Note: Descriptions are shown in the official language in which they were submitted.
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
METHODS AND SYSTEMS OF TREATING A WELLBORE
BACKGROUND OF INVENTION
Field of the Invention
[00011 Embodiments disclosed herein relate generally to methods and systems of
treating a wellbore, and more particularly to the removal of filtercakes which
form in
wellbores.
Background Art
[00021 Hydrocarbons (oil, natural gas, etc.) are typically obtained from a
subterranean
geologic formation (i.e., a "reservoir") by drilling a well that penetrates
the
hydrocarbon-bearing formation. In order for hydrocarbons to be "produced,"
that is,
travel from the formation to the wellbore (and ultimately to the surface),
there must
be a sufficiently unimpeded flowpath from the formation to the wellbore. One
key
parameter that influences the rate of production is the permeability of the
formation
along the flowpath that the hydrocarbon must travel to reach the wellbore.
Sometimes, the formation rock has a naturally low permeability; other times,
the
permeability is reduced during, for instance, drilling the well.
[00031 During the drilling of a wellbore, various fluids are typically used in
the well
for a variety of functions. When a well is drilled, a drilling fluid is often
circulated
into the hole to contact the region of a drill bit, for a number of reasons
such as: to
cool the drill bit, to carry the rock cuttings away from the point of
drilling, and to
maintain a hydrostatic pressure on the formation wall to prevent production
during
drilling. The fluids may be circulated through a drill pipe and drill bit into
the
wellbore, and then may subsequently flow upward through wellbore to the
surface.
During this circulation, the drilling fluid may act to remove drill cuttings
from the
bottom of the hole to the surface, to suspend cuttings and weighting material
when
circulation is interrupted, to control subsurface pressures, to maintain the
integrity of
the wellbore until the well section is cased and cemented, to isolate the
fluids from
the formation by providing sufficient hydrostatic pressure to prevent the
ingress of
formation fluids into the wellbore, to cool and lubricate the drill string and
bit,
and/or to maximize penetration rate.
1
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0004] During well operations, drilling fluid can be lost by leaking into the
formation.
To prevent this, the drilling fluid is often intentionally modified so that a
small
amount leaks off and forms a coating on the wellbore surface (often referred
to as a
"filtercake") and thereby protecting the formation. Filtercakes are formed
when
particles suspended in a wellbore fluid coat and plug the pores in the
subterranean
formation such that the filtercake prevents or reduce both the loss of fluids
into the
formation and the influx of fluids present in the formation. A number of ways
of
forming filtercakes are known in the art, including the use of bridging
particles,
cuttings created by the drilling process, polymeric additives, and
precipitates.
[0005] Upon completion of drilling, the filtercake may stabilize the wellbore
during
subsequent completion operations such as placement of a gravel pack in the
wellbore.
Additionally, during completion operations, when fluid loss is suspected, a
fluid loss
pill of polymers may be "spotted" or placed in the wellbore. Other completion
fluids
may be injected behind the fluid loss pill into a position within the wellbore
which is
immediately above a portion of the formation where fluid loss is suspected.
Injection
of fluids into the wellbore is then stopped, and fluid loss will then move the
pill
toward the fluid loss location to coat the formation and prevent or reduce
future fluid
loss.
[0006] After any completion operations have been accomplished, the filtercake
(formed during drilling and/or completion) on the side walls of the wellbore
must
typically be removed, because remaining residue of the filtercake may
negatively
impact production. That is, although filtercake formation and use of fluid
loss pills
are essential to drilling and completion operations, the barriers may be a
significant
impediment to the production of hydrocarbons or other fluids from the well,
if, for
example, the rock formation is still plugged by the barrier. Because
filtercake is
compact, it often adheres strongly to the formation and may not be readily or
completely flushed out of the formation by fluid action alone.
[0007] Thus, the filtercake must be removed during the initial state of
production,
either physically or chemically (i.e., via acids, oxidizers, and/or enzymes).
The
amount and type of drill solids affects the effectiveness of these clean up
treatments.
Also affecting the effectiveness of the clean up of the wellbore prior to
production is
2
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
the presence of polymeric additives, which may be resistant to degradation
using
conventional filtercake breakers.
[00081 The problems of efficient well clean-up and completion are a
significant issue
in all wells, and especially in open-hole horizontal well completions. The
productivity of a well is somewhat dependent on effectively and efficiently
removing
the filter cake while minimizing the potential of water blocking, plugging, or
otherwise damaging the natural flow channels of the formation, as well as
those of the
completion assembly.
