Note: Descriptions are shown in the official language in which they were submitted.
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-1-
COLOSTRUM-DERIVED FRACTION FOR WOUND
HEALING AND SKIN CARE
FIELD OF INVENTION
[0001] The present invention relates to a colostrum-derived mixture enriched
in
growth factors. Particularly, the invention provides a bovine colostrum
fraction for
the treatment of skin injury, diseases, wounds or ulcers. More particularly
the
invention provides a topical or oral composition that includes a
pharmaceutically
effective amount of bioactive growth factors and antimicrobial peptides
originating
from bovine colostrum, for the treatment of skin injury, diseases, wounds or
ulcers.
BACKGROUND
Skin function
[0002] Skin, as the outermost organ in the human body, continuously confronts
the
external environment and serves as a primary defense system or protective
barrier
against the environment (Lee et al. 2006). In addition to keeping the harsh
external
environment away from our own critically sensitive internal biological
environments,
skin acts as a regulator of body temperature and a sealant against fluid loss.
The
protective functions of skin can be classified as physical, thermal, immune,
ultra-
violet, oxidant radical, antimicrobial and a permeability barrier.
[0003] Following an injury (ulcer, cut, laceration, gash, tear, scrape,
abrasion,
scratch, floor burn, bruise, bite, UV radiation or excessive pressure), loss
of the
integrity of large portions of the skin may lead to major disability or even
death due
to an increased susceptibility to fungus, bacteria, and viral infections.
Skin wound healing
[0004] A scar is the end result or end point of the body to close a wound, and
it is a
normal process that occurs whenever an injury involves the dermis. The ideal
end
point is total regeneration, with new tissue having the same structural,
aesthetical,
and functional attributes as the original uninjured skin. However, in man and
domestic animals, unstructured scarring in the skin after trauma, surgery,
burns or
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-2-
sports injury is a major medical problem, often resulting in adverse
aesthetics, loss
of function, restriction of tissue movement and/or growth and adverse
physiological
effects. Current treatments are empirical, unreliable and unpredictable; there
are no
prescription drugs for the prevention or treatment of scarring (Bayat et al.
2003).
[0005] Skin wounds normally heal in a very orderly and efficient manner
characterized by four distinct, but overlapping phases; 1) hemostasis, 2)
inflammation, 3) proliferation and 4) remodeling (Singer & Clark 1999).
1) Hemostasis
[0006] Tissue repair begins with clot formation and platelet degranulation,
which
release the growth factors necessary for wound repair. Platelet-derived growth
factors are biologically active substances that enhance tissue repair
mechanisms
such as chemotaxis, cell proliferation, angiogenesis, extracellular matrix
deposition,
and remodeling (Werner & Grose 2003).
2) Inflammation
[0007] Infiltrating neutrophils cleanse the wounded area of foreign particles
and
bacteria and are extruded with eschar or phagocytosed by macrophages. In
response to specific chemoattractants, such as fragments of extracellular-
matrix
proteins, growth factors, and monocytes chemoattractants protein 1, monocytes
also infiltrate the wound site and become activated macrophages that release
various growth factors (Werner & Grose 2003).
3) Proliferation
[0008] Within hours after injury, the proliferation phase begins. The
proliferative
phase is characterized by the increased formation of granulation tissue where
fibroblasts lay bed of collagen and produces new capillaries, the contraction
of the
wound (wound edges pull together to reduce defect) and finally
epithelialization of
the affected area. Growth factors in concert with extracellular-matrix
molecules,
presumably stimulate fibroblasts of the tissue around the wound to proliferate
(Werner & Grose 2003). The formation of new blood vessels is necessary to
sustain
the newly formed granulation tissue. Wound contraction involves a complex and
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-3-
superbly orchestrated interaction of cells, extracellular matrix, and
cytokines. The
contraction probably requires stimulation by growth factors (Werner & Grose
2003).
4) Remodeling
[0009] Collagen remodeling during the transition from granulation tissue to
scar is
dependent on continued synthesis and catabolism of collagen at a low rate. The
degradation of collagen in the wound is controlled by several proteolytic
enzymes
termed matrix metalloproteinases, which are secreted by macrophages, epidermal
cells, and endothelial cells, as well as fibroblasts.
[0010] Chronic wounds may be stopped in any of the four phases, commonly;
however, disruption occurs in the inflammatory or proliferative phases. Many
mediators including inflammatory cells, growth factors, proteases and cellular
and
extracellular elements play an important role in different stages of the
healing
process. Alterations in one or more of these components may account for the
impaired healing observed in chronic wounds.
Growth factors and wound healing
[0011] As suggested above, and as reviewed by Werner & Grose (2003), growth
factors are involved in all steps of wound healing. Furthermore, multiple
studies
have demonstrated a beneficial effect of exogenous growth factors like PDGF,
FGF,
EGF, VEGF, TGF and IGF for wound healing (Werner & Grose 2003). In fact, PDGF
was the first growth factor to be approved for the treatment of human ulcers
(Regranex gel ).
Insulin-like growth factor
[0012] IGF-I and -II are small peptides, approximately 7 kDa in size, that are
structurally similar to insulin. IGF-I and -II are growth factors that have
both
mitogenic and metabolic actions that participate in the growth, survival and
differentiation of a number of cell types and tissues (Cohick & Clemmos 1993,
Stewart & Rotwein 1996). IGFs are unique among growth factors in that they can
act both synergistically, as a hormone, and locally, as autocrine/paracrine
factors
(Cohick & Clemmos 1993, Stewart & Rotwein 1996, Butler & LeRoith 2001).
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-4-
Although liver is the principal source of circulating IGFs, it is now believed
that brain,
muscle and bone also produce IGFs (Cohick & Clemmos 1993, Stewart & Rotwein
1996, Butler & LeRoith 2001).
[0013] In vivo IGFs are found complexed with specific IGF-binding proteins
(IGFBPs) (Shimasaki & Ling 1991). Most IGF-I present in the circulation as
part of a
150-kDa complex, comprising IGFBP-3 and an acid-labile subunit, which is
thought
to regulate the bioavailability of IGF to the tissues and to prolong the half-
life of IGFs
(Jones & Clemmons 1995).
[0014] IGFs are specifically expressed in granulation tissue and several
studies
have suggested a role of the IGF system in the wound healing abnormalities
associated with diabetes and glucocorticoids treatment (Bitar &Labbad 1996,
Bitar
2000, Blakytny et al. 2000). More specifically, these studies suggested that a
reduced expression of IGFs and/or their receptors leads to impaired wound
healing,
although this hypothesis has yet to be confirmed by functional studies.
Additionally,
IGFs act in synergy with other growth factors to promote healing (Werner &
Grose
2003).
