Note: Descriptions are shown in the official language in which they were submitted.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 1 -
Implant for parastomal hernia
The present invention relates to an implant suitable for use in the
prevention and/or treatment of hernias that may occur in the area of a
stoma, particularly one formed in the abdominal wall.
Stomas are openings formed in a wall, for example the abdominal wall, for
joining a hollow organ, for example the intestine, to the skin. Such an
operation proves necessary, for example in cases of cancer of the rectum
or Crohn's disease, to create an artificial anus for example, during which
operation the diseased part of the intestine is resected and the healthy
intestine is exteriorized at the skin. In this case, the stoma is formed in
the
abdominal wall. Figure 1 is a schematic illustration of the human digestive
tract. This diagram shows the stomach 1, the small intestine 2 and the
colon 3. The broken lines represent the part 3a of the colon that is diseased
and has been removed during the surgical procedure. The healthy part 3b
of the colon now opens to the outside at the stoma 4 formed in the
abdominal wall. Depending on the extent of the diseased part of the colon,
the stomas can be formed in the area of the ileum 5 (ileostomy) or of the
colon (colostomy), as shown in Figure 1.
Stomas can also be formed in the area of the ureters (ureterostomy).
After operations of this kind, hernias may develop around the stoma, that is
to say in the area of the peristomal wall. A weakening of the wall around
the stoma may therefore result in the appearance of a parastomal hernia.
To treat these parastomal hernias, prostheses are implanted that are
designed to strengthen the abdominal wall inside the patient, in the area of
the stoma. The implantation of these prostheses can be intraperitoneal,
that is to say within the actual abdominal wall, or retroperitoneal, resting
against the abdominal wall.
Prostheses for treating parastomal hernias have been described in the
document W02004/071349. However, these prostheses are not entirely
satisfactory, particularly since they are not adapted to all types of stomas
that are formed, particularly indirect stomas.
The reason is that, for example in the case of the colon, several stoma
configurations can be formed: the direct stoma, as shown in Figure 2, in
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
2 -
which the colon 3 issuing from the abdominal cavity 8 is perpendicular to
the abdominal wall 7 and hence to the skin 6 prior to its exteriorization, or
the indirect stoma, as shown in Figure 3, in which the colon 3 is caused to
form a bend within the abdominal cavity 8 prior to its exteriorization, the
colon thus having a part 3c parallel to the abdominal wall 7. The indirect
stoma avoids a situation where the internal part of the colon in the area of
the stoma becomes invaginated and exteriorizes.
There is therefore a need for a parastomal prosthesis able to protect the
1o intestine and hollow organs and to effectively strengthen the abdominal
wall regardless of the type of stoma that has been formed.
The present invention aims to meet this need by making available an
implant that has specific surfaces able to protect the hollow organs, such
as the intestine, regardless of the stoma that has been formed, and at the
same time to effectively strengthen the abdominal wall.
The subject matter of the present invention is an implant for the prevention
or treatment of a hernia formed in the abdominal wall in the proximity of a
stoma of an organ, comprising a layer of a porous structure whose surface
intended to face the abdominal cavity is covered by a first film of anti-
adhesive material, characterized in that said porous structure comprises a
first part intended to be in contact with the stoma organ and having a first
thickness El, and a second part having a second thickness E2 greater than
said first thickness El, said first part being covered, on its surface
intended
to face the abdominal wall, by a second film of anti-adhesive material.
Thus, in the implant according to the invention, the first part of the porous
structure, the part intended to be in contact with the stoma organ, for
3o example in contact with the intestine, is covered by a film of anti-
adhesive
material on both of its surfaces. In one embodiment of the invention, the
first and second films of anti-adhesive material are joined to form just one
film, and the first part of porous structure is totally enclosed within the
film
of anti-adhesive material. As will become clear from the explanations given
later with reference to Figures 13 to 15, the stoma organ, for example the
intestine, is protected irrespective of whether the stoma is a direct or
indirect one, because the part of the implant able to come into contact with
it is covered by a film of anti-adhesive material.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 3 -
In the present application, an "implant" is understood as a biocompatible
medical device that can be implanted in the human or animal body.
Within the meaning of the present application, the word "porous" is
understood as the characteristic according to which a structure has pores
or meshes, pockets, holes or orifices, that are open and are distributed
uniformly or irregularly and promote all cell colonization. The pores can be
present in all types of configurations, for example as spheres, channels,
hexagonal forms.
According to one embodiment of the invention, the porous structure
comprises a sponge, a fibrous matrix or a combination of a sponge and of a
fibrous matrix. For example, the sponge can be obtained by Iyophilization
of a gel, with pores being created during the Iyophilization. The fibrous
matrix can be any arrangement of yarns or yarn portions creating pores
between the yarns and/or yarn portions. For example, the fibrous matrix
can be a textile, for example obtained by knitting or weaving or according to
a technique for producing a nonwoven.
In one embodiment of the invention, the porous structure, for example the
sponge and/or the fibrous matrix, has pores with dimensions ranging from
approximately 0.1 to approximately 3 mm.
