Note: Descriptions are shown in the official language in which they were submitted.
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
ENANTIOMERICALLY ENRICHED IMIDAZOAZEPINONE COMPOUNDS
BACKGROUND OF THE INVENTION
[0001] Upon encountering antigen, naive CD4+ T helper precursor (Thp) cells
are
differentiated into two distinct subsets, Type 1 T helper (Th 1) and Type 2 T
helper (Th2).
These differentiated Th cells are defined both by their distinct functional
abilities and by
unique cytokine profiles. Specifically, Thl cells produce interferon-gamma,
interleukin (IL)-
2, and tumor necrosis factor (TNF)-beta, which activate macrophages and are
responsible for
cell-mediated immunity and phagocyte-dependent protective responses. In
contrast, Th2
cells are known to produce IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13, which are
responsible for
strong antibody production, eosinophil activation, and inhibition of several
macrophage
functions, thus providing phagocyte-independent protective responses.
Accordingly, Thl
and Th2 cells are associated with different immunopathological responses.
[00021 In addition, the development of each type of Th cell is mediated by a
different
cytokine pathway. Specifically, it has been shown that IL-4 promotes Th2
differentiation
and simultaneously blocks Thl development. In contrast, IL-12, IL-18 and IFN-
gamma are
the cytokines critical for the development of Thl cells. Accordingly, the
cytokines
themselves form a positive and negative feedback system that drives Th
polarization and
keeps a balance between Thl and Th2.
10003] Thl cells are involved in the pathogenesis of a variety of organ-
specific
autoimmune disorders, Crohn's disease, Helicobacter pylori-induced peptic
ulcer, acute
kidney allograft rejection, and unexplained recurrent abortions. In contrast,
allergen-specific
Th2 responses are responsible for atopic disorders in genetically susceptible
individuals.
Moreover, Th2 responses against still unknown antigens predominate in Omenn's
syndrome,
idiopathic pulmonary fibrosis, and progressive systemic sclerosis:
[0004] There remains a high unmet medical need to develop new therapeutic
treatments
that are useful in treating the various conditions associated with imbalanced
Thl/Th2 cellular
differentiation. For many of these conditions the currently available
treatment options are
inadequate. Accordingly, the Thl/Th2 paradigm provides a rationale for the
development of
strategies for the therapy of allergic and autoimmune disorders.
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
SUMMARY OF THE INVENTION
[0005] A first aspect of the present invention is an enantiomerically pure
compound
(sometimes referred to as an "active compound" herein) of Formula I:
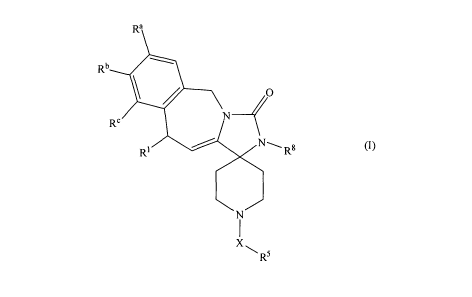
Ra
Rb O
Re N
RI NLRB
~I) N
X~
RS
or more particularly Formula la or Formula Ib:
Ra Ra
Rb Rb
o
Rc N Rc N
Rl\\N.Rs R1 NLRB
(Ia) N O b)
N
X\ s
Rs
[0006] wherein:
[0007] R' is C1_3 alkyl;
[0008] X is methylene, ethylene, propylene, ethenylene, propenylene, or
butenylene;
[0009] R5 is phenyl, pyrrolyl, benzimidazolyl, oxazolyl, isoxazolyl,
imidazothiazolyl,
quinolinyl, isoquinolinyl, indazolyl, pyridinyl, imidazopyridinyl, indolyl,
benzotriazolyl,
imidazolyl, benzofuranyl, benzothiadiazolyl, pyridimidinyl, benzopyranonyl,
thiazolyl,
2
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
thiadiazolyl, furyl, thienyl, pyrazolyl, quinoxalinyl, or naphthyl, and
substituted with
between 0 and 5 substituents independently selected from CI-a alkyl, C1_3
alkoxy, hydroxyl,
CI-3 alkylthio, cyclopropyl, cyclopropylmethyl, trifluoromethoxy, 5-
methylisoxazolyl,
pyrazolyl, benzyloxy, acetyl, (cyanyl)CI.3 alkyl, (phenyl)C2.3 alkenyl; and
halo;
100101 R8 is H, methyl, ethyl, propyl, (CI-3 alkoxy)C1.3 alkyl, (CI-3
alkylthio)CI.3 alkyl,
C1_3 hydroxyalkyl, phenyl, benzyl, furyl, pyrrolyl, imidazolyI, pyrazolyl,
pyrrolyl,
isothiazolyl, isooxazolyl, pyridyl, and thienyl;
[0011] wherein R8 is substituted with between 0 and 3 substituents
independently
selected from methyl, ethyl, halo, hydroxyl, C1.3 alkoxy, C1_3 alkylthio,
(C1.3 alkoxy)C1_3
alkyl, (C1.3 alkylthio)C1.3 alkyl, C1_3 hydroxyalkyl, (C1.3
mercaptoalkyl)phenyl, benzyl, furyl,
imidazolyl, pyrazolyl, pyrrolyl, isothiazolyl, isooxazolyl, pyridyl, and
thienyl; and
[00121 each of Ra, Rb, and R` is independently selected from hydrogen,
hydroxyl,
methoxy, benzyloxy, fluoro, chloro, amino, methylamino, dimethylamino, and
phenoxy;
[0013] or one pair selected from Ra and Rb, and Rb and R', taken together, is -
O-(CH2)-
0- or -O-CH2-CH2-O-;
[0014) or a pharmaceutically acceptable salt, a C1.6 alkyl ester or amide, or
a C2.6 alkenyl
ester or amide thereof.
[00151 A second aspect of the present invention is a composition comprising an
active
compound as described herein in a pharmaceutically acceptable carrier.
[0016] A third aspect of the present invention is a method of treating
rheumatoid arthritis
in a subject in need thereof, comprising administering to the subject an
active compound as
described herein in a treatment effective amount, along with the use of an
active compound as
described herein for the manufacture of a medicament for treating rheumatoid
arthritis in a
subject in need thereof.
[0017] A fourth aspect of the present invention is a method of treating
multiple sclerosis
in a subject in need thereof, comprising administering to said subject. an
active compound as
described herein in a treatment effective amount, along with the use of an
active compound as
described herein for the manufacture of a medicament for treating multiple
sclerosis in a
subject in need thereof.
[00181 A fifth aspect of the invention is a method of treating an autoimmune
disease in a
subject in need thereof, comprising administering to the subject an active
compound as
described herein in a treatment effective amount, along with the use of an
active compound as
described herein for the manufacture of a medicament for treating an
autoimmune disease in
a subject in need thereof, wherein the autoimmune disease is selected from the
group
3
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
consisting of systemic lupus erythematosus, type I diabetes mellitus,
psoriasis, and
atherosclerosis.
[0019] Other aspects of the present invention are disclosed herein and
discussed in
greater detail below.
DETAILED DESCRIPTION OF CERTAIN EMBODIMENTS OF THE INVENTION
A. Definitions
[0020] "Enantiomerically pure" as used herein means a stereomerically pure
compound,
or composition of a compound, the compound having one chiral center.
[0021] "Stereomerically pure" as used herein means a compound or composition
thereof
that comprises one stereoisomer of a compound and is substantially free of
other
stereoisomers of that compound. For example, a stereomerically pure
composition of a
compound having one chiral center will be substantially free of the opposite
enantiomer of
the compound. A stereomerically pure composition of a compound having two
chiral centers
will be substantially free of other diastereomers of the compound. A typical
stereomerically
pure compound comprises greater than about 80% by weight of one stereoisomer
of the,
compound and less than about 20% by weight of other stereoisomers of the
compound, more
preferably greater than about 90% by weight of one stereoisomer of the
compound and less
than about 10% by weight of the other stereoisomers of the compound, even more
preferably
greater than about 95% by weight of one stereoisomer of the compound and less
than about
5% by weight of the other stereoisomers of the compound, and most preferably
greater than
about 97% by weight of one stereoisomer of the compound and less than about 3%
by weight
of the other stereoisomers of the compound. See, e.g., US Patent No.
7,189,715.
[0022] "Stable", as used herein, refers to compounds that are not
substantially altered
when subjected to conditions to allow for their production, detection, and
preferably their
recovery, purification, and use for one or more of the purposes disclosed
herein. In some
embodiments, a stable compound or chemically feasible compound is one that is
not
substantially altered when kept at a temperature of 40 C or less, in the
absence of moisture or
other chemically reactive conditions, for at least a week.
[0023] "Alkyl" or "alkyl group," as used herein, means a straight-chain (i.e.,
unbranched), branched, or cyclic hydrocarbon chain that is completely
saturated. In certain
embodiments, alkyl groups contain 1-3 carbon atoms. In still other
embodiments, alkyl
groups contain 2-3 carbon atoms, and in yet other embodiments alkyl groups
contain 1-2
4
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
carbon atoms. In certain embodiments, the term "alkyl" or "alkyl group" refers
to a
cycloalkyl group, also known as carbocycle. Exemplary C1.3 alkyl groups
include methyl,
ethyl, propyl, isopropyl, and cyclopropyl.
[0024] "Alkenyl" or "alkenyl group," as used herein, refers to a straight-
chain (i.e.,
unbranched), branched, or cyclic hydrocarbon chain that has one or more double
bonds. In
certain embodiments, alkenyl groups contain 2-4 carbon atoms. In still other
embodiments,
alkenyl groups contain 3-4 carbon atoms, and in yet other embodiments alkenyl
groups
contain 2-3 carbon atoms. According to another aspect, the term alkenyl refers
to a straight
chain hydrocarbon having two double bonds, also referred to as "diene." In
other
embodiments, the term "alkenyl" or "alkenyl group" refers to a cycloalkenyl
group.
Exemplary C2_4 alkenyl groups include -CH=CH2, -CH2CH=CH2 (also referred to as
allyl), -
CH=CHCH3, -CH2CH2CH=CH2, -CH2CH=CHCH3, -CH=CH2CH2CH3, -CH=CH2CH=CH2,
and cyclobutenyl.
[0025] "Alkoxy", or "alkylthio", as used herein, refers to an alkyl group, as
previously
defined, attached to the principal carbon chain through an oxygen ("alkoxy")
or sulfur
("alkylthio") atom.
[0026] "Methylene", "ethylene", and "propylene" as used herein refer to the
bivalent
moieties -CH2-, -CH2CH2-, and -CH2CH2CH2-, respectively.
[0027] "Ethenylene", "propenylene", and "butenylene" as used herein refer to
the
bivalent moieties -CH=CH-, -CH=CHCH2-, -CH2CH=CH-, -CH=CHCH2CH2-,
-CH2CH=CH2CH2-, and -CH2CH2CH=CH-, where each ethenylene, propenylene, and
butenylene group can be in the cis or trans configuration. In certain
embodiments, an
ethenylene, propenylene, or butenylene group can be in the trans
configuration.
[0028] "Alkylidene" refers to a bivalent hydrocarbon group formed by mono or
dialkyl
substitution of methylene. In certain embodiments, an alkylidene group has 1-6
carbon
atoms. In other embodiments, an alkylidene group has 2-6, 1-5, 2-4, or 1-3
carbon atoms.
Such groups include propylidene (CH3CH2CH=), ethylidene (CH3CH=), and
isopropylidene
(CH3(CH3)CH=), and the like.
[0029] "Alkenylidene" refers to a bivalent hydrocarbon group having one or
more double
bonds formed by mono or dialkenyl substitution of methylene. In certain
embodiments, an
alkenylidene group has 2-6 carbon atoms. In other embodiments, an alkenylidene
group has
2-6, 2-5, 2-4, or 2-3 carbon atoms. According to one aspect, an alkenylidene
has two double
bonds. Exemplary alkenylidene groups include CH3CH=C=, CH2=CHCH=,
CH2=CHCH2CH=, and CH2=CHCH2CH=CHCH=.
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
[0030] "C1.6 alkyl ester or amide" refers to a C1-6 alkyl ester or a C1.6
alkyl amide where
each C1.6 alkyl group is as defined above. Such C1_6 alkyl ester groups are of
the formula (C1.
6 alkyl)OC(=0)- or (C1.6 alkyl)C(=0)O-. Such C1.6 alkyl amide groups are of
the formula
(C1_6 alkyl)NHC(=O)- or (C14 alkyl)C(=O)NH-.
[0031] "C2_6 alkenyl ester or amide" refers to a C2_6 alkenyl ester or a C2_6
alkenyl amide
where each C2.6 alkenyl group is as defined above. Such C2_6 alkenyl ester
groups are of the
formula (C24 alkenyl)OC(=O)- or (C2.6 alkenyl)C(=0)O-. Such C2-6 alkenyl amide
groups
are of the formula (C2.6 alkenyl)NHC(=O)- or (C24 alkenyl)C(=O)NH-.
[0032] "Treatment," "treat," and "treating" refer to reversing, alleviating,
delaying the
onset of, inhibiting the progress of, or preventing a disease or disorder as
described herein. In
some embodiments, treatment may be administered after one or more symptoms
have
developed. In other embodiments, treatment may be administered in the absence
of
symptoms. For example, treatment may be administered to a susceptible
individual prior to
the onset of symptoms (e.g., in light of a history of symptoms and/or in light
of genetic or
other susceptibility factors). Treatment may also be continued after symptoms
have resolved,
for example to prevent or delay their recurrence.
[0033] "Patient" or "subject", as used herein, means an animal subject,
preferably a
mammalian subject (e.g., dog, cat, horse, cow, sheep, goat, monkey, etc.), and
particularly
human subjects (including both male and female subjects, and including
neonatal, infant,
juvenile, adolescent, adult and geriatric subjects).
