Note: Descriptions are shown in the official language in which they were submitted.
CA 02717766 2010-10-15
STRIPPING ABSORPTION MODULE
This application claims priority on US Provisional Patent Application No.
61/381,333 filed
September 9, 2010, incorporated herein by reference.
FIELD OF THE INVENTION
The present invention relates generally to the field of fluid separation.
BACKGROUND OF THE INVENTION
In the field of fluid separation, it is known to utilize a brine, such as a
LiBr brine, for the
absorption of a process vapor and the consequential generation of heat. It is
also known to
utilize a pump to drive a heat-carrying fluid around a heat exchange circuit
to carry the heat
generated by the absorber to an evaporator or boiler to produce the process
vapor.
SUMMARY OF THE INVENTION
A process for use with a flow of a liquid mixture that is separable by
vaporization into a flow
of vapor and a depleted flow of liquid forms one aspect of the invention. The
process
comprises: a vaporization step, wherein a portion of said liquid mixture flow
is vaporized to
produce said flow of vapor and said depleted flow of liquid; an absorption
step, wherein (i)
the flow of vapor is introduced to a flow of brine which is adapted to
exothermically absorb
one or more components from the vapor and (ii) heat is withdrawn, to produce
at least a flow
of heat and a flow of brine which is enriched in the one or more components;
and a heat
transfer step, wherein the heat withdrawn in the absorption step is
transferred, to drive the
vaporization in the vaporization step. The transfer of heat to drive the
vaporization is
associated with the phase change of a working fluid from a gaseous state into
a liquid state.
The withdrawal of heat in the absorption step involves the phase change of the
working fluid
from the liquid state into the gaseous state. In the liquid state, the working
fluid flows only by
one or more of gravity, convection and wicking.
1
CA 02717766 2010-10-15
In the gaseous state, the working fluid flows only by one or more of diffusion
and convection.
Apparatus forms another aspect of the invention. The apparatus is for use with
a flow of a
liquid mixture that is separable by vaporization into a flow of vapor and a
depleted flow of
liquid. The apparatus comprises a structure which, in use:
= defines a first volume wherein said liquid mixture is received and separated
into said
flow of vapor and said depleted flow of liquid;
= defines a first liquid passage by which said depleted flow leaves the first
volume;
= defines a vapor passage by which said flow of vapor leaves the first volume;
= defines a second volume to which the vapor passage leads;
= includes heat and mass transfer apparatus disposed at least in part in the
second
volume, the heat and mass transfer apparatus: (i) receiving a flow of brine
adapted to
exothermically absorb one or more components from the vapor; (ii) introducing
the
flow of brine to the vapor; and (iii) withdrawing heat from the second volume,
to
produce at least a flow of heat and a flow of brine which is enriched in the
one or
more components;
= defines a second liquid passage by which the flow of brine which is enriched
in the
one or more components leaves the second volume; and
= includes heat movement apparatus for transferring the flow of heat to the
first volume
to provide for said separation.
In the apparatus, in use, the transfer of heat into the first volume is
associated with the phase
change of a working fluid from a gaseous state into a liquid state; the
withdrawal of the heat
from the second volume involves the phase change of the working fluid from the
liquid state
to the gaseous state; in the liquid state, the working fluid flows only by one
or more of gravity,
convection and wicking; and in the gaseous state, the working fluid flows only
by one or more
of diffusion and convection.
2
CA 02717766 2010-10-15
According to another aspect of the invention, the heat movement apparatus and
part of the
heat and mass transfer apparatus can be defined by one or more heat pipes,
each of said
one or more heat pipes having a heat receiving part disposed in the second
volume and a
heat delivering part disposed in the first volume to provide for said heat
transfer.
According to another aspect of the invention, the one or more heat pipes can
be stacked
such that that portion of the heat pipes disposed in the first volume operate
in use as a
packed vaporization column and that portion of the heat pipes disposed in the
second
volume operate in use as a packed absorption column.
According to another aspect of the invention, in use, the vapor leaving the
first volume can
be in substantial vapor-liquid equilibrium with the liquid mixture entering
the first volume.
According to another aspect of the invention, in use, the temperature of the
depleted flow of
liquid leaving the first volume can be lower than the temperature of the
liquid mixture entering
the first volume.
According to another aspect of the invention, in use, the pressure in the
first volume and the
temperature of the liquid mixture entering the first volume can be such that
substantially all of
the heat transferred to the first volume results in vaporization of the liquid
mixture.
According to another aspect of the invention: the structure can further define
a vent leading
from the second volume; and in use, at least a substantial portion of the
vapor can be
absorbed in the second volume, the balance leaving the second volume via the
vent.
