Note: Descriptions are shown in the official language in which they were submitted.
CA 02733294 2011-03-04
25771-786E
-1-
Pharmaceutical Combination of a Tiotropium Salt and Ciclesonide
This is a divisional application of Canadian Patent Application Serial
No. 2,614,631, which is a divisional application of Canadian Patent Serial
No. 2,436,540 filed October 23, 2001.
The present invention relates to novel pharmaceutical compositions
based on anticholinergics and corticosteroids, processes for preparing them
and their
use in the treatment of respiratory diseases.
The subject matter of application No. 2,436,540 ("parent application")
was restricted to pharmaceutical compositions in the form of inhalable
powders,
wherein the anticholinergic is crystalline tiotropium bromide monohydrate. The
subject of divisional application No. 2,614,631 ("first divisional
application") is directed
to pharmaceutical compositions in the form of inhalable solutions or
suspensions.
The subject matter of the subject divisional application ("second divisional")
is
restricted to a pharmaceutical combination of a tiotropium salt (1) with the
steroid
ciclesonide (2), optionally in the form of the enantiomers, mixtures of the
enantiomers
or in the form of the racemates thereof, optionally in the form of the
solvates or
hydrates and optionally together with a pharmaceutically acceptable excipient;
and
related use embodiments. However, it should be understood that the expression
"the
invention" and the like, when used herein, encompasses the subject matter of
the
parent and both the first and second divisional applications.
Description of the invention
The present invention relates to novel pharmaceutical compositions
based on anticholinergics and corticosteroids, processes for preparing them
and their
use in the treatment of respiratory diseases.
CA 02733294 2011-03-04
25771-786E
-Ia-
Surprisingly, it has been found that an unexpectedly beneficial
therapeutic effect, particularly a synergistic effect can be observed in the
treatment of
inflammatory or obstructive diseases of the respiratory tract if one or more
anticholinergics are used with one or more corticosteroids. In view of this
synergistic
effect the pharmaceutical combinations according to the invention can be used
in
smaller doses than would be the case with the individual compounds used in
monotherapy in the usual way. This reduces unwanted side effects such as may
occur when corticosteroids are administered, for example.
The effects mentioned above are observed both when the two active
substances are administered simultaneously in a single active substance
formulation
and when they are administered successively in separate formulations.
According to
the invention, it is preferable to administer the two active substance
ingredients
simultaneously in a single formulation.
CA 02733294 2011-03-04
2 -
Within the scope of the present invention the term
anticholinergics 1 denotes salts which are preferably
selected from among tiotropium salts, oxitropium salts
and ipratropium salts, most preferably tiotropium salts.
In the above-mentioned salts the cations tiotropium,
oxitropium and ipratropium are the pharmacologically
active ingredients. Within the scope of the present
patent application, an explicit reference to the above
cations is indicated by the use of the number it. Any
reference to compounds 1 naturally also includes a
reference to the ingredients 1' (tiotropium, oxitropium
or ipratropium).
By the salts 1 which may be used within the scope of the
present invention are meant the compounds which contain,
in addition to tiotropium, oxitropium or ipratropium as
counter-ion (anion), chloride, bromide, iodide, sulphate,
methanesulphonate or para-toluenesulphonate. Within the
scope of the present invention, the methanesulphonate,
chloride, bromide and iodide are preferred of all the
salts 1, the methanesulphonate and bromide being of
particular importance. Of outstanding importance
according to the.invention are salts 1 selected from
among tiotropium bromide,.oxitropium bromide and
ipratropium bromide. Tiotropium bromide is particularly
preferred.
Within the scope of the present invention, the word
corticosteroids (hereinafter 2) denotes compounds
selected from among flunisolide, beclomethasone,
triamcinolone, budesonide, fluticasone, mometasone,
ciclesonide, rofleponide, GW 215864, KSR 592, ST-126 and
dexamethasone.. Preferably, compounds 2 is selected from
among flunisolide, beclomethasone, triamcinolone,
budesonide, fluticasone, mometasone, ciclesonide and
dexamethasone. Most preferably, compound 2 is selected
CA 02733294 2011-03-04
3 -
from among budesonide, fluticasone, mometasone and
ciclesonide. In some cases, within the scope of the
present patent application, the term steroids 2 may also
be used on its own instead of the word corticosteroids 2.
Any reference to steroids 2 within the scope of the
present invention includes a reference to salts or
derivatives 2' which may be formed from the steroids.
Examples of possible salts or derivatives 2'
include: sodium salts, sulphobenzoates, phosphates,
isonicotinates, acetates, propionates, dihydrogen
phosphates, palmitates, pivalates or furoates. In some
cases the compounds of formula 2 may also occur in the
form of their hydrates.
The pharmaceutical combinations of 1 and 2 according to
the invention are preferably administered by inhalation.
Suitable inhalable powders packed into suitable capsules
(inhalettes) may be administered using suitable powder
inhalers. Alternatively, the drug may be inhaled by the
application of suitable inhalation aerosols. These
include inhalation aerosols which contain HFA134a, HFA227
or a mixture thereof as propellant gas. The drug may
also be inhaled using suitable solutions of the
pharmaceutical combination consisting of 'l and 2.
In one ae~ct, therefore, the invention relates to a
pharmaceutical composition which contains a combination
of .1 and 2.
In another aspect the present invention relates to a
pharmaceutical composition which contains one or more
salts 1 and one or more compounds 2, optionally in the
form of their solvates or hydrates. The active
substances maybe combined in a single preparation or
contained in two separate formulations. Pharmaceutical
CA 02733294 2011-03-04
- 4 -
compositions which contain the active substances 1 and 2 in
a single preparation are preferred according to the
invention.
