Note: Descriptions are shown in the official language in which they were submitted.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
Immunoglobulin aggregates
The current invention is in the field of protein concentration, to be more
precise it
relates to the use of tangential flow filtration (TFF) for immunoglobulin
concentration and immunoglobulin aggregate removal.
Background of the Invention
Proteins and especially immunoglobulins play an important role in today's
medical
portfolio. Expression systems for the production of recombinant polypeptides
are
well-known in the state of the art and are described by, e.g., Marino, M.H.,
Biopharm. 2 (1989) 18-33; Goeddel, D.V., et al., Methods Enzymol. 185 (1990) 3-
7;
Wurm, F., and Bernard, A., Curr. Opin. Biotechnol. 10 (1999) 156-159.
Polypeptides for use in pharmaceutical applications are mainly produced in
mammalian cells such as CHO cells, NSO cells, Sp2/0 cells, COS cells, HEK
cells,
BHK cells, PER.C6 cells, and the like.
For human application every pharmaceutical substance has to meet distinct
criteria.
To ensure the safety of biopharmaceutical agents to humans, for example,
nucleic
acids, viruses, and host cell proteins, which would cause severe harm, have to
be
removed. To meet the regulatory specification one or more purification steps
have
to follow the manufacturing process. Among other, purity, throughput, and
yield
play an important role in determining an appropriate purification process.
Due to their chemical and physical properties, such as molecular weight and
domain architecture, including secondary modifications, the downstream
processing of immunoglobulins is very complicated. For example, concentrated
solutions are required not only for formulated drugs but also for
intermediates in
downstream processing (DSP) to achieve low volumes for economic handling and
application storage. Furthermore, fast concentration processes are favored to
ensure smooth processes and short operating times. In this context imperfect
tangential flow filtration (TFF) processes, especially after final
purification steps,
can cause sustained damage even affecting the final drug product. The
correlation
between shear stress and aggregation in tangential flow concentration
processes for
monoclonal antibody (mAb) intermediate solutions was investigated by Ahrer,
K.,
et al., J. Membr. Sci. 274 (2006) 108-115. The influence on scalable process
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-2-
performance by selecting defined flow and pressure parameters was monitored
(see
e.g. Dosmar, M., et al., Bioprocess Int. 3 (2005) 40-50; Luo, R., et al.,
Bioprocess
Int. 4 (2006) 44-46). Mahler, H.-C., et al., (Eur. J. Pharmaceut.
Biopharmaceut. 59
(2005) 407-417) reported the induction and analysis of aggregates in a liquid
IgGI-antibody formulation formed by different agitation stress methods. In
US 6,252,055 a concentrated monoclonal antibody preparation is reported. A
method for producing a concentrated antibody preparation is reported in
US 2006/0182740. A combined process including an ultrafiltration, a
diafiltration,
and a second ultrafiltration sequence is reported in US 2006/0051347. In
EP 0 907 378 is reported a process for concentrating an antibody preparation
using
a cross-flow ultrafiltration with a fixed recirculation rate of 250 ml/min.
Summary of the Invention
One aspect of the current invention is a method for obtaining a concentrated
immunoglobulin solution by tangential flow filtration, characterized in that
said
method comprises the following steps:
i) providing a solution containing an immunoglobulin to be
concentrated wherein said immunoglobulin is present in said
solution in monomeric and polymeric form, whereby the fraction of
the polymeric soluble immunoglobulin form present in said
provided solution has a first value of more than 2.5 % when
determined by size exclusion chromatography,
ii) concentrating said solution provided under i) by employing a
tangential flow filtration,
iii) removing said polymeric immunoglobulin by filtration after the end
of the tangential flow filtration and thereby obtaining a concentrated
immunoglobulin solution,
whereby the fraction of said polymeric soluble form of said immunoglobulin is
after step ii) larger than said first value and smaller than a second value
which is
1.25 times the first value.
Another aspect of the current invention is a method for obtaining a
concentrated
immunoglobulin solution by tangential flow filtration, characterized in that
said
method comprises the following steps:
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-3-
i) providing a solution containing an immunoglobulin to be
concentrated wherein said immunoglobulin is present in said
solution in monomeric and polymeric form, whereby the fraction of
the polymeric soluble immunoglobulin form present in said
provided solution is more than 2.5 % when determined by size
exclusion chromatography with a first number of particles with a size
of more than 1 pm in said solution determined by light obscuration,
ii) concentrating said solution provided under i) by employing a
tangential flow filtration with a constant Ap of 3.0 bar thereby
obtaining a concentrated immunoglobulin solution with a second
number of particles with a size of more than 1 pm in said solution
determined by light obscuration,
whereby said second number of particles with a size of more than 1 m is less
than
200 times said first number of particles with a size of more than 1 m.
