Note: Descriptions are shown in the official language in which they were submitted.
CA 02703464 2010-05-07
REMOVAL OF HYDROGEN SULFIDE FROM WATER
The present invention relates to methods and systems for removing hydrogen
sulfide from water.
BACKGROUND OF THE INVENTION
Water that contains significant amounts of hydrogen sulfide is commonly
referred
to as sour water. Sour water can occur from a number of different industrial
processes as
well as occurring naturally in some water sources, such as wells. Besides the
smell and
affect on the taste of the water, hydrogen sulfide can cause corrosion and can
have a
number of environmental and health effects.
For example, it would be desirable to be able to use well water in many
drilling
and fracturing operations in hydrocarbon formations. However, some well water
in
certain areas contains significant amounts of hydrogen sulfide, which leads to
corrosion
problems and the like.
There are a number of ways hydrogen sulfide can be removed from water, but
many of these have significant disadvantages. For example, triazine can be
added to
water to remove hydrogen sulfide from the water. However, the high consumption
of
triazine that is necessary is costly and the overall reaction is relatively
slow. Oxidants
can be also be used to remove hydrogen sulfide from water, however, oxidants
can form
a solid product and can cause problems with corrosion. Caustic products can be
used but
they can result in high solids production. Zinc salts and chelates can be
used. However,
WSLegal\049798\00270\ 6019111v1 1
CA 02703464 2010-05-07
these have the disadvantages of producing solids and the process is
reversible. Iron
chelates can also be used, but they have the same disadvantages as zinc salts
and chelates.
Thus, there is a need for an efficient and less costly method and system for
removing hydrogen sulfide from water.
SUMMARY OF THE INVENTION
In a first aspect, a method of removing hydrogen sulfide from sour water is
provided, comprising providing water containing a first amount of hydrogen
sulfide and
having a first pH; adding a stripper gas to the water to form a mixture;
agitating the
mixture; and separating a gaseous portion from the mixture to form a water
product, the
water product containing a second amount of hydrogen sulfide that is less than
the first
amount of hydrogen sulfide.
In a second aspect, a system for removing hydrogen sulfide from sour water is
provided comprising a feed inlet for supplying water containing a first amount
of hydrogen
sulfide and having a first pH; a stripper gas feed inlet for supplying
stripper gas to the
water; at least one mixer for mixing the water and the stripper gas; and at
least one
separator downstream from the at least one mixer for separating a gaseous
portion from the
mixture to produce water having a second amount of hydrogen sulfide that is
less than the
first amount of hydrogen sulfide.
Thus, in the present application a system and method for removing hydrogen
sulfide from sour water is provided. Sour water containing hydrogen sulfide is
combined
WSLegal\049798\00270\ 6019111v1 2
CA 02703464 2010-05-07
with a stripping gas, for example, methane, carbon dioxide, or a combination
of the two. In
one embodiment, the pH of the sour water is lowered by the addition of an
acid, for
example, hydrochloric acid. The sour water is then passed through at least one
mixer/separator combination, and, optionally, a series of two or more
mixers/separators.
When the sour water is vigorously mixed with the stripping gas in the mixer,
the hydrogen
sulfide separates from the water phase into the gaseous phase. Thus, when the
mixed water
is subsequently passed to an associated separator, the gas, which now includes
a substantial
amount of hydrogen sulfide gas, can be separated from the water.
After the gas is removed in the separator, the remaining water can be removed
from the separator and, optionally, more stripping gas can be added to this
water and then
the water and stripping gas passed through the next mixer/separator in the
series. The gas
removed by each separator can be used for producing a final gas product, while
the
remaining water from each separator can be routed to the next mixer and
separator in the
series, if necessary. The water remaining in the last separator forms a water
product with
much, if not all, of the hydrogen sulfide having been removed.
