Note: Descriptions are shown in the official language in which they were submitted.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
1
PACKAGE FOR DISPENSING A PERSONAL CARE PRODUCT
FIELD OF THE INVENTION
The present invention is directed to packages for dispensing a liquid personal
care
product.
BACKGROUND OF THE INVENTION
Liquid personal care products are available in a wide variety of packages,
including
bottles, jars, tubes, and cans. Liquid personal care products meant for
application to the skin are
traditionally dispensed from a container onto users' hands and then applied to
the skin by hand.
Examples of such products include lotion, facial cleanser, and shaving cream.
This application
method however can be messy, and that portion of the product that does not get
transferred from
users' fingers is wasted.
Few liquid personal care products on the market have applicators or lathering
aids.
While patents relating to brush attachments for shaving cream in traditional
aerosol cans do
exist, there are no well-known lathering aids for shaving cream currently
available for consumer
purchase. U.S. Patent No. 4,603,992 (Kavoussi) (the "'992 Patent") is an
example of a
conventional aerosol shaving cream container with a shaving brush attachment.
The `992 Patent
is believed representative of the prior art: the container is cylindrical and
the shaving brush
attachment has a cylindrical fan shape that is rounded at the top. This sort
of applicator may not
work well to lather product onto smaller skin areas, such as the space between
the nose and
upper lip; an applicator with a shape other than cylindrical may work better.
But, if the shape of
the applicator is not cylindrical, it may be hard for the user to view the
orientation of the
applicator, particularly when the applicator contains foamed product and is
placed against the
skin. Accordingly, there is a need to inform users of the orientation of the
applicator as they
hold it close to the skin.
To solve these problems, a package with skin-friendly dispensing and lathering
capabilities may be used to apply and lather product directly onto the skin.
There is a desire to
develop a package that allows the product to be dispensed directly from a
container onto the
skin. A need exists for a container that better fits into a user's hand. The
package may comprise
a displacement member located remote from the portion of the package that
contacts the skin.
There exists a need for a flow-restricting member to keep water and other
impurities out of the
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
2
dispenser and container as well as prevent the product from flowing out of the
container unless
force is consciously exerted on the container to dispense the product. Also, a
need still exists for
an applicator and container that are ergonomically designed to lather product
on various skin
areas. As a result, if an applicator and/or container are not the traditional
round shape, a need
exists to inform the user of the positioning of the applicator in relation to
the positioning of the
container so the user may better line up the applicator with the area of skin
to be lathered. These
are all objects of the present invention; embodiments of the present invention
may combine
various objects mentioned. A particular embodiment may, but need not, embody
every object of
the invention.
SUMMARY OF THE INVENTION
The present invention is directed to a package for dispensing a liquid
personal care
product. A first exemplary embodiment comprises a body for grasping with a
user's hand,
wherein the body comprises a horizontal plane defining a first cross-sectional
shape. The
embodiment comprises an applicator comprising an applicator base and a skin-
contacting
portion extending from the applicator base to a first height. The embodiment
also comprises a
dispensing conduit through which a liquid personal care product is dispensable
from the body,
wherein the dispensing conduit comprises a proximal end and a distal end,
wherein the proximal
end comprises an inlet that is in fluid communication with the body, wherein
the distal end
extends from the applicator base to a second height that is less than the
first height. A flow-
restricting member is associated with the dispensing conduit.
A second exemplary embodiment comprises all of the elements of the first
exemplary
embodiment. In addition, the body comprises a horizontal plane defining a
first cross-sectional
shape and the skin-contacting portion comprises a horizontal plane defining a
second cross-
sectional shape. The distal end of the dispensing conduit extends from the
applicator base to a
second height that is about half the first height. The flow-restricting member
is located at or
near the distal end of the dispensing conduit. The flow-restricting member
comprises at least
two opposing members, wherein the members converge into a closed state and
diverge into an
open state, wherein the members are biased to the closed state to inhibit
water from entering the
dispensing conduit, and wherein the members may be forced into the open state
when a volume
of liquid personal care product is communicated from the body through the
dispensing conduit.
Each of the first cross-sectional shape and the second cross-sectional shape
comprises a major
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
3
dimension oriented along a first axis and a minor dimension oriented along a
second axis, and
wherein the major dimensions are greater than the minor dimensions.