A further aspect of the current invention is a method for producing a
heterologous
immunoglobulin comprising the following steps:
a) providing a recombinant mammalian cell comprising one or more
nucleic acids encoding a heterologous immunoglobulin,
b) cultivating said cell under conditions suitable for the expression of
the heterologous immunoglobulin,
c) recovering the heterologous immunoglobulin from the recombinant
mammalian cell or the culture medium,
d) concentrating the obtained aqueous, buffered solution comprising
the heterologous immunoglobulin using a tangential flow filtration
method with a constant Ap of 3.0 bar, whereby the number of
particles in said concentrated solution with a size of more than 1 m
is smaller than 200 times the number of particles with a size of more
than 1 pm in the solution prior to the concentrating when
determining the number of particles by light obscuration.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-4-
Still a further aspect of the current invention is a method for removing
immunoglobulin aggregates from an immunoglobulin solution, characterized in
that said method comprises the following steps:
i) providing a solution containing said immunoglobulin to be
concentrated, wherein said immunoglobulin is present in said
solution in monomeric and polymeric form, or
providing a solution containing an immunoglobulin to be
concentrated, wherein in said solution the formation of said
immunoglobulin in polymeric form is induced by the application of
heat,
ii) concentrating said solution provided under i) by employing a
tangential flow filtration with a constant Ap of 3.0 bar, and a
constant transmembrane pressure of 0.6 bar,
iii) removing immunoglobulin aggregates from the immunoglobulin
solution obtained in step ii) by filtration with a 0.2 m pore size
filter,
whereby the number of particles in said concentrated solution of step ii) with
a size
of more than 1 pm is smaller than 200 times the number of particles with a
size of
more than 1 pm in the solution prior to the concentrating when determining the
number of particles by light obscuration.
A final aspect of the current invention is a method for the reduction of the
formation of immunoglobulin in polymeric soluble form during a concentration
step with tangential flow filtration, wherein the reduction is achieved by the
addition, supplementation, or generation of immunoglobulin in polymeric
soluble
form prior to the start of the concentration step.
In one embodiment of the method according to the invention the number of
particles in said concentrated solution of with a size of more than 5 m is
smaller
than 100 times the number of particles with a size of more than 5 m in the
solution prior to the concentrating when determining the number of particles
by
light obscuration. In a further embodiment the fraction of said polymeric
soluble
immunoglobulin form is more than 5 % when determined as the area under the
curve of the peaks eluted prior to the peak of the monomeric immunoglobulin in
a
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-5-
size exclusion chromatogram of said solution. In another embodiment comprises
the method according to the invention prior to or after step d) the following
step:
e) purifying the aqueous, buffered solution containing the heterologous
immunoglobulin.
In one embodiment the heterologous immunoglobulin is a complete
immunoglobulin, or an immunoglobulin fragment, or an immunoglobulin
conjugate. In a further embodiment the mammalian cell is a CHO cell, a BHK
cell,
or a PER.C6 cell. In another embodiment the determining by light obscuration
is
at a protein concentration of 90 mg/ml. In one embodiment the fraction of said
polymeric soluble form of said immunoglobulin is larger than said first value
and
smaller than a second value which is 1.10 times the first value. In one
embodiment
one aspect of the invention is a method for obtaining a concentrated
immunoglobulin solution free of insoluble immunoglobulin aggregates by
tangential flow filtration and said concentrated immunoglobulin solution is
free of
insoluble immunoglobulin aggregates. In another embodiment said method is for
obtaining a concentrated immunoglobulin solution free of insoluble
immunoglobulin aggregates and soluble immunoglobulin aggregates of more than
5 m by tangential flow filtration, in which said concentrated immunoglobulin
solution is free of insoluble immunoglobulin aggregates and free of soluble
immunoglobulin aggregates of more than 5 pm and said removing said polymeric
immunoglobulin is by filtration with a filter with 1.0 m pore size of less,
in one
embodiment of 0.2 m pore size. In a further embodiment the concentration of
the
immunoglobulin after the concentrating is more than 80 mg/ml, in another
embodiment more than 90 mg/ml, in still another embodiment more than 100
mg/ml. In one embodiment the concentration of the immunoglobulin after the
concentrating is less than 275 mg/ml, or less than 180 mg/ml, or less than 130
mg/ml. In another embodiment said filtrating the concentrated immunoglobulin
solution is with a filter with a pore size of 1 m or less in order to remove
the
immunoglobulin in soluble aggregated form of more than 5 m and to remove the
immunoglobulin in insoluble aggregated form. In one embodiment said polymeric
immunoglobulin form is soluble aggregated and insoluble aggregated
immunoglobulin. In one embodiment said immunoglobulin in polymeric form is a
soluble aggregated immunoglobulin form. In one embodiment the fraction of the
polymeric soluble immunoglobulin form is less than 25 %, in another embodiment
less than 15 %, and in still another embodiment less than 10 %.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-6-
Detailed Description of the Invention
The current invention provides a method for obtaining a concentrated
immunoglobulin solution free of immunoglobulin aggregates by tangential flow
filtration comprising the following steps:
i) providing a solution containing an immunoglobulin to be concentrated
wherein said immunoglobulin is present in said solution in monomeric
and polymeric form, whereby the fraction of said soluble polymeric
form is more than 2.5 % when determined as the area under the curve
of the peaks eluted prior to the peak of the monomeric
immunoglobulin in a size exclusion chromatogram of said provided
solution, i.e. of the soluble high molecular weight (HMW) forms,
ii) concentrating said solution provided under i) by employing a tangential
flow filtration, whereby during the tangential flow filtration the number
of immunoglobulin molecules contained in the immunoglobulin in
polymeric form increases, preferably until insoluble particles are
formed,
iii) removing said insoluble, immunoglobulin in polymeric form by a
filtration step after the end of the tangential flow filtration and thereby
obtaining a concentrated immunoglobulin solution free of
immunoglobulin aggregates.
An anti-IL-1R antibody (see WO 2005/023872) was available in sufficient
quantities
in our laboratories at the time of the invention and, therefore, the current
invention
is exemplified with this immunoglobulin. The invention is likewise in general
practicable with other immunoglobulins. This exemplified description is done
only
by way of example and not by way of limitation of the invention. These
examples
are provided to aid the understanding of the present invention, the true scope
of
which is set forth in the appended claims.
