Note: Descriptions are shown in the official language in which they were submitted.
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
SCREENING METHOD FOR IDENTIFYING PATIENTS AT RISK OF ADVERSE
HEPATOLOGIC EVENTS
CROSS REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of U.S. Provisional Application No.
61/092,686,
filed August 28, 2008, which is incorporated herein by reference in its
entirety.
FIELD OF THE INVENTION
This present invention provides screening methods and kits for identifying
patients
who are at risk for liver injuries, particularly at an increased risk of
showing liver toxicity
upon administration of medications. The methods and kits are useful for
identifying patients
at risk of drug-induced liver injury to exclude such patients from certain
treatment protocols.
BACKGROUND OF THE INVENTION
Drugs sometimes cause serious injuries to the livers of patients, with loss of
hepatic
function leading to illness, disability, hospitalization, and even life
threatening liver failure
and death or need for liver transplantation. As the world population ages,
more and more
drugs are being prescribed and often combined with self-prescribed over-the-
counter
medications, so-called "dietary supplements," special diets and alcohol.
Exposure to
environmental chemicals is also rising. The liver is the principal organ for
metabolizing,
inactivating, and disposing of all of these toxins. Their metabolites may
injure the liver cells,
and complex drug-drug interactions complicate the situation. The combination
of all of these
risk factors has increased the incidence of liver injury.
Liver injury due to prescription and nonprescription medications is a growing
medical, scientific, and public health problem in the United States. In the
United States, drug-
induced liver injury (DILI) is now the leading cause of acute liver failure
(ALF), exceeding
all other causes combined (see WM Lee, et al. Acute Liver Failure Study
Group). DILI is
the single most common reason for Food and Drug Administration regulatory
actions
concerning drugs, including failure to gain approval for marketing, removal
from the
marketplace, and restriction of prescribing indications. Worldwide, the
estimated global
annual incidence rate of DILI is 13.9-24.0 per 100,000 inhabitants, and DILI
accounts for an
estimated 3%-9% of all adverse drug reactions reported to health authorities
(see Aithal GP,
1
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
et al. (1999) Br Med J 319: 1541-5; Friis and Andreasen (1992) Jlntern Med
232: 133-138;
and Dossing and Anderson (1982) Scan JGastroenterol 17:205-211).
Hepatotoxicity has been consistently the most important single cause of
withdrawals
and marked limitations of use of drugs or refusal to approve them. In the
1950's, iproniazid
(Marsilid) was probably the most hepatotoxic drug ever marketed, but
isoniazid, from the
same period, has been found to cause serious hepatotoxicity in about 0.1% of
recipients.
Benoxaprofen (Oraflex), ticrynafen (Selacryn), bromfenac (Duract) and
troglitizone (Rezulin)
all were withdrawn because of hepatotoxicity, and ibufenac, perhexilene and
dilevalol, all
marketed abroad, were never approved in the United States because of this
issue.
Hepatotoxicity has also caused important limitations of use for many drugs,
including
isoniazid, labetalol, dantrolene, felbamate, pemoline, tolcapone, and
trovafloxacin.
While in most cases, hepatotoxicity is recognized late, as the incidence is
often low or
dependent on other circumstances and neither animal nor human experience
before marketing
yields recognized signals of hepatotoxic potential. Post-marketing
surveillance now detects
serious hepatotoxins in months (bromfenac, tolcapone, troglitizone,
trovafloxacin), in marked
contrast to the years of delay in the past (iproniazid, isoniazid), but it is
obviously preferable
to discover them before marketing.
The use of complementary and alternative medicines has also been increasing in
Western countries, and there are numerous reports of hepatotoxicity from such
products in
both animal models as well as in humans (see Zimmerman HJ. (1999)
Hepatotoxicity: the
Adverse Effects of Drugs and other Chemicals on the Liver, 2nd ed. Lippincott
Williams &
Wilkins, Philadelphia, PA, 1999, pp. 731). Examples of alternative products
that have a well-
established potential to cause liver injury include pyrrolizidine alkaloids
(Comfrey), chaparral
leaf, germander, pennyroyal (squawmint oil), mistletoe, kava, and weight-loss
preparations
containing usnic acid (Favreau JT, et al. (2002) Ann Intern Med 136:590-5).
It has become clear that certain individuals are much more susceptible to drug-
induced liver damage than are others, and uncommon but severe idiosyncratic
liver damage
requires special consideration as a safety problem. Not only are people
genetically diverse,
which affects the way they metabolize drugs and other chemicals, but each
person's life
experience is different. Drug-induced liver injury is the leading cause of
acute liver failure
among patients presenting for evaluation at liver transplant centers in the
United States, and
the leading single cause for having to remove approved drugs from the market.
The ability of certain signals for identifying patients who are suffering from
DILI has
been suggested, notably transaminase elevations of various degrees (3 fold, 5
fold, etc.) and
2
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
frequencies (2%, 3%, etc.) and serum transaminase elevations accompanied by
elevated
bilirubin (see Zimmerman (1978) Drugs 16:25-45). Generally, patients provide,
at regular
intervals, serum samples for testing activities of alanine aminotransferases
(ALT, also known
as SGPT), aspartate aminotransferases (AST, also known as SGOT), alkaline
phosphatase
(ALP), total bilirubin (Bt), serum albumin and, much less commonly, blood
prothrombin
time. However, although these markers may be useful for identifying drugs that
should not be
approved or should be closely monitored, these signals are not useful for
predicting the
patients who should be excluded from receiving a potentially hepatotoxic drug
before putting
those patients at risk for an adverse event.
Preclinical studies often fail to predict liver toxicity levels, particularly
those of low
incidence or of lesser severity. Animal studies, although useful in
identifying severe
toxicities, fail in predicting these rare hepatic events. The situations that
cause individuals to
be at increased risk for a DILI are likely due to combinations of
environmental factors that
are impossible to recreate in a laboratory, such as drug compliance,
additional or alternative
medicine combinations, environmental exposures and genetic predisposition. It
has been
shown repeatedly that animal models simply fail to account for these
variables.
Although the pre-approval clinical phases of drug development represent a key
arena
for identification of hepatotoxic potential, clinical trials may not present
any evidence of liver
toxicity if the testing population is too small, too limited, or generally not
representative of
the subject populations that are ultimately exposed to the drug. At this
stage, drugs with
significant toxic potential are easily identified but those with low toxic
potential may be less
easily recognized. As a general rule, if an overt adverse event occurs in 1
per 1000 people, a
study must include at least 3000 individuals, which is a typical pre-approval
size. An
incidence of less than 1 per 1000 may never appear in the pre-approval
setting. Furthermore,
if the adverse reaction is delayed, the clinical trials may have included a
far smaller number
of exposed individuals at risk for sufficient duration. Furthermore, the
frequency of testing
and rules for stopping the drug may confound the identification of a
significant signal.
It is recognized that pre-existing liver disease is likely a risk factor for
DILI.
However, reliable indicators of liver disease that could predict subjects at
risk are lacking.
Subjects with severe liver disease usually are excluded from clinical studies,
but subjects with
mild elevations of biomarkers such as serum alanine aminotransferase (ALT) (in
the range of
2-3 times the upper limit of normal, i.e. subjects with mild liver disease)
are usually included.
ALT values are not predictive of DILI because in general such patients are not
at increased
risk of suffering idiosyncratic drug reactions, although their hepatic
response to an
3
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
idiosyncratic reaction may be exaggerated. The FDA has emphasized that
aminotransferase
abnormalities that are less than 3x ULN are common in untreated and placebo-
treated
subjects and are not informative about the potential for the development of
severe DILI.
Therefore, it has become standard practice to look at greater deviations such
as
aminotransferase values greater than 3X, greater than 5X, or greater than lOX
upper limit of
normal (ULN). Because these abnormalities can occur in placebo-treated groups,
it is
important to compare their rate in drug-exposed subject groups relative to
control groups
looking for an increased rate of aminotransferase elevation throughout the
overall study
population compared to control" (see FDA Guidance Document on DILI).
