Note: Descriptions are shown in the official language in which they were submitted.
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
TITLE OF THE INVENTION
Method, apparatus and kits for forming structural members within the cardiac
venous system
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Patent Application
Serial
No. 61/123,700, filed April 10, 2008, which hereby is incorporated herein in
its
entirety by reference thereto.
BACKGROUND OF THE INVENTION
[001] Field of the Invention
[002] The present invention relates to treatment of cardiac conditions in
living beings, and more particularly to forming structural members within the
cardiac venous system for the treatment of cardiac conditions in living
beings.
[003] Description of Related Art
[004] Cardiovascular disease ("CVD") is the leading cause of death in
the United States; see, e.g., C. Lenfant, Fixing the Failing Heart,
Circulation,.
Vol. 95, 1997, pages 771-772; American Heart Association, Heart and Stroke
Statistical Update, 2001; C. Lenfant, Cardiovascular Research: An NIH
Perspective, Cardiovasc. Surg., Vol. 5, 1997; pages 4-5; J.N. Cohn et al.,
Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel
on Heart Failure Research, Circulation, Vol. 95, 1997, pages 766-770.
[005] Heart failure ("HF") is generally defined as a change in the
pumping function of the heart accompanied by typical signs or symptoms.
Heart failure is a progressive disorder whereby the hemodynamic and
symptomatic states of the patient worsen over time despite the absence of
-1-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
clinically apparent adverse events. The symptomatic deterioration is often
accompanied by progressive left ventricular ("LV") chamber remodeling.
[006] Preventing or reversing remodeling has emerged as desirable in
the treatment of cardiomyopathy. Cardiomyopathy is a general term for
disease of heart muscle regardless of the underlying etiology, which may be,
for example, ischemic, hypertensive, dilated, hypertrophic, infiltrative,
restrictive, viral, postpartum, valvular, or idiopathic. Cardomyopathy
typically
results in heart failure.
[007] At the present time, the most effective treatment for patients in
end-stage heart failure is heart transplantation. However, given the chronic
shortage of donor hearts, alternate strategies are needed to improve the lives
of those with heart failure. Moreover, transplantation is not the most
suitable
treatment option for patients with milder forms of the disease. Other
treatment
approaches include the delivery of drugs to the site of action through the
bloodstream, and the injection of cells into ischemic myocardium to improve
cardiac function. An example of an approach for treating cardiovascular
problems with intramyocardial scaffolding is disclosed in United States Patent
Application Publication No. 2005/0271631, published December 8, 2005 in the
name of Lee et al. and entitled "Material compositions and related systems and
methods for treating cardiac conditions." Tissue engineering approaches for
cardiac therapy that are generally intended to repair lost or damaged tissue
through the use of cellular transplantation and biomaterial scaffolds have
also
been disclosed. One example of this approach involves suturing fetal
cardiomyocyte-seeded alginate gels to the epicardial surface in order to
preserve LV function. Another treatment approach involves the use of
mechanical external constraints to limit, stop, or even reverse negative left
ventricular remodeling. One previously disclosed study included suturing a
polymeric mesh to the epicardial surface for the intended purpose of providing
an external support to prevent LV dilation and deterioration of LV function
post-
MI. See Kelley ST, Malekan R, Gorman JH 3rd et al., Restraining infarct
expansion preserves left ventricle geometry and function after acute
-2-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
anteroapical infarction, Circulation 1999; 99:135-42. Another previously
disclosed device that has been investigated provides a plurality of
sutures.that
are implanted in an open-chest procedure across the ventricle under tension to
provide a change in the ventricle shape and a decrease in chamber diameter.
This trans-cavitary suture network is intended to decrease the radius of the
ventricle to thus reduce ventricular wall stress. Another previously disclosed
device under clinical investigation is generally a mesh structure that is
implanted as a jacket around the heart and adjusted to provide a snug fit
during
open-chest surgery. It is intended that the jacket restrains the heart from
further
enlargement. See, for example, Hani N. Sabbah, Reversal of Chronic
Molecular and Cellular Abnormalities Due to Heart Failure by Passive
Mechanical Ventricular Containment, Circ. Res., Vol. 93, 2003, pages 1095-
1101; Sharad Rastogi et al., Reversal of Maladaptive Gene Program in Left
Ventricular Myocardium of Dogs with Heart Failure Following Long-Term
Therapy with the Acorn Cardiac Support Devide, Heart Failure Reviews, Vol.
10, 2005, pages 157-163. Still another approach being investigated provides a
nitinol mesh as a similar external restraining device to that described above;
however, the super-elastic system is intended to assist in systolic
contraction,
and is generally intended for use via thorascopically guided minimally
invasive
delivery. Still another system being investigated includes a rigid ring that
is
implanted during open-chest surgery as another external constraining device to
the ventricle. This ring is intended to decrease ventricular wall stress and
prevent further enlargement of the heart by reducing the radius and modifying
the shape of the ventricle. Examples of devices and methods similar to one or
more of those discussed above have been disclosed by various companies,
including the following: "Acorn;" "Myocor;" "Paracor;" "Cardioclasp;" and
"Hearten." The Cardioclasp device is disclosed in an article by Abul Kashem et
al., CardioClasp: A New Passive Device to Re-Shape Cardiac Enlargement,
ASAIO Journal, 2002.
[008] Myocardial infarction ("MI") is a medical emergency in which
some of the heart's blood supply is suddenly and severely reduced or cut off,
causing the myocardium to die because it is deprived of its oxygen supply. A
-3-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
myocardial infarction may progressively advance into heart failure. Scar
tissue
formation and aneurismal thinning of the infarct region often occur in
patients
who survive myocardial infarctions. It is believed that the death of
cardiomyocytes results in negative left ventricular (LV) remodeling which
leads
to increased wall stress in the remaining viable myocardium. This process
results in a sequence of molecular, cellular, and physiological responses
which
lead to LV dilation. Negative LV remodeling is generally considered an
independent contributor to the progression of heart failure.
[009] Mitral. regurgitation ("MR") is incompetency of the mitral valve
causing flow from the left ventricle (LV) into the left atrium during systole.
Common causes include mitral valve prolapse, ischemic papillary muscle
dysfunction, rheumatic fever, and annular dilation secondary to LV systolic
dysfunction and dilation.
[010] Despite advances in the treatment of heart failure, aneurismal
thinning and mitral regurgitation, further improvement in the speed of
treatment
and reduction of the complexity and intrusiveness of treatment techniques and
devices is desirable. Generally, improved treatment techniques and devices
are desirable for the treatment of all forms of cardiomyopathy, including
early
forms of the disease.
BRIEF SUMMARY OF THE INVENTION
[011] Each of the various embodiments of the present inventions
overcome one or more of the needs and shortcomings discussed above.
Additional improvements and advantages may be recognized by those of
ordinary skill in the art upon study of the present disclosure.
[012] One embodiment of the invention is an apparatus to form
structural members in the cardiac venous system in order to reinforce the
myocardium are provided. In various aspects the apparatus may include a
catheter tube. The catheter tube defines a distal end, a proximal end, an
outer
-4-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
surface, and an inner surface, and the inner surface defines a lumen. The
apparatus may include a barrier. In various aspects, the barrier is disposed
generally about the distal end of the catheter tube. The barrier is
transformable
between a collapsed position and an expanded position. The barrier in the
collapsed position is deliverable into the vein segment and the barrier in the
expanded positions occludes the vein segment. The barrier cooperates with the
catheter tube to allow occluding agent to be delivered through the lumen into
portions of the vein segment distal of the barrier.
[013] Another embodiment of the invention is a catheter for establishing
an occlusion within a cardiac vein, comprising an elongated catheter body
having a distal end and a proximal end and comprising an injectate lumen
extending longitudinally'within the catheter body; a barrier disposed about a
periphery of the catheter body in proximity to the distal end thereof and
about
the injectate lumen; the barrier being controllably transformable between a
collapsed position for movement of the catheter body within the cardiac vein,
and an expanded position for occluding the cardiac vein in cooperation with
the
catheter body; and an injectate port disposed in-the catheter body distally of
the
barrier, the injectate lumen being in fluid communication with the injectate
port.
[014] Another embodiment of the invention is a catheter for establishing
an occlusion within a cardiac vein, comprising means for advancing a distal
end
of a catheter to a site within the cardiac vein; means for establishing a
first
venous occlusion about the catheter near a distal end thereof to occlude the
cardiac vein at the site, in cooperation with the catheter; means for
introducing
an occluding agent into the cardiac vein at the site to form at the site a
second
venous occlusion generally contiguous to the first venous occlusion; and
means for withdrawing the distal end of the catheter from the site following
the
occluding agent introducing step.
[015] Another embodiment of the invention is a method for forming
structural members in the cardiac venous system, which includes occluding a
vein segment by transforming a barrier disposed about the distal end of a
-5-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
catheter tube from a collapsed position into an expanded position at a vein
segment proximal end of the vein segment, and delivering a occluding agent
through a lumen defined by the catheter tube into the vein segment distal of
the
barrier.
[016] Another embodiment of the invention is a method for establishing
an occlusion within a segment of a cardiac vein, comprising advancing a distal
end of a catheter to a site within the cardiac vein; establishing a first
venous
occlusion about the catheter in proximity to a distal end thereof to occlude
the
cardiac vein at the site, in cooperation with the catheter; introducing an
occluding agent into the cardiac vein at the site to form at the site a second
venous occlusion generally contiguous to the first venous occlusion; and
withdrawing the distal end of the catheter from the site following the
occluding
agent introducing step.
[017] Another embodiment of the invention is a method for establishing
an occlusion within a cardiac vein, comprising positioning a distal end of a
catheter outer tube within the vein; expanding a barrier disposed about the
catheter outer tube near the distal end thereof for occluding the vein at a
first
location with an expanded barrier, in cooperation with the catheter;
positioning
a distal end of a catheter inner tube within the vein and spaced apart from
the
barrier, the distal end of the catheter inner tube having an occluder coupled
thereto; expanding the occluder within the vein for occluding the vein with an
expanded occluder at a second location spaced-away from the first location;
introducing occluding agent from the catheter into the vein between the
expanded barrier at the first location and the expanded occluder at the second
location; releasing the expanded occluder from the distal end of the inner
tube;
collapsing the barrier; and withdrawing the catheter from the vein.
[018] Another embodiment of the invention is a heart prosthetic for
treating a heart in a diseased condition, comprising a first occlusion of a
first
composition disposed within a part of a cardiac vein; and a second occlusion
of
a second composition different than the first composition disposed within a
part
-6-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
of the cardiac vein contiguous to the first occlusion; the first and second
occlusions being essentially in a solid state for provide structural support
to the
heart.
[019] Another embodiment of the invention is a kit which, in various
aspects, includes an occluding agent and a catheter. The occluding agent
solidifies from the liquid state to a solid state within a vein segment to
form at
least a portion of a structural member. The catheter includes a catheter tube
and a barrier. The catheter tube has a proximal end and a distal end and
defines a lumen. The barrier is disposed about the distal end and the barrier
is
transformable between a collapsed position and an expanded position. The
catheter is positionable within a vein segment, and the catheter is configured
to
deliver the occluding agent into portions of the vein segment distal of the
barrier.
[020] Another embodiment of the invention is a kit comprising a source
of an injectable occluding agent; and a catheter. The catheter comprises an
elongated catheter body, a coupler, and an injection port. The catheter body
has a distal end and a proximal end and comprising an injectate lumen
extending longitudinally within the catheter body, and a barrier disposed
about
a periphery of the catheter body in proximity to the distal end thereof and
about
the injectate lumen, the barrier being controllably transformable between a
collapsed position for movement of the catheter body within the cardiac vein,
and an expanded position for occluding the cardiac vein in cooperation with
the
catheter body. The coupler is in fluid communication with the injectate lumen,
the occluding agent source being adapted for coupling to the first coupler.
The
injectate port is disposed in the catheter body distally of the barrier, the
injectate lumen being in fluid communication with the injectate port.
[021] Other features and advantages of the inventions will become
apparent from the following detailed description and from the claims.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
-7-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[022] Figure 1 illustrates in perspective view an exemplary
implementation of a catheter.
[023] Figure 2A illustrates in perspective view an exploded distal
portion of the catheter of Figure 1 in a first operating condition.
[024] Figure 2B illustrates in perspective view an exploded distal
portion of the catheter of Figure 1 in a second operating condition.
[025] Figure 2C illustrates in perspective view an exploded distal
portion of the catheter of Figure 1 in a third operating condition.
[026] Figure 2D illustrates in a cross-sectional view a distal portion of
the catheter of Figure 1.
[027] Figure 2E illustrates in a cross-sectional view another distal
portion of the catheter of Figure 1.
[028] Figure 3 illustrates in a cross-sectional view a distal portion of the
catheter of Figure 1.
[029] Figure 4A is a schematic illustration of a catheter in a first state of
deployment.
[030] . Figure 4B is a schematic illustration of a catheter in a second
state of deployment.
[031] Figure 4C is a schematic illustration of a catheter in a third state
of deployment.
[032] Figure 4D is a schematic illustration of a catheter in a fourth state
of deployment.
-8-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[033] Figure 4E is a schematic illustration of a catheter in a fifth state of
deployment.
[034] Figure 4F is.a schematic illustration of a catheter in a sixth state
of deployment.
[035] Figure 4G is a schematic illustration of a catheter in a seventh
state of deployment.
[036] Figure 4H is a schematic illustration of a catheter in a eighth state
of deployment.
[037] Figure 41 is a schematic illustration of a catheter in a ninth state of
deployment.
[038] Figure 4J is a schematic illustration of a catheter in a tenth state
of deployment.
[039] Figure 5 illustrates in a cross-sectional view one example of a
structural member that resides in a vein.
[040] Figure 6 illustrates in a cross-sectional view another example of a
structural member that resides in a vein.
[041] Figure 7 illustrates in a cross-sectional view yet another example
of a structural member that resides in a vein.
[042] Figure 8A illustrates in a cross-sectional view a distal portion of
an exemplary catheter having a barrier in a collapsed position.
[043] Figure 8B illustrates in a cross-sectional view the catheter of
Figure 1 OA with the barrier in an expanded position.
-9-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[044] Figure 9A illustrates in perspective view a distal portion of an
exemplary embodiment of a catheter in a first operating condition.
[045] Figure 9B illustrates in perspective view the catheter of Figure 9B
in a second operating condition.
[046] The Figures are to facilitate explanation of the present invention.
The number, position, relationship and dimensions of the parts shown in the
Figures to form the various implementations described herein, as well as
dimensions and dimensional proportions to conform to specific force, weight,
strength, flow and similar requirements, are explained herein or are
understandable to a person of ordinary skill in the art upon reading this
patent.
Where used in various Figures, the same numerals designate the same or
similar parts. Furthermore, when the terms "top," "bottom," "right," "left,"
"forward," "rear," "first," "second," "inside," "outside," and similar terms
are
used, the terms should be understood in reference to the orientation of the
structures shown in the drawings and utilized to facilitate understanding.