The terms "tangential flow filtration" or "TFF", which are used
interchangeably
within the current invention, denote a filtration process wherein a solution
containing a polypeptide to be concentrated flows along, i.e. tangential, to
the
surface of a filtration membrane. The filtration membrane has a pore size with
a
certain cut off value. In one embodiment the cut off value is in the range of
from
20 kDa to 50 kDa, in another embodiment of 30 kDa. This filtration process is
a
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-7-
kind of an ultrafiltration process. The term "cross-flow" denotes the flow of
the
solution to be concentrated tangential to the membrane (retentate flow).
The terms "transmembrane pressure" or "TMP", which are used interchangeably
within the current invention, denote the pressure which is applied to drive
the
solvent and components smaller than the cut-off value of the filtration
membrane
through the pores of the filtration membrane. In one embodiment the
transmembrane pressure in the methods according to the current invention is
0.6 bar. The transmembrane pressure is an average pressure of the inlet,
outlet and
permeate and can be calculated as:
TMP = (Pm + Paõr
2 - P permeate
The term "immunoglobulin" refers to a protein consisting of one or more
polypeptide(s) substantially encoded by immunoglobulin genes. The recognized
immunoglobulin genes include the different constant region genes as well as
the
myriad immunoglobulin variable region genes. Immunoglobulins may exist in a
variety of formats, including, for example, Fv, Fab, and F(ab)2 as well as
single
chains (scFv) or diabodies (e.g. Huston, J.S., et al., Proc. Natl. Acad. Sci.
USA 85
(1988) 5879-5883; Bird, R.E., et al., Science 242 (1988) 423-426; in general,
Hood,
L.E., et al., Immunology, The Benjamin N.Y., 2nd edition (1984); and
Hunkapiller,
T., and Hood, L., Nature 323 (1986) 15-16).
The term "complete immunoglobulin" denotes an immunoglobulin which
comprises two so called light immunoglobulin chain polypeptides (light chains)
and two so called heavy immunoglobulin chain polypeptides (heavy chains). Each
of the heavy and light immunoglobulin chain polypeptides of a complete
immunoglobulin contains a variable domain (variable region) (generally the
amino
terminal portion of the polypeptide chain) comprising binding regions that are
able
to interact with an antigen. Each of the heavy and light immunoglobulin chain
polypeptides of a complete immunoglobulin comprises a constant region
(generally
the carboxyl terminal portion). The constant region of the heavy chain
mediates the
binding of the antibody i) to cells bearing a Fc gamma receptor (Fc'R), such
as
phagocytic cells, or ii) to cells bearing the neonatal Fc receptor (FcRn) also
known
as Brambell receptor. It also mediates the binding to some factors including
factors
of the classical complement system such as component (Clq). The variable
domain
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-8-
of an immunoglobulin's light or heavy chain in turn comprises different
segments,
i.e. four framework regions (FR) and three hypervariable regions (CDR).
The term "immunoglobulin fragment" denotes a polypeptide comprising at least
one domain selected from the variable domain, the CH1 domain, the hinge-
region,
the CH2 domain, the CH3 domain, or the CH4 domain of a heavy chain, or the
variable domain or the CL domain of a light chain. Also enclosed are
derivatives and
variants thereof. For example, a variable domain, in which one or more amino
acids
or amino acid regions are deleted, may be present.
The term "immunoglobulin conjugate" denotes a polypeptide comprising at least
one domain of an immunoglobulin heavy or light chain conjugated via a peptide
bond to a further polypeptide. The further polypeptide is a non-immunoglobulin
peptide, such as a hormone, or growth receptor, or antifusogenic peptide, or
complement factor, or the like. In one embodiment said immunoglobulin
conjugate contains an immunoglobulin molecule covalently linked to two or four
non-immunoglobulin polypeptides.
General chromatographic methods and their use are known to a person skilled in
the art. See for example, Chromatography, 5th edition, Heftmann, E. (ed.),
Part A:
Fundamentals and Techniques, Elsevier Science Publishing Company, New York,
(1992); Deyl, Z. (ed.), Advanced Chromatographic and Electromigration Methods
in Biosciences, Elsevier Science By, Amsterdam, The Netherlands, (1998);
Poole, C.
F., and Poole, S. K., Chromatography Today, Elsevier Science Publishing
Company,
New York, (1991); Scopes, R.K., Protein Purification: Principles and Practice,
Springer Verlag, New York, (1982); Sambrook, J., et al., (ed.), Molecular
Cloning: A
Laboratory Manual, second edition, Cold Spring Harbor Laboratory Press, Cold
Spring Harbor, N.Y., (1989); or Current Protocols in Molecular Biology,
Ausubel,
F. M., et al., (eds), John Wiley & Sons, Inc., New York.
For the purification of recombinantly produced heterologous immunoglobulins
often a combination of different column chromatography steps is employed.
Generally a protein A affinity chromatography is followed by one or two
additional
separation steps. The final purification step is a so called "polishing step"
for the
removal of trace impurities and contaminants like aggregated immunoglobulins,
residual HCP (host cell protein), DNA (host cell nucleic acid), viruses, or
endotoxins. For this polishing step often an anion exchange material in a flow-
through mode is used.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-9-
Different methods are well established and widespread used for protein
recovery
and purification, such as affinity chromatography with microbial proteins
(e.g.
protein A or protein G affinity chromatography), ion exchange chromatography
(e.g. cation exchange (carboxymethyl resins), anion exchange (amino ethyl
resins)
and mixed-mode exchange), thiophilic adsorption (e.g. with beta-
mercaptoethanol
and other SH ligands), hydrophobic interaction or aromatic adsorption
chromatography (e.g. with phenyl-sepharose, aza-arenophilic resins, or
m-aminophenylboronic acid), metal chelate affinity chromatography (e.g. with
Ni(II)- and Cu(II)-affinity material), size exclusion chromatography, and
electrophoretical methods (such as gel electrophoresis, capillary
electrophoresis)
(Vijayalakshmi, M.A., Appl. Biochem. Biotech. 75 (1998) 93-102).