BRIEF DESCRIPTION OF THE DRAWINGS
While the specification concludes with claims particularly pointing out and
distinctly
claiming the present invention, it is believed the same will be better
understood from the
following description taken in conjunction with the accompanying drawings in
which:
FIG. 1 is a perspective view of the top portion of a first exemplary
embodiment of the
present invention.
FIG. 2 is a front view of the embodiment shown in FIG. 1.
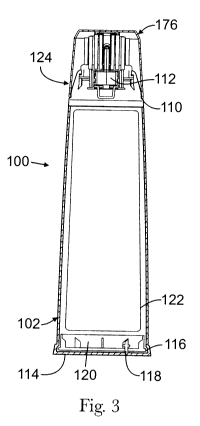
FIG. 3 is a vertical cross-sectional view of the embodiment shown in FIG. 1,
taken along
the line I-I.
FIG. 4 is a view of the top portion of the embodiment shown in FIG.3.
FIG. 5 is a vertical cross-sectional view of the top portion of the embodiment
shown in
FIG. 1, taken along the line II-II.
FIG. 6 is a front view of a second exemplary embodiment of the present
invention.
FIG. 7 is a side view of the embodiment shown in FIG. 6.
FIG. 8 is a vertical cross-sectional view of the embodiment shown in FIG. 6,
taken along
the line III-III.
FIG. 9 is a view of the top portion of the embodiment shown in FIG. 8.
FIG. 10 is a vertical cross-sectional view of the top portion of the
embodiment shown in
FIG. 6, taken along the line IV-IV.
FIG. 11 is a perspective view of the top portion of the embodiment shown in
FIG. 6.
FIG. 12 is a horizontal cross-sectional, perspective view of the embodiment
shown in
FIG. 8, taken along the line V-V.
FIG. 13 is a view of the top portion of the embodiment shown in FIG. 9.
FIGS. 14a-f are examples of cross-sectional shapes the package may comprise.
DETAILED DESCRIPTION OF THE INVENTION
The present invention may be understood more readily by reference to the
following
detailed description of illustrative and preferred embodiments. It is to be
understood that the
scope of the claims is not limited to the specific ingredients, methods,
conditions, devices, or
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
4
parameters described herein, and that the terminology used herein is not
intended to be limiting
of the claimed invention. Also, as used in the specification, including the
appended claims, the
singular forms "a," "an," and "the" include the plural, and reference to a
particular numerical
value includes at least that particular value, unless the context clearly
dictates otherwise. When
a range of values is expressed, another embodiment includes from the one
particular value
and/or to the other particular value. Similarly, when values are expressed as
approximations, by
use of the antecedent basis "about," it will be understood that the particular
values form another
embodiment. All ranges are inclusive and combinable.
All percentages and ratios used herein are by weight of the total composition,
and all
measurements made are at 25 C, unless otherwise designated.
The packages of the present invention can comprise, consist of, and consist
essentially of
the features of the invention described herein, as well as any of the
additional or optional
ingredients, components, steps, or limitations described herein.
The term "latherable" as used herein refers to a liquid personal care product
that is able
to form a foam or froth when agitated or spread onto the skin. The liquid
personal care product
may lather by entrapping air, by comprising a surfactant-type substance, or by
another means
known to those of ordinary skill in the art. Water addition may or may not be
necessary to
obtain a foam or froth, but it may enhance benefits or results depending on
the type of product
used. Agitation or spreading may be by hand, but is preferably by applicator.
Agitation or
spreading may be rapid or slow, and may comprise irregular and regular
movements.
The term "lathering" as used herein refers to the act of agitating or
spreading onto the
skin a liquid personal care product to produce a foam or froth. For instance,
a product may be
dispensed as a gel and then lathered into a foam. If a product is already in
the form of a foam or
froth when dispensed from its package, lathering may comprise spreading
product onto the skin
to cover an area of skin.
