Note: Descriptions are shown in the official language in which they were submitted.
CA 02718294 2013-08-20
NON-LINEAR DELIVERY DEVICE AND OCULAR IMPLANT FOR LOWERING
INTRAOCULAR PRESSURE
Cross-Reference to Related Applications
The present application is a divisional of Canadian Patent Application No.
2,442,652 filed April 8, 2002.
Background of the Invention
Field of the Invention
The invention relates generally to medical devices and methods for reducing
the
intraocular pressure in an animal eye and, more particularly, to shunt type
devices for
permitting aqueous outflow from the eye's anterior chamber and associated
methods
thereof for the treatment of glaucoma.
Description of the Related Art
The human eye is a specialized sensory organ capable of light reception and
able to receive visual images. The trabecular meshwork serves as a drainage
channel
and is located in anterior chamber angle formed between the iris and the
cornea. The
trabecular meshwork maintains a balanced pressure in the anterior chamber of
the eye
by draining aqueous humor from the anterior chamber.
About two percent of people in the United States have glaucoma. Glaucoma is a
group of eye diseases encompassing a broad spectrum of clinical presentations,
etiologies, and treatment modalities. Glaucoma causes pathological changes in
the optic
nerve, visible on the optic disk, and it causes corresponding visual field
loss, resulting in
blindness if untreated. Lowering intraocular pressure is the major treatment
goal in all
glaucomas.
In glaucomas associated with an elevation in eye pressure (intraocular
hypertension), the source of resistance to outflow is mainly in the trabecular
meshwork.
The tissue of the trabecular meshwork allows the aqueous humor ("aqueous") to
enter
Schlemm's canal, which then empties into aqueous collector channels in the
posterior
wall of Schlemm's canal and then into the aqueous veins, which form the
episcleral
venous system. Aqueous humor is a transparent liquid that fills the region
between the
cornea, at the front of the eye, and the lens. The aqueous humor is
continuously
- 1 -
= CA 02718294 2013-08-20
secreted by the ciliary body around the lens, so there is a constant flow of
aqueous
humor from the ciliary body to the eye's front chamber. The eye's pressure is
determined
by a balance between the production of aqueous and its exit through the
trabecular
meshwork (major route) or uveal scheral outflow (minor route). The trabecular
meshwork
is located between the outer rim of the iris and the back of the cornea, in
the anterior
chamber angle. The portion of the trabecular meshwork adjacent to Schlemm's
canal
(the juxtacanilicular meshwork) causes most of the resistance to aqueous
outflow.
Glaucoma is grossly classified into two categories: closed-angle glaucoma,
also
know as angle closure glaucoma, and open-angle glaucoma. Closed-angle glaucoma
is
caused by closure of the anterior chamber angle by contact between the iris
and the
inner surface of the trabecular meshwork. Closure of this
- la -
CA 02718294 2010-10-15
anatomical angle prevents normal drainage of aqueous humor from the anterior
chamber of the eye.
Open-angle glaucoma is any glaucoma in which the angle of the anterior chamber
remains open, but
the exit of aqueous through the trabecular meshwork is diminished. The exact
cause for diminished filtration
is unknown for most cases of open-angle glaucoma. Primary open-angle glaucoma
is the most common of
- 2 -
CA 02718294 2010-10-15
the glaucomas, and it is often asymptomatic in the early to moderately
advanced stage. Patients may suffer
substantial, irreversible vision loss prior to diagnosis and treatment.
However, there are secondary open-
angle glaucomas which may include edema or swelling of the trabecular spaces
(e.g., from corticosteroid
use), abnormal pigment dispersion, or diseases such as hyperthyroidism that
produce vascular congestion.
Current therapies for glaucoma are directed at decreasing intraocular
pressure. Medical therapy
includes topical ophthalmic drops or oral medications that reduce the
production or increase the outflow of
aqueous. However, these drug therapies for glaucoma are sometimes associated
with significant side effects,
such as headache, blurred vision, allergic reactions, death from
cardiopulmonary complications, and potential
interactions with other drugs. When drug therapy fails, surgical therapy is
used. Surgical therapy for open-
angle glaucoma consists of laser trabeculoplasty, trabeculectomy, and
implantation of aqueous shunts after
failure of trabeculectomy or if trabeculectomy is unlikely to succeed.
Trabeculectomy is a major surgery that
is widely used and is augmented with topically applied anticancer drugs, such
as 5-flurouracil or mitomycin-C
to decrease scarring and increase the likelihood of surgical success.
Approximately 100,000 trabeculectomies are performed on Medicare-age patients
per year in the
United States. This number would likely increase if the morbidity associated
with trabeculectomy could be
decreased. The current morbidity associated with trabeculectomy consists of
failure (10-15%); infection (a life
long risk of 2-5%); choroidal hemorrhage, a severe internal hemorrhage from
low intraocular pressure,
resulting in visual loss (1%); cataract formation; and hypotony maculopathy
(potentially reversible visual loss
from low intraocular pressure).
For these reasons, surgeons have tried for decades to develop a workable
surgery for the trabecular
meshwork.
The surgical techniques that have been tried and practiced are
goniotomy/trabeculotomy and other
mechanical disruptions of the trabecular meshwork, such as trabeculopuncture,
goniophotoablation, laser
trabecular ablation, and goniocurretage. These are all major operations and
are briefly described below.
Goniotomygrabeculotomy. Goniotomy and trabeculotomy are simple and directed
techniques of
microsurgical dissection with mechanical disruption of the trabecular
meshwork. These initially had early
favorable responses in the treatment of open-angle glaucoma. However, long-
term review of surgical results
showed only limited success in adults. In retrospect, these procedures
probably failed due to cellular repair
and fibrosis mechanisms and a process of 'filling in." Filling in is a
detrimental effect of collapsing and closing
in of the created opening in the trabecular meshwork. Once the created
openings close, the pressure builds
back up and the surgery fails.
Trabeculopuncture: Q-switched Neodynium (Nd) YAG lasers also have been
investigated as an
optically invasive technique for creating full-thickness holes in trabecular
meshwork. However, the relatively
small hole created by this trabeculopuncture technique exhibits a filling-in
effect and fails.
- 3 -
CA 02718294 2010-10-15
Goniophotoablation/Laser Trabecular Ablation: Goniophotoablation is disclosed
by Berlin in U.S.
Patent No. 4,846,172 and involves the use of an excimer laser to treat
glaucoma by ablating the trabecular
meshwork. This was demonstrated not to succeed by clinical trial. Hill et al.
used an Erbium:YAG laser to
create full-thickness holes through trabecular meshwork (Hill et at., Lasers
in Surgery and Medicine 11:341-
346, 1991). This technique was investigated in a primate model and a limited
human clinical trial at the
University of California, Irvine. Although morbidity was zero in both trials,
success rates did not warrant
further human trials. Failure was again from filling in of surgically created
defects in the trabecular meshwork
by repair mechanisms. Neither of these is a viable surgical technique for the
treatment of glaucoma.
Goniocurretage: This is an ab intemo (from the inside), mechanically
disruptive technique that uses
an instrument similar to a cyclodialysis spatula with a microcurrette at the
tip. Initial results were similar to
trabeculotomy: it failed due to repair mechanisms and a process of filling in.
Although trabeculectomy is the most commonly performed filtering surgery,
viscocanulostomy (VC)
and non-penetrating trabeculectomy (NPT) are two new variations of filtering
surgery. These are ab extern
(from the outside), major ocular procedures in which Schlemm's canal is
surgically exposed by making a large
and very deep scleral flap. In the VC procedure, Schlemm's canal is cannulated
and viscoelastic substance
injected (which dilates Schlemm's canal and the aqueous collector channels).
In the NPT procedure, the inner
wall of Schlemm's canal is stripped off after surgically exposing the canal.
Trabeculectomy, VC, and NPT involve the formation of an opening or hole under
the conjunctiva and
'scleral flap into the anterior chamber, such that aqueous humor is drained
onto the surface of the eye or into
the tissues located within the lateral wall of the eye. These surgical
operations are major procedures with
significant ocular morbidity. When trabeculectomy, VC, and NPT are thought to
have a low chance for
success, a number of implantable drainage devices have been used to ensure
that the desired filtration and
outflow of aqueous humor through the surgical opening will continue. The risk
of placing a glaucoma
drainage device also includes hemorrhage, infection, and diplopia (double
vision).
Examples of implantable shunts and surgical methods for maintaining an opening
for the release of
aqueous humor from the anterior chamber of the eye to the sclera or space
beneath the conjunctiva have
been discbsed in, for example, U.S. Patent No. 6,059,772 to Hsia et at., and
U.S. Patent No. 6,050,970 to
Baerveldt.
AN of the above surgeries and variations thereof have numerous disadvantages
and moderate
success rates. They involve substantial trauma to the eye and require great
surgical skill in creating a hole
through the full thickness of the sclera into the subconjunctival space. The
procedures are generally
performed in an operating room and have a prolonged recovery time for vision.
The complications of existing filtration surgery have prompted ophthalmic
surgeons to find other
approaches to lowering intraocular pressure.
- 4 -
CA 02718294 2010-10-15
The trabecular meshwork and juxtacanilicular tissue together provide the
majority of resistance to the outflow of aqueous and, as such, are logical
targets for
surgical removal in the treatment of open-angle glaucoma. In addition, minimal
amounts of tissue are altered and existing physiologic outflow pathways are
utilized.
As reported in Arch. Ophthalm. (2000) 118: 412, glaucoma remains a leading
cause of blindness, and filtration surgery remains an effective, important
option in
controlling the disease. However, modifying existing filtering surgery
techniques in any
profound way to increase their effectiveness appears to have reached a dead
end. The
article further states that the time has come to search for new surgical
approaches that
to may provide better and safer care for patients with glaucoma.