The term õheterologous immunoglobulin" denotes an immunoglobulin which is
not naturally produced by a mammalian cell. The immunoglobulin produced
according to the method of the invention is produced by recombinant means.
Such
methods are widely known in the state of the art and comprise protein
expression
in eukaryotic cells with subsequent recovery and isolation of the heterologous
immunoglobulin, and usually purification to a pharmaceutically acceptable
purity.
For the production, i.e. expression, of an immunoglobulin a nucleic acid
encoding
the light chain and a nucleic acid encoding the heavy chain are inserted each
into an
expression cassette by standard methods. Nucleic acids encoding immunoglobulin
light and heavy chains are readily isolated and sequenced using conventional
procedures. Hybridoma cells can serve as a source of such nucleic acids. The
expression cassettes may be inserted into an expression plasmid(s), which is
(are)
then transfected into a cell, which does not otherwise produce
immunoglobulins.
Expression is performed in appropriate prokaryotic or eukaryotic cells and the
immunoglobulin is recovered from the cells after lysis or from the culture
supernatant.
The term "solution containing an immunoglobulin to be concentrated" as used
within the current application denotes an aqueous, buffered solution
containing a
complete immunoglobulin, an immunoglobulin fragment, or an immunoglobulin
conjugate. This solution may be, e.g., a culture supernatant, or a column
chromatography eluate, or a polished immunoglobulin solution.
"Heterologous DNA" or õheterologous polypeptide" refers to a DNA molecule or a
polypeptide, or a population of DNA molecules or a population of polypeptides,
that do not exist naturally within a given host cell. DNA molecules
heterologous to
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-10-
a particular host cell may contain DNA derived from the host cell species
(i.e.
endogenous DNA) so long as that host DNA is combined with non-host DNA (i.e.
exogenous DNA). For example, a DNA molecule containing a non-host DNA
segment encoding a polypeptide operably linked to a host DNA segment
comprising a promoter is considered to be a heterologous DNA molecule.
Conversely, a heterologous DNA molecule can comprise an endogenous structural
gene operably linked with an exogenous promoter.
A peptide or polypeptide encoded by a non-host DNA molecule is a
"heterologous"
peptide or polypeptide.
The term "under conditions suitable for the expression of the heterologous
immunoglobulin" denotes conditions which are used for the cultivation of a
mammalian cell expressing an immunoglobulin and which are known to or can
easily be determined by a person skilled in the art. It is also known to a
person
skilled in the art that these conditions may vary depending on the type of
mammalian cell cultivated and type of immunoglobulin expressed. In general the
mammalian cell is cultivated at a temperature of from 20 C to 40 C, and for
a
period of time sufficient to allow effective protein production of the
immunoglobulin, e.g. of from 4 to 28 days.
The current invention provides a method for obtaining a concentrated
immunoglobulin solution substantially free of immunoglobulin aggregates by
tangential flow filtration comprising the following steps:
i) providing a solution containing an immunoglobulin to be concentrated
wherein said immunoglobulin is present in said solution in monomeric
and polymeric form, whereby the fraction of said soluble polymeric
form is more than 2.5 % when determined as the area under the curve
of the peaks eluted prior to the peak of the monomeric
immunoglobulin in a size exclusion chromatogram of said solution,
ii) concentrating said solution provided under i) by employing a tangential
flow filtration, whereby said soluble polymeric form of said
immunoglobulin does not increase by more than 25 % when
determined as the area under the curve of the peaks eluted prior to the
peak of the monomeric immunoglobulin in a size exclusion
chromatogram of said concentrated solution,
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-11-
iii) removing polymeric insoluble immunoglobulin by filtration after the
end of the tangential flow filtration and thereby obtaining a
concentrated immunoglobulin solution substantially free of
immunoglobulin aggregates.
In other words, the current invention comprises a method for obtaining a
concentrated immunoglobulin solution by tangential flow filtration,
characterized
in that said method comprises the following steps:
i) providing a solution containing an immunoglobulin to be
concentrated wherein said immunoglobulin is present in said
solution in monomeric and polymeric form, whereby the fraction of
the polymeric soluble immunoglobulin form present in said
provided solution has a first value of more than 2.5 % when
determined by size exclusion chromatography,
ii) concentrating said solution provided under i) by employing a
tangential flow filtration,
iii) removing said polymeric immunoglobulin by filtration after the end
of the tangential flow filtration and thereby obtaining a concentrated
immunoglobulin solution,
whereby the fraction of said polymeric soluble form of said immunoglobulin is
after step ii) larger than said first value and smaller than a second value
which is
1.25 times the first value.
The term "substantially free" denotes that a preparation of an immunoglobulin
contains at least 50 % (w/w) of the immunoglobulin in monomeric form, in one
embodiment at least 75 % of the immunoglobulin in monomeric form, in another
embodiment at least 90 % of the immunoglobulin in monomeric form, or in a
further embodiment more than 95 % of the immunoglobulin in monomeric form.
