Note: Descriptions are shown in the official language in which they were submitted.
CA 02718659 2015-03-10
LOW TEMPERATURE COATED PARTICLES FOR USE AS PROPPANTS OR
IN GRAVEL PACKS, METHODS FOR MAKING AND USING THE SAME
FIELD OF THE INVENTION
[0002] The disclosure relates to coated particles and to methods for making
and
using the same. In particular, this disclosure relates to coated particles
that are used in
well stimulation treatments as proppants or in gravel packs, and made by
coating a particle
with a curable liquid resin at ambient temperature, applying a reactive powder
(e.g., a
resole powder, a novolak powder, a polyester powder, an acrylic polymer
powder, a
urethane powder or an epoxy powder) to the coated particle and mixing until
dry at low
temperature. If desired the particles may also be used in coated sand
applications for the
foundry industry.
BACKGROUND OF THE INVENTION
[0003] The term "proppant" is indicative of particulate material
which is injected
into fractures in subterranean formations surrounding oil wells, gas wells,
water wells, and
other similar bore holes to provide support to hold (prop) these fractures
open and allow
gas or liquid to flow through the fracture to the bore hole or from the
formation.
Proppants are commonly used to prop open fractures formed in subterranean
formations
such as oil and natural gas wells during hydraulic fracturing.
[0004] Uncoated and/or coated particles are often used as proppants to keep
open
fractures imposed by hydraulic fracturing upon a subterranean formation, e.g.,
an oil or
gas bearing strata, or as filtering media in gravel packs.
[0005] The uncoated proppants are typically particles of sand,
ceramics, glass
beads, walnut shells, etc. as known in the art. Particles used to prop
fractures generally
comprise sand or sintered ceramic particles. The advantage of sand is that it
is
- 1 -
CA 02718659 2010-10-25
inexpensive. Its disadvantages are its relatively low strength (high crush
values) and
lower flow capacities than sintered ceramic particles. Sintered ceramic
particles are
disadvantageous in that the sintering is carried out at high temperatures,
resulting in high
energy costs to produce, and expensive raw materials are used.
[0006] The coated proppants have individual particles coated with a resin.
The
individual particles are typically particles of sand, ceramics, glass beads,
walnut shells,
etc. as known in the art. The proppant coatings may be precured or curable.
The precured
proppants include a substrate core and a coating of resin cured prior to
insertion into the
subterranean formation. The curable proppants include a substrate core and a
coating of
resin cured downhole to form a consolidated proppant pack. Resin formulations
typically
used for curable coatings on proppant substrates (sand, ceramic, etc.) result
in a highly
crosslinked coating on the surface of the substrates.
[0007]
Curable resin coated proppants and precured resin coated proppants have
been commercially available for use as propping agents. A curable proppant has
a resin
coating that includes a resin that is usually at least partially, and but not
fully, cured. In
contrast, a "precured" proppant has a cured resin coating. The terms "cured"
and
"curable" are defined for the present specification by three tests
historically employed in
the art.
[0008]
a) Temperature Stick Point Test: placing coated material on a heated melt
point bar and determining the lowest temperature at which the coated material
adheres to
the melt point bar. A "sticking temperature" of greater than 350 F, typically
indicates a
cured material, depending upon the resin system used.
[0009]
b) Acetone Extraction Test: an acetone extraction method, as described
below, to dissolve the fraction of resin within the coating that is uncured. A
weight loss of
<5% typically indicates that the particle has a procured coating.
[0010]
c) Compressive Strength Test: no bonding, or no consolidation of the
coated particles, following wet compression at 1000 psi at 200 F for a period
of as much
as 24 hours, which typically indicates a coating that was precured in the
manufacturing
process.
- 2 -
DOCSMTL. 4070637 \ 1
CA 02718659 2010-10-25
[0011] Unless otherwise indicated, the terms cured and curable are
defined by the
Compressive Strength Test.
[0012] Proppants are generally used to increase production of oil
and/or gas by
providing a conductive channel in the formation. Fracturing of the
subterranean formation
is conducted to increase oil and/or gas production. Fracturing is caused by
injecting a
viscous fracturing fluid or a foam at a high pressure (hereinafter injection
pressure) into
the well to create a fracture. A similar effect can be achieved by pumping a
thin fluid
(water containing a low concentration of polymer) at a high injection rate. As
the fracture
is formed, a particulate material, referred to as a "propping agent" or
"proppant" is placed
in the formation to maintain the fracture in a propped condition when the
injection
pressure is released. As the fracture forms, the proppants are carried into
the fracture by
suspending them in additional fluid or foam to fill the fracture with a slurry
of proppant in
the fluid or foam. Upon release of the pressure, the proppants form a pack
that serves to
hold open the fractures. The propped fracture thus provides a highly
conductive channel
in the formation. The degree of stimulation afforded by the hydraulic fracture
treatment is
largely dependent upon formation parameters, the fracture's permeability, the
propped
fracture length, propped fracture height and the fracture's propped width.
[0013] Gravel packing treatments are used to reduce the migration of
unconsolidated formation sands/fines into the well bore. In gravel packing
operations, the
coated and/or uncoated particles suspended in a carrier fluid are pumped into
a well bore
in which the gravel pack is to be placed. The carrier fluid leaks off into the
subterranean
zone and/or is returned to the surface while the particles are left in the
annulus between the
production string and the casing or outside the casing in the subterranean
zone adjacent to
the wellbore.
[0014] Gravel pack operations generally involve placing a gravel pack
screen in
the well bore and packing the surrounding annulus between the screen and the
well bore
with the particles. The gravel pack screen is generally a type of filter
assembly used to
support and retain the particles placed during the gravel pack operation. A
wide range of
sizes and screen configurations are available to suit the characteristics of a
particular well
bore, the production fluid, and the subterranean formation sands. Such gravel
packs may
- 3 -
DOCSMTL 4070637\1
CA 02718659 2015-03-10
be used to stabilize the formation while causing minimal impairment to well
productivity.
The gravel pack acts as a filter to separate formation sands from produced
fluids while
permitting the produced oil and/or gas to flow into the well bore. The
particles act to
prevent formation sands from plugging the screen or migrating with the
produced fluids,
and the screen acts to prevent fines from being produced to the surface and
out of the well.
[0015]
Gravel packing may also be used to protect the well borewall production
integrity by employing a tightly packed deposit of aggregate comprising sand,
gravel or
both between the borewall and the production pipe thereby avoiding the time
and expense
of setting a steel casing from the surface to the production zone which may be
many
thousands of feet below the surface. The gravel packing is inherently
permeable to the
desired hydrocarbon fluid and provides structural reinforcement to the
borewall against an
interior collapse or flow degradation. Such well completion systems are called
"open
hole" completions. The apparatus and process by which a packed deposit of
gravel is
placed between the borehole wall and the production pipe is encompassed within
the
definition of an "open hole gravel pack system." Unfortunately, prior art open
hole gravel
pack systems. for placing and packing gravel along a hydrocarbon production
zone, have
been attended by a considerable risk of precipitating a borehole wall collapse
due to
fluctuations in the borehole pressure along the production zone.
These pressure
fluctuations are generated by surface manipulations of the downhole tools in
direct fluid
circulation within the well and completion string. Further discussion of
gravel packs is
presented by US Patent No. 6,382,319.
[0016]
In some situations the processes of hydraulic fracturing and gravel packing
are combined into a single treatment to provide stimulated production and an
annular
gravel pack to reduce formation sand production. Such treatments are often
referred to as
"frac pack" operations. In some cases, the treatments are completed with a
gravel pack
screen assembly in place, and the hydraulic fracturing treatment being pumped
through the
annular space between the casing and screen. In such a situation, the
hydraulic fracturing
treatment usually ends in a screen out condition creating an annular gravel
pack between
the screen and casing. This allows both the hydraulic fracturing treatment and
gravel pack
to be placed in a single operation.
- 4 -
CA 02718659 2015-03-10
[0017] Moreover, sand control is another consideration when
extracting
hydrocarbons such as natural gas and crude oil from the earth's subsurface
formations,
from boreholes drilled into hydrocarbon bearing production zones. Production
of oil, gas
and water from unconsolidated or weakly consolidated formations is normally
accompanied by the production of formation sand particles along with the
produced fluids.
The production of sand with the well fluids poses serious problems such as the
erosion of
sub-surface and surface production facilities and the accumulation of the sand
in the
wellbore and surface separators. Several methods such as gravel packing,
screens and
plastic consolidation have been in use for many years with varying success.
However,
these methods have several-technical and cost limitations. Further discussion
of sand
control is presented by US Patent No. 6,364,019.
[0018] When the oilfield industry "fractures" hydrocarbon bearing
formations, the
use of proppants to retain the high surface area created by the fracture has
become
common practice. It is highly desirable that the proppant particles are of
high performance
and can be produced in highly efficient processes (are economically
attractive). It is
further desirable to develop coated particles that can be produced at remote
sites, such as
field applied at or near the wellsite.
- 5 -
CA 02718659 2015-03-10
SUMMARY OF THE INVENTION
[0018a] In accordance with one aspect of the present invention, there is
provided a
free flowing coated particles having a particle size range of about 6 mesh to
about 200
mesh, each particle comprising: a substrate selected from the group consisting
of: a
particulate substrate comprising an inorganic material and optionally an at
least partially
cured coating, a particulate substrate comprising an organic material and
optionally an at
least partially cured coating, a composite particle comprising a substantially
homogeneous
formed particle comprising a first portion of a binder and filler particles
dispersed
throughout said first portion of binder, wherein said first portion is at
least partly cured,
wherein the particle size of the filler particles ranges from about 0.5 to
about 60 um; and a
hybrid particle comprising a composite layer disposed on an inorganic
particulate core, the
composite layer comprising an at least partially cured organic coating and
filler particles,
wherein the particle size of the filler particles ranges from about 0.5 to
about 60 um; and a
coating disposed upon the substrate, the coating comprising a continuous phase
comprising a curable liquid resin and a reactive powder embedded or adhered to
the
continuous phase, wherein the curable liquid resin comprises at least one
member selected
from the group consisting of resole phenolic-formaldehyde resins, polyester
resins, acrylic
polymer resins, urethane resins, epoxy resins, melamine resins and anhydride
resins, and
wherein the reactive powder comprises at least one member selected from the
group
consisting of resole phenolic-formaldehyde resins, novolak phenolic-
formaldehyde resins,
polyester resins, acrylic polymer resins, urethane resins and epoxy resins,
wherein the
reactive powder is reactive with the curable liquid.
10018b1 In accordance with another aspect of the present invention, there
is
provided a free flowing coated particles having a particle size range of about
6 mesh to
about 200 mesh, each particle comprising: a substrate selected from the group
consisting
of: a particulate substrate comprising an inorganic material and optionally an
at least
partially cured coating, a particulate substrate comprising an organic
material and
optionally an at least partially cured coating, a composite particle
comprising a
substantially homogeneous formed particle comprising a first portion of a
binder and filler
particles dispersed throughout said first portion of binder, wherein said
first portion is at
least partly cured, wherein the particle size of the filler particles ranges
from about 0.5 to
about 60 p.m; and a hybrid particle comprising a composite layer disposed on
an inorganic
- 5a -
CA 02718659 2015-03-10
particulate core, the composite layer comprising an at least partially cured
organic coating
and filler particles, wherein the particle size of the filler particles ranges
from about 0.5 to
about 60 lam; and a coating disposed upon the substrate, the coating
comprising a
continuous phase comprising a curable liquid resin and a reactive powder
embedded or
adhered to the continuous phase, wherein the curable liquid resin comprises at
least one
member selected from the group consisting of resole phenolic-formaldehyde
resins,
polyester resins, acrylic polymer resins, urethane resins, epoxy resins,
melamine resins and
anhydride resins, wherein the reactive powder comprises an epoxy resin, and
wherein the
reactive powder is reactive with the curable liquid.
[0018c] In
accordance with yet another aspect of the present invention, there is
provided a free flowing coated particles having a particle size range of about
6 mesh to
about 200 mesh, each particle comprising: a substrate selected from the group
consisting
of: a particulate substrate comprising an inorganic material and optionally an
at least
partially cured coating, a particulate substrate comprising an organic
material and
optionally an at least partially cured coating, a composite particle
comprising a
substantially homogeneous formed particle comprising a first portion of a
binder and filler
particles dispersed throughout said first portion of binder, wherein said
first portion is at
least partly cured, wherein the particle size of the filler particles ranges
from about 0.5 to
about 60 lam; and a hybrid particle comprising a composite layer disposed on
an inorganic
particulate core, the composite layer comprising an at least partially cured
organic coating
and filler particles, wherein the particle size of the filler particles ranges
from about 0.5 to
about 60 lam; and a coating disposed upon the substrate, the coating
comprising a
continuous phase comprising a curable liquid resin and a reactive powder
embedded or
adhered to the continuous phase, wherein the coated particles further comprise
a surface
treatment selected from the group that consists of a non-reactive powder, a
resin or
resin/curing agent combination, or both, disposed on the coating, wherein the
curable
liquid resin comprises at least one member selected from the group consisting
of resole
phenolic-formaldehyde resins, polyester resins, acrylic polymer resins,
urethane resins,
epoxy resins, melamine resins and anhydride resins, wherein the reactive
powder
comprises at least one member selected from the group consisting of resole
phenolic-
formaldehyde resins, novolak phenolic-formaldehyde resins, polyester resins,
acrylic
polymer resins, urethane resins and epoxy resins, and wherein the reactive
powder is
reactive with the curable liquid.
- 5b -
CA 02718659 2015-03-10
[0019] In one embodiment, there is provided free flowing coated
particles having a
particle size range of about 6 mesh to about 200 mesh (3360 - 74 m), each
particle
includes (1) a substrate selected from (i) a particulate substrate comprising
an inorganic
material and optionally an at least partially cured coating, (ii) a
particulate substrate
comprising an organic material and optionally an at least partially cured
coating, (iii) a
composite particle comprising a substantially homogeneous formed particle
comprising a
first portion of a binder and filler particles dispersed throughout said first
portion of
binder, wherein said first portion is at least partly cured, wherein the
particle size of the
filler particles ranges from about 0.5 to about 60 jim, or (iv) a hybrid
particle comprising a
composite layer disposed on an inorganic particulate core, the composite layer
comprising
an at least partially cured organic coating and filler particles, wherein the
particle size of
the filler particles ranges from about 0.5 to about 60 lam; and (2) a coating
disposed upon
the substrate, the coating comprising a continuous phase comprising a curable
liquid resin
and a reactive powder embedded or adhered to the continuous phase.
[0020] In another embodiment, the curable liquid resin in the above
described free
flowing coated particles includes resole phenolic-formaldehyde resins,
polyester or
unsaturated polyester resins, acrylic polymer resins, urethane resins, epoxy
resins,
melamine resins, anhydride resins or any combination or subset thereof
[0021] In another embodiment, the reactive powder in the above described
free
flowing coated particles includes resole phenolic-formaldehyde resins, novolak
phenolic-
formaldehyde resins, polyester resins, acrylic polymer resins, urethane
resins, epoxy resins
or any combination or subset thereof
[0022] In another embodiment, the above described free flowing coated
particles
further include a surface treatment which includes resole phenolic-
formaldehyde resins,
polyester or unsaturated polyester resins, acrylic polymer resins, urethane
resins, epoxy
resins, melamine resins, anhydride resins or any combination or subset thereof
[0023] In another embodiment, there is provided a method of preparing
the above
described free flowing coated particles which includes mixing the substrate
with the
- 6 -
CA 02718659 2010-10-25
curable liquid resin at a temperature of between about 50 F to about 150 F to
form the
coating comprising the continuous phase, then admixing the reactive powder
such that it is
embedded in or adhered to the continuous phase.
[0024] In another embodiment, the above method further includes the
step of
applying a surface treatment to the free flowing coated particles.
[0025] The powders employed in the method may be reactive or non-
reactive.
[0026] The reactive powders in the products and/or methods are
reactive with at
least the above-mentioned continuous phase. Employing reactive powders
advantageously
improves unconfined compressive strength properties of the particle. Non-
reactive
powders, for example, silica flour are inert with respect to the continuous
phase, and may
be added to improve processing or storage properties.
[0027] Typically the reactive powders comprise at least one member of
the group
consisting of resole phenolic-formaldehyde resins, novolak phenolic-
formaldehyde resins,
polyester resins, acrylic polymer resins, urethane resins and epoxy resins.
The method of
making utilizes the low temperature application of organic resins to sand and
ceramic
substrates in cycles requiring only a matter of minutes that yields high
performance coated
particles for the oilfield (and foundry) industry. The powder is added to
effectively dry
the applied liquid coating and allow the coated particles to be separated and
free flowing.
[0028] The substrate is defined as the portion of the particle coated
by the one or
more outer coatings of the present invention. The substrate can be present in
the coated
particles in an amount of about 85 to about 99.5 weight percent (wt. %), based
on the total
weight of the coated particles. In one embodiment, in which the outer coating
is placed
directly on a sand or ceramic particle, the substrate is present in an amount
of about 95 to
99.5 wt%, based on the total weight of the coated particles.
[0029] The outer resin coating typically is between 0.5 to 15% of the total
weight
of the particle. In another embodiment, the resin coating is between 0.5 to
6.0% of the
total weight of the particle.
- 7 -
DOCSMTL: 4070637\1
CA 02718659 2010-10-25
[0030] In one embodiment, the outer coating is placed directly on a
single
inorganic particle. Typically the particulate substrate comprising an
inorganic material is
a sand or a ceramic particulate substrate. A preferred inorganic substrate is
40/70 sand. In
embodiments employing the inorganic particulate substrate coated with the one
or more
layers of coating comprising a continuous phase comprising curable liquid
resin and
reactive powder particles, preferably the dry free flowing particle has a loss
on ignition
(LOT) of about 0.3 to about 8%, about 0.3 to about 5%, about 0.5 to 5%, about
0.75 to 4%
or about 0.75 to 3%. Unless otherwise stated, all percentages disclosed in
this
specification are weight percents.
[0031] In embodiments employing an organic particulate substrate,
preferably the
dry free flowing particle has a LOT of about 0.3 to about 5%, not including
the LOI
attributable to the ignition of the organic particulate substrate.
[0032] In embodiments of a coated particle in which the substrate is
a composite
particle, then the LOI would be the combination of the LOI of the composite
substrate
(which would normally be in the 12-15% range based on the total weight of the
substrate,
after it has been cured) plus the LOI of the resin coating on the substrate
(0.5 to 5% by
weight based on the total weight of the coated particle). In such embodiments
the overall
LOI is typically about 12 % to about 20 % (including the LOI attributable to
the organic
binder of the composite substrate and the coating, but not including the LOI,
if any,
attributable to the filler of the composite substrate). Typically, the filler
particles are about
70 to 90 weight percent of the composite particle. Typically, the composite
substrate is
about 95 to about 99.5 wt% of the coated particle of this invention.
[0033] The composite substrate may comprise about 10 to about 90
weight percent
(wt. %), typically about 70 to about 90 wt. %, inorganic filler materials
based on the total
weight of the composite substrate. In an embodiment, the inorganic materials
can be
present in an amount of about 20 to about 80 weight percent (wt. %), based on
the total
weight of the composite substrate. In another embodiment, the inorganic
materials can be
present in an amount of about 30 to about 70 weight percent (wt. %), based on
the total
weight of the composite substrate. In yet another embodiment, the inorganic
materials can
- 8 -
DOCSMTL: 4070637\I
CA 02718659 2010-10-25
be present in an amount of about 40 to about 60 weight percent (wt. %), based
on the total
weight of the composite substrate.
[0034] Typically, the composite particle has a sphericity of at least
about 0.7.
[0035] In embodiments of a coated particle in which the substrate is
a hybrid
particle (inorganic particulate core having a composite layer of the organic
coating and
inorganic filler), then the LOT would be the combination of the LOT of the
hybrid substrate
(which would normally be in the about 5 to about 20% range based on the total
weight of
the substrate, after it has been cured) plus the LOI of the resin coating on
the substrate (0.5
to 5% by weight based on the total weight of the coated particle). In such
embodiments
the overall LOI is typically about 5.5% to about 25% (including the LOT
attributable to the
organic binder of the hybrid substrate and the coating). Typically, the cured
or at least
partially cured composite layer is about 25 to about 40 weight % of the hybrid
particle.
Typically, the hybrid particle is about 95 to 99 weight % of the coated
particle of this
invention.
[0036] The ratio of components, order of additions and time of the addition
and
mixing are selected to form such free flowing particles. For example, if the
powder is
mixed with the inorganic uncoated substrate particles before the curable
liquid resin is
applied, then adequate coating does not occur. Also, the ratio of curable
liquid resin and
powder is selected to achieve proper coating. Too much powder results in
excess loose
powder, and too much liquid curable resin delays drying out and becoming free
flowing.
Either situation will contribute to particle agglomerations (multiple
particles sticking
together) and affect storage stability of the finished product.
[0037] The present invention relates to a method of forming a
proppant pack or a
gravel pack comprising suspending the above-described free flowing particles
in a carrier
fluid to form a suspension and injecting the suspension into a subterranean
formation.
[0038] The present invention also relates to a proppant or gravel
pack particle
comprising a substrate having a coating of curable liquid resin containing
reactive powder
embedded in the curable resin coating.
- 9 -
DOCSMIL 4070637\1
CA 02718659 2010-10-25
[0039] Coating sand or ceramic substrates with a curable liquid resin
at room
temperature, followed by the introduction of a powdered phenol-formaldehyde
novolak
resin (with or without curatives such as hexamethylenetetramine) yields a high
performance, free flowing resin coated particle that can be used as an
oilfield proppant.
[0040] The liquid resin coating is reactive with the reactive powder. For
example,
a resole coating may assist with curing the novolak powder and/or the powder
may contain
hexamethylenetetramine (HEXA) to assist in curing the resole coating.
Typically the
reactive powder or non-reactive powder has an average particle size of about
200 mesh
(74 microns) or smaller, or about 230 mesh (63 microns) or smaller; or about
270 mesh
(about 53 microns) or smaller. For example, typical particle size of powdered
resin ranges
from 5 to 35 microns with a small amount of fines. Preferably the reactive
powder
comprises a novolak powder or a resole powder. Generally at least a majority
of the
powder in or on the coating comprises reactive resin powder. The non-reactive
powders
and reactive powders do not dissolve or do not appreciably dissolve in aqueous
servicing
fluids and oil-based servicing fluids.
[0041] In the outer curable coating the typical ratio of liquid resin
to powder (total
of reactive powder and optional inorganic powder) is approximately 1:3 to 1:6.
The
weight ratio of the liquid resin to powder of the outer coating is preferably
12-30% liquid
resin and 70-88% powder. When this is applied to the substrate, some of the
liquid resin
evaporates, so on a dry solids basis, we have a ratio of 8-20% solids from the
original
liquid resin and 80-92% solids from the powder. Thus, for example, for a
particle having
an inorganic particulate substrate and an LOI of about 3 weight percent, the
total powder
on the final particles would be 80-90% X 3% = 2.4-2.7 weight %. The liquid to
powder
ratio may change depending on surface area of the particle being covered,
liquid resin's
flow properties and powdered resin particle size distribution. Conditions and
weight ratios
are determined to obtain so the resulting product particles are dry and free
flowing with
little or no free excess powder.
[0042] The curable coatings can be applied at/near the wellsite. In
this process, the
operations may be a continuous addition to a moving bed of the substrate.
However,
producing the coated particles by this process is within the scope of this
invention whether
- 10 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
precoated onto the proppant at the sand coating plant or various remote
locations, such as
a part of a transload/inventory warehouse, at or near a wellsite. The process
is also a quick
and economical way to expand production capacity and with low capital costs.
[0043] The present invention is advantageous because the method
results in a
curable coated particle that binds downhole. Also, the present method can be
performed
in remote locations in a low cost plant. Freight savings may also exist by
minimizing the
costs associated with transporting substrates. Also, this no (or low)-heating
process
achieves energy savings over comparable processes which heat phenol-
formaldehyde
resins to melt the coating onto the substrate, to drive off the aqueous
portion of a liquid
resin or which apply heat to dry or cure the resin coating. Also, by avoiding
heating the
present method minimizes emissions of volatiles which must be dealt with in a
process
which heats phenol-formaldehyde resins to melt the coating onto the substrate,
to drive off
the aqueous portion of a liquid resin or which apply heat to dry or cure the
resin coating.
BRIEF DESCRIPTION OF THE FIGURES
[0044] The following is a brief description of figures wherein like
numbering
indicates like elements.
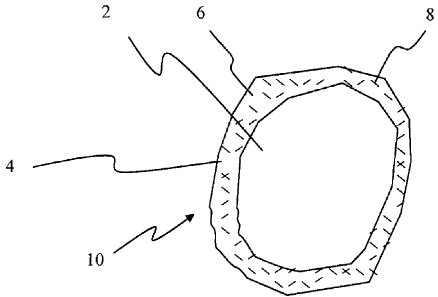
[0045] FIG. 1 depicts an embodiment of a coated particle comprising a
solid
inorganic or solid organic substrate upon which is disposed a resole coating
that comprises
a reactive powder.
[0046] FIG. 2 depicts another embodiment of a coated particle that
comprises a
solid inorganic substrate upon which is disposed a resole coating that
comprises a reactive
powder and inorganic or inert organic fillers.
[0047] FIG. 3 depicts another embodiment of a coated particle that
comprises a
substrate, wherein the substrate comprises the composite particle which is an
agglomerate
of inorganic particles and binder upon which is disposed a resole coating that
comprises a
reactive powder.
[0048] FIG. 4 depicts another embodiment of a coated particle that
comprises a
substrate, wherein the substrate comprises the hybrid particle comprising a
composite
- 11 -
DOCSMTL, 4070637\1
CA 02718659 2010-10-25
layer disposed on an inorganic particulate core, the composite layer
comprising a cured
organic coating and an inorganic filler upon which is disposed a resole
coating that
comprises a reactive powder.
[0049] FIG. 5 shows a photograph of particles of Sample A "as is"
produced in a
laboratory at a magnification of about 10X.
[0050] FIG. 6 shows a photograph of particles of Sample B "as is"
produced in a
laboratory at a magnification of about 10X.
[0051] FIG. 7 shows a photograph of a slug of Sample B particles
formed after a
1K psi UCS test at 200 degrees F at a magnification of about 10X.
[0052] FIG. 8 shows a photograph of Sample B particles after a hot tensile
test at a
magnification of about 10X.
[0053] FIG. 9 shows a sample of unpowdered resole from a comparative
example
at a magnification of about 12X.
[0054] FIG. 10 shows a sample of the product produced by a process
which
reversed the order of coating and powder sample from a comparative example a
magnification of about 30X.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[0055] As used herein, the terms "first," "second," and the like do
not denote any
order or importance, but rather are used to distinguish one element from
another, and the
terms "the", "a" and "an" do not denote a limitation of quantity, but rather
denote the
presence of at least one of the referenced items. Furthermore, all ranges
disclosed herein
are inclusive of the endpoints and independently combinable.
[0056] The present proppant or gravel pack particle comprises a
particle substrate
having a coating of curable liquid resin containing reactive powder embedded
in the
curable liquid resin coating. The reactive powder may be a resole phenolic-
formaldehyde
resin powder, a novolak phenolic-formaldehyde resin powder, a polyester resin
powder, an
- 12 -
DOCSMTI, 4070637\1
CA 02718659 2010-10-25
acrylic polymer resin powder, a urethane resin powder, or an epoxy resin
powder,
including any combination or subset of such reactive powders.
[0057] Typically for proppant, gravel pack or foundry sand,
individual particles of
the particulate substrate have a particle size in the range of USA Standard
Testing screen
numbers from about 6 to 200 mesh, e.g., 20 to 40 mesh. Typically for proppant
or gravel
pack individual particles of the particulate substrate have a particle size in
the range of
USA Standard Testing screen numbers from about 8 to about 100 mesh (i.e.
screen
openings of about 0.0937 inch to about 0.0059 inch), 20 to 80 mesh, or
preferably 40 to 70
mesh. Typical individual particles of the particulate substrate have a
diameter of about
0.01 to about 0.04 inches. Typically for foundry applications the substrate is
sand or
ceramic with particle size ranging from 30 to 140 mesh.
[0058] For example, the substrates 2, 21, 42 of, FIGs. 1-4 can have
average
particle sizes of about 100 micrometers to about 1,400 micrometers (about 140
mesh to
about 14 mesh) or about 300 micrometers to about 600 micrometers (about 50
mesh to
about 30 mesh) or about 400 micrometers to about 850 micrometers (about 40
mesh to
about 20 mesh).
[0059] The organic coating disposed upon the substrate is curable
prior to its use
as a proppant or in a gravel pack.
[0060] FIG. 1 shows an exemplary embodiment of the coated particle 10
comprises a particulate substrate 2 upon which is disposed an organic coating
4. The
particulate substrate 2 can comprise an organic material and/or an inorganic
material. The
substrate 2 preferably comprises a single inorganic particle. The organic
coating 4
comprises a curable liquid resin as a continuous phase 6 and a reactive powder
8
embedded or adhered to the continuous phase 6. If desired, a non-reactive
powder such as
inert inorganic or inert organic filler powders, such as silica flour, may be
employed
together with or in place of the reactive powder 8. Reactive powders are
preferred
because they advantageously may improve the unconfined compressive strength of
the
particle 10.
- 13 -
DOCSMTL. 4070637\1
CA 02718659 2010-10-25
[0061] FIG. 2 depicts a coated particle 12 which is the embodiment of
FIG. 1
modified to further include inert inorganic or inert organic filler particles
14 embedded or
adhered to the continuous curable liquid resin phase 6.
[0062] FIG. 3 depicts another embodiment of a coated particle 20 that
comprises a
substrate 21 and a coating 4 disposed on the substrate 21. The substrate 21
comprises an
agglomerate of inorganic particles 22 and binder 24. The coating 4 comprises
the reactive
powder 8 and continuous curable liquid resin phase 6. If desired a non-
reactive powder
such as inert inorganic or inert organic filler particles, such as silica
flour, may be
employed together with or in place of the reactive powder 8. Reactive powders
advantageously may improve the unconfined compressive strength of the particle
20. In
one embodiment, the addition of a non-reactive powder improves the storage
properties of
the coated particles.
[0063] FIG. 4 shows another exemplary embodiment comprising a coated
particle
40 which comprises a substrate 42 having an inorganic particle 44 as a core
and an at least
partially cured coating 46 which comprises inorganic or organic fillers 48. A
coating 52 is
deposited on this substrate 42. Coating 52 comprises a curable liquid resin
continuous
phase 54 and reactive resin powder 56. If desired a non-reactive powder such
as inert
inorganic or inert organic filler particles, such as silica flour, may be
employed together
with or in place of the reactive powder 56. Reactive powders advantageously
may
improve the unconfined compressive strength of the particle 40. Preferably,
the powder
56 comprises a reactive novolak or resole. If desired non-reactive powders
(not shown),
such as silica flour, may be provided in or on coating 52. In one embodiment,
at least a
majority of powder in or on the coating 52 comprises reactive resin powder.
A. Single Particle Substrate
[0064] As shown, for example, in FIGs. 1 and 2, the substrate may be a
single
particle. The substrate can be any of the organic or inorganic particulate
solid materials
normally used as propping agents, gravel pack or for sand control. For
example, suitable
particulate material, i.e., includes sand, naturally occurring mineral fibers,
such as zircon
and mullite, ceramic, such as sintered bauxite, or sintered alumina, other non-
ceramic
refractories such as milled or glass beads, or walnut shells. The substrates
can have any
- 14 -
DOCSMTL 4070637\]
CA 02718659 2015-03-10
desired shape such as spherical, egg shaped, cubical, polygonal, or the like.
It is generally
desirable for the substrates to be spherical in shape. Substrates can be
porous or non-
porous. The substrates do not melt at a temperature below 200 F or 225 F;
typically the
substrates do not melt at a temperature below 450 F or 550 F. The substrate
particles are
hard and resist deforming or can be deformable. Deforming is different from
crushing
wherein the particle deteriorates. Optionally the single particle substrate
may have an at
least partially cured resin coating.
[0065] US Patent Application Publication No. 2006/0078682 to McDaniel
et al.,
also discloses particulate substrates, comprising silica and alumina in a
silica to alumina
weight ratio of about 2.2 to about 5 and a bulk density of less than or equal
to about 1
gram per cubic centimeter, suitable for use as a single particle substrate in
the present
invention.
[0066] Examples of other inorganic materials that can be used in the
substrate are
inorganic oxides, inorganic carbides, inorganic nitrides, inorganic
hydroxides, inorganic
oxides having hydroxide coatings. inorganic carbonitrides, inorganic
oxynitrides,
inorganic borides, inorganic borocarbides, or the like, or a combination
comprising at least
one of the foregoing inorganic materials. Examples of suitable inorganic
materials are
metal oxides, metal carbides, metal nitrides, metal hydroxides, metal oxides
having
hydroxide coatings, metal carbonitrides, metal oxynitrides, metal borides,
metal
borocarbides, or the like, or a combination comprising at least one of the
foregoing
inorganic materials. Metallic cations used in the foregoing inorganic
materials can be
from transition metals, alkali metals, alkaline earth metals, rare earth
metals, or the like, or
a combination comprising at least one of the foregoing metals.
[0067] Examples of suitable inorganic oxides include silica (Si02),
alumina
(A1203), titania (Ti02), zirconia (Zr02), ceria (Ce02), manganese oxide
(Mn02), zinc
oxide (Zn0), iron oxides (e.g., FeO, a-Fe203, y-Fe2O3, Fe304, or the like),
calcium oxide
(CaO), manganese dioxide (Mn02 and Mn304), or a combination comprising at
least one
of the foregoing inorganic oxides. Examples of suitable inorganic carbides
include silicon
carbide (SiC), titanium carbide (TiC), tantalum carbide (TaC), tungsten
carbide (WC),
hafnium carbide (HfC), or the like, or a combination comprising at least one
of the
- 15 -
CA 02718659 2010-10-25
foregoing carbides. Examples of suitable nitrides include silicon nitrides
(Si3N4), titanium
nitride (TiN), or the like, or a combination comprising at least one of the
foregoing.
Examples of suitable borides are lanthanum boride (LaB6), chromium borides
(CrB and
CrB2), molybdenum borides (MoB2, Mo2B5 and MoB), tungsten boride (W2B5), or
the
like, or a combination comprising at least one of the foregoing borides.
Exemplary
inorganic substrates are those that comprise silica and/or alumina.
[0068] Other examples of suitable inorganic materials that can be
used in the
substrate are silica (sand), aeschynite (rare earth yttrium titanium niobium
oxide
hydroxide), anatase (titanium oxide), bindheimite (lead antimony oxide
hydroxide),
bixbyite (manganese iron oxide), brookite (titanium oxide), chrysoberyl
(beryllium
aluminum oxide), columbite (iron manganese niobium tantalum oxide), corundum
(aluminum oxide), cuprite (copper oxide), eincenite (rare earth yttrium
niobium tantalum
titanium oxide), fergusonite (rare earth iron titanium oxide), hausmannite
(manganese
oxide), hematite (iron oxide), ilmenite (iron titanium oxide), perovskite
(calcium titanium
oxide), periclase (magnesium oxide), polycrase (rare earth yttrium titanium
niobium
tantalum oxide), pseudobrookite (iron titanium oxide), members of the
pyrochlore group
such as, for example, betafite (rare earths calcium sodium uranium titanium
niobium
tantalum oxide hydroxide), microlite (calcium sodium tantalum oxide hydroxide
fluoride),
pyrochlore (sodium calcium niobium oxide hydroxide fluoride), or the like, or
a
combination comprising at least one of the foregoing pyrochlore group members;
ramsdellite (manganese oxide), romanechite (hydrated barium manganese oxide),
members of the rutile group, such as, for example, cassiterite (tin oxide),
plattnerite (lead
oxide), pyrolusite (manganese oxide), rutile (titanium oxide), stishovite
(silicon oxide), or
the like, or a combination comprising at least one of the foregoing rutile
group members;
samarskite-(Y) (rare earth yttrium iron titanium oxide), senarmontite
(antimony oxide),
members of the spinel group such as chromite (iron chromium oxide),
franklinite (zinc
manganese iron oxide), gahnite (zinc aluminum oxide), magnesiochromite
(magnesium
chromium oxide), magnetite (iron oxide), and spine! (magnesium aluminum
oxide), or the
like, or a combination comprising at least one of the foregoing spinel group
members;
taaffeite (beryllium magnesium aluminum oxide), tantalite (iron manganese
tantalum
niobium oxide), tapiolite (iron manganese tantalum niobium oxide), uraninite
(uranium
- 16 -
DOCSMTL: 4070637\I
CA 02718659 2010-10-25
oxide), valentinite (antimony oxide), zincite (zinc manganese oxide),
hydroxides, such as,
for example, brucite (magnesium hydroxide), gibbsite (aluminum hydroxide),
goethite
(iron oxide hydroxide), limonite (hydrated iron oxide hydroxide), manganite
(manganese
oxide hydroxide), psilomelane (barium manganese oxide hydroxide), romeite
(calcium
sodium iron manganese antimony titanium oxide hydroxide), stetefeldtite
(silver antimony
oxide hydroxide), stibiconite (antimony oxide hydroxide), or the like, or a
combination
comprising at least one of the foregoing inorganic materials.
[0069] Suitable examples of materials that are modified and used in
the substrate
are exfoliated clays (e.g., expanded vermiculite), exfoliated graphite, blown
glass or silica,
hollow glass spheres, foamed glass spheres, cenospheres, foamed slag, sintered
bauxite,
sintered alumina, or the like, or a combination comprising one of the
foregoing materials.
Exemplary inorganic substrates may be derived from sand, milled glass beads,
sintered
bauxite, sintered alumina, mineral fibers such as zircon and mullite, or the
like, or a
combination comprising one of the inorganic substrates. Hollow glass spheres
can be
commercially obtained from Diversified Proppants.
[0070] Suitable examples of organic materials that are used as the
substrate are
ground or crushed nut shells, ground or crushed seed shells, ground or crushed
fruit pits,
processed wood, ground or crushed animal bones, or a combination comprising at
least
one of the naturally occurring fillers. For example, suitable organic
materials are naturally
occurring organic fillers comprise crushed or ground walnut, crushed or ground
pecan,
crushed or ground almond, crushed or ground ivory nut, crushed or ground
brazil nut, or a
combination comprising at least one of the foregoing nuts. Other examples of
suitable
organic materials are crushed and ground seeds of plum, crushed and ground
seeds of
peach, crushed and ground seeds of cherry, crushed or ground olive hulls,
crushed and
ground seeds of apricot, ground or crushed seed shells maize, processed wood
materials
from oak, hickory, walnut, poplar and mahogany trees that have been processed
by
grinding or chipping.
[0071] In another exemplary embodiment, the inorganic substrate has a
bulk
density of about 0.6 to about 1.0 g/cm3 and an apparent density of about 1.3
to about 2.0
g/cm3. The inorganic substrates display a crush test percentage of less than
or equal to
- 17 -
DOCSMTE.: 4070637\1
CA 02718659 2015-03-10
about 20% when subjected to a crush test at 2000 psi as per API RP 60. The
inorganic
substrate has a roundness of about 0.6 to about 0.9 and a sphericity of about
0.6 to about
0.9.
100721 The density of the substrate can be chosen depending upon the
application
for which the proppant is being used. It is desirable to choose substrates
that can impart to
the proppant an apparent density of 1 to 4 g/cm3. The apparent density is
defined as the
density of the entire proppant (i.e., the weight per unit volume of the entire
material
including voids inherent in the proppant). In one embodiment, the substrate
has an
apparent density of about 1.4 to about 1.9 g/cm3. In another embodiment, the
substrate has
an apparent density of about 1.5 to about 1.85 g/cm3. In yet another
embodiment, the
substrate has an apparent density of about 1.6 to about 1.80 g/cm3. An
exemplary
apparent density for the substrate is about 1.80 g/cm3. Substrates coated with
this process,
such as sand have an apparent density of +/- 2.65 g/cm3 and various forms of
ceramics
have a density of 2.5 ¨ 3.4 gm/cc.
B. Composite Particle Substrate
100731 As shown for example in FIG. 3, noted above, the substrate 21
may
comprise a deformable composite particle comprising a homogeneous particle
comprising
fine filler particles 22 held together by a cured or at least partially cured
binder 24.
Various embodiments of these composite particles are further described below
and in US
Patent No. 6,406,789, US Patent No. 6,632,527 and US Patent No. 6,582,819, and
US
Patent Application Publication No. 2006/0078682 to McDaniel et al.
[0074] In such a case, the individual particles that combine to form
the substrate
can have average particle sizes of about 2 to about 30 micrometers. In one
embodiment,
the particles that agglomerate to form the substrate 21 may have average
particle sizes of
less than or equal to about 28 micrometers, or less than or equal to about 25
micrometers,
or less than or equal to about 20 micrometers, or less than or equal to about
15
micrometers. Bimodal or higher particle size distributions may be used.
- 18-
CA 02718659 2010-10-25
[0075]
The filler 21 can be particles or fibrous fillers. Fibrous fillers generally
have an aspect ratio greater than 1. As used herein, "fibrous" fillers may
therefore exist in
the form of whiskers, needles, rods, tubes, strands, elongated platelets,
lamellar platelets,
ellipsoids, micro fibers, nanofibers and nanotubes, elongated fullerenes, and
the like.
Where such fillers exist in aggregate form, an aggregate having an aspect
ratio greater than
1 will also suffice for the purpose of this invention. Examples of such
fillers well known
in the art include those described in "Plastic Additives Handbook, 5th
Edition" Hans
Zweifel, Ed, Carl Hanser Verlag Publishers, Munich, 2001. Non-limiting
examples of
suitable fibrous fillers include short inorganic fibers, including processed
mineral fibers
such as those derived from blends comprising at least one of aluminum
silicates,
aluminum oxides, magnesium oxides, and calcium sulfate hemihydrate, boron
fibers,
ceramic fibers such as silicon carbide, and fibers from mixed oxides of
aluminum, boron
and silicon sold under the trade name NEXTEL by 3M Co., St. Paul, MN, USA.
Also
included among fibrous fillers are single crystal fibers or "whiskers"
including silicon
carbide, alumina, boron carbide, iron, nickel, copper. Fibrous fillers such as
glass fibers,
basalt fibers, including textile glass fibers and quartz may also be included.
[0076]
Also included are natural organic fibers such as, for example, wood flour
obtained by pulverizing wood, and fibrous products such as cellulose, cotton,
sisal, jute,
cloth, hemp cloth, felt, and natural cellulosic fabrics such as Kraft paper,
cotton paper and
glass fiber containing paper, starch, cork flour, lignin, ground nut shells,
corn, rice grain
husks, or the like, or a combination comprising at least one of the foregoing.
[0077]
In addition, synthetic reinforcing fibers may be used in the composite
substrate. This includes organic materials capable of forming fibers such as
phenolic
fibers, polyethylene terephthalate, polybutylene terephthalate and other
polyesters,
polyarylates, polyethylene, polyvinylalcohol, polytetrafluoroethylene, acrylic
resins, high
tenacity fibers with high thermal stability including aromatic polyamides,
polyaramid
fibers such as those commercially available from Du Pont de Nemours under the
trade
name KEVLAR, polybenzimidazole, polyimide fibers such as those available from
Dow
Chemical Co. under the trade names POLYIMIDE 2080 and PBZ fiber,
polyphenylene
sulfide, polyether ether ketone, polyimide, polybenzoxazole, aromatic
polyimides or
- 19 -
DOCSMTL. 4070637\1
CA 02718659 2010-10-25
polyetherimides, and the like. Combinations of any of the foregoing fibers may
also be
used. Exemplary fibers are phenolic resin fibers.
[0078] In an exemplary embodiment, phenolic resin fiber or glass
fibers can be
used as the fibrous filler in the composite substrate. Useful glass fibers can
be formed
from any type of fiberizable glass composition and include those prepared from
fiberizable
glass compositions commonly known as "E-glass," "A-glass," "C-glass," "D-
glass," "R-
glass," "S-glass," as well as E-glass derivatives that are fluorine-free
and/or boron-free.
AR-glass can be used for its alkali resistance. Commercially produced glass
fibers
generally having nominal filament diameters of about 4.0 to about 35.0
micrometers, and
most commonly produced E-glass fibers having nominal filament diameters of
about 9.0
to about 30.0 micrometers may be included in the composite substrate. Use of
non-round
fiber cross sections are also possible. The glass fibers may be sized or
unsized. Sized
glass fibers can be coated on at least a portion of their surfaces with a
sizing composition
selected for compatibility with the coating that is disposed upon the
substrate. The sizing
composition facilitates wet-out and wet-through of the coating upon the fiber
strands and
assists in attaining desired physical properties in the composite.
[0079] The glass fibers are preferably glass strands that have been
sized. In
preparing the glass fibers, a number of filaments can be formed
simultaneously, sized with
a silane coating agent and then bundled into what is called a strand.
Alternatively the
strand itself may be first formed of filaments and then sized. The amount of
sizing
employed is generally that amount which is sufficient to bind the glass
filaments into a
continuous strand and ranges from about 0.1 to about 5 wt%, and more typically
ranges
from about 0.1 to 2 wt% based on the weight of the glass fibers. Generally,
this may be
about 1.0 wt% based on the weight of the glass filament. Glass fibers in the
form of
chopped strands about one-fourth inch long or less and preferably about one-
eighth inch
long may also be used. They may also be longer than about one-fourth inch in
length if
desired.
[0080] Fibers used in the composite substrate can have lengths of
about 6 to about
3200 gm. In one embodiment, fiber lengths are about 10 to about 1600 gm. In
another
- 20 -
DOCSMTL: 4070637\1
CA 02718659 2015-03-10
embodiment, fiber lengths are about 10 to about 800 [tm. Exemplary fibers are
shorter
than the greatest diameter of the composite substrate.
[0081] Fiber diameters (or, for fibers of non-circular cross-section,
a hypothetical
dimension equal to the diameter of a hypothetical circle having an area equal
to the cross-
sectional area of the fiber) are about 1 to about 20 i_tm. Aspect ratio
(length to diameter
ratio) can be in amounts of about 5 to about 175. The fiber may have a round,
oval,
square, rectangular or other appropriate cross-section. The fibers may be
straight,
crimped, curled or a combination thereof.
[0082] An exemplary filler used in the organic coating is silica
flour. The silica
flour generally has particle sizes of less than or equal to about 20
micrometers. In one
embodiment, the silica flour has particle sizes of less than or equal to about
10
micrometers. In another embodiment, the silica flour has particle sizes of
less than or
equal to about 5 micrometers. An example of commercially available silica
flour is
SIKRON SF 242 commercially available from Quarzwerke GmbH, Frechen, Germany.
C. Hybrid Particle Substrate
[0083] As shown in FIG. 4, another type of substrate is a hybrid
particle substrate
42 having an inorganic particle 44 as a core and a cured or at least partially
cured coating
(composite layer) 46 which comprises inorganic fillers or organic fillers 48.
The organic
coating 46 can be applied in a single layer or in multiple layers if desired.
[0084] The fillers 48 in the composite layer 46 of the hybrid particle
substrate 42
may be the same as described above for the composite particle substrate.
[0085] Various embodiments of these hybrid particles are further
described in U.S.
Application Serial No 11/230,693 filed September 20, 2005 (US Patent
Application
Publication No. 2006/0078682 Al to McDaniel et al.).
[0086] In the exemplary embodiment of FIG. 4 when the substrate of the
coated
particle comprises a single particle, an exemplary synthetically produced,
inorganic
substrate comprises one or more of silica (Si02), alumina (A1203), titanium
dioxide (h02),
-21 -
CA 02718659 2010-10-25
ferric oxide (Fe203), calcium oxide (CaO), magnesium oxide (MgO), potassium
dioxide
(K20) and sodium oxide (Na20). The inorganic substrate can also comprise
sulfite ions,
chloride ions, water, and carbon dioxide in trace amounts of less than or
equal to about 2
wt%, based on the weight of the substrate.
[0087] Synthetically produced organic substrates can comprise thermoplastic
polymers, thermosetting polymers, or a combination comprising a thermosetting
polymer
and a thermoplastic polymer. Examples of suitable organic materials that can
be used as
the substrate are polymer precursors (e.g., low molecular weight species such
as
monomers, dimers, trimers, or the like), oligomers, polymers, copolymers such
as block
copolymers, star block copolymers, terpolymers, random copolymers, alternating
copolymers, graft copolymers, or the like; dendrimers, ionomers, or the like,
or a
combination comprising at least one of the foregoing. When the substrate
comprises a
thermosetting polymer, it is desirable for the organic materials to undergo
curing
(crosslinking) upon the application of either thermal energy, electromagnetic
radiation, or
a combination comprising at least one of the foregoing. Initiators may be used
to induce
the curing. Other additives that promote or control curing such as
accelerators, inhibitors,
or the like, can also be used.
[0088] Examples of suitable thermosetting polymers for use in the
cured (non-
reactive) coating layer 46 of the substrate are epoxies, acrylate resins,
methacrylate resins,
phenol-formaldehydes, epoxy-modified novolacs, furans, urea-aldehydes,
melamine-
aldehydes, polyester resins, alkyd resins, phenol formaldehyde novolacs,
phenol
formaldehyde resoles, phenol-aldehydes, resole and novolac resins, epoxy
modified
phenolics, polyacetals, polysiloxanes, polyurethanes, or the like, or a
combination
comprising at least one of the foregoing thermosetting polymers.
D. Curable Outer Layer Resins and Powders
[0089] The outer organic coating comprises a curable liquid resin
polymer as a
continuous phase and a reactive and/or non-reactive powder. By outer organic
coating is
meant the outermost continuous phase resin coating of the particle and any
powder
embedded or protruding from the continuous phase.
- 22 -
DOCSMTL. 4070637\1
CA 02718659 2010-10-25
100901 The outer coating curable liquid resins of the present
invention are low
viscosity liquid resins which are able to be coated onto a substrate at low
temperatures,
from about 50 F (10 C) to about 150 F (66 C), preferably about 70 F (21 C) to
about
120 F (49 C), as described elsewhere in this specification and exhibit latency
or cure to
deliver full performance when applied in the subterranean formation. However,
they are
not cold set resins which can react at a temperature of 65 C or less without
the use of
additional heat. The latency associated with the use of these resole coating
resins is a
feature which distinguishes these resole resins from cold set resins such as,
for example,
the cold set resins, e.g., alkaline modified resoles, described in US patent
application
publication no. 2006/0078682 Al to McDaniel et al., paragraph 0043. Alkaline
modified
resole is made by adding potassium hydroxide or sodium hydroxide to resole
resin such
that a sufficient portion of the resin is converted to an alkaline salt of the
resin which is
capable of being cured by treating with esters without elevated temperature.
The outer
coating resoles employed in the present invention do not contain these
alkaline salts. The
outer coating resole resins of the present invention are latent but heat
curable such that
they can be cured by the elevated (above 70 C, typically above 80 C)
temperatures found
in a subterranean formation.
[0091] The reactive powders comprise one or more of the following:
novolak (with
or without hexamethylenetetramine), resoles, hydroxy functional polyesters
(reactive with
resoles), hydroxy functional polyacrylates (reactive with resoles), and
functionalized
polyurethanes that will be reactive with resoles, such as those having an
amine
functionality or a hydroxy functionality. The reactive powders may also
comprise epoxy
resins. Typically, the reactive powder has an average particle size of about
200 mesh (74
microns) or smaller.
100921 The non-reactive powders may be any inorganic or organic powders
which
are not chemically reactive with the continuous curable liquid resin coating.
Examples of
inorganic non-reactive powders include silica flour or ground glass or
minerals. Examples
of non-reactive organic powders include crushed nut shells or other natural
organic
materials such as, for example, wood flour obtained by pulverizing wood, and
fibrous
products such as cellulose, cotton, sisal, jute, cloth, hemp cloth, felt, and
natural cellulosic
fabrics such as Kraft paper, cotton paper and glass fiber containing paper,
starch, cork
- 23 -
DOCSNITI, 4070637\1
CA 02718659 2010-10-25
flour, lignin, ground nut shells, corn, rice grain husks, or the like, or a
combination
comprising at least one of the foregoing. Typically the non-reactive powder
has an
average particle size of about 200 mesh (74 microns) or smaller.
1. Resole Resins
[0093] An embodiment of the present invention employs a coating which
includes
phenol-aldehyde resole polymer provided as a solution or a dispersion. Resole
resin may
also be used as powder for embedding or adhering to the resole-containing
coating.
Typically the resole powder has an average particle size of about 200 mesh or
smaller and
is recovered via spray dried techniques to retain the reactivity of the
resole.
[0094] Resoles include phenol formaldehyde resoles, phenol formaldehyde and
furfuryl alcohol or furfuryl aldehyde resoles, or phenol formaldehyde resole
resins
substituted with alkylphenols or cashew nut oil. Solvent borne and aqueous
resoles are
included.
[0095] The resole resin used for the coating is liquid when applied
to the substrate
and thus has a molecular weight suitable to be a liquid. A typical molecular
weight
average for liquid resole ranges from about 400 to about 2000. The coating
resin is
curable. The coating resole resins are supplied as a wet aqueous solution and
are dried by
the present process to be in an uncured (non-cross-linked) state. A preferred
example of a
resole is manufactured by Hexion Specialty Chemicals, Inc. and is designated
as OWR-
262E.
[0096] The resole resin used for the powder is solid when applied to
the coated
substrate and thus has a molecular weight suitable to be a solid. A typical
molecular
weight average for solid resole ranges from about 500 to about 5000. The
powder resole
is curable. The resole powder may be applied in an uncured state. Preferably
the resole
powder to be reactive towards itself and the liquid resole adhesive (uncured
when
applied).
[0097] Preferably the resole resins are low free phenol resole resins
having less
than 3 wt. %, more preferably less than 2 wt. % free phenol.
- 24 -
DOCSMTL 4070637\1
CA 02718659 2015-03-10
[0098] The phenol-aldehyde resole resin has a phenol:aldehyde molar
ratio from
about 1:1 to about 1:3, typically from about 1:1 to about 1:1.95. A preferred
mode of
preparing the resole resin is to combine phenol with a source of aldehyde such
as
formaldehyde, acetaldehyde, propionaldehyde, furfural, benzaldehyde or
paraformaldehyde under alkaline catalysis. During such reaction, the aldehyde
is present
in molar excess. It is preferred that the resole resin have a molar ratio of
phenol to
formaldehyde from about 1:1.1 to 1:1.6. A typical way to make resoles is to
put a phenol
in a reactor, add an alkaline catalyst, such as sodium hydroxide or calcium
hydroxide, and
aldehyde, such as a 50 weight % solution of formaldehyde, and react the
ingredients under
elevated temperature until the desired viscosity or free formaldehyde is
achieved. Water
content is adjusted by distillation. Elasticizers or plastizers, such as
bisphenol A or
cashew nut oil, may also be present to enhance the binder elasticity or
plasticity. Other
known additives may also be present.
[0099] The resoles may be conventional resoles or modified resoles.
Modified
resoles are disclosed by U.S. Patent No. 5,218,038. Such modified resoles are
prepared by
reacting aldehyde with a blend of unsubstituted phenol and at least one
phenolic material
selected from the group consisting of arylphenol, alkylphenol, alkoxyphenol,
and
aryloxyphenol. Modified resole resins include alkoxy modified resole resins.
Of alkoxy
modified resole resins, methoxy modified resole resins are preferred. However,
the
phenolic resole resin which is most preferred is the modified orthobenzylic
ether-
containing resole resin prepared by the reaction of a phenol and an aldehyde
in the
presence of an aliphatic hydroxy compound containing two or more hydroxy
groups per
molecule. In one preferred modification of the process, the reaction is also
carried out in
the presence of a monohydric alcohol.
101001 Phenols and aldehydes suitable for preparing the modified
orthobenzylic
ether-containing phenolic resole resins are generally any of the phenols and
aldehydes
which may be utilized in the formation of phenolic resins. Metal ion catalysts
useful in
production of the modified phenolic resins include salts of the divalent ions
of Mn, Zn,
Cd, Mg, Co, Ni, Fe, Pb, Ca and Ba. Tetra alkoxy titanium compounds of the
formula
Ti(OR)4 where R is an alkyl group containing from 3 to 8 carbon atoms, are
also useful
catalysts for this reaction. A preferred catalyst is zinc acetate.
- 25 -
CA 02718659 2010-10-25
101011 A molar excess of aldehyde per mole of phenol is used to make
the
modified resole resins. Preferably the molar ratio of phenol to aldehyde is in
the range of
from about 1:1.1 to about 1:2.2. The phenol and aldehyde are reacted in the
presence of
the divalent metal ion catalyst at pH below about 7. To the reaction mixture
is added an
aliphatic hydroxy compound which contains two or more hydroxy groups per
molecule.
The hydroxy compound is added at a molar ratio of hydroxy compound to phenol
of from
about 0.001:1 to about 0.03:1.
[0102] Useful hydroxy compounds which contain two or more hydroxy
groups per
molecule are those having a hydroxyl number of from about 200 to about 1850.
The
hydroxyl number is determined by the standard acetic anhydride method and is
expressed
in terms of mg KOH/g of hydroxy compound. Suitable hydroxy compounds include
ethylene glycol, propylene glycol, 1,3-propanediol, diethylene glycol,
triethylene glycol,
glycerol, sorbitol and polyether polyols having hydroxyl numbers greater than
about 200.
[0103] After the aliphatic hydroxy compound containing two or more
hydroxy
groups per molecule is added to the reaction mixture, heating is continued
until from about
80% to about 98% of the aldehyde has reacted. The modified phenolic resole may
be
"capped" to be an alkoxy modified phenolic resole resin. In capping, a hydroxy
group is
converted to an alkoxy group by conventional methods that would be apparent to
one
skilled in the art given the teachings of the present disclosure.
[0104] Resoles also include a terpolymer of phenol, furfuryl alcohol (or
furfuryl
aldehyde) and formaldehyde.
[0105] A phenol-formaldehyde-furfuryl alcohol terpolymer is prepared
from the
catalytic reaction of phenol, formaldehyde and furfuryl alcohol, wherein the
catalyst is a
water soluble multivalent metal salt, and wherein the reaction is carried out
under
essentially hydrous conditions. The common water soluble salts of multivalent
metal ions
which can be used as the catalyst in the present invention are less costly
than the organic
solvent soluble salts at equal equivalents of metal ion that are used in the
process disclosed
in U.S. Pat. No. 4,255,554 to Wuskell. The use of a water soluble multivalent
metal salt
eliminates the necessity for controlling the reaction pH in the manner
necessary with an
acid catalyst. However, the multivalent metal salt catalyzed reaction must be
operated at a
-26 -
DOCSMTL: 4070637 \ 1
CA 02718659 2010-10-25
pH of less than 7Ø When uncontaminated phenol, formalin, furfuryl alcohol
and zinc or
lead acetate are mixed in the proper proportions, the pH is always less than
7Ø
[0106] The water soluble multivalent metal salts used as the
catalysts to make this
terpolymer include the multivalent ions of manganese, zinc, cadmium,
magnesium, cobalt,
nickel, tin, copper, iron, lead, and calcium. Preferred catalysts are zinc
acetate or lead
acetate, and mixtures thereof.
[0107] The terpolymer reaction can be carried out by initially
reacting furfuryl
alcohol and formaldehyde at temperatures of about 85 to 105 C, at atmospheric
pressure,
then adding phenol and continuing the reaction to a viscosity of about 100 to
10,000,
preferably about 200 to 5,000 centipoises, measured at a temperature of about
25 C.
However, the reaction can be conducted at elevated temperatures of up to about
140 C in
pressurized reaction vessels, taking care to ensure that the reaction mixture
does not boil
under these elevated conditions. The reaction can also be carried out by
initially reacting
phenol and formaldehyde, then adding the furfuryl alcohol and continuing the
reaction to a
viscosity of about 100 to 10,000 cps, preferably about 200 to 5,000 cps,
measured at about
C. Alternatively, the reaction can be carried out by reacting phenol, furfuryl
alcohol
and formaldehyde simultaneously in the presence of the water soluble
multivalent metal
salt catalysts. The resulting phenol-formaldehyde-furfuryl alcohol terpolymer
can be used
as is or diluted with any suitable solvent, including furfuryl alcohol or
water.
20 [0108] In general, the mole ratio of phenol to furfuryl alcohol
can vary from about
0.1:1 to about 10:1, respectively. The mole ratio of formaldehyde to
phenol+furfuryl
alcohol can vary from about 0.5:1 to 2:1, respectively in moles of CH2 0 :
phenol +
furfuryl alcohol. The amount of catalyst can vary from about 0.2% to about 8%
by weight
of the total amount of phenol and furfuryl alcohol.
25 [0109] Although the reaction has been described in terms of
formaldehyde, other
aldehydes of the general formula: R-CHO can also be used, wherein R is a
hydrocarbon
radical containing about 1-8 carbon atoms such as acetaldehyde,
propionaldehyde,
furfuraldehyde, paraformaldehyde, the solid low molecular weight polymer of
- 27 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
formaldehyde, and the like. The preferred form of formaldehyde is in the
hydrous state,
such as formalin.
[0110] Furfuryl alcohol or substituted furfuryl alcohol compounds can
be used
with the formula I:
C C
R3
C - C - CH2 OH
where R3 can be an alkyl, aryl, alkenyl, alkylol, alkoxy, aryloxy, halogen,
hydrogen or
hydroxy radical. The preferred compound is furfuryl alcohol.
[0111] In addition, although phenol is the preferred phenolic reactant,
other
substituted phenols can also be used, especially those phenols having the
formula II:
RO R5
HO _____________________________ R6
[0112] wherein R4, R5 and R6 can independently be hydrogen,
hydrocarbon
radicals, oxyhydrocarbon radicals, hydroxy radicals or halogen, and
substituted such that
either the two ortho, one ortho and the para, or the two ortho and the para
positions are
unsubstituted. In general, the phenols that can be used are those which are
suitable for
making phenolic resins. Some examples are o-cresol, m-cresol, p-cresol, octyl
phenol,
nonyl phenol, 3,5-dimethoxy phenol, p-tert-butylphenol, p-butoxyphenol,
resorcinol, 3,5-
xylenol, 3-5-diethylphenol, catechol, 3,5-dibutylphenol and the like.
[0113] After being applied as coatings, these terpolymers may be
cured with
curatives such as acid catalyst such as ammonium chloride or ammonium sulfate.
- 28 -
DOCSMTL- 4070637\1
CA 02718659 2015-07-06
Terpolymers are also disclosed by US Patent No. 7,153,575 granted December 26,
2006.
[0114] If desired the resole or resole powder may contain a curative,
for example
hexamethylenetetramine.
2. Polyester and Unsaturated Polyester Resins
[0115] Another embodiment of this invention employs a coating which
includes a
liquid polyester or an unsaturated polyester resin as the continuous phase.
Polyester or an
unsaturated polyester resin may also be used as a reactive powder for
embedding or
adhering to the continuous phase.
[0116] The term "polyester", as used herein, encompasses both
"homopolyesters"
and "copolyesters" and means a synthetic polymer prepared by the
polycondensation of
difunctional carboxylic acid with at least one difunctional hydroxyl compound,
e.g., a diol
or glycol component. Typical polyesters are those containing unsaturated
(vinyl)
endgroups which cure through the use of peroxide catalysts. These polyesters
may be
blended with other monomers to incorporate a desired property. Polymerization
catalysts
such as benzoyl peroxide may also use metal catalysts to accelerate cure, such
as cobalt
salts.
[0117] In one embodiment, the polyester resins useful as a reactive
powder include
hydroxy functional polyacrylates reactive with resoles. In another embodiment,
the
reactive powder includes polyhydroxy polyesters. Polyhydroxy polyester
polymers
(functionality of 2 or more) result from the reaction of polycarboxylic acids
or anhydrides
(typically isophthalic acid, phthalic acid or anhydride, maleic acid or
anhydride, fumaric
acid, sebacic acid, azelaic acid, adipic acid, trimellitic acid or anhydride,
etc.) with
polyhydroxy substances such as ethylene glycol, propylene glycol, neopentyl
glycol,
butylene glycol, 1,4-butanediol, hexylene glycol, 1,6-hexanediol, the
polyglycols such as
diethylene glycol or triethylene glycol, etc., the triols such as glycerine,
trimethylol ethane,
trimethylol propane, etc. and other higher functional alcohols such as
pentaerythritol,
sorbitol, mannitol, and the like. Polyhydroxy polyesters are further described
in US Patent
No. 4,920,199. Optimization of the melt/flow properties
- 29 -
CA 02718659 2010-10-25
of the resin and powder combination is beneficial for storage and performance
of the
coated proppant.
3. Acrylic Polymers
[0118] Another embodiment of the present invention utilizes liquid
acrylic
polymers as the continuous phase. Acrylic polymer resins may also be used as a
reactive
powder for embedding or adhering to the continuous phase.
[0119] Acrylate polymers (solutions and dispersions thereof) for use
as a curable
liquid resin in the continuous phase are polymers commonly called acrylics,
polyacrylates,
or acrylate polymers. Some acrylate monomers (the components of the polymers)
used to
form acrylate polymers may be acrylic acid, butyl acrylate, 2-ethylhexyl
acrylate, methyl
acrylate, ethyl acrylate, acrylonitrile, n-butanol, methyl methacrylate, 2-
hydroxyethyl
acrylate, 2-hydroxypropyl acrylate and TMPTA. The acrylate ion (CH2=CHC00-) is
the
ion of acrylic acid. Acrylates are the salts and esters of acrylic acid. They
are also known
as propenoates (since acrylic acid is also known as 2-propenoic acid).
Acrylates contain
vinyl groups, that is, two carbon atoms double bonded to each other, directly
attached to
the carbonyl carbon. Acrylates and methacrylates (the salts and esters of
methacrylic acid)
are common monomers in acrylate polymers. Others monomers common to acrylate
polymers (styrene) may also be incorporated.
[0120] In one embodiment, the acrylates useful as a reactive powder
include
hydroxy functional polyacrylates or amine functional polyacrylates, reactive
with resoles.
Optimization of the melt/flow properties of the resin and powder combination
is beneficial
for storage and performance of the coated proppant.
4. Urethane Resins
[0121] Another embodiment of this invention employs a coating which
includes a
liquid urethane resin as the continuous phase. Urethane resins may also be
used as a
reactive powder for embedding or adhering to the continuous phase.
[0122] Polyurethane resins are made by mixing a polyisocyanate
component, a
polyhydroxy component or a polyamine component and a catalyst. Typically the
- 30 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
polyhydroxy component is a polyhydroxy phenolic component dissolved in
solvent. The
polyamine component can be multifunctional and selected to produce still
reactive,
oligomerized liquid polyurethanes (solutions and dispersions thereof).
Generally the
solvents are mixtures of hydrocarbon and polar organic solvents such as
organic esters.
[0123] The polyhydroxy component is generally a phenolic resole resin or
alkoxy
modified resole resin as described above.
[0124] The isocyanate component may vary widely and has a
functionality of 2 or
more. As defined herein, polyisocyanates include isocyanates having such
functionality of
2 or more, e.g., diisocyanates, triisocyanates, etc. Exemplary useful
isocyanates are
organic polyisocyanates such as tolylene-2,4-diisocyanate, tolylene-2,6-
diisocyanate, and
mixtures thereof, particularly crude mixtures thereof that are commercially
available.
Other typical polyisocyanates include methylene-bis-(4-phenyl isocyanate), n-
hexyl
diisocyanate, naphthalene-1,5-diisocyanate, cyclopentylene-1,3-diisocyanate, p-
phenylene
diisocyanate, tolylene-2,4,6-triisocyanate, and triphenylmethane-4,4',4"-
triisocyanate.
Higher isocyanates are provided by the liquid reaction products of (1)
diisocyanates and
(2) polyols or polyamines and the like. In addition, isothiocyanates and
mixtures of
isocyanates can be employed. Also contemplated are the many impure or crude
polyisocyanates that are commercially available. Especially preferred for use
in the
invention are the polyaryl polyisocyanates having the following general
Formula VI:
NCO NCO NCO
411 CX2 CX2
VI
wherein R is selected from the group consisting of hydrogen, chlorine,
bromine, and alkyl
groups having 1 to 5 carbon atoms; X is selected from the group consisting of
hydrogen,
alkyl groups having 1 to 10 carbon atoms and phenyl; and n has an average
value of
generally about 0 to about 3. The preferred polyisocyanate may vary with the
particular
-31 -
DOCSMTL: 4070637\1
CA 02718659 2010-10-25
system in which the powder is employed. Urethanes are disclosed, for example,
by US
Patent No. 5,733,952 to Geoffrey.
[0125] Exemplary hydrocarbon solvents include aromatic hydrocarbons
such as
benzene, toluene, xylene, ethyl benzene, high boiling aromatic hydrocarbon
mixtures,
heavy naphthas and the like. Optimization of the melt/flow properties of the
resin and
powder combination is beneficial for storage and performance of the coated
proppant.
5. Epoxy Resins
[0126] Another embodiment of this invention employs a coating which
includes a
liquid epoxy resins (solutions and dispersions thereof) as the continuous
phase. Epoxy
resins may also be used as a reactive powder for embedding or adhering to the
continuous
phase.
[0127] Epoxy resins are commercially available and prepared from
either glycidyl
materials such as the ethers, produced by the reaction of chlorohydrin with a
phenol or
alcohol, or epoxies, such as the product from the reaction of peracetic acid
with a linear or
cycloaliphatic olefin. The epoxy resin molecule is characterized by the
reactive epoxy or
ethoxline groups of Formula I:
/0 \
C _________________________ C-
which serve as terminal linear polymerization points. Crosslinking or curing
is
accomplished through these groups or through hydroxyls or other groups
present. The
well-known epoxy resins are usually prepared by the base-catalyzed reaction
between an
epoxide, such as epichlorohydrin and a polyhydroxy compound, such as bisphenol
A.
Suitable cationic curable epoxides include monocycloaliphatic epoxides and
biscycloaliphatic epoxides.
[0128] In one embodiment, the epoxy resins can be selected from
glycidyl ethers
made from bisphenol A and epichlorohydrin. In another embodiment, the liquid
epoxy
- 32 -
DOCSMTL: 4070637\1
CA 02718659 2015-07-06
resin has a typical viscosity of about 200 to about 20,000 centipoises, and an
epoxide
equivalent weight of about 170 to about 500 and weight average molecular
weight of
about 350 to about 4000. Liquid epoxy resin products include, for example EPON
815
epoxy resin, commercially available, from Hexion Specialty Chemicals Inc.
[0129] In one embodiment, epoxy resins may be converted to thermoset solids
by a
variety of crosslinking mechanisms. The curatives, or curing agents, used to
complete the
conversion can be truly catalytic or multifunctional crosslinking agents that
become
chemically bound in the final three-dimensional structure. Curing agents
include, but are
not limited to, 1) alkaline curing agents, such as Lewis bases, primary and
secondary
aliphatic amines, amine adducts, cyclic amines, aromatic amines, polyamides,
and other
amines, such as dicydiandiamide and imidazoles; 2) acid curing agents, such as
Lewis
acids, phenols, organic acids, cyclic anhydrides, polysulfides and mercaptans.
The
preferred curing agent for use in this technology is Sigma Aldrich's
tetrafiuoroboric acid
(48% solution in water).
TM
[0130] Powder epoxy resins include, for example, EPON 2008, commercially
available Hexion Specialty Chemicals Inc. Optimization of the melt/flow
properties of the
resin and powder is beneficial for storage and performance of the coated
proppant.
6. Melamine Resins
[0131] Another embodiment of this invention utilizes a liquid
melamine resins as
the continuous phase. Melamine resins, with or without free methylol groups,
are capable
of curing, and may be accelerated by heat and or acids. Melamine resins
without free
methylol have --OR groups rather than --OH groups. Thus, for example, the
unreacted,
uncured, A-stage melamine resin can be coated onto substrates, providing the
adhesive
and reactive continuous phase to produce a tacky or high solids surface on the
substrate.
The continuous, reactive liquid phase can then function to adhere reactive dry
powders to
the surface, and the combination will then be heat cured when placed into the
downhole
fracture where the components react and result in amino methyl linkages. Astro
Mel 601
(TM-Hexion Specialty Chemicals) is a material of choice for this type of
application
because of the high solids composition with low viscosity, water insolubility,
and stability.
-33 -
CA 02718659 2010-10-25
[0132] An example of another melamine resin is Astro Mel NW-3A (TM-
Hexion
Specialty Chemicals), comprising a liquid, partially methylated melamine
resin. It is
particularly useful to crosslink hydroxyl-functional polymers (reactive
powders) and
capable of self condensing to provide excellent coating properties. The
balance of ¨OH
groups and ¨OR groups on this melamine resin can be adjusted to provide the
optimum
coating performance, depending on the total requirements of the liquid
adhesive resin and
powder resin combinations which produce the final coating on the substrate.
7. Anhydride Resins
[0133] Another embodiment of this invention utilizes a liquid
anhydride resin as
the continuous phase. Styrene maleic anhydride, also known as SMA or SMAnh, is
an
example of an anhydride resin polymer, and is composed of styrene and maleic
anhydride
monomers. The monomers are built in randomly making it a random copolymer,
formed
by a radical polymerization using an organic peroxide as the initiator. The
main
characteristics of SMA copolymer are its transparent appearance, high heat
resistance,
high dimensional stability, and the specific reactivity of the anhydride
groups. The latter
feature results in the solubility of SMA in alkaline (water-based) solutions
and dispersions.
SMA is available in a broad range of molecular weights and maleic anhydride
(MA)
contents. Low molecular weight oligomers are available (and converted to
solutions and
dispersions) that will function as the reactive adhesive layer on the
substrates to provide a
reactive coating that adheres the powder selected for the application. The
unique
chemistry of SMA allows a wide range of choices for the selection of the
reactive powder
to be used for the coating of the substrates. The solubility of SMA in
alkaline solutions
makes it suitable for various applications in the field of sizings (paper),
binders and
coatings. The specific reactivity of SMA makes it a suitable agent for
compatibilizing
normally incompatible polymers.
8. Novolak Polymer-Containing Resins
[0134] An embodiment of the present invention employs powder which
includes
phenol-aldehyde novolak polymer.
- 34 -
DOCSMTL. 4070637d
CA 02718659 2015-03-10
[0135] The novolak may be any novolak employed with proppants. The
novolak
may be obtained by the reaction of a phenolic compound and an aldehyde in a
strongly
acidic pH region. Suitable acid catalysts include the strong mineral acids
such as sulfuric
acid, phosphoric acid and hydrochloric acid as well as organic acid catalysts
such as oxalic
acid, or para-toluenesulfonic acid. An alternative way to make novolaks is to
react a
phenol and an aldehyde in the presence of divalent inorganic salts such as
zinc acetate,
zinc borate, manganese salts, cobalt salts, etc. The selection of catalyst may
be important
for directing the production of novolaks which have various ratios of ortho or
para
substitution by aldehyde on the phenolic ring, e.g., zinc acetate favors ortho
substitution.
Novolaks enriched in ortho substitution, i.e., high-ortho novolaks, may be
preferred
because of greater reactivity in further cross-linking for polymer
development. High ortho
novolaks are discussed by Knop and Pilato, Phenolic Resins, p. 50-51 (1985)
(Springer-
Verlag). High-ortho novolaks are defined as novolaks wherein at least 60% of
the total of
the resin ortho substitution and para substitution is ortho substitution,
preferably at least
about 70% of this total substitution is ortho substitution.
[0136] The novolak polymer typically comprises phenol and aldehyde in
a molar
ratio from about 1:0.85 to about 1:0.4. Any suitable aldehyde may be used for
this
purpose. The aldehyde may be formalin, paraformaldehyde, formaldehyde,
acetaldehyde,
furfUral, benzaldehyde or other aldehyde sources. Formaldehyde itself is
preferred.
[0137] Preferably the novolak resins are low free phenol novolak resins
having
less than 1 wt. %, more preferably less than 0.6 wt. % free phenol.
[0138] The molecular weight of the novolak will vary from about 500
to 10,000,
preferably 1,000 to 5,000 depending on their intended use. The molecular
weight of the
novolaks or other polymers in this description of the present invention are on
a weight
average molecular weight basis unless otherwise indicated. High-ortho novolak
resins are
especially preferred.
[0139] The novolak resin compositions typically comprise at least 10
weight
percent novolak polymer, preferably at least about 20 weight percent novolak
polymer,
most preferably about 50 to about 70 weight percent novolak polymer. The
remainder of
- 35 -
CA 02718659 2010-10-25
the resin composition could include crosslinking agents, modifiers or other
appropriate
ingredients. The phenolic moiety of the novolak polymer is selected from
phenols of
Formula III or bisphenols of Formula IV, respectively:
R1
= III, and
HO
IV.
R1
x
HO OH
101401 R and R1 are independently alkyl, aryl, arylalkyl or H. In
Formula III, R
and RI are preferably meta to the respective hydroxy group on the respective
aromatic
ring. Unless otherwise defined, alkyl is defined as having 1 to 6 carbon
atoms, and aryl is
defined as having 6 carbon atoms in its ring. In Formula IV, X is a direct
bond, sulfonyl,
alkylidene unsubstituted or substituted with halogen, cycloalkylidene, or
halogenated
cycloalkylidene. Alkylidene is a divalent organic radical of Formula V:
-C-
R3 V.
[0141] When X is alkylidene, R' and R3 are selected independently
from H, alkyl,
aryl, arylalkyl, halogenated alkyl, halogenated aryl and halogenated
arylalkyl. When X is
halogenated alkylidene, one or more of the hydrogen atoms of the alkylidene
moiety of
Formula V are replaced by a halogen atom. Preferably the halogen is fluorine
or chlorine.
- 36 -
DOCSMTL: 4070637\1
CA 02718659 2015-07-06
Also, halogenated cycloalkylidene is preferably substituted by fluorine or
chlorine on the
cycloalkylidene moiety.
[0142] A typical phenol of Formula III is phenol, per se. Typical
bisphenols of
Formula IV include Bisphenol A, Bisphenol C, Bisphenol E, Bisphenol F,
Bisphenol S, or
Bisphenol Z.
[0143] The novolak polymers may contain any one of the phenols of
Formula III,
bisphenols of Formula IV, or combinations of one or more of the phenols of
Formula III
and/or one or more of the bisphenols of Formula IV.
[0144] For practical purposes, phenolic novolaks do not harden upon
heating, but
remain soluble and fusible unless a hardener (curing agent) is present. Thus,
in curing a
novolak resin, a curing agent is used to overcome the deficiency of alkylene-
bridging
groups to convert the resin to an insoluble infusible condition. Appropriate
crosslinking
agents include hexamethylenetetramine (HEXA), paraformaldehyde, oxazolidines,
melamine resin or other aldehyde donors and/or the above-described resole
polymers.
Each of these crosslinkers can be used by itself or in combinations with other
crosslinkers.
The resole polymer may contain substituted or unsubstituted phenol. An example
of a
TM
preferred novolak powder is DURITE SD-536C, commercially available from Hexion
Specialty Chemicals, Inc. This powdered novolak contains 10 weight percent
HEXA as
its sole curing agent.
[0145] A novolak resin powder composition of this invention typically
comprises
up to about 25 weight percent HEXA and/or up to about 90 weight percent resole
polymers based on the total weight of coating composition. Where HEXA is the
sole
crosslinking agent, the HEXA comprises from about 5 to about 25 weight percent
of the
resin. Where the phenol-aldehyde resole polymer is the sole crosslinking
agent, the resin
contains from about 20 to about 90 weight percent of the resole polymer. The
composition may also comprise combinations of these crosslinkers. To make
phenolic
novolak polymers with one or more phenols of Formula III, the phenol is mixed
with
acidic catalyst and heated. Then an aldehyde, such as a 50 weight % solution
of
formaldehyde is added to the hot phenol and catalyst at elevated temperature.
Water made
- 37 -
CA 02718659 2010-10-25
by the reaction is removed by distillation to result in molten novolak. The
molten novolak
is then cooled and flaked and ground to a powder.
[0146] To make novolak polymers with bisphenols of Formula IV, the
bisphenol is
mixed with a solvent, such as n-butyl acetate, at elevated temperature. An
acid catalyst
such as oxalic acid or methane sulfonic acid is then added and mixed with the
bisphenol
and then an aldehyde, typically formaldehyde, is added. The reactants are then
refluxed.
It is noted that the preparation of the novolak resin can occur under acidic
catalysis, or
divalent metal catalysis (e.g., Zn, Mn), wherein the bisphenol is present in
greater than
equimolar amount relative to the source of aldehyde. After reflux, water is
collected by
azeotropic distillation with n-butyl acetate. After removal of the water and n-
butyl acetate,
the resin is flaked to yield resin products. Alternatively, the polymers can
be made using
water as a solvent.
[0147] The novolak polymer may optionally be further modified by the
addition of
VINSOL , epoxy resins, bisphenol, waxes, or other known resin additives. One
mode of
preparing an alkylphenol-modified phenol novolak polymer is to combine an
alkylphenol
and phenol at a molar ratio above 0.05:1. This combination is reacted with a
source of
formaldehyde under acidic catalysis, or divalent metal catalysis (e.g., Zn,
Mn). During
this reaction, the combination of alkylphenol and phenol is present in molar
excess relative
to the formaldehyde present.
[0148] If desired, phenol-aldehyde novolaks or bisphenol-aldehyde novolaks
may
be modified by reacting these novolaks with an additional quantity of aldehyde
using a
basic catalyst. Typical catalysts used are sodium hydroxide, potassium
hydroxide, barium
hydroxide, calcium hydroxide (or lime), ammonium hydroxide and amines. In the
case of
phenol-aldehyde polymers or bisphenol-aldehyde polymers, the molar ratio of
added
aldehyde to phenolic moiety, based on the phenolic moiety monomeric units in
the
novolak, ranges from 0.4:1 to 3:1, preferably from 0.8:1 to 2:1. This achieves
a
crosslinkable (reactive) polymer having different chemical structures and
generally higher
molecular weights than the resole polymers obtained by a single step process
which
involves initially mixing bisphenol monomers and aldehyde with an alkaline
catalyst at the
same molar ratio of the combined aldehyde and bisphenol. Furthermore, it is
feasible to
- 38 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
use different aldehydes at different stages of the polymer preparation. These
polymers can
be used alone or with other polymers, such as phenol-aldehyde novolaks,
bisphenol-
aldehyde novolak, or combinations thereof, as a crosslinking agent, or as a
component of
crosslinking agents. When the aldehyde-modified polymers are employed as
crosslinking
agents, they may be used with other typical crosslinking agents such as those
described
above for novolak polymers.
[0149] In one embodiment, the novolac resins may be those having
melting points
above 80 C, and preferably in the range from 80 C to 130 C. Even though lower
melt
point resins can be used, such resins may produce an undesirable tendency to
lead to
caking in the final product. In general, the higher the melting point, the
lower the
tendency to cake. Melting points as high as 150 C to 180 C can be used, but
could be
associated with penalties of increased energy costs and difficulties in
processing and
handling. Such resins may also generate a lower bonding strength at downhole
temperatures. Optimization of the melt/flow properties of the curable resin
and powder
combination is beneficial for storage and performance of the coated proppant.
[0150] In another embodiment, the novolac resins may be those having
softening
onset points, as measured by TMA, above 170 F, and preferably in the range
from 170 F
to 240 F. In general, the inventors have determined that the higher the
softening onset
point of the resin, the lower the tendency of the coated particle to cake.
Novolac resins
having softening onset points as high as 240 F to 300 F can be used, but
increased energy
costs and difficulties in processing and handling the final product may be
incurred.
Optimization of the softening/flow properties of the curable resin and powder
combination
is beneficial for storage and performance of the coated proppant.
E. Additives
[0151] Additives are used for special cases for special requirements. The
resin
coatings of the invention may include a wide variety of additive materials.
[0152] The resin coating may include one or more other additives such
as a
coupling agent, typically added to the liquid resin or applied to the
substrate prior to the
- 39 -
DOCSMT1, 4070637\1
CA 02718659 2015-07-06
addition of the liquid resin used to form the continuous layer, such as a
silane to promote
adhesion of the coating to substrate.
[0153] Such coupling agents include, for example, organo silanes
which are
known coupling agents. Examples of useful coupling agents of this type include
amino
silanes, epoxy silanes, mercapto silanes, hydroxy silanes and ureido silanes.
The use of
organofunctional silanes as coupling agents to improve interfacial organic-
inorganic
adhesion is especially preferred. These organofunctional silanes are
characterized by the
following formula VII:
R13¨Si ¨ (OR14)3 VII,
where R13 represents a reactive organic function and OR14 represents a readily
labile
alkoxy group such as OCH3 or 0C2H5. Particularly useful for coupling phenolic
or furan
resins to silica are the amino functional silanes of which Union Carbide A1100
(gamma
aminopropyltriethoxysilane) is an example. The silane can be premixed with the
resin or
added to the mixer separately.
[0154] The organic coating can optionally contain additives such as
silicone
lubricants, surfactants, wetting agents, dyes, pigments, flow modifiers (such
as flow
control agents and flow enhancers), hardeners, crosslinking agents, foaming
agents,
initiators, thermal stabilizers, light stabilizers, antioxidants, flame
retardants, anti-drip
agents, antiozonants, stabilizers, anti-corrosion additives, mold release
agents, fillers, anti-
static agents, waxes, dyes and the like, or combination comprising at least
one of the
TM
foregoing. One particularly useful additive to aid in the coating process is
XIAMETER
PMX-200, 350cs, a silicone lubricant commercially available from Dow Corning
Corporation.
[0155] The surfactants may be anionic, nonionic, cationic, amphoteric
or mixtures
thereof. Certain surfactants also operate as flow control agents. Other
additives include
humidity resistant additives or hot strength additives. Of course, the
additives may be
added in combination or singly.
- 40 -
CA 02718659 2015-07-06
[0156]
If desired, the organic coating can optionally contain an anti-dusting
additive thermoplastic elastomer to reduce the creation of dust relative to a
particle which
is the same except for lacking the thermoplastic elastomer. Some anti-dusting
additives
TM
include ethylene, butylacrylate copolymers (such as ENABLE copolymers
commercially
available from ExxonMobil Corporation). Other examples exist, such as NBR
(rubber)
modified novolaks and resoles that can function as "impact modifiers."
[0157]
If desired, the organic coating can optionally contain an impact modifier.
An impact modifier can impart elastic properties to the organic coating.
Suitable impact
modifiers include natural and synthetic elastomeric polymers, typically
derived from such
monomers as olefins (e.g., ethylene, propylene, 1-butene and 4-methyl-I -
pentene),
alkenylaromatic monomers (e.g., styrene and a -methylstyrene), conjugated
dienes (e.g.,
butadiene, isoprene and chloroprene), and vinylic carboxylic acids and their
derivatives
(e.g., vinyl acetate, acrylic acid, alkylacrylic acids, ethyl acrylate, methyl
methacrylate and
acrylonitrile). They include homopolymers and random, block, radial block,
graft and
core-shell copolymers or a combination comprising at least one of the
foregoing.
[01581
A particularly useful class of impact modifiers comprises the AB (diblock)
and ABA (triblock) copolymers and core-shell graft copolymers of
alkenylaromatic and
diene compounds, especially those comprising styrene and either butadiene or
isoprene
blocks. The conjugated diene blocks may be partially or entirely hydrogenated,
whereupon they may be represented as ethylene-propylene blocks and the like
and have
properties similar to those of olefin block copolymers. Examples of suitable
triblock
copolymers of this type are polystyrene-polybutadiene-polystyrene (SBS),
hydrogenated
polystyrene-polybutadiene-polystyrene (SEBS), polystyrene-polyisoprene-
polystyrene
(SIS), poly(a-methylstyrene)-polybutadiene-poly(a-methylstyrene) and poly(a-
methylstyrene)-polyi soprene-poly(a-methyl styrene). Particularly preferred
triblock
TM TM
copolymers are HYCAR, commercially available from Noveon, or KRATON D, and
TM
KRATON G, commercially available from Kraton Polymers LLC.
[0159]
Also suitable as impact modifiers are core-shell type graft copolymers and
ionomer resins, which may be wholly or partially neutralized with metal ions.
In general,
the core-shell type graft copolymers have a predominantly conjugated diene or
crosslinked
-41-
CA 02718659 2010-10-25
acrylate rubbery core and one or more shells polymerized thereon and derived
from
monoalkenylaromatic and/or acrylic monomers alone or in combination with other
vinyl
monomers. Other impact modifiers include the above-described types containing
units
having polar groups or active functional groups, as well as miscellaneous
polymers such
as Thiokol rubber, polysulfide rubber, polyurethane rubber, polyether rubber
(e.g.,
polypropylene oxide), epichlorohydrin rubber, ethylene-propylene rubber,
thermoplastic
polyester elastomers, thermoplastic ether-ester elastomers, and the like, as
well as
mixtures comprising any one of the foregoing. A suitable impact modifier
amongst the
ionomer resins is SURLYN available from Du Pont.
[0160] When multiple layers are used in the organic coating, the impact
modifiers
may be used in any of the layers. It is generally desirable to use the impact
modifiers in
the layer that is disposed upon the substrate. Impact modifiers may be used in
amounts
greater than or equal to about 0.5, preferably greater than or equal to about
1.0, more
preferably greater than or equal to about 1.5 wt% based upon the total weight
of the
organic coating. In general it is desirable to have the impact modifier
present in an
amount of less than or equal to about 20, preferably less than or equal to
about 15, more
preferably less than or equal to about 10 wt% of the total weight of the
organic coating.
F. Manufacturing Coated Particles
[0161] To make a coated proppant, or particle for gravel pack, the
appropriate
substrate (for example, single particle, composite particle or hybrid
particle), liquid resin,
and dry resin powder are mixed at conditions to provide a curable coating
composition. In
the embodiments employing composite particles or hybrid particles as
substrates the
organic material used in the curable outer coating may be the same or
different as that
used in the composite substrate or hybrid substrate, with the proviso that the
coating resin
material is curable and the resin of the composite substrate or hybrid
substrate is at least
partially cured.
[0162] The substrates, along with the desired thermosetting polymer
or
thermosetting polymer precursor are first taken in a mixing device and mixed
to form a
suitable first mixture at a temperature from about 50 F (10 C) to about 150 F
(66 C),
preferably about 70 F (21 C) to about 120 F (49 C). The thermosetting curable
resins
-42 -
DOCSMTL: 4070637\1
CA 02718659 2015-07-06
that constitute the continuous phase of the coating are liquids at room
temperature. The
substrates are not normally preheated prior to being mixed with the liquid
thermosetting
polymer. Upon mixing, the liquid thermosetting curable polymer is disposed
upon the
substrates to form an organic coating. It is desirable to add a coupling agent
to the mix at
some point before or while the substrate and liquid resin are being mixed.
Suitable
coupling agents are described in this description.
[0163] The mixing can take place in a device that uses shear force,
extensional
force, compressive force, ultrasonic energy, electromagnetic energy, thermal
energy or a
combination comprising at least one of the foregoing forces and energies and
is conducted
in processing equipment wherein the aforementioned forces are exerted by a
single screw,
multiple screws, intermeshing co-rotating or counter rotating screws, non-
intermeshing co-
rotating or counter rotating screws, reciprocating screws, screws with pins,
barrels with
pins, screen packs, rolls, rams, helical rotors, or a combination comprising
at least one of
TM
the foregoing. Exemplary mixing devices are buss kneaders, helicones, EIRICH
mixer,
TM TM TM
WARING blenders, HENSCHEL mixers, Barber Green batch mixers, ribbon blenders,
extruders or the like.
[0164] Then the non-reactive powder and/or reactive powder is applied
to the first
mixture of coated particles and mixed for sufficient time to form a free
flowing second
mixture of curable resin coated particles having non-reactive powder and/or
reactive,
preferably novolak or resole, powder embedded in and adhering on the coating.
The
amount of liquid coating resin and the amount of powder is selected depending
upon the
desired amount of coating to be applied. Typically the ratio of liquid resin
and powder
may vary depending upon the temperature and times of addition of the various
ingredients.
The typical ratio may be adjusted to avoid extremes of processability, namely
sticky
particles at one extreme and dusty particles at the other extreme. Product
performance
may depend upon the applied resin level of the combined liquid resin and
powder as well
as the resin and powder selected.
[0165] If desired one or more coatings of liquid resin and powder may
be applied.
Additional coatings may be applied by coating the particle having the single
resin and
powder coating with an additional liquid resin coating and then applying an
additional
- 43 -
CA 02718659 2015-07-06
portion of powder to the additional liquid coating, and repeating this as
desired.
Preferably sufficient resin is applied to achieve a loss on ignition (combined
coating
weight) of from about 0.3 to about 5 weight percent, preferably about 0.3 to
about 4
weight percent due to the one or more outer layers of curable resole coating
with reactive
powder.
[0166] Typically a silane is added to the sand in a mixer and, about
10 to 20, e.g.,
seconds after the silane, a liquid resin is added. The silane could also be
premixed into
the liquid resin. For example, in one embodiment, 3.0 to 10.4 grams of liquid
resole
(which is 65% solids) is used per 1000 grams of sand substrate. In another
embodiment
10 3.0 to 5.0 grams of liquid resole (which is 65% solids) is used per 1000
grams of sand
substrate. Then about 30 to 60 seconds after the liquid resin is added the
reactive powder
is added.
[0167] Preferably powder is added 60 seconds after the first silane,
which is 45
seconds after the liquid resin. However, these addition times are sensitive to
mixing speed
15 and ambient temp and mixer design. In a particularly useful example for
the coated
particles produced at laboratory conditions (23C with a Hobart mixer), silane
is added to
the substrate and mixed for 15 seconds; followed by the addition of liquid
resin while
mixing continues for an additional 45 seconds; followed by the powder addition
which
continues for an additional 240 seconds (300 seconds total cycle) before the
dry,
freeflowing particles are finished.
[0168] It is desirable to add a lubricant to the mix at some point
before the product
"breaks down" into free flowing particles. The lubricant is preferably liquid
at the mixing
temperature and has a sufficiently high boiling point so it is not lost during
the mixing
process. Suitable lubricants include liquid silicone such as Dow Corning
Silicone 200,
TM
mineral oil, paraffin wax, petrolatum, cocamidopropyl-hydroysultaine
(CHEMBETAINE
CAS commercially available from Lubrizol Corporation, or the synthetic
lubricant
TM
ACRAWAX CT, a bis-stearamide of a diamine, commercially available from Glyco
Chemicals, Inc.). The amount of lubricant can vary from about 0.01 or 0.03% to
about
0.5% by weight based upon the weight of the particulate material. In another
embodiment,
- 44 -
CA 02718659 2010-10-25
the amount of lubricant can vary from about 0.05% to about 0.25% by weight
based upon
the weight of the particulate material.
[0169] Additionally low levels of non-reactive organic or inorganic
filler powders
such as silica flour, wood flours or talc can be added as processing aids
after admixing the
reactive powder to the coated substrates or concurrently with admixing the
reactive
powder to the coated substrates. The non-reactive filler powders, if present,
are in an
amount of less than about 10% of the amount of the reactive powders. The non-
reactive
organic or inorganic filler powders typically have average particle sizes of
about 2 to
about 30 micrometers.
[0170] In one embodiment, to obtain improved storage properties, a surface
treatment can be applied to the coated particle that consists of a non-
reactive powder,
optionally a resin or resin/curing agent combination, or both. These
components react to
form a protective "surface treatment" on the reactive resins. This approach
aids in
generating a free flowing product and enhances the products ability to be
stored at
conditions of elevated temperature and humidity without forming excessive
clumping that
can create issues with the ability to load out the product for transfer to the
well site or
create issues in removing product from field bins as the product is added to
the fracturing
fluid to be transported down the well and out into the created fractures. An
example of an
effective "surface treatment" would be a small amount of a fast reacting resin
that can be
added along with a proportional amount of a fast reacting curing agent to the
surface of the
curable coated substrate. In one embodiment, the surface treatment is added in
an amount
of between about 0.05 and 1.5 wt%, based upon the weight of the substrate. In
another
embodiment, the surface treatment is added in an amount of between about 0.05
and 1.0
wt%, based upon the weight of the substrate. In another embodiment, the
surface
treatment is added in an amount of between about 0.05 and 0.5 wt%, based upon
the
weight of the substrate. The amount of curing agent needed depends upon the
resin and
may be calculated as is known in the art. In one embodiment, the curing agent
is capable
of substantially curing the resin in 60 seconds or less. In another
embodiment, the curing
agent in capable of substantially curing the resin in less then 10% of the
total mixing time.
-45 -
DOCSMT1, 4070637\1
CA 02718659 2015-07-06
[0171] In another embodiment, the surface treatment is about 0.1 wt%,
based upon
TM
the weight of the substrate of an epoxy resin, such as for example EPON 815
resin,
commercially available from Hexion Specialty Chemicals, Inc., which is cured
by the
addition of 0.012 weight percent of tetrafluoroboric acid, commercially
available from
TM
Sigma Aldrich Co. as a 48% solution in water. In another embodiment, EPON 815
epoxy
resin is added 30-45 seconds after the powdered novolak resin with the
aforementioned
TM
acid curing agent being added 15-30 seconds after the EPON 815 epoxy resin.
[0172] The particles having curable coatings are then recovered.
[0173] In one exemplary manner of manufacturing the coated particles,
a mixture
comprising the substrate, the thermosetting polymer or the thermosetting
polymer
precursor that will be used for the organic coating, the novolak powder or
resole powder
and any optional additives are introduced into a mixer such as an EIRICH
mixer. In one
embodiment, to coat and then form a composite particle, the mixing is first
carried out at a
first speed for a given time. Following this the mixing speed is changed. The
change in
the speed of mixing promotes the formation of a layer of the organic coating
around the
substrate to make particles of the desired size (i.e., about 200 to about 800
micrometers.
In another embodiment, to only develop a coating around a substrate particle,
changing
mixing speeds may not be beneficial. To obtain a desired density for the
coated particles,
process parameters can be varied. For example, the amount of filler or the
amount of
organic material can be increased to change the density of the coated
particles.
G. Particle Parameters
[0174] The following parameters may be useful when characterizing
particles of
the present invention.
1. Amount of Resin
[0175] The amount of resin is determinable by measuring Loss-on-Ignition
(LOI).
LOT is typically determined in a two hour furnace test, starting by pre-
conditioning a series
of crucibles with lids in a furnace pre-heated to 1700 F (927 C). The crucible
with the lid
is then placed in the furnace at 1700 F (927 C), the furnace is allowed to
heat back up to
- 46 -
CA 02718659 2010-10-25
1700 F (927 C), and the crucible with the lid is maintained at 1700 F (927 C)
for 15
minutes. The pre-conditioned crucibles and lids are then placed in a
desiccator containing
standard desiccants and allowed to cool to room temperature. Then, the
conditioned
crucible with the lid is weighed and approximately 8 grams of resin-coated
sand are placed
in the crucible. Then, the crucible with the lid and the sample is reweighed.
The covered
crucible and sample are then placed in the furnace at 1700 F (927 C), the
furnace is
allowed to heat back up to 1700 F (927 C), and the samples are kept in the
furnace for 2
hours after the furnace temperature has returned to 1700 F (927 C). The
crucible with lid
and sample are then transferred to the desiccator and allowed to cool to room
temperature.
The cooled crucible with lid containing the sample of sand is then re-weighed
using the
analytical balance, and the loss-on-ignition for each sample is calculated as
the difference
between the original and final sample weight.
2. Particle Size
[0176] The coated particle generally has an average particle size of
about 200 to
about 2,000 micrometers (about 70 mesh to about 10 mesh). In one embodiment,
the
coated particle has an average particle size of about 425 to about 850
micrometers (about
40 mesh to about 20 mesh). In another embodiment, the coated particle has an
average
particle size of about 212 to about 425 micrometers (about 70 mesh to about 40
mesh).
The coated particles can have bimodal or higher distributions. Typically the
reactive
and/or non-reactive powder has an average particle size of about 200 mesh
(about 70
mesh) or smaller.
3. Density
[0177] It is desirable for the coated particles to have a bulk
density of about 0.75 to
about 0.95 g/cm3. In one embodiment, the coated particles have a bulk density
of about
0.8 to about 0.9 g/cm3. In one embodiment, the coated particles have a bulk
density of
about 1.7 to about 3.6 g/cm3. The coated particle has an apparent density of
about 1 to
about 4 grams per cubic centimeter (g/cc) as determined by API RP 58 with
isobutanol. In
one embodiment, the coated particle has an apparent density of about 1.1 to
about 3 g/cc.
In another embodiment, the coated particle has an apparent density of about
1.15 to about
2 g/cc. It is desirable for the coated particles to have an apparent density
of about 1.6 to
- 47 -
DOCSMT1. 4070637\1
CA 02718659 2010-10-25
about 3.6 g/cm3. Density can be varied by either altering the density of the
cores/substrates or by altering the choice of filler or doing both.
4. Unconfined Compressive Strength
[0178] Compressive strength of curable proppants is defined as that
measured
according to the following procedure, known as the Unconfined Compressive
Strength or
UCS test. In this test, a 2 weight percent KC1 solution (doped with a small
amount of
detergent to enhance wettability) is added to proppant. The KC1 solution and
proppant
(about 6 to 18, typically 12 lbs. proppant per gallon KC1) are gently agitated
to wet the
proppant. Remove entrained air bubbles, if any. If necessary use a wetting
agent to
remove the bubbles. This slurry (about 100-200 grams depending on density) is
transferred into duplicate 1.25 inch OD X 10 inch stainless steel cylinders,
equipped with
valves on the top and bottom to bleed liquid and gas pressure as required, a
pressure gauge
reading 0-2000 psi, and a floating piston to transfer pressure to the sample.
Typically at
least 3, preferably at least 6 specimen molds are loaded to give a length
greater than two
times the diameter of the finished slug. The bottom valve is opened during the
application
of stress, allowing fluid to drain from the slurry, and then closed during the
application of
temperature. The cylinder is connected to a nitrogen cylinder and 1000 psi is
imposed on
the cylinder, transmitted by the sliding pistons to the sample, and then top
valve is shut
and bottom valve remains open. (As test temperature is approached near to the
fluid valve
on the mold, the bottom valve (fluid valve) is closed. Closing the fluid valve
too soon
may generate enough pressure, as the cell is heating, to prevent/reduce the
intended
closure stress applied to the proppant slug. Closing the valve too late may
allow loss of
too much fluid from the slug by evaporation or boiling).
[0179] The duplicate cylinders containing the sample are transferred
to an oven
preheated to the desired setpoint, i.e., 200+1 F, and remain in the oven for
24 hours.
Maintain stress and temperature during the cure time. Stress should be
maintained +10%.
During the curing process in the oven, loose curable proppant particles become
a
consolidated mass. At the end of the 24 hours, the cylinders are removed,
venting off
pressure and fluid rapidly, and the approximately one inch by six inch
consolidated slug
sample is pressed from the cylinder. The sample is allowed to cool and air dry
for about
- 48 -
DOCSMT1.= 4070637\1
CA 02718659 2010-10-25
24 hours, and cut (typically sawed) into compression slugs of diameter: length
ratio (D :
L) of about 1:2.5 or greater. Air drying is performed at a temperature of less
than about 49
degrees C (120 degrees F). Typically, both ends of each slug are smoothed to
give flat
parallel surfaces.
[0180] The compression slugs are mounted in a hydraulic press and force is
applied between parallel platens at a rate of about 4000 lbsf./minute until
the slug breaks.
For slugs with compressive strength less than 500 psi, use a loading rate of
1000
lbsaminute. The force required to break the slug is recorded, replicates are
documented,
and the compressive strength for each sample is calculated using the formula
below. An
average of the replicates is used to define the value for this resin coated
proppant sample.
(Fc, psi) = 4 x Fg / {(p x d2) [0.88 + (0.24d/h)]1
wherein
Fe = compressive strength (psi)
Fg = hydraulic gauge reading (lb force)
p = pi (3.14)
d = diameter of the slug (inches)
h = length of slug (inches)
[0181] Compressive strength of the slugs is determined using a
hydraulic press,
i.e., Carver Hydraulic Press, model #3912, Wabash, Indiana. Typical
compressive
strengths of proppants of the present invention range from 50 to 3000 psi or
higher.
However, the reproducibility of the UCS test is probably 10% at best.
Typically, the
individual resinous layers of the invention have UCS strengths greater than
500 psi, as
detailed below. It is also noted that the Compressive Strength Test can be
used to indicate
if a coating is cured or curable. No bonding, or no consolidation of the
coated particles,
following wet compression at 1000 psi at 200 F for a period of as much as 24
hours,
indicates a cured material.
5. Roundness
- 49 -
DOCSMTL. 4070637\1
CA 02718659 2010-10-25
[0182] It is desirable for the coated particle to have a roundness of
about 0.7 to
about 0.9. An exemplary roundness is about 0.8. It is also desirable for the
coated particle
to have a sphericity of about 0.7 to about 0.9 as measured were measured
according to API
RP 58 (American Petroleum Industry Recommended Procedure 58).
6. Acidity of Water Extracts
101831 The following description relates to a test procedure that
measures the
"acidity of water extracts of resin coated proppant". It is not a measure of
the acid
solubility of a resin coated proppant. It relates to a measure of how much
impact the water
extractables (from the coating) can have on the pH of water (or the pH of a
frac fluid
system).
[0184] The acidity test (as it relates to resin coated proppants) is
a measure of the
acidity levels of water extracts of the resin coated proppants. This test
relates to the effect
that the resin coated proppant (and the water extractable components found in
its coating)
will have on the pH of the fracturing fluid system that will be used to
transport the
proppant out into the hydraulically induced fracture.
[0185] This is determined as follows. Prepare a large, about 1000 mL,
quantity of
slowly boiling distilled or deionized water, using the first hot plate and the
large beaker.
Adjust the heat to give a low or slow steady boiling action. The temperature
should be
about 212 F (100 C) depending on the altitude. Set the heat selector to high
on the second
hot plate. Weigh 50 g resin coated proppant into a 250 mL graduated beaker.
Place the
beaker of resin coated proppant on the second hot plate. Rapidly, add boiling
deionized
(distilled) water to the 125 mL mark on the beaker of resin coated proppant
and stir once
to remove air bubbles. Allow the mixture to come to a boil, about 15 to 30
seconds is
required. Continue to boil for 3 minutes. Place the beaker in an ice bath and
stir until the
water temperature is 70 - 80 F (21-27 C). Curable and partially cured resin
coated
proppant will solidify to give a solid mass. It is necessary to break the mass
with a spatula
while the suspension is cooling. Stir first with the spatula to break up the
mass of resin
coated proppant, then stir with the thermometer. Stir enough to minimize the
time
required for full cooling while the water in the ice bath is at least as high
as the liquid in
the beaker.
- 50 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
[0186] After the suspension has cooled back to room temperature, add
deionized
water back to the beaker, 125 mL mark to replace any water lost during
boiling; and
immediately measure the pH of the water layer using a standardized pH meter.
Record the
initial pH to 0.05 units. While measuring pH the pH electrode is not in the
proppant layer.
Using 0.1 N sodium hydroxide titrate the pH of the suspension to pH = 9.00.
Record the
volume of the titrant, required to reach the pH endpoint, to the nearest 0.05
mL. Record
the endpoint pH to the nearest 0.05 units.
7. Acetone Extraction Test
101871 The Acetone Extraction Test is another method to determine if a
coating or
coatings are curable. The acetone extraction method dissolves the fraction of
resin that is
uncured. This test is performed by placing a dried pre-weighed sample, about
50 grams,
of resin coated particles (with a known resin coating content) in a Soxhlet
thimble and
refluxing acetone condensate over the material for 2 hours. After drying the
treated
sample, the change in resin content is reported as percent acetone
extractables.
Specifically, because uncured resin is soluble in acetone, and cured resin is
not soluble in
acetone, the acetone condensate reflux will remove only the uncured fraction.
By
weighing the sample both before and after acetone reflux and determining a
percentage
change, the degree of cure is calculated. For example, the weight loss for a
typical cured
resin coated sand may only be 5% of the LOT of the sample. Thus, a sample
having a 2.0
grams LOI may have a 0.1 gram acetone extractable. In contrast, the weight
loss for a
fully curable resin coated sand will be the LOI of the sample. Thus, for a
sample having a
2.0 grams LOI, removing 2.0 grams by acetone extraction would reflect that the
sample is
100% curable.
8. Temperature Stick Point Test
101881 The Temperature Stick Point Test is another indicator of
whether a coating
is curable. It is performed by placing coated material on a heated melt point
bar and
determining the lowest temperature at which the coated material sticks. A
"sticking
temperature" of greater than 350 F at the hottest end of the bar, typically
indicates a cured
- 51 -
DOCSMTL 4070637\1
CA 02718659 2015-07-06
material, depending upon the resin system used. The melt point bar is a brass
metal bar
(18 inches long and 2 inches wide) with an electric heating element at one
end. Therefore,
a temperature gradient can be established across the length of the bar and the
temperature
across the bar is monitored with thermometers or thermocouples.
[0189] Using a funnel, a uniform strip of about 100 grams of resin coated
substrate, e.g., sand, is laid on the heated bar and cured for 60 seconds.
Then the bar is
tipped to allow any uncured proppant to fall off. Melt point is the lowest
temperature at
which the resin coated sand forms a continuous mass and does not fall from the
bar once it
is tipped to ninety degrees. Typically, the cured coating has a sticking
temperature in the
range from about 150 to about 300 F, for example about 200 to about 250 F.
9. Wettability of Particles in Water
[0190] Wettability to determine the quantity of selected
surfactant(s) required to
wet proppant(s) is performed to determine the quantity of surfactant(s)
required for the
reduction of aeration/air entrainment to zero.
[0191] Prepare diluted surfactant solution and fill a 25 mL glass burette.
A dilution
factor of 1:100 is typical. However, many surfactants may be tested as is.
Then, add 200
mL of 2% KC1 to a 300 mL Berzelius (tall form) beaker (deionized H20 may be
used).
TM
Adjust the beaker under a VARIAC or stirrer with built-in speed control so the
blade is
about 1/4" above the bottom. The beaker should be clamped in place using a
ring stand
and clamp. Then adjust the burette to an appropriate position, set the stirrer
switch to OFF
and adjust the speed control to its highest position, which will not eject the
contents of the
beaker (sand in the water). Then, start the stirrer and add the appropriate
amount of
proppant to be tested.
[0192] Typical proppant loading ranges are listed in TABLE A:
TABLE A - Proppant Loading Ranges
lbn,/gal q/200 mL
2 48
- 52 -
CA 02718659 2010-10-25
4 96
6 144
8 192
240
12 288 preferred
[0193] Stir for 5 seconds, and then stop and observe the air bubbles
adhering to the
proppant grain surfaces. If no bubbles are visible, the proppant is considered
fully wetted.
If there are air bubbles then add 1/4 mL of surfactant, restart the stirrer
for 10 seconds, and
5 then again observe the air bubbles adhering to the proppant surface. If
bubbles are again
observed, then repeat the step of adding surfactant stirring and observing
until most of the
bubbles have disappeared, then reduce the incremental surfactant to 1/8 mL.
When the
bubbles are no longer observed, record the volume of surfactant required for
wetting the
proppant.
10 [0194] Repeat the test as follows to more nearly duplicate the
usage conditions and
procedure in the field.
[0195] Prepare another sample of water, and add to the water the
exact amount of
diluted surfactant (determined by the first procedure for when the proppant
was fully
wetted). Then place the beaker under the stirrer and start the stirrer. Add
the proper
amount of proppant. Stir for 10 seconds, and then stop the stirrer. Observe
and record the
relative quantity of air bubbles on the surfaces of the proppant. If there are
any bubbles
continue titration as before until they are gone and no additional surfactant
is required.
Record the additional volume of surfactant required.
[0196] Calculate the volume of surfactant required to completely wet
the proppant.
Vv, (name of surfactant), gal/1000 gal =1000 x ((Vsurf x FD) / Vfluid )
at X lb,,, prop/gal.
Vm, (name of surfactant), gal/1000 gal = 119.831 x ((Vsurf x FD) Mptop )
for each lb. prop/gal.
- 53 -
DOCSMTL 4070637\1
CA 02718659 2015-07-06
where,
Vv is volume of surfactant to wet proppant, gal/1000 gal at X lbw, prop/gal.
Vm is volume of surfactant to wet proppant, gal/1000 gal/lb, prop/gal
FD is dilution factor, volume surfactant/volume diluent, dimensionless
Vsurf = experimental volume of diluted surfactant, mL
Mprop '---- mass of proppant tested, g
Vfluld = volume of water in the proppant/water mixture, mL
10. Turbidity Test
[0197] The particles are subjected to a Turbidity Test as follows.
Weigh 15.0
grams of deionized/distilled water, doped with 0.1% HO surfactant, 15 grams
into a clean
sample cell (Hach catalog #21228 or equivalent) and replace the screw cap of
the cell.
FS0 is duPont Fluorosurfactant ZONYL TM FSO. Wipe the outside of the cell with
lint
free paper. Make sure no air bubbles adhere to the walls of the cell. 4) Place
the cell into
TM
the turbidimeter (HACH Model 2100P) and read the turbidity in NTU units. Weigh
5.00
grams of the sample to be measured and place this in the cell. Using the
Vortex mixer
TM
(Thermolyne Maxi-Mix 1 or equivalent), agitate the sample/water mixture for 10
seconds.
Again, clean the outside of the cell with lint free paper. Place the
sample/cell back into the
turbidimeter and read the turbidity, 30 seconds after the Vortex mixing ended.
Record the
turbidity in NTU units for this sample as "dust content".
[0198] Preferably the particles of the present invention achieve a
turbidity
measurement of less than 100 NTU after being subjected to 30 minutes ball mill
time, less
than 200 NTU after being subjected to 60 minutes ball mill time, and/or less
than 300
NTU after being subjected to 150 at 60 minutes ball mill time. The coated
particles
generally have a turbidity of less than or equal to about 250 as measured
according to API
RP 56.
11. Caking (Storage Stability) Test
- 54 -
CA 02718659 2010-10-25
[0199]
The coated particles are subjected to an elevated temperature/humidity
exposure (under controlled conditions) in order to determine the amount of
clumping
(bonding together of coated particles) that will occur in a storage situation.
A 50 gram
sample of product is placed in a cardboard cup having a 2 inch ID and 3 inch
depth. On
top of the sample is placed a 1 kilogram weight having an OD of 1.875 inch
(so that the
weight fits snugly inside the sample cup). To prepare a desiccator for the
caking test a
saturated solution of sodium chloride is prepared. A portion of the salt
solution ( 200 ml)
is placed in an open container that is situated inside the desiccator.
Positioned over (but
not touching) the salt solution is the sample platform. The presence of the
saturated salt
solution in the desiccator will insure the caking test is performed at a 75%
humidity
level. The sample cup containing the coated product and kilogram weight are
placed on
the sample platform and the desiccator is sealed. The sealed desiccator
(containing the salt
solution and sample cup) is then placed in a constant temperature oven set to
maintain a
test temperature (usually 130 F). The desiccator remains sealed at the test
temperature
for a minimum of 12 hours. At the prescribed time the desiccator is removed
from the
oven and the sample cup removed from the desiccator. The one kilogram weight
is taken
off the sample and the cup held at approximately a 45 angle with vertical
while
continually rotating the cup for a minimum of 15 seconds. A visual inspection
is made of
the sample and a caking value is given according to the following table:
Caking Value Appearance Description
0 product is free flowing
0.5
product breaks away from the wall of the sample cup and only small
clumps remain
1
product does not break away from walls of sample cup but can be
penetrated easily with a tongue depressor
2
Product does not break away from the walls of the sample cup and
pressure must exerted to penetrate the sample's surface
- 55 -
DOCSMTL 4070637\1
CA 02718659 2015-03-10
4
Product does not break away from cup walls and significant
pressure must be exerted to penetrate the surface of the sample
H. Use of Particles As Proppant
[0200]
The particles, as described in this invention comprise curable coatings.
Thus, they can be injected into a subterranean formation and the coatings cure
in the
subterranean formation. They may be injected into the subterranean formation
as the sole
proppant in a 100% proppant pack (in the hydraulic fracture) or as a part
replacement of
existing commercial available ceramic and/or sand-based proppants, resin-
coated and/or
uncoated, or as blends between those, e.g., coated particles are 10 to 50
weight % of the
proppant injected into the well. For example, after first pumping a precured
proppant or
uncoated proppant is placed in a well, a curable proppant (of the present
invention) can be
placed in the fracture that is closest to the wellbore or fracture openings.
This type
fracturing treatment in done without stopping to change the proppant and is
know in the
industry as a "tail-in treatment".
[0201] In the case of curable proppants, the method may comprise curing the
curable resin composition by exposing the resin composition to sufficient heat
and
pressure in the subterranean formation to cause curing of the resins and
consolidation of
the curable proppant of the present invention. In some cases an activator can
be used to
facilitate consolidation of curable proppant. In another embodiment employing
a curable
resin composition on the proppant, the method further comprises low
temperature acid
catalyzed curing at temperatures as low as 70 degrees F. An example of low
temperature
acid catalyzed curing is disclosed by U.S. Patent No. 4,785,884.
[0202]
The curable coated particles of the invention are especially advantageous
whether the coated particles are used alone as a proppant, or together with
other proppants
as a tail end after using uncoated proppant or precured coated proppant or
another curable
proppant to be in the portion of the fracture nearest the wellbore.
- 56 -
CA 02718659 2010-10-25
I. Use of Coated Particles as Gravel Packing or for Sand Control
[0203] It is known that oil or gas well boreholes are provided with
gravel packing
about their bore holes. Another aspect of the present invention is that these
gravel packs
may be provided with the coated particles of the present invention.
[0204] These coated particles would be provided in the standard sizes known
for
gravel used in gravel packs. Typically the strength requirements for a
proppant particle
used in packed fractures are higher than for gravel packing. The gravel pack
may serve
for sand control to prevent flow of formations fines from the formation into
the well bore.
[0205] In gravel packing operations, the coated particles can be
suspended in a
carrier fluid and pumped into a well bore in which the gravel pack is to be
placed. The
carrier fluid leaks off into the subterranean zone and/or is returned to the
surface while the
coated particles are left in the subterranean zone. The resultant gravel pack
acts as a filter
to separate formation sands from produced fluids while permitting the produced
oil and/or
gas to flow into the well bore. A method of forming a gravel pack therefore
comprises
suspending the coated particles in a carrier fluid to form a suspension;
pumping the
suspension into a well bore; and draining the carrier fluid to form a gravel
pack. Once in
place the injected particles cure to form a permeable solid barrier is formed
which
restrains the movement of the sand.
