Note: Descriptions are shown in the official language in which they were submitted.
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
BLOCK-POLYMER MEMBRANES
FOR ATTENUATION OF SCAR TISSUE
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
61/059,795,
filed June 8, 2008 and entitled Block-Polymer Membranes for Attenuation of
Scar Tissue
(Att. Docket MB811OPR), is a continuation-in-part of U.S. Application No.
12/199,760, filed
August 27, 2008 and entitled Resorbable Barrier Micro-Membranes for
Attenuation of Scar
Tissue During Healing (Att. Docket MB8039P), and is related to U.S.
Application No.
10/385,399, filed March 10, 2003 and entitled Resorbable Barrier Micro-
Membranes for
Attenuation of Scar Tissue During Healing (Att. Docket MA9496CON), now U.S.
Patent No.
6,673,362, the contents each and all of which are expressly incorporated
herein by reference.
This application is also related to U.S. Application No. 10/631,980, filed
July 31,
2003 (Att. Docket MA9604P), U.S. Application No. 11/203,660, filed August 12,
2005 (Att.
Docket MB9828P), U.S. Application No. 10/0 19,797, filed July 26, 2002 (Att.
Docket
MB9962P), U.S. Provisional Application No. 60/966,782, filed August 27, 2007
(Att. Docket
MB8039PR), and U.S. Provisional Application No. 60/966,861, filed August 29,
2007 (Att.
Docket MB8039PR2). The foregoing applications are commonly assigned and the
entire
contents of each and all of them are expressly incorporated herein by
reference.
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates generally to medical implants and, more
particularly, to
resorbable membranes and methods of using the membranes and of their use as
medical
implants.
2. Description of Related Art
A major clinical problem relating to surgical repair or inflammatory disease
is
adhesion which occurs during the initial phases of the healing process after
surgery or
disease. Adhesion is a condition which involves the formation of abnormal
tissue linkages
caused by the formation of fibrous scar tissue. These linkages can, for
example, impair
bodily function, produce infertility, obstruct the intestines and other
portions of the
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
gastrointestinal tract (bowel obstruction) and produce general discomfort,
e.g. pelvic pain.
The condition can in some instances be life threatening. One of the most
common forms of
adhesion occurs as a result of surgical interventions, although adhesion may
occur as a result
of other processes or events such as pelvic inflammatory disease, Khron's
disease, peritonitis,
mechanical injury, radiation treatment and the presence of foreign material.
Various attempts have been made to prevent adhesions, particularly
postoperative
adhesions. For example, the use of peritoneal lavage, heparinized solutions,
procoagulants,
modification of surgical techniques such as the use of microscopic or
laparoscopic surgical
techniques, the elimination of talc from surgical gloves, the use of smaller
sutures and the use
of physical barriers (membranes, gels or solutions) aiming to minimize
apposition of serosal
surfaces, have all been attempted. Unfortunately, limited success has been
seen with these
methods. Additionally, barrier materials, in various forms such as membranes
and viscous
intraperitoneal solutions designed to limit tissue apposition, have also met
with limited
success. These barrier materials can include cellulosic barriers,
polytetrafluoroethylene
materials, and dextran solutions.
U.S. Patent No. 5,795,584 to Tokahura et al. discloses anti-adhesion or scar
tissue
reduction films or membranes, and U.S. Patent No. 6,136,333 to Cohn et al.
discloses similar
structures. In the Tokahura et al. patent, a bioabsorbable polymer is
copolymerized with a
suitable carbonate and then formed into a non-porous single layer adhesion
barrier such as a
film. In the Cohn et al. patent, a polymeric hydrogel for anti-adhesion is
formed without
crosslinking by using urethane chemistry. Both of these patents involved
relatively complex
chemical formulas and/or reactions resulting in particular structures for use
as surgical
adhesion barriers. There continues to be a need to for an improved membrane.
SUMMARY OF THE INVENTION
The present invention provides an improved resorbable micro-membrane that can
be
used in various surgical contexts, for example, to inhibit, retard, or prevent
tissue adhesions
and reduce scarring, e.g., during tissue healing, and then be absorbed or
dissolved after an
appropriate period of time. The membranes can formed to have very thin
thicknesses, for
example, thicknesses between about 0.010 mm and about 0.300 mm, while
maintaining
adequate strength.
The present invention provides an improved resorbable micro-membrane that can
be
readily and reliably formed and positioned on, around, or in proximity to
anatomical
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
structures comprising hard or soft tissues. The membrane can be used in
various surgical
contexts, for example, to retard or prevent tissue adhesions and reduce
scarring.
Furthermore, the co-polymers of the present invention may facilitate provision
of relatively
simple chemical reactions and/or formulations, and/or may facilitate provision
of one or more
of enhanced or more controllable mechanical strength and/or accelerated or
more controllable
degradation relative to other, e.g., mother, poly(esters).
In accordance with one exemplary implementation of the present invention a
resorbable micro-membrane can be provided comprising a substantially uniform
composition
of a dualblock copolymer. The dualblock copolymer can comprise a first block
that may
comprise, consist essentially of, or consist of one or more polylactide and/or
polyglycolide
(e.g., PLA, PGA, or PLGA) and a second block that may comprise, consist
essentially of, or
consist of one or more a polyethylene glycol (e.g., PEG). The first block,
denoted as a
PLA/PGA block, may comprise a hydrophobic and biodegradable PLA/PGA block, and
the
second block, denoted as a PEG block, may comprise a hydrophilic PEG block.