Most significant hepatotoxins cause predominantly hepatocellular injury
indicated by
leakage of ALT from injured liver cells without prominent evidence of
hepatobiliary
obstruction. The ability to cause some hepatocellular injury is not a reliable
predictor of a
drug's potential for severe DILI. Many drugs that cause transient rises in
serum
aminotransferase activity do not cause progressive or severe DILI even if drug
administration
is continued. Many drugs show increased ALT signal without conferring a risk
of severe
injury (e.g., tacrine, statins, aspirin, heparin) indicating low specificity
for an excess of
aminotransferase elevations alone. It is only those drugs that cause
hepatocellular injury
extensive enough to affect the liver's functional ability to clear bilirubin
from the plasma or
to synthesize prothrombin and other coagulation factors that cause severe
DILI.
There remains a need for a test that can readily predict patients who are at
risk of
suffering from DILI, particularly DILI of low severity or frequency, before
administration of
a potentially toxic drug.
An object of the present invention is to provide methods and kits for
identifying
patients at risk of DILI and for providing treatment regimens that incorporate
such
information.
SUMMARY OF THE INVENTION
The present invention is founded on the recognition that levels of certain
lipoproteins
in a patient can be used to predict the risk that a patient will develop a
drug induced liver
injury after administration of a drug. In particular, levels of apolipoprotein
Al (ApoAl) in
serum relate to this risk. Redox signaling pathways play an important role in
both normal
and pathological cell function in tissues including the liver. Numerous
studies suggest that the
regulation of these signals may underlie the molecular and biochemical
pathogenesis leading
4
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
to clinical liver disease. Although the liver has a significant reserve
capacity, over time, this
reserve capacity can be diminished by various stress conditions including
underlying disease
states such as type 2 diabetes mellitus or atherosclerosis and exposure to
both
pharmaceuticals and environmental toxins. Each of these factors, taken in
isolation, may
have no obvious adverse effect on the liver. However, a combination of stress
factors
occurring simultaneously may overcome hepatic reserve capacity resulting in
hepatocyte
injury.
The present specification provides evidence that patients at risk for liver
injury exhibit
a compensated hepatic response, particularly a compensated response to oxidant
signals, even
in the absence of clinically defined laboratory abnormalities typically
associated with liver
injury. With otherwise normal laboratory values, modest elevations in ApoAl
expression to
concentrations greater than the upper limit of normal can reflect a
compensated hepatic stress
response that identifies individual patients at risk for progression to
hepatic abnormalities in
response to pharmacological agents.
Therefore, in one embodiment, a method of identifying a patient at risk of a
liver
injury, and in particular a drug-induced liver injury, is provided comprising
1) measuring the
level of ApoAl in a bodily fluid from the patient; and 2) comparing the
measured level of
ApoAl in the sample with a reference measurement in a population. In certain
instances, a
patient who has one or more samples in which the measured level of ApoAl is
greater than
an upper limit of normal (ULN) in a reference population is considered at
increased risk of
liver injury, in particular at greater risk of drug-induced liver injury. In
some embodiments,
the drug induced liver injury is from an antioxidant drug. In certain other
instances, the drug
induced liver injury is from a drug that increases PPAR activity. In
particular embodiments,
if the measured level of ApoAl in the bodily fluid is less than or equal to
the ULN, a
pharmacological agent is administered to the patient and if the measured level
of ApoAl is
greater than the ULN, a pharmacological agent is not administered.
In certain embodiments, measurement of ApoAl or structural modifications of
ApoAl are associated with redox related conditions, and in particular
inflammatory
conditions. In certain instances, the measurement is related to disorders such
as diabetes. In
some embodiments, the measurement is of levels of ApoAl. In other embodiments,
the
measurement is of ApoAl structural modifications such as lipid modification.
In some
embodiments, the ApoAl measurement above a ULN indicates a patient is in need
of
treatment for a condition.
In some embodiments, the methods further comprise measuring a level of ALT in
a
5
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
sample from the patient and comparing that to a reference ALT level in the
population. In
these instances, a measurement of ALT that exceeds ULN is also used to
classify the patients
as at increased risk of an adverse liver injury. In certain instances, the
measurement of ALT
is taken before any drug is administered. In these instances, the ALT level
can be used as a
further exclusionary criteria to identify patients who are at increased risk
of a drug induced
liver injury. In some instances, an ALT level of greater than ULN is
identified, but in other
instances an ALT level of at least 1.5 or at least 2.0 or greater ULN is
provided as criteria for
exclusion. In certain instances, ALT levels are measured in a patient
receiving a drug after a
period of time, such as at one week, two weeks, three weeks, four weeks, five
weeks or more
after commencing a therapeutic regimen. Patients whose ALT level is measured
as
exceeding ULN may be considered at increased risk of developing, having, or
suffering from,
liver toxicity. In particular embodiments, if the measured ALT level is less
than or equal to
the ULN, a pharmacological agent is administered to the patient and if the
measured ALT
level is greater than the ULN, a pharmacological agent is not administered.
A patient who has one or more samples in which measured ApoAl level exceeds
the
reference, such as a ULN in a population, may also be considered to have
increased
inflammatory activity. Such a patient may be considered at risk for additional
disorders,
including inflammatory disorders such as rheumatoid arthritis. In certain
instances, the
patient is at risk of or suffering from a disorder in glucose metabolism. Such
a disorder may
be diabetes mellitus and in particular may be type 2 diabetes mellitus.
In certain instances, a treatment protocol will be designed based on the
results of an
ApoAI measurement. This treatment protocol may require that antioxidant drugs
not be
given to a patient whose ApoAI measurement exceeds the reference value, such
as an ULN,
or it may be that the patient is closely monitored for hepatotoxicity. In
addition, the treatment
protocol may require an adjustment in external factors, such as diet or
exercise, to decrease
additional exposure to environmental toxins that may exacerbate liver injury.
In another embodiment, a method of identifying patients for drug treatment is
provided comprising measuring ApoAl levels in a bodily fluid; comparing the
measured
value with a reference measurement of ApoAl levels in a population; and only
providing
drug treatment if ApoAl levels in the fluid are less than or equal to the
reference value. In
certain instances, the reference value is an ULN in a reference population.
In specific embodiments, the patient sample is a serum sample. In other
embodiments, the sample is a plasma sample.
In some embodiments, the ULN is about 165mg/dL of ApoAl in the sample. In
other
6
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
embodiments it is between 150 and 200 mg/dL, between 155 and 195 mg/dL,
between 160
and 190 mg/dL, between 165 and 185 mg/dL. In some embodiments, a patient is
considered
at risk of liver injury if the measured level of ApoAI is greater than 150
mg/dL or greater
than 155 mg/dL or greater than 160 mg/dL or greater than 165 mg/dL or greater
than 170
mg/dL.
In yet another embodiment, a kit is provided for identification of a patient
at risk of a
drug induced liver injury comprising a detection system to measure a level of
ApoAl in a
patient sample and a system to compare the measured levels to a normal level
in a population.
In some embodiments, the detection system can be a labeled antibody to ApoAl
or an
ELISA kit comprising a measuring antibody to ApoAl and a labeled secondary
antibody. In
other embodiments, the detection system can be a binding partner other than an
antibody to
ApoAl. In yet further embodiments, the detection system can detect levels of
the ApoAl
gene product, such as by RT-PCR. The comparison system can be a separate
detection kit in
which the level of ApoAI is standardized to correspond to an upper limit of
normal. The
readout can be on a colorimetric scale or can be based on a direct comparison
of the level of
signal from the detection systems. In other embodiments, a chart is included
in the kit that
allows comparison of the measured ApoA l levels in the sample with an upper
limit of normal
in the population. In certain instances, a visual readout is included which
provides a marker
if the ApoAl level in the sample is greater than 1.0 times the upper limit of
normal. In
specific embodiments, the kit includes a detection apparatus that provides a
marker if the
measured level of ApoAl in the sample is greater than 165 mg/dl.
The methods and kits of the present invention may also be useful for
monitoring and
diagnosing various liver diseases, including early stage tissue injury/organ
rejection, certain
forms of viral infection, drug toxicity, and alterations in liver function.
The methods provide
information not currently available in the clinical arena, and are rapid and
reproducible. The
methods and kits are especially useful to evaluate therapeutic agents and
drugs for their
toxicity with respect to liver damage. The early detection of liver disease by
the methods of
the present invention can additionally permit earlier clinical intervention if
adverse reactions
do occur.
