Note: Descriptions are shown in the official language in which they were submitted.
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 1
CAVITATION ASSISTED SONOCHEMICAL HYDROGEN PRODUCTION SYSTEM
Field of the Invention
[0001 ] The present invention generally relates to efficient generation of
hydrogen and more
specifically to in-situ hydrogen generation.
Background of the Invention
[0002] Water is composed of two parts hydrogen and one part oxygen by mass or
volume.
Decomposed by any means, two moles of water will produce one mole of oxygen
gas (02) and
two moles of hydrogen gas (H2) at a given input of energy El. When combined
together
through any means, hydrogen and oxygen react to form water, releasing a given
output of
energy E2. By all known principles of physics and chemistry, El > E2 and thus
by
thermodynamics the process is not favored in direct action. For hydrogen to be
useful as an
energy source and economical to use, a means must be created to either reduce
the
dissociation energy of water, or provide energy in some other fashion in the
process, for
example with catalytic enhancement, or all the above.
[0003] Hydrogen can be manufactured by a variety of means (including, but not
limited to
chemical, electrical, thermal, radiolysis, etc.) from a variety of chemical
substances
(including, but not limited to, water, hydrocarbons, plants, rocks, etc.). In
the present
invention water is used as the hydrogen source and a catalytic combination of
electrolysis and
cavitation is used to generate the hydrogen. The method of cavitation may be
by a variety of
means (acoustical, hydrodynamic inertial, non-inertial, mechanical,
electromagnetic, etc.), or
any combination thereof.
[0004] Hydrogen, being the most abundant element on earth as well as in the
Universe, holds
particular promise as a fuel source, both on earth as well as in space.
Hydrogen can power
homes and factories, transportation modes (planes, trains, and vehicles).
Thus, hydrogen can
serve to eliminate carbon fuels completely in the electrical cycle, thus
bringing about a net
subtraction by the contribution of anthropomorphic processes to terrestrial
climate change.
There are four significant "hurdles" cited by numerous reviews to the use of
hydrogen. Each
is noted as follows.
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 2
[0005] 1. Production-How to produce massive amounts of hydrogen in an
efficient, safe,
environmentally `friendly' fashion.
[0006] 2. Storage-How to store the low density, flammable gas.
[0007] 3. Distribution-Hydrogen, being difficult to store, is thus difficult
to transport.
[0008] 4. Use-How can hydrogen be used is a bigger hurdle in light of the
prior two
items.
[0009] Accordingly what is needed is a method and system to overcome the
problems
encountered in the prior art and to provide an economical method and apparatus
to produce
hydrogen.
Summary of the Invention
[0010] A method and an apparatus to generate hydrogen gas as H2 from a
hydrogen
containing liquid such as water. In one embodiment, the structure is a
electrolytic cell
configured with catalytic enhancements to maximize the volume and mass of
hydrogen
produced, and minimize the energy input, thus minimizing cost of operation.
This device is
particularly configured to enhance catalytically the decomposition of water
and the formation
of hydrogen gas by: 1) the container apparatus configuration of electric and
magnetic fields;
2) the use of sonochemistry and cavitation; and 3) the use of applicable
solutes and solvents
in the device that change the pH, ionic state, and the chemical potential of
the device solution.
[0011] The cavitation may be generated by a variety of means including but not
limited to,
acoustic energy, hydrodynamic (inertial, non-inertial), mechanical,
electromagnetic energy,
etc., or any combination thereof.
[0012] There are four significant "hurdles" cited by numerous reviews to the
use of hydrogen.
Each is noted as follows.
[0013] 1. Production-How to produce massive amounts of hydrogen in an
efficient, safe,
environmentally `friendly' fashion. This patent is capable of producing
hydrogen from water,
and by any fashion in its recombination with oxygen to re-from water,
producing no pollution
whatsoever and returning water back to its original form.
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 3
[0014] 2. Storage-How to store the low density, flammable gas. This patent
eliminates the
need for storage, by creating a scalable process to generate hydrogen from
water in-situ
wherever it is needed. It thus eliminates the need for dangerous, costly, and
hazardous storage
and transport issues.
[0015] 3. Distribution-Hydrogen, being difficult to store, is thus difficult
to transport.
