Note: Descriptions are shown in the official language in which they were submitted.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
1
2-FLUOI QPYRAZOLO 1 5- PYRIMID1NES AS PROTEIN KINASE INHIBITORS
The present invention relates to novel pyrazolo 1,5-a]pyrimidine compounds
useful as protein kinase inhibitors, pharmaceutical compositions containing
the
compounds, and methods of treatment using the compounds and compositions to
treat diseases such as, for example, cancer, inflammation, arthritis, viral
diseases,
neurodegenerative diseases such as Alzheimer's disease, cardiovascular
diseases,
and fungal diseases. The compounds disclosed herein are especially useful as
cyclin
dependent kinase inhibitors, such as, for example, CDK-1 and CDK-2 inhibitors
and
Checkpoint inhibitors such as CHK-1 inhibitors.
BACKGROUND OF THE INVENTION
Cell growth and differentiation is a highly controlled process which, when
lost,
can lead to aberrant cell function, often resulting in a disease state.
Protein
phosphorylation is one of the main post translational mechanisms used to
control
cellular function. Protein kinases catalyze the phosphorylation of serine,
threonine
and tyrosine residues using either ATP or GTP. An analysis of the human genome
has
revealed that there are predicted to be - 500 protein kinases (Manning G.,
Whyte
D.B., Martinez R., Hunter T., Sudarsanam S., Science 298, 1912, 2002; Kostich
M,
English J, Madison V, Gheyas F, Wang L, Qiu P, Greene J, Laz TM., Genome Biol,
3(9), 2002). When phosphorylation regulation by these kinases is lost, a
number of
diseases may occur, including diabetes, Alzheimer's, inflammation, and cancer
(Cohen P., Eur. J. Biochem. 268, 5001-5010, 2001; Cohen P., Nat, Rev. Drug
Discovery 1, 309-315, 2002.) g
iyk~ `ijfe ..e uÃar si ca ..,l .k ,11 T. '~`'. ~ : ''. `+rr_ :fin a d apop-..
., ..,"Cdr key
rll cycle, which
division by regulating passage through the G1, S. G2, and M phases of DNA
synthesis
and mitosis. Protein kinase inhibitors, regulators or modulators, alter the
function of
kinases such as cyclin-dependent kinases (CDKs), mitogen activated protein
kinase
`'. , K ERK , d an s ithase ;ri se 3 '3SK. r a' ..J : -.,':pc f } . `.,
.,,rHK
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
2
Aurora C etc), and the like. Examples of protein kinase inhibitors are
described in
W002/22610 Al and by Y. Mettey et al in J. Med. Chem., (2003) 46 222-236.
Progression through the eukaryotic cell cycle is controlled by the cyclin
dependent kinase (CDK) family of kinases. CDKs are primarily serine/threonine
kinases and they bind to several different regulatory subunits called cyclins.
Different
CDKfcyclin heterodimers regulate a variety of processes in the cell cycle,
thus, it is
believed that CDK4/cyclin D and CDK2/cyclin E regulate control through G1 into
the
onset of the S phase. The down regulation of cyclin D and cyclin E and the up
regulation of cyclin A to form heterodimers with CDK2 and CDK1 promotes
passage
through the S-phase into G2. Finally, activated complexes of CDK1
(Cdc2)/cyclin B
and possibly CDK1 (Cdc2)/cyclin A are thought to promote the transition from
G2 into
the M-phase. (reviewed by Murray A., Cell 116, 221-234, 2004). Some of the
known
substrates for the CDKs are the tumor suppressor retinoblastoma protein (RB)
and
related family members p107 and p130 (Grana X., Garriga J., and Mayol X.,
Oncogene 17, 3365-3383, 1998). Phosphorylation of RB by CDK4 or CDK2 induces
the release of E2F transcription factors which in turn promote the expression
of
regulatory proteins to stimulate cell cycle progression and cell growth. In
human
tumors, the control of the RB function has been observed to be disrupted
through
mutation of the RB gene, CDK4 amplification, cyclin D and cyclin E over
expression,
inactivation of the CDK4 specific protein inhibitor p16lNK4A and a disruption
in the
level of the CDK inhibitor p27KIP1 (Sherr, C., Roberts J., Genes Dev. 13, 1501-
1512,
1999; Hall M., Peters G., Adv. Cancer Res. 68, 67-108, 1996; Stewart T.,
Wesfall M.,
Pietenpol J., Trends Pharmacol. Sci. 24, 139-145, 2003). These functional
disruptions
are believed to contribute to the development of breast, colon, gastric,
prostate,
nonsr all cell lure;, ovarian and other human cancers (Tsihiias J., Kapusta
..,
Slingcri4t -:d J. i . Rev. Med. 50, 401-423, 1999; Lloyd R., Erickson L., Jin
L., Kulig
E., Qian X., Cheville J., Scheithauser B., Am. J. Patrol. 154(4), 313-323,
1999).
The fact that uncontrolled regulation of the cell cycle pathway is thought to
be a
source of human cancers leads one to believe that inhibition of unregulated
CDK
o
ld be r<latment of cancers. A
u
lar6 nun, r- torts have bee sward developing
CDK c AT ~. t ,, e in out only a few r-.. / .j ales have progressed
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
3
into human clinical trials. These include flavopiridol, roscovitine (CYC-202)
and the 2-
aminothiazole derivative BMS-387032 (Zhai, S., Senderowicz A., Sausville E.,
Figg
W., Ann. Pharmacother. 36, 905-911, 2002; McClue S., Blake D., Clarke R.,
Cummings L., Fischer P., MacKenzie M., Stewart K., Wang S., Zhelev N., Zheleva
D.,
Lane D., Int. J. Cancer 102(5), 463-468, 2002; Misra R., et al., J.Med. Chem.
47,
1719-1728, 2004)
Another series of protein kinases are those that play an important role as a
checkpoint in cell cycle progression. Checkpoints prevent cell cycle
progression at
inappropriate times, such as in response to DNA damage, and maintain the
metabolic
balance of cells while the cell is arrested, and in some instances can induce
apoptosis
(programmed cell death) when the requirements of the checkpoint have not been
met.
Checkpoint control can occur in the G1 phase (prior to DNA synthesis) and in
G2,
prior to entry into mitosis.
One series of checkpoints monitors the integrity of the genome and, upon
sensing DNA damage, these "DNA damage checkpoints" block cell cycle
progression
in G1 & G2 phases, and slow progression through S phase. This action enables
DNA
repair processes to complete their tasks before replication of the genome and
subsequent separation of this genetic material into new daughter cells takes
place.
Inactivation of CHK1 has been shown to transduce signals from the DNA-damage
sensory complex to inhibit activation of the cyclin B/Cdc2 kinase, which
promotes
mitotic entry, and abrogate G2 arrest induced by DNA damage inflicted by
either
anticancer agents or endogenous DNA damage, as well as result in preferential
killing
of the resulting checkpoint defective cells. See, e.g., Peng et al., Science,
277, 1501-
1505 (1997); Sanchez et al., Science, 277, 1497-1501 (1997), Nurse, Cell 91.
865-
867 (1997); Kleinert, Science. 277, 1450-1451 (1997), Walworth et al,, "tip,
363,
368-371 (1993); and Al-Khodairy et al., lolec, BO L Cell., 5, 147-160 (1994).
Selective manipulation of checkpoint control in cancer cells could afford
broad
utilization in cancer chemotherapeutic and radiotherapy regimens and may, in
addition, offer a common hallmark of human cancer "genomic instability" #o be
t s~ ~.r born of cancer cells, A n .. f
place CHR1 l"A ge cft: ., ., 6troi. The
- of 1 of 1 . _.. d k, ;c., as CDDS1/CHK2, a
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
4
kinase recently discovered to cooperate with CHK1 in regulating S phase
progression
(see Zeng et al., Nature, 395, 547-510 (1998); Matsuoka, Science, 282, 1893-
1897
(1998)), could provide valuable new therapeutic entities for the treatment of
cancer.
There is a need to develop CDK and CHK1 inhibitors for the treatment of
human diseases, therefore, it is an objective of this invention to describe
compounds
that would be useful for the prevention or alteration of these diseases.
SUMMARY OF THE INVENTION
The invention relates to novel compounds and compositions containing those
compounds as well as methods of using the compounds. The compounds are
heterocyclic molecules that are useful in therapeutic applications, including
modulation
of disease or disease symptoms in a subject (for example, cat, dog, horse, or
human).
These diseases include Alzheimer's disease, cancer, diabetes, and
inflammation. The
compounds (including stereoisomers thereof) are synthesized either singly or
in a
combinatorial fashion to give structurally and stereochemically diverse
libraries of
compounds.
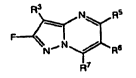
In certain embodiments, the compounds are fluoro substituted pyrazolo
pyrimidine compounds. In one embodiment, provided herein are compounds of
formula (I):
R3
Ni R
F-- Ir
N,N R'
R7
Formula (I)
or a pharraceuticafy acceptable sa't, solvate or ester thereof, wherein.
R`' is hydrogen, alkyl, cycioa k yl, cycienyl, alkynyl, trifluroalkyl,
difluroalkyl,
monofluroalkyl, heterocyclyl, heterocyclenyl, aryl, heteroaryl, halo, cyano,
_0_
trihaloalkyl, NR8R9, COO, CONR8R9, -OR8, -SR8, -S02R9, -S02NR8R9, -NR8S02R9, -
NR8COR9, or NR8CONR8R9, wherein each of said alkyl, cycloalkyl, cyclenyl,
alkynyl,
trif ... ' f , 4 Ik 3 :tern
-
.. .... qa
..,.$ from ~gp~ the
- _C.,.. _.[.. ~..~_ -eleroad the
1,
121 I rc ry
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
alkenyl, cyclenyl, heterocyclenyl, halo, trihaloalkyl, alkoxyl, hydroxyalkyl,
trihaloalkoxyl
and CN;
R5 and R7 are each selected from the group consisting of hydrogen, alkyl,
aminoalkyl, alkenyl, alkenyl, aryl, heterocyclyl, heterocyclenyl, cycloalkyl,
cyclenyl,
5 cycloalkylalkyl, cyclenylalkyl, cycloalkylalkenyl, cyclenylalkenyl,
heteroaryl,
heteroarylalkyl, heteroarylalkenyl, arylalkyl, arylalkenyl, heterocyclylalkyl,
heterocycle nylalkyl, heterocyclylalkenyl, heterocyclenylalkenyl, -S-
heterocyclyl, -S-
aminoalkyl, -S-heterocyclenyl, NR8R9, NRBCOR9, NR"SO2R9, CORI, C02R8,
CONRIR9, CH2OR8, ORI, SR8, and S02R8, wherein each of said alkyl, alkenyl,
alkynyl, aryl, heterocyclyl, heterocyclenyl, cycloalkyl, cyclenyl,
cycl'oalkylalkyl,
cyclenylalkyl, cycloalkylalkenyl, cyclenylalkenyl, heteroaryl,
heteroarylalkyl,
heteroarylalkenyl, arylalkyl, arylalkenyl, heterocyclylalkyl,
heterocyclenylalkyl,
heterocyclylalkenyl, heterocyclenylaikenyl, -S-heterocyclyl and -S-
heterocyclenyl can
be unsubstituted or substituted with at least one moiety, which can be the
same or
different, independently selected from the group consisting of halogen, alkyl,
trihaloalkyl, alkenyl, dihaloalkyl, monohaloalkyl, hydroxyalkyl, ORB, -0,
NR8R9, SRI,
S02R9, CN, S02NR"R9, and NO2;
R6 is hydrogen, halo, trihaloalkyl, alkyl, alkenyl, aryl, cyclenyl,
cycloalkyl,
heteroaryl, heterocyclenyl, heterocyclyl, heteroarylalkyl, heterocycle
nylalkyl,
heterocyclyfalkyl, arylalkyl, cyclenylalkyl, cyclylalkyl, NRIR9, NRICOR9,
NR8S02R9,
COR8, C02R8, CONR8R9, CH2OR8, ORI, SR8, or S02R8, wherein each of said alkyl,
alkenyl, aryl, cyclenyl, cycloalkyl, heteroaryl, heterocyclenyl, heterocyclyl,
heteroarylalkyl, heterocycienylalkyl, heterocyclylalkyl, arylalkyl,
cycienylalkyl, and
cyclylalkyl can be unsubstiuted or substituted with one or more moieties
independently
2.5 selected from the group consisting of halogen, alkyl, OR8, CN,
02R , CONRIR9, -SR8, S021 8, S02N1 8R5, NO2, NR8S02R~, NRBCORJ, and
NRBCONR8R9;
R8 and R9 are each independently selected from the group consisting of
hydrogen, trihaloalkyl, dihaloalkyl, monohaloalkyl, alkyl, alkenyl, aryl,
heteroar yl,
yi,
he c. _ c er AR , dies ~.e ji, fr eter'6ocyc c F`Y k :. eterc4.. Y -`C-l
F'ke `.)$;
and he eroarylalkyl..r. va ch of said K r , uikenyl, aryl,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
6
heteroaryl, arylalkyl, cycloalkyl, cyclenyl, cycloalkylalkyl, cyclenylalkyl,
cyclenylalkenyl,
heterocyclenyl, heterocyclyl, heterocycle nylalkyl, heterocyclenylalkenyl,
heterocyclylalkyl, and heteroarylalkyl can be unsubstituted or substituted
with at least
one moiety, which can be the same or different, independently selected from
the group
consisting of halo, alkyl, cycloalkyl, cyclenyl, aryl, heterocyclyl,
heterocyclenyl,
heteroaryl, trihaloalkyl, CN, hydroxyl, alkoxyl and NO2.
Pharmaceutical compositions formulated for administration by an appropriate
route and means containing effective concentrations of one or more of the
compounds
provided herein, or pharmaceutically acceptable derivatives thereof, that
deliver
amounts effective for the treatment, prevention, or amelioration of one or
more
symptoms of diseases or disorders that are modulated or otherwise affected by
CDK-
2 or CHK-1, are also provided. The effective amounts and concentrations are
effective for ameliorating any of the symptoms of any of the diseases or
disorders.
Methods of treatment, prevention, or amelioration of one or more symptoms of
a disease or disorder that is modulated or otherwise affected by CDK-2 or CHK-
1 is
implicated, are provided. Such methods include methods of treatment,
prevention and
amelioration of one or more symptoms of inflammatory disease,
neurodegenerative
disease, cancer and diabetes using one or more of the compounds provided
herein, or
pharmaceutically acceptable derivatives thereof. Non-limiting examples of
inflammatory disease are acute pancreatitis, chronic pancreatitis, asthma,
allergies,
and adult respiratory distress syndrome. Non-limiting examples of
neurodegenerative
disease are acute Alzheimer's disease, Parkinson's disease, cerebral ischemia,
and
other neurodegenerative diseases. Non-limiting examples of the diabetes are
diabetes
mellitus and diabetes insipidus, eg., type I diabetes and type 2 diabetes.
F 4 practicing the methods, effective C : r is of the ,car comb _
coruL li "i r g therapeutically effective concentrations of the compounds,
which are
formulated for systemic delivery, including parenteral, oral, or intravenous
delivery, or
for local or topical application, for the treatment of CDK-2 or CHK-1 mediated
diseases or disorders, including, but not iii,mited to, inflammatory diseases,
acute ancreatitis; cfri
pancreatitis, E. 't' ~. c? v i u e. t1
disease, P ! son's .-
di"- l .~ f i .., :r 5 JC ,~ p di ,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
7
type 1 diabetes, type 2 diabetes, breast cancer, stomach cancer, cancer of the
ovaries, cancer of the colon, lung cancer, brain cancer, cancer of the larynx,
cancer of
the lymphatic system, cancer of the Benito-urinary tract including the bladder
and the
prostate, bone cancer and cancer of the pancreas, are administered to an
individual
exhibiting the symptoms of these diseases or disorders.
The amounts are effective to ameliorate or eliminate one or more symptoms of
the diseases or disorders.
Articles of manufacture containing packaging material, a compound or
composition, or pharmaceutically acceptable derivative thereof, provided
herein, which
is effective for modulating the activity of CDK-2 or CHK-1, mediated diseases
or
disorders are provided, within the packaging material, and a label that
indicates that
the compound or composition, or pharmaceutically acceptable derivative
thereof, is
used for modulating the activity of CDK-2 or CHK-1, mediated diseases or
disorders,
are provided.
The compounds according to the invention can have pharmacological
properties; in particular, the compounds of Formula I can be inhibitors of
protein
kinases such as, for example, CDK1, CDK2, CDK3, CDK4, CDK5, CDK6 and CDK7,
CDK8, mitogen activated protein kinase (MAPKIERK), glycogen synthase kinase 3
(GSK3beta), Pim-1 kinases, Chk kinases (such as Chk1 and Chk2), tyrosine
kinases,
such as the HER subfamily (including, for example, EGFR (HER1), HER2, HER3 and
HER4), the insulin subfamily (including, for example, INS-R, IGF-IR, IR, and
IR-R), the
PDGF subfamily (including, for example, PDGF-alpha and beta receptors, CSFIR,
s-
kit and FLK-II), the FLK family (including, for example, kinase insert domain
receptor
(KDR), fetal liver kinase-1 (FLK-1), fetal liver kinase-4 (FLK-4) and the fms-
like tyrosine
kinase- $ (fit-1)), non-receptor protein tyrosine kinases, for exE F- e g_ CK,
Src, Frk, Btk,
CSK, Lfap70, Fes/Fps, Fak, Jak, Ack, and LIMK, growth factor receptor tyrosine
kinases such as VEGF-R2, FGF-R, TEK, Akt kinases, Aurora kinases (Aurora A,
Aurora B, Aurora C) and the like.