Therefore, there is a great clinical need for a method of treating glaucoma
that is
faster, safer, and less expensive than currently available modalities.
Summary of the Invention
The trabecular meshwork and juxtacanilicular tissue together provide the
majority of resistance to the outflow of aqueous and, as such, are logical
targets for
surgical approach in the treatment of glaucoma. Various embodiments of
glaucoma
shunts are disclosed herein for aqueous to exit through the trabecular
meshwork (major
route) or uveal scleral outflow (minor route) or other route effective to
reduce
intraocular pressure (10P).
Glaucoma surgical morbidity may be greatly decreased if one were to bypass
the focal resistance to outflow of aqueous only at the point of resistance,
and to utilize
remaining, healthy aqueous outflow mechanisms. This is in part because
episcleral
aqueous humor exerts a backpressure that prevents intraocular pressure from
going too
low, and one could thereby avoid hypotony. Thus, such a surgery may reduce the
risk
of hypotony-related maculopathy and choroidal hemorrhage. Furthermore, visual
recovery would be very rapid, and the risk of infection would be very small,
reflecting
a reduction in incidence from 2-5% to about 0.05%.
US 6,638,239 issued October 28, 2003 entitled APPARATUS AND METHOD
FOR TREATING GLAUCOMA, and US 6,736,791, issued May 18, 2004, entitled
GLAUCOMA TREATMENT DEVICE, disclose devices and methods of placing a
- 5 -
CA 02718294 2010-10-15
trabecular shunt ab intern , i.e., from inside the anterior chamber through
the trabecular
meshwork, into Schlemm's canal. The invention encompasses both ab intern and
ab
externo glaucoma shunts or stents and methods thereof.
Techniques performed in accordance with aspects herein may be referred to
generally as "trabecular bypass surgery." Advantages of this type of surgery
include
lowering intraocular pressure in a manner which is simple, effective, disease
site-
specific, and can potentially be performed on an outpatient basis.
Generally, trabecular bypass surgery (TBS) creates an opening, a slit, or a
hole
through trabecular meshwork with minor microsurgery. TBS has the advantage of
a
to much lower risk of choroidal hemorrhage and infection than prior
techniques, and it
uses existing physiologic outflow mechanisms. In some aspects, this surgery
can
potentially be performed under topical or local anesthesia on an outpatient
basis with
rapid visual recovery. To prevent "filling in" of the hole, a biocompatible
elongated
device is placed within the hole and serves as a stent. U.S. 6,638,239 issued
October 28,
2003, discloses trabecular bypass surgery.
As described in U.S. 6,638,239, issued October 28, 2003, and U.S. 6,736,791,
issued May 18, 2004, a trabecular shunt or stent for transporting aqueous
humor is
provided. The trabecular stent includes a hollow, elongate tubular element,
having an
inlet section and an outlet section. The outlet section may optionally include
two
segments or elements, adapted to be positioned and stabilized in side
Schelmm's canal.
In one embodiment, the device appears as a 'or shaped device.
In one aspect of the invention, a delivery apparatus (or "applicator") is used
for
placing a trabecular stent through a trabecular meshwork of an eye. Certain
embodiments of such a delivery apparatus are disclosed in copending U.S.
Application
No. 10/101,548, published September 19, 2002 as US 2002/0133168 Al (Inventors:
Gregory T. Smedley, Irvine, California, Morteza Gharib, Pasadena, California,
Hosheng Tu, Newport Beach, California; Attorney Docket No.: GLAUKO. 012A),
filed
March 18, 2002, entitled APPLICATOR AND METHODS FOR PLACING A
TRABECULAR SHUNT FOR GLAUCOMA TREATMENT. The stent has an inlet
section and an outlet section. The delivery apparatus includes a handpiece, an
elongate
tip, a holder and an actuator. The handpiece has a distal end and a proximal
end. The
- 6 -
CA 02718294 2010-10-15
elongate tip is connected to the distal end of the handpiece. The elongate tip
has a distal
portion and is configured to be placed through a corneal incision and into an
anterior
chamber of the eye. The holder is attached to the distal portion of the
elongate tip. The
holder is configured to hold and release the inlet section of the trabecular
stent. The
actuator is on the handpiece and actuates the holder to release the inlet
section of the
trabecular stent from the holder. When the trabecular stent is deployed from
the
delivery apparatus into the eye, the outlet section is positioned in
substantially opposite
directions inside Schlemm's canal. In one embodiment, a deployment mechanism
within the delivery apparatus includes a push-pull type plunger.
to Some
aspects of the invention relate to devices for reducing intraocular pressure
by providing outflow of aqueous from an anterior chamber of an eye. The device
generally comprises an elongated tubular member and cutting means. The tubular
member is adapted for extending through a trabecular meshwork of the eye. The
tubular
member generally comprises a lumen having an inlet port and at least one
outlet port
s for
- 7 -
CA 02718294 2013-08-20
providing a flow pathway. The cutting means is mechanically connected to or is
an
integral part of the tubular member for creating an incision in the trabecular
meshwork
for receiving at least a portion of the tubular member.
In one aspect, a self-trephining glaucoma stent is provided for reducing
and/or
balancing intraocular pressure in an eye. The stent generally comprises a
snorkel and a
curved blade. The snorkel generally comprises an upper seat for stabilizing
said stent
within the eye, a shank and a lumen. The shank is mechanically connected to
the seat
and is adapted for extending through a trabecular meshwork of the eye. The
lumen
extends through the snorkel and has at least one inlet flow port and at least
one outlet
flow port. The blade is mechanically connected to the snorkel. The blade
generally
comprises a cutting tip proximate a distal-most point of the blade for making
an incision
in the trabecular meshwork for receiving the shank.
In another aspect, the invention provides use of a delivery device and an
implant
for lowering intraocular pressure within an eye, wherein: the delivery device
has at least
a non-linear portion and is configured to position the implant so that it
fluidicly
communicates with a physiologic outflow pathway of the eye, and the implant
has at
least one inlet and at least one outlet connected to the inlet by at least one
pathway
through the implant, wherein at least one outlet of the implant is configured
to be
disposed in the physiologic outflow pathway following implantation of the
implant in the
eye and when the implant is in use.
In another aspect, the invention provides an implant for reducing intraocular
pressure by providing outflow of aqueous humor from the anterior chamber of an
eye,
comprising: a seat for stabilizing the implant within the eye; a shank having
a longitudinal
axis and being connected to the seat and adapted to extend through a tissue of
the eye;
a lumen extending through the seat and shank and having at least one inlet
port and at
least one outlet port; an outlet section at the outlet port end of the shank
having at least
two outlet channels; wherein the implant is configured to be placed in the eye
such that
the outlet section resides in a physiologic outflow pathway of the eye, the
shank extends
through the tissue of the eye and the seat resides in the anterior chamber of
the eye, so
that aqueous humor can, in use, flow from the anterior chamber into the lumen
through
the inlet port and then through the outlet port and the outlet channels into
the physiologic
outflow pathway.
- 8 -
= CA 02718294 2012-10-12
In another aspect, the invention provides an implant for treating glaucoma,
the
implant comprising: an inlet portion configured to be positioned in the
anterior chamber
of an eye; and an outlet portion in fluid communication with the inlet
portion, the outlet
portion configured to be positioned at least partially in a physiologic
outflow pathway of
the eye; wherein the outlet portion comprises a head portion comprising a
first outlet
opening along a surface of said head portion, said first outlet opening being
in fluid
communication with said outlet and inlet portions; and wherein a maximum
dimension of
said head portion is no greater that about a factor of two compared to a
minimum
distance between a distal end of said head portion and a proximal portion of
said head
portion.
In another aspect, the invention provides a system for treating an ocular
disorder,
comprising: an implant having an inlet portion and an outlet portion, a lumen
extending
between the inlet portion and the outlet portion and communicating with inlet
and outlet
ports, the implant sized so that in use aqueous humor flows from an anterior
chamber of
an eye into the lumen through the inlet port and then through the outlet port
and into a
uveal scleral outflow path of the eye; and a delivery device configured to
advance the
implant from within the anterior chamber, with the outlet portion leading the
inlet portion,
to a location where the outlet port communicates with the uveal scleral
outflow path and
the inlet port communicates with the anterior chamber.
Some aspects of the invention relate to methods of implanting a trabecular
stent
device in an eye. In one aspect, the device has a snorkel mechanically
connected to a
blade. The blade is advanced through a trabecular meshwork of the eye to cut
the
trabecular meshwork and form an incision therein. At least a portion of the
snorkel is
inserted in the incision to implant the device in the eye.
In one aspect, the invention provides a method of lowering intraocular
pressure
within an eye of a mammal, comprising: providing a delivery device having at
least a
non-linear portion; providing an implant having at least one inlet and at
least one outlet
connected to the inlet by at least one pathway through the implant; inserting
the non-
linear portion of the delivery device into an anterior chamber of the eye;
using the
delivery device to move the implant through a portion of the anterior chamber
and into
eye tissue to a position where the inlet is disposed within the anterior
chamber and the
outlet is disposed to drain fluid to a physiologic outflow pathway of the eye.
- 9 -
CA 02718294 2012-10-12
=
In another aspect, the invention provides use of a delivery device and an
implant
for lowering intraocular pressure within an eye of a mammal, wherein: the
delivery
device has at least a non-linear portion and is configured to position the
implant so that it
fluidicly communicates with a physiologic outflow pathway of the eye, and the
implant
has at least one inlet and at least one outlet connected to the inlet by at
least one
pathway through the implant, and the implant further has a cutting end
configured to
penetrate eye tissue.
