Note: Descriptions are shown in the official language in which they were submitted.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TRANSGENIC PLANTS WITH ENHANCED GROWTH CHARACTERISTICS
STATEMENT AS TO RIGHTS TO INVENTIONS MADE UNDER
FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT
This invention was made with government support under Contract No. W-740 -
ENG- 6 awarded by the United States Department of Energy to The Regents of
The University of California, and Contract No. DE-AC -06NA 9 , awarded by
the United States Department of Energy to Los Alamos National Security, LLC.
The government has certain rights in this invention.
RELATED APPLICATIONS
This application claims priority to United States Provisional Application No.
611190, 20 filed August 29, 2008.
l
BACKGROUND OF THE INVENTION
As the human population increases worldwide, and available farmland continues
to be destroyed or otherwise compromised, the need for more effective and
sustainable agriculture systems is of paramount interest to the human race.
Improving crop yields, protein content, and plant growth rates represent major
objectives in the development of agriculture systems that can more effectively
respond to the challenges presented.
In recent years, the importance of improved crop production technologies has
only
increased as yields for many well-developed crops have tended to plateau. Many
agricultural activities are time sensitive, with costs and returns being
dependent
upon rapid turnover of crops or upon time to market. Therefore, rapid plant
growth
is an economically important goal for many agricultural businesses that
involve
high-value crops such as grains, vegetables, berries and other fruits,
0
Genetic engineering has and continues to play an increasingly important yet
controversial role in the development of sustainable agriculture technologies.
A
large number of genetically modified plants and related technologies have been
developed in recent years, many of which are in widespread use today
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
(Factsheet: Genetically Modified Crops in the United States, Pew Initiative on
Food and Biotechnology, August 2004, (p wa biotec .org/resour s/fact ets).
The adoption of transgenic plant varieties is now very substantial and is on
the
rise, with approximately 250 million acres planted with transgenic plants in
2006.
While acceptance of transgenic plant technologies may be gradually increasing,
particularly in the United States, Canada and Australia, many regions of the
World
remain slow to adopt genetically modified plants in agriculture, notably
Europe,
Therefore, consonant with pursuing the objectives of responsible and
sustainable
agriculture, there- is a strong interest in the development of genetically
engineered
plants that do not introduce toxins or other potentially problematic
substances into
plants and/or the environment. There is also a strong interest in minimizing
the
cost of achieving objectives such as improving herbicide tolerance, pest and
disease resistance, and overall crop yields. Accordingly, there remains a need
for
transgenic plants that can meet these objectives.
The goal of rapid plant growth has been pursued through numerous studies of
various plant regulatory systems, many of which remain incompletely
understood..
In particular, the plant regulatory mechanisms that coordinate carbon and
nitrogen
:20 metabolism are not fully elucidated. These regulatory mechanisms are
presumed
to have a fundamental impact on plant growth and development.
The metabolism of carbon and nitrogen in photosynthetic organisms must be
regulated in a coordinated manner to assure efficient use of plant resources
and
energy. Current understanding of carbon and nitrogen metabolism includes
details
of certain steps and metabolic pathways which are subsystems of larger
systems.
In photosynthetic organisms, carbon metabolism begins with CO2 fixation, which
proceeds via two major processes, termed C-3 and C-- metabolism. In plants
with
C-3 metabolism, the enzyme ribulose bisphosphate carboxylase (RuBisCo)
:30 catalyzes the combination of C 2 with ribulose bisphosphate to produce 3e-
phosphoglycerate, a three carbon compound (C-3) that the plant uses to
synthesize carbon-containing compounds. in plants with C-4 metabolism, CO2 is
combined with phosphoenol pyruvate to form acids containing four carbons (C4),
2
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
in a reaction catalyzed by the enzyme phosphoenol pyruvate carboxylase. The
acids are transferred to bundle sheath cells, where they are decarboxylated to
release GO2, which is then combined with ribulose bisphosphate in the same
reaction employed by C-3 plants.
Numerous studies have found that various metabolites are important in plant
regulation of nitrogen metabolism. These compounds include the organic acid
malate and the amino acids glutamate and 0lutamine. Nitrogen is assimilated by
photosynthetic organisms via the action of the enzyme glutamine synthetase
(GS)
which catalyzes the combination of ammonia with glutamate to form glutamine.
OS plays a key role in the assimilation of nitrogen in plants by catalyzing
the
addition of ammonium to glutamate to form glutamine in an ATP-dependent
reaction (Miflin and Habash, 2002, Journal of Experimental Botany, Vol. 53,
No.
370, pp. 070-987). S also reassimilates ammonia released as a result of 15
photorespiration and the breakdown of proteins and nitrogen transport
compounds. S enzymes may be divided into two general classes, one
representing the cytoplasmic form (GS1) and the other representing the
plastidic
(i.e.; chloroplastic) form (052).
:20 Previous work has demonstrated that increased expression levels of GSI
result in
increased levels of S activity and plant growth, although reports are
inconsistent.
For example, Fuentes et 1, reported that CaMV S35 promoter driven
overexpression of Alfalfa 0S1 (cytoplasmic form) in tobacco resulted in
increased'
levels of : expression and GS activity in leaf tissue, increased growth under
25 nitrogen starvation, but no effect on growth under optimal nitrogen
fertilization
conditions (Fuentes et a1., 2001, J. Exp. Botany 52* 1071-81), Temple at al.
reported that transgenic tobacco plants overexpressing the full length Alfalfa
GS1
coding sequence contained greatly elevated levels of S transcript, and S
polypeptide which assembled into active enzyme, but did not report phenotypic
30 effects on growth (Temple at al., 1993, Molecular and General Genetics 230;
315-
325). Corruzi at at have reported that transgenic tobacco overexpressing a pea
cytosolic 0S1 transgene under the control of the CaMV S35 promoter show
increased S activity, increased cytosolic S protein, and improved growth
3
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
characteristics (U.S. Patent No. 5,197,547). Unkefer et at. have more recently
reported that transgenic tobacco plants overexpressing the Alfalfa GS1 in
foliar
tissues, which had been screened for increased leaf-to-root GS activity
following
genetic segregation by selfing to achieve increased OS1 transgene copy number,
were found to produce increased 2-hydroxy-5-oxoproline levels in their foliar
portions, which was found to lead to markedly increased growth rates over
wildtype tobacco plants (see, U. S, Patent Nos. 6,555,500; 6,593,275; and
6,831,040).
it) Unkefer at W. have further described the use of 2-hydroxy-5-oxo roline
(also
known as 2 -oxogi utara mate) to improve. plant growth (U.S. Patent Nos.
6,556,500;
5,593,275; 6,831,040). In particular, Unkefer et at, disclose that increased
concentrations of.2-hydroxy-5-oxoproline in foliar tissues (relative to root
tissues)
triggers a cascade of events that result in increased plant growth
characteristics.
Unkefer et al, describe methods by which the foliar concentration of 2-hydroxy-
5-
oxopreline may be increased in order to trigger increased plant growth
characteristics, specifically, by applying a solution of 2-hydroxy-5-
oxoproline
directly to the follar portions of the plant and over-expressing glutamine
synthatase preferentially in leaf tissues.
A number of transaminase and hydrolyase enzymes known to be involved in the
synthesis of 2-hydroxy-5-oxoproline in animals have been identified in animal
liver
and kidney tissues (Cooper and Meister, 1977, CRC Critical Reviews in
Biochemistry, pages 231-303; Meister, 1952, J. Biochem. 197: 304), In plants,
the biochemical synthesis of 2-hydroxy-5-oxoproline has been known but has
been poorly characterized. Moreover, the function of 2-hydroxy-5 oxoproline in
plants and the significance of its pool size (tissue concentration) are
unknown.
Finally, the art provides no specific guidance as to precisely what
transaminase(s)
or hydrolase(s) may exist and/or be active in catalyzing the synthesis of 2-
:30 hydroxy-5-oxoproline in plants, and no such plant transarninases have been
reported, isolated or characterized.
4
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SUMMARY OF THE INVENTION
The invention relates to transgenic plants exhibiting dramatically enhanced
growth
rates, greater seed and fruit/pod yields, earlier and more productive
flowering,.
more efficient nitrogen utilization, increased tolerance to high salt
conditions, and
increased biomass yields. In one embodiment, transgenic plants engineered to
over-express both glutamine phenylpyruvate transaminase (OPT) and gÃutamine
synthetase (GS) are provided. The GPT+GS double-transgenic plants of the
invention consistently exhibit enhanced growth characteristics, with TO
generation
it) lines showing an increase in biomass over wild type counterparts of
between 50%
and 300%. Generations that result from serial crosses and/or selfing typically
perform even better, with some of the double-transgenic plats achieving an
astounding four-fold biomass increase over wild type plants. Similarly, flower
and
fruit or pod yields are also tremendously improved, with TO generation lines
typically showing 50% to 70% increases over their wild type counterparts, and
in
some cases showing a 100% increase. Transg nic plants exhibiting such
enhanced growth phenotypic characteristics have been successfully generated
across a spectrum of individual plant species, using various transformation
methodologies, different expression vectors and promoters, and heterologous
and
:20 homologous transgene sequences from a variety of species, as exemplified
by the
numerous working examples provided herein. This invention, therefore, provides
a fundamental break-though technology that has the potential to transform
virtually all areas of agriculture.
Applicants have identified the enzyme glutamine phenylpyruvate transaminase
( PT) as a catalyst of 2-hydroxy-5--oxoproline (-oxoglutararnate) synthesis in
plants. 2-ox lutara mate is a powerful signal metabolite which regulates the
function of a large number of genes involved in the photosynthesis apparatus,
carbon fixation and nitrogen metabolism, The invention provides isolated
nucleic
:30 acid molecules encoding GPT, and discloses the novel finding that the
encoded
enzyme is directly involved in the synthesis of 2-hy roxy-5 oxoproline. This
aspect of the invention is exemplified herein by the disclosure of GPT
polynucleotides encoding GPTs from several species, including Arabidopsis,
5
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Grape, Rice, Soybean, Barley, Bamboo and a non-plant homolog from Zebra fish,
most of which have been expressed as recombinant GPTs and confirmed as
having GPT activity.
The invention further provides transgenic plants which express both: a GPT
transgene and a GS trans gene. The expression of these two trans eves in such
"double-transgene" plants results in a substantially increased rate of carbon
dioxide fixation and an extremely potent growth enhancing effect, as these
plants
exhibit very significantly and sometimes tremendously enhanced growth rates
and.
flower/fruit/pod/seed yields. Methods for the generation of such growth-
enhanced
tranaenic plants are provided.
By preferentially increasing the concentration of the signal metabolite 2-
oxo lutaraÃmate (i.e., in foliar tissues), the transgenic plants of the
invention are
capable of producing higher overall yields over shorter periods of time, and
therefore may provide agricultural industries with enhanced productivity
across a
wide range of crops, Importantly, unlike many transgenic plants described to
date, the invention utilizes natural plant genes encoding a natural plant
enzyme.
The enhanced growth characteristics of the transgenic plants of the invention
is
:20 achieved essentially by introducing additional GPT and GS capacity into
the plant.
Thus, the transgenic plants of the invention do not express any toxic
substances,
growth hormones, viral or bacterial gene products, and are therefore free of
many
of the concerns that have heretofore impeded the adoption of transgenic plants
in
certain parts of the World.
In one embodiment, the invention provides a transgenic plant comprising a GPT
transgene and a GS transgene, wherein said GPT transgene and said GS
transgene are operably linked to a plant promoter. In a specific embodiment,
the
GS transgene is a GS1 transgene. In another specific embodiment, the GPT
:30 transgene encodes a polypeptide having an amino acid sequence selected
from
the group consisting of (a) SEQ ID NO, SEQ ID NO: 9, SEQ ID NO: 15, SEQ ID
NO, 19, SEQ ID NO: 21, SEQ ID NO 24, SEQ ID NO, 30, SEQ ID N : 1, SEQ ID
NO,, 32, SEQ ID N : 33, SEQ ID NO, 34, SEQ ID NO: 35 and SEQ ID NO, 36,
6
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
and (b) an amino acid sequence that is at least 75% identical to any one of
SEQ
ID NO: 2; SEQ ID NO, 9; SEQ ID NO, 15, SEQ ID NO 19, SEQ ID NO: 21, SEQ.
ID NO 24, QIDNQ: 0,S SEQ ID NO: 31QIDNO: 32QIDNO:33,S Q
ID NO: 34, SEQ ID NQ: 35 and SEQ ID NO: 36 and has OPT activity. In yet
another specific embodiment, the GS transgene encodes a polypeptide having an
amino acid sequence selected form the group consisting of (a) SEQ ID NO: 4 and
SEQ ID NO, 7 from residue 11, and (b) an amino acid sequence that is at least
76% identical to SEQ ID NO, 4 or SEQ ID NO: 7. In some embodiments, the GP T
and S transgenes are incorporated into the genome of the plant. The transgenic
plant of the invention may be a monocotyledonous or a dicotyledonous plant.
The invention also provides progeny of any generation of the transgenlc plants
of
the invention, wherein said progeny comprises a GPT transgene and a OS
transgene, as well as a seed of any generation of the transgenic plants of the
invention, wherein said seed comprises said OPT transgene and said GS
trangene. The transgenic plants of the invention may display one or more
enhanced growth characteristics rate when compared to an analogous wild-type
or untransformed plant, including without limitation increased growth rate,
biomass yield, seed yield, flower or flower bud yield, fruit or pod yield,
larger
:20 leaves, and may also display increased levels of OPT and/or QS activity,
and/or
increased levels of 2-oxo l taramate. In some embodiments, the trans epic
plants of the invention display increased nitrogen use efficiency or increased
tolerance to salt or saline conditions,
Methods for producing the transgenie plants of the invention and seeds thereof
are also provided, including methods for producing a plant having enhanced
growth properties, increased nitrogen use efficiency and increased tolerance
to
germination or growth in salt or saline conditions, relative to an analogous
wild:
type or untransformed plant.
:30
7
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
BRIEF DESCRIPTION OF THE DRAWINGS
The patent or application file contains at least one drawing executed in
color.
Copies of this patent or patent application publication with color drawing(s)
will be
provided by the Office upon request and payment of the necessary fee.
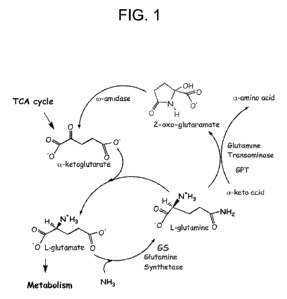
FIG. `Ã. Nitrogen assimilation and 2-oxoglutaramate biosynthesis: schematic of
metabolic pathway.
FIG. 2. Photograph showing comparison of transgenic tobacco plants over-
expressing either O 1 or OPT, compared to wild type tobacco plant. From left
to
right: wild type plant, Alfalfa 081 transgene, Arabidopsis OPT transgene. See
Examples 3 and 5, infra.
FIG. 3. Photograph showing comparison of transgenic Micro-Tom tomato plants
over-expressing either G S1 or OPT, compared to wild type tomato plant. From
left to right: wild type plant, Alfb, a GSI transgene, Arabidopsis OPT
transgene.
See Examples 4 and 6, infra.
FIG. 4. Photographs showing comparisons of leaf sizes between wild type and
G S1 or OPT transgenic tobacco plants. A Comparison between leaves from
G S1 transgenic tobacco (bottom leaf) and wild type (top leaf), B: Comparison
between leaves from OPT transgenic tobacco (bottom leaf) and wild type (top
leaf).
FIG. 5, Photographs showing comparisons of transg nic tobacco plants
2 15
generated from various crosses between G S1 and OPT transgenic tobacco lines
with wild type and single transgene plants. A-C= Cross 2, 3 and 7,
respectively.
See Example 7, infra.
FIG. 6. Photographs showing comparisons of leaf sizes between wild type and
crosses between 081 and OPT transgenic tobacco plants. A: Comparison
between leaves from GSXGPT Cross 3 (bottom leaf) and wild type (top leaf). B
Comparison between leaves from GSXGPT Cross 7 (bottom leaf) and wild type
(top leaf). See Example 7, infra.
8
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
FIG. 7. Photograph of transgenic pepper plant (right) and wild type control
pepper
plant (left), showing larger pepper fruit yield in the transgenic plant
relative to the
wild type control plant. See Example 8, infraõ
FIG. 8. Transgenic bean plants compared to wild type control bean plants
(several transgenic lines expressing Arabidopsis GPT and GS transgenes).
Upper Left: plant heights on various days; Upper right: flower bud numbers;
Lower
left: flower numbers; Lower right: bean pod numbers. Wildtype is the control,
and
lines 2A, 4A and 58 are all transgenic plant lines. See Example 9, infra.
FIG. 9. Photograph of transgenic bean plant (right) and wild type control bean
plant (left), showing increased growth in the transgenic plant relative to the
wild
type control plant. Transgenic line expressing Arabidopsis GPT and GS
transgenes. See Example g, infra.
FIG. 10. Transgenic bean plants pods, flowers and flower buds compared to wild
type control bean plants (transgenic line expressing grape GPT and Arabidopsis
GS transgenes). See Example 10, infra.
FIG. 11. Photograph of tr nsgenic bean plant (right) and wild type control
bean:
plant (left), showing increased growth in the transgenic plant relative to the
wild
type control plant. Transgenic line expressing Grape GPT and Arabidopsis GS
transgenes. See Example 10, infra,
FIG. 12. Transgenic Cowpea Line A plants compared to wild type control Cowpea
plants (transgenic line expressing Arabidopsis GPT and GS transgenes), showing
that the transgenic plants grow faster and flower and set pods sooner than
wild
type control plants. (A) Relative height and longest leaf measurements as of
May
:30 21, (B) Relative trifolate leafs and flower buds as of June 18, (C)
Relative
numbers of flowers, flower buds and pea pods as of June 22. See Example 11,
infra,
g
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
FIG, 13. Photograph of transgenic Cowpea Line A plant (right) and wild type
control Co pea plant (left), showing increased growth in the trans epic plant
relative to the wild type control plant. Tranagenic line expressing
Arabidopsis
OPT and GS transgenes. See Example 11, infra.
FIG. 14, Transgenic Cowpea Line plants compared to wild type control Cowpea
plants (transgenic line expressing Grape OPT and Arabidopsis S trans ene ),
showing that the transgenic plants grow faster and flower and set pods sooner
than wild type control plants. (A) plant heights, (B) flowers and pea pod
numbers,
(C) leaf bud and trifolate numbers. See Example 12, infra.
FIG. 15, Photograph of transgenic Cowpea Line G plant (right) and wild type
control Cowpea plant (left), showing increased growth in the transgenic plant
relative to the wild type control plant. Transgenic line expressing Grape GPT
and
Arabidopsis GS transgenes. See Example 12, infra.
FIG. 16. Photograph of transgenic Cantaloupe plant (right) and wild type
control
Cantaloupe plant (left), showing increased growth in the tranagenic plant
relative
to the wild type control plant. Transgenic line expressing Arabidopsis GPT and
:20 GS transgenes. See Example 14, infra.
FIG. 17. Photograph of transgenic Pumpkin plants (right) and wild type control
Pumpkin plants (left), showing increased growth in the transgenic plants
relative
to the wild type control plants. Transgenic lines expressing Arabidopsis GPT
and
OS transgenes. See Example 15, infra.
FÃG. 18. Photograph of transgenic Arabidopsis plants (right) and wild type
control
Arabidopsis plants (left), showing increased growth in the transgenic plants
relative to the wild type control plants. Transgenic lines expressing
Arabidopsis
:30 OPT and S transgenes. See Example 16, infra.
FÃG. 19. Transgenic tomato plants expressing Arabidopsis OPT and OS
transg nes compared to control tomato plants. (A) Photograph of transgenic
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
tomato plant leaves (right) vs. wild type control leaves (left) showing larger
leaves
in the transgenic plant. (B) Photograph of transgenic tomato plants (right)
and
wild type control plants (left), showing increased growth in the transgenic
plants
relative to the wild type control plants., See Example 17, infra.
FIG. 20. Photograph of transgenic Camelina plant (right) and wild type control
Camelina plant (left), showing increased growth in the transgenic plant
relative to
the wild type control plant. Transgenic line expressing Arabidopsis CPT and GS
transggenes. See Example 18, infra.
DETAILED DESCRIPTION OF THE INVENTION
DEFINITIONS
Unless otherwise defined, all terms of art, notations and other scientific
terminology used herein are intended to have the meanings commonly
understood by those of skill in the art to which this invention pertains. In
some
cases, terms with commonly understood meanings are defined herein for clarity
andlor for ready reference, and the inclusion of such definitions herein
should not
necessarily be construed to represent a substantial difference over what is
generally understood in the art. The techniques and procedures described or
referenced herein are generally well understood and commonly employed using
conventional methodology by those skilled in the art, such as, for example,
the
widely utilized molecular cloning methodologies described in Sambrook et al,,
Molecular Cloning: A Laboratory Manual 3rd. edition (2001) Cold Spring Harbor
Laboratory Press, Cold Spring Harbor, N.Y.: Current Protocols in Molecular
Biology (Ausbel et al., eds., John Ailey & Sons, Inc. 2001; Trans epic Plants:
Methods and Protocols (Leandro Pena, ed., Humana Press, Is' edition; 2004);
and, A robacter u, Protocols (Wan, ed., Humana Press, 2"a edition, 2006). As
appropriate, procedures involving the use of commercially available kits and
reagents are generally carried out in accordance with manufacturer defined'
protocols and/or parameters unless otherwise noted.
The term "nucleic acid" refers to deoxyribonucleotides or ribonucleotides and
45 polymers thereof (<'polynucleotides") in either single- or double-stranded
form.
11
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Unless specifically limited, the term 'polynucleotide" encompasses nucleic
acids
containing known analogues of natural nucleotides which have similar binding
properties as the reference nucleic acid and are metabolized in a manner
similar
to naturally occurring nucleotides. Unless otherwise indicated, a particular n
.ucleic
acid sequence also implicitly encompasses conservatively modified variants
thereof (e.g. degenerate colon substitutions) and complementary sequences and
as well as the sequence explicitly indicated; Specifically, degenerate radon
substitutions may be achieved by generating sequences in which the third
position
of one or more selected for all) colons is substituted with mixed-base and/or
deoxyinosine residues (Batzer at al., 1991, Nucleic Acid Res. 19: 5081;
Ohtsuka
at al., 1985 J. Biol. Chem. 260: 2605-2608: and Cassol at al., 1992; Rossolini
et
al., 1994, Mol. Cell. Probes 8: 91-98), The term nucleic acid is used
interchangeably with gene, cDNA, and mRNA encoded by a gene.
The term "promoter" refers to an array of nucleic acid control sequences that
direct transcription of an operably linked nucleic acid. As used herein, a
"plant
promoter" is a promoter that functions in plants. Promoters include necessary
nucleic acid sequences near the start site of transcription, such as, in the
case of
a polymerase 11 type promoter, a TATA element. A promoter also optionally
:20 includes distal enhancer or repressor elements, which can be located as
much as
several thousand base pairs from the start site of transcription, A
"constitutive"
promoter is a promoter that is active under most environmental and::
developmental conditions. An "inducible" promoter is a promoter that is active
under environmental or developmental regulation. The term "operably lined"
refers to a functional linkage between a nucleic acid expression control
sequence
(such as a promoter, or array of transcription factor binding sites) and a
second
nucleic acid sequence, wherein the expression control sequence directs
transcription of the nucleic acid corresponding to the second sequence.
:30 The terms "polypeptide,ry "peptide" and "protein" are used interchangeably
herein
to refer to a polymer of amino acid residues. The terms apply to amino acid
polymers in which one or more amino acid residue is an artificial chemical
mimetic
1
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
of a corresponding naturally occurring amino acid, as well as to naturally
occurring
amino acid polymers and non-naturally occurring amino acid polymers.
The term "amino acid" refers to naturally occurring and synthetic amino acids,
as
well as amino acid analogs and amino acid mimetics that function in a manner
similar to the naturally occurring amino acids. Naturally occurring amino
acids are
those encoded by the genetic code, as well as those amino acids that are later
modified, e. g,, hydroxyproline, y-carboxyglutamate, and 0-phosphoserine.
Amino
acid analogs refers to compounds that have the same basic chemical structure
as
a naturally occurring amino acid, Lie., an a carbon that is bound to a
hydrogen, a
carboxyl group, an amino group, and an R group, e.g., homoserine, norleucine,
methionine sulfoxide, methionine methyl sulfonium. Such analogs have modified
R groups (e.g., norleucine) or modified peptide backbones, but retain the same
basic chemical structure as a naturally occurring amino acid. Amino acid
mimetics refers to chemical compounds that have a structure that is different
from
the general chemical structure of an amino acid, but that functions in a
manner
similar to a naturally occurring amino acid.
Amino acids may be referred to herein by either their commonly known three
letter
:20 symbols or by the one-letter symbols recommended by the IUP -IUB
Biochemical Nomenclature Commission. Nucleotides, likewise, may be referred
to by their commonly accepted single-letter codes.
The term "plant" includes whole plants, plant organs (e.g., leaves, stems,
flowers,
roots, etc.), seeds and plant cells and progeny thereof. The class of plants
which
can be used in the method of the invention is generally as broad as the class
of
higher plants amenable to transformation techniques., including angiosperms
(monocotyledonous and dicotyledonous plants), as well as gymnosperms. It
includes plants of a variety of ploidy levels, including polyploid; diploid,
haploid
:30 and hemizygous.
The terms "GPT polynucleotide" and "GPT nucleic acid" are used interchangeably
herein, and refer to a full length or partial length polynucleotide sequence
of a
13
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
gene which encodes a polypeptide involved in catalyzing the synthesis of 2-
oxoglutaramate, and includes polynucleotides containing both translated
(coding)
and un-translated sequences, as well as the complements thereof. The term
"GPT coding sequence" refers to the part of the gene which is transcribed
and'.
encodes a GPT protein. The term "targeting sequence" refers to the amino
terminal part of a protein which directs the protein into a subcellular
compartment
of a cell, such as a chloroplast in a plant cell. GPT polynucl otides are
further
defined by their ability to hybridize under defined conditions to the GPT
polynucleotides specifically disclosed herein, or to PCR products derived
therefrom.
