Note: Descriptions are shown in the official language in which they were submitted.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
1
A pre-filled container insert for mixing two or more
ingredients for use in a pharmaceutical container system
Technical Field
The present invention relates to a pharmaceutical multi-chamber container
system for
mixing two or more ingredients of a pharmaceutical product, and more
particularly to a
pre-filled container insert to be used with such a system.
Background of the Invention
Solid active pharmaceutical ingredients (API) or formulated products generally
have better
shelf-life, i.e. degrade at a slower rate than solutions (or
dispersions/emulsions) of the same
compound. Therefore, it is commonplace to use dry powder, for example to
freeze-dry or
spray-dry pharmaceutical products to extend shelf-life of a pharmaceutical
product to
practical length, typically at least 18-24 months. At time of use, such a
solid product must
be dissolved in a suitable solvent before it can be administered to the
patient, usually via
is oral or parenteral routes or by nasal delivery. The dissolution of the
solid in a solvent is
typically made via transfer of the solvent from a vial or bag using a syringe.
Although
such a process is a standard procedure amongst hospital staff, it does take
training and skill
to do it in a safe and efficient manner without risk of needle-stick injury or
compromising
product sterility and thereby patient safety.
Patent abstract of Japan, JP2000037441A relates to an infusion container
system having a
solid drug storing part connected to a liquid solution container. A fluid
communication
channel is established between the two containers by pressing the solid
medicine storing
part to the solution container side and a partitioning member is opened and
moved from a
seal part. After making the solid drug storage container and the solution
container open for
free passage the solid drug dissolves in the liquid solution by shaking the
whole transfusion
container. The solution is now ready to be administered to a patient and a
puncture needle
is introduced into an infusion solution take off connection provided in the
top of the solid
drug storage container. A drawback with this device is that it requires a
complicated
construction of the solid drug storing part comprising several parts and which
will involve
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
2
a number of production steps. A further drawback is that two steps need to be
carried out
before the solution can be administered to a patient, namely first allow for
mixing of the
two ingredients and then introducing a needle to connect for example an
infusion set.
The Object of the Invention
It is an object of the present invention, in preferred embodiments at least,
to provide a
container system in which mixing of two or more ingredients of a
pharmaceutical product,
e.g. a solid and solvent or two different liquids, is made using a simple,
effective and
intuitive procedure with minimum risks for hospital staff and patients.
It is a further object of the present invention, in preferred embodiments at
least, to provide
a robust concept to be used in connection with blow-fill-seal (BFS) equipment
having a
construction involving few parts as well as few production steps.
is Summary of the Invention
In accordance with the present invention, from a first broad aspect at least,
there is
provided a pre-filled container insert for use in a pharmaceutical container
system for
mixing two or more ingredients of a pharmaceutical product. The pre-filled
container
insert comprises;
- a moulded container casing having walls, bottom and an open top for
receiving a solid
component,
- a resilient stopper for sealing the open top of the moulded container
casing,
- a bottom disc formed by a weakening rim in the bottom periphery of the
moulded
container casing, wherein the bottom disc is openable to allow mixing of the
pre-filled
solid component with a diluents fluid of the pharmaceutical container system
by
introducing a spike through the resilient stopper applying a pushing force on
the bottom
disc, by which force the weakening rim is broken and the container insert is
opened.
In accordance with the present invention, from a further broad aspect at
least, there is
provided a pre-filled container insert for use in a pharmaceutical container
system for
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
3
mixing two or more ingredients of a pharmaceutical product. The pre-filled
container insert
comprises;
- a moulded container casing having walls, bottom and an open top for
receiving an inner
open cylinder and a solid component,
- a resilient stopper for sealing the open top of the moulded container
casing,
- a bottom disc formed by a weakening rim in the bottom periphery of the
moulded
container casing, wherein the bottom disc is openable to allow mixing of the
pre-filled
solid component with a diluents fluid of the pharmaceutical container system
by applying a
pushing force onto the top of the moulded container, thereby compressing the
casing and
forcing the inner cylinder towards the bottom disc, by which force the
weakening rim is
broken and the container insert is opened.