The novel compounds of Formula I are expected to be useful in the therapy of
uch a ves, fungal
ti-
p,oliferative (e.g. ,.~ r retinopat~ ,,), 9lopec :ar iiov ,s Disease.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
8
Many of these diseases and disorders are listed in U.B. 5,413,974 cited
earlier, the
disclosure of which is incorporated herein.
More specifically, the compounds of Formula I can be useful in the treatment
of
a variety of cancers, including (but not limited to) the following:
carcinoma, including that of the bladder, breast, colon, kidney, liver, lung,
including
small cell lung cancer, esophagus, gall bladder, ovary, pancreas, stomach,
cervix,
thyroid, prostate, and skin, including squamous cell carcinoma; hematopoietic
tumors
of lymphoid lineage, including leukemia, acute lymphocytic leukemia, acute
lymphoblastic leukemia, B-cell lymphoma, T- cell lymphoma, Hodgkins lymphoma,
non-Hodgkins lymphoma, hairy cell lymphoma and Burkett's lymphoma;
hematopoietic tumors of myeloid lineage, including acute and chronic
myelogenous leukemias, myelodysplastic syndrome and promyelocytic leukemia;
tumors of mesenchymal origin, including fibrosarcoma and
rhabdomyosarcoma;
tumors of the central and peripheral nervous system, including astrocytoma,
neuroblastoma, glioma and schwannomas; and
other tumors, including melanoma, seminoma, teratocarcinoma, osteosarcoma,
xenoderoma pigmentosum, keratoctanthoma, thyroid follicular cancer and
Kaposi's
sarcoma.
Due to the key role of CDKs in the regulation of cellular proliferation in
general,
inhibitors could act as reversible cytostatic agents which may be useful in
the
treatment of any disease process which features abnormal cellular
proliferation, e.g.,
benign prostate hyperplasia, familial adenomatosis polyposis, neuro-
fi.bromatosis,
atherosclerosis, pulmonary fibrosis, arthritis, psoriasis, glomerulonephritis,
restenosis
following angioplasty or vascular surgery, hyper r ) , '-scar format ^r.
bowel disease, transplantation rejection, endotoxc shock, and fungal infection
s.
Compounds of Formula l can also be useful in the treatment of Alzheimer's
disease, as suggested by the recent finding that CDK5 is involved in the
phosphorylation of tau protein (J. Biochem, (1995) 117, 741-749).
mula l m~ uia.
Th,
~ k c
Se:_ .
Cc, - : c u nds of F as
r #, . be uuse" ~.., .: .. , ; : cancer (including but not
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
9
limited to those types mentioned hereinabove), viral infections (including but
not
limited to herpevirus, poxvirus, Epstein- Barr virus, Sindbis virus and
adenovirus),
prevention of AIDS development in HIV-infected individuals, autoimmune
diseases
(including but not limited to systemic lupus, erythematosus, autoimmune
mediated
glomerulonephritis, rheumatoid arthritis, psoriasis, inflammatory bowel
disease, and
autoimmune diabetes mellitus), neurodegenerative disorders (including but not
limited
to Alzheimer's disease, AIDS-related dementia, Parkinson's disease,
amyotrophic
lateral sclerosis, retinitis pigmentosa, spinal muscular atrophy and
cerebellar
degeneration), myelodysplastic syndromes, aplastic anemia, ischemic injury
associated with myocardial infarctions, stroke and reperfusion injury,
arrhythmia,
atherosclerosis, toxin-induced or alcohol related liver diseases,
hematological
diseases (including but not limited to chronic anemia and aplastic anemia),
degenerative diseases of the musculoskeletal system (including but not limited
to
osteoporosis and arthritis) aspirin-sensitive rhinosinusitis, cystic fibrosis,
multiple
sclerosis, kidney diseases and cancer pain.
Compounds of Formula 1, as inhibitors of the CDKs, can modulate the level of
cellular RNA and DNA synthesis. These agents would therefore be useful in the
treatment of viral infections (including but not limited to HIV, human
papilloma virus,
herpesvirus, poxvirus, Epstein-Barr virus, Sindbis virus and adenovirus).
Compounds of Formula I may also be useful in the chemoprevention of cancer.
Chemoprevention is defined as inhibiting the development of invasive cancer by
either
blocking the initiating mutagenic event or by blocking the progression of pre-
malignant
cells that have already suffered an insult or inhibiting tumor relapse.
Compounds of Formula I may also be useful in inhibiting tumor angiogenesis
and metastasis.
Cor-, Fo ids of Formula I may also act as inhibitors of other protein kinases,
e.g., protein kinase C, her2, raf 1, MEK1, MAP kinase, EGF receptor, PDGF
receptor,
IGF receptor, P13 kinase, weel kinase, Src; Abl and thus be effective in the
treatment
of diseases associated with other protein kinases,
Thu , . 8 a method of treating a m ff -A k human) ring OD.; CHK-1 by a
air :}U~ compound of Formula
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
I, or a pharmaceutically acceptable salt or solvate of said compound to the
mammal.
The pharmacological properties of the compounds of this invention may be
confirmed by a number of pharmacological assays. The exemplified
pharmacological
assays which are described later have been carried out with the compounds
5 according to the invention and their salts.
This invention is also directed to pharmaceutical compositions which comprise
at least one compound of Formula 1, or a pharmaceutically acceptable salt or
solvate
of said compound and at least one pharmaceutically acceptable carrier.
10 DETAILED DESCRIPTION
A. Definitions
Unless defined otherwise, all technical and scientific terms used herein have
the same meaning as is commonly understood by one of ordinary skill in the art
to
which this invention belongs. All patents, applications, published
applications and
other publications are incorporated by reference in their entirety. In the
event that
there are a plurality of definitions for a term herein, those in this section
prevail unless
stated otherwise.
As used above, and throughout this disclosure, the following terms, unless
otherwise indicated, shall be understood to have the following meanings:
"Patient" includes both human and animals.
"Mammal" means humans and other mammalian animals,
"Alkyl" means an aliphatic hydrocarbon group which may be straight or
branched and comprising about 1 to about 20 carbon atoms in the chain.
Preferred
alkyl groups contain about to about 12 carbon atoms in the chain. More
preferred
alkyl groups about 1 to about 3 cart vi atoms in the chain. Branched means
that one or more lower alkyl groups such as methyl, ethyl or propyl, are
attached to a
linear alkyl chain. "Lower alkyl" means a group having about 1 to about 6
carbon
atoms in the chain which may be straight or branched. "Alkyl" may be
unsubstituted
or ;_ tiona!ly substituted by one c sub ; =. nick h- the same or
¾i b; sE, each substit ue t being independe4 / ;a led from tie group < -
sisting of
halo, alkyl, aryl cycf .,:~:J . -:vano, hydroxv, ~.Yy, alkylthio, amino, 1 .,
g
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
11
NH(cycloalkyl), -N(alkyl)2: carboxy and -C(O)O-alkyl. Non-limiting examples of
suitable alkyl groups include methyl, ethyl, n-propyl, isopropyl and t-butyl.
"Alkenyl" means an aliphatic hydrocarbon group containing at least one carbon-
carbon double bond and which may be straight or branched and comprising about
2 to
about 15 carbon atoms in the chain. Preferred alkenyl groups have about 2 to
about
12 carbon atoms in the chain; and more preferably about 2 to about 6 carbon
atoms in
the chain. Branched means that one or more lower alkyl groups such as methyl,
ethyl
or propyl, are attached to a linear alkenyl chain. "Lower alkenyl" means about
2 to
about 6 carbon atoms in the chain which may be straight or branched. "Alkenyl"
may
be unsubstituted or optionally substituted by one or more substituents which
may be
the same or different, each substituent being independently selected from the
group
consisting of halo, alkyl. aryl, cycloalkyl, cyano, alkoxy and -S(alkyl). Non-
limiting
examples of suitable alkenyl groups include ethenyl, propenyl, n-butenyl, 3-
methylbut-
2-enyl, n-pentenyl, octenyl and decenyl.
"Alkylene" means a difunctional group obtained by removal of a hydrogen atom
from an alkyl group that is defined above. Non-limiting examples of alkylene
include
methylene, ethylene and propylene.
"Alkynyl" means an aliphatic hydrocarbon group containing at least one carbon-
carbon triple bond and which may be straight or branched and comprising about
2 to
about 15 carbon atoms in the chain. Preferred alkynyl groups have about 2 to
about
12 carbon atoms in the chain; and more preferably about 2 to about 4 carbon
atoms in
the chain. Branched means that one or more lower alkyl groups such as methyl,
ethyl
or propyl, are attached to a linear alkynyl chain. "Lower alkynyl" means about
2 to
about 6 carbon atoms in the chain which may be straight or branched. Non-
limiting
examples of suitable alkynyl groups include ethynyl, propynyl, 2-butynyl and 3-
:eihy:butynyl. "Alkynyl" may be unsubstituted or optionally substituted by one
or more
substituents which may be the same or different, each substituent being
independently selected from the group consisting of alkyl, aryl and
cycloalkyl.
"Aryl" means an aromatic monocyclic or multicyclic ring system comprising
about 6 to about 14 carbon atoms; prefe . AD-out 6 to about 10 carbon atoma.
The
aryl group can be optionally s _Ibz:...; t ,c or more "ring system subst t
'::~
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
12
which may be the same or different, and are as defined herein. Non-limiting
examples
of suitable aryl groups include phenyl and naphthyl.
"Heteroaryl" means an aromatic monocyclic or multicyclic ring system
comprising about 5 to about 14 ring atoms, preferably about 5 to about 10 ring
atoms,
in which one or more of the ring atoms is an element other than carbon, for
example
nitrogen, oxygen or sulfur, alone or in combination. Preferred heteroaryls
contain
about 5 to about 6 ring atoms. The "heteroaryl" can be optionally substituted
by one or
more "ring system substituents" which may be the same or different, and are as
defined herein. The prefix aza, oxa or thia before the heteroaryl root name
means that
at least a nitrogen, oxygen or sulfur atom respectively, is present as a ring
atom. A
nitrogen atom of a heteroaryl can be optionally oxidized to the corresponding
N-oxide.
Non-limiting examples of suitable heteroaryls include pyridyl, pyrazinyl,
furanyl,
thienyl, pyrimidinyl, pyridone (including N-substituted pyridones),
isoxazolyl,
isothiazolyl, oxazolyl, thiazolyl, pyrazolyl, furazanyl, pyrrolyl, pyrazolyl,
triazolyl, 1,2,4-
thiadiazolyl, pyrazinyl, pyridazinyl, quinoxalinyl, phthalazinyl, oxindolyl,
imidazo[1,2-
a]pyridinyl, imidazo[2,1-b]thiazolyl, benzofurazanyl, indolyl, azaindolyl,
benzimidazolyl,
benzothienyl, quinolinyl, imidazolyl, thienopyridyl, quinazolinyl,
thienopyrimidyl,
pyrrolopyridyl, imidazopyridyl, isoquinolinyl, benzoazaindolyl, 1,2,4-
triazinyl,
benzothiazolyl and the like. The term "heteroaryl" also refers to partially
saturated
heteroaryl moieties such as, for example, tetrahydroisoquinolyi, tetra
hydroqui no ly I and
the like.
"Aralkyl" or "arylalkyl"' means an aryl-alkyl- group in which the aryl and
alkyl are
as previously described. Preferred aralkyls comprise a lower alkyl group. Non-
limiting
examples of suitable aralkyl groups include benzyl, 2-phenethyl and
naphthalenylmethyl. The bond to the parent moiety is thra;~' ,le alkyl.
"Arylaikenyl" means an aryl group linked to the parent moiety through an
alkenyl moiety, defined above.
"Alkylaryl" means an alkyl-aryl- group in which the alkyl and aryl are as
previously described. Preferred alkylaryls comprise a lower alkyl group. Non-
limiting
-.- p#e of = is tolyl. T bond to the parem o f sz ~
tie aryl,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
13
"Cycloalkyl" or cyclyl means a non-aromatic mono- or multicyclic ring system
comprising about 3 to about 10 carbon atoms, preferably about 5 to about 10
carbon
atoms. Preferred cycloalkyl rings contain about 5 to about 7 ring atoms. The
cyclyalkyl
can be optionally substituted with one or more "ring system substituents"
which may
be the same or different, and are as defined above. Non-limiting examples of
suitable
monocyclic cycloalkyls include cyclopropyl, cyclopentyl, cyclohexyl,
cycloheptyl and
the like. Non-limiting examples of suitable multicyclic cycloalkyls include 1 -
decalinyl,
norbornyl, adamantyl and the like.
"Cycloalkylalkyl" or cyclylalkyl means a cycloalkyl moiety as defined above
linked via an alkyl moiety (defined above) to a parent core. Non-limiting
examples of
suitable cycloalkylalkyls include cyclohexylmethyl, adamantylmethyl and the
like.
"Cycloalkenyl" or cyclenyl means a non-aromatic mono or multicyclic ring
system comprising about 3 to about 10 carbon atoms, preferably about 5 to
about 10
carbon atoms which contains at least one carbon-carbon double bond. Preferred
cycloalkenyl rings contain about 5 to about 7 ring atoms. The cycloalkenyl can
be
optionally substituted with one or more "ring system substituents" which may
be the
same or different, and are as defined above. Non-limiting examples of suitable
monocyclic cycloalkenyls include cyclopentenyl, cyclohexenyl, cyclohepta-1,3-
dienyl,
and the like. Non-limiting example of a suitable multicyclic cycloalkenyl is
norbornylenyl.
"Cycloalkenylalkyl" or cyclenylalkyl means a cycloalkenyl moiety as defined
above linked via an alkyl moiety (defined above) to a parent core. Non-
limiting
examples of suitable cycloalkenylalkyls include cyclopentenylmethyl,
cyclohexenylrethyl and the like.
"CycloalkenylalkenyF" or "Cycloaikylalkenyl" means a cycloa< c fcioalkyi
moiety respectively, as defined above, linked via an alkenyl moiety as defined
above.
"Halogen" or halo means fluorine, chlorine, bromine, or iodine. Preferred are
fluorine, chlorine and bromine.
"Ring system substituent" means a substituent attached to an aromatic or non-
0 arc ...g < s h wws a , agent on the
P:rq S _ .1 subs t e , same or different, each being
pe ;e , w_ ._ , from coo-, e<:.;:irtg of ..' , alkenyl, alkynyl, aryl,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
14
heteroaryl, aralkyl, alkylaryl, heteroaralkyl, heteroarylalkenyl,
heteroarylalkynyl,
alkyiheteroaryl, hydroxy, hydroxyalkyl, alkoxy, aryloxy, aralkoxy, aryl,
aroyl, halo,
nitro, cyano, carboxy, alkoxycarbonyl, aryloxycarbonyl, a ralkoxycarbonyl,
alkylsulfonyl,
arylsulfonyl, heteroaryisulfonyl, alkylthio, arylthio, heteroarylthio,
aralkylthio,
heteroaralkylthio, cycloalkyl, heterocyclyl, -C(=N-CN)-NH2, -C(=NH)-NH2, -
C(=NH)-
NH(alkyl), Y1Y2N-, Y1Y2N-alkyl-, Y1Y2NC(O)-, Y1Y2NSO2- and -SO2NYIY2, wherein
YI
and Y2 can be the same or different and are independently selected from the
group
consisting of hydrogen, alkyl, aryl, cycloalkyl, and aralkyl. "Ring system
substituent"
may also mean a single moiety which simultaneously replaces two available
hydrogens on two adjacent carbon atoms (one H on each carbon) on a ring
system.
Examples of such moiety are methylene dioxy, ethylenedioxy, -C(CH3)2- and the
like
which form moieties such as, for example:
ro
b n and
"Heteroarylalkyl" or heteroarylalkenyl means a heteroaryl moiety as defined
above linked via an alkyl or alkenyl moiety respectively (defined above) to a
parent
core. Non-limiting examples of suitable heteroaryls include 2-pyridinylmethyl,
quinolinylmethyl and the like.
"Heterocyclyl" or heterocycloalkyl means a non-aromatic saturated monocyclic
or multicyclic ring system comprising about 3 to about 10 ring atoms,
preferably about
5 to about 10 ring atoms, in which one or more of the atoms in the ring system
is an
element other than carbon, for example nitrogen, oxygen or sulfur, alone or in
combination. There are no adjacent oxygen and/or sulfur atoms present in the
ring
system. Preferred heterocyclyls contain about 5 to about 6 ring atoms. The
prefix aza,
oxa or Chia before the heterocyclyi root name means that at least a nitrogen,
oxygen or
sulfur atom respectively is present as a ring atom. Any -NH in a heterocyclyl
ring may
exist protected such as, for example, as an -N(Boc), -N(CBz), -N(Tos) group
and the
like-, such protections are also considered part of this in. Anton. The
heterocyclyi can
be F s~ E .w d by one 1- r-,-,.e 91rir which may be the
same lerert. are as defined herein. J= m of the
heterocyclyl can b e c_- ", ona Hy oxidized to the o -: S-oxide or S, S-
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
dioxide. Non-limiting examples of suitable monocyclic heterocyclyl rings
include
piperidyl, pyrrolidinyl, piperazinyl, morpholinyl, thiomorpholinyl,
thiazolidinyl, 1,4-
dioxanyl, tetrahydrofuranyl, tetrahydrothiophenyl, lactam, lactone, and the
like.
"Heterocyclyi" may also mean a single moiety (e.g., carbonyl) which
simultaneously
5 replaces two available hydrogens on the same carbon atom on a ring system.
Example of such moiety is pyrrolidone:
H
C N
"Heterocyclylalkyl" or heterocycloalkylalkylmeans a heterocyclyl moiety as
defined above linked via an alkyl moiety (defined above) to a parent core. Non-
limiting
10 examples of suitable heterocyclylalkyls include piperidinylmethyl,
piperazinylmethyl
and the like.