[0034] "Pharmaceutically acceptable carrier" as used herein refers to a
nontoxic carrier,
adjuvant, or vehicle that does not destroy the pharmacological activity of the
compound with
which it is formulated. Pharmaceutically acceptable carriers, adjuvants or
vehicles that may
be used in the compositions of this invention include, but are not limited to,
ion exchangers,
alumina, aluminum stearate, lecithin, serum proteins, such as human serum
albumin, buffer
substances such as phosphates, glycine, sorbic acid, potassium sorbate,
partial glyceride`
mixtures of saturated vegetable fatty acids, water, salts or electrolytes,
such as protamine
sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium
chloride, zinc
salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone,
cellulose-based
substances, polyethylene glycol, cyclodextrins, sodium carboxymethylcellulose,
polyacrylates, waxes, polyethylene-polyoxypropylene-block polymers,
polyethylene glycol
and wool fat.
[0035] Unless indicated otherwise, nomenclature used to describe chemical
groups or
moieties as used herein follow the convention where, reading the,name from
left to right, the
6
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
point of attachment to the rest. of the molecule is at the right-hand side of
the name. For
example, the group "(C1_3 alkoxy)C1.3 alkyl," is attached to the rest of the
molecule at the
alkyl end. Further examples include methoxyethyl, where the point of
attachment is at the
ethyl end, and methylamino, where the point of attachment is at the amine end.
[0036] Unless indicated otherwise, where a bivalent group is described by its
chemical
formula, including two terminal bond moieties indicated by "-," it will be
understood that the
attachment is read from left to right.
[0037] Unless otherwise stated, structures depicted herein are also meant to
include all
enantiomeric, diastereomeric, and geometric (or conformational)) forms of the
structure; for
example, the R and S configurations for each asymmetric center, (Z) and (E)
double bond
isomers, and (Z) and (E) conformational isomers. Therefore, single
stereochemical isomers
as well as enantiomeric, diastereomeric, and geometric (or conformational)
mixtures of the
present compounds are within the scope of the invention. Unless otherwise
stated, all
tautomeric forms of the compounds of the invention are within the scope of the
invention.
Additionally, unless otherwise stated, structures depicted herein are also
meant to include
compounds that differ only in the presence of one or more isotopically
enriched atoms. For,
example, compounds having the present structures except for the replacement of
hydrogen by
deuterium or tritium, or the replacement of a carbon by a 13C- or 14C-enriched
carbon are
within the scope of this invention. Such compounds are useful, for example, as
analytical
tools or probes in biological assays.
B. Active compounds.
[0038] As described herein, active compounds of the invention may. optionally
be
substituted with one or more substituents, such as are illustrated generally
above, or as
exemplified by particular classes, subclasses, and species of the invention.
In general, the
term "substituted" refers to the replacement of hydrogen radicals in a given
structure with the
radical of a specified substituent. Unless otherwise indicated, a substituted
group may have a
substituent at each substitutable position of the group, and when more than
one position in
any given structure may be substituted with more than one substituent selected
from a
specified group, the substituent may be either the same or different at every
position.
[0039] As noted above, the present invention provides enantiomerically pure
compounds,
or active compounds, of Formula I:
7
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Ra
R b / O
Re N
R' NRE
(~) N
R5
or more particularly Formula la or Formula Ib:
Ra
Ra
Rb Rb
R N Re N
N----RE R' N\R8
(Ia) N (Ib) N
Xi
SRS Rs
[0040] wherein:
R' is C1.3 alkyl;
X is methylene, ethylene, propylene, ethenylene, propenylene, or butenylene;
R5 is phenyl, pyrrolyl, benzimidazolyl, oxazolyl, isoxazolyl,
imidazothiazolyl,
quinolinyl, isoquinolinyl, indazolyl, pyridinyl, imidazopyridinyl, indolyl,
benzotriazolyl, imidazolyl, benzofuranyl, benzothiadiazolyl, pyridimidinyl,
benzopyranonyl, thiazolyl, thiadiazoly], furyl, thienyl, pyrazolyl,
quinoxalinyl, or
naphthyl, and substituted with between 0 and 5 substituents independently
selected
from C alkyl, C1.3 alkoxy, hydroxyl, C1.3 alkylthio, cyclopropyl,
cyclopropylmethyl,
trifluoromethoxy, 5-methylisoxazolyl, pyrazolyl, benzyloxy, acetyl,
(cyanyl)C1.3
alkyl, (phenyl)C2.3 alkenyl; and halo;
8
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
R8 is H, methyl, ethyl, propyl, (CI-3 alkoxy)C1_3 alkyl, (C1_3 alkylthio)C1.3
alkyl, C1.3 hydroxyalkyl, phenyl, benzyl, furyl, pyrrolyl, imidazolyl,
pyrazolyl,
pyrrolyl, isothiazolyl, isooxazolyl, pyridyl, and thienyl;
wherein R8 is substituted with between 0 and 3 substituents independently
selected from methyl, ethyl, halo, hydroxyl, C1_3 alkoxy, C1_3 alkylthio,
(C1_3
alkoxy)C1_3 alkyl, (C1_3 alkylthio)C1.3 alkyl, CI-3 hydroxyalkyl, (C1.3
mercaptoalkyl)phenyl, benzyl, furyl, imidazolyl, pyrazolyl, pyrrolyl,
isothiazolyl,
isooxazolyl, pyridyl, and thienyl; and
each of Ra, Rb, and R is independently selected from hydrogen, hydroxyl,
methoxy, benzyloxy, fluoro, chloro, amino, methylamino, dimethylamino, and
phenoxy;
or one pair selected from Ra and Rb, and Rb and R`, taken together, is -0-
(CH2)-O- or -O-CH2-CH2-O-;
or a pharmaceutically acceptable salt, a C1_6 alkyl ester or amide, or a C2-6
alkenyl ester or
amide thereof.
[00411 In some embodiments of the foregoing:
R' is C1_2 alkyl;
R5 is phenyl, pyrrolyl, benzimidazolyl, oxazolyl, isoxazolyl,
imidazothiazoly],
quinolinyl, isoquinolinyl, indazolyl, pyridinyl, imidazopyridinyl, indolyl,
benzotriazolyl, imidazolyl, benzofuranyl, benzothiadiazolyl, pyridimidinyl,
benzopyranonyl, thiazolyl, thiadiazolyl, furyl, thienyl, pyrazolyl,
quinoxalinyl, or
naphthyl, and substituted with between 0 and 5 substituents independently
selected
from C1.4 alkyl, C1.3 alkoxy, hydroxyl, C1_3 alkylthio, cyclopropyl,
cyclopropylmethyl,
trifluoromethoxy, 5-methylisoxazolyl, pyrazolyl, benzyloxy, acetyl,
(cyanyl)C1_3
alkyl, (phenyl)C2_3 alkenyl; and halo;
R8 is, methyl, ethyl, or propyl, wherein R8 is substituted with from 0 and 3
hydroxyl substituents;
X is methylene or ethylene; and
Ra Rb and R` are each independently selected from the group consisting of H
and methoxy.
or a pharmaceutically acceptable salt, a C1_6 alkyl ester or amide, or a C2_6
alkenyl ester or
amide thereof.
[0042] In some embodiments of the foregoing:
R' is methyl;
9
CA 02704454 2010-04-30
WO 2009/064274 PCT[US2007/024011
R5 is phenyl, pyrrolyl or pyrazolyl, each of which is substituted 0, 1 or 2
times
with methyl;
R8 is ethyl;
X is methylene;
Ra and R` are each methoxy; and
Rb is H;
or pharmaceutically acceptable salt thereof.
[0043] In particular embodiments of the foregoing, the compound is:
H3CO
H3CO
N-~ 0
H3CO
N-'-11 H3CO 819762-01 N 819924
N
N
1 / O
O
o N- O N
Nl_z
819973
919971
N N
N-
_N N-
or a pharmaceutically acceptable salt thereof.
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
[0044] - Active compounds of the present invention include pharmaceutically
acceptable.
salts of the foregoing. Pharmaceutically acceptable salts include those
derived from
pharmaceutically acceptable inorganic and organic acids and bases. Examples of
suitable
acid salts include acetate, adipate, alginate, aspartate, benzoate,
benzenesulfonate, bisulfate,
butyrate, citrate, camphorate, camphorsulfonate, cyclopentanepropionate, .
digluconate,
dodecylsulfate, ethanesulfonate, formate, fumarate, glucoheptanoate,
glycerophosphate,
glycolate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide,
hydroiodide, 2-
hydroxyethanesulfonate, lactate, maleate, malonate, methanesulfonate, 2-
naphthalenesulfonate, nicotinate, nitrate, oxalate, palmoate, pectinate,
persulfate, 3-
phenylpropionate, phosphate, picrate, pivalate, propionate, salicylate,
succinate, sulfate,
tartrate, thiocyanate, tosylate and undecanoate. Other acids, such as oxalic,
while not in
themselves pharmaceutically acceptable, may be employed in the preparation of
salts useful
as intermediates in obtaining the compounds of the invention and their
pharmaceutically
acceptable acid addition salts.
[0045] Salts derived from appropriate bases include alkali metal (e.g., sodium
and,
potassium), alkaline earth metal (e.g., magnesium), ammonium and N+(CI-a
alkyl)4 salts..
This invention also envisions the quaternization of any basic nitrogen-
containing groups of
the compounds disclosed herein. Water- or oil-soluble or dispersible products
may be
obtained by such quaternization.
C. Pharmaceutical formulations.
[00461 Active compounds of the present invention can be combined with a
pharmaceutically acceptable carrier to provide pharmaceutical formulations
thereof. The
particular choice of carrier and formulation will depend upon the particular
route of
administration for which the composition is intended.
[0047] The compositions of the present invention may be suitable for oral,
parenteral,
inhalation spray, topical, rectal, nasal, buccal, vaginal or implanted
reservoir administration,
etc. Preferably, the compositions are administered orally, intraperitoneally
or intravenously.
Sterile injectable forms of the compositions of this invention may be aqueous
or oleaginous
suspension. These suspensions may be formulated according to techniques known
in the art
using suitable dispersing or wetting agents and suspending agents. The sterile
injectable
preparation may also be a sterile injectable solution or suspension in a
nontoxic parenterally
acceptable diluent or solvent, for example as a solution in 1,3-butanediol.
Among the
acceptable vehicles and solvents that may be employed are water, Ringer's
solution and
11
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
isotonic sodium chloride solution. In addition, sterile, fixed oils are
conventionally employed
as a solvent or suspending medium.
[0048] For this purpose, any bland fixed oil may be employed including
synthetic mono-
or di-glycerides. Fatty acids, such as oleic acid and its glyceride
derivatives are useful in the
preparation of injectables, as are natural pharmaceutically acceptable oils,
such as olive oil or
castor. oil, especially in their polyoxyethylated versions. These oil
solutions or suspensions
may also contain a long-chain alcohol diluent or dispersant, such as
carboxymethyl cellulose
or similar dispersing agents that are commonly used in the formulation of
pharmaceutically
acceptable dosage forms including emulsions and suspensions. Other commonly
used
surfactants, such as Tweens, Spans and other emulsifying agents or
bioavailability enhancers
which are commonly used in the manufacture of pharmaceutically acceptable
solid, liquid, or
other dosage forms may also.be used for the purposes of formulation.
[0049] The pharmaceutically acceptable compositions of this invention may be
orally
administered in any orally acceptable dosage form including, but not limited
to, capsules,
tablets, aqueous suspensions or solutions. In the case of tablets for oral
use, carriers
commonly used include lactose and corn starch. Lubricating agents, such as
magnesium
stearate, are also typically added. For oral administration in a capsule form,
useful diluents
include lactose and dried cornstarch. When aqueous suspensions are required
for oral use,
the active ingredient is combined with emulsifying and suspending agents. If
desired, certain
sweetening, flavoring or coloring agents may also be added.
[0050] Alternatively, the pharmaceutically acceptable compositions of this
invention may
be administered in the form of suppositories for rectal administration. These
can be prepared
by mixing the agent with a suitable non-irritating excipient that is solid at
room temperature
but liquid at rectal temperature and therefore will melt in the rectum to
release the drug. Such
materials include cocoa butter, beeswax and polyethylene glycols.
[0051] The pharmaceutically acceptable compositions of this invention may also
be
administered topically, especially when the target of treatment includes areas
or organs
readily accessible by topical application, including diseases of the eye, the
skin, or the lower
intestinal tract. Suitable topical formulations are readily prepared for each
of these areas or
organs.
[0052] Topical application for the lower intestinal tract can be effected in a
rectal
suppository formulation (see above) or in a suitable enema formulation.
Topically
transdermal patches may also be used.
12
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
[0053] For topical applications, the pharmaceutically acceptable compositions
may be
formulated in a suitable ointment containing the active component suspended or
dissolved in
one or more carriers. Carriers for topical administration of the compounds of
this invention
include, but are not limited to, mineral oil, liquid petrolatum, white
petrolatum, propylene
glycol, polyoxyethylene, polyoxypropylene compound, emulsifying wax and water.
Alternatively, the pharmaceutically acceptable compositions can be formulated
in a suitable
lotion or cream containing the active components suspended or dissolved in one
or more
pharmaceutically acceptable carriers. Suitable carriers include, but are not
limited to, mineral
oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl
alcohol, 2
octyldodecanol, benzyl alcohol and water.
[0054] For ophthalmic use, the pharmaceutically acceptable compositions may be
formulated as micronized suspensions in isotonic, pH adjusted sterile saline,
or, preferably, as
solutions in isotonic, pH adjusted sterile saline, either with or without a
preservative such as
benzylalkonium chloride. Alternatively, for ophthalmic uses, the
pharmaceutically
acceptable compositions may be formulated in an ointment such as petrolatum.