An advantage of preferred embodiments of the present invention is that there
is provided a
pre-filled container insert that is constructed from a minimum of parts and at
the same time
is has efficient and robust construction. A further advantage of preferred
embodiments of the
present invention is that there is provided a pre-filled container insert that
easily can be
opened to provide mixing of contents in the insert and the liquids container.
A still further
advantage is that the pre-filled container insert is manufactured as a
separate device and
can be incorporated into different types of blow-fill-seal containers forming
a
pharmaceutical multi-chamber container system. Yet another advantage is that
the
separate device can be aseptically filled, sealed and processed (e.g.
terminally sterilised)
under controlled conditions before the next unit operation of actually
mounting the device
into the diluents container.
According to preferred embodiments of the invention, the weakening rim in the
bottom
disc is arranged around the periphery of the bottom of the container casing
forming a
hinged disc such that the bottom disc remains connected to the container
casing when the
weakening rim is broken and the container is opened.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
4
In this way the bottom disc is prevented from falling out into the diluents
container after
opening of the pre-filled container insert.
According to preferred embodiments of the invention, the resilient stopper is
secured in
place by a clamp ring to hermetically seal the top opening.
The clamp ring locks and fixes the resilient stopper in the open top of the
container casing
and the moulded casing is hermetically sealed.
According to preferred embodiments of the invention, the bottom disc is
covered by a
moisture barrier foil.
According to preferred embodiments of the invention, the resilient stopper is
covered by a
moisture barrier foil.
By covering the container insert with a moisture barrier foil, both on the
bottom side or on
the resilient top side, the pre-filled content has a maximum of protection
from the outside
environment, i.e. light, moisture and/or oxygen.
According to preferred embodiments of the invention, the pre-filled container
insert is
aseptically mounted into the top of a BFS-container forming a multi-chamber
pharmaceutical container system.
According to preferred embodiments of the invention, the moulded container
casing has
pleated sidewalls forming a bellows container insert.
By forming the container casing as a bellows container an easily deformable
casing is
formed in one single piece.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
According to preferred embodiments of the invention, the pre-filled container
insert is
capable of use with one or more delivery systems, such as a nasal delivery
system.
Preferably the pre-filled container insert is connectable to or within, or
otherwise can be
incorporated with, for example, a standard nasal delivery mechanism such as a
nasal spray.
5
According to preferred embodiments of the invention, there is provided a
pharmaceutical
container system for mixing two or more ingredients of a pharmaceutical
product,
comprising
- a first container comprising a diluents liquid,
- a second container insert aseptically mounted into the top of the first
container, the
second container comprising
- a moulded container casing for receiving a solid component,
- a resilient stopper for sealing the open top of the moulded container
casing,
- a bottom disc formed by a weakening rim in the bottom periphery of the
moulded
is container casing, wherein the bottom disc is openable to allow mixing of
the pre-filled
solid component with a diluents fluid of the pharmaceutical container system
by
introducing a spike through the resilient stopper applying a pushing force on
the bottom
disc, by which force the weakening rim is broken and the container insert is
opened.
According to preferred embodiments of the invention, there is provided a
pharmaceutical
container system for mixing two or more ingredients of a pharmaceutical
product
comprising
- a first container comprising a diluents liquid,
- a second container insert aseptically mounted into the top of the first
container, the
second container comprising
- a moulded container casing having walls, bottom and an open top for
receiving an inner
open cylinder and a solid component,
- a resilient stopper for sealing the open top of the moulded container
casing,
- a bottom disc formed by a weakening rim in the bottom periphery of the
moulded
container casing, wherein the bottom disc is openable to allow mixing of the
pre-filled
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
6
solid component with a diluents fluid of the pharmaceutical container system
by applying a
pushing force onto the top of the moulded container, thereby compressing the
casing and
forcing the inner cylinder towards the bottom disc, by which force the
weakening rim is
broken and the container insert is opened.