The following exemplary packages further describe and demonstrate embodiments
within the scope of the present invention. The examples are given solely for
the purpose of
illustration and are not to be construed as limitations of the present
invention as many variations
thereof are possible without departing from the spirit and scope of the
invention. It is therefore
intended to cover in the appended claims all such changes and modifications
that are within the
scope of this invention.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
A first exemplary package 100 is shown in FIGS. 1 to 5. FIG.1 illustrates the
top portion
of package 100, comprising a body 102, and an applicator 124. Body 102 is able
to be grasped
with a user's hand; it comprises a horizontal plane defining a first cross-
sectional shape 104.
Cross-sectional shape 104 comprises a major dimension 106 oriented along a
first axis and a
minor dimension 108 oriented along a second axis. Body 102 may be provided in
a variety of
forms or shapes and may be made of various materials. Examples of suitable
plastic materials
include high density polyethylene ("HDPE"), low density polyethylene ("LDPE"),
polyethylene
terephthalate ("PET"), polypropylene ("PP"), polyvinyl chloride,
polycarbonate, nylon, and
fluorinated ethylene propylene. Body 102 may be made via a number of processes
known in the
art, such as blow molding, injection molding, and the like. Body 102 may be
comprised of
transparent, translucent, or opaque materials, or it may be comprised of a
combination of
materials with these properties. In a preferred embodiment, body 102 is opaque
and made of
thermoplastic resin via an extrusion blow molding process. In other
embodiments, body 102
may be transparent to show unique color, texture, pattern, or inclusions of a
liquid personal care
product; for example, moisturizing beads or white color to signify suitable
for use on sensitive
skin.
Many prior art packages have applicators which are attachable, but not
integral to the
package. Applicator 124 may be attachable or removeably attached to body 102,
such as with
screw-threads, a snap-fit collar, or the like. In a preferred embodiment,
applicator 124 is integral
with body 102, which means that the applicator is assembled with the body
during
manufacturing and is not intended to be removed by a consumer during the
product lifetime.
Applicator 124 comprises a skin-contacting portion 126 comprising a horizontal
plane defining a
second cross-sectional shape 128. Cross-sectional shape 128 comprises a major
dimension 130
oriented along a first axis and a minor dimension 132 oriented along a second
axis. Skin-
contacting portion 126 may comprise bristles, a sponge, a loofah, a shower
puff, or another
material or combination of materials that is capable of lathering a liquid
personal care product.
Skin-contacting portion 126 may be rinsed after use.
In a preferred embodiment, at least a part of skin-contacting portion 126 is
designed to fit
between a user's nose and upper lip. As such, minor dimension 132 of skin-
contacting portion
126 may be less than or equal to about 0.75 inches. In another embodiment,
minor dimension
132 of skin-contacting portion 126 may be less than or equal to about 0.5
inches. In a preferred
embodiment, major dimension 130 of skin-contacting portion 126 is less than or
equal to about
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
6
1.5 inches. Major dimension 130 and minor dimension 132 may vary depending on
the part of
the skin skin-contacting portion 126 is meant to contact. For example,
dimensions 130,132 may
be larger if skin-contacting portion 126 is meant for users' legs, or smaller
if skin-contacting
portion 126 is meant for users' underarms or bikini area.
In one embodiment, such as shown in FIG. 2, major dimension 106 of body 102
substantially aligns with major dimension 130 of skin-contacting portion 126.
Also as shown in
FIGS. 1 and 2, first cross-sectional shape 104 and second cross-sectional
shape 128 are in
vertical alignment. In other embodiments, cross-sectional shape 104 and cross-
sectional shape
128 may not be in vertical alignment. For example, skin-contacting portion 126
may be offset
from body 102 so that skin-contacting portion 126 is not positioned vertically
above body 102,
but rather extends from the side of body 102. Skin-contacting portion 126 may
also extend
horizontally from body 102, or from any angle in between; skin-contacting
portion 126 may be
fixed at a certain angle or it may be adjustable by users. Cross-sectional
shapes 104,128 of body
102 and applicator 124, respectively, may take different forms. In various
embodiments, as
shown in FIGS. 14a-f, first cross-sectional shape 104 and second cross-
sectional shape 128 may
be oblong circles, squares, or triangles; further exemplary shapes include
teardrop and pear. The
shapes 104,128 may substantially match or not match. For example, in one
embodiment, cross-
sectional shape 104 may have an oval shape while cross-sectional shape 128 may
have a
teardrop shape. Cross-sectional shapes 104,128 may be symmetrical or
asymmetrical. FIG. 1
illustrates cross-sectional shape 104 and cross-sectional shape 128 as
symmetrical, oblong
circles. In further embodiments, package 100 may comprise more than two
distinct cross-
sectional shapes.