[0015] The role of IGFBPs is less clear. It has been shown that the co
administration of IGFBP-I with IGF-I can enhance the stimulatory wound-healing
properties of IGF-l (Tsuboi et al. 1995) and IGF complexes with IGFBP-3 has a
greater effect on wound tissue formation than IGF alone (Campbell et al.
1999),
suggesting a vital role of IGFBPs in the regulation of IGF-I action at the
cellular
level.
Antimicrobial peptides and wound healing
[0016] Antimicrobial peptides (AMPs) are predominantly small cationic
polypeptides
that are classified together due to their capacity to inhibit the growth of
microbes. As
effectors of innate immunity, AMPs directly kill a broad spectrum of bacteria,
fungi,
and viruses. In addition, these peptides modify the local inflammation
response and
activate mechanisms of cellular and adaptive immunity (Reddy et al. 2004).
[0017] Human skin produces antimicrobial agents that form an innate epithelial
chemical shield. These endogenous AMPs are molecules produced by the
epithelial
DOCSQU E: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-5-
surface of the host. In addition skin peptides play an integral role in
stimulating and
regulating wound healing and inflammation. Two major classes of peptides have
been identified in mammalian skin; (1) defensins and (2) cathelicidins.
[0018] The defensins are cationic peptides (MW 3-5 kDa) divided in three
subfamilies, a-, R-, and circular 0-defensins based on the alignment of the
disulphide bonds. AMPs of the defensins family exhibit broad activity against
gram-
negative bacteria, fungi, mycobacteria and enveloped viruses and have been
isolated form neutrophils granules, macrophages and some specialized
epithelial
cells of the small intestine (Yang et al. 2007).
[0019] More specifically, a- and 3-defensins show a broad antibacterial
activity
against gram positive and negative bacteria (Ericksen et al. 2005, Ganz et al.
1985),
and have antifungal activity (Hoover et al. 2003). Defensins also have
antiviral
properties against adenovirus (Bastian & Schafer 2001), papilloma virus (Buck
et al.
2006), human immunodeficiency virus (HIV) (Chang et al. 2005), and herpes
simplex virus (Hazrati et al. 2006). Although directly antimicrobial in vitro,
the effect
of defensins on mammalian cells is an important component of how these
peptides
affect immunity. a-, R-defensins modify cell migration and maturation. R-
defensin are
chemoattractive for immature-dentritic cells and memory T-cells (Yang et al.
1999),
and also induce cytokines and other molecules secreted from host cells. a-
defensin
up-regulate the expression of TNF-a and IL-(3 in monocytes (Chaly et al.
2000).
[0020] The amphipathic structure and cationic charge of cathelicidin enable
the
latter to interact in the aqueous environment, the lipid-rich membrane, and
bind
negatively charged bacterial membranes. Cathelicidin peptides have broad
antimicrobial activity against gram-positive and negative bacteria (Nizet et
al. 2001),
vaccinia virus (Howell et al. 2004), and fungi (Lopez-Garcia 2005).
Cathelicidin
peptides are cationic and, like defensins, thought to directly bind to anionic
cell wall
and membrane of the microbe, increasing the permeability of the microbe cell
wall.
Cathelicidin also induces cellular signaling and activates keratinocytes and
leucocytes. Cathelicidin are chemoattractive to neutrophils, monocytes, and T
cells
and also promote angiogenesis (De Y et al. 2000). Transactivation of epidermal
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-6-
growth factor receptors by cathelicidin was observed in human epidermal
keratinocytes, and this induces keratinocytes migration (Tokumaru et al.
2005).
[0021] All Proline rich peptides (PRPs) isolated from mammals derive from the
cathelicidin. PRPs are very small peptide chains (3.5-6 kDa) of less then a
dozen
amino acids, with proline predominating (up to 50%). PRPs isolated from early
sheep colostrum have been shown to have regulatory effects on the immune
response (Wieczorek et al. 1979). PRP acts both in vitro and in vivo, and is
not
species specific. PRP increases permeability of skin vessels (Wieczorek et al.
1979), and causes differentiation of murine thymocytes into functionally
active T
cells (Januz & Lisowski 1993). It can simultaneously change surface markers
and
functions of the cell (Wieczorek 1989).
[0022] Evidence is accumulating that AMPs enter cells without membrane lysis
and,
once in the cytoplasm, bind to, and inhibit the activity of specific molecular
targets
essential to bacterial growth, thereby causing cell death. AMPs from mammals,
exerts other potentially exploitable biological activities, such as induction
of
syndecan expression in mesenchymal cells and inhibition of the NADPH oxidase
activity of neutrophils, suggesting a role of this peptide in wound healing
and
inflammation (Gennaro et al. 2002).
Colostrum
[0023] Colostrum is the early milk produced by mammals during the first
several
days post-parturition. Colostrum provides passive immunity to protect the
newborn
from opportunistic infections while the immune system is developing, as well
as to
facilitate the growth and immune maturation of the digestive tract and most
certainly
other tissues (Gopal & Gill 2000).
[0024] Colostrum has a nutrient profile and immunological composition that
differs
substantially from mature milk. In addition to macronutrients found in milk
such as
protein, carbohydrates, fat and micronutrients including vitamins and
minerals,
colostrum is rich in immunoglobulins, growth factors cytokines and nucleosides
but
also in oligosaccharides, antimicrobials, and immune-regulating factors (Thapa
2005). The typical composition of bovine colostrum is enumerated in Table 1.
DOCSQUG: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-7-
Colostrum as a source of growth factors
[0025] The presence of growth factors was first demonstrated in human
colostrum
and milk during the 1980's and then in bovine colostrum, milk and whey
(Gauthier et
al. 2006). Of significant interest is the fact that bovine growth factors
found in
colostrum are almost identical to that of human in both structures and
functions.
Specifically, colostrum represents an important source of EGF, IGF-I and II,
FGF,
PDGF, TGF, but also of interleukins and interferons (Table 1).
Colostrum as a source of antimicrobial peptides
[0026] Colostrum provides numerous antimicrobial factors that exhibit both
specific
and nonspecific bacteriostatic/bactericidal properties. Immunoglobulins are
among
the first line of protection that are delivered to the neonate through
suckling and
provide passively acquired immunity. Other proteins, such as the iron-binding
protein, lactoferrin (Clare et al. 2003), and enzymes, including lysozyme, and
lactoperoxidase play more of a direct role in inhibiting bacterial invasion
(Clare &
Swaisgood 2000). Recently, soluble defensins, cathelicidins, and toll-like
receptors
(TLRs) have been identified in human milk (Armogida et al. 2004).
[0027] Immunocompetent cell types, including macrophages, granulocytes, T and
B-lymphocytes are also present in colostrum. One thing of interest is the fact
that
the total antibacterial effect of colostrum is greater than the sum of the
individual
contributions of immunoglobulin and nonimmunoglobulin defense proteins. This
is
most likely due, at least in part, to their synergy.
