Note: Descriptions are shown in the official language in which they were submitted.
CA 02701136 2010-03-29
WO 2009/067779 PCT/CA2007/002161
VAPOUR PHASE ESTERIFICATION OF FREE FATTY ACIDS
Field of the Invention
The present invention relates to a method of producing biodiesel from
triglycerides combining thermal cracking and esterification.
Background of the Invention
In recent years, the area of biodiesels has drawn a great deal of attention.
Biodiesels are fuels produced from the esterification of biomass-derived oils
with
alcohol. Biodiesel can be produced from triglyceride sources such as canola,
corn,
soybean, palm etc.
Another potential source for biodiesels are the waste triglycerides from
animal
rendering facilities and waste cooking oils, such as those found as restaurant
trap
greases. However, this potential is presently still under-explored and waste
triglycerides, trap grease in particular, are most commonly dumped into
landfills.
Waste triglycerides often have high contaminants content, including bacteria,
which
must effectively be removed before processing. Furthermore, waste
triglycerides tend
to have a high content of free fatty acid (FFA), anywhere in the range of from
50% to
100%. Mixtures of free fatty acids and triglycerides have been found to be
very
difficult to convert to useful fuels by any traditional methods.
Traditional methods of producing biodiesels include transesterification and
esterification with alcohol using either an acid or base catalyst. However,
the high
FFA content in waste triglycerides causes undesirable soap formation in base
catalyzed esterification processes, rendering this process inoperable.
Waste triglycerides are also often heavily contaminated by, for example,
bacteria, detergents, silts and pesticides. These contaminants must be removed
before
esterification can take place, without adding significant additional cost to
the overall
processes.
One known method of processing high FFA feedstocks involves adding
glycerol to the feedstock to convert FFA's to mono- and diglycerides, followed
by
conventional alkali-catalyzed esterification. This method addresses the issue
of high
FFA content but does not treat or remove contaminants. A second method
involves
pre-treating an FFA-rich triglyceride feedstock with an acid catalyst to
convert FFA to
alkyl-esters and reduce FFA concentrations to less than about 0.5%, followed
by
1
CA 02701136 2011-02-02
traditional base-catalyzed esterification. This method again, only deals with
the issue
of high FFA content and not high contaminant levels.
An alternate method involves performing both esterification and
transesterification of triglycerides using a strong acid such as H2SO4 or
sulphamic
acid; however the product clean up is cumbersome and usually involves
neutralization
of the acidic catalyst and the removal of resulting salts. Acid ion-exchange
resins are
another option, but due to possible resin degradation esterification must be
carried out
below the resin degradation temperature, which significantly slows down the
process.
As well, water formation by FFA esterification prevents this process from
going to
completion.
Reaction time required for typical bio-diesel production by acid
esterification
ranges from 10 hours to 20 hours, which makes acid esterification of fatty
acids an
industrially unattractive route for fuel production. When esterification
temperature is
raised above 100 C, satisfactory conversion can be achieved within 8 to 12
hours.
However, carrying out acid esterification at above water's boiling point
diminishes
alcohol solubility in the methyl-esters. This is undesirable since, in order
to bring
esterification to near completion a high alcohol concentration must be
maintained in
the reactant mixture and this becomes difficult due to limited alcohol
solubility.
Thermal cracking of clean triglycerides under typical cracking conditions with
and without catalyst has been attempted, but this process was found to yield
mainly
naphtha, not diesel fuels. Furthermore, in typical thermal cracking of clean
or waste
triglycerides in the presence of a catalyst, there is a tendency for coke
formation to
occur on the catalyst, resulting in rapid deactivation.
It is therefore greatly desirable to find a method of converting
triglycerides,
and in particular low quality and waste triglyceride feedstocks, to biodiesel
that is
both efficient and economical. It is also desirable to find ways of dealing
with
contaminants and high FFA content in waste triglyceride feedstocks so that
they can
be converted into usable fuels.
Summary of the Invention
The present invention thus provides a method of producing biodiesel from
a triglyceride feedstock, comprising pretreating the triglyceride feedstock by
thermal cracking to remove contaminants and convert triglycerides, to form
2
CA 02701136 2011-02-02
a middle distillate fraction rich in free fatty acids. The middle distillate
fraction can
then be treated by vapour phase esterification under vacuum and in the
presence of an
alcohol and a solid acid catalyst to produce a mixed biodiesel/diesel stream.