Referring to FIG. 3, body 102 has an outlet 112 that is in fluid communication
with
applicator 124. Body 102 comprises a flat surface 114 that package 100 rests
on when it is not
in use. In a preferred embodiment, the bottom portion of body 102 may comprise
a lower gasket
116, an umbrella valve 118, and a bottom or bottom plate 120. Lower gasket 116
is preferably
made of a thermoplastic elastomer ("TPE") and bottom plate 120 is preferably
made of PP. It
should be noted that these aforementioned features, as well as other features
described
throughout, are not limiting on the scope of the appended claims where such
features are not
explicitly recited.
FIG. 4 illustrates applicator 124. In one embodiment, skin-contacting portion
126
extends to a first height 134 from applicator base 136, wherein first height
134 is equal to the
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
7
highest point of skin-contacting portion 126. In other embodiments, skin-
contacting portion 126
may extend to various heights in various zones, so there may be more than one
height of interest.
In a preferred embodiment, applicator base 136 is made out of PP and comprises
a plurality of
fiber receiving holes 140 to receive fibers 142 to form skin-contacting
portion 126. Applicator
base 136 comprises an orifice 144 to enable dispensing of product onto skin-
contacting portion
126. To prevent dispensed product from getting stuck in the bottom half of
skin-contacting
portion 126 or clogging outlet 112, one or more dispensing conduits 146 help
direct the product
to a preferred location within skin-contacting portion 126. This makes more
efficient use of the
product because more product is available for lathering onto the skin rather
than filling up the
lower portion of skin-contacting portion 126. FIG. 4 shows dispensing conduit
146 having a
proximal end 148 and a distal end 154. Proximal end 148 has an inlet 150 that
is in fluid
communication with outlet 112 of body 102 and a compression seal 152. In a
preferred
embodiment, a sufficient length of dispensing conduit 146 extends into skin-
contacting portion
126, with distal end 154 located somewhat close to a surface that contacts the
skin, thereby
dispensing more product closer to the skin. However, dispensing conduit 146
should not extend
too far into skin-contacting portion 126 or it might too aggressively contact
or scratch a user's
skin when in use. As such, in one embodiment, distal end 154 extends from
applicator base 136
to a second height 156 that is less than first height 134 of skin-contacting
portion 126. In this
configuration, distal end 154 may still contact a user's skin during use, but
desirably not to the
point of causing significant irritation to one's skin. Dispensing conduit 146
may be comprised
of a variety of materials. For example, dispensing conduit 146 may be made of
hard plastic and
precautions may be taken by the manufacturer or user to prevent dispensing
conduit 146 from
contacting the skin and causing discomfort, scratches, or injury to the skin.
However, in a
preferred embodiment, dispensing conduit 146 is made of a softer material; one
exemplary
material is TPE.
A safety cap 158 may be provided to cover dispensing conduit 146. Safety cap
158
prevents damage or disfigurement to skin-contacting portion 126 and it may
also prevent
leakage of product from dispensing conduit 146. In a particular embodiment,
safety cap 158 is a
temporary cover and may be removed and discarded by the consumer after
purchase. In a
preferred embodiment, safety cap 158 is made of PP.
Package 100 optionally comprises an over cap 176 that is removeably attached
to body
102 or applicator 124. Over cap 176 may comprise an outer ring 178 to compress
safety cap 158
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
8
and an inner ring 180 to contain safety cap 158. In a particular embodiment, a
ledge 182 on over
cap 176 allows over cap 176 to snap into a notch 110 of body 102. Over cap 176
may also
comprise a flat surface 184, allowing package 100 to be inverted, thus resting
on flat surface 184
when not in use. In one embodiment, over cap 176 is made of PP.
In particular embodiments, as shown in FIG. 5, a valve 186 may be disposed
between
body 102 and applicator 124. In a preferred embodiment, valve 186 comprises an
upper gasket
188 made of TPE or rubber, and a transition piece 190, a one-way valve 192,
and a valve cover
194, all three made of PP.
