Note: Descriptions are shown in the official language in which they were submitted.
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
L-CARNITINE AND ALKANOYL L-CARNITINE PHYTATES
AND PROCESS FOR PREPARING THE SAME
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims priority to U.S. Provisional Application
number 61/061,956, entitled "L-Camitine and Alkanoyl L-Carnitine Phytates and
Process for Preparing Same", filed June 16, 2008. The entire content of this
provisional patent application is incorporated herein by reference.
TECHNICAL FIELD
[0002] The present invention relates to novel salt form clusters of L-
carnitine
and alkanoyl L-carnitine, i.e., L-carnitine phytate and alkanoyl L-carnitine
phytates, and the process for preparing the same.
BACKGROUND
[0003] It is well known that L-carnitine and its alkanoyl derivatives lend
themselves to various therapeutical and nutritional uses. L-carnitine and its
alkanoyl derivatives inner salts are represented by formula:
CH3 OR 0
H3C-N+)LO_
CH3
L-Carnitine or Alkanoyl L-Carnitine
1
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
wherein R represents either a hydrogen atom or an alkanoyl group.
[0004] L-Carnitine is a cofactor required for transformation of free long-
chain fatty acids into acylcamitines, and for their subsequent transport into
the
mitochondrial matrix, where they undergo bate-oxidation for cellular energy
production. Mitochondrial fatty oxidation is the primary fuel source in heart
and
skeletal muscles, pointing to the relative importance of the nutrient for
proper
function in tissue. L-Carnitine and its alkanoyl derivatives also have
important
antioxidant effects, as demonstrated by their protective effect against
lipoperoxidation of phospholipid cell membranes caused by oxidative stress
induced at the myocardial and endothelial cell level. Conditions which appear
to benefit from L-carnitine and its alkanoyl derivatives include anorexia,
chronic
fatigue, coronary vascular disease, diphtheria, hypoglycemia, male
infertility,
muscular myopathies, Rett Syndrome, Alzheimer's disease, mood
enhancement, cognitive improvement, and sports performance. See, e.g.,
Gregory S. Kelly, "L-Carnitine: Therapeutic Applications of a Conditionally-
Essential Amino Acid", Alternative Medicine Review, 3 (5): 345-360 (1998).
[0005] While there are various therapeuticals and nutritional benefits of L-
carnitine and its alkanoyl derivatives, much research has been carried out to
improve their physical, chemical, and biological properties.
[0006] Research has primarily focused on the solutions of the physical and
chemical drawbacks of L-carnitine and its alkanoyl derivatives inner salts
because their hygroscopic physical characteristic creates complex problems
involving the processing and storing of both the raw materials and the
finished
products, and their inadequate chemical stability leads to the release of
traces
of trimethylamine and its concomitant unpleasant fishy smell.
[0007] In these previous research, various salt forms of L-carnitine and its
alkanoyl derivatives having "pharmacologically acceptable" acids as anion but
without unwanted toxic or side effects have been produced, with the knowledge
that the salts of L-carnitine and its alkanoyl derivates known to-date present
the
same therapeutical and nutritional benefits as do the so-called inner salts.
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
Selecting suitable acid is the major endeavor in screening salt forms of L-
carnitine and its alkanoyl derivatives having improved properties compared to
inner salts. While various mineral acids have been tested, including
hydrochloric acid, sulfuric acid, phosphoric acid, a larger number of organic
acids have been tested including fumaric acid, tartaric acid, lactic acid,
citric
acid, malic acid, oxalic acid, orotic acid and mucic acid. While the
combination
of these acids with the inner salts of L-carnitine and its alkanoyl
derivatives
more or less satisfactorily solved the problems associated with inner salts,
these
salts focused on a technological solution to the purely physical or chemical
drawbacks associated with L-carnitine and its alkanoyl derivatives' inner
salts.
[0008] Research has been made to produce salt forms of L-carnitine and its
alkanoyl derivatives with the anion moiety itself being endowed with
interesting
pharmacological and/or nutritional characteristics and, if possible, to
synergistically enhance the therapeutical and/or nutritional properties of L-
carnitine and its alkanoyl derivatives.
[0009] U.S. Patent Application Publication, No. US2006/0241181 Al
entitled "Alpha-Ketoglutarates of Active Ingredients and Compositions
Containing Same" (Publication Date: Oct. 26, 2006) to Pietro Pola et al.
discloses novel salt forms of L-camitine and its alkanoyl derivatives combined
with alpha-ketoglutaric acid. Alpha-ketoglutaric acid, which is a precursor to
L-
glutamine, plays an important metabolic role and has been successfully applied
in cardiac surgery due to its important role in the Krebs cycle and, hence, in
myocardial metabolism. However, according to the specification of this patent
application, only honey-like pasty mass is obtained as a salt product of L-
carnitine alpha-ketoglutarate and not any solid form.
[0010] Amino acids possess various therapeutical and nutritional attributes.
U.S. Patent No. 6,703,042 B1, entitled "Salts of L-Carnitine and Lower
Alkanoyl
L-Carnitine", (Issued: March 9, 2004) to Atonietta Buononato discloses salts
of
L-carnitine and alkanoyl L-carnitine with amino acids, such as leucine,
isoleucine, valine, cysteine, arginine and glycine to enhance therapeutical
3
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
and/or nutritional efficacy with respect to their inner salts. However, as
disclosed in this patent, the anion moiety (i.e., the amino acid moiety) of
the
salts had to be salified at the amino group with hydrochloric, or hydrobromic,
and/or phosphoric acid.
[0011] In the efforts to develop new generation salt forms of L-camitine and
alkanoyl L-carnitines which not only just solve the physical and chemical
drawbacks of the inner salts, but also enhance the therapeutical and/or
nutritional efficacy of the inner salts, endeavors should still focus on the
screening of a sophisticated acid.