In another aspect the present invention relates to
a pharmaceutical composition which contains, in addition to
therapeutically effective quantities of 1 and 2, a
pharmaceutically acceptable excipient. In another aspect
the present invention relates to a pharmaceutical
composition which does not contain any pharmaceutically
acceptable excipient in addition to therapeutically
effective quantities of 1 and 2.
The present invention also relates to the use of
1 and 2 for preparing a pharmaceutical composition
containing therapeutically effective quantities of 1 and 2
for treating inflammatory or obstructive diseases of the
respiratory tract, particularly asthma or chronic
obstructive pulmonary diseases (COPD) by simultaneous or
successive administration. Moreover, the pharmaceutical
combinations according to the invention may be used to
prepare a drug for treating cystic fibrosis or allergic
alveolitis (Farmer's Lung), for example, by simultaneous or
successive administration. The only reason for not using
the active substance combinations according to the invention
is if treatment with steroids is contraindicated for
therapeutic reasons.
According to one aspect of the invention of the
parent application, there is provided a pharmaceutical
composition in the form of an inhalable powder comprising
(1) crystalline tiotropium bromide monohydrate and (2) one
or more compounds selected from steroids, enantiomers
CA 02733294 2011-03-04
25771-786E
-4a-
thereof, mixtures of enantiomers, racemates thereof, solvates thereof and
hydrates
thereof.
According to another aspect of the invention of the parent application,
there is provided use of crystalline tiotropium bromide monohydrate and
component
(2) as defined herein in preparation of a pharmaceutical composition that is
an
inhalable powder for treating an inflammatory or obstructive disease of the
respiratory
tract.
According to one aspect of the invention of the first divisional
application, there is provided a pharmaceutical composition in the form of a
propellant-containing inhalable aerosol comprising an inhalable solution or
suspension of a tiotropium salt (1) and one or more steroids (2), optionally
in the form
of enantiomers thereof, mixtures of the enantiomers thereof, racemates
thereof,
solvates thereof and hydrates thereof, wherein 1 and 2 are in dissolved or
suspended
form.
According to another aspect of the invention of the first divisional
application, the pharmaceutical composition described herein may be used for
treating an inflammatory or obstructive disease of the respiratory tract.
According to an aspect of the second divisional application, there is
provided a pharmaceutical combination of a tiotropium salt (1) with the
steroid
ciclesonide (2), optionally in the form of the enantiomers, mixtures of the
enantiomers
or in the form of the racemates thereof, optionally in the form of the
solvates or
hydrates and optionally together with a pharmaceutically acceptable excipient.
The present invention further relates to the simultaneous or successive
use of therapeutically effective doses of the combination of the above
pharmaceutical
compositions 1 and 2 for treating inflammatory or obstructive respiratory
tract
diseases, particularly asthma or chronic obstructive pulmonary diseases
(COPD),
provided that treatment with steroids is not contraindicated for therapeutic
reasons,
by simultaneous or successive
CA 02733294 2011-03-04
-
administration. The present invention also relates to
the simultaneous or successive use of therapeutically
effective doses of the combination of the above
pharmaceutical compositions 1 and 2 for treating cystic
5 fibrosis or allergic alveolitis (Farmer's Lung), for
example.
In the active substance combinations of 1 and 2 according
to the invention, ingredients 1 and 2 may be present in
the form of their enantiomers, mixtures of enantiomers or
in the form of racemates.
The proportions in which the two active substances 1 and-
2 may be used in the active substance combinations
according to the invention are variable. Active
substances 1 and 2 may possibly be present in the form of
their solvates or hydrates. Depending on the choice of
the compounds 1 and 2, the weight ratios which may be
used within the scope of the present invention vary on
the basis of the different molecular weights of the
various compounds and their different potencies. As a
rule, the pharmaceutical combinations according to the
invention may contain compounds 1 and 2 in ratios by
weight ranging from 1:300 to 50:1, preferably from 1:250
to 40:1. In the particularly preferred pharmaceutical
combinations which contain tiotropium salt as compound 1
and a compound selected from among budesonide,
fluticasone, mometasone and ciclesonide as the steroid 2,
the weight ratios of 1 to.2 are most preferably in a
range in which tiotropium 1' and 2 are present in
proportions of 1:150 to 30:1, more preferably from 1:50
to 20:1.
For example, without restricting the scope of the
invention thereto, preferred combinations of 1 and 2
according to the invention may contain 11 and steroid 2
CA 02733294 2011-03-04
6 -
in the following weight ratios: 1:50; 1:49; 1:48; 1:47;
1:46; 1:45; 1:44; 1:43; 1:42; 1:41; 1:40; 1:39; 1:38;
1:37; 1:36; 1:35; 1:34; 1:33; 1:32; 1:31; 1:30; 1:29;
1:28; 1:27; 1:26; 1:25; 1:24; 1:23; 1:22; 1:21; 1:20;
1:19; 1:18; 1:17; 1:16; 1:15; 1:14; 1:13; 1:12; 1:11;
1:10; 1:9; 1:8; 1:7; 1:6; 1:5; 1:4; 1:3; 1:2; 1:1; 2:1;
3:1; 4:1; 5:1; 6:1; 7:1; 8:1; 9:1; 10:1; 11:1; 12:1;
13:1; 14:1; 15:1; 16:1; 17:1; 18:1; 19:1; 201.
