Note: Descriptions are shown in the official language in which they were submitted.
CA 02712213 2016-09-22
METHOD TO PERFORM LIMITED TWO DIMENSIONAL SEPARATION
OF PROTEINS AND OTHER BIOLOGICALS
BACKGROUND OF THE INVENTION
[0001] The present invention is in the technical field of two-dimensional
separation
of proteins and other biologicals and relates particularly to apparatus and a
method
* for the rapid and reproducible separation of species in a liquid medium.
[0002] The separation and characterization of proteins is ubiquitous
throughout the
life sciences. Two of the most popular electrophoresis separation techniques
are: 1)
gel isoelectric focusing (IEF), where the separation mechanism is based on
protein
surface charge providing isoelectric point (pI) separation and 2) sodium
dodecyl
sulfate (SDS) gel electrophoresis where the separation mechanism is based on
molecular weight (MW). These two techniques are most commonly performed
individually.
[0003] Isoelectric focusing (IEF) is a special electrophoretic technique for
separating
amphoteric substances such as peptides and proteins in an electric field,
across which
there is both voltage and a pH gradient, acidic in the region of the anode and
alkaline
near the cathode. Each substance in the mixture will migrate to a position in
the
separation column where the surrounding pH corresponds to its isoelectric
point.
There, in zwitterion form with no net charge, molecules of that substance
cease to
move in the electric field. Different amphorteric substances are thereby
focused into
narrow stationary bands.
[0004] In IEF separation, it is well known that proteins having molecular
weight
differences or conformational differences may possess similar pI values and
therefore
focus at the same location. In order to then separate these co-focused
proteins, a
technique called two-dimensional (2D) gel electrophoresis has been employed.
2D
- 1 -
CA 02712213 2010-08-05
gel electrophoresis combines two orthogonal separation techniques - gel IEF
and SDS
gel - to create a technique that dramatically increases separation resolution
and
provides for the separation of co-focused IEF protein zones. 2D gel
electrophoresis is
generally carried out in a polyacrylamide slab gel and although it has become
a
workhorse in the field of proteomics, owing to the high degree of resolution
which can
be obtained thereby, it is very labour-intensive, time consuming and non-
quantitative.
Moreover, although 2D gel electrophoresis does afford the highest degree of
molecular weight resolution of known electrophoretic separation techniques, it
has not
yet been possible to automate that process nor quantify the resolved component
proteins or other analytes. These and other drawbacks have motivated
researchers
to combine two orthogonal separation techniques in the liquid phase, using a
capillary
or coplanar microchannel format. While these are necessarily "limited
resolution"
techniques, relative to 2D gel electrophoresis, they are much simpler and
faster to
use and are of adequate resolution for many purposes.
[0005] It is known to combine capillary or channel isoelectric focusing (cIEF)
with
non-porous reverse phase microliquid chromatography (RPLC) in a two-
dimensional
layout, to obtain useful online detection and quantitation. However, the
interface
between the first and second separation dimension has hitherto been carried
out only
at the outlet end of the IEF separation capillary or channel. It is known that
the
separation and pH gradient obtained in cIEF may be disturbed when mobilizing
focused protein zones to reach the outlet end. A as result, it is more
challenging to
transfer separated zones from the first separation dimension to the second
separation
dimension in the orthogonal capillary or microchannel format than in apparatus
for 2D
gel electrophoresis. Fluid connections and for control of nanoliter volumes
are
required, making for complex analytical design and operation.
BRIEF SUMMARY OF THE INVENTION
- 2 -
CA 02712213 2010-08-05
[0006] This invention describes improved method and apparatus for carrying out
limited electrophoretic separation in the liquid phase. The objective of the
invention
is to provide a simple method and apparatus for limited "2D" separation using
both
capillary or channel IEF separation and capillary zone electrophoresis (CZE)
separation within the same capillary or channel. The present invention also
integrates real-time, whole-channel electrophoresis detection with automatic
sample
injection, automatic cIEF separation, separation zone manipulation and on-line
electrolyte selection, to achieve a separation resolution superior to that
obtained
using an orthogonal capillary arrangement.
