Note: Descriptions are shown in the official language in which they were submitted.
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
INTEGRATED HEART MONITORING DEVICE AND
METHOD OF USING SAME
CLAIM OF PRIORITY
This application claims priority from U.S. Pat. Appl. No. 12/119,315, entitled
"OPTICAL SENSOR APPARATUS AND METHOD OF USING SAME," U.S. Pat.
Appl. No. 12/119,339, entitled "DOPPLER MOTION SENSOR APPARATUS AND
METHOD OF USING SAME," U.S. Pat. Appl. No. 12/119,325, entitled
"INTEGRATED HEART MONITORING DEVICE AND METHOD OF USING SAME,"
U.S. Pat. Appl. No. 12/119,462, entitled "METHOD AND SYSTEM FOR
0 MONITORING A HEALTH CONDITION," all filed on May 12, 2008, and U.S. Pat.
Appl. No. 12/206,885, entitled "DOPPLER MOTION SENSOR APPARATUS AND
METHOD OF USING SAME," filed on September 9, 2008, all by the same inventor
hereto, and all applications incorporated herein by reference in their
entirety.
FIELD OF THE INVENTION
The present invention relates to sensing devices and, more specifically, to
devices for monitoring cardiac behaviour.
BACKGROUND AND SUMMARY OF THE INVENTION
Cardiovascular disease is a large, growing health problem world wide. Some
studies indicate that approximately 15% of the Western World suffers from one
or
n more cardiovascular disease. In the United States, nearly 25% of the
population is
affected, resulting in more than six million hospitalizations every year.
Various devices exist for monitoring certain parameters relating to cardiac
performance. In some instances, in vivo parameters of a patient may need to be
monitored over a period of time: for example, such monitoring may be necessary
in a
subject who has occasional irregular cardiac beats. Heart arrhythmias are
changes
in the normal sequence of electrical impulses that cause the heart to pump
blood
through the body. As such abnormal heart rhythms may only occur sporadically,
continuous monitoring may be required for detection. By providing continuous
monitoring, medical personnel determine if there is a tendency for production
of
n sustained irregular beats in a life-endangering fashion. Medical personnel
also use
the monitoring results to establish a proper course of treatment.
One prior art device that measures heart rate is the "Reveal" monitor by
Medtronic (Minneapolis, MN, USA). This device comprises an implantable heart
-1-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
monitor used, for example, in determining if syncope (fainting) in a subject
is related
to a heart rhythm problem. The Reveal monitor continuously monitors the rate
and
rhythm of the heart for up to 14 months. After waking from a fainting episode,
the
subject places a first recorder device external to the skin over the implanted
Reveal
monitor and presses a button to transfer data from the monitor to the
recorder. The
subject gives the first recorder to a physician who provides the subject with
a second
recorder to use for continued data acquisition. The physician then analyzes
the
information stored on the first recorder to determine whether abnormal heart
rhythm
has been recorded. The use of the recorder is neither automatic nor autonomic,
and
0 therefore requires either the subject to be conscious or another person's
intervention.
Another known type of implantable monitoring device is a transponder-type
device, in which a transponder is implanted in a patient and is subsequently
accessed with a hand-held electromagnetic reader in a non-invasive manner. An
example of the latter type of device is described in US Patent 5,833,603.
In many circumstances, medical personnel are interested in collecting a
variety of different types of data relating to the behaviour of the heart and
the
condition of the patient. Moreover, as mentioned above, it is desirable to
obtain as
much relevant data as possible without requiring the patient to visit health
care
provider. Relevant information may include the oxygen saturation level of
blood
n flowing through the aorta, blood pressure, heart rate, blood flow, stroke
volume,
cardiac output, the electrical activity of the heart (for generating
electrocardiogram
(ECG) data), and body temperature.
An integrated heart monitoring device for acquiring signals and transmitting
data is disclosed herein. In one embodiment of the invention, the monitoring
device
includes an optical sensor assembly including a plurality of photon emitters
and a
plurality of photon detectors for detecting a plurality of optical signals.
The emitters
and detectors face the aorta. A computing device operates the plurality of
emitters
and detectors and processes the plurality of optical signals to obtain optical
measurement values representing the location and size of the aorta and the
oxygen
n saturation of the blood flowing through the aorta.
The monitoring device further includes a Doppler sensor for emitting and
detecting a plurality of ultrasonic waves. The computing device also operates
the
Doppler sensor and, with the aid of the optical measurement values obtained
using
the optical sensor assembly, processes the plurality of ultrasonic waves to
obtain
-2-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
Doppler measurement values representing heart rate, blood flow, stroke volume,
blood pressure, and cardiac output.
The monitoring device further includes an ECG sensor for detecting the
electrical signals which cause the heart to pump. Additionally, the monitoring
device
includes a temperature sensor for measuring the temperature of the patient. An
energy storage device powers the computing device, the various sensors, and a
communication device which is configured to transmit the collected data, or
information relating to the collected data, according to a predetermined
schedule or
upon the occurrence of an event, such as abnormal data or a request for data
from
0 an external device. The sensors, the computing device, the communication
device,
and the energy storage device are enclosed in a housing, which may be worn by
the
patient or implanted.