"Heterocyclenyl" or heterocycloalkenyl means a non-aromatic monocyclic or
multicyclic ring system comprising about 3 to about 10 ring atoms, preferably
about 5
to about 10 ring atoms, in which one or more of the atoms in the ring system
is an
15 element other than carbon, for example nitrogen, oxygen or sulfur atom,
alone or in
combination, and which contains at least one carbon-carbon double bond or
carbon-
nitrogen double bond. There are no adjacent oxygen and/or sulfur atoms present
in
the ring system. Preferred heterocyclenyl rings contain about 5 to about 6
ring atoms.
The prefix aza, oxa or aria before the heterocyclenyl root name means that at
least a
nitrogen, oxygen or sulfur atom respectively is present as a ring atom. The
heterocyclenyl can be optionally substituted by one or more ring system suV, r
, -,Lq,,
w e e "ring system substituent" is as defined above, The nitrogen or sulfur
atom of
the heterocyclenyl can be optionally oxidized to the corresponding N-oxide, S-
oxide or
S,S-dioxide. Non-limiting examples of suitable heterocyclenyl groups include
1,2,3,4-
tetrahydropyridine, 1,24-hydropyridyl, 1,4-d hydropyridyl, 1,2,3,6-tetra
hydropyridine,
1, _ . 2 2-pyraz linyl,
dihyd:oirie,d z ii i H-
p.Y ran, dihyd-,7,f a-
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
16
dihydrothiophenyl, dihydrothiopyranyl, and the like. "Heterocyclenyl" may also
mean a
single moiety (e.g., carbonyl) which simultaneously replaces two available
hydrogens
on the same carbon atom on a ring system. Example of such moiety is
pyrrolidinone:
H
N
Q
"Heterocycle nylalkyl" or heterocycloalkenylalkyl means a heterocyclenyl
moiety
as defined above linked via an alkyl moiety (defined above) to a parent core.
"Heteroalkyl" means is a saturated or unsaturated chain containing carbon and
at least one heteroatom, wherein one or more of the chain atoms is an element
other
than carbon, for example nitrogen, oxygen or sulfur, alone or in combination,
wherein
no two heteroatoms are adjacent. Heteroalkyl chains contain from 2 to 15
member
atoms (carbon and heteroatoms) in the chain, preferably 2 to 10, more
preferably 2 to
5. For example, alkoxy (i.e., --O-alkyl or --O-heteroalkyl) radicals are
included in
heteroalkyl. Heteroalkyl chains may be straight or branched. Preferred
branched
heteroalkyl have one or two branches, preferably one branch. Preferred
heteroalkyl
1.5 are saturated. Unsaturated heteroalkyl have one or more carbon-carbon
double
bonds and/or one or more carbon-carbon triple bonds. Preferred unsaturated
heteroalkyls have one or two double bonds or one triple bond, more preferably
one
double bond. Heteroalkyl chains may be unsubstituted or substituted with from
1 to 4
substituents. Preferred substituted heteroalkyl are mono-, di-, or tri-
substituted.
Heteroalkyl may be substituted with lower alkyl, haloalkyl, halo, hydroxy,
aryloxy
heteroaryloxy, acyloxy, carboxy, monocyclic aryl, heteroaryl, cycloF 1. hete
;.rl,
spirocycle, amino, acylarnino, amido, keto, thioketo, cyano, or any
combination
thereof.
It should be noted that in hetero-atom containing ring systems of this
invention,
there are no hydroxyl groups on carbon atoms adjacent to a N, 0 or S, as well
as
there are no N or on carbon to _ Thus, for
example, in the ring:
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
17
4
C>2
N
H
there is no -OH attached directly to carbons marked 2 and 5.
It should also be noted that tautomeric forms such as, for example, the
moieties:
N 4
5 H and N OH
are considered equivalent in certain embodiments of this invention.
"Alkynylalkyl" means an alkynyl-alkyl- group in which the alkynyl and alkyl
are
as previously described. Preferred alkynylalkyls contain a lower alkynyl and a
lower
alkyl group. The bond to the parent moiety is through the alkyl. Non-limiting
examples
of suitable alkynylalkyl groups include propargylmethyl.
"Heteroaralkyl" means a heteroaryl-alkyl- group in which the heteroaryl and
alkyl are as previously described. Preferred heteroaralkyls contain a lower
alkyl group.
Non-limiting examples of suitable aralkyl groups include pyridylmethyl, and
quinolin-3-
ylmethyl. The bond to the parent moiety is through the alkyl.
"Hydroxyalkyl" means a HO-alkyl- group in which alkyl is as previously
defined.
Preferred hydroxyalkyls contain lower alkyl. Non-limiting examples of suitable
hydroxyalkyl groups include hydroxymethyl and 2-hydroxyethyl.
"Aryl" means an H-C(O)-, alkyl-C(O)- or cycloalkyl-C(O)-, group in which the
various groups are as previously described. The bond to the parent moiety is
through
the carbonyl. Preferred acyls contain a lower alkyl. Nona'Fn ing examples of
suitable
elude formy' , 1 -opanoyl.
"Aroyl" means an aryl-C(O)- group in which the aryl group is as previously
described. The bond to the parent moiety is through the carbonyl. Non-limiting
examples of suitable groups include benzoyl and 1- naphthoyl.
"Alkoxyi or "..X.aaoky' Cra i s ! O c , :n which the alkyl ' , . p is as
akKoxy groups incl de
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
18
methoxy, ethoxy, n-propoxy, isopropoxy and n-butoxy. The bond to the parent
moiety
is through the ether oxygen.
"Alkoxyoxo" is similar to alkoxycarbonyl (e.g., -CO2R), but the alkoxy group
additionally may include polyether functionality.
"Oxaalkynyl" indicates an alkynyl ether (e.g. propargyloxy group) linked to
the
parent moiety via the oxygen of oxaalkynyl.
"Aryloxy" means an aryl-O- group in which the aryl group is as previously
described. Non-limiting examples of suitable aryloxy groups include phenoxy
and
naphthoxy. The bond to the parent moiety is through the ether oxygen.
"Aralkyloxy" means an aralkyl-O- group in which the aralkyl group is as
previously described. Non-limiting examples of suitable aralkyloxy groups
include
benzyloxy and 1- or 2-naphthalenemethoxy. The bond to the parent moiety is
through
the ether oxygen.
"Alkylthio" means an alkyl-S- group in which the alkyl group is as previously
described. Non-limiting examples of suitable alkylthio groups include
methylthio and
ethylthio. The bond to the parent moiety is through the sulfur.
"Arylthio" means an aryl-S- group in which the aryl group is as previously
described. Non-limiting examples of suitable arylthio groups include
phenylthio and
naphthylthio. The bond to the parent moiety is through the sulfur.
"Aralkylthio" means an aralkyl-S- group in which the aralkyl group is as
previously described. Non-limiting example of a suitable aralkylthio group is
benzylthio. The bond to the parent moiety is through the sulfur.
"Alkoxycarbonyl" means an alkyl-O-CO- group. Non-limiting examples of
suitable alkoxycarbonyl groups include methoxycarbonyi and ethoxycarbonyl. The
bond to the parent moiety is through the cart r L
"} Aryloxycarbonyl" means an aryl-O-C(C_ group. Non-,-r.ng examples of
suitable aryloxycarbonyl groups include phenoxycarbonyl and naphthoxycarbonyl.
The
bond to the parent moiety is through the carbonyl.
"Araikoxyca`oonyl" means an aralkyl-O-C(O)- group. Non-limiting example of a
. . ; is beFez. -. no Si parent
ro gh f' k>.. [_
is
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
19
"Alkylsulfonyl" means an alkyl-S(02)- group. Preferred groups are those in
which the alkyl group is lower alkyl. The bond to the parent moiety is through
the
sulfonyl.
"Arylsulfonyl" means an aryl-S(02)- group. The bond to the parent moiety is
through the sulfonyl.
The term "substituted" means that one or more hydrogens on the designated
atom is replaced with a selection from the indicated group, provided that the
designated atom's normal valency under the existing circumstances is not
exceeded,
and that the substitution results in a stable compound. Combinations of
substituents
and/or variables are permissible only if such combinations result in stable
compounds.
By "stable compound' or "stable structure" is meant a compound that is
sufficiently
robust to survive isolation to a useful degree of purity from a reaction
mixture, and
formulation into an efficacious therapeutic agent.
The term "optionally substituted" means optional substitution with the
specified
groups, radicals or moieties.
The term "purifiedõ "in purified form" or "in isolated and purified form" for
a
compound refers to the physical state of said compound after being isolated
from a
synthetic process or natural source or combination thereof. Thus, the term
"purified",
"in purified form" or "in isolated and purified form" for a compound refers to
the
physical state of said compound after being obtained from a purification
process or
processes described herein or well known to the skilled artisan, in sufficient
purity to
be characterizable by standard analytical techniques described herein or well
known
to the skilled artisan.
It should also be noted that any carbon as well as heteroatom with unsatisfied
valences in the text, schemes, exa,, - e s and Tables herein is a -` t e,,e
the
:_ i t n~ m per of hydrogen atar (s) to satisfy the valences.
When a functional group in a compound is termed" protected", this means that
the group is in modified form to preclude undesired side reactions at the
protected site
when the compound is subjected to a reaction. Suitable protecting groups will
be
r i :. h; c_:, with ordina, dirt a by reference to stanÃar
texib ~ s such as, k - le, T. ` . Greene eta/, Protective Groups in organic
Synthesis 0 991). #f v York.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
When any variable (e.g., aryl, heterocycle, R2, etc.) occurs more than one
time
in any constituent or in Formula 1, its definition on each occurrence is
independent of
its definition at every other occurrence.
As used herein, the term "composition" is intended to encompass a product
5 comprising the specified ingredients in the specified amounts, as well as
any product
which results, directly or indirectly, from combination of the specified
ingredients in the
specified amounts.
Prodrugs and solvates of the compounds of the invention are also
contemplated herein. A discussion of prodrugs is provided in T. Higuchi and V.
Stella,
10 Pro-drugs as Novel Delivery Systems (1987) 14 of the A.C.S. Symposium
Series, and
in Bioreversible Carriers in Drug Design, (1987) Edward B. Roche, ed.,
American
Pharmaceutical Association and Pergamon Press. The term "prodrug" means a
compound (e.g, a drug precursor) that is transformed in vivo to yield a
compound of
Formula (1) or a pharmaceutically acceptable salt, hydrate or solvate of the
compound.
15 The transformation may occur by various mechanisms (e.g., by metabolic or
chemical
processes), such as, for example, through hydrolysis in blood. A discussion of
the
use of prodrugs is provided by T. Higuchi and W. Stella, "Pro-drugs as Novel
Delivery
Systems," Vol. 14 of the A.C.S. Symposium Series, and in Bioreversible
Carriers in
Drug Design, ed. Edward B. Roche, American Pharmaceutical Association and
20 Pergamon Press, 1987.
For example, if a compound of Formula (1) or a pharmaceutically acceptable
salt, hydrate or solvate of the compound contains a carboxylic acid functional
group, a
prodrug can comprise an ester formed by the replacement of the hydrogen atom
of
the acid group with a group such as, for example, (C1-C8)alkyl, (C2-
C12 alkanoyloxymetl yl, 1-(alkanoyloxy ethyl having from 4 to 9 carbon atoms,
1-
methyl- l-(alkanoyloxy)-ethyl having from 5 to 10 carbon atoms,
alkoxycarbonyloxymethyl having from 3 to 6 carbon atoms, 1 -(a I koxyca rbo
nyloxy) ethyl
having from 4 to 7 carbon atoms, 1-methyl-1 -(alkoxycarbonyloxy)ethyl having
from 5
to 8 carbon atoms, N-(alkoxycarbonyl)aminomethyl having from 3 to 9 carbon
atoms,
3t 1 ( `7 having f rom 4 to 10 carbon s, 3 p thalsdyl, 4-
crotonoiactonyl, gamma-o , , ...I .cton-4-y l di-N,N-(C C a!k C ; 2-C.w)alkyl
(such
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
21
as j3-dimethylaminoethyl), carbamoyl-(C,-C2)alkyl, N,N-di (C1-
C2)alkylcarbamoyl-(C1-
C2)alkyl and piperidino-, pyrrolidino- or morpholino(C2-C3)alkyl, and the
like.
Similarly, if a compound of Formula (1) contains an alcohol functional group,
a
prodrug can be formed by the replacement of the hydrogen atom of the alcohol
group
with a group such as, for example, (C1-C6)alkanoyloxymethyl, 1-((Ci -
C6)alkanoyloxy)ethyl, 1-methyl-l-((C,-C6)alkanoyloxy)ethyl, (C,-
C6)alkoxycarbonyloxymethyl, N-(C1-C6)alkoxycarbonylaminomethyl, succinoyl, (C,-
C6)alkanoyl, u-amino(C1-C4)alkanyl, arylacyl and a-aminoacyl, or u-aminoacyl-a-
aminoacyl, where each a-aminoacyl group is independently selected from the
naturally
occurring L-amino acids, P(O)(OH)2, -P(O)(O(C1-C6)alkyl)2 or glycosyl (the
radical
resulting from the removal of a hydroxyl group of the hemiacetal form of a
carbohydrate), and the like.
If a compound of Formula (l) incorporates an amine functional group, a prodrug
can be formed by the replacement of a hydrogen atom in the amine group with a
group such as, for example, R-carbonyl, RO-carbonyl, NRR'-carbonyl where R and
R'
are each independently (C1-C13)alkyl, (C3-C7) cycloalkyl, benzyl, or R-
carbonyl is a
natural u-aminoacyl or natural cr-aminoacyl, -C(OH)C(O)OY' wherein Y' is H,
(C1-
C6)alkyl or benzyl, _C(OY2)Y3 wherein Y2 is (C1-C4) alkyl and Y3 is (C1-
C6)alkyl,
carboxy (C,-C6)alkyl, amino(C1-C4)alkyl or mono-N--or di-N,N-(C1-
C6)alkylaminoalkyl,
-C(Y4)Y5 wherein Y4 is H or methyl and Y5 is mono-N- or di-N,N-(C,-
C6)alkylamino
morpholino, piperidin-1-yl or pyrrolidin-l -yl, and the like.
One or more compounds of the invention may exist in unsolvated as well as
solvated forms with pharmaceutically acceptable solvents such as water,
ethanol, and
gthe like, and it is intended that the invention embrace both solvated and
unsolvated
one or more solvent molecules. "'-"s physical associatcn involves varying
degrees of
ionic and covalent bonding, including hydrogen bonding. In certain instances
the
solvate will be capable of isolation, for example when one or more solvent
molecules
are incorporated in the crystal lattice of the crystalline solid. "Solvate"
encompasses
t s -:phase and isolatadler
S y ( ......U `'.anolates, a s ; a. e
yyh 5 .. tl Eli
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
22
One or more compounds of the invention may optionally be converted to a
solvate. Preparation of solvates is generally known. Thus, for example, M.
Caira et al,
J. Pharmaceutical Sci., 93(3), 601-611 (2004) describe the preparation of the
solvates
of the antifungal fluconazole in ethyl acetate as well as from water. Similar
preparations of solvates, hemisolvate, hydrates and the like are described by
E. C.
van Tonder et al, AAPS PharmSciTech., all, article 12 (2004); and A. L.
Bingham et
al, Chem. Commur7., 603-604 (2001). A typical, non-limiting, process involves
dissolving the inventive compound in desired amounts of the desired solvent
(organic
or water or mixtures thereof) at a higher than ambient temperature, and
cooling the
solution at a rate sufficient to form crystals which are then isolated by
standard
methods. Analytical techniques such as, for example 1. R. spectroscopy, show
the
presence of the solvent (or water) in the crystals as a solvate (or hydrate).
"Effective amount" or "therapeutically effective amount" is meant to describe
an
amount of compound or a composition of the present invention effective in
inhibiting
the above-noted diseases and thus producing the desired therapeutic,
ameliorative,
inhibitory or preventative effect.
The compounds of Formula I can form salts which are also within the scope of
this invention. Reference to a compound of Formula I herein is understood to
include
reference to salts thereof, unless otherwise indicated. The term "salt(s)", as
employed
herein, denotes acidic salts formed with inorganic and/or organic acids, as
well as
basic salts formed with inorganic and/or organic bases. In addition, when a
compound
of Formula I contains both a basic moiety, such as, but not limited to a
pyridine or
imidazole, and an acidic moiety, such as, but not limited to a carboxylic
acid,
zwitterions ("inner salts") may be formed and are included within the term
"salt(s)" as
used herein, Pharmaceutically acceptable (i.e., non-toxic, physiologically
acceptable)
sans are preferred, although other salts are also useful. Salts of the
compounds of the
Formula I may be formed, for example, by reacting a compound of Formula I with
an
amount of acid or base, such as an equivalent amount, in a medium such as one
in
which the salt precipitates or in an aqueous medium followed by
lyophilization,
10 $gq y@ Exer 4 ~+ ascorbates, benzoates.
_::s, camphorates,
camphom.3lfonates, furna aces, hy ; b omides, hydroiodides, lactates,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
23
maleates, methanesulfonates, naphtha lenesulfonates, nitrates, oxalates,
phosphates,
propionates, salicylates, succinates, sulfates, tartarates, thiocyanates,
toluenesulfonates (also known as tosylates,) and the like. Additionally, acids
which are
generally considered suitable for the formation of pharmaceutically useful
salts from
basic pharmaceutical compounds are discussed, for example, by P. Stahl et al,
Camille G. (eds.) Handbook of Pharmaceutical Salts. Properties, Selection and
Use.