The pharmaceutical compositions according to the
invention containing the combinations of 1 and 2 are
normally administered so that 1 and 2 are present
together in doses of 0.01 to 10000 g, preferably from 0.1
to 2000 g, more preferably from 1 to 1000 g, better still
from 5 to 500 g, preferably, according to the invention,
from 10 to 300 g, better still 20 to 2,00 g per single
dose. For example, combinations of 11 and 2 according to
the invention contain a quantity of tiotropium 1' and
steroid 2 such that the total dosage per single dose is
about 20 g, 25 g, 30 g, 351Lg, 45 g, 50 g, 55 g, 60 g,
65 g, 70 g, 75 g, 809g, 859g, 90 g, 9511g, 100 g, 105 g,
110 g, '115 g, 120 g, 1251Lg, 130 g, 1351Lg, 140 g, 145 g,
150 g, 1551Lg, 160 9, 165 g, 170 g, 175 g, 180 g, 185 g,
190 g, 195 g, 200 g, 205 g, 210 g, 215 g, 220 g, 225. g,
.230 g, 235 g, 240 g, 245 g, 250 g, 255 g, 260 g, 265 g,
270 g, 2751Lg or similar. In these dosage ranges, active
substances 1' and.2 may be present in the weight ratios
mentioned earlier.
For example, without restricting the scope of the
invention thereto, the combinations of 1 and 2 according
to the invention may contain a quantity of tiotropium 1'
and steroid 2 such that, for each single dose, 5 g of 1'
and 25 g of 2, 5 g of 1' and 50 g of 2, 5 g of 1' and
100 g of 2, 5 g of 1' and 125 g of 2, 5 g of 1' and 200 g
of 2, 5 g of 1' and 250 g of 2, 10 g of 1' and 25 g of 2,
CA 02733294 2011-03-04
7 -
g of 1' and 50gg of 2, 10 g of 1' and 100 g of 2, 10/19
of 1' and 125 g of 2, 10 g of 1' and 200 g of 2, 1O 9 of
1' and 250 g of 2, 18 g of 1' and 25 g of 2, 18 g of 1'
and 50 g of 2, 18 g of 1' and 100 g of 2, 18 g of 1' and
5 125 g of 2, 18 g of 1' and 200 g of .2, 18 g of 1' and
250 g of 2, 20 g of 1' and 25/19 of 2, 20 g of 1' and 50 g
of 2, 20 g of 1' and 100 g of 2, 20 g of 1' and 125 g of
2, 20 g of 1' and 200 9 of 2, 20 g of 1' and 250 g of 2,
36 g of 1' and 25 g of 2, 36 g of 1' and 50 g of 2, 36 g
10 of 1' and 100 g of 2, 3.6 g of 1' and 125 g of 2, 36 g of
1' and 200 g of 2, 36 g of 1' and 250 g of 2, 40 g of 1'
and 25 g of 2, 40 g of 1' and 50 g. of 2, 40 g of 1' and
100 g of 2, 40 g of 1' and 1251Lg of 2, 40 g of 1' and
200 g of 2 or 40 g of 1' and 250 g of 2 are administered.
If the active substance combination in which 1 denotes
tiotropium bromide is used as the preferred combination
of .1 and 2 according to the invention, the quantities of
active substance 1' and 2 administered per single dose
mentioned by way of example correspond to the following
quantities of 1 and 2 administered per single dose: 6 g
of 1 and 25 g of 2, 6 g of 1 and 50 g of 2, 6 g of 1 and
100 g of 2, 6 g of 1 and 125 g of 2, 6 g of 1 and 200 g
of 2, 6 g of 1 and 250 g of 2, 12 g of 1 and 25 g of 2,
12 g of 1 and 50 g of 2, 12 g of 1 and 100 g of 2, 12 g
of 1 and 125 g of 2, 12 g of 1 and 200 g of 2, 12 g of 1
and 250 g of 2, 21.7 g of 1 and 25 g of 2, 21.7 g of 1
and 50 g of 2, 21.7/19 of 1 and 100 g of 2, 21.7 g of 1
and 125 g of 2, 21.7 g of l and 200gg of 2, 2.1.7 g of 1
and 250 g of 2, 24.1 g of 1 and 25 g of 2, 24.1 g of 1
and 50 g of 2, 24.1 g of 1 and 100 g of .2, 24.1/1g of I
and 125 g of 2, 24.1/19 of 1 and 200 g of 2, 24.1/19 of 1 ..,
and 250 g of 2, 43.3 g of I and 25 g of 2, 43.3gg of 1
and 50/1g of 2, 43.3/1g of 1 and 100/1g of 2, 43.3 g of 1
and 125/1g of 2, 43.3 g of I and 200/1g of 2, 43.3/1g of 1
and 250 g of 2, 48..1/1g of 1 and 25/1g of 2, 48.1/1g of 1
CA 02733294 2011-03-04
8 -
and 50 g of 2, 48.1 g of J. and 100 g of 2, 48.1 9 of 1
and 125 g of 2, 48.1 g of 1 and 200 g of .2 or 48.1 g of 1
and 250 g of.2.