The
mixed biodiesel/diesel stream can then be treated with a basic solution to
convert
residual free fatty acids to non-foaming metallic soaps, which non-foaming
metallic
soaps can be separated by vacuum distillation, centrifugation, filtering or
combinations thereof.
The present invention also provides a method of producing a biodiesel/naphtha
mixture from a triglyceride feedstock. The method involves first pretreating
the
triglyceride feedstock by thermal cracking to remove contaminants and convert
triglycerides, to produce a middle distillate fraction rich in free fatty
acids, a naphtha
stream and a gas stream. Next, the naphtha stream and middle distillate
fraction are
treated by vapour phase esterification under vacuum and in the presence of an
alcohol
and a solid acid catalyst to produce a mixed biodiesel/naphtha stream. The
mixed
biodiesel/naphtha stream can then be treated with a basic solution to convert
residual
free fatty acids to non-foaming metallic soaps, which non-foaming metallic
soaps are
separated by vacuum distillation, centrifugation, filtering or combinations
thereof.
Brief Description of the Drawings
The present invention will now be described in further detail with reference
to
the following drawings, in which:
Fig. 1 is a flow sheet of a first preferred process for carrying out the
present invention.
Detailed Description of the Preferred Embodiments
The present process employs a novel combination of thermal cracking
followed by solid acid esterification under vacuum and elevated temperatures
to
convert triglycerides, and particularly low quality and waste triglycerides,
into usable
biodiesel. In the present process, thermal cracking is used as a pre-treatment
step to
break down the triglycerides into a broad range of free fatty acids and lower
3
CA 02701136 2011-02-02
molecular weight components. Thermal cracking also serves to remove
contaminants
found in waste triglycerides, which can cause problems downstream. The
resulting
product from the cracking step can then be treated by solid acid
esterification under
vacuum and elevated temperatures to convert fatty acids into alkyl esters
(biodiesel).
The esterification is carried out in the vapour phase.
For the purposes of the present invention, thermal cracking is considered to
loosely cover the process of breaking down large molecules into smaller
molecules at
a predetermined temperature and pressure.
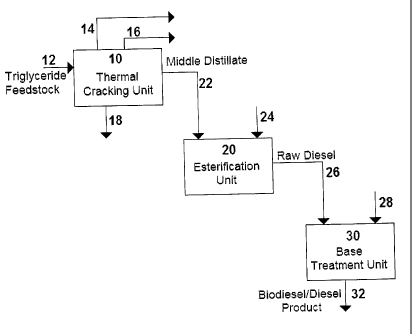
A flow diagram of the process steps and streams of one embodiment of the
present invention is shown in Fig. 1. A feedstock 12 of triglycerides, and
particularly
low quality or waste triglycerides, is fed to a thermal cracking unit 10. The
feedstock
12 can be any variety of triglyceride including oils such as canola, soy,
corn, palm,
cottonseed, mustard seed, fish or algae oils and waste or low quality
triglycerides such
as restaurant trap greases, waste greases from animal rendering facilities and
other
forms of waste oils and greases and low-quality vegetable oils. The feedstock
stream
12 can be heterogeneous in nature and can contain water and other
contaminants. The
triglyceride feedstock stream 12 can also have free fatty acid (FFA) content
as high as
50 to 100 wt.%. In an optional embodiment (not shown), the triglyceride
feedstock
12 may be filtered to remove any macroscopic contaminant particles prior to
thermal
cracking.
In the thermal cracking unit 10, triglycerides in the feedstock stream 12 are
destroyed since they are converted into free fatty acids, thus forming a
mixture of free
fatty acids and conventional hydrocarbons, such as paraffins, olefins and
aromatics.
Thermal cracking is preferably carried out at mild cracking conditions which,
for the
purposes of the present invention, are described as an operating temperature
preferably in the range of from 390 to 460 C, more preferably from 410 to 430
C, and
preferably at an operating pressure of from 0 to 60 psig (6.9 to 515 kPa),
more
preferably from 30 to 40 psig (308 to 377 kPa). Thermal cracking produces
various
fractions including gases 14, naphtha 16, middle distillate 22, and residue
18. Gases
mainly comprise of CO, CO2, hydrogen, methane, ethane, ethylene, propane, and
propylene. Contaminants from the feedstock 12 end up in the residue stream 18.