By integrating the plurality of sensors and other components mentioned
above, embodiments of the present invention permit a single device, mounted at
one
location on the patient's body, to accurately measure a comprehensive set of
parameters relating to the behaviour of the heart, including cardiac output.
Moreover, the integrated monitoring device described herein may perform
analyses
of the parameters and perform functions in response to the "on-board"
analyses, as
opposed to other sensing devices that export raw data for analysis by another
n device. As indicated above, the integrated monitoring device according to
embodiments of the invention also communicates with other devices, wirelessly
or
otherwise, providing information and receiving commands and data. As such, the
monitoring device collects, analyzes, and communicates data without any human
intervention.
The features of this invention, and the manner of attaining them, will become
more apparent and the invention itself will be better understood by reference
to the
following description of embodiments of the invention taken in conjunction
with the
accompanying drawings.
Brief Description of the Drawings:
n Figure 1A is a schematic side view of a monitoring device according to one
embodiment of the invention.
Figure 1 B is an outwardly-facing view of the monitoring device of Figure 1.
Figure 1 C is a perspective view of the monitoring device of Figure 1.
-3-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
Figure 2 is a schematic side view of the monitoring device of Figure 1 and a
vessel.
Figure 3 is a schematic side view of a Doppler sensor according to one
embodiment of the invention.
Figure 4 is a conceptual view of fluid flowing through a vessel.
Figure 5 is a schematic representation of a temperature sensing circuit.
Figure 6 is a conceptual view of a computing device according to one
embodiment of the present invention.
Figure 7 is a conceptual view of a system adapted to transmit and receive
0 communication signals from the monitoring device of Figure 1.
Corresponding reference characters indicate corresponding parts throughout
the several views. Although the drawings represent embodiments of the present
invention, the drawings are not necessarily to scale and certain features may
be
exaggerated in order to better illustrate and explain the present invention.
The
exemplifications set out herein illustrate embodiments of the invention in
several
forms and such exemplification is not to be construed as limiting the scope of
the
invention in any manner.
DETAILED DESCRIPTION OF EMBODIMENTS OF THE INVENTION
The embodiments discussed below are not intended to be exhaustive or limit
n the invention to the precise forms disclosed in the following detailed
description.
Rather, the embodiments are chosen and described so that others skilled in the
art
may utilize their teachings.
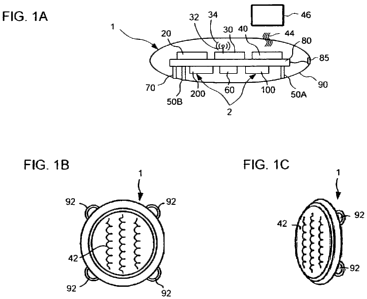
Fig. 1A depicts an integrated monitoring device according to one embodiment
of the present invention. The monitoring device 1 generally includes a
plurality of
components including an optical sensor assembly 2, a Doppler sensor 60, an ECG
sensor including probes 50A and 50B (hereinafter collectively referred to as
ECG
sensor 50), a temperature sensor 70, a computing device 20, a communication
device 30, and an energy storage device 40, each of the components mounted on
a
board 80 and being in electronic communication with computing device 20. The
n components are enclosed in a housing 90.
Throughout this application, references made to optical sensor assembly 2
refer to the optical sensor assembly 2 described in the Optical Sensor
Apparatus
application incorporated herein by reference above. Also, references to the
Doppler
sensor 60 refer to the Doppler sensor 60 described in the Doppler Motion
Sensor
-4-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
application incorporated by reference above. The full description of optical
sensor
assembly 2 and Doppler sensor 60 will not be repeated in this application.
In one embodiment according to the invention, monitoring device 1 is adapted
to measure the physiological behaviour of a patient's heart. By "patient" it
is meant a
person or animal. Although the invention disclosed herein is described in the
medical context, the teachings disclosed herein may be applicable in other
contexts
where compact data acquisition assemblies are desirable to perform
measurements
over time.
In one embodiment according to the invention, monitoring device 1 is
0 implanted subcutaneously in the patient's body. It should be understood,
however,
that monitoring device 1 may be implanted at different locations using various
implantation techniques. For example, monitoring device 1 may be implanted
within
the chest cavity beneath the rib cage. Housing 90 may be formed in the shape
of a
circular or oval disc, with dimensions roughly the same as two stacked quarter
dollar
coins. More specifically, housing 90 may be approximately three centimetres in
diameter and approximately one centimetre thick. Of course, housing 90 may be
configured in a variety of other shapes and sizes, depending upon the
application.
Housing 90 may include four outwardly projecting loops 92, shown in Figs. 1 B
and
1 C, for receiving sutures in order to fix the assembly subcutaneously within
the
n patient's body. More or fewer loops 92 may be provided depending upon the
shape
of housing 90. When so fixed, optical sensor assembly 2, Doppler sensor 60,
ECG
sensor 50, and temperature sensor 70 are positioned facing inwardly while an
energy coupler 42, which is described with particularity below, faces
outwardly.
