Note: Descriptions are shown in the official language in which they were submitted.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
1
NOVEL LUPANE DERIVATIVES
This application is related to application Serial No. 61/018,759, filed
January 3, 2008, and to application Serial No. 61 /039,660, filed March 26,
2008,
the entire disclosure of which is incorporated by reference.
Infection by the Human immunodeficiency virus (HIV) can lead to the
Acquired ImmunoDeficiency Syndrome (AIDS), an incurable and life threatening
condition which requires life-long treatment. It is estimated that the
HIV/AIDS
pandemic has resulted in the deaths of more than 25 million people since it
was
first recognized in 1981 and according to a UNAIDS report, an estimated 40
million
people worldwide are infected with HIV and about 2.5 million lost their. lives
to
AIDS in 2005. There is presently no effective vaccine for HIV. HIV primarily
infects
T cells, macrophages and other important components of the immune system
resulting in the gradual loss of cell-mediated immunity and as result, HIV
patients
become increasingly more susceptible to numerous opportunistic infections and
tumors and if left untreated, death usually results within 10 years following
infection.
The viral life cycle initiates with attachment of HIV gp120 surface protein to
the CD4 receptors present of the T-cells. This event triggers a conformational
change which exposes an additional binding site on gp120 and results with an
interaction with the chemokine co-receptors (CCR5 and CXCR4). Another
conformational change arising from co-receptor binding results in fusion of
the
cellular and viral membranes and release of the virion into the cell. After
uncoating and release of the viral genome in the cytoplasm, viral reverse
transcriptase (RT) then converts RNA into double stranded DNA which is then
integrated into the host genome by the action of HIV integrase. The proviral
DNA is
then transcribed and translated by host cellular system to express HIV RNA and
HIV
proteins which are then directed to the cell membrane where they assemble and
bud as immature virions. During or soon after the budding process, the viral
protease cleaves specific sites in Gag and Gag-Pot releasing essential viral
proteins
and enzymes such as capsid, nucleocapsid, reverse transcriptase, integrase and
spacer peptides SP1 and SP2. This last step is crucial for generating
functional viral
enzymes and also for the formation of the mature conical HIV capsid.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
2
A number of antiviral agents have been developed to interfere with various
stages of viral replication. For example, viral entry can be blocked with T-20
or
Maraviroc and post entry steps such as reverse transcription can be blocked
with
nucleoside RT inhibitors (examples: Lamivudine, Tenofovir, Zidovudine,
Didanosine, Emtricitabine, Abacavir) or nonnucleoside RT inhibitors (examples:
Nevirapine, Efavirenz and Delavirdine). Integration can be blocked by
Rategravir
and HIV proteolytic activity can be inhibited by protease inhibitors such as
Saquinavir, Indinavir, Amprenavir, Darunavir, Lopinavir, Atazanavir, and
Nelfinavir.
Other experimental agents such as Vicriviroc (CCR5), Elvitegravir (integrase),
Etravirine (RT), Apricitabine (RT), Bevirimat (maturation) are presently under
investigation. The use of combinations of antiretroviral agents have been
particularly effective in halting replication to undetectable levels and have
led to
markedly improved health and life span of HIV/AIDS patients. Nevertheless the
appearance of drug resistant viruses after long term therapy is a major
concern and
there is still a major need for additional drugs in order to provide
additional
options for these patients facing these issues.
Triterpenoid derivatives have been shown to possess anti-retrovirat
properties. For example, moronic acid (D. Yu, et al. J. Med. Chem. 2006, 49,
5462-
5469), oleanolic acid (H. -Assefa, et at. Bioorg. Med. Chem. Lett. 1999, 9,
1889-
1894), platanic acid (T. Fujioka, et at. J. Nat. Prod. 1994, 57, 243-247),
betulonic
acid (0. B. Flekhter, et al. Russ. J. Bioorg. Chem. 2004, 30, 80-88) and
betulinic
acid (I.-C. Sun, et at. Bioorg. Med. Chem. Lett. 1998, 8, 1267-1272)
derivatives
were shown to have anti-HIV-1 activities. Other triterpenes arising from the
modification of natural product precursors such as betulin have been
described, for
example 21-keto derivatives shown in references (M. Urban, et at. J. Nat.
Prod.
2007, 70, 526-532; M. Urban, et al. Synthesis 2006, 23, 3979-3986; J. Sarek,
et at.
Bioorg. Med. Chem. Lett. 2005,'l 5, 4196-4200; J. Sarek, et al. J. Med. Chem.
2003,
46, 5402-5414; M. Hajduch, J. Sarek WO 2001/090046). However, data pertaining
to their anti-HIV properties are either absent or are related to uses other
than for
the treatment of HIV/AIDS conditions. Furthermore, the cytotoxicity of these
compounds is unsuitable for the treatment of a chronic disease such as
HIV/AIDS.
This invention relates to 21-keto triterpenes and the discovery that these
novel modified triterpenoid derivatives possess significant anti-HIV activity.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
3
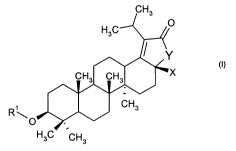
The present invention relates to a compound of formula (I):
CH3
H3C 0
Y
CH3 H3 X
R' CH3
\0
H3C CH3
(I)
wherein;
R1 is H, hydroxy protecting group or
O IO
HO A
A is C1_8 alkyl, C2_8 alkenyl, or -(CH2)1_20(CH2)1.2-;
Y is C=O or C-Ry1Ry2i
Ry1 and Rye are each independently H or -CH3;
X is NR2R3;
R2 R2 R3 R2
N 0, N N, N\ "O
Y R6 Y R3 /S-R
O 0 0 5
R R R3-, N, R3.
R 0 R6
12 12 12
O
NYR4 N 0 N
0 0 , or 0
R2 is H, C1_12 alkyl which is unsubstituted or substituted one or more times
by R10, C2_12 alkenyl which is unsubstituted or substituted one or more times
by R10,
or C2_12 alkynyl which is unsubstituted or substituted one or more times by
R10;
R3 and R3' are each independently H, C1.12 alkyl which is unsubstituted or
substituted one or more times by R10, C2_12 alkenyl which is unsubstituted or
substituted one or more times by R10, C2_12 alkynyl which is unsubstituted or
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
4
substituted one or more times by R10, C6_14 aryl which is unsubstituted or
substituted one or more times by R", C7_16 aralkyl which is unsubstituted or
substituted one or more times by R11, 5-12 member heteroaryl which is
unsubstituted or substituted one or more times by R11, 6-18 member
heteroaralkyl
which is unsubstituted or substituted one or more times by R11, 3-12 member
heterocycle which is unsubstituted or substituted one or more times by R12, or
4-18
member heterocycle-alkyl which is unsubstituted or substituted one or more
times
by R12;
R3 and R3' can also be taken together to form 5-12 member heteroaryl
which is unsubstituted or substituted one or more times by R11, or a 3-12
member
heterocycle which is unsubstituted or substituted one or more times by R12;
R4 is C1_12 alkyl which is unsubstituted or substituted one or more times by
R10, C2_12 alkenyl which is unsubstituted or substituted one or more times by
R10, C2.
12 alkynyl which is unsubstituted or substituted one or more times by R10,
C6_14 aryl
which is unsubstituted or substituted one or more times by R", C7.16 aralkyl
which is
unsubstituted or substituted one or more times by R11, 5-12 member heteroaryl
which is unsubstituted or substituted one or more times by R11, 6-18 member
heteroaralkyl which is unsubstituted or substituted one or more times by R11,
3-12
member heterocycle which is unsubstituted or substituted one or more times by
R12, or 4-18 member heterocycle-alkyl which is unsubstituted or substituted
one or
more times by R12;
R5 and R6 are each independently C1.12 alkyl which is unsubstituted or
substituted one or more times by R10, C2.12 alkenyl which is unsubstituted or
substituted one or more times by R10, C2.12 alkynyl which is unsubstituted or
substituted one or more times by R10, C6.14 aryl which is unsubstituted or
substituted one or more times by R11, C7_16 aralkyl which is unsubstituted or
substituted one or more times by R11, 5-12 member heteroaryl which is
unsubstituted or substituted one or more times by R11, 6-18 member
heteroaralkyl
which is unsubstituted or substituted one or more times by R11, 3-12 member
heterocycle which is unsubstituted or substituted one or more times by R12, or
4-18
member heterocycle-alkyl which is unsubstituted or substituted one or more
times
by R12;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
R10 is halogen, oxo, C1.6 alkoxy, -NH2, -NH(C1.4 alkyl), -N(C1.4 alkyt)2i -
C(O)NH2, -
C(O)NH(C1.4 alkyl), -C(O)N(C1.4 alkyt)2, -NHC(O)H, -N(C1.4 alkyl)C(O)H, -
N(C1.4
alkyl)C(O)C1_4 alkyl, -NHC(O)C1.4 alkyl, -NHC(O)0C1_4 alkyl, N(C1.4
alkyl)C(O)OC1.4 alkyl, -NHC(O)NH2, ,-N(C1.4 alkyl)C(O)NH2i -NHC(O)NHC1.4
alkyl, -N(C1.4 alkyl)C(O)NHC1.4 alkyl,-N(C1_4 alkyl)C(O)N(C1.4 alkyt)2i -
NHC(O)N(C1_4 alkyl)2i -C(O)H, -C(O)C1_4 alkyl, C(O)OH, -C(O)OC1.4 alkyl, -
OC(O)C1_4 alkyl, -OC(O)NH(C1_4 alkyl), -OC(O)N(C1_4 alkyl)2i -C(NOH)C1_4
alkyl, -
C(NOH)H, -C(NOCt.4 alkyl)C1.4 alkyl, -C(NOC1_4 alkyl)H, hydroxyl, nitro,
azido,
cyano, -S(0)0.3H, -S(0)0_3C1.4 alkyl, -SO2NH2, -SO2NH(C1.4 alkyl), -SO2N(C1.4
alkyth, -N(C1_4 alky[)S02C1.4 alkyl, -NHSO2C1_4 alkyl, -P(O)(OH)2, -P(O)(OC1.
4alkyl)OH, -P(O)(0C1_4alkyt)2i amidino, or guanidino;
R" is halogen, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2.6 alkynyl,
C1.6 alkoxy, -
NH2, -NH(C1.4 a(kyl), -N(C1.4 alkyl)2, -C(O)NH2, -C(O)NH(C1_4 alkyl), -
C(O)N(C1_4
alkyth, -NHC(O)H, -N(C1.4 alkyl)C(O)H, -N(Ct.4 a(kyl)C(O)C1.4 alkyl, -
NHC(O)C1.
4 alkyl, -NHC(O)0C1_4 alkyl, -N(C1_4 alkyl)C(O)OC1.4 alkyl, -NHC(O)NH2, ,-
N(C1.4
alkyl)C(O)NH2i -NHC(O)NHC1.4 alkyl, -N(C1.4 alkyl)C(O)NHCt.4 alkyl,-N(C1.4
alkyt)C(O)N(C1_4 alkyth, -NHC(O)N(C1_4 alkyl)2i -C(O)H, -C(O)C1_4 alkyl,
C(O)OH, -C(O)0C1_4 alkyl, -OC(O)C1.4 alkyl, -OC(O)NH(C1.4 alkyl), -OC(O)N(C1.4
alkyth, -C(NOH)C1.4 alkyl, -C(NOH)H, -C(NOC1.4 alkyt)C1_4 alkyl, -C(NOC1_4
alkyt)H, hydroxyl, nitro, azido, cyano, -S(0)0_3H, -S(0)0.3C1.4 alkyl, -
S02NH2i -
S02NH(C1_4 alkyl), -SO2N(C1.4 alkyth, -N(C1.4 alkyl)S02C1.4 alkyl, -NHS02Ct.4
alkyl, -P(O)(OH)2, -P(O)(OC1.4alkyl)OH, -P(O)(OCt.4alkyl)2i amidino, or
guanidino; and
R12 is halogen, oxo, C1.6 alkyl, halogenated C1.6 alkyl, C2_6 alkenyl, C2.6
alkynyl, C1.6
alkoxy, -NH2, -NH(C1_4 alkyl), -N(C1.4 alkyl)2i -C(O)NH2, -C(O)NH(C1_4 alkyl),
-
C(O)N(C1_4 alkyl)2i -NHC(O)H, -N(C1_4 alkyl)C(O)H, -N(C1.4 alkyl)C(O)C1.4
alkyl, -
NHC(O)Ct_4 alkyl, -NHC(O)OC1.4 alkyl, -N(C1.4 alkyl)C(O)OC1.4 alkyl, -
NHC(O)NH2, ,-N(C1_4 alkyl)C(O)NH2, NHC(O)NHC1.4 alkyl, -N(C1.4
alkyl)C(O)NHC1_4 alkyl,-N(C1.4 alkyl)C(O)N(C1_4 alkyth, -NHC(O)N(C1.4 alkyl)2i
-
C(O)H, -C(O)C1.4 alkyl, C(O)OH, -C(O)OC1.4 alkyl, -OC(O)Ct.4 alkyl, -
OC(O)NH(C1_4 alkyl), -OC(O)N(C1.4 atkyl)2, -C(NOH)Ct_4 alkyl, -C(NOH)H, -
C(NOC1_4 alkyl)C1_4 alkyl, -C(NOC1.4 alkyl)H, hydroxyl, nitro, azido, cyano, -
S(0)0_3H, -S(0)0.3C1.4 alkyl, -S02NH2i -S02NH(Ct_4 alkyl), -SO2N(C1.4 alkyth, -
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
6
N(C1.4 alkyl)S02C1_4 alkyl, -NHS02C1.4 alkyl, -P(O)(OH)2, -P(0)(OC1.4alkyl)OH,
-
P(O)(OC1.4alkyl)2, amidino, or guanidino;
or a pharmaceutically acceptable salt thereof.
In further embodiments, the compounds of the inventions are represented
by formula (I) wherein the following embodiments are present alone or in
combination:
Y is C=O.
Y is C-Ry1Ry2 and Ry1 and R,r2 are each -CH3.
Y is C-R,,1Ry2 and RY1 and Rye are each H.
R1 is
O OO O
" O HOOC . HOOC O
HOOC
O O
HOOC
O = HOOC O HOOCO0
O O
HOOC O ; HOOC O
HOOC O
HOOC 0 ; or
HOOC OO
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
7
R, is succinyl, glutaryl, 3'-methylglutaryl, 3'-methylsuccinyl, 3',3'-
dimethylsuccinyl, 3',3'- dimethylglutaryl, 2',2'-dimethylmalonyl, 2',3'-
dihydroxysuccinyl, 2',3'-dimethylsuccinyl, 2',2',3',3'-tetramethylsuccinyl, 2'-
methylsuccinyl, or 2',2'- dimethylsuccinyl.
R, is succinyl, glutaryl, 3'-methylglutaryl, 3'-methylsuccinyt, 3',3'-
dimethylsuccinyl, 3',3'- dimethylglutaryl, 2',2'-dimethylmalonyl, 2',3'-
dihydroxysuccinyl, 2',2',3',3'-tetramethylsuccinyl or 2',2'- dimethylsuccinyl.
R, is 3',3'-dimethylsuccinyt.
In a further embodiment, R, is H, or a hydroxy protecting group.
In a further embodiment, R, is H.
R2 is H or C,_12 alkyl which is unsubstituted or substituted one or more times
by R10.
R2 is H or C1_6 alkyl which is unsubstituted or substituted one or more times
by R10.
R2 is H, methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl,
cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
R2 is methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, or tert-butyl.
R2 is H.
R3' is H.
R3 and R3' can also be taken together to form a 5-6 member heteroaryl
which is unsubstituted or substituted one or more times by R" or a 5-6 member
heterocycle which is unsubstituted or substituted one or more times by R12.
R3 and R3' can also be taken together to form a piperidyl, a piperazinyl, or a
morpholinyl which is unsubstituted or substituted one or more times by R".
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
8
R3 is C1_12 alkyl which is unsubstituted or substituted one or more times by
RIO, C2.12 alkenyl which is unsubstituted or substituted one or more times by
R10, C2.
12 alkynyl which is unsubstituted or substituted one or more times by R10, C6-
14 aryl
which is unsubstituted or substituted one or more times by R", C7_16 aralkyl
which is
unsubstituted or substituted one or more times by R", 5-12 member heteroaryl
which is unsubstituted or substituted one or more times by R11, 6-18 member
heteroaralkyl which is unsubstituted or substituted one or more times by R", 3-
12
member heterocycle which is unsubstituted or substituted one or more times by
R12, or 4-18 member heterocycle-alkyl which is unsubstituted or substituted
one or
more times by Rte.
R3 is C1.12 alkyl which is unsubstituted or substituted one or more times by
R10, C6 aryl which is unsubstituted or substituted one or more times by R",
C7_9
aralkyl which is unsubstituted or substituted one or more times by R", 5-6
member
heteroaryl which is unsubstituted or substituted one or more times by R", 7-8
member heteroaralkyl which is unsubstituted or substituted one or more times
by
R", 5-6 member heterocycle which is unsubstituted or substituted one or more
times by R12, or 7-8 member heterocycle-alkyl which is unsubstituted or
substituted
one or more times by R12.
R3 is C1.6 alkyl which is unsubstituted or substituted one or more times by
RIO, phenyl which is unsubstituted or substituted one or more times by R",
benzyl
which is unsubstituted or substituted one or more times by R", 5-6 member
heteroaryl which is unsubstituted or substituted one or more times by R", 7-8
member heteroaralkyl which is unsubstituted or substituted one or more times
by
R", 5-6 member heterocycle which is unsubstituted or substituted one or more
times by R12, or 7-8 member heterocycle-alkyl which is unsubstituted or
substituted
one or more times by R12.
R4 is C1_12 alkyl which is unsubstituted or substituted one or more times by
R10, C2.12 alkenyl which is unsubstituted or substituted one or more times by
RIO, C2-
12 alkynyl which is unsubstituted or substituted one or more times by R10,
C6_14 aryl
which is unsubstituted or substituted one or more times by R", C7_16 aralkyl
which is
unsubstituted or substituted one or more times by R", 5-12 member heteroaryl
which is unsubstituted or substituted one or more times by R", 6-18 member
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
9
heteroaralkyl which is unsubstituted or substituted one or more times by R", 3-
12
member heterocycle which is unsubstituted or substituted one or more times by
R12, or 4-18 member heterocycle-alkyl which is unsubstituted or substituted
one or
more times by R12.
R4 is C1_12 alkyl which is unsubstituted or substituted one or more times by
R10, C6 aryl which is unsubstituted or substituted one or more times by R11,
C7.9
aralkyl which is unsubstituted or substituted one or more times by R11, 5-6
member
heteroaryl which is unsubstituted or substituted one or more times by R", 7-8
member heteroaralkyl which is unsubstituted or substituted one or more times
by
R11, 5-6 member heterocycle which is unsubstituted or substituted one or more
times by R12, or 7-8 member heterocycle-alkyl which is unsubstituted or
substituted
one or more times by R12.
R4 is C1_6 alkyl which is unsubstituted or substituted one or more times by
R10, phenyl which is unsubstituted or substituted one or more times by R11,
benzyl
which is unsubstituted or substituted one or more times by R11, 5-6 member
heteroaryl which is unsubstituted or substituted one or more times by R", 7-8
member heteroaralkyl which is unsubstituted or substituted one or more times
by
20, R1t, 5-6 member heterocycle which is unsubstituted or substituted one or
more
times by R12, or 7-8 member heterocycle-alkyl which is unsubstituted or
substituted
one or more times by R12.
R3, R4, R5 or R6 are Ct_12 alkyl which is unsubstituted or substituted one or
more times by R10.
R3, R4i R5 or R6 are C1.6 alkyl which is unsubstituted or substituted one or
more times by R10.
30 R3, R4, R5 or R6 are each independently methyl, ethyl, propyl, isopropyl,
butyl, sec-butyl, tert-butyl, cyclopropyt, cyctobutyl, cyctopentyl, or
cyclohexyl.
R3, R4, R5 or R6 are each independently is methyl, ethyl, propyl, isopropyl,
butyl, sec-butyl, or tert-butyl.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
R3, R4, R5 or R6 are phenyl which is unsubstituted or substituted one or more
times by R11.
R3, R4, R5 or R6 are phenyl.
R3, R4, R5 or R6 are 5-6 member heteroaryl which is unsubstituted or
substituted one or more times by R".
R3, R4, R5 or R6 are is pyridyl which is unsubstituted or substituted one or
10 more times by R".
R3, R4, R5 or R6 are is 7-8 member heteroaralkyl which is unsubstituted or
substituted one or more times by R".
R3, R4, R5 or R6 are -CH2-pyridyl which is unsubstituted or substituted one or
more times by R".
R3, R4, R5 or R6 are 5-6 member heterocycle which is unsubstituted or
substituted one or more times by R12.
R3, R4, R5 or R6 are piperidine which is unsubstituted or substituted one or
more times by R12.
R3, R4, R5 or R6 are 7-8 member heterocycle-alkyl which is unsubstituted or
substituted one or more times by R12
R3, R4, R5 or R6 are -CH2-piperidine which is unsubstituted or substituted one
or more times by R12.
R3, R4, R5 or R6 are each independently ethyl, iso-propyl, tert-butyl,
cyclopentyl, -CH2-cyclopentyl, cyclohexyl, -CH2-cyclohexyl, phenyl, benzyl,
pyridinyl, -CH2-pyridinyl, piperidynyl, piperazinyl, thienyl, morpholino,
oxadiazole,
pyrimidinyl, pyranyl, pyrazinyl,, thiazole, and pyrazole, which are
unsubstituted or
substituted by one or more substituents chosen from a halogen, C1.4 alkyl,
C1.4
alkyloxy, CF3, COC1.4 alkyl, COOH, COOC1.4 alkyl, cyano, NH2, nitro, NH(C1_6
alkyl),
and N(C1.6 a(kyt)2.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
11
R3 is 5-6 member heteroaryl which is unsubstituted or substituted one or
more times by R".
R3 is 5-6 member heterocycle which is unsubstituted or substituted one or
more times by R12.
R3 is pyridine which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R3 is pyrimidine which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1.3 alkoxy, or halogenated C1_3 alkyl.
R3 is pyrazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1_3 alkyl.
R3 is phenyl which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R3 is benzyl which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R3 is oxadiazole which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R3 is imidazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1_3 alkoxy, or halogenated C1.3 alkyl.
R3 is pyrrolidine which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1_3 alkoxy, or halogenated C1.3 alkyl.
R3 is piperidine which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1_3 alkoxy, or halogenated C1.3 alkyl.
R3 is cyclohexyl which is unsubstituted or substituted one or more times by
halogen, C1_3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
12
R3 is pyridine.
R3 is methylpyridine.
R3 is pyrimidine.
R3 is pyrazine.
R3 is pyrazole.
R3 is methylpyrazole.
R3 is thiadiazole.
R3 is methylthiadiazole.
R3 is oxadiazole.
R3 is methyloxadiazole.
R3 is piperidine.
R3 is methylpiperidine.
R3 is N-acetyl piperidine.
R3 is cyclohexyl.
R3 is difluorocyclohexyl.
R4 is 5-6 member heteroaryl which is unsubstituted or substituted one or
more times by R".
R4 is 5-6 member heterocycle which is unsubstituted or substituted one or
12
more times by R.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
13
R4 is pyridine which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is pyrimidine which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is pyrazine which which is unsubstituted or substituted one or more times
by halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1_3 alkyl.
R4 is pyrazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is phenyl which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is benzyl which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 atkoxy, or halogenated C1.3 alkyl.
R4 is thiazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is oxadiazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1_3 alkyl.
R4 is imidazole which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1_3 alkoxy, or halogenated C1.3 alkyl.
R4 is pyrrolidine which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
R4 is piperidine which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1_3 alkoxy, or halogenated C1.3 alkyl.
R4 is cyclohexyl which is unsubstituted or substituted one or more times by
halogen, C1.3 alkyl, C1.3 alkoxy, or halogenated C1.3 alkyl.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
14
R4 is pyridine.
R4 is methylpyridine.
R4 is pyrimidine.
R4 is pyrazine.
R4 is pyrazole.
R4 is methylpyrazole.
R4 is phenyl
R4 is fluorophenyl.
R4 is benzyl.
R4 is fluorobenzyl
R4 is thiazole.
R4 is methylthiazole.
R4 is oxadiazole.
R4 is methyloxadiazole.
R4 is imidazole.
R4 is methylimidazole.
R4 is piperidine.
R4 is methylpiperidine.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
R4 is N-acetyl piperidine.
R4 is thienyl.
R5 is C,_12 alkyl which is unsubstituted or substituted one or more times by
R10.
R5 is C1.6 alkyl which is unsubstituted or substituted one or more times by
10 R10.
R5 is methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl,
cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
R5 is methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, or tert-butyl.
R6 is C1.12 alkyl which is unsubstituted or substituted one or more times by
R10.
R6 is C1.6 alkyl which is unsubstituted or substituted one or more times by
R10.
R6 is methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, tert-butyl,
cyclopropyl, cyclobutyl, cyclopentyl, or cyclohexyl.
R6 is methyl, ethyl, propyl, isopropyl, butyl, sec-butyl, or tert-butyl.
R10 is halogen, oxo, C1.6 alkoxy, -NH2, -NH(C,.4 alkyl), -N(C1.4 alkyl)2i -
CONH2, -
CONH(C1_4 alkyl), -CON(C1.4 alkyl)2, -NHCOH, -N(C1.4 alkyl)COH, N(C,_4
alkyl)COC1.4
alkyl, -NHCOC1_4 alkyl, -NHCOOC1.4 alkyl, -NHCONHC1_4 alkyl, -N(C1_4
alkyl)CONHCt_4
alkyl,-N(C1_4 alkyl)CON(C1.4 alkyl)2, -NHCON(C1_4 alkyl)2, -C(O)H, -C(O)C1_4
alkyl,
carboxy, -C(O)0C1_4 alkyl, -C(NOH)C1.4 alkyl, -C(NOH)H, -C(NOC1.4 alkyl)C1_4
alkyl, -
C(NOC1_4 alkyl)H, hydroxyl, nitro, azido, cyano, -S(0)0.2H, -S(0)0.2C1.4
alkyl, -SO2NH2,
-SO2NH(C1_4 alkyl), -SO2N(C1.4 alkyl)2i -N(C1_4 alkyl)S02C1.4 alkyl, -
NHSO2C1_4 alkyl, -
P(O)(OH)2 or P(O)(0C1_4alkyl)2i
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
16
R10 is halogen, oxo, C1.6 alkoxy,-NH2, -NH(C1.4 alkyl), -N(C1.4 alkyl)2, -
CONH2, -CONH(Ct.4 alkyl), -CON(C1.4 alkyl)2i -NHCOH, -N(C1.4 alkyl)COH, -
N(C1_4
alkyl)COCt.4 alkyl, -NHCOC1.4 alkyl, -NHCOOC1.4 alkyl, -NHCONHC1_4 alkyl, -
N(C1_4
atkyl)CONHC1_4 alkyl,-N(C1.4 alkyl)CON(C1.4 alkyt)2i -NHCON(C1.4 alkyl)2i -
C(O)H, -
C(O)C1.4 alkyl, carboxy, -C(O)0C1_4 alkyl, -C(NOH)C1.4 alkyl,-C(NOH)H,
hydroxyl,
nitro, azido, cyano, -S(0)0.2H, -S(0)0.2C1_4 alkyl, -SO2NH2i -SO2NH(C1.4
alkyl), -
SO2N(C1.4 alkyl)2i -N(C1.4 alkyl)SO2C1.4 alkyl, -NHSO2C1_4 alkyl, or -
P(O)(OH)2.
Rt0 is halogen, oxo, -NH2, -NH(C1_4 alkyl), -N(C1.4 alkyt)2, -CONH2, -
CONH(C1.4
alkyl), -CON(C1_4 alkyl)2, -NHCOH, -N(C1_4 alkyl)COH, -N(C1.4 atkyl)COC1_4
alkyl, -
NH0001.4 alkyl, -NHCOOC1_4 alkyl, -NHCONHCt.4 alkyl, -C(O)H, -C(O)C1'_4 alkyl,
carboxy, -C(O)0C1.4 alkyl, hydroxyl, C1.4 alkoxy, nitro, nitroso, azido, or
cyano.
R10 is halogen, oxo, -NH2, -NH(C1_4 alkyl), -N(C1.4 alkyl)21 -CONH2, -
CONH(C1_4
alkyl), -CON(C1.4 alkyl)2, -NHCOH, -N(C1_4 alkyl)COH, -N(C1_4 alkyl)COC1.4
alkyl, -
NHCOC1.4 alkyl, -NHCOOC1.4 alkyl, -NHCONHC1_4 alkyl, -C(O)H, -C(O)C1.4 alkyl,
carboxy, -C(O)0C1_4 alkyl, hydroxyl, Ct.4 alkoxy, nitro, azido, or cyano.
R10 is halogen, oxo, -NH2, -NH(C1.4 alkyl), -N(C1.4 alkyt)2, -CONH2, -
CONH(C1_4
alkyl), -CON(C1.4 alkyth, -NHCOH, -N(C1_4 alkyl)COH, -N(C1_4 atkyl)COC1_4
alkyl, -
NH0001.4 alkyl, -NHCOOC1_4 alkyl, -NHCONHC1.4 alkyl, -C(O)H, -C(O)C1.4 alkyl,
carboxy, -C(O)0C1_4 alkyl, hydroxyl, or C1.4 alkoxy.