Some embodiments of the present invention may employ one or more flow-
restricting
members 160 associated with dispensing conduit 146. In a preferred embodiment,
flow-
restricting member 160 is positioned at or near distal end 154 of dispensing
conduit 146. Flow-
restricting member 160 acts to inhibit and/or prevent water from entering
dispensing conduit
146, while also preventing product from flowing out of package unless force is
consciously
exerted on a displacement member to dispense the product. Dispensing of the
product may then
be more accurately controlled and unwanted dispensing of the product may be
substantially
prevented. "Flow-restricting member" 160 as used herein means any of numerous
mechanical
devices by which the flow of a liquid personal care product may be started,
stopped, or regulated
by a movable part that opens, shuts, or partially obstructs one or more ports,
orifices or
passageways. Flow-restricting member 160 may be manually openable or pressure
activated.
Flow-restricting members 160 may be any valve known in the art including but
not limited to bi-
directional, uni-directional, duckbill, claw, umbrella, cross-slit, and slit
valves.
In one particular embodiment, flow-restricting member 160 has at least two
opposing
members 162, wherein members 162 converge into a closed or substantially
closed state, and
diverge into an open state. Members 162 are biased to the closed state to
inhibit and/or prevent
water or impurities from entering and clogging dispensing conduit 146, or to
inhibit
contamination or dilution of the product within dispensing conduit 146 or body
102. For
example, a user may rinse skin-contacting portion 126 under running water
after use; the water
can clog dispensing conduit 146 or dilute or contaminate the product if it
gets inside body 102.
Another reason members 162 are biased to the closed state is to prevent
unwanted leakage of the
product if package 100 is inverted while not in use. Members 162 may be forced
into the open
state when a volume of product is communicated from body 102 through
dispensing conduit 146
in response to manual forces being applied to a displacement member. Flow-
restricting member
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
9
160 returns to its closed position upon removal of the manual displacement
forces which results
in stopping the dispensing of the product through dispensing conduit 146.
In another particular embodiment, flow-restricting member 160 comprises a slit
valve
164. "Slit valve" 164 as used herein means a valve whose members are formed
from incision(s)
that have a closed and open position as described above. Preferably, slit
valve 164 is made of a
relatively flexible material, such as silicone rubber, polyvinyl chloride,
urethane, ethylene vinyl
acetate, styrene-butadiene copolymer, and other materials known to those of
ordinary skill in the
art. In one embodiment, slit valve 164 is made of silicone. The stiffness of
slit valve 164 in one
embodiment is sufficient to prevent the slit from opening under the
hydrostatic pressure of a
liquid personal care product when package 100 is placed in an inverted
position.
Body 102 may contain a liquid personal care product. Alternatively, body 102
may
comprise a flexible bag 122 to contain a liquid personal care product;
flexible bag 122 fits
within body 102 and acts as a bladder. In a preferred embodiment, flexible bag
122 attaches to
transition piece 190. Flexible bag 122 may be made out of HDPE, LDPE, mylar
film, or other
suitable material known to one of ordinary skill in the art.
Package 100 comprises a means to dispense a product contained within package
100. In
one embodiment, body 102 is flexible and resilient, and it is inwardly
deformable along its
minor dimension 108 in order to urge a volume of a liquid personal care
product from within
body 102 to skin-contacting portion 126.
In other embodiments, package 100 comprises a displacement member 166 that
urges the
product out of body 102 and onto skin-contacting portion 126. Displacement
member 166 may
be located away from skin-contacting portion 126 to allow product to be
manually dispensed by
the user while skin-contacting portion 126 is in contact with the skin. In a
preferred
embodiment, such as in FIG. 2, displacement member 166 comprises at least a
portion, or
sidewall, of body 102; wherein the sidewall (not shown) of body 102 is
flexible, resilient, and
deformable along its minor dimension 108. In one embodiment, two sidewalls are
located along
either side of body 102 along major dimension 106. In another embodiment,
displacement
member 166 is a piston (not shown) that is vertically moveable upward from
bottom 120 of
body 102. In one embodiment, the piston or similar member may extend partially
within body
102 to dispense a liquid personal care product. A further example of
displacement member 166
is a trigger, as shown in FIGS. 6-12 and discussed more fully below.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
A second exemplary package 200 is shown in FIGS. 6 to 13. Package 200 has many
of
the same features as package 100; most features are numbered consistently
between the two,
with a difference in the hundreds numeral, for example, body 102 of package
100 vs. body 202
of package 200. The descriptions, dimensions, and variations of the features
of package 100
may be applied to the corresponding features of package 200 discussed below,
unless otherwise
noted. In a preferred embodiment, body 202 is opaque and made of thermoplastic
resin via an
extrusion blow molding process.
