Note: Descriptions are shown in the official language in which they were submitted.
CA 02717104 2010-08-26
102 CA024P 1
STAINLESS STEEL USED FOR OIL COUNTRY TUBULAR GOODS
TECHNICAL FIELD
The present invention relates to stainless steel and more specifically
to stainless steel used for oil country tubular goods for use in gas wells or
oil wells.
BACKGROUND ART
Oil or natural gas produced from oil wells or gas wells contains
associated corroding gas as such as carbon dioxide gas and hydrogen sulfide.
Therefore, oil country tubular goods used for producing oil or natural gas
need high corrosion resistance.
Carbon steel or low alloy steel has been used as a steel for oil
country tubular goods. As the goods have come to be used in a tougher
corroding environment in an oil well or a gas well, SUS420 martensitic
stainless steel (13% Cr-based steel) having a Cr content of about 13% or
stainless steel having high corrosion resistance such as improved 13% Cr-
based steel produced by adding Ni to the 13% Cr-based steel has been used.
Recently, deep oil or gas well drilling has created a demand for oil
country tubular goods having higher strength for such deep oil or gas wells.
Furthermore, in a deep oil or gas well, a high temperature chloride aqueous
solution environment as high as 150 C or more including hydrogen sulfide
and carbon dioxide gas is created, and even higher corrosion resistance
than the conventional oil country tubular good is required. In such a high
temperature chloride aqueous solution environment including hydrogen
sulfide and carbon dioxide gas, two-phase stainless steel having corrosion
resistance and strength higher than conventional stainless steel may be
used. The two-phase stainless steel however contains a large amount of
alloy elements and therefore the manufacturing cost is high.
JP 2005-336595 A (hereinafter referred to as "Patent Document 1"),
JP 2006-16637 A (hereinafter referred to as "Patent Document 2"), and JP
2007-332442 A (hereinafter referred to as "Patent Document 3") propose the
- 1 -
CA 02717104 2010-08-26
102CA024P1
use of stainless steel pipes containing less alloy elements than the two-
phase stainless steel and having high strength and high corrosion
resistance in a high temperature chloride aqueous solution environment
including carbon dioxide gas. The stainless steel pipes disclosed by these
patent documents each have a greater Cr content than the conventional
13% Cr-based steel, such that the corrosion resistance can be improved.
More specifically, in the disclosure of Patent Document 1, the Cr
content of the stainless steel pipe is from 15.5% to 18% which is greater
than that of the conventional 13% Cr-based steel. Furthermore, when
Cr+Mo-1-0.3Si-43.5C-0.4Mn-Ni-0.3Cu-911.5 is established, the steel has a
two-phase structure including a ferrite phase and a martensite phase, so
that the hot workability of the oil country tubular good is improved. The
two-phase structure could lower the corrosion resistance, while when Ni,
Mo, and Cu that improve the corrosion resistance are added such that
Cr+0.65Ni+0.6Mo+0.55Cu-20C19.5 is established, the reduction in the
corrosion resistance of the oil country tubular good is prevented.
Similarly, according to the disclosure of Patent Document 2, the Cr
content of the stainless steel is from 15.5% to 18% and Ni that improves the
corrosion resistance is contained. The chemical composition of the
stainless steel disclosed by the document is similar to that in Patent
Document 1, but Mo is not an essential element, and therefore a less costly
alloy design is proposed. In addition, Cu is also an optional element.
The stainless steel disclosed by Patent Document 3 contains 14% to
18% Cr as well as Ni, Mo, and Cu, so that high corrosion resistance is
obtained. Furthermore, the steel includes a martensite phase and 3% to
15% austenite phase by volume, and therefore the toughness improves.
The kinds of stainless steel disclosed by Patent Documents 1 to 3
surely contain a larger amount of Cr than the conventional 13% Cr-based
steel and alloy elements such as Ni, Mo, and Cu are added, so that the
corrosion rate in a high temperature corroding environment is reduced.
For example, in an embodiment in Patent Document 1, using a 20 wt%
NaCl aqueous solution at 230 C in a 100 atm CO2 atmosphere, the
corrosion rate (mm/yr) was examined and it was established that the
- 2 -
CA 02717104 2010-08-26
102CA024P1
corrosion rate was reduced (see Table 2 in Patent Document 1).