If the active substance combination in which 1 is
tiotropium bromide monohydrate is used as the preferred
combination of 1 and 2 according to the invention, the
quantities of 11 and .2 administered per single dose
specified by way of example hereinbefore correspond to
the following quantities of 1 and 2 administered per
single dose: 6.2 g of 1 and 25 g of 2, 6.2 g of 1 and
50 g of 2, 6.2 g of 1 and 100 9 of 2, 6.2 g of 1 and
125 g of 2, 6.2 g of.1 and 200 g of 2, 6.2 g of 1 and
250 g of 2, 12.5 g of 1 and 25 g of 2, 12.5 g of 1 and
50 g of 2, 12.5 g of 1 and 100 g of 2, 12.5 g of 1 and
125 g of 2, 12.5 g of 1 and 200 g of 2_, 12.5 g of 1 and
250 g of 2, 22.5 g of 1 and 25 g of 2, 22.5 g of 1 and
50 g of 2, 22.5 g of 1 and 100 g of 2, 22.5 g of 1 and
125 g of 2, 22.5 g of 1 and 200 g of 2, 22.5 g of 1 and
250 g of 2, 25 g of 1 and 25 g of 2, 25 g of 1 and 50 g
of 2, 25 g of 1 and 100 g of 2, 25 g of 1 and 125 g of 2,
g of 1 and 200 g of 2, 25 g of 1 and 250 g of 2, 45 g
of 1 and 25 g of 2, 45 g of 1 and 50 g of 2, 45 g of 1
and 100 g of 2, 45 g of 1 and 125 g of 2, 45 g of 1 and
200 g of 2, 45 g of 1 and 250 g of .2, 50 g of 1 and 25 g
25 of 2, 50 g of 1 and 50 g of 2, 50 g of 1 and 100 g of 2,
50 g of 1 and 125 g of .2, 50 g of 1 and 200 g of 2 or
50 g of 1 and 250 g of 2.
The active substance combinations of 1 and 2 according to
the invention are preferably administered by inhalation.
For this purpose, ingredients 1 and 2 have to be made
available in forms suitable for inhalation. Inhalable
preparations include inhalable powders,
propellant-containing metering aerosols or
propellant-free inhalable solutions. Inhalable powders
CA 02733294 2011-03-04
- 9 -
according to the invention containing the combination of
active substances 1 and 2 may consist of the active
substances on their own or of a mixture of the active
substances with physiologically acceptable excipients.
Within the scope of the present invention, the term
propellant-free inhalable solutions also includes
concentrates or sterile inhalable solutions ready for
use. The preparations according to the invention may
contain the combination of active substances 1 and 2
either together in one formulation or in two separate
formulations. These formulations which may be used
within the scope of the present invention are described
in more detail in the next part of the specification.
A) Inhalable powder containing the combinations of active
substances 1 and 2 according to the invention:
The inhalable powders according to the invention may
contain 1 and 2 either on their own or in admixture with
suitable physiologically acceptable excipients.
If the active substances 1 and 2 are present in admixture
with physiologically acceptable excipients, the following
physiologically acceptable excipients may be used to
prepare these inhalable powders according to the
invention: monosaccharides (e.g. glucose or arabinose),
disaccharides (e.g. lactose, saccharose, maltose), oligo-
and polysaccharides (e.g. dextrane), polyalcohols (e.g.
sorbitol, mannitol, xylitol), salts (e.g. sodium
chloride, calcium carbonate) or mixtures of these
excipients with one another. Preferably, mono- or
disaccharides are used, while the use of lactose or
glucose is preferred, particularly, but not exclusively,
in the form of their hydrates. For the purposes. of the
invention, lactose is the particularly preferred
excipient, while lactose monohydrate is most particularly
preferred.
CA 02733294 2011-03-04
- 10 -
Within the scope of the inhalable powders according to
the invention the excipients have a-maximum average
particle size of up to 250 m, preferably between 10 and
150 m,.most preferably between 15 and 80 m. It may
sometimes seem appropriate to add finer excipient
fractions with an average particle size of 1 to 9 m to
the excipient mentioned above. These finer excipients
are also selected from the group of possible excipients
listed hereinbefore. Finally, in order to prepare the
irihalable powders according to the invention, micronised
active substance l and 2, preferably with an average
particle size of 0.5 to 10 m, more preferably from.1 to
5 m, is added to the excipient mixture. . Processes for
producing the inhalable powders according to the
invention by grinding and micronising and by finally
mixing the ingredients together are known from the prior
art. The inhalable powders according to the invention
may be prepared and administered either in the form of a
single powder mixture which contains both 1 and 2 or in
the form of separate inhalable powders which contain only
1 or 2.
The irihalable powders according to the invention may be
administered using inhalers known from the prior art.
Inhalable powders according to the invention which
contain a physiologically acceptable excipient in
addition to 1 and 2 may be administered, for example, by
means of inhalers which deliver a single dose from a--
supply using a-measuring chamber as described in
US 4570630A, or by other means as described in
DE 36 25 685 A. Preferably, the inhalable powders
according to the invention which contain physiologically
acceptable excipient in addition to 1 and 2 are packed
.into capsules (to produce so-called inhalettes) which are
used in inhalers as described, for example, in
WO 94/28958.
CA 02733294 2011-03-04
11
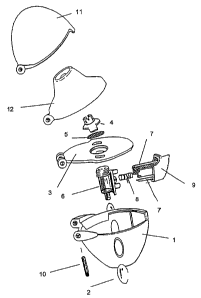
A particularly preferred inhaler for using the
pharmaceutical combination according to the invention in
inhalettes is shown in Figure 1.
This inhaler (Handihaler'm)for inhaling powdered
pharmaceutical compositions from capsules is
characterised by a housing 1 containing two windows 2, a
deck.3' in which. there are air inlet portions and which is
provided with a screen 5 secured via a screen housing 4,
an inhalation chamber 6 connected to the deck 3 on which
there is a push button 9 provided with two
sharpened pins 7 and movable counter to a spring 8, and a
mouthpiece 12 which is connected to the housing 1, the
deck 3. and a cover 11 via a spindle 10 to enable it to be
flipped open or shut.
If the inhalable powders according to the invention are
packed into capsules (inhalers) for the .preferred use
described above, the quantities packed into each capsule
should be 1 to 30mg, preferably 3 to 20mg, more
particularly 5 to 10mg of inhalable powder per capsule.
