Note: Descriptions are shown in the official language in which they were submitted.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 1 -
Atherectomy Devices and Methods
Related Applications
This application claims the benefit of United
States ,Provisional Patent Application Serial No.
61/043,998, filed April 10, 2008, and entitled
"Atherectomy Devices and Methods," which is incorporated
herein by reference.
This application is also a continuation- in-
part of co-pending United States Patent Application
Serial No. 12/288,593, filed October 22, 2008, and
entitled "Atherectomy Devices and Methods," which claims
the benefit of United States Provisional Patent
Application Serial No. 60/981,735, filed October 22,
2007, and entitled "Atherectomy Devices and Methods,"
which are incorporated herein by reference.
Field of the Invention
The devices and methods described below
generally relate to treatment of occluded body lumens.
In particular, the present devices and method relate to
improved devices for removal of the occluding material
from the blood vessels as well as other body parts. Such
devices include features for improved positioning within
the vessel or body part allowing for the targeted removal
of tissue or sweeping of a cutting mechanism in an arc-
shaped path.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
2 -
Background of the Invention
Atherosclerosis is a progressive disease. In
this disease, lesions of the arteries are formed by
accumulation of plaque and neointimal hyperplasia causing
an obstruction of blood flow. Often plaque is friable
and may dislodge naturally or during an endovascular
procedure, leading to embolization of a downstream
vessel.
Endovascular clearing procedures to reduce or
remove the obstructions to restore luminal diameter
allows for increased blood flow to normal levels are well
known. Removing the plaque has the effect of removing
diseased tissue and helps to reverse the disease.
Maintaining luminal diameter for a period of time
(several to many weeks) allows remodeling of the vessel
from the-previous pathological state to a more normal
state. Finally, it is the goal of an endovascular to
prevent short term complications. such as embolization or
perforation of the vessel and long term complications
such as ischemia from thrombosis or restenosis.
Various treatment modalities may help to
accomplish treatment goals. In atherectomy, plaque is
cut away, or excised. Various configurations are used
including a rotating cylindrical shaver or a fluted
cutter. The devices may include shielding by a housing
for safety. The devices may also remove debris via
trapping the debris in the catheter, in a downstream
filter, or aspirating the debris. In some cases a burr
may be used instead of a cutter, particularly to grind
heavily calcified lesions into very small particle sizes.
Aspiration may also be used with a burr-type atherectomy
device.
Balloon angioplasty is another type of
endovascular procedure. Balloon angioplasty expands and
opens the artery by both displacing the plaque and
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
3 -
compressing it. Balloon angioplasty is known to cause
barotrauma to the vessel from the high pressures required
to compress the plaque. This trauma leads to an
unacceptably high rate of,restenosis. Furthermore, this
procedure may not be efficient for treatment of elastic-
type plaque tissue, where such tissue can spring back to
occlude the lumen.
When clearing such obstructions it is
desirable to protect the vessel wall or wall of the body
lumen being cleared and to debulk substantially all of a
lesion. In additional cases, the procedure that clears
obstructions may also be coupled with placement of an
implant within the lumen. For example, it may be
desirable to deploy a stent to maintain patency of a
vessel for a period of time and/or to achieve local drug
delivery by having the stent elute a drug or other
bioactive substance.
On their own, stents fail to perform well in
the peripheral vasculature for a variety of reasons. A
stent with the necessary structural integrity to supply
sufficient radial force to reopen the artery often does
not perform well in the harsh mechanical environment of
the peripheral vasculature. For example, the peripheral
vasculature encounters a significant amount of
compression, torsion, extension, and bending. Such an
environment may lead to stent failure (strut cracking,
stent crushing, etc.) that eventually compromises the
ability of the stent to maintain lumen diameter over the
long-term. On the other hand, a stent that is able to
withstand the harsh mechanical aspects of the periphery
often will not supply enough radial force to open the
vessel satisfactorily. In many cases, medical
practitioners desire the ability to combine endovascular
clearing procedures with stenting. Such stenting may
occur prior to, after, or both before and after the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 4 -
endovascular clearing procedure.
Accordingly,a need remains for devices that
allow for improved atherectomy devices that are able to
navigate through tortous anatomy and clear materials from
body lumens (such as blood vessels) where the device
includes features to allow for a safe, efficient and
controlled fashion of shaving or grinding material within
the body lumen while minimizing procedure times. In
addition, there remains a need for devices that allow
steering of the distal portion of the device while
navigating through tortuous anatomy. The ability to
steer assists the physician in accessing tortuous anatomy
and can further assist in delivering a guidewire into the
entrance - -of angled or tortuous vessel
bifurcation/segments. This is possible because
variations of the steerable atherectomy catheter
described herein can also function as a `shuttle
catheter', where the physician can aim the distal tip
into the vessel to be accessed and advancing the
guidewire into that vessel from within the catheter.
There also remains a need for devices that are
configured to steer but will remain in a straight
configuration when not being articulated. It is
generally known that conventional catheters that take a
shape often bias to one side either through repeated
articulation or even after being left in packing for any
given period of time. Accordingly, when such steering
features are combined with tissue debulking devices,
there remains a risk of injury if the tissue debulking
device has an undesirable bend when the device is
supposed to be in a straight configuration.
The debulking devices described herein address
the problems noted above as well as provide significant
improved features to allow a physician to steer a
debulking device through tortuous anatomy and remove
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 5 -
tissue at a target site.
Summary of the Invention
Devices and methods described herein provide
debulking devices having improved means of clearing
obstructions within body lumens, especially the
vasculature. In many variations the devices are suited
for navigating through tortuous vessels. The features of
the devices and methods allow for controlled removal of
occlusive materials and navigation through tortuous and
diseased vessels. In some variations, the methods and
devices also have features to convey the materials away
from the operative site without the need to remove the
devices from the body lumen. Additional aspects include
controlled rates of tissue removal as well as other
safety features to prevent accidental cutting of the
lumen wall. Although the devices and methods described
herein discuss removal of materials from a blood vessel,
in certain cases the devices and methods have
applicability in other body parts as well. It should be
noted that the variations and features of the devices
described below may be incorporated selectively or in
combination with a basic device configuration that
includes a flexible body having a cutter, where the
cutter includes a housing and a cutter, where the housing
and cutter are able to rotate relative to each other.
Variations include a cutter that rotates within the
housing, a housing that rotates about the cutter, and
combinations thereof.
One variation of the device described herein
includes a device configured to remove material from body
structures. The device may be a vascular device and have
the required structure and configuration to navigate
tortuous anatomy. Alternatively, the device may be a
cutter that has features that are desired when used in
other parts of the anatomy.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 6 -
In any case, a variation of the device
comprises a catheter body having a proximal end and a
distal end and a catheter lumen extending therethrough, a
cutting assembly including a housing and a rotatable
cutter located within the housing, the cutting assembly
affixed to the distal end of the catheter, where the
housing includes at least one opening and the cutter
includes at least one cutting edge, a sweep frame located
adjacent to the cutting assembly, the sweep frame being
coupled to the catheter and rotatable independently of
the rotatable cutter, where the sweep frame comprises at
least a weakened section on a first radial side such that
compression of the sweep frame causes deflection towards
the first radial side resulting in deflection of the
distal end of the catheter body, and where rotation of
the deflected sweep frame causes the cutting assembly to
move in an arcuate path relative to an axis of a proximal
end of the sweep frame, and a rotatable torque shaft
extending through the catheter lumen and sweep frame and
having a first end coupled to the rotatable cutter and a
second end adapted to couple to a rotating mechanism.
As noted below, the sweep frame can have any
number of configurations. However, the sweep frame shall
allow for bending of the distal portion of the catheter
as well as rotation of the distal portion of the catheter
independently of the torque shaft and rotatable cutter.
In some variations, the sweep frame rotates independently
of the catheter body and in other variations, the sweep
frame rotates with the catheter body. In other
variations, a distal portion of the catheter body rotates
with the sweep frame while a proximal portion of the
catheter body remains stationary. In addition, devices
of the present invention can have any number of sweep
frames located about a length of the catheter body where
each sweep frame allows bending of the associated segment
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
7 -
of the catheter. These sweep frames can bend and be
rotated independently of each other. Alternatively,
bending or rotation of the sweep frames can be linked if
so desired.
The systems of the present invention can
further include a handle coupled to the proximal end,
where the sweep frame is rotatable independently of the
handle. Typically, the sweep frame is actuated by a
sweep member or sweep shaft. The sweep shaft is
fabricated such that it can translate axial force as well
as rotational movement from the handle or proximal end of
the device to the sweep frame.
In some variations, the sweep frame is
configured to limit deflection of the cutting assembly to
a pre-determined distance away from the axis of the
proximal end of the sweep frame at a maximum angle of
deflection. In additional variations, the bending
stiffness and resulting potential apposition force can be
varied with the deflection angle or displacement of the
cutting assembly and with axial position along the sweep
frame.
In additional variations, the weakened section
of the sweep frame comprises a varying column strength
that increases in a circumferential direction away from
the first radial side to prevent radial twisting of the
sweep frame when deflected. Such a configuration is
intended to prevent twisting or torsion of the weakened
section of the sweep frame upon bending. In one
variation, the sweep frame comprises struts to accomplish
such preferential bending towards the first radial side
and increasing column strength away from the first radial
side.
In most variations the sweep frame is located
entirely within the catheter body. However, in
additional variations, the sweep frame may be exposed or
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
8 -
on an exterior of the catheter. In any case, the sweep
frame is coupled to the catheter to permit bending and
steering of the catheter.
The sweep frame structure described herein can
be combined with any number of cutting assemblies as also
described or as known to those skilled in the art.
For example, in a variation, the cutter can
comprise a plurality of fluted cutting edges located on
both a near fluted cutting portion and a far fluted
cutting portion, where the near fluted cutting portion
and the far fluted cutting portion are spaced along an
axis of the cutter and the far fluted cutting portion has
fewer fluted cutting edges than the near fluted cutting
portion, where on rotation of the cutter the fluted
cutting edges remove material from the body lumen.
The cutting assemblies can include a cutting
housing having a plurality of openings along an exterior
surface of the housing. Alternatively the housing can be
a cylindrical housing having an open front face. Such an
open faced housing can either rotate (either with the
rotatable cutter or in an opposite direction) in which
case the housing functions as a cutter. Alternatively,
the open faced housing can remain stationary.
In additional variations of the device, the
cutting assembly can include a dilator member extending
distally from a front of the housing, the dilator member
having a passage extending therethrough and being in
fluid communication with the catheter lumen, where the
dilator member comprises a tapered shape having a smaller
diameter surface at a distal tip and a larger diameter
surface adjacent to the front of the housing, such that
as the dilator member advances through material, the
dilator member dilates material away into the opening in
the housing.
The present invention also includes methods
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
9 -
for debulking occlusive material from within the body.
Such methods may include advancing a catheter having an
elongate member with a debulking assembly affixed to a
distal end of the elongate member within the body lumen,
positioning the debulking assembly adjacent to the
occlusive material in the body lumen, the debulking
assembly having a cutter and a bending frame coupled to a
distal portion of the catheter and proximate to the
debulking assembly, where the bending frame comprises at
least a section having a reduced column strength on a
first radial side of the bending frame, deflecting the
bending frame in a direction of the first radial side by
advancing a sweep member at the proximal end of the
catheter, where deflecting the bending frame causes the
debulking assembly to also deflect in the direction of
the first radial side, rotating a torque shaft extending
through the catheter and coupled to at least the cutter
to debulk the occlusive material, and rotating the sweep
member independently of the torque shaft to rotate the
bending frame and cause the debulking assembly to sweep
in an arcuate path relative to an axis of a proximal end
of the bending frame.
As discussed herein, variations of the novel
devices include one or more sweep frames and/or sweep
tubes to cause deflection of the distal portion (and
other portions) of the debulking device. The sweep frame
improves conventional devices since it allows the
catheter to stay straight when in the straight position.
In other words, the sweep frame prevents the debulking
catheter from developing an undesirable "bend" when the
device is intended to be in a straight position. Such
undesired set bends are common with conventional
steerable catheters. Avoiding the undesirable set bend
reduces the chance that the debulking device creates
unwanted collateral damage to healthy tissue. For
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 10 -
example, a conventional device that assumes a bend
(either after multiple flexing, from an extended time in
packaging, from exposure to heat) can come to rest
against healthy tissue when the physician assumes that
the device is straight. Clearly, activation of the
conventional device in such a circumstance prevents the
physician from limiting the debulking to the target
tissue.
Aside from ease of construction (e.g., a
simple and inexpensive construction) the sweep frame
provides excellent column strength for improved forward
cutting speed in straight and in deflected positions.
This structure was found to prevent a failure mode where
the sheath collapses onto and spiral wraps around a
torque shaft. Moreover, the sweep frame provides
excellent apposition force for better cutting at
diameters larger than the catheter.
In addition, providing a sweep frame that must
be compressed to deflect allows for selectively "tuning"
the construction so that as the bending portion of the
sweep frame reaches the desired maximum desired
deflection, the segments forming the bending portion can
mechanically interfere to prevent further bending.
In another variation, the sweep frames of the
present devices can contain features so that a physician
can determine the orientation of the bend of the device
from a non-invasive imaging means. For example, the
sweep frame or catheter coupled to the sweep frame can
include one or more visualization mark(s) allowing for
non-invasive determination of an orientation and
direction of articulation of the sweep frame. The
visualization mark can be shaped with asymmetry out of
the bending plane that acts as a radiopaque marker
(either a cutout or a protrusion) to show direction of
device tip into/out of fluoroscopy plane when deflected.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 11 -
Marker could also be the addition of a stripe / band /
wire etc of radiopaque material like tantalum, gold,
platinum, etc.
In an additional variation to the method or
device, the sweep member can be locked relative to the
device to prevent the bending frame from further bending
or unbending. It may also independently lock relative to
the device to prevent sweep.
The devices and methods also include
delivering fluid through a fluid port. The fluid may
include a drug or other substance to aid in the
procedure.
In another variation of a method for removing
tissue within a body passage, the method can include
advancing a catheter having debulking assembly affixed to
a distal end of the catheter in the body, positioning.the
debulking assembly adjacent to the tissue in the body,
applying a distal force at a proximal end of the catheter
to deflect a bending frame coupled to distal portion of
the catheter, rotating the bending frame while deflecting
the bending frame to sweep the debulking assembly in an
arcuate path relative to an axis of a proximal end of the
bending frame, rotating a torque shaft extending through
the catheter and coupled to at least the cutter to remove
the tissue, and rotating the sweep shaft independently of
the torque shaft to rotate the bending frame and cause
the debulking assembly to sweep in an arcuate path
relative to an axis of a proximal end of the bending
frame.
Another variation of the method is to deflect
the distal end and advance the catheter to cut in an
axial direction. The axial cut pattern can be repeated at
subsequent radial positions to remove tissue.
Another variation of the method is to position
and deflect a second bending or sweep frame along the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 12 -
catheter body to advance the debulking assembly in the
direction set by the first sweep frame to increase the
reach of the debulking assembly. The second sweep frame
can provide a reaction force to the apposition force of
the cutter approximated against plaque or tissue without
requiring a reaction force from the catheter body
interacting with the vessel wall. The second bending
frame can also be used to allow precise control of the
cutter angle with respect to the tissue to be debulked.
A second sweep shaft can be rotated to sweep the
debulking assembly.
As discussed herein, some variations of the
devices have the ability to articulate. This
articulation allows for steering the device to the target
site as well as creating a sweeping motion of tissue
removal. This ability to steer can be useful when
attempting to navigate a guidewire through tortuous
anatomy. For example, a physician often encounters
resistance when advancing a guidewire through tortuous
anatomy, either due to occlusions within the vessel or
the tortuous nature of the vasculature. When the
physician encounters such resistance, the guidewire can
be withdrawn within or slightly extending from a
debulking catheter. The physician can then steer the
debulking catheter to redirect the -guidewire for
advancement. Once the guidewire is in place, the
physician can then activate the cutting mechanism to
selectively remove tissue.
The devices described herein may have a cutter
assembly having a portion of its housing having a curved
surface and where the opening forms a plane across the
curved surface such that as the cutting surface rotates
across the opening, a portion of the cutting surface
extends out of the housing through the opening. The
cutter assembly may also have various other features as
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 13 -
described below that improve the safety of the device as
it is articulated while cutting. Furthermore the cutter
may have a number of features to impel or drive cut
tissue into the cutter assembly for eventual removal by
one or more conveying members.
As noted, the devices described herein may
have one or more conveying members that convey materials
and/or fluids through the device. Such a feature is
useful to remove cut tissue and debris from the site
during the procedure. In some variations, the device may
include multiple conveyors to deliver fluids and remove
debris. However, the devices of the present invention
may also have containers for use in capturing debris or
other materials generated during the procedure.
Another feature for use with the inventions
herein is the use of a grinding burr rotatably coupled to
a tip of the device. The burr can be useful to remove
tissue that is otherwise not conducive to cutting with
the cutter assembly.
The devices described herein may use a
guidewire for advancement through the body. In such
cases the devices will have guide-wire lumens located
within or about the catheter. Alternatively, a guide-
wire section may be affixed to a portion of the device.
Devices of the present invention typically
include a torque shaft to deliver rotational movement to
components in the cutter assembly. The torque shaft may
include one or more lumens. Alternatively, the torque
shaft may be a solid or hollow member. Variations of the
torque shaft also include those aspects known in
catheter-type devices such as counter-wound coils,
stiffening members, etc. In some variations, the torque
shaft may have the conveying member integrally formed
about the exterior or an interior surface of the shaft.
Alternatively, or in combination, the conveying member
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 14 -
may be placed on (or within) the torque shaft as
described herein.
As noted herein, combinations of aspects of
the devices, systems, and methods described herein may be
combined as needed. Furthermore, combinations of the
devices, systems and methods themselves are within the
scope of the disclosure.
Brief Description of the Drawings
Fig. 1A illustrates an exemplary variation of
a device according to the present invention;
Fig. 1B shows an exploded view of the device
of Fig. 1A;
Fig. 1C shows a cross sectional view of the
cutting assembly;
Fig. 1D shows an exploded view of the cutting
assembly of Fig. 1A;
Figs. 2A shows the cutting edges through
openings of a housing;
Fig. 2B shows a side view of the cutting
assembly;
Fig. 2C illustrates a positive rake angle;
Fig. 3A illustrates a variation having a
dilation-member;
Figs. 3B-3D show conceptually the use of a
debulking device having a dilating member;
Figs. 4A-4B show a variation of a shielded
cutter having a plurality of front cutting surfaces, rear
cutting surfaces, and fluted cutting surfaces;
Figs. 5A-5B show another shielded cutter
having a plurality of front cutting surfaces and fluted
cutting surfaces;
Figs. 6A-6D show a cutter assembly having an
open ended housing;
Fig. 6E shows an exploded view of the cutter
assembly of Fig. 6C;
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 15 -
Fig. 6F shows a cutter assembly with the open
ended housing removing material from a lumen wall;
Figs. 6G-6H shows a respective perspective and
cross sectional side view of a variation of an open ended
cutter housing with an inner bevel;
Fig. 7A shows a tissue debulking device having
a sweep frame in an unflexed position;
Fig. 7B shows the tissue debulking device of
Fig. 7A where the sweep frame is flexed or compressed to
articulate the catheter;
Figs. 7C-7E show additional variations of
sweep members for use with the debulking devices
described herein.
Figs. 7F-7G show additional possible
variations a catheter body or sweep member;
Figs. 7H-7I show variations of a sweep frame
having a visualization feature that permits a physician
to determine orientation and direction of articulation of
the cutting assembly when the device is viewed under non-
invasive imaging;
Fig. 8A shows a variation of a device
configured for rapid exchange;
Fig. 8B illustrates an example of centering a
tip of a cutting assembly over a guide wire;
Fig. 9A shows a conveyor within the-
'catheter--body and sweep frame;
Fig. 9B shows a partial cross sectional view
of a variation of a torque shaft having counter wound
coils;
Fig. 9C shows a second conveyor within a
torque shaft;
Fig. 10A illustrates articulation of a tip of
the device;
Fig. 1OB-10D shows sweeping of the cutting
assembly;
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 16 -
Fig. 11A shows placement of housing windows to
prevent damage to the vessel walls;
Figs. 11B_-11C shows placement of features of
the cutter assembly that prevent damage to the vessel
walls;
Figs. 12A-12B show a control system for
rotating and articulating the cutter assembly;
Fig. 12C shows a cross sectional view of a
portion of the catheter hub mechanism that removes debris
from the device;
Figs. 12D-12F shows a variation of a control
knob having indexing features;
Fig. 13 shows a device with a burr tip;
Figs. 14A-14C provide examples of fluid
delivery systems;
Fig. 15 shows the device placed within a stent
or coil;
Figs. 16A-16B show variations of devices for
removing tissue from body lumens;
Figs. 17A-17F show additional variations for
centering devices within a lumen;
Figs. 18A-18C illustrate use of a debulking
device to assist in the navigation of a guidewire through
tortuous anatomy.
Description of the Preferred Embodiment
Although the disclosure hereof is detailed and
exact to enable those skilled in the art to practice the
invention, the physical embodiments herein disclosed
merely exemplify the invention which may be embodied in
other specific structures. while the preferred
embodiment has been described, the details may be changed
without departing from the invention, which is defined by
the claims.
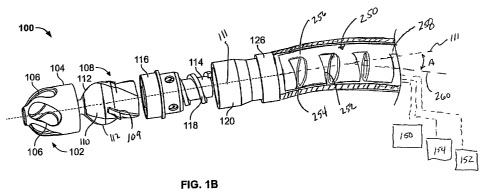
Fig. lA illustrates an exemplary variation of
a device 100 according to the present invention. As
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 17 -
shown the device 100 includes a cutter assembly 102
affixed to a catheter or catheter body 120. The catheter
body 120 can be a reinforced sheath (e.g., a polymeric
material with a braid). It is noted that the cutter
assembly shown in the figures exemplary purposes only.
The scope of this disclosure includes the combination of
the various embodiments, or single elements of various
embodiments, where possible, as well as the combination
of certain aspects of the various embodiments.
Fig. lA shows a variation of a tissue removal
or debulking device 100 where the cutter assembly 102 is
within the housing 104. In this variation, the cutter
assembly contains a first set of cutting edges 112 and a
second set of cutting edges 109, where the first cutting
edges 112 extend along the entire length of the cutting
assembly 102 (i.e., the entire length that is exposed in
the openings 106 of the housing 104). In contrast, the
second set of cutting edges 109 (in the figure only one
such second cutting edge is visible) extend only along a
portion. However, variations of the methods and devices
described herein can include any number of cutter
configurations as described herein or as known by those
skilled in the art. Furthermore, although the
illustrated device shows a plurality of openings 106 in
the housing 104, alternative cutting assemblies can
include a housing having a single opening on a distal
face. Such open faced cutters are shown below.
Fig. 1A also shows the device 100 having a
catheter body 120 extending from a distal portion 122 to
a proximal portion (not shown). As discussed below, the
catheter body 120 can be coupled to a rotating mechanism
or motor 150 that ultimately drives the cutter assembly
102 via a torque shaft 114 as shown in Fig. 1B.
Fig. 1A further illustrates a variation of a
sweep frame 250 located within the catheter body 250.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 18 -
Additional details regarding various sweep frames are
provided below. In any case, the sweep frame 250 permits
the distal portion 122 of the catheter 120 to bend or
articulate in response to a distally directed force
typically applied at a proximal portion of the catheter
or at a handle of the device. For purposes of clarity,
the sweep frame 250 is shown without the torque shaft 114
extending therethrough. However, the torque shaft shall
extend through the sweep frame 250 to drive rotation of
the rotatable cutter 108.
In the illustrated variation, the sweep frame
250 comprises a tube structure having a plurality of
serrations, slots, or semi-circumferential openings 252.
Overall, the area having the openings 252 on the sweep
frame 250 weaken the frame 250 by providing a section of
reduced column strength on a first radial side 254 of the
sweep frame (i.e., the sides containing the openings).
The portion 256 of the sweep frame 250 that is not
weakened maintains a column strength that is greater than
that of the first radial side 254 of the sweep frame 250.
This constructions permits deflection of the distal
portion of the device when an axial force is applied to
the sweep frame 250 driving it against a fixed section
(e.g., either the cutter assembly, a portion of the
catheter body 120, etc.) As shown in Fig. 1B, this axial
force compresses the sweep frame 250 causing the area
with the weakened column strength to compress (i.e., the
sides of the sweep frame 250 adjacent to the openings 252
move towards one another on the first radial side 254).
This in turn causes the deflection of the spine or
strengthened side 256 in a direction towards the first
radial side 254. Because the sweep frame 250 is coupled
to the catheter (either it is fully or partially
encapsulated within the catheter body 120, the deflection
of the sweep frame 250 causes deflection of the distal
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 19 -
end of the catheter body and cutter assembly 102 in a
direction towards the first radial side 254 causing an
axis of the cutter assembly 102 to form an angle A with
an axis of the proximal end 258 of the sweep frame 250.
The sweep frame 250 is rotatable independently
of the rotatable cutter 108 and torque shaft 114. In
certain variations, the sweep frame 250 is independently
rotatable from the catheter body 120 as well. In such
configurations, as the deflected sweep frame 250 rotates,
the cutting assembly and/or distal catheter portion move
in an arcuate path relative to an axis 260 of a proximal
end 258 of the sweep frame 250. The of the sweep frame
250 can also be configured to rotate with the catheter
body 120. In this latter configuration, the cutter
assembly 102 can also rotate with the sweep frame 250
while the rotatable cutter 108 still is able to rotate
independently of the sweep frame 250.
Fig. 1B also shows a variation of the cutting
edges comprising a first set of cutting edges 112 that
extend along (or substantially along) the cutter 108 and
a second cutting edge 109 that extends only along a
portion of the cutter 108. Although the number of
cutting edges can vary, typically the cutting edges will
be symmetric about an axis 111 of the cutter 108. For
example, in one variation, the illustrated cutter 108
will have a pair of second cutting edges 109
symmetrically located about the cutter 108 and a pair of
first cutting edges 112 symmetrically located about the
axis 111 of the cutter 108. Accordingly, such a
construction results in two cutting edges 112 located on
a far or distal end of the cutter 108 and four cutting
edges 109 and 112 located on a near or proximal end of
the cutter 108.
Providing a cutter 108 with fewer cutting
edges on a first cutting portion and an increased number
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 20 -
of cutting edges on a second cutting portion, as shown,
allows for a more--aggressive. cutting device. As
illustrated in the figures, the cutter can be configured
with cutting edges 109, 112 that are adjacent to grooves,
channels, or flutes (where the combination is referred to
as a "cutting flute"). The flute provides a path for the
cut material to egress from the treatment site through
the debulking device. By reducing the number of flutes
on a far end of the cutter, the flutes can be made
deeper. The deeper flutes allow the cutting edge
adjacent to the flute to remove greater amounts of
material. However, increasing the size of the material
can also increase the chances that the material becomes
stuck or moves slowly through the catheter during
removal.. To alleviate this potential problem and
increase the efficiency of transporting the material
through the catheter, the cutter can be configured with
an increased number of cutting edges towards a rear of
the cutter that reduce the size of the cut material.
Fig. 1B also shows the cutter coupled to a
rotating mechanism 150. In this variation the rotating
mechanism couples to the cutter via a torque shaft 114
that transmits rotational energy from the rotating
mechanism 150 (e.g., an electric, pneumatic, fluid, gas,
or other motor) to the cutter 108. Variations of the
devices include use of a rotating mechanism 150 located
entirely within the body of the device 100. In one
variation, the rotating mechanism 150 may be outside of
the surgical field (i.e., in a non-sterile zone) while a
portion of the device (e.g., the torque shaft - not
shown) extends outside of the surgical field and couples
to the rotating mechanism. The rotating mechanism can be
a motor drive unit. In one working example, a motor
drive unit having 4.5V and capable of producing cutting
speeds up to 25K rpm was used. Another example of a
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 21 -
motor drive unit included supplying the motor at 6V
nominal, running at about 12,000 RPM with higher torque.
This was accomplished by changing the gear ratio from
3:1 to 1:1.
The device 100 may also include a vacuum
source or pump 152 to assist in evacuation of debris
created by operation of the device. Any number of pumps
or vacuum sources may be used in combination with the
device. For example, a peristaltic pump may be used to
drive materials from the device and into a waste
container. Fig. 1B also shows the device 100 coupled to
a fluid source 154. As with the rotating mechanism, the
vacuum source and/or fluid source may be coupled to the
device from outside the surgical field.
It may be advantageous to rotatably couple the
torque shaft to the drive unit electromagnetically,
without physical contact. For example, the torque shaft
114 can have magnetic poles installed at the proximal
end, within a tubular structure that is attached to the
sheath around the torque shaft. The stationary portion
of the motor can be built into a handle that surrounds
the tubular structure. This allows the continuous
aspiration through the sheath without the use of high
speed rotating seals.
The device may also include a ferrule 116, as
shown in Fig. 1B, that permits coupling of the catheter
body 120 to the cutter assembly 102. The ferrule 116 may
serve as a bearing surface for rotation of the cutter 108
within the cutter assembly 102. In the illustrated
variation, the torque shaft 114 rotates inside the outer
catheter body 120, sweep frame 250 and ferrule 116 to
rotate the cutter and pull or aspirate tissue debris in a
proximal direction. The clearance between the catheter
tube and conveying member 118, as well as the pitch and
thread depth of the conveying member 118, are chosen to
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 22 -
provide the desired pumping effectiveness.
In one variation of the device, the housing
104 is connected to the catheter body 120 via the ferrule
116 and thus is static. The cutter 108 rotates relative
to the housing 104 such that the cutting surface 112 on
the cutter 108 shears or cleaves tissue and trap the
tissue inside the housing 104 so that it can be evacuated
in a proximal direction using the impeller action of the
helical flutes and vacuum from the torque shaft. In
alternate variations, such as where the housing includes
a forward cutting surface, the housing 104 rotates as
well as the cutter. Accordingly, the ferrule can serve
as a bearing surface for both the housing and cutter.
The ferrule 116 can have a distal bearing
surface to bear against the proximal surface of the
cutter 108 and keeps the cutter axially stable in the
housing 104. In cases where the housing is stationary,
the ferrule 116 can be rigidly bonded/linked to the
housing 104 using solder, brazing, welding, adhesives
(epoxy), swaging, crimped, press-fit, screwed on, snap-
locked or otherwise affixed. As shown, the ferrule 116
can have holes or other rough features that allow for
joining with the catheter body. While adhesives and heat
fusing may be employed in the construction, such features
.25 are not required. Often adhesives are unreliable for a
small surface contact and heat fusing can cause the tube
to degrade. The use of a mechanical locking ring 126
allows the cutting assembly 102 to be short. Such a
feature is important for maximizing the flexibility of
the distal section of the catheter as it is required to
navigate tortuosity in blood vessels. In one variation,
a ring or band (126) can be swaged onto the catheter body
120 and over the ferrule 116. This drives portions of
the ring/band as well as the catheter body into the
openings of the ferrule allowing for increased strength
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 23 -
between the cutter assembly 102 and catheter body 120.
As shown in Fig. 1C, in certain variations,
the housing 104 can have a distal nose with a center
lumen 142.for receiving a mating piece 140 of the cutter
108. Such features assist in centering the cutter 104
concentrically inside the housing 104. As noted below,
variations of the devices include the addition of a burr
element (as shown below) for grinding hard tissue such as
calcified plaque or a dilator member for separating
materials towards the openings 106.
The geometry of the cutter 108 and housing 104
can be used to tailor the desired degree of cutting. The
housing 104 and orientation of the openings 106 can be
used to limit the depth of cutting by the cutter 108. In
addition, the distal end of the housing 104 may be domed
shaped while the proximal end may have a cylindrical or
other shape. For example, by creating larger windows
106 in the housing a larger portion of cutter 108 may be
exposed and the rate of cutting increased (for a given
rotation speed). By placing the cutting window 106 on a
convex portion or side wall of the housing, the debulking
effectiveness is much less sensitive to the alignment of
the cutter housing to the lesion, than if the window were
on the cylindrical portion of the housing. This is a key
performance limitation of traditional directional
atherectomy catheters. In addition, placement of the
window on the convex portion of the housing creates a
secant effect (as described below).
Fig. 1D illustrates an exploded view of a
cutter assembly 102 and ferrule 116. In this variation,
the cutter assembly 102 includes a housing 104 having
three openings 106 symmetrically placed about a sidewall
105 of the housing. Fig. 1D also shows a variation of
cutter 108 that comprises a far or distal portion 90
mounted on near or proximal portion 92 (where the near
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 24 -
cutter portion can also be referred to as a cutter core
adapter). The near cutter portion 92 contains a shaft 94
terminating in a mating piece 140 for coupling the cutter
108 to the housing 104 (where the mating piece 140 nests
within an opening in a front face of the housing 104.
The cutter 108 can also include a passage 96 to allow for
passing of a guidewire through the device.
Although the inventive device includes cutters
formed from in a unitary body, providing-the cutter 108
with far and near 90, 92 cutter portions allows for
optimal selection of materials. In addition, as shown, a
first cutting edge 112 can extend along both cutter
portions 90, 92 while a secondary cutting edge 109
extends only along the near cutter portion 92. Given
this configuration, when the cutter portions 90, 92 join
to form the cutter 108 the far portion 90 of the cutter
only contains two fluted cutting edges while the near
cutting portion 92 includes four fluted cutting edges.
Naturally, any number of fluted cutting portions are
within the scope of the invention. However, variations
include fewer cutting edges on a distal end of the cutter
relative to the number of cutting edges on a proximal end
of the cutter. Moreover, the cutting edges may or may
not be symmetrically located about the cutter.
Figs. 2A-6H illustrated below show various
examples of cutting assemblies that can be incorporated
with the steerable tissue removal catheters employing a
sweep frame.
Fig. 2A illustrates the cutting assembly shown
in Figs. 1A through 1D where the openings 106 form
helical slots in the housing 104. The openings 106 may
or may not be aligned with the cutting edges 109, 112 of
the cutter 108. For aggressive cutting, the slots 106
and cutting edges 109, 112 can be aligned to maximize
exposure of the tissue to cutting edges. In other words,
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 25 -
the cutting edges 109, 112 and openings 106 can be in
alignment so all cutting edges 109, 112 are exposed at
the same time to allow simultaneous cutting.
Alternatively, alignment of the openings and edges 109,
112 may be configured so that fewer than all the cutting
edges 109, 112 are exposed at the same time. For
example, the alignment may be such that when one cutting
edge is exposed by an opening 106, the remaining cutting
edges are shielded within the housing 104. Variations of
such a configuration allow for any number of cutting
edges to be exposed at any given time. In addition, the
variation depicted in Fig. 2A shows a window or opening
106 large enough to expose both the first 112 and second
109 cutting edges. However, in alternate variations, the
windows can be configured to only expose the cutting
edges 112 on the far end of the cutter 108.
In another variation, to even out the torque
profile of the device when cutting, the-cutter 108 can be
configured such that the number edges/cutting surfaces
109, 112 of the flutes 110 that are aligned with the
housing openings 106 does not vary throughout the
rotational cycle. This prevents the catheter -from being-
overloaded with torque spikes and cyclic torque
variations due to multiple cutting edges/flutes engaging
with tissue in synchrony. In other words, the length of
the cutting surface 112 exposed through the openings 106
of the housing 104 remains the same or constant.
In the variation shown in Fig. 2B, the cutting
edges 109, 112 are configured to capture debris within
the flute 110 as the cutter 108 rotates. Typically, the
cutter 108 may be designed with a secant effect. This
effect allows for a positive tissue engagement by the
cutter 108. As the cutter' 108 rotates through the
opening, the cutting edge moves through an arc, where at
the peak of the arc the cutting edge slightly protrudes
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 26 -
above a plane of the opening. The amount of positive
tissue engagement can be controlled through selection of
the protrusion distance through appropriate design of the
housing geometry (for example, by a combination of
location and size of the window and radius of curvature
of the housing). The cutting edge 109 or 112 can extend
out of the housing 104 through the window 106 as it
rotates. This structure can also be designed to drive or
impel the debris to the conveying member 118. In this
case, the flutes 110 within the cutter 108 are helically
slotted to remain in fluid communication with the
conveying member 118. Variations of the device 100 can
also include a vacuum source 152 fluidly coupled to the
conveying member 118. In order to improve the impelling
force generated by the cutters, variations of the cutter
have helical flutes 110 and sharp cutting edges 112 that
are parallel to each other and are wound from proximal to
distal in the same sense as the rotation of the cutter.
When the cutter rotates, it becomes an impeller causing
tissue debris to move proximally for evacuation.
As shown in Fig. 2C, variations of the device
may have cutting edges 109, 112 with positive rake angles
a- that is the cutting edge is. pointed in the same
direction as that of the cutter rotation. This
configuration maximizes the effectiveness of the
impelling and cutting action (by biting into tissue and
avoiding tissue deflection) . The cutter, as described
above, is preferably made of hard, wear-resistant
material such as hardened tool or stainless steels,
Tungsten carbide, cobalt chromium, or titanium alloys
with or without wear resistant coatings, such as Titanium
Nitride. However, any material commonly used for similar
surgical applications may be employed for the cutter.
The outer surfaces of the proximal end of the cutter 108
are typically blunt and are designed to bear against the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
27 -
housing 104. Typically, these surfaces should be
parallel to the inner surface of the housing.
Figs. 2A-2B also show a surface of the cutter
108 having a curved-in profile distally and is close to
the housing 104 surface. Note that housing openings 106
with this curved profile allows the cutting edge 112 to
protrude beyond the housing's outer surface. In other
words, the openings 106 form a secant on the curved
surface of the housing 104. Such a feature allows
improved cutting of harder/stiffer material like
calcified or stiff fibrous tissue where such tissue does
not protrude into the housing 104.
By controlling the number of cutting edges
109, 112 that are exposed through openings 106 in the
housing 104, it is possible to control the relative
amount of cutting engagement (both length of cutting and
depth of cut, together which control the volume of tissue
removed per unit rotation of the cutter). These features
allow independent control of the maximum torque load
imposed on the device 100. By carefully selecting the
geometry of the flutes and or cutting edges 112 relative
to the openings 106 in the housing, it is possible to
further control the balance of torque. For example, the
torque load imposed on the device is caused by the
shearing of tissue when the cutter edge is exposed by
passing through the housing window. If all cutter edges
simultaneously shear, as for example when the number of
housing windows is an even multiple of cutter edges, the
torque varies cyclically with rotation of the cutter. By
adjusting the number of cutters and windows so one is not
an even multiple of the other (for example, by using 5
windows on the housing and 4 cutting edges on the
cutter), it is possible to have a more uniform torque
(tissue removal from shearing action) during each cycle
of the cutter.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
28 -
Fig. 3A shows a variation of a cutter assembly
102 where a housing 104 of the assembly 102 includes a
conical, tapered, or dilator extension 133 extending from
a front face of the housing 104. The dilator extension
133 serves a number of purposes namely that it can keep
the cutting assembly 102 from damaging a vessel wall. In
addition, the added structural reinforcement of the front
face- of the housing 104 reduces the chance that the
rotating cutter 108 actually cuts through the housing 104
if the struts were to deflect inward. However, one
important feature of the dilator extension 133 is that it
provides a tapered surface from a guidewire to the
openings 106 in the housing 104. Accordingly, as the
dilator extension 133 advances through occlusive
material, the dilator extension 133 forces or dilates
material away from a guidewire towards the openings 106
and cutting edges. In order to dilate material away from
a center of the device, the dilator extension 133 must
have sufficient radial strength. In one example, the
dilator extension 133 and housing 104 can be fabricated
from a single piece of material as discussed herein.
The dilator extension 133 typically includes
an opening 130 for passage of a guidewire. In addition,
in most variations, a front end 135 of the dilator
extension 133 will be rounded to assist in moving the
occlusive material over a surface of the dilator 133.
Furthermore, the surface of the dilator extension 133 can
be smooth to permit sweeping of the cutting assembly 102
as discussed below. Alternatively, the dilator extension
133 can have a number of longitudinal grooves to direct
material into the openings 106. In additional
variations, the dilator extension 133 may not include an
opening 130. In such a case, the dilator extension 133
would fully taper to a closed tip.
Figs. 3B to 3D conceptually illustrate use of
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 29 -
a debulking device having a dilating member 133. In this
variation, the device 100 is advanced over a guidewire
128. However, use of a guidewire 128 is optional. As
the device 100 approaches the plaque or occlusive
material 4, the dilating member 133 forces the plaque 4
away from a center of the debulking device 100 and
towards openings 106 in the cutting assembly 102 as shown
in Fig. 3C. Clearly, the dilating member 133 must have
sufficiently radial strength so that it forces the
obstruction towards the openings 106. However, in those
variations where the dilating member 133 is conical or
tapered, the plaque material 4 is gradually moved towards
the openings 106. In those devices not having a dilating
member 133, the physician must apply excessive force to
move the cutter against the plaque 4. In some excessive
cases, the cutter actually shears through the housing
leading to failure of the device. Fig. 3D illustrates a
situation where the debulking device 100 traverses the
entire occlusion 4. However, as noted below, the device
may be configured for sweeping within the vessel. As
such, the physician may choose to sweep the device 100
within the occlusion to open the occlusion during
traversal of the occlusion or after a path is created
through the occlusion. In either case, the nature of the
dilation member 133 also functions to keep the cutting
assembly 102 spaced apart from a wall of the vessel 2.
Figs. 4A and 4B show an additional variation
of a cutting assembly 102 for use with various debulking
devices. Fig 4B shows a side view of the cutter
assembly 102 of Fig. 4A. In this example, the cutting
assembly 102 includes larger windows 106 to accommodate a
cutter 108 that includes a plurality of directional
cutting surfaces 112, 113, 115. As the cutter 108
rotates within the housing 104, the fluted cutting edge
112 cuts in a direction that is tangential to a
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 30 -
rotational direction of the cutter 108. In other words,
the fluted cutting edges 112 cut material that is about
the perimeter of the cutter 108 as it spins. The cutter
108 also includes on or more forward and rearward cutting
surfaces 113, 115. These cutting surfaces 113, 115
engage tissue when the catheter is run in a forward
direction or rearward direction. The ability to engage
and remove engagements in the multiple directions have
been shown to be important for effective debulking.
However, a variation of a cutter 108 in the present
invention can include a cutter 108 with one or two
directional cutting surfaces. For example, the fluted
cutting edges 112 can be combined with either the forward
113 or rearward 115 cutting surfaces. The ability to
debulk in a forward, rearward and rotational directions
also reduces the chance that the cutter assembly deflects
from stubborn or hard tissue.
Figs. 5A and 5B show another variation of a
cutter assembly 102 having a forward cutting surface 113
on a front of the cutter 108. In this variation, the
cutter housing 104 includes two large openings 106 that
allow the forward cutting surface 113 to engage tissue
when moved in a distal direction. The cutter 108 also
includes a plurality of fluted cutting edges 112.
Figs. 6A and 6C illustrates another variation
of cutter assemblies 102 where the housing 104 includes
an opening 107 located on a front face of a cylindrical
housing 104. The cylindrical housing 104 containing a
cutter 108 therein. In such a variation, the front edge
of the housing 104 can function as a front or forward
cutting surface. As shown, the front cutting surface 113
can be beveled on an outside surface of the housing 104.
Such a beveled feature reduces the risk of the cutting
surface 113 from gouging or otherwise damaging the wall
of a vessel. As noted above, the forward cutting surface
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 31 -
113 engages and removes tissue or plaque 4 when the
device is advanced in a distal direction within a body
lumen 2 as shown in below. As discussed herein (see Fig.
11A), features of the device, including a guidewire 128
assist in preventing the device from excessively cutting
the lumen wall 2.
The cutter 108 construction can be similar to
that shown above. Namely, where the cutter has a varying
number of cutting edges on different portions.
Alternatively, the cutter 108 can be a conventional
fluted cutter. In one variation, the cutter 108 will be
tapered or rounded such that the front of the cutter
comprises a rounded or partial-ball shape.
The housing 104 can either be configured to
rotate with the cutter 108 or can be stationary and
function as a scraping, scooping, or chisel type surface.
For example, Figs. 6A and 6B show a variation where the
housing 104 can be affixed to the cutter 108 allowing for
rotation of the entire cutting assembly 102 about the
catheter body (not shown) or ferrule 116. In the
illustrated example, the cutting assembly 102 includes-
adjoining recessed pin cavities 103 for securing the
housing 104 to the cutter 108. Fig. 6B shows a cross
sectional view of the cutter assembly 102 of Fig. 6A. As
illustrated, in this particular variation, the entire
cutting assembly 102 rotates relative to the ferrule 116
which provides a bearing surface for the rotational
housing 108. The proximal or near portion 92 of the
cutter 108 rotates within the ferrule while the proximal
end of the housing 104 rotates about the ferrule 116.
The housing 104 can be linked to the cutter
108 in a variety of ways as is well understood by those
skilled in the art. For example the housing 104 can be
directly linked or affixed to the cutter 108 via
connection points 103 so that both rotate together.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 32 -
Alternatively, the housing 104 can be geared to rotate
faster or slower than the cutter 108. In yet another
variation, the gearing can be chosen to permit the
housing 104 to rotate in an opposite direction than the
cutter 108.
Variations of the cutting assemblies include
cutters 108 that protrude partially from the forward
cutting surface 113 of the housing 104. In other
variations, the cutter 108 can extend further from the
housing 104 or the assemblies can comprise cutters 108
that are totally recessed within the housing 108. In
certain variations, it was identified that aligning the
cutting surface 113 of the housing 104 with the deepest
part of the flute on the cutter 108 allows for improved
clearing of debris, especially where a single or double
fluted cutting edge configuration is used on a distal
portion of the cutter.
In any case, the fluted cutting edge 112
impels tissue debris back into the catheter. The outer
diameter of the housing, proximal to the forward cutting
surface 113 can be smooth to protect the lumen wall from
the cutting action of the cutting edges. When the
cutting assembly 102 is deflected, the outer diameter of
the housing 102 becomes flush against the lumen wall and
prevents the cutting edges from engaging the vessel wall.
As the cutter assembly is advanced forward, it removes
plaque 4 protruding from the lumen 2 wall and tissue
debris is impelled backwards by the fluted edge 112 of
the cutter 108.
Figs. 6C and 6D illustrate a variation of a
cutting assembly 102 where a housing 104 of the cutting
assembly 102 remains stationary about a catheter body
(not shown) or ferrule 116 while the cutter 108 rotates
within the ferrule.
Fig. 6D illustrates a partial cross sectional
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 33 -
view of the cutting assembly 102 of Fig. 6C where the
inner portion of the ferrule 116 provides a bearing
surface for the proximal end 92 of the cutter 108. The
housing 104 is affixed to the ferrule 116 and may also
function as a bearing surface for the rotating cutter
108.
Fig. 6E shows an exploded view of the cutting
assembly of Fig. 6C. Again, the cutter 108 can include a
distal or far cutting portion 90 and a proximal or near
cutting portion 92. The illustrated configuration
provides a device having fewer cutting edges 112 on a
distal portion 90 of the cutter and increased cutting
edges 109 and 112 on a proximal cutting portion 92.
However, variations include a traditional fluted cutter
as well. The housing 104 is mounted about the cutter
portions 90 and 92 and optionally secured to either the
catheter body (not shown) or ferrule 116. As noted
above, the housing 104 can also be affixed to the cutter
so that it rotates with the cutter.
In alternate variations, the cutter assembly
102 the mating surface 140 can function as a blunt bumper
at the very tip of the cutter 108 that acts as a buffer
to prevent accidental cutting into the guidewire or the
vessel wall given the cutter assemblies' open distal
design. In additional variations, the housing 104 could
be expandable (such as a basket or mesh). As the cutter
108 gyrates inside the housing, the housing expands to
cut a larger diameter.
Fig. 6F illustrates a cutting assembly 102
having a forward cutting surface 113 at a distal opening
117 of a housing 104. The housing 104 rotates along with
the cutter 108 to assist in removal of tissue. As noted
above, the forward cutting surface 113 engages and
removes tissue or plaque 4 when the device is advanced in
a distal direction within a body lumen 2 as shown in Fig.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 34 -
5E. As discussed below, features of the device,
including a guidewire 128 assist in preventing the device
from excessively cutting the lumen wall 2.
Figs. 6H and 61 show a respective perspective
view and cross-sectional side view of another variation
of an open ended cutter housing 104. As shown, the
cutter housing 104 includes an opening 107 located on a
front face of a cylindrical housing 104. In this
variation, the front edge of the housing 104 can function
as a front or forward cutting surface and has a beveled
surface 177 on an inside surface of the housing 104.
Such a beveled feature reduces the risk of the cutting
surface 113 from driving into the wall of a vessel. As
shown, some variations of the cutter housing 104 include
a bearing surface 178 located within the housing 104. In
an additional variation, to control the degree to which
the cutting assembly removes tissue, the distal end or
cutting surface 177 of the housing 104 can be scalloped
or serrated. For example, instead of being uniform, the
cutting surface 177 can vary along a circumference of the
housing in an axial direction (e.g., the serrated edges
of the cutter extend along an axial length of the
housing).
The tissue debulking catheters described
herein can perform biopsies, tumor removal, fibroid
treatment, debulking of unwanted hyperplastic tissues
such as enlarged prostate tissue, or other unwanted
tissue such as herniated spinal disc material. The
flexible, low profile catheter allows for ease of access
to the treatment site and minimizes trauma or collateral
damage to surrounding healthy tissue. with the continuous
aspiration capability, contamination of the surrounding
tissue during device introduction, treatment and removal
is reduced or even eliminated. In addition, aspiration
can be used to transfer biopsy tissue samples to outside
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 35 -
the body for testing with the catheter remains in situ.
This helps the physician make real time decision in
advancing treatment of malignant tissue. The shield on
the cutter assembly maintains controlled excision of
tissue by limiting the depth of cutter engagement and
thereby prevents the physician from inadvertently cutting
into healthy surrounding tissue. The tip steering
capability of the cutter allows the physician to direct
the cutter towards desired site of tissue removal and
minimizing collateral tissue damage. Finally, by
deflecting the cutter and rotating the deflection to
sweep in an arc, the catheter can excise large tumors or
tissue lumps larger than the diameter of the catheter.
Thus, excision of large tumors can be achieved through a
small access channel and thereby minimizing trauma to the
patient.
The construction of the cutting assembly can
provide for additional modes of energy delivery. For
example, the catheter excises tissue in vascularized
regions excessive bleeding can occur (e.g., lung biopsy
and excision). Accordingly, energy can be delivered to
the target site via a conductive cutter assembly (i.e.
shield or even cutter). Sound energy (ultrasound)
electrical energy (radio frequency current) , or even
microwaves can be used for this purpose. These energy
sources delivered through the cutter can also be used to
denature tissue (collagen), shrink tissue, or ablate
tissue.
Coatings can be applied to the moving
components in the catheter to reduce friction. In one
embodiment, the sheaths and the torque shaft are coated
with a hydrophilic coating (polyvinyl alchohol) to reduce
friction between the moving components in the catheter.
The coatings can also be hydrophobic (e.g. parylene,
PTFE). The coatings can be impregnated with heparin to
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 36 -
reduce blood clotting on surface during use.
Figs. 7A through 7E illustrate additional
variations of sweep frames for use with the cutting
assemblies and catheters described herein. For purposes
of showing the sweep frame, the torque shaft is omitted
from the drawings. However, as noted above in Fig. 1B, a
torque shaft will extend through the sweep frame where
the torque shaft and sweep frame can rotate independently
from one another.
Fig. 7A shows a distal view of a debulking
catheter 100 where the catheter body 120 is partially
removed to show a variation of a sweep frame 250. In
this variation, the sweep frame 250 is constructed from a
laser cut tube or sweep tube having serrations, openings,
or slots 252. The openings 252 create a weakened section
along a first radial side 254 of the sweep tube 250. The
side opposite 256 to the first radial side 254 comprises
an area of increased column strength. Accordingly, as a
physician applies an axial force at the proximal end of
the catheter 100, typically via a sweep member as
discussed below, the force causes the sweep tube 250 to
compress against a fixed area within the catheter 100.
As the force compresses the sweep frame 250, the sweep
frame 250 is forced to compress at the weakened section
along the first radial side 254 causing bending at the
continuous area or spine 256 of the sweep frame 250 in
the direction indicated by the arrow 262. The fixation
area (the area against which the sweep frame encounters
resistance) can be the cutter assembly or a distal area
on the catheter body 120. However, any area will suffice
so long as the sweep frame 250 is able to bend upon the
application of force.
The spacing and size of the openings 252 can
be selected to allow a pre-determined bend upon
deformation of the sweep frame 250. For example, the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 37.-
openings can be selected to limit deflection of the
distal end of the catheter to less than 90 degrees or to
any angular bend to provide an added safety measure when
the device is used within a vessel. Moreover, the
spacing between adjacent openings 252 and/or the size of
openings can vary in the sweep frame 250. For example,
the spacing and/or size of the openings 252 can increase
or decrease along the length of the sweep frame 250. In
an additional variation, the spacing and the size of the
openings can vary inversely along the length of the sweep
frame 250.
In the illustrated variation, the size of the
openings in the sweep tube 250 decrease in a direction
away from the first radial side 254 of the sweep tube
250. This configuration was found to minimize
interference with the torque shaft (not shown.)
In addition, the sweep frames 250 described
herein can have any number of features to assist in
joining the sweep frame 250 to the catheter 100. For
example, in those cases where the sweep frame is
constructed from a super-elastic or shape memory alloy,
the frame 250 can include one or more openings 253
located in a sidewall to increase the bond between the
superelastic/shape memory alloy component and a regular
metallic shaft.
Fig. 7B illustrates the tissue debulking
catheter 100 upon the application of force indicated in
the direction of arrow 264. As noted above, force 264 is
applied by the physician at the proximal end or handle of
the system 100. In some variations, the force is applied
through the use of a sweep member 270 that is axially
moveable within the catheter body 120. The sweep member
can comprise a tubular structure or a spline or wire that
has sufficient column strength to compress as well as
rotate the sweep frame 250. Because the distal end of
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 38 -
the sweep frame is prevented from moving distally
(typically because the cutter assembly is affixed to the
catheter body 120), the sweep frame bends at the spine
256 in the direction of the first radial side 254. As
shown, the spacing of the openings 252 simply decreases
at the first radial side 254. This causes articulation
of the cutting assembly 102 so that an axis of the
cutting assembly becomes offset from an axis of the
proximal end 258 of the sweep frame 250 as denoted by
angle A. As noted herein, the angle A is not limited to
that shown. Instead, the angle can be predetermined,
depending on the construction of a particular sweep frame
250 to provide any angle that is suited for a target
vessel or body lumen.
In one variation, the sweep member 270 (also
called a sweep shaft) can be fabricated as a hypo-tube
structure (constructed from a super-elastic allow or a
medical grade stainless steel). The sweep member 270 can
have varying degrees of flexibility to allow the catheter
100 to be more flexible at a distal portion and rigid at
a proximal portion. This allows for improved navigation
through tortuous anatomy as well as improved transmission
of torque generated at the proximal end of the device.
In additional variations, the sweep-member should not be
prone to excessive compression or elongation given that
it must transmit the rotational force to the sweep frame.
Upon articulation of the cutting assembly 102,
the physician can further rotate the sweep member 270 as
shown by arrow 280. Rotation of the sweep member 270
causes rotation of the sweep frame 250 when articulated
causing movement of the cutting assembly 102 in an arc-
type motion about an axis of the proximal end of the
sweep frame 258. This movement causes the cutting
assembly to move through an arc having a radius denoted
by 282. In some variations of the device, the sweep
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 39 -
frame 250 and sweep member 270 can rotate independently
of the catheter body 120. However, allowing the catheter
body 120 to rotate with the sweep frame 250 and sweep
member 270 reduces the resistance on the sweep member 270
as it rotates. In this latter case, the catheter body
120 as well as the cutter housing 104 rotate with the
sweep frame 250. However, the rotatable cutter (and the
torque shaft - not shown) still rotate independently of
the sweep frame 250. Also as noted above, this ability
to sweep the cutting assembly 102 in an arc or a circle
larger than a diameter of the cutter 102 allows the
physician to create a significantly larger opening in the
target site than the diameter of the cutting assembly
itself. Such a feature eliminates the need to exchange
the device for a separate cutting instrument having a
larger cutting head. Not only does such a feature save.
procedure time, but the device is able to create variable
sized openings in body lumens.
Fig. 7B also illustrates a variation of the
sweep member 270 that can be applied to any variation of
the device shown herein. In some cases it may be
desirable to disengage the sweep member 270 from the
sweep frame 250. In such a case, the sweep member 270
can be axially slidable to disengage the sweep frame 250.
However, upon re-engagement with the sweep frame 250,
the sweep member 270 must also be able to rotate the
sweep frame 250. Accordingly, the sweep frame 250 and
sweep member 270 can include one or more keys and key-
ways. Although the illustration shows the sweep frame
250 as having a keyway 266 at a proximal end 258 and the
sweep member 270 as having a key 272, any type of
configuration that allows translation of rotation is
within the scope of this disclosure.
Fig. 7C illustrates a variation of a device
100 having sweep frame 250 with a weakened section 268
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 40 -
having a varying column strength. In this variation, the
column strength of the sweep frame 250 increases in a
circumferential direction away from the first radial side
254. The increase in column strength prevents radial
twisting'of the sweep frame 250 as it deflects. In the
illustrated variation, the sweep frame 250 comprises a
plurality of reinforcement arms, ribs, or struts 274
within the openings 250 on the sweep frame 250 where the
arms, ribs, or struts 274 are configured to
preferentially bend towards the spine 256 as the sweep
frame 250 bends. In this variation, the portion
containing the arms, ribs, or struts 274 that is adjacent
to (but spaced from) the first radial side comprises a
second column strength that is greater than the column
strength of the radial side but less than a column
strength of the remaining spine 256. Again, the varying
column strength is intended to prevent twisting of the
sweep frame 250 upon deflection.
Fig. 7D shows another variation of a sweep
frame 250. In this variation, the sweep frame comprises
a plurality of rings 276 spaced apart to create the
openings 252 within the sweep frame 250. The rings can
be joined at the spine area 256 via a separate member, a
polymer coating, or a separate frame that is ultimately
joined to the rings. As noted above, the rings can be
spaced or vary in size to achieve the desired pre-
determined curvature upon compression of the sweep frame
250.
Fig. 7E shows another variation of a sweep
frame 250 comprises a woven, coiled, braided or laser cut
mesh structure similar to that of a vascular stent. The
sweep frame structure can comprise a wire or ribbon
material having a reinforced section to function as the
spine 256. For example, one side of the stent structure
sweep frame 250 can be treated via a coating, fixture or
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 41 -
any other means to increase a column strength of the
section. Accordingly, this area of the stent structure
sweep frame 250 functions as a spine 256 of the sweep
frame 250. Although the spine 256 of Figs. 7D and 7E are
shown to be along a bottom portion of the respective
sweep frames, the sweep frames can be manufactured to
provide varying regions of column strength as described
above.
It is understood that the sweep frames can
vary from those that are shown to be any structure that
allows for preferential bending and rotation when placed
within the catheter 100. The sweep frame can be
fabricated from a variety of materials including a shape
memory alloy, a super elastic alloy, a medical grade
stainless steel, or other polymeric material. The
material of the sweep-frame 250 can.be radiopaque, or can
be altered to be radiopaque. In such cases, the
physician will be able to observe the degree of
articulation of the device by observing the curve of the
sweep frame 250 prior to cutting tissue.
In general, for proper debulking of tissue
within vessels, a debulking device should have a catheter
that is able to support the cutter assembly with
sufficient apposition force (bending stiffness). The
catheter body must be torqueable enough (i.e., have
sufficient torsional stiffness) so that the physician can
point the cutter to desired the angular position within
the vessel. The debulking device must also be pushable
enough (i.e., have sufficient column stiffness) to allow
proper cutting as the physician advances the device
through tissue. However, these needs must be balanced
against making a device that is too stiff to reliably
access tortuous or angled anatomy. In order to balance
these requirements, a variation of a debulking device can
have a more flexible distal tip location (within the last
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 42 -
10cm) to improve. the navigation (trackability over
guidewire) in tortuous anatomy. Because the overall
stiffness (in compression and torque) depends upon the
full length of the catheter,,but navigation is influenced
mainly by the distal tip region, this method is one way
to optimize several variables at the same time.
An additional design for increased torque and
push is to construct the catheter body and/or sweep
member from a braid over a wound coil, with an optional
polymeric jacket covering. This composite construction
may be over a polymer liner made of a material such as
PTFE. Yet another variation includes a catheter shaft
and/or sweep member fabricated from a metal tube having
selective cuts along the length of the tube (e.g.,
stainless steel or nitinol) to create the desired profile
of stiffness (bending, torsion, and compression) along
the length of the catheter. This slotted metal tube can
be lined or jacketed with polymeric material, and further
may be treated to produce hydrophilic, hydrophobic, or
drug binding (heparin, antimicrobial) properties. The
configurations described herein apply to any debulking
device described herein.
Figs. 7F and 7G illustrate two possible
variations of a composite construction that can be
employed in fabricating either a sweep member or a
catheter body for use in the debulking devices described
herein. Fig. 7F shows a composite construction 290 of a
slotted tube 292 (where the tube can be selected from a
polymer, a metal - such as stainless steel, or a shape
memory alloy - such as a super-elastic Nitinol tube, or a
combination therein). The pattern of slots along the
tube can be tailored to achieve the desired properties
such as graded stiffness along the long axis and/or the
short axis of the shaft. The construction 290 can
optionally include polymeric coatings, sleeves, or liners
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
43 -
298 in the inner and outer surfaces of the tube. Fig. 7F
also shows a tube 292 as having a first region 294 and a
second region 296 where the frequency of the slots varies
between regions. Any number of slotted tube
configurations, such as those found in medical devices
designed for navigation to tortuous areas, can be
employed in the designs herein. Such designs, when
combined in debulking catheters with sweep frames as
described herein, provide significant and unexpected
improvements in steering and cutting of tissue.
Fig. 7G illustrates yet another variation of a
composite construction 300 that can be employed in sweep
members and catheter bodies for use with variations of
the debulking devices described herein. As illustrated,
the construction 300 includes a coil member 302 covered
by a braid 304. The coil and braid can each be
fabricated from any material commonly known in the field
of braided/coiled catheters. For example, the coil 302
can be wound from a super-elastic wire or ribbon. While
the braid can comprise a plurality of super elastic or
stainless steel filaments braided or woven together.
Fig. 7G also shows the braid 304 covered by a polymeric
coating, sleeve, or liner 306.
In an additional variation, the sweep frame
and/or sweep member can comprise a spiral cut tube
covered by a liner or polymeric layer. In such a case,
the angle of the spiral as well as the width can be
selected to impart desired characteristics on the device.
For example, the spiral can be selected to maximize
pushability of the device while maintaining a near one-
to-one relationship between the cutting assembly and
proximal end of the device when rotating or sweeping the
cutting assembly.
Figs. 7H-7I show variations of a sweep frame
250 having a visualization feature 284 that permits a
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 44 -
physician to determine orientation and direction of
articulation of the cutting assembly when the device is
viewed under non-invasive imaging. In Fig. 7H shows one
variation of the visualization feature 284 as being a
notch or opening on a side of the sweep frame 250 that is
perpendicular to the direction in which the frame bends.
In one example, the visualization mark is placed 90
degrees relative to the spine 256. Although the feature
284 is shown on the right side of the sweep frame 250,
any side may be used so long as the location and
orientation of the feature 284 conveys to the physician
the orientation and direction of bend of the sweep frame
250 via non-invasive imaging. Fig. 71 illustrates
another variation of an orientation feature 284
comprising a marking substance (either a radiopaque
additive or a highly radiopaque metal deposited on the
sweep frame 250). In any case, the visualization feature
must provide sufficient contrast against the frame 250
when viewed in a non-invasive imaging modality. The
feature can also comprise a structure selected from the
group consisting of a notch, opening, tab, protrusion, or
deposition.
As shown, both visualization features 284 are
on the right-hand side of the sweep frame 250 when the
spine 256 of the frame 250 is directly adjacent to the
physician. In this position, articulation of the sweep
frame (that occurs in a direction away from the spine),
causes the sweep frame 250 to deflect away from the
physician. Accordingly, when the physician observes the
visualization marks 284 to the right of the device, the
physician will know that flexure of the sweep frame 250
will occur directly away from the physician. Clearly,
the present invention includes any number of
visualization features or placement of such features on
any portion of the sweep frame so long as the physician
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 45 -
will be able to determine the orientation and direction
of bend of the sweep frame from viewing the visualization
mark(s) 284.
Fig. 8A illustrates a variation of a device
100 configured for rapid exchange. As shown, the device
100 includes a short passage, lumen, or other track 136
for the purpose of advancing the device 100 over a
guidewire 128. However, the track 136 does not extend
along the entire length of the device 100. Moreover, an
additional portion of the track 136 may be located at a
distal end of the catheter to center a guidewire 128.
This feature permits rapid decoupling of the
device 100 and guidewire 128 by merely holding the
guidewire still. and pulling or pushing the catheter 100
over the guidewire. One benefit of such a feature is
that the guidewire 128 may remain close to the site while
being decoupled from the device 100. Accordingly, the
surgeon can advance additional devices over the guidewire
and to the site in a rapid fashion. This configuration
allows for quick separation of the catheter from the wire
and introduction of another catheter over the wire since
most of the wire is outside of the catheter.
As shown in Fig. 8B, centering the tip of the
cutting assembly 102 over a guide wire 128 improves the
control, access and positioning of the cutting assembly
102 relative to a body lumen or vessel 2. To accomplish
this, the cutting assembly 102 can have a central lumen
to accommodate a guide wire 128. Variations of the
device 100 include a central guide wire lumen that runs
the length of the catheter through all central components
including the torque shaft and the cutter. As noted
above, a guidewire 128 can be affixed to the housing 104
or other non-rotational component of the cutting assembly
102. In such a case, the guidewire 128 may preferably be
a short segment that assists with navigation of the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
46 -
device through an occluded portion of a body lumen.
However, the devices 100 can also operate without a
guidewire since.the head is steerable like a guidewire.
Fig. 9A illustrates a partial cross-sectional
view of a variation of a device 100 showing the placement
of a torque shaft 114 within the catheter body 120 and
sweep frame 250. As shown, this variation of the device
100 includes a conveyor member 11-8 'located within the
device 100 and on an exterior surface of the torque shaft
114. The conveyor member 118 may be an auger type system
or an Archimedes-type screw that conveys the debris and
material generated during the procedure away from the
operative site. In any case, the conveying member 118
will have a raised surface or blade that drives materials
in a proximal direction away from the operative site.
Such materials may be conveyed to a receptacle outside of
the body or such materials may be stored within the
device 100. In one variation, the torque shaft 114 and
conveying member 118 extend along the length of the
catheter. As shown, the torque shaft 114 and conveyor
118 fit within the sweep frame 250. In some variations
of the device, a cover or film can be placed between the
sweep frame 250 and torque shaft 114 to prevent debris
from becoming trapped within the serrations, slots or
openings 252 of the sweep frame 250. The cover or film
also acts as a smooth, low friction surface.
Fig. 9B shows a partial sectional view of an
example of a torque shaft 114 for coupling to a cutter
assembly. To aid in removal of materials, the torque
shaft can be a set of counter-wound coils, with the outer
coil wound at the proper (greater) pitch to form the
conveying member 118. Winding the coils counter to each
other automatically reinforces the torque shaft 114
during rotation. Alternatively, the torque shaft 114 may
be made out of a rigid plastic, rendered flexible by
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 47 -
incorporation of a spiral relief or groove which acts as
a conveying member 118. Although the shaft may be
fabricated from any standard material, variations of the
shaft include a metal braid embedded_in polymer (PEBAX,
polyurethane, polyethylene, fluoropolymers, parylene,
polyimide, PEEK, PET) or one or more metal coils embedded
in a polymer such as PEBAX, polyurethane, polyethylene,
fluoropolymers parylene, polyimide, PEEK, PET. These
constructions maximize torsional strength and stiffness,
as well as column strength for "pushability", and
minimize bending stiffness for flexibility. Such
features are important for navigation of the catheter
through tortuous vessels but allow for smooth
transmission of torque over the long length of the
catheter. In the multi-coil construction, the inner coil
should be wound in the same sense as that of the rotation
so that it would tend to open up under torque resistance.
This ensures that the guidewire lumen remain patent
during rotation. The next coil should be wound opposite
the inner to counter the expansion to keep the inner coil
from binding up against the outer catheter tube.
Fig. 9B also shows a torque shaft 114 having a
central lumen 130. Typically the lumen will be used to
deliver a guidewire. In such cases, the central lumen
may be coated with a lubricious material (such as a
hydrophilic coating or Parylene) or made of a lubricious
material such as PTFE to avoid binding with the
guidewire. However, in some variations a guidewire
section is affixed to a distal end of the housing.
Moreover, the central lumen of the torque shaft 114 may
also be used to deliver fluids to the operative site
simultaneously with the guidewire or in place of the
guidewire.
In some variations, the conveying member 118
may be integral to the shaft 114 (such as by cutting the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 48 -
conveying member 118 into the torque shaft 114 or by
extruding the torque shaft 114 directly with a helical
groove or protrusion. In an additional variation as
shown in Fig. 9C, an additional conveying member 118 may
be incorporated on an inside of the torque shaft, where
the internal conveying member is wound opposite to that
of the external conveying member 118. Such a
configuration allows for aspiration and debris (via the
external conveying member 118) and infusion (via the
internal conveying member 118). Such a dual action can
enhance the ability to excise and aspirate plaque by:
(1) thinning the blood, whether by viscosity alone or
with the addition of anti-coagulants such as heparin or
warfarin (cumadin), and/or anti-platetlet drugs such as
Clopidogrel, (2) improving the pumpability (aspirability)
of the excised plaque by converting it into a solid-
liquid slurry that exhibits greater pumping efficiency,
and (3) establishing a flow-controlled secondary method
of trapping emboli that are not sheared directly into the
housing, by establishing a local recirculation zone.
As noted above, the conveying member 118 can
be wound in the same directional sense as the cutter 108
and in the same direction of rotation to effect
aspiration of tissue debris. The impeller action of the
cutter 108 moves the tissue debris from inside the
housing 104 openings 106 into the torque shaft. The
pitch of the cutting edges 112 may be matched in to that
of the conveying member 118 to further optimize
aspiration. Alternatively, the pitch of the conveying
member 118 may be changed to increase the speed at which
material moves once it enters the conveying member 118.
As discussed herein, debris can be evacuated outside the
body by the conveying member 118 action along the length
of the catheter and with or without supplement of the
vacuum 152 pump connected to the catheter handle.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 49 -
Alternatively, the debris may be accumulated in a
reservoir within the device.
Fig. 10A illustrates an example of a variation
of a device 100 being steered when using the sweep frame
and sweep member described above. The ability to steer
the tip of the device 100 is useful under a number of
conditions. For example, when debulking an eccentric
lesion as shown, the cutting assembly 102 should be
pointed towards the side of the vessel 2 having the
greater amount of stenotic material 4. Naturally, this
orientation helps prevent cutting into the bare
wall/vessel 2 and focuses the cutting on stenotic tissue
4. When in a curved section of the vessel 2, without the
ability to steer, the cutting assembly 102 would tend to
bias towards the outside of the curve. As shown in Fig.
10A, steering allows the cutting assembly 102 to point
inward to avoid accidental cutting of vessel wall 2.
The ability to steer the device 100 also
allows for a sweeping motion when cutting occlusive
material. Fig. 10B shows the rotation of the cutting
assembly 102. As shown in Fig. 10C, when the cutting
assembly 102 deflects relative to the axis of the
catheter, rotation of the deflected portion 102 creates a
sweeping motion. It is' noted that rotation or
articulation of the cutting assembly also includes
rotation or articulation of the catheter to allow the
cutting assembly to deflect relative to an axis of the
catheter. Fig. 10D shows a front view taken along an
axis of the vessel to illustrate the sweeping motion
causing the cutting assembly 102 to "sweep" over a larger
region than the diameter of the cutting assembly. In
most cases, when articulated, the device will be rotated
to sweep over an arc or even a full circle. The rotation
of the cutter may or may not be independent of the
rotation of the device. A user of the device may couple
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 50 -
the sweeping motion of the cutting assembly with axial
translation of the catheter for efficient creation of a
larger diameter opening over a length of the occluded
vessel. The combination of=movement can be performed
when the device is placed over a guidewire, for example
by the use of a lead screw in the proximal handle
assembly of the device. In another aspect of the devices
described herein, the angle of'articulation may be fixed
so that the device sweeps in a uniform manner when
rotated.
Fig. 10C also shows a variation of a debulking
device having-a catheter body 120 where a first or distal
portion 122 of the catheter body rotates 280 as the
cutting assembly sweeps in an arc. The second portion
137 of the catheter remains stationary. Accordingly, the
two part catheter may be joined to permit the relative
movement between sections. The second portion 137 may
incorporate a sweep frame and sweep shaft.
In addition, the shape of the housing 104 as
well as the location of the windows 106 can be chosen so
that when the device 100 is substantially aligned with
the lesion, or engages it at less than some critical
attack angle, it will cut effectively. However, when
pivoted at an angle greater than the critical angle, the
cutting edges or grinding element will not engage the
lesion as shown in Fig. 11A. This means that at large
deflections, as the catheter tip approaches the vessel
wall, it automatically reduces its depth of cut and
ultimately will not cut when the critical angle is
exceeded. For example, the cutter distal tip is blunt
and does not cut. As the catheter tip is deflected
outward, the blunt tip contacts the vessel and keeps the
cutting edges proximal to the tip from contacting the
vessel wall. Also the wire in combination with the
device can also act as a buffer to prevent the cutting
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 51 -
edges from reaching the vessel. As shown, the portion of
the guidewire that extends from the housing 104 will bend
at a minimum bend radius. This permits a portion of the
wire closest to the housing to act as a bumper and
prevents the cutter and windows from engaging the walls
of the vessel. In certain variations, wires with varying
bend radii can be selected to offer varying degrees of
protection by spacing the cutting head away from the
tissue wall.
Figs. 11B and 11C show a cutter assembly
design that is specialized for forward cutting. This
particular variation includes an open ended housing where
the cutter extends from the housing (as shown above).
However, a blunt bumper 119 at the tip of the cutter 108
acts as a buffer to prevent accidental cutting into the
guidewire 144 or excessively into the lumen wall 2. In
addition, this design can optionally incorporate a static
housing portion 121 on a back end of the cutter assembly
102 that partially shields the `cutter from deep side cuts
into the lumen wall 2.
As shown above, the catheter body 120 can
remains stationary while the inner sweep frame 250 and
sweep member 270 rotate to move the cutting assembly 102
in an arc or orbit within the lumen. Alternatively, the
sweep frame 250 and sweep member 270 can rotate with the
catheter body 120 but independently of the cutting
assembly and torque shaft. The outer sheath is
preferably composed of a metal braid sandwiched in a
polymeric matrix of such materials as high density
polyethylene (HDPE), polyethylene (PE), fluoro-polymer
(PTFE), nylon, polyether-block amide (PEBAX),
polyurethane, and/or silicone. The sheath is stiffer
proximally than distally. This can be achieved by using
softer grades of polymers distally and/or having no metal
braid distally.
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 52 -
Fig. 12A and 12B illustrate one variation of a
control system or fixture. As shown, the control system
200 includes a sweep control knob 202 coupled to the
sweep-member as discussed above. The sweep control knob
202 can axially advance the sweep member to cause
deflection of the sweep frame.. In addition, the sweep
control knob 202 can rotate independently relative torque
shaft and rotatable cutter in the cutting assembly 102.
Again, the sweep sheath can be composed of a super-
elastic alloy, a medical grade stainless steel, a metal
braid sandwiched in a polymeric matrix of such materials
as polyethylene (PE), fluoro-polymer (PTFE), nylon,
and/or. polyether-block amide (PEBAX), polyurethane,
and/or silicone. The sweep sheath can also be made of
counter wound metal coils. Its distal end is curved and
is preferably made of a material that can withstand high
degree of flex and retain its curved shape. Such
material may include polymers such as PE, nylon,
Polyetheretherketone (PEEK), Nickel Titanium (Nitinol),
or spring steel.
To allow the cutter assembly to be straight
and undeflected 102, the sweep sheath is withdrawn
proximally by the sweep control knob 202. This causes
removal of the axial force from the sweep frame (in some
variations, the sweep frame can be set in a straight
configuration). As shown in Fig. 12A, distal movement of
the sweep control knob 202 advances the sweep sheath to
deflect the catheter tip. The degree of the deflection
is controlled by the amount the sweep sheath is advanced.
The axial advancement of the sweep sheath is limited by
the maximum deflection of the sweep frame.
As shown in Fig. 12B, the sweep control knob
202 can be rotated to sweep the cutting assembly 102 in
an arc manner. Although sweeping of the cutting assembly
102 can occur via manual operation. Variations of the
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 53 -
device include sweep members that can be selectively
coupled to a motor to activate an automated rotation.
This allows the physician to have a smooth, continuous,
automated means to sweep the cutter without any manual
effort.
Figs. 12A and 12 B also show the catheter 120
as having a flush port 129. The flush port 129 provides
a means for injecting a fluid such as heparinized saline
or any other medicine into the catheter body 120 to keep
blood and tissue debris from clogging the space between
components in the device. The flush port .129 can also
help lubricate moving components within the device. One
desirable fluid path is along the length of the catheter
in the space between the catheter body 120 and sweep
member 270. Drugs or fluids can be introduced via the
flush port 129 for flow out of one'or more openings 131
near the catheter tip or cutting assembly 102. Drugs
flushing out near the cutting assembly can then infuse
into the vessel wall. Using a stenosis-inhibiting drug
like paclitaxel or rapamycin could help prevent
restenosis after the atherectomy procedure.
Turning now to a variation of the catheter 100
and control system 200, the entire system is arranged
from distal to proximal with a cutter assembly 102, a
catheter body 120, a flush port 129, a control system 200
for tip deflection and sweep control, a hub 204 or other
connection for providing aspiration of the cut materials
as well as a drive gear 206 to turn the torque shaft and
cutter. The gear 206 is connected to a rigid drive shaft
208 encased within the hub 204 as shown in Fig. 12C. The
drive shaft 208 can take a form of a hollow tube with a
central lumen for passage of the guidewire and is
centered within a lumen in the hub 204 and fixed axially
by a pair of bearings 210. A seal 211adjacent to the
bearing 210 prevents aspirated tissue debris from leaking
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 54 -
proximally through the bearing 210. A transfer propeller
212 is rigidly attached to the distal portion of the
drive shaft 208 to pump aspirated tissue debris 8 from
the catheter out into an attached aspiration reservoir.
The drive shaft 208 is connected to flexible torque shaft
114 that extends the length of the catheter body for the
purpose of transfer torque from the drive shaft to the
cutter. As noted above, the torque shaft 114 has helical
grooves on its outer diameter and central guidewire
lumen. During a procedure run, a motor drives the gear
206 to rotate. This causes rotation of the drive shaft
208, the transfer propeller 212, the torque shaft 114,
and the cutter (not shown) all in the same rotational
sense. Thus the cutter assembly effectively cuts plaque
and drives the debris back into the helical groove on the
torque shaft 114. The rotating helical grooves winds the
debris back into the hub 204, which is then transferred
to the aspiration reservoir by the transfer propeller
212. The propeller 212 can take the form of a screw or a
series of circumferentially arranged angled fan blades.
The cutter is preferably rotated at a speed of 10,000-
25,000 rpm. An alternative design would have the
aspiration reservoir built into the hub of the catheter.
As noted above, selecting a desired profile
for bending, torsion and axial strength characteristics
when designing the catheter body and/or sweep member
improves the overall function of the debulking catheter.
Aside from the improved ability to advance the cutting
assembly and sweep the cutting assembly in an arc-type
motion, the proper characteristics improve the ability of
the physician to steer the device. For example,
selection of the proper characteristics reduces the
chance that the distal portion of the device rotates more
or less than the proximal end or control knob.
It was found that the devices of the present
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
55 -
invention allow a physician to accurately determine the
rotation of the cutting assembly since the rotation of
the cutting assembly closely corresponds to the rotation
of the proximal end or control knob. Such close
correspondence is not available unless the catheter body
and/or sweep member has sufficient bending, torsion and
axial strength characteristics. Accordingly, a further
aspect of the debulking devices occurs when these
catheter bodies/sweep members are coupled to a system
having a sweep control knob 202 that enables indexing and
monitoring of the orientation of the cutter assembly.
Clearly, this one-to-one relationship can be lost when
the distal end or cutting assembly encounters sufficient
resistance against or within a lesion, occlusion, or
diseased tissue. However, in such cases, the device is
still able to debulk tissue and perform its function even
though the response may not be one-to-one. In any case,
the ability to maintain a near one-to-one relationship
and minimize rotational misalignment between the ends of
the device allows for steering of the debulking device
towards-the treatment site.
Fig. 12D shows a conceptual illustration a
control knob 202 allowing for indexing of the cutting
assembly. As shown, the control knob 200 includes an
orientation marker 214 that shall correspond to the
weakened section of the sweep frame (not shown) As
discussed above, the orientation marker 214 shall also
correspond to a side of the sweep frame that is opposite
to a spine of the sweep frame. Because the orientation
marker 214 is aligned with the sweep frame in such a
manner, the physician knows that the device shall bend in
a direction corresponding to the orientation marker 214.
This allows the physician to identify the orientation of
the cutting assembly as it sweeps within the body by
observing the orientation of the orientation marker as
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 56 -
the physician rotates the sweep control knob 202. Even
when the one-to-one relationship is lost (as noted
above), the indexing knob adds a fine control to direct
the distal end in defined steps or increments. This
control can be useful because the physician can direct
the cutter within the immediate vicinity to work on areas
that need to be resected, versus losing position due to
excessive movement. An atherectomy or tissue debulking
device having features that allow pushability as well as
torsional strength allow the physician greater feedback
and control when trying to steer the cutting assembly
towards a desired path within the body.
The sweep control knob 202 can also include a
plurality of indexing stops or divots 216. Although this
variation of the device contains divots, These indexing
stops 216 can have a twofold benefit. First, they allow
incremental rotational indexing as the physician rotates
the knob 202. This incremental indexing is permitted due
to the bending, torsion and axial strength
characteristics of the device permitting little or no
misalignment between the ends of the device. A secondary
advantage of the indexing stops 216 is that they allow
incremental axial indexing as the physician advances the
knob 202 in an axial direction to bend or steer the
distal end of. the debulking catheter by moving the sweep
member 270 in an axially distal direction.
As shown, any number of positions 218, 220,
222, 224 can be created on the knob 202. As shown in
Fig. 12E, a spring pin 226 can provide tactile feedback
to the physician as the knob 202 rotates. Once the
physician desires to bend or steer the debulking device
by moving the knob 202 in an axial direction 228, the
physician shall feel movement of the knob into the second
220 and third 222 stop positions as shown in Fig. 12F.
Additional components may be incorporated into
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 57 -
the devices described herein. For example, it can be
desirable to incorporate transducers into the distal
region of the catheter to characterize the plaque or to
assess plaque and wall thickness and vessel diameter for
treatment planning; also transducers may be desired to
indicate the progression of. debulking or proximity of
cutter to vessel wall. For example, pressure sensors
mounted on the catheter housing can sense the increase in
contact force encountered in the event that the housing
is pressed against the vessel wall. Temperature sensors
can be used to detect vulnerable plaque. Ultrasound
transducers can be used to image luminal area, plaque
thickness or volume, and wall thickness. Optical
coherence tomography can be used to make plaque and wall
thickness measurements. Electrodes can be used for
sensing the impedance of contacted tissue, which allows
discrimination between types of plaque and also vessel
wall. Electrodes can also be used to deliver impulses of
energy, for example to assess innervation, to either
stimulate or inactivate smooth muscle, or to characterize
the plaque (composition, thickness, etc.). For example,
transient spasm may be introduced to bring the vessel to
a smaller diameter easier to debulk, then reversed either
electrically or pharmaceutically. Electrical energy may
also be delivered to improve the delivery of drugs or
biologic agents, by causing the cell membrane to open in
response to the electric stimulation (electroporation).
One method of characterization by electrical measurement
is electrical impedance tomography.
As shown in Fig. 13, a cutter assembly 102 can
also have a burr protruding out its nose. Although the
burr 180 may have any type of abrasive surface, in one
variation, this burr is blunt and has fine grit (such as
diamond grit) to allow for grinding of heavily calcified
tissue without injuring adjacent soft tissue. This
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 58 -
combination of a burr and cutter allow the distal
assembly to remove hard stenotic tissue (calcified
plaque) using the burr while the sharp-edged shaving
cutter removes softer tissue such as fibrous, fatty
tissue, smooth muscle proliferation, or thrombus. In
variations, the burr can also have helical flutes to help
with aspiration, or the burr can be incorporated to a
portion of the cutting edge (for example, the most distal
aspect of the cutter).
Infusing solutions (flush) into the target
treatment site may be desirable. Infused cool saline can
prevent heating of blood and other tissue, which reduces
the possibility of thrombus or other tissue damage.
Heparinized saline can also prevent thrombus and thin out
the blood to help maximize effectiveness of aspiration.
The flush can also include drugs such as Clopidogrel,
Rapamycin, Paclitaxel or other restenosis-inhibitors.
This may help to prevent restenosis and may result in
better long term patency. The flush may include
paralytics or long-acting smooth muscle relaxants to
prevent acute recoil of the vessel. Figs. 14A-14C
illustrate variations of flushing out the device 100.
The flush can be infused through the guide wire lumen
(Fig. 14A), a side lumen in the catheter shaft (Fig. 14B)
or tube, the space between the flexing sheath and the
catheter and/or the sideports in the guidewire (Fig.14C).
Flush can come out of a port at the distal end of the
cutter pointing the flush proximally to facility
aspiration. Alternatively, by instilling the flush out
the distal end of the cutter housing over the rounded
surface, the flow may be directed rearward by the Coanda
effect. The restenosis-inhibitors can be carried by
microcapsules with tissue adhesives or velcro-like
features on the surface to stick to inner vessel surface
so that the drug adheres to the treatment site, and to
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 59 -
provide a time-release controlled by the resorption or
dissolving of the coating to further improve efficacy.
Such velcro-like 'features may be constructed with
nanoscale structures made of organic or inorganic
materials. Reducing the volume of foreign matter and
exposing remaining tissue and extracellular matrix to
drugs, stimulation, or sensors can make any of these
techniques more effective.
Another way to infuse fluid is to supply
pressurized fluid at the proximal portion of the
guidewire lumen (gravity or pressure feed) intravenous
bag, for example. A hemostatic seal with a side branch
is useful for this purpose; tuohy-borst adapters are one
example of a means to implement this.
Balancing the relative amount of infusion
versus fluid volume aspirated allows control over the
vessel diameter; aspirating more fluid than is instilled
will evacuate the vessel, shrinking its diameter, and
allow cutting of lesion at a greater diameter than the
atherectomy catheter. This has been a problem for
certain open cutter designs that use aspiration, because
the aggressive aspiration required to trap the embolic
particles evacuates and collapses the artery around the
cutter blades; this is both a performance issue because
the cutter can bog down from too high torque load, and
the cutter can easily perforate the vessel. The shielded
design described here obviates both problems, and further
requires less aggressive aspiration to be effective,
giving a wider range of control to the user.
The devices of the present invention may also
be used in conjunction with other structures placed in
the body lumens. For example, as shown in Fig. 15, one
way to protect the vessel and also allow for maximum
plaque volume reduction is to deploy a protective
structure such as a stent, thin expandable coil or an
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 60 -
expandable mesh 182 within a lesion. As this structure
expands after deployment, the thin wire coil or the
struts push radially outward through the plaque until it
becomes substantially flush with the vessel wall. This
expansion of thin members requires minimal displacement
of plaque volume and minimizes barotrauma produced in
balloon angioplasty or balloon expanded stent delivery.
Once the protective structure has expanded fully,
atherectomy can be performed to cut away the plaque
inside to open up the lumen. The vessel wall is protected
by the expanded structure because the structure members
(coil or struts) resist cutting by the atherectomy
cutter, and are disposed in a way that they cannot
invaginate into the cutter housing (and thereby be
grabbed by the cutter)'. It is also possible to adjust
the angle of the windows on the atherectomy catheter
cutter housing so that they do not align with the struts
or coils; the adjustment to orientation may be accounted
for in the coil or strut design, in the cutter housing
design, or both. Furthermore, the protective member can
be relatively flexible and have a low profile (thin
elements), so that it may be left in place as a stent.
Because the stent in this case relies mainly upon
atherectomy to restore lumen patency, it may be designed
to exert far less radial force as it is deployed. This
allows usage of greater range of materials, some of which
may not have as high of stiffness and strength such as
bioresorbable polymers and metal alloys. Also, this
allows a more resilient design, amenable to the
mechanical forces in the peripheral arteries. It also
minimizes flow disruption, to minimize hemodynamic
complications such as thrombosis related to the
relatively low flows found in the periphery. It is also
possible to perform atherectomy prior to placing the
protective structure, whether or not atherectomy is
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 61 -
performed after placing the structure.
Additional variations of systems include
devices 100 having a cutting assembly 170 comprising
spinning turbine-like coring cutter 172 as shown above
and as shown in Fig. 16A. Fig. 16B shows a side view of
the coring cutter 170. In use, the coring cutter can be
hydraulically pushed to drive the sharp edge through
tissue. The turbine like cutters has helical blades 174
on the inside of the sharp cylinder housing 176 (shell).
The coring cutter 170 may also have spokes or centering
devices 184 as shown to in Figs. 17A to 17F center the
shell about the guidewire. This helps to keep the cut of
the plaque centered about the vessel wall for safety.
The spokes also act as an impeller to pull stenotic
tissue back and this helps to drive the cutter forward as
well as achieve aspiration to minimize embolization.
It is also possible to use the devices and
methods described here to restore patency to arterial
lesions in the coronary circulation and in the
cerebrovascular circulation, both by debulking de novo
lesions and by debulking in stent restenosis.
The devices and methods described herein also
work particularly well in lesions that are challenging to
treat with other methods: at bifurcations, in tortuous
arteries, and in arteries which are subject to
biomechanical stresses (such as in the knee or other
joints).
In a further variation of the devices
described here, the motor drive unit may be powered by a
controller that varies the speed and torque supplied to
the catheter to optimize cutting efficiency or to
automatically orbit the cutter using variable speed with
a fixed flexible distal length of catheter (or providing
further orbiting control by controlling the length of the
distal flexible section of the catheter).
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 62 -
It is also possible to use feedback control to
operate the catheter in a vessel safe mode, so that the
rate of cutting is decreased as the vessel wall is
approached. This may be accomplished through speed
control, or by reducing the degree to which the cutting
blades penetrate above the housing window by retracting
the cutter axially within the housing. Feedback
variables could be by optical (infrared) or ultrasound
transducer, or by other transducers (pressure, electrical
impedance, etc.), or by monitoring motor performance.
Feedback variables may also be used in safety algorithms
to stop the cutter, for example in a torque overload
situation.
The atherectomy catheter may be further
configured with a balloon proximal to the cutter, for
adjunctive angioplasty or stent.delivery. The catheter
may optionally be configured to deliver self-expanding
stents. This provides convenience to the user and
greater assurance of adjunctive therapy at the intended
location where atherectomy was performed.
Further methods include use of similar devices
to debulk stenosis in AV hemodialysis access sites
(fistulae and synthetic grafts), as well as to remove
thrombus. By removing the cutter housing and recessing
the fluted cutter within the catheter sheath, a suitable
non-cutting thrombectomy catheter may be constructed.
Other methods of use include excising bone,
cartilage, connective tissue, or muscle during minimally
invasive surgical procedures. For example, a catheter
that includes cutting and burr elements may be used to
gain access to the spine for performing laminectomy or
facetectomy procedures to alleviate spinal stenosis. For
this application, the catheter may be further designed to
deploy through a rigid cannula over part of its length,
or have a rigid portion itself, to aid in surgical
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 63 -
insertion and navigation.
It is advantageous to couple atherectomy with
stenting. By removing material,.debulking the lesion, a
lesser radial force is required to further open the
artery and maintain lumen diameter. The amount of
debulking can be tuned to perform well in concert with
the mechanical characteristics of the selected stent.
For stents that supply greater expansion and radial
force, relatively "less atherectomy is required for
satisfactory result. An alternative treatment approach
is to debulk the lesion substantially, which will allow
placement of a stent optimized for the mechanical
conditions inherent in the peripheral anatomy. In
essence, the stent can support itself against the vessel
wall and supply mild radial force to preserve luminal
patency. The stent may be bioresorbable, and/or drug
eluting, with the resorption or elution happening over a
period for days to up to 12 weeks or more. A period of 4
to 12 weeks matches well with the time course of
remodeling and return to stability as seen in the classic
wound healing response, and in particular the known
remodeling time course of arteries following stent
procedures. In addition, the stent geometry can be
optimized to minimize thrombosis by inducing swirl in the
blood flow. This has the effect of minimizing or
eliminating stagnant or recirculating flow that leads to
thrombus formation. Spiral construction of at least the
proximal (upstream) portion of the stent will achieve
this. It is also beneficial to ensure that flow
immediately distal to the stent does not create any
stagnant or recirculation zones, and swirl is a way to
prevent this also.
Any of the atherectomy devices 100 described
herein can be used as a tool to treat chronic total
occlusions (CTO) or a complete blockage of the artery.
CA 02720761 2010-10-06
WO 2009/126309 - PCT/US2009/002253
- 64 -
The frontward cutting and tip-steering capabilities
allows the physician to controllably create a channel
through the blockage. In one such method for creating
this channel (recanalization) the physician places the
device 100 proximal edge of a blockage 10. The following
applications contain additional details on such a device
useful to treat CTO as well as additional features on
various debulking devices. Such patent applications
include: U.S. Patent Application No. 11/551,191, U.S.
Patent Application No. 11/551,193, U.S. Patent
Application No. 11/551,198, and U.S. Patent Application
No. 11/551,203 each filed October 19, 2006; U.S.* Patent
Application No. 11/567,715 filed December 06, 2006; U.S.
Patent Application No. 11/771,865 filed June 29, 2007;
and U.S. Provisional Application No. 60/981,735 filed
October 22, 2007 each of which is incorporated by
reference.
In another variation of the invention, the
steerable debulking device 100 can improve the ability of
a physician attempting to navigate a guidewire 144
through branching, tortuous or otherwise obstructed
anatomy. In the variation shown in Fig. 18A, as a
physician navigates a guidewire 144 through the anatomy,
the tortuous nature of the anatomy or obstructions 4
within the vessel 2 may prevent advancement of the
guidewire 144 as shown. In such a case, the steerable
debulking device 100 of the present invention permits a
physician to withdraw the guidewire within or just distal
to the debulking device 100 (as shown in Fig. 18B.) The
device 100 can then be advanced to a branching point or
beyond the tortuous location or obstruction, and
articulated (as shown in Fig. 18C) so that the physician
can then advance the guidewire 144 beyond the
obstruction, sharp bend or into the desired branch.
It is noted that the descriptions above are
CA 02720761 2010-10-06
WO 2009/126309 PCT/US2009/002253
- 65 -
intended to provide exemplary embodiments of the devices
and methods. It is. understood that, the invention
includes combinations of aspects of embodiments or
combinations of the embodiments themselves. Such
variations and combinations are within the scope of this
disclosure.
The foregoing is considered as illustrative
only of the principles of the invention. Furthermore,
since numerous modifications and changes will readily
occur to those skilled in the art, it is not desired to
limit the invention to the exact construction and
operation shown and described. While the preferred
embodiment has been described, the details may be changed
without departing from the invention, which is defined by
the claims.