A "GPT transgene" is a nucleic acid molecule comprising a GPT polynucleotide
which is exogenous to transgenic plant, or plant embryo, organ or seed,
harboring
the nucleic acid molecule, or which is exogenous to an ancestor plant, or
plant
embryo, organ or seed thereof, of a transgenic plant harboring the GPT
polynucleotide.
The terms "GS polynucleotide" and "GS nucleic acid" are used interchangeably
herein, and refer to a full length or partial length polynucleotide sequence
of a
:20 gene which encodes a glutamine synthetase protein, and includes
polynucleotides
containing both translated (coding) and un-translated sequences, as well as
the
complements thereof. The term "G5 coding sequence' refers to the part of the
gene which is transcribed and encodes a GS protein. The terms "G 1
polynucleotide" and "GS1 nucleic acid" are used interchangeably herein, and
refer
to a full length or partial length polynucdotidesequence of a gene which
encodes
a glutamine synthetase isoform 1 protein, and includes polynucleotides
containing
both translated (coding) and un-translated sequences, as well as the
complements thereof. The term "GS1 coding sequence" refers to the part of the
gene which is transcribed and encodes a G1 protein.
:30
A "GS transgene" is a nucleic acid molecule comprising a GS polynucleotide
which is exogenous to transgenic plant, or plant embryo, organ or seed,
harboring
the nucleic acid molecule, or which is exogenous to an ancestor plant, or
plant
14
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
embryo, organ or seed thereof, of a transgenic plant harboring the GPT
polynucleotide. A " S1 transgene" is a nucleic acid molecule comprising a S1
polynucleotide which is exogenous to transgenic plant, or plant embryo, organ
or
seed, harboring the nucleic acid molecule. or which is exogenous to an
ancestor
plant, or plant embryo, organ or seed thereof, of a transgenic plant harboring
the
GPT polynucleotide.
Exemplary GPT polynucleotides of the invention are presented herein, and
include GPT coding sequences for Arabidopsis, Rice, Barley, Bamboo, Soybean,
Grape, and Zebra Fish PTs.
Partial length OPT polynucleotides include polynucÃeotide sequences encoding N-
or C-terminal truncations of OPT, mature GPT (without targeting sequence) as
well as sequences encoding domains of GPT. Exemplary OPT polynucleotides
encoding N-terminal truncations of GPT include Arabidopsis -30, -45 and -56
constructs, in which coding sequences for the first 30, 45, and 56
respectively,
amino acids of the full length CPT structure of SEQ ID NO, 2 are eliminated.
In employing the OPT polynucleotides of the invention in the generation of
:20 transformed cells and transgenic plants, one of skill will recognize that
the
inserted polynucleotide sequence need not be identical, but may be only
"substantially identical" to a sequence of the gene from which it was
derived., as
further defined below. The term "GPT polynucleotide" specifically encompasses
such substantially identical variants. Similarly, one of skill will recognize
that
because of codon degeneracy, a number of polynucleotide sequences will encode
the same polypeptide, and all such poà nucleotide sequences are meant to be
included in the term PT polynucleotide. In addition, the term specifically
includes those sequences substantially identical (determined as described
below)
with an GPT polynucleotide sequence disclosed herein and that encode
:30 polypeptides that are either mutants of wild type OPT polypeptides or
retain the
function of the PT polypeptide (e.g., resulting from conservative
substitutions of
amino acids in a OPT polypeptid ). The term "GPT polynucleotide" therefore
also
includes such substantially identical variants.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The term "conservatively modified variants" applies to both amino acid and
nucleic
acid sequences. With respect to particular nucleic acid sequences,
conservatively
modified variants refers to those nucleic acids which encode identical or
essentially identical amino acid sequences, or where the nucleic acid does not
encode an amino acid sequence, to essentially identical sequences. Because of
the degeneracy of the genetic code, a large number of functionally identical
nucleic acids encode any given protein. For instance, the codons GCA, CC,
GCG and GCU all encode the amino acid alanine. Thus, at every position where
an alanine is specified by a colon, the colon can be altered to any of the
corresponding dons described without altering the encoded polypeptide. Such
nucleic acid variations are "silent variations;" which are one species of
conservatively modified variations. Every nucleic acid sequence herein which
encodes a polypaptide also describes every possible silent variation of the
nucleic
acid. One of skill will recognize that each don in a nucleic acid (except AU,
which is ordinarily the only colon for methionine, and TOG, which is
ordinarily the
only colon for tryptophan) can be modified to yield a functionally identical
molecule. Accordingly, each silent variation of a nucleic acid which encodes a
polypeptide is implicit in each described sequence.
As to amino acid sequences, one of skill will recognize that individual
substitutions, deletions or additions to a nucleic acid, peptide, polypeptide,
or
protein sequence which alters, adds or deletes a single amino acid or a small
percentage of amino acids in the encoded sequence is a 'conservatively
modified
variant' where the alteration results in the substitution of an amino acid
with a
chemically similar amino acid. Conservative substitution tables providing
functionally similar amino acids are well known in the art. Such
conservatively
modified variants are in addition to and do not exclude polymorphic variants,
interspecies homologs, and alleles of the invention.
:30
The following eight groups each contain amino acids that are conservative
substitutions for one another; 1) Alanine (A), Glycine (O); 2) Aspartic acid
(D),
Olutamic acid (E), 3) Asparagine (N). Olutamine (0) 4) Arginine (R), Lysine
(K);
16
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
5) Isoleucine (1), Leucine (L), Methionine (M)" Valhne (V), 6) Phenyl latrine
(F),
Tyrosine (Y), Tryptophan ('): 7) Serine (S), Threonine (T); and 8) Cysteine
(C),
Methionine (M) (see, e.g., Creighton, Proteins (1984)).
Macromolecular structures such as polypeptide structures can be described in
terms of various levels of organization. For a general discussion of this
organization, see, e.g., Alberts et at., Molecular Biologyy of the Cell (3`d
ed., 1994)
and Cantor and Schimmel, Biophysical Cp ist y Part 1: The Conformation of
Biological Macromolecules (1980). "Primary structure" refers to the amino acid
sequence of a particular peptide. "Secondary structure" refers to locally
ordered',
three dimensional structures within a polypeptide. These structures are
commonly known as domains, Domains are portions of a polypeptide that form a
compact unit of the polypeptide and are typically 25 to approximately 500
amino
acids long. Typical domains are made up of sections of lesser organization
such
as stretches of-sheet and ;x-helps. "Tertiary structure" refers to the
complete
three dimensional structure of a polypeptÃde monomer; "Quaternary structure"
refers to the three dimensional structure formed by the noncovalent
association of
independent tertiary units, Anisotropic terms are also known as energy terms.
The term "isolated" refers to material which is substantially or essentially
free from
components which normally accompany the material as it is found in its native
or
naiu:ral state. However, the term "isolated" is not intended refer to the
components present in an electrophoretic gel or other separation medium, An
isolated component is free from such separation media and in a form ready for
use in another application or already in use in the new applicationlmilieu. An
"isolated" antibody is one that has been identified and separated and/or
recovered
from a component of its natural environment. Contaminant components of its
natural environment are materials that would interfere with diagnostic or
therapeutic uses for the antibody, and may include enzymes, hormones, and
other proteinaceous or nonrproteinaceous solutes. In preferred embodiments,
the
antibody will be purified (1) to greater than 95% by weight of antibody as
determined by the Lowry method, and most preferably more than 99% by weight
(2) to a degree sufficient to obtain at least 15 residues of N-terminal or
internal
17
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
amino acid sequence by use of a spinning cup sequenator, or (3) to homogeneity
by SOS-PAGE under reducing or nonreducing conditions using Coomassie blue
or, preferably, silver stain. Isolated antibody includes the antibody in situ
within
recombinant cells since at least one component of the antibody's natural
environment will not be present. Ordinarily, however, isolated antibody will
be
prepared by at least one purification step.
The term "heterologous" when used with reference to portions of a nucleic acid
indicates that the nucleic acid comprises two or more subsequences that are
not
found in the same relationship to each other in nature. For instance, a
nucleic
acid is typically recombinantly produced, having two or more sequences from
unrelated genes arranged to make a new functional nucleic acid, e.g., a
nucleic
acid encoding a protein from one source and a nucleic acid encoding a peptide
sequence from another source. Similarly, a heterologous protein indicates that
the protein comprises two or more subsequences that are not found in the same
relationship to each other in nature (e.g- a fusion protein).
The terms "identical" or percent "identity," in the context of two or more
nucleic
acids or polypeptide sequences; refer to two or more sequences or subsequences
:20 that are the same or have a specified percentage of amino acid residues or
nucleotides that are the same (Le., about 70% identity, preferably 75%, 80%,
85%, 90%, or 95% identity over a specified region, when compared and aligned
for maximum correspondence over a comparison window, or designated region as
measured using a sequence comparison algorithms.õ or by manual alignment and
visual inspection. This definition also refers to the complement of a test
sequence,
which has substantial sequence or subsequence complementarity when the test
sequence has substantial identity to a reference sequence. This definition
also
refers to the complement of a test sequence, which has substantial sequence or
subsequence complementarity when the test sequence has substantial identity to
:30 a reference sequence.
When percentage of sequence identity is used in reference to polypeptides, it
is
recognized that residue positions that are not identical often differ by
conservative
18
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
amino acid substitutions, where amino acids residues are substituted for other
amino acid residues with similar chemical properties (e.g., charge or
hydrophobicity) and therefore do not change the functional properties of the
polypeptide. Where sequences differ in conservative substitutions, the percent
sequence identity may be adjusted upwards to correct for the conservative
nature
of the substitution.
For sequence comparison, typically one sequence acts as a reference sequence,
to which test sequences are compared. When using a sequence comparison
algorithm, test and reference sequences are entered into a computer,
subsequence coordinates are designated, if necessary, and sequence algorithm
program parameters are designated. Default program parameters can be used,
or alternative parameters can be designated. The sequence comparison
algorithm then calculates the percent sequence identities for the test
sequences
relative to the reference sequence, based on the program parameters.
A "comparison window", as used herein, includes reference to a segment of any
one of the number of contiguous positions selected from the group consisting
of
from 20 to 600, usually about 50 to about 200, more usually about 100 to about
:20 150 in which a sequence may be compared to a reference sequence of the
same
number of contiguous positions after the two sequences are optimally aligned..
Methods of alignment of sequences for comparison are well-known in the art.
Optimal alignment of sequences for comparison can be conducted, e.g., by the
local homology algorithm of Smith & Waterman, 1981, Adv, Appl. Math. 2:482, by
the homology alignment algorithm of Needleman & Wunsch, 1970, J. Mol. Biol.
48.443, by the search for similarity method of Pearson & Lippman, 1988, Proc.
Nat'l, Aced. Sci. USA 85:2441, by computerized implementations of these
algorithms (GAP, B ST IT, PASTA, and TFASTA in the Wisconsin Genetics
Software Package, Genetics Computer Group, 575 Science Dr., Madison, WI), or
:30 by manual alignment and visual inspection (see, e.g., Current Protocols in
Molecular Biology (Ausubel et al., eds. 1995 supplement)).
19
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
A preferred example of algorithm that is suitable for determining percent
sequence identity and sequence similarity are the BLAST and BLAST 2.0
algorithms, which are described in Altschul et at, 1977, Nuc. Acids Res.
25:3389-
3402 and Altschul et at, 1990, J. Mol. Biol. 215:403-410, respectively. BLAST
and BLAST 2.0 are used, typically with the default parameters described
herein,
to determine percent sequence identity for the nucleic acids and proteins of
the
invention. Software for performing BLAST analyses is publicly available
through
the National Center for Biotechnology Information. This algorithm involves
first
identifying high scoring sequence pairs (HSPs) by identifying short words of
length W in the query sequence, which either match or satisfy some positive-
valued threshold score T when aligned with a word of the same length: in a
database sequence. T is referred to as the neighborhood word score threshold
(Altschul et e1:, supra). These initial neighborhood word hits act as seeds
for
initiating searches to find longer HSPs containing them. The word hits are
extended in both directions along each sequence for as far as the cumulative
alignment score can be increased. Cumulative scores are calculated using, for
nucleotide sequences, the parameters M (reward score for a pair of matching
residues; always > 0) and N (penalty score for mismatching residues, always <
0).
For amino acid sequences, a scoring matrix is used to calculate the cumulative
:20 score. Extension of the word hits in each direction are halted when: the
cumulative alignment score falls off by the quantity X from its maximum
achieved
value; the cumulative score goes to zero or below, due to the accumulation of
one
or more negative-scoring residue alignments; or the end of either sequence is
reached. The BLAST algorithm parameters W, T, and X determine the sensitivity
and speed of the alignment. The BLASTN program (for nucleotide sequences)
uses as defaults a word length (W) of 11, an expectation (E) of 10, M=5, N=-4
and
a comparison of both strands. For amino acid sequences, the BLASTP program
uses as defaults a word length of 3, and expectation (E) of 10, and the
BLOSUM62 scoring à ratrix (see Henikoff & Henikoff, Prot. Nat!. Aced Sci.. USA
:30 89:10915 (1989)) alignments (B) of 50, expectation (E) of 10, M=5, N=-4,
and: a
comparison of both strands.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The BLAST algorithm also performs a statistical analysis of the similarity
between
two sequences (see, e. Ã.., Karlin & Altschul, 1993, Proc. Nat'l. Acad. Scl.
USA
90:5870-5707).. One measure of similarity provided by the BLAST algorithm is
the
smallest sum probability (P(N)1, which provides an indication of the
probability by
which a match between two nucleotide or amino acid sequences would occur by
chance, For example, a nucleic acid is considered similar to a reference
sequence if the smallest sum probability in a comparison of the test nucleic
acid to
the reference nucleic acid is less than about 0., more preferably less than
about
0.01, and most preferably less than about 0.001.
The phrase "stringent hybridization conditions" refers to conditions under
which a
probe will hybridize to its target subsequence, typically in a complex mixture
of
nucleic acid, but to no other sequences. Stringent conditions are sequence-
dependent and will be different in different circumstances. Longer sequences
hybridize specifically at higher temperatures. An extensive guide to the
hybridization of nucleic acids is found in Tijssen, Techniques in Biochemistry
and
Molecular Biology-Hybridization with Nucleic Probes, "Overview of principles
of
hybridization and the strategy of nucleic acid assays" (1993). Generally,
highly
stringent conditions are selected to be about 5-10 C.. lower than the thermal
melting point (Tm) for the specific sequence at a defined ionic strength pH.
Low
210 stringency conditions are generally selected to be about 15-30','C, below
the Tm_
Tm is the temperature (under defined ionic strength, pH, and nucleic
concentration) at which 50% of the probes complementary to the target
hybridize
to the target sequence at equilibrium (as the target sequences are present in
excess, at Tm, 150% of the probes are occupied at equilibriums). Stringent
conditions will be those in which the salt concentration is less than about I
.O
sodium ion, typically about 0.01 to 1.OM sodium ion concentration (or other
salts)
at pH 7.0 to 8.3 and the temperature is at least about 30'_' for short probes
(e.g.;
10 to 50 nucleotides) and at least about 60"S for long probes (e.g., greater
than
50 nucleotides). Stringent conditions may also be achieved with the addition
of
destabilizing agents such as formamide. For selective or specific
hybridization, a
positive signal is at least two times background, preferably 10 times
background
hybridization.
21
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Nucleic acids that do not hybridize to each other under stringent conditions
are
still substantially identical if the polypeptides which they encode are
substantially
identical. This occurs, for example, when a copy of a nucleic acid is created
using
the maximum codon degeneracy permitted by the genetic code. In such cased.
the nucleic acids typically hybridize under moderately stringent hybridization
conditions.
Genomic DNA or cDNA comprising GPT polynucleotides may be identified in
standard Southern blots under stringent conditions using the GIRT
polyn:ucleotide
sequences disclosed here. For this purpose, suitable stringent conditions for
such
hybridizations are those which include a hybridization in a buffer of 40%
formamlde, I M hla 1, 1 % SOS at 37"'C, and at least one wash in 0,2 X SSC at
a
temperature of at least about 50'C, usually about 55'C to about 60'C, for 20
minutes, or equivalent conditions. A positive hybridization is at least twice
background. Those of ordinary skill will readily recognize that alternative
hybridization and wash conditions may be utilized to provide conditions of
similar
stringency.
A further indication that two polynucleotides are substantially identical is
if the
reference sequence, amplified by a pair of oligonucleotide primers, can then
be
used as a probe under stringent hybridization conditions to isolate the test
sequence from a cDNA or genomic library, or to identify the test sequence in,
e.g.,
a northern or Southern blot.
TI NS ENIC PLAITS,
The invention provides novel transgenic plants exhibiting substantially
enhanced
agronomic characteristics, including faster growth, greater mature plant fresh
weight and total biomass, earlier and more abundant flowering, and greater
fruit,
pod and seed yields. The transgenic plants of the invention are generated by
introducing into a plant one or more expressible genetic constructs capable of
driving the expression of one or more polynucleotides encoding glutamine
synthetase (GS) and glutamine phenylpyruvate transaminase (GPT). In an
exemplary embodiment, single-transgene parental lines carrying either a GPT or
2
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
GS1 transgene coding sequence are generated, preferably selfed until
homozygous for the transgene, then crossed to generate progeny plants
containing both transgene ,
In stable transformation embodiments of the invention, one or more copies of
the
expressible genetic construct become integrated into the host plant genome,
thereby providing increased GS and GPT enzyme capacity into the plant, which
serves to mediate increased synthesis of 2-oxoglutaramate, which in turn
signals
metabolic gene expression, resulting in increased plant growth: and the
enhancement other agronomic characteristics. 2-oxoglutaramate is a metabolite
which is an extremely potent effector of gene expression, metabolism and plant
growth (U. S. Patent No. 6, 555,500), and which may play a pivotal role in the
coordination of the carbon and nitrogen metabolism systems (Lancien et al,,
2000,
Enzyme Redundancy and the Importance of 2-Oxo Ãluterate in Higher Plants
Ammonium Assimilation, Plant Physiol. 123: 817-824). See, also, the schematic
of the 2-oxoglutaramate pathway shown in FIG. 1.
In one aspect of the invention, applicants have isolated a nucleic acid
molecule
encoding the Arabidopsisglutamine phenylpyruvate transaminase (G PT) enzyme
:20 (see Example 1, infra), and have demonstrated for the first time that the
expressed recombinant enzyme is active and capable of catalyzing the synthesis
of the signal metabolite, 2-oxoglutararnate (Example 2, infra), Further,
applicants
have demonstrated for the first time that over-expression of the Arabidopsis
glutamine transaminase gene in a transformed heterologous plant results in
enhanced CO2 fixation rates and increased growth characteristics (Example 3,
infra).
Applicants' previous work demonstrated that over-expression of Alfalfa GS1
gene
under the control of a strong constitutive promoter results in transgenic
tobacco
:30 plants with higher levels of GS activity in the leaves. These plants
outgrow their
wild-type counterparts, fix O faster, contain increased concentrations of
total
protein, as well as increased concentrations of glutamine and 2-
oxoglutaramate,
and show increased rates of uptake of nitrate through their roots.
23
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
As disclosed herein (see Example 3, infra), over-expression of a transgene
comprising the full-length Arabidopsis GPT coding sequence in transg nic
tobacco plants also results in faster CO,2 fixation, and increased levels of
total
protein, glutami e and 2-oxoglutaramate. These transgenic plants also grow
faster than wild-type plants (FIG, 2). Similarly, in preliminary studies
conducted'
with tomato plants (see Example 4, infra), tomato plants transformed with the
Arabidopsis GPT transgene showed significant enhancement of growth rate,
flowering, and seed yield in relation to wild type control plants (FIG, 3 and
Example 4, infra).
In one particular embodiment, exemplified herein by way of Examples 3, 5 and
7,
infra, a first set of parental single-transgene tobacco plant lines carrying
the Alfalfa
f gene, including 5' and 3' untr nslated regions, were generated using
Agrobacterium mediated gene transformation, under selective pressure, together
with screening for the fastest growing phenotype, and selfing to
transgenefphenotype homo ygosity (see Example 5, infra). A second set of
parental single--transgene tobacco plant lines carrying the full length coding
sequence of Arabidopsis GPT were generated in the same manner (Example 3,
infra). High growth rate performing plants from each of the parental lines
were
then sexually crossed to yield progeny lines (Example 7, infra).
The resulting progeny from multiple crosses of Arabidopsis 031 and GPT
transg:enic tobacco plants produce far better and quite surprising increases
in
2:5 growth rates over the single-transgene parental lines as well as wildtype
plants.
FIG. 5 shows photographs of double-transgene progeny from single-transgene
1 X PT plant crosses, relative to wild type and single-transgene parental
plants. FIG. 6 shows photographs comparing leaf sizes of doubletransgene
progeny and wild type plants. Experimentally observed growth rates in these
double transgenic plants ranged between 200% and 300% over wild-type pants
(Example 7, infra), Moreover, total biomass levels increased substantially in
the
double-transgene plants, with whole plant fresh weights typically being about
two
to three tunes the wild-iy pe plant weights. Similarly, seed yields showed
similar
24
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
increases in the double-transgene plants, with seed pod production typically
two
to three times the wild type average, and overall seed yields exceeding
wild$yp
plant yields by 300-40M
.
In addition to the tranagenc tobacco plants referenced above, various other
species of transgenic plants comprising OPT and S transgenes are specifically
exemplified herein. As exemplified herein, transgenic plants showing enhanced
growth characteristics have been generated in two species of Tomato (see
Examples 4 and 17), Pepper (Example 8), Beans (Examples 9 and 10), Cowpea
(Examples 11 and 12), Alfalfa (Example 13), Cantaloupe (Example 14), Pumpkin
(Example 15), Arabidopsis (Example 16) and Camilena (Example 18), These
transgenic plants of the invention were generated using a variety of
transformation
methodologies, including Agrohacterium-mediated callus, floral dip, seed
inoculation, pod inoculation, and direct flower inoculation, as well as
combinations
thereof, and via sexual crosses of single transgene plants, as exemplified
herein.
Different GPT and CB transgenes were successfully employed in generating the
transgenic plants of the invention, as exemplified herein.
The invention also provides methods of generating a transgenic plant having
enhanced growth and other agronomic characteristics. In one embodiment, a
method of generating a transgenic plant having enhanced growth and other
agronomic characteristics comprises introducing into a plant cell an
expression
cassette comprising a nucleic acid molecule encoding a OPT transgene, under
the control of a suitable promoter capable of driving the expression of the
2:5 transgene, so as to yield a transformed plant cell, and obtaining a
transgenic plant
which expresses the encoded OPT, In another embodiment, a method of
generating a t ansgenic plant having enhanced growth and other agronomic
characteristics comprises introducing into a plant cell one or more nucleic
acid
constructs or expression cassettes comprising nucleic acid molecules encoding
a
OPT transgene and an CS transgene, under the control of one or more suitable
promoters (and, optionally, other regulatory elements) capable of driving the
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
expression of the transgenes, so as to yield a plant cell transformed thereby,
and
obtaining a transgenic plant which expresses the OPT and S transgenes.
Based on the results disclosed herein, t is clear that any number of GPT and S
polynucleotides may be used to generate the transgenic plants of the
invention.
Both OSI and OPT proteins are highly conserved among various plant species,
and it is evident from the experimental data disclosed. herein that closely-
related
non-plant OPTS may be used as well (e.g., Danio rerio OPT). With respect to
OPT, numerous GPT polynucleotides derived from different species have been
shown to be active and useful as :PT transgenes. Similarly, different S
polynucleotides may be used, including without limitation any plant 0S1
encoding
polynucleotide that generates OS activity in a host cell transformed with an
expressible S construct.
1.5 In a specific embodiment, the OPT transgene is a OPT polynucleotide
encoding
an rabidopsisderived OPT, such as the OPT of SEQ ID NO: 2, SEQ ID NO: 21
and SECS ID NO: 30, and the OS transgene is a OS polynucleotide encoding an
Alfalfa derived GS1 (i,ev, SEQ ID NO, 4) or an Arabidopsis derived GS1 (SEQ ID
N : 7). The OPT transgene may be encoded by the nucleotide sequence of SEQ
ID NO, 1; a nucleotide sequence having at least 75% and more preferably at
least
80% identity to SEQ ID NO, 1, and encoding a polypeptide having OPT activity;
a
nucleotide sequence encoding the polypeptide of SEQ ID NO, 2, or a polypeptide
having at least 75% and more preferably at least 80% sequence identity thereto
which has OPT activity; and a nucleotide sequence encoding the polypeptid'de
of
SEQ ID NO: 2 truncated at its amino terminus by between 30 to 56 amino acid
residues, or a polypeptide having at least 75% and more preferably at least
80%
sequence identity thereto which has OPT activity. The 0S1 transgene may be
encoded by the polynucleotide of SEQ ID NO. 3 or SEQ ID NO. 6 or a nucleotide
sequence having at least 75% and more preferably at least 80% identity to SEQ
ID NO,, 3 or SEQ ID NO: 6, and encoding a polypeptide having OPT activity; and
a
nucleotide sequence encoding the polypeptide of SEQ ID NO: 4 or 7, or a
polypeptide having at least 75% and more preferably at least 80% sequence
identity thereto which has S activity.
26
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
In another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Grape derived GPT, such as the Grape GPTs of SEQ ID NO, 9 and
SEQ ID NO: 31, and the GS transgene is a GS1 polynucleotide. The GPT
transgene may be encoded by the nucleotide sequence of SEQ ID NO. 8; a
nucleotide sequence having at least 76% and more preferably at least 80%
identity to SEQ ID NQ. 8, and encoding a polypeptide having GPT activity; a
nucleotide sequence encoding the polypeptide of SEQ ID NO 9 or SEQ ID NO:
31, or a polypeptide having at least 75% and more preferably at least 80%
sequence identity thereto which has GPT activity.
In yet another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Rice derived GPT, such as the Rice GPTs of SEQ ID NO 11 and
SEQ ID NO, 32, and the GS transgene is a GS1 polynucleotide. The GPT
transgene may be encoded by the nucleotide sequence of SEQ ID NO,, 10; a
nucleotide sequence having at least 75% and more preferably at least 80%
identity to SEQ ID NQ: 10, and encoding a polypeptide having GPT activity; a
nucleotide sequence encoding the polypeptide of SEQ ID NQ: 11 or SEQ ID NO:
32, or a polypeptide having at least 75% and more preferably at least 80%
sequence identity thereto which has GPT activity.
