Note: Descriptions are shown in the official language in which they were submitted.
CA 02709670 2010-08-12
FIELD OF THE INVENTION. The invention broadly relates to the field of
medicine, and
more particularly relates to the field of the evaluation of sensory
perception.
CA 02709670 2010-08-12
FIELD-BACKGROUND. Complaints of altered sensation are common in our society.
Sensory alterations occur in many medical conditions and injuries. These
altered
sensations can be grouped into two categories: negative and positive
phenomena.
Negative phenomena include anesthesia, analgesia, proprioceptive loss, and
others.
Positive phenomena include hyperpathia, dysesthesias and others. Currently,
the
evaluation of these phenomena relies primarily on the clinical examination.
For
instance, a subject may complain of numbness and a healthcare professional
will
"examine" the patient at the bedside. The professional will stimulate the body
region
encompassed within the complaint. Dependent upon the stimulation intensity,
the
patient will verbally affirm or deny feeling the provoking stimulus. The
intensity of the
sensory aberration and its root substrates are then inferred by the
professional. This
non-specific process is extremely dependent on the accuracy of the claimant
and the
judgment of the observer.
From a scientific perspective, aberrant sensation is very complex. It has
multiple
substrates including (a] the triggering condition or injury itself; usually a
nerve or nerve
root condition; [b] central nervous system adaptive mechanisms that influence
sensory
encoding; [c] perceptual elements; [d] emotional determinates; and [e]
depictive
constituents. These interactive phenomena render the bedside clinical
evaluation
inaccurate. This ambiguity has many confounding implications in circumstances
where
precision is important such as research (pharmaceutical and otherwise] and
medicolegal situations. In these situations especially, it would be extremely
desirable to
be separate the pure neurological factors from the other confounding elements.
A common example of this conundrum is diabetic neuropathy. The patient may
simply
complain of discomfort and numbness [e.g., "I feel that I am always walking in
sand"].
The examining professional may find a mixture of sensory loss, proprioceptive
loss, and
hyperpathic sensations involving the distal extremities. Variably the
patient's
verbalized complaints often do not correlate well with the clinical findings.
The
explanation for this discordance lies in the complexity of the neurology
itself plus the
other determinants described above.
Currently, the above clinical evaluation is often supplemented by
neurodiagnostic and
imaging tests. MRI evaluations may look for abnormal nerve signals or edema
(such as
in carpal tunnel syndrome --another common entity]. Nerve conduction studies
and
somatosensory evoked potentials are used to assess electrophysiological
correlates of
the injuries. Neither of these strategies has good concordance with the
severity or types
of sensory experience. Again, the reasons for this discrepancy are complicated
but have
to do with the limitations of the current technology.
There is accumulating basic science information that many of these abnormal
sensations are subserved biologically by differential adaptations of the
peripheral nerves
and their central sensory connections. Many stimulation modes of stimulation
are used
clinically to study and evaluate these diverse aberrant experiences, including
dynamic
mechanical, pressure, vibration, joint position, electric current, and thermal
stimulus.
Most of these modal inputs can be quantitatively measured and are readily
available.
The problem with the current assessment is how to unequivocally grade the
patient's
physiologic response. Current schemes rely purely on a verbal response. These
usually
involve either some assessment of perception thresholds or some patient
grading
system [such as an analog scale]. Two limitations of these methods are that
the results
fluctuate caused by: [a] patient-to-patient based on usual inter-subject
variation, and
[b] session-to-session deviations based on changes in mood, diurnal status,
etc.
Furthermore, these methods attempt to focus on the neurological components and
do
nothing to assess the emotional, perceptual, and depictive constituent of the
articulated
experience,
CA 02709670 2010-08-12
To overcome these deficiencies, the present invention describes a methodology
that
incorporates the available stimulation modes with non-verbal human somatic and
autonomic reactions. It also describes a new concept in judging human
responses.