In one aspect, the present invention provides a method for detecting liver
damage or
potential for liver damage in a subject by measuring an ApoAl level in a
sample from the
subject and comparing the level to a normal level. If the ApoAl level exceeds
an upper limit
of normal (ULN), then the patient is considered at greater likelihood of
suffering from or at
risk of suffering from liver damage. Such early diagnosis can be useful in
providing
7
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
motivation for early intervention and to provide information to analyze any
proposed medical
regimens.
BRIEF DESCRIPTION OF THE FIGURES
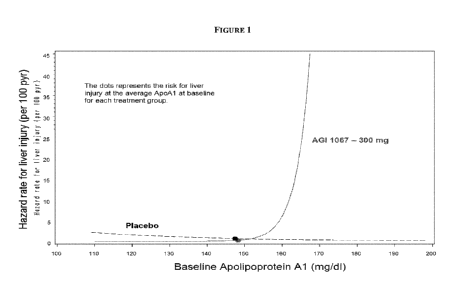
Figure 1 is a graph of Hazard rate for Liver injury as a function of ApoAl
levels in a
patient population and shows age-adjusted effect for the 5th to 95th
percentile range of
baseline ApoAl on subsequent liver events using a Cox Proportional Hazards
Model. As a
point of reference, the ULN for ApoAI was 165 mg/dL for this trial. The solid
line and the
dashed line represent AGI-1067 and placebo data, respectively.
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides methods and compositions useful, e.g. for
clinical
screening, diagnosis and prognosis of liver response in a mammalian subject,
for monitoring
the results of liver response therapy, for identifying patients most likely to
have an adverse
response to a particular therapeutic treatment and for drug screening and drug
development.
In a particular embodiment, the invention provides methods for determining
patients who are
at risk for developing drug-induced hepatotoxicity. For clarity of disclosure,
and not by way
of limitation, the invention will be described with respect to the analysis of
blood or liver
tissue samples. However, as one skilled in the art will appreciate, based on
the present
description, the assays and techniques described herein can be applied to
other types of
samples containing lipoproteins, including a body fluid (e.g. blood or a
fraction of blood
comprising serum or plasma or both, spinal fluid, urine or saliva), a tissue
sample from a
subject at risk of having or developing a liver response (e.g. a biopsy such
as a liver biopsy)
or homogenate thereof
As used herein the phrase "adverse hepatologic event" or the like includes
hepatotoxicity, liver damage and liver disease.
In certain embodiments, the methods and kits of the invention are useful in
identifying
patients at risk of adverse events in response to anti-oxidant agents.
Plasma lipoproteins are carriers of lipids from the sites of synthesis and
absorption to
the sites of storage and/or utilization. Lipoproteins are spherical particles
with triglycerides
and cholesterol esters in their core and a layer of phospholipids,
nonesterified cholesterol and
apolipoproteins on the surface. They are categorized into five major classes
based on their
hydrated density as very large, triglyceride-rich particles known as
chylomicrons (less than
0.95 g/ml), very low density lipoproteins (VLDL, 0.95 to 1.006 g/ml),
intermediate-density
8
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
lipoproteins (IDL, 1.006 to 1.019 g/ml), low-density lipoproteins (LDL, 1.019
to 1.063 g/ml)
and, high-density lipoproteins (HDL, 1.063 to 1.2 10 g/ml). Plasma
lipoproteins can be also
classified on the basis of their electrophoretic mobility. HDL co-migrate with
alpha-
globulins, LDL with beta-globulins, VLDL between alpha and beta-globulins with
so called
pre-beta globulins, whereas chylomicrons remain at the point of application.
(Osborne, J. D.
and Brewer, B. Jr. Adv. Prot. Chem. 31:253-337 (1977); Smith, L. C. et al.
Ann. Rev.
Biochem., 47:751-777 (1978)).
Apolipoproteins are protein components of lipoproteins with three major
functions:
(1) maintaining the stability of lipoprotein particles, (2) acting as
cofactors for enzymes that
act on lipoproteins, and (3) removing lipoproteins from circulation by
receptor-mediated
mechanisms. The four groups of apolipoproteins are apolipoproteins A (Apo A),
B (Apo B),
C (Apo C) and E (Apo E). Each of the three groups A, B and C consists of two
or more
distinct proteins. These are for Apo A: Apo A-I, Apo A-II, and Apo A-IV, for
Apo B: Apo
B-100 and Apo B-48; and for Apo C: Apo C-I, Apo C-II and Apo C-III. Apo E
includes
several isoforms. These apolipoproteins are contemplated for use in the
methods described
herein.
Apo A-I is the major protein constituent of lipoproteins in the high density
range. Apo
A-I may also be the ligand that binds to a proposed hepatic receptor for HDL
removal. A
number of studies support the clinical sensitivity and specificity of Apo A-I
as a negative risk
factor for atherosclerosis (Avogaro, P. et al., Lancet, 1:901-903 (1979);
Maciejko, J. J. et al.,
N. Engl. J. Med., 309:385-389 (1983)). Some investigators have also described
Apo A-I/Apo
B ratio as a useful index of atherosclerotic risk (Kwiterovich, P. O. et al.,
Am. J. Cardiol.,
69:1015-1021 (1992); Kuyl, J. M. and Mendelsohn, D., Clin. Biochem., 25:313-
316 (1992)).
ApoAl comprises 65% of the apolipoprotein of high density lipoprotein (HDL),
providing the structural scaffold for its formation. It is also a co-factor
for lecithin
cholesterol acyl transferase (LCAT), required for esterification of
cholesterol to cholesteryl
esters. HDL-cholesterol is involved in the reverse transport of cholesterol
from peripheral
tissues to the liver, from where it can be excreted. Hence ApoAl deficiency
confers
increased risk of coronary artery and peripheral vascular disease, even in the
absence of other
coronary risk factors. Patients with significant arteriosclerosis generally
have lower plasma
ApoAl concentrations than a normal population. Specific genetic abnormalities
of the
ApoAl gene may be associated with reduced levels of ApoAl and HDL. Reduced
ApoAl
values are also associated with smoking, diets rich in carbohydrates and/or
polyunsaturated
fats, dyslipoproteinaemias (eg familial hypo-alphalipoproteinaemia),
uncontrolled diabetes,
9
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
liver disease, chronic renal failure, and some therapies (beta blockers,
diuretics, progestins,
androgens).
Raised ApoAl concentrations are associated with pregnancy, familial
hyperalphalipoproteinaemia, and with drugs such as carbamazepine, phenytoin,
phenobarbitone, oestrogens, oral contraceptives, ethanol, niacin, fibrates and
statins. Most
genetic hypoalphalipoproteinaemias are caused by mutations in enzymes, and
transporters
involved in reverse cholesterol transport. Mutations in ApoAl are rare and
associated with
amyloidosis, peripheral neuropathy and both increased and decreased risks of
atherosclerosis.
Transcriptional regulation of ApoAI is upregulated by the peroxisome-
proliferator
activated receptor a (PPAR-a). Within the cell, PPAR-a is activated by ligands
such as
oxidized free fatty acids that not only mediate cellular redox signalling but
also represent
hepatocellular responses to stress. ApoAl has well established anti-oxidant
and anti-
inflammatory activities both in vitro and in vivo that can serve to inhibit
redox sensitive
signals driving its hepatic expression. It has now been recognized that the
presence of
modestly elevated ApoAl levels define a patient subgroup, especially in type 2
diabetes
mellitus, characterized by endogenous antioxidant and anti-inflammatory
compensation to
hepatic stress associated with low level inflammatory, oxidant and/or
pharmacologic
challenges. Drugs that confer additional oxidant-like stress that cannot be
further
compensated by the liver can therefore lead to hepatocyte injury, or drugs
that augment
endogenous direct and indirect antioxidant mechanisms may lead to
overcompensation.
ApoB-100 is an integral component of the four major atherogenic lipoproteins:
VLDL, IDL, LDL and Lp(a). Apo B-100 is distinguished from Apo B-48, which is
found
only in lipoproteins of intestinal origin, such as chylomicrons and
chylomicron remnants.
Apo B-48 is usually undetectable in the systemic circulation, except in rare
subjects with
Type I, III, or V hyperlipidemia. Apo B's initial function in VLDL and IDL
appears to be
structural; however, with exposure of binding domains on LDL, it becomes
responsible for
interaction with high-affinity LDL receptors on cell surfaces, which results
in uptake and
removal of LDL from the circulation. Several studies have shown that an
increased Apo B
level in blood is a reliable marker for coronary atherosclerosis (Sniderman,
A. et al., Proc.
Natl. Acad. Sci. USA, 77:604-608 (1980); Kwiterovich, P. O. et al., Am. J.