Again, this patent eliminates the need for storage and thus transport, by
creating a scalable
process to generate hydrogen from water in-situ wherever it is needed. There
is no need for
dangerous, costly, and hazardous storage, distribution, and transport issues.
[0016] 4. Use-How can hydrogen be used is a bigger hurdle in light of the
prior two
items. With the elimination of those two items, the relative cost of the use
of fuel cells
becomes economical even to the middle class. Without the need for refueling,
or by
minimizing the need for refueling, the ability to use fuel cells will become
ubiquitous to
modem life.
[0017] A method and apparatus of producing hydrogen is disclosed comprising
applying an
electrical current to flow through an aqueous solution. Cavitation is
generated within the
aqueous solution, where the cavitation lowers an amount of energy required to
break chemical
bonds of said aqueous solution.
[0018] The foregoing and other features and advantages of the present
invention will be
apparent from the following more particular description of the preferred
embodiments of the
invention, as illustrated in the accompanying drawings.
Brief Description of the Drawings
[0019] The subject matter, which is regarded as the invention, is particularly
pointed out and
distinctly claimed in the claims at the conclusion of the specification. The
foregoing and other
features, and advantages of the invention will be apparent from the following
detailed
description taken in conjunction with the accompanying drawings in which:
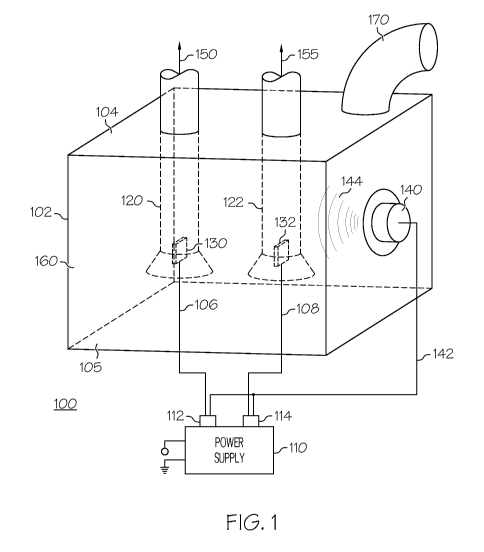
[0020] FIG. 1 is a diagram of a first embodiment of a hydrogen production
system according
to the present invention.
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 4
[0021] FIG. 2 is a diagram of a second embodiment of a hydrogen production
system
according to the present invention
[0022] FIG. 3 is a diagram of a conical funnel member of FIG. 2.
[0023] FIG. 4 is a diagram of a third embodiment of a hydrogen production
system according
to the present invention
[0024] FIG. 5 is a diagram of a first cavitation subsystem according to the
present invention.
[0025] FIG. 6 is a diagram of a second cavitation subsystem according to the
present
invention.
[0026] FIG. 7 is a diagram of the major factors affecting hydrogen production.
Description Of The Preferred Embodiments
[0027] It should be understood that these embodiments are only examples of the
many
advantageous uses of the innovative teachings herein. In general, statements
made in the
specification of the present application do not necessarily limit any of the
various claimed
inventions. Moreover, some statements may apply to some inventive features but
not to
others. In general, unless otherwise indicated, singular elements may be in
the plural and vice
versa with no loss of generality.
[0028] In this patent the following definitions apply when these words are
used:
[0029] Cavitation- Cavitation is the phenomenon of formation (irregardless of
mechanism) of
vapor bubbles in a fluid, in the region where the pressure of the fluid falls
below its vapor
pressure. Cavitation can be divided into two classes of behavior: inertial (or
transient)
cavitation, and non-inertial cavitation. Inertial cavitation is the process
where a void or bubble
in a liquid rapidly collapses, producing a shock wave. Non-inertial cavitation
is the process
where a bubble in a fluid is forced to oscillate in size or shape due to some
form of energy
(such as acoustic fields) input.
[0030] Acoustic Energy- For the purposes of this patent, acoustic energy
refers to all
frequencies, as well as any radiation of any frequency or wavelength in the
electromagnetic
spectrum. Also for the purposes of this patent, acoustic energy, as well as
any radiation of any
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 5
frequency or wavelength in the electromagnetic spectrum, may be employed as a
single
frequency (wavelength) or any frequency combination thereof (as a discrete
sum, difference,
harmonics, sub-harmonics, overtones, series, etc.).