Clinical uses of colostrum
[0028] The use of colostrum for the treatment of illness and for the
maintenance of
well-being dates back thousands of years. The Ayurvedic physicians and the
Rishis
of India have been using colostrum for medicinal purposes since cows have
become
domesticated. The remarkable thing about colostrum is that all these
substances act
synergistically in such a way as to enhance the overall effect of each
individual
component.
[0029] Available evidence suggests a beneficial effect of oral supplementation
of
colostrum in improving body composition, aspects of athletic performance,
diarrhea
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
in person with immune-deficiency syndromes, NSAID-induced gastrointestinal
disturbances and aspects of the acute phase response that occurs secondary to
surgery (Kelly 2003). Furthermore, some authors have claimed that topical
application of colostrum has benefic effects on surface wound and other skin
related
diseases. Other milk-derived products when taken orally or when applied
topically
have demonstrated therapeutic benefits (Kelly 2003).
Table 1. Composition of human and bovine colostrum. afrom Solomon 2002, bfrom
Gauthier et al. 2006.
Components
Water 78 %a
Carbohydrates 3.1 % a
Fat 3.6 % a
Protein 14.3 % a
Immunoglobulins
IgG 77 mg/ml a
IgM 4.9 mg/ml a
sIgA 4.4 mg/mI a
Lactoferrin + a
Lysozyme + a
Lactoperoxidase + a
Growth factors
IGF-l - 32-2000 ng/mlb
IGF-II - 150-600 ng/mlb
TGF-(31 - 12-43 ng/mlb
TGF-(32 -- 300 ng/mlb
EGF -4-325 ng/mlb
SUMMARY OF THE INVENTION
[0030] One aspect of the present invention is to provide a process for the
preparation or the production of a composition enriched in growth factors from
colostrum, and its uses thereof for the treatment of surface wounds and
ulcers.
[0031] More particularly, the process of the invention comprises the following
steps
of:
a') optionally thawing frozen whole colostrum to a temperature of at least
about 4 C;
DOCSQUE; 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-9-
a) precipitating casein from said colostrum and separating curds from
colostrum whey;
b) heating said colostrum whey to a temperature of greater than 40 C;
c) submitting said heated whey to ultra-filtration with a molecular weight cut-
off of about 14kDa or less and collecting permeate;
d) acidifying said permeate obtained in step c) to a pH of about 3 or above.
[0032] Optionally, the acidified permeate obtained in step d) is further
concentrated
with nanofiltration to obtain a liquid composition. Particularly, this liquid
composition
comprises at least 20 ng/ml of IGF-1; less than 5% (w/w) of protein and is
substantially free of lysozyme and TGF-13.
[0033] Further optionally, this liquid composition is freeze-dried to obtain a
solid
composition. Particularly, this solid composition comprises at least 1 ng of
IGF-1 per
mg of solid; less than 5% (w/w) of protein and is substantially free of
lysozyme and
TGF-(3.
[0034] A particularly aspect of the invention provide a composition enriched
in
antimicrobial peptides of molecular weight of less than about 15kDa,
preferably less
than 12 kDa, more preferably less than 1 OkDa. Particularly, the composition
of the
invention comprises physiological amount of these low molecular weight
peptides.
[0035] More particularly, the composition of the invention is substantially
free of
lysozyme and/or TGF-13.
[0036] Particularly, the composition of the invention comprises a total
protein
concentration of less than 5% (w/w), preferably less than 2% (w/w).
[0037] More particularly, the liquid composition of the invention comprises at
least
20 ng/ml of IGF-1, particularly, at least about 40 ng/ml.
[0038] More particularly, the solid composition of the invention comprises at
least 1
ng of IGF-1 per mg of solid, particularly, at least about 1.8 ng/mg.
[0039] A further aspect of the invention provides the use of the composition
as
defined herein for the manufacture of a formulation for the treatment of a
wound
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-10-
and/or facilitating and/or accelerating wound healing while inducing minimal
collagen contraction. Particularly, the composition is a topical or an oral
formulation.
[0040] A further aspect of the invention provides a method for healing a wound
while inducing minimal collagen contraction, said method comprising treating
said
wound with the formulation as defined herein.
[0041] Another aspect of the present invention is to provide a method for
treating
skin injury and ulcers comprising application to the wound with the liquid
composition "as is" or with the topical or oral formulation as defined herein.
[0042] Accordingly, one aspect of the present invention contemplates a method
for
accelerating wound healing and improving aesthetical presentation of the scars
formed in the wound healing process, the method comprising application on the
wounded skin an effective amount of a composition or formulation capable of
improving wound healing properties.
[0043] Someone skilled in the art will recognize the type of skin injury or
ulcers that
can be treated with the method and/or composition and/or formulation of the
present invention and may include, amongst others, skin surface wounds,
surgery
wounds, burns, diabetic ulcers and pressure sores.
DETAILED DESCRIPTION OF THE INVENTION
[0044] The present invention now will be described more fully hereinafter with
reference to the accompanying drawings, in which preferred embodiments of the
invention are shown. This invention, may, however, be embodied in many
different
forms and should not be construed as limited to the embodiments set forth
herein;
rather, these embodiments are provided so that this disclosure will be
thorough and
complete, and will fully convey the scope of the invention to those skilled in
the art.
Brief description of the drawings
[0045] Figure 1. Effect of colostrum-derived fraction on fibroblasts metabolic
activity
(XTT assay).
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-11-
[0046] Figure 2. Effect of colostrum-derived fraction on collagen contraction.
[0047] Figure 3. Effect of colostrum-derived fraction on collagen
synthesis/deposition and soluble collagen release.
[0048] Figure 4. Effect of colostrums-derived fraction on wound contraction.
[0049] Figure 5. Effect of colostrums-derived fraction on wound tissue areas.
[0050] Figure 6. Effect of colostrums-derived fraction on wound thickness.
[0051] Figure 7. Effect of colostrums-derived fraction on collagen deposition
[0052] Figure 8. Effect of colostrums-derived fraction on collagen density.
[0053] Figure 9. Effect of colostrums-derived fraction on tissue ingrowth in
PVA
sponge.
[0054] Figure 10. Effect of colostrums-derived fraction on scar formation.
[0055] Figure 11. Effect of colostrums-derived fraction on the prevention of
hypertrophic scar.
Definitions
[0056] For the purpose of the present invention the following terms are
defined
below. The expression "effective amount" as used herein is intended to mean an
amount sufficient to induce a beneficial or desired clinical result. An
effective
amount can be administered in one or more doses. For purposes of this
invention,
an effective amount of growth factors and/or dairy derived proteins, or other
composition is an amount that induces a treatment or prophylactic response
against
at least one wound healing and/or collagen "anti-contraction" responsible
factor, in
vitro and/or in vivo.