(2002) Zurich: Wiley-VCH; S. Berge et al, Journal of Pharmaceutical Sciences
(1977)
66(1) 1-19; P. Gould, International J. of Pharmaceutics (1986) 33 201-217;
Anderson
eta!, The Practice of Medicinal Chemistry (1996), Academic Press, New York;
and in
The Orange Book (Food & Drug Administration, Washington, D.C. on their
website).
These disclosures are incorporated herein by reference thereto.
Exemplary basic salts include ammonium salts, alkali metal salts such as
sodium, lithium, and potassium salts, alkaline earth metal salts such as
calcium and
magnesium salts, salts with organic bases (for example, organic amines) such
as
dicyclohexylamines, t-butyl amines, and salts with amino acids such as
arginine,
lysine and the like. Basic nitrogen-containing groups may be quarternized with
agents
such as lower alkyl halides (e.g. methyl, ethyl, and butyl chlorides, bromides
and
iodides), dialkyl sulfates (e.g. dimethyl, diethyl, and dibutyl sulfates),
long chain
halides (e.g. decyl, lauryl, and stearyl chlorides, bromides and iodides),
aralkyl halides
(e.g. benzyl and phenethyl bromides), and others.
All such acid salts and base salts are intended to be pharmaceutically
acceptable salts within the scope of the invention and all acid and base salts
are
considered equivalent to the free forms of the corresponding compounds for
purposes
of the invention.
Pharmaceutically acceptable esters of the present compounds include the
roiiowing groups: (1) carboxylic acid esters obtained by esterification of the
hydroxy
groups, in which the non-carbonyl moiety of the carboxylic acid portion of the
ester
grouping is selected from straight or branched chain alkyl (for example,
acetyl, n-
propyl, t-butyl, or n-butyl), alkoxyalkyl (for example, methoxymethyl),
aralkyl (for
ylox ik for ex< . ~ o yl), aryl (for
ptk 4 ; ,i X C _4alkytl, t ccxy or
a (2) suif for yl (for ;. r r i
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
24
methanesulfonyl); (3) amino acid esters (for example, L-valyl or L-isoleucyl);
(4)
phosphonate esters and (5) mono-, di- or triphosphate esters. The phosphate
esters
may be further esterified by, for example, a C1-20 alcohol or reactive
derivative thereof,
or by a 2,3-di (C6-24)acyl glycerol.
Compounds of Formula I, and salts, solvates, esters and prodrugs thereof, may
exist in their tautomeric form (for example, as an amide or imino ether). All
such
tautomeric forms are contemplated herein as part of the present invention.
The compounds of Formula (1) may contain asymmetric or chiral centers, and,
therefore, exist in different stereoisomeric forms. It is intended that all
stereoisomeric
forms of the compounds of Formula (I) as well as mixtures thereof, including
racemic
mixtures, form part of the present invention. In addition, the present
invention
embraces all geometric and positional isomers. For example, if a compound of
Formula (1) incorporates a double bond or a fused ring, both the cis- and
trans-forms,
as well as mixtures, are embraced within the scope of the invention.
Diastereomeric mixtures can be separated into their individual diastereomers
on the basis of their physical chemical differences by methods well known to
those
skilled in the art, such as, for example, by chromatography and/or fractional
crystallization. Enantiomers can be separated by converting the enantiomeric
mixture
into a diastereomeric mixture by reaction with an appropriate optically active
compound (e.g., chiral auxiliary such as a chiral alcohol or Mosher's acid
chloride),
separating the diastereomers and converting (e.g., hydrolyzing) the individual
diastereomers to the corresponding pure enantiomers. Also, some of the
compounds
of Formula (1) may be atropisomers (e.g., substituted biaryls) and are
considered as
part of this invention. Enantiomers can also be separated by use of chiral
HPLC
column.
it is also possible that the compounds of Formula (1) may exist in different
tautomeric forms, and all such forms are embraced within the scope of the
invention.
Also, for example, all keto-enol and imine-enamine forms of the compounds are
included in the invention.
3 p 6a 3 y4 f. e7rQs for b~.isomers . _ t omersygand ~~t,e like)
p
of L e p rewsent cum pour, s ` i3 e salts, 5 ('.-t.~o_, esters and pro
of the compounds as oAj :-1-:E `. d esters cf the prodrugs), r as
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
those which may exist due to asymmetric carbons on various substituents,
including
enantiomeric forms (which may exist even in the absence of asymmetric
carbons),
rotameric forms, atropisomers, and diastereomeric forms, are contemplated
within the
scope of this invention, as are positional isomers (such as, for example, 4-
pyridyl and
5 3-pyridyl). (For example, if a compound of Formula (l) incorporates a double
bond or a
fused ring, both the cis- and trans-forms, as well as mixtures, are embraced
within the
scope of the invention. Also, for example, all keto-enol and imine-enamine
forms of
the compounds are included in the invention.) Individual stereoisomers of the
compounds of the invention may, for example, be substantially free of other
isomers,
10 or may be admixed, for example, as racemates or with all other, or other
selected,
stereoisomers. The chiral centers of the present invention can have the S or R
configuration as defined by the 1UPAC 1974 Recommendations. The use of the
terms
"salt", "solvate", "ester", "prodrug" and the like, is intended to equally
apply to the salt,
solvate, ester and prodrug of enantiomers, stereoisomers, rotamers, tautomers,
15 positional isomers, racemates or prodrugs of the inventive compounds.
The present invention also embraces isotopically-labelled compounds of the
present invention which are identical to those recited herein, but for the
fact that one
or more atoms are replaced by an atom having an atomic mass or mass number
different from the atomic mass or mass number usually found in nature.
Examples of
20 isotopes that can be incorporated into compounds of the invention include
isotopes of
hydrogen, carbon, nitrogen, oxygen, phosphorus, fluorine and chlorine, such as
2H,
3H, 13C, 14C, 15N, 180, 170, 3'P, 32P, 35S,'8F, and 36Cl, respectively.
Certain isotopically-labelled compounds of Formula (l) (e.g., those labeled
with
3H and 14C) are useful in compound and/or substrate tissue distribution
assays.
25 Tritiated (i.e., 3H) and carbon-14 (i,e., 14C isotopes are particularly
preferred for their
ease of preparation and detectabiiity. Further, substitution with heavier
isotopes such
as deuterium (i.e., 2H) may afford certain therapeutic advantages resulting
from
greater metabolic stability (e.g., increased in vivo half-life or reduced
dosage
requirements) and hence may be preferred it some circumstances. Isotopically
$t of F caL be prepared by E ..:''g pa f &s
analogous to those disclosed in the Schemes and/or in the Example ; c ~
Jbelow, by
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
26
substituting an appropriate isotopically labelled reagent for a non-
isotopically labelled
reagent.
Polymorphic forms of the compounds of Formula 1, and of the salts, solvates,
esters and prodrugs of the compounds of Formula 1, are intended to be included
in the
present invention.
As used herein, amelioration of the symptoms of a particular disorder by
administration of a particular compound or pharmaceutical composition refers
to any
lessening, whether permanent or temporary, lasting or transient that can be
attributed
to or associated with administration of the composition.
As used herein, IC50 refers to an amount, concentration or dosage of a
particular test compound that achieves a 50% inhibition of a maximal response,
such
as modulation of CDK-2 kinase activity, in an assay that measures such
response.
As used herein, EC50 refers to a dosage, concentration or amount of a
particular test compound that elicits a dose-dependent response at 50% of
maximal
expression of a particular response that is induced, provoked or potentiated
by the
particular test compound.
In the case of amino acid residues, such residues may be of either the L- or D-
form. The configuration for naturally occurring amino acid residues is
generally L.
When not specified the residue is the L form. As used herein, the term "amino
acid"
refers to a-amino acids which are racemic, or of either the D- or L-
configuration. The
designation "d" preceding an amino acid designation (e.g., dAla, dSer, dVal,
etc.)
refers to the D-isomer of the amino acid. The designation "dl" preceding an
amino
acid designation (e.g., d1Pip) refers to a mixture of the L- and D-isomers of
the amino
acid. it is to be understood that the chiral centers of the compounds provided
herein
may undergo epimerization in vivo. As such, one of skill in the a s that
administration of a compound in its (R) form is equivalent, for compounds that
undergo epimerization in vivo, to administration of the compound in its (S)
form.
As used herein, substantially pure means sufficiently homogeneous to appear
`.,ee of readily detectable impurities as determined bj standard methods of
analysis,
3 &
(TLCi nd==ormance liquid
ct. ~) ,:uu rY5 C.'. e of skill in the art
to assess suc C11 a ion would not
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
27
detectably alter the physical and chemical properties, such as enzymatic and
biological activities, of the substance. Methods for purification of the
compounds to
produce substantially chemically pure compounds are known to those of skill in
the
art. A substantially chemically pure compound may, however, be a mixture of
stereoisomers. In such instances, further purification might increase the
specific
activity of the compound.
The term "purified", "in purified form" or "in isolated and purified form" for
a
compound refers to the physical state of said compound after being isolated
from a
synthetic process or natural source or combination thereof. Thus, the term
"purified",
"in purified form" or "in isolated and purified form" for a compound refers to
the
physical state of said compound after being obtained from a purification
process or
processes described herein or well known to the skilled artisan, in sufficient
purity to
be characterizable by standard analytical techniques described herein or well
known
to the skilled artisan.
As used herein, "haloalkoxy" refers to RO- in which R is a haloalkyl group.
As used herein, "heterocyclyloxy", refers to RO- in which R is a heterocyclyl
group, heteroaryloxy refers to RO- in which R is a heteroaryl group.
As used herein, "substituted alkyl," "substituted alkenyl," "substituted
alkynyl,"
"substituted cycloalkyl," "substituted cycloalkenyl," "substituted
cycloalkynyl,"
"substituted aryl," "substituted heteroaryl," "substituted heterocyclyl,"
"substituted
alkylene," "substituted alkenylene," "substituted alkynylene," "substituted
cycloalkylene," "substituted cycloalkenylene," "substituted cycloalkynylene,"
"substituted arylene," "substituted heteroarylene" and "substituted
heterocyclylene"
refer to alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl,
aryl, heteroaryl,
heterocyclyl, alkylene, alkenylene, alkynylene, cycloalkylene, cycloalken': ie
cycloalkynylene, arylene, heteroarylene and heterocyclylene groups,
respectively, that
are substituted with one or more substituents, in certain embodiments one,
two, three
or four substituents, where the substituents are as defined herein, for
example, in one
embodiment selected from O'.
As herein, "amid." refers _ group - "Thioarn.ido"
refers :r;t
refers to the divalent group -%C.1(S)NH
OO(O)NH-, "Thiaamido" refers to the :pup
refers
G; N
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
28
to the divalent group -SC(S)NH-. "Ureido" refers to the divalent group -
HNC(O)NH-.
"Thioureido" refers to the divalent group -HNC(S)NH-.
Where the number of any given substituent is not specified (e.g., haloalkyl),
there may be one or more substituents present. For example, "haloalkyl" may
include
one or more of the same or different halogens. As another example,
"C1_3alkoxyphenyl" may include one or more of the same or different alkoxy
groups
containing one, two or three carbons.
As used herein, the abbreviations for any protective groups, amino acids and
other compounds, are, unless indicated otherwise, in accord with their common
usage, recognized abbreviations, or the IUPAC-IUB Commission on Biochemical
Nomenclature (see, (1972) Biochem. 11:942-944). Certain of the abbreviations
used
herein are listed below.
B. Compounds
In one embodiment, the compounds provided herein for use in the
compositions and methods provided herein have formula 1, where the variables
are as
described below. All combinations of such embodiments are within the scope of
the
instant disclosure.
In one embodiment, provided herein are compounds of formula (1):
R3 N R5
NN 6
R7
Formula (1)
or a pharmaceutically acceptable salts, solvates, esters, prodrugs and
stereoisomers
thereof, where R3, R5, R6 and R7 are select ,
wherein:
R3 is hydrogen, alkyl, cycloalkyl, cyclenyl, alkynyl, trifluroalkyl,
difluroalkyl,
monofluroalkyl, heterocyclyl, heterocyclenyl, aryl, heteroaryl, halo, cyano, -
0-
trihaloalkyl, NR8R9, CO:_R8, CONR8R , -OR", -SR", -S02R8, -S02NR8R9, -
NR8SC2R9, -
R CC , ' 't werein each . z_ . ycià ~y1; c e l
:; of uroalkyl lY, erocyclyl, ,tE -c e _'l, aryl,
1 .hied or subs sc with ~- c c moieties
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
29
independently selected from the group consisting of alkyl, cycloalkyl, aryl,
heteroaryl,
alkenyl, cyclenyl, heterocyclenyl, halo, trihaloalkyl, alkoxyl, hydroxyalkyl,
trihaloalkoxyl
and CN;
R5 and R7 are each selected from the group consisting of hydrogen, alkyl,
aminoalkyl, alkenyl, alkynyl, aryl, heterocyclyl, heterocyclenyl, cycloalkyl,
cyclenyl,
cycloalkylalkyl, cyclenylalkyl, cycloalkylalkenyl, cyclenylalkenyi,
heteroaryl,
heteroarylalkyl, heteroarylaikenyl, arylalkyl, arylalkenyl, heterocyclylalkyl,
heterocyclenylalkyl, heterocyclylaikenyl, heterocyclenylalkenyl, -S-
heterocyclyl, -S-
aminoalkyl, -S-heterocyclenyl, NRBR9, NRBCOR9, NR"SO2R9, COR8, C02R8,
CONR8R9, CH2OR8, ORB, SR8, and S02R8, wherein each of said alkyl, alkenyl,
alkynyl, aryl, heterocyclyl, heterocyclenyl, cycloalkyl, cyclenyl,
cycloalkylalkyl,
cyclenylalkyl, cycloalkylalkenyl, cyclenylalkenyl, heteroaryl,
heteroarylalkyl,
heteroarylalkenyl, arylalkyl, arylalkenyl, heterocyclylalkyl,
heterocyclenylalkyl,
heterocyclylalkenyl, heterocyclenylalkenyl, -S-heterocyclyl and -S-
heterocyclenyl can
be unsubstituted or substituted with at least one moiety, which can be the
same or
different, independently selected from the group consisting of halogen, alkyl,
trihaloalkyl, alkenyl, dihaloalkyl, monohaloalkyl, hydroxyalkyl, OR8, -0,
NR8R9, SR8,
S02R9, CN, S02NR"R9, and NO2;
R6 is hydrogen, halo, trihaloalkyl, alkyl, alkenyl, aryl, cyclenyl,
cycloalkyl,
heteroaryl, heterocyclenyl, heterocyclyl, heteroarylalkyl, heterocycle
nylalkyi,
heterocyclylalkyl, arylalkyl, cyclenylalkyl, cyclylalkyl, NR8R9, NR8COR9,
NR8S02R9,
CORE, COO', CONR8R9, CH2OR8, OR8, SR8, or S02R8, wherein each of said alkyl,
alkenyl, aryl, cyclenyl, cycloalkyl, heteroaryl, heterocyclenyl, heterocyclyl,
heteroarylalkyl, heterocyclenylalkyl, heterocyclylalkyl, arylalkyl,
cyclenylalkyl, and
cyclylaikyl can be unsubstiuted or substituted with one or more c ieties
independently
selected fr ~:.<< J Soup consisting of halogen, C~,~y ..ycioalky,, naoalkyl,
OR8, CN,
NRBR9, C02R8, CONR8R9, -SR8, SO2R8, S02NR8R., NO2, NR8S02R9, NR8COR9, and
NRBCONR8R9;
R8 and R9 are each independertly selected from the group consisting of
L he
{ . , l ÃYF _. _ ,e! k('3 a,_ Ja 1r
argil
cyclc k cycle 9yi, ;.; c alky ti's ;ienylalkyl.
vclenyl h r Oyc; , ,t ycle heterocy. 5 ,. , I
J Y
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
heterocyclylalkyl, and heteroarylalkyl, wherein each of said alkyl, alkenyl,
aryl,
heteroaryl, arylalkyl, cycloalkyl, cyclenyl, cycloalkylalkyl, cyclenylalkyl,
cyclenylalkenyl,
heterocyclenyl, heterocyclyl, heterocyclenylalkyl, heterocyclenylalkenyl,
heterocyclylalkyl, and heteroarylalkyl can be unsubstituted or substituted
with at least
5 one moiety, which can be the same or different, independently selected from
the group
consisting of halo, alkyl, cycloalkyl, cyclenyl, aryl, heterocyclyl,
heterocyclenyl,
heteroaryl, trihaloalkyl, CN, hydroxyl, alkoxyl and NO2.
In one embodiment R3 is selected from the group consisting of heteroaryl,
alkyl,
halogen and cycloalkyl, wherein said heteroaryl can be unsubstituted or
substituted
10 with alkyl.
N 0
3 or s
In another embodiment, R3 is
In yet another embodiment, R3 is
N N
=_'N\ ~'
N-CH3 t3 \ CH3
'z or
In another embodiment, R3 is ethyl.
15 In still another embodiment, R3 is cyclopropyl.
In yet still another embodiment, R3 is bromine.
In another embodiment, R5 is selected from the group consisting of
_~_H
2
/`~/NH
-~-S hater c: aryl,
O
[ , or I . ! erc n
hoterocyiyi can be unsubstituted or substituted with hydroxyalkyl and said
aryt can be
20 unsubstituted or substituted with halogen.
In another embodiment, R5 is
or
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
31
__N
In yet another embodiment, R5 is OH or HO
In another embodiment, R6 is halogen or (C,-C6)alkyl.
In yet another embodiment, R6 is bromine, methyl or ethyl.
