Note: Descriptions are shown in the official language in which they were submitted.
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
METHODS AND COMPOSITIONS FOR USE OF CYCLIC ANALOGUES OF
HISTATIN
Field of the Invention
[0001] The present invention relates to cyclic analogues of histatin, and to
their use in novel
compositions and methods of treatment.
Background of the Invention
[0002] Fungal and bacterial infections are a rising health risk as evidenced
by the emergence
of 9 million patients afflicted with these infections in the seven major
markets worldwide.
Patients at risk include those undergoing surgery, leukemic and HIV/AIDS
patients and those
who are immuno-compromised due to cancer, surgery or organ transplantation.
This is
partially as a result of the develoment of antibiotic-resistance in micro-
organisms.
[0003] As a result, there is a growing unmet need in connection with anti-
microbial agents,
and in particular, anti-fungal and anti-bacterial agents, which represents a
market opportunity
to develop non-toxic anti-fungal agents with high potency in humans.
Summary of the Invention
[0004] Cyclic analogues of histatin have now been determined to be useful to
treat anti-
microbial infection in humans either alone or in combination with other
antimicrobial agents.
The cyclic analogues are advantageously more potent but less toxic than
currently used anti-
microbial agents. In addition, compositions comprising the cyclic analogue
with other anti-
microbial agents are synergistic, exhibiting greater than expected activity.
[0005] Thus, in one aspect of the present invention, there is provided a
method of treating a
microbial infection in a human, comprising administering to the human a
therapeutically
effective amount of a cyclic analogue of histatin.
[0006] In another aspect of the invention, an anti-microbial composition for
use in treating a
microbial infection in a human is provided comprising a cyclic analogue of
histatin in
combination with a pharmaceutically acceptable carrier.
[0007] In another aspect, a composition for treating a microbial infection is
provided
comprising a cyclic analogue of histatin and a second anti-microbial agent.
1
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
[0008] In further aspects, kits and articles of manufacture are provided. Kits
comprise cyclic
analogues of histatin as well as compliance means such as instructions for
use. An article of
manufacture in accordance with the invention comprises packaging within which
is an anti-
microbial composition comprising a cyclic analogue of histatin. The packaging
is labelled to
indicate that the composition is suitable for treating a microbial infection
in a human.
[0009] In another aspect, a method of treating a microbial infection in a
mammal is provided
comprising administering to the mammal an anti-microbial agent in combination
with a
carrier molecule that targets microbial mitochondria.
[0010] In another aspect, a method of inhibiting microbial mycelial growth is
provided
comprising contacting a microorganism with a cyclic analogue of histatin.
[0011 ] In a further aspect, a method of treating a microbial infection in a
mammal is provided
comprising administering to the mammal a cyclic analogue of histatin that
targets a protein
selected from the group consisting of. microtubial-associated protein (YTMI),
septin
(CDC3), spindle dynamic control protein (SLK19), regulation of G-protein
function (CRPI),
HSP70 family member (SSA2), enolase I (ENOI), HSP member (SSE1), 26S
proteosomal
subunit (RPT5), HSP90, mitochondrial HSP protein (SSCI), betal subunit of
ATPase
complex (ATP2), mitochondrial aconitase/hydratase (ACOI) and microsomal ATPase
(CDC48), RAV1, HSP60, dihydrolipoamide dehydrogenase (LPD1), 60S ribosomal
subunit
protein L3 (RPL3) and seryl-tRNA synthetase (SESI).
[0012] In another aspect, a method of screening candidate anti-microbial
compounds is
provided. The method comprises the steps of.
1) contacting a candidate compound with at least one target selected from the
group
consisting of. microtubial-associated protein (YTM1), septin (CDC3), spindle
dynamic
control protein (SLK19), regulation of G-protein function (CRP!), HSP70 family
member
(SSA2), enolase I (ENO1), HSP member (SSEI), 26S proteosomal subunit (RPT5),
HSP90,
mitochondrial HSP protein (SSC1), betal subunit of ATPase complex (ATP2),
mitochondrial
aconitase/hydratase (ACO1) and microsomal ATPase (CDC48), RAV1, HSP60,
dihydrolipoamide dehydrogenase (LPD1), 60S ribosomal subunit protein L3 (RPL3)
and
seryl-tRNA synthetase (SESI); and
2
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
2) detecting whether or not said compound associates or interacts with one of
said
targets, wherein interaction with a target is indicative that said compound
may have anti-
microbial activity.
[0013] These and other aspects, features and advantages of the invention will
become
apparent from the following detailed description, claims and drawings.
Brief Description of the Drawings
[0014] Figure 1 illustrates the amino acid sequences of several histatins;
[0015] Figure 2 graphically illustrates the effect of temperature on the
activity of a cyclic
analogue of histatin (A) and the activity of 5 M and 50 M doses of the cyclic
analogue (B);
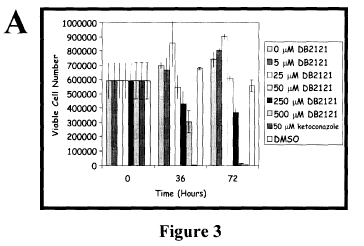
[0016] Figure 3 graphically illustrates the lack of toxicity of a cyclic
histatin analogue on
human primary cultured cells as compared with the toxicity of ketoconazole;
and
[0017] Figure 4 is a pie-chart illustrating the classes of microbial proteins
with which a cyclic
analogue of histatin associates.