[0057] The terms "individual" or "subject" treated according to this invention
is a
vertebrate, preferably a mammal, more preferably a human. Mammals include, but
are not limited to, farm animals, sport animals, rodents, primates, and pets.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-12-
[0058] The term "substantially free" as used herein is intended to mean an
amount
that is substantially reduced from the original amount in which this
constituent is
found in colostrum. Particularly, when referring to lysozyme of TGF-R, the
term
"substantially free" means less than 10% of the original amount, preferably,
less
than 5% of the original amount, more preferably, less than 2% of the original
amount, most preferably in trace amount.
[0059] The terms "polypeptide" and "peptide" are used interchangeably to refer
to
polymers of amino acids of molecular weight of about 15kDa or less, and may be
interrupted by non-amino acids.
[0060] The term "protein" refers to polymers of amino acids of molecular
weight of
more than 15kDa, and may be interrupted by non-amino acids.
Description of particular embodiments
[0061] Particularly, the present invention provides the process as defined
herein,
wherein the colostrum in step a) is obtained from thawing frozen whole
colostrum to
a temperature of at least about 4 C; preferably about 10 C.
[0062] Particularly, the present invention provides the process as defined
herein,
wherein, in step a), the casein and curds are separated by treatment with
microbial
rennet; or by acid treatment, preferably at pH 4.6.
[0063] Particularly, the present invention provides the process as defined
herein
wherein the acidified permeate obtained in step d) is concentrated to obtain a
concentrated liquid fraction.
[0064] Particularly, the present invention provides the process as defined
herein,
wherein the concentrated liquid fraction is freeze-dried to obtain a solid
fraction
(powder).
[0065] Particularly, the present invention provides the process as defined
herein,
wherein in step b), said colostrum whey is heated to a temperature of at least
45 C,
preferably at a temperature of at least about 50 C.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-13-
[0066] Particularly, the present invention provides the process as defined
herein,
wherein in step c), said heated whey is submitted to ultra-filtration with a
molecular
weight cut-off of about 12kDa or less; preferably about 1 OkDa.
[0067] Particularly, the present invention provides the process as defined
herein,
wherein in step d) the permeate is acidified at a pH of about 3.5; preferably
at a pH
of 3.2.
[0068] Particularly, the present invention provides the process as defined
herein,
wherein the acidified permeate is concentrated with nanofiltration or any
other
concentration method.
[0069] Particularly, the present invention provides the process as defined
herein,
wherein said colostrum is obtained from: cow, ewe, jenny (donkey's female);
goat or
buffalo.
[0070] In a particular aspect of the invention, there is provided a
composition
derived from colostrum comprising at least 60% of antimicrobial peptides of MW
less than 500Da; IGF-1 and a total protein concentration of less than 5%
(w/w).
[0071] Particularly, the present invention provides the composition as defined
herein, comprising at least 70% of antimicrobial peptides of MW less than
500Da
and further comprising at least 20% (w/w) of antimicrobial peptides of
molecular
weight between 500Da and 1 OkDa of which between 2% and 10% (w/w) is
constituted of IGF-1.
[0072] Particularly, the present invention provides the composition as defined
herein, wherein the composition is liquid and comprises at least 20 ng/ml of
IGF-1; a
total protein concentration of less than 2% (w/w) and is substantially free of
lysozyme and TGF-R. Particularly, the present invention provides the
composition as
defined herein, wherein IGF-1 is present at at least about 40ng/ml.
[0073] Particularly, the present invention provides the composition as defined
herein, wherein the composition is solid (powder) and comprises at least 1 ng
of
IGF-1 per mg of solid; a total protein concentration of less than 2% (w/w) of
protein
and is substantially free of lysozyme and TGF-R. Particularly, the present
invention
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-14-
provides the composition as defined herein, wherein IGF-1 is present at at
least
about 1.6ng/mg total solid.
[0074] Particularly, the present invention provides the composition as defined
herein, wherein said peptides below 500Da are present in physiologically
effective
amounts.
[0075] Particularly, the present invention provides the composition as defined
herein, wherein said peptide of MW less than 500Da is selected from the group
consisting of: PDGF; FGF; EGF; VEGF; defensins, cathelicidins, anti-microbial
peptides (AMP) and proline- rich peptides (PRP).
[0076] Particularly, the present invention provides a formulation comprising a
composition as defined herein, further comprising a physiologically acceptable
excipient; particularly, the formulation is topical or an oral formulation.
EXAMPLES
Example 1-Preparation of composition
[0077] Frozen whole bovine colostrum (less than 48 hrs post-parturition) is
slowly
thawed to 10 C until complete melting. The fat fraction is then separated from
this
raw colostrum using a milk separator. The skimmed colostrum is then heated to
35 C and caseins are precipitated with the addition of double strength
microbial
rennet (Danisco, Madison, WI, USA) for 20 minutes. The curds are separated by
centrifugation from colostrum whey at 65 C. After the casein fines are removed
at
37 C from the colostrum whey with a milk separator, the colostrum whey is
heated
to 50 C and submitted to an ultrafiltration step using a 10,000 molecular
weight cut
off (MWCO) spinal wound membrane (Parker Process Advanced Filtration, Oxnard,
CA, USA). The ultrafiltration permeate is collected and acidified to pH 3.2.
The
acidified permeate is then concentrated with nanofiltration. Finally, the
concentrated
acidified permeate is freeze-dried.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
- 15-
Example 2-Analysis and composition
Liquid Solid
Proteins <2% <2%
IGF-l 43 ng/mL 1.95 ng/mg total solids
Antimicrobial Presence Presence
peptides (<10 kDa) (<10 kDa)
Molecular weight distribution (HPSEC)
[0078] Samples were solubilized in HPLC grade water and then filtrated on
0.2pm
pore size filters. Samples were then passed trough a TSK-GEL, Guard SWXL (6.0
i.d. x 40 mm) pre-column (Tosoh Biosep LLC, Montgomeryville, PA, USA) and a
TSK-GEL, G2000 SWXL (7.8 i.d. x 300 mm) column (Tosoh Biosep LLC,
Montgomeryville, PA, USA) at a flow rate of 0.6mL/minute using a Waters HPLC
(Mississauga, Ont., Can) containing two pumps (model 600), a controller (model
600E) and a UV detector (model 486). The results were analyzed with Millennium
32 software.