[0031] First Embodiment of Hydrogen Production System
[0032] FIG. 1 is a cross sectional side view of the hydrogen production system
100 according
to the present invention. Hydrogen production system 100 consists of a
container apparatus
102 in the fashion of an electrolytic cell capable of storing a volume of a
solution 160.
Solution 160 is comprised of a solvent and solute. The solvent is preferably
water or another
aqueous solution containing hydrogen. The solute is a chemical compound
capable of
carrying an electrical charge i.e. an electrolyte. The sides of container
apparatus 102 are
preferably non-electrically conductive. Two electrically-conductive pieces 130
and 132 are
held above the bottom member 105 of container apparatus 120 by supporting
members 106
and 108, respectively. The electrically-conductive piece 130 is connected to
the negative
terminal 112 of power supply 110. Thus, the electrically-conductive piece 130
is a cathode.
Likewise, the electrically conductive piece 132 is connected to the positive
terminal 114 of
power supply 110. Thus, the electrically-conductive piece 132 is an anode. A
hollow,
cylindrical tube 120 is connected to and passes though top member 104 of
container apparatus
102. The bottom of tube 120 is flared outward and positioned so that the
bottom of tube 120
is below the bottom of cathode 130 but not touching bottom member 105 of
container
apparatus 102. Likewise, a hollow, cylindrical tube 122 is connected to and
passes through
top member 104 of container apparatus 102. The bottom of tube 122 is flared
outward and
positioned so that the bottom of tube 122 is below the bottom of anode 132 but
not touching
bottom member 105 of container apparatus 102. Finally, a transducer 140 is
connected to one
side of container apparatus 102. Wires 142 connect transducer 140 to power
supply 110.
[0033] As previously mentioned, power supply 110 causes cathode 130 to be
negatively
charged and anode 132 to be positively charged. As a result, an electrical
current is created
between cathode 130 and anode 132. The electrical current electrolyzes
solution 160 and
causes hydrogen to form around cathode 130 and oxygen to form around anode
132. Tube
120 funnels the hydrogen out of container apparatus 102 for use further use
(shown by arrow
150), such as to provide fuel for hydrogen fuel cells or to directly power an
engine. Likewise
tube 122 funnels the oxygen out of container apparatus 102 (shown by arrow
155). As
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 6
solution 160 is electrolyzed and the constituent gases are removed from the
system 100,
additional solution can be added through an inlet 170.
[0034] Transducer 140 produces acoustic energy waves 144 which transmit
through and
cause cavitation in solution 160. This cavitation decreases the energy
required to break the
chemical bonds of solution 160. As a result, in the presence of cavitation, a
greater amount of
hydrogen is produced at cathode 130 at a given voltage than in the absence of
cavitation.
Alternatively, in the presence of cavitation, the same amount of hydrogen is
produced at
cathode 130 at a lower voltage than in the absence of cavitation.
[0035] Hydrogen production system 100 is designed to be portable. In one
embodiment,
hydrogen production system 100 is sized approximately 8" in length by 8" in
width by 8" in
height so that it can fit as an engine component in a vehicle. However, it is
clear to one
skilled in the art that hydrogen production system 100 and its components can
be scaled larger
or smaller without affecting the spirit and scope of the present invention.
Likewise, it is clear
to one skilled in the art that hydrogen production system 100 and its
components can take on
many different shapes without affecting the spirit and scope of the present
invention. FIG. 1
shows one embodiment of the present invention where container apparatus 102 is
shaped to
allow maximum transmittal of sound waves 144 though solution 160. Finally, it
is clear to
one skilled in the art that any number of transducers 140 may be placed at
various locations
on container apparatus 102 and used to produce acoustic energy waves 144 in
order to
maximize the creation of cavitation within solution 160.