In one embodiment of the invention, the porous structure comprises a
textile. For example, the porous structure can be composed of a textile.
According to one embodiment of the invention, said thickness El of the first
part of the porous structure ranges from approximately 0.15 to 0.50 mm. A
relatively small thickness of this kind allows the abdominal wall to be
strengthened without any risk of damaging the stoma organ, for example
the intestine, which is in contact with the implant.
Said first part of porous structure is preferably a textile in the form of a
knit.
This knit is preferably a two-dimensional knit, that is to say preferably a
knit
having a thickness less than or equal to 5 times the mean diameter of the
yarns from which it is made, for example knitted on a warp knitting machine
or raschel machine with the aid of two guide bars forming a knit with two
surfaces, said knit being free of sheets of connecting yarns between its two
opposite surfaces.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 4 -
When the first part of porous structure is a two-dimensional knit as defined
above, the pores are formed by the empty spaces situated between the
constituent yarns of the knit, for example the meshes.
The constituent yarns of the knit that form the first part of porous structure
can be chosen from among yarns made of biocompatible materials,
bioabsorbable materials, non-bioabsorbable materials and their mixtures.
In the present application, the word "bioabsorbable" is understood as the
characteristic according to which a material is absorbed by the biological
tissues and disappears in vivo at the end of a given period, which can vary
for example from one day to several months, depending on the chemical
nature of the material.
Thus, examples of bioabsorbable materials suitable for the yarns forming
the first part of porous structure are polylactic acid (PLA), polysaccharides,
polycaprolactones (PCL), polydioxanones (PDO), trimethylene carbonates
(TMC), polyvinyl alcohol (PVA), polyhydroxyalkanoates (PHA), polyamides,
polyethers, oxidized cellulose, polyglycolic acid (PGA), copolymers of these
materials and their mixtures.
Examples of non-bioabsorbable materials suitable for the yarns forming the
first part of porous structure are polypropylenes, polyesters such as
polyethylene terephthalates, polyamides, polyvinylidene fluoride, and their
mixtures.
The yarns forming the first part of porous structure of the implant can, for
example, be chosen from among monofilament yarns, multifilament yarns
3o and their combinations.
The multifilament yarn count preferably varies from 40 to 110 dtex.
The monofilament yarns preferably have a diameter ranging from 0.06 to
0.15 mm.
In one embodiment of the invention, the yarns forming the first part of
porous structure are monofilament yarns. Such monofilament yarns pose
less risk of sepsis than do multifilament yarns.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
5,-
In one embodiment of the invention, the monofilament yarns are of
polyethylene terephthalate.
A monofilament yarn suitable for the first textile part of the implant
according to the invention is, for example, a monofilament yarn with a
diameter of approximately 0.08 mm, of polyethylene terephthalate.
The porous structure of the implant according to the invention comprises a
second part with a thickness E2 greater than the thickness El of the first
part. The second part of porous structure is essentially designed to act as a
reinforcement of the abdominal wall.
Thus, the value of the thickness E2 of the second part of porous structure
can vary depending on the value of the thickness El of the first part of the
structure, the value of the thickness E2 of the second part of porous
structure needing to be greater than that of the value of the thickness El of
the first part of porous structure. The reason is that the second part of
porous structure preferably has mechanical strength superior to that of the
first part of porous structure. For example, said second thickness E2 of the
second part of the porous structure can range from approximately 0.40 to
3.00 mm.
As will become clear from the description that follows, the surface of the
layer of porous structure intended to be placed facing the abdominal cavity
is covered by a film of anti-adhesive material which prevents the organs
and other viscera of the abdominal cavity from attaching themselves to the
implant. This surface will be referred to hereinafter as the closed surface of
the implant. By contrast, the surface of the second part of porous structure
intended to be placed facing the abdominal wall is not covered by a film of
anti-adhesive material and remains open to all cell colonization at the time
of implantation. This surface will be referred to hereinafter as the open
surface of the second part of porous structure. This surface of the second
part of porous structure is intended to be placed resting against the
abdominal wall. To permit better fixing of the implant to the abdominal wall,
the open surface of the second part of porous structure can comprise
fastening means, for example self-fixing ones, inherent to this surface.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
6 -
Thus, by virtue of its porous character and its thickness, the second part of
porous structure of the implant according to the invention is especially
adapted to promote tissue growth via its open surface after implantation.
The cells of the abdominal wall deeply colonize the second part of porous
structure by way of its open surface placed facing the abdominal wall.
In one embodiment of the invention, the second part of porous structure is
a textile in the form of a three-dimensional knit, for example as described in
applications W099/06080 and W099/05990. Within the meaning of the
lo present application, the term "three-dimensional knit" is understood as an
assembly or arrangement of monofilament or multifilament yarns or a
combination of these, obtained by knitting and having two opposite
surfaces that are separated by a significant thickness, preferably of greater
than or equal to 0.50 mm, said thickness comprising connecting yarns and
pores.
Such a three-dimensional knit can be knitted, for example, on a warp
knitting machine or double-bed raschel machine with the aid of several
guide bars forming a knit that comprises two opposite surfaces and a
spacer. In the present application, the word "spacer" is understood as the
set or sets of yarns that connect the two surfaces of a three-dimensional
knit to each other, thereby constituting the thickness of a knit, as is
described in W099/06080 or in W099/05990.