R10 is halogen, oxo, -NH2, -NH(C1.4 alkyl), -N(C1.4 alkyl)2, -CONH2, -
CONH(C1.4
alkyl), -CON(C1.4 atkyl)2, -N(C1_4 alkyl)COC1.4 alkyl, -NHCOC1_4 alkyl,
carboxy, -
C(O)OC1.4 alkyl, hydroxyl, C1.4 alkoxy, or cyano.
R" is halogen, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2.6 alkynyl,
C1.6
alkoxy,-NH2, -NH(C1.4 alkyl), -N(C1.4 alkyth, -CONH2, -CONH(C1.4 alkyl), -
CON(C1.4
alkyth, -NHCOH, -N(C1.4 alkyl)COH, N(C1_4 atkyl)COC1_4 alkyl, -NHCOCt_4 alkyl,
-
NHCOOC1.4 alkyl, -NHCONHC1_4 alkyl, -N(C1.4 alkyl)CONHC1_4 alkyl, N(C1.4
alkyt)CON(C1_4 alkyl)2i -NHCON(C1.4 alkyth, -C(O)H, -C(O)Ct.4 alkyl, carboxy, -
C(O)0C1_4 alkyl, -C(NOH)C1.4 alkyl, -C(NOH)H, -C(NOCt_4 alkyl)C1.4 alkyl, -
C(NOC1.4
alkyl)H, hydroxyl, nitro, azido, cyano, -S(0)0.2H, -S(0)0.2C1.4 alkyl, -
SO2NH2, -
SO2NH(C1.4 alkyl), -SO2N(C1.4 alkyl)2, -N(C1.4 alkyl)S02C1.4 alkyl, -NHSO2C1_4
alkyl, -
P(O)(OH)2 or P(O)(OC1.4alkyl)2i
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
17
_
R" is halogen, C1_6 alkyl, halogenated C1_6 alkyl, C2.6 alkenyl, C2_6 alkynyl,
C1
6 alkoxy, -NH2, -NH(C1.4 alkyl), -N(C1_4 alkyl)2i -CONH2, -CONH(C1.4 alkyl), -
CON(C1.
4 alkyl)2, -NHCOH, -N(C1.4 alkyl)COH, -N(C1.4 alkyl)COC1.4 alkyl, -NHCOC1.4
alkyl, -
NH000C1.4 alkyl, -NHCONHC1_4 alkyl, -N(C1_4 alkyl)CONHC1_4 alkyl, -N(C1_4
alkyl)CON(C1_4 alkyl)2, -NHCON(C1.4 alkyl)2, -C(Q)H, -C(O)C1.4 alkyl, carboxy,
-
C(0)0 C1_4 alkyl,-C(NOH) C1.4 alkyl, -C(NOH)H, hydroxyl, nitro, azido, cyano, -
S(0)0_2H, -S(0)0_2C1.4 alkyl, -SO2NH2, -S02NH(C,_4 a(kyl), -SO2N(C,_4 alkyl)2,
-N(C1_4
alkyl)S02C1.4 alkyl, -NHS02C1_4 alkyl, or -P(O)(OH)2.
Rt1 is halogen, C1.6 alkyl, halogenated C1_6 alkyl, C2.6 alkenyl, C2_6
alkynyl,-NH2i -
NH(C1_4 alkyl), -N(C1_4 atkyl)2, -CONH2, -CONH(C1.4 alkyl), -CON(C1_4 alkyl)2,
-NHCOH, -
N(C1_4 alkyl)COH, -N(C1_4 alkyl)COC1.4 alkyl, -NHCOC1_4 alkyl, -NHCOOC1_4
alkyl, -
NHCONHC1.4 alkyl, -C(O)H, -C(O)C1_4 alkyl, carboxy, -C(O)0C1_4 alkyl,
hydroxyl, C1.6
alkoxy, nitro, nitroso, azido, or cyano.
R11 is halogen, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2.6
alkynyl,-NH2i -
NH(C1.4 alkyl), -N(C1.4 alkyl)2, -CONH2, -CONH(C1.4 alkyl), -CON(C1_4 alkyl)2,
-NHCOH,
N(C1_4 alkyl)COH, -N(C1_4 alkyl)COC1.4 alkyl, NHCOC1_4 alkyl, -NHCOOC1.4
alkyl, -
NHCONHC1_4 alkyl, -C(O)H, -C(O)C1.4 alkyl, carboxy, -C(O)0C1.4 alkyl,
hydroxyl, C1.6
alkoxy, nitro, azido, or cyano.
R11 is halogen, C1.6 alkyl, halogenated C1_6 alkyl, C2.6 alkenyl, C2_6
alkynyl, -NH2,
-NH(C1.4 alkyl), -N(C1_4 alkyl)2, -CONH2, -CONH(C1.4 alkyl), -CON(C1.4
alkyl)2, -NHCOH,
-N(C1.4 alkyl)COH, -N(C1.4 alkyl)COCt.4 alkyl, -NHCOC1.4 alkyl, -NHCOOC1.4
alkyl, -
NHCONHC1_4 alkyl, -C(O)H, -C(O)C1_4 alkyl, carboxy, -C(O)OC1.4 alkyl,
hydroxyl, or CI-6
alkoxy.
R11 is halogen, C1_6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2_6
alkynyl, -NH2i
-NH(C1.4 alkyl), -N(C1.4 alkyl)2, -CONH2, -CONH(C1_4 alkyl), -CON(C1.4
alkyl)2, -N(C1.4
alkyl)COC1.4 alkyl, -NHCOC1_4 alkyl, carboxy, -C(O)0C1.4 alkyl, hydroxyl, or
C1.6
alkoxy.
R12 is halogen, oxo, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2_6
alkynyl,
C1.6 alkoxy,-NH2, -NH(C1.4 alkyl), -N(C1.4 alkyl)2, -CONH2, -CONH(C1.4 alkyl),
-CON(C1.4
alkyl)2, -NHCOH, -N(C1.4 alkyl)COH, -N(C1.4 alkyl)COC1_4 alkyl, -NHCOC1.4
alkyl, -
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
18
NHCOOC1.4 alkyl, -NHCONHC1_4 alkyl, -N(C1_4 alkyl)CONHC1.4 alkyl, -N(C1.4
alkyl)CON(C1_4 alkyl)2i -NHCON(C1.4 alkyth, -C(O)H, -C(O)C1.4 alkyl, carboxy, -
C(O)OC1_4 alkyl, -C(NOH)C1.4 alkyl, -C(NOH)H, -C(NOC1.4 alkyl)C1.4 alkyl, -
C(NOC1.4
alkyl)H, hydroxyl, nitro, azido, cyano, -S(0)0_2H, -S(0)0.2C1.4 alkyl, -
SO2NH2, -
S02NH(C1_4 alkyl), -SO2N(C1.4 alkyth, -N(C1.4 alkyl)S02C1_4 alkyl, -NHSO2C1.4
alkyl, -
P(O)(OH)2 or P(O)(OC1.4alkyl)2.
R12 is halogen, oxo, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2.6
alkynyl, C1.6 alkoxy, -NH2, -NH(C1_4 alkyl), -N(C1_4 alkyl)2, -CONH2, -
CONH(C1.4
alkyl), -CON(C1.4 alkyth, -NHCOH, -N(C1.4 alkyl)COH, -N(C1.4 alkyl)COC1.4
alkyl, -
NH0001_4 alkyl, -NHCOOC1.4 alkyl, -NHCONHC1.4 alkyl, -N(C1_4 alkyl)CONHC1.4
alkyl,
-N(C1.4 alkyl)CON(C1.4 alkyth, -NHCON(C1_4 alkyth, -C(O)H, -C(O)C1.4 alkyl,
carboxy, -C(0)O C1.4 alkyl, -C(NOH) C1.4 alkyl, -C(NOH)H, hydroxyl, nitro,
azido,
cyano, -S(0)0.2H, -S(0)0_2C1_4 alkyl, -SO2NH2, -S02NH(C,_4 alkyl), -SO2N(C1.4
alkyl)2,
-N(C1.4 alkyl)S02C1_4 alkyl, -NHS02C,_4 alkyl, or -P(O)(OH)2.
R12 is halogen, oxo, C1.6 alkyl, halogenated C1.6 alkyl, C2.6 alkenyl, C2.6
alkynyl, -
NH2, -NH(C1.4 alkyl), -N(C1.4 alkyth, -CONH2, -CONH(C1_4 alkyl), -CON(Ct.4
alkyth, -
NHCOH, -N(C1.4 alkyl)COH, -N(C1_4 alkyl)COC1.4 alkyl, -NHCOC1_4 alkyl, -
NHCOOCt_4
alkyl, -NHCONHC1_4 alkyl, -C(O)H, -C(O)C1_4 alkyl, carboxy, -C(O)0C1_4 alkyl,
hydroxyl, C1.6 alkoxy, nitro, nitroso, azido, or cyano.
R12 is halogen, oxo, C1.6 alkyl, halogenated C1.6 alkyl, C2_6 alkenyl, C2.6
alkynyl, -
NH2, -NH(Ct.4 alkyl), -N(C1.4 alkyth, -CONH2, -CONH(C1_4 alkyl), -CON(C1_4
alkyl)2i -
NHCOH, -N(C1_4 alkyl)COH, -N(C1_4 alkyl)COC1_4 alkyl, -NHCOC1_4 alkyl, -
NHCOOC1.4
alkyl, -NHCONHC1.4 alkyl, -C(O)H, -C(O)C1_4 alkyl, carboxy, -C(O)0C1_4 alkyl,
hydroxyl, C1_6 alkoxy, nitro, azido, or cyano.
R 12 is halogen, oxo, C1.6 alkyl, halogenated C1_6 alkyl, C2.6 alkenyl, C2_6
alkynyl, -
NH2, -NH(C1.4 alkyl), -N(C1.4 alkyl)2i -CONH2, -CONH(C1_4 alkyl), -CON(C1.4
alkyth, -
NHCOH, -N(C1_4 alkyl)COH, -N(Ct_4 alkyl)COC1.4 alkyl, -NHCOCt.4 alkyl, -
NHCOOC1.4
alkyl, -NHCONHC1.4 alkyl, -C(O)H, -C(O)C1_4 alkyl, carboxy, -C(O)OC1.4 alkyl,
hydroxyl, or C1.6 alkoxy.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
19
R 12 is halogen, oxo, C1.6 alkyl, halogenated C1_6 alkyl, -NH,, -NH(C1.4
alkyl), -
N(C,_4 alkyl)2, -CONH2, -CONH(C1_4 alkyl), -CON(C1.4 alkyl)2, -N(C1.4
alkyl)COC1_4 alkyl, -
NH0001_4 alkyl, carboxy, -C(O)0C1_4 alkyl, hydroxyl, or C1_6 alkoxy.
In a further embodiment, the present invention relates to a compound
of formula (II) or formula (Ila):
CH3 CH3
H3C H3C O
Y "R3 Y N\
/ 3
CH3 CH3 N N CH3 CH3 N R3
R2 R3 R2 O
CH3 CH3
Rl"O Ril O
H3C CH3 H3C CH3
(II) (Ila)
or a pharmaceutically acceptable salt thereof, wherein R1, R2, R3, and R3'
are defined above.
In a further embodiment, the present invention relates to a compound
of formula (II)
CH3
H3C 0
O
3 C H 3 'J~ ~R3
CH N N
R2 R3
CH3
R~~O
H3C c H3
(II)
or a pharmaceutically acceptable salt thereof, wherein R1, R2, R3, and R3'
are defined above.
In a further embodiment, the present invention relates to a compound
of formula (III) :
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
CH3
H3C O
Y ~
3 R4
4
CH3 CH
RZ
CH3
R~~O
H3C'' C',H3
(III)
or a pharmaceutically acceptable salt thereof, wherein R1, R2, and R4 are
defined above.
In a further embodiment, the present invention relates to a compound
of formula (IV)
CH3
H3C O
O
N O, R6
C H
3 C H 3 1
R2
CH3
R1 O
H3C CH3
(IV)
or a pharmaceutically acceptable salt thereof, wherein R,, R2, and R6 are
defined above.
It will be appreciated by those skilled in the art that the compounds in
accordance with the present invention can exists as stereoisomers, for
example,
optical (+ and -), geometrical (cis and trans) and conformational isomers
(axial and
equatorial). All such stereoisomers are included in the scope of the present
invention.
It will be appreciated by those skilled in the art that the compounds in
accordance with the present invention can contain a chiral center. The
compounds
of formula (I) may thus exist in the form of two different optical isomers
(i.e. (+) or
(-) enantiomers). All such enantiomers and mixtures thereof including racemic
mixtures are included within the scope of the invention. The single optical
isomers
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
21
or enantiomers can be obtained by methods well known in the art, such as
chiral
HPLC, enzymatic resolution and chiral auxiliary.
In one embodiment, the compounds of the present invention are provided in
the form of a single enantiomer at least 95%, at least 97% and at least 99%
free of
the corresponding enantiomer.
In a further embodiment the compound of the present invention are in the
form of the (+) enantiomer at least 95% free of the corresponding (-)
enantiomer.
In a further embodiment the compound of the present invention are in the
form of the (+) enantiomer at least 97% free of the corresponding (-)
enantiomer.
In a further embodiment the compound of the present invention are in the
form of the (+) enantiomer at least 99% free of the corresponding (-)
enantiomer.
In a further embodiment, the compounds of the present invention are in the
form of the (-) enantiomer at least 95% free of the corresponding (+)
enantiomer.
In a further embodiment the compound of the present invention are in the
form of the (-) enantiomer at least 97% free of the corresponding (+)
enantiomer.
In a further embodiment the compound of the present invention are in the
form of the (-) enantiomer at least 99% free of the corresponding (+)
enantiomer.
There is also provided pharmaceutically acceptable salts of the compounds
of the present invention. By the term pharmaceutically acceptable salts of
compounds are meant those derived from pharmaceutically acceptable inorganic
and organic acids and bases. Examples of suitable acids include hydrochloric,
hydrobromic, sulphuric, nitric, perchloric, fumaric, maleic, phosphoric,
glycotlic,
tactic, salicylic, succinic, toluene-p-sulphonic, tartaric, acetic,
triftuoroacetic,
citric, methanesulphonic, formic, benzoic, matonic, naphthalene-2-sulphonic
and
benzenesutphonic acids. Other acids such as oxalic, while not themselves
pharmaceutically acceptable, may be useful as intermediates in obtaining the
compounds of the invention and their pharmaceutically acceptable acid addition
salts.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
22
Salts derived from amino acids are also included (e.g. L-Arginine, L-Lysine).
Salts derived from appropriate bases include alkali metals (e.g. sodium,
lithium, potassium), alkaline earth metals (e.g. calcium, magnesium),
ammonium,
NR4+ (where R is C1-4 alkyl) salts, choline, meglumine and tromethamine.
A reference hereinafter to a compound according to the invention includes
that compound and its pharmaceutically acceptable salts.
In one embodiment of the invention, the pharmaceutically acceptable salt
is a sodium salt.
In one embodiment of the invention, the pharmaceutically acceptable salt
is a lithium salt.
In one embodiment of the invention, the pharmaceutically acceptable salt
is a potassium salt.
In one embodiment of the invention, the pharmaceutically acceptable salt
is a tromethamine salt.
In one embodiment of the invention, the pharmaceutically acceptable salt
is an L-Arginine salt.
In one embodiment of the invention, the pharmaceutically acceptable salt
is a meglumine salt.
It will be appreciated by those skilled in the art that the compounds in
accordance with the present invention can exist in different polymorphic
forms. As
known in the art, polymorphism is the ability of a compound to crystallize as
more
than one distinct crystalline or "polymorphic" species. A polymorph is a solid
crystalline phase of a compound with at least two different arrangements or
polymorphic forms of that compound molecule in the solid state. Polymorphic
forms of any given compound are defined by the same chemical formula or
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
23
composition and are as distinct in chemical structure as crystalline
structures of
two different chemical compounds.
It will further be appreciated by those skilled in the art that the compounds
in accordance with the present invention can exist in different solvate forms,
for
example hydrates. Solvates of the compounds of the invention may also form
when
solvent molecules are incorporated into the crystalline lattice structure of
the
compound molecule during the crystallization process.
Unless otherwise defined, all technical and scientific terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art
to which this invention belongs. All publications, patent applications,
patents, and
other references mentioned herein are incorporated by reference in their
entirety.
In case of conflict, the present specification, including definitions, will
control. In
addition, the materials, methods, and examples are illustrative only and not
intended to be limiting.
The term "alkyl" represents a linear, branched or cyclic hydrocarbon moiety.
The terms "alkenyl" and "alkynyl" represent a linear, branched or cyclic
hydrocarbon moiety which has one or more double bonds or triple bonds in the
chain, respectively. Examples of alkyl, alkenyl, and alkynyl groups include
but are
not limited to methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl,
tert-
butyl, pentyl, isopentyl, neopentyl, tert-pentyl, hexyl, isohexyl, neohexyl,
alkyl,
vinyl, acetylenyl, ethylenyl, propenyl, isopropenyl, butenyl, isobutenyl,
butadienyl,
pentenyl, pentadienyl, hexenyl, hexadienyl, hexatienyl, heptenyl, heptadienyl,
heptatrienyl, octenyl, octadienyl, octatrienyl, octatetraenyl, propynyl,
butynyl,
pentynyl, hexynyl, cyclopropyl, cyclobutyl, cyclopentyl, cyctohexenyt,
cyclohexdienyl and cyclohexyl. Where indicated the "alkyl," "alkenyl," and
"alkynyl"
can be optionally substituted such as in the case of haloalkyls in which one
or more
hydrogen atom is replaced by a halogen, e.g., an alkylhalide. Examples of
haloalkyls include but are not limited to trifluoromethyl, difluoromethyl,
fluoromethyl, trichloromethyl, dichloromethyl, chloromethyl, trifluoroethyl,
difluoroethyl, fluoroethyl, trichloroethyl, dichloroethyl, chloroethyl,
chlorofluoromethyl, chlorodifluoromethyl, dichlorofluoroethyl. Aside from
halogens, where indicated, the alkyl, alkenyl or alkynyl groups can also be
optionally substituted by, for example, oxo, -NRdRei -CONRdRe, =NO-Re, -
NRRdCORe1
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
24
carboxy, -C(=NRd)NReRf, azido, cyano, C1_6 alkyloxy, C2_6 alkenyloxy, C2.6
alkynyloxy,
--N(Rd)C(=NRe)-NRfRg, hydroxyl, nitro, nitroso, -N(Rh)CONRIRJ, -S(0)o.2Ra, -
C(O)Ra, -
C(O)ORa , -SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbk, -CRaN=ORb, -OCONReRf and/or
NRaCOORb, wherein Ra-Rj are each independently H, CI-4 alkyl, C2.4 alkenyl, or
C2-4
alkynyl.
The terms "cycloalkyl" and "cycloalkenyl" represent a cyclic hydrocarbon
alkyl or alkenyl, respectively, and are meant to include monocyclic (e.g.,
cyclohexyl), spiro (e.g., spiro [2.3]hexanyl), fused (e.g.,
bicyclo[4.4.0]decanyl),
and bridged (e.g., bicyclo[2.2.1]heptanyl) hydrocarbon moieties. Where
indicated,
the "cycloalkyl", and "cycloalkenyl" groups can also be optionally substituted
as
defined in "alkyl" and "alkenyl" definition,
The terms "alkoxy," "alkenyloxy," and "alkynytoxy" represent an alkyl,
alkenyl or alkynyl moiety, respectively, which is covalently bonded to the
adjacent atom through an oxygen atom. Like the alkyl, alkenyl and alkynyl
groups, where indicated the alkoxy (-0-alkyl), alkenyloxy (-0-alkenyl) and
alkynyloxy (-0-alkynyl) groups can also be optionally substituted. Examples
include but are not limited to methoxy, ethoxy, propoxy, isopropoxy, butoxy,
isobutoxy, sec-butoxy, tert-butoxy, pentyloxy, isopentyloxy, neopentyloxy,
tert-pentyloxy, hexyloxy, isohexyloxy, trifluoromethoxy and neohexyloxy.
The alkoxy, alkenyloxy, and alkynyloxy groups can be optionally substituted
by, for example, halogens, oxo, -NRdRe, -CONRdRef -NRdCORe, carboxy, -
C(=NRd)NReRf, azido, cyano, -N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, nitroso, C1_6
alkyl, C2.6 alkenyl, C2.6 alkynyl, -N(Rh)CONR;R;, -S(0)0_2Ra, -C(O)Ra, -
C(O)ORa,
=NO-Re, -SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbRc, CRaN=ORb, -OCONReRf and/or -
NRa000Rb, wherein Ra-Rj are each independently H, C1.4 alkyl, C2.4 alkenyl, or
C2.4 alkynyl.
The term "aryl" represents a carbocyclic moiety containing at least one
benzenoid-type ring (i.e., may be monocyclic or polycyclic), and which where
indicated may be optionally substituted with one or more substituents.
Examples include but are not limited to phenyl, tolyl, dimethylphenyl,
aminophenyl, anilinyl, naphthyl, anthryl, phenanthryl or biphenyl. The aryl
groups can be optionally substituted by, for example, halogens, -NRdRe, -
CONRdRe, -NRdCORe, carboxy, -C(=NRd)NReRf, azido, cyano, -
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, nitroso, -N(Rh)CONR;R;, C1.6 alkyl, C2.6
alkenyl, C2.6 alkynyl, C1.6 alkyloxy, C2.6 alkenyloxy, C2.6 alkynyloxy,
S(0)0.2Ra,
optionally substituted 5-12 member heteroaryl, optionally substituted 6-18
member heteroaralkyl, optionally substituted 3-12 member heterocycle,
optionally substituted 4-18 member heterocycle-alkyl, -C(O)Ra, -C(O)ORa, -
SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbRc, -CRaN=ORb, -OCONReRf and/or -NRaCOORb,
wherein Ra-R; are each independently H, C1.4 alkyl, C2.4 alkenyL, or C2.4
alkynyl.
10 The terms "aryloxy," represent an aryl moiety substituted with an
oxygen, wherein the point of attachement to the molecule it substitutes is on
the oxygen. Where indicated the aryloxy group (-0-aryl) can also be
optionally substituted by one or more substituents, for example, halogens, -
NRdRe, -CONRdRe, -NRdCORei carboxy, -C(=NRd)NReRf, azido, cyano, -
N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, -N(Rh)CONR;R;, CI-6 alkyl, C2.6 alkenyl,
C2-6
alkynyl, C1.6 alkyloxy, C2_6 alkenyloxy, C2_6 alkynyloxy, S(O)0.2Ra,
optionally
substituted 5-12 member heteroaryl, optionally substituted 6-18 member
heteroaralkyl, optionally substituted 3-12 member heterocycle, optionally
substituted 4-18 member heterocycle-alkyl, C(O)Ra, C(O)ORa, SO2NRaRb,
20 NRaSO2Rb, NRaSO2NRbRc, CRaN=ORb, -OCONReRf or NRaCOORb, wherein Ra-Rj are
each independently H, C1.4 alkyl, C2.4 alkenyl, or C2_4 alkynyl.
The term "aralkyl" represents an aryl group attached to the adjacent
atom by an alkyl, alkenyl, or alkynyl. Like the aryl groups, where indicated
the aralkyl groups can also be optionally substituted. Examples include but
are not limited to benzyl, benzhydryl, trityl, phenethyl, 3-phenylpropyl, 2-
phenylpropyl, 4-phenylbutyl and naphthylmethyl. Where indicated, the
aralkyl groups can be optionally substituted by, for example, halogens, -
NRdRe, -CONRdRe7 -NRdCORef carboxy, -C(=NRd)NReRf, azido, cyano, -
N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, nitroso, -N(Rh)CONR;R;, C1.6 alkyl, C2-6
alkenyl, C2_6 alkynyl, C1.6 alkyloxy, C2.6 alkenyloxy, C2.6 alkynyloxy,
S(0)0_2Ra,
optionally substituted 5-12 member heteroaryl, optionally substituted 6-18
member heteroaralkyl, optionally substituted 3-12 member heterocycle,
optionally substituted 4-18 member heterocycle-alkyl, -C(O)Ra, -C(O)ORa, -
SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbRc, -CRaN=ORb, -OCONReRf and/or -NRaCOORb,
wherein Ra-R; are each independently H, C1_4 alkyl, C2_4 alkenyl, or C2.4
alkynyl.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
26
The term "heterocycle" represents an optionally substituted, non
aromatic, saturated or partially saturated wherein said cyclic moiety is
interrupted by at least one heteroatom selected from oxygen (0), sulfur (S)
or nitrogen (N). It is understood that in a 3-12 member heterocycle moiety,
the 3-12 member represents the total of the ring atoms present in the
heterocycle moiety. Heterocycles may be monocyclic or polycyclic rings.
Examples include but are not limited to azetidinyl, dioxolanyl, morpholinyl,
morpholino, oxetanyl, piperazinyl, piperidyl, piperidino, cyclopentapyrazolyl,
cyclopentaoxazinyl, cyctopentafuranyl, tetrahydrofuranyl,
tetrahydrothiofuranyl, tetrahydrothiofuranyl, tetrahydropyranyl,
tetrahydrothiopyranyl, tetrahydrothiopyranyl dioxyde, thiazolinyl, oxazolinyl,
pyranyl, thiopyranyl, aziridinyl, azepinyt, dioxazepinyl, diazepinyt,
oxyranyl,
oxazinyl, pyrrolidinyl, thiopyranyl, thiotane, pyrazolidinyl, dioxanyl, and
imidazolidinyl. Where indicated, the heterocyclic groups can be optionally
substituted by, for example, halogens, oxo, -NRdRe, -CONRdRe, =NO-Re, -
NRdCORe, carboxy, -C(=NRd)NReRf, azido, cyano, -N(Rd)C(=NRe)NRfRg, hydroxyl,
nitro, nitroso, -N(Rh)CONR;R;, C1.6 alkyl, C2-6 alkenyl, C2.6 alkynyl, C7.12
aratkyl,
C6-12 aryl, C1-6 alkyloxy, C2-6 alkenyloxy, C2-6 alkynytoxy, S(0)o-2Ra, C6-1o
aryl, C6.10
aryloxy, C7-10 arylalkyt, C6.10 aryl-C1-10 alkyloxy, -C(O)Ra, -C(O)ORa, -
SO2NRaRb, -
NRaSO2Rb, -NRaSO2NRbRc, -CRaN=ORb, -OCONReRf and/or -NRaCOORb, wherein
Ra-R; are each independently H, C1-4 alkyl, C2-4 alkenyl or C2.4 alkynyl.
The term "heterocycle-alkyl" represents an optionally substituted
heterocycle group attached to the adjacent atom by an alkyl, alkenyl, or
alkynyl group. It is understood that in a 5-18 member heterocycle-alkyl
moiety, the 5-18 member represents the total of the ring atoms present in
the heterocycle moiety and the carbon atoms present in the alkyl, alkenyl or
alkynyl group. For example, the following groups are encompassed by a 7
member heterocycle-alkyl (* represents the attachment point):
N N CH3
S O
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
27
Where indicated the heterocycle-alkyl groups can be optionally
substituted by, for example, halogens, oxo, -NRdRe, -CONRdRe, -NRdCORe,
carboxy, -C(=NRd)NReRf, azido, cyano, -N(Rd)C(=NRe)NRfRg, hydroxyl, nitro,
nitroso, -N(Rh)CONRIR;, C1-6 alkyl, C2.6 alkenyl, C2.6 alkynyl, C1.6 alkyloxy,
C2.6
alkenyloxy, C2-6 alkynyloxy, S(O)o-2Ra, C6.1o aryl, C6-10 aryloxy, C7-10
arylalkyl, C6.
aryl-C1.10 alkyloxy, -C(O)Ra, -C(O)ORa, =NO-Re, -SO2NR,Rb, -NRaSO2Rb, -
NRaSO2NRbRc, -CRaN=ORb, -OCONReRf and/or -NRaCOORb, wherein R,,-R; are
each independently H, C1.4 alkyl, C2.4 alkenyl or C2.4 alkynyl.
10 The term "heteroaryt" represents an optionally substituted aromatic
cyclic moiety wherein said cyclic moiety is interrupted by at least one
heteroatom selected from oxygen (0), sulfur (S) or nitrogen (N). It is
understood that in a 5-12 member heteroaryl moiety, the 5-12 member
represents the total of the ring atoms present in the heteroaryl moiety.