However, it was found based on the inventors' investigation that the
use of stainless steel having a high Cr content in a high temperature
chloride aqueous solution environment containing carbon dioxide gas
lowers the corrosion rate but SCC (Stress Corrosion Cracking) is more
likely to be caused.
In conventional stainless steel such as 13% Cr steel, the corrosion
rate is extremely high in a high temperature chloride aqueous solution
environment. Therefore, while general corrosion is generated, SCC that is
local cracking is not generated. On the other hand, when the Cr content is
larger than that in the conventional stainless steel, the corrosion rate is
lowered as disclosed by Patent Documents 1 to 3. The reduction in the
corrosion rate is caused by a passive film that forms on the surface of the
stainless steel. However, the passive film is locally weakened and
destroyed in a high temperature environment. The destroyed part is more
likely to dissolve, and this dissolution is probably the cause for SCC.
Therefore, in stainless steel used in a high temperature chloride
aqueous solution environment containing carbon dioxide gas, it is necessary
not only to reduce the corrosion rate but also to improve the SCC resistance.
SUMMARY OF THE INVENTION
It is an object of the present invention to provide stainless steel for
an oil country tubular goods having high corrosion resistance in a carbon
dioxide gas contained, high temperature chloride aqueous solution
environment at 150 C or higher. More specifically, it is to provide stainless
steel for an oil country tubular goods having a reduced corrosion rate and
high SCC resistance in a carbon dioxide gas contained, high temperature
chloride aqueous solution environment.
The inventors considered that it would be necessary to add at least
16 mass % Cr and a small amount of Mo to steel in order to reduce the
corrosion rate in a carbon dioxide gas contained, high temperature chloride
aqueous solution environment at 150 C or higher. However, Cr and Mo are
- 3 -
CA 02717104 2010-08-26
102CA024P1
ferrite forming elements and therefore if at least 16 mass % Cr and a small
amount of Mo are contained, a major part of the structure of the steel
becomes a ferrite phase and therefore high strength cannot be obtained.
On the other hand, an austenite phase at high temperatures is
stabilized by adding Ni that is an austenite forming element, so that a
martensite phase is formed by quenching and a high strength steel
structure is obtained. However, if the amount of Ni is too large, the
starting temperature for martensite transformation (Ms point) is lowered
and therefore martensite transformation is not generated even at room
temperatures, so that high strength is not provided. Therefore, when the
Ni content is appropriately adjusted, a structure mainly including a
martensite phase and about at least 10% ferrite phase by volume is formed
and high strength can be provided.
The copper (Cu) effectively enhances a ferrite phase, and therefore a
high strength structure can be provided by adding Cu. In addition, Cu
reduces the corrosion rate in a high temperature chloride aqueous solution
environment and improves the SCC resistance.
Based on the foregoing findings, the inventors concluded that
stainless steel having prescribed strength and reduced corrosion rate can be
provided when the steel contains 16% to 18% Cr, more than 2% and not
more than 4% Mo, 3.5% to 7% Ni, and 1.5% to 4% Cu.
The inventors also found that by adding at least a prescribed
amount of an earth rare metal (REM) in the chemical composition described
above, high SCC resistance results even in a carbon dioxide gas contained,
high temperature chloride aqueous solution environment. Now, this will
be described in detail.
The inventors prepared stainless steel having the chemical
compositions in Table 1 and these kinds of stainless steel were evaluated for
their SCC resistance.