In accordance with another feature of the present invention, a resorbable
micro-
membrane is provided comprising, consisting essentially of, or consisting of a
substantially
uniform composition of a tri block copolymer, which may comprise a first block
that may
comprise, consist essentially of, or consist of a polylactide and/or a
polyglycolide (e.g., PLA,
PGA, or PLGA), a second block that may comprise, consist essentially of, or
consist of one
or more polyethylene glycol (e.g., PEG), and a third block that may comprise,
consist
essentially of, or consist of a polylactide and/or a polyglycolide (e.g., PLA,
PGA, or PLGA).
The first and third blocks, each denoted as a PLA/PGA block, preferably may
comprise one
or more hydrophobic and biodegradable PLA/PGA blocks, and the second block,
denoted as
a PEG block, preferably may comprise one or more hydrophilic PEG block.
When the first and third blocks are the same or share one or more common
characteristics, they may both be referred to as "A" blocks, and the second
block may be
referred to as a "B" block.
The first PLA/PGA block and the second PEG block together may form a PLA/PGA-
PEG (i.e., A-B) copolymer, and addition of the third PLA/PGA block may
altogether form a
PLA/PGA-PEG-PLA/PGA (i.e., A-B-A) copolymer. These PLA/PGA-PEG (and/or
PLA/PGA-PEG-PLA/PGA) copolymer membranes can be formed, for example, by
extrusion
at, for example, an initial, relatively high viscosity property. The initially
high viscosity
property may facilitate reliable formation of the membrane by, for example,
attenuating the
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
occurrence of, for example, breaking or tearing of the membrane, during the
extrusion
process. After processing and sterilization, the viscosity or viscosity
property of the
polymer(s) comprising the membrane may typically be lower. Other viscosity
properties
(e.g., relatively high viscosity properties) can be used according to other
aspects of the
invention, in order, for example, to increase the strength of the PLA/PGA-PEG
(and/or
PLA/PGA-PEG-PLA/PGA) copolymer material during the manufacturing process, such
as an
extrusion process. In modified embodiments, the initial viscosity property may
not be
relatively high. An extrusion manufacturing process may provide the membrane
with a
biased molecular orientation.
According to another feature, a membrane has a first substantially-smooth
surface and
a second substantially-smooth surface, is non-porous, and is about 0.01 mm to
about 0.300
mm thick as measured between the first substantially-smooth surface and the
second
substantially-smooth surface. The membrane thus can possess a varying cross-
sectional
thickness. For example, the membrane can comprise at least one relatively
thick portion,
which can form at least a segment of an edge of the membrane. In other
embodiments, the
membrane may have a uniform thickness.
While the apparatus and method have or will be described for the sake of
grammatical
fluidity with functional explanations, it is to be expressly understood that
the claims, unless
indicated otherwise, are not to be construed as limited in any way by the
construction of "means"
or "steps" limitations, but are to be accorded the full scope of the meaning
and equivalents of the
definition provided by the claims under the judicial doctrine of equivalents.
Any feature or combination of features described herein are included within
the scope
of the present invention provided that the features included in any such
combination are not
mutually inconsistent as will be apparent from the context, this
specification, and the
knowledge of one of ordinary skill in the art. In addition, any feature or
combination of
features described herein may be specifically excluded from any embodiment of
the present
invention. For purposes of summarizing the present invention, certain aspects,
advantages
and novel features of the present invention are described. Of course, it is to
be understood
that not necessarily all such aspects, advantages or features will be embodied
in any
particular implementation of the present invention. Additional advantages and
aspects of the
present invention are apparent in the following detailed description and
claims that follow.
CA 02731404 2011-01-19
Printed 07-10-2010' DESCPAMD,= PCT/IB 2009/OtPCT/IB 2009/006 229)9
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. I illustrates an ABA-tri block copolymer showing a basic structure of an
embodiment of PLA-PEG-PLA (ABA) and the reduction in molecular weight of the
polymer over
time.
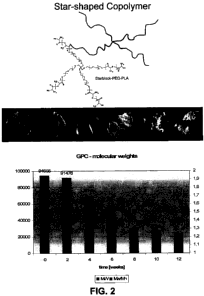
FIG. 2 illustrates an embodiment of a starblock copolymer showing a basic
structure of an
embodiment of a star shaped PEG with PLA and/or PGA/PEG arms, and the
reduction in the
molecular weight of the polymer over time.
FIG. 3 illustrates a BAB-tri block copolymer and the reduction in the
molecular weight of
the polymer over time.
FIG. 4 illustrates a specific ABA-tri block copolymer and the reduction in the
molecular
weight of the polymer over time.
FIG. 5 illustrates a specific PLA and PEG admixture and the reduction in the
molecular
weight of the polymer over time.
FIG. 6 illustrates a specific PLA and ABA triblock copolymer mixture and the
reduction
in molecular weight over time.
DETAILED DESCRIPTION OF THE INVENTION
Reference will now be made in detail to the presently preferred embodiments of
the
invention, examples of which are illustrated in the accompanying drawings.
Wherever possible,
the same or similar reference numbers are used in the drawings and the
description to refer to the
same or like parts. It should be noted that the drawings are in simplified
form and are not to
precise scale. In reference to the disclosure herein, for purposes of
convenience and clarity only,
directional terms, such as, top, bottom, left, right, up, down, over, above,
below, beneath, rear,
and front, are used with respect to the accompanying drawings. Such
directional terms should not
be construed to limit the scope of the invention in any manner.
