Note: Descriptions are shown in the official language in which they were submitted.
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
N-OXIDES OF VENLAFAXINE AND O-DESMETHYLVENLAFAXINE AS PRODRUGS
INDEX page
Title of the invention 1
Index 1
Technical field 1
Background art 2
Disclosure 3
Definitions 6
Example 1: Analytical methods 8
Example 2: Syntheses of specific compounds 11
Example 3: Pharmacological methods 16
Example 4: Pharmacokinetic and pharmacological test results 18
Example 5: Pharmaceutical preparations 20
Bibliography 23
Claims 24
Abstract 28
TECHNICAL FIELD
This invention relates to the fields of pharmaceutical and organic chemistry,
and provides
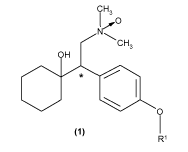
venlafaxine-N-oxide and O-desmethylvenlafaxine-N-oxide, having formula (1):
iH3
O
CH3
OH
O
RI
wherein the asterisk (*) marks the asymmetric carbon atom, R' is H or CH3, and
tautomers,
stereoisomers, hydrates and solvates thereof, as prodrugs of venlafaxine and
its major (active)
metabolite 0-desmethylvenlafaxine respectively, as well as pharmaceutical
compositions
containing this compound, methods for preparing it, and methods for preparing
compositions.
BACKGROUND ART
Venlafaxine is a phenethylamine bicyclic derivative, chemically unrelated to
tricyclic, tetracyclic
or other available antidepressant agents. It has been reported that its (-)-
enantiomer is a more
potent inhibitor of norepinephrine synaptosomal uptake while its (+)-
enantiomer is more
selective in inhibiting serotonin uptake (Howell, 1994). Venlafaxine is
marketed as racemate.
1
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
?H3
CH3
OH
O
I
CH3
( )-1-(2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-cyclohexanol, venlafaxine
(U.S. Pat. No. 4,761,501; Pento, 1988)
The mechanism of venlafaxine's antidepressant action in humans is believed to
be associated
with its potentiation of neurotransmitter activity in the CNS. Preclinical
studies have shown that
venlafaxine and its major metabolite, 0-desmethylvenlafaxine, are potent
inhibitors of neuronal
serotonin and norepinephrine reuptake and weak inhibitors of dopamine
reuptake. 0-desmethyl-
venlafaxine is the only major active metabolite. Other metabolites are N-
desmethylvenlafaxine,
and N,O-didesmethylvenlafaxine (Klamerus, 1992). 0-desmethylvenlafaxine
succinate is in a
late stage of its development, and recently received an approvable letter from
the FDA for the
treatment of Major Depressive Disorder. The compound is also in development as
treatment of
vasomotor symptoms associated with menopause.
N-oxides are known since 1894. By now it is very well known that N-oxides are
metabolites of
many tertiary amines, and in most cases are also intermediates between
tertiary amines and
their N-dealkylated analogs. Most, but not all, tertiary amine drugs give rise
to N-oxides. This is
for instance the case with morphine, imipramine, promazine, cinnarizine and
nicotine. How
much N-oxidation takes place varies from trace amounts to a nearly
quantitative conversion.
Some N-oxides were shown to be more potent than their corresponding tertiary
amines. The
most famous example of these is chlordiazepoxide (Librium ), one of the most
frequently used
drugs in psychiatric and general medicine. In many more cases however, N-
oxides were found
to be less potent than their corresponding tertiary amines, and N-oxidation is
most commonly
regarded to be metabolic deactivation. Whilst N-oxides are easily reduced to
their
corresponding tertiary amines by chemical means, in the human body this
happens to varying
degrees. Some N-oxides undergo nearly quantitative reductive conversion to the
corresponding
tertiary amines and in other cases the conversion is a mere trace reaction or
even completely
absent (Bickel, 1969). Thus, the formation of N-oxides and their corresponding
tertiary amines is
unpredictable. Once formed, N-oxides may be more active than their
corresponding tertiary
2
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
amines, less active or even completely inactive. N-oxides may be reduced to
the corresponding
tertiary amines or not. When they are, the reaction may be a mere trace or
nearly quantitative.
Since Paracelsus ('Sola dosis facit venenum') it is generally accepted that
therapeutic as well as
toxic effects of drugs are related to their concentration at the relevant
target sites. Because
generally speaking the latter are not easily accessible, blood plasma levels
are used as
approximations of relevant drug concentrations. During drug development a
window of suitable
plasma concentrations are defined providing a lower limit or range for
efficacy, and an upper
range at which side effects start to become apparent. In ideal situations the
two concentrations
are so far apart that it is easy to administer the drug in such a way that it
is effective, yet does
not give rise to side effects. In reality, situations are hardly ever ideal,
and most drugs show side
effects. In most cases the occurrence of side effects can be linked to peak
plasma
concentrations exceeding the lower level associated with the occurrence of
side effects.
Venlafaxine produces peak plasma concentrations resulting in side effects. The
most commonly
observed adverse events associated with the use of venlafaxine (incidence of
5% or greater)
and not seen at an equivalent incidence among placebo-treated patients (i.e.,
incidence for
venlafaxine at least twice that for placebo), include sustained hypertension,
headache, asthenia,
sweating, nausea, constipation, somnolence, dry mouth, dizziness, insomnia,
nervousness,
anxiety, blurred or blurry vision, and abnormal ejaculation/orgasm or
impotence in males
(Physicians' Desk Reference, 1999; Sinclair, 1998). These adverse effects can
significantly limit
the dose level, frequency, and duration of drug therapy. Adverse events can be
attenuated
using extended-release formulations (venlafaxine XR), but different compounds
can solve the
problem, too. It would thus be desirable to find a compound with the
advantages of venlafaxine
while avoiding its disadvantages. Prodrugs have an identical pharmacological
profile, but a
more favourable pharmacokinetic profile.
DISCLOSURE
When administrated orally, venlafaxine-N-oxide and O-desmethylvenlafaxine-N-
oxide act as
prodrugs: they are rapidly converted to their parent compounds venlafaxine and
0-desmethyl-
venlafaxine respectively. The invention relates to N-oxides having formula
(1):
CH3
O
\CH3
OH
*
C
RI
3
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
wherein the asterisk (*) marks the asymmetric carbon atom, R' is H or CH3, and
to tautomers,
stereoisomers, hydrates and solvates thereof. The N-oxides of the invention
may be
substantially free of venlafaxine and 0-desmethylvenlafaxine, and tautomers,
stereoisomers,
salts, hydrates and solvates thereof. Venlafaxine N-oxide and 0-des-
methylvenlafaxine N-oxide
can be prepared by oxidizing venlafaxine or 0-desmethylvenlafaxine with a
suitable oxidizing
agent, for instance with m-CPBA. The invention relates to racemates, mixtures
of diastereomers
and the individual stereoisomers of the N-oxides of the invention, as well as
to hydrates and
solvates thereof.
The invention particularly relates to N-oxides having formula (1) wherein R'
is CH3.
Another preferred embodiment of the invention are N-oxides having formula (1)
wherein R' is H.
Yet other preferred embodiments are (S)- and (R)-enantiomers of N-oxides
having formula (1).