In yet another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Soybean derived GPT, such as the Soybean GPTs of SEQ ID NO,, 13,
SEQ IS NCB: 33 or SEQ ID NO, 33 with a further Isoleucine at the N-terminus of
the sequence, and the S transgene is a GS1 polynucleotide. The GPT
transgene may be encoded by the nucleotide sequence of SEQ ID NO, 12; a
nucleotide sequence having at least 75% and more preferably at least 80%
identity to SEQ ID NO: 12, and encoding a polypeptide having GPT activity;-. a
nucleotide sequence encoding the polypeptide of SEQ ID NO: 13 or SEQ ID NO,
33 or SEQ ID NO, 33 with a further Isoleucine at the N-terminus of the
sequence,
.30 or a polypeptide having at least 75% and more preferably at least 80%
sequence
identity thereto which has GPT activity.
27
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
In yet another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Barley derived GPT, such as the Barley GPTs of SEQ ID NO, 15 and
SEQ ID NO; 34, and the GS transgene is a GS1 polynucleotide. The GPT
transgene may be encoded by the nucleotide sequence of SEQ ID NO: 14; a
nucleotide sequence having at least 75% and more preferably at least 80%
identity to SEQ ID NO: 10, and encoding a polypeptide having GPT activity, a
nucleotide sequence encoding the polypeptide of SEQ ID NO: 15 or SEQ ID NO,
34, or a polypeptide having at least à 5% and more preferably at least 80%
sequence identity thereto which has GPT activity.
In yet another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Zebra fish derived GPT, such as the Zebra fish GPTs of SEQ ID NO.
17 and SEQ ID NO, 35, and the GS transgene is a GS1 polynuleotide. The GPT
transgene may be encoded by the nucleotide sequence of SEQ ID NO,, 16; a
nucleotide sequence having at least 75% and more preferably at least 80%
identity to SEQ ID NQ: 16, and encoding a polypeptide having GPT activity; a
nucleotide sequence encoding the polypeptide of SEQ ID NQ: 17 or SEQ ID NO:
35, or a polypeptide having at least 75% and more preferably at least 80%
sequence identity thereto which has GPT activity.
In yet another specific embodiment, the GPT transgene is a GPT polynucleotide
encoding a Bamboo derived GPT, such as the Bamboo GPT of SEQ ID NO. 36,
and the GS transgene is a GS1 polynucleotide. The GIRT transgene may be
encoded by a nucleotide sequence encoding the polypeptide of SEQ ID NO, 88,
or a polypeptide having at least 75% and more preferably at least 80% sequence
identity thereto which has GPT activity.
Other GPT polynucleotide suitable for use as GPT transgenes in the practice of
the invention may be obtained by various means, as will be appreciated by one
:30 skilled in the art, tested for the ability to direct the expression of a
GPT with GPT
activity in a recombinant expression system (i.e., E. coil (see Examples 20-
23), in
a transient in p/ante expression system (see Example 19), or in a transgenic
plant
(see Examples 1-18).
28
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TRANSGENE CONSTRUCTS/ EXPRESSION VECTORS
In order to generate the transgenic plants of the invention, the gene coding
sequence for the desired trans ene(s) must be incorporated into a nucleic
acid'.
construct (also interchangeably referred to herein as a (transgene) expression
vector, expression cassette, expression construct or expressible genetic
construct) which can direct the expression of the transgene sequence in
transformed plant cells. Such nucleic acid constructs carrying the
transgene(s) of
interest may be introduced into a plant cell or cells using a number of
methods
known in the art, including but not limited to electroporation, DNA
bombardment or
biolistic approaches, micro nj ction, and via the use of various DNA-based
vectors
such as Agro acterium himefaciens and Agrobacterium rhizogenes vectors.
Once introduced into the transformed plant cell, the nucleic acid construct
may
direct the expression of the incorporated transgene(s) (i.e., GPT), either in
a
transient or stable fashion. Stable expression is preferred, and is achieved
by
utilizing plant transformation vectors which are able to direct the
chromosomal
integration of the transgene construct. Once a plant cell has been
successfully
transformed, it may be cultivated to regenerate a transgenic plant.
:20 A large number of expression vectors suitable for driving the constitutive
or
induced expression of inserted genes in transformed plants are known. In
addition, various transient expression vectors and systems are known. To a
large
extent, appropriate expression vectors are selected for use in a particular
method
of gene transformation (see, infra), Broadly speaking., a typical plant
expression
vector for generating transgenic plants will comprise the transgene of
interest
under the expression regulatory control of a promoter, a selectable marker for
assisting in the selection of transformants, and a transcriptional terminator
sequence.
:30 More specifically, the basic elements of a nucleic acid construct for use
in
generating the transgenic plants of the invention are. a suitable promoter
capable
of directing the functional expression of the transgene(s) in a transformed
plant
cell, the transgene (s) (i.e., GPT coding sequence) operably linked to the
29
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
promoter, preferably a suitable transcription termination sequence (Le.,
nopaline
synthetic enzyme gene terminator) operably linked to the transgene, and
typically
other elements useful for controlling the expression of the transgene, as well
as
one or more selectable marker genes suitable for selecting the desired
transgenic product (i.e., antibiotic resistance genes).
As Agrobacterium furnefaciens is the primary transformation system used to
generate transgenic plants, there are numerous vectors designed for
Agrobacterium transformation, For stable transformation, Agrobacterium systems
1.0 utilize "binary" vectors that permit plasmid manipulation in both E. cols
and
Agrobacterium, and typically contain one or more selectable markers to recover
transformed plants (Hellens at at, 2000, Technical focus: A guide to
Agrobacteriur r binary Ti vectors. Trends Plant Sci 5:446-451). Binary vectors
for
use in Agrobacterlum transformation systems typically comprise the borders of
T-
DNA, multiple cloning sites, replication functions for Escherichia coil and A.
tumefaciens, and selectable marker and reporter genes.
So-called "super-binary" vectors provide higher transformation efficiencies,
and
generally comprise additional virulence genes from a Ti (Komari et at, 2006,
Methods iÃol. Biel, 343. 15-41). Super binary vectors are typically used in
plants
which exhibit lower transformation efficiencies, such as cereals. Such
additional
virulence genes include without limitation virB, v/rE, and virG (Vain et at,
2004,
The effect of additional virulence genes on transformaton efficiency,
transgene
integration and expression in rice plants using the pGreenlpoup dual binary
vector system. Transgenic Res. 13: 593-603; Srivatanakul et at, 2000,
Additional
virulence genes influence transgene expression: transgene copy number,
integration pattern and expression. J. Plant Physiol. 157, 685-690; Park et
at,
2000, Shorter T-DNA or additional virulence genes improve Agrobacterium-
mediated transformation. Theor. Appl. Genet. 101, 1015-1020; Jin et at, 1937,
Genes responsible for the superviru/ence phenotype of Arobacterium
tumefaciens A281. J. Bacteriol. 169: 4417-4425).
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
In the embodiments exemplified herein (see Examples, infra), expression
vectors
which place the inserted transgene() under the control of the constitutive
CaMV
35S promoter and the RuBi co promoter are employed. A number of expression
vectors which utilize the CaMV 35S and RuBsCo promoter are known and/or
commercially available and/or derivable using ordinary skill in the art,
PLANT PROMOTERS
The term 'promoter' is used to designate a region in the genome sequence
upstream of a gene transcription start site (TS S), although sequences
downstream of TSS may also affect transcription initiation as well. Promoter
elements select the transcription initiation point, transcription specificity
and rate.
Depending on the distance from the T5, the terms of 'proximal promoter'
(several hundreds nucleotides around the T SS) and 'distal promoter'
(thousands
and more nucleotides upstream of the TSS) are also used. Both proximal and
distal promoters include sets of various elements participating in the complex
process of cell-, issue-, organ-, developmental stage and environmental
factors-
specific regulation of transcription. Most promoter elements regulating TSS
selection are localized in the proximal promoter.
A large number of promoters which are functional in plants are known in the
art.
In constructing GPT and GS transgene constructs, the selected promoter(s) may
be constitutive, non-specific promoters such as the Cauliflower Mosaic Virus
35S
ribosomal promoter (CaMV 3 promoter), which is widely employed for the
expression of transgenes in plants. Examples of other strong constitutive
promoters include without limitation the rice actin 1 promoter, the aMV 19S
promoter, the Ti plasmid nopaline synthase promoter, the alcohol dehydrogenase
promoter and the sucrose synthase promoter.
Alternatively, in some embodiments, it may be desirable to select a promoter
based upon the desired plant cells to be transformed by the transgene
construct,
the desired expression level of the transgene, the desired tissue or
subcellular
compartment for transgene expression, the developmental stage targeted, and
31
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
the like.
For example, when expression in photosynthetic tissues and compartments is
desired, a promoter of the ribulose bisphosphate carboxylase (RuBisCo) gene
may be employed. In the Examples which follow, expressible nucleic acid
constructs comprising GPT and GS1 transgenes under the control of a tomato
RuBisCo promoter were prepared and used in the generation of trans genic
plants
or to assay for GPT activity in pia za or in E. col .
When the expression in seeds is desired, promoters of various seed storage
protein genes may be employed. For expression in fruits, a fruit specific
promoter
such as tomato 2A11 may be used. Examples of other tissue specific promoters
include the promoters encoding lectin (Vodkin et a1., 1983, Cell 34:1023x31;.
Lindstrom at al,, 1990, Developmental Genetics 11:160-167), corn alcohol
dehydrogenase 1 (Vogel et al, 1989, J. Cell. Biochem. (Suppl. 0) 13:Part D
Dennis et at, 1984, Nucl. Acids Res_, 12(9): 3983-4000), corn light harvesting
complex (Simpson, 1986, Science, 233 34-38; Bansal at at, 1992, Proc. Natl.
Acad. Sci, USA, 89: 3654-3658), corn heat shock protein (Odell at at, 1988,
Nature, 313: 810-812; Rochester et at, 1986, EMBO J., 8: 451-458), pea small
:20 subunit RuBP carboxylase (Paulsen at at, 1986, Mol. Gen. Genet., 206(2):
193-
200; Cashmere at al., 1983, Gen. Eng. Plants, Plenum Press, New York, pp 29-
38), Ti pl smld mannoplne synthase and Ti plasm id nopal ne synthase
(Langrld'ge
et at, 1989, Proc. Natl. Acad. Sci. USA, 86: 3219-3223), petunia chalcone
isomerase (Van Tunen et at, 1988, FMBO J. 7(8):. 1257-1263), bean glycine rich
protein 1 (Keller at at, 1989, EMBO J. 8(5): 1309-1314), truncated CaMV 35s
(Odell et at, 1985, supra), potato patatin (Wenzier et at, 1989, Plant Mol.
Biol. 12'
41-50), root cell (Conkling et al., 1990, Plant Physiol. 93, 1203-1211), maize
zein
(Reina at at, 1990, Nucl. Acids Res. 18(21): 6426; Kriz et al., 1987, Mol.
Gen.
Genet. 207(1): 90-98;' Wandelt and Fix, 1989, Nuc. Acids Res. 17(6): 2354;
:30 Langridge and Fein, 1983, Cell 34: 1015-1022., Reina at at, 1990, Nucl.
Acids
Res. 18(21): 6426), globulin-1 (Belanger and Kriz, 1991, Genetics 129: 863-
872),
ct-t bulin (Carpenter at at, 1992, Plant Cell 4(): 667-571, Uribe et at, 1998,
Plant
Mot Biol. 37(6): 1069-1078), cab (Sullivan, et at, 1989, Mel. Gen. Genet.
215(3):
32
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
431-440), PEPCase (Hudspeth and Grula, 1989, Plant Mol, Biol. 12: 579-589), f
gene complex (Chandler et al., 1989, The Plant Cell 1 1175-1183), chalcone
synthase (Franken et al., 1991, EMBO J. 10(9): 2605-2612) and 9lutamine
synthetase promoters (U.S. Pat. No. 5,391,725; Edwards at at., 1990, Proc.
Natl.
Acad. ScÃ. USA 87; 3459-3463; Brears et al., 1991, Plant J. 1(2): 235-244).
in addition to constitutive promoters, various inducible promoter sequences
may
be employed in cases where it is desirable to regulate transgene expression as
the transgenÃc plant regenerates, matures, flowers, etc. Examples of such
inducible promoters include promoters of heat shock genes, protection
responding
genes (Ã.e., phenylalanine ammonia lyase; see, for example Bevan at al., 1989,
EMBO J. 8(7): 899-906), wound responding genes (i.e., cell wall protein
genes),
chemically inducible genes (i.e., nitrate reductase, chitina e) and dark
inducible
genes (i.e., asparagine synthetase see, for example U.S. Patent No.
5,256,558).
Also, a number of plant nuclear genes are activated by lightõ including gene
families encoding the major chlorophyll a/b binding proteins (cab) as well as
the
small subunit of ribulose- 1,5-bi phos hate carboxylase (rboS) (see, for
example,
Tobin and Silverthorne, 1985, Annu. Rev. Plant PhysioÃ, 36,,569-593, Dean et
al.,
1989, Annu. Rev. Plant PhysioÃ. 40: 415-439,),
Other inducible promoters include ABA- and turgor-inducible promoters, the
auxin-binding protein gene promoter (Schwob at al., 1993, Plant J.
4(3):423432),
the UDP glucose flavonoid glycosyl-transferase gene promoter (Ralston et al.,
1988, Genetics 119(1): 185-197); the MPI proteinase inhibitor promoter
(Cordero
at aÃ., 1994, Plant J. 6(2); 141-150), the glyceraldehyde-3-phosphate
dehydrogenase gene promoter (Kohler at al., 1995, Plant Mol, Biol. 29(6). 1293-
1298; Quigley et al., 1989, J. Mel. Evol.. 29(5); 412421; Martinez at
aÃ.,1989, J.
à ol. Biol. 208(4): 551-565) and light inducible plastid glutamine synthetase
gene
from pea (U.S. Pat. No. 5,391,725, Edwards et al., 1990, supra).
:30
For a review of plant promoters used in plant transgenic plant technology, see
Potenza et al., 2004, In Vitro Cell. bevel. Blot - Plant, 40(1): 1-22. For a
review of
33
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
synthetic plant promoter engineering, see, for example, Venter, 1., 2007,
Trend's
Plant Sci., 12(3): 118-124.
GLUTAMINE PHENYLPYRUVATE TRANSAMINASE (GPT) TRANS ENE
The present invention discloses for the first time that plants contain a
glutamine
phenylpyruvate transaminase (GPT) enzyme which is directly functional in the
synthesis of the signal metabolite 2-hydroxy-5-oxoprolÃne, Until now no plant
transaminase with a defined function has been described. Applicants have
isolated and tested GPT polynucleotide coding sequences derived from several
plant and animal species, and have successfully incorporated the gene into
heterologous transgenic host plants which exhibit markedly improved growth
characteristics, including faster growth, higher foliar protein content,
increased
glutamine synthetase activity in foliar tissue, and faster G fixation rates.
In the practice of the invention, the OPT gene functions as one of at least
two
transgenes incorporated into the transgenic plants of the invention, the other
being the glutamine sythetase gene (see infra).
It is expected that all plant species contain a OPT which functions in the
same
metabolic pathway, involving the biosynthesis of the signal metabolite 2-
hydroxyy
5ooproiIne. Thus, in the practice of the invention, any plant gene encoding a
OPT homolog or functional variants thereof may be useful in the generation of
trap genic plants of this invention, Moreover, given the structural similarity
between various plant OPT protein structures and the putative ( and
biologically
active) OPT homolog from Danio reria (Zebra fish) (see Example 22), other non-
plant OPT homology may be used in preparing OPT transgenes for use in
generating the transgenic plants of the invention.
When individually compared (by. BLAST alignment) to the Arabidopsis mature
protein sequence provided in SEQ ID NO: 30, the following sequence identities
and homologies (BLAST `positives', including similar amino acids) were
obtained
for the following mature OPT protein sequences:
34
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
[SEQ ID] or FIG. NO. ORIGIN % IDENTITY %POSITIVE
(31] Grape 84 93
[32] Rice 83 91
[33] Soybean 83 93
[34] Barley 82 91
[3] Zebra fish 83 g
[36] Bamboo 81 90
FIG. 2 Corn 79 0
l0 FIG. 2 Castor 84 93
1.2 Poplar 85 93
Underscoring the conserved nature of the structure of the GPT protein across
most plant species, the conservation seen within the above plant species
extends
15 to the non-human putative GPTs from Zebra fish and hlamydomonas. In the
case of Zebra fish, the extent of identity is very high (83% amino acid
sequence
dentiy with the mature ArabÃdopsis GPT of E ID NO: 30, and 92% homologous
taking similar amino acid residues into account). The Zebra fish mature GPT
was
confirmed by expressing it in E coil and demonstrating biological activity
:20 (synthesis of 2-oxoglutaramate).
n order to determine whether putative GPT homologs would be suitable for
generating the growth-enhanced transgenic plants of the invention, one need
nitially express the coding sequence thereof in E. co/i or another suitable
host
25 and determine whether the 2-oxoglutaramate signal metabolite is synthesized
at
increased levels (see Examples 19-23). Where such an increase is
demonstrated., the coding sequence may then be introduced into both
homologous plant hosts and heterologous plant hosts, and growth
characteristics
evaluated. Any assay that is capable of detecting 2-oxoglutaramate with
.30 specificity may be used for this purpose, including without limitation the
NMR and
HPLC assays described in Example 2, infra. In addition, assays which measure
GPT activity directly may be employed, such as the GPT activity assay
described
n Example 7.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Any plant GPT wits 2-oxogl taramate synthesis activity may be used to
transform
plant cells in order to generate transgenic plants of the invention. There
appears
to be a high level of structural homology among plant species, which appears
to
extend beyond plants, as evidenced by the close homology between various plant
GPT proteins and the putative Zebra fish GPT homolog. Therefore, various plant
GPT genes may be used to generate growth-enhanced transgenic plants in a
variety of heterologous plant species. In addition, GPT transgenes expressed
in a
homologous plant would be expected to result in the desired enhanced-growth
characteristics as well (Le., rice glutamine transaminase over-expressed in
tranag.enic rice plants), although it is possible that regulation within a
homologous
cell may attenuate the expression of the transgene in some fashion that may
not
be operable in a heterologous cell.
GLUTAMINE SYNT HETASE: GS TRANSGEN .:
In the practice of the invention, the glutamine synthetase (GS) gene functions
as
one of at least two transgenes incorporated into the transgenic plants of the
invention (GPT being the other of the two).
Glutamine synthetase plays a key role in nitrogen metabolism in plants, as
well as
in animals and bacteria. The GS enzyme catalyzes the addition of ammonium to
glutamate to synthesize glut mine in an ATP-dependent reaction. GS enzymes
215 from assorted species show highly conserved amino acid residues considered
to
be important for active site function, indicating that GS enzymes function
similarly
(for review, see Eisenberg at al., Biochimica et Biophysica Acta, 1477:122
145,
2000).
G is distributed in different subcellular locations (chloroplast and
cytoplasm) and
is found in various plant tissues, including leaf, root, shoot, seeds and
fruits.
There are two major isoforms of plant G : the cystolic isoform (G 1) and the
plastidic (chloroplastic) isoform (GS2). GS2 is principally found in leaf
tissue and
functions in the assimilation of ammonia produced by photorespiration. or by
3
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
nitrate reduction. GS1 is mainly found in leaf and root tissue, typically
exists in a
number of different isoforms in higher plants, and functions to assimilate
ammonia
produced by all other physiological processes (Coruzzi, 1991, Plant Science
74:
145-155; McGrath and Coruzzi, 1991, Plant J. 1(3): 275-280; Lam at at, 1996,
Ann. Rev., Plant Physloly Plant Mot Biol. 47: 569-593; Stitt, 1999, Curr. Op.
Plant
Biol. 2: 1788-186 Oliveira at at, 2001, Brazilian J. Med. Biol. Res. 34: 567-
575).
Multiple S genes are associated with a complex promoter repertoire which
enable the expression of CS in an organ and tissue specific manner, as well as
in
an environmental factor-dependent manner.
Plant glut mine synthetase consists of eight subunits, and the native enzyme
in
plants has a molecular mass ranging from 320 to 380 kD, each subunit having a
molecular mass of between 38 and 45 kD.. The GSI genes of several plants,
especially legumes, have been cloned and sequenced (Tischer at at, 1986, M'ol
Gen Genet. 203, 221-229; Gebhardt at al.. 1986, EMBO 15.1429-1435; Tinge'
at al., 1987, EMBO J. 6: 1-9, T in ey at at, 1988, J Biol Chem. 263; 9661-
9657;
Bennett at al., 1989, Plant Mel Biol. 12: 553-565; Boron and Legocki, 1993,
Gene
136: 95-102, Roche at at, 1993, Plant Mol Biol. 22, 971-983; Marsolier at at,
1995, Plant Mol Biol. 27: 1-16; Temple at at, 1995, Mol Plant-Microbe
Interact. 8.
:20 218-227), All have been found to be encoded by nuclear genes (for review,
see,
Morey at at, 2002, Plant Physiol. 128(1) 182-193).
Chloroplastic GS2 appears to be encoded by a single gene, while various
cystoloic GS1 isoforms are encoded within multigene families (Tingey at al.,
1987,
supra; Sakamoto at at, 1989, Plant Mol. Biol. 13; 611-614, Brears at al, 1991,
supra, U at al., 1993, Plant biol. Biol., 23.401-407, Dubois at al., 1996,
Plant Mol.
Biol, 31:803-817; Lam at al., 1996, supra). GS1 multigene families appear to
encode different subunits which may combine to form homo- or hetero-octamers,
and the different members show a unique expression pattern suggesting that the
:39 gene members are differentially regulated, which may relate to the various
functional roles of glutamine synthetase plays in overall nitrogen metabolism
(Gebhardt at at, 1986, supra; Tingey at al., 1987, supra; Bennett at at, 1989,
supra, Walker and Coruzzi, 1989, supra; Peterman and Goodman, 1991, Mol Gen
37
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Genet. 1991,330.145-154,, Marsoli r et al., 1995, supra; Temple et at., 1995,
supra; Dubois et at., 1996, supra).
In one embodiment, a GS1 gene coding sequence is employed to generate GS
transg.ene constructs. In particular embodiments, further described in the
Examples, infra, the Alfalfa or Arabidopsis GS1 gene coding sequence is used
to
generate a transgene construct that may be used to generate a transgenic plant
expressing the GS1 transgene..s an example, such a Instruct may be used to
transform Agro acteria. The transformed Agrobacteria are then used to generate
TO transgenic plants. Example 5 demonstrates the generation of TO S1
transg.enic tobacco plants using this approach. Similarly, Examples 6 and 17
demonstrates the generation of TO GS1 transgenic tomato plants, Example
demonstrates the generation of T0, GS1 transgenic pepper plants, Examples 9
and
10 demonstrate the generation of TO GS1 transgenic bean plants, Examples 11
and 12 demonstrate the generation of TO S1 transgenic co pea plants, Example
13 demonstrates the generation of T0z GSI transgenic alfalfa plants, Example
14
demonstrates the generation of if0 GS1 transgenic cantaloupe plants, Example
15
demonstrates the generation of TO G1 transgenic pumpkin plants, Example 16
demonstrates the generation of Tr G S1 transgenic Arabidopsis plants, and
:20 Example 18 demonstrates the generation of Ti GS1 transgenic Cantaloupe
plants.
TRANSCRIPTION TERMINATORS.:
In preferred embodiments, a 3' transcription termination sequence is
incorporated
downstream of the transgene in order to direct the termination of
transcription and
permit correct polyadenylation of the mRNA transcript. Suitable transcription
terminators are those which are known to function in plants, including without
limitation, the nopaline synthase(NOS) and octopine synthase (OCS) genes of
Agrobacferium tumefaciens, the T7 transcript from the octopine synthase gene,
the 3' end of the protease inhibitor I or 11 genes from potato or tomato, the
CaMV
36S terminator, the trnl terminator and the pea rbcS E9 terminator. In
addition, a
gene's native transcription terminator may be used. In specific embodiments,
described by way of the Examples, infra, the nopaline synthase transcription
38
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
terminator is employed.
SELECTABLE MARKERS:
Selectable markers are typically included in transgene expression vectors in
order
to provide a means for selecting transformants. While various types of markers
are available, various negative selection markers are typically utilized,
including
those which confer resistance to a selection agent that inhibits or kills
unt nsfermed cells, such as genes which impart resistance to an antibiotic
(such
as kanamycin, gentamycin, anamycin, hygromycin and hygromycinB) or
resistance to a herbicide (such as sulfonyiurea, gulfosinate, phosphinothricÃn
and
glyphosate). Screenable markers include, for example, genes encoding
glucuronidase (Jefferson, 1987, Plant Mol. Biol. Rep 5: 387405), genes
encoding
luciferase (Ow et aL, 1986, Science 234. 855-859) and various genes encoding
proteins involved in the production or control of anthocyanin pigments (See,
for
example, U.S. Patent 6,573,432). The E. coil glucuronidase gene (gus, gusA or
uldA) has become a widely used selection marker in plant transgenics, largely
because of the glucuronidase enzyme's stability, high sensitivity and ease of
detection (e.g., fluorometric, spectrophotometric, various histochemical
methods).
Moreover, there is essentially no detectable glucuronidase in most higher
plant
species.
TRANSFORMATION METHODOLOGIES AND YSTEÃM .-
'Various methods for introducing the transgene expression
vector constructs of the
invention into a plant or plant cell are well known to those skilled in the
art, and
any capable of transforming the target plant or plant cell may be utilized.
Agrobacterium-mediated transformation is perhaps the most common method',
utilized in plant transgeni , and protocols for Agrob cter um-mediated
transformation of a large number of plants are extensively described in the
literature (see, for example, Ag obacterium Protocols, Wan, ed., Humana Press,
2 edition, 2006). Agrobacterium to eraci ns is a Gram negative soil bacteria
that causes tumors (Crown Gall disease) in a great many divot species, via the
39
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
insertion of a small segment of tumor-inducing DNA ( "T-DNA.", 'transfer DNA')
into
the plant cell, which is incorporated at a semi-random location into the plant
genome, and which eventually may become stably incorporated there. Directly
repeated DNA sequences, called T-DNA borders, define the left and the right
ends of the T-DNA. The T-DNA can be physically separated from the remainder
of the Ti-plasmid, creating a `binary vector' system.