FIGS. 7 and 8 depict package 200 comprising a body 202 and an applicator 224.
Body
202 of package 200 comprises a horizontal plane defining a first cross-
sectional shape 204. First
cross-sectional shape 204 comprises a major dimension 206 oriented along a
first axis, and a
minor dimension 208 oriented along a second axis, as shown in FIGS. 7 and 8.
Applicator 224
comprises a skin-contacting portion 226, wherein skin-contacting portion 226
comprises a
horizontal plane defining a second cross-sectional shape 228. Second cross-
sectional shape 228
comprises a major dimension 230 oriented along a first axis and a minor
dimension 232 oriented
along a second axis, as shown in FIGS. 7 and 8. In a preferred embodiment,
major dimension
206 of body 202 substantially aligns with minor dimension 230 of skin-
contacting portion 226,
and first cross-sectional shape 204 and second cross-sectional shape 228 are
in vertical
alignment. Also in a preferred embodiment, package 200 has first cross-
sectional 204 and
second cross-sectional 228 shapes that are symmetrical, oblong circles. Body
202 comprises a
flat surface 220. In a preferred embodiment, body 202 comprises an upper body
210 and a
lower body 216.
FIG. 9 illustrates body 202 having an outlet 214 that is in fluid
communication with
applicator 224. Upper body 210 comprises a notch 212, where an optional over-
cap 276 snaps
on. Lower body 216 comprises a body cavity 218, wherein a flexible bag 222
fillable with a
liquid personal care product fits within body cavity 218. FIG. 9 also
illustrates a dispensing
conduit 246, a flow-restricting member 260, a displacement member 266, and
optional valve
286 of package 200.
As seen in FIG. 10, skin-contacting portion 226 of applicator 224 extends to a
first height
234 from an applicator base 236. In a preferred embodiment, applicator base
236 comprises a
plurality of fiber receiving holes 240 that receive fibers 242 to form skin-
contacting portion 226.
Applicator base 236 comprises an orifice 244 to enable dispensing of product
onto skin-
contacting portion 226.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
11
FIG. 11 illustrates dispensing conduit 246, and its proximal end 248 and
distal end 254.
Proximal end 248 has an inlet 250 and a compression seal 252. Distal end 254
has a second
height 256. A safety cap 258 may be provided to cover dispensing conduit 246
and prevent
leakage. Flow-restricting member 260 is associated with dispensing conduit
246. In a preferred
embodiment, flow-restricting member 260 comprises opposing members 262. In
other
embodiments, flow-restricting member 260 comprises a valve, such as a slit
valve 264.
In particular embodiments, as shown in FIGS. 9-11, a valve 286 may be disposed
between body 202 and applicator 224. In a preferred embodiment, best seen in
FIG. 10, valve
286 may comprise a stationary piston 288, a compression spring 290, a collar
292, a ball 296,
and a one-way valve 298. In one embodiment, stationary piston 288 and collar
292 are made of
PP, while compression spring 290 and ball 296 are made of stainless steel.
FIGS. 8-12 depict displacement member 266 which is associated with body 202.