In another embodiment of a monitoring device 1 according to the invention,
monitoring device 1 is integrated with an implanted cardiac device such as a
pacemaker, a Cardiac Resynchronization Therapy (CRT) device, an implantable
cardioverter defibrillator (ICD), etc. In such an embodiment, monitoring
device 1
may communicate with the implanted cardiac device and provide information from
the implanted cardiac device as well as from its own sensors to external
devices in
n the manner described in the System for Monitoring application incorporated
herein
by reference above. As many implanted cardiac devices are currently well-
understood and routinely prescribed, integration of monitoring device 1 into
such
other devices may provide an effective means for achieving market acceptance.
-5-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
The above-described integration may be achieved by combining the
components of monitoring device 1 and the cardiac device. If the cardiac
device
includes a computing device, for example, the algorithms that carry out the
functions
according to the invention may be incorporated with the computing device of
the
cardiac device instead of adding a second computing device. In a similar
manner,
energy storage and communication devices may be combined to avoid duplication
and lower cost. In one embodiment, some components of monitoring device 1 are
included within housing 90 and some components are included with the cardiac
device. The cardiac device and the components in housing 90 are operably
0 connected.
In another embodiment, monitoring device 1 is positioned externally to the
patient's body. A support member is provided to support monitoring device 1
externally to the body. The support member may be permanently or temporarily
coupled to monitoring device 1. In one embodiment, the support member
comprises
an adhesive layer for adhesively coupling the support member to the patient's
body.
In another embodiment, the support member comprises a belt, which may be
elastic,
for holding monitoring device 1 against the patient's body.
Monitoring device 1 may be implanted or positioned on the patient with the aid
of an external mapping system such as an ultrasound machine. Proper placement
n ensures that the aorta is located within the sensing range of the various
sensors of
monitoring device 1. For example, monitoring device 1 may be positioned on the
chest or back of the patient in a location that reduces interference by the
ribs of the
measurements acquired in the manner described herein.
1. OPTICAL SENSOR
As described in full detail in the Optical Sensor Apparatus application,
optical
sensor assembly 2, among other things, senses the oxygen saturation level of
the
patient's blood conveyed through the aorta. Sensing assembly 2 emits beams of
electromagnetic energy in the infrared (IR) range of the electromagnetic
spectrum
and detects IR signals reflected from haemoglobin in the aorta, which is the
iron-
containing oxygen-transport metalloprotein in red blood cells.
Fig. 2 illustrates the relationship between the aorta 3 conveying blood 4
having haemoglobin in red blood cells 5 and a pair of photocells, emitter 101
and
detector 201, included in emitter array 100 and detector array 200 of sensor
assembly 2, respectively. Emitter array 100 may include sixteen emitters 101-
116
-6-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
(only emitter 101 is shown) and detector array 200 may include sixteen
detectors
201-216 (only detector 201 is shown), each being paired with an emitter.
Sensor
assembly 2 and arrays 100, 200 may be selected from the 8572 family of optical
sensors manufactured by Motorola. Emitter 101 emits a beam of photons,
including
photon 101', some of which pass through aorta 3, and others of which are
reflected
off of red blood cells 5, including photon 101'. As described in the Optical
Sensor
Apparatus application, computing device 20 controls the operation of emitter
101 and
measures the time required for detector 201 to detect the reflected beam, and
interprets signals provided by detector 201 to determine the power or
intensity of the
0 reflected beam. This information, along with the known characteristics of
the emitted
beam, permits computing device 20 to determine a variety of characteristics of
aorta
3 and blood 4.
According to one embodiment of the invention, computing device 20
processes signals received from detector array 200 to calculate the time
required for
the emitted beam to travel from the emitter, to the aorta 3, and to the
detector as a
reflected beam. As the speed of the beam is known and the distance between the
emitter and detector is known, a simple calculation yields the distance from
monitoring device 1 to the aorta 3. Computing device 20 also uses the signals
from
detector array 200 to determine the power of the reflected beam received by
each
n detector in detector array 200. These power values are then used to
determine the
diameter of aorta 3 using the methods and principles described in the Optical
Sensor
Apparatus application.
It should be understood that in determining the diameter of the aorta in the
manner described above, each of emitters 101-116 are activated individually,
in
rapid succession. In one embodiment of the invention, the beams are emitted
individually in a row-by-row scanning fashion, beginning with emitter 101,
which is
followed sequentially until emitter 116 is activated. In other embodiments,
other
sequences are followed.
As indicated above, optical sensor assembly 2 also provides monitoring
n device 1 with the ability to determine the oxygen saturation level of blood
carried by
the aorta. When making an oxygen saturation measurement, sensing device 1
activates all emitters 101-116 of emitter array 100 simultaneously. Having
already
determined the size and location of the aorta as described above, the expected
size
or area of the reflected beam received by detector array 200 may be computed
by
-7-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
computing device 20. The intensity or power of the reflected beam is, however,
unknown prior to this measurement. Detector array 200 receives the reflected
beam
and provides a signal indicating the intensity of the reflected beam. As the
intensity
of the emitted beam is known, the intensity measurement of the reflected beam
permits computation of the oxygen saturation percentage of the blood in the
aorta.