[00091 Accordingly, there exists a continuing need for systems and methods
that
effectively and efficiently clean the wellbore.
SUMMARY OF INVENTION
[00101 In one aspect, embodiments disclosed herein relate to methods of
treating a
wellbore including emplacing at least one electrolytic tool in a desired
section of the
wellbore, applying an electric charge to wellbore fluids present in the
desired section
of the wellbore, and generating oxidants in situ by electrolyzing components
of the
wellbore fluids.
[00111 In another aspect, embodiments disclosed herein relate to methods of
breaking
a filtercake formed in a wellbore, including generating oxidants in situ by
electrolyzing components of a wellbore fluid present in the wellbore; and
allowing the
oxidants to degrade filtercake components.
[00121 In another aspect, embodiments disclosed herein relate to systems for
breaking
a filtercake formed on a surface of a wellbore, including a wellbore having a
filtercake formed thereon; a fluid supply source for supplying an aqueous
solution
into the wellbore; and at least one electrolytic tool for generating oxidants
in the
wellbore.
[00131 Other aspects and advantages of the invention will be apparent from the
following description and the appended claims.
3
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
BRIEF DESCRIPTION OF DRAWINGS
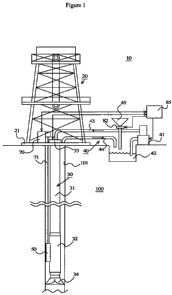
[0014] Figure 1 is a schematic drawing of one embodiment of a drilling system.
[0015] Figure 2 is a schematic view of an electrolytic tool, according to
embodiments
disclosed herein.
[0016] Figure 3 is a block diagram of an oxidant generation system, according
to
embodiments disclosed herein.
[0017] Figure 4 is a flow chart showing a process of a filtercake treatment,
according
to embodiments disclosed herein.
DETAILED DESCRIPTION
[0018] Generally, embodiments disclosed herein relate to the use of
electrolytic tools
downhole. In one aspect, embodiments disclosed herein relate to methods of
treating
a wellbore including emplacing an electrolytic tool in a desired section of
the
wellbore, applying an electric charge to wellbore fluids present, and
generating
oxidants in situ by electrolyzing components of the wellbore fluid. In another
aspect,
embodiments disclosed herein relate to methods of breaking a filtercake formed
in a
wellbore, including generating oxidants in situ by electrolyzing components of
a
wellbore fluid present in the wellbore, and allowing the oxidants to degrade
filtercake
components. In yet another aspect, embodiments disclosed herein relate to
systems
for breaking a filtercake formed on a surface of a wellbore, including a
wellbore
having a filtercake formed thereon, a fluid supply source for supplying an
aqueous
solution into the wellbore and an electrolytic tool for generating oxidants in
the
wellbore.
[0019] Removal of filtercakes is a key concern in well completion operations
as
incomplete removal of a filtercake can negatively affect subsequent
hydrocarbon
production. The applicants have advantageously found that an electrolytic tool
may
be used downhole to generate oxidants in situ which are able to degrade
filtercakes.
Use of such tools may provide desired control over the timing of the breaking
of the
filtercake, and alternatively may provide for generating oxidants in situ to
allow for
control of bacterial populations in the wellbore.
4
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0020] Methods and systems disclosed herein may be used in any drilling system
known in the art. Referring initially to Figure 1, a schematic drawing of a
typical
drilling system is shown. A drilling system 10 is provided for drilling a
wellbore into
an earthen formation 100 to exploit natural resources such as oil. The
drilling system
includes a derrick 20, a drill string assembly 30, a fluid circulation system
40, an
electrolytic tool 50, a winch unit 70, and a control unit 85. The derrick 20
is built on a
derrick floor 21 placed on the ground. The derrick 20 supports the drill
string
assembly 30 which is inserted into a wellbore 101 and carries out a drilling
operation.
[0021] The drill string assembly 30 includes a drill string 31, a bottom hole
assembly
32, and a drive system 33. During an operation for drilling the wellbore 101,
the drill
pipe 31 is rotated by the drive system 33, and this rotation is transmitted
through the
bottom hole assembly 32 to the drill bit 34.
[0022] The fluid circulation system 40 includes a fluid pump 41, a mud pit 42,
a
supply line 43, and a return line 44. The fluid circulation system 40
circulates a
wellbore fluid through the drill string assembly 30 and into the wellbore 101.
