancers, isotonic
agents, buffering agents,
antioxidants, local anesthetics, suspending and dispersing agents, wetting or
emulsifying agents,
complexing agents, sequestering or chelating agents, cryoprotectants,
lyoprotectants, thickening
agents, pH adjusting agents, and inert gases.
[00329] Suitable aqueous vehicles include, but are not limited to, water,
saline, physiological saline or
phosphate buffered saline (PBS), sodium chloride injection, Ringers injection,
isotonic dextrose
injection, sterile water injection, dextrose and lactated Ringers injection.
Non-aqueous vehicles
include, but are not limited to, fixed oils of vegetable origin, castor oil,
corn oil, cottonseed oil, olive
oil, peanut oil, peppermint oil, safflower oil, sesame oil, soybean oil,
hydrogenated vegetable oils,
hydrogenated soybean oil, and medium-chain triglycerides of coconut oil, and
palm seed oil. Water-
miscible vehicles include, but are not limited to, ethanol, 1,3-butanediol,
liquid polyethylene glycol
(e.g., polyethylene glycol 300 and polyethylene glycol 400), propylene glycol,
glycerin,lV-methyl-2-
pyrrolidone, dimethylacetamide, and dimethylsulfoxide.
[00330] Suitable antimicrobial agents or preservatives include, but are not
limited to, phenols, cresols,
mercurials, benzyl alcohol, chiorobutanol, methyl and propyl p-
hydroxybenzates, thimerosal,
benzalkonium chloride, benzethonium chloride, methyl- and propyl-parabens, and
sorbic acid.
Suitable isotonic agents include, but are not limited to, sodium chloride,
glycerin, and dextrose.
Suitable buffering agents include, but are not limited to, phosphate and
citrate. Suitable antioxidants
are those as described herein, including bisulfite and sodium metabisulfite.
Suitable local anesthetics
include, but are not limited to, procaine hydrochloride. Suitable suspending
and dispersing agents are
those as described herein, including sodium carboxymethylcelluose,
hydroxypropyl methylcellulose,
and polyvinylpyrrolidone. Suitable emulsifying agents include those described
herein, including
polyoxyethylene sorbitan monolaurate, polyoxyethylene sorbitan monooleate 80,
and triethanolamine
oleate. Suitable sequestering or chelating agents include, but are not limited
to EDTA. Suitable pH
adjusting agents include, but are not limited to, sodium hydroxide,
hydrochloric acid, citric acid, and
lactic acid. Suitable complexing agents include, but are not limited to,
cyclodextrins, including a-
cyclodextrin, [3-cyclodextrin, hydroxypropyl-(3-cyclodextrin, sulfobutylether-
[i-cyclodextrin, and
sulfobutylether 7-[i-cyclodextrin (CAPTISOL , CyDex, Lenexa, KS).
[00331] In some embodiments, the pharmaceutical compositions provided herein
may be formulated
for single or multiple dosage administration. The single dosage formulations
are packaged in an
ampule, a vial, or a syringe. The multiple dosage parenteral formulations must
contain an
antimicrobial agent at bacteriostatic or fungistatic concentrations. All
parenteral formulations must be
sterile, as known and practiced in the art.
[00332] In one embodiment, the pharmaceutical compositions are provided as
ready-to-use sterile
solutions. In another embodiment, the pharmaceutical compositions are provided
as sterile dry soluble
products, including lyophilized powders and hypodermic tablets, to be
reconstituted with a vehicle
prior to use. In yet another embodiment, the pharmaceutical compositions are
provided as ready-to-
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
use sterile suspensions. In yet another embodiment, the pharmaceutical
compositions are provided as
sterile dry insoluble products to be reconstituted with a vehicle prior to
use. In still another
embodiment, the pharmaceutical compositions are provided as ready-to-use
sterile emulsions.
[00333] The pharmaceutical compositions provided herein may be formulated as
immediate or
5 modified release dosage forms, including delayed-, sustained, pulsed-,
controlled, targeted-, and
programmed-release forms.
[00334] The pharmaceutical compositions may be formulated as a suspension,
solid, semi-solid, or
thixotropic liquid, for administration as an implanted depot. In one
embodiment, the pharmaceutical
compositions provided herein are dispersed in a solid inner matrix, which is
surrounded by an outer
10 polymeric membrane that is insoluble in body fluids but allows the active
ingredient in the
pharmaceutical compositions diffuse through.
[00335] Suitable inner matrixes include polymethylmethacrylate,
polybutylmethacrylate, plasticized or
unplasticized polyvinylchloride, plasticized nylon, plasticized
polyethyleneterephthalate, natural
rubber, polyisoprene, polyisobutylene, polybutadiene, polyethylene, ethylene-
vinylacetate copolymers,
15 silicone rubbers, polydimethylsiloxanes, silicone carbonate copolymers,
hydrophilic polymers, such as
hydrogels of esters of acrylic and methacrylic acid, collagen, cross-linked
polyvinylalcohol, and cross-
linked partially hydrolyzed polyvinyl acetate.
Suitable outer polymeric membranes include polyethylene, polypropylene,
ethylene/propylene
copolymers, ethylene/ethyl acrylate copolymers, ethylene/vinylacetate
copolymers, silicone rubbers,
20 polydimethyl siloxanes, neoprene rubber, chlorinated polyethylene,
polyvinylchloride, vinylchloride
copolymers with vinyl acetate, vinylidene chloride, ethylene and propylene,
ionomer polyethylene
terephthalate, butyl rubber epichlorohydrin rubbers, ethylene/vinyl alcohol
copolymer, ethylene/vinyl
acetate/vinyl alcohol terpolymer, and ethylene/vinyloxyethanol copolymer.
Modified Release
25 [00336] In other embodiments, the pharmaceutical compositions provided
herein may be formulated as
a modified release dosage form. As used herein, the term "modified release"
refers to a dosage form
in which the rate or place of release of the active ingredient(s) is different
from that of an immediate
dosage form when administered by the same route. Modified release dosage forms
include delayed-,
extended-, prolonged-, sustained-, pulsatile-, controlled-, accelerated- and
fast-, targeted-,
30 programmed-release, and gastric retention dosage forms. The pharmaceutical
compositions in
modified release dosage forms can be prepared using a variety of modified
release devices and
methods known to those skilled in the art, including, but not limited to,
matrix controlled release
devices, osmotic controlled release devices, multiparticulate controlled
release devices, ion-exchange
resins, enteric coatings, multilayered coatings, microspheres, liposomes, and
combinations thereof.