These capsules contain, according to the invention,
either together or separately, the doses of 11 and 2
mentioned hereinbef.ore for each single dos.e..
.B) Propellant gas-driven inhalation aerosols containing
the combinations of active substances 1 and 2:
Inhalation aerosols containing propellant gas according
to the invention may contain substances 1 and 2 dissolved
in the propellant gas or in dispersed form. 1 and 2 may
be present in separate formulations or in a single
preparation, in which 1 and 2 are either both dissolved,
both dispersed or only one component is dissolved and the
other is dispersed. The propellant gases which may be
used to prepare the inhalation aerosols according to the
invention are known from the prior art. Suitable
CA 02733294 2011-03-04
- 12 -
propellant gases are selected from among hydrocarbons
such as n-propane., n-butane or isobutane and
halohydrocarbons such as fluorinated derivatives of
methane, ethane, propane, butane, cyclopropane or
cyclobutane. The propellant gases mentioned above may be
used on their own or in mixtures thereof. Particularly
preferred propellant gases are halogenated alkane
derivatives selected from TG134a and TG227. Of the
halogenated hydrocarbons mentioned above, TG134a
(1,1,1,2-tetrafluoroethane) and TG227 (1,1,1,2,3,3,3-
heptafluoropropane) and mixtures thereof are preferred.
according to the invention.
The propellant-driven inhalation aerosols according to
the invention may also contain other ingredients such as
co-solvents, stabilisers, surfactants, antioxidants,
lubricants and pH adjusters. All these ingredients are
known in the art.
The inhalation aerosols containing propellant gas
according to the invention may contain up to 5 wt.-% of
active substance 1 and/or 2. Aerosols according to'the
invention contain, for example, 0.002 to 5 wt.-%, 0.01 to
3 wt.-%, 0.015 to 2 wt.-%, 0.1 to 2 wt.-%, 0.5 to 2 wt.-%
or 0.5 to 1 wt.-% of active substance 1 and/or 2.
If the active substances 1 and/or 2 are present in
dispersed form, the particles of active substance
preferably have an average particle size of up to 10 m,
preferably from 0.1 to 5 m, more preferably from 1 to
5 m.
The propellant-driven inhalation aerosols according to
the invention mentioned above may be administered using
inhalers known in the art (MDIs = metered dose inhalers).
Accordingly, in another aspect, the present invention
CA 02733294 2011-03-04
- 13 -
relates to pharmaceutical compositions in the form of
propellant-driven aerosols as hereinbefore described
combined with one or more inhalers suitable for
administering these aerosols. In addition, the present
invention relates to inhalers which are characterised in
that they contain the propellant gas-containing aerosols
described above according to the invention.' The present
invention also relates to cartridges which are fitted
with a suitable valve and can be used in a suitable
inhaler and which contain one of the above-mentioned
propellant gas-containing inhalation aerosols according
to the invention. Suitable cartridges and methods of
filling these cartridges with the inhalable aerosols
containing propellant gas according to the invention are
known from the prior art.
C) Propellant-free inhalable solutions or suspensions
containing the combinations of active substances 1 and 2
according to the invention.
It is particularly preferred to. use the active substance
'combination according to the invention in the form of
propellant-free inhalable solutions and suspensions. The
solvent used may be an aqueous or alcoholic, preferably
an ethanolic solution. The solvent may be water on its
own or a mixture of water and ethanol. The relative
proportion of ethanol compared with water is not limited
but the maximum is up to 70 percent by volume, more
particularly up to 60 percent by volume and most
preferably up to 30 percent by volume. The remainder of
the volume is made up of water. The solutions or
suspensions containing 1 and 2, separately or together,
are adjusted to a pH of 2 to 7, preferably '2 to 5, using
suitable acids. The pH may be adjusted using acids
selected from inorganic or organic acids. Examples of
suitable inorganic acids include hydrochloric acid,
hydrobromic acid, nitric acid, sulphuric acid and/or
CA 02733294 2011-03-04
- 14 -
phosphoric acid. Examples of particularly suitable.
organic acids include ascorbic acid, citric acid, malic
acid, tartartic acid, maleic acid, succinic acid, fumaric
acid, acetic acid, formic acid and/or propionic acid etc.
Preferred inorganic acids are hydrochloric and sulphuric
acids. It is also possible to use the acids which have
already formed an acid addition salt with one of the
active substances. Of the organic acids, ascorbic acid,
fumaric acid and citric acid are preferred. If desired,
mixtures of the above acids may be used, particularly in
the case of acids which have other properties in addition
to their acidifying qualities, e.g. as flavourings,
antioxidants or complexing agents, such as citric acid or
ascorbic acid, for example. According to the invention,
it is particularly preferred to use hydrochloric acid to-
adjust the pH.
According to the invention, the addition of editic acid
(EDTA) or one of the known salts thereof, sodium editate,
as stabiliser or complexing agent is unnecessary in the
present formulation. Other embodiments may contain this
compound or these compounds. In a preferred embodiment
the content based on sodium editate is less than
100mg/100ml, preferably less than 50mg/ml, more
preferably less than 20mg/ml. Generally, inhalable
2.5 solutions in which the content of sodium editate is from
0 to 10mg/100ml are preferred.