The term "does not increase by more than 10 %" as used within this application
denotes that the fraction of the immunoglobulin in polymeric soluble form does
not increase by more than 10 %. For example if the fraction of the
immunoglobulin
in polymeric soluble form is 7.5 % prior to the tangential flow filtration
determined
by size exclusion chromatography this term denotes that the fraction does not
increase by more than 0.75 %, that is to 8.25 % at maximum.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
- 12-
The term õimmunoglobulin in monomeric form" as used within this application
denotes immunoglobulin molecules which are not associated either covalently or
non-covalently with one or more other immunoglobulin molecules. This does not
exclude that the immunoglobulin molecule is associated either covalently or
non-
covalently with one or more not-immunoglobulin molecules, such as
carbohydrates, chromatin etc.
The terms õimmunoglobulin in polymeric form" and õimmunoglobulin in
aggregated form" as used within this application denotes immunoglobulin
molecules which are associated either covalently or non-covalently with one or
more immunoglobulin molecules. These associated immunoglobulin molecules
may be of the same immunoglobulin molecule or different immunoglobulin
molecules. This does not exclude that the immunoglobulin molecule is
associated
either covalently or non-covalently with one or more not-immunoglobulin
molecules, such as carbohydrates, chromatin etc. The term "polymeric soluble
form" or "high molecular weight (HMW) form", which can be used
interchangeably within this application, denote polymeric, i.e. aggregated,
immunoglobulin, whereby said aggregate is still soluble in an aqueous buffered
solution. The term "polymeric insoluble form" denotes polymeric, i.e.
aggregated,
immunoglobulin whereby said aggregate is not soluble in an aqueous buffered
solution.
In step i) of the method according to the invention is the immunoglobulin
present
in monomeric form and in polymeric form, whereby these two forms are soluble
in
the solution. In step ii) of the method is the provided immunoglobulin
solution
containing the immunoglobulin in monomeric and polymeric form concentrated
using a tangential flow filtration method. It has now surprisingly been found
that if
a solution contains an immunoglobulin in polymeric but soluble form prior to a
concentration step with tangential flow filtration the number of
immunoglobulin
molecules, i.e. the size of the particle, contained in the immunoglobulin in
polymeric form increases until the immunoglobulin in polymeric form is no
longer
soluble in the solution. Concomitantly almost no new soluble immunoglobulin in
polymeric form is formed during the tangential flow filtration. Thus, it has
been
found that in immunoglobulin solutions containing immunoglobulin in polymeric
soluble form prior to a concentration step with tangential flow filtration the
contained immunoglobulin in polymeric soluble form further agglomerates
additional immunoglobulin molecules from the solution resulting in a reduced
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
- 13-
formation of new immunoglobulin in polymeric soluble form. Thus, this results
in
immunoglobulin in polymeric insoluble form, which can be removed from the
concentrated immunoglobulin solution after the concentration step by a simple
filtration step.
Another aspect of the current invention is a method for obtaining a
concentrated
immunoglobulin solution by tangential flow filtration comprising the following
steps:
i) providing a solution containing an immunoglobulin to be concentrated
wherein said immunoglobulin is present in said solution in monomeric
and polymeric form,
ii) concentrating said solution provided under i) by employing a
tangential flow filtration with a Ap of 3.0 bar,
whereby the number of particles with a size of more than 1 pm in said solution
does
not increase during the concentrating step by more than a factor of 200 when
the
number of particles is determined by light obscuration. In one embodiment the
number of particles is determined by light obscuration at a protein
concentration of
90 mg/ml. In one embodiment the number of particles with a size of more than
5 pm does not increase by more than a factor of 100 when the number of
particles is
determined by light obscuration. In another embodiment is the fraction of said
polymeric form of said immunoglobulin more than 5 % when determined as the
area under the curve of the peaks eluted prior to the peak of the monomeric
immunoglobulin in a size exclusion chromatogram of said solution. In a further
embodiment is the concentration of the immunoglobulin after the concentrating
more than 80 mg/ml, in one embodiment 90 mg/ml or more. In another
embodiment the transmembrane pressure during the tangential flow filtration is
constant at 0.6 bar.
Another aspect of the invention is a method for producing a heterologous
immunoglobulin comprising the following steps:
a) providing a recombinant mammalian cell comprising one or more
nucleic acids encoding a heterologous immunoglobulin,
b) cultivating said cell under conditions suitable for the expression of
the heterologous immunoglobulin,
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-14-
c) recovering the heterologous immunoglobulin from the recombinant
mammalian cell or the culture medium,
d) concentrating the recovered aqueous, buffered solution comprising
the heterologous immunoglobulin using a tangential flow filtration
method with a constant Op of 3.0 bar, whereby the number of
particles in said solution with a size of more than 1 m does not
increase by more than a factor of 200 when the number of particles is
determined by light obscuration.
In one embodiment the number of particles is determined at a protein
concentration of 90 mg/ml. In a further embodiment the heterologous
immunoglobulin is a complete immunoglobulin, or an immunoglobulin fragment,
or an immunoglobulin conjugate. In still another embodiment the mammalian cell
is a CHO cell, a BHK cell, or a PER.C6 cell.
The term "recombinant mammalian cell" refers to a cell into which a nucleic
acid,
e.g. encoding a heterologous polypeptide, can be or is introduced /
transfected. The
term õcell" includes cells which are used for the expression of nucleic acids.
In one
embodiment the mammalian cell is a CHO cell (e.g. CHO K1, CHO DG44), or a
BHK cell, or a NSO cell, or a SP2/0 cell, or a HEK 293 cell, or a HEK 293 EBNA
cell,
or a PER.C6 cell, or a COS cells. In another embodiment the mammalian cell is
a
CHO cell, or a BHK cell, or a PER.C6 cell. As used herein, the expression
"cell"
includes the subject cell and its progeny. Thus, the term "recombinant cell"
includes the primary transfected cell and cultures including the progeny cells
derived there from without regard to the number of transfers. It is also
understood
that all progeny may not be precisely identical in DNA content, due to
deliberate or
inadvertent mutations. Variant progeny that have the same function or
biological
activity as the originally transformed cell are included.