A standard procedure for a nurse preparing an intra-venous (IV)-container is
to visually
inspect, and in some procedures to spike, the IV-container before starting
infusion.
Therefore, using a pharmaceutical container system according to embodiments of
the
present invention, the standard working procedure is not changed. In preferred
embodiments, by simply pushing the container top to allow mixing and then
ensure the
complete mixing operation by visual inspection, a simple an intuitive
procedure is
obtained. In addition, no separate mixing step using hypodermic needles are
necessary,
eliminating the risk of needle-stick injuries. Mixing is made without risk of
microbial
contamination since the solid and liquid chambers are both parts of the same
hermetically
is sealed container. In other embodiments, similar steps are carried out but
for preparing a
nasal delivery device such as a nasal spray, which typically comprises a
container, and a
spray or other nasal delivery mechanism attached thereto.
Medicaments suitable for administration via a pharmaceutical multi-chamber
container
system for mixing two or more ingredients of a pharmaceutical product, and
more
particularly to be pre-filled with the container insert of the present
invention include for
example proton pump inhibitors, for example omeprazol, anticancer medicaments,
antibiotics, immunotherapies, vaccines, antiviral medicaments, polypeptides
and peptides,
for example, peptide hormones and growth factors, polypeptide vaccines,
enzymes
endorphines, lipoproteins and polypeptides involved in the blood coagulation
cascade.
Brief Description of the Drawings
The present invention will now be described, for exemplary purposes, in more
detail by
way of embodiments and with reference to the enclosed drawings, in which:
Figures 1 a- I g illustrate schematically the manufacturing steps of a pre-
filled container
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
7
insert in accordance with a preferred embodiment of the present invention,
and,
Figures 2a and 2b schematically illustrate the opening procedure of the pre-
filled container
insert, and,
Figures 3a-3d show a pharmaceutical container system for mixing two or more
ingredients
s of a pharmaceutical product, which system consists of a BFS-container filled
with a
diluents liquid and a pre-filled insert sealed to the container, and,
Figures 4a-4h illustrate schematically the manufacturing steps of a further
pre-filled
container insert in accordance with a preferred embodiment of the present
invention, and,
Figures 5a-5c schematically illustrate the opening procedure of the pre-filled
container
insert of Figures 4a-4h, and;
Figures 6a-6d show a pharmaceutical container system for mixing two or more
ingredients
of a pharmaceutical product, which system consists of a BFS-container filled
with a
diluents liquid and a pre-filled insert sealed to the container.
is Detailed Description of Preferred Embodiments
Figures 1 a to 1 g and 4a to 4h schematically illustrate how pre-filled
container inserts can
be manufactured. The container inserts, being manufactured separately e.g. by
injection
moulding can use any type thermoplastic material, e.g. high-barrier
engineering plastics if
necessary to impart high-barrier properties, e.g. with light, moisture or
oxygen sensitive
compounds.
Figure 1 a shows the moulded container casing 1 having walls 2, bottom 3 and
an open top
4 for receiving a medicament component. The container casing 1 which is made
using e.g.
injection moulding preferably has a cylindrical geometry and the bottom 3 has
a suitable
weakening rim 5 in the bottom periphery. The weakening rim 5 will make it
possible to
push open the bottom disc 3 to allow material transfer between the pre-filled
insert and a
liquid container. Preferably, a small portion of the weakening rim is kept
thicker,
functioning as a hinge 6 so as to prevent the bottom disc 3 to completely
disengage from
the solids container on opening.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
8
To further augment the moisture or gas barrier of the container insert and
thus to protect
the content, a barrier foil 7, e.g. an aluminium foil can be used to cover the
bottom disc 3,
which can be seen in Figure lb.
