Note: Descriptions are shown in the official language in which they were submitted.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-'-
SEPARATION OF WATER FROM HYDROCARBONS
FIELD OF THE INVENTION
[0001] This invention relates to a method for the separation of water from
hydrocarbons, especially liquid hydrocarbons such as petroleum naphthas,
natural
gas condensates, petroleum fuels such as gasoline, middle distillates such as
road
diesel fuel and kerojet, particularly under low temperature conditions. The
water
removed may be present as such in the hydrocarbons or mixed with other water-
miscible materials or contaminants which may be removed simultaneously with
the
water.
BACKGROUND OF THE INVENTION
[0002] Significant amounts of water become mixed with hydrocarbon streams
during production and processing. Petroleum refinery streams, for example, may
be
treated with water, steam or various aqueous solutions during processing in
order to
carry out the processing and to meet various quality specifications. Steam
stripping,
caustic treating and amine treating are frequently used in conventional
refinery
processing and although much of the water introduced in this way can be
removed
by simple settling procedures, a certain amount of water remains in the fuel
after
removal of the bulk of the water. Excess amounts of water frequently adversely
affect the properties and quality of hydrocarbon fuels, for example, by
creating haze
in fuels which would otherwise be clear, accelerating rust and other forms of
corrosion on containers and equipment, and by the formation of ice crystals at
low
temperatures which may lead to plugging of filters and other equipment, for
example, fuel injectors. Water may also contain contaminants such as acids
which
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-2-
may lead to accelerated corrosion. It is therefore usually necessary to
separate any
remaining water from petroleum fuels and other products in order to meet
various
product specifications; the separation may be carried out out at the refinery,
at the
distribution terminal or at the location of use, for example, the airport.
[00031 Product specifications frequently require relatively low levels of
water in
order to avoid the problems mentioned above, for example, ASTM D 2709 for
diesel fuel oils sets a 0.5 % volume maximum limit on water in sediment for
diesel
fuel oils varying from light distillate fuels for road diesels (1D fuel) up to
heavy
distillate fuels (4D fuel) for low and medium speed diesels operating at
constant
speed and load. Similar specifications may be found for other hydrocarbon
fuels
including motor gasoline and middle distillate products including home heating
oil,
aviation kerosene and vaporizing oil. Products sold in cold climates are
particularly
subject to problems arising from the freezing of water and the consequence
formation of ice crystals at temperatures below freezing, the problems of
product
quality control are therefore exacerbated in such climates.
[00041 The production of petroleum hydrocarbons from subterranean formations
may also result in hydrocarbon streams which are contaminated by water, either
alone or mixed with other contaminants. While water, e.g. brine, may normally
be
readily separated from liquid crudes, problems may be encountered with the
separation of water from other produced fluids, for example, natural gas
condensates. which are relatively light, low boiling hydrocarbon fractions
produced
from natural gas wells. One instance of this problem is in the production of
natural
gas which has a relatively high water content which leads to undesirable
hydrate
formation; hydrates normally require removal prior to the shipping of the gas
because of their propensity to plug equipment and flowlines. Water removal may
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-3-
usually be effected by the addition of a dehydrating agent or hydrate
suppressor
such as ethylene glycol followed by separation of the water/glycol phase from
the
hydrocarbon liquids in the conventional manner. Large quantities of water
which
are encountered in some gas fields, especially when low ambient temperatures
are
prevalent, may however make normal processing techniques ineffective or of
limited utility. An effective method of water separation would therefore be
useful
for applications such as this.
[0005] . While chemical methods may be used to remove water from the main
body of the fuel, they have generally received less commercial acceptance on
the
large scale used in refinery operations because of the cost factor. Chemical
methods, it eluding salt drying, require the replacement or regeneration of
the
reagents used in the process and the reagents themselves and their products
formed
by interaction with the water frequently introduce their own complications in
subsequent processing. Because the cost of the reagents is directly
proportional to
the amount of water in the product, physical methods of separation have
normally
been preferred since their operational cost is not so directly related to the
amount of
water which needs to be separated.