BRIEF DESCRIPTION OF THE DRAWINGS
Referring to the drawings wherein like reference numerals indicate similar
parts
throughout the several views, several aspects of the present invention are
illustrated by
way of example, and not by way of limitation, in detail in the figures,
wherein:
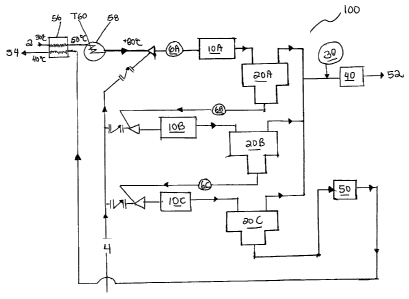
Fig. 1 is a process diagram of a system for removing hydrogen sulfide from
water.
WSLegal\049798\00270\ 6019111v1 3
CA 02703464 2010-05-07
DESCRIPTION OF VARIOUS EMBODIMENTS
The detailed description set forth below in connection with the appended
drawing
is intended as a description of various embodiments of the present invention
and is not
intended to represent the only embodiments contemplated by the inventor. The
detailed
description includes specific details for the purpose of providing a
comprehensive
understanding of the present invention. However, it will be apparent to those
skilled in
the art that the present invention may be practiced without these specific
details.
Fig. 1 is a schematic illustration of a system 100 for removing hydrogen
sulfide
(H2S) from water. The system 100 includes a feed inlet 2, a stripper gas feed
inlet 4, a
number of pH adjustment modules 6A, 6B, 6C for adding acid to the feed, a
number of
mixers 10A, IOB, 10C, a number of separators 20A, 20B, 20C, a triazine
injection point
30, a gas compressor 40, a water polisher 50, a water product outlet 54 and a
gas product
outlet 52.
Sour water (water containing a first amount of hydrogen sulfide) is introduced
into the system 100 through the feed inlet 2. The sour water can be from an
industrial
process such as a hydrocarbon upgrading process, natural source such as a well
or any
other suitable source of hydrogen sulfide containing water.
A stripper gas can be provided via stripper gas feed inlet 4 for supplying a
stripper
gas that will be mixed with the sour water. The stripper gas can be methane,
CO2, a
mixture of methane and C02, steam, nitrogen, a mixture of any of these gases,
etc.
WSLegal\049798\00270\ 60191110 4
CA 02703464 2010-05-07
A pH adjustment module 6A can also be provided to allow for the injection of
an
acid, such as hydrochloric acid, C02/carbonic acid, organic acids, such as
formic acid,
acetic acid, etc., into the sour water entering the system 100 through the
feed inlet 2 and
the stripper gas entering the system 100 through the stripper gas feed inlet
4. The pH
adjustment module 6A and the acid injected into the sour water and stripper
gas can be
used to lower the pH of the sour water from a first pH to a desired lower
second pH.
At neutral (-7) to alkaline pH, hydrogen sulfide in water tends to dissolve
and
convert into hydrosulfide ions (HS-) and/or sulfide ions (S-). However, in
water having a
more acidic pH (< 7) the hydrosulfide ions tend to convert to hydrogen sulfide
gas (H2S).
By using the pH adjustment module 6A to reduce the pH level of the sour water,
some of
the hydrosulfide ions present in the sour water will convert into the hydrogen
sulfide gas
(H2S), which H2S gas will then separate from the water phase into the gaseous
phase.
In one embodiment, the pH adjustment module 6A can reduce the pH of the
mixture of sour water and stripper gas to a pH in the range of 5 to 7.
The sour water from the feed inlet 2 may first pass through heat exchanger 56
where it can be preheated to about 50 C. The preheated sour water can be
further heated
in heater 58 to about 80 C, if desired. Stripping gas from the stripper gas
feed inlet 4 can
be fed to the heated (or non-heated, if heaters/heat exchangers are not used)
and the sour
water/stripping gas mixture can then be fed through a number of stages, where
each stage
has a mixer I OA, I OB, IOC and a corresponding separator 20A, 20B, 20C.
Each mixer 10A, 10B, 1OC can be any type of mixer suitable for sufficiently
agitating the mixture of water and stripping gas, however, in one aspect, the
mixers I OA,
WS Legal\04979 8\00270\ 6019111v1 5
CA 02703464 2010-05-07
IOB, IOC are static mixers. Static mixers are used for mixing two fluids and
typically
contain mixer elements or baffles that agitate the two fluids as they are
passed through
the static mixer. An example of a static mixer that can be used is SulzerTM
Mixer SMV,
but other static mixers known in the art can also be used.