In yet another aspect, the invention provides use of a delivery device and an
implant that drains aqueous humor from an anterior chamber of an eye to a
uveal scleral
outflow path of the eye for implanting the implant into eye tissue from a
location within
the anterior chamber, wherein at least one outlet of the implant is configured
to be
disposed in the uveal scleral outflow path following implantation of the
implant in the eye
and when the implant is in use.
Some aspects provide a self-trephining glaucoma stent and methods thereof
which advantageously allow for a "one-step" procedure in which the incision
and
placement of the stent are accomplished by a single device and operation. This
desirably allows for a faster, safer, and less expensive surgical procedure.
In any of the
embodiments, fiducial markings, indicia, or the like and/or positioning of the
stent device
in a preloaded applicator may be used for proper orientation and alignment of
the device
during implantation.
Among the advantages of trabecular bypass surgery is its simplicity. The
microsurgery may potentially be performed on an outpatient basis with rapid
visual
recovery and greatly decreased morbidity. There is a lower risk of infection
and choroidal
hemorrhage, and there is a faster recovery, than with previous techniques.
For purposes of summarizing the invention, certain aspects, advantages and
novel features of the invention have been described herein above. Of course,
it is to be
understood that not necessarily all such advantages may be achieved in
accordance
with any particular embodiment of the invention. Thus, the invention may be
embodied
or carried out in a manner that achieves or optimizes one advantage or group
of
advantages as taught or suggested herein without necessarily achieving other
advantages as may be taught or suggested herein.
All of these embodiments are intended to be within the scope of the invention
herein disclosed. These and other embodiments of the invention will become
readily
- 10-
CA 02718294 2012-10-12
apparent to those skilled in the art from the following detailed description
of the preferred
embodiments having reference to the attached figures, the invention not being
limited to
any particular preferred embodiment(s) disclosed.
- 1 Oa -
CA 02718294 2010-10-15
Brief Description of the Drawings
Having thus summarized the general nature of the invention and some of its
features and
advantages, certain preferred embodiments and modifications thereof will
become apparent to those skilled in
the art from the detailed description herein having reference to the figures
that follow, of which:
FIG. 1 is a coronal cross-sectional view of an eye;
FIG. 2 is an enlarged cross-sectional view of an anterior chamber angle of the
eye of FIG. 1;
FIG. 3 is a simplified partial view of an eye illustrating the implantation of
a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 4 is a side elevation view of the stent of FIG. 3;
FIG. 5 is a top plan view of the stent of FIG. 3;
FIG. 6 is a bottom plan view of the stent of FIG. 3;
FIG. 7 is a front end view of the stent of FIG. 3 (along line 7-7 of FIG. 4);
FIG. 8 is a rear end view of the stent of FIG. 3 (along line 8-8 of FIG. 4);
FIG. 9 is an enlarged top plan view of a cutting tip of the stent of FIG. 3;
FIG. 10 is a top plan view of one exemplary embodiment of a snorkel top
seating surface;
FIG. 11 is a top plan view of another exemplary embodiment of a snorkel top
seating surface;
FIG. 12 is a top plan view of yet another exemplary embodiment of a snorkel
top seating surface;
FIG. 13 is a top plan view of still another exemplary embodiment of a snorkel
top seating surface;
FIG. 14 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with another embodiment of the
invention;
FIG. 15 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with a further embodiment of the
invention;
FIG. 16 is a side elevation view of a glaucoma stent having features and
advantages in accordance
with one embodiment of the invention;
FIG. 17 is atop plan view of the stent of FIG. 16;
FIG. 18 is a bottom plan view of the stent of FIG. 16;
FIG. 19 is a front end view along line 19-19 of FIG. 16;
FIG. 20 is a rear end view along line 20-20 of FIG. 16;
FIG. 21 is a side elevation view of a glaucoma stent having features and
advantages in accordance
with one embodiment of the invention;
FIG. 22 is a top plan view of the stent of FIG. 21;
FIG. 23 is a bottom plan view of the stent of FIG. 21;
FIG. 24 is a front end view along line 24-24 of FIG. 21;
FIG. 25 is a rear end view along line 25-25 of FIG. 21;
-11-
CA 02718294 2010-10-15
FIG. 26 is a front elevation view of a glaucoma stent having features and
advantages in accordance
with one embodiment of the invention;
FIG. 27 is a side elevation view along line 27-27 of FIG. 26;
FIG. 28 is a rear end view along line 28-28 of FIG. 26;
FIG. 29 is a simplified partial view of an eye illustrating the temporal
implantation of a glaucoma stent
using a delivery apparatus having features and advantages in accordance with
one embodiment of the
invention;
FIG. 30 is an oblique elevational view of an articulating arm stent
delneryiretrieval apparatus having
features and advantages in accordance with one embodiment of the invention;
FIG. 31 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent using a
delivery apparatus crossing through the eye anterior chamber;
FIG. 32 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 33 is a detailed enlarged view of the barbed pin of FIG. 32;
FIG. 34 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 35 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 36 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 37 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 38 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 39 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 40 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 41 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 42 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with one embodiment of the invention;
FIG. 43 is a simplified partial view of an eye illustrating the implantation
of a valved tube stent device
having features and advantages in accordance with one embodiment of the
invention;
-12-
CA 02718294 2010-10-15
FIG. 44 is a simplified partial view of an eye illustrating the implantation
of an osmotic membrane
device having features and advantages in accordance with one embodiment of the
invention;
FIG. 45 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent using ab
extemo procedure having features and advantages in accordance with one
embodiment of the invention;
FIG. 46 is a simplified partial view of an eye illustrating the implantation
of a glaucoma stent having
features and advantages in accordance with a modified embodiment of the
invention; and
FIG. 47 is a simplified partial view of an eye illustrating the implantation
of a drug release implant
having features and advantages in accordance with one embodiment of the
invention.
Detailed Description of the Preferred Embodiments
The preferred embodiments of the invention described herein relate
particularly to surgical and
therapeutic treatment of glaucoma through reduction of intraocular pressure.
While the description sets forth
various embodiment specific details, it will be appreciated that the
description is illustrative only and should
not be construed in any way as limiting the invention. Furthermore, various
applications of the invention, and
modifications thereto, which may occur to those who are skilled in the art,
are also encompassed by the
general concepts described herein.
FIG. 1 is a cross-sectional view of an eye 10, while FIG. 2 is a close-up view
showing the relative
anatomical locations of a trabecular meshwork 21, an anterior chamber 20, and
Schlemm's canal 22. A
sclera 11 is a thick collagenous tissue which covers the entire eye 10 except
a portion which is covered by a
4. comea 12.
Referring to FIGS. 1 and 2, the cornea 121$ a thin transparent tissue that
focuses and transmits light
into the eye and through a pupil 14, which is a circular hole in the center of
an iris 13 (colored portion of the
eye). The cornea 12 merges into the sclera 11 at a juncture referred to as a
limbus 15. A ciliary body 16
extends along the interior of the sclera 11 and is coextensive with a choroid
17. The choroid 17 is a vascular
layer of the eye 10, located between the sclera 1I and a retina 18. An optic
nerve 19 transmits visual
information to the brain and is the anatomic structure that is progressively
destroyed by glaucoma.
Still referring to FIGS. 1 and 2, the anterior chamber 20 of the eye 10, which
is bound anteriorly by
the cornea 12 and posteriorly by the iris 13 and a lens 26, is filled with
aqueous humor (hereinafter referred to
as *aqueous's). Aqueous is produced primarily by the ciliary body 16, then
moves anteriorly through the pupil
14 and reaches an anterior chamber angle 25, formed between the iris 13 and
the cornea 12.
As best illustrated by the drawing of FIG. 2, in a normal eye, aqueous is
removed from the anterior
chamber 20 through the trabecular meshwork 21. Aqueous passes through the
trabecular meshwork 21 into
Schlemm's canal 22 and thereafter through a plurality of aqueous veins 23,
which merge with blood-carrying
veins, and into systemic venous circulation. Intraocular pressure is
maintained by an intricate balance
between secretion and outflow of aqueous in the manner described above.
Glaucoma is, in most cases,
characterized by an excessive buildup of aqueous in the anterior chamber 20
which leads to an increase in
- 13-
CA 02718294 2010-10-15
intraocular pressure. Fluids are relatively incompressible, and thus
intraocular pressure is distributed
relatively uniformly throughout the eye 10.
As shown in FIG. 2, the trabecular meshwork 21 is adjacent a small portion of
the sclera 11. Exterior
to the sclera 11 is a conjunctiva 24. Traditional procedures that create a
hole or opening for implanting a
device through the tissues of the conjunctiva 24 and sclera 11 involve
extensive surgery by an ab extern
procedure, as compared to surgery for implanting a device, as described
herein, which ultimately resides
entirely within the confines of the sclera ii and cornea 12.
Self-treohinino Glaucoma Stent
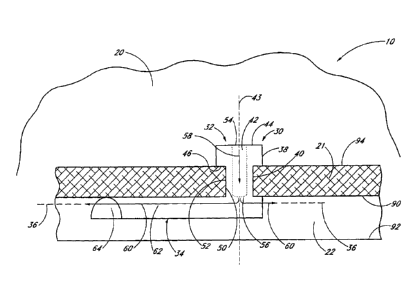
FIG. 3 generally illustrates the use of one embodiment of a trabecular
stenting device 30 for
establishing an outflow pathway, passing through the trabecular meshwork 21,
which is discussed in greater
detail below. FIGS. 4-9 are different views of the stent 30. Advantageously,
and as discussed in further detail
later herein, the self-trephining-stent allows a one-step procedure to make an
incision in the trabecular mesh
21 and place the stent or implant 30 at the desired or predetermined position
within the eye 10. Desirably,
this facilitates and simplifies the overall surgical procedure.