Heteroaryls may be monocyclic or polycyclic rings. Examples include but are
not limited to dithiadiazinyl, furanyl, isooxazolyl, isothiazolyl, imidazolyt,
oxadiazolyl, dioxazole, oxatriazole, oxazolyl, pyrazinyl, pyridazinyl,
pyrimidinyt, pyridyt, pyrazolyt, pyrrotyl, thiatriazotyt, tetrazolyl,
thiadiazolyl,
triazolyt, thiazolyl, thienyl, tetrazinyt, thiadiazinyl, triazinyt, thiazinyt,
furoisoxazolyl, imidazothiazolyl, thienoisothiazolyl, thienothiazolyl,
imidazopyrazolyl, pyrrolopyrrolyl, thienothienyl, thiadiazolopyrimidinyl,
thiazolothiazinyl, thiazolopyrimidinyl, thiazolopyridinyl, oxazolopyrimidinyl,
oxazolopyridyl, benzoxazolyl, benzisothiazolyl, benzothiazolyl,
imidazopyrazinyl, purinyl, pyrazolopyrimidinyl, imidazopyridinyl,
benzimidazolyl, indazolyl, benzoxathiolyl, benzodioxolyl, benzodithiolyt,
indolizinyl, indolinyl, isoindolinyl, furopyrimidinyt, furopyridyl,
benzofuranyl,
isobenzofuranyl, thienopyrimidinyl, thienopyridyl, benzothienyt,
benzoxazinyt, benzothiazinyt, quinazotinyt, naphthyridinyt, quinolinyt,
isoquinolinyl, benzopyranyl, pyridopyridazinyl and pyridopyrimidinyl. Where
indicated the heteroaryl groups can be optionally substituted by, for
example, halogens, -NRdRe, -CONRdRe, -NRdCORe, carboxy, -C(=NRd)NReRf,
azido, cyano, -N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, nitroso, -N(Rh)CONRIRj,
C1.6
alkyl, C2.6 alkenyl, C2.6 alkynyl, C1-6 alkyloxy, C2-6 alkenyloxy, C2.6
alkynyloxy,
S(O)o-2Ra, C6-1o aryl, C6-1o aryloxy, C7-10 arylalkyl, C6-1o aryl-C1-10
alkyloxy, -
C(0)Ra, -C(O)ORa, -SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbR,, -CRaN=ORb, -OCONReRf
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
28
and/or -NRaCOORb, wherein Ra-R; are each independently H, C1-4 alkyl, C2.4
alkenyl or C2-4 alkynyl.
The term "heteroaralkyl" represents an optionally substituted
heteroaryl group attached to the adjacent atom by an alkyl, alkenyl, or
alkynyl group. Where indicated the heteroaralkyl groups can be optionally
substituted by, for example, halogens,-NRdRe, -CONRdRe, -NRdCORe, carboxy, -
C(=NRd)NReRf, azido, cyano, -N(Rd)C(=NRe)NRfRg, hydroxyl, nitro, nitroso, -
N(Rh)CONR;R;, C1-6 alkyl, C2.6 alkenyl, C2-6 alkynyl, C1.6 alkyloxy, C2.6
alkenyloxy,
C2-6 alkynyloxy, S(0)o-2Ra, C6-10 aryl, C6.10 arytoxy, C7-10 arylalkyl, C6.10
aryl-Ct.10
alkyloxy, -C(O)Ra, -C(O)ORa, -SO2NRaRb, -NRaSO2Rb, -NRaSO2NRbRc, -CRaN=ORb, -
OCONReRf and/or -NRaCOORb, wherein Ra-R; are each independently H, C1.4
alkyl, C2.4 alkenyl, or C2.4 alkynyl. It is understood that in a 6-18 member
heteroaralkyl moiety, the 5-18 member represents the total of the ring atoms
present in the heteroaryl moiety and the carbon atoms present in the alkyl,
alkenyl or alkynyl group. For example, the following groups are encompassed
by a 7 member heteroaralkyl (* represents the attachment point):
N~ N CH3
"Halogen atom" is specifically a fluorine atom, chlorine atom, bromine
atom or iodine atom.
The term "oxo" represents =0.
A dash ("-") that is not between two letters or symbols is used to indicate a
point of attachement for a substitutent. For example, -CONRdRe is attached
through the carbon of the amide.
A bond represented by a combination of a solid and dashed line, ie.
may be either a single or double bond.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
29
The term "guanidino" represents -N(Rd)C(=NRe)NRfRg wherein Rd, Re, Rf
and Rg are each independently selected from H, C1.10 alkyl, C2.10 alkenyl,
C2_10
alkynyl, C6.12 aryl, and C7.12 aralkyl, or Rf and Rg are taken together with
the
nitrogen to which they are attached to form an optionally substituted 4 to 10
member heterocycle or an optionally substituted 5-12 member heteroaryl.
The term "amidino" represents -C(=NRd)NReRf wherein Rd, Re and Rf
are each independently selected from H, C1_10 alkyl, C2_10 alkenyl, C2.10
alkynyl, C6.12 aryl, and C7.12 aralkyl, or Re and Rf are taken together with
the
nitrogen to which they are attached to form an optionally substituted 4 to 10
member heterocycle or an optionally substituted 5-12 member heteroaryl.
The term "hydroxyl protecting group" is well known in the field of
organic chemistry. Such protecting groups may be found in "Protective
Groups in Organic Synthesis" second edition, Wiley-interscience pulblication,
by T.W. Greene and P.G.M. Wuts. Examples of hydroxy protecting groups
include but are not limited to benzyl, acetyl, benzoyl, pivaloyl and
isopropyloxycarbonyl.
When there is a sulfur atom present, the sulfur atom can be at different
oxidation levels, i.e., S, SO, or SO2. All such oxidation levels are within
the scope
of the present invention.
The term "independently" means that a substituent can be the same or a
different definition for each item.
In still another aspect, there is provided a method for prevention or
treatment of HIV infections in a subject in need of such treatment comprising
administering to the subject a therapeutically effective amount of a compound
of
formula (I) or composition of the invention.
In still another aspect, there is provided a method for delaying the onset of
AIDS or treating AIDS in a subject in need of such treatment comprising
administering to the subject a therapeutically effective amount of a compound
of
formula (I) or composition of the invention.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
In another embodiment, there is provided the use of a compound,
composition or combination of the invention for the manufacture of a
medicament
for treating or preventing HIV infections in a subject in need of such
treatment.
In another embodiment, there is provided the use of a compound,
composition or combination of the invention for the manufacture of a
medicament
for blocking cellular entry of HIV in a subject.
In another embodiment, there is provided the use of a compound,
10 composition or combination of the invention for the manufacture of a
medicament
for delaying the onset of AIDS or treating AIDS in a subject in need of such
treatment.
In still another aspect, there is provided a method for blocking cellular
entry of HIV in a subject or for the prevention or treatment of HIV infections
in a
subject in need of such treatment comprising administering to the subject a
pharmaceutical combination comprising at least one compound of formula (I) and
at least one further therapeutic agent.
20 In still another aspect, there is provided a method for delaying the onset
of
AIDS or treating AIDS in a subject in need of such treatment comprising
administering to the subject a pharmaceutical combination comprising. at least
one
compound of formula (I) and at least one further therapeutic agent.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one further therapeutic agent which is an antiviral
agent.
In one embodiment, the pharmaceutical combination of this invention may
contain at least one further antiviral agent which is chosen from nucleoside
and
30 nucleotide analog reverse transcriptase inhibitors, non-nucleoside reverse
transcriptase inhibitors, protease inhibitors, attachment and fusion
inhibitors,
integrase inhibitors, and maturation inhibitors.
In one embodiment, the pharmaceutical combinations of this invention may
contain at least one other antiviral agent which is a nucleoside and
nucleotide
analog reverse transcriptase inhibitors chosen from 3TC (lamivudine,
Epivir(D), AZT
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
31
(zidovudine, Retrovir(D), Emtricitabine (Coviracil , formerly FTC), d4T (2',3'-
dideoxy-2',3'-didehydro-thymidine, stavudine and Zerit ), tenofovir (Viread ),
2',3'-dideoxyinosine (ddl, didanosine, Videx ), 2',3'-dideoxycytidine (ddC,
zalcitabine, Hivid ), Combivir (AZT/3TC or zidovudine/lamivudine
combination),
Trivizir (AZT/3TC/abacavir or zidovudine/lamivudine/abacavir combination),
abacavir (1592U89, Ziagen ), Epzicom (abacavir and lamivudine), Truvada
(Tenofovir and emtricitabine), SPD-754 (apricitabine), Elvucitabine (ACH-
126,443,
(Beta-L-Fd4C), Alovudine (MIV-310), DAPD (amdoxovir), Racivir, phosphazid,
stampidine, CMX-157, PPI-801/802 (formerly MIV-410), MIV-210, fozivudine
tidoxil,
KP-1461, Fosalvudine (HDP 99.0003), 9-[(2-hydroxymethyl)-1,3-dioxolan-4-
yl]guanine, and 2-amino-9-[(2-hydroxymethyl)-1,3-dioxolan-4-yl]adenine.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is a non-nucleoside
reverse
transcriptase inhibitor chosen from Nevirapine (Viramune , NVP, BI-RG-587),
delavirdine (Rescriptor , DLV), efavirenz (DMP 266, Sustiva ), (+)-Calanolide
A,
Capravirine (AG1549, formerly S-1153), DPC083, MIV-150, TMC120, Intelence
(etravirine , TMC125), TMC-278 or BHAP (delavirdine), calanolides, GW695634,
RDEA806, RDEA427, RDEA640, UK-453061, BILR355, VRX 840773 and L-697,661 (2-
Pyridinone 3benzoxazolMeNH derivative).
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is a protease inhibitor
chosen
from nelfinavir (Viracept , NFV), amprenavir (141W94, Agenerase ), indinavir
(MK-639, IDV, Crixivan ), saquinavir (Invirase , Fortovase , SQV), ritonavir
(Norvir , RTV), lopinavir (ABT-378, Kaletra(D), Atazanavir (Reyataz ,
BMS232632),
mozenavir (DMP-450), fosamprenavir (GW433908), R0033-4649, Tipranavir
(Aptivus , PNU-140690), Darunavir (Prezista , TMC114), SPI-256, Brecanavir
(GW640385), P-1946, MK-8122 (formerly PPL-100)and VX-385.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is an attachment and
fusion
inhibitor chosen from T-20 (enfuvirtide, Fuzeon ), T-1249, TRI-999, TRI-1144,
Schering C (SCH-C), Vicriviroc (Schering D, SCH-D), FP21399, PRO-140, PRO 542,
PRO 452, TNX-355, Aplaviroc (GW873140, AK602), TBR-220 (formerly TAK-220),
TBR-652 (formerly TAK-652), PF-232798, Maraviroc (Selzentry , UK-427,857) or
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
32
soluble CD4, CD4 fragments, CD4-hybrid molecules, BMS-806, BMS-488043,
AMD3100, AMD070, AMD887, INCB9471, INCB15050, KRH-2731, KRH-3140, SJ-3366,
SP-01A, sifuvirtide, and KRH-3955.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is an integrase inhibitor
chosen from S-1360, L-870,810, elvitegravir (GS9137, JKT 303), GS9137, L-
870,812,
raltegravir (Isentress6, MK-0518), MK-2048, GSK1349572, and C-2507.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is a maturation inhibitor
chosen from Vivecon (MPC-9055) and Bevirimat PA-457.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is a zinc finger
inhibitor and is
azodicarbonamide (ADA).
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is an antisense drug and
is
HGTV43.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent which is an immunomodulator,
immune stimulator or cytokine chosen from interleukin-2 (IL-2, Aldesleukin,
Proleukin), granulocyte macrophage colony stimulating factor (GM-CSF),
erythropoietin, Multikine, Ampligen, thymomodutin, thymopentin, foscarnet,
HE2000, Reticulose, Murabutide, Resveratrol,. HRG214, HIV-1 Immunogen
(Remune), WHO and EP HIV-1090.
In another embodiment, the pharmaceutical combination of this invention
may contain at least one other antiviral agent chosen from: 2',3'-
dideoxyadenosine,
3'-deoxythymidine, 2',3'-dideoxy-2',3'-didehydrocytidine and ribavirin;
acyclic
nucleosides such as acyclovir, and ganciclovir; interferons such as alpha-,
beta-and
gamma-interferon; glucuronation inhibitors such as probenecid; and TIBO drugs,
HEPT, Pictovir (VGX-410) and TSAO derivatives.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
33
In another embodiment, the pharmaceutical combination of this invention
may contain an inhibitor of the cytochrome P450.
In another embodiment, the pharmaceutical combination of this invention
may contain an inhibitor of the cytochrome P450 chosen from atazanavir,
clarithromycin, indinavir, itraconazole, ketoconazole, nefazodone, nelfinavir,
ritonavir, saquinavir, tetithromycin, amprenavir, erythromycin, fluconazole,
fosamprenavir, grapefruit juice, fluvoxamine, fluoxetine, macrolide
antibiotics, sertraline sulfaphenazole, Troleandomycin, cyclosporine,
clomethiazole, atazanavir, mibefradil, vitamin E, bergamottin,
dihydroxybergamottin, and pharmaceutically acceptable salts thereof.
In another embodiment, the pharmaceutical combination of this invention
may contain an inhibitor of the cytochrome P450 which is ritonavir or a
pharmaceutically acceptable salt thereof.
The combinations referred to above may conveniently be presented for use
in the form of a pharmaceutical formulation and thus pharmaceutical
formulations
comprising a combination as defined above together with a pharmaceutically
acceptable carrier thereof comprises a further aspect of the invention.
The individual components of such combinations may be administered
either sequentially or simultaneously in separate or combined pharmaceutical
formulations.
In a further embodiment, the compound of formula (I) and at least one
further therapeutic agent are administered sequentially.
In a further embodiment, the compound of formula (I) and at least one
further therapeutic agent are administered simultaneously.
Thus, a further embodiment of the invention is a kit for use in administering
a combination, the kit comprising: a first containment means for storing a
compound according to formula I in the form of a pharmaceutical formulation
further comprising a pharmaceutically acceptable carrier; and a second
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
34
containment means for storing at least one further therapeutic agent in the
form of
a pharmaceutical formulation further comprising a pharmaceutically acceptable
carrier.
In one embodiment, the present invention further provides a
pharmaceutical composition comprising at least one compound having the formula
(I) or a pharmaceutically acceptable salt thereof, or a pharmaceutically
acceptable
hydrate thereof, or a pharmaceutically acceptable solvate thereof, and at
least
one pharmaceutically acceptable carrier or excipient.
The terms "host" or "patient" or "subject" means a human, male or
female, for example, a child, an adolescent, or an adult.
It will be appreciated that the amount of a compound of the invention
required for use in treatment will vary not only with the particular compound
selected but also with the route of administration, the nature of the
condition for
which treatment is required and the age and condition of the patient and will
be
ultimately at the discretion of the attendant physician. In general, however,
a
suitable dose will be in the range of from about 0.1 to about 750 mg/kg of
body
weight per day, for example, in the range of 0.5 to 60 mg/kg/day, or, for
example,
in the range of 1 to 20 mg/kg/day.
The desired dose may conveniently be presented in a single dose or as
divided dose administered at appropriate intervals, for example as two, three,
four
or more doses per day.
The compound is conveniently administered in unit dosage form; for
.example containing 10 to 1500 mg, conveniently 20 to 1000 mg, most
conveniently
50 to 700 mg of active ingredient per unit dosage form.
Ideally the active ingredient should be administered to achieve peak plasma
concentrations of the active compound of from about 1 to about 75pM, about 2
to
50 pM, about 3 to about 30 pM. This may be achieved, for example, by the
intravenous injection of a 0.1 to 5% solution of the active ingredient,
optionally in
saline, or orally administered as a bolus containing about 1 to about 500 mg
of the
active ingredient. Desirable blood levels may be maintained by a continuous
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
infusion to provide about 0.01 to about 5.0 mg/kg/hour or by intermittent
infusions
containing about 0.4 to about 15 mg/kg of the active ingredient.
When a compound of the present invention or a pharmaceutically
acceptable salt thereof is used in combination with a second therapeutic agent
active against the same virus, the dose of each compound may be either the
same
as or differ from that when the compound is used alone. Appropriate doses will
be
readily appreciated by those skilled in the art.
10 While it is possible that, for use in therapy, a compound of the invention
may be administered as the raw chemical, it is preferable to present the
active
ingredient as a pharmaceutical composition. The invention thus further
provides a
pharmaceutical composition comprising a compound of the present invention or a
pharmaceutically acceptable salt thereof together with one or more
pharmaceutically acceptable carriers therefore and, optionally, other
therapeutic
and/or prophylactic ingredients. The carrier(s) must be "acceptable" in the
sense
of being compatible with the other ingredients of the formulation and not
deleterious to the recipient thereof.
20 Pharmaceutical compositions include those suitable for oral, rectal, nasal,
topical (including buccal and sub-lingual), transdermaL, vaginal or parenteral
(including intramuscular, sub-cutaneous and intravenous) administration or in
a
form suitable for administration by inhalation or insufflation. The
formulations
may, where appropriate, be conveniently presented in discrete dosage units and
may be prepared by any of the methods well known in the art of pharmacy. All
methods include the step of bringing into association the active compound with
liquid carriers or finely divided solid carriers or both and then, if
necessary,
shaping the product into the desired formulation.
30 Pharmaceutical compositions suitable for oral administration may
conveniently be presented as discrete units such as capsules, cachets or
tablets
each containing a predetermined amount of the active ingredient; as a powder
or
granules; as a solution, a suspension, or as an emulsion. The active
ingredient may
also be presented as a bolus, electuary or paste. Tablets and capsules for
oral
administration may contain conventional excipients such as binding agents,
fillers,
lubricants, disintegrants, or wetting agents. The tablets may be coated
according
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
36
to methods well known in the art. Oral liquid preparations may be in the form
of,
for example, aqueous or oily suspensions, solutions, emulsions, syrups or
elixirs, or
may be presented as a dry product for constitution with water or other
suitable
vehicle before use. Such liquid preparations may contain conventional
additives
such as suspending agents, emulsifying agents, non-aqueous vehicles (which may
include edible oils), or preservatives.
The compounds according to the invention may also be formulated for
parenteral administration (e.g. by injection, for example, bolus injection or
continuous infusion) and may be presented in unit dose form in ampoules, pre-
filled syringes, small volume infusion or in multi-dose containers with an
added
preservative. The compositions may take such forms as suspensions, solutions,
or
emulsions in oily or aqueous vehicles, and may contain formulatory agents such
as
suspending, stabilizing and/or dispersing agents. Alternatively, the active
ingredient may be in powder form, obtained by aseptic isolation of sterile
solid or
by lyophilization from solution, for constitution with a suitable vehicle,
e.g. sterile,
pyrogen-free water, before use.
For topical administration to the epidermis, the compounds according to
the invention may be formulated as ointments, creams or lotions, or as a
transdermal patch. Such transdermal patches may contain penetration enhancers
such as linalool, carvacrol, thymol, citral, menthol and t-anethole. Ointments
and
creams may, for example, be formulated with an aqueous or oily base with the
addition of suitable thickening and/or gelling agents. Lotions may be
formulated
with an aqueous or oily base and will in general also contain one or more
emulsifying agents, stabilizing agents, dispersing agents, suspending agents,
thickening agents, or coloring agents.
Compositions suitable for topical administration in, the mouth include
lozenges comprising active ingredient in a flavored base, usually sucrose and
acacia
or tragacanth; pastilles comprising the active ingredient in an inert base
such as
gelatin and glycerin or sucrose and acacia; and mouthwashes comprising the
active
ingredient in a suitable liquid carrier.
Pharmaceutical compositions suitable for rectal administration wherein the
carrier is a solid are, for example, presented as unit dose suppositories.
Suitable
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
37
carriers include cocoa butter and other materials commonly used in the art,
and
the suppositories may be conveniently formed by admixture of the active
compound with the softened or melted carrier(s) followed by chilling and
shaping
in moulds.
Compositions suitable for vaginal administration may be presented as
pessaries, tampons, creams, gels, pastes, foams or sprays containing in
addition to
the active ingredient such carriers as are known in the art to be appropriate.
For intra-nasal administration the compounds of the invention may be used
as a liquid spray or dispersible powder or in the form of drops. Drops may be
formulated with an aqueous or non-aqueous base also comprising one more
dispersing agents, solubilizing agents, or suspending agents. Liquid sprays
are
conveniently delivered from pressurized packs..
For administration by inhalation the compounds according to the invention
are conveniently delivered from an insufflator, nebulizer or a pressurized
pack or
other convenient means of delivering an aerosol spray. Pressurized packs may
comprise a suitable propellant such as dichlorodifluoromethane,
trichlorofluoromethane, dichlorotetrafluoroethane, carbon dioxide or other
suitable gas. In the case of a pressurized aerosol the dosage unit may be
determined by providing a valve to deliver a metered amount.
Alternatively, for administration by inhalation or insufflation, the
compounds according to the invention may take the form of a dry powder
composition, for example a powder mix of the compound and a suitable powder
base such as lactose or starch. The powder composition may be presented in
unit
dosage form in, for example, capsules or cartridges or, e.g., gelatin or
blister
packs from which the powder may be administered with the aid of an inhalator
or
insufflator.
When desired the above described formulations adapted to give sustained
release of the active ingredient may be employed.