Venlafaxine-N-oxide and compositions comprising them are useful in treating
affections or
diseases effectively treatable-albeit with side effects- with venlafaxine:
depression, including
major depressive disorder, generalized anxiety disorder, obsessive compulsive
disorder, social
anxiety disorder, panic disorder, general depressive disorders, diabetic
neuropathy, migraine
and vasomotor symptoms associated with menopause, a.k.a. `hot flashes'.
The invention also comprises:
pharmaceutical compositions for treating, for example, a disorder or condition
treatable
by venlafaxine, the compositions comprising an N-oxide of formula (1), and a
pharmaceutically
acceptable carrier;
methods of treating a disorder or condition treatable by venlafaxine, the
methods
comprising administering to a mammal in need of such treating an N-oxide of
formula (1);
methods of treating a disorder or condition treatable by venlafaxine, the
methods
comprising administering to a mammal in need of such treating an N-oxide of
formula (1);
pharmaceutical compositions for treating a disorder or condition treatable by
venlafaxine,
the compositions comprising an N-oxide of formula (1), and a pharmaceutically
acceptable
carrier;
methods for treating a disorder or condition treatable by venlafaxine, the
methods
comprising administering to a patient in need of such treating an N-oxide of
formula (1).
The invention also provides the use of an N-oxide of formula (1), for the
manufacture of
medicament.
The invention further relates to combination therapies wherein a compound of
the
invention, or a pharmaceutical composition or formulation comprising a
compound of the
invention, is administered concurrently or sequentially or as a combined
preparation with
4
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
another therapeutic agent or agents, for instance venlafaxine or 0-desmethyl-
venlafaxine, for
treating one or more of the conditions listed. Such other therapeutic agent(s)
may be
administered prior to, simultaneously with, or following the administration of
the compounds of
the invention.
The invention also provides compounds, pharmaceutical compositions, kits and
methods
for treating a disorder or condition treatable by venlafaxine, the method
comprising
administering to a patient in need of such treating an N-oxide of formula (1).
The invention also provides methods of preparing the compounds of the
invention and
the intermediates used in those methods.
The compounds of the present invention contain an asymmetric center. This will
produce
two optical isomers. All of the possible optical isomers and diastereomers, in
mixtures and as
pure or partially purified compounds, belong to this invention. The present
invention
comprehends all such isomeric forms of these compounds. Formula (1) shows the
structure of
the class of compounds without preferred stereochemistry. The independent
syntheses of these
diastereomers, or their chromatographic separations, may be achieved as known
in the art by
appropriate modification of the methodology disclosed therein. Their absolute
stereochemistry
may be determined by the X-ray crystallography of crystalline products or
crystalline
intermediates, which are derivatized, if necessary, with a reagent containing
an asymmetric
center of known absolute configuration. Racemic mixtures of the compounds can
be separated
into the individual enantiomers by methods well-known in the art, such as the
coupling of a
racemic mixture of compounds to an enantiomerically pure compound to form a
diastereomeric
mixture, followed by separation of the individual diastereomers by standard
methods, such as
fractional crystallization or chromatography. The diasteromeric derivatives
may then be
converted to the pure enantiomers by cleavage of the added chiral residue. The
racemic mixture
of the compounds can also be separated directly by chromatographic methods
utilizing chiral
stationary phases: Methods well-known in the art. Alternatively, any
enantiomer of a compound
may be obtained by stereoselective synthesis using optically pure starting
materials or reagents
of known configuration by methods well-known in the art.
Some of the crystalline forms for the compounds may exist as polymorphs: as
such
intended to belong to the invention. In addition, some of the compounds may
form solvates with
water (i.e. hydrates), or common organic solvents. Such solvates also fall
within the scope of
this invention.
Isotopically-labeled N-oxides of formula (1), detectable by PET or SPECT, also
fall within
the scope of the invention. The same applies to N-oxides of formula (1)
labeled with [13C]-, [14C]-
,[3H]-, [18F]-, [1251]- or other isotopically enriched atoms, suitable for
receptor binding or
metabolism studies.
5
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
The chance finding that N-oxides of venlafaxine and 0-desmethylvenlafaxine are
useful
as prodrugs of their respective parent compounds, offers possibilities to use
these compounds
as alternatives, with the clinical benefits of an extended duration of action
and a blunted peak
plasma concentration, leading to an enhanced side-effect profile. Thus in some
embodiments
of the present invention, compounds of the present invention may be provided
substantially free
of parent compound 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-
cyclohexanol, venlafaxine,
or 0-desmethylvenlafaxine. By substantially free is meant that compound of the
present
invention contains less than about 50%, 40%, 30%, 20%, 10%, 1%, 0.5% or is,
within
detectable limits, free of venlafaxine or 0-desmethylvenlafaxine as an
impurity.
Pharmaceutical compositions containing N-oxides of venlafaxine and/or 0-
desmethylvenla-
faxine which are substantially free of venlafaxine and/or 0-
desmethylvenlafaxine are envisioned
in accordance with the present invention.
DEFINITIONS
As used herein, the term "venlafaxine" means the racemic compound (R,S)-1-[2-
(dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol.
Any compound metabolized in vivo to provide the bioactive agent (i.e., the
compound of
formula (1)) is a prodrug within the scope and spirit of the application.
Prodrugs are therapeutic
agents, inactive per se but transformed into one or more active metabolites.
Thus, in the
methods of treatment of the present invention, the term "administering" shall
encompass
treating the various disorders described with the compound specifically
disclosed, or with a
compound that not specifically disclosed, but that converts to the specified
compound in vivo
after administration to the patient. Prodrugs are bioreversible derivatives of
drug molecules used
to overcome some barriers to the utility of the parent drug molecule. These
barriers include, but
are not limited to, solubility, permeability, stability, presystemic
metabolism and targeting
limitations (Bundgaard, 1985; King, 1994; Stella, 2004; Ettmayer, 2004;
Jarvinen, 2005).
Prodrugs, i.e. compounds that when administered to humans by any known route,
are
metabolised to compounds having formula (1), belong to the invention. In
particular this relates
to the hydroxy group, which can be reacted with organic acids to yield
compounds having
formula (1) wherein an additional group is present that is easily removed
after administration, for
instance, but not limited to amidine, enamine, a Mannich base, a hydroxyl-
methylene derivative,
an O-(acyloxymethylene carbamate) derivative, carbamate, ester, amide or
enaminone.
The term "polymorphism" is defined as the ability of a compound to exist in
more than
one crystal form, a so-called polymorph. Polymorphism is a frequently
occurring phenomenon.
Polymorphism is affected by several crystallization conditions such as
temperature, level of
supersaturation, the presence of impurities, polarity of solvent, rate of
cooling. Polymorphs can
6
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
be characterized by several methods such as solid state NMR, solubility tests,
DSC or melting
point determination, IR or Raman spectroscopy.
To provide a more concise description, some of the quantitative expressions
given
herein are not qualified with the term "about". It is understood that whether
the term "about" is
used explicitly or not, every quantity given herein is meant to refer to the
actual given value, and
it is also meant to refer to the approximation to such given value that would
reasonably be
inferred based on the ordinary skill in the art, including approximations due
to the experimental
and/or measurement conditions for such given value. Throughout the description
and the claims
of this specification the word "comprise" and variations of the word, such as
"comprising" and
"comprises" is not intended to exclude other additives, components, integers
or steps. The term
"composition" as used herein encompasses a product comprising specified
ingredients in
predetermined amounts or proportions, as well as any product that results,
directly or indirectly,
from combining specified ingredients in specified amounts. In relation to
pharmaceutical
compositions, this term encompasses a product comprising one or more active
ingredients, and
an optional carrier comprising inert ingredients, as well as any product that
results, directly or
indirectly, from combination, complexation or aggregation of any two or more
of the ingredients,
or from dissociation of one or more of the ingredients, or from other types of
reactions or
interactions of one or more of the ingredients. In general, pharmaceutical
compositions are
prepared by uniformly and intimately bringing the active ingredient into
association with a liquid
carrier or a finely divided solid carrier or both, and then, if necessary,
shaping the product into
the desired formulation. The pharmaceutical composition includes enough of the
active object
compound to produce the desired effect upon the progress or condition of
diseases.