Specifically, the fluid pump 41 pumps wellbore fluid, which is reserved in the
mud pit
42, through the supply line 43, and then, the wellbore fluid is injected into
the drill
string 31. The wellbore fluid injected into drill string 31 is then discharged
from the
drill bit 34 to the bottom of the wellbore 101 and returns to the mud pit 42
through the
return line 44.
[0023] When drilling a wellbore 101, fluids that exit drill bit 34 and
circulate through
the wellbore 101 may form a thin, low-permeability filtercake to seal
permeable
formations 100 penetrated by the bit 34. A variety of drilling fluids
including oil-
based and water-based wellbore fluids may be used to drill a wellbore 101.
These
well fluids may consist of synthetic polymers or biopolymers (such as to
increase the
rheological properties (e.g. plastic viscosity, yield point value, gel
strength) of the
drilling mud), clays, polymeric thinners, flocculants, and organic colloids
added
thereto to obtain the required viscosity and filtration properties. Heavy
minerals, such
as barite or carbonate, may also be added to increase density. Further, solids
from the
formation are incorporated into the mud and often become dispersed in the mud
as a
consequence of drilling.
5
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0024] Because such additives or solids may form a part of the filtercake due
to their
presence in the fluid, some additives may be added to specifically impart
desired
properties to the filtercake, to prevent both the loss of fluids from the
wellbore into
the formation and the influx of fluids that may be present in the formation
into the
wellbore. For example, various polymeric additives may also act as fluid loss
control
agents to prevent or reduce the loss of wellbore fluid to the surrounding
formation by
reducing the permeability of filtercakes formed on the newly exposed rock
surface.
Most of the polymeric additives employed in drilling muds are resistant to
biodegradation, thereby extending the utility of the additives for the useful
life of the
mud. Specific examples of biodegradation-resistant polymeric additives include
biopolymers; synthetic polymers, such as polyacrylamides and other acrylamide-
based polymers; cellulose derivatives, such as dialkylcarboxymethylcellulose,
hydroxyethylcellulose; and the sodium salts of carboxy-methylcellulose,
chemically
modified starch, guar gum, phosphomannans, scleroglucans, glucans, and
dextrans.
Further, in addition to such polymeric additives, bridging agents such as
calcium
carbonate or fibrous materials may be added to bridge fractures or pores in a
formation. While the filtercake serves an important role in drilling
operations, the
barrier can be a significant impediment to the production of hydrocarbons from
the
formation. Thus, once drilling and completion operations are complete, and
production is desired, this coating or filtercake must be removed.
[0025] Removal of filtercakes is therefore a key concern in well completion
operations. Typical prior art techniques involve using breaking agents such as
enzymes, oxidants or acids to remove filtercakes downhole. Examples of such
techniques may be found in US Patent Nos. 1,984,668, 4,609,475, 4,941,537, 5,
247,
995, 6,861,394, and 5,607,905. However, the use of these various breaking
agents
may have disadvantages. For example, enzymes may be expensive and sensitive to
the harsh downhole environment, and acids tend to be costly, inefficient and
time
consuming. Also, prior art breaking agents may work too slowly or too quickly
and
therefore may not allow control over timing of the breaking of the filtercake.
[0026] The applicants have advantageously found that oxidants for degrading a
filtercake in accordance with embodiments disclosed herein, may be generated
in situ
downhole by use of an electrolytic tool. Referring now to Figure 2, a
schematic of a
6
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
simple electrolytic cell 51 according to some embodiments disclosed herein is
shown.
The electrolytic cell 51 includes at least one inlet port 54, through which a
brine
solution present in the wellbore may enter electrolytic cell 51, and at least
one outlet
port 56, through which generated oxidants may exit into the wellbore. The
electrolytic cell 51 may contain at least one reaction chamber 57, for the
housing of
electrodes. The electrodes may be of any type or configuration known in the
art. The
electrolytic cell may contain at least two electrodes wherein at least one
electrode is a
positive electrode or an anode 58, and at least one electrode is a negative
electrode or
a cathode 59. The electrolytic tool may further include at least one control
circuit (not
shown) for selectively providing an electrical potential between the at least
one
cathode and the at least one anode, and an energy source (not shown) in
electrical
contact with the control circuit for delivering a controlled electrical charge
to the
control circuit. The at least one control circuit may be in electrical contact
with the
cathode 59 and the anode 58. Further, one skilled in the art would appreciate
that no
limitation on the arrangement of electrolytic cells may find use in accordance
with
embodiments of the present disclosure. Non-limiting examples of various
electrolytic
cells that may be used and/or modified for use downhole in the methods and
system
of the present disclosure include those described in U.S. Patent Nos.