As introduced above, asking subjects to grade a constant grade of sensory
input is
fraught with subject inconsistency. One person's grade of mild may be another
person's moderate or severe. Thus, in sensory loss situations, one stoic
individual may
describe his carpal tunnel numbness as minimal while another histrionic person
may
describe the same loss as severe, The same variability occurs with the use of
perception thresholds. The concept of perception threshold follows: a sensory
mode's
intensity is gradually increased [from zero upwards] until the person first
perceives it.
The perception threshold has two limitations. It is quite variable from
individual to
individual due to biological diversity. Secondly, it cannot directly evaluate
positive
phenomena such as paresthesias, dysesthesias, etc.
SUMMARY OF THE INVENTION. Briefly, according to an embodiment of the present
invention, a method for evaluating verbalized human abnormal sensory
experiences is
expressed. The invention uses commercially available stimulation devices [as
described
above] and commercially available autonomic and muscular recording equipment.
As
summarized above, the methodology combines these two systems in novel ways to
provide a new, comprehensive assessment of the abnormal sensory experience.
Conceptually, the method uses a new verbal stimulation response goal called
the
Aganaktic Recognition Levels for ARL]. ARL is very distinct from perception
threshold.
The subject is asked to identify the intensity of stimulus mode that first
becomes
irritating; this stimulus is applied for a brief time frame. Based on the
principles
introduced above, the ARL is increased in situations were negative phenomena
are
tested whereas it is reduced in situations where positive phenomena are
studied. ARL
therefore provides more information the perception threshold that cannot
directly
assess positive abnormal sensory phenomena such as paresthesias.
The second conceptual innovation involving the use of ARL stimulation is that
it evokes
non-verbal neurological reactions, specifically autonomic and somatic outputs,
The
autonomic outflows will include heart rate, skin conductive responses, blood
volume
pulse, and others. The somatic output will include surface EMG activity.2
Based on the
ubiquitous biological facts, a threatening physical disturbance will cause a
change in
somatic and autonomic homeostasis. On the other hand, since ARL is an
incipient
irritative input, it will induce a temporally-linked, transient perturbation
in autonomic
levels. On the other hand, it is insufficient to cause anticipatory or
prolonged responses
that would be usually associated with potentially destructive or damaging
inputs [with
higher stimulus intensities]. Thus, the ARL corresponds to a well-defined
intermediate
sensory input that should helps distinguish the various elements of a
subject's
abnormal sensory experience.
An example should suffice to illustrate the principles. Consider a musician
who
develops unilateral carpal tunnel syndrome with biological components of
paresthesias
and numbness. Because the condition menaces professional goals, the musician's
natural perception of the problem could be that it is severe although its
biological
elements are mild; therefore he grades both the paresthesias and numbness as
8/ 10 on
The term "aganaktic" derives from the Greek for physical irritation.
2Clearly other autonomic phenomena could be considered in future variations on
the invention. The listed
variables are sufficient for most neuropathic conditions-
CA 02709670 2010-08-12
an analog scale. When his ARL stimulus is applied, his autonomic-somatic
responses
will transiently and reactively change and recover coincident with stimulation
onset and
duration. This temporal concordance demonstrates a purely physical-sensory
type of
subject profile. The ARL stimulus for numbness will be tactile pressure; its
value will
be mildly increased consistent with mild tactile anesthesia. The ARL stimulus
for
paresthesias will be electrical stimulation [of a known type]; its value will
be reduced
compared to a control value consistent with mild hyperesthesia. The
discrepancy
between the ARL test values and the patient's verbalized severe sensory
experience is
explained by perceptual or descriptive factors of a psychosocial type. These
insights
are not readily available by current clinical and research tools.