Under normal operating conditions, monitoring device 1 may perform an oxygen
saturation measurement once or twice per day.
In one embodiment, monitoring device 1 also calculates cardiac pulse. As
discussed previously, detectors 201-216 produce power signals representative
of
0 iron content in blood. As the heart pumps oxygenated blood through the
aorta, the
power signals fluctuate. A plurality of power signals may be obtained in rapid
succession to capture the power measurement fluctuation. More specifically, by
performing many oxygen saturation measurements (e.g., ten times per second),
over
a period of time (e.g., fifteen seconds), the saturation measurements will
exhibit a
pattern or periodicity that represents the beating of the heart. Computing
device 20
may determine a curve to fit the saturation measurements, such as a sinusoidal
curve, which corresponds directly to the cardiac cycle. Computing device 20
may
determine the frequency of peak values of the curve to determine its period.
Each
period represents a cardiac cycle. By multiplying the number of cardiac cycles
in the
n sample period (e.g., fifteen seconds) by an appropriate factor, computing
device 20
may determine pulse rate in terms of cardiac cycles per minute. In one
embodiment,
computing device 20 stores cardiac pulse values as normal reference values and
detects an abnormal or irregular cardiac rhythm by comparing cardiac pulse
values
to reference values.
2. DOPPLER SENSOR
In an embodiment where monitoring device 1 includes Doppler sensor 60,
aorta diameter and location provided by optical sensor assembly 2 may be used
to
calculate the velocity of blood flowing through the aorta, the volume of blood
flowing
through the aorta, the patient's blood pressure, and the cardiac output. These
n parameters may be used to calculate and diagnose an abnormal condition
relating to
cardiac output. As is fully described in the Doppler Motion Sensor application
referenced above, one embodiment of Doppler sensor 60 includes three
transducers
for insonating the aorta and receiving reflected ultrasonic waves. The
velocity of the
blood in the aorta is determined by directing the ultrasonic waves towards the
blood
-8-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
at a known angle, measuring the frequency shift of the reflected ultrasound
energy,
and then calculating the velocity of the blood. More specifically, the Doppler
frequency shift is proportional to the component of the velocity vector that
is parallel
to the insonifying wave. The velocity v of the blood is determined by the
following
equation:
v=fd c /(2 f cos 0)
where c is the velocity of sound in blood, f is the frequency of the
insonifying
wave, 0 is the angle between the wave and the velocity vector, and fd is
Doppler
frequency shift.
n Doppler sensor 60, which may be a continuous wave sensor or a pulsed wave
sensor, measures frequency shift by comparing phase shifts between
subsequently
received waves according to principles that are well known in the art. As the
distance between Doppler sensor 60 and the aorta has already been determined
by
optical sensor assembly 2, and the speed of sound through tissue is known, the
frequency shift permits computing device 20 to determine the actual velocity
of blood
in the aorta.
Fig. 3 illustrates Doppler sensor 60 including linear array transducers A, B
and
C according to one embodiment of the invention. Each of transducers A, B and C
is
operably connected with a driver device (not shown) that powers the
transducer.
n Transducers A, B and C are disposed at an angle relative to each other.
Transducers B and C are disposed at a 45 degree angle relative to transducer A
and
at 90 degrees relative to each other. Other relative angles among the
transducers
may be used. Each of transducers A, B and C may be driven at a different
frequency to distinguish the source of the reflected waves received by Doppler
sensor 60. For convenience, each transducer in a linear array is referred to
herein
as a transducer segment. In the embodiment shown, each linear array transducer
comprises five transducer segments. Transducer segments may be operably
connected to be activated separately or concurrently. Separate activation of
one or
more transducer segments is desirable to limit power consumption.
n As shown, transducer A includes segments Al-A5, transducer B includes
segments B1 -155, and transducer C includes segments C1 -C5. Each segment may
transmit and receive ultrasonic energy in the form of waves. The arrows
originating
at each segment and projecting perpendicularly to the segment represent the
direction of waves transmitted by each segment. Further, arrows 72, 74, and 76
-9-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
represents the directions of waves produced by transducers A, B and C, in
aggregate, respectively. In one embodiment, one or more segments of transducer
A
are energized at a frequency of 5 Mhz, one or more segments of transducer B
are
energized at a frequency of 4.5 Mhz, and one or more segments of transducer C
are
energized at a frequency of 5.5 Mhz. Frequency selection is a function of the
distance between the transducer and the target fluid and is selected
accordingly. A
reflected wave may be measured at each segment of a linear array transducer.
Each segment may be energized sequentially and may be energized a plurality of
times.
n The Doppler shift, or frequency shift, is proportional to the component of
the
velocity vector parallel to the impinging wave. Since the Doppler shift
depends from
the cosine of the angle 0 between the wave and the velocity vector, and the
cosine
function ranges between 0 and 1, signals produced by waves oriented parallel
to the
velocity vector produce selected signals and, as the angle 0 increases, it
becomes
increasingly more difficult to determine the Doppler shift from those signals.