[0006] Various physical separations have been used including centrifugal
separation and filtration, for example, using sand filters. A technique which
has,
however, become commercially attractive in recent years is liquid/liquid
coalescence. See, for example, Refining Details: Advances in Liquid/Liquid
Coalescing Technology, Gardner, Today's Refinery, March 1997. The method of
coalescing a liquid suspended in another immiscible phase using a coalescing
device frequently referred to as a coalescer, has been found useful for
removing
liquids both from the gaseous phase as in aerosols and from suspensions of one
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-4-
liquid in another liquid with which it is immiscible but may be soluble to a
limited
degree. Coalescing devices are particularly effective where the volume of
liquid to
be removed is small in comparison to the volume of the phase from which it is
removed so that the technique is of potential application for the separation
of small
quantities of water from hydrocarbon fuels. The Gardener article discusses the
factors that are relevant to the coalescence of droplets of the discontinuous
phase
from the continuous phase and the ease or difficulty of separation of the
immiscible
phases. These factors include the physical properties of the phases such as
density,
viscosity, surface tension and interfacial tension. In addition, the
properties of the
system such as drop size, curvature of the liquid/liquid interface,
temperature,
concentration gradients and vibrations may also affect the effectiveness of
the
coalescence. As noted in U.S. Patent No. 5, 443,724 (Williamson) any or all of
these factors may be significant in a particular situation but the density,
drop size
and interfacial tension of the two liquids appear to be the most significant
factors as
well as those over the least amount of control can be exercised in affecting
the
separations.
[00071 The type of coalescer employed for the separation depends on the
difficulty of separation or coalescence as influenced by the various relevant
factors
outlined above. The type of fluids being separated frequently determines the
nature
of the packing used in the coalescence device. Glass fibers have found
widespread
industrial application in commercial devices. Frequently, however, the
presence of
surfactants in water/hydrocarbon emulsions lowers the interfacial tension to a
value
less than about 20 dynes/cm at which the emulsions are stable enough to resist
being broken through processing in conventional mesh packing/glass fiber
coalescers as well as by other techniques. While electrostatic precipitators
may be
effective on such emulsions down to interfacial tensions below 10 dyne/cm,
their
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-5-
use is rather less favored than the relatively cheaper coalescence method.
Surfactants disarm conventional glass filters coalescers by bonding with glass
fibers, allowing water molecules to flow through the coalescers with the
hydrocarbons. Frequent changes of the cartridge material in the coalescers may
obviate this problem but the increased labor and disposal costs associated
with
frequent cartridge change out are undesirable as is the continued need to
monitor
the quality of the product to ensure that appropriate specifications are being
met.
The use of various polymeric materials such as phenolic or acrylic resins
which act
primarily as binding agents for glass fiber packings may be effective to
reduce
disarming of coalescers to a significant extent, but the problem remains.
[0008] U.S. 5,443,724 discloses a coalescer-separator apparatus which enables
longer coalescer cartridge life to be obtained as a result of improved flow
distribution within the device. The device is stated to be particularly
suitable for the
separation of water from organic liquids such as fuels and is capable of
achieving
extended life using a more compact unit with the same or improved level of
performance compared to larger conventional units. As described in U.S.
5,443,724, the coalescence is carried out using a packing material which has a
critical wetting surface energy which is intermediately critical wetting
surface
tension (CWST) of the discontinuous and continuous phase liquids. This results
in
the formation of droplets of the discontinuous phase, after which the mixture
of the
continuous phase liquid and the droplets of the discontinuous liquid are
conducted
to a separating element which permits the continuous phase liquid (petroleum
fuel)
to pass but substantially resist or prevent passage of the discontinuous phase
which
can then be separately collected and taken away from the bulk of the product.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-6-
[0009] Various porous media with differing surface energies are mentioned in
U.S. 5,443,724 including polytetrafluoroethylene (PTFE),
polybutyleneteraphalate
(PBT) and other polyfluorinated polymers such as fluorinated ethylene and
propylene (FEP) resins. These materials which provide the requisite surface
energy
to the coalescence/separation filters may be used in the form of a coating of
a
backing such as glass fiber, stainless steel screens or pleated paper packs.
Other
media suitable for use as the functional or discontinuous phase barrier
material of
the separating element are disclosed, for example, in U.S. 4,716,074 (Hurley)
and
U.S. Patent No. 4,759,782 (Miller); reference is made to these patents for
details of
suitable materials for providing the requisite surface properties in
coalescence/separation devices.
[0010] Normally, separation of liquids by the coalescence technique requires
three stages to be successful. First of all, filtration is required to remove
fine
particles such as iron oxide and iron sulfide that stabilize emulsions and for
this
purpose, mesh, screen, packed and sand filters are normally satisfactory.