35 The release rate of the active ingredient(s) can also be modified by
varying the particle sizes and
polymorphorism of the active ingredient(s).
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
71
[00337] Examples of modified release include, but are not limited to, those
described in U.S. Pat. Nos.:
3,845,770; 3,916,899; 3,536,809; 3,598,123; 4,008,719; 5,674,533; 5,059,595;
5,591,767; 5,120,548;
5,073,543; 5,639,476; 5,354,556; 5,639,480; 5,733,566; 5,739,108; 5,891,474;
5,922,356; 5,972,891;
5,980,945; 5,993,855; 6,045,830; 6,087,324; 6,113,943; 6,197,350; 6,248,363;
6,264,970; 6,267,981;
6,376,461; 6,419,961; 6,589,548; 6,613,358; and 6,699,500.
Matrix Controlled Release Devices
[00338] In some embodiments, the pharmaceutical compositions provided herein
in a modified release
dosage form may be fabricated using a matrix controlled release device known
to those skilled in the
art (see, Takada et al in "Encyclopedia of Controlled Drug Delivery," Vol. 2,
Mathiowitz ed., Wiley,
1999).
[00339] In one embodiment, the pharmaceutical compositions provided herein in
a modified release
dosage form is formulated using an erodible matrix device, which is water-
swellable, erodible, or
soluble polymers, including synthetic polymers, and naturally occurring
polymers and derivatives,
such as polysaccharides and proteins.
[00340] Materials useful in forming an erodible matrix include, but are not
limited to, chitin, chitosan,
dextran, and pullulan; gum agar, gum arabic, gum karaya, locust bean gum, gum
tragacanth,
carrageenans, gum ghatti, guar gum, xanthan gum, and scleroglucan; starches,
such as dextrin and
maltodextrin; hydrophilic colloids, such as pectin; phosphatides, such as
lecithin; alginates; propylene
glycol alginate; gelatin; collagen; and cellulosics, such as ethyl cellulose
(EC), methylethyl cellulose
(MEC), carboxymethyl cellulose (CMC), CMEC, hydroxyethyl cellulose (HEC),
hydroxypropyl
cellulose (HPC), cellulose acetate (CA), cellulose propionate (CP), cellulose
butyrate (CB), cellulose
acetate butyrate (CAB), CAP, CAT, hydroxypropyl methyl cellulose (HPMC),
HPMCP, HPMCAS,
hydroxypropyl methyl cellulose acetate trimellitate (HPMCAT), and ethylhydroxy
ethylcellulose
(EHEC); polyvinyl pyrrolidone; polyvinyl alcohol; polyvinyl acetate; glycerol
fatty acid esters;
polyacrylamide; polyacrylic acid; copolymers of ethacrylic acid or methacrylic
acid (EUDRAGIT ,
Rohm America, Inc., Piscataway, NJ); poly(2-hydroxyethyl-methacrylate);
polylactides; copolymers
of L-glutamic acid and ethyl-L-glutamate; degradable lactic acid-glycolic acid
copolymers; poly-D-(-
)-3-hydroxybutyric acid; and other acrylic acid derivatives, such as
homopolymers and copolymers of
butylmethacrylate, methylmethacrylate, ethylmethacrylate, ethylacrylate, (2-
dimethylaminoethyl)methacrylate, and (trimethylaminoethyl)methacrylate
chloride.
[00341] In further embodiments, the pharmaceutical compositions are formulated
with a non-erodible
matrix device. The active ingredient(s) is dissolved or dispersed in an inert
matrix and is released
primarily by diffusion through the inert matrix once administered. Materials
suitable for use as a non-
erodible matrix device included, but are not limited to, insoluble plastics,
such as polyethylene,
polypropylene, polyisoprene, polyisobutylene, polybutadiene,
polymethylmethacrylate,
polybutylmethacrylate, chlorinated polyethylene, polyvinylchloride, methyl
acrylate-methyl
methacrylate copolymers, ethylene-vinylacetate copolymers, ethylene/propylene
copolymers,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
72
ethylene/ethyl acrylate copolymers, vinylchloride copolymers with vinyl
acetate, vinylidene chloride,
ethylene and propylene, ionomer polyethylene terephthalate, butyl rubber
epichlorohydrin rubbers,
ethylene/vinyl alcohol copolymer, ethylene/vinyl acetate/vinyl alcohol
terpolymer, and
ethylene/vinyloxyethanol copolymer, polyvinyl chloride, plasticized nylon,
plasticized
polyethyleneterephthalate, natural rubber, silicone rubbers,
polydimethylsiloxanes, silicone carbonate
copolymers, and ; hydrophilic polymers, such as ethyl cellulose, cellulose
acetate, crospovidone, and
cross-linked partially hydrolyzed polyvinyl acetate,; and fatty compounds,
such as carnauba wax,
microcrystalline wax, and triglycerides.
[00342] In a matrix controlled release system, the desired release kinetics
can be controlled, for
example, via the polymer type employed, the polymer viscosity, the particle
sizes of the polymer
and/or the active ingredient(s), the ratio of the active ingredient(s) versus
the polymer, and other
excipients or carriers in the compositions.
[00343] In other embodiments, the pharmaceutical compositions provided herein
in a modified release
dosage form may be prepared by methods known to those skilled in the art,
including direct
compression, dry or wet granulation followed by compression, melt-granulation
followed by
compression.
2. Osmotic Controlled Release Devices
[00344] In some embodiments, the pharmaceutical compositions provided herein
in a modified release
dosage form may be fabricated using an osmotic controlled release device,
including one-chamber
system, two-chamber system, asymmetric membrane technology (AMT), and
extruding core system
(ECS). In general, such devices have at least two components: (a) the core
which contains the active
ingredient(s); and (b) a semipermeable membrane with at least one delivery
port, which encapsulates
the core. The semipermeable membrane controls the influx of water to the core
from an aqueous
environment of use so as to cause drug release by extrusion through the
delivery port(s).
[00345] In addition to the active ingredient(s), the core of the osmotic
device optionally includes an
osmotic agent, which creates a driving force for transport of water from the
environment of use into
the core of the device. One class of osmotic agents water-swellable
hydrophilic polymers, which are
also referred to as "osmopolymers" and "hydrogels," including, but not limited
to, hydrophilic vinyl
and acrylic polymers, polysaccharides such as calcium alginate, polyethylene
oxide (PEO),
polyethylene glycol (PEG), polypropylene glycol (PPG), poly(2-hydroxyethyl
methacrylate),
poly(acrylic) acid, poly(methacrylic) acid, polyvinylpyrrolidone (PVP),
crosslinked PVP, polyvinyl
alcohol (PVA), PVA/PVP copolymers, PVA/PVP copolymers with hydrophobic
monomers such as
methyl methacrylate and vinyl acetate, hydrophilic polyurethanes containing
large PEO blocks,
sodium croscarmellose, carrageenan, hydroxyethyl cellulose (HEC),
hydroxypropyl cellulose (HPC),
hydroxypropyl methyl cellulose (HPMC), carboxymethyl cellulose (CMC) and
carboxyethyl, cellulose
(CEC), sodium alginate, polycarbophil, gelatin, xanthan gum, and sodium starch
glycolate.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
73
[00346] The other class of osmotic agents are osmogens, which are capable of
imbibing water to affect
an osmotic pressure gradient across the barrier of the surrounding coating.
Suitable osmogens include,
but are not limited to, inorganic salts, such as magnesium sulfate, magnesium
chloride, calcium
chloride, sodium chloride, lithium chloride, potassium sulfate, potassium
phosphates, sodium
carbonate, sodium sulfite, lithium sulfate, potassium chloride, and sodium
sulfate; sugars, such as
dextrose, fructose, glucose, inositol, lactose, maltose, mannitol, raffinose,
sorbitol, sucrose, trehalose,
and xylitol; organic acids, such as ascorbic acid, benzoic acid, fumaric acid,
citric acid, maleic acid,
sebacic acid, sorbic acid, adipic acid, edetic acid, glutamic acid, p-
tolunesulfonic acid, succinic acid,
and tartaric acid; urea; and mixtures thereof.
[00347] Osmotic agents of different dissolution rates may be employed to
influence how rapidly the
active ingredient(s) is initially delivered from the dosage form. For example,
amorphous sugars, such
as Mannogeme EZ (SPI Pharma, Lewes, DE) can be used to provide faster delivery
during the first
couple of hours to promptly produce the desired therapeutic effect, and
gradually and continually
release of the remaining amount to maintain the desired level of therapeutic
or prophylactic effect over
an extended period of time. In this case, the active ingredient(s) is released
at such a rate to replace
the amount of the active ingredient metabolized and excreted.
[00348] The core may also include a wide variety of other excipients and
carriers as described herein
to enhance the performance of the dosage form or to promote stability or
processing.
[00349] Materials useful in forming the semi-permeable membrane include
various grades of acrylics,
vinyls, ethers, polyamides, polyesters, and cellulosic derivatives that are
water-permeable and water-
insoluble at physiologically relevant pHs, or are susceptible to being
rendered water-insoluble by
chemical alteration, such as crosslinking. Examples of suitable polymers
useful in forming the
coating, include plasticized, unplasticized, and reinforced cellulose acetate
(CA), cellulose diacetate,
cellulose triacetate, CA propionate, cellulose nitrate, cellulose acetate
butyrate (CAB), CA ethyl
carbamate, CAP, CA methyl carbamate, CA succinate, cellulose acetate
trimellitate (CAT), CA
dimethylaminoacetate, CA ethyl carbonate, CA chloroacetate, CA ethyl oxalate,
CA methyl sulfonate,
CA butyl sulfonate, CA p-toluene sulfonate, agar acetate, amylose triacetate,
beta glucan acetate, beta
glucan triacetate, acetaldehyde dimethyl acetate, triacetate of locust bean
gum, hydroxylated ethylene-
vinylacetate, EC, PEG, PPG, PEG/PPG copolymers, PVP, HEC, HPC, CMC, CMEC,
HPMC,
HPMCP, HPMCAS, HPMCAT, poly(acrylic) acids and esters and poly-(methacrylic)
acids and esters
and copolymers thereof, starch, dextran, dextrin, chitosan, collagen, gelatin,
polyalkenes, polyethers,
polysulfones, polyethersulfones, polystyrenes, polyvinyl halides, polyvinyl
esters and ethers, natural
waxes, and synthetic waxes.
[00350] Semi-permeable membrane may also be a hydrophobic microporous
membrane, wherein the
pores are substantially filled with a gas and are not wetted by the aqueous
medium but are permeable
to water vapor, as disclosed in U.S. Pat. No. 5,798,119. Such hydrophobic but
water-vapor permeable
membrane are typically composed of hydrophobic polymers such as polyalkenes,
polyethylene,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
74
polypropylene, polytetrafluoroethylene, polyacrylic acid derivatives,
polyethers, polysulfones,
polyethersulfones, polystyrenes, polyvinyl halides, polyvinylidene fluoride,
polyvinyl esters and
ethers, natural waxes, and synthetic waxes.
[00351] The delivery port(s) on the semi-permeable membrane may be formed post-
coating by
mechanical or laser drilling. Delivery port(s) may also be formed in situ by
erosion of a plug of water-
soluble material or by rupture of a thinner portion of the membrane over an
indentation in the core. In
addition, delivery ports may be formed during coating process, as in the case
of asymmetric membrane
coatings of the type disclosed in U.S. Pat. Nos. 5,612,059 and 5,698,220.
[00352] The total amount of the active ingredient(s) released and the release
rate can substantially by
modulated via the thickness and porosity of the semi-permeable membrane, the
composition of the
core, and the number, size, and position of the delivery ports.
[00353] The pharmaceutical compositions in an osmotic controlled-release
dosage form may further
comprise additional conventional excipients or carriers as described herein to
promote performance or
processing of the formulation.
[00354] The osmotic controlled-release dosage forms can be prepared according
to conventional
methods and techniques known to those skilled in the art (see, Remington: The
Science and Practice of
Pharmacy, supra; Santus and Baker, J. Controlled Release 1995, 35, 1-2 1;
Verma et al., Drug
Development and Industrial Pharmacy 2000, 26, 695-708; Verma et al., J.
Controlled Release 2002,
79, 7-27).
[00355] In other embodiments, the pharmaceutical compositions provided herein
are formulated as
AMT controlled-release dosage form, which comprises an asymmetric osmotic
membrane that coats a
core comprising the active ingredient(s) and other pharmaceutically acceptable
excipients or carriers.
See, U.S. Pat. No. 5,612,059 and WO 2002/17918. The AMT controlled-release
dosage forms can be
prepared according to conventional methods and techniques known to those
skilled in the art,
including direct compression, dry granulation, wet granulation, and a dip-
coating method.
[00356] In certain embodiments, the pharmaceutical compositions provided
herein are formulated as
ESC controlled-release dosage form, which comprises an osmotic membrane that
coats a core
comprising the active ingredient(s), a hydroxylethyl cellulose, and other
pharmaceutically acceptable
excipients or carriers.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
3. Multiparticulate Controlled Release Devices
[00357] In some embodiments, the pharmaceutical compositions provided herein
in a modified release
dosage form may be fabricated a multiparticulate controlled release device,
which comprises a
multiplicity of particles, granules, or pellets, ranging from about 10 m to
about 3 mm, about 50 .m to
5 about 2.5 mm, or from about 100 m to about 1 mm in diameter. Such
multiparticulates may be made
by the processes know to those skilled in the art, including wet-and dry-
granulation,
extrusion/spheronization, roller-compaction, melt-congealing, and by spray-
coating seed cores. See,
for example, Multiparticulate Oral Drug Delivery; Marcel Dekker: 1994; and
Pharmaceutical
Pelletization Technology; Marcel Dekker: 1989.
10 [00358] Other excipients or carriers as described herein may be blended
with the pharmaceutical
compositions to aid in processing and forming the multiparticulates. The
resulting particles may
themselves constitute the multiparticulate device or may be coated by various
film-forming materials,
such as enteric polymers, water-swellable, and water-soluble polymers. The
multiparticulates can be
further processed as a capsule or a tablet.
15 4. Targeted Delivery
[00359] In some embodiments, the pharmaceutical compositions provided herein
may also be
formulated to be targeted to a particular tissue, receptor, or other area of
the body of the subject to be
treated, including liposome-, resealed erythrocyte-, and antibody-based
delivery systems. Examples
include, but are not limited to, U.S. Pat. Nos. 6,316,652; 6,274,552;
6,271,359; 6,253,872; 6,139,865;
20 6,131,570; 6,120,751; 6,071,495; 6,060,082; 6,048,736; 6,039,975;
6,004,534; 5,985,307; 5,972,366;
5,900,252; 5,840,674; 5,759,542; and 5,709,874, all of which are incorporated
herein by their entirety.
Immediate Release
[00360] In some embodiments, the pharmaceutical compositions provided herein
in an immediate
release dosage form are capable of releasing not less than 75 % of the
therapeutically active ingredient
25 or combination and/or meet the disintegration or dissolution requirements
for immediate release
tablets of the particular therapeutic agents or combination included in the
tablet core, as set forth in
USP XXII, 1990 (The United States Pharmacopeia.)
Topical Administration
[00361] In other embodiments, the pharmaceutical compositions provided herein
may be administered
30 topically to the skin, orifices, or mucosa. The topical administration, as
used herein, include
(intra)dermal, conjuctival, intracomeal, intraocular, ophthalmic, auricular,
transdermal, nasal, vaginal,
uretheral, respiratory, and rectal administration.
[00362] In further embodiments, the pharmaceutical compositions provided
herein may be formulated
in any dosage forms that are suitable for topical administration for local or
systemic effect, including
35 emulsions, solutions, suspensions, creams, gels, hydrogels, ointments,
dusting powders, dressings,
elixirs, lotions, suspensions, tinctures, pastes, foams, films, aerosols,
irrigations, sprays, suppositories,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
76
bandages, dermal patches. The topical formulation of the pharmaceutical
compositions provided
herein may also comprise liposomes, micelles, microspheres, nanosystems, and
mixtures thereof.
[00363] Pharmaceutically acceptable carriers and excipients suitable for use
in the topical formulations
provided herein include, but are not limited to, aqueous vehicles, water-
miscible vehicles, non-
aqueous vehicles, antimicrobial agents or preservatives against the growth of
microorganisms,
stabilizers, solubility enhancers, isotonic agents, buffering agents,
antioxidants, local anesthetics,
suspending and dispersing agents, wetting or emulsifying agents, complexing
agents, sequestering or
chelating agents, penetration enhancers, cryopretectants, lyoprotectants,
thickening agents, and inert
gases.
[00364] In some embodiments, the pharmaceutical compositions may also be
administered topically by
electroporation, iontophoresis, phonophoresis, sonophoresis and microneedle or
needle-free injection,
such as POWDERJECTTM (Chiron Corp., Emeryville, CA), and BIOJECTTM (Bioject
Medical
Technologies Inc., Tualatin, OR).
[00365] The pharmaceutical compositions provided herein may be provided in the
forms of ointments,
creams, and gels. Suitable ointment vehicles include oleaginous or hydrocarbon
vehicles, including
such as lard, benzoinated lard, olive oil, cottonseed oil, and other oils,
white petrolatum; emulsifiable
or absorption vehicles, such as hydrophilic petrolatum, hydroxystearin
sulfate, and anhydrous lanolin;
water-removable vehicles, such as hydrophilic ointment; water-soluble ointment
vehicles, including
polyethylene glycols of varying molecular weight; emulsion vehicles, either
water-in-oil (W/O)
emulsions or oil-in-water (O/W) emulsions, including cetyl alcohol, glyceryl
monostearate, lanolin,
and stearic acid (see, Remington: The Science and Practice of Pharmacy,
supra). These vehicles are
emollient but generally require addition of antioxidants and preservatives.
[00366] Suitable cream base can be oil-in-water or water-in-oil. Cream
vehicles may be water-
washable, and contain an oil phase, an emulsifier, and an aqueous phase. The
oil phase is also called
the "internal" phase, which is generally comprised of petrolatum and a fatty
alcohol such as cetyl or
stearyl alcohol. The aqueous phase usually, although not necessarily, exceeds
the oil phase in volume,
and generally contains a humectant. The emulsifier in a cream formulation may
be a nonionic,
anionic, cationic, or amphoteric surfactant.
[00367] Gels are semisolid, suspension-type systems. Single-phase gels contain
organic
macromolecules distributed substantially uniformly throughout the liquid
carrier. Suitable gelling
agents include crosslinked acrylic acid polymers, such as carbomers,
carboxypolyalkylenes,
Carbopol ; hydrophilic polymers, such as polyethylene oxides, polyoxyethylene-
polyoxypropylene
copolymers, and polyvinylalcohol; cellulosic polymers, such as hydroxypropyl
cellulose, hydroxyethyl
cellulose, hydroxypropyl methylcellulose, hydroxypropyl methylcellulose
phthalate, and
methylcellulose; gums, such as tragacanth and xanthan gum; sodium alginate;
and gelatin. In order to
prepare a uniform gel, dispersing agents such as alcohol or glycerin can be
added, or the gelling agent
can be dispersed by trituration, mechanical mixing, and/or stirring.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
77
[00368] The pharmaceutical compositions provided herein may be administered
rectally, urethrally,
vaginally, or perivaginally in the forms of suppositories, pessaries, bougies,
poultices or cataplasm,
pastes, powders, dressings, creams, plasters, contraceptives, ointments,
solutions, emulsions,
suspensions, tampons, gels, foams, sprays, or enemas. These dosage forms can
be manufactured
using conventional processes as described in Remington: The Science and
Practice of Pharmacy,
supra.
[00369] Rectal, urethral, and vaginal suppositories are solid bodies for
insertion into body orifices,
which are solid at ordinary temperatures but melt or soften at body
temperature to release the active
ingredient(s) inside the orifices. Pharmaceutically acceptable carriers
utilized in rectal and vaginal
suppositories include bases or vehicles, such as stiffening agents, which
produce a melting point in the
proximity of body temperature, when formulated with the pharmaceutical
compositions provided
herein; and antioxidants as described herein, including bisulfite and sodium
metabisulfite. Suitable
vehicles include, but are not limited to, cocoa butter (theobroma oil),
glycerin-gelatin, carbowax
(polyoxyethylene glycol), spennaceti, paraffin, white and yellow wax, and
appropriate mixtures of
mono-, di- and triglycerides of fatty acids, hydrogels, such as polyvinyl
alcohol, hydroxyethyl
methacrylate, polyacrylic acid; glycerinated gelatin. Combinations of the
various vehicles may be
used. Rectal and vaginal suppositories may be prepared by the compressed
method or molding. The
typical weight of a rectal and vaginal suppository is about 2 to about 3 g.
[00370] The pharmaceutical compositions provided herein may be administered
ophthalmically in the
forms of solutions, suspensions, ointments, emulsions, gel-forming solutions,
powders for solutions,
gels, ocular inserts, and implants.
[00371] The pharmaceutical compositions provided herein may be administered
intranasally or by
inhalation to the respiratory tract. The pharmaceutical compositions may be
provided in the form of
an aerosol or solution for delivery using a pressurized container, pump,
spray, atomizer, such as an
atomizer using electrohydrodynamics to produce a fine mist, or nebulizer,
alone or in combination
with a suitable propellant, such as 1, 1, 1,2-tetrafluoroethane or 1, 1,
1,2,3,3,3 -heptafluoropropane. The
pharmaceutical compositions may also be provided as a dry powder for
insufflation, alone or in
combination with an inert carrier such as lactose or phospholipids; and nasal
drops. For intranasal use,
the powder may comprise a bioadhesive agent, including chitosan or
cyclodextrin.
[00372] Solutions or suspensions for use in a pressurized container, pump,
spray, atomizer, or
nebulizer may be formulated to contain ethanol, aqueous ethanol, or a suitable
alternative agent for
dispersing, solubilizing, or extending release of the active ingredient
provided herein, a propellant as
solvent; and/or an surfactant, such as sorbitan trioleate, oleic acid, or an
oligolactic acid.
[00373] In another embodiment, the pharmaceutical compositions provided herein
may be micronized
to a size suitable for delivery by inhalation, such as about 50 micrometers or
less, or about 10
micrometers or less. Particles of such sizes may be prepared using a
comminuting method known to
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
78
those skilled in the art, such as spiral jet milling, fluid bed jet milling,
supercritical fluid processing to
form nanoparticles, high pressure homogenization, or spray drying.
[00374] Capsules, blisters and cartridges for use in an inhaler or insufflator
may be formulated to
contain a powder mix of the pharmaceutical compositions provided herein; a
suitable powder base,
such as lactose or starch; and a performance modifier, such as l-leucine,
mannitol, or magnesium
stearate. The lactose may be anhydrous or in the form of the monohydrate.
Other suitable excipients
include dextran, glucose, maltose, sorbitol, xylitol, fructose, sucrose, and
trehalose. The
pharmaceutical compositions provided herein for inhaled/intranasal
administration may further
comprise a suitable flavor, such as menthol and levomenthol, or sweeteners,
such as saccharin or
saccharin sodium.
[00375] In one embodiment, the pharmaceutical compositions provided herein for
topical
administration may be formulated to be immediate release or modified release,
including delayed-,
sustained-, pulsed-, controlled-, targeted, and programmed release.
EXAMPLES
Example 1
Synthesis of 2-(4-ethoxyphenXl)-4-methy1-l-(4-sulfamoylphenyl)-pyrrole
R2 NC R2
~\ ~R2CH0 ~\ \ N TMS-CN I\ \~ R3 Ra
- / CHO
i ~
R 02S R102S'~ R102S
A B C
R2 ~ R3 2 R3
R
R N R4 R R4
I / OH
R102S Rl02S
D E
Scheme 1.
[00376] Substituted benzaldehyde undergoes dehydration condensation by
reaction with aniline
compound A in an inert solvent at a temperature of between 5 C to 200 C to
give aldimine
compound B. Trimethylsilyl cyanide is then reacted with aldimine compound B in
the presence of a
Lewis acid to afford anilinonitrile C. An a-(3-unsaturated aldehyde is then
reacted with anilinonitrile
C to afford compound D which then undergoes dehydration and
dehydrogencyanation under basic
conditions in a modification of the method described in Ann. Chem. 589, 176
(1954).
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
79
Example 2
Synthesis of Erlotinib
OH ci
O0 / N SOC12 N
p \ N J DMF, CH3C12 N
F G
OH NH
O/ I ~
\ NJ
NH2 NH
H I
Scheme 2.
[00377] Starting material compound H is heated in a suspension of metal alkali
and solvent, then
heated to give compound I. Starting material alcohol F, is placed in a solvent
mixture of thionyl
chloride, methylene chloride and dimethylformamide to give the chloride G.
Chloride G is then
coupled with compound I to give erlotinib J.
Example 3
Pharmacokinetics and Metabolism of 2-(4-ethoxyphenyI)-4-methyl-l-(4-
sulfamoyIphenyl)-pyrrole
[00378] Orally administered 2-(4-ethoxyphenyl)-4-methyl- 1-(4-sulfamoylphenyl)-
pyrrole was rapidly
absorbed in all species examined (mice, rats, dogs, and monkeys). Peak plasma
concentrations were
achieved between 1 and 3 hours after a dose of 5 mg/kg. The elimination half
life (t %) was 4-5 hours
in rodents and dogs, and approximately 2 hours in monkeys. Oral availability
was greatest in rodent,
and was reduced in dogs and monkeys (59 and 34% respectively).
Pharmacokinetics in human
subjects demonstrated a linear dose exposure relationship from doses of 2 mg
to 800 mg given orally.
The half-life in human subjects is 15-18 hours.
Example 4
Toxicology of 2-(4-ethoxyphenyl)-4-methyl-1_(4-sulfamovlphenyl - rrole
[00379] Toxicological evaluation of 2-(4-ethoxyphenyl)-4-methyl- 1 -(4-
sulfamoylphenyl)-pyrrole in
mice, rats, dogs and monkeys revealed expected findings related to inhibition
of cyclooxygenase and
consistent with animal safety observations with other COX-2 selective
inhibitors. In single dose
studies, the minimum lethal dose of 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole was
600 mg/kg in rats and >2000 mg/kg in dogs. An endoscopy study conducted in
human subjects
demonstrated no increase in gastric or duodenal toxicity compared to placebo.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
Example 5
Biological Evaluation
COX-2 Selective Inhibitors
HT-29 Model:
5 1003801 Mice are injected subcutaneously in the left paw (1x106 tumor cells
suspended in 30%
Matrigel) and tumor volume is evaluated using a phlethysmometer twice a week
for 30-60 days.
Implantation of human colon cancer cells (HT-29) into nude mice produces
tumors that will reach 0.6-
2 ml between 30-50 days. Blood is drawn twice during the experiment in a 24 h
protocol to assess
plasma concentration and total exposure by AUC analysis. The data is expressed
as the mean+/-SEM.
10 Student's and Mann-Whitney tests are used to assess differences between
means using the InStat
software package.
[00381] A. Mice injected with HT-29 cancer cells are treated with cytoxin i.p
at doses of 50 mg/kg on
days 5, 7 and 9 in the presence or absence of a composition comprising a
combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole with erlotinib in the
diet. The efficacy of both
15 agents are determined by measuring tumor volume. The results from these
studies may demonstrate
that a composition comprising a combination of 2-(4-ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-
pyrrole with erlotinib administered in the diet to tumor bearing mice can
delay the growth of tumors
and metastasis when administered as sole therapy.
[00382] B. In a second assay, mice are injected with HT-29 cancer cells are
then treated with 5-FU on
20 days 12 through 15. Mice injected with HT-29 cancer cells are treated with
5-FU i.p at doses of 50
mg/kg on days 12, 13, 14, and 15 in the presence or absence of a composition
comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole with
erlotinib in the diet.
The efficacy of both agents are determined by measuring tumor volume.
Treatment using the
composition may reduce tumor volume by up to 70%. In the same assay, 5-FU
decreases tumor
25 volume by 61%. Further, the composition and 5-FU may decrease tumor volume
by 83%.
[00383] C. In a third assay, mice injected with HT-29 colon cancer cells are
treated with 5-FU i.p 50
mg/kg on days 14 through 17 in the presence or absence of a composition
comprising a combination
of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole with erlotinib
(1600 ppm) and
valdecoxib (160 ppm) in the diet. The efficacy of both agents are determined
by measuring tumor
30 volume. Treatment with 5-FU may result in a 35% reduction in tumor volume.
Treatment with the
composition and valdecoxib may reduce tumor volume by 52% and 69%,
respectively. In the same
assay, the combination of 5-FU and the composition may decrease tumor volume
by 72% while the
combination of 5-FU and valdecoxib may decrease tumor volume by 74%.
Example 6
35 In Vitro Inhibition of EGFR Kinase Activity
[00384] The in vitro activity of the combinations described herein in
inhibiting the receptor tyrosine
kinase may be determined by the following procedure. The activity of the
combinations of the present
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
81
disclosure, in vitro, can be determined by the amount of inhibition of the
phosphorylation of an
exogenous substrate (e.g., Lys3 -- Gastrin or polyGluTyr (4:1) random
copolymer (Posner et al., J.
Biol. Chem., 1992, 267 (29), 2063 8-472)) on tyrosine by epidermal growth
factor receptor kinase by a
test compound relative to a control. Affinity purified, soluble human EGF
receptor (96 ng) is obtained
according to the procedure in G. N. Gill, W. Weber, Methods in Enzymology,
1987, 146, 82-8 from
A431 cells (American Type Culture Collection, Rockville, Md.) and preincubated
in a microfuge tube
with EGF (2 g/ml) in phosphorylation buffer+vanadate (PBV: 50 mM HEPES, pH
7.4; 125 mM
NaCI; 24 mM MgCIZ; 100 M sodium orthovanadate), in a total volume of 10 l,
for 20-30 minutes at
room temperature. The composition comprising a combination of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole with erlotinib, dissolved in dimethylsulfoxide
(DMSO), is diluted in PBV,
and 10 l is mixed with the EGF receptor/EGF mix, and incubated for 10-30
minutes at 30 C. The
phosphorylation reaction is initiated by addition of 20 131 P-ATP/substrate
mix (120 M Lys3 -
Gastrin (sequence in single letter code for amino acids, KKKGPWLEEEEEAYGWLDF),
50 mM
Hepes pH 7.4, 40 p.M ATP, 2 pCi .gamma.-[33 P]-ATP) to the EGFr/EGF mix and
incubated for 20
minutes at room temperature. The reaction is stopped by addition of 10 l stop
solution (0.5 M
EDTA, pH 8; 2 mM ATP) and 6 gl 2N HCI. The tubes are centrifuged at 14,000
RPM, 4 C., for 10
minutes. 35 l of supernatant from each tube is pipetted onto a 2.5 cm circle
of Whatman P81 paper,
bulk washed four times in 5% acetic acid, 1 liter per wash, and then air
dried. This results in the
binding of substrate to the paper with loss of free ATP on washing. The [33 P]
incorporated is
measured by liquid scintillation counting. Incorporation in the absence of
substrate (e.g., lys3-gastrin)
is subtracted from all values as a background and percent inhibition is
calculated relative to controls
without the composition present. Such assays, carried out with a range of
doses of test combinations,
allow the determination of an approximate IC50 value for the in vitro
inhibition of EGFR kinase
activity. Other methods for determining the activity of the combinations
presented herein are
described in U.S. Pat. No. 5,747,498, the disclosure of which is incorporated
herein.
Example 7
Pharmaceutical Compositions and Dosage Forms
[00385] Dosage formulations comprising pharmaceutical excipients and carriers
and a pharmaceutical
composition comprising a combination of erlotinib (A) and 2-(4-ethoxyphenyl)-4-
methyl- 1 -(4-
sulfamoylphenyl)-pyrrole (B) include:
Combination Amount of A per tablet (mg) Amount of B per tablet (mg)
A/B 25 1, 5, 10, 25, 50, 100, 200, 300, 400,
600, 800, 1000, 1200
A/B 100 1, 5, 10, 25, 50, 100, 200, 300, 400,
600, 800, 1000, 1200
A/B 150 1, 5, 10, 25, 50, 100, 200, 300, 400,
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
82
600, 800, 1000, 1200
A/B 200 1, 5, 10, 25, 50, 100, 200, 300, 400,
600, 800, 1000, 1200
A/B 300 1, 5, 10, 25, 50, 100, 200, 300, 400,
600, 800, 1000, 1200
A/B 450 1, 5, 10, 25, 50, 100, 200, 300, 400,
600,800,1000,1200
[00386] Dosage formulations described herein, including the formulations set
forth in the above table,
may be administered in a single fixed dose comprising a combination of 2-(4-
ethoxyphenyl)-4-methyl-
1-(4-sulfamoylphenyl)-pyrrole and erlotinib or as a separate administration of
a single dose of 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole and a single dose of
erlotinib.
Example 8
Biological Evaluation
COX-2 Selective Inhibitors
A431 Squamous Vulvar Carcinoma Model
Mice are injected subcutaneously in the left paw (1x106 tumor cells suspended
in 30% Matrigel) and
tumor volume is evaluated using a phlethysmometer twice a week for 30-60 days.
Implantation of
squamous vulvar carcinoma (A43 1) into nude mice produces tumors that reach
0.6-2 ml between 30-
50 days. Blood is drawn twice during the experiment in a 24 h protocol to
assess plasma
concentration and total exposure by AUC analysis. The data is expressed as the
mean+/-SEM.
Student's and Mann-Whitney tests are used to assess differences between means
using the InStat
software package.
Mice injected with A431 cancer cells are treated with a composition comprising
one of the following:
1. erlotinib (75 or 100 mg/kg ); 2. 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-pyrrole (3, 10,
30, or 100 mg/kg); 3. celecoxib (10, 30, 100, or 300 mg/kg); 4. erlotinib (75
mg/kg) and 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole (3, 10, or 30 mg/kg); 4.
erlotinib (75 mg/kg)
and celecoxib (10, 30, or 100 mg/kg). The efficacy is determined by measuring
tumor volume. The
results from these studies, shown in Figure 3, demonstrate that a composition
comprising a
combination of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole with
erlotinib
administered to tumor bearing mice delayed the growth of tumors by 55%
compared to erlotinib alone.
The combination of erlotinib and celecoxib demonstrated a tumor growth delay
of only 36%.
Example 9
Study of 2- 4-ethox hen 1-4-meth 1-1- 4-sulfamo 1 hen 1- rrole in combination
with erlotinib in
metastatic or recurrent non-small cell lung cancer (NSCLC) patients
Methods: Subjects with recurrent or metastatic NSCLC were treated with
erlotinib (150 mg/day PO)
and escalating doses of 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole (100 - 1200
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
83
mg/day PO). Pharmacokinetics for 2-(4-ethoxyphenyl)-4-methyl-1-(4-
sulfamoylphenyl)-pyrrole and
erlotinib were evaluated.
Results: Nineteen subjects were treated: 3 at 2-(4-ethoxyphenyl)-4-methyl- 1 -
(4-sulfamoylphenyl)-
pyrrole 100 mg; 4 at 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole
200 mg; 12 at 2-(4-
ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole 400 mg. The optimal
biologic dose selected
was 400 mg. 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-pyrrole
pharmacokinetics reveal a
median Tmax of 2 hours (range 1.5-4), mean (SD) T1/2 of 11.8 (5.3) hours, Cmax
of 313.0 (99.6)
ng/ml and AUC (0-t) of 2816 (1204) ng-h/ml.
The results show that 2-(4-ethoxyphenyl)-4-methyl-l-(4-sulfamoylphenyl)-
pyrrole and erlotinib have
been safely administered in this study.
Example 10
Study of 2-(4-ethoxyphenyl -4-meth yl- I 44-sulfamoylphenvl)-pyrrole in
combination with erlotinib
in metastatic or recurrent non-small cell lung cancer (NSCLC) patients
This study compares the anti-tumor efficacy of 2-(4-ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-
pyrrole and erlotinib with placebo and erlotinib as measured by time to
disease progression. The
experimental arm consists of 400 mg/day 2-(4-ethoxyphenyl)-4-methyl-l-(4-
sulfamoylphenyl)-
pyrrole, in combination with 150 mg/day erlotinib. The placebo arm consists of
400 mg/day placebo
tablets, in combination with 150 mg/day erlotinib.