Displacement member 266 is inwardly moveable in order to urge a volume of a
liquid personal
care product from within flexible bag 222 to skin-contacting portion 226. In a
preferred
embodiment, displacement member 266 is a trigger 268 that is depressible along
body's 202
major dimension 206. In one embodiment, a rib 272 from upper body 210 locks
trigger 268 in
place. In FIG. 11, a trigger holder 274 extends off applicator base 236 to
hold trigger 268 in
place; trigger 268 has two arms which hug the collar 292. Two L ribs 270 and
the tail of trigger
268 are visible in body cavity 218, as seen in FIG. 12. L ribs 270 are
optional; their purpose is
to prevent trigger 268 from damaging or pinching flexible bag 222. In
operation, trigger 268
displaces a moving cylinder 294 which in turn displaces a volume of the
product from flexible
bag 222 to skin-contacting portion 226. In a particular embodiment, trigger
268, L ribs 270, rib
272, trigger holder 274, and moving cylinder 294 are made of PP.
FIGS. 9 and 13 best show an optional over cap 276 that is removeably attached
over
applicator 224. Over cap 276 comprises an outer ring 278 to compress safety
cap 258 and an
inner ring 280 to contain safety cap 258. A ledge 282 on over cap 276, visible
in FIG. 9, allows
over cap 276 to snap into notch 212 of body 202. Over cap 276 may also
comprise a flat surface
284, allowing package 200 to be inverted, thus resting on flat surface 284
when not in use.
In an exemplary method of use, after obtaining package 100,200, a user may
remove
optional over cap 176,276 and safety cap 158,258. If preferred, the user may
wet skin-
contacting portion 126,226. The user may then hold skin-contacting portion
126,226 against the
desired skin surface and deform body 102,202 or move displacement member
186,266 inwardly.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
12
In response, an amount of product is forced out of package 100,200 via
dispensing conduit
146,246 to skin-contacting portion 126,226. As the user moves skin-contacting
portion 126,226
around on the skin surface, the product dispensed is lathered by skin-
contacting portion 126,226.
In an example where the product is a shave prep composition, the user may
create a lather with
skin-contacting portion 126,226, then use a razor to shave the prepared area.
In an example
where the product is a facial or body cleanser, skin-contacting portion
126,226 may be used to
exfoliate and cleanse the skin while creating a lather. In particular
embodiments, when the first
axes of body 102,202 and skin-contacting portion 126,226 are in alignment, the
user may more
easily determine the orientation of skin-contacting portion 126,226 because
body 102,202
visually intimates the positioning of skin-contacting portion 126,226. This
may be important,
for example, if a user is trying to lather the area between the nose and upper
lip.
It is contemplated that the package 100,200 of the present invention may
include
alternate mechanisms for dispensing the personal care compositions of the
present invention.
These include electronic pumps, manual pumps, screw-containing mechanisms,
aerosols,
pressurized gasses, trigger pumps, and the like. One of ordinary skill would
readily be able to
integrate the mechanism for dispensing the personal care composition into the
present invention.
Packages 100,200 of the present invention may contain a wide variety of liquid
personal
care products, preferably those that are latherable by skin-contacting portion
126,226. Exemplary
product forms include gels, creams, foaming and non-foaming liquids,
mechanically pumpable
liquids, non-aerosol gels, aerosol gels, aerosol foams, pastes, serums, and
sprays. Examples of
suitable products to be contained in body 102,202 or flexible bag 122,222
include body wash,
body lotion, facial cleanser, facial lotion, shampoo, conditioner, deodorant,
shave gel or cream,
self tanner, nail polish, nail polish remover, and other personal care
products. In a preferred
embodiment, the liquid personal care product is a shave prep composition. The
following six
examples of shave prep products are given solely for the purpose of
illustration and are not to be
construed to limit scope of the present invention as many variations thereof
are possible without
departing from the spirit and scope of the invention.