[0007] The quotation marks about "2D" above reflect the fact that the present
invention uses two different and sequential electrophoretic techniques, but
not
orthogonal capillaries as in the known arrangements described above. The term
"2D"
is, a convenient shorthand term for designating a method and apparatus
employing
two-stage electrophoretic separation, and will be used in the remainder of the
specification and in the claims without quotation marks.
BRIEF DESCRIPTION OF THE DRAWINGS
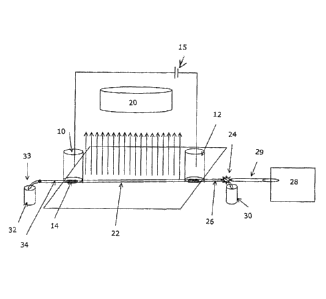
[0010] Figure 1 is a schematic representation of a first embodiment of
apparatus for performing limited 2D separation using electrophoresis and
controlled
hydrodynamic flow.
[0011] Figure 2 illustrates schematically a physiochemical mechanism
postulated to explain the separation of proteins in the presence of a
hydrodynamic
flow as in the method of the invention.
- 3 -
CA 02712213 2010-08-05
[0012] Figure 3 is a schematic representation of a second embodiment of
apparatus according to the invention for performing limited 2D separation
using
electrophoresis and chemical mobilization.
[0013] Figure 4 illustrates graphically the separation of two proteins
having the
same pI value but different charge responses to pH, using the method of the
invention.
[0014] Figure 5 illustrates graphically the results of a separation
effected by
using apparatus according to the first embodiment of the invention, showing a
single
peak of tryptosinogen and pI Marker 9.46 mixture when hydrodynamic flow is
minimized, and split peak of tryptosinogen and pI Marker 9.46 when
hydrodynamic
flow is toward the cathode.
[0015] Figure 6 illustrates graphically the results of a separation
effected by
apparatus according to the second embodiment of the invention, showing two
peaks
of transferrin prior to anodic mobilization and four peaks of transferring
subsequent
to anodic chemical mobilization.
DETAILED DESCRIPTION OF THE INVENTION
[0016] Figure 1 shows a first embodiment of the apparatus. A microfluid
device
is provided, including an anolyte tank 10 and a catholyte tank 12 such that
electrolyes in the tanks are isolated from the sample mixture by ion
conductive
barriers 14 (such as semipereamble membranes). A high voltage supply connected
across two electrodes that are immersed in the respective tanks. A CCD imaging
camera 20 is focused so that it can detect light passing through or emitted
from the
entire length of a horizontal capillary separation channel 22. The camera 20
is able
to display and capture pictures in real-time, or at least very rapidly. A
light source
and collimation means (not shown) are provided for applying a sheet of light
(arrows
- 4 -
CA 02712213 2016-09-22
L) to pass through or emit from the entire length of separation channel 22. A
real
time CCD sensor camera/sensor arrangement like that used with the apparatus of
the
present invention is described in more detail in US patent No. 6,852,206,
having a
common inventor and the same assignee as the present application. US patent
6,852,206 discloses detection and
measurement apparatus of analyte separation zones in a capillary.
(0017] A switch valve 24 is connected to the microfluidic device such that
an
inlet flow channel portion 26 at one end of the separation channel may be
selectively
connected to either an autosampler 28 for sample injection, or to the fluid
medium
contents of an inlet vial 30. A hydrodynamic flow across separation channel 22
can
be induced and controlled by vertical up or down fine-control motion of a
hydrodynamic flow vial 32 containing fluid medium, the contents of which are
connected by means of hydrodynamic flow control valve 33 with an outlet flow
channel portion 34 of the separation channel.
[0018] With the switch valve 24 position set for fluid connection of the
inlet
channel portion 26 of the separation channel to the autosampler 28, and with a
shut-
off valvefor autosampler connection tube 29 open, a sample containing a
mixture of
proteins , carrier ampholytes and a sieving solution such as methyl cellulose
is
injected into the separation channel by the autosampler until the sample
mixture
volume fills the separation channel to overflow. The position of the switch
valve is
then set to connect the inlet vial with the separation channel and the high
voltage is
turned on by means of HV switch 36. An electric field is thereby established
across
the separation channel and a linear pH gradient is formed by the carrier
ampholytes.