In Figure 1 c the moulded container casing is pre-filled with a content 8, a
powder or other
suitable solid (or semi-solid) in a well-defined, clean environment (e.g.
aseptic filling in
isolator or other suitable uni directional air flow hood (UDF)). According to
another
aspect of this invention not shown in the drawings, the moulded container
casing is
pre-filled with a semi-solid content or a liquid, which is to be mixed with
the diluents prior
to infusion.
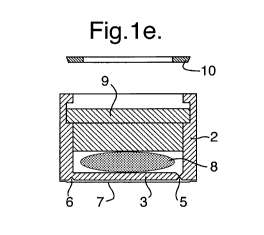
Figures 1 d, 1 e and 1 f show how the container insert is hermetically closed
using a resilient
stopper 9, e.g. a standard rubber stopper or thermoplastic elastomer (TPE)
part, which is
mounted through the open top and secured by a clamp ring 10 to ensure a safe
and tight
is fastening of the stopper 9. As shown in Figure lg, a barrier foil 15, i.e.
an aluminium foil,
can be used to cover the resilient stopper 9 and clamp ring 10 to further
augment the
moisture or gas barrier of the pre-filled container insert 17. The open centre
of the clamp
ring 10 reveals a circular area of the resilient stopper where the spike (or a
puncture
needle) can be entered through the stopper. The pre-filled container insert 17
can be
sterilised either in-line or in a separate step after filling/closing using
standard methods
such as e-beam or gamma irradiation. Such a sterile pre-filled container
insert can then be
transported to a suitable standard filling line for liquids to be mounted to a
blow-fill-seal
container or other suitable liquids plastic container. The container may
comprise a
component of a nasal delivery device, which preferably contains the
appropriate liquid.
Figure 2a schematically illustrate how a spike 20 is pushed through the
resilient stopper 9
of the pre-filled container insert and in 2b how the pushing force from the
spike 20 breaks
the weakened rim 5 in bottom periphery (as well as the barrier foil 7) and the
content 8 of
the pre-filled container insert is free to leave the container.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
9
Figure 3a illustrates an embodiment of a pharmaceutical container system 21
wherein a
pre-filled container insert 17 according to the present invention is mounted
into the top of a
blow-fill-seal container 22. In the embodiment of Figures 3a to 3d, the system
21
comprises means suitable for intravenous delivery of a medicament, but the
system may
comprise any other, suitable pharmaceutical system, such as a container (e.g.
a bottle, vial
or tube) for use with a nasal delivery mechanism, e.g. a spray or dropper that
connects to
the container. The pre-filled container insert 17 of the Figure 3a embodiment
filled with a
content 8 will be enclosed into the liquid blow-fill-seal container and the
top portion of the
pre-filled container is revealed to the user by opening a twist-off top 23 of
the blow-fill-
seal container 22, which is shown in Figure 3b. In a preferred embodiment,
also shown in
Figure 3b, a moisture barrier seal 15 covers the top, i.e. a resilient stopper
9 of the pre-
filled container under the twist-off top 23. After the moisture barrier seal
15 has been
removed the content 8 of the pre-filled container insert 17 is ready to be
mixed with the
diluents fluid in the blow-fill-seal container 22. Figure 3c shows how a spike
20 punctures
is the resilient stopper 9 and enters into the pre-filled container 17. The
pushing force from
the spike 20 acts on the bottom disc 3and the weakened rim 5 in the periphery
of the disc
breaks up. The disc 3 is prevented from falling out into the blow-fill-seal
container 22 by a
hinge 6 connecting the bottom disc to the wall 2 of the container insert. The
content of the
pre-filled container can now be mixed with the diluents fluid of the blow-fill-
seal container
by gentle shaking the container. In Figure 3d a bottom flap 25 is pulled up to
allow
hanging of the container system near a patient while administering the
substance to the
patient. According to another aspect of the present invention not shown in the
drawings,
the pre-filled container insert is welded into a flexible bag wall being
filled with the
diluents liquid.
Figure 4a shows another embodiment of the present invention. Like reference
numerals
are used to indicate components similar to those of the embodiment of Figures
1 to 3. The
moulded container casing 1 has walls 2, bottom 3 and an open top 4 for
receiving a
medicament component. The container casing 1 which is made using e.g.
injection
moulding preferably has a cylindrical geometry and differs from the previous
embodiment
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
in that the walls 2 are pleated such that the container casing has the form of
a bellows. The
bottom 3 has a suitable weakening rim 5 in the bottom periphery. Preferably, a
small
portion of the weakening rim is kept thicker, functioning as a hinge 6 so as
to prevent the
bottom disc 3 to completely disengage from the solids container on opening. To
further
5 augment the moisture or gas barrier of the container insert and thus to
protect the content, a
barrier foil 7, e.g. an aluminium foil can be used to cover the bottom disc 3,
which can be
seen in Figure 4b.
In Figure 4c an inner cylinder 30 is placed inside the moulded container
casing and, as
10 shown in Figure 4d, the inner cylinder placed within the container walls is
then pre-filled
with a content 8, a powder or other suitable solid (or semi-solid) in a well-
defined, clean
environment (e.g. aseptic filling in isolator or other suitable uni
directional air flow hood
(UDF)). According to another aspect of this invention not shown in the
drawings, the
inner cylinder with container is pre-filled with a semi-solid content or a
liquid which is to
is be mixed with the diluents prior to infusion. The weakening rim 5 will make
it possible to
push open the bottom disc 3 using the inner cylinder 30 to allow material
transfer between
the pre-filled insert and a liquid container. The inner cylinder 30 is tapered
and oriented in
relation to the weakening rim so that the tapered end push first at the
position directly
opposite the hinge part of the weakening rim along the periphery.
Figures 4e and 4f show how the container insert is hermetically closed using a
resilient
stopper 9, e.g. a standard rubber stopper or thermoplastic elastomer (TPE)
part, which is
mounted through the open top and secured by a clamp ring 10 to ensure a safe
and tight
fastening of the stopper 9. As shown in Figure 4h, a barrier foil 15, i.e. an
aluminium foil,
can be used to cover the resilient stopper 9 and clamp ring 10 to further
augment the
moisture or gas barrier of the pre-filled container insert 17. Alternatively,
the aluminium
foil is replaced with a standard tamper-evident protective seal. The open
centre of the
clamp ring 10 reveals a circular area of the resilient stopper where the spike
(or a puncture
needle) can be entered through the stopper. The pre-filled container insert 17
can be
sterilised either in-line (at filling) or in a separate step after
filling/closing using standard
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
11
methods such as e-beam or gamma irradiation. Such a sterile pre-filled
container insert
can then be transported to a suitable standard filling line for liquids to be
mounted to a
blow-fill-seal container or other suitable liquids plastic container.
Figure 5a schematically illustrates how a pressing force is applied to the top
of the bellows
container insert 17 and how the pushing force compresses the walls 2 of the
container
insert. In Figure 5b the inner cylinder 30 presses on the bottom 3 and breaks
the weakened
rim 5 in bottom periphery (as well as the barrier foil 7) and the content 8 of
the pre-filled
container insert is free to leave the container and be mixed with a diluent
fluid (not shown).
In Figure 5c a spike 20 is introduced through the rubber stopper 9 to make the
container
ready for administration to a patient.