Compounds according to the present invention include:
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
38
Cpd# Name
14-1 3/30-(3', 3'-Dimethylsuccinyl)-17/3-tert-butyloxycarbonylamino-
21-oxolup-18-ene;
14-2 3/3-0-(cis-Cyclohexane-3'-carboxylic acid-1'-carboxyl)-17/-
[N -tert-butyloxycarbonyl-amino]-21-oxolup-18-ene;
3/3-0-[(1 ' R, 3' S)-1 ', 2', 2' -Trimethyl-cyclopentane-3' -carboxylic
14-3 acid- 1'-carboxyl]-17/3[N-tert-butyloxycarbonyl-amino]-21-
oxolup-18-ene;
15-1 3/3 0-(3',3'-Dimethylsuccinyl)-17/3 amino-21-oxolup-18-ene;
15-2 3/3-0-(cis -Cyclohexane-3'-carboxylic acid-1'-carboxyl)-17/3
amino-21-oxolup-18-ene;
15-3 3Q-0-[(1'R,3'S)-1',2',2'-Trimethyl-cyclopentane-3'-carboxylic
acid- l' -carboxyl] -17,6-amino-21 -oxotup- 1 8-ene;
16-1 3/3 0-(3',3'-Dimethylsuccinyl)-17/3 (1-tert-
butyloxycarbonylpiperidine-4-amino]-21-oxolup-18-ene;
16-2 3/30-(3',3'-Dimethylsuccinyl)-17/3(methylamino)-21-oxolup-
18-ene;
17-1 3/3-0-(3',3'-Dimethylsuccinyl)-17/3 [N'-(benzyl)ureido]-21-
oxolup-l8-ene;
17-2 3/3 0-(3',3'-Dimethylsuccinyl)-17/3[N'-(ethyl)ureido]-21-
oxolup-l8-ene;
17-3 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(phenyl)ureido]-21-
oxolup-18-ene;
17-4 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(isopropyl)ureido]-21-
oxolup-18-ene;
17-5 3/30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(tert-butyl)ureido]-21-
oxolup-18-ene;
17-6 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(pyridin-3-yl)ureido]-21-
oxolup-18-ene;
17-7 3/30-(3',3'-Dimethylsuccinyl)-17/-[N'-(cyclohexyl)ureido]-21-
oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
39
17-8 3/30-(3',3'-Dimethylsuccinyl)-17/3[N'-
(cyclohexylmethyl)ureido]-21-oxolup-18-ene;
17-9 3/30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-tert-
butyloxycarbonyt-piperidin-4-yl)-ureido]-21-oxolup-18-ene;
17-10 3/30-(3',3'-Dimethylsuccinyl)-17/l-(N'-piperidin-4-yl-ureido)-
21-oxolup-18-ene;
17-11 3/3-0-(3' , 3'-Dimethylsuccinyl)-17/3 [N'-(1-acetyl piperidin-4-yl)-
ureido]-21-oxolup-18-ene;
17-12 3/30-(3',3'-Dimethylsuccinyl)-17/3[N'-(pyridin-3-
y1methyl)ureido] -21-oxolup-18-ene;
17-13 3/30-(3',3'-Dimethylsuccinyl)-17,8 [(morpholine-4-carbonyl)-
amino]-21-oxolup-18-ene;
17-14 3/30-(3',3'-Dimethylsuccinyl)-17/3[(4-tert-butyloxycarbonyl-
piperazine-1-carbonyl)-amino]-21-oxolup-18-ene;
17-15 3/30-(3',3'-Dimethylsuccinyt)-17/3-[(piperazine-1-carbonyl)-
amino]-21-oxolup-18-ene;
17-16 3/30-(3',3'-Dimethylsuccinyl)-17/3[(4-methyl-piperazine-1-
carbonyl)-ami no] -21-oxolup-18-ene;
17-17 3/3 0-(3',3'-Dimethylsuccinyl)-17/3 [(4-acetyl-piperazine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-18 3/-0-(3',3'-Dimethylsuccinyl)-173 [N'-isopropyl-N'-methyl-
ureido]-21-oxolup-18-ene;
17-19 3/30-(3',3'-Dimethylsuccinyl)-17/3-(N'-5-methyl-
[1,3,4]oxadiazol-2-yl-ureido)-21-oxolup-18-ene;
3/-0-(3', 3'-Dimethylsuccinyl)-17/3[(4-tert-
17-20 butoxycarbonylamino-piperidine-1-carbonyl)-amino]-21-
oxolup-18-ene;
17-21 3/30-(3',3'-Dimethylsuccinyl)-178 (N'-cyclopropylmethyl-
ureido)-21-oxolup-18-ene;
17-22 3/3 0-(3',3'-Dimethylsuccinyl)-17,Q(N'-cyctopropyl-ureido)-21-
oxotup-18-ene;
17-23 3/30-(3',3'-Dimethylsuccinyl)-17/3[(4-amino-piperidine-1-
carbonyl)-amino]-21-oxolup-18-ene trifluoroacetate;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
17-24 3/3-0-(3',3'-Dimethylsuccinyl)-17,Q[(4-amino-piperidine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-25 330-(3',3'-Dimethylsuccinyl)-17,13-(N'-pyridin-2-yl-ureido)-21-
oxolup-18-ene;
17-26 3,13 0-(3',3'-Dimethylsuccinyl)-17/3(N'-pyridin-4-yl-ureido)-21-
oxotup-18-ene;
17-27 3/30-(3',3'-Dimethylsuccinyl)-17,&[N'-(pyridin-2-
ytmethyl)ureido]-21-oxolup-1 8-ene;
17-28 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-methyl-piperidin-4-
ylmethyl)-ureido]-21-oxolup-l8-ene;
17-29 3/30-(3',3'-Dimethylsuccinyl)-17/3[(4-isopropyl-piperazine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-30 3/3-0-(3',3'-Dimethylsuccinyl)-17/3[N'-(1-methyl-piperidin-4-
yl)- ureido]-21-oxolup-18-ene;
17-31 3/3-0-(3', 3'-Dimethylsuccinyl)-17/3-[N'-(pyridin-4-
ytmethyl)ureido]-21-oxolup-18-ene;
17-32 3,Q 0-(3',3'-Dimethylsuccinyl)-17#-(N'-pyrimidin-2-yl-ureido)-
21-oxolup-18-ene;
17-33 3,Q0-(3',3'-Dimethylsuccinyl)-17p-[N'-(1-tert-
butyloxycarbonyl-azetidin-3-yl)- ureido]-21-oxolup-18-ene;
3/3 0-(3',3'-Dimethylsuccinyl)-17,(3-[(3-(S)-1-tert-
17-34 butyloxycarbonyl-pyrrolidin-2-ytmethyl)-ureido]-21-oxolup-18-
ene;
17-35 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-[azetidin-3-yl-ureido)]-21-
oxolup-18-ene;
3/30- (3', 3' -Dimethylsuccinyl)-173- [(4- (tert-butyloxycarbonyl-
17-36 methyl-amino)-piperidine-1-carbonyl)-amino]-21-oxolup-18-
ene;
17-37 31.30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-methyl-1 H-pyrazol-4-
yt)ureido]-21-oxolup-18-ene;
17-38 3/3-0-(3',3'-Dimethylsuccinyl)-17,13 (3-(S)-1-pyrrolidin-2-
ytmethyl-ureido)-21-oxolup-18-ene;
17-39 3/3-0- (3',3'-Dimethylsuccinyl)-17,(3[(4-methylamino-piperidine-
1-carbonyl)-amino]-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
41
3/30-(3',3'-Dimethylsuccinyl)-17/3[((R)-3-tert-
17-40 butyloxycarbonyl amino- pyrrolidine-1-carbonyl)-amino]-21-
oxotup-18-ene;
3/30-(3',3' Dimethylsuccinyl)-17/3-[((S)-3-tert-
17-41 butyloxycarbonyl amino- pyrrolidine-1-carbonyl)-amino]-21-
oxolup-l8-ene;
17-42 3/30-(3',3'-Dimethylsuccinyl)-17/J-[((R)-3-amino-pyrrolidine-1-
carbonyt)-amino]-21-oxolup-1 8-ene;
17-43 3/3 0-(3',3'-Dimethylsuccinyl)-17/3 [((S)-3-amino-pyrrolidine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-44 3/30-(3',3'-Dimethylsuccinyl)-17/3-(N'-pyrimidin-5-yl-ureido)-
21-oxolup-18-ene;
17-45 3/30-(3',3'-Dimethylsuccinyl)-17/3(N'-5-methyl-
[1, 3, 4]thiadiazol-2-yl-ureido)-21-oxolup-18-ene;
17-46 3,8-0- (3',3'-Dimethylsuccinyl)-17/3-(N'-pyrimidin-4-yl-ureido)-
21-oxolup-18-ene;
17-47 3/30-(3',3'-Dimethylsuccinyl)-17/-(N'-2-methylpyridin-4-yl-
ureido)-21-oxolup-18-ene;
17-48 3/3-0- (3',3'-Dimethylsuccinyl)-173 [N'N'-dimethyl-ureido]-21-
oxolup-18-ene;
17-50 3/30-(3',3'-Dimethylsuccinyl)-17/3[N'-((S)-2,2,2-trifluoro-1-
methyl-ethyl)-ureido]-21-oxolup-18-ene;
17-51 3/30-(3',3'-Dimethylsuccinyl)-17/3[N'-((R)-2,2,2-trifluoro-1-
methyl- ethyl) -ureido] -21-oxolup-18-ene;
17-52 3/30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(pyrimidin-4-
ylmethyl)ureido]-21-oxolup-18-ene;
17-53 3/30-(3',3'-Dimethylsuccinyl)-17/3[N'-(1-methyl-1 -pyridin-2-
yl-ethyt)ureido]-21-oxolup-18-ene;
17-54 3/3-0-(3',3'-Dimethylsuccinyl)-17/3 [N'-((S)-1-cyclohexyl-
ethyl)ureido]-21-oxolup-18-ene;
17-55 3/30-(3',3'-Dimethylsuccinyl)-17/-[N'-((R)-1-cyclohexyl-
ethyl)ureido] -21 -oxolup- 1 8-ene;
17-56 3/30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(2,2,2-trifluoro-1,1-
dimethyl- ethyl) ureido]-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
42
17-57 3#-0-(3',3'-Dimethylsuccinyl)-17#-[N'-.(4,4-
difluorocyclohexyl)ureido]-21-oxolup-18-ene;
17-58 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-(3,3-difluoro-pyrrolidine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-59 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[(4,4-difluoro-piperidine-1-
carbonyl)-amino]-21-oxolup-18-ene;
17-60 3/3-0-(3',3'-Dimethylsuccinyl)-17,6-[N'-(4,4-difluoro-cyclohexyl-
methyl)ureido]-21-oxolup-18-ene;
17-61 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-((S)-1-phenyl-ethyl)-
ureido]-21-oxolup-18-ene;
17-62 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-((R)-1-phenyl -ethyl)-
ureido]-21-oxolup-18-ene;
17-63 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-methyl-1 -phenyl-
ethyl)-ureido]-21-oxolup-18-ene;
17-64 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(thiophen-2-ylmethyl)-
ureido]-21-oxolup-1 8-ene;
17-65 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(2-methyl-
[1,3,4]oxadiazol-2-ylmethyl)-ureido]-21-oxolup-18-ene;
17-66 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-phenyl-cyclopropyl)-
ureido]-21-oxolup-18-ene;
17-67 3/.3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(pyrimidin-2-
y1methyl)ureido] -21-oxolup-18-ene;
17-68 3/3-0-(3',3'-Dimethylsuccinyl)-l7/3-[N'-(pyrimidin-5-
ylmethyl)ureido]-21-oxolup-18-ene;
17-69 3/3-0-(3',3'-Dimethylsuccinyl)-l7/3-[N'-(1-pyridin-2-yl-
cyclopropyl)-ureido]-21-oxolup-18-ene;
17-70 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(l-methyl-1 -cyclohexyl-
ethyl)-ureido]-21-oxolup-18-ene;
17-72 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(1-pyridin-2-yl-ethyl)-
ureido]-21-oxolup-18-ene;
17-73 3/3-0-(3',3'-Dimethylsuccinyl)-1 7,6-[N'-(1-methyl-1 H-imidazol-
2-yl-methyl)-ureido]-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
43
17-74 3#30-(3',3'-Dimethylsuccinyl)-17/3-[N'-(4-methyl-4H-
[1,2,4]triazol-3-yi-methyl)-ureido]-21-oxolup-18-ene;
17-75 3/3-0- (3',3'-Dimethylsuccinyl)-17/3 [N'-(thiophen-3-ytmethyl)-
ureido]-21-oxolup-18-ene;
17-76 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-[N'-(2-methy[-2H-pyrazol-3-
yl-methyl)-ureido]-21 oxolup-1 8-ene;
18-1 3,130-(3',3'-Dimethylsuccinyl)-17/3-methylsulfonylamino-21-
oxotup-18-ene;
19-1 3/30-(3', 3'-Dimethylsuccinyl)-l 7/3acetylamino-21-oxolup-18-
ene;
19-2 33-0-(3',3'-Dimethylsuccinyl)-17/3benzamido-21-oxolup-18-
ene;
19-3 3/3-0,1 7/3-N-bis(3',3'-dimethylsuccinyl)-21-oxolup-18-ene;
19-4 3/-0-(3',3'-Dimethylsuccinyl)-17/3-(5-methyl-
[1,3,4]oxadiazole-2-amido)-21-oxolup-18-ene;
19-5 3/30-(3',3'-Dimethylsuccinyl)-17/3(1-tert-
butyloxycarbonylpiperidine-4-amido)-21-oxolup-18-ene;
19-6 3/30-(3',3'-Dimethylsuccinyl)-17/3(piperidine-4-amido)-21-
oxolup-18-ene;
19-7 3/3 0-(3',3'-Dimethylsuccinyl)-17/3(1-acetylpiperidine-4-
amido)-21-oxolup-18-ene;
19-8 3f-0-(3',3'-Dimethylsuccinyl)-l 7/3 (pyridin-3-yl)amido-21-
oxotup-18-ene;
19-9 3/3-0- (3',3'-Dimethylsuccinyl)-17/3(4-fluorobenzyl)amido-21-
oxolup-18-ene;
19-10 3/30-(3',3'-Dimethylsuccinyl)-17/3benzamido-21-oxolup-18-
ene;
19-11 3,130-(3',3'-Dimethylsuccinyl)-17,13(2-fluorobenzamido)-21-
oxolup-18-ene;
19-12 3/3-0-(3',3'-Dimethy(succinyl)-17/3(3-fluorobenzamido)-21-
oxotup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
44
19-13 3/3-0-(3',3'-Dimethylsuccinyl)-17,0(4-fluorobenzamido)-21-
oxolup-18-ene;
19-14 3fl-0-(3',3'-Dimethylsuccinyl)-17/3 (pyridin4-yl)amido-21-
oxolup-18-ene;
19-15 330-(3',3'-Dimethylsuccinyl)-17/3(pyridin-2-yl)amido-21-
oxolup-18-ene;
19-16 3,00-(3',3'-Dimethylsuccinyl)-17,6-(pyrazin-2-yl)amido-21-
oxolup-18-ene;
19-17 3,8- 0-(3',3'-Dimethylsuccinyl)-17/3(1-methyl- 1 H-pyrazole-3-
amido)-21-oxolup-18-ene;
19-18 3/30-(3',3'-Dimethylsuccinyl)-17/3(1-methyl- 1 H-imidazole-5-
amido)-21-oxolup-l'8-ene;
19-19 3/30-(3',3'-Dimethylsuccinyl)-17/-(1-methyl-1 H-imidazole-4-
amido)-21-oxolup-18-ene;
19-20 3,8- 0-(3',3'-Dimethylsuccinyl)-17/3(2-thiophene)amido-21-
oxolup-18-ene;
19-21 3/30-(3',3'-Dimethylsuccinyl)-17/3-(1,3-thiazole-2-amido)-21-
oxolup-18-ene;
19-22 3,00-(3',3'-Dimethylsuccinyl)-17/3(pyrimidi ne-2-amido)-21-
oxolup-l8-ene;
19-23 3,00-(3',3'-Dimethylsuccinyl)-1718-(pyri midi ne-5-amido)-21-
oxolup-18-ene;
19-24 3/30-(3',3'-Dimethylsuccinyl)-17 3 (5-methylisoxazole-3-
amido)-21-oxolup-18-ene;
19-25 3/3 0-(3',3'-Dimethylsuccinyl)-1718-(pyrimidi ne-4-amido)-21-
oxolup-18-ene;
19-26 3,00-(3',3'-Dimethylsuccinyl)-17/3(1-methyl- 1 H-imidazole-2-
amido)-21-oxolup-18-ene;
19-27 3/3 0-(3',3'-Dimethylsuccinyl)-17#3(1,3-thiazole-4-amido)-21-
oxolup-18-ene;
19-28 3/30-(3',3'-Dimethylsuccinyl)-17,3(1,3-thiazole-5-amido)-21-
oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
19-29 3/.-0-(3',3'-Dimethylsuccinyl)-17#-phenylacetamido-21-oxolup-
18-ene;
19-30 3/-0-(3',3'-Dimethytsuccinyt)-17/3-tetrahydro-pyran-4-
carbonylamino-21-oxolup-18-ene;
19-31 3/3-0- (3', 3'-Dimethylsuccinyl)-17/3-isopropytamido-21-oxotup-
18-ene;
19-32 3/-0-(3',3'-Dimethylsuccinyl)-17/itert-butylamido-21-oxolup-
18-ene;
19-33 3/-0-(3',3'-Dimethylsuccinyl)-1 7/3 cyclohexylamido-21-oxolup-
18-ene;
19-34 3/30-(3',3'-Dimethylsuccinyl)-17,8-(1 -methyl-1 H-pyrazole-5-
amido)-21 -oxotup-1 8-ene;
19-35 3/30-(3',3'-Dimethylsuccinyl)-17/3(1-methyl-1 H-pyrazole-4-
amido)-21-oxolup-1 8-ene;
19-36 3/30-(3',3'-Dimethylsucciny()-17/3(1-methylpiperidine-4-
amido)-21-oxolup-18-ene;
19-37 3/30-(3',3'-Dimethylsuccinyl)-17/3(3-pyrrolidin-1-yl-
propionamido)-21-oxolup-18-ene;
19-38 3/30-(3',3'-Dimethylsuccinyl)-17/3(2-pyrrolidin-1-yl-
acetamido)-21-oxolup-18-ene;
19-39 3/30-(3',3'-Dimethylsuccinyt)-17/3(4-pyrrolidin-1-yl-
butyramido)-21-oxolup-18-ene;
19-40 3/30-(3',3'-Dimethylsuccinyl)-17/3(3-piperidin-1-yl-
propionamido)-21-oxolup-1 8-ene;
19-41 3/3 0-(3',3'-Dimethylsuccinyl)-17/3 [3-(2-oxo-pyrrolidin-1-yi)-
propionamido]-21-oxolup-1 8-ene;
19-42 3/30-(3',3'-Dimethylsuccinyl)-17/3(5)-1-methyl-pyrrolidine-2-
amido]-21-oxolup-18-ene;
19-43 3/30-(3',3'-Dimethylsuccinyl)-17,8 (4-methytbenzamido)-21-
oxotup-18-ene;
19-44 3/30-(3',3'-Dimethylsuccinyl)-17/3(3-methylbenzamido)-21-
oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
46
19-45 3/-0-(3',3'-Dimethylsuccinyl)-17/3-(2-methylbenzamido)-21-
oxolup-18-ene;
19-46 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-(2-methylpyridin-3-
yl )ami do-21-oxolup-18-ene;
19-47 3/30-(3',3'-Dimethylsuccinyl)-17/3 (5-methylpyridin-3-
yl)amido-21-oxolup-18-ene;
19-48 3/-0-(3',3'-Dimethylsuccinyl)-17/3(6-methylpyridin-3-
yl)amido-21-oxolup-18-ene;
19-49 3/30-(3',3'-Dimethylsuccinyl)-17/3(4-methylpyridin-3-
yt)amido-21-oxolup-18-ene;
19-50 3/3-0-(3',3'-Dimethylsuccinyl)-17/3 (4-hydroxypyridin-3-
yl)amido-21-oxolup-18-ene;
19-51 3/3-0-(3',3'-Dimethylsuccinyl)-17/3 (2-hydroxypyridin-3-
yt)amido-21-oxolup-18-ene;
19-52 3/30-(3',3'-Dimethylsuccinyl)-17/3(2-methylpyridin-4-
yt )amido-21-oxolup-18-ene;
19-53 3/30-(3',3'-Dimethylsuccinyl)-17/.3-(2-dimethylamino-
acetamido)-21-oxolup-18-ene;
19-54 3/3 0-(3',3'-Dimethylsuccinyl)-17/3(4-methylpiperazine-1-
acetamido)-21-oxolup-18-ene;
19-55 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-(R)-1-methyl-pyrrolidine-2-
amido]-21-oxolup-18-ene;
19-56 3/3-0-(3',3'-Dimethylsuccinyl)-17/-(S)-1-isopropyl-pyrrolidine-
2-amido]-21-oxolup-18-ene;
19-57 3/30-(3',3'-Dimethylsuccinyl)-17/3 (S)-1-tert-butytoxycarbonyt-
pyrrolidine-2-amido]-21-oxolup-18-ene;
19-58 3/-0-(3',3'-Dimethylsuccinyl)-17/3-(R)-1-tert-butytoxycarbonyt-
pyrrolidine-2-amido]-21-oxolup-18-ene;
19-59 3/30-(3',3'-Dimethylsucciny()-173 ((RS)-2-dimethytamino-
propionamido)-21-oxolup-18-ene;
19-60 3/30-(3',3'-Dimethylsuccinyl)-17/3(1-imidazole-acetamido)-21-
oxotup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
47
19-61 3/30-(3', 3'-Dimethylsuccinyl)-17/3(2-carboxy-benzamido)-21-
oxolup-18-ene;
19-62 3/30-(3',3'-Dimethylsuccinyl)-17/3(5-cyclopropyl-
[1,3,4]oxadiazole-2-amido)-21-oxolup-18-ene;
19-63 3/3-0-(3',3'-Dimethylsuccinyl)-17/3 (5-phenyl-[1,3,4]oxadiazole-
2-amido)-21-oxolup-18-ene;
19-64 3/30-(3',3'-Dimethylsuccinyl)-17/3-(2,6-dimethylpyridin-4-
yl)amido-21-oxolup-18-ene;
19-65 3/30-(3',3'-Dimethylsuccinyl)-17/3-((S)-1-methylpiperidine-2-
amido)-21-oxolup-18-ene;
19-66 3/30-(3',3'-Dimethylsuccinyl)-17/3-((R)-1-methylpiperidine-2-
amido)-21-oxolup-18-ene;
19-67 3/3-0-(3',3'-Dimethylsuccinyl)-17/3(3-methyl-
[1,2,4]oxadiazole-5-amido)-21-oxolup-18-ene;
19-68 3/30-(3',3'-Dimethylsuccinyl)-17/3-(5-methyl-
[1,2,4]oxadiazole-3-amido)-21-oxolup-18-ene;
19-69 3/30-(3',3'-Dimethylsuccinyl)-17/3 (5-methyl-
[1, 3,4]oxadiazole-2-methyl-amido)-21-oxolup-18-ene;
19-70 3/30-(3',3'-Dimethylsuccinyl)-173 (8/3oxa-
bicyclo[3.2.1 ]octane-3-amido)-2t-oxolup-18-ene;
19-71 3/30-(3',3'-Dimethylsuccinyl)-17/3 (8a-oxa-
bicyclo[3.2.1 ]octane-3-amido)-21-oxolup-18-ene;
19-72 3/30-(3',3'-Dimethylsuccinyl)-17/3(2-methyl-1,3-thiazole-4-
amido)-21-oxolup-18-ene;
19-73 3/30-(3',3'-Dimethylsuccinyl)-17/3 (1-azetidin-acetamido)-21-
oxolup-18-ene;
19-74 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-(N',N'-dimethyl-oxalamido)
21-oxolup-18-ene;
19-75 3/30-(3',3'-Dimethylglutaryl)-17/3-(5-methyl- [1,3,4]oxadiazole-
2-amido)-21-oxolup-18-ene;
19-76 3/30-(3',3'-Dimethylsuccinyl)-17/3(2-methyl- l,3-oxazole-4-
amido)-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
48
19-77 3/30-(3',3'-Dimethylsuccinyl)-17/3(5-methyl-thiophene-2-
amido)-21-oxolup-18-ene;
19-78 3/30-(3',3'-Dimethylsuccinyl)-17,8-(thiophene-3-amido)-21-
oxolup-18-ene;
19-79 3/30-(3',3'-Dimethylsuccinyl)-17,6-(5-ethyl-[1,3,4]-oxadiazole-
2-amido)-21-oxolup-18-ene;
19-80 3/30-(3',3'-Dimethylsuccinyl)-17,(3(5-methyl-[1,3]-oxazole-2-
amido)-21-oxolup-18-ene;
19-81 3/3-0-(cis-Cyclohexane-3'-carboxylic acid-1'-carboxyl)-17,(3[(5-
methyl- [1, 3,4]oxadiazol-2-carbonyl)-amino]-21-oxolup-18-ene;
19-82 3/30-(3',3'-Dimethylsuccinyl)-17/3(1 H-pyrazole-4-amido)-21-
oxolup-18-ene;
19-84 3/3-0-(3',3'-Dimethylsuccinyl)-17,(3(5-isopropyl-[1,3,4]-
oxadiazole-2-amido)-21-oxolup-18-ene;
20-1 3/3-0-(3', 3'-Dimethylsuccinyl)-17/3methoxycarbonylamino-21-
oxolup-18-ene;
20-2 3/3-0-(3',3'-Dimethylsuccinyl)-17/3isopropyloxycarbonylamino-
21-oxolup-18-ene;
20-3 3/3 0-(3',3'-Dimethylsuccinyl)-17/3
cyclopropylmethoxycarbonylamino-21-oxolup-18-ene;
20-4 3/30-(3',3'-Dimethylsuccinyl)-17/3
cyclopentyloxycarbonylamino-2l-oxolup-18-ene;
20-5 3/30-(3',3'-Dimethylsuccinyl)-17/3
cyclohexyloxyca rbonylami no-21-oxolup-18-ene;
20-6 3/30-(3',3'-Dimethylsuccinyl)-17/3[(1-carboxylic acid tert-
butyl ester) -piperidine-4-oxycarbonylamino]-21-oxolup-18-ene;
20-7 3/30-(3',3'-Dimethylsuccinyl)-17/3
cyclohexylmethoxycarbonylamino-21-oxolup-18-ene;
20-8 3/30-(3',3'-Dimethylsuccinyl)-17/3 benzyloxycarbonylamino-21-
oxolup-18-ene;
20-9 3/30-(3',3'-Dimethylsuccinyl)-17/3
cyclobutyloxycarbonylamino-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
49
20-10 3/3-0-(3',3'-Dimethylsuccinyl)-17,3-(1-
cyclopropylethoxycarbonylamino)-21-oxolup-18-ene;
20-11 3/3 0-(3',3'-Dimethylsuccinyl)-17,(3 (1-methyl-
cyclopropylmethoxycarbonylamino)-21-oxolup-18-ene;
20-12 3/30-(3',3'-Dimethylsuccinyl)-17,3-(1-amino
cyclopropylmethoxycarbonylamino)-21-oxolup-18-ene;
20-13 3/30-(3',3'-Dimethylsuccinyl)-17/3 ((R)-1-methylpyrrolidine-3-
oxycarbonylamino)-21-oxolup-18-ene;
20-14 3/30-(3',3'-Dimethylsuccinyl)-17/3 (tetrahydro-thiopyran-4-
oxycarbonylamino)-21 -oxolup-18-ene;
20-15 3/3-0-(3',3'-Dimethylsuccinyl)-17/3-(1,1-dioxo-tetrahydro-
thiopyran-4-oxycarbonylami no)-21-oxolup-18-ene;
20-16 3/30-(3',3'-Dimethylsuccinyl)-17/3-(piperidine-4-
oxycarbonylamino)-21-oxolup-18-ene;
20-17 3/3-0- (3',3'-Dimethylsuccinyl)-17,8-(1 -methylpiperidine-4-
oxycarbonylamino)-21 -oxolup-18-ene;
20-18 3/30-(3',3'-Dimethylsuccinyl)-17,3-(1-methylpiperidine-4-
methoxycarbonylamino)-21-oxolup-18-ene;
20-19 3/3 0-(3',3'-Dimethylsuccinyl)-17/3(1-methylpiperidine-3-
methoxycarbonylamino)-21-oxolup-18-ene;
20-20 3/3 0-(3',3'-Dimethylsuccinyl)-17/3-((S)1-methylpyrrolidine-2-
methoxycarbonylami no)-21-oxolup-18-ene;
20-21 3/-0-(3',3'-Dimethylsuccinyl)-l 7/3 (1-methylpyrrolidine-3-
methoxycarbonylamino)-21-oxolup-18-ene;
20-22 3/-0-(3',3'-Dimethylsuccinyl)-17/3(2-cyclopropy)-1-
ethoxycarbonylamino)-21-oxolup-18-ene;
20-23 3/30-(3',3'-Dimethylsuccinyl)-17,3-(2-pyrrolidin-1-
ethoxycarbonylamino)-21-oxolup-18-ene;
20-24 3/30-(3',3'-Dimethylsuccinyl)-17/3(2-(2-oxo-pyrrolidin)-1-
ethoxycarbonylamino)-21-oxolup-18-ene;
24-1 1 7/36enzamido-3/3hyd roxy-21-oxolup-18-ene;
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
24-2 17,&(pyridin-2-yl)amido-3/3-hydroxy-21-oxolup-18-ene; and
25-1 17/3-(isopropylcarbonylamino)-3,8-hydroxy-21-oxolup-18-ene;
and pharmaceutically acceptable salts thereof.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
51
EXAMPLES
The following general schemes and examples are provided to illustrate
various embodiments of the present invention and shall not be considered as
limiting in scope. It will be appreciated by those of skill in the art that
other
compounds of the present invention can be obtained by substituting the
generically
or specifically described reactants and/or operating conditions used in the
following examples.
In the foregoing and in the following examples, all temperatures are set
forth uncorrected in degrees Celsius; and, unless otherwise indicated, all
parts and
percentages are by weight.
Analytical HPLC is carried out under standard conditions using a
Phenomenex Gemini C18 column, 250 x 4.6 mm, 3 m, 11OA for the methods A, B,
C, D and E, a Varian pursuit XRs C18 column, 50 x 4.6 mm, 3 m, for the
methods
F, G, H and I and a Waters SymmetryShield C18 column, 250 x 4.6 mm, 5 m for
the method J. Elution is performed using a linear gradient with a flow rate of
1
mL/min. as described in the following table (Solvent A is 0.01% TFA in H20;
solvent
B is 0.01% TFA in CH3CN):
Methods A B C D E
Solvent B 50 to 90% 60 to 100% 30 to 70% 20 to 60% 40 to 80%
over 40 min over 40 min over 40 min over 40 min over 40 min
Methods F G H I J
Solvent B 50 to 95% 15 to 45% 30 to 75% 15 to 60% 80 to 90%
over 15 min over 15 min over 15 min over 15 min over 20 min
The following abbreviations may be used as follows:
Ac acetyl
AcOEt Ethyl acetate
AcOH Acetic acid
Ac20 Acetic anhydride
(Boc)20 di-tert-butyldicarbonate
br broad
DABCO 1,4-diazabicyclo[2.2. 2] octane
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
52
DCM dichloromethane
DIPEA Diisopropylethylamine
DMAP 4- Dimethylaminopyridine
DPPA Diphenylphosphoryl azide
NaOAc Sodium acetate
PCC Pyridinium chlorochromate
q Quadruplet
Sept. Septuplet
TEA Triethylamine
TFA Trifluoroacetic acid
THE Tetrahydrofuran
Hal Halogen
J
'.'
H H
H Pyridine, Ac2O HBr/AcOH
O Toluene, AcOH, Ac2O
H OH DMAP O H
HO H 0 H 0
2
O
' HI
Na2Cr2O7.2H2O H
0 H 0 Toluene, NaOAc, AcOH, Ac2O O H O
0 O 0 0
A H 3 H 4
0 0
KOH H Ru02.H2O, Na104 H
O
Toluene, EtOH AcOEt, H2O, TFA
H OH H OH
0 H 5 0 H 6
0 0
KOH H
O DPPA,TEA H
McOH, THE Toluene NCO
OH
H - H
HO H 7 HO 8
H
Scheme 1
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
53
Lup-20(29)-ene-3/3,28-diyl diacetate 2
To a mixture of betulin 1 (100 g, 0.225 mot) in 120 mL of anhydrous pyridine
is
added DMAP (2.68 g, 0.022 mot) and 48 mL (0.495 mot) of acetic anhydride. The
reaction mixture is stirred at room temperature for 5 hours and diluted with
iced
water. The mixture is then extracted with DCM (3 x 200 ml-) and the combined
organic layers are washed back with aqueous HCl 1 N (3 x 200 mL), brine and
dried
over sodium sulfate. The pale yellow solid is taken up with methanol (400 mL),
filtered off and rinsed with methanol (2 x 400 mL) to give the title compound
2
(102.35 g, 86.3%) as a colorless solid.
Lup-18-ene-3/3,28-diyl diacetate 3
A solution of 90 mL of HBr in acetic acid (33%) is added to a mixture of 2
(45.03 g,
85.48 mmol) in 90 mL of toluene, 90 mL of acetic anhydride and 90 mL of acetic
acid previously heated at 90 C. The reaction mixture is stirred and heated at
this
temperature for 4 hours. After cooling, 46 g of sodium acetate is added and
the
mixture is evaporated to dryness. The pate brownish residue is re-evaporated
from
methanol (50 mL) and the residue is triturated with methanol, filtered off and
washed with methanol to obtain 42.35 g of a pale brownish solid. After
recrystallization in ethyl acetate (0.5 L) and cooling on ice for 0.5 hour,
the title
compound 3 (25.78 g, 60.4%) is isolated as a colorless solid.
21-Oxo-Lup-18-ene-3/3,28-diyl diacetate 4
A mixture of 3 (22 g, 41.76 mmol), sodium acetate (19.5 g, 238 mmol) and
sodium
dichromate dihydrate (14.9 g, 50.1 mmol) in 280 mL of anhydrous toluene, 350
mL
of acetic acid and 76 mL of acetic anhydride is stirred overnight at 60 C.
After
cooling, water (500 mL) and ethyl acetate (350 mL) are added and the layers
are
separated. The organic layer is washed successively with water (500 mL), a
saturated solution of sodium carbonate (3 x 250 mL), water (500 mL) and brine
(3 x
200 mL), dried over sodium sulfate and concentrated in vacuum. The gummy
yellow solid is triturated with methanol and filtered off to yield the title
compound
4 (21.41 g, 94.8%) as a colorless solid.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
54
28-Hydroxy-21-oxolup-18-en-3Q vl acetate 5
A solution of compound 4 (12.07 g, 22.3 mmol) and potassium hydroxide (1.49 g,
26.76 mmol) in a mixture 1:1 of toluene and ethanol (0.72 L) is stirred
vigorously at
room temperature for 1 hour. The reaction mixture is neutralized with aqueous
HCl
1 N (27 mL) and evaporated to dryness. The solid is taken up with water and a
minimum of acetone then filtered off. The precipitate is washed with water and
dried to yield the title compound 5 (10.24 g, 92%) as a colorless solid.
3f -Acetoxy-2l-oxolup-18-en-28-oic acid 6
A suspension of compound 5 (10.24 g, 20.5 mmol) in ethyl acetate (720 mL) is
added to a mixture of ruthenium oxide (IV) hydrate (272 mg, 2.05 mmol) and
sodium periodate (26.3 g, 123 mmol) in water (650 ml-) and TFA (11 mL). The
biphasic mixture is stirred vigorously overnight at room temperature. Ethanol
(100
mL) is added and the separated organic layer is filtered through a short
column of
silica gel. Water (400 mL) is added and the organic layer is dried over sodium
sulfate, and concentrated in vacuo. The yellow solid is taken up with diethyl
ether,
filtered off and washed with diethyl ether to give the title compound 6 (4.96
g,
47.2%) as a colorless solid.
316-Hydroxy-21-oxolup-18-en-28-oic acid 7
To a solution of compound 6 (300 mg, 0.58 mmol) in 3 mL of methanol and 10 mL
of THE is added an aqueous 4N solution of potassium hydroxide (1.45 mL, 5.8
mmot). The reaction mixture is stirred for 2 days at room temperature,
neutralized
with HCl 1 N (6 mL) and extracted with DCM (20 mL). The organic layer is
washed
with water (3 x 20 mL), brine (20 mL) and dried over sodium sulfate to yield
the
title compound 7 (201.4 mg, 73.7%).
'H NMR (400 MHz, CDCl3): 6 [ppm] 3.20 (m, 2H), 2.74 (d x d, 1 H), 2.57 (d, 1
H), 2.46
(m, 1H), 2.17 (d, 1H), 2.06 (br d, 1H), 1.90 - 1.24 (m, 15H), 1.21 (s, 3H),
1.19 (s,
3H), 1.04 (s, 3H), 0.98 - 0.83 (m, 2H), 0.96 (s, 3H), 0.93 (s, 3H), 0.87 (s,
3H), 0.76
(s, 3H), 0.70 (br d, 1H).
LC/MS: m/z = 471.46 (M+H+).
30-Hydroxy-21-oxolup-18-en-170-isocyanate 8
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
To a stirring suspension of compound 7 (124 mg, 0.26 mmol) in dry toluene (2.2
mL) is added TEA (40 L, 0.32 mmol) and DPPA (68 L, 0.32 mmol). The mixture
is
stirred for 24 hours at room temperature and concentrated to dryness. The
residue
is purified by flash chromatography on silica gel (ethyl acetate/hexanes 0% to
20%)
to afford the title compound 8 (70.3 mg, 57%) as a white solid.