Thus, in the case where the. second part of porous structure is a three-
dimensional knit as described above, the knitting structure can define within
the thickness of the knit a multiplicity of transverse channels or pockets
that
may or may not be. mutually parallel. These pockets or channels can be
interconnected and thus allow the colonizing cells to pass from one pocket
or channel to another. A second part of porous structure of this type
promotes good tissue growth after implantation.
The yarns constituting the second part of porous structure of the implant
according to the invention can be chosen from among yarns made of
biocompatible materials, bioabsorbable materials, non-bioabsorbable
materials and their mixtures, already listed above for the first part of
porous
structure.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
7 -
Thus, examples of bioabsorbable materials suitable for the yarns forming
the second part of porous structure are polylactic acid (PLA),
polysaccharides, polycaprolactones (PCL), polydioxanones (PDO),
trimethylene carbonates (TMC), polyvinyl alcohol (PVA),
polyhydroxyalkanoates (PHA), polyamides, polyethers, oxidized cellulose,
polyglycolic acid (PGA), copolymers of these materials and their mixtures.
Examples of non-bioabsorbable materials suitable for the yarns forming the
second part of porous structure are polypropylenes, polyesters such as
polyethylene terephthalates, polyamides, polyvinylidene fluoride, and their
mixtures.
The yarns forming the second part of the porous structure can, for
example, be chosen from among monofilament yarns, multifilament yarns
and their combinations.
The multifilament yarn count preferably varies from 40 to 110 dtex.
The monofilament yarns preferably have a diameter ranging from 0.06 to
0.15 mm.
In one embodiment of the invention, the yarns forming the first part of
porous structure are monofilament yarns. Such monofilament yarns pose
less risk of sepsis than do multifilament yarns. For example, the
monofilament yarns are of polyethylene terephthalate.
A monofilament yarn suitable for the second part of porous structure of the
implant according to the invention is, for example, a monofilament yarn with
a diameter of approximately 0.08 mm, of polyethylene terephthalate.
In one embodiment of the invention, said second part of porous structure
has, on its open surface intended to face the abdominal wall, means of
fastening said second part to said abdominal wall. These fastening means
can be chosen from among elements that are integrally formed on said
second textile part, such as loops and barbs, or from among elements
joined to the surface of the second textile part, such as a rough covering,
hooks, threads or clips fixed on the surface of the second textile part.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 8 -
In one embodiment of the invention, said fastening means are chosen from
among loops, barbs and their mixtures. In such a case, the loops and barbs
can be obtained from yarns or portions of yarns that are woven and/or
knitted directly for example, with the three-dimensional knit forming the
second part of porous structure. For example, in order to obtain barbs, it is
possible to use hot-melt yarns such as are described in the application
WOO1/81667.
In the embodiment of the invention in which the first part of porous structure
is in the form of a two-dimensional knit and the second part of porous
structure is in the from of a three-dimensional knit, the two knits, i.e. two-
dimensional and three-dimensional, can be manufactured separately then
joined together by at least one seam, for example, in order to form the layer
of porous structure of the implant.
In another embodiment, the two-dimensional knit and the three-dimensional
knit are knitted together on the same knitting machine and constitute a
textile made in one piece, for example by using supplementary guide bars
for the three-dimensional knit and/or different yarn runs for producing each
of the two knits. In such an embodiment of the invention, the porous
structure layer of the implant according to the invention is composed of a
textile formed in one piece, said textile having a two-dimensional zone,
corresponding to the first part of the porous structure, and one or more
three-dimensional zones, corresponding to the second part of the porous
structure. In such an embodiment, it is possible to form a selvage at the
passage from a two-dimensional zone to a three-dimensional zone with a
view to forming a smooth connection between the two parts, such that the
difference in thickness between the two parts does not form a step that
could damage the biological tissue situated in the proximity of the implant.
The layer of porous structure of the implant according to the invention is
covered, on its second surface intended to face the abdominal cavity, by a
first film of anti-adhesive material. Moreover, the first part of porous
structure is covered, on its surface intended to face the abdominal wall, by
a second film of anti-adhesive material.
Within the meaning of the present application, the term "anti-adhesive
material" is understood as a smooth and non-porous biocompatible
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
9 -
material that prevents the organs and other viscera of the abdominal cavity
from attaching themselves to the implant.
The anti-adhesive material forming the first film can be identical to or
different from the material forming the second film.
In one embodiment of the invention, the anti-adhesive material constituting
the first and/or second film(s) is chosen from among bioabsorbable
materials, non-bioabsorbable materials and their mixtures.
In one embodiment of the invention, the bioabsorbable materials suitable
for the first and/or second film(s) of anti-adhesive material are chosen from
among collagens, oxidized celluloses, polyarylates, trimethylene
carbonates, caprolactones, dioxanones, glycolic acid, lactic acid,
glycolides, lactides, polysaccharides, for example chitosans, polyglucuronic
acids, hylauronic acids, dextrans and their mixtures.