4
CA 02701136 2010-03-29
WO 2009/067779 PCT/CA2007/002161
It was noted that the mild thermal cracking conditions used in the present
invention to crack the feedstock 12 produces a mainly diesel-like fraction,
having a
boiling range of between 165 C and 345 C, rather than naphtha (IBP to 165 C),
as
was produced from thermal cracking of triglycerides at higher temperatures and
pressures.
The middle distillate fraction 22 makes up more than half of the thermally
cracked product and has been found to have suitable characteristics for
further
treatment by esterification. The middle distillate fraction 22 is rich in C 16
and C 18
fatty acids, comprising free fatty acids formed from thermal cracking of
triglycerides,
the original free fatty acids present in the feedstock and conventional
hydrocarbons.
Middle distillates typically encompass a range of petroleum equivalent
fractions from
kerosene to lubricating oil and include light fuel oils and diesel fuel. In
one
embodiment of the present invention the middle distillate fraction 22 was
found to
have a boiling point range of from 150 to 360 C, and more preferably from 165
to
345 T. The middle distillate fraction 22 still has some fuel quality issues
such as
high viscosity, high acid number, high cloud point and high concentrations of
nitrogen
and/or sulphur.
The present invention incorporates a vapour phase esterification process that
overcomes the alcohol solubility issue and thus substantially accelerates acid
esterification rates. In the present invention, acid esterification is
operated under
vacuum and high temperature, higher than normally used in liquid
esterification, to
achieve free fatty acids conversion higher than 97% in less than 10 minutes.
High
temperature accelerates the reaction rate and high vacuum and temperature
ensure all
components are in vapour phase. By operating in the vapour phase, it is
possible to
increase alcohol concentration by simply increasing the feed rate of the
alcohol, since
the alcohol solubility is no longer an issue.
The middle distillate fraction 22 is fed to an esterification unit 20, where
vapour phase esterification is carried out in the presence of an alcohol
stream 24 and a
solid acid catalyst to produce alkyl esters (biodiesel). The esterification
process is
carried out at a temperature preferably ranging from 150 to 350 C, more
preferably
from 200 to 250 C. The esterification process operates under a vacuum,
preferably in
the range of 0.1 to 1.16 psia (6 to 60 mmHg), and is more preferably 0.1 to
0.58 psia
(6 to 30 mmHg). The alcohol stream 24 can be any suitable alcohol known in the
art,
5
CA 02701136 2010-03-29
WO 2009/067779 PCT/CA2007/002161
or mixtures thereof. The alcohol stream 24 is preferably methanol. The ratio
of
middle distillate stream 22 to alcohol 24 is preferably in the range of from
3:1 to 0.1:1
and is more preferably in the range of from 2:1 to 1:1.
Residence time in the esterification unit 20 can range from 6 to 425 minutes
and preferably ranges from 6 to 43 minutes. For the present purposes,
residence time
is defined by dividing the catalyst volume by the total liquid feed rate.
The ability to conduct the esterification at higher temperatures is further
advantageous since this circumvents the catalysis quenching by water. Since
water is
a co-product of acid esterification, it can detrimentally quench the
esterification
reaction if not removed continuously. In the present invention, as water forms
by
esterification, it immediately evaporates from the catalyst surface, thereby
avoiding
deactivation of the esterification catalyst.
The solid acid catalyst is preferably chosen from super acids such as, for
example Ti02 solid support doped with Zr(S04)2, Sn02 doped with sulphuric
acid,
and sulphated zirconium oxide (Zr02/SO42-). Other solid acids suitable for the
current
application are superacids including sulphated iron oxide or halogenated
alumina,
sulphated tin oxide, trifluoromethyl-imines (R1CF3CNR2, where R1 and R2 are
hydrocarbon chains), tungstated zirconia-alumina (W/SiZr-Al), silica-supported
aluminum chloride.
Free fatty acids can be acid esterified by the following reaction, here shown
with the alcohol optionally being methanol:
H+
RCOOH + CH3OH RCOOCH3 +H2O
The water byproduct can inhibit the reaction, and may prevent esterification
from proceeding to completion. As mentioned above, esterification at high
temperatures and under vacuum conditions has been surprisingly found to
alleviate
this problem in the present invention.