Cardiol., 71:631-
639 (1993); McGill et al. Coron. Artery Dis., 4:261-270 (1993); Tornvall, P.
et al.,
Circulation, 88:2180-2189 (1993)).
Techniques used for both Apo A-I and B include immunological procedures using
antibodies directed against Apo A-I or B and include radio-immunoassay (RIA),
enzyme
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
immunoassay (ELISA), competitive or capture systems, fluorescence immunoassay,
radial
immunodiffusion, nephelometry, turbidimetry and electroimmunoassay.
Kits and methods using antibodies which are immunoreactive with specific
apolipoproteins are used to determine the concentrations of apolipoproteins
such as ApoAI in
human blood, serum or plasma sample to determine an individual's risk of an
averse hepatic
event after administration of a drug. Useful monoclonal antibodies (MAbs) that
may be used
in these kits and methods are described for example in U.S. Patent No.
7,098,036 that
specifically bind to epitopes present in apolipoproteins and lipoproteins,
enabling rapid and
reliable determinations of levels of specific blood lipoprotein and/or
apolipoprotein levels,
including Apo B-100, Apo A-I, Apo A-11, Apo C-111, and Apo E.
Serum ApoAl (and ApoB) levels are increasingly recognized as better indicators
of
atherosclerotic risk than cholesterol and triglycerides alone. Atherosclerotic
patients are
better distinguished from normal individuals by the finding of increased
plasma ApoB or
decreased plasma ApoAl than by a raised LDL- and low HDL-cholesterol. The
ratio of
ApoAl to ApoB is considered to provide a particularly good index of
cardiovascular risk as
compared to the individual values.
Methods
The present invention encompasses methods of determining or predicting if a
drug,
compound, or other therapeutic agent for use in the treatment for a disease or
other medical
condition will be likely to have hepatotoxic effects, e. g. idiosyncratic
hepatotoxicity, in vivo.
Ideally, such methods are performed prior to administration of the drug (or
combination of
drugs) to a patient or patient population.
In one aspect, the present invention provides a method for detecting liver
damage in a
subject by measuring an apolipoprotein level such as ApoAl level in a sample
from the
subject and comparing the level to a normal level. If the apolipoprotein level
exceeds an
upper limit of normal level, then the patient is considered at greater
likelihood of suffering
from or at risk of suffering from liver damage. Such early diagnosis can be
useful in
providing motivation for early intervention, and to provide information to
analyze any
proposed medical regimens. Certain subjects may also be excluded from
treatment with a
drug if they demonstrate a risk of liver damage.
In certain embodiments, measurements of apolipoprotein such as ApoAI are
supplemented by measurements of ALT and total bilirubin, to identify patients
currently
suffering from hepatic events. As used herein, the term ULN refers to a
predetermined
11
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
apolipoprotein level that is identified from normal individuals in a
population. In related
embodiments, ULN may be measured from a sample of individuals in a geographic
region,
ethnic population or defined by other criteria. The population is then used to
identify a
threshold ULN for later comparison to an apolipoprotein level from a test
patient. The ULN
is the threshold value within which 95 percent of a healthy normal population
falls and is thus
determined as the value by which 5% or 5% or less of the normal population
exceeds this
value.
As referred to herein, the term "population" or "patient population" refers to
a group
of two or more patients. The patient population can be a few patients, a dozen
patients,
hundreds of patients, or thousands of patients. The population can be defined
as patients in
need of treatment for a particular condition, for example diabetes or
atherosclerosis. The
population can be, for example, those involved in a study wherein some
patients are
administered a therapeutic agent and others are administered a placebo. The
term "patient
population" is not meant to be limiting. For example, the term also
encompasses a "reference
population" or two or more people who will not undergo treatment for a
condition.
In certain instances, elevations of ALT above 1.5 times ULN, above 2 times
ULN,
above 2.5 times ULN, above 3 times ULN, above 3.5 times ULN, above 4 times
ULN, above
4.5 times ULN, or above 5 times ULN are diagnostic of hepatic events. In
certain other
instances, total bilirubin levels (TBL) of greater than 1 times ULN, greater
than 1.5 times
ULN and in particular greater than 2 times ULN are also diagnostic of hepatic
events. In
particular, the combination of ALT and TBL above ULN are diagnostic of hepatic
events.
In certain embodiments, a patient is categorized as at risk if the measured
apolipoprotein level exceeds 1.0 of the upper limit of normal (ULN) in a
population, or
exceeds 1.1 ULN, or 1.2 ULN, or 1.3 ULN, or 1.4 ULN, or 1.5 ULN in a
population. In
certain embodiments, the ULN is measured in a geographic population. In
certain other
embodiments, the ULN is measured in a sample of individuals having a disorder.
In
particular, in certain embodiments, the ULN is measured based on a measurement
of
individuals having diabetes. In certain embodiments, these patients are
diagnosed based on a
glycemic parameter, such as a glucose level above 7.0 mmol/L, or a hemoglobin
Alc
(HbAlc) value greater than 7%.
In other embodiments, the patient is diagnosed as at risk if the measured
apolipoprotein level exceeds at least one standard deviation from normal. In
certain
instances, this can be at least 1 or at least 1.5 or at least 2 or greater
standard deviations. The
standard deviation can be calculated based on a sample of at least 100 or at
least 500 or at
12
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
least 1000 individuals.
Different apolipoproteins have different reference levels based on their
normal serum
concentration. In a particular embodiment, the reference level (also the ULN)
of ApoA 1 is
about 150mg/dL, about 155mg/dL, about 160mg/dL, about 165mg/dL, about
170mg/dL,
about 175mg/dL, about 180mg/dL, about 185mg/dL or about about 190mg/dL. In
other
subembodiments, the reference level is between 150 and 200 mg/dL, between 155
and 195
mg/dL, between 160 and 190 mg/dL, between 165 and 185 mg/dL. greater than 150
mg/dL
or greater than 155 mg/dL or greater than 160 mg/dL or greater than 165 mg/dL
or greater
than 170 mg/dL.
In a particular embodiment, the reference level of Apo-A-II is about 30 mg/dL,
about
35 mg/dL, about 40 mg/dL, about 45 mg/dL, or about 50 mg/dL. In other
embodiments, the
reference level is between 10-50 mg/dL, between 20-40 mg/dL or greater than 50
mg/dL.
In a particular embodiment, the reference level of Apo-A-IV is about 30 mg/dL,
about 35 mg/dL, about 40 mg/dL, about 45 mg/dL, or about 50 mg/dL. In other
embodiments, the reference level is between 10-50 mg/dL, between 20-40 mg/dL
or greater
than 50 mg/dL.
In a particular embodiment, the reference level of Apo-B is about 120 mg/dL,
about
125 mg/dL, about 130 mg/dL, about 135 mg/dL, or about 145 mg/dL. In other
embodiments,
the reference level is between 100-150 mg/dL, between 120-140 mg/dL or greater
than 150
mg/dL.
In a particular embodiment, the reference level of Apo-C-II is about 5 mg/dL,
about 7
mg/dL, about 8 mg/dL, about 10 mg/dL, or about 15 mg/dL. In other embodiments,
the
reference level is between 3-8 mg/dL, between 4-6 mg/dL or greater than 10
mg/dL.
In a particular embodiment, the reference level of Apo-C-III is about 10
mg/dL,
about 12 mg/dL, about 15 mg/dL, about 17 mg/dL, or about 20 mg/dL. In other
embodiments, the reference level is between 5-15 mg/dL, between 8-12 mg/dL or
greater
than 20 mg/dL.
In a particular embodiment, the reference level of Apo-E is about 5 mg/dL,
about 7
mg/dL, about 8 mg/dL, about 10 mg/dL, or about 15 mg/dL. In other embodiments,
the
reference level is between 3-8 mg/dL, between 4-6 mg/dL or greater than 10
mg/dL.