[0036] Second Embodiment of Hydrogen Production System
[0037] FIG. 2 is a cross sectional side view of another embodiment, referred
to as hydrogen
production system 200, of the present invention. Hydrogen production system
200 consists of
a container apparatus 202 in the fashion of an electrolytic cell capable of
storing a solution
160. The sides of container apparatus 102 are preferably non-electrically
conductive. A
hollow, cylindrical, electrically conductive piece 230 is held above the
bottom member 207 of
container apparatus 202 by supporting members 232. A second electrically
conductive
member 234 is held above the bottom member 207 of container apparatus 202 by
supporting
member 205. Electrically conductive piece 230 is connected to the positive
terminal 214 of
power supply 210. Thus, electrically conductive piece 230 is an anode.
Likewise, electrically
conductive piece 234 is connected to the negative terminal 212 of power supply
210. Thus,
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 7
electrically conductive piece 234 is a cathode. A hollow, cylindrical tube 220
is connected to
and passes through top member 206 of container apparatus 202. The bottom of
tube 220 is
flared outward and positioned so that some portion of cathode 234 is within
the tube 220.
Finally, a transducer 240 is connected to one side of container apparatus 202.
Wires 242
connect transducer 240 to power supply 210.
[0038] Power supply 210 causes cathode 234 to be negatively charged and anode
230 to be
positively charged. As a result, an electrical current is created between
cathode 234 and
anode 230. The cylindrical shape of anode 230 and the position of cathode 234
along the axis
of anode 230 takes advantage of the electrical field produced by cathode 234
and anode 230
and helps to maximize the flow of electricity between cathode 234 and anode
230.
[0039] As previously described, the electrical current flowing between cathode
234 and
anode 230 electrolyzes solution 160 and causes hydrogen to form around cathode
234 and
oxygen to form around anode 230. Tube 250 funnels the hydrogen out of
container apparatus
202 for further use (shown by arrow 250). Referring to FIG. 3, a conical piece
310 is placed
on top of anode 230. Conical piece 310 funnels oxygen out of container
apparatus 202 (shown
by arrow 340). Referring back to FIG. 2, as solution 160 is electrolyzed and
the constituent
gases are removed from the system 100, additional solution can be added
through an inlet
280.
[0040] Hydrogen production system 200 is the same as hydrogen production
system 100 in
that transducer 240 produces sound waves 244 which transmit through and cause
cavitation in
solution 160. This cavitation decreases the energy required to break the
chemical bonds of
solution 160 via electrolysis. As a result, in the presence of cavitation, a
greater amount of
hydrogen is produced at cathode 234 at a given voltage than in the absence of
cavitation.
Alternatively, in the presence of cavitation, the same amount of hydrogen is
produced at
cathode 234 at a lower voltage than in the absence of cavitation.
[0041] Hydrogen production system 200 is designed to be portable. In one
embodiment,
hydrogen production system 200 is sized approximately 8" in length by 8" in
width by 8" in
height so that it can fit as an engine component in a vehicle. However, it is
clear to one
skilled in the art that hydrogen production system 200 and its components can
be scaled larger
or smaller without affecting the spirit and scope of the present invention.
Likewise, it is clear
to one skilled in the art that hydrogen production system 200 and its
components can take on
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 8
many different shapes without affecting the spirit and scope of the present
invention. FIG. 2
shows one embodiment of the present invention where container apparatus 202 is
shaped to
allow maximum transmittal of acoustic energy waves 244 though solution 160.
Finally, it is
clear to one skilled in the art that numerous transducers 240 may be placed at
various
locations on container apparatus 202 and used to produce acoustic energy waves
244 in order
to maximize the creation of cavitation within solution 160.
[0042] Third Embodiment of Hydrogen Production System
[0043] FIG. 4 is a cross sectional side view of another embodiment, referred
to as hydrogen
production system 400, of the present invention. Hydrogen production system
400 consists of
a cylindrically-shaped container apparatus 402 in the fashion of an
electrolytic cell capable of
storing a solution 160. Container apparatus 402 has an electrically conductive
inner wall 403
and a non-electrically conductive outer wall 470. An electrically conducive
piece 430 is held
above the bottom member 407 of container apparatus 402 by supporting member
405.
