Note: Descriptions are shown in the official language in which they were submitted.
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
4-IMIDAZOLIDINONES AS KV1.5 POTASSIUM CHANNEL INHIBITORS
FIELD OF THE INVENTION
The present invention relates to compounds that are effective as Kv1.5
potassium
channel inhibitors. The present invention also relates to compositions
comprising
certain Kv1.5 potassium channel inhibitors, and to methods for treating
cardiac
arrhythmia.
BACKGROUND OF THE INVENTION
Atrial fibrillation (AF) is a frequently encountered cardiac arrhythmia in the
clinical
setting. It affects nearly 3 million people in the United States and its
prevalence
increases with the aging of the population. AF is most often treated with
class III
antiarrhythmic agents, acting at both the atrial and ventricular levels.
Commonly used
or prescribed antiarrhythmic drugs inhibit various potassium channels, and
prolong
ventricular repolarization. Prolongation of ventricular repolarization can in
turn
precipitate the occurrence of life-threatening-ventricular arrhythmias, mainly
Torsades de Pointes (TdP).
Certain atrial-selective antiarrhythmic agents offer one possibility of
increased
therapeutic efficacy and safety by minimizing cardiac proarrhythmia inherent
in
conventional antiarrhythmic therapies.
There is an unmetneed to provide certain new compounds that function as
effective
atrial-selective antiarrhythmic agents and which do not affect ventricular
rhythm. In
addition, there is an unmet need to provide certain new compounds that
function as
effective atrial-selective antiarrhythmic agents and that are compatible with
other
cardiac devices, cardiac protocols, therapies, and medications. References
related to
Kv1.5 potassium channel include: Brendel, J., et al., Curr. Med. Chem. 2003,
1, 273-
287; Firth, A. L., et al., 2008, 33, 31-47; Vidaillet, H., et al., Am. J. Med.
2002, 113,
365-370; Tsang, T. S. M., et al., Am. J. Med. 2002, 113, 432-435; Yang, Q., et
al.,
- 1 -
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Expert Opin. Ther. Patents 2007, 17, 1443-1456; Regan, C. P., et al., J.
Cardiovasc.
Pharmacol. 2007, 49, 236-245; Nattel, S. Physiol. Rev. 2007, 87, 425-456;
Tombola,
F., et al., Annu. Rev. Cell Dev. Biol. 2006, 22, 23-52; and Wirth, K. J., et
al., J.
Cardiovasc. Pharmacol. 2007, 49, 197-206.
SUMMARY OF THE INVENTION
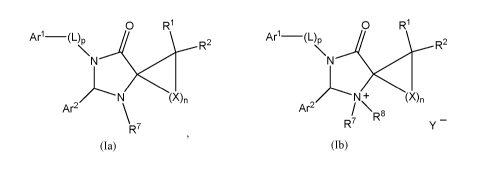
The present invention provides compounds of Formula (la) or (lb):
O R1 O R1
Art-(L), R2 Ar1-(L)\ R2
N N
~N (X)n ~N+ (X)n
Ar2 \ Ar2 \R$
R7 R7 Y
(ta) (1b)
or pharmaceutically acceptable salts thereof,
wherein:
Art is a C6-C16 aryl ring or a 5-14 membered heteroaryl ring, each of which is
optionally substituted with 1, 2, 3 or 4 substituents independently selected
from C1-6
alkyl, F, Cl, Br, I, C3-6 cycloalkyl C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl),
N(C1.6 alkyl)2,
NO2, C1.3 haloalkyl, SH, SC1.6 alkyl and CN;
Arz is a C6-C16 aryl ring or a 5-14 membered heteroaryl ring, each of which is
optionally substituted with 1, 2, 3 or 4 substituents independently selected
from C1-6
-2-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
alkyl, F, Cl, Br, I, C3-6 cycloalkyl, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl),
N(C1.6 alkyl)2,
NO2, C1.3 haloalkyl, SH, -SC1.6 alkyl and CN;
each X is independently -CR3R4-;
each L is independently -CR5R6-;
R1, R2, R3, R4, R5 and R6 are each independently selected from H, halogen and
C1-6
alkyl optionally substituted with 1, 2, 3 or 4 substituents independently
selected from
C1-6 alkyl, F,CI, Br, I, C1-6 alkoxy, OH, NH2, NO2, C1.3 haloalkyl, SH, S-C1.6
alkyl and
CN;
R7 and R8 are each independently selected from H and C1-6 alkyl optionally
substituted with 1, 2, 3 or 4 substituents independently selected from F, Cl,
Br, I, C1-6
alkoxy, OH, NH2, NO2, C1.3 haloalkyl, SH, -SC1.6 alkyl, CN, C3.10 cycloalkyl,
a 3-10
membered heterocyclyl ring, C6-, C16-aryl ring, and a 5-10 membered heteroaryl
ring;
Y is a counterion;
n is 1, 2, 3, 4 or 5; and
p is 0, 1, 2, 3 or 4.
The present invention also provides compositions comprising an effective
amount of
one or more compounds of Formula (la) or (lb) and one or more excipients.
The present invention also provides a method for treating or preventing
cardiac
arrhythmias, for example atrial arrhythmia, including but not limited to,
atrial
fibrillation and atrial flutter, said method comprising administering to a
subject an
effective amount of a compound of Formula (la) or (lb) according to the
present
invention.
-3-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
The present invention also provides a method for treating or preventing
cardiac
arrhythmias, for example atrial arrhythmia, including but not limited to,
atrial
fibrillation and atrial flutter, wherein said method comprises administering
to a subject
a composition comprising an effective amount of one or more compounds of
Formula
(Ia) or (lb) according to the present invention and one or more excipients.
The present invention also provides methods for treating or preventing
diseases or
conditions associated with cardiac arrhythmias, including but not limited to,
thromboembolism, stroke, and heart failure. In some embodiments, the methods
comprise administering to a subject an effective amount of a compound of
Formula
(la) or (lb) according to the present invention.
The present invention further provides methods for treating or preventing
diseases or
conditions associated with cardiac arrhythmias, including but not limited to,
thromboembolism, stroke, and heart failure, wherein said method comprises
administering to a subject a composition comprising an effective amount of one
or
more compounds of Formula (la) or (lb) according to the present invention and
one
or more excipients.
The present invention also provides a method for inducing cardioversion, said
method comprising administering to a subject an effective amount of a compound
Formula (la) or (lb) according to the present invention.
The present invention also provides a method for inhibiting Kv1.5 potassium
channel
in a subject comprising administering a therapeutically effective amount of a
compound of Formula (Ia) or (lb) to the subject.
The present invention also provides a method for treating or preventing a
disorder
associated with inhibition of Kv1.5 potassium channel in a subject comprising
administering a therapeutically effective amount of a compound of Formula (Ia)
or
(lb) to the subject. As an example, these compounds are useful in treating
atrial
arrhythmia, thromboembolism, stroke or cardiac failure.
-4-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
These and other objects, features, and advantages will become apparent to
those
skilled in the art from a reading of the following detailed description and
the
appended claims.
DETAILED DESCRIPTION OF THE INVENTION
Kv1.5 potassium channel inhibitors of the present invention are capable of
treating
and preventing arrhythmia in the atrial portion of the human heart or in the
heart of
certain animals. It has been discovered that functional Kv1.5 potassium
channels
are found in human atrial tissue but not in human ventricular myocytes.
Without
wishing to be limited by theory, it is believed the inhibition of the Kv1.5
voltage-gated
Shaker-like potassium (K') ion channel can ameliorate, abate, or otherwise
cause to
be controlled, atrial fibrillation and flutter without prolonging ventricular
repolarization.
DEFINITIONS
As used herein, all percentages, ratios and proportions herein are by weight,
unless
otherwise specified. All temperatures are in degrees Celsius ( C), unless
otherwise
specified. All documents cited are in relevant part; the citation of any
document is
not to be construed as an admission that it is prior art with respect to the
present
invention.
Throughout the description, where compositions are described as having,
including,
or comprising specific components, or where processes are described as having,
including, or comprising specific process steps, it is contemplated that
compositions
of the present invention also consist essentially of, or consist of, the
recited
components, and that the processes of the present teachings also consist
essentially
of, or consist of, the recited processing steps.
In this specification, where an element or component is said to be included in
and/or
selected from a list of recited elements or components, it should be
understood that
the element or component can be any one of the recited elements or components
-5-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
and can be selected from a group consisting of two or more of the recited
elements
or components.
The use of the singular herein includes the plural (and vice versa) unless
specifically
stated otherwise. In addition, where the use of the term "about" is before a
quantitative value, the present invention also includes the specific
quantitative value
itself, unless specifically stated otherwise.