Methods of Detection
In certain embodiments, levels of apolipoprotein can be measured in serum or
plasma
or other bodily fluidsamples from the patient. The apolipoprotein levels can
be measured by
13
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
any suitable means, but for example, can be measured using an antibody to an
epitope of the
apolipoprotein protein. In some embodiments, the levels of apolipoprotein are
measured
using an antibody assay such as ELISA. ELISA kits for quantitative
determination of native
and recombinant human apolipoprotein in plasma or serum samples are
commercially
available, such as from Mabtech AB. Such a kit can contain a capture Ab, such
as a
monoclonal antibody, a labeled detection mAb, astreptavidin-enzyme conjugate
HRP and a
purified apolipoprotein as a standard.
In other embodiments, apolipoprotein gene expression is measured using, for
example, RT-PCR. In certain instances, ApoAl transcription can be altered by
the
underlying disorders and the levels of mRNA in an individual's sample can be
predictive of
the individual's risk of a DILI.
In one embodiment, a method of identifying a patient at risk of a liver
injury, and in
particular a drug-induced liver injury, is provided comprising 1) measuring
the level of
apolipoprotein in a bodily fluid from the patient; and 2) comparing the
measured level of
apolipoprotein in the sample with an ULN in the patient population. A value
greater than the
ULN is a predetermined level of apolipoprotein that is used as a reference
level for
determining risk of a liver injury after a patient is administered a drug. In
one embodiment
the apolipoprotein is ApoA I. Other apolipoproteins are contemplated in the
methods
described herein.
In one embodiment, the drug is a monoester of probucol, for example the
monosuccinic acid ester of probucol.
In another embodiment, a method of identifying a patient at risk of a liver
injury, and
in particular a drug-induced liver injury, is provided comprising 1) measuring
the level of
ApoAl in a bodily fluid from the patient; and 2) comparing the measured level
of ApoAl in
the sample to the reference level of ApoA 1. If the measured level of ApoA 1
is higher that
the reference level, the patient may be at greater risk for liver injury than
a patient with an
ApoAl level less than or equal to the reference level. It is then possible to
exclude a patient
from drug treatment using this information.
Methods are also provided for assessing or screening for liver injury, damage
or
disease in a human is provided by 1) measuring the level of apolipoprotein,
such as ApoAl in
a bodily fluid from the patient; and 2) comparing the measured level of
apolipoprotein in the
sample with an ULN in the patient population, where an apolipoprotein level
higher than the
ULN indicates liver injury, damage or disease.
In another embodiment, a method is provided for assessing or screening for
liver
14
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
injury, damage or disease in a human is provided by 1) measuring the level of
apolipoprotein
such as ApoA 1 in a bodily fluid from the patient; and 2) comparing the
measured level of
apolipoprotein in the sample to a predetermined reference level of
apolipoprotein where an
apolipoprotein level higher than the ULN indicates liver injury, damage or
disease.
In one embodiment, a method is provided for diagnosing hepatic events in a
human, is
provided by 1) measuring the level of apolipoprotein in a bodily fluid from
the patient; and 2)
comparing the measured level of apolipoprotein in the sample with an ULN in
the patient
population where an apolipoprotein higher than the ULN indicates a hepatic
event.
In another embodiment, a method is provided for assessing or screening for
liver
injury, damage or disease in a human is provided by 1) measuring the level of
apolipoprotein
in a bodily fluid from the patient; and 2) comparing the measured level of
ApoAl in the
sample to a predetermined reference level of apolipoprotein where an
apolipoprotein level
higher than the ULN indicates liver injury, damage or disease.
In certain instances, a patient who has one or more samples in which the
measured
level of apolipoprotein such as ApoAI is greater than ULN is considered at
increased risk of
liver injury, in particular at greater risk of drug-induced liver injury.
A patient who has one or more samples in which measured apolipoprotein level,
such
as ApoAl, exceeds ULN may also be considered to have increased inflammatory
activity.
Such a patient may be considered at risk for additional disorders, including
inflammatory
disorders such as rheumatoid arthritis. In certain instances, the patient is
at risk of or
suffering from a disorder in glucose metabolism. Such a disorder may be
diabetes mellitus
and in particular may be type 2 diabetes mellitus.
In certain instances, a treatment protocol will be designed based on the
results of an
ApoAI measurement. This treatment protocol may require that antioxidant drugs
not be
given to a patient whose ApoAl measurement exceeds ULN, or it may be that the
patient is
closely monitored for hepatotoxicity. In addition, the treatment protocol may
require an
adjustment in external factors, such as diet or exercise, to decrease
additional exposure to
environmental toxins that may exacerbate liver injury.
In another embodiment, a method of identifying patients for drug treatment is
provided comprising measuring ApoAl levels in a bodily fluid; comparing the
measured
value with an ULN of ApoAl levels in a population; and only providing drug
treatment if
ApoAl levels in the fluid are less than or equal to ULN.
In specific embodiments, the patient sample is a serum sample. In other
embodiments, the sample is a plasma sample.
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
In some embodiments, the methods further comprise measuring a level of ALT in
a
sample from the patient and comparing that to a reference ALT level in the
population. In
these instances, a measurement of ALT that exceeds ULN is also used to
classify the patients
as at increased risk of an adverse liver injury. In certain instances, the
measurement of ALT
is taken before any drug is administered. In these instances, the ALT level
can be used as a
further exclusionary criteria to identify patients who are at increased risk
of a drug induced
liver injury. In some instances, an ALT level of greater than ULN is
identified, but in other
instances an ALT level of at least 1.5 or at least 2.0 or greater ULN is
provided as criteria for
exclusion. In certain instances, ALT levels are measured in a patient
receiving a drug after a
period of time, such as at one week, two weeks, three weeks, four weeks, five
weeks or more
after commencing a therapeutic regimen. Patients whose ALT level is measured
as
exceeding ULN may be considered at increased risk of, or suffering from, liver
toxicity.
Drug-induced liver injury can occur in patients who have been treated with one
or
more drugs. A wide variety of drugs can induce liver injuries or damage,
including but not
limited to PPAR agonists, anti-inflammatory drugs, HIV protease inhibitors,
neurological
drugs, estrogenic and anti-estrogenic drugs, anti-angina drugs, muscle
relaxants, anti-
psychotic drugs, antihistamines, and other drugs, compounds, and therapeutic
agents. In
certain embodiments, the drug is an anti-cancer, anti-bacterial, anti-fungal,
anti-viral, anti-
hypertension, anti-depression, anti-anxiety, and anti- arthritis agent. In
another embodiment,
the drug is for the treatment of allergies, diabetes, hypercholesteremia,
osteoporosis,
Alzheimer's disease, Parkinson's disease, and/or other neurodegenerative
diseases, and
obesity.
Non-limiting examples of PPAR agonists include Pioglitazone, Rosiglitazone,
Tesaglitazar, Ragaglitazar, Troglitazone, Farglitazar, Ciglitazone, Azelaoyl
PAF, 2-
Bromohexadecanoic acid, Clofibrate, 15-Deoxy-d12, 14-prostaglandin,
Fenofibrate, Fmoc-
Leu-OH, GW1929, GW7647, 8 (S)-Hydroxy- (5Z, 9E, 11Z, 14Z) -eicosatetraenoic
acid (8 (S)
-HETE), Leukotriene B4, LY-171,883 (Tomelukast), Prostaglandin A2,
Prostaglandin J2,
Tetradecylthioacetic acid (TTA), WY-14643 (Pirinixic acid), and NN622 (Novo
Nordisk,
A/S), and related substances.
Non-limiting examples of anti- anxiety and anti-psychotic drugs include
Hydroxyzine
Hydrochloride, Lorazepam, Buspirone Hydrochloride, Pazepam, Chlordiazepoxide,
Meprobamate, Oxazepam, Trifluoperazine, Clorazepate Dipotassium, Diazepam,
Clozapine,
Prochlorperazine, Haloperidol, Thioridazine, Thiothixene, Risperidone,
Trifluoperazine
Hydrochloride, Chlorpromazine, and related substances. Non-limiting examples
of HIV
16
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
protease inhibitors include Saquinavir, Amprenavir, Ritonavir, Nelfinavir,
Indinavir,
Atazanavir (BMS232632; Bristol-Myers Squibb), Fosamprenavir (GW433908 ;
GlaxoSmithKline), L-756,423 (Merck), Mozenavir (DMP450; Triangle
Pharmaceuticals),
Tipranavir (PNU-140690; Boehringer Ingelheim); R0033-4649 (Roche) TMC114
(Tibotec
Virco), and related substances.
