Note: Descriptions are shown in the official language in which they were submitted.
CA 02714094 2010-08-30
I = .t
69331-63D
METHODS AND APPARATUS FOR CONDUCTING MULTIPLE MEASUREMENTS
ON A SAMPLE
This application is a division of Canadian patent
application SN 2,459,893 filed September 10, 2002 (the
parent application).
It should be understood that the expression "the
present invention" or the like used in this specification
encompasses not only the subject matter of this divisional
application but that of the parent application also.
1. FIELD OF THE INVENTION
This application relates to reagents apparatus,
systems, kits and methods for conducting multiple chemical,
biochemical and/or biological assays on a sample.
2. BACKGROUND OF THE INVENTION
At this time, there are a number of commercially
available instruments that utilize electrochemiluminescence
(ECL) for analytical measurements including drug screening.
Species that can be induced to emit ECL (ECL-active species)
have been used as ECL labels. Examples of ECL labels
include: i) organometallic compounds where the metal is
from, for example, the noble metals of group VIII, including
Ru-containing and Os-containing organometallic compounds
such as the tris-bipyridyl-ruthenium (RuBpy) moiety and ii)
luminol and related compounds. Species that participate
with the ECL label in the ECL process are referred to
1
CA 02714094 2010-08-30
6 9 3 3 1 - 6 313
herein as ECL coreactants. Commonly used coreactants include tertiary amines
(e.g., see U.S.
Patent No. 5,846,485), oxalate, and persulfate for ECL from RuBpy and hydrogen
peroxide for
ECL from luminol (see, e.g., U.S. Patent No. 5,240,863). The light generated
by ECL labels can
be used as a reporter signal in diagnostic procedures (Bard et al., U.S.
Patent No. 5,238,808)-
For instance, an ECL label can be covalently coupled to a
binding agent such as an antibody, nucleic acid probe, receptor or ligand; the
participation of the
binding reagent in a binding interaction can be monitored by measuring ECL
emitted from the
ECL label. Alternatively, the ECL signal from an ECL-active compound may be
indicative of
the chemical environment (see, e.g., U.S. Patent No. 5,641,623 which describes
ECL assays that
monitor the formation or destruction of ECL coreactants). For more background
on ECL, ECL
labels, ECL assays and instrumentation for conducting ECL assays see U.S.
Patents Nos.
5,093,268; 5,147,806; 5,324,457; 5,591,581; 5,597,910; 5,641,623; 5,643,713;
5,679,519;
5,705,402; 5,846,485; 5,866,434; 5,786,141; 5,731,147; 6,066,448; 6,136,268;
5,776,672;
5,308,754; 5,240,863; 6,207,369; 6,214,552 and 5,589,136 and Published PCT
Nos.
W099/63347; W000/03233; W099/53962; W099/32662; W099/14599; W098/12539;
W097/36931 and W098/57154.
Commercially available ECL instruments have demonstrated exceptional
performance.
They have become widely used for reasons including their excellent
sensitivity, dynamic range,
precision, and tolerance of complex sample matrices. The commercially
available
instrumentation uses flow cell-based designs with permanent reusable flow
cells. Recently, ECL
instrumentation has been disclosed that uses reagents immobilized on the
electrode used to
induce ECL (see, e.g., U.S. Pat. Nos. 6,140,045; 6,066,448; 6,090,545;
6,207,369 and Published
2
CA 02714094 2012-09-27
= =
69331-63D
PCT Appl. No. W098/12539). Multi-well plates having integrated electrodes
suitable for
such ECL measurements have also been recently disclosed (see, e.g., copending
U.S. Patent
Nos. 7,842,246 and 6,977,722.
These multi-well plates having integrated electrodes include plates having
multiple
assay domains within a well.
The use of multi-well assay plates allows for the parallel processing and
analysis of
multiple samples distributed in multiple wells of a plate. Typically, samples
and reagents are
stored, processed and/or analyzed in multi-well assay plates (also known as
microplates or
microtiter plates). Multi-well assay plates can take a variety of forms, sizes
and shapes. For
convenience, some standards have appeared for some instrumentation used to
process samples
for high throughput assays. Assays carried out in standardized plate formats
can take advantage
of readily available equipment for storing and moving these plates as well as
readily available
equipment for rapidly dispensing liquids in and out of the plates. Some well
established multi-
well plate formats include those found on 96-well plates (12 x 8 array of
wells), 384-well plates
(24 x 16 array of wells) and 1536-well plate (48 x 32 array of well). The
Society for
Biomolecular Screening has published recommended mieroplate specifications for
a variety of
plate formats.
3. SUMMARY OF THE INVENTION
3
CA 02714094 2014-05-20
69331-63D
The present invention includes apparatus, systems, system components,
reagents, kits and methods for performing a plurality of assays on a sample.
The invention
includes assay modules having one or more assay cells (e.g. , wells,
compartments, chambers,
channels, flow cells, etc.) that comprise a plurality of assay domains (e.g. ,
discrete locations
on a module surface where an assay reaction occurs and/or where an assay
signal is emitted
for carrying out a plurality of assay measurements. The assay cell is,
preferably, adapted to
hold a volume of fluid in contact with assay domains within the assay cell
without contacting
assay domains in other assay cells of an assay module. In preferred
embodiments, the assay
modules are multi-well plates, the plates comprising a plurality of wells, one
or more of the
wells comprising a plurality of assay domains (referred to herein as Multi-
Domain Multi-Well
Plates or MDMW Plates). Preferably, the plates are designed to be compatible
with plate
handling equipment (e.g., fluid dispensers, plate washers, plate stackers,
plate movers, and/or
plate readers) designed for use with standard format multi-well plates.
According to one aspect of the present invention, there is provided a method
of
determining the effect of an inhibitor on a plurality of enzymes, wherein said
plurality of
enzymes each exhibit a distinct enzymatic activity, which comprises:
contacting a sample
containing the inhibitor with a plurality of assay domains located within a
well of a multi-well
plate, said plurality of assay domains each having enzyme substrates
immobilized thereon and
each assay domain differing in selectivity for each distinct enzymatic
activity, wherein said
well of said multi-well plate further comprises the plurality of enzymes
provided as dried
reagents, and detecting the effect of the inhibitor on the plurality of
enzymes.
4
CA 02714094 2012-09-27
=
69331-63D
The assays of the invention are preferably coupled to a detection step that
involves the use of an electrode, the generation of light, and the measurement
of the generated
light. Examples of processes that may be used in such a detection step include
electrochemiluminescence (also referred to as electrogenerated
chemiluminescence),
electroluminescence, and chemiluminescence triggered by an electrochemically-
generated
species. For the purposes of the application and for convenience, these three
processes will be
referred to as "electrode induced luminescence". Electrochemiluminescence
involves
electrogenerated species and the emission of light. For example,
electrochemiluminescence
may involve luminescence generated by a process in which one or more reactants
are
generated
4a
CA 02714094 2012-09-27
69331-63D
electrochemically and undergo one or more chemical reactions to produce
species that emits
light, preferably repeatedly. The invention also relates to assays and
measurements that do not
require the use of an electrode, for example, the assays of the invention may
be based on
measurements of cherniluminescence, fluorescence, bioluminescence,
phosphorescence, optical
density and processes that involve the emission of light from a scintillant.
The invention also
relates to assays and measurements that do not involve luminescence, for
example, the assays of
the invention may be based on measurements of electrochemical processes (e.g.,
processes
involving the measurement or generation of current or voltage), electrical
processes (e.g.,
processes involving the measurement of resistance or impedance), surface
plasmon resonance or
optical interference effects.
Accordingly, in certain preferred embodiments of the invention, the assay
modules and/or
MDMW Plates are adapted to allow assay measurements to be conducted using
electrode
induced luminescence measurements (most preferably, electrochemiltuninescence
measurements), e.g., as described in copending US Patent No. 7,842,246 and
6,977,722.
16 Advantageously, assay domains
patterned on a surface of a well (e.g., on an electrode in a well adapted for
conducting electrode
induced luminescence measurements) are defined by physical boundaries which
can include
ledges or depressions in the surface, patterned materials deposited or printed
on the surface, and
or interfaces between regions of the surface that vary in a physical property
(e.g., wettability).
Such physical boundaries simplify the patterning of reagents on =surfaces of a
well by confining
and preventing the spreading of small drops of reagents applied to an assay
domain.
=
5
CA 02714094 2010-08-30
r - (1)
V 0 03/023360
PCT/US02/28652
By providing two levels of multiplexing (multiple wells per plate and multiple
domains
per well), MDMW Plates provide a variety of advantages over conventional multi-
well plates
that only have one assay domain per well. For example, a MDMW Plate having N
wells and M
assay domains per well allows a panel of M assays to be run on a plurality of
N samples.
Conducting the same series of assays on conventional N-well plates would
require M plates, M
times more sample and reagents, and considerably more pipetting and plate
handling steps to
achieve the same performance. Conducting the same series of assays on
conventional array
"chips" would involve the handling and movement of N chips and would likely
not be
compatible with standard plate handling equipment designed for use with multi-
well plates.
Conducting the same series of assays on a single ultra-high density multi-well
plate with Mx N
wells would generally lead to reduced assay sensitivity (sample volume and,
therefore, number
of analyte molecules, tends to scale inversely with the density of wells on a
plate) as well as to
other problems associated with ultra-high density plate formats (e.g.,
expensive and complicated
fluid dispensing equipment, lack of mixing, evaporative losses, trapping of
air bubbles, inability
to carry out wash steps, etc.).
The invention includes assay formats that take advantage of multiplexing
available
through the use of assay cells comprising multiple assay domains and, in
particular, through the
use of MDMW Plates. Some examples of preferred assay formats are described
below. It is
understood that while some of the forms are described in terms of MDMW Plates,
they can be
applied to other assay modules comprising assay cells with multiple domains.
It is also
understood that the multiplicity of assay domains available within an assay
cell allows many of
the formats described below to be combined within one cell.
CA 02714094 2010-08-30
1
. =
WO 03/023360
PCT/US02/286S2
In one preferred assay format, a plurality of analytes or activities are
measured within one
well of a MDMW plate. For example, panels of assays may be developed for
measuring a
plurality of analytes or activities associated with a particular biological
system (e.g., panels of
immunoassays or hybridization assays for monitoring cytokine mRNA or protein
levels), disease
state (e.g., panels of assays for cardiac markers, for identifying allergens
responsible for allergic
reactions, for identifying infectious organisms, etc.), tissue type, organism,
class of protein,
enzyme or biological molecule, etc. In one embodiment, a panel of assays is
used to provide a
fingerprint for identifying a biological system (e.g., a pattern of analyte
levels associated with a
particular cell type, organelle type, organism type, tissue type, bacteria or
virus). For example, a
plurality of assays for different components found within a genus of
biological systems can be
used to identify species or subspecies within that genus. In another
embodiment, a differential
measurement involving a plurality of assays for different components within a
biological system
is used to identify the state of the biological system (e.g., diseased vs.
normal state, activated vs.
normal state, etc.) or to identify the components within a biological system
that are affected by
an external condition or stimulus (e.g., changes in the distribution of
components associated with
development of a disease state, addition of a stimulatory species, addition of
a potential drug
candidate, changes in environmental conditions such as pH, temperature, etc.).
Assay panels
may also be used to determine the function of one or more proteins. For
example, a protein may
be screened against a patterned library of enzyme substrates and/or potential
binding partners to
identify enzymatic or binding activities. Conversely, a patterned library of
proteins may be
exposed to a known biological material to determine if any of the proteins
binds to, reacts with or
is otherwise transformed by the biological material.
CA 02714094 2010-08-30
t
WO 03/023360
PCT/US02/28652
In another preferred assay format, some fraction of the assay domains present
in a well
are devoted to internal standards, controls or calibrators. For example, one
or more assay
domains may be left uncoated or may be coated with a blocking agent or a
biomaterial not
expected to participate in a reaction with a sample; such assay domains may be
used to measure
and/or correct for non-specific binding of labels to surface in the well. In
another example, one
or more assay domains is coated with a labeled reagent (e.g., a reagent
labeled with an ECL
label); such assay domains may be used to measure and/or correct for
conditions that may affect
the generation and measurement of signal (e.g., ECL) from a label (e.g., pH,
temperature,
chemical interferents, colored species, etc.). In another example, one or more
assay domains are
used to carry out a control assay for a control analyte that is spiked into
the assay mixture.
Preferably, the control assay is similar in format to assays carried out on
other assay domains.
Control assays may be used to measure and/or correct for non-specific binding,
conditions that
affect the generation of signal from a label and conditions that affect assay
reactions (variations
in incubation time, temperature, mixing, etc.).
In another preferred assay format the same analyte or activity is measured in
multiple
domains within a well; such redundancy can allow for greater statistical
confidence in an assay
result. Such multiple measurements of the same analyte may involve the use of
multiple roughly
identical assay domains or, alternatively, may involve the use of assay
domains that vary in some
property (for example, domain size, domain location, surface density of an
assay reagent,
blocking agent, assay reagent affinity, assay reagent specificity, assay
format, sensitivity to
interferents, sensitivity to ternperature, assay kinetics, sensitivity to
optical distortion, etc.) so as
to account for, detect, and/or compensate for a source of assay error (e.g.,
inconsistent or non-
8
CA 02714094 2010-08-30
= w =
WO 03/023360
PCT/US02/28652
homogenous mixing, steric crowding of assay reagents in an assay domain, non-
specific binding,
matrix effects, interfering species, imprecise temperature control, imprecise
timing of assay
steps, exceeding of assay dynamic range, variation in fluid volume or meniscus
shape, etc.).
In another preferred assay format, the same analyte or activity is measured in
multiple
domains within a well, the domains being comprised on individually addressable
electrodes. In
such a system one may measure the kinetics of an assay reaction by
sequentially applying
electrical energy to individual assay domains at selected times and measuring
the change in
electrical current, electrical potential, or, preferably, electrode induced
luminescence (most
preferably, ECL) over time. By measuring different time points at different
assay domains, it is
not necessary to repeatedly apply possibly damaging electrical energy to the
same assay domain.
In another preferred assay format, the same analyte is measured at different
assay
domains within a well, the different assay domains being designed to measure a
different
property or activity of the analyte. In one embodiment, an enzyme with
multiple different
activities is measured in a well comprising different assay domains that
differ in their selectivity
for each enzymatic activity of the enzyme (e.g., assay domains that comprise
substrates for
selected enzymatic activities and/or assay domains that are capable of
capturing and measuring
the substrates or products of selected enzymatic activity), that are designed
to measure binding
activities of the enzyme (e.g., assay domains comprising potential binding
partners of the
enzyme or that are designed to capture the enzyme so as to allow the
measurement of interaction
with potential binding partners in solution) and/or assay domains designed to
measure the ability
of the enzyme to act as a substrate for a second enzyme (e.g., binding domains
designed to allow
for a specific binding assay of the product of the action of the second enzyme
on the first
, .
CA 02714094 2010-08-30
W0413/023360
PCT/US02/28652
enzyme). In another embodiment, a well comprises a domain for measuring the
amount of an
enzyme (e.g., via a binding assay such as an immunoassay) and one or more
other domains for
measuring one or more activities associated with the enzyme; this embodiment
allows the
measured activity to be referenced to the amount of enzyme. The inclusion of
assay domains
capable of capturing an enzyme of interest has the added advantage of allowing
the purification
of the enzyme from a crude sample within the assay well. In yet another
embodiment of the
invention, a well comprises an assay domain capable of capturing an enzyme of
interest and one
or more additional assay domains for measuring an activity of the enzyme of
interest. Methods
using such a well may include a wash step for purifying the enzyme from
impurities in a crude
enzyme preparation.
In another preferred assay format, the number of measurements that can be
carried out in
one well is increased by co-immobilizing a plurality of assay reagents in each
domain within the
well. For example, one can screen for the binding partner of a biomaterial of
interest by
patterning a library of M potential binding partners on M assay domains in a
well, exposing the
well to a sample containing a labeled biomaterial and looking for the assay
domain that produces
a signal indicative of a binding event. Alternatively, one can pattern a
library of Mx I potential
binding partners by co-immobilizing I potential binding partners in each assay
domain. A signal
at a specific assay domain would indicate that one of I potential binding
partners has binding
activity; the identity of the binding partner could be determined by then
individually testing each
component of that assay domain. Advantageously, an assay kit may contain a
first MDMW plate
that multiplexes assay reagents within assay domains of a well and a set of
additional MDMW
plates that are patterned so as to allow the testing of each individual
component of an assay
it
CA 02714094 2010-08-30
=
WO 03/023360
PCT/US02/286S2
domain in the first plate (e.g., a second MDMW Plate having a well with a
plurality of assay
domains each comprising one component of an assay domain on the first plate).
In another preferred embodiment, the number of assay components that can be
patterned
and uniquely identified on an array of M domains(where M is an integer greater
than three) is
increased by patterning each reagent into a unique group of assay domains. For
example, one
can pattern a library of up to Z=(M!)/[2!(M-2)!] potential binding partners
(preferably, > M
binding partners) so that up to (M-1) binding partners are inunobilized in
each domain but one or
more (preferably, all) binding partners are immobilized in a unique pair of
domains (the other
binding partners, preferably, being immobilized in unique sets of one domain.
In this case, those
one or more binding partners can be identified by looking for pairs of assay
domains producing
signals indicative of a binding event. By way of example, wells comprising 4,
7, 10, and 25
assay domains have, respectively, 6, 21, 45 and 300 unique pairs of domains
per well. Similarly,
one can pattern a library of up to (M!)/[ZI(M-Z)!] potential binding partners
(preferably, > M
binding partners) so that one or more (preferably, all) binding partners are
immobilized in a
unique group of Z domains. The number of components that can be screened in a
given well can
be further increased by patterning some components in groups of Z1 domains,
others in groups of
Z2 domains and so on, where Z1, Z2, ... are integers greater than or equal to
one and less than or
equal to M.
In another preferred assay format, potentially cross-reacting analytes are
measured in
different domains in the same well. For example, two similar analytes (a first
analyte and a
second analyte) may be measured using two assay domains comprising binding
reagents (a first
domain selective for the first analyte and a second domain selective for the
second analyte) even
1 i-,
CA 02714094 2010-08-30
NV 0 03/023360
PCT/US02/28652
if the binding reagents are only partially selective for each analyte. By
carrying out the assays in
the same well, the binding of the second analyte to the second domain reduces
its effective
concentration in solution and reduces its ability to interfere with the
measurement of the first
analyte at the first domain. To the extent that such effects do not completely
eliminate cross-
reactions, the ability to measure both cross-reacting species allows for the
mathematical
deconvolution of signals so as to further reduce the effect of cross-reactions
on assay results.
Such deconvolutions can, e.g., be based on empirical calibrations (e.g., using
a two dimensional
matrix of calibrators varying the concentrations of both analytes, preferably
the calibrators are
chosen and the results modeled using Design of Experiment techniques) or on
theoretical models
(e.g., models derived using the thermodynamic and/or kinetic parameters
associated with each
possible binding interaction). Optionally, only the first analyte is measured
and the second
domain serves only to sequester the second analyte and prevent it from
interfering with the
measurement of the first analyte. The methods described above for reducing
cross-reactions and
interferences can be used to i) reduce and/or account for interfering
substances in crude
biological samples (e.g., blood, plasma, serum, tissue extracts, cell
extracts) such as bilirubin,
lipid, hemaglobin and/or ii) to aid in preventing and/or to prevent assay
interference and cross-
reactions from closely related species, e.g., to aid in measuring and
distinguishing between
closely related drugs and related metabolites, steroidal hormones and related
metabolites,
vitamins and related metabolites, modified forms of proteins, nucleic acids
and saccharides (e.g.,
different phosphorylation states of an analyte, between different degradation
states of an analyte,
different bound states of an analyte, etc.), etc.
12
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/286:C2
In another preferred assay format, a plurality of different assay domains in a
well are
adapted to measure different forms of an analyte of interest. By way of
example, domains may
be adapted to measure free and bound fonns of an analyte of interest (e.g.,
free PSA vs. bound =
PSA), to measure unmodified and/or modified forms of an analyte of interest
(examples of
modifications that can be measured include, but are not limited to,
phosphorylation,
ubiquitnation, prenylation, myristoylation, glyosidation), and/or to measure
cleavage or
degradation products of an analyte (e.g., protease, nuclease or glycosidase
products).
Alternatively, one assay domain generically measures the total amount of
multiple forms of an
analyte and a second assay domain is specific for one form of the analyte
(e.g., for measuring
free PSA vs. total PSA).