Molecular weight distribution ^ Immunel solid
^ Immunel liquid
70 -
%) 50
40 -
20
0 FE LE-Ai
>50000 20000- 10000- 5000- 2000- 1000- 500- <500 Da
Da 50000 20000 10000 5000 Da 2000 Da 1000 Da
Da Da Da
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
- 16-
Example 3-Fibroblast functions
[0079] Human fibroblasts were derived from foreskins and used at passages
between 10 and 25. They were maintained in Dulbecco's modified Eagle's medium
(DMEM, Sigma Chemical Co.) supplemented with 5% FBS and
antibiotics/antifungic. For the cultures in the presence of the compositions
to be
tested the concentration of FBS was decreased to 0.5%. In monolayer and 3-D
culture experiments, fibroblasts were seeded for a few hours in the presence
of
5%FBS and then rinsed and the medium was changed to 0.5%FBS in the presence
of the different concentrations of the compositions to be tested. Cultures
were
performed in 5% CO2 and humid atmosphere.
[0080] The XTT Cell Proliferation Assay is a colorimetric assay system which
measures the reduction of a tetrazolium component (XTT) into soluble formazan
product by the mitochondria of viable cells. The samples are read using an
ELISA
plate reader at a wavelength of 450nm. The amount of color produced is
directly
proportional to the number of viable cells.
Metabolic activity measurements (XTT assay) in monolayer cell cultures
[0081] XTT (2, 3-bis{2methoxy-4-nitro-5-sulfophenyl}-2H-tetrazolium-5-
carboxyanilide inner salt) assay was performed according to the manufacturer's
procedure (TOX2, Sigma-Aldrich Canada Ltd., Oakville, Ontario). For the XTT
assay, cells were seeded at 2.5x104 cells/cm2 for fibroblasts and endothelial
cells
both in 24 multi-well plates. After 2-4 hrs, cell cultures were rinsed once
and media
supplemented with only 0.5% FBS containing the different dilutions (1:1000,
1:100,
1:10 and 1:1) of each composition to be tested were introduced. Cells were
grown
for 4 days with one medium change at day 2. However, each composition was
added every day in culture medium. Afterwards, cell cultures were rinsed in
PBS. A
PBS solution of XTT (1 mg/ml) was mixed with a PBS solution of
phenazinemethosulfate and incubated at 37 C for 1 hr. Optical densities were
then
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-17-
measured with a fluorometer (Spectra Max 340Pe molecular Devices, Sunnyvale,
CA) set at 450 nm absorbance, and values were subtracted from the blank
values.
For each condition, cultures were performed in triplicate. In addition, a
standard
curve of increasing cell densities was used to correlate the optical density
value with
the cell numbers.
[0082] As demonstrated in Figure 1, the 1:1 dilution of the fraction enhanced
significantly the cell growth compared to control value (P<0.05). The lowest
dilution
tested (1:0.5) did also enhance cell growth significantly in comparison to the
control
(P<0.05). The highest dilutions tested (1:10 and 1:5) were not different from
the
control value. These data suggest that the present colostrum-derived fraction
increased fibroblast metabolic activity in a bell-shape manner with a peak
activity at
a dilution of 1:1.
Example 4-Collagen contraction
[0083] Fibroblasts were mixed in collagen solution prior to gel formation at a
density
of 5 x 104 cells per 500p1 gel in wells of 24 multiwell plates. Collagen gels
were
made by mixing a rat tail tendon collagen solution (3.0 mg/ml) with a solution
containing culture medium (DMEM 5x sans NaHCO3), FBS, 0.26M NaHCO3, 1N
NaOH and the suspension of cells as previously described by Elsdale & Bard
(1972). Briefly the formula was the following:
1 ml DMEM 5x without NaHCO3
500pl FBS
500pl NaHCO3 at 0.26M
2Opl NaOH at 1N
100pl H2O
Add 0.88m1 cell dispersion (5.7 x 105 cells/ml) and then 2m1 rat tail
collagen. The
mixed solution was transferred to wells. Gels were formed after 15mn
incubation.
After 2-4hrs of incubation in medium with serum (5%)-supplemented medium, the
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-18-
medium was changed, cell cultures rinsed and the different compositions were
diluted in culture medium supplemented with 0.5% FBS. Composition was
introduced daily for 4 consecutive days. Triplicate samples were analyzed. By
24
hrs the gels were released from wells and left floating in order to trigger
the collagen
contraction by fibroblasts. Medium was changed at day 2. After 4 days, images
of
the gels were taken and quantified using an image analysis system (Image J).
The
values of the areas occupied by gels were expressed in mm2 according to scale
setting.
[0084] As shown in Figure 2, all dilutions prevent collagen contraction,
compared to
control value (P<0.05), which suggest that in presence of the colostrum-
derived
fraction collagen will not contract to form an hypertrophic scar.
Example 5-Collagen synthesis/deposition
[0085] Fibroblasts were seeded at 1x105 cells per well of 6 multi-well plates
in
serum-supplemented medium. As described earlier, medium was changed after 2-
4hrs of incubation with a low-serum-supplemented (0.5% FBS) medium containing
the composition to be tested. Fibroblast cell cultures were labeled by
introducing
1.5pCi/ml [3H]-proline (Perkin Elmer) in medium after 4 hrs post-cell seeding
and
then every other day at medium changes for 7 days in monolayer cell cultures.
The
labeling medium contained 1 Opg/ml of ascorbate. At each medium change, media
were pooled (medium pool) corresponding to most soluble collagen that has been
released form the cells. At day 5, media were pooled with the previous
respective
pools, and the cell-matrix layers were scraped from wells with a rubber
policeman
and collected to be pooled (cell-matrix pool). The latter consisted mainly of
insoluble
collagen that has been deposited by the cells forming the extracellular matrix
around
the fibroblasts. To avoid any biodegradation during these maneuvers, both
pools
were treated with a protease inhibitor cocktail (EDTA, NEM, PAB, and PMSF in 1
M
TrisHCl at pH 7.5). Pools were then promptly frozen until further use. The two
frozen
pools were thawed, vortexed, and then dialyzed against water for 3-4 days
until the
free radioactivity was released as determined by periodic counts. Dialyzed
pools
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-19-
were frozen to be freeze-dried in a freeze-drying apparatus (Labconco). Dried
samples were rehydrated in a 4%SDS solution in 5mM TRIS (pH 8.0) while keeping
an equal final volume to all samples. For each condition, duplicate samples
from the
cell-matrix pool and those from the medium pool were counted separately in a
liquid
scintillation counter (Ultima Gold, Perkin-Elmer). Values were counted in dpm.
The
compositions were changed on a daily basis for 6 days.
[0086] As shown in Figure 3, the two highest dilutions tests (1:10 and 1:5) of
the
colostrum-derived fraction increased amount of collagen and deposition in the
cell-
matrix pool rather than releasing it in soluble form.