In the illustrated embodiment of FIGS. 3-9, the shunt or stent 30 generally
comprises a snorkel 32
and a main body portion or blade 34. The snorkel 32 and blade 34 are
mechanically connected to or in
mechanical communication with one another. The stent 30 and/or the body
portkon 34 have a generally
longitudinal axis 36.
In the illustrated embodiment of FIGS. 3-9, the stent 30 comprises an integral
unit. In modified
embodiments, the stent 30 may comprise an assembly of individual pieces or
components. For example, the
stent 30 may comprise an assembly of the snorkel 32 and blade 34.
In the illustrated embodiment of FIGS. 3-9, the snorkel 32 is in the form of a
generally elongate
tubular member and generally comprises an upper seat, head or cap portion 38,
a shank portion 40 and a
lumen or passage 42 extending therethrough. The seat 38 is mechanically
connected to or in mechanical
communication with the shank 40 which is also mechanically connected to or in
mechanical communication
with the blade 34. The snorkel 32 and/or the lumen 42 have a generally
longitudinal axis 43.
In the illustrated embodiment of FIGS. 3-9, the seat 38 is generally circular
in shape and has an
upper surface 44 and a lower surface 46 which, as shown in FIG. 3, abuts or
rests against the trabecular
meshwork 21 to stabilize the glaucoma stent 30 within the eye 10. In modified
embodiments, the seat 38 may
efficaciously be shaped in other suitable manners, as required or desired,
giving due consideration to the
goals of stabilizing the glaucoma stent 30 within the eye 10 and/or of
achieving one or more of the benefits
and advantages as taught or suggested herein. For example, the seat 38 may be
shaped in other polygonal
or non-polygonal shapes and/or comprise one or more ridges which extend
radially outwards, among other
suitable retention devices.
- 14 -
CA 02718294 2010-10-15
In the illustrated embodiment of FIGS. 3-9, and as best seen in the top view
of FIG. 5, the seat top
surface 44 comprises fiducial marks or indicia 48. These marks or indicia 48
facilitate and ensure proper
orientation and alignment of the stent 30 when implanted in the eye 10. The
marks or indicia 48 may
= comprise visual differentiation means such as color contrast or be in the
form of ribs, grooves, or the like.
Alternatively, or in addition, the marks 48 may provide tactile sensory
feedback to the surgeon by
= incorporating a radiopaque detectable or ultrasound imaginable substrate
at about the mark 48. Also, the
seat 38 and/or the seat top surface 44 may be configured in predetermined
shapes aligned with the blade 34
and/or longitudinal axis 36 to provide for proper orientation of the stent
device 30 within the eye 10. For
example, the seat top surface 44 may be oval or ellipsoidal (FIG. 10),
rectangular (FIG. 11), hexagonal (FIG.
12), among other suitable shapes (e.g. FIG. 13).
In the illustrated embodiment of FIGS. 3-9, and as indicated above, the seat
bottom surface 46 abuts
or rests against the trabecular meshwork 21 to stabilize and retain the
glaucoma stent 30 within the eye 10.
For stabilization purposes, the seat bottom surface 46 may comprise a stubbed
surface, a ribbed surface, a
surface with pillars, a textured surface, or the like.
In the illustrated embodiment of FIGS. 3-9, the snorkel shank 40 is generally
cylindrical in shape.
With the stent 30 implanted, as shown in FIG. 3, the shank 40 is generally
positioned in an incision or cavity
50 formed in the trabecular meshwork 21 by the self-trephining stent 30.
Advantageously, and as discussed
further below, this single step of forming the cavity 50 by the stent 30
itself and placing the stent 30 in the
,
desired position facilitates and expedites the overaU surgical
procedure. In modified embodiments, the
snorkel shank 40 may efficaciously be shaped in other suitable manners, as
required or desired. For
example, the shank 40 may be in the shape of other polygonal or non-polygonal
shapes, such as, oval,
eMposoidal, and the like.
In the illustrated embodiment of FIGS. 3-9, and as best seen in FIG. 3, the
shank 40 has an outer
surface 52 in contact with the trabecular meshwork 21 surrounding the cavity
50. For stabilization purposes,
the shank outer surface 52 may comprise a stubbed surface, a ribbed surface, a
surface with pillars, a
textured surface, or the like.
In the illustrated embodiment of FIGS. 3-9, the snorkel lumen 42 has an inlet
port, opening or orifice
54 at the seat top surface 44 and an outlet port, opening or orifice 56 at the
junction of the shank 40 and blade
34. The lumen 42 is generally cylindrical in shape, that is, it has a
generally circular cross-section, and its
ports 54, 56 are generally circular in shape. In modified embodiments, the
lumen 42 and ports 54, 56 may be
efficaciously shaped in other manners, as required or desired, giving due
consideration to the goals of
providing sufficient aqueous outflow and/or of achieving one or more of the
benefits arid advantages as taught
or suggested herein. For example, the lumen 42 and/or one or both ports 54, 56
may be shaped in the form
of ovals, ellipsoids, and the like, or the lumen 42 may have a tapered or
stepped configuration.
-15-
CA 02718294 2010-10-15
Referring in particular to FIG. 3, aqueous from the anterior chamber 20 flows
into the lumen 42
through the inlet port 54 (as generally indicated by arrow 58) and out of the
outlet port 56 and into Schlemm's
canal 22 (as generally indicated by arrows 60) to lower and/or balance the
intraocular pressure (lOP). In
another embodiment, as discussed in further detail below, one or more of the
outlet ports may be configured
to face in the general direction of the stent longitudinal axis 36. In
modified embodiments, the snorkel 32 may
comprise more than one lumen, as needed or desired, to facilitate multiple
aqueous outflow transportation into
Schlemm's canal 22.
In the illustrated embodiment of FIGS. 3-9, the blade longitudinal axis 36 and
the snorkel longitudinal
axis 43 are generally perpendicular to one another. Stated differently, the
projections of the axes 36, 43 on a
common plane which is not perpendicular to either of the axes 36, 43 intersect
at 90 . The blade longitudinal
axis 36 and the snorkel longitudinal axis 43 may intersect one another or may
be offset from one another.
In the illustrated embodiment of FIGS. 3-9, the main body portion or blade 34
is a generally curved
elongated sheet- or plate-like structure with an upper curved surface 62 and a
lower curved surface 64 which
defines a trough or open face channel 66. The perimeter of the blade 34 is
generally defined by a curved
proximal edge 68 proximate to the snorkel 32, a curved distal edge 70 spaced
from the proximal edge 68 by a
pair of generally straight lateral edges 72, 74 with the first lateral edge 72
extending beyond the second lateral
edge 74 and intersecting with the distal edge 70 at a distal-most point 76 of
the blade 34 proximate a blade
cutting tip 78.
In the illustrated embodiment of FIGS. 3-9, and as shown in the enlarged view
of FIG. 9, the cutting
tip 78 comprises a first cutting edge 80 on the distal edge 70 and a second
cutting edge 82 on the lateral edge
72. The cutting edges 80, 82 preferably extend from the distal-most point 76
of the blade 34 and comprise at
least a respective portion of the distal edge 70 and lateral edge 72. The
respective cutting edges 80, 82 are
formed at the sharp edges of respective beveled or tapered surfaces 84, 86. In
one embodiment, the
remainder of the distal edge 70 and lateral edge 72 are dull or rounded. In
one embodiment, the tip 78
proximate to the distal-most end 76 is curved slightly inwards, as indicated
generally by the arrow 88 in FIG. 5
and arrow 88 (pointed perpendicular and into the plane of the paper) in FIG.
9, relative to the adjacent
curvature of the blade 34.
In modified embodiments, suitable cutting edges may be provided on selected
portions of one or
more selected blade edges 68, 70, 72, 74 with efficacy, as needed or desired,
giving due consideration to the
goals of providing suitable cutting means on the stent 30 for effectively
cutting through the trabecular
meshwork 21 (FIG. 3) and/or of achieving one or more of the benefits and
advantages as taught or suggested
herein.
Referring in particular to FIG. 9, in one embodiment, the ratio between the
lengths of the cutting
edges 80, 82 is about 2:1. In another embodiment, the ratio between the
lengths of the cutting edges 80, 82
is about 1:1. In yet another embodiment, the ratio between the lengths of the
cutting edges 80, 82 is about
-16-
_
CA 02718294 2010-10-15
1:2. In modified embodiments, the lengths of the cutting edges 80, 82 may be
efficaciously selected in other
manners, as required or desired, giving due consideration to the goals of
providing suitable cutting means on
the stent 30 for effectively cutting through the trabecular meshwork 21 (FIG.
3) and/or of achieving one or
more of the benefits and advantages as taught or suggested herein.
=
Still referring in particular to FIG. 9, in one embodiment, the ratio between
the lengths of the cutting
edges 80, 82 is in the range from about 2:1 to about 1:2. In another
embodiment, the ratio between the
lengths of the cutting edges 80, 82 is in the range from about 5:1 to about
1:5. In yet another embodiment,
the ratio between the lengths of the cutting edges 80, 82 is in the range from
about 10:1 to about 1:10. In
modified embodiments, the lengths of the cutting edges 80, 82 may be
efficaciously selected in other
manners, as required or desired, giving due consideration to the goals of
providing suitable cutting means on
the stent 30 for effectively cutting through the trabecular meshwork 21 (FIG.
3) and/or of achieving one or
more of the benefits and advantages as taught or suggested herein.
As shown in the top view of FIG. 9, the cutting edge 80 (and/or the distal end
70) and the cutting
edge 82 (and/or the lateral edge 72) intersect at an angle O. Stated
differently, 0 is the angle between the
projections of the cutting edge 80 (and/or the distal end 70) and the cutting
edge 82 (and/or the lateral edge
72) on a common plane which is not perpendicular to either of these edges.