Detailed Description
[0018] Provided are methods and compositions for use in treating microbial
infections in
humans comprising the administration of a cyclic analogue of histatin or a
variant or
derivative thereof.
[0019] Cyclic analogues of histatin that may be used in the compositions and
methods of the
present invention are described and characterized in U.S. Patent No.6,555,650
and in Brewer,
et al. (2002) Biochemistry 41:5526-5536, both of which are hereby incorporated
by reference
in their entireties. Generally, as used herein, the term "cyclic analogue of
histatin" refers to
cyclic analogues exhibiting anti-microbial activity that are formed from any
histatin, or
derivative or variant thereof, of suitable length to stably cyclize, including
for example,
histatin-1 (H-1) to histatin-12 (H-12), as set out in Figure 1, particularly
histatins H-1 to H-6,
and more particularly, histatins H-1, H-3 and H-5. The term "variant" as it is
used with
respect to histatin, refers to a molecule that exhibits substantial sequence
homology with a
histatin, for example, at least about 60% homology, preferably at least about
80% homology,
and more preferably at least about 90 - 95% homology. Examples of suitable
variants include
3
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
histatins in which lysine, glutamic acid or cysteine residues are introduced
into the histatin to
replace one or more existing amino acid residues in order to provide a variant
that is readily
cyclized to form a cyclic histatin analogue. The term "derivative" as used
herein with respect
to histatin refers to a histatin molecule that includes one or more
modifications at a reactive
site thereon, such as at a free carboxyl or amine group or other side chain
group. Such
modifications may be implemented in order to confer on the histatin analogue
desirable
properties such as increased stability, or improved cellular uptake. One
cyclic histatin
analogue in accordance with the invention is referred to herein as DB2121 and
has the amino
acid sequence, RHHCYKRKFHEKHHCHRGY (SEQ ID NO: 1).
[0020] The preparation of such cyclic analogues of histatin is described in
U.S. Patent
No.6,555,650, and generally involves standard methods of peptide synthesis,
followed by
amino acid substitutions, where desired, to facilitate cyclization. The linear
histatin peptide is
then cyclized under appropriate conditions for cyclization, as will be
appreciated by one of
skill in the art and as described in U.S. Patent No. 6,555,650, which will
depend on the amino
acid residues involved in the cyclization reaction.
[0021] In accordance with a method of the invention, cyclic analogues of
histatin have been
determined to be useful to treat microbial infections, including for example
bacterial
infections and fungal infections. Thus, the present cyclic histatin analogues
may be used to
treat human bacterial infections caused by, for example, a member of the genus
Streptococcus, Staphylococcus, Bordetella, Corynebacterium, Mycobacterium,
Neisseria,
Haemophilus, Actinomycetes, Streptomycetes, Nocardia, Enterobacter, Yersinia,
Fancisella,
Pasturella, Moraxella, Acinetobacter, Erysipelothrix, Branhamella,
Actinobacillus,
Streptobacillus, Listeria, Calymmatobacterium, Brucella, Bacillus,
Clostridium, Treponema,
Escherichia, Salmonella, Kleibsiella, Vibrio, Proteus, Erwinia, Borrelia,
Leptospira,
Spirillum, Campylobacter, Shigella, Legionella, Pseudomonas, Aeromonas,
Rickettsia,
Chlamydia, Borrelia and Mycoplasma, and further including, but not limited to,
a member of
the species or group, Group A Streptococcus, Group B Streptococcus, Group C
Streptococcus, Group D Streptococcus, Group G Streptococcus, Streptococcus
pneumoniae,
Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus faecalis,
Streptococcus
faecium, Streptococcus durans, Neisseria gonorrheae, Neisseria meningitidis,
Staphylococcus
aureus, Staphylococcus epidermidis, Corynebacterium diptheriae, Gardnerella
vaginalis,
Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium ulcerans,
4
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
Mycobacterium leprae, Actinomyctes israelii, Listeria monocytogenes,
Bordetella pertusis,
Bordatella parapertusis, Bordetella bronchiseptica, Escherichia coli, Shigella
dysenteriae,
Haemophilus influenzae, Haemophilus aegyptius, Haemophilus parainfluenzae,
Haemophilus
ducreyi, Bordetella, Salmonella typhi, Citrobacter freundii, Proteus
mirabilis, Proteus
vulgaris, Yersinia pestis, Kleibsiella pneumoniae, Serratia marcessens,
Serratia liquefaciens,
Vibrio cholera, Shigella dysenterii, Shigella flexneri, Pseudomonas
aeruginosa, Franscisella
tularensis, Brucella abortis, Bacillus anthracis, Bacillus cereus, Clostridium
perfringens,
Clostridium tetani, Clostridium botulinum, Treponema pallidum, Rickettsia
rickettsii,
Helicobacter pylori or Chlamydia trachomitis. Non-limiting examples of
diseases resulting
from a bacterial infection in a human include otitis media, conjunctivitis,
pneumonia,
bacteremia, meningitis, sinusitis, pleural empyema and endocarditis, and
meningitis, such as
for example infection of cerebrospinal fluid.