'H NMR (400 MHz, CDCl3): 6 [ppm] 3.19 (m, 1H), 3.11 (m, 1H), 3.01 (d x d, 1H),
2.58 (d, 1 H), 2.40 (d x d, 1 H), 2.11 - 1.22 (m, 17H), 1.17 (m, 9H), 0.96 (s,
3H), 0.90
(s, 3H), 0.89 (s, 3H), 0.76 (s, 3H), 0.68 (m, 1H).
LC/MS: m/z = 468.57 (M+H+).
0 0
H H
0 DPPA, TEA O Toluene
O H OH toluene O H N3
O 0
H H
6 9
O O
H H
KOH
NCO HCl NH2 HCI
O DCM 0 McOH,THF
/\ H H
O O
H 10 H 11
O O
H H 0 0>=0
NH2 HCl (Boc)ZO, TEA NHBOC A
H = DCM H = Pyridine
HO HO
H 12 H 13
O O
H H
HCl
NHBOC Dioxane NH2 HCI
O O 5-0 L H H
HO 'k A'O H HOIAH
14 15
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
56
Scheme 2
30-Acetoxy-21-oxolup-18-en-28-oic acid azide 9
To a stirring suspension of compound 6 (1.11 g, 2.17 mmol) in dry toluene (16
mL)
is added TEA (0.33 mL, 2.6 mmol) and DPPA (0.56 mL, 2.6 mmol). The mixture is
stirred for 3 hours at room temperature and concentrated to dryness. The
residue
is purified by flash chromatography on silica gel (ethyl acetate/hexanes 0% to
20%)
to afford the title compound 9 (967 mg, 83%) as a white solid.
1H NMR (400 MHz, CDCl3): 6 [ppm] 4.45(d x d, 1H), 3.18 (m, 1H), 2.60 (d x d,
1H),
2.48 (d, 1 H), 2.44 (m, 1 H), 2.11 (d, 1 H), 2.21 (s, 3H), 2.0 (m, 1 H), 1.92 -
1.22 (m,
14H), 1.20 (d, 3H), 1.18 (d, 3H), 1.04 (m, 1H), 1.03 (s, 3H), 0.90 (s, 3H),
0.89 (s,
3H), 0.83 (s, 3H), 0.82 (s, 3H), 0.79 (m, 1H).
LC/MS: m/z = 538.58 (M+H+).
3j9-Acetoxy-21-oxolup-18-en-17/3-isocyanate 10
A solution of compound 9 (960 mg, 1.78 mmol) in toluene (18 mL) is stirred at
80 C
for 2 hours and concentrated to dryness to afford the title compound 10 (784
mg,
86%) as a white solid.
'H NMR (400 MHz, CDCl3): 6 [ppm] 4.46(d x d, 1H), 3.11 (m, 1H), 3.01 (d x d,
1H),
2.58 (d, 1H), 2.41 (d, 1H), 2.09 (m, 1H), 2.04 (s, 3H), 2.0 - 1.24 (m, 15H),
1.18 (d,
3H), 1.70 (m, 6H), 1.04 (m, 1H), 0.92 (s, 3H), 0.89 (s, 3H), 0.84 (s, 3H),
0.83 (s,
3H), 0.80 (m, 1 H).
LC/MS: m/z = 510.48 (M+H+).
3/3-Acetoxy-1713 amino-21-oxolup-18-ene hydrochloride 11
To a stirring solution of compound 10 (784 mg, 1.54 mmol) in DCM (15 mL) is
added
concentrated HCl (5 mL). The biphasic mixture is stirred 48 hours at room
temperature, and then concentrated to dryness to give the title compound 11
(765
mg, 96%) as a white solid.
'H NMR (400 MHz, DMSO-d6): 6 [ppm] 8.40 (br s, 2H), 4.37(d x d, 1H), 3.16 (m,
1H),
2.83 (d x d, 1 H), 2.43 (m, 2H), 2.08 (m, 1 H), 1.97 (s, 3H), 1.92 - 1.0 (m,
25H), 0.86
(s, 3H), 0.82 (s, 3H), 0.81 (m, 1H), 0.77 (s, 6H).
17~3Amino-313hydroxy-21-oxolup-18-ene hydrochloride 12
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
57
To a solution of compound 11 (760 mg, 1.46 mmol) in 3.8 mL of methanol and
11.2
mL of THE is added an aqueous 4N solution of potassium hydroxide (3.6 mL, 14.6
mmol). The reaction mixture is stirred for 24 hours at room temperature and
acidified with 4N HCt. The precipitate formed is collected by filtration to
yield the
title compound 12 (705 mg, 100%) as a white solid.
1H NMR (400 MHz, DMSO-d6): S [ppm] 4.28 (d x d, 1 H), 3.14 (m, 1 H), 2.95 (m,
1 H),
2.86 (br d, 1 H), 2.48 (m, 2H), 2.42 (d, 2H), 2.06 (m, 1 H), 1.90 - 1.07 (m,
24H), 0.91
(m, 1H), 0.84 (s, 3H), 0.81 (s, 3H), 0.80 (s, 3H), 0.65 (m, 1H), 0.63 (s, 3H).
LC/MS: m/z = 442.56 (M+H+).
170-tert-Butytoxycarbonylamino-313-hydroxy-21-oxolup-18-ene 13
To a stirring suspension of compound 12 (600 mg, 1.25 mmol) in DCM (13 mL) is
added successively TEA (0.4 mL, 3.14 mmol) and (Boc)20 (411 mg, 1.88 mmol).
The
solution is stirred overnight at room temperature then another 1.56 mmol of
TEA
and 0.94 mmol of (Boc)20 is added and stirring is continued for 3 hours. The
mixture is diluted with DCM and washed with HCl (1N) and water, dried over
sodium sulfate and concentrated to dryness. The residue is purified by flash
chromatography on silica gel (methanol/DCM 0% to 8%) to afford the title
compound 13 as foam (564 mg, 83%).
1H NMR (400 MHz, CDCl3): S [ppm] 4.62 (br s, 1H), 3.19 (d x d, 1H),3.11 (m,
1H),
2.83 (br d, 1H), 2.62 (d, 1H), 2.23 (br d, I H), 1.88 (m, 3H), 1.73 (m, 1H),
1.68 -
1.10 (m, 30H), 0.98 (m, 1H), 0.96 (s, 3H), 0.92 (s, 3H), 0.88 (s, 3H), 0.76
(s, 3H),
0.70 (m, 1H).
LC/MS: m/z = 542.61 (M+H+).
39-043', 3'- Dimethylsuccinyl)-17/_ -tert-butyloxycarbonylamino-21-oxolup-l8-
ene
14-1
0
H 0
N-O
0
HOr = H
O
H
O
A stirring solution of compound 13 (557 mg, 1.03 mmol), DMAP (151 mg, 1.23
mmol) and 2,2-dimethylsuccinic anhydride (527 mg, 4.12 mmol) in dry pyridine
(11
mL) is heated overnight at 120 C. Another 3.12 mmol of 2,2-dimethylsuccinic
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
58
anhydride is added and heating at 120 C is continued for 4 hours. The mixture
is
cooled down to room temperature and concentrated to dryness. The residue is
diluted in ethyl acetate, washed twice with HCl 1N, water and brine, dried
over
sodium sulfate and concentrated to dryness. The residue is purified by flash
column
chromatography on silica gel (ethyl acetate/hexanes 0% to 40%) to yield the
title
compound 14-1 as a white solid (467 mg, 68%).
'H NMR (400 MHz, CDCl3): S [ppm] 4.50 (d x d, 1H), 3.11 (m, 1H), 2.84 (br s,
1H),
2.60 (m, 3H), 2.24 (br s, 1H), 1.98 - 0.95 (m, 39H), 0.92 (s, 3H), 0.90 (s,
3H), 0.83
(s, 3H), 0.80 (s, 3H), 0.79 (m, 2H).
LC/MS: m/z = 670.69 (M+H+).
30-0-(3',3'-Dimethylsuccinyl)-170-amino-21-oxolup-18-ene hydrochloride 15-1
O
NH2 CIH
0
H
HO
0 H
0
To a stirring solution of compound 14-1 (414 mg, 0.62 mmol) in dioxane (5 mL)
is
added 4N HCl in dioxane (1.5 mL). The mixture is stirred 24 hours at room
temperature. Another 1.5 mL of 4N HCl in dioxane is added and the mixture is
stirred for 2 days and then concentrated to dryness to give the title compound
15-
1 (390 mg, 100%) as a white solid. An aliquot (80 mg) is purified by flash
column
chromatography on silica gel (methanol/DCM 0% to 10%) to give the title
compound
15-1 as a white solid (38 mg).
'H NMR (400 MHz, DMSO-d6): 6 [ppm] 4.36 (d x d, 1 H), 3.18 (d x d, 1 H), 3.05
(m,
1H), 2.48 (m, 2H), 2.13 (d, 2H), 2.0 - 1.18 (m, 16H), 1.17 - 0.94 (m, 17H),
0.84 (s,
3H), 0.82 (s, 3H), 0.76 (s, 6H).
LC/MS: m/z = 570.59 (M+H+).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
59
O RZ H o
H / reductive H ~
_ amination N-R2
NH' CI H
~ H or H
HO A O H R -X HO A O =
2 H
base
15 where X= Br, I, CI, OMs, OTs, 16
Scheme 3
General procedure for the preparation of compound 16:
An alkyl R2 group is introduced to compound 15 by conventional reductive
amination with an aldehyde or a ketone (see A.F. Abdel-Magid, et at. J. Org.
Chem.
(1996), 61, 3849-3862) or by alkytation with an alkyl halide in presence of a
base
such as TEA, DI PEA or NaH in a solvent such as THE or DMF to give compound
16.
3j3-O- (3',3'-Dimethylsuccinyl)-17 (1-tert-butyloxycarbonylpiperidine-4-aminol-
21-oxolup-18-ene 16-1
0
N O
OH 0
H
O H N
HOYV__j_O
O
To a stirring suspension of compound 15-1 (54.6 mg, 0.09 mmol) and N-Boc-4-
piperidine (21.5 mg, 0.11 mmol) in dichloroethane (0.6 mL) is added TEA (17
L,
0.13 mmol). After stirring the mixture at room temperature for 1 hour, sodium
triacetoxyborohydride (28.6 mg, 0.13 mmol) is added and the reaction is
stirred for
24 hours at room temperature. The mixture is diluted with ethyl acetate,
washed
with aqueous sodium bicarbonate, water and brine, dried over sodium sulfate
and
concentrated to dryness. The residue is purified by flash column
chromatography
on silica gel (methanol/DCM 0% to 10%) to give the title compound 16-1 as a
white
solid (39 mg, 68%).
1H NMR (400 MHz, DMSO-d6): 8 [ppm] 4.49 (m, 1 H), 4.10 (q, 1 H), 3.90 (m, 4H),
3.18
(m, I H), 3.0 (t, 1H), 2.77 (m, 1H), 2.60 (m, 2H), 2.35 (m, I H), 2.08 (m,
2H), 1.84
(m, 3H), 1.78 - 0.94 (m, 47H), 0.90 (s, 3H), 0.88 (s, 3H), 0.81 (m, 7H).
LC/MS: m/z = 753.7 (M+H+).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
0
H 0
R3
0 0 H _= 2 3
HOAx0 H
17
R3NCO 0
or O\~O
R3R3N000I R5SO2CI N'S,R5
O OH, HOA0 N-R2 18
OH,
H
HOAAO O
R4000I H 0
15 or 16 R2 = H or alkyl or R4000H N R4
R2
H
or R4= )L )L HOA0 H
O O R4
R6000CI 0 0 19
or R4"O"R4
0
H 0
NO-R6
H = R2
HO~AA0
H
Scheme 4
General procedures
Ureas 17 are made by treatment of compound 15 or 16 with an isocyanate (1 to 3
eq.), carbamoyl chloride or phosgene or triphosgene followed by an amine in a
solvent such as toluene or THF.
Sulfonamides 18 are obtained by coupling compound 15 or 16 with the
appropriate
sulfonyl chloride (1 to 3 eq.) in solvents such as THE or DCM and in the
presence of
a base such as TEA or DIPEA.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
61
Amides 19 are prepared by coupling compound 15 or 16 with the appropriate acyl
chloride (1 to 3 eq.), preactivated carboxylic acid, mixed anhydride or
anhydride in
solvents such as THE or DCM and in the presence of a base such as TEA or
DIPEA.
Carbamates 20 are obtained by reacting compound 15 or 16 with the appropriate
chloroformate (1 to 3 eq.) in solvents such as THE or DCM and in the presence
of a
base such as TEA or DIPEA.
3/i 0-(3',3'-Dimethylsuccinvl)-l7,3-[N'-(benzyl)ureidol-21-oxolup-18-ene 17-1
0
OHH 0
N 'K N-'~O
0 H H
H01 '~jo
O
To a stirring suspension of compound 15-1 (49.2 mg, 0.08 mmol) in DCM (1 mL)
is
added TEA (23 L, 0.18 mmol) and benzyl isocyanate (15 L, 0.12 mmol). The
mixture is stirred for 24 hours at room temperature, diluted with DCM, washed
twice with water and brine, dried over sodium sulfate and concentrated to
dryness.
The residue is purified by flash column chromatography on silica gel
(methanol/DCM 0% to 10%) to give the title compound 17-1 as a white solid (39
mg,
68%).
1H NMR (400 MHz, DMSO-d6): 6 [ppm] 12.2 (br s, 1H), 7.25 (m, 2H), 7.20 (m,
3H),
6.28 (s, 1 H), 6.21 (t, 1 H), 4.35 (d x d, 1 H), 4.20 (d x d, 1 H), 4.07 (d x
d, 1 H), 3.04
(m, 1 H), 2.83 (d x d, 1 H), 2.43 (m, 2H), 2.09 (d, 2H), 1.90 (m, 2H), 1.75 -
0.94 (m,
30H), 0.87 (s, 3H), 0.85 (s, 3H), 0.80 (m, 1H), 0.76 (s, 6H).
LC/MS: m/z = 703.68 (M+H').
30-0-(3' 3'-Dimethylsuccinvl)-170-methylsulfonylamino-21-oxolup-18-ene 18-1
0
/ 00
0 Oj H
N'S
HO O
1~ 0
O
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
62
A solution of compound 15-1 (48.6 mg, 0.08 mmol) in DCM (0.8 mL) is treated
with
TEA (51 L, 0.4 mmol) and. methanesulfonyl chloride (25 L, 0.128 mmol). The
mixture is stirred in microwave at 100 C for 5 minutes. The mixture is diluted
with
DCM, washed twice with water and brine, dried over sodium sulfate. The residue
is
purified by flash column chromatography on silica get (methanol/DCM 0% to 10%)
to
yield the title compound 18-1 (26.5 mg, 51%) as a pale yellow solid.
'H NMR (400 MHz, CDCl3): 8 [ppm] 4.62 (s, 1 H), 4.48 (d x d, 1 H), 3.14 (m, 1
H), 3.05
(m, 1 H), 2.98 (s, 3H), 2.88 (d, 1 H), 2.60 (q, 2H), 2.33 (d, 1 H), 2.27 (br
d, 1 H), 2.02
- 1.82 (m, 2H), 1.78 - 1.1 (m, 28H), 1.02 (m, 1H), 0.91 (s, 3H), 0.90 (s, 3H),
0.81
(m, 7H).
LC/MS: m/z = 648.6 (M+H').
313-0-(3',3'-Dimethylsuccinyt)-17,3-acetylamino-21-oxolup-18-ene 19-1
O
H O
N
O = H
H O "j H
O
O
To a stirring solution of compound 15-1 (50 mg, 0.08 mmol) in THE (1 mL) is
added
TEA (23 L, 0.18 mmol) and acetyl chloride (9 L, 0.12 mmol). The mixture is
stirred for 2 hours at room temperature, diluted with ethyl acetate, washed
twice
with water and brine, dried over sodium sulfate and concentrated to dryness.
The
residue is purified by flash column chromatography on silica gel (methanol/DCM
0%
to 5%) to give the title compound 19-1 as a white solid (25 mg, 50%).
'H NMR (400 MHz, CDCl3): 6 [ppm] 5.43 (s, 1 H)., 4.48 (d x d, 1 H), 3.12 (m, 1
H), 2.75
(t, 1 H), 2.69 (d, 1 H), 2.65 (d, 1 H), 2.55 (d, 1 H), 2.30 (m, 2H), 1.97 (s,
3H), 1.94 -
1.12 (m, 28H), 1.10 (s, 3H), 1.01 (m, 1H), 0.92 (s, 3H), 0.89 (s, 3H), 0.83
(s, 3H),
0.81 (s, 3H).
LC/MS: m/z = 612.64 (M+H`).
3Q 0-(3',3'-Dimethylsuccinyl)-17,8 methoxycarbonylamino-21-oxolup-18-ene 20-1
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
63
0
H 0
N0-
0 H
H01r H
O H
0
To a stirring suspension of compound 15-1 (51.6 mg, 0.085 mmol) in DCM (1 mL)
is
added TEA (24 L, 0.19 mmol) and methylchloroformate (10 L, 0.13 mmol). The
mixture is stirred for 2 hours at room temperature, diluted with DCM, washed
twice with water and brine, dried over sodium sulfate and concentrated to
dryness.
The residue is purified by flash column chromatography on silica gel
(methanol/DCM 0% to 10%) and repurified by reverse-phase HPLC (55 to 85% CH3CN
in H2O (3 mmol HCl) over 60 min at 244 nm) to give the title compound 20-1 as
a
white solid (1.5 mg, 3%).
1H NMR (400 MHz, CDCl3): 6 [ppm] 4.73 (br s, 1H), 4.44 (d x d, 1H), 3.55 (s,
3H),
3.07 (m, 1H), 2.76 (t, 1H), 2.54 (m, 3H), 2.17 (m, 2H), 1.90 - 1.08 (m, 27H),
1.05
(s, 3H), 0.95 (m, 1H), 0.86 (s, 3H), 0.84 (s, 3H), 0.77 (s, 3H), 0.75 (s, 3H),
0.74 (m,
1 H).
LC/M5: m/z = 628.64 (M+H+).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
64
O
0
H
H O
AcCI, MeOH NH3 CI
N 0 0 0
H H
II =
, H = O~A O
HO A AO Fi H 35
14 ~0 R2-X
RZ H or base
reductive where X= Br, I, CI, OMs
amination
O 0
H H
NR2
0 0 H LiOH 0 I0 O H
HO Al AAO = H OAAO
H 16 6
0 0
CI)LR4 or HO R4
O
H I 0
N R4
H R2
HOJLAAO
H 19
Scheme 5
General procedure for the preparation of compound 19:
Step 1: Compound 14 is treated with acetyl chloride in methanol and stirred at
room temperature to give compound 35.
Step 2: An alkyl R2 group is introduced to compound 35 by conventional
reductive
amination with an aldehyde or a ketone or by alkylation with an alkyl halide
in
presence of a base such as TEA, DIPEA or NaH in a solvent such as THE or DMF
to
give compound 36.
Step 3: The deprotection of the ester group occurs in solvents such as
methanol,
THE or dioxane using an aqueous solution of inorganic base such as lithium
hydroxide or potassium hydroxide (3 to 10 eq.) at temperature ranging from 20
to
60 C.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
Step 4: Amides 19 are prepared by coupling compound 16 with the appropriate
acyl chloride (1 to 3 eq.), preactivated carboxylic acid, mixed anhydride or
anhydride in solvents such as THF, DMF or DCM and in the presence of a base
such
as TEA or DI PEA.
3,&O-(3',3'-Dimethylsuccinyl)-17 3 [methyl-(5-methyl-[1,3,4]oxadiazole-2-
carbonyl)-amino]-21-oxolup-18-ene 19-69
O
OH / N 0
O
O H N
H0 ,N
~0
H
O
Step 1: To an ice-cold stirring suspension of 14-1 (146.8 mg, 0.22 mmol) in
MeOH
(2 mL) is added acetyl chloride (0.16 mL, 2.19 mmol). The mixture is stirred
at
room temperature overnight and the solvent is removed by evaporation. The
residue is purified by flash column chromatography on silica gel (methanol/DCM
0%
to 8%) to yield the title compound 3,3-0-(3',3'-Dimethylsuccinyl-methyl-ester)-
17,-
amino-21-oxolup-18-ene hydrochloride 35-1 (118 mg, 86%).
1H NMR (400 MHz, CDCl3): S [ppm] 4.48 (d x d, 1H), 3.67 (s, 3H), 3.30 (m, 2H),
2.58
(q, 2H), 2.45 (q, 2H), 2.00-1.20 (m, 15H), 1.25 (s, 3H), 1.24 (s, 3H), 1.17
(d, 3H),
1.15 (d, 3H), 1.14 (s, 3H), 1.00 (m, 1H), 0.82 (s, 3H), 0.80 (s, 3H), 0.79 (m,
I H).
Step 2: To an ice-cold stirring solution of 35-1 (101 mg, 0.164 mmol) in dry
THE
(1.6 mL) is added sodium hydride (14 mg, 0.345 mmol, as a 60% dispersion in
mineral oil). The mixture is stirred 15 minutes at 0 C then methyl iodide
(0.012
mL, 0.196 mmol) is added. The resulting mixture is stirred at 0 C for 30
minutes
then at room temperature overnight. The mixture is diluted with ethyl acetate,
washed with aqueous ammonium chloride, water, aqueous sodium thiosulfate 5%
and brine, dried over sodium sulfate. The residue is purified by flash column
chromatography on silica gel (methanol/DCM 0% to 5%) to yield 3/i-0-(3',3'-,
Dimethylsucci nyl-methyl-ester)-17,3-N-methyl-amino-21-oxolup-18-ene
hydrochloride 36-1 (30 mg, 30%) as an oil.
1 H NMR (400 MHz, CDCl3): 8 [ppm] 4.48 (d x d, 1H), 3.67 (s, 2H), 3.13 (sept.,
1H),
2.62 (d, 1 H), 2.55 (d, 1 H), 2.37 (d, 1 H), 2.11 (s, 3H), 1.98 (d, 1 H), 1.97
(t x d, 1 H),
1.87 (m, 3H), 1.72 (d x t, 1H), 1.68-0.70 (m, 14H), 1.26 (s, 3H), 1.25 (s,
3H), 1.19
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
66
(d, 3H), 1.17 (d, 3H), 1.13 (s, 3H), 0.90 (s, 3H), 0.89 (s, 3H), 0.82 (s, 3H),
0.80 (s,
3H).
LC/MS: m/z = 598.45 (M+H+).
Step 3: To a stirring solution of 36-1 (80 mg, 0.134 mmol) in 1,4-dioxane (1
mL)
and water (0.5 mL) is added LiOH 1N (0.401 mL, 0.401 mmol). The mixture is
stirred overnight at room temperature, neutralized to pH 7 with HCl 1 N,
extracted
with ethyl acetate (1X), DCM (containing few drops of methanol) (3X). The
organic
extracts are combined and dried over sodium sulfate. The residue is purified
by
flash column chromatography on silica gel (methanol/DCM 0% to 10%) to yield 16-
2
(43,mg, 55%) as a white solid.
1 H NMR (400 MHz, CD30D): 8 [ppm] 4.46.(d x d, 1 H), 3.25 (sept., 1 H), 2.89
(m, 1 H),
2.58 (d, 1H), 2.53 (d, 1H), 2.38 (d, 1H), 2.12 (s, 3H), 2.07 (d, 1H), 2.01-
1.93 (m,
4H), 1.79 (d x t, 1H), 1.70-1.27 (m, 11H), 1.24 (s, 3H), 1.23 (s, 3H), 1.20
(s, 3H),
1.18 (d, 3H), 1.17 (d, 3H), 1.14-0.98 (m, 2H), 0.96 (s, 6H), 0.86 (s, 3H),
0.85 (s,
3H), 0.84 (m, 1 H).
LC/MS: m/z = 584.52 (M+H+).
HPLC (D) RT = 26.63 min.
Step 4: To an ice-cold stirring solution of potassium 5-methyl-
[1,3,4]oxadiazole-2-
carboxylate (13.4 mg, 0.081 mmol) in dry acetonitrile (1.0 mL) is added a
catalytic
amount of DMF followed by oxalyl chloride (0.037 mL, 0.074 mmol). The mixture
is
stirred 50 minutes at 0 C, it is then added to an ice-cold stirring
suspension of 16-
2 (42.9 mg, 0.074 mmol) and TEA (0.021 mL, 0.147 mmol) in dry THE (1.3 mL).
The resulting mixture is stirred 3.5 hours at room temperature, diluted with
ethyl
acetate, washed with water and brine, dried over sodium sulfate. The residue
is
purified by flash column chromatography on silica gel (methanol/DCM 0% to 10%)
to
yield the title compound 19-69 (8.2 mg, 16%) as white solid.
1H NMR (400 MHz, CDCl3): 6 [ppm] 4.48 (d x d, 1H), 3.89 (br s, 3H), 3.16
(sept.,
1H), 2.64 (d, 2H), 2.59 (s, 3H), 2.54(d, 1H), 2.44 (br s, 2H), 2.00-0.70 (m,
19H),
1.29 (s, 3H), 1.28 (s, 3H), 1.22 (t, 6H), 1.05 (s, 3H), 0.90 (s, 3H), 0.84 (s,
3H), 0.82
(s, 3H), 0.79 (s, 3H).
LC/MS: m/z = 694.97 (M+H+).
HPLC (A) RT = 24.93 min.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
67
O O
H a H
NH+CI HCkR4 HR4
H or H
~OAAAO p ~OAAAO
H H 37
H
CI~
35 R4
LIOH
0
OH ~ 0
H R4
IIII H
OAAAO =
H
19
SScheme 6
General procedure
Step 1: Amides 19 are prepared by coupling compound 35 with the appropriate
acyl chloride, preactivated carboxylic acid, mixed anhydride or anhydride in
solvents such as THE, DCM or DMF and in the presence of a base such as TEA or
DI PEA.
Step 2: The deprotection occurs in solvents such as methanol, THE or dioxane
using
an aqueous solution of inorganic base such as lithium hydroxide or potassium
hydroxide (3 to 10 eq.) at temperature ranging from 20 to 60 C.
3Q-0-(3',3'-Dimethylsuccinyl)-17,6 (4-hydroxypyridine-3-yl)amido-21-oxolup-18-
ene
19-50
0
H O OH
N
H
HO _
O =
H
O
Step 1: To a stirring suspension of compound 35-1 (85.3 mg, 0.14 mmol) and 4-
hydroxynicotinic acid (28.7 mg, 0.21 mmol) in DMF (1.5 mL) is added TEA (53
L,
0.41 mmol) and HATU (78.4 mg, 0.21 mmol). The mixture is stirred for 2 hours
at
room temperature. Water is added slowly and the precipitate formed is
collected
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
68
by filtration and purified by flash column chromatography on silica gel
(methanol/DCM 0% to 8%) to yield the title compound 30-0-(3',3'-
Dimethylsuccinyl
methyl-ester)-171.i-(4-hydroxypyridine-3-yl)amido-21-oxolup-18-ene 37-1 (70
mg).
'H NMR (400 MHz, CDCl3): S [ppm] 11.6 (br s, 1H), 10.92 (br s, 1H), 8.80 (s,
1H),
7.61 (d, 1 H), 6.57 (m, 1 H), 4.44 (d x d, 1 H), 3.66 (s, 3H), 3.15 (m, 1 H),
2.89 (t,
1H), 2.79 (d, 1H), 2.57 (q, 2H), 2.24 (m, 3H), 1.88 (m, 2H), 1.74-0.72 (m,
14H),
1.24 (s, 3H), 1.23 (s, 3H), 1.18 (d, 3H), 1.15 (d, 3H), 1.08 (s, 3H), 0.94 (s,
3H), 0.83
(s, 3H), 0.81 (s, 3H), 0.78 (s, 3H).
LC/MS: m/z = 705.98 (M+H+).
Step 2: To a stirring solution of 37-1 (70 mg, 0.10 mmol) in 1,4-dioxane (0.8
mL)
and water (0.2 mL) is added LiOH=H20 (12.5 mg, 0.30 mmol). The mixture is
stirred
overnight at room temperature, acidifed to pH 3 with HCl 1 N, and the
precipitate
formed is collected by filtration. The product is purified by flash column
chromatography on silica gel (methanol/DCM 0% to 15%) to yield 19-50 (31 mg)
as a
white solid.
'H NMR (400 MHz, CDCl3): S [ppm] 11.7 (br s, 1H), 10.80 (s, 1H), 8.42 (d x d,
1H),
7.38 (m, 1 H), 6.52 (d x d, 1 H), 4.42 (d x d, 1 H), 3.12 (m, 1 H), 2.93 (m, 1
H), 2.80
(d, 1H), 2.54 (q, 2H), 2.40 (m, 1H), 2.29 (d, 1H), 2.07 (m, 5H), 1.88 (m, 2H),
1.70-
0.70 (m, 10H), 1.22 (s, 3H), 1.21 (s, 3H), 1.18 (d, 3H), 1.16 (d, 3H), 1.03
(s, 3H),
0.90 (s, 3H), 0.80 (s, 3H), 0.78 (s, 3H), 0.76 (s, 3H).