[0012] Phytic acid, also known as inositol hexaphosphate, myo-inositol
hexaphosphate, and IP6, is a 6-phosphate ester of inositol as represented by
the molecular formula:
H203PO OP03H2 H2O3PO
OP03H2 H2O3PO OPO3H2
OPO3H2
H203P0 OP03H2
OP03H2 OP03H2 OP03H2
Phytic Acid
[0013] Phytic acid is naturally occurring in substantial amounts in whole
grain, cereals, legumes, nuts, and seeds, and is the primary energy source for
germinating plants. Phytic acid and its lower phosphorylated forms are also
found in most mammalian cells, where they assist in regulating a variety of
important cellular functions. Phytic acid functions as an antioxidant by
chelating
divalent cations such as copper and iron, preventing the generation of
reactive
oxygen species responsible for cell injury and carcinogenesis. Both in vivo
and
in vitro studies utilizing IP6 have revealed a significant anticancer activity
with a
variety of tumor types, possibly via inhibition of tumor cell growth and
differentiation. In vitro studies with colon, liver, and rhabdomyosarcoma cell
lines, and animal models of mammary, colon, intestinal, and liver cancer, as
q
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
well as rhabdomyosarcoma, have all demonstrated IP6's anticancer properties.
Other properties of 1P6 include an anti-platelet aggregating and lipid-
lowering
effect, suggesting a potential health benefit for the cardiovascular system;
inhibition of HIV-1 virus replication; modulation of insulin secretion in
pancreatic
beta cells; and inhibition of urinary calcium oxalate crystallization, thereby
preventing renal stone development. See e.g. Monograph, Inositol
Hexaphosphate", Alternative Medicine Review, 7 (3): 244-248 (2002).
[0014] Other notable functions of phytic acid include the deodorant effect of
body odor, bad breath or uraroma; the prevention of acute alcoholism; and the
enrichment of the taste of meat and fish. These properties of phytic acid
provide its pharmaceutical and/or nutritional added value.
[0015] The biochemistry and pharmacokinetics of phytic acid have also
been studied. Inositol phosphates are synthesized from the parent molecule
inositol and daily dietary consumption of inositol is estimated to be one
gram.
Once inositol reaches the cells of the intestinal tract it is phosphorylated
to
create inositol hexaphosphate (IP6), and then subsequently dephosphorylated
to its lower forms, such as inositol pentaphosphate (1P5), inositol
tetraphosphate (P4), inositol triphosphate (IP3), inositol monophosphate
(IP1),
which play important roles in signal transduction. Independent of the route of
administration, IP6 has been discovered to be absorbed almost instantly,
transported intracellularly and dephosphorylted into lower inositol
phosphates.
IP6 can reach targeted tumor tissue as early as one hour post-administration.
When incubated with a human mammary cancer cell line, low levels of IP6 were
detected as early as one minute post-incubation.
[0016] Based on its dietary derivation (i.e., it is non-toxic), its chemical
properties (e.g., six phosphates attached in one inositol molecule), and its
various biological activities, phytic acid is a novel acid to react with L-
carnitine
and alkanoyl L-carnitine inner salts to produce L-carnitine phytate and
alkanoyl
L-carnitine phytates. It is apparently an innovation in the evolution of salt
forms
of L-carnitine and salt forms of alkanoyl L-carnitine.
S.
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
OBJECTS OF THE INVENTION
[0017] An object of the present invention is to provide a novel generation of
L-carnitine and alkanoyl L-carnitine salt form derivatives derived from their
corresponding inner salts and phytic acid, i.e., L-carnitine phytate and
alkanoyl
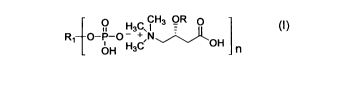
L-carnitine phytates. These salts are represented by General Formula (I):
CH3 OR 0
1o H3C, l +
R, O-P-O - N~j)OH
OH H3C n
General Formula (I)
wherein n = 1-6; R1 is the phytate anion; R is hydrogen, or straight or
branched-
chain alkanoyl group having 2-12 carbon atoms; preferably, the alkanoyl group
is a lower alkanoyl group having 2-5 carbon atoms; and more preferably, the
alkanoyl group is selected from acetyl, propionyl, butyryl, isobutyryl,
valeryl and
isovaleryl groups.
[0018] Another object of the invention is to supply a process for the
preparations of the salts represented by the formula shown in General Formula
(I).
[0019] A further object of the invention is to provide the use of L-carnitine
phytate and alkanoyl L-carnitine phytates.
DETAILED DESCRIPTION OF THE INVENTION
[0020] The following detailed description is directed to certain specific
embodiments of the invention. However, the invention can be embodied in a
multitude of different ways as defined and covered by the claims.
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0021] L-Carnitine phytate and alkanoyl L-carnitine phytates
[0022] As illustrated by the formula of phytic acid, phytic acid is a 6-
phosphate ester of inositol with each phosphate group possessing 2 proton
dissociation sites. There are total of 12 proton dissociation sites in one
phytic
acid molecule; six of which are strongly acidic with an approximate pKa value
of
1.5; three sites are weakly acidic with pKa values 5.7, 6.8 and 7.6; and the
remaining three sites are very weakly acidic, with pKa values greater than 10.
See Costello, A.J.R., et al., ,31P-Nuclear Magnetic Resonance-pH Titrations of
myo-lnositol Hexaphosphate", Carbohydrate Research, 46: 159-171 (1976).
The six strongly acidic protons (with a pKa value of 1.5) are the first
dissociation
protons of each of the six phosphate groups in the phytic acid molecule. This
dissociation ability is similar to the proton dissociations of phosphoric
acid, i.e.,
pKa1 (2.12) < pKa2 (7.21) < pKa3(12.67). Dissociation of the protons of phytic
acid leaves the molecule with several negative charges, which can attract
positively charged molecules to generate phytate.