In another preferred assay format, the starting material/substrate and product
(and,
optionally, intermediates and/or side products) in a reaction of interest are
measured at different
assay domains of a well of a MDMW Plate. In one embodiment, measurements in
different
wells are carried out for different reaction times, allowing for a complete
kinetic characterization
of the reaction. In a second embodiment, assays for the starting material and
product (or any two
species produced and/or consumed in the reaction) show some level of cross-
reactivity; as
described above, measurement of both species can be used to reduce the effect
of the cross-
reactivity. In a third embodiment, measurement of both starting material and
product allows one
to correct for variations in the original amount of the starting material.
Such corrections are
especially important when following reactions or activities in complex
biological systems such
as cells or tissue. For example, in following the phosphorylation of a
cellular receptor in
response to activation of the cell, it is desirable to correct the measured
amount of
13 -
_ -
CA 02714094 2010-08-30
vv0 03/023360
PCT/US02/28652
phosphorylated receptor to account for variations in the level of receptor
protein expression in
the cell line. Measurement of phosphorylated and nonphosphorylated forms of
the receptor
allows the extent of phosphorylation to be expressed as a percentage.
Alternatively, the same
information can be obtained through measurements of total receptor and
phosphorylated
receptor.
In another preferred assay format, a well comprises a first assay domain
containing a
labeled substrate for a cleavage reaction and a second assay domain containing
a binding reagent
capable of capturing the product of the cleavage reaction. Preferably, the
substrate is linked to a
label (preferably an ECL label) such that the cleavage reaction results in the
release, from the
first assay domain of a cleavage product linked to the label. The extent of
the cleavage reaction
may be followed by measuring the drop of signal from the first assay domain
and the increase in
signal from the second assay domain. By way of example, the binding reagent
may be an
antibody (e.g., an antibody specific for a peptide released by proteolytic
activity) or a nucleic
acid probe (e.g., a probe specific against an oligonucleotide released by a
nuclease activity)
directed against the cleavage product. Alternatively, the substrate may he
further linked to a
capture moiety (e.g., a hapten or biotin) such that the released cleavage
product comprises both a
label and a capture moiety. In this alternate embodiment, the binding reagent
can be a binding
reagent directed against the capture moiety (e.g., an antibody directed
against the hapten, avidin,
or streptavidin).
In another preferred assay format, a library of enzymes is co-immobilized with
binding
reagents capable of capturing enzyme products so as to form an array of assay
domains having
assay domains that contain both an enzyme and a binding reagent capable of
capturing a product
14
CA 02714094 2010-08-30
< = -
WO 03/023360
PCT/US02/28652
=
of the enzyme. Such an array allows the signals derived from the enzyme
reactions to be
produced in a pattern that corresponds to the arrangement of enzymes. In one
embodiment, the
enzymes are paired with binding reagents that are preferentially specific for
the product of that
enzyme. In another embodiment, the binding reagents are capable of binding the
products of a
plurality of enzymes in the well. In such a case, the assay domains are spaced
appropriately and
carried out under appropriate conditions (e.g., in the absence of mixing) to
increase the
probability that a product produced in one domain will bind binding reagents
in that same
domain before it has the opportunity to diffuse to a different domain. For
example, a library of
tyrosine kinases may be patterned into an array of assay domains, each domain
also comprising
an anti-phosphotyrosine antibody. Introduction of one or more tyrosine kinase
substrates
(preferably, linked to a label, most preferably, linked to an ECL label) leads
to phosphorylation
of the substrates and capture of the labeled product by the anti-
phosphotyrosine antibody. The
= labeled product produced in a domain will be preferentially captured by
antibodies in the same
domain, ensuring that the signal generated in a domain is representative of
the activity of the
enzyme in that domain.
In another preferred assay format, multiple assay domains are used to aid in
screening
antibodies (or other binding reagents) for a binding reagent with a desired
specificity for a
binding species. Samples (e.g., supematants from hybridoma cultures) are
contacted with a
plurality of assay domains. One assay domain comprises the binding species.
The others
include controls for specificity and cross-reactivity (e.g., closely related
substances, potential
assay inteiferents, a carrier protein used in an immunization procedure used
to generate the
binding reagents, linkers used in generating carrier protein-hapten
conjugates, etc.). In one
t5'
CA 02714094 2010-08-30
. = = .
WO 03/023360
PCT/11S02/28652
embodiment, the binding of a binding reagent can be detected using a labeled
secondary binding
reagent that broadly binds a class of binding reagents (e.g., an anti-species
antibody). In another
embodiment, a plurality of binding domains comprise a panel of anti-species
antibodies directed
against different antibody classes (alternatively, any antibody class specific
binding reagent may
be used) and the binding domains are contacted with a hybridoma supernatant
(or other sample
containing antibodies) in order to determine the class of the antibody in the
supernatant. In one
preferred embodiment, the binding of binding reagents to specific domains is
detected using a
labeled secondary binding reagent that broadly binds a class of binding
reagents (e.g., an anti-
species antibody) so as to measure the amount of all antibodies in the sample.
Alternatively, a
labeled hapten may be used as the detection reagent so that only the class of
antibodies having a
desired specificity is determined.
In another preferred assay format, multiple assay domains are used to expand
the
dynamic range of an assay beyond what can be achieved using a single assay
domain. For
example, a binding assay may involve the use of a plurality of binding domains
comprising
binding reagents that differ in their affinity for the analyte of interest.
The domain with the
highest affinity binding reagent is used to measure low concentrations of
analyte. Domains with
intermediate or weak affinity binding reagents are used for samples having
intermediate or high
concentrations of analyte. In the case of assay domains with intermediate or
weak affinity
binding reagents, the binding reagents are, preferably, selected to have
dissociation constants
roughly centered in the range of analyte concentrations to be measured by that
assay domain.
In the specific case of sandwich binding assays that experience a hook effect
at high
concentrations, the dynamic range of the assay may be expanded by pairing the
sandwich
16
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
immunoassay (conducted in a first assay domain) with a competitive assay for
the same analyte
(conducted in a second assay domain). Preferably, the competitive assay
involves the
competition of analyte in a sample with an immobilized analog of the analyte
for binding to a
labeled anti-analyte antibody. More preferably, the analog of the analyte does
not comprise the
epitope recognized by the capture antibody in the sandwich assay (e.g., the
analog of the analyte
may be a peptide fragment derived from a protein analyte that does not include
the epitope
recognized by the capture antibody). The sandwich and competitive assays may
use the same
labeled detection antibody. Advantageously, the amount of detection antibody
is roughly equal
to the sum of the amount of analog of the analyte and capture antibody. For
amounts of analyte
lower than the amount of capture antibody, the sandwich assay will give a
signal roughly linearly
dependent on the concentration of analyte and the competitive assay will be
roughly independent
of analyte concentration. Amounts of analyte higher than the amount of capture
antibody will
lead to decreases is signal from both the sandwich assay (due to hook effect,
i.e., the increase in
the probability that the analyte will be bound to only one of the detection or
capture antibody and
not both at the same time) and the competitive assay (due to competition). In
this region, the
competitive assay may be used to quantify analyte or to simply warn that the
dynamic range of
the sandwich assay has been exceeded. In an alternate embodiment, capture
reagents are chosen
that differ in binding kinetics; the binding time and kinetic constants are
chosen so that i) low
concentrations of analyte are measured in fast binding assay domains and ii)
high concentrations
of analyte (exceeding the binding capacity of the assay domains) are measured
in kinetically
controlled binding reactions at slow binding domains.
CA 02714094 2010-08-30
=
VI' 0 03/023360
PCT/US(J2/28652
In another preferred assay format, one or more assay domains in a well are
used for
purposes other than as a solid phase for a solid phase assay. By way of
example: i) an assay
domain may comprise binding reagents for sequestering an assay interferent;
ii) an assay domain
may comprise binding reagents for capturing and purifying a biological
material (e.g., a protein
of unknown function, an enzyme, an enzyme substrate, a binding partner in a
binding reaction,
etc.) from a crude preparation such as blood, serum, cell lysates, tissue
samples, etc. and/or iii)
an assay domain may be used as a location for storing dried reagents to be
rehydrated and
dissolved during the course of an assay (e.g., binding reagents, enzymes,
enzyme substrates,
controls, calibrators, buffers, blocking agents, detergents, labeled-reagents,
ECL coreactants,
inhibitors, drug candidates, etc.). Preferably, the dried reagents in one
assay domain are
prevented, during preparation and storage of an assay well, from contacting
the other assay
domains in a well so as to prevent unwanted interactions between reagents. In
such case, the
dried reagents do not contact other assay domains until sufficient sample
volume is added to the
well to spread the sample across all the domains. Advantageously, when storing
dried reagents
in an assay domain, the assay domain is surrounded by a physical boundary
(e.g., ledges or
depressions on a surface of the well, patterned materials deposited or printed
on the surface, and
or interfaces between regions of the surface that vary in a physical property
such as wettability)
that allows small drops of fluids to be confined on the assay domain but also
allows a larger
volume of fluid to spread over multiple domains. In one embodiment of a
competitive binding
assay, one assay domain of a well comprises an immobilized binding reagent and
another assay
domain comprises a dried labeled competitor; this arrangement prevents the
competitor from
binding to the binding reagent prior to the addition of sample. In one
embodiment of a sandwich
18
CA 02714094 2010-08-30
= =
WO 03/023360
PCT/US02/28652
binding assay, a first assay domain of a well comprises an immobilized capture
binding reagent
and a second assay domain comprises a dried labeled binding reagent; this
arrangement prevents
the labeled binding reagent from binding non-specifically to the first assay
domain, e.g., during
drying or storage of the reagents. In one embodiment of an enzyme inhibition
assay, a first assay
domain of a well comprises an enzyme substrate (dried on or immobilized in the
assay domain)
and a second assay domain comprises a dried enzyme; this arrangement prevents
the enzyme
from acting on the substrate prior to the addition of the sample containing an
inhibitor.
The invention includes assay modules and MDMW Plates adapted to carry out
assays
using one or more assay formats of the invention, methods of using the modules
or plates,
methods of making the modules or plates, kits including the plates and one or
more reagents used
in an assay, and systems including plates and apparatuses for reading plates.
The invention
includes the measurement of analytes or chemical, biological or biochemical
activities using the
modules, plates or methods of the invention. The invention also includes the
measurement or
identification (e.g., in a screen of a library of potential drugs) of
modulators (e.g., inhibitors or
enhancers) of such chemical, biological or biochemical activities. The
invention also includes
the application of the plates, modules or methods of the invention to the
characterization of a
=
=
protein. For example a protein may be screened against a library of biological
materials to
identify biological materials that bind the protein, accept the protein as an
enzymatic substrate,
are modified by an enzymatic activity of the protein, or otherwise interact
with the protein.
Conversely, a biological material may be screened against a library of
proteins to identify the
proteins that bind the biological material, accept the biological material as
an enzymatic
19
-
CA 02714094 2010-08-30
. ,
69331-63D
substrate, are modified by an activity of the biological
material, or otherwise interact with the biological
material.
In one aspect, the invention relates to a method
of determining the effect of an inhibitor on a plurality of
enzymes, which comprises: contacting a sample containing the
inhibitor and the plurality of enzymes with a plurality of
assay domains having substrates for the enzymes immobilized
thereon, and detecting the effect of the inhibitor on the
plurality of enzymes.
In another aspect, the invention relates to a
method of conducting an assay, which comprises: contacting a
sample containing a plurality of enzymes with an assay
module having a plurality of assay domains comprising
substrates corresponding to the enzymes immobilized thereon,
and detecting the enzymatic reaction of the substrates at
the assay domains.
In another aspect, the invention relates to an
assay domain having immobilized thereon an enzyme and a
reagent capable of binding a product of an enzymatic
reaction initiated by the enzyme.
CA 02714094 2010-08-30
69331-63D
4. DESCRIPTION OF THE FIGURES
Fig. IA is a schematic representation, according to one embodiment of the
invention, of a panel
of binding assays.
Fig. 1B is a schematic representation, according to one embodiment of the
invention, of a panel
of sandwich binding assays.
Fig. IC is a schematic representation, according to one embodiment of the
invention, of a panel
of competitive binding assays.
Fig. ID is a schematic representation, according to one embodiment of the
invention, of a panel
of enzyme assays. '
Fig. 2 is a schematic representation, according to one embodiment of the
invention, of an assay
panel that includes a test binding assay, a control for non-specific binding,
a control for the efficiency of
signal generation and transmission, and a control binding assay.
Figure 3 is a schematic representation, according to one embodiment of the
invention, of an
assay panel that includes assays for several activities of an enzyme with a
plurality of activities.
00 Figure 4 is a schematic representation, according to one embodiment
of the invention, of an
assay panel comprising a binding assay for an enzyme and a binding assay for
an enzyme product
20a
CA 02714094 2010-08-30
- WO 03/023360
PCT/US02/286-52
Figure 5 is a schematic representation, according to one embodiment of the
invention, of an
assay panel comprising binding assays for the substrate and product of an
enzymatic reaction.
Figure 6 is a schematic representation, according to one embodiment of the
invention, of an
assay panel for a cleaving enzyme comprising an assay domain having a labeled
substrate for the
enzyme and an assay domain comprising a binding reagent capable of capturing a
labeled product of the
invention.
Figure 7 is a schematic representation, according to one embodiment of the
invention, of an
assay panel comprising an array of assay domains comprising different enzymes,
the enzymes being co-
immobilized for binding reagents capable of binding enzymatic products.
Figures 8A-C are schematic representations of expanded dynamic range binding
assays,
according to preferred embodiments of the invention, comprising three assay
domains of varying affinity
for the analyte.
Figures 9A-B are schematic representations of expanded dynamic range binding
assays,
according to preferred embodiments of the invention, comprising a sandwich
binding assay and a
competitive binding assay for the same analyte.
Figure 10A shows a layered view of MDMW plate 1000, a MDMW plate that is
adapted for
electrode induced chemiluminescence measurements.
Figure 10B shows a stylized cross sectional view of 3 wells of MDMW plate
1000, a MDMW
plate that is adapted for electrode induced chemiluminescence measurements.
Figure 10C shows dielectric layer 1140, a modification of dielectric layer
1040 shown in Figures
10A and 10B.
=
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
Figure 10D shows a stylized cross-sectional view of 3 wells of MDMW plate
1100, a
modification of plate 1000 employing dielectric layer 1140
Figure 11 shows a view of a MDMW plate adapted for electrode induced
chemiluminescence
measurements.
Figure 12 is a schematic description of an assay for two activities of HIV RT
enzyme.
Figures 13A and B are graphical representations of the inhibition of two
activities of HIV RT by
an inhibitor.
Figure 14 is a graphical representation of the selectivity of a MDMW Plate
designed to measure
4 different infectious agents.
Figure 15 is a table showing the selectivity of a MDMW Plate designed to
measure two different
lcinase activities.
Figure 16 plots signal as a function of the concentration of bovine IgG that
is labeled with biotin
and a sulfonated derivative of Ru(bpy)3. Data is plotted for MDMW Plates
having avidin-coated assay
domains that vary in number and size.
Figures 17A-D demonstrate the independent measurement by ECL sandwich
immunoassay of
four analytes (IL-113, IL-6, INF-a and IFN-y) in wells of a multi-well assay
plate. The working
electrode in each well is patterned with four assay domains, each assay domain
comprising a capture
antibody specific for one of the analytes. The plots show the ECL signal
emitted from each assay
domain as a function of the concentration of each analyte.
Figure 18 is a CCD camera image showing the independent measurement by ECL
sandwich
immunoassay of four analytes (IL-113, IL-6, INF-a and IFN-y) in wells of a
multi-well assay plate. The
working electrode in each well is patterned with four assay domains, each
assay domain comprising a
22 -
õ
CA 02714094 2010-08-30
= = =
WO 03/023360
PCT/US02/28652
capture antibody specific for one of the analytes. The figure shows an image
of the ECL emitted from a
sector of wells used to assay samples containing varying mixtures of the four
analytes. The highlighted
well is annotated to show the arrangement of the four assay domains. That
specific well was used to
assay a sample having 250 pg/mL each of IL-113 and TNF-a and 8 pg/mL each of
IL-6 and IFN-y.
Figure 19A is a schematical representation of a 4-spot well adapted for an
assay for EGF induced
Receptor Autophosphorylation at Tyrosine 1173 using MSDTM Standard 4-spot
Multi-ArrayTM Plates
according to one embodiment of the invention. Figures 19B-D are CCD images of
wells of the plate
having different concentrations of EGF.
5. DETAILED DESCRIPTION OF THE INVENTION
The assay domains of the invention may be adapted to carry out assays in a
wide range of
formats. Preferably, assay measurements are coupled to the capture or release
of detectable label
(e.g., an enzyme, particle, photoluminescent species, chemiluminescent
species,
electrochemiluminescent species, electroactive species, radioactive species,
magnetic species,
etc.) from a solid phase, preferably, a surface of an assay domain.
Preferably, the label is
detectable by electrode induced luminescence (most preferably,
electrochemiluminescence) and
the solid phase is an electrode adapted to induce electrode induced
luminescence (preferably,
electrochemiluminescence). By analogy, the assay concepts described herein can
also be applied
to solid phase assay formats that do not require the use of a label such as
surface plasmon
resonance and optical interference techniques.
Figures 1A-D are schematic representations that show selected examples of
assay panels
that may be carried out in assay cells (preferably, wells of a MDMW Plate)
comprising multiple
23,P
CA 02714094 2010-08-30
= = . =
WO 03/023360
PCT/US02/28652
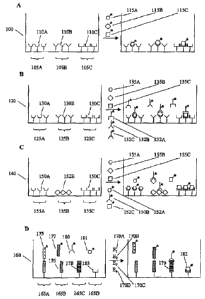
assay domains. Figure IA illustrates a panel of binding assays carried out in
a well 100 of a
MDMW Plate having assay domains 105A-C, comprising immobilized binding
reagents 110A-C
directed against labeled analytes 115A-C. Suitable binding reagent/analyte
pairs are known in
the art and include antibody/hapten, antibody/antigen, receptor/ligand,
nucleic acid
sequence/complementary sequence, lectin/sugar, nucleic acid/nucleic acid
binding protein,
protein/protein (e.g., proteins that dimerize, aggregate form binding
complexes), etc.
Figure 1B illustrates a panel of sandwich binding assays carried out in a well
120 of a
MDMW Plate having assay domains 125A-C comprising immobilized capture binding
reagents
130A-C and soluble detection binding reagents 132A-C directed against analytes
135A-C. In
one preferred embodiment, the panel is a panel of sandwich immunoassays. In
another preferred
embodiment, the panel is a panel of sandwich nucleic acid hybridization
assays.
Figure 1C illustrates a panel of competitive binding assays carried out in a
well 140 of a
MDMW Plate having assay domains 145A-C. Analytes 155A-C compete with
competitors
152A-C for binding to binding reagents 150A-C. If the competitor of an analyte
is labeled, the
corresponding binding reagent is immobilized, or visa versa. In one preferred
embodiment, the
panel is a panel of competitive immunoassays.
Figure 1D illustrates a panel of enzyme assays carried out in well 160 of a
MDMW Plate.
Enzyme 170A cleaves labeled substrate 175 (immobilized in assay domain 165A)
to release a
labeled product from the assay domain. Enzyme 170B joins substrate 176
(immobilized in assay
domain 165B) and labeled substrate 177 to link the label to the assay domain.
Enzyme 170C
modifies substrate 178 (immobilized in assay domain 165C) to make a product
179 that is
recognized by labeled binding reagent 180. Enzyme 170D catalyzes the
conversion of labeled
23
CA 02714094 2010-08-30
. =
WO 03/023360
PCT/US02/28652
substrate 181 to labeled product 182. Labeled product 182 is then captured by
binding reagent
183 (immobilized in assay domain 165D). In an alternate embodiment, the label
is omitted from
substrate 181 and product 182 and product 182 is detected by addition of a
labeled detection
binding reagent to form a sandwich complex. Enzymes (and other chemical,
biochemical, and/or
biological activities) that can be measured by one or all of the formats
described in Figure 1D
include, but are not limited to, nucleic acid polymerases, nucleic acid
ligases, helicases,
integrases, nucleases, proteases, protein synthesis, glycosidases,
phosphatases, kinases,
prenylation enzymes, myristoylation enzymes, etc.