As is
fully described in the Doppler Motion Sensor application, the K-shape
arrangement
of transducers A, B, C ensures that at least one of the transducers is
oriented at an
acceptable angle 0. Three transducers enable Doppler sensor 60 to obtain a
sufficient number of signals even when the relative position of Doppler sensor
60
n and aorta 3 change slightly with time or other factors such as a patient's
activity level
and posture. When broad waves are transmitted, reflected waves may be received
by each transducer. However, since waves have frequencies corresponding to
each
transmitting transducer, Doppler sensor 60 is able to select which signals to
filter
based on the relative position of the corresponding transmitting transducer
and its
transmission frequency.
As discussed previously, the calculation of blood velocity requires knowledge
of the incident angle 0 between the emitted waves and aorta 3. Incident angle
and
other data characterizing the relative position of aorta 3 and Doppler sensor
60 may
be obtained in various ways. Once obtained, it may be stored in memory 26 (see
n Fig. 6) as reference values. In one embodiment, the relative position data
is
provided to computing device 20 by optical sensor assembly 2. With this
information, computing device 20 computes a blood velocity value by comparing
the
frequency of the transmitted and the received waves according to well known
frequency-shift and angle algorithms or tables.
-10-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
The velocity of the blood flowing through the aorta is different depending
upon
which portions of the blood are being measured. According to well-understood
principles of fluid dynamics, fluid flowing near the outer wall of a vessel
flows more
slowly than fluid flowing through the central axis of the vessel because of
shear
stresses between the fluid and the outer wall. The distance and diameter
measurements provided by optical sensor apparatus 2 permit computing device 20
to determine, from the measurements of Doppler sensor 60, the location of
aorta 3
within the reflected wave detected by Doppler sensor 60. Each of the five
velocity
measurements in each of the three sets of measurements taken in the manner
0 described in the Doppler Motion Sensor application is averaged to determine
an
approximate overall blood flow through aorta 3 and to account for the velocity
profile
across the diameter of aorta 3. The sets of velocity measurements are taken at
the
maximum (systolic) and minimum (diastolic) flow conditions of the cardiac
cycle.
Accordingly, by averaging the five velocity measurements for each of the three
sets
of maximum flow measurements, and averaging the three results from those
calculations, computing device 20 determines an average maximum flow
measurement. An average minimum flow measurement is computed in a similar
fashion. Finally, the average minimum and average maximum blood flow
measurements are averaged to determine the mean blood flow for the patient.
n Next, computing device 20 may compute stroke volume by simply multiplying
the mean blood flow measurement described above by the area of aorta 3 (i.e.,
mean blood flow * rrr2), where r is the radius of aorta 3. Of course, the
radius of
aorta 3 is simply one half of the aorta diameter, which is determined using
optical
sensor assembly 2 in the manner described above.
3. ECG SENSOR
ECG sensor 50 is, in one embodiment of the invention, a single lead device
including an anode probe 50A and a cathode probe 50B (collectively referred to
herein as "ECG sensor 50"). ECG sensor 50 uses probes 50A, 50B to detect
changes in voltage of the electrical impulses provided to the heart muscles.
As is
n well understood in the art, these electrical signals which trigger heart
beats normally
start at the top of the heart in the right atrium, and travel from the top of
the heart to
the bottom. They cause the heart muscle to contract as they travel through the
heart. As the heart contracts, it pumps blood out to the rest of the body. By
monitoring the electrical activity of the heart over time, ECG sensor 50
permits
-11-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
computing device 20 to determine how fast the heart is beating (i.e., pulse)
by
determining the number of cardiac cycles per minute (or fraction of a minute).
The
pulse measurement may be used with the stroke volume discussed above to
determine cardiac output. More specifically, the stroke volume measurement may
be multiplied by the number of strokes per minute (i.e., pulse) to determine
the total
volume of blood displaced per minute (i.e., cardiac output).
In one embodiment of the invention, the pulse measured by ECG sensor 50 is
compared to the pulse measured by optical sensor assembly 2 as described in
the
Optical Sensor Apparatus application. If the measurements differ by more than
a
0 predetermined amount, the ECG pulse measurement may be discarded under the
assumption that electrical interference or some other disturbance caused
errors in
the detected signals. In this manner, optical sensor assembly 2 functions as a
backup pulse measurement device for ECG sensor 50.
ECG sensor 50 provides voltage measurements to computing device 20,
which in turn processes the data by filtering out frequencies outside the
known range
of frequencies generated by the heart's electrical activity. ECG sensor 50 is
also
mounted within housing 90 in a manner and location that electrically isolates
ECG
sensor 50 from the other electronic components of monitoring device 1 to
minimize
the electrical interference caused by those other electronic devices. In one
n embodiment of the invention, the output of ECG sensor 50 is passed through a
band
pass filter having a lower cut-off frequency and an upper cut-off frequency.
Additionally, computing device 20 may further process the data to produce a
smooth
ECG trace by applying any of a number of suitable digital smoothing functions.