According to another aspect of the invention, the apparatus can further
comprise desorption
apparatus for receiving the flow of brine produced by the heat and mass
transfer apparatus
and producing: the flow of brine adapted to exothermically absorb said one or
more
components from the vapor; and a product stream.
3
CA 02717766 2010-10-15
According to another aspect of the invention, the apparatus can further
comprise: a
secondary absorber which, in use: (i) receives the balance of the vapor; and
(ii) introduces
the balance of the vapor to a secondary flow of brine which is adapted to
exothermically
absorb the one or more components, to produce a diluted brine.
According to another aspect of the invention, the desorption apparatus can
further receive
the diluted brine and further produces the secondary flow of brine.
According to another aspect of the invention, in use: the pressures in the
first volume and
second volume can be reduced in comparison to atmospheric pressure; at least
the majority
of the vapor can be absorbed in the second volume; and a vacuum pump can
provide for at
least the non-condensables of the vapor to be voided from the apparatus.
According to another aspect of the invention, the first volume can be defined
by one or more
first voids and the second volume can be defined by one or more second voids.
According to another aspect of the invention, each of the one or more first
voids and each of
the one or more second voids can be defined by a respective vessel; and piping
can define
the vapor passage.
According to another aspect of the invention, each of the one or more first
voids and each of
the one or more second voids can be defined in a vessel.
According to another aspect of the invention, piping exterior to the vessel
can define the
vapor passage.
According to another aspect of the invention: the vessel can be
compartmentalized by
bulkheads to define the one or more first voids and one or more second voids
and; one or
more apertures defined in the bulkheads can define the vapor passage.
The apparatus can form part of a bioproduct production facility, which forms
another aspect
of the invention. The facility comprises, in addition to the apparatus, an
arrangement
4
CA 02717766 2010-10-15
wherein, in use, catabolism of a broth takes place on a continuous basis. The
apparatus is
coupled to the arrangement to: withdraw a flow of the broth on a continuous
basis; remove a
catabolic inhibitor from the withdrawn broth to produce an inhibitor-
containing flow and a
remainder flow; and return the remainder flow to the arrangement.
According to another aspect of the invention, the catabolism can be
fermentation and the
inhibitor can be alcohol.
According to another aspect of the invention, the inhibitor-containing flow
can have a higher
concentration of the inhibitor than does the broth.
According to another aspect of the invention, in use, a bleed stream of the
broth can be
withdrawn to avoid toxin buildup; the bleed stream can be fermented in
batches; and the
facility can further comprise further apparatus for receiving the product of a
batch
fermentation and producing (i) a stream of whole stillage from which ethanol
has been
substantially removed and (ii) brine enriched in ethanol which is fed to the
desorption
apparatus and separated.
According to another aspect of the invention,in use: the broth withdrawn from
the
arrangement can have a temperature of about 28-32 C and an ethanol
concentration of
about 4-10%; the remainder flow can have a temperature of about 2-4 C lower
than that of
the withdrawn flow, and have an ethanol concentration of about 2-6% less than
that of the
withdrawn flow; and the pressure in the first volume can be about 30-100 Torr.
According to another aspect of the invention, in use: the broth withdrawn from
the
arrangement can have a temperature of about 30 C and an ethanol concentration
of about
7%; the remainder flow can have a temperature of about 28 C and an ethanol
concentration
of about 2%; and the pressure in the first volume can be about 30Torr.
5
CA 02717766 2010-10-15
According to another aspect of the invention, the heat pipes can be arranged
parallel to a
common axis and the structure can be adapted for pivotal movement about a
horizontal axis
which is orientated normally to the common axis.
The apparatus of the invention can, according to yet another aspect of the
invention, form
part of a bio-product production facility which comprises an arrangement
wherein, in use,
catabolism of a broth takes place on a batch basis. In this facility, the
apparatus is coupled
to the arrangement to: withdraw a flow of the broth; remove a catabolic
inhibitor from the
withdrawn broth to produce an inhibitor-containing flow and a remainder flow;
and return the
remainder flow to the arrangement.
Other advantages, features and characteristics of the present invention will
become more
apparent upon consideration of the following detailed description and the
appended
drawings, the latter being briefly described hereinafter, it being understood
in the drawings,
like reference numerals denote like structures throughout.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a schematic view of a stripping/absorption module according to an
exemplary embodiment of the invention;
Figure 2 is a schematic view of the module of Figure 1 in an exemplary use;
Figure 3 is a view, similar to Figure 2, of another exemplary use of the
structure of
Figure 1;
Figure 4 is a another schematic view showing another exemplary use of the
structure
of Figure 1;
Figure 5 is a schematic view of an ethanol production facility employing two
modules
according to Figure 1;
6
CA 02717766 2010-10-15
Figure 6 is a simplified schematic view of the ethanol production facility of
Figure 5 in
combination with a simplified view of the structure of Figure 4;
Figure 7 is a schematic view of an alternate embodiment of the module of
Figure 1;
Figure 8 is a schematic view of a yet further embodiment of the module of
Figure 1;
Figure 9 is a cross-sectional view of a yet further embodiment of the module
of
Figure 1; and
Figure 10 is a schematic view of another embodiment of the invention.