[0022] The cyclic histatin analogues may also be used to treat fungal
infections in humans
caused by, for example, Cryptococcus spp., Candida spp., Aspergillus spp.,
Histoplasma spp.,
Coccidioides spp., Paracoccidioides spp. Blastomyces spp., Fusarium spp.,
Sporothrix spp.,
Trichosporon spp., Torulopsis spp., Rhizopus spp., Pseudallescheria spp.,
Dermatophytes
spp., Paeciliomyces spp., Alternaria spp., Curvularia spp., Exophiala spp.,
Wangiella spp.,
Penicillium spp., Saccharomyces spp., Dematiaceous fungi and Pneumocystis
carinii. Non-
limiting examples of human disease resulting from a fungal infection include
cryptococcal
meningitis, athlete's foot, yeast infection, mold and mildew related
illnesses, thrush,
histoplasmosis, blastomycosis, onychomyosis and Tinea infections such as Tinea
capitis,
Tinea versicolor and Tinea pedis.
[0023] Therapeutically effective dosages of cyclic histatin analogues are
administered to a
human to treat a microbial infection. The term "therapeutically effective" as
it is used herein
with respect to dosages refers to a dosage that is effective to treat a given
microbial infection
without causing unacceptable adverse side effects. The term "administered"
refers to any
appropriate means of providing the cyclic histatin dosage to a recipient, and
will depend on
the dosage form being used as will be described. For example, the dosage may
be
administered orally, by injection, mucosally and topically as will be
described in more detail.
The term "treat" refers to at least partial inhibition of the microorganism
causing the infection
which may result in amelioration of one or more symptoms of the infection.
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
[0024] Therapeutically effective dosages according to the method, thus, are in
the range of
0.01 ng to about 10 g per kg body weight, specifically in the range of about 1
ng to about 0.1
g per kg, and more specifically in the range of about 100 ng to about 10 mg
per kg.
However, as one of skill in the art will appreciate, the effective therapeutic
dosage of the
histatin cyclic analogues will vary depending on the symptoms, age and body
weight of the
patient being treated, the nature and severity of the infection to be treated
or prevented and
the route of administration. The present histatin analogues may be
administered in a single
dose or in divided doses.
[0025] The cyclic histatin analogues may be administered in the treatment of a
microbial
infection in a human alone or in a composition combined with a
pharmaceutically acceptable
adjuvant or carrier. The expression "pharmaceutically acceptable" means
acceptable for use
in the pharmaceutical arts, i.e. not being unacceptably toxic, or otherwise
unsuitable for
administration to a human. Examples of pharmaceutically acceptable adjuvants
include, but
are not limited to, diluents, excipients and the like. Reference may be made
to "Remington's:
The Science and Practice of Pharmacy", 21st Ed., Lippincott Williams &
Wilkins, 2005, for
guidance on drug formulations generally. The selection of adjuvant depends on
the intended
mode of administration of the composition. In one embodiment of the invention,
the
compounds are formulated for administration by infusion, or by injection
either
subcutaneously or intravenously, and are accordingly utilized as aqueous
solutions in sterile
and pyrogen-free form and optionally buffered or made isotonic. Thus, the
compounds may
be administered in distilled water or, more desirably, in saline, phosphate-
buffered saline or
5% dextrose solution. Compositions for oral administration via tablet,
capsule, lozenge,
solution or suspension in an aqueous or non-aqueous liquid, an oil-in-water or
water-in-oil
liquid emulsion, an elixir or syrup are prepared using adjuvants including
sugars, such as
lactose, glucose and sucrose; starches such as corn starch and potato starch;
cellulose and
derivatives thereof, including sodium carboxymethylcellulose, ethylcellulose
and cellulose
acetates; powdered tragancanth; malt; gelatin; talc; stearic acids; magnesium
stearate;
calcium sulfate; vegetable oils, such as peanut oils, cotton seed oil, sesame
oil, olive oil and
corn oil; polyols such as propylene glycol, glycerine, sorbital, mannitol and
polyethylene
glycol; agar; alginic acids; water; isotonic saline and phosphate buffer
solutions. Wetting
agents, lubricants such as sodium lauryl sulfate, stabilizers, tableting
agents, disintegrating
agents, anti-oxidants, preservatives, colouring agents and flavouring agents
may also be
present. In another embodiment, the cyclic analogue may be formulated for
application
6
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
topically as a cream, lotion or ointment. For such topical application, the
cyclic analogue is
combined with an appropriate base such as a triglyceride base. Such creams,
lotions and
ointments may also contain a surface active agent and other cosmetic additives
such as skin
softeners and the like as well as fragrance. Aerosol formulations, for
example, for nasal
delivery, may also be prepared in which suitable propellant adjuvants are
used.