The mixture of sour water and stripper gas from the feed inlet 2 and the gas
stripper feed inlet 4 can be routed through the first mixer 1 OA after the pH
of the mixture
has been adjusted by the pH adjustment module 6A. The first mixer 1OA can mix
the
sour water and the stripping gas, dispersing the stripping gas in the sour
water.
Additionally, the agitation of the mixture can cause the dissolved hydrogen
sulfide (H2S)
to move into the gas phase. The agitation of the sour gas and stripping gas
can cause the
hydrogen sulfide (H2S) to break out of solution and transfer from the liquid
phase into the
gas phase of the stripping gas.
A first separator 20A can be paired with the first mixer 1 OA so that the
water and
hydrogen sulfide-containing gas can be routed to the first separator 20A after
formation
in the first mixer 1 OA. The first separator 20A can separate a gas portion
from the water,
with the gas portion containing stripping gas and gaseous hydrogen sulfide.
This gas
portion can be routed away to form the resulting gas product 52.
The water from the first separator 20A, while containing less hydrogen sulfide
than the sour water being input into the system 100, can still contain some
hydrogen
sulfide dissolved in the water. This remaining water can be routed to a
subsequent stage
with a second mixer 10B and a second separator 20B to remove some of the
hydrogen
sulfide remaining in the water.
WSLegal\049798\00270\ 6019111v1 6
CA 02703464 2010-05-07
In one aspect, a pH adjustment module 6B can be used to lower the pH of the
water before it is routed to the second mixer l OB.
The second mixer l OB can be used to mix water from the first separator 20A
with
stripper gas from the stripper gas feed inlet 4 and then the mixture can be
routed to the
second separator 20B to separate another gas portion from the water.
Additional stripper
gas can be added to the water before it passes through the second mixer 20B to
replenish
the stripper gas that was removed from the water in the first separator 20A.
The
additional gas portion separated from the water in the second separator 20B
can be routed
away to form the resulting gas product 52. This additional gas portion can
comprise
stripper gas removed from the water and gaseous hydrogen sulfide.
The water from the second separator can be routed through a last stage
containing
a third mixer l OC and a third separator 20C. Additional stripper gas from the
stripper gas
feed inlet 4 can be added to the water from the second separator 20B before
the mixture
of water and stripper gas is passed through the third mixer 1OC to be agitated
before
being fed to the third separator 20C. A gas portion can be removed from the
water in the
third separator 20C and routed to form the resulting gas product 52. This gas
portion can
comprise stripper gas and hydrogen sulfide.
In one aspect, a pH adjustment module 6C can be used to lower the pH of the
water before it is passed to the third mixer l OC.
From the third separator 20C, remaining water can be used to form the final
water
product containing significantly reduced levels of hydrogen sulfide (sweet
water) in
relation to the sour water that was input into the system 100.
WSLegal\049798\00270\ 6019111v1 7
CA 02703464 2010-05-07
Optionally, a water polisher 50, such as a water polisher that uses a chemical
treatment, can be provided to improve the quality of the final water product
54. This may
be useful where the water product 54 is intended to be used as drinking water.
For
example, this chemical treatment can be acrolein, chlorine dioxide, chlorine,
triazine, iron
oxides (solid) or other suitable chemicals for treating the water.
Although Fig. 1 illustrates three stages of agitating and separating, where
each
stage has a mixer I OA, I OB, I OC and a corresponding separator 20A, 20B,
20C, more or
fewer stages using mixers and separators could be used depending on the level
of
hydrogen sulfide in the sour water and/or the level of hydrogen sulfide that
is acceptable
in the final water product for a given purpose.
The gas portions obtained from the three separators 20A, 20B, 20C can be
combined to form a gas product containing stripping gas removed from the water
and
gaseous hydrogen sulfide. This gas product can then be recycled, collected for
storage,
transported to another location in a gas line, etc. Optionally, the gas
product obtained
from the separators 20A, 20B, 20C can be "sweetened", i.e., the hydrogen
sulfide
removed, by further treatment with a chemical sweetener such as triazine,
e.g., SCRUB-
ITTM. Other methods for sweetening sour gas can also be used; see, for
example, U.S.
