Note: Descriptions are shown in the official language in which they were submitted.
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
METHOD AND DEVICES FOR PRODUCING AIR SENSITIVE ELECTRODE
MATERIALS FOR LITHIUM ION BATTERY APPLICATIONS
FIELD OF THE INVENTION
The present invention is concerned with reaction chambers to be utilized for
the mass
production of air sensitive materials, especially for the synthesis of
electrode materials for
lithium batteries.
BACKGROUND OF THE INVENTION
Oxidation and reduction reactions are commonly utilized for the synthesis of
inorganic
crystalline materials. This is especially true for the synthesis of electrode
materials for Li-ion
batteries including cathode and anode materials. Conventionally, cathode
materials such as
lithium cobalt oxide, lithium nickel oxide, lithium manganese oxide and the
mixed oxides are
synthesized under oxidative environments. These materials are more readily
obtainable since
control of an oxidative heat treatment environment (e.g. heat treatment in
open air
environment) is not difficult. In contrast, a reductive environment is less
feasible since
control of a reductive heat treatment atmosphere is difficult. The difficulty
stems from the
fact that during the heat treatment steps of the synthesis, especially at
elevated temperatures
(e.g. >500 C), a slight leakage of air during the heat treatment would be
detrimental for the
reaction and therefore degrade the quality of the synthesized materials. The
difficulties in
controlling a reductive atmosphere make mass production unlikely or very
expensive. One
example is the synthesis of lithium iron phosphate that is conventionally
synthesized in a
reducing or inert atmosphere. A LiFePO4 type cathode material has been
discussed for
replacing LiCo02 for lithium ion battery applications because of the
potentially lower cost
(Fe replacing Co) and the safer operating characteristics of the material (no
decomposition of
the material during charging). However, processing issues such as high
temperature heat
treatment (>600 C) under an inert or reducing atmosphere makes the material
expensive and
CA 02705260 2012-11-19
68549-24
it is not widely accepted. Until the present, the maintenance of a reducing or
an inert
atmosphere at a high temperature was still a key factor limiting good control
of the quality of
the synthesized materials. To ensure a complete seal of the furnace,
especially when heat
treated at high temperatures, is very difficult.
Prior arts such as U.S. Patent Nos. 5,910,382, 6,723,470, 6,730,281,
6,815,122,
6,884,544, and 6,913,855, in general, teach methods and precursors utilized
for the formation
of stoichiometric LiFePO4, or the substitution of cations for iron. The above
mentioned
patents only show how the materials are synthesized. None of the prior art
teaches how to
control the heat treatment environment efficiently and cost effectively.
SUMMARY OF THE INVENTION
Some embodiments of the present invention may provide methods and devices
for controlling a heat treatment environment that can be widely applicable to
the synthesis of
materials to form electrode materials. Some embodiments of the invention may
also provide
methods and devices that are cost effective and insure good quality of the
synthesized
material.
According to one aspect of the present invention, there is provided a system
for
use in a synthesizing process for synthesizing precursors to form a
synthesized product at
elevated temperatures, comprising materials of the synthesizing process, a
furnace that is open
to air of the atmosphere, with gases within the furnace chamber consisting
essentially of gases
resulting from heating the materials of the synthesizing process and air of
the atmosphere
entering the furnace, a vessel, having at least one opening, for containing
the materials of the
synthesizing process, and a solid reductive material, wherein said materials
of the
synthesizing process are completely separated from the air of the atmosphere
within the
furnace by at least one of the vessel and the solid reductive material.
According to another aspect of the present invention, there is provided a
process for use in a synthesizing process for synthesizing precursors to form
a synthesized
product at elevated temperatures within a furnace absent a controlled
atmosphere, comprising
2
CA 02705260 2012-03-08
74445-88
providing a furnace that is open to air of the atmosphere, with gases within
the furnace
consisting essentially of gases resulting from heating the materials of the
synthesizing process
and air of the atmosphere entering the furnace, placing precursors into a
vessel, having at least
one opening, so as to have the precursors contained in the vessel, for the
synthesizing process,
placing a solid reductive material in combination with the vessel so that
materials of the
synthesizing process are separated from the air of the atmosphere of the
furnace by at least
one of the vessel and the solid reductive material, placing the contained
precursors in the
furnace, and heating the contained precursors to a synthesizing temperature to
form a
synthesized product.