- 4 -
CA 02717104 2010-08-26
102CA024P1
Table 1
Steel Chemical composition (unit: mass %, the balance consisting of Fe and
impurities)
No. C Si Mn P S r Cu Cr Ni Mo sol.A1 Ca N 0 REM
Al 0.019 0.31 0.51 0.016 0.0009 1.9 17.1 3.9 2.4 0.029 0.0010 0.020 0.003
0.0001
A2 0.018 0.30 0.55 0.015 0.0010 2.0 17.2 4.2 2.5 0.030 0.0008 0.018 0.005
0.0002
A3 0.021 0.29 0.52 0.017 0.0010 2.1 16.9 4.1 2.5 0.029 0.0013 0.016 0.006
0.0005
A4 0.020 0.29 0.49 0.016 0.0008 2.0 17.0 4.0 2.6 0.028 0.0016 0.019 0.003
0.0011
A5 Ø019 0.31 0.51 0.015 0.0010 1.9 17.2 4.1 2.4 0.032 0.0011 0.022 0.005
0.0028
A6 0.019 0.31 0.50 0.016 0.0009 1.9 17.1 4.1 2.4 0.029 0.0009 0.018 0.003 0.03
Referring to Table 1, in the stainless steel grades with Nos. Al to A6,
the chemical compositions are the same except for REM. The REM
content is different among the numbered stainless steel grades in the range
from 0.0001% to 0.03%. Furthermore, these numbered stainless steel
grades were subjected to quenching-tempering such that the yield stress of
each kind of stainless steel was adjusted in the range from 860 MPa to 900
MPa. The structures of these numbered stainless steel grades include, in
volume percentage, 60% martensite phase, 30% ferrite phase, and 10%
austenite phase.
A specimen for four-point bending test having a length of 75 mm, a
width of 10 mm, and a thickness of 2 mm was sampled from each of the
numbered stainless steel grades. The sampled specimens were subjected
to a bending load by four-point bending. At the time, the bending amount
of each specimen was determined according to ASTM G39 such that stress
applied on each specimen was equal to the yield stress of each specimen.
Each bent specimen was immersed for one month in a 25 wt% NaC1
aqueous solution in an autoclave at 204 C (400F) having CO2 enclosed
therein under a pressure of 30 atm. After the immersion for one month,
each specimen was examined for the presence of SCC. More specifically, a
longitudinal section of each specimen was observed with a 100x
magnification optical microscope and determined for the presence/absence
of SCC by visual inspection.
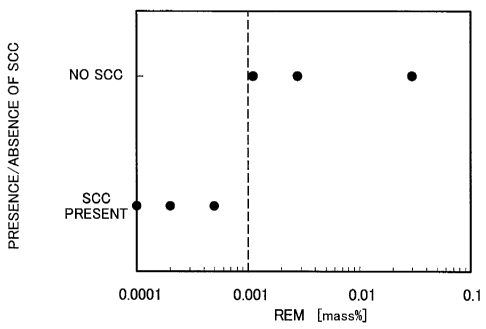
The test result is given in Fig. 1. In Fig. 1, the abscissa represents
- 5 -
CA 02717104 2010-08-26
102CA024P1
the REM content (% by mass) and the ordinate represents the
presence/absence of SCC. In Fig. 1, "=" in the "SCC present" on the
ordinate indicates the presence of SCC, while "0" in the "no SCC" indicates
the absence of SCC. As can be clearly seen from Fig. 1, when the REM
content was not less than 0.001%, no SCC was generated even in a carbon
dioxide contained, high temperature chloride aqueous solution environment.
While how REM improves the SCC resistance is not clearly known, this
may be for the following reason.
As a result of microscopic observation of stainless steel specimens
that had SCC in the above-described tests, it was found that SCC was
originated from a pit and propagated along an prior austenite grain
boundary in a martensite predominant structure. This may suggest that
the accumulation behaviors of dislocations toward the prior austenite
boundary under stress and crack propagation are somehow correlated.
Then, REM probably has some effect on the accumulation behaviors of
dislocations toward the prior austenite boundary and the SCC resistance of
the stainless steel containing at least 0.001% REM may be improved. Note
that the stainless steel grades with Nos. Al to A3 contained 0.0008% to
0.0013% Ca but their REM contents were less than 0.001% and therefore
SCC was generated. Therefore, at least 0.001% REM content contributed
to the improvement of the SCC resistance more than Ca did.
The inventors have completed the following invention based on the
foregoing findings.
Stainless steel used for an oil country tubular goods according to the
invention includes, in percent by mass, 0.001% to 0.05% C, 0.05% to 1% Si,
at most 2% Mn, at most 0.03% P, less than 0.002% S, 16% to 18% Cr, 3.5%
to 7% Ni, more than 2% and at most 4% Mo, 1.5% to 4% Cu, 0.001% to 0.3%
rare earth metal, 0.001% to 0.1% sol. Al, 0.0001% to 0.01% Ca, at most
0.05% 0, and at most 0.05% N, and the balance consists of Fe and
impurities.