[0023] When phytic acid reacts with the inner salts of L-carnitine or its
alkanoyl derivatives, each negatively charged phosphate group will preferably
incorporate one inner salt at its quaternary ammonium cation and the
corresponding phosphate dissociated proton incorporates the carboxyl anion of
the inner salt. While there are 12 dissociation sites in one phytic acid
molecule,
theoretically, only up to 6 molecules of L-carnitine inner salt or its
alkanoyl
derivative inner salt can be incorporated. Because as described above, there
are 6 phosphate groups in one phytic acid molecule, only the first
dissociation
site of each phosphate group is acidic enough (pKa 1.5) to incorporate with a
inner salt to generate a corresponding salt. The other dissociation sites are
too
weakly acidic (with pKa values of 5.7, 6.8, 7.6, and in some instances greater
than 10) to form stable ionic bonds with the quaternary ammonium cation of L-
carnitine or its alkanoyl derivatives' inner salt, because the pKa value of L-
carnitine's inner salt is 3.8. See, Cogt C., et al, Enantiomeric Separation
of
D/L-Carnitine Using HPLC and CZE after Derivatization", Chromatographia, Vol.
1
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
40 (5/6): 287-295, (1995). So, each phosphate group of phytic acid can only
incorporate 1 inner salt, and a total of 6 phosphate groups of phytic acid can
incorporate a total of 6 inner salts of L-carnitine or its alkanoyl
derivatives.
[0024] One phytic acid molecule can preferably incorporate 1 to 6
molecules of L-carnitine or its alkanoyl derivatives' inner salts depending on
the
mole ratio of added inner salts. Theoretically, when equal molar ratio of
inner
salt and phytic acid are added together, then the salt product will be L-
carnitine
phytate (in a 1:1 ratio), or alkanoyl L-carnitine phytate (in a 1:1 ratio);
and
similarly, when 2, 3, 4, 5 times the number of moles of inner salt are added,
individually, the phytate product will be 2:1, 3:1, 4:1, and 5:1 respectively.
But
practically, since there are 6 same strong acidic dissociation sites in one
phytic
acid molecule, when an equal mole number of inner salt and phytic acid moles
are added together, the phytate product is a mixture of 1:1 to 6:1 mole ratio
more or less randomly created, and there is unreacted phytic acid leftover.
Therefore, practically only when a 6:1 mole ratio of L-carnitine or its
alkanoyl
derivative inner salt and phytic acid are added together will 6 phosphate
groups
of phytic acid molecules fully react to obtain L-carnitine or alkanoyl L-
carnitine
phytate (in a 6:1 ratio). Thus, in practice a 6:1 ratio of phytate can be
produced.
[0025] According to one embodiment, the mole ratios between L-carnitine
cation moiety or its alkanoyl derivatives cation moiety and the phytic acid
anion
moiety of L-carnitine phytate or alkanoyl L-carnitine phytate can be 1:1, 2:1,
3:1,
4:1, 5:1, and 6:1, corresponding salts as represented by General Formula (I).
According to one embodiment, the mole ratio is 6:1 with the salts being L-
carnitine phytate (6:1) and alkanoyl L-carnitine phytates (6:1) as represented
by
General Formula (II):
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
OH
0
ROro....
-N_ RO O
1 O / + OH
0 OR O H- HOB ,NO:POH O=P-OH N
0 OPA
HO3PO OPO3F~ CH3 OR 0 - ~ O u :"0 O- OH
H3C-N HO` r0 OR
-HO3POOPOOHP03H- CH3 0 )* 8 RO N-POHO-P`O HOb~N vIOH
p=
HO -N
OR
O
HO
General Formula (II)
wherein R is either hydrogen, or a straight or branched-chain alkanoyl group
having 2-12 carbon atoms. According to one embodiment, the alkanoyl group is
a lower alkanoyl group having 2-5 carbon atoms. According to one embodiment,
the alkanoyl group is selected from acetyl, propionyl, butyryl, isobutyryl,
valeryl
and isovaleryl groups.
[0026] L-carnitine and alkanoyl L-carnitine phytates (in a 6:1 mole ratio)
possess many desirable characteristics:
[0027] 1. They are new, pure, and structurally well defined chemical
compounds.
[0028] 2. They contain 6 L-carnitine or alkanoyl L-carnitine molecules
clustered around one phytic acid anion in one salt form of phytate (in a 6:1
mole
ratio) molecule. In comparison with all the other L-carnitine or alkanoyl L-
carnitine salt forms, a 6:1 mole ratio is the biggest mole ratio achieved, so
far.
[0029] 3. Since phytic acid is 6-phosphate ester of inositol, the stereo
configuration of the salt form (6:1) is dendritic in six-directions, as a
novel ionic
bonded dendrimer molecule, with expected synergistic efficacy.
[0030] 3.1. According to one embodiment, L-carnitine phytate (in a 6:1
mole ratio) (C48H1o8N6042P6, molecular weight 1627.24) is a six-directional
dendrimer as shown by Formula (1):
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
OH
O
HO.....
-N- HO 0
10-
/O .OH 0=P-OH N+ ` OH
HOB 1 . N OH 6 O, 0-
"` ~~~ 00 0 O'POH
HO -0 O'P \ + OH
HO N / \0 J 0 HO `O 0__N+ 0
+' OH
7 \ HO P
O HO -N-
,,,OH
0
HO
L-Carnitine Phytate (6:1)
Formula (1)
[0031] 3.2. According to one embodiment, acetyl L-carnitine phytate (in a
6:1 mole ratio) (C60H12oN6048P6, molecular weight 1879.44) is a six-
directional
dendrimer as shown by Formula (2):
OH
O
AcOin.