Electrically conductive inner wall 403 is connected to the positive terminal
414 of power
supply 410. Thus, conductive inner wall 403 is an anode. Electrically
conductive piece 430
is connected to the negative terminal 412 of power supply 410. Thus,
electrically conductive
piece 430 is a cathode. A hollow, cylindrical tube 420 is connected to and
passes through the
top member 480 of container apparatus 402. The bottom of tube 420 is flared
outward and
position so that some portion of cathode 430 is within tube 420. Finally, a
transducer 440 is
connected to bottom member 407 of container apparatus 402. Wires 444 connect
transducer
440 to power supply 410.
[0044] Power supply 410 causes cathode 430 to be negatively charged and anode
403 to be
positively charged. As a result, an electrical current is created between
cathode 430 and
anode 403. The cylindrical shape of anode 403 and the position of cathode 430
along the axis
of anode 403 takes advantage of the electrical field produced by cathode 430
and anode 403
and helps to maximize the flow of electricity between cathode 430 and anode
403.
[0045] As previously described, the electrical current flowing between cathode
430 and
anode 403 electrolyzes solution 160 and causes hydrogen to form around cathode
430 and
oxygen to form around anode 403. Tube 420 funnels the hydrogen out of
container apparatus
402 for further use (shown by arrow 450). Conically-shaped top member 480 of
container
apparatus 402 funnels oxygen out of container apparatus 402 (shown by arrow
455). As
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 9
solution 160 is electrolyzed and the constituent gases are removed from the
system 400,
additional solution can be added through an inlet 490.
[0046] Hydrogen production system 400 is the same as hydrogen production
systems 100 and
200 in that transducer 440 produces acoustic energy waves 442 which transmit
through and
cause cavitation in solution 160. This cavitation decreases the energy
required to break the
chemical bonds of solution 160 via electrolysis. As a result, in the presence
of cavitation, a
greater amount of hydrogen is produced at cathode 430 at a given voltage than
in the absence
of cavitation. Alternatively, in the presence of cavitation, the same amount
of hydrogen is
produced at cathode 430 at a lower voltage than in the absence of cavitation.
[0047] Hydrogen production system 400 is designed to be portable. In one
embodiment,
hydrogen production system 400 is sized approximately 8" in length by 8" in
width by 8" in
height so that it can fit as an engine component in a vehicle. However, it is
clear to one
skilled in the art that hydrogen production system 400 and its components can
be scaled larger
or smaller without affecting the spirit and scope of the present invention.
Likewise, it is clear
to one skilled in the art that hydrogen production system 400 and its
components can take on
many different shapes without affecting the spirit and scope of the present
invention. Finally,
it is clear to one skilled in the art that any number of transducers 440 may
be placed on
container apparatus 402 and used to produce sound waves 442 in order to
maximize the
creation of cavitation within solution 160.
[0048] Throughout the descriptions of hydrogen production systems 100, 200,
and 400, a
cylindrical tube, tube 120, 250, and 420, is used to capture hydrogen formed
around the
cathode and direct the hydrogen out of the systems. It will be clear to one
skilled in the art
that tubes 120, 250, and 450 can be replaced by any means to capture and
direct the hydrogen.
Such means include, but are not limited to, tubes and similarly shaped
conduits, membrane
filtering, diffusive evaporation, differential pressures, and channeling
solution flow.
[0049] Embodiments of Cavitation Sub-System
[0050] Throughout the descriptions of hydrogen production systems 100, 200,
and 400,
transducers 140, 240, and 440 are used to produce acoustic energy waves 144,
244, and 442
which cause cavitation within solution 160. It will be clear to one skilled in
the art that
transducers 140, 240, and 440 can be replaced by any means for generating
cavitation. Such
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 10
means for creating cavitation include, but are not limited to, acoustic means,
mechanical
means, hydrodynamic means, electromagnetic means, and ionizing radiation
means.
[0051] Figures 1, 2 and 4 show embodiments of the present invention where the
cavitation is
produced by a specific acoustic means, namely, by using a transducer to pass
acoustic energy
waves through solution 160. However, other acoustic means can be used to
produce the
cavitation. It will be understood by one having skill in the art that such
acoustic means
includes, but is not limited to, transducers, microphones, and speakers.