Although the disclosure herein refers to certain illustrated embodiments, it
is to be
understood that these embodiments are presented by way of example and not by
way of
limitation. The intent of this disclosure, while discussing exemplary
embodiments, is that the
following detailed description be construed to cover all modifications,
alternatives, and
equivalents of the embodiments as may fall within the spirit and scope of the
invention as defined
by the appended claims.
Barrier membranes of the present invention may be constructed from various
biodegradable materials, such as resorbable polymers. In accordance with one
embodiment, non-
limiting polymers which may be used to form barrier membranes of the present
invention can
include a dualblock copolymer. As embodied herein, the dualblock copolymer can
comprise a
first block that may include, consist essentially of, or consist of a
polylactide and/or a
polyglycolide (e.g., PLA, PGA, or PLGA) and a second block that may include,
consist
essentially of, or consist of a polyethylene glycol (e.g., PEG). The first
block, denoted as a
5
1/3; AMENDED SHEET 02-11-2009'
CA 02731404 2011-01-19
,Phinted. 07-1.0-2010 DESCPAMD PCT/IB 2009/OIPC1/IB 20p9/006229)
g
PLA/PGA block, can comprise one or more of a hydrophobic and a biodegradable
PLA/PGA
block, and the second block, denoted as a PEG block, can comprises a
hydrophilic PEG block.
The first PLA/PGA block may be referred to as an "A" block, and the second PEG
block may be
referred to as a "B" block. The first PLA/PGA block and the second PEG block
together may
form a PLA/PGA-PEG (i.e., A-B. or AB) dualblock copolymer.
Other non-limiting block polymers which may be used to form barrier membranes
of the
present invention include a triblock copolymer or a starblock copolymer. As
embodied herein, the
triblock copolymer can comprise a first block that may include or consist of a
polylactide and/or a
polyglycolide (e.g., PLA, PGA, or PLGA), a second block that may include or
consist of a
polyethylene glycol (e.g., PEG), and third block that may include or consist
of a polylactide
and/or a polyglycolide (e.g., PLA, PGA, or PLGA). The first block, denoted as
a PLA/PGA block,
can comprise a hydrophobic and biodegradable PLA/PGA block, the second block,
denoted as a
PEG block, can comprise a hydrophilic PEG block, and the third block, denoted
as a PLA/PGA
block, can comprise a hydrophobic and biodegradable PLA/PGA block. When the
first PLA/PGA
block and third PLA/PGA block are the same or share one or more common
characteristics, they
may each be referred to as an "A" block, and the second PEG block may be
referred to as a "B"
block. The first PLA/PGA block, the second PEG block, and the third first
PLA/PGA block
together may form a PLA/PGA-PEG-PLA/PGA (i.e., A-B-A, or ABA) triblock
copolymer (see,
e.g., FIG. 1).
The combination block copolymer may, alternatively, be characterized as a
PEGPLA/
PGA-PEG (i.e., B-A-B, or BAB) triblock copolymer.
In other implementations, the combination block copolymer may be a 4plus
(i.e., four or
more blocks) block copolymer, comprising, for example, a PEG block (i.e., B
block) that is
formed with, coupled to, or disposed between three or more PLA/PGA blocks
(i.e., A blocks).
The 4plus block copolymer may, alternatively, comprise, for example, a PLA/PGA
block (i.e., A
block) that is formed with, coupled to, or disposed between three or more PEG
blocks (i.e., B
blocks).
The 4plus block copolymer may comprise a PEG block (i.e., B block) having one
or more
of a symmetrical shape and a star shape, with regions (e.g., arms, branches,
or points) coupling
(e.g., being connected to or with) three or more PLA/PGA (or PLA and/or
PGA/PEG) blocks (i.e.,
A blocks), which number may comprise, for example, four. See, for example,
FIG. 2 for an
embodiment of a starblock polymer. In a preferred implementation, the number
of regions equals
the number of PLA/PGA blocks. Alternatively, the 4plus block copolymer can
comprise a
PLA/PGA block (i.e., A block) having one or more of a symmetrical shape and a
star shape, with
regions (e.g., arms, branches, or points) coupling (e.g., being connected to
or with) three or more
PEG blocks (i.e., B blocks). The number of regions can, as in the preceding
example, equal the
number of PEG blocks and, in a particular example, can comprise four.
6
2/3 AMENDED SHEET 02-11-2009'
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
The combination block copolymer membranes can be formed by extrusion at an
initial, relatively high viscosity property. The initially high viscosity
property may facilitate
reliable formation of the membrane by attenuating the occurrence of, for
example, breaking
or tearing of the membrane, during the extrusion process. After processing and
sterilization,
the viscosity property of the membrane will typically be lower. Other
relatively high
viscosity properties can be used according to other aspects of the invention,
in order, for
example, to increase the strength of the material. The extrusion procedures
advantageously
can provide for efficient production of the membranes. Moreover, membranes
which are
manufactured by such extrusion techniques can be free from solvent trappings
in the
membrane and, furthermore, can be provided with, for example, a molecular
bias, including a
predetermined molecular bias. Monoaxial or biaxial extrusion may be employed
to
manufacture the membranes.
Compositions of the combination block copolymer can be extruded to form
membranes of the present invention. In certain embodiments, PLA/PGA-PEG block
copolymers taking the forms of one or more of the following polymers: 1.