4,761,208,
5,385,711, 6,261,464, 6,524,475, 6,558,537, 6,736,966, 6,805,787, 7,005,075,
and
7,008,523, all of which are herein incorporated by reference. One of skill in
the art
would also recognize that electrolytic cells may be incorporated into hardware
typically used in downhole. For example, completion hardware such as slotted
liners
and sand screens may be used as electrodes for the generation of oxidants
within the
wellbore in some embodiments of the present disclosure.
[00271 As briefly mentioned, a brine solution may enter and generated oxidants
may
exit the electrolytic cell 51. For optimal generation of oxidants within the
wellbore
by the electrolytic cell 51, there must be an electrolyte solution capable of
transmitting an electrical charge upon which the electrolytic cell 51 may act.
The
capacity to transmit an electrical charge is known to be related to the ionic
character
of the electrolyte. Thus, when using the tool downhole to generate oxidants,
the
wellbore fluid may act as an electrolyte. Use of the wellbore fluid as an
electrolyte
is environmentally friendly and provides cost savings because no additional
fluids
need to be introduced into the wellbore.
7
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0028] In particular, the wellbore fluid acting as an electrolyte may be a
water-based
fluid. The wellbore fluid may include an aqueous solution as the base fluid
including
at least one of fresh water, sea water, brine, mixtures of water and water-
soluble
organic compounds and mixtures thereof. For example, the aqueous solution may
be
formulated with mixtures of desired salts in fresh water. Such salts may
include, but
are not limited to alkali metal chlorides, hydroxides, or carboxylates, for
example. In
various embodiments, the wellbore fluids disclosed herein may include
seawater,
aqueous solutions wherein the salt concentration is less than that of sea
water, or
aqueous solutions wherein the salt concentration is greater than that of sea
water.
Salts that may be found in seawater include, but are not limited to, sodium,
calcium,
aluminum, magnesium, potassium, strontium, lithium, and salts of chlorides,
bromides, carbonates, iodides, chlorates, bromates, formates, nitrates,
oxides, sulfates,
silicates, phosphates and fluorides. Salts that may be incorporated in brines
include
any one or more of those present in natural seawater or any other organic or
inorganic
dissolved salts.
[0029] Additionally, brines that may be used in the drilling fluids disclosed
herein
may be natural or synthetic, with synthetic brines tending to be much simpler
in
constitution. In a particular embodiment, a brine may include halide or
carboxylate
salts of mono- or divalent cations of metals, such as cesium, potassium,
calcium, zinc,
and/or sodium. The presence of these salts enhances the ionic character of the
wellbore fluid, thereby increasing its ability to transmit an electric charge
and
enhancing its properties as an electrolyte.
[0030] Referring back to Figure 2, an electrical potential may be provided by
a
control unit (shown in Figure 3 as 85), and may be conducted between the
electrodes
58 and 59 by the wellbore fluid. A controlled electrical charge passes through
the
wellbore fluid from the at least one cathode 59 to the at least one anode 58,
thereby
generating at least one oxidant in the electrolytic solution. When the
wellbore fluid
flows through the reaction chamber 57 of the electrolytic cell 51, and an
electrical
current is passed between the anode 58 and the cathode 59, several chemical
reactions occur that involve the water, as well as one or more of the other
salts or
ions contained in the wellbore fluid.
8
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0031] The electrical current polarizes the electrodes 58, 59 and causes
dissociation of
the wellbore fluid into component ions. For example, where the wellbore fluid
includes a solution of sodium chloride (NaCI), the NaCl brine may dissociate
into
sodium and chlorine ions which would migrate to the cathode and to the anode,
respectively:
[0032] NaCl(aq) Na+(aq) + Cl (aq)
[0033] The anode is known to be electron deficient, and without being bound by
any
particular theory, it is believed that the anode withdraws electrons from the
water
and other ions adjacent to the anode, which results in the formation of
oxidative
species in the wellbore electrolyte. For instance, the following chlorine
generating
reaction may occur at the anode surface:
[0034] 2Cl-(aq) 4 C12(g) + 2e-
[0035] The chlorine gas (Cl2) generated by the chlorine reaction may dissolve
in the
water to generate hypochlorite ions (OCl-) which are an oxidative species
useful in
embodiments of this disclosure:
[0036] C12(g) + H20(l) 4 20C1-(,,q) + 2H+(aq)
[0037] Note that several other potential chlorine-oxygen reactions (e. g.
chlorine
dioxide) may also take place.