DETAILED DESCRIPTION
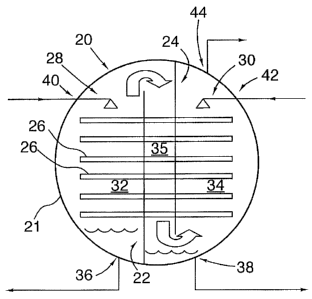
A stripping/absorption module (SAM) is shown in Figure 1 in schematic form and
designated
with general reference numeral 20.
This module comprises: a vessel 21, a pair of bulkheads 22,24, a plurality of
heat pipes 26
and a pair of distributors 28,30.
Vessel 21 is a robust vessel, suitable for operation at reduced pressures, for
example, 30
Torr.
The pair of bulkheads comprises a first bulkhead 22 and a second bulkhead 24.
The first
bulkhead 22 extends upwardly from the base of the vessel and terminates
beneath the top of
the vessel. The second bulkhead 24 is disposed in spaced relation from the
first, extends
downwardly from the top of the vessel and terminates above the base. Through
this
arrangement, first 32 and second 34 voids are defined interiorly of the
vessel, which are
coupled to one another by a conduit 35 defined by the space between the
bulkheads 22,24.
7
CA 02717766 2010-10-15
The vessel is punctuated by a plurality of ports 36-44, one lower port 36,38
at the base of
each void, one upper port 40,42 adjacent the top of each void and one
uppermost port 44
proximal the top of the second void 34.
The plurality of heat pipes 26 extend from the first void 32 to the second
void 34 and are for
carrying heat from the second void 34 to the first void 32. The heat pipes 26
are of
conventional construction and as such are not described herein in detail.
The pair of distributors 28,30 extend one each from the upper ports 40,42 of
the first and
second voids 32, 34 and are adapted for wetting the heat pipes 26.
From this, it should be understood that the major functional features of the
illustrated SAM
are:
= first void 32;
= second void 34;
= the conduit 35 connecting the first and second voids;
= the lower ports 36,38;
= the upper ports 40,42;
= the uppermost port 44;
= the heat pipes 26; and
= the distributors 28,30
Figure 2, which is a schematic embodiment of an exemplary separator apparatus
for use with
a flow of a mixed liquid that is separable by vaporization into a flow of
vapor and a depleted
flow of liquid, shows the manner in which such major functional features
operate in use.
Herein, it will be seen that the module 20 is shown along with a secondary
absorber 46 and a
desorption apparatus 48.
8
CA 02717766 2010-10-15
Turning first to the module 20, it will be understood that the first void 32
forms a first volume.
This is where the flow of mixed liquid is received and partially vaporized
into the
aforementioned flows of vapor and depleted flow of liquid. The manner in which
vaporization
is carried out is described below, in the description relating to the heat
pipes.
The lower port 42 at the base of the first volume defines a first liquid
passage by which said
depleted flow of liquid leaves the first volume 32.
The conduit 35 defines a vapor passage by which said flow of vapor leaves the
first volume
32.
The second void 34 defines a second volume to which the vapor passage 35
leads.
The uppermost port 44 defines a vent.
The distributors 28,30 and heat pipes 26 together define heat and mass
transfer apparatus
and heat movement apparatus. The heat and mass transfer apparatus: (i)
receives a flow of
brine adapted to exothermically absorb one or more components from the vapor;
(ii)
introduces the flow of brine to the vapor (i.e. the brine is sprayed or
dropped into the second
volume 34 onto the heat pipes 26); and (iii) withdraws heat from the second
volume, to
produce at least a flow of heat and a flow of brine which is enriched in the
one or more
components. The heat movement apparatus transfers the flow of heat to the
first volume 32
to provide for said separation, and as such, each of the heat pipes 26 has a
heat receiving
part disposed in the second volume and a heat delivering part disposed in the
first volume.
The brine can be, for example, only, LiBr solution having a lithium bromide
mass
concentration between 40% to 70%, preferably between 45% to 65%. However, any
absorbent fluid known in the art would be suitable.
9
CA 02717766 2010-10-15
The lower port 38 at the base of the second volume 34 defines a second liquid
passage by
which the flow of brine which is enriched in the one or more components leaves
the second
volume 34.