Poly(L-lactide-
co-PEG), 2. Poly(L-lactide-co-DL-lactide-co-PEG), and 3. Poly(L-lactide-co-
glycolide-co-
PEG); PLA/PGA-PEG-PLA/PGA block copolymers taking the forms of. 4. Poly(L-
lactide-
co-PEG-co-L-lactide), 5. Poly(L-lactide-co-PEG-co-L-lactide-co-DL-lactide), 6.
Poly(L-
lactide-co-PEG-co-L-lactide-co-glycolide), 7. Poly(L-lactide-co-DL-lactide-co-
PEG-co-L-
lactide-co-DL-lactide), 8. Poly(L-lactide-co-DL-lactide-co-PEG-co-L-lactide-co-
glycolide),
9. Poly(L-lactide-co-glycolide-co-PEG-co-L-lactide-co-glycolide), and 10.
other forms from
combinations and/or permutations of the above (optionally combined with any
one or more
other items disclosed or referenced herein) for starblock and/or 4plus block
copolymers, can
be manufactured or obtained. For instance, such items may be manufactured or
obtained,
without limitation, from Boehringer Ingelheim KG of Germany, for extrusion
into the
membranes of the present invention.
Exemplary chemical structures, and synthesis and nomenclature conventions to
be used
herein follow, wherein:
CA 02731404 2011-01-19
P.i'inted: 07-10-201.0 DESCPAMDf PCT/IB 2009/0(PCT/IB 2009/006 229+)9
Scheme A
0
H3C
O
O
CH3 f0~.
O 0
catalyst
A B
H3C H H H
0 O
If XC\ II
0\C_0 i- \Cz [1-/Y [fo\/o/}R1
C
H3C H H H / \H
A B
R,= CH3 for diblock
R1= A for triblock
Scheme B shows the incorporation of polyethylene glycol (PEG) units into a
block co-polymer
with PLGA, again by the action of the catalyst. PEG also has low systemic
toxicity, and is
currently used in various medical and pharmaceutical agents.
The resulting block co-polymer can be represented schematically as follows:
Scheme B
RLLRLLLRLLR .LLRR-O-[-CH2-CH2-O-]õR
A B
Commercially obtained PLGA:PEG block co-polymers include the RESOMER PEG
products
from Boehringer Ingelheim.
8
3/3 AMENDED SHEET 02-11-2009
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
One preferred (though non-exclusive) product is RESOMER PEG Sample MD Type
LRP d 70 5 5, wherein LR stands for RESOMER Acronym LR (A-block), P stands for
PEG (B-
block), 70 stands for the mole ratio within the A-block, the first 5 stands
for the weight percent
PEG, and the second 5 stands for the molecular weight of the PEG divided by
one thousand.
Typical non-limiting examples of PLA/PGA-PEG (and/or PLA/PGA-PEG-
PLA/PGA) copolymers are as follows: For controlled release functionalities
(CR), the
polymer will typically contain from about 5% to about 15% PEG. For medical
devices
(MD) the polymer will typically contain less than about 5% PEG. For controlled
release the
A block may contain, e.g., D,L-lactide-co-glycolide (RG). For Medical Devices,
the A Block
may contain, e.g., L lactide (L), L-lactide-co-D,L-lactide (LR), or L Lactide-
co-glycolide
(LG).
FIGS. 1-6 elucidate certain compositions and characteristics of contemplated
embodiments according to the present invention. A membrane of the present
invention can
have at least one substantially smooth-surface. Preferably, a membrane of the
present
invention has two (opposing) substantially smooth surfaces. As measured
between the
opposing surfaces, a membrane of the present membrane can have a thickness of
about 0.01
mm to about 0.3 mm and, more preferably, about 0.01 mm to about 0.1 mm. In a
preferred
embodiment, a membrane of the present invention has a thickness of about 0.0
15 mm to
about 0.025 mm. In another preferred embodiment, a membrane of the present
invention has
a maximum thickness of about 0.02 mm. A preferred micro-membrane of the
present
invention can comprise one or more of a substantially uniform composition and
a biased
molecular orientation in the membrane as a consequence, for example, of
extrusion.
As used herein, the term "non-porous" refers to a material which is generally
water
tight and, in accordance with a preferred embodiment, not fluid permeable.
However, in a
modified embodiment of the invention micro-pores (which are fluid permeable
but not cell
permeable) may exist in the micro-membrane of the present invention, to the
extent, for
example, that they do not substantially disrupt the smoothness of the surfaces
of the
resorbable micro-membrane to cause scarring of tissue. In substantially
modified
embodiments for certain applications, pores which are cell permeable but not
vessel
permeable may be manufactured and used.
As presently embodied, many of the thinner membrane thicknesses can be
sufficiently
contoured even in the absence of heating to glass transition temperature. As
presently
embodied, the resorption of the resorbable membrane can be between
approximately 2 and 24
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
months. In one embodiment, membranes of the present invention can be capable
of resorbing
(i.e., being absorbed by the mammalian body) within a period, for example, of
about 10 to 20
weeks, or of about 20 to 30 weeks, or, according to other implementations, up
to about 18
months, or up to about 24 months or more from an initial implantation of the
membrane into
the mammalian body. The resorbable membrane can be resorbed within the body of
the
patient to a point where substantial strength is no longer present within a
period of
approximately 1 year. Complete resorption of the resorbable membrane may
subsequently
occur after a total period of 1.5 to 2 years has elapsed since the initial
implantation. In other
embodiments, the resorbable membrane may comprise in whole or part non-
resorbable plastic
or metallic materials.