Filtration
is followed by the coalescence step which, in the case of water and
hydrocarbon
fuels, is normally accomplished by the use of fluoropolymer membranes which
are
effective emulsion breakers in liquids with an interfacial tension of greater
than
about 1 dyne/cm. Separation takes place when the coalesced water droplets are
repelled by a hydrophobic barrier membrane, again normally formed from a
polymeric material such as fluoropolymer, which permits the hydrocarbon fuel
to
flow through the cartridge while preventing transfer of the water across the
membrane.
[0011] Regardless of the details of the coalescence/separation techniques used
one problem that remains, particularly in cold climates, is the problem of
icing.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-7-
When the fuel which is to be treated contains relatively high levels of water,
low
temperatures cause the water to freeze and form ice crystals which plug
filters,
including the prefilters used in coalescence/separation. Accordingly,
regardless of
the potential for utilizing the coalescence/separation technique, problems
arising
from the presence of water in the feed still remain.
SUMMARY OF THE INVENTION
[00121 A method has been devised for the removal of dissolved water or water
and ice from hydrocarbon liquid products in a manner which enables the
hydrocarbon products to be readily treated by the coalescence/separation
technique
while reducing the potential for plugging filters and other equipment with ice
crystals or solid deposits. , According to the present invention, dissolved
water or
water/ice is removed from liquid hydrocarbons by contacting the a feed stream
of
the hydrocarbon with a liquid treating agent having an affinity for water
prior to
subjecting the hydrocarbon/treating agent mixture to
coalescence/separation.'to
remove the water from the hydrocarbon. The treating agent is separated,
together
with water removed from the feed stream, from the hydrocarbon product by the
coalescence/separation step and recirculated to the feed. The composition of
the
circulating aqueous phase comprising the treating agent and removed water is
controlled to achieve the desired level of water removal to meet relevant
product
specifications. Consistent with the removal of the water during the
coalescence/separation, the water concentration of the circulating treating
agent/water blend will tend to increase gradually with transfer of the water
in the
feed to the circulating fluid. This progressive increase in water content can
be
compensated by controlled addition of pure treating agent solvent to the
recirculating fluid coupled with accumulation of the treating agent/water
mixture
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-8-
and continuous or periodic dumping of excess mixture. Alternatively, the
circulating mixture may be subjected to continuous or batch regeneration or
disposed of in any other way which is convenient and economical.
[00131 The treating agent which finds a wide degree of utility as well as
being
economically favorable comprises a mixture of (i) water with (ii) a water-
hydrocarbon co-solvent. The preferred class of co-solvents are the alcohols,
especially methanol, but other water-miscible organic compounds may also be
used,
as described below. Other, generally less favorable treating agents which may
be
used include strong aqueous salt solutions, as well as organic and inorganic
liquids
such as amines or even acids, particularly if it is desired to remove a
contaminant
from the hydrocarbon which can be reacted with the treating agent or a
component
of it.
DRAWINGS
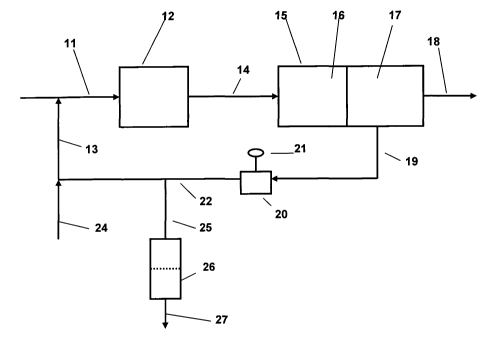
[00141 The single figure of the accompanying drawings is a schematic flowchart
of a system for removing water from hydrocarbon liquid products using a
coalescence/separation technique.