-N- AcO 0
0 OAc 10-
+ OH
HO 0=P-OH N
, +N~O_OH O O\ .O
O\0 0' R\
HO ,0*0, O - \ + PAC
O'P` O PLO _N 0
ACO N ( OHO-P=0 HO
0) OH
F_
HO -N+
,,OAc
0
HO
Acetyl L-Carnitine Phytate (6:1)
Formula (2)
[0032] 3.3. According to one embodiment, propionyl L-carnitine phytate (in
a 6:1 mole ratio) (C66H132N6046P6, molecular weight 1963.61) is a six-
directional
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
dendrimer as shown by Formula (3):
OH
o & 0 )
~O
p
O -N-
O
o tO-
O 0--P-OH N+ OH
HO +N\p OH 0 O\\ .O
-O O_POH O
/ it
O HOB .O O ~0 \ O~
O,P\p 0 PLO ~N+ - O
").'0 +N \ HO-$ O HO / OH
O~ \O -
HO -N+
O \`
HO
Propionyl L-Carnitine Phytate (6:1)
Formula (3)
[0033] 3.4. According to one embodiment, butyryl L-carnitine phytate (in a
6:1 mole ratio) (C72H144N6O48P6, molecular weight 2046.76) is a six-
directional
dendrimer as shown by Formula (4):
OH
O
0
O N- O\\\\
0 0 0 _ OH
O=P-OH N+
HO +N~O~ ~H 0l6 ~
p` ~POH
HO O O` \
0, H. _N
HO-P;O H
HO -rrH+
O
O
HO
Butyryl L-Carnitine Phytate (6:1)
Formula (4)
11
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0034] 3.5. According to one embodiment, isobutyryl L-carnitine phytate
(in a 6:1 mole ratio) (C72H144N6048P6, molecular weight 2046.76) is a six-
directional dendrimer as shown by Formula (5):
OH
O OI"..
O
0 -N- 0 0
O O 1O- OH
/~ ~ O=P-OH
HO '\ 1 .N\0\ OH i0 O\\ ,O- \
~/ p \O O-POH
O HO O O,O \ O
P'O Oj HOP-0 -N` IQ
O` HO-P~O
0 OH
HO -N O
HO
Isobutyryl L-Camitine Phytate (6:1)
Formula (5)
[0035] 3.6. According to one embodiment, valeryl L-camitine phytate (in a
6:1 mole ratio) (C78H156N6048P6, molecular weight 2129.76) is a six-
directional
dendrimer as shown by Formula (6):
O OH
O
O -N- O
+Q. OH
O O 10 iN
0=P OH
HO +N\0- OH O OP'0 \
/ O-OH O
OP~O'P - N+' O
O +N O OOH O
MO-P OH
O/1J}-J O-
H -N+ O
O
O
HO
Valeryl L-Carnitine Phytate (6:1)
Formula (6)
b0.
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0036] 3.7. According to one embodiment, isovaleryl L-carnitine phytate
(in a 6:1 mole ratio) (C78H156N6048P6, molecular weight 2129.76) is a six-
directional dendrimer as shown by Formula (7):
OH
O
00-
0 -N- O
O O 10 - ~; OH
~/ 0=P 0 OH N+
HO's 1 ,N\O~ $
~,O-
~/ / O `O O'POH Ol
\ O HOPI O-P \ + O
/ O +N~\O OOHO O
HO-P' OH
O/1JY \O-
O
HO -N~'()O-y
HO
Isovaleryl L-Carnitine Phytate (6:1)
Formula (7)
[0037] 4. Both cation moiety (i.e., L-carnitine or alkanoyl L-carnitines) and
anion moiety (i.e., phytate) have various biologically beneficial properties,
some
of which are similar or the same, such as the antioxidant property as well as
cardiovascular and immune system benefits. These biologically beneficial
properties can reasonably be expected to be due to the synergistic efficacy of
their salt form complex.
[0038] 5. Phytic acid is a liquid substance (syrup), strongly acidic and not
convenient for storage, processing, and consuming. However, when it is
composed with L-carnitine or its alkanoyl derivatives inner salt, the salt
form
phytates are weakly acidic, and according to one embodiment, L-carnitine
phytate (in a 6:1 mole ratio) and lower alkanoyl L-carnitine phytate (in a 6:1
mole ration), such as acetyl L-carnitine phytate (in a 6:1 mole ratio) and
propionyl L-carnitine phytate (in a 6:1 mole ratio), are solid, and, thus easy
to
handle and use.
13
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0039] 6. L-carnitine and its alkanoyl derivative inner salts are strongly
hygroscopic, but according to one embodiment, their phytates (in a 6:1 mole
ratio) are less hygroscopic, which is acceptable for processing and storage.
[0040] 7. L-carnitine and its alkanoyl derivative hydrochlorides have
unpleasant and irritating hydrochloric smells, which according to one
embodiment, their phytates (in a 6:1 mole ratio) do not have.
[0041] 8. According to one embodiment, L-camitine and alkanoyl L-
carnitine phytates (in a 6:1 mole ratio) are stable and almost odorless,
without
the unpleasant fishy smell given off by the inner salts (which is the emission
of
traces of amine that is usually generated by the decomposition of inner
salts).
[0042] 9. According to one embodiment, both L-carnitine or its alkanoyl
derivatives and phytic acid as well as their salt form complexes are non-toxic
and safe to consume.