The stainless steel according to the invention preferably further
includes, in place of a part of Fe, at least one selected from the group
consisting of at most 0.5% Ti, at most 0.5% Zr, at most 0.5% Hf, at most
- 6 -
CA 02717104 2010-08-26
102CA024P1
0.5% V, and at most 0.5% Nb.
In this way, the generation of a pit attributable from a Cr depleted
layer can be reduced.
The stainless steel described above preferably has a structure
including, in volume percentage, 10% to 60% ferrite phase and 2% to 10%
residual austenite phase.
The stainless steel according to the invention preferably has a yield
stress of at least 654 MPa.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1 is a graph showing the relation between the contents of rare
earth metals in stainless steel and SCC.
BEST MODE TO CARRY OUT THE INVENTION
Now, embodiments of the invention will be described in detail.
Stainless steel according to the invention is applicable to oil country tuber
goods for use in a carbon dioxide gas contained, high temperature chloride
aqueous solution environment at 150 C or more. Hereinafter, this carbon
dioxide gas contained, high temperature chloride aqueous solution
environment at 150 C or more will be simply referred to as "high
temperature chloride aqueous solution environment."
1. Chemical Composition
The stainless steel according to the invention has the following
chemical composition. Hereinafter, "%" related to elements means "% by
mass."
C: 0.001% to 0.05%
Carbon (C) forms carbide with Cr and lowers the corrosion
resistance of steel in a high temperature chloride aqueous solution
environment. Therefore, the C content is preferably as small as possible.
Therefore, the upper limit for the C content is 0.05%. Note that the lower
limit for the C content that can substantially be controlled is 0.001%.
Si: 0.05% to 1%.
Silicon (Si) deoxidizes steel in refining process. To obtain the effect,
.7 -
CA 02717104 2010-08-26
102 CA024P 1
the lower limit for the Si content is 0.05%. On the other hand, an
excessive Si content not only saturates the deoxidizing effect but also lowers
the hot workability of the steel. Therefore, the upper limit for the Si
content is 1%.
Mn: 2% or less
Manganese (Mn) improves the strength of the steel. However, an
excessive Mn content is more likely to cause segregation in the steel. The
segregation in the steel lowers the toughness of the steel and also lowers
the SCC resistance in a high temperature chloride aqueous solution
environment. Therefore, the Mn content is not more than 2%. The Mn
content is preferably not less than 0.2% in order to improve the strength.
However, if the Mn content is less than 0.2%, the strength of the steel is
improved to some extent.
P: 0.03% or less
Phosphorus (P) is an impurity and lowers the SSC (sulfide stress
cracking) resistance and the SCC resistance in a high temperature chloride
aqueous solution environment. Therefore, the P content is preferably as
small as possible. The P content is therefore not more than 0.03%.
S: less than 0.002%
Sulfur (5) combines with Mn or the like and forms an inclusion.
The formed inclusion becomes an origin for a pit or SCC and lowers the
corrosion resistance of the steel. In addition, S lowers the hot workability
of the steel. Therefore, the S content is preferably as small as possible.
Therefore, the S content is less than 0.002%.
Cr: 16% to 18%
Chromium (Cr) is an essential element that improves the corrosion
resistance in a high temperature chloride aqueous solution environment.
In order to achieve high SCC resistance in the high temperature chloride
aqueous solution environment, the lower limit for the Cr content is 16%.
On the other hand, since Cr is a ferrite forming element, an excessive Cr
content increases the ratio of a ferrite phase in the steel structure and
lowers the strength of the steel. Furthermore, it lowers the ratio of a
residual austenite phase, which lowers the toughness of the steel.
- 8 -
CA 02717104 2010-08-26
102 CA024P 1
Therefore, the upper limit for the Cr content is 18%. The Cr content is
preferably from 16.5% to 17.5%.