DETAILED DESCRIPTION
[00151 The present invention is applicable to the separation of water, either
alone or mixed with other contaminants, from hydrocarbon- liquids. The method
is
particularly applicable to the separation of water from light hydrocarbon
liquids of
relatively low viscosity, comparable to that of the water to be separated. The
method is of particular applicability to the separation of water from refinery
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-9-
hydrocarbon fuels including gasoline (including heavy gasoline and light
gasoline),
middle distillates such as home heating oil, vaporizing oil, road diesel
including all
ASTM D2 diesels, kerosene type aviation fuels, as well as potentially to other
liquid
hydrocarbon product streams which require removal of water in order to meet
product specifications or other service or commercial requirements. Normally,
the
amount of water which is present in these materials prior to separation will
be
relatively small, typically not more than about 5 volume percent, but product
specifications will normally require a much lower water content in order to be
acceptable. For example, as noted above, D2 diesel fuel is required to contain
no
more than 0.2 % combined water and sediment and similar requirements will be
encountered with aviation kerosenes in view of the very low temperatures
encountered by military and commercial jet aircraft at high altitudes. The
present
separation technique is not dependent upon the chemical composition of the
hydrocarbon fuel except to the extent that the chemical composition affects
physical
properties such as specific gravity, interfacial surface tension, miscibility
with water
and viscosity. The chemical composition may also affect the degree to which
surfactants added during processing or spontaneously formed during the
processing
(for example, during caustic washing) and the effect the surfactants may have
on the
other properties, especially emulsion stability, micelle formation, reverse
micelle
formation.
[0016] The present method is also applicable to the separation of water from
natural gas liquids also known as natural as condensates. These low viscosity
hydrocarbon liquids generally comprise propane, butane and possibly higher
hydrocarbons separated from the lower boiling methane and ethane in natural
gas
from subterranean wells. Natural gas may, as noted above, need to be treated
at or
near the we! ihead to remove either produced water or water combined with
various
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-10-
chemicals such as hydrate suppressors, for instance, ethylene glycol, which
have
been used to treat the produced gas and which have separated out with the
liquids as
a result of their boiling in the temperature range set for the liquids. Thus,
the
present invention provides an effective method for the removal of water/glycol
(ethylene glycol) mixtures from natural gas liquids.
[0017] In the present method, a liquid treating agent which has an affinity
for
the water in the hydrocarbon feed is mixed with the liquid hydrocarbon before
the
fuel is subjected to coalescence/separation treatment. The treating agent
causes the
water and possibly other contaminants to form an aqueous mixture which, when
in
fully coalesced form, is substantially immiscible with the hydrocarbon
although
initially it may be suspended in the majority hydrocarbon phase and not
readily
separable from it by other means. It is this aqueous mixture which is then
separated
from the hydrocarbon in the coalescence/separation step. During the
coalescence/separation treatment, the treating agent and water are separated
in the
form of a single coalesced phase which is substantially immiscible with the
hydrocarbon majority component and recirculated for further addition to the
feed.
An illustrative schematic of the process configuration is shown in the
attached
figure by way of example.
[0018] The treating agent has for its required effect to have an affinity for
the
water which is to be removed from the hydrocarbon feed. It may also desirably
have an affinity for any other contaminants in the feed which should be
removed at
this time. For example, if the feed also contains an acidic contaminant such
as
hydrogen sulfide, the use of an amine as the treating agent may be used to
effect
removal of the hydrogen sulfide as well as of the water. Conversely, if the
feed is,
for example, a refinery stream containing basic contaminants such as alkalis
from
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-11-
caustic treatment, the use of an acidic treating agent may be effective to
remove
both water and the residual alkali. The reactive components of the treating
agent
may make up the entire treating agent or may be added as additive components.
[00191 As described in greater detail below, a very useful treating agent is a
mixture of water and a co-solvent, miscible with both the alcohol and with the
hydrocarbon. This has been found to be very effective in removing water from
refinery liquid fuel products such as gasoline and middle distillates such as
road
diesel, kerojet or heating oil. As noted below, it may also be used with
natural gas
condensates. One preferred embodiment of the invention is shown in the drawing
and is described below by reference to the treatment of a refinery fuel with
such a
treating agent.
[00201 In the figure, a refinery fuel such as mogas, road diesel or kerojet is
introduced by way of line 11 to prefilter 12 with the alcohol/water mixture
being
added through line 13. Prefilter 12 is suitably a mesh or screen filter with
additional
packing, e.g. compressed glass fibers or polymer (nylon, polyolefin) mesh,
adapted
to remove fine particulate matter such as iron oxide, silica, which may
stabilize
emulsions and possibly damage the coalescer/separation units. Use of a high
efficiency prefilter such as a sand filter may result in some removal of
water.