[0038] The protons generated (H) may in turn combine with free electrons at
the
electron-rich cathode to generate hydrogen gas, which may be vented from the
electrolytic tool by any means known in the art:
[0039] 2H+(aq) + 2e- 4 H2(g)
[0040] While the chemistry of oxidant generation has been illustrated by using
NaCl
brines as an example, one skilled in the art would appreciate that these
principles
apply to the generation of oxidants from any ionic solution by electrolysis.
The
present disclosure relates to the production of one or more oxidants and may
include, for example, hypochlorite, chlorine, bromine, chlorine dioxide,
ozone,
hydrogen peroxide, and other chioro-oxygenated and bromo-oxygenated species.
9
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0041] Flow dynamics, which include the movement of molecules in a flowing
solution by turbulence, predict that the conversion of salts will increase as
the
solution flow path nears the anode surface layer. Consequently, in some
embodiments, methods and systems of the present disclosure preferably maximize
the flow of the wellbore electrolyte over the anode in order to maximize the
generation of oxidants. Flow of the wellbore fluid may be enhanced by any
means
known in the art, for example mixers such as propellers, etc.
[0042] In particular, pumping devices 60, 61 may be set between the positive
electrode 58 and the negative electrode 59. The pumping devices may have
propeller blades, valves, or any means known in the art to generate a fluid
stream in
the reaction chamber 57 so that the wellbore fluid surrounding the
electrolytic tool is
induced into the reaction chamber 57 of the electrolytic cell 51 through inlet
port 54,
passes through the reaction chamber 57 of the electrolytic cell 51, and is
released
from the outlet port 56. The inlet port 54 may include an inlet port mechanism
such
as a valve, or any other mechanism known in the art to seal the inlet port
after the
wellbore fluid has entered the cell. Once generated, the oxidant-rich wellbore
fluid
may exit the electrolytic cell 51 via the outlet port 56.
[0043] The local concentration of oxidants present in the exiting wellbore
fluid may
be measured by any instrument known in the art, for example, an oxidant
sensor.
Once the oxidant sensor has detected that the local concentration of oxidant
is
sufficient to break the filtercake, the electrical potential applied across
the electrodes
of the electrolytic cell may be removed and the electrolytic tool may then be
removed from the wellbore.
[0044] The oxidants now present in the wellbore fluid may degrade the
filtercake by
any mechanism known in the art. For example, it is known that filtercakes may
comprise polymers such as polysaccharides. Oxidants are known to attack the
glycosidic linkage between the rings, causing chain scission. Accordingly, as
the
polymer breaks down to shorter chains, the filtercake degrades, and may be
removed
by the circulating wellbore fluid. The oxidant becomes reduced by this
process, and
the reduced form may be reoxidized by the electrolytic tool, if deemed
necessary.
Alternatively, one skilled in the art would appreciate that the electrolytic
tool may
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
continuously (or intermittently) generate oxidants until it has been
determined that
the filtercake has been sufficiently removed.
[0045] The applicants have also found that the ability to generate oxidants in
situ for
the breaking of filtercakes provides advantageous control over the timing of
the
breaking of the filtercake. Because the electrolytic tool may be emplaced at
the site
of the filtercake desired to be removed (e.g., at the producing interval),
thereby
generating an oxidant-rich environment in close proximity to the filtercake,
the
timing of the breaking of the filtercake may be triggered by the providing of
an
electrical potential across the electrodes of the electrolytic cell. For
example, this
technique may provide greater controllability as compared to conventional
emplacement of breaker fluids, which may react too fast or too slow depending
on
the presence or absence of delay mechanisms.
[0046] Additionally, the applicants have further found that an electrolytic
tool may be
placed downhole to generate oxidants in situ which are able to kill bacteria
which
may be present in the wellbore. The drilling process initiates communication
between the surface and the subsurface oilfield environments. During drilling,
wellbore fluids may be circulated from the surface to the bit to remove
cuttings, and
to control formation pressures downhole. In this process, chemicals and
bacteria
from the surface may be circulated into the deep subsurface energy-rich, oil-
bearing
strata, and the hydrocarbon laden cuttings may be brought into the oxygen-
rich,
moderate temperature surface environment. Through this mechanical process,
microbiological activity may be initiated in the surface and subsurface
environments. While this typically does not occur normally, this may lead to
bacterial contamination of the wellbore.