It should be understood that the order of steps or order for performing
certain actions
is immaterial so long as the present teachings remain operable. Moreover, two
or
more steps or actions can be conducted simultaneously.
As used herein, unless otherwise noted, "alkyl" whether used alone or as part
of a
substituent group refers to a saturated straight and branched carbon chain
having 1
to 20 carbon atoms or any number within this range, for example, 1 to 6 carbon
atoms or 1 to 4 carbon atoms. Designated numbers of carbon atoms (e.g. C1.6)
shall
refer independently to the number of carbon atoms in an alkyl moiety or to the
alkyl
portion of a larger alkyl-containing substituent. Non-limiting examples of
alkyl groups
include methyl, ethyl, n-propyl, iso-propyl, n-butyl, sec-butyl, iso-butyl,
tent-butyl, and
the like. Where so indicated, alkyl groups can be optionally substituted. In
substituent groups with multiple alkyl groups such as N(C1.6alkyl)2, the alkyl
groups
may be the same or different.
As used herein, unless otherwise noted, "alkoxy" refers to groups of formula -
Oalkyl.
Designated numbers of carbon atoms (e.g. -OC1.6) shall refer independently to
the
number of carbon atoms in the alkoxy group. Non-limiting examples of alkyl
groups
include methoxy, ethoxy, n-propoxy, iso-propoxy, n-butoxy, sec-butoxy, iso-
butoxy,
tert-butoxy, and the like. Where so indicated, alkoxy groups can be optionally
substituted.
As used herein, the terms "alkenyl" and "alkynyl" groups, whether used alone
or as
part of a substituent group, refer to straight and branched carbon chains
having 2 or
-6-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
more carbon atoms, preferably 2 to 20, having at least one carbon-carbon
double
bond ("alkenyl") or at least one carbon-carbon triple bond ("alkynyl"). Where
so
indicated, alkenyl and alkynyl groups can be optionally substituted.
Nonlimiting
examples of alkenyl groups include ethenyl, 3-propenyl, 1-propenyl (also 2-
methylethenyl), isopropenyl (also 2-methylethen-2-yl), buten-4-yl, and the
like.
Nonlimiting examples of alkynyl groups include ethynyl, prop-2-ynyl (also
propargyl),
propyn-1-yl, and 2-methyl-hex-4-yn-1-yl.
As used herein, "cycloalkyl," whether used alone or as part of another group,
refers
to a non-aromatic hydrocarbon ring including cyclized alkyl, alkenyl, or
alkynyl
groups, e.g., having from 3 to 14 ring carbon atoms, for example, from 3 to 7
or 3 to 6
ring carbon atoms, and optionally containing one or more (e.g., 1, 2, or 3)
double or
triple bonds. Cycloalkyl groups can be monocyclic (e.g., cyclohexyl) or
polycyclic
(e.g., containing fused, bridged, and/or Spiro ring systems), wherein the
carbon
atoms are located inside or outside of the ring system. Any suitable ring
position of
the cycloalkyl group can be covalently linked to the defined chemical
structure.
Where so indicated, cycloalkyl rings can be optionally substituted.
Nonlimiting
examples of cycloalkyl groups include: cyclopropyl, cyclopropenyl, cyclobutyl,
cyclobutenyl, cyclopentyl, cyclopentenyl, cyclopentadienyl, cyclohexyl,
cyclohexenyl,
cycloheptyl, cyclooctanyl, decalinyl, octahydropentalenyl, octahydro-1H-
indenyl,
3a,4,5,6,7,7a-hexahydro-3H-inden-4-yl, decahydroazulenyl;
bicyclo[6.2.0]decanyl,
decahydronaphthalenyl, and dodecahydro-lH-fluorenyl. The term "cycloalkyl"
also
includes carbocyclic rings which are bicyclic hydrocarbon rings, non-limiting
examples of which include, bicyclo-[2.1.1]hexanyl, bicyclo[2.2.1]heptanyl,
bicyclo[3.1.1]heptanyl, 1,3-dimethyl[2.2.1]heptan-2-yl, bicyclo[2.2.2]octanyl,
and
bicyclo[3.3.3]undecanyl.
"Haloalkyl" is intended to include both branched and straight-chain saturated
aliphatic hydrocarbon groups having the specified number of carbon atoms,
substituted with 1 or more halogen atoms. As used herein, halogen refers to F,
Cl,
Br and I. Haloalkyl groups include perhaloalkyl groups, wherein all hydrogens
of an
alkyl group have been replaced with halogens (e.g., -CF3, -CF2CF3). The
halogens
can be the same (e.g., CHF2, -CF3) or different (e.g., CF2CI). Where so
indicated,
-7-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
haloalkyl groups can optionally be substituted with one or more substituents
in
addition to halogen. Examples of haloalkyl groups include, but are not limited
to,
fluoromethyl, dichloroethyl, trifluoromethyl, trichloromethyl,
pentafluoroethyl, and
pentachloroethyl groups.
The term "aryl," wherein used alone or as part of another group, is defined
herein as
an aromatic monocyclic ring of 6 carbons or an aromatic polycyclic ring of
from 10 to
14 carbons. Aryl groups include but are not limited to, for example, phenyl or
naphthyl (e.g., naphthylen-1-yl or naphthylen-2-yl). Where so indicated, aryl
groups
may be optionally substituted with one or more substituents. Aryl groups also
include, but are not limited to for example, phenyl or naphthyl rings fused
with one or
more saturated or partially saturated carbon rings (e.g., bicyclo[4.2.0]octa-
1,3,5-
trienyl, indanyl), which can be substituted at one or more carbon atoms of the
aromatic and/or saturated or partially saturated rings.
The terms "heterocyclic," "heterocycle," and "heterocyclyl," whether used
alone or as
part of another group, are defined herein as groups having one or more rings
(e.g., 1,
2 or 3 rings) and having from 3 to 20 atoms (e.g., 3 to 10 atoms, 3 to 6
atoms)
wherein at least one atom in at least one ring is a heteroatom selected from
nitrogen
(N), oxygen (0), and sulfur (S), and wherein the ring that includes the
heteroatom is
non-aromatic. In heterocyclyl groups that include 2 or more fused rings, the
non-
heteroatom bearing ring may be aryl (e.g., indolinyl, tetrahydroquinolinyl,
chromanyl).
Exemplary heterocyclyl groups have from 3 to 14 ring atoms of which from 1 to
5 are
heteroatoms independently selected from nitrogen (N), oxygen (0), or sulfur
(S).
One or more N or S atoms in a heterocyclyl group can be oxidized (e.g., N-O-,
S(O),
SO2). Where so indicated, heterocyclyl groups can be optionally substituted.
Non-limiting examples of monocyclic heterocyclyl groups include, for example:
diazirinyl, aziridinyl, urazolyl, azetidinyl, pyrazolidinyl, imidazolidinyl,
oxazolidinyl,
isoxazolinyl, isoxazolyl, thiazolidinyl, isothiazolyl, isothiazolinyl
oxathiazolidinonyl,
oxazolidinonyl, hydantoinyl, tetrahydrofuranyl, pyrrolidinyl, morpholinyl,
piperazinyl,
piperidinyl, dihydropyranyl, tetrahydropyranyl, piperidin-2-onyl
(valerolactam),
2,3,4,5-tetrahydro-1H-azepinyl, 2,3-dihydro-1H-indole, and 1,2,3,4-tetrahydro-
-8-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
quinoline. Non-limiting examples of heterocyclic groups having 2 or more rings
include, for example: hexahydro-lH-pyrrolizinyl, 3a,4,5,6,7,7a-hexahydro-1H-
benzo[d]imidazolyl, 3a,4,5,6,7,7a-hexahydro-1H-indolyl, 1,2,3,4-
tetrahydroquinolinyl,
chromanyl, isochromanyl, indolinyl, isoindolinyl, and decahydro-1H-
cycloocta[b]pyrrolyl.