In one embodiment of the invention, the non- bioabsorbable materials
suitable for the first and/or second film of anti-adhesive material are chosen
from among polytetrafluoroethylene, polyethylene glycols, polysiloxanes,
polyurethanes, stainless steels, derivatives of precious metals and their
mixtures.
In one embodiment of the invention, the material constituting the first and/or
second film(s) of anti-adhesive material is a hydrophilic bioabsorbable
material, preferably chosen from the group formed by collagens,
polysaccharides and their mixtures. Of the collagens that can be used
according to the invention, the following may be mentioned:
1) collagen whose helical structure is at least partially denatured by heat,
without hydrolytic degradation, and whose method of preparation is
described in W099/06080,
2) native collagen, not heated, filmed with or without glycerol, crosslinked
by gamma irradiation or by other chemical or physical means,
3) and/or their mixtures.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 10 -
Of the polysaccharides that can be used as absorbable hydrophilic material
according to the invention, the following may be mentioned: oxidized
cellulose, hylauronic acid, starch, chitosan, crosslinked dextrans and/or
their mixtures. All these materials are well known to persons skilled in the
art. An oxidized cellulose suitable for the present invention is the product
sold under the brand name "Interceed " by Ethicon. A hyaluronic acid
suitable for the present invention is the product sold under-the brand name
"Hyalobarrier " by Fidia Advanced Biopolymers, or the product sold under
the brand name "Seprafilm " by Genzyme.
In one embodiment of the invention, the first film and the second film form a
single and unique film, the first film then completely coating the first part
of
porous structure and thus covering this porous structure part both on its
surface intended to face the abdominal cavity and also on its surface
intended to face the abdominal wall. Thus, said first part of porous structure
is totally enclosed in the film of anti-adhesive material before implantation
and at the moment of implantation.
Thus, at the moment of implantation, and whatever the embodiment of the
invention, the two surfaces of the first part of porous structure are occluded
by a continuous film of anti-adhesive material.
The first part of the porous structure of the implant according to the
invention, regardless of whether it is totally coated by the first film of
anti-
adhesive material or whether each of its surfaces are covered, one by the
first film of anti-adhesive material, the other by the second film of anti-
adhesive material, is thus protected at least during the initial phase of
cicatrization, i.e. is not exposed to the inflammatory cells such as
granulocytes, monocytes, macrophages, or the multinucleated giant cells
that are generally activated by the surgical procedure. Nor is it exposed to
the bacteria that may be present. The reason for this is that, at least during
the initial phase of cicatrization, which may last approximately 5 to 10 days,
only the film or films of anti-adhesive material are accessible to the various
factors such as proteins, enzymes, cytokines or inflammatory cells, in the
first textile part.
In the case where the film or films of anti-adhesive material are made of
non-absorbable materials, they thus protect the first part of porous structure
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 11 -
before and after implantation, throughout the period of implantation of the
implant.
Furthermore, by virtue of the film or films of anti-adhesive material, the
surrounding fragile tissues, such as the hollow viscera for example, are
protected in particular from the formation of severe postsurgical fibrous
adhesions.
In the case where the anti-adhesive material comprises a bioabsorbable
material, it is preferable to choose a bioabsorbable material that is not
absorbed until after a few days, such that the film of anti-adhesive material
can perform its function of protecting the stoma organ, for example the
intestine, and the hollow organs during the days following the operation,
and until the cellular recolonization of the implant in turn protects the
fragile
organs.
The thickness of the first anti-adhesive film is preferably much less than the
thickness E2 of the second part of porous structure. In fact, the film of anti-
adhesive material must preferably not occlude the open surface of the
second part of the porous structure, so as to permit cellular recolonization
of the second part of porous structure after implantation.
The first film of anti-adhesive material is preferably continuous, smooth and
non-porous, covering the whole surface of the porous structure intended to
be placed facing the abdominal cavity. In one embodiment, the first film of
anti-adhesive material extends past the edges of the layer of porous
structure. Thus, the implant is protected from contact with the viscera. The
first film of anti-adhesive material can, for example, extend past the edges
of the layer of porous structure by a distance ranging from 3 to 10
millimetres.
The first film of anti-adhesive material is preferably joined to the surface
of
the layer of porous structure intended to be placed facing the abdominal
cavity by means of surface penetration, keeping open the porosity on the
opposite surface of the second part of the porous structure, that is to say
the open surface, intended to be placed facing the abdominal wall.
The implant according to the invention can be used via the laparoscopic
route. If necessary, for example when the first and second films of anti-
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 12 -
adhesive material are made of dried collagen, it is preferable to rehydrate
the implant at the time of use, in order to make it flexible and easier to
use.
The implant according to the invention can, for example, be prepared
according to the following method:
a) a textile is prepared that has two-dimensional zones and three-
dimensional zones, as has been described above,
I o b) a solution of an anti-adhesive material is prepared,
c) the solution obtained at b) is poured into a mould,
d) the textile is then applied to the solution, the surface of the textile
intended to face the abdominal cavity being placed on said solution in such
a way that said solution impregnates the two-dimensional zones of said
textile completely,
e) it is left to dry.