Useful panels include panels of assays for analytes or activities associated
with a specific
biochemical system, biochemical pathway, tissue, organism, cell type,
organelle, disease state,
class of receptors, class of enzymes, etc. Preferred panels include
immunoassay for cytolcines
and/or their receptors (e.g., one or more of INF-a, TNF-13, IL2, IL4, IL6,
ILE),
IL12, IFN-y, etc.), growth factors and/or their receptors (e.g., one or more
of EGF, VGF, TGF,
VEGF, etc.), second messengers (e.g., cAMP, cGMP, phosphorylated forms of
inositol and
phosphatidyl inositol, etc.) drugs of abuse, therapeutic drugs, auto-
antibodies (e.g., one or more
antibodies directed against the Sm, RNP, SS-A, SS-B Jo-1, and Sc1-70
antigens), allergen specific
antibodies, tumor markers, cardiac markers (e.g., one or more of Troponiti T,
Troponin I,
myoglobin, CKMB, etc.), markers associated with hemostasis (e.g., one or more
of Fibrin
monomer, D-dimer, thrombin-antithrombin complex, prothrombin fragments 1 & 2,
anti-Factor
Xa, etc.), markers of acute viral hepatitis infection (e.g., one or more of
IgM antibody to hepatitis
A virus, IgM antibody to hepatitis B core antigen, hepatitis B surface
antigen, antibody to
hepatitis C virus, etc.), markers of Alzheimers Disease (P-amyloid, tau-
protein, etc.), markers of
Ify
CA 02714094 2010-08-30
= . =
WO 03/023360
PCT/US02/28652
osteoporosis (e.g., one or more of cross-linked N or C-telopeptides, total
deoxypyridinoline, free
deoxypyridinoline, osteocalcin, alkaline phosphatase, C-terminal propeptide of
type I collagen,
bone-specific alkaline phosphatase, etc.), markers of fertility (e.g., one or
more of Estradiol,
progesterone, follicle stimulating hormone (FSH), luetenizing hormone (LH),
prolactin, P-hCG,
testosterone, etc.), markers of congestive heart failure (e.g., one or more of
p-natriuretic protein
(BNP), a-natriuretic protein (ANP), endothelin, aldosterone, etc.), markers of
thyroid disorders
(e.g., one or more of thyroid stimulating hormone (TSH), Total T3, Free T3,
Total T4, Free T4,
and reverse T3), and markers of prostrate cancer (e.g., one or more of total
PSA, free PSA,
complexed PSA, prostatic acid phosphatase, creatine kinase, etc.). Preferred
panels also include
nucleic acid arrays for measuring mRNA levels of mRNA coding for cytolcines,
growth factors,
components of the apoptosis pathway, expression of the P450 enzymes,
expression of tumor
related genes, etc. Preferred panels also include nucleic acid arrays for
genotyping individuals
(e.g., SNP analysis), pathogens, tumor cells, etc. Preferred panels also
include libraries of
enzymes and/or enzyme substrates (e.g., substrates and/or enzymes associated
with
ubiquitination, protease activity, kinase activity, phosphatase activity,
nucleic acid processing
activity, GTPase activity, guanine nucleotide exchange activity, GTPase
activating activity, etc.).
Preferred panels also include libraries of receptors or ligands (e.g., panels
of G-protein coupled
receptors, tyrosine kinase receptors, nuclear hormone receptors, cell adhesion
molecules
(integrins, VCAM, CD4, CD8), major histocompatibility complex proteins,
nicotinic receptors,
etc.). Preferred panels also include libraries of cells, cell membranes,
membrane fragments,
reconstituted membranes, organelles, etc. from different sources (e.g., from
different cell types,
cell lines, tissues, organisms, activation states, etc.).
26
CA 02714094 2010-08-30
. . =
WO 03/023360
PCT/US02/28652
Applications of panels include the determination of a state of a biological
system, the
detection or identification of disease state. The determination of analytes
associated with a state
of a biological system (e.g., by differential measurements of a plurality of
analytes in samples
derived from normal or diseased biological systems or from normal and
activated biological
systems, etc.). *Panels may also be employed in drug screening. Through the
use of panels, the
effect of a potential drug on a plurality of biological activities (e.g.,
binding interactions or
enzymatic activities) can be determined in one well of a MDMW Plate. Panels
may also be used
to speed up the characterization of a protein. For example a protein may be
screened against a
library of biological materials to identify biological materials that bind the
protein, accept the
protein as an enzymatic substrate, are modified by an enzymatic activity of
the protein, or
otherwise interact with the protein. Conversely, a biological material may be
screened against a
library of proteins to identify the proteins that bind the biological
material, accept the biological
material as an enzymatic substrate, are modified by an activity of the
biological material, or
otherwise interact with the biological material.
Some assay domains in an assay cell or well may be reserved for assay controls
or
calibrators. Figure 2 is a schematic representation, according to one
embodiment of the
invention, of an assay panel that includes a test binding assay for an analyte
of interest, a control
for non-specific binding, a control for the efficiency of signal generation
and transmission, and a
control binding assay. Figure 2 shows a well 200 of a MDMW Plate having assay
domains
210A-D. Assay domain 210A comprises a capture binding reagent 215 (e.g., an
antibody or a
nucleic acid) specific for the analyte of interest 217. Assay domain 210B
comprises a blocking
agent 225 (e.g., BSA, or bovine IgG) that, preferably, was also used to block
open sites in assay
-
2i
CA 02714094 2010-08-30
. =
vVO 03/023360
PCT/US02/28652
domain 210A. Alternatively, blocking agent 225 is a reagent with similar
properties to capture
binding reagent 215 except that it is not expected to interact with samples
introduced into the
assay. Assay domain 210C comprises a labeled reagent 235. Assay domain 210D
comprises a
capture binding reagent 245 specific for control analyte 247. The well also
comprises labeled
detection antibody 219 that is specific for analyte 215, labeled detection
antibody 249 that is
specific for control analyte 247, an unknown quantity of analyte 217, and a
predetermined
amount of control analyte 247. Formation of a sandwich complex in assay domain
210A allows
for measurement of the analyte. Assay domain 210B is used to measure the
amount of
background signal including non-specific binding of the labeled reagents.
Assay domain 210C is
used to control for factors that influence the efficiency of signal generation
by the label and the
efficiency of signal detection. Measurement of a sandwich complex in assay
domain 210D is
used to control for factors that influence the efficiency of binding
reactions. In alternate
embodiments, assay domains 210A and/or 210D comprise reagents for conducting
other types of
measurements such as other binding assay formats or enzymatic activity assays.
Figure 3 illustrates an assay, according to one embodiment of the invention,
for the
activities of an enzyme with multiple activities. The assay is carried out in
well 300 of a
MDMW Plate. Enzyme 370 cleaves labeled substrate 375 (immobilized in assay
domain 365A)
to release a labeled product from the assay domain. Enzyme 370 also joins
substrate 376
(immobilized in assay domain 365B) and labeled substrate 377 to link the label
to the assay
domain. Enzyme 370 also modifies substrate 378 (immobilized in assay domain
365C) to make
a product 379 that is recognized by labeled binding reagent 380. Enzyme 370
also catalyzes the
conversion of labeled substrate 381 to labeled product 382. Labeled product
382 is then captured
za
CA 02714094 2010-08-30
. =
WO 03/023360
PCT/US02/28652
by binding reagent 383 (immobilized in assay domain 365D). In an alternate
embodiment, the
label is omitted from substrate 381 and product 382 is detected by addition of
a labeled detection
binding reagent to form a sandwich complex.
Figure 4 illustrates an assay, according to one embodiment of the invention,
of an
enzymatic activity. Well 400 of a MDMW Plate comprises assay domain 410 having
an
immobilized capture binding reagent 412 (e.g., an antibody) capable of binding
enzyme 415 and
assay domain 420 comprising an immobilized binding reagent 425 (e.g., an
antibody) capable of
binding a product of enzyme 415. Addition of a sample containing the enzyme
leads to the
capture of the enzyme in assay domain 420. Optionally, a wash step may be
introduced to
remove interfering substances in the enzyme sample. Addition of a labeled
substrate results in
the generation of a labeled product that is captured and measured in assay
domain 420. Addition
of labeled detection reagent 417 (e.g., an antibody) allows for the
measurement of the amount of
enzyme 415 in assay domain 410 via sandwich binding assay. This measurement
allows the
measured enzymatic activity to be referenced to the amount of enzyme.
Alternatively, enzyme
410 is labeled and labeled detection reagent 417 may be omitted. In another
alternative
embodiment, labeled detection reagent 417 is omitted and enzyme 415 is
captured and,
optionally, purified but not directly measured. In a preferred embodiment of
the assay, enzyme
415 is a phosphatase or kinase and binding reagent 425 is an antibody that
preferentially binds to
one of a phospho-peptide or its nonphosphorylated form.
90 Figure 5 illustrates an assay, according to one embodiment of the
invention, where an
enzymatic activity is measured by measuring the consumption of substrate and
the generation of
product. Well 500 in a MDMW Plate comprises an assay domain 510A comprising
immobilized
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
capture binding reagent 512 specific for labeled enzyme substrate 515 and an
assay domain 510B
comprising immobilized capture binding reagent 517 specific for labeled enzyme
product 520.
A sample comprising a mixture of labeled substrate 515 and labeled product 520
resulting from
the action of an enzyme on the enzyme substrate is introduced into well 500.
Measurement of
substrate and product by binding assay allows the extent of conversion to be
calculated even if
the initial amount of substrate was unknown. Alternatively, substrate 515 is
not labeled and
substrate 515 and product 520 are measured via sandwich binding assay or
competitive binding
assay. In a preferred embodiment of the invention, the enzyme is a lcinase or
phosphatase and
the binding reagents are antibodies specific for the phosphorylated or non-
phosphorylated form
of a peptide or protein. Alternatively, one capture reagent is specific for
either product or
substrate and the other capture reagent binds both equally. This panel allows
the measurement of
product or substrate in one domain and the combined total of product and
substrate in the other
domain.
Figure 6 illustrates an assay, according to one embodiment of the invention,
for an
enzyme with a cleaving activity. Well 600 of a MDMW Plate comprises assay
domain 610
comprising an immobilized labeled substrate 615 and assay domain 620
comprising an
immobilized binding reagent 622 that is specific for labeled enzyme product
625. Enzyme 630
cleaves substrate 615 forming product 625 which is captured in assay domain
620. The assay
format allows the measurement of both the consumption of substrate and the
production of
product. Optionally, substrate 615 is not labeled and product 625 is measured
via a sandwich or
competitive binding assay. In a preferred embodiment, enzyme 630 is a
protease, substrate 615
is a labeled peptide, and binding reagent 622 is an antibody specific for the
peptide.
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
Alternatively, substrate 615 also comprises a capture moiety and binding
reagent 622 is specific
for the capture moiety.
Figure 7 illustrates an assay format, according to one embodiment of the
invention, for
measuring the activity of an immobilized array of enzymes. Well 700 of a MDMW
Plate
comprises assay domains 710A-C which comprise immobilized enzymes 715A-C and
immobilized binding reagents 720A-C, the binding reagents being specific for a
product of the
enzyme co-immobilized in the same assay domain. Introduction of labeled
substrates 725A-C
(which may the same or different) leads to the generation of labeled products
730A-C (which
may be the same or different). The close proximity of the enzymes to binding
reagents leads to
preferential capture of products in the assay domain in which they were
produced (as opposed to
the diffusion and capture of products in adjacent domains). Preferably, the
assay domains are in
slight depressions in the bottom of the well so as to inhibit convection near
the surface of the
domains and to inhibit horizontal diffusion away from the surface of the
domains. In an alternate
embodiment, the substrates are not labeled and the product is measured via a
sandwich or
competitive binding assay. In one preferred embodiment, the enzymes are
ldnases, the substrates
have consensus sequences for specific members of the kinase library, and the
binding reagents
are antibodies specific for the product of the enzymes with which they are co-
immobilized.
Alternatively, the binding reagents are broadly specific for phosphopeptides
(e.g., an anti-
phosphotyrosine or an anti-phosphoserine antibody). In another preferred
embodiment, the
enzymes are kinases, the substrates are the same and are a generic kinase
substrate, and the
binding reagents are the same and are binding reagents broadly specific for
phosphopeptides
(e.g., an anti-phosphotyrosine or an anti-phosphoserine antibody).
31'
CA 02714094 2010-08-30
'WO 03/023360
PCT/US02/28652
Figures 8A-C illustrate expanded dynamic range binding assays, according to
preferred
embodiments of the invention, comprising three assay domains of varying
affinity for the analyte
of interest. This assay format is particularly advantageous when the dynamic
range must extend
to analyte concentrations that are greater than the binding capacity of assay
domains in a well.
Well 800 in a MDMW Plate comprises assay domains 810A-C for measuring analyte
820, the
well comprising i) assay domain 810A comprising immobilized binding reagent
815A, binding
reagent 815A having a dissociation constant for analyte = Ica, ii) assay
domain 810B comprising
immobilized binding reagent 815B, binding reagent 815B having a dissociation
constant for
analyte = dand iii) assay domain 810C comprising immobilized binding reagent
815C,
binding reagent 815C having a dissociation constant for analyte = IC4c,
wherein Kda < b <
and wherein 1(db and KZ are, preferably, greater than the concentration of
analyte needed to
saturate assay domain 810A. A labeled analyte 820 is introduced into the well.
Preferably, the
dissociation constants differ by a factor of 10 or more so that: i) when the
concentration of
analyte is << Kdb, only assay domain 810A will be significantly populated
(Fig. 8A); ii) when the
concentration of analyte is ¨1(db, assay domain 810A will be saturated, assay
domain 810B will
be partially populated, and assay domain 810C will be negligibly populated
(Fig. 8B); and iii)
when the concentration of analyte is ¨ KZ, assay domains 810A and 810B will be
saturated and
assay domain 810C will be partially populated (Fig. 8C). In each concentration
range, the signal
from the partially populated assay domain is used to quantitate analyte.
Optionally, assay domain
810C is omitted or additional assay domains are included for extending the
dynamic range into
additional concentration ranges.
CA 02714094 2010-08-30
. . . =
WO 03/023360
PCT/US02/28652
Figures 9A-B illustrate expanded dynamic range binding assays, according to
preferred
embodiment of the inventions, comprising a sandwich binding assay and a
competitive binding
assay for the same analyte. This assay format is particularly advantageous
when the dynamic
range must extend to analyte concentrations that are greater than the binding
capacity of assay
domains in a well. Well 900 in a MDMW Plate comprises i) assay domain 910
comprising an
immobilized capture binding reagent 912 that is specific for analyte 915 and
ii) assay domain
920 comprising an immobilized competitor 925 that competes with analyte 915
for binding to
labeled binding reagent 917. Introduction of analyte 915 and labeled binding
reagent 917 leads
to the binding of labeled binding reagent 917 to assay domain 910 via a
sandwich complex and
to assay domain 920 via direct binding. Preferably, the amount of capture
binding reagent 912 is
roughly the same as the amount of competitor 925 and is roughly half of the
amount of labeled
binding reagent 917. For amounts of analyte less than the amount of capture
binding reagent
(i.e., the binding capacity of assay domain 910), the amount of label bound to
assay domain 910
is roughly proportional to the amount of analyte and the amount of label bound
to assay domain
920 is roughly constant and saturated (Fig. 9A). For amounts of analyte
greater than the amount
of capture binding reagent, the amount of label bound to assay domain 910
decrease with
increasing analyte due to the "hook effect" and the amount of label bound to
assay domain 920
also decreases due to competitive binding (Fig. 9B). The competitive assay can
therefore be
used to quantitate analyte at high concentrations of analyte or simply to
provide warning that the
sandwich assay has exceeded its dynamic range. In a preferred embodiment,
analyte 915 is a
protein and binding reagents 912 and 917 are antibodies specific for different
epitopes on analyte
915. Competitor 925 may be a labeled version of analyte 915 or, more
preferably, is a peptide
CA 02714094 2012-09-27
=
69331-63D
that binds to binding reagent 917 but not binding reagent 912 (thereby,
reducing the possibility
of binding reagent 912 or competitor 925 leaching from the surface and
binding).
According to preferred embodiments of the invention, the assay domains of the
invention
are incorporated in assay modules or plates adapted for electrode induced
luminescence
(preferably, electrochemiluminescence) assays, e.g., assay domains are
supported on one or more
integrated electrodes within an assay cell (e.g., the well of a MDMW plate).
Suitable assay
modules and well plates, and methods of using such assay modules and plates
and systems
incorporating the same set forth in U.S. Patent Nos. 7,842,246 and 6,977,722
(see Sections 3, 4 and 5.1-5-6). According
to one preferred embodiment of the invention, an assay module or plate
comprises one or more
(preferably two or more, 6 or more, 24 or more, 96 or more, 384 or more, 1536
or more or 9600
or more) assay wells, assay chambers and/or assay domains (e.g., discrete
locations on a module
surface where an assay reaction occurs and/or where an assay signal is
emitted; typically an
= electrode surface, preferably a working electrode surface). According to
an even more preferred
embodiment, the assay module is a multi-well assay plate having a standard
well configuration
(e.g., 6 well, 24 well, 96 well, 384 well, 1536 well, 6144 well or 9600 well).
The wells of such
plates can further comprise a plurality (e.g., 2 or more, 4 or more, 7 or
more, 25 or more, 64 or
more, 100 or more, etc.) of discrete assay domains.
One aspect of the invention relates to improved assay modules (e.g., plates)
adapted for
use in assays, preferably luminescence assays, more preferably electrode
induced luminescence
assays, even more preferably electrochemiluminescence assays. The assay
modules of the
34
CA 02714094 2010-08-30
. =
WO (13/023360
PCT/US02/286S2
invention are preferably suitable not only for ECL assays, but also suitable
for fluorescence
assays, chemiluminescence assays, bioluminescence assays, phosphorescence
assays, optical
transmittance assays (e.g., measurements of optical density or light
scattering) and
electrochemical assays (e.g., wherein the measurement involves measuring
current or voltage).
According to one preferred embodiment of the invention, an assay module or
plate
comprises one or more (preferably two or more, 6 or more, 24 or more, 96 or
more, 384 or more,
1536 or more or 9600 or more) assay wells, assay chambers and/or assay domains
(e.g., discrete
locations on a module surface where an assay reaction occurs and/or where an
assay signal is
emitted; typically an electrode surface, preferably a working electrode
surface). According to a
particularly preferred embodiment, the assay plate is a multi-well assay plate
having a standard
well configuration (e.g., 6 well, 24 well, 96 well, 384 well, 1536 well, 6144
well or 9600 well).
An electrode induced luminescence well (preferably electrochemiluminescence
well (i.e.,
a well adapted for electrochemiluminescence)) or electrode induced
luminescence domain
(preferably electrochemiluminescence assay domain (i.e., an assay domain
adapted for
electrochemiluminescence assays)) may include a first electrode surface (such
as a working
electrode surface) and, preferably also includes a second electrode surface
(such as a counter
electrode surface).
The invention also relates to a multi-well module, preferably an assay plate,
for
conducting one or more assays, the module having a plurality of wells (and/or
chambers),
wherein two or more of the plurality of wells (and/or chambers) comprise at
least one first
electrode surface and, preferably at least one counter electrode surface.
According to a preferred
embodiment, two or more of the plurality of wells (and/or chambers) comprise a
working
35 -
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
electrode surface and, preferably a counter electrode surface, adapted to
induce luminescence in
the wells. The invention also relates to a multi-well module, preferably a
plate, for conducting
one or more assays, the module having a plurality of wells, wherein one or
more of the plurality
of wells comprise a working electrode surface and a counter electrode surface
adapted to induce
luminescence in the wells. Preferably, all or substantially all of the wells
comprise an electrode
surface.
Another embodiment relates to a multi-well assay module, preferably an assay
plate, for
conducting electrode induced luminescence (preferably
electrochemiluminescence) assays, the
module, preferably a plate, having a plurality of wells, wherein each of the
plurality of wells
comprises at least one first electrode surface (e.g., a working electrode)
and, preferably, at least
one second electrode surface (e.g., a counter electrode).
Another embodiment relates to an assay plate for conducting one or more
electrode
induced luminescence (preferably electrochemiluminescence) assays, the plate
having a plurality
of wells or assay regions comprising electrode surfaces, wherein the electrode
surfaces consist
essentially of at least one working electrode surface and at least one counter
electrode surface.
Preferably, the assay regions or assay wells are free of reference electrodes
allowing for a
greater density of assay domains and simplified instrumentation for inducing
and measuring
luminescence.
One aspect of the invention relates to improved assay modules (e.g., plates)
adapted for
use in assays, preferably luminescence assays, more preferably electrode
induced luminescence
assays, even more preferably electrochemiluminescence assays. The assay
modules of the
invention are preferably suitable not only for ECL assays, but also suitable
for fluorescence
CA 02714094 2010-08-30
. . =
WO 03/023360
PCT/US02/28652
assays, chemiluminescence assays, bioluminescence assays, phosphorescence
assays, optical
transmittance assays (e.g., measurements of optical density or light
scattering) and
electrochemical assays (e.g., wherein the measurement involves measuring
current or voltage).