The term "heteroaryl," whether used alone or as part of another group, is
defined
herein as a single or fused ring system having from 5 to 20 atoms (e.g., 5 to
10
atoms, 5 to 6 atoms) wherein at least one atom in at least one ring is a
heteroatom
selected from nitrogen (N), oxygen (0), and sulfur (S), and wherein further at
least
one of the rings that includes a heteroatom is aromatic. In heteroaryl groups
that
include 2 or more fused rings, the non-heteroatom bearing ring may be a
carbocycle
(e.g., 6,7-Dihydro-5H-cyclopentapyrimidine) or aryl (e.g., benzofuranyl,
benzothiophenyl, indolyl). Exemplary heteroaryl groups have from 5 to 14 ring
atoms
and contain from 1 to 5 ring heteroatoms independently selected from nitrogen
(N),
oxygen (0), and sulfur (S). One or more N or S atoms in a heteroaryl group can
be
oxidized (e.g., N-O-, S(O), SO2). Where so indicated, heteroaryl groups can be
substituted. Non-limiting examples of monocyclic heteroaryl rings include, for
example: 1,2,3,4-tetrazolyl, [1,2,3]triazolyl, [1,2,4]triazolyl, triazinyl,
thiazolyl, 1H-
imidazolyl, oxazolyl, furanyl, thiopheneyl, pyrimidinyl, and pyridinyl. Non-
limiting
examples of heteroaryl rings containing 2 or more fused rings include:
benzofuranyl,
benzothiophenyl, benzoxazolyl, benzthiazolyl, benztriazolyl, cinnolinyl,
naphthyridinyl,
phenanthridinyl, 7H-purinyl, 9H-purinyl, 5H-pyrrolo[3,2-d]pyrimidinyl, 7H-
pyrrolo[2,3-
d]pyrimidinyl, pyrido[2,3-dlpyrimidinyl, 2-phenylbenzo[d]thiazolyl, 1H-
indolyl, 4,5,6,7-
tetrahydro-1-H-indolyl, quinoxalinyl, 5-methylquinoxalinyl, quinazolinyl,
quinolinyl, and
isoquinolinyl.
One non-limiting example of a heteroaryl group as described above is Cl-C5
heteroaryl, which is a monocyclic aromatic ring having 1 to 5 carbon ring
atoms and
at least one additional ring atom that is a heteroatom (preferably 1 to 4
additional ring
atoms that are heteroatoms) independently selected from nitrogen (N), oxygen
(0),
and sulfur (S). Examples of C1-C5 heteroaryl include, but are not limited to
for
example, triazinyl, thiazol-2-yl, thiazol-4-yl, imidazol-l-yl, 1H-imidazol-2-
yl, 1H-
-9-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
imidazol-4-yl, isoxazolin-5-yl, furan-2-yl, furan-3-yl, thiophen-2-yl,
thiophen-4-yl,
pyrimidin-2-yl, pyrimidin-4-yl, pyrimidin-5-yl, pyridin-2-yl, pyridin-3-yl,
and pyridin-4-yl.
For the purposes of the present invention, fused ring groups, spirocyclic
rings,
bicyclic rings and the like, which comprise a single heteroatom will be
considered to
belong to the cyclic family corresponding to the heteroatom containing ring.
For
example, 1,2,3,4-tetrahydroquinoline having the formula:
CO
H
is, for the purposes of the present invention, considered a heterocyclyl
group. 6,7-
Dihydro-5H-cyclopentapyrimidine having the formula:
k' N
is, for the purposes of the present invention, considered a heteroaryl group.
When a
fused ring unit contains heteroatoms in both a saturated and an aryl ring, the
aryl ring
will predominate and determine the type of category to which the ring is
assigned.
For example, 1,2,3,4-tetrahydro-[1,8]naphthyridine having the formula:
H
N~
is, for the purposes of the present invention, considered a heteroaryl group.
-10-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
The terms "treat" and "treating," as used herein, refer to partially or
completely
alleviating, inhibiting, ameliorating and/or relieving a condition from which
a patient is
suspected to suffer.
As used herein, "therapeutically effective" refers to a substance or an amount
that
elicits a desirable biological activity or effect.
Except when noted, the terms "subject" or "patient" are used interchangeably
and
refer to mammals such as human patients and non-human primates, as well as
experimental animals such as rabbits, rats, and mice, and other animals.
Accordingly, the term "subject" or "patient" as used herein means any
mammalian
patient or subject to which the compounds of the invention can be
administered. In
an exemplary embodiment of the present invention, to identify subject patients
for
treatment according to the methods of the invention, accepted screening
methods
are employed to determine risk factors associated with a targeted or suspected
disease or condition or to determine the status of an existing disease or
condition in a
subject. These screening methods include, but are not limited to for example,
conventional work-ups to determine risk factors that may be associated with
the
targeted or suspected disease or condition. These and other routine methods
allow
the clinician to select patients in need of therapy using the methods and
compounds
of the present invention.
The term "substituted" is used throughout the specification. The term
"substituted" is
defined herein as a moiety, whether acyclic or cyclic, which has one or more
(e.g. 1-
10) hydrogen atoms replaced by a substituent as defined herein below.
Substituents
include those that are capable of replacing one or two hydrogen atoms of a
single
moiety at a time, and also those that can replace two hydrogen atoms on two
adjacent carbons to form said substituent. For example, substituents that
replace
single hydrogen atoms includes, for example, halogen, hydroxyl, and the like.
A two
hydrogen atom replacement includes carbonyl, oximino, and the like.
Substituents
that replace two hydrogen atoms from adjacent carbon atoms include, for
example,
epoxy, and the like. When a moiety is described as "substituted" any number of
its
hydrogen atoms can be replaced, as described above. For example,
difluoromethyl
-11-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
is a substituted C1 alkyl; trifluoromethyl is a substituted C1 alkyl; 4-
hydroxyphenyl is a
substituted aryl ring; (N,N-dimethyl-5-amino)octanyl is a substituted C8
alkyl; 3-
guanidinopropyl is a substituted C3 alkyl; and 2-carboxypyridinyl is a
substituted
heteroaryl.
At various places in the present specification, substituents of compounds are
disclosed in groups or in ranges. It is specifically intended that the
description
include each and every individual subcombination of the members of such groups
and ranges. For example, the term "C1.6 alkyl" is specifically intended to
individually
disclose C1, C2, C3, C4, C5, C6, C1-C6, C1-C5, C1-C4, C1-C3, C1-C2, C2-C6, C2-
C5, C2-C4,
C2-C3, C3-C6, C3-C5, C3-C4, C4-C6, C4-C5, and C5-C6 alkyl.
In one aspect, the present invention provides compounds of Formula (la) or
(lb):
0 R1 0 R1
Art-(L)\ R2 Ar1-(L)p R2
N1\ N
/
/ N (X)n Xt, N+ (X)n
Ar2 Ar2 I \R$
R7 R7 Y
(Ia) (Ib)
or pharmaceutically acceptable salts thereof,
wherein:
Art is a C6-C10 aryl ring or a 5-14 membered heteroaryl ring, each of which is
optionally substituted with 1, 2, 3 or 4 substituents independently selected
from C1-6
alkyl, F, Cl, Br, I, C3-6 cycloalkyl, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl),
N(C1.6 alkyl)2,
NO2, C1.3 haloalkyl, SH, SC1.6 alkyl and CN;
-12-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Are is a C6-C1o aryl ring or a 5-14 membered heteroaryl ring, each of which is
optionally substituted with 1, 2, 3 or 4 substituents independently selected
from C1-6
alkyl, F, Cl, Br, I, C3-6 cycloalkyl, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl),
N(C1.6 alkyl)2,
NO2, C1.3 haloalkyl, SH, -SC1.6 alkyl and CN;
each X is independently -CR3R4-;
each L is independently -CR5R6-;
R1, R2, R3, R4, R5 and R6 are independently selected from H, halogen and C1-6
alkyl
optionally substituted with 1, 2, 3 or 4 substituents independently selected
from C1-6
alkyl, F, Cl, Br, I, C1-6 alkoxy, OH, NH2, NO2, C1.3 haloalkyl, SH, S-C1.6
alkyl and CN;
R7 and R8 are each independently selected from H and C1-6 alkyl optionally
substituted with 1, 2, 3 or 4 substituents independently selected from F, Cl,
Br, I, C1-6
alkoxy, OH, NH2, NO2, C1.3 haloalkyl, SH, SC1.6 alkyl, CN, C3.10 cycloalkyl, a
3-10
membered heterocyclyl ring, C6-, C1o-aryl ring, and a 5-10 membered heteroaryl
ring;
Y is a counterion;
n is 1, 2, 3, 4 or 5; and
p is 0, 1, 2, 3 or 4.