The cIEF process begins and upon completion, proteins are focused and
separated
into zones according to their pI when both electro-osmotic flow and
hydrodynamic
flow are stable. The entire IEF process is continuously monitored and the
images of
the separation trace are continuously captured (recorded) in real-time by the
whole-
channel CCD imaging camera of the CCD sensor unit. At this point, the first
- 5 -
CA 02712213 2010-08-05
dimensional separation (cIEF) is complete and the second dimensional
separation is
initiated.
[0019] The second dimensional separation is applied to the IEF focused
zones
(proteins) by the application of a controlled hydrodynamic flow. The
hydrodynamic
flow is induced by a microgravitational force arising in the separation
channel 22
resulting from the finely controlled up or down motion of the hydrodynamic
flow vial.
When hydrodynamic flow is introduced into the separation channel following IEF
focusing, the pH gradient will be affected and additional sample mixture will
enter the
separation channel. As more sample mixture is continuously injected into the
separation channel owing to the hydrodynamic flow, the focused zones at the
far end
of the separation channel (along the direction of hydrodynamic flow) are
continuously
pushed out. For example, if the outlet vial 32 is raised slightly, then the
hydrodynamic flow direction proceeds from the anodic (outlet end) to the
cathodic
end (inlet end). More sample mixture is introduced from the anodic end, and
the
most basic zones focused at the cathodic end will be pushed out of the
separation
capillary (over the ion conductive barrier area, see Figure 2). Since this
hydrodynamic
flow coexists with an electric field, the separation zone resolution and shape
is
preserved when the hydrodynamic flow is limited and carefully controlled and
the
newly injected sample mixture ampholytes are focused into their pI position.
The
movement of relatively larger molecular weight proteins (protein A in Fig 2)
is slower
than that of smaller ones (protein B in Fig 2) in a sieving solution such as
methylcellulose. As a result, a limited second dimensional separation of cIEF
zones
(proteins) due to mass difference is achieved. Again, the entire second
dimension
separation process is continuously monitored and the images of the separation
trace
are continuously captured (recorded) in real-time by the whole-channel, CCD
imaging
camera.