[00211 After passing through prefilter 12, the blend of hydrocarbon, alcohol
and
water passes through line 14 to coalescence/separation unit 15. Coalescer unit
15 is
divided into two stages, comprising a first or coalescence stage 16 and a
second or
separation stage 17. In the coalescence stage, the suspended particles of
water are
subjected to coalescence into larger droplets in the presence of a suitable
coalescing
medium through which the liquids pass in order to effect the desired
coalescence of
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-12-
the water, now with the added co-solvent. In separation stage 17, the combined
fluids pass over a separation membrane which is selected to have a surface
energy
favoring passage of the hydrocarbon phase through the walls of the separation
membrane while excluding the aqueous phase comprising the co-solvent and the
water. The liquid hydrocarbon fuel, now containing only a small and acceptable
amount of water passes out of the coalescence/separation unit through line 18
to
product storage while the separated co-solvent/water phase, now containing
water
removed from the original fuel feed, is removed through line 19 for
recirculation to
the feed. A control valve 20 is provided in the recycle loop under control of
manual
or automatic controller 21 to permit actuation of the recycle when required.
Recirculation is generally not required for operation above freezing point (0
C)
when only the normal coalescence of free water is required, with no hazard of
ice
crystal formation. When seasonal ambient temperatures drop below freezing,
however, and icing and filter plugging are. prevalent, injection and
recirculation of
the co-solvent/water blend can be initiated in order to prevent filter and
equipment
plugging by ice crystals. Actuation of the injection of the co-solvent/water
blend
into the feed and recirculation can be initiated either manually or
automatically in
response to ambient temperature sensors or, preferably, by a pressure sensor
on a
filter responsive to pressure increase upon plugging with ice crystals.
[00221 The alcohol/water blend passes from control valve 20 to injection line
13
through line 22 with additional co-solvent being injected to the circuit
through line
24 in order maintain the desired co-solvent/water ratio for effective
coalescence and
separation. As water is progressively removed from the fuel feed, and makeup
co-
solvent is added, excess co-solvent/water mixture may be purged through the
circuit
through line 25 and to dump tank 26 from which the blend may be removed
through
line 27. While it is acceptable to allow an accumulation of water in the
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
- 13-
recirculation loop up to a permitted maximum set by processing requirements
(permissible water concentration in fuel, acceptable co-solvent loss to fuel),
it will
normally be preferred to maintain the loop at a constant composition ratio
between
the co-solvent and the water by periodic or continuous addition of co-solvent
accompanied by removal of excess circulating liquid. Removal of the excess co-
solvent/water may be carried out continuously or periodically or, as an
alternative,
the gross composition of the recycle loop may be controlled by removal of the
water
from the co-solvent, for example by distillation, or reverse osmosis to the
extent
required to maintain constant composition in the recycle loop. Reclamation of
the
co-solvent in this way is potentially attractive since the regenerated co-
solvent is
recycled to the process while removing trace contaminants such as salt or
trace
soaps/surfactants arising from the processing. As an alternative, the co-
solvent/water may, depending on the nature of the co-solvent, be disposed of
as a
relatively clean combustible mixture. With methanol as the co-solvent, for
example, the methanol/water blend may be used as a low energy content fuel in
certain burner and industrial processes, especially if the energy content is
maintained above about 18,000 kJ/kg. The exact choice of method by which the
composition of the recycle stream is maintained at the desired value is not,
however, important and may be selected according to convenience, local
economics
and other considerations.
[00231 The same technique may be used to separate water/glycol mixtures from
natural gas condensates. In this case, similar considerations apply except
that the
residual water content in the gas may not be as low as required for high
quality fuel
products, being set, however, by pipelining specifications which may vary
according to the temperature conditions prevailing along the pipeline with
more
stringent specifications prevailing in the colder climates and for undersea
pipelines.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-14-
[0024] The co-solvent which is used in the blend with the water to promote
removal of the water from the hydrocarbon feed is a liquid which is miscible
with
both water and the hydrocarbon majority component, at least to a limited
extent.
Organic liquids such as oxygenates are generally suitable and preferred for
this
purpose in view of their availability, cost and functioning in the present
process.