Additional information is built into the invention's other features. The
combination of a
definitively measured input with quantifiable autonomic-somatic reactions
provides
additional information. Many medical conditions are tainted by comorbid
emotional
factors such as anxiety and depression that often go under-recognized by
healthcare
professionals. This is certainly true in neurological syndromes. The test
protocol is
designed to monitor and quantify subject's autonomic and somatic reactions
before,
during, and after the ictal provocation. If the subject's autonomic parameters
modulate
before or after the ictal event, that indicates an emotive substrate to the
individual's
clinical presentation. This information cannot be derived from clinical and
research
tools presently in use.
The final component of the invention protocol is the consistent use of control
and test
sites in pairs. It is well known that multiple biological factors influence
perception
thresholds including age and gender. There are other potentially confounding
variables
such as race, occupation, comorbid medical conditions, medications, and
culture. To
carefully account for this diversity, it is customary to have large normative
populations
and then attempt to compare the proband subject to the corresponding control
group.
A more direct approach is to use a patient as his or her own control. This
technique
mitigates the biological diversity normally caused by the listed variables.
In summary, the current methodology describes multiple new features never
before
incorporated into the evaluation of the human abnormal sensations. These
features
include {a] the development of the Aganaktxc Recognition Level [ARL]; [b] the
unique
combination of different measurable sensory stimulation modes with defined
quantifiable autonomic and somatic reactions; [c] the monitoring of autonomic-
somatic
reactions before and after the stimulation ictus; and [d] the use of pair
control-test site
results. These combined features provide insights into the multiple facets
comprising a
patient's experience of abnormal sensation. As illustrated above, the proper
use of
multiple stimulus modes at ARL intensity allows assessment of positive and
negative
sensory phenomena; the concomitant use of a verbalized analog scale permits
appraisal
of the sociodynamic-descriptive axis; the monitoring of autonomic-somatic
reactions
before and after the stimulation ictus evaluates the emotional components; and
the
combination of paired control-test sites mitigates confounding subject
variables such as
age, gender, comorbid medical conditions and medications, etc.
BRIEF DESCRIPTION OF THE FIGURES.
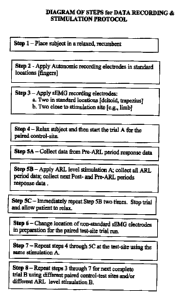
A. Figure One - Block Diagram of the Trial Methodology. This diagram details
the
steps followed during the current embodiment of this methodology. These steps
are
summarized briefly here and are described in detail during the next section.
Step 1
occurs after a directed history and physical exam by the technician [a
physical therapist
or similarly trained individual]. The patient is placed in a relaxed recumbent
position
with relevant body areas exposed for the trial. During this activity, data
will be
CA 02709670 2010-08-12
gathered concerning the present intensity of the subject's abnormal sensory
experience.
Step 2 is the placement and calibration of the Autonomic recording electrodes
to
monitor changes in skin conductance, heart rate, blood volume pulse,
temperature and
other autonomic variables]. Step 3 is the placement and calibration of several
surface
electromyography electrodes; these electrodes will collect data before,
during, and after
the ARL administration period. During Step 4, the test trial is explained to
the subject
who is placed in a relaxed state. During Step 5, the actual control-site trial
is run,
Step 5 A is the first ARL administration period; as mentioned reaction data is
recorded
before, during, and after the ARL administration which can be variable in time
length.
Step 5B is a rest period wherein the subject is allowed to return to a resting
state. Step
5C repeats Steps SA and 5B to make sure that the ARL administration data is
reproducible and reliable. Step 6 allows the subject to relax; electrodes can
be
repositioned as warranted. Step 7 repeats Step 4 through SC specifically at
the paired
test site [using the same stimulation mode and comparable electrode
placements]. Step
8 repeats Steps 3 through 7 at a different pair of control-test site trials
and/or different
stimulation modes as clinically warranted by the differential diagnosis.