[0043] Preparation of L-carnitine and alkanoyl L-carnitine phytates
[0044] According to one embodiment, L-carnitine phytates are produced by
a reaction between an L-camitine inner salt, or an alkanoyl L-carnitine inner
salt,
and phytic acid as shown by reaction Scheme (I):
OPO3H2 CH3 OR 0 O CH3 OR 0
H2O3PO OPO3H2 H3C.N = ~~ HZC~ I +
-I n = i ~~` Rj O-P -O - N
H203P0 OP03H2 H3C O OH H3C OH In
OP03H2
Scheme (I)
wherein n = 1-6; R1 is the phytate anion; and R is hydrogen, or a straight or
branched-chain alkanoyl group having 2-12 carbon atoms. According to one
embodiment, the alkanoyl group is a lower alkanoyl group having 2 - 5 carbon
atoms. According to one embodiment, the alkanoyl group is selected from
acetyl, propionyl, butyryl, isobutyryl, valeryl and isovaleryl groups.
14
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0045] According to one embodiment, L-carnitine or alkanoyl L-carnitine
inner salt is added to an aqueous solution of phytic acid while stirring.
According to one embodiment, the mole ratio between the combined L-carnitine
or alkanoyl L-camitine inner salt and phytic acid may range from a 1:1 mole
ratio to a 6:1 mole ratio with the ratio depending on the desired purpose.
According to one embodiment, after L-carnitine or alkanoyl L-carnitine inner
salt
is added to an aqueous solution of phytic acid and stirred about 15 minutes a
clear solution is obtained. According to one embodiment, after L-carnitine or
alkanoyl L-carnitine inner salt is added to an aqueous solution of phytic acid
and
stirred about 15 minutes a clear solution is obtained which is further stirred
for
another 20 minutes at the same conditions and then was dried in a vacuum to
obtain the resulting product.
[0046] According to one embodiment, L-carnitine or alkanoyl L-carnitine
phytate (in a 6:1 mole ratio) is prepared as shown by reaction Scheme (II):
OH
O
RO,......
-N R
O OR OH
HO 1 H =P-OH N+ O\
O
N-O_ PH 6
`O OI OH
HYPO` J~'OPO3H2 . Ha OR O of
JT` 7 '1 -I- 6- N~~ _ HO O_P' OR
H3Ccl
Y
HPO H3C O O~P" TTTO O HO O
OP03H2 3HZ ROB N\ OHO P= OH
HO -N+
OR
O
HO
Scheme (II)
wherein R represents either a hydrogen or an alkanoyl group (either a straight
or branched-chain alkanoyl group) having 2 -12 carbon atoms. According to
one embodiment, the alkanoyl group is a lower alkanoyl group having 2 - 5
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
carbon atoms. According to one embodiment, the alkanoyl group is selected
from acetyl, propionyl, butyryl, isobutyryl, valeryl and isovaleryl groups.
[0047] According to one embodiment, a 6 times mole ratio of L-carnitine or
alkanoyl L-carnitine inner salt is added to a 1 time mole ratio of 50% aqueous
solution of phytic acid (having a pH value lower than 1) and stirred at 10-50
degrees Celsius. According to one embodiment, a 6 times mole ratio of L-
carnitine or alkanoyl L-carnitine inner salt is added to a 1 time mole ratio
of 50%
aqueous solution of phytic acid (having a pH value lower than 1) and stirred
at
10-50 degrees Celsius for about 15 minutes until a clear solution (having a pH
value of 3-4) is obtained. According to one embodiment, the clear solution
(having a pH value of 3-4) is stirred for an additional 20 minutes and then
concentrated under a vacuum at 40-70 degrees Celsius. According to one
embodiment, the residue is repeatedly taken up with anhydrous ethanol.
According to one embodiment, the final residue is dried in a vacuum oven at 40-
70 degrees Celsius to obtain the resultant product.
[0048] According to one embodiment, L-carnitine and alkanoyl L-carnitine
phytate can be used for pharmaceutical, nutriceutical, and cosmetic purposes,
including but not limited to antioxidants, improvement for immunity,
anticancer,
a treatment and/or cure of disease (for example, for cardiovascular disease,
strokes, Alzheimer's disease, Down's syndrome, and various neuropathies),
boosting brain functions, improving learning and memory capacities (including
age associated memory impairment), an anti-aging supplement, athletic
performance, weight loss, and an animal feed additive.
[0049] Since phytic acid (IP6) is a 6-phosphate form of inositol, it has been
found to be absorbed almost instantly, transported intracellularly and
dephosphorylated into lower inositol phosphates, which play important roles in
signal transduction, so it is reasonable to conclude that inositol
monophosphate
(IP1), inositol diphosphate (IP2), inoistol triphosphate (IP3), inositol
tetraphosphate (IP4) , and inositol pentaphosphate (IP5), all can be used as
acids to react with inner salts of L-carnitine and alkanoyl L-carnitine to
form the
tb
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
anion moiety of the salts. Accordingly, L-carnitine and alkanoyl L-carnitine
inositol monphosphates, L-carnitine and alkanoyl L-carnitine inositol
diphosphates, L-carnitine and alkanoyl L-carnitine inositol triphosphates, L-
carnitine and alkanoyl L-carnitine inositol tetra phosphates, L-carnitine and
alkanoyl L-carnitine inositol pentaphosphates are within the scope of certain
particular embodiments of the present invention.
[0050] According to one embodiment, while the mole ratio between L-
carnitine (or alkanoyl L-carnitine) and phytic acid is selected from within a
range
of a mole ratio of 1:1 to 6:1, for L-carnitine phytate salt and alkanoyl L-
carnitine
phytate salt, since there are 12 dissociation sites in one phytic acid
molecule, a
mole ratio larger than 6:1 (for example, 7:1 to 12:1) is also included in the
scope
of an embodiment.
[0051] The invention is further explained by the following example
embodiments, which are provided for illustrative purpose only and are not to
be
construed as limiting the scope of the invention.