[0055] The pharmaceutically acceptable compositions of this invention may also
be
administered by nasal aerosol or inhalation. Such compositions are prepared
according to
techniques well-known in the art of pharmaceutical formulation and may be
prepared as
solutions in saline, employing benzyl alcohol or other suitable preservatives,
absorption
promoters to enhance bioavailability, fluorocarbons, and/or other conventional
solubilizing or
dispersing agents.
[0056] Most preferably, the pharmaceutically acceptable compositions of this
invention
are formulated for oral administration.
D. Subjects and methods of use.
[0057] Active compounds of the present invention may be administered to
patients or
subjects to treat a variety of different condition, particularly patients or
subjects afflicted
with:
(a) rheumatoid arthritis;
(b) multiple sclerosis;
(c) systemic lupus erythematosus (see, e.g., T-bet regulates IgG class
switching and
pathogenic auto Ab production, Proc. Natl. Acad. Sci. USA 99(8): 5545-50
(2002);
Imbalance of Thl/Th2 transcription factors in patients with lupus nephritis,
Rheumatology
(Oxford) 45(8): 951-7 (2006));
13
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
(d) type 1 diabetes (see, e.g., Identification of a novel type 1 diabetes
susceptibility
gene, T-bet, Human Genetics 111(3): 177-84 (2004); T-bet controls
autoaggressive CD8
lymphocyte response in type I diabetes, J. Exp. Med. 199(8): 1153-62 (2004));
(e) psoriasis (see, e.g., J Mol. Med 81(8): 471-80 (2003)); and
(f) atherosclerosis (see, e.g., Proc. Natl. Acad. Sci. USA 102(5): 1596-601
(2005)).
[0058] Active compounds may be administered to subjects by any suitable route,
including orally, parenterally, by inhalation spray, topically, rectally,
nasally, buccally,
vaginally or via an implanted reservoir. The term "parenteral" as used herein
includes
subcutaneous, intravenous, intramuscular, intra-articular, intra-synovial,
intrasternal,
intrathecal, intrahepatic, intralesional and intracranial injection or
infusion techniques.
Preferably, the compositions are administered orally, intraperitoneally or
intravenously.
[0059] The active compounds are administered to the subjects in a treatment
effective, or
therapeutically effective, amount. The amount of the compounds of the present
invention that
may be combined with the carrier materials to produce a composition in a
single dosage form
will vary depending upon the host treated, and the particular route of
administration.
Preferably, the compositions should be formulated so that a dosage of between
0.01 - 100
mg/kg.body weight/day of the inhibitor can be administered to a patient
receiving these
compositions. In certain embodiments, the compositions of the present
invention provide a
dosage of between 0.01 mg and 50 mg is provided. In other embodiments, a
dosage of
between 0.1 and 25 mg or between 5 mg and 40 mg is provided.
[0060] It should also be understood that a specific dosage and treatment
regimen for any
particular patient will depend upon a variety of factors, including the
activity of the specific
compound employed, the age, body weight, general health, sex, diet, time of
administration,
rate of excretion, drug combination, and the judgment of the treating
physician and the
severity of the particular disease being treated. The amount of a compound of
the present
invention in the composition will also depend upon the particular compound in
the
composition.
[0061] In order that the invention described herein may be-more fully
understood, the
following examples are set forth. It should be understood that these examples
are for
illustrative purposes only and are not to be construed as limiting this
invention in any manner.
14
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
EXAMPLES 1-41
Synthesis of Compounds
[0062] Microwave assisted reactions were carried out using an Emrys Liberator
instrument supplied by Biotage Corporation. Solvent removal was carried out
using either a
Buchi rotary evaporator or a Genevac centrifugal evaporator. Analytical and
preparative
chromatography was carried out using a Waters autopurification instrument
using either
normal phase or reverse phase HPLC columns, under either acidic, neutral, or
basic
conditions. Compounds were estimated to be >90% pure, as determined by area
percent of
ELSD chromatograms. NMR spectra were recorded using a Varian 300 MHz
spectrometer.
[0063] General methods and experimentals for preparing compounds of the
present
invention are set forth below. In certain cases, a particular compound is
described by way of
example. However, it will be appreciated that in each case a series of
compounds of the
present invention were prepared in accordance with the schemes and
experimentals described
below.
Scheme 1
O
HN~
O NH
(NH4)2CO3, KCN 0
N H2O, McOH
N
I I
Boo Boo
ER-811160
[0064] ER-811160. As depicted in Scheme 1 above, a solution of potassium
cyanide
(22.5 g, 0.335 mol) in water (50 mL) was added dropwise over 5 minutes to a
solution of 1-
Boc-piperidone (32.48 g, 0.1598 mol) and ammonium carbonate (33.8 g, 0.351
mol) in water
(90 mL) and methanol (110 mL). An off-white precipitate began to form soon
after addition
was complete. The reaction flask was sealed and the suspension stirred at room
temperature
for 72 hours. The resultant pale yellow precipitate was filtered and was
washed with small
portions of water to give ER-811160 (37.1 g, 86%) as a colorless solid.
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 2
MeO
We
H Me N~p
O NH Br I i O NH
OMe
N K2CO3, acetone N
I reflux I
Boc Boc
ER-811160 ER-818039
[0065] ER-818039. As depicted in Scheme 2 above, a suspension of ER-811160
(30.0 g,
0.111 mol), 3,5-dimethoxybenzyl bromide (30.9 g, 0.134 mol), and potassium
carbonate
(18.5 g, 0.134 mol) in acetone (555 mL) was heated under reflux overnight. The
reaction
solution was cooled to room temperature, filtered and concentrated in vacuo.
The crude
orange residue was dissolved in a minimal amount of MTBE (250 mL). A small
amount of
hexanes was added (50 mL) and the product allowed to precipitate out (over -2
hours) as a
colorless solid which was isolated by vacuum filtration. The filter cake was
washed with
small amounts of MTBE, and dried in vacuo to provide ER-818039 (39.6g, 85%) as
a
colorless solid.
Scheme 3
MeO MeO
OMe OMe
O O
HCI, dioxane
NH O NH
N N
I H HCI
Boc
ER-818039 ER-823143-01
[0066] ER-823143-01. As depicted in Scheme 3 above, to a 1-neck round-bottom
flask
containing ER-818039 (2.15 g, 0.00512 mol) was slowly added a solution of 4N
HCl in 1,4-
dioxane (3.8 mL, 0.049 mol). The starting material slowly dissolved over 20
minutes and a
16
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
colorless precipitate formed after 30 minutes. MTBE (3 ml) was then added.
After 2 hours,
the reaction was filtered and washed with MTBE, which provided ER-823143-01
(1.81 g,
99%) as a colorless solid.
Scheme 4
MeO
MeO / \ OMe
OMe 0
O N \
NH
N ` 3,5-dimethoxybenzaldehyde 0
0 NH NaBH(OAc)3, DMF
N
N OMe
H HCI
ER-823143-01 ER-817098 OMe
[0067] ER-817098: As depicted in Scheme 4 above, to a suspension of ER-823143-
01
(41.5 mg, 0.000117 mol) and 4A molecular sieves in 1,2-dimethoxyethane (0.5
mL, 0.004
mol) under an atmosphere of nitrogen was added 3,5-dimethoxybenzaldehyde (21.3
mg,
0.000128 mol) followed by triethylamine (16.2 L, 0.000117 mol). The reaction
was stirred
for 1 hour. Sodium triacetoxyborohydride (34.6 mg, 0.000163 mol) was added,
and the
reaction was stirred overnight. Silica gel flash chromatography yielded ER-
817098 (45.3 mg,
83%) as a colorless solid.
Scheme 5
MeO MeO
OMe OMe
o O 1.0 O N H NMM of LiHMDS in THE O ~\0
N N
q OMe q OMe
OMe OMe
ER-817098 ER-817116
[0068] ER-817116: As depicted in Scheme 5 above, to a solution of ER-817098-00
(50.0
mg, 0.000106 mol) and 1-bromo-2-methoxyethane (15.6 L, 0.000160 mol) in N-
methylpyrrolidinone (1.0 mL, 0.010 mol) was added 1.0 M lithium
hexamethyldisilazide
17
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
solution in tetrahydrofuran (0.16 mL). The temperature was increased to at 80
C and the
reaction mixture stirred overnight. The reaction mixture was cooled to room
temperature,
quenched with water and then extracted several times with MTBE. The MTBE
extracts were
combined and washed with water (2x) and brine (lx). The organic layer was
dried over
magnesium sulfate, filtered, and concentrated in vacuo. Flash chromatography
provided ER-
817116 (32.2 mg, 58%) as colorless oil.
Scheme 6
MeO Meo
OMe OMe
/O O
NH lodoethane Nom/
NaH O
DMF
N N
OMe I OMe
OMe OMe
ER-817098 ER-817118
[0069] ER-817118: As depicted in Scheme 6 above, to a solution of ER-817098
(2.85 g)-
0.00607 mol)- ind metliylformamide (15 mL) was added sodium hydride (364 mg,
0.00910 mol) followed by iodoethane (758 L, 0.00910 mol). The reaction
mixture was
stirred overnight. Water was very slowly added and the reaction mixture was
extracted
several times with MTBE. The MTBE extracts were combined and washed with water
(2x)
and brine (lx). The organic layer was dried over magnesium sulfate, filtered,
and
concentrated in vacuo. Flash chromatography using ethyl acetate as eluent
provided ER-
817118 (2.89 g, 96%) as a colorless oil.
18
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 7
MeO MeO
-OMe L MgBr--~--"
0 THF, -78 C to r.t. ` 0
Me0 N
N ii. TFA, 78 C to 400C NH
0 NH
iii. Boc2O, Et3N, MeOH
N
H HCI Boc
ER-823143-01 ER-823914
[00701 ER-823914: As depicted in Scheme 7 above, to a solution of ER-823143-01
(5.03
g, 0.0141 mol) in tetrahydrofuran (30.0 mL, 0.370 mol) at -78 C was slowly
added 1.0 M of
allylmagnesium bromide in ether (71 mL). The reaction mixture was warmed to
room
temperature and stirred overnight. The reaction mixture was cooled to -78 C,
treated
dropwise with trifluoroacetic acid (21.8 mL, 0.283 mol), and then concentrated
in vacuo to a
small residual volume. Triethylamine was added to neutralize residual TFA and
the mixture
then concentrated in vacuo to dryness. The residual red oil was dissolved in
methanol (138
mL, 3.41 mol) and treated with di-tert-butyldicarbonate (3.34 g, 0.0148 mol)
followed by
triethylamine (2.38 mL, 0.0169 mol) and stirred overnight at room temperature.
The reaction
mixture was concentrated in vacuo and purified by flash chromatography
(eluent: 50%
hexanes in ethyl acetate) to provide ER-823914 (3.25 g, 52%) as a colorless
solid.
Scheme 8
MeO
Meo
q O Etl
MeO N~ D
NaH, DMF - MeO `
NH
N/
N
Boc - N
Boc
ER-823914
ER-823915
[0071] ER-823915: As depicted in Scheme 8 above, to a solution of ER-823914
(2.20 g,
0.00496 mol) in N,N-Dimethylformamide (12.4 mL, 0.160 mol) was added sodium
hydride
(298 mg, 0.00744 mol) followed by iodoethane (607 p.L, 0.00744 mol). The
reaction mixture
was stirred overnight then quenched with water and extracted several times
with MTBE. The
19
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
MTBE extracts were combined and washed with water and brine. The organic layer
was
dried over magnesium sulfate, filtered, and concentrated in vacuo. Flash
chromatography
(eluent: 40% hexanes in ethyl acetate) provided ER-823915 (0.80 g, 34%) as a
colorless
foam.
Scheme 9
MeO MeO
`
O HCI/dioxane - 0
Me0 \ MeO
N-/ Nom/
N N
Boc H HCI
ER-823915 ER-823917-01
(0072] ER-823917-01: As depicted in Scheme 9 above, ER-823915 (799.2 mg,
0.001695
mol) was dissolved in a solution of 4 M hydrogen chloride in 1,4-dioxane (10
mL). The
reaction mixture was stirred overnight and then concentrated in vacuo to
provide ER-823917-
01 (0.69g, quantitative) as an orange solid.
Scheme 10
MeO MeO MeO
j0 resolution by \ O 0
MeO N- MeO N--~ + MeO N
i Nom/ chiral HPLC Nom/ i Nom/
N
N N
Boc Boc Boc
ER-823915 ER-824184 ER-824185
[0073] ER-824184 & ER-824185: As depicted in Scheme 10 above, a solution of ER-
823915 (200 mg) in acetonitrile (1 ml) was injected onto a CHIRALPAK AS-H SFC
column (30 mm x 250 mm, 5 micron particle size) and eluted with 95 : 5 n-
heptane : i-
propanol at a flow rate of 40 ml/min. Eluted fractions were detected using a
UV detector
with the wavelength set at 290 nm. The first eluting fraction was isolated and
concentrated
by rotary evaporation in vacuo to afford ER-824184; the second eluting
fraction was isolated
and concentrated by rotary evaporation in vacuo to afford ER-824185.
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 11
MeO MeO
O HCI/dioxane \ //O
Me0 N-~ Me0 N-('
i Nom/ `N, /
N N
H HCI
Boc
ER-824184 ER-824188-01
ER-824188-01: As depicted in Scheme 11 above, ER-824184 (25.33 g, 0.05371 mol)
was
dissolved in a solution of 4 M hydrogen chloride in 1,4-dioxane (135 mL). The
reaction
mixture was stirred overnight and then concentrated in vacuo to provide ER-
824188-01 (21.9
g, quantitative) as an orange solid. Single crystal X-ray diffraction analysis
of ER-824188-01
showed the absolute configuration of the stereocenter to be S, as depicted in
Scheme 11.