The output of ECG sensor 50 also permits computing device 20 to identify the
maximum and minimum blood flow through the aorta, which are used in the stroke
volume and cardiac output calculations described above. Additionally, It shows
the
heart's rhythm (steady or irregular) and where in the body the heart beat is
being
recorded. It also records the strength and timing of the electrical signals as
they
pass through each part of the heart.
n Referring now to Fig. 4, ECG sensor 50 and Doppler sensor 60 are used in
combination to determine the blood pressure of the patient directly from the
aorta 3.
One skilled in the art may, using the ECG trace provided by ECG sensor 50,
determine with accuracy the maximum blood flow position of the cardiac cycle
and
the minimum blood flow position. Doppler sensor 60 facilitates determination
of the
-12-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
speed or velocity of blood in aorta 3 under the maximum and minimum blood flow
conditions in the manner described above. Computing device 20 converts the
velocity measurements under each condition into pressure measurements using
the
known internal surface area of aorta 3 (determined from the diameter
measurements
facilitated by optical sensor assembly 2) according to principles dictated by
Bernoulli's equation. These pressure measurements reflect the dynamic pressure
of
the blood flowing through aorta 3 under the maximum and minimum flow
conditions.
More specifically, at T, of Fig. 4, the dynamic pressure PD, corresponds to
the
pressure determined from the velocity measurements taken under maximum blood
0 flow conditions. At T2, PD2 corresponds to the pressure determined from the
velocity
measurements taken under minimum blood flow conditions. It is well known that
the
total pressure of a fluid flowing through a vessel is the sum of the dynamic
pressure
and the static pressure. In the case of the aorta 3, the static pressure
(depicted as
force arrows directed outwardly against the outer wall of the aorta 3) under
maximum
flow conditions (PS1) directly corresponds to the systolic blood pressure
measurement and the static pressure under minimum flow conditions (PS2)
directly
corresponds to the diastolic blood pressure measurement.
The systolic and diastolic blood pressure measurements may be derived by
further computing the total pressure (PT) of blood flowing through aorta 3 and
taking
n advantage of the fact that the total pressure through the aorta at T, (i.e.,
PT,) must
be the same as the total pressure at T2 (i.e., PT2). Total pressure is derived
by
computing the change in pressure from the minimum flow conditions to the
maximum
flow conditions. This change or acceleration, in conjunction with the stroke
volume
and the known elasticity of aorta 3, permits computing device 20 to determine
total
pressure according to well known principles in the art. Thus, at time T1, the
equation
PT, = PS1 + PD, may be solved for PS1 and at time T2, the equation PT2 = PS2 +
PD2 may be solved for PS2. As indicated above, PS1 and PS2 are the systolic
and
diastolic blood pressure measurements, respectively.
It should be understood that although the blood pressure computation
n described above refers to determining blood pressure in aorta 3, the same
process
may be carried out to determine blood pressure in the pulmonary artery,
assuming
the pulmonary artery is within the sensing range of monitoring device 1. As
described in the Optical Sensor Apparatus application, monitoring device 1
distinguishes between the pulmonary artery and aorta 3 by measuring the oxygen
-13-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
saturation of both, and determining which vessel carries blood with higher
oxygen
saturation. That vessel must be aorta 3. In another embodiment of the
invention,
monitoring device 1 instead identifies the vessel with lower oxygen saturation
as the
vessel of interest (i.e., the pulmonary artery). The location and size of the
pulmonary
artery is then determined in the same manner as described with reference to
aorta 3.
With the geometry of the pulmonary artery defined, the pressure of the blood
flowing
through the pulmonary artery is measured as described above with reference to
aorta 3.
In one embodiment of the invention shown in Fig. 1A, housing 90 includes a
0 connector 85 to permit connection of additional ECG leads. Connector 85 is
electrically coupled to ECG sensor 50 and other components of monitoring
device 1
through board 80. When additional ECG leads are connected to connector 85,
connector 85 passes signals from the additional leads to ECG sensor 50. As is
known in the art, the additional leads may be affixed to various locations on
the
chest, back, arms, or legs of the patient, and each lead includes a receiver
for
detecting electrical activity. As indicated above, connector 85 may also
function as a
I/O port for monitoring device 1, permitting downloading of data to a docking
station
304 and uploading of data and instructions during a programming operation.
4. TEMPERATURE SENSOR
n A variety of different devices may function as temperature sensor 70.
Temperature sensor 70 is, in one embodiment of the invention, a readily
available
resistance temperature detector (RTD). In general, temperature sensor 70 may
include a metallic component (wire wound or thin film) with the physical
property of
an electrical resistance which varies with changes in temperature. Typically,
the
higher the temperature in the environment of temperature sensor 70, the
greater the
electrical resistance across the metallic component of temperature sensor 70.
Temperature sensors having a platinum metallic component may be desirable
because of the nearly linear relationship between resistance and temperature
platinum exhibits over a fairly wide temperature range. Of course, one skilled
in the
n art could readily adapt a temperature sensor having a non-linear
temperature/resistance curve, so long as the sensor's behaviour over the
temperature range of interest is suitably repeatable.