Ni: 3.5% to 7%
Nickel (Ni) improves the corrosion resistance in a high temperature
chloride aqueous solution environment and also improves the toughness of
the steel. In order to obtain these effects, the lower limit for the Ni
content
is 3.5%. On the other hand, Ni is an austenite forming element and an
excessive Ni content increases the ratio of a residual austenite phase in the
structure of the steel, which lowers the strength of the steel. Therefore,
the upper limit for the Ni content is 7%. The Ni content is preferably from
3.5% to 6.5%, more preferably from 3.8% to 5.8%.
Cu: 1.5% to 4%
Copper (Cu) lowers the dissolution rate of the steel in a high
temperature chloride aqueous solution environment and also improves the
SCC resistance of the steel. In addition, Cu strengthens a ferrite phase in
the structure of the steel. In order to obtain these effects, the lower limit
for the Cu content is 1.5%. On the other hand, an excessive Cu content
lowers the hot workability of the steel. Therefore, the upper limit for the
Cu content is 4%. The Cu content is preferably from 1.5% to 3.0%, more
preferably from 1.5% to 2.5%.
Mo: more than 2% and not more than 4%
Molybdenum (Mo) improves the pitting corrosion resistance and the
SCC resistance of the steel when it coexists with Cr. In order to obtain the
effects, the Mo content is more than 2%. On the other hand, Mo is a ferrite
forming element and therefore an excessive Mo content increases the ratio
of a ferrite phase in the structure of the steel, which lowers the strength.
Therefore, the Mo content is not more than 4%. The Mo content is
preferably from 2.1% to 3.3%, more preferably from 2.3% to 3.0%.
Sol. Al: 0.001% to 0.1%
Aluminum (Al) deoxidizes steel in refining process. In order to
obtain the effect, the lower limit for the Al content is 0.001%. On the other
hand, an excessive Al content causes a large amount of an alumina
inclusion to be generated in the steel, which lowers the toughness of the
- 9 -
CA 02717104 2010-08-26
102CA024P1
steel. Therefore, the upper limit for the Al content is 0.1%. Note that the
Al content in the specification means the content of acid soluble aluminum
(sol. Al).
Ca: 0.0001% to 0.01%
Calcium (Ca) deoxidizes steel in refining process. In addition, Ca
improves the hot workability. In order to obtain these effects, the lower
limit for the Ca content is 0.0001%. On the other hand, an excessive Ca
content causes a large amount of an inclusion such as CaO to be generated
in the steel, which lowers the toughness of the steel. Furthermore, the
inclusion such as CaO forms an origin for a pit. Therefore, the upper limit
for the Ca content is 0.01%.
N: 0.05% or less
Nitrogen (N) stabilizes an austenite phase and also improves the
pitting corrosion resistance. On the other hand, an excessive N content
causes various nitrides to be formed in the steel, which lowers the
toughness of the steel. Therefore, the N content is not more than 0.05%.
In order to effectively obtain the effect, the lower limit for the N content
is
preferably 0.005%.
0: 0.05% or less
Oxygen (0) is an impurity and combines with another element to
form oxide, which lowers the toughness and the corrosion resistance of the
steel. Therefore, the 0 content is preferably as small as possible.
Therefore, the 0 content is not more than 0.05%.
Rare Earth Metals: 0.001% to 0.3%
Rare earth metals (REM) are important elements according to the
invention. The REM improve the SCC resistance in a high temperature
chloride aqueous solution environment as described above. In order to
obtain the effect, the lower limit for the REM content is 0.001%. On the
other hand, an excessive REM content saturates the effect. Therefore, the
upper limit for the REM content is 0.3%. The REM content is preferably
from 0.001% to 0.1%, more preferably from 0.001% to 0.01%.
Note that the REM according to the invention refer to yttrium (Y)
with atomic number 39 and lanthanoids from lanthanum (La) with atomic
- 10 -
CA 02717104 2010-08-26
102CA024P1
number 57 to lutetium (Lu) with atomic number 71.
The stainless steel according to the invention contains at least one
of the above REM. Therefore, the REM content means the total content of
at least one selected from the plurality of REM described above.
The balance of the chemical composition includes Fe and impurities.
The stainless steel according to the invention contains at least one
selected from the group consisting of Ti, Zr, Hf, V, and Nb in place of a part
of Fe if necessary.
Ti: 0.5% or less
Zr: 0.5% or less
Hf: 0.5% or less
V: 0.5% or less
Nb: 0.5% or less
Titanium (Ti), zirconium (Zr), hafnium (Hf), vanadium (V), and
niobium (Nb) are not essential elements and added as optional elements.
These elements each fix C and reduce the generation of Cr carbide.
Therefore, the generation of a pit attributable to a Cr depleted layer formed
around Cr carbide is reduced and the SCC sensitivity is reduced. However,
an excessive content of any of these elements lowers the toughness of the
steel. Therefore, the upper limits for the contents of these elements are
each 0.5%. In order to effectively obtain the above-described effect, the
lower limits for the contents of these elements are each preferably 0.005%.
Note however that if the contents of these elements are less than the
preferable lower limit, the above-described effect is obtained to some extent.
2. Manufacturing Method
The stainless steel according to the invention can have the following
structure by carrying out quenching-tempering as heat treatment, so that
the corrosion resistance as intended and strength necessary when it is used
as oil country tubular goods can be provided. Now, a method of
manufacturing a stainless steel pipe according to the invention will be
described by way of example.
Steel having the above-described chemical composition is melted
and made into a billet. The produced billet is subjected to hot working and
- 11 -
CA 02717104 2010-08-26
102CA024P1
made into a stainless steel pipe. A Mannesmann method for example is
employed as the hot working to make a seamless steel pipe. Note that the
hot working may be hot extruding or hot forging.
The produced stainless steel pipe is subjected to quenching and
tempering. At the time, the preferable quenching temperature is from
900 C to 1200 C, and the preferable tempering temperature is from 450 C
to 650 C.
3. Structure
The structure of the stainless steel produced by the above-described
method includes, in percent by volume, 10% to 60% ferrite phase and 2% to
10% residual austenite phase.
Now, the volume percentage of the ferrite phase is obtained by the
following method. A specimen having its surface polished is etched using a
mixture solution of aqua regia and glycerin. Using the etched specimen,
the area ratio of the ferrite phase at the specimen surface is measured by a
point-counting method according to JISG0555. The measured area ratio is
used as a volume percentage. The volume percentage of the residual
austenite phase is measured by X-ray diffraction.
Note that in the structure of the stainless steel, the portion other
than the ferrite phase and the residual austenite phase is mainly a
tempered martensite phase. Carbide, nitride, boride and a Cu phase may
be included other than the martensite phase.
The stainless steel according to the invention has the above
described structure, so that the yield stress is not less than 654 MPa (that
corresponds to 95 ksi). The yield stress may be adjusted to 758 MPa (that
corresponds to 110 ksi) or more and further to 862 MPa (that corresponds to
125 ksi) or more. The yield stress in this specification refers to 0.2% offset
yield stress based on the ASTM standard.
The stainless steel according to the invention has high toughness
since it contains the residual austenite phase as much as the above-
described volume percentage in the structure.
Examples
A plurality of kinds of stainless steel having various chemical
- 12 -
CA 02717104 2010-08-26
102CA024P1
compositions were produced and examined for their SCC resistance in a
high temperature chloride aqueous solution environment.
Manufacture of Specimens
A plurality of kinds of stainless steel having the chemical
compositions in Table 2 were melted.