Patent No. 4,978,512, incorporated herein by reference.
The triazine injection point 30 can be used to inject triazine into the gas
product to
remove some of the hydrogen sulfide and then, optionally, passing the treated
gas
through a compressor 40 to form the final gas product 52. The final gas
product 52 can
WSLegal\049798\00270\ 6019111v1 8
CA 02703464 2010-05-07
be pipelined for further use. It is also understood that that the sour gas can
be treated in
other in-line injection systems, H2S scrubber systems or chemical solvent
systems.
In operation, system 100 can be used to remove hydrogen sulfide from water.
Sour water can be input into the system at the feed inlet 2 where it can be
combined with
a stripper gas from the stripper gas feed inlet 4. The mixture of sour water
and stripper
gas can then have the pH adjusted, such as by the addition of an acid, to
reduce the pH
and decrease the amount of the hydrogen sulfide that is the form of hydrogen
sulfide ions
in the sour water.
The pH reduced mixture of sour water and stripping gas can be passed through a
series of agitating and separating stages, where each stage agitates the
mixture of the
stripper gas and the water and then separates a gas portion from the water. In
each stage,
the mixture of water and stripping gas is passed through one of the mixers I
OA, I OB, I OC
to agitate the mixture before it is passed to the corresponding separator 20A,
20B, 20C,
where a gas portion is removed from the water. The water removed from the
separator
20A or 20B is then mixed with more stripping gas and passed through the
subsequent
mixer IOB or 1OC, before it is passed to the corresponding separator 20B or
20C and an
additional gas portion is separated from the water. When the water reaches the
last
separator 20C, the water remaining after another gas portion has been
separated off, can
form the final water product 54 which has a second amount of hydrogen sulfide
which is
less than the first amount of hydrogen sulfide in the sour water that was
input into the
system 100. In some cases, the water product 54 can contain little or no
hydrogen sulfide
in it (e.g., the water product 54 can be sweet water).
WSLegal\049798\00270\ 6019111v1 9
CA 02703464 2010-05-07
The water from the last separator 20C can also be further treated, such as by
using
a water polisher 50, to further increase the quality of the final water
product 52.
The gas product obtained from the various separators 20A, 20B, 20C will
typically contain stripping gas and gaseous hydrogen sulfide that has been
removed from
the water. These gas products can be combined to form a final gas product,
however, in
one aspect, the gas products collected from the separators 20A, 20B, 20C can
be further
treated to remove some or all of the hydrogen sulfide, such as by injecting
triazine into
the gas products, and thus improve the quality of the final gas product 52.
The gas
portions can also be passed through a gas compressor 40, such as when storage
and/or
transport of the gas products is desired without further treatment.
Example 1
Samples of inlet water were subjected to four (4) separate stripping steps
using
methane as the stripping gas under varying conditions, namely, (i) 30 C (base
case); (ii)
30 C and addition of 40m3/d HCI; (iii) 60 C and addition of 40m3/d HCI; (iv)
80 C and
addition of 40m3/d HCI; and (v) 30 C and increasing stripping gas to 10
million standard
cubic feet per day (MMSCFD) per stage. The amount of H2S in both the water
phases
and gas phases was determined immediately prior to entering the first stripper
and then
after each of the four stripping steps. The amount of H2S is given in
kgmol/hr, as the unit
is being operated on a continuous basis. Table 1 gives the results of the
above five
different conditions considered.