[0052] An example of a mechanical means to cause cavitation within hydrogen
production
systems 100, 200, and 400 includes, but is not limited to, a propeller system
contained within
container apparatus 102, 202, and 402, which causes cavitation as the
propeller spins on its
axis. FIG. 5 shows a cross sectional view of such a propeller system. As
shown, propeller
blades 520 spin about the axis of propeller system 510 causing cavitation to
be produced in
solution 160. Propeller system 510 may be powered by power source 110, 210, or
410. It
will be understood by one having skill in the art that other mechanical means
can be used to
produce the cavitation. Such mechanical means include, but are not limited to,
a propeller
system, pistons, shock tubes, and light gas guns.
[0053] An example of a hydrodynamic means to cause cavitation within hydrogen
production
systems 100, 200, and 400 includes, but is not limited to, the injection of a
compressed gas,
for example, compressed air, into container apparatus 102, 202, and 402 to
cause cavitation.
FIG. 6 shows a cross sectional view of such a compressed gas injection system.
As shown,
compressed gas injection system 610 is affixed to container apparatus 102,
202, or 402.
Compressed gas travels (indicated by arrows 640) from a compressor (not shown)
through
tube 630 to compressed gas injection system 610. The compressed gas flows
through tubes
620 and is introduced into solution 160 as bubbles, i.e. cavitation. In one
embodiment,
compressed gas injection system 610 may be separated from solution 160 by a
porous
membrane that permits the transfer of the compressed gas through the membrane
while
preventing solution 160 from entering compressed air system 610. An example of
such a
membrane is Gore-Tex. It will be understood by those having skill in the art
that other
hydrodynamic means can be used to produce the cavitation. Such hydrodynamic
means
include, but are not limited to, a compressed gas injector system and any
device capable of
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 11
transferring momentum into solution 160 without transferring mass into
solution 160, for
example, a shock plate or paint shaker.
[0054] An example of an electromagnetic means to cause cavitation within the
hydrogen
production systems 100, 200, and 400 includes, but is not limited to, a laser
beam directed to
pass into solution 160 so as to produce a shock wave that causes cavitation
within solution
160. It will be understood by those having skill in the art that other
electromagnetic means
can be used to produce cavitation. Such electromagnetic means include, but are
not limited
to, a laser beam, x-rays, gamma rays, high speed electrons, electric arc,
magnetic
compression, plasma generation, and electromagnetic radiation arising from any
type of
electron or proton reaction.
[0055] Finally, an example of an ionizing radiation means to cause cavitation
within the
hydrogen production systems 100, 200, and 400 includes, but is not limited to,
passing high
energy protons into solution 160 where cavitation is formed around the
protons. Generally,
ionizing radiation is any radiation that is capable of removing an electron
from a chemical
bond. Therefore, it will be understood by those having skill in the art that
such ionizing
radiation means include, but are not limited to, all electromagnetic radiation
greater in energy
than ultraviolet radiation and high energy particles such as photons, protons,
neutrons, and
charged and uncharged nuclei.
[0056] Throughout the descriptions of hydrogen production systems 100, 200,
and 400, as
well as the examples of the various means of causing cavitation, cavitation is
said to occur
within solution 160. It will be understood by those having skill in the art
that causing
cavitation "within" solution 160 means causing cavitation within the
electrolytic zone.
[0057] FIG. 7 is a diagram of the major factors affecting the production of
hydrogen
according to the present invention. Solution factors 710 are the major factors
affecting
solution 160. These solutions factors include a solvent and solute. As
previously described,
the solvent is water or another aqueous solution containing hydrogen. The
solute is a
chemical compound, such as acid (such as HI or HC1), base (NaOH), or salt
(such as KI or
Nal), and is held at a particular density per volume of solvent in order to
maximize the
electrical conductivity of the solution. The solution has a particular pH, and
it is held at a
particular temperature and pressure, whether in hydrogen production system
100, 200, or 400,
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 12
to minimize the energy required to break the chemical bonds of the solvent.
Finally, the
solution has a particular ionic and covalent state (chemical potential).