As shown in Fig. 5, temperature sensor 70 is coupled to a constant current
source 100, which is also located within housing 90. Current source 100
maintains a
-14-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
constant current through temperature sensor 70 as the resistance of
temperature
sensor 70 changes with temperature. Accordingly, the voltage across
temperature
sensor 70 (VT) changes in direct proportion to the changes in temperature.
More
specifically, according to Ohm's law, Voltage = current'` resistance. With a
constant
current source, changes in resistance due to temperature changes are detected
as
changes in VT. In one embodiment of the invention, VT is passed through analog-
to-
digital converter 22, which is read by computing device 20. Computing device
20
then determines the measured temperature and stores the temperature
measurement in memory 26.
n It should be understood that although Fig. 5 depicts a constant-current,
voltage measurement circuit for use in conjunction with temperature sensor 70,
a
variety of different circuits could readily be adapted for use with
temperature sensor
70, including circuits that measure changes in current passing through
temperature
sensor 70.
Temperature sensor 70 is mounted within housing 90 such that the
temperature sensitive component of temperature sensor 70 is adjacent the outer
surface of housing 90, and substantially thermally isolated from any thermal
energy
that may be created by the operation of the other electronic components
mounted
within housing 90. In this way, temperature sensor 70 is positioned to detect
n temperature changes in the body of the patient (either when monitoring
device 1 is
implanted or when it is worn by the patient) as opposed to changes in
temperature of
the electronics of monitoring device 1. It should be understood, however, that
temperature sensor 70 may also be calibrated to compensate for changes in
detected temperature due to thermal energy from monitoring device 1. Memory 26
of computing device 20 includes a look-up table relating the digital voltage
across
temperature sensor 70 to temperature, according to the specified operational
characteristics of temperature sensor 70. Computing device 20 periodically
reads
the digital voltage signal, accesses the look-up table in memory 26, and
determines
the current body temperature of the patient. The temperature is stored in
memory 26
n and may be transmitted from monitoring device 1 in the manner described
below
with reference to communication device 30.
2. COMPUTING DEVICE
Computing device 20 comprises a plurality of components. While
components are described herein as if they were independent components, the
-15-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
components may be combined in a single device such as an application specific
integrated circuit. As shown in Fig. 6, computing device 20 includes an A/D
converter 22 (which also converts optical signals to digital signals), a
processor 24, a
memory 26, a program 28, inputs 23, and outputs 25. Memory 26 may include, but
is not limited to, RAM, ROM, EEPROM, flash memory or other memory technology.
A/D converter 22, processor 24 and memory 26 may be constructed in an
integrated
circuit. The integrated circuit may further include emitter array 100,
detector array
200, and communication device 30.
Program 28 represents computer instructions directing processor 24 to
0 perform tasks responsive to data. Program 28 resides in memory 26. Data,
including reference data and measurement data, also resides in memory 26.
Reference data may be stored in ROM or it may be stored in RAM so that it may
be
modified over time, either in response to external inputs or in response to
characteristics of measurement data collected over time. Protocols for
responding to
measurement values may also be provided. Protocols may be stored in permanent
memory or may be stored in non-permanent memory such as RAM and are
described in further detail in the System for Monitoring application reference
above.
Computing device 20 may be configured to cause communication device 30
to transmit an alert if an abnormal condition is detected, particularly a
condition
n determined to be a serious or dangerous condition according to a prescribed
protocol. The alert may be used to actuate an alarm or to alert the patient to
take
remedial action. A remedial action may be terminating or reducing physical
activity.
The alert may also provide global positioning (GPS) information to an
emergency
service. Referring to Fig. 7, the abnormal condition, when found to be
present, may
also be displayed on a computer 36 and/or transmitted via communication device
30
to a caregiver. The alert may comprise a text message or a code corresponding
to
the condition. Computing device 20 may also initiate a new measurement cycle
and
measure on a continuous basis in response to the detection of an abnormal
condition.
n Computing device 20 may also initiate a treatment. Monitoring device 1 may
receive, through communication device 30, an external command to perform a
treatment in response to the alert. Optionally, based on the protocol, an
abnormal
condition may also be used to direct a device adapted to provide treatment to
deliver
-16-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
such treatment. Treatment may include, for example, an electric shock or a
drug
delivery.
Parameter values and/or other information may be communicated to an
external device. The parameter values may be stored in memory 26 and
transmitted
wirelessly by communication device 30. The communication signal from
communication device 30 may be activated on a periodic basis (e.g., once per
day,
once per week, etc.), in response to an abnormal condition, in response to an
externally received command, whenever memory usage exceeds a predetermined
amount, or whenever the energy storage level is determined to be low, the
latter two
0 conditions established to prevent data loss as a result of memory overflow
or energy
loss. It should also be understood that monitoring device 1 may include
communication devices in addition to communication device 30. For example,
where
communication device 30 is a cellular modem, monitoring device 1 may also
include
a backup Bluetooth or RF communication device. Such a backup device may be
desirable in situations where, after one or more attempts, it becomes apparent
that
the cellular modem is unable to transmit information (e.g., due to low
available
power, poor network coverage, etc.). In such a situation, computing device 20
may
activate the backup communication device to transmit information or an alert
to an
alternate external receiving device.
n Alternatively or in addition to the above-described transmissions, computing
device 20 may be programmed to respond to requests for data received by
communication device 30 (e.g., from a health care provider) by causing
communication device 30 to transmit the requested data or information
representing
the requested data.