Agrobacteriur transformation may be used for stably transforming dicots,
monocots, and cells thereof (Rogers et al_ 1986, Methods Enzymot, 118: 627-
641; Hernalsteen et al., 1984, EMBO J., 3: 3039-3041; Hoykass-Van Slogteren et
al., 1984, Nature, 311: 763-764; Grimsley et al., 1987, Nature 325: 167-1679;
Boulton et al., 1989, Plant Mol. Biol. 12: 31-40, Gould et at, 1991, Plant
Ph:yslol.
g ; 426-434). Various methods for introducing DNA into Agrobacteria are known,
including electroporation, freeze/thaw methods, and triparental mating. The
most
efficient method of placing foreign DNA into Agrobacterium is via
electroporation
(Wise at at, 2006, Three Methods for the Introduction of Foreign DNA into
Agrobacterium, Methods in Molecular Biology, vol. 343: Agroba terlum
Protocols,
2/a, volume 1; Ed,, Wang, Humana Press Inc., Totowa, NJ, pp. 43-53). In
addition, given that a large percentage of T-DN.As do not integrate,
210 Agrobacteertum-mediated transformation may be used to obtain: transient
expression of a transgene via the transcriptional competency of unincorporated
transgene construct molecules (Helens at at, 2005, Plant Methods 1:13),
A large number of Agrobacter 'um transformation vectors and methods have been
described (Karimi at at, 2002, Trends Plant Sci. 7(5): 193-5), and many such
vectors may be obtained commercially (for example, Ãnvitrogen). In addition, a
growing number of 'open-source" Agrobacterrum transformation vectors are
available (for example, pcambia vectors; Cambia, Canberra, Australia). See,
also,
subsection herein on TI AN GENE CONSTRUCTS, supra. In a specific
embodiment described further in the Examples, a pMON315-based vector was
used in the leaf disc transformation system of Horsch et. at (Horsch at
aÃ.,1995.
Science 227.,1229-1231) to generate growth enhanced transgenic tobacco and
tomato plants.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Other commonly used transformation methods that may be employed in
generating the transgenic plants of the invention include without limitation
microprojectile bombardment, or biolistic transformation methods, protoplast
transformation of naked DNA by calcium, polyethylene glycol (PEG) or
electroporation (Paszkowski at at, 1984, EMBO J. 3: 2727-2722; Potrykus et at,
1985, Mol. Gen, Genet. 199:. 169-177; Fromm at at, 1985, Proc. Nat. Acad. Sci.
USA 82: 5824-5828; Shimamoto at al., 1989, Nature, 338: 274.276.
Biolistic transformation involves injecting millions of DNA-coated metal
particles
into target cells or tissues using a biolistic device (or "gene gun"), several
kinds of
which are available commercially; once inside the cell, the DNA elutes off the
particles and a portion may be stably incorporated into one or more of the
cell's
chromosomes (for review, see Kikkert at at, 2005, Stable Transformation of
Plant
Cells by Particle Bombardment Biobstic , in, Methods in Molecular Biology,
vol.
286: Transgenic Plants: Methods and Protocols, Ed. L. Pena, Humana Press inc.,
Totowa, NJ).
Electroporation is a technique that utilizes short, high-intensity electric
fields to
:20 permeabilize reversibly the lipid bilayers of cell membranes (see, for
example,
Fisk and Dandekar, 2005, Introduction and Expression of Transgenes in Plant
Protoplasts, in, Methods in Molecular Biology, vol. 286, Trans epic Plants.
Methods and Protocols, Ed, L. Pena,, Humana Press Inc.,, Totowa, NJ, pp. 79-
90;
Fromm at al-1987, Electroporation of DNA and RNA into plant protoplastt , in
Methods in Enzymology, Vol. 153, Wu and Grossman, eds., Academic Press,
London, UK, pp. 351-366; Joersbo and Brunstedt, 1991. Electropor tfo .
mechanism and transient expression; stable transformation and biological
effects
in plant protoplasts. Physiol. Plant. 81, 256-264; Bates, 1994, Genetic
transformation of plants by protoplast electroporation. Mol. Biotech. 2: 136-
145;
.30 Dillen at al., 1998, Electroporation-mediated DNA transfer to plant
protoplasts and
intact plant tissues for transient gene expression assays, in Cell Biology,
Vol. 4,
ed., Cells, Academic Press, London, UK, pp. 92-99), The technique operates by
creating aqueous pores in the bacterial membrane, which are of sufficiently
large
41
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
size to allow DNA molecules (and other macromolecules) to enter the cell,
where
the transgene expression construct (as T-DNA) may be stably incorporated into
plant enomic DNA, leading to the generation of transformed cells that can
subsequently be regenerated into trans genic plants.
Newer transformation methods include so-called :.floral dip" methods, which
offer
the promise of simplicity, without requiring plant tissue culture, as is the
case with
all other commonly used transformation methodologies (Bent et al., 2006,
Arabidopsis thahfana Floral Dip 2/e, Transformation Method,. -Methods Mol
Biol, vol.
Humana Press Inc,,
It) 343, r G d uiii Protocols, volume ; ,r Wanq
Totowa, NJ, pp. 87-103; Clough and Bent, 1998, Floral dip: a simplified method
for Agrobacter/u-mediated transformat/on of Arabidopsis that/ana, Plant J. I:
735-743). However, with the exception of Arabidopsls, these methods have not
been widely used across a broad spectrum of different plant species. Briefly,
floral dip transformation is accomplished by dipping or spraying ftowerng
plants in
with an appropriate strain of Agrobacterium tumefaciens. Seeds collected from
these T0, plants are then germinated under selection to identify trans epic TJ
individuals. Example 16 demonstrated floral dip inoculation of Arabidopsis to
generate transgenic Arabidopsis plants.
Other transformation methods include those in which the developing seeds or
seedlings of plants are transformed using vectors such as Agrobacterium
vectors.
For example, as exemplified in Example 8, such vectors may be used to
transform
developing seeds by injecting a suspension or mixture of the vector ii,e.,
Agrobacteriaj directly into the seed cavity of developing pods (i.e., pepper
pods,
bean pods, pea pods and the like). Seedlings may be transformed as described
for Alfalfa in Example 13. Germinating seeds may be transformed as described
for Camelina in Example 18. Intra-fruit methods, in which the vector is
injected:
into fruit or developing fruit, may be used as described for Cantaloupe melons
in
.30 Example 14 and pumpkins in Example 15.
Still other transformation methods include those in which the flower structure
is
targeted for vector inoculation, such as the flower inoculation methods
described
4
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
for beans in Examples 9 and 10, peas in Examples 11 and 12 and tomatoes in
Example 17.
The foregoing plant transformation methodologies may be used to introduce
transgenes into a number of different plant cells and tissues, including
without
limitation, whole plants, tissue and organ expÃants including chloroplasts,
flowering tissues and cells, protoplasts, meristern cells, callus, immature
embryos
and gametic cells such as mierospores, pollen, sperm and egg cells, tissue
cultured cells of any of the foregoing, any other cells from which: a fertile
regenerated transgenic plant may be generated. Callus is initiated from tissue
sources including, but not limited to, immature embryos, seedling apical
meristems, microspores and the lice. Cells capable of proliferating as callus
are
also recipient cells for genetic transformation.
Methods of regenerating individual plants from transformed plant cells,
tissues or
organs are known and are described for numerous plant species.
As an illustration, transformed pÃantlets (derived from transformed cells or
tissues)
are cultured in a root-permissive growth medium supplemented with the
selective
:20 agent used in the transformation strategy (i.e., and antibiotic such as
kanamycin).
Once rooted, transformed plantlets are then transferred to soil and allowed to
grow to maturity. Upon flowering, the mature plants are preferably selfed
(self-
fertilized), and the resultant seeds harvested and used to grow subsequent
generations. Examples 3 6 describe the regeneration of transgenic tobacco and
tomato plants.
T0, transgenic plants may be used to generate subsequent generations (e.g.,
T1,
T2, etc.) by selfing of primary or secondary transformants, or by sexual
crossing of
primary or secondary transformants with other plants (transformed or
:30 untransformed). For example, as described in Example 7, infra, individual
plants
over expressing the Alfalfa GSI gene and outperforming wiidtype plants were
crossed with individual plants over-expressing the Arabidopsis GPT gene and
outperforming wildtype plants, by simple sexual crossing using manual pollen
43
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
transfer. Reciprocal crosses were made such that each plant served as the male
in a set of crosses and each plant served as the female in a second set of
crosses. During the mature plant growth stage, the plants are typically
examined:
for growth phenotype, C02 fixation rate, etc. (see following subsection).
SELECTION OF GROWTH-ENHANCED TP N EI IC PLANT :
Transgenic plants may be selected, screened and characterized using standard
methodologies, The preferred transgenic plants of the invention will exhibit
one or
more phenotypic characteristics indicative of enhanced growth and/or other
desirable agronomic properties. Transgenic plants are typically regenerated
under selective pressure in order to select transformants prior to creating
subsequent transgenic plant generations. In addition, the selective pressure
used
may be employed beyond To, generations in order to ensure the presence of the
desired transgene expression construct or cassette.
TO transformed plant cells, caili, tissues or plants may be identified and
isolated by
selecting or screening for the genetic composition of and/or the phenotypic
characteristics encoded by marker genes contained in the transgene expression
construct used for the transformation. For example, selection may be conducted
by growing potentially-transformed plants. tissues or cells in a growth medium
containing a repressive amount of antibiotic or herbicide to which the
transforming
genetic construct can impart resistance. Further, the transformed plant cells,
tissues and plants can be identified by screening for the activity of marker
genes
215 (such as -glucuronidase) which may be present in the transgene expression
construct.
Various physical and biochemical methods may be employed for identifying
plants
containing the desired transgene expression construct, as is well known.
Examples of such methods include Southern blot analysis or various nucleic
acid
amplification methods (Le., PCR) for identifying the transgene, transgene
expression construct or elements thereof; Northern blotting, S1 RNase
protection,
reverse transcriptase PCR (RT-PCR) amplification for detecting and determining
the RNA transcription products; and protein gel electrophoresis, Western
blotting,
44
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
immunoprecipitation, enzyme immunoassay, and the like for identifying the
protein
encoded and expressed by the transgene.
In another approach, expression levels of genes, proteins and/or metabolic
compounds that are know to be modulated by transgene expression in the target
plant may be used to identify transformants. In one embodiment of the present
invention, increased levels of the signal metabolite 2-oxoglutaramate may be
used
to screen for desirable transformants, as exemplified in the Examples.
Similarly,
increased levels of GPT and/or activity may be assayed, as exemplified in the
Examples.
Ultimately, the transformed plants of the invention may be screened for
enhanced
growth and/or other desirable agronomic characteristics, Indeed, some degree
of
phenotypic screening is generally desirable in order to identify transformed
lines
with the fastest growth rates, the highest seed yields, etc., particularly
when
identifying plants for subsequent selfing, cross-breeding and back-crossing.
Various parameters may be used for this purpose, including without limitation,
growth rates, total fresh weights, dry weights, seed and fruit yields (number,
weight), seed and/or seed pod sizes, seed pod yields (e.g., number, weight),
leaf
:20 sizes, plant sizes, increased flowering, time to flowering, overall
protein content (in
seeds, fruits, plant tissues), specific protein content (i,e., GS), nitrogen
content,
free amino acid, and specific metabolic compound levels (i.e., 2-oxog lutara
mate),
Generally, these phenotypic measurements are compared with those obtained'.
from a parental identical or analogous plant line, an untransformed identical
or
analogous plant, or an identical or analogous wild-type plant (i.e., a normal
or
parental plant). Preferably, and at least initially, the measurement of the
chosen
phenotypic characteristic(s) in the target transgenic plant is done in
parallel with
measurement of the same characteristic(s) in a normal or parental plant.
Typically, multiple plants are used to establish the phenotypic desirability
and/or
:30 superiority of the transgenic plant in respect of any particular
phenotypic
characteristic.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Preferably, initial transformants are selected and then used to generate T and
subsequent generations by selling (self-fertilization), until the transgene
genotype
breeds true (i.e., the plant is homozygous for the transgene). In practice,
this is
accomplished by selfing for 3 or 4 generations, screening at each generation
for
the desired traits and selling those individuals. As exemplified herein,
transgenic
plant lines propagated through at least one sexual generation (Tobacco,
Arabidopsis, Tomato) demonstrated higher transgene product activities compared
to lines that did not have the benefit of sexual reproduction and the
concomitant
increase in transgene copy number.
Stable transgenic lines may be crossed and back-crossed to create varieties
with
any number of desired traits, including those with stacked transgenes,
multiple
copies of a transgene, etc. Additionally, stable transgenic plants may be
further
modified genetically, by transforming such plants with further transgenes or
additional copies of the parental transgene. Also contemplated are transgenic
plants created by single transformation events which introduce multiple copies
of
a given transgene or multiple transgenes. Various common breeding methods
are well know to those skilled in the art (see, e.g., Breeding Methods for
Cultivar
Development; Wilcox J. ed., American Society of Agronomy, Madison Wis.
:20 (1987)) .
In a another aspect, the invention provides transgenic plants characterized by
increased nitrogen use efficiency, Nitrogen use efficiency may be expressed as
plant yield per given amount of nitrogen. In the Examples provided herein, the
transgene and control plants all received the same nutrient solutions in the
same
amounts. The transgenic plants were consistently characterized by higher
yields,
and thus have higher nitrogen use efficiencies.
In yet another aspect, the invention provides transenic plants and seeds
thereof
:30 with increased tolerance to high salt growth conditions. This aspect of
the
invention is exemplified by Example 24, which describes the germination of
transgenic tobacco plant seeds in very high salt conditions( g mM NaCI)..
While
counterpart wild type tobacco seeds germinated at a rate of only about 10%, on
46
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
average, the transgenic tobacco seeds achieved nearly the same rate of
germination obtained under no salt conditions for both transgenic and wild
type
seeds, or about 92%.
EXAMPLES
Various aspects of the invention are further described and illustrated by way
of the
several examples which follow, none of which are intended to limit the scope
of
the invention.
1.13 EXAMPLE 1: ISLOATION OF AP SIDOP IS GLUAMINE PH YLPYR VATS
TRA. SAMINASE (GPT) GENE:
In an attempt to locate a plant enzyme that is directly involved in the
synthesis of
the signal metabolite 2-oxoglutaramate, applicants hypothesized that the
putative
plant enzyme might bear some degree of structural relationship to a human
protein that had been characterized as being involved in the synthesis of 2-
oxoglutaramate. The human protein, glutamine transaminase K (E. C. 2.6.1,64)
(also referred in the literature as cysteine conjugate -lyase, kyneurenine
aminotransferase, glutamine phenylpyruvate transaminase, and other names),
:20 had been shown to be involved in processing of cysteine conjugates of
halogenated xenobiotics (Perry et al., 1995, FEBS Letters 360:277-280). Rather
than having an activity involved in nitrogen metabolism, however, human
cysteine
conjugate 1"s-.lyase has a detoxifying activity in humans, and in animals.
Nevertheless, the potential involvement of this protein in the synthesis of 2-
oxoglutaramate was of interest.
Using the protein sequence of human cysteine conjugate i&--lyase, a search
against the TIGR Abidosis plant database of protein sequences identified one
potentially related sequence, a polypeptide encoded by a partial sequence at
the
Arabido sis gene locus at Atl 87767 , sharing approximately 36% sequence
homology/identity across aligned regions.
47
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The full sling region of the gene was then amplified from an Arabidopsis cDNA
library (Stratagene) with the following primer pair,
5'-CCCATCGATGTACC TGGACATAAATGGTGTGATG-3'
5'- GATGGTACCTCAGACTTTTCTCTTAAGCTTCTGCTTC_3'
These primers were designed to incorporate CÃ;a à (ATCGAT and Kpn I
(GGTACC) restriction sites to facilitate subsequent subcloning into expression
vectors for generating transgenic plants. Takara ExTaq DNA polymerise enzyme
was used for high fidelity PCR using the following conditions initial
denaturing
94C for 4 minutes, 30 cycles of 94C 30 second, annealing at 55C for 30
seconds,
extension at 72C for 90 seconds, with a final extension of 72C for 7 minutes.
The
amplification product was digested with CÃa à and Kpn I restriction enzymes,
isolated from an agarose gel electrophoresis and ligated into vector pMon3l6
(Rogers, et. a!, 1987 Methods in Enzymology 153:253-277) which contains the
cauliflower mosaic virus (CaMV, also CMS') 35S constitutive promoter and the
nopaline synthase (NOS) 3' terminator. The ligation was transformed into DH5a
cells and transformants sequenced to verify the insert.
A 13 kb cDN.A was isolated and sequenced, and found to encode a full length
protein of 440 amino acids in length, including a putative chloroplast signal
sequence.
EXAMPLE 2: PRODUCTION OF BIOLOGICALLY ACTIVE RECOMBINANT
Ai BIDl PSIS GLUTAMINE PHENYLPYRUVATE TRANSAMINASE (GPT);
To test whether the protein encoded by the cDNA isolated as described: in
Example 1, supra, is capable of catalyzing the synthesis of 2- oxoglutaramate,
the
cDNA was expressed in E. coli, purified, and assayed for its ability to
synthesize
.30 2-oxogÃutaramate using a standard method,
NMR.Assay for 2 xoglutarar ate,
Briefly, the resulting purified protein was added to a reaction mixture
containing
150 mM Tris-HCI, pH 8,5, 1 mM beta mercaptoethanol, 200 mM glutamine, 100
48
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
mgt glyoxylate and 200 microM pyridoxal 5'-phosphate. The reaction mixture
without added test protein was used as a control. Test and control reaction
mixtures were incubated at 37 C for 20 hours, and then clarified by
centrifugation
to remove precipitated material. Supernatants were tested for the presence and
amount of 2-oxoglutaramate using C NMR with authentic chemically synthesized
2noxogiutaramate as a reference, The products of the reaction are 2-
oxoglutaramate and glycine, while the substrates (glutamine and glyoxylate)
diminish in abundance. The cyclic 2-oxoglutaramate gives rise to a distinctive
signal allowing it to be readily distinguished from the open chain glutamine
precursor.
HPL Assay for 24-olutaramate.
An alternative assay for GPT activity uses HPLC to determine 2-oxogÃutaramate
production, following a modification of Calderon et al., 1985, J Bacterial
161(2).
807-809. Briefly, a modified extraction buffer consisting of 25 M Tris-HCI pH
8.5, 1 mM EDTA, 20,uM FAD, 10 mM Cysteine, and -1. % (vffv) Mercaptoet anol.
Tissue samples from the test material (i.e., plant tissue) are added to the
extraction buffer at approximately a 1/3 ratio (w/v), incubated for 30 minutes
at
37"'C, and stopped with 200td of 20% TCA. After about 5 minutes, the assay
mixture is centrifuged and the supernatant used to quantify 2-oxoglutaramate
by
HPLC, using an ION-300 7.0mm ID X 30 cm L column, with a mobile phase in
0.011 h2SO4, a flow rate of approximately 0.2 ml /min, at 40'C. Injection
volume
is approximately 20 td, and retention time between about 38 and 39 minutes.
Detection is achieved with 210nm UV light.
Results Using NM Assa .
This experiment revealed that the test protein was able to catalyze the
synthesis
of 2- oxoglutaramate. Therefore, these data indicate that the isolated cDNA
encodes a glutamine phenylpyruvate transaminase that is directly involved in
the
30 synthesis of 2-oxoglutaramate in plants. Accordingly, the test protein was
designated A ra idfop s glutamine phenylpyruvate transaminase, or "GPT"
49
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The nucleotide sequence of the Arabidopsis OPT coding sequence is shown in
the Table of Sequences, SEQ ID NO, 1. The translated amino acid sequence of
the GPT protein is shown in SEQ ID NO, 2,
EXAMPLE 3; CREATION OF TRANSGENIC TOBACCO PLANTS OVER-
EXPRESSING ARABIDOPSIS OPT:
Generation of Plant Expression- Vector MGN-PJU:
Briefly, the plant expression vector pM on31 -PJU was constructed as follows.
The isolated cDNA encoding Arabidopsis GPT (Example 1) was cloned into the
Clal- :pni polylinker site of the pMON31 vector, which places the GPT gene
under the control of the constitutive cauliflower mosaic virus (CaMV) 35S
promoter and the nopaline synthase (NOS) transcriptional terminator. A
kanamycin resistance gene was included to provide a selectable marker.
A robacte,Iurx -Mediated Plant Transformations:
pMON-PJU and a control vector pMon316 (without inserted DNA) were
transferred to Agrobacteri m tumefaciens strain pTiTT37ASE using a standard
.20 electroporation method (McCormac et at, 1998, Molecular Biotechnology
9:155-
159), followed by plating on LB plates containing the antibiotics
spectinormycin
(100 micro gm 1 ml) and kanamycin (50 micro gm I ml). Antibiotic resistant
colonies of Agrobacterium were examined by PCR to assure that they contained
plamid.,
Nicotiana tabac m cv. Xanthi plants were transformed with pMON-PJU
transformed Agrobacteria using the leaf disc transformation system of Horsch
et.
at (Dorsch et al.,199 , Science 227.1229-1231).. Briefly, sterile leaf disks
were
inoculated and cultured for 2 days, then transferred to selective MS media
containing 100 pg/ml kanamycin and 500 pg/rnl clafaran. Transforman:ts were
confirmed by their ability to form roots in the selective media.
Generation of GIST Traansgenic Tobacco Plants:
Sterile leaf segments were allowed to develop callus on Murashige & Skoog
(M S) media from which the transformant plantlets emerged. These plantlets
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
were then transferred to the rooting-permissive selection medium (M&S medium
with kanamycin as the selection agent). The healthy, and now rooted,
transformed tobacco plantlets were then transferred to soil and allowed to
grow to
maturity and upon flowering the plants were selfed and the resultant seeds
were
harvested. During the growth stage the plants had been examined for growth
phenotype and the CO2 0fixation rate was measured for many of the young
transgenic plants.
Production of TI and T2 Generation GPT Trans epic Plants:
Seeds harvested form the TO generation of the transgenic tobacco plants were
germinated on M&S media containing kanamycin (100 mg / L) to enrich for the
transg.ene. At least one fourth of the seeds did not germinate on this media
(kanamycin is expected to inhibit germination of the seeds without resistance
that
would have been produced as a result of normal genetic segregation of the
gene)
and more than half of the remaining seeds were removed because of
demonstrated sensitivity (even mild) to the kanamycin.
The surviving plants (T1 generation) were thriving and these plants were then
selfed to produce seeds for the T2 generation. Seeds from the T1 generation
were
:20 germinated on MS media supplemented for the Ãransformant lines with
kanamycin
(10mg/liter). After 14 days they were transferred to sand and provided quarter
strength Hoagland's nutrient solution supplemented with 25 mM potassium
nitrate.
They were allowed to grow at 24CC with a photoperiod of 18 h light and 8 hr
dark
with a light intensity of 900 micromoles per meter squared per second. They
were
harvested 14 days after being transferred to the sand culture.
Characterization of GPT Transgenic Pl.a nts:
Harvested transgenic plants (both GPT transgenes and vector control
transgenes)
were analyzed for glutamine sythetase activity in root and leaf, whole plant
fresh
weight, total protein in root and leaf, and CO2 fixation rate (Knight et al.,
1988,
Plant Physiol. 88: 333). Non-transformed, wild-type A. turefacieris plants
were
also analyzed across the same parameters in order to establish a baseline
control.
51
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Growth characteristic results are tabulated below in Table 1. Additionally, a
photograph of the GPT transgenic plant compared to a wild type control plant
is
shown in FIG. 2 (together with SI trans e is tobacco plant, see Example 5).
Across all parameters evaluated, the GPT transgenic tobacco plants showed'.
enhanced growth characteristics. In particular the GPT transgenic plants
exhibited a greater than 50% increase in the rate of CO2 0fixation, and a
greater
than two-fold increase in glutarnine synthetase activity in leaf tissue,
relative to
wild type control plants. In addition, the leaf-to-root GS ratio increased by
almost three-fold in the transaminase transgenic plants relative to wild type
control,
Fresh weight and total protein quantity also increased in the transgenic
plants, by
about 50% and 80% (leaf), respectively, relative to the wild type control.
These
data demonstrate that tobacco plants overexpressing the Abidopsis GPT
trangene achieve significantly enhanced growth and CO2 0fixation rates.
Table I
Protein mg/.ram fresh weight Leaf Root
Wild type _ control 8.3 2.3
Uric PNI-8 a second control 8,9 2.98
Line PN9 9 13, 3.2
lutrtmi a nthetase activity, micromoies/min/mg protein
L'ild teatlc of leaf root= 4.1:1 ) 4.3 #
PN1-8 (Ratio of leaf : root = 4,2: 1) 5.2 1.3
PN9-9 (Ratio of leaf. root w 19.9 :1) 10.5 0.97
Whole # lane Fresh Wer9ht-,..
- --------- --------- ---------
''ildlyp-e---------------------------------------------------------------------
-------------- ------------------------------- 217 - --------------------------
-----
PÃ I-8 26.1
P9-S 33.1
-------------------------------------------------------------------------------
------------
CO2 Fixation Rate, umolefrt 2/sec
Wild t pe 8.4
PÃ 1.8 8.9
PÃN9-9 12.9
Data= average of three plants
Wild type --- Control plants; not regenerated or frt nstormed.
PNI lines were produced by regeneration aftertransfornnation using a construct
without inserted gone.
A control against the processes of regeneration and transformation.
PN 9 lines were produced by regeneration after transformation usÃog a co
nstruct : itl the Arribidopsis
OPT gene.