Non-limiting examples of anti-inflammatory drugs include Diclofenac,
Diflunisal,
Etodolac, Fenoprofen, Flurbiprofen, Ibuprofen, Indomethacin, Ketoprofen,
Ketorolac,
Meclofenamate, Mefenamic Acid, Nabumetone, Naproxen, Oxaprozin, Piroxicam,
Sulindac,
Tolmetin, and related substances. Non-limiting examples of antihistimines
include Azelastine
(Astelin), Fexofenadine (e. g., Allegra), Cetirizine (e. g., Zyrtec,
Desloratadine (e. g.,
Clarinex), Loratadine (e. g., Claritin, Alavert), Astemizole, Azatadine,
Brompheniramine,
Chlorpheniramine, Clemastine, Cyproheptadine, Dexchlorpheniramine,
Dimenhydrinate,
Diphenhydramine, Doxylamine, Hydroxyzine, Phenindamine, Pyrilamine,
Terfenadine,
Tripelennamine, Triprolidine, Methdilazine, Promethazine, Trimeprazine,
Diphenhydramine
Liquid, and related substances. Non-limiting examples of muscle relaxants
include
Dantrolene (e. g., Dantrium), Baclofen (e. g., Lioresal, Carisoprodol (e. g.,
Soma;),
Chlorphenesin (e. g., Maolate;), Chlorzoxazone (e. g., Paraflex),
Cisatracurium,
Cyclobenzaprine (e. g., Flexerilt)), Dantrolene, Diazepam (e. g., Valium;),
Metaxalone (e. g.,
Skelaxin;), Gallamine, Methocarbamol (e. g., Robaxin;), Mivacurium,
Orphenadrine (e. g.,
Norflex), Pancuronium, Rocuronium, Tizanidine, Suxamethonium, Vecuronium, and
related
drugs.
Non-limiting examples of estrogens and anti-estrogens include conjugated
estrogens
(e. g., Premarin, esterified estrogens (e. g., Estratabg, Menestg,
Estratest;), synthetic
conjugated estrogens (e. g., Cenestin), Estropipate (e. g., Ogen, Ortho-Est),
Ethinyl Estradiol
(e. g., Estinyl), Desogestrel, Diethylstilbestrol (e. g., Stilphostrol),
Dienestrol (e. g. , Ortho
Dienestrol), Chlorotrianisene (Tace, Estradiol (e. g., Estrace, Alora,
Climara, Vivelle),
Estradiol Cypionate (e. g., Depo-Estradiolg, Depogens, Dura-Estring, Estra-De,
Estro-Cyp,
Estroject-LA, Estronol-LA), Estropipate, Ethacrynic Acid, Ethynodiol
Diacetate,
Levonorgestrel, Medroxyprogesterone, Medroxyprogesterone Acetate, Mestranol,
Norethindrone, Norgestimate, Norgestrel, Tamoxifen (e. g., Nolvadex),
Toremifene (e. g.,
Fareston;), Raloxifene (e. g., Evista), Megestrol Acetate (Megace,
Aminogluthethimide (e. g.,
Cytadren), Anastrozole (e. g., Arimidex;), Letrozole (e. g., Femara,
Exemestane (e. g.,
Aromasin), Goserelin (e. g., Zoladex, Leuprolide (e. g., Lupron), and related
substances.
Non-limiting examples of anti-angina drugs include Calan SR, Isoptin, Isoptin
SR,
17
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
Verelan, Nicardipine Hydrochloride, Diltiazem Hydrochloride, Nadolol,
Isosorbide
Mononitrate, Isosorbide Dinitrate, Metroprolol Tartrate, Nitroglycerin,
Amlodipine Besylate,
Nifedipine, Atenolol, and related drugs.
In some embodiments, the drug induced liver injury is from an antioxidant
drug. In
certain other instances, the drug induced liver injury is from a drug that
increases PPAR
activity.
These methods may be used to identify a patient at risk for an adverse hepatic
event or
presently having an adverse hepatic event when administered a drug to treat a
disease. The
disease is not critical for the methods described herein and diseases for
which drugs are
administered may be grouped into three main categories: neoplastic disease,
inflammatory
disease, and degenerative disease.
Examples of diseases include, but are not limited to, metabolic diseases
(e.g., obesity,
cachexia, diabetes, anorexia, etc.), cardiovascular diseases (e.g.,
atherosclerosis,
ischemia/reperfusion, hypertension, myocardial infarction, restenosis,
cardiomyopathies,
arterial inflammation, angina, etc.), immunological disorders (e.g., chronic
inflammatory
diseases and disorders, such as Crohn's disease, inflammatory bowel disease,
reactive
arthritis, rheumatoid arthritis, osteoarthritis, including Lyme disease,
insulin-dependent
diabetes, organ-specific autoimmunity, including multiple sclerosis,
Hashimoto's thyroiditis
and Grave's disease, contact dermatitis, psoriasis, graft rejection, graft
versus host disease,
sarcoidosis, atopic conditions, such as asthma and allergy, including allergic
rhinitis,
gastrointestinal allergies, including food allergies, eosinophilia,
conjunctivitis, glomerular
nephritis, certain pathogen susceptibilities such as helminthic (e.g.,
leishmaniasis) and certain
viral infections, including HIV, and bacterial infections, including
tuberculosis and
lepromatous leprosy, etc.), myopathies (e.g. polymyositis, muscular dystrophy,
central core
disease, centronuclear (myotubular) myopathy, myotonia congenita, nemaline
myopathy,
paramyotonia congenita, periodic paralysis, mitochondrial myopathies, etc.),
nervous system
disorders (e.g., neuropathies, Alzheimer's disease, Parkinson's disease,
Huntington's disease,
amyotropic lateral sclerosis, motor neuron disease, traumatic nerve injury,
multiple sclerosis,
acute disseminated encephalomyelitis, acute necrotizing hemorrhagic
leukoencephalitis,
dysmyelination disease, mitochondrial disease, migrainous disorder, bacterial
infection,
fungal infection, stroke, aging, dementia, peripheral nervous system diseases
and mental
disorders such as depression and schizophrenia, etc.), oncological disorders
(e.g., leukemia,
brain cancer, prostate cancer, liver cancer, ovarian cancer, stomach cancer,
colorectal cancer,
throat cancer, breast cancer, skin cancer, melanoma, lung cancer, sarcoma,
cervical cancer,
18
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
testicular cancer, bladder cancer, endocrine cancer, endometrial cancer,
esophageal cancer,
glioma, lymphoma, neuroblastoma, osteosarcoma, pancreatic cancer, pituitary
cancer, renal
cancer, and the like) and ophthalmic diseases (e.g. retinitis pigmentosum and
macular
degeneration). The term also includes disorders, which result from oxidative
stress, inherited
cancer syndromes, and metabolic diseases.
The methods and kits of the present invention may also be useful for
monitoring and
diagnosing various liver diseases, including early stage tissue injury/organ
rejection, certain
forms of viral infection, drug toxicity, and alterations in liver function.
The methods provide
information not currently available in the clinical arena, and are rapid and
reproducible. The
methods and kits are especially useful to evaluate therapeutic agents and
drugs for their
toxicity with respect to liver damage. The early detection of liver disease by
the methods of
the present invention can additionally permit earlier clinical intervention if
adverse reactions
do occur.
Kits
The present invention also provides kits for determining or predicting in vivo
hepatoxicity in a patient or patient population prior to or during
administration of a drug,
compound, or other therapeutic agent. Such kits are useful in clinical or pre-
clinical settings,
and can be used concurrently with various stages of patient trials.
In one embodiment, a kit is provided for identification of a patient at risk
of a drug
induced liver injury comprising a detection system to measure a level of
apolipoprotein such
as ApoAl in a patient sample and a system to compare the measured levels to a
normal level
in a population. The patient sample may be in the form of a bodily fluid, such
as blood and
blood plasma, mucus, saliva, serum, or urine. In some embodiments, the
detection system in
the kit can be a labeled antibody to ApoAl or an ELISA kit comprising a
measuring antibody
to ApoAl and a labeled secondary antibody. In other embodiments, the detection
system can
be a binding partner other than an antibody to apolipoprotein. In yet further
embodiments,
the detection system can detect levels of the apolipoprotein gene product,
such as by RT-
PCR. The comparison system can be a separate detection kit in which the level
of ApoAl is
standardized to correspond to an upper limit of normal. The readout can be on
a colorimetric
scale or can be based on a direct comparison of the level of signal from the
detection systems.