Scheme 12
MeO MeO
O HCI/dioxane O
Me0 \ N Me0 / N
Nom/ / Nom./
N N
i
H HCI
Boc
ER-824185 ER-824280-01
ER-824280-01: As depicted in Scheme 12 above, ER-824185 (457.2 mg, 0.0009695
mol)
was dissolved in a solution of 4 M hydrogen chloride in 1,4-dioxane (2.5 mL).
The reaction
mixture was stirred overnight and then concentrated in vacuo to provide ER-
824280-01
(383.2 mg, 97%) as an orange solid. Single crystal X-ray diffraction analysis
of a Mosher
amide derivative of ER-824188-01 showed the absolute configuration of the
stereocenter to
be R, as depicted in Scheme 11.
21
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 13
MeO
MeO
\ O Me0 N
MeO N- f Nom/
N-methylpyrrole-2-ca rba Idehyde
N NaBH(OAc)3, DMF N
H HCI
ER-824188-01 ER-819924
ER-819924: As depicted in Scheme 13 above, ER-824188-01 (62.4 mg, 0.000153
mol) and
N-methylpyrrole-2-carbaldehyde (0.000229 mol) were dissolved/suspended in N,N-
dimethylformamide (0.62 mL). After stirring for 30 minutes, sodium
triacetoxyborohydride
(47.8 mg, 0.000214 mol) was added. The reaction mixture was stirred overnight
then
purified by reverse phase chromatography to afford ER-819924 (71.1 mg, 83.4%)
as an oil.
Scheme 14
MeO
MeO
\ , O Me0 N
Me0 N-~ N,/
Nom/
N-methylpyrrole-2-ca rba Idehyde
N NaBH(OAc)3, DMF I -r '
H HCI ~
ER-824280-01 ER-819925
ER-819925: As depicted in Scheme 14 above, ER-824280-01 (59.5 mg, 0.000146 mol
and
N-methylpyrrole-2-carbaldehyde (0.000219 mol) were dissolved/suspended in N,N'-
dimethylformamide (0.60 mL). After stirring for 30 minutes, sodium
triacetoxyborohydride
(45.6 mg, 0.000204 mol) was added. The reaction mixture was stirred overnight
then
purified by reverse phase chromatography to afford ER-819925 (51.9 mg, 76.6%)
as an oil.
22
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Scheme 15
Me
Me
1/ 0
0 MeO N
MeO N~ N,/
N
3,5-dime thylbenryl bromide
N
N DBU,DMF
H HCI I \
ER-824188-01 ER-819762
[00741 ER-819762: As depicted in Scheme 15 above, a solution of ER-824188-01
(5.7 g,
0.0140 mol), 1,8-diazabicyclo[5.4.0]undec-7-ene (4.4 mL, 0.029 mol) and 3,5-
dimethylbenzyl bromide (4.7 g, 0.024 mol) in N,N-dimethylformamide (50 mL) was
heated
at 97 C overnight. An aqueous work-up and purification by flash chromatography
provided
ER-819762 (4.86 g, 71 %) as colorless solid.
Scheme 16
MeD MeO
MeO N~ MeO N-
i N/ N
HCI
N HCI N
ER-819762 ER-819762-01
[00751 ER-819762-01: As depicted in Scheme 16 above, a solution of ER-819762
(4.77
g, 0.00974 mol), Acetonitrile (10 mL) and 1M HCl in Water (11 mL) was stirred
at room
temperature for approximately 5 minutes. The solution was concentrated to
provide ER-
819762-01 (5.1 g, quantitative) as a colorless crystalline solid -after
lyophilization. Single
crystal X-ray diffraction analysis of ER-819762-01 showed the absolute
configuration of the
stereocenter to be S, as depicted in Scheme 16.
23
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Scheme 17
MeO
MeO
O MeO N
Me0 N'~ Nom/
N/
3,5-dimethylbenzyl chloride
N
N DBU, DMF
H HCI
ER-824280-01 ER-819763
[0076] ER-819763: As depicted in Scheme 17 above, a solution of ER-824280-01
(66.9
g, 0.1640 mol), 1,8-diazabicyclo[5.4.0]undec-7-ene (54 mL, 0.361 mol) and 3,5-
dimethylbenzyl chloride (42.4 g, 0.213 mol) in N-Methylpyrrolidinone (669 mL)
was heated
at 72 C for 2 hours. After cooling, water was added to precipitate the desired
product.
Filtration and drying under vacuum provided ER-819763 (74.4g, 92%) as
colorless solid.
Scheme 18
MeO
MeO / \ We
OMe Br
O
O N__f
N- f O NH
O NH DBU, DMF
N
N
H HCI
ER-823143-01 ER-824102
ER-824102: As depicted in Scheme 18 above, to a solution of ER-823143-01 (4.00
g, 0.0112
mol) in N,N-dimethylformamide (25 mL) at room temperature was added alpha-
bromomesitylene (3.13 g, 0.0157 mol) followed by DBU (4.37 mL, 0.0292 mol).
After
stirring for 1 hour, reaction was quenched with half-saturated aq. NH4Cl,
diluted with ethyl
acetate, and stirred for lh to give two clear layers. Organic layer was
separated, aq. layer was
extracted with ethyl acetate (2x). Combined extracts were dried over Na2SO4,
filtered, and
concentrated in vacuo. Crystallization from MTBE afforded ER-824102 (4.30 g,
87%) as a
colorless solid.
24
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 19
MeO MeO
OMe yOMe
O O
N BrMgHO N
NH NH
0 THF. -650C to 0 C
N N
I?,- I?,-
ER-824102 ER-819929
ER-819929: As depicted in Scheme 19 above, to a solution of ER-824102 (3.72 g,
0.0085
mol) in tetrahydrofuran (35 mL) at -65 C was added' 1.0 M allylmagnesium
bromide in ether
(25.5 mL, 0.0255 mol) over 10 min keeping internal temperature below -50 C.
The reaction
mixture was allowed to warm to 0 C. After 3 h at 0 C, reaction was quenched
with saturated
aq. NH4C1, diluted with ethyl acetate and water, stirred for 10 min to give
two clear layers.
Organic layer was separated, aq. layer was extracted with ethyl acetate.
Combined extracts
were washed with water, brine, dried over Na2SO4, filtered, concentrated in
vacuo to give
crude product ER-819929 (4.15 g, quantitative) as a colorless solid that was
used for next
step without further purification.
Scheme 20
MeO
OMe MeO
HO N-{' TFA MeO N4
NH NH
N
N
ER-819929 ER-819930
ER-819930: As depicted in Scheme 20 above, a solution of ER-819929 (37 mg,
0.000077
mol) in trifluoroacetic acid (0.5 mL) was stirred at room temperature for 16
hours. Dark
brown-red reaction mixture was diluted with EtOAc (5 mL), neutralized with sat
aq NaHCO3
(5 mL, careful: gas evolution). Two-layer mixture was stirred for 10 min to
give two clear,
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
almost colorless layers. The organic layer was separated; the aq layer was
extracted with
EtOAc. Combined organic extracts were dried over Na2SO4, filtered,
concentrated in vacuo.
Purification by flash chromatography eluting with 1:1 Heptane-EtOAc, 1:3
Heptane-EtOAc,
100% EtOAc afforded ER-819930 (26 mg, 73%) as a colorless solid.
Scheme 21
Me Me Me
MeO / \ II MeO MeO N
NH N + N_-,
UHMDS
THF, DMF
N N N
ER-819930 ER-820006 ER-820007
ER-820006 and ER-820007: As depicted in Scheme 21 above, to a solution of ER-
819930
(110 mg, 0.000238 mol) and methallyl bromide (72 L, 0.000715 mol) in DMF (1.5
mL,)
was added 1.0 M lithium hexamethyldisilazide solution in tetrahydrofuran (0.52
mL, 0.00052
mol). After stirring for 18 h at rt, reaction mixture was diluted with MTBE,
quenched with
half-saturated aq NH4CI. Aq. layer was separated, extracted with MTBE.
Combined extracts
were dried over Na2SO4, filtered, concentrated in vacuo. Purification by flash
chromatography eluting with 3:2 Heptane-EtOAc, 1:1 Heptane-EtOAc furnished
racemic
product (68 mg, 55%) as a colorless oil. Racemic product (55 mg) was subjected
to chiral
HPLC on Chiralpak AS column eluting with heptane-isopropanol (9:1) to afford
first eluting
enantiomer ER-820006 (21 mg, 38%, [a)D = +83.7 (c=0.35, CHC13) and second
eluting
enantiomer ER-820007 (23 mg, 42%, [a]D = -74.2 (c=0.38, CHC13). Absolute
stereochemistry was assigned tentatively based on analogy in optical rotation
and chiral
HPLC retention time with ER-819762/ER-819763 pair of enantiomers.
26
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 22
MeD MeO Me
1 ~f0 1 .(/0 1 X10
1 MeO
MeO N 1 g{\~0 0 MeO N40
NH i N-f OH N-// -OH
LiHMDS, THF, DMF
N N N
ER-619930 ER-819786 ER-81 9787
ER-819786 and ER-819787: As depicted in Scheme 22 above, a 5 mL microwave
reactor
vial equipped with a stir bar was charged with ER-819930 (110 mg, 0.000238
mol), DMF
(1.5 mL), 2-(2-bromoethoxy)tetrahydro-2H-pyran (108 p.L, 0.000715 mol) and
1.00 M of
lithium hexamethyldisilazide in tetrahydrofuran (520 pL, 0.00052 mol). The
reactor vial was
microwaved at 200 C for 15 min. More 2-(2-bromoethoxy)tetrahydro-2H-pyran (108
}iL,
0.000715 mol) and 1.00 M of lithium hexamethyldisilazide in tetrahydrofuran
(520 .iL,
0.00052 mol) were added, and reaction mixture was heated by microwave
irradiation at
200 C for another 15 min. Purification by preparative reverse phase HPLC
provided racemic
product (25 mg, 21%) as a colorless glassy oil. Racemic product (17 mg) was
subjected to
chiral HPLC on Chiralpac AS column eluting with heptane-isopropanol (9:1) to
afford first
eluting enantiomer ER-819786 (7.2 mg, 42%, [a]D = +72.0 (c=0.1, CHC13) and
second
eluting enantiomer ER-819787 (7.5 mg, 44%, [a]D = -73.0 CHC13). Absolute
stereochemistry was assigned tentatively based on analogy in optical rotation
and chiral
HPLC retention time with ER-819762/ER-819763 pair of enantiomers.
27
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Scheme 23
Me
1 / 0
MeO N TsOl/~0
NH
LIHMDS, THF, DMF
N
ER-819930
Me Me Me
O~ \ 0 j,0H OOH
MeO ( ,O MeMeO 1 1 ,
N + N +
N N N
ER-819993 ER-81 9788 ER-819789
ER-819993 and ER-819994: As depicted in Scheme 23 above, a 5 mL microwave
reactor
vial equipped with'a stir bar was charged with ER-819930 (110 mg, 0.000238
mol), DMF
(1.5 mL), ((4S)-2,2-dimethyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate
(205 mg,
0.000715 mol) and 1.00 M of lithium hexamethyldisilazide in tetrahydrofuran
(520 L,
0.00052 mol). The reactor vial was heated by microwave irradiation at 200 C
for 15 min.
More ((4S)-2,2-dimethyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate (157
mg,
0.000548 mol) and 1.00 M of lithium hexamethyldisilazide in tetrahydrofuran
(477 L,
0.000477 mol) were added, and reaction mixture was heated by microwave
irradiation at
200 C for another 15 min. Purification by preparative reverse phase HPLC
provided
acetonide ER-819993 (40 mg, 30%) and diol material (18 mg, 14%) as 1:1
mixtures of
diastereomers. Separation of diastereomeric diols by chiral HPLC on Chiralpac
AS column
eluting with heptane-isopropanol (9:1) afforded the first eluting diastereomer
ER-819788 (5.0
mg) and the second eluting diastereomer ER-819789 (5.2 mg). Absolute
stereochemistry was
assigned tentatively based on analogy in chiral HPLC retention time with ER-
819762/ER-
819763 pair of enantiomers.
28
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Scheme 24
Me
Me
O Meo N
MeO N N-/
N-/
N
H O
MeO F
ER-824220-00 ER-819990 F F
[00771 ER-81990: As depicted in Scheme 24 above, a solution of ER-824220-00
(51.8
mg, 0.000139 mol), triethylamine (97 L, 0.00070 mol), 4-dimethylaminopyridine
(3.4 mg,
0.000028 mol) and (R)-(-)-a-Methoxy-a-trifluoromethylphenylacetyl chloride
(0.052 mL,
0.00028 mol) in Methylene Chloride (500 .tL) was stirred at room temperature
for 5 hours.
Purification by flash chromatography, followed by crystallization from ethyl
acetate/heptane/pentane provided ER-819990 (49.2 mg, 60%) as crystals.
[00781 Compounds that are exemplified in subsequent sections, and in Tables 1-
2 below,
but not depicted explicitly above, can be synthesized using general methods
consistent with
Scheme 13 and/or Scheme 15. For compounds exemplified in hydrochloride salt
form, these
can be prepared by subjecting the corresponding free base to the general
conditions described
in Scheme 16.