52
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 4: GENERATION OF TRANSGENIC TOMATO PLANTS CARRYING
ARABIDOPSIS OPT TRANSGENE:
Transgenic Ly opersicorr esculenturn (Micro-Tom Tomato) plants carrying the
Arabidopsis GPT transgene were generated using the vectors and methods
described in Example 3. To transgenic tomato plants were generated and grown
to maturity=, Initial growth characteristic data of the GPT transgenic tomato
plants
is presented in Table 1Ã. The transgenic plants showed significant enhancement
of growth rate, flowering, and seed yield in relation to wild type control
plants. In
addition, the transgenic plants developed multiple main stems, whereas wild
type
plants developed with a single main stem. A photograph of a GPT transgenic
tomato plant compared to a wild type plant is presented in FIG. 3 (together
with:
G S1 transgenic tomato plants, see Example 6).
l TABLE 11
Growth Wildtype GPT Transgenic
Characteristics Tomato Tomato
Stem height, cm 6.5 18, 12, 11 major stems
Stems 1 3 rya or, 6 other
--------------
Buds
Flowers 2 16
8 12
Fruit 0 3
EXAMPLE 5- GENERATION OF TRANSGENIC TOBACCO PLANTS
OVEREXPRESSING ALFALFA G S1
Generation of Plant Expression Vector GS111:
Transgenic tobacco plants overexpressing the Alfalfa G1 gene were generated
as previously described (Temple et aÃ., 1993, Mol. Gen. Genetics 236. 315-
325).
Briefly, the plant expression vector pGS111 was constructed by inserting the
entire coding sequence together with extensive regions of both the 5" and 3'
untranslated regions of the Alfalfa GS1 gene [SEQ ID NO, 3] (DasSarma at al.,
1986, Science, Vol 232, Issue 4755, 1242-1244) into pÃ' ON316 (Rogers et al.,
1987, supra), placing the transgene under the control of the constitutive
.30 cauliflower mosaic virus (CaMV) 35S promoter and the nopaline synthase
(NOS)
53
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
transcriptional terminator. A kanamycin resistance gene was included to
provide
a selectable marker.
Generation of 1 Transformants:
p 111 was transferred to Agrobacterlurn turnefaci~ens strain pTiTT37ASE using
triparental mating as described (Rogers at al., 1987, supra, Unkefer at al.,
U. S.
Patent No, 6,555,500). Nicotiana tabacum cv, Xanthi plants were transformed
with pG 111 transformed Agrobacteria using the leaf disc transformation system
of Horsch at. at (Horsch et al.,1995, Science 227.1229-1231). Transformants
were selected and regenerated an MS medium containing 10OpglÃml kanarmycin.
Shoots were rooted on the same medium (with kanamycin, absent hormones) and
transferred to potting soil:perlite:vermiculite (3.1:1), grown to maturity,
and allowed'.
to self. Seeds were harvested from this TO generation, and subsequence
generations produced by selfing and continuing selection with kanamycin. The
best growth performers were used to yield a T3 for crossing with the best
performing GPT over-expressing lines identified as described in Example 3, A
photograph of the GS1 transgenic plant compared to a wild type control plant
is
shown in FIG, 2 (together with GPT transgenic tobacco plant, see Example 3)
7
EXAMPLE 6 GENERATION OF TRANSGENIC TOMATO PLANTS CARRYING
ALFALFA GS1 TRANSGENE:
Transgenic Ly per icon esculentu (Micro-Tom Tomato) plants carrying the
Alfalfa G S1 transgene were generated using the vector described in Example 5
and a transformation protocol essentially as described (Sun et at, 2006. Plant
Cell
Physiol. 46(3) 426-31). TO transgenic tomato plants were generated and grown
to
maturity. Initial growth characteristic data of the GPT transgenic tomato
plants is
presented in Table Ill. The transgenic plants showed significant enhancement
of
growth rate, flowering, and seed yield in relation to wild type control
plants. In
addition, the transgenic plants developed multiple main stems, whereas wild:
type
plants developed with a single main stem. A photograph of a G1 transgenic
tomato plant compared to a wild type plant is presented in FIG. 3 (together
with
GPT transgenic tomato plant, see Example 4).
54
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TABLE Ili
Growth Wildtype GS1 Transgenic
Characteristics Tomato Tomato
Stem height, cm 6.5 16, 7, 5 major stems
Sterns 1 3 major, 3 med, 1 srn
Buds 2 2
Flowers 8 13
Fruit 0 4
EXAMPLE 7. GENERATION OF DOUBLE TRANSGENIC TOBACCO PLANTS
CARRYING GSI AND (APT TRANSGENES:
In an effort to determine whether the combination of GS 1 and GPT transgenes
in
a single transgenic plant might improve the extent to which growth and other
agronomic characteristics may be enhanced, a number of sexual crosses
between high producing lines of the single trans gene (GS1 or GPT) transgenic
plants were carried out. The results obtained are dramatic, as these crosses
repeatedly generated progeny plants having surprising and heretofore unknown
increases in growth rates, biomass yield, and seed production.
Materials and Methods:
Single-transene, trans genic tobacco plants overexpressing CPT or GS1 were
generated as described in Examples 3 and 4, respectively. Several of fastest
growing T2 generation GPT transgenic plant lines were crossed with the fastest
growing T3 generation GS1 transgenic plant lines using reciprocal crosses. The
progeny were then selected on kanamycin containing M&S media as described in
Example 3, and their growth, flowering and seed yields examined.
Tissue extractions for CPT and GS activities: GPT activity was extracted from
fresh plant tissue after grinding in cod 100 mM Tris--HCI, pH 7.6, containing
1 mm
ethylenedhaminetetraacetic, 200 mM pyridoxal phosphate and 6 mlvl,
mercaptoethanol in a ratio of 3 ml per gram of tissue. The extract was
clarified: by
centrifugation and used in the assay. GS activity was extracted from fresh:
plant
tissue after grinding in cold 50 mM imidazole, pH 7,5 containing 10 mM MCI ,
and 12.5 mM mercaptoethaÃol in a ratio of 3 ml per gram of tissue. The extract
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
was clarified by centrifugation and used in the assay. GPT activity was
assayed
as described in Calderon and Mora, 1985, Journal Bacteriology 161.807-809. GS
activity was measured as described in Shapiro and Stadtmann, 1970, Methods in
Enzymology 17A: 910-922. Both assays involve an incubation with substrates
and cofactor at the proper pH. Detection was by HPLC.
Results.
The results are presented in two ways. First, specific growth characteristics
are
tabulated in Tables IV A and 11.8 (biomass, seed yields, growth rate, GS
activity,
GPT activity, 2-oxogÃutaramate activity, etc). Second, photographs of progeny
plants and their leaves are shown in comparison to single-transgene and wild
type
plants and leaves are presented in FIG. 5 and FIG. 6, which show much larger
whole plants, larger leaves, and earlier and/or more abundant flowering in
comparison to the parental single-transgene plants and wild type control
plants.
Referring to Table IVA, double-transgene progeny plants form these crosses
showed tremendous increases total biomass (fresh weight), with fresh weights
ranging from 45-89 grams per individual progeny plant, compared to a range of
only 19-24 grams per individual wild type plant, representing on average,
about a
two- to three-fold increase over wild type plants, and representing at the
high end,
an astounding four-fold increase in biomass over wild type plants. Taking the
24
individual double-transgene progeny plants evaluated, the average individual
plant biomass was about 2.75 times that of the average wild type control
plant.
Four of the progeny lines showed approximately 2.5 fold greater average per
plant
fresh weights, while two lines showed over three-fold greater fresh weights in
comparison to wild type plants.
in comparison to the single-transgene parental lines, the double-transene
progeny plants also shoed far more than an additive growth enhancement.
Whereas GPT single-transgene lines show as much as about a 50% increase
over wild type biomass, and GS1 .single-transgene lines as much as a 86%
increase, progeny plants averaged almost a 200% increase over wild type
plants.
56
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Similarly, the double transgene progeny plants flowered earlier and more
prolifically than either the wild type or single transgene parental lines, and
produced a far greater number of seed pods as well as total number of seeds
per
plant. Referring again to Table l.A, on average, the double-transgene progeny
produced over twice the number of seed pods produced by wild type plants, with
two of the high producer plants generating over three times the number of seed
pods compared to wild type. Total seed yield in progeny plants, measured on a
per plant weight basis, ranged from about double to nearly quadruple the
number
produced in wild type plants.
57
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
S-112;983
TABLE N.A
PLANT LINE FRESH WEIGHT SEED PODS SEED YIELD GS ACTIVITY
Ole plant ds/ ant lent LEAF ROOT LIR RATIO
Wild 1Vpe Tobacco
Wild type 1 18.73 26 0.967
Wild t)w 2 24.33 24 1.07
Wild type 3 23.6 32 0.9
Wild type 4 18.95 32 1.125
WT Average 21.4025 28.5 10155 7.75 1A5 5.34
Cross I
X1L1a x PA9-9ff
1 59.21 62 2.7811
2 65.71 56
3 55.36 72
4 46.8 56
Cross I Average 56.77 61.5 14.98 1.05 14.27
Compared to WT +265% +216% +274% +193% -28% +267%
Cross 2
PA9-2 x L9
1 70.83 61 1.76
2 49.17 58 3.12
3 50.23 90 NA
4 45.77
Cross 2 Average 54 58.3 2A4 16.32 1.81 9.02
Compared to WT +252% +205% +240% +211% +125% +169%
Cross 3
PA9-9ff xLla
1 89.1 77 3.687
2 78.18
3 58.34
4 61.79
Cross 3 Average 71.85 77 (one plant) 3.678 (one plant) 15.92 1.38 11.54
Compared to WT +336% +270% +362% +205% -5% +216%
58
SUBSTITUTE SHEET (RULE 26)
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
PLANT LINE FRESH WEIGHT SEED PODS SEED YIELD GS ACTIVITY
g/whole plant #podsiplant g/plant LEAF ROOT LIR RATIO
Cross 5
PA9-1Oaa x L1a
1 65.34 45 2.947
2 53.28 64 3.3314
3 49.85 42 1.5667
4 44.63 42 2.5013
Cross 5 Average 53.275 48.25 2.86928 13.03 1.8 7.24
Compared to WT +244% +169% +283% +168%
Cross 6
PA9-17b x L1a
1 56.7 64 2.492
2 55.05 66 2.162
3 51.51 59 1.8572
4 45.38 72 4.742
Cross 6 Average 52.16 65.25 2.8133 14.114.7 1.1.1124 13.29
Compared to WT +244% +229% +277% 52
Cross 7
PA9-20aa x L 1 b
1 76.26 67 2.0535
2 66.27 42 1.505
3 72.26 72 2.3914
4 63.91 91 2.87
Cross 7 Average 69.675 68 2.204975 14.12 1.24 11.39
Compared to WT +326% +239% +217%
Control PA9-9ff
1 32.18 N/A
2 32.64 N/A
3 34.67 N/A
4 25.18 N/A
Average 31.17 N/A 11.57 1.14 10.15
Compared to WT +148%
59
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
PLANT LINE FRESH WEIGHT SEED PODS SEED YIELD GS ACTIVITY
g/whole plant #pods/plant g/plant LEAF ROOT L/R RATIO
Control GS L1a
1 41.74 N/A
2 36.24 N/A
3 33.8 N/A
4 30.48 N/A
Average 35.57 N/A 13.15 1.23 10.69
Compared to WT +166%
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Table IV.B shows growth rate, biomass and yield, and biochemical
characteristics of
Line XX (Line 3 further selfed) compared to the single transgene line
expressing GS1
and wild type control tobacco. All parameters are greatly increased in the
double
transgenic plant (Line XX). Notably, 2-oxoglutaramate activity was almost 17
fold
higher, and seed yield and foliar biomass was three-fold higher, in Line XX
plants
versus control plants.
TABLE IV.B
Specific GPT 2-
Activity Activity oxoglu- Trans
Plant Growth th Foliar Fruit Seed
Type Rate Biomass Flowers Yield g umol/ nmol/Ft taramate Gene
m /d FWt, g /Buds min/gFW /gFWt nmol/gF Assay
9/g t Wt
Wildtype, 228 21.40 28.5 1.02 7.75 16.9 68.9 No
avg
Line 1 GS 269 35.57 NM NM 11.6 NM 414 Yes
Line XX 339 59.71 62.9 2.94 16.3 243.9 1,153.6 Yes
NM Not Measured
EXAMPLE 8: GENERATION OF DOUBLE TRANSGENIC PEPPER PLANTS
CARRYING GS1 AND GPT TRANSGENES:
In this example., Big Jim chili pepper plants (New Mexico varietal) were
transformed
with the Arabidopsis GPT full length coding sequence of SEQ ID NO: 1 under the
control of the CMV 35S promoter, and the Arabidopsis GS1 coding sequence of
SEQ
ID NO: 6 under the control of the RuBisCo promoter, using Agrobacterium-
mediated
transfer to seed pods. After 3 days, seeds were harvested and used to generate
TO
plants and screened for transformants. The resulting double-transgenic plants
showed higher pod yields, faster growth rates, and greater biomass yields in
comparison to the control plants.
61
SUBSTITUTE SHEET (RULE 26)
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Materials and Methods:
Solanaceae apis cu+ Pepper plants ("Big Jim" varietal) were transformed with
the
Arabidopsis GPT full length coding sequence of SEQ ID NO: 1 under the control
of
the CM V 35promoter within the expression vector pMON (see Example 3), and the
Arabidopsis GS1 coding sequence of SEQ ID NO: 6 under the control of the
RuBisCo
promoter within the expression vector pCambia 1201 (Tomato rubisco rbcS3C
promoter: Kyozulka et al., 1993, Plant Physical.. 103- 991-1000; SEQ ID NO:
22;
vector construct of Q ID NO, 6), using Agrobacterium-mediated transfer to seed
-1.0 pods.
For this and all subsequent examples, the Cambia 1201 or 1305.1 vectors were
constructed according to standard cloning methods (Sambrook et 1., 1989,
supra,
Saiki et al., 1988, Science 239: 487-491). The vector is supplied with a 35S
CaMV
promoter; that promoter was replaced with RcbS-3C promoter from tomato to
control
the expression of the target gene, The Cambia 1201 vectors contain bacterial
chlorophenicol and plant hygromycin resistance selectable marker genes. The
Cambia 1305.1 vectors contain bacterial chlorophenicol and hygromycin
resistance
selectable marker genes.
The transgene expression vectors pMON (GPT transgene) and arbia 1201 (GS
transgene) were transferred to separate Agrà bacterium tumefaciens strain
LBA4404
cultures using a standard electroporation method (McCormac et el.. 1998,
Molecular
Biotechnology 9:155-159). Transformed Agrobacteriurn were selected on media
containing 50 p9/ml of either streptamycin for pMON constructs or
chloroarnpheni.co
for the Cambia constructs. Transformed Agrobacterium cells were grown in LB
culture media containing 25 pg/ml of antibiotic for 36 hours. At the end of
the 36 hr
growth period cells were collected by centrifugation and cells from each
transformation were resuspended in 100 ml LB broth without antibiotic.
62
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Pepper plants were then transformed with a mixture of the resulting
Agrobacterium
cell suspensions using a transformation protocol in which the Agrobacterium is
injected directly into the seed cavity of developing pods. Briefly, developing
pods
were injected with the 200 ml mixture in order to inoculate immature seeds
with the
Agrobacteria essentially as described (Wang and Waterhouse, 1997, Plant Mot.
Biol.
Reporter 15: 209-215). in order to induce Agrobacteria virulence and improve
transformation efficiencies, 10 pg/ l acetosyringonone was added to the
Agrobacteria cultures prior to pod inoculations (see, Sheikholeslam and Weeks,
1986. Plant Idol. Biol. 8: 291-298).
Using a syringe, pods were injected with a liberal quantity of the
Agrobacterium
vector mixture, and left to incubate for about 3 days. Seeds were then
harvested and
germinated, and developing plants observed for phenotypic characteristics
including
growth and antibiotic resistance. Plants carrying the transgenes were green,
whereas
untransformed plants showed signs of chlorosis in leaf tips. Vigorous growing
transformants were grown and compared to wild type pepper plants grown under
identical conditions.
Results:
The results are presented in FIG. 7 and Table V. FIG. shows a photograph of a
GPT+GS double transonic pepper plant compared to a control plant grown for the
same time under identical conditions. This photograph shows tremendous pepper
yield in the transgenic line compared to the control plant.
Table V presents biomass yield and GS activity, as well as transgene
genotyping, in
the transgenic lines compared to the wild type control. Referring to Table V,
double-
transgene progeny plants showed tremendous increases total biomass (fresh
weight), with fresh weights, ranging from 393 w 662 grams per individual
transgenic
plant, compared to an average of 328 grams per wild type plant. Transgenic
line A
63
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
produced more than twice the total biomass of the controls. Moreover, pepper
yields
in the transgenie lines were greatly improved over wild type plants, and were
50%
greater than control plants (on average). Notably, one of the transgene fines
produced twice as many peppers as the control plant average.
TABLE V. TRANSGENIC PEPPER GROWTH/BIOMASS AND REPRODUCTION
...............................................................................
...............................................................................
...................................................................... .
Biomass, Yield GS activity Transgene
Plant type Polar Fresh Peppers, g Umoles/min Presence
Wt t /gFt Assay
Wildtype, avg 328.2 817 1.09 Negative
Line A2 457.3 184.2 1.57 GPT'- Yes
.:..........................................................................:..
..............>............................................;...................
.........................;.............................................
Line A5 661.7 148.1 1.8 GPT - Yes
.. ......... .......................... .::: ...................... ........
.... .......
Line 1 493A .....
93 x..... 141.0 1.3 GPT - Yes
............................::...........................................::....
........................................:......................................
......:............................................:
..................
Line B4 393.1 136.0 1.6 GPT Yes
Lne C1 509A 152.0 1.55 GPT - Yes
...............................................................................
...............................................................................
..........................................................
Pt Fresh Weight; It Dry Weight
EXAMPLE 9; GENERATION OF DOUBLE TRANS+GENIC BEAN PLANTS
CARRYING ARABIDOPSIS GSI AND GPT TRANS+GENES.
In this example, yellow wax bean plants (Ptiaseolus vtutgar s) were
transformed with
the Arabidopsis GPT full length coding sequence of SEQ ID NO: 1 under the
control
of the CMV 35S promoter within the expression vector p Cambia 1201, and the
Arabidopsis GS1 coding sequence of SEQ ID NO, 6 under the control of the
RuBiso
promoter within the expression vector pCambia 1201, using Agrobacterium-
mediated
transfer into flowers.
Materials and Methods:
The transgene expression vectors p Cambia 1201-GPT (vector construct of SEQ ID
NO, 27) and p Cambia 1201-GS (vector construct of SEQ ID N : 6) were
transferred
to separate Agrobacterium tumefaciens strain LBA4404 cultures using a standard
64
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
electroporation method (McCormac et al., 1998, Molecular Biotechnology 9:1, 5-
1 9 .
Transformed Agrobacterium were selected on media containing 50 pg/mi of
chloroamphnicol. Transformed Agrobacterium cells were grown in LB culture
media
containing 25 pg/m1 of antibiotic for 36 hours. At the end of the 36 hr growth
period
cells were collected by centrifugation and cells from each transformation were
resuspended in 100 ml LB broth without antibiotic.
Bean plants were then transformed with a mixture of the resulting
Agrobacterium cell
suspensions using a transformation protocol in which the Agrobacteria is
injected
directly into the flower structure (Yasseem, 2009, Plant l1 o!. Biol. Reporter
27: 20-
28), In order to induce Agrobacteria virulence and improve transformation
efficiencies, 10 pg/ml acetosyringonone was added to the Agrobacteria cultures
prior
to flower inoculation. Briefly, once flowers bloomed, the outer structure
encapsulating the reproductive organs was gently opened with forceps in order
to
permit the introduction of the.Agrobacteria mixture, which was added to the
flower
structure sufficient to flood the anthers.
Plants were grown until bean pods developed, and seeds were harvested and used
to generate transgenic plants. Transgenic plants were then grown together with
control bean plants under identical conditions, photographed and
phenotypically
characterized. Growth rates were measured for both transgenic and control
plants.
In this and all examples, Glutamine synthetase (GS) activity was assayed
according
to the methods in Shapiro and Stadtmann, 1970, Methods in Enzymology 17A- 910-
922; and, Glutamine phenylpyruvate transaminase (GPT) activity was assayed
according to the methods in Calderon et al.:, 1985, J. Bacteriol. 161. 807-
809, See
details in Example 7, Methods, sera.
Results:
The results are presented in FIG. 8, FIG. 9 and Table VI,
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
FIG. 8 shows GPT+ S transgenic bean line A growth rate data relative to
control
plants, including plant heights on various days into cultivation, well as
numbers of
flower buds, flowers, and bean pods. These data show that the GPT+GS double
transgenic bean plants outgrew their counterpart control plants. The
transgenic
plants grew taller, flowered earlier and produced more flower buds and
flowers, and
developed bean pods and produced more bean pods that the wild type control
plants.
TABLE V1: TRANSGENIC BEANS LINE A
G PT Apt vet B Activity
Plant Type Bean Pod nmoles/h/gF umoles1min Antibiotic
Yield FWt, g Wt
/gF`t Resistance
Wildtype, avg 126.6 101,9 25. Negative
.................................. ................ ......
.:: .........
2A 211.E ....::::: N1~1:: NM
::..............................................::.............:..........:....
..............::............................................<..................
........................:...........................................;:
4A 207.7 NM NM +
....
513 205.7 9841 101.3 +
WT Wildtype; t Fresh Weight; NM Not Measured
Table VI presents bean pod yield, GPT and activity, as well as antibiotic
resistance status, in the transgenic lines compared to the wild type control
(average
of several robust control plants{ control plants that did not grow well were
excluded
from the analyses). Referring to Table Vl, double-transgene progeny plants
showed
substantial bean pod biomass increases (fresh pod weight) in comparison to the
control plants, with bean pod biomass yields consistently above 200 grams per
individual transgenic plant, compared to an average of 127 grams per wild type
plant,
representing an over 60% increase in pod yield in the double transgene lines
relative
to control plant(s).
66
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Lastly, FIG, 9 shows a photograph of a G T+ B double transgenic bean plant
compared to a control plant grown for the same time under identical
conditions,
showing increased growth in the transgenic plant.
EXAMPLE 10. GENERATION OF DOUBLE TRANSGENIC BEAN PLANTS
CARRYING ARABIDOPSIS GSI AND GRAPE GPT TRANSGENES.
In this example, yellow wax bean plants (Phaseolus vulg rrls) were transformed
with
the Grape GPT full length coding sequence of SEQ ID NO: 8 under the control of
the
RuBisCo promoter within the expression vector pCambia 1305.1, and the
Arabidopsis GB1 coding sequence of SEQ ID NO, under the control of the RuBisCo
promoter within the expression vector p Cambia 1201, using Agrobacterium-
mediated
transfer into developing pods.
Materials and Methods:
The transgene expression vectors p Cambia 1 01_GPT(grape (vector construct of
SEQ ID NO. 8) and p Cambia 1201-GS (vector construct of SEQ ID NO 6) were
transferred to separate Agrobacterlum tumefaciens strain LBA4404 cultures
using a
standard electroporation method (Mc ormac et al. 1998, Molecular Biotechnology
9:155-159), Transformed Agrobacterium were selected on media containing 50
pg/mi of chioroamphenicol, Transformed Agrobacterium cells were grown in LB
culture media containing 25 p /ml of antibiotic for 36 hours. At the end of
the 36 hr
growth period cells were collected by centrifugation and cells from each
2 25
transformation were resuspended in 100 ml LB broth without antibiotic.
Bean plants were then transformed with a mixture of the resulting
Agrobacterium cell
suspensions using a transformation protocol in which the Agrobacteria is
injected
10 directly into the flower structure. In order to induce Agrobacteria
virulence and
improve transformation efficiencies, 10 pglml acetosyringonone was added to
the
67
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Agrobacteria cultures prior to flower inoculation. Briefly, once flowers
bloomed, the
outer structure encapsulating the reproductive organs was gently opened with
forceps in order to permit the introduction of the Agrobacteria mixture, which
was
added to the flower structure sufficient to flood the anthers.
Plants were grown until bean pods developed, and seeds were harvested and used
to generate transgenic plants. Transgenic plants were then grown together with
control bean plants under identical conditions, photographed and
phenotypically
characterized. Growth rates were measured for both transgenic and control
plants.
Results:
The results are presented in FIG. 10, PIG. 11 and Table VII.
FIG. 10 shows GPT+GS transgenic bean line growth rate data relative to control
plants, specifically including numbers of flower buds, flower's, and bean
pods. These
data show that the GPT+GS double transgenic bean plants outgrew their
counterpart
control plants. Notably, the transgenic plants produced substantially more
bean pods
that the wild type control plants.
TABLE VII: TRANS GENIC BEANS LINE G: POD YIELDS
...............................................................................
...............................................................................
...................................................................... .
Plant Type Bean Pod Yield FWt, g Antibiotic Resistance
...............................................................................
...............................................................................
.....................................................................
Wild type a ................................ 157"
NegatÃve....................... ..:.
......................................................... ......
a-
GI 200,5
G 2 178.3
WT Wildtype; FWt Fresh Weight; NM Not Measured
5
Table VII presents bean pod yield and antibiotic resistance status, in the
transgenic
lines compared to the wild type control (average of several robust control
plants;
control plants that did not grow well were excluded from the analyses).
Referring to
Table `11, double-transgene progeny plants showed substantial bean pod biomass
68
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
increases (fresh pod weight) in comparison to the control plants, with bean
pod
biomass yields of 200.5 (line GI) and 1 78 grams (line G2) per individual
transgenic
plant, compared to an average of '158 grams per individual wild type plant,
representing approximately a 27% increase in pod yield in the double trans
gene lines
relative to control plants.
Lastly, FIG. 11 shows a photograph of a GPT+GS double transgenic bean plant
compared to a control plant grown for the same time under identical
conditions. The
transgenic plant shows substantially increased size and biomass, larger leaves
and a
more mature flowering compared to the control plant.
EXAMPLE 11: GENERATION OF DOUBLE TRANS GENIC CO 'PEA PLANTS
CARRYING ARABIDOPSIS GSi AND GPT TRANSGENES
-15 In this example, common Cowpea plants were transformed with the
Arabidopsis GPT
full length coding sequence of SEQ ID NO: 1 under the control of the CMV S
promoter within the expression vector pMON, and the Arabidopsis GS1 coding
sequence of SEQ ID NO: 6 under the control of the RuB s o promoter within the
expression vector p Cambia -1201, using .grobacterium-mediated transfer into
flowers. Materials and methods were as in Example 9, supra.