The term "buffered" as used within this application denotes a solution in
which
changes of pH due to the addition or release of acidic or basic substances is
leveled
by a buffer substance. Any buffer substance resulting in such an effect can be
used.
In one embodiment pharmaceutically acceptable buffer substances are used, such
as
e.g. phosphoric acid or salts thereof, acetic acid or salts thereof, citric
acid or salts
thereof, morpholine or salts thereof, 2-(N-morpholino) ethanesulfonic acid or
salts
thereof, histidine or salts thereof, glycine or salts thereof, arginine or
salts thereof,
or TRIS (hydroxymethyl aminomethane) or salts thereof. In one embodiment the
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
- 15-
buffer substance is phosphoric acid or salts thereof, acetic acid or salts
thereof, or
citric acid or salts thereof, or histidine or salts thereof, or arginine or
salts thereof.
Optionally the buffered solution may comprise an additional salt, such as e.g.
sodium chloride, and/or sodium sulphate, and/or potassium chloride, and/or
potassium sulfate, and/or sodium citrate, and/or potassium citrate. In one
embodiment of the invention the pH value of the buffered aqueous solution is
of
from pH 3.0 to pH 10.0, in another embodiment of from pH 3.0 to pH 7.0, in a
further embodiment of from pH 4.0 to pH 6.0, and in still another embodiment
of
from pH 4.5 to pH 5.5.
In another embodiment comprises the method prior to, i.e. before, or after
step d)
the following step:
e) purifying the aqueous, buffered solution containing the heterologous
immunoglobulin.
The purification in step e) can be by different methods and techniques, such
as a
chromatography step, or a combination of different or similar chromatography
steps, or precipitation, or salting out, or ultrafiltration, or diafiltration,
or
lyophilization, or buffer change, or combinations thereof, or the like.
In another embodiment the heterologous immunoglobulin is a complete
immunoglobulin, or an immunoglobulin fragment, or an immunoglobulin
conjugate. In one embodiment the mammalian cell is a CHO cell, a BHK cell, a
NSO
cell, a Sp2/0 cell, a COS cell, a HEK cell, or a PER.C6 cell.
Still another aspect of the current invention is a method for obtaining a
concentrated immunoglobulin solution, comprising the following steps:
i) providing a solution containing an immunoglobulin to be concentrated
wherein said immunoglobulin is present in said solution in monomeric and
polymeric form,
ii) concentrating said solution by employing a tangential flow filtration, and
producing thereby a concentrated immunoglobulin solution,
iii) filtrating the concentrated immunoglobulin solution obtained under ii)
in order to remove the immunoglobulin in polymeric form.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-16-
Thus, it has been found that during the tangential flow filtration of
immunoglobulin solutions containing said immunoglobulin in monomeric form
and in polymeric form the formation of additional soluble immunoglobulin in
polymeric form is reduced whereas the already contained immunoglobulin in
polymeric form which is soluble in the solution is transferred to an
immunoglobulin in polymeric form which is insoluble in the solution. Thus, the
number of immunoglobulin molecules associated with each other in the
immunoglobulin in polymeric form increases during the concentration of the
immunoglobulin solution with tangential flow filtration until the
immunoglobulin
in polymeric form is no longer soluble in the solution. Thus, another aspect
of the
current invention is a method for the reduction of the formation of soluble
immunoglobulin in polymeric form during a concentration step with tangential
flow filtration, wherein the reduction is achieved by the addition,
supplementation,
or generation of soluble immunoglobulin in polymeric form prior to the start
of the
tangential flow filtration. The immunoglobulin in polymeric form may be, e.g.,
generated by heat stress.
The following examples and figures are provided to aid the understanding of
the
present invention, the true scope of which is set forth in the appended
claims. It is
understood that modifications can be made in the procedures set forth without
departing from the spirit of the invention.
Description of the Figures
Figure 1 Turbidity during concentration via different concentration
modes; X-axis: concentration in mg/ml, Y-axis: turbidity
determined at 350 nm.
Figure 2 Number of particles > 1 m per ml solution during concentration
via different concentration modes; X-axis: concentration factor
(CF), Y-axis: 106 particles/ml > 1 m.
Figure 3 Stained insoluble aggregates out of concentrated anti-IL-1R
antibody solutions (left: Ap 3.0 bar; right: Ap 1.2 bar).
Figure 4 Increase in high molecular weight forms (HMWs) with respect to
the HMWs present in the sample before concentration; X-axis: 1:
with low HMW content (approximately 0.6 %) prior to the TFF
with Op 1.2 bar; 2: with high HMW content (approximately
7.2 %) prior to the TFF with Op 1.2 bar; 3: with low HMW
content prior to the TFF with Op 3.0 bar; 4: with high HMW
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
- 17-
content prior to the TFF with zp 3.0 bar; Y-axis increase in
HMWs in %.