In other embodiments, a chart is included in the kit that allows comparison of
the measured
ApoAI levels in the sample with an upper limit of normal in the population.
In certain instances, a visual readout is included which provides a marker
signal if the
19
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
ApoAl level in the sample is greater than 1.0 times the upper limit of normal.
In specific
embodiments, the kit includes a detection apparatus that provides a marker
signal if the
measured level of ApoAl in the sample is greater than 165 mg/dl. In one
embodiment, the
predetermined level is a part of the kit so that a minimum concentration of
apolipoprotein is
required for the kit to identify a positive result. Only individuals having an
apolipoprotein
concentration greater than the predetermined level will show a positive result
when using the
kit.
In one embodiment, the kits contain compositions of strips of a solid phase
material
coated with one or more of the antibodies and are referred to herein as
"dipsticks". The
dipsticks specifically bind an apolipoprotein when dipped into a protein
sample. The amount
of apolipoprotein bound on the dipstick is quantitated using an appropriate
method, for
example, by staining with a lipid stain or reaction with a second labeled
antibody. The
intensity of the stain on the dipstick is proportional to the concentration of
the apolipoprotein
circulating in the blood and can be quantitated by comparison with standards
containing
known amounts of lipid. The dipsticks can be provided alone or in kits which
enable the lay
person to carry out the assay without the need of a physician or technical
laboratory. In one
embodiment, the concentration of anti-apolipoprotein antibody, or other
binding element on
the dipstick is only sufficient to detect a concentration of apolipoprotein
greater than the
predetermined level. In this regard, a positive result on the dipstick will
only appear when a
concentration of apolipoprotein in the test sample exceeds the predetermined
level.
Monoclonal antibodies to apolipoproteins can be used not only as components of
dipsticks, but also in a variety of other dignostic kits, including enzyme
immunoassays,
radioimmunoassays as well as fluorescent and chemiluminescent immunoassays to
determine
apolipoproteins in biological samples with which they are immunoreactive.
Antibodies can be bound to a solid phase material for use in assays described
herein.
Various types of adsorptive materials, such as nitrocellulose, ImmobilonTM ,
polyvinyldiene
difluoride (all from BioRad, Hercules, Calif.) can be used as a solid phase
material to bind
the anti-lipoprotein antibodies. Other solid phase materials, including resins
and well-plates
or other materials made of polystyrene, polypropylene or other synthetic
polymeric materials
can also be used. In the preferred embodiment for assaying apolipoprotein
concentrations,
pieces or strips of these materials are coated with one or more antibodies, or
functional
fragments thereof, directed against specific epitopes of apolipoproteins for
use in patient
samples. The dipsticks may also be attached to one end of a longer strip of a
solid support
material, such as plastic, which can serve as a handle for dipping a dipstick
into a solution or
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
sample, such as a sample of whole blood, blood plasma, or blood serum. The
plastic handle
can also serve as a tether so that multiple dipsticks can be attached to a
common support.
Such a multi-strip design may be particularly useful in a set-up for testing
multiple
apolipoproteins simultaneously.
Although various sizes of dipsticks are possible, in one embodiment, pieces of
the
solid phase material that are coated with antibody have the general dimensions
of 0.5 cm x
0.5 cm and can be attached to the longer solid support strips having general
dimensions of 0.5
cm x 5 cm. Such dimensions permit an accurate determination of apolipoprotein
levels in as
little as 100 pL of blood.
The dipsticks useful in the claimed methods contain one or more regions
containing
immobilized antibodies specific for particular epitopes on apolipoproteins or
lipoproteins.
Examples of antibody-conjugated diagnostic dipticks are described for example,
but not
limited to, U.S. Patent Nos. 7,098,036; 6,808,889, and 6,087,185.
A dipstick may contain more than one antibody so that the single dipstick can
be used
to detect more than one apolipoprotein. For example, two or more separate
pieces of a solid
phase material, each coated with an antibody directed against a particular
apolipoprotein or
lipoprotein, can be attached to a longer strip of solid support to produce a
dipstick with two or
more separate areas, each specific for a particular apolipoprotein. The means
to attach the
solid phase material to a solid support should not impair the function of the
molecules coated
on the solid phase material and must be secure enough to withstand soaking in
whole blood,
serum, plasma, and the other solutions described herein which are used to
wash, stain, and
preserve the dipsticks. A preferred method of attaching antibody-coated solid
phase material
to a longer strip of solid support is to use a glue or cement such an acrylate
adhesive (for
example, SUPER GLUETM, Super Glue Corporation, Hollis, N.Y.; DUROTM, Loctite
Corporation, Cleveland, Ohio).
Dipsticks can be designed for quantification of one or more apolipoproteins in
a
sample from a test patient. In one embodiment, dipsticks designed for
quantification of a
apolipoprotein contain a single antigen-binding area which is dipped into a
sample, stained
for bound lipid lipoproteins or apolipoprotein, and visually compared with a
set of printed
colored standards to determine the concentration of the particular lipoprotein
or
apolipoprotein.
In addition, dipsticks can be designed for detecting a change in the relative
level of
particular apolipoproteins in a sample. Dipsticks can be designed for
detecting a change in
the relative level of specific apolipoproteins which contain two antigen-
binding areas, each
21
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
area coated with a different antibody. After processing the dipstick to detect
the
apolipoprotein antigens bound by each antibody, the relative intensities of
the colors in the
two areas of the dipstick are compared as an indication of the relative
concentrations of the
two antigens in the blood.
A determination of relative levels of specific apolipoproteins can also be
made by
simultaneously using two separate dipsticks. However, a single dipstick with
two antigen
binding areas is generally easier to use, especially for the lay person, and
an assessment of
relative color intensities in two areas in close proximity on a single
dipstick is relatively easy
to make even for the untrained observer.
In another embodiment, dipsticks are made that contain distinct areas or spots
of
known amounts of molecules whose levels are to be determined by the dipstick.
For example,
known amounts of lipid, lipoproteins and/or apolipoproteins are placed on the
dipsticks using
methods such as those used for attaching antibodies to the solid phase
material described
above. Such known amounts of lipids, lipoproteins, and apolipoproteins present
on dipsticks
act as "internal standards", whose staining intensity can be compared to that
in the antigen-
binding areas of the dipstick in order to estimate the amount of antigen bound
by the
antibodies on the dipstick.
Important reagents in these methods include antibodies or functional fragments
of the
antibodies, which specifically recognize and bind a particular lipoprotein,
leaving other
lipoproteins in the sample unadsorbed. In order to assay a sample of whole
blood, serum or
plasma for apolipoproteins, dipsticks are incubated with EDTA-treated or
heparinized blood
for 2 to 5 minutes at room temperature. After incubation, each strip is washed
to remove
unbound blood, (for example, under tap water for 0.5 to 1 minute at
temperatures not
exceeding 40C. The dipsticks are then stained, for example, by immersing the
dipsticks in a
solution of stain such as Sudan Red 7B for 2 to 5 minutes at room temperature
to stain the
lipid present in the bound lipoprotein particles. Excess stain is then removed
by an additional
wash. Residual moisture or stain may be drawn off by touching an absorbent
towel with the
edge of dipstick. The "face" of the dipstick, that is, the side of the
dipstick containing
immobilized antibody, should not be blotted, which might disturb the
immobilized antibody
and/or bound antigen. After drying, the intensity of the staining can be
compared with
standardized colored strips to determine the concentration of lipoprotein in
the blood.
A number of other lipid stains such as Oil Red 0 or Sudan Black B can be also
used
for staining of dipsticks. However, in the preferred embodiment, Sudan Red 7B,
also known
as Fat Red 7B (Sigma, St. Louis, Mo.), dissolved in a mixture of methanol and
NaOH is used
22
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
because of its high color intensity. In another embodiment lipoproteins are
stained prior to
being bound to antibody ("pre-stained"), such as antibody on a dipstick, using
any of the
above mentioned lipid stains dissolved in propylene glycol (Wollenweber, J.
and Kahlke, W.,
Clin. Chim. Acta, 29:411-420 (1970)). The pre-stained blood, plasma or serum
sample is then
incubated, for example, with anti-LDL or anti-HDL dipsticks. After washing and
drying, the
quantity of pre-stained lipoprotein captured by the dipstick is determined
visually according
to the intensity of the color, for example, by comparison with a set of
printed colored
standards.