Results:
The results are presented in FIGS. 12 and 13, and Table VI. FIG. 12 shows
relative
growth rates for the GPT+GS transgenic Cowpea line A and wild type control
Cowpea at several intervals during cultivation, including (FIG. 12A) height
and
longest leaf measurements, (FIG. 12B) tr folate leafs and flower buds, and
(FIG. 12C)
flowers, flower buds and pea pods. These data show that the GPT+GS double
transgenic Cowpea plants outgrew their counterpart control plants. The
transgenic
69
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
plants grew faster and taller, had longer leaves, and set flowers and pods
sooner
than wile type control plants.
TABLE VIII: TRANSGENIC COWPEA LINE A
Pod GPT Activity GS Activity
Pea
Plant Type Meld, nmoles/h/gF umoi/min/gF Antibiotic
Fit, t t Resistance
Wildtype, avg 741 44.4 28.3 Negative
:4A 112,3 NM 41.3... +
:..............................................::..............................
.............::.......................................................
.......................................
........................................
...............................................................................
...............................................................................
..................................................................... .
38 11&8 ?36,2 54.9
..
........
WT Wildtype; FWt Fresh Weight; NM Not Measured
Table Vlll presents pea pod yield, GPT and activity, as well as antibiotic
resistance status, in the transgenic lines compared to the wild type control
(average
of several robust control plants, control plants that did not grow well were
excluded
1.0 from the analyses). Referring to Table Vill, double-transgene progeny
plants showed
substantial pea pod biomass increases (fresh pod weight} in comparison to the
control plants, with average transgenic plant pea pod biomass yields nearly
52%
greater than the yields measured in control plants).
Lastly, FIG. 13 shows a photograph of a GPT+GS double transgenic bean plant
compared to a control plant grown for the same time under identical
conditions,
showing increased biomass and pod yield in the transgenic plant relative to
the wild
type control plant.
EXAMPLE 12-, GENERATION OF DOUBLE TRANBGENIC COWPEA PLANTS
CARRYING ARABIDOPSIS GS1 AND GRAPE GPT TRANSGENE :
In this example, common Cowpea plants were transformed with the Grape GPT full
length coding sequence of SEQ ID NO, 8 under the control of the RuBisCo
promoter
within the expression vector pCambia 1305.1 (vector construct of SEQ 1D NO,
8),
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
and the Arabidopsis G S1 coding sequence of SEQ ID NO. 6 under the control of
the
RuBisCo promoter within the expression vector pCambia 1201 (vector construct
of
SEQ ID NO, 6), using Agrobacterium-mediated transfer into flowers. Materials
and
methods were as in Example 11, supra.
Results:
The results are presented in FIGS. 14 and 15, and Table IX
1.0 FIG. 14 shows relative growth rates for à re GPT+GS trans epic Cowpea line
G and
wild type control Cowpea, These data show that the transgenic plants are
consistently higher (FIG. 1 A), produce substantially more flowers, flower
buds and
pea pods (FIG. 1413), and develop trifolates and leaf buds faster (FIG. 14C).
TABLE IX: TRANSGENIC COWPEA LINE 0
...............................................................................
...............................................................................
...................................................................... .
Pod Yield, GPT Activity GS Activity
Antibiotic
Plant Type Ft Vt, nmoleslh/gF umol/min/gF Resistance
WT Wt
Wildty e, avg 597 44 4 26.7 Negative
G 102.0 ......... 555.E 34.x... .........
.. .. ...............
WT Wildtype; FWt Fresh Weight, NM l Not Measured
Table IX presents pea pod yield, COPT and GS activity, as well as antibiotic
resistance
status, in the trans epic lines compared to the wild type control (average of
several
robust control plants: control plants that did not grow well were excluded
from the
analyses). Referring to Table IX, double-transgene progeny plants showed
substantial pea pod biomass increases (fresh pod weight) in comparison to the
control plants, with average pea pod biomass yields 70% greater in the
transgenic
plants compared to control plants).
71
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Lastly, FIG, 15 shows a photograph of a GPT+GS double transgenic pea plant
compared to a control plant grown for the same time under identical
conditions,
showing increased height, biomass and leaf size in the transgenic plant
relative to
the wild type control plant.
EXAMPLE 13: GENERATION OF DOUBLE TRANSGENIC ALFALFA PLANTS
CARRYING ARABIDOPSIS GSI AND CPT TRANSGENES:
In this example, Alfalfa plants ( ed/sago sativa, var Ladak) were transformed
with
the Arabidopsis GPT full length coding sequence of SEQ ID N: 1 under the
control
of the CMV 35S promoter within the expression vector pMON316 (see Example 3,
supra), and the Arabidopsis GS1 coding sequence of SEQ ID NO, 6 under the
control
of the RuBisCo promoter within the expression vector pOambla 1201 (vector
construct of SEQ ID NO: 6), using Agrobacterium-mediated transfer into
seedling
plants. Agrobacterium vectors and mixtures were prepared for seedling
inoculations
as described in Example 11, supra.
Seedling Inoculations:
When Alfalfa seedlings were still less than about 1/2 inch tall, they were
soaked in
paper toweling that had been flooded with the Agrobacteria mixture containing
both
transgene constructs, Theseedlings were left in the paper toweling for two to
three
days, removed and then planted in potting soil. Resulting TO and control
plants were
then grown for the first 30 days in a growth chamber, thereafter cultivated in
a
greenhouse, and then harvested 42 days after sprouting. At this point, only
the
transgenic Alfalfa line displayed flowers, as the wild type plants only
displayed
immature flower buds. The plants were characterized as to flowering status and
total
biomass.
72
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Results:
The results are presented in Table X. The data shows that the transgenic
Alfalfa
plants grew faster, flowered sooner, and yielded on average about a 62%
biomass
increase relative to the control plants.
TABLE X: TRANSGENIC ALFALFA VS. CONTROL
...............................................................................
...............................................................................
...................................................................... .
...............................................................................
...............................................................................
..................................................................... .
Flowering Stage
Plant Type Biomass at Sacrifice, g
Small defined buds
Wildtype, avg 6.03 No buds swelling.
No flowers
Transgene #5 10-38 4 Open flowers
...........
Transgene I 1 9.03 Flower buds swelling....
Transgene #13 9,95 Flower buds swelling
EXAMPLE 14: GENERATION OF DOUBLE TRANSGENIC CANTALOUPE
PLANTS CARRYING ARABIDOPSIS GSI AND GPT TR:A.NSGENES:
In this example, Cantaloupe plants (Cucumis melt var common) were transformed
with the Arabidopsis GPT full length coding sequence of SEQ ID NO, 1 under the
control of the CMV 35S promoter within the expression vector pMON316 (see
Example 3, supra), and the Arabidopsis GS1 coding sequence of SEQ ID NO, 6
under the control of the RuBisCo promoter within the expression vector pCambia
1201 (vector construct of SEQ ID NO, 6), using Agrobacterirm-mediated transfer
via
injection into developing melons. Agrobacterium vectors and mixtures were
prepared
for intra-melon inoculations as described in Example 8, supra. Inoculations
into
developing melons were carried out essentially as described in Example 8. The
plants were characterized as to flowering status and total biomass relative to
control
melon plants grown under identical conditions.
73
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The results are presented in FIG. 16 and Table Xl. Referring to Table XI, the
transgenic plants showed substantial foliar plant biomass increases in
comparison to
the control plants, with an average increase in biomass of 63%. Moreover, a
tremendous increase in flower and flower bud yields was observed in all five
transgenic lines. Control plants displayed no flowers and only 5 buds at
sacrifice, on
average. In sharp contrast, the transgenic plants displayed between 2 and 5
flowers
per plant, and between 21 and 30 flower buds, per plant, indicating a
substantially
higher growth rate and flower yield, Increased flower yield would be expected
to
translate into correspondingly higher melon yields in the transgenic plants.
Referring
to FIG. 16 (a photograph comparing transgenic Cantaloupe plants to control
Cantaloupe plants), the transgenic Cantaloupe plants show dramatically
increased
height, overall biomass and flowering status relative to the control plants.
TABLE XI: TRANGENIC CANTALOUPE VERSUS CONTROL
Plant Type Biomass Flowers / Flower Antibiotic
Foliar F Wt, g Buds at Sacrifice Resistance
Wildtype, avg 22.8 0/5 Negative
...............................................................................
...............................................................................
...............................................................
Line 1 37.0 3/21
......... ......... ......... ........ .............. ......... .. .........
........
Line 2 35.E 2/30 +
...............................................................................
...............................................................................
......................................................................
Line 3 37,1 3/27
.............:
Line 4 40,6 5/26 +
.........
Line 5 35.7 4/30 +
........ ......... ......... .............. .... ......... ......... .........
Ft Fresh Weight
EXAMPLE 15: GENERATION OF DOUBLE TRANSGENIC PUMPKIN PLANTS
CARRYING ARABIDOPSIS GS1 AND GPT TRANSGENES:
In this example, common Pumpkin plants (Cucurbita maxima) were transformed
with
the Arabidopsis CPT full length coding sequence of SEC ID N ; I under the
control
74
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
of the CMV promoter within the expression vector pMON316 (see Example 3,
supra), and the Arabidopsis I coding sequence of SEQ ID N : 6 under the
control
of the RuBisCo promoter within the expression vector p ambia 1201 (vector
construct of SEQ ID NO. 6), using Agrobacterium-mediated transfer via
injection into
developing pumpkins, essentially as described in Example 14, supra. The tr ns
epic
and control pumpkin plants were grown under identical conditions until the
emergence of flower buds in the control plants, then all plants were
characterized as
to flowering status and total biomass.
The results are presented in FIG. 17 and Table Il. Referring to Table XII, the
transgenic plants showed substantial foliar plant biomass increases in
comparison to
the control plants, with an increase in average biomass yield of 67% over
control
plants. Moreover, an increase in flower bud yields was observed in four of the
five
trans epic lines in comparison to control. Control plants displayed only 4
buds at
sacrifice (average).. In contrast, four transgenic plant lines displayed
between 8 and
15 flowers buds per plant, representing a two- to nearly four-fold yield
increase.
TABLE XII: TRANGENIC PUMPKIN VERSUS CONTROL
...............................................................................
...............................................................................
......................................................................
Plant Type Biomass Hower Buds at Antibiotic
Folhar F't Sacrifice Resistance
...............................................................................
...............................................................................
...................................................................
Wildtype, avg 473 42 Negative
Line I (Photo) 82.3 8
:.......:
..................................................:............................
...............................................................................
;:......................................................;:
Line2 74.3 8 +
...............................................................................
...............................................................................
................................................................
Line 3 80.3 9
...............................................................................
..................................
.....................................................
........................................................
Line 4 (Photo) 77,8 4
...............................................................................
...............................................................................
..................................................................
Line 5 884,5 15 +
::.........................................................::..................
..:............................................................................
.............;:......................................................;:
F Wt Fresh Weight;
Referring to FIG. 17 (a photograph comparing transgenic pumpkin plants to
control
plants), the transgenic pumpkin plants show substantially increased plant
size,
overall biomass and leaf sizes and numbers relative to the control plants.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 16: GENERATION OF DOUBLE TRANS GENIC ARABIDOPSIS
PLANTS CARRYING ARABIDOPSIS GSI AND OPT TRANSGENES:
In this example, Arabidopsis t altana plants were transformed with the
truncated
Arabidopsis COPT coding sequence of SEQ ID NO. 18 under the control of the CMV
35S promoter within the expression vector pMON316 (see Example 3, supra), and
transgenic plants thereafter transformed with the Arabidopsis GS1 coding
sequence
of SEQ ID NO: 6 under the control of the RuBisCo promoter within the
expression
vector pCambia 1201 (vector construct of SECS ID NO, 6), using Agrobacterium-
1.0 mediated "floral dip" transfer as described (Harrison et at, 2006, Plant
Methods 2.19_
Clough and Bent, 1998, Plant J. 1 6 :735--743). Agrobacterium vectors pMON 16
carrying GPT and pCambia 1201 carrying GS1 were prepared as described in
Examples 3 and 11, respectively.
Transformation of two different cultures of Agrobacterium with either a pMon
316
Arabidopsis GTP construct or with a Cambia 1201 + Arabidopsis GS construct was
done by electroporat on using the method of Weigel and Glazebrook 2002. The
transformed Agrobacterium were then grown under antibiotic selection,
collected by
centrifugation resuspended in LB broth with antibiotic and used in the floral
dip of
Arabidopsis inflorescence. Floral dipped Arabidopsis plants were taken to
maturity
and self-fertilized and seeds were collected. Seeds from twice dipped plants
were
first geminated on a media containing 20ug/ml of kanamycin and by following
regular
selection procedures surviving seedlings were transferred to media containing
20 ug
of hygromycin. Plants (3) surviving the selection process on both antibiotics
were
self-fertilized and seeds were collected. Seeds from the TI generation were
germinated on MS media containing 20 ug/mI of hygromycin and surviving
seedlings
were taken to maturity, self-fertilized and seeds collected. This seed
population the
T2 generation was then used for subsequent growth studies.
The results are presented in FIG. 18 and Table X111. Referring to Table III,
which
shows data from g wild type and 6 transgenic Arabidopsis plants (averaged),
the
76
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
transgenic plants displayed increased levels of both GPT and GS activity. GPT
activity was over twenty-fold higher than the control plants. Moreover, the
transgenic
plant fresh foliar weight average was well over four-fold that of the wild
type control
plant average. A photograph of young transgene Arabidopsis plants in
comparison
to wild type control Arabidopsis plants grown under identical conditions is
shown in
FIG. 18, and reveals a consistent and very significant increase in transgenic
plants
relative to the control plant.
TABLE XIII: TRANSCENIC ARABIDOPSIS VERSUS CONTROL
GS Activity
Plant type Biomass, g GPTActÃvÃty umollmin/gl= Antibiotic
Fresh foliar wt nmol/h!g Wt It Resistance
Wildtype, avg.. 0,246 ......... 18 4 7 :..... begatÃve.........
Transgene 1,166 895.6 18.2 Positive
. :::
......::.::......:::.:..................................:::........::::::::....
...:::: * * *
*..:......................::::::::...............:..............:::::::::......
......::::.......:::::
EXAMPLE 17. GENERATION OF TRANSGENIC TOMATO PLANTS CARRYING
ARABIDOPSIS COPT AND GSI TRANSGENES:
In this example, tomato plants (Solanum Jycopersicon, "money Maker" variety)
were
transformed with the Arabidopsis GPT full length coding sequence of SEQ ID NO:
I
under the control of the CMV 35S promoter within the expression vector pMON316
(see Example 3, supra), and the Arabidopsis 1 coding sequence of SEQ ID NO, 6
under the control of the RuÃBisCo promoter within the expression vector
pCambia
1201 (vector construct of SEQ ID NO. 6). Single transgene PT) transgenic
tomato
plants were generated and grown to flowering essentially as described in
Example 4.
The Arabidopsis G S1 transgene was then introduced into the single-transgene
TO
plants using Agrobacterium-mediated transfer via injection directly into
flowers (as
described in Example 8). The transgenic and control tomato plants were grown
under identical conditions and characterized as to growth phenotype
characteristics.
Resulting TO double-transgene plants were then grown to maturity, photographed
along with control tomato plants, and phenotypically characterized.
77
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
The results are presented in FIG, 19 and in Table IXX Referring to Table IX X,
double-transgene tomato plants showed substantial foliar plant biomass
increases in
comparison to the control plants, with an increase in average biomass yield of
45%
over control. Moreover, as much as a 70% increase in tomato fruit yield was
observed in the transgenic lines compared to control plants (e.g., 51 tomatoes
harvested from Line 4C, versus and average of approximately 30 tomatoes from
control plants). A much higher level of GPT activity was observed in the
transgenic
plants (e.g., line 4C displaying an approximately 32-fold higher GPT activity
in
comparison to the average GPT activity measured in control plants). GS
activity was
also higher in the transgenic plants relative to control plants (almost double
in Line
4C),
With respect to growth phenotype, and referring to FIG. 19, the transgenic
tomato
plants displayed substantially larger leaves compared to control plants (FIG
19A). In
addition, it can be seen that the transgenic tomato plants were substantially
larger,
taller and of a greater overall biomass (see FIG. 196).
-)o
TABLE IXX: TRANSCENI{C TOMATO GROWTH AND REPRODUCTION
Total OPT GS Activity
Biomass Tomatoes Activity
Plant Type Foliar FWt, Harvested nmoles/h n Presence
until /gFWt /gFWt essay
Sacrifice
.....................:. ......... ......... .......... ........ ...
Wildtype, 891 287 30,2 1417 Negative
avg
Line 1288 43 9181 1 +
18 26.4 +
Line 4C 1146 51 7
78
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 18: GENERATION OF TRANSGENIC CAMILENA PLANTS CARRYING
ARABIDOPSIS GPT AND GSI TRANSGENES:
In this example, Camelina plants (Car elina satIva, Vat MT 303) were
transformed
with the ArabidopsÃs GPT full length coding sequence of SEQ ID NO: 1 under the
control of the R:uÃB s o promoter within the expression vector pC nbà 1201,
and the
Arabldopsls GS1 coding sequence of SEQ ID NO: 6 Ã nder the control of the
RuBisCo
promoter within the expression vector p Cambia 1201, using grobacterium-
edlated
transfer into germinating seeds according to the method described in Ghee et
al.,
1989, Plant Physiol. 91. 1 1 -1218. Agrobacteriurn vectors and mixtures were
prepared for seed inoculations as described in Example 11, supra.
Transgenic and control Camelina plants were grown under identical conditions
(30
days in a growth chamber and then moved to greenhouse cultivation) for 39
days,
and characterized as to biomass, growth characteristics and flowering stage.
The results are presented in Table XX and FIG, 20. Referring to Table X X, it
can be
seen that total biomass in the transgenic plants was, on average, almost
double
control plant biomass. Canopy diameter was also significantly improved in the
transgenic plants. FIG. 20 shows a photograph of tr nsgenic Camelina compared
to
control_ The transgenic plant is noticeably larger and displays more advanced
flowering status,
TABLE XXr TRANSGENIC CAIt ELINA VERSUS CONTROL
........ . .............
Height i Canopy Biomass
Plant Type Flowering Stage
Diameter, inches _....................
g..................................................
+'ildt pe, avg 1414 8.35 Partial flowering
Transdene C--1 15,5/5 16.54 F01 flowering
Transgene C- 14/7 14,80 Initial flowering
79
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 19: ACTIVITY OF BARLEY OFT TRANSGENE IN PLANTA
In this example, the putative coding sequence for Barley GPT was isolated and
expressed from a transgene construct using an in planta transient expression
assay.
Biologically active recombinant Barley GPT was produced, and catalyzed the
increased synthesis of 2- oxoglutaramate, as confirmed by HPLC.
The Barley (Hordeur vuf are) GPT coding sequence was determined and
synthesized. The DNA sequence of the Barley 'T coding sequence used in this
1.0 example is provided in SEQ ID NO 14, and the encoded GPT protein amino
acid
sequence is presented in SEQ ID NO: 15,
The coding sequence for Barley GIFT was inserted into the 1305A cambia vector,
and transferred to A robacterum tume acien strain LBA4O4 using a standard
electroporation method (McCormac et al., 1998, Molecular Biotechnology 9:155-
159),
followed by plating on LB plates containing hygromycin (50 micro gm J ml).
Antibiotic
resistant colonies of Agrobacteriur were selected for analysis.
The transient tobacco leaf expression assay consisted of injecting a
suspension of
transformed Agrobacterium (1,5-2.0 OD 650) into rapidly growing tobacco
leaves.
Intradermal injections were made in a grid across the leaf surface to assure
that a
significant amount of the leaf surface would be exposed to the Agrobacteriurn.
The
plant was then allowed to grow for 3-5 days when the tissue was extracted as
described for all other tissue extractions and the GPT activity measured.
PGPT activity in the inoculated leaf tissue (1217 nanomolesI FWt/h) was three-
fold the
level measured in the control plant leaf tissue (407 nanomoles1 F Jtih),
indicating
that the Hordeur. OPT construct can direct the expression of functional GPT in
a
trans genic plant.
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 20: ISOLATION AND EXPRESSION OF RECOMBINANT RICE GPT
GENE CODING SEQUENCE AND ANALYSIS OF BIOLOGICAL ACTIVITY
In this example, the putative coding sequence for rice GPT was isolated and
expressed in E. coll. Biologically active recombinant rice OPT was produced,
and
catalyzed the increased synthesis of 2- oxogl taramate, as confirmed by HPLC.
Materials and Methods.
Rice OPT coding se uence and expression in E, coli:
1.0 The rice (O za sat v a) GPT coding sequence was determined and
synthesized,
inserted into a PET28 vector, and expressed in E. coil. Briefly, E. coli cells
were
transformed with the expression vector and transformants grown overnight in LB
broth diluted and grown to OD 8.4, expression induced with isopropyl-: -l -
thiogalactoside (0.4 micromolar), grown for 3 hr and harvested. A total of 25
X 106
cells were then assayed for biological activity using the NMt assay, below.
Untransformed, wild type E. coil cells were assayed as a control. An
additional
control used E coli cells transformed with an empty vector.
The DNA sequence of the rice CPT coding sequence used in this example is
provided in SEQ ID N : 10, and the encoded OPT protein amino acid sequence is
presented in SEQ ID NO, 11,
H'.PL.C Assay for 2-o.xoolutaramate:
HPLC was used to determine 2Moxoglutara ate production in GPT-overexpressing
E.
roll cells, following a modification of Calderon at al., 1985, J lacteriol
131(2): 807-
889. Briefly, a modified extraction buffer consisting of 25 mM Tris-HCI pH 8,
5, 1 mM
EDTA, 20 jx Pyridoxal phosphate, 10 mM Cysteine, and -1,5% (vtv)
Mercaptoethanol was used. Samples (lysate from E. coil cells, 25 X 106 cells)
were
added to the extraction buffer at approximately a 1/3 ratio (w/ v), incubated
for 30
minutes at 37'C, and stopped with 2OOE.d of 20% TCA. After about 5 minutes,
the
81
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
assay mixture is centrifuged and the supernatant used to quantify 2-
oxoglutaramate
by HPLC, using an ION-381 7.8mm ID X 30 cm L column, with a mobile phase in
0.01N h2SO4, a flow rate of approximately 0.2 ml/min, at 40'C. Injection
volume is
approximately 20 t:d, and retention time between about 38 and 89 minutes.
Detection
is achieved with 21 Onm UV light.
N'MR analysis comparison with authentic 2-oxoglutaramate was used to establish
that the Arabidopisis full length sequence expresses a GPT with 2-
oxoglutaramate
synthesis activity. Briefly, authentic 2-oxoglutarmate (structure confirmed
with NMR)
made by chemical synthesis to validate the HPLC assay, above, by confirming
that
the product of the assay (molecule synthesized in response to the expressed
GPT)
and the authentic 2-oxoglutaramate elute at the same retention time. In
addition,
when mixed together the assay product and the authentic compound elute as a
single peak. Furthermore, the validation of the HPLC assay also included
monitoring
the disappearance of the substrate glutamine and showing that there was a 1:1
molar
stoechiometry between glutarnine consumed to 2-oxoglutaramte produced, The
assay procedure always included two controls, one without the enzyme added and
one without the glutarnine added. The first shows that the production of the 2-
oxoglutaramate was dependent upon having the enzyme present, and the second
shows that the production of the 2-oxoglutaramate was dependent upon the
substrate glutamine.
Results:
Expression of the rice GPT coding sequence of SEQ ID N : 10 resulted in the
over-
expression of recombinant GPT protein having 2-oxoglutaramate synthesis-
catalyzing bioactivity. Specifically, 1.72 nanomoles of 2-oxoglutaramate
activity was
observed in the E. coil cells overexpressing the recombinant rice OPT,
compared to
only 0,02 nanomoles of 2-oxoglutaramate activity in control E. coif cells, an
86-fold
activity level increase over control.
82
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
EXAMPLE 21: ISOLATION AND EXPRESSION OF RECOMBINANT SOYBEAN
OPT GENE CODING SEQUENCE AND ANALYSIS OF BIOLOGICAL ACTIVITY
In this example, the putative coding sequence for soybean OPT was isolated and
expressed in E co/i. Biologically active recombinant soybean OPT was produced,
and catalyzed the increased synthesis of 2- oxoglutaramate, as confirmed by
HPLC,
Materials and Methods:.
1.0 Soybean OPT coding se. uence and expression in E c ok.
The soybean (Glycine max) OPT coding sequence was determined and synthesized,
inserted into a PET28 vector, and expressed in E. co%. Briefly, E. colt cells
were
transformed with the expression vector and transformants grown overnight in LB
broth diluted and grown to OD 0.4, expression induced with isopropyl YD#
thiogalactoside (0.4 micromolar), grown for 3 hr and harvested. A total of 25
X 106
cells were then assayed for biological activity using the NMR assay, below,
U'ntra sformed, wild type E. co/i cells were assayed as a control. An
additional'
control used E coli cells transformed with an empty vector.
The DNA sequence of the soybean OPT coding sequence used in this example is
provided in SEQ ID NO, 12, and the encoded OPT protein amino acid sequence is
presented in SECS ID NO 15,
HPLC Assay for 2-oxociutararnate:
H':PLC was used to determine 2-oxoglutaramate production in GPT-overexpressing
E.
coli cells, as described in Example 20, supra.
Results:
3 0 Expression of the soybean OPT coding sequence of SECS ID NO, 12 resulted
in the
over-expression of recombinant OPT protein having 2-oxoglutaramate synthesis-
83
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
catalyzing bioactivity. Specifically, 31.9 nanomoles of 2-oxoglutaramate
activity was
observed in the E. co/i cells overexpressing the recombinant soybean GPT,
compared to only 0,02 nanomoles of 2-oxoglutaramate activity in control E.
call cel.l'ls,
a nearly 16OO-fold activity level increase over control.
EXAMPLE 22: ISOLATION AND EXPRESSION OF RECOMBINANT ZEBRA FISH
OPT GENE CODING SEQUENCE AND ANALYSIS OF BIOLOGICAL ACTIVITY
1.0 In this example, the putative coding sequence for Zebra fish GPT was
isolated and
expressed in ,E, co/l. Biologically active recombinant Zebra fish GPT was
produced,
and catalyzed the increased synthesis of 2- oxo lutaramate, as confirmed by
NMR.