[0058] Power factors 720 are the major factors affecting the delivery of power
to cathodes
130, 234, and 430, and anodes 132, 230, and 403. It will be readily apparent
to one skilled in
the art that the power factors 720 include voltage applied, current applied,
and total power
applied. Additionally, although hydrogen production systems 100, 200, and 400
have been
shown with a single cathode and single anode, it is apparent to one skilled in
the art that the
number of voltage/current applications points can be increased without
affecting the spirit and
scope of the present invention. Likewise it is apparent to one skilled in the
art that the sizes
and shapes of cathodes 130, 234, and 430 and anodes 132, 230, and 403 can
change without
affecting the spirit and scope of the present invention. Finally, it is
apparent to one skilled in
the art that power supplies 110, 210, and 410 can be any power producing
device, such as a
battery, solar panel, or fuel cell.
[0059] Material Composition factors 730 are the major factors affecting the
materials of the
hydrogen production systems 100, 200, and 400. The materials comprising
cathodes 130,
234, and 430, and anodes 132, 230, and 403 are selected to maximize electrical
conductivity.
Such materials include, but are not limited to, metals such as copper,
platinum, and high order
non-linear crystals including, but not limited to, lithium niobate and lithium
tantalate.
[0060] The catalytic factors 740 employed to enhance and catalyze the
production of
hydrogen are the major factors affecting the energy balance within solution
160. The non-
energy input catalytic factors lowering the necessary electrolytic input
energy AE, to AE2
include but are not limited to: (1) process temperature (as a function of
AEcav, AE2, partial
molar concentrations of species), (2) container properties (composition,
shape), (3) solution
properties (solute/solvent composition [ species, concentrations, etc.], pH,
chemical potential,
pressure, catalytic agents added [supported catalysts, gases such as noble
gases, etc.]), (4)
electrode properties (composition [elemental, isotopic, chemical], shape,
microsurface
[crystal planes, etc.], macrosurface [holes, edges, etc.], and (5) structure
of applied
electromagnetic field [energized, unenergized]).
[0061] Referring to Table 1, a set of equations is set forth showing that even
in the presence
of cavitation, the energy required to perform the electrolysis of solution 160
to produce
hydrogen is greater than the energy that is produced when that hydrogen is
recombined with
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 13
oxygen. Thus, it is apparent to one skilled in the art that the teachings
described herein are
not directed to a perpetual energy device. Rather, because of the net energy
loss that results
from the electrolysis of solution 160, energy is introduced into systems 100,
200, and 400 as
represented by power supplies 110, 210, and 410 to drive the electrolysis and
catalytic
processes.
1 Electrolysis (decomposition) of water requires energy input: E dec
2 H2O (1) -* 2 H2 (g) + 02 (g) E aec
E aec / 2 = E 1 ---> energy consumed per mole H2O or H2
Formation of water requires energy output: E form
2
2 H2 (g) + 02 (g) -* 2 H2O (1) E form
E form / 2 = E 2 - energy released per mole H2O or or H2
By the First Law of Thermodynamics, electrolysis is not fully reversible since
the
3 heat and entropy losses cannot be fully accounted for. Thus, we have the
result:
E 1 > E 2 always.
As a result, the process of electrolysis/water reformation, as well as the
process
described herein cannot be termed a "Perpetual Motion (or Energy) Machine" of
any
kind.
4 The thermodynamic efficiency relation = E 2 / E 1 X 100% gives a guide to
the relative efficiency of the electrolysis/water reformation process. An
eventual
efficiency of 80% or more is possible.
E 1 (energy consumed per mole H2O or H2 to decompose water to H2 gas) may be
represented in the present invention by the quantity E 3 , which is:
E 3 = E electrolysis + E cavitation + E other
where the electrolysis term represents only the electrical energy input from
the
electrodes as electrolysis, the cavitation term represents only the electrical
energy
input from acoustical energy (or any means) to cause or sustain cavitation,
and the
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 14
`other' term represents any energy input for heating, cooling, stirring, or
measurement. Here energy is represented as the total energy (power) input as
the
function of current and voltage by Ohm's Law.
In the absence of catalytic factors 740, E electrolysis ~ E 1. However, for
the
6 process described herein to be valid, E electrolysis must be less than E 1
E 1 > E electrolysis
since the process described herein is a catalytic process which lowers the
necessary
energy to form hydrogen gas. Thus, the overall equation is:
[ E 3 = E electrolysis + E cavitation + E other ] G E 2
which requires the value E 3 to approach E 2 . Since E 1 > E 2 always,
the equation E 1 > E 3 is valid.
