Note: Descriptions are shown in the official language in which they were submitted.
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
INFECTIOUS DISEASE TESTING OF MENSTRUAL FLUID,
ENDOMETRIAL/MENSTRUAL CELLS, AMNIOTIC FLUID, UMBILICAL CORD BLOOD
OR OTHER SAMPLES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the priorities of U.S. Provisional Patent
Application Serial No.
60/993,748, filed September 14, 2007, entitled "Infectious Disease Testing of
Menstrual Fluid,
Endometrial/Menstrual Cells, Amniotic Fluid, Umbilical Cord Blood or Other
Samples," and
U.S. Provisional Patent Application Serial No. , filed September 9, 2008,
entitled
"Infectious Disease Testing of Menstrual Fluid, Endometrial/Menstrual Cells,
Amniotic Fluid,
Umbilical Cord Blood or Other Samples," the entireties of which are
incorporated herein by
reference.
FIELD OF THE INVENTION
[00021 The invention relates generally to infectious disease testing and
specifically to
infectious disease testing of samples of bodily fluid, tissues and/or cells or
cellular components
obtained or procured from, for example, any one of a specimen of menstrual
fluid, endometrial
menstrual cells, umbilical cord blood, or amniotic fluid.
BACKGROUND OF THE INVENTION
[0003] There are many different types of infectious diseases in humans caused
by viruses,
bacteria and other agents. By way of non-limiting example, infectious diseases
include, but are
not limited to, Hepatitis A, Hepatitis B, Hepatitis C, Cytomegalovirus, Human
T-cell
Lymphotropic Virus Type 1, Human T-cell Lymphotropic Virus Type II, Human
Immunodeficiency Virus Type 1, Human Immunodeficiency Virus Type II, West Nile
Virus,
1
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
Trypansoma cruzi, Syphilis, and Treponema pallidum. Several tests have been
developed to
determine whether a human has an infectious disease.
[0004] Infectious disease testing involves a wide array of test methodologies
used to evaluate
the presence and absence of infectious agents in a specimen collected from a
human. Forms of
enzyme immunoassays are relied upon for methods for infectious disease testing
of venous
and/or arterial blood samples. For example, testing methods may incorporate
the enzyme
immunoassay (EIA) and enzyme-linked immunosorbent assay (ELISA). The EIA and
ELISA
tests are used to detect and to quantify antigens and antibodies present in a
venous and/or arterial
blood sample. Because sensitivities of most enzyme immunoassays are high, they
are typically
used for screening samples for the presence of infectious diseases.
100051 Many infectious disease tests employing enzyme immunoassay methods use
a solid
phase of an inactivated infectious agent coated onto micro wells and a
detection platform. The
solid phase test systems employ detection methods based on the adherence of
red cell antibodies
on the inactivated agent's antigen coated onto the surface of microtitration
wells on a microtiter
plate. The test procedure may be either a two or three step solid phase for
red cell adherence tests
carried out in the microtitration wells coated with the inactivated agent's
antigen. Serum or
plasma samples obtained from a venous or arterial blood specimen are added to
the antigen
coated wells. The serum or plasma samples are incubated with antibodies
specific for the antigen
that bind to the immobilized antigen. Unbound antibodies are washed from the
wells and
replaced with a suspension of coated indicator cells. A test result is
considered positive when the
usual migration of the indicator red cells to the bottom of the well is
impeded by bridges formed
between the red cells and the antigen-bound antibodies. The result causes the
indicator red cells
to adhere over the surface of the microtitration well. A test result is
considered negative when
2
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
there is an absence of antigen-antibody bridges allowing the indicator red
cells to pellet to the
bottom of the well as a packed, well-defined cell button. For the tests with
multiple phases, the
phases represent stepwise sample incubations, washings, and resulting
assessments. In addition,
many tests, whether for a virus, bacteria or other infectious agents, may use
a colorimetric
indicator to denote the test results.
[00061 Regardless of the test used for infectious disease testing, the
specimen or sample type
for testing is prescribed. Infectious disease screening tests licensed by the
Food and Drug
Administration (FDA) prescribe specific types of specimens to be used with
licensed test kits.
These specimens are serum, plasma, or cadaveric serum specimens which are
obtained from
venous or arterial puncture. Certain kit inserts expressly preclude use of a
kit for testing
saliva/oral fluid or urine samples.
[0007] Developments in the stem cell industry have identified new sources of
stem cells from
body fluids and tissue sources. For example, stem cells may be harvested from
menstrual fluid,
amniotic fluid, and umbilical cord blood. Infectious disease tests and assays
are not available for
use in infectious disease testing of menstrual fluid and endometrial/menstrual
cells obtained from
menstrual fluid, amniotic fluid samples, and/or umbilical cord blood.
[0008] Menstrual fluid, endometrial/menstrual cells and umbilical cord blood
is a readily
available specimen source that may be used for infectious disease testing. An
amniotic fluid
specimen would be a by-product of a previously-collected amniotic fluid sample
used for another
primary test assessing another parameter. Umbilical cord blood is another
specimen that has been
shown to be easily procured and provides a suitable amount of sample for
testing. Indeed, the
process of procuring endometrial/menstrual fluid and/or cells during the
menstrual cycle,
3
CA 02699491 2010-03-12
1 ~
WO 2009/035706 PCT/US2008/010751
amniotic fluid from a previously-collected sample, and umbilical cord blood
generally imparts
little to no risk to the donor associated with collection techniques.
[0009] Menstrual fluid, endometrial/menstrual cells, amniotic fluid samples,
and/or umbilical
cord blood specimens are beneficial because each type of sample may serve as a
single source
for infectious disease testing. A single source sample does not require
analysis of any other
comparative sample, and its does not require analysis of an indirect sample.
As an example of
such indirect testing, and under current protocols in the cord blood industry,
the presence or
absence of infectious diseases in umbilical cord blood is determined
indirectly from the results of
testing of the mother's venous blood sample. A correlation of the infectious
disease test results is
made from the mother's blood relating to the umbilical cord blood. The cord
blood is never
directly tested.
[0010] The present invention provides methods, processes, and systems for
direct testing of
umbilical cord blood, menstrual fluid, endometrial/menstrual cellular
suspensions, amniotic
fluid, and/or other bodily fluids, tissues or cells for the present of
infectious diseases or markers
associated with infectious diseases such an infectious disease antigens and
human antibodies
created in an immune response to an infectious disease. Use of a single source
specimen of
umbilical cord blood, menstrual fluid, endometriallmenstrual cells, amniotic
fluid, and/or other
bodily fluids, tissues or cells samples provides for such direct infectious
disease testing.
[0011] Accordingly, there is a present need for methods and processes for
testing specimens of
menstrual fluid, endometrial/menstrual cells suspensions, amniotic fluid,
and/or umbilical cord
blood for infectious disease. Thus, there is a need for methods and processes
for infectious
disease testing of these types of biological samples.
SUMMARY OF THE INVENTION
4
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0012] The present provides methods and processes for infectious disease
testing of menstrual
fluid, endometrial/menstrual cells, amniotic fluid, and/or umbilical cord
blood.
[0013] The invention includes methods and processes for procuring and
processing blood,
fluids, cells, and tissues obtained during menstruation, and includes the
testing for the presence
of infectious diseases and agents in the menstrual fluid and/or
endometrial/menstrual cells in
suspension. For example, the invention comprises methods and processes for
procuring and
processing blood, fluids, and tissues obtained during menstruation, and
testing or analyzing the
blood, fluid, and/or tissues to determine the presence of any infectious
disease and infectious
agents in the menstrual fluid and/or endometrial/menstrual cells.
[0014] The invention includes methods and processes for processing amniotic
fluid obtained
as a by-product of amniotic fluid sampling collected for the primary purpose
of other clinical or
research assessments, and includes the testing for the presence of infectious
diseases and agents
in the sample of amniotic fluid. For example, the invention comprises methods
and processes for
processing amniotic fluid samples obtained as a by-product of amniotic fluid
samples collected
for the primary purpose of other clinical or research assessments, and testing
or analyzing the
blood, fluid, and/or tissues to determine the presence of any infectious
disease and infectious
agents in the amniotic fluid.
[0015] The invention includes methods and processes for processing umbilical
cord blood
obtained at childbirth, and includes the testing for the presence of
infectious agents/diseases in
the umbilical cord blood. For example, the invention comprises methods and
processes for
procuring and processing umbilical cord blood obtained at childbirth, and
testing or analyzing
the blood, fluid, and/or tissues to determine the presence of any infectious
disease and infectious
agents in the umbilical cord blood.
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0016] In an embodiment, the present invention provides a method for analyzing
a non-venous
and non-arterial puncture human fluid or cell sample to detect the presence of
at least one
infectious disease. The method comprises the step of first obtaining a
sufficient volume of the
non-venous and non-arterial puncture human fluid or cell sample. The non-
venous and non-
arterial puncture human fluid or cell sample may be procured from any one of a
specimen of
menstrual fluid, endometrial menstrual cells, umbilical cord blood, or
amniotic fluid. The method
comprises the following step of testing the sufficient volume of the non-
venous and non-arterial
human fluid or cell sample for at least one infectious disease.
[0017] The present invention provides additional steps of performing
confirmatory testing of
an arterial or venous blood sample obtained from a human and comparing
confirmatory testing
results to test results for the non-venous and non-arterial puncture human
fluid or cell sample.
[0018] The present invention provides that the infectious disease tested for
in the non-venous
and non-arterial human fluid or cell sample comprises any one of Hepatitis A,
Hepatitis B,
Hepatitis C, Cytomegalovirus, Human T-cell Lymphotropic Virus Type 1, Human T-
cell
Lymphotropic Virus Type II, Human Immunodeficiency Virus Type 1, Human
Immunodeficiency Virus Type II, West Nile Virus, Trypansoma cruzi, Syphilis,
and Treponema
pallidum. Biological samples may be tested for other infectious diseases.
[0019] The present invention provides for testing that may comprise analyzing
the biological
sample for at least one form of antigen or antibody associated with the
presence of at least one
infectious disease in a human.
[0020] In another embodiment, the present invention provides a method for
direct testing of a
menstrual fluid sample from a human for the presence of at least one
infectious disease. The
method comprises isolating a sufficient volume of the menstrual fluid sample
from a pre-
6
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
collected specimen. The method comprises analyzing the menstrual fluid sample
for the presence
of at least one or more infectious diseases.
[0021] The present invention provides that the infectious diseases that may be
directly tested
for in the menstrual fluid sample comprises any one of Hepatitis A, Hepatitis
B, Hepatitis C,
Cytomegalovirus, Human T-cell Lymphotropic Virus Type 1, Human T-cell
Lymphotropic Virus
Type II, Human Immunodeficiency Virus Type 1, Human Immunodeficiency Virus
Type II,
West Nile Virus, Trypansoma cruzi, Syphilis, and Treponema pallidum.
[0022] The present invention provides that the step of analyzing the menstrual
fluid sample
comprises determining the presence of at least one form of antigen associated
with an infectious
disease or an antibody created by a human's immune response to the presence of
an infectious
disease in the human's body.
[0023] In a further embodiment, the present invention provides a method for
direct testing of a
human body fluid or cell sample for the presence of at least one infectious
disease. The method
provides preparing a sufficient volume of human body fluid or cell sample for
analysis. The
method also provides analyzing human body fluid or cell sample for antigens or
antibodies
associated with an infectious disease.
[0024] The present invention provides that the human body fluid or cell sample
may be
procured from any one of a specimen of menstrual fluid, endometrial menstrual
cells, umbilical
cord blood, or amniotic fluid.
[0025] The present invention provides that the infectious disease tested for
in the human body
fluid or cell sample comprises any one of Hepatitis A, Hepatitis B, Hepatitis
C,
Cytomegalovirus, Human T-cell Lymphotropic Virus Type 1, Human T-cell
Lymphotropic Virus
Type II, Human Immunodeficiency Virus Type 1, Human Immunodeficiency Virus
Type II,
7
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
West Nile Virus, Trypansoma cruzi, Syphilis, and Treponema pallidum. The human
body fluid
or cell sample may be tested for other infectious diseases.
[0026] The present invention provides that the testing may comprise a
screening test or a
confirmatory test.
[0027] The present invention provides that the testing may comprise analyzing
the human
body fluid or cell sample for the presence and/or quantity of an antigen
produced by an
infectious disease or the presence and/or quantity of a human antibody
associated with the
presence of an infectious disease in a human.
[0028] The present invention provides an additional step of correlating the
screening test
results with the confirmatory -test results. The screening test results may
comprise test results
obtained by the embodiments of the present invention for non-venous and non-
arterial puncture
human fluid or cell samples, menstrual fluid samples, and human body fluid or
cell samples. The
confirmatory test results may be obtained from additional testing of non-
venous and non-arterial
human fluid or cell samples, menstrual fluid samples, and human body fluid or
cell samples or
alternatively corresponding venous or arterial blood samples.
[0029] The present invention provides for determining the presence or absence
of infectious
disease antigens or human antibodies created by an imrnune response to
infectious disease
antigens.
BRIEF DESCRIPTION OF THE DRAWINGS
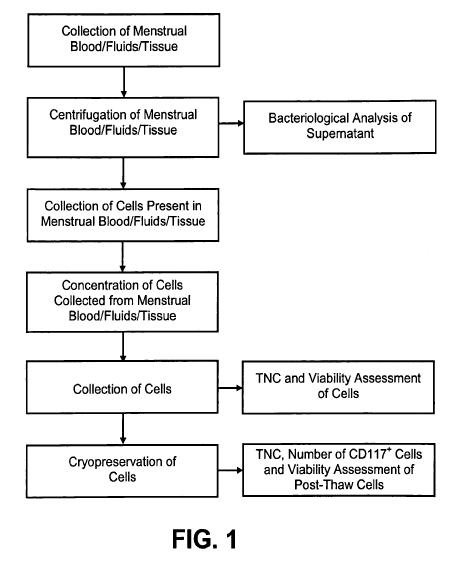
[0030] FIG. 1 shows a flow chart depicting an overview of an embodiment of a
method to
procure and to process menstrual blood, fluids, tissue and cells;
[0031] FIG. 2 shows a flow chart depicting an overview of an embodiment of a
method to
procure and process menstrual blood, fluids, tissue and cells;
8
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0032] FIG. 3 shows a flow chart depicting another embodiment for procuring
and processing
menstrual blood, fluids, tissue and cells and testing for infectious diseases;
[0033] FIG. 4 shows a flow chart depicting an overview of a method of the
present invention
for procuring and processing umbilical cord blood and testing for infectious
diseases; and
[0034] FIG. 5 shows a flow chart depicting an overview of a method of the
present invention
for procuring and processing amniotic fluid and testing for infectious
diseases.
DESCRIPTION OF THE EMBODIMENTS OF THE INVENTION
100351 In reference to FIGS. 1 through 5, infectious disease testing methods
and processes for
menstrual fluid, endometrial/menstrual cells, amniotic fluid, and/or umbilical
cord blood are
provided by the present invention.
[0036] Methods are provided for obtaining a sample of inenstrual fluid,
endometrial/menstrual
cells, amniotic fluid samples, umbilical cord blood or other bodily fluid or
tissue. The methods
comprise the further step of testing a suitable volume of sample with
infectious disease testing
methods. The infectious disease testing methods may be selected for testing
for infectious
diseases of interest. The infectious disease testing methods may be commercial
tests.
[0037] A method is provided for analyzing a non-venous and non-arterial
puncture human
fluid or cell sample to detect the presence of at least one infectious
disease. The method
comprises first obtaining a sufficient volume of the non-venous and non-
arterial puncture human
fluid or cell sample. The non-venous and non-arterial human fluid or cell
sample may be
procured from any one of a specimen of menstrual fluid, endometrial menstrual
cells, umbilical
cord blood, or amniotic fluid.
[0038] The method comprises the additional step of testing the sufficient
volume of the non-
venous and non-arterial human fluid or cell sample for an infectious disease.
The testing may be
9
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
focused on analyzing the biological sample for an antigen or antibody
associated with the
presence of an infectious disease in a human. The infectious disease testing
may be in the nature
of a screening test or a confirmatory test.
100391 The method may comprise an additional step of performing confirmatory
testing of an
arterial or venous blood sample obtained from a human and comparing
confirmatory testing
results to test results for the non-venous and non-arterial human fluid or
cell sample. The test
results are reported to the patient, sample donor, health care provider,
and/or stem cell banking
provider.
[0040] Methods are provided for direct testing of a menstrual fluid sample
from a human for
the presence of at least one infectious disease. The method comprises
isolating a sufficient
volume of the menstrual fluid sample from a pre-collected specimen. The method
comprises
analyzing the menstrual fluid sample for the presence of an infectious
disease. The analysis of
the menstrual fluid sample may comprise a screening test or a confirmatory
test. The menstrual
fluid sample is analyzed to determine the presence or absence of an antigen
associated with an
infectious disease or an antibody created by a human's immune response to the
presence of an
infectious disease.
[0041] Methods are provided for direct testing of a human body fluid or cell
sample for the
presence of at least one infectious disease. The method provides preparing a
sufficient volume of
human body fluid or cell sample for analysis. The human body fluid or cell
sample may be
procured from any one of a specimen of menstrual fluid, endometrial menstrual
cells, umbilical
cord blood, or amniotic fluid. The method also provides analyzing human body
fluid or cell
sample for antigens or antibodies associated with an infectious disease. The
analysis may
comprise a screening test or a confirmatory test. The testing is focused on
analyzing the human
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
body fluid or cell sample for the presence and/or quantity of an antigen
produced by an
infectious disease or the presence and/or quantity of a human antibody
associated with the
presence of an infectious disease in a human.
[0042] The methods of the present invention provide for infectious disease
testing. Infectious
diseases that are tested for by way of the present invention include, but are
not limited to,
Hepatitis A, Hepatitis B, Hepatitis C, Cytomegalovirus, Human T-cell
Lymphotropic Virus Type
1, Human T-cell Lymphotropic Virus Type II, Human Immunodeficiency Virus Type
1, Human
Immunodeficiency Virus Type II, West Nile Virus, Trypansoma cruzi, Syphilis,
and Treponema
pallidum. The present invention may test for other infectious diseases.
[0043] Sources of Fluid or Cell Sample for Infectious Disease Testing
[0044] The present invention provides for several sources of human fluid or
cell samples for
infectious disease testing. The sample source may be characterized as a non-
venous and non-
arterial puncture human fluid or cell specimen. The sample source may also be
characterized as a
human body fluid or cell sample. In particular, the sample source comprises
menstrual fluid that
may be menstrual flow collected during menstruation, amniotic fluid collected
during
amniocentesis, or umbilical cord blood collected during birth. Specimens of
each of these sample
sources that are initially collected may be tested for infectious diseases.
Alternatively, the sample
sources may be processed to obtain concentrated volumes of plasma, serum,
and/or cellular
suspensions that may be tested. If needed for infectious disease testing, the
cells of the cellular
suspension may be lysed to obtain cellular components for infectious disease
testing.
[0045] Menstrual Fluid
[0046] Referring now to FIGS. 1 through 3, the invention comprises methods for
procuring
menstrual fluid specimens. Menstrual fluid specimens comprise menstrual blood,
fluids, cells,
11
CA 02699491 2010-03-12
. .,
WO 2009/035706 PCT/US2008/010751
and tissues obtained during menstruation. A menstrual fluid specimen may be
collected from the
menstrual fluid and then analyzing or tested to determine the presence of at
least one infectious
disease antigen associated with an infectious disease or the presence of human
antibodies
produced by an immune response to an infectious disease that are present in
the menstrual fluid.
[0047] Menstrual fluid samples may be collected in a variety of different ways
for infectious
disease testing. In an embodiment, the menstrual flow may be collected
according to the
processes and methods described in U.S. Patent Application Serial No.
12/074,423, entitled
"Procurement, Isolation, and Cryopreservation of Endometrial/Menstrual Cells"
filed March 2,
2008 which is incorporated herein in its entirety by reference. Other
processes and methods for
collecting a menstrual fluid sample may be used so long as a sufficient volume
of menstrual flow
sample is collected to perform the step of testing the menstrual fluid sample
for the presence of
an infectious disease.
[0048] In an embodiment, a donor may use a procurement kit to collect a may
comprise media
tubes that contain collection media in a rack covered in Parafilm, at least
one collection device
and instructions (i,e., INSTEAD as described in FIG. 2 or the DIVA cup as
described in FIG. 3),
antiseptic cleaning pads, small plastic bags, small biohazard bag (to place
specimens in for return
to laboratory), Parafilm, nanocooler, and a collection form. The collection
kit may remain at
room temperature prior to use. Alternatively, the collection kit may be
maintained at a
refrigerated temperature of about 1 C to about 10 C prior to use.
[0049] The menstrual flow sample may be collected in a collection cup designed
for menstrual
flow collection. The collection of menstrual flow may take place on one of the
heaviest days of
the donor's menstrual period which may be the first or second day. The
collection cup may be
12
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
sterilized prior to use and the donor should follow the manufacturer's
instructions for insertion of
the collection cup into the vagina.
[0050] The collection cup may be inserted into the vagina. The general area
around the vagina
may be cleansed with an aseptic cleaning pad prior to insertion of the
collection cup. The
collection cup may remain inside the vagina for about up to 3 hours or less or
any other suitable
amount of time necessary to collect a menstrual flow sample in a sufficient
volume to allocate
some of the menstrual flow sample for infectious disease testing. After a
suitable amount of time
has passed with the collection cup positioned inside the vagina, a donor may
then remove the cup
and pour the contents comprising menstrual flow contents into a 50 milliliter
collection tube
holding collection media. The collection media may comprise about 10
milliliters of an isotonic
solution with an anticoagulant. For example, but not as a limitation, Hank's
Balanced Salts
Solution may be used as an isotonic solution, and Heparin (10 units per
milliliter) may be used as
an anticoagulant. Other collection media may be used including, but not
limited to, the collection
media described in U.S. Patent Application Serial No. 12/074,423. The
collection tube should be
closed and sealed.
[0051] Multiple menstrual flow samples may be collected using a new collection
cup each
time. If multiple menstrual flow samples are collected, the entire sample of
menstrual flow in a
single collection cup should be placed in one 50 milliliter collection tube
containing collection
media. One collection tube should be used for each sample of menstrual flow.
Whether a single
sample or multiple samples are collected, the sample or samples may be
maintained at room
temperature (about 15 C to about 25 C) prior to shipment to the laboratory so
long as the sample
or samples arrive at the laboratory within about 24 hours to about 72 hours of
collection.
Alternatively, and in cases where multiple samples are collected, collection
tubes containing
13
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
samples may be refrigerated at about 1 C to about 10 C until all samples have
been collected
prior to shipment. Alternative shipping and handling methods and processes for
menstrual fluid
are provided in U.S. Patent Application Serial No. 12/074,423 and may be used
in the present
invention. The package containing the menstrual flow sample or samples may be
maintained at
room or ambient air temperature (about 15 C to about 25 C) throughout shipment
and should
arrive at the laboratory within about 24 hours of collection and may be
refrigerated upon arrival
at the laboratory. Alternatively, the package containing the menstrual flow
sample or samples
may be maintained at a temperature of about 1 C to about 10 C throughout
shipment.
(0052] A peripheral blood sample may also be collected from the donor for
screening or
comparative infectious disease testing purposes. The peripheral blood sample
may be collected
according to standard phlebotomy techniques in an evacuated blood collection
system holder and
the evacuated tube with EDTA (dipottasium salt of ethylenediaminetetraacetic
acid). The
peripheral blood sample may also be shipped along with the menstrual flow
sample to a
laboratory as previously described.
[0053] The present invention comprises the step of analyzing and determining
the presence of
any infectious disease and infectious agents in the menstrual flow sample.
Upon arrival of the
menstrual flow sample or samples at a laboratory, the collection tube, or
tubes if multiple
menstrual flow samples are collected, are disinfected with IPA or Cavicide,
for example, and
transferring each collection tube to a biologically safety cabinet under
sterile conditions. Each
collection tube may be placed on ice or maintained at a temperature between
about 1 C to about
C throughout processing in preparation for aliquoting a sample into a transfer
tube for
infectious disease testing.
14
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0054] The menstrual flow sample may be processed by removing an aliquot of
about 5
milliliters from the sample and placing the sample in a transfer tube, such as
a 6 milliliter transfer
tube or other tube suitably size to hold a sufficient volume of sample for
testing. As an
alternative and as described in FIG. 3, the menstrual flow sample may be
centrifuged at about
2000 RPM for about 4 C. Centrifugation may also take place at room
temperature. Once the
centrifugation is completed, a 10 milliliter sample may be collected from the
supernatant and
placed in a transfer tube.
[0055] The transfer tube with either an uncentrifuged menstrual flow sample or
supernatant
from a centrifuged menstrual flow sample may be labeled and prepared for
infectious disease
testing. The transfer tube may be maintained at room temperature if infectious
disease testing
will occur at a time period less than about 7 days. Altematively, the transfer
tube may be
refrigerated at about 1 C to about 10 C for up to about 7 days prior to
infectious disease testing.
the transfer tube may be maintained at a temperature between about 1 C to
about 10 C
throughout shipment to an off site infectious disease testing service
provider. Alternatively, the
transfer tube may be shipped at room temperature.
[0056] In a further embodiment, a pre-processing sample of supematant obtained
from a
menstrual flow sample specimen that was procured and processed in connection
with methods
and processes of menstrual stem cell collection, such as those generally
outlined and described in
FIGS. 1 and 2 or as described in U.S. Patent Application Serial No. 12/074,423
may be obtained
for infectious disease testing.
[0057] In another embodiment, a suitable volume of a post-processing menstrual
flow sample
obtained according to the methods and processes of U.S. Patent Application
Serial No.
12/074,423 may be obtained for infectious disease testing.
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0058] The transfer tube containing an aliquot of menstrual flow, centrifuged
menstrual flow,
supernatant from a pre-processing sample or from post-processing sample may be
tested for
infectious disease markers of interest. For example, an order form for an
infectious disease
testing may be completed to order infectious marker testing for infectious
diseases of interest if
testing is to occur off site by a laboratory or a service provider that
performs infectious disease
testing services. Alternatively, the transfer tube containing the menstrual
flow sample may be
tested for infectious diseases at the menstrual flow sample-receiving
laboratory. A transfer tube
may be refrigerated at about 1 C to about 10 C for up to about 7 days prior to
infectious disease
testing. A transfer tube may be maintained at a temperature between about 1 C
to about 10 C
throughout shipment to an off site infectious disease testing service
provider. Altematively, the
transfer tube may be shipped at room temperature.
[0059] Alternative forms of collecting, transporting, preparing, and testing a
menstrual flow
sample are provided in U.S. Patent Application Serial No. 12/074,423 and may
be used for
obtaining a menstrual flow sample for infectious disease testing according to
the invention.
[0060] Umbilical Cord Blood Samples
[0061] Referring to FIG. 4, the present invention provides methods and
processes for
obtaining an umbilical cord blood sample during at childbirth, and then
analyzing or testing the
umbilical cord blood sample to determine the presence of any infectious
disease antigens or
human antibodies resulting from an immune response to the infectious disease
antigen in the
umbilical cord blood sample.
[0062] Umbilical cord blood may be obtained at childbirth using any manner of
umbilical cord
blood collection methods. In an embodiment, a cord blood collection kit (U-
Cord Collection Kit
Contents: Blue Basin with Clear Top, Mother's Blood Draw Kit, DonorCareTM
Needle Guard,
16
, CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
Alcohol Wipes, Tincture of Iodine Swabstick, Baxter Blood Collection Bag (250
mL) with CPD
anticoagulant and 16 gauge needle, C-Section Adapter Kit (Sterile), Plastic
zip bag with adhesive
backing and absorbent towels) may be used in accordance with manufacturer's
instructions. For
example, cord blood collection for a vaginal delivery may occur by double
clamping the cord
and cutting the umbilical cord. The blue plastic DonorCare needle guard may be
clamped around
the collection tubing. After delivery of a baby(s) and prior to expulsion of a
placenta, about 4-6"
of umbilical cord may be cleansed with an alcohol wipe followed by a Tincture
of Iodine swab.
For maximum volume, a needle may be inserted at an insertion site just above
the clamp that
remains on the umbilical cord. Using gravity, collect as much blood as
possible into the bag. At
least about 80 mL (including 35 mL of anticoagulant) of collection may be
needed for
processing. When collection is complete, the bag may be at least 1/3 full.
When collection is
completed, the blood may be milked in the tubing down into the bag. The bag
may be gently
inverted several times to mix the umbilical cord blood and CPD anticoagulant.
For multiple
births, individual cord blood kits should be used for each baby.
[0063] If collection of an umbilical cord blood sample occurs after cesarean
delivery, a C-
Section Adapter Kit may be used for double clamping the cord after birth and
inserting a needle
into the umbilical vein to collect umbilical cord blood. As much blood as is
possible may be
collected in the bag. At least about 80 mL (including 35 mL of anticoagulant)
of collection may
be needed for processing. Gently invert bag several times to mix the cord
blood and CPD
anticoagulant. For multiple births, individual cord blood kits should be used
for each baby.
[00641 If placenta is detached, the umbilical cord may be cleansed. A needle
may be inserted
into the umbilical vein attached to the placenta. The detached placenta may be
elevated to
facilitate collection of cord blood. At least about 80 mL (including 35 mL of
anticoagulant) of
17
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
collection may be needed for processing. For multiple births, individual cord
blood kits should
be used for each baby.
[0065] A collected umbilical cord blood sample may be prepared for shipment by
wrapping a
labeled cord blood bag with the absorbent towels. The cord blood bag may be
placed in a large
plastic zip bag that is closed. An adhesive backing may be removed from the
large plastic zip bag
then the bag may be pressed to bottom of a container to secure the collected
blood sample for
shipment. A lid may be placed on the container and secured with tape. AirNet
or another suitable
courier may be contacted within about 2 hours of birth(s) to arrange shipment.
The cord blood
shipment should arrive in a laboratory within at least about 48 hours of
collection.
[0066] The cord blood bag should be transferred into a laboratory, sterilized,
and prepared, for
processing in a biological safety cabinet. A sample of the cord blood may be
drawn for infectious
disease testing. Alternatively, a sample of cord blood may be obtained after
removal of the red
cells from the umbilical cord blood sample. The cord blood sample used for
infectious disease
testing may be about one milliliter of cord blood for a red cell sample and up
to about three
milliliters for a plasma sample.
[0067] The sample of cord blood will be transferred to a sterile collection
tube coated with
EDTA. The sample of cord blood or cord blood plasma may be transferred to two
or more 6
milliliter tubes for testing. The tubes may or may not contain an
anticoagulant, such a Heparin.
[0068] Amniotic Fluid
[0069] Referring to FIG. 5, the present invention comprises methods and
processes for
obtaining amniotic fluid samples as a by-product of amniotic fluid samples
collected for the
primary purpose of other clinical or research assessments, and analyzing or
testing the amniotic
18
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
fluid sample to determine the presence of any infectious disease antigens or
human antibodies
created by an immune response to the infectious disease antigens in the sample
of amniotic fluid.
[0070] The invention provides processing amniotic fluid samples obtained as a
by-product of
amniotic fluid samples collected for the primary purpose of other clinical or
research
assessments. Amniotic fluid is collected as a medical procedure used for
prenatal diagnosis, in
which a small amount is extracted from the amnion around the developing fetus.
Amniocentesis
may be performed when there is enough fluid surrounding the fetus. The sample
may be
removed via a syringe and guidance via ultrasound. Amniocentesis may be
performed as early as
about 13 weeks of gestation, but may also be collected between about 15 weeks
to about 20
weeks of gestation. When the amniotic fluid sample is collected by
amniocentesis for genetic
testing, about 10 milliliters of the amniotic fluid may be aliquoted into 2-6
milliliter collection
tubes coated with EDTA, or other suitable anticoagulant for infectious disease
testing.
[0071] Other Fluids, Tissues and Cells
[0072] As yet a further example, the invention comprises methods and processes
for procuring
and processing any other human bodily fluid, cell or tissue and analyzing and
determining the
presence of any infectious disease and infectious agents in the human bodily
fluid, cell or tissue.
For example, cellular suspensions of fetal placental cells and matemal
placental cells may be
tested for infectious diseases. The placental samples may be obtained by
methods disclosed in
U.S. Patent Application Publication No. 20080064098, entitled "Procurement,
Isolation and
Cryopreservation of Matemal Placental Cells," published on March 13, 2008 and
U.S. Patent
Application Publication No. 20080050814, entitled "Procurement, Isolation and
Cryopreservation of Fetal Placental Cells," published on February 28, 2008. A
pre-processing or
post-processing maternal placental stem cell sample or a fetal placental stem
cell sample, as
19
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
described in the preceding published U.S. patent applications may be tested
for the presence of
an infectious disease antigen or human antibody created by an immune response
to the infectious
disease antigen present in either type of placental stem cell sample.
[0073] Infectious Disease Testing Methods
[0074] The methods and processes of the present invention also provide steps
for analyzing or
.testing non-venous and non-arterial puncture human fluid or cells samples and
human body fluid
or cell samples to determine the presence of any infectious disease antigens
or human antibodies
created by an immune response to the infectious disease antigens. The sources
of the non-venous
and non-arterial puncture human fluid or cells samples and human body fluid or
cell samples
may comprise menstrual fluid that may be collected during menstruation,
amniotic fluid
collected during amniocentesis, or umbilical cord blood collected during
birth.
[0075] The present invention provides 'that the sample of the non-venous and
non-arterial
puncture human fluid or cells samples or human body fluid or cell samples in
the transfer tube is
tested or analyzed for infectious diseases. According to the present
invention, the transfer tube
containing any one of menstrual flow, centrifuged menstrual flow, supernatant
from a pre-
, processing sample or from post-processing sample, cord blood, cord blood
plasma, amniotic
fluid sample, pre-processing or post-processing maternal placental stem cell
sample, or a fetal
placental stem cell sample is analyzed or tested for the infectious disease of
interest.
[0076] The present invention provides that infectious disease testing of any
one of menstrual
flow, centrifuged menstrual flow, supernatant from a pre-processing sample or
from post-
processing sample, cord blood, cord blood plasma, amniotic fluid sample, pre-
processing or post-
processing maternal placental stem cell sample, or a fetal placental stem cell
sample may use
commercially-available testing methods, other FDA approved, licensed or
commercially
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
acceptable infectious disease testing methodologies, or other testing methods
for infectious
diseases. Specimen volumes for infectious testing would be based on required
volumes for
commercially-available tests or as otherwise required.
[0077] The testing may be performed by any infectious disease testing
laboratory. For
example, an order form may be completed to order infectious marker testing for
infectious
diseases of interest for testing to occur off site by a laboratory or a
service provider.
Alternatively, the transfer tube containing the menstrual flow sample may be
tested for infectious
diseases at the laboratory where the body fluid or cell sample was shipped for
processing. A
transfer tube with a specimen may be refrigerated at about 1 C to about 10 C
for up to about 7
days prior to infectious disease testing. A transfer tube may be maintained at
a temperature
between about 1 C to about 10 C throughout shipment to an off site infectious
disease testing
service provider. Alternatively, the transfer tube may be shipped at room
temperature.
[00781 By way of non-limiting example, the infectious diseases that may be
tested for in the
specimens include, but are not limited to, Hepatitis C Virus, Hepatitis B
Virus Core Antigen,
Hepatitis B Surface Antigen, HIV - I/II, Cytomegalovirus, HTLV UII, West Nile
Virus, Syphilis,
and Trepanome pallidum, and any other infectious diseases.
[0079] The specimen of menstrual flow, centrifuged menstrual flow, supernatant
from a pre-
processing sample or a post-processing sample, cord blood, cord blood plasma,
anuiiotic fluid
sample, pre-processing or post-processing maternal placental stem cell sample,
or a fetal
placental stem cell sample is analyzed by infectious disease testing
methodologies to detect the
presence of at least one or more infectious disease antigen and/or
complementary human
antibodies.
[0080] Hepatitis C
21
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0081] The infectious disease test for the Hepatitis C virus may be used to
test for the presence
of Hepatitis C virus (HCV). A test method used to detect HCV may be an ELISA
test for the
detection of human antibodies to Hepatitis C Virus (Anti-HCV) in human serum
or plasma. The
test utilizes microwells coated with recombinant hepatitis C virus encoded
antigens as the solid
phase. When enzyme substrate is applied, the presence of antigen or antibody
can be detected by
development of a colormetric end-product. A commercially-available test is the
Ortho ELISA
Test System. This test may be used to test a sample for infectious disease.
Other HCV diagnostic
tests may be used to test for HCV in specimens.
[0082] The assay procedure is a three-stage test carried out in a microwell
coated with a
combination of recombinant hepatitis C virus (rHCV) antigen (antigens: C22-3,
c200 and NS5).
In the first stage, a diluted test specimen of about 10 microliters is
incubated for about 60
minutes in the test well. If antibody reactive to any of the three antigens is
present, complexes
will not be formed. In the subsequent washing step, unbound serum or plasma
proteins will be
removed. In the second stage, about 200 microliters of murine monoclonal
antibody conjugated
to horseradish peroxidase is added to the microwell. The conjugate binds
specifically to the
human IgG portion of the antigen-antibody complexes. If antigen-antibody
complexes are not
present, the unbound conjugate will be removed by subsequent washing. In the
third stage, about
200 microliters of an enzyme detection system composed of o-phenylenediamine
(OPD) and
hydrogen peroxide is added to the test well. If bound conjugate is present,
the OPD will be
oxidized, resulting in a colormetric end-product. In this reaction, peroxidase
is divalently
oxidized by hydrogen peroxide to form an intermediate compound, which is in
turn, reduced to
its initial state by subsequent interaction with hydrogen ion donating OPD.
The resulting
oxidized form of OPD has an orange color. About 50 microliters of 4N Sulfuric
acid is then
22
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
added to stop the reaction. The color intensity depends on the amount of bound
conjugate in the
well, and, therefore, color intensity is a function of the concentration of
anti-HCV present in the
specimen. The color intensity is measured with a microwell reader (photometer)
designed to
measure light absorbance in a microwell. Results of the test are interpreted
by the absorbance
value of the color change, based on a determined cutoff absorbance value.
Specimens with
absorbance values less than the cutoff are considered non-reactive or negative
for the Hepatitis C
Virus and those above the cutoff absorbance value are considered reactive or
positive for
Hepatitis C Virus.
[0083] Hepatitis B Virus Core Antigen
100841 Hepatitis B Virus Core Antigen (recombinant) is an enzyme-linked
immunosorbent
assay for the detection of antibody to Hepatitis B Virus Core Antigen (anti-
HBc) in Human
Serum or Plasma. A variety of serologic markers appear following infection
with hepatitis B
virus (HBV). The first marker to appear is usually hepatitis B surface antigen
(HBsAg).
Antibodies to hepatitis B core antigen (anti-HBc) appear next, and remain
detectable following
the clearance of HBsAg and into convalescence. The determination of anti-HBc
in serum and
plasma may be used as an aid to monitor the progress of HBV infection. Anti-
HBc appears in
virtually all individuals infected with HBV and is an accurate serological
marker of recent and
past infection. A commercially available test is the Ortho ELISA Test System.
This test may be
used to test a specimen for infectious disease. Other suitable tests may be
used to test for
Hepatitis B.
[0085] The enzyme-linked immunosorbent assay for detection of Hepatitis B
Virus Core
Antigen may be a three-stage test carried out in a microwell coated with
recombinant-derived
hepatitis B core antigen (HBc). In the first stage, a test specimen of about
10 microliters is placed
23
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
directly in the test well containing specimen diluent and incubated for about
60 minutes. If anti-
HBc is present in the specimen, antigen-antibody complexes will form on the
microwell surface.
If anti-HBc is not present, complexes will not form and the unbound serum or
plasma proteins
will be removed in the washing step. In the second stage, about 200
microliters of antibody
conjugate is added to the test well and incubated for about 60 minutes. The
antibody conjugate is
a mixture of murine monoclonal antibodies specific for human IgG and IgM. The
conjugate will
bind specifically to the antibody portion of the antigen-antibody complexes.
If antigen-antibody
complexes are not present, the unbound conjugate will be removed by washing.
In the third
stage, an enzyme detection system composed of about 200 microliters of o-
phenylenediamine
(OPD) and hydrogen peroxide is added to the test well and incubated for about
60 minutes: If
bound conjugate is present, the OPD will be oxidized, resulting in a colored
end-product. 50
microliters of 4N Sulfuric acid is then added to stop the reaction. The color
intensity depends on
the amount of bound conjugate and therefore is a function of the concentration
of anti-HBc
present in the specimen. The color intensity is measured with a microwell
reader. Results of the
test are interpreted by the absorbance value of the color change, based on a
determined cutoff
absorbance value. Specimens with absorbance values less than the cutoff are
considered non-
reactive or negative for the Hepatitis B Virus Core Antigen. Those above the
cutoff absorbance
value are considered reactive or positive of Hepatitis B Virus Core Antigen.
[0086] Another possible test is a test for the antibody to Hepatitis B Surface
Antigen (Murine
Monoclonal) Peroxidase Conjugate antibody to HBsAg ELISA (Enzyme-Linked
Immunosorbent
Assay) is used for the detection of Hepatitis B Surface Antigen (HBsAg) in
human serum or
plasma. Such a test may be used as a screening test and an aid in diagnosis of
potential hepatitis
B infection. The test utilizes microwells coated with antibody to HBsAg as a
solid phase. When
24
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
enzyme substrate is applied, the presence of antigen or antibody can be
detected by development
of a colored end-product. A commercially available test is the Ortho Antibody
to HBsAg ELISA.
Other suitable tests may be used to test for Hepatitis B.
[0087] The test is a two-stage assay carried out in a microwell coated with
antibody to
HBsAg. In the first stage, working conjugate comprised of antibody to HBsAg,
and diluted in
conjugate diluent that is blue in color, is added to the test well. The test
specimen is then added
to the test well and a SOM (sample omission monitoring) read is performed. The
plate is
incubated for a specified length of time. If HBsAg is present in the specimen,
it will bind to the
antibody coated on the well and simultaneously bind to the conjugate to form
immobilized
antibody-HBsAg-conjugate complexes. If HBsAg is not present, these complexes
will not be
formed. Unbound serum or plasma proteins will be removed in the subsequent
washing steps. In
the second stage, an enzyme detection system composed of o-phenylenediamine
(OPD) and
hydrogen peroxide is added to the test well. If bound conjugate is present,
the OPD will be
oxidized, resulting in a colored end-product. In this reaction, peroxidase is
divalently oxidized by
hydrogen peroxide to form an intermediate compound, which is in tum, reduced
to its initial state
by subsequent interaction with hydrogen ion donating OPD. The resulting
oxidized form of OPD
has an orange color. Sulfuric acid is then added to stop the reaction. The
color intensity depends
on the amount of bound conjugate in the well. Therefore, color intensity is a
function of the
concentration of HBsAg present in the specimen. The color intensity may be
measured with a
microwell reader at 490 or 492 nm. Results of the test are interpreted by the
absorbance value of
the color change, based on a determined cutoff absorbance value. Specimens
with absorbance
values less than the cutoff are considered non-reactive or negative for the
Hepatitis B Surface
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
Antigen. Those above the cutoff absorbance value are considered reactive or
positive of Hepatitis
B Surface Antigen.
[0088) Human Immunodeficiency Virus - 1/2
[0089) The test for the Human Inununodeficiency Virus (HIV) is a synthetic
peptide enzyme
immunoassay (EIA) for the detection of the antibody to Human Immunodeficiency
Virus Types
I and/or 2(HIV-1 and HIV-2). The test uses microwells that are coated with a
mixture of four
peptides from the virus. A commercially-available test is the Bio-Rad HN-1/HIV-
2 EIA test kid.
This test may be used to test a sample for infectious disease. Other suitable
tests may be used to
test for HIV - 1/2.
[0090] Specimens are evaluated for the presence of HIV-1 and HIV-2 antibodies'
by
interaction with the adsorbed peptides in the wells. Specimens to be tested
are diluted in
specimen diluent, added to each well, and the plates containing the wells are
incubated and
washed. If antibodies to either HIV-1 or HIV-2 are present, they bind to the
adsorbed antigen and
are not removed by washing. The working conjugate solution, peroxidase-labeled
goat anti-
human immunoglobulin, is then added to the wells and will bind to the antibody-
antigen
complex, if present. Unbound conjugate is removed by a wash step. Next,
working chromogen
solution is added to the plate and allowed to incubate. A blue or blue-green
color develops in
proportion to the amount of antibody that has been bound to the antigen-coated
plate. The
enzyme reaction is stopped by the addition of acid, which results in a color
change to yellow.
The optical absorbance of controls and specimens is determined with a
spectrophotometer with
wavelength set at 450 nm. Results of the test are interpreted by the
absorbance value of the color
change, based on a determined cutoff absorbance value. Specimens with
absorbance values less
than the cutoff are considered non-reactive or negative for the Human
Immunodeficiency Virus.
26
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
Those above the cutoff absorbance value are considered reactive or positive of
Human
Immunodeficiency Virus.
[0091] Cytomegalovirus
[0092] Cytomegalovirus (CMV) is a common human viral pathogen which belongs to
the
family of herpes viruses. The presence of CMV antibodies in an individual
indicates prior
infection by the virus. The test for CMV is a qualitative solid phase red cell
adherence test
system for the detection of antibodies (IgG plus IgM) to Cytomegalovirus (CMV)
in human
serum or plasma. A commercially-available test is the Capture CMV from
Immuocor. Other
suitable tests may be used to test for CMV.
(0093] The assay procedure is a two step solid phase red cell adherence test
carried out in
microtitration wells coated with inactivated CMV virus. Serum or plasma
samples are added to
the viral-coated wells. The specimen are incubated for five minutes; during
which antibodies
specific for CMV proteins bind to immobilized viral proteins. Unbound
immunoglbbulins are
washed from the wells and replaced with a suspension of anti-IgG-plus anti-IgM-
coated indicator
red cells. Centrifugation brings the indicator red cells in contact with
antibodies bound to the
immobilized viral proteins. In the case of a positive test, the migration of
the indicator red cells
to the bottom of the wells is impeded as the anti-IgG and anti-IgM bridges are
formed between
the indicator red cells and the viral bound antibodies. As a consequence, the
indicator red cells
adhere over the surface of the microtitration well. In contrast, in the
absence of viral antigen-
antibody interactions (i.e., a negative test) the indicator red cells are not
impeded during their
migration, and pellet to the bottom of the well as a packed, well-defined cell
button.
[0094] Human T-cell Lymphotropic Virus (HTLV UIl)
27
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[0095] Human T-cell Lymphotropic Virus (HTLV I/II) tests detects antibody to
the HTLV-I
and HTLV-II viruses. HTLV-I has been associated with adult T-cell
leukemia/lymphoma (ATL)
and HTLV-I is associated with myelopathy/tropical spastic paraparesis
(HAM/TSP). The test for
HTLV I/II is an in vitro enzyme immunoassay (EIA) for the qualitative
detection of antibodies to
Human T-Lymphotropic Virus Type I and Type II in human serum or plasma. A
commercially-
available test is the Inno-Lia HTLV UII. Other suitable tests may be used to
test for Human T-
cell Lymphotropic Virus (HTLV I/II).
[0096] The assay utilizes a bead as a solid phase, coated with detergent-
solubilized and
sonicated HTLV proteins, to bind antibodies to the HTLV from human serum or
plasma. Goat
antibodies directed against human immunoglobulins, conjugated with horseradish
peroxidase,
are then incubated with the bead. Finally, the beads are incubated with o-
phylenediamine (OPD)
substrate solution containing hydrogen peroxide. A yellow-orange color
develops if antibodies
present in the sample bind to the bead.
[0097] The test specimen is diluted in specimen diluent and incubated with a
polystyrene bead
coasted with detergent solubilized HTLV-I and HTLV-II proteins (inactivated).
Specific
antibodies present in the sample bind to the HTLV-I and HTLV-II antigens on
the bead.
Unbound materials are removed by washing the beads. Goat antibody directed
against human
IgG that has been conjugated with horseradish peroxidase (anti-human IgG:
HRPO) is then
incubated with the beads and binds to the human IgG on the solid phase.
Unbound conjugate is
removed by washing the beads. The beads are then incubated with o-
phenylenediamine (OPD)
substrate solution containing hydrogen peroxide. The reaction of OPD substrate
solution with
HRPO yields a yellow-orange color. The intensity of the color formed is
proportional to the
amount of HTLV-I and/or HTLV-II antibody present in the sample. The enzyme
reaction is
28
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751.
stopped by the addition of sulfuric acid and the intensity of the color
developed is read using a
spectrophotometer. Results of the test are interpreted by the absorbance value
of the color
change, based on a determined cutoff absorbance value. Specimens with
absorbance values less
than the cutoff are considered non-reactive or negative for the Human T-
Lymphotropic Virus.
Those above the cutoff absorbance value are considered reactive or positive of
Human T-
Lymphotropic Virus.
[0098] West Nile Virus
[0099] West Nile Virus (WNV) is a mosquito-borne flavivirus that is associated
with human
disease ranging from mild flu-like symptoms to severe neurological
degeneration. The test for
WNV may be the commercially available Procleix WNV assay, which involves three
main steps,
occurring in a single tube: sample preparation; WNV RNA target amplification
by transcription-
mediated amplification (TMA); and detection of the amplification products by
the hybridization
protection assay. Other suitable tests may be used to test for West Nile
Virus.
[00100] During sample preparation, RNA is isolated from specimens via the use
of target
capture. The specimen is treated with a detergent to solubilize the viral
envelope, denature
proteins and release viral genomic RNA. Oligonucleotides that are homologous
to highly
conserved regions of WNV are hybridized to RNA or DNA target, if present in
the specimen,
and captured onto magnetic microparticles that are separated from the specimen
in a magnetic
filed. Wash steps are utilized to remove extraneous components from the
reaction tube. Magnetic
separation and wash steps are performed with a target capture system.
[00101] Target amplification occurs via TMA, which is a transcription-based
nucleic acid
amplification method that utilizes two enzymes. Reverse transcriptase is used
to generate a DNA
29
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
copy. A polymerase then produces multiple copies of RNA from the DNA copy
template. The
Procleix WNV assay utilizes the TMA method to amplify regions of WNV RNA.
[00102] Detection is achieved by HPA, using single-stranded nucleic acid
probes with
chemiluminescent labels that are complementary to the amplicon. The labeled
nucleic acid
probes hybridize specifically to the amplicon. The selection reagent
differentiates between
hybridized and unhybridized probes by inactivating the label on unhybridized
probes. During the
detection step, the chemiluminescent signal produced by the hybridized probe
is measured.
Internal control is added to each test specimen, control, and assay calibrator
via the working
target capture reagent. A specimen is nonreactive, or negative, for the WNV if
the signal is less
than the cutoff. A specimen is reactive, or positive, for WNV if the signal is
more than the cutoff.
[00103] Syphilis
[00104] The etiologic agent of Syphilis is the microorganism Treponema
pallidum. Tests for
syphilis detect antibody formed against Treponema pallidum by a human. The
test reacts with
non-treponemal lipid antigens. A commercially-available test is the
fluorescent treponemal
antibody absorption (FTA-ABS) test. Other suitable tests may be used to test
for Syphilis.
[00105] The FTA-ABS test reaction detects circulating antibodies against the
etiologic agent
of syphilis. The primary reaction involves antibodies which attach to antigens
along the surface
and intemal structure of the microorganism. This reaction occurs during the
incubation step of
the test while the serum or specimen is diluted 1:5 in sorbent and covers the
smears of the
microorganism. The sorbent is prepared from saprophytic Reiter treponeme
culture which
contains substances that remove non-specific antibodies to "group treponemal
antigens" found in
normal individuals, but does not significantly absorb the antibodies against
the virulent
treponema in the diseased population. A rinsing period follows the primary
incubation, which
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
removes all unbound serum antibody. A secondary reaction and incubation period
then follows.
The reagent used in the secondary reactions is a fluorescence labeled anti-
human conjugate,
which covers the smear. The antigen surface is then thoroughly rinsed free of
unbound conjugate
and viewed under an appropriate fluorescence microscope. Fluorescence
intensity of patient
serum is recorded relative to control standards which establish the
specificity and sensitivity of
the test procedure. The intensity of fluorescence is graded on a scale of 4+
to negative (no
fluorescence).
[00106] Preliminary Study
[00107] A preliminary infectious disease study was performed on two menstrual
flow
specimens collected by a donor of menstrual fluid for menstrual stem cell
collection and
preservation. Established, licensed infectious disease testing methods were
used for infectious
diseases of interest and were employed by a third-party infectious disease
testing facility.
Menstrual flow comprising menstrual blood, fluid, cells and tissue was
collected by the donor
according to collection methods disclosed in U.S. Patent Application Serial
No. 12/074,423. The
donor also provided a comparative, venous specimen of blood according to
standard phlebotomy
techniques for testing for infectious disease.
[00108] The menstrual flow samples were collected from the donor using
individual
procurement kits comprising media tubes in a rack covered in parafilm, at
least one collection
device and instructions for use (i.e., DivaCup), antiseptic cleaning pads,
small plastic bags, small
biohazard bag for shipment of specimens to laboratory, parafilm, nanocooler,
and a collection
form. The collection kit remained at a refrigerated temperature of about 1 C
to about 10 C prior
to use. The collection took place on one of the heaviest flowing days of the
donor's menstrual
cycle, which was usually the first and/or second day. The donor used a DIVA
collection cup. The
31
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
-collection cup was sterilized prior to use, and the donor followed the
manufacturer's instructions
for use. Prior to collection, the donor washed her hands and used aseptic
technique as much as
possible during the collection of the menstrual flow.
[00109] The collection cup remained inside the vagina for less than about 3
hours to collect a
menstrual flow sample for infectious disease testing. The donor removed the
collection cup and
poured the collected menstrual fluid specimen into about 10 milliliters of a
isotonic solution
(Hank's Balanced Salts Solution) with an anticoagulant (Heparin 10 units per
milliliter) in a
sterile 50 milliliter conical tube. The menstrual flow sample was poured into
the conical tube
while avoiding contact with the rim of the tube. The cap of the conical tube
was screwed onto the
conical tube and then was wrapped with a plastic parafilm to prevent leakage
of the menstrual
flow sample. 1fie conical tube was inverted several times to mix the menstrual
flow sample and
the isotonic solution and anticoagulant.
[00110] The collection steps were repeated for the second sample. The first
sample was
refrigerated at a temperature of about 2 C to about 8 C until the second
sample was collected.
The conical tubes containing the menstrual flow samples and the venous sample
were placed into
a small plastic bag and in a thermal box with containing ice. The menstrual
flow samples and
venous sample were maintained at a temperature of about 1 C to about 10 C for
the duration of
shipment. The package containing the menstrual flow samples and venous sample
were
transported to the laboratory within at least about 48 hours of collection.
[00111] When the specimens arrived in the laboratory, each menstrual flow
sample was
separated into two EDTA-coated tubes. Each of the sets of two tubes were
labeled and an order
form was filled out to order infectious marker testing of the menstrual
samples and a venous
sample from the donor. The tests ordered included commercially-available
infectious disease
32
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
tests for Hepatitis B Surface Antigen, Hepatitis B Core Antibody, Hepatitis C
Virus, Human
Immunodeficiency Virus 1& 2, Human T-Lymphotropic Virus UII, Cytomegalovirus
and
Syphilis.
(00112] The results of the testing of the menstrual specimens showing
comparative
relationships for the preliminary test data is as follows.
[001131 TABLE 1: Infectious disease test results: Venous sample vs Menstrual
Sample -
Qualitative and Quantitative Results
Specimen 1
Test Specimenl - Venous Specimenl - Menstrual
HBsAg Negative Nonreactive Negative Nonreactive
HIV 1/2 Negative Nonreactive Negative Nonreactive
HBc Negative Nonreactive Negative Nonreactive
HTLV I/II Negative Nonreactive Negative Nonreactive
HCV Negative Nonreactive Negative Nonreactive
Specimen 2
Venous HBsAg HBc HCV HIV I/2 HTLV I/II
Neg ctrl 0.031 0.083 0.048 0.061 0.039
Specimen ID74 0.018 0.047 0.009 0.056 0.061
Pos ctrl 1.378 1.365 1.696 1.575 0.693
Cutoff value 0.096 0.494 0.644 0.302 0.37
Menstrual A HBsAg HBc HCV HIV 1/2 HTLV I/II
Neg ctrl 0.023 0.083 0.047 0.041 -0.039
Specimen ID75 0.029 0.052 0.014 0.028 0.042
33
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
Pos ctrl 1.378 1.365 1.71 1.265 0.727
Cutoff value 0.096 0.494 0.663 0.304 0.366
Menstrual B HBsAg HBc HCV HIV I/2 HTLV I/II
Neg ctrl 0.042 0.057 0.044 0.027 0.026
Specimen ID618 0.055 0.076 0.006 0.042 0.034
Pos ctrl 1.38 1.09 1.749 1.494 0.856
Cutoff value 0.103 0.46 0.645 0.283 0.356
Qualitative Results, Specimen 2:
Test Results
HBsAg Negative Nonreactive
HN 1/2 Negative Nonreactive
HBc Negative Nonreactive
HTLV UII Negative Nonreactive
HCV Negative Nonreactive
[00114] Comparison Study for Peripheral Blood Samples and Menstrual Blood
Samples
[00115] A study was performed using the methods of the present invention to
analyze the
infectious disease marker testing for Peripheral blood (PB) samples compared
to menstrual blood
(MB) samples which are also referred to as "M2" samples. 34 samples of paired
PB and MB
samples were tested. Menstrual flow specimens and corresponding peripheral
blood samples
were collected according to the present invention. Suitable volumes of
menstrual flow specimens
34
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
and peripheral blood specimens were tested or analyzed to determine the
presence of infectious
diseases using commercial infectious disease tests.
[00116] Infectious Disease Tests for the Comparison Study
[00117] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Hepatitis B Virus Core Antigen (anti-HBc) with
the Ortho ELISA
test system (Ortho (2006). Hepatitis B Virus Core Antigen (Recombinant). ELISA
Test System.
Raritan, N.J.) The testing comprised an Enzyme-linked immunosorbent assay
(ELISA) in a
three-stage test carried out in microwell coated with recombinant-derived
hepatitis B core
antigen (rHBcAg). In the first stage, each sufficient volume of test specimen
of menstrual flow
and peripheral blood was separately incubated in the test well with added
diluent. During the
second stage, an antibody conjugate specific for human IgG and IgM was added
to the well. An
enzyme detection system was added in the third stage. The detection system was
comprised of
hydrogen peroxide and o-phenylenediamine. Two paired peripheral blood and
menstrual fluid
samples tested positive to the HBc antigen, and the other paired peripheral
blood and menstrual
fluid samples tested negative for HBc antigen according to testing protocols.
Exemplary data is
shown in Table 2.
[00118] TABLE 2: Positive and Negative Results of Paired Testing of PB/MB for
Hepatitis B
Virus Core Antigen
7H56
M2-028IDT 1.074 Reactive
PB-028IDT 9.907 Reactive
M2-0241DT 0.042 NR
M2-0241DT 0.131 NR
[00119] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Human T-Lymphotropic Virus Types I and II using
the ABBOTT
PRISM test (Abbott Laboratories (2007). Abbott Prism HTLV-I/HTLV-II. Abbott
Park, IL). The
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
ABBOTT PRISM System is an automated immunoassay analyzer for performing
chemiluminescent immunoassays (ChLIA). Each paired menstrual flow and
peripheral blood
specimen were separately incubated with microparticles coated with sonicated
and detergent-
inactivated HTLV-I and HTLV-II antigens. The microparticles were then filtered
out of the
mixture and incubated with a probe consisting of biotinylated HTLV-I and HTLV-
II proteins.
Acridinium-labeled anti-biotin conjugate was added to the microparticles to
bind any present
probe. After incubation, the unbound conjugate was washed away. Alkaline
hydrogen peroxide
solution was added to generate any chemiluminescent signal. The resultant
photons were
counted. The amount of any light emitted is proportional to the amount of anti-
HTLV-I and/or
anti-HTLV-II in the sample. No paired peripheral blood and menstrual fluid
samples tested
positive for anti-HTLV-I and/or anti-HTLV-II. Exemplary data is shown in Table
3.
,[00120] TABLE 3: Positive and Negative Result of Paired Testing of PB/MB for
Human T-
Lymphotropic Virus Types I and II
HTL~~1~1~/#IT
M2-OO1IDT 0.029 NR
PB-OOlIDT 0.065 NR
[00121] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Antibody to Hepatitis B surface antigen (mouse
monoclonal IgM)
using the ABBOTT PRISM system. Each paired menstrual flow and peripheral blood
specimen
were separately incubated with microparticles coated with mouse monoclonal
anti-HBc. The
microparticles were then filtered out of the mixture and incubated with
acridinium-labeled goat
polyclonal anti-HBc conjugate. After this incubation, the unbound conjugate
was washed away.
Alkaline hydrogen peroxide solution was added to generate a chemiluminescent
signal. The
resultant photons were counted. The amount of light emitted is proportional to
the amount of
HBsAg in the sample. One menstrual flow sample tested positive for HBsAg and
its paired
36
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
peripheral blood sample tested negative for HBsAg. All other paired samples
tested negative for
HBsAg. Exemplary data is shown in Table 4.
[00122] TABLE 4: Positive and Negative Result of Paired Testing of PB/MB for
Hepatitis B
surface antigen
....P_.. . _ . .. ._ . . . _. >. . _ . .,....x. . . z _,.._ _ . _ .
M2-030IDT Repeatedly Reactive
PB-030IDT 0.069 NR
M2-0391DT 0.057 NR
PB-0391DT 0.030 NR
1001231 The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Hepatitis C Virus (Anti-HCV) using the ORTHO
ELISA Test
System (Ortho (2006). Hepatitis C Virus Encoded Antigen (Recombinant c22-2,
c200, and NS5).
ELISA Test System. Raritan, N.J.). The three-stage test was carried out in
microwells coated
with a combination of three recombinant hepatitis C virus (rHCV) antigens: c22-
2, c200, and
NS5. In the first stage, diluted specimens of each of the paired menstrual
flow and peripheral
blood specimens were incubated in the test well. During the second stage,
murine monoclonal
antibody conjugated to horseradish peroxidase were added to the wells. An
enzyme detection
system was added in the third stage and comprised hydrogen peroxide and o-
phenylenediamine
(OPD). The end-product was measured with a microwell reader. The amount of
bound conjugate
is proportional to the color intensity and is therefore a function of the
concentration of anti-HCV
present in the specimen. One paired menstrual flow specimen and peripheral
blood specimen
tested positive for HCV. All other paired samples tested negative. Exemplary
data is shown in
Table 5.
[00124] TABLE 5: Positive and Negative Result of Paired Testing of PB/MB for
Hepatitis C
Virus
am le
M2-017IDT 9.714 Reactive
37
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
PB-017IDT 9.718 Reactive
M2-016IDT 0.004 NR
PB-016IDT 0.016 NR
[00125] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Human Immunodeficiency Virus Types I and 2 using
a BIO-RAD
HIV-1/HIV-2 test (Bio-Rad (2007). Human Immunodeficiency Virus Types 1 and 2
(Recombinant Synthetic Peptides). Redmond, WA). The Bio-Rad test is an enzyme
immunoassay
based on the principle of the direct antibody sandwich technique. Solid phase
microwell strip
plates were coated with purified antigens: gp160 and p24 recombinant proteins
derived from
HIV-1; a peptide representing the immunodominant region of the HIV-2
transmembrane
glycoprotein, gp36; and a synthetic polypeptide mimicking an artificial HIV-1
group 0 specific
epitope. Paired menstrual flow and peripheral blood samples and controls were
added to the
microwells along with a specimen diluent containing a dye which changes color
from purple to
blue during this combination. The wells were incubated and washed. A green
conjugate solution
was added and the wells were incubated. The conjugate contains peroxidase-
conjugated antigens
(peptides mimicking various immunodominant epitopes of the HIV-1 and HIV-2
transmembrane
glycoproteins, and a p24 recombinant protein). Working TMB solution was then
added and
allowed to incubate. A blue-green color develops in proportion to the amount
of HIV antibody
present in the sample. No paired samples tested positive for HIV-1 or HIV-2.
Exemplary data is
shown in Table 6.
[00126] TABLE 6: Positive and Negative Result of Paired Testing of PB/MB for
Human
Immunodeficiency Virus Types I and 2
~~.
M2-016IDT 0.024 NR
PB-016IDT 0.028 NR
38
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
[00127] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of Treponema pallidum using the OLYMPUS PK TP System
(Olympus. Olympus PK TP System Microhemagglutination Test for Detection of
Treponema
pallidum Antibodies using the Olympus PK Instrument. Center Valley, PA). This
system is based
on the principle of agglutination and pattern recognition. Each of the paired
menstrual flow and
peripheral blood specimens were diluted in a diluent composed of phosphate-
buffered saline
containing normal rabbit testicular extract and cell components of sonicated
Reiter T. phagedenis
Sensitized cells were then added to a test mixture comprised of fixed chicken
erythrocytes
sensitized with components of the pathogenic T. pallidum. The reactants were
allowed to settle
in terraced microwells. Hemagglutination occurs in the presence of T. pallidum
antibodies in the
specimen. The OLYMPUS PK instrument was used to read the wells for
agglutinated and
unagglutinated patterns. A reactive test is a homogenous layer of cells; a
nonreactive test would
result in a compact dense button surrounded by a clear zone. No paired
menstrual flow and
peripheral blood specimens tested positive for Treponema pallidum. Exemplary
data is shown in
Table 7.
[00128] TABLE 7: Positive and Negative Result of Paired Testing of PB/MB for
Treponema
pallidum (Syphilis)
M2-OO1IDT Ne R
PB-001 IDT Neg/NR
[00129] The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed for the presence of antibodies to Hepatitis B surface antigen (mouse
monoclonal) using
a BIO-RAD system (Bio-Rad (2007). Antibody to Hepatitis B Surface Antigen
(Mouse
Monoclonal). Redmond, WA). This system is a qualitative enzyme immunoassay for
the
detection of Hepatitis B surface antigen (HBsAG) in human serum or plasma.
Wells of the
39
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
microwell strip plates were coated with mouse monoclonal antibody to HBsAg
(anti-HBs).
Paired menstrual flow and peripheral blood specimens and appropriate controls
were then added
to the wells and incubated. Washing was followed by the addition of conjugate
solution
(peroxidase-conjugated mouse monoclonal antibodies directed against HBsAg).
Working TMB
Solution was added to the plate and incubated. A blue or blue-green color
develops in proportion
to the amount of HBsAG present in the sample.
[001301 The paired menstrual flow specimen and peripheral blood specimen were
tested or
analyzed using the HIV-1 Discriminatory Assay with the PROCLEIX ULTRIO system.
The
PROCLEIX ULTRIO Assay is a qualitative in vitro nucleic acid assay system for
the detection
of HIV-1 RNA, HCV RNA, and HBV DNA. The assay contains reagents which may be
used for
simultaneous detection of all three viruses or the individual viruses: HIV-1,
HCV, and HBV. The
first step of the assay was preparation of paired menstrual flow and
peripheral blood specimens;
in which viral RNA and DNA were isolated via the use of target capture. The
specimens were
treated with a detergent to solubilize the viral envelope, denature proteins
and release viral
genomic RNA and/or DNA. Oligonucleotides homologous to highly conserved
regions of HIV-
1, HCV, and HBV were hybridized to the HN-1 RNA, HCV RNA, or HBV DNA target,
if
present, in the test specimens. The hybridized targets were then captured onto
magnetic
microparticles that were separated from the specimen in a magnetic field, and
then washed.
Target amplification occurred via TMA, a transcription-based nucleic aoid
amplification method
that utilizes two enzymes, MMLV reverse transcriptase and T7 RNA polymerase.
Reverse
transcriptase was used to generate a DNA copy of the target sequence. The
assay utilizes the
TMA method to amplify regions of HIV-1 RNA, HCV RNA, and/or HBV DNA. Detection
was
achieved by HPA using single-stranded nucleic acid probes with
chemiluminescent labels that
CA 02699491 2010-03-12
WO 2009/035706 PCT/US2008/010751
are complementary to the amplicon. The labeled nucleic acid probes hybridized
specifically to
the amplicon. The selection reagent differentiates between hybridized and
unhybridized probes
by inactivating the label on unhybridized probes. During the detection step,
the
chemiluminescent signal produced by the hybridized probe was measured in a
luminometer and
was reported as Relative Light Units (RLU). Intemal Control was added to each
test specimen,
control (if used), or assay calibrator tube via the working Target Capture
Reagent that contains
the Intemal Control. The Internal Control in this reagent controls for
specimen processing,
amplification, and detection steps. Internal Control signal in each tube or
assay reaction is
discriminated from the HIV-1/HCV/HBV signal by the differential kinetics of
light emission
from probes with different labels. Intemal Control-specific amplicon was
detected using a probe
with rapid emission of light (flasher signal). Amplicon specific to HIV-
1/HCV/HBV is detected
using probes with relatively slower kinetics of light emission (glower
signal). The Dual Kinetic
Assay (DKA) is a method used to differentiate between the signals from flasher
and glower
labels. When used for the simultaneous detection of HIV-1, HCV, and HBV, the
assay
differentiates between Internal Control and combined HIV-1/HCV/HBV signals but
does not
discriminate between individual HIV-1, HCV, and HBV signals. No paired
menstrual flow or
peripheral blood samples tested positive for any of the infectious diseases.
[00131] While preferred embodiments of the present invention have been shown
and
described, it will be apparent to those skilled in the art that many changes
and modifications may
be made without departing from the invention in its broader aspects. The
appended claims are
intended to cover, therefore, all such changes and modifications as fall
within the true spirit and
scope of the invention.
41