According to some embodiments, Art is a substituted C6-C1o-aryl ring or a 5-14
membered heteroaryl ring, each of which is substituted with at least one
substituent
independently selected from C1-6 alkyl, F, Cl, Br, I, C3-6 cycloalkyl, C1-6
alkoxy, OH,
NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, SC1.6 alkyl and
CN. In
some embodiments, each aryl or heteroaryl ring is further optionally
substituted with
1, 2, 3 or 4 substituents independently selected from C1-6 alkyl, F, Cl, Br,
I, C3-6
cycloalkyl, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3
haloalkyl,
SH, SC1.6 alkyl and CN. In some embodiments, Ar2 is a C6-C1o-aryl ring or a 5-
14
-13-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
membered heteroaryl ring, substituted with at least one substituent
independently
selected from C1-6 alkyl, F, Cl, Br, I, C1-6 alkoxy, OH, NH2, NH(C1_6 alkyl),
N(C1.6
alkyl)2, NO2, C1.3 haloalkyl, SH, SC1.6 alkyl and CN. In some embodiments,
each aryl
or heteroaryl ring is further optionally substituted with 1, 2, 3 or 4
substituents
independently selected from C1-6 alkyl, F, Cl, Br, I, C1-6 alkyl, OH, NH2,
NH(C1_6 alkyl),
N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, -SC1.6 alkyl and CN. In some
embodiments,
both Art and Are are independently a C6-C1o-aryl ring or a 5-14 membered
heteroaryl
ring, substituted with at least one substituent independently selected from C1-
6 alkyl,
F, Cl, Br, I, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3
haloalkyl,
SH, SC1.6 alkyl and CN. In yet further embodiments, each aryl or heteroaryl
ring is
further optionally substituted with 1, 2, 3 or 4 substituents independently
selected
from C1-6 alkyl, F, Cl, Br, I, C1-6 alkyl, OH, NI-1z, NH(C1.6 alkyl), N(C1.6
alkyl)2, NO2, C1-
3 haloalkyl, SH, -SC1.6 alkyl and CN.
In some embodiments, each R1, R2, R3 and R4, is H. In some such embodiments,
R5
and R6 are each H.
In some embodiments, L is CH2. In some further embodiments, p is 2.
In some embodiments of the compounds of Formula (Ia) or (lb), Art is phenyl
optionally substituted with 1, 2 or 3 substituents independently selected from
C1-6
alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2,
C1.3
haloalkyl, SH, SC1.6 alkyl and CN. In some embodiments, Art is phenyl
optionally
substituted with 1, 2 or 3 substituents independently selected from C1-6
alkyl,
halogen, C1-6 alkoxy, OH, and CF3. In some embodiments, Art is phenyl
optionally
substituted with 1, 2 or 3 substituents independently selected from methyl, F,
Cl and
methoxy. In some embodiments, Art is phenyl optionally substituted with 1, 2
or 3
methoxy groups. In some embodiments, Art is para-substituted phenyl. In some
embodiments, Art is 4-methoxyphenyl.
In some embodiments of the compounds of Formula (la) or (lb), Arz is phenyl
optionally substituted with 1, 2, or 3 substituents independently selected
from C1-6
alkyl, halogen, C3-6 cycloalkyl, C1-6 alkoxy, OH, NHZ, NH(C1.6 alkyl), N(C1.6
alkyl)2,
-14-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
NO2, C13 haloalkyl, SH, -S-C1.6 alkyl and CN. In some embodiments, Are is
phenyl
optionally substituted with 1, 2 or 3 substitutents independently selected
from C1-6
alkyl, halogen, N(C1.6 alkyl)2, and CF3. In some embodiments, Ar2 is para-
substituted
phenyl. In some embodiments, Are is phenyl substituted at the 4-position with
methyl,
ethyl, isopropyl, t-butyl, F, Cl, CF3, dimethylamino, diethylamino, or
diisopropylamino.
In some embodiments of the compounds of Formula (Ia) or (lb), Ar 2 is 5-14
membered heteroaryl ring optionally substituted with 1, 2, 3 or 4 substituents
independently selected from C1-6 alkyl, halogen, C3-6 cycloalkyl, C1-6 alkoxy,
OH, NH-
2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, S-C1.6 alkyl and
CN. In some
such embodiments, Ar2 is pyrimidinyl optionally substituted with 1, 2, or 3
substituents independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH,
NH2,
NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, -S-C1.6 alkyl and CN.
In some
such embodiments, Are is pyrimidinyl optionally sibstituted with 1, 2 or 3
substitutents
independently selected from NH2, NH(C1.3 alkyl), and N(C1.3 alkyl)2. In some
such
embodiments, Ar2 is pyrimidin-5-yl optionally substituted 1 substitutent
selected from
C1-6 alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2,
NO2, C1.3
haloalkyl, SH, -S-C1.6 alkyl and CN.
In some embodiments of the compounds of Formula (la) or (lb), Art is 5-14
membered heteroaryl optionally substituted with 1, 2, 3 or 4 substituents
independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1_6
alkyl),
N(C1-6 alkyl)(C1.6 alkyl), NO2, C1.3 haloalkyl, S-C1.6 alkyl and CN. In some
embodiments, Art is pyrimidinyl optionally substituted with 1, 2, or 3
substituents
independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1.6
alkyl),
N(C1.6 alkyl)(C1.6 alkyl), NO2, C1.3 haloalkyl, S-C1.6 alkyl and CN; or with
1, 2 or 3
substitutents independently selected from C1-3 alkyl, halogen, C1-3 alkoxy,
OH, NH2,
NH(C1.3 alkyl), N(C1.3 alkyl)(C1.3 alkyl), NO2, and C1.3 haloalkyl; or with 1,
2 or 3
substitutents independently selected from C1-3 alkyl, halogen, C1.2 alkoxy,
OH, NH2,
NH(C1.6 alkyl), N(C1.6 alkyl)(C1.6 alkyl) and CF3; or with 1 substitutent
selected from
C1-3 alkyl, halogen, -OC1.2 alkyl, OH, NH2, NH(C1.6 alkyl), N(C1.3 alkyl)(C1.3
alkyl) and
CF3. In some embodiments, Art is pyrimidin-5-yl optionally sibstituted with
NH2,
NH(C1.6 alkyl), or N(C1.6 alkyl)(C1.6 alkyl). In some embodiments, Art is
pyrimidin-5-yl
-15-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
optionally substituted with diethylamino. In some embodiments, Art is
pyrimidin-5-yl
optionally substituted with NH2, NH(C1.6 alkyl), or N(C1.6 alkyl)2. In some
embodiments, Art is pyrimidin-5-yl optionally substituted with diethylamino.
In some embodiments of the compounds of Formula (Ia) or (lb), R7 is H, C1-6
alkyl
optionally substituted with halogen, C3-16 cycloalkyl, a 3-10 membered
heterocyclyl,
C6-C10 aryl, or a 5-10 membered heteroaryl. In some such embodiments, R' is H,
C1-
6 alkyl or cyclopropylmethyl. In some embodiments, R7 and R8 are each
independently C1-6 alkyl. R7 and R8 are each methyl. In some such embodiments,
n
is 1, 2, 3 or 4. In some such embodiments, R3, at each occurrence, is H, and
R4, at
each occurrence, is H.
In some embodiments of the compounds of Formula (Ia) or (lb), p is 2; each L
is -
CH2-; and Art is phenyl optionally substituted with 1, 2 or 3 substituents
independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1.6
alkyl),
N(C1.6 alkyl)2, and C1.3 haloalkyl . In some such embodiments, Art is 4-
methoxyphenyl, Are is phenyl optionally substituted with 1, 2, 3 or 4
substituents
independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH, NH2, NH(C1.5
alkyl),
N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, S-Ci_6 alkyl and CN. In some such
embodiments, Ar2 is phenyl substituted at the 4-position with methyl, ethyl,
isopropyl,
t-butyl, F, Cl, CF3, dimethylamino, diethylamino, or diisopropylamino. In some
embodiments, Are is a 5-14 membered heteroaryl ring optionally substituted
with 1, 2,
3 or 4 substituents independently selected from C1-6 alkyl, halogen, C1-6
alkoxy, OH,
NH2, NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, -S-Ci_6 alkyl
and CN. In
some embodiments, Ar2 is pyrimidinyl optionally substituted with 1, 2, or 3
substituents independently selected from C1-6 alkyl, halogen, C1-6 alkoxy, OH,
NH2,
NH(C1.6 alkyl), N(C1.6 alkyl)2, NO2, C1.3 haloalkyl, SH, S-C1.6 alkyl and CN.
In some
embodiments, R' is H, C1-6 alkyl, or cyclopropylmethyl. In some embodiments,
R'
and R8 are each independently C1-6 alkyl, n is 1, 2, 3 or 4. In some
embodiments, R3,
at each occurrence, is H, and R4, at each occurrence, is H.