[0047] Further, organic polymers present as viscosifiers and fluid loss
control agents
in a wellbore fluid tend to be of plant or microbiological origin and may act
as a ready
food source for growth of naturally occurring oilfield bacteria. If bacterial
growth is
excessive, the consumption of these organic wellbore fluid components may
result in
a loss of the rheological properties of the mud, microbial corrosion of well
tubulars
and screens, biomass plugging in injection wells and the formation, and
hydrogen
sulfide production deep in the formation. If left untreated, it is possible
that bacterial
contamination may cause a breakdown of wellbore integrity.
11
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
[0048] Thus, in accordance with certain embodiments, oxidants generated in
situ in a
wellbore from an electrolyte solution may be used to kill bacteria downhole.
Without wishing to be bound by theory, it is believed that oxidants may attack
components of the bacterial cell wall, such as peptidoglycans and other
polysaccharides. Accordingly, methods and systems disclosed herein may
generate
oxidants in situ for the reduction of bacterial populations downhole.
[0049] Electrolytic tools of use in embodiments disclosed herein may be placed
in the
wellbore by any means known in the art. For example, the various embodiments
of
the present disclosure may work by placing at least part of or the entire
electrolytic
tool in the wellbore. Placement may occur at any stage of wellbore operations.
Typically, the electrolytic tool may be placed in the wellbore during
completion and
before production. However, one skilled in the art would appreciate that no
limitation
exists on the present disclosure. For example, it is envisioned that after
production
has begun, it may be determined that residual filtercake may remain on the
wellbore
walls impeding production, thereby requiring a subsequent cleaning operation,
such as
by use of the electrolytic tools disclosed herein. Further, if a wellbore
stabilizing gel
is misplaced during drilling operations, it is envisioned that electrolytic
tools
disclosed herein may be used to trigger breaking of the gel in the
inappropriate
location so that it may be placed in the desired location. Additionally, if
the tool is
being used to control bacterial growth, it is envisioned that it may be
desirable to form
oxidants at any stage, including drilling.
[0050] Thus, when generation of oxidants is desired, the electrolytic tool, or
portions
thereof, may be placed in the desired section of the well. This provides
advantageous control over axial placement. With prior art breaker fluids,
problems
may arise with respect to the proper placement of the breaker fluid, that is,
ensuring
that it is delivered to the entire desired zone (that is, the zone that needs
filtercake
removal). It is foreseeable in some cases that the portions of the filtercake
that
encounter the breaker fluid first may react and break apart more quickly than
other
portions of the filtercake do, with the potential that some fluid loss may be
experienced in the region in which the filter cake has quickly broken up. Use
of an
electrolytic tool with adequate dimensions may advantageously allow generation
of
oxidants over all of the filtercake, so that most of the filtercake may be
broken at
12
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
about the same time. Alternatively, several electrolytic cells may be emplaced
in
proximity to the filtercake to advantageously allow for generation of oxidants
over
all of the filtercake.
[0051] In embodiments disclosed herein, the desired depth and/or lateral
positioning
of the electrolytic tool in the wellbore may be advantageously controlled by
the use
of any equipment known in the art such as winches etc. Further, the depth and
lateral positioning of the electrolytic tool in the wellbore may be measured
by any
instrumentation known in the art, such as depth gauges, sensors, cameras etc.
Once
optimal placement of the electrolytic tool has been achieved, the oxidants may
then
be generated in situ at the desired section of the wellbore, thereby achieving
paramount axial distribution of the oxidant breaker.
[0052] Referring now to Figure 3, a block diagram of an exemplary electrolytic
tool
according to embodiments herein is shown. The electrolytic tool includes an
oxidant
generation system 80. The oxidant generation system 80 includes the oxidant
generator 50, a control unit 85, the winch unit 70, a power supply unit 81,
and a valve
actuator 82. The oxidant generator 50 includes an electrolytic cell 51, an
oxidant
sensor 52, and optionally a hydraulic power generator 53. In some cases, the
oxidant
generator 50 may comprise multiple electrolytic cells 51, which may be
electrically
connected to each other in series or in parallel, to allow for the breaking of
filtercakes
over larger intervals. Alternatively, multiple oxidant generators 50 may be
used in a
single operation depending on the length of interval to be broken and/or
dimensions
of the tool. The oxidant generator 50 is suspended in the wellbore 101 by a
cable 71.