In another embodiment, R7 is -NHR8, wherein R6 is selected from the group
consisting of hydrogen, heteroaryl and heteroarylalkyl, wherein said
heteroarylalkyl
can be unsubstituted or substituted with one or more moieties independently
selected
from the group consisting of hydroxyl, oxo, alkyl, and alkoxyl and said
heteroaryl can
be unsubstituted or substituted with alkyl.
In another embodiment, R8 is
=S
N
In yet another embodiment, R8 is H3C
N
In still yet another embodiment, R8 is
N-CH3 or N=OH
C/ N-O
In still another embodiment,, R8 is 0 H3C"/O
In another e,. a rest a o .:x:und having the formula.
N RS
N
N R6
NB
or esters, .
h~~.r ,'rugs and stereolsc mers
3l !_. R g'''; 'a :~.+eat ;ted t-
r~~ R~, R ~$, R~ i.i .: ;.. ~r ~tra 14+ cr 4av3h i other
her
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
32
wherein:
R3 is selected from the group consisting of heteroaryl, alkyl, halogen and
cycloalkyl,
wherein said heteroaryl can be unsubstituted or substituted with alkyl;
_~_H
N-P
R5 is selected from the group consisting of heterocyclyl, aryl, OH
5 , or wherein said heterocylyl can be unsubstituted or
substituted with hydroxyalkyl and said aryl can be unsubstituted or
substituted with
halogen;
R6 is halogen or (C,-C6)alkyl;
R8 is selected from the group consisting of hydrogen, heteroaryl and
heteroarylalkyl,
wherein said heteroarylalkyl can be unsubstituted or substituted with one or
more
moieties independently selected from the group consisting of hydroxyl, oxo,
alkyl, and
alkoxyl and said heteroaryl can be unsubstituted or substituted with alkyl.
In another embodiment, a compound having the formula:
R3
N R5
NfN R6
NHR8
or a pharmaceutically acceptable salts, solvates, esters, prodrugs and
stereoisomers
thereof, where R3, R5, R6 and R7 are selected independently of each other
wherein:
R3 is selected from the group consisting of
N"'--CHI 0 LCH ,
ethyl, cyclopropyl, and bromine,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
33
R is selected from the group consisting of phenyl, H,
N
OH O OH , or
NH, wherein said phenyl can be unsubstituted or substituted with fluorine;
R6 is bromine, methyl or ethyl;
R8 is
N
N
N
hydrogen, H3C /
s 1 WCH3 or OH
N -OH
0 H3C IO
Non-limiting examples of compounds of Formula I include:
OH
N-N S
D~N
HN era HN N HN
8r ,N- N Br N
1.0 NH2 o ti H, NHS
N
N
S N
NH.
N/~~" N Br'N
NH2 NH2 NH2 s INH2
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
34
-"IH '-'H N
HN 3! e Nht BEd s . V S~iN OH NH
N_ N N_
l3r 1 Lt{ F N Y f N Y #~ \.
NH2 NH2 NH2 NH2 'O
~Br k'~ ~ati H
O., N
Ori l
OH Jib OH~-NH OH,, NH (3H~ IH
f
0 0 0 vN'0
N N` N'
6r i NYN , F ,N
4N`N N OH (NH NH
NH (NH
1 ~
N, 0
UN C ~O or
or a pharmaceutically acceptable salt, solvate or ester thereof.
In another embodiment a pharmaceutical composition, comprising a compound
of Formula 1, and a pharmaceutically acceptable carrier.
In another embodiment the pharmaceutical composition containing the
compounds of Formula I that is formulated for single dosage administration.
In another embodiment, use of a compound of Formula I in the preparation of a
medicament for the treatment of a CDK-2 mediated disease.
In another embodiment, a method of treatment, prevention, or amelioration of
one or more symptoms of a disease or disorder that is modulated or otherwise
affected by CDK-2, comprising administering to a patient in need thereof an
effective
o pound of Formula i ore pharmace :~1
in another embodiment, use of a compound of Formula I in the preparation of a
medicament for the treatment of a CHK-1 mediated disease.
In another embodiment, a method of treatment, prevention, or amelioration of
on c + r 'c e s r;ptoms of a disease. c disorder tha is . or. uu. ated 0':
an eff , . , .
ou; -Et of a coo,-,;pound of Formuia 1 of a pharmaceuticaily acceptable salt
thereof.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
In another embodiment, the disease or disorder that is treated is selected
from
the group inflammatory disease, neurodegenerative disease, cancer and
diabetes.
In another embodiment, the disease treated is an inflammatory disease
selected from the group consisting of acute pancreatitis, chronic
pancreatitis, asthma,
5 allergies, and adult respiratory distress syndrome.
In another embodiment, the disease treated is a neurodegenerative disease
selected from the group consisting of acute Alzheimer's disease, Parkinson's
disease,
cerebral ischemia, and other neurodegenerative diseases.
In another embodiment the disease treated is diabetes selected from diabetes
10 mellitus and diabetes insipidus.
In another embodiment the diabetes treated is selected from type 1 diabetes
and type 2 diabetes.
In another embodiment the disease treated is a cancer selected from the group
consisting of:
15 tumor of the bladder, breast (including BRCA-mutated breast cancer),
colorectal,
colon, kidney, liver, lung, small cell lung cancer, non-small cell lung
cancer, head and
neck, esophagus, bladder, gall bladder, ovary, pancreas, stomach, cervix,
thyroid,
prostate, and skin, including squamous cell carcinoma;
leukemia, acute lymphocytic leukemia, acute lymphoblastic leukemia, chronic
20 lymphocytic leukemia ("CLL"), acute and chronic myelogenous leukemia,
myelodysplastic syndrome and promyelocytic leukemia;
B-cell lymphoma, T- cell lymphoma, Hodgkins lymphoma, nonHodgkins
lymphoma, hairy cell lymphoma, mantle cell lymphoma, myeloma and Burkett`s
lymphoma;
25 fibrosarcoma, rhabdomyosarcoma;
head and neck. mantle cell lymphoma, myeloma;
astrocytoma, neuroblastoma, glioma, glioblastoma, malignant glial tumors,
astrocytoma, hepatocellular carcinoma, gastrointestinal stromal tumors
("GIST") and
schwannomas;
30 cart- inrn osteosarcoma,
x. urea pigmentosum, sera octanthor - :,:cif follicular cancer and l aposi`s
-S C, M a,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
36
In another embodiment a method of treating one or more diseases associated
with cyclin dependent kinase in a mammal, comprising administering to said
mammal
an amount of a first compound, which is a compound of Formula I, or a
pharmaceutically acceptable salt, solvate, ester or prodrug thereof;
and
an amount of at least one second compound, said second compound being an
anti-cancer agent;
wherein the amounts of the first compound and said second compound
result in a therapeutic effect.
In another embodiment a method of treatment comprising administering to said
mammal an amount of a first compound, which is a compound of Formula I, an
amount of at least one second compound, said second compound being an anti-
cancer agent and further comprising radiation therapy.
In another embodiment wherein said anti-cancer agent is selected from the
group consisting of a cytostatic agent, cytostatic agent, cisplatin,
doxorubicin,
taxotere, taxol, etoposide, irinotecan, camptostar, topotecan, paclitaxel,
docetaxel,
epothilones, tamoxifen, 5-fluorouracil, methoxtrexate, temozolomide,
cyclophosphamide, SCH 66336, 8115777, L778,123, BMS 214662, Iressa, Tarceva,
antibodies to EGFR, Gleevec, intron, ara-C, adriamycin, cytoxan, gemcitabine,
Uracil
mustard, Chlormethine, Ifosfamide, Melphalan, Chlorambucil, Pipobroma,
Triethylenemelamine, Triethylenethiophosphoramine, Busulfan, Carmustine,
Lomustine, Streptozocin, Dacarbazine, Floxuridine, Cytarabine, 6-
Mercaptopurine,
6-Thioguanine, Fludarabine phosphate, oxaliplatin, leucovirin, ELOXATINTM,
Pentostatine, Vinblastine, Vincristine, Vindesine, Bleomycin, Dactinomycin,
Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mithramycin, Deoxycofor,
Mitorycin-C, L-Asparaginase, Teniposide 17a-Ethinylestradiol,
Diethylstilbestrol,
Testosterone, Prednisone, Fluoxymesterone, Dromostanolone propionate,
Testolactone, Megestro I acetate, Methylprednisolone, Methyltestosterone,
Prednisolone, Triamcinolone, Chlorotrianisene, Hydroxyprogesterone,
Aminoglutetlimid, Estr :: Medroxypro .. u~~eacetate, Leuprolide,
`~, 1 v, Tyr E: , goserelin, Cisplatin, Carboplatin, Hydroxyurea, Arnsacr-e,
Pry b r Mitoxantrone, Levamisole, Navelbene, Anastrazole,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
37
Letrazole, Capecitabine, Reloxafino, Droloxafine, Hexamethylmelamine, Avastin,
herceptin, Bexxar, Velcade, Zevalin, Trisenox, Xeloda, Vinoreibine, Porfimer,
Erbitux,
Liposomal, Thiotepa, Altretamine, Melphalan, Trastuzumab, Lerozole,
Fulvestrant,
Exemestane, Fulvestrant, lfosfomide, Rituximab, C225, Campath, Clofarabine,
cladribino, aphidicolon, rituxan, sunitinib, dasatinib, tezacitabine, SmIl,
fludarabine,
pentostatin, triapine, didox, trimidox, amidox, 3-AP, and MDL-181,731.
In another embodiment a pharmaceutical composition comprising a
therapeutically effective amount of at least one compound of Formula I, or a
pharmaceutically acceptable salt, solvate, ester or prodrug thereof, in
combination
with at least one pharmaceutically acceptable carrier.
in another embodiment, the pharmaceutical composition of containing the
compound of Formula I or pharmaceutically acceptable salt, solvate, ester or
prodrug
thereof, additionally comprising one or more anti-cancer agents selected from
the
group consisting of cisplatin, doxorubicin, taxotere, taxol, etoposide,
irinotecan,
camptostar, topotecan, paclitaxel, docetaxel, epothilones, tamoxifen, 5-
fluorouracil,
methoxtrexate, temozolomide, cyclophosphamide, SCH 66336, R115777, L778,123,
BMS 214662, lressa, Tarceva, antibodies to EGFR, Gleevec, intron, ara-C,
adriamycin, cytoxan, gemcitabine, Uracil mustard, Chlormethine, ifosfamide,
Melphalan; Chlorambucil, Pipobroman, Triethylenemelamine,
Triethylenethiophosphoramine, Busulfan, Carmustine, Lomustine, Streptozocin,
Dacarbazine, Floxuridine, Cytarabine, 6-Mercaptopurine, 6-Thioguanine,
Fludarabine
phosphate, oxaliplatin, leucovirin, ELOXATINTM, Pentostatine, Vinblastine,
Vincristine,
Vindesine, Bleomycin, Dactinomycin, Daunorubicin, Doxorubicin, Epirubicin,
Idarubicin, Mithramycin, Deoxycoformycin, Mitomycin-C, L-Asparaginase,
Teniposide
17, Ethinylestradioi, Diethyistiibestrol, Testosterone, 'rednisone, =.'`;_,.A-
x:ymesterone,
Dromostanolone propionate. Testolactone, Megestrolacetate, Methylprednisolone,
Methyltestosterone, Prednisolone, Triamcinoione, Chlorotrianisene,
Hydroxyprogesterone, Aminoglutethimide, Estramustine,
Medro.xyprogesteroneacetate, Leuprolide, Flutamide, Toremifene, goserelin,
Cispiatin,
m s .: *w g p . S w! Ã ii a,: S P ,
Lejf .:woe, Naveb L trazole, Cape Relcxa~;Ae,
Lk: ~.~ `one, l exa -- . ' r~ ;astir, l e cept r , >.x: tE , Velcad ; Zeval r
,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
38
Trisenox, Xeloda, Vinorelbine, Porfimer, Erbitux, Liposomal, Thiotepa,
Altretamine,
..elphalan, Trastuzumab, Lerozole, Fulvestrant, Exemestane, Fulvestrant,
Ifosfomide,
Rituximab, C225, Campath, Clofarabine, cladribine, aphidicolon, rituxan,
sunitinih,
dasatinib, tezacitabine, SmI1, fludarabine, pentostatin, triapine, didox,
trimidox,
amidox, 3-AP, and MDL-101,731.
In another embodiment, a method of inhibiting one or more cyclin dependent
kinases in a patient, comprising administering a therapeutically effective
amount of the
pharmaceutical composition comprising a therapeutically effective amount of at
least
one compound of Formula I, or a pharmaceutically acceptable salt, solvate,
ester or
prodrug thereof, in combination with at least one pharmaceutically acceptable
carrier
to said patient.
In another embodiment, a method of inhibiting one or more Checkpoint kinases
in a patient, comprising administering a therapeutically effective amount of
the
pharmaceutical composition comprising a therapeutically effective amount of at
least
1.5 one compound of Formula I, or a pharmaceutically acceptable salt, solvate,
ester or
prodrug thereof, in combination with at least one pharmaceutically acceptable
carrier
to said patient.
A method of treating one or more diseases by inhibiting one or more kinases,
wherein said kinases are selected from the group consisting of cyclin
dependent
kinases, Checkpoint kinases, tyrosine kinases and Pim-1 kinases, comprising
administering a therapeutically effective amount of at least one compound of
Formula
I or a pharmaceutically acceptable salt, solvate, ester or prodrug thereof to
a patient in
need of such treatment.
In another embodiment, a compound of Formula I in purified form.
C. Preparation of the Compounds
The compounds described herein can be obtained from commercial sources or
synthesized by convenUional methods as shown below using commercially
available
F,- t ;,tom, the compounds can be .gig the
1<<: scheme pr be~ow.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
39
Compounds of Formula l where R5 is a C-linked substituent (e.g. compound 1a),
can
be prepared as illustrated in Scheme 1, starting from 2-fluoro-5-aminopyrazole
(J.
Heterocyclic Chem, 1978, 15, 1447. and Tetrahedron Letters 1979, 34, 3179.)
and the
corresponding beta-ketoester:
Scheme 1
H2N j F AEQH ~/~ R
F
H Fib Q N-N reflex N /--N PQCl,
H
0 N,N-dÃrnethylanil ie
2 3
1 . NBS, CH3CN N RBr
N RS 2. E+eleQNa,MeC}H N!N Re
Ci
NN i Rs
4
We
5 1 . Suzuki coupling
N- N
2 RZH
R3
N NH (Z= NH00, S) N R5
F \ N F \
N- Br NBS N-N Rs
1. R ,=H NH2 Rf,_Br 1,0 R~
la (Fi, =NHFi, OR, 5R)
Compounds of Formula l where R5 is a N-linked substituent (e.g. compound 1 b),
can
be prepared as illustrated in Scheme 2 from 2-fluoro-5-aminopyrazole and
appropriately substituted dimethyl malonate:
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
Scheme 2
O H2N CI
+ f #. re#lux N` OH PQCi3
C3 N-N McUNa N'N R6 N,N dimethylaniline R6
R6 H McOr H cl
6 2 7 8
RZH
H [ (z= NH, 0, S)
N N Br 1. NBS
F N,N Br Suzuki N;`R6 R5 CH3CN /`~~NJ CC
Fes`
NH C-linked N N~2. NaHCO3 N"N Rs
lb substituent R7 NMP 9 R7
I 1.0 (R7 =NHR, OR, SR) R R`NH (R7 =NHR, OR, SR)
N (R5 = NHR.R")
5 The compounds described herein can be separated from a. reaction mixture
and further purified by a method such as column chromatography, high-pressure
liquid
chromatography, or recrystallization. As can be appreciated by the skilled
artisan,
further methods of synthesizing the compounds of the formulae herein will be
evident
to those of ordinary skill in the art. Additionally, the various synthetic
steps may be
10 performed in an alternate sequence or order to give the desired compounds.
Synthetic chemistry transformations and protecting group methodologies
(protection
and deprotection) useful in synthesizing the compounds described herein are
known
in the art and include, for example, those such as described in R. Larock,
Comprehensive Organic Transformations, VCH Publishers (1989); T.W. Greene and
15 P.G.M. Wuts, Protective Groups in Organic Synthesis, 2d. Ed., John Wiley
and Sons
11991); L. Fieser and M. Fieser, Pieser and Fieser's Reagents for Organic
Synthesis,
John Wiley and Sons (12 L. F' r c c _ Y. f R for
Organic Synthesis, John Wiley and Sons ( 995), and subsequent editions
thereof.
The compounds according to the invention can have pharmacological
20 properties; in particular, the compounds of Formula l can be inhibitors of
protein
kinases such as, for example, the inhibitors of the cyclin-dependent kinases,
Checkpoint kinase, r itog -; Vated protein k; P 'ER gll tease
kinasc: GSK beta) and' 'he ; W. The cyclirt depe 1r:eE:i _~ kinases (C s) 'nc!
ode, for
exam p e, C D02 (..Cf K1), C K2, CDK4, CDK5, CDK6, COK7 and CDK8. One such
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
41
Checkpoint kinase is CHK-1. The novel compounds of Formula I are expected to
be
useful in the therapy of proliferative diseases such as cancer, autoimmune
diseases,
viral diseases, fungal diseases, neurological/neurodegenerative disorders,
arthritis,
inflammation, anti-proliferative (e.g., ocular retinopathy), neuronal,
alopecia and
cardiovascular disease. Many of these diseases and disorders are listed in
U.S.
6,413,974 cited earlier, the disclosure of which is incorporated herein.