With such a method it is possible to obtain an implant according to the
invention in which the first film and the second film form a single and
unique film.
Alternatively, step d) is replaced by step d') in which the solution of anti-
adhesive material only superficially impregnates a single surface of the
two-dimensional zones, thereby forming the first film. The procedure is then
supplemented by an additional step in which the opposite surface of the
two-dimensional zones is impregnated by the same solution of anti-
3o adhesive material or by another solution of another anti-adhesive material
in order to form the second film.
Methods of covering/coating that can be used according to the present
invention are described in documents W099/06080 and W02004/043294.
The implant according to the invention can have any shape adapted to the
anatomy of the patient and/or to the surgical technique envisaged. For
example, the shape of the implant can be round, oval, square or
rectangular.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 13 -
In one embodiment, the implant has a generally elongate shape, for
example oval or rectangular. For example, the length of the implant can
range from 12 to 30 cm and its width can range from 10 to 20 cm.
In another embodiment, the implant has a generally round shape. For
example, the diameter of the implant can range from 5 to 20 cm.
In one embodiment of the invention, said first part of porous structure has
lo the form of a central strip, and, for example, the width of the central
strip
can range from 2 to 10 cm.
In another embodiment of the invention, said first part of porous structure
has the form of a disc, and, for example, the diameter of the disc can range
from 2 to 10 cm.
In one embodiment of the invention, at least one orifice is formed at the
centre of the first part of the porous structure in order to provide a passage
for the stoma organ, for example the intestine, during implantation of the
implant. Alternatively, at least one orifice is formed within the first part
of
the porous structure, said orifice being offset relative to the centre of the
implant. For certain types of surgery, for example ureterostomies, the
implant can have two orifices. In one embodiment of the invention, the
orifice or orifices can be connected to an edge of the implant by way of a
slit. For example, the dimensions of the orifices can range from 0.5 to 8 cm.
The orifice or orifices can be offset relative to the centre of the implant.
The advantages of the present invention, and variants thereof, will become
evident from the following detailed description and from the attached
3o drawings, in which:
Figure 1 is a schematic illustration of the human digestive tract, in which a
stoma has been formed,
Figure 2 is a schematic illustration of a direct stoma,
Figure 3 is a schematic illustration of an indirect stoma,
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 14 -
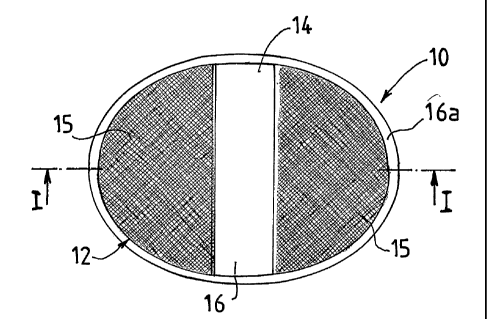
Figure 4 is a plan view of a first embodiment of an implant according to the
invention,
Figure 5 is a plan view of a second embodiment of an implant according to
the invention,
Figure 6 is a simplified schematic cross-sectional view of the implant from
Figure 4,
Figure 7 is a photograph taken with a Hitachi S-800 FEG scanning electron
microscope, magnification x40, showing an embodiment of the first part of
porous structure of an implant according to the invention,
Figure 8 is a photograph taken with a Hitachi S-800 FEG scanning electron
microscope, magnification x250, showing the first part of porous structure
from Figure 7 once enclosed in the film of anti-adhesive material,
Figure 9 is a photograph taken with a Hitachi S-800 FEG scanning electron
microscope, magnification x20, showing an embodiment of the second part
of porous structure of an implant according to the invention, covered on
one surface by the first film of anti-adhesive material,
Figures 10, 10A, 11 and 12 show embodiments of the knitting structure
suitable for producing a textile for an implant'according to the invention,
Figure 13 is a cross-sectional view of an implant according to the invention
once it has been implanted after a direct colostomy,
Figure 14 is a schematic plan view of another embodiment of an implant
3o according to the invention once it has been implanted after an indirect
colostomy,
Figure 15 is a cross-sectional view of the implant from Figure 14 along the
line II in Figure 14.
Referring to Figures 4 and 6, an implant 10 according to the invention is
shown which comprises a layer of porous structure in the form of a
biocompatible textile 11. As will be seen more clearly from Figures 13 and
15, the layer of porous structure or textile 11 comprises a first surface 12
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 15 -
intended to be placed facing the abdominal wall after implantation, and a
second surface opposite the first surface 12, this second surface 13 being
intended to be placed facing the abdominal cavity after implantation.
As will be seen clearly from Figure 4, which is a plan view of an implant
according to the invention, the layer of porous structure comprises a first
textile part 14 and a second textile part 15, the first textile part and the
second textile part together forming the biocompatible textile 11 (see Figure
6). As will be seen more clearly from Figures 13-15 regarding the first
lo surface 12 of the biocompatible textile 11, the first part 14 of textile is
able
to come into contact with the intestine, and the second part 15 of textile is
intended to be placed facing the abdominal wall once the implant 10
according to the invention is implanted in the patient.