By virtue of use of the heat pipes, it will be understood that: that the
transfer of heat into the
first volume is associated with the phase change of a working fluid, in this
case, water, from a
gaseous state into a liquid state; the withdrawal of the heat from the second
volume involves
the vaporization of the working fluid from the liquid state into the gaseous
state; the working
fluid in the liquid state flows only by one or more of gravity and wicking;
and the working fluid
in this gaseous state flows only by one or more of diffusion and convection.
Working fluids
other than water can and would be used, depending upon the application:
ammonia and
commercial refrigerant fluids are but two examples. The choice of working
fluid is a matter
of routine to persons of ordinary skill and as such is not described herein.
The heat pipes 26 are stacked such that that portion of the heat pipes
disposed in the first
volume 32 operate in use as a packed evaporation column and that portion of
the heat pipes
disposed in the second volume 34 operate in use as a packed absorption column.
Accordingly:
= the vapor leaving the first volume 32 is in substantial vapor-liquid
equilibrium with the
mixed liquid entering the first volume 32;
= at least a substantial portion of the vapor is absorbed in the second volume
34, with
the balance leaving the second volume via the vent 44.
The secondary absorber 46: (i) receives the balance of the vapor, i.e. that
portion not
absorbed in the SAM; and (ii) introduces the balance of the vapor to a
secondary flow of
brine which is adapted to exothermically absorb the one or more components.
This
produces a diluted brine and also produces a gas stream composed of non-
absorbable
gases and any non-absorbed absorbables, the latter being vacated from the
secondary
absorber along arrow 50.
CA 02717766 2010-10-15
The desorption apparatus 48, i.e. a boiler or a distillation apparatus,
receives the flow of
brine produced by the heat and mass transfer apparatus and the diluted brine
produced by
the secondary absorber 46 and produces:
= the flow of brine 52 adapted to exothermically absorb at least one or more
components from the vapor; and the secondary flow of brine 54; and
a product stream 56.
Figure 3 shows a variation of the structure of Figure 2, for use in
circumstances wherein the
pressures in the first volume and second volume are reduced in comparison to
atmospheric
pressure. In this application, at least the majority of the enriched vapor is
absorbed in the
second volume and a vacuum pump 58 provides for the non-condensables and any
unabsorbed condensables in the vapor to be voided from the apparatus.
Turning now to Figure 4, same will be understood to show in schematic form a
plant that
could be usefully used for concentrating apple juice. This plant is generally
similar to the
structure of Figure 3, i.e. in that it includes a SAM 20, a secondary absorber
46 and a
vacuum pump 58, but the desorption apparatus 48 has, rather than a
distillation device, a
two-stage desorber [since the purpose in this application is not to
fractionate the mixed liquid
but merely to concentrate the juice.] The two-stage desorber includes a number
of
economizers 60, to advantageously pass heat between various parts of the
process, a pair of
boilers 62 and variety of pumps 64. Predictions have been made in respect of
the operation
of this system, the values being set forth on Table 1, below.
11
CA 02717766 2010-10-15
Stream Flow rate lb/hr Temp C %LiBr
Water LiBr Total
1 100 0 100 30 0
2 60 0 60 30 0
3 40 0 40 30 0
4A 80 120 200 165 60
4B 80 120 200 70 60
5A 40 120 240 55 50
5B 40 120 240 85 50
6 98 120 218 90 54.2
7 18.7 0 18.7 30 0
8 21.3 0 21.3 105 0
9 25.6 0 25.6 178 0
As required <30
Table 1
5 The predicted energy input (in the form of 125 psig steam) fed via stream
12, is 557 Btu/Ib
water evaporated. This contrasts favorably to simple evaporation efficiency
[about 1000
Btu/Ib]. At the same time, the facility is predicted to be relatively
inexpensive to construct
and operate, as will be evidence to persons of ordinary skill in the art on
review of the
schematic.