Compositions of the present invention may also be administered as a bolus,
electuary, or
paste. Compositions for mucosal administration are also encompassed, including
oral, nasal,
rectal or vaginal administration for the treatment of infections which affect
these areas. Such
compositions generally include one or more suitable non-irritating excipients
or carriers
comprising, for example, cocoa butter, polyethylene glycol, a suppository wax,
a salicylate or
other suitable carriers. Other adjuvants may also be added to the composition
regardless of
how it is to be administered which, for example, may aid to extend the shelf-
life thereof.
[0026] Thus, the present cyclic histatin analogues may be included in
compositions in which
an anti-microbial is advantageous such as medicines to treat particular
infections as described
above, other healthcare products such as wound treatments, eye drops,
toothpaste, mouth
washes or rinses, soaps, bath gel, shampoo, body lotion and beauty products
such as makeup
or other cosmetics.
[0027] In another aspect, the present cyclic analogues of histatin may be
administered to a
mammal in need of treatment for an microbial infection combined with another
active
ingredient, for example, a second antimicrobial agent such as an antifungal or
antibacterial
agent. Antibacterial agents that may be used as a second antimicrobial agent
in the
compositions and methods of the present invention include, but are not limited
to,
cephalosporins including cephalosporins I generation such as Cefadroxil,
Cefazolin,
Cephalexin, Cephalothin, Cephapirin, and Cephradine; cephalosporins II
generation such as
Cefaclor, Cefamandol, Cefonicid, Cefotetan, Cefoxitin, Cefprozil,
Ceftmetazole, Cefuroxime,
Cefuroxime axetil, and Loracarbef; cephalosporins III generation such as
Cefdinir,
Ceftibuten, Cefditoren, Cefetamet, Cefpodoxime, Cefprozil, Cefuroxime
(axetil), Cefuroxime
(sodium), Cefoperazone, Cefixime, Cefotaxime, Cefpodoxime proxetil,
Ceftazidime,
Ceftizoxime, and Ceftriaxone; and cephalosporins IV generation such as
Cefepime;
quinolones and fluoroquinolones such as Cinoxacin, Ciprofloxacin, Enoxacin,
Gatifloxacin,
Grepafloxacin, Levofloxacin, Lomefloxacin, Moxifloxacin, Nalidixic acid,
Norfloxacin,
Ofloxacin, Sparfloxacin, Trovafloxacin, Oxolinic acid, Gemifloxacin, and
Perfloxacin;
7
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
penicillins such as Amoxicillin, Ampicillin, Bacampicillin, Carbenicillin
Indanyl,
Mezlocillin, Piperacillin, and Ticarcillin; penicillin/beta lactamase
inhibitors such as
Amoxicillin-Clavulanic Acid, Ampicillin-Sulbactam, Benzylpenicillin,
Cloxacillin,
Dicloxacillin, Methicillin, Oxacillin, Penicillin G (Benzathine, Potassium,
Procaine),
Penicillin V, Piperacillin+Tazobactam, Ticarcillin+Clavulanic Acid, and
Nafcillin;carbepenems such as Imipenem-Cilastatin and Meropenem; a monobactam
such as
Aztreonam; macrolides and lincosamines such as Azithromycin, Clarithromycin,
Clindamycin, Dirithromycin, Erythromycin, Lincomycin, and Troleandomycin;
glycopeptides such as Teicoplanin and Vancomycin; rifampins such as Rifabutin,
Rifampin,
and Rifapentine; oxazolidonones such as Linezolid; tetracyclines such as
Demeclocycline,
Doxycycline, Methacycline, Minocycline, Oxytetracycline, Tetracycline, and
Chlortetracycline; aminoglycosides such as Amikacin, Gentamicin, Kanamycin,
Neomycin,
Netilmicin, Streptomycin, Tobramycin, and Paromomycin; streptogramins such as
Quinopristin+Dalfopristin; sulfonamides such as Mafenide, Silver Sulfadiazine,
Sulfacetamide, Sulfadiazine, Sulfamethoxazole, Sulfasalazine, Sulfisoxazole,
Trimethoprim-
Sulfamethoxazole, and Sulfamethizole; and other antibiotic agents such as
Bacitracin,
Chloramphenicol, Colistemetate, Fosfomycin, Isoniazid, Methenamine,
Metronidazol,
Mupirocin, Nitrofurantoin, Nitrofurazone, Novobiocin, Polymyxin B,
Spectinomycin,
Trimethoprim, Colistin, Cycloserine, Capreomycin, Pyrazinamide, Para-
aminosalicyclic acid,
and Erythromycin ethylsuccinate + sulfisoxazole .
[0028] Examples of antifungal agents that may be used as the second
antimicrobial agent in
the compositions and methods of the present invention include, but are not
limited to, azoles
such as fluconazole, voriconazole, clotrimazole, itraconazole, ketoconazole,
miconazole, ER
30346, SCH 56592; polyenes such as amphotericin B, nystatin or liposomal and
lipid forms
thereof such as Abelcet, AmBisome and Amphocil; purine or pyrimidine
nucleotide
inhibitors such as flucytosine; or polyoxins such as nikkomycins, in
particular nikkomycin Z
or other chitin inhibitors, elongation factor inhibitors such as sordarin and
analogs thereof,
mannan inhibitors such as predamycin, bactericidal/permeability-inducing (BPI)
protein
products such as XMP.97 or XMP.127.