Generally, there are two kinds of catalytic factors: non-energy input
catalytic factors
7 which are based on no energy input (e.g. electrode materials,
configurations, etc.);
and energy input catalytic factors which are based on energy input (e.g.
cavitation,
heating, cooling, stirring, etc). Examples of both kinds of catalytic factors
are set
forth in catalytic factors 740.
Table 1.
[0062] Referring back to FIG. 7, the energy input factors 750 lowering the
electrolytic input
energy AE1 to AE2 include, but are not limited to: (1) AEother (energy
necessary for the
temperature control and measurement, mechanical, stirring, etc.), and (2)
AEcav (cavitator
properties [size, shape, composition], configuration [number, density per unit
area/volume,
etc.], power input [f (V, I)], acoustic frequency spectrum input,
electromagnetic frequency
spectrum input). As described above, a cavitator can be any device capable of
causing
cavitation.
[0063] It has been advantageously shown that the following factors in one
embodiment,
hydrogen production system 400, greatly increase hydrogen production in the
present
invention: (1) the use of a specific acoustical spectrum to maximize
cavitation in solution
160; (2) the use of sodium or potassium iodide salt in solution 160 to
maximize the
conductivity and chemical potential of solution 160; (3) the dissolution of an
effective amount
of noble gas into solution 160, such that the noble gas is completely
dissolved in the solution,
to electromagnetically enhance the production of cavitation thus maximizing
the generation of
hydrogen gas - in the present embodiment, the noble gas is preferably argon
and an effective
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 15
amount of noble gas to be completely dissolved in solution 160 is up to five
percent (5%) at
Standard Temperature and Pressure; (4) the shape and configuration of the
electrodes, which
for hydrogen production system 400 comprise the electrically conductive inner
wall 403 and
electrically conductive inner piece 430, to (i) maximize the mechanical
separation of the
hydrogen and oxygen gas products and (ii) maximize the electrolysis electric
field by use of
the cylindrical electrode configuration (which maximizes the electric field by
a multiplicative
ratio of the inner and outer radii); and (5) the shape of the container, for
example, hydrogen
production system 400 comprises an electrically conductive inner wall 403
contained within
an non-electrically conductive outer wall 470 so as to electrically isolate
the function of the
hydrogen production system 400 from the outside world.
[0064] Likewise, although it is clear to one skilled in the art that the
solution 160 may be
exposed to any temperature and/or pressure and that solution 160 may be
contained within
either a sealed or unsealed container, it has been advantageously shown for
one embodiment,
hydrogen system 400, that the hydrogen production using the teachings
described herein is
preferably performed in a sealed, but not pressurized, container at
approximately Standard
Temperature and Pressure (STP).
[0065] Additionally, it is self evident that the teachings and embodiments set
forth herein are
focused on minimizing the amount of input energy while maximizing the output
of hydrogen
gas. The most important factor affecting the total input energy is
electrolysis voltage. Thus,
it is self evident that requiring less input voltage for the same given amount
(or greater) of
hydrogen gas generated will result in requiring less input energy, thus, less
input power. As a
result of requiring less input power, the input-output thermodynamic
difference is minimized
and as a result a larger fraction of input power can be generated by energy
sources such as
solar cells, recharged batteries, etc., thus maximizing overall efficiency and
quantity of
hydrogen generated.
[0066] Although a specific embodiment of the invention has been disclosed, it
will be
understood by those having skill in the art that changes can be made to this
specific
embodiment without departing from the spirit and scope of the invention.
Likewise, it will be
understood by those having skill in the art that the teachings herein can be
scaled in size to
increase or decrease hydrogen production without affecting the scope and
spirit of the present
invention. The scope of the invention is not to be restricted, therefore, to
the specific
CA 02729837 2010-12-31
WO 2010/002781 PCT/US2009/049040
9028-10001 16
embodiment, and it is intended that the appended claims cover any and all such
applications,
modifications, and embodiments within the scope of the present invention.
[0067] What is claimed is: