Note: Descriptions are shown in the official language in which they were submitted.
CA 02693390 2015-12-04
30725-1660
DERIVATIVES OF N-(ARYLAMINO) SULFONAMIDES INCLUDING POLYMORPHS AS
=
INHIBITORS OF MEK AS WELL AS COMPOSITIONS, METHODS OF USE AND METHODS FOR
PREPARING THE SAME
FIELD OF THE INVENTION
This invention concerns N-(2-arylamino) aryl sulfonamide compounds which are
inhibitors of MEK including
crystalline polymorphic forms which exhibit a specific powder x-ray
diffraction profile and/or a specific differential
scanning calorimetry profile. This invention also concerns pharmaceutical
compositions comprising the compounds
described herein and methods of use of the compounds and compositions
described herein, including the use in the
treatment and/or prevention of cancer, hyperproliferative diseases and
inflanunatory conditions. The invention also
=
concerns methods of making the compunds and compositions described herein.
This application relates to a plurality of inventions. Therefore, reference to
"the present invention"
or the like as used throughout this specification may refer to the invention
as claimed, or to one of the
Is other inventions disclosed herein.
=
BACKGROUND OF THE INVENTION
Oncogenes -- genes that contribute to the production of cancers -- are
generally mutated forms of certain normal
cellular genes ("proto-oncogenes"). Oncogenes often encode abnormal versions
of signal pathway components, such as
receptor tyrosine kina.ses, scrine-threonine kinases, or downstream signaling
molecules. The central downstream
signaling molecules are the Ras proteins, which are anchored on the inner
surfaces of cytoplasmic membranes, and which
hydrolyze bound guanosine triphosphate (GTP) to guanosine diphosphate (GDP).
When activated by a growth factor,
growth factor receptors initiate a chain of reactions that leads to the
activation of guanine nucleotide exchange activity on
Ras. Ras alternates between an active "on" state with a bound GTP (hereafter
"Ras.GTP'') and an inactive "off state with
a bound GDP. The active "on" state, Ras.GTP, binds to and activates proteins
that control the growth and differentiation
of cells.
For example, in the "rnitogen-activatedprotein kinase (MAP kinase) cascade,"
Ras.GTP leads to the activation of a
cascade of serine/threonine kinases. One of several groups of kinases known to
require a Ras.GTP for their own
activation is the Raf family. The Raf proteins activate "MEKI" and "MEK2,"
abbreviations for mitogen-activated ERK,
activating kinases (where ERK is extracellular signal-regulated protein
kinase, another designation for IvIAPK). MEK1
and MEK2 are dual-function serine/threonine and tyrosine protein kinases and
are also known as MAP kinase kinases.
Thus, Ras.GTP activates Raf, which activates MEKI and MEK2, which activate MAP
kinase (MAPK). Activation of
MAP kinase by mitogens appears to be essential for proliferation, and
constitutive activation of this kinaseis sufficient to
induce cellular transformation. Blockade of downstream Ras signaling, as by
use of a dominant negative Raf-1 protein,
can completely inhibit mitogenesis, whether induced from cell surface
receptors or from oncogenic Ras mutants.
-Page 1-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The interaction of Raf and Ras is a key regulatory step in the control of cell
proliferation. To date, no substrates of
MEK other than MAPK have been identified; however, recent reports indicate
that MEK may also be activated by other
upstream signal proteins such as MEK kinase or MEKK1 and PKC. Activated MAPK
translocates and accumulates in
the nucleus, where it can phosphorylate and activate transcription factors
such as Elk-1 and Sapla, leading to the
enhanced expression of genes such as that for c-fos.
Once activated, Raf and other Icinases phosphorylate MEK on two neighboring
serine residues, S218 and s222 in the
case of MEK1. These phosphorylations are required for activation of MEK as a
kinase. In turn, MEK phosphorylates
MAP kinase on two residues separated by a single amino acid: a tyrosine, Y185
and a threonine, MEK appears to
associate strongly with MAP kinase prior to phosphorylating it, suggesting
that phosphorylation of MAP kinase by MEK
may require a prior strong interaction between the two proteins. Two factors ¨
MEK's unusual specificity and its
requirement for a strong interaction with MAP kinase prior to phosphorylation -
- suggest that MEK's mechanism of
action may differ sufficiently from the mechanisms of other protein kinases as
to allow for selective inhibitors of MEK.
Possibly, such inhibitors would operate through allosteric mechanisms rather
than through the more usual mechanism
involving blockage of an ATP binding site.
Thus, MEK] and MEK2 are validated and accepted targets for anti-proliferative
therapies, even when the
oncogenic mutation does not affect MEK structure or expression. See, e.g.,
U.S. Patent Publications 2003/0149015 by
Barrett et aL and 2004/0029898 by Boyle et al.
Several examples of 1-substituted-2(p-substituted-phenylamino)-aryl inhibitors
of MEK have been reported. U.S.
Patent Nos. 6,440,966 and 6,750,217 and corresponding publication WO 00/42003
described carboxylic and hydroxamic
acid esters and N-substituted amide derivatives of sulfonamide-substituted-2(4-
iodophenylarnino)-berizoic acid esters and
N-substituted benzamides as functioning as MEK inhibitors. The sulfonamide may
also be N-substituted.
U.S. Patent 6,545,030 and corresponding publication WO 00/42029 describe MEK
inhibitors that are 1-
heterocycly1-2(4-iodophenylamino)-benzene, where the heterocycle is a five-
membered nitrogen-containing ring such as
pyrazole, triazole, oxazole, isoxazole, and isoxazolinone. The more recent
U.S. Patent Publication 2005/004186
describes related compounds in which the 4-iodo substituent of the '030 patent
is replaced by a very broad genus of
moieties including alkyl, alkoxy, acyloxy, alkenyl, carbamoyl, carbamoylalkyl,
carboxyl, carboxylalkyl, N-
acylsulfonamido, and others.
U.S. Patent 6,469,004 and corresponding publication WO 00/42022 describe
carboxylic and hydroxamic acid
esters of a group of heterocyclo-condensed phenylene compounds, i.e.,
benzimiclazoles, benzooxazoles, benzothiazoles,
benzothiadiazoles, quinazolines, etc. The heterocycles are 7-F-6-(4-iodo-
phenylamino)-5-carboxylic acid esters,
carboxylic acid amides or hydroxamic acid esters. More recent publication U.S.
2005/0026970 described similar
compounds in which the 4-iodo substituent was replaced by a very broad genus
of structures. Related compounds are
described in patent publications WO 03/077855, WO 03/77914 and US
2005/0554701. Further examples of 2-(4-
iodophenylamino)-phenylhydroxamic acid esters which are reported to be useful
as MEK inhibitors can be found in WO
2005/028426.
Patent Publication WO 02/06213 and corresponding U.S. Application Ser. No.
10/333,399 (U.S. 2004/0054172)
describe hydroxy-substituted acid esters of 1-oxamic acid- 2(4-
halophenylamino)-3,4-difluoroberizene. U.S. Patent No.
6,891,066 and corresponding publication WO 03/62191 describe similar compounds
wherein the 4-halo substituent is
replaced by a very broad genus of structures. Among the substituents in the 4-
position were methyl, ethyl, ethynyl, and
2-hydroxyethyl. Specific related compounds are described in U.S. Patent No.
6,770,778.
- Page 2-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Patent Publication WO 04/083167, published September 30, 2004, (in Japanese)
discloses more than two thousand
¨ but provides NMR data for only 400 ¨1-(N-substituted sulfonyl urea)-2(2,4-
dihalophenylamino)-3,4-difluorobenzenes
and asserts that they useful as MEK inhibitors. Data indicating inhibition of
MEK were presented for a subgroup of just
twelve. In addition to a secondary or tertiary amine, these twelve compounds
all contained one of the following groups:
an N, N-disubstituted sulfonyl urea, N-piperazinesuIfonamide, N-
piperidinesulfonamide or N-pyrrolidinesulfonamide.
The MEK cascade has also been implicated in inflammatory diseases and
disorders. U.S. Application Publication
No. 2006/0030610 to Koch et al., U.S. Application Publication No. 2006/0140872
to Furue at al. This includes both
acute and chronic inflammation disorders. Examples of such disorders are
allergic contact dermatitis, rheumatoid
arthritis, osteoarthiitis, inflammatory bowel diseases, chronic obstructive
pulmonary disorder, psoriasis, multiple
sclerosis, asthma, diseases and disorders related to diabetic complications,
and inflammatory complications of the
cardiovascular system such as acute coronary syndrome. Among inflammatory
bowel diseases are Crohn's disease and
ulcerative colitis.
MEKI and MEK2 are validated and accepted targets for anti-proliferative
therapies, even when the oncogenic
mutation does not affect MEK structure or expression. See, e.g., U.S. Patent
Publications 2003/0149015 by Barrett et al.
and 2004/0029898 by Boyle et al.
SUMMARY OF THE INVENTION
Provided herein are compounds of formula I, or pharmaceutically acceptable
salts, solvates, polymorphs, esters,
tautomers or prodrugs thereof:
GO
NH H X
R0 ,N,
formula I
wherein
Z is H or F;
X is F, Cl, CH3, CH2OH, CH2F, CHF2, or CF3;
Y is I, Br, Cl, CF3, C1-C3 alkyl, C2-C3 alkenyl, C2-C3 alkynyl, cyclopropyl,
OMe, OEt, SMe, phenyl or Het,
where Het is a 5- to 10- membered mono- or bicyclic heterocyclic group, which
group is saturated, olefmic, or
aromatic, containing 1-5 ring heteroatoms selected independently from N, 0,
and S; where
all said phenyl or Het groups are optionally substituted with F, Cl, Br, I,
acetyl, methyl, CN, NO2, CO2H,
C1-C3 alkyl, C1-C3 alkoxy, C1-C3 Ci-C3 alkyl-C(=S)-, C1-C3 alkoxy-
C(=S)-, C1-C3 alkyl-C(0)O-,
Ci-C3 alkyl-0-(C=0)-, C1-C3 alkyl-C(=0)NH-, C1-C3 alkyl-C(=NH)NH-, C1-C3 alkyl-
NH-(C=O)-, di-C1-C3
alkyl-N-(C=0)-, C1-C3 alkyl-C(=0)N(C1-C3 alkyl)-, C1-C3 alkyl-S(=0)2NH- or
trifluoromethyl;
all said methyl, ethyl, Cf-C3 alkyl, and cyclopropyl groups are optionally
substituted with OH;
all said methyl groups are optionally substituted with one, two, or three F
atoms;
R is H, F, Cl, Br, I, CH3NH-, (CH3)2N-, Cl-Cs alkyl, C1-C4 alkoxy, C3-C6
cycloalkyl, C2-C6 alkenyl, C2-05
alkynyl, phenyl, monosubstituted phenyl, 0(C1-C4 alkyl),
0-C(=0)(CI-C4 alkyl) or C(=0)0(C1-C4 alkyl); where
said alkyl, alkoxy, cycloalkyl, alkenyl, alkynyl and phenyl groups are
optionally substituted with 1-3
substituents selected independently from F, Cl, Br, I, OH, CN, cyanomethyl,
nitro, phenyl and trifluoromethyl;
- Page 3 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
said C1-C6 alkyl and C1-C4 alkoxy groups also optionally substituted with
OCH3or OCH2CH3;
G is Gi, 02, Rig', Rib, Ric, Rid, Rle, Ari, Ai-2 or Ar3; where
GI is C1-C6 alkyl optionally substituted with one amino, C1-C3 alkylamino, or
dialkylamino group, said
dialkylamino group comprising two C1-C4 alkyl groups which may be identical or
non-identical; or
Gi is a C3-05 diamino alkyl group;
G2 is a 5- or 6- membered ring, which is saturated, unsaturated, or aromatic,
containing 1-3 ring
heteroatoms selected independently from N, 0, and S, optionally substituted
with 1-3 substituents selected
independently from F, CI, OH, 0(C1-C3 alkyl), OCH3, OCH2CH3, CH3C(=0)NH,
CH3C(=0)0, CN, CF3, and a
5-membered aromatic heterocyclic group containing 1-4 ring heteroatoms
selected independently from N, 0,
and S;
Ric is methyl, optionally substituted with 1-3 fluorine atoms or 1-3 chlorine
atoms, or with OH,
cyclopropoxy, or Ci- C3 alkoxy, where said cyclopropoxy group or the Ci- C3
alkyl moieties of said Ci- C3
alkoxy groups are optionally substituted with one hydroxy or methoxy group,
and where all C5- alkyl groups
within said C1- C4 alkoxy are optionally further substituted with a second OH
group;
Rib is CH(CH3)-C1.3 alkyl or C3-C6 cycloalkyl, said alkyl and cycloalkyl
groups optionally substituted
with 1-3 substituents selected independently from F, Cl, Br, I, OH, OCH3, and
CN;
Ric is (CH2)nO.R'; where
rn is 0 or 1; and where
when m is 0, n is 1 or 2;
when m is 1, n is 2 or 3;
R' is C1-C6 alkyl, optionally substituted with 1-3 substituents selected
independently from F, Cl, OH,
OCH3, OCH2CH3, and C3-C6 cycloalkyl;
Rid is C(A)(A)(B)-; where
B is H or C14 alkyl, optionally substituted with one or two OH groups;
A and A' are independently H or C14 alkyl, optionally substituted with one or
two OH groups; or
A and A', together with the carbon atom to which they are attached, form a 3-
to 6- member saturated
ring;
Ric is
(CH2)q¨
R2-6
Rle
where
q is 1 or 2;
R2 and R3 are each independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-butyl
or methylsulfonyl;
R4 is H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3, OCH2F, OCHF2, OCF3, ethyl, n-
propyl, isopropyl,
cyclopropyl, isobutyl, sec-butyl, tert-butyl, methylsulfonyl, nitro,
acetamido, amidinyl, cyano, carbamoyl,
methylcarbamoyl, dimethylcarbamoyl, 1,3,4-oxadiazol-2-yl, 5-methyl-I,3,4-
oxadiazol, 1,3,4-thiadiazol, 5-
methy1-1,3,4-thiadiazol 1H-tetrazolyl, N-morpholyl carbonyl amino, N-
morpholylsulfonyl and N-
pyrrolidinylcarbonylamino;
R5 is H, F, Cl or methyl;
- Page 4 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
R6 is H, F, Cl or methyl;
Ari is
R2-6
Ari
where
U and V are, independently, N, CR2 or CR3;
R2, R3 and R4 are, independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, see-butyl, tert-
butyl, acetamido, amidinyl, cyano,
carbamoyl, methylcarbamoyl, dimethylcarbamoyl, 1,3,4-oxadiazol-2-34, 5-methyl-
1,3,4-oxadiazolyi, 1,3,4-
thiadiazolyl, 5-methyl-1,3,4-thiadiazolyl, 1H-tetrazolyl, N-
morpholylcarbonylamino, N-morpholylsulfonyl,
N-pyrrolidinylcarbonylamino, and methylsulfonyl;
R5 and R6 are, independently, H, F, Cl or methyl.;
Ar2 is
R7-8
U
Ar2
where
the dashed line represents alternative formal locations for the second ring
double bond;
U is -S-, -0- or -N =, and where
when U is -0- or -S-, V is -CH=, -CC1= or -N =;
when U is -N =, V is -CH=, -CC1=, or -N=;
R7 is H or methyl;
Rg is H, acetamido, methyl, F or Cl;
Ar3 is
R7
N
Rg
Ar3
where
U is -NH-, -NCH3- or -0-;
R7 and R8 are, independently, H, F, Cl, or methyl.
In some embodiments, the invention provides a compound of formula 1, selected
from the compounds below:
FOr IVIA
F
401 N nith
1111111-1111 F I
- Page 5 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
OH 77 OH 7-7 OH \--7
HO.J..õ_õ...-7I . 0
HO...,....},..õ2c,s-z,0 HO,s,0 H0,....),...A;t0 ry <
=-:-. NH F
0 NH H F 0- '''NH H F 0- ''.-NH H F H
N
= 0 Me0 0 N 40 F
" N ribi N
0 10
F 1 F I 411111frill F 11111 1 F
1
F F F F
,
HO
Floif:.1.y 0
1-10,S Ha"--"'-'-'7:...S H0a5,,s0
,.,-5-- CC NH F 0- NNW F
J. ''NH H F H H 0-
''NH
H F
Oo 6 N a N
0 40 Me0 so N 40
Me0 ao N so
-.W.- F -.W-- 1 F I F I F
I
F F F and F
.
In some embodiments, the invention provides a compound of formula I, selected
from:
9H 771-I
HO.,õ...--1-.õ)C. -1,0
0- ....'NH F e.,-S
H =-= NH
H F
Me0 0F N so F N
I 40 00
F I
F and F , where the 2-0H carbon is
in the R
configuration.
In some embodiments, the invention provides a compound of formula I, selected
from:
OH 7.7 9 77H
0
,,,:S.5,-
NNH H F c., NHH F
Me0 so N 40 F N
40 40
F I F I
F and F 5
where the 2-0H carbon is in the S
configuration.
In some embodiments, the invention provides a composition comprising a
compound of formula I, selected from
those shown below, where the 2-0H carbon is in the R configuration,
substantially free of the S- isomer.
OH OH µ-=
H0.5õ.L0 HOcAx0
NNH H F0- NH H F
meo 0 N 0 FN
F I 4040
F 1
F F
, =
In some embodiments, the invention provides a composition comprising a
compound of formula I, selected from
those shown below, where the 2-0H carbon is in the S configuration,
substantially free of the R- isomer.
9H 77
om
HO.õ...õ...c,,..X.,,..s.:_0
(:).'NNH F
0- NH H F FE
F 0 NI 0
Me0 40 N 40
F I F I
F F
, .
In some embodiments, this invention provides a compound of formula I, where Y
is phenyl, pyridyl, or pyrazolyl.
In another subgeneric embodiment, this invention provides a compound of
formula I, where Y is substituted phenyl,
pyridyl, or pyrazolyl. In yet another subgeneric embodiment, this invention
provides a compound of formula I, where Y
is Br or I. In one subgeneric embodiment, this invention provides a compound
of formula I, where G is 1-piperidyl, 2-
- Page 6 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
piperidyl, 3-piperidyl, or 4-piperidyl. In another subgeneric embodiment, this
invention provides a compound of formula
I, where G is 1-piperazyl or 2-piperazyl. In another subgeneric embodiment,
this invention provides a compound of
formula I, where G is morpholyl. In another subgeneric embodiment, this
invention provides a compound of formula I,
where G is N-methyl-2-aminoethyl. In one subgeneric embodiment, this invention
provides a compound of formula I,
where G is N-methyl-3-amino-n-propyl. In another subgeneric embodiment, this
invention provides a compound of
formula I, where G is (CH3)2N-CH2CH2-NH-(CH2)õ-, where n is 1, 2, or 3. In
another subgeneric embodiment, this
invention provides a compound of formula I, where G is (CH3CH2)2N-CH2CH2-NH-
(CH2).-, where n is 1 or 2. In a more
specific subgeneric embodiment, this invention provides a compound of formula
I, where G is 1-piperidyl, 2-piperidyl, 3-
piperidyl, or 4-piperidyl; R is H, halo, or methoxy; X is F; and Y is I. In
another more specific subgeneric embodiment,
this invention provides a compound of formula I, where G is 1-piperazyl or 2-
piperazyl; R is H, halo, or methoxy; X is
F; and Y is I In another more specific subgeneric embodiment, this invention
provides a compound of formula I, where G
is morpholy1; R is H, halo, or methoxy; X is F; and Y is I. In another more
specific subgeneric embodiment, this
invention provides a compound of formula I, where G is N-methyl-2-aminoethyl;
R is H, halo, or methoxy; X is F; and
Y is I In another more specific subgeneric embodiment, this invention provides
a compound of formula I, where G is N-
methyl-3-amino-n-propyl; R is H, halo, or methoxy; X is F; and Y is I. In
another more specific subgeneric embodiment,
this invention provides a compound of formula I, where G is (CH3)2N-CH2CH2-NH-
(CH2)õ-, where n is 1, 2, or 3; R is
H, halo, or methoxy; X is F; and Y is I. In another more specific subgeneric
embodiment, this invention provides a
compound of formula I, where G is (CH3CH2)2N-CH2CH2-NH-(CH2)n-, where n is I
or 2; R is H, halo, or methoxy; X is
F; and Y is I.
In some embodiments, the invention provides a pharmaceutical composition
comprising a compound of formula I
or a pharmaceutically acceptable salt, solvate, polymorph, ester, tautomer or
prodrug thereof. In some embodiments the
pharmaceutical composition further comprises at least one pharmaceutically
acceptable carrier.
In some embodiments, the invention provides a pharmaceutical composition
comprising a compound selected
HOJO.77H
''NH F 0p.i
FIN, Me0 00 N
1 1
from: F and F , or a pharmaceutically
acceptable salt, solvate,
polyrnorph, ester, tautomer or prodrug thereof. In some embodiments, the
pharmaceutical composition further comprises
at least one pharmaceutically acceptable carrier. In some embodiments, the
compound is in the R configuration. In some
embodiments, the compound is in the R configuration, substantially free of the
S- isomer. In some embodiments, the
compound is in the S configuration.
In some embodiments, the compound is in the S configuration, substantially
free of the R- isomer. In some
771-1
0- NH H F
F isc io
embodiments, the compound is: F . In some embodiments, the compound is:
HO
NH
Me0 N so
-Page 7-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
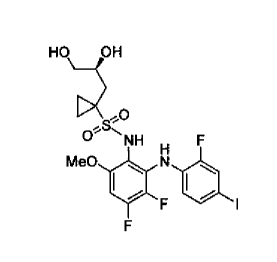
The invention also relates to a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl) cycIoproparie-l-
sulfonamide (also referred to herein as
"Compound A" and "N-(-)-(3,4-difluoro-2-(2-fluoro-4-iodopheny1amino)-6-
methoxypheny1)-1-(2,3-dihydroxypropy1)
HO OH
0
Li' NHH F
Med io N io
cyclopropane-l-sulfonamide"): F , that exhibits a specific powder x-
ray diffraction pattern. In
some embodiments, the powder x-ray diffraction pattern contains at least 50%
of the peaks shown in FIG. 5. In some
embodiments, the powder x-ray diffraction pattern contains at least 70% of the
peaks shown in FIG. 5. In some
embodiments, the powder x-ray diffraction pattern contains at least 90% of the
peaks shown in FIG. 5. In some
embodiments, the powder x-ray diffraction pattern is substantially the same as
the powder x-ray diffraction pattern shown
in FIG. 5. Compound A has been characterized as the "S" isomer by making the R
and S-MTPA esters at the secondary
alcohol and comparing the proton chemical shift difference. See, e.g., Dale,
IA.; Mosher, H.S., J. Am. Chem. Soc., 1973,
95, 512 and Ohtani et aL, J. Am. Chem. Soc., 1991, 113,4092.
The invention also relates to N-(R)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl) cyclopropane-l-sulfonamide (also referred to herein as
"Compound B" and "N-(+)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide"):
H0v.dFl
u NH
Med N
Compound B has been characterized as the "R" isomer by making the R and S-MTPA
esters at
the secondary alcohol and comparing the proton chemical shift difference. See,
e.g., Dale, J.A.; Mosher, H.S., J. Am.
Chem. Soc., 1973, 95, 512 and Ohtani et at, J. Am. Chem. Soc., 1991, 113,
4092.
The invention also relates to a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
HH
I>? 0
u- 'NH H F
Me N
1111111-1-111 F 4111111"
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-l-
sulfonamide: F that
exhibits a specific differential scanning calorimetry pattern. In some
embodiments, the differential scannine calorimetry
pattern is substantially the same as the differential scanning calorimetry
pattern shown in FIG. 6.
The invention also relates to pharmaceutical compositions comprising an
effective amount of crystalline
polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheriy1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide and a pharmaceutically acceptable carrier or
vehicle.
In some embodiments, the crystalline polymorph form A of N-(S)-(3,4-difluoro-2-
(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)-I-(2,3-dihydroxypropyl) cyclopropane-l-sulfonamide is useful
for treating or preventing cancer or an
inflammatory disease. The invention further relates to methods for treating or
preventing cancer or an inflammatory
disease, comprising administering an effective amount of a crystalline
polymorph form A of N-(S)-(3,4-difluoro-2-(2-
- Page 8 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
fluoro-4-iodophenylamino)-6-methoxyphenyl)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide to a subject in need
thereof.
In other aspects, the present invention is directed to pharmaceutical
compositions comprising effective amounts of
a compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof. In
some embodiments, the pharmaceutical compositions further comprise a
pharmaceutically acceptable carrier. Such
compositions may contain adjuvants, excipients, and preservatives, agents for
delaying absorption, fillers, binders,
adsorbents, buffers, disintegrating agents, solubilizing agents, other
carriers, and other inert ingredients. Methods of
formulation of such compositions are well-known in the art.
In other aspects, the present invention is directed to a pharmaceutical
composition comprising a compound of
formula I or a pharmaceutically acceptable salt, solvate, polymorph, ester,
tautomer or prodrug thereof. In some
embodiments, the pharmaceutical composition is in a form suitable for oral
administration. In further or additional
embodiments, the pharmaceutical composition is in the form of a tablet,
capsule, pill, powder, sustained release
formulation, solution, suspension, for parenteral injection as a sterile
solution, suspension or emulsion, for topical
administration as an ointment or cream or for rectal administration as a
suppository. In further or additional
embodiments, the pharmaceutical composition is in unit dosage forms suitable
for single administration of precise
dosages. In further or additional embodiments the amount of compound of
formula I is in the range of about 0.001 to
about 1000 mg/kg body weight/day. In further or additional embodiments the
amount of compound of formula I is in the
range of about 0.5 to about 50 mg/kg/day. In further or additional embodiments
the amount of compound of formula I is
about 0.001 to about 7 g/day. In further or additional embodiments the amount
of compound of formula I is about 0.002
to about 6 g/day. In further or additional embodiments the amount of compound
of formula I is about 0.005 to about 5
g/day. In further or additional embodiments the amount of compound of formula
I is about 0.01 to about 5 g/day. In
further or additional embodiments the amount of compound of formula I is about
0.02 to about 5 g/day. In further or
additional embodiments the amount of compound of formula us about 0.05 to
about 2.5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments, dosage levels below the lower limit of
the aforesaid range may be more than
adequate. In further or additional embodiments, dosage levels above the upper
limit of the aforesaid range may be
required. In further or additional embodiments the compound of formula I is
administered in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day.
In some embodiments, the pharmaceutical composition is for administration to a
mammal. In further or additional
embodiments, the mammal is human.
In further or additional embodiments, the pharmaceutical composition further
comprises a pharmaceutical carrier,
excipient and/or adjuvant. In further or additional embodiments, the
pharmaceutical composition further comprises at
least one therapeutic agent In further or additional embodiments, the
therapeutic agent is selected from the group of
cytotoxic agents, anti-angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-
neoplastic agent is selected from the group of consisting of alkylating
agents, anti-metabolites, epidophyllotoxins;
antineoplastic enzymes, topoisomerase inhibitors, procarbazines,
mitoxantrones, platinum coordination complexes,
biological response modifiers and growth inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic
-Page 9-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
growth factors. In further or additional embodiments, the therapeutic agent is
taxol, bortezomib or both. In further or
additional embodiments, the pharmaceutical composition is administered in
combination with an additional therapy. In
further or additional embodiments, the additional therapy is radiation
therapy, chemotherapy, surgery or any combination
thereof. In further or additional embodiments, the pharmaceutical composition
comprises a pharmaceutically acceptable
salt of a compound of formula I.
Provided herein are compositions and methods for using a composition
comprising a compound selected from:
NH F 0' 'NH H F
Me0 riti F
Mir F
and F
. In some embodiments, the 2-0H carbon on the compound is in the R
configuration. In some embodiments, the 2-0H carbon on the compound is in the
S configuration. In some
embodiments, composition is substantially free of the S- isomer of the
compound. In some embodiments, the
10 composition is substantially free of the R- isomer of the compound. In
some embodiments, the compound contains less
than 10% of the S- isomer of the compound. In some embodiments, the compound
contains less than 10% of the R-
isomer of the compound. In some embodiments, the compound contains less than
5% of the S- isomer of the compound.
In some embodiments, the compound contains less than 5% of the R- isomer of
the compound. In some embodiments,
the compound contains less than 1% of the S- isomer of the compound. In some
embodiments, the compound contains
15 less than 1% of the R- isomer of the compound.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
0 NH
Me0 00 N
comprising about 1-100 mg of a compound having the following structure:
F . In some embodiments,
the composition allows for modified release of the compound. In some
embodiments, the composition allows for
sustained release of the compound. In some embodiments, the composition allows
for delayed release of the compound.
20 In some embodiments, the compound is present in an amount of about 1-50
mgs. In some embodiments, the compound is
present in an amount of about 1-10 mgs. In some embodiments, the compound is
present in an amount of about 10-20
mgs. In some embodiments, the compound is present in an amount of about 20-40
mgs. In some embodiments, the
compound is present in an amount of about 40-50 mgs.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HOH
cr NH
me. N
25 comprising: about 1-50
mg of a compound having the following structure F , wherein the
composition
allows for modified release of the drug. In some embodiments, the composition
further comprises microcrystalline
cellulose. In some embodiments, the composition further comprises
croscarmellose sodium. In some embodiments, the
composition further comprises sodium lauryl sulfate. In some embodiments, the
composition further comprises
magnesium stearate.
-Page 10-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Also provided here in are compositions comprising about ling of a compound
having the following structure:
HO OH
0
o' NH F
Me0 N 40
. In some embodiments, the composition further comprises about 222.2mg of
microcrystalline
cellulose. In some embodiments, the composition further comprises about 12.0mg
of croscarmellose sodium. In some
embodiments, the composition further comprises about 2.4mg of sodium lauryl
sulfate. In some embodiments, the
composition further comprises about 2.4mg of magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO OH
0
o' NH
Me0 N 40
comprising about 10mg of a compound having the following structure
F . In some embodiments, the
composition further comprises about 213.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO OH
ci" 'NH F
Me
comprising about 20mg of a compound having the following structure:
F . In some embodiments, the
composition further comprises about 203.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO OH
0õS:NH F
Me0 40
comprising about 40mg of a compound having the following structure
F . In some embodiments, the
composition further comprises about 183.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
- Page 11 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
H0414
1111.
0 'NH F
Me0 N
F
comprising: about 0.4% by weight of a compound having the following structure
F , and about 99.6% by
weight of a pharmaceutically acceptable carrier or vehicle. In some
embodiments, the pharmaceutically acceptable carrier
or vehicle comprises microcrystalline cellulose. In some embodiments, the
microcrystalline cellulose is about 92.6% by
weight of the composition. In some embodiments, the composition further
comprises about 5% by weight croscarmellose
sodium. In some embodiments, the composition further comprises about 1% by
weight sodium lauryl sulfate. In some
embodiments, the composition further comprises about 1% by weight magnesium
stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO OH
0NH
Me0 N
F
comprising about 4.2% by weight of a compound having the following structure
F , and about 95.8 %
by weight of a pharmaceutically acceptable carrier or vehicle. In some
embodiments, the pharmaceutically acceptable
carrier or vehicle comprises microcrystalline cellulose. In some embodiments,
the microcrystalline cellulose is about
88.8% by weight of the composition. In some embodiments, the composition
further comprises about 5% by weight
croscarmellose sodium. In some embodiments, the composition further comprises
about 1% by weight sodium lauryl
sulfate. In some embodiments, the composition further comprises about 1% by
weight magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO OH
F
Me0
1111LIF F
comprising from about 2% to about 10% by weight of a compound having the
following structure F , and
from about 98% to about 90% by weight of a pharmaceutically acceptable carrier
or vehicle. In some embodiments, the
pharmaceutically acceptable carrier or vehicle further comprises
microcrystalline cellulose. In some embodiments, the
microcrystalline cellulose is from about 85% to about 95% by weight of the
composition. In some embodiments, the
composition further comprises from about 1% to about 6% by weight
croscarmellose sodium. In some embodiments, the
composition further comprises from about 0.1% to about 2% by weight sodium
lauryl sulfate. In some embodiments, the
composition further comprises from about 0.25% to about 1.5% by weight
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO\ .,PH
DIP? 0
0' 14H
Me op N 40
comprising about lmg of a compound having the following structure F . In
some embodiments, the
composition further comprises about 222.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
-Page 12-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
Ho;
0õs': NH
Me0 N
F lir I
comprising about 10mg of a compound having the following structure: In some
embodiments, the
composition further comprises about 213.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO PH
S-
cr' NH H F
Me0 N
comprising about 20mg of a compound having the following structure: F .
In some embodiments, the
composition further comprises about 203.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO pH
o
0NH
ivle0 N
comprising about 40mg of a compound having the following structure:
F . In some embodiments, the
composition further comprises about 183.2mg of microcrystalline cellulose. In
some embodiments, the composition
further comprises about 12.0mg of croscarmellose sodium. In some embodiments,
the composition further comprises
about 2.4mg of sodium lauryl sulfate. In some embodiments, the composition
further comprises about 2.4mg of
magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO pH
\P 0
Me0 40 N
comprising about 0.4% by weight of a compound having the following structure
F , and about 99.6%
by weight of a pharmaceutically acceptable carrier or vehicle. In some
embodiments, the pharmaceutically acceptable
carrier or vehicle comprises microcrystalline cellulose. In some embodiments,
the microcrystalline cellulose is about
92.6% by weight of the composition. In some embodiments, the composition
further comprises about 5% by weight
-Page 13-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
croscarmellose sodium. In some embodiments, the composition further comprises
about 1% by weight sodium lauryl
sulfate. In some embodiments, the composition further comprises about 1% by
weight magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO pH
ID? 0
0
Me0 40 N 40
1
comprising about 4.2% by weight of a compound having the following structure
F , and about 95.8 %
by weight of a pharmaceutically acceptable carrier or vehicle. In some
embodiments, the pharmaceutically acceptable
carrier or vehicle comprises microcrystalline cellulose. In some embodiments,
the microcrystalline cellulose is about
88.8% by weight of the composition. In some embodiments, the composition
further comprises about 5% by weight
croscarmellose sodium. In some embodiments, the composition further comprises
about 1% by weight sodium lauryl
sulfate. In some embodiments, the composition further comprises about 1% by
weight magnesium stearate.
Also provided herein are compositions and methods of treating cancer or
inflammation with compositions
HO pH
\Põo
.s.
0, NH
Me0 N
"PI F
comprising from about 2% to about 10% by weight of a compound having the
following structure
and from about 98% to about 90% by weight of a pharmaceutically acceptable
carrier or vehicle. In some embodiments,
the pharmaceutically acceptable carrier or vehicle comprises microcrystalline
cellulose. In some embodiments, the
microcrystalline cellulose is from about 85% to about 95% by weight of the
composition. In some embodiments, the
composition further comprises from about 1% to about 6% by weight
croscarmellose sodium. In some embodiments, the
composition further comprises from about 0.1% to about 2% by weight sodium
lauryl sulfate. In some embodiments, the
composition further comprises from about 0.25% to about 1.5% by weight
magnesium stearate.
Also provided herein is a crystalline polymorph Form A of N-(-)-(3,4-difluoro-
2-(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-l-sulfonamide that
exhibits a powder x-ray diffraction pattern
comprising at least 50% of the peaks identified in the powder x-ray
diffraction pattern shown in FIG. 5 and compositions
comprising this compound.. In some embodiments, the crystalline polymorph Form
A, wherein the powder x-ray
diffraction pattern comprises at least 70% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG. 5.
In some embodiments, the powder x-ray diffraction pattern comprises at least
90% of the peaks identified in the powder
x-ray diffraction pattern shown in FIG. 5. In some embodiments, the powder x-
ray diffraction pattern substantially the
same as the powder x-ray diffraction pattern shown in FIG. 5. In some
embodiments, the crystalline polymorph has a
melting point onset as determined by differential scanning calorimetry at
about 143 C. In some embodiments, the
crystalline polymorph is substantially free of water. In some embodiments, the
crystalline polymorph is substantially free
of solvent.
Also provided herein is a crystalline polymorph Form A of N-(-)-(3,4-difluoro-
2-(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-l-sulfonamide that
exhibits a differential scanning calorimetry
pattern substantially the same as the differential scanning calorimetry
pattern shown in FIG. 6 and compositions
comprising this compound. In some embodiments, the crystalline polymorph has a
melting point onset as determined by
differential scanning calorimetry at about 143 C. In some embodiments, the
crystalline polymorph of claim 67 or 68,
- Page 14-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
wherein the crystalline polymorph is substantially free of water. In some
embodiments, the crystalline polymorph of any
of claims 67-69, wherein the crystalline polymorph is substantially free of
solvent.
Also provided herein is a polymorphic form of N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-1-sulfonamide made by a
method comprising the step of
crystallizing amorphous N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyI)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide and compositions comprising this compound. In some
embodiments, the crystallization step
comprises crystallizing from a mixture of ethyl acetate and heptane. In some
embodiments, the mixture of ethyl acetate
and heptane is in a ratio of from about 1-4 parts ethyl acetate to about 2-10
parts heptane. In some embodiments, the
mixture of ethyl acetate and heptane is in a ratio of from about 2 parts ethyl
acetate to about 5 parts heptane.
Also provided herein are methods for inhibiting MEK enzymes comprising
contacting said MEK enzyme with a
compound or composition described herein, wherein the compound is present in
an amount sufficient to inhibit said
enzyme by at least 25%. In some embodiments, the MEK enzyme is MEK kinase. In
some embodiments, said contacting
occurs within a cell.
Also provided herein are methods for treating a MEK mediated disorder in an
individual suffering from said
disorder, comprising administering to said individual an effective amount of a
compound or composition described
herein. In some embodiments, the MEK inhibitor is administered in combination
with an additional therapy. In some
embodiments, the additional therapy is radiation therapy, non-MEK kinase
inhibitor therapy, chemotherapy, surgery,
Glucocorticoid, methotrexate, biological response modifiers, or any
combination thereof. In some embodiments, the
MEK mediated disorder is selected from the group consisting of inflammatory
diseases, infections, autoiinmune
disorders, stroke, ischemia, cardiac disorder, neurological disorders,
fibrogenetic disorders, proliferative disorders,
hyperproliferative disorders, tumors, leukemias, neoplasms, cancers,
carcinomas, metabolic diseases and malignant
diseases. In some embodiments, the MEK mediated disorder is a
hyperproliferative disease. In some embodiments, the
MEK mediated disorder is cancer, tumors, leukemias, neoplasms, or carcinomas.
In some embodiments, the MEK
mediated disorder is an inflammatory disease. In some embodiments, said
inflammatory disease is rheumatoid arthritis
or multiple sclerosis.
Also provided herein are methods for the treatment or prophylaxis of a
proliferative disease in an individual
comprising administering to said individual an effective amount of a compound
or composition described herein. In some
embodiments, the proliferative disease is cancer, psoriasis, restenosis,
disease, or atherosclerosis. In some embodiments,
the proliferative disease is cancer. In some embodiments, the cancer is brain
cancer, breast cancer, lung cancer, ovarian
cancer, pancreatic cancer, prostate cancer, renal cancer, colorectal cancer,
leukemia, myeloid leukemia, glioblastoma,
follicular lymphona, pre-B acute leukemia, chronic lymphocytic B-leukemia,
stomach cancer, mesothelioma or small cell
lung cancer. In some embodiments, the method further comprises administering
at least one therapeutic agent. In some
embodiments, this step comprises the administration of at least one additional
cancer therapy. In some embodiments, the
additional therapy is radiation therapy, non-MEK kinase inhibitor therapy,
chemotherapy, surgery, Glucocorticoid,
methotrexate, biological response modifiers, or any combination thereof.
Also provided herein are methods for the treatment or prophylaxis of an
inflammatory disease in an individual
comprising administering to said individual an effective amount of a
composition comprising a compound described
herein. In some embodiments, the inflammatory disease is rheumatoid arthritis
or multiple sclerosis.
Also provided herein are methods for degrading, inhibiting the growth of or
killing cancer cells comprising
contacting the cells with an amount of the compound or composition described
herein effective to degrade, inhibit the
-Page 15-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
growth of or kill cancer cells. In some embodiments, the cancer cells comprise
brain, breast, lung, ovarian, pancreatic,
prostate, renal, stomach or colorectal cancer cells.
Also provided herein are methods of inhibiting tumor size increase, reducing
the size of a tumor, reducing tumor
proliferation or preventing tumor proliferation in an individual comprising
administering to said individual an effective
amount of the compound or composition described herein to inhibit tumor size
increase, reduce the size of a tumor,
reduce tumor proliferation or prevent tumor proliferation. In some
embodiments, the tumor occurs in the brain, breast,
lung, ovaries, pancreas, prostate, kidney, stomach, colon or rectum.
Also provided herein are methods for treating or preventing anIcylosing
spondylitis, gout, tendonitis, bursitis or
sciatica, comprising administering to a subject in need thereof an effective
amount of a compound of formula (I) or a
X
R0
N
Z Y
pharmaceutical salt thereof: F ; wherein:
Z is H or F;
X is F, Cl, CH3, CH2OH, CH2F, CHF2, or CF3;
Y is I, Br, Cl, CF3, C1-C3 alkyl, 02-C3 alkenyl, C2-C3 alkynyl, cyclopropyl,
OMe, OEt, SMe, phenyl or Het,
where Het is a 5- to 10- membered mono- or bicyclic heterocyclic group, which
group is saturated, olefunc, or
aromatic, containing 1-5 ring heteroatoms selected independently from N, 0,
and S; where
all said phenyl or Het groups are optionally substituted with F, Cl, Br, I,
acetyl, methyl, CN, NO2, CO2H,
C1-C3 alkyl, C1-C3 alkoxy, C1-C3 alkyl-C(=0)-, C1-C3 alkyl-C(=S)-, C1-C3
alkoxy-C(=S)-, C1-C3 alkyl-C(-0)0-,
C1-C3 alkyl-0-(C=0)-, C1-C3 alkyl-C(0)NB-, C1-C3 alkyl-C(=NH)NH-, C1-C3 alkyl-
NH-(C=0)-, di-C1-C3
alkyl-N-(C=0)-, C1-C3 alkyl-C(-0)N(C1-C3 alkyl)-, C1-C3 alkyl-S(=0)2NH- or
trifluoromethyl;
all said methyl, ethyl, C1-C3 alkyl, and cyclopropyl groups are optionally
substituted with OH;
all said methyl groups are optionally substituted with one, two, or three F
atoms;
R is H, F, Cl, Br, I, CH3NH-, (CH3)2N-, C1-C6 alkyl, C1-C4 alkoxy, C3-C6
cycloalkyl, C2-C6 aikenyl, C2-C6
alkynyl, phenyl, monosubstituted phenyl, 0(C1-C4 alkyl),
0-C(=0)(C1-C4 alkyl) or C(=0)0(C1-C4 alkyl); where
said alkyl, alkoxy, cycloalkyl, alkenyl, alkynyl and phenyl groups are
optionally substituted with 1-3
substituents selected independently from F, Cl, Br, I, OH, CN, cyanomethyl,
nitro, phenyl and trifluoromethyl;
said C1-C6 alkyl and C1-C4 alkoxy groups also optionally substituted with
OCH3or OCH2C1-13;
G is GI, G2, Ria, Rib, Riõ Rid, Rie, Arli Ar2 or Ar3; where
Gi is C1-C6 alkyl optionally substituted with one amino, C1-C3 alkylamino, or
dialkylamino group, said
diallcylamino group comprising two CI-Ca alkyl groups which may be identical
or non-identical; or
G1 is a C3-C8 diamino alkyl group;
G2 is a 5- or 6- membered ring, which is saturated, unsaturated, or aromatic,
containing 1-3 ring
heteroatoms selected independently from N, 0, and S, optionally substituted
with 1-3 substituents selected
independently from F, Cl, OH, 0(C1-C3 alkyl), OCH3, OCH2CH3, CH3C(=0)NH,
CH3C(=0)0, CN, CF3, and a
5-membered aromatic heterocyclic group containing 1-4 ring heteroatoms
selected independently from N, 0,
and S;
Ria is methyl, optionally substituted with 1-3 fluorine atoms or 1-3 chlorine
atoms, or with OH,
cyclopropoxy, or Ci- C3 alkoxy, where said cyclopropoxy group or the Ci- C3
alkyl moieties of said C1- Ca
-Page 16-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
alkoxy groups are optionally substituted with one hydroxy or methoxy group,
and where all C3- alkyl groups
within said Ci- C4 alkoxy are optionally further substituted with a second OH
group;
Rib is CH(CH3)-C1.3 alkyl or C3-C6 cycloalkyl, said alkyl and cycloalkyl
groups optionally substituted
with 1-3 substituents selected independently from F, Cl, Br, 1, OH, OCH3, and
CN;
R1c is (CH2).0õ,R1; where
m is 0 or 1; and where
when m is 0, n is 1 or 2;
when m is 1, n is 2 or 3;
R' is C1-C6 alkyl, optionally substituted with 1-3 substituents selected
independently from F, Cl, OH,
OCH3, OCH2CH3, and C3-C6 cycloalkyl;
Rid is C(A)(A1)(B)-; where
B is H or C1.4 alkyl, optionally substituted with one or two OH groups;
A and A' are independently H or C14 alkyl, optionally substituted with one or
two OH groups; or
A and A', together with the carbon atom to which they are attached, form a 3-
to 6- member saturated
ring;
R1, is
(CH2)
Rõ
where
ci is 1 or 2;
112 and R3 are each independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-butyl
or rnethylsulfonyl;
R4 is H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3, OCH2F, OCHF2, OCF3, ethyl, n-
propyl, isopropyl,
cyclopropyl, isobutyl, sec-butyl, tert-butyl, methylsulfonyl, nitro,
acetamido, amidinyl, cyano, carbamoyl,
methylcarbamoyl, dimethylcarbamoyl, 1,3,4-oxadiazol-2-yl, 5-methyl-1,3,4-
oxadiazol, 1,3,4-thiadia.zol, 5-
methy1-1,3,4-thiadiazol 1H-tetrazolyl, N-morpholyI carbonyl amino, N-
morpholylsulfonyl and N-
pyn-olidinylcarbonylamino;
R5 is H, F, Cl or methyl;
R6 is F, Cl or methyl;
Art is
R2-6
ri -1
where
U and V are, independently, N, CR2 or CR3;
K2, R3 and R4 are, independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-
butyl, acetamido, amidinyl, cyano,
carbamoyl, methylcarbamoyl, dimethylcarbamoyl, 1,3,4-oxadiazol-2-yl, 5-methyl-
1,3,4-oxadiazolyl, 1,3,4-
-Page 17-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
thiadiazolyl, 5-methyl-1,3,4-thiadiazolyl, 11-1-tetrazolyl, N-
morpholylcarbonylamino, N-morpholylsulfonyl,
N-pyrrolidinylcarbonylarnino, and methylsulfonyl;
R5 and Rg are, independently, H, F, Cl or methyl;
Ar2 is
Er,V R
.:.õ., 7-8
U-,---..
Ar2
where
the dashed line represents alternative formal locations for the second ring
double bond;
U is -S-, -0- or -N =, and where
when U is -0- or -S-, V is -CI-1=, -CC1= or -N =;
when U is -N =, V is -CI-1=, -CC1=, or -N=;
R7 is H or methyl;
R8 is H, acetamido, methyl, F or Cl;
Ar3 is
R7
N''-------
li
R8
Ar3
where
U is -NH-, -NCH3- or -0-;
R7 and Rg are, independently, H, F, Cl, or methyl. In some embodiments, the
compound is selected
from:
>,,s,o A.,..4.0
0-- 'NH F 0- 'NH'' F eNi.3
,.... -NH
0- NNH F H H H
H N N
Ali N di FIN, 0 0 0 0
glir F LIIIIP I F I F 1 F 1
F5 F F F
OH .77
H 0 ......> \ ......õ)(00 H 0 ji ......7 0
0%S\ NH F 9H 77
HO0 911 77
HO,.....)....}1.,
0- µ...NH H F
H NNH F - NNH
F
N
0 Si Me0 is N so
F H
N H
N
0 1101
F 1 F I 0 0
F I F I
F F F F
911 77 HO
HO.,...õ).-....___A,...õ,.0
HO..----...Y....e
HOF'"---7,5-' HO..3?
0.--NH H F Cr NH H F
0- NH H F 0-
NH F
N so N
0 Si Me Meg 01, N so H
di N ill
F 1 F 1 F I 11111"
F tillir 1
F F F and F .In
, ,
-Page 18-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
OH\-7
HOJ
NNHF NH HF
Me0 so N FIN,
some embodiments, the compound is selected from F and F
; where
the 2-0H carbon is in the R configuration. In some embodiments, the compound
of formula (I), or a pharmaceutical salt
0
HO
(f'NN
NNH H
Me N 401
thereof, is selected from F and F ; where the 2-
0H carbon is in
771-I
NNH
Me0 N io
the S configuration. In some embodiments, the compound is F . In some
embodiments, the
7-71-1
NNH H F
Me0 is N io
compound is
Provided herein are also methods for treating stomach cancer by administering
a therapeutically effective amount
of a compound or composition described herein. Provided herein are also
methods for treating leukemiam melanoma, or
hepatoma by administering a therapeutically effective amount of a compound or
composition described herein.
Provided herein are also methods for treating non-small cell lung cancer by
administering a therapeutically
effective amount of a compound or composition described herein. Provided
herein are also methods for treating colon
cancer by administering a therapeutically effective amount of a compound or
composition described herein. Provided
herein are also methods for treating CNS cancer by administering a
therapeutically effective amount of a compound or
composition described herein. Provided herein are also methods for treating
ovarian cancer by administering a
therapeutically effective amount of a compound or composition described
herein. Provided herein are also methods for
treating renal cancer by administering a therapeutically effective amount of a
compound or composition described herein.
Provided herein are also methods for treating prostate cancer by administering
a therapeutically effective amount of a
compound or composition described herein. Provided herein are also methods for
treating breast cancer by administering
a therapeutically effective amount of a compound or composition described
herein. In various embodiments, these
methods further comprise administering at least one additional therapeutic
agent. In some embodiments, at least one
additional cancer therapy is performed. In some embodiments, the additional
cancer therapy is radiation therapy,
chemotherapy, surgery, or any combination thereof
Also provided herein are methods for treating or preventing psoriasis by
administering a therapeutically effective
amount of a compound or composition described herein in a topical dosage form.
In various embodiments, the compositions are administered orally. In some
embodiments, the composition is
administered once a day or twice a day. In some embodiments, the composition
is administered once a day for at least
one week.
In some embodiments, upon oral administration of the composition, Tmõõ of the
compound is achieved between 1
hour and 3 hours after administration of the composition to a fasted subject.
In some embodiments, upon administration
-Page 19-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
to a subject, the compound reaches a Cmõõ between about 0.01 ug,/ml to about
1.0 pg/ml on day 1. hi some embodiments,
upon administration to a subject, the compound reaches a C,,,, between about
0.01 pg/ml to about 0.8 pg/ml on day 1. In
some embodiments, upon administration to a subject, the compound reaches a Cm
ax between about 0.03 pg/m1 to about
0.5 118/m1 on day 1. In some embodiments, the compound has an AUC between
about 0.1 jig luirnL to about 5.0 pg
hr/mL from 0-12 hours. In some embodiments, the compound has an AUC between
about 0.1 pg hr/ml, to about 4.0 pg
hr/mL. In some embodiments, the compound has an AUC between about 0.5 pg hr/mL
to about 3.0 pg hr/mL. In some
embodiments, the compound has a T,,,õõ between 0.5 and 5.0 hours. In some
embodiments, the compound has a Tmax
between 1.0 and 3.0 hours. In some embodiments, the compound has a Tinaõ
between 1.0 and 2.5 hours. In some
embodiments, the compound has a plasma concentration greater than about 0.01
mg/mL after 5 hours after a single dose.
In some embodiments, the compound has a plasma concentration greater than
about 0.01 mg/mL after 10 hours after a
single dose. In some embodiments, the compound has a plasma concentration
greater than about 0.01 mg/I'LL after 15
hours after a single dose.
In some embodiments, upon administration to a group of 10 subjects, the
compound reaches a mean C..õ between
about 0.01 pg/m1 to about 1.01.1g/m1 on day 1. In some embodiments, upon
administration to a group of 10 subjects, the
compound reaches a mean C,õ,õõ between about 0.01 pg/m1 to about 0.8 g/m1 on
day 1. In some embodiments, upon
administration to a group of 10 subjects, the compound reaches a mean Cm ax
between about 0.03 pg/m1 to about 0.5
pg/rril on day 1. In some embodiments, the compound has a mean AUC between
about 0.1 jig hr/mL to about 5.0 pg
hr/mL. In some embodiments, the compound has a mean AUC between about 0.1 jig
hr/mL to about 4.0 jig hr/mL. In
some embodiments, the compound has a mean AUC between about 0.5 pg hr/mL to
about 3.0 Mg hr/mL. In some
embodiments, the compound has a mean TE,õõ between 0.5 and 5.0 hours. In some
embodiments, the compound has a
mean Tõ,õõ between 1.0 and 3.0 hours. In some embodiments, the compound has a
mean Tõ,aõ between 1.0 and 2.5 hours.
Also provided herein are methods for decreasing tumor volume by administering
the compounds and compositions
described herein. In some embodiments, after daily administration of the drug
for 5 days, the tumor decreases in volume
by at least about 25%. In some embodiments, after daily administration of the
drug for 5 days, the tumor decreases in
volume by at least about 50%. In some embodiments, after daily administration
of the drug for 5 days, the tumor
decreases in volume by at least about 20-70%. In some embodiments, after daily
administration of the drug for 15 days,
the tumor decreases in volume by at least about 25%. In some embodiments,
after daily administration of the drug for 15
days, the tumor decreases in volume by at least about 50%. In some
embodiments, after daily administration of the drug
for 15 days, the tumor decreases in volume by at least about 20-70%. In some
embodiments, after daily administration of
the drug for 30 days, the tumor decreases in volume by at least about 25%. In
some embodiments, after daily
administration of the drug for 30 days, the tumor decreases in volume by at
least about 50%. In some embodiments, after
daily administration of the drug for 30 days, the tumor decreases in volume by
at least about 20-70%.
Also provided herein are methods for inhibiting tumor growth by administering
the compounds and compounds
described herein. In some embodiments, after administration of the drug, the
tumor growth is inhibited by at least about
20%. In some embodiments, after administration of the drug, the tumor growth
is inhibited by at least about 40%. In
some embodiments, after administration of the drug, the tumor growth is
inhibited by at least about 60%. In some
embodiments, after administration of the drug, the tumor growth is inhibited
by at least about 80%. In some
embodiments, after administration of the drug, the tumor growth is inhibited
by between about 20% to about 100%. In
some embodiments, after administration of the drug, the tumor growth is
substantially inhibited.
In some embodiments, the composition is administered twice a day. In some
embodiments, the composition is
administered once a day.
-Page 20-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, the MEK inhibitor does not interfere with the
coadministration of a second tumor
suprressing agent.
In some embodiments, the composition is in the form of a tablet, a capsule, a
gel cap, a caplet, a pellet, or a bead.
In some embodiments, the composition is in the form of a capsule or tablet
dosage form has a total weight of about 50 mg
to about 1000 mg. In some embodiments, the composition is in the form of a
capsule or tablet has a total weight selected
from the group consisting of 50 mg, 75mg, 100 mg, 150 mg, 200 mg, 250 mg, 300
mg, 350 mg, 400 mg, 450 mg, and
500 mg. In some embodiments, the composition is in the form of a capsule or
tablet has a total weight of about 240 mg.
In some embodiments, the composition further comprises at least one filler
selected from microcrystalline
cellulose, silicified microcrystalline cellulose, lactose, a compressible
sugar, xylitol, sorbitol, mannitol, pregelatinized
starch, maltodextrin, calcium phosphate, calcium carbonate, starch and a
calcium silicate.
In some embodiments, the composition further comprises at least one
disintegrant selected from croscarmellose
sodium, sodium starch glycolate, crospovidone, rnethylcellulose, alginic acid,
sodium alginate, starch derivatives,
betonite and veegum.
In some embodiments, the composition further comprises at least one lubricant
selected from magnesium stearate,
metallic stearates, talc, sodium stearyl fumarate and stearic acid.
In some embodiments, the composition further comprises at least one wetting
agent or surfactant selected from
sodium lauryl sulfate, glycerol, sorbitan oleates, sorbitan stearates,
polyoxyethylenated sorbitan laurate, pahnitate,
stearate, oleate or hexaolate, polyoxyethylene stearyl alcohol and sorbitan
monolaurate.
Provided herein are compositions in the form of a capsule or tablet and the
capsule or tablet releases at least 60
percent of the drug within 30 minutes using U.S. Pharmacopeia (USP) Apparatus
II at 50 rpm with 1% sodium lauryl
sulfate in water as the dissolution medium. In some embodiments, the
composition is in the form of a capsule or tablet
and the capsule or tablet releases about 60-100 percent of the drug within 30
minutes using U.S. Pharmacopeia (USP)
Apparatus II at 50 rpm with 1% sodium lauryl sulfate in water as the
dissolution medium. In some embodiments, the
composition is in the form of a capsule or tablet and the capsule or tablet
releases about 60-90 percent of the drug within
30 minutes using US. Pharmacopeia (USP) Apparatus II at 50 rpm with 1% sodium
lauryl sulfate in water as the
dissolution medium. In some embodiments, the composition is in the form of a
capsule or tablet and the capsule or tablet
releases about 60-80 percent of the drug within 30 minutes using US.
Pharmacopeia (USP) Apparatus II at 50 rpm with
1% sodium lauryl sulfate in water as the dissolution medium.
Provided herein are also batches of capsules or tablets, each comprising from
about 1 to about 50 nag of a
compound described herein and having a USP acceptance value for content
uniformity of less than about 15.
Methods of Treatment
The invention relates to methods for treating or preventing cancer, comprising
administering to a subject in need
an effective amount of a pharmaceutical composition comprising a compound of
formula (I), as described herein. In
various embodiments, the compounds and compositions useful in these methods
are as described by the genus of formula
(I) or any sub-genus or species exemplified throughout the present application
falling within formula (I).
The invention relates to methods for treating or preventing an inflammation
disease, comprising administering to a
subject in need an effective amount of a pharmaceutical composition comprising
a compound of formula (I), as described
herein. In various embodiments, the compounds and compositions useful in these
methods are as described by the genus
of formula (I) or any sub-genus or species exemplified throughout the present
application falling within formula (I).
In some embodiments, the invention relates to methods for treating or
preventing ankylosing spondylitis, gout,
tendonitis, bursitis or sciatica, comprising administering to a subject in
need thereof an effective amount of a
- Page 21 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
pharmaceutical composition comprising a compound of formula (I), as described
herein. In various embodiments, the
compounds and compositions useful in these methods are as described by the
genus of formula (I) or any sub-genus or
species exemplified throughout the present application falling within formula
(I).
In some aspects, the present invention is also directed to a method of
treating a disease in an individual suffering
from said disease comprising administering to said individual an effective
amount of a composition comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof.
In other aspects, the present invention is directed to a method of treating a
disorder in a mammal, comprising
administering to said mammal a therapeutically effective amount of the
compound of formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof
In other aspects, the present invention is directed to a method of treating a
disorder in a human, comprising
administering to said mammal a therapeutically effective amount of the
compound of formula I Or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof
In other aspects, the present invention is directed to a method of treating a
hyperproliferative disorder in a
mammal, including a human, comprising administering to said mammal a
therapeutically effective amount of the
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof
In other aspects, the present invention is directed to a method of treating an
inflammatory disease, condition, or
disorder in a mammal, including a human, comprising administering to said
mammal a therapeutically effective amount
of the compound of formula I, or a pharmaceutically acceptable salt, ester,
prodrug, solvate, hydrate or derivative thereof.
In other aspects, the present invention is directed to a method of treating a
disorder or condition which is
modulated by the MEK cascade in a mammal, including a human, comprising
administering to said mammal an amount
of the compound of formula I, or a pharmaceutically acceptable salt, ester,
prodrug, solvate, hydrate or derivative thereof,
effective to modulate said cascade. The appropriate dosage for a particular
patient can be determined, according to
known methods, by those skilled in the art.
Inhibition of MEK Enzyme
In other aspects, the present invention is directed to a method for inhibiting
a MEK enzyme. In some
embodiments, the method comprises contacting said MEK enzyme with an amount of
a composition comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof,
sufficient to inhibit said enzyme, wherein said enzyme is inhibited. hi
further or additional embodiments the enzyme is at
least about 1% inhibited. In further or additional embodiments the enzyme is
at least about 2% inhibited. In further or
additional embodiments the enzyme is at least about 3% inhibited. In further
or additional embodiments the enzyme is at
least about 4% inhibited. In further or additional embodiments the enzyme is
at least about 5% inhibited. In further or
additional embodiments the enzyme is at least about 10% inhibited. In further
or additional embodiments the enzyme is
at least about 20% inhibited. In further or additional embodiments the enzyme
is at least about 25% inhibited. In further
or additional embodiments the enzyme is at least about 30% inhibited. In
further or additional embodiments the enzyme
is at least about 40% inhibited. In further or additional embodiments the
enzyme is at least about 50% inhibited. In
further or additional embodiments the enzyme is at least about 60% inhibited.
In further or additional embodiments the
enzyme is at least about 70% inhibited. In further or additional embodiments
the enzyme is at least about 75% inhibited.
In further or additional embodiments the enzyme is at least about 80%
inhibited. In further Or additional embodiments the
enzyme is at least about 90% inhibited. In further or additional embodiments
the enzyme is essentially completely
inhibited. In further or additional embodiments the MEK enzyme is MEK kinase.
In further or additional embodiments
the MEK enzyme is MEKI. In further or additional embodiments the MEK enzyme is
MEK2. In further or additional
- Page 22 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments the contacting occurs within a cell. In further or additional
embodiments the cell is a mammalian cell. In
further or additional embodiments the mammalian cell is a human cell. In
further or additional embodiments, the MEK
enzyme is inhibited with a composition comprising a pharmaceutically
acceptable salt of a compound of formula I.
MEK Mediated Disorder
In other aspects, the present invention is directed to a method of treatment
of a MEK mediated disorder in an
individual suffering from said disorder comprising administering to said
individual an effective amount of a composition
comprising a compound of formula J or a pharmaceutically acceptable salt,
solvate, polymorph, ester, tautomer or
prodrug thereof. In some embodiments, the composition comprising a compound of
formula I is administered orally,
intraduodenally, parenterally (including intravenous, subcutaneous,
intramuscular, intravascular or by infusion), topically
or rectally. In some embodiments, the pharmaceutical composition is in a form
suitable for oral administration. In further
or additional embodiments, the pharmaceutical composition is in the form of a
tablet, capsule, pill, powder, sustained
release formulations, solution, suspension, for parenteral injection as a
sterile solution, suspension or emulsion, for
topical administration as an ointment or cream or for rectal administration as
a suppository. In further or additional
embodiments, the pharmaceutical composition is in unit dosage forms suitable
for single administration of precise
dosages. In further or additional embodiments, the pharmaceutical composition
further comprises a pharmaceutical
carrier, excipient and/or adjuvant.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.001 to about
1000 mg/kg body weight/day. In further or additional embodiments the amount of
compound of formula I is in the range
of about 0.5 to about 50 mg/kg/day. In further or additional embodiments the
amount of compound of formula I is about
0.001 to about 7 g/day. In further or additional embodiments the amount of
compound of formula I is about 0.01 to about
7 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.02 to about 5 g/day. In
further or additional embodiments the amount of compound of formula I is about
0.05 to about 2.5 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.1 to
about 1 g/day. In further or additional
embodiments, dosage levels below the lower limit of the aforesaid range may be
more than adequate. In further or
additional embodiments, dosage levels above the upper limit of the aforesaid
range may be required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the MEK
mediated disorder is a mammal. In further or additional embodiments, the
individual is a human.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
- Page 23 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezomib or both.
In some embodiments, the MEK mediated disorder is selected from the group
consisting of inflammatory diseases,
infections, autoimmune disorders, stroke, ischemia, cardiac disorder,
neurological disorders, fibrogenetic disorders,
proliferative disorders, hyperproliferative disorders, non-cancer
hyperproliferative disorders, tumors, leukemias,
neoplasms, cancers, carcinomas, metabolic diseases, malignant disease,
vascular restenosis, psoriasis, atherosclerosis,
rheumatoid arthritis, osteoarthritis, heart failure, chronic pain, neuropathic
pain, dry eye, closed angle glaucoma and wide
angle glaucoma. In further or additional embodiments, the MEK mediated
disorder is an inflammatory disease. In further
or additional embodiments, the MEK mediated disorder is a hyperproliferative
disease. In further or additional
embodiments, the MEK mediated disorder is selected from the group consisting
of tumors, leukemias, neoplasms,
cancers, carcinomas and malignant disease. In further or additional
embodiments, the cancer is brain cancer, breast
cancer, lung cancer, ovarian cancer, pancreatic cancer, prostate cancer,
stomach cancer, renal cancer, colorectal cancer or
leukemia. In further or additional embodiments, the fibrogenetic disorder is
scleroderma, polymyositis, systemic lupus,
rheumatoid arthritis, liver cirrhosis, keloid formation, interstitial
nephritis or pulmonary fibrosis. In further or additional
embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a compound of
formula I is administered.
Achieving Effects
In other aspects, the present invention is directed to a method for achieving
an effect in a patient comprising the
administration of an effective amount of a composition comprising a compound
of formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof, to a
patient, wherein the effect is selected from
the group consisting of inhibition of various cancers, immunological diseases,
and inflammatory diseases. In some
embodiments, the effect is inhibition of various cancers. In further or
additional embodiments, the effect is inhibition of
immunological diseases. In further or additional embodiments, the effect is
inhibition inflammatory diseases.
In some embodiments, the composition comprising a compound of formula us
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula! is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
- Page 24-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from cancer is a
mammal. In further or additional embodiments, the individual is a human. In
further or additional embodiments, an
effective amount of a composition comprising a pharmaceutically acceptable
salt of a compound of formula I is
administered.
In other aspects, the present invention is directed to a method for degrading,
inhibiting the growth of or killing a
cancer cell comprising contacting said cell with an amount of a composition
effective to degrade, inhibit the growth of or
to kill said cell, the composition comprising a compound of formula I or a
pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof. In some embodiments, the cancer
cells comprise brain, breast, lung,
ovarian, pancreatic, prostate, renal, or colorectal cancer cells. In further
or additional embodiments, the composition is
administered with at least one therapeutic agent. In further or additional
embodiments, the therapeutic agent is taxol,
bortezomib or both. In further or additional embodiments, the therapeutic
agent is selected from the group consisting of
cytotoxic agents, anti-angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-
neoplastic agents selected from the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins;
antineoplastic enzymes, topoisomerase inhibitors, procarbazines,
mitoxantrones, platinum coordination complexes,
biological response modifiers and growth inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic
growth factors. In some embodiments, the cancer cells are degraded. In further
or additional embodiments, 1% of the
cancer cells are degraded. In further or additional embodiments, 2% of the
cancer cells are degraded. In further or
additional embodiments, 3% of the cancer cells are degraded. In further or
additional embodiments, 4% of the cancer
cells are degraded. In further or additional embodiments, 5% of the cancer
cells are degraded. In further or additional
embodiments, 10% of the cancer cells are degraded. In further or additional
embodiments, 20% of the cancer cells are
degraded. In further or additional embodiments, 25% of the cancer cells are
degraded. In further or additional
embodiments, 30% of the cancer cells are degraded. In further or additional
embodiments, 40% of the cancer cells are
degraded. In further or additional embodiments, 50% of the cancer cells are
degraded. In further or additional
embodiments, 60% of the cancer cells are degraded. In further or additional
embodiments, 70% of the cancer cells are
degraded. In further or additional embodiments, 75% of the cancer cells are
degraded. In further or additional
embodiments, 80% of the cancer cells are degraded. In further or additional
embodiments, 90% of the cancer cells are
degraded. In further or additional embodiments, 100% of the cancer cells are
degraded. In firther or additional
embodiments, essentially all of the cancer cells are degraded. In various
embodiments, the aforementioned degradation
occurs in one day, five days, ten days, one month, two months, six months or
one year.
In some embodiments, the cancer cells are killed. In further or additional
embodiments, 1% of the cancer cells are
killed. In further or additional embodiments, 2% of the cancer cells are
killed. In further or additional embodiments, 3%
of the cancer cells are killed. In further or additional embodiments, 4% of
the cancer cells are killed. In further or
additional embodiments, 5% of the cancer cells are killed. In further or
additional embodiments, 10% of the cancer cells
are killed. In further or additional embodiments, 20% of the cancer cells are
killed. In further or additional embodiments,
25% of the cancer cells are killed. In further or additional embodiments, 30%
of the cancer cells are killed. In further or
additional embodiments, 40% of the cancer cells are killed. In further or
additional embodiments, 50% of the cancer cells
are killed. In further or additional embodiments, 60% of the cancer cells are
killed. In further or additional embodiments,
70% of the cancer cells are killed. In further or additional embodiments, 75%
of the cancer cells are killed. In further or
additional embodiments, 80% of the cancer cells are killed. In further or
additional embodiments, 90% of the cancer cells
-Page 25-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
are killed. In further or additional embodiments, 100% of the cancer cells are
killed. In flu-ther or additional
embodiments, essentially all of the cancer cells are killed. In various
embodiments, the aforementioned cancer cell
killing occurs in one day, five days, ten days, one month, two months, six
months or one year.
In further or additional embodiments, the growth of the cancer cells is
inhibited. In further or additional
embodiments, the growth of the cancer cells is about 1% inhibited. In further
or additional embodiments, the growth of
the cancer cells is about 2% inhibited. In further or additional embodiments,
the growth of the cancer cells is about 3%
inhibited. In further or additional embodiments, the growth of the cancer
cells is about 4% inhibited. In further or
additional embodiments, the growth of the cancer cells is about 5% inhibited.
In further or additional embodiments, the
growth of the cancer cells is about 10% inhibited. In further or additional
embodiments, the growth of the cancer cells is
about 20% inhibited. In further or additional embodiments, the growth of the
cancer cells is about 25% inhibited. In
further or additional embodiments, the growth of the cancer cells is about 30%
inhibited. In further or additional
embodiments, the growth of the cancer cells is about 40% inhibited. In further
or additional embodiments, the growth of
the cancer cells is about 50% inhibited. In further or additional embodiments,
the growth of the cancer cells is about 60%
inhibited. In further or additional embodiments, the growth of the cancer
cells is about 70% inhibited. In further or
additional embodiments, the growth of the cancer cells is about 75% inhibited.
In further or additional embodiments, the
growth of the cancer cells is about 80% inhibited. In further or additional
embodiments, the growth of the cancer cells is
about 90% inhibited. In further or additional embodiments, the growth of the
cancer cells is about 100% inhibited. In
various embodiments, the aforementioned inhibition occurs in one day, five
days, ten days, one month, two months, six
months or one year.
In other aspects, the present invention is directed to a method of reducing
the size of a tumor, inhibiting tumor size
increase, reducing tumor proliferation or preventing tumor proliferation in an
individual, comprising administering to
said individual an effective amount of a composition comprising a compound of
formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof. In
some embodiments, the size of a tumor is
reduced. In further or additional embodiments, the size of a tumor is reduced
by at least 1%. In further or additional
embodiments, the size of a tumor is reduced by at least 2%. In further or
additional embodiments, the size of a tumor is
reduced by at least 3%. In further or additional embodiments, the size of a
tumor is reduced by at least 4%. In further or
additional embodiments, the size of a tumor is reduced by at least 5%. In
further or additional embodiments, the size of a
tumor is reduced by at least 10%. In further or additional embodiments, the
size of a tumor is reduced by at least 20%. In
further or additional embodiments, the size of a tumor is reduced by at least
25%. In further or additional embodiments,
the size of a tumor is reduced by at least 30%. In further or additional
embodiments, the size of a tumor is reduced by at
least 40%. In further or additional embodiments, the size of a tumor is
reduced by at least 50%. In further or additional
embodiments, the size of a tumor is reduced by at least 60%. In further or
additional embodiments, the size of a tumor is
reduced by at least 70%. In further or additional embodiments, the size of a
tumor is reduced by at least 75%. In further
or additional embodiments, the size of a tumor is reduced by at least 80%. In
further or additional embodiments, the size
of a tumor is reduced by at least 85%. In further or additional embodiments,
the size of a tumor is reduced by at least
90%. In further or additional embodiments, the size of a tumor is reduced by
at least 95%. In further or additional
embodiments, the tumor is eradicated. In some embodiments, the size of a tumor
does not increase. In various
embodiments, the aforementioned effects on tumor size occurs in one day, five
days, ten days, one month, two months,
six months or one year.
In some embodiments, tumor proliferation is reduced. In some embodiments,
tumor proliferation is reduced by at
least 1 %. In some embodiments, tumor proliferation is reduced by at least 2
%. In some embodiments, tumor
- Page 26-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
proliferation is reduced by at least 3 %. In some embodiments, tumor
proliferation is reduced by at least 4 %. In some
embodiments, tumor proliferation is reduced by at least 5 %. In some
embodiments, tumor proliferation is reduced by at
least 10 %. In some embodiments, tumor proliferation is reduced by at least 20
%. In some embodiments, tumor
proliferation is reduced by at least 25 %. In some embodiments, tumor
proliferation is reduced by at least 30 %. In some
embodiments, tumor proliferation is reduced by at least 40 %. In some
embodiments, tumor proliferation is reduced by at
least 50 %. In some embodiments, tumor proliferation is reduced by at least 60
%. In some embodiments, tumor
proliferation is reduced by at least 70 %. In some embodiments, tumor
proliferation is reduced by at least 75 %. In some
embodiments, tumor proliferation is reduced by at least 75 %. In some
embodiments, tumor proliferation is reduced by at
least 80 %. In some embodiments, tumor proliferation is reduced by at least 90
%. In some embodiments, tumor
proliferation is reduced by at least 95 %. In some embodiments, tumor
proliferation is prevented. In various
embodiments, the aforementioned effects on cell proliferation occurs in one
day, five days, ten days, one month, two
months, six months or one year.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or any combination thereo. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, rnitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezornib or both.
In some embodiments, the composition is administered orally, intraduodenaliy,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula! is about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula 1 is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In farther or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula! is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from cancer is a
mammal. In further or additional embodiments, the individual is a human. In
further or additional embodiments, an
- Page 27 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
effective amount of a composition comprising a pharmaceutically acceptable
salt of a compound of formula I is
administered.
Proliferative Diseases
In other aspects, the present invention is directed to a method for the
treatment or prophylaxis of a proliferative
disease in an individual comprising administering to said individual an
effective amount of a composition comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof. In
some embodiments, the proliferative disease is cancer, psoriasis, restenosis,
autoirmnune disease, or atherosclerosis. In
further or additional embodiments, the proliferative disease is a
hyperproliferative disease. In further or additional
embodiments, the proliferative disease is selected from the group consisting
of tumors, leukemias, neoplasms, cancers,
carcinomas and malignant disease. In further or additional embodiments, the
cancer is brain cancer, breast cancer, lung
cancer, ovarian cancer, pancreatic cancer, prostate cancer, renal cancer,
colorectal cancer, stomach cancer, head and neck
cancer or leukemia. In further or additional embodiments, the fibrogenetic
disorder is scleroderma, polymyositis,
systemic lupus, rheumatoid arthritis, liver cirrhosis, keloid formation,
interstitial nephritis or pulmonary fibrosis. In
further or additional embodiments, stomach cancer, brain cancer, breast
cancer, lung cancer, non small cell lung cancer,
ovarian cancer, pancreatic cancer, liver cancer, prostate cancer, renal
cancer, colorectal cancer or leukemia. In further or
additional embodiments, the cancer is brain cancer or adrenocorticai
carcinoma. In further or additional embodiments,
the cancer is breast cancer. In further or additional embodiments, the cancer
is ovarian cancer. In further or additional
embodiments, the cancer is pancreatic cancer. In further or additional
embodiments, the cancer is prostate cancer. In
further or additional embodiments, the cancer is renal cancer. In further or
additional embodiments, the cancer is
colorectal cancer. In further or additional embodiments, the cancer is myeloid
leukemia. In further or additional
embodiments, the cancer is glioblastoma. In further or additional embodiments,
the cancer is follicular lymphona. In
further or additional embodiments, the cancer is pre-B acute leukemia. In
further or additional embodiments, the cancer
is chronic lymphocytic B-leukemia. In further or additional embodiments, the
cancer is mesothelionia. In further or
additional embodiments, the cancer is small cell lung cancer. In further
embodiments, the cancer is stomach cancer.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery, or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonallanti-hormonal
therapeutic agents, and haematopoietic growth factors.
In further or additional embodiments, the therapeutic agent is selected from
taxol, bortezomib or both. In some
embodiments, the composition is administered orally, intraduoderially,
parenterally (including intravenous, subcutaneous,
intramuscular, intravascular or by infusion), topically or rectally.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.001 to about
1000 mg/kg body weight/day. In further or additional embodiments the amount of
compound of formula I is in the range
of about 0.5 to about 50 mg/kg/day. In further or additional embodiments the
amount of compound of formula I is about
0.001 to about 7 g/day. In further or additional embodiments the amount of
compound of formula I is about 0.01 to about
7 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.02 to about 5 g/day. In
Page 28-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
further or additional embodiments the amount of compound of formula I is about
0.05 to about 2.5 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.1 to
about 1 g/day. In further or additional
embodiments, dosage levels below the lower limit of the aforesaid range may be
more than adequate. In further or
additional embodiments, dosage levels above the upper limit of the aforesaid
range may be required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula 1 is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the
proliferative disease is a mammal. In further or additional embodiments, the
individual is a human. In further or
additional embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a
compound of formula I is administered.
Inflammatory Diseases
In other aspects, the present invention is directed to a method for the
treatment or prophylaxis of an inflammatory
disease in an individual comprising administering to said individual an
effective amount of a composition comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodnig thereof. In
further or additional embodiments, the inflammatory disease is selected from
chronic inflammatory diseases, rheumatoid
arthritis, rheumatoid arthritis, spondyloarthropathies, gouty arthritis,
osteoarthritis, juvenile arthritis, acute rheumatic
arthritis, enteropathic arthritis, neuropathic arthritis, psoriatic arthritis,
pyogenic arthritis, atherosclerosis, systemic lupus
erythematosus, inflammatory bowel disease, irritable bowel syndrome,
ulcerative colitis, reflux esophagitis, Crohn's
disease, gastritis, asthma, allergies, respiratory distress syndrome,
pancreatitis, chronic obstructive pulmonary disease,
pulmonary fibrosis, psoriasis, eczema or scleroderma.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the composition
comprising a compound of formula I is
administered in combination with at least one therapeutic agent.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
-Page 29-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the
inflammatory disease is a mammal. In further or additional embodiments, the
individual is a human. In further or
additional embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a
compound of formula I is administered.
Cancer
In other aspects, the present invention is directed to a method for the
treatment or prophylaxis of cancer in an
individual comprising administering to said individual an effective amount of
a composition comprising a compound of
formula I or a pharmaceutically acceptable salt, solvate, polymorph, ester,
tautomer or prodrug thereof. In further or
additional embodiments, the cancer is brain cancer, breast cancer, stomach
cancer, lung cancer, ovarian cancer,
pancreatic cancer, prostate cancer, renal cancer, colorectal cancer or
leukemia. In further or additional embodiments, the
fibrogenetic disorder is scleroderma, polymyositis, systemic lupus, rheumatoid
arthritis, liver cirrhosis, keloid formation,
interstitial nephritis or pulmonary fibrosis. In further or additional
embodiments, the cancer is brain cancer, breast cancer,
lung cancer, ovarian cancer, pancreatic cancer, prostate cancer, renal cancer,
stomach cancer, colorectal cancer or
leukemia. In further or additional embodiments, the cancer is brain cancer or
adrenocortical carcinoma. In further or
additional embodiments, the cancer is breast cancer. In further or additional
embodiments, the cancer is ovarian cancer.
In further or additional embodiments, the cancer is pancreatic cancer. In
further or additional embodiments, the cancer is
prostate cancer. In further or additional embodiments, the cancer is renal
cancer. In further or additional embodiments,
the cancer is colorectal cancer. In further or additional embodiments, the
cancer is myeloid leukemia. In further or
additional embodiments, the cancer is glioblastoma. In further or additional
embodiments, the cancer is follicular
lymphona. In further or additional embodiments, the cancer is pre-B acute
leukemia. In further or additional
embodiments, the cancer is chronic lymphocyte B-leukemia. In further or
additional embodiments, the cancer is
mesothelioma. In further or additional embodiments, the cancer is small cell
lung cancer. In some embodiments, the
cancer is stomach cancer.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery, or any combination thereof In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of allcylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezornib or both.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.02 to
about 5 g/day. In further or additional
-Page 30-
CA 02693390 2015-12-04
30725-1660
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate.
In further or additional embodiments, dosage levels above the upper limit of
the aforesaid range may be required_
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In further or
additional embodiments the compound of formula I is administered in multiple
doses, more than once per day. In further
or additional embodiments the compound of formula I is administered twice
daily. In further or additional embodiments
the compound of formula I is administered three times per day. In further or
additional embodiments the compound of
formula 1 is administered four times per day. In further or additional
embodiments the compound of formula I is
administered more than four times per day. In some embodiments, the individual
suffering from cancer is a mammal. In
further or additional embodiments, the individual is a human. In further or
additional embodiments, an effective amount
=
of a composition comprising a pharmaceutically acceptable salt of a compound
of formula I is administered.
BRIEF DESCRIPTION OF THE DRAWINGS
The novel features of the invention are set forth with particularity in the
appended claims. A better understanding
of the features and advantages of the present invention will be obtained by
reference to the following detailed description=
that sets forth illusti alive embodiments, in which the principles of the
invention are utilized, and the accompanying
drawings of which:
Figure 1 shows graphs of average tumor volume against time (days) in mice
implanted with A375 Melanoma,
Co1o205 Colon Tumor, A431 Epidermoid Tumor or HT-29 Colon Tumor cells. Mice
were dosed orally (25mg/kg,
50mg/kg or 100mg/kg), once a day, for 14 days.
Figure 2 shows a graph of % Tumor growth inhibition (%TGI) in A375 Xenograll
mice dosed 50mg/kg QD,
25mg/kg BID, 50mg/kg QD and 12.5ingikg BID.
Figure 3 shows a graph of plasma concentration (log nM) against pERK %
inhibition in female nu/nu mice =
implanted with Co1o205 tumor cells. Mice were given a single dose of 2.5, 5,
10, or 25 mg/kg.
Figure 4 shows a graph of plasma concentration (ng/mL) against time (hours) in
humans after administration of a
single dose 2mg (2 x lmg capsules), 4mg (4 x lmg capsules) or 6ing (6 x lmg
capsules).
Figure 5 is a graph of a powder x-ray diffraction (PXR)) pattern of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide Form A, generated using a
Joel XRG-3000 diffiactometer. The graph plots the intensity of the peaks as
defined by counts per second versus the
diffraction angle 20 in degrees_
Figure 6 is a graph of a modulated Differential Scanning Calorirnerry (DSC)
thermogram of N-(S)-(3,4-difluoro-
2-(2-flusaro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide Form A
generated using a TA Instruments differential scanning calorimeter Q1000. The
graph plots the normalized heat flow in
units of Watts/gam (Wig) versus the measured sample temperature in C.
Figure 7 is a graph of the PXRD patterns of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide Form A (top)
and N-(S)-(3,4-dilluoro-2-(2-tiuoro-
- Page 31 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcycIopropane-1-
sulfonamide amorphous (bottom),
generated using a Inel XRG-3000 diffractometer. The graph plots the intensity
of the peaks as defined by counts per
second versus the diffraction angle 20 in degrees.
Figure 8 shows a Dynamic Vapor Sorption/Desorption (DVS) isotherm of N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-I-
sulfonamide Form A generated using a
VTI SGA-100 Vapor Sorption Analyzer.
Figure 9 shows a Thermogravimetry (TG) thermogram of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide Form A)
generated using a TA Instrument 2950
thermogravimetric analyzer.
Figure 10(a) and Figure 10(b) show growth arrest of Log phase dividing A375
cells exposed to increasing
concentrations of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropypcycloproparie-1-sulfonamide. Cells were analyzed for ATP
content. 100% growth arrest was
determined using 1pM N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide.
Figure 11 shows a 48 hr AK assay in A375 cells. Log phase dividing A375 cells
were exposed to N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide and
PD-325901 for 48 hr and analyzed for AK release.
Figures 12A-12C show N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide growth inhibition of (A) human
colorectal carcinoma Co1o205 cells ((I50
= 11 nM); (B) A375 cells (GI 22 nM) and (C) inhibition of MDA-MB231 cells
which do not show N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylarnino)-6-methoxyphenyI)- I -(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide -
induced growth arrest in 2-dimensional anchorage dependent assays.
Figure 13A shows inhibition of growth of human colorectal carcinoma Colo205
cells, with GI50 values at 6 riM
and 11 nM respectively.
Figure 13B shows inhibition of growth of A375 cells with GI50 values at 5 riM
and 22 nM.
Figures14A and Figure 14B show the effect of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane- 1-sulfonamide on cell
cycle progression, demonstrating that
exposure of A375 cells to N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylarnino)-
6-tnethoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide causes arrest in the GI phase of
the cell cycle, indicated by the depletion
of cells in both the G2 and S phases.
Figure 15A and Figure 15B show the effect of N-(S)-(3,4-ditluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide on the
stomach cancer (gastric adenocarcinoma)
cell line AGS after 3 days (Figure 15A) and 6 days (Figure 15B). The y axis is
the cell number relative to vehicle
and the x axis is uM of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide.
Figure 16 shows the mean liver weights in tumor bearing mice after treatment
with N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-
1-sulfonamide (2mg/kg, once
daily, po; 10mg/kg, once daily, po and 50mg/kg, once daily, po).
Figure 17 shows liver tumor weights in tumor bearing mice after treaement with
N-(S)-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide (2mg/kg, once daily, po;
10mg/kg, once daily, po and 50mg/Icg, once daily, po).
-Page 32-
CA 02693390 2015-12-04
30725-1660
Figure 18 shows the average tumor weights in after treactnent with N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)- I -(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide (2mg/kg; Om g/kg ; and
50mg/kg).
Figure 19 shows the inhibition of 1is746t Cell proliferation in a graph of
cell number (relative to vehicle) vs
concentration of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylaraino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide.
Figure 20A depicts a graph comparing the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-icxiophenylamino)-6-methoxypheny0-1-(2,3-
dihydroxypropyl)cyclopropane-1-sullonamide at day
5 of treatment of non-small cell lung cancer (NSCLC) 1v1V522 cells.
Figure 2013 is a graph demonstrating the respective apoptosis levels at
increasing concentrations N-(S)-(3,4-
ditluoro-2-(2-fluoro-4-iodophenylarnino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at day
5 of treatment of non-small cell lung cancer (NSCLC) H358 cells.
Figure 20C is a graph demonstrating the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4-
difinoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide at day
6 of treatment of non-small cell lung cancer (NSCLC) A549 cells.
Figure 2011 is a graph demonstrating the respective apoptosis leveLs at
increasing concentrations of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylatnino)-6-methoxypheny1)- I -(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide at day.
5 of treatment of non-small cell lung cancer (NSCLC) H727 cells.
Figure 20E is a graph demonstrating the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-i-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide at day
5 of treatment of colon HT29 cells.
Figure 20F is a graph demonstrating the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide at day
6 of treatment of colon HCT116 cells.
Figure 20G is a graph demonstrating the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4- =
difluoro-2-(2-fluoro-4-iodophenylamino)-6-rnethoxypheny1)- I -(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at day
5 of treatment of colon HUH7 Hepatoma cells.
Figure 20H is a graph depicting the respective apoptosis levels at increasing
concentrations of N-(S)-(3,4-
diflu oro-2-(2-fluoro-4-iodophenyl arnino)-6-methoxyphenyI)- I -(2,3-
dihydroxypropyl)cyclopropane-1-su lfonam i de at day
5 of treatment of Sarcoma-L/2-0S cells.
Figure 201 is a graph demonstrating the respective apoptosis levels at
increasing concentrations of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-io d ophenylamin o)-6-rnethoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide at day
5 of treatment of Cilioma D37 cells.
-Page 33-
CA 02693390 2015-12-04
30725-1660
10 DETAILED DESCRIPTION OF THE INVENTION
The section headings used herein are for organizational purposes only and are
not to be construed as limiting the
subject matter described.
Certain Chemical Terminology
Unless defined otherwise, all technical and scientific terms used herein have
the same meaning as is commonly
understood by one of skill in the art to which the claimed subject matter
belongs.
In the event that there is a plurality of definitions for terms herein, those
in this section prevail.
Where reference is made to a URL or other such identifier or address, it is
understood that such identifiers can change
and particular information on the internet can come and go, but equivalent
information can be found by searching the
intez-net or other appropriate reference source. Reference thereto evidences
the availability and public dissemination of
such information.
It is to be understood that the foregoing general description and the
following detailed description are exemplary
and explanatory only and are not restrictive of any subject matter claimed. In
this application, the use of the singular
includes the plural unless specifically stated otherwise. It must be noted
that, as used in the specification and the
appended claims, the singular forms "a", "an" and "the" include plural
referents unless the context clearly dictates
otherwise. It should also be noted that use of "or" means "and/or" unless
stated otherwise. Furthermore, use of the term
"including" as well as other forms, such as "include", "includes", and
"included" is not limiting.
Definition of standard chemistry terms may be found in reference works,
including Carey and Sundberg
"ADVANCED ORGANIC CHEMISTRY ei ED." Vols. A (2000) and B (2001), Plenum Press,
New York. Unless otherwise
indicated, conventional methods of mass spectroscopy, N/v1R, IIPLC, lR and
UV/Vis spectroscopy and pharmacology,
within the skill of the art are employed. Unless specific definitions are
provided, the nomenclature employed in
connection with, and the laboratory procedures and techniques of, analytical
chemistry, synthetic organic chemistry, and
medicinal and pharmaceutical chemistry described herein are those known in the
art. Standard techniques can be used for
chemical syntheses, chemical analyses, pharmaceutical preparation,
formulation, and delivery, and treatment of patients.
Reactions and purification techniques can be performed e.g., using kits of
manufacturer's specifications Or as commonly
accomplished in the art or as described herein. The foregoing techniques and
procedures can be generally performed of
conventional methods well known in the art and as described in various general
and more specific references that are
cited and discussed throughout the present specification. Throughout the
specification, groups and substituents thereof
can be chosen by one skilled in the field to provide stable moieties and
compounds.
- Pane 34 -
=
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Where substituent groups are specified by their conventional chemical
formulas, written from left to right, they
equally encompass the chemically identical substituents that would result from
writing the structure from right to left. As
a non-limiting example, -CH20- is equivalent to ¨0CH2-.
Unless otherwise noted, the use of general chemical terms, such as though not
limited to "alkyl," "amine," "aryl,"
are equivalent to their optionally substituted forms. For example, "alkyl," as
used herein, includes optionally substituted
alkyl.
The compounds presented herein may possess one or more stereocenters and each
center may exist in the R or S
configuration, or combinations thereof. Likewise, the compounds presented
herein may possess one or more double
bonds and each may exist in the E (trans) or Z (cis) configuration, or
combinations thereof. Presentation of one particular
stereoisomer, regioisomer, diastereomer, enantiomer or epimer should be
understood to include all possible
stereoisomers, regioisomers, diastereomers, enantiomers or epimers and
mixtures thereof. Thus, the compounds
presented herein include all separate configurational stereoisomeric,
regioisomerie, diastereomeric, enantiomeric, and
epimeric forms as well as the corresponding mixtures thereof. Presentation of
one particular chemical structure or
chemical name for a compound which contains one or more chiral centers, but
which does not designate a particular
stereochemistry, should be understood to include all possible stereoisomers,
including mixtures of all possible
stereoisomers, pure forms or substantially pure forms of one particular
stereoisomer and pure forms or substantially pure
forms of the alternate stereoisomer. Techniques for inverting or leaving
unchanged a particular stereocenter, and those
for resolving mixtures of stereoisomers are well known in the art and it is
well within the ability of one of skill in the art
to choose an appropriate method for a particular situation. See, for example,
Furniss et al. (eds.), VOGEL'S
ENCYCLOPEDIA OF PRACTICAL ORGANIC CHEMISTRY 5TH ED., Longman Scientific
and Technical Ltd.,
Essex, 1991, 809-816; and Heller, Ace. Chem. Res. 1990, 23, 128.
The terms "moiety", "chemical moiety", "group" and "chemical group", as used
herein refer to a specific segment
or functional group of a molecule. Chemical moieties are often recognized
chemical entities embedded in or appended to
a molecule.
The term "bond" or "single bond" refers to a chemical bond between two atoms,
or two moieties when the atoms
joined by the bond are considered to be part of larger substructure.
The term "optional" or "optionally" means that the subsequently described
event or circumstance may or may not
occur, and that the description includes instances where said event or
circumstance occurs and instances in which it does
not. For example, "optionally substituted alkyl" means either "alkyl" or
"substituted alkyl" as defined below. Further, an
optionally substituted group may be un-substituted (e.g., -CH2CH3), fully
substituted (e.g., -CF2CF3), mono-substituted
(e.g., -CH2CH2F) or substituted at a level anywhere in-between fully
substituted and mono-substituted (e.g., -CH2CHF2, -
CH2CF3, -CF2CH3, -CFHCHF2, etc). It will be understood by those skilled in the
art with respect to any group containing
one or more substituents that such groups are not intended to introduce any
substitution or substitution patterns (e.g.,
substituted alkyl includes optionally substituted cycloalkyl groups, which in
turn are defined as including optionally
substituted alkyl groups, potentially ad infinitum) that are sterically
impractical and/or synthetically non-feasible. Thus,
any substituents described should generally be understood as having a maximum
molecular weight of about 1,000
daltons, and more typically, up to about 500 daltons (except in those
instances where macromolecular substituents are
clearly intended, e.g., polypeptides, polysaccharides, polyethylene glycols,
DNA, RNA and the like).
Unless otherwise noted, the use of general chemical terms, such as though not
limited to "alkyl," "amine," "aryl,"
are unsubstituted.
-Page 35-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
As used herein, C1-Cõ includes C1-C2, C1-C3. . Ci-C.x. By way of example only,
a group designated as "C1-C4"
indicates that there are one to four carbon atoms in the moiety, i.e. groups
containing 1 carbon atom, 2 carbon atoms, 3
carbon atoms or 4 carbon atoms, as well as the ranges C1-C2 and Ci-C3. Thus,
by way of example only, "C1-C4 alkyl"
indicates that there are one to four carbon atoms in the alkyl group, i.e.,
the alkyl group is selected from among methyl,
ethyl, propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, and t-butyl.
Whenever it appears herein, a numerical range such as
"1 to 10" refers to each integer in the given range; e.g., "1 to 10 carbon
atoms" means that the group may have 1 carbon
atom, 2 carbon atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms, 6 carbon
atoms, 7 carbon atoms, 8 carbon atoms,
9 carbon atoms, or 10 carbon atoms.
The term "A and A', together with the carbon atom to which they are attached,
form a 3- to 6- member saturated
ring ", as used herein, refers to the following structures for compounds of
formula I:
B
4:0 C1:0
0" NH 0' NH 0" NH 0' NH
The terms "heteroatom" or "hetero" as used herein, alone or in combination,
refer to an atom other than carbon or
hydrogen. Heteroatoms are may be independently selected from among oxygen,
nitrogen, sulfur, phosphorous, silicon,
selenium and tin but are not limited to these atoms. In embodiments in which
two or more heteroatoms are present, the
two or more heteroatoms can be the same as each another, or some or all of the
two or more heteroatoms can each be
different from the others.
The term "alkyl" as used herein, alone or in combination, refers to a straight-
chain or branched-chain saturated
hydrocarbon monoradical having from one to about ten carbon atoms, or one to
six carbon atoms. Examples include, but
are not limited to methyl, ethyl, n-propyl, isopropyl, 2-methyl-1-propyl, 2-
methyl-2-propyl, 2-methyl-1-butyl, 3-methyl-
1-butyl, 2-methyl-3-butyl, 2,2-dimethyl-1-propyl, 2-methyl-l-pentyl, 3-methyl-
l-pentyl, 4-m ethyl-l-pentyl, 2-methy1-2-
pentyl, 3-methyl-2-pentyl, 4-methyl-2-pentyl, 2,2-dimethy1-1-butyl, 3,3-
dimethyl-l-butyl, 2-ethyl-I-butyl, n-butyl,
isobutyl, sec-butyl, t-butyl, n-pentyl, isopentyl, neopentyl, tert-amyl and
hexyl, and longer alkyl groups, such as heptyl,
octyl and the like. Whenever it appears herein, a numerical range such as "C1-
C6 alkyl" or "Ci.6 alkyl", means that the
alkyl group may consist of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, 4
carbon atoms, 5 carbon atoms or 6 carbon
atoms. In one embodiment, the "alkyl" is substituted. Unless otherwise
indicated, the "alkyl" is unsubstititued.
The term "alkenyl" as used herein, alone or in combination, refers to a
straight-chain or branched-chain
hydrocarbon monoradical having one or more carbon-carbon double-bonds and
having from two to about ten carbon
atoms, or two to about six carbon atoms. The group may be in either the cis or
trans conformation about the double
bond(s), and should be understood to include both isomers. Examples include,
but are not limited to ethenyl (-CH=CH2),
1-propenyl (-CH2CH=CH2), isopropenyl [-C(CH3)=CH2], butenyl, 1,3-butadienyl
and the like. Whenever it appears
herein, a numerical range such as "C2-C6 alkenyl" or "C2.6 alkenyl", means
that the alkenyl group may consist of 2 carbon
atoms, 3 carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms. In
one embodiment, the "alkenyl" is
substituted. Unless otherwise indicated, the "alkenyl" is unsubstititued.
The term "alkynyl" as used herein, alone or in combination, refers to a
straight-chain or branched-chain
hydrocarbon monoradical having one or more carbon-carbon triple-bonds and
having from two to about ten carbon
atoms, or from two to about six carbon atoms. Examples include, but are not
limited to ethynyl, 2-propynyl, 2-butynyl,
1,3-butadiynyl and the like. Whenever it appears herein, a numerical range
such as "C2-C6 alkynyl" or "C2_6 alkynyl",
means that the alkynyl group may consist of 2 carbon atoms, 3 carbon atoms, 4
carbon atoms, 5 carbon atoms or 6 carbon
atoms. In one embodiment, the "alkynyl" is substituted. Unless otherwise
indicated, the "alkynyl" is unsubstititued.
-Page 36-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The terms "heteroalkyl", "heteroalkenyl" and "heteroalkynyl" as used herein,
alone or in combination, refer to
alkyl, alkenyl and alkynyl structures respectively, as described above, in
which one or more of the skeletal chain carbon
atoms (and any associated hydrogen atoms, as appropriate) are each
independently replaced with a heteroatom (i.e. an
atom other than carbon, such as though not limited to oxygen, nitrogen,
sulfur, silicon, phosphorous, tin or combinations
thereof), or heteroatomic group such as though not limited to -0-0-, -S-S-, -0-
S-, -S-0-, =N-N=, -N=N-, -N=N-NH-, -
P(0)2-, -0-P(0)2-, -P(0)2-0-, -S(0)-, -S(0)2-, -SnH2- and the like.
The terms "haloallcyl", "haloalkenyl" and "haloallcynyl" as used herein, alone
or in combination, refer to alkyl,
alkenyl and alkynyl groups respectively, as defmed above, in which one or more
hydrogen atoms is replaced by fluorine,
chlorine, bromine or iodine atoms, or combinations thereof. In some
embodiments two or more hydrogen atoms may be
replaced with halogen atoms that are the same as each another (e.g.
difluoromethyl); in other embodiments two or more
hydrogen atoms may be replaced with halogen atoms that are not all the same as
each other (e.g. 1-chloro-l-fluoro-l-
iodoethyD. Non-limiting examples of haloalkyi groups are fluoromethyl,
chlorornethyl and brornoethyl. A non-limiting
example of a haloalkenyl group is bromoethenyl. A non-limiting example of a
haloallcynyl group is chloroethynyl.
The term "carbon chain" as used herein, alone or in combination, refers to any
alkyl, alkenyl, alkynyl, heteroalkyl,
heteroalkenyl or heteroalkynyl group, which is linear, cyclic, or any
combination thereof. If the chain is part of a linker
and that linker comprises one or more rings as part of the core backbone, for
purposes of calculating chain length, the
"chain" only includes those carbon atoms that compose the bottom or top of a
given ring and not both, and where the top
and bottom of the ring(s) are not equivalent in length, the shorter distance
shall be used in determining the chain length.
If the chain contains heteroatoms as part of the backbone, those atoms are not
calculated as part of the carbon chain
length.
The terms "cycle", "cyclic", "ring" and "membered ring" as used herein, alone
or in combination, refer to any
covalently closed structure, including alicyclic, heterocyclic, aromatic,
heteroaromatic and polycyclic fused or non-fused
ring systems as described herein. Rings can be optionally substituted. Rings
can form part of a fused ring system. The
term "membered" is meant to denote the number of skeletal atoms that
constitute the ring. Thus, by way of example only,
cyclohexane, pyridine, pyran and pyrimidine are six-membered rings and
cyclopentane, pyrrole, tetrahydrofuran and
thiophene are five-membered rings.
The term "fused" as used herein, alone or in combination, refers to cyclic
structures in which two or more rings
share one or more bonds.
The term "cycloalkyl" as used herein, alone or in combination, refers to a
saturated, hydrocarbon monoradical ring,
containing from three to about fifteen ring carbon atoms or from three to
about ten ring carbon atoms, though may
include additional, non-ring carbon atoms as substituents (e.g.
methylcyclopropyl). Whenever it appears herein, a
numerical range such as "C3-C6 cycloalkyl" or "C3_,6 cycloalkyl ", means that
the cycloalkyl group may consist of 3
carbon atoms, 4 carbon atoms, 5 carbon atoms or 6 carbon atoms, i.e., is
cyclopropyl, cycIobutyl, cyclopentyl or
cyclohepty, although the present definition also covers the occurrence of the
term" cycloalkyl" where no numerical
range is designated. The term includes fused, non-fused, bridged and spiro
radicals. A fused cycloalkyl may contain from
two to four fused rings where the ring of attachment is a cycloalkyl ring, and
the other individual rings may be alicyclic,
heterocyclic, aromatic, heteroaromatic or any combination thereof. Examples
include, but are not limited to cyclopropyI,
cyclopentyl, cyclohexyl, decalinyl, and bicyclo [2.2.1] heptyl and adamantyl
ring systems. Illustrative examples include,
but are not limited to the following moieties:
Page 37-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
CI> 0=1 CC
11:7
and the like.
In one embodiment, the "cycloalkyl" is substituted. Unless otherwise
indicated, the "cycloalkyl" is unsubstititued.
The terms "non-aromatic heterocycly1" and "heteroalicyclyr as used herein,
alone or in combination, refer to a
saturated, partially unsaturated, or fully unsaturated nonaromatic ring
monoradicals containing from three to about
twenty ring atoms, where one or more of the ring atoms are an atom other than
carbon, independently selected from
among oxygen, nitrogen, sulfur, phosphorous, silicon, selenium and tin but are
not limited to these atoms. In
embodiments in which two or more heteroatoms are present in the ring, the two
or more heteroatoms can be the same as
each another, or some or all of the two or more heteroatoms can each be
different from the others. The terms include
fused, non-fused, bridged and spiro radicals. A fused non-aromatic
heterocyclic radical may contain from two to four
fused rings where the attaching ring is a non-aromatic heterocycle, and the
other individual rings may be alicyclic,
heterocyclic, aromatic, heteroaromatic or any combination thereof. Fused ring
systems may be fused across a single bond
or a double bond, as well as across bonds that are carbon-carbon, carbon-
hetero atom or hetero atom-hetero atom. The
terms also include radicals having from three to about twelve skeletal ring
atoms, as well as those having from three to
about ten skeletal ring atoms. Attachment of a non-aromatic heterocyclic
subunit to its parent molecule can be via a
heteroatom or a carbon atom. Likewise, additional substitution can be via a
heteroatom or a carbon atom. As a non-
limiting example, an imida7olidine non-aromatic heterocycle may be attached to
a parent molecule via either of its N
atoms (imidazolidin-1-y1 or imida7olidin-3-y1) or any of its carbon atoms
(imidazolidin-2-yl, imidazolidin-4-y1 or
imidazolidin-5-y1). In certain embodiments, non-aromatic heterocycles contain
one or more carbonyl or thiocarbonyl
groups such as, for example, oxo- and thio-containing groups. Examples
include, but are not limited to pyrrolidinyl,
tetrahydrofuranyl, dihydrofuranyl, tetrahydrothienyl, tetrahydropyranyl,
dihydropyranyl, tetrahydrothiopyranyl,
piperidino, morpholino, thiomorpholino, thioxanyl, piperazinyl, azetidinyl,
oxetanyl, thietanyl, homopiperidinyl,
oxepanyl, thiepanyl, oxazepinyl, diazepinyl, thiazepinyl, 1,2,3,6-
tetrahydropyridinyl, 2-pyrrolinyl, 3-pyrrolinyl,
indolinyl, 2H-pyranyl, 4H-pyranyl, dioxanyl, 1,3-dioxolanyl, pyrazolinyl,
dithianyl, dithiolanyl, dihydropyranyl,
dihydrothienyl, dihydrofuranyl, pyrazolidinyl, imila7olinyl, imidazolidinyl, 3-
azabicyclo[3.1.01hexanyl, 3-
azabicyclo[4.1.0]heptanyl, 3H-indoly1 and quinolizinyl. Illustrative examples
of heterocycloalkyl groups, also referred to
as non-aromatic heterocycles, include:
, \--0 0,N,
I I I
0 s ii
c
1-NH HN-NH
0
0
N 0
0 00 0 0õ0 0 0 0
crko ' HVIINNH ' (lc ' , HN 0 ,
and the like.
-Page 38-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The terms also include all ring forms of the carbohydrates, including but not
limited to the monosaccharides, the
disaccharides and the oligosaccharides. In one embodiment, the "non-aromatic
heterocycly1" or "heteroalicycly1" is
substituted. Unless otherwise indicated, the "non-aromatic heterocyclyr or
"heteroalicyclyr is unsubstititued.
The term "aryl" as used herein, alone or in combination, refers to an aromatic
hydrocarbon radical of six to about
twenty ring carbon atoms, and includes fused and non-fused aryl rings. A fused
aryl ring radical contains from two to
four fused rings where the ring of attachment is an aryl ring, and the other
individual rings may be alicyclic, heterocyclic,
aromatic, heteroaromatic or any combination thereof. Further, the term aryl
includes fused and non-fused rings
containing from six to about twelve ring carbon atoms, as well as those
containing from six to about ten ring carbon
atoms. A non-limiting example of a single ring aryl group includes phenyl; a
fused ring aryl group includes naphthyl,
phenanthrenyl, anthracenyl, azulenyl; and a non-fused bi-aryl group includes
biphenyl. In one embodiment, the "aryl" is
substituted. Unless otherwise indicated, the "aryl" is unsubstititued.
The term "heteroaryl" as used herein, alone or in combination, refers to an
aromatic monoradicals containing from
about five to about twenty skeletal ring atoms, where one or more of the ring
atoms is a heteroatom independently
selected from among oxygen, nitrogen, sulfur, phosphorous, silicon, selenium
and tin but not limited to these atoms and
with the proviso that the ring of said group does not contain two adjacent 0
or S atoms. In embodiments in which two or
more heteroatoms are present in the ring, the two or more heteroatoms can be
the same as each another, or some or all of
the two or more heteroatoms can each be different from the others. The term
heteroaryl includes fused and non-fused
heteroaryl radicals having at least one heteroatom. The term heteroaryl also
includes fused and non-fused heteroaryls
having from five to about twelve skeletal ring atoms, as well as those having
from five to about ten skeletal ring atoms.
Bonding to a heteroaryl group can be via a carbon atom or a heteroatom. Thus,
as a non-limiting example, an imidazole
group may be attached to a parent molecule via any of its carbon atoms
(imidazol-2-yl, imidazol-4-y1 or imidazol-5-y1),
or its nitrogen atoms (imidazol-1-y1 or imidazol-3-yD. Likewise, a heteroaryl
group may be further substituted via any or
all of its carbon atoms, and/or any or all of its heteroatoms. A fused
heteroaryl radical may contain from two to four
fused rings where the ring of attachment is a heteroaromatic ring and the
other individual rings may be alicyclic,
heterocyclic, aromatic, heteroaromatic or any combination thereof. A non-
limiting example of a single ring heteroaryl
group includes pyridyl; fused ring heteroaryl groups include benzimidazolyl,
quinolinyl, acridinyl; and a non-fused bi-
heteroaryl group includes bipyridinyl. Further examples of heteroaryls
include, without limitation, furanyl, thienyl,
oxazolyl, acridinyl, phenazinyl, benzimidazolyl, benzofuranyl, benzoxazolyl,
benzothiazolyl, benzothiadiazolyl,
benzothiophenyl, benzoxadiazolyl, benzotriazolyl, imidazolyl, indolyl,
isoxazolyl, isoquinolinyl, indolizinyl, isothiazolyI,
isoindolyloxadiazolyl, indazolyl, pyridyl, pyridazyl, pyrimidyl, pyrazinyl,
pyrrolyl, pyrazinyl, pyrazolyl, purinyl,
phthalazinyl, pteridinyl, quinolinyl, quinazolinyl, quinoxalinyl, triazolyl,
tetrazolyl, thiazolyl, triazinyl, thiadiazolyl and
the like, and their oxides, such as for example pyridyl-N-oxide. Illustrative
examples of heteroaryl groups include the
following moieties:
NH , /INH/IN
N N N ______________________________________
s
/
is( s
=
N/ Ne 40 _lb
N N 11111111 7 and the
like.
In one embodiment, the "heteroaryl" is substituted. Unless otherwise
indicated, the "heteroaryl" is unsubstititued.
-Page 39-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The term "heterocycly1" as used herein, alone or in combination, refers
collectively to heteroalicyclyI and
heteroaryI groups. Herein, whenever the number of carbon atoms in a
heterocycle is indicated (e.g., C1-C6 heterocycle),
at least one non-carbon atom (the heteroatom) must be present in the ring.
Designations such as "C1-C6 heterocycle" refer
only to the number of carbon atoms in the ring and do not refer to the total
number of atoms in the ring. Designations
such as "4-6 membered heterocycle" refer to the total number of atoms that are
contained in the ring (i.e., a four, five, or
six membered ring, in which at least one atom is a carbon atom, at least one
atom is a heteroatom and the remaining two
to four atoms are either carbon atoms or heteroatoms). For heterocycles having
two or more heteroatoms, those two or
more heteroatoms can be the same or different from one another. Non-aromatic
heterocyclic groups include groups
having only three atoms in the ring, while aromatic heterocyclic groups must
have at least five atoms in the ring. Bonding
(i.e. attachment to a parent molecule or further substitution) to a
heterocycle can be via a heteroatom or a carbon atom.
In one embodiment, the "heterocycly1" is substituted. Unless otherwise
indicated, the "heterocycyl" is unsubstititued.
The terms "halogen", "halo" or "halide" as used herein, alone or in
combination refer to fluoro, chloro, bromo
and/or iodo.
The term "amino" as used herein, alone or in combination, refers to the
monoradical -NH2.
The term "alkylamino" as used herein, alone or in combination, refers to the
monoradical -NH(alkyl) where alkyl
is as defined herein.
The term "diallglamino" as used herein, alone or in combination, refers to the
monoradical -N(allcyl)(alkyl) where
each alkyl may be identical or non-identical and is as defmed herein.
The term "diamino alkyl" as used herein, alone or in combination, refers to an
alkyl group containing two amine
groups, wherein said amine groups may be substituents on the alkyl group which
may be amino, alkylamino, or
dialkylamino groups, or wherein one or both of said amine groups may form part
of an alkyl chain to form -allcylene-N(H
or alkyl)-alkylene-N(H or alkyl or alkylene-)(H or alkyl or alkylene-).
The term "hydroxy" as used herein, alone or in combination, refers to the
monoradical -OH.
The term "cyano" as used herein, alone or in combination, refers to the
monoradical -CN.
The term "cyanomethyl" as used herein, alone or in combination, refers to the
monoradical -CH2CN.
The term "nitro" as used herein, alone or in combination, refers to the
monoradical -NO2.
The term "oxy" as used herein, alone or in combination, refers to the
diradical -0-.
The term "oxo" as used herein, alone or in combination, refers to the
diradical =0.
The term "carbonyl" as used herein, alone or in combination, refers to the
diradical -C(=0)-, which may also be
written as -C(0)-.
The terms "carboxy" or "carboxyl" as used herein, alone or in combination,
refer to the moiety -C(0)0H, which
may also be written as -COOH.
The term "alkoxy" as used herein, alone or in combination, refers to an alkyl
ether radical, -0-alkyl, including the
groups -0-aliphatic and -0-carbocyclyl, wherein the alkyl, aliphatic and
carbocyclyl groups may be optionally
substituted, and wherein the terms alkyl, aliphatic and carbocyclyl are as
defined herein. Non-limiting examples of
alkoxy radicals include methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, iso-
butoxy, sec-butoxy, tert-butoxy and the
like.
The term "sulfmyl" as used herein, alone or in combination, refers to the
diradical -S(=0)-.
The term "sulfonyl" as used herein, alone or in combination, refers to the
diradical -S(=0)2-.
The terms "sulfonamide", "sulfonamido" and "sulfonamidyl" as used herein,
alone or in combination, refer to the
diradical groups -S(0)2-NH- and ¨NH-S(=0)2-=
-Page 40-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The terms "sulfamide", "sulfamido" and "sulfamidyl" as used herein, alone or
in combination, refer to the diradical
group -NH-S(----0)2-NH-.
The term "reactant," as used herein, refers to a nucleophiie or electrophile
used to create covalent linkages.
It is to be understood that in instances where two or more radicals are used
in succession to define a substituent
attached to a structure, the first named radical is considered to be terminal
and the last named radical is considered to be
attached to the structure in question. Thus, for example, the radical
arylallcyl is attached to the structure in question by the
alkyl group.
Certain Pharmaceutical Terminology
The term "MEK inhibitor" as used herein refers to a compound that exhibits an
IC 50 with respect to MEK activity,
of no more than about 100gM or not more than about 50 M, as measured in the
Mekl kinase assay described generally
herein. "IC50" is that concentration of inhibitor which reduces the activity
of an enzyme (e.g., MEK) to half-maximal
level. Compounds described herein have been discovered to exhibit inhibition
against MEK. Compounds of the present
invention preferably exhibit an IC50with respect to MEK of no more than about
10gM, more preferably, no more than
about 511M, even more preferably not more than about 1gM, and most preferably,
not more than about 200nM, as
measured in the Mekl kinase assay described herein.
The term "subject", "patient" or "individual" as used herein in reference to
individuals suffering from a disorder,
and the like, encompasses mammals and non-mammals. Examples of mammals
include, but are not limited to, any
member of the Mammalian class: humans, non-human primates such as chimpanzees,
and other apes and monkey
species; farm animals such as cattle, horses, sheep, goats, swine; domestic
animals such as rabbits, dogs, and cats;
laboratory animals including rodents, such as rats, mice and guinea pigs, and
the like. Examples of non-mammals
include, but are not limited to, birds, fish and the like. In one embodiment
of the methods and compositions provided
herein, the mammal is a human.
The terms "treat," "treating" or "treatment," and other grammatical
equivalents as used herein, include alleviating,
abating or ameliorating a disease or condition symptoms, preventing additional
symptoms, ameliorating or preventing the
underlying metabolic causes of symptoms, inhibiting the disease or condition,
e.g., arresting the development of the
disease or condition, relieving the disease or condition, causing regression
of the disease or condition, relieving a
condition caused by the disease or condition, or stopping the symptoms of the
disease or condition, and are intended to
include prophylaxis. The terms further include achieving a therapeutic benefit
and/or a prophylactic benefit. By
therapeutic benefit is meant eradication or amelioration of the underlying
disorder being treated. Also, a therapeutic
benefit is achieved with the eradication or amelioration of one or more of the
physiological symptoms associated with the
underlying disorder such that an improvement is observed in the patient,
notwithstanding that the patient may still be
afflicted with the underlying disorder. For prophylactic benefit, the
compositions may be administered to a patient at risk
of developing a particular disease, or to a patient reporting one or more of
the physiological symptoms of a disease, even
though a diagnosis of this disease may not have been made.
The terms "effective amount", "therapeutically effective amount" or
"pharmaceutically effective amount" as used
herein, refer to an amount of at least one agent or compound being
administered that is sufficient to treat or prevent the
particular disease or condition. The result can be reduction and/or
alleviation of the signs, symptoms, or causes of a
disease, or any other desired alteration of a biological system. For example,
an "effective amount" for therapeutic uses is
the amount of the composition comprising a compound as disclosed herein
required to provide a clinically significant
decrease in a disease. An appropriate "effective" amount in any individual
case may be determined using techniques,
such as a dose escalation study.
-Page 41-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The terms "substantially free of water" and "substantially free of solvent" as
used herein, refer to crystalline
polymorph forms comprising less than 0.01, 0.1, 0.2, 0.3, 0.4, 0.5, 1 or 2% by
weight of water or solvent respectively.
The term "substantially the same as" as used herein, refers to a powder x-ray
diffraction pattern or differential
scanning calorimetry pattern that may be non-identical to those depicted
herein, but that falls within the limits of
experimental error, when considered by one of ordinary skill in the art.
The terms "administer," "administering", "administration," and the like, as
used herein, refer to the methods that
may be used to enable delivery of compounds or compositions to the desired
site of biological action. These methods
include, but are not limited to oral routes, intraduodenal routes, parenteral
injection (including intravenous, subcutaneous,
intraperitoneal, intramuscular, intravascular or infusion), topical and rectal
administration. Those of skill in the art are
familiar with administration techniques that can be employed with the
compounds and methods described herein, e.g., as
discussed in Goodman and Gilman, The Pharmacological Basis of Therapeutics,
current ed.; Pergamon; and
Remington's, Pharmaceutical Sciences (current edition), Mack Publishing Co.,
Easton, Pa. In preferred embodiments, the
compounds and compositions described herein are administered orally.
The term "acceptable" as used herein, with respect to a formulation,
composition or ingredient, means having no
persistent detrimental effect on the general health of the subject being
treated.
The term "pharmaceutically acceptable" as used herein, refers to a material,
such as a carrier or diluent, which does
not abrogate the biological activity or properties of the compounds described
herein, and is relatively nontoxic, i.e., the
material may be administered to an individual without causing undesirable
biological effects or interacting in a
deleterious manner with any of the components of the composition in which it
is contained.
The term "pharmaceutical composition," as used herein, refers to a
biologically active compound, optionally mixed
with at least one pharmaceutically acceptable chemical component, such as,
though not limited to carriers, stabilizers,
diluents, dispersing agents, suspending agents, thickening agents, and/or
excipients.
The term "carrier" as used herein, refers to relatively nontoxic chemical
compounds or agents that facilitate the
incorporation of a compound into cells or tissues.
The term "agonist," as used herein, refers to a molecule such as a compound, a
drug, an enzyme activator or a
hormone modulator which enhances the activity of another molecule or the
activity of a receptor site.
The term "antagonist," as used herein, refers to a molecule such as a
compound, a drug, an enzyme inhibitor, or a
hormone modulator, which diminishes, or prevents the action of another
molecule or the activity of a receptor site.
The term "modulate," as used herein, means to interact with a target either
directly or indirectly so as to alter the
activity of the target, including, by way of example only, to enhance the
activity of the target, to inhibit the activity of the
target, to limit the activity of the target, or to extend the activity of the
target.
The term "modulator," as used herein, refers to a molecule that interacts with
a target either directly or indirectly.
The interactions include, but are not limited to, the interactions of an
agonist and an antagonist.
The term "pharmaceutically acceptable derivative or prodrug" as used herein,
refers to any pharmaceutically
acceptable salt, ester, salt of an ester or other derivative of a compound of
formula 1, which, upon administration to a
recipient, is capable of providing, either directly or indirectly, a compound
of this invention or a pharmaceutically active
metabolite or residue thereof. Particularly favored derivatives or prodrugs
are those that increase the bioavailability of the
compounds of this invention when such compounds are administered to a patient
(e.g., by allowing orally administered
compound to be more readily absorbed into blood) or which enhance delivery of
the parent compound to a biological
compartment (e.g., the brain or lymphatic system).
-Page 42-
CA 02693390 2015-12-04
30725-1660
As used herein, a "prodmg" is a compound that may be converted under
physiological conditions or by solvolysis
to the specified compound or to a pharmaceutically acceptable salt of such
compound. Prodrugs include compounds
wherein an amino acid residue, or a polypeptide chain of two or more amino
acid residues, is covalently joined through
an amide or ester bond to a free amino, hydroxy, or carboxylic acid group of
compounds of Formulas I. The amino acid
residues contemplated include but are not limited to the 20 naturally-
occurring amino acids. Other suitable amino acids
include 4-hydroxyproline, hydroxylysine, demosine, isodemosine, 3-methyl
histidine, norvaline, I3-alanine, 7-
aminobutyric acid, cirtulline, honnocysteine, homoserine, ornithine and
methionine sulfone. Additional types of prodrugs
are well known in the art.
Pharmaceutically acceptable prodrugs of the compounds described herein
include, but are not limited to, esters,
carbonates, thiocarbonates, N-acyl derivatives, N-acyloxyalkyl derivatives,
quaternary derivatives of tertiary amines, N-
Mannich bases, Schiff bases, amino acid conjugates, phosphate esters, metal
salts and sulfonme esters. Various forms of
prodrugs are well known in the art. See for example Design of Prodrugs,
13uridgaard, A. Ed., Elseview, 1985 and Method
in Enzymology, Widder, K. etal., Ed.; Academic, 1985, vol. 42, p. 309-396;
Bundgaard, H. "Design and Application of
Prodrugs" in A Textbook of Drug Design and Development, Krosgaard-Larsen and
H. Bundgaard, Ed., 1991, Chapter 5,
p. 113-191; and Bundgaard, R., Advanced Drug Delivery Review, 1992, 8, 1-38.
The prodrugs described herein include, but are not limited to, the following
gimps and combinations of these
groups; amine derived prodrugs:
R )L ,R ,R
¨N ¨N 0 --N S ¨ N-11'0" ¨N S ¨NOR ¨N 0 0-'13
N ¨ et' R R N -.1".SAR R \ISA R
H H 141 111
N
R S
R ,R )1, .)( R R
¨N 0 S ¨N 0 ¨Nos ¨N S ¨N S
$¨so
111
Hydroxy prodrugs include, but are not limited to acyloxyalkyl esters,
alkoxycarbonyloxyalkyl esters, alkyl esters, aryl
esters and disulfide containing esters.
The term "pharmaceutically acceptable salt" as used herein, includes salts
that retain the biological effectiveness of
the free acids and bases of the specified compound and that are not
biologically or otherwise undesirable. Compounds
described may possess acidic or basic groups and therefore may react with any
of a number of inorganic or organic bases,
and inorganic and organic acids, to form a pharmaceutically acceptable salt.
Examples of pharmaceutically acceptable
salts include those salts prepared by reaction of the compounds described
herein with a mineral or organic acid or an
inorganic base, such salts including, acetate, acrylate, adipate, alginate,
aspartate, benzoate, henzenesulfonate, bisulfate,
bisulfite, bromide, butyrate, butyn-1,4-dioate, camphorate, carnphorsulfonate,
caproate, caprylate, chlorides,
chlorobenzoate, chloride, citrate, cyclopentanepropionatc, decanoate,
digluconate, dihyclrogenphosphate, dinitrobenzoate,
dodecylsulfate, ethanesulfonate, tbrtnate, furnarate, glucoheptanoate,
glycerophosphate, glycolate, hemisulfate,
heptanoate, hexanoate, hexyrie-1,6-dioate, hych-oxybenzoate, y-
hydroxybutyrate, hydrochloride, hydrobromide,
hydroiodide, 2-hydroxyethane,sulfonate, iodide, isobutyrate, lactate, maleate,
malonate, methanesulfonate, tnandelate.
metaphosphate, inethanesulfonate, methoxybenzoate, methylbenzoate,
rnonohydrogeriphosphate, 1-napthale.mesulfortate,
2-napthalenesulfonate, nicotinate, nitrate, oxalates, palmoate, pectinate,
persulfate, pbeaylacetates, phanylpropionates,
phenylpropionate, phosphate, picrate, pivalate, propionate, pyrosulfate,
pyrophosphate, propiolate, propionates, phthalate,
- Page 43 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
, phenylbutyrate, propanesulfonate, pyrophosphates, salicylate, succinate,
sulfate, sulfite, succinate, suberate, sebacate,
sulfonate, tartrate, thiocyanate, tosylate undeconate and xylenesulfonate.
Other acids, such as oxalic, while not in
themselves pharmaceutically acceptable, may be employed in the preparation of
salts useful as intermediates in obtaining
the compounds of the invention and their pharmaceutically acceptable acid
addition salts. (See for example Berge et al.,
J Pharra Sc!. 1977, 66, 1-19.) Further, those compounds described herein which
may comprise a free acid group may
react with a suitable base, such as the hydroxide, carbonate or bicarbonate of
a pharmaceutically acceptable metal cation,
with ammonia, or with a pharmaceutically acceptable organic primary, secondary
or tertiary amine. Representative alkali
or alkaline earth salts include the lithium, sodium, potassium, calcium,
magnesium, and aluminum salts and the like.
Illustrative examples of bases include sodium hydroxide, potassium hydroxide,
choline hydroxide, sodium carbonate,
N-1-(C1.4 alky1)4, and the like. Representative organic amines useful for the
formation of base addition salts include
ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine,
piperazine and the like. It should be
understood that the compounds described herein also include the quaternization
of any basic nitrogen-containing groups
they may contain. Water or oil-soluble or dispersible products may be obtained
by such quatemization. See, for example,
Berge etal., supra. These salts can be prepared in situ during the final
isolation and purification of the compounds of the
invention, or by separately reacting a purified compound in its free base form
with a suitable organic or inorganic acid,
and isolating the salt thus formed.
The terms "enhance" or "enhancing," as used herein, means to increase or
prolong either in potency or duration a
desired effect. Thus, in regard to enhancing the effect of therapeutic agents,
the term "enhancing" refers to the ability to
increase or prolong, either in potency or duration, the effect of other
therapeutic agents on a system. An "enhancing-
effective amount," as used herein, refers to an amount adequate to enhance the
effect of another therapeutic agent in a
desired system.
The terms "pharmaceutical combination", "administering an additional therapy",
"administering an additional
therapeutic agent" and the like, as used herein, refer to a pharmaceutical
therapy resulting from the mixing or combining
of more than one active ingredient and includes both fixed and non-fixed
combinations of the active ingredients. The
term "fixed combination" means that at least one of the compounds described
herein, and at least one co-agent, are both
administered to a patient simultaneously in the form of a single entity or
dosage. The term "non-fixed combination"
means that at least one of the compounds described herein, and at least one co-
agent, are administered to a patient as
separate entities either simultaneously, concurrently or sequentially with
variable intervening time limits, wherein such
administration provides effective levels of the two or more compounds in the
body of the patient. These also apply to
cocktail therapies, e.g. the administration of three or more active
ingredients.
The terms "co-administration", "administered in combination with" and their
grammatical equivalents or the like,
as used herein, are meant to encompass administration of the selected
therapeutic agents to a single patient, and are
intended to include treatment regimens in which the agents are administered by
the same or different route of
administration or at the same or different times. In some embodiments the
compounds described herein will be co-
administered with other agents. These terms encompass administration of two or
more agents to an animal so that both
agents and/or their metabolites are present in the animal at the same time.
They include simultaneous administration in
separate compositions, administration at different times in separate
compositions, and/or administration in a composition
in which both agents are present. Thus, in some embodiments, the compounds of
the invention and the other agent(s) are
administered in a single composition. In some embodiments, compounds of the
invention and the other agent(s) are
admixed in the composition.
- Page 44 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The term "metabolite," as used herein, refers to a derivative of a compound
which is formed when the compound is
metabolized.
The term "active metabolite," as used herein, refers to a biologically active
derivative of a compound that is
formed when the compound is metabolized.
The term "metabolized," as used herein, refers to the sum of the processes
(including, but not limited to, hydrolysis
reactions and reactions catalyzed by enzymes) by which a particular substance
is changed by an organism. Thus,
enzymes may produce specific structural alterations to a compound. For
example, cytochrome P450 catalyzes a variety of
oxidative and reductive reactions while uridine diphosphate
glucuronyltransferases catalyze the transfer of an activated
glucuronic-acid molecule to aromatic alcohols, aliphatic alcohols, carboxylic
acids, amines and free sulphydryl groups.
Further information on metabolism may be obtained from The Pharmacological
Basis of Therapeutics, 9th Edition,
McGraw-Hill (1996).
Compounds
Described herein are compounds of formula I, and pharmaceutically acceptable
salts, solvates, polymorphs, esters,
amides, tautomers or prodrugs thereof:
0 NH GO
R so N 40
formula I
wherein
Z is H or F;
X is F, Cl, CH3, CH2OH, CH2F, CHF2, or CF3;
Y is I, Br, Cl, CF3, C1-C3 alkyl, C2-C3 alkenyl, C2-C3 alkynyl, cyclopropyl,
OMe, OEt, SMe, phenyl or Het,
where Het is a 5- to 10- membered mono- or bicyclic heterocyclic group, which
group is saturated, olefmic, or
aromatic, containing 1-5 ring heteroatoms selected independently from N, 0,
and S; where
all said phenyl or Het groups are optionally substituted with F, Cl, Br, I,
acetyl, methyl, CN, NO2, CO2H,
C1-C3 alkyl, C1-C3 alkoxy, C1-C3 alkyl-C(=0)-, CI-Ca alkyl-C(=S)-, C1-C3
alkoxy-C(=S)-, C1-C3 alkyl-C(=0)0-,
C1-C3 alkyl-0-(C=0)-, C1-C3 allcyl-C(=0)NH-, C1-C3 alkyl-C(=NH)NH-, C1-C3
alkyl-NH-(C=0)-, di-C1-C3
alkyl-N-(C=0)-, C1-C3 alkyl-C(=0)N(CI-C3 alkyl)-, C1-C3 alkyl-S(=0)2NH- or
trifluoromethyl;
all said methyl, ethyl, C1-C3 alkyl, and cyclopropyl groups are optionally
substituted with OH;
all said methyl groups are optionally substituted with one, two, or three F
atoms;
R is H, F, Cl, Br, I, CH3NII-, (CH3)2N-, C1-C6 alkyl, CI-CI alkoxy, C3-C6
cycloalkyi, C2-C6 alkenyl, C2-C6
alkynyl, phenyl, monosubstituted phenyl, 0(C1-C4 allcyl),
0-C(=0)(C1-C4 alkyl) or C(=0)0(C1-C4 alkyl); where
said alkyl, alkoxy, cycloalkyl, alkenyl, alkynyl and phenyl groups are
optionally substituted with 1-3
substituents selected independently from F, Cl, Br, I, OH, CN, cyanomethyl,
nitro, phenyl and trifluoromethyl;
said C1-C6 alkyl and C1-C4 alkoxy groups also optionally substituted with
OCH3or OCH20-13;
G is 01, 02, Rla, Rib, R1,, Rid, R1,, Art, Ar2 or Ar3; where
Gi is C1-C6 alkyl optionally substituted with one amino, C1-C3 allcylamino, or
dialkylamino group, said
dialkylamino group comprising two CI-C.4 alkyl groups which may be identical
or non-identical; or
GI is a C3-C8 diamino alkyl group;
-Page 45 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
02 is a 5- or 6- membered ring, which is saturated, unsaturated, or aromatic,
containing 1-3 ring
heteroatoms selected independently from N, 0, and S, optionally substituted
with 1-3 substituents selected
independently from F, Cl, OH, 0(C1-C3 alkyl), OCH3, OCH2CH3, CH3C(--0)NH,
CH3C(-0)0, CN, CF3, and a
5-membered aromatic heterocyclic group containing 1-4 ring heteroatoms
selected independently from N, 0,
and S;
R1õ is methyl, optionally substituted with 1-3 fluorine atoms or 1-3 chlorine
atoms, or with OH,
cyclopropoxy, or Cr C3 alkoxy, where said cyclopropoxy group or the Ci- C3
alkyl moieties of said Ci- C3
alkoxy groups are optionally substituted with one hydroxy or methoxy group,
and where all C3- alkyl groups
within said C i- C4 alkoxy are optionally further substituted with a second OH
group;
Rib is CH(CH3)-C1.3 alkyl or C3-C6 cycloalkyl, said alkyl and cycloalkyl
groups optionally substituted
with 1-3 substituents selected independently from F, Cl, Br, I, OH, OCH3, and
CN;
is (CH2).0,õR'; where
m is 0 or 1; and where
when m is 0, n is 1 or 2;
when m is 1, n is 2 or 3;
R' is C1-C6 alkyl, optionally substituted with 1-3 substituents selected
independently from F, Cl, OH,
OCH3, OCH2CH3, and C3-C6 cycloalkyl;
Rid is C(A)(N)(13)-; where
B is H or Cmalkyl, optionally substituted with one or two OH groups;
A and A' are independently H or C1.4allcyl, optionally substituted with one or
two OH groups; or
A and A', together with the carbon atom to which they are attached, form a 3-
to 6- member saturated
ring;
R/e is
-
R2-6
Rie
where
q is 1 or 2;
R2 and R3 are each independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-butyl
or inethylsulfonyl;
R4 is H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3, OCH2F, OCHP2, OCF3, ethyl, n-
propyl, isopropyl,
cyclopropyl, isobutyl, sec-butyl, tert-butyl, methylsulfonyl, nitro,
acetamido, amidinyl, cyano, carbamoyl,
methylcarbamoyl, dimethylcarbamoyl, 1,3,4-oxadiazoI-2-yl, 5-methyl-1,3 ,4-
oxadiazol, 1,3,4-thiadiazol, 5-
methy1-1,3,4-thiadiazoI 1H-teti-azolyl, N-morpholyl carbonyl amino, N-
morpholylsulfonyl and N-
pyrrolidinylcarbonylamino;
R5 is H, F, Cl or methyl;
R6 is H, F, Cl or methyl;
Ari is
R2-6
\
-Page 46-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
where
U and V are, independently, N, CR2 or CR3;
R2, R3 and R4 are, independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3 OCH3,
OCH2F, OCHF2,
OCF3, ethyl, n-propyl, isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-
butyl, acetamido, amidinyl, cyano,
carbamoyl, methylcarbamoyl, dimethylcarbamoy1,1,3,4-oxadiazol-2-yl, 5-methyl-
1,3,4-oxadiazolyl, 1,3,4-
thiadiazolyl, 5-methyl-1,3,4-thiadiazolyl, 1H-tetrazolyl, N-
morpholylcarbonylamino, N-morpholylsulfonyl,
N-pyrrolidinylcarbonylamino, and methylsulfonyl;
R5 and R6 are, independently, H, F, CI or methyl;
Ar2 is
R7_8
Ar2
where
the dashed line represents alternative formal locations for the second ring
double bond;
U is -S-, -0- or -N =, and where
when U is -0- or -S-, V is -CH=, -CC1= or -N =;
when U is -N =, V is -CFI, -CC1=, or -N=;
R7 is H or methyl;
R8 is H, acetamido, methyl, F or Cl;
Ar3 is
R7
R8
Ar3
where
U is -NH-, -NCH3- or -0-;
R7 and Rg are, independently, H, F, Cl, or methyl.
In addition to the definitions given herein for the groups G, R , X, Y and Z,
additional substitutions which could be
contemplated by those of skill in the chemical and pharmaceutical arts are
included.
In some embodiments, the invention provides a compound of formula I, where G
is GI or 02. In other
embodiments, G is GI. In further or additional embodiments, G is G2.
In some embodiments, the invention provides a compound of formula I, where G
is RI,. Rib, R10 Rid, Rle Ari, Ar2
or Ar3. In further or additional embodiments, G is RI., Rib, Ric, Rid or R1e.
In further or additional embodiments, G is Rieõ
In further or additional embodiments, G is Rib. In further or additional
embodiments, G is Ric. In further or additional
embodiments, G is Rid. In further or additional embodiments, G is R1e. In
further or additional embodiments, G is Ari,
Ar2 or Ar3. In further or additional embodiments, G is Art. In further or
additional embodiments, G is Ar2. In further or
additional embodiments, G is Ar3
In some embodiments provided are compounds of formula I, or their
pharmaceutically acceptable salts. In further
or additional embodiments, provided herein are compounds of formula I, or
their solvates. In further or additional
- Page 47 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments, provided herein are compounds of formula I or their polymorphs.
In further or additional embodiments,
provided herein are compounds of formula I, or their esters. In further or
additional embodiments, provided herein are
compounds of formula I, or their amides. In further or additional embodiments,
provided herein are compounds of
formula I or their tautomers. In further or additional embodiments, provided
herein are compounds of formula I or their
prodrugs.
In some embodiments, Z is H. In some embodiments, Z is F. In some embodiments,
X is F. In some embodiments,
X is Cl. In some embodiments, X is CH3. In some embodiments, X is CH2OH. In
some embodiments, X is CH2F. In
some embodiments, X is CRF2. In some embodiments, X is CF3. In some
embodiments, X is F, Cl, or CH3.
In some embodiments, G is G1 or 02, X is F, Cl, or CH3; Y is I, Br, Cl, CF3,
C1-C3 alkyl, phenyl, pyridyl, pyrrolyl,
pyrazolyl, said phenyl, pyridyl, pyrrolyl, and pyrazolyl groups optionally
substituted with F, Cl, Br, I, acetyl, methyl, CN,
NO2, CO2H, Ci-C3 alkyl, C1-C3 alkoxy, C1-C3
C1-C3 alkyl-C(=S)-, C1-C3 alkoxy-C(=S)-, C1-C3 alkyl-
C(=0)0-, C1-C3 alkyl-0-(C=0)-, C1-C3 alkyl-C(0)NU-, C1-C3 alkyl-C(=NH)NH-, C1-
C3 alkyl-NH-(C=0)-, di-C1-C3
alkyl-N-(C=O)-, C1-C3 alkyl-C(=0)N(Ci-C3 alkyl)-, C1-C3 alkyl-S(=0)2NH- or
trifluoromethyl; and Z is H or F. In
further or additional embodiments, G is Gi or 02, and R is F, Cl, C1-C4 alkyl
or C1-C4 alkoxy, said C1-C4 alkyl group
and the C1-C4 alkyl moiety of said Ci-C4 alkoxy group optionally substituted
with F, Cl, OCH3, or OCH2CH3. In further
or additional embodiments, G is Gi or 02, and R is H, F, Cl, C1-C4 alkyl,
methoxy, ethoxy, or 2-methoxy-ethoxy.
In some embodiments, Gi is N-methyl-2-aminoethyl. In further or additional
embodiments, Giis (CH3)2N-
CH2CH2-NH-(CH2)11-, where n is 1, 2, or 3. In further or additional
embodiments, Glis (CH3)2N-CH2CH2-NH-(CH2)-,
where n is 1, 2, or 3, and X is F. In further or additional embodiments, Giis
(CH3)2N-CH2CH2-NI-1-(CH2)-, where n is I,
2,or3,XisFandZisF.
In some embodiments, 02 is I -piperidyl, 2-piperidyl, 3-piperidyl, or 4-
piperidyl. In further or additional
embodiments, 02 is morpholyl, 1-piperazyl, or 2-piperazyl.
In some embodiments, G is Ria, Rib, Rio, Rid, Rie, Ari, Ar2 or Ar3 and X is F,
Cl, or CH3. In further or additional
embodiments, G is Ria, Rib, R10, Rid, Rie, Ari, Ar2 or Ar3, X is F, Cl, or CH3
and Y is I, Br, Cl, CF3, or Ci-C3 alkyl In
further or additional embodiments, G is Ria, Rib, RIG, Rid, Riõ Ari, Ar2 or
Ar3, X is F, Cl, or CH3, Y is I, Br, Cl, CF3, or
C1-C3 alkyl and Z is H or F
In further or additional embodiments, G is Ria, Rib, Ric, Rid, Rle, Art, Ar2
or Ar3 and R is F, Cl, C1-C4 alkyl Or
C4 alkoxy, said C1-C4 alkyl group and the Ci-C4 alkyl moiety of said C1-C4
alkoxy group optionally substituted with F,
Cl, OCH3, or OCH2CH3. In further or additional embodiments, G is Ria, Rib,
RIG, Rid, Riõ Ari, Ar2 or Ar3 and R is H, F,
Cl, C1-C4 alkyl, methoxy, ethoxy, or 2- methoxy-ethoxy.
In some embodiments, G is Ria; and Z is F. In further or additional
embodiments, G is Ria where Ric, is CH3, R is
H; and Y is Br, I, CF3, or CH3.In some embodiments, G is Rib and Z is F. In
further or additional embodiments, G is Rib,
Z is F, and R is H, F, or OCH3. In further or additional embodiments, G is
Rib, Z is F, R is H, F, or OCH3, and X is F or
CH3. In further or additional embodiments, G is Rib, Z is F, R is H, F, or
OCH3, X is F or CH3 and Y is Br, I or CH3. In
further or additional embodiments, G is Rib where Rib is C3-C6 cycloalkyl. In
further or additional embodiments, G is Rib
where Rib is substituted C3-C6 eycloalkyl. In further or additional
embodiments, G is Rib where Rib is unsubstituted C3-
C6 eyeloalkyl. In further or additional embodiments, Cl is Rib where Rib is
unsubstituted C3-C6 eyeloalkyl and R is H. In
further or additional embodiments, G is Rib where Rib is isopropyl or
cyclopropyl.
In some embodiments, G is Rk, and Y is I, Br, CH3, or CF3. In further or
additional embodiments, G is Rio, Y is I,
Br, CH3, or CF3, and Z is F. In further or additional embodiments, G is Ric, Y
is I, Br, CH3, or CF3. Z is F and m is zero.
Page 48-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, G is Rid and BY is fluoro, chloro, methyl, ethyl, propyl,
isopropyl, sec-butyl, iso-butyl, tert-
butyl, cyclopropyl, cyclobutyl, fluoromethyl, methoxy, fluoromethoxy,
methylamino or dimethylamino. In further or
additional embodiments, G is Rid, R is fluoro, chloro, methyl, ethyl, propyl,
isopropyl, sec-butyl, iso-butyl, tert-butyl,
cyclopropyl, cyclobutyl, fluoromethyl, methoxy, fluoromethoxy, methylamino or
dimethylamino and X is F, CI, CH3, or
mono-, di- or tri- fluoromethyl. In further or additional embodiments, G is
Rid, R is fluoro, chloro, methyl, ethyl, propyl,
isopropyl, sec-butyl, iso-butyl, tert-butyl, cyclopropyl, cyclobutyl,
fluoromethyl, methoxy, fluoromethoxy, methylamino
or dimethylamino, X is F, Cl, CH3, or mono-, di- or tri- fluoromethyl and Y is
I, Br, CI, or mono-, di- or tri-
fluoromethyl. In further or additional embodiments, G is Rid, R is fluoro,
chloro, methyl, ethyl, propyl, isopropyl, sec-
butyl, iso-butyl, tert-butyl, cyclopropyl, cyclobutyl, fluoromethyl, methoxy,
fluoromethoxy, methylamino or
dimethylamino, X is F, Cl, CH3, or mono-, di- or tri- fluoromethyl, Y is I,
Br, Cl, or mono-, di- or tri- fluoromethyl and Z
is H or F. In further or additional embodiments, G is Rid and R is F, Cl,
methyl, ethyl, methoxy, ethoxy, or 2- methoxy-
ethoxy.
In further or additional embodiments, G is Rid, R is F, Cl, methyl, ethyl,
methoxy, ethoxy, or 2- methoxy-ethoxy
and X is F, Cl, or CH3. In further or additional embodiments, G is Rid, R is
F, Cl, methyl, ethyl, methoxy, ethoxy, or 2-
methoxy-ethoxy, X is F, Cl, or CH3 and Y is I, Br, Cl, or mono-, di- or tri-
fluoromethyl. In further or additional
embodiments, G is Rid, R is F, Cl, methyl, ethyl, methoxy, ethoxy, or 2-
methoxy-ethoxy, X is F, Cl, or CH3, Y is I, Br,
Cl, or mono-, di- or tri- fluoromethyl and Z is H or F. In further or
additional embodiments, G is Rid and R is H; X is F,
Cl, CH3, or mono-, di- or tri- fluoromethyl. In further or additional
embodiments, G is Rid, R is H; X is F, Cl, CH3, or
mono-, di- or tri- fluoromethyl and Y is I, Br, Cl, or mono-, di- or tri-
fluoromethyl. In further or additional embodiments,
G is Rid, RD is H; X is F, CI, CH3, or mono-, di- or tri- fluoromethyl, Y is
I, Br, Cl, or mono-, di- or tri- fluoromethyl and
Z is H or F.
In further or additional embodiments, G is Rid where Rid is C(A)(A') is C1-C6
cycloalkyl. In further or additional
embodiments, G is Rid where Rid is C(A)(A') is Ci-C6 cycloalkyl and B is H. In
further or additional embodiments, G is
Rid where Rid is C(A)(A') is C1-C6 cycloalkyl and B is methyl, ethyl, 2-
hydroxyethyl, n-propyl, 3- hydroxypropyl, 2,3-
dihydroxypropyl, 3,4-dihydroxybutyl, isopropyl, 1-methyl-2-hydroxy ethyl, n-
butyl, sec-butyl, isobutyl, or 2-
hydroxymethy1-3-hydroxy propyl.
In further or additional embodiments, G is Rid where Rid is C(A)(A') is Ci-C6
cycloalkyl and B is 2,3-
dihydroxypropyl or 3,4-dihydroxybutyl. In further or additional embodiments, G
is Rid where Rid is C(A)(A') is C1-C6
cycloalkyl and B is 2,3-dihydroxypropyl or 3,4-dihydroxybutyl, in which the
chiral carbon in B is in the R configuration.
In further or additional embodiments, G is Rid where Rid is C(A)(A') is C1-C6
cycloalkyl and B is 2,3-dihydroxypropyl or
3,4-dihydroxybutyl, in which the chiral carbon in B is in the S configuration.
In further or additional embodiments, G is
Rid where Rid is C(A)(A') is Ci-C6 cycloalkyl and B is methyl, optionally
substituted with one OH group, or C2-C4 alkyl,
optionally substituted with one or two OH groups. In further or additional
embodiments, G is Rid where Rid is C(A)(A')
is C1-C6 cycloalkyl and R is fluoro, chloro, methyl, ethyl, propyl,
isopropyl, sec-butyl, iso-butyl, tert-butyl, cyclopropyl,
cyclobutyl, fluoromethyl, methoxy, fluoromethoxy, methylamino or
dimethylamino. In further or additional
embodiments, G is Rid where Rid is C(A)(A') is Ci-C6 cycloalkyl and R is F,
Cl, methyl, ethyl, methoxy, ethoxy, or 2-
methoxy-ethoxy. In further or additional embodiments, G is Rid where Rid is
C(A)(A') is Ci-C6 cycloalkyl and R is H; X
is F, Cl, CH3, or mono-, di- or tri- fluoromethyl.
In further or additional embodiments, the invention provides a composition
comprising a compound of formula I,
where G is Rid where Rid is C(A)(A') is C1-C6 cycloalkyl and B is 2,3-
dihydroxypropyl or 3,4-dihydroxybutyl, in which
the chiral carbon in B is in the R configuration, which is substantially free
of the S isomer. In further or additional
-Page 49-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments, the invention provides a composition comprising a compound of
formula I, where G is Rid where Rid is
C(A)(A') is C1-C6 cycloalkyl and B is 2,3-dihydroxypropyl, in which the chiral
carbon in B is in the R configuration,
which is substantially free of the S isomer. In further or additional
embodiments, the invention provides a composition
comprising a compound of formula I, where G is Rid where Rid is C(A)(A') is C1-
C6 cycloalkyl and B is 3,4-
dihydroxybutyl, in which the chiral carbon in B is in the R configuration,
which is substantially free of the S isomer. In
further or additional embodiments, the invention provides a composition
comprising a compound of formula I, where G
is Rid where Rid is C(A)(A') is C1-C6 cycloalkyl and B is 2,3-dihydroxypropyl
or 3,4-dihydroxybutyl, in which the chiral
carbon in B is in the S configuration, which is substantially free of the R
isomer. In further or additional embodiments,
the invention provides a composition comprising a compound of formula I, where
G is Rid where Rid is C(A)(A') is Gi-
ll) C6 cycloalkyl and B is 2,3-dihydroxypropyl, in which the chiral carbon
in B is in the S configuration, which is
substantially free of the R isomer. In further or additional embodiments, the
invention provides a composition comprising
a compound of formula I, where G is Rid where Rid is C(A)(A') is Ci-C6
cycloalkyl and B is 3,4-dihydroxybutyl, in
which the chiral carbon in B is in the S configuration, which is substantially
free of the R isomer.
In further or additional embodiments, G is Rid where Rid is C(A)(A') is
cyclopropyl. In further or additional
embodiments, G is Rid where Rid is C(A)(A) is cyclopropyl and B is H. In
further or additional embodiments, G is Rid
where Rid is C(A)(A') is cyclopropyl and B is methyl, ethyl, 2-hydroxyethyl, n-
propyl, 3- hydroxypropyl, 2,3-
dihydroxypropyl, 3,4-dihydroxybutyl, isopropyl, 1-methyl-2-hydroxy ethyl, n-
butyl, sec-butyl, isobutyl, or 2-
hydroxymethy1-3-hydroxy prop)/ In further or additional embodiments, G is Rid
where Rid is C(A)(A') is cyclopropyl
and B is 2,3-dihydroxypropyl or 3,4-dihydroxybutyl. In further or additional
embodiments, G is Rid where Rid is
C(A)(A') is cyclopropyl and B is 2,3-dihydroxypropyl or 3,4-dihydroxybutyl, in
which the chiral carbon in B is in the R
configuration. In further or additional embodiments, G is Rid where Rid is
C(A)(A') is cyclopropyl and B is 2,3-
dihydroxypropyl or 3,4-dihydroxybutyl, in which the chiral carbon in B is in
the S configuration. In further or additional
embodiments, G is Rid where Rid is C(A)(A) is cyclopropyl and B is methyl,
optionally substituted with one OH group,
or C2-C4 alkyl, optionally substituted with one or two OH groups. In further
or additional embodiments, G is Rid where
Rid is C(A)(A') is cyclopropyl and R is fluoro, chloro, methyl, ethyl,
propyl, isopropyl, sec-butyl, iso-butyl, tert-butyl,
cyclopropyl, cyclobutyl, fluoromethyl, methoxy, fluoromethoxy, methylamino or
dimethylamino. In further or additional
embodiments, (3 is Rid where Rid is C(A)(A') is cyclopropyl and R is F, Cl,
methyl, ethyl, methoxy, ethoxy, or 2-
methoxy-ethoxy. In further or additional embodiments, G is Rid where Rid is
C(A)(A') is cyclopropyl and R is H; X is F,
Cl, CH3, or mono-, di- or tri- fluoromethyl.
In further or additional embodiments, the invention provides a composition
comprising a compound of formula I,
where G is Rid where Rid is C(A)(A') is cyclopropyl and B is 2,3-
dihydroxypropyl or 3,4-dihydroxybutyl, in which the
chiral carbon in B is in the R configuration, which is substantially free of
the S isomer. In further or additional
embodiments, the invention provides a composition comprising a compound of
formula I, where G is Rid where Rid is
C(A)(A') is cyclopropyl and B is 2,3-dihydroxypropyl, in which the chiral
carbon in B is in the R configuration, which is
substantially free of the S isomer. In further or additional embodiments, the
invention provides a composition comprising
a compound of formula I, where G is Rid where Rid is C(A)(A') is cyclopropyl
and B is 3,4-dihydroxybutyl, in which the
chiral carbon in B is in the R configuration, which is substantially free of
the S isomer. In further or additional
embodiments, the invention provides a composition comprising a compound of
formula I, where G is Rid where Rid is
C(A)(A') is cyclopropyl and B is 2,3-dihydroxypropyl or 3,4-dihydroxybutyl, in
which the chiral carbon in B is in the S
configuration, which is substantially free of the R isomer. In further or
additional embodiments, the invention provides a
composition comprising a compound of formula I, where G is Rid where Rid is
C(A)(A') is cyclopropyl and B is 2,3-
-Page 50-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
dihydroxypropyl, in which the chiral carbon in B is in the S configuration,
which is substantially free of the R isomer. In
further or additional embodiments, the invention provides a composition
comprising a compound of formula I, where G
is Rid where Rid is C(A)(A) is cyclopropyl and B is 3,4-dihydroxybutyl, in
which the chiral carbon in B is in the S
configuration, which is substantially free of the R isomer.
In some embodiments, G is RI, and n is 1. In further or additional
embodiments, G is R1e, R is H, R.4.6 are H, R2
and R3 are, independently, H, F, Cl, Br, CH3, CH2F, CHF2, CF3, 0 CH3, OCH2F,
OCHF2, OCF3, ethyl, n-propyl,
isopropyl, cyclopropyl, isobutyl, sec-butyl, tert-butyl, and methylsulfonyl, X
is F and Y is I.
In some embodiments, G is Ari where Arlis phenyl optionally substituted with
one group selected from acetamido,
amidinyl, cyano, carbamoyl, methylcarbamoyl, dimethylcarbamoyl, 1,3,4-
oxadiazol-2-yk 5-methyl-1,3,4-oxadiazolyl,
1,3,4-thiadiazolyI, 5 -methyl- 1,3,4- thiadiazolyl, IH-tetrazolyl, N-
morpholylcarbonylamino, N-morpholylsulfonyl, N-
pyrrolidinylcarbonylamino, and methylsulfonyl, optionally substituted with 1-3
substituents selected independently from
F, Cl, and CH3. In further or additional embodiments, G is Ai) where Arlis
phenyl optionally substituted with one group
selected from acetamido, amidinyl, cyano, carbamoyl, methylcarbamoyl,
dimethylcarbamoyl, 1,3,4-oxadiazol-2-yl, 5-
methy1-1,3,4-oxadiazolyl, 1,3,4-thiadiazolyI, 5 -methyl- 1,3,4-
thiadiazoly1,1H-tetrazolyl, N-morpholylcarbonylamino, N-
tnorpholylsulfonyl, N- pyrrolidinylcarbonylamino, and methylsulfonyl,
optionally substituted with 1-3 substituents
selected independently from F, Cl, and CH3, R is H, X is F, Cl, or methyl and
Y is Br, I, CF3, C1-C3 alkyl, C2-C3 alkenyl,
R2
R3
C2-C3 alkynyl, cyclopropyl, 0 CH3, OCH2CH3 or SCH3. In some embodiments, G is
Ari where Ari is and
where R2 and R3 are, independently, H, F, Cl, CH3, CF3, OCH3. In further or
additional embodiments, G is Ari where Ari
R2
..3
is and where R2 and R3 are, independently, H. F, CI, CH3, CF3,
OCH3, X is F or CH3, Y is I, Br, or Cl; and
Z is F. In further or additional embodiments, G is ATI where Arlis phenyl or
mono-substituted phenyl. In further or
additional embodiments, G is Art where Arils phenyl or mono-substituted
phenyl, X is F or CH3, Y is I, Br, or Cl, Z is F;
and R is F, methyl, ethyl, methoxy, or 2-methoxy-ethoxy. In further or
additional embodiments, G is Ari where U is N
or CR2 and V is N. In further or additional embodiments, G is An_ where U is N
or CR2 and V is CR. In further or
additional embodiments, G is Ari where U is N or CR2, V is CR, R is H, X is
F, Cl, or methyl and Y is Br, I, CF3, C1-C3
alkyl, C2-C3 alkenyl, C2-C3 alkynyl, cyclopropyl, OCH3, OCH2CH3 or SCH3.
R7
0
V
R8-
,
In some embodiments, G is Ar2 where Ar2 is U
where R1 is H or methyl and Rs is H, acetamido,
R7
V
R8-4
methyl, F or Cl. In further or additional embodiments, G is Ar2 where Ar2 is
U where R7 is H or methyl, Rs is
H, acetamido, methyl, F or Cl, R is H, X is F, Cl, or methyl, Y is Br, I,
CF3, Ci-C3 alkyl, C2-C3 alkenyl, C2-C3 alkynyl,
cyclopropyl, OCH3, OCH2CH3 or SCH3, and Z is F. In further or additional
embodiments, G is Ar2 where Ar2 is
R7
v¨(
R8-
U where
U is S or 0, V is CH=, and Rs is H or CH3, R7 is H or methyl, R8 is H,
acetamido, methyl, F or Cl,
R is H, X is F, Cl, or methyl, Y is Br, I, CF3, C1-C3 alkyl, C2-C3 alkenyl,
C2-C3 alkynyl, cyclopropyl, OCH3, OCH2CH3
-Page 51-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
or SCH3 and Z is F. In further or additional embodiments, R is H. In further
or additional embodiments, R is H, X is F
or CI and Y is Br, I, CH2CH3 or SCH3
In some embodiments, G is Ar3 where U is -0-.
In further or additional embodiments, G is RI., where R1. is defined as above.
In further or additional
embodiments, G is RI., and R is H, where Riõ is defined as above. In further
or additional embodiments, G is Ria and R
is as defined above, other than H, and Ria is defined as above. In further or
additional embodiments, G is Ria, where Ria
is methyl, monohalomethyl, C1-C3 alkoxymethyl, or cyclopropoxymethyl. In
further or additional embodiments, G is RI.,
where RIa is methyl, monohalomethyl, C1-C3 alkoxymethyl, or cyclopropoxy
methyl and where R is F, Cl, C1-C3 alkyl,
monochIoro C1-C3 alkyl, C1-C3 alkoxy, trifluoro methoxy, or 2-methoxy-ethoxy.
In further or additional embodiments, G is Rib, where Rib is defined as above.
In further or additional
embodiments, G is Rib, and R is H, where Rib is defined as above. In further
or additional embodiments, G is Rib, R is
H and Z is F, where Rib is defined as above. In further or additional
embodiments, G is Rib and R is as defined above,
other than H, and Rib is defined as above. In further or additional
embodiments, G is Rib, where Rib is isopropyl, 2-butyl,
2-pentyl, cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl, all optionally
substituted with 1 or 2 substituents selected
independently from F, Cl, OH, and OCH3; Y is Br, I, methyl, or
trifluoromethyl. In further or additional embodiments, G
is Rib, where Rib is isopropyl, 2-butyl, 2-pentyl, cyclopropyl, cyclobutyl,
cyclopentyl, or cyclohexyl, optionally
substituted with 1 or 2 substituents selected independently from F, Cl, OH,
and OCH3; Y is Br, 1, methyl, or
trifluoromethyl; and R is F, Cl, C1-C3 alkyl, monochloro C1-C3 alkyl, Ci-C3
alkoxy, trifluoromethoxy, or 2-methoxy-
ethoxy. In further or additional embodiments, G is Rib, where Rib is
isopropyl, 2-butyl, 2-pentyl, cyclopropyl, cyclobutyl,
cyclopentyl, or cyclohexyl, all optionally substituted with one Cl or with 1
or 2 OH groups; and Y is Br, I, methyl, or
trifluoromethyl. In further or additional embodiments, G is Rib, where Rib is
isopropyl, 2-butyl, 2-pentyl, cyclopropyl,
cyclobutyl, cyclopentyl, or cyclohexyl, all optionally substituted with one Cl
or with 1 or 2 OH groups; Y is Br, I,
methyl, or trifluoromethyl; and R is F, Cl, C 1-C3 alkyl, rnonochloro C1-C3
alkyl, C1-C3 alkoxy, trifluoromethoxy, or 2-
methoxy-ethoxy.
In further or additional embodiments, G is Ric, where Rie is defined as above.
In further or additional
embodiments, G is Ric, and R is H, where Ric is defined as above. In further
or additional embodiments, G is Ric and R
is as defined above, other than H, and Ric is defined as above. In further or
additional embodiments, G is Ric, and R is
H, where Rio is (C112)n0õ,11.1, where m is 0 or 1, n is 2 or 3 when m is 1,
and n is 1 or 2 when m is 0, and IV is Ci-C6 alkyl,
optionally substituted with 1-3 substituents selected independently from F,
Cl, OH, OCH3, OCH2CH3, and C3-C6
cycloalkyl. In another more specific subgeneric embodiment, m is zero, n is 1
or 2, and It. is Ci-C4 alkyl, optionally
substituted as described above. In another more specific subgeneric
embodiment, m is 1, n is 2 or 3, and R' is C1-C4 alkyl,
optionally substituted as described above. In a still more specific subgeneric
embodiment, m is zero, n is 1 or 2, and R' is
Ci-C. alkyl, optionally substituted with 1 -3 groups selected from OH, OCH3,
Cl, and cyclopropyl.
In further or additional embodiments, G is Rid, where Rid is defined as above.
In further or additional
embodiments, G is Rid, and R is H, where Rid is defined as above. In further
or additional embodiments, G is Rid and R
is as defined above, other than H, and Rid is defmed as above. In further or
additional embodiments, G is Rid, and R is
H, where Rid is C(A)(N)(B)- where B, A, and A' are, independently, H or C1.4
alkyl, optionally substituted with one or
two OH groups or halogen atoms, or A and Ai, together with the carbon atom to
which they are attached, form a 3- to 6-
member saturated ring, said ring optionally containing one or two heteroatoms
selected, independently, from 0, N, and S
and optionally substituted with one or two groups selected independently from
methyl, ethyl, fluoro, chloro, bromo and
iodo.
- Page 52 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In further or additional embodiments, G is Rth, where Rie is defined as above.
In further or additional
embodiments, G is Rk, and R is H, where RI, is defined as above. In further
or additional embodiments, G is Rie and R
is as defined above, other than H, and Rie is defined as above.
In further or additional embodiments, G is Arl, where Arils defined as above.
In further or additional
embodiments, G is Aril, and R is H, where Arlis defined as above. In further
or additional embodiments, G is Arland R
is as defined above, other than H, and Ariis defined as above.
In further or additional embodiments, G is Ar2, where Ar2 is defined as above.
In further or additional
embodiments, G is Ar2, and R is H, where Ar2 defined as above. In further or
additional embodiments, G is Ar2 and R is
as defined above, other than H, and Ar2 is defined as above.
In further or additional embodiments, X is F, Cl, or CH3; Y is I, Br, Cl, CF3
or C1-C3 alkyl, and Z is H or F. In
further or additional embodiments, X is F, Cl, or CH3: Y is I, Br, Cl, CF3, or
C1-C3 alkyl, Z is H or F, and R is halogen,
C1-C6 alkyl, monohalo C1-C6 alkyl, C3-C6 cycloalkyl, C2-C6 alkenyl, C2-C6
alkynyl, phenyl, monosubstituted phenyl,
OR3, 0-C(=0)R4, or C(-0)0R5. In further or additional embodiments, X is F, Cl,
or CH3: Y is I, Br, Cl, CF3, or C1-C3
alkyl, Z is H or F, and R is furyl, thienyl, thiazolyl, isothiazolyl,
oxazolyl, isoxazolyl, pprolyl, or pyrazolyl. In further or
additional embodiments, X is F, Cl, or CH3: Y is I, Br, Cl, CF3, or C1-C3
alkyl, Z is H or F, and R is F, Cl, C1-C4 alkyl,
Ci-C3 alkoxy, trifluoromethoxy, or 2- methoxy-ethoxy.
In another more specific subgeneric embodiment, Rld is cycloalkyl or 1-alkyl-
cycloalkyl, in which the 1-alkyl
group is optionally substituted with one or two OH groups or with one or two
halogen atoms.
In another more specific subgeneric embodiment, R is halogen, C1-C6 alkyl,
monohalo C1-C6 alkyl, C3-C6
cycloalkyl, C2-C6 alkenyl, C2-C6 alkynyl, phenyl, monosubstituted phenyl, OR3,
O-C(=0)R4, or C(=0)0R5; and Rid is
cycloalkyl or 1-alkyl-cycloalkyl, in which the 1-alkyl group is optionally
substituted with one or two OH groups or with
one or two halogen atoms.
In another more specific subgeneric embodiment, R is furyl, thienyl,
thiazolyl, isothiazolyl, oxazolyl, isoxazolyl,
pyrrolyl, or pyrazolyl; and Rid is cycloalkyl or 1-alkyl-cycloalkyI, in which
the 1-alkyl group is optionally substituted
with one or two OH groups or one or two halogen atoms.
In another more specific subgeneric embodiment, Rid is cycloalkyl or 1-alkyl-
cycloalkyl, in which the 1-alkyl
group is optionally substituted with one or two OH groups, and where Y is Br,
I, methyl, or trifluoromethyl. In another
more specific subgeneric embodiment, Rid is cycloalkyl or 1-alkyl-cycloalkyl,
in which the 1-alkyl group is optionally
substituted with one or two fluorine or chlorine atoms, and where Y is Br, I,
methyl, or trifluoromethyl. In another more
specific subgeneric embodiment, Rid is cycloalkyl or (1 -alley 1)-cycloalkyl,
in which the 1-alkyI group is optionally
substituted with one or two OH groups, and where R ' is F, Cl, C1-C3 alkyl,
monochloro C1-C3 alkyl, C1-C3 alkoxy,
trifluoromethoxy, or 2-methoxy-ethoxy. In another more specific subgeneric
embodiment, Rid is tetrahydrofuryl,
tetrahydrothienyl, pyrrolidyl, piperidyl, piperazinyl, or morpholyl, each
optionally substituted as described above, and
where Y is Br, I, methyl, or trifluoromethyl. In another more specific
subgeneric embodiment, Rid is oxazolidinyl,
thiazolidinyl, isoxazolidinyl, isothiazolidinyl, tetrahydrofuryl,
tetrahydrothienyl, pyrrolidyl, piperidyl, piperazinyl, or
morpholyl, each optionally substituted as described above, and where Y is Br,
I, methyl, or trifluoromethyl. In another
more specific subgeneric embodiment, Rid is cyclopropyl or 1-alkyl-
cyclopropyl, in which the I-alkyl group is optionally
substituted with one or two OH groups, and where R ' is F, Cl, methyl, ethyl,
chloromethyl, C1-C2 alkoxy,
trifluoromethoxy, or 2-methoxy-ethoxy. In an even more specific embodiment,
Rid is 1-(monohydroxyallcyl) cycloalkyl.
In another more specific embodiment, Rid is 1-(monohydroxyalkyl) cycloalkyl,
where R ' is F, Cl, methyl, ethyl,
chloromethyl, C1-C2 alkoxy, trifluoromethoxy, or 2-methoxy-ethoxy. In an even
more specific embodiment, Rid is 1-
- Page 53 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
(dihydroxyalkyl) cycloalkyl. In another more specific embodiment, Rid is 1-
(dihydroxyalkyl) cycloalkyl, where R ' is F,
Cl, methyl, ethyl, chloromethyl, C1-C2 alkoxy, trifluoromethoxy, or 2-methoxy-
ethoxy.
In a more specific subgeneric embodiment U is CR2 and V is N. In another more
specific, subgeneric embodiment,
U and V are both N. In a more specific, subgeneric embodiment, U is CR2 and V
is CR3.
In a still more specific subgeneric embodiment, this invention provides a
compound of formula I, where G is Ari
and Ari is phenyl or monosubstituted phenyl, R is F, methyl, ethyl, C1-C3
alkoxy, trifluoromethoxy, or 2-methoxy-
ethoxy; X is F, Cl, or CH3; Y is I; and Z is F. In another subgeneric
embodiment, this invention provides a compound of
formula I, where G is Ari, where Ari is phenyl or monosubstituted phenyl, R
is halogen, C1-C6 alkyl, C3-C6 cycloalkyl,
C2-C6 alkenyl, C2-C6 alkynyl, all such alkyl, cycloalkyl, alkenyl, and alkynyl
groups optionally substituted with 1-3
substituents selected independently from halogen, OH, CN, cyanomethyl, nitro,
phenyl, and trifluoromethyl; or R is
phenyl, OR3, furyl, thienyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl,
pyrrolyl, or pyrazolyl. In a more specific
subgeneric embodiment, this invention provides a compound of formula I, where
A is Ari, where Ari is phenyl or
monosubstituted phenyl, R is F, Cl, C1-C3 alkyl, C1-C3 alkoxy, 2-
methoxyethoxy, C2-C3 alkenyl, C2-C3 alkynyl,
trifluoromethyl, phenyl, fury], or thienyl, thiazolyl, isothiazolyl, oxazolyl,
isoxazolyl, pyrrolyl, or pyrazolyl; X is F, Cl, or
methyl; Y is I, Br, Cl, CF3, or CI-C3 alkyl; and Z is F.
In another still more specific subgeneric embodiment, this invention provides
a compound of formula I, where G is
Ari, where Ari is phenyl or monosubstituted phenyl, R is H; X is F, Cl, or
CH3; Y is Br or I; and Z is F.
In another subgeneric embodiment his invention provides a compound of formula
I, where G is Ar2, where Ar2 is
2-thienyl, 2-furyl, 3 -thienyl, 3 -furyl, 2-pyrrolyl, or 3 -pyrrolyl, all
optionally substituted with methoxycarbonyl,
methylcarbamoyl, acetamido, acetyl, methyl, ethyl, trifluoromethyl, or
halogen. In a more specific subgeneric
embodiment his invention provides a compound of formula I, where G is Ar2,
where Ar2 is 2-thienyl, 2-furyl, 3-thienyl,
3-furyl, 2-pyrrolyl, or 3- pyrrolyl, all optionally substituted with
methoxycarbonyl, methylcarbamoyl, acetamido, acetyl,
methyl, ethyl, trifluoromethyl, or halogen; R is other than H; X is F, Cl, or
CH3: Y is I, Br, Cl, CF3, or C1-C3 alkyl, and Z
is H or F. In another subgeneric embodiment this invention provides a compound
of formula I, where G is Ar2, where Ar2
is 2-thienyl, 2-furyl, 3-thienyl, 3-furyl, 2-pyrrolyl, or 3-pyrroIy1, all
optionally substituted with methoxycarbonyl,
methylcarbamoyl, acetamido, acetyl, methyl, ethyl, trifluoromethyl, or
halogen; R is F, Cl, C1-C3 alkyl, monochloro Ci-
C3 alkyl, C1-C3 alkoxy, trifluoromethoxy, methyloxy-methoxy, or 2-methoxy-
ethoxy; X is F, Cl, or CH3: Y is I, Br, Cl,
CF3, or CI-C3 alkyl, and Z is 1-1 or F. In another subgeneric embodiment his
invention provides a compound of formula I,
where G is Ar2, where Ar2 is 2-thienyl, 2-furyl, 3-thienyl, 3-furyl, 2-
pyrrolyl, or 3-pyrrolyl, all optionally substituted with
methoxycarbonyl, methylcarbamoyl, acetamido, acetyl, methyl, ethyl,
trifluoromethyl, or halogen; R is H; X is F, Cl, or
CH3: Y is I, Br, Cl, CF3, or C1-C3 alkyl, and Z is H or F. In another
subgeneric embodiment his invention provides a
compound of formula I, where G is Ar2, where Ar2 is thiazolyl, isothiazolyl,
oxazolyl, isoxazolyl, pyrrolyl, or pyrazolyl,
all optionally substituted with methoxycarbonyl, methylcarbamoyl, acetamido,
acetyl, methyl, ethyl, trifluoromethyl, or
halogen; R is H or methoxy; X is F, Cl, or CH3: Y is I, Br, Cl, CF3, or C1-C3
alkyl, and Z is H or F.
In some embodiments, the compound of formula (I), or a pharmaceutical salt
thereof, is selected from
,09 \-711
s-=
Cr F NNH F F
N
10 !FSI
F
9 9
- Page 54 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
0 7-7H 0, 77H 0. \-7H 0 77H
H0,.....9,,,,,X. 0
---Ce' Ha)--õ,..õX0 HO,._.-1-õõ)(,,,s,0 HO....,..--
1,..A....s,-,0
F 0-- NNH H F 0- N NH F 0--
N.NH F
H H
Me so N 40 N
F 0 so N
0 40 ...o.....,,..0 40 N 40
F I F I F 1 r
1
F F F r
HO
HO"
,s,0 -'----.7 H HO c,,0
---"--7 H0a)Z
...5"-
F 01'.'NNH F 0- ...'NH r
H H
N 0 Me0 0 N so Me0 0 N 0
F I F I r I
F F and F
In some embodiments, the invention provides a compound of formula I, or a
pharmaceutical salt thereof, selected
0 ,H
HOõ.....,,X.õ7õ --...0
0.-
HO )(,µeNH F ,,-.1.S.õ
'-' NHH F H
Me 0 N 0 FIN,
F I F i
from: r and F , where the 2-0H carbon is
in the R
5 configuration.
In some embodiments, the invention provides a compound of formula I, or a
pharmaceutical salt thereof, selected
9 77H
HO),0
.õ.........õ.v0H ,se... HO.õ...õ--1....,..A. .s-D
ON NH FH H 0 NH F
Me0 io N 0 F 0 N 40
F I F 1
from: r and F , where the 2-0H carbon is
in the S
configuration.
In further or additional embodiments, the compound of formula (I), or a
pharmaceutical salt thereof,
õ;.s.-,.....0
0 NHF
H
Me0 so N 40
F I
10 is F
In further or additional embodiments, the compound of formula (I), or a
pharmaceutical salt thereof,
1-10-.......0
, .,r...S
,-, NHH F
Me0 so N 0
F I
iS F
In some embodiments, the invention provides a composition comprising a
compound of formula I, selected from
those shown below, where the 2-0H carbon is in the R configuration,
substantially free of the S- isomer.
HO..õ...--1.--7c.,:.0 HO0
Ct.' NHH F &'..' H NH F
F
Me0 so N 40 F101 N I 10
F I
15 F F
, =
-Page 55-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, the invention provides a composition comprising a
compound of formula I, selected from
those shown below, where the 2-0H carbon is in the S configuration,
substantially free of the R- isomer.
HOJv-7H
9 77H
L.4.0
NH
Me0 so N
In some embodiments, this invention provides a compound of formula I, where Y
is phenyl, pyridyl, or pyrazolyl.
In another subgeneric embodiment, this invention provides a compound of
formula I, where Y is substituted phenyl,
pyridyl, or pyrazolyl. In yet another subgeneric embodiment, this invention
provides a compound of formula I, where Y
is Br or I. In one subgeneric embodiment, this invention provides a compound
of formula I, where G is 1-piperidyl, 2-
piperidyl, 3-piperidyl, or 4-piperidyl. In another subgeneric embodiment, this
invention provides a compound of formula
1, where G is 1-piperazyl or 2-piperazyl. In another subgeneric embodiment,
this invention provides a compound of
formula I, where G is morpholyl. In another subgeneric embodiment, this
invention provides a compound of formula I,
where G is N-methyl-2-aminoethyl. In one subgeneric embodiment, this invention
provides a compound of formula I,
where G is N-methyl-3-amino-n-propyl. In another subgeneric embodiment, this
invention provides a compound of
formula I, where G is (CH3)2N-CH2CH2-NH-(CI-12)n-, where n is 1, 2, or 3. In
another subgeneric embodiment, this
invention provides a compound of formula I, where G is (CH3CH2)2N-CH2CH2-NH-
(CH2)-, where n is 1 or 2. In a more
specific subgeneric embodiment, this invention provides a compound of formula
I, where G is 1-piperidyl, 2-piperidyl, 3-
piperidyl, or 4-piperidyl; le is H, halo, or methoxy; X is F; and Y is I. In
another more specific subgeneric embodiment,
this invention provides a compound of formula I, where G is 1-piperazyl or 2-
piperazyl; R is H, halo, or methoxy; X is
F; and Y is I In another more specific subgeneric embodiment, this invention
provides a compound of formula I, where G
is morpholyl; BY is H, halo, or methoxy; X is F; and Y is I. In another more
specific subgeneric embodiment, this
invention provides a compound of formula I, where G is N-methyl-2-aminoethyl;
R. is H, halo, or methoxy; X is F; and
Y is I In another more specific subgeneric embodiment, this invention provides
a compound of formula I, where G is N-
methyl-3-amino-n-propyl; R is H, halo, or methoxy; X is F; and Y is I. In
another more specific subgeneric embodiment,
this invention provides a compound of formula I, where G is (CH3)2N-CH2CH2-NH-
(CH2)-, where n is 1, 2, or 3; R is
H, halo, or methoxy; X is F; and Y is I. In another more specific subgeneric
embodiment, this invention provides a
compound of formula I, where U is (CH3CH2)2N-CH2CH2-NH-(C142)n-, where n is 1
or 2; R is H, halo, or methoxy; X is
F; and Y is I.
In some embodiments, the invention provides a pharmaceutical composition
comprising a compound of formula I
or a pharmaceutically acceptable salt, solvate, polymorph, ester, amide,
tautomer or prodrug thereof. In some
embodiments the pharmaceutical composition further comprises at least one
pharmaceutically acceptable carrier.
In some embodiments, the invention provides a pharmaceutical composition
comprising a compound selected
771-I 0
0- NH H F NH
FN 40 40 Me0 N
from: F and F
, or a pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug thereof. In some embodiments, the
pharmaceutical composition further
comprises at least one pharmaceutically acceptable carrier. In some
embodiments, the compound is in the R
-Page 56-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
configuration. In some embodiments, the compound is in the R configuration,
substantially free of the S- isomer. In some
embodiments, the compound is in the S configuration. In some embodiments, the
compound is in the S configuration,
0, 7-7H
0-. NH F
H
F N fil
IF F 11111)11 I
substantially free of the R- isomer. In some embodiments, the compound is:
F . In some
9 77H
0.- s'NH F
H
Me0 Ail N rib
4111" F 111111" f
embodiments, the compound is: F . In
some embodiments, the compound is:
9F1 77 HO....,..õZR.,...0
HO...õ...A..õ.X...õs,-.0 0.--r-NH F
0- NHF H
H
Me0 il r4 ith Me0 0 N di
'FP F 411111)1P I F 111111-1" I
F . In some embodiments, the compound is: F .
Tables With Non-limiting Examples of Compounds of Formula I
The tables below show examples of individual compounds provided or
contemplated by this invention. These
examples should in no way be construed as limiting.
Table 1 shows embodiments of this invention which are compounds of formula I,
wherein R is as defmed herein,
G is Ria where Ria is as defined in the table and X, Y and Z are defined in
the table.
Table 1
. _
Ria X Y Ria X
Y
CH2F Cl
I
CH3 F I CH2F F
Br
CH3 CI I CH2F CI
Br
CH3 _ F Br CH2F F
CH3
CH3 CI Br CH2F CI
CH3
CH3 F CH3 CH2F F
CF3
CH3 CI CH3 CH2F CI
CF3
CH3 F CF3 CF3 F
I
CH3 CI CF3 CF3 Cl
I
CH S . F CECH CF3 F
Br
CH3 CI C2CH CF3 Cl
Br
CH3 . F SCH3 CF3 F
CH3
CH3 CI SCH3 CF3 CI
CH3
CH3 F (CH2)2CH3 CF3 F
CF3
CH3 CI (CH2)2CH3 _ CF3 CI
CF3
CH3 F CH2CH3 CH2CI F
I
CH3 CI CH2CH3 CH2CI CI
I
CH3 F CH2OH CH2CI F
Br
CH3 CI CH2OH CH2CI CI
Br
CH3 F 1> CH2CI F_
CH3
CH3 Cl C> CH2CI Cl
CH3
CH3 CH3 CH=CF12 CH2CI F
CF3
CH3 CH3 CECH CH2CI CI
CF3
CH3 CH3 SCH3 CHCl2 F I
CH2F F 1 _ CHCl2 CI I
-Page 57-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
,
Ria X Y Rio X Y
CHCl2 F Br CH20 ¨ CI CH3
CHCl2 CI Br F CF3
CHCl2 F CH3 -
CI CF3
CHCl2 Cl CH
.
CHCl2 F CF3 C H2 O -.,....Ø,-,..0 H F
I
CHCl2 CI CF3 CH.20 ..,......----..,,cm CI
I
CCI3 F I CH20 ---"--'0H F Br
CCI3 CI I
CH213'----"Th014 CI Br
CCI3 F Br
CH2 "---------oli F CH3
CCI3 CI Br
CCI3 . F CH3 Cli2o.õ....õ---..,0H CI
CH3
CCI3 CI CH3 C h12 0 -,...,-=----0 m F
CF3
CCI3 F CF3 c F120 0 H CI CF3
CCI3 CI CF3
0H20 ..,µ,.ome F I
CH2OH F I
CH2OH CI I CH20 ,,--,01.4e CI I
CH2OH , F Br CH20 ,..õ..,,,cime F
Br
CH2OH CI Br CH20 .....,,,,,ome CI
Br
CH2OH F CH3 CH2o ,,,,....-,0.4e F
CH3
CH2OH CI CH3 ,
CH20 .,,,,,--õ01.1e CI CH3
CH2OH F CF3
CH2OH CI- CF3 Ch2o,,õ-^,ome F CF3
CH20Me F I cH2o'--e---`-ome Cl CF3
CH20Me Cl I 0H F I
CH
CH20Me F Br OH
CH20Me CI Br cii2o_c011 CI I
CH20Me F CH3
OH
CH20Me Cl CH3 ii-OH F Br
CH20Me F CF3 GH20
CH20Me CI CF3 \-OH
CH
CH20Me F C - CI Br
ECH CH2
CH20Me CI SCH3OH
' -
CH20Me CH3 CF3 20_cOH F CH3
CH
CH20Me CH3 CECH OH
CH20Et F I e0H CI CH3
CH20Et CI I CH20_
OH
CH20Et F Br , ,..C1H F CF3
CH20Et CI Br CH2c
CH20Et F CH3 OH
r-OH CI CF3
CH20Et CI CH3 CH20
CH20Et F CF3 \-OH
CH20Et CI CF3 CH3 , F phenyl
CH20-4 F I CH3 CI phenyl
CH20-4 CI I CH3 CH3 phenyl
CH20- F Br CH3 F 3-pyri dyl
C H20- <I CI Br CH3 CI 3-
pyridyl
CH20-<1 F CH3 CH3 CH3 4-pyridyl
CH20-1 , CI CH3 CHs F
pyrazolyl
CH20-4 F CF3 CH3 CI pyrazolyl
CH20-4 CI CF3 CH3 F _ 4-pyridyl
CH20 ¨. F I CH3 CI 4-
pyri dyl
CH3 CH3 2-(CH3-
S02-
CH20 ¨<, Cl I NH)-phenyl
_
CH20 ¨. F Br CH3 CH3 3-(CH3-
S02-
CH20 ¨ CI Br NH)-
phenyl ,
. CH3 CI 3-(C H3-502-
CH20 ¨<, F CH3 NH)
phenyl
-Page 58-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Ria X
CH3 F 3-(CH-SO2-
NH)-phenyl
- Page 59 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Table 2 shows embodiments of this invention which are compounds of formula I,
wherein R is as defined herein,
G is Rib where Rib is as defined in the table and X, Y and Z are defined in
the table.
Table 2
Rib X /1( . Z Rib X Y
Z
D F I F
0 F I
F
1> CI I F
I> F Br F 0 CI I
F
._
I> CI Br F
0 F Br
F
D F _ CH3 F
D CI CH3 F 0 CI
Br F
I> F CF3 F
0 F CH3
F
D CI CF3 , F
I> F CECH F 0 CI CH3
F
I> CI CECH F
0 F CF3
F
,
D F SCH3 F
I> =
CI SCH3 F 0 CI CF3
F
D F CH2OH F
C> CI D
F
D CI CH2OH F
c5.0:13 F (CH2)30H F F (CH2)2CH3
F
C.
D CI (CH2)30H F
r
> F (CH2)2CH3 F
_.(1-1 CI CECH
F
D CI (CH2)2CH3 F
L.<
D F CH2CH3 F
D CI CH2CH3 F ,S--> . CH3
SCH3 F
I> F (CH2)2CH3 F
..,...tool-i3 CI CF3
F
D CI (CH2)2C H3 F
D CH3 I F
q0H CH3 CH3
F
D CH3 Br F
I> CH3 CH3 F
I> CH3 CF3 F
D CH3 CH2CH3 F
ocH, F CH2OH
F
D CH3 (CH2)2CH3 F
r.el CI (CH2)30H
F
I> CH3 CECH F
=
I> CH3 SCH3 F
1¨<
C14 CH3 (CH2)2CH3 F ........t0CHa F OCH2CH3
F
>6' 1--)-=
Net CH3 I F
F I F
F CH=CH2 F
0 Cl I
F
I> CI CH=CH2 F.
I> CH3 CH=CH2 F 0 F Br
F
D F I> F
a CI Br
F
D F OCH3 F
I> CI (CH2)2CH2OH F CD F
CH3 F
O F I F
CD CI CH3
F
O I F CD
. - F CF3
F
CI F
O F Br F 0 CI
CF3 F
0 CI Br F
I> F phenyl
F
0 F CH3 F
D CI phenyl
F
0' CI CH3 F D F - 3-pyridyl
F
O F CF3 F D CI
3-pyridyl F
> F pyrazol-4-y1
F
0 CI CF3 F
I>. CI pyrazol-4-
y1 F
-Page 60-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Rib X Y Z
[:- F 4-pyridyl F
I> CI 4-pyridyl F
I> F 1-methyl- F
pyrazol-4-y1
N CI 1-methyl- F
pyrazo1-4-y1
I> F pyrazol-3-y1 F
N CI pyrazol-3-y1 _ F
I> F 2-(CH3-S02- F
NH)-phenyl
1> Cl 2-(CH3-S02- F
NH)-phenyl
_
I> F 3-(CH3-S02- F
NH)-phenyl
Ir.> CI 3-(CH3-S02- F
NH)-phenyl
C> CH3 2-(CH3-S02- F
NH)-phenyl
I> CH3 3-(CH3-S02- F
NH)-phenyl
C> F 4-CF30-phenyl F
I> CI 4-.CFO-phenyl F
1> CH3 4-CF30-phenyl F
C1 CI 2-(CH3-S02- F
44>i
NH)-phenyl
,
O F phenyl F
0 phenyl
'
0 Cl 3-pyridyl F
O F 3-pyridyl F
O CI pyrazol-4-y1 F
O F pyrazol-4-y1 F
,
0 Cl 4-pyridyl F
O F 4-pyridyl F
O CI 1-methyl- F
pyrazol-4-y1
O CH3 1-methyl- F
pyrazol-4-y1
0 F pyrazol-3-y1 F
-
C> CI pyrazol-3-y1 F
_
0 F 2-(CH3-S02-
F
NH)-phenyl
0 CI 2-(CH3-S02-
F
NH)-phenyl
0 F phenyl F
_
0 CI phenyl F
0 F 3-pyridyl F
C> CI 3-pyridyl F
0 CI pyrazol-3-y1 F
-Page 61-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Table 3 shows embodiments of this invention which are compounds of formula I,
wherein R is as defined herein,
G is Ric where Ric is as defined in the table and X, Y and Z are defined in
the table.
Table 3
Ric X Y Z Ric _ X Y
Z
..._
CH2CH3 F I F n-butyl CH3 OCH2CH3
F
CH2CH3 CI I F n-butyl F
OCH2CH2OH ' F
CH2CH3 F Br F n-butyl F CF3
F
CH2CH3 CI Br F n-butyl CI CF3
F
CI-12CH3 F- CH3 F sec-butyl F
I F
CH2CH3 CI CH3 F sec-butyl _ Cl
I .. , F
CH2CH3 F CF3 F sec-butyl F
Br F
CH2CH3 CI CF3 F sec-butyl Cl
Br F
CH2CH3 CH3 CH3 F , sec-butyl F
CH3 F
CH2CH3 CH3 CH3 F sec-butyl Cl
CH3 F
CH2CH3 CH3 CECH _ F sec-butyl F
CF3 F
CH2CH3 CH3 SCH3 F sec-butyl
CI __ CF3 F
CH2CH3 F CECH F CH2CF3 F I
F
CH2CH3 CI SCH3 F CH2CF3 Cl I
F
CH2CH3 F t> _ F CH2CF3 F Br
F
CH2CH3 CI I> F CH2CF3 CI Br
F
CH2CH3 CH3 I> F CH2CF3 F CH3
F
CH(CH3)2 F OCH3 F CH2CF3 CI . CH3
F
CH(CH3)2 CI OCH3 F CH2CF3 F CF3
F _
CH(CH3)2 F I _ F CH2CF3 Cl CF
F
CH(CH3)2 CI I F CH2CCI3 F I
F
CH(CH3)2 F Br F CH2CCI3 Cl I
F
CH(CH3)2 CI Br_ F CH2CCI3 F Br
F
CH(CH3)2 F CH3 F CH2CCI3 Cl Br
F
CH(CH3)2 , CI CH3 F CH2CCI3 F CH3
F
CH(CH3)2 F CH2CH3 F CH2CCI3 Cl CH3
F
CH(CH3)2 CI CH2CH3 F CH2CCI3 F CF3
F
CH(CH3)2 CH3 CH2CH3 F CH2CCI3 Cl CF3
_ F
CH(CH3)2 CI CH2CH3 F CH2-<1 F I
F
CH(CH3)2 Fl CH(CH3)2 F CH2-4 Cl
I F
CH(CH3)2 CI CH(CH3)2 F CH2-4 F
Br _ F
CH(CH3)2 F CF3 _ F CH2- CI Br
F
CH(CH3)2 CI CH3 F CH2-4 F CH3
F
CH(CH3)2 CH3 Br F CH2-4 Cl CH3
F
CH(CH3)2 CH3 CECH F CH2-4 F CF3
F
CH(CH3)2 CH3 SCH3 F CH2-.4 Cl CF3
F
CH(CH3)2 CH3_
I> F CH2CH2F F I
F
CH(CH3)2 F CH2OH F CH2CH2F Cl I
F
CH(CH3)2 CIOH
-= F CH2CH2F F Br
F
CH2CH2F Cl Br F
CH2CH2F F CH3F
n-butyl F I F
_ CH2CH2F CI CH3 F
n-butyl CI I F
CH2CH2F F CF3 F
n-butyl F Br F
CH2CH2F Cl CF3 _ F
n-butyl CI Br F
CH2CH2CI F I F
n-butyl F CH3 F
CH2CH2CI Cl I F
n-butyl Cl CH3 F
CH2CH2CI F Br F
_ n-butyl F OCH3 F - -
CH2CH2CI Cl Br F
n-butyl Cl OCH3 F
- CH2CH2CI F CH3
F
n-butyl CH3 OCH3 F
CH2CH2CI Cl CH3 F
n-butyl Cl OCH2CH3 F
CH2CH2CI F CF3 F
, n-butyl F OCH2CH3 F ,
CH2CH2CI Cl CF F
- Page 62 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Ric X Y Z Ric X Y
Z
CH2CH2CH2CI F I F cH2C1-1[30-< F
I F
CH2CH2CH2CI CI I F
CI I F
- CH2CH2CH2CI F _ Br F CH3CH20-<
CH2CH2CH2CI CI _ Br F , cH2CH20- F
Br F
CH2CH2CH2CI F CH3 F CH2C Cl Br
F
1120-
, CH2CH2CH2CI CI CH F
..
CH2CH2CH2CI F CF3 F CH2CH20-< F
CH3 F
., ,.
CH2CH2CH2CI CI CF , F
CH2CH20-< CI CH3 F
CH2CH2OH F I F .
.
CH2CH2OH CI I F cH2CH20-- F
CF3 F
_
CH2CH2OH F Br F CI2CH20-< Cl
CF3 F
CH2CH2OH , CI Br F
F .
I F
- CH2CH20-<1
CH2CH2OH F CH3 F .
CH2CH2OH CI CH3 F C142CH20-< CI
I F
...
CH2CH2OH F CF3 F
CH2C1120< F Br F
CH2CH2OH CI CF3 F
CH2CH2CH2OH F _ I F CH2CH20- CI
Br F
_
CH2CH2CH2OH Cl I F CH2CH20.< F
CH3 F
CH2CH2CH2OH F Br F
- CH2CH20 CI
CH3 F
CH2CH2CH2OH CI Br F -
. CH2CH2CH2OH F CH3 F CH2CH20 --< F
CF3 F
CH2CH2CH2OH CI CH3 F CH2CH20-1 CI
CF3 F
CH2CH2CH2OH F CF3 F
CH2CH2CH20Et F I
F
CH2CH2CH2OH .õ CI CF F
CH2CH2CH20Et CI I
F
(CH2)40H F I F .
CH2CH2CH20Et F Br
.
F _
(CH2)40H CI I F
CH2CH2CH20Et CI _
Br
F
(CH2)40H F Br F
CH2CH2CH20Et F _
CH3
F
(CH2)40H CI Br F
CH2CH2CH20Et Cl CH3
F
(CH2)40H F CH3 F ,
CH2CH2CH20Et F _
CF3
F
(CH2)40H CI CH3 F
CH2CH2CH20Et CI CF3 F
(CH2)40H F CF3 , F _
_
(CH2)40H Cl CF3 F cH2c1-12c1r-0- F
I F
_
CH2CH2OCH3 F I F cH2cH,ciii--0- CI
I F
CH2CH2OCH3 CI I F ---
CH2CH2OCH3 .õ F Br F cHicH,cHi-o-- F
Br F
_
_.
CH2CH2OCH3 CI Br F cH2cH2crii--0- CI
Br F
CH2CH2OCH3 F CH3 F
CH2CH2OCH3 , CI CH3 F ci-icH,c1-11--0-
F CH3 F
_
_.
CH2CH2OCH3 F CF3 F CH2CH2C-0¨ Cl
CH3 F
CH2CH2OCH3 CI CF F
(CH2)30CH3 F I F cH2cH20-1F- F CF3
F- _
_.
(CH2)30CH3 CI I F cH2cH2cHro--( CI
CF3 F
(CH2)30CH3 F Br F __.
cii,cH,cii F I
F
i-o--4
(CH2)30CH3 CI Br F -
(CH2)30CH3 F CH3 F cH2cH2a1-0--<1 CI
I F
(CH2)30CH3 CI CH3 F cH2cH2cHT-0--(1 F
Br F
_
(CH2)30CH3 F CF3 FBr
F
_
(CH2)30CH3 CI CF3 F cii,crizeHr-o- -....4-1
CI
F CH3 F
cii,cHsHT-0--<:::1
CH2CH20Et F I F
CH2CH20Et CI I F ci-6cHacHi-0 CI CH3
F-4 _
CH2CH20Et F Br F cH,cii,c11-0--<1
F CF3 F
CH2CH20Et CI Br F , CH2C1-12.CH1c)¨
CI CF3 F
CH2CH20Et F CH3 F
cn2c112-0,-...com F I F
CH2CH20Et CI CH3 F OH
CH2CH20Et F CF3 FCI I
F
cii,cliro-"C
CH2CH20Et CI CF3 F OH
-Page 63-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Ric X Y Z Ric X Y Z
,
cri,c112-0"."CH F Br F CHICHI CI 3-
pyridyl F
¨
OH , CH2CH3 F 3-pyridyl F
cn,cutro-"C" CI Br F CH2CH3 CI 4-
pyridyl F
OH CH2CH3 F pyrazolyl F
..
en2042-0.....--,(oH F CHs F
OH
CH2CH3 CI
pyrazolyl F
-
CH2C112-0C1 CI CH3 F CH2CH3 CH3 4-
pyridyl F
OH C H2C H3 CH3 4-pyridyl F
cia2cii2-0--"CH F CF3 F CH2CH3 CH3 2-(CH3-
S02- F
OH NH)-phenyl
cn2c112-0"CH CI CF3 F CH2CH3 CH3 3-(CH3-
S02- F
OH NH)-phenyl
F I F
cH2-51 ":,---ori CH2CH3 F 3-(CH3-S02-
F
OH CI I F NH)-phenyl
cH2-A.--MH CH2CH3 CI 3-(CH3-S02-
F
(1:t
F Br F NH)-phenyl
CH/'-OH--"`OH CH2CH3 F
phenyl F
OH CI Br F
cH2-A---Thri CH2CH3 CI
phenyl F
F CH3 F CH2CH3 CH3
phenyl F
2'
CH21--1 ThH _
3-pyridyl
CI CH3 F
._.
CH{ CL-1 'OH CH(CH3)2 F 3-
pyndyl F
F CF3 F CH(CH3)2 CI 4-
pyridyl F
cH2L'oH CH(CH3)2 F
pyrazolyl F
CI CF3 F CH(CH3)2 CI
pyrazolyl F
0.42...r
.,,OH
F I F CH(CH3)2 F 4-
pyridyl F
ci,i, 01.i
CH(CH3)2 CI_ 4-
pyridyl F
OH CI I F CH(CH3)2 F 2-(CH3-S02- F
01-12--"ty"--OH NH)-phenyl
.
OH CH(CH3)2 CI 3-(CH3-S02-
F
OH CH3 I F NH)-phenyl
C H2'1 ' OH
1- CH(CH3)2 F 3-(CH3-S02- F
F Br F NH)-phenyl
CH2-5"14cOH
CH(CH3)2 CI 3-(CH3-S02-
F
CI Br F NH)-phenyl
CH2-"I'OH CH(CH3)2 CH3
phenyl F
OH CH3 Br F CH(CH3)2 CI
phenyl-F
CH2---kri'OH
CH(CH3)2 Fl
phenyl F
OH F CH3 F . CH(CH3)2 CI 3-pyridyl
F
CHeY-OH 3-
pyridyl
OH
CI CH3 F CH(CH3)2 F 4-
pyridyl F
CHX-101;OH CH(CH3)2 CI
pyrazolyl F
OH CH3 CH3 F CH(CH3)2 CH3 pyrazolyl
F
01-12-ti-OH CH(CH3)2 CH3 4-
pyridyl F
F CECH F CH(CH3)2 CH3 4-
pyridyl F
ci.12,:i 0H
CH(CH3)2 CH3 2-(CH3-502-
F
NH)-phenyl
F SCH3 F
CHI-O.Li-r-HOH CH(CH3)2 F 3-(CH3-S02-
F
OH NH)-phenyl
OH F CH2CH2CH3 F
01-12--A-r-'0H CH(CH3)2 CI 3-(0H3-S02-
F
OH NH)-phenyl
OH CI CH2CH(OH)CH3 F 3-(CH3-S02-
cH2"Cfri " NH)-phenyl
F CH(CH3)2 F n-butyl F
phenyl F
CH2-"Y-OH
OH n-butyl CI
phenyl F
CI CF3 F n-butyl F
phenyl F
OFIXOH-OH n-butyl _ CI 3-
pyridyl F
___ CH2CH3 F phenyl F n-butyl F 3-
pyridyl F
CH2CH3 CI phenyl F n-butyl CI 4-pyridyl F
CH2CH3 F phenyl F n-butyl F pyrazolyl F
- Page 64 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Ric X Y Z Ric X Y Z
n-butyl CI pyrazolyl F CH2CH2F CI phenyl F
n-butyl CH3 4-pyridyl F CH2CH2F F phenyl F
n-butyl CI 4-pyridyl F CH2CH2F CI phenyl F
n-butyl F 2-(CH3-S02- F CH2CH2F F 3-pyridyl F
NH)-phenyl CH2CH2F CI 3-pyridyi F
n-butyl CH3 3-(CH3-S02- F CH2CH2F F 4-pyridyl F
NH)-phenyl . CH2CH2F CI pyrazolyl F
n-butyl F 3-(CH3-S02- F pyrazolyl
NH)-phenyl
CH2CH2CI F 4-pyridyl F
3-(CH3-S02-
NH)-phenyl CH2CH2CI CI 4-pyridyl F
CH2CH2CI F 2-(CH3-
S02- F
n-butyl F phenyl F
NH)-phenyl
n-butyl CI phenyl F
CH2CH2CI CI 3-(CH3-
S02- F
phenyl NH)-
phenyl
sec-butyl F 3-pyridyl F CH2CH2CI F 3-(CH3-
S02- F
_
sec-butyl CI 3-pyridyl F NH)-
phenyl
,
sec-butyl F 4-pyridyl F CH2CH2CI CI 3-(CH3-
S02- F
sec-butyl CI pyrazolyl F NH)-
phenyl
_
sec-butyl F pyrazolyl F CH2CH2CI F phenyl F
sec-butyl CI 4-pyridyl F CH2CH2CI CI phenyl F
sec-butyl . F 4-pyridyl F phenyl
,
sec-butyl CI CF3 F CH2CH2CH2CI F 3-pyridyl F
CH2CF3 F phenyl F CH2CH2CH2CI CI 3-pyridyl F
CH2CF3 CI phenyl F CH2CH2CH2CI F 4-pyridyl F
CH2CF3 F phenyl F CH2CH2CH2CI CI pyrazolyl F
CH2CF3 CI 3-pyridyl F CH2CH2CH2CI F pyrazolyl F
CH2CF3 F 3-pyridyl F CH2CH2CH2CI CI 4-pyridyl F
CH2CF3 CI 4-pyridyl F CH2CH2CH2CI F 4-pyridyl F
CH2CF3 F pyrazolyl F CH2CH2CH2CI CI 2-(CH3-
S02- F
CH2CF3 Cl pyrazolyl F NH)-
phenyl
, 4-pyridyl 3-(CH3-
S02-
CH2CCI3 F 4-pyridyl F NH)-
phenyl
CH2CCI3 Cl 2-(CH3-S02- F CH2CH2OH F 3-(CH3-
S02- F
NH)-phenyl NH)-
phenyl
CH2CCI3 F 3-(CH3-S02- F CH2CH2OH CI 3-(CH3-
S02- F
NH)-phenyl NH)-
phenyl
CH2CCI3 CI 3-(CH3-S02- F CH2CH2OH F phenyl F
NH)-phenyl CH2CH2OH CI phenyl F
CH2CCI3 F 3-(CH3-S02- F CH2CH2OH F phenyl F
NH)-phenyl CH2CH2OH CI 3-pyridyl F
CH2CCI3 _ CI phenyl F CH2CH2OH F 3-pyridyl F
CH2CCI3 F phenyl F CH2CH2OH CI 4-pyridyl F
CH2CCI3 CI phenyl F pyrazolyl
3-pyridyl CH2CH2CH2OH F pyrazolyl F
CH2-1 F 3-pyridyl F CH2CH2CH2OH CI 4-pyridyl F
CH2-1 Cl 4-pyridyl F CH2CH2CH2OH F 4-pyridyl F
CH2-1 F pyrazolyl F CH2CH2CH2OH CI 2-(CH3-
802- F
CH2-4 CI pyrazolyl F NH)-
phenyl
CH2-4 F 4-pyridyl F CH2CH2CH2OH F 3-(CH3-
S02- F
CH2-4 CI 4-pyridyl F NH)-
phenyl
CH2-4 F 2-(CH3-S02- F CH2CH2CH2OH CI 3-(CH3-
S02- F
NH)-phenyl NH)-
phenyl
CH2-1 CI 3-(CH3-S02- F CH2CH2CH2OH F 3-(CH3-
S02- F
NH)-phenyl ¨ , NH)-
phenyl
3-(CH3-S02- CH2CH2CH2OH CI phenyl F
NH)-phenyl phenyl
CH2CH2F F 3-(CH3-502- F (CH2)40H F phenyl F
NH)-phenyl (CH2)40H CI 3-pyridyl F
_ _
-Page 65-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Ric X Y Z
(CH2)40H F 3-pyridyl F
(CH2)40H CI 4-pyridyl F
(CH2)40H F pyrazolyl F
(CH2)40H CI pyrazolyl F
(CH2)40H F 4-pyridyl F
(CH2)40H CI 4-pyridyl F
2-(CH3-S02-
NH)-phenyl
CH2CH2OCH3 F 3-(CH3-S02- F
NH)-phenyl
CH2CH2OCH3 CI 3-(CH3-S02- F
NH)-phenyl
CH2CH2OCH3 F 3-(CH3-S02- F
NH)-phenyl
CH2CH2OCH3 CI phenyl F
CH2CH2OCH3 _ F phenyl F
CH2CH2OCH3 CI phenyl F
CH2CH2OCH3 _ F 3-pyridyl F
CH2CH2OCH3 CI 3-pyridyl F
4-pyridyI
(CH2)30CH3 F pyrazolyl F
(CH2)30CH3 CI pyrazolyl F
(C I-12)30C H3 F 4-pyridyl F
(C H2)30C H3 CI 4-pyridyi F
(CH2)30CH3 F 2-(CH3-S02- F
NH)-phenyl
(CH2)30CH3 CI 3-(CH3-S02- F
NH)-phenyl
(CH2)30CH3 F 3-(CH3-S02- F
, NH)-phenyl
(CH2)30CH3 Cl 3-(CH3-S02- F
NH)-phenyl
phenyl
CH2CH20Et F phenyl F
CH2CH20Et CI phenyl F
CH2CH20Et F 3-pyridyl F
CH2CH20Et Cl 3-pyridyl F
CH2CH20Et F 4-pyridyl F
CH2CH20Et Cl pyrazolyl F
CH2CH20Et F pyrazolyl F
CH2CH20Et Cl 4-pyridyl F
-Page 66-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Tables 4a and 4b show embodiments of this invention which are compounds of
formula I, where G,--- Rid, Z is F, X
is F and Rid and R are defined in the table. Each line in the table
corresponds to five species which differ only at position
Y.
Table 4a
A'
B
A\,'V,0
0- NH F
H
R SI N 40
F Ya.b, o or d
F
Ya = CH3; Yb = Br; Y, = I; Yd = Ci;
CMPD # A, A' B le
1(a-d) H, H H OCH3
2(a-d) _ H, H- H NHCH3
3(a-d) H, H H CH2CH3
4(a-d) H, H H CH2CH=CH2
5(a-d) H, H H CN
6(a-d) H, H H CF3
7(a-d) H, H H F
8(a-d} H, H H C6H6
9(a-d) H, H -CH2CH(OH)CH2OH .... OCH3
10(a-d) H, I-I -CH2CH(OH)CH2OH NHCH3
11(a-d) H, H -CH2CH(OH)CH2OH CH2CH3
, 12(a-d) -(CH2)2- -CH2(C3H5) OCH3
13(a-d) -(CH2)2- -CH2(C3H5) NHCH3
14(a-d) -(CH2)2- -CH2(C3H5) CH2CH3
15(a-d) -(CH2)2- CH3 F
16(a-d) -(CH2)2- -CH2CH2OH F
17(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH F
. 18(a-d) CH3, H -(CH2)2CH(OH)CH2OH F
19(a-d) -(CH2)2- CH3 OCH3
20(a-d) -(CH2)2- -CH2CH2OH OCH3
21(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH OCH3
22(a-d) CH3, H -(CH2)2CH(OH)CH2OH OCH3
23(a-d) -(CH2)2- CH3 H
24(a-d) -(CH2)2- -CH2CH2OH H
25(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH H
26(a-d) CH3, H -(CH2)2CH(OH)CH2OH H
27(a-d) H, H H OCH3
28(a-d) H, H H NHCH3
29(a-d) H, H H CH2CH3
30(a-d) H, H H CH2CH=CH2
_ 31(a-d) H, H H CN
32(a-d) H, H H CF3
33(a-d) H, H H F
34(a-d) H, 1-1 H C8H6
35(a-d) H, H -CH2CH(OH)CH2OH OCH3
36(a-d) H, H -CH2CH(OH)CH2OH NHCH3 .
37(a-d) H, H -CH2CH(OH)CH2OH_ CH2CH3
38(a-d) -(CH2)2- -CH2(C3H5) OCH3
39(a-d) -(CH2)2- -CH2(C3H5) NHCH3
40(a-d) -(CH2)2- -CH2(C3H5) CH2CH3
41(a-d) -(CH2)2- CH3 F
"
, -Page 67-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
_
CMPD # A, A' B R
_
42(a-d) -(CH2)2- -CH2CH2OH F
43(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH F
44(a-d) CH3, H , -(CH2)2CH(OH)CH2OH F
45(a-d) -(CH2)2- CH OCH3
46(a-d) -(CH2)2- -CH2CH2OH OCH3
47(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH OCH3
48(a-d) CH3, H -(CH2)2CH(OH)CH2OH . OCH3
_
49(a-d) , -(CH2)2- . CH3 H
50(a-d) -(CH2)2- . -CH2CH2OH H
51(a-d) -(CH2)2- -(CH2)2CH(OH)CH2OH H
52(a-d) CH3, H -(CH2)2CH(OH)CH2OH H
Table 4b
CllAPD # A, A' B R
1(a-d) H, H H . 2-furanyl
2(a-d) H, H H 1,2,3 triazoly1-4-y1
3(a-d) H, H H 4-imidazoly1
4(a-d) H, H H 2-furanyl
5(a-d) H, H H 1,2,3 triazoly1-4-y1
6(a-d) , H, H H 4-imidazoly1
7(a-d) H, H - 2-furanyl
(CH2)2CH(OH)CH2OH
8(a-d) H, H - 1,2,3 triazoly1-4-y1
(CH2)2CH(OH)CH2OH
9(a-d) H, H - 4-imidazoly1
(CH2)2CH(OH)CH2OH
10(a-d) -(CH2)2- , -CH2(C3H5) 2-furanyl _
11(a-d) -(CH2)2- -CH2(C3H5) 1,2,3 triazoly1-4-0
12(a-d) -(CH2)2- -CH2(C3H5) 4-imidazoly1
13(a-d) -(CH2)2- CH3 4-thiazoly1
14(a-d) -(CH2)2- -CH2CH2OH 4-thiazoly1
15(a-d) -(CH2)2- - 4-thiazoly1
(CH2)2C1t0H)CH2OH
16(a-d) CH3, H - 4-thiazoly1
(CH2)2CH(OH)CH2OH
17(a-d) -(CH2)2- C1-13 2-oxazoly1 _
18(a-d) -(CH2)2- -CH2CH2OH 2-oxazoly1
19(a-d) -(CH2)2- - 2-oxazoly1
(CH2)2CH(OH)CH2OH
20(a-d) CH3, H - 2-oxazoly1
(CH2)2CH(OH)CH2OH
-
21(a-d) H, H H 2-furanyl
22(a-d) H, H , H 1,2,3 triazoly1-4-y1
23(a-d) H, H H 4-imidazoly1
24(a-d) H, H H 2-furanyl
25(a-d) H, H H 1,2,3 triazoly1-4-y1
26(a-d) H, H H 4-imidazoly1
27(a-d) H, H -CH2CH(OH)CH2OH 2-furanyi
28(a-d) H, H -CH2CH(OH)CH2OH 1,2,3 triazoly1-4-y1
29(a-d) H, H -CH2CH(OH)CH2OH 4-imidazoly1
30(a-d) -(CH2)2- -CH2(C3H5) 2-furanyl
31(a-d) -(CH2)2- -CH2(C3H5) 1,2,3 triazoly1-4-y1
32(a-d) -(CH2)2- -CH2(C3H5) 4-imidazoly1
33(a-d) -(CH2)2- CH 4-thiazoly1
34(a-d) -(CH2)2- -CH2C1-120H 4-thiazoly1
35(a-d) -(CH2)2- - 4-thiazoly1
1 I (CH2)2CH(OH)CH2OH
- Page 68 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
CMPD # A, A B R
36(a-d) CH3, H - 4-thiazoly1
(CH2)2CH(OH)CH2OH
37(a-d) -(CH2)2- CH 2-oxazoly1
38(a-d) -(CH2)2- -CH2CH2OH 2-
oxazoly1
39(a-d) -(CH2)2- - 2-oxazoly1
(CH2)2CH(OH)CH2OH
40(a-d) CH3, H - 2-oxazoly1
(CH2)2CH(OH)CH2OH .
Table 5a shows embodiments of this invention which are compounds of formula I,
where G is Ari, Ar2 or Rid, and
where R is H, Z is F and G and X are defined in the table. Each line in the
table corresponds to five species (Ya, Yb, Y.,
Ya and Ye) which differ only at position Y, where Ye = SCH3; Yb = Br; Y, = I;
Yd = Cl; Y, ¨ CH3.
Table 5a
0
11
N1--NH X
or II H
N 0
Ar2 'a
or
Rid litF
Ya,b,c, d, ore
F
Y.= SCH3; Yb = Br; Y, = I; Yd = Cl; Y, = CH3
Compound Compound
# G = Rid, Ari, or Ar2 X # G =
Rid, Ari, or Ar2 X
1 (a-e) _ phenyl CI 31 (a-e) 2-F-3-Cl-
phenyl CI
2 (a-e) phenyl F 32 (a-e) 2-F-3-Cl-
phenyl F
3 (a-e) 2-F-phenyl CI 33 (a-e) 2-F-4-Cl-
phenyl CI
4 (a-e) 2-F-phenyl F 34 (a-e) 2-F-4-Cl-
phenyl F
5 (a-e) 3-F-phenyl CI 35 (a-e) 2-F-5-Cl-
phenyl CI
6 (a-e) 3-F-phenyl F .. 36 (a-e)
2-F-5-Cl-phenyl _ F
7 (a-e) 4-F-phenyl CI . 37 (a-e) 3-
cyano-4-F-phenyl CI
8 (a-e) 4-F-phenyl F 38 (a-e) 3-
cyano-4-F-phenyl F
9 (a-e) 2,4-di-F-phenyl CI 39 (a-e) 2-Cl-phenyl
CI
(a-e) 2,4-di-F-phenyl F 40 (a-e) 2-Cl-phenyl
F
11 (a-e) 2,5-di-F-phenyl CI 41 (a-e) 3-Cl-phenyl
CI
12 (a-e) 2,5-di-F-phenyl F 42 (a-e) 3-CI-phenyl
F
13 (a-e) 2,6-di-F-phenyl CI 43 (a-e) 4-Cl-phenyl
CI
14 (a-e) 2,6-di-F-phenyl F 44 (a-e) 4-Cl-phenyl
F
(a-e) 3,4-di-F-phenyl CI 45 (a-e) 2,3-di-Cl-phenyl
CI
16 (a-e) 3,4-di-F-phenyl F 46 (a-e) 2,3-di-Cl-
phenyl F
17 (a-e) 3,5-di-F-phenyl CI_ 47 (a-e)
2,5-di-Cl-phenyl CI
18 (a-e) 3,5-di-F-phenyl F 48 (a-e) 2,5-di-Cl-
phenyl F
19 (a-e) 2,6-di-F-phenyl CI 49 (a-e) 2,6-di-Cl-
phenyl CI
(a-e) 2,6-di-F-phenyl F 50 (a-e) 2,6-di-Cl-phenyl
F
21 (a-e) 2,3,4-tri-F-phenyl CI 51 (a-e) 3,5-di-CI-
phenyl CI
22 (a-e) 2,3,4-tri-F-phenyl F 52 (a-e) 3,5-di-Cl-
phenyl F
23 (a-e) 3,4,5-tri-F-phenyl . CI 53 (a-e) 2,4-di-Cl-
phenyl CI
24 (a-e) 3,4,5-tri-F-phenyl , F 54 (a-e) 2,4-di-Cl-
phenyl F
(a-e) penta-F-phenyl CI 55 (a-e) 3,4-di-Cl-phenyl
CI
26 (a-e) penta-F-phenyl F 56 (a-e) _
3,4-di-Cl-phenyl F
27 (a-e) 3-CI-4-F-phenyl CI 57 (a-e) 2,4,6-tri-Cl-
phenyl CI
28 (a-e) 3-CI-4-F-phenyl. F 58 (a-e)
2,4,6-tri-Cl-phenyl F
29 (a-e) 2-CI-4-F-phenyl CI 59 (a-e) ,
2-CI-4-CF3-phenyl CI
(a-e) 2-CI-4-F-phenyl F 60 (a-e) 2-CI-4-
CF3-phenyl F
- Page 69 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Compound Compound
# G = Rid, Ari, or Ar2 X
# G = Rid, AN or Ar2 X
61 (a-e) 2-CF3-phenyl CI 111 (a-e) 3-C1-44(N-
CI
62 (a-e) 2-CF3-phenyl F
pyrrolidinylcarbonyl)aminolphenyl _
63 (a-e) µ 3-CF3-phenyl CI 112 (a-e) 3-C1-4-[(N-
F
64 (a-e) 3-CF3-phenyl F
pyrrolidinylcarbonyl)aminolphenyl ,
65 (a-e) 4-CF3-phenyl CI 113 (a-e) 3,5-
dimethylisoxazoly1 CI
66 (a-e) 4-CF3-phenyl F 114 (a-e) 3,5-
dimethylisoxazoly1 F
67 (a-e) 2-CF30 phenyl CI _ 115 (a-e) 4-
(N-morpholinylsulfonyl)phenyl CI
68 (a-e) 2-CF30 phenyl F 116 (a-e) 4-
(N-morpholinylsulfonyl)phenyl F
69 (a-e) 3-CF30 phenyl CI 117 (a-e) 3-F-benzyl
CI
70 (a-e) 3-CF30 phenyl _ F 118 (a-e) 3-F-benzyl
F
71 (a-e) 4-CF30 phenyl CI 119 (a-e) 4-F-benzyl
CI
72 (a-e) 4-CFs0 phenyl F 120 (a-e) 4-F-benzyl
F
73 (a-e) 2-CHF20 phenyl CI 121 (a-e) 3-F-phenyl-ethyl
CI
74 (a-e) 2-CHF20 phenyl F 122 (a-e) 3-F-phenyl-ethyl
F
75 (a-e) 2-methyl-5-nitro-phenyl CI
123 (a-e) 4-F-phenyl-ethyl CI
76 (a-e) 2-methyl-5-nitro-phenyl F
124 (a-e) 4-F-phenyl-ethyl F
77 (a-e) 2-cyano-phenyl CI 125 (a-e) 8-quinolinyl
CI
78 (a-e) 2-cyano-phenyl F 126 (a-e) 8-quinolinyl
F
79 (a-e) 3-cyano-phenyl CI 127 (a-e) 2-thienyl
_ CI
80 (a-e) 3-cyano-phenyl F 128 (a-e) 2-thienyl
F
81 (a-e) 4-cyano-phenyl CI 129 (a-e) 2,3-di-C1-thien-5-y1
CI
82 (a-e) 4-cyano-phenyl F 130 (a-e) 2,3-di-C1-thien-5-y1
F
83 (a-e) 4-methoxy-phenyl CI 131 (a-e)
1,3,5 trimethy1-1H-pyrazoly1 CI
84 (a-e) 4-methoxy-phenyl F 132 (a-e) .
1,3,5 trimethy1-1H-pyrazoly1 F
85 (a-e) 3,4-dimethoxy-phenyl CI
133 (a-e) 1,3-climethy1-5-C1-1H-pyrazoly1 CI
86 (a-e) 3,4-dimethoxy-phenyl F
134 (a-e) 1,3-dimethy1-5-C1-1H-pyrazoly1 F
87 (a-e) 3-carbamyl-phenyl CI 135 (a-e)
1 -methy1-3CF3-1H-pyrazol-4-y1 CI
88 (a-e) 3-carbamyl-phenyl F 136 (a-e)
1-methy1-3CF3-1H-pyrazol-4-y1 F
89 (a-e) 3-carboxyl-phenyl CI
137 (a-e) 2-acetamido-4-methyl-thiazol-5-y1 CI
90 (a-e) 3-carboxyl-phenyl F
138 (a-e) 2-acetannido-4-methyl-thiazo1-5-y1 F
91 (a-e) 3-(N,N-dimethylcarbamoyl)phenyl CI 139 (a-e)
2,4-dimethyl-thiazol-5-y1 CI
92 (a-e) 3-(N,N-dimethylcarbamoyl)phenyl F 140 (a-e)
2,4-dimethyl-thiazol-5-y1 F
93 (a-e) 4-methylsulfonyl-phenyl CI
141 (a-e) 1,2-dimethy1-11-1-imidazol-4-y1 CI
94 (a-e) 4-methylsulfonyl-phenyl F
142 (a-e) 1,2-dimethy1-1H-imidazol-4-y1 F
95 (a-e) 3-(1,3,4 oxadiazo1-2-yl)phenyl CI
96 (a-e) 3-(1,3,4 oxadiazol-2-yl)phenyl F
97 (a-e) 3-(1,3,4 thiadiazol-2-yl)phenyl CI
98 (a-e) 3-(1,3,4 thiadiazol-2-yl)phenyl F
99 (a-e) 3-(5-methy1-1-1,3,4- CI
oxadiazol)phenyl
100 (a-e) 3-(5-methyl-1-1,3,4- F
oxadiazol)phenyl
101 (a-e) 3-(5-methy1-1-1,3,4- Cl
thiadiazol)phenyl
. .
102 (a-e) 3-(5-methy1-1-1,3,4- F
thiadiazol)phenyl
103 (a-e) 3-amidinyl-phenyl CI
104 (a-e) 3-amidinyl-phenyl F
105 (a-e) 3-(1H-tetrazolyl)phenyl CI .
106 (a-e) 3-(1H-tetrazolyl)phenyl F
107 (a-e) 4-acetamido-phenyl CI
108 (a-e) 4-acetamido-phenyl F
109 (a-e) 3-CI-4-[(N- CI
morpholinylcarbonyl)amino]phenyl
110 (a-e) 3-CI-4-[(N- F
morpholinylcarbonyl)amino]phenyl
... ,
- Page 70 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Table 5b shows embodiments of this invention which are compounds of formula I,
where G is Ari, Ar2 or Rid, and
where R is H, Z is F and G and X are defined in the table. Each line in the
table corresponds to five species (Yõ Yb, Ye,
Yd and Ye) which differ only at position Y, where Y, = phenyl; Yi, = 3-
substituted phenyl; Y, = 3-pyridyl; Yd = 4-pyridyl;
Ye= 3-pyrazolyl.
Table 5b
0
II
Ari¨S¨NH X
or II H
Ar2 0, N 40
or
Rid F
Ye,b,c, d, ore
F
Y. = phenyl; Yb = 3-substituted phenyl; Y, = 3-pyridyl; Yd = 4-pyridyl; Ye = 3-
pyrazoly1
Compound # G = Rich Ari, or Ar2 X Compound # G = Rich Ari, or
Ar2 X
_
1 (a-c) phenyl CI 41 (a-e) . 3-Cl-phenyl
Cl
2 (a-e) phenyl F 42 (a-c) 3-Cl-phenyl
F
3 (a-e) 2-F-phenyl Cl 43 (a-c) 4-Cl-phenyl
Cl
4 (a-e) 2-F-phenyl F 44 (a-e) 4-Cl-phenyl
F
5 (a-e) 3-F-phenyl Cl 45 (a-c) 2,3-
di-Cl-phenyl Cl
6 (a-c) 3-F-phenyl F 46 (a-e) 2,3-
di-Cl-phenyl F
7 (a-c) 4-F-phenyl Cl - 47 (a-c) 2,5-
di-CI-phenyl CI
8 (a-e) 4-F-phenyl F 48 (a-c) 2,5-
di-Cl-phenyl F
-
9 (a-c) 2,4-di-F-phenyl CI 49 (a-e) 2,6-
di-Cl-phenyl CI
(a-c) 2,4-di-F-phenyl F 50 (a-e) 2,6-di-
Cl-phenyl F
11 (a-c) 2,5-di-F-phenyl Cl 51 (a-c) 3,5-
di-Cl-phenyl Cl
12 (a-c) 2,5-di-F-phenyl F 52 (a-c) 3,5-
di-Cl-phenyl F
13 (a-c) 2,6-di-F-phenyl Cl 53 (a-e) 2,4-
di-Cl-phenyl Cl
14 (a-c) 2,6-di-F-phenyl F 54 (a-c) 2,4-
di-Cl-phenyl F
(a-c) 3,4-di-F-phenyl CI 55 (a-c) 3,4-di-
Cl-phenyl Cl
16 (a-c) 3,4-di-F-phenyl F 56 (a-c) 3,4-
di-Cl-phenyl F
_ 17 (a-c) 3,5-di-F-phenyl Cl 57 (a-c) 2,4,6-tri-Cl-
phenyl Cl
18 (a-c) 3,5-di-F-phenyl F 58 (a-c) 2,4,6-tri-Cl-
phenyl F
19 (a-c) 2,6-di-F-phenyl Cl 59 (a-c) 2-C1-4-CF3-
phenyl j Cl
(a-c) 2,6-di-F-phenyl F 60 (a-c) 2-C1-4-CF3-phenyl
F
21 (a-c) 2,3,4-tri-F-phenyl Cl 61 (a-c) 2-CF3-phenyl
Cl
22 (a-c) 2,3,4-tri-F-phenyl F 62 (a-c) 2-CF3-phenyl
F
23 (a-e) 3,4,5-rri-F-phenyl Cl 63 (a-c) 3-CF3-phenyl
Cl
24 (a-c) 3,4,5-tri-F-phenyl F 64 (a-c) 3-CF3-phenyl
F
(a-c) penta-F-phenyl Cl 65 (a-c) 4-CF3-phenyl
Cl
26 (a-c) penta-F-phenylF 66 (a-c) 4-CF3-phenyl
F
27 (a-c) 3-C1-4-F-phenyl - Cl 67 (a-c) 2-
CF30 phenyl Cl
28 (a-c) 3-C1-4-F-phenyl F 68 (a-c) 2-
CF30 phenyl F
29 (a-c) 2-C1-4-F-phenyl Cl 69 (a-c) 3-
CF30 phenyl Cl
(a-c) 2-C1-4-F-phenyl F 70 (a-e) 3-CF30
phenyl F
31 (a-c) 2-F-3-C1-phenyl Cl 71 (a-c) 4-
CF30 phenyl _ Cl
32 (a-c) 2-F-3-Cl-phenyl F 72 (a-c) 4-
CF30 phenyl F
33 (a-c) 2-F-4-Cl-phenyl Cl . 73 (a-c) 4-
CHF20-phenyl Cl
34 (a-c) 2-F-4-Cl-phenyl F 74 (a-e) 4-
CHF20-phenyl F
(a-c) 2-F-5-Cl-phenyl Cl 75 (a-c) 2-methyl-5-nitro-
phenyl Cl
_ 36 (a-c) 2-F-5-Cl-phenyl F 76 (a-c) 2-methyl-5-nitro-
phenyl F
37 (a-e) 3-cyano-4-F-phenyl Cl 77 (a-c)
2-cyano-phenylCl
38 (a-c) 3-cyano- 4-F-phenyl F . 78 (a-c) 2-
cyano-phenyl - F
39 (a-c) 2-Cl-phenyl Cl 79 (a-c) 3-
cyano-phenyl Cl
(a-c) 2-CI-phenyl F 80 (a-e) 3-
cyano-phenyl F
-Page 71-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Compound # G = Rid, Arl, or Ar2 X
Compound # G = Rid, An, or Ar2 X
81 (a-e) 4-cyano-phenyl. Cl 120 (a-e) 4-F-benzyl
F
82 (a-e) . 4-cyano-phenyl F 121 (a-e) 3-F-phenyl-ethyl
Cl
83 (a-c) 4-methoxy-phenyl Cl 122 (a-c)
3-F-phenyl-ethyl F
84 (a-e) 4-methoxy-phenyl F 123 (a-c)
4-F-phenyl-ethyl Cl
85 (a-c) 3,4-dimethoxy-phenyl Cl
124 (a-c) 4-F-phenyl-ethyl F
86 (a-c) 3,4-dimethoxy-phenyl F
125 (a-e) 8-quinolinyl Cl
87 (a-c) 3-carbamyl-phenyl Cl 126 (a-e)
8-quinolinyl F
88 (a-c) 3-carbamyl-phenyl F 127 (a-c)
2-thienyl Cl
89 (a-c) 3-carboxyl-phenyl Cl 128 (a-c)
2-thienyl F
90 (a-c) 3-carboxyl- phenyl F 129 (a-c)
2,3-di-C1-thien-5-y1 Cl
91 (a-c) 3-(N,N- Cl 130 (a-c) 2,3-di-C1-thien-5-y1
F
dimethylcarbamoyl)phenyl 131 (a-c) 1,3,5 trimethy1-1H-
CI
92 (a-c) 3-(N,N- F pyrazolyl
dimethylcarbarnoyl)phenyl 132 (a-c) 1,3,5 trimethyl-1H-
F
93 (a-c) , 4-methylsulfonyl-phenyl Cl
pyrazolyl
94 (a-c) 4-methylsulfonyl-phenyl F 133 (a-c)
1,3-dimethy1-5-C1-1H- Cl
95 (a-c) 3-(1,3,4 oxadiazol-2- Cl
pyrazolyl
yl)phenyl 134 (a-e) 1,3-dimethyI-5-C1-1H-
F
_
96 (a-c) 3-(1,3,4 oxadiazol-2- F
pyrazolyl
yl)phenyl 135 (a-c) 1-methy1-3-CF3-1H-
Cl
97 (a-c) 3-(1,3,4 thiadiazol-2- Cl pyrazol-4-
y1
yl)phenyl 136 (a-c) 1-methy1-3-CF3-1I-1-
F
98 (a-c) 3-(1,3,4 thiadiazol-2- F pyrazol-4-y1
yl)phenyl 137 (a-c) 2-acetamido-4-methyl-
CI
99 (a-c) 3-(5-methy1-1,3,4- Cl
thiazol-5-y1
oxadiazol)phenyl 138 (a-c) 2-acetamido-4-methyl- F
'
100 (a-c) 3-(5-methy1-1,3,4- F
thiazol-5-y1
oxadiazol)phenyl 139 (a-c) 2,4-dimethyl-thiazol-5-y1 CI
101 (a-c) 3-(5-methy1-1,3,4- Cl 140 (a-c)
2,4-dimethyl-thiazol-5-y1 F
thiadiazol)phenyl _ 141 (a-c) 1,2-dimethy1-1H-imidazol-
Cl
102 (a-c) 3-(5-methy1-1,3,4- F 4-y1
thiadiazol)phenyl 142 (a-c) 1,2-dimethy1-1H-imidazol-
F
103 (a-c) 3-amidinyl-phenyl Cl 4-y1
104 (a-c) 3-amidinyl-phenyl F 143 (a-c)
1-(2-hydroxyethyl) F
105 (a-c) 3-(1H-tetrazolyl)phenyl Cl cyclopropyl
106 (a-e) 3-(1H-tetrazolyl)phenyl . F 144 (a-c)
1-(3-hydroxypropyl) F
107 (a-c) 4-acetamido-phenyl Cl cyclopropyl
108 (a-c) 4-acetamido-phenyl F 145 (a-c) 1-(2,3-
dibydroxypropyl) F
109 (a-c) 3-0-4-[(N- Cl cyclopropyl
molpholinykarbonyl) 146 (a-c) 1-(3,4-dihydroxybutyl) F
aminolphenyl cyclopropyl
110 (a-c) 3-C1-4-[(N- F 147 (a-c) 1-(2,3-
dihydroxypropyl) F
morpholinylcarbonyl) cyclobutyl
amincdphenyl
111 (a-c) 3-C1-4-[(N- Cl
pyrrolidinykarbonyl)
amino]phenyl
112 (a-c) 3-C1-4-[(N- F
pyrrolidinylcarbonyl)
amino]phenyl
113 (a-c) 3,5-dimethylisoxazoly1 Cl
114 (a-c) 3,5-dimethylisoxazoly1 F
115 (a-c) 4-(N- Cl
, morpholinylsulfortypphenyl
116 (a-c) 4-(N- F
morpholinylsulfonyl)phenyl
'
117 (a-c) 3-F-benzyl Cl
118 (a-c) 3-F-benzyl F
119 (a-c) 4-F-benzyl Cl
- Page 72 -
CA 02693390 2015-12-04
=
30725-1660
Synthetk Procedures
In another aspect, methods for synthesizing the compounds described herein are
provided. In some embodiments,
the compounds described herein can be made by the methods described below. The
procedures and examples below are
intended to illustrate those methods. Neither the procedures nor the examples
should be construed as limiting the
invention in any way. Compounds described herein may also be synthesized using
standard synthetic techniques known
to those of skill in the art or using methods known in the art in combination
with methods described herein. In additions,
solvents, temperatures and other reaction conditions presented herein may vary
according to the practice and knowledge
of those of skill in the art.
The starting materials for the synthesis of the compounds as described herein
may be obtained from commercial
sources, such as Aldrich Chemical Co. (Milwaukee, Wis.), Sigma Chemical Co.
(St. Louis, Mo.), or the starting materials
can be synthesized. The compounds described herein, and other related
compounds having different substituents can be
synthesized using techniques and materials known to those of skill in tbe art,
such as described, for example, in March,
ADVANCED ORGANIC CHEMISTRY 4th Ed., (Wiley 1992); Carey and Sundberg, ADVANCED
ORGANIC CHEMISTRY el Ed.,
Vols. A and B (Plenum 2000,2001), and Green and Wuts, PROTECTIVE GROUPS IN
ORGANIC SY1111IESIS3'd Ed.,
(Wiley 1999). General methods for the preparation of compound as
disclosed herein may be derived from known reactions in the field, and the
reactions may be modified by the use of
appropriate reagents and conditions, as would be recognized by the skilled
person, for the introduction of the various
moieties found in the formulae as provided herein. As a guide the following
synthetic methods may be utilized.
Formation of Covalent Linkages by Reaction of Electropinle with a Nuclet_Thile
The compounds described herein can be modified using various electrophiles or
nucleophiles to form new
functional groups or substituents. The table below entitled "Examples of
Covalent Linkages and Precursors Thereof' lists
selected examples of covalent linkages and precursor functional groups which
yield and can be used as guidance toward.
the variety of electrophiles and nucleophiles combinations available.
Precursor functional groups are shown as
electrophilic groups and nucleophilic groups.
Covalent Linkage Eleetrophile Nucleophile
Product
____________________ Carboxamides Activated esters
Amines/aniliaies
Carboxamides Acyl azides Amines/anilines
Carboxamides Acyl halides Amines/anilines
Esters Acyl halides Alcohols/phenols
Esters Acyl nitriles Alcohols/phenols
=
Carboxamides /key! nitriles Amines/anilines
Imines Aldeltydes Amines/anilines
Hydrazones Aldehydes or Hydrazines
ketones
Oximes Aldehydes or Hydroxylarnines
ketones
Alkyl amines Alkyl halides Amines/anilines
Esters Alkyl halides Carboxylic acids
Thioethers Alkyl halides Thiols
Ethers Alkyl halides Alcohols/phenols
Thioethers ______________________________________ Alkyl sulfonates Thiols
Esters __________________________________________ Alkyl sulfonates
Carboxylic acids
Ethers Alkyl sulfonates
Alcohols/phenols
Esters Anhydrides Alcohols/phenols
Carboxamides Anhydrides Am ineslan Hines
Thiophenols Aryl halides Thiols
Aryl amines Aryl halides Amities I
Thioethers Aziridines Thiols
- Page 73 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Boronate esters Boronates Glycols
Carboxamides Carboxylic acids Amines/anilines
Esters Carboxylic acids Alcohols
Hydrazines Hydrazides Carboxylic acids
N-acylureas or Carbodiimides Carboxylic acids
Anhydrides
Esters Diazoalkanes Carboxylic acids
Thioethers poxides Thiols
Thioethers Haloacetamides Thiols
Ammotriazines Halotriazines Amines/anilines
Triazinyl ethers Halotriazines Alcohols/phenols
Amidines Imido esters Amines/anilines
Ureas Isocyanates Amines/anilines
Urethanes Isocyanates Alcohols/phenols
Thioureas Isothiocyanates Amines/anilines
Thioethers Maleimides Thiols
Phosphite esters Phosphoramidites Alcohols
Si1)(1 ethers Silyl halides Alcohols
Alkyl amines Sulfonate esters Amines/anilines
Thioethers Sulfonate esters Thiols
Esters Sulfonate esters Carboxylic
acids
Ethers Sulfonate esters Alcohols
Sulfonamides Sulfonyl halides Amines/anilines
Sulfonate esters Sulfonyl halides
Phenols/alcohols
Examples of Covalent Linkages and Precursors Thereof
Use of Protecting Groups
In the reactions described, it may be necessary to protect reactive functional
groups, for example hydroxy, amino,
imino, thio or carboxy groups, where these are desired in the final product,
to avoid their unwanted participation in the
reactions. Protecting groups are used to block some or all reactive moieties
and prevent such groups from participating
in chemical reactions until the protective group is removed. In some
embodiments, each protective group is removable
by a different means. Protective groups that are cleaved under totally
disparate reaction conditions fulfill the requirement
of differential removal. Protective groups can be removed by acid, base, and
hydrogenolysis. Groups such as trityl,
dimethoxytrityl, acetal and t-butyldimethylsilyl are acid labile and may be
used to protect carboxy and hydroxy reactive
moieties in the presence of amino groups protected with Cbz groups, which are
removable by hydrogenolysis, and Fmoc
groups, which are base labile. Carboxylic acid and hydroxy reactive moieties
may be blocked with base labile groups
such as, but not limited to, methyl, ethyl, and acetyl in the presence of
amines blocked with acid labile groups such as t-
butyl carbarnate or with carbamates that are both acid and base stable but
hydrolytically removable.
Carboxylic acid and hydroxy reactive moieties may also be blocked with
hydrolytically removable protective
groups such as the benzyl group, while amine groups capable of hydrogen
bonding with acids may be blocked with base
labile groups such as Fmoc. Carboxylic acid reactive moieties may be protected
by conversion to simple ester
compounds as exemplified herein, or they may be blocked with oxidatively-
removable protective groups such as 2,4-
dimethoxybenzyl, while co-existing amino groups may be blocked with fluoride
labile say! earbamates.
Ally] blocking groups are useful in then presence of acid- and base-
protecting groups since the former are stable
and can be subsequently removed by metal or pi-acid catalysts. For example, an
allyl-blocked carboxylic acid can be
deprotected with a Pd-catalyzed reaction in the presence of acid labile t-
butyl carbamate or base-labile acetate amine
protecting groups. Yet another form of protecting group is a resin to which a
compound or intermediate may be attached.
As long as the residue is attached to the resin, that functional group is
blocked and cannot react. Once released from the
resin, the functional group is available to react.
Protecting or blocking groups may be selected from:
-Page 74-
. CA 02693390 2015-12-04
30725-1660
,...-
methyl (Me) Ethyl (El) 1-Butyl (t-Bu)
Ally1 Benzyl (On) .
0
0 0,1r-\ ,0 \
(I3u y- ----, -K.
is o /Ph
Ph+ I
A-/
0 0 Ph
Acetyl Alloc Boc Cbx TrItyl
0
cr, / o_K/ , 1
_si__0, / y....
_--..
1
pMBn TBDMS Teoc
Fmoc
Other protecting croups, plus a detailed description of techniques applicable
to the creation of protecting groups
and their removal are described in Greene and Wuts, Protective Groups in
Organic Synthesis, 3rd Ed., John Wiley &
Sons, New York, NY, 1999, and Kocienski, Protective Groups, Thieme Verlag, New
York, NY, 1994. .
Making compounds of formula I
Compounds of this invention can be made by a variety of methods. The
procedures below are intended to illustrate
those methods, and the examples given are intended to illustrate the scope of
this invention. Neither the methods not the
examples should be construed as limiting the invention in any way.
1. The preparation of compound of formula VI is outlined below .
uti2 õ
NO2 NO2
1/0,fai F BaEs Fto l 0,Nr.,.,,, /X
4
reductIon B0 .!.4õ..4....,....),( 1 0,
,
X
l-1214 ,.....a F
IV
,.. ./
,...,
Y
It t) Pyridine
protection R-
1¨C1 or Etzt4, Cli2C12
0 v
0µ 0 0 0
II
110 , NrX Et0N, CH2012 Ro
.....1, ....,,1%),AX
' I
N...
it F Y Fto 0
"õ X
....= ====( ,Cii
F=-=%%:::, .
R-1--CI F
F F
0
VB VIII VI
v
Scheme I above illustrates a method for making the sulfonamide derivatives of
formula VI. 1,2 Diamine
derivative (formula IV) can be easily prepared in two steps from the desired
nitro derivatives (formula I). Compounds of
formula rv can be reacted with the sulfonyl chloride derivatives (formula V,
see next scheme) to form the desired
sulfonamide. Alternatively, the 1,2 diamine derivatives IV can be protected to
for an imidazolidone (formula VII),
before being reacted with the corresponding sulfonyl chloride. Deprotection of
the 1,2 diamine VIII under basic =
conditions provided the desired material VI.
-Page 75-
.
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
H. The general route to synthesis compound of general formula V is outlined
below
n-EitiOHRBr
13
,A, n_Be n_.
0".0 pyridine 0 n-Buti
0 0
IX XX XI
KSCN SOCl2
0 0 0 0
xii
Scheme II above shows one example of the preparation of complex sunny'
chloride. Compound XX can he
synthesized from IX, allcylated, and converted to the potassium salt XII.
Treatment of the salt with SOC12 or POC13
affords the desired compounds. Other more specific procedures to prepare
unique sulfonyl chloride derivatives are
reported in the experimental section.
HI. The general route to synthesis compound of general formula XIII is
outlines Scheme 3.
0,70
R. si "Pd" coupling Ro N
Ar
VI XIII
Scheme III above illustrates the preparation of sulfonamide derivatives of
general formula XIII. For example,
these compounds can be easily obtained by reacting the compound VI with a
boronic acid using a palladium catalyst
under Suzuki conditions.
IV. The general route to synthesis compound of general formula XIII is
outlines Scheme 4.
R1
NH2 H ,C)VP R2 e -NH
X ¨ RiR2NH
õ0 so N Roe, 40 Nr1
j
F
IV XIV XV
Scheme IV above illustrates the preparation of sulfonamide derivatives of
general formula XV. The vinyl
sulfonamide (XIV) is reacted with amines to form derivatives of general
formulas XV.
Further Forms of Compounds of Formula I
Isomers of compounds of formula I
The compounds described herein may exist as geometric isomers. The compounds
described herein may possess
one or more double bonds. The compounds presented herein include all cis,
trans, syn, anti, entgegen (E), and zusammen
(Z) isomers as well as the corresponding mixtures thereof. In some situations,
compounds may exist as tautomers. The
compounds described herein include all possible tautomers within the formulas
described herein. The compounds
described herein may possess one or more chiral centers and each center may
exist in the R or S configuration. The
compounds described herein include all diastereomeric, enantiomeric, and
epimeric forms as well as the corresponding
mixtures thereof. In additional embodiments of the compounds and methods
provided herein, mixtures of enantiomers
and/or diastereoisomers, resulting from a single preparative step,
combination, or interconversion may also be useful for
the applications described herein. The compounds described herein can be
prepared as their individual stereoisomers by
reacting a racemic mixture of the compound with an optically active resolving
agent to form a pair of diastereoisomeric
compounds, separating the diastereomers and recovering the optically pure
enantiomers. Resolution of enantiomers can
-Page 76-
= CA 02693390 2015-12-04
30725-1660
be carried out using covalent cliastereomerie derivatives of the compounds
described herein, or dissociable complexes
may be used (e.g,, crystalline diastereorneric salts). Diastereomers have
distinct physical properties (e.g., melting points,
boiling points, solubilities, reactivity, etc.) and can be readily separated
by taking advantage of these dissimilarities. The
diastereorners can be separated by chiral chromatography, or
separation/resolution techniques based upon differences in
solubility. The optically pure enantiomer is then recovered, along with the
resolving agent, by any practical means that
would not result in racemization. A more detailed description of the
techniques applicable to the resolution of
stereoisomers of compounds from their racemic mixture can be found in Jean
Jacques, Andre Collet, Samuel H. Wile'',
"Enantiomers, Racemates and Resolutions," John Wiley And Sons, Inc., 1981,
Labeled compounds of formula I
Also described herein are isotopically-labeled compounds of formula I and
methods of treating disorders. For
example, the invention provides for methods of treating diseases, by
administering isotopically-labeled compounds of
formula I. The isotopically-labeled compounds of formula I can be administered
as pharmaceutical compositions. Thus,
compounds of formula I also include isotopically-labeled compounds, which are
identical to those recited herein, but for
the fact that one or more atoms are replaced by an atom having an atomic mass
or mass number different from the atomic
mass or mass number usually found in nature. Examples of isotopes that can be
incorporated into compounds of the
invention include isotopes of hydrogen, carbon, nitrogen, oxygen, phosphorous,
sulfur, fluorine and chloride, such as 2H,
3B, 13C, 14C, eN, 180, 1707 31p, UP, 35,,,
"F, and 36C1 , respectively. Compounds described herein, pharmaceutically
acceptable salts, thereof which contain the aforementioned isotopes andlor
other isotopes of other atoms are within the
scope of this invention_ Certain isotopically-labeled compounds of formula I,
for example those into which radioactive
isotopes such as 3H and "C are incorporated, are useful in drug and/or
substrate tissue distribution assays. Tritiated, i. e.,
3H and carbon-14, i. e.,
u isotopes are often easily prepared and detectabilited. Further, substitution
with heavier
isotopes such as deuterium, i. e., 2.H, can afford certain therapeutic
advantages resulting from greater metabolic stability,.
for example increased in vivo half-life or reduced dosage requirements and,
hence, may be desirable in some
circumstances. Isotopically labeled compounds and pharmaceutically acceptable
salts thereof can generally be prepared
by carrying out procedures described herein, by substituting a readily
available isotopically labeled reagent for a non-
isotopically labeled reagent.
The compounds described herein may be labeled by other means, including, but
not limited to, the use of
chromophores or fluorescent moieties, bioluminescent labels, or
chemilumineseent labels.
Pharmaceutically acceptable salts of compounds of formula I
Also described herein are pharmaceutically acceptable salts of compounds of
formula land methods of treating
disorders. For example, the invention provides for methods of treating
diseases, by administering pharmaceutically
acceptable salts of compounds of formula I. The pharmaceutically acceptable
salts of compounds of formula lean be
administered as pharmaceutical compositions.
Thus, the compounds described herein can be prepared as pharmaceutically
acceptable salts formed when an acidic
proton present in the parent compound either is replaced by a metal ion, for
example an alkali metal ion, an alkaline earth
ion, or an aluminum ion; or coordinates with an organic base. Base addition
salts can also be prepared by reacting the
free acid form of the compounds described herein with a pharmaceutically
acceptable inorganic or organic base,
including, but not limited to organic bases such as etbanolainine,
diethancilamine, triethanolamine, trornethamine, N-
methylglucamine, and the like and inorganic bases such as aluminum hydroxide,
Calcium hydroxide, potassium
- Page 77 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
hydroxide, sodium carbonate, sodium hydroxide, and the like. In addition, the
salt forms of the disclosed compounds can
be prepared using salts of the starting materials or intermediates.
Further, the compounds described herein can be prepared as pharmaceutically
acceptable salts formed by reacting
the free base form of the compound with a pharmaceutically acceptable
inorganic or organic acid, including, but not
limited to, inorganic acids such as hydrochloric acid, hydrobromic acid,
sulfuric acid, nitric acid, phosphoric acid
metaphosphoric acid, and the like; and organic acids such as acetic acid,
propionic acid, hexanoic acid,
cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic
acid, succinic acid, malic acid, maleic acid,
furnaric acid, Q-toluenesulfonic acid, tartaric acid, trifluoroacetic acid,
citric acid, benzoic acid, 3-(4-
hydroxybenzoyl)benzoic acid, cinnamic acid, mandelic acid, arylsulfonic acid,
methanesulfonic acid, ethanesulfonic acid,
1,2-ethanedisulfonic acid, 2-hydroxyethanesuIfonic acid, benzenesulfonic acid,
2-naphthalenesulfonic acid, 4-
methylbicyclo-[2.2.2]oct-2-ene-1-carboxylic acid, glucoheptonic acid, 4,4'-
methylenebis-(3-hydroxy-2-ene-1 -carboxylic
acid), 3-phenylpropionic acid, trimethylacetic acid, tertiary butylacetic
acid, lauryl sulfuric acid, gluconic acid, glutamic
acid, hydroxynaphthoic acid, salicylic acid, stearic acid, and muconic acid.
Solvates of compounds of formula I
Also described herein are solvates of compounds of formula I and methods of
treating disorders. For example, the
invention provides for methods of treating diseases, by administering solvates
of compounds of formula I. The solvates
of compounds of formula I can be administered as pharmaceutical compositions.
Solvates contain either stoichiometric or non-stoichiometric amounts of a
solvent, and may be formed during the
process of crystallization with pharmaceutically acceptable solvents such as
water, ethanol, and the like. Hydrates are
formed when the solvent is water, or alcoholates are formed when the solvent
is alcohol. Solvates of the compounds
described herein can be conveniently prepared or formed during the processes
described herein. By way of example only,
hydrates of the compounds described herein can be conveniently prepared by
recrystallization from an aqueous/organic
solvent mixture, using organic solvents including, but not limited to,
dioxane, tetrahydrofuran or methanol. In addition,
the compounds provided herein can exist in unsolvated as well as solvated
forms. In general, the solvated forms are
considered equivalent to the unsolvated forms for the purposes of the
compounds and methods provided herein.
Polymorphs of compounds of formula I
Also described herein are polymorphs of compounds of formula I and methods of
treating disorders. For example,
the invention provides for methods of treating diseases, by administering
polymorphs of compounds of formula I. The
polymorphs of compounds of formula I can be administered as pharmaceutical
compositions.
Thus, the compounds described herein include all their crystalline forms,
known as polymorphs. Polymorphs
include the different crystal packing arrangements of the same elemental
composition of a compound. Polymorphs may
have different X-ray diffraction patterns, infrared spectra, melting points,
density, hardness, crystal shape, optical and
electrical properties, stability, and solubility. Various factors such as the
recrystallization solvent, rate of crystallization,
and storage temperature may cause a single crystal form to dominate.
Crystalline Polymorph Form of N-(S)-(3,41-difluoro-2-(2-flooro-4-
iodophenylamino)-6-methorypheny1)-1-(2,3-
dihydroxypropyl) eyclopropane-I-sulfonamide
- Page 78 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The invention also relates to a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
HOOH
D141) 0
Me0 io N 40
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane- 1-
sulfonamide: F that
exhibits a specific powder x-ray diffraction pattern. In some embodiments, the
powder x-ray diffraction pattern contains
at least about 50% of the peaks shown in FIG. 5. In some embodiments, the
powder x-ray diffraction pattern contains at
least about 70% of the peaks shown in FIG. 5. In some embodiments, the powder
x-ray diffraction pattern contains at
least about 90% of the peaks shown in FIG. 5. In some embodiments, the powder
x-ray diffraction pattern is
substantially the same the powder x-ray diffraction pattern shown in FIG. 5.
The invention also relates to a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyl)-1-(2,3-dihydroxypropyl) cyclopropane- 1-
sulfonamide that exhibits a specific
differential scanning calorimetry pattern. In some embodiments, the specific
differential scannin calorimetry patem is
substantially the same as the differential scanning calorimetry pattern shown
in FIG. 6. In some embodiments the
crystalline polymorph form A has a melting point onset as determined by
differential scanning calorimetry at about
I43 C.
The invention also relates to a polymorphic form of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane- 1-sulfonamide made by a
method comprising the step of
crystallizing amorphous N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide from a solvent. The invention also relates to a
polymorphic form of N-(S)-(3,4-difluoro-2-
(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane- 1-sulfonamide made by a
method comprising the step of crystallizing amorphous N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-l-sulfonamide from a
mixture of hexane and ethyl acetate.
The invention also relates to pharmaceutical compositions comprising an
effective amount of crystalline
polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide and a pharmaceutically acceptable carrier or
vehicle. Other aspects of the invention relate
to a pharmaceutical composition comprising the crystalline polymorph form A
and at least one excipient or carrier.
The crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-I-
(2,3-dihydroxypropyl) cyclopropane-1-sulfonamide is useful for treating or
preventing cancer or an inflammatory
disease. The invention further relates to methods for treating or preventing
cancer or an inflammatory disease,
comprising administering an effective amount of a crystalline polymorph form A
of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane- 1-
sulfonamide to a subject in need thereof.
Yet other aspects of the invention relate to methods for the treatment or
prophylaxis of an inflammatory disease
comprising administering to a subject in need thereof an effective amount of
the crystalline polymorph. Further aspects
of the invention relate to methods for the treatment or prophylaxis of a
proliferative disease comprising administering to
a subject in need thereof an effective amount of the crystalline polymorph.
Prodrugs of compounds of formula I
Also described herein are prodrugs of compounds of formula I and methods of
treating disorders. For example, the
invention provides for methods of treating diseases, by administering prodrugs
of compounds of formula I. The prodrugs
of compounds of formula I can be administered as pharmaceutical compositions.
Page 79-
CA 02693390 2015-12-04
30725-1660
Prodrugs are generally drug precursors that, following administration to a
subject and subsequent absorption, are
converted to an active, or a more active species via some process, such as
conversion by a metabolic pathway. Some
prodrugs have a chemical group present on the prodrug that renders it less
active and/or confers solubility or some other
property to the drug. Once the chemical group has been cleaved anclior
modified from the prodrug the active drug is
generated. Prodrugs are often useful because, in some situations, they may be
easier to administer than the parent drug.
They may, for instance, be bioavailable by oral administration whereas the
parent is not. The prodrug may also have
improved solubility in pharmaceutical compositions over the parent drug. An
example, without limitation, of a prodrug
would be a compound as described herein which is administered as an ester (the
"prodrug") to facilitate transmittal across
a cell membrane where water solubility is detrimental to mobility but which
then is metabolically hydrolyzed to the
carboxylic acid, the active entity, once inside the cell where water-
solubility is beneficial. A further example of a prodrug
might be a short peptide (polyarnino acid) bonded to an acid group where the
peptide is metabolized to reveal the active
moiety.
Prodrugs may be designed as reversible drug derivatives, for use as modifiers
to enhance drug transport to site-
specific tissues. The design of prodrugs to date has been to increase the
effective water solubility of the therapeutic
compound for targeting to regions where water is the principal solvent. See,
e.g., Fedorak et al., Am. J. Physiol.,
269:G210-218 (1995); MeLoed et al., Gastroenterol, 106:405-413 (1994);
Hochhaus et al., Blamed Chrom., 6:283-286
(1992); J. Larsen and H. Bundgaard, Mt. J. Pharmaceutics, 37, 87 (1987); J.
Larsen et al., Int. J. Pharmaceutics, 47, 103
(1988); Sinkula eta)., J. Pharm. Sci., 64:181-210 (1975); T. Higuchi and V.
Stella, Pro-drugs as Novel Delivery Systems,
Vol. 14 of the A.C_S. Symposium Series; and Edward B. Roche, Ilioreversible
Carriers in Drug Design, American
Pharmaceutical Association and Pergamon Press, 1987,
Additionally, prodrug derivatives of compounds described herein can be
prepared by methods known to those of
ordinary skill in the art (e.g., for further details see Saulnier et al.,
(1994), Bioorganic and Medicinal Chemistry Letters,
Vol. 4, p. 1985). By way of example only, appropriate prodrugs can be prepared
by reacting a non-derivatized compound
of formula I with a suitable carbamylating agent, such as, but not limited to,
1,1-acyloxyalkylcarbanochloridate, pan.-
nitrophenyl carbonate, or the like. Prodrug forms of the herein described
compounds, wherein the prodrug is metabolized
in vivo to produce a derivative as set forth herein are included within the
scope of the claims. Indeed, some of the herein-
described compounds may be a prodrug for another derivative or active
compound.
In some embodiments, prodrugs include compounds wherein an amino acid residue,
or a polypeptide chain of two
or more (e. g., two, three or four) amino acid residues is covalently joined
through an amide or ester bond to a free
amino, hydroxy or carboxylic acid group of compounds of the present invention.
The amino acid residues include but arc
not limited to the 20 naturally occurring amino acids commonly designated by
three letter symbols and also includes 4-
hydroxyproline, hydroxylysine, demosine, isoclernosine, 3-methylhisticline,
norvaline, beta-alanine, ganuna-aminobutyric
acid, cirtalline, homocysteine, homoserine, omithine and rnethionine sulfone.
Additional types of prodrugs are also
encompassed.
Compounds of formula I having free amino, amido, hydroxy or carboxylic groups
can be converted into prodrugs.
For instance, free carboxyl groups can be derivatized as amides or alkyl
esters. Free hydroxy groups may be derivatized
using groups including but not limited to hemisuccinates, phosphate esters,
dimethylaininoacetates, and
phosphoryloxymethyloxycarbonyis, as outlined in Advanced Drug Delivery Reviews
1996, 19,115. Carbamate prodrugs
of hydroxy and amino groups are also included, as are carbonate prodrugs,
sulfonate esters and sulfate esters of hydroxy
groups.
- Page 30 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Derivatization of hydroxy groups as (acyloxy) methyl and (acyloxy) ethyl
ethers wherein the acyl group may be an
alkyl ester, optionally substituted with groups including but not limited to
ether, amine and carboxylic acid
functionalities, or where the acyl group is an amino acid ester as described
above, are also encompassed. Prodrugs of this
type are described in J. Med. Chem. 1996, 39, 10. Free amines can also be
derivatized as amides, sulfonamides or
phosphonatnides. All of these prodrug moieties may incorporate groups
including but not limited to ether, amine and
carboxylic acid ftmctionalities.
Sites on the aromatic ring portions of compounds of formula I may be
susceptible to various metabolic reactions,
therefore incorporation of appropriate substituents on the aromatic ring
structures, can reduce, minimize or eliminate this
metabolic pathway.
Pharmaceutical Compositions
Described herein are pharmaceutical compositions. In some embodiments, the
pharmaceutical compositions
comprise an effective amount of a compound formula I, or a pharmaceutically
acceptable salt, thereof. In some
embodiments, the pharmaceutical compositions comprise an effective amount of a
compound formula I, or a
pharmaceutically acceptable salt, solvate, polymorph, ester, amide, tautomer,
prodrug, hydrate, or derivative thereof and
at least one pharmaceutically acceptable carrier. In some embodiments the
pharmaceutical compositions are for the
treatment of disorders. In some embodiments the pharmaceutical compositions
are for the treatment of disorders in a
mammal. lit some embodiments the pharmaceutical compositions are for the
treatment of disorders in a human.
In further aspects, the present invention is directed to a pharmaceutical
composition comprising a compound of
formula I or a pharmaceutically acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof. In some
embodiments, the pharmaceutical compositions further comprise a
pharmaceutically acceptable carrier. Such
compositions may contain adjuvants, excipients, and preservatives, agents for
delaying absorption, fillers, binders,
adsorbents, buffers, disintegrating agents, solubilizing agents, other
carriers, and other inert ingredients. Methods of
formulation of such compositions are well-known in the art.
In some embodiments, the pharmaceutical composition is in a form suitable for
oral administration. In further or
additional embodiments, the pharmaceutical composition is in the form of a
tablet, capsule, pill, powder, sustained
release formulation, solution, suspension, for parenteral injection as a
sterile solution, suspension or emulsion, for topical
administration as an ointment or cream or for rectal administration as a
suppository.
In further or additional embodiments, the pharmaceutical composition is in
unit dosage forms suitable for single
administration of precise dosages. In further or additional embodiments the
amount of compound of formula I is in the
range of about 0.001 to about 1000 mg/kg body weight/day. In further or
additional embodiments the amount of
compound of formula I is in the range of about 0.5 to about 50 mg/kg/day. In
further or additional embodiments the
amount of compound of formula I is about 0.001 to about 7 g/day. In further or
additional embodiments the amount of
compound of formula! is about 0.002 to about 6 g/day. In further or additional
embodiments the amount of compound of
formula I is about 0.005 to about 5 g/day. In further or additional
embodiments the amount of compound of formula I is
about 0.01 to about 5 g/day. In further or additional embodiments the amount
of compound of formula I is about 0.02 to
about 5 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.05 to about 2.5
g/day. In further or additional embodiments the amount of compound of formula
I is about 0.1 to about 1 g/day. In
further or additional embodiments, dosage levels below the lower limit of the
aforesaid range may be more than
adequate. In further or additional embodiments, dosage levels above the upper
limit of the aforesaid range may be
required.
-Page 81-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the pharmaceutical composition is for
administration to a mammal. In further or additional embodiments, the mammal
is human.
In further or additional embodiments, the pharmaceutical composition further
comprises a pharmaceutical carrier,
excipient and/or adjuvant. In further or additional embodiments, the
pharmaceutical composition further comprises at
least one therapeutic agent. In further or additional embodiments, the
therapeutic agent is selected from the group of
cytotoxic agents, anti-angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-
neoplastic agent is selected from the group of consisting of alkylating
agents, anti-metabolites, epidophyllotoxins;
antineoplastic enzymes, topoisomerase inhibitors, procarbazines,
mitoxantrones, platinum coordination complexes,
biological response modifiers and growth inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic
growth factors. In further or additional embodiments, the therapeutic agent is
taxol, bortezomib or both. In further or
additional embodiments, the pharmaceutical composition is administered in
combination with an additional therapy. In
further or additional embodiments, the additional therapy is radiation
therapy, chemotherapy, surgery, or any
combination thereof. In further or additional embodiments, the pharmaceutical
composition comprises a
pharmaceutically acceptable salt of a compound of formula I.
HO\ H
DI*1
0NH
Me 0 N
The invention also relates to a composition comprising F . In some
embodiments, the 2-0H
carbon on the compound is in the R configuration. In some embodiments,
composition is substantially free of the S-
isomer of the compound. In some embodiments, the compound contains less than
10% of the S- isomer of the
compound. In some embodiments, the compound contains less than 5% of the S-
isomer of the compound. In some
embodiments, the compound contains less than 1% of the S- isomer of the
compound. In some embodiments, the
compound is in the R configuration.
In some embodiments, the 2-0H carbon on the compound is in the S
configuration. In some embodiments,
composition is substantially free of the R- isomer of the compound. In some
embodiments, the compound contains less
than 10% of the R- isomer of the compound. In some embodiments, the compound
contains less than 5% of the R-
isomer of the compound. In some embodiments, the compound contains less than
1% of the R- isomer of the compound.
In some embodiments, the compound is in the S configuration.
In some embodiments, the composition contains at least about 50% of a compound
which exhibits a powder x-ray
diffraction pattern comprising at least 50% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG.
5. In some embodiments, the powder x-ray diffraction pattern comprises at
least 70% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG. 5. In some embodiments, the
powder x-ray diffraction pattern comprises
at least 90% of the peaks identified in the In some embodiments, the powder x-
ray diffraction pattern substantially the
same as the powder x-ray diffraction pattern shown in FIG. 5.
-Page 82-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, the composition contains at least about 75% of a compound
which exhibits a powder x-ray
diffraction pattern comprising at least 50% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG.
5. In some embodiments, the powder x-ray diffraction pattern comprises at
least 70% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG. 5. In some embodiments, the
powder x-ray diffraction pattern comprises
at least 90% of the peaks identified in the In some embodiments, the powder x-
ray diffraction pattern substantially the
same as the powder x-ray diffraction pattern shown in FIG. 5.
In some embodiments, the composition contains at least about 90% of a compound
which exhibits a powder x-ray
diffraction pattern comprising at least 50% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG.
5. In some embodiments, the powder x-ray diffraction pattern comprises at
least 70% of the peaks identified in the
powder x-ray diffraction pattern shown in FIG. 5. In some embodiments, the
powder x-ray diffraction pattern comprises
at least 90% of the peaks identified in the In some embodiments, the powder x-
ray diffraction pattern substantially the
same as the powder x-ray diffraction pattern shown in FIG. 5.
In some embodiments, substantially all of the compound in the composition
exhibits a powder x-ray diffraction
pattern comprising at least 50% of the peaks identified in the powder x-ray
diffraction pattern shown in FIG. 5. In some
embodiments, the powder x-ray diffraction pattern comprises at least 70% of
the peaks identified in the powder x-ray
diffraction pattern shown in FIG. 5. In some embodiments, the powder x-ray
diffraction pattern comprises at least 90% of
the peaks identified in the In some embodiments, the powder x-ray diffraction
pattern substantially the same as the
powder x-ray diffraction pattern shown in FIG. 5.
In some embodiments, the crystalline polymorph present in the composition has
a melting point onset as
determined by differential scanning calorimetry at about 143 C. In some
embodiments, the crystalline polymorph is
substantially free of water. In some embodiments, the crystalline polymorph is
substantially free of solvent.
In some embodiments, the composition contains at least about 50% of a compound
which exhibits a differential
scanning calorimetry pattern substantially the same as the differential
scanning calorimetry pattern shown in FIG. 6. In
some embodiments, the crystalline polymorph has a melting point onset as
determined by differential scanning
calorimetry at about 143 C. In some embodiments, the crystalline polymorph is
substantially free of water. In some
embodiments, the crystalline polymorph is substantially free of solvent.
In some embodiments, the composition contains at least about 75% of a compound
which exhibits a differential
scanning calorimetry pattern substantially the same as the differential
scanning calorimetry pattern shown in FIG. 6. In
some embodiments, the crystalline polymorph has a melting point onset as
determined by differential scanning
calorimetry at about 143 C. In some embodiments, the crystalline polymorph is
substantially free of water. In some
embodiments, the crystalline polymorph is substantially free of solvent.
In some embodiments, the composition contains at least about 90% of a compound
which exhibits a differential
scanning calorimetry pattern substantially the same as the differential
scanning calorimetry pattern shown in FIG. 6. In
some embodiments, the crystalline polymorph has a melting point onset as
determined by differential scanning
calorimetry at about 143 C. In some embodiments, the crystalline polymorph is
substantially free of water. In some
embodiments, the crystalline polymorph is substantially free of solvent.
In some embodiments, substantially all the compound in the composition
exhibits a differential scanning
calorimetry pattern substantially the same as the differential scanning
calorimetry pattern shown in FIG. 6. In some
embodiments, the crystalline polymorph has a melting point onset as determined
by differential scanning calorimetry at
about 143 C. In some embodiments, the crystalline polymorph is substantially
free of water. In some embodiments, the
crystalline polymorph is substantially free of solvent.
- Page 83 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, the polymorphic form of N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
rnethoxypheny1)-l-(2,3-dihydroxypropyl) cyclopropane-l-sulfonamide made by a
method comprising the step of
crystallizing amorphous N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
rnethoxyPheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide. In some embodiments, the crystallization step
comprises crystallizing from a mixture of
ethyl acetate and heptane, for example, a mixture of ethyl acetate and heptane
is in a ratio of from about 1-4 parts ethyl
acetate to about 2-10 parts heptane or more specifically, a ratio of from
about 2 parts ethyl acetate to about 5 parts
heptane.
In some embodiments, the compound is formulated for the immediate release of
the compound. In some
embodiments, the compound is formulated for the sustained release of the
compound. In other embodiments, the
compound is formulated for the extended release of the compound.
In some embodiments, the composition is in a tablet dosage form. In other
embodiments, the composition is in a
cpasule dosage form. The composition can be made into a capsule or tablet
dosage form and a wide range of alternative
compositions and manufacturing approaches can be used, References: (1)
Remington, The Science and Practice of
Pharmacy, 20th Edition, 2000, (2) Pharmaceutical Dosage Forms Tablets Volumes
1-3, 1989 and (3) Modern
Pharmaceuticals 4th Edition, 2002. A range of manufacturing approaches can be
employed including dry blending, wet
granulation, roller compaction, extrusion, spheronization, coating, and spray
drying processes. Soft gel formulation and
manufacturing approaches are also possible.
In some embodiments, the composition includes a filler or diluent. In various
embodiments, the filler or diluent is
selected from microcrystalline cellulose, silicified microcrystalline
cellulose, lactose, mannitol, compressible sugar,
calcium phosphate, calcium sulfate, calcium carbonate, calcium silicate and
starch. In other embodiments, the filler or
diluent is microcrystalline cellulose.
In some embodiments, the composition includes a disintegrant. In various
embodiments, the disintegrant is
selected from croscarmellose sodium, sodium starch glycolate, crospovidone,
methylcellulose, alginic acid, sodium
alginate, starch derivatives, betonite and veegum. In some embodiment, the
disintegrant is croscarrnellose sodium.
In some embodiments, the composition includes a lubricant. In various
embodiments, the lubricant is selected
from magnesium stearate, metallic stearates, talc, sodium stearyl fumarate and
stearic acid. In some embodiments, the
lubricant is magnesium stearate.
In some embodiments, the composition includes a wetting agent or surfactant.
In various embodiments, the
wetting agent or surfactant is selected from sodium lauryl sulfate, glycerol,
sorbitan oleates, sorbitan stearates,
polyoxyethylenated sorbitan laurate, palmitate, stearate, oleate or hexaolate,
polyoxyethylene stearyl alcohol and sorbitan
monolaurate. In some embodiments, the wetting agent or surfactant is sodium
lauryl sulfate.
Additional excipients such as glidants, flavors, and colorants can also be
added. Additional alternative excipients
can be found in The Handbook of Pharmaceutical Excipients, 5' Edition, 2005
and the FDA Inactive Ingredient
database.
The invention also relates to a composition comprising:
H0\411
0116)
0
Me0 N
about lrng of a compound of structure: (as defined in any
of the above
embodiments);
- Page 84 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
about 222.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
HO\._0H
0 NH
Me0 N
1
about 10mg of a compound of structure: F (as defined in any
of the above
embodiments);
about 213.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
H0\
(OH
s,NH
Me0 N
about 20mg of a compound of structure: F (as defined in any
of the above
embodiments);
about 203.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
HC)),.0
0NH
meo N so
about 40mg of a compound of structure: F (as defined in any of the above
embodiments);
about 183.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
-Page 85-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
The invention also relates to a composition comprising: about 0.4% by weight
of a compound of structure:
HOqH
1111 ,0
0
Me0 so N io
(as defined in any of the above embodiments), and about 99.6% by weight of a
pharmaceutically
acceptable carrier or vehicle. In some embodiments, the pharmaceutically
acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or additional embodiments, the
microcrystalline cellulose is about 92.6% by weight
of the composition. In further or additional embodiments, the composition
further comprises: about 5% by weight
croscannellose sodium; about 1% by weight sodium lauryl sulfate; and about 1%
by weight magnesium stearate.
The invention also relates to a composition comprising: about 4.2% by weight
of a compound of structure:
HC)\-),/
i=S''
0 F
Me0 N
(as defined in any of the above embodiments); and about 95.8 % by weight of a
pharmaceutically
acceptable carrier or vehicle. In some embodiments, the pharmaceutically
acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or additional embodiments, the
microcrystalline cellulose is about 88.8% by weight
of the composition. In further or additional embodiments, the composition
further comprises: about 5% by weight
croscarmellose sodium; about 1% by weight sodium lauryl sulfate; and about 1%
by weight magnesium stearate.
The invention also relates to a composition comprising: from about 2% to about
10% by weight of a compound of
HCI\:10
o'S NH
Me N 40
structure: F
(as defined in any of the above embodiments); and from about 98% to about
90% by
weight of a pharmaceutically acceptable carrier or vehicle. In some
embodiments, the pharmaceutically acceptable
carrier or vehicle comprises microcrystalline cellulose. In further or
additional embodiments, the microcrystalline
cellulose is from about 85% to about 95% by weight of the composition. In
further or additional embodiments, the
composition further comprises: from about 1% to about 6% by weight
croscannellose sodium; from about 0.1% to about
2% by weight sodium lauryl sulfate; and from about 0.25% to about 1.5% by
weight magnesium stearate. In some
embodiments, the pharmaceutically acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or
additional embodiments, the microcrystalline cellulose is from about 85% to
about 95% by weight of the composition. In
further or additional embodiments, the composition further comprises: from
about 1% to about 6% by weight
croscarmellose sodium; and from about 0.25% to about 1.5% by weight magnesium
stearate.
The invention also relates to a composition comprising:
HO OH
0
,
cy NH
Me0 N
=
about ling of a compound of structure:
- Page 86 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
about 222.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
HO OH
0
0ANH
[MO N
=
about 10mg of a compound of structure:
about 213.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
HO OH
0
0 NH
Me N
1111111' F 411111131
=
about 20mg of a compound of structure: F9
about 203.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
1-100H
0
0' NH
Me 40 N so
about 40mg of a compound of structure: F=
about 183.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising: about 0.4% by weight
of a compound of structure:
HO OH
0
0, NH
Med N so
, and about 99.6% by weight of a pharmaceutically acceptable carrier or
vehicle. In some
-Page 87-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments, the pharmaceutically acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or
additional embodiments, the microcrystalline cellulose is about 92.6% by
weight of the composition. In further or
additional embodiments, the composition further comprises: about 5% by weight
croscarmellose sodium; about 1% by
weight sodium lauryl sulfate; and about 1% by weight magnesium stearate.
The invention also relates to a composition comprising: about 4.2% by weight
of a compound of structure:
HO\OH
0
0' NH H F
Me0 N is
, and about 95.8 % by weight of a pharmaceutically acceptable carrier or
vehicle. In some
embodiments, the pharmaceutically acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or
additional embodiments, the microcrystalline cellulose is about 88.8% by
weight of the composition. In further or
additional embodiments, the composition further comprises: about 5% by weight
croscarmellose sodium; about 1% by
weight sodium lauryl sulfate; and about 1% by weight magnesium stearate.
The invention also relates to a composition comprising: from about 2% to about
10% by weight of a compound of
HRH
Fl F
,St NH
Me0 N
structure: F , and from about 98% to about 90% by weight of a
pharmaceutically acceptable carrier or
vehicle. In some embodiments, the pharmaceutically acceptable carrier or
vehicle comprises microcrystalline cellulose.
In further or additional embodiments, the microcrystalline cellulose is from
about 85% to about 95% by weight of the
composition. In further or additional embodiments, the composition further
comprises: from about 1% to about 6% by
weight croscarmellose sodium; from about 0.1% to about 2% by weight sodium
lauryl sulfate; and from about 0.25% to
about 1.5% by weight magnesium stearate. In some embodiments, the
pharmaceutically acceptable carrier or vehicle
comprises microcrystalline cellulose. In further or additional embodiments,
the microcrystalline cellulose is from about
85% to about 95% by weight of the composition. in further or additional
embodiments, the composition further
comprises: from about 1% to about 6% by weight croscarmellose sodium; and from
about 0.25% to about 1.5% by
weight magnesium stearate.
The invention also relates to a composition comprising:
NO pH
\P,0
0õS:NH
WO N so
=
about lmg of a compound of structure:
about 222.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
Page 88-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
HO zOH
'p
0.= NH
Me0 N 40
=
about 10mg of a compound of structure:
about 213.2mg of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
Ho\41-1
Dilln" 0
H
Me N
,=
about 20mg of a compound of structure:
about 203.2mg of microcrystalline cellulose;
about 12.0mg of croscamiellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising:
HO ..0H
0,5:NH
Me0 40 N
about 40mg of a compound of structure: F=
about 183.2ing of microcrystalline cellulose;
about 12.0mg of croscarmellose sodium;
about 2.4mg of sodium lauryl sulfate; and
about 2.4mg of magnesium stearate.
The invention also relates to a composition comprising: about 0.4% by weight
of a compound of structure:
HO pH
\P,c.
04"SNH
Me0 N so
, and about 99.6% by weight of a pharmaceutically acceptable carrier or
vehicle. In some
embodiments, the pharmaceutically acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or
additional embodiments, the microcrystalline cellulose is about 92.6% by
weight of the composition. In further or
additional embodiments, the composition further comprises: about 5% by weight
croscarmellose sodium; about 1% by
weight sodium lauryl sulfate; and about 1% by weight magnesium stearate.
-Page 89-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The invention also relates to a composition comprising: about 4.2% by weight
of a compound of structure:
HO pH
\P 0
0" NH F
Me0 40 tkiJ 40
, and about 95.8 % by weight of a pharmaceutically acceptable carrier or
vehicle. In some
embodiments, the pharmaceutically acceptable carrier or vehicle comprises
microcrystalline cellulose. In further or
additional embodiments, the microcrystalline cellulose is about 88.8% by
weight of the composition. In further or
additional embodiments, the composition further comprises: about 5% by weight
croscarmellose sodium; about 1% by
weight sodium lauryl sulfate; and about 1% by weight magnesium stearate.
The invention also relates to a composition comprising: from about 2% to about
10% by weight of a compound of
>Lo
0NH
Fl
Me N
structure: F , and from about 98% to about 90% by weight of a
pharmaceutically acceptable carrier or
vehicle. In some embodiments, the pharmaceutically acceptable carrier or
vehicle comprises microcrystalline cellulose.
In further or additional embodiments, the microcrystalline cellulose is from
about 85% to about 95% by weight of the
composition. In further or additional embodiments, the composition further
comprises: from about 1% to about 6% by
weight croscarmellose sodium; from about 0.1% to about 2% by weight sodium
lauryl sulfate; and from about 0.25% to
about 1.5% by weight magnesium stearate. In some embodiments, the
pharmaceutically acceptable carrier or vehicle
comprises microcrystalline cellulose. In further or additional embodiments,
the microcrystalline cellulose is from about
85% to about 95% by weight of the composition. In further or additional
embodiments, the composition further
comprises: from about 1% to about 6% by weight croscarmellose sodium; and from
about 0.25% to about 1.5% by
weight magnesium stearate.
Also described herein are pharmaceutical compositions comprising an effective
amount of a crystalline polymorph
form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide. In some embodiments, the
pharmaceutical compositions comprise an
effective amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide, and at least
one pharmaceutically acceptable
carrier. In some embodiments the pharmaceutical compositions are for the
treatment of disorders. In some embodiments
the pharmaceutical compositions are for the treatment of disorders in a
mammal. In some embodiments the
pharmaceutical compositions are for the treatment of disorders in a human. In
some embodiments the pharmaceutical
compositions are for the treatment or prophylaxis of inflammatory diseases. In
some embodiments the pharmaceutical
compositions are for the treatment or prophylaxis of proliferative diseases.
Methods of Use of the Compounds, Including Polymorph Forms, and the
Compositions
In other aspects, the present invention is directed to a method for achieving
an effect in a patient comprising the
administration of an effective amount of a compound of formula I or a
pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug thereof, to a patient, wherein
the effect is selected from the group
consisting of inhibition of various cancers, immunological diseases, and
inflammatory diseases. In some embodiments,
the compound or pharmaceutically acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof is
-Page 90-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
administered as a component of a composition that further comprises a
pharmaceutically acceptable carrier or vehicle. In
some embodiments, the effect is inhibition of various cancers. In further or
additional embodiments, the effect is
inhibition of immunological diseases. In further or additional embodiments,
the effect is inhibition inflammatory
diseases.
Any of the compositions described and claimed herein may be used in the
methods provided in this section.
In some embodiments, the composition comprising a compound of formula us
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy, or
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula 1 is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In some embodiments of the compositions and methods provided herein, provided
are MEK protein kinase
inhibitors further comprising a compound of formula 1, wherein the compound of
formula I is present in an amount of
about 0.1 mg to about 200 mg. In other embodiments, the MEK protein kinase
inhibitor comprises a compound of
formula I and is present in an amout of about 0.2 mg to about 100 mg. In other
embodiments, the MEK protein kinase
inhibitor comprises a compound of formula I and is present in an amout of
about 0.3 mg to about 90 mg. In other
embodiments, the MEK protein kinase inhibitor comprises a compound of formula
I and is present in an amout of about
0.4 mg to about 80 mg. In other embodiments, the MEK protein kinase inhibitor
comprises a compound of formula I and
is present in an amout of about 0.5 mg to about 70 mg. In other embodiments,
the MEK protein kinase inhibitor
comprises a compound of formula I and is present in an amout of about 0.4 mg
to about 80 mg. In other embodiments,
the MEK protein kinase inhibitor comprises a compound of formula I and is
present in an amout of about 0.5 mg to about
70 mg. In other embodiments, the MEK protein kinase inhibitor comprises a
compound of formula I and is present in an
amout of about 1 mg to about 60 mg. In other embodiments, the MEK protein
kinase inhibitor comprises a compound of
formula I and is present in an amout of about 1.5 mg to about 50 mg. In other
embodiments, the MEK protein kinase
inhibitor comprises a compound of formula I and is present in an amout of
about 2 mg to about 45 mg. In other
embodiments, the MEK protein kinase inhibitor comprises a compound of formula
I and is present in an amout of about
2.5 mg to about 40 mg. In further embodiments, MEK protein kinase inhibitor
further comprising the compound of
- Page 91 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
OH .77
0' 'NH H F
Me0 la, N
411" F
formula I present in the dosage amounts provided herein is selected from the
group consisting of:
HO,,Z..7 0
nee
- 'NH F
F ak, N
F 4111
and
In some embodiments of the compositions and methods provided herein, provided
are MEK protein kinase
inhibitors further comprising a compound of formula I, wherein the compound of
formula I is present in an amount of
about 0.1 mg, about 0.2 mg, about 0.25 mg, about 0.3 mg, about 0.4 mg, about
0.5 mg, about 0.6 mg, about 0.7 mg, about
0.8 mg, about 0.9 mg, about 1 mg, about 1.5 mg, about 2 mg, about 2.5 mg,
about 3 mg, about 3.5 mg, about 4.0 mg,
about 4.5 mg, about 5 mg, about 5.5 mg, about 6 mg, about 6.5 mg, about 7 mg,
about 7.5 mg, about 8 mg, about 8.5 mg,
about 9 mg, about 9.5 mg, about 10 mg, about 10.5 mg, about 11 mg, about 11.5
mg, about 12 mg, about 12.5 mg, and/or
about 13 mg, about 14 mg, or about 15 mg. In further embodiments, the compound
of formula I present in the dosage
OH 77
H0j5Z.1
C( 'NH F 0- 'NH
F
Mee N FN
= 10
4117 F
amounts provided herein is selected from the group consisting of: and
In some embodiments of the compositions and methods provided herein, provided
are MEK protein kinase
inhibitors further comprising a compound of formula I, wherein the compound of
formula I is present in an amount of
about 15 mg, about 20 mg, about 25 mg, about 30 mg, about 35 mg, about 40 mg,
about 45 mg, about 50 mg, about 55
mg, about 60 mg, about 65 mg, about 75 mg, about 80 mg, about 85 mg, about 90
mg, about 95 mg, about 100 mg, about
110 mg, about 120 mg, about 125 mg, about 130 mg, about 140 mg, about 150 mg,
about 160 mg, about 170 mg, about
175 mg, about 180 mg, about 190 mg, or about 200 mg. In further embodiments,
the compound of formula I present in
z 0
HO
0 'NH H F
Me0 N
F
the dosage amounts provided herein is selected from the group consisting of:
F and
HO
jO
- 'NH F
F N maim
411111 I
F F
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from cancer is a
mammal. In further or additional embodiments, the individual is a human. In
further or additional embodiments, an
effective amount of a composition comprising a pharmaceutically acceptable
salt of a compound of formula I is
administered.
-Page 92-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some aspects, the present invention is directed to a method of treating a
disease in an individual suffering from
said disease comprising administering to said individual an effective amount
of a composition comprising a compound of
formula I or a pharmaceutically acceptable salt, solvate, polymorph, ester,
tautomer or prodrug thereof.
In other aspects, the present invention is directed to a method of treating a
disorder in a mammal, comprising
administering to said mammal a therapeutically effective amount of the
compound of formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof
In other aspects, the present invention is directed to a method of treating a
disorder in a human, comprising
administering to said mammal a therapeutically effective amount of the
compound of formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester, tautomer or prodrug thereof.
MEK Modulated Disease and Disorders
Also described herein are methods of modulating MEK activity by contacting MEK
with an amount of a
compound of formula I sufficient to modulate the activity of MEK. Modulate can
be inhibiting or activating MEK
activity. In some embodiments, the invention provides methods of inhibiting
MEK activity by contacting MEK with an
amount of a compound of formula I sufficient to inhibit the activity of MEK.
hi some embodiments, the invention
provides methods of inhibiting MEK activity in a solution by contacting said
solution with an amount of a compound of
formula I sufficient to inhibit the activity of MEK in said solution. In some
embodiments, the invention provides methods
of inhibiting MEK activity in a cell by contacting said cell with an amount of
a compound described herein sufficient to
inhibit the activity of MEK in said cell. In some embodiments, the invention
provides methods of inhibiting MEK
activity in a tissue by contacting said tissue with an amount of a compound
described herein sufficient to inhibit the
activity of MEK in said tissue. In some embodiments, the invention provides
methods of inhibiting MEK activity in an
organism by contacting said organism with an amount of a compound described
herein sufficient to inhibit the activity of
MEK in said organism. In some embodiments, the invention provides methods of
inhibiting MEK activity in an animal
by contacting said animal with an amount of a compound described herein
sufficient to inhibit the activity of MEK in
said animal. In some embodiments, the invention provides methods of inhibiting
MEK activity in a mammal by
contacting said mammal with an amount of a compound described herein
sufficient to inhibit the activity of MEK in said
mammal. In some embodiments, the invention provides methods of inhibiting MEK
activity in a human by contacting
said human with an amount of a compound described herein sufficient to inhibit
the activity of MEK in said human.
Compounds of formula I, and compositions containing a compound of formula I,
and pharmaceutically acceptable
salts, solvates, polymorphs, esters, amides, tautomers or prodrugs thereof,
may modulate the activity of MEK enzymes;
and, as such, are useful for treating diseases or conditions in which aberrant
MEK enzyme activity contributes to the
pathology and/or symptoms of a disease or condition.
In some aspects, the present invention is directed to a method of treating a
disorder or condition which is
modulated by the MEK cascade in a mammal, including a human, comprising
administering to said mammal an amount
of the compound of formula I, or a pharmaceutically acceptable salt, ester,
prodrug, solvate, hydrate or derivative thereof,
effective to modulate said cascade. The appropriate dosage for a particular
patient can be determined, according to
known methods, by those skilled in the art.
In other aspects, the present invention is directed to a method for inhibiting
a MEK enzyme. In some
embodiments, the method comprises contacting said MEK enzyme with an amount of
a composition comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug
thereof; sufficient to inhibit said enzyme, wherein said enzyme is inhibited.
In further or additional embodiments the
enzyme is at least about 1% inhibited. In further or additional embodiments
the enzyme is at least about 2% inhibited. In
-Page 93-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
further or additional embodiments the enzyme is at least about 3% inhibited.
In further or additional embodiments the
enzyme is at least about 4% inhibited. In further or additional embodiments
the enzyme is at least about 5% inhibited. In
further or additional embodiments the enzyme is at least about 10% inhibited.
In further or additional embodiments the
enzyme is at least about 20% inhibited. In further or additional embodiments
the enzyme is at least about 25% inhibited.
In further or additional embodiments the enzyme is at least about 30%
inhibited. In further or additional embodiments the
enzyme is at least about 40% inhibited. In further or additional embodiments
the enzyme is at least about 50% inhibited.
In further or additional embodiments the enzyme is at least about 60%
inhibited. In further or additional embodiments the
enzyme is at least about 70% inhibited. In further or additional embodiments
the enzyme is at least about 75% inhibited.
In further or additional embodiments the enzyme is at least about 80%
inhibited. In further or additional embodiments the
enzyme is at least about 90% inhibited. In further or additional embodiments
the enzyme is essentially completely
inhibited. In further or additional embodiments the MEK enzyme is MEK kinase.
In further or additional embodiments
the MEK enzyme is MEK1. In further or additional embodiments the MEK enzyme is
MEK2. In further or additional
embodiments the contacting occurs within a cell. In further or additional
embodiments the cell is a mammalian cell. In
further or additional embodiments the mammalian cell is a human cell. In
further or additional embodiments, the MEK
enzyme is inhibited with a composition comprising a pharmaceutically
acceptable salt of a compound of formula I.
In further or additional aspects, the present invention is directed to a
method of treatment of a MEK mediated
disorder in an individual suffering from said disorder comprising
administering to said individual an effective amount of
a composition comprising a compound of formula I or a pharmaceutically
acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof. In some embodiments, the composition
comprising a compound of formula I is
administered orally, intraduodenally, parenterally (including intravenous,
subcutaneous, intramuscular, intravascular or
by infusion), topically or rectally. In some embodiments, the pharmaceutical
composition is in a form suitable for oral
administration. In further or additional embodiments, the pharmaceutical
composition is in the form of a tablet, capsule,
pill, powder, sustained release formulations, solution, suspension, for
parenteral injection as a sterile solution, suspension
or emulsion, for topical administration as an ointment or cream or for rectal
administration as a suppository. In further or
additional embodiments, the pharmaceutical composition is in unit dosage forms
suitable for single administration of
precise dosages. In further or additional embodiments, the pharmaceutical
composition further comprises a
pharmaceutical carrier, excipient and/or adjuvant.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.001 to about
1000 mg/kg body weight/day. In further or additional embodiments the amount of
compound of formula I is in the range
of about 0.5 to about 50 mg/kWday. In further or additional embodiments the
amount of compound of formula I is about
0.001 to about 7 g/day. In further or additional embodiments the amount of
compound of formula I is about 0.01 to about
7 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.02 to about 5 g/day. In
further or additional embodiments the amount of compound of formula I is about
0.05 to about 2.5 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.1 to
about 1 g/day. In further or additional
embodiments, dosage levels below the lower limit of the aforesaid range may be
more than adequate. In further or
additional embodiments, dosage levels above the upper limit of the aforesaid
range may be required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
-Page 94-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the MEK
mediated disorder is a mammal. In further or additional embodiments, the
individual is a human.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
arigiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonallanti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezomib or both.
In some embodiments, the MEK mediated disorder is selected from the group
consisting of inflammatory diseases,
infections, autoimmune disorders, stroke, ischemia, cardiac disorder,
neurological disorders, fibrogenetic disorders,
proliferative disorders, hyperproliferative disorders, non-cancer
hyperproliferative disorders, tumors, leukemias,
neoplasms, cancers, carcinomas, metabolic diseases, malignant disease,
vascular restenosis, psoriasis, atherosclerosis,
rheumatoid arthritis, osteoarthritis, heart failure, chronic pain, neuropathic
pain, dry eye, closed angle glaucoma and wide
angle glaucoma. In further or additional embodiments, the MEK mediated
disorder is an inflammatory disease. In further
or additional embodiments, the MEK mediated disorder is a hyperproliferative
disease. In further or additional
embodiments, the MEK mediated disorder is selected from the group consisting
of tumors, leukemias, neoplasms,
cancers, carcinomas and malignant disease. In further or additional
embodiments, stomach cancer, brain cancer, breast
cancer, lung cancer, ovarian cancer, pancreatic cancer, prostate cancer, renal
cancer, colorectal cancer or leukemia. In
further or additional embodiments, the fibrogenetic disorder is scleroderma,
polymyositis, systemic lupus, rheumatoid
arthritis, liver cirrhosis, keloid formation, interstitial nephritis or
pulmonary fibrosis. In further or additional
embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a compound of
formula I is administered.
The invention also relates to methods of modulating MEK activity by contacting
MEK with an amount of a
crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide sufficient to modulate the activity
of MEK. Modulate can be inhibiting or
activating MEK activity. In some embodiments, the invention provides methods
of inhibiting MEK activity by contacting
MEK with an amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-
(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide sufficient to
inhibit the activity of MEK. In some
embodiments, the invention provides methods of inhibiting MEK activity in a
solution by contacting said solution with
an amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-dihydroxypropypcyclopropane-l-sulfonamide sufficient to inhibit the
activity of MEK in said solution. In some
embodiments, the invention provides methods of inhibiting MEK activity in a
cell by contacting said cell with an amount
of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide sufficient to inhibit the activity
of MEK in said cell. In some
embodiments, the invention provides methods of inhibiting MEK activity in a
tissue by contacting said tissue with an
amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyI)-1-
(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide sufficient to inhibit the
activity of MEK in said tissue. In some
-Page 95-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments, the invention provides methods of inhibiting MEK activity in an
organism by contacting said organism
with an amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide sufficient to
inhibit the activity of MEK in said
organism. In some embodiments, the invention provides methods of inhibiting
MEK activity in an animal by contacting
said animal with an ofamount a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide sufficient to
inhibit the activity of MEK in said
animal. In some embodiments, the invention provides methods of inhibiting MEK
activity in a mammal by contacting
said mammal with an amount of a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide sufficient
to inhibit the activity of MEK in said
mammal. In some embodiments, the invention provides methods of inhibiting MEK
activity in a human by contacting
said human with an amount of a crystalline polymorph form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylanaino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide sufficient to
inhibit the activity of MEK in said
human.
Cancer
In other aspects, the present invention is directed to a method for the
treatment, prevention or prophylaxis of
cancer in an individual comprising administering to said individual an
effective amount of a compound of formula I or a
pharmaceutically acceptable salt, solvate, polymorph, ester, amide, tautomer
or prodrug thereof. In some embodiments,
the compound or pharmaceutically acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof is
administered as a component of a composition that farther comprises a
pharmaceutically acceptable carrier or vehicle. In
further or additional embodiments, the cancer is brain cancer, breast cancer,
lung cancer, ovarian cancer, pancreatic
cancer, prostate cancer, renal cancer, colorectal cancer, stomach cancer, or
leukemia. In further or additional
embodiments, the fibrogenetic disorder is scleroderma, polymyositis, systemic
lupus, rheumatoid arthritis, liver cirrhosis,
keloid formation, interstitial nephritis or pulmonary fibrosis. In further or
additional embodiments, the cancer is brain
cancer, breast cancer, lung cancer, ovarian cancer, pancreatic cancer,
prostate cancer, renal cancer, colorectal cancer,
leukemia, melanoma, thyroid cancer, or basal cell carcinoma. In further or
additional embodiments, the cancer is brain
cancer or adrenocortical carcinoma. In further or additional embodiments, the
cancer is breast cancer. In further or
additional embodiments, the cancer is ovarian cancer. In further or additional
embodiments, the cancer is pancreatic
cancer. In further or additional embodiments, the cancer is prostate cancer.
In further or additional embodiments, the
cancer is renal cancer. In further or additional embodiments, the cancer is
colorectal cancer. In further or additional
embodiments, the cancer is myeloid leukemia. In further or additional
embodiments, the cancer is glioblastoma. In
further or additional embodiments, the cancer is follicular lymphona. In
further or additional embodiments, the cancer is
pre-B acute leukemia. In further or additional embodiments, the cancer is
chronic lymphocytic B-leukemia. In further or
additional embodiments, the cancer is mesothelioma. In further or additional
embodiments, the cancer is small cell lung
cancer. In some embodiments, the cancer is stomach cancer.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
- Page 96 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezomib or both.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.001 to about
1000 mg/kg body weight/day. In further or additional embodiments the amount of
compound of formula I is in the range
of about 0.5 to about 50 mg/kg/day. In further or additional embodiments the
amount of compound of formula I is about
0.001 to about 7 g/day. In further or additional embodiments the amount of
compound of formula I is about 0.01 to about
7 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.02 to about 5 g/day. In
further or additional embodiments the amount of compound of formula I is about
0.05 to about 2.5 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.1 to
about 1 g/day. In further or additional
embodiments, dosage levels below the lower limit of the aforesaid range may be
more than adequate. In further or
additional embodiments, dosage levels above the upper limit of the aforesaid
range may be required.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the compound of formula I is administered in a single dose, once
daily. In further or additional
embodiments the compound of formula I is administered in multiple doses, more
than once per day. In further or
additional embodiments the compound of formula I is administered twice daily.
In further or additional embodiments the
compound of formula I is administered three times per day. In further or
additional embodiments the compound of
formula I is administered four times per day. In further or additional
embodiments the compound of formula I is
administered more than four times per day. In some embodiments, the individual
suffering from cancer is a mammal. In
further or additional embodiments, the individual is a human. In further or
additional embodiments, an effective amount
of a composition comprising a pharmaceutically acceptable salt of a compound
of formula I is administered.
Abnormal Cell Growth
Also described herein are compounds, pharmaceutical compositions and methods
for inhibiting abnormal cell
growth. In some embodiments, the abnormal cell growth occurs in a mammal.
Methods for inhibiting abnormal cell
growth comprise administering an effective amount of a compound of formula I,
or a pharmaceutically acceptable salt,
solvate, polymorph, ester, amide, tautomer, prodrug, hydrate, or derivative
thereof, wherein abnormal cell growth is
inhibited. Methods for inhibiting abnormal cell growth in a mammal comprise
administering to the mammal an amount
of a compound of formula I, or a pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer, prodrug,
hydrate, or derivative thereof, wherein the amounts of the compound, or salt,
is effective in inhibiting abnormal cell
growth in the mammal.
In some embodiments, the methods comprise administering an effective amount of
a compound of formula I, or a
pharmaceutically acceptable salt, solvate, polymorph, ester, amide, tautomer,
prodrug, hydrate, or derivative thereof, in
combination with an amount of a chemotherapeutic, wherein the amounts of the
compound, or its salt, solvate,
polymorph, ester, amide, tautomer, prodrug, hydrate, or derivative thereof and
the chemotherapeutic are together
effective in inhibiting abnormal cell growth. Many chemotherapeutics are
presently known in the art and can be used in
combination with the compounds of the invention. In some embodiments, the
chemotherapeutic is selected from the
group consisting of mitotic inhibitors, alkylating agents, anti-metabolites,
intercalating antibiotics, growth factor
inhibitors, cell cycle inhibitors, enzymes, topoisomerase inhibitors,
biological response modifiers, anti-hormones,
angiogenesis inhibitors, and anti-androgens.
-Page 97-
CA 02693390 2015-12-04
30725-1660
Also described are methods for inhibiting abnormal cell growth in a mammal
comprising administering to the
mammal an amount of a compound of formula I, or a pharmaceutically acceptable
salt, solvate, polymorph, ester, amide,
tautomer, prodrug, hydrate, or derivative thereof, in combination with
radiation therapy, wherein the amounts of the -
compound, or its salt, solvate, polymorph, ester, amide, tautomer, prodrug,
hydrate, or is derivative thereof is in
combination with the radiation therapy effective in inhibiting abnormal cell
growth or treating the hyperproliferative
disorder in the mammal. Techniques for administering radiation therapy are
known in the art, and these techniques can be
used in the combination therapy described herein. The administration of the
compound of formula 1 in this combination
therapy can be determined as described herein.
The invention also relates to a method of and to a pharmaceutical composition
of inhibiting abnormal cell growth
in a mammal which comprises an amount of a compound of formula I, or a
pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer, prodrug, hydrate, or derivative thereof, or
an isotopically-labeled derivative thereof,
and an amount of one or more substances selected from anti-angiogenesis
agents, signal nansduction inhibitors, and
antiproliferative agents.
Anti-angiogenesis agents, such as MMP-2 (matrix-metalloprotienase 2)
inhibitors, MMP-9 (matrix-
metalloprotienase 9) inhibitors, and COX-11 (cyclooxygenase 11) inhibitors,
can be used in conjunction with a
compound of the present invention and pharmaceutical compositions described
herein. Examples of useful COX-II
inhibitors include CELEBREXIm (atecoxib), valdecoxib, and rofecoxib. Examples
of useful matrix metalloproteinase
inhibitors arc described in WO 96/33172 (published October 24,1996), WO
96/27583 (published March 7,1996),
European Patent Publication No. 0818442, European Patent Publication No.
1004578,
WO 98/07697 (published February 26, 1998), WO 98/03516 (published January 29,
1998), WO
98/34918 (published August 13,1998), WO 98/34913 (published August 13,1998),
WO 98/33768 (published August
6,1998), WO 98/30566 (published July 16, 1998), European Patent Publication
606,046 (published July 13,1994),
European Patent Publication 931, 788 (published July 28,1999), WO 90/05719
(published May 31,1990), WO 99/52910
(published October 21,1999), WO 99/52889 (published October 21, 1999), WO
99/29667 (published June 17,1999),
WO 99/007675, United States Patent No. 6,511,993, United States Patent
No. 7,030,242, United States Patent 5,863,949 (issued January 26, 1999),
United States Patent 5,861,510 (issued January 19, 1999), and European
Patent Publication 780,386 (published June 25, 1997).
Some MMP-2 and MMP-9 inhibitors have little or no
activity inhibiting MMP-1, while some selectively inhibit MIVIP-2 and/or AMP-9
relative to the other matrix-
metalloproteinases (i. e., MAP-I, MMP-4, MMP-5, MMP-6, MMP- 7, MMP-8, MMP-
I 0, MMP-11, MMP-12,
andMMP-13). Some specific examples of M1VIP inhibitors useful in the present
invention are AG-3340, RO 32-3555, and
RS 13-0830.
In other aspects, the present invention is directed to a method for degrading,
inhibiting the growth of or killing a
cancer cell comprising contacting said cell with an amount of a composition
effective to degrade, inhibit the growth of or
to kill said cell, the composition comprising a compound of formula 1 or a
pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug thereof. In some embodiments, the
cancer cells comprise brain, breast,
lung, ovarian, pancreatic, prostate, renal, or colorectal cancer cells.
In further or additional embodiments, the composition is administered with at
least one therapeutic agent. In
further or additional embodiments, the therapeutic agent is taxol, bortezornib
or both. In further or additional
embodiments, the therapeutic agent is selected from the group consisting of
cytotoxic agents, anti-angiogenesis agents
-Page 98-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
and anti-neoplastic agents. In further or additional embodiments, the anti-
neoplastic agents selected from the group of
consisting of alkylating agents, anti-metabolites, epidophyllotoxins;
antineoplastic enzymes, topoisornerase inhibitors,
procarbazines, mitoxantrones, platinum coordination complexes, biological
response modifiers and growth inhibitors,
hormonal/anti-hormonal therapeutic agents, and haematopoietic growth factors.
In some embodiments, the cancer cells are degraded. In further or additional
embodiments, 1% of the cancer cells
are degraded. In further or additional embodiments, 2% of the cancer cells are
degraded. In further or additional
embodiments, 3% of the cancer cells are degraded. In further or additional
embodiments, 4% of the cancer cells are
degraded. In further or additional embodiments, 5% of the cancer cells are
degraded. In further or additional
embodiments, 10% of the cancer cells are degraded. In further or additional
embodiments, 20% of the cancer cells are
degraded. In further or additional embodiments, 25% of the cancer cells are
degraded. In further or additional
embodiments, 30% of the cancer cells are degraded. In further or additional
embodiments, 40% of the cancer cells are
degraded. In further or additional embodiments, 50% of the cancer cells are
degraded. In further or additional
embodiments, 60% of the cancer cells are degraded. In further or additional
embodiments, 70% of the cancer cells are
degraded. In further or additional embodiments, 75% of the cancer cells are
degraded. In further or additional
embodiments, 80% of the cancer cells are degraded. In further or additional
embodiments, 90% of the cancer cells are
degraded. In further or additional embodiments, 100% of the cancer cells are
degraded. In further or additional
embodiments, essentially all of the cancer cells are degraded.
In some embodiments, the cancer cells are killed. In further or additional
embodiments, 1% of the cancer cells are
killed. In further or additional embodiments, 2% of the cancer cells are
killed. In further or additional embodiments, 3%
of the cancer cells are killed. In further or additional embodiments, 4% of
the cancer cells are killed. In further or
additional embodiments, 5% of the cancer cells are killed. In further or
additional embodiments, 10% of the cancer cells
are killed. In further or additional embodiments, 20% of the cancer cells are
killed. In further or additional embodiments,
25% of the cancer cells are killed. In further or additional embodiments, 30%
of the cancer cells are killed. In further or
additional embodiments, 40% of the cancer cells are killed. In further or
additional embodiments, 50% of the cancer cells
are killed. In further or additional embodiments, 60% of the cancer cells are
killed. In further Or additional embodiments,
70% of the cancer cells are killed. In further or additional embodiments, 75%
of the cancer cells are killed. In further or
additional embodiments, 80% of the cancer cells are killed. In further or
additional embodiments, 90% of the cancer cells
are killed. In further or additional embodiments, 100% of the cancer cells are
killed. In further or additional
embodiments, essentially all of the cancer cells are killed.
In further or additional embodiments, the growth of the cancer cells is
inhibited. In further or additional
embodiments, the growth of the cancer cells is about 1% inhibited. In further
or additional embodiments, the growth of
the cancer cells is about 2% inhibited. In further or additional embodiments,
the growth of the cancer cells is about 3%
inhibited. In further or additional embodiments, the growth of the cancer
cells is about 4% inhibited. In further or
additional embodiments, the growth of the cancer cells is about 5% inhibited.
In further or additional embodiments, the
growth of the cancer cells is about 10% inhibited. In further or additional
embodiments, the growth of the cancer cells is
about 20% inhibited. In further or additional embodiments, the growth of the
cancer cells is about 25% inhibited. In
further or additional embodiments, the growth of the cancer cells is about 30%
inhibited. In further or additional
embodiments, the growth of the cancer cells is about 40% inhibited. In further
or additional embodiments, the growth of
the cancer cells is about 50% inhibited. In further or additional embodiments,
the growth of the cancer cells is about 60%
inhibited. In further or additional embodiments, the growth of the cancer
cells is about 70% inhibited. In further or
additional embodiments, the growth of the cancer cells is about 75% inhibited.
In further or additional embodiments, the
-Page 99-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
growth of the cancer cells is about 80% inhibited. In further or additional
embodiments, the growth of the cancer cells is
about 90% inhibited. In further or additional embodiments, the growth of the
cancer cells is about 100% inhibited. In
further or additional embodiments, a composition comprising a pharmaceutically
acceptable salt of a compound of
formula I is used.
Also described herein are methods for inhibiting abnormal cell growth. In some
embodiments, the abnormal cell
growth occurs in a mammal. Methods for inhibiting abnormal cell growth
comprise administering an effective amount of
a crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyl)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide, wherein abnormal cell growth is
inhibited. Methods for inhibiting
abnormal cell growth in a mammal comprise administering to the mammal an
amount of a crystalline polymorph form A
of N-(S)-(3 ,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)4-(2,3-
dihydroxypropyl)cycIopropane-1-
sulfonamide, wherein the amount of a crystalline polymorph form A of N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide is effective in inhibiting
abnormal cell growth in the mammal.
In some embodiments, the methods comprise administering an effective amount of
a crystalline polymorph form A
of N-(S)-(3,4-difluoro-2-(2 -fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cycl opropane-1-
sulfonamide in combination with an amount of a chemotherapeutic, wherein the
amounts of the crystalline polymorph
form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide and of the chemotherapeutic are
together effective in inhibiting abnormal
cell growth.
Many chemotherapeutics are presently known in the art and can be used in
combination with the compounds and
compositions of the invention. In some embodiments, the chemotherapeutic is
selected from the group consisting of
mitotic inhibitors, alkylating agents, anti-metabolites, intercalating
antibiotics, growth factor inhibitors, cell cycle
inhibitors, enzymes, topoisomerase inhibitors, biological response modifiers,
anti-hormones, angiogenesis inhibitors, and
anti-androgens.
In some embodiments, the methods for inhibiting abnormal cell growth in a
mammal comprise administering to
the mammal an amount of a crystalline polymorph form A of N-(S)-(3,4-difluoro-
2-(2-fluoro-4-iodophenyiamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide in combination
with radiation therapy, wherein the
amount of crystalline polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-rnethoxyphenyl)-1-
(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide in combination with the
radiation therapy is effective in inhibiting
abnormal cell growth. Techniques for administering radiation therapy are known
in the art, and these techniques can be
used in the combination therapy described herein.
Treatment of a Hyperproliferative Disorder
In other aspects, the present invention is directed to a method of treating a
hypeiproliferative disorder in a
mammal, including a human, comprising administering to said mammal a
therapeutically effective amount of the
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, tautomer or prodrug thereof.
In other aspects, the present invention is directed to a method for the
treatment, prevention or prophylaxis of a
proliferative disease in an individual comprising administering to said
individual an effective amount of a compound of
formula I, or a pharmaceutically acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof. In some
embodiments, the compound or pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug
thereof is administered as a component of a composition that further comprises
a pharmaceutically acceptable carrier or
vehicle. In some embodiments, the proliferative disease is cancer, psoriasis,
restenosis, autoimmune disease, or
-Page 100-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
atherosclerosis. In further or additional embodiments, the proliferative
disease is a hyperproliferative disease. In further
or additional embodiments, the proliferative disease is selected from the
group consisting of tumors, leukemias,
neoplasms, cancers, carcinomas and malignant disease. In further or additional
embodiments, the cancer is brain cancer,
breast cancer, lung cancer, ovarian cancer, pancreatic cancer, prostate
cancer, renal cancer, stomach cancer, colorectal
cancer or leukemia. In further or additional embodiments, the fibrogenetic
disorder is scleroderma, polymyositis,
systemic lupus, rheumatoid arthritis, liver cirrhosis, keloid formation,
interstitial nephritis or pulmonary fibrosis. In
further or additional embodiments, the cancer is brain cancer, breast cancer,
lung cancer, ovarian cancer, stomach cancer,
pancreatic cancer, prostate cancer, renal cancer, colorectal cancer or
leukemia. In further or additional embodiments, the
cancer is brain cancer or adrenocortical carcinoma. In further or additional
embodiments, the cancer is breast cancer. In
further or additional embodiments, the cancer is ovarian cancer. In further or
additional embodiments, the cancer is
pancreatic cancer. In further or additional embodiments, the cancer is
prostate cancer. In further or additional
embodiments, the cancer is renal cancer. In further or additional embodiments,
the cancer is colorectal cancer. In further
or additional embodiments, the cancer is myeloid leukemia. In further or
additional embodiments, the cancer is
glioblastoma. In further or additional embodiments, the cancer is follicular
lymphona. In further or additional
embodiments, the cancer is pre-B acute leukemia. In further or additional
embodiments, the cancer is chronic
lymphocytic B-leukemia. In further or additional embodiments, the cancer is
mesothelioma. In further or additional
embodiments, the cancer is small cell lung cancer. In some embodiments, the
cancer is stomach cancer.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
surgery or a combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of alkylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezomib or both.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula us about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
-Page 101-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the
proliferative disease is a mammal. In further or additional embodiments, the
individual is a human. In further or
additional embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a
compound of formula I is administered.
Tumor Size
In other aspects, the present invention is directed to a method of reducing
the size of a tumor, inhibiting tumor size
increase, reducing tumor proliferation or preventing tumor proliferation in an
individual, comprising administering to
said individual an effective amount of a compound of formula I or a
pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or prodrug thereof. In some embodiments, the
compound or pharmaceutically
acceptable salt, solvate, polymorph, ester, amide, tautomer or prodrug thereof
is administered as a component of a
composition that further comprises a pharmaceutically acceptable carrier or
vehicle. In some embodiments, the size of a
tumor is reduced. In further or additional embodiments, the size of a tumor is
reduced by at least 1%. In further or
additional embodiments, the size of a tumor is reduced by at least 2%. In
further or additional embodiments, the size of a
tumor is reduced by at least 3%. In further or additional embodiments, the
size of a tumor is reduced by at least 4%. In
further or additional embodiments, the size of a tumor is reduced by at least
5%. In further or additional embodiments,
the size of a tumor is reduced by at least 10%. In further or additional
embodiments, the size of a tumor is reduced by at
least 20%. In further or additional embodiments, the size of a tumor is
reduced by at least 25%. In further or additional
embodiments, the size of a tumor is reduced by at least 30%. In further or
additional embodiments, the size of a tumor is
reduced by at least 40%. In further or additional embodiments, the size of a
tumor is reduced by at least 50%. In further
or additional embodiments, the size of a tumor is reduced by at least 60%. In
further or additional embodiments, the size
of a tumor is reduced by at least 70%. In further or additional embodiments,
the size of a tumor is reduced by at least
75%. In further or additional embodiments, the size of a tumor is reduced by
at least 80%. In further or additional
embodiments, the size of a tumor is reduced by at least 85%. In further or
additional embodiments, the size of a tumor is
reduced by at least 90%. In further or additional embodiments, the size of a
tumor is reduced by at least 95%. In further
or additional embodiments, the tumor is eradicated. In some embodiments, the
size of a tumor does not increase.
In some embodiments, tumor proliferation is reduced. In some embodiments,
tumor proliferation is reduced by at
least I %. In some embodiments, tumor proliferation is reduced by at least 2
%. In some embodiments, tumor
proliferation is reduced by at least 3 %. In some embodiments, tumor
proliferation is reduced by at least 4 %. In some
embodiments, tumor proliferation is reduced by at least 5 %. In some
embodiments, tumor proliferation is reduced by at
least 10 %. In some embodiments, tumor proliferation is reduced by at least 20
%. In some embodiments, tumor
proliferation is reduced by at least 25 %. In some embodiments, tumor
proliferation is reduced by at least 30 %. In some
embodiments, tumor proliferation is reduced by at least 40 %. In some
embodiments, tumor proliferation is reduced by at
least 50 %. In some embodiments, tumor proliferation is reduced by at least 60
%. In some embodiments, tumor
proliferation is reduced by at least 70 %. In some embodiments, tumor
proliferation is reduced by at least 75 %. In some
embodiments, tumor proliferation is reduced by at least 75 %. In some
embodiments, tumor proliferation is reduced by at
least 80 %. In some embodiments, tumor proliferation is reduced by at least 90
%. In some embodiments, tumor
proliferation is reduced by at least 95 %. In some embodiments, tumor
proliferation is prevented.
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the additional
therapy is radiation therapy, chemotherapy,
-Page 102-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
surgery or any combination thereof. In further or additional embodiments, the
composition comprising a compound of
formula I is administered in combination with at least one therapeutic agent.
In further or additional embodiments, the
therapeutic agent is selected from the group of cytotoxic agents, anti-
angiogenesis agents and anti-neoplastic agents. In
further or additional embodiments, the anti-neoplastic agent is selected from
the group of consisting of allcylating agents,
anti-metabolites, epidophyllotoxins; antineoplastic enzymes, topoisomerase
inhibitors, procarbazines, mitoxantrones,
platinum coordination complexes, biological response modifiers and growth
inhibitors, hormonal/anti-hormonal
therapeutic agents, and haematopoietic growth factors. In further or
additional embodiments, the therapeutic agent is
selected from taxol, bortezomib or both.
In some embodiments, the composition is administered orally, intraduodenally,
parenterally (including
intravenous, subcutaneous, intramuscular, intravascular or by infusion),
topically or rectally. In further or additional
embodiments the amount of compound of formula I is in the range of about 0.001
to about 1000 mg/kg body weight/day.
In further or additional embodiments the amount of compound of formula I is in
the range of about 0.5 to about 50
mg/kg/day. In further or additional embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day.
In further or additional embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or
additional embodiments the amount of compound of formula I is about 0.02 to
about 5 g/day. In further or additional
embodiments the amount of compound of formula I is about 0.05 to about 2.5
g/day. In further or additional
embodiments the amount of compound of formula I is about 0.1 to about 1 g/day.
In further or additional embodiments,
dosage levels below the lower limit of the aforesaid range may be more than
adequate. In further or additional
embodiments, dosage levels above the upper limit of the aforesaid range may be
required.
In further or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from cancer is a
mammal. In further or additional embodiments, the individual is a human. In
further or additional embodiments, an
effective amount of a composition comprising a pharmaceutically acceptable
salt of a compound of formula I is
administered.
Inflammatory Disease
In other aspects, the present invention is directed to a method for the
treatment, prevention or prohylaxis of an
inflammatory disease in an individual comprising administering to said
individual an effective amount of compound of
formula I or a pharmaceutically acceptable salt, solvate, polymorph, ester,
amide, tautomer or prodrug thereof. In some
embodiments, the compound or pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer or proclrug
thereof is administered as a component of a composition that further comprises
a pharmaceutically acceptable carrier or
vehicle. In further or additional embodiments, the inflammatory disease is
selected from chronic inflammatory diseases,
rheumatoid arthritis, rheumatoid arthritis, spondyloarthropathies, ankylosing
spondylitis, gout, tendonitis, bursitis,
sciatica, gouty arthritis, osteoarthritis, juvenile arthritis, acute rheumatic
arthritis, enteropathic arthritis, neuropathic
arthritis, psoriatic arthritis, pyogenic arthritis, atherosclerosis, systemic
lupus erythematosus, inflammatory bowel
disease, irritable bowel syndrome, ulcerative colitis, reflux esophagitis,
Crohn's disease, gastritis, asthma, allergies,
respiratory distress syndrome, pancreatitis, chronic obstructive pulmonary
disease, pulmonary fibrosis, psoriasis, eczema
or scleroderma.
-Page 103-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
In some embodiments, the composition comprising a compound of formula I is
administered in combination with
an additional therapy. In further or additional embodiments, the composition
comprising a compound of formula I is
administered in combination with at least one therapeutic agent. In some
embodiments, the composition is administered
orally, intraduodenally, parenterally (including intravenous, subcutaneous,
intramuscular, intravascular or by infusion),
topically or rectally. In further or additional embodiments the amount of
compound of formula I is in the range of about
0.001 to about 1000 mg/kg body weight/day. In further or additional
embodiments the amount of compound of formula I
is in the range of about 0.5 to about 50 mg/kg/day. In further or additional
embodiments the amount of compound of
formula I is about 0.001 to about 7 g/day. In further or additional
embodiments the amount of compound of formula I is
about 0.01 to about 7 g/day. In further or additional embodiments the amount
of compound of formula I is about 0.02 to
about 5 g/day. In further or additional embodiments the amount of compound of
formula I is about 0.05 to about 2.5
g/day. In further or additional embodiments the amount of compound of formula
I is about 0.1 to about 1 g/day. In
further or additional embodiments, dosage levels below the lower limit of the
aforesaid range may be more than
adequate. In further or additional embodiments, dosage levels above the upper
limit of the aforesaid range may be
required.
In further Or additional embodiments the compound of formula I is administered
in a single dose, once daily. In
further or additional embodiments the compound of formula I is administered in
multiple doses, more than once per day.
In further or additional embodiments the compound of formula I is administered
twice daily. In further or additional
embodiments the compound of formula I is administered three times per day. In
further or additional embodiments the
compound of formula I is administered four times per day. In further or
additional embodiments the compound of
formula I is administered more than four times per day. In some embodiments,
the individual suffering from the
inflammatory disease is a mammal. In further or additional embodiments, the
individual is a human. In further or
additional embodiments, an effective amount of a composition comprising a
pharmaceutically acceptable salt of a
compound of formula I is administered.
Modes of Administration
Described herein are compounds of formula I or a pharmaceutically acceptable
salt solvate, polymorph, ester,
amide, tautomer, prodrug, hydrate, or derivative thereof. Also described, are
pharmaceutical compositions comprising a
compound of formula I or a pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer, prodrug,
hydrate, or derivative thereof. The compounds and compositions described
herein may be administered either alone or in
combination with pharmaceutically acceptable carriers, excipients or diluents,
in a pharmaceutical composition,
according to standard pharmaceutical practice.
Also described herein are pharmaceutical compositions comprising crystalline
polymorph N-(S)-(3,4-difluoro-2-
(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide (Form A). The
compounds and compositions described herein may be administered either alone
or in combination with
pharmaceutically acceptable carriers, excipients or diluents, in a
pharmaceutical composition, according to standard
pharmaceutical practice. Administration can be effected by any method that
enables delivery of the compounds to the site
of action. These methods include, though are not limited to delivery via
enteral routes (including oral, gastric or duodenal
feeding tube, rectal suppository and rectal enema), parenteral routes
(injection or infusion, including intraarterial,
intracardiac, intradennai, intraduodenal, intramedullary, intramuscular,
intraosseous, intraperitoneal, intrathecal,
intravascular, intravenous, intravitreal, epidural and subcutaneous),
inhalational, transdermal, transmucosal, sublingual,
buccal and topical (including epicutaneous, dermal, enema, eye drops, ear
drops, intranasal, vaginal) administration,
although the most suitable route may depend upon for example the condition and
disorder of the recipient. Those of skill
-Page 104-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
in the art will be familiar with administration techniques that can be
employed with the compounds and methods of the
invention. By way of example only, compounds described herein can be
administered locally to the area in need of
treatment, by for example, local infusion during surgery, topical application
such as creams or ointments, injection,
catheter, or implant, said implant made for example, out of a porous, non-
porous, or gelatinous material, including
membranes, such as sialastic membranes, or fibers. The administration can also
be by direct injection at the site of a
diseased tissue or organ.
Administration of the compounds and compositions described herein can be
effected by any method that enables
delivery of the compounds to the site of action. These methods include oral
routes, intraduodenal routes, parenteral
injection (including intravenous, subcutaneous, intraperitoneal,
intramuscular, intravascular or infusion), topical, and
rectal administration. For example, compounds described herein can be
administered locally to the area in need of
treatment. This may be achieved by, for example, but not limited to, local
infusion during surgery, topical application,
e.g., cream, ointment, injection, catheter, or implant, said implant made,
e.g., out of a porous, non-porous, or gelatinous
material, including membranes, such as sialastic membranes, or fibers. The
administration can also be by direct injection
at the site (or former site) of a tumor or neoplastic or pre-neoplastic
tissue. Those of ordinary skill in the art are familiar
with formulation and administration techniques that can be employed with the
compounds and methods of the invention,
e.g., as discussed in Goodman and Gilman, The Pharmacological Basis of
Therapeutics, current ed.; Pergamon; and
Remington's, Pharmaceutical Sciences (current edition), Mack Publishing Co.,
Easton, Pa.
The formulations include those suitable for oral, parenteral (including
subcutaneous, intradermal, intramuscular,
intravenous, intraarticular, and intramedullary), intraperitoneal,
transmucosal, transdermal, rectal and topical (including
dermal, buccal, sublingual and intraocular) administration although the most
suitable route may depend upon for example
the condition and disorder of the recipient. The formulations may conveniently
be presented in unit dosage form and may
be prepared by any of the methods well known in the art of pharmacy. All
methods include the step of bringing into
association a compound of the subject invention or a pharmaceutically
acceptable salt, solvate, polyrnorph, ester, amide,
tautomer, prodrug, hydrate, or derivative thereof ("active ingredient") with
the carrier which constitutes one or more
accessory ingredients. In general, the formulations are prepared by uniformly
and intimately bringing into association the
active ingredient with liquid carriers or finely divided solid carriers or
both and then, if necessary, shaping the product
into the desired formulation.
Formulations suitable for oral administration may be presented as discrete
units such as capsules, cachets or tablets
each containing a predetermined amount of the active ingredient; as a powder
or granules; as a solution or a suspension
in an aqueous liquid or a non-aqueous liquid; or as an oil-in-water liquid
emulsion or a water-in-oil liquid emulsion. The
active ingredient may also be presented as a bolus, electuary or paste.
Pharmaceutical preparations which are useful for oral administration include
tablets, push-fit capsules made of
gelatin, as well as soft, sealed capsules made of gelatin and a plasticizer,
such as glycerol or sorbitol. Tablets may be
made by compression or molding, optionally with one or more accessory
ingredients. Compressed tablets may be
prepared by compressing in a suitable machine the active ingredient in a free-
flowing form such as a powder or granules,
optionally mixed with binders, inert diluents, or lubricating, surface active
or dispersing agents. Molded tablets may be
made by molding in a suitable machine a mixture of the powdered compound
moistened with an inert liquid diluent. The
tablets may optionally be coated or scored and may be formulated so as to
provide slow or controlled release of the active
ingredient therein. All formulations for oral administration should be in
dosages suitable for such administration. The
push-fit capsules or tablets can contain the active ingredient; in admixture
with a filler such as microcrystalline cellulose,
silicified microcrystalline cellulose, pregelatinized starch, lactose,
dicalcium phosphate, or compressible sugar ; a binder
- Page 105 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
such as hypromellose, povidone or starch paste; a disintegrant such as
croscarmellose sodium, crospovidone or sodium
starch glycolate; a surfactant such as sodium lauryl sulfate and/or lubricants
and processing aides such as talc,magriesium
stearate, stearic acid or colloidal silicion dioxide and, optionally,
stabilizers. In soft capsules, the active compounds may
be dissolved or suspended in suitable liquids, such as fatty oils, liquid
paraffin, or liquid polyethylene glycols. In
addition, stabilizers may be added. Dragee cores are provided with suitable
coatings. For this purpose, concentrated sugar
solutions are useful, which may optionally contain gum arabic, talc, polyvinyl
pyrrolidone, carbopol gel, polyethylene
glycol, and/or titanium dioxide, lacquer solutions, and suitable organic
solvents or solvent mixtures. Dyestuffs or
pigments may be added to the tablets or Dragee coatings for identification or
to characterize different combinations of
active compound doses.
Pharmaceutical preparations may be formulated for parenteral administration by
injection, e.g., by bolus injection
or continuous infusion. Formulations for injection may be presented in unit
dosage form, e.g., in ampoules or in multi-
dose containers, with an added preservative. The compositions may take such
forms as suspensions, solutions or
emulsions in oily or aqueous vehicles, and may contain formulatory agents such
as suspending, stabilizing and/or
dispersing agents. The formulations may be presented in unit-dose or multi-
dose containers, for example sealed ampoules
and vials, and may be stored in powder form or in a freeze-dried (lyophilized)
condition requiring only the addition of the
sterile liquid carrier, for example, saline or sterile pyrogen-free water,
immediately prior to use. Extemporaneous
injection solutions and suspensions may be prepared from sterile powders,
granules and tablets of the kind previously
described.
Formulations for parenteral administration include aqueous and non-aqueous
(oily) sterile injection solutions of the
active compounds which may contain antioxidants, buffers, bacteriostats and
solutes which render the formulation
isotonic with the blood of the intended recipient; and aqueous and non-aqueous
sterile suspensions which may include
suspending agents and thickening agents. Suitable lipophilic solvents or
vehicles include fatty oils such as sesame oil, or
synthetic fatty acid esters, such as ethyl oleate or triglycerides, or
liposomes. Aqueous injection suspensions may contain
substances which increase the viscosity of the suspension, such as sodium
carboxymethyl cellulose, sorbitol, or dextran.
Optionally, the suspension may also contain suitable stabilizers or agents
which increase the solubility of the compounds
to allow for the preparation of highly concentrated solutions.
Pharmaceutical preparations may also be formulated as a depot preparation.
Such long acting formulations may be
administered by implantation (for example subcutaneously or intramuscularly)
or by intramuscular injection. Thus, for
example, the compounds may be formulated with suitable polymeric or
hydrophobic materials (for example as an
emulsion in an acceptable oil) or ion exchange resins, or as sparingly soluble
derivatives, for example, as a sparingly
soluble salt.
For buccal or sublingual administration, the compositions may take the form of
tablets, lozenges, pastilles, or gels
formulated in conventional manner. Such compositions may comprise the active
ingredient in a flavored basis such as
sucrose and acacia or tragacanth.
Pharmaceutical preparations may also be formulated in rectal compositions such
as suppositories or retention
enemas, e.g., containing conventional suppository bases such as cocoa butter,
polyethylene glycol, or other glycerides.
Pharmaceutical preparations may be administered topically, that is by non-
systemic administration. This includes
the application of a compound of the present invention externally to the
epidermis or the buccal cavity and the instillation
of such a compound into the ear, eye and nose, such that the compound does not
significantly enter the blood stream. In
contrast, systemic administration refers to oral, intravenous, intraperitoneal
and intramuscular administration.
-Page 106-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Pharmaceutical preparations suitable for topical administration include liquid
or semi-liquid preparations suitable
for penetration through the skin to the site of inflammation such as gels,
liniments, lotions, creams, ointments or pastes,
and drops suitable for administration to the eye, ear or nose. The active
ingredient may comprise, for topical
administration, from 0.001% to 10% w/w, for instance from 1% to 2% by weight
of the formulation. It may however
comprise as much as 10% w/w or may comprise less than 5% w/w, or from 0.1% to
1% w/w of the formulation.
Pharmaceutical preparations for administration by inhalation are conveniently
delivered from an insufflator,
nebulizer pressurized packs or other convenient means of delivering an aerosol
spray. Pressurized packs may comprise a
suitable propellant such as dichiorodifluoromethane, trichlorofluoromethane,
dichlorotetrafluoroethane, carbon dioxide
or other suitable gas. In the case of a pressurized aerosol, the dosage unit
may be determined by providing a valve to
deliver a metered amount. Alternatively, for administration by inhalation or
insufflation, pharmaceutical preparations
may take the form of a dry powder composition, for example a powder mix of the
compound and a suitable powder base
such as lactose or starch. The powder composition may be presented in unit
dosage form, in for example, capsules,
cartridges, gelatin or blister packs from which the powder may be administered
with the aid of an inhalator or insufflator.
It should be understood that in addition to the ingredients particularly
mentioned above, the compounds and
compositions described herein may include other agents conventional in the art
having regard to the type of formulation
in question, for example those suitable for oral administration may include
flavoring agents.
Formulations
It should be noted that any of the compositions and compounds described herein
may be used in any of the
formulations discussed in this section, which is not intended to be limiting
and should not be so construed.
The compounds or compositions described herein can be delivered in a vesicle,
e.g., a liposome (see, for example,
Langer, Science 1990, 249,1527-1533; Treat et al., Liposomes in the Therapy
ofinfectious Disease and Cancer, Lopez-
Bernstein and Fidler, Ed., Liss, N.Y., pp. 353-365, 1989).The compounds and
pharmaceutical compositions described
herein can also be delivered in a controlled release system. In one
embodiment, a pump may be used (see, Sefton, 1987,
CRC Crit. Ref. Biomed. Eng. 14:201; Buchwald et al. Surgery, 1980 88,507;
Saudek et al. N Engl. J. Med. 1989, 321,
(574). Additionally, a controlled release system can be placed in proximity of
the therapeutic target. (See, Goodson,
Medical Applications of Controlled Release, 1984, Vol. 2, pp. 115-138). The
pharmaceutical compositions described
herein can also contain the active ingredient in a form suitable for oral use,
for example, as tablets, troches, lozenges,
aqueous or oily suspensions, dispersible powders or granules, emulsions, hard
or soft capsules, or syrups or elixirs.
Compositions intended for oral use may be prepared according to any method
known to the art for the manufacture of
pharmaceutical compositions, and such compositions may contain one or more
agents selected from the group consisting
of sweetening agents, flavoring agents, coloring agents and preserving agents
in order to provide pharmaceutically
elegant and palatable preparations. Tablets contain the active ingredient in
admixture with non-toxic pharmaceutically
acceptable excipients which are suitable for the manufacture of tablets. These
excipients may be, for example, inert
diluents, such as calcium carbonate, sodium carbonate, lactose, calcium
phosphate or sodium phosphate; granulating and
disintegrating agents; fillers such as microcrystalline cellulose, silicified
microcrystalline cellulose, pregelatinized starch,
lactose, dicalcium phosphate, or compressible sugar; binders such as
hypromellose, povidone or starch paste;
disintegrants such as croscarmellose sodium, crospovidone or sodium starch
glycolate; a surfactant such as sodium lauryl
sulfate and/or lubricants and processing aides such as talc, sodium
croscarinellose, corn starch, or alginic acid; binding
agents, for example starch, gelatin, polyvinyl-pyrrolidone or acacia, and
lubricating agents, for example magnesium
stearate, stearic acid or colloidal silicion dioxide and, optionally, talc.
The tablets may be un-coated or coated by known
techniques to mask the taste of the drug or delay disintegration and
absorption in the gastrointestinal tract and thereby
-Page 107-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
provide a sustained action over a longer period. For example, a water soluble
taste masking material such as
hydroxypropylmethyl-cellulose or hydroxypropylcellulose, or a time delay
material such as ethyl cellulose, or cellulose
acetate butyrate may be employed as appropriate. Formulations for oral use may
also be presented as hard gelatin
capsules wherein the active ingredient is mixed with an inert solid diluent,
for example, calcium carbonate, calcium
phosphate or kaolin, or as soft gelatin capsules wherein the active ingredient
is mixed with water soluble carrier such as
polyethyleneglycol or an oil medium, for example peanut oil, liquid paraffin,
or olive oil. The capsule and tablet dosage
forms may be prepared by various processing techniques including dry blending
and wet granulation techniques. In the
dry blending method of manufacture the drug substance may be incorporated into
the dosage form by dry blending with
the excipients followed by encapsulation into a capsule shell or compression
into a tablet form. The dry blending
operation may be approached in a stepwise manner and include screening steps
between the blending steps to facilitate
formation of a uniform blend. In the wet granulation method of manufacture the
drug substance may be added to the dry
excipients and mixed prior to the addition of the binder solution or the drug
substance may be dissolved and added as a
solution as part of granulation. In the wet granulation technique the
surfactant, if used, may be added to the dry
excipients or added to the binder solution and incorporated in a solution
form. Capsule dosage forms may also be
manufactured by dissolving the drug substance in a material that can be filled
into and is compatible with hard gelatin
capsule shells that can be subsequently banded and sealed. Capsule and tablet
dosage forms may also be produced by
dissolving the drug substance in a material such a molten form of a high
molecular weight polyethylene glycol and
cooling to a solid form, milling and incorporating this material into
conventional capsule and tablet manufacturing
processes.
Aqueous suspensions contain the active material in admixture with excipients
suitable for the manufacture of
aqueous suspensions. Such excipients are suspending agents, for example sodium
carboxymethylcellulose,
methylcellulose, hydroxypropyhnethyl-cellulose, sodium alginate, polyvinyl-
pyrrolidone, gum tragacanth and gum
acacia; dispersing or wetting agents may be a naturally-occurring phosphatide,
for example lecithin, or condensation
products of an alkylene oxide with fatty acids, for example polyoxyethylene
stearate, or condensation products of
ethylene oxide with long chain aliphatic alcohols, for example
heptadecaethylene-oxycetanol, or condensation products
of ethylene oxide with partial esters derived from fatty acids and a hexitol
such as polyoxyethylene sorbitol monooleate,
or condensation products of ethylene oxide with partial esters derived from
fatty acids and hexitol anhydrides, for
example polyethylene sorbitan monooleate. The aqueous suspensions may also
contain one or more preservatives, for
example ethyl, or n-propyl p-hydroxybenzoate, one or more coloring agents, one
or more flavoring agents, and one or
more sweetening agents, such as sucrose, saccharin or aspartame.
Oily suspensions may be formulated by suspending the active ingredient in a
vegetable oil, for example arachis oil,
olive oil, sesame oil or coconut oil, or in mineral oil such as liquid
paraffin. The oily suspensions may contain a
thickening agent, for example beeswax, hard paraffin or cetyl alcohol.
Sweetening agents such as those set forth above,
and flavoring agents may be added to provide a palatable oral preparation.
These compositions may be preserved by the
addition of an anti-oxidant such as butylated hydroxyanisol or alpha-
tocopherol.
Dispersible powders and granules suitable for preparation of an aqueous
suspension by the addition of water
provide the active ingredient in admixture with a dispersing or wetting agent,
suspending agent and one or more
preservatives. Suitable dispersing or wetting agents and suspending agents are
exemplified by those already mentioned
above. Additional excipients, for example sweetening, flavoring and coloring
agents, may also be present. These
compositions may be preserved by the addition of an anti-oxidant such as
ascorbic acid.
-Page 108-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Pharmaceutical compositions may also be in the form of an oil-in-water
emulsions. The oily phase may be a
vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example liquid paraffin or mixtures of these.
Suitable emulsifying agents may be naturally-occurring phosphatides, for
example soy bean lecithin, and esters or partial
esters derived from fatty acids and hexitol anhydrides, for example sorbitan
monooleate, and condensation products of
the said partial esters with ethylene oxide, for example polyoxyethylene
sorbitan monooleate. The emulsions may also
contain sweetening agents, flavoring agents, preservatives and antioxidants.
Syrups and elixirs may be formulated with sweetening agents, for example
glycerol, propylene glycol, sorbitol or
sucrose. Such formulations may also contain a demulcent, a preservative,
flavoring and coloring agents and antioxidant.
Pharmaceutical compositions may be in the form of a sterile injectable aqueous
solution. Among the acceptable
vehicles and solvents that may be employed are water, Ringer's solution and
isotonic sodium chloride solution. The
sterile injectable preparation may also be a sterile injectable oil-in-water
microemulsion where the active ingredient is
dissolved in the oily phase. For example, the active ingredient may be first
dissolved in a mixture of soybean oil and
lecithin. The oil solution then introduced into a water and glycerol mixture
and processed to form a microemulsion. The
injectable solutions or microemulsions may be introduced into a patient's
blood-stream by local bolus injection.
Alternatively, it may be advantageous to administer the solution or
microemulsion in such a way as to maintain a
constant circulating concentration of the instant compound. In order to
maintain such a constant concentration, a
continuous intravenous delivery device may be utilized. An example of such a
device is the Deltec CA1DDPLUSTM
model 5400 intravenous pump. The pharmaceutical compositions may be in the
form of a sterile injectable aqueous or
oleagenous suspension for intramuscular and subcutaneous administration. This
suspension may be formulated according
to the known art using those suitable dispersing or wetting agents and
suspending agents which have been mentioned
above. The sterile injectable preparation may also be a sterile injectable
solution or suspension in a non-toxic
parenterally-acceptable diluent or solvent, for example as a solution in 1,3-
butane diol. In addition, sterile, fixed oils are
conventionally employed as a solvent or suspending medium. For this purpose
any bland fixed oil may be employed
including synthetic mono- or diglycerides. In addition, fatty acids such as
oleic acid find use in the preparation of
injectables.
Pharmaceutical compositions may also be administered in the form of
suppositories for rectal administration of the
drug. These compositions can be prepared by mixing the inhibitors with a
suitable non-irritating excipient which is solid
at ordinary temperatures but liquid at the rectal temperature and will
therefore melt in the rectum to release the drug.
Such materials include cocoa butter, glycerinated gelatin, hydrogenated
vegetable oils, mixtures of polyethylene glycols
of various molecular weights and fatty acid esters of polyethylene glycol.
For topical use, creams, ointments, jellies, solutions or suspensions, etc.,
containing a compound or composition of
the invention are useful for topical administration. As used herein, topical
application can include mouth washes and
gargles.
Pharmaceutical compositions may be administered in intranasal form via topical
use of suitable intranasai vehicles
and delivery devices, or via transdermal routes, using those forms of
transdermal skin patches well known to those of
ordinary skill in the art.
The formulations may conveniently be presented in unit dosage form and may be
prepared by any of the methods
well known in the art of pharmacy. All methods include the step of bringing
into association a compound of the subject
invention or a pharmaceutically acceptable salt, ester, prodrug or solvate
thereof ("active ingredient") with the carrier
which constitutes one or more accessory ingredients. In general, the
formulations are prepared by uniformly and
intimately bringing into association the active ingredient with liquid =Tiers
or finely divided solid carriers or both and
-Page 109-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
then, if necessary, shaping the product into the desired formulation. Methods
of preparing various pharmaceutical
compositions with a specific amount of active compound are known, or will be
apparent, to those skilled in this art. To
be administered in the form of transdermal delivery, the dosage form will, of
course, be continuous rather than
intermittent throughout the dosage regimen.
Doses
The amount of pharmaceutical compositions administered will firstly be
dependent on the mammal being treated.
In the instances where pharmaceutical compositions are administered to a human
subject, the daily dosage will normally
be determined by the prescribing physician with the dosage generally varying
according to the age, sex, diet, weight,
general health and response of the individual patient, the severity of the
patient's symptoms, the precise indication or
condition being treated, the severity of the indication or condition being
treated, time of administration, route of
administration, the disposition of the composition, rate of excretion, drug
combination, and the discretion of the
prescribing physician. Also, the route of administration may vary depending on
the condition and its severity.The
pharmaceutical composition may be in unit dosage form. In such form, the
preparation is subdivided into unit doses
containing appropriate quantities of the active component, e.g., an effective
amount to achieve the desired purpose.
Determination of the proper dosage for a particular situation is within the
skill of the art. Generally, treatment is initiated
with smaller dosages which are less than the optimum dose of the compound.
Thereafter, the dosage is increased by small
amounts until the optimum effect under the circumstances is reached. For
convenience, the total daily dosage may be
divided and administered in portions during the day if desired. The amount and
frequency of administration of the
compounds described herein, and if applicable other therapeutic agents and/or
therapies, will be regulated according to
the judgment of the attending clinician (physician) considering such factors
as described above. Thus the amount of
pharmaceutical composition to be administered may vary widely. Administration
may occur in an amount of between
about 0.001 mg/kg of body weight to about 100 mg/kg of body weight per day
(administered in single or divided doses),
or at least about 0.1 mg/kg of body weight per day. A particular therapeutic
dosage can include, e.g., from about 0.01 mg
to about 7000 mg of compound, or, e.g., from about 0.05 mg to about 2500 mg.
The quantity of active compound in a
unit dose of preparation may be varied or adjusted from about 0.1 mg to 1000
mg, from about 1 mg to 300 mg, or 10 mg
to 200 mg, according to the particular application. In some instances, dosage
levels below the lower limit of the aforesaid
range may be more than adequate, while in other cases still larger doses may
be employed without causing any harmful
side effect, e.g. by dividing such larger doses into several small doses for
administration throughout the day. The amount
administered will vary depending on the particular IC50 value of the compound
used. In combinational applications in
which the compound is not the sole therapy, it may be possible to administer
lesser amounts of compound and still have
therapeutic or prophylactic effect.
Additional dosing is provided throughout the specification and claims.
Dosage Forms
The pharmaceutical composition may, for example, be in a form suitable for
oral administration as a tablet,
capsule, pill, powder, sustained release formulations, solution, suspension,
for parenteral injection as a sterile solution,
suspension or emulsion, for topical administration as an ointment or cream or
for rectal administration as a suppository.
The pharmaceutical composition may be in unit dosage forms suitable for single
administration of precise dosages. The
pharmaceutical composition will include a conventional pharmaceutical carrier
or excipient and a compound according to
the invention as an active ingredient. In addition, it may include other
medicinal or pharmaceutical agents, carriers,
adjuvants, etc.
-Page 110-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Exemplary parenteral administration forms include solutions or suspensions of
active compounds in sterile
aqueous solutions, for example, aqueous propylene glycol or dextrose
solutions. Such dosage forms can be suitably
buffered, if desired.
Suitable pharmaceutical carriers include inert diluents or fillers, water and
various organic solvents. The
pharmaceutical compositions may, if desired, contain additional ingredients
such as flavorings, binders, excipients and
the like. Thus for oral administration, tablets containing various excipients,
such as citric acid may be employed together
with various disintegrants such as starch, alginic acid and certain complex
silicates and with binding agents such as
sucrose, gelatin and acacia. Additionally, lubricating agents such as
magnesium stearate, sodium lauryl sulfate and talc
are often useful for tableting purposes. Solid compositions of a similar type
may also be employed in soft and hard filled
gelatin capsules, including lactose or milk sugar and high molecular weight
polyethylene glycols. When aqueous
suspensions or elixirs are desired for oral administration the active compound
therein may be combined with various
sweetening or flavoring agents, coloring matters or dyes and, if desired,
emulsifying agents or suspending agents,
together with diluents such as water, ethanol, propylene glycol, glycerin, or
combinations thereof.
Methods of preparing various pharmaceutical compositions with a specific
amount of active compound are known,
Or will be apparent, to those skilled in this art. For examples, see
Remington's Pharmaceutical Sciences, Mack Publishing
Company, Ester, Pa., 18th Edition (1990).
Combination Therapies
The compounds described herein or a pharmaceutically acceptable salt, solvate,
polymorph, ester, amide, tautomer,
proclrug, hydrate, or derivative thereof may be administered as a sole
therapy. The compounds described herein or a
pharmaceutically acceptable salt, solvate, polymorph, ester, amide, tautomer,
prodrug, hydrate, or derivative thereof may
also be administered in combination with another therapy or therapies.
Also described herein is N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cycloproparte-1-sulfonamide (Form A) which may be administered
as a sole therapy. Crystalline
polymorph form A of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide may also be administered in
combination with another therapy or therapies.
By way of example only, if one of the side effects experienced by a patient
upon receiving one of the compounds
described herein is hypertension, then it may be appropriate to administer an
anti-hypertensive agent in combination with
the compound. Or, by way of example only, the therapeutic effectiveness of one
of the compounds described herein may
be enhanced by administration of an adjuvant (i.e., by itself the adjuvant may
only have minimal therapeutic benefit, but
in combination with another therapeutic agent, the overall therapeutic benefit
to the patient is enhanced). Or, by way of
example only, the benefit experienced by a patient may be increased by
administering one of the compounds described
herein with another therapeutic agent (which also includes a therapeutic
regimen) that also has therapeutic benefit. By
way of example only, in a treatment for diabetes involving administration of
one of the compounds described herein,
increased therapeutic benefit may result by also providing the patient with
another therapeutic agent for diabetes. In any
case, regardless of the disease, disorder or condition being treated, the
overall benefit experienced by the patient may
simply be additive of the two therapeutic agents or the patient may experience
a synergistic benefit.
Other therapies include, but are not limited to administration of other
therapeutic agents, radiation therapy or both.
In the instances where the compounds described herein are administered with
other therapeutic agents, the compounds
described herein need not be administered in the same pharmaceutical
composition as other therapeutic agents, and may,
because of different physical and chemical characteristics, be administered by
a different route. For example, the
compounds/compositions may be administered orally to generate and maintain
good blood levels thereof, while the other
-Page 111-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
therapeutic agent may be administered intravenously. The determination of the
mode of administration and the
advisability of administration, where possible, in the same pharmaceutical
composition, is well within the knowledge of
the skilled clinician. The initial administration can be made according to
established protocols known in the art, and then,
based upon the observed effects, the dosage, modes of administration and times
of administration can be modified by the
skilled clinician. The particular choice of compound (and where appropriate,
other therapeutic agent and/or radiation)
will depend upon the diagnosis of the attending physicians and their judgment
of the condition of the patient and the
appropriate treatment protocol. Other therapeutic agents may include
chemotherapeutic agents, such as anti-tumor
substances, for example those selected from, mitotic inhibitors, for example
vinblastine; alkylating agents, for example
cis-platin, carboplatin and cyclophosphamide; anti-metabolites, for example 5-
fluorouracil, cytosine arabinside and
hydroxyurea, or, for example, an anti-metabolite disclosed in European Patent
Application No. 239362 such as N- (5- [N-
(3, 4-dihydro-2-methyl-4- oxoquinazolin-6-yhnethyl)-N-methylamino1-2-thenoy1)-
L-glutamic acid; growth factor
inhibitors; cell cycle inhibitors; intercalating antibiotics, for example
adriamycin and bleomycin; enzymes, for example,
interferon; and anti-hormones, for example anti- estrogens such as NolvadexTM
(tanaoxifen) or, for example anti-
androgens such as CasodexTm (4'-cyano-3- (4-fluorophenylsulphony1)-2-hydroxy-2-
methyl-3'- (trifluoromethyl)
propionanilide). Such conjoint treatment may be achieved by way of the
simultaneous, sequential or separate dosing of
the individual components of treatment.
The compounds and compositions described herein (and where appropriate
chemotherapeutic agent and/or
radiation) may be administered concurrently (e.g., simultaneously, essentially
simultaneously or within the same
treatment protocol) or sequentially, depending upon the nature of the disease,
the condition of the patient, and the actual
choice of chemotherapeutic agent and/or radiation to be administered in
conjunction (i.e., within a single treatment
protocol) with the compound/composition.
In combinational applications and uses, the compound/composition and the
chemotherapeutic agent and/or
radiation need not be administered simultaneously or essentially
simultaneously, and the initial order of administration of
the compound/composition, and the chemotherapeutic agent and/or radiation, may
not be important. Thus, the
compounds/compositions of the invention may be administered first followed by
the administration of the
chemotherapeutic agent and/or radiation; or the chemotherapeutic agent and/or
radiation may be administered rust
followed by the administration of the compounds/compositions of the invention.
This alternate administration may be
repeated during a single treatment protocol. The determination of the order of
administration, and the number of
repetitions of administration of each therapeutic agent during a treatment
protocol, is well within the knowledge of the
skilled physician after evaluation of the disease being treated and the
condition of the patient. For example, the
chemotherapeutic agent and/or radiation may be administered first, especially
if it is a cytotoxic agent, and then the
treatment continued with the administration of the compounds/compositions of
the invention followed, where determined
advantageous, by the administration of the chemotherapeutic agent and/or
radiation, and so on until the treatment
protocol is complete. Thus, in accordance with experience and knowledge, the
practicing physician can modify each
protocol for the administration of a compound/composition for treatment
according to the individual patient's needs, as
the treatment proceeds. The attending clinician, in judging whether treatment
is effective at the dosage administered, will
consider the general well-being of the patient as well as more definite signs
such as relief of disease-related symptoms,
inhibition of tumor growth, actual shrinkage of the tumor, or inhibition of
metastasis. Size of the tumor can be measured
by standard methods such as radiological studies, e.g., CAT or MAI scan, and
successive measurements can be used to
judge whether or not growth of the tumor has been retarded or even reversed.
Relief of disease-related symptoms such as
pain, and improvement in overall condition can also be used to help judge
effectiveness of treatment.
-Page 112-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Specific, non-limiting examples of possible combination therapies include use
of the compounds of the invention
with agents found in the following pharmacotherapeutic classifications as
indicated below. These lists should not be
construed to be closed, but should instead serve as illustrative examples
common to the relevant therapeutic area at
present. Moreover, combination regimens may include a variety of routes of
administration and should include oral,
intravenous, intraoeular, subcutaneous, dermal, and inhaled topical.
For the treatment of oncologic diseases, proliferative disorders, and cancers,
compounds according to the present
invention may be administered with an agent selected from the group
comprising: aromatase inhibitors, antiestrogen,
anti-androgen, corticosteroids, gonadorelin agonists, topoisomerase land 2
inhibitors, microtubule active agents,
alkylating agents, nitrosoureas, antineoplastic antimetabolites, platinum
containing compounds, lipid or protein kinase
targeting agents, IMiDs, protein or lipid phosphatase targeting agents, anti-
angiogenic agents, Akt inhibitors, IGF-I
inhibitors, FGF3 modulators, mTOR inhibitors, Smac rnimetics, HDAC inhibitors,
agents that induce cell differentiation,
bradykinin 1 receptor antagonists, angiotensin II antagonists, cyclooxygenase
inhibitors, heparanase inhibitors,
lymphokine inhibitors, cytokine inhibitors, IKK inhibitors, P38MAPK
inhibitors, ARRY-797, HSP90 inhibitors,
multlikinase inhibitors, bisphosphanates, rapamycin derivatives, anti-
apoptotic pathway inhibitors, apoptotic pathway
agonists, PPAR agonists, RAR agonists, inhibitors of Ras isoforms, telomerase
inhibitors, protease inhibitors,
metalloproteinase inhibitors, aminopeptidase inhibitors, SHIP activators - AQX-
MN100, Humax-CD20 (ofatumurnab),
CD20 antagonists, 1L2-diptheria toxin fusions.
For the treatment of oncologic diseases, proliferative disorders, and cancers,
compounds according to the present
invention may be administered with an agent selected from the group
comprising: dacarbazine (DTIC), actinonaycins C2,
C3, D, and F1, cyclophosphamide, melphalan, estramustine, maytansinol,
rifamycin, streptovaricirt, doxorubicin,
daunorubicin, epirubiein, idarubicin, detorubicin, carminomycin, idarubicin,
epirubicin, esorubicin, mitoxantrone,
bleomycins A, A2, and B, camptothecin, Irinotecan®, Topotecan®, 9-
aminocamptothecin, 10,11 -
methyIenedioxycamptothecin, 9-nitrocamptothecin, bortezomib, temozolomide,
TAS103, NPI0052, combretastatin,
combretastatin A-2, combretastatin A-4, calicheamicins, neocareinostatins,
epothilones A B, C, and semi-synthetic
variants, Herceptin®, Rituxan®, CD40 antibodies, asparaginase,
interleukins, interferons, leuprolide, and
pegaspargase, 5-fluorouracil, fluorodeoxyuridine, ptorafur, 5'-
deoxyfluorouridine, UFT, MITC, S-1 capecitabine,
diethylstilbestrol, tamoxifen, toremeftne, tolmudex, thymitaq, flutarnide,
fluoxymesterone, bicalutamide, fmasteride,
estradiol, trioxifene, dexamethasone, leuproelin acetate, estramustine,
droloxifene, medroxyprogesterone, megesterol
acetate, aminoglutethimide, testolactone, testosterone, diethylstilbestrol,
hydroxyprogesterone, mitomycins A, B and C,
porfiromyein, cisplatin, carboplatin, oxaliplatin, tetraplatin, platinum-DACH,
ormaplatin, thalidomide, lenalidomide, CI-
973, telomestatin, CHIR258, Rad 001, SAHA, Tubacin, 17-AAG, sorafenib, JM-216,
podophyllotoxin,
epipodophyllotoxin, etoposide, teniposide, Tarceva®, Iressa®,
Imatinib®, Miltefosine®,
Perifosine®, aminopterin, methotrexate, methopterin, dichloro-
methotrexate, 6-mereaptopurine, thioguanine,
azattuoprine, allopurinol, cladribine, fludarabine, pentostatin, 2-
chloroadenosine, deoxycytidine, cytosine arabinoside,
cytarabine, azacitidine, 5-azacytosine, gencitabine, 5-azacytosine-
arabinoside, vincristine, vinblastine, vinorelbine,
leurosine, leurosidine and vindesine, paclitaxel, taxotere and docetaxel.
For the treatment of inflammatory diseases or pain, compounds and
pharmaceutically acceptable salts of the
compounds according to the present invention may be administered with an agent
selected from the group comprising:
corticosteroids, non-steroidal anti-inflammatories, muscle relaxants and
combinations thereof with other agents,
anaesthetics and combinations thereof with other agents, expectorants and
combinations thereof with other agents,
antidepressants, anticonvulsants and combinations thereof; antihypertensives,
opioids, topical eannabinoids, capsaicin,
-Page 113-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
betamethasone dipropionate (augmented and nonaugemnted), betamethasone
valerate, clobetasol propionate, prednisone,
methyl prednisolone, diflorasone diacetate, halobetasol propionate,
amcinonide, dexamethasone, dexosimethasone,
fluocinolone acetononide, fluocinonide, halocinonide, clocortalone pivalate,
dexosimetasone, flurandrenalide, salicylates,
ibuprofen, ketoprofen, etodolac, diclofenac, meclofenamate sodium, naproxen,
piroxicam, celecoxib, cyclobenzaprine,
baclofen, cyclobenzaprine/lidocaine, baclofen/cyclobenzaprine,
cyclobenzaprine/lidocaine/ketoprofen, lidocaine,
lidocaine/deoxy-D-glucose, prilocaine, EMLA Cream (Eutectic Mixture of Local
Anesthetics (lidocaine 2.5% and
prilocaine 2.5%), guaifenesin, guaifenesin/ketoprofen/cyclobenzaprine,
amitryptiline, doxepin, desipramine, imipramine,
amoxapine, clomipramine, nortriptyline, protriptyline, duloxetine,
mirtazepine, nisoxetine, maprotiline, reboxetine,
fluoxetine, fluvoxamine, carbamazepine, felbamate, lamotrigine, topiramate,
tiagabine, oxcarbazepine, carbamezipine,
zonisamide, mexiletine, gabapentin/clonidine, gabapentin/carbamazepine,
carbamazepine/cyclobenz,aprine,
antihypertensives including clonidirte, codeine, loperamide, tramadol,
morphine, fentanyl, oxycodone, hydrocodone,
levorphanol, butorphanol, menthol, oil of wintergreen, camphor, eucalyptus
oil, turpentine oil; CB1/CB2 ligands,
acetaminophen, infliximab, nitric oxide synthase inhibitors, particularly
inhibitors of inducible nitric oxide synthase,
PDE4 inhibitors ¨ similar mechanism to Ibudilast (AV-411) , CDC-801, JNK
inhibitors - CC-401, Combination
TNF/PDE4 inhibitors ¨ CDC-998, IL1 antagonists e.g. Analcinra ¨ Kineret, AMG
108, (mAb) that targets IL-1, SHIP
activators - AQX-MN100, C5 antagonists, C5a inhibitors, Pexelizumab,
Pyrimidine synthesis inhibitors, lymphokine
inhibitors, cytokine inhibitors, IKK inhibitors, P38MAPK inhibitors, ARRY-797,
HSP90 inhibitors, muhlikinase
inhibitors, bisphosphanates, PPAR agonists, Coxl and cox 2 inhibitors, Anti-
CD4 therapy, B-cell inhibitors, COX/LOX
dual inhibitors, Immunosuppressive agents, iNOS inhibitors, NSAIDs, sPLA2
inhibitors, Colchicine, allopurinol,
oxypurinol, Gold, Ridaura ¨ Auranofm, febuxostat, Puricase, PEG-unease
formulations, Benzbromarone, Long-acting
beta-2 agonists (LABAs), salmeterol (Serevent Diskus) and formoterol
(Foradil), Leukotriene modifiers include
montelukast (Singulair) and zafirlukast (Accolate). Inhaled cromolyn (Intal)
or nedocromil (Tilade), Theophylline. Short-
acting beta-2 agonists, Ipratropium (Atrovent), Immunotherapy-(Allergy-
desensitization shots), Anti-IgE monoclonal
antibodies ¨ Xolair, Common DMARDs include hydroxychloroquine (Plaqueni1), the
gold compound auranofm
(Ridaura), sulfasalazine (Azulfidine), minocycline (Dynacin, Minocin) and
rnethotrexate (Rheumatrex), leflunomide
(Arava), azathioprine (Imuran), cyclosporine (Neoral, Sandimmune) and
cyclophosphamide (Cytoxan), Antibiotics,
CD80 antagonists, costimulatory factor antagonists, Humax-CD20 (ofatumumab);
CD20 antagonists, MEK inhibitors,
,NF kappa B inhibitors, anti B-cell antibodies, denosumab, mAb that
specifically targets the receptor activator of nuclear
factor kappa B ligand (RANICL). IL17 inactivating anti-bodies, IL-17 receptor
antagonists/inhibitors, CTLA inhibitors,
CD20 inhibitors, soluble VEGFR-1 receptors, anti-VEGFR-1 receptor antibodies,
anti-VEGF antibodies, integrin
receptor antagonist, Selectin inhibitors, P-selectin and E-selectin
inhibitors, Phospholipase A2 Inhibitors, Lipoxygenase
Inhibitors, RANKL and RANK antagonists/antibodies, Osteoprotegerin
antagonists, Lymphotoxin inhibitors, B-
lymphocyte stimulator, MCP-1 inhibitors, MIF inhibitors, inhibitors of: CD2,
CD3, CD4 , CD25 , CD40 and CD40
Ligand CD152 (CTLA4), Macrolide immunosuppressants, Selective inhibitors of
nucleotide metabolism, Inhibitors of
chemotaxis, CXC receptor and CXC ligand inhibitors, Chemolcine Antagonists,
leukocyte chemotaxis inhibitors
Adhesion Molecule blockers, Selectins Lymphocyte Function Antigen-1 (LFA-1,
CD11a) antagonists, Very Late
Antigen-4 (VLA-4) antagonists, Matrix Metalloprotease Inhibitors, Elastase
Inhibitors, Cathepsin Inhibitors.
For the treatment of ophthalmologic disorders and diseases of the eye,
compounds and pharmaceutically
acceptable salts of the compounds according to the present invention may be
administered with an agent selected from
the group comprising: beta-blockers, carbonic anhydrase inhibitors, .alpha.-
and .beta.-adrenergic antagonists including
Page 114-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
al-adrenergic antagonists, .alpha.2 agonists, miotics, prostaglandin analogs,
corticosteroids, and immunosuppressant
agents.
For the treatment of ophthalmologic disorders and diseases of the eye,
compounds pharmaceutically acceptable
salts of the compounds according to the present invention may be administered
with an agent selected from the group
comprising: timoIol, betaxolol, levobetaxolol, carteolol, levobunolol,
propranolol, brinzolamide, dorzolamide, nipradiloI,
iopidine, brimonidine, pilocarpine, epinephrine, latanoprost, travoprost,
bimatoprost, unoprostone, dexamethasone,
prednisone, methylprednisolone, azathioprine, cyclosporine, and
immunoglobulins.
For the treatment of autoimmune disorders, compounds pharmaceutically
acceptable salts of the compounds
according to the present invention may be administered with an agent selected
from the group comprising:
corticosteroids, immunosuppressants, prostaglandin analogs and
antimetabolites.
For the treatment of autoimmune disorders, compounds according to the present
invention may be administered
with an agent selected from the group comprising: dexamethasome, prednisone,
methylprednisolone, azathioprine,
cyclosporine, itninunoglobulins, latanoprost, travoprost, bimatoprost,
unoprostone, infliximab, rutuximab, methotrexate,
non-steroidal anti-inflammatories, muscle relaxants and combinations thereof
with other agents, anaesthetics and
combinations thereof with other agents, expectorants and combinations thereof
with other agents, antidepressants,
anticonvulsants and combinations thereof; antihypertensives, opioicis, topical
caimabinoids, and other agents, such as
capsaicin, betamethasone dipropionate (augmented and nonaugemnted),
betamethasone valerate, clobetasol propionate,
prednisone, methyl prednisolone, diflorasone diacetate, halobetasol
propionate, amcinonide, dexamethasone,
dexosimethasone, fluocinolone acetononide, fluocirionide, halocinonide,
clocortalone pivalate, dexosimetasone,
flurandrenalide, salicylates, ibuprofen, ketoprofen, etodolac, diclofenac,
meclofenamate sodium, riaproxen, piroxicam,
celecoxib, cyclobenzaprine, baclofen, cyclobenzaprine/lidocaine,
bacIofert/cyclobenzaprine,
cyclobenzaprine/lidocaine/ketoprofen, lidocaine, lidocaine/deoxy-D-glucose,
prilocaine, EMLA Cream (Eutectic Mixture
of Local Anesthetics (lidocaine 2.5% and prilocaine 2.5%), guaifenesin,
guaifenesin/ketoprofen/cyclobenzaprine,
amityptiline, doxepin, desipramine, iiniprainine, amoxapine, clomipramine,
nortriptyline, protriptyline, duloxetine,
mirtazepine, nisoxetine, maprotiline, reboxetine, fluoxetine, fluvoxamine,
carbamazepine, felbamate, lamotrigine,
topiramate, tiagabine, oxcarbazepine, carbamezipine, zonisamide, mexiletine,
gabapentin/clonidine,
gabapentin/carbamazepine, carbamazepine/cyclobenzaprine, antihypertensives
including clonidine, codeine, loperamide,
tramadol, morphine, fentanyl, oxycodone, hydrocodone, levorphanol,
butorphanol, menthol, oil of wintergreen, camphor,
eucalyptus oil, turpentine oil; CB1/CB2 ligands, acetaminophen, infliximab;
nitric oxide synthase inhibitors, particularly
inhibitors of inducible nitric oxide synthase; and other agents, such as
capsaicin. PDE4 inhibitors ¨ similar mechanism to
Ibudflast (AV-411) , CDC-801, JNK inhibitors - CC-401, Combination TNF/PDE4
inhibitors ¨ CDC-998, ILI
antagonists e.g. Anakinra ¨ Kineret, AMG 108, (mAb) that targets IL-1, SHIP
activators - AQX-MN100, C5
antagonists, C5a inhibitors, Pexelizumab, Pyrimidine synthesis inhibitors,
lymphokine inhibitors, cytokine inhibitors,
HU( inhibitors, P38MAPK inhibitors, ARRY-797, HSP90 inhibitors, multlikinase
inhibitors, bisphosphanates, PPAR
agonists, Coxl and cox 2 inhibitors, Anti-CD4 therapy, B-cell inhibitors,
COX/LOX dual inhibitors,
Inununosuppressive agents, iNOS inhibitors, NSAIDs, sPLA2 inhibitors,
Colchicine, allopurinol, oxypiffinol, Gold,
Ridaura ¨ Auranofln, febuxostat, Puricase, PEG-uricase formulations,
Benzbromarone, Long-acting beta-2 agonists
(LABAs), salmeterol (Serevent Diskus) and formoterol (Foradil), Leukotriene
modifiers include montelukast (Singulair)
and zafirlukast (Accolate). Inhaled cromolyn (Intal) or nedocromil (Tilade),
Theophylline. Short-acting beta-2 agonists,
Ipratropium (Atrovent), Imnaunotherapy-(Allergy-desensitization shots), Anti-
IgE monoclonal antibodies ¨ Xolair,
Common DMARDs include hydroxychloroquine (Plaquenil), the gold compound
auranofm (Ridaura), sulfasalazine
-Page 115-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
(Azulfidine), minocycline (Dynacin, Minocin) and methotrexate (Rheumatrex),
leflunomide (Arava), azathioprine
(Imuran), cyclosporine (Neoral, Sandimmune) and cyclophosphamide (Cytoxan),
Antibiotics, CD80 antagonists,
costimulatory factor antagonists, Humax-CD20 (ofatumumab); CD20 antagonists,
MEK inhibitors, NF kappa B
inhibitors, anti B-cell antibodies, denosumab, inAb that specifically targets
the receptor activator of nuclear factor kappa
B ligand (RANKL). IL17 inactivating anti-bodies, 1L-17 receptor
antagonists/inhibitors, CTLA inhibitors, CD20
inhibitors, soluble VEGFR-1 receptors, anti-VEGFR-1 receptor antibodies, anti-
VEGF antibodies, integrin receptor
antagonist, Selectin inhibitors, P-selectin and E-selectin inhibitors,
Phospholipase A2 Inhibitors , Lipoxygenase
Inhibitors, RANKL and RANK antagonists/antibodies, Osteoprotegerin
antagonists, Lymphotoxin inhibitors, B-
lymphocyte stimulator, MCP-1 inhibitors, MIF inhibitors, inhibitors of: CD2,
CD3, CD4 , CD25 , CD40 and CD40
Ligand CD152 (CTLA4), Macrolide inununosuppressants, Selective inhibitors of
nucleotide metabolism, Inhibitors of
chemotaxis, CXC receptor and CXC ligand inhibitors, Chemokine Antagonists,
leukocyte chemotaxis inhibitors
Adhesion Molecule blockers, Selectins Lymphocyte Function Antigen-1 (LFA-1,
CD11a) antagonists, Very Late
Antigen-4 (VLA-4) antagonists, Matrix Metalloprotease Inhibitors, Elastase
Inhibitors, Cathepsin Inhibitors.
For the treatment of metabolic disorders, compounds and pharmaceutically
acceptable salts of the compounds
according to the present invention may be administered with an agent selected
from the group comprising: insulin,
insulin derivatives and mimetics, insulin secretagogues, insulin sensitizers,
biguanide agents, alpha-glucosidase
inhibitors, insulinotropic sulfonylurea receptor ligands, protein tyrosine
phosphatase-1B (PTP-1B) inhibitors, GSK3
(glycogen synthase kinase-3) inhibitors, GLP-1 (glucagon like peptide-1), GLP-
I analogs, DPKV (dipeptidyl peptidase
IV) inhibitors, RXR Iigands sodium-dependent glucose co-transporter
inhibitors, glycogen phosphorylase A inhibitors, an
AGE breaker, PPAR modulators, LXR and FXR modulators, non-glitazone type PPARS
agonist, selective glucocorticoid
antagonists, metformin, Glipizide, glyburide, Amaryl, meglitirtides,
nateglinide, repaglinide, PT-112, SB-517955,
SB4195052, SB-216763, NN-57-05441, NN-57-05445, GW-0791, AGN-194204,
T-1095, BAY R3401,
acarbose Exeridin-4, DPP728, LAF237, vildagliptin , MK-0431, saxagliptin,
GSK23A, pioglitazone, rosiglitazone, (R)-1-
{445-methyl-2-(4-trifluoromethyl-phenyl)-oxazol-4-ylmethoxyl-benze-
nesulfony112,3-dihydro-1H-indole-2-carboxylic
acid described in the patent application WO 03/043985, as compound 19 of
Example 4, and 0I-262570.
Diseases
Described herein are methods of treating a disease in an individual suffering
from said disease comprising
administering to said individual an effective amount of a compound of formula
I or a pharmaceutically acceptable salt,
solvate, polymorph, ester, amide, tautomer, procirug, hydrate, or derivative
thereof.
Also described herein are methods of treating a disease or disorder in an
individual suffering from said disease Or
disorder comprising administering to said individual an effective amount of N-
(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyI)-1-(2,3-dihydroxypropyl)cyclopropane-l-
sulfonamide (Form A). The invention
extends to the use of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide (Form A) in the manufacture of a
medicament for treating a disease or
disorder.
In some embodiments, the invention relates to the prophylaxis or treatment of
any disease or disorder in which
MEK lcinase plays a role including, without limitation: oncologic,
hematologic, inflammatory, ophthalmologic,
neurological, immunologic, cardiovascular, and dermatologic diseases as well
as diseases caused by excessive or
unregulated pro-inflammatory cytokine production including for example
excessive or unregulated TNF, IL-1, IL-6 and
1L-8 production in a human, or other mammal. The invention extends to such a
use and to the use of the compounds for
the manufacture of a medicament for treating such cytokine-mediated diseases
or disorders. Further, the invention
Page 116-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
extends to the administration to a human an effective amount of a MEK
inhibitor for treating any such disease or
disorder.
Diseases or disorders in which MEK kinase plays a role, either directly or via
pro-inflammatory cytokines
including the cytokines TNF, IL-1, IL-6 and IL-8, include, without limitation:
dry eye, glaucoma, autoimmune diseases,
inflammatory diseases, destructive-bone disorders, proliferative disorders,
neurodegenerative disorders, viral diseases,
allergies, infectious diseases, heart attacks, angiogenic disorders,
reperfusion/ischemia in stroke, vascular hyperplasia,
organ hypoxia, cardiac hypertrophy, thrombin-induced platelet aggregation, and
conditions associated with prostaglandin
endoperoxidase synthetase-2 (COX-2).
In certain aspects of the invention, the disease is a hyperproliferative
condition of the human or animal body,
including, but not limited to cancer, hyperplasias, restenosis, inflammation,
immune disorders, cardiac hypertrophy,
atherosclerosis, pain, migraine, angiogenesis-related conditions or disorders,
proliferation induced after medical
conditions, including but not limited to surgery, angioplasty, or other
conditions.
In further embodiments, said hyperproliferative condition is selected from the
group consisting of hematologic and
nonhematologic cancers. In yet further embodiments, said hematologic cancer is
selected from the group consisting of
multiple myeloma, leukemias, and lymphomas. In yet further embodiments, said
leukemia is selected from the group
consisting of acute and chronic leukemias. In yet further embodiments, said
acute leukemia is selected from the group
consisting of acute lymphocytic leukemia (ALL) and acute nonlyrnphocytic
leukemia (ANLL). In yet further
embodiments, said chronic leukemia is selected from the group consisting of
chronic lymphocytic leukemia (CLL) and
chronic myelogenous leukemia (CML). In further embodiments, said lymphoma is
selected from the group consisting of
Hodgkin's lymphoma and non-Hodgkin's lymphoma. In further embodiments, said
hematologic cancer is multiple
myeloma. In other embodiments, said hematologic cancer is of low,
intermediate, or high grade. In other embodiments,
said nonhematologic cancer is selected from the group consisting of: brain
cancer, cancers of the head and neck, lung
cancer, breast cancer, cancers of the reproductive system, cancers of the
digestive system, pancreatic cancer, and cancers
of the urinary system. In further embodiments, said cancer of the digestive
system is a cancer of the upper digestive tract
or colorectal cancer. In further embodiments, said cancer of the urinary
system is bladder cancer or renal cell carcinoma.
In further embodiments, said cancer of the reproductive system is prostate
cancer.
Additional types of cancers which may be treated using the compounds and
methods described herein include:
cancers of oral cavity and pharynx, cancers of the respiratory system, cancers
of bones and joints, cancers of soft tissue,
skin cancers, cancers of the genital system, cancers of the eye and orbit,
cancers of the nervous system, cancers of the
lymphatic system, and cancers of the endocrine system. In certain embodiments,
these cancer s may be selected from the
group consisting of: cancer of the tongue, mouth, pharynx, or other oral
cavity; esophageal cancer, stomach cancer, or
cancer of the small intestine; colon cancer or rectal, anal, or anorectaI
cancer; cancer of the liver, intrahepatic bile duct,
gallbladder, pancreas, or other biliary or digestive organs; laryngeal,
bronchial, and other cancers of the respiratory
organs; heart cancer, melanoma, basal cell carcinoma, squamous cell carcinoma,
other non-epithelial skin cancer; uterine
or cervical cancer; uterine corpus cancer; ovarian, vulvar, vaginal, or other
female genital cancer; prostate, testicular,
penile or other male genital cancer; urinary bladder cancer; cancer of the
kidney; renal, pelvic, or urethral cancer or other
cancer of the genito-urinary organs; thyroid cancer or other endocrine cancer;
chronic lymphocytic leukemia; and
cutaneous T-cell lymphoma, both granulocytic and monocytic.
Yet other types of cancers which may be treated using the compounds and
methods described herein include:
adenocarcinoma, angiosarcoma, astrocytoma, acoustic neuroma, anaplastic
astrocytoma, basal cell carcinoma,
blastogliorna, chondrosarcoma, choriocarcinoma, chordoma, craniopharyngioma,
cutaneous melanoma,
-Page 117-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
cystadenocarcinoma, endotheliosarcoma, embryonal carcinoma, ependymoma,
Ewing's tumor, epithelial carcinoma,
fibrosarcoma, gastric cancer, genitourinary tract cancers, glioblastoma
multiforme, heinangioblastoma, hepatocaular
carcinoma, hepatoma, Kaposi's sarcoma, large cell carcinoma, leiomyosarcoma,
liposarcoma, lymphangiosarcoma,
lymphangioendotheliosarcoma, medullary thyroid carcinoma, medulloblastoma,
meningioma mesothelioma, inyelomas,
myxosarcoma neuroblastoma, neurofibrosarcoma, oligodendroglioma, osteogenic
sarcoma, epithelial ovarian cancer,
papillary carcinoma, papillary adenocarcinomas, parathyroid tumors,
pheochromocytoma, pinealoma, plasmacytomas,
retinoblastoma, rhabdomyosarcoma, sebaceous gland carcinoma, seminoma, skin
cancers, melanoma, small cell lung
carcinoma, squamous cell carcinoma, sweat gland carcinoma, synovioma, thyroid
cancer, uveal melanoma, and Wilm's
tumor.
Also described are methods for the treatment of a hyperproliferative disorder
in a mammal that comprise
administering to said mammal a therapeutically effective amount of a compound
of formula I, or a pharmaceutically
acceptable salt, solvate, polymorph, ester, amide, tautomer, prodrug, hydrate,
or derivative thereof, in combination with
an anti-tumor agent. In some embodiments, the anti-tumor agent is selected
from the group consisting of mitotic
inhibitors, alkylating agents, anti- metabolites, intercalating antibiotics,
growth factor inhibitors, cell cycle inhibitors,
enzyme inhibitors, topoisomerase inhibitors, biological response modifiers,
anti- hormones, angiogenesis inhibitors, anti-
androgens, SHIP activators - AQX-MN100, Humax-CD20 (ofatumumab), CD20
antagonists, 1L2-diptheria toxin fusions.
The disease to be treated using the compounds, compositions and methods
described herein may be a hematologic
disorder. In certain embodiments, said hematologic disorder is selected from
the group consisting of sickle cell anemia,
myelodysplastic disorders (MDS), and myeloproliferative disorders. In further
embodiments, said myeloproliferative
disorder is selected from the group consisting of polycythemia vera,
myelofibrosis and essential thrombocythemia.
The compounds, compositions and methods described herein may be useful as anti-
inflammatory agents with the
additional benefit of having significantly less harmful side effects. The
compounds, compositions and methods described
herein are useful to treat arthritis, including but not limited to rheumatoid
arthritis, spondyloarthropathies, ankylosing
spondylitis, gout, gouty arthritis, osteoarthritis, systemic lupus
erythematosus, juvenile arthritis, acute rheumatic arthritis,
enteropathic arthritis, neuropathic arthritis, psoriatic arthritis, and
pyogenic arthritis. The compounds, compositions and
methods described herein are also useful in treating osteoporosis and other
related bone disorders. These compounds,
compositions and methods described herein are also useful to teat
gastrointestinal conditions such as reflux esophagitis,
diarrhea, inflammatory bowel disease, Crohn's disease, gastritis, irritable
bowel syndrome and ulcerative colitis. The
compounds, compositions and methods described herein may also be used in the
treatment of pulmonary inflammation,
such as that associated with viral infections and cystic fibrosis. In
addition, the compounds, compositions and methods
described herein are also useful in organ transplant patients either alone or
in combination with conventional
immunomodulators. Yet further, the compounds, compositions and methods
described herein are useful in the treatment
of pruritis and vitaligo. In particular, compounds, compositions and methods
described herein are useful in treating the
particular inflammatory disease rheumatoid arthritis.
Further inflammatory diseases which may be prevented or treated include,
without limitation: asthma, allergies,
respiratory distress syndrome or acute or chronic pancreatitis. Furthermore,
respiratory system diseases may be prevented
or treated including but not limited to chronic obstructive pulmonary disease,
and pulmonary fibrosis. In addition, MEK
kinase inhibitors described herein are also associated with prostaglandin
endoperoxidase synthetase-2 (COX-2)
production. Pro-inflammatory mediators of the cyclooxygenase pathway derived
from arachidonic acid, such as
prostaglandins, are produced by inducible COX-2 enzyme. Regulation of COX-2
would regulate these pro-inflammatory
mediators, which affect a wide variety of cells and are important and critical
inflammatory mediators of a wide variety of
-Page 118-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
disease states and conditions. In particular, these inflammatory mediators
have been implicated in pain, such as in the
sensitization of pain receptors, and edema. Accordingly, additional MEK kinase-
mediated conditions which may be
prevented or treated include edema, analgesia, fever and pain such as
neuromuscular pain, headache, dental pain, arthritis
pain and pain caused by cancer.
Further, the disease to be treated by the compounds, compositions and methods
described herein may be an
ophthalmologic disorder. Ophthalmologic diseases and other diseases in which
angiogenesis plays a role in pathogenesis,
may be treated or prevented and include, without limitation, dry eye
(including Sjogren's syndrome), macular
degeneration, closed and wide angle glaucoma, retinal ganglion degeneration,
occular ischemia, retinitis, retinopathies,
uveitis, ocular photophobia, and of inflammation and pain associated with
acute injury to the eye tissue. The compounds,
compositions and methods described herein are useful to treat glaucomatous
retinopathy and/or diabetic retinopathy. The
compounds, compositions and methods described herein are also useful to treat
post-operative inflammation or pain as
from ophthalmic surgery such as cataract surgery and refractive surgery.. In
further embodiments, said ophthalmologic
disorder is selected from the group consisting of dry eye, closed angle
glaucoma and wide angle glaucoma.
Further, the disease to be treated by the compounds, compositions and methods
described herein may be an
autoimmune disease. Autoimmune diseases which may be prevented or treated
include, but are not limited to: rheumatoid
arthritis, inflammatory bowel disease, inflammatory pain, ulcerative colitis,
Crohn's disease, periodontal disease,
temporomandibular joint disease, multiple sclerosis, diabetes,
glomerulonephritis, systemic lupus erythematosus,
scleroderma, chronic thyroiditis, Grave's disease, hemolytic anemia,
autoimmune gastritis, autoimmune neutropenia,
thrombocytopenia, chronic active hepatitis, myasthenia gravis, atopic
dermatitis, graft vs. host disease, and psoriasis.
Inflammatory diseases which may be prevented or treated include, but are not
limited to: asthma, allergies, respiratory
distress syndrome or acute or chronic pancreatitis. In particular, compounds,
compositions and methods described herein
are useful in treating the particular autoimmune diseases rheumatoid arthritis
and multiple sclerosis.
Further, the disease to be treated by the compounds, compositions and methods
described herein may be a
dermatologic disorder. In certain embodiments, said dermatologic disorder is
selected from the group including, without
limitation, melanoma, basel cell carcinoma, squamous cell carcinoma, and other
non-epithelial skin cancer as well as
psoriasis and persistent itch, and other diseases related to skin and skin
structure, may be treated or prevented with MEK
kinase inhibitors of this invention.
Metabolic diseases which may be treated or prevented include, without
limitation, metabolic syndrome, insulin
resistance, and Type 1 and Type 2 diabetes. In addition, the compositions
described herein may be useful to treat insulin
resistance and other metabolic disorders such as atherosclerosis that are
typically associated with an exaggerated
inflammatory signaling.
The compounds, compositions and methods described herein are also useful in
treating tissue damage in such
diseases as vascular diseases, migraine headaches, periarteritis nodosa,
thyroiditis, aplastic anemia, Hodgkin's disease,
sclerodoma, rheumatic fever, type I diabetes, neuromuscular junction disease
including myasthenia gravis, white matter
disease including multiple sclerosis, sarcoidosis, nephritis, nephrotic
syndrome, Beheet's syndrome, polymyositis,
gingivitis, periodontis, hypersensitivity, swelling occurring after injury,
ischernias including myocardial ischemia,
cardiovascular ischemia, and ischemia secondary to cardiac arrest, and the
like. The compounds, compositions and
methods described herein may also be useful to treat allergic rhinitis,
respiratory distress syndrome, endotoxin shock
syndrome, and atherosclerosis.
Further, the disease to be treated by the compounds, compositions and methods
described herein may be a
cardiovascular condition. In certain embodiments, said cardiovascular
condition is selected from the group consisting of
-Page 119-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
atherosclerosis, cardiac hypertrophy, idiopathic cardiomyopathies, heart
failure, angiogenesis-related conditions or
disorders, and proliferation induced after medical conditions, including, but
not limited to restenosis resulting from
surgery and angioplasty.
Further, the disease to be treated by the compounds, compositions and methods
described herein may be a
neurological disorder. In certain embodiments, said neurologic disorder is
selected from the group consisting of
Parkinson's disease, Alzheimer's disease, Alzheimer's dementia, and central
nervous system damage resulting from
stroke, ischemia and trauma. In other embodiments, said neurological disorder
is selected from the group consisting of
epilepsy, neuropathic pain, depression and bipolar disorders.
Further, the disease to be treated by the compounds, compositions and methods
described herein may cancer such
as acute myeloid leukemia, thymus, brain, lung, squamous cell, skin, eye,
retinoblastoma, intraocular melanoma, oral
cavity and oropharyngeal, bladder, gastric, stomach, pancreatic, bladder,
breast, cervical, head, neck, renal, kidney, liver,
ovarian, prostate, colorectal, esophageal, testicular, gynecological, thyroid,
CNS, PNS, AIDS related AIDS-Related (e.g.
Lymphoma and Kaposi's Sarcoma) or Viral-Induced cancer. In some embodiments,
the compounds and compositions are
for the treatment of a non-cancerous hyperproliferative disorder such as
benign hyperplasia of the skin (e. g., psoriasis),
restenosis, or prostate (e. g., benign prostatic hypertrophy (BPH)).
Further, the disease to be treated by the compounds, compositions and methods
described herein may pancreatitis,
kidney disease (including proliferative glomerulonephritis and diabetes-
induced renal disease), pain, a disease related to
vasculogenesis or angiogenesis, tumor angiogenesis, chronic inflammatory
disease such as rheumatoid arthritis,
inflammatory bowel disease, atherosclerosis, skin diseases such as psoriasis,
eczema, and scleroderma, diabetes, diabetic
retinopathy, retinopathy of prematurity, age-related macular degeneration,
hemangioma, tendonitis, bursitis, sciatica,
glioma, melanoma, Kaposi's sarcoma and ovarian, breast, lung, pancreatic,
prostate, colon and epidermoid cancer in a
mammal.
Further, the disease to be treated by the compounds, compositions and methods
described herein may the
prevention of blastocyte implantation in a mammal.
Patients that can be treated with the compounds described herein, or their
pharmaceutically acceptable salts,
solvate, polymorphs, esters, amides, tautomers, prodrugs, hydrates, or
derivatives according to the methods of this
invention include, for example, patients that have been diagnosed as having
psoriasis; restenosis; atherosclerosis; BPH;
breast cancer such as a ductal carcinoma in duct tissue in a mammary gland,
medullary carcinomas, colloid carcinomas,
tubular carcinomas, and inflammatory breast cancer; ovarian cancer, including
epithelial ovarian tumors such as
adenocarcinoma in the ovary and an adenocarcinoma that has migrated from the
ovary into the abdominal cavity; uterine
cancer; cervical cancer such as adenocarcinoma in the cervix epithelial
including squamous cell carcinoma and
adenocarcinomas; prostate cancer, such as a prostate cancer selected from the
following: an adenocarcinoma or an
adenocarinoma that has migrated to the bone; pancreatic cancer such as
epitheliod carcinoma in the pancreatic duct tissue
and an adenocarcinoma in a pancreatic duct; bladder cancer such as a
transitional cell carcinoma in urinary bladder,
urothelial carcinomas (transitional cell carcinomas), tumors in the urothelial
cells that line the bladder, squamous cell
carcinomas, adenocarcinomas, and small cell cancers; leukemia such as acute
myeloid leukemia (AML), acute
lymphocytic leukemia, chronic lymphocytic leukemia, chronic myeloid leukemia,
hairy cell leukemia, myelodysplasia,
and myeloproliferative disorders; bone cancer; lung cancer such as non-small
cell lung cancer (NSCLC), which is
divided into squamous cell carcinomas, adenocarcinomas, and large cell
undifferentiated carcinomas, and small cell lung
cancer; skin cancer such as basal cell carcinoma, melanoma, squamous cell
carcinoma and actinic keratosis, which is a
skin condition that sometimes develops into squamous cell carcinoma; eye
retinoblastoma; cutaneous or intraocular (eye)
-Page 120-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
melanoma; primary liver cancer (cancer that begins in the liver); kidney
cancer; thyroid cancer such as papillary,
follicular, medullary and anaplastic; AIDS-related lymphoma such as diffuse
large B-cell lymphoma, B-cell
immunoblastic lymphoma and small non-cleaved cell lymphoma; Kaposi's Sarcoma;
viral-induced cancers including
hepatitis B virus (HBV), hepatitis C virus (HC'V), and hepatocellular
carcinoma; human lymphotropic virus-type 1
(HTLV-1) and adult T-cell leukemia/lymphoma; and human papilloma virus (HPV)
and cervical cancer; central nervous
system cancers (CNS) such as primary brain tumor, which includes gliomas
(astrocytoma, anaplastic astrocytoma, or
glioblastoma multiforme), Oligodendroglioma, Ependymoma, Meningioma, Lymphoma,
Schwarmoma, and
Medulloblastoma; peripheral nervous system (PNS) cancers such as acoustic
neuromas and malignant peripheral nerve
sheath tumor (MPNST) including neurofibromas and schwannomas, malignant
fibrous cytoma, malignant fibrous
histiocytoma, malignant meningioma, malignant mesothelioma, and malignant
mixed MUllerian tumor; oral cavity and
oropharyngeal cancer such as, hypopharyngeal cancer, laryngeal cancer,
nasopharyngeal cancer, and oropharyngeal
cancer; stomach cancer such as lymphomas, gastric stromai tumors, and
carcinoid tumors; testicular cancer such as germ
cell tumors (GCTs), which include seminomas and nonseminornas, and gonadal
stromal tumors, which include Leydig
cell tumors and Sertoli cell tumors; thymus cancer such as to thymomas, thymic
carcinomas, Hodgkin disease, non-
Hodgkin lymphomas carcinoids or carcinoid tumors; rectal cancer; and colon
cancer.
Kits
The compounds, compositions and methods described herein provide kits for the
treatment of disorders, such as
the ones described herein. These kits comprise a compound, compounds or
compositions described herein in a container
and, optionally, instructions teaching the use of the kit according to the
various methods and approaches described
herein. Such kits may also include information, such as scientific literature
references, package insert materials, clinical
trial results, and/or summaries of these and the like, which indicate or
establish the activities and/or advantages of the
composition, and/or which describe dosing, administration, side effects, drug
interactions, or other information useful to
the health care provider.. Such information may be based on the results of
various studies, for example, studies using
experimental animals involving in vivo models and studies based on human
clinical trials. Kits described herein can be
provided, marketed and/or promoted to health providers, including physicians,
nurses, pharmacists, formulary officials,
and the like. Kits may also, in some embodiments, be marketed directly to the
consumer.
The compounds described herein can be utilized for diagnostics and as research
reagents. For example, the
compounds described herein, either alone or in combination with other
compounds, can be used as tools in differential
and/or combinatorial analyses to elucidate expression patterns of genes
expressed within cells and tissues. As one non-
limiting example, expression patterns within cells or tissues treated with one
or more compounds are compared to control
cells or tissues not treated with compounds and the patterns produced are
analyzed for differential levels of gene
expression as they pertain, for example, to disease association, signaling
pathway, cellular localization, expression level,
size, structure or function of the genes examined. These analyses can be
performed on stimulated or unstimulated cells
and in the presence or absence of other compounds which affect expression
patterns.
Besides being useful for human treatment, the compounds and formulations of
the present invention are also useful
for veterinary treatment of companion animals (eg dogs, cats), exotic animals
and farm animals (eg horses), including
mammals, rodents, and the like.
The examples and preparations provided below further illustrate and exemplify
the compounds of the present
invention and methods of preparing such compounds. It is to be understood that
the scope of the present invention is not
limited in any way by the scope of the following examples and preparations.
-Page 121-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
EXAMPLES
General Exemplary Procedures for the Synthesis of Sulfonamides
Procedure A: To a solution of the amine (1 eq) in anhydrous dichloromethane (3
rnL/ mrnole) was added
anhydrous triethylamine (5 eq). To this solution was added the sulfonyl
chloride (1 eq) and the solution was stirred at
room temperature for 16 h. The solvent was evaporated and the residue was
purified by flash column chromatography on
silica.
Procedure B: To a stirred solution of the amine (1 eq) in anhydrous pyridine
(5mllmmole) was added the sulfonyl
chloride (1 - 5 eq). The reaction mixture was stirred at 40 C for 48 hours.
The reaction mixture was partitioned with
water and Et0Ac. The organic layer was washed with brine, dried (MGS04) and
concentrated under reduced pressure.
The residue was purified by flash column chromatography on silica.
Procedure C: Substitution of the iodo-atom:
A suspension containing 1 eq. aryl iodide, 1.5 equiv. of the boronic acid or
boronic ester, 0.25 eq. PdC12(dppt) x
DCM and 10 eq. anhydrous K2CO3 powder in a deoxygenated mixture of dioxane and
water (3:1) was heated in a
microwave reactor for 60 min at 115 C. It was extracted using aq. NFI4C1 /
THF, and the organic fraction was dried
using Na2SO4. The crude reaction products were purified using flash-column
chromatography (Si, Et0Ac / Hexanes, or
CHC13 / Me0H). Yields: 20-40%.
Procedure D: Synthesis of N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny0-
2-
(alkylamino)ethanesulfonamide:
2-Chloro-ethanesulfonyl chloride (0.1 ml, 1 mmol) was added to a solution of
5,6-difluoro-M-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine (0.364 g, 1 mmol) and triethylamine (0.28 ml, 2
mmol) in CH2C12 (5 ml) and the
reaction mixture was stirred at room temperature for 16h. Then it's treated
with an excess amine (10 eq) either in solution
or as a neat liquid. The reaction mixture stirred at room temperature for
additional 6h. The reaction mixture diluted with
CH2C12 (10 ml) and water (10 m1). The organic layer was sequentially washed
with dil. HC1 (2x20 ml, 2N) and saturated
NaHCO3 (2x10 ml) solution. Then the CH2C12 layer dried (MgSO4) and evaporated
to obtain the crude product. The
impure product was purified under preparative HPLC conditions to obtain the
pure products in 50-60 % yield.
Example 1
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)methanesulfonamide:
Step A: 2,3-Difluoro-N-(2-fluoro-4-iodophenv1)-6-nitroaniline:
N 2 H
ni
1
To a solution of 2-fluoro-4-iodoaniline (11.40 g, 47 =rot) in 100 ml anhydrous
THF at 0 C, 47 ml of a 1M
solution of LHMDS in THF (47 mmol) was added dropwise. The color of the
solution turned dark purple. The solution
was transferred via cannula to a dropping funnel, and the solution (containing
the amine free base) was added in small
portions to a solution of 2,3,4-trifluoronitrobenzene (8.321 g, 47.0 mmol) in
anhydrous THF (50 ml) at 0 C. After
completion of addition the mixture was stirred under argon at room temperature
for 15 hours. The volume of the solvent
was reduced, followed by extraction using ethyl acetate and brine. The organic
layer was dried over sodium sulfate, the
solvent was removed, and the obtained dark oil was purified by flash
chromatography (Et0Ac / hexane 1:5, Rf = 0.58)
yielding the crude product, which became a brown solid upon drying in vacua
(yield: 6.23 g, 33.6%): tor/z = 393 [M-1] .
Step B: 5,6-Difluoro-N1-(2-fluoro-4-iodophenvbbenzene-1,2-diamine:
- Page 122-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
NH2 F
N
1
To a solution of nitro-diarylamine (6.23 g, 15.8 mmol) in 300 ml ethanol was
added iron powder (13.74 g,
246 mmol) and amrnonitun chloride (13.59 g, 254 mmol) and the mixture was
heated with stirring at 100 C oil
bath temperature for 14 hours. It was filtered and the residue washed two
times with ethanol. The ethanol was
removed in vacuo, and the residue was extracted using ethyl acetate / 1M NaOH
solution. During the
extraction, more precipitate was formed which was filtered and discarded. The
combined organic layers were
washed with brine and dried over sodium sulfate. The solvent was removed, and
the crude product was
recrystallized from CHC13 / hexane (1:50). The product was obtained as brown
needles (2.094 g, 66%,), Rf =
0.44 (Et0Ac / Hex 1:3), 1H-NMR (500 MHz, CDC13), 8 = 7.40-7.38 (dd, 1H, J=
11.3 Hz, J= 1.5 Hz), 7.25-7.23
(d, 1H, J= 8.5 Hz), 6.97-6.92 (q, Ill, J= 9 Hz), 6.51-6.48 (m, 1H), 6.24-6.21
(t, 1H, J= 9 Hz), 5.3 (s, 1H, NH,
br), 3.80 (s, 2H, NH2, br), LRMS (ESI): m/z = 365 [M+H].
Step C: N-(3A-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyllmethanesulfonamide:
,,cep
e'NH H F
N
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with methanesulfonyl chloride to obtain the desired product. 1H NMR: (500 MHz,
CDC13): 8 = 7.38-7.37 (d, 1H), 7.35-
7.34 (m, 1H), 7.27-7.26 (m, 1H), 7.20-7.0 (q, 1H), 6.68 (s, 1H, br), 6.15-6.12
(q, 1H), 5.65 (s, 1H, br), 2.95 (s, 3H); m/z =
441 [M-11.
Example 2
2 N-(3,4-difluoro-2-(2-fluoro-4-
lodophienylamino)phenyl)cyclopropanesuIfonamide:
L., to
0 N H F
1.1
1
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with cyclopropanesulfonyl chloride to obtain the desired product. 11-I NMR:
(500 MHz, CDC13): = 7.38-7.37 (d, 1H),
7.35-7.34 (m, 1H), 121-1 Id (m, IH), 7.20-7.0 (q, 111), 6.68 (s, 1H, br), 6.15-
6.12 (q, 1H), 5.65 (s, 1H, br), 3.25-3.20 (m,
1H), 2.4-2.3 (m, 2H), 2.0-1.8 (m, 2H); m/z = 467 [M-1].
Example 3
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)propane-2-suIfonamide:
0NH F
*
-Page 123-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with isopropyIsulfonyl chloride to obtain the desired product. Yield: 39%. 11-
1-NMR (500 MHz, CDC13): 8 = 7.50-7.43
(m, 1H), 7.35-7.34 (m, 1H), 7.27-7.26 (m, 111), 7.15-7.09 (q, 1H, J= 1.6 Hz),
6.62 (s, 111, br), 6.22-6.18 (q, 1H, J= 1.5
Hz), 5.65 (s, 1H, br), 3.30-3.28 (m, 11-1), 1.38-1.37 (d, 6H, J= 1.2 Hz); m/z
= 469 [M- I].
Example 4
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)butane-l-sulfonamide:
'NH H F
40
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with n-butylsulfonyl chloride to obtain the desired product. Yield: 55%. 111-
NMR (500 MHz, CDC13): 8 = 7.50-7.43 (m,
10 1H), 7.35-7.34 (m, 1H), 7.27-7.26 (m, 1H), 7.15-7.09 (q, 111, J= 1.6
Hz), 6.62 (s, 111, br), 6.22-6.18 (q, 1H, J=1.5 Hz)5
5.65 (s, 114, br), 3.06-3.031 (t, 21-1, J= 1.4 Hz), 1.75-1.71 (m, 2H), 1.38-
1.36 (m, 2H), 0.87-0.86 (t, 311, J= 1.3 Hz); in/z =
483 [M-1]
Example 5
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyI)-2,2,2-trifluoro ethane
sulfonamide:
,0
Fsc- ;S-
0" 'NH H F
N
11111" F 41111}111 I
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenypbenzene-1,2-diamine was reacted
with 1,1,1-trifluoroethylsulfonyl chloride to obtain the desired product.
Yield: 28%. m/z = 509 [M-I].
Example 6
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)butane-2-sulfonaniide:
F
F
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with sec-butylsulfonyl chloride to obtain the desired product. Yield: 22%. 'I-
I-NMR (500 MHz, Me0H[d4]): S = 7.60-
7.40 (m, 3H), 7.18-7.00 (q, 1H), 6.55-6.45 (m, 1H), 3.55-3.50 (m, 1H), 2.20-
2.00 (m, 1H), 1.80-1.60 (m, 111), 1.43-1.40
(d, 3H), 1.06-1.04 (t, 3H); m/z = 483 [M-l].
Example 7
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-N-methyl cyclopropane
sulfonamide:
A.,4P
õ.
H F
40 N
1
-Page 124-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
To a solution of N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)phcnyl)cycloproPane-sulfonamide (see Example
2) (283.9 mg, 0.61 minol) in 3 ml anhydrous THF was added at -78 C a IM
solution of LHMDS (0.6 ml, 0.6 mol) and the
solution was stirred for 10 min at this temperature. Then, methyl iodide (0.8
ml, 1.824 g, 12.9 mmol) was added and the
mixture was warmed to room temperature and stirred for 7 h. The solvent was
removed and the residue extracted using
Et0Ac and brine. The organic fractions were dried using Na2SO4 and the solvent
was removed. The obtained crude
product was purified using flash-column chromatography (Si, Et0Ac/Hexanes 1:2,
Rf = 0.45). Yield: 205 mg, 70%).
1H-NMR (500 MHz, CDC13): 6 = 7.41-7.39 (d, 1H, J= 10 Hz), 7.30-7.29 (d, 1H, J=
8.0 Hz), 7.23-7.20 (m, 1H), 6.98-
6.93 (q, 1H, J= 8.5 Hz), 6.60 (s, 1H, br), 6.51-6.47 (m, 111), 3.23 (s, 3H),
2.46-2.42 (m, 111), 1.19-1.16 (m, 2H), 1.04-
1.02 (m, 2H); m/z = 481 [M-11.
Example 8
I-Chloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl) methane
sulfonamide:
0,..6:1NH F
N
11111}1 F I
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with chloromethanesulfonyl chloride to obtain the desired product, m/z = 475
[M-1]-.
Example 9
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)-2-methylpropane-2-
sulfonamide:
9
1.44 F
1401 =
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with 2-methylpropane-2-sulfonyl chloride (synthesized according to the
literature procedure) to obtain the desired
product. 111 NMR (300 MHz., CDCI3): 5 7.50 (m, 1H), 7.43 (dd, J= 1.8 & 10.5
Hz, 111), 7.28 (br s, 1H), 7.10 (dd, J= 9.0
& 17.7 Hz, 1H), 6.48 (br s, D20 exchangeable, 1H), 6.19 (t, J= 7.8 & 9.6 Hz,
1H), 5.58 (br s, D20 exchangeable, 1H),
1.39 (s, 9H); m/z = 383 [M-1] .
Example 10
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)cyclopentanesulfonamide:
9
OINH H F
1.
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with cyclopentanesulfonyl chloride to obtain the desired product 11-1 NMR (300
MHz, CDC13): 6 7.42 (dd, J= 2.1 & 10.5
Hz, 1H), 7.36 (ddd, J= 2.4, 4.8, & 9.3 Hz, 11-1), 7.25 (m, 2H), 7.10 (dd, J=
9.6 & 17.7 Hz, 1H), 6.67 (br s, D20
exchangeable, 1H), 6.20 (dt, J= 1.5, 8.4 & 17.4 Hz, 1H), 3.53 (p, 1H), 1.80
(in, 8H); m/z = 495 [M-1] .
-Page 125-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 11
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)cyclohexanesulfonamide:
9
F
F 1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with cyclohexanesulfonyl chloride to obtain the desired product. NMR (300
MHz, CDC13): 7.43 (dd, J=1.5 & 10.2
Hz, 1H), 7.37 (ddd, J= 2.4,4.8 & 9.6 Hz, 1H), 7.27 (m, 1H), 7.11 (dd, J= 9.3 &
18.0 Hz, Ill), 6.64 (br s, 1H), 6.18 (dt, J
= 1.5, 9.0 & 17.4 Hz, 1H), 5.63 (br s, 1H), 2.95 (triplet of triplet, 2.10-
1.16 (m, 10H); m/z = 509 [M-1]-
Example 12
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-inethyleyelopropane-1-
sulfonamide:
Step A: n-Butyl 3 -chloro-l-propanesulfonate:
0'
O-nBu
Triethylamine (28 ml, 200 mmol) in CH2C12 (50 ml) was slowly added to an ice-
cooled solution of 3-chloro-1-
propanesulfonyl chloride (36.6g, 200 mmol) and 1-butanol (18.4 g, 240 m mol)
in CH2C12 (250 ml) and stirring was
continued for 16h. The mixture was diluted with CH2C12 (200 ml), washed
(aqueous HC1) and dried (MgSO4) and the
solvent was evaporated to obtain the titled product 1 (40.85 g, 95%) in crude
form as slightly yellow oil which was used
for the next reaction without further purification. ITINMR (CDC13)) 8 0.94 (t,
J= 7.5 Hz, 311), 1.44 (sextet, 211), 1.72
(quintet, 2H), 2.31 (quintet, 2H), 3.27(t, J= 6.9 Hz, 2H), 3.68 (t, J= 6.3
Hz), 4.23 (t, J= 6.6 Hz, 2H).
Step B: 1-Butyl cyclopropanesulfonate:
0-nBu
Solutions of 1-butyl 3-chloro-l-propanesulforiate (4.6 g, 21.39 mmol in 25 ml
THF) and of butyllithium (14.7 ml,
23.53 mmol, 1.6M, THF) were simultaneously added to THF (150 ml) at -78 C
under nitrogen atmosphere. The solution
was allowed to warm to 0 C and then quenched with water (2 m1). The volatiles
evaporated under reduced pressure and
the residue extracted with CH2C12 (150 ml). The extract was washed with water
and dried (MgSO4) and evaporated to
give crude desired product (3.23 g, 78.22%) in almost pure form as pale yellow
oil which was used for next step without
further purification. II-1 NMR (300 MHz, CDC13) 5 0.94 (t, J = 7.5 Hz, 3H),
1.07 (m, 2H), 1.25 (n, 2H), 1.45(sextet, 2H),
1.74(quintet, 2H), 2.45 (heptet, 1H), 4.23 (t, J = 6.6 Hz, 211).
Step C: Butyl 1-Methyl-cyclopropanesulfonate:
Lo
0-nBu
To a solution of 1 -Butyl cyclopropanesulfonate (1 g, 5.58 mmol) in THE (15
ml) butyllithium solution ( 3.84 ml,
6,14 mmol, 1.6M, THF) was slowly added at -78 C under nitrogen atmosphere.
After 15 minutes Mel (0.72 ml, 11.16
mmol) was added and the solution was allowed to warm to 0 C and quenched with
water (1 ml). The volatiles
evaporated under reduced pressure and the residue extracted with CH2C12 (100
ml). The extract was washed with water,
-Page 126-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
dried (MgSO4) and evaporated. The residue was purified over silica gel
chromatography (eluants: hexane/ CH2C12) to
obtain the titled product (0.59 g, 55.0%) as a colorless oil. 11-1 NMR (300
MHz, CDC13)) 5 0.84 (tn, 2H), 0.95 (t, J = 7.2
Hz, 3H), 1.43 (m, 4H), 1.53 (s, 3H), 1.74(m, 211), 4.21 ((t, J = 6.6 Hz, 2H).
Step D: 1-Potassium 1-Methyl-cycloprooanesulfonate:
1-\<"
$e
0
OK
A mixture of 1-Butyl 1-Methyl-cyclopropanesulfonate (0.386 g, 2 mmol) and
potassium thiocyanate (0.194 g,
2 nitnol) in DME (5 ml) and water (5 ml) was refluxed for 16h. The volatiles
were evaporated to obtain the crude
sulfonate (0.348g, quantitative) which was dried under vacuum at 50 C for 16h.
The crude product was used in the next
reaction without further purification. 1H NMR (300 MHz, D20) 5 0.56 (t, J= 6.3
Hz, 211), 0.96 (t, J = 6.3 Hz, 2H), 1.26
(s, 3H).
Step E: 1-Methyl-cyclopropanesulfonylchloride:
6</Sso
0'
CI
A solution of 1-potassium 1-methyl-cyclopropanesulfonate (0.348 g, 2 mmol),
thionyl chloride (5 ml) and DMF
(5 drops) was refluxed at 60 C for 16h. The volatiles evaporated under reduced
pressure and the residue extracted with
CH2C12 (50 m1). The extract was washed with water, dried (MgSO4.) and
evaporated to obtain the crude product as
yellow gummy oil which was used in the next reaction without further
purification.
Step F: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-
methylcyclopropane-1-sulfonamide:
6'NH H F
110 110
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with 1-methyl-cyclopropanesulfonylchloride to obtain the desired product.
IFINMR (300 MHz, CDC13): 8 7.42 (dd,
1.8 & 10.5 Hz, 1H), 7.36 (ddd, J= 2.4, 4.5 & 9.0 Hz, 1H), 7.27 (d, J= 6.0 Hz,
1H), 7.07 (dd, J= 9.3 & 17.7 Hz, 1H),
6.24 (dt, J= 2.1, 8.7 & 17.4 Hz, 1H), 5.86 (hr s, 111), 1.43 (s, 3H), 1.33 (t,
J= 5.4 Hz, 2H), 0.75 (dd, J= 5.1 & 6.3 Hz,
2H); m/z = 481 [M-11-.
Example 13
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-
sulfonamide:
Step A: Butyl c_yclopropanesulfonate:
So
CrO
Cyclopropanesulfonyl chloride (5 g, 35 mmol, 1 eq) was dissolved in an excess
BuOH (20 ml), the reaction
mixture was cooled at -10 C and pyridine (5.8 mL, 70 mmol, 2 eq) was slowly
added dropwise. The mixture was slowly
warmed at room temperature and stirred overnight. The solvent was removed
under reduced pressure and the resulting
white solid was dissolved in CHC13. The organic phase was washed with water,
brine and dried (MgSO4) and
-Page 127-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
concentrated to give an oil (4.8 g, 24.9 mmol, 71%). 'H NMR (300 MHz, CDC13):
8 4.25 (t, 2H), 2.46 (m, 1H), 1.74 (m,
2H), 1.45 (m, 2H), 1.25 (dd, 2H), 1.09 (dd, 2H), .93 (t, 3H).
Step B: Butyl 1-allylcyclopropane-1-sulfonate:
0 0
To a solution of 1-butyl cyclopropanesulfonate (4.8 g, 24.9 mmol) in THF at -
78 C was added simultaneously
butyllithium solution (15.6 ml, 24.9 mmol, 1.6M, THF) and ally! iodide (24.9
mmol) under nitrogen atmosphere. The
reaction mixture was stirred 2 hours at -78 C and 3 hours at room temperature.
The volatiles were evaporated under
reduced pressure and the residue extracted with CH2C12 (100 ml). The extract
was washed with water, dried (MgSO4)
and evaporated. The residue was purified over silica gel chromatography
(eluants: hexane/ CH2C12) to obtain the titled
product (3.75 g, 69.0%) as a colorless oil. 11-1 NMR (300 MHz, CDC13): 6 5.6
(m, 1H), 5.13-5.08 (t, 2H), 4.21 (t,
2.65 (d, 2H), 1.7 (m, 211), 1.4 (m, 4H), .93 (m, 511).
Step C: Potassium 1-allylcyclopropane-l-sulfonate:
LrO-Ki
o'5'1D
A mixture of 1-butyl 1-methyl-cyclopropanesulfonate (3.75 g, 17.2 mmol) and
potassium thioeyanate (1.7 g,
17.2 mmol) in DME (20 ml) and water (20 ml) was refluxed for 16h. The
volatiles were evaporated to obtain the crude
sulfonate (3.44g, quantitative) which was dried under vacuum at 50 C for 16h.
The crude product was used in the next
reaction without further purification. IFINMR (CDC13): 8 5.6 (m, 111), 4.91-
4.85 (dd, 2H), 2.471-2.397 (d, 2H), 0.756
(m, 211), 0.322 (m, 2H).
Step D: 1-allylcyclopropane-l-sulfonyl chloride:
cI
/s.
00
A solution of potassium l-allylcyclopropane-l-sulfonate (3.44 g, 17.2 mmol),
thionyl chloride (10 ml) and DMF
(5 drops) was refluxed at 60 C for 16h. The volatiles evaporated under reduced
pressure and the residue extracted with
CH2C12 (50 m1). The extract was washed with water, dried (MgSO4) and
evaporated to obtain the crude product as
yellow gummy oil which was washed with hexane and used in the next reaction
without further purification (2.7 g, 15
mmol, 87%). IHNMR (300 MHz, CDC13): ö 5.728 (m, 111), 5.191 (t, 211), 2.9 (d,
2H), 0.756 (m, 2H), 0.322 (m, 2H).
Step E: 1-allyl-N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)cyclopropane- 1-sulfonamide:
A'N1-1 H F
N
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenypbenzene-1,2-diamine was reacted
with 1-allylcyciopropane-l-sulfonyl chloride to obtain the desired product.
m/z = 507 [M-l].
-Page 128-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step F: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-142,3-
dihydroxypropyncyclopropane-1-
sulfonamide:
HO
NH H F
N
1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)cyclopropane-1-
sulfonamide (0,77 g, 1.52 nunol)
5 and 4-methylmorpholine N-oxide (0,18 g, 1.52 nnnol) were dissolved in THF
(50 mL). Osmium tetroxide was added at
room temperature (0.152 mmol, 0.965 mL, 4% in H20) and the reaction mixture
was stirred at room temperature for
16 hours. Et0Ac was added, the organic phase was washed with water, dried
(MgSO4) and concentrated under reduced
pressure. The residue was purified over silica gel chromatography (eluants:
Et0Ac/ Me0H) to obtain the titled product
(0.65 g, 79%). 1H NMR (300 MHz, CDC13 + D20): 5 7.38 (dd, J= 1.8 & 10.5 Hz,
1H), 7.36 (ddd, J= 2.4, 5.1 & 9.3 Hz,
10 1H), 7.25 (d, J= 8.7 Hz, 1H), 7.02 (dd, J= 9.0 & 17.7 Hz, 1H), 6.27 (dt,
J= 3.0, 8.7 & 17.4 Hz, 1H), 3.92 (m, 1H), 3.54
(dd, J= 3.9 & 11.1 Hz, 1H), 3.39 (dd, J= 6.6 & 11.1 Hz, 1H), 2.16 (dd, J= 9.6
& 15.9 Hz, 1H), 1.59 (d, J= 14.1 Hz,
111), 1.41 (m, 1H), 1.26 (m, 1H), 0.83 (in, 2H); m/z = 542 [M-l].
Example 14
(S)-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-
dihydroxypropyl)eyelopropane-l-
15 sulfonamide:
S'
E5-NriH F
FI
The pure S isomer was obtained by chiral HPLC separation of the racemic
mixture (example 13). '14 NMR (300
MHz, CDC13 + D20): 5 7.38 (dd, J= 1.8 & 10.5 Hz, 1H), 7.36 (ddd, J= 2.4, 5.1 &
9.3 Hz, 1H), 7.25 (d, J= 8.7 Hz, 1H),
7.02 (dd, J= 9.0 & 17.7 Hz, 1H), 6.27 (dt, J= 3.0, 8.7 & 17.4 Hz, 1H), 3.92
(m, 111), 3.54 (dd, J= 3.9 & 11.1 Hz, 1H),
20 3.39 (dd, J= 6.6 & 11.1 Hz, 1H), 2.16 (dd, J= 9.6 & 15.9 Hz, 1H), 1.59
(d, J= 14.1 Hz, 111), 1.41 (m, 1H), 1.26 (m,
1H), 0.83 (m, 2H); m/z = 542 FM-I].
Example 15
(R)-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-
sulfonamide:
HojE,X1
hNM F
0 0
40 40
25 FI
The pure R isomer was obtained by chiral HPLC separation of the racemic
mixture (example 13). 11-INMR (300
MHz, CDC13+ D20): 57.38 (dd, J= 1.8 & 10.5 Hz, 1H), 7.36 (ddd, J= 2.4, 5.1 &
9.3 Hz, 1H), 7.25 (d, J= 8.7 Hz, 11),
7.02 (dd, J= 9.0 & 17.7 Hz, 1H), 6.27 (dt, J= 3.0, 8.7 & 17.4 Hz, 1H), 3.92
(m, 1H), 3.54 (dd, J= 3.9 & 11.1 Hz, 1H),
3.39 (dd, J= 6.6 & 11.1 Hz, 1H), 2.16 (dd, J= 9.6 & 15.9 Hz, 111), 1.59 (d, J=
14.1 Hz, 1H), 1.41 (m, 1H), 1.26 (m,
30 1H), 0.83 (m, 2H); m/z = 542 IM-11-.
-Page 129-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 16
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyt)-1-(2-
hydroxyethyl)eyelopropane-1-sulfonamide:
Step A: 2-d -bromocyclopropypethanol:
Br
OH
To a solution of neat diethyl zinc (3.3 ml, 3.977 g, 30 mmol) in 100 ml
anhydrous DCM was added very slowly
trifluoroacetic acid (2.31 ml, 3.4188 g, 30 mmol) dropwise at 0 C. (Caution:
Violent gas evolution, exothermic!). After
completed addition of the TFA, the suspension was stirred for 20 min at the
same temperature, followed by the addition
of diiodo methane (2.45 ml, 8.134 g, 30.4 mmol). It was further stirred at 0 C
for 20 min, and then a solution of 3-
bromobut-3-en-l-ol (1 ml, 1.523 g, 10.1 mmol) in 10 ml DCM was added at the
same temperature. After complete
addition, the mixture was warmed to room temperature and stirred for 4 hours.
The mixture was quenched with 100 ml
Me0H and 40 ml brine, and it was further stirred for 30 min. The solvents were
reduced, and the residue extracted using
CHC13 / aq. NH4C1. The organic layers were collected, washed with brine and
water, and the solvent was removed to
give 2-(l-bromocyclopropy1)-ethanol in sufficient purity (1.6564 g, 100%). 1H-
NMR (500 MHz, CDC13): = 3.90-3.83
(t, 211), 1.91-1.87 (t, 2H), 1.71 (s, 1H, br), 1.14-1.09 (m, 2H), 0.83-0.79
(m, 211).
Step B: TBS protected 2-(1-bromocyclopropyflethanol:
OTBs
To a solution of the cyclopropyl alcohol (Step A) (1.303 g, 7.95 mmol) in 30
ml anhydrous DCM was added
anhydrous pyridine (1.2 ml, 1,1736 g, 14.8 mmol) and TBSOTf (2.7 ml, 3.1077 g,
11.76 mop and the solution was
stirred at room temperature for 16h. It was extracted with CHC13 / brine and
the organic fraction was dried with MgSO4.
The solvent was reduced and the crude product purified using flash-column
chromatography (Si, CHC13 / hexanes 1:10,
Rf = 0.4). Yield: 0.796 g, 36%. 1H-NMR. (500 MHz, CDC13): 6 = 3.95-3.75 (t,
2H), 1.95-1.85 (t, 2H), 1.15-1.05 (m, 211),
0.95-0.80 (m, H1-1), 0.15-0.05 (s, 6H).
Step C: TBS protected 2-(1-chlorosulfonylcyclopropyflethanol:
OTBS
To a solution of the cyclopropyl bromide prepared in step B (1.1227 g, 4.04
mmol) in 15 ml anhydrous diethyl
ether was added a 1.7 M solution of t-BuLi in pentane (4.8 ml, 8.16 mmol) at -
78 C. The solution was stirred for 30 min
at this temperature, and was then transferred via a transfer canola into a
solution of freshly distilled sulfuryl chloride
(0.65 ml, 1.029 g, 8.1 mmol) in 8 ml diethyl ether at -78 C. The yellow
suspension was warmed to room temperature.
The solvent was removed, and the residue was dried in vacuo to remove
excessive sulfuryl chloride. Then, the residue
was extracted two times with hexane, and after filtration the solvent was
evaporated in vacua to give the suifonyl
chloride in sufficient purity as a colorless oil. Yield: 870 mg (72%). 111-NMR
(300 MHz, CDC13): 8 3.95-3.85 (t, 211),
2.35-2.25 (t, 2H), 1.80-1.70 (m, 2H), 1.45-1.38 (m, 211), 0.90 (s, 9H), 0.10
(s, 611).
-Page 130-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step D: TB S-protected N-(3 .4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-
1-(2-
hydroxyethyl)cyclopropane-1 -sulfonamide:
(01138
AA2,13
ci" 'NH F
According to the general procedure B, 5,6-difiuoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with the cyclpropylsulfonyl chloride prepared in step C to obtain the desired
product. 'H-NMR (300 MHz, CDC13):
7.44-7.39 (dd, 1H), 7.32-7.24 (m, 2H), 7.1-6.98 (q, 1H), 6.34-6.24 (m, 1H),
6.16 (s, 111, br), 3.85-3.75 (t, 211), 2.15-2.00
(t, 2H), 1.35-1.20 (m, 2H), 0.95-0.75 (m, 1 1H), 0.10 (s, 6H); in/z = 625 [M-
1]-.
Step E: N-(3,4-difluoro-2-(2-fluoro-4-iodophenvlamino)pheny1)-1-(2-
hvdroxyethybcyclopropane-1-
sulfonamide:
(pH
cr. NH Ei F
10
To a solution of the TBS-protected sulfonamide prepared in step D (21 mg,
0.033 mmol) in 1 ml THF was added
0.1 ml aq.1.2N HCI solution at 0 C and the solution was stirred for 2 h. The
solvents were reduced and the residue was
extracted using aq. NaHCO3 solution and Et0Ac. The organic fractions were
dried with MgSO4 and the volatiles were
removed. The crude product was purified using flash-column chromatography (Si,
CHC13 / Me0H 10:1, RI = 0.45) to
give the pure product. Yield: 16.9 mg (100%). 1-11-NMR (300 MHz, CDC13): 6 =
7.44-7.39 (dd, 1H), 7.32-7.24 (m, 2H),
7.1-6.98 (q, 111), 6.34-6.24 (m, 1H), 6.16 (s, 1H, br), 3.85-3.75 (1, 211),
2.15-2.00 (t, 211), 1.35-1.20 (m, 2H), 0.95-0.85
(m, 2H); m/z = 511 [M-1] .
Example 17
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-3-hydroxypropane-1-
sulfonamide:
0
13 NH
To a solution of 3-chloro-N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)pheny1)-propane- 1-sulfonamide (69.4
mg, 0.138 mmol) in a mixture of 8 ml 1,4-dioxane and 2 ml H20 was added KOH
powder (0.674 g, 12.0 mmol) and the
mixture was heated to the reflux temperature for 3 days. It was extracted
using Et0Ac / brine, the organic fraction was
dried with Na2SO4 and the volatiles were removed. The residue was purified
using flash-column chromatography (Si,
DCM / Me0H 5:1, Rf = 0.3). Yield: 41 mg (62%). 11-I-NMR (500 MHz, Me0H [d41):
6 = 7.38- 7.21 (d, 1H), 7.23-7.21
(d, 1H), 7.06-7.00 (q, 111), 6.52-6.50 (m, 111), 6.17-6.13 (t, 1H), 3.30-3.27
(t, 2H), 2.86-2.83 (t, 211), 2.05-2.00 (m, 211);
rn/z = 485 [M-1] .
-Page 131-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Example 18
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-2-methyl-5-
(trifluoromethyl)furan-3-sulfonamide:
HC
CFs
0 `NH F
* 1.1
According to the general procedure B, 5,6-difiuoro-N1-(2-fiuoro-4-
iodophenyl)benzene-1,2-diamine (0.182 mmol)
was reacted with 2-methyl-5-(trifluoromethypfuran-3-sulfonyl chloride (0.5
mmol) to form N-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)pheny1)-2-methy1-5-(trifluoromethyl)furan-3-sulfonamide. 114
NMR (CDC13) 8 2.2 (s, 311), 5.3 (s,
111), 6.0 (dt, 1H), 6.8 (s, 1H), 6.95 (s, 1H), 7.0-7.3 (in, 314), 7.4 (dd,
1H).
Example 19
N-(5-(N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)sulfamoyi)-
methylthiazol-2-yDacetamide:
HN
0 'NH F
N
11111)111 F I
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine (0.182 mmol)
was reacted with 2-acetamido-4-methylthiazole-5-sulfonyl chloride (0.5 mmol)
to obtain N-(5-(N-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)phenyl)sulfamoy1)-4-methylthiazol-2-ypacetamide . 1H
NMR (CDC13)) S 2.1 (s, 311), 2.2 (s,
3H), 5.9 (dt, 1H), 6.05 (s, 114), 7.0-7.6 (m, 3H), 7.4 (dd, 1H), 8.0 (s, 111).
Example 20
5-(5-Chloro-1,2,4-thiadiazot-3-y1)-N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyl) thiophene-2-
sulfonamide:
0 'NH F
N
F 11111"
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine (0.182 mmol)
was reacted with 5-(5-chloro-1,2,4-thiadiazol-3-ypthiophene-2-sulfonyl
chloride (0.5 mmol) to obtain 5-(5-chloro-1,2,4-
thiadiazol-3-y1)-N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)thiophene-2-sulfonamide. 11-1 NMR (300 MHz,
CDC13)) 5 5.8 (dt, 1H), 5.95 (s, 111), 6.95 (d, 1H),7.4 (m, 2H), 7.6 (d, 1H),
7.8 (s,11-1).
-Page 132-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Example 21
N-(34-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-3,5dimethylisoxazole-4-
sulfonamide:
0 sh1H F
= 40
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-iodophenyl)
benzene-1,2-diamine (0.182
mmol) was reacted with 3,5-dimethylisoxazole-4-sulfonyl chloride (0.5 mmol) to
obtain N-(3,4-difluoro-2-(2-fluoro-4-
iodophenyl amino)pheny1)-3,5dimethylisoxazole-4-sulfonamide. 111 NMR (300 MHz,
CDC13)) 6 2.2 (s, 311), 2.4 (s, 3H),
5.8 (s, 11-1), 6.0 (dt, 1H), 5.95 (s, 1H), 6.9 (s, 1H),7.0 (q, 1H), 7.2 (m,
3H), 7.4 (dd, 111).
Example 22
5-Chloro-N-(34-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1,3-dimethy1-1H-
pyrazole-4-sulfonamide:
F
1+1
01
si
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-iodophenyD
benzene-1,2-diamine (0.182
mmol) was reacted with 5-chloro-1,3-dimethy1-1H-pyrazole-4-sulfonyl chloride
(0.5 mmol) to obtain 5-chloro-N-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino) phenyl)-1,3-dimethy1-1H-pyrazole-4-
sulfonamide. 1H NMR (300 MHz,
CDC13)) 6 2.1 (s, 3H), 3.6 (s, 3H), 5.8 (s, 1H), 5.95 (dt, 1H), 7.0 (q, 1H),
7.2 (d, 1H), 7.3 (m, 2H), 7.4 (dd, 1H).
Example 23
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-2,5-dimethylfuran-3-
sulfonamide:
0 'NH F
10 40
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-iodophenyl)
benzene-1,2-diamine (0.182
mmol) was reacted with 2,5-dimethylfuran-3-sulfonyl chloride (0.5 mmol) to
obtain N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino) phenyl)-2,5-dimethylfuran-3-sulfonamide. II-1 NMR (300 MHz,
CDC13)) 6 2.2 (s, 3H), 2.3 (s, 3H), 5.8
(s, 1H), 6.0 (di, 1H), 6.8 (s, 1H), 7.0 (q, 111), 7.2 (d, 1H), 7.3 (m, 2H),
7.4 (dd, 11-1).
Example 24
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-methy1-3-
(trifluoromethyl)-1H-pyrazoIe-4-
sulfonamide:
CNH F
101
-Page 133-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-iodophenyl)
benzene-1,2-diamine (0.182
mmol) was reacted with 1-methyl-3-(trifluoromethyl)-1H-pyrazole-4-sulfonyl
chloride (0.5 mmol) to obtain N-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-methyl-3-(trifluoromethyl)-1H-
pyrazole-4-sulfonamide. 1H NMR
(300 MHz, CDC13)) 5 3.8 (s, 3H), 5.7 (s, 111), 6.0 (dt, 1H), 7.0 (q, 1H), 7.2
(m, 2H), 7.4 (dd, 1H), 7.8 (s, 1H).
Example 25
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-2,4-dimethylthiazole-5-
sulfonamide:
01-4\
0 'NH F
40
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-iodophenyD
benzene-1,2-diamine (0.182
mmol) was reacted with 2,4-climethylthiazole-5-sulfonyl chloride (0.5 mmol) to
obtain N-(3,4-difluoro-2-(2-fluoro-4-
10 iodophenylamino)pheny1)-2,4-dimethylthiazole-5-sulfonamide. 111 NMR (300
MHz, CDC13)) 8 2.3 (s, 311), 2.6 (s, 3H),
5.7 (s, 1H), 5.9 (dt, 1H), 7.1 (q, 1H), 7.2 (d, 111), 7.3 (m, IH), 7.4 (d,
1H), 7.4 (s, 1H).
Example 26
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1,2-dimethy1-1H-
imidazole-4-sulfonamide:
Nµ
0NH
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodopheriyObenzene-1,2-diamine was reacted
with 1,2-dimethy1-1H-imidazole-4-sulfonyl chloride to obtain the title
compound. 1H NMR (300 MHz, CDC13): 8 7.95
(br s, 1H), 7.37 (dd, J= 1.8 & 10.8 Hz, 111), 7.32-7.14 (in, 3H), 6.98 (dd, J=
9.6 & 17.7 Hz, 1H), 5.87 (dt, õI= 4.2, 9.0 &
17.4 Hz, 1H), 5.55 (hr s, 1H), 3.49 (s, 3H), 2.31 (s, 3H).
Example 27
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)thiophene-3-sulfonamide:
0
0-8 NH F
8 =
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyObenzene-1,2-diamine was reacted
with thiophene-3-sulfonyl chloride to obtain the title compound. 1H NMR (300
MHz, CDC13): ö 8.00 (dd, J= 1.2 & 3.3
Hz, 111), 7.45 (dd, J= 0.9 & 5.1 Hz, 111), 7.35 (m, 2H), 7.27 (m, 2H), 6.91
(dd, J= 9.3 & 17.1 Hz, 1H), 6.64 (ddd, J=
2.1, 4.8 & 8.7 Hz, 1H), 6.34 (dt, J= 5.4, 8.7 & 14.1 Hz, 111), 5.98 (br d, J=
2.1 Hz, D20 exchangeable, 1H).
-Page 134-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 28
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)furan-2-sulfonamide:
9
crt-NH HF
01
1
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with furan-2-sulfonyl chloride to obtain the title compound. 1H NMR (300 MHz,
CDC13): 6 7.53 (br s, D20
exchangeable, 1H), 7.38 (dd, J= 1.8 & 10.5 Hz, 7.30 (d, J= 8.4 Hz, 1H),
7.21 (d, J= 3.0 Hz, 1H), 6.96 (dd, J= 8.7
& 16.5 Hz, 1H), 6.87 (ddd, J= 1.8, 5.1 & 9.0 Hz, 1H), 6.53 (dd, J= 1.8 & 3.6
Hz, 1H), 6.44 (dt, J=5.1, 8.7 & 13.8 Hz,
1H), 6.22 (br s, D20 exchangeable, 111).
Example 29
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyI)-5-methylthiophene-2-
sulfonamide:
9
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-1,2-diamine was reacted
with 5-methylthiopherie-2-sulfony1 chloride to obtain the title compound. 1H
NMR (300 MHz, CDC13): 8 7.34 (dd, J=
0.9 & 10.2 Hz, 1H), 7.30 (ddd, J= 2.1, 4.8 & 9.0 Hz, 1H), 7.25 (d, J= 3.9 Hz,
1H), 7.07 (m, 2H), 6.65 (dd, J= 1.2 & 3.9
Hz, 1H), 5.89 (dt, J= 2.4, 8.7 & 17.4 Hz, 1H), 5.54 (br s, D20 exchangeable,
1H), 2.46 (s, 3H).
Example 30
5-Chloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)thiophene-2-
sulfonamide:
9
ch.....,cLsitNH
40 40
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene 1 ,2-diamine was reacted
with 5-chlorothiophene-2-sulfonyl chloride to obtain the title compound.
111.NMR (300 MHz, CDC13): 8 7.38 (dd, J=
1.5& 10.2 Hz, 1H), 7.32 (ddd, J= 2.1, 5.1 & 9.3 Hz, 1H), 7.25 (d, J= 3.9 Hz,
1H), 7.10 (dd, J= 9.0 & 18.6 Hz, 3H),
6.84 (d, J= 4.2 Hz, 1H), 5.86 (dt, J= 1.8, 8.7 & 17.4 Hz, 1H), 5.49 (br s, D20
exchangeable, 1H).
Example 31
5-Bromo-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenypthiophene-2-
sulfonamide:
9
B r N F
\ 1 0 H
1
-Page 135-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2-diamine was reacted
with 5-bromothiophene-2-sulfonyl chloride to obtain the title compound. 1H NMR
(300 MHz, CDC13): ö 7.39-7.29 (m,
2H), 7.20-7.05 (m, 3H), 6.96 (d, J= 3.6 Hz, 1H), 5.85(dt, J= 2.1, 9.0 & 17.4
Hz, 1H), 5.54 (br s, 1H).
Example 32
4-Bromo-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)thiophene-3-
sulfonamide:
-(731' NH
f o
FI
s 1401 110
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene1,2-dia,mine was reacted
with 4-bromothiophene-3-sulfonyl chloride to obtain the title compound. 1H NMR
(300 MHz, CDC13): 8 7.48 (br m,
2H), 7.39 (dd, J= 1.8 & 10.5 Hz, 111), 7.28 (ddd, J= 2.4,4.8 & 9.0 Hz, 1H),
7.17 (d, J= 8.4 Hz, 1H), 7.02 (m, 111), 6.02
(dt, J= 2.4, 8.7 & 17.4 Hz, 1H), 5.68 (br s, 1H).
Example 33
4-Bromo-5-ehloro-N-(3,4-difluoro-2-(2-11uoro-4-
iodophenylamino)phenyl)thiophene-2- sulfonamide:
9
F
Br
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2-diamine was reacted
with 4-bromo-5-chlorothiophene-2-sulfonyl chloride to obtain the title
compound. 1H NMR (300 MHz, CDC13): 6 7.42-
7.34 (m, 2H), 7.25 (br m, 3H), 7.13 (dd, J= 9.0 & 17.1 Hz, 1H), 6.02 (dt, J=
2.4, 6.6 & 17.4 Hz, 1H), 5.52 (br s, 1H).
Example 34
3-Bromo-5-chloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)
phenyl)thiophene-2- sulfonamide:
0
CI 3 g
)CeWNHH F
Br 6-1
F I
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2-diamine was reacted
with 3-bromo-5-chlorothiopherie-2-sulfonyl chloride to obtain the title
compound. 111 NMR (300 MHz, CDCb): 6 7.41
(dd, J= 2.1 & 10.5 Hz, IH), 7.35 (br m, 2H), 7.31 (dd, J= 2.1 & 4.2 Hz, 1H),
7.19 (d, J= 8.7 Hz, 1H), 7.08 (dd, ./.= 9.0
& 17.4 Hz, 1H), 6.02(dt, J= 2.1, 8.4& 17.1 Hz, 1H), 5.59 (br s, 1H).
Example 35
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)-2,5-dimethylthiophene-3-
sulfonamide:
9
--s?- N1H
S / 0 0
110
1
-Page 136-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2-diamine was reacted
with 2,5-dimethyIthiophene-3-sulfonyl chloride to obtain the title compound.
'11 NMR (300 MHz, CDC13): 6 7.39 (dd, J
--- 1.8 & 10.2 Hz, 1H), 7.24-7.16 (hr m, 2H), 7.13 (dd, .1= 9.0 & 17.4 Hz,
1H), 6.77 (d, J= 9.6 Hz, 1H), 5.98 (dt, J= 2.4,
8.7 & 17.4 Hz, 1H), 5.55 (hr s, 1H), 2.33 (s, 6H).
Example 36
2,5-Dichloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)thioPhene-3-
sulfonamide:
9
A`INIH
SI 0 F
CI so
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2-diamine was reacted
with 2,5-dichlorothiophene-3-sulfonyl chloride to obtain the title compound.
11-1 NMR (300 MHz, CDC13): 6 7.41(dd, J=
1.5 & 10.5 Hz, 1H), 7.28-7.20 (m, 2H), 7.08 (dd, J= 9.0 & 17,4 Hz, 211), 6.99
(s, 1H), 6.03 (dt, J----- 2.1, 8.7 & 17.4 Hz,
111), 5.56 (br s, 1H).
Example 37
Methyl 3-(N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)
sulfamoyl)thlophene-2-earboxylate:
/1?-11'NH
S / 0 r
me02C io 401
According to the general procedure B, 5,6-diffuoro-N1-(2-fluoro-4-iodophenyl)
benzene 1,2-diamine was reacted
with methyl 3-(chlorosulfonyl)thiophene-2-carboxylate to obtain the title
compound. 'H NMR (300 MHz, CDC13):
8.58 (s, 1H), 7.43 (dd, J= 5.1 & 10.8 Hz, 2H), 7.35 (dd, J= 1.8 & 10.2 Hz,
1H), 7.31 (ddd, .1¨ 2.1, 4.2 & 9.3 Hz, 1H),
7.04 (m, 2H), 5.88 (dt, J= 2.7, 8.7 & 17.4 Hz, 1H), 5.65 (br s, 1H), 3.85 (s,
3H).
Example 38
Methyl 5-(N-(3,4-difluoro-2-(2-1Iuoro-4-iodophenylamino)phenyl)sulfamoy1)-l-
methyl- IH-pyrrole-2-
carboxylate:
cr4Fi
MeD2C--UINHIH F
40 10
According to the general procedure B, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene 1 ,2-diamine was reacted
with methyl 5-(chlorosulfony1)-1-methyl-1H-pyrrole-2-carboxylate to obtain the
title compound. 11-1NMR (300 MHz,
CDC13): 87.37 (dd, J= 1.8 & 10.5 Hz, 1H), 7.29 (m, 211), 7.12-6.94 (m, 4H),
5.87 (dt, J= 1.8, 8.4 & 17.4 Hz, 111), 5.56
s, 1H), 3.65 (s, 3H), 3.75 (s, 3H).
-Page 137-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 39
N-(3,4-difluoro-2-(2-fluoro-4-iodophenyiamino)phenyt)-5-methylisoxazale-4-
sulfonamide:
NH H F
R-31,
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzenel,2- diamine was reacted
5 with the corresponding sulfbnyl chloride to obtain the title compound.
Yield: 22%. m/z = 508 EM-If.
Example 40
3-Chloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)propane-I-
sulfonamide:
CI
NH F
=
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyl)benzene-I,2-diamine was reacted
10 with 3-chloroproparie-1-sulfonyl chloride to obtain the desired product.
'11NMR (500 MHz, CDC13): 6 = 7.39-7.38 (d,
1H), 7.35-7.34 (m, 1H), 7.27-7.26 (m, 111), 7.10-7.0 (q, 1H), 6.63 (s, 1H,
br), 6.15-6.11 (q, 1H), 5.60 (s, 1H, br), 3.60-
3.56 (t, 2H), 3.22-3.20 (m, 2H), 2.22-2.16 (m, 211).
Example 41
N-(2-(4-chloro-2-fluorophenylamino)-3,4-difluorophenyl)
cyclopropanesulfonamide:
0,y
0 'NH F
= =
CI
See example 1. NMR (300 MHz, CDC13) 8 0.85-0.95 (m, 211), 1.05-1.15 (ra,
2H), 2.2-2.4 (m, 111), 5.8 (s, 1H),
6.3 (t, 1H), 6.6-7.4 (m, 5H); m/z = 375 [M-1]-.
Example 42
N-(3,4-difluoro-2-(4-iodo-2-methylphenylamino)phenyl)cyclopropanesulfonamide:
oy
0 'NH CH3
20
See example 1. 11-1 NMR (CDC13) 8 0.80-1.0(m, 211), 1.05-1.20(m, 2H), 1.55 (s,
311), 2.4-2.5 (m, 1H), 5.6(s,
1H), 6.2 (dd, 1H), 6.4 (s, 11-1), 7.1 (q, 1H), 7.3-7.4 (m, 2H), 7.5 (s, 1H);
m/z = 463 [M-11-.
-Page 138
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 43
N-(2-(4-tert-buty1-2-chloropheny1andno)-3,4-difluorophenyl)
eyclopropanesulfonamide:
0'NH CI
See example 1. 1H NMR (300 MHz, CDC13) 8 0.9-1.0 (m, 2H), 1.05-1.20 (m, 2H),
1.3 (s, 9H), 2.4-2.5 (m, 1H),
5.8 (s, 11-1), 6.3 (dd, 1H), 6.6 (s, 1H), 7.0-7.2 (m, 2H), 7.3-7.4 (m, 2H);
m/z = 413 [M-1]-.
Example 44
N-(2-(2,4-dichlorophenylamino)-3,4-difluorophenyl)cyclopropanesulfonamide:
07
CNH CI
CI
See example 1. 114 NMR (300 MHz, CDC13) 8 0.9-1.0 (m, 2H), 1.05-1.20 (m, 2H),
2.4-2.5 (m, 1H), 6.0 (s, 1H),
10 6.3 (dd, 1H), 6.6 (s, 1H), 7.0-7.2 (m, 2H), 7.3-7.4 (m, 2H); m/z = 392
[M-1] .
Example 45
3-Chloro-N-43,4-difluoro-2-(2-fluoro-4-trifluoromethyl)
phenylamino)phenyl)propane- 1-sulfonamide:
CI&NH H F
CF3
See example 1. 11-1NMR (300 MHz, CDC13): ö 7.39-7.26 (m, 2H), 7.25 (m, 1H),
7.18 (dd, J= 9.0 & 17.7 Hz, 1H),
6.78 (br s, D20 exchangeable, 1H), 6.50 (t, J= 8.1 Hz, 11-1), 6.00 (br d, D20
exchangeable, J= 1.5 Hz, 1H), 3.63 (t, J=
6.0 & 6.3 Hz, 2H), 3.29 (t, J= 7.2 & 7.8 Hz, 2H), 2.26 (quintet, 2H); m/z =
445 [M-1] .
Example 46
N-(3,4-difluoro-2-(2-ehloro-4-trilluoromethyt)phenylamino)methanesulfonamide:
5,
NH H CI
0 N
10 40
CF3
See example 1. 11-1NMR (300 MI-lz, CDC13): 8 7.65 (d, J=7.8 Hz, 1H), 7.33 (m,
211), 7.19 (dd, J= 9.3 & 17.4 Hz,
1H), 6.90 (br s, D20 exchangeable, 111), 6.45 (dd, J=1.5 & 8.4 Hz, 1H), 6.39
(br s, D20 exchangeable, 1H), 3.02 (s,
31-1); m/z = 399 [M-1].
-Page 139-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 47
3-ChIoro-N-(3,4-difluoro-2-(2-chIoro-4-trifluoromethyl)
phenylamino)phenyl)propane-l-sulfonamide:
0
H
0
CF3
See example 1. '11 NMR (300 MHz, CDC13): 8 7.66 (d, J= 1.5 Hz, 1H), 7.36 (m,
211), 7.19 (dd, J= 9.0 & 17.4
Hz, 1H), 6.91 (br s, D20 exchangeable, 1H), 6.50 (dd, J= 8.4 & 1.5 Hz, 1H),
6.37 (s, D20 exchangeable, 1H), 3.62 (t, J =
6.0 Hz, 2H), 3.29 (t, J = 7.5 & 7.8 Hz, 2H), 2.27 (quintet, 211); rn/z = 462
[M-1] .
Example 48
3-Chloro-N-(3,4-difluoro-2-(2-bromo-4-trifluoromethyl)
phenylannino)phenyl)propane-i-sulfonamide:
ry Br
0
101
OF3
See example 1. NMR (300 MHz, CDC13): 8 7.82 (s, 111), 7.38 (m, 211), 7.20
(dd, J= 9.0 & 17.7 Hz, 111), 6.62
(br s, D20 exchangeable, 111), 6.43 (d, J= 8.4 Hz, 1H), 6.23 (s, D20
exchangeable, 1H), 3.65 (t, J = 6.0 Hz, 211), 3.30 (t,
J= 7.5 Hz, 2H), 2.28 (quintet, 2H); miz = 506 [M-1] .
Example 49
Cyclopropanesulfonic acid (3,4,6-trifluoro-2-(2-fluoro-4-iodo-phenylamino)-
phenyl)-amide:
Step A: (2-F1uoro-4-iodo-pheny1)-(2,3,5-trifluoro-6-nitro-pheny1)-amine:
NO2 H F
F opc
A stirred solution of 2-fluoro-4-iodoaniline (3.64 gm, 15.37 mmol) in dry THF
(100 ml) under nitrogen was cooled
to -78 C and a solution of 1.0 M lithium hexa methyl disilazide (LiN(SiMe3)2)
"LHMDS" (15.37 ml, 15.37 mmol) was
added slowly. This reaction mixture was kept stirring at -78 C for another
hour and then 2,3,4,6-tetrafluoronitrobenzene
was added. The reaction mixture was allowed to warm to room temperature and
stirring continued for another 16 hours.
Ethyl acetate (200 ml) was added to the reaction mixture and was washed with
water. Organic layer was dried over
sodium sulfate and further purified by column chromatography to provide yellow
solid (3.75 gm, yield: 59.24%). M-Fr:
410.9. IFI NMR (DMSO, 300 MHz): 6.85 (t, 1H); 7.38 (d, 1H); 7.62 (m, 2H); 8.78
(s, 1H).
Step B: 3 ,4,6-Trifluoro-N2-(2-Fluoro-4-iodo-pheny1)-benzene-1,2-diamine:
NH2H F
Fk
25
To the stirred solution of (2-fluoro-4-iodo-pheny1)-(2,3,5-trifluoro-6-nitro-
phenyl)-amine 3 (5.2 gm, 12.62 mmol)
in Et0H (200 ml), ammonium chloride (10.12 gm, 189.3 mmol) and iron powder
(10.57 gin, 189.3 minol) was added.
This reaction mixture was kept stirring at reflux for 16 hours. Reaction
mixture was allowed to cool and was filtered
over celite and concentrated to dryness. The residue obtained was taken into
Et0Ac and was washed with water. The
-Page 140-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Et0Ac layer was dried over sodium sulfate and further purified by
crystallization from Et0H to provide off-white solid
(3.2 gm, yield: 66.39%). M-1-1+: 381.1. 11-1NMR (DMSO, 300 MHz): 5.0 (s, 2H);
6.2 (t, 1H); 7,2 -7.3 (m, 2H); 7.45 (s,
1H); 7.5 (d, 1H).
Step C: 4.6.7-Trifluoro-1-(2-Fluoro-4-iodo-phenyl)-1,3,-dihydrobenzoimidazole-
2- one:
H
sN4 F
F [1/41
To the stirred solution of 3,4,6-trifluoro-N2-(2-Fluoro-4-iodo-phenyl)-benzene-
1,2- diamine 3 (0.285 gin, 0.74
mmol) in CH2C12 (2 ml), 1,1'-carbonyldiimidazole (0.125 gm, 0.75 mmol) was
added. This reaction mixture was kept
stirring at room temperature for 16 hours when product precipitated out. The
white solid was filtered and used further
without any purification. (0.2 gm, yield: 65.85%): m/z = 407 [M-11-.
Step D/E: Cyclopropanesulfonic acid (3.4,6-trifluoro-2-(2-fluoro-4-iodo-
phenylamino)-phenyl)-amide:
0,A
H
FIN,
F
A stirred solution of 4,6,7-trifluoro-1-(2-fluoro-4-iodo-pheny1)-1,3,-
dihydrobenzimidazol-2-one (0.2 gm, 0.41
mmol) in dry THF (4 ml) under nitrogen was cooled to -78 C and a solution of
1.0 M LiHMDS (0.41 ml, 0.41 mmol)
was added slowly. (2 ml) followed by addition of cyclopropanesulfonyl chloride
(0.050 ml, 0.49 mmol). This reaction
mixture was kept stirring at room temperature for 16 hours, concentrated to
dryness and was taken into Et0Ac. The
Et0Ac was washed with water, dried over sodium sulfate and concentrated to
dryness. The residue obtained 1-
cyclopropanesulfony1-4,5,7-trifluoro-3-(2- fluoro-4-iodo-pheny1)-1,3-dihydro-
benzimidazol-2-one 5 was taken into
dioxane (2 ml) and to this 1.0 N NaOH (0.5m1) was added and kept stirring at
room 50 C for 16 hours. TLC indicated
incomplete reaction, the product was purified by HPLC to provide off-white
solid (4.4 mg) M+H+: 484.7, M-H+: 486.7.
'1-INMR (CDC13, 300 MHZ): 0.9 - 1.1 - (m, 2H); 1.1 - 1.2 (m, 2H); 2.45 -2.55
(m, IH); 6.05 (s, 1H); 6.44 - 6.54 (m, 1
H); 7.1 (s, 11-1); 7.4 - 7.7 (d, IH); 7.38 - 7.44 (dd, 1H); m/z = 485 [M-l].
Example 50
N-(3,4-difluoro-2-(4-fluoro-2-lodopbenylamino)-6-ethoxyphenyl) cyelopropane
sulfonamide:
Step A: (2J-Difluoro-5-methoxy-6-nitro-pheny1)-(2-fluoro-4-iodo-pheriy1)-
amine:
N 2 H F
Me igh N iiIM
MP I
A stirred solution of (2-fluoro-4-iodo-pheny1)-(2,3,5-trifluoro-6-nitro-
pheny1)-amine (1.23 gm, 3 mmol) in dry
THF (25 ml) under nitrogen was cooled to -78 C and a solution of 25% Na0Me
(0.68 ml, 0.3 mmol) was added slowly.
Reaction mixture was allowed to warm to room temperature and stirring
continued for another 16 hours. TLC indicated
incomplete reaction. Ethyl acetate (100 ml) was added to the reaction mixture
and was washed with water. Organic
layer was dried over sodium sulfate and further purified by column
chromatography to provide yellow solid (0.6 gm,
yield: 47.6%). m/z = 424 [M=Hr.
-Page 141-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step B: 5,6-Difluoro-N1-(4-fluoro-2-iodophenyl)-3-methoxybenzene-1,2-diamine:
NH2 H F
NleD N
WFIIMI4
To the stirred solution of (2,3-difluoro-5-methoxy-6-nitro-pheny1)-(2-fluoro-4-
iodo-pheny1)-amine (0.57 gm, 1.34
mmol) in Et0H (20 ml), ammonium chloride (1.18 gm, 20.16 mmol) and iron powder
(1.15 gm, 21.44 mmol) was added.
This reaction mixture was kept stirring at reflux for 16 hours. Reaction
mixture was allowed to cool and was filtered
over celite and concentrated to dryness. The residue obtained was taken into
Et0Ac and was washed with water. The
Et0Ac layer was dried over sodium sulfate and further purified by
crystallization from Et0H to provide off-white solid
(0.47 gm, yield: 90.3%). M-1-r: 393.2. 'I-1 NMR (DMSO, 300 MHz): 3.76 (s, 3H);
6.1 (t, 1H); 6.8 -7.0 (m, 1H); 7.2 (d,
1H); 7.35 (s, 1H); 7.42 (d, 1H).
Step C: 6,7-Difluoro-1-(4-fluoro-2-iodophenv1)-4-methoxy-1H-benzold]imidazol-
2(3H)-one:
H p
F
F
To the stirred solution of 5,6-difluoro-N1-(4-fluoro-2-iodopheny1)-3-
rnethoxybenzene-1,2-diamine (0.17 gin, 0.43
mmol) in CH2C12 (2 ml), 1,1'-Carbonyldiimidazole (0.085 gm, 0.53 mmol) was
added. This reaction mixture was kept
stirring at room temperature for 16 hours when product precipitated out. The
white solid was filtered and used further
without any purification. (0.089 gm); m/z = 419 [M-11
Step D/F: N-(3,4-difluoro-2-(4-fluoro-2-iodophenylamino)-6-
methoxyphenyl)cyclopropanesulfonamide:
R ,0
,H
N H F
Me()
j
F 111111 I
A stirred solution of1-(cyclopropylsulfony1)-4,5-difluoro-3-(2-fluoro-4-
iodopheny1)-7-methoxy-1H-
benzordlimidazol-2(3H)-one (0.89 gm, 0.17 mmol) in dry THF (4 ml) under
nitrogen was cooled to -78 C and a solution
of 1.0 M LiHMDS (0.17 ml, 0.17 mmol) was added slowly. (2 ml) followed by
addition of cyclopropanesulfonyl
chloride (0.021 ml, 0.21 mmol). This reaction mixture was kept stirring at
room temperature for 16 hours, concentrated
to dryness and was taken into Et0Ac. The Et0Ac was washed with water, dried
over sodium sulfate and concentrated to
dryness. The resulting 1-(cyclopropyIsulfony1)-4,5- difluoro-3-(2-fluoro-4-
iodopheny1)-7-methoxy-11-1-benzo[d]imidazol-
2(3H)-one was taken into dioxane (2 ml) and to this 1.0 N NaOH (0.5m1) was
added and kept stirring at room 50 C for
16 hours. TLC indicated incomplete reaction, the product was purified by HPLC
to provide off-white solid (2.5 mg)
M+H4: 484.7, M-1-1+: 497.3. '11NMR (CDC13, 300 MHz): 0.85 -0.95 (m, 2H); 1.05 -
1.15 (m, 2H); 2.4 - 2.5 (m, 1H); 3.9
(s, 31-1); 6.1 (s, 1H); 6.4 - 6.6 (m, 2 II); 7.3 (m, 1H); 7.35 - 7.4 (dd, 1H);
nilz = 497 [M-11-.
-Page 142-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 51
Methylsulfonic acid (3,4-difluoro-2-(2-fluoro-4-iodo-phenylamino)-6-methoxy-
phenyl)-amide:
0-,AI4H
Me0 N
A stirred solution of 5,6-difluoro-N1-(4-fluoro-2-iodopheny1)-3-methoxybenzene-
1,2-diamine (0.150 gm, 0.38
mmol) in dry C112C12 (4 m1), TEA (.264 ml, 1.9 mmol) and methanesulfonyl
chloride was added slowly. This reaction
mixture was kept stirring at room temperature for 16 hours, TLC indicated
incomplete reaction along with starting
material two products were observed. The reaction mixture was washed with
water, organic layer was dried over sodium
sulfate and concentrated to dryness, the product was purified by column
chromatography. The minor product was found
to be the expected compound (6.4 mg). M-H+: 471.5. NMR (CDC13, 300 MHz):
3.9 (s, 311); 6.05 (s, 1H); 6.4 - 6.5
(m, 1H); 6.5 - 6.6 (m, 1H); 7.2 (s, 1H); 7.28 (d, 111); 7.35 - 7.4 (d, 111);
m/z = 471 EM-11-.
Example 52
1-(2,3-Dihydroxy-propy1)-cyclopropanesulfonic acid 13,4,6-trifluoro-2-(4-
fluoro-2-iodo-phenylamino)-
phenylFainide:
Step A: 1-AIlyl-cyclopropanesulfonic acid [3.4.6-trifluoro-2-(2-fluoro-4-iodo-
phenylamino)phenyli-
amide:
6 10-NH h.ii 7
F I
According to the general procedure B, 1-allyl-cyclopropanesulfonyl chloride
was reacted with 3,5,6-trifluoro-N1-
(2-fluoro-4-iodophenyl)benzene-1,2-diamine to obtain the title product.
'FINNIR (CDC13, 300 MHz): 8 7.41 (dd, 1H),
7.38 (dd, 1H), 7.09 (s, 111), 6.78 (m, 1H), 6.49 (m, 1H), 5.96 (s, 1H), 5.86
(m, 1H), 5.18 (d, 211), 2.76 (d, 2H), 1.23 (m,
2H), 0.872 (m, 2H).
Step B: 1-(2,3-Dihydroxypropyr)-N-(3,4,6-trifluoro-2-(2-fluoro-4-
iodophenylamino)phenvbcyclopropane-l-sulfonamide:
1-10
6-NH 1.1 F
F
F
1-Allyi-cyclopropanesulfonic acid [3,4,6-trifluoro-2-(2-fluoro-4-iodo-phenyl
amino)- phenyl]-amide (110 mg, 0.21
mmol) and 4-methyhnorpholine N-oxide (24.6 mg, 0.21 mmol) was dissolved in THF
(8 mL). Osmium tetroxide was
added at room temperature (0.021 mmol, 0.153 inL, 4% in H20) and the reaction
mixture was stirred at room temperature
for 16 hours. Et0Ac was added, the organic phase was washed with water, dried
(MgSO4) and concentrated under
reduced pressure. The residue was purified over silica gel chromatography
(eluants: Et0Ae/ Me0H) to obtain the titled
product (0.89 g, 75 %). ITINMR (CDC12, 300 MHz): 8 7.39 (dd, J = 1.5 & 10.6
Hz, 1H), 7.29 (d, 1= 8.8 Hz, IH), 7.28
(s, 1H), 6.97 (s, 1H), 6.76 (m, 1H), 6.49 (m, 1H), 4.13 (m, 1H), 3.66 (dd, J =
3.7 & 11.4 Hz, 111), 3.53 (dd. J 6.7 & 11.2
-Page 143-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Hz, 1H), 2.50(dd, J = 10.0 & 16.1 Hz, 1H), 1.6 (m,1H), 1.46(m, 111), 1.28 (m,
1H), 1.20 (m, 2H), 0.92(m, 211); m/z =-
559 [M-1] .
Example 53
(S)-1-(2,3-dihydroxypropy1)-N-(3,4,6-trifluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)cyclopropane-1-
sulfonamide:
HO1..P
g-NF, H F
Fl,11111151 F I
The pure S isomer was obtained by chiral HPLC separation of the racemic
mixture (example 52). 111NMR
(CDC13, 300 MHz): ö 7.39 (dd, J = 1.5 & 10.6 Hz5 111), 7.29 (d, J = 8.8 Hz,
1H), 7.28 (s, 111), 6.97 (s, 1H), 6.76 (m, 1H),
6.49 (m, 1H), 4.13 (m, 1H), 3.66 (dd, J = 3.7 & 11.4 Hz5 1H), 3.53 (dd, J =
6.7 & 11.2 Hz, 1H), 2.50(dd, .1= 10.0 & 16.1
Hz, 111), 1.6 (m,1H), 1.46 (m, 1H), 1.28 (m, 111), 1.20 (m, 2H), 0.92 (m, 2H);
rth = 559 [M-1]-.
Example 54
(R)-1-(2,3-dihydroxypropy1)-N-(3,4,6-trifluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)cycloproPane-1-
sulfonamide:
gri
P
giv1H H F
F
41111)1 F 4111"
The pure R isomer was obtained by chiral HPLC separation of the racemic
mixture (example 52). '1-INMR
(CDC13, 300 MHz): 8 7.39 (dd, J = 1.5 & 10.6 Hz, 1H), 7.29 (d, J = 8.8 Hz,
1H), 7.28 (s, 1H), 6.97 (s, 1H), 6.76 (m, 1H),
6.49 (m, 1H), 4.13 (m, 1H), 3.66 (dd, J = 3.7 & 11.4 Hz, 111), 3.53 (dd, J =
6.7 & 11.2 Hz, 1H), 2.50(dd, J = 10.0 & 16.1
Hz, 1H), 1.6 (m, 1H), 1.46 (m, 1H), 1.28 (m, 111), 1.20 (m, 2H), 0.92 (m, 2H);
m/z = 559 [M-1]-.
Example 55
N-(3,4-ditluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-
dihydroxypropyl)eyelopropane-l-sulfonamide:
Step A: 1-A11y1-N-3,4-difluoro-2-(2-fluoro-4-iodophenvlamino)-6-
methoxypheny1)cyc1opropanc-1-
sulfonamide:
NH H F
r"'"- N gag
11111" F 11111111'111 I
According to the general procedure B, 1-allyl-cyclopropanesulfonyl chloride
was reacted with 5,6-difluoro-N1-(2-
fluoro-4-iodopheny1)-3-methoxybenzene-1,2-diamine to obtain the title product.
ill NMR (CDCI3, 300 MHz): 8 7.417
(dd, 111), 7.309(s, 1H), 7.25 (m, 1.11), 6.89 (m, 1H), 6.52(m, 1H), 6.427 (m,
1H), 6.03 (s, 11-1), 5.668 (m, 111), 5.11 (t, 1H),
3.9 (s, 3H), 2.75 (d, 211), 1.21 (m, 211), 0.767 (m, 2H).
-Page 144-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step B: N-(3.4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-
dihydroxyproPyl) cyclopropane-
1-sulfonamide:
HO
tNH H F
Me0 N
111111P F 411113.11
1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl)
cyclopropane-l-sulfonamide (97 mg,
0.18 mmol) and 4-methyhnorpholine N-oxide (21 mg, 0.18 mmol) were dissolved in
THF (8 mL). Osmium tetroxide
was added at room temperature (0.018 mmol, 0.13 rnL, 4% in H20) and the
reaction mixture was stirred at room
temperature for 16 hours. Et0Ac was added, the organic phase was washed with
water, dried (MgSO4) and concentrated
under reduced pressure. The residue was purified over silica gel
chromatography (eluants: Et0Ac/ Me0H) to obtain the
titled product (0.80 g, 78%). 1H NMR (CDC13, 300 MHz): 67.38 (dd, J = 1.7 &
10.3 Hz,1H), 7.26 (m, 111), 7.14 (s,
1H), 6.87 (s, 111), 6.53 (dd, J = 6.8 & 11.4 Hz, 1H), 6.43 (m, 1H), 4.06 (m,
1H), 3.89 (s, 3H), 3.63 (dd, I = 3.7 & 11.1 Hz,
1H), 3.49 (dd, J= 6.4 & 11.1 Hz, 1H), 2.3 (dd, J = 9.7 & 16.1 Hz, 1H), 1.77
(dd, J = 1.9 & 16.0 Hz, 1H), 1.37 (m, 1H),
1.25 (m, 1H), 1.21 (m, 211), 0.86 (m, 2H); m/z = 571 [M-l].
Example 56
(S)-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide:
HO
tNH H F
MeON
F 41111" I
The pure S isomer was obtained by chiral HPLC separation of the racemic
mixture (example 55). 1H NMR
(CDC13, 300 MHz): 67.38 (dd, I = 1.7 & 10.3 Hz,1H), 7.26 (m, 111), 7.14 (s,
111), 6.87 (s, 1H), 6.53 (dd, J= 6.8 & 11.4
Hz, 1H), 6.43 (m, 1H), 4.06 (m, 1H), 3.89 (s, 3H), 3.63 (dd, J = 3.7 & 11.1
Hz, 111), 3.49 (dd, J = 6.4 & 11.1 Hz, 111), 2.3
(dd, J = 9.7 & 16.1 Hz, 111), 1.77 (dd, J = 1.9 & 16.0 Hz, 11-1), 1.37 (m,
1H), 1.25 (m, 1H), 1.21 (m, 211), 0.86 (m, 211);
nilz = 571 [M-1]-.
Example 57
(R)-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide:
0
N F
0
Me0
F I
The pure R isomer was obtained by chiral HPLC separation of the racemic
mixture (example 55). 1H NMR
(CDC13, 300 MHz): 67.38 (dd, J = 1.7 & 10.3 Hz,1H), 7.26 (m, 1H), 7.14 (s,
1H), 6.87 (s, 111), 6.53 (dd, J = 6.8 & 11.4
Hz, 1H), 6.43 (m, 1H), 4.06 (in, 1H), 3.89 (s, 3H), 3.63 (dd, J = 3.7 & 11.1
Hz, 1H), 3.49 (dd, 3 = 6.4 & 11.1 Hz, 1H), 2.3
(dd, J = 9.7 & 16.1 Hz, 11-1), 1.77 (dd, J = 1.9 & 16.0 Hz, 111), 1.37 (m,
1H), 1.25 (m, 1H), 1.21 (m, 2H), 0.86 (m, 2H);
m/z = 571 [M-1 ].
-Page 145-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 58
1-(2-hydroxyethyl)-N-(3,4,6-trifluoro-2-(2-fluoro-4-iodophenylamino)phenyl)
eyelopropane-1-sulfonamide:
Step A: TBS-protected 1-(2-hydroxyethyl)-N-(3.4,6-trifluoro-2-(2-fluoro-4-
iodopheny1amino) phenyl)
cyclopropane-l-sulfonamide:
czOTBS
ALL,<Ice
NH H F
F fai N
4111113111 F 4111111= -1111
According to the general procedure B, the sulfonyl chloride prepared in step C
of example 16 was reacted with
5,6-difluoro-N1-(2-fluoro-4-iodopheriy1)-3-fluorobenzene-1,2- diamine to
obtain the title product Yield: 13%. 111-NMR
(300 MHz, CDC13): 6 = 7.51 (s, 1H, br), 7.37-7.35 (d, 1H), 7.27-7.25 (d, 1H),
6.94 (s, 1H, br), 6.78-6.68 (m, 1H), 6.46-
6.44(m, 1H), 3.90-3.88 (t, 21-1), 2.12-2.10(t, 2H), 1.31-1.28(m, 2H), 0.91-
0.89(m, 2H), 0.86 (s, 9H), 0.05 (s, 6H); m/z =
643 [M-1]
Step B: 1-(2-hydroxyethyl)-N-(3,4,6-trifluoro-2-(2-fluoro-4-iodophenylamino)-
phenv1) cyclopropane-1-
sulfonamide:
(01-1
Cr" H F
N
F 411111= -111
Same procedure as in step E, example 16. Yield: 100%. 111-NMR (300 MHz,
CDC13): 8 = 7.51 (s, 111, br), 7.37-
7.35 (d, 111), 7.27-7.25 (d, 1H), 6.94 (s, 1H, br), 6.78-6.68 (m, 1H), 6.46-
6.44 (m, 1H), 3.90-3.88 (t, 2H), 2.12-2.10 (t,
211), 1.31-1.28 (m, 2H), 0.91-0.89 (m, 2H); m/z = 529 [M-1] .
Example 59
N-(34-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2-
hydroxyethyl)eyelopropane-1-
sulfonamide:
Step A: TBS-protected N-(3,4-dif1uoro-2-(2-fluoro-4-iodophenylamino'1-6-
methoxypheny1)-1-(2-
hydroxyethyl)cyclopropane-1-sulfonamide:
(OTBS
A.K)0
AYH
11
F= 111}1111 i
According to the general procedure B, the sulfonyl chloride prepared in step C
of example 16 was reacted with
5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-methoxy-benzene- 1,2-diamine to
obtain the title product. Yield: 37%. 111-
NMR (300 MHz, CDC13): ö= 7.40-7.34 (dd, 1H), 7.23-7.21 (m, 1H), 6.61 (s, 1H,
br), 6.57-6.49 (dd, 1H), 6.48-6.39 (m,
1H), 3.9-3.7 (m, 5H), 2.15-2.05 (t, 2H), 1,30-1.20 (m, 2H), 0.95-0.80 (m,
11H), 0.05 (s, 6H); miz = 655 [M-1] .
-Page 146-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step B: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2-
hydroxyethyncyclopropane-1-sulfonamide:
(OH
'
At5(/C)
F
H
0 N
110 0
F I
F
Same procedure as in step E, example 16. Yield: 100%. 1H-NMR (300 MHz, CDC13):
6 = 7.40-7.34 (dd, 1H),
7.23-7.21 (m, 1H), 6.61 (s, 1H, br), 6.57-6.49 (dd, 1H), 6.48-6.39 (m, 1H),
3.9-3.7 (m, 5H), 2.15-2.05 (t, 2H), 1.30-1.20
(m, 211), 0.95-0.80 (m, 2H); m/z= 541 [M-1f.
Example 60
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyI)-1-(3-hydroxy-2-
(hydroxymethyl)propyl)eyelopropane-1.-sulfonamide:
Step A: Dimethyl 2-(2-bromoallyl)malonate:
0 0
Me OMe
"ji""
Br
To a suspension of sodium hydride (5.0 g, 125 mmol) in HMPA (50 ml, distilled
from calcium hydride) was added
a solution of dimethyl malonate (11.7 ml, 100 mmol) in HMPA (5 ml) at 0 C
under argon. The mixture was heated to
50 C and stirred 1 hour. Following this the solution was again cooled to 0 C,
and a solution of 2,3-dibromopropene
(12.2 ml, 100 mmol) in HMPA (5 ml) was added to the reaction mixture. Next,
the solution was warmed to 40 C and
stirred for 1 hour. The reaction mixture was quenched with aq. HC1 (10%, 88
ml) and extracted with ether (3 x 45 ml).
The organic fractions were collected, dried over MgS0-4, and the solvent was
removed in vacua. The crude oil was
purified via silica gel chromatography (eluants: chloroform/hexane) to obtain
the titled product as a colorless oil (16.3 g,
65%). 'H-NMR (300 MHz, CDC13) 6 5.70 (d, .1= 1.8 Hz, 1 H), 5.48 (d, J = 1.8
Hz, 111), 3.63 (t, J = 7.5 Hz, 1 H), 3.76 (s,
6 H), 3.04 (d, J = 7.5 Hz, 2 H).
Step B: 2-(2-Bromoallyl)propane-1.3-diol:
OH OR
''...r.
Br
Lithium aluminum hydride (1.9 g, 7.65 mmol) was slurried in anhydrous diethyl
ether (50 ml) and cooled to -78 C
in a dry ice/acetone bath. A solution of the product from step A (0.639 g,
16.84 mmol) in dry ether (26 ml) was then
added dropwise. After the malonate was added, the solution was allowed warm to
room temperature and stirring was
continued for 3 hours. The reaction was quenched with brine (50 ml), extracted
with ethyl acetate (3 x 25 ml) and dried
over MgSO4. The solvent was removed in vacuo to give the desired product (1.3
g, 86%) which was used for the next
step without further purification. 1H-NMR (300 MHz, CDCI3) 5 5.66 (d, J = 1.2
Hz, 1 H), 5.48 (d, J = 1.5 Hz, 1H), 3.86
(m, 2 H), 3.73 (m, 211), 2.51 (d, J = 7.5 Hz, 2 H), 2.40 (br s,2 H), 2.15 (m,
1 H).
-Page 147-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step C: Di-tert-butvldimethvlsily1 protected 2-(2-bromoallyllpropane-1.3-diol:
TBSO-\
TBSO-if
The product from step B (2.8 g, 14.20 mmol) was dissolved in anhydrous THF
(140 ml). Anhydrous pyridine (2.5
ml, 31.24 mmol) was added, and the solution was cooled to 0 C. tert-
Butyldinaethylsilyltriflate (7.2 ml, 31.24 mmol)
was added dropwise, and upon completion, the reaction solution was heated to
35 C. After stirring for 6 days, the
reaction was quenched with 100 ml brine, extracted with ethyl acetate (3 x 50
ml) and dried over MgSO4. The combined
organic phases were evaporated to obtain the crude product (5.5 g, 91%) as a
yellow oil which was used in the next step
without further purification. 1H-NMR (300 MHz, CDC13) 6 5.54 (d, J = 0.9 Hz, 1
H), 5.40 (d, J = 1.2 Hz, 111), 3.55 (d, J
= 5.4,4 H), 2.40 (d, J = 6.9 Hz, 2 H), 1.97 (m, 1 H), 0.85 (s, 18 H), 0.02 (s,
9 H).
Step D: Di-tert-butyldimethylsilyl_protected 241-bromocyclopro = 1 meth 1 aro
eane-1 3-diol:
TBSO--\\ <51-Br
TBSO--7
A reaction flask was charged with anhydrous CH2C12 (10 ml) and diethyl zinc
(1.0 M in hexanes, 4.65 ml, 4.65
mmol) at 0 C. Trifluoroacetic acid (0.358 ml, 4.65 mmol) was added dropwise
and the solution was allowed to stir for
minutes. Diiodomethane (0.375 ml, 4.65 mmol) was then added and the solution
was stirred for another 20 minutes.
15 Finally, the product from step C (0.492 g, 1.16 mmol) was added and the
solution was allowed to warm to ambient
temperature, stirring for 16 hours. The reaction was quenched with saturated
aqueous NH4CI. The layers were
partitioned and the aqueous phase was extracted with chloroform (3 x 5 ml).
The combined organic phases were washed
with brine (10 ml), dried over MgSO4, and the volatiles were removed in vacuo.
The resulting crude was purified via
silica gel chromatography (eluants: chloroform/hexanes) to provide the product
as a clear oil (0.280 g, 64%). 111-NMR
20 (300 MHz, CDC13) 83.66 (d, J = 5.4, 4 H), 2.08 (m, 1 H), 1.64 (d, I =
6.9, 2 H), 1.13 (m, 2 H), 0.88 (s, 18 H), 0.81 (m, 2
H), 0.04 (s, 9 H).
Step E: Di-tert-butyldimethylsilyl protected 1-(3-hydroxy-2-
(hydroxymethyl)propyl)cyclopropane-1-
sulfonyl chloride:
(1 TBS0)5---C1
8
TBSO
The product from step D (0.507 g, 1.16 mmol) was dissolved in anhydrous ether
(6 ml) and the reaction solution
was cooled to -78 C. Following this, tert-butyllithium (1.7 M in pentane, 1.50
ml, 2.55 mmol) was added dropwise over
5 minutes. After stirring for 0.5 hours, the lithiated product was transferred
via cannula to a stirred solution of suffuryl
chloride (0.206 ml, 2.55 mmol) in dry ether (6 ml) at -78 C. Once the transfer
is complete, the solution was allowed to
warm to room temperature, the solvent was evaporated and the resulting white
solid was slurried in dry hexanes. This
slurry was immediately filtered through celite, and all volatiles were removed
in vacuo. The resulting crude product
(0.376 g, 71%) was isolated as a yellow oil and was used in the following step
without further purification. 11-I-NMR
(300 MHz, CDC13) 6 3.60 (m, 4 H), 2.16 (m, 1 H), 2.03 (d, 2 H), 0.88 (s, 18
H), 0.04 (s, 9 H).
-Page 148-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step F: Di-tert-butyldimethylsityl protected N-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(3-hydroxy-2-(hydroxymethyl)propyl) cyclopropane-l-
sulfonamide:
TBSõTBS
0 0
0' NH F
rah, N
F 11111"
5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-methoxybenzene-1,2-diamine (8.8 mg,
0.022 mmoD was dissolved in
anhydrous pyridine (0.5 ml) under an argon atmosphere. The product from step E
(20.5 mg, 0.045 mmol), dissolved in
dry pyridine (0.5 ml), was added to the reaction flask and the mixture was
heated at 80 C for 21 hours. The solvent was
removed in vacuo and the resulting crude was purified via silica gel
chromatography (eluents: ethyl acetate/hexanes) to
provide the title compound (2.75 mg, 15%). m/z 813.5 (M-1).
Step G: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(3-
hydroxy-2-
(hydroxymethyl)propyl)cycloproparie-1-sulfonamide:
OH OH
01 NH 1_1 F
0
F = 00
The product from step F (27.9 mg, 0.0342 mmol) was dissolved in TIM (1 ml) and
treated with aqueous HC1 (1.2
N, 0.2 ml) at 0 C. The resulting solution was stirred for 4 hours. Following
this, the reaction was quenched with
saturated aqueous NaHCO3, extracted with ethyl acetate, dried over MgSO4 and
the volatiles were removed in vacuo.
The resulting crude was purified via silica gel chromatography (eluents:
methanol/chloroform) followed by LC-MS
purification to provide the title compound (11.8 mg, 59%). 'H-NMR (300 MHz,
CD30D) 8 7.32 (dd, 1 H), 7.21 (d, 1 H),
6.76 (dd, 1 H), 6.33 (m, 1 H), 3.82 (s, 3 H), 3.52 (d, 4 H), 2.01 (m, 1 H),
1.88 (d, 2 H), 1.07 (m, 2 H), 0.75 (m, 2 H), rn/z
585.3
Example 61
N-(34-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl) cyclobutane
sulfonamide:
Step A: Cyclobutanesulfonyl chloride:
SO2C1
To a suspension of Mg turnings (0.790 g, 32.5 mmol) in 20 ml anhydrous diethyl
ether was added a solution of
cyciobutylbromide (1.8 ml, 2.5722 g, 19.1 mmol) in 20 ml diethyl ether in
small portions with strong stirring. After the
initial exothermic reaction had ceased, the mixture was further heated to the
reflux temperature for 30 min. The
suspension was cooled down to room temperature and the supernatant was added
in small portions to an ice-cold solution
of sulfuryl chloride (4.6 ml, 7.728 g, 57.2 mrnol) in 30 ml anhydrous DCM.
After complete addition, the suspension was
warmed to room temperature and the volatiles were removed in vacuo. The
residue was dried in oil-pump vacua for 15
min, then it was extracted with hexane (150 m1). The hexane suspension was
filtered and the hexane was removed in
-Page 149-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
vacuo to give the crude product as dark purple oil which was used for the next
step without further purification. There is
still some unreacted cyclopropylbromide present. Crude yield: 1.1 g (38%).
Step B: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyl)cyclobutanesulfonamide:
NH H F
0
Me IP N 1111
According to the general procedure B, the cyclobutylsulfonyl chloride prepared
in the step above was reacted with
5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-methoxy-benzene- 1,2-diamine to
obtain the title product. Yield: 75%. 1H-
NMR (300 MHz, CDC13): 6 = 7.44 (s, 111, br), 7.41-7.36 (dd, in), 7.24-7.23 (m,
1H), 6.54-6.38 (m, 2H), 5.90 (s, 1H, br),
3.85-3.75 (m, 5H), 2.60-2.40 (m, 2H), 2.25-2.15 (m, 1H), 2.15-1.95 (m, 2H);
m/z = 511 [M-1].
Example 62
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methylpheny1)-1-(2,3-
dihydroxypropyl)eyelopropane-1-
sulfonamide:
Step A: (3,4,5-Trifluorophenyl)methanol:
HO 110
To a cooled (-5 C) solution of 3,4,5 -trifluorobenzaldehyde (7.0 g, 43.75
mmol) in a mixture ( 50 ml, 9:1) of THE
and water NaBH4 (1.662 g, 43.75 mmol) was slowly added in portions over a
period of 30 mm. The reaction mixture
was allowed to attain room temperature over a period of 2h and carefully
poured into ice-cold dil HC1 (200 ml, IN). The
oily layer was extracted into CH2C12 (250 ml) and the organic layer washed
with water (200 ml), dried (MgSO4) and
evaporated. The crude product (7.08 g, quantitative) obtained was taken
forward without further purification.
Step B: 5-(Bromomethyl)-1,2,3-trifluorobenzene:
F
Br
To a solution of the (3,4,5-Trifluorophenyl)methanol (40 mmol) in CH2C12 (150
ml), a solution of thionyl bromide
(6.16 ml, 80 mmol) in CH2Cl2 (50 nil) was added slowly. The reaction mixture
stirred at room temperature for 16h and
poured into ice-water (200 ml). The organic layer was separated and washed
with saturated NaHCO3 (2x200 ml), water
(200 ml), dried (MgSO4) and evaporated to obtain the corresponding bromo
compound as a pale yellow oil in
quantitative yield. The crude product was carried forward for the next
reaction without further purification.
Step C: 1,2,3-Trifluoro-5-methylbenzene:
H3C 401 F
The above bromo compound (40 mmol) was mixed with triethylsilane (48 mmol) and
the reaction mixture was
treated with solid PdC12 (4 mmol) in small portions. After a few minutes a
vigorous exothermic reaction was ensued and
care was taken to reflux the contents of the flask by placing a reflux
condenser. The reaction mixture was stirred at room
-Page 150-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
temperature for additional 6h and the contents were allowed to settle over
16h. Then the crude liquid product was
decanted carefully and carried forward for the next reaction without further
purification, it was assumed tat the reaction
proceeds in quantitative yield.
Step D: 1,2,3-Trifluoro-5-methy1-4-nitrobenzene:
NO2
H3C F
1,2,3-Trifluoro-5-methylbenzene (40 mmol) was added to conc. H2SO4 (50 ml) at
0-5 C. Then the reaction
mixture was slowly treated with conc. HNO3 (3.39 ml, 48.44 mmol, 90%) while
maintaining the internal temperature
below 20 C. The reaction mixture was stirred at room temperature for 16h and
poured onto ice (300 g) and the oily
layer was extracted with CH2C12 (2x125 ml). The organic layer was washed with
water (2x200 ml), brine (200 ml) and
dried (MgSO4) and evaporated to obtain the crude product which was purified
over flash silica gel chromatography to
obtain the title product (6.5 g, 85%). 11-1-NMR. (300 MHz, CDC13): 8 6.96
(septet, 111), 2.39 (s, 3H). 19FNMR (CDC13): 8
-128.18,- 141.50, -159.05.
Step E: 2,3-Difluoro-N-(2-fluoro-4-iodopheny1)-5-methy1-6-nitroani1ine:
No2 F
HaC N
4111)111 F 41111"
2-Fluoro-4-iodoaniline and 1,2,3-trifluoro-5-methyl-4-nitrobenzene were
reacted using the condition described in
Example 1 (Step A) to form the title compound. M-H+: 407.9
Step F: 5,6-Difluoro-N1-(2-fluoro-4-io dopheny1)-3 -methylb enzerte-1.2-
diamine :
NH2 F
H3C
110 40
2,3-Difluoro-N-(2-fluoro-4-iodopheny1)-5-methyl-6-nitroanifine was reduced
using the condition described in
Example 1 (step B) to form the title compound. M-H+: 377.4
Step G: 1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methylpherwl)cyclopropane-1-
sulfonamide:
H3C N aft"
1111"11 F I
According to the general procedure B, 1-allyl-cyclopropanesulfonyl chloride
(142 mg, 142 mg) was reacted with
5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-methylbenzene-1,2- diamine (150 mg,
0.4 mmol) to obtain the title product
(100 mg, 47%); riz/z = 521 [M-1]-.
-Page 151-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step H: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylarnino)-6-methylphenyl)-1(2,3-
dthydroxypropyl)cyc lopropane-1 -sulfonamide:
0 NH H F
1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methylphenyl)
cyclopropane-l-sulfonamide ( 150 mg,
5 0.29 mmol) and 4-methylmorpholine N-oxide (33 mg, 0.29 nunoI) was
dissolved in THF (5 mL). Osmium tetroxide was
added at room temperature (0.029 mmol, 0.18 mL, 4% in H20) and the reaction
mixture was stirred at room temperature
for 16 hours. Et0Ac was added, the organic phase was washed with water, dried
(MgSO4) and concentrated under
reduced pressure. The residue was purified over silica gel chromatography
(eluants: Et0Ac/ Me0H) to obtain the titled
product (0.110 g, 68 %). 'H-NMR (300 MHz, CDC13): 8 7.07 (m, 111), 6.97 (hr m,
211), 6.84 (m, 211), 6.60 (br m, 2H),
10 3.98 (hr m, 1H), 3.58 (m, 111), 3.43 (m, 1H), 3.20 (d, J= 3.9 Hz, 111),
2.42 (s, 311), 2.31 (dd, J= 9.9 & 15.6 Hz, 1H),
2.01 (br t, 1H), 2.31 (dd, J= 9.9 & 15.6 Hz, 1H), 1.66 (dd, J= 2.1 & 15.9 Hz,
1H), 1.52 (m, 1H), 1.40 (m, 1H), 0.91 (m,
2H).
Example 63
1-(2,3-Dihydroxypropyl)-N-(6-ethyl-3,4-difluoro-2-(2-fluoro-4-iodophenylamino)
phenyl) cyclopropane-1-
15 sulfonamide:
Step A: 1-(3,4.5-Trifluorophenypethanol:
101
An ethereal solution (17.41 ml, 52.24 mmol, 3M) of MeMgBr was slowly added at -
78 C to a solution of 3,4,5-
trifluorobenzaldehyde (6.96 g, 43.53 mmol) in THF (125 m1). The reaction
mixture was stirred at room temperature for
20 16h and was cooled (0 C) and was quenched, sequentially, with excess
ethyl acetate (10 ml) and water (5 ml). Excess
anhydrous MgSO4 (5 g) was added and stirred for 30 minutes at room
temperature. The suspension was filtered over
celite and the solids were washed with ethyl acetate (2x25 ml). The combined
filtrate was evaporated to obtain the
product in quantitative yield (7.65 g).
Step B: 5-(1-Bromoethyl)-1,2,3-trifluorobenzene:
Br 40
To a solution of the 1-(3,4,5-Trifluorophenypethanol: (7.65 g, 43.5 mmol) in
CH2C12 (250 ml), a solution of
thionyl bromide (18.1 g, 87 mmol) in CH2C12 (50 ml) was added slowly. The
reaction mixture stirred at room
temperature for 16h and poured into ice-water (200 m1). The organic layer was
separated and washed with saturated
NaHCO3 (2x200 ml), water (200 ml), dried (MgSO4) and evaporated to obtain the
corresponding bromo compound as a
pale yellow oil in quantitative yield (10.4 g). The crude product was carried
forward for the next reaction without further
purification.
-Page 152-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step C: 5-Ethyl- 1.2,3 -trifluorobenzene:
CH3
soi F
The above bromo compound (9.65 g, 40.4 mmol) was mixed with triethylsilane (41
mmol) and the reaction
mixture was treated with solid PdC12 (177 mg, 4 mmol) in small portions. After
a few minutes a vigorous exothermic
reaction was ensued and care was taken to reflux the contents of the flask by
placing a reflux condenser. The reaction
mixture was stirred at room temperature for additional 6h and the contents
were allowed to settle over 16h. Then the
crude liquid product was decanted carefully and carried forward for the next
reaction without further purification. It was
assumed tat the reaction proceeds in quantitative yield.
Step D: 1-Ethy1-3,4,5-trifluoro-2-nitrobenzene:
C Ei3 NO2
F
1,2,3-Tritluoro-5-methylbenzene (6.46 g, 40.4 mmol) was added to conc. H2SO4
(50 ml) at 0-5 C. Then the
reaction mixture was slowly treated with conc. 11NO3 (3.39 ml, 48.44 mmol,
90%) while maintaining the internal
temperature below 20 C. The reaction mixture was stirred at room temperature
for 16h and poured onto ice (300 g) and
the oily layer was extracted with CH2C12 (2x125 ml). The organic layer was
washed with water (2x200 ml), brine (200
ml) and dried (MgSO4) and evaporated to obtain the crude product which was
purified over flash silica gel
chromatography to obtain the title product (6.6 g, 79%). 1H NMR (CDCI3): 6
6.98 (septet, 1H), 2.68 (q, 2H), 1.26 (t, J=
7.8 & 7.2 Hz, 3H).
Step E: 3-Ethyl-5.6-difluoro-N-(2-fluoro-4-iodopheny1)-2 nitroaniline:
NO2 F
H
N 40
2-Fluoro-4-iodoaniline (2.05 g, 10 mmol) and 1-ethyl-3,4,5-trifluoro-2-
nitrobenzene (2.37 g, 10 mmol) were
reacted using the condition described in example 1 (Step A) to form the title
compound (2.47 g, 60%); ni/z = 407 [M-1]-.
Step F: 3-Ethyl-5,6-difluoro-N1-(2-fluoro-4-iodophenyDbenzene-1,2-diamine:
NH2 11
F 1
1,2,3-Trifluoro-5-methyl-4-nitrobenzene (2.47 g, 5.85 mmol) was reduced using
the condition described in
example 1 (Step B) to form the title compound. M-H+: 393
-Page 153-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step G: 1-Allyl-N-(6-ethy1-3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)cycloproparie-1-
sulfonamide:
crls,NH H F
N
111" F 11111111
According to the general procedure B, 1-allyl-cyclopropanesulfonyl chloride
(230 mg, 1.27 mmol) was reacted
with 5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-methylbenzene-1,2-diamine (100
mg, 0.255 mmol) to obtain the title
product (72 mg, 53%); m/z = 535 [M-1].
Step H: 1-(2,3-Dihydroxypropy1)-N-(6-ethy1-3A-difluoro-2-(2-fluoro-4-
iodophenylamino)phenyl)cyclopropane-l-sulfonamide:
v._7H
,p
d NH H F
110 1.1
1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methylphenyl)
cyclopropane-l-sulfonamide ( 70 mg,
0.13 mmol) and 4-methylmorpholine N-oxide (15 mg, 0.13 mmol) was dissolved in
THF (2 mL). Osmium tetroxide was
added at room temperature (0.013 mmol) 0.075 mL, 4% in H20) and the reaction
mixture was stirred at room
temperature for 16 hours. Et0Ac was added, the organic phase was washed with
water, dried (MgSO4) and concentrated
under reduced pressure. The residue was purified over silica gel
chromatography (eluants: Et0Ac/Me0H) to obtain the
titled product. 11-1 NMR (300 MHz, CDC13): 5 7.38 (dd, J= 2.1 & 10.8 Hz, 1H),
7.27 (m, 211), 7.12 (br s, 1H), 6.91 (dd,
J= 8.1 & 10.8 Hz, 1H), 6.69 (br s, 1H), 6.36 (dt, J= 4.8, 8.7 & 13.5 Hz, 1H),
4.00 (m, 1H), 3.62 (dd, J= 3.6 & 10.5 Hz,
1H), 3.47 (br m, 2H), 2.81 (q, 2H), 2.40 (dd, f= 10.2 & 15.9 Hz, 1H), 1.73 (br
m, 2H), 1.58 (m, 111), 1.43 (m, 1H), 0.94
(m, 2H).
Example 64
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-(2-methoxyethoxy)pheny1)-1-
(2,3-
dihydroxypropyl)eyelopropane-l-sulfonamide:
Step A: 1,2,3-Trifluoro-5-(2-methoxyethoxy)-4-nitrobenzene:
NO2
is
To a mixture of 3,4,5-trifluoro-2-nitrophenol (1.93, 10 mmol), Ph3P (3.93 g,
15 mmol), and 2-methoxy-ethanol
(1.18 ml, 15 mmol) in anhydrous THF (25 ml) a solution of diisopropyl
azodicarboxylate (2.91 ml, 15 mmol) in THF (5
ml) was added at 0 C and the reaction mixture was stirred at room temperature
for 16h. The volatiles were evaporated
and the residue was dissolved in C112C12 (100 ml) and the organic layer was
washed with water (100 ml), brine (100 ml)
dried (MgSO4) and evaporated. The residue obtained was purified over flash
silica gel chromatography to obtain the
titled product in 68% (1.70 g) yield. II-1NMR (300 MHz, CDC13): 5 6.78 (ddd,
J= 2.4, 6.0, 11.7 Hz, 111), 4.19 (t, J= 4.5
Hz, 2H), 3.72 (t, J= 4.5 Hz, 2H), 3.39 (s, 3H).
-Page 154-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Step B: 2,3-Difluoro-N-(2-fluoro-4-iodopheny1)-5-(2-methoxyethoxy)-6-
nitroaniline:
NO2 H F
N
111" F
2-Fluoro-4-iodoaniline (1.6 g, 6.8 mmol) and 1,2,3-trifluoro-5-(2-
methoxyethoxy)-4-nitrobenzene (1.7 g, 6.8
mmol) were reacted using the condition described in Example 1 (Step A) to form
the title compound (1.02 g, 32%); m/z =
467[M-l].
Step C: 5,6-Difluoro-N1-(2-fluoro-4-iodophenyl)-3-(2-methoxyethoxv)benzene-1,2-
diamine:
NH2 F
40 N
1
2,3-Difiuoro-N-(2-fluoro-4-iodopheny1)-5-(2-methoxyethoxy)-6-nitroaniline
(1.017 g, 2.17 mmol) was reduced
using the condition described in Example 1 (Step B) to form the title
compound; m/z = 337 [M-1].
Step D: 1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-(2-
methoxyethoxy)phenyl)cyclopropane-l-sulfonamide:
r
¨'NH H F
0
40 1,4 õI
According to the general procedure B,1-allyl-cyclopropanesulfonyl chloride
(450 mg, 2.5 mmol) was reacted with
5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-(2-rnethoxyethoxy)benzene-1,2-
diamine (219 mg, 2.5 mmol) to obtain the
title product (230 mg, 78%); m/z = 581 [MAL
Step E: N-(3,4-clifluoro-2-(2-fluoro-4-iodophenylamino)-6-(2-
methoxyethoxy)pheny1)-1-(2,3-
dihydroxypropyllcyclopropane-1-sulfonamide:
p
0 NH H
raw tai
F
1-allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-(2-
methoxyethoxy)phenybcyclopropane-l-sulfonamide
(230 mg, 0.395 mmol) and 4-methylmorpholine N-oxide (46 mg, 0.395 mmol) was
dissolved in THF (2 mL). Osmium
tetroxide was added at room temperature (0.039 mmol, 0.25 mL, 4% in H20) and
the reaction mixture was stirred at
room temperature for 16 hours. Et0Ac was added, the organic phase was washed
with water, dried (MgSO4) and
concentrated under reduced pressure. The residue was purified over silica gel
chromatography (eluants: Et0Ae/MeOH)
to obtain the titled product. 1HNMR (300 MHz, CDC13): 8 7.36 (dd, J= 1.8 &
10.5 Hz, 111), 7.27 (m, 2H), 6.56 (dd, J=
6.9 & 11.4 Hz, 1H), 6.40 (dt, J= 5.7, 7.5 & 12.9 Hz, 1H), 4.17 (m, 2H), 4.01
(m, 111), 3.78 (m, 2H), 3.60 (dd, 3.6 &
11.1 Hz, 1H), 3.47 (m, 111), 3.45 (s, 3H), 2.36 (dd, .1= 9.6 & 15.9 Hz, 111),
1.78 (dd, J= 2.4 & 15.6 Hz, 1H), 1.45-1.25
(m, 2H), 0.89 (m, 2H).
- Page 155 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Example 65
2,4-dichloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl) benzene
sulfonamide:
Ati GI
NH
* *
1
Synthesized by method A using the appropriate sulfonyl chloride, m/z = 571 [M-
1].
Example 66
2-chloro-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-4-
(trifluoromethyl)benzenesulfonamide:
õc 01
110
Synthesized by method A using the appropriate sulfonyl chloride, tn/z = 605 [M-
1].
Example 67
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyI)-2-(trifluoromethoxy)
benzene sulfonamide:
YP3
oi NH
01
1
Synthesized by method A using the appropriate sulfonyl chloride, m/z = 587 [M-
1].
Example 68
4-(N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)sulfamoyl)benzoic
acid:
F100C
0
!H
110 t
Synthesized by method A using the appropriate sulfonyl chloride, m/z = 584 [M-
11.
Example 69
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pbenyl)benzenesulfonamide:
40 p
,s,
oi NH H F
N 1101
Synthesized by method A using the appropriate sulfonyl chloride, m/z = 503 [M-
1].
-Page 156-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 70
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)plieny1)-2-fluorobenzene
sulfonamide:
Fo
,
Synthesized by method A using the appropriate sulfonyl chloride, m/z = 521 [M-
11.
General procedure D: substitution of the iodine atom:
A suspension containing 1 eq. aryl iodide, 1.5 equiv. of the boronic acid or
boronic ester, 0.25 eq. PdC12(dppf) x
DCM and 10 eq. anhydrous K2CO3 powder in a deoxygenated mixture of dioxane and
water (3:1) was heated in a
microwave reactor for 60 min at 115 C. It was extracted using aq. NH4C1/THF,
and the organic fraction was dried using
Na2SO4. The crude reaction products were purified using flash-column
chromatography (Si, Et0Ac/Hexanes, or
CHC13/Me0H). Yields: 20-40%.
Example 71
N-(3,4-difluoro-2-(2-fluoro-4-methylphenylamino)phenyDcyclopropanesulfonamide;
0, NH
110
CH3
General procedure D: 11-1.-NMR (500 MHz, CDCI3): = 7.38-7.36 (m, 1H), 7.06-
7.03 (q, 111), 6.92-6.90 (1H),
6.73-6.72 (d, 111), 6.63 (s, IH, br), 6.37-6.33 (t, 1H), 5.54 (s, 111, br),
2.42-2.39 (m, 1H), 2.25 (s, 3H), 1.14-1.11 (in, 211),
0.94-0.90 (m, 2H); m/z = 355 [M-1].
Where racemic mixtures of chiral compounds have been resolved into separate
enantiomers, the phrase
"substantially free" of the epimer, as used herein, means an enantiomeric
excess of at least 90%.
Example 72
N-(3,4-difluoro-2-(2-fluoro-4-(1H-pyrazol-4-yOphenylamino)phenyl)cyclopropane
sulfonamide
Step A: 2,3-Difluoro-N-(2-fluoro-4-iodopheny1)-6-nitroaniline:
NO2 H F
N
To a solution of 2-fluoro-4-iodoaniline (11.40 g, 47 mmol) in 100 ml anhydrous
TI-IF at 0 C, 47 ml of a
1M solution of LHMDS in TI-IF (47 mmol) was added dropwise. The color of the
solution turned dark purple.
The solution was transferred via cannula to a dropping funnel, and the
solution (containing the amine free base)
was added in small portions to a solution of 2,3,4-trifluoronitrobenzene
(8.321 g, 47.0 mmol) in anhydrous THF
(50 ml) at 0 C. After completion of addition the mixture was stirred under
argon at room temperature for 15
hours. The volume of the solvent was reduced, followed by extraction using
ethyl acetate and brine. The organic
layer was dried over sodium sulfate, the solvent was removed, and the obtained
dark oil was purified by flash
-Page 157-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
chromatography (Et0Ac / hexane 1:5, Rf = 0.58) yielding the crude product,
which became a brown solid upon
drying in vacua (yield: 6.23 g, 33.6%). m/z = 393 IM-11.
Step 8: 5,6-Difluoro-N1-(2-fluoro-4-iodophenyl)benzene-1,2-diamine
NH2 H F
N
F
To a solution of nitro-diarylamine (6.23 g, 15.8 mmoI) in 300 ml ethanol was
added iron powder (13.74 g,
246 mmol) and ammonium chloride (13.59 g, 254 nunol) and the mixture was
heated with stirring at 100 C oil
bath temperature for 14 hours. It was filtered and the residue washed two
times with ethanol. The ethanol was
removed in vacuo, and the residue was extracted using ethyl acetate / 1M NaOH
solution. During the extraction,
more precipitate was formed which was filtered and discarded. The combined
organic layers were washed with
brine and dried over sodium sulfate. The solvent was removed, and the crude
product was recrystallized from
CHC13 / hexane (1:50). The product was obtained as brown needles (2.094 g,
66%,). Rf = 0.44 (Et0Ac / Hex
1:3). 1H-NMR (500 MHz, CDC13): 8 = 7.40-7.38 (dd, 111, J= 11.3 Hz, J=1.5 Hz).
7.25-7.23 (d, 1H, J= 8.5
Hz), 6.97-6.92 (q, IH, J= 9 Hz), 6.51-6.48 (m, 1H), 6.24-6.21 (t, 1H, J= 9
Hz), 5.3 (s, 1H, NH, br), 3.80 (s, 211,
NH2, br); LRMS (EST): m/z = 365 jM+Hr.
Step C: N-(3A-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)cyclopropane
sulfonamide:
NHH F
40 N 40
According to the general procedure A, 5,6-difluoro-N1-(2-fluoro-4-
iodophenyObenzene-1,2-diamine was
reacted with cyclopropanesulfonyl chloride to obtain the desired product. (500
MHz, CDC13): 8 = 7.38-7.37 (d,
1H), 7.35-7.34 (m, 1H), 7.27-7.26 (m, 111), 7.20-7.0 (q, 1H), 6.68 (s, 1H,
br), 6.15-6.12 (q, 1H), 5.65 (s, 1H, br),
3.25-3.20 (m, 1H), 2.4-2.3 (m, 211), 2.0-1.8 (m, 2H); m/z = 467 [M-lf.
Step D: N-(3,4-difluoro-2-(2-fluoro-4-(1H-pyrazol-4-yflphenylamino)phenyl)
cyclopropanesulfonamide:
d NH H F
N
11151 F IN
General procedure C: 1H-NMR (500 MHz, CDCI3): 6= 8.00-7.90 (m, 2H), 7.30-7.20
(m, 2H), 7.15-7.10 (m, 1H),
7.05-7.00 (m, 111), 6.70-6.60 (m, 1H), 2.40-2.35 (m, 1H), 1.05-1.0 (m, 2H),
0.95-0.85 (m, 21-1); = 407 [M-11-.
Example 73
N-(3,4-difluoro-2-(2-fluoro-4-(1-methy1-1H-pyrazol-4-yl)phenylamino)phenyl)
cyclopropanesulfonamide
p
cr NH H F
N
-Page 158-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
General procedure C: 111-NMR (500 MHz, CDC13): S = 7.95 (s, 1H), 7.75 (s, 1H),
7.30-7.20 (m, 2H), 7.15-7.10
(m, 111), 7.05-7.00 (m, 111), 6.70-6.60 (in, 1H), 3.95 (s, 3H), 2.40-2.35 (m,
1H), 1.05-1.0 (m, 211), 0.95-0.85 (m, 211); m/z
=421 [A4-1T
Example 74
N-(3,4-difluoro-2-(2-fluoro-4-(1H-pyrazol-3-yl)phenylamino)phenyi)
cyclopropanesulfonamide
Z\P
dr'NH F
N
411111" F I \
N,N
General procedure C: 111-NMR (500 MHz, CDC13): 5 .= 7.90 (s, 111), 7.80 (s,
1H), 7.30-7.20 (m, 2H), 7.15-7.10
(m, 1H), 7.05-7.00 (m, 1H), 6.70-6.60 (in, 111), 3.95 (s, 3H), 2.40-2.35 (m,
1H), 1.05-1.0 (m, 2H), 0.95-0.85 (m, 211); m/z
=407 [M-11
Example 75
N-(3,4-difluoro-2-(2-fluoro-4-(pyridin-4-yl)phenylamino)phenyl)
cyclopropanesulfonamide
s,
6, NH H F
N
4111}111 F
General procedure C: 1H-NMR (500 MHz, CDC13): S = 8.62-8.61 (d, 211), 7.43-
7.41 (m, 411), 7.23-7.22 (m, 1H),
7.16-7.11 (q, 1H), 6.61-6.58 (t, 1H), 6.11 (s, 1H, br), 2.53-2.50 (m, 1H),
1.21-1.10 (m, 2H), 1.02-0.99 (m, 2H); m/z = 418
[114-1r.
Example 76
N-(3,4-difluoro-2-(2-fluoro-4-(pyridin-3-yl)phenyiamino)phenyl)
cyclopropanesulfonamide
Zs/P
0-NH F
0
N fith
111111" F 111111"
General procedure C: 111-NMR (500 MHz, [D6]-DMS0): 5 = 9.45 (s, 1H), 8.91 (s,
111), 8.54 (s, 1H), 8.07-8.06
(d, 1H), 7.76-7.70 (m, 2H), 7.46-7.34 (m, 2H), 7.34-7.33 (d, 2H), 6.80-6.78
(m, 1H), 0.86-0.79 (m, 4H); = 418 [M-1]-
Example 77
N-(2-(4-cyano-2-fluorophenylamino)-3,4-difluorophenypcyclopropanesulfonamide
Ap
s,
6, NH H F
1111 N 110
N
A suspension containing the aryl iodide (75.5 mg, 0.161 namol), CuCN (46.6 mg,
0.520 mmol and Pd(OAc)2 (0.47
mg) in 1 ml anhydrous DMF was heated to 130 it for 60 min. in a microwave
reactor. The mixture was extracted using
-Page 159-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
brine / THF, and the organic fractions were dried using Na2SO4. Subsequent
flash-column chromatography gave the
product as a dark red semi-solid (Rf = 0.42 (Et0Ac/Hexanes 1:1). Yield: 15 %.
m/z = 366 [M-1r.
Example 78
N-(3,4-difluoro-2-(3-fluorohipheny1-4-ylamino)phenyl)cyclopropanesulfonamide
AP
rNH
40 N 40
110
General procedure C: 1H-NMR (500 MHz, CDC13): 5 = 7.55-7.53 (m, 2H), 7.45-7.3
(m, 5H), 7.20-7.15 (d, 1H),
7.13-7.10 (q, 1H), 6.70 (s, in, br),6.60-6.55 (t, 1H), 5.75 (s, 1H, br), 2.53-
2.50 (m, 1H), 1.21-1.10 (m, 2H), 1.02-0.99 (m,
2H); m/z = 417 [M-1]-.
Example 79
N-(2-(3'-acety1-3-fluorobipheny1-4-ylanitino)-3,4-difluorophenyl)
cyclopropanesulfonamide
,s-
0,NHH F
N
111111)1 F till}111 0
1.1
General procedure C: 1H-NMR (500 MHz, CDC13): ö = 8.6 (s, 1H), 7.86-7.85 (d,
111), 7.68-7.66 (d,1H), 7.49-7.46
(t, 1H), 7.38-7.33 (m, 211), 7.20-7.18 (d, 1H), 7.09-7.03 (q, 111), 6.90 (s,
1H, br), 6.57-6.54 (t, 1H), 5.90 (s, 1H), br), 2.61
(s, 3H), 2.46-2.43 (m, 1H), 1.15-1.13 (m, 2H), 0.94-0.91 (m, 2H); m/z = 459 [M-
1].
Example 80
N-(2-(4'eyano-3-fluorobiphenyl-4-ylamino)-3,4-difluorophenyi)
cyclopropanesulfonamide
L\it
dr'NH
101 1101
General procedure C 1H-NMR (500 MHz, CDC13): 5 = 7.68-7.66 (m, 2H), 7.58-7.57
(m, 2H), 7.38-7.35 (m, 2H),
20 7.20-7.18 (d, 1H), 7.18-7.02 (q, 1H), 6.67 (s, 1H, br), 6.58-6.54 (t,
1H), 5.99 (s, 1H, br), 2.47-2.44 (m, 1H), 1.15-1.13 (m,
2H), 0.94-0.91 (m, 2H); mlz 442 [M-1T.
Example 81
N-(2-(3,4'-difluorobipheny1-4-ylamino)-3,4-
difluorophenyl)cyclopropanesulfonamide
o
0/ NH H F
N
F
110
-Page 160 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
General procedure C: 11-1-NMR (500 MHz, CDC13): 8 - 7.44-7.37 (m, 3H), 7.29-
7.27 (d,111), 7.11-7.05 (m, 411),
6.70 (s, 1H, br), 6.53-6.50 (t, 111), 5.81 (s, 1H, br), 2.47-2.44 (m, 1H),
1.15-1.13 (m, 211), 0.94-0.91 (m, 211); m/z = 435
IM-if.
Example 82
N-(3,4-difluoro-2-(3-fluoro-4'-(methylsutfonamido)bipheny1-4-ylamino)phenyl)
cyclopropanesulfonamide
0
0, NH H F
N
General procedure C: 1H-NMR (500 MHz, [D61-DMS0): = 9.39 (s, 1H, br), 7.63-
7.60 (m, 3H), 7.53-7.50 (d,
111), 7.30-7.23 (m, 411), 7.74-7.65 (m, 1H), 2.99 (s, 311), 0.80-0.73 (m, 4H);
rnlz = 510 (M-1]-.
Example 83
N-(3,4-difluoro-2-(2-fluoro-4-methylphenylamino)phenyl)cyclopropanesulfonamide
________________________________________ P
S,
6/ NH
11
FO
CH
General procedure C: 11-1-NMR (500 MHz, CDC13): 6 = 7.38-7.36 (in, 111), 7.06-
7.03 (q, 1H), 6.92-6.90 (1H),
6.73-6.72 (d, 1H), 6.63 (s, 111, br), 6.37-6.33 (t, 111), 5.54 (s, 1H, br),
2.42-2.39 (m, 1H), 2.25 (s, 311), 1.14-1.11 (m, 2H),
0.94-0.90 (m, 2H); m/z = 355 IM-1T.
Example 84
4'-(6-(cyclopropanesulfonamido)-2,3-difluorophenylamino)-3'-fluorobipheny1-3-
carboxylic acid
Ap
ci NH H F
fai N
41111111 F 11111F1 OH
is 0
General procedure C: 11-1-NMR (500 MHz, [D41-Me0H): = 8.21 (s, 1H), 7.93-7.91
(d, 111), 7.73-7.72 (d, 1H),
7.47-7.43 (m, 211), 7.33-7.31 (d, 2H), 7.15-7.12 (q, 1H), 6.71-6.68 (m, 1H),
2.51-2.46 (m, 1H), 0.94-0.93 (In, 2H), 0.88-
0.87 (m, 2H); m/z = 499 [MAT.
Example 85
N-(3,4-difluoro-2-(3-fluoro-3'-(methylsulfonamido)bipheny1-4-ylamino)phenyl)
cyclopropanesulfonamide
_____________________________________ 0
NH H F
N
41111111 F 111111}.1111
Nx-
F O' 0
General procedure C: 'H-NMR (500 MHz, [D4]-Me0H): 8 = 7.92 (s, 1H), 7.46-7.34
(m, 51-1), 7.34-7.31 (d, 1H),
7.29-7.22 (m, 1H), 7.16-7.15 (q, 111), 6.74-6.71 (m, 111), 2.80 (s, 311), 2.54-
2.51 (m, 1H), 0.94-0.92 (m, 2H), 0.91-0.90
(m, 2H); m/z = 510 [M-l].
-Page 161-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 86
N-(3,4-difluoro-2-(3-fluoro-2'-(methylsulfonamido)bipheny1-4-ylamino)phenyl)
cyclopropanesulfonamide
01 NH H p
40 hiN?S
F
General procedure C: 11-1-NMR (500 MHz, [D4]-Me0H): ö= 7.50-7.49 (d, 1H), 7.40-
7.32 (m, 4H), 7.29-7.28 (d,
111), 7.26-7.10 (m, 2H), 6.73-6.71 (m, 1H), 2.80 (s, 3H), 2.51-2.49 (m, 111),
0.94-0.92 (m, 211), 0.91-0.90 (m, 2H); nilz =
510 [M-lf.
Example 87
N-(3,4-difluoro-2-(3-fluoro-W-(trifluoromethoxy)biphenyl-4-ylamino)phenyl)
cyclopropanesulfonamide
A ,o
%0P/--NH 1.4 F
4111111..1-11P ,CF3
0
10
General procedure C: 11-1-NMR (500 MHz, [D4]-Me0H): 6 = 7.69-7.67 (d, 2H),
7.46-7.43 (d, 1H), 7.36-7.33 (m,
4H), 7.30-7.29 (q, 1}1), 6.73-6.72 (m, 1H), 2.51-2.49 (m, 1H), 0.94-0.92 (m,
2H), 0.91-0.90 (m, 2H); m/z = 501 [M-1T.
Example 88
N-(3,4-Difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)-2-(methylamino)
ethanesulfonamide
9
F
HO 1
101
15
General procedure D. 111 NMR (300 MHz, CDC13): 5 9.01 (br s, D20
exchangeable, 111), 7.36 (dd, J= 2.1 & 10.5
Hz, 1H), 7.27 (m, 1H), 7.17 (m, 1H), 7.03 (dd, J= 9.0 & 16.8 Hz, 1H), 6.48 (s,
D20 exchangeable, 1H), 6.31 (dt, J= 3.0,
8.7 & 17.4 Hz, 1H), 3.45 (br t. 2H). 3.31 (br s, 211), 2.65 (s. 311). 1.80(br
s, D20 exchangeable, 1H).
Example 89
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-2-(2-
(dimethylamino)ethylamino)
20 ethanesulfonamide
9
F
H 0 N
40 40
General procedure D. 1H NMR (300 MHz, CDC13): 6 7.35 (m, 1H), 7.25 (m, 111),
7.18 (d, J= 8.7 Hz, 1H), 7.02 (dd,
J= 8.7 & 18.0 Hz, 1H), 6.38 (tn, 1H), 6.18 (dd, J= 8.7 & 17.1 Hz, 111), 3.62
(t, J=5.7 & 6.3 Hz, 2H), 3.35 (m, 2H), 3.26
(m, 2H), 3.26 (t, J= 5.7 & 6.6 Hz, 211), 3.11 (t, J= 5.1& 6.0 Hz, 211), 2.85
(s, 61-1).
-Page 162-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Example 90
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl)-2-(ethyl(methyDamino)
ethanesulfonamide
9
H F
0 N
General procedure D. II-1 NMR (300 MHz, (CDC13 D20)): 8 7.39 (dd, J= 1.5 &
10.5 Hz, 1H), 7.31 (m, 2H), 7.07
(dd, J = 9.0 & 17.4 Hz, 111), 6.30 (dl, J = 2.4, 9.0 & 17.4 Hz, 1H), 3.55 (t,
J = 6.9 & 7.8 Hz, 2H), 3.38 (br t, J= 6.0 & 8.7
Hz, 2H), 3.05 (q, 21-1), 2.69 (s, 3H), 1.31 (t, J= 7.2 Hz, 311).
Example 91
N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-2-(4-methylpiperazin-1-
y1) ethanesulfonamide
H F
101
General procedure D. II-I NMR (300 MHz, CD30D): ö 7.45 (dd, J= 2.1 & 10.8 Hz,
1H), 7.30 (m, 2H), 7.16 (dd, J
= 9.6 & 17.7 Hz, 1H), 6.39 (dt, .1= 3.3, 9.3 & 17.7 Hz, 1H), 3.26 (m, J= 7.5
Hz, 211), 3.10 br m, 6H), 2.87 (s, 311), 2.82
(t, J= 7.5 Hz, 2H), 2.48 (br in, 4H).
In vitro Biological Activity
Example 92
Generation of 1050 Data
Materials and preparation of reagents: Human GST-MEK1 and the constitutively
active allele GST-MEK14A
(harboring the mutations Ser218Asp and Ser222Asp) were subcloned into the
yeast expression vector pGEM4Z
(Promega, Madison, WI) from the wild type human MEK1 cDNA. GST-MEK1cA was
expressed in Escherichia coli and
partially purified using Glutathione Sepharose 4B affmity resin (Amersharn
Pharmacia Biotech, Piscataway, NJ). The
ERK2 allele was subcIoned from MAPK2fErk2 cDNA (wild type) in pUSEamp (Upstate
Biotechnology, Inc., Waltham,
MA) into the vector pET2la (Novagen, Madison, WI) resulting in an N-terminal
histidine-tagged mouse ERK2 allele.
ERK2 was expressed and purified to homogeneity [Zhang, 1993 #33]. Myelin basic
protein (MBP) was purchased from
Gibco BRL (Rockville, MD). EasyTides adenosine 5'-triphosphate (ATP) ([y-3311)
(NEN Perkin Elmer, Wellesley, MA)
was the source of radiolabel for all kinase reactions. Activated Raf-1
(truncated) and activated MAPKinase 2/ERK2
were purchased from Upstate, Inc. (Lake Placid, NY). 4-20% Criterion Precast
gels were purchased from Bio-Rad
(Hercules, CA).
Determination of enzymatic activity: Compounds were diluted from
dimethylsulfoxide (DMSO) stocks into
lxHMNDE (20 mM HEPES pH 7.2, 1 mM MgC12, 100 mM NaC1, 1.25 mM DTT, 0.2 mM
EDTA). A typical 25-
microliter assay contained 0.002 nanomoles MEK1 CA, 0.02 nanomoles ERK2, 0.25
nanomoles MBP, 0.25 nanornoles
unlabeled ATP, and 0.1 uCi [y3313] ATP. The screening assay essentially
comprised four additions. Five ul of diluted
compound were dispensed to 96-well assay plates. Ten ul of 2.5x enzyme
cocktail (MEK1cA and ERK2 only) were then
added to each well followed by a pre-incubation for 30 minutes at ambient
temperature. Ten ill of 2.5x substrate cocktail
(labeled and unlabeled ATP plus MBP) were then added, followed by incubation
for 60 minutes at ambient temperature.
-Page 163-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Finally, 100 ul of 10% trichloroacetic acid (TCA) were added and incubated for
30 minutes at room temperature to halt
the reaction and precipitate radiolabeled protein products. Reaction products
were harvested on glass fiber 96 well filter
plates prewetted with water and 1% pyrophosphate. The filter plate was then
washed 5 times with water. Water was
displaced by absolute ethanol and the plate was allowed to air dry for 30
minutes at room temperature. A back seal was
applied manually and 40 pi of scintillation cocktail were dispensed to each
well. A top seal was applied and the plate
was counted in the TopCount for two seconds per well.
For certain experiments a truncated version of MEK that requires activation by
Raf kinase were used.
Example 93
Generation of EC50 Data
Effects of compounds in the cell were determined by Western blotting for
phosphorylated ERK. MDA-MB-231
breast cancer cells were plated in a 48 well plate at 20,000 cells per well
and grown in a 37 humidified CO2 incubator.
The following day, the growth media (DMEM + 10% fetal bovine serum) was
removed and replaced with starve media
(DMEM + 0.1% fetal bovine serum). Cells were incubated in the starve media for
sixteen hours and then treated with a
range of compound concentrations for thirty minutes. After incubation with
compound, cells were stimulated with
10Ong/m1EGF for five minutes. The cells were then lysed and analyzed by
Western blot using a monoclonal antibody
raised to phosphotylated ERK. The signal was amplified using a secondary
antibody conjugated to a near -IR dye and
detected on a Licor Odyssey scanner. The intensity of signal was quantitated
and this data was used to generate dose
response curves and EC50 calculations.
Legend: A, EC50 = < 2.0nM; B, EC50 = 2.0-15nM; C, EC50= 15nM-100nM;
D, EC50 > 100nM, IC50 < 201.tM; F, EC50 > 100nM, IC50 > 201.tM
Compound Activity Compound
Activity
Number Structure pAl Number Structure
p114
1000 =,.. if A 1004 &I
C
0e-,NH F N
0 NH
0 NH 0
F I F H
00 100
F I
F
1001 Zec) A 1005 <3...õ .
c
/,
0 NH S
// N
40 8 40
r 1 0 N H
NH
F 1401 101
F I
1002 JP B F
1006
i
<1 hip
C
0 N f
NH
10, 0 4 N
0 NH
F i NH
1003 C 00 40
..--------,g F I
d/ NH
, a
1007
C
si
F 0 NH
Olt N IS
F I
F
-Page 164-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Compound Activity Compound
Activity
Number Structure , IIM Number , Structure
10080 cy C 1017 o B
V. e i
pip' NNH
H
140 40 NH
4 0
F 1
F I
F - F
1009
.
C 1018
HO A
1 1.,
(Racemic) HO
(3 1
NH F 0
//
NH 5
0 NH
NH F
F I 0 0
- F I
10100
It A F
orz..--SN 1019 OH A
I NH Hoõ........_b...
(Racemic)
zo NH
0 I.1
F Jo
0/7('NH F
,--U 40 NH so
F I
I-
1020 HO A
1011 ___(-1 , jo C (Racemic)
`.13.'=
FN
0 NH F
NH
40 01
\NH F
F
1012 0 F N
`9,/,' F B 001 10
F 1
r
0 NH
NH 1021
C HO,. j&11 A
001 *
F I (S isomer)
Compound A
4"
F 0 11 F
1013 6.6(/
o B .--.41)
NH
// N 40 110
F I
0 NH
.........= 40 NH 0 1022 pH B
HO ;
F
(R isomer)
I ''----- e
F
Compound B
4 N
1014 C 0 NH
SI /
- 0 am NH so
4-,
0 NH "IP F I
los NH 401
1023 H) F B
itc/Hro.,õ,
I (R isomer)
1015 F
* D
0._
D NH F NH F
N
0 * F 40 NH *I
F I F I
F
1016 C
0,49
'".
o NH
osi NH ail
r i
-Page 165-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Compound Activity Compound
Activity
Number Structure tiM Number Structure
11M
1024 rio B 1031 oH A
Fii) i
(S isomer)
o'"--
NH,---
1.1 F 40 10
NH
40 10 F F 1
F 1
F
1025 ca, R B
/7N-
0 NH F
---0 ....... NH
..,.....,
F I
1026 :: IA A
o--;--
11 F
0 NH
.--= I. * I
F
1027 z:scsoH A
cy.--,...s.-:--0
\
N F
NH
140 10
F 1
F
1028 OH OH A
H r
o H
, i 0 401
F I
F
1029C
d'.... \
F
ila NH 401
1/1111j F I
F
1030 0H C
1-10 0
I"
0';'? \
NH F
......c...",õ0
iiih Nil Ali
4111111111 F 11111)11 I
F
-Page 166-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Legend: A, EC50 = < 2.0 nM; B, EC50= 2.0-15 nM; C, EC50= 15 nM-100 nM;
D, EC50 =100 nM ¨200 nM; E, EC50 > 200nM; ND = not yet determined
_
MBA I
MDA
pERK
pERK
ELISA
ELISA
CPD # Structure EC50 CPD # Structure
EC50
¨
0497618 A, 0 E 0499268ND
r-A /0
- NH F 0- '1,ii-{F
H H
N
0 10 it. N Akii
F I \,N 4" F ll111"
F N F 0
H F
0497620 A. o E 0499271E
,...S'''
µ0' NH FCNNH r F
40 N. 40 H
F
I \ N N
40 0 0 9
F
I N-S-
H H"
0 AD -
0530701 o.,-,5õ
o D
0 NH F \---s NH
14 F
H
N
SO N \ 10
0 I
FCI A-SiNi F F
I \
F N--N-
H 0530716 A5,0 ND
0497688 0 E cr ''N1-1 I-1 F
0 10
F H 9
so N-g¨
j ''NH H F
0 N I.
F F
0530717 ,L.0 ND
F .-- N
I 0" ''NHH F
0497689 A E N
40 40
(-,eD F a H F
- NH F 4111r
0
N 0 0561599 - 0., o ocF,
C
F r)SH F
F I 141,,,,-- H
N
N
0497692 A, 0 E 101 101
F I
r,
- -NNH H F F
N
1401 101 di 0561608 o.,...õ.õ0
C
F
NIP NH N-- H F
N
F Me00C Si 1401
0499266 A...,s,...0 E F I
F
Cr '''NH F
H 0620926 meooc NIrO E
N
0 "NH H F
F N
0499267 A, 0 ND F I
(:)...S4'.'NH F
F
H
N
0
F iii
F
4111r
CN
-Page 167-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
MDA
pERK
ELISA
CPD # Structure EC50
0620927
NH H F
S N
01
0621002 0.zsel.0
HNI1 so
0621016
'1\111
1--rly
41111r F 4111111friP
0621026
Nr NH Ft F
dmh N so
1111111 F 1
0621029 D
Lj" NH Li F
`11 le 40
0621030 oo ND
I NH F
- Page 168 -
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
In vivo Biological Activity
Example 94
The compounds and compositions described herein are useful for the treatment
or prophylaxis of one or more
diseases including but not limited to cancer, inflammatory bowel disease
(IBD), psoriasis and rheumatoid arthritis (RA).
The compounds and compositions described herein are also useful for the once-
or twice-daily oral treatment or
prophylaxis of one or more diseases including but not limited to cancer, IBD,
psoriasis and RA.
In vivo tests of the compound of the structure below (Compound A, prepared as
described herein), are described in
HO Ott
IV? 0
0, NH F
Me0 46
11111111' f
this example:
Human tumors were implanted in nu/nu mice. Compound A was administered orally
for 14 days once tumors
were approximately 100 mm3 in size. Tumor growth inhibition (TGI) was
determined after 14 days of treatment as the
reduction in the size of tumors in treated groups versus vehicle controls. The
time to endpoint (TTE) was calculated as
the time for the tumor to reach the specified endpoint volume or the last day
of the study, whichever came first.
Treatment outcome was determined from percent tumor growth delay (%TGD),
defined as the percent increase in median
TTE of treated versus vehicle-treated control mice. Animals were also
monitored for regression responses. Levels of
pERK in tumors and brain were determined by Western blots and correlated with
plasma levels of Compound A for the
pharrnacodynamic/pharmacolcinetic study. A number of tumor models were
evaluated with different doses and dosing
regimens. Treatment with 25 or 50 mg/kg once daily (QD) showed statistically
significant %TGD in A375 melanoma
tumors, Co1o205 colon cancer tumors, and A431 epidermoid tumors. Statistically
significant TGI was observed for oral
dosing at 25 mg/kg QD for these tumor models as well as in HT29 colon cancer
tumors. The effect of different dosing
regimens was evaluated in A375 xenog,rafts. Although 100 mg/kg Compound A
given orally once every two days
showed statistically significant %TGD (91%), it was not as effective as QD
treatments at 25 mg/kg (143% TGD) or 50
mg/kg (233% TGD). Twice daily (BID) dosing was also more effective than QD
dosing as measured by %TGI. Dosing
at 12.5 mg/kg BID resulted in 79.5% TGI compared to 51.7% for 25 mg/kg QD of
Compound A. Dosing at 25 mg/kg
BID resulted in 110.1% TGI compared to 69.9% TGI for 50 mg/kg QD. A
pharmacodynamic/phannacokinetic study in
Colo205 xenografts show inhibition of pERK formation in tumors while minimal
inhibition was observed in brain
suggesting potent anti-tumor activity with limited CNS penetration.
Compound A is a potent inhibitor of MEK1/2 that suppresses tumor cell growth
in vitro and in vivo. BRAF status
determines sensitivity to growth inhibition by the compound in anchorage-
dependent growth but not anchorage-
independent growth or in xenografts. Maintaining adequate MEK inhibition
throughout the dosing interval appears to be
more important than peak levels due to the greater efficacy with more frequent
dosing. Compound A has a favorable pk
profile in humans, with the projected therapeutic dose, based on xenograll
results, of 20-40mg/day in humans.
Example 94A
Inhibition of Cancer Cell Growth (61150)
Anchorage-dependent growth inhibition was measured using CellTiterGlo reagent
after 48 hr treatment with
Compound A of cells grown in 384-well plates. Anchorage- independent growth
assays used MTS
(methanethiosulfonate) reagent after 7 days treatment of cells grown in media
containing 0.15% agarose or on non-
binding plates (A431). Growth inhibition values (GI50) are shown in the table
below.
Tumor Cell Line BRAF Anchorage-Dependent GI50 Anchorage-
Independent G150
-Page 169-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
status (TIM sd) (nM sd)
A375 Melanoma _ V600E 67 12 68 34
Co1o205 Colon WOOF 74 45 33 16
HT29 Colon V600E 70 12 Not determined
A431 Epidermoid Normal _ >10,000 65 19
Example 94B
Anti-Tumor Xenograft Activity
Female nu/nu mice were implanted with A375 Melanoma, Co1o205 Colon Tumor, A431
Epidermoid Tumor or
HT-29 Colon Tumor cells, which were allowed to grow to 100-200 mm3. Compound A
or vehicle was administered
orally (25mg/kg, 50mg/kg or 100mg/kg), once a day, for 14 days. Average tumor
volumes were graphed for vehicle and
treated groups and are shown in Fig. 1.
Example 94C
Tumor Growth Inhibition (TGI) 25 mg/kg QD
Tumor Growth Inhibition for the groups treated with 25 mg/kg Compound A were
calculated for the indicated
tumor xenografts. Tumor Growth Inhibition was measured at the end of once
daily dosing for 14 days and calculated
according to:
%TGI = 100 x 1 - (treated tumor volume fõ,õ1¨ tumor volu_i_mne. aw)
(vehicle treated tumor voltunefõ,õµ ¨ tumor volumeinitio)
The range for A375 and Colo205 represent values from 2 separate studies.
Tumor Xenograft % TGI P value
A375 Melanoma 52-72** <0.001
Co1o205 Colon 70-123** <0.001
HT29 Colon 56 <0.001
A431 Epidermoid 67 <0.001
**Regressions noted during course of experiment
Example 94D
ED50 in Colo205 Xenografts
Male nu/nu mice were implanted with Co1o205 tumor cells. After 10 days animals
were randomized by tumor size
(range 126-256 mm3) and treated with paclitaxel (IV, Q0Dx5), vehicle or
Compound A (PO, QDx14).
Pharmacokinetic parameters were obtained from dosing Balb/c mice with 25 mg/kg
Compound A and
extrapolating values for the lower dose groups and shown in the table below.
Initial Day 15
Tumor Tumor
Treatment Regimen Volume Volume % Cõõ,x Cm,õ
AUC
Group n Agent mg/kg (mm3) (mm3) TGI (.1g/mL) (pg/mL) (ug-hr/mL)
1 10 Vehicle 185 11.1 2093 174
2 10 Paclitaxel 30 184 9.8 113 9.6 104*
3 10 2.5 184 9.8 1187 127 47* 0.99 0.003
5.5
4 10 Compound 5 183.8 9.8 1175 104 48* 1.97 0.006
11.0
5 10 A 10 185.1 11.7 1045 160 55* 3.94 0.012
22.0
6 10 25 185.1 11.7 762 81 70* 9.85 0.029 55.0
*P<0.001
Example 94E
Tumor Growth Inhibition with A375 Xenografts
A375 Xenograft mice were administered Compound A 50mg/kg QD, 25mg/kg BID,
50mg/kg QD and 12.5mg/kg
BID. The VoTGI was calculated and graphed and is shown in Fig. 2.
-Page 170-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Example 94F
Plasma Concentrations in Mice
Female nu/nu mice were implanted with A375 tumor cells, which were allowed to
grow to 100-200 mm3.
Compound A or vehicle was administered orally once a day (QD) or twice a day
(BID) (50mg/kg QD, 25mg/kg BID,
50mg/kg QD and 12.5mg/kg BID). Tumor Growth Inhibition was measured at the end
of once daily dosing for 14 days
and calculated according to:
%TGI = 100 x 1 - (treated tumor volumefioi¨ tumor volumemataa
(vehicle treated tumor volumefium ¨ tumor volumeg)
AUC (ughr/m1) 132.5 117.0 66.5
78.0
Cff,õõ (ug/m1) 23.8 10.2 11.9 7.8
C,õõ, (fig/m1) 0.06 1.24 0.03
0.49
Cõ,in Free Fraction (ng/ml) 0.117 2.48 0.059 0.986
Statistical Significance = Logrank test
Example 94G
Mouse Xenograft Tumors and Inhibition of Brain MEK Activity
Female nu/nu mice implanted with Co1o205 tumor cells were given a single dose
of vehicle or Compound A at 2.5,
5, 10, or 25 mg/kg. Compound levels were determined in plasma samples and pERK
levels were determined in tumor
and brain samples collected at 2, 6, 12, and 24 hr post-dose. The pERK levels
from Western blots were quantified using
the LI-COR Odyssey, normalized to total ERK levels and compared to vehicle-
treated levels to determine % MEK
inhibition. MEK inhibition in tumor or brain for each mouse was graphed with
the corresponding plasma concentration
of Compound A in the animal. Non-linear regression gave an EC50 of 73nM for
MEK inhibition in tumors. The brain
EC50 was >5000 nM.
A graph of plasma concentration (log nM) against pERK % inhibition is shown in
Fig. 3.
Preparation of capsules
Example 95A
Blue size 1 hard gelatin capsules were prepared containing a dry powder blend
composition in 1 mg and 10 mg
HO4H
,St
0' NH F
N
F 4111-1-111
strengths of Compound A (see table shown in example 93 above) of structure:
Compound A was prepared as described herein, and then micronized using a fluid
energy mill (Spiral Jet Mill,
electronically grounded, with a grinding chamber diameter of 50mm; a 500. 4 x
0.8mm nozzle ring; an injector nozzle
diameter of 0.8mm and injector nozzle distance of 3mm).. Compound A and a
portion of the microcrystalline cellulose
were mixed and screened through a #20 mesh screen and added to a diffusion-
tumble blender (V-blender). The remaining
Microcrystalline Cellulose was screened through a #20 mesh screen, added to
the materials in the blender and blended.
The Croscarmellose Sodium and Sodium Lauryl Sulfate were screened through a
#20 mesh screen, added to the materials
in the blender and blended. The powder blend was passed through a rotating
impeller mill (Quadro CoMil) and added
back to the blender and blending continued. The Magnesium Stearate was
screened through a #20 mesh screen and
blended with the milled powder blend. The powder blend was filled into size 1
capsules. The 10 mg capsules were
banded for identification.
The composition of the capsules is shown in the table below:
Component 1 mg capsule _ 10 mg capsule I
-Page 171
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
mg/unit % mg/unit (1/0
Compound A 1.0 0.4 10.0 4.2
Microcrystalline Cellulose, NF (Avicel PH302) 222.2 92.6 213.2
88.8
Croscarmellose Sodium, NF (Ac-Di-Sol) 12.0 5.0 12.0 5.0
Sodium Lauryl Sulfate, NF 2.4 1.0 2.4 1.0
Magnesium Stearate, NF 2.4 1.0 2.4 1.0
Totar 240.0 100.0 240.0
100.0
Blue Size 1 Hard Gelatin Capsule Shell 1 1
'Target fill weight adjusted based on actual potency of blend.
Typical batch formula for a 10,000 batch of 1 mg capsules were as follows:
Quantity per batch (g)
Batch Formula Components
(for 10,000 units)
Compound A 10.0
Microcrystalline Cellulose, NF (Avicel PH302) 2222
Croscarmellose Sodium, NF (Ac-Di-Sol) 120.0
Sodium Lauryl Sulfate, NF 24.0
Magnesium Stearate, NF 24.0
Total Fill Weight'. 2400
Blue Size 1 Hard Gelatin Capsule Shell 10,000
'Target fill weight adjusted based on actual potency of blend.
Typical batch formula for a 10,000 batch of 10 mg capsules were as follows:
Quantity per batch (g)
Batch Formula Components
(for 10,000 units)
Compound A 100.0
Microcrystalline Cellulose, NF (Avicel PH302) 2132
Croscarmellose Sodium, NF (Ac-Di-Sol) 120.0
Sodium Lauryl Sulfate, NF 24.0
Magnesium Stearate, NF 24.0
Total Fill Weight' 2400
Blue Size 1 Hard Gelatin Capsule Shellb 10,000
'Target fill weight adjusted based on actual potency of blend.
Example 95B
Blue size 1 hard gelatin capsules are prepared containing a dry powder blend
composition in 1 mg and 10 mg
HO pH
111`. 0
,NH S*
1.1 F
Me= N
40 40
strengths of Compound B (see table shown in example 93 above) of structure:
Compound B is prepared as described herein, and micronized using a fluid
energy mill (Spiral Jet Mill,
electronically grounded, with a grinding chamber diameter of 50min; a 500. 4 x
0.8mm nozzle ring; an injector nozzle
diameter of 0.8mm and injector nozzle distance of 3mm).. Compound 13 and a
portion of the microciystalline cellulose
are mixed, screened through a #20 mesh screen and added to a diffusion-tumble
blender (V-blender). The remaining
Microcrystalline Cellulose is screened through a #20 mesh screen, added to the
materials in the blender and blended. The
Croscarmellose Sodium and Sodium Lauryl Sulfate are screened through a #20
mesh screen, added to the materials in the
blender and blended. The powder blend is passed through a rotating impeller
mill (Quadro CoMil), added back to the
blender and blending continued. The Magnesium Stearate is screened through a
420 mesh screen and blended with the
milled powder blend. The powder blend is filled into size 1 capsules. The 10
mg capsules are banded for identification.
The composition of the capsules is shown in the table below:
Component j 1 mg capsule 10 m
ca sule
-Page 172-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
mg/unit % mg/unit %
Compound B 1.0 0.4 10.0 4.2
_
Microcrystalline Cellulose, NF (Avicel PH302) 222.2 92.6 213.2
88.8
Croscarmellose Sodium, NF (Ac-Di-Sol) 12.0 5.0 12.0 5.0
_
Sodium Lauryl Sulfate, NF 2.4 1.0 2.4 1.0
,
Magnesium Stearate, NF 2.4 1.0 2.4 1.0
Total' 240.0 100.0 240.0
100.0
' Blue Size 1 Hard Gelatin Capsule
Shell 1 1
In vivo Activity in Humans
Example 96
Administration of the capsules described in example 95A in Human Cancer
Patients
Human cancer patients were administered a single dose of the 1 mg or 10mg
capsule composition described above
in example 95A. For a 2mg dose, patients were given 2 x lmg capsules; for a
4mg dose, patients were given 4 x lmg
capsules; for a 6mg dose, patients were given 6 x lmg capsules; for a 10mg
dose, patients were given 1 x 10mg capsule;
for a 20mg dose, patients were given 2 x 10mg capsules.
The concentration-time profiles were monitored and are shown in Fig. 4 and in
the table below:
Dose D ay T . C Cl2hr AUC0-12hr AUCT
(hr)
(mg) (rig/mL) (11g/1111-) (ng=hr/mL)
(ngthr/mL)
2 1 2.0 0.111 0.0378 0.700 NA
,
35 - 2.0 0.202 0.0756 NA 2.07
_
4 1 1.5 0.292 0.134 - 2.26 NA
35 1.0 0.544 0.310 NA 5.12
35 NA 1.57 1.01 NA 14.3
35 NA 3.28 2.19 NA _ 29.5
Crystalline Polvmorob Forms
Example 97: Preparation of N-(3.4-difluoro-2-(2-fluoro-4-
iodophenylamino)pheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide
N-(3.4-difluoro-2-(2-fluoro-4-iodophenylamino)pheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide
was prepared according to previously described procedures (see published
international patent application WO
2007/014011) and as outlined below.
NO2 F NO2 H F NO2 H F NH2 H
F
F it F +H2N it LHMDS F ril N Ai Na0Me Me0 iii N iii H2 Me0 N
Fiti
4111111)V. F 411111friP I 4111113-1 F
111111)-IP I 1111"1 F 41111111)-111
F F F F
.-------7 -ID 0 -7H
HO..õ.õ--10
õ---..Y. ..0 --- - NNH F 0- NNH F
/ , H H
0-SCci Me0 di N 0s04 Me0 di N AI
411111fril F 41111"1-1. I 11111111-1111 F
1111111" I
F F
Step A: 2-Fluoro-N-(2,3,5-trifluoro-6-nitropheny1)-4-iodobenzertarnine
A solution of 1.0 M lithium hexa methyl disilazide (LiN(SiMe3)2) "LHMDS"
(15.37mL, 15.37mmol) was slowly
added to a stirred solution of 2-fluoro-4-iodoaniline (3.64g, 15.37mmol) in
dry TI-IF (100 mL) under nitrogen at -78 C
and stirring continued at -78 C for another hour. 2,3,4,6-
Tetrafluoronitrobenzene was added, and the reaction mixture
was allowed to warm to room temperature and stirring continued for another 16
hours. Ethyl acetate (200 mL) was added
and the organic phase was washed with water, dried over sodium sulfate and
further purified by column chromatography
-Page 173-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
to provide the product as a yellow solid (3.75g, 59.24%). M-11+: 410.9. 1H NMR
(DMSO, 300 MHz): 6.85 (t, 1H); 7.38
(d, 1H); 7.62 (m, 211); 8.78 (s, 1H).
Step B: 2-Fluoro-N-(2,3-difluoro-5-methoxy-6-nitropheny1)-4-iodobenzenamine
A stirred solution of (2-fluoro-4-iodo-phenyl)-(2,3,5-trifluoro-6-nitro-
phenyl)-amine (1.23g, 3 mmol) in dry THF
(25 ml) under nitrogen was cooled to -78 C and a solution of 25% sodium
inethoxide (0.68 ml, 0.3 mmol) was added
slowly. The reaction mixture was allowed to warm to room temperature and
stirring continued for another 16 hours.
TLC indicated incomplete reaction. Ethyl acetate (100 mL) was added to the
reaction mixture and the organic layer was
washed with water, dried over sodium sulfate and further purified by column
chromatography to provide the desired
compound as a yellow solid (0.6g, 47.6%). nilz = 424 [M+H].
Step C: 5,6-Difluoro-N1-(2-fluoro-4-iodopheny1)-3-methoxybenzene-1,2-diamine
Ammonium chloride (1.18g, 20.16 mmol) and iron powder (1.15g, 21.44 mmol) were
added to a stirred solution of
(2,3-difluoro-5-methoxy-6-nitro-pheny1)-(2-fluoro-4-iodo-pheny1)-amine (0.57g,
1.34 mmol) in ethanol (20 mL). The
mixture was stirred at reflux for 16 hours, cooled to room temperature,
filtered over celite and the filtrate concentrated to
dryness. The resulting residue was taken into ethyl acetate, washed with
water, dried over sodium sulfate and further
purified by crystallization from ethanol to provide the product as an off-
white solid (0.47g, 90.3%). M-H+: 393.2. 11-1
NMR (DMSO, 300 MHz): 3.76 (s, 3H); 6.1 (t, 111); 6.8 -7.0 (m, 1H); 7.2 (d,
1H); 7.35 (s, 1H); 7.42 (d, 1H).
Step D: 1-Allyl-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyl) cyclopropane-l-sulfonamide
To a stirred solution of 5,6-difluoro-N1-(2-fluoro-4-iodopheny1)-3-
methoxybenzene-1,2-diamine (1 eq) in
anhydrous pyridine (5m1/mmole) was added 1-allyl-cyclopropanesulfonyl chloride
(1 - 5 eq). The reaction mixture was
stirred at 40 C for 48 hours. The reaction mixture was partitioned with water
and ethyl acetate. The organic layer was
washed with brine, dried (MgSO4) and concentrated under reduced pressure. The
residue was purified by flash column
chromatography on silica to obtain the title product. 1H NMR (CDC13, 300 MHz):
8 7.417 (dd, 1H), 7.309(s, 1H), 7.25
(m, 1H), 6.89 (m, 1H), 6.52(m, 111), 6.427 (m, 1H), 6.03 (s, 1H), 5.668 (in,
1H), 5.11 (t, 1H), 3.9 (s, 3H), 2.75 (d, 211),
1.21 (m, 2H), 0.767 (m, 2H).
Step E: N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)
cyclopropane-1-sulfonamide
1-Allyi-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl)
cyclopropane-l-sulfonamide (97mg,
0.18 mmol) and 4-methylmorpholine N-oxide (21mg, 0.18 minol) were dissolved in
TI-EF (8 mL). Osmium tetroxide was
added at room temperature (0.018 mmol, 0.13 mL, 4% in H20) and the reaction
mixture was stirred at room temperature
for 16 hours. Ethyl acetate was added, and the organic phase was washed with
water, dried (MgSO4) and concentrated
under reduced pressure. The residue was purified over silica gel
chromatography (eluants: Et0Ac/ Me0H) to obtain the
titled product (0.80 g, 78 %). 11INMR (CDC13, 300 MHz): 8 7.38 (dd, J = 1.7 &
10.3 Hz,1H), 7.26 (m, 1H), 7.14 (s,
1H), 6.87 (s, 111), 6.53 (dd, J = 6.8 & 11.4 Hz, 1H), 6.43 (in, 111), 4.06 (m,
111), 3.89 (s, 3H), 3.63 (dd, J = 3.7 & 11.1 Hz,
1H), 3.49 (dd, J = 6.4 & 11.1 Hz, 1H), 2.3 (dd, J = 9.7 & 16.1 Hz, 1H), 1.77
(dd, J= 1.9 & 16.0 Hz, 1H), 1.37 (m, 1H),
1.25 (m, 1H), 1.21 (m, 2H), 0.86 (m, 2H); m/z = 571 [M-l].
Example 98: Preparation of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)-1-(2,3-
dihydroxypropyl) cyclopropane-l-sulfonamide
-Page 174-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
HO
g'NH H F
PAe0 N
F
The pure S isomer was obtained by chiral HPLC separation of the racemic
mixture. 11-1NMR (CDCI3, 300 MHz): 6
7.38 (dd, J = 1.7 & 10.3 Hz,1H), 7.26 (m, 1H), 7.14 (s, 1H), 6.87 (s, 1H),
6.53 (dd, J= 6.8 & 11.4 Hz, 111), 6.43 (m, 1H),
4.06 (m, 1H), 3.89 (s, 311), 3.63 (dd, J = 3.7 & 11.1 Hz, 1H), 3.49 (dd, J =
6.4 & 11.1 Hz, 1H), 2.3 (dd, J = 9.7 & 16.1 Hz,
111), 1.77 (dd, J = 1.9 & 16.0 Hz, 1H), 1.37 (m, 1H), 1.25 (m, 1H), 1.21 (m,
2H), 0.86 (m, 2H); m/z = 571 [M-1] .
Example 99: Preparation of N-(R)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-
6-methoxypheny1)4-(2,3-
dihydroxypropyl) cyclopropane-l-sulfonamide
OH r7
g'NH H F
sA N
-e- lig 11111
11111" F 11151
The pure R isomer was obtained by chiral HPLC separation of the racernic
mixture. 11-1 NMR (CDC13, 300 MHz):
67.38 (dd, J = 1.7 & 10.3 Hz,1H), 7.26 (m, 1H), 7.14 (s, 1H), 6.87 (s, 1H),
6.53 (dd, 1=6.8 & 11.4 Hz, 1H), 6.43 (m,
1H), 4.06 (m, 1H), 3.89 (s, 311), 3.63 (dd, I = 3.7 & 11.1 Hz, 1H), 3.49 (dd,
J = 6.4 & 11.1 Hz, 1H), 2.3 (dd, J = 9.7 &
16.1 Hz, 1H), 1.77 (dd, J = 1.9 & 16.0 Hz, 1H), 1.37 (m, 1H), 1.25 (m, 1H),
1.21 (m, 2H), 0.86 (m, 211); tn/z = 571 [M-
1]-.
Example 100: Preparation of crystalline polymorph Form A of N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-l-
sulfonamide
Preparation i) N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide (216.10g) was charged to a 4L Erlenmeyer flask
equipped with a large magnetic stir bar and
a magnetic stirrer/hot plate. Ethyl Acetate (ca. 600 rnL, purchased from
Fisher) was added. Heating and stirring were
initiated to form a brown suspension. The mixture was brought to a low reflux
and additional ethyl acetate (ca. 200 mL)
was added to effect complete dissolution giving a dark brown solution. Heptane
(purchased from Acros) was slowly
added portionwise to the refluxing solution at a rate that all precipitates
that formed on each addition were quickly
dissolved and reflux maintained. Upon the addition of 2 L of heptanes to the
solution the solids formed dissolved very
slowly at reflux. Heating was stopped and the crystallization mixture allowed
to equilibrate to room temperature with
stirring over 16 h. A thick layer of crystalline material developed around the
surface of the glass over the aging period.
The resulting suspension was equilibrated in an ice/water bath with stirring.
The suspension was filtered on a 25 cm
Buchner funnel dressed with Whatman #1 filter media. The collected crystals
were washed with heptanes (1 L) and
allowed to air dry under vacuum. The crystals were further dried at 40 C/ <1
torr over 20 h to yield the product as a pink
crystalline solid (160.99 g, 77.2%).
Preparation ii) N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide (13.2g) and ethyl acetate (30mL) were charged to an
Erlenmeyer flask equipped with a
large magnetic stir bar and a magnetic stirrer/hot plate. Stirring and heating
to low reflux were initiated to effect complete
dissolution giving a dark brown solution. Heptanes were slowly added
portionwise to the refluxing solution at a rate that
all precipitates that formed on each addition were quickly dissolved and
reflux maintained, until the addition of heptanes
to the solution caused the solids formed to dissolve very slowly at reflux (--
90mL heptanes). Heating was stopped and the
crystallization mixture allowed to equilibrate to room temperature with
stirring over 16 h. A thick layer of crystalline
-Page 175-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
material developed around the surface of the glass over the aging period. The
resulting suspension was equilibrated in an
ice/water bath with stirring. The suspension was filtered on Buchner funnel
dressed with Whatman #1 filter media. The
collected crystals were washed with heptanes, and allowed to air dry under
vacuum. The crystals were further dried at
40 C/ <1 ton- over 20 h to yield the product as a pink crystalline solid.
Preparation iii) N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)
cyclopropane-l-sulfonamide (44.8g) and ethyl acetate (750mL) were charged to
an Erlenmeyer flask equipped with a
large magnetic stir bar and a magnetic stirrer/hot plate. Stirring and heating
to low reflux were initiated to effect complete
dissolution giving a dark brown solution. Hexanes were slowly added
portionwise to the refluxing solution at a rate that
all precipitates that formed on each addition were quickly dissolved and
reflux maintained, until the addition of hexanes
to the solution caused the solids formed to dissolve very slowly at reflux (--
2L hexanes). Heating was stopped and the
crystallization mixture allowed to equilibrate to room temperature with
stirring over 16 h. A thick layer of crystalline
material developed around the surface of the glass over the aging period. The
resulting suspension was equilibrated in an
ice/water bath with stirring. The suspension was filtered on Buchner funnel
dressed with Whatnaan #1 filter media. The
collected crystals were washed, and allowed to air dry under vacuum. The
crystals were further dried at 40 C/ <1 ton
over 20 h to yield the product as a pink crystalline solid.
Example 101: Preparation of crystalline polymorph of N-(R)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl) cyclopropane-1-sulfonamide
N-(R)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl) cyclopropane- I -
sulfonamide (216.10g) is charged to a 4L Erlenmeyer flask equipped with a
large magnetic stir bar and a magnetic
stirrer/hot plate. Ethyl Acetate (ca. 600 mL) is added. Heating and stirring
are initiated to form a brown suspension. The
mixture is brought to a low reflux and additional ethyl acetate (ca. 200 mL)
is added to effect complete dissolution giving
a dark brown solution. Heptane is charged to the solution slowly portionwise
to the refluxing solution at a rate that all
precipitates that form on each addition are quickly dissolved and reflux is
maintained. Upon the addition of 2 L of
heptanes to the solution the solids formed dissolve very slowly at reflux.
Heating is stopped and the crystallization
mixture is allowed to equilibrate to room temperature with stirring over 16 h.
A thick layer of crystalline material
develops around the surface of the glass over the aging period. The resulting
suspension is equilibrated in an ice/water
bath with stirring. The suspension is filtered on a 25 cm Buchner funnel
dressed with Whatrnan #1 filter media. The
collected crystals are washed with heptanes (1 L) and allowed to air dry under
vacuum. The crystals are further dried at
40 C/ <1 ton over 20 h.
Example 102: Generation of IC50 Data
Materials and preparation of reagents: Human GST-MEK1 and the constitutively
active allele GST-MEK1 CA
(harboring the mutations Ser218Asp and Ser222Asp) were subcloned into the
yeast expression vector pGEM4Z
(Promega, Madison, WI) from the wild type human MEK1 cDNA. GST-MEK11A was
expressed in Escherichia coil and
partially purified using Glutathione Sepharose 4B affinity resin (Amersham
Pharmacia Biotech, Piscataway, NJ). The
ERIC2 allele was subcloned from MAPK2/Erk2 cDNA (wild type) in pUSEarnp
(Upstate Biotechnology, Inc., Waltham,
MA) into the vector pET2la (Novagen, Madison, WI) resulting in an N-terminal
histidine-tagged mouse ERK2 allele.
ERIC2 was expressed and purified to homogeneity [Zhang, 1993 #33]. Myelin
basic protein (MBP) was purchased from
Gibco BRL (Rockville, MD). EasyTides adenosine 5'-triphosphate (ATP) ([1/-
33P]) (NEN Perkin Elmer, Wellesley, MA)
was the source of radiolabel for all kinase reactions. Activated Raf-1
(truncated) and activated MAPKinase 2/ERK2
were purchased from Upstate, Inc. (Lake Placid, NY). 4-20% Criterion Precast
gels were purchased from Bio-Rad
(Hercules, CA).
-Page 176-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Determination of enzymatic activity: Compounds were diluted from
dimethylsulfoxide (DMSO) stocks into
lxHMNDE (20 mM HEPES pH 7.2, 1 mM MgC12, 100 mM NaC1, 1.25 mM DTT, 0.2 mM
EDTA). A typical 25-
microliter assay contained 0.002 nanomoles MEK1 CA, 0.02 nanomoles ERK2, 0.25
nanomoles MBP, 0.25 nanomoles
unlabeled ATP, and 0.1 pCi ell ATP. The screening assay essentially comprised
four additions. Five ul of diluted
compound were dispensed to 96-well assay plates. Ten ul of 2.5x enzyme
cocktail (MEK1 CA and ERK2 only) were then
added to each well followed by a pre-incubation for 30 minutes at ambient
temperature. Ten ill of 2.5x substrate cocktail
(labeled and unlabeled ATP plus MBP) were then added, followed by incubation
for 60 minutes at ambient temperature.
Finally, 100 Id of 10% trichloroacetic acid (TCA) were added and incubated for
30 minutes at room temperature to halt
the reaction and precipitate radiolabeled protein products. Reaction products
were harvested on glass fiber 96 well filter
plates prewetted with water and 1% pyrophosphate. The filter plate was then
washed 5 times with water. Water was
displaced by absolute ethanol and the plate was allowed to air dry for 30
minutes at room temperature. A back seal was
applied manually and 40 ul of scintillation cocktail were dispensed to each
well. A top seal was applied and the plate
was counted in the TopCount for two seconds per well. For certain experiments
a truncated version of MEK that requires
activation by Raf kinase were used.
Example 103: Generation of EC50 Data
Effects of compounds in the cell were determined by Western blotting for
phosphorylated ERK. MDA-MB-231
breast cancer cells were plated in a 48 well plate at 20,000 cells per well
and grown in a 37 humidified CO2 incubator.
The following day, the growth media (DMEM + 10% fetal bovine serum) was
removed and replaced with starve media
(DMEM + 0.1% fetal bovine serum). Cells were incubated in the starve media for
sixteen hours and then treated with a
range of compound concentrations for thirty minutes. After incubation with
compound, cells were stimulated with
I 0Ong/m1 EGF for five minutes. The cells were then lysed and analyzed by
Western blot using a monoclonal antibody
raised to phosphoryIated ERK. The signal was amplified using a secondary
antibody conjugated to a near -IR dye and
detected on a Licor Odyssey scanner. The intensity of signal was quantitated
and this data was used to generate dose
response curves and EC50 calculations.
Example 104: Activity data of compounds
The compounds described in examples 1, 2 and 3 were tested in the assays
described above. The results are
summarized in the table below (A, EC50 = < 2.0nM; B, EC50= 2.0-15nM):
Compound
Number Structure Activity
HQ
Eg, 97
NH F A
(Racemic) 0
ari NH riai
F 41P)
0
Eg. 98
(S isomer) 0 NH F A
00 140
1
-Page 177-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Compound
Number Structure Activity
OH
HO
Eg. 99 N
0 NH
(R isomer)
,o *F1H
Example 105: XRPD Data
XPRD was performed on a 1nel XRG-3000 diffractometer, equipped with a curved
position-sensitive detector with
a 20 range of 1200. Real time data was collected using Cu Ka radiation at a
resolution of 0.03 020. The tube voltage and
amperage were set to 40kV and 30mA, respectively. Patterns are displayed from
2.5 to 40 '20 to facilitate direct pattern
comparisons. Samples of (S)-N-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl) cyclopropane-1-sulfonamide (synthesized as described herein)
were prepared for analysis by packing
them into thin-walled glass capillaries. Each capillary was moved onto a
goniometer head that is motorized to permit
spinning of the capillary during data acquisition. The samples were analyzed
for 5 minutes. Instrument calibration was
preformed daily using a silicon reference standard. FIG. 5 is a graph of a
powder x-ray diffraction (PXRD) pattern of N-
(S)-(3,4-difluoro-2-(2-fluoro-4-iodopheny1amino)-6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-
sulfonamide Form A. FIG. 7 is a graph of the powder x-ray diffraction (PXRD)
patterns of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-
1-sulfonamide Form A (top) and
Amorphous (bottom).
Example 106: Differential Scanning Calorimetry (DSC)
Analyses were carried out on a TA Instruments differential scanning
calorimeter Q1000. The instrument was
calibrated using indium as the reference material. The sample was placed into
a standard aluminum DSC pan with a non-
crimped lid configuration, and the weight accurately recorded. To determine
the glass transition temperature (Ts) of
amorphous material, the sample cell was cycled several times between -40 C and
140 C. The final temperature was
ramped to 150 C. The Ts is reported from the inflection point of the last
cycle transition. FIG. 6 is a graph of a modulated
DSC thermogram of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-l-sulfonamide (Form A). The graph plots the
normalized heat flow in Watts/gram (Wig)
versus the measured sample temperature in C.
Example 107: Dynamic Vapor Sorption/Desorption (DVS)
Moisture sorption/desorption data were collected on a VTI SGA-100 Vapor
Sorption Analyzer. Sorption and
desorption data were collected over a range of 5% to 95% relative humidity
(RH) at 10% RH intervals under a nitrogen
purge. Samples were not dried prior to analysis. Equilibrium criteria used for
analysis were less than 0.0100% weight
change in 5 minutes, with a maximum equilibration time of 3 hours if the
weight criterion was not met. Data were not
corrected for the initial moisture content of the samples. Sodium chloride and
polyvinypyrrolidine were used as
calibration standards. FIG. 8 shows a DVS isotherm of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxyphenyl)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide (Form A). The
material exhibits a negligible
weight change during the experiment.
Example 108: Thermogravimetry (TG)
-Page 178-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Analyses were carried out on a TA Instrument 2950 thermogravimetric analyzer.
The calibration standards were
nickel and AlumelTM. Each sample was placed in an aluminum sample pan and
inserted into the TG furnace. Samples
were equilibrated at 25 C, and then heated under a stream of nitrogen at a
heating rate of 10 C/min, up to a final
temperature of 350 C. FIG. 9 shows a TG thermogram of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
naethoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide (Form A)
demonstrating negligible weight loss up
to 140 C, indicating polymorph, form A is unsolvated.
Example 109: In Vitro Cancer Screen
The human tumor cell lines were grown in RPMI 1640 medium containing 5% fetal
bovine serum and 2 mM
glutamine. Cells are inoculated into 96 well microtiter plates in 100 L at
plating densities ranging from 5,000 to 40,000
cells/well depending on the doubling time of individual cell lines. After cell
inoculation, the microtiter plates are
incubated at 37 C, 5 % CO2, 95 % air and 100 % relative humidity for 24 h
prior to addition of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-nriethoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane- 1-sulfonamide.
After 24 h, two plates of each cell line were fixed in situ with TCA, to
represent a measurement of the cell
population for each cell line at the time of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-
(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide addition (Tz). N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyl)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide was
solubilized in dimethyl sulfoxide at 400-fold
the desired final maximum test concentration and stored frozen prior to use.
At the time of addition, an aliquot of frozen
concentrate is thawed and diluted to twice the desired final maximum test
concentration with complete medium
containing 50 g/mlgentarnicin. Additional four, 10-fold or 1/4 log serial
dilutions are made to provide a total of five
concentrations plus control. Aliquots of 100 111 of these different dilutions
are added to the appropriate microtiter wells
already containing 100 [11 of medium, resulting in the required final
concentrations.
Following addition of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide, the plates were incubated for an
additional 48 h at 37 C, 5 % CO2, 95 %
air, and 100 % relative humidity. For adherent cells, the assay is terminated
by the addition of cold TCA. Cells are fixed
in situ by the gentle addition of 50 I of cold 50 % (w/v) TCA (final
concentration, 10 % TCA) and incubated for 60
minutes at 4 C. The supernatant is discarded, and the plates are washed five
times with tap water and air dried.
Sulforhodamine B (SRB) solution (100 I) at 0.4 % (w/v) in 1 % acetic acid is
added to each well, and plates are
incubated for 10 minutes at room temperature. After staining, unbound dye is
removed by washing five times with 1 %
acetic acid and the plates are air dried. Bound stain is subsequently
solubilized with 10 mM trizma base, and the
absorbance is read on an automated plate reader at a wavelength of 515 run.
For suspension cells, the methodology is the
same except that the assay is terminated by fixing settled cells at the bottom
of the wells by gently adding 50 1 of 80 %
TCA (final concentration, 16 % TCA). Using the seven absorbance measurements
[time zero, (Tz), control growth, (C),
and test growth in the presence of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at the five concentration levels
(Ti)], the percentage growth was calculated
at each of the drug concentrations levels. Percentage growth inhibition is
calculated as:
Percentage growth inhibition =(Ti-Tz) x 100
(concentrations for which Ti>/=Tz) (C-Tz)
Percentage growth inhibition --(Ti-Tz) x 100
(concentrations for which Ti<Tz) Tz
Three dose response parameters were calculated. Growth inhibition of 50 %
(GI50) was calculated from [(Ti-
Tz)/(C-Tz)] x 100 = 50, which is the concentration resulting in a 50%
reduction in the net protein increase (as measured
by SRB staining) in control cells during the drug incubation. The N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
- Page 179-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide concentration
resulting in total growth inhibition
(TO!) was calculated from Ti = Tz. The LC50 (concentration of drug resulting
in a 50% reduction in the measured
protein at the end of the drug treatment as compared to that at the beginning)
indicating a net loss of cells following
treatment is calculated from [(Ti-Tz)/Tz] x 100 -50. Values were calculated
for each of these three parameters if the
level of activity was reached; however, if the effect was not reached or
wasexceeded, the value for that parameter is
expressed as greater or less than the maximum or minimum concentration tested.
Panels corresponding to Leukemia, Non-Small Cell Lung Cancer, Colon Cancer,
CNS Cancer, melanoma, ovarian
cancer, renal cancer, prostate cancer and breast cancer, for the cell lines
indicated were examined and the results are
shown below.
G150 LC50 TGI
Panel Cell Line (AM) (PM) (AM)
Leukemia CCRF-CEM
17.378 100.000 60.256
Leukemia HL-
60(TB) 0.010 100.000 100.000
Leukemia K-562 6.607
100.000 100.000
Leukemia MOLT-4
10.965 100.000 69.183
Leukemia RPMI-
8226 26.915 100.000 100.000
Leukemia SR 38.019
100.000 100.000
Non-Small Cell Lung Cancer A549/ATCC 0.589 100.000 64.565
Non-Small Cell Lung Cancer EKVX 0.214 61.660 13.804
Non-Small Cell Lung Cancer , HOP-62 0.069 42.658 12.589
Non-Small Cell Lung Cancer HOP-92 0.047 58.884 0.324
Non-Small Cell Lung Cancer NCI-H226 3.311 74.131 24.547
Non-Small Cell Lung Cancer NCI-H23 0.056 74.131 2.884
Non-Small Cell Lung Cancer NCI-H3221v1 0.162 46.774
15.488
Non-Small Cell Lung Cancer NCI-H460 3.631 52.481 19.498
Non-Small Cell Lung Cancer NCI-H522 5.248 100.000
29.512
Colon Cancer HCC-2998 0.010 0.457 0.035
Colon Cancer HCT-116 0.195 67.608 12.589
Colon Cancer HCT-15 0.603 60.256 16.982
Colon Cancer HT29 0.026 29.512 3.090
Colon Cancer KM12 0.229 48.978 13.490
Colon Cancer SW-620 0.039 66.069 12.589
CNS Cancer SF-268 2.570 100.000
25.704
CNS Cancer SF-295 9.333 53.703 23.442
CNS Cancer SF-539 1.514 60.256 20.417
CNS Cancer SNB-19 0.251 75.858 24.547
CNS Cancer SNB-75 0.302 , 34.674 4.467
CNS Cancer U251 0.891 44.668 17.378
Melanoma LOX 1MVI 0.195 38.905 10.715
Melanoma MALME-3M 0.010 19.953 0.014
Melanoma M14 0.015 29.512 0.166
Melanoma SK-MEL-28 0.028 22.387 0.214
Melanoma SK-MEL-5 0.062 38.905 13.804
Melanoma UACC-257
, 0.020 66.069 10.233
Melanoma UACC-62 0.014 20.893 0.170
-Page 180-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
GI50 LC50 TGI
Panel Cell Line (1134) (IIM)
(PM) ,
Ovarian Cancer IGROV1 0.018 19.055
0.295
Ovarian Cancer OVCAR-3 2.512 48.978
17.783
Ovarian Cancer OVCAR-4 0.562 72.444
16.218
Ovarian Cancer OVCAR-5 0.017 40.738
12.023
Ovarian Cancer SK-OV-3 12.882 100.000
41.687
Renal Cancer 786-0 5.129 63.096 23.442
Renal Cancer A498 0.191 44.668 4.169
Renal Cancer ACHN 0.275 83.176 21.878
Renal Cancer CAKI-1 0.389 100.000
26.915
Renal Cancer SN12C 0.851 47.863 18.621
Renal Cancer 1K-10 0.224 100.000
23.442
Renal Cancer U0-31 0.158 40.738 11.482
Prostate Cancer PC-3 8.128 100.000
37.154
Prostate Cancer DU-145 2.138 95.499
22.387
Breast Cancer MCF7 10.965 85.114
30.903
Breast Cancer NCl/ADR-RES 3.467 100.000
25.704
Breast Cancer MDA-MB-231 0.069 35.481 10.471
Breast Cancer HS 578T 0.617 85.114
13.490
Breast Cancer MDA-MB-435 0.035 41.687 12.303
Breast Cancer BT-549 5.754 47.863
20.893
Breast Cancer T-47D 4.898 100.000
38.019
Breast Cancer MDA-MB-468 0.019 54.954 10.233
Example 110: In Vitro Anti-Proliferative Activity
In the present example, the following effects of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide were
examined: (1) activity (GI50) against the
growth of several tumor cell lines harboring different mutations; (2) activity
(GI50) against the growth of several B-Raf
mutant cell lines; (3) effects on anchorage independent cell growth; (4)
effects on the cell cycle; and (5) toxic effects on
primary liver and kidney cells.
Cell Culture/Growth Inhibition Assay
Human melanoma A375 cells and human colon cancer Co1o205 cells were obtained
from ATCC (Manassas, VA).
A375 cells were maintained in DMEM supplemented with 10% fetal bovine serum,
glutamine (2 mM), penicillin (100
U/ml), and streptomycin (100 ug/m1). Cells were maintained at 37 C, 5% CO2,
and 100% humidity. Co1o205 cells were
maintained in RPMI supplemented with 10% fetal bovine serum, glutamine (2 mM),
penicillin (100 U/ml), and
streptomycin (100 ug/m1). For growth inhibition experiments, cells were plated
in white 384-well rnicroplates at 1000
cells/20 'Dwell. After 24 hr, 5 ul of a 5X drug stock solution was added. All
drugs were initially prepared as 200X
stocks in DMSO, such that final DMSO concentration was 0.5%. Cells were
incubated for 48 hr at 37 C and ATP levels
were determined using CeIlTiterGlo (Promega, Madison, WI). Adenylate kinase
(AK) release was determined using
Toxilight (Cambrex, Walkersville, MD). Non-linear curve-fitting was performed
using GraphPad Prism 4 (GraphPad
Software, San Diego, CA). 4-Amino-842R,3R,4S,5R)-3,4-dihydroxy-5-
(hydroxymethyl)tetrahydrofuran-2-
yl)pyrido[2,3-d]pyrimidin-5(8H)-one (VRX-14686) is a cytotoxic agent used as a
reference compound.
Page 181-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Growth inhibition (%) = (Vehicle Only control (RLU)- N-(S)-(3,4-difluoro-2-(2-
fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide RLU)/(Vehicle
Only control RLU-1 pM N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide RLU); based on growth arrest induced
by N-(S)-(3,4-difluoro-2-
(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide where
ATP levels are measured.
Cell Viability (%) = (N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide RLU-10 M VRX-14686 RLU)/(Vehicle
Only control RLU-10
M Tamoxifen RLU); based on cell killing induced by VRX-14686 where ATP levels
are measured.
Cell Killing (%) (N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane- 1-sulfonamide RLU- Vehicle Only control RLU)/(10
M Tamoxifen RLU -
Vehicle Only control RLU); based on cell killing induced by Tamoxifen where AK
release is measured.
RLU = Relative Luminescence Units
Evaluation of Cell Cycle Arrest
A375 cells were plated in 96-well microplates at 10,000 cells/200 l/well.
After 24 hr cells were approximately
50% confluent and 50 I of a 5X drug stock solution was added. After another
24 hr, cells were trypsinized, fixed in 200
pl Prefer (Anatech, Battle Creek, MI), and stored at 4 C overnight. Cells
were then rinsed in PBS, permeabilized and
stained in 0.1% Triton X-100, 200 lig/m1 DNase-free RNase, and 25
pg/m1propidium iodide (Molecular Probes,
Sunnyvale, CA), and analyzed on the Guava PCA-96 (Guava Technologies, Foster
City, CA). Data were analyzed using
ModFit LT (version 3.0, Verity, Topsham, ME).
(1) Evaluation of anchorage independent cell growth inhibition
Wells of an "ultra low binding" plate (Corning, Acton MA) were filled with 60
pl of a 0.15% agarose solution in
complete RPMI. Then, 60 pl complete RPMI containing 9000 Co1o205 cells in
0.15% agarose was added per well.
After 24 hr, 60 pi of a 3X drug solution in agarose free complete RPMI was
added. After 7 days, 36 al 6X MTS reagent
(CellTiter 96 Aqueous, Promega, Madison, WI) was added per well. After 2 hr at
37 C, absorbance at 490 tun was
determined on the M5 plate reader (Molecular Devices, Sunnyvale, CA). Non-
linear curve-fitting was performed using
GraphPad Prism 4.
(2) Growth Inhibition (GI50) against MEK-dependent cancer cell growth
Log phase dividing B-Raf mutant cells A375 (human melanoma), A431 (melanoma),
Co1o205 (colon carcinoma),
HT29 (colorectal adenocarcinoma), MDA-MB231 (breast adenocarcinoma), and BxPC3
(pancreatic adenocarcinoma)
were exposed to N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropypeyclopropane-1-sulfonamide for 48 hr and analyzed for ATP
content. 100% growth arrest was
determined using 1 tiM N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide.
The table below shows the mean GI50 values from at least three experiments,
for each cell line and show that N-
(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-
sulfonamide caused growth inhibition in three B-Raf mutant cell lines (A375,
Colo205, and HT29), as well as one
ras/raf/MEKJMAPK pathway wild-type cell line (A431) with a mean potency of 79
nM ( 9 nM).
Cell Line Mean StDev C.V.
A375 71nM 12.1nM 17%
A431 86nM 25.4nM 30%
C01 205 89nIvl 40 .1nM 45%
- Page 182 -
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
HT29 70nM 12.2nM 18%
MDA >1uM
BxPC3 >lum
In a separate study, log phase dividing B-Raf mutant cells A375 (human
melanoma), SK Mein (human
melanoma), and Co1o205 (human colon carcinoma) were exposed to N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide for 48 hr and analyzed for
ATP content. The table below shows the GI50 for each cell line indicating N-
(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphertyl)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide caused growth inhibition
with a potency approximating its EC50 value for IVIEK inhibition.
Cell Line GI50 (nM)
A375 56
SK Mel 28 105
Colo205 27
Figures 10A and 10B show growth arrest of Log phase dividing A375 cells
exposed to increasing concentrations
of N-(S)- (3,4-di fluoro-2-(2- fluoro-4- iodophenylamino)-6-methoxyphenyI)-1-
(2,3- dihydroxypropyl)cye loprop ane- 1-
sulfonamide.. Cells were analyzed for ATP content. 100% growth arrest was
determined using 1 M N-(S)-(3,4-difluoro-
2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide.
Cell supernatants were analyzed for cytotoxic lysis by measuring adenylate
lcinase (AK) release. Log phase
dividing A375 cells were exposed to N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1 -sulfonamide and PD-325901 for 48 hr. (100%
cell killing was determined using 20
tamoxifen.) The results are shown in Figure 11. This data indicates that N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide causes a non-toxic growth
arrest in several susceptible human cancer cell lines, demonstrated by i)
growth arrest measurements (ATP quantitation);
and ii) lack of cytotoxic cell lysis (AK release). The lack of AK release was
confirmed for all cell lines tested.
Anchora2e Independent Growth Inhibition
Anchorage independent growth of Co1o205, A375, and MDA-MB231 cells exposed to
N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-
1-sulfonamide for 7 days, was
quantitatively assessed in a 96-well microplate format. Viability was
determined by MTS assay. G150 values are shown
below:
Cell Line Mean StDev C.V.
Co1o205 4011M 8.1nM 20%
A375 84nM 17.2nM 21%
MDA-MB231 81nM 55.6nM 69%
Figures 12A-12C show N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyI)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide growth inhibition of (A) human
colorectal carcinoma Colo205 cells (0I50
= 11 n.M); (B) A375 cells (GI50 = 22 nM) and (C) inhibition of MDA-MB231 cells
which do not show N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophertylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cycIopropane-1-sulfonamide -
induced growth arrest in 2-dimensional anchorage dependent assays.
Log phase dividing A375 cells were exposed to N-(S)-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)-6-
methoxyphenyl)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide (I uM) for 48
hr and the cell supernatants analyzed
for growth inhibition (ATP content) and cytotoxic lysis (AK release). 100%
viability (ATP assay) was determined in
-Page 183-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
vehicle only control wells. The table below shows the results indicating N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyI)-1-(2,3-dihydroxypropypcyclopropane-l-
sulfonamide causes non-toxic growth
arrest in B-Raf mutant human melanoma A375 cells.
% Control
ATP, Cell viability 27%
AK, Cell killing 4%
Anchora2e Independent Growth Inhibition
Anchorage independent growth was quantitatively assessed in a 96-well
microplate format. Figure 13A shows
inhibition of growth of human colorectal carcinoma Co1o205 cells, with 0150
values at 6 nM and 11 nM respectively.
Figure 13B shows inhibition of growth of A375 cells with G150 values at 5 nM
and 22 nM.
Cell Cycle Analysis of N-(S)-(3.4-ditluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyl)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide Induced Growth Arrest
MEK inhibition has been shown to induce Gl/S phase cell cycle arrest in A375
cells.
Log phase dividing A375 cells were exposed to N-(S)-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide for 24 hr and
the percentages of cells which
stained for phase dependent amounts of intracellular DNA were determined using
flow cytometry.
The table below shows percentage distribution of cells in respective growth
phases in N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-
l-sulfonamide and control (vehicle
only) treated cells.
Phase %
Cl S
G2
Control 61.8 27.1
11.1
N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6- 111 84.7 11.8
3.5
rnethoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide nM
37 74.3 18.7
7.0
nM
Figure 14A and Figure 14B show the effect of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide on cell cycle
progression, demonstrating that
exposure of A375 cells to N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylarnino)-
6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide causes arrest in the 01 phase of the
cell cycle, indicated by the depletion
of cells in both the 02 and S phases.
Evaluation of Primary Hepatocyte and Renal Cell Toxicity
Cryopreserved rat hepatocytes were obtained from CellzDirect (Austin, TX) and
plated on collagen coated 96-well
plates according to manufacturer's instructions. Drug was added 4 hr after
plating (final DMSO concentration (1.5%).
Plated human hepatocytes were obtained from CellzDirect and processed
according to manufacturer's instructions.
Cryopreserved human renal proximal tubule epithelial cells (RPTEC) were
obtained from Cambrex and were
processed according to manufacturer's instructions. Cells were expanded for 4
days and then plated in 96-well plates at
50,000 cells/well for drug exposure.
After 48 hr, supernatant AK levels were determined using Toxilight, and
cellular ATP levels were determined
using CellTiterGlo. Full kill values were determined using 15 uM VRX-14686.
The results are shown in below. Very little cell lysis was observed. Minimal
toxicity (81% survival) was seen at 30
IIM N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-
- Page 184-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
sulfonamide in freshly plated primary human hepatocytes. RPTEC cells showed a
dose dependent ATP depletion and
evident cell lysis at 30 M.
ATP (% Cell Survival) AK release (% Cell
Survival)
Hepatocytes RPTEC Hepatocytes RPTEC
Compound A Rat Human Human Rat Human
Human
30.0 57% 81% 34% 91% 109%
41%
10.0 72% 107% 85% 93% 112%
99%
3.3 87% 104% 91% 97% 102%
95%
1.1 114% 108% 94% 96% 92% 96%
The above data illustrates that (1) N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-inethoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide inhibits cell growth and division
in select human cancer cells with Glso
values ranging from 70-89 nM in anchorage dependent proliferation assays
without causing toxicity as determined by
cell lysis assay; (2) N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenyIamino)-6-
methoxyphenyI)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide inhibits cell growth and division
in select human cancer cells with G150
values of 51 nM and 22 nM in anchorage dependent and independent proliferation
assays respectively; (3) N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyl)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide causes
01 arrest and inhibits anchorage independent growth in A375 cells, providing
evidence of anticancer activity in a
physiologically relevant in vitro model; and (4) N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide shows little cytotoxicity
against primary normal human
hepatocytes, human renal proximal tubule epithelial cells and rat hepatocytes.
Example 111: Phannacokinetics of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide in Cancer
Patients following Multiple
Doses
Dose Tn,,,,, C,õx AUC T t112*
Rõ Rac
N C24hr (Pg/mL) Cõ,õ
AUCT
(mg) (hr) (pg/niL) (pg-hr/mL) (hr)
2
1.33 0.0504 0.00938 0.517 11.4 1.76 1.90
3
(21.7) (49.2) (82.8) (61.2) (38.8) (35.6) (23.9)
4
1.50 0.105 0.0313 1.39 14.9 1.49 1.91
3
(33.3) (41.0) (41.1) (42.7) (0.992) (21.6) (36.1)
6 3 1.50 0.205 0.0489 2.22 15.6 1.58
2.07
(33.3) (16.6) (12.2) (5.79) (23.8) (38.5) (23.5)
Rag: accumulation index
* Inaccurate estimate due to limited sampling time
Following multiple dosing of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyI)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at 2, 4, or 6 mg/subject, N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
ioclophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide was readily absorbed with
mean T. ranging between 1.33 to 1.50 hr. Mean C., CT, and AUC values increased
with dose in a dose-proportional
manner. Accumulation indices range between 1.49 to 1.76 for Cõ,õõ and 1.90 to
2.07 for AUC, respectively, indicating
moderate accumulation. Although the half-life cannot be accurately measured
due to limited sampling time following
multiple doses, the hale-life was expected to be longer than 22 hr following
multiple doses based on the accumulation
indices. These half-life values are significantly longer than observed in the
mouse efficacy model which a typical range
of 2 - 3 hr was seen. In addition, encouraging peak-to-trough ratios were seen
in all doses.
Example 112: Pharmacokinetics of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-I-sulfonamide in Healthy
Volunteers following Multiple
Doses
-Page 185-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Dose Tinaõ Cmax AUCT Rae
Rac
C24hr (lAghilL) (hr) Clliaõ
AUCT
(mg) (hr) (IleimL) (pg-hr/mL)
2.00 0.182 0.0318 1.02(1.80) 14.6 1.14 1.29
6
(61.2) (35.5) (53.0) (43.7) (39.7) (15.2) (19.0)
(13.4)
2.25 0.313 0.0350 2.60 13.4 1.23 1.24
6
(39.1) (17.6) (36.5) (22.0) (21.9) (24.1) (6.51)
accumulation index
Inaccurate estimate due to limited sampling time
Following multiple dosing of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
5 dihydroxypropybcyclopropane-l-sulfonamide at 10 or 20 mg/subject, N-(S)-
(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1 -
sulfonamide was readily absorbed with
mean T. ranging between 2.00 to 2.25 hr. Mean C., Cõ and AUC values increased
with dose. Accumulation indices
range between 1.14 to 1.23 for Cõ,õx and 1.24 to 1.29 for AUC, respectively,
indicating insignificant accumulation. Half-
lives were similar for two dose regimens ranging between 13 and 15 hr. These
half-life values are shorter than observed
10 in the cancer patients.
Example 113: In Vitro Anti-Proliferative Activity
The effect of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny0-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide on inhibition of cell proliferation
was examined in a cell line derived from
a human gastric carcinoma ("stomach cancer") in a cell proliferation assay.
15 Cell culture/ Growth Inhibition Assay: Human gastric carcinoma Hs746t
cells were obtained from ATCC
(Manassas, VA). Hs746t cells were maintained in DMEM supplemented with 10%
fetal bovine serum, penicillin (100
U/ml), and streptomycin (100 ug/m1). Cells were maintained at 37 C, 5% CO2,
and 100% humidity. For cell
proliferation experiments, cells were plated in white 96-well plates with
clear bases at 3000 cells/100 ul/well. After 24
hr, cell media was removed and replaced with media containing N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
20 methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide at
various doses. Following incubation for 48
hours at 37 C, ATP levels were determined using CellTiterGlo (Promega,
Madison, WI) and reading luminescence
values using a In Biosystems Analyst HT (Sunnyvale, CA). The ATP level for
each dose was determined in triplicate
using independent wells.
Relative cell number =(mean RLU (N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide
treated))/(mean RLU Vehicle Only
control).
Figure 19 shows a graph of cell number (relative to vehicle) vs concentration
of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide and demonstrates that N-(S)-
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheriy1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide
inhibits the proliferation of human gastric carcinoma Hs746t cells after 48
hours treatment
Example 114: In Vitro Anti-Proliferative Activity
The effect of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylarnino)-6-
inethoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide on inhibition of cell proliferation
was examined in a cell line derived from
a human gastric adenocarcinoma ("stomach cancer") in a cell proliferation
assay.
Cell culture/ Growth Inhibition Assay
Human gastric adenocarcinoma AGS cells were obtained from ATCC (Manassas, VA).
AGS cells were
maintained in DMEM/F12 supplemented with 10% fetal bovine serum, penicillin
(100 U/ml), and streptomycin (100
-Page 186-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
pg/ml). Cells were maintained at 37 C, 5% CO2, and 100% humidity. For cell
proliferation experiments, cells were
plated in white 96-well plates with clear bases at 3000 cells/100 pl/well.
After 24 hr, cell media was removed and
replaced with media containing N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyl)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at various doses. Following
incubation for 3 days at 37 C, ATP levels
were determined using CellTiterGlo (Promega, Madison, WI) and reading
luminescence values using a LJL Biosystems
Analyst HT (Sunnyvale, CA). The ATP level for each dose was determined in
triplicate using independent wells. In
another experiment, 1000 cells/100u1/well were plated and the cells were
treated for 6 days and assayed as before.
Relative cell number--,(mean RLU (N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide
treated))/(mean RLU Vehicle Only
control).
Figure 15A and Figure 15B shows a graphical plot of N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide concentration
vs cell number (relative to vehicle)
after (A) 3 days and (B) 6 days exposure to N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-
(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide, demonstrating that N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylarnino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide inhibits the proliferation of
the human gastric adenocarcinoma AGS cell line.
Example 115: Growth Response of Orthotopic Human Hep3B Tumors in Nude Mice
treated with
different amounts of N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide
The dose response efficacy of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide ("Compound A"), in inhibiting the
development of orthotopic Hep3B2.1-7
human hepatic carcinoma was assessed in BALB/c nu/nu mice, in comparison with
an optimal dose of 5-Fluorouracil
(75mg/kg).
Animals: Female BALB/c nu/nu mice (University of Adelaide, Waite Campus, SA,
Australia), aged 10-
14 weeks, with a body weight range: of 19.1-29.94g (mean 22.95g) were used for
the study. The mice were
divided into 6 Study groups (4 treatment groups and 2 control groups) as
follows:
Number of Mice per Group: 10 in Groups 1 to 5 inclusive
15 in 'Take-Rate' Control Group (Group 6)
The mice were kept in a controlled environment (targeted ranges: temperature
21 3 C, humidity 30-70%, 10-15
air changes per hour) under barrier (quarantine) conditions with a 12hour
light/12hour dark cycle. Temperature and
relative humidity were monitored continuously. A commercial rodent diet (Rat
and Mouse Cubes, Speciality Feeds Pty
Ltd, Glen Forrest, Western Australia) and tap water were provided to the
animals ad libitum. Both food and water
supplies were sterilized by autoclaving.
Tumor Inoculation: Hep3B human hepatic carcinoma cells (Passage 2 from working
stock VP-Stock 353) were
cultured in RPMI1640 cell culture medium, which was supplemented with 10% PBS
and penicillin-streptomycin
(50IU/mL final concentration). The cells were harvested by trypsinisation,
washed twice in HBSS and counted. The cells
were then resuspended in HBSS:Matrigel (1:1, v/v) and adjusted to a final
volume containing lx108 cells/mL. Prior to
inoculation, the incision site was liberally swabbed with alcohol and an
incision made through the abdominal wall to
expose the liver. The needle was introduced through the surface of the liver
where 101iL of cells (1x106 cells) were
discharged. The needle was held in this position for approximately 30 seconds
to allow the Matrigell'to polymerize in
order to avoid leakage of tumor cells into the abdominal cavity.
-Page 187-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
Treatment commenced 14 days post-inoculation. On Day 7 of the study (21 days
post-inoculation), all mice from
the 'Take-Rate' control group were culled and the livers visually assessed for
the presence of tumors.
Materials: The following were obtained from the respective suppliers.
Sterile saline solution (0.9% NaCl(aq)) was obtained from Baxter Healthcare
Australia, Old Toongabbie, NSW,
Australia. CremophorEL was obtained from Sigma-Aldrich Pty Ltd, Castle Hill,
NSW, Australia. 5-Fluorouracil, clinical
formulation, clear, colorless liquid was obtained from Mayne Pharma Pty Ltd.
RPM11640 cell culture medium, FBS and
HBSS were obtained from Invitrogen Australia Pty Ltd, Mt Waverley, VIC,
Australia. Penicillin-streptomycin and
Trypan Blue were obtained from Sigma-Aldrich, Castle Hill, NSW, Australia.
Hep3B2.1-7 human hepatic carcinoma
cells were sourced from American Type Culture Collection (ATCC), Rockville,
MD, USA. Matrigel was obtained from
BD Biosciences, North Ryde, NSW, Australia.
The use of Matrigee in the inoculation suspension improves the take rate of
the tumor and decrease tumor size
variability, and the growth of the Hep3B2.1-7 human hepatic carcinoma is more
stable when inoculated in the presence
of this extracellular matrix.
Compound Preparation and Administration: CremophorEL:Saline (1:9, v/v; vehicle
control), N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophertylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide
("Compound A") or 5-Fluorouracil (compound control) were administered
according to the schedule below:
Group Compound Dose (mg/kg) Scheduled Treatment Treatment Administered
1
Vehicle Once daily for 21 Days Once daily for 19
Days
10mL/Icg
Control (Day 0 to 20) (Day 0 to 18)
0.2mL @ Once daily for 21 Days Once
daily for 19 Days
2 Compound A
10mL/kg=2mg/kg (Day 0 to 20) (Day 0 to 18)
3
1.0mL @ Once daily for 21 Days Once
daily for 19 Days
Compound A
10mL/kg=10mg/kg (Day 0 to 20) (Day 0 to 18)
4 C d 5.0mL @
Once daily for 21 Days Once daily for 19 Days
ompoun A
10mL/kg=50mg/kg (Day 0 to 20) (Day 0 to 18)
7.5mL @ Once weekly for 21 Days Once weekly
for three
5 5-Fluorouracil
10mL/kg=75mg/kg (Day 0,7 and 14) weeks (Day 0, 7
and 14)
'Take-Rate'
6 No treatment
Control
The Vehicle Control, CremophorEL:Saline (1:9, v/v), was administered p.o. in a
dosing volume of 10mL/kg, once
daily for 21 consecutive days (Day 0 to 20).
N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenyIamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-
sulfonamide, was formulated in CremophorEL:Saline (1:9, v/v). A stock solution
was prepared weekly and stored at 4 C.
Dosing solutions were prepared on each day of administration. N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-sulfonamide was
administered p.o. in a dosing volume of
10mL/kg, once daily for 21 days (Day 0 to 20). The compound was administered
at doses of 2, 10 and 50mg/kg.
5-Fluorouracil clinical formulation was diluted in sterile saline and
administered i.v. via the tail vein at a
concentration of 75mg/kg, in a dosing volume of 10mUkg, once per week for
three weeks (on Day 0, 7 and 14).
No treatment was administered to the mice in Group 6 ('Take-Rate' Control). On
Day 7 of the study (21 days post-
inoculation), the mice were culled and the liver exposed to determine the
'Take-Rate' and the size of the tumors in the
liver wall.
-Page 188-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Each animal's body weight was measured immediately prior to dosing. The volume
administered to each mouse
was calculated and adjusted based on the body weight.
Tumor Measurements: Liver and tumor wet weight were measured when each were
excised post-mortem on the
termination day of the study. At the termination of the study, livers were
excised from all mice in each study group and
weighed. The number of visible tumors, if present, was counted. These tumors
were removed from the liver and weighed.
Data Measurement and Sample Collection Schedule
Data Measurement Schedule
Body weight Day 0, then three times per week (Monday, Wednesday
and Friday), and on the
termination day of the study for Groups 1 to 5 inclusive.
Liver weight and tumor Wet weight of excised liver and tumor all mice in
Groups 1 to 5 inclusive, post-
weight
mortem on the termination day of the study, and from one mouse in Group
5 which
died on the final treatment day.
Sample Collection
Livers and tumors From all mice in Group 6 (Take-Rate' Control) on
Day 7 of the study.
Livers and tumors
From all mice in Groups 1 to 5 inclusive, post-mortem on the termination
day of
the study, and from one mouse in Group 5 which died on the final treatment
day.
Liver
From all mice in Groups 1 to 5 inclusive, post-mortem on the termination
day of
the study, and from one mouse in Group 5 which died on the final treatment
day.
Data Acquisition and Calculation: Each animal's transponder (Bar Code Data
Systems Ply Ltd, Botany Bay,
NSW) was scanned using a barcode reader (LabMax I, DataMars, Switzerland)
immediately prior to acquisition of data.
All measurements were acquired with the same handheld calipers (Absolute
Digimatic Model CD-6" CS, Mitutoyo
Corporation, Japan). The data was synchronized with vivoPharm's secure
relational database using Pendragon Forms 4.0
(Pendragone Software Corporation, Libertyville, IL, U.S.A.) as transfer
software. AIDAM v2.4 was used for data reports
and data calculation.
Statistical and Calculations: All statistical calculations were performed
using SigmaStat 3Ø (SPSS Australasia
Pty Ltd, North Sydney, NSW, Australia).
A two-sample t-test was used to determine the significance in body weight
change within a treatment group
between Day 0 and the termination day of the study. Where the data failed the
Normality test or the Equal Variance Test,
a Mann-Whitney Rank Sum Test was performed.
A One-Way Analysis of Variance (ANOVA) (All Pairwise Multiple Comparison
Procedure and Multiple
Comparison versus Control Group) was performed on liver weight and tumor
weight data at the end of the study. Where
this test did not pass the Equal Variance test, the Kruskal-Wallis One-Way
Analysis of Variance (ANOVA) on ranks was
performed. The same statistical analyses were performed on the data for the
tumor-bearing mice in the study.
A p value of less than 0.05 was considered significant.
Liver Weight and Tumor Weight Data for Tumor-Bearing Mice and Average Weight
of Liver and Tumors per
Mouse per Group for Tumor-Bearing Mice
Average Liver Average Tumor
No. of Mice with
Group TreatmentSEM SEM
Weight (g) Weight (g)
Tumors (out of 10)
Vehicle
1 4.560 0.673 3.382 0.979 4
Control
= Compound A
2 2.775 0.475 1.776 0.576 6
@ 2 mg/kg
3 Compound A 2.551 0.446 1.407 0.465 7
-Page 189-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
@ 10 Ing/kg
Compound A
4 1.677 0.161 0.624 0.257 4
50mg/kg
5FUTM @ 75
1.217 0.051 0.143 0.078 4
mg/kg
Samples were not collected from the mouse in Group 5 (5-Fluorouracil at
75mg/kg) which was culled during the
study period. Due to the presence of large tumors in some of the mice, as
indicated by a swollen appearance of the
abdomen, the study was terminated 18 days post-initial treatment.
5 A dose-dependent trend in the decrease in liver and tumor weight is
evident in the N-(S)-(3,4-difluoro-2-(2-fluoro-
4-iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-
sulfonamide-treated groups. When
considering only the tumor-bearing mice, the average weight of the liver in
the groups treated with N-(S)-(3,4-difluoro-2-
(2-fiuoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide at the highest
dose (Group 4 at 50mg/kg) and 5-Fluorouracil (Group 5 at 75mg/kg) was found to
be significantly different to the
Vehicle Control group (Group 1; p < 0.05). Also, the average weight of the
tumors in the groups treated with N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-1-sulfonamide
(Groups 3 and 4 at 10 mg/kg and 50mg/kg respectively) and 5-Fluorouracil
(Group 5 at 75mg/kg) was found to be
significantly different to the Vehicle Control group.
These results are presented graphically in Figure 16 (Mean Liver weight ¨
tumor-bearing mice only) and Figure
17 (liver tumor weights ¨ tumor bearing mice only)
Example 116: Growth Response of Orthotopic Human HT-29 Colon Tumors in Nude
Mice
treated with different amounts of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-
(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide
The dose response efficacy of N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide ("Compound A"), in inhibiting the
development of the orthotopic HT-29
human colorectal adenocarcinoma was assessed in BALB/c nu/nu mice, in
comparison with an optimal dose of 5-
Fluorouracil (75mg/kg).
Animals: Female BALB/c nu/nu mice (University of Adelaide, Waite Campus, SA,
Australia), aged 7-
12 weeks, with a body weight range: of 16.58-25.39g (mean 21.52g) were used
for the study. The mice were
divided into 6 Study groups (4 treatment groups and 2 control groups) as
follows:
Number of Mice per Group: 10 in Groups 1 to 5 inclusive
9 in 'Take-Rate' Control Group (Group 6)
The mice were kept in a controlled environment (targeted ranges: temperature
21 3 C, humidity 30-70%, 10-15
air changes per hour) under barrier (quarantine) conditions with a 12hour
light/12hour dark cycle. Temperature and
relative humidity were monitored continuously. A commercial rodent diet (Rat
and Mouse Cubes, Speciality Feeds Pty
Ltd, Glen Forrest, Western Australia) and tap water were provided to the
animals ad libitum. Both food and water
supplies were sterilized by autoclaving.
Tumor Inoculation: HT-29 human colorectal adenocarcinoma cells (Passage 4 from
working stock VP-Stock 325)
were cultured in RPMI1640 cell culture medium, which was supplemented with 10%
FBS and penicillin-streptomycin
(501U/naL fmal concentration). The cells were harvested by trypsinisation,
washed twice in HBSS and counted. The cells
were then resuspended in HBSS and adjusted to a final volume containing 2x108
cells/mL. Prior to inoculation, the
incision site was liberally swabbed with alcohol and an incision made through
the abdominal wall to expose the caecum
-Page 190-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
wall. The needle was introduced through the surface of the caecum wall where
54 of cells (1x106 cells) were
discharged.
Materials: The following were obtained from the respective suppliers.
Sterile saline solution (0.9% NaCkaq)) was obtained from Baxter Healthcare
Australia, Old Toongabbie, NSW,
Australia. CremophorEL was obtained from Sigma-Aldrich Pty Ltd, Castle Hill,
NSW, Australia. 5-Fluorouracil, clinical
formulation, clear, colorless liquid was obtained from Mayne Pharma Pty Ltd.
RPMI1640 cell culture medium, FBS and
HBSS were obtained from Invitrogen Australia Pty Ltd, Mt Waverley, VIC,
Australia. Penicillin-streptomycin and
Trypan Blue were obtained from Sigma-Aldrich, Castle Hill, NSW, Australia. HT-
29 human colorectal adenocarcinoma
cells were sourced from American Type Culture Collection (ATCC), Rockville,
MD, USA.
Compound Preparation and Administration: CremophorEL:Saline (1:9, v/v; vehicle
control), N-(S)-
(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-1-sulfonamide
or 5-Fluorouracil (compound control) were administered according to the
schedule below:
Group Compound Dose (mg/kg) Scheduled
Treatment Treatments Administered
Once daily for 21 Days Once daily for 21 Days (Day
1 Vehicle Control 10mL/kg
(Day 0 to 20) 0 to
20)
rnpound A
2 Co 0.2mL @ Once daily for 21 Days Once daily for 10 Days
(Day
10mL/kg=2mg,/kg (Day 0 to 20) 0 to
9)
3 C d A 1.0mL @ Once daily for 21 Days Once daily for 21 Days
(Day
ompoun
10mL/kg=10mg/kg (Day 0 to 20) 0 to
20)
4 C 5.0mL @ Once daily for 21 Days Once daily for 8 Days
(Day
ompound A
10mL/kg=50mg/kg (Day 0 to 20) 0 to
7)
7.5mL @ Once weekly for three Once weekly
for 3 weeks
5 5-Fluorouracil
10mL/kg=75mg,/kg weeks (Day 0,7 and 14) (Day 0, 7 and
14)
'Take-Rate'
6 No treatment
Control
The Vehicle Control, CremophoreEL:Saline (1:9, v/v), was administered p.o. in
a dosing volume of 10mL/kg,
once daily for 21 consecutive days (Day 0 to 20).
N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylam ino)-6-methoxypheny1)-1 -(2,3-
dihydroxypropyl)cyclopropane-1-
sulfonamide was formulated in CreinophorEL:Saline (1:9, v/v). A stock solution
was prepared weekly and stored at 4 C.
Dosing solutions were prepared on each day of administration. N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxyphenyI)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide was
administered p.o. at doses of 2, 10 and
50mg/kg, in a dosing volume of 10mL/kg, once daily for 21 days (Day 0 to 20).
5-Fluorouracil clinical formulation was diluted in sterile saline and
administered i.v. via the tail vein at a
concentration of 75mg/kg, in a dosing volume of 10mL/kg, once per week for
three weeks (on Day 0, 7 and 14).
No treatment was administered to the mice in Group 6 ('Take-Rate' Control). On
Day 7 of the study (21 days post-
inoculation), the mice were culled and the colon exposed to determine the take-
rate and size of the tumors in the caecum
wall.
Each animal's body weight was measured immediately prior to dosing. The volume
administered to each mouse
was calculated and adjusted based on the body weight.
Tumor Measurements: Caecum and tumor wet weight were measured when each were
excised post-mortem on
the termination day of the study. At the termination of the study, the caecum
was excised from all mice in each study
group and weighed with the tumors intact. The tumors were then excised from
the caecum and weighed.
-Page 191-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
The livers was also excised from all mice in each group at the termination of
the study and fixed in 10% buffered
formaiin. Five liver samples from the Vehicle Control group were embedded in
paraffin, sectioned and stained with
haematoxylin and eosin (H&E) for histological assessment for morphological
changes.
Data Measurement and Sample Collection Schedule
Data Measurement Schedule
Body weight
Day 0, then three times per week (Monday, Wednesday and Friday), and on the
termination day of the study for Groups 1 to 5 inclusive.
Caecum weight and Excised caecum and tumor from each mouse at
termination in Groups 1 to 5 inclusive
tumor weight
Sample Collection
Caecum and tumors From all mice in Group 6 ('Take-Rate' Control) post-
mortem, on Day 7 of the study.
Caecum and tumors From all mice in Groups 1 to 5 inclusive post-mortem,
on the termination day of the study,
and from mice which died during the study period.
Liver From all mice in Groups 1 to 5 inclusive post-mortem,
on the termination day of the study
and from mice which died during the study period.
Data Acquisition and Calculation: Each animal's transponder (Bar Code Data
Systems Pty Ltd, Botany Bay,
NSW) was scanned using a barcode reader (LabMax I, DataMars, Switzerland)
immediately prior to acquisition of data.
All measurements were acquired with the same handheld calipers (Absolute
Digimatic Model CD-6" CS, Mitutoyo
Corporation, Japan). The data was synchronized with vivoPharm's secure
relational database using Pendragon Forms 4.0
(Pendragono, Software Corporation, Libertyville, IL, U.S.A.) as transfer
software. AIDAM v2.4 was used for data
reports and data calculations.
Statistical and Calculations: All statistical calculations were performed
using SigmaStat 3Ø (SPSS Australasia
Pty Ltd, North Sydney, NSW, Australia).
A two-sample t-test was used to determine the significance in body weight
change within a treatment group
between Day 0 and the termination day of the study. In the groups treated with
N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-
sulfonamide at 2 and 50 mg/kg, treatment
was discontinued early due to excessive body weight loss. In these groups, a
two-sample t-test was used to determine the
significance in body weight change within a treatment group between Day 0 and
the final treatment day of the study, and
between the last treatment day and the termination day of the study. Where the
data did not pass the Normality test or the
Equal Variance Test, a Mann-Whitney Rank Sum Test was performed.
A One-Way Analysis of Variance (ANOVA) (All Pairwise Multiple Comparison
Procedure and Multiple
Comparison versus Control Group) was performed on the caecum weight and tumor
weight data at the end of the study.
Where the data did not pass the Normality test the values were converted to
the natural logarithm prior to performing the
procedure.
A p value of less than 0.05 was considered significant.
Observations: Average body weight loss was measured in all study groups,
including the Vehicle Control group.
Diarrhoea and signs of dehydration (loss of skin elasticity) were observed in
all of the study groups, including the
Vehicle Control. Severe body weight loss early during the study period led to
the cessation of treatment in the groups
receiving N-(S)-(3,4-difluoro-2-(2-fiuoro-4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-dihydroxypropypcyclopropane-
1-sulfonamide at the lowest (2mg/kg) and highest dose (50mg/kg) on Day 9 and
Day 7 of the study, respectively. As
body weight loss was less severe in the group receiving N-(S)-(3,4-difluoro-2-
(2-ftuoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide at 10mg/kg,
all treatments for this group were
-Page 192-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
administered as scheduled. The average body weight losses at the end of the
study for this group and the 5-Fluorouracil
treatment group were significant.
Although the take rate of the HT-29 tumors in the 'Take -Rate' group 21 days
after inoculation was
100%, the size of these tumors was much lower than anticipated. This may have
contributed to there being no
significant difference in average caecum and tumor weights between N-(S)-(3,4-
difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-l-
sulfonamide ¨ treated Groups and the
Vehicle Control Group. There was also no effect of 5-Fluorouracil on the
weight of the caecum and HT-29
tumors.
Body Weight Measurements ( SEM (Final Treatment Days and Study End Date)
Host Response
Delta Body % Delta
Weight (g) Body Delta Body
% Delta Survival
Grp Compound Dose (mg/kg), Route, Schedule Weight (g)
Number
( SEM) Weight ( SEM) Body Weight
(No.
Final Final
Final Study
Final Study
Alive/
Treatment Treatment
Day
Day
Total)
Day Day
Once daily for 21 days
1 Vehicle Control - p.o. -0.8 0.4
-3.6 8/10
(Day 0 to 20)
Once daily for ten Days -2.4 + 1.4
2 Compound A 2 p.o. -11.1 0.1 0.9
0.4 4/10
(Day 0 to 9) (Day 9)
Once daily for 21 days
3 Compound A 10 p.o. -1.5+0.3
-6.9 7/10
(Day 0 to 20)
Once daily for eight -3.8 0.5
4 Compound A 50 p.o. -17.8 -1.3 0.9
-5.9 7/10
Days (Day 0 to 7) (Day 7)
Once weekly for three
5 5-Fluorouracil 75 iv.
-3.3 0.4 -15.2 8/10
weeks (Day 0, 7 and 14)
Body weight data was not collected for Group 6 ('Take-Rate' Control). The
group was culled on Day 7 of the study
(Day 21 post-inoculation) to assess visually whether the tumors were growing
adequately for the purpose of the study.
Treatment was discontinued in Group 2 (N-(8)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropypcyclopropane-1-su1fonamide at 2 mg/kg) on
Day 9 of the study and in
Group 4 (N-(8)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1
-(2,3-
clihydroxypropyDcyclopropane- 1-sulfonamide at 50mg/kg) on Day 7 of the study,
as the mice were losing
excessive body weight. The remaining groups all received all of the scheduled
treatments during the study
period.
The average tumor weight in each group is shown in Figure 18. The average
weight of tumor for each group
includes only those which survived until the final day of the study. Values
for mice which died during the study period
are not included in the calculated average values.
Caecum Weight and Tumor Weight Data
Average Caecum
Average Tumour
Group Treatment Animal ID Caecum Weight (g)
Tumour Weight (g) SEM SEM
Weight (g)
Weight (g)
Vehicle Control 173811 0.290 0.070 0.413 0.081
0.149 0.080
(CremophorIEL:Sali 170774 0.331 0.160
Page 193-
CA 02 6 933 9 0 2010-01-18
WO 2009/018233 PCT/US2008/071392
ne) 170729 0267 0.017
173673 0.307 0.005
171429 0.311 0.326
175732 0.946 0.627
171539 0.286 0.038
173576 0.287 0.002
170836 0.397 0.000
172014 0.506 0.176
175867 0.347 0.001
170936 0.170 0.001
172003 0.150 0.000
Compound A @2 176472 0.205 0.005 .
2 0.296 0.033 0.033
0.030
mg/kg 171338 0.377 0.001
170825 0.233 0.122
174466 0.251 0.005
171587 0322 0.003
. -
173623 0.287 0.000
170793 0.198 0.000
172304 0.258 0.257
175676 0.111 0.008
Compound A @ 171003 0.263 0.422
3 0.244 0.025 0.078
0.059
10mg/kg 171466 0.237 0.001
176386 0.234 0.014
170858 0.289 0.004
175862 0.320 0.100
171364 0.251 0.000
175697 0.230 0.002
=
171349 0.254 0.002
174272 0.238 0.000
176335 0.201 0.004
Compound A @ 171041 0.337 0.166
4 0.290 0.038 0.122
0.092
50mg/kg 174536 0.169 0.655
175656 0.328 0.001
173626 0.217 0.001
171437 0.312 0.001
174501 0.463 0.026
, .
511.1"4 at 75 mg/kg 171322 0.355 0.245 A 0.391 0.050
0.069 0.041
175559 0.199 0.001
176302 0.360 0.000
176241 0.284 0.000
175857 0.421 0.010
176242 0.706 0.329
174165 0.415 0.130
-Page 194-
CA 0 2 6 93 3 90 2 01 0-01-1 8
WO 2009/018233 PCT/US2008/071392
176417 0.327 0.079
170592 0.237 0.000
171501 0377 0.003
The shaded boxes indicate samples collected from mice which died during the
study period. Calculated average
values for caecum weight and tumor weight exclude these values. Trends
indicate a reduction in HT-29 tumor and
caecum weight data after treatment with 10 mg/kg N-(S)-(3,4-difluoro-2-(2-
fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide.
Example 117: Tumor growth delay in nude mice bearing human A375 melanoma
xenogyafts
Six groups (n=9) of tumored mice were used. Control groups included one
receiving the 10% Cremophor
EL/saline vehicle by oral gavage (po), once-daily for 14 days (qd x14), and a
second given paclitaxel as a reference agent
at 30 mg,/kg by tail vein injection (iv), every other day for five doses (qod
x5). The four experimental groups received
oral N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-inethoxypheny1)-1-
(2,3-dihydroxypropypcyclopropane-1-
sulfonamide ("Compound A") at 25 mg/kg or 50 mg/kg, qd x14, or at 12.5 or 25
mg/kg, bid x14. Treatment outcome was
assessed by TGD, defined as the difference in median time to endpoint tumor
volume in a treatment group compared to
the control group. Toxicity was assessed by body weight measurements and
clinical observations.
Animals: Female athymic nude mice (nu/nu, Harlan) were 10 to 11 weeks old and
had a body weight (BW) range
of 19.3 to 25.5 gams on Day 1 of the study. The animals were fed ad libitum
water (reverse osmosis, 1 ppm Cl) and
NIH 31 Modified and Irradiated Lab Diet* consisting of 18.0% crude protein,
5.0% crude fat, and 5.0% crude fiber. The
mice were housed on irradiated ALPHA-Dri bed-o'cobe Laboratory Animal Bedding
in static rnicroisolators on a 12-
hour light cycle at 21-22 C (70-72 F) and 40-60% humidity. The
recommendations of the Guide for Care and Use of
Laboratory Animals with respect to restraint, husbandry, surgical procedures,
feed and fluid regulation, and veterinary
care were adhered to.
Tumor Implantation: Xenografts were initiated from A375 human melanoma tumors
by serial transplantation in
athymic nude mice. An A375 tumor fragment 1 min3) was implanted subcutaneously
into the right flank of each test
mouse, and tumor growth was monitored as the average size approached 100 - 150
mm3. Thirteen days later, designated
as Day 1 of the study, animals were placed into six groups each consisting of
nine mice (reduced from ten) with
individual tumor volumes ranging from 63 to 221 mm3 and group mean tumor
volumes of 125.3 to 125.9 mm3. Tumor
volume was calculated using the formula: Tumor Volume (mm3) =2 X 1, where w =
width and / = length in mm of an
2
A375 tumor.
Materials: N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-
1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide was dissolved at 5 mg/mL in 10%
Cremophor EL in saline with
sonication, shaking, and heating to 35 C to assist in dissolution. The 5 mg/mL
solution served as the dosing solution for
treatment at 50 mg/kg, and dosing solutions for 25 mg/kg and 12.5 mg/kg
treatment were prepared by serial dilution.
Dosing solutions were stored for up to one week at room temperature protected
from light.
Paclitaxel (NPI) dosing solutions were prepared from a 30 mg/mL stock for each
day's use by diluting to 3 mg/mL
in 5% ethanol, 5% Cremophor EL in 5% dextrose in water (D5W). Paclitaxel
dosing was at 30 mg/kg.
Treatment: The table below shows the treatment regimen.
Treatment Regimen
Group u Agent mg/kg I Route Schedule
-Page 195-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
1 9 Vehicle po qd x 14
2 9 Paclitaxel 30 iv god x 14
3 9 Compound A 50 po qd x 14
4 9 Compound A 25 pa bid x 14 first
day
1 dose
9 Compound A 25 po qd x 14
6 9 Compound A 12.5 po bid x 14 first
day
1 dose
Mice in Group 1 received vehicle consisting of 10% Cremophor EL in saline by
oral gavage (po) daily for fourteen
doses (qd x14), and served as a control for tumor progression. Group 2 animals
were administered intravenous (iv)
paclitaxel as a reference agent at 30 nag/kg, once every other day for five
doses (qod x5). Group 3 ¨6 mice received oral
5 N-(S)-(3 ,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxyphenyI)-1 -
(2,3-dihydroxypropyl)cyc lopropane-1 -
sulfonamide at the following respective schedules: 50 mg/kg, qd x14; 25 mg/kg,
twice-daily for 14 days with a single
dose given on the first and last days (bid x14); 25 mg/kg, qd x14; and 12.5
mg/kg, bid x14. All doses were given in
volumes of 0.2 mL per 20 g of body weight, and were scaled to the body weight
of the animal.
Endpoint: Tumors in all groups were measured twice weekly using calipers. Each
animal was euthanized when its
tumor reached the endpoint size of 2000 mm3 or on the final day of the study
(Day 60), whichever came first. The time
to endpoint (TE) for each mouse was calculated from the following equation:
TTE (days)
log (endpoint volume, mm3) b where b is the intercept and m is the
slope of the line obtained by linear
=
regression of a log-transformed tumor growth data set.
The data set was comprised of the first observation that exceeded the study
endpoint volume and the three
consecutive observations that immediately preceded the attainment of the
endpoint volume. Animals that do not reach
the endpoint are assigned a 1 FE value equal to the last day of the study.
Animals classified as NTR (non-treatment-
related) deaths due to accident (NTRa) or due to unknown causes (NTRu) are
excluded from TTE calculations (and all
further analyses). Animals classified as TR (treatment-related) deaths or NTRm
(non-treatment-related due to
metastasis) are assigned a TTE value equal to the day of death.
Treatment outcome was determined from tumor growth delay (TGD), defined as the
increase in the median time to
endpoint (TTE) in a treatment group compared to the control group: TGD T ¨ C,
expressed in days, or as a percentage
of the median TTE of the control group: %TGD = T - C x 100, where: T = median
TTE for a treatment group, C = median
TTE for the control group (Group 1).
Treatment may cause partial regression (PR) or complete regression (CR) of the
tumor in an animal. In a PR
response, the tumor volume is 50% or less of its Day 1 volume for three
consecutive measurements during the course of
the study, and equal to or greater than 13.5 mm3 for one or more of these
three measurements. In a CR response, the
tumor volume is less than 13.5 mm3 for three consecutive measurements during
the course of the study. An animal with a
CR response at the termination of a study is additionally classified as a
tumor-free survivor (TFS). Tumor regressions
were monitored and recorded.
Side Effects: Animals were weighed daily for the first five days of the study
and then twice weekly. The mice
were observed frequently for overt signs of any adverse, treatment-related
side effects, and clinical signs were recorded
when observed. Acceptable tolerability is defined as a group mean body-weight
loss of less than 20% during the test and
not more than one treatment-related death in a group of animals. Any dosing
regimen that does not meet these criteria is
considered above the maximum tolerated dose (MTD). A death is classified as TR
if attributable to treatment side effects
-Page 196-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
as evidenced by clinical signs and/or necropsy, or may be classified as TR if
due to unknown causes during the dosing
period or within 10 days of the last dose. A death is classified as NTR if
there is no evidence that death was related to
treatment side effects.
Statistical and Graphical Analyses: The logrank test was used to analyze the
significance of the differences
between the TTE values of treated and control groups. Two-tailed statistical
analyses were conducted at significance
level P = 0.05.
Median tumor growth curves show group median tumor volumes as a function of
time. When an animal
exited the study due to tumor size or TR death, the final tumor volume
recorded for the animal was included
with the data used to calculate the group median tumor volume at subsequent
time points. Curves were
truncated after 50% of the animals in a group had exited the study due to
tumor progression. Kaplan-Meier
plots were constructed to show the percentage of animals remaining in the
study as a function of time, and
used the same data set as the logrank test. Prism (GraphPad) for Windows 3.03
was used for all graphic
presentations and statistical analyses.
Treatment Response Summary
Group Median T-C %TGD Statistical MTV (n) Regressions Mean
RW
TTE Significance Day 60 Nadir
PR CR TFS
1 22.8 0 0 0
2 28.8 6.0 26 ** 0 0 0 -5.3%
Day 15
3 27.5 4.7 21 ** 1 0 0
4 59.9 37.1 163 *** 0(4) 4 5 4 -0.6%
Day 15
5 25.6 2.8 12 Ns 0 0 0
6 27.5 4.7 21 1 0 0
Growth of A375 Tumors in Control Mice (Group 1): Animals in Group 1 received
the 10% Cremophor EL/saline
vehicle, po, gd x14. Tumors in the control mice grew progressively to the 2000
min3 endpoint volume with a median
TTE of 22.8 days, establishing a maximum possible T-C in the study of 37.1
days, or 163% TGD.
Effect of Treatment with Paclita.ycel (Group 2): Group 2 animals were
administered paclitaxel as a reference
agent, 30 mg/kg, iv, god x5. All nine of the animals achieved the tumor volume
endpoint. Tumor growth paralleled and
was slightly right-shifted compared with the control group. The median TTE
value was 28.8 days, corresponding to 26%
TGD, a significant result by Logrank analysis (Table 2, P = 0.0088 (31 vs.
G2). No tumor regressions were associated
with paclitaxel treatment.
Effect of Treatment with N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny0-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide (Groups 3- 6): Groups 3 ¨6 received
oral dosing with N-(S)-(3,4-
difluoro-2-(2-fluoro-4-iodophenylamino)-6-methoxypheny1)-1-(2,3-
dihydroxypropyl)cyclopropane-l-sulfonamide as
monotherapy. Group 3 animals were administered 50 mg/kg on a qd x14 schedule.
The nine tumors in the group achieved
the volume endpoint. Median tumor volume for the group underwent little net
change for the first ¨ 10 days, then
increased for the duration of the study. A single animal experienced tumor PR.
The median TTE value was 27.5 days, or
21% TGD, a significant result (P = 0.0054 G1 vs. G).
Animals in Group 4 received 25 mg/kg N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-
(2,3-dihydroxypropyl)cyclopropane-l-sulfonamide on a bid x14 schedule. Four of
the nine animals in the group
remained on Day 60, all TES. An additional 2/9 animals had tumors reaching the
volume endpoint on the day before
study's end. The group had 4/9 PR, 5/9 CR and 4/9 TES. Median tumor volume
dropped beginning in the first few days
-Page 197-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
of the study and continued for about 30 days. Tumor regrowth in 5/9 animals
accounted for a resurgence of median tumor
growth beginning on about Day 32 and continuing to the end of the study. The
group median TTE value was 59.9 days,
representing the maximum possible 163% TGD (P < 0.0001, Table Al).
Mice in Group 5 also received 25 mg/kg N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxyphenyI)-
1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide, but on a less intense qd
x14 schedule. All nine animals in Group 5
reached the tumor volume endpoint, with no tumor regressions. Tumor growth
tracked closely with that of the control
group. The median TTE was 25.6 days, or 12% TGD, a non-significant result (P =
0.0662 01 vs. G5).
Group 6 animals were administered 12.5 mg/kg N-(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylarnino)-6-
methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-sulfonamide on a bid x14
schedule. All tumors in the group
achieved the volume endpoint. As with Group 4, median tumor volume in Group 6
dropped early in the study, but this
reduction was sustained for only about nine days and was associated with a
single PR response. Tumor volume increased
from Day 10 to study end. The median TTE for the group was 27.5 days,
corresponding to a significant 21% TGD (P =
0.0424 G1 vs. G6).
In summary, N-(S)-(3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-6-
methoxypheny1)-1-(2,3-
dihydroxypropypcyclopropane-l-sulfonamide displayed dose-related antitumor
activity against human A375 melanoma
xenografts with both once-daily and twice-daily oral dosing. Twice-daily
dosing was superior to once-daily in the
magnitude of TGD produced and in the numbers of objective responses. Thus, N-
(S)-(3,4-difluoro-2-(2-fluoro-4-
iodophenylamino)-6-methoxypheny1)-1-(2,3-dihydroxypropyl)cyclopropane-1-
sulfonamide antitumor activity is both
dose- and schedule-dependent.
Example 118: Activity Against Subcutaneous COLO 205 Human Colon Carcinoma
Xenografts
Animals: Female athymic nude mice (nu/nu, Harlan) were 12 to 13 weeks old and
had a body weight (BW)
range of 18.3 to 27.3 grams on Day 1 of the study. The animals were fed ad
libitum water (reverse osmosis, 1 ppm Cl)
and NIB 31 Modified and Irradiated Lab Diet consisting of 18.0% crude
protein, 5.0% crude fat, and 5.0% crude fiber.
The mice were housed on irradiated ALPHA-Dri bed-o'cobs Laboratory Animal
Bedding in static microisolators on a
12-hour light cycle at 21-22 C (70-72 F) and 40-60% humidity. The
recommendations of the Guide for Care and
Use of Laboratory Animals with respect to restraint, husbandry, surgical
procedures, feed and fluid regulation,
and veterinary care were adhered to.
Tumor Implantation: Xenografts were initiated from COLO 205 human colon
carcinoma cells. Tumor cells
were cultured 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin
G sodium, 100 tig,/mL streptomycin
sulfate, 0.25 ttg/mL amphotericin B, and 25 p.g/mL gentamicin, 2 ni.M
glutamine, 1 niM sodium pyruvate, 10 mM
HEPES and 0.075% sodium bicarbonate. Cell cultures were maintained in tissue
culture flasks in a humidified incubator
at 37 C, in an atmosphere of 5% CO2 and 95% air. On the day of tumor cell
implant, Colo 205 cells were harvested
during logarithmic growth and resuspended in 50% Mantel matrix (BD
Biosciences) in PBS at a concentration of 5 x
106 cells/mL. Each test mouse received 1 x 106 Colo 205 cells implanted
subcutaneously in the right flank, and the
growth of tumors was monitored as the average size approached 80 - 120 ram3.
Fourteen days later, designated as Day 1
of the study, animals were placed into eight groups (n = 9) with individual
tumor volumes ranging from 63 to 196 rom3
and group mean tumor volumes of 118-119 mrn3. Tumor volume was calculated
using the formula:
Tumor Volume (mm3) ¨ __ / , where w = width and / = length in mm of a COLO 205
tumor. Tumor weight may be
2
estimated with the assumption that 1 mg is equivalent to 1 mm3 of tumor
volume.
-Page 198-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Materials: Dosing solutions of Compound A were prepared fresh daily by
dissolving the required amount of
compound in 100% Cremophor EL, and then diluting ten-fold with normal saline.
Final dosing solution concentrations
were 2.5, 5, 10, or 20 mg/mL, in order to provide respective doses of 25, 50,
100 or 200 mg/kg in a dosing volume of 10
mL/kg. Paclitaxel (Natural Pharmaceuticals, Inc.) was prepared fresh on each
day of dosing in a vehicle consisting of
5% ethanol and 5% Cremophor EL in 90% D5W (5% EC vehicle).
Treatment: The table below shows the treatment regimen.
Treatment Regimen
Group n Agent mg/kg Route Schedule
1 9 Vehicle po qd x 14
2 9 Paclitaxel 30 iv qod x 14
3 9 Compound A 50 po qd x 14
4 9 Compound A 25 po bid x 14 first day
1 dose
5 9 Compound A 25 po qd x 14
6 9 Compound A 12.5 po bid x 14 first day
1 dose
Group 1 received the formulation vehicle (10% Cremophor EL in saline), and
served as the tumor growth
control group. Group 2 received the reference drug paclitaxel administered on
its optimal schedule in nude mice (30
mg/kg iv. qod x5). Groups 3 ¨6 received 25, 50, 100 and 200 mg/kg doses,
respectively, of Compound A administered
p.o. qd x14, with dosing in Group 6 (200 mg/kg) discontinued after six days
due to toxicity. All doses were scaled to the
weight of the animal (0.2 mL per 20 grams body weight).
Endpoint: Tumors were measured twice each week using calipers. Each animal was
euthanized when its tumor
reached the pre-determined endpoint size of 2000 mm3 or on the final day of
the study (Day 74), whichever came first.
However, control tumors did not exhibit logarithmic growth characteristics
after attaining a size of approximately 800
mm3. Therefore endpoint tumor size of 800 mrn3 was used for analysis of tumor
growth delay (TGD). The time to
endpoint (TTE) for each mouse was calculated from the following equation:
log10 (endpoint volume, inin3) ¨ b
TTE (days) = ______________________ , where b is the intercept and m is the
slope of the line obtained by linear
regression of a log-transformed tumor growth data set. The data set was
comprised of the first observation that exceeded
the study endpoint volume and the three consecutive observations that
immediately preceded the attainment of the
endpoint volume. Animals that do not reach the endpoint are assigned a TTE
value equal to the last day of the study.
Animals classified as NTR (non-treatment-related) deaths due to accident
(NTRa) or due unknown causes (NTRu) are
excluded from TTE calculations (and all further analyses). Animals classified
as TR (treatment-related) deaths or NTRin
(non-treatment-related death due to metastasis) are assigned a TTE value equal
to the day of death.
Treatment outcome was evaluated by tumor growth delay (TGD), which is defined
as the increase in the median
time to endpoint (TTE) in a treatment group compared to the control group: TGD
= T C, expressed in days, or as a
T
percentage of the median TTE of the control group: %TGD ¨ Cx 100, where: T
= median TTE for a treatment
group, C --- median TTE for the control group.
The control group was specified as Group 1 mice.
Treatment may cause partial regression (PR) or complete regression (CR) of the
tumor in an animal. In a PR
response, the tumor volume is 50% or less of its Day 1 volume for three
consecutive measurements during the course of
the study, and equal to or greater than 13.5 nun3 for one or more of these
three measurements. In a CR response, the
-Page 199-
CA 02693390 2010-01-18
WO 2009/018233 PCT/US2008/071392
tumor volume is less than 13.5 mm3 for three consecutive measurements during
the course of the study. Regression
responses were monitored and recorded.
Side Effects: Animals were weighed daily for the first five days of the study
and then twice weekly. The mice
were observed frequently for overt signs of any adverse, treatment-related
side effects, and clinical signs of toxicity were
recorded when observed. Acceptable toxicity is defined as a group mean body-
weight loss of less than 20% during the
study and not more than one treatment-related (TR) death among ten treated
animals, and any dosing regimen that results
in greater toxicity is considered above the maximum tolerated dose (MTD). A
death is classified as TR if attributable to
treatment side effects as evidenced by clinical signs and/or necropsy, or may
be assessed as TR if due to unknown causes
during the dosing period or within 10 days of the last dose. A death is
classified as an NTR if there is no evidence that
death was related to treatment side effects. Animals were monitored for side
effects by frequent observation and BW
measurements. BW changes were unremarkable, and all treatments were acceptably
tolerated, except Group 6. Six once
daily p.o. doses of 200 mg/kg Compound Aresulted in one TR death assessed on
Day 7 and two additional TR deaths on
Day 8. All mice in Group 6 exhibited clinical symptoms of toxicity including
hunched postures, hypoactivity, and loose
stools.
Statistical and Graphical Analyses: The logrank test was used to analyze the
significance of the differences
between the TTE values of treated and control groups. Two-tailed statistical
analyses were conducted at significance
level P = 0.05.
Median tumor growth curves show group median tumor volumes plotted on a log
scale as a function of time.
When an animal exited the study due to tumor size or TR death, the final tumor
volume recorded for the animal was
included with the data used to calculate the group median tumor volume at
subsequent time points. Curves were
truncated after 50% of the animals in a group had exited the study due to
tumor progression or after the second TR death
in a group. Kaplan-Meier plots were constructed to show the percentage of
animals remaining in the study as a function
of time, and used the same data set as the logrank test. Prism (GraphPad) for
Windows 3.03 was used for all graphic
presentations and statistical analyses.
Treatment Response Summary
Grp Median T-C /0TGD SS MTV (n) No of MeanBW
Day 74 PR CR IFS TR NTR
41.0 - 322(2) 0 0 0 0 0
2 60.0 19.0 46% ns 0(3) 1 0 0 2 2
3 47.9 6.9 17% ns 0(3) 1 0 0 2 2
4 59.1 18.1 44% ns 195 (1) 4 0 0 0 0
5 74.0 33.0 80% ns 320 (5) 4 0 0 1
6 57.8 16.8 41% ne 0 (3) 1 3 0 2 2
0.1%
Day 22
Growth of COLO 205 Tumors in Control Mice (Group 1)
Group 1 tumors exhibited slow, heterogeneous growth. The tumors of 7/9 vehicle-
treated Group 1 control mice
attained the 800 min3 tumor volume endpoint and two mice remained at study's
end. The Group 1 median TIE was 41.0
days, and therefore the maximum TGD possible in this 74-day study was 33.0
days (80%).
Effect of Treatment with Paclitaxel (Group 2)
Eight Group 2 mice (n = 9) that received treatment with paclitaxel remained in
the study on Day 74 with an
MTV of 143 nam3. This corresponds to the maximum possible TGD (33.0 days or
80%) and statistically significant
activity (P = 0.002). Five PR responses were documented. The median tumor
growth curve shows a decrease in MTV
through Day 19, followed by little change until Day 47 when tumor growth
resumed.
Page 200-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
Effect of Treatment with Compound A (Groups 3-6)
Groups 3, 4, 5 produced median TTEs of 47.9, 59.1 and 74.0 days, respectively.
Groups 3 and 4 had non-
significant logrank results, and the Group 5 logrank test attained borderline
significance (P = 0.058). These treatments
produced dose-dependent numbers of regressions, however, the type of
regression response (PR vs. CR) and the numbers
of 74-day survivors per group did not correlate with dose. The median tumor
growth curves indicate similar activities for
the three dose levels early in the study (through Day 29), followed by dose-
dependent delays in tumor re-growth. Group
6 produced three TR deaths, and dosing was stopped after Day 6. The 200 mg/kg
treatment was therefore deemed above
the MTD and not evaIuable for TGD.
Compound A demonstrated dose-dependent activity against COLO 205 colon
carcinoma xenografts. When
administered at 25 mg/kg, Compound A exhibited a TGD of 3%. At 50 mg/kg,
Compound A produced a TGD of 46%.
The 100 mg/kg treatment was acceptably tolerated and, like the paclitaxel
treatment, resulted in the maximum TGD
possible in the experiment with a similar number of regression responses. The
200 mg/kg treatment produced 3/9 TR
deaths and was above the MTD. A more pronounced initial decrease in tumor
burden for Compound A compared to
paclitaxel was observed; however, the duration of the effect was shorter.
Tumor re-growth in the 25 and 50 mg/kg
groups initially proceeded at a more rapid pace compared to controls, and by
the end of study, MTVs approached those
of controls. The 100 mg/kg treatment did not exhibit this rapid re-growth, but
did show faster tumor growth compared to
that of paclitaxel.
Example 119: Human Clinical Trial
A randomized, Double-blind, open label, historical control, single group
assignment, safety/efficacy human
phase I clinical trial with compound A vs placebo in Patients with chemo-naive
advanced or metastatic pancreatic cancer
will be performed.
The primary purpose of the study is to evaluate the safety and tolerability of
compound A. A secondary
outcome will be to evaluate the response rate, clinical benefit, and tumor
shrinkage after treatment with compound A.
Further, the study will be designed to evaluate time to disease progeression
and overall survival of patients with the
pancreatic cancer. In addition, pharmacodynamic changes in tumor vascular
parameters will be evaluated (including, e.g.
blood flow, blood volume, time to peak ROC-receiver operator characteristics
curve) by DCE-MRI.
Moreover, the biologic markers such as MEK1 and ME1C2 genetic plymorphisms and
serum proteomics will be
used to correlate outcomes. This will also permit the resectability rates of
tumors after treatment to be determined, as
well as the MID for compound A to be evaluated.
During the study, compound A will be administered in varying does of about
about 1 mg, about 1.5 mg, about 2
mg, about 2.5 mg, about 3 mg, about 3,5 mg, about 4.0 mg, about 4.5 mg, about
5 mg, about 5.5 mg, about 6 mg, about
6.5 mg, about 7 mg, about 7.5 mg, about 8 mg, about 8.5 mg, about 9 mg, about
9.5 mg, about 10 mg, about 10.5 mg,
about 11 mg, about 11.5 mg, about 12 mg, about 12.5 mg, about 13 mg, about
13.5 mg, about 14 mg, about 14.5, or
about 15 mg.
Inclusion criteria for the study will be based on the follwoing factors:
= Histologically/pathologically confirmed locally advanced unresectable or
borderline unresectable pancreatic
cancer, and no evidence of metastatic disease.
= Diagnosis of locally advanced unresectable pancreatic cancer based on
assessment by dual-phase CT scan
and/or endoscopic ultrasound (BUS) (EUS described in Appendix F).
-Page 201-
CA 02693390 2010-01-18
WO 2009/018233
PCT/US2008/071392
= Measurable disease according to RECIST and obtained by dual-phase CT scan
within 14 days prior to being
registered for protocol therapy.
= Tumor size greater than or equal to 2 cm on dual-phase computed
tomography scan.
= Adequate organ function documented within 14 days of registration as
evidenced by:absolute neutrophil count >
1500/mm3; platelet count;100,000/mm3; hemoglobin 9 gm/dL without transfusion
requirement in the prior 4
weeks; total bilirubin < 1.5 times upper limit of normal (ULN); transaminases
(AST and/or ALT) 2.5 x ULN;
PT (or INR) < 1.5 x ULN and aPTT within normal limits (patients who receive
anticoagulation treatment with
an agent such as warfarin or heparin will be allowed to participate; for
patients on warfarin, close monitoring of
at least weekly evaluations will be performed until INR is stable based on a
measurement at predose, as defined
by the local standard of care; Creatinine clearance of > 60 ml/ min calculated
using the Cockcroft-Gault
formula.
Exclusion Criteria will include: prior treatment with compound A within 6
months prior to registration; clinical
evidence of duodenal mucosal invasion by tumor (as documented by endoscopy or
endoscopic ultrasound); minor
surgical procedure (e.g fine needle aspiration or needle biopsy) within 14
days of study registration; major surgical
procedure, significant traumatic injury, or serious non-healing wound, ulcer
or bone fracture within 21 days of study
registration; any of the following within 6 months prior to study drug
administration: severe/unstable angina (anginal
symptoms at rest), new onset angina (began within the last 3 months) or
myocardial infarction, congestive heart failure,
cardiac ventricular arrhythmias requiring anti-arrhythmic therapy; history of
thrombotic or embolic events such as
cerebrovascular accident or transient ischemic attack within the past 6
months; history of aneurysm or arteriovenous
malformation; known human immunodeficiency virus (HIV) infection or chronic
Hepatitis B or C; active clinically
serious infection greater than CTCAE grade 2; receipt of any investigational
agent within 4 weeks of study registration;
uncontrolled hypertension defined as systolic blood pressure greater than 150
minHg or diastolic pressure greater than 90
nunHg, despite optimal medical management; pulmonary hemorrhage/bleeding event
greater than CTCAE Grade 2
within 4 weeks of study registration; any other hemorrhage/bleeding event
greater than CTCAE Grade 3 within 4 weeks
of study registration; evidence or history of bleeding diathesis or
coagulopathy; chronic, daily treatment with aspirin or
other nonsteroidal anti-inflammatory medications; use of St. John's Wort,
rifampin (rifampicin), ketoconazole,
itraconazole, ritonavir, or grapefruit juice; known or suspected allergy to
compound A; any condition that impairs
patient's ability to swallow whole pill; any malabsorption problem; other
severe, acute or chronic medical or psychiatric
condition, or laboratory abnormality that may increase the risk associated
with study participation or study drug
administration, or may interfere with the interpretation of study results, and
in the judgment of the investigator would
make the patient inappropriate for entry into this study; history of collagen
vascular disease; any contraindication to
undergo magnetic resonance imaging.
Example 120: Human Clinical Trial
A randomized, Double-blind, open label, historical control, single group
assignment, safety/efficacy human
phase I clinical trial with compound A in Patients with chemo-naive advanced
or metastatic stomach cancer will be
performed in the same manner as that prescribed in Example 117, except the
enrolled patients with be diagnosed either
lymphoma, gastric stromal tumors, or carcinoid tumors of the stomach.
Example 121: Carrageenan-Induced Paw Edema (CPE) in Rats
-Page 202-
=
= CA 02693390 2015-12-04
30725-1660
Compound A (6, 20 & 60 mg/kg) or indornethacin (3 mg/kg) were administered
orally 2 hr prior to injection of
I% suspension of carrageenan into the right hind foot pad of male Sprague-
Dawley rats (N=6 per treatment group). The
hind paw edema was measured 3 hr later by assessing paw volume by
plethysmography. Reduction of hind paw edema
by 30% or greater indicates significant acute anti-inflammatory activity,
Indomethacin (Indo) was used as a positive
control drug. Increases in paw volume were shown in each of the treatment
groups demonstrating that oral =
administration of Compound A resulted in significant anti-inflammatory
activity in the rat carrageenan paw edema model
in all dose groups.
Example 122: Rat Adjuvant Arthritis Inflammation Assay
In the rat adjuvant induced arthritis model, Complete Freund's Adjuvant (CFA)
is injected into the right hind
paw of rats to induce pathologies similar to rheumatoid arthritis in humans.
Compound A was administered orally for 5
consecutive days at 2, 6, and 20 mg/kg. Dexamethasone at S mg/kg was also
administered orally for 5 days. Enbrel at 10
mg/kg was administered by subcutaneous injection on days 1 and 4. CFA was
injected into the right hind paw one hour
after the first dose on day 1. The percent inhibition of right hind paw
swelling relative to vehicle treated controls on days
1 and 5 were determined for the acute phase while the percent inhibition of
left hind paw swelling relative to vehicle
treated controls on days 14 and 18 were determined for the delayed phase.
Polyarthritis was scored as the presence of
swelling in front paws, tail, nose or ear.
The percent inhibition of swelling relative to control for the different
treatment groups was tested.
Compound A at 20 mg/kg showed significant reduction in swelling in both the
acute and delayed
phases. For the polyarthritis scoring, all 6 animals in the vehicle treated
group had swelling in the front paws and tail. For
the 20 mg/kg Compound A group, 2 of 6 did not have front paw swelling and 4 of
6 did not have swelling in the tail. For
the enbrel group, no animals were protected from front paw swelling and 3 of 6
animals did not have swelling in the tail:
Example 123: Inhibition of Collagen-Antibody lnduced-Arthritis (CAM) in Mice
Male Balb/c mice (N=8 per treatment group) were injected intravenously (tail
vein) with 2 mg of collagen
antibody cocktail (Chondrex) on day 0. RDEA119 (1, 3 & 10 mg/kg QD) or
dexamethasone (1 mg/kg QD) were
administered orally from days 0-4, while Enbrel was injected subcutaneously on
days 1 and 3. Art intraperitoneal
injection of LPS (50 pg) was given on day 3 to all mice except naive animals.
The determination of the arthritic scores on
all limbs were determined (maximum score 16). Significant anti-inflammatory
activity was
noted for all test articles and reference drugs. Enbrel and dexamethasone were
used as positive controls.
=
Example 124: In Vivo Cell Proliferation Assay
A method for determining cell proliferation counts in cancerous cells treated
with a MEK protein kinase
inhibitor, is understood in the art and is described in Kenny, L.M. et al.,
Positron Emission Tomography
(PET) Imaging of Cell Proliferation in Oncology, Clinical Oncology, 16:176-185
(2004). A MEK
protein kinase inhibitor (e.g. Compound A) is examined in vivo to determine
their effect on
proliferation of cancerous coils. 50 patients are voluntarily enrolled in the
study, all of which are suffering from
pancreatic cancer at a similar stage of cancerous development. 25 patients are
administered a combination of compound
A. The final 25 patients are administered placebo. Each patient is
administered a daily dose for 14 days with a radio
labeled tracer, e.g. labeled fluoro-2-deoxy-DF-glucose (FDG).
=
After 14 days of treatment, a trained physician using a non-invasive positron
emission tomography (PET)
imaging apparatus detects tumor cell proliferation. Moreover, the trained
physician will determine cell proliferation
counts of both tumor and normal cell tissue for patients treated with Compound
A and placebo. The results will indicate
a decrease in cell proliferation counts between the MEK protein kinase
inhibitor (e.g. Compound A) and placebo. This
-Page 203-
=
CA 02693390 2015-12-04
30725-1660
assay for the determining cell proliferation counts using labeled tracers and
PET imaging is referred to herein as an "in
= vivo cell proliferation method." Other in vivo cell proliferation methods
are known in the art.
Similar analysis can be used to determine decrease in tumor size.
Example 125: In VIvo Apoptosis Assay
MEK inhibitor, e.g., Compound A is examined in vivo to determine its effect on
apoptosis of cancer cells. 40
patients are voluntarily enrolled in the study, all of which are suffering
from pancreatic cancer at a similar stage of
cancerous development. 20 patients are administered compound A and 20 patients
are administered placebo. Each
patient is adrninsitered a daily dose for 14 days.
After 14 days, each patient will consume a detectable lipopolysaecharide
binding protein (LBP)
= reagent coupled to a label. In accordance with WO/2006/054068, each patient
is
than placed in the field of a scanning apparatus whereby the scaning apparatus
detects the consumed reagent bound to
dead cells. The number of dead cells can be correlated to a level apoptosis of
each patient. The apoptosis levels in
patients administered the combinations and those administered the single
entity agents can be compared against each
others, as well as with respect to the cohort group administered placebo. This
assay for the detection of apoptosis levels
using a lipopolysaccharide binding protein and scanning apparatus is referred
to as the herein as an "in vivo apoptosis
method."
=
Example 126: Dissolution Studies
Capsules containing Compound A were prepared as described in the above
examples. The following dissolution
data was obtained using the USP<711> method for dissolution.
lmg from 10mg form
Time (min) % Release (%R5D) % Release (%RSD)
15 78 (8.3) 80 (7.3)
30 82(71) 87(92)
45 82 (6.7) 92 (9.6)
60 88(63) 92(72)
=
70 86(57) 95(54)
=
=
- Page 204 -