The micro-membranes may be used in a number of surgical applications,
including:
surgical repair of fracture orbital floors, surgical repair of the nasal
septum and perforated ear
drum micro-membrane, as a protective sheathing to facilitate osteogenesis,
surgical repair of
the urethral anatomy and repair of urethral strictures, prevention of
synostosis in completed
corrective surgery for cranial fusions and forearm fractures, lessening of
soft-tissue fibrosis
or bony growth, as a temporary covering for prenatal rupture omphalocele
during staged
repair procedures, guided tissue regeneration between the teeth and gingival
margin,
tympanic membrane repairs, ducal coverings and neural repair, heart vessel
repair, hernia
repair, tendon anastomoses, temporary joint spacers, wound dressings, scar
coverings, and as
a covering for gastroschisis. The micro-membrane of the present invention can
be
particularly suitable for preventing tissue from abnormally fibrotically
joining together
following surgery, which can lead to abnormal scarring and/or interfere with
normal
physiological functioning. In some cases, such scarring can force and/or
interfere with
follow-up, corrective, or other surgical operations.
The very thin construction of these membranes is believed to substantially
accelerate
the rate of absorption of the membranes, compared to rates of absorption of
thicker
membrane implants of the same material. It is believed, however, that
resorption into the
body too quickly of the membrane may, in some instances, yield undesirable
drops in local
pH levels, thus introducing/elevating, for example, local inflammation,
discomfort and/or
foreign antibody responses. Further, a resulting uneven (e.g., cracked,
broken, roughened or
flaked) surface of a micro-membrane degrading too early may undesirably cause
tissue
turbulence between the tissues before, for example, adequate healing has
occurred,
potentially resulting in tissue inflammation and/or scarring, as well as
risking the formation
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
of tissue adhesions, thus defeating a purpose of the membrane. In other
instances, a different
(e.g., more rapid) resorption may be desired in one or more areas of a
patient, and/or at one or
more points in time of one or more surgical procedures, so that, in accordance
with an aspect
of the present invention, rates of absorption may be varied, temporally and/or
spatially, or
contour varied, by varying the materials of the membrane or parts thereof.
Micro-membranes in accordance with an aspect of the present invention may be
provided in rectangular shapes that are for example several centimeters on
each side, or can
be cut and formed into other specific shapes, configurations and sizes, by the
manufacturer
before packaging and sterilization. In modified embodiments, various known
formulations
and copolymers of, for example, polylactides may affect the physical
properties of the micro-
membrane. The micro-membranes of the present invention may be sufficiently
flexible to
conform over and/or around anatomical structures, although some heating in a
hot water bath
may be necessary for thicker configurations. In modified embodiments, certain
polylactides
which may become somewhat more rigid and brittle at thicknesses above, for
example, 0.25
mm and which may be softened by formation with other polymers, copolymers
and/or other
monomers, e.g., epsilon-caprolactone, for example, may be implemented to form
micro-
membranes.
Moreover, in accordance with another aspect of the present invention, the
micro-
membrane may comprise a substance for cellular control, such as at least one
of a
chemotactic substance for influencing cell-migration, an inhibitory substance
for influencing
cell-migration, a mitogenic growth factor for influencing cell proliferation
and a growth
factor for influencing cell differentiation. Such substances may be disposed
on and/or
impregnated within the membrane, but may also be coated on one or more
surfaces of the
membrane. In addition, substances may be contained in discrete units on or in
the membrane,
which may be effective to facilitate selective release of the substances when
the membrane is
inserted into a patient. Other configurations for accommodating different
anatomical
structures may be formed. For example, configurations may be designed to be
formed into,
for example, cone structures to fit around base portions with protrusions
extending through
the centers of the membranes. Suture perforations may be formed around
perimeters of the
membranes, and cell and vessel permeable pores may be included as well.
In general, any particulars, features or combinations thereof (in whole or in
part, in
structure or step), described or referenced herein, may be combined with any
particulars,
features or combinations thereof (in whole or in part, in structure or step),
described or
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
referenced in any of the documents mentioned herein, including without
limitation U.S.
Application No. 11/203,660 and U.S. Provisional Application No. 60/966,861
and/or, U.S.
Application No. 10/0 19,797 (in whole or in part, in any combination or
permutation that
would be viewed by one skilled in the art to be possible or modifiable to be
possible, in
structure or step, provided that the particulars or features included in any
such combination
are not mutually inconsistent. Each of these patent applications is expressly
incorporated by
reference herein.
In accordance with one implementation of the present invention, the pre-formed
micro-membranes can be preformed and sealed in sterilized packages for
subsequent use by
the surgeon. Since one objective of the micro-membranes of the present
invention can be to
reduce sharp edges and surfaces, preformation of the membranes is believed to
help, in some
instances, facilitate, albeit to a relatively small degree, rounding of the
edges for less rubbing,
tissue turbulence and inflammation. That is, the surfaces and any sharp edges
of the micro-
membranes are believed to be capable of ever so slightly potentially degrading
over time in
response to exposure of the membranes to moisture in the air, to thereby form
rounder edges.
This is believed to be an extremely minor effect. Moreover, any initial
heating to glass
temperature of the pre-cut membranes just before implanting may conceivably
further round
any sharp edges. Furthermore, the very micro-membranes of the present
invention may be
particularly susceptible, at least theoretically, to these phenomena, and,
perhaps to a more
noticeable extent, are susceptible to tearing or damage from handling, thus
rendering the pre-
forming of the micro-membranes potentially beneficial for preserving the
integrity thereof.