Accordingly, the pharmaceutical compositions of the present invention
encompass any
composition made by admixing a compound of the present invention and a
pharmaceutically
acceptable carrier.
Within the context of this application, the term `combination preparation'
comprises
both true combinations, meaning an N-oxide of formula (1), and other
medicaments physically
combined in one preparation such as a tablet or injection fluid, as well as
`kit-of-parts',
comprising an N-oxide of formula (1), and venlafaxine or another medicament in
separate
dosage forms, together with instructions for use, optionally with further
means for facilitating
compliance with the administration of the component compounds, e.g. label or
drawings. With
true combinations, the pharmacotherapy by definition is simultaneous. The
contents of `kit-of-
parts', can be administered either simultaneously or at different time
intervals. Therapy being
either concomitant or sequential will be dependant on the characteristics of
the other
medicaments used, characteristics like onset and duration of action, plasma
levels, clearance,
etc., as well as on the disease, its stage, and characteristics of the
individual patient.
7
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
By "pharmaceutically acceptable" it is meant the carrier, diluent or excipient
must be
compatible with the other ingredients of the formulation and not deleterious
to the recipient
thereof.
Dose: recommended treatment dose is the same as for venlafaxine: 75 mg per
day,
administered in two or three divided doses, taken with food. Pharmacokinetic,
pharmacodynamic, and other considerations may alter the dose actually
administered to a
higher or lower value. The dose of the compound to be administered will depend
on the relevant
indication, the age, weight and sex of the patient and may be determined by a
physician. The
dosage will preferably be in the range of from 0.01 mg/kg to 10 mg/kg. The
typical daily dose of
the active ingredients varies within a wide range and will depend on various
factors such as the
relevant indication, the route of administration, the age, weight and sex of
the patient and may
be determined by a physician. In general, oral and parenteral dosages will be
in the range of 0.1
to 1,000 mg per day of total active ingredients.
The term "therapeutically effective amount" as used herein refers to an amount
of a
therapeutic agent to treat or prevent a condition treatable by administrating
a composition of the
invention. That amount is the amount sufficient to exhibit a detectable
therapeutic, preventative
or ameliorative response in a tissue system, animal or human. The effect may
include, for
example, treating or preventing the conditions listed herein. The precise
effective amount for a
subject will depend upon the subject's size and health, the nature and extent
of the condition
being treated, recommendations of the treating physician (researcher,
veterinarian, medical
doctor or other clinician), and the therapeutics, or combination of
therapeutics, selected for
administration. Thus, it is not useful to specify an exact effective amount in
advance.
The term "treatment" as used herein refers to any treatment of a mammalian,
preferably
human condition or disease, and includes: (1) preventing the disease or
condition from
occurring in a subject predisposed to the disease, but not yet diagnosed as
having it, (2)
inhibiting the disease or condition, i.e., arresting its development, (3)
relieving the disease or
condition, i.e., causing the condition to regress, or (4) stopping the
symptoms of the disease. As
used herein, the term "medical therapy" intendeds to include prophylactic,
diagnostic and
therapeutic regimens carried out in vivo or ex vivo on humans or other
mammals. The term
"subject" as used herein, refers to an animal, preferably a mammal, most
preferably a human,
who has been the object of treatment, observation or experiment.
EXAMPLE 1: ANALYTICAL METHODS
Nuclear magnetic resonance spectra ('H NMR and 13C NMR, APT) were determined
in the
indicated solvent using a Bruker DRX 600 ('H: 600 MHz, 13C: 150 MHz) at 300 K,
unless
indicated otherwise. The spectra were determined in deuterated DMSO, obtained
from
Cambridge Isotope Laboratories Ltd. Chemical shifts (b) are given in ppm
downfield from
8
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
tetramethylsilane ('H). Coupling constants J are given in Hz. Peakshapes in
the NMR spectra
are indicated with the symbols `q' (quartet), `dq' (double quartet), `t'
(triplet), `dt' (double triplet),
`d' (doublet), `dd' (double doublet), `s' (singlet), `bs' (broad singlet) and
`m' (multiplet). NH and
OH signals were identified after mixing the sample with a drop of D20.
Melting points were recorded on a Buchi B-545 melting point apparatus.
Flash chromatography refers to purification using the indicated eluent and
silica gel (either
Acros: 0.030-0.075 mm or Merck silica gel 60: 0.040-0.063 mm).
Reactions were monitored by using thin-layer chromatography (TLC) on silica
coated plastic
sheets (Merck precoated silica gel 60 F254) with the indicated eluent. Spots
were visualised by
UV light (254 nm) or 12.
Liquid Chromatography- Mass Spectrometrry (LC-MS) was performed using a system
consisting of 2 Perkin Elmer series 200 micro pumps. The pumps are connected
to each other
by a 50 pl tee mixer, connected to a Gilson 215 auto sampler. The method is as
follows:
step total time flow (pl/min) A(%) B(%)
0 0 2000 95 5
1 1.8 2000 0 100
2 2.5 2000 0 100
3 2.7 2000 95 5
4 3.0 2000 95 5
A= 100% Water with 0.025% HCOOH and 10mmol NH4HCOO pH= +/- 3
B= 100% ACN with 0.025% HCOOH
The auto sampler has a 2 pl injection loop. The auto sampler is connected to a
Waters Atlantis
C18 30*4.6 mm column with 3 pm particles. The column is thermo stated in a
Perkin Elmer
series 200 column oven at 40 C. The column is connected to a Perkin Elmer
series 200 UV
meter with a 2.7 pl flowcel. The wavelength is set to 254 nm. The UV meter is
connected to a
Sciex API 150EX mass spectrometer. The mass spectrometer has the following
parameters:
Scanrange:150-900 a.m.u.; polarity: positive; scan mode: profile ; resolution
Q1: UNIT ; step
size: 0.10 a.m.u.; time per scan: 0.500 sec; NEB: 10; CUR: 10 IS: 5200; TEM:
325; DF: 30; FP:
225 and EP: 10. The light scattering detector is connected to the Sciex API
150. The light
scattering detector is a Sedere Sedex 55 operating at 50 C and 3 bar N2. The
complete system
is controlled by a G3 powermac.
9
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
Venlafaxine and its N-oxide were analyzed in mouse plasma and brain samples
using a
generic bioanalytical method comprising protein precipitation and HPLC with
MS/MS detection.
Proteins in 100 pl plasma were precipitated with acetonitrile, and 5 pl
samples of the
obtained solutions were analyzed. Complete brains were homogenized and
centrifuged, and 10
pl samples of the supernatant were analyzed.