Example 11
Study of 2-(4-ethoxyphenyl)-4-meth yl- 1 -(4-sulfamoIphenyI)-pyrrole in
combination with erlotinib
and gemcitabine in advanced pancreatic cancer patients
This study compares the anti-tumor efficacy of 2-(4-ethoxyphenyl)-4-methyl-l-
(4-sulfamoylphenyl)-
pyrrole and erlotinib/gemcitabine with placebo and erlotinib/gemcitabine as
measured by time to
disease progression. The experimental arm will consist of 400 mg/day 2-(4-
ethoxyphenyl)-4-methyl-
1-(4-sulfamoylphenyl)-pyrrole, in combination with 100 mg/day erlotinib, and
gemcitabine
administered as an intravenous infusion of a 1000 mg/m2 dose over 30 minutes
once a week for up to 7
weeks followed by one week of rest and then subsequent cycles of weekly
infusions for 3 weeks of a 4
week cycle. The placebo arm consists of 400 mg/day placebo tablets, in
combination with 100 mg/day
erlotinib, and gemcitabine administered as an intravenous infusion of a 1000
mg/m2 dose over 30
minutes once a week for up to 7 weeks followed by one week of rest and then
subsequent cycles of
weekly infusions for 3 weeks of a 4 week cycle.
Example 12
Treatment of Non-Small Cell Lung Cancer
[00387]A method for treating a subject having non-small cell lung cancer
comprising administering to
the subject a therapeutically effective amount of a combination comprising 2-
(4-ethoxyphenyl)-4-
methyl 1-(4-sulfamoylphenyl)-pyrrole and erlotinib or their respective
pharmaceutically acceptable
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
84
salt, solvate or prodrug is contemplated. The subject is treated using a
single dosage form of a
combination of 2-(4-ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole in
about 100, about 200,
about 300, about 400, about 600, about 800, and about 1000 and about 1200 mg
aliquots and erlotinib
in about a 150 mg aliquot. The treatment of the subject using a separate
administration of 2-(4-
ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole in about 200, about 400,
and about 800 mg
aliquots and erlotinib in about a 150 mg aliquot is also contemplated. The
maximum tolerated dose of
this combination will be an endpoint for this study.
Example 13
Treatment of Breast Cancer
[00388]A method for treating a subject having breast cancer comprising
administering to the subject a
therapeutically effective amount of a combination comprising 2-(4-
ethoxyphenyl)-4-methyl 1-(4-
sulfamoylphenyl)-pyrrole and erlotinib or their respective pharmaceutically
acceptable salt, solvate or
prodrug is contemplated. Combined treatment with erlotinib and 2-(4-
ethoxyphenyl)-4-methyl 1-(4-
sulfamoylphenyl)-pyrrole is expected to achieve increased tumor inhibition
compared with erlotinib
administered as a single agent.
Example 14
Treatment of Colorectal Cancer
[00389] A method for treating a subject having colorectal cancer comprising
administering to the
subject a therapeutically effective amount of a combination comprising 2-(4-
ethoxyphenyl)-4-methyl
1-(4-sulfamoylphenyl)-pyrrole and erlotinib or their respective
pharmaceutically acceptable salt,
solvate or prodrug is contemplated. Combined treatment with erlotinib and 2-(4-
ethoxyphenyl)-4-
methyl 1-(4-sulfamoylphenyl)-pyrrole are expected to achieve significantly
increased tumor inhibition
compared with either 2-(4-ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole
or erlotinib
administered as single agents.
Example 15
Treatment of Glioma
[00390]A method for treating a subject having glioma comprising administering
to the subject a
therapeutically effective amount of a combination comprising 2-(4-
ethoxyphenyl)-4-methyl 1-(4-
sulfamoylphenyl)-pyrrole and erlotinib or their respective pharmaceutically
acceptable salt, solvate or
prodrug is contemplated. The subject is treated using a single dosage form of
a combination of 2-(4-
ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole in about 1, about 5,
about 10, about 25, about
50, about 100, about 200, about 300, about 400, about 600, about 800, about
1000, and about 1200 mg
aliquots and erlotinib in about a 25 mg aliquot. The treatment of the subject
using a separate
administration of 2-(4-ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole in
about 1, about 5,
about 10, about 25, about 50, about 100, about 200, about 300, about 400,
about 600, about 800, about
1000, and about 1200 mg aliquots and erlotinib in about 100, 150, 200, 300, or
450 mg aliquot is also
contemplated. The treatment will also be administered in subjects with 2nd
temozolomide failures.
CA 02692977 2010-01-12
WO 2009/009778 PCT/US2008/069894
Example 16
Treatment of Head and Neck Cancer
[00391]A method for treating a subject having head and neck cancer comprising
administering to the
subject a therapeutically effective amount of a combination comprising 2-(4-
ethoxyphenyl)-4-methyl
5 1-(4-sulfamoylphenyl)-pyrrole and erlotinib or their respective
pharmaceutically acceptable salt,
solvate or prodrug is contemplated. The subject is treated using a single
dosage form of a combination
of 2-(4-ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole in about 1, about
5, about 10, about
25, about 50, about 100, about 200, about 300, about 400, about 600, about
800, about 1000, and about
1200 mg aliquots and erlotinib in about a 25 mg aliquot. The treatment of the
subject using a separate
10 administration of 2-(4-ethoxyphenyl)-4-methyl 1-(4-sulfamoylphenyl)-pyrrole
in about 1, about 5,
about 10, about 25, about 50, about 100, about 200, about 300, about 400,
about 600, about 800, about
1000, and about 1200 mg aliquots and erlotinib in about 100, 150, 200, 300, or
450 mg aliquot is also
contemplated. The treatment will also be administered in conjunction with one
or one or more
chemotherapy agents (e.g., paclitaxel [Taxol ], docetaxel [Taxotere ],
gemcitabine [Gemza.r ],
15 doxorubicin [Doxil ]) which may be further combined with established
chemotherapeutic agents
(e.g., methotrexate [Trexall , Methotrex ]). In another embodiment, therapy
for the treatment of
head and neck cancer according to the invention utilizes a combination of
therapeutically effective
amount of a composition comprising a combination of 2-(4-ethoxyphenyl)-4-
methyl 1-(4-
sulfamoylphenyl)-pyrrole and erlotinib and Erbitux .