LC/MS: m/z = 691.86 (M+H+).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
69
0 0
H
NHZ N- 0
H CIH O O
HOxAxO H HO~AxO H
15 H 26
R3 R3NH
O
HI 0
N' R3
O O H = H R
HO 'k AAO Fi
17
Scheme 7
Isocyanates 26 are made by treatment of compound 15 with phosgene or
triphosgene. Ureas 17 are obtained by treatment of the isocyanates 26 with an
amine in a solvent such as toluene or THE in the presence of a base such as
TEA or
DI PEA.
3/3-0- (3',3'-Dimethylsuccinyl)-17 3 [N'-(1-tert-butyloxycarbonyl-piperidin-4-
yl)-
ureido]-21-oxolup-18-ene 17-9
o O
H / O
O~
0H1 N
N)LN H H
O
HO~
O
O
Step 1: To an ice-cold stirring solution of triphosgene (335 mg, 1.129 mmol)
in dry
THE (5 mL) is added drop wise over 10 minutes a solution of compound 15-1 (456
mg, 0.753 mmol) and DIPEA (0.33 mL, 1.881 mmol) in dry THE (6 mL). The
resulting
mixture is stirred at room temperature for 3 hours. Water is added drop wise
at
0 C and the mixture is diluted with ethyl acetate, washed with water and
brine,
dried over sodium sulfate. The residue is purified by flash column
chromatography
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
on silica gel (ethyl acetate/hexanes 0% to 80%) to yield 3#-0-(3',3'-
dimethylsuccinyl)-21-oxolup-18-ene-17,&isocyanate 26-1 (419 mg) as a foam.
'H NMR (400 MHz, CDCl3): 6 [ppm] 4.49 (d x d, 1H), 3.11 (sept., 1H), 2.99 (d x
d,
1 H), 2.67 (d, 1 H), 2.59 (d, 1 H), 2.54 (d, 1 H), 2.41 (d, 1 H), 2.10 (m, 1
H), 2.04-1.80
(m, 3H), 1.75 (d x t, 1H), 1.70-1.46 (m, 6H), 1.39-1.24 (m, 5H), 1.30 (s, 3H),
1.28
(s, 3H), 1.18 (d, 3H), 1.17 (d, 3H), 1.16 (s, 3H), 1.01 (t x d, 1 H), 0.91 (s,
3H), 0.89
(s, 3H), 0.83 (s, 3H), 0.81 (s, 3H), 0.78 (m, 1 H).
IR (v, cm'): 2257 (NCO).
10 Step 2: To a stirring solution of 3/i-O-(3',3'-dimethylsuccinyl)-21-oxolup-
18-ene-
17fl-isocyanate 26-1 (151 mg, 0.253 mmol) in dry toluene (6 mL) is added 4-
amino-
1-Boc-piperidine (203 mg, 1.014 mmol). The mixture is stirred 2 hours at 80 C,
cooled down and diluted with ethyl acetate, washed with HCl 1 N, water and
brine,
dried over sodium sulfate. The residue is purified by flash column
chromatography
on silica gel (methanol/DCM 0% to 10%) to yield the title compound 17-9 (173
mg)
as a white solid.
'H NMR (400 MHz, CDCl3): 6 [ppm] 5.40 (br s, 1 H), 4.56 (br s, 1 H), 4.47 (d x
d, 1 H),
3.97 (br s, 2H), 3.66 (br s, 1 H), 3.16 (sept., 1 H), 2.91 (d, 1 H), 2.78 (t,
2H), 2.66 (d,
1 H), 2.59 (d, 1 H), 2.52 (d, 1 H), 2.28 (d, 1 H), 2.13 (d, 1 H), 1.99 (m, 1
H), 1.90-0.70
20 (m, 19H), 1.42 (s, 9H), 1.28 (s, 3H), 1.27 (s, 3H), 1.21 (d, 3H), 1.17 (d,
3H), 1.10
(s, 3H), 0.92 (s, 3H), 0.89 (s, 3H), 0.82 (s, 3H), 0.80 (s, 3H).
LC/MS: m/z = 797.44 (M+H').
HPLC (Method A) RT = 29.8 min.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
71
o 0
H H
1) R2CHO
NH2 NH
CIH or I
H 2) R2X (X = Hal, OMs, OTs) H
RIO RIO H 21
H 11 or 12
R1 = H, Ac
0 0
OH / 0 H 0
R3NCO or R 1) KOH if R1 = Ac 'k R 3
11 or 12 N N' 3 N N
or 21 R3R3 NCOCI - _ R2 R 3 2) Pyridine, DMAP 0 0 H R2 R'3
H
0
RIO - 22 Oy 0 HOA0 17
H H
A
0 0
H H 0
11 or 12 RSOCI O%O 1)KOHKR1=AC / N,S,R
,2 S,
or 21 N R5 5
R 2) Pyridine, DMAP 0I I0I R2
H 2 0 I H
RIO H 23 Oy 70 HO A O H .18
A
O
0
0H / 0
H ' 0
11 or 12 R4COOH or II 1) KOH if R1 = Ac N R4
or 21 R R4 2) Pyridine, DMAP 0 0 H R2
R4000I or H = R2 x x -
R4COOCOR4 R'0 - 24 O O HO A O H 19
H
A
0 0
H O H ~ 0
11 or 12 R6000CI or 1) KOH if R1 = Ac
N O N0
or 21
R6000OCOR6 H = R2 R6 2) Pyridine, DMAP H _ R2 R6
R1O H 25 Oy ~j0 HO A O H
A 20
Scheme 8
General procedure for the preparation of compound 21:
An alkyl R2 group is introduced to compound 11 or 12 by conventional reductive
amination with an aldehyde (see A.F. Abdel-Magid, et at. J. Org. Chem. (1996),
61,
3849-3862) or by alkylation with an alkyl halide in presence of a base such as
TEA,
DIPEA or NaH in a solvent such as THE or DMF to give compound 21.
General procedure for the synthesis of ureas 17
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
72
Step 1: A solution of compound 11, 12 or 21 and the appropriate isocyanate or
carbamoyl chloride in solvents such as toluene, dichloromethane or chloroform
is
stirred for 4 to 20 hours at room temperature or under reflux. The residue
obtained
is purified by flash column chromatography.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 22 (R1 = H).
Step 2: A solution of urea 22 (R1 = H), a base such as DMAP, TEA, DABCO or
DIPEA
and the appropriate anhydride (2 to 10 equivalents) in solvents such as
pyridine,
TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 4 to 24 hours. The
mixture is concentrated, washed with acid and purified by flash column
chromatography on silica get to yield compound 17.
General procedure for the synthesis of sulfonamides 18
Step 1: A solution of compound 11, 12 or 21 and the appropriate- sulfonyt
chloride
in solvents such as THE or DCM and in the presence of a base such as TEA or
DIPEA
is stirred for 4 to 20 hours at room temperature or under reflux. The residue
obtained is purified by flash chromatography on silica gel to afford the
desired
amide 23.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 23 (R1 = H).
Step 2: A solution of sulfonamide 23 (R1 = H), a base such as DMAP, TEA, DABCO
or
DIPEA and the appropriate anhydride (2 to 10 equivalents) in solvents such as
pyridine, TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 4 to 24
hours.
The mixture is = concentrated, washed with acid and purified by flash column
chromatography on silica gel to yield compound 18.
General procedure for the synthesis of amides 19
Step 1: A solution of compound 11, 12 or 21 and the appropriate acyl chloride
or
anhydride in solvents such as THE or DCM and in the presence of a base such as
TEA
or DIPEA is stirred for 4 to 20 hours at room temperature or under reflux. The
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
73
residue obtained is purified by flash chromatography on silica gel to afford
the
desired amide 24.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 24 (R1 = H).
Step 2:_A solution of amide 24 (R1 = H), a base such as DMAP, TEA, DABCO or
DIPEA
and the appropriate anhydride (2 to 10 equivalents) in solvents such as
pyridine,
TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 4 to 24 hours. The
mixture is concentrated, washed with acid and purified by flash column
chromatography on silica gel to yield compound 19.
General procedure for the synthesis of carbamates 20
Step 1: To a stirring solution of compound 11, 12 or 21 in toluene or benzene
is
added the desired chtoroformate or anhydride (3 equivalents). The resulting
mixture is stirred 2 to 4 hours under reflux. After standard acidic workup,
the
residue obtained is purified by flash chromatography on silica gel to afford
the
desired carbamate 25.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 25 (R1 = H).
Step 2: A stirring solution of carbamate 25 (R1 = H), a base such as DMAP,
TEA,
DABCO or DIPEA and the appropriate anhydride (2 to 10 equivalents) in solvents
such as pyridine, TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 4
to 24
hours. The mixture is concentrated, washed with acid and purified by flash
column
chromatography on silica gel to yield compound 20.
17f3-Benzamido-36-hydroxy-21-oxolup-18-ene 24-1
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
74
O
H O
H
H =
HO H
To a stirring suspension of compound 12 (62.7 mg, 0.13 mmol) in THE (1.3 ml-)
is
added TEA (37 L, 0.28 mmol) and benzoyl chloride (23 L, 0.20 mmol). The
mixture is stirred for 3 hours at room temperature, diluted with ethyl
acetate,
washed twice with 1 N HCI, water and brine, dried over sodium sulfate and
concentrated to dryness. The residue is purified by flash column
chromatography
on silica gel (methanol/DCM 0% to 4%) to give the title compound 24-1 as a
white
solid (55 mg, 77%).
'H NMR (400 MHz, CDCI3): 8 [ppm] 7.73 (m, 2H), 7.50 (m, 1 H), 7.43 (m, 2H),
6.13
(s, 1H), 3.19 (m, 2H), 2.90 (m, 1H), 2.87 (d, 1H), 2.40 (m, 1H), 2.35 (d, 1H),
1.94
(m, 2H), 1.80 - 1.20 (m, 20H), 1.14 (s, 3H), 0.96 (m, 6H), 0.86 (m, 3H), 0.75
(s,
3H), 0.70 (m, 1 H).
3/30-(3',3'-Dimethylsuccinyl)'-17/3 benzamido-21-oxolup-18-ene 19-2
O
OH O
O H HO lr~~L O O
A stirring solution of compound 24-1 (55 mg, 0.10 mmol), DMAP (14.8 mg, 0.12
mmol) and 2,2-dimethylsuccinic anhydride (38.7 mg, 0.30 mmol) in dry pyridine
(1.0 mL) is heated at 120 C for 4 hours. Another 0.30 mmol of 2,2-
dimethylsuccinic
anhydride is added and heating at 120 C is continued for 24 hours. The mixture
is
cooled down to room temperature and concentrated to dryness. The residue is
diluted in ethyl acetate, washed twice with HCl IN, water and brine, dried
over
sodium sulfate and concentrated to dryness. The residue is purified by flash
column
chromatography on silica gel (methanol/DCM 0% to 10%) to yield the title
compound 19-2 as a white solid (48 mg, 71%).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
'H NMR (400 MHz, CDCl3): S [ppm] 7.71 (m, 2H), 7.50 (m, 1H), 7.42 (m, 2H) 6.11
(s, 1 H), 4.48 (d x d, 1 H), 3.17 (m, 1 H), 2.90 (d, 1 H), 2.83 (d, 1 H), 2.66
(d, 1 H),
2.55 (d, 1 H), 2.41 (d, 1 H), 2.36 (d, 1 H), 1.95 (m, 3H), 1.76 - 1.16 (m,
26H), 1.13 (s,
3H), 0.97 (s, 3H), 0.87 (s, 3H), 0.83 (s, 3H), 0.79 (s, 3H).
LC/MS: m/z = 674.70 (M+H+).
17f3-(pyridin-2-yl)amido-313-hydroxy-21-oxolup-18-ene 24-2
O
H O
N \
H
H
HO Z
To a stirring suspension of compound 12 (107.7 mg, 0.22 mmol) and picolinic
acid
10 (41.3 mg, 0.34 mmol) in DMF (2.2 mL) is added TEA (71 L, 0.56 mmol) and
HATU
(127.6 mg, 0.34 mmol). The mixture is stirred for 2 hours at room temperature.
Water is added slowly and the precipitate formed is collected by filtration to
give
the title compound 24-2 as an off-white solid (120 mg).
'H NMR (400 MHz, CDCl3): S [ppm] 8.54 (m, 1 H), 8.33 (br s, 1 H), 8.13 (m, 1
H), 7.83
(t x d, 1 H), 7.43 (d x d, 1 H), 3.19 (m, 2H), 2.86 (t, 2H), 2.47 (m, 1 H),
2.37 (d, 1 H),
1.96 (m, 3H), 1.76-1.16 (m, 18H), 1.04-0.86 (m, 1OH), 0.83 (s, 3H), 0.78 -
0.66 (m,
4H).
30-0- (3' , 3' -Dimethylsuccinyl)-17/3-(pyridin-2-yl)amido-21-oxolup-18-ene
20 hydrochloride 19-15
O
H O CIH
N
O H
H
H01r\~
O H
O
A stirring solution of compound 24-2 (113 mg, 0.21 mmol), DMAP (30.3 mg, 0.25
mmol) and 2,2-dimethylsuccinic anhydride (105.9 mg, 0.83 mmol) in dry pyridine
(2.0 mL) is heated at 130 C for 4 hours. Another 0.42 mmol of 2,2-
dimethylsuccinic
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
76
anhydride is added and heating at 130 C is continued for 24 hours. The mixture
is
cooled down to room temperature and concentrated to dryness. The residue is
diluted in ethyl acetate, washed twice with HCl 1N, water and brine, dried
over
sodium sulfate and concentrated to dryness. The residue is purified by flash
column
chromatography on silica gel (methanol/DCM 0% to 10%) to yield the title
compound 19-15 as a pate yellow solid (137.6 mg).
'H NMR (400 MHz, CDCl3): 6 [ppm] 8.52 (m, 1 H), 8.32 (br s, 1 H), 8.12 (m, 1
H), 7.82
(t x d, 1 H), 7.42 (d x d, 1 H), 4.46 (d x d, 1 H), 3.17 (m, 1 H), 2.85 (m,
2H), 2.60 (q,
2H), 2.47 (m, 1H), 2.37 (d, 1H), 1.96 (m, 3H), 1.74-1.16 (m, 24H), 1.02-0.98
(m,
4H), 0.95 (s, 3H), 0.84 - 0.72 (m, 10H).
LC/MS: m/z = 675.7 (M+H+).
Ureas 17 and carbamates 20 can also be prepared from the isocyanate 8 as
described in scheme 9.
O
H
NCO
H
R1O H H R3N~R3 8 or 10 1) R6OH
R1 =H, Ac 2) KOH if R1 = Ac
2)KOHifR1=Ac
O 0
H O O
H~NR3 HO-R6
H R3 H
HO H 22 HO H 25
O O Pyridine 0 /j/ amp Pyridine
O~ 7
DMAP A DMAP
A
O 0
OH O H O
NNRs
HO-R6
O O Fi R. O O -
HO AO s HO Ik A~O H
7 H 20
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
77
Scheme 9
General procedure for the synthesis of ureas 17
Step 1: A solution of isocyanate 8 or 10 and the desired amine R3'R3NH in
solvents
such as benzene, toluene or chloroform is stirred for 4 to 20 hours at room
temperature or under reflux. The residue obtained is purified by flash
chromatography on silica gel to afford the desired urea intermediate 22.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 22 (R1 = H).
Step 2: A solution of urea 22 (R1 = H), a base such as DMAP, TEA, DABCO or
DIPEA
and the appropriate anhydride (2 to 10 equivalents) in solvents such as
pyridine,
TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 10 to 24 hours. The
mixture is concentrated, washed with acid and purified by flash column
chromatography on silica gel to yield compound 17.
General procedure for the synthesis of carbamates 20
Step 1: To a stirring solution of isocyanate 8 or 10 in toluene or benzene is
added
the desired alcohol (3 to 10 equivalents) and a catalyst such as titanium (IV)
tert-
butoxide. The resulting mixture is stirred 2 to 4 hours at room temperature.
After
standard workup, the residue obtained is purified by flash chromatography on
silica
gel to afford the desired carbamate intermediate 25.
If R1 = Ac then deprotection occurs in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 25 (R1 = H).
Step 2: A stirring solution of carbamate 25 (R1 = H), a base such as DMAP,
TEA,
DABCO or DIPEA and the appropriate anhydride (2 to 10 equivalents) in solvents
such as pyridine, TEA or toluene (0.2-1.0 M) is heated from 90 to 120 C for 10
to 24
hours. The mixture is concentrated, washed with acid and purified by flash
column
chromatography on silica gel to yield compound 20.
17/3-(isopropytcarbonylamino)-3p-hydroxy-21-oxolup-18-ene 25-1
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
78
O
OH / O
N O
H
H
HO
H
Step 1: To a stirring solution of compound 10 (300 mg, 0.59 mmol) in toluene
(3
mL) is added isopropanot (68 L, 0.88 mmol) and titanium (IV) tert-butoxide
(23
L, 0.06 mmol). The mixture is stirred for 1 hour at room temperature. A
saturated
solution of ammonium chloride and ethyl acetate are added and the organic
layer
is dried over sodium sulfate and concentrated to dryness. The residue is
purified by
flash column chromatography on silica gel (ethyl acetate/hexanes 0% to 50%) to
yield the 17)9-(isopropylcarbonylamino)-3/acetoxy-21-oxolup-18-ene as white
foam
(349 mg).
'H NMR (400 MHz, CDCl3): 8 [ppm] 4.85 (m, 1 H), 4.73 (br s, 1 H) 4.45 (d x d,
1 H),
3.12 (m, 1H), 2.83 (m, 1H), 2.59 (m, 1H), 2.23 (d, 1H), 2.02,(s, 3H), 1.88 (m,
3H),
1.78-1.13 (m, 25H), 1.11 (s, 3H), 1.03 (m, 1H), 0.91 (s, 3H), 0.90 (s, 3H),
0.83 .(s,
3H), 0.82 (s, 3H), 0.79 (m, 1 H).
Step 2: To a solution of 17#-(isopropylcarbonylamino)-3,(-acetoxy-21-oxolup-18-
ene
(349 mg, 0.6 mmol) in 2 mL of methanol and 6 mL of THE is added an aqueous 4N
solution of potassium hydroxide (1.5 mL, 6 mmol). The reaction mixture is
stirred
for 24 hours at room temperature, acidified with 4N HCl and extracted with
ethyl
acetate. The organic layer is dried over sodium sulfate and concentrated to
dryness
to yield the title compound 25-1 (330 mg).
'H NMR (400 MHz, CDCl3): 6 [ppm] 4.80 (br s, 1 H), 4.65 (br s, 1 H) 3.15 (d x
d, 1 H),
3.05 (m, 1 H), 2.80 (m, 1 H), 2.55 (m, 1 H), 2.20 (d, 1 H), 1.85 (m, 4H), 1.60-
1.08 (m,
27H), 1.06 (s, 3H), 0.94 (m, 1H), 0.91 (s, 3H), 0.88 (s, 3H), 0.83 (s, 3H),
0.70 (s,
3H), 0.65 (m, 1 H).
30-0- (3' , 3' -Dimethylsuccinyl)-17/3-(isopropylcarbonylamino)-21-oxolup-18-
ene
20-2
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
79
O
H O
N)~O
O = H
HOY / K H .
O
A stirring solution of compound 25-1 (327 mg, 0.6 mmol), DMAP (91 mg, 0.74
mmol) and 2,2-dimethylsuccinic anhydride (476 mg, 3.7 mmol) in dry pyridine
(6.0
ml-) is heated at 130 C for 24 hours. The mixture is cooled down to room
temperature and concentrated to dryness. The residue is diluted in ethyl
acetate,
washed twice with HCl 1 N, water and brine, dried over sodium sulfate and
concentrated to dryness. The residue is purified by flash column
chromatography
on silica gel (methanol/DCM 0% to 10%) and crystallized in water/methanol to
yield
the title compound 20-2 as a white solid (286 mg).
'H NMR (400 MHz, CDCl3): 8 [ppm] 4.87 (br s, 1 H), 4.70 (br s, 1 H), 4.48 (d x
d, 1 H),
3.12 (m, 1H), 2.83 (m, 1H), 2.60 (m, 3H), 2.25 (m, 1H), 1.89 (m, 3H), 1.76-
0.94 (m,
39H), 0.92 (s, 3H), 0.89 (s, 3H), 0.85-0.75 (m, 7H).
LC/MS; m/z = 656.7 (M+H').
Table 1 illustrates some intermediateswhich are synthesized using the
procedures
described herein.
Cpd# Structure Name Analytical data
O
3p-0-acetyl-173-
11 q q o NH,HCI amino-21-oxotup- LCMS (M'/M+H'): 485.56
" 18-ene
hydrochloride
XH
H 3p-hydroxy-17p-.
12 NH2 HCI amino-21-oxolup- LC/MS (M'/M+H'): 442.56
18-ene
HO H H hydrochloride
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
OH 0 3p-hydroxy-17p-
22-1 H N (benzyl)ureido]-21 - LC/MS (M+/M+H+): 575.66
Ho oxolup-18-ene
H
0 0 3p-hydroxy-17p-
H H [N'-3p-hydroxy-
22 2 H H'. = oxotup-18-ene LC/MS (M+/M+H+): 911.39
H H 17p-ureido]-21-
"' a H H oxolup-18-ene
1H NMR (400 MHz, CDCl3): 6 [ppm] 7.73
(m, 2H), 7.50 (m, 1H), 7.43 (m, 2H), 6.13
H / 30-hydroxy-17p- (s, 1H), 3.19 (m, 2H), 2.90 (m, 1H), 2.8
24-1 H I ` benzamido-21- (d, 1 H), 2.40 (m, 1 H), 2.35 (d, 1 H), 1.9
H oxotup-18-ene (m, 2H), 1.80-1.20 (m, 20H), 1.14 (s, 3H),
HO a 0.96 (m, 6H), 0.86 (m, 3H), 0.75 (s, 3H),
0.70 (m, 1H).
1H NMR (400 MHz, CDCl3): S [ppm] 8.5
o (m, 1H), 8.33 (br s, 1H), 8.12 (m, 1H),
H 3p-hydroxy-17p- 7.83 (m, 1H), 7.43 (m, 1H), 3.19 (m, 2H),
24 2 " (pyridin-2- 2.86 (m, 2H), 2.47 (m, 1 H), 2.38 (d, 1 H),
" I . yl)amido-21- 1.96 (m, 3H), 1.78-0.64 (m, 14H), 1.25 (d,
HO " oxotup-18-ene 3H), 1.23 (d, 3H), 1.03 (s, 3H), 0.97 (s,
" 3H), 0.95 (s, 3H), 0.83 (s, 3H), 0.73 (s,
3H).
0 3p-hydroxy-17p-(5-
H / methyl- HPLC : 24.98
24-3 HEN [1,3,4]oxadiazole- Method (E)
H = ~ 2 amido) 21 LC/MS (M+/M+H+): 552.34
HO H oxotup-18-ene
O3p-hydroxy-17p-(1-
0
" ~ 00
H OH
24-4 H" ~1 tert- butytoxycarbonytpi LC/MS (M+/M+H+): 553.61
peridine-4-amido)- (-Boo)
H a 0),l 21-oxolup-18-ene
0
H 3p-hydroxy-17p-
24 5 H (pyridin-3- LC/MS (M+/M+H+): 547.50
N yl)amido-21-
HO oxolup-18-ene
H
0
" / I F 3p-hydroxy-17p-(4-
24-6 fluorobenzyl)amido LC/MS (M+/M+H+): 578.50
H -21-oxolup-18-ene
HO
H
H NMR (400 MHz, CDCl3): S [ppm] 8.7
" / 3p-hydroxy-17p- (m, 2H), 7.55 (m, 2H),6.21 (br s, 1 H), 3.18
24-7 H (pyridin-4- (m, 2H), 2.86 (m, 2H), 2.40 (m, 2H), 1.9
H - iN yl)amido-21- (m, 3H), 1.79-0.68 (m, 14H), 1.23 (d, 3H),
Ho oxolup-18-ene 1.21 (d, 3H), 1.13 (s, 3H), 0.98 (s, 3H),
" 0.96 (s, 3H), 0.86 (s, 3H), 0.75 (s, 3H).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
81
'H NMR (400 MHz, CDCl3): S [ppm] 9.35 (d,
0 33 hydroxy 173 1H), 8.75 (d, 1H), 7.52 (d x d, 1H), 3.18
" N(m, 2H), 2.83 (m, 2H), 2.46 (m, 1H), 2.38
(pyrazin 2
24 8 " (d, 1H), 1.94 (m, 3H), 1.78-0.68 (m, 14H),
H yl)amido-21- 1.25 (d, 3H), 1.23 (d, 3H), 1.02 (s, 3H),
Ho oxolup 18 ene
H 0.98 (s, 3H), 0.95 (s, 3H), 0.83 (s, 3H),
0.74 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 7.33 (b
0 30-hydroxy-17)3-(1- s, 1H), 6.95 (br s, 1H), 6.73 (br s, 1H),
"", methyl-1H- 3.90 (s, 3H), 3.16 (m, 3H), 2.83 (m, 2H),
249 " pyrazole 3 amido) 2.38 (m, 2H), 1.94 (m, 3H), 1.78-0.66 (m,
H0 21-oxolup 18 ene 13H), 1.23 (d, 3H), 1.21 (d, 3H), 1.06 (s,
H 3H), 0.95 (s, 6H), 0.84 (s, 3H), 0.74 (s,
3H).
1H NMR (400 MHz, CDCl3): S [ppm] 7.48 (b
0 30-hydroxy-173-(1- s, 1H), 7.41 (br s, 1H), 5.92 (br s, 1H),
H o 1 methyl-1 H- 3.85 (s, 3H), 3.17 (m, 2H), 2.83 (m, 2H),
24-10 H IlyN)
imidazole-5- 2.38 (m, 2H), 1.92 (m, 3H), 1.78-0.68 (m,
H - amido)-21-oxolup- 14H), 1.22 (d, 3H), 1.21 (d, 3H), 1.12 (s,
HO
XH 18-ene 3H), 0.95 (s, 6H), 0.86 (s, 3H), 0.75 (s,
3H).
0 33-hydroxy-17p-(1 H NMR (400 MHz, CDCl3): 6 [ppm] 7.45 (b
H 0 methyl-1 H- s, 1 H), 7.33 (br s, 1 H), 7.18 (br s, 1 H),
24-11 N imidazoLe-4- 3.70 (s, 3H), 3.16 (m, 2H), 2.83 (m, 2H),
H " amido)-21-oxolup- 2.38 (m, 2H), 1.93 (m, 3H), 1.78-0.68 (m,
HO 18-ene 14H), 1.22 (br s, 6H), 1.04 (s, 3H), 0.94 (s,
" hydrochloride 6H), 0.82 (s, 3H), 0.73 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 7.48
o (m, 1H), 7.46 (m, 1H), 7.06 (d x d, 1H),
H 0 3p-hydroxy-173-(2- 5.97 (br s, 1H), 3.17 (m, 2H), 2.85 (m,
24-12 H thiophene)amido- 2H), 2.36 (m, 2H), 1.95 (m, 3H), 1.78-0.6
H 21-oxolup-18-ene (m,. 14H), 1.23 (s, 3H), 1.21 (s, 3H), 1.11
H0 H (s, 3H),0.96 (s, 3H), 0.95 (s, 3H), 0.85 (s,
3H), 0.74 (s, 3H).
'H NMR (400 MHz, CDCl3): S [ppm] 7.84 (d,
H 33-hydroxy-173- 1 H), 7.56 (d, 1 H), 7.41 (br s, 1 H), 3.1
24 13 " N (1,3-thiazole-2- (m, 2H), 2.85 (m, 2H), 2.40 (m, 2H), 1.95
" S~ amido)-21-oxolup- (m, 3H), 1.80-0.67 (m, 14H), 1.23 (s, 3H),
Ho OH 18-ene 1.21 (s, 3H), 1.12 (s, 3H), 0.98 (s, 3H),
0.94 (s, 3H), 0.83 (s, 3H), 0.73 (s, 3H).