According to one preferred embodiment of the invention, an assay module or
plate
comprises one or more (preferably two or more, 6 or more, 24 or more, 96 or
more, 384 or more,
1536 or more or 9600 or more) assay wells, assay chambers and/or assay domains
(e.g., discrete
locations on a module surface where an assay reaction occurs and/or where an
assay signal is
emitted; typically an electrode surface, preferably a working electrode
surface). According to a
particularly preferred embodiment, the assay plate is a multi-well assay plate
having a standard
well configuration (e.g., 6 well, 24 well, 96 well, 384 well, 1536 well, 6144
well or 9600 well).
An electrode induced luminescence well (preferably electrochemiluminescence
well (i.e.,
a well adapted for electrochemiluminescence)) or electrode induced
luminescence domain
(preferably electrochemiluminescence assay domain (i.e., an assay domain
adapted for
electrochemiluminescence assays)) may include a first electrode surface (such
as a working
electrode surface) and, preferably also includes a second electrode surface
(such as a counter
electrode surface).
The invention also relates to a multi-well module, preferably an assay plate,
for
conducting one or more assays, the module having a plurality of wells (and/or
chambers),
wherein two or more of the plurality of wells (and/or chambers) comprise at
least one first
electrode surface and, preferably at least one counter electrode surface.
According to a preferred
. embodiment, two or more of the plurality of wells (and/or chambers) comprise
a working
electrode surface and, preferably a counter electrode surface, adapted to
induce luminescence in
37'
CA 02714094 2010-08-30
. =
WO 03/023360
PCT/US02/28652
the wells. The invention also relates to a multi-well module, preferably a
plate, for conducting
one or more assays, the module having a plurality of wells, wherein one or
more of the plurality
of wells comprise a working electrode surface and a counter electrode surface
adapted to induce
luminescence in the wells. Preferably, all or substantially all of the wells
comprise an electrode
surface.
Another embodiment relates to a multi-well assay module, preferably an assay
plate, for
conducting electrode induced luminescence (preferably
electrochemiluminescence) assays, the
module, preferably a plate, having a plurality of wells, wherein each of the
plurality of wells
comprises at least one first electrode surface (e.g., a working electrode)
and, preferably, at least
one second electrode surface (e.g., a counter electrode).
Another embodiment relates to an assay plate for conducting one or more
electrode
induced luminescence (preferably electrochemiluminescence) assays, the plate
having a plurality
of wells or assay regions comprising electrode surfaces, wherein the electrode
surfaces consist
essentially of at least one working electrode surface and at least one counter
electrode surface.
Preferably, the assay regions or assay wells are free of reference electrodes
allowing for a
greater density of assay domains and simplified instrumentation for inducing
and measuring
luminescence.
The working electrode surface area may be smaller, the same or larger than the
counter
electrode surface area. In some embodiments, the working electrode surface is
preferably much
larger than the counter electrode surface. This configuration allows for a
greater working
electrode surface on which to immobilize assay reagents. Preferably, the
surface ratio of the
working electrode surface to the counter electrode surface is at least 2 to 1,
more preferably at
CA 02714094 2010-08-30
. = ,
-WO 03/023360
PCT/US02/28-652
least 5 to 1, even more preferably at least 10 to 1, still more preferred at
least 50 to 1, even more
preferably at least 100 to 1 and most preferred at least 500 to 1.
Surprisingly, the assay modules
of the invention provide for the performance of electrochemiluminescence
assays with very little
counter electrode surface. Preferably, the working electrode is substantially
centered within the
well so as to maximize the percentage of ECL emitted from the electrode that
can be captured by
a light detector placed above the well.
According to another embodiment, the first electrode surface (e.g., working
electrode
surface) is centered at the bottom of each well and the second electrode
surface (e.g., counter
electrode surface) is adjacent the periphery of the bottom of each well. In
some embodiments,
the working electrode surface is centered at the bottom of each well and is
completely
surrounded by the counter electrode surface.
Alternatively, for some applications it is desirable that working electrode
surfaces be
small, e.g., relative to the surface area of a well or well bottom. In some
applications, this
configuration may reduce non-specific signals. According to one embodiment of
the invention,
the multi-well assay module has a plurality of wells, each well having a well
bottom comprising
a first electrode surface, a second electrode surface and a dielectric surface
(preferably the
dielectric surface is the surface of the bottom of the well between the first
electrode surface and
the second electrode surface), wherein the ratio of the first electrode
surface and the dielectric
surface (or alternatively the surface of the well bottom) is less than 1 to 5,
preferably 1 to 10,
more preferably 1 to 30.
According to one preferred embodiment of the invention, the assay module
comprises a
first electrode surface (preferably a working electrode surface) that is
bounded by a dielectric
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
surface, the dielectric surface being raised or lowered (preferably, raised)
and/or of different
hydrophobicity (preferably, more hydrophobic) than the electrode surface.
Preferably, the
dielectric boundary is higher, relative to the electrode surface, by 0.5 -100
micrometers, or more
preferably by 2-30 micrometers, or most preferably by 8-12 micrometers. Even
more
preferably, the dielectric boundary has a sharply defmed edge (i.e., providing
a steep boundary
wall and/or a sharp angle at the interface between the electrode and the
dielectric boundary).
Preferably, the first electrode surface has a contact angle for water 10
degrees less than the
dielectric surface, preferably 15 degrees less, more preferably 20 degrees
less, more preferably
30 degrees less, even more preferably 40 degrees less, and most preferred 50
degrees less. One
advantage of having a dielectric surface that is raised and/or more
hydrophobic than the electrode
surface is in the reagent deposition process where the dielectric boundary may
be used to confine
a reagent within the boundary of the electrode surface. In particular, having
a sharply defined
edge with a steep boundary wall and/or a sharp angle at the interface between
the electrode and
dielectric boundary is especially useful for "pinning" drops of solution and
confining them to the
electrode surface.
According to another embodiment, an assay module comprises one or more
(preferably
two or more) wells, the wells having one or more first electrode surfaces
(preferably one or more
working electrode surfaces) and a plurality of assay domains immobilized
therein. Preferably, at
least two of the plurality of the assay domains comprises different binding
reagents. Preferably,
each well comprises at least four, more preferably at least seven, even more
preferably at least
ten assay domains and most preferred at least 15 assay domains. One preferred
embodiment is a
24 well plate wherein each well comprises at least 16, preferably at least 25,
more preferably at
40'
=
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
least 64, even more preferably at least 100 assay domains per well and most
preferably at least
250 assay domains per well.
Another embodiment of the invention relates to a multi-well module (preferably
a multi-
well plate) having a plurality of wells, wherein the wells comprise a
plurality of working
electrode surfaces having assay domains immobilized thereon. Preferably, the
assay domains are
independently addressable. For example, a well may comprise a plurality of
assay domains,
wherein each assay domain comprises an electrode which is independently
addressable from the
other assay domains within the well. In another example, a group of wells may
each comprise a
plurality of assay domains, wherein each assay domain comprises an electrode
which is
independently addressable from the other assay domains within the well, but
which is jointly
addressable with an assay domain in each of the other wells.
Alternatively, for some applications it is desirable that working electrode
surfaces be
small, e.g., relative to the surface area of a well or well bottom. In some
applications, this
configuration may reduce non-specific signals. According to one embodiment of
the invention,
the multi-well assay module has a plurality of wells, each well having a well
bottom comprising
a first electrode surface, a second electrode surface and a dielectric surface
(preferably the
dielectric surface is the surface of the bottom of the well between the first
electrode surface and
the second electrode surface), wherein the ratio of the first electrode
surface and the dielectric
surface (or alternatively the surface of the well bottom) is less than 1 to 5,
preferably 1 to 10,
more preferably 1 to 30.
According to one preferred embodiment of the invention, the assay module
comprises a
first electrode surface (preferably a working electrode surface) that is
bounded by a dielectric
CA 02714094 2010-08-30
,
WO 03/023360
PCT/US02/28652
surface, the dielectric surface being raised or lowered (preferably, raised)
and/or of different
hydrophobicity (preferably, more hydrophobic) than the electrode surface.
Preferably, the
dielectric boundary is higher, relative to the electrode surface, by 0.5 -.100
micrometers, or more
preferably by 2-30 micrometers, or most preferably by 8-12 micrometers. Even
more
preferably, the dielectric boundary has a sharply defined edge (i.e.,
providing a steep boundary
wall and/or a sharp angle at the interface between the electrode and the
dielectric boundary).
Preferably, the first electrode surface has a contact angle for water 10
degrees less than the
dielectric surface, preferably 15 degrees less, more preferably 20 degrees
less, more preferably
30 degrees less, even more preferably 40 degrees less, and most preferred 50
degrees less. One
advantage of having a dielectric surface that is raised and/or more
hydrophobic than the electrode
surface is in the reagent deposition process where the dielectric boundary may
be used to confine
a reagent within the boundary of the electrode surface. In particular, having
a sharply defined
edge with a steep boundary wall and/or a sharp angle at the interface between
the electrode and
dielectric boundary is especially useful for "pinning" drops of solution and
confining them to the
electrode surface.
According to another embodiment, an assay module comprises one or more
(preferably
two or more) wells, the wells having one or more first electrode surfaces
(preferably one or more
working electrode surfaces) and a plurality of assay domains immobilized
therein. Preferably, at
least two of the plurality of the assay domains comprises different binding
reagents. Preferably,
each well comprises at least four, more preferably at least seven, even more
preferably at least
ten assay domains and most preferred at least 15 assay domains. One preferred
embodiment is a
24 well plate wherein each well comprises at least 16, preferably at least 25,
more preferably at
42=
CA 02714094 2010-08-30
sVO 03/023360
PCT/US02/28652
least 64, even more preferably at least 100 assay domains per well and most
preferably at least
250 assay domains per well.
Another embodiment of the invention relates to a multi-well module (preferably
a multi-
well plate) having a plurality of wells, wherein the wells comprise a
plurality of working
electrode surfaces having assay domains immobilized thereon. Preferably, the
assay domains are
independently addressable. For example, a well may comprise a plurality of
assay domains,
wherein each assay domain comprises an electrode which is independently
addressable from the
other assay domains within the well. In another example, a group of wells may
each comprise a
plurality of assay domains, wherein each assay domain comprises an electrode
which is
independently addressable from the other assay domains within the well, but
which is jointly
addressable with an assay domain in each of the other wells.
The invention also relates to methods and apparatus for the measurement of
signals from assay modules and MDMW plates of the invention. The preferred
apparatus of the
invention can be used to induce and measure luminescence in assays conducted
in assay
modules, preferably in multi-well assay plates. It may incorporate, for
example, one or more
photodetectors; a light tight enclosure; electrical connectors for contacting
the assay modules;
mechanisms to transport multi-well assay modules into and out of the apparatus
(and in
particular, into and out of light tight enclosures); mechanisms to align and
orient multi-well
assay modules with the photodetector(s) and with electrical contacts;
mechanisms to track and
identify modules (e.g. bar code readers); mechanisms to make electrical
connections to modules,
one or more sources of electrical energy for inducing luminescence in the
modules; and
appropriate electronics and software.
43 -
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
The apparatus may also include mechanisms to store, stack, move and/or
distribute one or
more assay modules (e.g. multi-well plate stackers). The apparatus may
advantageously use
arrays of photodetectors (e.g. arrays of photodiodes) or imaging
photodetectors (e.g. CCD
cameras) to measure light. These detectors allow the apparatus to measure the
light from
multiple wells, assay domains, and/or assay cells simultaneously and/or to
image the intensity
and spatial distribution of light emitted from an individual well, assay cell
and/or assay domain.
The apparatus can preferably measure light from one or more sectors of an
assay module,
preferably a multi-well assay plate. In some embodiments, a sector comprises a
group of wells,
assay domains and/or assay cells numbering between one and a number fewer than
the total
number of wells (and/or chambers) in the assay module (e.g. a row, column, or
two-dimensional
sub-array of wells in a multi-well plate). In preferred embodiments, a sector
comprises between
4 percent and 50 percent of the wells of a multi-well plate. In especially
preferred embodiments,
multi-well assay plates are divided into columnar sectors (each sector having
one row or column
of wells) or square sectors (e.g., a standard sized multi-well plate can be
divided into six square
sectors of equal size). In some embodiments, a sector may comprise one or more
wells with
more than one fluid containment region within the wells. The apparatus,
preferably, is adapted
to sequentially induce ECL in and/or sequentially measure ECL from the sectors
in a given
module, preferably plate.
One aspect of the invention relates to the immobilization of materials in
assay domains
on electrodes having improved electrode compositions and surfaces and assay
modules
comprising these electrode compositions and surfaces. Electrodes in the
present invention are
preferably comprised of a conductive material. The electrode may comprise a
metal such as
4-t
CA 02714094 2010-08-30
= = . =
WO 03/023360
PCT/US02/28652
gold, silver, platinum, nickel, steel, iridium, copper, aluminum, a conductive
alloy, or the like.
They may also comprise oxide coated metals (e.g. aluminum oxide coated
aluminum).
Electrodes may comprise non-metallic conductors such as conductive forms of
molecular carbon.
Electrodes may also be comprised of semiconducting materials (e.g. silicon,
germanium) or
semi-conducting films such as indium tin oxide (ITO), antimony tin oxide (ATO)
and the like.
Electrodes may also be comprised of mixtures of materials containing
conducting composites,
inks, pastes, polymer blends, metal/non-metal composites and the like. Such
mixtures may
include conductive or semi-conductive materials mixed with non-conductive
materials.
Preferably, electrode materials are substantially free of silicone-based
materials.
Electrodes (in particular working electrodes) used in assay modules of the
invention are
advantageously able to induce luminescence from luminescent species.
Preferable materials for
working electrodes are materials able to induce electrochemiluminescence from
Ruthenium-iris-
bipyridine in the presence of tertiary alkyl amines (such as tripropyl amine).
Examples of such
preferred materials include platinum, gold, ITO, carbon, carbon-polymer
composites, and
conductive polymers.
Preferably, electrodes are comprised of carbon-based materials such as carbon,
carbon
black, graphitic carbon, carbon nanotubes, carbon fibrils, graphite, carbon
fibers and mixtures
thereof. Advantageously, they may be comprised of conducting carbon-polymer
composites,
conducting particles dispersed in a matrix (e.g. carbon inks, carbon pastes,
metal inks), and/or
conducting polymers. One preferred embodiment of the invention is an assay
module, preferably
a multi-well plate, having electrodes (e.g., working and/or counter
electrodes) that comprise
carbon, preferably carbon layers, more preferably screen-printed layers of
carbon inks. Some
CA 02714094 2010-08-30
. . =
WO 03/023360
PCT/US02/28652
useful carbon inks include materials produced by Acheson Colloids Co. (e.g.,
Acheson 440B,
423ss, PF407A, PF407C, PM-003A, 30D071, 435A, Electrodag 505SS, and
AquadagTm), E. I.
Du Pont de Nemours and Co. (e.g., Dupont 7105, 7101, 7102, 7103, 7144, 7082,
7861D, and
CB050), Conductive Compounds Inc (e.g., C-100), and Ercon Inc. (e.g., G-451).
In another preferred embodiment, the electrodes of the invention comprise
carbon fibrils.
The terms "carbon fibrils", "carbon nanotubes", single wall nanotubes (SWN1),
multiwall
nanotubes (MWNT), "graphitic nanotubes", "graphitic fibrils", "carbon
tubules", "fibrils" and
"buckeytubes", all of which terms may be used to describe a broad class of
carbon materials (see
Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C.; "Science of Fullerenes and
Carbon
Nanotubes", Academic Press, San Diego, CA., 1996, and references cited
therein). The terms
"fibrils" and "carbon fibrils" are used throughout this application to include
this broad class of
carbon-based materials. Individual carbon fibrils as disclosed in U.S. Patent
Nos. 4,663,230;
5,165,909; and 5,171,560 are particularly advantageous. They may have
diameters that range
from about 3.5 nm to 70 nm, and length greater than 102 times the diameter, an
outer region of
multiple, essentially continuous, layers of ordered carbon atoms and a
distinct inner core region.
Simply for illustrative purposes, a typical diameter for a carbon fibril may
be approximately
between about 7 and 25 nm, and a typical range of lengths may be 1000 nm to
10,000 rim.
Carbon fibrils may also have a single layer of carbon atoms and diameters in
the range of 1 nm ¨
2 run. Electrodes of the invention may comprise one or more carbon fibrils,
e.g., in the form of a
fibril mat, a fibril aggregate, a fibril ink, a fibril composite (e.g., a
conductive composite
comprising fibrils dispersed in an oil, paste, ceramic, polymer, etc.). One
preferred embodiment
of the invention relates to a multi-well plate comprising a substrate
comprising a carbon
46-
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/286S2
nanotube-containing composite (preferably, carbon nanotubes dispersed in a
polymeric matrix),
wherein the surface of the substrate is etched to expose the carbon nanotubes,
thereby forming
one or more working electrodes.
Electrodes may be formed into patterns by a molding process (i.e., during
fabrication of
the electrodes), by patterned deposition, by patterned printing, by selective
etching, through a
cutting process such as die cutting or laser drilling, and/or by techniques
known in the art of
electronics microfabrication. Electrodes may be self supporting or may be
supported on another
material, e.g. on films, plastic sheets, adhesive films, paper, backings,
meshes, felts, fibrous
materials, gels, solids (e.g. metals, ceramics, glasses), elastomers, liquids,
tapes, adhesives, other
electrodes, dielectric materials and the like. The support may be rigid or
flexible, flat or
deformed, transparent, translucent, opaque or reflective. Preferably, the
support comprises a flat
sheet of plastic such as acetate or polystyrene. Electrode materials may be
applied to a support
by a variety of coating and deposition processes known in the art such as
painting, spray-coating,
screen-printing, ink-jet printing, laser printing, spin-coating, evaporative
coating, chemical vapor
deposition, etc. Supported electrodes may be patterned using photolithographic
techniques (e.g.,
established techniques in the microfabrication of electronics), by selective
etching, and/or by
selective deposition (e.g., by evaporative or CVD processes carried out
through a mask). In a
preferred embodiment, electrodes are comprised of extruded films of conducting
carbon/polymer
composites. In another preferred embodiment, electrodes are comprised of a
screen printed
conducting ink deposited on a substrate. Electrodes may be supported by
another conducting
material. Advantageously, screen printed carbon ink electrodes are printed
over a conducting
metal ink (e.g., silver ink) layer so as to improve the conductivity of the
electrodes.
CA 02714094 2010-08-30
, .
WO 03/023360
PCT/US02/28652
According to one preferred embodiment of the invention, the electrode surface
(preferably a working electrode surface of an assay module or assay plate) is
bounded by a
dielectric surface, the dielectric surface being raised or lowered
(preferably, raised) and/or of
different hydrophobicity (preferably, more hydrophobic) than the electrode
surface. Preferably,
the dielectric boundary is higher, relative to the electrode surface, by 0.5 -
100 micrometers, or
more preferably by 2-30 micrometers, or most preferably by 8-12 micrometers.
Even more
preferably, the dielectric boundary has a sharply defined edge (i.e.,
providing a steep boundary
wall and/or a sharp angle at the interface between the electrode and the
dielectric boundary).
Preferably, the first electrode surface has a contact angle for water 10
degrees less than
the dielectric surface, preferably 15 degrees less, more preferably 20 degrees
less, more
preferably 30 degrees less, even more preferably 40 degrees less, and most
preferred 50 degrees
less. One advantage of having a dielectric surface that is raised and/or more
hydrophobic than
the electrode surface is in the reagent deposition process where the
dielectric boundary may be
used to confine a reagent within the boundary of the electrode surface. In
particular, having a
sharply defined edge with a steep boundary wall and/or a sharp angle at the
interface between the
electrode and dielectric boundary is especially useful for "pinning" drops of
solution and
confining them to the electrode surface. In an especially preferred embodiment
of the invention,
the dielectric boundary is formed by printing a patterned dielectric ink on
and/or around the
electrode, the pattern designed so as to expose one or more assay domains on
the electrode.
Electrodes may be modified by chemical or mechanical treatment to improve the
immobilization of reagents. The surface may be treated to introduce functional
groups for
immobilization of reagents or to enhance its adsorptive properties. Surface
treatment may also
CA 02714094 2010-08-30
. .
WO 03/023360 _
PCT/US02/28652
be used to influence properties of the electrode surface, e.g., the spreading
of water on the
surface or the kinetics of electrochemical processes at the surface of the
electrode. Techniques
that may be used include exposure to electromagnetic radiation, ionizing
radiation, plasmas or
chemical reagents such as oxidizing agents, electrophiles, nucleophiles,
reducing agents, strong
acids, strong bases and/or combinations thereof. Treatments that etch one or
more components
of the electrodes may be particularly beneficial by increasing the roughness
and therefore the
surface area of the electrodes. In the case of composite electrodes having
conductive particles or
fibers (e.g., carbon particles or fibrils) in a polymeric matrix or binder,
selective etching of the
polymer may be used to expose the conductive particles or fibers.