Table 1. Analytical Data for Exemplary Compounds of Formula I
Example # Structure ER-# Analytical Data
NMR H
(400 MHz, CD3OD) S 7.18 (s, 2H), 7.15
M (s, 114), 6.49 (d, J=2.6 Hz, I H), 6.42 (d,
_ J=2.3 Hz, I H), 5.18 (br s, 1 H), 4.70 (d,
J=14.4 Hz, 114), 4.62 (d, J=14.4 Hz, I H),
Meo N--~ 4.32 (s, 2H), 4.27-4.19 (m, I H), 3.80 (s,
N~/ 3H), 3.78 (s, 3H), 3.50-3.38 (m, 4H),
1 819762-01 3.26 (br s, 2H), 2.36 (s, 6H), 2.31-2.17
N HC1 Salt (m, 2H), 1.97 (br d, J=14.4 Hz, IH), 1.79
(br d, J=14.4 Hz, IH), 1.38 (d, J=7.3 Hz,
3H), 1.16(t,J=7.0Hz,3H)
M/Z (ES+)
CaIc.: 489.30
Found: 490.40 (M+H)
29
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Analytical HPLC:
Method A I
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 11.21 min
Chiral HPLC
Method C I
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.819 min
NMR H
(400 MHz, CDC13) 8 6.94 (s, 2H), 6.90
(s, 1 H), 6.44 (d, J=2.6 Hz, I H), 6.41 (d,
J=2.3 Hz, 1H), 5.02 (d, J=8.5 Hz, IH),
4.81 (d, J=14.1 Hz, 1H), 4.58 (d, J= 14.4
Hz, I H) 4.17-4.09 (m, I H), 3.79 (s, 314),
3.78 (s, 3H), 3.49 (s, 2H), 3.51-3.26
(m,1 H), 3.26-3.17 (m, I H), 2.79-2.76 (m,
Me IH), 2.71-2.68 (m, 1H), 2.56-2.46 (m,
2H), 2.31 (s, 61-f), 2.00-1.86 (m 2H),
o 1.68-1.58 (m, 2H), 1.35 (d, J=7.3 Hz,
MeO --- f/ 3H), 1.15 (t, J=7.2 Hz, 3H)
2 81+97.63-00
Salt Free M/Z (ES+)
N
Calc.: 489.30
Found: 490.40 (M+H)
Analytical HPLC:
Method A I
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 11.16 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.786 min
NMR H
(400 MHz, CDC13) 5 12.68 (br s, 1 H),
7.25 (s, 2H), 7.10 (s, 1 H), 6.42 (d, 3=2.4
M Hz, I H), 6.41 (d, J=2.4 Hz, I H), 4.79 (d,
J=14.4 Hz, 1 H), 4.7 5 (d, J=8.8 Hz, I H),
4.61 (d, J=14.4 Hz, I H), 4.05-4.14 (m,
moo -~ N N -OH 3H), 3.81 (s, 3H), 3.79 (s, 3H), 3.76 (m,
819786-01 2H), 3.58 (m, 2H), 3.48 (d, J=10.0 Hz,
3 N HCI Salt IH), 3.35 (d, J=10.8 Hz, IH), 3.11 (q,
J=10 HZ, 2H), 2.82-3.00 (m, 2H), 2.37
(s, 6H), 1.89 (d, J=14.2 Hz, IH), 1.73 (d,
13.9 Hz, 114), 1.34 (d, J=7.2 Hz, 3H)
M/Z (ES+)
Ca1c.: 505.29
Found: 506.40 (M+H)
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) Sum
Retention Time: 9.88 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.687 min
NMR H
(400 MHz, DMSO) S 12.67 (br s, 1H),
7.27 (s, 2H), 7.11 (s, I H), 6.43 (d, J=2.4
Hz, 1H), 6.41 (d, J=2.4 Hz, IH), 4.75-
4.81 (m, 2H), 4.61 (d, J=14.4 Hz, I H),
4.10 (br s, 3 H), 3.81 (s, 314), 3.79 (s, 3 H),
3.77 (m, 2H), 3.58 (br s, 2H), 3.49 (br s,
Me 1 H), 3.35 (br. s, I H), 2.87-3.11 (m, 4H),
2.37 (s, 6H), 1.89 (br s, 1H), 1.73 (d,
Me0\ N-{ 0 11.2 Hz, 1H), 1.35 (d, J=5.2 Hz, 314)
N--,/'OH
4 819787-01 M/Z (ES+)
N HCl Salt. CaIc.: 505.29
Found: 506.40 (M+H)
Analytical HPLC:
Method A I
SunFire MS CIS (4.6x100mm) 5um
Retention Time: 9.87 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.435 min
NMR'H
(400 MHz, CDCl3) S 12.29 (br s, 1 H),
7.24 (s, 214), 7.10 (s, I H), 6.43 (d, J=2.4
Hz, I H), 6.41 (d, J=2.4 Hz, I H), 4.80 (d,
Me J=8.8 Hz, 1H), 4.78 (d, J=14.4 Hz, IH),
4.64 (d, J=14.4 Hz, IH), 4.04-4.16 (m,
\ HO 3H), 3.81 (s, 3H), 3.79 (s, 3H), 3.77-3.85
M~ ~" }JOH (m, 2H), 3.61 (d, J=5.6 Hz, 2H), 3.37
819788-01 3.54 (m, 3H), 2.91-3.11 (m, 4H), 2.36 (s,
N HCl Salt 6H), 1.92 (d, J=11.6 Hz, I H), 1.72 (d,
13.2 Hz, IH), 1.37 (d, J=7.3 Hz, 3H)
M/Z (ES+)
Calc.: 535.30
Found: 536.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 4.6xI00mm 5um
31
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Retention Time: 9.27 min
Chiral HPLC
Method C I
CHIRALPAK IA (0.46x25 cm)
Retention Time: 8.471 min
NMR H
(400 MHz, CDCI3) S 12.32 (br s, I H),
7.24 (s, 2H), 7.11 (s, I H), 6.44 (d, J=2.4
Hz, I H), 6.42 (d, J=2.4 Hz, I H), 4.80 (d,
J=8.8 Hz, I H), 4.80 (d, J=14.6 Hz, 1 H),
4.63 (d, J=14.4 Hz, IH), 4.05-4.16 (m,
3H), 3.81 (s, 3H), 3.80 (s, 3H), 3.76-3.79
(m, 2H), 3.60 (d, J=5.6 Hz, 2H), 3.38-
Me 3.53 (m, 3H), 2.94-3.08 (m, 4H), 2.37 (s,
o H 6H), 1.87 (d, J=11.2 Hz, IH), 1.78 (d,
moo -~ Q off 13.2 Hz, 1H), 1.36 (d, J=7.3 Hz, 3H)
6 " 819789-01 M/Z(ES+
HCl Salt )
N Cale.: 535.30
Found: 536.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 9.27 min
Chiral HPLC
Method C I
CHIRALPAK IA (0.46x25 cm)
Retention Time: 5.591 min
NMR H
(400 MHz, CD3OD) S 6.83-6.81 (m, 114),
6.45 (d, J=2.3 Hz, IH), 6.38 (d, J=2.3
Hz, 2H), 6.12 (t, J=3.2 Hz, IH), 5.17 (br"
s, I H), 4.67 (d, J=14.4 Hz, 1 H), 4.59 (d,
Meo J=14.4 Hz, 1H), 4.44 (s, 2H), 4.24-4.17
(m, 1 H), 3.74 (t, J=7.8 Hz, 61-1), 3.62-
3.54 0 3.54 (m, 2H), 3.46-3.35 (m, 2H), 2.21 (br
-0 s, 2H), 2.62 (s, 3H), 2.23-2.14 (m, 2H),
Meo i Nom/ 819924-01 1.95 (br d, J=13.8 Hz, I H), 1.78 (br d,
7 HCl Salt J=13.5 Hz, 1H), 1.35 (d, J=7.3 Hz, 3H),
1.13 (t, J=7.0 Hz, 314)
N N M/Z (ES+)
Cale.: 464.28
Found: 465.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS-C18 (4.6x100mm) 5um
Retention Time: 9.59 min
32
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example # Structure ER-# Analytical Data
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 7.790 min
NMR H
(400 MHz, CD3OD) S 6.83-6.81 (m, 1 H),
6.45 (d, J=2.3 Hz, 1H), 6.38 (d, 3=2.3
Hz, 2H), 6.11 (t, J=3.2 Hz, 1H), 5.17 (br
s, 111), 4.67 (d, J=14.4 Hz, 1 H), 4.59 (d,
J=14.4 Hz, IH), 4.34 (s, 2H), 4.24-4.17
(m, I H), 3.74 (t, J=7.9 Hz, 614), 3.67-
3.36 (m, 4H), 3.19 (br s, 2H), 2.62 (s,
Meo 3H), 2.27-2.14 (m, 2H), 1.95 (br d,
J=]2.6Hz, IH), 1.78(brd,J=11.4 Hz,
/ 0 1 H), 1.35 (d, J=7.3 Hz, 3H), 1.13 (t,
Meo N ' J=7.0 Hz, 3H)
",/ 819925-00
8 Salt Free M/Z (ES+)
Cale.: 464.28
N
N Found: 465.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xlOOmm) 5um
Retention Time: 9.58 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.821 min
NMR H
(400 MHz, CD3OD).S 7.91-7.87 (m, 1H),
7.85-7.81 (m, 114), 7.67-7.60 (m, 2H),
6.46 (d, J=2.3 Hz, 1H), 6.39 (d, J=2.3
Hz, 1H), 4.80 (s, IH), 4.68 (d, J=14.4
MeO Hz, 1H), 4.60 (d, J=14.1 Hz, IH), 4.23
(q, J=7.3, 14.6 Hz, I H), 4.15 (s, 21-t),
0 3.76 (s, 3H), 3.74 (s, 3H), 3.58-3.41 (m,
MeO "-' 4H), 3.33-3.28 (m, 2H), 2.62 (s, 3H),
",/ 819926-01 2.44-2.33 (m, 2H), 1.93 (br d, J=16.1 Hz,
9 HCl Salt 1H), 1.76 (br d, J=14.4 Hz, IH), 1.37 (d,
J=7.3 Hz, 3H), 1.14 (t, J=7.0 Hz, 3H)
N /
rl " MlZ (ES+)
N / \ Cale.: 515.29
Found: 5 1 6.36 (M+H)
Analytical I-IPLC:
Method Al
SunFire MS C18 (4.6xlOOmm) 5um
Retention Time: 8.28 min
33
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Chiral HPLC
Method C I
CHIRALPAK IA (0.46x25 cm)
Retention Time: 7.461 min
NMR 1H
(400 MHz, CD3OD) S 7.96-7.92 (m, IH),
7.90-7.86 (m, 1H), 7.71-7.64 (m, 2H),
6.50 (d, J=2.3 Hz, I H), 6.43 (d, J=2.3
Hz, IH), 4.86 (s, 1H), 4.72 (d, J=14.4
Hz, 1 H), 4.64 (d, J=14.1 Hz, I H), 4.27
(q, J=7.2, 14.5 Hz, I H), 4.20 (s, 2H),
3.80 (s, 3H), 3.78 (s, 3H), 3.68-3.39 (m,
MeO 4H), 3.37-3.31 (m, 2H), 2.66 (s, 3H),
2.49-2.42 (m, 2H), 1.98 (br d, J=14.6 Hz,
0 1H), 1.81 (br d, J=14.4 Hz, IH), 1.41(d,
Meow J=7.3 Hz, 3H), 1.18 (t, J=7.0 Hz, 3H)
819927-01
HCI Salt M/Z (ES+)
N / Calc.:515.29
N Found: 516.36 (M+H)
NI
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 8.28 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 5.670 min
NMR'H
(400 MHz, CD3OD) S 7.02 (d, J=7.0 Hz,.