Liquid Chromatography - Tandem Mass Spectrometry (LC-MS/MS) was performed
using a
Sciex AP14000 LC-MS/MS. Samples were quantified using extracted calibration
samples,
treated the same as the study samples, in the range of 1 - 5000 ng/ml and 5.0 -
5000 ng/brain
for plasma and brain samples, respectively. Compound peak area was used for
quantification.
Calibration curves were fitted to the model y = A + Bx + Cx2 (y is the peak
area of the analyte, x
is the nominal calibration level in ng/ml (plasma) or ng/g (brain), A is the
intercept, B is the slope
and C is the description of the curvature). 1/x2 weighing was used. LC-MS/MS
system
performance was monitored using a reference solution injected at standard
intervals. The
method was not validated in detail, therefore the reported concentrations were
good
estimations. The Lower Limit Of Quantification (LLOQ) was established at 1.00
ng/ml and 5.00
ng/brain, for plasma and brain samples, respectively. Values below the LLOQ
were given as
best estimate. Reversed phase HPLC was performed using gradient elution by a
Hypersil BDS
C18 100 x 4.6 mm 3 pm analytical column, at 45 C and with a flow of 1.00
ml/min:
Solvent
Time(min) %A %B %C %D
0.00 10.0 70.0 0.0 20.0
1.00 10.0 70.0 0.0 20.0
2.00 10.0 10.0 0.0 80.0
4.00 10.0 10.0 0.0 80.0
4.10 10.0 70.0 0.0 20.0
7.00 10.0 0.0 0.0 20.0
Solvent A 100 mM NH4FA/ 1% FA
Solvent B Milli-Q water
Solvent C methanol
Solvent D acetonitrile
Detection on MS/MS was done using positive MRM ionization. Measured ions were:
venlafaxine venlafaxine-N-oxide
Q1 278.3 294.5
Q3 121.1 121.1
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
EXAMPLE 2: SYNTHESES OF SPECIFIC COMPOUNDS
(R,S)-1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-cyclohexanol
(venlafaxine), and
(R,S)-1-[2-(dimethylamino)-1-(4-hydroxyphenyl)ethyl]-cyclohexanol (0-
desmethylvenla-
faxine) were synthesized as described in EP 1 721 889. An alternative to the
latter is given
below.
(R,S)-1-[2-oxido-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-cyclohexanol
(venlafaxine N-
oxide):
Venlafaxine (0.28 g, 1.02 mmol) was dissolved in 20 ml DCM and cooled to -10
C. To the
reaction mixture was added meta-chloroperbenzoic acid (m-CPBA, 0.8 g, 2.02
mmol) and the
solution was stirred at -10 C for 30 minutes. Solid K2CO3 (2 g) was added and
the resulting
mixture was stirred for another 30 minutes at 0 C. The reaction mixture was
filtrated (glass
funnel), and the precipitate was washed carefully with DCM. The resulting
solution was
concentrated and purified by flash chromatography (Si02, DCM/MeOH (95/5
followed by 9/1) to
yield the title compound as a solid (0.22 g, 74%). mp 145 C. LCMS ; Rt : 1.12
min, ([M+H]+ =
294).'H- NMR (600 MHz, D6DMSO) : b 7.12 (bd, J = 8 Hz, 2H), 6.86 (bd, J = 8
Hz, 2H), 3.95-
3.89 (m, 1 H), 3.73 (s, 3H), 3.56-3.52 (m, 1 H), 3.28-3.25 (m, 1 H), 3.14 (s,
3H), 2.95 (s, 3H), 1.69-
1.53 (m, 3H), 1.47-1.42 (m, 1 H), 1.38-1.32 (m, 2H), 1.31-1.25 (m, 1 H), 1.02
(dt, J = 11 Hz, 4 Hz,
1 H), 0.87 (dt, J = 11 Hz, 4 Hz, 1 H), 0.77-0.69 (m, 1 H).
(R,S)-1-[2-oxido-(dimethylamino)-1-(4-hydroxyphenyl)ethyl]-cyclohexanol (0-
desmethyl-
venlafaxine N-oxide) can be prepared by the same method.
(S)- and (R)- enantiomers of venlafaxine, their respective N-oxides, and the 0-
desmethyl
analogues were synthesized as depicted in the scheme below.
11
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
LAH
HO HO NO
H2N HCO2H Me2N
+
H2CO I / I /
OMe 9 OMe 10 OMe 11
resolution
HO OH
OO
O O
O O
HO HO HO O HO
Me2N Me2N Me2N Me2N
I m-CPBA + m-CPBA
0 I \ . I \ \ -- I \
OMe OMe OMe OMe
3 1 HPPh2 2 4
n-BuLi
P
HO HO HO O HO
Me2N Me2N Me2N/ Me2N
I m-CPBA + m-CPBA
0 I \ . I \ \ -- I \
OH OH OH OH
7 5 6 8
12
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
HO HO HO O HO
Me2N Me2N Me2N Me2N~
I
O
OMe OMe OMe OMe
(S)-venlafaxine (S)-venlafaxine oxide (R)-venlafaxine (R)-venlafaxine oxide
HO HO HO O HO
Me2N Me2N Me2N Me2N~
O
OH OH OH OH
0-desmethyl- 0-desmethyl- 0-desmethyl- 0-desmethyl-
(S)-venlafaxine (S)-venlafaxine oxide (R)-venlafaxine (R)-venlafaxine oxide
(R,S)-1-(2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl)cyclohexanol (venlafaxine,
10):
Cyclohexanol (33g, 0.13 mol) was dissolved in formic acid (99%, 54 mL, 1.43
mol) and water
(330 mL) by addition of formaldehyde (37%, 41 mL, 1.48 mol). The mixture was
refluxed for 2 h.
Reaction mixture was concentrated to 150 mL (pH - 1.0), water (100 ml) was
added and the
mixture was extracted with ethyl acetate (4x 100 mL). The aqueous layer was
cooled in an ice
bath and basified to pH - 10 by addition of 50% NaOH. The mixture was
extracted with ethyl
acetate (3 x 100 mL), dried over Na2SO4 and concentrated. Yield:
This material (23.8 g, mainly compound 11) was suspended in diethyl ether (500
mL) and
treated with lithium aluminum hydride (3.8g, 0.1 mol). The suspension was
stirred for 18 h at rt.
5 N KOH (16 mL) was added carefully, and the mixture was stirred for 15 min.
Solids were
removed by filtering over Celite, and washed (diethyl ether, 300 mL). The
filtrates were were
dried (sodium sulfate) and concentrated. Yield: 21.4 g of compound 10 (60 %)
as a white solid.
'H-NMR (300 MHz, CDC13): 67.05 (d, 2H, J = 8.8 Hz), 6.81 (d, 2H, J = 8.8 Hz),
3.79 (s, 3H),
3.27(t,1H,J=12.6),2.93(dd,1H,2J=3.2Hz,3J=12.5Hz),2.32(s,6H),2.30(dd,1H,2J=
3.2 Hz, 3J 12.5 Hz), 1.82-1.61 (m, 3H), 1.60-1.45 (m, 3H), 1.42-1.22 (m, 2H),
1.01-0.78 (m, 2H).
R-venlafaxine (compound 2 in the scheme above):
(R,S)-Venlafaxine (23.4 g, 84 mmol) was dissolved in ethyl acetate (160 mL).
To the solution
was added a solution of D-ditoluyl-tartaric acid (18.7g, 48 mmol) in ethyl
acetate (130 mL).