0 33-O-acetyl-173-
" (5-methyl- HPLC : 39.41
24 14 H AN [1,3,4]oxadiazole- Method (E)
~ 2 amido) 21 LC/MS (M`/M+H`): 594.33
0 a oxolup-18-ene
o H NMR (400 MHz, CDCl3): S [ppm] 9.33 (s,
0 30-hydroxy-173- 1H), 9.06 (s, 2H), 6.13 (brs, 1H), 3.17 (m,
24-15 (pyrimidine-5- 2H), 2.80 (m, 2H), 2.41 (m, 2H), 1.95 (m,
H ) amido)-21-oxolup- 2H), 1.78-0.66 (m, 15H), 1.23 (s, 3H), 'Ir
N 18-ene 1.21 (s, 3H), 1.05 (s, 3H), 0.98 (s, 3H),
HO 0.96 (s, 3H), 0.86 (s, 3H), 0.75 (s, 3H).
o H -NMR (400 MHz, CDCl3): 6 [ppm] 6.86 (b
0 3p-hydroxy-17p-(5- s, 1H), 6.38 (d, 1H), 3.17 (m, 2H), 2.81
methylisoxazole-3- (m, 2H), 2.46 (s, 3H), 2.38 (m, 2H), 1.92
24 16 H" Y o amido)-21-oxolup- (m, 2H), 1.78-0.66 (m, 14H), 1.23 (s, 3H),
18-ene
1.20 (s, 3H), 1.07 (s, 3H), 0.95 (s, 6H),
H0 0.84 (s, 3H), 0.74 (s, 3H).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
82
'H NMR (400 MHz, CDCl3): S [ppm] 9.24 (d,
O 33-hydroxy-173- 1 H), 8.96 (d, 1 H), 8.22 (br s, 1 H), 8.05 (d
00 (pyrimidine-4- x d, 1H), 3.18 (m, 2H), 2.83 (m, 2H), 2.41
24-17 _ H' N `N amido) 21 oxolup (m, 2H), 1.95 (m, 2H), 1.80-0.66 (m,
18-ene 15H), 1.24 (s, 3H), 1.22 (s, 3H), 1.03 (s
Ho ,
3H), 0.97 (s, 3H), 0.95 (s, 3H), 0.83 (s,
3H), 0.74 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 7.53 (b
o 33-hydroxy-17(3-(1- s, 1H), 6.96 (d, 1H), 6.93 (d, 1H), 4.00 (s,
0II methyl-1 H- 3H), 3.16 (m, 2H), 2.89 (m, 1H), 2.80 (d,
24-18 NN imidazole-2- 1 H), 2.53 (m, 1 H), 2.38 (d, 1 H), 1.94 (m,
" INS amido)-21-oxolup- 3H), 1.78-0.68 (m, 14H), 1.22 (d, 3H),
H0 18-ene 1.20 (d, 3H), 1.09 (s, 3H), 0.95 (s, 6H),
0.85 (s, 3H), 0.74 (s, 3H).
o H NMR (400 MHz, CDCl3): S [ppm] 8.73 (d,
0 33-hydroxy-17[3- I H), 8.11 (d, 1H), 7.56 (br s, 1H), 3.1
(1,3-thiazole-4- (m, 2H), 2.83 (m, 2H), 2.39 (m, 2H), 1.95
24 19 H s> amido)-21-oxolup- (m, 3H), 1.79-0.66 (m, 14H), 1.24 (s, 3H),
18-ene 1.22 (s, 3H), 1.04 (s, 3H), 0.96 (s, 3H),
HO 0.95 (s, 3H), 0.83 (s, 3H), 0.74 (s, 3H).
0 H NMR (400 MHz, CDCl3): S [ppm] 8.87 (s,
0 33-hydroxy-173- 1H), 8.21 (s, 1H), 6.04 (br s, 1H), 3.17 (m,
24-20 (1,3-thiazole-5- 2H), 2.83 (m, 2H), 2.38 (m, 2H), 1.95 (m,
H JN amido)-21-oxolup- 3H), 1.79-0.66 (m, 14H), 1.23 (s, 3H),
H0 18-ene 1.21 (s, 3H), 1.10 (s, 3H), 0.97 (s, 3H),
0.95 (s, 3H), 0.85 (s, 3H), 0.74 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 7.43 (d,
33 hydroxy 173 (1 1 H), 6.46 (d, 1 H), 5.97 (br s, 1 H), 4.10 (s,
" N methyl-1H 3H), 3.18 (m, 3H), 2.83 (m, 2H), 2.38 (m,
24-21 N 2H), 1.94 (m, 3H), 1.78-0.66 (m, 14H),
H _ pyrazole 5 amido) 1.23 (d, 3H), 1.21 (d, 3H), 1.13 (s, 3H),
NO H 21-oxolup-18-ene 0.96 (s, 3H), 0.95 (s, 3H), 0.87 (s, 3H),
0.75 (s, 3H).
0 H NMR (400 MHz, CDCl3): S [ppm] 7.78 (s,
H QQ 33-hydroxy-173-(1- 1 H), 7.66 (d, 1 H), 5.73 (br s, 1 H), 3.90 (s,
24-22 N_ methyl-1H- 3H), 3.17 (m, 2H), 2.83 (m, 2H), 2.34 (m,
N pyrazole-4-amido)- 2H), 1.93 (m, 3H), 1.78-0.66 (m, 14H),
HO " 21-oxolup-18-ene 1.23 (d, 3H), 1.21 (d, 3H), 1.07 (s, 3H),
" 0.95 (s, 6H), 0.84 (s, 3H), 0.74 (s, 3H).
0
H 0 33-hydroxy-173-(3- HPLC : 36.91
24-23 H methylbenzamido)- Method (E)
H 21-oxotup-18-ene LC/MS (M'/M+H'): 560.32
HO
H
O 33-hydroxy-17,8-
" (2,6- HPLC : 16.51
24 24 H VIIN dimethylpyridin-4- Method (C)
H yl)amido-21- LC/MS (M+/M+H'): 575.49
HO H oxotup-18-ene
0 33-hydroxy-173-
" ~ ((RS)1- HPLC : 21.51; 22.02(1:1)
24-25 " Y 1 methylpiperidine- Method (D)
H 2-amido)-21 - LC/MS (M+/M+H+): 567.51
H H oxolup-18-ene
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
83
'H NMR (400 MHz, CDCL3): S [ppm] 7.21 (s,
o 3p-hydroxy-17p-[2- 1 H), 3.18 (m, 2H), 2.93 (d, 2H), 2.70 (m,
" " (4- 2H), 2.60 (m, 4H), 2.45 (m, 3H), 2.30 (s,
24-26 methylpiperazin-1- 3H), 2.25 (m, 2H), 2.00-0.68 (m, 18H),
H yl)-acetamido]-21- 1.21 (d, 3H), 1.20 (d, 3H), 1.05 (s, 3H),
HO H oxolup-18-ene 0.96 (s, 3H), 0.93 (s, 3H), 0.87 (s, 3H),
0.75 (s, 3H).
o 'H NMR (400 MHz, CDCl3): 6 [ppm] 7.24 (s,
H o 1 H), 3.18 (m, 2H), 2.88 (d, 2H), 2.78 (m,
3p-hydroxy-17(3-(2- 1H 2.70 d 1H 2.35 m 2H), 2.31 s
24-27 dimethylamino- )' ( )' ( )' ( '
H acetamido) 21 6H), 2.00 0.68 (m, 17H), 1.21 (d, 3H),
1.20 (d, 3H), 1.09 (s, 3H), 0.96 (s, 3H),
Ho H oxolup 18 ene 0.93 (s, 3H), 0.87 (s, 3H), 0.75 (s, 3H).
0 0 3p-hydroxy-17(3-(2
" HPLC : 20.69
24 28 r,)V methylpyridin-4- ( )
" ,N yl)amido-21- Method C
Ho " oxolup-18-ene LC/MS (M'/M+H'): 561.44
OH Yl 3p-hydroxy-17(3
((RS)2- HPLC :11.19; 11.54
24-29 Method (C)
dimethylamino-
propionamido)-21 - LC/MS (M'/M+H'): 541.40
HO
oxolup-18-ene
0
" 0 ~N 3p-hydroxy-17p-(2- HPLC : 11.44
24-30 imidazol-1-yl- Method (C)
H acetamido)-21- LC/MS (M'/M+H'): 550.39
Ho H oxolup-18-ene
O0
H 3p-hydroxy-173-(6- HPLC : 13.96
24 31 N methylpyridin 3 ( )
" yl)amido-21
HO N oxotup-18-ene LC/MS^(M'/M+H'): 561.32
H
0
03p-hydroxy-17p-(4- HPLC : 12.53
OHH
24 32 N methylpyridin-3-
N
" 6 yl)amido 21 LC/MSnM'I/M+ (HE)
): 561.32
HO oxolup-18-ene
0
H 3p-hydroxy-17p-(5- HPLC : 17.94
24-33 methylpyridin 3
" N yl)amido-21 LC/MSMM'hM+(HE)
): 561.31
oxolup-18-ene
HO
H
0
H 0 3p-hydroxy-17p-(2- HPLC : 10.57
24-34 methylpyridin-3-
" yl)amido-21- Method (E)
HO H N oxolup-18-ene LC/MS (M'/M+H'): 561.31
H
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
84
0
H I ~6 3p-hydroxy-17(3-(2- HPLC : 35.40
24-35 H methylbenzamido)- Method (E)
H 21 oxolup 18 ene LC/MS (M'/M+H+): 560.30
H
H
H NMR (400 MHz, CDCl3): S [ppm] 9.30 (b
3,13-hydroxy-17,13-(3- s, 1H), 3.18 (d x d, 1H), 3.12 (m, 1H),
OH 2.73 (m, 3H), 2.55 (m, 3H), 2.32 (m, 3H),
N N pyrrolidin-1-yl24 36 propionamido)-21 2.28 (m, 4H), 2.05 (s, 3H), 1.94-
0.66 (m,
oxolup 18 ene 24H), 1.20 (d, 3H), 1.17 (d, 3H), 1.10 (s,
Ho H 3H), 0.96 (s, 3H), 0.91 (s, 3H), 0.87 (s,
3H), 0.75 (s, 3H).
0
H 03/3-hydroxy-1713-(2- 'H NMR (400 MHz, CDCl3): S [ppm] 7.30 (b
24-37 N IIv ND pyrrolidin-1-yl- s, 1 H), 3.19 (m, 2H), 3.02 (d, 2H), 2.69
" acetamido)-21- (m, 2H), 2.58 (m, 4H), 2.26 (m, 2H), 1.95-
HO " oxotup-18-ene 0.62 (m, 42H).
H
H / 0 NO 3Q-hydroxy-17Q-(4- 'H NMR (400 MHz, CDC13): S [ppm] 6.83 (b
24 38 N pyrrolidin-1-yl- s, 1H), 3.19 (m, 1H), 3.11 (m, I H), 2.77
" butyramido)-21- (m, 1H), 2.66 (d, 1H), 2.48 (m, 5H), 2.28
HO H H oxolup-18-ene (m, 3H), 2.05 (s, 3H), 2.00 0.66 (m, 46H).
O H NMR (400 MHz, CDCl3): S [ppm] 9.30 (b
H 3Q-hydroxy-17/3-(3- s, 1H), 3.15 (m, 2H), 2.84 (m, 1H), 2.70-
24-3 9 N ~N piperidin-1-yl- 2.26 (m, 10H), 1.92 (m, 3H), 1.78-0.66
" propionamido)-21- (m, 21H), 1.20 (d, 3H), 1.18 (d, 3H), 1.09
HO H H oxolup-18-ene (s, 3H), 0.96 (s, 3H.), 0.92 (s, 3H), 0.87 (s,
3H), 0.75 (s, 3H).
H NMR (400 MHz, CDCl3): S [ppm] 6.63 (b
H / 3,Q-hydroxy-17Q-[3- s, 1H), 3.62-3.38 (m, 4H), 3.18 (m, 5H),
24-40 NJ-IN (2-oxo-pyrrolidin-1- 2.60 (d, 1H), 2.48 (m, 2H), 2.32 (m, 3H),
~S-i yl)-propionamido]- 1.94-0.66 (m, 18H), 1.19 (d, 3H), 1.17 (d,
HO - " 03D
21-oxolup-18-ene 3H), 1.09 (s, 3H), 0.95 (s, 3H), 0.91 (s,
" 3H), 0.87 (s, 3H), 0.75 (s, 3H).
0 3/3-hydroxy-17l-
" j [(S) 1 methyl
24 41 H~ NO pyrrolidine-2- LC/MS (M'/M+H'): 553.27
H - " amido]-21-oxolup-
HO 18-ene
H
0
H 0 3,(3-hydroxy-17,0-(1-
24-42 N ko isopropylazetidine 3 amido) 21 LC/MS (M'/M+H'): 567.30
HO H oxolup-18-ene
H
0
H 0 3p-hydroxy-170-(3-
24 43 H thiophene)amido- LC/MS (M'/M+H'): 552.41
H - 21-oxolup-18-ene
HO
H
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
0 30-hydroxy-17(3-(5- HPLC : 30.83
24-44 methyl-[1,3]-
" oxazole 2 amido) LC/MS~(M+l/M+HE): 551.41
HO " 21-oxolup-18-ene
0
~ON 30-hydroxy-170- HPLC : 11.62
25-1 o isopropytoxycarbon ( )
" ylamino-21-oxolup- Method J
Ho 18-ene LC/MS (M /M+H ): 528.26
O 3R-0-acetyl-170-
H / tert-
25-2 butytoxycarbonyta LCIMS (M'/M+H+): 584.68
H mino]-21-oxolup-
18-ene
O 3(3-hydroxy-170-
H o tert-
25-3 Hx butyloxycarbonyla LC/MS (M'/M+H`): 542.61
H mino]-21-oxolup-
HO 18-ene
0
H 0 l 30-0-acetyl-170-
25-4 Hxo'\ isopropytoxycarbon LC/MS (M'/M+H+): 584.54
ylamino-21-oxotup-
H
18-ene
Fi
1H NMR (400 MHz, CDCl3): 6 [ppm] 4.83 (b
(0 s, 1 H) 4.48 (d x d, 1 H), 3.83 (d, 2H), 3.1
l 0 30.0-acetyl-170
ropylmethox (m, 1H), 2.85 (m, 1H), 2.66 (d, 1H), 2.26
25-5 Hx cyclop
cyclop opy1met (d, 1H), 2.04 (s, 3H), 1.91-0.80 (m, 19H),
21-oxotup-18-ene arbnytam no- 1.22 (s, 3H), 1.21 (s, 3H), 1.13 (s, 3H),
0.93 (s, 3H), 0.92 (s, 3H), 0.85 (s, 3H),
0.84 (s, 3H), 0.53 (d, 2H), 0.25 (d, 2H).
1H NMR (400 MHz, CDCl3): S [ppm] 5.04
30-0-acetyl-170- (m, 1H), 4.74 (br s, 1H) 4.44 (d x d, 1H),
/ cyclopentyl7f3 b 3.10 (m, I H), 2.82(m, I H), 2.60 (d, I H),
25-6 Hx 10 onylamino 21 2.22 (d, 1H), 2.02 (s, 3H), 1.89-0.77 (m,
oxotup-18-ene 27H), 1.16 (s, 3H), 1.13 (s, 3H), 1.10 (s,
3H), 0.90 (s, 3H), 0.89 (s, 3H), 0.82 (s,
3H), 0.81 (s, 3H).
1H NMR (400 MHz, CDCl3): 6 [ppm] 5.04
30-hydroxy-170- (m, 1H), 4.75 (br s, 1H), 3.15 (m, 2H),
25 7 0 cyclopentytoxycarb 2.84(m, 1H), 2.60 (d, 1H), 2.22 (d, 1H),
H onylamino 21 1.89-0.77 (m, 26H), 1.18 (d, 3H), 1.16 (d,
oxolup-18-ene 3H), 1.10 (s, 3H), 0.90 (s, 3H), 0.89 (s,
Ho 3H), 0.82 (s, 3H), 0.81 (s, 3H), 0.70 (m,
1 H).
1H NMR (400 MHz, CDCl3): 8 [ppm] 4.72 (s,
(0 30-0-acetyl-170- 1H), 4.60 (m, 1H) 4.45 (d x d, 1H), 3.11
25 8 l cyclohexyloxycarbo (m, 1H), 2.82(m, 1H), 2.62 (d, 1H), 2.23
O nylamino-21 (d, 1H), 2.02 (s, 3H), 1.90-0.77 (m, 29H),
O H
oxolup 18 ene 1.20 (s, 3H), 1.19 (s, 3H), 1.10 (s, 3H),
0.91 (s, 3H), 0.89 (s, 3H), 0.83 (s, 3H),
0.82 (s, 3H).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
86
H NMR (400 MHz, CDCl3): 8 [ppm] 4.57 (b
0 30-hydroxy-17p- s, 1H), 3.17 (d x d, 1H), 3.11 (m, 1H),
0 2.83 (m, 1H), 2.62 (d, 1H), 2.22 (d, 1H),
25-9 NA0 n cyclohexyloxycarbo 2.02 (s, 3H), 1.88-1.20 (m, 29H), 1.18 (s,
nylamino-21- 3H), 1.16 (s, 3H), 1.09 (s, 3H), 0.94 (s,
Ho oxolup 18 ene 3H), 0.91 (s, 3H), 0.86 (s, 3H), 0.74 (s,
3H).
3p-0-acetyl-17p H NMR (400 MHz, CDCl3): 8 [ppm] 4.76 (s,
(1-tert- 1H), 4.74 (m, 1H) 4.45 (d x d, 1H), 3.72
(m, 2H), 3.13 (m, 1H), 3.12 (m, 2H),
o ~N - butoxycarbonylpipe 2.80(m, 1H), 2.61 (d, 1H), 2.25 (d, 1H),
25-10 Nxo ridin-4
yloxycarbonylamin 2.03 (s, 3H), 1.89 0.78 (m, 23H), 1.42 (s,
o)-21-oxolup-18- 9H), 1.20 (s, 3H), 1.18 (s, 3H), 1.10 (s,
3H), 0.92 (s, 3H), 0.90 (s, 3H), 0.83 (s,
ene 3H), 0.82 (s, 3H).
H NMR (400 MHz, CDCl3): 8 [ppm] 4.76 (s,
(o 3p-0-acetyl-17p- 1H), 4.45 (d x d, 1H), 3.81 (d, 2H), 3.41
cyclohexylmethoxy (d, 1H), 3.11 (m, 1H), 2.82(m, 1H), 2.62
25-11 H ~ carbonylamino-21 (d, 1H), 2.24 (d, 1H), 2.01 (s, 3H), 1.89
oxolup-180.78 (m, 29H), 1.20 (s, 3H), 1.18 (s, 3H),
1.10 (s, 3H), 0.91 (s, 3H), 0.89 (s, 3H),
0.83 (s, 3H), 0.82 (s, 3H).
H NMR (400 MHz, CDCl3): 8 [ppm] 4.82 (s,
3p-hydroxy-170- 1H), 3.81 (d, 2H), 3.40 (d, 1H), 3.18 (d x
o
cyclohexylmethoxy d, 1H), 3.10 (m, 1H), 2.82(m, 1H), 2.62
25-12 H carbonylamino-21- (d, 1H), 2.24 (d, 1H), 1.87-0.78 (m, 29H),
oxolup 18 ene 1.18 (s, 3H), 1.17 (s, 3H), 1.09 (s, 3H),
HO 0.94 (s, 3H), 0.91 (s, 3H), 0.86 (s, 3H),
0.74 (s, 3H).
H NMR (400 MHz, CDCl3): 5 [ppm] 7.32,
3p-0-acetyl-170- (m, 5H), 5.03 (s, 2H), 4.84 (b s, 1H), 4.45
o (d x d, 1H), 3.11 (m, 1H), 2.80 (m, 1H),
25-13 NJLO benzy(oxycarbonyla 2.63 (d, 1H), 2.25 (d, 1H), 2.03 (s, 3H),
mino-21-oxotup-18- 1.87-0.77 (m, 19H), 1.20 (s, 3H), 1.18 (s,
ene 3H), 1.06 (s, 3H), 0.91 (s, 3H), 0.90 (s,
3H), 0.83 (s, 3H), 0.82 (s, 3H).
H NMR (400 MHz, CDCl3): 8 [ppm] 4.77 (b
s, 1H) 4.48 (d x d, 1H), 4.15 (m, 1H), 3.15
3p-0-acetyl-17p- (m, 1H), 2.86 (m, 1H), 2.66 (d, 1H), 2.26
cyclopropyimethox (d, 1H), 2.05 (s, 3H), 1.94-0.81 (m, 19H),
25 14 H ycarbonylamino- 1.63 (s, 3H), 1.23 (s, 3H), 1.22 (s, 3H),
21-oxotup-18-ene 1.14 (s, 3H), 0.95 (s, 3H), 0.93 (s, 3H),
0.86 (s, 3H), 0.85 (s, 3H), 0.39 (m, 3H),
0.21 (m, 1 H).
H NMR (400 MHz, CDCl3): 6 [ppm] 4.85
0 (br s, 1 H) 4.48 (d x d, 1 H), 3.83 (s, 2H),
3(3-0-acetyl-17p- 3.15 (m, 1 H), 2.82 (m, 1 H), 2.69 (d, 1 H),
25-15 JL0 cyclopropyimethox 2.28 (d, 1 H), 2.04 (s, 3H), 1.93-0.80 (m,
HI ycarbonylamino- 18H), 1.23 (s, 3H), 1.21 (s, 3H), 1.14 (s,
21-oxolup-18-ene 3H), 1.09 (s, 3H), 0.94 (s, 3H), 0.92 (s,
3H), 0.85 (s, 3H), 0.84 (s, 3H), 0.45 (d,
2H), 0.33 (d, 2H).
H NMR (400 MHz, CDC(3): 5 [ppm] 4.87
3Q-acetoxy-17,Q (m, 1H), 4.78 (br s, 1H) 4.44 (d x d, 1H),
r fJ cyclobutyloxycarbo 3.11 (m, I H), 2.82(m, 1H), 2.62 (d, I H),
25-16 H 'flIO nylamino-21 2.22 (d, 1H), 2.01 (s, 3H), 2.00-0.77 (m,
oxolup-18-ene 25H), 1.14 (s, 3H), 1.11 (s, 3H), 1.10 (s,
3H), 0.90 (s, 3H), 0.89 (s, 3H), 0.82 (s,
3H), 0.81 (s, 3H).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
87
1H NMR (400 MHz, CDCl3): S [ppm] 4.87 (s,
0 3,8-acetoxy-17,6- 1 H), 4.60 (m, 1 H) 4.43 (d x d, 1 H), 3.60
o r`s tetrahydro- (m, 1 H), 3.10 (m, 1 H), 2.80(m, 1 H), 2.60
25-17 Hx0 thiopyran-4- (m, 3H), 2.53 (d, 1H), 2.23(d, 1H), 2.01
yloxycarbonylamin (s, 3H), 1.87-0.76 (m, 23H), 1.18 (s, 3H),
o-21-oxolup-18-ene 1.16 (s, 3H), 1.08 (s, 3H), 0.90 (s, 3H),
0.88 (s, 3H), 0.81 (s, 3H), 0.80 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 4.77 (s,
0 1H), 4.47 (m, 1H) 4.45 (d x d, 1H), 3.11
0 3Q-acetoxy-17f3-(1- (m, 1H), 2.81 (m, 1H), 2.64 (m, 1H), 2.59
"xo " methyl-piperidin 4 (d, 1H), 2.26 (d, 1H), 2.24(s, 3H), 2.14
25-18 H yloxycarbonylamin
o o)-21-oxolup-18- (m, 2H), 2.02 (s, 3H), 1.88-0.77 (m, 23H),
1.20 (s, 3H), 1.18 (s, 3H), 1.10 (s, 3H),
ene 0.91 (s, 3H), 0.89 (s, 3H), 0.82 (s, 3H),
0.81 (s, 3H).
1H NMR (400 MHz, CDCl3): 8 [ppm] 4.96 (b
o 3,6-acetoxy-17,8- s, 1 H), 4.44 (d x d, 1 H) 4.10 (m, 1 H), 3.95
((S)-1-Methyl- (d x d, 1H), 3.13 (m, 2H), 2.80 (m, 1H),
25-19 Jl pyrrolidin-2-yl)- 2.60 (d, 1H), 2.35 (s, 4H), 2.11 (m, 2H),),
H methoxycarbonyta 2.01 (s, 3H), 1.90-0.77 (m, 21H), 1.19 (d,
mino-21-oxolup-18- 3H), 1.17 (d, 3H), 1.08 (s, 3H), 0.90 (s,
N ene 3H), 0.89 (s, 3H), 0.82 (s, 3H), 0.81 (s,
3H).
H NMR (400 MHz, CDCl3): 8 [ppm] 5.20 (b
3p-acetoxy-17,8-(2- s, 1H), 4.46 (d x d, 1H), 4.15 (br s, 2H),
0 pyrrolidin-1- 3.14 (m, 1H), 2.85 (m, 1H), 2.65 (m, 7H),
25-20 Hx0 ylethoxycarbonyla 2.28 (m, 4H), 2.05 (s, 3H), 1.88-0.80 (m,
= mino)-21-oxolup- 19H), 1.22 (d, 3H), 1.20 (d, 3H), 1.11 (s,
o N U 18-ene 3H), 0.93 (s, 3H), 0.92 (s, 3H), 0.85 (s,
3H), 0.84 (s, 3H).
1H NMR (400 MHz, CDCl3): S [ppm] 4.74 (s,
(0 3p-acetoxy-17,6- 1H) 4.43 (d x d, 1H), 4.04 (d, 2H), 3.10
opylethoxyc (m, 1 H), 2.81 (m, 1 H), 2.61 (d, 1 H), 2.2
25-21 o HA0 cyclop cyclopropyiethox (d, 1H), 2.00 (s, 3H), 1.86-0.77 (m, 21H),
- oxolup-18-ene 1.18 (s, 3H), 1.16 (s, 3H), 1.08 (s, 3H),
0.89 (s, 3H), 0.87 (s, 3H), 0.80 (s, 3H),
0.79 (s, 3H), 0.38 (m, 2H), 0.00 (m, 2H).
3/3-0-acetyl-17,8 'H NMR (400 MHz, CDCl3): S [ppm] 4.86 (b
(1-tert- s, 1 H) 4.45 (d x d, 1 H), 3.55 (d, 2H), 3.1
j "A0~ butoxycarbonylami (m, 1 H), 2.82 (m, 1 H), 2.64 (d, 1 H), 2.25
25-22 " o no- (d, 1H), 2.02 (s, 3H), 1.89-1.16 (m, 18H),
cyclopropylmethox 1.41 (s, 9H) 1.20 (s, 3H), 1.18 (s, 3H),
ycarbonytamino)- 1.11 (s, 3H), , 0.92 (s, 3H), 0.89 (s, 3H),
21-oxolup-18-ene 0.83 (s, 3H), 0.82 (s, 3H), 0.80 (m, 4H).
0 3/3-acetoxy-17,6-(1 H NMR (400 MHz, CDCl3): S [ppm] 5.26
0 methyl-pyrrolidin- (m, 1H), 5.10 (br s, 1H), 4.89 (d x d, 1H),
4 X CN- 3.11 (m, 1H), 2.85 (m, 2H), 2.60 (d, 1H),
25-23 0 H yloxycarbonylamin 2.45-2.20 (m, 8H), 2.02 (s, 3H), 1.92-0.72
A0 o) 21 oxolup 18 (m, 22H), 1.19 (s, 3H), 1.17 (s, 3H), 1.08
ene (s, 3H), 0.90 (s, 3H), 0.89 (s, 3H), 0.82 (s,
3H), 0.81 (s, 3H).
0 3(3-0-acetyl-17(3- H NMR (400 MHz, CDCl3): S [ppm] 4.83 (b
0 (1-methylpiperidin- s, 1H), 4.48 (d x d, 1H), 3.88 (m, 2H),
"xo 4- 3.12 (m, 1H), 2.85 (m, 3H), 2.60 (d, 1H),
25-24 0 H " 2.28 (s, 3H), 2.20 (m, 2H), 2.02 (s, 3H),
ylmethoxycarbonyl 1.99-0.77 (m, 24H), 1.19 (d, 3H), 1.17 (d,
amino) 21 oxolup 3H), 1.09 (s, 3H), 0.90 (s, 3H), 0.89 (s,
18-ene 3H), 0.82 (s, 3H), 0.81 (s, 3H).
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
88
1H NMR (400 MHz, CDC13): 6 [ppm] 4.88 (b
0 30-0-acetyl-17R- s, 1H), 4.45 (d x d, 1H), 3.92 (m, 1H),
0 (1-methylpiperidin- 3.82 (m, 1H), 3.10 (m, 1H), 2.80 (m, 3H),
25 25 Nx N- 3- 2.60 (d, 1H), 2.28 (s, 3H), 2.20 (m, 2H),
= ylmethoxycarbonyl 2.02 (s, 3H), 1.99-0.75 (m, 24H), 1.19 (d,
amino)-21-oxolup- 3H), 1.17 (d, 3H), 1.10 (s, 3H), 0.90 (s,
18-ene 3H), 0.88 (s, 3H), 0.82 (s, 3H), 0.81 (s,
3H).
3,8-acetoxy-17Q-(1 H NMR (400 MHz, CDCl3): 6 [ppm] 5.75 (b
s, 1H), 4.45 (dx d, 1H), 3.90 (m, 2H) 3.2
O Methyl-pyrrolidin (m, 1H), 2.85 (m, 1H), 2.75-2.40 (m, 7H),
25-26 o Hx methoxycarbonyla 2.35 (s, 3H), 2.20 (m, 4H), 2.00 (s, 3H),
~o 10 mino-21-oxolup-18- 1.99-0.76 (m, 16H), 1.18 (s, 3H), 1.16 (s,
ene 3H), 1.09 (s, 3H), 0.89 (s, 3H), 0.88 (s,
3H), 0.81 (s, 3H), 0.80 (s, 3H).
1H NMR (400 MHz, CDCl3): 6 [ppm] 4.86 (b
0 3Q acetoxy 17Q [3 s, 1H), 4.45 (d x d, 1H), 4.15 (m, 2H) 3.43
(m, 4H), 3.12 (m, 1 H), 2.80 (m, 1 H), 2.6
-
25-27 N NO (2-oxo pyrrolidin-1 (d, 1 H), 2.34 (m, 1 H), 2.25 (m, 2H), 2.0
yl)-propionamido]- (s, 3H), 2.00-0.77 (m, 20H), 1.20 (s, 3H),
A0 0- N 21 -oxolup- 1 8-ene 1.18 (s, 3H), 1.10 (s, 3H), 0.90 (s, 3H),
0.89 (s, 3H), 0.83 (s, 3H), 0.82 (s, 3H).