2H), 6.47 (d, J=2.3 Hz, 1H), 6.41 (d,
J=2.3 Hz, I H), 5.10 (d, J=8.8 Hz, I H),"
4.68 (d, J=14.4 Hz, 1H), 4.57 (d, J=14.4
MeO Hz, 1 H), 4.19-4.12 (m, 1 H), 3.79 (s, 3H),
3.77 (s, 3H), 3.51 (s, 2H), 3.28-3.18 (m;`
2H), 2.82-2.73 (m, 2H), 2.65-2.54 (m,
Meo N-~ 2H), 2.24 (d, J=1.8 Hz, 6H), 2.07-1.90
N,/ 819931-00 (m, 2H), 1.69 (br d, J=12.0 Hz, 1H), 1.54
11 Salt Free (br d, J=13.5 Hz, 1H), 1.34 (d, J=7.3 Hz,
N 3H),1.14 (t, J=7.0 Hz, 3H)
M/Z (ES+)
Ca1c.:507.29
Found: 508.42 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 11.22 min
34
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.958 min
NMR H
(400 MHz, CDC13) S 6.92 (d, J=7.0 Hz,
2H), 6.42 (d, J=2.3 Hz, 1 H), 6.39 (d,
J=2.3 Hz, 1 H), 4.99 (d, J=8.5 Hz, 1 H),
4.79 (d, J=14.1 Hz, 11-1), 4.56 (d, J=14.4
Hz, 1 H), 4.15-4.08 (m, ] H), 3.77 (d,
J=3.2 Hz, 6H), 3.42 (s, 2H), 3.29-3.24
MeO (m, 1H), 3.24-3.15 (m, 1H), 2.75-2.65
(m, 2H), 2.53-2.43 (m, 2H), 2.23 (d,
o J=2.] Hz, 6H), 1.97-1.83 (m, 2H), J = 7 . 0 N-/ 819943-00
12 Salt Free M/Z (ES+)
N Calc.: 507.29
Found: 508.36 (M+H)
~ F
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 11.20 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.684 min
NMR H
(400 MHz, CDC13) S 7.29 (br d, J=6.7,
114), 7.24-7.21 (m; 1 H), 7.12 (t, J=9.1
Hz, 114), 6.42 (d, J=2.3 Hz, 114), 6.39 (d,
J=2.3 Hz, 1 H), 4.98 (d, J=8.5 Hz, 1 H),
4.79 (d, J=14.4 Hz, 1H), 4.56 (d, J=14.4
MeO Hz, ] H), 4.15-4.08 (m, 1 H), 3.77 (d,
\ o J=2.3 Hz, 6H), 3.52 (s, 2H), 3.31-3.24
(m, 1 H), 3.24-3.15 (m, 1 H), 2.73-2.62
Meo
N-/ 819933-00 (m, 2H), 2.57-2.48 (m, 2H), ] .73-1.83
13 Salt Free (m, 2H), 1.68-1.55 (m, 2H), 1.33 (d,
J=7.0 Hz, 3H), 1.15 (t, J=7.0 Hz, 3H)
N
O F
F M/Z (ES+)
a F F Calc.: 563.24
Found: 564.30 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 11.45 min
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-4 Analytical Data
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 5.872 min
NMR H
(400 MHz, CDCl3) 5 7.29 (br d, J=7.3,
1 H), 7.24-7.21 (m, 1 H), 7.12 (t, J=8.9
Hz, IH), 6.42 (d, J=2.3 Hz, I H), 6.39 (d,
J=2.6 Hz, 1 H), 4.98 (d, J=8.5 Hz, I H),
4.79 (d, J=14.4 Hz, 1H), 4.56 (d, J=14.4
Hz, I H), 4.15-4.08 (m, 1 H), 3.77 (d,
J=2.1 Hz, 6H), 3.52 (s, 2H), 3.31-3.24
MeO (m, I H), 3.22-3.15 (m, 11-1), 2.73-2.62
(in, 2H), 2.57-2.48 (m, 2H), 1.97-1.83
o (in, 2H), 1.68-1.58 (in, 2H), 1.33 (d,
Meo / N-- J=7.30 Hz, 3H), 1.15 (t, J=7.0 Hz, 3H)
14 -/ 819945-00
Salt Free MIZ (ES+)
N Calc.: 563.24
o~F Found: 564.30 (M+H)
/ F F
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 11.77 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.144 min
NMR'H
(400 MHz, CD3OD) S 7.24 (q, J=8.3,
15.1 Hz, 114), 6.91 (t, J=8.8 Hz, I H),
6.47 (d, J=2.3 Hz, I H), 6.41 (d, J=2.3
Hz, I H), 5.00 (d, J=8.8 Hz, 1 H), 4.67 (d,
MeO J=14.1 Hz, I H), 4.5 7 (d, J=14.1 Hz, 111),
4.14-4.06 (m, 1H), 3.79 (s, 3H), 3.78 (s,
~0 3H), 3.76 (s, 2H), 3.29-3.17 (m, 2H),
Meo N 2.89-2.81 (m, 2H), 2.75-2.70 (m,2H),
N-/ 819934-00 2.27 (s, 3H), 2.06-1.95 (m, 2H), 1.68 (br
15 Salt Free d, J=13.5 Hz, I H), 1.56 (br d, J=13.5 Hz,
1H), 1.31 (d, J=7.3 Hz, 3H), 1.13 (t,
N F J=7.0 Hz, 3H)
M/Z (ES+)
F Calc.: 511.26
Found: 512.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 4.6xI00mm 5um
36
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example # Structure ER-# Analytical Data
Retention Time: 10.52 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.734 min
NMR H
(400 MHz, CDC13) 8 7.07 (q, J=8.3, 14.8
Hz, I H), 6.77 (t, J=8.1 Hz, 1H), 6.41 (d,
J=2.3 Hz, 1 H), 6.3 8 (d, J=2.3 Hz, I H),
4.89 (d, J=8.5 Hz, 1 H), 4.78 (d, J=14.1
Hz, 1 H), 4.54 (d, J=14.4 Hz, I H), 4.10-
4.02 (m, 1 H), 3.77 (d, J=2.6 Hz, 6H),
3.68 (s, 2H), 3.29-3.21 (m, IH), 3.19-
Meo 3.10 (m, 1 H), 2.81-2.71 (m, 2H), 2.61 (q,
J=12.0, 23.1 Hz, 2H), 2.23 (s, 3H), 1.96-
0 1.83 (m, 2H), 1.65-1.55 (m, 2H), 1.30 (d,
q
/
Meo N~ / J=7.3 Hz, 3H), 1.10 (t, J=7.0 Hz, 3H)
16 NI /
Salt Free M/Z (ES+)
N F Cale.: 511.26
Found: 512.39 (M+H)
I\
F Analytical HPLC:
Method Al
SunFire MS C18 (4.6x]00mm) 5um
Retention Time: 10.45 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.799 min
NMR 'H
(400 MHz, CD3OD) 8 7.22 (d, J=7.0 Hz,
1H), 7.15-7.11 (m, 1H), 6.98 (t, J--9.2
Hz, 1 H), 6.48 (d, J=2.3 Hz, I H), 6.41 (d,
Meo J=2.6 Hz, I H), 5.05 (d, J=8.5 Hz, I H),
4.68 (d, J=14.4 Hz, IH), 4.57 (d, J=14.4
/ o Hz, I H), 4.16-4.09 (m, 1 H), 3.79 (s, 3 H),
N~j 3.77 (s, 3H), 3.66 (s, 2H), 3.28-3.20 (m,
Meo \` 2H), 2.87-2.79 (m, 2H), 2.72-2.63 (m,
NI/ 819935-00 2H), 2.33 (s, 3H), 2.06-1.94 (m, 2H),
17 Salt Free 1.69 (br d, J=13.5 Hz, IH), 1.56 (br d,
L N F J=13.5 Hz, IH), 1.32 (d, J=7.3 Hz, 3H),
1.14 (t, J=7.0 Hz, 314)
M/Z (ES+)
Cale.: 493.27
Found: 494.37 (M+H)
Analytical HPLC:
Method Al
37
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
SunFire MS CIS (4.6xl00mm) Sum
Retention Time: 10.48 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.585 min
NMR H ,
(400 MHz, CD3OD) S 7.23-7.21 (m, I H),
7.15-7.11 (m, I H), 7.98 (t, J=9.2 Hz,
I H), 6.47 (d, J=2.3 Hz, I H), 6.41 (d,
J=2.3 Hz, 114), 5.05 (d, J=8.8 Hz, 114),
4.68 (d, J=14.4 Hz, 1H), 4.57 (d, J=14.4
Hz, I H), 4.16-4.09 (m, I H), 3.79 (s, 3 H),
3.77 (s, 3H), 3.66 (s, 2H), 3.28-3.18 (m,
Meo 2H), 2.87-2.79 (m, 2H), 2.72-2.61 (m,
2H), 2.33 (s, 3H), 2.06-1.94 (m, 2H),
/ o 1.69 (br d, J=11.7 Hz, 1H), 1.56 (br d,
Meo N-~ J=13.8 Hz, 1H), 1.32 (d, J=7.3 Hz, 3H),
18 NI/ 819947-00 1.14 (t, J=7.0 Hz, 3H)
N F Salt Free M/Z (ES+)
Cale.: 493.27
Found: 494.41 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 10.52 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.695 min
NMR H
(400 MHz, CD3OD) S 7.09-7.07 (m, 1 H),
7.02-6.97 (m, I H), 6.88-6.84 (m, 1 H),
MeO 6.44 (d, J=2.3 Hz, IH), 6.38 (d, J=2.6
Hz, IH), S.10 (d, J=8.8 Hz, IH), 4.65 (d,
o J=14.4 Hz, I H), 4.55 (d, J=14.4 Hz, I H),
4.16-4.09 (m, 1H), 3.84 (s, 3H), 3.75 (d,
Meo
N/ J=9.1 Hz, 6H), 3.55 (s, 2H), 3.26-3.19
19 819936-00 (m, 2H), 2.81-2.72 (m, 2H), 2.63-2.527
Salt Free (m, 2H), 2.03-1.90 (m, 2H), 1.67 (br d,
N J= 13.5 Hz, I H), 1.54 (br d, J= 13.5 Hz,
OMe IH), 1.31 (d, J=7.3 Hz, 3H), 1.11 (t,
F J=7.0 Hz, 3H)
M/Z (ES+)
Cale.: 509.27
Found: 510.47 (M+H)
38
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example # Structure ER-# Analytical Data
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 9.71 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 6.902 min
NMR H
(400 MHz, CD3OD) S 7.13-7.10 (m, 1 H),
7.06-7.01 (m, 1H), 6.91-6.88 (m, 114),
6.48 (d, J=2.6 Hz, IH), 6.41 (d, J=2.6
Hz, I H), 5.13 (d, J=8.8 Hz, 1 H), 4.69 (d,
J=14.4 Hz, 1H), 4.58 (d, J=14.1 Hz, IH),
4.20-4.13 (m, 1H), 3.88 (s, 3H), 3.80 (s,
3H), 3.77 (s, 3H), 3.58 (s, 2H), 3.28-3.20
MeO (m, 2H), 2.85-2.75 (m, 2H), 2.67-2.56
(m, 2H), 2.06-1.93 (m, 2H), 1.71 (br d,
0 J=11.7 Hz, IH), 1.57 (br d, J=11.7 Hz,
Meo /i I/ 1H), 1.35 (d, J=7.0 Hz, 3H), 1.15 (t,
N 819948-00 J=7.0 Hz, 3H)
20 Salt Free
N M/Z (ES+)
We Cale.: 509.27
Found: 510.47 (M+H)
F
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 9.70 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 4.781 min
NMR H
(400 MHz, CD3OD) S 6.54 (d, J=2.1 Hz,
-_O 2H), 6.48 (d, J=2.3 Hz, 1H), 6.42-6.40
(m, 2H), 5.12 (d, J=8.5 Hz, I H), 4.68 (d,
J=14.1 Hz, IH), 4.58 (d, J=14.1 Hz, 1H),
4.20-4.12 (m, lH), 3.80 (s, 3H), 3.78 (d,
0, N
N J=0.9 Hz, 9H), 3.56 (s, 2H), 3.29-3.20
829893-00
21 (m, 2H), 2.85-2.77 (m, 2H), 2.67-2.56
Salt Free (m, 2H), 2.07-1.95 (m, 2H), 1.72-1.68
N(m, 1 H), 1.58-1.55 (m, 1 H), 1.34 (d,
(~ 0 J=7.3 Hz, 3H), 1.15 (t, J=7.0 Hz, 3H)
i
M/Z (ES+)
0"
Cale.: 521.29
Found: 522.39 (M+H)
39
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 10.22 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 8.084 min
NMR'H
(400 MHz, CDC13) S 6.49 (d, J=2.3 Hz,
2H), 6.42 (d, J=2.6 Hz, 1 H), 6.39 (d,
J=2.6 Hz, 1 H), 6.34 (t, J=2.2 Hz, 1 H),
5.00 (d, J=8.5 Hz, I H), 4.79 (d, J=14.4
Hz, 1 H), 4.56 (d, J=14.4 Hz, 1 H), 4.15-
4.08 (m, 1H), 3.78 (s, 6H), 3.77 (d, J=2.1
-- Hz, 6H), 3.49 (s, 2H), 3.33-3.24 (m, 1H),
3.24-3.15 (m, 1H), 2.77-2.67 (m, 2H),
2.55-2.46 (m, 2H), 1.99-1.85 (m, 2H),
1.66-1.57 (m, 2H), 1.33 (d, J=7.0 Hz,
3H), 1.14 (t, J=7.0 Hz, 3H)
22 819950-00
Salt Free MJZ (ES+)
N Calc.: 521.29
" Found: 522.38 (M+H)
Analytical HPLC:
" Method A ]
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 10.25 min
Chiral HPLC
Method C I
CHIRALPAK IA (0.46x25 cm)
Retention Time: 5.601 min
NMR H
(400 MHz, DMSO) S 12.85 (br s, 11-1),
7.24 (s, 21-1), 7.10 (s, I H), 6.40-6.42 (m,
Me 2H), 4.79-4.85 (m, 3H), 4.54-4.67 (m,
2H), 3.86-4.14 (m, 51-1), 3.80 (s, 314),
Meo " ~ 3.79 (s, 3H), 3.30-3.49 (m, 2H), 2.82-
3.14 (m, 4H), 2.37 (s, 6H), 1.80 (d,
820006-01 J=13.7 Hz, 1H), 1.76 (s, 3H), 1.68 (d,
23 14.2 Hz, 1 H), 1.34 (d, J=7.1 Hz, 3H)
N HCl Salt
M/Z (ES+)
Calc.: 515.31
Found: 516.42 (M+H)
Analytical HPLC:
Method A I
SunFire MS C18 4.6xl00mm 5um
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example # Structure ER-# Analytical Data
Retention Time. 12.04 min
Chiral HPLC
Method CI
CHIRALPAK IA (0.46x25 cm)
Retention Time: 7.576 min
NMR 1H
(400 MHz, DMSO) S 12.84 (br s, 1 H),
7.24 (s, 2H), 7.10 (s, 1 H), 6.41-6.42 (m,
2H), 4.79-4.85 (m, 3H), 4.54-4.67 (m,
2H), 3.86-4.14 (m, 5H), 3.80 (s, 3H),
3.79 (s, 3H), 3.29-3.46 (m, 2H), 2.82-
Me 3.14 (m, 4H), 2.36 (s, 6H), 1.80 (d,
J=13.9 Hz, IH), 1.76 (s, 3H), 1.69 (d,
"-~ 0 14.4 Hz, IH), 1.34 (d, J=7.1 Hz, 3H)
Me0
820007-01 M/Z(ES+)
24 HCl Salt Calc.: 515.31
N Found: 516.42 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) Sum
Retention Time: 12.02 min
Chiral HPLC
Method Cl
CHIRALPAK IA (0.46x25 cm)
Retention Time: 5.074 min
NMR H
(400 MHz, CD3OD) S 8.34 (s, I H), 6.46