Similarly, when the terms "proximal," "distal," and similar positional terms
are
used, the terms should be understood in reference to the structures shown in
the drawings and utilized to facilitate understanding.
DETAILED DESCRIPTION OF THE INVENTION, INCLUDING THE BEST
MODE
[047] ,Occluding agent may be delivered into one or more selected
sections of the cardiac venous system by a catheter to form one or more
structural members configured to reinforce the myocardium in order to prevent,
moderate, stop, or reverse negative cardiac remodeling due to various adverse
cardiac conditions, both acute and chronic. The cardiac conditions that may be
treated using the apparatus and methods described herein may include
cardiomyopathy, myocardial infarctions, acute myocardial infarctions,
-10-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
arrhythmias, valvular insufficiency, congestive heart failure, mitral
regurgitation
and other heart valve abnormalities, other cardiac complications, and
combinations thereof. Kits for treating the.cardiac conditions using the
methods described herein are also contemplated.
[048] The myocardium is composed of interlacing bundles of cardiac
muscle fibers arranged spirally around the circumference of the heart. These
cardiac muscle fibers receive blood through coronary circulation. The coronary
arteries branch from the aorta just beyond the aortic valve to supply blood to
the cardiac muscle fibers, and the coronary veins empty into the right atrium
via
the coronary sinus. The myocardium is well supplied with a highly distributed
system of coronary arteries and veins. Typically, each coronary artery as it
courses along the surface of the heart-has coronary veins that course
generally
alongside. This is also generally true of the smaller branches of the main
coronary arteries, including those that penetrate into the myocardium and
perfuse the deeper layers of the muscle of the heart. Small veins such as
venules return the blood to larger cardiac veins. Thus the venous system
network of the heart is distributed throughout the thickness of the heart
muscle
and is present everywhere arteries are present.
[049] Material may be implanted or injected into cardiac veins as
discrete masses at various sites in the cardiac venous system, where it
occludes the vein at the site of injection but also disperses in the vein and
also
into venules and possibly even the capillaries in fluid communication with the
site of injection in order to reinforce the myocardium for the purpose of
preventing, moderating, stopping or reversing negative cardiac remodeling due
to various adverse cardiac conditions, both acute and chronic, or for the
purpose of treating localize anomalies of the heart, or for both purposes.
Cardiac conditions that may be treated using such techniques include
cardiomyopathy, myocardial infarctions, acute myocardial infarctions,
arrhythmias, valvular insufficiency, congestive heart failure, Mitral
regurgitation
and other heart valve abnormalities, and other cardiac complications. Kits for
treating the cardiac conditions using the techniques described herein are also
-11-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
contemplated. Exemplary techniques are described in US Patent Application
Publication No. US 2008/0269720, published October 30, 2008 (Sabbah,
"Cardiac Repair, Resizing and Reshaping Using the Venous System of the
Heart"), which hereby is incorporated herein in its entirety by reference
thereto.
[050] Figure 1 shows a catheter 10 that is particularly advantageous for
delivering occluding agent into the venous system of the heart. In various
aspects, occluding agent is delivered into various vein segments by the
catheter 10 to form structural members. The vein segments, in various
aspects, may include a major part of a cardiac vein, or may include small
portions of a cardiac vein such as a distal portion, a proximal portion, or a
medial portion. In various aspects, occluding agent may be disposed in a
series of segments within a cardiac vein, thereby forming a series of
structural
members within the particular cardiac vein. The occluding agent, in various
aspects, may be disposed in vein segments within several cardiac veins and
may be disposed within several segments of the several cardiac veins.
[051] Although one or more vein segments of the cardiac venous
system are occluded by the structural members formed from the occluding
agent, occlusion of the vein segments is not adverse to treatment. Veins have
much thinner walls with less smooth muscle than arteries. Relative to
arteries,
veins have very little elasticity because venous connective tissue contains
considerably more collagen fibers than elastin fibers. Moreover, venous
smooth muscle has little inherent myogenic tone. Accordingly, veins are highly
distensible and have little elastic recoil, so that non-occluded veins in
proximity
to occluded veins can easily distend to accommodate additional volumes of
blood diverted from the occluded vein segment 400 with only a small increase
in venous pressure.
[052] The occluding agent may be delivered into vein segments of the
cardiac venous system by the catheter 10 to form structural members
configured to treat a localized heart anomaly, the heart generally, the
ventricle(s), or the atria. Where a generalized treatment is desired, mapping
-12-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
need not be performed to select the sections into which the occluding agent is
delivered. A generalized approach is particularly applicable to the
ventricles.
Where a localized treatment is desired, the site of the heart disorder such as
a
myocardial infract may be identified, and vein segments of the cardiac venous
system encompassing the localized heart disorder may be selected. Occluding
agent may be delivered by the catheter 10 into the vein segments to form
structural members to reshape and/or remodel the atria, and in particular an
enlarged left atrium, and/or to aid in prevention of atrial fibrillation
and/or other
atria-related conditions. Suitable techniques for identifying various heart
disorders such as thin walled regions or aneurysms requiring treatment may
include MRI, echocaridogram, and other imaging and mapping modalities as
would be recognized by those of ordinary skill in the art upon study of this
disclosure.
[0531 Identifying the vein segments of the cardiac venous system into
which the occluding agent may be delivered to form structural members may be
done empirically. Alternatively, computer-aided selection may be practiced if
desired. In one technique, finite element model simulation is used to model a
region of the heart such as the left ventricle. For example, using an imaging
or
mapping technique, parameters of the patient's left ventricle, including the
location, extent and thickness of damaged wall areas, are measured and added
to the model. The formation of structural members in selected vein segments
of the cardiac venous system may be simulated by changing the transmural
coordinates of epicardial and endocardial mesh nodes in border zone elements
corresponding to the selected segments, along with changing the contractility
of
the elements. The selected vein segments may be changed over successive
simulations to identify an optimal set of vein segments of the cardiac venous
system to receive occluding agent for the formation of structural members. A
suitable finite element model simulation is disclosed in an article by Samuel
T.
Wall et al., Theoretical Impact of the Injection of Material Into the
Myocardium:
A Finite Element Model Simulation, in Circulation AHA 106.657270, November
27, 2006, which hereby is incorporated herein in its entirety by reference
thereto.
-13-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[054] While some cardiac conditions may be treated in one procedure,
other cardiac conditions may be treated by successive deliveries of the
occluding agent into the cardiac venous system over time. For example, in
some aspects, the occluding agent may be delivered into one or more vein
segments of the cardiac venous system to form structural member(s), and the
effect studied before delivery of the occluding agent into additional vein
segment(s) of the cardiac venous system to form additional structural
member(s). In various aspects, the delivery of the occluding agent into one or
more vein segment(s) of the cardiac venous system to form structural
member(s) may be configured to fine tune the beneficial results of prior
deliveries.
[055] The occluding agent 100 (see Figures 4E-4J and 5-7) is suitable
for forming a rigid or semi-rigid structural member 500 (see Figures 4J and 5-
7)
within sections of the cardiac venous system to support the wall of the heart
in
such a way as to prevent, moderate, stop or reverse negative cardiac
remodeling. A suitable occluding agent 100 is one that may be delivered in a
low viscosity liquid state into one or more vein segments 400 of the cardiac
venous system, and that would solidify into a rigid or semi-rigid solid state
(includiing gel) to support the wall of the heart. Accordingly, the occluding
agent
100 is transformable between at least two states, a liquid state and a solid
state, and may solidify from the liquid state into the solid state. In the
liquid
state, the occluding agent 100 may pass through the lumen 15 of the catheter
into the vein segment 400 and may flow throughout the vein segment 400 to
occupy the vein segment 400. The occluding agent 100 may solidify from the
liquid state into the solid state, and, in the solid state, the occluding
agent 100
may form at least a portion of the structural member 500 to reinforce the
myocardium in order to prevent, moderate, stop, or reverse negative cardiac
remodeling.
[056] Exemplary occluding agent 100 includes natural and synthetic
polymers (any FDA approved polymer for human implantation),. fibrin sealants,
alginates, collagens, sugars, hydrogels, self-assembling peptides, PLGA, PEG,
-14-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
coagulation protein based sealants, hyaluronic acid, alginate and chitosan
hydrogels and beads, alginate material with covalently attached peptides,
alginate beads coated with chitosan material, self-assembling peptide scaffold
hydrogels, and so forth, either alone or in combinations of two or more.
Suitable biopolymer materials are commercially available from a variety of
commercial sources, including the NovaMatrix Unit of FMC Biopolymer
'Corporation, 1735 Market Street, Philadelphia, PA 19103 and Omrix
Biopharmaceuticals, 630 5th Avenue, 22nd Floor, New York, NY 10111. This
list of occluding agent 100 is illustrative, and the occluding agent 100 may
be
essentially any FDA approved material that has a degree of purity, preparation
time, ease of expression (viscosity), reaction rate (cure time), strength
(energy
to failure), compliance, water uptake, burst strength, tissue adherence,
endurance, degradation rate, and so forth, suitable for forming a supportive
structure upon delivery into the selected vein segment 400. The various
properties of the occluding agent 100 such as stiffness, compliance, and
resorption rate may be tailored to the particular condition(s) being treated
and
may be tailored for the size of the vein segment 400 into which the agent is
to
be delivered.
[057] The occluding agent 100 may serve as a platform for delivery of
other therapeutic materials, including living cells (including, for example,
myocytes, fibroblasts, fibrocytes or profibrotic blood' progenitor cells, stem
cells,
and muscle cells), growth factor (including, for example, angiogenic factors
such as VEGF, FGF, and HGF; chemotractants; stem cell derived factor; and
TGF-b), stem cell products, peptides, proteins, genes, chondrocytes, insoluble
molecules, other biologics, and so forth, alone or in combinations of two or
more.
[058] The catheter 10, in various aspects, may be used to deliver the
occluding agent 100 into the vein segment 400 and, in various aspects, may be
configured to prevent entrainment of the occluding agent 100 in the venous
flow and subsequent conveyance of the occluding agent 100 into the right
atrium of the heart. An implementation of the catheter 10 is illustrated in
Figure
-15-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
1. The catheter 10 includes catheter tube 12 with distal end 18 and proximal
end 16. The distal end 18 is configured to be positionable within the vein
segment 400 of the cardiac venous system at which the occluding agent 100 is
to be delivered. A lumen 15 is disposed within the catheter tube 12 and
terminates at an aperture 19 at the distal end 18 to deliver the occluding
agent
100 into the vein segment 400. If a multiple-component occluding agent is
used, multiple lumen may be provided that terminate in respective apertures
or,
if desired, in- a mixing chamber that communicates with an aperture. The
barrier 60 is secured circumferentially about the distal end 18 of the
catheter
tube 12. Radiopaque materials may be disposed proximate the.distal end 18 in
various implementations in order to facilitate navigation of the distal end 18
through the various bodily passages to the vein segment 400 by the physician,
and the distal end 18 is atraumatic to avoid damaging the various bodily
passages including the vein segment 400.
[059] Figure 1 also shows that the proximal end 16 of the catheter tube
12 may terminate at a hub 24. The hub 24 and adjacent portions of the catheter
tube 12 may include a strain relief 26 configured to relieve torsional strain.
The
hub 24 may include features through which,.inter alia, a guide wire may be
inserted and/or extracted and through which fluids including the occluding
agent 100 in the liquid state may be communicated to the one or more lumen
15, and the hub 24 is otherwise generally configured to cooperate with the
proximal end 16 of the catheter tube 12 as would be understood by one of
ordinary skill in the art upon study of this disclosure. For example, the hub
24
may include an inflation port 28 having a coupling, such as a luer-lock type
fitting, for connecting one or more of the lumen 15 defined by the catheter 10
to
a source of fluid. In various implementations, the hub 24 may include a
guidewire port 32. The guidewire port 32 may be in communication with one of
the one or more lumen 15 defined by the catheter 10 to receive the guidewire
over which the catheter 10 may be passed in order to deliver the catheter 10
into position within the cardiac vein of a patient. The guidewire port 32 may
include a hemostatic valve, which allows the guidewire to traverse and slide
-16-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
within the lumen 15*while resisting the flow of blood or other fluids through
the
lumen 15 and guidewire port 32.
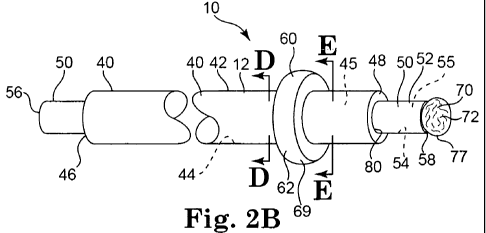
[060] Figures 2A, 2B, 2C, 2D, and 2E illustrate an implementation of
portions of the catheter 10 in various operating conditions. This illustrated
implementation of the catheter 10 includes the catheter tube 12 formed as an
outer tube 40 and an inner tube 50. The outer tube 40 defines an outer tube
outer surface 42 and an outer tube inner surface 44. The outer tube inner
surface 44 defines an outer lumen 45 that passes generally from an outer tube
proximal end 46 to an outer tube distal end 48 to communicate fluid and/or to
communicate the inner tube 50 generally from the outer tube proximal end 46
to the outer tube distal end 48.
[061] The outer tube 40 may be made from a range of materials. For
example, in one implementation, the outer tube 40 may be a metal, such as, for
example, stainless steel or nitinol. In another implementation, the outer tube
40
can be made from one or more polymers such as polyethylene, nylon,.
polyimide, among others. Combinations,of materials such as the above
materials may also be employed, and the material(s) may be varied along the
length of the outer tube 40. The materials and dimensions are generally
selected to provide a desired balance of longitudinal stiffness and torsional
rigidity based on the characteristics of the outer tube 40 to allow the outer
tube
40 to be positioned within the vein segment 400 of the patient.
[062] As illustrated, the barrier 60 extends circumferentially around the
outer tube outer surface 42 generally proximate the outer tube distal end 48
to
block blood flow between the outer tube outer surface 42 and the vein inner
surface 404 (see Figures 4A-4J). The barrier 60, as illustrated, defines a
barrier outer surface 62 and a barrier inner surface 64, and the barrier inner
surface 64 defines a barrier chamber 65 (Figure 3). The barrier 60 may be
constructed of a variety of different materials, including, for example,
Nylon,
PEEK, Pebax, among others. Portions of'the barrier outer surface 62 are
-17-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
engaged with the outer tube outer surface 42 to prohibit the flow of blood
between the barrier outer surface 62 and the outer tube outer surface 42.
[063] The barrier 60 is transformable between at least a collapsed
position 67 as illustrated in. Figure 2A, and an expanded position 69 as
illustrated in Figure 2B. In the illustrated implementation, the outer tube 40
defines a barrier lumen 245 (Figure 3) that extends along at least a portion
of
the outer tube 40 to transmit fluid to/from the barrier 60 in order to
inflate/deflate the barrier. The barrier lumen 245, as illustrated in Figure
2D,
has a crescent shape to maximize the flow cross-section. In other
implementations, the barrier lumen 245 may have a circular cross-section or
other cross-sectional shape, and a plurality of barrier lumen could be
provided
in various implementations.