Many detectable labels, reporters, moieties are known in the art and can be
used with
the invention. For example, the detectable moiety may be chromogenic,
fluorogenic, or
luminescent, or may be a member of a specific binding pair, a substance
detectable by an
antibody in any of the known immunoassay methods. There are many different
labels and
methods of labeling known to those of ordinary skill in the art. Examples of
the types of
labels which can be used in the present invention include enzymes,
radioisotopes, fluorescent
compounds, colloidal metals, chemiluminescent compounds, phosphorescent
compounds,
and bioluminescent compounds. Those of ordinary skill in the art will know of
other suitable
labels for binding to apolipoprotein such as ApoAl, a compound that binds
apolipoprotein or
an antibody that binds apolipoprotein, or will be able to ascertain such,
using routine
experimentation.
Modifications and variations of the present invention will be obvious to those
skilled
in the art from the foregoing. All of these embodiments are considered to fall
within the
scope of this invention.
EXAMPLES
Analyses were conducted to identify potential risk factors for drug-induced
liver
toxicity in clinical studies of the monosuccinic acid ester of probucol (AGI-
1067). AGI-1067
represents a new class of therapies targeting certain chronic diseases such as
type II diabetes
mellitus and atherosclerosis. AGI-1067 functions both as a direct antioxidant
and an inducer
of endogenous, antioxidant processes such as hemeoxygenase-1 and thioredoxin.
In a large clinical study (more than 6000 pts, 2 year average exposure), AGI-
1067 was
shown to reduce hard cardiovascular endpoints (a composite of cardiovascular
death, non-
fatal myocardial infarction (MI) and non-fatal stroke) in well-treated
patients with pre-
existing coronary artery disease and to lower HbAlc levels in a large subset
of these patients
with type 2 diabetes mellitus. In the same study, a small number of the 3078
patients
23
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
randomized to treatment with AGI-1067 exhibited reversible elevations in liver
function tests
with eight of those patients demonstrating elevated ALT and bilirubin compared
with four
patients in the placebo arm.
Example 1: ALT elevation alone predicts hepatotoxicity poorly
There were 36 patients who had peak elevations of ATL between 3X and <5X ULN
in the combined dataset of diabetes mellitus patients. A summary of the data
by treatment
group is shown in Table 1. The incidence of ALT elevations in this range was
comparable
for the AGI-1067 and placebo treated groups of patients. ALT levels therefore
appear to
have limited value in assessing potential for hepatotoxicity.
Table 1.
Number of
Patients
Percent of ALT Percent
Randomized 3X to <5X ULN of ALT
Diabetes Patients With Elevations
TBL<2X ULN
Placebo 43% 19 53%
AGI-1067 57% 17 47%
75 m 9% 3 8%
150 mg 9% 4 11%
300 m 39% 10 28%
Total 36
Example 2: ALT elevations in combination with total bilirubin levels is a
useful
indicator of hepatic events
There were 24 diabetes patients in the combined dataset who had hepatic events
as
using a criteria of either ALT >5X ULN plus TBL <2X ULN or ALT >3X ULN plus
TBL
>2X ULN criteria. Table 2 summarizes the patients by treatment arm and by dose
of AGI-
1067. At randomization, 57% of the patients were in the AGI-1067 arm and 43%
were in the
placebo arm resulting in an AGI-1067 to placebo ratio of 1.3. Of the 24
hepatic events, 17
occurred in AGI-1067 treated patients and 7 occurred in placebo treated
patients.
24
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
Table 2.
BEFORE USE OF THE RISK IDENTIFICATION TOOL
Number of Hepatic Events
Percent of ALT >5X ULN ALT >3X ULN Total Percent of
Randomized With Plus Hepatic
Diabetes TBL <2X ULN TBL >2X ULN Events
Patients
Placebo 43% 4 3 7 29%
AGI-1067 57% 13 4 17 71%
75 mg 9% 4 0 4 17%
150 m 9% 2 0 2 8%
300 m 39% 7 4 11 46%
Total 17 7 24
Example 3: ApoAl levels are directly correlated to adverse liver events.
ApoAl measurement was both sensitive and specific for identifying subsequent
hepatic events. The events were defined as a measurement of ALT >5X ULN with
TBL <2X
ULN or ALT >3X ULN with TBL >2X ULN. Figure 1 was generated from type 2
diabetes
mellitus patient data shows age-adjusted effect for the 5th to 95th percentile
range of baseline
ApoAl on subsequent liver events using a Cox Proportional Hazards Model. As a
point of
reference, the ULN for ApoAl was 165 mg/dL for this trial. The solid line and
the dashed
line represent AGI-1067 and placebo data, respectively.
Example 4: Measurement ofApoAl provides a useful tool for identifying patients
at
risk of drug induced liver toxicities
As noted in Example 2, hepatic events in the sample populations were
distributed
71% (17/24) for AGI-1067 treated patients and 29% (7/24) for patients
receiving placebo
resulting in an AGI-1067 to placebo ratio of 2.4. When patients in whom ApoAl
measurements before administration of any drug exceeded ULN (here, 165 mg/dl)
were
excluded from the sample, the number of hepatic events achieved parity for the
AGI-1067
and placebo treatment arms.
There were 14 patients in the combined dataset who had hepatic events using
the ALT
>5X ULN plus TBL <2X ULN or ALT >3X ULN plus TBL >2X ULN criteria. Table 3
summarizes the patients by treatment arm and by dose of AGI-1067. As noted in
Example 2,
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
at randomization the ratio of AGI-1067 to placebo patients was 1.3, the same
value as the
randomization ratio for AGI-1067 compared to placebo.
When patients with elevated ApoAl levels or ALT levels of greater than 2.0
times
ULN were excluded, 10 hepatic events were eliminated. Of these, 90% were in
the AGI-
1067 treatment arm. Of the remaining 14 hepatic events, 8 were in AGI-1067
treated patients
while 6 were in placebo treated patients. These hepatic events were
distributed 57% (8/14)
for AGI-1067 patients and 43% (6/14) for patients receiving placebo. This
resulted in an
AGI-1067 to placebo ratio of 1.3.
Table 3.
USING THE RISK IDENTIFICATION TOOL
Number of Hepatic Events
Percent of ALT >5X ULN ALT >3X ULN Total Percent
Randomized With Plus of
Diabetes TBL <2X ULN TBL >2X ULN Hepatic
Patients Events
Placebo 43% 3 3 6(114%) 43%
AGI-1067 57% 6 2 8(153%) 57%
75 m 9% 2 0 2 14%
150 mg 9% 1 0 1 7%
300 m 39% 3 2 5 36%
Total 9 5 14 (J,42%)
Thus, application of the exclusionary criteria resulted in very low rates of
hepatic
events with an incidence of 0.4% for both the AGI- 1067 (8/1851) and placebo
(6/1419)
groups.
Table 4 summarizes the impact of the exclusionary criteria (primarily ApoAI
elevation) on the number, frequency distribution and ratio of hepatic events
for the AGI-1067
and placebo groups in the 3270 type 2 diabetes mellitus patients contained in
the datasets.
26
CA 02735582 2011-02-28
WO 2010/025410 PCT/US2009/055430
Table 4.
Before Use of the Risk Using the Risk
Identification Tool Identification Tool
Percent of Number of Percent of Number of Percent of
Randomize Hepatic Hepatic Hepatic Hepatic
d Diabetes Events Events Events Events
Patients
Placebo 43 % 7 29 % 6 43 %
AGI-1067 57% 17 71% 8 57%
Ratio
1067/Placebo 2.4 1.3
Hepatic
Events
Table 5 shows that using Baseline ApoAl as a "Predictive Biomarker" would have
resulted in seven times more excluded hepatic events for the AGI-1067 patients
than for the
placebo patients. ALT >2X ULN could also be used as a secondary indicator for
exclusion of
patients.
Table 5.
Number of Hepatic Events Excluded
Biomarker AGI-1067 Placebo
Apo Al >165m /dl 7 1
Baseline ALT >2X ULN 1 0
Month 1 ALT >2X ULN 1 0
Totals 9 1
27