While oxygenate esters such as the esters of lower fatty acids and lower
alcohols
such as ethyl acetate, ethyl propionate, propyl acetate, ethyl butyrate, amyl
acetate,
esters of the lower alkanols such as methanol, ethanol, propanol and the lower
oxo-
alcohols with lower fatty acids such as hexanoic acid, octanoic acid (e.g. 2-
ethyl
hexanoic acid), as well as other oxygenates such as the ketones, such as
acetone,
methyl ethyl ketone (MEK), methyl propyl ketone, aldehydes, ethers such as
dimethyl ether and methyl propyl ether, may be used, the preferred co-solvents
are
alcohols, especially the lower alkanols such as methanol, ethanol and
propanol.
Alcohols are particularly suited to the removal of water/glycol mixtures from
natural gas condensates in view of their miscibility for with both the water
and the
glycol hydrate suppressor.
[0025] The extent to which the co-solvent is required to be miscible with both
water and the hydrocarbon is not important as long as the selected co-solvent
is
miscible with water in all proportions which are used in the process and that
the co-
solvent has an affinity for water. It is the co-solvent's affinity for water
which
effects the dehydration of the hydrocarbon e.g. the fuel or NGL (natural gas
liquids)
and its miscibility with the water which enables the blend of co-solvent and
water to
be effectively removed from the hydrocarbon by the coalescence technique. The
extent to which the co-solvent may be miscible with the hydrocarbon may affect
the
extent to which the dehydration is completed, a factor which may be
significant
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
- 15-
with fuels. It may also affect the losses of the co-solvent to the hydrocarbon
(see
below). In order to maintain the loss of co-solvent to the hydrocarbon within
acceptable limits a limited miscibility of the co-solvent/water mixture with
the
hydrocarbon is desirable. For this reason, blends of methanol with water
particularly commend themselves since blends of these components with high
water
contents are almost insoluble with hydrocarbon fuels including gasoline as
well as
NGL.
[00261 The lower alkanols which from the preferred class of co-solvents for
use
in the present process are those which have a limited solubility in liquid
refinery
hydrocarbon fuels and NGL. Since the compositions of fuel and NGL may vary,
the selected alcohol may vary also. As with other potential co-solvents, the
alcohols will be selected for their mutual solubility in hydrocarbons and
water as
well as their convenient availability and favorable economics. The alcohols
may be
monohydric, dihydric or higher alcohols although monohydric and dihydric
alcohols (glycols) will normally be preferred. Other functional groups may
also'be
present on the alcohols, for example, ether groups, although halogens will
normally
not be preferred for reasons of cost, toxicity, and corrosion potential;
hydroxyamines should normally be excluded in view of their potential to act as
surfactants for the hydrocarbon/water system. The preferred monohydric
alcohols
are methanol and ethanol although propanol may also be used to advantage and
glycols such as ethyleneglycol, propyleneglycol, dipropyleneglycol, as well as
glycol ethers such as ethyleneglycol methylether, diethyleneglycol methylether
and
others will be found suitable. Alcohols with relatively higher solubilities in
the
hydrocarbon phase may be used at the expense of excessive losses to treated
fuels
and NGL unless they are combined with relatively high amounts of water, a
measure which detracts from the dehydration which is the object of the
process.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-16-
The loss of alcohol or other selected co-solvent to the fuel is not, however,
in it self
necessarily undesirable since the alcohol may act as a deicer in the fuel
product,
both during distribution and subsequent use, so eliminating the need for a
separate
deicing additive injection.
[00271 As noted above, however, it is possible and on occasion may be
desirable to use alternative treating agents. In order to remove water from
hydrocarbon products it is possible, for example, to envisage the use of
strong
aqueous salt solutions. These have the required affinity for water as a result
of their
high concentration of mineral salt and, as a result of the removal of the
water from
the hydrocarbon feed, would become progressively more dilute until their
effectiveness ceases. By control of the composition of the circulating fluid
stream,
e.g. by the addition of more salt and withdrawal of excess volume, it is
possible to
utilize these salt solutions in the same way as the alcohol/water mixtures
referred to
above. Sodium chloride solutions will not be found to be optimal in this
application
but strong solutions of other salts such as calcium chloride or sodium sulfate
will be
more effective. The use of strong solutions may be found very effective in
warmer
climates where freezing problems will not be encountered. The use of salt
solutions
may also be economically favorable. The same simple conventional methods may
be used to maintain the concentration of the salt in the solution to restore
its
functionality as the dehydrating agent, e.g. by the addition of fresh salt and
removal
of a portion of the circulating volume of liquid or by regenerating the
solution, for
example, by distillation or membrane separation (osmosis). Alternatively, salt
solutions may be cheap enough to be used on a once-through basis with
regeneration, particularly if used only intermittently.