Co-solvents and/or other excipients may be added to the
propellant-free inhalable solutions according to the
invention. Preferred co-solvents are those which contain
hydroxyl groups or other polar groups, e.g. alcohols -
particularly isopropyl alcohol, glycols - particularly
propyleneglycol, polyethyleneglycol, polypropyleneglycol,
glycolether, glycerol, polyoxyethylene alcohols and
polyoxyethylene fatty acid esters. The terms excipients
CA 02733294 2011-03-04
- 15 -
and additives in this context denote any
pharmacologically acceptable substance which is not an
active substance but which can be formulated with the
active substance or substances in the pharmacologically
suitable solvent in order to improve the qualitative
properties of the active substance formulation-
Preferably, these substances have no pharmacological
effect or, in connection with the desired therapy, no
appreciable or at least no undesirable pharmacological
effect. The excipients and additives include, for
example, surfactants such as soya lecithin, oleic acid,
sorbitan esters, such as polysorbates,
polyvinylpyrrolidone, other stabilisers, complexing
agents, antioxidants and/or. preservatives which guarantee
or prolong the shelf life of the finished pharmaceutical
formulation, flavourings, vitamins and/or other additives
known in the art. The additives also include
pharmacologically acceptable salts such as sodium
chloride as isotonic agents..
The preferred excipients include antioxidants such as
ascorbic acid, for example, provided that it has not
already been used to adjust the pH, vitamin A, vitamin E,
tocopherols and similar vitamins and provitamins
occurring in the human body.
Preservatives may be used to protect the formulation from
contamination with pathogens. Suitable preservatives are
those which are known in the art, particularly cetyl
pyridinium chloride, benzalkonium chloride or benzoic
acid or benzoates such as sodium benzoate in the
concentration known from the prior art. The
preservatives mentioned above are preferably present in
concentrations of up to 50mg/100ml, more preferably
between 5 and 20mg/100mi.
CA 02733294 2011-03-04
- 16 -
Preferred formulations contain, in addition to the
solvent water and the combination of active substances 1
and 2, only benzalkonium chloride and sodium editate. In
another preferred embodiment, no sodium editate is
5- present.
The propellant-free inhalable solutions according to the
invention are administered in particular using inhalers
of the kind which are capable of nebulising a small
amount of a liquid formulation in the therapeutic dose
within a few seconds to produce an aerosol suitable for
therapeutic inhalation. Within the scope of the present
invention, preferred inhalers are those in which a
quantity of less than 100 L, preferably less than 50 L,
more preferably between 10 and 30 L_of active substance
solution can be nebulised in preferably one spray action
to form an aerosol with an average particle size of less
than 20 m, preferably less than 10 m, in such a way that
the inhalable part of the aerosol corresponds to the
therapeutically effective quantity.
An apparatus of this kind for propellant-free delivery of
a metered quantity of a liquid pharmaceutical composition
for inhalation is described for example in International
Patent Application WO 91/14468 and also in WO 97/12687
(cf.. in particular Figures 6a and 6b).. The nebulisers
(devices) described therein are known by the name
Respimat .
This nebuliser (Respimat ) can advantageously be used to
produce the inhalable aerosols according to the invention
containing the combination of active substances 1 and 2.
Because of its cylindrical shape and handy size of less
than 9 to 15 cm long and 2 to 4 cm wide, this device can
be carried at all times by the patient. The nebuliser
sprays a defined volume of pharmaceutical formulation
CA 02733294 2011-03-04
- 17 -
using high pressures through small nozzles so as to
produce inhalable aerosols.
The preferred atomiser essentially consists of an upper
housing part,- a pump housing, a nozzle, a locking
mechanism, a spring housing, a spring and a storage
container, characterised by
- a pump housing which is secured in the upper housing
part and which comprises at one end a nozzle body
with the nozzle or nozzle arrangement,
- a hollow plunger with valve body,
- a power takeoff flange in which the hollow plunger'
is secured and which is located in the upper housing
part,
- a-locking mechanism situated in the upper housing
part,
- a spring housing with the spring contained therein,
which is rotatably mounted on the upper housing 'part
by means of a rotary bearing,
- a lower housing part which is fitted onto the spring
20. housing in the axial direction.
The hollow plunger with valve body corresponds to a
device disclosed in WO 97/12687. It projects partially
into the cylinder of the pump housing and is axially
movable within the cylinder. Reference is made in
2.5 particular to Figures 1 to 4, especially Figure 3, and
the relevant parts of the description. The hollow
plunger with valve body exerts a pressure of 5 to 60 Mpa
(about 50 to 600 bar), preferably 10 to 60 Mpa (about 100
to 600 bar) on the fluid, the measured amount of active
30 substance solution, at its high pressure end at the
moment when the spring is actuated. Volumes of 10 to 50
microlitres are preferred, while volumes of 10 to 20
microlitres are particularly preferred and a volume of 15
microlitres per spray is most particularly preferred.
CA 02733294 2011-03-04
- 18 -
The valve body is preferably mounted at the end of the
hollow plunger facing the valve body.
The nozzle in the nozzle body is preferably
microstructured, i.e. produced by microtechnology.
Microstructured valve bodies are disclosed for example in
WO-94/07607; reference is hereby made to the contents of
this specification., particularly Figure 1 therein and the
associated description..
The valve body consists for example of two sheets of
glass and/or silicon firmly joined together, at least one
of which has one or more microstructured channels which
connect the nozzle inlet end to the nozzle outlet end.
At the nozzle outlet end there is at least one round or
non-round opening 2 to 10 microns deep and 5 to 15
microns wide, the depth preferably being 4.5 to 6.5'
microns while the length is preferably 7 to 9 microns.