0
0
H H
H 0 0 0
NHBOC NHBOC
NHBOC 0 + 0 H
~~\///
H HO ~( O HO` ^ J~ O H
HO 0 v H 14-1
4H 40~ /X\ 14-4
13
HCI
40NH,HCI
O HOYVj 0
15-1
0
Scheme 10
31 O- (3' , 3' - Di methylsucci nyl) -17/3-tert- butyloxycarbonylami no-21-
oxolup-18-ene
14-1
0
OH 0
H OH
N-k
O -
HO~~ _ H
O
To a stirring mixture of compound 13 (43 g, 0.079 mot), DMAP (11.7 g, 0.095
mot)
in TEA (396 mL) is added 2,2-dimethylsuccinic anhydride (25.4 g, 0.198 mot).
The
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
89
reaction mixture is heated at 95 C overnight. The mixture is cooled down to
room
temperature and concentrated to dryness. The residue is dissolved in ethyl
acetate
(400 mL) and washed twice with a saturated solution of NaHCO3 (100 mL). The
mixture is diluted with 100 mL of EtOAc and 1N HCl (100 mL) is added while
stirring. The mixture is cooled in an ice-bath and stirred for 30 min. The
precipitate formed is collected by filtration to yield the title compound 14-1
as a
white solid (39 g) in a ratio of 97:3 of 14-1:14-4 respectively. The organic
layer of
the filtrate is dried over sodium sulfate and concentrated to dryness. The
solid
obtained (16 g) is recristaltized twice with ethyl methyl ketone to give the
title
compound 14-1 (3.6g) in a ratio of 98:2 of 14-1:14-4 respectively.
HPLC analysis: 14-1 RT : 13.4 min, 14-4 RT : 14.1 min using method (J)
3/3-0-(3',3'-Dimethylsuccinyt)-173-amino-21-oxolup-18-ene hydrochloride 15-1
0
HI
NH2 CIH
O
H
HO
YV_j O H
O
A solution of compound 14-1 (53.6 g, 0.08 mot) in 4N HCt in dioxane (536 mL)
is
stirred at room temperature overnight. The mixture is concentrated to dryness
and triturated with hexanes to give the title compound 15-1 (46 g, 95%) as a
white
solid.
'H NMR (400 MHz, DMSO-d6): 6 [ppm] 4.36 (d x d, 1 H), 3.18 (d x d, 1 H), 3.05
(m,
1H), 2.48 (m, 2H), 2.13 (d, 2H), 2.0 - 1.18 (m, 16H), 1.17 - 0.94 (m, 17H),
0.84 (s,
3H), 0.82 (s, 3H), 0.76 (s, 6H).
The compounds of the present invention wherein Y is C(O) can be prepared as
generally described in schemes 11, 12, 13 or 14.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
O O
H Se02 H 0
NH dioxane NH
H 00 II H OO
HO A O HO A O
14 27
O
H / 0
HCI H NH2 HCI
O O -
H
HOAA O
28
Scheme 11
General procedure:
Step 1: Selenium dioxide (4 to 6 eq.) is added to a solution of compound 14
previously dissolved in dioxane, acetic acid and acetic anhydride. The
reaction
mixture is refluxed overnight, then cooled to room temperature and filtered
through Celite. The residue is dissolved in DCM, washed with water, brine,
dried
10 over sodium sulfate and evaporated to dryness. The crude material is
purified by
flash chromatography on silica gel to yield the compound 27.
Step 2: The compound 27 is deprotected in solvents such as THE or dioxane and
4N
HCI. The mixture is stirred for 12 to 24 hours at room temperature then
concentrated to dryness to give 28.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
91
O
H / 0
NH
H .R3
N
HOA~O = O N
H R3'
29
R3NCO OH
or 0
R3R3 NH2
R5SO2Cl H
HO JLA 0 R5
O'H 0 S,
0
H2
IH
HOxAxO H OH 28 RACOCI 0
or R4000H H
O O
or 0 0 HOA0 R4
H
R4 O R4
R6000CI 31
O 0
H
O
HOAO O
H R6
32
Scheme 12
General procedures:
Ureas 29 are made by treatment of compound 28 with an isocyanate (1 to 3 eq.)
or
phosgene or triphosgene followed by an amine (R3R3'NH2) in a solvent such as
toluene or THF.
10 Sulfonamides 30. are obtained by coupling compound 28 with the appropriate
sulfonyl chloride (1 to 3 eq.) in solvents such as THE or DCM and in the
presence of
a base such as TEA or DIPEA.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
92
Amides 31 are prepared by coupling compound 28 with the appropriate acyl
chloride (1 to 3 eq.), preactivated carboxylic acid, mixed anhydride or
anhydride in
solvents such as THE or DCM and in the presence of a base such as TEA or
DIPEA.
Carbamates 32 are obtained by reacting compound 28 with the appropriate
chloroformate (1 to 3 eq.) in solvents such as THE or DCM and in the presence
of a
base such as TEA or DIPEA.
O 0
H SeO
2 H 0
X dioxane X
O O O O
H
HOAA~O HO AA ~ H
O
H H
17-20 29-32
Scheme 13
General procedure:
Selenium dioxide (4 to 6 eq.) is added to a solution of compound 17, 18, 19 or
20
previously dissolved in dioxane, acetic acid and acetic anhydride. The
reaction
mixture is refluxed overnight, then cooled to room temperature and filtered
through Celite. The residue is dissolved in DCM, washed with water, brine,
dried
over sodium sulfate and evaporated to dryness. The crude material is purified
by
flash chromatography on silica gel to yield the compound 29, 30, 31 or 32,
respectively.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
93
O O
H Se02 O NaOH or KOH
X p dioxane O Solvent
O 4 p H H
22, 23, 24 or 25 33
OHO 0 O
H 0
Ao x
0 0 Pyridine, DMAP H HO A O
HO H
H
34 29-32
Scheme 14
General procedure:
Step 1: Selenium dioxide (4 to 6 eq.) is added to a solution of compound 22,
23, 24
or 25 previously dissolved in dioxane, acetic acid and acetic anhydride. The
reaction mixture is refluxed overnight, then cooled to room temperature and
filtered through Celite. The residue is dissolved in DCM, washed with water,
brine,
dried over sodium sulfate and evaporated to dryness. The crude material is
purified
by flash chromatography on silica gel to yield the compound 33.
Step 2: The ester 33 is deprotected in solvents such as methanol, THE or
dioxane
using an aqueous solution of inorganic base such as sodium hydroxide or
potassium
hydroxide (10 to 20 eq.) at temperature ranging from 20 to 60 C to give the
alcohol 34.
Step 3: To the alcohol 34 in solvents such as pyridine, TEA or toluene (0.2-
1.0 M) is
added a cyclic anhydride (2 to 10 eq.) and base such as DMAP, TEA, DABCO or
DIPEA (1.1 to 2 eq.). The reaction mixture is heated at temperature ranging
from
90 to 140 C until completion to yield after standard acidic aqueous work up
and
purification by flash chromatography on silica gel the acid 29, 30, 31 or 32.
HIV Replication Activity
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
94
HIV-1 Replication in MT2 cell line with and without 30% human serum: The cells
are
infected at a Multiciplicity of Infection (MOI) of 0.5 for 3h and then washed
twice
with complete media to remove residual virus. Cells are then resuspended at
0.5 x
106/ml in complete medium (RPMI, 10% FBS, 1% sodium pyruvate), and seeded into
96-well plates (6.25 x 104/well). The cells are cultured in the presence or
absence
of various concentrations of test compounds in serial dilutions for 3 days at
37 C.
The test compounds are serially diluted in complete medium supplemented or not
with 30% human serum. After 3 days, 100 pL of cultured medium with cells are
replaced with 120 pL of freshly diluted test compounds in complete medium
containing or not 30% Human serum. The level of HIV-1 replication is
determined at
days 5 after infection by the presence of viral RT activity in harvested
supernatant
fluid. The IC50 and IC90 values for the virus replication are determined by
using
GRAPHPAD PRISM software.
PBMCs are separated from healthy donors' blood by standard density gradient
centrifugation, resuspended at a cell density of 1.5 X 106 cells/ml in culture
medium containing 2 pg/mL of phytohaemagglutinin (PHA), and thereafter
incubated for 3 days at 37 C in a humidified 5% CO2 atmosphere. The PHA-
stimulated PBMCs are adjusted at a concentration of 5x106/mL and then infected
with HIV-1111B at a MOI of 5.0 for 3 hours at 37 C in a humidified 5% CO2
atmosphere
and then washed to remove any residual virus. Thereafter, cells are
resuspended
in culture medium supplemented with interleukin-2 (IL-2) at a concentration of
50
units/mL (2X) and seeded at a density of 0.2 X 106 cells/well into 96-well
plates in
the absence or presence of various concentrations of the test compound. Then,
infected-cells are cultured for 4 days at 37 C in a humidified 5% CO2
atmosphere
in the absence or presence of 30% human serum after which an aliquot of
cultured
medium supernatant is replaced with fresh medium supplemented with human
serum (when necessary) containing the serially diluted test compound. The IC50
and IC90 values for the virus replication are determined at day 6 post-
infection by
measuring the reverse transcriptase activity in the harvested supernatant by
using
GRAPHPAD PRISM software.
The IC50 of the compounds tested in accordance with the HIV replication
activity
assay MT2 (HIV111B) are represented in Table 1.
Table 2 of compounds illustrates some of the compounds of the present
invention
which are synthesized and tested using the procedures described herein.
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
Retention time (tR) for each compound are measured using the standard
analytical
HPLC methods described above.
Table 2.
MT2 MT2 (HIVIIIB)
Cpd# Structure (HIV,,,B) with HS 30% (MtR (min)
ethod) M /M+H
IC50 range IC50 range
0
H 0
14-1 N0 +++ +++ 670.69
HO H
0 H
'H NMR (400 MHz,
CH3OD): 8 [ppm] 6.90
(br s, 1 H), 4.46 (dxd,
1 H), 3.17 (sept., 1 H),
2.96 (dxd, 1 H), 2.50 (d,
0 I H), 2.37 (m, I H), 2.18
H I (d, 1 H), 2.17 (br s, 2H),
14-2 O NH + 2.04-1.81 (m, 5H), 1.78
0 H O (dxt, I H), 1.74-0.80 (m,
H00 H 19H), 1.40 (s, 9H), 1.18
(t, 9H), 0.99 (s, 3H),
0.96 (s, 3H), 0.88 (s,
3H), 0.85 (s, 3H).
O
H
14-3 0 0'NH 0 +++ 3(B)9 725.16
H
O
H
HO
0
H
15-1 1NH2CIH +++ +++ 570.59
O H
HO O
H
H
0
579.42
15-2 NHz +
0II ^^ }0II~ (M-NH2)
HO X O
GH
0
.56 625.55
15-3 NHz +++ 20P
O O
HO O CIH
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
96
0
H / N/\
16-1 H~ +++ +++ 753.7
HO H YVJ O FI
0
H /
j~ . .
16-2 H +++ 26.63 584.5
0 (0)
HO H
0 H
O
0
H
17-1 Q H = HH I +++ +++ 703.68
HO`II_\/Jl 0
011 H
O
H
17-2 H N-^-, +++ +++ 21.6 641.6
HO H = (A)
O H
0
OH
17-3 H N' N H +++ +++ 30.9 689.6
0 (A)
Ho
o H
0
17-4 NxIIII N +++ +++ 7.63 655.7
\/Io H H (F)
HO \
(I _ O _
O
0
/ O
17-5 N N +++ +++ 9.12 671.9
HO Y (F)
Y o
O
17-6 N,N +++ +++ 13.6 691.9
\/ I0 H H CIH (G)
HO` x x
v 0
0
17-7 HxH~ +++ 9.81 695.9
(F)
HO
Y-Ilj 0
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
97
O
17-8 HxH +++ 10.64 709.9
HO`
T~ O
o 0
H IOUI H~
17-9 H H +++ 797.44
\/ I H
HO` x x _
lll( O H
O
0
17-10 H II ~NFi + 26.69 696.73
(D)
HO OO CIH
0
0 0
N
17-11 HxH +++ 3(C) 3.92 739.06
0
HO~
0
17-12 HxH +++ . )5 704.88
O A
Ho~ N
J( 0
0
H
17-13 H" N~ +++ 2092 683.81
\\// 0 H 00 (A)
HO
H
0
H
17-14 ' pxN~ +++ +++ 30.77 783.74
J~yj 0 YQ-I< (A)
0 H 0
0
H
17-15 O H~, NH +++ +++ 26.29 682.83
HO x H
CIH
XH
0
H
17-16 O N~ ~N +++ 26.50 696.86
H (D)
HO H
O H
0
H
17-17 \\// IoI H~N +++ 15.44
725.02
HO x H
O H O
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
98
0
17-18 I HN~ +++ 2~A~4 669.63
HO ` X10
0
O
N
LI- - +++ 3~E~1 695.75
17-19 \/ ' I HxN
HO`x0
0
0
0
17-20 HO V II H "K NH +++ 1 ~B~9 797.43
0
o 0 0
o IIII
17-21 NxN ++ 17(.1B)2
667.68
\/ 101 H H~ HO`s O
0
O
17-22 NH ) H +++ 15.72 653.60
\/ QO
(B)
HO
0
O
17-23 NHxN ++ 26.15 696.83
1/ 101 /D
HO ` x _ NHx l /
it V 0 TFA
0
jO
17-24 N~N ++ 12.53 696.96
H NH (G)
HO YUO~I~CIIH
0
0
17-25 NxN +++ 30.31
H
H H (E) 690.81
HO \/ IO `G
rlx H
O
+++ 20.22
17-26 H N" 'N IN
ry ry 690.4 \\// 0
H H ~C)
1
HO J~ 0
O
0
17-27 NN +++ 21.45 704.4
H H N IIJ
P
HO
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
99
0
0 N + 11.9)5 724.97
17-28 H xH
0 GH
0
H
17-29 \\// 0 H~ ~ +++ 18.32 724.5
HO OH 0 0
17-30 / NJN J +++ 11.76
~+) 710.97
0 H H GH (l7
HO \~ õ 0 _
0
0
17-31 \\//aII / HxH N +++ 1 8.26 704.88
HO` lj
lift vv 0
0
17-32 NN +++ 25.18
H
691.79
\/ OI H H 8A)
_ H - F1
HO
O H
O
O 0
17-33 H NON N x +++ 2/5.95 769.38
HO&O OH 0
ry / 0
17-34 q~q +++ 23.89
HO\/ O 797.64
'II O H F1
O
0 O17-35 q H
H / HxHaH ++ 1(D)2 668.85
HO H
O H
0
0
17-36 H" +++ 23.59 811.73
()
HO` x J~0
110(( ~V 0
4i
H
17-37 H~NH +++ 12.85 693.91
H (H)
HO x 0
H N-N
0 CIH
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
100
0
OH ' CIM 28.20
H OH
N" N~ / 1 +++ 8.u) 20
697.05
H
HO O U
H
0
17-39 N N ++ 16.15
711.13
`/ 01I
HO` _ x N
lI O
GH
0
17-40 \/V0O " NQ +++ 26.03 783.57
HO T(`s} NH (A)
0 0
O
17-41 0 " No +++ 26.28 783.58
HO` x NH (A)
II O o
0 0
O
17-42 N N ++ 15.39 682.95
o ( )
HO s o GH NHz
0
O
17-43 N N +++ 15.57
682.93
~)
HO`U }~
1I v O CH NHz
0
O
17-44 H pxq1 +++ 6.00
HO 691.96
II H (F)
O H
0
0
N
0 17-45
NNJ'S +++ 30.73 712.09
HO 0
o v
CIH
H
17-46 p~q~ +++ 13.25 692.03
HO x H
O H
O
0 Cm
H
17-47 N N +++ 8.85 705.08
\\// I0 H H
H (H)
HO
H
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
101
0
17-48 HA, N +++ 8.11 641.78
`/o (F)
HO `s x
1f v O
0
O F
4~0 0 F~F
17-50 \/ oI HxH ++t 9.39 710.06
HO
jf " O
O
0
F
0 F F
17-51 HxH +++ 9.73 710.06
(F)
HO
0
0
/ 0 CIH
17-52 HxH' " ttt 2(E~8 706.07
HO`x x ~=
11 v 0
0
/ 0
17-53 HxH +++ 24.08 706.07
HO 1/ II (E)
`x x 04 0
17-54 0 / HxH +++ 2820 724.22
HO` x0
0
0
17-55 H
\/ xN +++ 2('~7 724.23
o' g
HO n _ `s x 4j
0 F
0 F
17-56 0 HxH +++ 10.80 724.19
HO~
0 F
0
17-57 NH ) HN +++ 9.55 732.27
(F)
H0~
0
0
0
17-58 H~N +++ 9=FO 704.08
F F ( )
HO II`U xO F
V
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
102
0
28.13
17-59 N N37 +++ (A) 718.14
HO `1/x0 F
0
O
27.66
17-60 \/ 1 HH-J F +++ (A) 746.33
HO`s x0 ~XF
0
0
17-61 HxH +++ 1(0..48 718.19
HO
n0
0
17-62 `/ oI HxH - +++ 10.(F)03 718.19
HO ` ~ x0
0
0
17-63 0 HxH +++ 11.(F)44 732.29
Ho~O
0
0
0 9.17
17-64 o HxH +++ (F) 710.09
H0
0
0
17-65 NlkN N +++ 15.39 710.13
`/ II
HO`xx0
0
0
17-66 N +++ 10.69 730.27
(F)
H0
0
0
0 CIH
+++ S(F) 706.11
17-67 N NH N-
N
HO \ \ /o0
0
0
O CIH
17-68 HH1 - J +++ 23.59 706.04
o
HOr\ ~/ N
0
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
103
0
17-69 0 / HxH +++ . 17E10 731.26
HO ` ~ x0 CIH
0 v
0
17-70 Nx''IIN + 31.66 738.28
H H (B)
HO`xx
1T v 0
0
0
17-72 I N +++ 10.00 719.18
HO CIH
O
H O
17-73 0 H x NH +++ 1.5 708.07
\ / 'I H N)
HO, 0 CIH NJ
O
0
H 0
17-74 Nx INIH + 10.03
709.07
\/0 H H \ N (H)
HO~~ Jl0 CIH NT-,/ N
0
/ 0
17-75 HxH
HO S +++ .0 710.01
` x
lj " O
0
H O
5.76
17-76 \\// pII HNH~ +++ (
G) 708.08
HO`s H l
111111 p CIH N
0
H 0/0
18-1 N N +++ +++ 648.6
0 H H
Y\Ijo
O
0
H /
19-1 pI H +++ +++ 612.64
HO H
fill __ O
0
0
H
19-2 \/ pIO H +++ +++ 674.70
HO`IIIIy ~l H 0 H
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
104
0
H 0
19-3 H " 1~~H +++ 21.8 698.8
\\// 0
HOO _ H - (A)
O
0
H 0 ~
19-4 H ~N
N +++ +++ 32.5 680.6
_ " - (E)
HOI O H
0
OH 0
19-5 HtN +++ +++ 31.8 681.7
,~ ~( u H (A) (M Boc)
0 H
0
H O
19-6 H N H Ha +++ + 26.4 681.6
HO
yvi 0 H
0
OH 00
19-7 H ~1N o +++ +++ 24.9 723.6
(E)
H0~0 H
0
OH
19-8 H +++ +++ 26.5 675.6
\ / Io _
HO` x x0 _ H cw (E)
FI
O
F
H ~
19-9 H +++ +++ 29.9 706.6
\\// o H (A)
HO
H
0
0
H 0
19-10 \\// 0 H +++ +++ ND ND
NeO x H
o
O
0
H O F
19-11 H b +++ +++ 33.6 691.0
HO H (A) YV-j O H
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
105
0
H 0
19-12 o H F +++ 32.4
(A) 692.8
HO x H
O H
O
0
H 0
19-13 H +++ +++ 31.8 691.0
HO x H
0 H
0
H
19-14 H iN cIH +++ 24.4
675.7
H0 O H
0 H
CIH
H / 0
19-15 \/ 101 H +++ 675.7
HO ,o H
IIII __ O
0
OH / CIH
H OH
19-16 p "~ +++ 38.1 676.7
N (E)
HO
0 H
0
H 0 CH
678.8
19-17 H N- +++ 25.9
(A)
HO\ x H
V O H
O
H 0
19-18 o H N +++ (H) 678.7
H0 _ H CIH
0 H
O
0
H O
19-19 H " +++ 10.9 678.7
HO - H - CIH
O H
0
H 0
19-20 \\ // 0I H S +++ (A~2 680.7
HO - H
H
0
H OO
19-21 \\ // 0 H s~ +++ 32.7 681.7
HO - H
H
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
106
0
0 CIH
H " +++ (E)2 676.7
19-22
\\u// O
HO I _ NJ
(I O
II
O
O
19-23 H 0 J +++ +++ 30.~E~0 676.8
0
HO 0 N
O
O
31.5
19-24 H -" +++ +++ (A) 679.8
0
HO
O
0
0
19-25 Nk CIH +++ 38.6 676.8
O H N_, N
HO~
O
0
~ 35.5
19-26 o " "~ +++ (E) 678.8
\/ 0
HO CIH
O
0
0 30.6
19-27 H"~ +++ (A) 681.7
0 3
HO
0
O
23.9
+++ 681.7
19-28 "
0 I~ s--// (A)
HO
O
0
O
0 30.8
19-29 H
HO ` +++ 0. 688.8
\\v// '0u1
II
O
O
O
19-30 N +++ 682.84
\/ 0 H
HO II
O
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
107
0
0
19-31 H +++ 8(F).32 640.5
O
HO
O o
0
0
47
19-32 H +++ 9.47 (F) 654.2
HO\~~p
(0 _
0
19-33 o 0 +++ +++ 10.37 680.75
H (F)
HO O0
0
I-C
19-34 H H ; +++ +++ (E)3 678.7
H0 H CIH
O H
0
H 28.3
19-35 H +++ (E) 678.7
\\// p Fi N
HO x CIH
O H
0
19-36 OH
H o +++ (77.2 695.8
HO x O H
0
O
19-37 H +++ 28.7 695.8
H
HO 0
H
O
O
H
\/ 0 "LN 19.0
19-38 H +++ 1. 681.7
HO x H
O H
O
O
H +++
19. 709.9
19-39 p OH
HO 0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
108
0
H 0
19-40 H)-~'" +++ 20.2
709.9
\\// 0 H
HO x 0
H
0
O
H 0
H
19-41 Ham'' +++ 1(H> 709.9
\\// o
HO 0
H
0
O
OH
19-42 H N +++ 1(Cj 681.7
H
HO YVJ H
O
H O
H +++ 33.7 688.4
19-43
\\// o
HO x H
H
0
H 0
+++ (B) 688.8
19-44
H
HO i0 0
H
0
H 0
32.4
19-45 H
+++ (A) 688.8
\\// o
HO H
O H
0
H 0 HCI
19-46 H ~" +++ ~A~ 689.8
\\// H
HO x 0
H
0
H HCI 25.4
19-47 H qj'N +++ (E) 689.8
\\// 0
HO x 0
H
0
H HCI 21.4
19-48 H +++ (E) 689.7
\\// O H
HO x 0
O
H HCI 19.6
19-49 H +++ (E) 689.9
\\// 0 H
HO 0
H
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
109
0
H
19-50 H "
+++ (E) 691.9
\\// O HO
x
HO
O H XH
0
0
H O H
+++ 29.0 691.9
19-51 .H ,
O H
HOLO
H
0
H
N ~" +++ 1(E~ 689.9
19-52
\/ o
HO / H
O
0
O
H O
"J", 19-53 H +++ (27.1
D) 655.8
\/ O H
HO x 0
H
O
N /
19-54 H HAJ +++ 26.5
711.1
\\// O H
HO 0
O
OHH
19-55 H N +++ (H) 681.9
HO \\//x0 0 HCI
H
O
0 7.92
19-56 H~ NUJ +++ (H) 701.09
0
HO
O CIH
O
O
0
N 32.08 667.71
19-57 HO o oyNN +++ (A) (M-Boc)
0
0
0
N 33.66 667.75
19-58 0 0- N +++ (A) (M-Boc)
H0
0 0
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
110
0 17.64
0 and
19-59 NKr +++ 18.01 669.86
H P
HO II\/ O - N,
O
O
0
x 17.92
678.87
19-60
HO0
O N
O
0
0 OH
19-61 +++. - 718.98
0 H j
/
HO
O
0
O
19-62 p +++ - 707.09
Io' N-N
HO` x
v O
O
O
19-63 0 p~; O, +++ - 743.31
N
MO
O
0
H O
H +++ 2(C4)9
19-64 704.06
\/O H
Nab x 0
H
O
0
H 28.56
19-65 " H +++ (D) 696.06
O H
Na OO
H
O
0
H / ) "~ t++ 2(Djb 696.06
19-66 p
O H
Na O 0
H
O
0
19-67 o p"~ +++ 27(A).57 680.9
N
HO NI` x
v O
O
0
0 25.38
19-68 o p~ +++ (A) 680.9
N-0
HO` x
O
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
111
0
N 24.93
19-69 +++ (A)
HO) 694.9
\\// IIII
~ ~
40 N
"'
0
~~x 19-70 19-70 N +++ (A) 709.15
O
yll~
0
o 26.06
19-71 H +++ (A) 709.15
HO
O O
0
34.2
19-72 H ",r +++ (A) 696.0
\/ o g
HO II
O
O
~
19-73 N +++ 17~. .228 667.93
O 0 HCI
0
19-74 H +++ 2A; 669.9 0
HO~
0
25.0
19-75 N ~}- +++ 5. 695.0
0 00 _ H N-N
HO v v 'o
0
9.68
19-76 H)" +++ (F) 679.9
HO~
0
0
19-77 \/ I H +++ 9.0 695.0
HO \
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
112
0
~
19-78 H +++ 29.~A~3 680.9
\\// oII _ S
HO`-~
0
25.9
19-79 o H N~ +++ (A) 695.0
HO
O
O
38.1
19-80 o Hr +++ (E) 679.9
HO~
0
0
19-81 H" Y ,>- ++ - 706.96
JJ0Jj ff~0 N -
HO
O
~ 24.12
19-82 H N +++ (E) 664.82
\ / O _ N
HO II H
O
O
O
O
19-84 01 H N_N +++ 1 ~0 709.07
HO \ ~ ~O
0
O
H O
20-1 No- +++ +++ 628.64
\/ 101 H
HO \ H
fl _ O
O
O
H 0
20-2 H~ +++ +++ 33.3 656.6
HO 0 =
FI
0
4~O 0 20-3 Hxo+++ +++ 668.73
o HO
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
113
0 n
20-4 Hxo^' +++ 682.83
\y/ 0u
HO Il o
O
O
O
20-5 N) 0O a +++ 696.93
0
HO _
O
O
0 0
O
20-6 N o +++ +++ 798.50
0
0
0
o
20-7 HxNlk o~ +++ +++ 711.03
0
Ho 0 ;x
0
0
0
20-8 xo~ +++ 704.92
n I~
0
O
20-9 / Hxo +++ - 668.65
0
H0
O
O
O
13.84
20-10 Nxo~ +++ 682.5
0
0
Y-vio4i
0
0
o
20-11 NxIf 0'~ +++ 682.79 0
O
Y v 0
0
0
0
20-12 Nxo +++ 12.32 683-5
Ip H HiN'
HO_ HCI
O
0
O
20-13 Hxp" CN +++ 29.0 697.91
p (E)
H0
O
0
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
114
0
0
20-14 Hxo +++ - 714.87
0
HO
O
O
0 0
O 6=0
20-15 Hx0 +++ .5 747.17
HO \
(I\/ O
0
0 HCI
o 'NH
20-16 NA +++ 1~.2..003 697.8
0 0
0
O
0
12.32
20-17 Nxo +++ (G) 711.93
O O
0
O
0
19.87
20-18 Hx +++ (C) 726.13
O N,
HO~
O
0
19.43
726.12
20-19 Hxo~ +++ P
/ 0
HO`{\~ N
O
O
0
29.73
20-20 \/0 I ONko NO +++ (D) 711.93
HO_ l x
O
O
0
0
20-21 H ~ +++ 712.04
HO` x x
O
0
O
20-22 HX -~ +++ - 682.73
o
HO
O
0
29.66
20-23 Hx0'-") +++ (D) 711.84
\/ 0
HO` x x
O
O
CA 02711424 2010-07-02
WO 2009/082819 PCT/CA2008/002291
115
0
20-24 I N- 0--N? +++ 6.19 726.06
O H
II O
NO` x
O
When the compounds are tested more than once, the average IC50 is provided.
MT2 (HIVIIIB) IC50 with or without HS
+ > 1000 nM
++ 200-999 nM
+++ < 199 nM
The preceding examples can be repeated with similar success by substituting
the
generically or specifically described reactants and/or operating conditions of
this
invention for those used in the preceding examples.
White the invention has been illustrated with respect to the production and of
particular compounds, it is apparent that variations and modifications of the
invention can be made without departing from the spirit or scope of the
invention.