[0087] The effect of the colostrum-derived fraction seems to have two distinct
activities depending on its dilutions. The proliferative activity is prone at
lower
dilutions, whereas collagen metabolic activity is expressed at higher
dilutions.
[0088] The colostrum-derived fraction appears to have interesting properties
not
only on fibroblast growth and synthetic activities, but also on the remodeling
of pre-
existing collagen. Collagen gel contraction is a phenomenon that has been
reported
as a physical arrangement of pre-existing collagen fibrils (Guidry and
Grinnell,
1985). This is due to direct contact between fibroblasts that adhere strongly
to the
extracellular matrix and remodel the fibrils. In addition, the collagen
synthesis in
collagen gel has been found slightly diminished, as well as the DNA
replication,
compared to that observed in monolayer cell culture (Mauch et al., 1988;
Nusgens
et al., 1984). This phenomenon may be largely due to the presence of collagen
fibrils around the cells that down-regulate cell activity as observed in
normal
connective tissue.
[0089] Inhibition or limitation of collagen gel contraction has been reported
previously with specific molecules such as prostaglandins El and E2, and the
incorporation of proteoglycans, heparin, or plasma fibronectin in the collagen
gel
(Anderson et al, 1990; Guidry and Grinnell, 1987). Serum deprivation is also
an
important factor as demonstrated also in our assessments. Moreover, it has
been
reported that high doses of bFGF inhibited collagen gel contraction (Finesmith
et al.
1990) whereas TGF-(3 facilitated it. Withouit wishing to be bound by theory,
it is
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-20-
thought that the present colostrum-derived fraction contains some of these
molecules that correspond to antagonist on the matrix contraction and
remodelling.
[0090] Since the collagen gel contraction assay is a model relative to the
physiopathology of wound healing, scar formation and tissue remodeling, the
research and development of agonists and antagonists is incomplete. The
present
fraction is a potential antagonist product, especially as it facilitates also
cell growth
and collagen synthesis as shown in specific in vitro experimental conditions.
This
composition has potential applications in plastic and reconstructive surgery
to treat
scar tissues. Indeed, recurrence of excessive remodeled scar tissues (e.g.,
keloids,
hypertrophic scar tissues) after surgery represents an important issue.
Furthermore,
such composition may find application not only in wound healing but also in
fibrotic
reaction. The present fraction may also find an application in skin ulcers
since
rebuilding collagen matrix in the wound is also important to consider in such
cases.
Example 6- Large open wounds on guinea pigs
[0091] The healing effect of compounds such as the colostrum-derived fraction
was
studied using a model of large full-thickness open wounds in guinea pigs. This
model allows investigating the long term wound healing effect of the colostrum-
derived fraction. Since the colostrum-derived fraction is in solution,
different dilutions
were applied daily for a period of 3 weeks.
[0092] Female Hartley guinea pigs weighing between 250-350g (3-4 weeks old)
were purchased at Charles River. Animals were kept in separate cages, with
water
and food ad libitum. All animal experiments were performed according to the
guidelines of the Canadian Council for Animal Care and approved by our
institutional Animal Care Committee.
[0093] The wound model consisted in the creation by scalpel incision of one
large
open full-thickness wound on each flank of each animal, close to the spinal
cord.
Each wound area had a 2.5 cm length along the cranio-caudal axis and 2cm
large.
The whole connective tissue was removed to insure no residue left at the
bottom.
The animals received, 1 hr before surgery, a subcutaneous injection of an
analgesic
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-21-
(Buprenorphine , 0.05mg/kg) and a Ringer's lactate solution at a volume
corresponding to 1/10`h of their weights. The animals were anesthetised by
isofluorane under oxygen. Surgery was performed in sterile conditions. The
skin
was aseptized with hibitane, 70% alcohol, and proviodine. Animals received for
2
days after surgery an analgesic at the same dose as described earlier at 12hrs
interval. Animals were weighed periodically at dressing change without the
dressing
to compare with the initial weight performed one day before surgery. All
animals
grew in an exponential manner as expected in a normal guinea pig development.
[0094] Thereafter, the respective wound was filled with the solutions to be
tested.
Five composition:PBS dilutions were tested: 1) 1:0.5, 2) 1:1, 3) 1:10, 4)
1:100 et 5)
1:1000. The control animals received PBS. The wounds were then covered with a
transparent self-adhesive polyurethane film (Tegaderm , 3M), followed by a
sterile
gaze. The trunk of each animal was wrapped with a self-adhesive elastic
bandage
(Elastoplast ) to maintain the bandage in place and to avoid any trauma. Each
animal was identified by a label on its cage. The bandage was left in place
and
periodically changed at days 5, 9, 14, and 19. A photograph of each wound was
taken at days 5, 9, 14 and 23 (last sacrifice). A cm scale ruler and an
identified label
were inserted in each photograph. Quantification of macroscopic view of the
wound
areas was performed directly on photographs.
Wound contraction
[0095] Wound contraction as measured represents the size of the wound as
viewed
from above the back of the animals as determined by tracing over the
photographs.
The trend curves of contraction were generally similar for all conditions,
with an
important drop by day 9 (Figure 4). At days 5 and 9, there were no significant
difference statistically (equal variance test failed). However, statistic
analyses
(ANOVA and Fisher LSD method) show a significant difference by day 14 in the
wounds treated with the 1:1 and 1:0.5 dilutions compared to control wounds and
those treated with 1:10 and 1:1000 dilutions (p<_0.05) By day 23, there was
also a
significant difference between the highest doses (1:0.5) and control wounds.
(Figure 4).
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-22-
Wound tissue areas
[0096] As shown in Figure 5 the treatment of large open wound with the
composition of the invention increases formation of wound tissue.
Quantification of
the surface areas occupied by the wound tissue by day 23 showed significant
increase with 1:1 dilution (Fisher LSD method of statistic analysis was used
with a
failed equal variance test).
Wound thickness
[0097] Quantification of the thickness of the wound tissue also showed a
significant
increase by day 16 with the wounds treated with 1:1 dilution compared to
control
and the lowest dose (1:1000). By day 23, the thickness of wounds treated with
a
1:100 dilution was significantly higher than the other conditions using the
Student-
Newman-Keuls method as statistical comparisons methods (normality and equal
variance test passed). (Figure 6)
Collagen deposition and density
[0098] The composition increased the areas occupied by collagen with a
tendency
to increase its density. By day 23, there was an increase in surface area
occupied
by collagen where the composition was applied at a dilution of 1:100 (Figure
7).
However, this increase in surface area was not significantly different
compared to
control (PBS). Treatment of large open wound with the composition also induced
an
increased of collagen density compared to control (Figure 8).