Ex. 1 Ex. 2 Ex. 3 Ex. 4 Ex. 5 Ex. 6
Ingredient Base Base Base Base Base Base
Water QS QS QS QS QS QS
Palmitic Acid 7.75 6.0 0 0 6.0 0
Triethanolamine 6.05 4.7 8.76 8.76 4.7 8.76
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
13
Stearic Acid 2.6 2.0 0 0 2 0
Myristic Acid 0 0 14.0 14.0 0 14.0
Mineral Oil kaydol 0 0 0 0 1.5 0
GlycerylOleate 2.0 2.0 1.5 1.5 2.5 1.5
Sorbitol (70% solution) 1.0 1.0 0 0 0.5 0
Hydroxyethylcellulose 0.5 0.3 0.75 0.75 0.3 0.75
Polyox WSR-301 (PEG-90M) 0.06 0.06 0 0 0.06 0
Polyox WSR-N-12K (PEG-23M) 0.05 0.05 0 0 0.05 0
Sodium Myristoyl Sarcosinate (30%) 1.0 0 6.67 6.67 0 6.67
Polymer LR30M cationic 0.1 0.2 0.45 0.45 0.2 0.45
Fragrance 0.85 0.85 0.85 0.85 0.85 0.85
PVP K60 (45% solution) 0 0 0.49 0.49 0 0.49
Glycerin 0.5 1.5 8.5 8.5 0.5 8.5
Colorant 0.002 0.002 0.002 0.002 0.002 0.002
Parts of Base Mixed w/Lathering 97.15 97.15 96 97.15 97.15 100
Agent
Volatile Lathering Agent 2.85 2.85 4.0 2.85 2.85 0
The above examples can be made as follows: Mix the water with glycerin and
then add to this
mixture a pre-blend of the LR30M and hydroxyethylcellulose powders. For
formulas containing
sodium myristoyl sacrosinate, add this component next. For formulas containing
PVP K60, add
this component next. Mix until homogeneous and start heating. Stop mixing, and
then add the
fatty acids. When the temperature reaches 75 C, add triethanolamine and mix
for approximately
more minutes. Cool to less than 35 C, and then add the fragrance and colorant.
For example
6, store in closed container until it is charged into an appropriate
dispenser. For examples 1-5,
combine the base composition with the lathering agent and charge the mixture
into containers or
packages capable of containing volatile agents.
As noted above, the applicator or skin-contacting portion thereof may comprise
a number
of different materials and material forms, such as, bristles, foam pads,
sponges, etc. Regarding
the use of bristles, material and physical properties of bristles can impact
skin feel and
latherability, for example. Table 1 and the following discussion illustrate
this notion.
Table 1 (Dimensions are in inches)
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
14
Bristle Material Bristle Diameter Bristle Length Skin-Contacting Skin-
Contacting
Major Dimension Minor Dimension
Nylon 0.006 0.68 1.12 0.73
Nylon 0.008 0.55 1.12 0.73
Nylon 0.006 0.7 1.17 0.73
Nylon 0.008 0.54 1.17 0.73
Nylon 0.008 0.65 1.19 0.66
Nylon 0.008 0.55 1.19 0.66
Nylon 0.003 0.7 1.18 0.73
Nylon 0.004 0.7 1.18 0.73
Nylon 0.003 0.5 1.18 0.73
Nylon 0.004 1.0 1.18 0.73
Silicone 0.06 0.53 0.73 0.63
Consumers perceived the nylon bristles having a bristle diameter of 0.006 or
0.008 inches to be
too stiff, and nylon bristles having a bristle diameter of 0.003 or 0.004 as
being in the right range
for softness. Bristles having a "mid-length" (that is those of 0.7 inches)
were preferred for
stiffness over those that were shorter or longer.
The dimensions and values disclosed herein are not to be understood as being
strictly
limited to the exact numerical values recited. Instead, unless otherwise
specified, each such
dimension is intended to mean both the recited value and a functionally
equivalent range
surrounding that value. For example, a dimension disclosed as "40 mm" is
intended to mean
"about 40 mm."
Every document cited herein, including any cross referenced or related patent
or
application, is hereby incorporated herein by reference in its entirety unless
expressly excluded
or otherwise limited. The citation of any document is not an admission that it
is prior art with
respect to any invention disclosed or claimed herein or that it alone, or in
any combination with
any other reference or references, teaches, suggests or discloses any such
invention. Further, to
the extent that any meaning or definition of a term in this document conflicts
with any meaning
or definition of the same term in a document incorporated by reference, the
meaning or
definition assigned to that term in this document shall govern.
CA 02715516 2010-08-13
WO 2009/124049 PCT/US2009/038953
While particular embodiments of the present invention have been illustrated
and
described, it would be obvious to those skilled in the art that various other
changes and
modifications can be made without departing from the spirit and scope of the
invention. It is
therefore intended to cover in the appended claims all such changes and
modifications that are
within the scope of this invention.