Within 10 min. the salt started to precipitate. The mixture was stirred for 4
hours at rt. The
precipitate was collected by filtering over a glass ilter, and washed with
ethyl acetate (2 x 100
13
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
mL). White crystalline solid. This solid was recrystallized from ethyl
acetate:methanol (6:1, 100
mL). The solids were collected on a glass ilter. Yield: 14.0 g. This material
was treated with 2N
NaOH (cold, 180 mL). The aqueous phase was extracted with ethyl acetate (3 x
200 mL). The
organic phase was washed with 2 N NaOH (cold, 75 mL) then with water until
washings were
neutral (pH 7). The organic phase was dried (sodium sulfte) and concentrated.
Yield: 8.1 g of R-
Venlafaxine (2) as white crystalline solid. m.p.:106 C - 109.5 C. [a]p23 =-
8.0 (c = 1.5, MeOH).
Chiral HPLC: 99 % e.e.'H-NMR (CDC13): see above.
S-Venlafaxine (1):
The mother liquor of the resolution (see above) was freed by washing with 1 N
NaOH (4 x 100
mL), with water (3 x 200 mL) and with brine (100 mL). The organic phase was
dried (sodium
sulphate) and concentrated. Oil, solidifies quickly. This material was re-
dissolved in ethyl
acetate (75 mL). A solution of L-ditoluyl tartaric acid (11.3 g, 29 mmol) in
ethyl acetate (75 mL)
was added. Precipitation started within 5 minutes. Ethyl acetate (50 mL) was
added and the
mixture was stirred for 72 hours at room temperature. Solids were collected on
a glass filter.
Yield: 14.2 g. This material was treated with 2N NaOH (cold, 180 mL). The
aqueous phase was
extracted with ethyl acetate (3 x 200 mL). The organic phase was washed with 2
N NaOH (cold,
75 mL) then with water until washings were neutral (pH 7). The organic phase
was dried
(sodium sulphate) and concentrated. Yield: 6.7 g of S-Venlafaxine (1) as white
crystalline solid.
m.p. 104.5 C - 106 C. [a]p23 =+13.9 (c = 1.6, MeOH). Chiral HPLC: 98 % e.e. 'H-
NMR
(CDC13): see above
S-Venlafaxine N-oxide (3)
Crude material (2.2 g, 7.5 mmol) has been obtained by FAI (106796), according
to the
procedure described for 4. Pure 3 was obtained by column chromatography
(gradient
dichloromethane:methanol, 9:1 4 dichloromethane: 3.5 M ammonia in methanol,
9:1). Yield:
1.70 g (5.8 mmol, 78 %) of 3 as a slightly yellow solid. [a]p23 =-20.8 (c =
1.0, MeOH).'H-NMR
(300 MHz, CDC13): S 7.09 (d, 2H, J = 8.5 Hz), 6.84 (d, 2H, J 8.5 Hz), 4.16 (m,
1 H), 3.79 (s,
3H), 3.51 (dd, 1 H, 2J = 3.8 Hz, 3J = 12.7 Hz), 3.41 (m, 1 H), 3.27 (s, 3H),
3.07 (s, 3H), 1.78-1.60
(m, 3H), 1.60-1.35 (m, 4H), 1.29-1.01 (m, 2H), 0.95-0.76 (m, 1 H).
R-Venlafaxine N-oxide (4)
R-Venlafaxine (2, 1.0 g, 3.4 mmol) was dissolved in dichloromethane (60 mL).
The solution was
cooled to -10 C. m-CPBA (fresh, 2.9 g, 7.3 mmol) was added. The suspension was
stirred for
30 min. at -10 C. TLC check revealed full conversion. K2CO3 (5.0 g, 36 mmol.)
was added, and
the mixture was stirred for 30 min. at 0 C. Dichloromethane (50 mL) was added
and the
suspension was filtered. The filtrate was dried and concentrated. The crude
product was purified
14
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
as above. YIELD: 0.9 g (3.0 mmol, 90 %) as a white solid. [a]DZ3: not
determined.'H-NMR (300
MHz, CDC13): see above.
O-desmethyl-S-Venlafaxine (5)
A solution of diphenylphosphine (18 mL, 0.1 mol) in dry tetrahydrofuran (120
mL) was, under
N2, cooled to -10 C. n-BuLi (2.5 M in hexanes, 50 mL) and additional
tetrahydrofuran (40 mL)
were added. The mixture was stirred for 30 min. at -10 C, then the temperature
was allowed to
raise to 0 C. At this temperature a solution of S-venlafaxine (1, 6.2 g, 23
mmol) in
tetrahydrofuran (60 mL) was added. The mixture was stirred for 2 hours, while
the temperature
was allowed to raise to room temperature, and subsequently at reflux
temperature for 16 hours.
The reaction mixture was cooled to room temperature, poured into 2N HCI (cold,
300 mL) and
stirred for 10 min. The aqueous phase was washed (ethyl acetate, 3 x 300 mL),
then neutralized
(pH 7) by means of slow (!) addition of NaHCO3 (s), and extracted (ethyl
acetate, 6 x 300 mL).
The organic phase was dried (Na2SO4) and concentrated in vacuo. The residue
was suspended
in ethyl acetate (100 mL) and stirred for 30 min. Solids were collected on a
glass filter, and
washed with ethyl acetate, until the smell of diphenylfosfine could no longer
be detected. White
solid. Yield: 4.6 g (17.5 mmol, 76 %). m.p. 237.3 C - 237.9 C. [a]p23 = +17.0
(c = 0.88, MeOH).
'H-NMR (300 MHz, DMSO-d6): S 9.12 (br, 1 H), 6.94 (d, 2H, J = 8.3 Hz), 6.62
(d, 2H, J = 8.3 Hz),
5.37 (br, 1 H), 2.98 (m, 1 H), 2.71 (t, 1 H, J = 5.8 Hz), 2.34 (m, 1 H), 2.14
(s, 6H), 1.64-1.22 (m,
7H), 1.20-0.78 (m, 3H).
O-desmethyl-R-Venlafaxine (6)
As above, starting with R-Venlafaxine (5.0 g, 18 mmol), using
diphenylphosphine (14 mL) and
n-BuLi in hexanes (2.5 M, 41 mL). Yield: 3.8 g (14.5 mmol, 80 %). m.p. 235.5 C
- 237.1 C .
[a]p23 = -21.3 (c = 0.9, MeOH). 'H-NMR (300 MHz, DMSO-d6): see above.
O-desmethyl-S-venlafaxine N-oxide (7)
O-desmethyl-S-Venlafaxine (5, 1.5 g, 5.7 mmol) was suspended in
dichloromethane (100 mL).
The suspension was cooled to -10 C. m-CPBA (4.8 g, 12 mmol) was added. The
suspension
was stirred for 60 min. at -10 C. TLC check revealed full conversion. K2CO3
(7.5 g, 54 mmol.)
was added, and the mixture was stirred for 30 min. at 0 C. Dichloromethane
(100 mL) was
added and the suspension was filtered. The residue was stirred in methanol
(300 mL), and
filtered again. The combined filtrates were concentrated in vacuo (Yield:7.1
g).
This material was purified by column chromatography: the crude product was
dissolved in
methanol (20 mL), brought on the column (silica in dichloromethane), eluted
with
dichloromethane (200 mL) and subsequently with dichloromethane: 7M NH3 in
methanol, 9: 1.