EXAMPLE EMBODIMENTS
Example Embodiment 1
[0052] Preparation of L-Carnitine Phytate (in a mole ratio of 1:1 - 6:1)
[0053] L-Carnitine Phytate (in a mole ratio of 1:1-6:1) is represented by
Formula (8):
0 CHs OH 0
HCI
R, O-P-O _2 N OH
OH H3C 1-6
L-Carnitine Phytate (1:1 - 6:1)
Formula (8)
wherein R, represents the phytate anion.
17
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
[0054] According to one embodiment, 32.2 grams (0.2 mole) of L-carnitine
inner salt (C7H15NO3, molecular weight 161.20) are added to a 50% aqueous
solution (pH value < 1) of 66.0 gram (0.1 mole) phytic acid and stirred at
room
temperature resulting in an exothermic reaction. According to one embodiment,
32.2 grams (0.2 mole) of L-carnitine inner salt (C7H15NO3, molecular weight
161.20) are added to a 50% aqueous solution (pH value < 1) of 66.0 gram (0.1
mole) phytic acid and after stirred at room temperature for about 15 minutes
creates a solution. According to one embodiment, the solution (having a pH
value <1) was further stirred 20 minutes. According to one embodiment, the
solution is concentrated on an evaporator under a vacuum at 50 C. According
to one embodiment, the residue is repeatedly (3 times) taken up using
anhydrous ethanol under a vacuum to dry the solution as much as possible.
According to one embodiment, the residue is further dried in a vacuum oven at
50 C to obtain 106.1 grams of residue from the mixture of L-carnitine phytate
(1:1-6:1) and phytic acid, which appears clear, thick and sticky.
Example Embodiment 2
[0055] Preparation of L-Carnitine Phytate (in a 6:1 mole ratio)
[0056] L-Carnitine Phytate (in a 6:1 mole ratio) (C48H108N6042P6, molecular
weight 1627.24) is represented by the formula shown by Formula (1).
[0057] According to one embodiment, 96.7 grams (0.6 mole) of L-carnitine
inner salt (C7H15NO3, molecular weight 161.20) was added to a 50% aqueous
solution (having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid and
stirred
at room temperature resulting in an exothermic reaction. According to one
embodiment, 96.7 grams (0.6 mole) of L-carnitine inner salt (C7H15NO3,
molecular weight 161.20) was added to a 50% aqueous solution (having a pH
value < 1) of 66.0 grams (0.1 mole) phytic acid and was stirred at room
temperature for about 15 minutes to create a solution. According to one
embodiment, the solution (having a pH value of about 4) was further stirred 20
1-1
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
minutes. According to one embodiment, the solution was concentrated on an
evaporator under a vacuum at 50 C. According to one embodiment, the
residue was repeatedly (3 times) taken up using anhydrous ethanol under a
vacuum to dry the solution as much as possible. According to one embodiment,
the residue is further dried in a vacuum oven at 50 C to obtain 166.2 grams of
white solid powder with an almost quantitative yield (i.e., the white solid
powder
contains 2% H20). According to one embodiment, this white solid powder has a
melting point of 121-125 degrees Celsius (dec.), is odorless (i.e., it does
not
have the unpleasant fishy smell of L-carnitine inner salt that is usually
generated by the decomposition of L-carnitine inner salt to emit amine), and
has
a pH value of 4 (c=1% H20),'HNMR (D20 ppm) 6 = 4.89, 4.46, and 4.24 (6H, m,
CH-O-P), 4.64 (6H, m, CH-OH), 3.45 (12H, m, CH2N), 3.20 (54H, s, NCH3),
2.59 (12H, m, CH2COOH).
Example Embodiment 3
[0058] Preparation of Acetyl L-Carnitine Phytate (in a 6:1 mole ratio)
[0059] Acetyl L-Carnitine Phytate (in a 6:1 mole ratio) (C60H120N6O48P6,
molecular weight 1879.44) is represented by Formula (2).
[0060] According to one embodiment, 122.09 grams (0.6 mole) of acetyl L-
carnitine inner salt (C9H17NO4, molecular weight 203.24) was added to a 50%
aqueous solution (having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid
and stirred at room temperature resulting in an exothermic reaction. According
to one embodiment, 122.09 grams (0.6 mole) of acetyl L-carnitine inner salt
(C9H17NO4, molecular weight 203.24) was added to a 50% aqueous solution
(having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid and was stirred
at
room temperature for about 15 minutes to create a solution. According to one
embodiment, the solution (having a pH value of about 4) was further stirred 20
minutes. According to one embodiment, the solution is concentrated on an
evaporator under a vacuum at 50 C. According to one embodiment, the
iI
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
residue was repeatedly (3 times) taken up with anhydrous ethanol in a vacuum
to dry the residue as much as possible. According to one embodiment, the
residue is further dried in a vacuum oven at 50 C to obtain 192.7 grams of
white
solid powder with an almost quantitative yield (i.e., the white solid powder
contains 2% H20). According to one embodiment, this white solid powder has a
melting point of 101-105 degrees Celsius (dec.), is odorless (i.e., it does
not
have the unpleasant hydrochloric smell of acetyl L-carnitine hydrochloride
that
is a commonly used salt form of acetyl L-carnitine), has a pH value of 4 (c=1
%
H20), 1HNMR (D20 ppm) b = 5.59 (6H, m, CH-OAc), 4.88, 4.45, and 4.22 (6H,
m, CH-O-P), 3.22 (12H, m, CH2N), 3.16 (54H, s, NCH3), 2.96 (12H, m,
CH2COOH), 2.11 (18H, s, CH3CO).
Example Embodiment 4
[0061] Preparation of Propionyl L-Carnitine Phytate (in a 6:1 mole ratio)
[0062] Propinoyl L-Carnitine Phytate (in a 6:1 mole ratio) (C66H132N6048P6,
molecular weight 1963.61) is represented by Formula (3).