12
CA 02717766 2010-10-15
Turning now to Figure 5, same will be understood to show in schematic form an
ethanol
production facility and will be seen to include:
= a corn milling facility 66, a cooking/liquefaction facility 68 and a
saccharification facility
70; these are all substantially conventional, in that they take corn and
create
therefrom a feedstock suitable for fermentation;
= a yeast conditioning facility 72, for producing a flow of water, enzymes and
yeast;
= a continuous stirred tank reactor (CSTR) 76: to which the feedstock, water,
etc, are
fed, in which fermentation continuously takes place and from which a bleed
stream
135 is drawn;
= a SAM device 20, coupled to the CSTR to: withdraw a flow of the fermentation
broth
on a continuous basis, preferentially remove alcohol from the withdrawn broth
to
produce an enriched alcohol (brine) flow 104 and a remainder flow 131; and
return
the remainder flow 131 to the CSTR 76;
= a batch tank 78, which receives and ferments the bleed stream 135 in batches
= a second SAM device 20 coupled to receive the product from the batch tank 78
and
produce (i) a stream of whole stillage from which ethanol has been
substantially
removed and (ii) brine enriched in ethanol
= a stillage processor 79
= a secondary absorber 46, for absorbing the remainder of the absorbables not
taken
up by the SAM devices 20;
= a vent scrubber 74 for extracting trace alcohol from, inter alia, the batch
tank 78 and
the secondary absorber 46 and diverting same back to the cooking facility 68,
before
exhausting non-condensables to atmosphere via stream 142
= 3 desorbers 84,82,80, arranged to create a three-stage desorber, to
regenerate the
brine, produce a concentrated ethanol stream; and produce a recycle water
stream;
= a condenser 90 and receiver 92, for condensing the recycle water stream and
returning same to the corn milling facility 68;
= a rectifier/dehydration facility 86;
= economizers 60 and pumps 64, for passing flows between the various elements;
and
= ethanol product storage facility 88.
13
CA 02717766 2010-10-15
Predicted operating conditions for various of the flows are indicated in Table
2.
FERMENTATION TRAIN Stream # Mass flow rate lb/hr percent percent Temperature
Stream name water sugars DGS ethanol total ethanol sugars Degrees C
Mash fed to main fermentor 137 224,400 66,000 37,620 0 328,020 0 20 30
Beer feed to SAM1 132 211,455 5,610 37,620 18,117 272,802 7 2 30
Beer recycled from SAM1 131 198,511 5,610 37,620 6,039 247,780 2 2 28
Main fermentor bleed 135 211,455 5,610 37,620 18,117 272,802 7 2 30
Fully fermented beer 136 211,455 0 37,620 20,922 269,997 8 0 32
BRINE TRAIN Stream If Mass flow rate lb/hr Stream Composition Temperature
Stream name
LiBr Water Ethanol Total % LiBr % Water % Ethanol Degrees C
SAM1 vapours 99a 0 12,944.63 12,078.00 25,022.63 0 51.73 48.27 30
SAM2 vapours 99b 0 19,927.34 20,922.00 40,849.34 0 48.78 51.22 30
Feed to Secondary Absorber 100 133,028 121,558 33,000 287,586 50 42 11.47 55
Product from Secondary Absorber 101 133,028 128,787 33,803 295,618 45 44 11.43
45
Strong Brine to SAM1 103 66,514 44,343 0 110,857 60 40 0 70
Strong Brine to SAM2 104 66,514 44,343 0 110,857 60 40 0 70
Low Pressure Desorber Product 107 133,028 88,686 0 221,714 60 40 0 95
High Pressure Desorber Poduct 112 133,028 105,799 3,426 242,254 55 44 1.41 260
Mid Pressure Desorber Product 113 133,028 118,271 15,898 267,197 50 44 6 175
Ethanol Laden Condensate 117 0 12,472 12,472 24,944 0 50 50 202
Mid Pressure Vapor 118 0 10,516 17,905 28,421 0 37 63 138
Ethanol Product as vapor 163 0 134 33,000 33,134 0 0 100 ambient
Persons of ordinary skill in the art will readily understand the operation of
the device in
consideration of these flows and the schematic. Accordingly, for brevity, a
detailed item-by-
item description is neither required nor provided.
14
CA 02717766 2010-10-15
However, Table 2 is notable at least for the following:
= product streams fed to the rectifier 86 are of concentrations suitable for
conventional
processing by pervaporation or molecular sieve techniques;
= calculations suggest that high quality heat requirements, i.e. fuel-
generated heat, for
the high pressure desorber 84, are 4,717 btu/gallon ethanol produced [up to
the
rectifier 86]; this contrasts favorably to common ethanol production
facilities, wherein
heat requirements up to rectification can reach as high as 18,000 btu/gallon
= the broth withdrawn from CSTR 76 has a temperature of about 30 C and the
remainder broth has a temperature of about 28 C; this arrangement is
advantageous, in that the broth is never elevated in temperature above about
30 C
[or supercooled], which would harm the live yeast.
Again, the facility is predicted to be relatively inexpensive to construct, as
will be evident to
persons of ordinary skill.
Without intending to be bound by theory, it is believed that the advantageous
energy and
construction cost requirements flow in part from:
= the pressure in the first volume 32 and the temperature of the mixed liquid
entering
the first volume 32 are such that substantially all of the heat transferred to
the first
volume 32 results in evaporation of the mixed liquid;
= the remainder broth has a temperature lower than that of the withdrawn
broth,
thereby reducing chilling loads on the CSTR;
= the use of multiple-effect desorption; and
= the relatively modest refrigeration loads associated with the vaporization
[which, in
areas where very cold cooling water is not available in abundance, i.e., as is
commonly the case, must be provided by mechanical means]
Figure 6 shows a simplified variation of the FIG. 5 structure, with further
detail in respect of
an advantageous method for stillage processing, utilizing a SAM according to
the invention.