Figure 6a illustrates an embodiment of a pharmaceutical container system 21
wherein a
pre-filled container insert 17 according to the present invention is mounted
into the top of a
is blow-fill-seal container 22. As discussed above, the system may comprise
any other,
suitable pharmaceutical system, such as a container for use with a nasal
delivery
mechanism. The pre-filled container insert 17 filled with a content 8 will be
enclosed into
the liquid blow-fill-seal container and the top portion of the pre-filled
container is revealed
to the user by opening a twist-off top 23 of the blow-fill-seal container 22,
which is shown
in Figure 6b. In a preferred embodiment, also shown in Figure 6b, a moisture
barrier seal
15 covers the top, i.e. a resilient stopper 9 of the pre-filled container
under the twist-off top
23. After removal of the twist-off top the content 8 of the pre-filled
container insert 17 is
ready to be mixed with the diluents fluid in the blow-fill-seal container 22.
Figure 6c
shows how the walls 2 of the bellows insert are compressed. The pushing force
makes the
inner cylinder 30 to break the weakened rim 5 in the periphery of the bottom
disc 3 and the
rim breaks up. The disc 3 is prevented from falling out into the blow-fill-
seal container 22
by a hinge 6 connecting the bottom disc to the wall 2 of the container insert.
The content
of the pre-filled container can now be mixed with the diluents fluid of the
blow-fill-seal
container by gentle shaking of the container. After visual inspection to
ensure complete
mixing of the contents and confirmation of the absence of particulates, the
moisture barrier
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
12
seal (or tamper-evident seal) can be removed. A spike 20 (or puncture needle)
may be
introduced through the stopper 9 to make the container ready to be
administered to a
patient. According to another aspect of the present invention not shown in the
drawings,
the pre-filled container insert is welded into a flexible bag wall being
filled with the
s diluents liquid.
To mix the content of the solid and liquid containers in the above intravenous
examples,
the user (typically a nurse) adopts a normal working procedure with
intravenous, i.e. IV-
infusions. A preferred use of a system in accordance with an embodiment of the
present
io invention as illustrated in Figures 1 to 3 is described below in
chronological order.
1) The nurse twists off the blow-fill seal container top and peels off the
moisture barrier
seal if there is one (and disinfects the rubber stopper top if that is
standard procedure).
2) A sterile IV-administration set bag is opened.
is 3) The IV-spike of the administration set is inserted into the rubber
stopper top, thereby
piercing the rubber stopper. As the spike is fully inserted, the bottom disc
of the solids
chamber is pushed open by the spike, and the solids and liquids chambers are
now
communicating to enable mixing.
4) The contents of the solids and liquids chambers are mixed by gently rocking
the
20 container.
5) The nurse visually inspects the contents of the container to ensure full
dissolution and
absence of particulate matter.
6) The needle is attached to the patient after purging, and IV-infusion is
ready to start.
25 A preferred use of a system in accordance with another embodiment of the
present
invention as illustrated in Figures 4 to 6 is described below in chronological
order.
1) The nurse twists off the blow-fill seal container top.
CA 02733173 2011-02-04
WO 2010/019096 PCT/SE2009/050922
13
2) The nurse compresses the bellows insert to make the pre-filled content fall
into the
diluents container and the solids and liquids chambers are now communicating
to enable
mixing.
3) The contents of the solids and liquids chambers are mixed by gently rocking
the
container.
4) The nurse peels off the moisture barrier (or protective) seal and
disinfects the rubber
stopper top if that is standard procedure
4) A sterile IV-administration set bag is opened. The IV-spike of the
administration set is
inserted into the rubber stopper top, thereby piercing the rubber stopper.
5) The nurse visually inspects the contents of the container to ensure full
dissolution and
absence of particulate matter.
6) The needle is attached to the patient after purging, and IV-infusion is
ready to start.
In other embodiments, for example where the pre-filled insert is for use with,
e.g., a nasal
delivery device, some of the above steps may be carried out, but with the
insert contained
within, or otherwise associated with, a suitable container such as a nasal
spray bottle or the
like.
Further, it will be understood that the present invention is not limited to
the described
embodiments but can be modified in many different ways without departing from
the
scope of the appended claims.