[0020] Figure 3 shows a second embodiment of the apparatus. The same
reference numerals are used to indicate components corresponding to those of
the
- 6 -
CA 02712213 2010-08-05
first apparatus embodiment (Fig. 1). The microfluid device contains an analyte
tank
10, a catholyte tank 12 and a chemical mobilization tank 38. The electrolyes
in the
three tanks are isolated from the sample mixture by ion conductive barriers
14. High
voltage supply is connected at one end to an electrode immersed in the anolyte
tank
and at the other end to HV switch 36 such that connection can be made to
either an
electrode immersed in the catholyte tank or an electrode immersed in the
chemical
mobilization tank. Real time CCD sensor 20 is focused such that it can detect
light
(arrows L) passing through or emitted from the entire length of separation
channel 22
and the camera is able to display and capture pictures in real-time, or at
least very
rapidly. Means (not shown) are provided in both the first and second
embodiments of
the invention for projecting a sheet of light to pass through or emit from the
entire
length of the separation channel. As with the first embodiment described above
switch valve 24 is connected to the microfluidic device such that the inlet
flow channel
26 may be connected to either autosampler 18 for sample injection or to an
inlet vial
30. The end of the outlet channel is immersed in an outlet vial.
[0021] The anolyte, catholyte and chemical mobilization tanks (10, 12,38)
are
filled with appropriate electrolytes and, with the switch valve position set
for
connection between the inlet of the separation channel and the autosampler and
the
shut-off valvle to capillary section 29 open, a sample containing a mixture of
proteins
, carrier ampholytes and a sieving solution such as methyl cellulose solution
is
injected into the separation channel by the autosampler until the sample
mixture
volume fills the separation channel to overflow. The switch valve position is
then set
for connection between inlet vial 30 and separation channel 22, the high
voltage is
turned on and the switch valve 24 is set such that the catholyte electrode is
contacted, an electric field established across the separation channel, and a
linear pH
gradient is formed by the carrier ampholytes. The cIEF process begins and upon
completion, proteins are focused and separated into zones according to their
pI when
both electro-osmotic flow and hydrodynamic flow are well controlled. The
entire cIEF
process is continuously monitored and the images of the separation trace are
- 7 -
CA 02712213 2010-08-05
continuously captured (recorded) in real-time by the whole-channel, CCD
imaging
camera. At this point, the first dimensional separation (cIEF) is complete and
the
second dimensional separation begins.
[0022] The second dimensional separation is achieved in this second
embodiment of the apparatus, not by controlled hydrodynamic pressure but by
chemical mobilization of the cIEF focused zones. An electric switch that is
selectively
operable to connect to anolyte electrode or the catholyte electrode is changed
to
connect to the chemical mobilization solution upon completion of cIEF.
Mobilization of
the focused zones will then occur. It is known that when non-acid solution is
used as
the anolyte, focused cIEF zones will migrate towards the anode (anodic
mobilization).
Whereas when non-base solution is used as the catholyte, focused cIEF zones
will
migrate towards the cathode (cathodic mobilization). Therefore, anodic
mobilization
may be achieved by switching the high voltage contact to the anode from the
acid
solution tank to the chemical mobilization tank that contains non-acid
solution, or
cathodic mobilization may be achieved by switching the high voltage contact to
the
cathode from the base solution tank to the chemical mobilization tank that
contains
non-base solution.
[0023] The rate of migration due to chemical mobilization is determined
by the
charge-to-mass ratio of the protein and the mobility of the protein in a
specific
sieving solution. For example, two exemplary proteins with the same pI value
have
different rates of migration in response to a pH change (Figure 4). As a
result, these
two proteins will not experience the same rate of motion during chemical
mobilization. In addition, when this movement is carried out in a sieving
solution,
proteins with different molecular weight or shape (conformation) may have
different
mobility. Therefore, proteins with the same pI, but have different mobility
change
with pH or different molecular weights or conformation can be separated with
limited
2D separation of cIEF zones using chemical mobilization. Again, the entire
second
dimension separation process is continuously monitored and the images of the
- 8 -
CA 02712213 2010-08-05
separation trace are continuously captured (recorded) in real-time by the
whole-
channel, CCD imaging camera.
[0024] cIEF is a steady state technique. Focusing and separation of
proteins is
achieved when transitional peaks or zones converge into stationary zones.
However,
if single-point detection is used, it is difficult to know the exact time when
all proteins
are focused, since the speed of protein focusing is affected by sample
conditions such
as: content of salt and carrier ampholytes in the sample, experimental
conditions
such as separation channel dimensions, electric field strength and electrolyte
concentration. As a result, two transitional peaks or zones for one protein
may be
detected when the protein is not yet focused. Further, an abnormal peak may be
observed due to protein aggregation or precipitation resulting from prolonged
protein
focusing. With whole-column detection, as used with the present invention,
however,
the separation and focusing of an individual protein can be monitored in real
time,
avoiding the problems of 2D separation of transitional peaks (premature
focusing)
and separation of precipitated proteins (over focusing). The pI value of the
protein is
calibrated and the second dimension separation is applied. With real-time,
whole
column detection, the protein separation can be monitored, providing better
protein
fingerprinting by allowing straightforward assignment of protein zones based
on pI
and relative molecular weight differences.
Example 1: Induced Hydrodynamic Flow as Second Dimension of Separation
[0025] Figure 5 illustrates hydrodynamic flow induced limited 2D
separation of
protein trypsinogen and a small molecular weight pI marker. In this
experiment,
trypsinogen and a small molecular pI marker were mixed with 8% pH 3-10
Pharmalyte and 0.35% methylcellulose. The sample mixture was injected into a
50
mm 100 pm inner diameter FC coated capillary with a micro autosampler.