One embodiment of the present invention may provide a unit, for use within a
furnace absent a controlled atmosphere, in a synthesizing process for
synthesizing precursors
to form a synthesized product at elevated temperatures. The unit has a vessel,
having at least
one opening, for containing materials of the synthesizing process, and a solid
reductive
material, wherein the materials of the synthesizing process are separated from
the atmosphere
of the furnace by either the vessel or the reductive material.
2a
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will become more readily apparent from the following description
of
preferred embodiments thereof shown, by way of example only, in the
accompanying
drawings, wherein:
Figs. 1(a) and 1(b) are illustrations of a first embodiment of the unit of the
invention;
Figs. 1(c) and 1(d) are illustrations of a second embodiment of the unit of
the
invention;
Fig. 1(e) is an illustration of a third embodiment of the unit of the
invention.
Fig. 2(a) is an illustration of units of the first and/or second embodiments
in a furnace
for carrying out a synthesizing process;
Fig. 2(b) is an illustration of units of the third embodiment in a furnace for
carrying
out a synthesizing process;
Fig. 3 is a graph of an x-ray diffraction pattern for a representative sample
of a
synthesized electrode material prepared using units of the invention;
Fig. 4 is a graph for showing battery test data for the same material as in
Fig. 3;
Fig. 5 is a graph of x-ray diffraction patterns for 5 similar synthesized
electrode
materials prepared using units of the invention; and
Fig. 6 is a graph for showing battery test data for 10 similar synthesized
electrode
materials prepared using units of the invention.
DETAILED DESCRIPTION OF THE INVENTION
Figs. 1(a) - 1(e) show schematic diagrams of individually sealed units (ISU)
containing
materials that are subjected to the synthesizing heat treatments. Designs of
furnaces that
contain the ISUs of different geometries are shown in Figs. 2(a) and 2(b).
In Figs. 1(a) and 1(b) the ISU 1 is a vessel having one end 2 completely
sealed while the
other end 3 is open to the atmosphere. Precursors to be synthesized to form an
electrode
3
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
material are contained at 4. The precursors, intermediate products, and
resulting material of
the synthesizing process are referred to as materials of the synthesizing
process throughout
the description. The materials of the synthesizing process, contained at 4,
are protected
from the atmosphere of the filrnace, into which ISUs are placed for heating,
by either the
material of the vessel 1, or a solid reductive material layer 5 that limits
the permeation of air
from the furnace atmosphere. It should be mentioned that since the reductive
material (e.g.
carbon black) is usually porous, the porosity of the reductive material layer
would allow the
permeation of any gas by-product released from the material being synthesized,
to the
atmosphere. In general, either the gas by-product or the oxidation of the
reductive material
would generate gas and keep the pressure within the ISU positive, compared to
the
atmosphere. However, if the material being synthesized does not generate gas
as a by-product,
a decrease of the porosity of the reductive material layer (by means of
tapping, for example)
would ensure separation from the atmosphere.
In Figs. 1(c) and 1(d) each ISU of a second embodiment is a vessel 1 having
both ends 6
open to the environment. Precursors to be synthesized to form an electrode
material are
contained at 4. The materials of the synthesizing process, contained at 4, are
protected from
the atmosphere of the furnace, into which ISUs are placed for heating, by
solid reductive
material layers 5 that limit the permeation of air from the furnace
atmosphere. As
mentioned above, the solid reductive material is usually porous to allow
permeation of any
gases resulting from the synthesizing process.
In both of the embodiments, a divider 11 can be used to separate the reductive
material 5
from the material 4 of the synthesizing process. The divider preferably is
inert to the
materials being separated and porous to any gases being generated. Also, as
shown in Figs.
1(a) - 1(d), at 7, a high-temperature durable glass fiber packing can be used
to hold all of the
materials in the vessels.
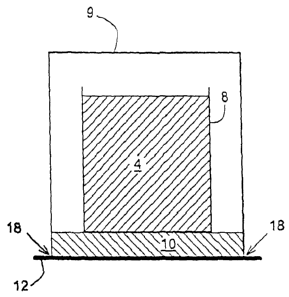
Similar characteristics can be observed in a third embodiment of an ISU shown
in Fig.
4
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
1(e). From Fig. 1(e), it can be seen that the materials to be synthesized 4
are contained in a
crucible 8. The path of airflow from any open side of a vessel 9 is controlled
by the
presence of reductive material 10. A bottom of the crucible separates the
reductive material
from the materials of the synthesizing process. A tray 12 facilitates handling
of the unit.