CA 02717766 2010-10-15
Briefly, CSTR 76 receives feedstocks 96 and produces strong beer 98 which is
fed to a SAM
apparatus 20. Weak beer 100 passes back from this SAM to CSTR 76. A bleed
stream 104
passes to batch tank 78. Strong beer 102 from batch tank 78 is fed to its own
SAM 20.
Whole stillage 108 from batch tank 78 is centrifuged 110 to produce wet cake
112 and thin
stillage 114, the latter being sent to yet another SAM 20, to produce syrup
116 which, along
with cake 112, is dried in a DDGS dryer 118. Dilute brine 120 produced by each
of the
SAMS is fed to still 94 for regeneration. Although still 94 shows all of the
diluted brines
converging, it should be understood that still apparatus 94 could have two
trains, thereby to
keep separate brine streams relatively higher concentration in ethanol and
brine streams
relatively barren of ethanol.
The predicted utility in respect of the aforementioned prophetic examples has
been verified
experimentally.
Experimental Results
Twenty heat pipes, each 7.0" in length and 0.25" in diameter, were mounted
horizontally, one
above the other,, to form an array about 10.0" in height. This assembly was
sandwiched
between transparent sheets of acrylic. Two separate, side-by-side chambers [an
evaporator
chamber and an absorber chamber] were formed between the sheets, with the heat
pipes
passing through both chambers. A 0.5" ID hose was used to connect the top part
of the
evaporator chamber to the bottom of the absorber chamber. At the top of each
chamber, a
crude liquid distributor was provided. At the top of each chamber, a 2 litre
flask, vented to
atmosphere was provided, and coupled to the liquid distributor of that chamber
via a flow
control valve. At the bottom of each chamber, a liquid exit port was provided,
coupled to a
collection flask. A vent at the top of the absorber chamber was coupled a
standard
laboratory vacuum pump with two lines of defense protecting it from water and
ethanol
vapours.
16
CA 02717766 2010-10-15
The first defense measure was a secondary absorber comprised of a flask partly
filled with a
strong cool LiBr solution. Gases en route to the vacuum pump were forced to
bubble through
the solution in the flask, stripping them of absorbable components. The second
stage of
defense was a liquid nitrogen cold trap.
Two runs were made. In each run, measured amounts of brine were provided in
the bubbler
tank and absorber-coupled flask and a measured amount of beer was provided in
the
evaporator-coupled flask; the flow control valves were opened; and temperature
and
pressure measurements were made as the liquids traversed the unit. Readings
were
terminated when one or both of the feed flasks had been drained.
Time Beer Beer Brine Brine System
Elapsed Input Output Input Output Pressure
min C C C C mmHg
1 26 24 49 23 41.7
2 27 27 60 42 37.2
3 27 27 62 48 33
4 27 27 63 . 52 29.1
5 27 27 64 52 27.7
6 27 28 64 55 27.7
7 27 29 65 53 28.6
8 27 31 66 56 28
Run 1 - Table 3
Bubbler starting weight 1303g
ending weight 1304g
Cold trap starting weight 0
Ending weight 0
Beer starting weight 83g ethanol + 952 g water = 1035g (8% EtOH)
ending weight 70g ethanol + 931 g water = 1000g (7% EtOH)
Brine starting weight 701g water + 1052g LiBr = 1753 g (60% LiBr)
Ending weight 13g ethanol + 713g H2O + 1052 LiBr = 1778 (59% LiBr)
17
CA 02717766 2010-10-15
This test confirmed that the SAM can preferentially remove ethanol from an
ethanol-water
mixture and simultaneously cool the ethanol water mixture. It also indicated
that a secondary
absorber is a useful way to remove residual water and ethanol vapors from the
vacuum train.