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-17-
[00281 Other materials which have an affinity for water may be used as the
treating agent. Liquid amines in particular, commend themselves for this
purpose,
especially when acidic contaminants such as hydrogen sulfide are to be removed
from the hydrocarbon. Amines may be as technically pure compounds or as
solutions in water as long as the required affinity for water is retained.
Suitable
amines for this purpose many include substituted amines such as
triethanolamine
and diethanolamine as well as simple amines such as monoethanolamine.. These
treating agents may be useful for removing water simultaneously with acidic
contaminants such as hydrogen sulfide or carbonyl sulfide from natural gas
liquids.
[00291 The amount of the treating agent, e.g. co-solvent/water blend whichtis
added relative to the feed and the relative amounts of co-solvent and water in
the
blend are codependent variables to a certain extent. As noted above, a loss of
the
co-solvent to the hydrocarbon does take place but this can be controlled by
increasing the amount of water in the blend which is injected into the fuel.
On the
other hand, the addition of more water detracts from the effectiveness of the
dehydration if resort is made to this expedient in order to limit the loss of
co-solvent
to the fuel. Another factor which requires consideration is the effect of the
co-
solvent upon the fuel product specifications. Large amounts of the lower
alcohols
such as methanol and ethanol or other volatile co-solvents, may degrade flash
point
of treated fuels to an extent that fuel product specifications are not met.
Thus,
increasing the relative amount of water in the mixture may be effective for
controlling changes in flash point during the treatment process although
possibly at
the expense of diminished effectiveness for water removal. The higher alcohols
such as dipropyleneglycol may, however, may be added to distillate fuels after
the
water removal process to act as high flash point deicers during subsequent
distribution and use. In general, however, the loss of co-solvent to natural
gas
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
- 18-
liquids will represent merely an economic loss of the process rather than
having a
major effect on the product (NGL) quality.
[0030] The amount of water in the co-solvent /water blend will depend upon the
selected cb-solvent, its solubility in the hydrocarbon components of the fuel
or other
hydrocarbon liquid, the ratio of water/co-solvent blend to the hydrocarbon,
operating temperature and other factors. Taking the lower alcohol, methanol,
as the
selected solvent, the ratio of methanol to water will normally vary between
20/80
and 80/20 by volume. This ratio will vary with other alcohols and other co-
solvents according to the specific alcohol or other co-solvent selected, its
solubility
characteristics with water and the hydrocarbon and the extent to which
transfer of
the alcohol to the hydrocarbon fuel can be tolerated and the amount of water
in the
initial hydrocarbon feed. Normally, however, with methanol as the alcohol, the
volume ratio of alcohol and water will be from about 75:25 to 25:75 with
approximately 50:50 blends being normally adequate.
[0031] The preferred alcohol for use in the present process is methanol which
is
selected not only for its low cost, low viscosity and boiling point (if
reclaiming is
desired) but also for its effectiveness in dissolving solid ice when present.
A 50:50
methanol/water blend has been found to be extremely effective and this ratio
is
sufficiently concentrated to dehydrate the fuel down to low water levels. The
freezing point of a 50/50 methanol/water mixture is below 40 C so the process
is
capable of operating at ambient temperature over wide limits from less than -
40 C
up to relatively high ambient temperatures. This reperesents a very useful
working
range, especially for use with fuel treatment in cold climates and for gas
condensate
treatment when low temperature gas pipeline specifications have to be met.
Methanol has the additional advantage that loss to the hydrocarbon fuel
majority
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
_19-
component is relatively small, as compared to higher alcohols and for this
reason,
process losses may be minimized. Combinations of alcohols such as methanol
with
dipropyleneglycol may be used when it is desired to achieve some losses to
fuels in
order to provide residual deicing capability for the treated fuel product.
[0032] The amount of the co-solvent/water blend which is added to the
hydrocarbon feed is typically less than 10% by volume of the total feed
although the
exact amount selected will be depend upon the degree of dehydration desired,
the
co-solvent e.g. alcohol selected, the ratio of water to co-solvent in the
blend and any
relevant product specifications such as flash point for treated fuel products.