The communication signal may be received by equipment near the patient
to alert the patient to the condition, or received remotely (such as over a
network) by
a healthcare provider, relative, or other predetermined recipient. Further
description
of a networked system including at least some of the principles of the present
invention is provided in the System for Monitoring application reference
above.
n 3. COMMUNICATION DEVICE
In one embodiment of the invention, communication device 30 is a two-way
communication device, e.g. via the cellular telephone system and/or the GPS
satellite system, such as NOKIA model number KNL1147-V. In an alternate
embodiment, communication device 30 is capable of transmitting information,
but
-17-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
does not receive information or commands. As shown in Fig. 1 A, communication
device 30 includes an antenna 32 for transmitting and receiving communication
signals. The communication signals, represented by symbol 34, travel
wirelessly to
and from one of a plurality of optional external communication devices.
Referring again to Fig. 7, an external communication device may be a
computer 302 or any electronic device capable of wirelessly receiving a
communication signal, such as telephone 306 which is exemplified as a cellular
phone. By communication signal is meant a signal that has one or more of its
characteristics set or changed to encode information in the signal. By way of
0 example, and not limitation, communication signals include acoustic, RF,
infrared,
other wireless media, and combinations of any of the above. An external
communication device may also be a relay unit located externally of the
patient's
body, e.g. clipped to the patient's belt. The relay unit may include a
receiver for
receiving the transmissions from communication device 30, and a transmitter
for re-
transmitting the communication signal to another external communication
device.
The relay unit may also be stationary and hardwired for connection to the
internet or
direct connection to a healthcare provider's computer. Likewise, the relay
unit may
receive a communication signal from a healthcare provider and transmit the
signal to
communication device 30.
n 4. ENERGY STORAGE DEVICE
Referring again to Figs. 1A-1C, a system for recharging energy storage
device 40 may be provided in one embodiment according to the invention.
Computing device 20 receives energy from energy storage device 40. Energy
storage device 40 includes an energy storage component such as a battery.
Optionally, monitoring device 1 may also include an energy coupler for
receiving
energy from an external source to charge energy storage device 40.
One example of an energy coupler is an electromagnetic device, such as
induction coils 42, for receiving external electromagnetic signals 44 and
converting
such signals into electrical energy for recharging the energy storage
component. An
n external electromagnetic device 46 generates electromagnetic signal 44 which
is
received and converted into electrical energy by energy storage device 40.
Energy
storage device 40 may provide a charge signal to computing device 20.
Computing
device 20 may compare the charge signal to a reference charge signal and
initiate a
low charge communication signal for alerting the patient and/or healthcare
providers.
-18-
CA 02722662 2010-10-26
WO 2009/138883 PCT/IB2009/006088
Alternatively, a detector, such as a voltage sensor, may be used to monitor
the
charge of energy storage device 40 and provide a signal to computing device 20
when the charge falls below a threshold. Electromagnetic device 46 may be
placed
near monitoring device 1 to charge energy storage device 40.
Energy may instead, or additionally, be provided in the form of ultrasonic
vibrations. For example, a piezoelectric transducer may be included in
monitoring
device 1. An ultrasonic vibration may be provided externally. The transducer
generates electricity when driven by ultrasonic vibrations. As indicated
herein,
energy or power may also be provided to monitoring device 1 through connector
85.
n While this invention has been described as having an exemplary design, the
present invention may be further modified within the spirit and scope of this
disclosure. This application is therefore intended to cover any variations,
uses, or
adaptations of the invention using its general principles. Further, this
application is
intended to cover such departures from the present disclosure as come within
known
or customary practice in the art to which this invention pertains. For
example, it
should be understood that each of or some of optical sensor assembly 2,
Doppler
sensor 60, ECG sensor 50, and temperature sensor 70 may be modular in design.
As such, a plurality of different Doppler sensors 60, for example, may be
produced to
have different performance characteristics (e.g., different output
frequencies).
n Depending upon the application, any of the plurality of the sensors may be
installed
in monitoring device 1 to achieve the desired performance. Once monitoring
device
1 is equipped with the selected sensors, computing device 20 may be programmed
to adapt the various algorithms to accommodate the selected sensors. In this
manner, a basic monitoring device 1 including computing device 20,
communication
device 30, etc., may be "custom" built with any of a variety of sensors and
programmed to operate with the selected sensors.
As another example, it should be understood that while optical sensor
assembly 2, Doppler sensor 60, and temperature sensor 70 are described herein
as
being activated to obtain measurements relatively infrequently (at least under
normal
n conditions) to conserve power, as battery technology improves, the frequency
of
activation of these sensors may be increased. Also, where monitoring device 1
is
worn externally, connector 85 may be used to supply power to monitoring device
1,
thereby eliminating the power consumption concern and permitting frequent, or
even
continuous, operation of these sensors.
-19-