WSLegal\049798\00270\ 6019111v1 10
CA 02703464 2010-05-07
Table 1
Methane Stripping Gas
30 C 30 C Temperature Temperature 30 C, Increase
Base Add to 60 C with to 80 C with Stripping Gas to
Case 40m3/d HCL HCL 10 MMSCFD
HCL per Stage
Inlet Sep Water H2S kgmol/hr 1.969 1.293 0.837 0.505 1.293
15` Stripper Water H2S kgmol/hr 0.334 0.216 0.077 0.029 0.120
2"d Stripper Water H2S kgmol/hr 0.058 0.038 0.008 0.002 0.012
3`d Stripper Water H2S kgmol/hr 0.011 0.007 0.001 0.000 0.001
4`h Stripper Water H2S kgmol/hr 0.002 0.001 0.000 0.000 0.000
Inlet Sep Gas Phase H2S kgmol/hr 0.531 1.207 1.663 1.995 1.207
1st Stripper Gas Phase H2S kgmol/hr 1.635 1.077 0.760 0.477 1.172
2d Stripper Gas Phase H2S kgmol/hr 0.276 0.178 0.069 0.027 0.108
3`d Stripper Gas Phase H2S kgmol/hr 0.048 0.031 0.007 0.002 0.011
4`h Stripper Gas Phase H2S kgmol/hr 0.008 0.006 0.001 0.000 0.001
HCL Rate m3/d 0 40 40 40 40
Water Rate m3/d 15988 15899 15899 15899 15899
Stripping Temp C 30 30 60 79 30
151 Stripper Gas Rate MMSCFD 5 5 5 5 10
2d Stripper Gas Rate MMSCFD 5 5 5 5 10
3`d Stripper Gas Rate MMSCFD 5 5 5 5 10
4th Stripper Gas Rate MMSCFD 5 5 5 5 10
Inlet Sep Pressure kPa(a) 96.5 96.5 96.5 96.5 96.5
155 Stripper Pressure kPa(a) 96.5 96.5 96.5 96.5 96.5
2"d Stripper Pressure kPa(a) 96.5 96.5 96.5 96.5 96.5
3`d Stripper Pressure kPa(a) 96.5 96.5 96.5 96.5 96.5
4th Stripper Pressure kPa(a) 96.5 96.5 96.5 96.5 96.5
It can be seen from the results presented in Table 1 that, even in the base
case
where approximately 79% of the total H2S of the inlet feed is in the water
phase, a
significant amount of H2S is stripped from the inlet water by using methane as
the
stripping gas and at least one stripper (i.e., after the first stripper, the
first stripper water
only contains approximately 13% of the original H2S present in the inlet feed
(water/gas
phases). Further, it can be seen that, as the temperature of the inlet water
increases and
the pH decreases with the addition of HCI, less of the total H2S of the inlet
feed is
WSLegal\049798\00270\ 6019111v1 11
CA 02703464 2010-05-07
initially present in the inlet water and therefore an even more significant
amount of H2S
is removed from the inlet water in the first stripping step.
For example, when 40 m3/d HCl is added to the inlet feed and the inlet feed is
then subjected to the first stripping stage, the first stripper water now only
contains
approximately 8.6% of the original H2S present in the feed. When 40 m3/d of
HCl is
added to the inlet feed and the temperature is raised to 80 C, the amount of
H2S present
in the water after the first stripper is reduced to approximately I%. Thus, by
using higher
temperatures and lower pH, essentially all of the H2S can be stripped from the
water after
only two stripper stages.
Finally, it can be seen from Table 1 that increasing the amount of stripping
gas
used from 5MMSCFD to 10 MMSCFD resulted in the first stripper water containing
less
than 5% of the original H2S present in the inlet feed. This is a significant
improvement
from the base case where the first stripper water still contained
approximately 13% of the
original H2S present in the feed.
The previous description of the disclosed embodiments is provided to enable
any
person skilled in the art to make or use the present invention. Various
modifications to
those embodiments will be readily apparent to those skilled in the art, and
the generic
principles defined herein may be applied to other embodiments without
departing from
the spirit or scope of the invention. Thus, the present invention is not
intended to be
limited to the embodiments shown herein, but is to be accorded the full scope
consistent
with the claims, wherein reference to an element in the singular, such as by
use of the
article "a" or "an" is not intended to mean "one and only one" unless
specifically so
WSLegal\049798\00270\ 6019111v1 12
CA 02703464 2010-05-07
stated, but rather "one or more". All structural and functional equivalents to
the elements
of the various embodiments described throughout the disclosure that are known
or later
come to be known to those of ordinary skill in the art are intended to be
encompassed by
the elements of the claims. Moreover, nothing disclosed herein is intended to
be
dedicated to the public regardless of whether such disclosure is explicitly
recited in the
claims.
WSLegal\049798\00270\ 6019111v1 13