[0029] The cyclic histatin analogue and the second antimicrobial agent may be
administered
to a mammal in the treatment of an infection either separately, or in
combination. The term
"mammal" is used herein to encompass both human and non-human mammals.
8
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
[0030] In addition, the cyclic histatin analogue may be combined with a second
anti-
microbial agent to provide an effective anti-microbial composition. The
composition may
additionally include pharmaceutically acceptable adjuvants to facilitate the
administration
thereof in a selected dosage form as described above. The amount of cyclic
histatin analogue
combined with the second antimicrobial agent will vary with the cyclic
histatin analogue
being used as well as the second antimicrobial agent being used, as will be
appreciated by
one of skill in the art. Generally, the amount of each will be an amount
suitable for
administration to a mammal, as determined using appropriate studies, that is
not toxic or
otherwise unacceptable. Thus, the dosage of cyclic histatin in the composition
may be
present in the range of 0.01 ng to about 10 g per kg body weight, while the
dosage of the
second antimicrobial agent will be present in a suitable dosage range as
determined for that
agent. In a preferred embodiment, the combination of a cyclic histatin
analogue and another
anti-microbial agent provides a composition that yields anti-microbial
activity that is greater
than the expected anti-microbial effect thereof, i.e. greater than the
expected additive effect of
the combination, and thus, is a synergistic composition. Accordingly, the
dosages of each of
the cyclic histatin and the second antimicrobial agent in the combined
composition may
advantageously be less than the dosage generally administered when the cyclic
histatin and
the second antimicrobial are used alone. This is particularly desirable to
reduce toxicity and
other undesirable affects that may be present when using full-strength dosages
of
antimicrobial agents.
[0031] In another aspect of the invention, kits are provided comprising an
anti-microbial
composition that includes a cyclic analogue of histatin in combination with a
pharmaceutically acceptable adjuvant or carrier, and compliance means such as
instructions
for its use to treat a microbial infection in a human. The instructions may
additionally
include an indication of recommended dosages to be used. Additional compliance
means
may also be included in such kits including any means which facilitate the
correct useage of
the composition. Such compliance means may include additional instructions,
dispensing
means, and the like. Alternatively, an article of manufacture is provided
comprising
packaging within which is contained the anti-microbial composition. In this
case, the
packaging is labeled at least to indicate that the composition is suitable for
treating a
microbial infection in a human, and may be further labeled to indicate
recommended dosages
and other conditions for use. In accordance with preceding aspects of the
invention, the kits
9
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
and articles of manufacture may additionally comprise a second antimicrobial
agent either
combined with the cyclic histatin analogue or separate thereform.
[0032] In another aspect of the present invention, methods of screening
compounds for use as
anti-microbial agents are provided that are based on the determination that
certain microbial
proteins are targets of cyclic analogues of histatin. These protein targets
include microtubial-
associated protein (YTM1), septin (CDC3), spindle dynamic control protein
(SLK19),
regulation of G-protein function (CRP1), HSP70 family member (SSA2), enolase I
(ENO1),
HSP member (SSE1), 26S proteosomal subunit (RPT5), HSP90, mitochondrial HSP
protein
(SSCI), betal subunit of ATPase complex (ATP2), mitochondrial
aconitase/hydratase
(ACOI) and microsomal ATPase (CDC48), RAV1, HSP60, dihydrolipoamide
dehydrogenase (LPD1), 60S ribosomal subunit protein L3 (RPL3) and seryl-tRNA
synthetase
(SES1). The systemics name of certain of these proteins from the Candida
genome database
(http://www.candidagenome.org) are as follows: YTM1 - orfl9.4815; cdc3 -
orfl9.1055;
SLK19 - orfl9.6763; CRP1 - orfl9.4784; SSA2 - orfl9.1065; ENOI - orfl9.395;
SSE1 -
orfl9.2435; SSCI - orfl9.1896; ATP2 - orfl9.5653; ACO1 - orfl9.6385; and CDC48
-
orfl 9.2340
[0033] The method of screening candidate anti-microbial agents includes
incubating the
candidate compound with one or more of said target proteins, either per se or
in a whole cell
environment, and determining whether or not the candidate associates with,
e.g. including via
covalent, electrostatic, hydrophobic, aromatic, ionic and dipolar
associations, via hydrogen
donating and accepting forces, or otherwise interacts with, the target. The
determination of
an association with a target protein is indicative that the candidate may have
anti-microbial
activity, and represents a candidate for further testing including, for
example, microbial cell
inhibition studies. Alternatively, it may be determined whether or not the
candidate
modulates the activity of the protein target using an appropriate assay for
this purpose as
would be appreciated by one of skill in the art. A determination that the
candidate modulates,
either by inhibition or activation, a target protein is indicative that the
candidate may have
anti-microbial activity and therefore warrants further study. Although the
candidate anti-
microbial agents suitable for screening may be any selected compound, cyclic
analogues of
histatin are particularly suitable for screening in this manner.