Compounds described herein can contain an asymmetric atom (also referred as a
chiral center), and some of the compounds can contain one or more asymmetric
-16-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
atoms or centers, which can thus give rise to optical isomers (enantiomers)
and
diastereomers. The present teachings and compounds disclosed herein include
such enantiomers and diastereomers, as well as the racemic and resolved,
enantiomerically pure R and S stereoisomers, as well as other mixtures of the
R and
S stereoisomers and pharmaceutically acceptable salts thereof. Optical isomers
can
be obtained in pure form by standard procedures known to those skilled in the
art,
which include, but are not limited to for example, chiral chromatography,
diastereomeric salt formation, kinetic resolution, and asymmetric synthesis.
The
present invention also includes cis and trans or E/Z isomers of compounds of
Formula (la) or (lb)containing alkenyl moieties (e.g., alkenes and imines). It
is also
understood that the present teachings encompass all possible regioisomers, and
mixtures thereof, which can be obtained in pure form by standard separation
procedures known to those skilled in the art, and include, but are not limited
to,
column chromatography, thin-layer chromatography, and high-performance liquid
chromatography.
Pharmaceutically acceptable salts of compounds of the present invention, which
can
have an acidic moiety, can be formed using organic and inorganic bases. Both
mono
and polyanionic salts are contemplated, depending on the number of acidic
hydrogens available for deprotonation. Suitable salts formed with bases
include
metal salts, such as alkali metal or alkaline earth metal salts, for example
sodium,
potassium, or magnesium salts; ammonia salts and organic amine salts, such as
those formed with morpholine, thiomorpholine, piperidine, pyrrolidine, a mono-
, di- or
tri-lower alkylamine (e.g., ethyl-tert-butyl-, diethyl-, diisopropyl-,
triethyl-, tributyl- or
dimethylpropylamine), or a mono-, di-, or trihydroxy lower alkylamine (e.g.,
mono-, di-
or triethanolamine). Specific non-limiting examples of inorganic bases include
NaHCO3, Na2CO3, KHCO3, K2CO3, Cs2CO3, LiOH, NaOH, KOH, NaH2PO4, Na2HPO4,
and Na3PO4. Internal salts also can be formed. Similarly, when a compound
disclosed herein contains a basic moiety, salts can be formed using organic
and
inorganic acids. For example, salts can be formed from the following acids:
acetic,
propionic, lactic, benzenesulfonic, benzoic, camphorsulfonic, citric,
tartaric, succinic,
dichloroacetic, ethenesulfonic, formic, fumaric, gluconic, glutamic, hippuric,
hydrobromic, hydrochloric, isethionic, lactic, maleic, malic, malonic,
mandelic,
-17-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
methanesulfonic, mucic, napthalenesulfonic, nitric, oxalic, pamoic,
pantothenic,
phosphoric, phthalic, propionic, succinic, sulfuric, tartaric,
toluenesulfonic,
camphorsulfonic, carbonic, as well as other known pharmaceutically acceptable
acids.
When any variable occurs more than one time in any constituent or in any
formula, its
definition in each occurrence is independent of its definition at every other
occurrence (e.g., in N(C1.6 alkyl)2 , each C1.6 alkyl may be the same or
different than
the other). Combinations of substituents and/or variables are permissible only
if such
combinations result in stable compounds.
The compounds described herein may be administered to humans and other animals
orally, parenterally, sublingually, by aerosolization or inhalation spray,
rectally,
intracisternally, intravaginally, intraperitoneally, bucally, intrathecally or
topically in
dosage unit formulations containing conventional nontoxic pharmaceutically
acceptable carriers, adjuvants, and vehicles as desired. The term parenteral
as used
herein includes subcutaneous injection, intravenous injection, intramuscular
injection,
intrasternal injection, or infusion techniques. Topical administration may
also involve
the use of transdermal administration such as transdermal patches or
ionophoresis
devices.
Methods of formulation are well known in the art and are disclosed, for
example, in
Remington: The Science and Practice of Pharmacy, Mack Publishing Company,
Easton, Pa., 21st Edition (2005), incorporated herein by reference.
Pharmaceutical compositions for use in the present invention can be in the
form of
sterile, non-pyrogenic liquid solutions or suspensions, coated capsules,
suppositories, lyophilized powders, transdermal patches or other forms known
in the
art.
Injectable preparations, for example, sterile injectable aqueous or oleaginous
suspensions may be formulated according to the known art using suitable
dispersing
-18-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
or wetting agents and suspending agents. The sterile injectable preparation
may
also be a sterile injectable solution, suspension or emulsion in a nontoxic
parenterally
acceptable diluent or solvent.
In addition, sterile, fixed oils are conventionally employed as a solvent or
suspending
medium. For this purpose any bland fixed oil may be employed including
synthetic
mono- or di-glycerides. In addition, fatty acids such as oleic acid find use
in the
preparation of injectables. The injectable formulations can be sterilized, for
example,
by filtration through a bacterial-retaining filter, or by incorporating
sterilizing agents in
the form of sterile solid compositions which can be dissolved or dispersed in
sterile
water or other sterile injectable medium prior to use.
Formulations comprising crystalline forms of the compositions described herein
for
slow absorption from subcutaneous or intramuscular injection are provided
herein.
Additionally, delayed absorption of a parenterally administered drug form may
be
accomplished by dissolving or suspending the compounds in an oil vehicle.
Injectable depot forms are made by forming microencapsule matrices of the drug
in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio
of drug to polymer and the nature of the particular polymer employed, the rate
of drug
release can be controlled. Examples of other biodegradable polymers include
poly(orthoesters) and poly(anhydrides). Depot injectable formulations may also
be
prepared by entrapping the drug in liposomes or microemulsions, which are
compatible with body tissues.
Solid dosage forms for oral administration include capsules, tablets, pills,
powders,
and granules. In such solid dosage forms, the active compound is mixed with at
least
one inert, pharmaceutically acceptable excipient or carrier such as sodium
citrate or
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose,
glucose, mannitol, and silicic acid, b) binders such as, for example,
carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose,
and
acacia, c) humectants such as glycerol, d) disintegrating agents such as agar-
agar,
calcium carbonate, potato or tapioca starch, alginic acid, certain silicates,
and sodium
carbonate, e) solution retarding agents such as paraffin, f) absorption
accelerators
-19-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
such as quaternary ammonium compounds, g) wetting agents such as, for example,
acetyl alcohol and glycerol monostearate, h) absorbents such as kaolin and
bentonite
clay, and i) lubricants such as talc, calcium stearate, magnesium stearate,
solid
polyethylene glycols, sodium lauryl sulfate, and mixtures thereof. In the case
of
capsules, tablets and pills, the dosage form may also comprise buffering
agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-
filled gelatin capsules using such excipients as lactose or milk sugar as well
as high
molecular weight polyethylene glycols and the like.
The solid dosage forms of tablets, capsules, pills, and granules can be
prepared with
coatings and shells such as enteric coatings and other coatings well known in
the
pharmaceutical formulating art. They may optionally contain opacifying agents
and
can also be of a composition that they release the active ingredient(s) only,
or
preferentially, in a certain part of the intestinal tract, optionally, in a
delayed manner.
Examples of embedding compositions that can be used include polymeric
substances and waxes.
The compounds described herein can also be in micro-encapsulated form with one
or
more excipients as noted above. The solid dosage forms of tablets, capsules,
pills,
and granules can be prepared with coatings and shells such as enteric
coatings,
release controlling coatings and other coatings well known in the
pharmaceutical
formulating art. In such solid dosage forms the active compound may be admixed
with at least one inert diluent such as sucrose, lactose or starch. Such
dosage forms
may also comprise, as is normal practice, additional substances other than
inert
diluents, e.g., tableting lubricants and other tableting aids such a magnesium
stearate and microcrystalline cellulose. In the case of capsules, tablets and
pills, the
dosage forms may also comprise buffering agents. They may optionally contain
opacifying agents and can also be of a composition that they release the
active
ingredient(s) only, or preferentially, in a certain part of the intestinal
tract, optionally,
in a delayed manner. Examples of embedding compositions that can be used
include polymeric substances and waxes.
-20-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, microemulsions, solutions, suspensions, syrups and elixirs. In
addition to
the active compounds, the liquid dosage forms may contain inert diluents
commonly
used in the art such as, for example, water or other solvents, solubilizing
agents and
emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, EtOAc,
benzyl
alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol,
dimethylformamide,
oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and
sesame oils),
glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid
esters of
sorbitan, and mixtures thereof. Besides inert diluents, the oral compositions
can also
include adjuvants such as wetting agents, emulsifying and suspending agents,
sweetening, flavoring, and perfuming agents.