One particularly useful embodiment is the modification of the electrode, and
more
broadly a material incorporated into the present invention by treatment with a
plasma,
specifically a low temperature plasma, also termed glow-discharge. The
treatment is carried out
in order to alter the surface characteristics of the electrode, which come in
contact with the
plasma during treatment. Plasma treatment may change, for example, the
physical properties,
chemical composition, or surface-chemical properties of the electrode. These
changes may, for
example, aid in the immobilization of reagents, reduce contaminants, improve
adhesion to other
materials, alter the wettability of the surface, facilitate deposition of
materials, create patterns,
and/or improve uniformity. Examples of useful plasmas include oxygen,
nitrogen, argon,
ammonia, hydrogen, fluorocarbons, water and combinations thereof. Oxygen
plasmas are
especially preferred for exposing carbon particles in carbon-polymer composite
materials.
Oxygen plasmas may also be used to introduce carboxylic acids or other
oxidized carbon
functionality into carbon or organic materials (these may be activated, e.g.,
as active esters or
49¨
CA 02714094 2010-08-30
. . ,
WO 03/023360
PCT/US02/28652
acyl chlorides) so as to allow for the coupling of reagents. Similarly,
ammonia-containing
plasmas may be used to introduce amino groups for use in coupling to assay
reagents.
Treatment of electrode surfaces may be advantageous so as to improve or
facilitate
immobilization, change the wetting properties of the electrode, increase
surface area, increase the
binding capacity for the immobilization of reagents (e.g., lipid, protein or
lipid/protein layers) or
the binding of analytes, and/or alter the kinetics of electrochemical
reactions at the electrode. In
some applications, however, it may be preferable to use untreated electrodes.
For example, we
have found that it is advantageous to etch carbon ink electrodes prior to
immobilization when the
application calls for a large dynamic range and therefore a high binding
capacity per area of
electrode. We have discovered that oxidative etching (e.g., by oxygen plasma)
has additional
advantages in that the potential for oxidation of tripropyl amine (TPA) and
the contact angle for
water are both reduced relative to the unetched ink. The low contact angle for
water allows
reagents to be adsorbed on the electrode by application of the reagents in a
small volume of
aqueous buffer and allowing the small volume to spread evenly over the
electrode surface.
Surprisingly, we have found that excellent assays may also be carried out on
unetched caroon ink
electrodes despite the presence of polymeric binders in the ink. In fact, in
some applications
requiring high sensitivity or low-non specific binding it is preferred to use
unetched carbon ink
electrodes so as to minimize the surface area of exposed carbon and therefore
minimize
background signals and loss of reagents from non-specific binding of reagents
to the exposed
carbon. Depending on the ink used and the process used to apply the ink, the
electrode surface
may not be easily wettable by aqueous solutions. We have found that we can
compensate for the
low wettability of the electrodes during the adsorption of reagents by adding
low concentrations
SO"
CA 02714094 2010-08-30
. =
WO 03/023360
PCT/US02/28652
of non-ionic detergents to the reagent solutions so as to facilitate the
spreading of the solutions
over the electrode surface. Even spreading is especially important during the
localized
immobilization of a reagent from a small volume of solution. For example, we
have found that
the addition of 0.005-0.04 % Triton X-100 allows for the spreading of protein
solutions over
unetched carbon ink surfaces without affecting the adsorption of the protein
to the electrode and
without disrupting the ability of a dielectric film applied on or adjacent to
the electrode
(preferably, a printed dielectric film with a thickness of 0.5 -100
micrometers, or more preferably
2-30 micrometers, or most preferably 8-12 micrometers and having a sharply
defined edge) to
confine fluids to the electrode surface. Preferably, when non-ionic detergents
such as Triton X-
100 are used to facilitate spreading of reagents (e.g., capture reagents) onto
unetched screen-
printed electrodes (i.e., so as to allow the immobilization of the reagents),
the solutions
containing the reagents are allowed to dry onto the electrode surface. It has
been found that this
drying step greatly improves the efficiency and reproducibility of the
immobilization process.
Electrodes can be derivatized with chemical functional groups that can be used
to attach
other materials to them. Materials may be attached covalently to these
functional groups, or they
may be adsorbed non-covalently to derivatized or underivatized electrodes.
Electrodes may be
prepared with chemical functional groups attached covalently to their surface.
These chemical
functional groups include but are not limited to COOH, OH, NH2, activated
carboxyls (e.g., N-
hydroxy succinimide (NHS)- esters), poly-(ethylene glycols), thiols, alkyl
((CH2)n) groups,
and/or combinations thereof). Certain chemical functional groups (e.g., COOH,
OH, NH2, SH,
activated carboxyls) may be used to couple reagents to electrodes. For further
reference to useful
immobilization and bioconjugation techniques see G. Hemianson, A. Mallia and
P. Smith,
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
Immobilized Affinity Ligand Techniques (Academic Press, San Diego, 1992) and
G. Hermanson,
Bioconjugate Techniques (Academic Press, San Diego, 1996).
In preferred embodiments, NHS-ester groups are used to attach other molecules
or
materials bearing a nucleophilic chemical functional group (e.g., an amine).
In a preferred
embodiment, the nucleophilic chemical functional group is present on and/or in
a biomolecule,
either naturally and/or by chemical derivatization. Examples of suitable
biomolecules include,
but are not limited to, amino acids, proteins and functional fragments
thereof, antibodies, binding
fragments of antibodies, enzymes, nucleic acids, and combinations thereof.
This is one of many
such possible techniques and is generally applicable to the examples given
here and many other
analogous materials and/or biomolecules. In a preferred embodiment, reagents
that may be used
for ECL may be attached to the electrode via NHS-ester groups.
It may be desirable to control the extent of non-specific binding of materials
to
electrodes. Simply by way of non-limiting examples, it may be desirable to
reduce or prevent
the non-specific adsorption of proteins, antibodies, fragments of antibodies,
cells, subcellular
particles, viruses, serum and/or one or more of its components, ECL labels
(e.g., Rull(bpy)3 and
Ruill(bpy)3 derivatives), oxalates, trialkylamines, antigens, analytes, and/or
combinations
thereof). In another example, it may be desirable to enhance the binding of
biomolecules.
One or more chemical moieties that reduce or prevent non-specific binding
(also known
as blocking groups) may be present in, on, or in proximity to an electrode.
Such moieties, e.g.,
PEG moieties and/or charged residues (e.g., phosphates, ammonium ions), may be
attached to or
coated on the electrode. Examples of useful blocking reagents include proteins
(e.g., serum
albumins and immunoglobins), nucleic acids, polyethylene oxides, polypropylene
oxides, block
CA 02714094 2010-08-30
= . . =
WO 03/023360
PCT/US02/28652
copolymers of polyethylene oxide and polypropylene oxide, polyethylene imines
and detergents
or surfactants (e.g., classes of non-ionic detergents/surfactants known by the
trade names of Brij,
Triton, Tween, Thesit, Lubrol, Genapol, Pluronic (e.g., F108), Tetronic,
Tergitol, and Span).
Materials used in electrodes may be treated with surfactants to reduce non-
specific
binding. For example, electrodes may be treated with surfactants and/or
detergents that are well
known to one of ordinary skill in the art (for example, the Tween, Triton,
Pluronics (e.g., F108),
Span, and Brij series of detergents). Solutions of PEGs and/or molecules which
behave in
similar fashion to PEG (e.g., oligo- or polysaccharides, other hydrophilic
oligomers or polymers)
("Polyethylene glycol chemistry: Biotechnical and Biomedical Applications",
Harris, J.M.
Editor, 1992, Plenum Press) may be used instead of and/or in conjunction with
surfactants and/or
detergents. Undesirable non-specific adsorption of certain entities such as
those listed above
may be blocked by competitive non-specific adsorption of a blocking agent,
e.g., by a protein
such as bovine serum albumin (BSA) or immunoglobulin G (IgG). One may adsorb
or
covalently attach an assay reagent on an electrode and subsequently treat the
electrode with a
blocking agent so as to block remaining unoccupied sites on the surface.
In preferred embodiments, it may be desirable to immobilize (by either
covalent or non-
covalent means) biomolecules or other media to carbon-containing materials,
e.g., carbon black,
fibrils, and/or carbon dispersed in another material. One may attach
antibodies, fragments of
antibodies, proteins, enzymes, enzyme substrates, inhibitors, cofactors,
antigens, haptens,
lipoproteins, liposaccharides, cells, sub-cellular components, cell receptors,
viruses, nucleic
acids, antigens, lipids, glycoproteins, carbohydrates, peptides, amino acids,
hormones, protein-
binding ligands, pharmacological agents, and/or combinations thereof. It may
also be desirable
-
CA 02714094 2010-08-30
. =
. .
69331-63D
to attach non-biological entities such as, but not limited to polymers,
elastomers, gels, coatings,
ECL tags, redox active species (e.g., tripropylarnine, oxalates), inorganic
materials, chelating
agents, linkers, etc. A plurality of species may be co-adsorbed to form a
mixed layer on the
surface of an electrode. Most preferably, biological materials (e.g.,
proteins) are immobilized on
carbon-containing electrodes by passive adsorption. Surprisingly, biological
membranes (e.g.,
cells, cell membranes, membrane fragments, membrane vesicles, lipsomes,
organelles, viruses,
bacteria, etc.) may be directly adsorbed on carbon without destroying the
activity of membrane
components or their accessibility to binding reagents (see, e.g., copending
U.S. Application No.
10/208,526 (entitled "Assay Electrodes Having Immobilized Lipid/Protein
Layers, Methods Of
Making The Same And Methods Of Using The Same For Luminescence Test
Measurements-),
tiled on July 29, 2002.
Electrodes used in the multi-well assay plates of the invention are typically
non-porous,
however, in some applications it is advantageous to use porous electrodes
(e.g., mats of carbon
fibers or fibrils, sintered metals, and metals films deposited on filtration
membranes, papers or
other porous substrates. These applications include those that employ
filtration of solutions
through the electrode so as to: i) increase mass transport to the electrode
surface (e.g., to increase
the kinetics of binding of molecules in solution to molecules on the electrode
surface); ii) capture
particles on the electrode surface; and/or iii) remove liquid from the well.
The assay modules of the present invention may use dielectric inks, films or
other
electrically insulating materials (hereinafter referred to as dielectrics).
Dielectrics in the present
invention may be used to prevent electrical connectivity between electrodes,
to define patterned
regions, to adhere materials together (i.e., as adhesives), to support
materials, to define assay
54
CA 02714094 2010-08-30
. -
WO 03/023360
PCT/US02/2i3652
domains, as masks, as indicia and/or to contain assay reagents and other
fluids. Dielectrics are
non-conducting and advantageously non-porous (i.e., do not permit transmission
of materials)
and resistant to dissolving or degrading in the presence of media encountered
in an electrode
induced luminescence measurement. The dielectrics in the present invention may
be liquids,
gels, solids or materials dispersed in a matrix. They may be deposited in
uncured form and cured
to become solid. They may be inks, solid films, tapes or sheets. Materials
used for dielectrics
include polymers, photoresists, plastics, adhesives, gels, glasses, non-
conducting inks, non-
conducting pastes, ceramics, papers, elastomers, silicones, thermoplastics.
Preferably, dielectric
materials of the invention are substantially free of silicones. Examples of
non-conducting inks
include UV curable dielectrics such as materials produced by Acheson Colloids
Co. (e.g.,
Acheson 451SS, 452SS, PF-021, ML25251, ML25240, 1vf125265, and Electrodag
38DJB16
clear) and E. I. du Pont de Nemours and Co. (e.g., Dupont: 5018, 3571, and
5017).
Dielectrics of the present invention may be applied by a variety of means, for
example,
printing, spraying, laminating, or may be affixed with adhesives, glues,
solvents or by use of
mechanical fasteners. Patterns and/or holes in dielectric layers may be formed
by molding
processes (i.e., during fabrication of the layer), by selective etching and/or
by a cutting process
such as die cutting or laser drilling. Dielectrics may be deposited and/or
etched in patterns
through the use of established photolithographic techniques (e.g., techniques
used in the
semiconductor electronics industry) and/or by patterned deposition using an
evaporative or CVD
process (e.g., by deposition through a mask). In a preferred embodiment, a
dielectric ink is
deposited on a substrate by printing (e.g., ink jet printing, laser printing
or, more preferably,
screen printing) and, optionally, UV cured. Preferably, the screen printed
dielectric is LTV
55 -
CA 02714094 2010-08-30
. . =
Vila 03/023360
PCT/US02/28652
curable allowing for improved edge definition than solvent based dielectrics.
In another
preferred embodiment, a non-conducting polymeric film is affixed to a support
using an
adhesive.
When using a dielectric ink printed on or adjacent an electrode to confivae
fluids to
regions of the electrode surface, the dielectric film preferably has a
thickness of 0.5 -100
micrometers, or more preferably 2-30 micrometers, or most preferably 8-12
micrometers and
also, preferably, has a sharply defined edge with steep walls.
The invention includes plate tops and assembled plates comprising a plate top
and,
preferably, a plate bottom defining well bottoms having one or more electrode
surfaces, most
preferably having one or more working electrode surfaces and, optionally, one
or more counter
electrode surfaces. Preferably, the plate top is a structure with holes,
wherein the structure may
be combined with a plate bottom to form a multi-well plate, the walls of the
wells of the plate
being at least partially defined by the inside surfaces of the holes through
the plate top. The
holes through the plate top may be a variety of shapes (e.g., round, oval,
square, rectangular,
triangular, star shaped, etc.). The holes may be of various sizes. They can
also have irregular
dimensions within a hole (e.g., the hole may become more narrow or more wide
at different
depths). For example, the hole may be shaped like a cone, becoming more narrow
at the bottom
so as to optimize the collection of light emitted from the well bottom. The
plate top may also
have structures or indicia thereon that aid in identifying the plate top,
distinguishing the plate top
from other configurations of plate top, or in aligning and handling the plate
top.
Advantageously, the dimensions and structure of the plate top are preferably
in accordance with,
or at least compatible with, industry standards for the footprints and shapes
of assay plates.
CA 02714094 2010-08-30
. . =
WO 03/023360
PCT/US02/28652
The plate top may be made from conducting or non-conducting materials.
Preferably, the
majority of the plate top is a unitary molded structure made from rigid
thermoplastic material
such as polystyrene, polyethylene or polypropylene. Optimally, this unitary
structure is formed
of (or, alternatively, coated with) inexpensive material that is generally
impervious to reactants,
can withstand modest levels of heat and light and is, preferably, resistant to
the adsorption of
biomolecules. Preferably, the plate top is substantially free of silicones.
Plate tops may be clear
or translucent. Different colored materials may be used to improve the results
of certain ECL
measurement processes.
It is preferable that the plate top comprise a material that does not transmit
light so as to
prevent cross-talk between wells. A highly reflective metallic coating or
constituent material
may provide an especially reflective interior surface for each of wells so as
to increase the
efficiency with which light can be transmitted to photodetectors. An opaque
white plastic
material such as a plastic filled with light scattering particles (e.g., lead
oxide, alumina, silica or,
preferably, titanium dioxide particles) may provide an interior surface for
the wells that is highly
light scattering thereby improving light gathering efficiency. In one
embodiment, preferred plate
tops comprise plastics (e.g., well walls) comprising such light scattering
particles at a
concentration of from 4-20 wt%, preferably 6-20%, more preferably 6-15%, even
more
preferably 6-12%, and most preferred approximately 9%. In an alternate
preferred embodiment,
the plate top comprises an opaque, preferably non-reflective, black material
to prevent the
reflection or scattering of ECL-generated light from different locations
within a well and to
prevent reflective interference during ECL test measurements. In general, when
imaging light
emitted from a well (e.g., when using a camera to produce an image of light
emitted from the
374
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
well) it is advantageous that the interior surface of the well (e.g., as
defined by a plate top)
comprise an absorptive (e.g., black) preferably non-scattering material since
the detection of
scattered light will reduce the fidelity of the image. In general, when
detecting light in a non-
imaging mode (e.g., when a single light detector is used to detect all the
light emitted from a
well) it is advantageous that the interior surface of the well comprise a
reflective or highly
scattering material so as to prevent the loss of light due to adsorption of
light at the well walls
and to maximize the collection of light at the detector.
The invention also includes assay module tops and assembled assay modules
comprising
an assay module top and a plate bottom or assay module substrate. The assay
module top may
be a plate top (as described above). The assay module top may have, e.g.,
holes, channels,
and/or wells that when mated to a plate bottom or assay module substrate
define wells and/or
chambers, such wells and/or chambers preferably comprising one or more
electrodes (and/or
assay domains) provided by the plate bottom or assay module substrate. The
assay module top
may have additional channels, tubes or other microfluidics so as to allow the
flow of samples
into, out of and/or between wells, flow cells and chambers of an assay module.
Figures 10A and 10B show a layered view and a stylized cross-sectional view,
respectively, of an embodiment of the multi-well assay plate of the invention.
Multi-well assay
plate 1000 is a laminar structure comprising, in sequence, a plate top 1020,
an adhesive layer
1030, a dielectric layer 1040, a conductive layer 1050, a substrate layer 1060
and a contact layer
1070. Holes 1022 and 1032 through plate top 1020 and adhesive layer 1030,
respectively, are
aligned so as to form a plurality of wells 1002 having well bottoms defined by
dielectric layer
1040, conductive layer 1050 and/or substrate layer 1060 and well walls defined
by the interior
CA 02714094 2010-08-30
. . = =
WO 03/023360
PCT/US02/286S2
surfaces of holes 1022 and 1032. Through-holes 1062 and 1064 through substrate
layer 1060
provide an electrical path between elements of conductive layer 1050 and
elements of contact
layer 1070. Details A-D show the pattern of layers 1070, 1060, 1050 and 1040
within a given
sector of plate 1000. Element 1080 shows layers 1070, 1060, 1050 and 1040
aligned and
stacked, in order from top to bottom-- 1040 (top), 1050, 1060, and 1070
(bottom) -- so as to
form a plate bottom with integrated electrodes.
Plate top 1020 is a plate top as described above. Adhesive layer 1030 is an
adhesive
suitable for forming a fluid-tight seal between plate top 1020 and dielectric
layer 1040,
conductive layer 1050 and/or substrate layer 1060. Adhesive layer 1030 may be
an adhesive
coating applied, e.g., by spray coating, onto plate top 1020. In a preferred
embodiment, adhesive
layer 1030 is a double sided adhesive tape (i.e., a plastic film coated on
both sides with
adhesive). Holes 1032 are preferably formed by a cutting process such as laser
drilling or die
cutting. Optionally, adhesive 1030 may be omitted (e.g., when the adjoining
layers have
adhesive properties or when sealing is accomplished without the use of
adhesives, e.g., by
clamping, heat sealing, sonic welding, solvent welding, etc.). Alternatively,
both plate top 1020
and adhesive layer 1030 may be omitted.
Conductive layer 1050 comprises materials suitable for use as working
electrodes and/or
counter electrodes in an ECL assay and is supported on substrate 1060, a non-
conductive
substrate such as a plastic sheet or film. Preferably, conductive layer 1054
is a conductive
coating such as a carbon ink and may be formed by a printing process such as
screen printing.
Conductive layer 1050 is sectioned, e.g., by screen printing in a defined
pattern, into 6
electrically isolated working electrode sections 1052 and 6 electrically
isolated counter electrode
CA 02714094 2010-08-30
. . .
WO 03/02336()
PCT/US(12/28652
sections 1054 so as to divide plate 1000 into 6 independently addressable
square sectors. As
shown in the figure, the sectioning is designed so that fluid in a given well
will be in contact with
at least one working electrode section and at least one counter electrode
section. The working
electrode sections may have a different composition than the counter electrode
sections so as to
optimize the performance of the electrodes or they may comprise the same
materials so as to
minimize the complexity of manufacturing, e.g., to reduce the number of
printing steps.
Preferably, they both comprise a carbon ink overlayer over a silver ink
underlayer; the carbon
ink providing the active electrode surface and the silver ink providing
sufficient conductivity so
that, during use of the plate in an assay, electrical potential is evenly
distributed throughout a
particular section. When forming such layers, e.g., by a two step printing
process, it is beneficial
that the overlayer be of slightly larger dimensions than the underlayer and
that it be of suitable
thickness to ensure that a sample in wells 1002 is not exposed to the
underlayer material. It may
be beneficial to print or deposit the overlayer in multiple layers so as to
ensure that the
underlayer is completely covered so that the underlayer does not interfere
with subsequent
processing steps or with ECL measurements (e.g., a preferred electrode
material comprises three
layers of carbon ink over a layer of silver ink, the layers most preferably
being deposited by
screen printing). Dielectric layer 1040 is an electrically insulating film,
preferably formed from
a dielectric ink by a printing process such as screen printing. Dielectric
layer 1040 is patterned
so as to define the surfaces of conductive layer 1050 that contact fluids in
wells 1002 (i.e., the
surfaces that are not covered). Holes 1042 in dielectric layer 1040 define
fluid containment
regions on the working electrode sections 1052 of conductive layer 1050. In
such fluid
containment regions, the dielectric layer acts as a barrier that can be used
to confine small
CA 02714094 2010-08-30
. . ,
WO 03/023360
PCT/US02/28652
volumes of fluids over the working electrode, e.g., to aid in depositing assay
reagents onto
selected assay domains within a well. Holes 1042 in dielectric layer 1040
define one fluid
containment regions and/or assay domains on the working electrode surface
within each well of
plate 1000. Optionally, dielectric layer 1040 may be omitted (in such a case,
reagents may still
be deposited into defined assay domains by controlled deposition, e.g., using
microdispensing or
pin transfer techniques).