More specifically, the compounds of Formula I can be useful in the treatment
of
a variety of cancers, including (but not limited to) the following:
carcinoma, including that of the bladder, breast, colon, kidney, liver, lung,
including
small cell lung cancer, esophagus, gall bladder, ovary, pancreas, stomach,
cervix,
thyroid, prostate, and skin, including squamous cell carcinoma; hematopoietic
tumors
of lymphoid lineage, including leukemia, acute lymphocytic leukemia, acute
lymphoblastic leukemia, B-cell lymphoma, T- cell lymphoma, Hodgkins lymphoma,
non-Hodgkins lymphoma, hairy cell lymphoma and Burkett's lymphoma;
hematopoietic tumors of myeloid lineage, including acute and chronic
myelogenous leukemias, myelodysplastic syndrome and promyelocytic leukemia;
tumors of mesenchymal origin, including fibrosarcoma and
rhabdomyosarcoma;
tumors of the central and peripheral nervous system, including astrocytoma,
neuroblastoma, glioma and schwannomas; and
other tumors, including melanoma, seminoma, teratocarcinoma, osteosarcoma,
xenoderoma pigmentosum, keratoctanthoma, thyroid follicular cancer and
Kaposi's
sarcoma.
Due to the key role of CDKs in the regulation of cellular proliferation in
general,
23 inhibitors could act as revs .,; h`e -yttosta.ti = t . may be The
treatment of any disease process which features abnormal cellular
proliferation, e.g.,
benign prostate hyperplasia, familial adenomatosis polyposis, neuro-
fibromatosis,
atherosclerosis, pulmonary fibrosis, arthritis, psoriasis, glomerulonephritis,
restenosis
following angioplasty or vascular surgery, hypertrophic scar formation,
inflammatory
cease tr f. f ation ~~ er~c otoxic sl ocl , aid f n ` g_
One series of checkpoints Ã. 4 he integrity of the =e an~
sensing DNA damage, these "DNA d; i:;a checkpoints" block ccH cycle
progression
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
42
in G, & G2 phases, and slow progression through S phase. This action enables
DNA
repair processes to complete their tasks before replication of the genome and
subsequent separation of this genetic material into new daughter cells takes
place.
Inactivation of CHK1 has been shown to transduce signals from the DNA-damage
sensory complex to inhibit activation of the cyclin B/Cdc2 kinase, which
promotes
mitotic entry, and abrogate G2 arrest induced by DNA damage inflicted by
either
anticancer agents or endogenous DNA damage, as well as result in preferential
killing
of the resulting checkpoint defective cells. See, e.g., Peng et al., Science,
277, 1501-
1505 (1997); Sanchez et al., Science, 277, 1497-1501 (1997), Nurse, Cell, 91,
865-
867 (1997); Weinert, Science, 277, 1450-1451 (1997); Walworth et al., Nature,
363,
368-371 (1993); and Al-Khodairy et al., Mo/ec. Biol. Cell., 5, 147-160 (1994).
Selective manipulation of checkpoint control in cancer cells could afford
broad
utilization in cancer chemotherapeutic and radiotherapy regimens and may, in
addition, offer a common hallmark of human cancer "genomic instability" to be
exploited as the selective basis for the destruction of cancer cells. A number
of
factors place CHK1 as a pivotal target in DNA-damage checkpoint control. The
elucidation of inhibitors of this and functionally related kinases such as
CDS1/CHK2, a
kinase recently discovered to cooperate with CHK1 in regulating S phase
progression
(see Zeng et al., Nature, 395, 507-510 (1998); Matsuoka, Science, 282, 1893-
1897
(1998)), could provide valuable new therapeutic entities for the treatment of
cancer.
Compounds of Formula I can also be useful in the treatment of Alzheimer's
disease, as suggested by the recent finding that CDK5 is involved in the
phosphorylation of tau protein (J. Biochem, (1995) 117, 741-749),
Compounds of Formula I may induce or inhibit apoptosis. The apoptotic
response is aberrant in a variety of human diseases, Compounds of Fora `
modulators of apoptosis, will be useful in the treatment of cancer (including
but not
limited to those types mentioned hereinabove), viral infections (including but
not
limited to herpevirus, poxvirus, Epstein- Barr virus, Sindbis virus and
adenovirus),
prevention of AIDS development in Hl infected individuals, autoimmune diseases
tC:._ lupus, m., ~r': osus, a..1 hne me an
0
i o m e r u I o n meumatcsd a hritis, psoriasis, infian d sease
autoimmune d (-:betes rnellitu:), Jrc-=degenerative disorders c! ;c;i;~:g but
not ,.. ., &
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
43
to Alzheimer's disease, AIDS-related dementia, Parkinson's disease,
amyotrophic
lateral sclerosis, retinitis pigmentosa, spinal muscular atrophy and
cerebellar
degeneration), myelodysplastic syndromes, aplastic anemia, ischemic injury
associated with myocardial infarctions, stroke and reperfusion injury,
arrhythmia,
atherosclerosis, toxin-induced or alcohol related liver diseases,
hematological
diseases (including but not limited to chronic anemia and aplastic anemia),
degenerative diseases of the musculoskeletal system (including but not limited
to
osteoporosis and arthritis) aspirin-sensitive rhinosinusitis, cystic fibrosis,
multiple
sclerosis, kidney diseases and cancer pain.
Compounds of Formula I, as inhibitors of the CDKs, can modulate the level of
cellular RNA and DNA synthesis. These agents would therefore be useful in the
treatment of viral infections (including but not limited to HIV, human
papilloma virus,
herpesvirus, poxvirus, Epstein-Barr virus, Sindbis virus and adenovirus).
Compounds of Formula I may also be useful in the chemoprevention of cancer.
.1.5 Chemoprevention is defined as inhibiting the development of invasive
cancer by either
blocking the initiating mutagenic event or by blocking the progression of pre-
malignant
cells that have already suffered an insult or inhibiting tumor relapse.
Compounds of Formula I may also be useful in inhibiting tumor angiogenesis
and metastasis.
Compounds of Formula I may also act as inhibitors of other protein kinases,
e.g., protein kinase C, her2, raf 1, MEK1, MAP kinase, EGF receptor, PDGF
receptor,
IGF receptor, P13 kinase, weel kinase, Src, Abl and thus be effective in the
treatment
of diseases associated with other protein kinases.
Thus, another aspect of this invention is a method of treating a mammal (e.g.,
human) having a disease or condition assoc -e with the CDKs by administ ~~
therapeutically effective amount of at least one compound of Formula 1, or a
pharmaceutically acceptable salt or solvate of said compound to the mammal.
The compounds of this invention may also be useful in combination with one or
more of anti-cancer treatments such as radiation therapy, and/or one or more
anti-
_:c '-gym the group consisting of cy: agents, cytotoxic
age; is (suc..n as for example, but not limited to, DNA interactive ctggents
(such as
_ rit ? or doxorubicin))= taxanes (e.g. taxotere, taxoi); topois4 -:e ii
inhibitors
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
44
(such as etoposide); topoisomerase I inhibitors (such as irinotecan (or CPT-1
1),
camptostar, or topotecan); tubulin interacting agents (such as paclitaxel,
docetaxel or
the epothilones); hormonal agents (such as tamoxifen); thymidilate synthase
inhibitors
(such as 5-fluorouracil); anti-metabolites (such as methoxtrexate); alkylating
agents
(such as temozolomide (TEMODARTM from Schering-Plough Corporation, Kenilworth,
New Jersey), cyclophosphamide); Farnesyl protein transferase inhibitors (such
as,
TM (4-[2-[4-[(11 R)-3,10-dibromo-8-chloro-6,11-dihydro-5H-
benzo[5,6]cyclohepta[1,2-b]pyridin-11-y1-]-1-pipe ridinyl]-2-oxoehtyl]-1-
pipe ridinecarboxamide, or SCH 66336 from Schering-Plough Corporation,
Kenilworth,
New Jersey), tipifarnib (Zarnestrao~ or R115777 from Janssen Pharmaceuticals),
L778,123 (a farnesyl protein transferase inhibitor from Merck & Company,
Whitehouse
Station, New Jersey), BUS 214662 (a farnesyl protein transferase inhibitor
from
Bristol-Myers Squibb Pharmaceuticals, Princeton, New Jersey); signal
transduction
inhibitors (such as, Iressa (from Astra Zeneca Pharmaceuticals, England),
Tarceva
(EGFR kinase inhibitors), antibodies to EGFR (e.g., C225), GLEEVECTM (C-abl
kinase
inhibitor from Novartis Pharmaceuticals, East Hanover, New Jersey);
interferons such
as, for example, intron (from Schering-Plough Corporation), Peg-Intron (from
Schering-Plough Corporation); hormonal therapy combinations; aromatase
combinations; ara-C, adriamycin, cytoxan, and gemcitabine.
Other anti-cancer (also known as anti-neoplastic) agents include but are not
limited to Uracil mustard, Chlormethine, Ifosfamide, Melphalan, Chlorambucil,
Pipobroman, Triethylenemelamine, Triethylenethiophosphoramine, Busulfan,
Carmustine, Lomustine, Streptozocin, Dacarbazine, Floxuridine, Cytarabine,
6-Mercaptopurine. 6-Thioguanine, Fludarabine phosphate, oxaliplatin,
leucovirin,
oxaiiplatin (ELOXATINT' from Sanofi-Synthelabo Pharmaeuticals, France),
Pentostatine, Vinblastine, Vincristine, Vindesine, Bleomycin, Dactinomycin,
Daunorubicin. Doxorubicin, Epirubicin, idarubicin, Mithramycin,
Deoxycoformycin,
Mitomycin-C, L-Asparaginase, Teniposide 17(x-Ethinylestradiol,
Diethylstilbestrol,
Testosterone, Predniysone~,gFluoxymesterone, DromostanoionepropioYnyate,
tor `, n. ', ~' 9rthaC at . M _. ``-}9 ate, a4 t 9L s o E ¾e¾on,
Pretartisc' ~G~ r e, Chior r sene; Hydroxyprogesterone,
Amin glute , Es[ramustinc 'E rccgxyprogesteroneacetate, Leuprolide,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
Flutamide, Toremifene, goserelin, Cisplatin, Carboplatin, Hydroxyurea,
Amsacrine,
Procarbazine, Mitotane, Mitoxantrone, Levamisole, Navelbene, Anastrazole,
Letrazole, Capecitabine, Reloxafine, Droloxafine, or Hexamethylmelamine,
Avastin,
herceptin, Bexxar, Velcade, Zevalin, Trisenox, Xeloda, Vinoreibine, Porfimer,
Erbitux,
5 Liposomal, Thiotepa, Altretamine, Melphalan, Trastuzumab, Lerozole,
Fulvestrant,
Exemestane, Fulvestrant, Ifosfomide, Rituximab, C225, Campath, Clofarabine,
cladribine, aphidicolon, rituxan, sunitinib, dasatinib, tezacitabine, SmI1,
fludarabine,
pentostatin, triapine, didox, trimidox, amidox, 3-AP, and MDL-101,731.
When administering a combination therapy to a patient in need of such
10 administration, the therapeutic agents in the combination, or a
pharmaceutical
composition or compositions comprising the therapeutic agents, may be
administered
in any order such as, for example, sequentially, concurrently, together,
simultaneously
and the like. The amounts of the various actives in such combination therapy
may be
different amounts (different dosage amounts) or same amounts (same dosage
15 amounts). Thus, for non-limiting illustration purposes, a compound of
Formula I and
an additional therapeutic agent may be present in fixed amounts (dosage
amounts) in
a single dosage unit (e.g., a capsule, a tablet and the like). A commercial
example of
such single dosage unit containing fixed amounts of two different active
compounds
for oral administration is VYTORINe (available from Merck Schering-Plough
20 Pharmaceuticals, Kenilworth, New Jersey).
If formulated as a fixed dose, such combination products employ the
compounds of this invention within the dosage range described herein and the
other
pharmaceutically active agent or treatment within its dosage range, For
example, the
CDC2 inhibitor olomucine has been found to act synergistically with known
cytotoxic
25 agents in inducing apoptosis (J. Ce// Sci., (1995) 108, 2897. Compounds of
Formula i
may also be administered sequentially with known anticancer or cytotoxic
agents
when a combination formulation is inappropriate. The invention is not limited
in the
sequence of administration; compounds of Formula I may be administered either
prior
to or after administration of the known anticancer or cytotoxic agent. For
example, the
3c cy' of he c~,,:oe gas.. is ; o tc . lfeced by the
setae aye c 0i :6 .listration with anticancer gc-7_}_s. Dancer Research,
11997) 57_
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
46
3375. Such techniques are within the skills of persons skilled in the art as
well as
attending physicians.
Accordingly, in an aspect, this invention includes combinations comprising an
amount of at least one compound of Formula 1, or a pharmaceutically acceptable
salt
or solvate thereof, and an amount of one or more anti-cancer treatments and
anti-
cancer agents listed above wherein the amounts of the compounds! treatments
result
in desired therapeutic effect.
The pharmacological properties of the compounds of this invention may be
confirmed by a number of pharmacological assays.
I[l This invention is also directed to pharmaceutical compositions which
comprise
at least one compound of Formula I, or a pharmaceutically acceptable salt or
solvate
of said compound and at least one pharmaceutically acceptable carrier.
D. Formulation of pharmaceutical compositions
The pharmaceutical compositions provided herein contain therapeutically
effective amounts of one or more of the compounds provided herein that are
useful in
the prevention, treatment, or amelioration of one or more of the symptoms of
diseases
or disorders associated with CDK-2 or CHK-1, in a pharmaceutically acceptable
carrier. Diseases or disorders associated with CDK-2 or CHK-1 include, but are
not
limited to, inflammatory diseases, neurodegenerative diseases, cancer and
diabetes.
Pharmaceutical carriers suitable for administration of the compounds provided
herein
include any such carriers known to those skilled in the art to be suitable for
the
particular mode of administration.
In addition, the compounds may be formulated as the sole pharmaceutically
active ingredient in the composition or may be combined with other active
ingredients.
The compositions contain one or more compounds ;r ed herein. The
compounds are, in one embodiment, formulated into suitable pharmaceutical
preparations such as solutions, suspensions, tablets, dispersible tablets,
pills,
capsules, powders, sustained release formulations or elixirs, for oral
administration or
in sterile solutions or suspensions for parenteral administration, as well as
transdermal
ep '~ inhalers. In one the c ,
desc'ibed above are formulated into compositions t._ dues
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
47
and procedures well known in the art (see, e.g., Ansel introduction to
Pharmaceutical
Dosage Forms, Fourth Edition 1985, 126).
In the compositions, effective concentrations of one or more compounds or
pharmaceutically acceptable derivatives thereof is (are) mixed with a suitable
pharmaceutical carrier. The compounds may be derivatized as the corresponding
salts, esters, enol ethers or esters, acetals, ketals, orthoesters,
hemiacetals,
hemiketals, acids, bases, solvates, hydrates or prodrugs prior to formulation,
as
described above. The concentrations of the compounds in the compositions are
effective for delivery of an amount, upon administration, that treats,
prevents, or
ameliorates one or more of the symptoms of diseases or disorders associated
with
CDK-2 or CHK-1 activity or in which CDK-2 or CHK-1 activity is implicated.
In one embodiment, the compositions are formulated for single dosage
administration. To formulate a composition, the weight fraction of compound is
dissolved, suspended, dispersed or otherwise mixed in a selected carrier at an
effective concentration such that the treated condition is relieved,
prevented, or one or
more symptoms are ameliorated.
The active compound is included in the pharmaceutically acceptable carrier in
an amount sufficient to exert a therapeutically useful effect in the absence
of
undesirable side effects on the patient treated. The therapeutically effective
concentration may be determined empirically by testing the compounds using in
vitro
and in vivo systems described herein and then extrapolated therefrom for
dosages for
humans.
The concentration of active compound in the pharmaceutical composition will
depend on absorption, inactivation and excretion rates of the active compound,
the
physicochemical characteristics of the compound, the dosage schedule, and
amount
administered as well as other factors known to those of skill in the art. For
example,
the amount that is delivered is sufficient to ameliorate one or more of the
symptoms of
diseases or disorders associated with CDK-2 or CHK-1 activity or in which CDK-
2 or
CHK-1 activity is implicated, as described herein.
n or:c r t, a osa void produce a serum
cotave ingredieni of from j;..1 to about 5
0
10 pg, m;. Ã he
ph t' , =a l Om positions, in anoth, r tiao me should provide a dosage of
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
48
from about 0.001 mg to about 2000 mg of compound per kilogram of body weight
per
day. Pharmaceutical dosage unit forms are prepared to provide from about 0.01
mg,
0.1 mg or 1 mg to about 500mg, 1000 mg or 2000 mg, and in one embodiment from
about 10 mg to about 500 mg of the active ingredient or a combination of
essential
ingredients per dosage unit form.
The active ingredient may be administered at once, or may be divided into a
number of smaller doses to be administered at intervals of time. It is
understood that
the precise dosage and duration of treatment is a function of the disease
being treated
and may be determined empirically using known testing protocols or by
extrapolation
from in vivo or in vitro test data. It is to be noted that concentrations and
dosage
values may also vary with the severity of the condition to be alleviated. It
is to be
further understood that for any particular subject, specific dosage regimens
should be
adjusted over time according to the individual need and the professional
judgment of
the person administering or supervising the administration of the
compositions, and
that the concentration ranges set forth herein are exemplary only and are not
intended
to limit the scope or practice of the claimed compositions.
In instances in which the compounds exhibit insufficient solubility, methods
for
solubilizing compounds may be used. Such methods are known to those of skill
in
this art, and include, but are not limited to, using cosolvents, such as
dimethylsulfoxide
(DMSO), using surfactants, such as TWEEN , or dissolution in aqueous sodium
bicarbonate. Derivatives of the compounds, such as prodrugs of the compounds
may
also be used in formulating effective pharmaceutical compositions.