The implant 10 shown in Figure 4 is oval in shape. Its length can range, for
example, from 15 to 30 cm, and its width can range, for example, from 12
to 20 cm. The shape of the implant can be adapted to the anatomy of the
patient. It can also vary depending on the surgical technique envisaged.
In one example not shown, the implant has a generally round shape. Its
diameter can then range from 5 to 20 cm, for example.
Referring to Figure 6, the implant 10 according to the invention is covered
on its second surface 13 by a film 16 of anti-adhesive material. The edge
16a of the film of anti-adhesive material extends past the second surface
13 of the textile 11, for example by a distance ranging from 3 to 10 mm.
Thus, the implant 10 is protected from contact with the viscera when it is
implanted.
3o Figure 6 is a simplified cross-sectional view of the implant from Figure 4
along line II, where the thickness of the film 16 is exaggerated to make
matters easier to understand. As will be seen clearly from Figure 6, the first
part 14 of textile and the second part 15 of textile each have a thickness,
namely a thickness El and a thickness E2, respectively. The value of the
thickness E2 of the second part 15 of textile is superior to the value of the
thickness El of the first part 14 of textile. Moreover, the film 16 completely
encompasses the first part 14 of textile but only penetrates superficially
into
the thickness E2 of the second part 15 of textile. In Figure 6, the thickness
of the film 16 is exaggerated. It must be understood that the film 16
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 16 -
penetrates into the second part 15 of textile only by a short distance, for
example by a distance corresponding to 2 to 10% of the thickness E2. In
the example shown, the value of the thickness El is 0.75 mm, while that of
the thickness E2 is 2.00 mm.
Thus, as will be seen clearly from Figure 6, the first part 14 of textile is
covered by film 16 of anti-adhesive material on its two surfaces, and this
first part 14 of textile is totally enclosed within the film 16 of anti-
adhesive
material.
By contrast, as regards the second part 15 of textile, its first surface 12,
intended to be placed facing the abdominal wall, is not covered by film 16
of anti-adhesive material. This surface 12 will be referred to hereinbelow as
the open surface of the second part 15 of textile. By contrast, the second
surface 13 intended to be placed facing the abdominal cavity, is covered by
film 16 of anti-adhesive material. This surface 13 will be referred to
hereinbelow as the closed surface of the second part of textile. Thus, the
film 16 of anti-adhesive material penetrates only superficially into the
second part 15 of textile, in the area of its closed surface 13, leaving open
the porosity of the first open surface 12 of the second textile part 15.
Figure 7 shows a view of the first part 14 of textile. In this example, the
first
part of textile is a knit obtained on a warp knitting machine or raschel
machine with two guide bars A and B, threaded regularly with one guide
full, one guide empty, using the knitting structure shown in Figure 10 for
bars A and B. The respective charts used for bars A and B are the
following:
Bar A: 4-4-5-4/4-4-4-3/3-3-2-1/1-1-0-1/1-1-1-2/2-2-3-4//
Bar B: 1-1-0-1/1-1-1-2/2-2-3-4/4-4-5-4/4-4-4-3/3-3-2-1//
The yarn used is preferably a monofilament yarn of polyethylene
terephthalate, diameter 0.08 mm and titre 69 dtex. The knit thus formed
comprises two opposite surfaces but is free of connecting sheets between
its two opposite surfaces. It is a two-dimensional knit according to the
present application.
The thickness of the first part of textile formed from such a knit is
approximately 0.25 mm.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 17 -
In the example shown, the knitting used for the first part of textile creates
pores, preferably with dimensions that can range from 0.1 to 3 mm,
preferably from 1.5 to 2 mm. At the moment of implantation, these pores
are not visible, nor are they accessible to tissue colonization, because the
whole of the first part of textile is confined in the film 16 of anti-adhesive
material. However, after a few days, as the film of anti-adhesive material is
absorbed and disappears after performing its function of limiting and/or
avoiding formation of adhesions during the first 10 days following the
implantation operation, the pores of the first part 14 of textile become
accessible to tissue colonization. When a yarn of polyethylene
terephthalate is used for producing the two-dimensional knit, this knit is
non-bioabsorbable and remains permanently at the implantation site.
In another embodiment of the invention, said first part 14 of textile is made
of a bioabsorbable material that is absorbed more slowly than the
bioabsorbable material constituting the film 16 of anti-adhesive material.
As is shown in Figure 8, which is a scanning electron microscope
photograph of a section of the implant according to one embodiment of the
invention in the area of the first textile part, the latter is enclosed in the
film
16 of anti-adhesive material. The coating of the first part 14 of textile by
the
film 16 of anti-adhesive material can be effected using any method known
to a person skilled in the art. In the example shown in Figure 8, the first
part
14 of textile is coated using the method described in the application
W02004/043294.
Thus, as will be seen clearly from Figure 8, the first part 14 of textile is
covered by the film of anti-adhesive material on its two surfaces, and the
porosity (see Figure 7) of the first part of textile is totally occluded at
the
moment of implantation. Thus, once covered with a film 16 of anti-adhesive
material, the two surfaces of the first part 14 of textile are smooth and non-
porous, as shown in Figure 8. The two surfaces of the first part 14 of textile
do not damage the organs situated in the proximity of this first part 14 of
textile, particularly the stoma organs.
The second part 15 of textile, of which the thickness is greater than that of
the first part 14 of textile, can be a knit which is obtained on a warp
knitting
machine or double-bed raschel machine and which has two opposite
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 18 -
surfaces connected to each other by connecting yarns, that is to say a
three-dimensional knit according to the present application. For example, a
first surface of the knit is produced with the two guide bars A and B already
mentioned above for producing the first part 14 of textile, these being
threaded identically and with the same charts as above. The second
surface of the knit is produced with two supplementary guide bars D and E,
threaded with one guide full, one guide empty, using the knitting structure
shown in Figure 10 for bars D and E. The respective charts used for bars D
and E are the following:
Bar D: 0-1-1-1/1-2-2-2/3-4-4-4/5-4-4-4/4-3-3-3/2-1-1-1//
Bar E: 5-4-4-4/4-3-3-3/2-1-1-1/0-1-1-1/1-2-2-2//3-4-4-4//
The connection of the two surfaces can be effected, for example, by
hooking one loop in two, or in three, or in four, or in five, or in six of one
of
the bars D or E, whose knitting structure will be adapted. For example, in
one embodiment of the invention, the connection of the two surfaces is
effected by hooking one loop in three of the bar E, which thus becomes bar
E', with the knitting structure shown in Figure 10A and according to the
following chart:
Bar E': 5-4-3-4/4-3-3-3/2-1-1-1/0-1-2-1/1-2-2-2/3-4-4-4//
In another embodiment, the connection of the two surfaces can be effected
with the aid of a fifth guide bar C, with the knitting structure shown in
Figure
11 and according to the following chart:
Bar C:
Thus, when the first part 14 of textile is in the form of a central strip
separating two lateral strips of the second part 15 of textile, as is shown in
Figures 4 and 5, the textile 11 can be produced in one piece, on the same
knitting machine.
With the guide bars A, B, D and E' described above:
- the whole of the first surface 13 of the textile 11 is produced with the two
guide bars A and B,
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 19 -
- along a first length, corresponding to the first lateral strip of the second
part 15 of textile, the guide bars D and E' are threaded one guide full, one
guide empty, in order to produce the second surface of the three-
dimensional knit forming said second part 15 of textile,
- then, along the length corresponding to the width of the central strip of
the
first part 14 of textile, the guide bars D and E' are left empty in order to
form
the two-dimensional knit,
- finally, along a length corresponding to the second lateral strip of the
second part 15 of textile, the guide bars D and E' are again threaded one
guide full, one guide empty, in order to produce the second surface of the
three-dimensional knit forming said second part 15 of textile.
In such a case, the optional fifth guide bar C is threaded only in the zones
of the three-dimensional knit.
Finally, in order to obtain a smooth join between the three-dimensional knit
forming the second part 15 of textile and the two-dimensional knit forming
the first part 14 of textile, it is possible to use, still on the same
knitting
machine, a supplementary guide bar F in order to finish the edges of the
three-dimensional knits, threaded in the area of these edges, according to
the knitting structure shown in Figure 12, using the following chart for
example:
Bar F: 1-0-1-1/1-2-1-1//
A monofilament yarn will preferably be chosen to produce the second part
15 of textile. This is because multifilament yarns pose greater risks of
3o bacteria developing in the interstices present between the various
filaments
of the yarn.
The yarn used is preferably a monofilament yarn of polyethylene
terephthalate, with a diameter of approximately 0.08 mm and titre 69 dtex.
The thickness of the second part 15 of textile, produced in the form of the
three-dimensional knit described above, is approximately 1.50 mm.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 20 -
As will be seen from Figure 9, the second part 15 of textile is covered, on
its surface intended to face the abdominal cavity, by the film 16 of anti-
adhesive material. The film 16 of anti-adhesive material penetrates only
superficially into the three-dimensional knit forming the second part 15 of
textile. Consequently, the surface of the second part 15 of textile intended
to face the abdominal wall is open, and its porosity is not occluded. This
open surface therefore promotes all cellular growth.
The superficial covering of the surface of the second part 15 of textile
intended to be placed facing the abdominal cavity can be carried out using
any method known to a person skilled in the art, for example using the
method described in the application W099/06080.
The material used for the film 16 of anti-adhesive material can, for
example, be collagen prepared in the manner described in the application
W099/06080.
The film 16 of anti-adhesive material may be applied to the surface of the
textile 11 intended to be placed facing the abdominal cavity, in the following
2 0 way:
- The solution of collagen is poured into a mould having the external
dimensions desired for the film. The textile produced above is then applied
to this solution, at the centre of the mould, the surface to be covered being
placed on the solution of collagen. The solution of collagen then penetrates
into the textile by capillary force, completely coating the first part of
textile
and covering the latter on the two opposite surfaces of the two-dimensional
knit forming it, and penetrating only by a small distance into the thickness
of the second part of textile, thus creating a superficial film for this three-
3o dimensional part. Once the collagen has dried, the film is cut around the
textile using a scalpel.