Contact layer 1070 is a conductive layer that allows for electrical connection
of the multi-
well assay plate to an external source of electrical energy. The contact layer
is sectioned in a
series of working electrode contacts 1072 and counter electrode contacts 1074
to allow
independent connection to specific sections of electrodes 1052 and 1054. The
contact layers are,
preferably, formed by printing, most preferably screen printing, a silver ink
under layer (to
provide high conductivity) followed by a carbon ink overlayer (to prevent
corrosion of the silver
ink and prevent any deleterious effects by the exposed silver on a subsequent
plasma processing
step). Holes 1062 and 1064 in substrate 1060 are, preferably, made by a
cutting process such as
die cutting or laser drilling. Holes 1062 are filled with a conductive
material to provide an
electrical connection between working electrode contacts 1072 and working
electrode sections
1052. Holes 1064 are filled with conductive material to provide an electrical
connection
between counter electrode contacts 1074 and counter electrode sections 1054.
Holes 1062 and
1064 are preferably filled with conductive material during the formation of
conductive layer
1050 or contact layer 1070, e.g., during the printing of a conductive ink on a
substrate, excess ink
is forced into holes in the substrate so as to fill the holes with the
conductive ink.
61 -
CA 02714094 2010-08-30
. .
\NO 03/023360
PCT/US02/28652
In operation, test samples are introduced into wells of plate 1000. A source
of electrical
energy is connected across one or more working electrode sections 1052 and one
or more
counter electrode sections 1054 (via one or more of working electrode contacts
1072 and one or
more of counter electrode contacts 1074, respectively). Application of
electrical energy across
these connections leads to the application of an electrochemical potential
across the test samples
via the exposed surfaces of electrode sections 1052 and 1054 (the application
of electrochemical
potential being confined to wells in sectors contacting working electrode and
counter electrode
sections that are in electrical connection to the source of electrical
energy).
The structure shown in Figures 10A and 10B is readily modified so as to be
applicable to
plates having different numbers of wells, different arrangements of wells
and/or different
arrangements of independently addressable sectors. Preferred embodiments
include 96-well
plates having 4, 7, or 10 assay domains per well and 24-well plates having 25,
64 or 100 wells
per plate. Figure 10C shows dielectric layer 1140, a modification of
dielectric layer 1040
designed to expose 4 "fluid containment regions" 1141 on the working electrode
surface of each
well (the figure is only shown for one sector of the plate). Figure 10D shows
a stylized cross-
sectional view of 3 wells of plate 1100 which is identical to plate 1000
except for the
replacement of dielectric layer 1040 with dielectric layer 1140.
Figure 11 shows, multi-well assay plate 1500, an embodiment of the invention
that is
particularly well suited for genomic or proteomic analysis. The size of the
wells is chosen so as
to optimize the efficiency of the imaging of luminescence generated from the
wells by the
imaging instrument (as described below). Multi-well assay plate 1500 is a
laminar structure
comprising, in sequence, plate top 1520, adhesive layer 1530, conductive tape
layer 1514B,
62
CA 02714094 2010-08-30
. = =
WO 03/023360
PCT/US02/28652
dielectric layer 1540, conductive layer 1552, substrate 1560, contact layer
1572 and conductive
tape layer 1514A. Element 1580 shows layers 1572, 1560, 1552 and 1540 aligned
and stacked,
in order from top to bottom, 1540 (top), 1552, 1560, 1572 (bottom). Conductive
tape layers
1514A and 1514B are provided by folding conductive tape 1510 around element
1580 at fold
1516. Holes 1522, 1532 and 1518 are aligned so as to form a plurality of wells
having well
bottoms defined by element 1580. Through-holes 1562 through substrate 1560
provide an
electrical path between conductive layer 1552 and contact layer 1572. Through
holes 1512
through conductive tape layer 1514A provide access to contact layer 1572 (and,
therefore a way
to contact conductive layer 1552). Plate top 1520 is analogous to plate top
1020 from Figure 10
except for the specific arrangement of wells. Adhesive layer 1530 is an
adhesive analogous to
adhesive layer 1030 in Figure 10 and may be omitted. Conductive tape 1510 is a
laminate
structure comprising a conductive film on an insulating and adhesive substrate
(preferably, a
plastic film coated on one side with an evaporated layer of aluminum and on
the other side with
an adhesive). Substrate 1560, conductive layer 1552, dielectric layer 1540 and
contact layer
1572 are similar in composition and preparation to substrate 1060, conductive
layer 1050,
dielectric layer 1040 and contact layer 1072 as described for Figure 10.
Conductive layer 1552
is sectioned into 6 square sections so as to divide plate 1500 into 6
independently addressable
sectors (each having one well). Holes 1542 through dielectric layer 1540,
define a large number
(preferably 10-50,000, more preferably 100-10,000; 256 are shown in the
figure) of fluid
containment regions in each well. Binding reagents such as specific nucleic
acid sequences or
specific proteins can be selectively introduced and or immobilized into
specific fluid
containment regions by selectively microdispensing the binding reagents into
the specific fluid
63¨
CA 02714094 2010-08-30
, . =
6 9 3 3 1 - 63D
containment regions.
While the figures illustrating embodiments of the plates of the invention have
shown
specific patterns for number, shape and distribution of wells, sectors and
fluid containment
regions/assay domains, it should be clear that the designs are adaptable so as
to allow for a wide
variation in these parameters.
= The assay domains and immobilized layers of the invention are useful for
carrying out a
wide variety of established assay formats, e.g., assays based on the
measurement of
electrochemical voltage and/or current or, preferably, an electrode-induced
luminescence, most
preferably, electrochemiluminescence. For examples of methods for conducting
ECL assays, the
reader is directed towards U.S. Patents Nos. 5,591,581; 5,641,623; 5,643,713;
5,705,402;
6,066,448; 6,165,708; 6,207,369; and 6,214,552 and Published PCT Applications
W087/06706
and W098/12539. Assays may be directed
to, but are not limited to, the measurement of the quantity of an analyte; the
measurement of a
property of a sample (e.g., temperature, luminescence, electrochemical
activity, color, turbidity,
etc.); the measurement of a chemical, biochemical and/or biological activity
(e.g., an enzymatic
activity); the measurement of a kinetic or thermodynamic parameter (e.g., the
rate or equilibrium
constant for a reaction), etc.
The embodiments of the invention can be used to test a variety of samples
which may
contain an analyte or activity of interest. Such samples may be in solid,
emulsion, suspension,
liquid, or gas form. They may be, but are not limited to, samples containing
or derived from, for
example, cells (live or dead) and cell-derived products, cell fragments, cell
fractions, cell lysates,
organelles, cell membranes, cell culture supernatants (including supernatants
from antibody
=
64
CA 02714094 2010-08-30
= . .
WO 03/023360
PCT/US02/28652
producing organisms such as hybridomas), waste or drinking water, food,
beverages,
pharmaceutical compositions, blood, serum, plasma, hair, sweat, urine, feces,
tissue, saliva,
mucous, oils, sewage, environmental samples, organic solvents or air. The
sample may further
comprise, for example, water, organic solvents (e.g., acetonitrile, dimethyl
sulfoxide, dimethyl
formamide, n-methyl-pyrrolidone or alcohols) or mixtures thereof.
Analytes that may be measured include, but are not limited to, whole cells,
cell surface
antigens, subcellular particles (e.g., organelles or membrane fragments),
viruses, prions, dust
mites or fragments thereof, viroids, antibodies, antigens, haptens, fatty
acids, nucleic acids (and
synthetic analogs), proteins (and synthetic analogs), lipoproteins,
polysaccharides, inhibitors,
cofactors, haptens, cell receptors, receptor ligands, lipopolysaccharides,
glycoproteins, peptides,
polypeptides, enzymes, enzyme substrates, enzyme products, second messengers,
cellular
metabolites, hormones, pharmacological agents, synthetic organic molecules,
organometallic
molecules, tranquilizers, barbiturates, alkaloids, steroids, vitamins, amino
acids, sugars, lectins,
recombinant or derived proteins, biotin, avidin, streptavidin, or inorganic
molecules present in
the sample. Activities that may be measured include, but are not limited to,
the activities of
phosphorylases, phosphatases, esterases, trans-glutaminases, nucleic acid
damaging activities,
transferases, oxidases, reductases, dehydrogenases, glycosidases, ribosomes,
protein processing
enzymes (e.g., proteases, kinases, protein phosphatases, ubiquitin-protein
ligases, etc.), nucleic
acid processing enzymes (e.g., polymerases, nucleases, integrases, ligases,
helicases,
telomerases, etc.), cellular receptor activation, second messenger system
activation, etc.
In one embodiment of the invention, a sample potentially containing a
luminescent,
chemiluminescent and/or redox-active substance (preferably an ECL-active
substance) is
65 -
CA 02714094 2010-08-30
=
WO 03/023360
PCT/US02/28652
introduced to an assay plate or one or more wells of an assay plate of the
invention and an
electrochemical or luminescent signal (preferably, electrochemiluminescence)
from the sample is
induced and measured from one or more assay domains so as to measure the
quantity of the
substance and/or identify the substance. In another embodiment of the
invention, a sample
containing a luminescent, chemiluminescent and/or redox-active substance
(preferably an ECL-
active substance) is introduced to an assay plate or one or more wells of an
assay plate of the
invention and an electrochemical or luminescent signal (preferably,
electrochemiluminescence)
from the sample is induced and measured from one or more assay domains so as
to measure the
presence of substances, chemical activities or biological activities that
affect the production of
the signal from the substance (e.g., the presence, production and/or
consumption of ECL
coreactants, hydrogen ions, luminescence quenchers, chemiluminescence
triggers, etc.). In other
embodiments of the invention, luminescent, chemiluminescent and/or redox-
active substances
(preferably ECL-active substances) are used as labels to allow the monitoring
of assay reagents
such as enzyme substrates or binding reagents. Assay formats for measuring
analytes through
the use of labeled binding reagents specific for the analyte include
homogeneous and
heterogeneous methods. Heterogeneous methods may include a wash step to
separate labels
(and/or labels attached to a material) on a solid phase/electrode from labels
in solution.
A wide variety of materials have been shown to emit electrode induced
luminescence,
particularly electrochemiluminescence, and may be used with the methods,
plates, kits, systems
and instruments of the invention. In preferred electrochemiluminescence
systems, ECL-active
materials and/or labels are regenerated after the emission of
electrochemiltuninescence and,
during an electrochemiluminescence experiment, may be repeatedly excited to an
excited state
oo
CA 02714094 2012-09-27
69331-63D
and/or induced to emit luminescence. For example, one class of ECL-active
materials are
believed to function via a mechanism that includes the steps of i) oxidation
of the material; ii)
reduction of the oxidized material by a strong reducing agent so as to produce
the material in an
excited state and iii) emission of a photon from the excited state so as to
regenerate the ECL-
active material. Preferably, the difference in redox potentials between the
ECL-active material
and the strong reducing agent is sufficient so that the energy released by
step (ii) is equal to or
greater than the energy of the photon. In an analogous mechanism, steps (i)
and (ii) may be
replaced by i) reduction of the material and ii) oxidation of the reduced
material by a strong
oxidizing agent. In some especially preferred systems, the mechanism is
believed to further
comprise the step of oxidizing an ECL coreactant so as to form the strong
reducing agent or,
analogously, reducing an ECL coreactant -to form the strong oxidizing agent.
Preferred luminescent materials and labels include luminescent organometallic
complexes of Ru, Os and Re. Some especially useful materials are polypyridyl
complexes of
ruthenium and osmium, in particular, complexes having the structure MLIL2L3
where M is
-
ruthenium or osmium, and L', I; and L each are bipyridine, phenanthroline,
substituted
bipyridine and/or substituted phenanthroline. We have found that the inclusion
of substituted
bipyridines or phenanthrolines presenting substituents comprising negatively
charged groups,
preferably sulfate groups and most preferably sulfonate groups (as described
in copending US
Patent No. 6,808,939 are especially preferred due to their resistance to
67
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
non-specific binding, in particular to electrodes comprising carbon, carbon
particles, carbon
fibrils, carbon composites, carbon fibril composites and/or carbon inks.
The invention also relates to detection methods using the electrodes of the
present
invention.
One aspect of the invention relates to methods of measuring an analyte of
interest,
wherein the analyte of interest is in-unobilized on an electrode (preferably
in an assay domain of
an assay cell or assay well). One embodiment comprises the steps of: i)
immobilizing the
analyte of interest on an electrode, preferably within an assay domain, e.g.,
by contacting the
electrode with a sample comprising the analyte of interest and ii) measuring
the analyte of
interest. The immobilization preferably proceeds via the formation of covalent
bonds to
functional groups on the electrode, or more preferably via the formation of
specific binding
interactions with binding reagents immobilized on the electrode, or most
preferably via passive
adsorption on the electrode.
Another aspect of the invention relates to methods of measuring an analyte of
interest that
binds to a biomaterial, wherein the biomaterial is immobilized on an electrode
(preferably in an
assay domain of an assay cell or assay well). One embodiment comprises the
steps of i)
contacting the biomaterial with a sample comprising the analyte; ii) forming a
complex on the
electrode comprising the analyte and the biomaterial and ii) measuring the
analyte of interest.
The biomaterial is preferably immobilized on the electrode via covalent bonds
to functional
groups on the electrode, or more preferably via specific binding interactions
with a capture
reagent immobilized on the electrode, or most preferably via passive
adsorption on the electrode.
Optionally, the assay method also comprises the step of immobilizing the
biomaterial on the
o8
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
electrode. This immobilization step can be carried out before, during and/or
after the step of
contacting the biomaterial with the sample.
Preferably, the aforementioned methods of measuring an analyte further
comprise the
steps of applying electrical energy (e.g., current or voltage) to the
electrode (preferably, under
conditions appropriate for inducing electrochemiluminescence, e.g., in the
presence of an ECL
coreactant) and measuring luminescence (preferably, electrochemiluminescence)
induced at the
electrode (e.g., from a luminescent species, preferably an
electrochemiluminescent species,
associated with the analyte), wherein the luminescence signal correlates to
the amount of analyte
present. Optionally, the method may comprise the step of introducing an ECL
coreactant prior to
the induction of luminescence. The luminescent species may be the analyte
itself or it may be a
luminescent species linked to the analyte. Such linkages may include i) a
covalent bond, ii) a
specific binding interaction (e.g., via a labeled antibody directed against
the analyte) and/or iii) a
non-specific binding interaction. The assay method, preferably, further
comprises the step of
forming the linkage between the label and the analyte, e.g., by contacting or
mixing the analyte
with a label or a labeled reagent such as a labeled binding reagent. The
formation of the linkage
may be carried out before, during and/or after the immobilization step. The
assay method may
also include one or more wash steps to remove material (e.g., analyte,
biomaterial, blocking
reagent, labeled reagent, etc.) that is not bound to the electrode.
Another aspect of the invention relates to methods of measuring a binding
interaction of a
biomaterial with a binding partner, wherein the biomaterial is immobilized on
an electrode
(preferably in an assay domain of an assay cell or assay well). One embodiment
comprises the
steps of i) contacting the biomaterial with a binding partner of the
biomaterial; ii) forming a
69µ--
CA 02714094 2010-08-30
. .
WO 03/023360
PCT/US02/28652
complex on the electrode comprising the biomaterial and the binding partner
and ii) measuring
the complex so as to measure the binding interaction. The biomaterial is
preferably immobilized
on the electrode via covalent bonds to functional groups on the electrode, or
more preferably via
specific binding interactions with a capture reagent immobilized on the
electrode, or most
preferably via passive adsorption on the electrode. Optionally, the assay
method also comprises
the step of immobilizing the biomaterial on the electrode. This immobilization
step can be
carried out before, during and/or after the step of contacting the biomaterial
with the binding
partner. The measurement of the binding interaction may be used in a variety
of applications
including, but not limited to, i) measuring the amount of the biomaterial; ii)
measuring the
amount of the binding partner and iii) measuring the affinity of a biomaterial
for binding partner.
The assay method may further comprise the step of contacting the biomaterial
and/or the binding
partner with an inhibitor of the binding interaction so that the extent of
binding is indicative, e.g.,
of the amount of the inhibitor or the inhibition constant of the inhibitor.
The inhibition assay
may also be used to screen compounds for inhibitors of the binding
interaction.
Preferably, the aforementioned method of measuring a binding interaction
further
comprises the steps of applying electrical energy (e.g., current or voltage)
to the electrode
(preferably, under conditions appropriate for inducing
electrochemiluminescence, e.g., in the
presence of an ECL coreactant) and measuring luminescence (preferably,
electrochemiluminescence) induced at the electrode (e.g., from a luminescent
species, preferably
an electrochemiluminescent species, associated with the binding partner),
wherein the
luminescence signal correlates to the number of binding interactions.
Optionally, the method
may comprise the step of introducing an ECL coreactant prior to the induction
of luminescence.
CA 02714094 2010-08-30
= , .
WO 03/023360
PCT/US02/28652
1
The luminescent species may be the binding partner itself or it may be a
luminescent species
linked to the binding partner. Such linkages may include i) a covalent bond,
ii) a specific
binding interaction (e.g., via a labeled antibody directed against the binding
partner) and/or iii) a
non-specific binding interaction. The assay method, preferably, further
comprises the step of
forming the linkage between the label and the binding partner, e.g., by
contacting or mixing the
binding partner with a label or a labeled reagent such as a labeled binding
reagent. The
formation of the linkage may be carried out before, during and/or after the
immobilization step.
The assay method may also include one or more wash steps to remove material
(e.g., binding
partner, biomaterial, blocking reagent, labeled reagent, etc.) that is not
bound to the electrode.
Another aspect of the invention relates to methods of measuring an activity or
process
that modifies a substance, the method comprising the steps of subjecting the
substance to a
sample comprising the activity or to conditions under which the process occurs
and measuring
the extent of the modification so as to measure the activity or process. The
extent of the
modification is, preferably, measured by selectively measuring the modified
substance and/or the
remaining unmodified substance according to the assay methods of the invention
(e.g., by using
labeled antibodies specific for the starting material or product). Optionally,
the activity or
process is carried out in the presence of an inhibitor of the activity or
process so that the extent of
modification is indicative, e.g., of the amount of the inhibitor or the
inhibition constant of the
inhibitor. The inhibition assay may also be used to screen compounds for
inhibitors of the
binding interaction and/or for measuring an activity or process that modifies
a binding partner of
an immobilized substance.
CA 02714094 2010-08-30
U0 03/023360
PCT/US02/28652
In one embodiment, a substance is immobilized on an electrode (preferably in
an assay
domain of an assay cell or assay well) and subjected to a modifying activity
or process, and
assayed to determine the extent of modification. In another embodiment, a
substance is
subjected to a modifying activity or process, immobilized on an electrode, and
assayed to
determine the extent of modification. In yet another embodiment, a cell is
subjected to a
modifying activity or process, the cell is lysed, a biological membrane or
other component
derived from the cell (e.g., an protein, nucleic acid, second messenger,
organelle, membrane
fragment, membrane vesicle, membrane ghost, membrane protein, membrane lipid,
etc) is
immobilized on an electrode, and assayed to determine the extent of
modification. Examples of
activities and processes that can be measured include kinase
activity/phosphorylation (including
autophosphorylation of membrane bound kinases), phosphatase
activity/dephosphorylation,
changes in membrane lipid composition or orientation (e.g., changes in
phosphatidyl serine
levels during apoptosis), hydrolysis or changes in phosphorylation state of
membrane
phosphatidyl inositols, prenylation or myristoylation of proteins, binding
and/or release of
soluble proteins and/or peripheral membrane proteins to biological membranes,
transfer of
proteins and/or lipids between biological membranes (e.g., between organelles
and/or between an
organelle and the cytoplasmic membrane), etc.