B. Figure Two. Block Diagram representing the physical set-up of one
embodiment of
this invention. The NeuroEvaluation recording system presently monitors skin
conductance, heart rate, and blood volume pulse although other autonomic
parameters
are possible. The NeuroEvaluation recording system also currently uses surface
EMG
recordings although needle electrodes may be used for certain applications.
The
stimulation modalities include: palpatory pressure; various electrical current
applications; joint proprioceptive by motion [e.g., goniometry or
inclinornetry]; and
others. As mentioned these are often combined in a single subject's session to
specifically evaluate the different aspects of the subject's abnormal sensory
experience[s]. The computer interface requires proprietary written soft-ware
to
specifically maximize data capture and analysis. Standard computer outputs are
applicable.
DETAILED DESCRIPTION.
According to the current embodiment of the present invention, a method for
evaluating
the human subject's abnormal sensory experience is disclosed. Unlike other e.-
dsting
methods, the current invention is designed to comprehensively assess
biological,
perceptual, and emotional aspects of that experience. This is accomplished by
combining several elements: [a] the invention of a new stimulation technique
termed
ART,; this allows the evaluation of both positive and negative abnormal
sensory
phenomena, while simultaneously avoiding the emotional and psychological
sequelae of
stronger stimulation intensities; [b] the innovative combination of verbal,
graded verbal,
and non-verbal data; the later non-verbal data includes somatic and automatic
reactions to ARL application; this allows the distinction between purely
physical-
sensory and perceptual-descriptive components of someone's complaint; [d] the
gathering of the autonomic-electromuscular data before, during and after the
ARL
application; this allows the differentiation between physical-sensory and
emotional
aspects of the patient's presentation; [e] the use of paired control-test
sites in a
scientific paradigm to mitigate inter-subject variability issues; and [f] the
use of
precisely defined time periods for accurate data collection and analysis
[described more
fully below].
This section will describe the actual steps of the methodology,
Step 1. Based on the referral information, a directed history and physical
exam, the
appropriate total protocol is selected. For illustrative purposes, assume the
referred
CA 02709670 2010-08-12
subject is sent with diagnosis of right upper extremity paresthetic numbness
after a
multiple sclerosis exacerbation; the patient is a musician by trade. The
intake sensory
information reveals that she is anxious, depressed, and grades her changes
[both
paresthesias and numbness] as severe. The intake physical examination finds
mildly
decreased light touch and mild hyperesthesia to pinwheel stimulation; joint
position
sense is severely affected even at the wrist. The subject's left arm is
asymptomatic and
normal by examination; it will serve for the control site. The stimulation
modes will
include electrical current stimulation to evaluate the paresthesias; pressure
to evaluate
the light touch loss; and range of motion to evaluate the proprioceptive
complaints-
Step 2. Autonomic recording electrodes are placed on the digits of the left
hand to
record general autonomic parameters including skin conduction response, heart
rate,
and blood volume pulse. Connections and recording stability are confirmed,
Step 3. Non-specific surface EMG electrodes are placed over the left deltoid
and
trapezius to monitor general muscle tensions. Because the first trial will
begin with the
left leg, site-specific sEMG electrodes are placed over appropriate forearm
muscles to
assess local muscle guarding and movement.
Step 4. The examination is explained to the subject. The first trial will
involve the
control left leg to reduce examination-related anxiety. Pressure is the first
selected
stimulation mode. The concept of ARL is carefully explained and demonstrated
to
minimize stimulation-related anxiety. Specifically, the subject is instructed
to press a
button and verbally state now when she first feels pressure-evoked physical
irritation.
As mentioned previously, using ARL application mitigates stimulation-induced
psychobiological reactions.3 It also eliminates the ambiguities associated
with higher
stimulation stimuli required to acquire verbal responses for moderate or
intolerable
experiences.4
Step 5. Step 5 constitutes the ARL application period. In the illustrative
subject [the
female with multiple sclerosis); the first control site will be the left
anterior forearm
using a pressure mode stimulus [to evaluate light touch examination findings).