YIELD: 1.15 g (4.1 mmol, 72 %) of compound 7 as a slightly yellow solid.
[a]p23 = -26.8 (c =
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
0.8, MeOH). 'H-NMR (300 MHz, DMSO-d6): S 9.66 (br, 1 H), 6.98 (d, 2H, J = 8.3
Hz), 6.69 (d,
2H, J = 8.3 Hz), 3.88 (m, 1 H), 3.55 (dd, 2J = 2.4 Hz, 3J = 12.7 Hz, 1 H),
3.34 (br, 1 H), 3.20 (dd, 2J
= 2.4 Hz, 3J = 12.7 Hz, 1 H), 3.14 (s, 3H), 2.95 (s, 3H), 1.69-1.20 (m, 6H),
1.11-0.66 (m, 4H).
O-Desmethyl-R-venlafaxine N-oxide (8)
As above, starting with O-desmethyl-R-Venlafaxine (6, 1.5 g, 5.7 mmol). Yield:
1.20 g (4.3
mmol, 75 %) as a slightly yellow solid. [a]p23 = +16.3 (c = 0.8, MeOH). 'H-NMR
(300 MHz,
DMSO-d6): see above.
EXAMPLE 3: PHARMACOLOGICAL METHODS
In vitro affinity for neurotransmitter reuptake sites were either obtained by
CEREP (128, rue
Danton, 92500 Rueil-Malmaison, France) or at Solvay Pharmaceuticals B.V. (C.J.
van
Houtenlaan 36, 1381 CP Weesp, The Netherlands), using well documented
procedures.
Measured were affinities for serotonin (Tatsumi, 1999), norepinephrine
(Pacholczyk, 1991) and
dopamine reuptake sites (Pristupa, 1994).
In vitro functional inhibition of [3H]-serotonin reuptake: Male rats (Wistar
Hsd/Cpb: WU;
175-200 g) were decapitated, the cerebral hemispheres were rapidly removed,
and a P2-
synaptosomal fraction was prepared. Synaptosomes were pre-incubated in absence
or
presence of the test compound for 15 min at 37 C, in a medium containing the
MAO inhibitor
pargyline (7 M). Subsequently, the synaptosomes were exposed to [3H]-
serotonin (0.2 mM
final concentration) for 10 min. [3H]-Serotonine uptake was stopped by
filtration with a harvester
and the non-incorporated radioactivity was removed by extensive washing.
Filterplates with
synaptosomes were dehydrated, and the amount of [3H]-serotonin present was
determined by
Betaplate liquid scintillation counting. Inhibitory effects on the uptake of
the [3H]-serotonin were
expressed as pIC50 value, that is the negative logarithm of the concentration
at which half
maximal inhibition of radiolabeled neurotransmitter uptake is achieved. pIC50
values given are
mean values of 2-9 experiments performed in duplicate. Testcompounds, 10-2 M
dissolved in
DMSO, were diluted in Krebs Ringer buffer to the testconcentrations of 10-$ to
10-5 M. Further
experimental details were as described (Coyle, 1969).
In vitro functional inhibition of [3H]-norepinephrine reuptake: Male rats
(Wistar Hsd/Cpb:
WU; 175-200 g) were decapitated, the hypothalamus was rapidly removed and a
crude
synaptosomal fraction was prepared. Synaptosomes were pre-incubated in absence
or
presence of the test compound for 10 min at 37 C, in a medium containing the
MAO inhibitor
pargyline (7 M). Subsequently, the synaptosomes were exposed to [3H]-
norepinephrine (0.4
16
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
mM final concentration) for 15 min. [3H]- Norepinephrine uptake was stopped by
filtration with a
harvester and the non-incorporated radioactivity was removed by an extensive
washing
programme. The filterplates with synaptosomes were dehydrated and the amount
of [3H]-
norepinephrine present was determined by Betaplate liquid scintillation
counting. Inhibitory
effects on the uptake of the [3H]- norepinephrine were expressed as pIC50
value, that is the
negative logarithm of the concentration at which half maximal inhibition of
radiolabeled
neurotransmitter uptake is achieved. pIC50 values given are mean values of 2-9
experiments
performed in duplicate. Testcompounds, 10-2 M dissolved in DMSO, were diluted
in Krebs
Ringer buffer to testconcentrations of 10-$ - 10-5 M. Further experimental
details were as
described (Coyle, 1969).
In vitro functional inhibition of [3H]-dopamine reuptake: Male rats (Wistar
Hsd/Cpb: WU;
175-200 g) were decapitated; the striatum was rapidly removed and a crude
synaptosomal
fraction (P2) was prepared by homogenization and centrifugation. Synaptosomes
were pre-
incubated in absence or presence of the test compound for 15 min at 37 C, in a
medium
containing the monoamine oxidase inhibitor pargyline (7x10-6 M) (Coyle, 1969).
Subsequently,
[3H]-dopamine (2x10-' M final concentration) was added and incubation was
continued for 10
min. [3H]-dopamine uptake was stopped by filtration and the synaptosomes were
washed four
times with phosphate buffered saline. The amount of [3H]-dopamine in the
synaptosomes was
determined by Betaplate liquid scintillation counting. Compounds were tested
in a concentration
range of 10-9 to 10-5 M. Inhibitory effects on the uptake of [3H]-dopamine
were expressed using
the pIC50 value (the negative logarithm of the concentration at which the drug
caused 50%
uptake inhibition). Inhibition of DA uptake was performed in duplicate.
The human colon model TIM2 (TNO Intestinal Model 2): is a dynamic model for
the human
large intestine that simulates in vivo conditions. It is an artificial
digestive system that has been
validated by many studies (Minekus, 1999).
Venlafaxine-N-oxides are prodrugs of the parent compound. They are useful in
the treatment of
diseases effectively treatable-albeit with side effects-with venlafaxine:
depression, including
major depressive disorder, generalized anxiety disorder, obsessive compulsive
disorder, social
anxiety disorder, panic disorder, general depressive disorders, diabetic
neuropathy, migraine
and vasomotor symptoms associated with menopause, a.k.a. `hot flashes'.
17
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
EXAMPLE 4: PHARMACOKINETIC AND PHARMACOLOGICAL TESTRESULTS
Venlafaxine and its N-oxide, formulated in 40% HPPCD or 1% methylcellulose,
respectively,
were individually administered (intravenously (i.v.) or orally (p.o.)) to male
NMRI mice (3 animals
per time point), after which their plasma and brain were analyzed by LC-MS
(method see
above) for both compounds. Data were averaged (n=3), and collected in table 1.
Table 1: plasma and brain concentrations of venlafaxine and its N-oxide
venlafaxine venlafaxine-N-oxide
plasma brain plasma brain
administered Time (h) [ng/ml] [ng/g] [ng/ml] [ng/g]
0.17 250 710 0.32 0.19
0.5 97 289 0.12 0
venlafaxine 1.0 1 49 200 0.10 0
mg/kg i.v. 3 4.7 15 0 0
7 0.30 1.7 0 0
24 0.37 0 0 0
0.17 630 1133 2.5 0
0.5 543 2267 7.0 0.37
venlafaxine 1 523 1800 3.0 0.09
mg/kg p.o. 3 38 137 0.31 0
7 3.6 15 0.06 0
24 0 0 0 0
0.17 42 56 623 3.3
0.5 22 52 173 58
venlafaxine-N- 1 8.0 26 39 8.1
oxide 1.0 mg/kg 3 0.59 3.0 7.3 0.44
i.v. 7 0 0.85 0.16 0.15
24 0 0 0 0
0.17 82 55 1040 14
0.5 72 167 840 33
venlafaxine-N- 1 117 353 183 6.7
oxide 10 mg/kg 3 77 280 38 1.8
P.O. 7 3.3 12 0.75 1.6
24 0.63 0 0.12 0
In mice, venlafaxine is only marginally metabolized to its N-oxide: The
concentration thereof in
10 the plasma never exceeds 1 - 2% of that of the parent compound, and in
brain only traces can
be found. When venlafaxine-N-oxide itself is administered it is reduced to the
parent compound.