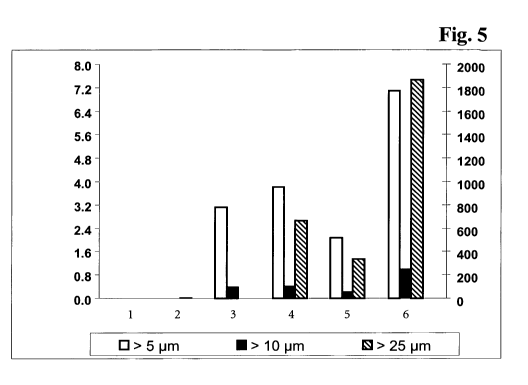
Figure 5 Number of particles per ml in the solutions before concentration
and after concentration with a Ap of 1.2 bar or 3.0 bar,
respectively; X-axis: 1: before concentration with low HMW
content; 2: before concentration with high HMW content; 3: after
concentration of solution with low HMW content with Ap of 1.2
bar; 4: after concentration of solution with high HMW content
with Op of 1.2 bar; 5: after concentration of solution with low
HMW content with Ap of 3.0 bar; 6: after concentration of
solution with high HMW content with Ap of 3.0 bar; left Y-axis:
105 particles per ml; right Y-axis: particles per ml for size > 25 m.
Figure 6 For material with a high initial high molecular weight form
content a species at about 5000 nm is observable beside decreased
intensity for the monomer (before concentration only one signal);
X-axis: particle size in nm; Y-axis: relative intensity in %; squares:
concentrate of a low HMW content solution at Op of 1.2 bar;
diamond: concentrate of a high HMW content solution at Ap of
1.2 bar; triangle: concentrate of a low HMW content solution at
Ap of 3.0 bar; circle: concentrate of a high HMW content solution
at Ap of 3.0 bar.
Example 1
Methods
a) Turbidity measurement.
The photometric absorbance was determined at 350 nm and 550 nm, where no
intrinsic chromophores in the antibody solution absorb (UV-VIS
spectrophotometer Evolution 500, Thermo Fisher Scientific, Waltham, USA). The
samples were measured undiluted. As a reference medium the appropriate buffer
solution was used. Every measurement was conducted three times.
b) Size-exclusion-HPLC.
The chromatography was conducted with a Tosoh Haas TSK 3000 SWXL column
on a Summit HPLC system (Dionex, Idstein, Germany). The elution peaks were
monitored at 280 nm by a UV diode array detector (Dionex). After dissolution
of
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-18-
the concentrated samples to 1 mg/ml the column was washed with a buffer
consisting of 200 mM potassium dihydrogen phosphate and 250 mM potassium
chloride pH 7.0 until a stable baseline was achieved. The analyzing runs were
performed under isocratic conditions using a flow rate of 0.5 ml/min. over
30 minutes at room temperature. The chromatograms were integrated manually
with Chromeleon (Dionex, Idstein, Germany). Aggregation in % was determined
by comparing the area under the curve (AUC) of high molecular weight forms
with
the AUC of the monomer peak.
c) Light obscuration.
To monitor the particle burden in a range of 1-200 pm a SVSS-C particle
analyzer
was used (PAMAS Partikelmess- and Analysesysteme, Rutesheim, Germany). The
system was calibrated according to the requirements of US Pharmacopeia Vol.
24,
<788>, with near-monosize polystyrene spheres. Three measurements of a volume
of 0.5 ml with a pre-flushing volume of 0.5 ml were performed. Results were
calculated as mean value and referred to a sample volume of 1.0 ml. The number
of
particles counted was within the sensor's concentration limit.
d) Dynamic light scattering (DLS)
DLS is a non-invasive technique for measuring particle size, typically in the
sub-
micron size range. In the current invention the Zetasizer Nano S apparatus
(Malvern Instruments, Worcestershire, UK) with a temperature controlled quartz
cuvette (25 C) was used for monitoring a size range between 1 nm and 6 m. The
intensity of the back scattered laser light was detected at an angle of 173 .
The
intensity fluctuates at a rate that is dependent upon the particle diffusion
speed,
which in turn is governed by particle size. Particle size data can therefore
be
generated from an analysis of the fluctuation in scattered light intensity
(Dahneke,
B.E. (ed), Measurement of Suspended Particles by Quasielectric Light
Scattering,
Wiley Inc. (1983); Pecora, R., Dynamic Light Scattering: Application of Photon
Correlation Spectroscopy, Plenum Press (1985)). The size distribution by
intensity
was calculated using the multiple narrow mode of the DTS software (Malvern).
Experiments were conducted with undiluted samples.
e) Staining method for detection of insoluble aggregates
The concentrated antibody solution was filtered through a 0.22 pm cellulose
acetate
filter membrane (Sartorius, Gottingen, Germany) and the retained particles
were
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-19-
stained with Reversible Protein Detection Kit solution from Sigma-Aldrich
(Steinheim, Germany). The membrane was examined after washing with buffer
under a stereomicroscope MZ 12 (Leica, Wetzlar, Germany) equipped with a
digital
camera DC 100 (Leica) under 80-times magnification (see e.g. Li, B., et al.,
J.
Pharmaceutical Sci. 96 (2007) 1840-1843).
Example 2
Tangential Flow Filtration
A conditioned and filtered histidine-buffered aqueous solution (pH 5.8) of an
anti-
IL-IR antibody was concentrated twenty fold up to 100 mg/ml by use of an
automated TFF system AKTAcrossflowTM (GE Healthcare, Amersham Bioscience
AB, Uppsala, Sweden) by employing a scalable flat sheet cassette (Sartorius,
Gottingen, Germany) with a HydrosartTM membrane of regenerated cellulose, with
a nominal molecular weight cut-off of 30 kDa, a membrane area of 0.02 m2 and a
total membrane loading of about 400 g/m2.
The target concentration was set to 90 mg/ml. Different Ap parameters were
tested
and were the following:
Method 1: transmembrane pressure = 0.6 bar
cross-flow = 90 ml/min
Ap = 1.2 bar
Method 2: transmembrane pressure = 0.6 bar
Ap = 3.0 bar.