Referring to in particular to FIG. 9, in one embodiment, the angle 8 is about
50 . In another
embodiment, the angle 0 is in the range from about 40 to about 60'. In yet
another embodiment, the angle 0
"
is in the range from about 30 to about 70 . In modified embodiments,
the angle 0 may be efficaciously
selected in other manners, as required or desired, giving due consideration to
the goals of providing suitable
cutting means on the stent 30 for effectively cutting through the trabecular
meshwork 21 (FIG. 3) and/or of
achieving one or more of the benefits and advantages as taught or suggested
herein.
The stent 30 of the embodiments disclosed herein can be dimensioned in a wide
variety of manners.
Referring in particular to FIG. 3, the depth of Schlemm's canal 22 is
typically about less than 400 microns
(p.m). Accordingly, the stunt blade 34 is dimensioned so that the height of
the blade 34 (referred to as H4, in
FIG. 4) is typically less than about 400 m. The snorkel shank 40 is
dimensioned so that it has a length
(referred to as Li in FIG. 4) typically in the range from about 150 p.m to
about 400 pen which is roughly the
typical range of the thickness of the trabecular meshwork 21.
Of course, as the skilled artisan will appreciate, that with the stent 30
implanted, the blade 34 may
rest at any suitable position within Schlemm's canal 22. For example, the
blade 34 may be adjacent to a front
wall 90 of Schlemm's canal 22 (as shown in FIG. 3), or adjacent to a back wall
92 of Schlemm's canal 22, or
at some intermediate location therebetween, as needed or desired. Also, the
snorkel shank 40 may extend
into Schlemm's canal 22. The length of the snorkel shank 40 and/or the
dimensions of the blade 34 may be
efficaciously adjusted to achieve the desired implant positioning.
- 17-
CA 02718294 2010-10-15
The trabecular stenting device 30 (FIGS. 3-9) of the exemplary embodiment may
be manufactured or
fabricated by a wide variety of techniques. These include, without limitation,
by molding, thermo-forming, or
other micro-machining techniques, among other suitable techniques.
The trabecular stenting device 30 preferably comprises a biocompatible
material such that
inflammation arising due to irritation between the outer surface of the device
30 and the surrounding tissue is
minimized. Biocompatible materials which may be used for the device 30
preferably include, but are not
limited to, titanium, titanium alloys, medical grade silicone, e.g.,
Silasticim, available from Dow Coming
Corporation of Midland, Michigan; and polyurethane, e.g., Pel!ethane", also
available from Dow Coming
Corporation.
In other embodiments, the stent device 30 may comprise other types of
biocompatible material, such
as, by way of example, polyvinyl alcohol, polyvinyl pyrolidone, collagen,
heparinized collagen,
polytetrafluoroethylene, expanded polytetrafludroethylene, fluorinated
polymer, fluorinated elastomer, flexible
fused silica, polyolefin, polyester, polysilicon, and/or a mixture of the
aforementioned biocompatible materials,
and the like. In still other embodiments, composite biocompatible material may
be used, wherein a surface
material may be used in addition to one or more of the aforementioned
materials. For example, such a
surface material may include polytetrafluoroethylene (PTFE) (such as
TeflonTm), polyimide, hydrogel, heparin,
therapeutic drugs (such as beta-adrenergic antagonists and other anti-glaucoma
drugs, or antibiotics), and
the ;ike.
In an exemplary embodiment of the trabecular meshwork surgery, the patient is
placed in the supine
position, prepped, draped and anesthetized as necessary. In one embodiment, a
small (less than about 1
4,
mm) incision, which may be self sealing is made through the cornea 12. The
corneal incision can be made in
a number of ways, for example, by using a micro-knife, among other tools.
An applicator or delivery apparatus is used to advance the glaucoma stent 30
through the corneal
incision and to the trabecular meshwork 21. Some embodiments of such a
delivery apparatus are disclosed
in copending U.S. Application No. 10/101,548, published September 19, 2002 as
US 2002/0133168 Al
(Inventors: Gregory T. Smedley, Irvine, California, Morteza Gharib, Pasadena,
California, Hosheng Tu,
Newport Beach, California; Attorney Docket No.: GLAUK0.012A), filed March 18,
2002, entitled
APPLICATOR AND METHODS FOR PLAC1NGA TRABECULAR SHUNT FOR GLAUCOMA
TREATMENT. Some embodiments of a delivery apparatus are also discussed in
further detail later
herein. Gonioscopic, microscopic, or endoscopic guidance may be used during
the trabecular meshwork
surgery.
With the device 30 held by the delivery apparatus, the blade 34 of the self-
trephining glaucoma stent
device 30 is used to cut and/or displace the material of the trabecular
meshwork 21. The snorkel shank 40
may also facilitate in removal of this material during implantation. The
delivery apparatus is withdrawn once
- 18-
CA 02718294 2010-10-15
the device 30 has been implanted in the eye 10. As shown in FIG. 3, once
proper implantation has been
accomplished the snorkel seat 38 rests on a top surface 94 of the trabecular
meshwork 21, the snorkel shank
40 extends through the cavity 50 (created by the device 30) in the trabecular
meshwork 21, and the blade
extends inside Schlemm's canal 22.
Advantageously, the embodiments of the self-trephining stent device of the
invention allow for a
"one-step procedure to make an incision in the trabecular meshwork and to
subsequently implant the stent in
the proper orientation and alignment within the eye to allow outflow of
aqueous from the anterior chamber
through the stent and into Schlemm's canal to lower and/or balance the
intraocular pressure (10P). Desirably,
this provides for a faster, safer, and less expensive surgical procedure.
Many complications can arise in trabecular meshwork surgeries, wherein a knife
is first used to
create an incision in the trabecular meshwork, followed by removal of the
knife and subsequent installation of
the stent. For instance, the knife may cause some bleeding which clouds up the
surgical site. This may
require more effort and time to clean the surgical site prior to placement of
the stent Moreover, this may
cause the intraocular pressure (10P) to rise or to faH undesireably. Thus,
undesirably, such a multiple step
procedure may demand crisis management which slows down the surgery, makes it
less safe, and more
expensive.
FIG. 14 is a simplified partial view of an eye 10 illustrating the
implantation of a self-trephining
glaucoma stent device 30a having features and advantages in accordance with
one embodiment. The stent
30a is generally similar to the stent 30 of FIGS. 3-9 except that its snorkel
32a comprises a longer shank 40a
which extends into Schlemm's canal 22 and a lumen 42a which bifurcates into
two output channels 45a.
In the illustrated embodiment of FIG. 14, the shank 40a terminates at the
blade 34. Aqueous flows
from the anterior chamber 20 into the lumen 42a through an inlet port 54a (as
generally indicated by arrow
58a). Aqueous then flows through the output channels 45a and out of respective
outlet ports 56a and into
Schlemm's canal 22 (as generally indicated by arrows 60a). The outlet channels
45a extend radially
outwards in generally opposed directions and the outlet ports 56a are
configured to face in the general
direction of the stent longitudinal axis 36 so that they open into Schlemm's
canal 22 and are in proper
orientation to allow aqueous outflow into Schlemm's canal 22 for lowering
and/or balancing the intraocular
pressure (10P). As indicated above, fiducial marks or indicia and/or
predetermined shapes of the snorkel seat
38 allow for proper orientation of the blade 34 and also the output channels
45a and respective ports 56a
within Schlemm's canal.
In the illustrated embodiment of FIG. 14, two outflow channels 45a are
provided. In another
embodiment, only one outflow channel 45a is provided. In yet another
embodiment, more than two outflow
channels 45a are provided. In modified embodiments, the lumen 42a may extend
all the way through to the
blade 34 and provide an outlet port as discussed above with reference to the
embodiment of FIGS. 3-9.
- 19-
CA 02718294 2010-10-15
FIG. 15 is a simplified partial view of an eye 10 illustrating the
implantation of a self-trephining
glaucoma stent device 30b having features and advantages in accordance with
one embodiment. The stent
30b is generally similar to the stent 30 of FIGS. 3-9 except that its snorkel
32b comprises a longer shank 40b
which extends into Schlemm's canal 22 and a lumen 42b which bifurcates into
two output channels 45b.
In the illustrated embodiment of FIG. 15, the shank 40b extends through the
blade 34. Aqueous
flows from the anterior chamber 20 into the lumen 42b through an inlet port
54b (as generally indicated by
arrow 58b). Aqueous then flows through the output channels 45b and out of
respective outlet ports 56b and
into Schlemm's canal 22 (as generally indicated by arrows 60b). The outlet
channels 45b extend radially
outwards in generally opposed directions and the outlet ports 56b are
configured to face in the general
direction of the stent longitudinal axis 36 so that they open into Schlemm's
canal 22 and are in proper
orientation to allow aqueous outflow into Schlemm's canal 22 for lowering
and/or balancing the intraocular
pressure (10P). As indicated above, Nuclei marks or indicia and/or
predetermined shapes of the snorkel seat
38 allow for proper orientation of the blade 34 and also the output channels
45b and respective ports 56b
within Schlemm's canal.
In the illustrated embodiment of FIG. 15, two outflow channels 45b are
provided. In another
embodiment, only one outflow channel 45b is provided. In yet another
embodiment, more than two outflow
channels 45b are provided. In modified embodiments, the lumen 42b may extend
all the way through to the
blade 34 and provide an outlet port as discussed above with reference to the
embodiment of FIGS. 3-9.