[064] With the barrier 60 in the collapsed position 67, the outer tube 40
may be positioned within the vein segment 400 such that the outer tube distal
end 48 is generally at the vein segment proximal end 406 (Figure 4J). Fluid
may be communicated via the barrier lumen 245 into the barrier chamber 65
through a barrier port 242 (Figure 3) to expand the barrier 60 into an
expanded
position (expanded position 69 is shown in the illustrated implementation of
Figure 3). In other implementations, the barrier 60 may be mechanically
expanded such as by the application of an electrical current to a shape memory
alloy, or may be otherwise configured to be transformable between at least a
collapsed position 67 and in an expanded position 69 in ways recognizable by
those of ordinary skill in the art upon study of this disclosure.
[065] In the expanded position 69, portions of the barrier outer surface
62 may be generally biased against the venous inner surface to anchor the
outer tube 40 to the venous inner surface 404 and to prevent blood flow from
passing between the venous inner surface 404 and the outer tube outer surface
42 in the proximal direction. With the barrier 60 in the expanded position 69,
fluid may be communicated from the barrier chamber 65 into the outer tube
outer lumen 45 to collapse the barrier 60 into the collapsed position 67 in
order
-18-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
to release the barrier outer surface 62 from the venous inner surface 404 so
that the outer tube 40 may be withdrawn.
[066] In the implementation of the catheter 10 illustrated in Figures 2A
and 2B, an inner tube 50 may be slidably received within the outer lumen 45 of
the outer tube 40. The inner tube 50 is configured to emplace one or more
occluders 70 within the vein segment 400. As illustrated, the inner tube 50
defines an inner tube outer surface 52 and an inner tube inner surface 54. In
this implementation, the inner tube inner surface 54 defines- an inner lumen
55
that passes generally from an inner tube proximal end 56 to an inner tube
distal
end 58 to communicate fluid generally from the inner tube proximal end 56 to
the inner tube distal end 58. The inner tube 50 may be made from a variety of
materials, such as, for example, stainless steel, nitinol, or one or more
polymers such as polyethylene, nylon, polyimide, among others, or
combinations thereof. The materials are generally selected to allow the inner
tube 50 to be advanced and withdrawn through the outer lumen 45 and to
communicate fluid, which may be under pressure, through the inner lumen 55.
[067] As illustrated, portions of the inner tube 50 generally proximate
the inner tube distal end 58 may extend forth from the outer aperture 80
defined by the terminus of the outer lumen 45 at the outer tube distal end 48,
as illustrated in Figure 2B. In various implementations, radiopaque materials
may be disposed proximate the outer tube distal end 48 and/or the inner tube
distal end 58 in order to facilitate manipulation of the outer tube 40 and/or
the
inner tube 50 by the physician. The outer tube distal end 48 and the inner
tube
distal end 58 are'generally configured to be atraumatic in order to avoid
damage to the vein segment and other bodily passages as the outer tube distal
end.48 and the inner tube distal end 58 are navigated into position by the
physician.
[068] The outer tube proximal end 46 and the inner tube proximal end
56 are generally illustrated in Figure 2B. As illustrated, the inner tube
proximal
end 56 extends proximally from the outer tube proximal end 46 so that a
-19-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
physician may grasp portions of the outer tube 40 distal to the outer tube
proximal end 46 and portions of the inner tube 50 distal to the inner tube
proximal end 56 in order to manipulate the outer tube 40 and the inner tube 50
with respect to one another. In various implementations, the outer tube
proximal end 46 and the inner tube proximal end 56 may cooperate with the
hub 24 to allow the outer tube 40 and the inner tube 50 to be manipulated with
respect to one another.
[069] An occluder 70 may be removably disposed upon the inner tube
distal end 58, as illustrated in Figures 2B and 2C. The occluder 70 may be a
balloon having an occluder outer surface 72 and an occluder inner surface 74,
and an occluder chamber 75 may be defined by the occluder inner surface 74
(Figure 3). Many different compliant or semi-compliant material are suitable
for
a balloon occluder, including, for example, nylon, polyamines, ethylene-vinyl
acetate, polyvinyl chloride, olefin copolymers or homopolymers, polyethylenes,
polyurethanes, and various blends. of polymers and copolymers. Many different
materials are suitable for expanding a balloon occluder by filling it,
including, for
example, hydrogels, silicones and epoxy, and various bioabsorbable materials
such as hyaluronic acid injectable fillers, human collagens, and human fibrin
sealant. Alternatively, the occluder 70 may be made of a suitable sponge
material such as collagen that expands automatically when released into the
vein, such materials being known to a person of ordinary skill in the art.
[070] Where the occluder 70 is a balloon-type occluder, it may be
inflatable between at least a contracted position 77 and a dilated position
79,
and the inner lumen 55 may fluidly communicate with the occluder chamber 75
to introduce fluid into the occluder chamber 75 in order to inflate the
occluder
70 from the contracted position 77 into the dilated position 79. With the
occluder 70 in the contracted position 77, the occluder 70 may be advanced
upon the inner tube distal end 58 through the outer lumen 45. The inner tube
distal end 58 may be extended forth from the outer aperture 80 and
manipulated to position the occluder 70 within the vein segment 400, and fluid
may be communicated from the inner lumen 55 into the occluder chamber 75 to
-20-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
inflate the occluder 70 from the contracted position 77 into the dilated
position
79, as illustrated in Figures 2B, 2C, and 3. In some implementations,
occluding
agent 100 may be communicated into the occluder chamber 75 to inflate the
occluder'70 into the dilated position 79. In the dilated position 79
illustrated in
Figure 2C, portions of the occluder outer surface 72 may be generally biased
against the interior venous surface to substantially block blood flow through
the
vein segment 400.
[071] The occluder 70 illustrated in Figures 2B and 2C has a generally
spherical shape in both the contracted position 77 and in the dilated position
79. In other implementations, the occluder 70 may be configured in a variety
of
shapes, and the shapes may vary between the contracted position 77 and the
dilated position.. In other implementations, the occluder may be configured to
spring mechanically from the contracted position 77 into the dilated position,
or
may configured to be alterable between at least the contracted position 77 and
the'dilated position 79 in ways recognizable by those of ordinary skill in the
art
upon study of this disclosure.
[072] As illustrated in Figure 2C, the inner tube 50 may be
disconnected from the occluder 70, which is in the dilated position 79, and
the
inner tube distal end 58 at least partially withdrawn toward the outer tube
distal
end 48. Occluding agent 100 may then be delivered through an inner aperture
90, which is defined by the terminus of the inner lumen 55 at the inner tube
distal end 58. In various implementations, the inner tube 50 may be traversed
between the occluder 70 and the outer tube distal end 48 as the occluding
agent 100 is delivered through the inner aperture 90. In various other
implementations, a plurality of apertures could be disposed generally about
the
inner tube distal end 58 through which the occluding agent 100 could be
delivered from the inner lumen 55 into thevein segment 400. In various other
implementations, the inner tube 50 may be withdrawn entire from the outer
lumen 45 and the occluding agent 100 delivered through, for example, the
outer lumen 45 through the outer aperture 80 and into the vein segment 400.
-21-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[073] Figure 2D illustrates a cross-section of the catheter tube 12
proximal of the barrier 60 that includes the crescent-shaped barrier lumen
245.