Step
five can be subdivided into three parts.
Step 5A[i]. The Immediate Pre- and Post-ARL periods. The Immediate Pre- and
Post-
ARL data collection constitute an innovation not seen previously in prior
stimulation
techniques for the study of human experience/ It is critical for the proper
identification
of significant psychobiological factors [such as generalized or event-related
amdety].5
3 Many people are naturally fearful of intense physical experiences and have
anticipatory and reactive
anxiety responses. This fear increases with rising stimulus intensity. Thus,
high stimulating intensities can
cause natural psychobiological responses that confound the separation between
domains. In relaxed
healthy individuals, ARL application does not evoke such rest period
perturbations.
Subjects vary greatly in the perceptions of what they personally deem as a
"moderate" or "unacceptably
severe" intensity, Take for example, most people will agree that an
temperature of approximately 82
degrees is warm, but there will be a great deal of variation as to what
ambient temperature is deemed hot or
intolerable. These perceptions are affected by multiple non-physical-sensory
factors such as personality
style, gender, cultural background, and other issues. There are also
influenced by descriptive style such as
stoic, histrionic, or emotive. Thus, the higher the stimulation intensity
becomes, individual subjective
factors become more and more influential.
Prior techniques do not access this data because they have usually relied on
purely verbal responses to
stimulation. The process described here requires the acquisition of non-verbal
reactions by a reliable and
reproducible data collection system.
CA 02709670 2010-08-12
Such patients will commonly show autonomic perturbations during the Pre- and
Post-
ARL periods not seen in healthy [e.g., non-anxious] volunteers; these
autonomic
perturbations may be accompanied by sEMG changes reflecting heightened muscle
tension. Similar findings may occur in subjects with strong sociodynamic
influences.6
Step SA(ii). The ARL test period. The ARL period is subdivided into five
discrete
subdivisions. These include: [1] a short pre-stimulus period lasting five
seconds to
collect baseline autonomic data for mathematical analysis; [2] an ARL ramping
phase
where the stimulation begins at zero intensity and gradually increases. This
will be of
variable length dependent on stimulation mode but lasts seconds; [3] an ARL
maintenance phase that begins when the subject presses a hand-held device that
signals that ARL intensity is reached; this maintenance period is brief
lasting 4
seconds; [4] an Initial recovery period lasting 10 seconds; and [5] a Final
recovery period
lasting approximately 15-20 seconds. These recovery periods allow the
equipment to
record the ARL-related autonomic and somatic changes and their return to
baseline
homeostasis. There is no parallel to this design in any prior assessment
technique,
This uniqueness is attributable to the combination of the verbal and non-
verbal
information gathering process which significant surpasses the analysis
gathered by
utilizing an isolated verbal response.?
Step 5B is an mtra-run rest period that allows the subject to completely
return to
baseline. During the rest period, autonomic and somatic data will be captured
as well.
This rest period data is extremely helpful in capturing biomarker information
concerning post-stimulation psychobiological and sociodynamic non-verbal
reactions.
It also guarantees ample time to allow the patient to return to homeostatic
autonomic
and muscular stability prior to the second ARL run.
Step SC repeats steps 5A[i], 5A[ii[, and 5B to ensure reproducibility of the
process, By
utilizing these repetitions, the data can be group using standard statistical
methods
such as means and standard deviations for more accurate mathematical analysis
later.
The successful completion of the repetition ends the analysis of the first
control site.
Step 6 is constitutes a break between sites. The patient is allowed to move,
reposition,
and talk. Electrodes are reset or moved as needed clinically.
Step 7 continues the first trial with the acquisition of ARL data at the first
test site. In
our illustration, this would be at the right anterior forearm [in a region
congruent with
the immediately previous control site]. The stimulus mode would again be
pressure.