Approximately one hour after i.v. administration of venlafaxine-N-oxide,
venlafaxine concen-
trations in plasma and brain exceed those of the N-oxide. The effects are more
pronounced
after oral administration: Venlafaxine concentrations in both plasma and brain
rise to levels that
are a factor 10 to 100 higher than those of the N-oxide.
18
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
Suspended in 1% methylcellulose, venlafaxine-N-oxide (1 mg) was inserted into
the lumen (120
ml) of the TIM2 model (see above, Minekus, 1999). Samples from the lumen and
the dialysate
(the latter being a model for the vascular bed of the intestines) were taken
at various time
intervals, and analyzed for venlafaxine-N-oxide and venlafaxine: Table 2:
Table 2 reduction of venlafaxine N-oxide in the human colon model TIM2
venlafaxine-N-oxide venlafaxine
lumen dialysate lumen dialysate
Time (h) [ng/ml] [ng/ml] [ng/ml] [ng/ml]
0 < 1.0 < 1.0 < 1.0 < 1.0
2 2.0 7.4 5,400 320
4 2.0 1.2 4,700 470
6 2.0 1.0 4,600 430
8 1.9 < 1.0 3,900 370
24 < 1.0 < 1.0 970 210
Form the results above it is clear that already within 2 hours after dosing
venlafaxine N-oxide
was nearly quantitatively reduced to venlafaxine. Because many studies
validated TIM2 as an in
vitro model with high predictive value for the gastrointestinal conditions in
living human beings, it
is predicted that also in man, after oral administration, venlafaxine N-oxide
will be reduced to
venlafaxine: that it will be a prodrug.
Table 3: plasma pharmacokinetics of venlafaxine and its N-oxide
venlafaxine Venlafaxine-N-oxide
Route of administration: i.v. p.o. i.v. p.o.
Dose (mg/kg) 1 10 1 10
C,,,ax (ng/ml) 407.9 (Co)* 630.0 1205.2 (Co)* 1040.0
T,,,ax (hr) 0.0 0.2 0.0 0.2
ti, (hr) 0.8 0.9 0.8 3.1
AUCO >end (ng/ml x hr) 194.3 943.4 361.6 841.6
- remark Tend = 24 hrs Tend = 7 hrs Tend = 7 hrs Tend = 24 hrs
AUCo - (ng/ml x hr) 194.8 948.0 361.7 842.2
- remark Tend = 00 Tend = 00 Tend = 00 Tend = oO
Clearance (ml/min/kg) 85.6 - 46.1 -
VD (ml/kg) 6200.0 - 3000.0 -
Bioavailability (%) 48.7 - 23.3 -
Brain/plasma ratio 3.1 3.4 0.1 0.0
* For i. v. administration, Cmax values were extrapolated to To (time zero)
19
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
From the data given above it is evident that clearance, volume of distribution
and
bioavailability of venlafaxine are twice as high as those of its pyridine N-
oxide. Clearly, the two
compounds have different pharmacokinetic properties. As is also clear from the
data given in
Table 1, venlafaxine-N-oxide hardly penetrates the brain: Hence dramatically
different
brain/plasma ratio's.
in vitro pharmacology of (O-desmethyl)venlafaxine, their N-oxides, and
enantiomers
dopamine noradrenaline serotonine
binding inhibition binding inhibition binding inhibition
compound pK; pIC50 pK; pIC50 pKi pIC50
(R,S)-venlafaxine 5.4 5.3 5.2 6.2 7.9 7.2
(S)-venlafaxine 4.8 5.0 < 4.5 6.1 8.0 7.3
(R)-venlafaxine 5.6 5.5 5.4 6.8 7.5 7.1
(S)-O-desmethyl-venlafaxine 4.9 5.0 4.6 6.4 8.0 7.3
(R)-O-desmethyl-venlafaxine 5.2 5.3 5.2 6.4 7.6 7.1
(R,S)-venlafaxine-N-oxide < 4.5 4.5 < 4.5 4.9 5.5 4.9
(S)-venlafaxine-N-oxide < 4.5 < 4.5 < 4.5 < 4.5 5.2 4.8
(R)-venlafaxine-N-oxide < 4.5 < 4.5 < 4.5 4.4 5.1 4.7
(S)-O-desmethyl-venlafaxine-N-oxide < 4.5 < 4.5 < 4.5 < 4.5 4.9 4.6
(R)-O-desmethyl-venlafaxine-N-oxide < 4.5 < 4.5 < 4.5 < 4.5 5.0 4.4
The in vitro pharmacological data compiled in the table above clearly indicate
that venlafaxine is
most potent as inhibitor of serotonine reuptake. Its (R)- and (S)-enantiomers
showed only
marginal differences. The major metabolite, 0-desmethylvenlafaxine, was found
to be
equipotent with venlafaxine, both as racemate, and in the form of its
individual enantiomers.
The N-oxides of venlafaxine and 0-desmethyl-venlafaxine, as racemates as well
as individual
(R)- or (S)-enantiomers were found to be virtually devoid of activity.
EXAMPLE 5: PHARMACEUTICAL PREPARATIONS
For clinical use, N-oxides of formula (1) are formulated into pharmaceutical
compositions that
are important and novel embodiments of the invention because they contain the
compounds,
more particularly specific compounds disclosed herein. Types of pharmaceutical
compositions
that may be used include: tablets, chewable tablets, capsules (including
microcapsules),
solutions, parenteral solutions, ointments (creams and gels), suppositories,
suspensions, and
other types disclosed herein, or are apparent to a person skilled in the art
from the specification
and general knowledge in the art. The active ingredient for instance, may also
be in the form of
an inclusion complex in cyclodextrins, their ethers or their esters. The
compositions are used for
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
oral, intravenous, subcutaneous, tracheal, bronchial, intranasal, pulmonary,
transdermal,
buccal, rectal, parenteral or other ways to administer. The pharmaceutical
formulation contains
at least one N-oxide of formula (1) in admixture with at least one
pharmaceutically acceptable
adjuvant, diluent and/or carrier. The total amount of active ingredients
suitably is in the range of
from about 0.1 %(w/w) to about 95% (w/w) of the formulation, suitably from
0.5% to 50% (w/w)
and preferably from 1% to 25% (w/w). In some embodiments, the amount of active
ingredient is
greater than about 95% (w/w) or less than about 0.1 % (w/w).