In accordance with an aspect of the present invention, a surgical prosthesis
(e.g., a
resorbable scar-tissue reduction micro-membrane system) can comprise an
adhesion-resistant
region (e.g., a biodegradable region, a biodegradable side, a membrane and/or
a micro-
membrane) as described herein, and further may comprise an optional tissue-
ingrowth region
(e.g., another membrane, a bridging membrane as referenced herein, a
biodegradable region
and/or a biodegradable side or mesh).
The surgical prosthesis (e.g., biodegradable surgical prosthesis) can be
constructed for
use in the repair of soft tissue defects, such as soft tissue defects
resulting from incisional and
other hernias and soft tissue defects resulting from extirpative tumor
surgery. The surgical
prosthesis may also be used in cancer surgeries, such as surgeries involving
sarcoma of the
extremities where saving a limb is a goal. Other applications of the surgical
prosthesis of the
present invention may include laparoscopic or standard hernia repair in the
groin area,
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
umbilical hernia repair, paracolostomy hernia repair, femora hernia repair,
lumbar hernia
repair, and the repair of other abdominal wall defects, thoracic wall defects
and
diaphragmatic hernias and defects.
According to an aspect of the present invention, the tissue-ingrowth region
and the
adhesion-resistant region may differ in both (A) surface appearance and (B)
surface function.
For example, the tissue-ingrowth region can be constructed with at least one
of a surface
topography (appearance) and a surface composition (function), either of which
may facilitate
strength, longevity or lack thereof, and/or a substantial fibroblastic
reaction in the host tissue
relative to for example the anti-adhesion region. On the other hand, the
adhesion-resistant
region can be constructed with at least one of a surface topography and a
surface
composition, either of which may facilitate, relative to the tissue-ingrowth
region, an anti-
adhesive effect between the biodegradable surgical implant and host tissues.
A. Surface Topography (Appearance):
The tissue-ingrowth region can be formed to have an open, non-smooth and/or
featured surface comprising, for example, alveoli and/or pores distributed
regularly or
irregularly. In further embodiments, the tissue-ingrowth region can be formed
to have,
additionally or alternatively, an uneven (e.g., cracked, broken, roughened or
flaked) surface
which, as with the above-described surfaces, may cause tissue turbulence
(e.g., potential
tissue inflammation and/or scarring) between host tissues and the tissue-
ingrowth region.
Over time, with respect to the tissue-ingrowth region, the patient's fibrous
and
collagenous tissue may substantially completely overgrow the tissue-ingrowth
region,
growing over and affixing the tissue-ingrowth region to the tissue. In one
implementation,
the tissue-ingrowth region comprises a plurality of alveoli or apertures
visible to the naked
eye, through or over which the host tissue can grow and achieve substantial
fixation.
As an example, pores may be formed into the tissue-ingrowth region by punching
or
otherwise machining, or by using laser energy. Non-smooth surfaces may be
formed, for
example, by abrading the tissue-ingrowth region with a relatively course
surface (e.g., having
a 40 or, preferably, higher grit sandpaper-like surface) or, alternatively,
non-smooth surfaces
may be generated by bringing the tissue-ingrowth region up to its softening or
melting
temperature and imprinting it with a template (to use the same example, a
sandpaper-like
surface). The imprinting may occur, for example, during an initial formation
process or at a
subsequent time.
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
On the other hand, the adhesion-resistant region can be formed to have a
closed,
continuous, smooth and/or non-porous surface. In an illustrative embodiment,
at least a
portion of the adhesion-resistant region is smooth comprising no
protuberances, alveoli or
vessel-permeable pores, so as to attenuate occurrences of adhesions between
the tissue-
ingrowth region and host tissues.
In a molding embodiment, one side of the press may be formed to generate any
of the
tissue-ingrowth region surfaces discussed above and the other side of the
press may be
formed to generate an adhesion-resistant region surface as discussed above.
Additional
features (e.g., roughening or forming apertures) may subsequently be added to
further define
the surface of, for example, the tissue-ingrowth region. In an extrusion
embodiment, one side
of the output orifice may be formed (e.g. ribbed) to generate a tissue-
ingrowth region
(wherein subsequent processing can further define the surface such as by
adding transverse
ribs/features and/or alveoli) and the other side of the orifice may be formed
to generate an
adhesion-resistant biodegradation region surface. In one embodiment, the
adhesion-resistant
region is extruded to have a smooth surface and in another embodiment the
adhesion-resistant
region is further processed (e.g., smoothed) after being extruded.
B. Surface Composition (Function):
As presently embodied, the tissue-ingrowth region comprises a first material,
and the
adhesion-resistant region comprises a second material which is different from
the first
material. In modified embodiments, the tissue-ingrowth region and the adhesion-
resistant
region may comprise the same or substantially the same materials. In other
embodiments, the
tissue-ingrowth region and the adhesion-resistant region may comprise
different materials
resulting from, for example, an additive having been introduced to at least
one of the tissue-
ingrowth region and the adhesion-resistant region.