[0034] The identification of the foregoing protein targets for cyclic
analogues of histatin also
provides a further method of treating a microbial infection in a mammal. The
method
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
includes the steps of administering an anti-microbial agent in combination
with a carrier
molecule that targets a protein selected from the group consisting of.
microtubial-associated
protein (YTM1), septin (CDC3), spindle dynamic control protein (SLK19),
regulation of G-
protein function (CRPI), HSP70 family member (SSA2), enolase I (ENO1), HSP
member
(SSE1), 26S proteosomal subunit (RPT5), HSP90, mitochondrial HSP protein
(SSCI), betal
subunit of ATPase complex (ATP2), mitochondrial aconitase/hydratase (ACO1) and
microsomal ATPase (CDC48), RAVI, HSP60, dihydrolipamide dehydrogenase (LPD1),
betal subunit of ATPase complex (ATP2), 60s ribosomal subunit protein L3
(RPL3) and
seryl-tRNA synthetase (SES 1). Although the carrier molecule may be any
molecule suitable
to associate with one or more of the target proteins, cyclic analogues of
histatin are
particularly suitable for this purpose.
[0035] Those skilled in the art will recognize, or be able to ascertain using
no more than
routine experimentation, many equivalents to the specific embodiments of the
invention
described herein. While specific embodiments of the subject invention have
been discussed,
the above specification is illustrative and not restrictive. Many variations
of the invention
will become apparent to those skilled in the art upon review of this
specification. The full
scope of the invention should be determined by reference to the claims, along
with their full
scope of equivalents, and the specification, along with such variations. Such
equivalents are
intended to be encompassed by the claims that follow.
[0036] Embodiments of the invention are described by reference to the
following specific
examples which are not to be construed as limiting.
Example 1 - Activity of DB2121 Against Fungi and Bacteria
[0037] The efficacy of the cyclic analogue, 13132121, to inhibit cell growth
was measured in
various fungal and bacterial organisms and the results were compared to
several other known
anti-fungal compounds such as ketoconazole (Table 1). The MIC of DB2121 in
fungal or
yeast strains was at least 100-fold more potent than either histatin H5 or the
previously
developed patented analogue, P-113. DB2121 was found to possess an approximate
40-fold
greater ability over histatin H5 to inhibit the Gram-positive Bacillus
subtilis.
11
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
Table 1.
DRUG uM MIC
ORGANISM DB2121 H5 P113 FLU ITRA KETO AMP-b
FUNGI
(Lab Strains)
Candida albicans (SC5314) 4.5 =350 >350 x97.9 3.1 5.5 1.2
(Clinical Strains)
Candida albicans (isolate #3) 8.0 >350 >350 297.9 57.09 59.41 0.6
Candida albicans (isolate #4) 4.5 >350 >350 297.9 27.09 29.41 4.5
Candida albicans (isolate 95) 4.5 -350 >350 297.9 x7.09 29.41 3.2
Carulida albicans (isolate #11) 6.5 >350 >350 297.9 5.5 3.4 50.625
Candida albicans (isolate #28) 4.5 = 350 >350 297.9 x7.09 29.41 x0.625
Candida albicans (isolate #77) 4.5 ->351) >350 297.9 27.09 x9.41 (N/D)
Candida albicans (isolate #98) 4.5 >350 ='350 597.9 57.09 6.2 x0.625
Candida albicans (isolate #99) 4.5 >350 >350 597.9 27.09 6.2 5.5
Candida albicans (isolate #AST97 7.8 >350 >350 597.9 5.4 7.2 4.5
Candida dubliniensis (isolate #10) 4.5 >350 >350 597.9 57.09 29.41 5.4
Candida tro icalis (isolate #93) 4.5 >350 >350 297.9 57.09 29.41 56.5
BACTERIA
(Lab Strains)
S. aureus (ATCC 29213) 12.5 (NID) (N/D) (N/D) (N 1)) (N/D) (N/D)
S. haernolpticus (ATCC 29970) 4.5 (ND) (N/D) (N/D) (N/D) (N/D) (N/D)
S. auretts (ATCC 43300) 5.5 (N/I)) (N/D) (N/D) (ND) (N/D) (N/D)
S. aureus (ATCC 25923) 4.5 (N./D) (N/D) (N%D) (N/D) (N%D) (N/D)
Bacillus subtilis 23.9 >350 >350 (NiD) (N/D) (N/)) (N/D)
(Clinical Strains)
MRSAS. aureus (STRAIN 1) 11.5 >350 -350 (ND) (ND) (N/D) (ND)
MRSAS. aureus (STRAIN 2) 11.5 >350 >350 (ND) (N/D) (N/D) (N/D)
1) 115=1listaim 115, KETO=Ketoconazole. FLUFtuconazole, ITRA=Itraconazole, AMP-
b--Amphoteracin-h,
Pl 13 (former Demegen product), MIC=Minimum lnhihitory concentration
2) All incubations were performed (it,, 37oC for one hour in 10 mM NaCI/50 mM
MOPS, pH 7.2. After this
time period. aligouts were spiked into YPD broth for determination of MIC
after 24 hours of growth (d,
30oC (fungi) or 37oC (bacteria)
3) ClinicalCanclidu isolates were oral swabs or a blood culture AST97) from
HIV-infected patients
Example 2 - Activity in Combination with another anti-microbial agent
[0038] In similar cell inhibition studies as those described in Example 1
above, DB2121 was
also shown to kill Candida albicans strains when used in combination with
other anti-
microbial agents, including ketoconazole, itraconazole and flucoconanzole.