Materials and Methods:
Zebra fish PT coding sequence and expression in E. cols:
The Zebra fish (Dania rerio) GPT coding sequence was determined and
synthesized,
inserted into a PET28 vector, and expressed in E. coll. Briefly, E. coil cells
were
transformed with the expression vector and transformants grown overnight in LB
broth diluted and grown to OD .4, expression induced with isopropyl-3-D-
thiogalac oside (G.4 micromolar), grown for 3 hr and harvested, A total of 25
X. 106
cells were then assayed for biological activity using the NMR assay, below.
Untransformed, wild type E. co/i cells were assayed as a control. An
additional
control used E cell cells transformed with an empty vector.
The DNA sequence of the Zebra fish OPT coding sequence used in this example is
provided in SEQ ID NO, 16, and the encoded GPT protein amino acid sequence is
presented in SEQ ID NO. 17,
84
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
H,PL Assay for .2-oxoglutaramate,
H'.PLG was used to determine 2-oxoglutaramate production in GPT-overexpressing
E'.
coil cells, as described in Example 20, supra
Results:
Expression of the Zebra fish GPT coding sequence of SEQ ID NO: 16 resulted in
the
over-expression of recombinant GPT protein having 2 oxoglutaram to synthesis-
catalyzing bioactivity. Specifically, 28.6 nanomoles of 2-oxoglutaramate
activity was
I.0 observed in the E. co/i cells overexpressing the recombinant Zebra fish
GPT,
compared to only 0.02 nanomoles of 2-oxoglutaramate activity in control E.
coil cells,
a more than I ,400-fold activity level increase over control.
EXAMPLE 23: GENERATION AND EXPRESSION RECOMBINANT
TRUNCATED ARABIDOPSIS GPT GENE CODING SEQUENCES AND ANALYSIS
OF BIOLOGICAL ACTIVITY
In this example, two different truncations of the Arabidopsis GPT coding
sequence
were designed and expressed in E. co/i, in order to evaluate the activity of
OPT
proteins in which the putative chloroplast signal peptide is absent or
truncated.
Recombinant truncated GPT proteins corresponding to the full length
Arabidopsis
GPT amino acid sequence SEQ ID NO, 1, truncated to delete either the first 80
amino-terminal amino acid residues, or the first 45 amino-terminal amino acid
residues, were successfully expressed and showed biological activity in
catalyzing
the increased synthesis of 2- oxoglutaramate, as confirmed by N MR.
Materials and Methods:
Truncated Arabidopsis GPT coding se uences and expression in E. colt
The DNA coding sequence of a truncation of the Arabidopsis thaliana GOT coding
sequence of SEQ ID NO: I was designed, synthesized, inserted into a PET28
vector,
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
and expressed in E. co/l. The DNA sequence of the truncated Arabidopsis GPT
coding sequence used in this example is provided in SEQ ID NO: 20 (-45
construct), and the corresponding truncated GPT protein amino acid sequence is
provided in SEQ ID NO. 21. Briefly, E. call cells were transformed with the
expression vector and transformants grown overnight in LB broth diluted and
grown
to OD 0.4, expression induced with isopropyl-.1 -D-thiogalactoside (.4
micromolar),
grown for 3 hr and harvested. A total of 25 X 06' cells were then assayed for
biological activity using HPLC as described in Example 20. Untransformed, wild
type
E. coil cells were assayed as a control. An additional control used E cold
cells
transformed with an empty vector.
Expression of the truncated -45 Arabidopsis GPT coding sequence of SEQ ID NO:
20
resulted in the over-expression of biologically active recombinant GPT protein
( 2-
oxoglutaramate synthesis-catalyzing bioactivity) Specifically, 16.1 nanomoles
of 2-
oxoglutaramate activity was observed in the E. coin cells overexpressing the
truncated -45 OPT, compared to only 0.02 nanomoles of 2-oxoglutaramate
activity in
control E. coil cells, a more than 00-fold activity level increase over
control. For
comparison, the full length Arabidopsis gene coding sequence expressed in the
same E. coil assay generated 2.8 nanomoles of 2-oxoglutaramate activity, or
roughly
less than one-fifth the activity observed from the truncated recombinant GPT
protein.
EXAMPLE 24: GPT + OS TRANSGENIC TOBACCO SEED GERMINATION
TOLERATES HIGH SALT CONCENTRATIONS
In this example, seeds form the double transgene tobacco line XX-3 (Cross 3 in
Table 4, see Example 7) were tested in a seed germination assay designed to
evaluate tolerance to high salt concentrations.
86
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
Materials and Methods:
Tobacco seeds from the wild type and XX-3 populations were surfaced sterilized
(5%
bleach solution for 5 minutes followed by a 10% ethanol wash for 3 minutes)
and
rinsed with sterile distilled water. The surface sterilized seeds were then
spread on
Murashige and Skoog media (10% agarose) without sucrose and containing either
0
or 200 mM Na 1. The seeds were allowed to germinate in darkness for 2 days
followed by 6 days under a 16:8 photoperiod at 24C, On day eight the rate of
germination was determined by measuring the percentage of seeds from the
control
1.0 or transgene plants that had germinated.
Results,
The results are tabulated in Table X1 below, The rate of germination of the
transgenic plant line seeds under zero salt conditions was the same as
observed with
wild type control plant seeds. In stark contrast, the germination rate of the
transgenic
plant line seeds under very high salt conditions far exceeded the rate seen in
wild
type control seeds. Whereas over 81 % of the transgenic plant seeds had
germinated
under the high salt conditions, only about 9% of the wild type control plant
seeds had
germinated by the same time point. These data indicate that the trans genic
seeds
are capable of germinating very well under high salt concentrations, an
important trait
for plant growth in areas of increasingly high water and/or soil salinity.
TABLE X1;
TRANSGENIC TOBACCO PLANTS GERMINATE AND TOLERATE HIGH SALT
Plant type Control JO NaCl. Tess 200 mM l aCi. a
Germination % Germination
ildt pe 9 2,87,94 g, 11,8
Trans : ene line X-3 92, 91, 94 84, 82, 78
87
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
All publications, patents, and patent applications cited in this specification
are herein
incorporated by reference as if each individual publication or patent
application were
specifically and individually indicated to be incorporated by reference.
The present invention is not to be limited in scope by the embodiments
disclosed
herein, which are intended as single illustrations of individual aspects of
the
invention, and any which are functionally equivalent are within the scope of
the
invention. Various modifications to the models and methods of the invention,
in
addition to those described herein, will become apparent to those skilled in
the art
from the foregoing description and teachings, and are similarly intended to
fall within
the scope of the invention. Such modifications or other embodiments can be
practiced without departing from the true scope and spirit of the invention.
88
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TABLE OF SEQUENCES:
SEQ ID NO: I Arabidopsis glutamine h yflpyfr- v t tr nsar inase DNA coding
sequence:
ATGTACCTGGACATAAATGGTGTGATGATCAAACAGTTTAGCTTCAAAGCCTCTC
TTCTCCCATTCTCTTCTAATTTCCGACAAAGCTCCGCCAAAATCCATCGTCCTAT
CGGAGCCACCATGACCACAGTTTCGACTCAGAACGAGTCTACTCAAAAACCCGT
CCAGGTGGCGAAGAGATTAGAGAAGTTCAAGACTACTATTTTCACTCAAATGAG
CATATTGGCAGTTAAACATGGAGCGATCAATTTAGGCCAAGGCTTTCCCAATTTC
GACGGTCCTGATTTTGTTAAAGAAGCTGCGATCCAAGCTATTAAAGATGGTAAAA
ACCAGTATGCTCGTGGATACGGCATTCCTCAGCTCAACTCTGCTATAGCTGCGC
GGTTTCGTGAAGATACGGGTCTTGTTGTTGATCCTGAGAAAGAAGTTACTGTTAC
ATCTGGTTGCACAGAAGCCATAGCTGCAGCTATGTTGGGTTTAATAAACCCTGG
TGATGAAGTCATTCTCTTTGCACCGTTTTATGATTCCTATGAAGCAACACTCTCTA
TGGCTGGTGCTAAAGTAAAAGGAATCACTTTACGTCCACCGGACTTCTCCATCC
CTTTGGAAGAGCTTAAAGCTGCGGTAACT,AACAAGACTCGAGCCATCCTTATGA
A.CACTCCGCAC,AACCCGAC CGGGAAGATGTTCACTAGGGAGGAG CTTGAAACC
ATTGCATCTCTCTGCATTGAAAACGATGTGCTTGTGTTCTCGGATGAAGTATACG
ATAAGCTTGCGTTTGAAATGGATCACATTTCTATAGCTTCTCTTCCCGGTATGTA
TGAAAGAACTGTGACCATGAATTCCCTGGGAAAGACTTTCTCTTTAACCGGATG
GAAGATCGGCTGGGCGATTGCGCCGCCTCATCTGACTTGGGGAGTTCGACAAG
CACACTCTTACCTCACATTCGCCACATCAACACCAGCACAATGGGCAGCCGTTG
CAGCTCTCAAGGCACCAGAGTCTTACTTCAAAGAGCTGAAAAGAGATTACAATG
TGAAAAAGGAGACTCTGGTTAAGGGTTTGAAGGAAGTCGGATTTACAGTGTTCC
CATCGAGCGGGACTTACTTTGTGGTTGCTGATCACACTCCATTTGGAATGGAGA
ACGATGTTGCTTTCTGTGAGTATCTTATTGAAGAAGTTGGGGTCGTTGCGATCC
CAACGAGCGTCTTTTATCTGAATCCAGAAGAAGGGAAGAATTTGGTTAGGTTTG
CGTTCTGTAAAGACGAAGAGACGTTGCGTGGTGCAATTGAGAGGATGAAGCAG
AAGCTTAAGAGAAAAGTCTGA
SECS ID NO. 2 Ar idopsis GPT amino acid sequence
ilYLÃDINGVMIKQFF F A LLPFS FRQSSAKIHRPIGATMTTV TQN STG PVGV
AKRLEKFKTTIFTQM ILAVKHGAINLGGGFPNFOGPDFVKEAAI AIKÃDGKN YARG
YGIPGLN AI.AAI FRET TGLVVDPEÃ EVTVT GCTEAIAAAMLGLII PGDEVÃLFAPFY
C YEATL 'IAGAKVKGITLRPPDF IPLEELKAAVTNKTI ILMNTPH PTGK FTRE
ELETIASLCIENDVLVFSDEVYDKLAFEMCHI IA LPG YERTVTMN LGKTFSLTG
KIG AIAPPHLT GVRQAHSYLTFAT TPAGWAAVAALKAPE ` FKELKRDYN ''K
I TLVI GLKEVGFTVFP SGTYFV'VADHTPFG EN VAFCEYLIEEVGV'VAIPTS 'F
YLNPEEGKNLVRFAFCKDEETLRGAIERMKQKLKI KV
89
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO: 3 Alfalfa GS1 DNA coding sequence (upper case) with 5' and 3"
untranslated sequences (indicated in lower case),
atttccgttttcgttttcatttg a at cg a atctttaggattcaataca
attttacta
CTTATCAACCTTGACCTCTCCGAAACCACCGAGAAAATCATCG CCG
AATACATATGGATTGGTGGATCTGGTTTGGACTTGAGGAGCAAAGC
AAGGACTCTACCAGGACCAGTTACTGACCCTTCACAGCTTCCCAAG
TGGAACTATGATGGTTCCAGCACAGGTCAAGCTCCTGGAGAAGAT
AGTGAAGTTATTATCTACCCACAAGCCATTTTCAAGGACCCATTTA
GAAG GGGTAACAATATCTTGGTTATGTGTGATGCATACACTCCAGC
TGGAGAGCCCATTCCCACCAACAAGAGACATGCAGCTGCCAAGAT
TTTCAGCCATCCTGATGTTGTTGCTGAAGTACCATGGTATGGTATT
GAGCAAGAATACACCTTGTTGCAGAAAGACATCAATTGGCCTCTTG
GTTGGCCAGTTGGTGGTTTTCCTGGACCTCAGGGACCATACTATTG
TO GAG CTGGTGCTGACAAGG CATTTGGCCGTGACATTGTTGACTC
A:CATTACAAAGCCTGTCTTTTGCCGGCATCAACATCATGGAAT
AATG GTGAAGTGATGCCTGGTCAATGGGAATTCCAAGTTTG GTCCCT
CAGTTGGTATCTCTGCTGGTGATGAGATATGGGTTGCTCGTTACAT
TTTGGAGAGGATCACTGAGGTTGCTGGTGTGGTGCTTTCCTTTGAC
CCAAAACCAATTAAGGGTGATTGGAATGGTGCTGGTGCTCACACAA
ATTACAGCACCAAGTCTATGAGAGAAGATGGTGGCTATGAAGTCAT
CTTGAAAGCAATTGAGAAGCTTGGGAAGAAGCACAAGGAGCACAT
TGCTGCTTATGGAGAAGGCAACGAGCGTAGATTGACAGGGCGACA
TGAGACAGCTGACATTAACACCTTCTTATGGGGTGTTGCAAACCGT
GGTGCGTCGATTAGAGTTGGAAGGGACACAGAGAAAGCAGGGAAA
GGTTATTTCGAGGATAGGAGGCCATCATCTAACATGGATCCATATG
TTGTTACTTCCATGATTGCAGACACCACCATTCTCTGGAAACCATA
Agccaccacacacacatgcattgaagtatttgaaagtcattgttgattccgcattagaatttgg
tcattgttttttctaggatttggatttgtgttattgttatggttcacactttgtttgtttgaatttgaggc
cttgttataggtttcatatttctttctcttgttctaagtaaatgteagaataataatgtaat
SEQ ID NO 4 Alfalfa GSI amino acid sequence
MSLLSOLINLDLSETTEI IIAEYIWIGGSGLDLR KA "L GPVTDPSGLP WÃ YDG
STGQAPGEDSEVIIYPQAIFKI PFRRGNNILVMCDAYTPAGEPIPTNKRHAAAKÃFSH
PDVVAE.VPWYGIEQEYTLL(KDINWPLGWPVGGFPGPQGPYYGGA AD AFGR I
VDSITYKACLYAGINI; GINGEVMPGQ `EFQVGPSVGISAGCEI VARYILERITEVA
GVVLSFI PKPIKGDi NGAGAHTNYSTKSMREDGGYE `ILKAIEKLGKKHK FIIAAYG
EGNEI RLTGRHETADII TFL #`GV'ANRGASIRVGR TEKAGKGYFEDRRPSSNMD P
YVV TSM I AITTI LWKP
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO: 5 Alfalfa G S1 DNA coding sequence (upper case) with 5' and 3'
untranslated sequences (Indicated in lower case) and vector sequences from
Cial to
Srnal/Sspl and S pl/Smal to Sall/Xhol (lower case, underlined).
atcgatgaattcgaqctcqqtacccatttccgttttcgttttcatttgattcattgaatcaaatcga
atcgaatctttaggattcaatacagattccttagattttactaagtttgaaaccaaaaccaaaa
cATGTCTCTCCTTTCAGATCTTATCAACCTTGACCTCTCCGAAACCA
CC GAGAAAATCATCGCCGAATACATATGGATTGGTGGATCTGGTTT
GGACTTGAGGAGCAAAGCAAGGACTCTACCAGGACCAGTTACTGA
CCCTTCACAGCTTCCCAAGTGGAACTATGATGGTTCCAGCACAGGT
CAAGCTCCTGGAGAAGATAGTGAAGTTATTATCTACCCACAAGCCA
TTTTCAAG GACCCATTTAGAAG G G GTAACAATATCTTG GTTATGTG
TGATGCATACACTCCAGCTGGAGAGCCCATTCCCACCAACAAGAG
AC ATGCAGCTGCCAAGATTTTCAGCCATCCTGATGTTGTTGCTGAA
GTACCATGGTATGGTATTGAGCAAGAATACACCTTGTTGCAGAAAG
ACATCAATTGGCCTCTTGGTTGGCCAGTTGGTGGTTTTCCTGGACC
TCAGGGACCATACTATTGTGGAGCTGGTGCTGACAAGGCATTTGG
CCGTG.ACATTGTTGACTCACATTACAAAGCCTGTCTTTA.TGCCGGC
ATCAACATCAGTGGAATCAATGGTGAAGTGATGCCTGGTCAATGGG
AATTCCAAGTTGGTCCCTCAGTTGGTATCTCTGCTGGTGATGAGAT
ATGGGTTGCTCGTTACATTTTGGAGAGGATCACTGAGGTTGCTGGT
GTGGTGCTTTCCTTTGACCCAAAACCAATTAAGGGTGATTGGAATG
GTGCTGGTGCTCACACAAATTACAGCACCAAGTCTATGAGAGAAGA
TGGTGGCTATGAAGTCATCTTGAAAGCAATTGAGAAGCTTGGGAAG
AAGCACAAGGAGCACATTGCTGCTTATGGAGAAGGCAACGAGCGT
AGATTGACAGGGCGACATGAGACAGCTGACATTAACACCTTCTTAT
GGGGTGTTGCAAACCGTGGTGCGTCGATTAGAGTTGGAAGGGACA
CAGAGAAAGCAGGGAAAGGTTATTTCGAGGATAGGAGGCCATCAT
CTAACATGGATCCATATGTTGTTACTTCCATGATTGCAGACACCAC
CATTCTCTGGAAACCATAAgccaccacacacacatgcattgaagtatttgaaagtc
attgttgattccgcattagaatttggtcattgttttttctaggatttggatttgtgttattgttatggttc
acactttgtttgtttgaatttgaggccttgttataggtttcatatttctttctcttgttctaagtaaatg
tcagaataataatgtaa#gggqatcctctaqaqtcqaq
SFG ID NO: 6 Ara ldcpsis GSI coding sequence
Cambia 1201 vector + rbcS3C+arabldcpsis GS1 Bold ATG is the start site,
AAAA.AAGAAAAAAAAAACATATCTTTGTTTGTCGTATGGGAAGTTTTGAGATAAGG
ACGAGTGAGGG.GTTAAAATTCAGTGGCCATTGATTTTGTAATGCCAAGAACCAC
AAAATCCAATGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCG
TTAGATAGGAAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTA
ACCAATTATTTCAGCACCA TGTCTCTGCTCTCAGATCTCGTTAACCTCAACCTCA
CCGATGCCACCGGGAAAATCATCGCCGAATACATATGGATCGGTGGATCTGGA
91
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
ATGGATATC.AGAAGCAAAGCCAGGACACTACCAGACCAGTGACTGATCCATCA
AAGCTTCCCAA.GTGGAACTACGACGGATCCAGCACCGGTCAGGCTTGCTGGAGA
AGACAGTGAAGTCATTCTATACCCTCAGGCAATATTCAAGGATCCCTTCAGGAAA
GGCAACAACATCCTGGTGATGTGTGATGCTTACACACCAGCTGGTGATCCTATT
CCAACC.AACAAGAGGCACAACGCTGCTAAGATCTTCAGCCACCCCGACGTTGC
CAAGGAGG.AGCCTTGGTATG G GATTGAGCAAGAATACACTTTGATGCAAAAGGA
TGTGAACTGGCCAATTGGTTGGCCTGTTGGTGGCTACCCTGGCCCTCAGGGAC
CTTACTACTGTGGTGTGGGAGCTGACAAAGCCATTGGTCGTGACATTGTGGATG
CTCACTACAAGGCCTGTCTTTACGCCGGTATTGGTATTTCTGGTATCAATGGAGA
AGTCATGCCAGGCCAGTGGGAGTTCCAAGTCGGCCCTGTTGAGGGTATTAGTT
CTGGTGATCAAGTCTGGGTTGCTCGATACCTTCTCGAGAGGATCACTGAGATCT
CTGGTGTAATTGTCAGCTTCGACCCGAAACCAGTCCCGGGTGACTGGAATGGA
GCTGGAGCTCACTGCAACTACAGCACTAAGACAATGAGAAACGATGGAGGATTA
GAAGTGATCAAGAAAGCGATAGGGAAGCTTCAGCTGAAACACAAAGAACACATT
GCTGCTTACGGTGAAGGAAACGAGCGTCGTCTCACTGGAAAGCACGAAACCGC
AGACATCAACACATTCTCTTGGGGAGTCGCGAACCGTGGAGCGTCAGTGAGAG
TGGGAC GTGACACAGAGAAG GAAG GTAAAGG GTACTTCGAAGACAG,AACG C CA
GC:TTCTAACATGGATCCTTACGTTGTCACCTCCATGATCGCTGAGACGACCATA
CTCGGTTGA
SEQ ID NO: 7 Arabidopsis G 1 amino acid sequence
Vector sequences at N-terminus in italics
DL `NRRTSM LL DLV LNLTCATGKIIAEYIWIGG GMDIR A TLPGPVTDP
LP tNYDG TGGAAG. D EVILYPQAIFKDPF GNNILVMCCAYTPAGL PIP'T
RHNAAKIE HPDVA EEP YGIEQEYTTLMQK VN PIG `PVGGYPGPQGPYYC
GVGAOKAIGRDIVDAHYKACLYAGIGISGINGEVMPGQ E GVGPVEGIS GDQV W
VARYL ERITEI GVIV FDPKPVPGDW GAGAHC YSTI TMRNDGGLEVII I AIG
LCL HKEHIAAYGEGNEPRLTGKHETAÃ INTF WG ':ANRG.ASVRVGRDTEI EGKG
'EECRRPA NMDPY VVTS IAETTILG
SEC ID NO: 8 Grape GPT DNA sequence
Showing Cambia 1305.1 with (3' end of) rbcS3C+Vitis (Grape). Bold ATG is the
start site, parentheses are the catl intron and the underlined actagt is the
spel cloning
site used to splice in the hordeum gene.
AAAAAAGAAAAAAAAAACATATCTTGTTTGTCAGTATGGGAAGTTTGAGATAAGG
ACGAGTGAG GG GTTtA.ATTCAGTG GCCATTGATTTTGTAATGCCAAGAACCAC
AAAATCCAATGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCG
TTAGATAGGAAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTA
ACCAATTATTTCAGCACCA TGGTAGATCTGAGG(GTAAATTTCTAGTTTTTCTCCT
92
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TCATTTTCTTGGTTAGGACCCTTTTCTCTTTTTATTTTTTTGAGCTTTGATCTTTCT
TTAAACTGATCTATTTTTTAATTGATTGGTTATGGTGTAAATATTACATAGCTTTAA
CTGATAATCTGATTACTTTATTTCGTGTGTCTATGATGATGATGATAGTTACAG)A
ACCGACGA 4 C TA G TATG CAG CTCTCTCAATGTACCTGGACATTCCCAGAGTTGC
TTAAAAGACCAGCCTTTTTAAGGAGGAGTATTGATAGTATTTCGAGTAGAAGTAG
GTCCAGCTCCAAGTATCCATCTTTCATGGCGTCCGCATCAACGGTCTCCGCTCC
AAATACGGAGGCTGAGCAGACCCATAACCCCCCTCAACCTCTACAGGTTGCAAA
GCGCTTGGAGAAATTCAAAACAACAATCTTTACTCAAATGAGCATGCTTGCCATC
AAACATGGAGCAATAAACCTTGGCCAAGGGTTTCCCAACTTTGATGGTCCTGAG
TTTGTCAAAGAAGCAGCAATTCAAGCCATTAAGGATGGGAAAAACCAATATGCTC
GTGGATATGGAGTTCCTGATCTCAACTCTGCTGTTGCTGATAGATTCAAGAAGG
ATACAGGACTCGTGGTGGACCCCGAGAAGGAAGTTACTGTTACTTCTGGATGTA
CAGAAGCAATTGCTGCTACTATGCTAGGCTTGATAAATCCTGGTGATGAGGTGA
TCCTCTTTGCTCCATTTTATGATTCCTATGAAGCCACTCTATCCATGGCTGGTGC
CCAAATAAAATCCATCACTTTACGTCCTCCGGATTTTGCTGTGCCCATGGATGAG
CTCAAGTCTGCAATCTCAAAGAATACCCGTGCA,ATCCTTATAAACACTCCCCATA
ACCCCACAGGAAAGATGTTCACAAGGGAGGAACTGAATGTGATTGCATCCCTCT
CATTGAGAATGATGTGTTGGTGTTTACTGATGAAGTTTACGACAAGTTTGGCTTT
CGAAATGG ATCACATTTCCATG GCTTCTCTTCCTG G GATGTACGAGAG GACCGT
GACTATGAATTCCTTAGGGAAAACTTTCTCCCTGACTGGATGGAAGATTGGTTG
GACAGTAGCTCCCCCACACCTGACATGGGGAGTGAGGCAAGCCCACTCATTCC
TCACGTTTGCTACCTGCACCCCAATGCAATGGGCAGCTGCAACAGCCCTCCGG
GCCCCAGACTCTTACTATGAAGAGCTAAAGAGAGATTACAGTGCAAAGAAGGCA
ATCCTGGTGGAGGGATTGAAGGCTGTCGGTTTCAGGGTATACCCATCAAGTGG
GACCTATTTTGTGGTGGTGGATCACACCCCATTTGGGTTGAAAGACGATATTGC
GTTTTGTGAGTATCTGATCAAGGAAGTTGGGGTGGTAGCAATTCCGACAAGCGT
TTTCTACTTACACCCAGAAGATGGAAAGAACCTTGTGAGGTTTACCTTCTGTAAA
GACGAGGGAACTCTGAGAGCTGCAGTTGAAAGGATGAAGGAGAAACTGAAGCC
TAAACAATAG G G G CAC GTGA
SEQ ID NO: 9 Grape GPT Ã o acid sequence
MVDLRNRRTSMQLSQCT 'I`FPELLKRPAFLRRSIDSISSRSRSSSKYPSFMASAST
5 V A NTEAEQTH PPQPL.QVAKRLEEKF :TTIFTQMSI LAII GAINLGQGFPNFDGP
EFVKEAAIQAIKDG NQ '`ARGYGVPDLN AVAORF KDTGLVV PEKEVTVTSGCT
EAIAATMLGLINPGDEVILFAPFYD aYFATLSMAGAQIKSITLRPPDFAVPMDELKSAI
SKNTRAILINTPHNPTGKMFTREELNVIASLCIENDVLVFTCEVYCKLAFEMDHISIMAS
LPGMYERTVTI NSLGKTFSLTG KIG WTVAPPHLTWGVRQAFISFLTFATCTPI Q `
AAATALRAPDS'Y'YEELKROYSAKKAIL.VEGLKAVGFRV PSSGTYFVVVDI TPFGLI
DDIAFCEYLIKEVGVVAIPTSVFYLHPEDGKNLVRFTFCKDEGTLRAA 'ERI KEKLKP
KQ
9
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO: 10 Rice GPT DNA sequence,
Rice GPT codon optimized for E. soli expression, untranslated sequences shown
in
lowercase
atgtg ATGA6,AA CTGGCA G TTTCT G : AC CCGGCA .C . CGCAA C
TC.A TG, AAT C GC'I C G GCt\, A C CGAGCTTT C T GCTGA a ,GC
LTG GTC TAG T GT GCGAGC TGCG T 'N GCGAGCCCG CA C CAG
CAGCACTGAGCCCGATGGC GCGCAAGCACCGTGGCAGCAG1A CGGTGC
AGCA AGCAGCAGCAGAAAAACAGCAGCAGCAGCCG TGCAGGTGGCGA
CGTCT IkAAAATTTAAAACCACCATTTTTACCCA ATGAGCATGCTGGCGATTA
AACATGGCGCGATTA.ACCTGGGCCAGGGC TCC.