Dosage forms for topical or transdermal administration of a compound of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions,
sprays, inhalants or patches. The active component is admixed under sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives
or buffers as may be required. Ophthalmic formulations, ear drops, and the
like are
also contemplated as being within the scope of this invention.
Compositions of the invention may also be formulated for delivery as a liquid
aerosol
or inhalable dry powder. Liquid aerosol formulations may be nebulized
predominantly into particle sizes that can be delivered to the terminal and
respiratory
bronchioles.
Effective amounts of the compounds of the invention generally include any
amount
sufficient to detectably modulate Kv1.5 potassium channel activity, or to
alleviate
symptoms of diseases associated with Kv1.5 potassium channel activity or
susceptible to Kv1.5 potassium channel activity modulation.
The amount of active ingredient that may be combined with the carrier
materials to
produce a single dosage form will vary depending upon the host treated and the
particular mode of administration. It will be understood, however, that the
specific
dose level for any particular subject will depend upon a variety of factors
including
-21-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
the activity of the specific compound employed, the age, body weight, general
health,
sex, diet, time of administration, route of administration, rate of excretion,
drug
combination, and the severity of the particular disease undergoing therapy.
The
therapeutically effective amount for a given situation can be readily
determined by
routine experimentation and is within the skill and judgment of the ordinary
clinician.
In another aspect of the invention, kits that include one or more compounds of
the
invention are provided. Representative kits include a compound described
herein
(e.g., a compound of Formula la or lb) and a package insert or other labeling
including directions for treating or preventing atrial arrhythmia,
thromboembolism,
stroke, or cardiac failure by administering an effective amount of a compound
of the
present invention.
In another aspect of the invention, kits that include one or more compounds of
the
invention are provided. Representative kits include a compound described
herein
(e.g., a compound of Formula la or lb) and a package insert or other labeling
including directions for inhibiting Kv1.5 potassium channel by administering
an
effective amount of a compound of the present invention.
In another aspect of the invention, kits that include one or more compounds of
the
invention are provided. Representative kits include a compound described
herein
(e.g., a compound of Formula la or lb) and a package insert or other labeling
including directions for inducing cardioversion by administering an effective
amount
of a compound of the present invention.
The Kv1.5 potassium channel inhibitors of the present invention are 5-
spirocyclic-4-
imidazolidinones, and include all enantiomeric and diasteriomeric forms and
salts of
compounds having the formula (Ia) or (lb):
-22-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
O R1 O R1
Art-(L)\ R2 Arl-(L)\ R2
N N
N (X)n N + W.
Ar2 \ Ar2 / \Ra
R7 R7 Y
(Ia) (Ib)
wherein the core scaffold is numbered in the following manner, shown for
Formula
(lb) as an example
0 R1
Art-(L)P R2
N3 a
'22 1
Ar2 ~ (X)n
R7 \R8 Y
5
For the purposes of the present invention, a compound depicted by the racemic
formula, for example:
0
will stand equally well for either of the two enantiomers of Formula:
-23-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
0
N
I ~ I ~ a
or
or mixtures thereof (or in the case where a second chiral center is present,
all
diastereomers and mixtures thereof).
Compounds of the present invention can be prepared in accordance with the
procedures outlined herein, from commercially available starting materials,
compounds known in the literature, or readily prepared intermediates, by
employing
standard synthetic methods and procedures known to those skilled in the art.
Standard synthetic methods and procedures for the preparation of organic
molecules
and functional group transformations and manipulations can be readily obtained
from
the relevant scientific literature or from standard textbooks in the field. It
will be
appreciated that where typical or preferred process conditions (i.e., reaction
temperatures, times, mole ratios of reactants, solvents, pressures, etc.) are
given,
other process conditions can also be used unless otherwise stated. Optimum
reaction conditions can vary with the particular reactants or solvent used.
Those
skilled in the art will recognize that the nature and order of the synthetic
steps
presented can be varied for the purpose of optimizing the formation of the
compounds described herein.
The processes described herein can be monitored according to any suitable
method
known in the art. For example, product formation can be monitored by
spectroscopic
means, such as nuclear magnetic resonance spectroscopy (e.g., 1H or 13C),
infrared
-24-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
spectroscopy, spectrophotometry (e.g., UV-visible), mass spectrometry, or by
chromatography such as high-performance liquid chromatograpy (HPLC), gas
chromatography (GC), gel-permeation chromatography (GPC), or thin layer
chromatography (TLC).
Preparation of the compounds can involve protection and deprotection of
various
chemical groups. The chemistry of protecting groups can be found, for example,
in
Greene at al., Protective Groups in Organic Synthesis, 4th Ed. (John Wiley &
Sons,
2007), the entire disclosure of which is incorporated by reference herein for
all
purposes.
The reactions or the processes described herein can be carried out in suitable
solvents, which can be readily selected by one skilled in the art of organic
synthesis.
Suitable solvents typically are substantially nonreactive with the reactants,
intermediates, and/or products at the temperatures at which the reactions are
carried
out, i.e., temperatures that can range from the solvent's freezing temperature
to the
solvent's boiling temperature. A given reaction can be carried out in one
solvent or a
mixture of more than one solvent. Depending on the particular reaction step,
suitable
solvents for a particular reaction step can be selected.
The compounds of these teachings can be prepared by methods known in the art.
The reagents used in the preparation of the compounds of these teachings can
be
either commercially obtained or can be prepared by standard procedures
described
in the literature. For example, compounds of the present invention can be
prepared
according to the method illustrated in Scheme 1:
-25-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
SCHEME 1
0 R1
0 R'
HO R2 Are-(Up z
1. A ion
H2N (X) n
PG
A B
Are-CHO
O R1 O RI
Arl(L)p R2 Arl-(L)p R2
R
N N
Alkylation
Arz N NX zH NX
Ar
R7
D C
In Scheme I, the amine-protected amino acid A contains an -NH-PG group.
Generally, the amine-protected amino acid may have the formula
0 R1
R2
HO
Z Wn
where -Z is any protected amino group. In the example of Scheme I, Z is -NH-
PG.
As another example, Z may be a protected amino group of the formula
-26-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
PG2\
N
I
PG,
wherein PG1 and PG2 are each a protecting group,
or of the formula
ZEN
I
G,
wherein PG, and PG2 are each a protecting group functionality, which may be,
as an
example, a carbonyl group. As an example, Z may be a phthalimido group.
Amine-protected amino acid A (wherein PG is an amine protecting group) is
treated
with amine Ar'-(L)P NH2 under amide coupling conditions. Suitable amide
coupling
conditions include the use of a coupling reagent such as a carbodiimide (e.g.,
N-(3-
dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (EDAC) or
dicyclohexylcarbodiimide (DCC)), or PyBOP. Optionally, the coupling conditions
comprise a coupling agent and a hydroxylated moiety (e.g., N-
hydroxybenzotriazole
(HOBt), (HOAt) or pentafluorophenol). Any suitable amide coupling conditions
known in the art may be employed. Removal of the protecting group PG yields
compound B. Any suitable amine protecting group (e.g., tert-butoxy carbonyl,
benzyloxy carbonyl, etc.) and corresponding deprotection conditions (e.g.,
treatment
with acid (e.g., HCI, TFA) or hydrogenation (e.g., palladium catalyzed
hydrogenation)) may be used (see, e.g., T.W. Greene, Protective Groups in
Organic
Synthesis, 4 TH Ed. (John Wiley and Sons, 2007). Compound B is condensed with
aldehyde Are-CHO in the presence of a base to produce 4-imidazolidinone C.
-27-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Alkylation of C yields 4-imidazolidinone D. Any suitable alkylation conditions
may be
employed, including, for example, treatment with an alkyl halide (e.g., alkyl
iodide,
alkyl bromide, alkyl chloride) and base (e.g., metal hydride, such as NaH) in
a
suitable solvent (e.g., THE, DMF).
Compounds of formula (I) containing a quaternized N-1 nitrogen can be
prepared, for
example, according to Scheme 2:
SCHEME 2:
0 Ri 0 Ri
Arl-(L)P R2 Arl-(L)P R2
Base
N Alkylating agent N
N (x)n /'-N (x)n
Ar2 H Ar2 8
R7 R
C E
Compound C may be treated with an appropriate base (e.g., sodium hydride) in
the
presence of at least two equivalents of an alkylating agent (e.g., alkyl
halide, alkyl
sulfonate, etc.) in a suitable solvent (e.g., DMF) to yield compound E.