Vessel 9 is not sealed tightly against tray 12 in order that gases can flow
freely to or from the
reductive material, as shown at 18.
Figs. 2(a) and 2(b) show the various embodiments of the invention as utilized
in a
furnace to carry out the synthesizing process.
In Fig. 2(a) first embodiments and/or second embodiments are shown in furnace
13.
Heating elements of the furnace are shown at 14.
In Fig. 2(b) four units of the third embodiment of the invention are shown at
15 in
furnace 16. Heating elements of the furnace are shown at 17. As mentioned
above, the
furnaces are not required to be sealed and a controlled inert or reducing
environment is not
necessary.
The common structures of the ISUs are as follows:
a. An ISU includes a space that contains the materials being subjected to the
synthesizing heat treatment;
b. An ISU includes a space that contains the reductive material;
= c. The reductive material is placed in the vessel in a manner as:
Uncontrolled atmosphere/reductive material/synthesized material (Figs. 1(a)
and
1(b)), or
uncontrolled atmosphere/reductive material/synthesized material/ reductive
materiaVuncontrolled atmosphere (Figs. 1(c) and 1(d));
d. The reductive material can be placed on top of the synthesized material as
shown in
Figs. 1(a) -1(d) or somewhere else in contact with the outer atmosphere as
shown in
Fig. 1 (e);
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
e. The ISU can dissipate gas generated by the synthesizing reaction.
In the embodiments of Figs. 1(b) and 1(d) the flow of gases is from the
materials of
the synthesizing process, through the reductive material to the uncontrolled
atmosphere, or
the reverse of same.
In the embodiments of Figs. 1(a) and 1(c) the flow of gases is from the
materials of
. the synthesizing process, through the separator, through the reductive
material to the
uncontrolled atmosphere, or the reverse of the same.
In the embodiment of Fig. 1(e) the flow of gases is from the materials of the
synthesizing process, through the separation between the crucible and the
vessel, through the
reductive material to the uncontrolled atmosphere, or the reverse of same.
Other advantages provided by the utilization of ISUs include:
A. No need for an inert atmosphere in the furnace, thus resulting in:
i. Easy scale up for production;
ii. Much lower cost of a furnace since a gas-tight furnace becomes
unnecessary;
iii. The cost of inert gas can be saved;
iv. Overall cost of the synthesis protocol is reduced; and
v. Easy control of the quality of the resultant synthesized materials.
Since one ISU can be considered as one furnace.
B. Good performance of the synthesized material as demonstrated in the
following examples.
C. Consistency in performance of the synthesized material, which is
extremely
important for battery applications.
Owing to the advantage of the controlled heat treatment environment provided
by the
ISUs, materials that require heat treatment under an inert atmosphere can be
obtained easily
and cost efficiently. Following are examples of materials synthesized in an
ISU of the
6
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
invention, in order to better describe use of the invention.
EXAMPLE 1. Synthesis of LiFePO4 using methods and devices of the invention
In order to demonstrate the novelty of the ISUs disclosed in the present
patent
application, the synthesis of conventional LiFePO4 in bulk quantity is used.
12kg (75 moles)
of Fe203 and 5.55kg (75 moles) of Li2CO3 and 1.8kg (150 moles) of Super P
(carbon black,
available from MMM Carbon, Belgium), molar ratio of (1:1:2), were mixed
together with the
addition of a suitable amount of water to form a paste. After mixing
thoroughly, the proper
stoichiometric amount of phosphoric acid was added and extended mixing was
utilized (6
hours). Finally, the slurry was dried in air at 150 C for 10 hours, followed
by further heat
treatment at 400 C for 10 hours until chunks of materials were obtained. The
as-prepared
material was then subjected to grinding and ball milling for about 12 hours.
The ground
powdery materials was then loaded into several ISUs as shown in Fig. 1(a) with
the addition
of a carbonaceous material placed directly on top of the ground powdery
material for heat
treatment. In practice, the carbonaceous material can be placed directly on
top of the
synthesized material or separated by a thin layer of porous glass fiber
fabrics or other inert
plate. The ISUs were then placed in a furnace as shown in Fig. 2(a).
The heat treatment was conducted at 650 C for 24 hours resulting in the
synthesized
material. After the heat treatment step, slight grinding and sieving were
conducted on the
synthesized material. The post-heat treated materials were then ready for
further tests, as will
be described below.