- 13 -
102CA024P1
Table 2
Steel
Chemical composition (unit: mass %, the balance consisting of Fe
and impurities)
No. C Si Mn P S Cu Cr Ni Mo sol.A1 Ca N 0
REM Ti Zr Hf V Nb
1
0.022 0.36 0.51 0.017 0.001 2.4 16.3 3.6 2.4 0.014 0.0003 0.019 0.005 0.03 a)
- -
2
0.011 0.39 0.65 0.018 0.0008 1.6 17.6 6.3 2.1 0.039 0.0005 0.044 0.018
0.002 a) - - - -
3
0.018 0.38 1.28 0.019 0.0009 3.3 17.2 3.9 2.2 0.029 0.001 0.011 0.003
0.001 a) - - - -
4
0.032 0.84 1.79 0.017 0.001 1.7 16.3 4.5 3.5 0.021 0.0015 0.016 0.004 0.008 c)
- - -
0.042 0.29 0.23 0.011 0.0015 3.5 17.8 6.2 3.6 0.033 0.0005 0.008 0.004 0.019
b) - - - -
6
0.039 0.11 0.59 0.019 0.0018 1.8 16.2 4.2 2.3 0.018 0.0008 0.015 0.005 0.22b)
- - - -
7
0.022 0.36 0.55 0.022 0.0009 2.3 16.4 4.5 2.6 0.015 0.0012 0.026 _0.004 0.015
c) - - - 0.05 - 0
8 0.025 0.52 0.68 0.028 0.0008 1.6 16.5 5.6 2.5 0.029 0.0029 0.022 0.008
0.011 c) 0.04 - - -
0
9
0.019 0.48 0.66 0.019 0.0009 1.9 17.2 6.1 2.5 0.036 0.00050.018 0.005
0.007 a) - 0.02 - 0.09 -
0.018 0.33 0.55 0.017 0.001 2.1 16.6 5.9 2.8 0.055 0.0002 0.019 0.003 0.002 a)
- - 0.01 0.08 - = 0
0
11 0.023 0.22 0.49 0.015 0.001 2.8 17.5 4.8 2.7 0.057 0.0009 0.0170.004
0.009c) - - - 0.11 0.08 0
co
12 0.026 0.28 0.48 0.011 0.0009 2.4 16.8 4.2 3.2 0.022 0.001 0.021 0.006
0.026b) 0.15 - - 0.04 0.13
13 0.021 0.35 0.44 0.018 0.0008 2.3 16.9 3.7 1.9 0.028 0.0012 0.019 0.005
0.011 - -
14
0.018 0.39 0.41 0.017 0.0008 2.3 15.1 3.8 2.3 0.019 0.00110.009 0.005
0.008 a) - - -
0.023 0.32 0.49 0.015 0.001 1.3 17.2 4.5 2.5 0.033 0.0009 0.018 0.011 0.015 a)
- - -
16 0.025 0.33 0.51 0.014 0.001 2.3 17.5 4.4 2.3 0.036 0.0009 0.015 0.004
- - - - -
17
0.021 0.35 0.55 0.014 0.0009 2.2 16.9 3.1 2.8 0.039 0.0012 0.014 0.004
0.014 a) - - - - -
An underlined numerical value is outside the range defined by the invention.
- 14 -
CA 02717104 2010-08-26
102 CA024P 1
The numerical values in Table 2 refer to the contents of
corresponding elements (% by mass). Among these chemical compositions,
the balance other than the elements described in Table 1 includes Fe and
impurities. The symbols a) to c) attached to the numerical values in the
"REM" column each represent the kind of REM included in the steel. More
specifically, a) means that the contained REM is neodymium (Nd). Similarly,
b) means that the contained REM is yttrium (Y) and c) means that the
contained REM is misch metal. The misch metal contains, in percent by
mass, 51.0% cerium (Ce), 25.5% lanthanum (La), 18.6% neodymium (Nd),
4.8% praseodymium (Pr) and 0.1% samarium (Sm).
With reference to Table 2, the steel kinds with Nos. 1 to 12 each had
a chemical composition within the range defined by the invention.
Regarding the steel with No. 13, its Mo content was less than the lower
limit defined by the invention. Regarding the steel with No. 14, its Cr
content was less than the lower limit defined by the invention. Regarding
the steel with No. 15, its Cu content was less than the lower limit defined
by the invention. The steel with No. 16 contained no REM. Regarding
the steel with No. 17, its Ni content was less than the lower limit defined by
the invention.
These numbered steels were each subjected to hot forging and hot
rolling, and a steal plate having a thickness of 12 mm was produced. The
numbered steel plates were subjected to quenching and tempering. In the
quenching processing, the steel plates were each heated for 15 minutes at a
quenching temperature from 980 C to 1200 C and then cooled with water.
In the tempering processing, the tempering temperature was from 500 C to
650 C. Through these steps, the yield stress of each steel plate was
adjusted to be in the range from 800 MPa to 950 MPa.
Structure Observation and Tensile Tests
The volume percentage (%) of the ferrite phase and the residual
austenite phase of each steel plate was obtained by the measuring method
described in 3.