[0063] According to one embodiment, 130.4 grams (0.6 mole) of propinoyl
L-carnitine inner salt (C1oHi9NO4, molecular weight 217.26) was added to a
50% aqueous solution (having a pH value < 1) of 66.0 grams (0.1 mole) phytic
acid and stirred at room temperature resulting in an exothermic reaction.
According to one embodiment, 130.4 grams (0.6 mole) of propinoyl L-carnitine
inner salt (C1oH19NO4, molecular weight 217.26) was added to a 50% aqueous
solution (having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid and was
stirred at room temperature for about 15 minutes to create a solution.
According to one embodiment, the solution (having a pH value of about 3.5)
was further stirred 20 minutes. According to one embodiment, the solution is
concentrated on an evaporator under a vacuum at 50 C. According to one
embodiment, the residue was repeatedly (3 times) taken up with anhydrous
ethanol in a vacuum to dry the residue as much as possible. According to one
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
embodiment, the residue is further dried in a vacuum oven at 50 C to obtain
201.3 grams of white solid powder with an almost quantitative yield (i.e., the
white solid powder contains 2% H20). According to one embodiment, this white
solid powder has a melting point of 85-90 degrees Celsius (dec.), is odorless
(i.e., it does not have the unpleasant hydrochloric smell of propionyl L-
carnitine
hydrochloride that is a commonly used salt form of propionyl L-carnitine), and
has a pH value of 4 (c=1 % H20), ' HNMR (D20 ppm) b= 5.60 (6H, m, CH-O-
Propionyl), 4.88, 4.46, and 4.22 (6H, m, CH-O-P), 3.22 (12H, m, CH2N), 3.16
(54H, s, NCH3), 2.96 (12H, m, CH2COOH), 2.41 (12H, q, CH3CH2CO), 1.07
(18H, t, CH3CH2CO).
Example Embodiment 5
[0064] Preparation of Butyryl L-Carnitine Phytate (in a 6:1 mole ratio)
[0065] Butyryl L-Carnitine Phytate (in a 6:1 mole ratio) (C72H144N6048P6,
molecular weight 2046.76) is represented by Formula (4).
[0066] According to one embodiment, 138.8 grams (0.6 mole) of butyryl L-
carnitine inner salt (C11H21NO4, molecular weight 231.26) was added to a 50%
aqueous solution (having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid
and stirred at room temperature resulting in an exothermic reaction. According
to one embodiment, 138.8 grams (0.6 mole) of butyryl L-carnitine inner salt
(C11H21NO4, molecular weight 231.26) was added to a 50% aqueous solution
(having a pH value < 1) of 66.0 grams (0.1 mole) phytic acid and was stirred
at
room temperature for about 15 minutes to create a solution. According to one
embodiment, the solution (having a pH value of about 3.8) was further stirred
20
minutes. According to one embodiment, the solution is concentrated on an
evaporator under a vacuum at 50 C. According to one embodiment, the
residue was repeatedly (3 times) taken up with anhydrous ethanol in a vacuum
to dry the residue as much as possible. According to one embodiment, the
residue is further dried in a vacuum oven at 50 C to obtain 209.5 grams of
I1
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
transparent gel-like product with an almost quantitative yield (i.e., the
transparent gel-like product contains 2.24% H20). According to one
embodiment, this transparent gel-like product has a pH value of 4 (c=1 % H20),
1HNMR (D20 ppm) 6=5.62 (6H, m, CH-O-Butyryl), 4.85, 4.43, and 4.21 (6H, m,
CH-O-P), 3.21 (12H, m, CH2N), 3.14 (54H, s, NCH3), 2.98 (12H, m, C j. COOH),
2.41 (12H, t, CH3CH2CH2CO), 1.21 (12H, m, CH3CH2CH2CO), 1.08 (18H, t,
CH3CH2CH2CO).
Example Embodiment 6
[0067] Preparation of Isobutyryl L-Carnitine Phytate (in a 6:1 mole ratio)
[0068] Isobutyryl L-Carnitine Phytate (in a 6:1 mole ratio) (C72Hu4-N6O48P6,
molecular weight 2046.76) is represented by Formula (5).
[0069] According to one embodiment, 34.7 grams (0.15 mole) of isobutyryl
L-carnitine inner salt (C11H21NO4, molecular weight 231.26) was added to a
50% aqueous solution (having a pH value < 1) of 16.5 grams (0.025 mole)
phytic acid and stirred at room temperature resulting in an exothermic
reaction.
According to one embodiment, 34.7 grams (0.15 mole) of isobutyryl L-carnitine
inner salt (C11 H2, NO4, molecular weight 231.26) was added to a 50% aqueous
solution (having a pH value < 1) of 16.5 grams (0.025 mole) phytic acid and
was
stirred at room temperature for about 15 minutes to create a solution.
According to one embodiment, the solution (having a pH value of about 3.7)
was further stirred 20 minutes. According to one embodiment, the solution is
concentrated on an evaporator under a vacuum at 50 C. According to one
embodiment, the residue was repeatedly (3 times) taken up with anhydrous
ethanol in a vacuum to dry the residue as much as possible. According to one
embodiment, the residue is further dried in a vacuum oven at 50 C to obtain
52.8 grams of transparent gel-like product with an almost quantitative yield
(i.e.,
the transparent gel-like product contains 3% H20). According to one
embodiment, this transparent gel-like product has a pH of 4 (c=1 % H20),
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
1HNMR (D20 ppm) 6 = 5.68 (6H, m, CH-O-isoButyryl), 4.88, 4.40, and 4.11 (6H,
m, CH-O-P), 3.21 (12H, m, CH2N), 3.14 (54H, s, NCH3), 2.98 (12H, m,
CH2COOH), 2.31 (6H, m, (CH3)2CHCO), 1.06 (36H, d, (qH3)2CHCO).