The utilization of ISUs is not limited to the synthesis of lithium iron
phosphate, or
limited to the choice of starting materials and precursor processing steps
described for the
synthesis of lithium iron phosphate of the present example.
X-ray diffraction pattern data of the synthesized material is shown in Fig. 3.
It is
observed that phase pure material was obtained using the processing 'methods
and devices
7 '
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
presented in this example, without the use and control of an inert gas, such
as nitrogen or
argon. Battery test data (obtained using a three electrode design test battery
and lithium is
utilized as the reference electrode) are shown in Fig. 4. From Fig. 4 it can
be seen that the
capacity is high during the first charge-discharge cycle (¨C/5 rate,
0.23mA/cm2). The
material synthesized in the present case is comparable or superior to the
prior art material
disclosed in U.S. Patent No. 6,723,470, which was obtained using an inert
atmosphere as a
heat treatment environment.
EXAMPLE 2. Demonstration of consistently the synthesized LiFePO4 using methods
and
devices of the invention
In the present example, ten batches of materials synthesized using the ISUs
shown in Fig. 1(a)
were tested for quality consistency. The precursor processing procedures for
each batch
were the same as the procedures described in example 1. The ten different
batches were
subjected to 10 identical heat treatment procedures in ISUs. From the ten
batches, five
batches were subjected to the x-ray diffraction= pattern analyses and the
results are shown in
Fig. 5. Also, a stack of the 1st cycle data for each batch is shown in Fig. 6.
More accurate
numerical data is provided in Table 1. From Fig. 5 it can be seen that all of
the materials are
phase pure in nature. The peak intensity and peak positions for each sample
are similar, as
shown and indicated in Fig. 5. In Fig. 6, the 1st charge and discharge plot
for each sample is
again very similar. The 1st charge capacity ranges from 132-137mAh/g and the
1st
discharge capacity ranges from 118-124mAh/g. All these data suggest that the
consistency
of the materials synthesized using the ISUs is insured.
=
8
CA 02705260 2010-05-07
WO 2009/064265 PCT/US2007/023784
Table 1. The detailed electrochemical data of the ten batches heat treated
using the ISUs.
I Batch Names lst charge lst discharge 1st charge 15t
discharge 1st cycle
capacity capacity average average Coulomb
(mAh/g) (mAh/g) voltage (V) voltage (V) efficiency
AE11021 133.97 118.69 3.5083 3.3800 0.8859
AE11031 132.15 118.64 3.5070 3.3805 0.8978
AE11041 137.30 124.11 3.5016 3.3845 0.9039
AE11051 135.29 118.60 3.5088 3.3778 0.8766
AE11061 133.03 119.06 3.5066 3.3810 0.8950
AE11121 132.14 118.75 3.5071 3.3608 0.8987
AE11131 133.19 120.19 3.5083 3.3791 0.9024
AE11141 135.69 122.59 3.5189 3.3794 0.9035
AE11151 136.43 122.55 3.5109 3.3776 0.8983
AE11161 134.71 120.52 3.5090 3.3778 0.8947
The devices of the present invention provide the following advantages. There
is no need for
the use of an inert gas in the furnace, such as nitrogen or argon, or forming
gas (nitrogen plus
hydrogen), thus a completely sealed furnace is not required. The ISUs are semi-
open to the
atmosphere of the furnace, thus sealing of the ISUs is not difficult. There is
a short thermal
diffusion distances from the heat source to the material being synthesized.
With use of the
reductive material, such as carbon black or carbonaceous materials for air
permeation
prevention, even if a small amount of air permeation occurs during heat
treatment, oxidation
of the carbonaceous material prevents further oxidation of the material being
synthesized.
The reductive material can be porous so to allow the dissipation of gas
produced by the
9
CA 02705260 2010-05-07
WO 2009/064265
PCT/US2007/023784
materials that are subjected to the heat treatment. The depth of the ISUs
shown in Fig. 1(a)
and 1(b) are adjustable for the prevention of oxidation, for example a longer
depth would
give a better-isolated environment. Also, the geometry of the ISUs is flexible
to
accommodate the design of the furnaces, such as shown in Figs. 2(a) and 2(b).
While specific materials, dimensional data, etc. have been set forth for
purposes of
describing embodiments of the invention, various modifications can be resorted
to, in light of
the above teachings, without departing from applicant's novel contributions;
therefore in
determining the scope of the present invention, reference shall be made to the
appended
claims.
=