The inner tube 50 is disposed within the outer lumen 45 such that the outer
tube inner surface 44 and the inner tube outer surface 52 define an annular
lumen 255 for the communication of fluid.
[074] Figure 2E illustrates a cross-section of the catheter tube 12 distal
of the barrier 60. The crescent-shaped barrier lumen 245 is not present in
this
view, having terminated at the barrier port 247 proximate the barrier (Figure
3).
The annular lumen 255 extends to the distal end 18 of the catheter tube 12 to
communicate fluid through the distal end of the catheter tube 12.
[075] Operation of an implementation of the catheter 10 to deliver the
occluding agent 100 into the vein segment 400 is generally illustrated in
Figures 4A to 4J. As illustrated in Figure 4A, the catheter 10 may be
navigated
through various bodily passages to place the outer tube distal end 48 into
position within the vein segment 400. The barrier 60 is in the collapsed
position
67 in Figure 4A, and blood may flow in the direction indicated between the
outer tube outer surface 42 and the vein inner surface 404.
[076] In Figure 4B, the barrier 60 has been transformed from the
collapsed position 67 into the expanded position 69. Portions of the barrier
outer surface 62 may bias against the vein inner surface 404 to occlude the
vein and to anchor the outer tube 40 within the vein segment 400.
[077] As illustrated in Figure 4C, the inner tube 50 is passed within the
outer lumen 45 and through the outer aperture 80 into the vein segment 400.
An occluder 170 in the contracted position 177 is secured to the inner tube
distal end 58, as illustrated.
[078] As illustrated in Figure 4D, the occluder 170 in an expanded
condition defines an occluder outer surface, an occluder inner surface 174,
and
an occluder chamber 175. The occluder 170 is operable to inflate between at
-22-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
least a contracted position 177 (Figure 4C) and a dilated position 179. The
inner tube 50 may be manipulated to position the occluder 170 within the vein
segment 400. In various implementations, portions of the occluder 170 and/or
portions of the inner tube 50 proximate the inner tube distal end 58 may be
coated with various radiopaque materials or otherwise adapted to facilitate
placement by the physician. When inflated into the dilated position 179, the
occluder 170 generally biases portions of the occluder outer surface against
the
vein inner surface 404 to occlude the vein and to lock the occluder 170 into
the
vein segment 400. In some implementations, a gas may be communicated into
the occluder chamber 175 to inflate the occluder 170. In other
implementations, a liquid such as saline. solution may be communicated into
the occluder chamber 175 to inflate the occluder 170. In other
implementations, the occluding agent 100 may communicated into the occluder
chamber 175 to inflate the occluder 170.
[079] As illustrated in Figures 4C and 4D, the occluder 170 in. the
contracted position 177 is positioned proximate the vein segment distal end
408 of the vein segment 400 and then the occluder 170 is inflated into the
dilated position 179 to engage the vein inner surface 404 in order to
generally
occlude the vein segment 400 at the vein segment distal end 408. Blood may
then be removed from the vein segment 400 between the occluder 170 and the
barrier 60 by being withdrawn through the annular lumen 255 (Figure 2D), as
indicated in Figure 4C. In other implementations (not shown), the inner tube
50
may be detached from the occluder 170 in the dilated position 179 and the
blood may be removed by being withdrawn from the vein segment 400 through
the inner aperture 90 (Figure 2C) and the inner lumen 55. In still other
implementations (not shown), the occluder 170 may be positioned proximate
the outer tube distal end 48, inflated into the dilated position 179 to engage
the
vein inner surface 404, and then pushed to the vein segment distal end 408 to
force blood out of the'vein segment 400 between the barrier 60 and the vein
segment distal end 408. In still other implementations, blood may be permitted
to remain in the vein segment 400 and is displaced and pushed back into the
-23-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
venules as the occluding agent 100 is introduced and flows into the vein and
venules.
[080] In Figure 4E, occluding agent 100 is delivered into the vein
segment 400 through an outer aperture 80 (Figure 2A). The occluder 170 and
the barrier 60 hold the occluding agent 100 within the vein segment 400 while
the occluding agent 100 is admitted into the vein segment 400 through the
outer aperture 80 after passing through the annular lumen 255 (Figure 2E).
The inner tube 50 remains secured to the occluder 170 to lock the occluder 170
into the vein segment 400 in the illustrated implementation. In other
implementations, the inner tube 50 may be detached from the occluder 170,
and the occluding agent 100 passed through the inner lumen 55 into the vein
segment 400 through the inner aperture 90. In still other implementations,
after
the inner tube 50 is detached from the occluder 170, the inner tube 50 is
substantially withdrawn from the outer lumen 45, and the occluding agent 100
is passed into the vein segment 400 through the outer aperture 80. In still
other
implementations, the occluding agent 100 may be passed through another
lumen within the inner tube 50 into the vein segment 400 through one or more
apertures in the wall of the inner tube 50, with the occluder 170 either
attached
or detached. In still other implementations, the separate components of a
multi-
component occluding agent may be introduced using a combination of the
aforementioned techniques.
[081] As illustrated in Figure 4F, the inner tube distal end 58 is
detached from the occluder 170 and withdrawn from the vein segment 400. In
various implementations, the inner tube 50 may be substantially or entirely
withdrawn from the outer lumen 45. The barrier 60 remains in the expanded
position 69 to secure the occluding agent 100 in the-vein segment 400. The
occluding agent 100 may be in the liquid state, may be solidifying into the
solid
state, or may be generally solidified into the solid state.
[082] As illustrated in Figure 4G, the outer tube distal end 48 with the
barrier 60 in the expanded position 69 is withdrawn somewhat in the proximal
-24-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
direction. As illustrated, the occluding agent 100 is at least partly
solidified into
the solid state so that the occluding agent 100 remains positioned in the vein
segment 400.
[083] As illustrated in Figure 4H, the inner tube 50 is passed through
the outer lumen 45 and extends forth from the outer aperture 80 (Figure 2A).
As illustrated, occluder 270 is secured to the inner tube distal end 58
(Figure
2B). The occluder 270 defines an occluder outer surface 272, an occluder
inner surface 274, and an occluder chamber 275. The occluder 270 is
operable to inflate between at least contracted position 277 and dilated
position -
279. The occluder 270 is. illustrated in the contracted position 77 in Figure
4H.
[084] As illustrated in Figure 41, the occluder 270 is inflated into a
dilated position 279 to retain the occluding agent 100 within the vein segment
400. As illustrated, the occluder 270 in the dilated position 279 is
positioned
such that, when dilated, portions of the occluder outer surface 272 are biased
against the occluding agent 100 to compress the occluding agent 100.
Portions of the occluder outer surface 272 are also biased against the vein
inner surface 404 to lock the occluder 270 into place. In some
implementations, a gas may be communicated into the occluder chamber 275
to inflate the occluder 270. In other implementations, a liquid such as saline
solution may be communicated into the occluder chamber 275 to inflate the
occluder 270. In still other implementations, the occluding agent 100 may
communicated into the occluder chamber 275 to inflate the occluder 270.
[085] Figure 4J ,shows the occluding agent 100 interposed between the
occluder 170 and the occluder 270 in the vein segment 400, after the inner
tube
distal end 58 has been detached from the occluder 270, the barrier 60 has
been deflated into a collapsed position 67, and the catheter 10 including the
inner tube 50 and the outer tube 40 has been withdrawn. The occluder 170
and the occluder 270 lock the occluding agent 100 in the solid state into the
vein segment 400 to form the structural member 500. In some
implementations, the occluding agent 100 may adhere to portions of the
-25-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
occluder outer surface 172 and to portions of the occluder outer surface 272
such that the occluder 170, the occluding agent 100, and the occluder 270 form
a generally unitary structural member 500 in the vein segment 400 between the
vein segment proximal end 406 and the vein segment distal end 408. Portions
of the occluder 270 define a structural member proximal end 506 and portions
of the occluder 170 define a structural member distal end 508, as illustrated.