Alternatively, the covering/coating method described in W02004/043294
can be used.
In another embodiment not shown here, the film 16 only superficially covers
the surface of the first part of textile, intended to be placed facing the
abdominal cavity, and does not encompass the two opposite surfaces of
this first part of textile. In such a case, the surface of the first part of
textile
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 21 -
intended to be placed facing the abdominal wall is covered with a second
film of anti-adhesive material. Thus, each of the two opposite surfaces of
the first part of textile is covered by a smooth and continuous film of anti-
adhesive material. Covering methods that can be used to form this second
film are also described in W02004/043294.
Figure 13 shows an implant according to the invention after it has been
implanted, in the case of a direct stoma. To do this, the implant according
to the invention shown in Figure 5 is used for example. In this figure, the
lo reference numbers designating the same elements as in Figure 4 have
been retained. The implant 10 in Figure 5 comprises an orifice 17 which
has been created at the centre of the implant 10 and at the centre of the
central strip formed by the first part 14 of textile. Such an orifice 17 can
have a diameter ranging from I to 8 cm. A slit 18 starting from the central
orifice 17 and opening out on an edge of the implant 10 allows the implant
to be adjusted around the colon 3 during implantation of the implant.
In one embodiment not shown here, the orifice 17 is offset relative to the
centre of the implant 10. It is also possible to have several orifices,
2o depending on the surgery envisaged.
Thus, in Figure 13, an implant 10 similar to that in Figure 5 has been
placed around the colon 3, which is at right angles to the abdominal wall 7
and to the skin 6. As will be seen from this figure, the first part 14 of
textile
covered entirely, that is to say on its two opposite surfaces, by the film 16
of anti-adhesive material is situated in direct proximity to the colon 3.
Thus,
the colon 3, which is a fragile organ, is not damaged by the implant 10. The
open surface of the second part 15 of textile, which is porous and promotes
cellular recolonization, is situated facing the abdominal wall 7. Thus, after
implantation, the cells of the abdominal wall can gradually colonize the
second part 15 of textile, for example the three-dimensional knit forming it.
It is possible to fix the implant 10 to the abdominal wall 7 using staples or
sutures. In addition, or alternatively, the open surface of the second part 15
of textile can intrinsically comprise barbs or loops, which will facilitate
its
natural attachment to the abdominal wall. Such an affixing knit is described
in the application WO01/81667.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 22 -
Finally, the second surface of the textile, completely covered by film 16 of
anti-adhesive material, is situated facing the abdominal cavity 8. Thus, the
hollow and fragile organs, the viscera, are not damaged by the implant.
Figures 14 and 15 show an implant according to the invention after it has
been implanted, in the case of an indirect stoma. To do this, the implant
according to the invention in Figure 4 is used, for example. Figure 14
shows a plan view of the implant 10 according to Figure 4 at its
implantation site in the area of the colon 3. For greater clarity, the skin
and
the abdominal wall have not been depicted. As will be seen from Figure 15,
which is a cross-sectional view of Figure 14 along line II-II and in which the
abdominal wall 7 and the skin 6 have been depicted, the colon 3 forms a
bend prior to exteriorization, and the implant 10 is placed inside this bend.
A part 3c of the colon is thus situated between the implant 10 and the
abdominal wall 7.
As will be seen from these two figures, the part 3c of the colon faces and is
able to come into contact with the first part 14 of textile covered on its two
opposite surfaces by the film 16 of anti-adhesive material. Thus, neither the
part 3c of the colon, situated between the implant 10 and the abdominal
wall 7, nor the part 3d of the colon corresponding to the second length of
the bend and able to lie under the implant 10 in the area of the abdominal
cavity 8, risks being damaged by the implant 10. This is because the parts
3c and 3d of the colon 3 are each facing a surface of the first part 14 of
textile covered by a film 16 of anti-adhesive material. Moreover, the
relatively small thickness El of this first part 14 of textile permits
flexible
and atraumatic support of the colon 3.
In an indirect stoma of this kind, the implant 10 essentially acts like a
3o hammock for the part 3c of the colon 3, and the implant 10 can be fixed to
the abdominal wall 7 via the open surface of the second part 15 of textile
placed facing the abdominal wall 7.
The present invention also relates to a method for treatment or prevention
of a hernia in the proximity of a stoma formed in the skin, comprising the
step of implanting an implant of the type described above in the area of the
stoma. In one embodiment of the invention, the implant is fixed to the
abdominal wall. The implant described above can be implanted by open
surgery or by laparoscopy.
CA 02706865 2010-05-26
WO 2009/071998 PCT/IB2008/003761
- 23 -
The implant according to the invention is used in particular in the treatment
of parastomal hernias. It is able to support and/or protect the organs that
are to be treated, such as the colon or ureters, without damaging them,
while at the same time effectively strengthening the wall in which the stoma
is formed, such as the abdominal wall, irrespective of the type of stoma
formed, i.e. direct stoma or indirect stoma.