According to an implementation of the present invention, the adhesion-
resistant
region is constructed to minimize an occurrence of adhesions of host tissues
(e.g., internal
body viscera) to the surgical prosthesis. In modified embodiments, the
adhesion-resistant
region and the tissue-ingrowth region of the surgical prosthesis may be formed
of the same
material or relatively less divergent materials, functionally speaking, and
the adhesion-
resistant region may be used in conjunction with an anti-inflammatory gel
agent applied, for
example, onto the adhesion-resistant region at a time of implantation of the
surgical
prosthesis. According to other broad embodiments, the adhesion-resistant
region and the
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
tissue-ingrowth region may be formed of any materials or combinations of
materials
disclosed herein (including embodiments wherein the two regions share the same
layer of
material) or their substantial equivalents, and the adhesion-resistant region
may be used in
conjunction with an anti-inflammatory gel agent applied, for example, onto the
adhesion-
resistant region at a time of implantation of the surgical prosthesis.
The tissue-ingrowth region can be formed of similar and/or different materials
to
those set forth above, to facilitate strength, longevity or lack thereof,
and/or direct post-
surgical cell colonization via, for example, invoking a substantial
fibroblastic reaction in the
host tissue. In an illustrated embodiment, the tissue-ingrowth region is
constructed to be
substantially incorporated into the host tissue and/or to substantially
increase the structural
integrity of the surgical prosthesis. Following implantation of the surgical
prosthesis, body
tissues (e.g., subcutaneous tissue and/or the exterior fascia) commence to
incorporate
themselves into the tissue-ingrowth region. While not wishing to be limited by
theory, it is
believed that the body, upon sensing the presence of the tissue-ingrowth
region of the present
invention, is disposed to send out fibrous tissue which grows in, around
and/or through and at
least partially entwines itself with the tissue-ingrowth region. In this
manner, the surgical
prosthesis can become securely attached to the host body tissue.
Regarding different materials, according to an aspect of the present
invention, the
tissue-ingrowth region can comprises a biodegradable (e.g., resorbable)
polymer composition
having one or more different characteristics than that or those of a
biodegradable (e.g.,
resorbable) polymer composition of the adhesion-resistant region. The
different
characteristics may include (la) time or rate of biodegradation affected by
additives, (lb)
time or rate of biodegradation affected by polymer structures/compositions,
(2) polymer
composition affecting strength or structural integrity, and (3) ability to
facilitate fibroblastic
reaction.
In accordance with a method of the present invention, the surgical prosthesis
can be
used to facilitate repair of, for example, a hernia in the ventral region of a
body. An
implanted surgical prosthesis having both an adhesion-resistant region
disposed on one side
and having a tissue-ingrowth region disposed on a second side of the surgical
prosthesis can
be provided. The abdominal wall can include muscle enclosed and held in place
by an
exterior fascia and an interior fascia. An interior layer, called the
peritoneum, can cover the
interior side of the interior fascia. The peritoneum is a softer, more pliable
layer of tissue that
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
forms a sack-like enclosure for the intestines and other internal viscera. A
layer of skin and a
layer of subcutaneous fat cover the exterior fascia.
Surgical repair of a soft tissue defect (e.g., a hernia) can be performed by
using, for
example, conventional techniques or advanced laparoscopic methods to close
substantially all
of a soft tissue defect. According to one implementation, an incision can be
made through
the skin and subcutaneous fat, after which the skin and fat can be peeled back
followed by
any protruding internal viscera (not shown) being positioned internal to the
hernia. In certain
implementations, an incision can be made in the peritoneum followed by
insertion of the
surgical prosthesis into the hernia opening so that the surgical prosthesis is
centrally located
in the hernia opening. One or both the tissue-ingrowth region and the adhesion-
resistant
region may be attached by, e.g., suturing to the same layer of the abdominal
wall, e.g., the
relatively-strong exterior fascia. Alternatively, the adhesion-resistant
region may be attached
to another member, such as the interior fascia and/or the peritoneum. The
tissue-ingrowth
region can be surgically attached to the exterior fascia while the adhesion-
resistant region can
be attached to the tissue-ingrowth region and/or optionally to the exterior
fascia using, e.g.,
heat bonding, suturing, and/or other affixation protocols disclosed herein or
their substantial
equivalents. Those possessing skill in the art will recognize that other
methods of
sizing/modifying/orientating/attaching a surgical prosthesis of this invention
may be
implemented according to the context of the particular surgical procedure.
The size of the surgical prosthesis typically will be determined by the size
of the
defect. Use of the surgical prosthesis in a tension-free closure may be
associated with less
pain and less incidence of post surgical fluid accumulation. Exemplary sutures
may be
implemented to at least partially secure the surgical prosthesis to the
abdominal wall
structure. The sutures can be implemented so that no lateral tension is
exerted on the exterior
fascia and/or muscle. When disrupted, the skin and fat may be returned to
their normal
positions, with, for example, the incisional edges of the skin and fat being
secured to one
another using suitable means such as subsurface sutures.
In modified embodiments of the present invention, one or both of the tissue-
ingrowth
region and the adhesion-resistant region of the surgical prosthesis, can be
heat bonded (or in a
modified embodiment, otherwise attached, such as by suturing). Heat bonding
may be
achieved, for example, with a bipolar electro-cautery device, ultrasonicly
welding, or similar
sealing between the tissue-ingrowth region and the adhesion-resistant region
and/or directly
to surrounding tissues. Such a device can be used to heat the surgical
prosthesis at various
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
locations, such as at edges and/or at points in the middle, at least above its
glass transition
temperature, and preferably above its softening point temperature. The
material is heated,
e.g., along with adjacent tissue, such that the two components bond together
at their interface.