Upon mixing or addition of the compound(s), the resulting mixture may be a
solution, suspension, emulsion or the like. The form of the resulting mixture
depends
upon a number of factors, including the intended mode of administration and
the
solubility of the compound in the selected carrier or vehicle. The effective
concentration is sufficient for ameliorating the symptoms of the disease,
disorder or
condition treated and may be empirically determined.
The pharmaceutical compositions are provided for administration to humans
r: s dosage forms; such as tablets, _ ses pills granules,
><T ntE g o LS ,aL~
pdwe on or sLÃ gs pe}ye 7:~ _-~. -d oral .lions, :nd oil-
t=r emulsions c! ; . ~g suitable the co . rn~ _ _ y
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
49
acceptable derivatives thereof. The pharmaceutically therapeutically active
compounds and derivatives thereof are, in one embodiment, formulated and
administered in unit-dosage forms or multiple-dosage forms. Unit-dose forms as
used
herein refers to physically discrete units suitable for human and animal
subjects and
packaged individually as is known in the art. Each unit-dose contains a
predetermined
quantity of the therapeutically active compound sufficient to produce the
desired
therapeutic effect, in association with the required pharmaceutical carrier,
vehicle or
diluent. Examples of unit-dose forms include ampoules and syringes and
individually
packaged tablets or capsules. Unit-dose forms may be administered in fractions
or
multiples thereof. A multiple-dose form is a plurality of identical unit-
dosage forms
packaged in a single container to be administered in segregated unit-dose
form.
Examples of multiple-dose forms include vials, bottles of tablets or capsules
or bottles
of pints or gallons. Hence, multiple dose form is a multiple of unit-doses
which are not
segregated in packaging.
Liquid pharmaceutically administrable compositions can, for example, be
prepared by dissolving, dispersing, or otherwise mixing an active compound as
defined above and optional pharmaceutical adjuvants in a carrier, such as, for
example, water, saline, aqueous dextrose, glycerol, glycols, ethanol, and the
like, to
thereby form a solution or suspension. If desired, the pharmaceutical
composition to
be administered may also contain minor amounts of nontoxic auxiliary
substances
such as wetting agents, emulsifying agents, solubilizing agents, pH buffering
agents
and the like, for example, acetate, sodium citrate, cyclodextrine derivatives,
sorbitan
monolaurate, triethanolamine sodium acetate, triethanolamine oleate, and other
such
agents.
Actual methods of preparing s dosage ` re known. be .pparent,
to those skilled in this art; for example, see Remington's Pharmaceutical
Sciences,
Mack Publishing Company, Easton, Pa., 15th Edition, 1975.
Dosage forms or compositions containing active ingredient in the range of
0.005% to 100% with the balance made up from non-toxic carrier may be
prepared.
3 E ..5 mrs are known a the art.
plated ... : i ,3n s i 3.001%-1001:- . _~ m::ve ingredient, in one
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
1. Compositions for oral administration
Oral pharmaceutical dosage forms are either solid, gel or liquid. The solid
dosage forms are tablets, capsules, granules, and bulk powders. Types of oral
tablets
include compressed, chewable lozenges and tablets which may be enteric-coated,
5 sugar-coated or film-coated. Capsules may be hard or soft gelatin capsules,
while
granules and powders may be provided in non-effervescent or effervescent form
with
the combination of other ingredients known to those skilled in the art.
a. Solid compositions for oral administration
In certain embodiments, the formulations are solid dosage forms, in one
10 embodiment, capsules or tablets. The tablets, pills, capsules, troches and
the like can
contain one or more of the following ingredients, or compounds of a similar
nature: a
binder; a lubricant; a diluent; a glidant; a disintegrating agent; a coloring
agent; a
sweetening agent; a flavoring agent; a wetting agent; an emetic coating; and a
film
coating. Examples of binders include microcrystalline cellulose, gum
tragacanth,
15 glucose solution, acacia mucilage, gelatin solution, molasses,
polvinylpyrrolidine,
povidone, crospovidones, sucrose and starch paste. Lubricants include talc,
starch,
magnesium or calcium stearate, lycopodium and stearic acid. Diluents include,
for
example, lactose, sucrose, starch, kaolin, salt, mannitol and dicalcium
phosphate.
Glidants include, but are not limited to, colloidal silicon dioxide.
Disintegrating agents
20 include crosscarmellose sodium, sodium starch glycolate, alginic acid, corn
starch,
potato starch, bentonite, methylcellulose, agar and carboxymethylcellulose.
Coloring
agents include, for example, any of the approved certified water soluble FD
and C
dyes, mixtures thereof; and water insoluble FD and C dyes suspended on alumina
hydrate. Sweetening agents include sucrose, lactose, mannitol and artificial
25 sweetening agents such as saccharin, and any number of spry dried flavors.
Flavoring agents include natural flavors extracted from plants such as fruits
and
synthetic blends of compounds which produce a pleasant sensation, such as, but
not
limited to peppermint and methyl salicylate. Wetting agents include propylene
glycol
monostearate, sorbitan monooleate, diethylene glycol monolaurate and
30 polyoxyeti=yler c- .: e'-ie,. Ernetic-coatin'- uc e fatty. ; fats, waxes.
a t. d shellac; and ce !'.use aceta0 ;: : tes. coatings include
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
51
hydroxyethylcelIulose, sodium ca rboxymethylceliulose, polyethylene glycol
4000 and
cellulose acetate phthalate.
The compound, or pharmaceutically acceptable derivative thereof, could be
provided in a composition that protects it from the acidic environment of the
stomach.
For example, the composition can be formulated in an enteric coating that
maintains
its integrity in the stomach and releases the active compound in the
intestine. The
composition may also be formulated in combination with an antacid or other
such
ingredient.
When the dosage unit form is a capsule, it can contain, in addition to
material of
the above type, a liquid carrier such as a fatty oil. In addition, dosage unit
forms can
contain various other materials which modify the physical form of the dosage
unit, for
example, coatings of sugar and other enteric agents. The compounds can also be
administered as a component of an elixir, suspension, syrup, wafer, sprinkle,
chewing
gum or the like. A syrup may contain, in addition to the active compounds,
sucrose as
a sweetening agent and certain preservatives, dyes and colorings and flavors.
The active materials can also be mixed with other active materials which do
not
impair the desired action, or with materials that supplement the desired
action, such
as antacids, H2 blockers, and diuretics. The active ingredient is a compound
or
pharmaceutically acceptable derivative thereof as described herein. Higher
concentrations, up to about 98% by weight of the active ingredient may be
included.
In all embodiments, tablets and capsules formulations may be coated as known
by those of skill in the art in order to modify or sustain dissolution of the
active
ingredient. Thus, for example, they may be coated with a conventional
enterically
digestible coating, such as phenylsalicylate, waxes and cellulose acetate
phthalate.
b. Liquid compositions for oral administration
Liquid oral dosage forms include aqueous solutions, emulsions, suspensions,
solutions and/or suspensions reconstituted from non-effervescent granules and
effervescent preparations reconstituted from effervescent granules. Aqueous
solutions
include, for example, elixirs and syrups. Emulsions are either oil-in-water or
water-in-
oil,
E x;rc- are clear, sweetened, hyorca b ;c Filarmaceutica,`6y
to ors 'ri elixirs include so r;s concentrated a ,.4 s
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
52
solutions of a sugar, for example, sucrose, and may contain a preservative. An
emulsion is a two-phase system in which one liquid is dispersed in the form of
small
globules throughout another liquid. Pharmaceutically acceptable carriers used
in
emulsions are non-aqueous liquids, emulsifying agents and preservatives.
Suspensions use pharmaceutically acceptable suspending agents and
preservatives.
Pharmaceutically acceptable substances used in non-effervescent granules, to
be
reconstituted into a liquid oral dosage form, include diluents, sweeteners and
wetting
agents. Pharmaceutically acceptable substances used in effervescent granules,
to be
reconstituted into a liquid oral dosage form, include organic acids and a
source of
carbon dioxide. Coloring and flavoring agents are used in all of the above
dosage
forms.
Solvents include glycerin, sorbitol, ethyl alcohol and syrup. Examples of
preservatives include glycerin, methyl and propylparaben, benzoic acid, sodium
benzoate and alcohol. Examples of non-aqueous liquids utilized in emulsions
include
mineral oil and cottonseed oil. Examples of emulsifying agents include
gelatin, acacia,
tragacanth, bentonite, and surfactants such as polyoxyethylene sorbitan
monooleate.
Suspending agents include sodium carboxymethylcellulose, pectin, tragacanth,
Veegum and acacia. Sweetening agents include sucrose, syrups, glycerin and
artificial sweetening agents such as saccharin. Wetting agents include
propylene
glycol monostearate, sorbitan monooleate, diethylene glycol monolaurate and
polyoxyethylene lauryl ether. Organic acids include citric and tartaric acid.
Sources of
carbon dioxide include sodium bicarbonate and sodium carbonate. Coloring
agents
include any of the approved certified water soluble FD and C dyes, and
mixtures
thereof. Flavoring agents include natural flavors extracted from plants such
fruits, and
synthetic blends of compounds which produce a pleasant taste sensation,
For a solid dosage form, the solution or suspension, in for example propylene
carbonate, vegetable oils or triglycerides, is in one embodiment encapsulated
in a
gelatin capsule. For a liquid dosage form, the solution, e.g., for example, in
a
polyethylene glycol, may be diluted with a sufficient quantity of a
pharmaceutically
accepta carrier, e.
g ., ~ ate r. to ~. _sas_. _ do ... ..
Alternatively, liquid or semi-solid oral fo , may be prepared oy
dispersing the active compound or sa in vegetable oils, glycols,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
53
triglycerides, propylene glycol esters (e.g., propylene carbonate) and other
such
carriers, and encapsulating these solutions or suspensions in hard or soft
gelatin
capsule shells. Briefly, such formulations include, but are not limited to,
those
containing a compound provided herein, a dialkylated mono- or poly-alkylene
glycol,
including, but not limited to, 1,2-dimeth.oxymethane, diglyme, triglyrne,
tetraglyme,
polyethylene glycol-350-dimethyl ether, polyethylene glycol-550-dimethyl
ether,
polyethylene glycol-750-dimethyl ether wherein 350, 550 and 750 refer to the
approximate average molecular weight of the polyethylene glycol, and one or
more
antioxidants, such as butylated hydroxytolueno (BHT), butylated hydroxyanisole
(BHA), propyl gallate, vitamin E, hydroquinone, hydroxycoumarins,
ethanolamine,
lecithin, cephalin, ascorbic acid, malic acid, sorbitol, phosphoric acid,
thiodipropionic
acid and its esters, and dithiocarbamates.
Other formulations include, but are not limited to, aqueous alcoholic
solutions
including a pharmaceutically acceptable acetal. Alcohols used in these
formulations
are any pharmaceutically acceptable water-miscible solvents having one or more
hydroxyl groups, including, but not limited to, propylene glycol and ethanol.
Acetals
include, but are not limited to, di(lower alkyl) acetals of lower alkyl
aldehydes such as
acetaldehyde diethyl acetal.
2. Injectables, solutions and emulsions
Parenteral administration, in one embodiment characterized by injection,
either
subcutaneously, intramuscularly or intravenously is also contemplated herein.
Injectables can be prepared in conventional forms, either as liquid solutions
or
suspensions, solid forms suitable for solution or suspension in liquid prior
to injection,
or as emulsions. The injectables, solutions and emulsions also contain one or
more
excipients, Suitable excipients are, for example, water, saline, dextrose, c
1viceroi or
e r iol. In addition, if desired, the pharmaceutical compositions to be
adrn.;nistered
may also contain minor amounts of non-toxic auxiliary substances such as
wetting or
emulsifying agents, pH buffering agents, stabilizers, solubility enhancers,
and other
such agents, such as for example, sodium acetate, sorbitan monolaurate,
L 1 ... 5, i..` _ . kxt7 in ,
n o a slow-release or sus ~i . t ; ' : 's that a
col ~t v:, of dosage is maintained is alo ;pit t.;d Briefly, a
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
54
compound provided herein is dispersed in a solid inner matrix, e.g.,
polymethylmethacrylate, polybutylmethacrylate, plasticized or unplasticized
polyvinylchloride, plasticized nylon, plasticized polyethyleneterephthalate,
natural
rubber, polyisoprene, polyisobutylene, polybutadiene, polyethylene, ethylene-
vinylacetate copolymers, silicone rubbers, polydimethylsiloxanes, silicone
carbonate
copolymers, hydrophilic polymers such as hydrogels of esters of acrylic and
methacrylic acid, collagen, cross-linked polyvinylalcohol and cross-linked
partially
hydrolyzed polyvinyl acetate, that is surrounded by an outer polymeric
membrane,
e.g., polyethylene, polypropylene, ethylene/propylene copolymers,
ethylene/ethyl
acrylate copolymers, ethylene/vinylacetate copolymers, silicone rubbers,
polydimethyl
siloxanes, neoprene rubber, chlorinated polyethylene, polyvinylchloride,
vinylchloride
copolymers with vinyl acetate, vinylidene chloride, ethylene and propylene,
ionomer
polyethylene terephthalate, butyl rubber epichlorohydrin rubbers,
ethylene/vinyl
alcohol copolymer, ethylene/vinyl acetate/vinyl alcohol terpolymer, and
1.5 ethylene/vinyloxyethanol copolymer, that is insoluble in body fluids. The
compound
diffuses through the outer polymeric membrane in a release rate controlling
step. The
percentage of active compound contained in such parenteral compositions is
highly
dependent on the specific nature thereof, as well as the activity of the
compound and
the needs of the subject.
Parenteral administration of the compositions includes intravenous,
subcutaneous and intramuscular administrations. Preparations for parenteral
administration include sterile solutions ready for injection, sterile dry
soluble products,
such as lyophilized powders, ready to be combined with a solvent just prior to
use,
including hypodermic tablets, sterile suspensions ready for injection, sterile
dry
-,5 insoluble products ready to be combined with a vehicle just prior to use
and sterile
emulsions. The solutions may be either aqueous or nonaqueous.
If administered intravenously, suitable carriers include physiological saline
or
phosphate buffered saline (PBS), and solutions containing thickening and
solubilizing
agents, such as glucose, polyethylene glycol, and polypropylene glycol and
mixtures
thereof.
Pharm:-, .. 'y a ,., .r ,aL used z w t p j- parations include
aqueous `.onaquec .......,.:`, c agents,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
buffers, antioxidants, local anesthetics, suspending and dispersing agents,
emulsifying
agents, sequestering or chelating agents and other pharmaceutically acceptable
substances.
Examples of aqueous vehicles include Sodium Chloride Injection, Ringers
5 Injection, Isotonic Dextrose Injection, Sterile Water Injection, Dextrose
and Lactated
Ringers Injection. Nortaqueous parenteral vehicles include fixed oils of
vegetable
origin, cottonseed oil, corn oil, sesame oil and peanut oil. Antimicrobial
agents in
bacteriostatic or fungistatic concentrations must be added to parenteral
preparations
packaged in multiple-dose containers which include phenols or cresols,
mercurials,
10 benzyl alcohol, chlorobutanol, methyl and propyl p-hydroxybenzoic acid
esters,
thimerosal, benzalkonium chloride and benzethonium chloride. Isotonic agents
include
sodium chloride and dextrose. Buffers include phosphate and citrate.
Antioxidants
include sodium bisulfate. Local anesthetics include procaine hydrochloride.
Suspending and dispersing agents include sodium carboxymethylcelluose,
15 hydroxypropyl methylcellulose and pofyvinylpyrrolidone. Emulsifying agents
include
Polysorbate 80 (TWEEN`" 80). A sequestering or chelating agent of metal ions
include
EDTA. Pharmaceutical carriers also include ethyl alcohol, polyethylene glycol
and
propylene glycol for water miscible vehicles; and sodium hydroxide,
hydrochloric acid,
citric acid or lactic acid for pH adjustment.
20 The concentration of the pharmaceutically active compound is adjusted so
that
an injection provides an effective amount to produce the desired
pharmacological
effect. The exact dose depends on the age, weight and condition of the patient
or
animal as is known in the art.
The unit-dose parenteral preparations are packaged in an ampoule, a vial or a
25 syringe with a needle. All preparations for par= r f , be sterile,
as is known and practiced in the art.
Illustratively, intravenous or intraarterial infusion of a sterile aqueous
solution
containing an active compound is an effective mode of administration. Another
embodiment is a sterile aqueous or oily solution or suspension containing an
active
material injected as necessary to produce the ~ c > '~ l gicai effect.
Injectabies are designed for local and sy 'emic ads,: ,F~tr '. n. in one
embodiment, a therapeutically effective dcs-~ e s formulated to ,: ntair a
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
56
concentration of at least about 0.1 % w/w up to about 90% w/w or more, in
certain
embodiments more than 1 % w/w of the active compound to the treated tissue(s).
The compound may be suspended in micronized or other suitable form or may
be derivatized to produce a more soluble active product or to produce a
prodrug. The
form of the resulting mixture depends upon a number of factors, including the
intended
mode of administration and the solubility of the compound in the selected
carrier or
vehicle. The effective concentration is sufficient for ameliorating the
symptoms of the
condition and may be empirically determined.
3. Lyophilized powders
Of interest herein are also lyophilized powders, which can be reconstituted
for
administration as solutions, emulsions and other mixtures. They may also be
reconstituted and formulated as solids or gels.
The sterile, lyophilized powder is prepared by dissolving a compound provided
herein, or a pharmaceutically acceptable derivative thereof, in a suitable
solvent. The
solvent may contain an excipient which improves the stability or other
pharmacological
component of the powder or reconstituted solution, prepared from the powder.