[0039] Generally, a logarithmatically grown culture of Candida albicans was
washed twice
in a solution containing 20 mM Tris-Cl, pH 7.2 and 20 mM NaCl and then
suspended in this
solution. Various concentrations of cyclic Histatin analogue and either
fluconazole,
itraconazole or ketoconazole were added immediately to this culture and shaken
vigorously at
37 C for 1 hour. Aliquots were spiked at a dilution of 1:100 into pre-warmed
YPD culture
media in 24-well dishes. The dishes were incubated in a 37 incubator for a
period of 24
hours to monitor growth and determine the Minimum Inhibitory Concentration
(MIC)
required for each compound either separately as controls and in combination.
12
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
[0040] Table 2 indicates that in a combination therapy using cyclic histatin
analogue and
either of the three tested antimicrobial compounds, concentrations of up to 12-
fold less of
cyclic histatin analogue and also the additional antimicrobial compounds were
required
compared to the drugs when administered separately.
Table 2.
TREATMENT MIC (DB2121a) .tM MIC (azoleb) M
DB2121 11 N/A
DB2121+Fluconazole 0.375 8.15
DB2121+Ketoconazole 0.375 0.078
DB2121+Itraconazole 0.375 0.059
Fluconazole N/A 97.8
Ketoconazole N/A 0.94
Itraconazole N/A 0.71
a) refers to cyclic Histatin analogue
b) refers to Fluconazole, Ketoconazole or Itraconazole
[0041 ] For these specific combinations, a suitable working range of each of
the cyclic histatin
and the second antimicrobial agent would be as follows:
i) an amount of cyclic Histatin analogue of equal to or greater than 0.37uM
used with
ketoconazole in an amount of equal to or greater than 0.078uM
ii) an amount of cyclic Histatin analogue of equal to or greater than 0.37uM
used with
fluconazole in an amount of equal to or greater than 8.15uM
iii) an amount of cyclic Histatin analogue of equal to or greater than 0.37uM
used
with
itraconazole in an amount of equal to or greater than 0.059uM
Example 3 - Inhibition of microbial mycelial growth
[0042] C. albicans (SC5314) fungi were cultured according to standard methods.
Once in
log phase, the fungal cells were resuspended in 10mM sodium phosphate and
incubated for
1.5 hours at 37 C in the presence of either (A) 50 gM ketoconazole, (B)
vehicle, (C) 50 tM
Histatin H5, or (D) 50 M DB2121. An aliquot of the mixture was then spiked
into YPD
containing 10% FBS and cultured at 37 C. At various time points, the cells
were imaged
13
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
under phase contract microscopy at 40 X magnification. The experiment was
performed at
last three times.
[0043] A significant inhibition of mycelial growth was noted when C. albicans
was pre-
incubated with DB2121. Moreover, DB2121 was effective to inhibit mycelial
growth pre-
and post-hyphae (data not shown) induction. Serial dilutions indicated that
mycelial growth
in the presence of DB2121 was approximately 500-1000 fold less than control
and with other
treatments, such as exposure to ketoconazole or histatin H5.
Example 4 - Conditions of inhibition of by DB2121
[0044] C. albicans (SC5314) were cultured according to standard procedures.
Once in log
phase, the fungal cells were resuspended in 10mM sodium phosphate and the
DB2121
peptide was tested for efficacy against C. albicans under various conditions.
DB2121 was
added to the fungal cells and incubated at either 4 C or 37 C for 1.5 hours
with shaking. In a
separate experiment, C. albicans was incubated with either 5 or 50 M DB2121
(f.c.) for a
total of 1.5 hours at 37 C. At certain time points, samples were taken to
monitor cell
viability. In a related set of experiments, (1) vehicle or (2, 3) Dansylated
DB2121 was
introduced to C. albicans under the conditions stated above. Following
exposure, the fungal
cells were counter-stained with SYTOX Green reagent to monitor for cell
viability. The
experiments were performed at least three times with similar results. Error
bars represent
standard deviation.
[0045] The activity of DB2121 was determined to be temperature-dependent, and
in
particular, more potent at physiological temperatures (Figure 2A) and acts
quickly to kill
Candida in as little as 5 minutes of exposure at certain concentrations (e.g.
50 M) (Figure
2B).
Example 5 - Toxicology of DB2121
[0046] Toxicological studies of DB2121 in human cells were also undertaken.
Primary
human neonatal foreskin epithelial cells were used to examine DB2121 toxicity,
using a
method as described in Min et al. 2004. Nat Biotechnol 22:717-23, the contents
of which are
incorporated herein by reference. Briefly, when the cells were in log phase,
(A) 50 M
DB2121, B) 50 M Histatin H5, (C) 50 M ketoconazole, or (D) DMSO was added to
the
culture medium. At 18 and 36 hours following addition of compound, the
foreskin epithelial
14
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
cells were imaged using phase contract microscopy at 20X magnification. (E)
Immediately
following the image analysis, proteins were extracted, separated on SDS-PAGE
gels, Western
blotted, and probed for Phosphorylated and non-phosphorylated ERK 1, 2 to
monitor the
proliferation of the foreskin keratinocytes. The experiments was repeated
three times.
[0047] Following the foregoing treatment, the human primary cells appeared
morphologically normal except when treated with ketoconazole. The toxicity of
ketoconazole to mammalian cells has been noted previously.
[0048] It was then investigated whether or not these phenotypes were reflected
at the
molecular level. The primary cells were lysed and monitored for a marker of
proliferation
namely phosphorylated ERK 1, 2. Significant inhibition of ERK 1, 2 activation
in the
ketoconazole-treated cells was observed as compared to control. Conversely,
activated ERK
1, 2 was not suppressed in DB2121-treated cells as compared to controls.
[0049] Both studies show that DB2121 is non-toxic to human primary cells at
working (e.g.
antimicrobial) concentrations of 50 pM .
[0050] To determine range of non-toxic concentrations of DB2121, various
concentration s
of DB2121, 50 M ketoconazole or DMSO as a control were separately added to
primary
human foreskin epithelial cell culture. At 0, 36 and 72 hours after addition,
the epithelial
cells were titriated off the culture plate and the viable cell number was
determined. The
experiment was performed in triplicate. At 72 hours, the foreskin epithelial
cells exposed to
the various compounds were imaged at 20X magnification using phase contrast
microscopy.
For cell counting, the epithelial cells were stained with trypan exclusion
stain to monitor cell
viability.
[0051] These results show minimal toxicity of DB2121 in primary cultured cells
and a
superior toxicity profile when compared to ketoconazole (Figure 3).
[0052] In addition, mice were able to withstand a concentration of 15mg/kg of
DB2121 when
injected via an intra-peritoneal route. When injected into adult rats, a
concentration of up to
and including 1.5 mg/kg caused no harm when injected intra-venously.
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
Example 6 - In Vitro Targets of DB2121
[0053] Confocal microscopy using fluorescent-tagged versions of DB2121
determined that
DB2121 does enter Candida albicans (SC5314) and that it locates specifically
within the
mitochondria of the organism (as determined by fluorescent probes specific for
yeast).
[0054] In an attempt to elucidate the mode of action of DB2121, Candida
albicans was
cultured in liquid media to an OD600 of -0.40-0.80 using standard protocols.
At this point,
the cells were harvested and the whole cell extract was collected. Equal
amounts of protein
were passed over glutathione-agarose columns containing purified GST or GST-
DB2121.
The column was extensively washed and the bound proteins were eluted with a
gradient of
free glutathione. Fractions were collected and the proteins from these
fractions were resolved
on 12% SDS-PAGE gels. The gels were stained. To identify the proteins of
interest, the
protein extracted, dried down and taken up in 0.2% formic acid. The peptides
obtained from
each protein band were then analyzed using LC/MS/MS on a Micromass. A pie
chart
indicating the classes of proteins identified in Candida albicans is shown in
Figure 4.
[0055] The DB2121 protein targets identified in Candida albicans (SC5314)
were:
microtubial-associated protein (YTM1), septin (CDC3), spindle dynamic control
protein
(SLK19), regulation of G-protein function (CRPI), HSP70 family member (SSA2),
enolase I
(ENOI), HSP member (SSE 1), 26S proteosomal subunit (RPT5), HSP90,
mitochondrial HSP
protein (SSC1), betal subunit of ATPase complex (ATP2), mitochondrial
aconitase/hydratase
(ACO1) and microsomal ATPase (CDC48), RAVI, HSP60, dihydrolipoamide
dehydrogenase (LPD1), 60S ribosomal subunit protein L3 (RPL3) and seryl-tRNA
synthetase
(SESI). Generally, the proteins listed above belong to different functional
groups - proteins
associated with morphogenesis/cell cycle/cell spindle/cytokinesis, proteins
associated with
the mitochondria, proteins associated with cell stress and metabolism, and
proteins associated
with translation control. These data are in good correlation with the data
obtained from
confocal microscopy.
Example 7 - Stability of DB2121
[0056] DB2121 cyclic analogue was determined to be stable in vitro in human
saliva for at
least 72 hours as determined by mass spectrometry. The cyclic analogue was
incubated in
human saliva at a concentration of 1 uM and at a temperature of 37 C in vitro.
At various
time points from 0 to 72 hours, 1.0 ul aliquots were taken and injected
directly into a
16
CA 02727939 2010-11-03
WO 2008/134882 PCT/CA2008/000853
Micromass Quattro Micro mass spectrometer. Data was collected for a total of 3
minutes.
The data was then processed using Mass Lynx 4.0 Analysis software.
[0057] The expected average mass of the cyclic histatin analogue in its active
form is
expected to be 2557.93. Cyclic histatin analogue was shown to be present in
the human
saliva up to at least 72 hours incubation. The peaks within an acceptable
error of
approximately I mass unit corresponding to cyclic histatin analogue were
2556.95 at 0 hours,
2557.65 at 24 hours, and 2557.10 at 72 hours incubation.
17