FIGS. 16-20 show different views of a self-trephining glaucoma stent device
30c having features and
advantages in accordance with one embodiment. The stent 30c is generally
similar to the stent 30 of FIGS. 3-
9 except that it has a modified blade configuration. The stent 30c comprises a
blade 34c which is a generally
curved elongated sheet- or plate-like structure with an upper curved surface
62c and a lower curved surface
64c which defines a trough or open face channel 66c. The perimeter of the
blade 34c is generally defined by
a curved proximal edge 68c proximate to the snorkel 32, a curved distal edge
70c spaced from the proximal
edge 68c by a pair of generally straight lateral edges 72c, 74c which are
generally parallel to one another and
have about the same length.
In the illustrated embodiment of FIGS. 16-20, the blade 34c comprises a
cutting tip 78c. The cutting
tip 78c preferably includes cutting edges formed on selected portions of the
distal edge 70c and adjacent
portions of the lateral edges 72c, 74c for cutting through the trabecular
meshwork for placement of the snorkel
32. The cutting edges are sharp edges of beveled or tapered surfaces as
discussed above in reference to
FIG. 9. The embodiment of FIGS. 16-20 may be efficaciously modified to
incorporate the snorkel
configuration of the embodiments of FIGS. 14 and 15.
FIGS. 21-25 show different views of a self-trephining glaucoma stent device
30d having features and
advantages in accordance with one embodiment. The stent 30d is generally
similar to the stent 30 of FIGS.
3-9 except that it has a modified blade configuration. The stent 30d comprises
a blade 34d which is a
- 20 -
CA 02718294 2010-10-15
generally curved elongated sheet- or plate-like structure with an upper curved
surface 62d and a lower curved
surface 64d which defines a trough or open face channel 66d. The perimeter of
the blade 34d is generally
defined by a curved proximal edge 68d proximate to the snorkel 32, a pair of
inwardly converging curved
distal edges 70d', 70d" spaced from the proximal edge 68d by a pair of
generally straight respective lateral
edges 72d, 74d which are generally parallel to one another and have about the
same length. The distal
edges 70d', 70d" intersect at a distal-most point 76d of the blade 34d
proximate a blade cutting tip 78d.
In the illustrated embodiment of FIGS. 21-25, the cutting tip 78d preferably
includes cutting edges
formed on the distal edges 70d', 70d" and extending from the distal-most point
76d of the blade 34d. In one
embodiment, the cutting edges extend along only a portion of respective distal
edges 70d', 70d". In another
embodiment, the cutting edges extend along substantially the entire length of
respective distal edges 70d',
70d. In yet another embodiment, at least portions of the lateral edges 72d,
74d proximate to respective distal
edges 70d', 70d" have cutting edges. In a further embodiment, the tip 78d
proximate to the distal-most end
76d is curved slightly inwards, as indicated generally by the arrow 88d in
FIG. 21 and arrow 88d (pointed
perpendicular and into the plane of the paper) in FIG. 22, relative to the
adjacent curvature of the blade 34d.
In the embodiment of FIGS. 21-25, the cutting edges are sharp edges of beveled
or tapered surfaces
as discussed above in reference to FIG. 9. The embodiment of FIGS. 21-25 may
be efficaciously modified to
incorporate the snorkel configuration of the embodiments of FIGS. 14 and 15.
FIGS. 26-28 show different views of a self-trephining glaucoma stent device
30e having features and
.a advantages in accordance with one embodiment. The stent device 30e
generally comprises a snorkel 32e
mechanically connected to or in mechanical communication with a blade or
cutting tip 34e. The snorkel 32e
has a seat, head or cap portion 38e mechanically connected to or in mechanical
communication with a shank
40e, as discussed above. The shank 40e has a distal end or base 47e. The
snorkel 32e further has a lumen
42e which bifurcates into a pair of outlet channels 45e, as discussed above in
connection with FIGS. 14 and
15. Other lumen and inlet and outlet port configurations as taught or
suggested herein may also be
efficaciously used, as needed or desired.
In the illustrated embodiment of FIGS. 26-28, the blade 34e extends downwardly
and outwardly from
the shank distal end 47e. The blade 34e is angled relative to a generally
longitudinal axis 43e of the snorkel
32e, as best seen in FIGS. 27 and 28. The blade 34e has a distal-most point
76e. The blade or cutting tip
34e has a pair of side edges 70e', 70e", including cutting edges, terminating
at the distal-most point 76e, as
best seen in FIG. 26. In one embodiment, the cutting edges are sharp edges of
beveled or tapered surfaces
as discussed above in reference to FIG. 9.
Referring to FIGS. 26-28, in one embodiment, the blade 34e includes cutting
edges formed on the
edges 70e', 70e" and extending from the distal-most point 76e of the blade
34d. In one embodiment, the
cutting edges extend along only a portion of respective distal edges 70e',
70e. In another embodiment, the
- 21 -
CA 02718294 2010-10-15
cutting edges extend along substantially the entire length of respective
distal edges 70e', 70e6. In yet another
embodiment, the blade or cutting tip 34e comprises a bent tip of needle, for
example, a 30 gauge needle.
In general, any of the blade configurations disclosed herein may be used in
conjunction with any of
the snorkel configurations disclosed herein or incorporated by reference
herein to provide a self-trephining
glaucoma stent device for making an incision in the trabecular meshwork for
receiving the corresponding
snorkel to provide a pathway for aqueous outflow from the eye anterior chamber
to Schlemm's canal, thereby
effectively lowering and/or balancing the intraocular pressure (10P). The self-
trephining ability of the device,
advantageously, allows for a None-step procedure in which the incision and
placement of the snorkel are
accomplished by a single device and operation. In any of the embodiments,
fiducial markings or indicia,
and/or preselected configuration of the snorkel seat, and/or positioning of
the stent device in a preloaded
applicator may be used for proper orientation and alignment of the device
during implantation.
Delivery Apparatus
In many cases, a surgeon works from a temporal incision when performing
cataract or goniometry
surgery. FIG. 29 illustrates a temporal implant procedure, wherein a delivery
apparatus or applicator' 100
having a curved tip 102 is used to deliver a stent 30 to a temporal side 27 of
the eye 10. An incision 28 is
made in the cornea 10, as discussed above. The apparatus 100 is then used to
introduce the stent 30
through the incision 28 and implant it within the eye 10.
Still referring in particular to FIG. 29, in one embodiment, a similarly
curved instrument would be
used to make the incision through the trabecular meshwork 24. In other
embodiments, a self-trephining stent
device 30 may be used to make this incision through the trabecular meshwork
21, as discussed above. The
temporal implantation procedure illustrated in FIG. 29 may be employed with
the any of the various stent
embodiments taught or suggested herein.
FIG. 30 illustrates one embodiment of an apparatus comprising an articulating
stent applicator or
retrieval device 100a In this embodiment, a proximal arm 106 is attached to a
distal arm 108 at a joint 112.
This joint 112 is movable such that an angle formed between the proximal arm
106 and the distal arm 108 can
change. One or more claws 114 can extend from the distal arm 108, in the case
of a stent retrieval device.
Similarly, this articulation mechanism may be used for the trabecular stent
applicator, and thus the articulating
applicator or retrieval device 100a may be either an applicator for the
trabecular stent, a retrieval device, or
both, in various embodiments. The embodiment of FIG. 30 may be employed with
the any of the various stent
embodiments taught or suggested herein.
FIG. 31 shows another illustrative method for placing any of the various stent
embodiments taught or
suggested herein at the implant site within the eye 10. A delivery apparatus
100b generally comprises a
syringe portion 116 and a cannula portion 118. The distal section of the
cannula 118 has at least one
irrigating hole 120 and a distal space 122 for holding the stent device 30.
The proximal end 124 of the lumen
of the distal space 122 is sealed from the remaining lumen of the cannula
portion 118. The delivery
- 22 -
CA 02718294 2010-10-15
apparatus of FIG. 30 may be employed with the any of the various stent
embodiments taught or suggested
herein.
In one aspect of the invention, a delivery apparatus (or *applicato() is used
for placing a trabecular
stent through a trabecular meshwork of an eye. Certain embodiments of such a
delivery apparatus are
disclosed in copending U.S. Application No. 10/101,548, published September
19, 2002 as US
2002/0133168 Al (Inventors: Gregory T. Smedley, Irvine, California, Morteza
Gharib, Pasadena,
California, Hosheng Tu, Newport Beach, California; Attorney Docket No.:
GLAUK0.012A), filed
March 18, 2002, entitled APPLICATOR AND METHODS FOR PLACING A TR1BECULAR
SHUNT FOR GLAUCOMA TREATMENT.
The stent has an inlet section and an outlet section. The defivery apparatus
includes a handpiece,
an elongate tip, a holder and an actuator. The handpiece has a distal end and
a proximal end. The elongate
tip is connected to the distal end of the handpiece. The elongate tip has a
distal portion and is configured to
be placed through a corneal incision and into an anterior chamber of the eye.
The holder is attached to the
distal portion of the elongate tip. The holder is configured to hold and
release the inlet section of the
trabecular stent. The actuator is on the handpiece and actuates the holder to
release the inlet section of the
trabecular stent from the holder. When the trabecular stent is deployed from
the delivery apparatus into the
eye, the outlet section is positioned in substantially opposite directions
inside Schlemm's canal. In one
embodiment, a deployment mechanism within the delivery apparatus includes a
push-pull type plunger.
In some embodiments, the holder comprises a clamp. In some embodiments, the
apparatus further
comprises a spring within the handpiece that is configured to be loaded when
the stent is being held by the
holder, the spring being at least partially unloaded upon actuating the
actuator, allowing for release of the
stent from the holder.
In various embodiments, the clamp compnses a plurality of claws configured to
exert a clamping
force onto the inlet section of the stent. The holder may also comprise a
plurality of flanges.
In some embodiments, the distal portion of the elongate tip is made of a
flexible material. This can
be a flexible wire. The distal portion can have a deflection range, preferably
of about 45 degrees from ,he
long axis of the handpiece.
The delivery apparatus can further comprise an irrigation port in the elongate
tip.
Some aspects include a method of placing a trabecular stent through a
trabecular meshwork of an
eye, the stent having an inlet section and an outlet section, including
advancing a delivery apparatus holding
the trabecular stent through an anterior chamber of the eye and into the
trabecular meshwork, placing part of
tne stent through the trabecular meshwork and into a Schlemm's canal of the
eye; and releasing the stent
from the delivery apparatus.
- 23 -
CA 02718294 2010-10-15
In various embodiments, the method includes using a delivery apparatus that
comprises a handpiece
having a distal end and a proximal end; an elongate tip connected to the
distal end of the handpiece, the
elongate tip having a distal portion and being configured to be placed through
a corneal incision and into an
anterior chamber of the eye; a holder attached to the distal portion of the
elongate tip, the holder configured to
hold and release the inlet section of the trabecular stent; and an actuator on
the handpiece that actuates the
holder to release the inlet section of the trabecular stent from the holder.
In one aspect, the trabecular stent is removably attached to a delivery
apparatus (also known as
uapplicator). When the trabecular stent is deployed from the delivery
apparatus into the eye, the outlet
section is positioned in substantially opposite directions inside Schlemm's
canal. In one embodiment, a
deployment mechanism within the delivery apparatus includes a push-pull type
plunger. In some
embodiments, the delivery applicator may be a guidewire, an expandable basket,
an inflatable balloon, or the
like.
Other Embodiments
Screw/Barb Anchored Stent:
FIGS. 32 and 33 illustrate a glaucoma stent device 30f having features and
advantages in
accordance with one embodiment. This embodiment of the trabecular stent 30f
includes a barbed or threaded
screw-like extension or pin 126 with barbs 128 for anchoring. The barbed pin
126 extends from a distal or
base portion 130 of the stent 301.
In use, the stent 30f (FIG. 32) is advanced through the trabecular meshwork 21
and across
Schlemm's canal 22. The barbed (or threaded) extension 126 penetrates into the
back wall 92 of Schlemm's
canal 22 up to the shoulder or base 130 that then rests on the back wall 92 of
the canal 22. The combination
of a shoulder 130 and a barbed pin 126 of a particular length limits the
penetration depth of the barbed pin
126 to a predetermined or preselected distance. In one embodiment, the length
of the pin 126 is about 0.5
mm or less. Advantageously, this barbed configuration provides a secure
anchoring of the stent 301. As
discussed above, correct orientation of the stent 30f is ensured by
appropriate fiducial marks, indicia or the
like and by positioning of the stent in a preloaded applicator.
Referring to FIG. 32, the aqueous flows from the anterior chamber 20, through
the lumen 42f, then
out through two side-ports 561 to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-pod 561. In
other embodiments, more then two
outlet ports 561, for example, six to eight ports (like a pin wheel
configuration), may be efficaciously used, as
needed or desired.
Still referring to FIG. 32, in one embodiment, the stent 30f is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30f
may be combined with any of the
blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
- 24 -
CA 02718294 2010-10-15
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Deeply Threaded Stent:
FIG. 34 illustrates a glaucoma stent device 30g having features and advantages
in accordance with
one embodiment The stent 30g has a head or seat 38g and a shank or main body
portion 40g with a base or
distal end 132. This embodiment of the trabecular stent 30g includes a deep
thread 134 (with threads 136) on
the main body 40g of the stent 30g below the head 38g. The threads may or may
not extend all the way to
the base 132.
In use, the stent 30g (FIG. 34) is advanced through the meshwork 21 through a
rotating motion, as
with a conventional screw. Advantageously, the deep threads 136 provide
retention and stabilization of the
stent 30g in the trabecular meshwork 21.
Referring to FIG. 34, the aqueous flows from the anterior chamber 20, through
the lumen 42g, then
out through two side-ports 56g to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-port 56g. In
other embodiments, more then two
outlet ports 56g may be efficaciously used, as needed or desired.
One suitable applicator or delivery apparatus for this stent 30g (FIG. 34)
includes a preset rotation,
for example, via a wound torsion spring or the like. The rotation is initiated
by a release trigger on the
applicator. A final twist of the applicator by the surgeon and observation of
suitable fiducial marks, indicia or
.14 the like ensure proper alignment of the side ports 56g with Schlemm's
canal 22.
Referring to FIG. 34, in one embodiment, the stent 30g is inserted through a
previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 309
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 211s made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Rivet Style Stent
FIG. 35 illustrates a glaucoma stent device 30h having features and advantages
in accordance with
one embodiment. The stent has a base or distal end 138. This embodiment of the
trabecular stent 30h has a
pair of flexible ribs 140. In the unused state, the ribs are initially
generally straight (that is, extend in the
general direction of arrow 142).
Referring to FIG. 35, upon insertion of the stent 30h through the trabecular
meshwork 21, the ends
144 of respective ribs 140 of the stent 30h come to rest on the back wall 92
of Schlemm's canal 22. Further
advancement of the stent 30h causes the ribs 140 to deform to the bent shape
as shown in the drawing of
FIG. 35. The ribs 140 are designed to first buckle near the base 138 of the
stent 30h. Then the buckling point
moves up the ribs 140 as the shank part 40h of the stent 30h is further
advanced through the trabecular
meshwork 21,
- 25 -
CA 02718294 2010-10-15
The lumen 42h (FIG. 35) in the stent 30h is a simple straight hole. The
aqueous flows from the
anterior chamber 20, through the lumen 42h, then out around the ribs 140 to
the collector channels further
along Schlemm's canal 22 in either direction.
Referring to FIG. 35, in one embodiment, the stent 30h is inserted through a
previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30h
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Grommet Style Stant:
FIG. 36 illustrates a glaucoma stent device 301 having features and advantages
in accordance with
one embodiment. This embodiment of the trabecular stent 301 includes a head or
seat 381, a tapered base
portion 146 and an intermediate narrower waist portion or shank 401.
In use, the stent 301 (FIG. 36) is advanced through the trabecular meshwork 21
and the base 146 is
pushed into Schlemm's canal 22. The stent 30i is pushed slightly further, if
necessary, until the meshwork 21
stretched by the tapered base 146 relaxes back and then contracts to engage
the smaller diameter portion
waist 401 of the stent 301. Advantageously, the combination of the larger
diameter head or seat 381 and base
146 of the stent 301 constrains undesirable stent movement. As discussed
above, correct orientation of the
stent 301 is ensured by appropriate fiducial marks, indicia or the like and by
positioning of the stent in a
preloaded applicator.
Referring to FIG. 36, the aqueous flows from the anterior chamber 20, through
the lumen 421, then
out through two side-ports 561 to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-port 561. In
other embodiments, more then two
outlet ports 561 may be efficaciously used, as needed or desired.
Still referring to FIG. 36, in one embodiment, the stent 301 is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 301
may be combined with any of the
blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Blointeractive Stent:
FIG. 37 illustrates a glaucoma stent device 30j having features and advantages
in accordance with
one embodiment. This embodiment of the trabecular stent 30j utilizes a region
of biointeractive material 148
that provides a site for the trabecular meshwork 21 to firmly grip the stent
30j by ingrowth of the tissue into the
biointeractive material 148. As shown in FIG. 37, preferably the
biointeractive layer 148 is applied to those
surfaces of the stent 301 which would abut against or come in contact with the
trabecular meshwork 21.
- 26 -
CA 02718294 2010-10-15
In one embodiment, the biointeractive layer 148 (FIG. 37) may be a region of
enhanced porosity with
a growth promoting chemical. In one embodiment, a type of bio-glue 150 that
dissolves over time is used to
hold the stent secure during the time between insertion and sufficient
ingrowth for stabilization. As discussed
above, correct orientation of the stent 30j is ensured by appropriate fiducial
marks, indicia or the like and by
positioning of the stent in a preloaded applicator.
Referring to FIG. 37, the aqueous flows from the anterior chamber 20, through
the lumen 421, then
out through two side-ports 56j to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-port 56j. In
other embodiments, more then two
outlet ports 561 may be efficaciously used, as needed or desired.
Still referring to FIG. 37, in one embodiment, the stent 301 is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 301
may be combined with any of the
blade configurations taught or suggested herein- to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Glued or Welded Stent:
FIG. 38 illustrates a glaucoma stent device 30k having features and advantages
in accordance with
one embodiment. This embodiment of the trabecular stent 30k is secured in
place by using a permanent
(non-dissolving) bio-glue 152 or a 'welding' process (e.g. heat) to form a
weld 152. The stent 30k has a head
or seat 38k and a lower surface 46k.
The stent 30k is advanced through the trabecular meshwork 21 until the head or
seat 38k comes to
rest on the trabecular meshwork 21, that is, the head lower surface 46k abuts
against the trabecular
meshwork 21, and the glue or weld 152 is applied or formed therebetween, as
shown in FIG. 38. As
discussed above, correct orientation of the stent 30k is ensured by
appropriate Nuclei marks, indicia or the
like and by positioning of the stent in a preloaded applicator.
Referring to FIG. 38, the aqueous flows from the anterior chamber 20, through
the lumen 42k, then
out through two side-ports 56k to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-port 56k. In
other embodiments, more then two
outlet ports 56k may be efficaciously used, as needed or desired.
Still referring to FIG. 38, in one embodiment, the stent 30k is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30k
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 211s made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
- 27 -
CA 02718294 2010-10-15
Hydrophilic Latching Stant:
FIG. 39 illustrates a glaucoma stent device 30m having features and advantages
in accordance with
one embodiment. This embodiment of the trabecular stent 30m is fabricated from
a hydrophilic material that
expands with absorption of water. Desirably, this would enable the device 30m
to be inserted through a
smaller incision in the trabecular meshwork 21. The subsequent expansion
(illustrated by the smaller arrows
154) of the stent 30m would advantageously enable it to latch in place in the
trabecular meshwork 21. As
discussed above, correct orientation of the stent 30m is ensured by
appropriate fiducial marks, indicia or the
like and by positioning of the stent in a preloaded applicator.
Referring to FIG. 39, the aqueous flows from the anterior chamber 20, through
the lumen 42m, then
out through two side-ports 56m to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-pod 56m. In
other embodiments, more then two
outlet ports 56m may be efficaciously used, as needed or desired.
Still referring to FIG. 39, in one embodiment, the stent 30m is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30m
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Photodynamic Stant:
FIG. 40 illustrates a glaucoma stent device 30n having features and advantages
in accordance with
one embodiment This embodiment of the trabecular stent 30n is fabricated from
a photodynamic material
that expands on exposure to light.
It is commonly known that there is a diurnal variation in the aqueous humor
production by the eye ¨
it is higher during the day than it is at night. The lumen 42n of the stent
30n responds to light entering the
cornea during the day by expanding and allowing higher flow of aqueous through
the lumen 42n and into
Schlemm's canal 22. This expansion is generally indicated by the smaller
arrows 156 (FIG. 40) which show
the lumen 42n (and ports) expanding or opening in response to light stimulus.
(The light or radiation energy E
is generally given by E = hv, where h is Planck's constant and v is the
frequency of the light provided.) At
night, in darkness, the lumen diameter decreases and reduces the flow allowed
through the lumen 42n. In
one embodiment, an excitation wavelength that is different from that commonly
encountered is provided on an
as-needed basis to provide higher flow of aqueous to Schlemm's canal 22.
This photodynamic implementation is shown in FIG. 40 for the self-latching
style of stent 30n, but
can be efficaciously used with any of the other stent embodiments, as needed
or desired. As discussed
above, correct orientation of the stent 30n is ensured by appropriate fiducial
marks, indicia or the like and by
positioning of the stent in a preloaded applicator.
- 28 -
CA 02718294 2010-10-15
Referring to FIG. 40, the aqueous flows from the anterior chamber 20, through
the lumen 42n, then
out through two side-ports 56n to be directed in both directions along
Schlemm's canal 22. Alternatively, flow
could be directed in only one direction through a single side-port 56n. In
other embodiments, more then two
outlet ports 56n may be efficaciously used, as needed or desired.
Still referring to FIG. 40, in one embodiment, the stent 30n is inserted
through a previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30n
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Collector Channel Alignment Stent:
FIG. 41 illustrates a glaucoma stent device 30p having features and advantages
in accordance with
one embodiment. This figure depicts an embodiment of a stent 30p that directs
aqueous from the anterior
chamber 20 directly into a collector channel 29 which empties into aqueous
veins. The stent 30p has a base
or distal end 160.
In the illustrated embodiment of FIG. 41, a removable alignment pin 158 is
utilized to align the stent
lumen 42p with the collector channel 29. In use, the pin 158 extends through
the stent lumen 42p and
protrudes through the base 160 and extends into the collector channel 29 to
center and/or align the stent 30p
over the collector channel 29. The stent 30p is then pressed firmly against
the back wall 92 of Schlemm's
canal 22. A permanent bio-glue 162 is used between the stent base and the back
wall 92 of Schlemm's canal.
22 to seat and securely hold the stent 30p in place. Once positioned, the pin
158 is withdrawn from the lumen
42p to allow the aqueous to flow directly from the anterior chamber 20 into
the collector duct 29. The collector
ducts are nominally 20 to 100 micrometers ( m) in diameter and are visualized
with a suitable microscopy
method (such as ultrasound biomicroscopy (UBM)) or laser imaging to provide
guidance for placement of the
stent 30p.
Referring to FIG. 41, in one embodiment, the stent 30p is inserted through a
previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30p
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Barbed Stent (Anterior Chamber to Collector Channel):
FIG. 42 illustrates a glaucoma stent device 30q having features and advantages
in accordance with
one embodiment. This figure depicts an embodiment of a stent 30q that directs
aqueous from the anterior
chamber 20 directly into a collector channel 29 which empties into aqueous
veins. The stent 30q has a base
or distal end 166 and the channel 29 has wall(s) 164.
- 29 -
CA 02718294 2010-10-15
In the illustrated embodiment of FIG. 42, a barbed, small-diameter extension
or pin 168 on the stent
base 166 is guided into the collector channel 29 and anchors on the wall(s)
164 of the channel 29. The pin
168 has barbs 170 which advantageously provide anchoring of the stent 30q. The
collector ducts 29 are
nominally 20 to 100 micrometers (pm) in diameter and are visualized with a
suitable microscopy method
(such as ultrasound biomicroscopy (WM)) or laser imaging to provide guidance
for placement of the stent
Referring to FIG. 42, in one embodiment, the stent 30q is inserted through a
previously made
incision in the trabecular meshwork 21. In other embodiments, the stent 30q
may be combined with any of
the blade configurations taught or suggested herein to provide self-trephining
capability. In these cases, the
incision through the trabecular meshwork 21 is made by the self-trephining
stent device which has a blade at
its base or proximate to the base.
Valved Tube Stent (Anterior Chamber to Choroid):
FIG. 43 illustrates a valved tube stent device 30r having features and
advantages in accordance with
one embodiment. This is an embodiment of a stent 30r that provides a channel
for flow between the anterior
chamber 20 and the highly vascular choroid 17. Clinically, the choroid 17 can
be at pressures lower than
those desired for the eye 10. Therefore, this stent 30r includes a valve with
an opening pressure equal to the
desired pressure difference between the choroid 17 and the anterior chamber 10
or a constriction that provide
the desired pressure drop.
Osmotic Membrane (Anterior Chamber to Chorold):
FIG. 44 illustrates a osmotic membrane device 30s having features and
advantages in accordance
= 20 with one embodiment This embodiment provides a channel for flow
between the anterior chamber 20 and
the highly vascular choroid 17. The osmotic membrane 30s is used to replace a
portion of the endothelial
layer of the choroid 17. Since the choroid 17 is highly vascular with blood
vessels, the concentration of water
on the choroid side is lower than in the anterior chamber 20 of the eye 10.
Therefore, the osmotic gradient
drives water from the anterior chamber 20 into the choroid 17.
Clinically, the choroid 17 (FIG. 44) can be at pressures lower than those
desired for the eye 10.
Therefore, desirably, both osmotic pressure and the physical pressure gradient
are in favor of flow into the
choroid 17. Flow control is provided by proper sizing of the area of the
membrane, ¨ the larger the
membrane area is the larger the flow rate will be. This advantageously enables
tailoring to tune the flow to
the desired physiological rates.
Ab Externo Insertion of Stent via Small Puncture:
FIG. 45 illustrates the implantation of a stent 30t using an ab extemo
procedure having features and
advantages in accordance with one embodiment. In the ab extemo procedure of
FIG. 45, the stent 30t is
inserted into Schlemm's canal 21 with the aid of an applicator or delivery
apparatus 100c that creates a small
puncture into the eye 10 from outside.
- 30-
CA 02718294 2010-10-15
Referring to FIG. 45, the stent 30t is housed in the applicator 100c, and
pushed out of the applicator
100c once the applicator tip is in position within the trabecular meshwork 21.
Since the tissue surrounding the
trabecular meshwork 21 is optically opaque, an imaging technique, such as
ultrasound biomicroscopy (UBM)
or a laser imaging technique, is utilized. The imaging provides guidance for
the insertion of the applicator tip
and the deployment of the stent 30t This technique can be used with a large
variety of stent embodiments
with slight modifications since the trabecular meshwork 21 is punctured from
the sclera, side rather than the
anterior chamber side in the ab extern insertion.
FIG. 46 a glaucoma stent device 30u having features and advantages in
accordance with a modified
embodiment. This grommet-style stent 30u for ab e:ctemo insertion is a
modification of the embodiment of
FIG. 36. In the embodiment of FIG. 46, the upper part or head 38u is tapered
while the lower part or base 172
is flat, as opposed to the embodiment of FIG. 36. The stent 30u is inserted
from the outside of the eye 10
through a puncture in the sdera. Many of the other embodiments of stents
taught or suggested herein can be
modified for similar implantation.
This ultra microscopic device 30u (FIG. 46) can be used with (1) a targeting
Lasik-type laser, or with
(2) contact on eyes or with (3) combined ultrasound microscope or (4) other
device insertor handpiece.
Targeted Drug Delivery to the Trabecular Meshwork:
FIG. 47 illustrates a targeted drug delivery implant 30v having features and
advantages in
accordance with one embodiment. This drawing is a depiction of a targeted drug
delivery concept. The slow
release implant 30v is implanted within the trabecular meshwork 21.
A drug that is designed to target the trabecular meshwork 21 to increase its
porosity, or improve the
active transport across the endothelial layer of Schlemm's canal 22 can be
stored in this small implant 30v
(FIG. 47). Advantageously, slow release of the drug promotes the desired
physiology at minimal dosage
levels since the drug is released into the very structure that it is designed
to modify:
While the components and techniques of the invention have been described with
a certain degree of
particularity, it is manifest that many changes may be made in the specific
designs, constructions and
methodology herein above described without departing from the spirit and scope
of this disclosure. It should
be understood that the invention is not limited to the embodiments set forth
herein for purposes of
exemplification, but is to be defined only by a fair reading of the appended
claims, including the full range of
equivalency to which each element thereof is entitled.
- 31 -