The present inventors have conducted acid esterification of middle distillate
derived from thermal cracking of triglycerides using the methods of the
present
invention. Results are given in Table 1 below.
6
CA 02701136 2010-03-29
WO 2009/067779 PCT/CA2007/002161
-o 0 3 3 3 3 3 3 3 3 0
'lull
a aar~Uaa~~s~~ aaaaa~a
w -
O O v~ M l~ õ~ ~
WSJ e a1 N
,fin Q o N M 00 M 00 ~1 M O O N y~j p
v w O O O o0 ~t a\ N oo r- 00 t- r-
w U N- N 1- 00 C 00 0 0 C' C 0 C C
O ~ rn
^ U a)
a) ~ 00 ~. cd r,
0
.~ N ~ ..d N O
C/]
r. Cj
72
Z3 U N N N N N N N N I In In 1
Z 00 Vn 00 00 00
~ HU 0.U-o
=s t
' V L V) to to - N U O
^ O N O O O O 0 0 0 0 0 0 0 ~~ C y
U U O ~n In In O O O kn Vn V) of (n V) O O t~- O
4-4 nz)
H H M N N N N N p N N N N N N N N
O/J ti
o N 3~~0
-o N p-o ox
v v v v `" v C40 v v v yUõ , S,,,, a) p
O O 00 O 000 ~- o
~CA Cl) C/) V~ CA N V~ V~
FU N N N N N N N N [n
O O 00 O 0 00 O
c, uv) v) N N HNt, N.
. dNHcC 1.0 o
N N - N
N N- 0 0 O
00 00 00
QQ C, Q, - - - d U
^" Q a) O O O O O O a) O O O -- r H
.Q Q a) ~p ' C "0 "a) w d . *
u,o 0 00 0 oR,0oo0000
7
CA 02701136 2010-03-29
WO 2009/067779 PCT/CA2007/002161
Esterification produces a raw diesel stream 26 of approximately 50% alkyl
esters (biodiesel) and 50% hydrocarbons. These hydrocarbons can include
tetradecane, pentadecane, 1-hexadecene, hexadecane, heptadecane, 1-octadecene,
octadecane, nonadecane, 1-eicosene, eicosane, heneicosane, 1-docosene,
docosane,
tricosane, tetracosane, pentacosane, hexacosane, heptacosane, octacosane,
nonacosane, triacontane, untriacontane, dotriacontane, tritriacontane,
tetratriacontane,
pentatriacontane, hexatriacontane, heptatriacontane, and octatriacontane.
It should be noted that, in addition to esterifying only the middle
distillates
fraction 22 from thermal cracking, it is also possible to esterify both the
naphtha
stream 16 and middle distillates fraction 22 from the thermal cracking step.
This
optional method circumvents an extra step of separating naphtha 16 from the
middle
distillates 22.
Depending on the type of catalyst used and the degree of esterification
achieved, the raw diesel stream 26 may exceed acidity limits allowed by ASTM
specifications for biodiesel, namely 0.5 mg KOH/g. To reduce acidity, the raw
diesel
stream 26 can optionally be fed to a base treatment unit 30, together with a
basic
solution 28. The basic solution 28 reacts with any unreacted fatty acids in
the raw
diesel stream 26 to produce non-foaming metallic soaps with low solubility in
bio-
diesel. These non-foaming metallic soaps can then be separated by conventional
known methods such as vacuum distillation, centrifugation, filtering or
combinations
thereof. The inventors note that the non-foaming metallic soaps, which include
salts
such as calcium, magnesium, potassium and lithium salts, have a high viscosity
and
can be sold as a valuable bio-lubricant by-product.
Base treatment is preferably carried out at temperatures of from 30 to 60 C,
and more preferably at temperatures of from 40 to 50 C and preferably at
atmospheric pressure. The basic solution is preferably chosen from lithium
hydroxide
(LiOH), potassium hydroxide (KOH), magnesium hydroxide (Mg(OH)2), and calcium
hydroxide (Ca(OH)2). Most preferred are LiOH and Ca(OH)2.
The base treatment step results in a mixed biodiesel/diesel product 32 that
has
been found to have excellent fuel properties.