(d, J=2.6 Hz, 114), 6.41 (d, J=2.3 Hz,
I H), 5.13 (d, J=8.8 Hz, 1 H), 4.69 (d,
J=14.4 Hz, 1H), 4.58 (d, J=14.4 Hz, IH),
4.21-4.14 (m, 1H), 3.78 (s, 3H), 3.76 (s,
0 3H), 3.58 (d, J=2.3 Hz, 2H), 3.30-3.16
(m, 2H), 2.93 (br d, J=12.0 Hz, I H), 2.84
0 (br d, J=11.1 Hz, I H), 2.79-2.65 (m, 2H),
--O N-~ 2.44 (s, 3H), 2.08-1.94 (m, 2H), 1.75 (br
N/ 819810-01 d, J=13.8 Hz, IH), 1.59 (br d, J=11.1 Hz,
25 HCl Salt IH), 1.34 (d, J=7.3 Hz, 3H), 1.14 (t,
N J=7.0 Hz, 3H)
N M/Z (ES+)
Calc.: 466.26
Found: 568.45 (M+H+101)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 8.00 min
41
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
NMR'H
(400 MHz, DMSO) S 7.82 (d, J=4.4
Hz, I H), 7.16 (d, J=4.7 Hz, 1 H), 6.49 (d,
J=2.3 Hz, 1 H), 6.3 9 (d, J=2.3 Hz, 1 H),
5.06(d,J=8.5Hz, IH),4.64(d,J=14.4
- 0 Hz, 1H),4.50(d,J=14.4 Hz, IH),4.10-
4.02 (m, I H), 3.76 (s, 3H), 3.74 (s, 3H),
0 3.31 (s, 2H), 3.17-3.07 (m, 2H), 2.76-
~0 N-~ 2.52 (m, 4H), 2.21 (s, 3H), 1.86-1.72 (m,
N--/ 811352-00 2H), I.56 (br d, J=11.1 Hz, I H), 1.40 (br
26 Salt Free d, J=12.3 Hz, IH), 1.28 (d, J=7.3 Hz,
3H), 1.03 (t, J=6.9 Hz, 3H)
N
N S M/Z (ES+)
Cale.: 521.25
N Found: 522.34 (M+H)
Analytical HPLC:
Method A I
SunFire MS Cl8 (4.6x100mm) 5um
Retention Time: 8.05 min
(400 MHz, CD3OD) 8 8.93-8.92 (m, 1H),
8.36-8.33 (m, 114), 7.91-7.86 (m, 2H),
7.61 (t, J=7.6 Hz, 1 H), 7.55-7.52 (m,
I H), 6.48 (d, 3=2.3 Hz, I H), 6.41 (d,
J=2.3 Hz, I H), 5.17 (d, J=8.5 Hz, I H),
-"0 4.68 (d, J=14.4 Hz, IH), 4.58 (d, J=14.4
Hz, I H), 4.32 (s, 2H), 4.20-4.12 (m, 1 H),
3.80 (s, 314), 3.77 (s, 3H), 3.30-3.19 (m,
0 2H), 2.99-2.86 (m, 2H), 2.86-2.74 (m,
N~ 2H), 2.10-1.97 (m, 2H), 1.69 (br d,
27 -,l 819955-01 J=13.5 Hz, IH), 1.56 (br d, J=1 1.7 Hz,
HCl Salt 1H), 1.34 (d, J=7.3 Hz, 3H), 1.13 (t,
N N J=7Ø Hz, 3H)
M/Z (ES+)
Cale.: 512.28
Found: 513.40 (M+H)
Analytical HPLC:
Method A I
SunFire MS CI8 (4.6xl00mm) 5um
Retention Time: 9.80 min
42
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
(500 MHz, CD3OD) S 7.60 (s, 1 H), 6.50
(d, J=2.3 Hz, 1 H), 6.43 (d, J=2.3 Hz,
I H), 5.22 (br s, 1 H), 4.71 (d, J=14.2 Hz,
1H), 4.64 (d, J=14.2 Hz, 1H), 4.30 (br s,
1 H), 4.25 (br s, 1 H), 3.83-3.77 (m, 9H),
3.57 (br s, 1 H), 3.44 (br s, 2H), 3.22 (br
s, 2H), 2.65 (s, 2H), 2.39 (s, 3H), 2.26-
1 O 2.13 (m, 2H), 2.00 (br d, J=11.4 Hz, 1 H),
~0 N- 819976-01 1.82 (br d, J=13.3 Hz, 1 H), 1.39 (d, J=6.9
28 N HCl Salt Hz, 3H), 1.17 (t, J=6.9 Hz, 3H)
N M/Z (ES+)
Calc.: 479.29
N'' Found: 480.45 (M+H)
N
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) Sum
Retention Time: 7.22 min
(400 MHz, CDCl3) S 6.60 (d, J=7.0 Hz,
2H), 6.45 (d, J=2.6 Hz, 114), 6.42 (d,
J=2.3 Hz, 1 H), 5.03 (d, J=8.8 Hz, 1 H),
-0 4.82 (d, J=14.1 Hz, 1 H), 4.59 (d, J=14.4
Hz, I H), 4.18-4.10 (m, l H), 3.90 (d,
J=0.9 Hz, 6H), 3.80 (d, J=2.9 Hz, 6H),
0 3.49 (s, 2H), 3.35-3.18 (m, 2H), 2.78-
2.67 N/ (m, 2H), 2.58-2.47 (m, 2H), 2.01-
824214-00 1.86 (m, 2H), 1.70-1.60 (m, 2H), 1.37 (d,
29 Salt Free J=7.3 Hz, 3H), 1.18 (t, J=7.0 Hz, 3H)
N
o~ M/Z (ES+)
Calc.: 539.28
F Found: 540.36 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 9.52 min
(400 MHz, DMSO) 5 9.26 (br s, l H),
7.90 (br s, 2H), 7.51 (br s, 1 H), 6.52 (d,
J=2.1 Hz, 1 H), 6.43 (d, J=2.3 Hz, I H),
4.97 (br s, 1 H), 4.66 (d, J=14.6 Hz, 1 H),
0 4.55 (d, J=14.1 Hz, 1 H), 4.19-4.11 (m,
'0 N--~ / 1 H), 3.79-3.72 (m, 6H), 3.47 (br s, 6H),
30 811305-01 3.21 (br d, J=5.9 Hz, 1H), 2.85 (br s,
HCl Salt 1H), 2.67 (s, 2H), 2.54 (s, 3H), 1.79 (br
d, J=13.5 Hz, 1H), 1.59 (br d, J=13.2 Hz,
N 1H), 1.36 (d, J=7.3 Hz, 3H), 1.01 (t,
N J=7.0 Hz, 3H)
M/Z (ES+)
Cale.: 515.29
43
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
Found: 516.43 (M+H)
Analytical HPLC:
Method A I
SunFire MS C 18 (4.6x 100mm) 5um
Retention Time: 8.23 min
O M/Z (ES+)
Cale.: 480.27
Found: 481.34 (M+H)
N 811283-01
31 HC1 Salt Analytical HPLC:
N Method Al
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 7.77 min
O
N~
(500 MHz, DMSO) 8 7.59 (d, J=6.9 Hz,
I H), 7.54 (d, J=7.3 Hz, I H), 7.29-7.25.
(m, I H), 7.23-7.20 (m, 1 H), 6.80 (s, 114),
6.48 (d, J=2.3 Hz, I H), 6.39 (d, J=2.3
Hz, I H), 4.99 (d, J=8.7 Hz, I H), 4.64 (d,
J=14.6 Hz, IH), 4.50 (d, J=14.2 Hz, 1H),
` 4.04-3.97 (m, IH), 3.76 (s, 3H), 3.75 (s,
3H), 3.73 (s, 2H), 3.20-3.09 (m, 2H),
2.84-2.59 (m, 4H), 1.92-1.82 (m, 2H),
` N,1 811308-01 1.58 (br d, J=13.3 Hz, 114), 1.44 (br d,
32 HCI Salt J=13.3 Hz, 1H), 1.24 (d, J=7.3 Hz, 3H),
1.03 (t, J=3.0 Hz, 3H)
N
M/Z (ES+)
I I Cale.: 501.26
Found: 603.38 (M+1-I+I01)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 10.12 min
(500 MHz, CD3OD) S 8.20 (d, J=9.2 Hz,
I H), 7.93 (d, J=6.4 Hz, 1 H), 7.82-7.79
(m, IH), 6.50 (d, J=2.3 Hz, IH), 6.43 (d,
J=2.3 Hz, 1H), 5.30 (br s, IH), 4.96 (br s,
2H), 4.71 (d, J=14.6 Hz, IH), 4.64 (d,
N,/ 811332-01 J=14.6 Hz, I H), 4.27 (br s, 1 H), 3.81 (s,
33 HC1 Salt 3H), 3.78 (s, 3H), 3.66 (br s, 4H), 3.19
HCl Salt
N (br s, 2H), 2.21 (br s, 2H), 1.99 (br s,
I H), 1.82 (br s, I H), 1.40 (br d, J=6.4
Hz,3H), 1.15(brs,3H)
S" N M/Z (ES+)
Cale.: 519.23
44
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-4 Analytical Data
Found: 621.40 (M+H+101)
Analytical HPLC:
Method Al
SunFire MS Cl8 (4.6x100mm) 5um
Retention Time: 10.32 min
(500 MHz, CD3OD) 6 7.42 (br s, I H),
_ 6.67 (br s, I H), 6.65 (s, IH), 6.50 (d,
J=2.3 Hz, 1H), 6.43 (d, J=2.7 Hz, 2H),
5.30 (br s, 11-1), 4.73-4.63 (m, 3H), 4.28
O (br s, 1H), 3.80 (s, 3H), 3.78 (s, 3H), 3.57
(br s, 3H), 3.20 (br s, 2H), 2.65 (s, 3H),
N 2.51 (s, 2H), 2.19 (br s, 2H), 2.01 (br s,
"-11
I H), 1.84 (br s, 1 H), 1.41 (br d, J=6.9
34 820017-01 Hz, 3H), 1.20-1.12 (m, 3H)
HCl Salt
N M/Z (ES+)
Cale.: 531.28
Found: 532.42 (M+H)
Ho
Analytical HPLC:
p~N Method Al
SunFire MS C18 (4.6xI00mm) 5um
Retention Time: 10.27 min
(400 MHz, CD3OD) S 8.22 (s, I H), 8.04-
8.03 (m, I H), 7.53-7.51 (m, 114), 6.49 (d,
J=2.6 Hz, I H), 6.42 (d, J=2.3 Hz, 114),
4.76-4.55 (m, 3H), 4.26-4.21 (m, IH),
-o 3.82-3.76 (m, 8H), 3.67 (br s, 2H), 3.59
(d, J=6.7 Hz, 2H), 3.29 (br s, 2H), 2.48-
\ 2.32 (m, 2H), 2.02 (br s, IH), 1.86 (br s,
IH), 1.38 (d, J=7.3 Hz, 3H), 1.17 (t,
36 0 "-1/ 811309-01 J=7.0 Hz, 3H)
HCI Salt
MIZ (ES+)
N
i Cale.: 507.23
" Found: 508.36 (M+H)
Analytical HPLC:
Method A I
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 6.95 min
~o (400 MHz, CDC13) S 6.72-6.70 (m, I H),
6.45 (d, J=2.3 Hz, I H), 6.42 (d, J=2.3
o Hz, IH), 6.09 (t, J=3.2 Hz, 1H), 6.05-
o " 6.04 (m, IH), 5.02 (d, J=8.8 Hz, IH),
37 ",,-,., 820008-00 4.82 (d, J=14.4 Hz, IH), 4.59 (d, J=14.4
N Salt Free Hz, IH), 4.25 (t, J=7.2 Hz, 2H), 4.20-
" 4.12 (m, IH), 3.80 (d, J=2.9 Hz, 6H),
" 3.52 (s, 2H), 3.31-3.22 (m, 1H), 3.22-
" 3.13 (m, I H), 2.94 (t, J=7.0 Hz, 21-1),
2.79-2.68 (m, 2H), 2.56-2.46 (m, 2H),
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example # Structure ER-# Analytical Data
1.91-1.77 (m, 2H), 1.73-1.62 (m, 2H),
1.37 (d, J=7.3 Hz, 3H), 1.17 (t, J=7.0 Hz,
3H)
M/Z (ES+)
Calc.: 503.29
Found: 504.39 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x] 00mm) 5um
Retention Time: 9.56 min
(400 MHz, CDC13) S 6.94 (s, 2H), 6.91
(s, 1 H), 6.60 (s, 1 H), 6.28 (s, 1 H), 5.05
Ho (d, J=8.3 Hz, 1 H), 4.81 (d, J=14.2 Hz,
1H), 4.59 (d, J=14.2 Hz, 1H), 4.11-4.03
(m, I H), 3.53 (s, 2H), 3.28-3.17 (m, 2H),
0 2.79-2.74 (m, 21T), 2.56-2.53 (m, 2H),
Holt N--~ 2.30 (s, 6H), 1.96-1.90 (m, 2H), 1.70-
Nom/ 1.59 (m, 2H), 1.40 (d, J=7.1 Hz, 3H),
38 819888-00 1.11 (t, J=6.8 Hz, 3H)
Salt Free
N M/Z (ES+)
Calc.: 461.27
Found: 462.37 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xlOOmm) 5um
Retention Time: 6.57 min
(400 MHz, CD3OD) S 7.71 (d, J=7.9 Hz,
2H), 7.58 (d, J=8.5 Hz, 4H), 7.36 (t,
J=7.6 Hz, 2H), 7.32-7.20 (m, 3H), 6.49
(d, J=2.3 Hz, I H), 6.42 (d, J=2.6 Hz,
1 H), 5.23 (d, J=8.8 Hz, 1 H), 4.71 (d,
-o J=14.4 Hz, 1H), 4.63 (d, J=14.4 Hz, 1H),
4.46 (s, 2H), 4.29-4.22 (m, 1H), 3.79 (s,
3H), 3.77 (s, 3H), 3.61-3.47 (m, 4H),
3.28-3.15 (m, 2H), 2.35-2.17 (m, 2H),
819814-01 1.99 (br d, J=13.8 Hz, 1H), 1.80 (br d,
39 HCl Salt J=14.1 Hz, 1H), 1.40 (d, J=7.3 Hz, 3H),
1.16 (t, J=7.0 Hz, 314)
M/Z (ES+)
Calc.: 563.31
Found: 564.36 (M+H)
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xlOOmm) 5um
Retention Time: 12.04 min
46
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example # Structure ER-# Analytical Data
(400 MHz, CD3OD) 8 7.46 (s, IH), 6.46
(d, J=2 Hz, 1H), 6.40 (d, J=2 Hz, 1H),
5.07 (d, J=7 Hz, 1H), 4.68 (d, J=14 Hz,
1 H), 4.56 (d, J=14 Hz, 1 H), 4.10-4.05
(m, 1 H), 3.80-3.72 (m, 9H), 3.43 (br s,
2H), 3.22-3.17 (m, 2H), 2.68-2.48 (m,
1 ~0 2H), 2.20 (s, 3H), 2.03-1.86 (m, 2H),
0 N 1.75-1.50 (m, 2H), 1.33 (d, J=7 Hz, 3H),
Nom/
40 819971-00 1.12 (t, J=6.9 Hz, 3H)
Salt Free
N M/Z (ES+)
Cale.: 479.29
/N- Found: 480.45 (M+H)
N
Analytical HPLC:
Method Al
SunFire MS C18 (4.6x100mm) 5um
Retention Time: 7.15 min
(400 MHz, CD3OD) S 6.45 (d, J=2 Hz,
1 H), 6.40 (d, J=2 Hz, 1 H), 5.95 (s, 1 H),
5.11 (d, J=7 Hz, 1H), 4.68 (d, J=14 Hz,
~0 1 H), 4.55 (d, J=14 Hz, 1 H), 4.22-4.10
(m, 1H), 3.80-3.69 (m, 9H), 3.60-6.49
(m, 2H), 3.30-3.12 (m, 2H), 2.84-2.49
0 \ N0 (m, 2H), 2.15 (s, 3H), 2.02-1.83 (m, 2H),
N--1 1.70-1.42 (m, 2H), 1.33 (d, J=7 Hz, 3H),
41 819973-00 1.10 (t, J=6.9 Hz, 3H)
Salt Free
N M/Z (ES+)
Cale.: 479.29
Found: 480.45(M+H)
/N-N
Analytical HPLC:
Method Al
SunFire MS C18 (4.6xl00mm) 5um
Retention Time: 7.98 min
Analytical methods:
Method Al
Solvent A: 0.2% Et3N in water
Solvent B: 0.2% Et3N in acetonitrile
Flow rate: 2.0 ml/min
Linear Gradient:
time
(min) I %A %B
0 70 30
2 70 30
9 5 95
14 5 95
47
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Method C1
Mobile Phase: 0.1% Et2NH in ethanol
Flow rate: 1.0 ml/min
Isocratic.