Also
relevant would be the ratio of water and glycol in treating natural gas
condensates
which have been treated with glycol hydrate suppressor. Normally, in the case
of
alcohol/water blends, the amount of the alcohol/water blend will be from 0.1
to
10% by volume of the total fuel feed and in most cases from 0.5 to 5% of the
feed
will be found sufficient. In many cases, about 1% by volume of the feed will
be
adequate with the 50/50 methanol/water blend selected for the dehydration.
[0033] The prefilters which are used ahead of the coalescer may be any
suitable
type of conventional filter, including sand filters, metal or polymer meshes,
or other
porous material capable of removing small solid particles which would tend to
stabilize the fuel/water emulsions and which might result in damage to the
more
delicate coalescer membranes. Polyester and nylon mesh filters are suitable,
typically with crush strengths in the range of 70-145 kg.cm 2 (75 - 150psi)
and other
non-woven filter materials may be used as convenient alternatives. The filter
material may be contained in a conventional filter housing and the filter
material in
any convenient configuration which provides the desired filter life,
filtration
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-20-
capacity and flow rate, for example, pleated mats, cylindrical sheets or mats,
helical
or spirally wound mats.
[00341 In a similar manner, the material of the coalescer and separation
elements in the coalescing unit and the separation unit may be provided in a
form
which provides the necessary mechanical strength, liquid flow rate and unit
life. In
the simplest form, the media serving as the coalescer and separator materials
may
be provided in sheet form which may be formed either as flat sheets, pleated
or
corrugated sheets or in other suitable arrangements e.g. cylindrically,
helically or
spirally wound sheets, as disclosed in U.S. 5,443,724 to which reference is
made for
a disclosure of suitable coalescer and separator materials and configurations*
for
them.
[00351 The coalescer promotes the coalescence of the discontinuous or highly
divided phase of the hydrocarbon/water mixture in which the water is in the
form' of
finely divided droplets which are immiscible with the hydrocarbon phase into
larger
and coarser droplets. The coalescing material is used in the form of a packing
in
which the material has a critical wetting surface energy intermediate the
surface
tensions of the liquids forming the continuous and discontinuous phases, that
is, of
the hydrocarbon majority component and the water which is to be removed. In
practice, this means that the medium needs a surface energy of less than about
72
dynes/cm. Similarly, the material of the separating element is selected so as
to have
a surface energy which permits passage of the majority hydrocarbon component
through the small pores of the separator material but to preclude transfer of
the
water across the wall. In this case, since water is the discontinuous phase
which is
to be separated (along with the alcohol/water injected) the separator
materials are
selected to have a critical surface energy (CWST) below the surface tension of
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-21-
water which is typically about 72 mN-m 1. As disclosed in U.S. 5,443,724,
materials preferred for use as the phase barrier material for the separator
include
silicones, such as silicone treated paper and more preferably fluoropolymeric
materials of which fluorocarbons or perfluorocarbons (perfluoro resins) are
particularly preferred. Examples of preferred materials for use as the packing
or
coating in the separator include polytetrafluoroethylene (PTFE) or other
polyfluorinated polymers such as fluorinated ethylenepropylene (FEP) resins.
As
noted, a preferred separator material includes a coating of one of these
materials on
a stainless steel screen or a pleated paper pack. Other suitable materials
include
those disclosed in U.S. 4,759,782 to which reference is made for a disclosure
of
such materials. Generally, the phase barrier material which acts to prevent
the
discontinuous phase passing through it (and is therefore appropriately
referred to as
the discontinuous phase barrier material) is selected to have pores smaller
than a
substantial amount of the droplets of the liquid which forms the discontinuous
phase. Typically, the pore size of the functional part of the separator
material is
selected to be from 5 to 140 microns, preferably 40 to 100 microns. When, as'
in
this case, the discontinuous phase is water, the pore size is preferably
approximately
80 microns.
[00361 The coalescing unit and the separation unit may suitably be contained
in
a housing which provides and adequate number of coalescing/separating elements
with these elements being suitably arranged inside the housing for reasons of
functionality and operating convenience. A suitable arrangement is shown in U.
S.
5,443,724, using coalesces and separator cartridge elements arranged in super
posed
relationship with one another in a cylindrical type housing which permits
ready
access to the cartridges when they require replacement. However, other
CA 02706772 2010-05-26
WO 2009/070255 PCT/US2008/013047
-22-
configurations may be used and reference is made to commercial suppliers of
this
equipment including Pall Corporation of East Hills, NY 11548.