The boiling point distribution of the resultant biodiesel/diesel product 32 is
found to be broader than that of biodiesel produced by conventional
transesterification
8
CA 02701136 2011-02-02
alone. The mixed biodiesel/diesel product 32 can be used both neat or can
optionally
be further blended with regular diesel.
The naphtha stream 16 from the thermal cracking unit 10 contains oxygenates
and can optionally be sold as a valuable by-product such as octane improver.
The
residue stream 18 can be discarded by well known means in the art.
The following examples serve to better illustrate portions of the process of
the
present invention, without limiting the scope thereof:
Esterification setup:
A metal screen was placed in the bottom of a stainless steel micro-reactor
(reactor
volume 10 mL) and covered with a thick layer of glass wool. The reactor was
loaded
with a measured amount of catalyst and was tightly shut. When the
predetermined
temperature was reached, vacuum was applied and two syringe pumps containing
feedstock and methanol respectively were started and the feeds entered the
microreactor.
Vapour leaving the reactor was condensed and analyzed for FFA conversion.
Example 1:
The esterification system consists of 1) a feed syringe pump, 2) a micro-
reactor
(10 mL), 3) a water-cooled condenser, 4) a room temperature trap, 5) an ice-
water
trap and 6) a mechanical vacuum pump. The vacuum pump attached to the exit
side of
the system maintained constant vacuum during the esterification. Thermally
cracked
palm oil and methanol were premixed at a weight ratio of 1:1 and loaded in an
8 mL
syringe pump. Calcined Ti02/Zr(SO4)2 in an amount of 4.1 g was charged in the
micro-reactor between the layers of compacted glass wool and used as the acid
catalyst. The catalyst occupied about 8 mL of the reactor volume. The reactor
was
heated to near 200 C, the vacuum pump was turned on and feed was started at a
feed
rate of 20 L/min. The system pressure was maintained at 57 mmHg (1.1 psia) by
bleeding a small stream of air into the vacuum pump inlet. Upon completion of
the
experiment after 7 h 45 mm, the total acid number (TAN) determination of the
product was performed. The total amount of cracked palm oil was 10.84 g and
the
total amount of reacted oil (free of methanol) was 8.7 g. The TAN number of
the feed
mixture was 120.0mg KOH/g and that of the product was 20.632mg KOH/g. Thus,
the free fatty acids conversion based on the TAN number was 82.8 %.
9
CA 02701136 2011-02-02
Example 2:
The esterification system consists of 1) a feed syringe pump, 2) a micro-
reactor
(10 mL), 3) a water-cooled condenser, 4) a room temperature trap, 5) a liquid-
nitrogen
trap, and 6) mechanical vacuum pump. The vacuum pump attached to the exit side
of
the system was intended to maintain the system pressure during esterification.
Thermally cracked palm oil and methanol were premixed at a weight ratio of 1:1
and
loaded in a 50 ml syringe pump. Calcined ZrO2/SO42- in an amount of 5.7g was
loaded
into the micro-reactor as the solid acid catalyst, between the layers of
compacted glass
wool. The catalyst occupied 8.7 mL of the reactor volume. The reactor was
heated to
near 250 C, the vacuum pump was turned on and feed was started at a feed rate
of
1000 L/min. The system pressure was maintained at 5 mmHg (0.1 psia) by
bleeding
a small stream of air into the vacuum pump inlet. Upon completion of the
experiment
after 50 min, the samples were collected, mass balance performed, and the
total acid
number (TAN) was determined. The total amount of input (feed) was 40.0 g, the
total
amount of output was 36.0 g including 0.8 g collected on the catalyst bed.
Thus, the
mass balance for this experiment was 90.0%. The TAN number of the feed was 113
mg KOH/g and that of the product was 2.219 mg KOH/g. Thus, the free fatty
acids
conversion based on the TAN number was 98.0%.
This detailed description of the process and methods is used to illustrate
certain
embodiments of the present invention. It will be apparent to those skilled in
the art
that various modifications can be made in the present process and methods and
that
various alternative embodiments can be utilized. Therefore, it will be
recognized that
various modifications can also be made to the applications to which the method
and
processes are applied without departing from the scope of the invention, which
is
limited only by the appended claims.