EXAMPLES 42-126
In Vitro Biological Activity
[0079] HEKT-bet-luc assay: This assay measures a T-bet dependent reporter
(luciferase)
activity in engineered HEK cells that express a human T-bet and a T-box
responsive element
driving luciferase reporter. HEKT-bet cells were plated at 2x104/well in 96-
well plate and
compound was added into cell culture for 24 hours. Luciferase activity was
measured by
adding 50 l of Steady-Glo reagent (Promega) and samples were read in Victor V
reader
(PerkinElmer). The activity of compound was determined by comparing compound
treated
samples to non-compound treated vehicle controls. The IC50 values were
calculated utilizing
a maximum value corresponding to the amount of luciferase in the absence of a
test
compound and a minimum value corresponding to a test compound value obtained
at
maximum inhibition.
[0080] Determination of Normalized HEKT-bet IC50 values: Compounds were
assayed in microtiter plates. Each plate included a reference compound which
was ER-
819544. The un-normalized IC50 value for a particular compound was divided by
the ICso
value determined for the reference compound in the same microtiter plate to
provide a
relative potency value. The relative potency value was then multiplied by the
established
potency of the reference compound to provide the normalized HEKT-bet IC50
value. In this
assay, the established potency for ER-819544 was 0.035 M. The IC50 values
provided
herein were obtained using this normalization method.
[0081] Exemplary compounds of the present invention were assayed according to
the
methods set forth above in the HEKT-bet-luc assay described above. Table 2
below set forth
exemplary compounds of the present invention having an IC50 of up to the
indicated amount
( M) as determined by the normalized HEKT-bet-luc assay described above.
48
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Table 2. IC50 Values of Exemplary Compounds
Example
Structure ER-Number C50 (pm)
MeO
Me0 N
42 ER-819762 0.04
N
MeO
Me0 N
N,/
43 ER-819763 0.55
N
MeO
Me0 N
/ N-/+OH
44 ER-819786 0.03
N
MeO
Me0 N
N-/-OH
45 ER-819787 0.17
N
49
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m
MeO
Meo N OH
1 __/OH
N
46 ER-819788 0.03
N
MeO
Meo N OH
N1 OH
47 ER-819789 0.17
N
MeO
1 , ~0
Meo N N
48 ~/ ER-819924 0.05
N
Meo
Meo N
49 N- ER-819925 0.32
N
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number IC50 ( m)
MeO
0
~
Me0 N
/ N/
50 ER-819926 0.04
N
N
N
MeO
,0
Me0 N --j
N,/
51 ER-819927 0.21
N
Y, N
N
MeO
~0
Me0 N
/ N,/
52 ER-819931 0.07
N
MeO
MeO , 0
N
53 N11 ER-819943 >10
N
51
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
MeO
Me0 N
54 ER-819933 1.15
N
\ O~F
F
/ F F
MeO
Me0 N
N,/
55 ER-819945 >10
N
\ O~F
F
/ F F
MeO
Me0 /
Nom/ ER-819934 0.10
56
N F
F
MeO
Me0 N
57 N,/ ER-819946 2.97
N F
F /
52
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
MeO
,R:
Me0
N,/
58 ER-819935 0.13
N F
MeO
~
Me0 N 0
N-/
59 ER-819947 2.6
N F
MeO
Me0 N
60 N,/ ER-819936 0.12
N
OMe
F
MeO
MeO N
61 N/ ER-819948 > 10
N
\ OMe
F
53
CA 02704454 2010-04-30
WO 2009/064274 PCTIUS2007/024011
Example
Structure ER-Number C50 ( m)
MeO
O
MeO N~JI--
62 ER-820006 0.06
N
MeO
Me0 N
63 N ER-820007 1.26
N
~O
O
O N
64 R-819810-01 0.012
N
N
O
54
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
--O
O
--O
N-,/
65 R-811352-02 0.013
N
N S
N
0
-,O N--'(
66 R-819955-01 0.020
N N
-0
O
N
67 R-819800-01 0.023
N
N
N
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 (pm)
-O
N-~( /
68 00. R-819976-01 0.023
N
N--'
N
-O
0
N
69 R-819953-01 0.026
N Nf
I IN
-O
O
N
N-,/
70 R-824214-00 0.029
N
F
O~
56
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
O
N
71 R-819973-01 0.030
N
/N --N
O
N-
N
00 72 R-811305-01 0.031
N
N
N
O
--O N
N-_/
73 R-819783-01 0.035
N
NH
57
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
xampl
Structure ER-Number C50 ( m)
-O
N O
74 R-819847-01 0.035
N
N
N-
N
--O
/O
~O N /
00.
75 R-811300-01 0.035
N
NH
-O
O
O N
76 Nom/ ER-811278-01 0.037
N
N
58
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
Nom(
N,/
77 R-819804-01 0.040
N
N
-O
N
78 R-811323-01 0.042
N
i
--o
O
N
79 R-811349-01 0.043
N
N--
59
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
N-`(
N-,/
80 R-819833-01 0.047
N
O N
O
81 R-819954-01 0.047
N
_O
82 R-819966-01 0.048
N
N
H
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
~O
O
-~O N
N,/
83 R-811283-01 0.053
N
O
N
O
84 R-819957-01 0.055
N
N
'J
N
O
O N--~(
\N ~~
85 R-811308-01 0.056
N
TO
61
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
-'O
O
O N-~
86 R-819837-01 0.056
N
N
-O
O
N-~
Noo
87 R-819832-01 0.057
N
N
-~O
O
O
N-,/
88 R-819826-01 0.067
N
/ \N
N~
H
62
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
-~O
O
O N
N-,/
89 R-819844-01 0.067
N
HN /
-O
O
N-
N
90 R-811332-01 0.067
N
N
N
N4 O
91 R-820004-01 0.067
N
I-- ri N O\
N
O\
63
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 (pm)
-O
N O
92 R-820017-01 0.069
N
nN/
jN
O
N-
N-,/
93 R-811297-01 0.074
N
N
NI
64
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 (pm
-~O
0
0 N-~
N-,/
"Oo 94 R-811317-01 0.074
N
~N
N\ /
-0
O
O N--~/(
N
95 R-811312-01 0.077
N
N
N
-0
0
~O N--
,
96 R-819958-01 0.079
N
N
N
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number IC50 ( m)
O
97 R-819842-01 0.081
N
S/>
N
-~O
O
~O N /
N
98 R-811365-01 0.084
N
S
--O
N O
N-,/
99 R-811284-01 0.088
N
N` \ N
66
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
N-~(
100 R-819820-01 0.090
N
H
N
N
N
-O
N
101 N-,/ R-819961-01 0.096
N
N~
---o O
O N-~
102 R-811306-01 0.10
N p
67
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m
O
N
N,/
103 R-811304-01 0.10
N
O
-~O
O
~~,,=/ Nom/
104 R-820009-00 0.11
N
N
N
O
_o N
105 R-811291-01 0.12
N 0
O
68
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
'O
O
O N
N-,/
106 R-819979-01 0.12
N
N
NC
O
N-
N---/
107 R-811292-01 0.13
N
N
N
O
O N
108 N---/ R-811309-01 0.13
N
N
69
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
O
--O N~
109 R-819985-01 0.13
N
N
N
-O
~.=' / Nom/
110 R-819965-01 0.13
N
H
N
N
-~O
O
O N-/
N,/
111 R-819808-01 0.14
CA 02704454 2010-04-30
WO 2009/064274 PCT/1JS2007/024011
Example
Structure ER-Number C50 ( m)
-0
O
N-~
/
Nv
112 R-820020-02 0.18
N
N
N~
N
0
0 N--~(
113 R-811346-02 0.20
N
- N
N////
1 /
F
-'0
O
0 N-
N
114 R-819780-01 0.20
N
H
71
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
-O
O
O N
N-,/
115 R-819981-01 0.20
N
Y--- N
O
---O
O
-~O /
Nom/
116 R-811279-01 0.21
N
S
NON
O
N /
/
N-,/
117 R-811358-01 0.22
N
N
72
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
-~O
O
-~O N-~
N-,/
118 R-819849-01 0.22
N
Li
F
C
F
N-~/
119 R-820008-00 0.24
N
N //
N
-~O
O
-~O N-/
N,/
120 R-811302-01 0.25
N N\
N
N
73
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 ( m)
-~O
N O
121 R-811301-01 0.26
N
S
N
-,O N-S(
\N
122 3R-811359-01 0.27
N
X
HO
O
H O N--Y
N-,/
123 R-819888-00 0.30
N
74
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
Example
Structure ER-Number C50 (ttm)
=O
O
O N
N-,l
124 R-819814-01 0.30
N
N--~(
N
125 R-819971-01 0.034
N
NI
EXAMPLE 126
In vivo Biological Activity: Active Immunization
Suppression of arthritis development in CIA. DBA1/J mice were immunized with
bCII/CFA at day 0 then boosted at day 21 with bCII/IFA. Arthritis development
was
monitored over the course of study. The arthritis score is as follows: 0 =
normal paw, score of
1 = 1-2 digit inflamed paws; score of 2 = 3 digits or 1-2 digit + wrist or
ankle inflamed, score
of 3 = hand + more than 2 digits inflamed; and score of 4 = multiple digits (3-
4) + important
wrist or ankle inflammation.
(A) Partial therapeutic evaluation of compounds. Active compound was given by
oral
dosing once daily at the dose indicated from day 20 after induction of
antibodies to collagen
CA 02704454 2010-04-30
WO 2009/064274 PCT/US2007/024011
II but before disease development. (B) Full therapeutic evaluation of
compound. Active
compound was given after disease was developed (from day 7 after the second
immunization). (C) X-ray analysis of mouse paws from full therapeutic CIA
study. X-ray
score is the index of measurement of combination of osteopenia, bone erosion
and new bone
formation. (D) Representative X-ray radiographs.
Data is given in Table 3 below. In general, these data compare favorably the
activity
of methotrexate in this model.
EXAMPLE 127
In vivo Biological Activity: Passive Immunization
Suppression of arthritis development in CAIA. BALB/c mice were injected i.v.
with I mg of anti-type II collagen antibody at day 0, and 3 days later 25 g
of LPS was
injected i.p. with active compound and methotrexate (MTX) was given once daily
PO from
day 0 to day 7. Arthritis score and body weight was monitored over the course
of study.
Data is given in Table 3 below. These data compare favorably to methotrexate,
which is not particularly active in this model.
Table 3.
Arthritis Model Approx ED50 (mg/kg)
Compound Active Immunization Passive Immunization
(CIA mouse) (CAIA mouse)
ER-819762-01 3 30-60
ER-819924-01 3-10 n.a.
ER-819973-01 3-10 n.a.
[00821 While we have described a number of embodiments of this invention, it
is
apparent that our basic examples may be altered to provide other embodiments
that utilize the
compounds and methods of this invention. Therefore, it will be appreciated
that the scope of
this invention is to be defined by the appended claims rather than by the
specific
embodiments that have been represented by way of example.
76