In the case of a plurality of nozzle openings, preferably
two, the directions of spraying of the nozzles in the
nozzle body may extend parallel to one another or may be
inclined relative to one another in the direction of the
nozzle opening. In a nozzle body with at least two
nozzle openings at the outlet end the directions of
spraying may be at an angle of 20 to 160 to one another,
preferably 60 to 150 , most preferably 80 to 100 . The
nozzle openings are preferably arranged at a spacing of
10 to 200 microns, more preferably at a spacing of 10 to
100 microns, most preferably 30 to 70 microns. Spacings
of 50 microns are most preferred. The directions of
spraying will therefore meet in the vicinity of the
nozzle openings.
The liquid pharmaceutical preparation strikes the nozzle
body with an entry pressure of up to 600 bar, preferably
CA 02733294 2011-03-04
- 19 -
200 to 300 bar, and is atomised into an inhalable aerosol
through the nozzle openings. The preferred particle or
droplet sizes of the aerosol are up to 20 microns,
preferably 3 to 10 microns.
The locking mechanism contains a spring, preferably a
cylindrical helical compression spring, as a store for
the mechanical energy. The spring acts on the power
takeoff flange as an actuating member the movement of
which is determined by the position of a locking member.
The travel of the power takeoff flange is precisely
limited by an upper and lower stop. The spring is
preferably biased, via a power step-up gear,. e.g. a
helical thrust gear, by an external torque which is
produced when the upper housing part is rotated counter
to the spring housing in the lower housing part. In this
case, the upper housing part and the power takeoff flange
have a single or multiple V-shaped gear.
The locking member with engaging locking surfaces is
arranged in a ring around the power takeoff flange. It
consists, for example, of a ring of plastic or metal
which is inherently radially elastically deformable. The
ring is arranged in a plane at right angles to the
atomiser axis. After the biasing of the spring, the
locking surfaces of the locking member move into the path
of the power takeoff flange and prevent the spring from
relaxing. The locking member is actuated by means of a
button. The actuating button is connected or coupled to
the locking member. In order to actuate the locking
mechanism, the actuating button is moved parallel to the
annular plane, preferably into the atomiser; this causes
the deformable ring to deform in the annual plane.
Details of the construction of the locking mechanism are
given in WO 97/20590.
CA 02733294 2011-03-04
- 20 -
The lower housing part is pushed axially over the spring
housing and covers the mounting, the drive of the spindle
and the storage container for the fluid.
When the atomiser is actuated the upper housing part is
rotated relative to the lower housing part, the lower
housing part taking the spring housing with it. The
spring is thereby compressed and biased by means of the
helical thrust gear and the locking mechanism engages
automatically. The angle of rotation is preferably a
whole-number fraction of 360 degrees, e.g. 180 degrees.
At. the same time as the spring is biased, the power
takeoff part in the upper housing part is moved along by
a given distance, the hollow plunger is withdrawn inside
the cylinder in the pump housing, as a result of which
some pf the fluid is sucked out of the storage container
and into the high pressure chamber in front of the
nozzle.
If desired, a number of exchangeable storage containers
which contain the fluid to be atomised may be pushed into
the atomiser one after another and used. in succession.
The storage container contains the aqueous aerosol
preparation according to the invention.
The atomising process is initiated by pressing gently on
the actuating button. As a result, the locking mechanism
opens up the path for the power takeoff member. The
biased spring pushes the plunger into the cylinder of the
pump housing. The fluid leaves the nozzle of the
atomiser in atomised form.
Further details of construction are disclosed in PCT
Applications WO 97/12683 and WO 97/20590, to which
reference is hereby made.
CA 02733294 2011-03-04
21 -
The components of the atomiser (nebuliser) are made of a
material which is suitable for its purpose. The housing
of the atomiser and - if its operation permits, other
parts as well are preferably made of plastics, e.g. by
injection moulding. For medicinal purposes,
physiologically safe materials are used.
Figures 2a/b attached to this patent application, which
are identical to Figures 6a/b of WO 97/12687, show the
nebuliser (Respimat ) which can advantageously be used
for inhaling the aqueous aerosol preparations according
to the invention.
Figure 2a shows a longitudinal section through the
atomiser with the spring biased while Figure 2b shows a
longitudinal section through the atomiser with the spring
relaxed.
The upper housing part (51) contains the pump housing
(52) on the end of which is mounted the holder (53) for
the atomiser nozzle. In the holder is the nozzle body
(54) and a filter (55). The hollow plunger (57) fixed in
the power takeoff flange (56) of the locking mechanism
projects partially into the cylinder of the pump housing.
At its end the hollow plunger carries the valve body
(58). The hollow plunger is sealed off by means of the
seal (59). Inside the upper housing part is the stop
(60) on which the power takeoff flange abuts when the
spring is relaxed. On the power takeoff flange is the
stop (61) on which the power takeoff flange abuts when
the spring is biased. After the biasing of the spring
the locking member (62) moves between the stop (61) and a
support (63).in the upper housing part. The actuating
button (64) is connected to the locking member. The
upper housing part ends in the mouthpiece (65) and is
CA 02733294 2011-03-04
- 22 -
sealed off by means of the protective cover (66) which
can be placed thereon.
The spring housing (67) with compression spring (68) is
rotatably mounted on the upper housing part by means of
the snap-in lugs (69) and rotary bearing. The lower
housing part (70) is pushed over the spring housing.
Inside the spring housing is the exchangeable storage
container (71) for the fluid (72) which is to be
atomised. The storage container is sealed off by the
stopper (73) through which the hollow plunger projects
into the storage container and is immersed at its end in
the fluid (supply of active substance solution)
The spindle (74) for the mechanical counter is mounted in
the covering of the spring housing. At the end of the
spindle facing the upper housing part is the drive pinion
(75). The slider (76) sits on the spindle.
The nebuliser described above is suitable for nebulising
the aerosol preparations according to the invention to
produce an aerosol suitable for inhalation.