Example Embodiment 7
[0070] Preparation of Valeryl L-Carnitine Phytate (in a 6:1 mole ratio)
[0071] Valeryl L-Carnitine Phytate (in a 6:1 mole ratio) (C78H156N6048P6,
molecular weight 2129.76) is represented by Formula (6).
[0072] According to one embodiment, 24.5 grams (0.1 mole) of valeryl L-
carnitine inner salt (C12H23NO4, molecular weight 245.32) was added to a 50%
aqueous solution (having a pH value < 1) of 11.0 grams (0.0166 mole) phytic
acid and stirred at room temperature resulting in an exothermic reaction.
According to one embodiment, 24.5 grams (0.1 mole) of valeryl L-carnitine
inner
salt (C12H23NO4, molecular weight 245.32) was added to a 50% aqueous
solution (having a pH value < 1) of 11.0 grams (0.0166 mole) phytic acid and
was stirred at room temperature for about 15 minutes to create a solution.
According to one embodiment, the solution (having a pH value of about 3.5)
was further stirred 20 minutes. According to one embodiment, the solution is
concentrated on an evaporator under a vacuum at 50 C. According to one
embodiment, the residue was repeatedly (3 times) taken up with anhydrous
ethanol in a vacuum to dry the residue as much as possible. According to one
embodiment, the residue is further dried in a vacuum oven at 50 C to obtain
36.56 grams of transparent gel-like product with an almost quantitative yield
(i.e.,
the transparent gel-like product contains 2.9% H20). According to one
embodiment, this transparent gel-like product has a pH value of 4 (c=1 % H20),
1HNMR (D20 ppm) 6 = 5.59 (6H, m, CH-O-Valeryl), 4.85, 4.43, and 4.21 (6H, m,
CH-O-P), 3.19 (12H, m, CH2N), 3.13 (54H, s, NCH3), 2.99 (12H, m, CH2COOH),
2.44 (12H, t, CH3CH2CH2CH2CO), 1.21-1.29 (24H, m, CH3CH2CH2CH2CO),
1.08 (18H, t, CH3CH2CH2CH2CO).
23
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
Example Embodiment 8
[0073] Preparation of Isovaleryl L-Carnitine Phytate (in a 6:1 mole ratio)
[0074] Isovaleryl L-Carnitine Phytate (in a 6:1 mole ratio) (C78H156N6048P6,
molecular weight 2129.76) is represented by Formula (7).
[0075] According to one embodiment, 12.25 grams (0.05 mole) of isovaleryl
L-carnitine inner salt (C12H23NO4, molecular weight 245.32) was added to a
50% aqueous solution (having a pH value < 1) of 5.5 grams (0.0083 mole)
phytic acid and stirred at room temperature resulting in an exothermic
reaction.
According to one embodiment, 12.25 grams (0.05 mole) of isovaleryl L-carnitine
inner salt (C12H23NO4, molecular weight 245.32) was added to a 50% aqueous
solution (having a pH value < 1) of 5.5 grams (0.0083 mole) phytic acid and
was
stirred at room temperature for about 15 minutes to create a solution.
According to one embodiment, the solution (having a pH value of about 3.5)
was further stirred 20 minutes. According to one embodiment, the solution is
concentrated on an evaporator under a vacuum at 50 C. According to one
embodiment, the residue was repeatedly (3 times) taken up with anhydrous
ethanol in a vacuum to dry the residue as much as possible. According to one
embodiment, the residue is further dried in a vacuum oven at 50 C to obtain
18.3 grams of transparent gel-like product with an almost quantitative yield
(i.e.,
the transparent gel-like product contains 3.0% H20). According to one
embodiment, this transparent gel-like product has a pH value of 4 (c=1% H20),
1HNMR (D20 ppm) 6 = 5.56 (6H, m, CH-O-isovaleryl), 4.86, 4.43, and 4.21 (6H,
m, CH-O-P), 3.18 (12H, m, CH2N), 3.11 (54H, s, NCH3), 2.98 (12H, m,
CH2COOH), 2.43 (12H, d, (CH3)2CH1CH2CO), 1.20-1.26 (6H, m,
(CH3)2CH1CH2CO), 1.05 (36H, d, (CH3)2CH1CH2CO).
-1LI
CA 02727918 2010-12-13
WO 2010/005465 PCT/US2009/003522
Example Embodiment 9
[0076] Acute Toxicity Study (LDso) of L-Carnitine Phytate (in a 6:1 mole
ratio)
[0077] The single dose oral acute toxicity of L-carnitine phytate (in a 6:1
mole ratio) was evaluated in mice. Five dosages (25, 20, 15, 10, and 5 g/Kg)
were orally administrated to five groups of mice in which each group had five
male and five female mice. After dosing, the five groups of mice were observed
and daily records (for 14 days) were made of their general conditions, toxic
response, and deaths. All of the dead mice were necropsied, in which each of
the body's thorace and abdomen were opened, and each heart, liver, spleen,
lung, kidney, and intestine were examined and recorded. Under the conditions
of this test, the acute oral LD50 of L-carnitine phytate (6:1) was determined
to be
14.86g/Kg in the mice. Moreover, there were not any obvious abnormalities
observed in the hearts, livers, spleens, lungs, kidneys, and intestines
studied.
[0078] While the above detailed description and example embodiments
have shown, described and pointed out novel features of the invention as
applied to various embodiments, it will be understood that various omissions,
substitutions, and changes in the form and details of the device or process
illustrated may be made by those skilled in the art without departing from the
spirit of the invention. As will be recognized, the present invention may be
embodied within a form that does not provide all of the features and benefits
set
forth herein, as some features may be used or practiced separately from
others.
The scope of the invention is indicated by the appended claims rather than by
the foregoing description and example embodiments. All changes which come
within the meaning and range of equivalency of the claims are to be embraced
within their scope.