The heat bonding may also be used initially, for example, to secure the tissue-
ingrowth
region to the adhesion-resistant region. Since the tissue-ingrowth region
serves more of a
load-bearing function, a few typical embodiments may exclude heat-bonding as
the sole
means for securing this region to host tissues. In other embodiments, the
technique of heat
bonding the surgical prosthesis to itself or body tissue may be combined with
another
attachment method for enhanced anchoring. For example, the surgical prosthesis
may be
temporarily affixed in position using two or more points of heat bonding using
an electro-
cautery device, and sutures, staples or glue can subsequently (or in other
embodiments,
alternatively) be added to secure the surgical prosthesis into place.
The tissue-ingrowth region and the adhesion-resistant region may be arranged
to form
more than one layer or substantially one layer, or the regions may both belong
to a single,
integrally formed layer. For example, the tissue-ingrowth region and the
opposing adhesion-
resistant region may be arranged in two layers, wherein one of the regions is
disposed on top
of, and opposite to, the other region.
In one embodiment, the tissue-ingrowth region and the adhesion-resistant
region may
be combined on a single side of the surgical prosthesis in, for example,
substantially one
layer, wherein the regions are adjacent each other on one side of the surgical
prosthesis. As a
slight deviation, a surgical prosthesis having a tissue-ingrowth region on at
least one (and
preferably, both) side(s) thereof may be manufactured using any of the
techniques described
herein and, subsequently, an adhesion-resistant region may be formed on, e.g.,
one side, by
smoothing, filling, or otherwise processing an area of the tissue-ingrowth
region with a
suitable material as disclosed herein or technique (e.g., coating or filling
with a liquid or
flowable polymer composition, and/or mechanically smoothing) to thereby form
an adhesion-
resistant region having adhesion-resistant properties relative to those of the
tissue-ingrowth
region.
Similarly, a patch of adhesion-resistant region may be sized and affixed
(e.g., heat
bonded, such as with a bipolar electro-cautery device, ultrasonicly welded, or
similarly
affixed) at a time of implantation directly to at least one of the tissue-
ingrowth region and
surrounding host tissues. In modified embodiments, the affixing may be
accomplished using,
for example, press or adhesive bonding, or sutures. In further embodiments, at
least part of
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
the affixing may occur at a time of manufacture of the surgical prosthesis
before packaging.
The patch of adhesion-resistant region alternatively may be partially affixed
(e.g., using
techniques enumerated in this paragraph) at, for example, a non-perimeter or
central area
thereof to an area (e.g., a non-perimeter or central area) of the tissue-
ingrowth region, so that
a surgeon can trim the adhesion-resistant region (and/or the tissue-ingrowth
region) at a time
of implantation while the adhesion-resistant biodegradable implant is affixed
to the tissue-
ingrowth region. For instance, a tissue-ingrowth region may substantially
surround an
adhesion-resistant region on one side of the surgical prosthesis, and only a
tissue-ingrowth
region may be formed on the other side of the surgical prosthesis. In such an
implementation, the adhesion-resistant region of the surgical prosthesis can
be sized and
shaped so as to substantially cover any opening created by the soft tissue
defect, with the
tissue-ingrowth regions facilitating surgical attachment to, and incorporation
into, the host
tissue on at least one side of, and, preferably, on both sides of, the
surgical prosthesis.
In modified embodiments, the tissue-ingrowth region and/or the adhesion-
resistant
region on a given surface or surfaces of the surgical prosthesis each may be
of any size or
shape suited to fit the particular soft tissue defect. For example, either of
the tissue-ingrowth
region and/or the adhesion-resistant region on a given surface of the surgical
prosthesis may
have shapes of ovals, rectangles and various complex or other shapes wherein,
for each such
implementation, the two regions may have essentially the same, or different,
proportions
and/or dimensions relative to one another.
In general, various techniques may be employed to produce the surgical
prosthesis,
which typically has one or two layers defining the tissue-ingrowth region and
the adhesion-
resistant region. Useful techniques include solvent evaporation methods, phase
separation
methods, interfacial methods, extrusion methods, molding methods, injection
molding
methods, heat press methods and the like as known to those skilled in the art.
The tissue-
ingrowth region and the adhesion-resistant region may comprise two distinct
layers or may be
integrally formed together as one layer.
The tissue-ingrowth region and the adhesion-resistant region may be partially
or
substantially entirely formed or joined together. Joining can be achieved by
mechanical
methods, such as by suturing or by the use of metal clips, for example,
hemoclips, or by other
methods, such as chemical or heat bonding.
The above-described embodiments have been provided by way of example, and the
present invention is not limited to these examples. Multiple variations and
modification to
CA 02731404 2011-01-19
WO 2010/001250 PCT/IB2009/006229
the disclosed embodiments will occur, to the extent not mutually exclusive, to
those skilled in
the art upon consideration of the foregoing description. Additionally, other
combinations,
omissions, substitutions and modifications will be apparent to the skilled
artisan in view of
the disclosure herein. As iterated above, any feature or combination of
features described
and referenced herein are included within the scope of the present invention
provided that the
features included in any such combination are not mutually inconsistent as
will be apparent
from the context, this specification, and the knowledge of one of ordinary
skill in the art. For
example, any of the implants and implant components, sub-components, or uses,
and any
particulars or features thereof, or other features, including method steps and
techniques, may
be used with any other structure and process described or referenced herein,
in whole or in
part, in any combination or permutation. Accordingly, the present invention is
not intended
to be limited by the disclosed embodiments, but is to be defined by reference
to the appended
claims.