[0206] For another example, the coated particles may be used by
filling a
cylindrical structure with the resin-containing particulate material, i.e.,
proppant, and
inserted into the wellbore. Once in place, the coated particles act as a
filter or screen to
eliminate the backwards flow of sand, other proppants, or subterranean
formation
particles. This is a significant advantage to eliminate the back flow of
particulates into
above ground equipment. This employs prepacked screens, in which the resin
coated
sand/ceramic is put through a cure before the screen assembly is placed in the
well.
[0207] The following examples, which are meant to be exemplary, not
limiting,
illustrate compositions and methods of manufacturing of some of the various
embodiments
of the coated particles described herein.
- 57 -
DOCSMTL 4070637\1
CA 02718659 2015-07-06
EXAMPLES
[0208] The following examples serve to illustrate the present
invention. Unless
otherwise indicated all parts and percentages are by weight, and all screen
mesh sizes are
U.S. Standard Screen sizes. In the Examples the silane is A1100 adhesion
promoter from
Union Carbide Corporation. The proppant was coated with liquid OWR-262E, a
commercial phenol-formaldehyde resole resin manufactured by Hexion Specialty
Chemicals, Inc., Louisville, Kentucky. The powder used with the proppant
(unless
TM
otherwise noted) was DURITE SD-909A (a phenol-formaldehyde novolak powder
(with
15% hexamethylenetetramine), a commercial phenol-formaldehyde novolak
manufactured
TM
by Hexion Specialty Chemicals, Inc., Louisville, Kentucky. The DURITE SD-909A
powder has a particle size range for passing through a 200 mesh screen.
EXAMPLE 1
[0209] This experiment was conducted to determine the properties of a
coated
particle of the present invention. The coating cycle was as follows. 1000
grams of
CARBOPROP 12/18 intermediate density ceramic particles at room temperature
were
added to a Hobart lab mixer. Then the mixer agitator was started. Then, 0.8
grams of
A1100 (aminopropyltriethoxysilane) was added and the timer is started, (0:00
minutes).
After 30 seconds, 10.0gms of OWR-262E (phenol-formaldehyde liquid resole) was
added.
TM
When the timer is at 2 minutes, 18.4 grams of DURITE SD-909A phenol-
formaldehyde
novolak powder (with 15% hexamethylenetetramine) was added with continued
mixing
(about 2.4% total organics on the particles). When the timer is at 12 minutes,
the mass
was free flowing and was removed from the mixer. This product was then tested
for 24
hour UCS bond strength at conditions of 1000 psi and 200 F, yielding 590 psi.
EXAMPLE 2
[0210] This experiment was conducted to determine the properties of a
coated
particle of the present invention. The coating cycle was as follows. 1000
grams of
TM
CARBOPROP 12/18 intermediate density ceramic particles at room temperature
were
- 58 -
CA 02718659 2015-07-06
added to a Hobart lab mixer. Then the mixer agitator was started. Then 0.8
grams of
A1100 (aminopropyltriethoxysilane) was added and the timer is started, (0
minutes). After
about 30 seconds, 10.0 gms of OWR-262E (phenol-formaldehyde liquid resole) was
added. When the timer is at 2 minutes, 33.2 grams of SD-672D powder (phenol-
formaldehyde novolak, no hexamethylenetetramine) was added with continued
mixing
(about 4% total organics on the particles); SD-672D powder had a particle size
of particle
size of +/- 100 mesh. When the timer was at 12 minutes, the mass was free
flowing and
was removed from the mixer. This product was then tested for 24 hour UCS bond
strength
at conditions of 1000 psi and 200 F, yielding 1075 psi.
EXAMPLE 3
[0211] A curable resin coating was developed by adding 0.4 grams of
coupling
agent (A-1100 silane) to 1 kilogram of substrate with constant agitation. A
liquid resole
(OWR-262E), available from Hexion Specialty Chemicals, Inc., Louisville,
Kentucky),
was added at 15 seconds into the cycle, after the silane. A powdered novolak
resin
TM
DURITE FD-900-A, (with 7% hexamethylenetetramine was used to prepare Samples
C, D
and G), or a powdered novolak resin, SD-909A, (with 15% hexamethylenetetramine
was
used to prepare Samples A, B, E, and F) each available from Hexion Specialty
Chemicals,
Inc., Louisville, Kentucky was then added at 1 minute into the cycle time. The
material
was mixed for 4 additional minutes and discharged from the mixing apparatus.
Using the
procedure above, analytical properties varied while evaluating alternative
resins, resin
levels, particle sizes and substrates. Concentration of hexamethylenetetramine
in the
novolak powder thus varied from 7 - 15%, dependant upon which powder was used
in
each formulation (see Tables 1, 2, and 3 for analytical data).
[0212] FIG. 5 shows a photograph of a sample of lab prepared
particles (as is) of
Sample A with about a 10X magnification.
[0213] FIG. 6 shows a photograph of a sample of lab prepared
particles (as is) of
Sample B with about a 10X magnification.
[0214] FIG. 7 shows a photograph of a slug of lab prepared particles
of Sample B
with about a 10X magnification after the 1000 psi unconfined compressive
strength test.
- 59 -
CA 02718659 2010-10-25
[0215] FIG. 8 shows a sample of lab prepared particles of Sample B
with about a
10X magnification after a hot tensile strength test.
[0216] A caking analysis was performed on certain material by placing
50 grams
of coated material in a cylindrical container with a 1 kilogram load on it and
placed in a
heated oven for 24 hours at temperatures ranging from 105-140 F (see TABLES 1,
2 and
3 for analysis data).
TABLE 1
Property Measured
Sample Number A B C D
Sand, API Mesh Size,
40/70 40/70 40/70 40/70
Nominal
Silane Addition A-1100,
0.4 / 0 0.4 / 0 0.4 / 0 0.4 / 0
wt.= grams / time = seconds
Resole Addition
OWR-262E, wt.= grams / 10.4 / 15 7.0 / 15 8.6 / 15 5.2 /
15
time = seconds
Powder Addition
SD-909A,wt.= grams / time 23.0 / 60 20.0 / 60
= seconds
Powder Addition
FD-900A,wt.= grams / time 21.5 / 60 11.5
/ 60
= seconds
Discharge, time = seconds 300 300 300 300
Resin Content, LOT, weight
2.98 2.14 2.46 1.19
%
Melt (Stick) Point, F[ C] 204 [96] 214 [101] < 185
[85] < 185 [85]
Hot Tensile Strength, psi 119 44
Particle Size Distribution
US Standard Sieve No.
[mm]
30 [0.589] 0.1 0.1 0.0 0.0
40 [0.42] 6.0 7.7 6.0 7.5
45 [0.351] 10.2 10.5 22.2 24.2
50 [0.297] 38.0 34.9 48.8 48.7
60 [0.249] 25.5 24.5 14.6 13.9
70 [0.211] 17.9 19.6 7.6 5.6
80 [0.150] 2.3 2.6 0.8 0.1
pan [<0.150] 0.0 0.1 0.0 0.0
Total 100.0 100.0 100.0 100.0
in-size (-40+70)
91.6 89.5 93.2 92.4
[-0.42+0.211]
- 60 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
TABLE 2
Sample Number A
Turbidity, NTU (FTU) 233 125
Unconfined Compressive
Strength
Closure Stress
at 200 F (93 C), 24 hr
in 2% KC1, 12 lbm/gal 405 [2795] 210 [1449] 305
[2105] 85 [587]
added at 0.0 psi [0.0
MPa]
Closure Stress
at 200 F (93 C), 24 hr
1325 231
in 2% KC1, 12 lbm/gal 508 [3505] 955 [6590]
[9143] [1594]
added at 1,000 psi [6.9
MPa]
Closure Stress
at 150 F [66 C], 24 hr
in 2% KC1, 12 lbm/gal 103 41
added at 0.0 psi [0.0
MPa]]
Closure Stress
at 150 F [66 C], 24 hr
in 2% KC1, 12 lbm/gal 146 81
added at 1,000 psi [6.9
MPa]
Caking Free
@ 105 F free flowing
Tendency flowing
Free
@ 125 F free flowing
flowing
Free
@ 140 F free flowing
flowing
Clusters,
1.0 1.0 1.0 1.0
weight %
Coating
Efficiency, 100.0 100.0 100.0 100.0
weight %
pH of Water
initial pH 8.9 8.8
Extract
mL 0.1 N
NaOH to 0.4 0.3
pH = 9
mL 0.1 N
NaOH to 4.6 4.4
pH =10
- 61 -
DOCSMTI, 4070637\1
CA 02718659 2010-10-25
TABLE 3
Sample
12/18 CarboProp 500g 1,000g 1,000g
A-1100 0.4g 0.4g 0.4g
OWR-262E 5.0 g 10.6 g 10.6 g
SD-909A 8.2 g 32.4 g
FD-900A 32.4 g
Melt (stick) point F
< 185 < 185 <185
2.14 3.80 3.75
wt %LOI
UCS,psi (1K) @ 200 F 490 2,000 1,750
UCS,psi (atm) @ 200 F 1,020 880
Hot Tensile, psi 224 232 121
Initial pH 8.43 8.72
milliliters to pH=9 0.9 0.7
milliliters to pH=10 7.6 5.6
pH (slurry water) 7.79 8.13
Cycle Addition Times
Time = 0: Add Coupling Agent
Time = 30 seconds: Add Resole
Time =2 minutes: Add PF powder
Time = 12 minutes: Discharge
SST (Starting Sand Temperature) = Ambient Temperature
[0217] The above data demonstrated that by coating sand or ceramic
substrates
with a liquid phenol-formaldehyde resole at room temperature, followed by the
introduction of a powdered phenol-formaldehyde novolak resin (with or without
hexamethylenetetramine curative) yields a high performance, free-flowing resin
coated
particle that can be used as an oilfield proppant.
EXAMPLE 4
[0218] This example shows delaying the addition of the powder (after
the silane
and resole) dries out the resole and causes the resole to lose its ability to
hold powder. The
- 62 -
DOCSMTL 4070637\1
CA 02718659 2010-10-25
addition of the novolak powder at delayed times shows the effect of free (non-
adhering)
powder to the substrate due to partial drying of the liquid resole as the
cycle time
progresses. After coating four separate batches with various addition times,
each material
was sieved through 100 and 200 mesh screens. The unadhered powder collected on
the
pan was weighed. TABLE 4 lists the powder addition times and shows the
unadhered
residual phenol formaldehyde resin powder resulting from each sample
preparation. The
other times in the Cycle Addition Time were as in TABLE 4.
[0219] 7.0 grams of a liquid resole (OWR-262E), available from Hexion
Specialty
Chemicals, Inc., Louisville, Kentucky), was added at 15 seconds into the
cycle, after the
silane. However, the time of addition of 20.0 grams of the powdered novolak
resin (FD-
900-A), available from Hexion Specialty Chemicals, Inc., Louisville, Kentucky)
was
varied. In the first run, the powder was added at 1 minute into the cycle
time. In
subsequent runs, the powder was added at a later time as shown in TABLE 4. The
results
below show an increasing amount of unadhered when powder addition was delayed.
TABLE 4
Powder addition time Unadhered residual PF powder
1 min 1.04g
2 min 2.01 g
3 min 2.96g
4 min 2.98g
Ingredients:
1,000 grams raw sand
7.0 grams of OWR-262E liquid resole
20.0 grams of FD-900-A powdered novolak resin
EXAMPLE 5
[0220] An additional coating test was performed to determine the
effects of
reversing the process, namely, by adding the powder to the sand substrate
before the liquid
resole. The phenol formaldehyde powder was added at 15 seconds followed by the
liquid
resole addition at 60 seconds with the same cycle time of 300 seconds. The
material
- 63 -
DOCSMTL 4070637\1
CA 02718659 2015-07-06
separated into three phases: the aggregates of resole plus sand; the unadhered
powder; and
the poorly, partially coated substrate.
[0221] FIG. 9 (with 12X magnification) shows aggregates (clusters) of
resole and
sand resulting from sifting a sample of the poorly partially coated substrate.
FIG. 9 shows
a number of aggregates containing a high amount of resin. This sample was
tested for loss
on ignition (LOI) and found to have an LOT of 19.1 wt. %. This indicated the
liquid resin
was not effective to coat the particles in a homogeneous layer. Rather than
coat the
substrate particles, the resole resin concentrated as aggregates with a few
grains and
powdered resin.
[0222] FIG. 10 (with 30X magnification) shows another sample of the poorly
partially coated substrate that was recovered "as is and not sifted. FIG. 10
shows a large
amount of powder not adhered to the substrate. This sample was tested for LOT
and found
to have an LOT of 1.99 wt. %. The presence of large amounts of unadhered
powder
indicates the inability to reach a homogeneous dry mix of powder and substrate
in advance
of the liquid resin addition. Once the liquid resin is added, it will only
ball-up as the
aggregates of FIG. 9, creating a situation where the substrate surface is not
adhesive to the
powder remaining.
EXAMPLE 6
[0223] This example illustrates the effect of a resin "surface
treatment" applied to
the curable coated proppant. A curable resin coating is applied to a sand
substrate by first
applying 0.75 grams of a coupling agent (A-1100) to a 1000 gram sample of sand
while
applying constant agitation. A liquid resole (OWR-262E) at the concentration
of 3 grams
is then added to the sand/coupling agent mixture (starting 15 seconds after
the addition of
the coupling agent. At the one minute mark (45 seconds after the addition of
the coupling
agent), 16 grams of the powdered novolak SD-536C (containing 10%
hexamethylenetetramine) is added to the mixture. Both of the aforementioned
resins are
available from Hexion Specialty Chemicals, Inc., Louisville, Kentucky. Thirty
seconds
TM
after the addition of the powdered resin, a silicone lubricant (XIAMETER PMX-
200) is
added to the mixture. Thirty seconds after the addition of the silicone, the
first component
TM
of the "surface treatment" EPON 815 (available from Hexion Specialty
Chemicals, Inc.,
- 64 -
CA 02718659 2015-07-06
Houston, Texas) at a concentration of 1 gram is applied to the coated sand.
Thirty seconds
after the addition first component of the surface treatment (150 seconds after
the addition
of the first component), 0.12 grams of tetrafluoroboric acid is added, as a
curing agent, to
crosslink and complete the surface treatment. Forty seconds after the addition
of the acid,
1 gram of a solid surfactant is added. At the 200 second mark (ten seconds
after the
addition of the dry surfactant), the coated sand exists the mixer. This coated
product is
designated as Sample A. Sample B is prepared with the same ingredients and
timing
TM
sequence but without the addition of the surface treatment components (EPON
815 and
tetrafluoroboric acid). Although the surface treatment additives were not
included, the
total mixing time was the same. To demonstrate the effect of the surface
treatment (as
related to the storage properties of the coated product), a caking test was
performed at 150
F and 75% humidity. Sample A (coated sand with surface treatment) was
determined to
have a "caking value" of 1(denoting a sample with soft clumps). Sample B
(subjected to
the same test conditions) gave a caking value of 4 (denoting clumps that
resisted breaking
up when pressure is applied).
EXAMPLE 7
This example illustrates the effect of using a high melt point reactive powder
resin
to improve storage stability (reduce "caking tendencies" in hot/humid
conditions). A
curable resin coating is applied to a sand substrate by first applying 0.75
grams of a
coupling agent (A-1100) to a 1000 gram sample of sand while applying constant
agitation.
A liquid resole (OWR-262E) at the concentration of 3 grams is then added to
the
sand/coupling agent mixture (starting 15 seconds after the addition of the
coupling agent).
At the one minute mark (45 seconds after the addition of the coupling agent),
16 grams of
the powdered novolak PD-6564 (containing 5% hexamethylenetetramine) is added
to the
mixture. The PD-6564 powder has a softening onset point (as measured by a
thermal
mechanical analyzer) of 222 F. Both of the aforementioned resins are
available from
Hexion Specialty Chemicals, Inc., Louisville, Kentucky. Thirty seconds after
the addition
TM
of the powdered resin, a silicone lubricant (XIAMETER PMX-200) is added to the
mixture. One hundred seconds after the addition of the silicone lubricant, 1
gram of a
solid surfactant is added. At the 200 second mark (ten seconds after the
addition of the
dry surfactant), the coated sand exists the mixer. This coated product is
designated as
- 65 -
CA 02718659 2015-07-06
Sample C. Sample B (with no surface treatment) is prepared with the
ingredients described
in Example 6 and the same timing sequence. To demonstrate the effect of the
high melt
point powder (as related to the storage properties of the coated product), a
caking test was
performed at 150 F and 75% humidity. Sample C (coated sand with high melt
point
resin) was determined to have a "caking value" of 0 (denoting a free flowing
sample).
Sample B (subjected to the same test conditions) gave a caking value of 4
(denoting
clumps that resisted breaking up when pressure is applied).
[0224] While the invention has been described with reference to
exemplary
embodiments, it will be understood by those skilled in the art that various
changes may be
made. The scope of the claims should not be limited by the preferred
embodiments set
forth in the examples, but should be given the broadest interpretation
consistent with the
description as a whole.
- 66 -