Example 7- Subcutaneous implantation of PVA sponges
[0099] The PVA (Polyvinyl alcohol) sponge model is used for studying
granulation
and reparative tissue ingrowth. Tissue grows within its interstices shortly
after
implantation. This model is highly reproducible and is a biologically valid
model for
studying healing responses.
[00100] Spraque Dawley rats were used for this implantation model. A dorsal
median incision (rostral) was performed in the skin down to the aponevrosis,
and
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-23-
then two subcutaneous pockets (one on each side) were made. Each wound area
was 1x1 cm. A PVA sponge was introduced in each pocket and the skin was
sutured
(4/0 monofilaments). Sponges were far from the skin incision to avoid any
interference. Specimens were retrieved at days 8 and 15 to see the speed of
tissue
ingrowth. After 8 and 15 days, implants were retrieved, fixed in formaldehyde
and
processed for histological sections which was stained with hematoxylin,
phloxin,
saffron and Hoechst 33342 to mark the nuclei and the sections of day 15 were
also
stained with picro-sirius to visualize the collagen deposition. Quantification
was
performed by measuring the length of tissue invasion from the host-implant
interface
to the invasive front, in multiple areas in a random manner. Moreover, using
Hoechst-stained tissue sections, the number of nuclei present in the sponge
was
counted using the image analysis system and reported to the total surface of
the
void spaces of PVA sponge present on each histological section. This ratio was
used for comparison between the different conditions.
[00101] As shown in Figure 9, the composition significantly (p=0.001)
increases the tissue ingrowth in PVA sponges. An increase of 42% of the tissue
growth in PVA sponges was observed in animal treated with the composition.
This
result demonstrates that the composition of the invention induces tissue
formation in
wounds.
Example 8- Hypertrophic scar
[00102] Wound healing is a fundamental complex-tissue reaction leading to
skin reconstitution. Alterations in the orchestrated wound healing process
result in
hypertrophic or keloid scarring. Although hypertrophic scarring commonly
occurs
following burns, many aspects such as incidence of an optimal treatment for
scar
hypertrophy remain unclear. Hypertrophic scar is associated with polarized Th2
systemic response to injury that leads to increased T cells and their Th2
fibrogenic
cytokines in tissues and the development of fibrosis and hypertrophic scars.
Concentration of the composition
DOCSQUE: 756309\l
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-24-
[001031 Bioactive compounds of the composition were concentrated by
dehydration with speed-vacuum. The samples were frozen and applied in a speed
vacuum with a pressure of 100 milliTorr. A concentration of almost 30 times
was
achieved. This concentrated fraction was named speed-vaccum IM.
[00104] The wound model consisted in the creation by scalpel incision of two
open full-thickness wounds on each flank of each animal, close to the spinal
cord.
Each wound area had a 1 x1 cm. If necessary, haemostasis was insured by
compression with a gaze and thermocoagulation. The whole connective tissue was
removed to ensure no residue left at the bottom. The day of surgery, the
animals
received, 1 hr before surgery, a subcutaneous injection of an analgesic
(Buprenorphine , 0.05mg/kg) and a Ringer's lactate solution at a volume
corresponding to 1/10th of their weights. For surgery, the animals were
anesthetised
by isofluorane under oxygen. Surgery was performed in sterile conditions. The
skin
was aseptized with hibitane, 70% alcohol, and proviodine. The respective wound
was covered with a polyurethane sheet (Tegaderm) and the animals wrapped in
gauzes and elastoplast to protect the wounds for 5 days. After 5 days the
dressing
was renewed in the same manner. After 15 days, the wounds were left at air-
free
environment until epithelialisation was almost completed (>_90%). This was
reached
at 19 days and iconography was performed as image prior to treatment. Wounds
on
each animal were then treated (day 19 corresponds to day 0 of treatment) by
speed-
vacuum IM and speed-vacuum BSA on one side and on the other side,
respectively.
Wounds were treated for 14 days by daily application (except Sundays) and
photographs were taken at day 7 (Day 26) and at sacrifice at day 14 (day 33).
Macroscopic evaluation of the wound area included the scar tissue, thickening
of the
scar, etc. and microscopic observation with quantification (see Figure 10 for
an
example).
[00105] Scar formation. As shown in Figure 10, treatment of a wound by the
composition reduces scar formation. An untreated wound may lead to the
formation
of a scar. Indeed, a scar was formed in 7 out of 8 wounds treated with control
vehicle (BSA) (Figure 10). However, following 14 days of treatment with the
composition, the majority of wounds (5 of 8) have no scar.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-25-
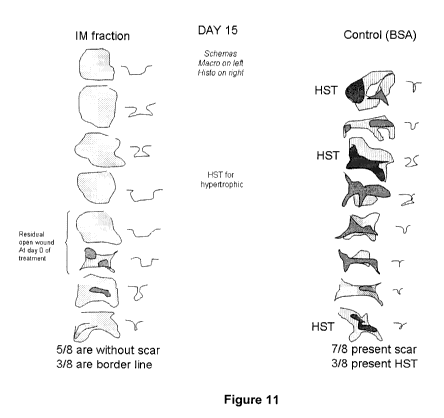
[00106] Hypertrophic wound. Treatment with the composition prevented the
formation of hypertrophic wound (Figure 11). No hypertrophic scar was observed
in
wounds treated with the composition. However, in wounds treated with the BSA
(control), hypertrophic wounds were formed (3 out of 8).
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-26-
[00107] REFERENCES
1. Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and
quantification of innate immune system mediators in human breast milk.
Allergy Asthma Proc. 2004; 25(5):297-304.
2. Bastian A, Schafer H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral
infection in vitro. Regul Pept. 2001; 101(1-3):157-61.
3. Bayat A, McGrouther DA, Ferguson MW. Skin scarring. BMJ 2003; 326:88-
92.
4. Bitar MS, Labbad ZN. Transforming growth factor-beta and insulin-like
growth factor-I in relation to diabetes-induced impairment of wound healing.
J Surg Res. 1996; 61(1):113-9.
5. Bitar MS. Insulin and glucocorticoid-dependent suppression of the IGF-I
system in diabetic wounds. Surgery. 2000; 127(6):687-95.
6. Blakytny R, Jude EB, Martin Gibson J, Boulton AJ, Ferguson MW. Lack of
insulin-like growth factor 1 (IGF1) in the basal keratinocyte layer of
diabetic
skin and diabetic foot ulcers. J Pathol. 2000; 190(5):589-94.
7. Buck CB, Day PM, Thompson CD, Lubkowski J, Lu W, Lowy DR, Schiller JT.
Human alpha-defensins block papillomavirus infection. Proc Natl Acad Sci U
S A. 2006; 103(5):1516-21.
8. Butler AA, LeRoith D. Minireview: tissue-specific versus generalized gene
targeting of the igf1 and igfl r genes and their roles in insulin-like growth
factor physiology. Endocrinology. 2001; 142(5):1685-8.