[086] Figure 5 illustrates an implementation of the structural member
500 that includes the occluder 70 in combination with the occluding agent 100.
In this implementation, the occluder 70 defines the structural member distal
end
508 and portions of the occluding agent 100 in the solid state define the
structural member proximal end 506.
[087] Figure 6 illustrates another implementation of the structural
member 500 that includes the occluding agent 100, with the structural member
distal end 508 and the structural member proximal end 506 defined by portions
of the occluding agent 100 in the solid state. In implementations wherein the
occluding agent-100 rapidly solidifies from the liquid state to the solid
state, the
occluder(s) 70 may not be needed to lock the occluding agent 100 into position
within the vein segment 400.
[088] As illustrated in Figure 7, occlusions may be formed from fast-
setting or fast-curing occluding agent, or by materials such as sponges. Where
fast-setting occluding agent is used, for example, the catheter 10 may be -
positioned with the inner tube 50 extended. However, instead of providing the
barrier 60 and the occluder 70, ports may be substituted for dispensing an
occluding agent 101.1 that is designed for rapid solidification from a fluid
state
to a solid state. The rapidly setting occluding agent 101.1 may be dispensed
into the vein segment 400 from the ports to form terminal occlusion 370 and
occlusion 470, and a second occluding agent 100.2 may be delivered into the
vein segment 400 between the terminal occlusions 370 and 470 to form a
primary occlusion 570. The occlusions 370, 470 and 570 in this
implementation are all substantially uniformly solid. In various
-26-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
implementations, different types of occluding agents may be used for the
occluding agents 101.1 and 101.2, and even for forming the occlusions 370
and 470 if desired, with the various types of occluding agent 100 being
selected
to produce specific desired therapeutic effects. The materials used in the
catheter should be selected to minimize bonding between the cured occluding
agents and the catheter, to allow for withdrawal of the catheter. A suitable
rapid-setting occluding agent is CoSeal surgical sealant, which is-available
from Angiotech Pharmaceuticals of Vancouver, British Columbia, Canada, and
Baxter Healthcare Corporation of Fremont, California.
[089] Figures 8A and 8B show a catheter 160 that has an alternative
implementation 162 of the barrier 60. Figure 8A shows the barrier 162
disposed about the distal end of the catheter tube 12 of the catheter 160 in a
collapsed condition. When in a collapsed condition, the barrier 162 may be
positioned in the vein segment 400, as illustrated in Figure 8A. The barrier
162, as illustrated in Figure 8B, may be transformed into an expanded
condition
to bias portions of the barrier outer surface 164 circumferentially against
the
vein inner surface 404. As illustrated in Figure 8B, the barrier 162 in the
expanded position is deployed generally across the vein segment in the form of
a diaphragm to occlude the vein segment 400. A first orifice 36 and a second
orifice 37 are opened in the expanded barrier 162 so that occluding agent 100
in the liquid state may flow through the lumen 15 of the catheter 10, through
the
first orifice 36 and the second orifice 37, and into the vein segment 400
distal of
the barrier 162. When the occluding agent 100 solidifies sufficiently, the
barrier
162, in this illustrated implementation, may be transformed into the
contracted
position and withdrawn with the catheter tube 12 from the vein segment 400. In
other implementations, the barrier 162 may be designed to be released from
the distal end of the catheter tube 12 to remain in the vein segment after the
catheter tube 12 is withdrawn. Illustratively, for this example the barrier
162
may be a sponge. In other implementations, the barrier 162 may be configured
in other ways to be attached to the catheter tube 12 generally proximate the
distal end to occlude the vein segment and to allow the delivery of occluding
agent 100 into the vein segment 400 distal of the barrier 160.
-27-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[090] A suitable catheter 340 for injecting occluding agent into the
cardiac venous system is shown in various operating conditions in FIGS. 9A
and 9B. FIG. 9A shows the catheter 340 in a condition suitable for being
advanced through a cardiac vein, and FIG. 9B shows the catheter 340 in a
deployed condition for defining a segment of a cardiac vein into which the
occluding agent may be introduced. This illustrated implementation of the
catheter 340 includes an outer tube 342 and an inner tube 350. The outer tube
342 defines a lumen 346 for communicating fluid and/or for communicating the
inner tube 350 into the vein. The outer tube 342 and the inner tube 350 may be
made from a variety of materials alone or in combination, including metals
such
as, for example, stainless steel or nitinol, and polymers such as
polyethylene,
nylon, and polyimide, among others.
[091] The catheter 340 includes an expandable barrier 344, shown
collapsed in FIG. 9A and expanded in FIG. 9B. The barrier 344 extends .
circumferentially around the outer tube 342 generally proximate the distal end
thereof to block blood flow through the vein. The barrier 344 may be
constructed of a variety of different materials, including, for example,
Nylon,
PEEK, and Pebax, among others. The outer tube 342 includes a lumen (not
shown) to transmit fluid to/from the barrier 344 in order to inflate/deflate
the
barrier 344. With the barrier 344 in its collapsed position, the catheter 340
may
be moved and positioned as desired within the cardiac venous system. When
the catheter. 340 is properly positioned, the barrier 344 is expanded to
engage
the wall of the vein, thereby stabilizing the distal end of the catheter 340,
blocking blood flow through the vein, and establishing one end of the segment
into which the occluding agent is to be introduced.
[092] The inner tube 350 is slidably received within the lumen 346 for
placing an occluder 360 within the vein in a spaced-apart relationship with
the
barrier 344. The occluder 360, which is removably disposed at the distal end
of
the inner tube 350, is passed through the lumen of the outer tube 342 in a
collapsed condition, is advanced through the vein a desired distance from the
distal end of the outer tube 342, and is expanded to engage the wall of the
vein
-28-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
for establishing the other end of the segment into which the occluding agent
is
to be introduced. Although shown in FIG. 9B as being generally spherical as
deployed, the occluder 360 may be any desired shape.
[093] The inner tube 350 includes two lumen (not shown). One of
these lumen is for transmitting fluid to/from the occluder 360 where the
occluder 360 is designed to be inflatable and releasable, or inflatable and
deflatable, or for containing a wire for mechanically releasing the occluder
360
where the occluder is designed to expand upon release, such as a sponge.
Where the occluder is designed to expand upon release, the release wire and
associated lumen may be eliminated if the release mechanism is triggered by
the pressure of occluding agent injectate in the other lumen. The other lumen
is for communicating fluid with ports 352, 354 and 356 (illustratively three
ports
are shown) on the inner tube 350. The ports 352, 354 and 356 are for
introducing occluding agent into the between the occluder 360 and the barrier
344, and may be used to suction blood from the volume if desired.
[094] To place occluding agent within a desired segment of the cardiac
venous system, the catheter 340 as shown in FIG. 9A is advanced to a desired
position within the cardiac venous system. The barrier 344 is expanded to
stabilize the distal end of the catheter 340, to define one end of the
segment,
and to block the flow of blood to the heart from the segment. The inner tube
350 is advanced a desired distance into the vein from the distal end of the
outer
tube 342, and the occluder 360 is expanded to define the other end of the
segment. Occluding agent is introduced into the segment through the ports
352, 354 and 356. The occluding agent is permitted to cure, and the inner tube
350 is retracted and removed from the catheter 340. The occluder 360 may be
collapsed for withdrawal, or may be'detached prior introduction of occluding
agent, or may automatically detach as retraction of the inner tube 350 begins.
The barrier 344 is collapsed and the catheter 340 is removed from the site.
Optionally, a second occluder similar to the occluder 360 may be placed at the
other end of the segment before the catheter 340 is removed.
-29-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
[095] Radiopaque materials may be disposed proximate the distal end
of the outer tube 342 and/or the distal end of the inner tube 350 to
facilitate or
confirm proper placement-of the distal end of the catheter 340 within the
desired segment of the cardiac venous system.
[096] The proximal ends (not shown) of the outer tube 342 and the
inner tube 350 extend from the proximal end of the catheter 340 and are
connected to a handle (not shown) to allow the a physician to control the
various functions performed by the catheter 340.
[097] Methods for forming a structural member 500 in the vein segment
400 to reinforce the myocardium include delivering the occluding agent 100
into
the vein segment 400, the occluding agent 100 solidifying within the vein
segment 400 thereby forming the structural member 500. Treating a particular
cardiac condition by selecting one or more vein segments 400, delivering the
occluding agent 100 into the one or more vein segments 400 thereby forming
one or more structural members 500 within the one or more vein segments 400
may be included in the methods. The catheter 10 may define one or more
lumen 15 for delivering the occluding agent 100 into the vein segment 400.
The methods, in various implementations, may include positioning at least one
occluder 70 within the vein thereby, occluding. the vein segment 400 and
retaining the occluding agent 100 within the vein segment 400. Positioning at
least one occluder 70 using the catheter 10 may be included in the methods.
The, methods may include disposing the occluding agent 100 within the vein
segment 400 between an occluder 170 and an occluder 270. Various
implementations of the methods may include the occluder 170, the occluding
agent 100, and the occluder 270 defining the structural member 500. Including
a therapeutic material in the occluding agent 100 maybe part of the methods in
various aspects.
[098] Delivering the occluding agent 100 into the vein segment 400 by
a particular implementation of the catheter 10 may proceed in the following
manner. The method may be initiated by placing the outer tube distal end 48
-30-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
within the vein segment 400 by navigating the catheter 10 through various
bodily lumen. Transforming the barrier 60 (Figures 4A-41) from the collapsed
position 67 into the expanded position 69, or the barrier 162 from the
collapsed
position (Figure BA) into the expanded position 169 (Figure 8B), thereby
biasing the barrier outer surface (62, 164) against the vein inner surface
404,
occluding the vein, and anchoring the outer tube 40 into position within the
vein
segment 400 may be part of the methods. The methods may include passing
the inner tube 50 through the outer lumen 45 and extending the inner tube
distal end 58 through the outer aperture 80 into the vein segment 400.
Positioning the occluder 170 within the vein segment 400 by manipulating the
inner tube 50, and biasing portions of the occluder outer surface 172 against
the vein inner surface 404 by inflating the occluder 170 from the contracted
position 177 into the dilated position 179 thereby occluding the vein and
locking
the occluder 170 into position within the vein segment 400 may be included in
the methods.
[099] The methods may include removing the blood from the vein
segment 400. The methods, in various aspects, may include inflating the
occluder 170 from the contracted position 177 into the dilated position 179
and
pushing the occluder 170 in the dilated position distally to push blood
distally
from the vein segment 400. In various aspects, the methods may include
withdrawing blood from the vein segment 400 through annular lumen 255
and/or through inner lumen 55.
[0100] The methods, in various aspects, may include delivering the
occluding agent 100 into the vein segment 400 through the outer aperture 80
via the annular.lumen 255, the occluder 170 and the barrier 60 holding the
occluding agent 100 within the vein segment 400. In other aspects, the
methods may include detaching the inner tube distal end 58 from the occluder
170, and delivering the occluding agent 100 via the inner lumen 55 through the
inner aperture 90 into the vein segment 400. In still other aspects, the
methods
may include detaching the inner tube 50 from the occluder 170, withdrawing the
-31-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
inner tube 50 from the outer lumen 45, and delivering the occluding agent 100
into the vein segment 400 through the outer aperture 80 via the outer lumen
45.
[0101] The methods may include detaching the inner tube distal end 58
from the occluder 170 and withdrawing the inner tube distal end 58 may be
included in the methods, the barrier 60 remaining in the expanded position 69
thereby securing the occluding agent 100 in the vein segment 400. In various
aspects, the inner tube 50 may be substantially or entirely withdrawn from the
outer lumen 45.
[0102] The methods may proceed by withdrawing the outer tube distal
end 48 somewhat in the proximal direction with the barrier 60 in the expanded
position 69. Passing the inner tube 50 through the outer lumen 45 and
extending the inner tube distal end 58 forth from the outer aperture 80 into
the
vein segment 400, and inflating a occluder 270 secured to the inner tube
distal
end 58 thereby retaining the occluding agent 100 between the occluder 170
and.the occluder 270 may be steps in the various methods. Compressing the
occluding agent 100 within the vein segment 400 by dilating the occluder 270
and locking the occluder 270 into position in the vein segment 400 by biasing
portions of the occluder outer surface 272 against the vein inner surface 404
may be part of the methods. The methods may include detaching the inner
tube distal end 58 from the occluder 270, deflating the barrier 60 into the
collapsed position 67, and withdrawing the catheter 10 including the inner
tube
50 and the outer tube 40 thereby locking the occluding agent 100 into the vein
segment 400 by interposing the occluding agent 100 between the occluder 170
and the occluder 270.
[0103] In a variation of the catheters 10 and 340 and the methods of
operating them, the inner tubes 50 and 350 may be fixed or have very limited
slidable motion relative to the outer tubes 40 and 342. With reference to the
catheter 340 (Figures 9A and 9B), for example, the distal end of the inner
tube
350 may be at or project from the distal end of the outer tube 342 even when
the catheter 340 is configured for being advanced through a cardiac vein. If
-32-
CA 02720047 2010-09-29
WO 2009/126314 PCT/US2009/002258
projecting from the distal end of the outer tube 342, the amount of projection
may be small, or may be on the order of the intended length of the vein
segment to be occluded. The expandable barrier 344 and the occluder 360 are
collapsed while the catheter 340 is being advanced through the cardiac vein.
When the catheter 340 is properly positioned, the barrier 344 is expanded to
engage the wall of the vein, thereby stabilizing the distal end of the
catheter
340, blocking blood flow through the vein, and establishing one end of the
segment into which the occluding agent is to be introduced. If the inner tube
350 is in this variation slidable with respect to the outer tube 342, it may
be
extended or retracted as needed to establish the proper segment length before
the occluder 360 is expanded. If the inner tube 350 is in this variation fixed
with
respect to the outer tube 342, the occluder 360 is expanded, which may but
need not be done concurrently with expansion of the barrier 344. In either
case,
expanding the occluder 360 establishes the other end of the segment into
which the occluding agent is to be introduced. To complete the occlusion, the
occluding agent is introduced, the inner tube 350 is detached from the
occluder
360 and either left projecting from the distal end of the outer tube 342 or
drawn
into the outer tube 342 either completely or partially, the barrier 344 is
collapsed, and the catheter 340 is removed.
[0104] The various exemplary implementations described herein are
illustrative of the invention. Variations and modifications of these
implementations are possible, and practical alternatives to and equivalents of
the various elements of the embodiments are contemplated. These and other
variations and modifications of the implementations disclosed herein may be
made without departing from the scope and spirit of the invention, as set
forth
in the following claims.
-33-