Turbidity measurements (Figure 1), LO results (Figure 2, Table 1) in the
course of
concentration process showed that enhanced formation of immunoglobulin in
aggregated, insoluble form was depending on the applied shear stress.
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-20-
E
v
bA
N M N O N (M
A
v
cn 0' Ln rn
M M \.p 01
m -4 00
N M O -
a~- N d~ N O~
Al
Ci
cd
u!
in 00 r+
c~ -- N d+ M `c en N
> 00 ~c 00
N d 00 N N O O
n N M N n
v
w N o 00 00
in rn
o 0000 00 \0
0
Ul)
-- Ln r4 00 00 N -4 =--i
00 in N M \O N
A -- N Cr, N 'C
G1 V "
'C O
u a bin ~ bin '
bQ bA v bA ~,
on on o
a bD bA O
cz O `~ o cz `~
w X
o .0 4, o o 3 ~n I- o O o 3 ~n
O v v. w v w w
0" 0-0 O O 0 v CIO O O
cl co
0 0 O ^n 0 0 O
wO O^ =- N O N O '~; C ~, o O o O
3 v O o~ 2 .O o
cd r.
u c~ ; o~ o Q. o u Cl U Cl a o A. o
v~ N O bA D O= D a a O bA O bA d d C
v~ n 0 0 0 0 r O O O O X x
4- 4~ bj~
o o`'w w o`' o bn
u w -0 u- H Cl H m -0 u-4 u w H cz H cl
0
uoilnlos jui / sajDipiid Jo zagwnu
u
h
v
H
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-21-
The visual easily detectable burden of insoluble immunoglobulin in polymeric
form
in concentrated solutions by using a filtration-staining method depends on
applied
shear stress and the used concentration method, supporting results obtained by
LO
and turbidity measurements (Fig. 3). The increase of immunoglobulin in
polymeric
form for the concentrated solutions depends on the status of the polymeric
precursors before concentration. When material is used, which already contains
the
immunoglobulin in polymeric form (as in the current example 7.5 % as
determined
by SEC), an increase in the amount of polymeric forms during tangential flow
filtration was not detectable by SEC (see Table 2).
Table 2: SEC data
HMW/ mean
HMW/ HMW/ mean increase value of
polymeric polymeric Polymeric value in increase main LMW
sample form form forms polymeric aggregates in Peak forms
Peak 1 Peak 2 aPeak I nd 2 forms [%] aggregates IL-1R
[%]
before 0.54 0.00 0.54 0.67 99.46 0.00
concentration,
solution 0.49 0.22 0.71 98.29 0.00
containing a
low number
of aggregated 0.74 0.02 0.76 99.14 0.04
forms
TFF with E'p 0.90 0.00 0.90 0.95 34.33 41.79 99.03 0,06
= 1.2 bar of
low number 0.96 0.03 0.99 47.76 98.93 0.00
aggregate 0.00 0.96 0.96 43.28 98.99 0.05
solution
before 5.07 2.20 7.27 7.23 92.59 0.15
concentration,
solution 4.69 2.50 7.19 92.64 0.18
containing a
high number
of aggregated
forms
TFF with Ap 5.05 2.61 7.66 7.29 5.95 0.83 92.17 0.16
= 1.2 bar of
high number 4.63 2.63 7.26 0.41 92.56 0.18
aggregate 4.49 2.46 6.95 -3.87 92.86 0.20
solution
before 5.27 2.41 7.68 7.44 92.02 0.30
concentration,
solution 4.69 2.50 7.19 92.64 0.18
containing a
high number
of aggregated
forms
CA 02706403 2010-05-20
WO 2009/068282 PCT/EP2008/010060
-22-
HMW/ mean
HMW/ HMW/ mean increase value of
polymeric polymeric polymeric value in increase main LMW
sample form form forms polymeric aggregates in Peak forms
Peak 1 Peak 2 aPeak I nd 2 forms [%] aggregates IL-1R
[%]
TFF with Ap 5.02 2.80 7.82 8.02 5.18 7.91 91.84 0.34
= 3.0 bar of
high number 5.09 2.92 8.01 7.73 91.81 0.19
aggregate 5.38 2.86 8.24 10.83 91.54 0.22
solution
before 0.59 0.15 0.74 0.73 99.17 0.09
concentration,
solution 0.49 0.22 0.71 98.29 0.00
containing a
low number
of aggregated
forms
TFF with Op 0.00 1.06 1.06 46.21 37.47 98.83 0.11
= 3.0 bar of
low number 0.00 0.98 0.98 35.17 98.83 0.19
aggregate 0.00 0.95 0.95 31.03 99.03 0.02
solution
In contrast to this the amount of high molecular weight forms/polymeric
soluble
forms increased when nearly monomeric material (0.6 % of immunoglobulin in
polymeric form as determined by SEC) was concentrated (Fig. 4). Regarding the
status of larger and insoluble aggregates after concentration a contrarily
relation
was observed. Solutions with a higher extend of polymeric form precursors
before
concentration showed more larger and insoluble aggregates compared to the
initially nearly monomeric solutions (Fig. 5). A shift from smaller, soluble
polymeric precursors, i.e. from polymeric forms in which only a few
immunoglobulin molecules are aggregated, to larger insoluble aggregates during
the
TFF process depending on the status of the polymeric precursors of the used
intermediate was found. DLS data supports this perspective (Fig. 6).
Not only unfavorable high pressure profiles in TFF but also contaminants like
soluble aggregates can cause an ongoing aggregation process with a shift of
aggregates to larger insoluble species and precipitates during concentration
by TFF.