Alternatively,
monoalkylated 4-imidazolidinone D may be treated with an appropriate base
(e.g.
sodium hydride) and an alkylating agent (e.g. alkyl halide, alkyl sulfonate,
etc.) in a
suitable solvent (e.g. DMF) to produce compound E.
EXAMPLES
The following non-limiting examples are presented merely to illustrate the
presentinvention. The skilled person will understand that there are numerous
equivalents and variations not exemplified but which still form part of the
present
teachings.
-28-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Example 1: Preparation of 6-(4-tert-butyl phenyl)-7-[2-(4-methoxyphenyl)ethyl]-
5-
methyl-5,7-diazaspiro[3.4]octan-8-one (Compound #9 in Table 1)
0
Io
NHz H
O I / N
/ O
NHBoc ~~NHzTFA NN
HO )PyBOP, DMF, DIPEA H 1) K2C03, McOH, Reflux
1 2) TFA 2 2) CH31, NaH, THE 3
Step 1: 860 mg (4 mmol) of Boc amino acid 1 and 4-methoxyphenethyl amine (604
mg, 4 mmol) were dissolved in 10 DMF at room temperature and 2.05 g (4 mmol)
of
PyBOP was added. The reaction pH was adjusted to 6.0 with DIPEA and the
reaction was stirred for 12 hours. The reaction was then diluted with EtOAc
and H2O.
The layers were separated, and the aqueous layer was extracted with EtOAc. The
combined organic layers were dried with Na2SO4, filtered and concentrated
under
vacuum. Chromatography with EtOAc/Hexane (0% EtOAc to 50% EtOAc/Hexane)
provided 1.40 g tert-butyl 1-(4-methoxyphenethylcarbamoyl)cyclobutylcarbamate
(95%) as white solid.
The white solid was dissolved in 50 ml CH2CI2. 13 ml trifluoroacetic acid was
added
and the reaction was stirred at room temperature for 3 hours. The solvent was
removed under vacuum to provide 1-amino-N-(4-
methoxyphenethyl)cyclobutanecarboxamide trifluoroacetate 2 (2.21 g) as a light
brown oil which was used without further purification.
Step 2: The light brown oil was dissolved in 20 ml of methanol, 676 mg of 4-t-
butylbenzaldehyde was added, followed by 1.38 g K2CO3, and the reaction was
heated to reflux. After 18 hours, the reaction was allowed to cool to room
temperature, the solution was filtered, and the resulting solution was
stripped of
solvent. The residual oil was re-dissolved in EtOAc, washed with H2O, dried
over
MgSO4, filtered and the solvent was removed under vacuum. Chromatography with
EtOAc/Hexane (0% EtOAc to 60% EtOAc/Hexane) provided 6-(4-tert-butylphenyl)-7-
(4-methoxyphenethyl)-5,7-diazaspiro[3.4]octan-8-one as a white solid.
-29-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
An amount, 392 mg (1 mmol), of the white solid was dissolved in THE (20 ml)
and 27
mg (1.2 mmol) of NaH was added, followed by 197 mg (1.4 mmol) of iodomethane.
The reaction was stirred for 48 hours, and then the solvents were removed
under
vacuum. The residual material was dissolved in EtOAc, washed with water, dried
over Na2SO4, filtered and stripped of solvents. Chromatography with
EtOAc/Hexane
(0% EtOAc to 50% EtOAc/Hexane) provided 264 mg of the desired 6-(4-tert-
butylphenyl)-7-[2-(4-methoxyphenyl)ethyl]-5-methyl-5,7-diazaspiro[3.4]octan-8-
one 3
as a clear oil.
Example 2: Preparation of 5-(4-tert-butylphenyl)-6-[2-(4-methoxyphenyl)ethyl]-
4,4-
dimethyl-7-oxo-6-aza-4-azoniaspiro[2.4]heptane (Compound #25 in Table 1)
NaH, CH31, DMF
O . ` N /v
NH N )
JTFA
4 5
A solution of 378 mg (1 mmol) of 4 (prepared according to the procedure of
Example
1), 35 mg (1.5 mmol) NaH and 284 mg (2 mmol) iodomethane in DMF was stirred at
room temperature for 24 hours. The mixture was diluted with 100 ml of EtOAC,
washed with H2O (2 x 50 ml), dried over Na2SO4, filtered and stripped to an
oil.
Reverse phase HPLC purification with CH3CN/H20 0.1% TFA provided 304 mg
(60%) of the desired 5-(4-tent-butylphenyl)-6-[2-(4-methoxyphenyl)ethyl]-4,4-
dimethyl-
7-oxo-6-aza-4-azoniaspiro[2.4]heptane.
Compounds 1-25 listed in Table I were prepared according to the procedures
described in Examples 1 and 2 above using the corresponding reagents (e.g.,
corresponding amino acid, amine, and aldehyde reagents). High-performance
liquid
chromatography (HPLC) was recorded with Column Aquasil C18 (Aquasil C18 HPLC
column, 50 mm length x 2 mm ID, 5 micron particle) using following conditions:
-30-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Mobile Phase A: 10 mM NH4OAC in 95% water / 5% CAN (Pipette 6.67 mL of 7.5 M
NH4OAC solution into 4743 mL H2O, then add 250 mL of ACN to the solution and
mixture). Mobile Phase B: 10 mM NH4OAC in 5% water / 95% CAN (Pipette 6.67 mL
of 7.5 M NH4OAC solution into 243 mL H2O. Then add 4750 mL of ACN to the
solution and mixture). Flow Rate: 0.800 mUmin, Column Temperature: 40 C,
Injection Volume: 5 mL and UV: monitor 214 nm and 254 nm.
Gradient Table:
Time(min) %B
0.0 0
2.5 100
4.0 100
4.1 0
5.5 0
Mass spectra were recorded using Agilent 1200 HPLC/time-of-flight mass
spectrometer 3x50 mm, 1.8 micron stable bond C18 column, T = 70 C, linear
gradient from 70/30 (A:B) to 5/95 (A:B) over 1.2 minutes; A) water w/ 0.1%
formic
acid, B) acetonitrile w/ 0.1% formic acid. Mass spectrometer was scanned from
m/z
100-1000.
Table I
Time
Structure Name Mass
(min)
5-(4-tert-butylphenyl)-6-[2-(4-
methoxyphenyl)ethyl]-4,6-
1 iazaspiro[2.4]heptan-7-one 379 3.
-31-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
\\ 5-(4-fluorophenyl)-6-[2-(4-
methoxyphenyl)ethyl]-4,6-
2 iazaspiro[2.4]heptan-7-one 341 2.
5-(4-chlorophenyl)-6-[2-(4-
methoxyphenyl)ethyl]-4,6-
3 - iazaspiro[2.4]heptan-7-one 357 2.
-[2-(4-methoxyphenyl)ethyl]-5-[4-
(trifluoromethyl)phenyl]-4,6-
4 iazaspiro[2.4]heptan-7-one 391 3.1
5-(4-tert-butylphenyl)-6-[2-(4-
methoxyphenyl )ethy l]-4-methyl-4, 6-
. iazaspiro[2.4]heptan-7-one 393 3.
5-(4-fluorophenyl)-6-[2-(4-
methoxyphenyl)ethyl]-4-methyl-4,6-
6 iazaspiro[2.4]heptan-7-one 355 3.1
-[2-(4-methoxyphenyl)ethyl]-4-methyl-
5-[4-(trifluo romethyl )phenyl]-4, 6-
7 iazaspiro[2.4]heptan-7-one 405 3.
-(4-tert-butylphenyl)-7-[2-(4-
methoxyphenyl)ethyl]-5, 7-
8 iazaspiro[3.4]octan-8-one 393 3.
-32-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
-(4-tert-butylphenyl)-7-[2-(4-
methoxyphenyl)ethyl]-5-methyl-5,7-
9 iazaspiro[3.4]octan-8-one 407 3.