If the formulation according to the invention is
nebulised using the method described above (Respimat )
the quantity delivered should correspond to a defined
quantity with a tolerance of not more than 25%,
preferably 20% of this amount in at least 97%, preferably
at least 98% of all operations of the inhaler (spray
actuations). Preferably, between 5 and 30 mg of
formulation, most preferably between 5 and 20 mg of
formulation are delivered as a defined mass on each
actuation.
However, the formulation according to the invention may
also be nebulised by means of inhalers other than those
CA 02733294 2011-03-04
- 23 -
described above, e.g. jet stream inhalers or other
stationary nebulisers.
Accordingly, in a further aspect, the invention relates
to pharmaceutical formulations in the form of
propellant-free inhalable solutions or suspensions as
described above combined with a device suitable for
administering these formulations, preferably in
conjunction with the Respimat . Preferably, the
invention relates to propellant-free inhalable solutions
or suspensions characterised by the combination of active
substances :1 and 2 according to the invention in
conjunction with the device known by the name Respimat .
In addition, the present invention relates to the
above-mentioned devices for inhalation, preferably the
Respimat , characterised in that they contain the
propellant-free inhalable solutions or.suspensions
according to the invention. as described hereinbefore.
The propellant-free inhalable solutions or suspensions
according to the invention may take the form of
concentrates or sterile inhalable solutions or
suspensions ready for use, as well as the above-mentioned
solutions and suspensions designed for use in a
Respimat . Formulations ready for use may be produced
from the concentrates, for example, by the addition of
isotonic saline solutions. Sterile formulations ready
for use may be administered using energy-operated fixed
or portable nebulisers which produce inhalable aerosols
by means of ultrasound or compressed air by the Venturi
principle or other principles.
Accordingly, in another aspect, the present invention
relates to pharmaceutical compositions in the form of
propellant-free inhalable solutions or suspensions as
described hereinbefore which take the form of
CA 02733294 2011-03-04
- 24 -
concentrates or sterile formulations ready for use,
combined with a device suitable for administering these
solutions, characterised in that the device is an energy-
operated free-standing or portable nebuliser which
produces inhalable aerosols by means of ultrasound or
compressed air by the Venturi principle or other methods.
The Examples which follow serve to illustrate the present
invention in more detail without restricting the scope of
the invention to the following embodiments by way of
example.
Starting materials
Ti o ropi um bromide: -
The tiotropium bromide used in the following formulations
examples may be obtained as described in European Patent
Application 418 716 Al.
In order to prepare the inhalable powders according to
the invention, crystalline tiotropium bromide monohydrate
may also be used.. This crystalline tiotropium bromide
monohydrate may be obtained by the method described
below.
15.0 kg of tiotropium bromide are placed in 25.7 kg of
water in a suitable reaction vessel. The mixture is
heated to 80-90 C and stirred at constant temperature
until a clear solution is formed. Activated charcoal
(0.8 kg) moistened with water is suspended in 4.4 kg of
water, this mixture is added to the solution containing
the tiotropium bromide and the resulting mixture is
rinsed with 4.3 kg of water. The mixture thus obtained
is stirred for at. least 15 minutes at 80-90 C and then
filtered through a heated filter into an apparatus
preheated to an external temperature of 70 C. The filter.
CA 02733294 2011-03-04
- 25 -
is rinsed with 8.6 kg of water. The contents of the
apparatus are cooled at 3-50C for every 20 minutes to a
temperature of 20-25 C. The apparatus is cooled further
to 10-15 C using cold water and crystallisation is
completed by stirring for at least another hour. The
crystals are isolated using a suction filter dryer, the
crystal slurry isolated is washed with 9 litres of cold
water (10-15 C) and cold acetone (10-15'C).' The crystals
obtained are dried at 25 C in a nitrogen current over a
period of 2 hours.
Yield: 13.4 kg of tiotropium bromide monohydrate (86% of
theory).
The crystalline tiotropium bromide monohydrate thus
obtained is micronised by known methods in order to
prepare the active substance in the form of the average
particle size corresponding to the specifications
according to the invention.
CA 02733294 2011-03-04
- 26 -
Examples of Formulations
A) Tnhal hl e powders:
1)
Ingredients g per capsule
Tiotropium bromide 21.7
Budesonide 200
Lactose 4778.3
Total 5000
2)
Ingredients g per capsule
Tiotropium bromide 21.7
Fluticasone propionate 125
Lactose 4853.3
Total 5000
3)
Ingredients pg per capsule
Tiotropium bromide x H2O 22.5
Mometasone furoate 250
Lactose 4727.5
Total 5000
4)
Ingredients g per capsule
Tiotropium bromide 21.7
Ciclesonide 250
Lactose 4728.3
Total 5000
CA 02733294 2011-03-04
- 27 -
R) PrnnFllant gag-containing aArnanls for inhalation:
1) Suspension aerosol:
Ingredients .wt.-%
Tiotropium bromide 0.029
Budesonide 0.4
Soya lecithin 0.2
TG 134a: TG227 = 2:3 ad 100.
2) Suspension aerosol:
Ingredients wt.-%
Tiotropium bromide 0.029
Fluticasone-propionate 0.3
Isopropyl myristate 0.1
TG 227 ad 100
3) Suspension aerosol:
Ingredients wt.-%
Tiotropium bromide 0.029
Mometasone-furoate 0.6
Isopropyl myristate 0.1
TG 227 ad 100
4) Suspension aerosol:
Ingredients wt.-%
Tiotropium bromide 0.029
Ciclesonide 0.4
Isopropyl myristate 0.1
TG 227 ad 100