9. Campbell PG, Durham SK, Hayes JD, Suwanichkul A, Powell DR. Insulin-
like growth factors-binding protein-3 binds to fibrinogen and fibrin J Biol
Chem. 1999; 274(42): 30215-30221.
DOCSQUG: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-27-
10. Clare DA, Catignani GL, Swaisgood HE. Biodefense properties of milk: the
role of antimicrobial proteins and peptides. Curr Pharm Des. 2003; 9(16):
1239-55.
11. Clare DA, Swaisgood HE. Bioactive milk peptides: a prospectus. J Dairy
Sci.
2000; 83(6):1187-95.
12. Chaly YV, Paleolog EM, Kolesnikova TS, Tikhonov II, Petratchenko EV,
Voitenok NN. Neutrophil alpha-defensin human neutrophil peptide modulates
cytokine production in human monocytes and adhesion molecule expression
in endothelial cells. Eur Cytokine Netw. 2000; 11 (2):257-66.
13. Chang TL, Vargas J Jr, DelPortillo A, Klotman ME. Dual role of alpha-
defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005; 115(3):765-73.
14. Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev
Physiol. 1993; 55:131-53.
15. De Yang, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J,
Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-
derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a
receptor to chemoattract human peripheral blood neutrophils, monocytes,
and T cells. J Exp Med. 2000; 192(7):1069-74.
16. Ericksen B, Wu Z, Lu W, Lehrer RI. Antibacterial activity and specificity
of
the six human {alpha}-defensins. Antimicrob Agents Chemother. 2005;
49(1):269-75.
17. Gauthier SF, Pouliot Y, Maubois JL. Growth factors from bovine milk and
colostrum : composition, extraction and biological activities. Lait. 2006; 86:
99-125.
18. Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer
RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin
Invest.
1985; 76(4):1427-35.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-28-
19. Gennaro R, Zanetti M, Benincasa M, Podda E, Miani M. Pro-rich
antimicrobial peptides from animals: structure, biological functions and
mechanism of action. Curr Pharm Des. 2002; 8(9):763-78.
20. Gopal PK, Gill HS. Oligosaccharides and glycoconjugates in bovine milk and
colostrum. Br J Nutr. 2000; 84 Suppl 1:S69-74.
21. Hazrati E, Galen B, Lu W, Wang W, Ouyang Y, Keller MJ, Lehrer RI, Herold
BC. Human alpha- and beta-defensins block multiple steps in herpes
simplex virus infection. J Immunol. 2006; 177(12):8658-66.
22. Hoover DM, Wu Z, Tucker K, Lu W, Lubkowski J. Antimicrobial
characterization of human beta-defensin 3 derivatives. Antimicrob Agents
Chemother. 2003; 47(9):2804-9.
23. Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective
killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J
Immunol. 2004; 172(3):1763-7.
24. Janusz M, Lisowski J. Proline-rich polypeptide (PRP)--an immunomodulatory
peptide from ovine colostrum. Arch Immunol Ther Exp (Warsz). 1993; 41(5-
6):275-9.
25. Jones JI, Clemmons DR. Insulin-like growth factors and their binding
proteins: biological actions. Endocr Rev. 1995 Feb;16(1):3-34.
26. Kelly GS. Bovine colostrums: a review of clinical uses. Altern Med Rev.
2003; 8(4):378-94.
27. Lee SH, Jeong SK, Ahn SK. An update of the defensive barrier function of
skin. Yonsei Med J 2006; 47(3): 293-306.
28. Lopez-Garcia B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of
cathelicidins and their potential role in Candida albicans skin infection. J
Invest Dermatol. 2005; 125(1):108-15.
29. Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA,
Pestonjamasp V, Piraino J, Huttner K, Gallo RL. Innate antimicrobial peptide
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
-29-
protects the skin from invasive bacterial infection. Nature. 2001;
414(6862):454-7.
30. Okuyama H, Urao M, Lee D, Drongowski RA, Coran AG. The effect of
epidermal growth factor on bacterial translocation in newborn rabbits. J
Pediatr Surg. 1998; 33(2):225-8.
31. Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premisese and
promises. Int J Antimicrobial Agents 2004: 24;536-547.
32. Schultz GS, White M, Mitchell R, Brown G, Lynch J, Twardzik DR, Todaro
GJ. Epithelial wound healing enhanced by transforming growth factor-alpha
and vaccinia growth factor. Science. 1987; 235(4786):350-2.
33. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;
341(10):738-46.
34. Shimasaki S, Ling N. Identification and molecular characterization of
insulin-
like growth factor binding proteins (IGFBP-1, -2, -3, -4, -5 and -6). Prog
Growth Factor Res. 1991;3(4):243-66.
35. Steed DL. Modifying the wound healing response with exogenous growth
factors. Clin Plast Surg. 1998; 25(3):397-405.
36. Stewart CE, Rotwein P. Insulin-like growth factor-II is an autocrine
survival
factor for differentiating myoblasts. J Biol Chem. 1996; 271(19):11330-8.
37. Thapa BR. Therapeutic potentials of bovine colostrums. Indian J Pediatr.
2005; 72(10):849-52.
38. Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K,
Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama
S, Yoshimura A, Sugai M, Hashimoto K. Induction of keratinocyte migration
via transactivation of the epidermal growth factor receptor by the
antimicrobial peptide LL-37. J Immunol. 2005; 175(7):4662-8.
DOCSQUE: 756309\1
CA 02722821 2010-10-27
WO 2009/135306 PCT/CA2009/000627
- 30 -
39. Tsuboi R, Shi CM, Sato C, Cox GN, Ogawa H. Co-administration of insulin-
like growth factor (IGF)-I and IGF-binding protein-1 stimulates wound healing
in animal models. J Invest Dermatol. 1995; 104(2):199-203.
40. Werner S, Grose R. Regulation of wound healing by growth factors and
cytokines. Physiol Rev. 2003; 83(3):835-70.
41. Wieczorek Z, Zimecki M, Janusz M, Staroscik K, Lisowski J. Praline-rich
polypeptide from ovine colostrum: its effect on skin permeability and on the
immune response. Immunology. 1979; 36(4):875-81.
42. Yang D, Chertov 0, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson
M, Schroder JM, Wang JM, Howard OM, Oppenheim JJ. Beta-defensins:
linking innate and adaptive immunity through dendritic and T cell CCR6.
Science. 1999; 286(5439):525-8.
43. Yang D, Liu ZH, Tewary P, Chen Q, de la Rosa G, Oppenheim JJ. Defensin
participation in innate and adaptive immunity. Curr Pharm Des. 2007; 13(30):
3131-9.
DOCSQUE: 756309\1