A winch unit 70 lifts and/or lowers the cable 71 to adjust depth position of
the oxidant
generator 50 in the wellbore 101. The control unit 85 includes, for example, a
CPU, a
ROM, a RAM, an input and an output port, a memory apparatus and the like (not
shown). The control unit 85 is electrically connected to at least the oxidant
generator
50, the winch unit 70, and power supply unit 81.
[0053] The control unit 85 operates the oxidant generator 50, the winch unit
70 and
valve actuator 82 by transmitting command signals (solid arrowed lines). The
command signals may be based on detection signals of the oxidant sensor 51
connected to the oxidant generator 50 and/or the depth gauge 72 connected to
the
winch unit 70. In some embodiments where the depth gauge 72 reports that the
13
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
oxidant generator 50 has not been sufficiently lowered, or alternatively, has
been
lowered too much, a feedback command signal may be sent to the winch unit 70
through the control unit 85 to adjust the depth of the oxidant generator 50
accordingly. In other embodiments, where the oxidant sensor 51 detects that
the
concentration of the oxidant may be less than desired, or alternatively, more
than
desired, a feedback command signal may be sent to the winch unit 70 through
the
control unit 85 to adjust the output of the oxidant generator accordingly. The
feedback command signal may be automated or input manually. Accordingly, the
power supply unit 81 supplies electrical power (broken arrowed lines) to
control unit
60, the oxidant generator 50, the winch unit 70 and the valve actuator 82,
based on
command signals transmitted by the control unit 85.
[00541 Referring now to Figure 4, a method of treating a wellbore is shown in
a flow
chart. In 1000, a wellbore fluid which is an electrolytic brine solution may
be
emplaced within a wellbore. One skilled in the art would appreciate that such
electrolytic brine solutions may have been the fluid used to drill the
wellbore or may
have been a subsequent fluid placed in the wellbore for completion operations,
for
example. In 2000, the electrolytic tool may be placed in the section of the
wellbore
where removal of the filtercake is desired. In 3000, applying voltage to the
electrodes generates oxidants in the brine solution in the electrolytic cell.
In 4000, the
wellbore is evaluated to assess the efficiency of the breaking of the
filtercake. If the
filtercake has been sufficiently removed to allow desired hydrocarbon
production, the
electrolytic tool is deactivated in 5000, and removed from the wellbore, as in
6000. If
the filtercake has not been sufficiently removed, the electrolytic tool may be
activated
once again by applying a voltage across the electrodes, as in 3000. This
iteration may
repeat until the filtercake has been sufficiently removed, and then the
electrolytic tool
may then be deactivated and removed from the wellbore as in 5000 and 6000,
respectively.
[00551 Advantageously, embodiments of the present disclosure provides for the
degradation of filtercakes by oxidants generated downhole, in situ, by use of
an
electrolytic tool. The in situ generation of oxidants may provide advantageous
control
over timing of breaking of the oxidative breaker in the wellbore. Further,
generating
oxidants in situ from relatively benign precursors such as brines may result
in less
14
CA 02718072 2010-09-09
WO 2009/112948 PCT/IB2009/005119
corrosion in the drill string assembly and is more environmentally friendly.
Even
further, generating oxidants in situ at the desired site may allow use of
smaller
volumes of chemicals such as oxidative breaker and other additives, and may be
more
cost-efficient, using species already present in a wellbore instead of
requiring a
subsequent pumping of a breaker fluid downhole. Applicants have further
advantageously found that generating oxidants downhole may allow for control
of
bacterial populations downhole. Control of bacterial populations downhole may
result in decreased microbial corrosion of tubular and screens, biomass
plugging, and
hydrogen sulfide production. As such, appreciable cost savings, environmental,
and
safety benefits may be actualized by use of embodiments of the methods and
systems
of the present disclosure.
[00561 While the invention has been described with respect to a limited number
of
embodiments, those skilled in the art, having benefit of this disclosure, will
appreciate
that other embodiments can be devised which do not depart from the scope of
the
invention as disclosed herein. Accordingly, the scope of the invention should
be
limited only by the attached claims.