Then, a round rod tensile test specimen was sampled from each of
the steel plates and subjected to a tensile test. The length-wise direction
- 15 -
CA 02717104 2010-08-26
102 CA024P 1
of the round rod tensile test specimen was arranged in the direction of
rolling the steel plate and the parallel part of the round rod tensile test
specimen had a diameter of 14 mm, and a length of 20 mm. The tensile
tests were carried out at room temperatures.
SCC Evaluation Tests
A four-point bending specimen having a length of 75 mm, a width of
mm, and a thickness of 2 mm was sampled from each of the steel plates.
Each of the sampled specimens was bent by four-point bending. At the
time, according to ASTM G39, the amount of bending of each of the
10 specimens was determined so that stress applied on each of the specimens
was equal to the yield stress of each of the specimens.
The bent specimens were each immersed for one month in a 25 wt%
NaC1 aqueous solution in an autoclave at 204 C (400F) having CO2 enclosed
therein under a pressure of 30 atm. After the immersion for one month,
the specimens were examined for the presence of SCC. More specifically, a
longitudinal section of each specimen was observed with a 100x
magnification optical microscope and examined for the presence/absence of
SCC by visual inspection. The weight of each specimen was measured
before and after the test. From the difference between the measured
weights, the weight loss of each specimen caused by corrosion was obtained
and the corrosion rate was calculated based on the weight loss.
Test Result
The test result is given in Table 3.
- 16 -
CA 02717104 2010-08-26
102CA024P1
Table 3
Ferrite Austenite
phase in phase in SCC Corrosion
Steel YS
volume volume evaluation rate
No. (MPa)
percentage percentage result (g/(m2.hr))
(%) (%)
1 924 25 2.1 NO SCC <0.1
2 915 26 5.2 NO SCC <0.1
3 901 28 5.6 NO SCC <0.1
4 893 35 3.2 NO SCC <0.1
940 15 4.2 NO SCC <0.1
6 886 38 2.2 NO SCC <0.1
7 922 20 4.1 NO SCC <0.1
8 928 21 4.5 NO SCC <0.1
9 926 24 3.6 NO SCC <0.1
933 19 6.8 NO SCC <0.1
11 911 28 5.2 NO SCC <0.1
12 903 33 3.3 NO SCC <0.1
SCC
13 880 38 2.3 <0.1
PRESENT
SCC
14 932 20 4.9 >0.1
PRESENT
SCC
873 42 2.0 <0.1
PRESENT
SCC
16 899 30 3.5 <0.1
PRESENT
SCC
17 880 35 2.8 <0.1
PRESENT
The "YS" column in Table 3 represents the yield stress (MPa) of
5 each of the numbered steel plates obtained by the tensile tests. The
"ferrite phase" and "residual austenite phase" columns represent the
volume percentages (%) of the ferrite phase and the residual austenite
phase in each of the steel plates. In the "SCC evaluation result" column,
the "NO SCC" indicates that there was no SCC generated at the four-point
- 17 -
CA 02717104 2012-05-28
bending test specimen, and the "SCC PRESENT" indicates that there was
SCC. In the "corrosion rate" column, the "<0.1" indicates that the corrosion
rate was less than 0.1 g/(m2-hr), while the "> 0.1" indicates that the
corrosion
rate was not less than 0.1 ggin2-hr).
With reference to Table 3, the steels with Nos. 1 to 12 did not have any
SCC and their corrosion rates were all less than 0.1 g/(m2-hr). Their yield
stress values were all 654 MPa or more.
On the other hand, the steels with Nos. 13, 15 and 17 had SCC because
their Mo, Cu, and Ni contents were small. The steel with No. 14 had SCC
because it contained only a small amount of Cr, and its corrosion rate was not
less than 0.1 g/(m2.hr). Furthermore, the steel with No. 16 had SCC because
it did not contain REM.
Applicable Field in the Industry
The stainless steel according to the invention can be applied as oil
country tubular goods and particularly suitably applied to an oil country
tubular good for use in a carbon dioxide gas contained, high temperature
chloride aqueous solution environment at 150 C or higher.
- 18-