The compounds of the invention can be brought into forms suitable for
administration by
means of usual processes using auxiliary substances such as liquid or solid,
powdered
ingredients, such as the pharmaceutically customary liquid or solid fillers
and extenders,
solvents, emulsifiers, lubricants, flavorings, colorings and/or buffer
substances. Frequently used
auxiliary substances include magnesium carbonate, titanium dioxide, lactose,
saccharose,
sorbitol, mannitol and other sugars or sugar alcohols, talc, lactoprotein,
gelatin, starch,
amylopectin, cellulose and its derivatives, animal and vegetable oils such as
fish liver oil,
sunflower, groundnut or sesame oil, polyethylene glycol and solvents such as,
for example,
sterile water and mono- or polyhydric alcohols such as glycerol, as well as
with disintegrating
agents and lubricating agents such as magnesium stearate, calcium stearate,
sodium stearyl
fumarate and polyethylene glycol waxes. The mixture may then be processed into
granules or
pressed into tablets. A tablet is prepared using the ingredients below:
Ingredient Quantity (mg/tablet)
venlafaxine N-oxide 10
Cellulose, microcrystalline 200
Silicon dioxide, fumed 10
Stearic acid 10
Total 230
The components are blended and compressed to form tablets each weighing 230
mg.
The active ingredients may be separately premixed with the other non-active
ingredients,
before being mixed to form a formulation. The active ingredients may also be
mixed with each
other, before being mixed with the non-active ingredients to form a
formulation.
Soft gelatin capsules may be prepared with capsules containing a mixture of
the active
ingredients of the invention, vegetable oil, fat, or other suitable vehicle
for soft gelatin capsules.
Hard gelatin capsules may contain granules of the active ingredients. Hard
gelatin capsules
may also contain the active ingredients together with solid powdered
ingredients such as
21
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
lactose, saccharose, sorbitol, mannitol, potato starch, corn starch,
amylopectin, cellulose
derivatives or gelatin.
Dosage units for rectal administration may be prepared (i) in the form of
suppositories
that contain the active substance mixed with a neutral fat base; (ii) in the
form of a gelatin rectal
capsule that contains the active substance in a mixture with a vegetable oil,
paraffin oil or other
suitable vehicle for gelatin rectal capsules; (iii) in the form of a ready-
made micro enema; or (iv)
in the form of a dry micro enema formulation to be reconstituted in a suitable
solvent just prior to
administration.
Liquid preparations may be prepared in the form of syrups, elixirs,
concentrated drops or
suspensions, e.g. solutions or suspensions containing the active ingredients
and the remainder
consisting, for example, of sugar or sugar alcohols and a mixture of ethanol,
water, glycerol,
propylene glycol and polyethylene glycol. If desired, such liquid preparations
may contain
coloring agents, flavoring agents, preservatives, saccharine and carboxymethyl
cellulose or
other thickening agents. Liquid preparations may also be prepared in the form
of a dry powder,
reconstituted with a suitable solvent prior to use. Solutions for parenteral
administration may be
prepared as a solution of a formulation of the invention in a pharmaceutically
acceptable
solvent. These solutions may also contain stabilizing ingredients,
preservatives and/or buffering
ingredients. Solutions for parenteral administration may also be prepared as a
dry preparation,
reconstituted with a suitable solvent before use.
Also provided according to the present invention are formulations and `kits of
parts'
comprising one or more containers filled with one or more of the ingredients
of a pharmaceutical
composition of the invention, for use in medical therapy. Associated with such
container(s) can
be various written materials such as instructions for use, or a notice in the
form prescribed by a
governmental agency regulating the manufacture, use or sale of pharmaceuticals
products,
which notice reflects approval by the agency of manufacture, use, or sale for
human or
veterinary administration. The use of formulations of the present invention in
the manufacture of
medicaments for use in treating depression, including major depressive
disorder, generalized
anxiety disorder, obsessive compulsive disorder, social anxiety disorder,
panic disorder, general
depressive disorders, diabetic neuropathy, migraine and vasomotor symptoms
associated with
menopause, a.k.a. `hot flashes', and methods of medical treatment or
comprising the
administration of a therapeutically effective total amount of at least one N-
oxide of formula (1),
either as such or, in the case of prodrugs, after administration, to a patient
suffering from
depression, including major depressive disorder, generalized anxiety disorder,
obsessive
compulsive disorder, social anxiety disorder, panic disorder, general
depressive disorders,
diabetic neuropathy, migraine and vasomotor symptoms associated with
menopause, a.k.a. `hot
flashes'.
22
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
By way of example and not of limitation, several pharmaceutical compositions
are given,
comprising preferred active compounds for systemic use or topical application.
Other
compounds of the invention or combinations thereof, may be used in place of
(or in addition to)
said compounds. The concentration of the active ingredient may be varied over
a wide range as
discussed herein. The amounts and types of ingredients that may be included
are well known in
the art.
BIBLIOGRAPHY
To the extend in which the following references are useful to one skilled in
the art, or to more
fully describe this invention, they are incorporated herein by reference.
Neither these, nor any
other documents or quotes cited herein, nor citations to any references, are
admitted to be prior
art documents or citations.
Bickel, M.H.,: "The pharmacology and Biochemistry of N-oxides", Pharmacol.
Reviews, 21(4),
325 - 355, 1969.
Bundgaard, H. (editor), "Design of Prodrugs", Elsevier, 1985.
Coyle, J.T. and S.H. Snyder, 1969,"Catecholamine uptake by synaptosomes in
homogenates of
rat brain; stereospecificity in different areas", J. Pharmacol. Exp. Ther.
170, 221-231, 1969.
Ettmayer, P. et al., "Lessons learned from marketed and investigational
prodrugs",
J.Med.Chem., 47, 2393-2404, 2004.
Howell, S. R. et al. Xenobiotica 24(4):315-327 (1994).
Janowsky, A. et al., J.Neurochem., 46, 1272-1276, 1986.
Jarvinen, T. et al., "Design and Pharmaceutical applications of prodrugs",
pages 733-796 in:
S.C. Gad (editor): "Drug Discovery Handbook", John Wiley & Sons Inc., New
Jersey, U.S.A.,
2005.
King, F.D., (editor), page 215 in: "Medicinal Chemistry: Principles and
Practice", 1994, ISBN 0-
85186-494-5.
Klamerus, K. J. et al. J. Clin. Pharmacol. 32:716-724 (1992).
Minekus, M., M. Smeets-Peter, A. Bernalier, S. Marol-Bonnin, R. Havenaar, P.
Marteau, M.
Alric, G. Fonty, and J. H. J. Huis in't Veld. `A computer-controlled system to
simulate
conditions of the large intestine with peristaltic mixing, water absorption
and absorption of
fermentation products'. Appl. Microbiol. Biotechnol. 53:108-114, 1999.
Pacholczyk, T. et al., Nature, 350, 350-354, 1991
Pento, J. T. Drugs of the Future 13(9):839-840 (1988).
Physicians' Desk Reference pp. 3293-3302 (53rd ed., 1999).
23
CA 02699961 2009-12-01
WO 2009/000797 PCT/EP2008/057939
Pristupa, Z.B. et al., Mol. Pharmacology., 45, 125-135, 1994.
Sinclair, J. et al. Rev. Contemp. Pharmacother. 9:333-344 (1998).
Stella,J., "Prodrugs as therapeutics", Expert Opin. Ther. Patents, 14(3), 277-
280, 2004.
Tatsumi, M., et al., Eur.J.Pharmacol.,368, 277-283, 1999
PATENTS AND PATENT APPLICATIONS:
EP 1 721 889
U.S. 4,761,501
24