One embodiment of the method of measuring an activity or process (or,
alternatively, an
inhibitor of an activity or process) that modifies a substance relates to
measuring an activity or
process that results from the activation of a membrane protein (e.g., as a
result of a change in the
physical or chemical environment, a change in membrane potential, the
aggregation of the
protein, the binding of a ligand to a membrane receptor, etc.). For example,
the activation of a
2'
CA 02714094 2010-08-30
. =
WO 03/023360
PCT/US02/28652
membrane protein may lead to phosphorylation of the protein or of other
components of the
membrane (the phosphorylated components being measured, e.g., using
phosphopeptide specific
antibodies); ii) the sequestration or binding (or, alternatively, the release)
to the membrane of
soluble cellular components such as peripheral membrane proteins or
cytoplasmic proteins (the
binding of soluble cellular components being measured, e.g., using antibodies
specific for the
components); iii) the up or down regulation of membrane proteins (the membrane
proteins being
measured, e.g., using antibodies specific for the specific membrane protein
being monitored),
etc.
Another aspect of the invention relates to kits for use in conducting assays,
preferably
luminescence assays, more preferably electrode induced luminescence assays,
and most
preferably electrochemiluminescence assays, comprising an assay module,
preferably an assay
plate, more preferably a multi-well assay plate, and at least one assay
component selected from
the group consisting of binding reagents, enzymes, enzyme substrates and other
reagents useful
in carrying out an assay. Examples include, but are not limited to, whole
cells, cell surface
antigens, subcellular particles (e.g., organelles or membrane fragments),
viruses, prions, dust
mites or fragments thereof, viroids, antibodies, antigens, haptens, fatty
acids, nucleic acids (and
synthetic analogs), proteins (and synthetic analogs), lipoproteins,
polysaccharides,
lipopolysaccharides, glycoproteins, peptides, polypeptides, enzymes (e.g.,
phosphorylases,
phosphatases, esterases, trans-glutaminases, transferases, oxidases,
reductases, dehydrogenases,
glycosidases, protein processing enzymes (e.g., proteases, kinases, protein
phosphatases,
ubiquitin-protein ligases, etc.), nucleic acid processing enzymes (e.g.,
polymerases, nucleases,
integrases, ligases, helicases, telomerases, etc.)), enzyme substrates (e.g.,
substrates of the
73.
CA 02714094 2010-08-30
. .
WO 03/023360
PCT/US02/28652
enzymes listed above), second messengers, cellular metabolites, hormones,
pharmacological
agents, tranquilizers, barbiturates, alkaloids, steroids, vitamins, amino
acids, sugars, lectins,
recombinant or derived proteins, biotin, avidin, streptavidin, luminescent
labels (preferably
electrochemiluminescent labels), electrochemiltu-ninescence coreactants, pH
buffers, blocking
agents, preservatives, stabilizing agents, detergents, dessicants, hygroscopic
agents, etc. Such
assay reagents may be unlabeled or labeled (preferably with a luminescent
label, most preferably
with an electrochemiluminescent label). One embodiment of the invention
includes a kit for use
in conducting assays, preferably luminescence assays, more preferably
electrode induced
luminescence assays, and most preferably electrochemiluminescence assays,
comprising an
assay module, preferably an assay plate, more preferably a multi-well assay
plate, and at least
one assay component selected from the group consisting of: (a) at least one
luminescent label
(preferably electrochemiluminescent label); (b) at least one
electrochemiluminescence
coreactant); (c) one or more binding reagents; (d) a pH buffer; (e) one or
more blocking reagents;
(f) preservatives; (g) stabilizing agents; (h) enzymes; (i) detergents; (j)
desiccants and (k)
hygroscopic agents.
Preferably, the kit comprises the assay module, preferably an assay plate, and
the assay
component(s) in one or more, preferably two or more, more preferably three or
more containers.
=
Preferably, the assay module is a multi-well plate is adapted for use in
conducting the
electrode induced luminescence assays (preferably electrochemiluminescence
assays) in sectors.
According to one embodiment, the kit comprises one or more of the assay
components in
one or more plate wells, preferably in dry form.
CA 02714094 2010-08-30
, =
=V0 03/023360
PCT/US02/28652
According to one embodiment, the assay components are in separate containers.
According to another embodiment, the kit includes a container comprising
binding reagents and
stabilizing agents. According to another embodiment, the well reagents may
include binding
reagents, stabilizing agents. Preferably, the kits do not contain any liquids
in the wells.
One preferred embodiment relates to a kit for use in conducting electrode
induced
luminescence assays (preferably electrochemiluminescence assays) comprising an
assay plate,
preferably a multi-well assay plate, and at least one assay component selected
from the group
consisting of at least one luminescent label (preferably
electrochemiluminescent label) and at
least one electrochemiluminescence coreactant).
Another embodiment relates to a kit comprising a multi-well plate and at least
one
electrode induced luminescent label (preferably electrochemiluminescent label)
and/or at least
one bioreagent and/or at least one blocking reagent (e.g., BSA).
According to one preferred embodiment, the kit comprises at least one
bioreagent,
preferably immobilized on the plate surface selected from: antibodies,
fragments of antibodies,
proteins, enzymes, enzyme substrates, inhibitors, cofactors, antigens,
haptens, lipoproteins,
liposaccharides, cells, sub-cellular components, cell receptors, viruses,
nucleic acids, antigens,
lipids, glycoproteins, carbohydrates, peptides, amino acids, hormones, protein-
binding ligands,
pharmacological agents, luminescent labels (preferably ECL labels) or
combinations thereof.
According to another preferred embodiment, the kit comprises at least one
biological
membrane or component thereof, preferably immobilized on the plate surface,
that comprises an
active protein selected from: single transmembrane receptors with intrinsic
tyrosine kinase
activity; non-tyrosine kinase transmembrane receptors (e.g., transferrin
receptor); G-protein
75, =
CA 02714094 2010-08-30
. ,
WO 03/023360
PCT/US02/28652
coupled receptors (GPCR); GPCR effector proteins (e.g., adenylate cyclase);
phosphoinositides
(e.g., phosphatidy inositol 4,5 bisphosphate (PIP2)); phospholipid or
sphingolipid composition,
identification, or function (i.e., changes in phosphotidylserine presence
during apoptosis);
organelle-bound GTPases/guanine nucleotide exchange factors (GEFs)/GTPase
activating
proteins (GAPs); cytokine/chemokine receptors; cell adhesion molecules (e.g.,
VCAM,
integrins); cytoplasmic peripheral membrane protein kinases (e.g., src);
intracellular protein
kinase adaptor/docking proteins (e.g., insulin receptor substrate 1, GRB2);
ion channels (e.g.,
nicotinic acetylcholine receptor, CFTR, etc.); passive transporters (e.g.,
glucose); active (ATP-
driven) transporters; ion-linked transporters (e.g., Na+/glucose);
glycosyltranferases/glycoprotein
modifying enzymes; nuclear membrane fragments; and soluble receptors.
Preferably, the kit includes immobilized reagents comprised of proteins,
nucleic acids, or
combinations thereof
According to one preferred embodiment, the plurality of wells includes at
least two
different bioreagents. For example, a well may include two or more assay
domains, wherein two
or more assay domains have different bioreagents.
Preferably, the kit comprises at least one electrochemiluminescence coreactant
and/or at
least one electrode induced luminescence label (preferably
electrochemiluminescent label).
Another aspect of the invention relates to improved methods and systems for
selecting or
identifying biologically active compounds and, optionally, incorporating such
biologically active
compounds into suitable carrier compositions in appropriate dosages. The
invention includes the
use of the assay electrodes, kits and/or methods of the invention to screen
for new drugs,
CA 02714094 2010-08-30
.
. -
WO 03/023360
PCT/US02/28652
preferably, by high-throughput screening (HIS), preferably involving screening
of greater than
50, more preferably 100, more preferably 500, even more preferably 1,000, and
most preferably
5,000. According to a particularly preferred embodiment, the screening
involves greater than
10,000, greater than 50,000, greater than 100,00, greater than 500,000 and/or
greater than
1,000,000 compounds.
One embodiment of the invention relates to a method for selecting or
identifying
biologically active compounds from a library of compounds, said method
comprising screening
said library of compounds for biological or biochemical activity, wherein said
screening includes
assaying the library of compounds for the biological or biochemical activity,
the assays being
conducted using the assay electrodes of the invention.
Preferably, the method further comprises identifying one or more active
compounds.
Preferably, the method further comprises testing said one or more active
compounds for
bioavailability, toxicity and/or biological activity in vivo. According to one
preferred
embodiment, the testing comprises further screening using the assay electrodes
of the invention.
Preferably, the method further comprises synthesizing analogues of said one or
more
active compounds. According to one preferred embodiment, the analogues are
screened for
bioavailability, biological activity and/or toxicity using the assay
electrodes of the invention.
According to a particularly preferred embodiment, the method further comprises
formulating the one or more compounds into drugs for administrating to humans
and/or animals.
Preferably, the formulating comprises determining the suitable amount of the
one or more active
compounds in the drug and mixing the suitable amount with one or excipients or
carriers.
Preferably, the excipient comprises sugar and/or starch.
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
Another embodiment of the invention relates to a method of analyzing one or
more
complex mixtures of biochemical substances to measure a plurality of binding
components
therein, comprising:
(a) contacting said mixtures with one or more assay electrodes having one
or more
lipid/protein layers immobilized thereon, preferably by adding said mixtures
to a multi-well plate
adapted for electrode induced luminescence assays (preferably
electrochemiluminescence
assays), wherein the wells of the plate include the assay electrodes;
(b) applying a voltage or current to the electrodes sufficient to induce
luminescence;
and
(c) measuring emitted luminescence.
Another embodiment of the invention relates to a method of analyzing the
output of one
or more combinatorial (biological and/or chemical) mixtures to measure a
plurality of binding
components therein, comprising:
(a) contacting said mixtures to one or more assay electrodes, preferably by
introducing said mixture into a multi-well plate adapted for electrode induced
luminescence
(preferably electrochemiluminescence) assays, said plate having a plurality of
wells comprising
one or more assay electrodes;
(b) applying a voltage or current to the electrodes sufficient to induce
luminescence;
and
(c) measuring emitted luminescence.
Another embodiment of the invention relates to a method for measuring a single
biochemical substance in a sample in a multiplicity of simultaneous assays,
comprising:
CA 02714094 2010-08-30
, .
WO 03/02336()
PCT/US02/28652
(a) contacting said sample with an assay electrode, preferably by
introducing said
sample into a multi-well plate adapted for electrode induced luminescence
(preferably
electrochemiluminescence) assays, said plate having a plurality of wells
comprising one or more
assay electrodes;
(b) applying a voltage or current to the electrodes sufficient to induce
luminescence;
and
(c) measuring emitted luminescence.
A further embodiment of the invention relates to a method of drug discovery
comprising:
(a) selecting a multiplicity of compounds for testing;
(b) screening said multiplicity of compounds for biological activity (using
any one of
the multi-well plates and/or apparatus described above) to find one or more
biologically active
compounds; and
(c) modifying said one or more biologically active compounds to
reduce toxicity
and/or enhance biological activity thereby forming one or more modified
biologically active
compounds.
Preferably, the method further comprises screening said modified biologically
active
compounds for biological activity and/or toxicity (using the assay electrodes
of the invention
described above).
Preferably, the method further comprises determining the appropriate dosage of
one or
more of said modified biologically active compounds. Preferably, the method
still further
comprises incorporating such dosage into a suitable carrier such as sugar or
starch to form a drug
in solid (e.g., pill or tablet) or liquid form.
79.t.
CA 02714094 2010-08-30
= ' " 69331-63D
Advantageously, the assay electrodes, assay modules and methods of the
invention may
be integrated into and/or used in a variety of screening and/or drug discovery
methods. Such
screening and/or drug discovery methods include those set forth in U.S. Patent
No. 5,565,325 to
Blake; U.S. Patent No. 5,593,135 to Chen et al.; U.S. Patent No. 5,521,135 to
Thastrup etal.;
U.S. Patent No. 5,684,711 to Agrafiotis et al.; U.S. Patent No. 5,639,603 to
Dower et al.; U.S.
Patent No. 5,569,588 to Ashby et al.; U.S. Patent No. 5,541,061; U.S. Patent
No. 5,574,656; and
U.S. Patent No. 5,783,431 to Peterson et al.
According to another embodiment, the invention further comprises identifying
adverse
effects associated with the drug and storing information relating to the
adverse effects in a
database. See, United States Patent No. 6,219,674 by Classen.
Another aspect of the invention relates to improved biologically active
compounds and/or
drugs made using the inventive methods.
6. EXAMPLES
The following examples are illustrative of some of the electrodes, plates,
kits and
methods falling within the scope of the present invention. They are, of
course, not to be
considered in any way limitative of the invention. Numerous changes and
modification can be
made with respect to the invention by one of ordinary skill in the art without
undue
experimentation.
CA 02714094 2010-08-30
= =
WO 03/023360
PCT/US02/28652
Example 1. Fabrication of Multi-Well Assay Plates Having Screen Printed
Electrodes.
Multi-layer plate bottoms were prepared by screen printing electrodes and
electrical
contacts on 0.007" thick Mylar polyester sheet. The Mylar sheet was first cut
with a CO2 laser
so to form conductive through-holes (i.e., holes that were subsequently made
conductive by
filling with conductive ink) as well as to form alignment holes that were used
to align the plate
bottom with the plate top. Electrical contacts were formed on the bottom of
the Mylar sheet by
screen printing an appropriately patterned silver ink layer (Acheson 479ss)
and a carbon ink
overlayer (Acheson 407c). The carbon ink layer was dimensioned slightly larger
(0.01 inches)
than the silver ink layer to prevent exposure of the edge of the silver film.
Working and counter
electrodes were formed on the top of the Mylar film in a similar fashion
except that three layers
of carbon ink were used to ensure that no silver remained exposed. The
conductive through-
holes filled with conductive ink during these screen-printing steps. A
dielectric ink was
subsequently printed over the electrode layers so as to define the active
exposed surface area of
the working electrode. Typically, nine plate bottoms were simultaneously
printed on an I 8"x12"
Mylar sheet. Typical registrational tolerances during the screen printing
steps were +1- 0.007-
0.008 inches on the top side of the substrate and +1- 0.010 inches on the
bottom side. The
separation between the printed counter and working electrode strips was kept
at > 0.010 inches to
prevent the formation of short circuits. Optionally, the working electrodes
were conditioned by
treating the patterned plate bottoms for 5 min. with an oxygen plasma (2000 W,
200 mtorr) in a
plasma chamber (Series B, Advanced Plasma Systems, St. Petersburg, FL)
modified with large
area flat electrodes.
CA 02714094 2010-08-30
. . .
WO 03/023360
PCT/US02/28652
Multi-well assay plates were assembled using the plate bottoms described above
and
injection molded plate tops. The dimensions of the plate tops met industry
standards as
established by the Society of Biomolecular Screening. The plate tops were
either made of black
plastic (polystyrene loaded with black pigment) or white plastic (polystyrene
loaded with
titanium dioxide). The bottom surfaces of the plate tops were contacted with
die-cut double
sided tape (1 mil PET coated on each side with 2 mil of acrylic pressure
sensitive adhesive) so as
to allow for sealing of the plate tops to the plate bottoms. The tape was cut
to form holes that
were slightly oversized relative to the holes in the plate tops. The plate
bottoms were fixed
(using the laser cut alignment holes) onto alignment pins on an X-Y table. The
plate bottoms
were optically aligned to the plate tops and then sealed together using a
pneumatic press (400
pounds, 10 s). Alignment was carried out sufficiently accurately so that the
exposed working
electrodes were centered within the wells (+/- 0.020 inches for 96-well plates
and +/- 0.015
inches for 384 well plates). These tolerances ensured that the exposed regions
of the working
electrodes were within the wells and that there were exposed counter electrode
surfaces on both
sides of the working electrode. In some examples, assay reagents were
deposited and dried on
the plate bottoms prior to assembly of the plate.
A variety of types of multi-well assay plates were prepared according to the
procedure
described above. A few specific plate designs are described in more detail
below to allow for
reference in subsequent examples. Plate B, a 96-well plate sectioned into 6
square sectors of 4 x
4 wells, was prepared using components and patterns as pictured in Figure 10A
and had a black
plate top. Plate C, a 96-well plate sectioned into 6 square sectors of 4 x 4
wells, was prepared
using components and patterns as pictured in Figure 10 (except that the
dielectric layer in Plate C
62 "
CA 02714094 2010-08-30
= = ,
WO 03/023360
PCT/US02/2802
is patterned so as to expose four isolated "fluid containment regions" on the
working electrode
surface within each well, see Figures 10C and 10D) and a black plate top.
Plate D was similar to
Plate C except that the dielectric layer was patterned so as to expose 7
isolated "fluid
containment regions" on the working electrode within each well. Plate E was
similar to Plate C
except that the dielectric layer was patterned so as to expose 10 isolated
"fluid containment
regions" on the working electrode within each well. Plate F, G and H were
analogous to Plate C,
except that they had 24 wells that were square in shape (the plates being
sectored into 6 square
sectors of 4 wells) and the dielectric layer was patterned to expose 25, 64 or
100 fluid
containment regions, respectively. In Figure 10A, details A, B, C and D show
for Plate B: the
printed contact layer, the Mylar film with through-holes, the printed
electrode layer and the
printed dielectric layer (in one sector of the plate) , respectively.
Example 2. ECL Measurements.
Plates were read on an instrument designed to make electrical contact to
individual
square sectors. The sector in electrical contact with the instrument was
aligned with a telecentric
lens (having a front element with a diameter of 4.1") coupled to a cooled CCD
camera
(VersArray: 1300F, Princeton Instruments) that was used to image ECL emitted
from the sector.
The camera employed a CCD chip with dimensions of roughly 2.6 cm x 2.6 cm and
having a
1340 x 1300 array of pixels. The pixel size was 0.02 mm x 0.022 mm. An optical
band pass
filter in the optical path was used to select for light matching the emission
profile of ruthenium-
tris-bipyridine. A translation table was used to translate the plate under the
telecentric lens so as
to allow all 6 sectors to be read. Image analysis software was used to
identify wells or assay
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
domains within wells and to quantitate ECL from specific wells or domains. ECL
from plates
having screen printed carbon working electrodes was induced using a linear
voltage scan from
roughly 2-5 V over 3 seconds unless otherwise indicated. ECL is reported as
the total integrated
light signal measured over the period of the voltage scan (after correcting
for background light
levels and detector offset).
Example 3. An ECL Assay Measuring Multiple Activities of an Enzyme in One Well
of an
MDMW Plate
Many nucleic acid processing enzymes have both nucleic acid synthesizing
(e.g.,
polymerase or ligase) activities and nuclease activities. One example is HIV
Reverse
Transcriptase (RT), an enzyme with both a RNA-dependent DNA polymerase (RDDP)
activity
and an RNAse H activity. The following example demonstrates an ECL assay for
measuring
both HIV RT activities in one well of an MDMW Plate.
The assay format is illustrated in Figure 12. The enzyme substrate is a 5"-
labeled (using
TAG phosphoramidite, IGEN International, Inc.) DNA primer bound to the 3 '-end
of an RNA
target sequence. The RDDP activity extends the DNA primer to make a
complementary copy of
the RNA sequence. The RNAse H activity selectively hydrolyzes RNA in RNA-DNA
duplexes.
RNAse activity is measured by hybridizing the labeled DNA product to an
immobilized probe
(3 "-B13) that is complementary to the DNA primer sequence (thus measuring the
RNAse
catalyzed exposure of the DNA primer). The RDDP activity is measured by
hybridizing the
labeled DNA product to an immobilized probe (5 "-B13) that is complementary to
the extended
DNA sequence (thus measuring the RDDP extension of the DNA primer).
84
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
The assay was conducted on a MDMW plate adapted for electrode induced
chemiluminescence measurements and having four fluid containment regions
exposed on the
working electrode surface of each well (Plate C of Example 1). The two probes
(3"-B13 and 5'-
B13) were biotin-labeled to facilitate immobilization. Each probe was pre-
bound to avidin.
Assay domains were formed by immobilizing each probe in one fluid containment
region of each
well by microdispensing the avidin-probe complexes onto the fluid containment
regions
(between 100-1000 nL containing 1 pmol of probe) using a non-contact
microdispensor (BioDot
or Cartesian Technologies) and allowing the solutions to dry. The two
additional fluid
containment regions were used as control domains (one was coated with avidin,
the other was
not treated). The plates were blocked with a solution containing BSA and
washed prior to use.
The assays were carried out by adding to the wells of plates 1 nmol of the
dNTPs, 5 pmol
of the substrate, 3 pmol of the enzyme and varying amounts of an RT inhibitor
in 100 uL of a
buffer containing 50mM Tris ph 8.0, 40mM KC1, 10m1M MgC12, 0.025% Triton X-
100, 2.5mM
DTT. The reaction mixture was incubated for 20 min at 22 C and then quenched
by the addition
of EDTA. The plates were incubated for an additional 2 hours to allow the
hybridization
reactions to proceed. Tripropylamine was added (ORIGEN Assay Buffer, IGEN
International
and the products assayed by electrochemiluminescence measurements. The ECL
signals were
corrected by subtracting assay background (measured in wells in which EDTA was
added prior
to the enzyme). Figures 13A-B show that the inhibitor aurintricarboxylic acid
(ATA; DuPont)
inhibits both RNAse H (Fig. 13B) and RDDP activities (Fig. 13A). By contrast,
125 uM ddCTP
(a chain terminating agent) completely inhibited the RDDP activity but had no
effect on the
RNAse H activity (data not shown).
CA 02714094 2010-08-30
. , =
WO 03/023360
PCT/US02/28652
Example 4. Detection of a Panel of Respiratory Disease Antigens
An MDMW plate adapted for ECL measurements and having 4 fluid containment
regions
on the working electrode surface exposed in each well (Plate C of Example 1)
was coated with
antibodies specific to four respiratory diseases: Influenza A, Influenza B,
Respiratory Syncytial
Virus (RSV), and Streptococcus Pyogenes (Strep A). Capture antibody solutions
(50 ug/ml in
phosphate buffered saline, PBS) were dispensed using a BioDot Dispenser onto
the fluid
containment regions within the wells (250 nl/spot) such that each well
contained one assay
domain coated with each antibody. The solutions were allowed to dry, at which
time a 5% BSA
solution was added (200 ul/well) and the plate refrigerated overnight. The
plate was washed
with PBS before use (4 x 250 ul/well).
Antigen solutions were prepared by diluting solutions of bacteria or purified
virus
obtained from commercial sources by 1000x or 100x, respectively, using PBS.
The approximate
titers after dilution were: 2.3 x1011 virus particles/ml Influenza A; 320 HA
units/0.05 ml, or 0.1
mg/ml protein for Influenza B; 6.6 x108 virus particles/ml RSV; 1.5 x104
CFU/ml Strep A.
To perform the assay, 100 ul of the diluted antigen solutions were combined
with 450 ul
of PBS containing 0.2% Tween-20. 75 ul of these solutions were combined with
10 ul of a
solution of the appropriate labeled antibody solution (sulfonated derivative
of Ru(bpy)3) such
that the final concentration was 3 ug/ml labeled antibody. 50 ul of this
solution was added to
individual wells of the washed plate and incubated for 8 minutes. The plate
was then washed
with PBS (4x 200 ul/well) and 100 ul of ORIGEN Assay Buffer (IGEN
International) was added
CA 02714094 2010-08-30
- = =
WO 03/023360
PCT/US02/28652
to each well. The plate was then analyzed using electrochemiluminescence
detection. Figure 14
shows that each antigen was selectively measured in the appropriate assay
domain.
Example 5. Measurement of Tyrosine Kinase and Serine/Threonine Kinase
Activities in a
Well of a MDMW Plate.
This example used an MDMW plate adapted for ECL measurements and having 4
fluid
containment regions on the working electrode surface exposed in each well
(Plate C of Example
1). Each fluid containment region received 250 nL of one of the following
solutions: 1 mg/ml
Poly-Glu:Tyr (4:1) (PGT) in PBS buffer with 0.0075% Triton; 1 mg/ml Myelin
Basic Protein
(MBP) in PBS buffer with 0.0075% Triton; 0.5 mg/ml Avidin in PBS buffer with
0.0075%
Triton; 5% BSA solution in PBS. The plate was then dried overnight and blocked
in a 5% BSA
solution at 4 C for 2 days. The plate was washed to remove blocking agent
prior to use.
For phosphorylation of PGT (tyrosine kinase assay) 0.1 mU/u1 of c-SRC was
used, for
phosphorylation of MBP (threonine kinase) 15 pg/1.11 of ERK-1 was used. The
capture efficiency
of the avidin-coated domain was determined by measuring the binding of bovine
IgG labeled
with biotin and a sulfonated form of Ru(bpy)3.
Each spot (PGT, MBP, Avidin and BSA) was exposed to a solution of each
enzyme/analyte (as well as to mixtures of the enzymes and analytes) in the
presence of labeled
(sulfonated derivative of Ru(bpy)3) antibodies directed against the kinase
products (anti-
phosphotyrosine and anti-phospho-MBP (or, alternatively, using unlabeled
primary antibodies
and labeled secondary antibodies). After incubating the plates to allow the
enzyme and binding
reactions to proceed, a TPA-containing buffer was added and the plates were
analyzed by ECL
S7
CA 02714094 2010-08-30
4
WO 03/023360
PCT/US02/28652
(no wash was required). Reported signals were corrected by subtracting
background measured in
the absence of enzyme/analyte. Each point includes an average of four
measurements for
background signal and 12 measurements for specific signal. The table in Figure
15 summarizes
results of this experiment.
The PGT domain only showed high signal in the presence of the tyrosine kinase
src. As
expected, the MPB gave high signal in the presence of the ERK-1, but also gave
elevated signals
in the presence of SRC, presumably because of the presence of several
tyrosines in MPB and the
relative non-specific nature of both SRC and the anti-phophotyrosine antibody.
The avidin
domain gave a good signal in the presence of the biotinylated analyte and did
not act as a
substrate for the kinases. This result demonstrates the utility of including a
binding domain, e.g.,
for capturing (and, optionally, purifying) lcinases to be tested from crude
samples. The BSA
spot did not provide a significant signal in the presence of the
analyte/enzymes and shows that
the blocking agent did not show non-specific reactions with the assay
reagents.
Example 6. Evaluation of the Detection Limits of MDMW Plates.
In this Example, Applicants measured the detection limits of the ECL
measurement of
bovine IgG labeled with biotin and a sulfonated derivative of Ru(bpy)3 (--=
2.3 labels per protein)
as a function of the area of the binding domain. Binding domains were formed
by coating Avidin
onto one or more of the exposed regions (fluid containment regions) of the
electrode (by
microdispensing avidin solutions and drying on the surface of the electrode).
Five plate types
were prepared:
CA 02714094 2010-08-30
L =
WO 03/023360
PCT/US02/28652
Standard 96: Plate type B from Example 1 having a single large binding domain
coated
with avidin.
4-Spot-1: Plate type C from Example 1 having 4 small fluid containment
regions, three
of which are coated with avidin to form a binding domain.
4-Spot-3: Plate type C from Example 1 having 4 small fluid containment
regions, only
one of which is coated with avidin to form binding domains.
7-Spot-1: Plate type D from Example 1 having 7 smaller fluid containment
regions, three
of which are coated with avidin to form a binding domain.
7-Spot-3: Plate type D from Example 1 having 7 smaller fluid containment
regions, only
one of which is coated with avidin to form binding domains.
After standard blocking and washing procedures, a serial dilution of tag-IgG-
biotin was
assayed in 50 microliter volumes with 2 hour incubation time using
intermittent shaking. The
plates were read with a 2.5-4.5 volt scan for 5 seconds. Figure 16 shows a log-
log plot of the
uncorrected data. Surprisingly, the detection limits are actually
significantly better for the multi-
array format than for the standard format. The relative detection limits
(relative to the standard
96 plate) calculated for each plate type are: standard 96 (1.0), 4-spot-1
(4.2), 4-spot-3 (1.4), 7-
spot-1 (4.4), 7-spot-3 (2.1). This is expected if most of the tag is captured
at the working
electrode spot. As the spot gets smaller, the total specific light emitted
should stay constant,
while the background signal decreases with area. On average for all
calibrators above the
detection limit, the signal for 1 of the 4 spots spotted is 2.7 times as high
as the average signal
when 3 spots are spotted. This indicates that most (-90%) of the tagged
molecules are being
39A
CA 02714094 2010-08-30
WO 03/023360
PCT/US02/28652
captured on the single spot. This example demonstrates that assays in MDMW
plates having
small assay domains can have the same or better performance as assays in
conventional single
domain plates.
Example 7. Multi-Analyte Immunoassay of MDMW Plates
Sandwich immunoassays for four different cytokines ¨ interleukin 113 (IL-113),
interleukin
6 (IL-6), interferon y (IFN-y) and tumor necrosis factor a (TNF-a) - were
carried out
simultaneously in the wells of plates manufactured according to the design and
procedure
described for Plate C in Example 1. Four capture antibodies (each selective
for one of the
analytes of interest) were patterned into distinct assay domains by
microdispensing solutions of
the antibodies on the fluid containment regions within each well (one antibody
per region) and
allowing the antibodies to adsorb to the surface of the working electrode.
Solutions (0.25 uL)
containing the antibody (at a concentration of 32 ug/mL for IL-113 and TNF-a
or 64 ug/mL for
IL-6 and IFN-y) and 0.1 % BSA in phosphate buffered saline were dispensed onto
the fluid
containment regions using a solenoid valve controlled microdispensor (Biodot
Dispensor,
Cartesian Technologies) and allowed to evaporate to dryness. The volume of the
antibodies was
sufficient to spread over all of the exposed electrode surface within a fluid
containment region
but was small enough so that the fluid did not spread past the boundary formed
by the dielectric
layer. After drying the antibody solution on the working electrode, the plate
tops were attached
and the excess unbound antibody was removed (and uncoated surfaces blocked) by
filling the
wells with a solution containing 5 % (w/v) bovine serum albumin (BSA) in
phosphate buffered
CA 02714094 2010-08-30
= . _ . =
WO 03/023360
PCT/US02/28652
saline (PBS). The plates were incubated with the blocking solution overnight
at 4 C and then
washed with PBS.
The assays were carried out by the steps of i) adding 0.02 mL of the sample to
the well
and incubating for 1 hour on a plate shaker; ii) washing the wells with PBS;
iii) adding 0.02 n..1.1,
of a solution containing 2,000 ng/mL each of four detection antibodies
(labeled with NHS ester
1) against the four analytes of interest and incubating for 1 hour on a plate
shaker; iv) washing
with PBS; v) introducing 0.1 mL of a solution containing tripropylamine in
phosphate buffer
(ORIGEN Assay Buffer, IGEN International) and vi) measuring ECL. Figures 17A-D
demonstrate that each of the analytes of interest can be independently
measured in a single
sample in a single well of a multi-well assay plate. The figures show ECL
emitted from each
assay domain as a function of the concentration of each analyte. The
introduction of a specific
analyte led to a linear increase in ECL with analyte concentration (relative
to the background
signal measured in the absence of any analyte) at assay domains having capture
antibodies
directed against that analyte, but did not affect the ECL at assay domains
having antibodies
directed against the other analytes. Figure 18 shows a CCD image of the ECL
emitted from a
sector of wells used to assay solutions containing mixtures of the four
analytes. The highlighted
well is annotated to show the arrangement of the four assay domains. That
specific well was
used to assay a sample having 250 pg/mL each of IL-lp and TNF-a and 8 pg/mL
each of IL-6
and IFN-y.
Example 8. Multi-Plex Assay for Total EGF Receptor and Auto-Phosphorylated EGF
Receptor
CA 02714094 2010-08-30
= = =
WO 03/023360
PCT/U S02/28652
This example shows an ECL assay that measures in one well of a MDMW Plate
total
(phosphorylated and non-phosphorylated) EGF Receptor (T-EGFR) and
phosphorylated EGF
Receptor (P-EGFR).
Preparation of Lysates for Multi-Plex:
1. A-431 cells were cultured in 150 mm tissue culture dishes and serum starved
overnight
(DMEM supplemented with 1% Penicillin-Streptomycin and 1% Sodium Pyruvate).
2. Following two rinses with serum-free media, one dish was stimulated with
200nM EGF
in serum-free media for 15 minutes at room temperature. The unstimulated plate
was
given serum-free media only.
3. The cells were rinsed with two volumes of PBS.
4. 2mls of a modified RIPA buffer (fresh sodium orthovanadate added the
morning of the
assay) was added to the dishes. RIPA buffer included: 1mM neat sodium
orthovanadate,
150mM NaC1, 50mM Tris, 6mM Deoxycholate, 0.5% NP40, in water with a fresh
protease inhibitor tablet, 1 tablet per 10mL buffer). Cells were incubated
with the R1PA
for 10 minutes on ice.
5. Supernatant was collected and quantitated using the Pierce BCA Protein
Assay.
Protocol for Multiplex Assay:
L Biotin-labeled antibodies for T-EGFR (specific for the cytoplasmic domain of
EGFR)
and P-EGFR (anti-phosphotyrosine) were prebound (1 hour) with one equivalent
of
avidin and deposited by microdispensing (one antibody per region, 0.5 pmol per
region in
CA 02714094 2010-08-30
= =
WO 03/023360
PCT/US02/28652
0.25 uL) onto two of the four fluid containment regions in each well of a MDMW
Plate
(Plate C of Example 1). The two remaining fluid containment regions were used
as
controls for non-specific binding and cross-reactions. One region was coated
with
Avidin only. The other was left bare but eventually blocked with BSA.
2. The antibodies were allowed to dry. The wells were then blocked for one
hour at room
temperature with 2001.11 per well of 5% BSA in water.
3. The plates were washed four times with dH20.
4. 50m/well of lysate was added to each well of the 96 well plates and
shaken
intermittently for one hour.
5_ The plates were washed four times with dH20.
6. The Sulfo-TagTm -labeled a-EGFR antibody (50 uL of 33 nM) was added and the
binding
reaction allowed to proceed for 1 hour at room temperature with shaking. The
plates were
washed four times with dH20.
7. 100111 per well of 100mM TPA with 400mM gly-gly assay buffer was added
just prior to
ECL analysis.
8. The plates were analyzed using ECL detection.
The table below compares the ECL signals measured from the T-EGFR and P-EGFR
assays for lysates from stimulated and unstimulated cells. As expected, over
the time course of
the experiment, the levels of T-EGFR do not change considerably on
stimulation, however, a
large increase in P-EGFR was observed.
CA 02714094 2010-08-30
. ,
WO 03/023360
PCT/US02/28652
Analyte
Sample T-EGFR P-EGFR
Unstimulated 24,200 57
Stimulated 23,545 122
Example 9. Multi-Plex Assay for Detection of Autophosphorylated and
Nonphosphorylated EGF Receptor
This example shows an ECL assay that measures both nonphosphorylated EGF
receptor
and EGF receptor that is phosphorylated at tyrosine 1173 in a single well of a
MDMW Plate.
A-431 cell lysates were prepared as described in Example 8, except that
separate dishes
of cells were stimulated with 0.2nM, 5riM and 200nM EGF.
Protocol for Multiplex Assay:
1. Antibodies specific for EGF receptor that is autophosphorylated at tyrosine
1173
(pY1173) and antibodies specific for EGF receptor that is not phosphorylated
at tyrosine
1173 (Y1173) were deposited by microdispensing (one antibody per region, 0.2
pmol per
region in 0.25 uL) and passively adsorbed onto two of the four fluid
containment regions
in each well of a MDMW Plate (Plate C of Example 1). The two remaining fluid
containment regions were used as controls for non-specific binding and cross-
reactions;
these regions were left bare but eventually blocked with BSA.
2. The antibodies were allowed to dry overnight. The wells were then blocked
for one hour
at room temperature with 200i.d per well of 5% BSA in water.
3. The plates were washed with PBS.
9-1'
CA 02714094 2010-08-30
vV0 03/023360
PCT/US02/286:2
4. 5 g/we1l of lysate was added to each well of the 96 well plates and the
plates were
shaken intermittently for three hours.
5. The plates were washed with PBS.
6. A Sulfo-TAG label labeled oc-EGFR antibody directed against the
extracellular domain of
the receptor (50 uL of a 33 nM solution) was added and the binding reaction
allowed to
proceed for 1 hour at room temperature with shaking. The plates were washed
four cycles
with PBS.
7. 150 1 per well of 100mM TPA with 400mM gly-gly assay buffer was added
just prior to
ECL analysis.
8. The plates were analyzed using ECL detection in a Sector HTSTm plate reader
(Meso
Scale Discovery).
Figures 19A-D demonstrate that the amount of autophosphorylation of the
tyrosine at
position 1173 on the EGF receptor can be controlled and quantified in a single
well of a multi-
well assay plate. The schematic in Fig. 19A shows placement of the pY1173 and
Y1173
antibodies on two diagonally opposed fluid containment regions in the same
well. The EGF
receptor contained in A-431 cell lysates binds to the appropriate surface
immobilized antibody.
Specifically, only the phosphorylated tyrosine at position 1173 binds the
pY1173 antibody, and
only the nonphosphorylated tyrosine at position 1173 binds to the Y1173
antibody. Competition
for binding of the receptor to more than one assay domain in a single well is
circumvented in this
format. The reporter a-EGFR antibody binds the extracellular domain of both
receptors. The
CCD images in Figures 19B-D show ECL emitted from each assay domain as a
function of
increasing EGF concentration. No detectable autophosphorylation of tyrosine
1173 was
_
CA 02714094 2010-08-30
=
vy 0 03/023360
PCT/US02/28652
observed at 0.2nM EGF, approximately 50% of the receptor was phosphorylated at
5nM EGF,
and approximately 90% was phosphorylated at 200nM EGF.
Example 10. Measurement of Tyrosine Kinase and Serine/Threonine 1Cinase
Activities in
a Well of a MDA1W Plate
This example used an MDMW plate adapted for ECL measurements and having 4
fluid
containment regions on the working electrode surface exposed in each well
(Plate C of Example
1). Each of the four fluid containment regions received 250 nL of one of the
four following
solutions: (i) 0.5 mg/ml Poly-Glu:Tyr (4:1) (PGT) in PBS buffer with 0.015%
Triton; (ii) 0.2
mg/ml Myelin Basic Protein (MBP) in PBS buffer with 0.015% Triton; (iii) 0.3
mg/ml
Streptavidin in PBS buffer with 0.015% Triton; (iv) 0.3 mg/ml BSA solution in
PBS with 0.15%
Triton. The plate was then dried for 1-1.5 hours at ambient conditions,
vigorously washed with
PBS containing 0.1% Triton, washed with water and blocked in a 5% BSA solution
for at least 2
hours at room temperature. The washing included a bottom wash using a 96-well
Plate Washer
from Biotech that allows the creation of a constant flow of wash solution in
the well and was
very efficient for washing out an excess of peptides/proteins from the
electrode surface. The
washing with the Triton-containing solution was followed by 3x washes to
remove traces of
Triton. After blocking, the plate was washed again to remove blocking agent
prior to use.
For phosphorylation of PUT (tyrosine lcinase assay), 0.05 m1.1/1.1.1 of c-SRC
was used; for
phosphorylation of MBP (threonine kinase), 2 nM of ERK-2 was used. The capture
efficiency of
the Streptavidin-coated domain was determined by measuring the binding of
bovine IgG labeled
with biotin and a sulfonated form of Ru(bpy)3 (Sulfo-TAGTm label by Meso Scale
Discovery).
CA 02714094 2010-08-30
, .
WO 03/023360
PCT/US02/286:s2
Each spot (PGT, MBP, Streptavidin and BSA) was exposed to a solution of
unlabeled
primary antibodies directed against phosphotyrosine and phosphorylated MBP and
labeled
secondary antibodies. After incubating the plates to allow the enzyme and
binding reactions to
proceed, a TPA-containing buffer was added and the plates were analyzed by ECL
(no wash was
required). Each point includes an average of 12 measurements with CV's of 7¨
10%. Table A
below summarizes the results obtained from this experiment.
Table A
Domain No Enzyme/No bIgG* Analyte bIgG* Only SRC-only
ERK2-only
SA 272 5,447 324 309
PGT 990 953 17,223 1,153
MBP 1,241 1,354 1,237 32,810
BSA - 138 134 168 209
The bold faced values are specific signals; the other numbers are ECL due to
non-specific
interactions.
The PGT and MBP domains only showed high signal in the presence of the
tyrosine
kinase SRC and Threonine kinase (ERK2), respectively. Titration curves of the
activity of both
kinases (SRC and ERK) exhibited nearly linear response on corresponding
domains. The
Streptavidin domain gave a good signal in the presence of the biotinylated
analyte and did not act
as a substrate for the kinases. This result demonstrates the utility of
including a binding domain,
e.g., for capturing (and, optionally, purifying) kinases to be tested from
crude samples. The BSA
spot did not provide a significant signal in the presence of the
analyte/enzymes and shows that
the blocking agent did not show non-specific reactions with the assay
reagents.
97--
CA 02714094 2010-08-30
* 69331-63D
The present invention is not to be limited in scope by the specific
embodiments described
herein. Indeed, various modifications of the invention in addition to those
described herein will
become apparent to those skilled in the art from the foregoing description and
accompanying
figures. Such modifications are intended to fall within the scope of the
claims.
98