Steps 5A through 5C are repeated exactly as described above. When this has
been
finished, the first pair control-test trial is done- In the final data
analysis, this specific
pairing will allow accurate control-to-test site comparisons yielding a
thorough
6 These inferences arise deductively- When a subject is resting, the autonomic
and electromuscular activity
should remain constant. Any perturbations cannot be due to external forces
since these are not present. By
cause-and-effect deduction, any such perturbations must arise from the
subject's internal matters
[psychobiological or sociodynamic in origin].
7 Without such monitoring, there is no system to record peri-stimulus response
data because no such data
exists. There are no independent methods to verify or refute that a physico-
sensory response has resulted
from the OIT stimulation.
CA 02709670 2010-08-12
assessment of the abnormal sensory experiences Steps 5 through 7 can therefore
be
viewed as a continuum to complete Trial One of the designed clinical study
Step 8 repeats steps 5 through 7 using different body sites and stimulation
modes as
warranted for a thorough clinical analysis of the patient's symptoms. In our
illustrative
case, there would be a second control-test trial [number two] using the medial
forearms
[left-control; right-test] but switching the stimulation mode to electrical
stimulation for
an analysis of the hyperesthesia to pin prick]. The third and last trial
repeats steps 5
through 7 using range of motion [indinometryj at the wrists. At the end of
trial three,
the entire study would be finished. The raw data will be graphically recorded
and
summarized; the information will be presented in a tabular format. The
interpreting
physician will therefore have access to the original raw data from the
recorded reaction
information and stimulation values; the data will be tabulated and a clinical
report
generated.
Table One depicts the tabular data from our hypothetical MS patient.
As previously mentioned, the power of this comparison lies in the fact that
each trial serves as its own
internal control. Thus, the results are not as affected by subject-to-subject
variables such as gender, age,
personality, cultural background, etc.
CA 02709670 2010-08-12
TABLE ONE TABULATED STUDY RESULTS
Trw Stimulus Stimulus VAS Mean ARL value ARL Rest
mode site score responses Responses
1 Pressure Con- L arm 4 kg/cm2 ++ +++
Test - R arm 8110 5 kg/cm2 ++ ++
2 Electrical Con - L am 3 mamps ++ ++
current Test - R arm 8/10 2 mumps ++ ++
3 Inelino- Con - L arm 70 deg Eat + +++
Metry Test- R arm 8/10 80 deg Lit + +++
These results are consistent with mild analgesia to light touch, moderate
hypersensitivity
[dysesthesias] to current, and mild-to-moderate proprioceptive loss [at the
wrist]. There is
a substantial amount of superimposed anxiety. These outcomes clarify the
complex
interactions between the patient's complaints of severe deficits. The actual
findings are
that the sensory loss and proprioceptive loss are mild; the discomfort is
moderate, and a
majority of the experience is due to a reactive anxiety/depression. Counseling
for the
anxiety and treatment of the dysesthesias with neuropathic medications will
offer the
patient a substantial improvement in her symptoms with her best outcome
[rather than
intensive rehabilitation].
This type of information would not be plausible with older test methods [such
as the
King technique]. They do not capture the PRC information nor do they
assiduously
compare control-test site responses in the above systematic fashion. As
explained
previously, these former tests were designed other purposes. They do not make
the
distinctions betweens the four domains of human pain experience; they do not
attempt
to evaluate different tissue odynic generators-
In summary, the current embodiment of the invention contains multiple novel
developments not previously seen. As this new technology is developed, the
precise
timing and structure of the actual trial protocols will modify but the
underlying
principles will remain the same; specifically, ARL level stimulation will be
used for most
applications; autonomic and electromuscular NeuroEvaluation tracings and data
will be
obtained; control-test condition comparisons will continue. On the other hand,
new
stimulation modes will be developed to address presently non-accessible issues
such as
gastrointestinal, urologic, and other interior body pain syndromes. As
dictated by
physiological reaction times and pragmatic considerations, modifications of
the actual
trial epochs are anticipated.