The heat transfer coefficient for the device in this run was calculated as 33
BTU/hr/ft2/ F
Time Beer Beer Brine Brine System
Elapsed Input Output Input Output Pressure
(min) C C 0C cc mmHg
1 35 22 50 37 20
2 36 25 63 44 25
3 36 27 68 49 25
4 36 27 69 52 30
36 27 71 53 30
6 37 30 72 55 30
7 37 30 72 55 40
8 36 30 72 54 42
9 37 31 72 53 40
37 31 72 53 40
11 37 31 73 53 40
12 36 31 73 52 40
13 36 32 73 53 40
14 36 33 76 58 40
36 33 77 60 45
16 35 34 78 61 43
17 34 34 78 63 43
18 34 34 79 64 45
5
Run 2 - Table 4
Bubbler starting weight 1303g
ending weight 1304g
Cold trap starting weight 0
Ending weight 16.5g ethanol + 16.5g water = 33g (50% EtOH)
Beer starting weight 481g ethanol + 1236 g water = 1717 (28% EtOH)
ending weight 307g ethanol + 1154 g water = 1462g (21 % EtOH)
Brine starting weight 1239g water + 1859LiBr = 3098g (60% LiBr)
Ending weight 11 5g EtOH + 1293g H2O + 1859g LiBr = 3267
(43% LIBr)
18
CA 02717766 2010-10-15
This.test also confirmed that the SAM device can preferentially remove ethanol
from an
ethanol-water mixture and simultaneously cool the ethanol water mixture. The
heat transfer
coefficient for the device in this run was calculated as 70 BTU/hr/ft2/ F. As
the liquid
distribution system in the test apparatus left unwetted much of the heat pipe
surface area,
this performance is viewed as relatively favourable. A more thorough liquid
distribution can
be expected to bring the coefficient in line with published values for
commercial systems,
which typically exceed 150 BTU/hr/ft2/ F.
Whereas Figure 1 shows a schematic SAM, it will be evident to persons of
ordinary skill in
the art that changes can be made.
Figure 7 shows one such possibility, namely, an arrangement wherein the heat
pipes 26 are
arranged parallel to a common axis X-X and the structure is adapted for
pivotal movement
about a horizontal axis Y-Y which is orientated normally to the common axis.
It is
contemplated that this arrangement could be used to periodically expedite
clearance of liquid
from the heat-delivering parts of the pipes.
Figure 8 shows another possibility, wherein the first volume 32 is defined by
one or more first
voids 32A, the second volume 34 is defined by one or more second voids 34A,
each of the
one or more first voids 32A and each of the one or more second voids 34A are
defined by a
respective vessel 130; and piping 132 defines the vapor passage 36. It is
contemplated that
this embodiment may have some usefulness in terms of reduced construction
costs, as well
as heat transfer efficiency [shorter heat pipes are normally better]. Discrete
vessels render
it possible to create substantial pressure differentials between the
absorption and
vaporization operations, and commensurate greater temperature differentials. A
greater
temperature differential would result in higher heat transfer through the heat
pipes; this could
have advantage in terms of capital costs, i.e. fewer heat pipes and smaller
vessels.
19
CA 02717766 2010-10-15
However, it should be understood that small pressure differentials could be
created even
within a SAM device of the type shown schematically in Figure 1, by the
interposition, for
example, of a pump or fan in the vapor passage.
Figure 9 shows yet another possible SAM structure, wherein the vessel 21 is
defined by a
horizontally-orientated cylindrical vessel, the vapor passage 35 [indicated by
arrows A] is
defined by external piping (not shown) and the first volume 32 and second
volume 34 are
separated from one another by a vertical bifurcating wall 140. The wall is
defined by upper
142 and lower 144 ridges extending interiorly from the tubular wall of the
vessel 21. A rubber
148 sheet spans between the ridges 142 and 144 and is sandwiched between steel
sheets
146 and 150 which are secured to one another by upper 156 and 152 lower webs.
The
rubber sheet 148 is perforated with holes to permit the heat pipes 26 to be
passed
therethrough in substantially hermetically sealed relation; the steel sheets
146,150 have
corresponding holes, of larger diameter, to permit free passage of the heat
pipes. Without
intending to be bound by theory, this arrangement is believed to be
advantageous from the
standpoint of relatively low construction costs and simplicity in terms of
maintenance; for
maintenance, the operator would merely be required to remove one end of the
vessel, and
slide the entire heat pipe assembly out horizontally. Various bearings or
rollers (not shown),
could also be employed, if desired, to further simply construction. Further,
whereas a single
bifurcating wall is shown, the vessel could be segmented by two walls, each
having heat
pipes formed therethrough, to produce a structure having similar functionality
to that shown
in Figure 8. In this two wall embodiment (not shown) the heat pipes could be
angled, so that
drop flow could travel back and forth in the chamber.
Further, whereas specific operating conditions are delineated in the
description relating to
Figures 4 and 5, it will be understood that wide variations are possible.
CA 02717766 2010-10-15
For example, in the context of an ethanol production facility, wherein the
viability of the yeast
is to be maintained on a continuous fermentation basis, at least the following
ranges are
contemplated to have utility:
= the broth withdrawn from the fermentation arrangement can have a temperature
of
about 28-32 C and an ethanol concentration of about 4-10%;
= the remainder broth can have a temperature of about 2-4 C lower than that of
the
withdrawn broth and have an ethanol concentration of about 2-4% less than that
of
the withdrawn broth; and
= the pressure in the first volume can be about 30-100 Torr.