-(4-tert-butylphenyl)-3-[2-(4-
methoxyphenyl)ethyl]-1, 3-
iazaspiro[4.4]nonan-4-one 407 3.5
-(4-tert- b uty l p h e n y l)-3- [2-(4-
methoxyphenyl)ethyl]-1-methyl-1,3-
11 iazaspiro[4.4]nonan-4-one 421 3.5
-(4-oh l o ro p h e n y l)-3-[ 2-(4-
methoxyphenyl)ethyl]-1-methyl-1, 3-
12 iazaspiro[4.4]nonan-4-one 399 3.5
3-[2-(4-methoxyphenyl)ethyl]-1-methyl-
-[4-(trifluoromethyl)phenyl]-1,3-
13 iazaspiro[4.4]nonan-4-one 433 3.3
-[4-(diethylamino)phenyl]-3-[2-(4-
methoxyphenyl)ethyl]-1-methyl-1, 3-
14 iazaspiro[4.4]nonan-4-one 436 3.4
-(4-tert-butylphenyl)-3-[2-(4-
methoxyphenyl)ethyl]-1,3-
iazaspiro[4.5]decan-4-one 421 3.6
-33-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
-(4-chlorophenyl)-3-[2-(4-
methoxyphenyl)ethyl]-1,3-
16 iazaspiro[4.5]decan-4-one 397 3.3
3-[2-(4-methoxyphenyl)ethyl]-2-[4-
(trifluoromethyl) pheny1]-1, 3-
17 iazaspiro[4.5]decan-4-one 433 3.3
o7 -(4-tert-butyl phenyl)-3-[2-(4-
methoxyphenyl)ethyl]-1-methyl-1,3-
18 iazaspiro[4.5]decan-4-one 435 3.9
-(4-c h l o ro phenyl)-3-[ 2-(4-
methoxyphenyl)ethyl]-1-methyl-1, 3-
19 iazaspiro[4.5]decan-4-one 413 3.5
3-[2-(4- m et h oxy phenyl)ethyl]-1-methyl-
-[4-(trifluoromethyl)phenyl]-1, 3-
20 iazaspiro[4.5]decan-4-one 447 3.5
-[4-(diethylamino)phenyl]-3-[2-(4-
methoxyphenyl)ethyl]-1-methyl-1, 3-
21 iazaspiro[4.5]decan-4-one 450 3.6
-[2-(diethylamino)pyrimidin-5-yl]-3-[2-
(4-methoxyphenyl )ethyl]-1-methyl-1, 3-
22 iazaspiro[4.5]decan-4-one 452 3.6
-34-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
1-(cyclopropylmethy1)-2-[4-
(diethyl amino)phenyl]-3-[2-(4-
methoxyphenyl)ethy]-1,3-
23 iazaspiro[4.5]decan-4-one 490 4
-(4-tert-butyl phenyl)-7-[2-(4-
methoxyphenyl)ethyl]-5,5-dimethyl-8-
24 oxo-7-aza-5-azoniaspiro[3.4]octane 421 3.
5-(4-tert-butyl phenyl)-6-[2-(4-
methoxyphenyl )ethyl]-4,4-d i methyl-7-
25 oxo-6-aza-4-azoniaspiro[2.4]heptane 407 3.6
KV1.5 PATCH CLAMP EP
Kv1.5 currents are recorded by the whole cell mode of patch clamp
electrophysiology. Kv1.5 is stably over expressed in HEK cells.
Microelectrodes are
pulled from borosilicate glass (TW150) and heat polished (tip resistance, 1.5
to 3
megaohms). The external solution is standard Tyrodes solution. The internal
(microelectrode) solution contained: 110 mM KCI, 5 mM K2ATP, 5 mM K4BAPTA, 1
mM MgCl2 and 10 mM HEPES, adjusted to pH 7.2 with KOH. Command potentials
are applied for I second to +60mV from a holding potential of -70 mV using
Axon
software (pClamp 8.1) and hardware (Axopatch 1D, 200B). Compounds are prepared
as 10-20mM DMSO stocks and diluted to appropriate test concentrations. After
stable currents are achieved, compounds are perfused onto the cells and the
cells
are pulsed every 5 seconds until no further changes in current are evident at
a given
compound concentration. Inhibition is measured at the end of the 1 second
pulses
and expressed relative to controls. Kv1.5 inhibition is estimated by single
point
determinations done at 1 M.
-35-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
Generally following this procedure, results for representative compounds
according
to the present invention are listed in Table II below.
Table II
Kõ1.5
Name Ainhib
ImM.
1. 5-(4-tent-butylphenyl)-6-[2-(4-methoxyphenyl)ethyl]-4,6- 4
iazaspi ro[2.4]hepta n-7-one
2. 5-(4-fluorophenyl)-6-[2-(4-methoxyphenyl)ethyl]-4,6-diazaspiro[2.4]heptan-7
9
one
3. 5-(4-chlorophenyl)-6-[2-(4- m et h oxy p h e n y l) et h y l]-4 , 6-d i az
as p i ro [ 2.4 ] h e pt a n-
7-one
4. -[2-(4-methoxyphenyl)ethyl]-5-[4-(trifluoromethyl)phenyl]-4,6-
iazaspi ro[2.4]hepta n-7-one
5. 5-(4-tent-butylphenyl)-6-[2-(4-methoxyphenyl)ethyl]-4-methyl-4,6- 82
iazaspi ro[2.4]hepta n-7-one
6. 5-(4-fluorophenyl)-6-[2-(4-methoxyphenyl)ethyl]-4-methyl-4,6- 9
iazaspi ro[2.4]hepta n-7-one
7. -[2-(4-methoxyphenyl)ethyl]-4-methyl-5-[4-(trifluoromethyl)phenyl]-4,6-
iazaspiro[2.4]heptan-7-one 1
8. -(4-tent-butylphenyl)-7-[2-(4-methoxyphenyl)ethyl]-5,7-diazaspiro[3.4]octan-
0
8-one
9. -(4-tert-butylphenyl)-7-[2-(4-methoxyphenyl)ethyl]-5-methyl-5,7-
iazaspiro[3.4]octan-8-one 85
10. -(4-tert-butylphenyl)-3-[2-(4-methoxyphenyl)ethyl]-1,3- 18
iazaspi ro[4.4]nona n-4-one
11 -(4-tert-butylphenyl)-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3-
iazaspiro[4.4]nonan-4-one 90
12. -(4-chlorophenyl)-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3- 6
iazaspi ro[4.4]nona n-4-one
13. 3-[2-(4-methoxyphenyl)ethyl]-1 -methyl-2-[4-(trifluoromethyl)phenyl]-1,3-
81
iazaspi ro[4.4]nona n-4-one
14 -[4-(diethylamino)phenyl]-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3-
iazaspiro[4.4]nonan-4-one 93
15. -(4-tert-butylphenyl)-3-[2-(4-methoxyphenyl)ethyl]-1,3- 0
iazaspiro[4.5]decan-4-one
16 -(4-chlorophenyl)-3-[2-(4-methoxyphenyl)ethyl]-1,3-diazaspiro[4.5]decan-4-
one
17. 3-[2-(4-methoxyphenyl)ethyl]-2-[4-(trifluoromethyl)phenyl]-1,3- 15
iazaspiro[4.5]decan-4-one
18 -(4-tert-butylphenyl)-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3- 74
iazaspiro[4.5]decan-4-one
19. -(4-chlorophenyl)-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3-
iazaspiro[4.5]decan-4-one 87
-36-
CA 02709187 2010-06-11
WO 2009/079630 PCT/US2008/087459
20 3-[2-(4-methoxyphenyl)ethyl]-1-methyl-2-[4-(trifluoromethyl)phenyl]-1,3- 91
iazaspiro[4.5]decan-4-one
21. -[4-(diethyl amino)phenyl]-3-[2-(4-methoxyphenyl)ethyl]-1-methyl-1,3- 86
iazaspi ro[4.5]decan-4-one
22.
-[2-(diethyl amino)pyrimidin-5-yl]-3-[2-(4-methoxyphenyl)ethyl]-1-methyl- 12
1, 3-d iazaspi ro[4.5]deca n-4-one
23. 1 -(cyclopropylmethyl)-2-[4-(d iethyla m ino) phenyl]-3-[2-(4- 12
methoxyphenyl)ethyl]-1, 3-diazas piro[4.5]decan-4-one
24 -(4-tert-butyl phenyl)-7-[2-(4-methoxyphenyl)ethyl]-5,5-dimethyl-8-oxo-7- 5
aza-5-azo n i as pi ro [ 3.4]octa n e
25. 5-(4-tert-butyl phenyl)-6-[2-(4-methoxyphenyl)ethyl]-4,4-dimethyl-7-oxo-6-
10
aza-4-azo n ias piro[2.4]heptane
Variations, modifications, and other implementations of what is described
herein will
occur to those skilled in the art without departing from the spirit and the
essential
characteristics of the present teachings. Accordingly, the scope of the
present
teachings is to be defined not by the preceding illustrative description but
instead by
the following claims, and all changes that come within the meaning and range
of
equivalency of the claims are intended to be embraced therein.
Each of the printed publications, including but not limited to patents, patent
applications, books, technical papers, trade publications and journal articles
described or referenced in this specification are incorporated by reference
herein,
included in their entire contents.
-37-