Excipients that may be used include, but are not limited to, dextrose,
sorbital, fructose,
corn syrup, xylitol, glycerin, glucose, sucrose or other suitable agent. The
solvent may
also contain a buffer, such as citrate, sodium or potassium phosphate or other
such
buffer known to those of skill in the art at, in one embodiment, about neutral
pH.
Subsequent sterile filtration of the solution followed by lyophilization under
standard
conditions known to those of skill in the art provides the desired
formulation. In one
embodiment, the resulting solution will be apportioned into vials for
lyophilization.
Each vial will contain a single dosage or multiple dosages of the compound.
The
iyophilized powder can be stored under appropriate conditions, such as at
about 4 "C
to room temperature.
Reconstitution of this lyophilized powder with water for injection provides a
formulation for use in parenteral administration. For reconstitution, the
lyophilized
powder is addedq to sterile water or other suitable carrier.
y ~The precise amount
:T _ _ p^ `mod c.7 a pon tb Ã,_, -) .,. f..j L,:: ..a ,,.y. :.Sir. x V-i-r+ 6t
an be
Topical administration
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
57
Topical mixtures are prepared as described for the local and systemic
administration. The resulting mixture may be a solution, suspension, emulsions
or
the like and are formulated as creams, gels, ointments, emulsions, solutions,
elixirs,
lotions, suspensions, tinctures, pastes, foams, aerosols, irrigations, sprays,
suppositories, bandages, dermal patches or any other formulations suitable for
topical
administration.
The compounds or pharmaceutically acceptable derivatives thereof may be
formulated as aerosols for topical application, such as by inhalation. These
formulations for administration to the respiratory tract can be in the form of
an aerosol
or solution for a nebulizer, or as a microfine powder for insuff lation, alone
or in
combination with an inert carrier such as lactose. In such a case, the
particles of the
formulation will, in one embodiment, have diameters of less than 50 microns,
in one
embodiment less than 10 microns.
The compounds may be formulated for local or topical application, such as for
topical application to the skin and mucous membranes, such as in the eye, in
the form
of gels, creams, and lotions and for application to the eye or for
intracisternal or
intraspinal application. Topical administration is contemplated for
transdermal delivery
and also for administration to the eyes or mucosa, or for inhalation
therapies. Nasal
solutions of the active compound alone or in combination with other
pharmaceutically
acceptable excipients can also be administered.
These solutions, particularly those intended for ophthalmic use, may be
formulated as 0.01 % - 10% isotonic solutions, pH about 5-7, with appropriate
salts.
5. Compositions for other routes of administration
Other routes of administration, such as transdermal patches, including
iontophoretic and electrophoretic devices, and rectal administration, are also
contemplated herein.
Transdermal patches, including iotophoretic and electrophoretic devices, are
well known to those of skill in the art.
For example, pharmaceutical dosage forms for rectal administration are rectal
suppositories, cad. _. a rtd
herein mean solid bodies for ins ini.o the rer en at body
temperature releasing one or m
l-IF
macobQ , o y active
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
58
ingredients. Pharmaceutically acceptable substances utilized in rectal
suppositories
are bases or vehicles and agents to raise the melting point. Examples of bases
include cocoa butter (theobroma oil), glycerin-gelatin, carbowax
(polyoxyethylene
glycol) and appropriate mixtures of mono-, di- and triglycerides of fatty
acids.
Combinations of the various bases may be used. Agents to raise the melting
point of
suppositories include spermaceti and wax. Rectal suppositories may be prepared
either by the compressed method or by molding. The weight of a rectal
suppository, in
one embodiment, is about 2 to 3 gm.
Tablets and capsules for rectal administration are manufactured using the
same pharmaceutically acceptable substance and by the same methods as for
formulations for oral administration.
6. Targeted Formulations
The compounds provided herein, or pharmaceutically acceptable derivatives
thereof, may also be formulated to be targeted to a particular tissue,
receptor, or other
area of the body of the subject to be treated. Many such targeting methods are
well
known to those of skill in the art. All such targeting methods are
contemplated herein
for use in the instant compositions.
In one embodiment, liposomal suspensions, including tissue-targeted
liposomes, such as tumor-targeted liposomes, may also be suitable as
pharmaceutically acceptable carriers. These may be prepared according to
methods
known to those skilled in the art. Briefly, liposomes such as multilamellar
vesicles
(MLV's) may be formed by drying down egg phosphatidyl choline and brain
phosphatidyl serine (7:3 molar ratio) on the inside of a flask. A solution of
a
compound provided herein in phosphate buffered saline lacking divalent cations
(PBS)
is added and the flask shaken until the lipid film is dispersed. The res
asides
are washed to remove unencapsulated compound, pelleted by centrifugation, and
then resuspended in PBS.
7. Articles of manufacture
The compounds or pharmaceutically acceptable derivatives may be packaged
30,
c r r aging material, a compound oe
Pharr _.ceptubie de,uca:~).. le'f provided herein, fy;, active for
mods il y CE K-2 or CHK-1 , or for treatment, prever W ; ;; F.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
59
amelioration of one or more symptoms of CDK-2 or CHK-1 mediated diseases or
disorders, or diseases or disorders in which CDK-2 or CHK-1 activity, is
implicated,
within the packaging material, and a label that indicates that the compound or
composition, or pharmaceutically acceptable derivative thereof, is used for
modulating
the activity of CDK-2 or CHK-1, or for treatment, prevention or amelioration
of one or
more symptoms of CDK-2 or CHK-1 mediated diseases or disorders, or diseases or
disorders in which CDK-2 or CHK-1 is implicated.
The articles of manufacture provided herein contain packaging materials.
Packaging materials for use in packaging pharmaceutical products are well
known to
those of skill in the art. Examples of pharmaceutical packaging materials
include, but
are not limited to, blister packs, bottles, tubes, inhalers, pumps, bags,
vials,
containers, syringes, bottles, and any packaging material suitable for a
selected
formulation and intended mode of administration and treatment. A wide array of
formulations of the compounds and compositions provided herein are
contemplated
as are a variety of treatments for any disease or disorder in which CDK-2 or
CHK-1 is
implicated as a mediator or contributor to the symptoms or cause.
EXAMPLES
Normal phase silica gel chromatography on a Biotage instrument was
accomplished using a Quad UV System (PIN 07052) utilizing KP-SIL 32-63 um
columns, 60A with flash cartridges 12+M or 25+M.
Commonly used abbreviations
AcOH Acetic acid
(Boc)20 1-tent-butyl-dicarbonate
DMAP 4-Dimethylaminopyridine
Et20 Diethyl ether
EtOAc Ethyl acetate
EtOH Ethanol
ÃCPBA meta-Chlorope ro:rf z,:ic acid
MeOH Methanol
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
NBS N-bromosuccinimide
NMP 1 -methyl-2-pyrrolid i none
Pd(OAc)2 Palladium acetate
POCK Phosphorus oxychloride
5 RT Room temperature
SEMCL 2-(Trimethylsilyl)ethoxymethyl chloride
Si02 Silica gel
10 PREPARATIVE EXAMPLE 1
BocN OH BocN O~
0 0 0
SOCl2 (18.5 ml-) was added slowly under N2 to a stirred mixture of the acid
15 (50.0 g, 218 mmol) and pyridine (44.0 ml-) in anhydrous CH2CI2 (300 mL).
The
mixture was stirred at 25 C for 20 min, then Meldrum's acid (35.0 g, 243 mmol)
and
DMAP (66.6 g, 546 mmol) were added and the mixture was stirred under N2 for 1
hr.
Then Et20 (2 L) was added, the mixture was washed with 1 M HCI (3x500 mL),
brine
(500 mL), and the organic layer was dried over Na2SO4, flitered, and the
solvent was
20 evaporated. The residue was dissolved in MeOH (580 mL), and the mixture was
refluxed for 4 hr. The solvent was evaporated and the residue was purified by
column
chromatography on silica gel with 10:1 CH2CI2/EtOAc as eluent. Pale yellow oil
(26.5
g, 43 %) was obtained.
25 PREPARATIVE EXAMPLE 2
H2N BocNN
BocN O -
N-NN`N
6
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
61
A mixture of the beta-ketoester from Preparative Example 1 (1.1 eq.) and 2-
fluoro-5-aminopyrazole (J. Heterocyclic Chem. 1978, 15, 1447. and Tetrahedron
Letters 1979, 34, 3179.) (1.0 eq.) in anhydrous toluene is stirred and
refluxed under
N2. The solvent is evaporated and the residue is purified by column
chromatography
on silica gel with CH2CI2/MeOH as eluent.
PREPARATIVE EXAMPLE 3
i
BocN N ~~ - BocN _N
"Y N- N N- N
OH Cl
A mixture of the product from Preparative Example 2 , N,N-dimethylaniline ,
and POCI3 is stirred at 25 C. Excess of POCI3 is evaporated and the residue is
poured into saturated aqueous NaHCO3. The mixture is extracted with CH2CI2
(3x200
mL), the combined extracts are dried over Na2SO4, filtered, and the solvent is
evaporated. The residue is purified by column chromatography on silica gel
with
CH2CI2/EtOAc as eluent.
PREPARATIVE EXAMPLE 4
BocN,,,-',Y N BocN N
N-N
N-N
N H2
A mixture of the product from Preparative Example 3, 2.0 M NH3 in 2-propanol,
and conc. aqueous NH4OH is stirred in a closed pressure vessel at 70 C. The
solvents are evaporated and the residue is purified by column chromatography
on
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
62
PREPARATIVE EXAMPLE 5
BOON N ocN __N
NH2 SEM"N,SEM
A mixture of the product from Preparative Example 4 (1.0 eq.), SEMCI (3.5
eq.), and diisopropylethylamine (7.0 eq.) in dry 1,2-dichloroethane is stirred
and
refluxed under N2. The mixture is then poured into saturated aqueous NaHCO3
solution, extracted with CH2CI2, dried over Na2SO4, and filtered. The solvents
are
evaporated and the residue is purified by column chromatography on silica gel
with
CH2CI2/EtOAc as eluent.
PREPARATIVE EXAMPLE 6
Br
BocN rN BocN N,' N F - F
N N
SEM"N'SEM SEM"N'SEM
A solution of NBS (0.9 eq.) in anhydrous CH3CN is added under N2 to a stirred
solution of the product from Preparative Example 5 (1.0 eq.) in anhydrous
CH3CN .
Th 4 x _ the .. >. _ dv ~r a,.
column chromatography on silica gel with hexane/EtOAc as eluent.
PREPARATIVE EXAMPLE 7
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
63
N N
BocNCI_ _N BC BocN N ,
N; tjt / F -
`~ -N
N
SEM"N,SEM SEM"'SEM
A mixture of the product from Preparative Example 6 (1.0 eq.), 1-methyl-4-
(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)-1 H-pyrazole (1.4 eq.),
Pd[PPh3]4 (0.10
eq.), and Na2CO3 (3.0 eq.) in 1,2-dirnethoxyethane and H2O is stirred and
refluxed
under N2. The solvents are evaporated and the residue is purified by column
chromatography on silica gel with hexane/EtOAc as eluent.
PREPARATIVE EXAMPLE 8
V, N
N-- N.
BOCK N HN N
N-N F N-N
SEWSEM NH2
A mixture of the product from Preparative Example 7 and 3N aqueous HCI
plus EtOH is stirred at 60 C. The solvents are evaporated, Na2CO3 and 6:1
mixture of
CH2Cl2/MeOH are added to the residue and the mixture is stirred under N2 for
15 min.
Then it is loaded onto a column and it is purified by column chromatography on
silica
gel with CH2C12/7N NH3 in MeOH as eluerlt.
PREPARATIVE EXAMPLE 9
KN,N~ N
NH2 NH2
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
64
A solution of Br2 (1.0 eq.) in dry CH2CI2 is added dropwise to a stirred
solution
of the amine from Preparative Example 8 (1.0 eq.) in tert-BuNH2 and CH2CI2.
The
mixture is stirred at 25 C, the solvents are evaporated and the residue is
purified by
column chromatography on silica gel with CH2CI2/MeOH as eluent.
PREPARATIVE EXAMPLE 10
Y YDu` H2N HO TN
HN- N F r.
O O
OBI
A mixture of diethyl malonate (2.0 eq.) and 2-fluoro-5-aminopyrazole (J.
Heterocyclic Chem. 1978, 15, 1447. and Tetrahedron Letters 1979, 34, 3179.)
(1.0
eq.) is stirred and refluxed under N2. MeONa in MeOH is then added to the
residue
and the mixture is refluxed under N2. The mixture is then acidified, the
precipitate is
filtered, washed with H2O, then with CH2CI2, and dried in a vacuum.
PREPARATIVE EXAMPLE 11
HOB N - C rN
N-N
OH CI
A mixture from Preparative Example 10,
N,N
and POC13 is stirred at 100 C. Excess of POCK is evaporated and the residue is
poured into saturated aqueous NaHCO3. The mixture is extracted with CH2CI2
(3x200
mL), the combined extracts are dried over Na2SO4. filtered, and the solvent is
evaporated. The residue is purified by cc;,, -.i chromatography on i~ . gel
with
CH
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
PREPARATIVE EXAMPLE 12
CI Qr N -C ~N
F F
CI HN
\.-N
5 A mixture of the product from Preparative Example 11, 3-aminomethylpyridine,
and triethylamine in dioxane is stirred at 100 C. The residue is poured into
saturated
aqueous NaHCO3 and the mixture is extracted with CH2CI2 (3x200 mL). The
combined extracts are dried over Na2SO4, filtered, and the solvent is
evaporated. The
residue is purified by column chromatography on silica gel with CH2CI2/EtOAc
as
10 eluent.
PREPARATIVE EXAMPLE 13
CI N~-- CI N
N F \,F
HN SEW N
N N
A mixture of the product from Preparative Example 12 (1.0 eq.), SEMCI (3.5
(7 in Jr is stirred and
,;7 1
reflu>.ed under N2.le mixture is then poured into saturated aqueous NaHCO3
solution, extracted with CH2Cl2, dried over Na2SO4, and filtered, The solvents
are
evaporated and the residue is purified by column chromatography on silica gel
with
CH2CI2/EtOAc as eluent.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
66
PREPARATIVE EXAMPLE 14
OH
"Nnl -F F
_N N-N
,N
SEM SEWN
i i
N N
A mixture of the product from Preparative Example 13, 2-
hydroxyethylpiperidine, and triethylamine in dioxane is stirred at 100 C. The
residue
is poured into saturated aqueous NaHCO3 and the mixture is extracted with
CH2CI2
(3x240 mL). The combined extracts are dried over Na2SO4, filtered, and the
solvent is
evaporated. The residue is purified by column chromatography on silica gel
with
CH2CI2/MeOH as eluent.
PREPARATIVE EXAMPLE 15
OH OH
Br
=NN CN N
Fj F
N N
Sf`M`N SEM`N
~ 1 Ff
l
A solution of NBS (0.9 eq.) in anhydrous CH3CN is added under N2 to a stirred
solution of the product from Preparative Example 14 (1.0 eq.) in anhydrous
CH3CN.
The mixture is stirred, the solvents are evaporated, and the residue is
purified by
column chromatography on silica gel with CH2C121EtOAc as eluent.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
67
PREPARATIVE EXAMPLE 16
OH OH A
NN N N N --~ ~ N N
SEW N SEM`N
N
A mixture of the product from Preparative Example 15 (1.0 eq.),
tributylethynyltin (1.4 eq.) and Pd[PPh3 4 (0.10 eq.) in dioxane is stirred
and refluxed
under N2. The solvents are evaporated and the residue is purified by column
chromatography on silica gel with CH2CI2/EtOAc as eluent.
PREPARATIVE EXAMPLE 17
Uri A
N N N
CNT
N N EN'N
SEW N SEM"N
N
5
A mixture of the product from Preparative Example 16 and Pd/C in EtOAc is
stirred under H2. The solvents are evaporated and the residue is purified by
column
chromatography on silica gel with CH2CI2/EtOAc as eluent.
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
68
PREPARATIVE EXAMPLE 18
OH OH
N N N N
s i
N-N N-
SEMN SEWN
O
N N5
A mixture of the product from Preparative Example 17 and MCPBA in CH2CI2 is
stirred under N2. The mixture is poured into saturated aqueous NaHCO3 and
extracted with CH2CI2 (3x200 mL). The combined extracts are dried over Na2SO4,
filtered, and the solvent is evaporated. The residue is purified by column
chromatography on silica gel with CH2CI2/MeOH as eluent.
PREPARATIVE EXAMPLE 19
OH OH
~N'r N N N
N1N NN
SEM"N~ HNC
N,O N O
A mixture of the product from Preparative Example 18 and 3N aqueous HCI
plus EtOH is stirred at 60 C. The solvents are evaporated, Na2CO3 and 6:1
mixture of
CH2CI2/MeOH are added to the residue and the mixture is stirred under N2 for
15 min.
Then is loaded onto a column and it is purified by column chromatography on
silica
ge v H as eiuent,
CA 02706946 2010-05-27
WO 2009/070567 PCT/US2008/084643
69
All references cited herein, whether in print, electronic, computer readable
storage media or other form, are expressly incorporated by reference in their
entirety,
including but not limited to, abstracts, articles, journals, publications,
texts, treatises,
internet web sites, databases, software packages, patents, and patent
publications. A
number of embodiments of the invention have been described. Nevertheless, it
will be
understood that various modifications may be made without departing from the
spirit
and scope of the invention. These modifications specifically include but are
not limited
to the addition of substituents to carbon or nitrogen atoms, or as otherwise
appropriate, as envisioned by and described in the specification; the
resulting
molecules are within the spirit and scope of the invention. Accordingly, other
embodiments are within the scope of the following claims and the Summary
(above).