As well, whereas the structure of Figure 5 is indicated to be useful for
ethanol production,
persons of ordinary skill in the art will readily recognize that the structure
could be readily
modified for handling other separations, notably but not limited to butanol
and methanol.
Indeed, the general structure of Figure 5 could be useful for any catabolic
reaction having a
catabolic inhibitor capable of removal by absorption. Further, whereas the
description
references continuous production, it should be understood that this is not
strictly necessary.
In a batch ethanol operation, broth could be withdrawn from the batch tank
while the
fermentation is underway and passed through a SAM device, to withdraw ethanol.
Removing ethanol from the batch would take stress off the yeast and could
decrease cycle
time and increase yield. As well, whereas in the context of ethanol and a LiBr
brine, the
thermodynamics are such that ethanol is withdrawn preferentially, i.e. at a
higher
concentration than the bulk, this is not strictly required for usefulness. In
the context of an
aqueous system, for example, wherein water and another component are being
withdrawn,
there could be occasions where it was acceptable that water was withdrawn in
preference to
the other component, and make-up water was added to balance flows. In this
further
regard, it should be understood that in this specification and the appended
claims, 'liquid
mixture' means a liquid with another material mixed together; the other
material may be
liquid, such as alcohol, but this is not necessarily the case, as evidenced
from, inter alia, the
apple juice concentrator example.
21
CA 02717766 2010-10-15
Further, whereas the secondary absorbers are shown in series with the SAM
devices, it will
be appreciated that this is not necessary. Secondary absorbers could be
deployed in
parallel, or omitted altogether in some situations.
Additionally, whereas the distributors are illustrated schematically as
perforated pipes, but it
will be understood that sprayers or distribution trays, such as used in packed
columns, could
be used. The particular form of distributor chosen will vary, inter alia, with
the geometry of
the reactor and is a matter of routine for persons of ordinary skill.
As yet another option, not shown, the structure of Fig. 5 could usefully be
used as an adjunct
to a conventional dry-mill corn ethanol plant. In plants of this type, the
bottoms of the beer
columns are typically sent to centrifuges, for the production of DDGS and
other co-products.
These bottoms contain unfermented C5 and C6 fermentable sugars, primarily
cellulose and
hemi-cellulose, which are difficult to process. Diversion of this bottoms
stream to the
structure of Fig. 5, for pre-treatment, hydrolization and fermentation, allows
additional ethanol
to be extracted from the original corn feedstock, and this incremental ethanol
production is
carried out in circumstances that obtain the general benefits in energy
efficiency previously
mentioned. Without intending to be bound by theory, it is believed that this
modification to
an existing plant in this way can increase ethanol yield per bushel of corn;
on a per bushel of
corn basis, increase revenue from ethanol sales that substantially offset
losses in revenue
from decreased sales of DDGS and other co-products; increase production
capacity of the
main plant (in that fermentation residence time can be reduced in the main
plant, since
unfermented sugars will be captured in the add-on plant); and generally
increase revenues
that offset increases in costs.
As another option, the SAM device could be replaced with a conventional liquid-
liquid heat
exchanger. FIG. 10 shows a tube 204 and shell 202 heat exchanger configured
for this
purpose. In this case, the flow of mixed liquid 242 would enter a manifold 206
on one side of
the heat exchanger and travel through tubes 204 to manifold 208. During this
travel, the
mixed liquid would be partially vaporized into a flow of vapor 230 and a
depleted flow of liquid
234. The flow of vapor 230 is directed back into the shell 202, at 232. An
entrainment
22
CA 02717766 2010-10-15
separator 210 ensures that only vapor is directed to the shell 202. Strong
brine 226 is
introduced into the shell such that vapor 232 is introduced to the brine 226.
Absorption of the
vapor 232 occurs, as in the case of the earlier-described embodiments,
producing a brine
enriched in ethanol which exits the shell at 228. Gases 236 leaving the shell
pass to an
entrainment separator 222, to return any entrained brine to the shell at 238,
and a vacuum
pump 224 draws non-condensables from the shell to exit at 240. A blower 220 is
used to
create a pressure differential in the vapor flows between 230 and 232, to
account for
pressure drop in the system. This alternative could have some advantage in
terms of capital
costs. However, the vapor 230 is in substantial vapor-liquid equilibrium with
the depleted
flow 234, i.e. higher water content, which has disadvantage in terms of
operating costs.
Yet further variations on all the above would be readily appreciated by
persons of ordinary
skill in the art. Accordingly, the invention should be understood as limited
only by the
accompanying claims, purposively construed.
23