Focusing
was conducted at a focusing voltage of 3000 V, with 80 mM H3PO4 as anolyte and
100
mM NaOH as catholyte. Detection was conducted with a real-time, whole column
UV
- 9 -
CA 02712213 2010-08-05
detector. The hydrodynamic flow is controlled by the water level difference in
the
hydrodynamic flow vial and the inlet vial.
[0026] It can be seen that when hydrodynamic flow was minimized (i.e.
under
first dimension cIEF separation conditions), there were two peaks in the
electrophorogram (trace a). The more acidic peak to the left of the
electrophorogram
(egram) contains the minor component of trypsinogen (pk 1) and the more basic
peak to the right of the egram contains the major component of trypsinogen (pk
2)
and the pI marker (pk3). When a hydrodynamic flow was introduced in the
direction
of the cathodic end (trace b), the minor component of trypsinogen (pk 1)
further
partially separated into two subcomponents, and the pI marker (pk 3) was
partially
separated from peak the major component of trypsinogen (pk 2). The pI marker
(pk
3) moved more quickly to a more basic position than the major trypsinogen
component (pk 2) due to its smaller molecular weight in a sieving solution.
When a
hydrodynamic flow was introduced in the direction of the anodic end (trace c),
again
because of the smaller MW of the pI marker (pk 3) compared to that of the
major
component of trypsinogen (pk 2), the pI marker shifted more quickly to a more
acidic
position than that of the major component of trypsinogen.
Example 2: Chemical Mobilization as Second Dimension of Separation
[0027] Figure 6 illustrates chemical mobilization induced limited 2D
separation
of transferrin, myoglobin and a small molecular weight pI marker (pI 4.22). In
this
experiment, transferrin and myoglobin and the pI marker were mixed with 8% pH
3-
Pharmalyte and 0.35% methylcellulose. The sample mixture was injected into a
50
mm 100 pm inner diameter FC coated capillary with a micro autosampler.
Focusing
was conducted at a focusing voltage of 3000 V, with 80 mM H3PO4 as anolyte and
100
mM NaOH as catholyte. Detection was conducted with a real-time, whole column
UV
detector. For anodic mobilization (trace b), the anolyte was replaced with 100
mM
NaOH upon completion of cIEF focusing. For cathodic mobilization (trace c),
the
catholyte was replaced with 80 mM H3PO4 upon completion of focusing.
- 10 -
CA 02712213 2010-08-05
In Trace a, it can be seen that when electroosmotic flow and hydrodynamic flow
are
well controlled (i.e. under first dimension cIEF separation conditions), the
transferrin
protein is partially resolved into two peaks and a minor myoglobin peak (pk 1)
is
noted. Under anodic mobilization (trace b), the transferrin protein is now
partially
resolved into 4 peaks and the minor myoglobin component is partially resolved
into 2
peaks (pk 1). When cathodic chemical mobilization was introduced (trace c),
the two
peaks of transferrin (trace a) are separated into two larger peaks and one
smaller
peak.
[0028] Neither chemical mobilization conditions produced any split or
partially
separation of the pI marker peak (pI 4.22) and the major myoglobin peak.
CONCLUSION
[0029] From the description and examples herein it will be seen that
applicants'
provides a rapid, reproducible and quantative limited 2D electrophoresis
separation.
Channel or capillary-based electrophoresis, unlike 2D gel electrophoresis
permits
automatic sample injection. No sample transfer or handling is involved and
either
hydrodynamic flow or chemical mobilization can be used, since both can be well
controlled. Applicants' arrangement allows "two-dimensional" electrophoresis
to be
carried out within a single separation channel and in a single analysis run.
The use of
real time, whole channel image detection affords very good reproducibility in
both
qualitative and quantative characterization.
[0030] While the foregoing written description of the invention enables
one of
ordinary skill to make and use what is considered presently-to be the best
mode
thereof, those of ordinary skill will understand and appreciate the existence
of
variations, combinations, and equivalents of the specific embodiment, method,
and
examples herein. The invention should therefore not be limited by the above
-11-
CA 02712213 2010-08-05
described embodiment, method, and examples, but by all embodiments and methods
within the scope and spirit of the invention as claimed.
- 12-