GAACTTTGATG CCCGGATTTTGTGAAAGAAGC GCGATTCAGGC ATTAACGC
GGGCAAAAA,CCAGTATGCGCGTGGCTATGGCGTGCCGGAACTGAACAGCGCGA
TTGCGGAACGTTTTGTGAAAGATAGCGGCCTGCAGGTGGATCCGGAAAAAGAA
GT ACCGT ACC:A CGGCTGCACC AAGCGATTGCGGC ACCATTCTGU CCT
GATTAAC C;GGG GATGAAGTG.AT T CT : ~ TG GC T T TTATGAT, GCTAT A
A. CC AC CT A . C'A l GG 3GGGLGCG,,\rAC GA \,AG( A i"I AuC u l G `CCz
CrG GG TTTTA GTG C TGGA.A >AACTGAA, G C T >AG . ,AA:AACA
CCGTGCGATTATGA'FT,kACACCCCGCATAACCCGACCGGCAAAATGTTTACCCG
TGAAGAACTGGAATTTATTGCGACCCTGTGCAAAGAAAACGATGTGCTGCTGTT
TGCGGATGAAGTGTATGATAAACTGGCGTTTGAAGCGGATCATATTAGCATGGC
GAGCATTCCCGGGCATGTATG, CGTACCGTGACCATGAACAGCCT GGCAAAA
CC:TTTAGCCTGAC CGGCTGGAAAATTGGCTGGGCGATTGCGCCGCCGCATCTG
ACCTGGGGCGTGCGTCAGGCACATAGCTTTCTGACCTTTGCA,ACCTGCACCCC
GATGCAGGCAGCCGCC CAGCA CACT CGTGCACCGGATAGCTATTATGAAG
AACTGCGTCGTGATTATG CGCGGA, .kAAAGCGCTGCTGGTGA.ACGGCCTGAAA
GATGCGGGCTTTATTGTGTATCCGAGCAGC GCACCTATTTTGTGATGGT GAT
CATACCCCGTTTG CTTTGATAACGATATT AATTTTGC AATATCTGATTCGTG
AAGTGGGCGTGGTGGCGA.TTCCGCCGAGCGTGTTTTATCTGA.ACCCGGAAGAT
GG C ,%AAACCT GTT CG2TTTT,A CTTTTGCAAAGATGATGAAACCCTGCGTGCG
GCGGTGGAACGTATGAA CCAAACT r, T.AAA.A. ,AAAGCTTgcggc cae,tcr: rc
accaccaccaccaccactga
SEQ ID NO: 11 Rice GPIT amino acid sequence
Includes amino terminal amino acids MWfor cloning and His tag sequences from
pet28 vector in italics.
MWM FLATPATATATRHEMPLNPS SA FLLSSLRRSLVA LRI ASPAAAAAL
SPMA ASTV NG KA a,AE QQQQPVQVAKRLEKFKTTIFTQ 'IS LAZE H All L
GQGFPNFDGPI NAG KNQYARGY VPELN AIAERFLKDS LQVDPE
EVTVTSGGTEAIA.ATILGLINPGDEVILFAPFYDSYEATL MA ANVKAITLRPPDF
VPLEELI A .V KNTRAIMINTPHNPTG MFTREELEFIATL KENDVLLFADEVYD L
AFEADHI S fASII GMY'ERTVTMN LGKTF LT W ,IG AIAPPHLT GVR{ AH FL
94
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TFATCTPMGAAAAAALRAPDSYYEELRRDYGAKKALLVNGLKDAGFIVYPSSGTYF
'Ã VDHTPFGFDNDI FCEYLIRE `G "'AÃPP V YL PEDGKNLVRFT 'CKDDETLR
AA\'EISMKTKLR .KKLAAML HHHHHH
SEQ ID NO: 12 Soybean GPT DNA sequence
TOPO 151 D WITH SOYBEAN for E coli expression
From starting dodo. Vector sequences are italicized
ATGCATCATCACCATCACCATGGTAAGCC TATCCCTAACCCTCTCCTCGGTCTC
GATTCTACGGAAAACCTGTATTTTCAGGGAATTGATCCC TTCACCGCGAAACGT
CTGGAAAAATTTCAGACCACCATTTTTACCCAGATGAGCCTGCTGGCGATTAAAC
ATGGCGCGATTAACCTGGGCCAGGGCTTTCCGAACTTTGATGGCCCGGAATTT
GTGAAAGAAGCGGCGATTCAGGCGATTCGTGATGGCAAAAACCAGTATGCGCG
TGGCTATGGCGTGCCGGATCTGAACATTGCGATTGCGGAACGTTTTAAAAAAGA
TACCGGCCTGGTGGTGGATCCGGAAAAAGAAATTACCGTGACCAGCGGCTGCA
CCGAAGCGATTGCGGCGACCATt ATTGGCCTGATTAACCCGGGCGATGAAGTG
ATTATGTTTGCGCCGTTTTATGATAGCTATGAAGCGACCCTGAGCATGGCGGGC
GCGAAAGTGAAAG GCATTACCCTGCGTCCGCCGGATTTTGCGGTG CCGCTG GA
AGAACTGAAAAGCACCATTAGCAAAAACACCCGTGCGATTCTGATTAACACCCC
GCATAACCCGACCGGCAAAATGTTTACCCGTGAAGAACTGAACTGCATTGCGAG
CCTGTGCATTGAAAACGATGTGCTGGTGTTTACCGATGAAGTGTATGATAAACT
GGCGTTTGATATGGAACATATTAGCATGGCGAGCCTGCCGGGCATGTTTGAACG
TACCGTGACCCTGAACAGCCTGGGCAAAACCTTTAGCCTGACCGGCTGGAAAAT
TGGCTGGGCGATTGCGCCGCCGCATCTGAGCTGGGGCGTGCGTCAGGCGCAT
GCGTTTCTGACCTTTGCAACCGCACATCCGTTTCAGTGCGCAGCAGCAGCAGCA
CTGCGTGCACCGGATAGCTATTATGTGGAACTGAAACGTGATTATATGGCGAAA
CGTGCGATTCTGATTGAAGGCCTGAAAGCGGTGGGCTTTAAAGTGTTTCCGAGC
AGCGGCACCTATTTTGTGGTGGTGGATCATACCCCGTTTGGCCTGGAAAACGAT
GTGGCGTTTTGCGAATATCTGGTGAAAGAAGTGGGCGTGGTGGCGATTCCGAC
CAGCGTGTTTTATCTGAACCCGGAAGAAGGCAAAAACCTGGTGCGTTTTACCTT
TTGCAAAGATGAAGAAACCATTCGTAGCGCGGTGGAACGTATGAAAGCGAAACT
GCGTAAAGTCGACTAA
SFQ ID NO: 13 Soybean GPT amino acid sequence
Translated protein product, vector sequences 'italicized
MHHHHH GKPIP 'PLLGLDSTENLYFQG/DPFTA :RLEISFQTTIFTQMSLL.AIK GAI
NLGQGFP IFDGPEFVKEAAIQAIRDGKNQYARGYGVPOLNIAIAERF KDTGLWDP
EKEIT T SGCTE IAATMIGLINPGDEVIMFAPFYD YEATL aMAGA VKGITLRPPDF
:AV'PLEELI STISKNTRAILINTPHNPTGKMFTREELNCIASLCiEND LVFTDEVYDKL
AFI MEHISMASLPGMFERTVTLNSL.GKTFSLTG KIG `AIAPPHL GV QAHAFL
TFATAHPFQCAAAA ,L,RAPDSYYV'ELKROYMA RAILIEGLKAVGFKVFPSS TYFV
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
VVDHTP GLEND'A. C YLV VGVVAIPT VFYL P EG NLVRFT `C DE TIR
AVERMKAKLRKVD
SEC ID NO, 14 Barley GPT DNA sequence
Coding sequence from start with intmmn removed
A TGGTAGATCTGAGGAACCGACGAA CTA+TATGGCATCCGCCCCCGCCTCCGC
CTCCGCGGCCCTCTCCACCGCCGCCCCCGCGACAACGGGGCCGCCAAGCC+
ACGGAGCAGCGGCCGGTACAGGTGGCTAAGCGATTGGAGAAGTTCAAAACAAC
AATTTTCACACAGATGAGCATGCTCGCAGTGAAGCATGGAGCAATAAACCTTGG
ACAGGGGTTTCCCAATTTTGATGGCCCTGACTTTGTCAAAGATGCTGCTATTGA
GGCTATCAAAGCTGGAAAGAATCAGTATGCAAGAGGATATGGTGTGCCTGAATT
GAACTCAGCTGTTGCTGAGAGATTTCTCAAGGACAGTGGATTGCACATCGATCC
TGATAAGGAAGTTACTGTTACATCTGGGTGCACAGAAGCAATAGCTGCAACGAT
ATTGGGTCTGATCAACCCTGGGGATGAAGTCATACTGTTTGCTCCATTCTATGAT
TCTTATGAGGCT CA+CTGTCCATGGCTGGTG GAATGTCAAAGCCATTACACTC
GGCC TCCGGACTTTGCAGTCCCTCTTGA,GAGCTAAAGGCTGCAGTCTCGAA
GAATACCAGAGCAATAATGATTAATACACCTCACAACCCTACCGGGAAAATGTTC
ACAAGGGAGGAACTTGAGTTCATTGCTGATCTCTGCAAGGAAAATGACGTGTTG
CTCTTTGCCGATGAGGTCTACGACAAGCTGGCGTTTGAGGCGGATCACATATCA
ATGGCTTCTATTCCTGGCATGTATGAGAGGACCGTCACTATGAACTCCCTGGGG
AAGACGTTCTCCTTGACCGGATGGAAGATCGGCTGGGCGATAGCACCACCGCA
CCTGACATGGGGCGTAAGGCAGGCACACTCCTTCCTCACATTCGCCACCTCCA
CGCCGATGCAATCAGCAGCGGCGGCGGCCCTGAGAGCACCGGACAGCTACTT
TGAGGAGCTGAAGAGGGACTACGGCGCAAAGAAAGCGCTGCTGGTGGACGGG
CTCAAGGCGGCGGGCTTCATCGTCTACCCTTCGAGCGGAACCTACTTCATCATG
GTCGACCACACCCCGTTCGGGTTCGACAACGACGTCGAGTTCTGCGAGTACTT
GATCCGCGAGGTCGGCGTCGTGGCCATCCCGCCAAGCGTGTTCTACCTGAACC
CGGAGGACGGGAAGAACCTGGTGAGGTTCACCTTCTGCAAGGACGACGACACG
CTAAGGGCGGCGGTGGACAGGATGAAGGCCAAGCTCAGGAAGAAATGA
SEQ ID NO, 15 Barley GPT amino acid sequence
Translated sequence from start site (matron removed)
MVDLRNRRT MASAPAGA AALSTAAPADI GAAK_PTEGRPVCVA RLEIMFKTTIFT
QMSMLAVKHGAINLGQGFPNFDGPDF KDAAIEAIKAG SAVA
ERFL:GSGLHIDPIKEVTVTSGCTEAIAATILGLINPGDEVILFAPFYDSYEATL SMAG
AEIVKAITLRPPDFAVPLEEL.KAAV KNTI AIMII TPHNPTGKMFTREELEFIADLC E
NDVLLFADEVYDI LAFEADHIS 'IASIPGMYERTVTM N SLGKTFSLTGWKIGWAIAPP
H'LT4fGVRQAHSFL.TFATSTPMQSAAAAALRAPDSYFEELKRDYGAKKALLVDGLI
AGFIVY SSGTYFIMVDHTPFGFDNDVEFCEYLIREVGVVAIPP VFYLNPEDG NLV
R FTFCKDDDT L RAAVD RM KAKLR
96
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO 16 Zebra fish GPT DNA sequence
Demo rerio sequence designed for expression in E coll. Bold, italicized
nucleotides
added for cloning or from pET28b vector.
A TGTCCGTGCGAAACGTCTGGAAAAATTTAAAACCACCATTTTTACCCAGATGA
GCATGCTG G CGATTAAACATGGCGCGATTAACCTGGGCCAGGGCTTTCCGAAC
TTTGATGGCCCGGATTTTGTGAAAGAAGCGGCGATTCAGGCGATTCGTGATGGC
AACAACCAGTATGCGCGTGGCTATGGCGTGCCGGATCTGAACATTGCGATTAG
CGAACGTTATAAAAAAGATACCGGCCTGGCGGTGGATCCGGAAAAAGAAATTAC
CGTGACCAGCGGCTGCACCGAAGCGATTGCGGCGACCGTGCTGGGCCTGATT
AACCCGGGCGATGAAGTGATTGTGTTTGCGCCGTTTTATGATAGCTATGAAGCG
ACCCTGAGCATGGCGGGCGCGAAAGTGAAAGGCATTACCCTGCGTCCGCCGG
ATTTTGCGCTGCCGATTGAAGAACTGAAAAGCACCATTAGCAAAAACACCCGTG
CGATTCTGCTGAACACCCCGCATAACCCGACCGGCAAAATGTTTACCCCGGAAG
AACTGAACACCATTGCGAG CCTGTG CATTGAAAACGATGTGCTGGTGTTTAGCG
A.TGAAGTGTATG,ATAAACTGGCGTTTGATATGGAACATATTAGCATTG C GAGCT
GCCGGGCATGTTTGAACGTACCGTGACCATGAACAGCCTGGGCAAAACCTTTA
GCCTGACCGGCTGGAAAATTGGCTGGGCGATTGCGCCGCCGCATCTGACCTGG
GGCGTGCGTCAGGCGCATGCGTTTCTGACCTTTGCAACCAGCAACCCGATGCA
GTGGGCAGCAGCAGTGGCACTGCGTGCACCGGATAGCTATTATACCGAACTGA
AACGTGATTATATGGCGAAACGTAGCATTCTGGTGGAAGGCCTGAAAGCGGTG
GGCTTTAAAGTGTTTCCGAGCAGCGGCACCTATTTTGTGGTGGTGGATCATACC
CCGTTTGGCCATGAAAACGATATTGCGTTTTGCGAATATCTGGTGAAAGAAGTG
GGCGTGGTGGCGATTCCGACCAGCGTGTTTTATCTGAACCCGGAAGAAGGCAA
AAACCTGGTGCGTTTTACCTTTTGCAAAGATGAAGGCACCCTGCGTGCGGCGGT
GGATCGTATGAAAGAAAAACTGCGTAAAGTCGACAAGCTTGCGGCCGCACTCG
AGCACCACCACCACCACCACTGA
SEQ ID NO 17 Zebra fish GPR amino acid sequence
Amino acid sequence of Dania redo cloned and expressed in E. co/i (bold,
italicized
amino acids are added from vector/ cloning and His tag on C-terminus)
M'SVAKRLE F TTIFTQl ML.AIKHGAINLGQGF `NFDG DFV E.AAI AIRDGN Q
YARGYGVPDLNIAISERYKKDTGLAVDPEKEITVTSGCTEAIAATVLGLINPGDEVI F
APFYQ YE:ATESMAGAKV GITLRPPI FALPIEELKSTISKNTRAILLNTPNI PTGKMF
TPEELITIALCIEIND'VLVFIEYKLAFDMEHÃSIALPGFERT'TI1NSLGKTFL
TG iKIGWAIAPPHLT !'GVRQAHAFLTFATSNPMQ AAA 'ALRAPDSYYTELKRDY
MAKRS1L EGLKA GFKVFPSSGTYFVVVDHTPFGH:EICIDIAFC YL KEVG AIPT
FYLNPEEG NL'VRFTFCKDEGTLRAAV'DRII EKLRKVDK"LAAAL 'K to HH-
7
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO: 18 Arabldopsls truncated GPT -30 construct DNA sequence
ArabÃosi. GPT with 30 amino acids removed from the targeting sequence.
ATGGCCAAAATCCATCGTCCTATCGGAGCCACCATGACCACAGTTTCGACTCAG
AACGAGTCTACTCAAAAACCCGTCCAGGTGGCGAAGAGATTAGAGAAGTTCAAG
ACTACTATTTTCACTCAAATGAG CATATTGGCAG TTAAACATGGAGCG ATCAATT
TAGGCCAAGGCTTTCCCAATTTCGACGGTCCTGATTTTGTTAAAGAAGCTGCGA
TCCAAGCTATTAAAGATGGTAAAAACCAGTATGCTCGTGGATACGGCATTCCTCA
GCTCAACTCTGCTATAGCTGCGCGGTTTCGTGAAGATACGGGTCTTGTTGTTGA
TCCTGAGAAAGAAGTTACTGTTACATCTGGTTGCACAGAAGCCATAGCTGCAGC
TATGTTGGGTTTAATAAACCCTGGTGATGAAGTCATTCTCTTTGCACCGTTTTAT
GATTCCTATGAAGCAACACTCTCTATGGCTGGTGCTAAAGTAAAAGGAATCACTT
TACGTCCACCGGACTTCTCCATCCCTTTGGAAGAGCTTAAAGCTGCGGTAACTA
ACAAGACTCGAGCCATCCTTATGAACACTCCGCACAACCCGACCGGGAAGATGT
TCACTAGGGAGGAGCTTGAAACCATTGCATCTCTCTGCATTGAAAACGATGTGC
TT GTGTTCTC G GAT G AAGTATAC GATAAG CTTG C GTTTGAAATG G ATC ACATTTC
TATAGCTTCTCTTCCCGGTATGTATGAAGAACTGTGACCATGAATTCCCTGGGA
AAGACTTTCTCTTTAACCGGATG GAAGATC GGCTG GGCGATTGCGCCGCCTCAT
CTGACTTGGGGAGTTCGACAAGCACACTCTTACCTCACATTCGCCACATCAACA
CCAGCACAATGGGCAGCCGTTGCAGCTCTCAAGGCACCAGAGTCTTACTTCAAA
GAGCTGAAAAGAGATTACAATGTGAAAAAGGAGACTCTGGTTAAGGGTTTGAAG
GAAGTCGGATTTACAGTGTTCCCATCGAGCGGGACTTACTTTGTGGTTGCTGAT
CACACTCCATTTGGAATGGAGAACGATGTTGCTTTCTGTGAGTATCTTATTGAAG
AAGTTGGGGTCGTTGCGATCCCAACGAGCGTCTTTTATCTGAATCCAGAAGAAG
GGAAGAATTTGGTTAGGTTTGCGTTCTGTAAAGACGAAGAGACGTTGC
GTGGTGCAATTGAGAGGATGAAGCAGAAGCTTAAGAGAAAAGTCTGA
SEQ ID NO: 19 Arabidopsis truncated GPT -30 construct amino acid sequence
MAKIHR.PIGATI TTV FTQMS ILAVKHGAINLGQG
FPNFQGPDFVKEAAIQAIKDGKNQYARGYGIPQLN AIAARFREDTGLVVDPEKEVT
VTSGCTEAIAAAMLGLINPGDEVILFAPFY YYEATLS MAGAKVKG ITLRPPDFSIPLE
IAS LCIENDVLVFCDEVYDKLAFE I
QHI IA LPGMYERTVTMNSLGKTF LTG 'KIG ''AIAPPHLTWGVRQAI YLTFAT
TPAQ AAVAALKAPE YFK.E.LKRCYNVKKETLVKGLKEVGFTVFPSSGTYFVVADH
TPFGMENDVAFCEYLIEEVGVVAIPTSVFYLNPEEGKNLVRFAFCKDEETLRGAIER
MKQKLKRKV
98
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NQ: 20: ArabIdo s s truncated GPT -45 construct DNA sequence
ArabÃdo sIs GPT with 45 residues in the targeting sequence removed
ATGGCGACTCAGAACGAGTCTACTCAAAAACCCGTC CAGGTGGCGAAGAGATTA
GAGAAGTTCAAGACTACTATTTTCACTCAAATGAGCATATTGGCAGTTAA.CATG
GAGCGATCAATTTAG G CCAAGGCTTTCC CAATTTCGACG GTCCTGATTTTGTTAA
AGAAGCTGCGATCCAAGCTATTAAAGATGGTAAAAACCAGTATGCTCGTGGATA
CGGCATTCCTCAGCTCAACTCTGCTATAGCTGCGCGGTTTCGTGAAGATACGGG
TCTTGTTGTTGATCCTGAGAAAGAAGTTACTGTTACATCTGGTTGCACAGAAGCC
ATAGCTGCAGCTATGTTGGGTTTAATAAACCCTGGTGATGAAGTCATTCTCTTTG
CACCGTTTTATGATTCCTATGAAGCAACACTCTCTATGGCTGGTGCTAAAGTAAA
AGGAATCACTTTACGTCCACCGGACTTCTCCATCCCTTTGGAAGAGCTTAAAGC
TGCGGTAACTAACAAGACTCGAGCCATCCTTATGAACACTCCGCACAACCCGAC
CGGGAAGATGTTCACTAGGGAGGAGCTTGAAACCATTGCATCTCTCTGCATTGA
AAACGATGTGCTTGTGTTCTCGGATGAAGTATACGATAAGCTTGCGTTTGAAATG
GATCACATTTCTATAGCTTCTCTTCCCGGTATGTATGAAAGAACTGTGACCATGA
A.TTCC+CTGGGA.GACTTTCTCTTTAA.CCGGATGGAAGATC GG CTGG GCGATTG
GGCCGCCTCATCTGACTTGGGGAGTTCGACAAGCACATCTTACCTCACATTCG
CCACATCAACACCAGCACAATGGGCAGCCGTTGCAGCTCTCAAGGCACCAGAG
TCTTACTTCAAAGAGCTGAAAAGAGATTACAATGTGAAAAAGGAGACTCTGGTTA
AGGGTTTGAAGGAAGTCGGATTTACAGTGTTCCCATCGAGCGGGACTTACTTTG
TGGTTGCTGATCACACTCCATTTGGAATGGAGAACGATGTTGCTTTCTGTGAGTA
TCTTATTGAAGAAGTTGGGGTCGTTGCGATCCCAACGAGCGTCTTTTATCTGAAT
CCAGAAGAAGGGAAGAATTTGGTTAG GTTTGCGTTCTGTAAAGACGAAGAGACG
TTGCGTGGTGCAATTGAGAGGATGAAGCAGAAGCTTAAGAGAAAAGTCTGA
SEQ ID NO: 21: ArabIdo sls truncated GPT -4construct amino acid sequence
MATQNE TQKPVQVAKRLEl FKTTIFTQM 1LAVKHGAINLGQGFPNFI GPDFVK.EA
AIQAIKCGI NGYARGYGIPQLN AIAAR RE TGLVV P KEVTVTSG TEAIAAAI L
GLINPG 3EViLFAPFYD YEATL MAGAKV GITLRPPDFSIPLEELI AAVTNKTAIL
MNTPHNPTGKMFTREELETIACLCIENDVL F DEVYDKLAFEM 3HI IASLPGMYEI
TVTMN LGKTF LTG KIG 'AIAPPHLT 'GVRQAHSYLTFAT TPAQ AAVAALKA
PE YFf ELKR.DYNVKKETL KGLKEVGFTV `P SGTYF' ACHTP GMENDVAFCE
YLIEEVGVVAIPT VFYLNPEEGKNLVRFAFCK EETLRGAIER IKQKLKRKV
SEQ ID NO: 22: Tomato Rub co promoter
TOMATO RBIsCo rbcS3C promoter sequence from lpnl to Ncol
G G TA C CGT TTGAATC CTCCTTAAAG TTTTTCTC TG GAG AAACT G TAG TART TTTAC
TTTGTTGTGTTCCCTTCATCTTTTGAATTAATGGCATTTGTTTTAATACTAATCTGC
TTCTGAAACTTGTAATGTATGTATATCAGTTTCTTATAATTTATCCAAGTAATATCT
99
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TCCATTCTCTATGCAATTGCCTGCATAAGCTCGACAAAAGAGTACATCAACCCCT
CCTCCTCTGGACTACTCTAGCTAAACTTGAATTTCCCCTTAAGATTATGAAATTG
ATATATCCTTAACAAACGACTCCTTCTGTTGGAAAATGTAGTACTTGTCTTTCTTC
TTTTGGGTATATATAG:TTTATATACACCATACTATGTACAACATCCAAGTAGAGTG
AAMTGGATACATGTACAAGACTTATTTGATTGATTGATGACTTGAGTTGCCTTAG
GAGTAACAAATTCTTAGGTCAATAAATCGTTGATTTGAAATTAATCTCTCTGTCTT
AGACAGATAGGAATTATGACTTCCAATGGTCCAGAAAGCAAAGTTCGCACTGAG
GGTATACTTGGAATTGAGACTTGCACAGGTCCAGAAACCAAAGTTCCCATCGAG
CTCTAAAATCACATCTTTGGAATGAAATTCAATTAGAGATAAGTTGCTTCATAGCA
TAGGTAAAATGGAAGATGTGAAGTAACCTGCAATAATCAGTGAAATGACATTAAT
ACACTAAATACTTCATATGTAATTATCCTTTCCAGGTTAACAATACTCTATAAAGT
AAGAATTATCAGAAATGGGCTCATCAAACTTTTGTACTATGTATTTCATATAAGGA
AGTATAACTATACATAAGTGTATACACAACTTTATTCCTATTTTGTAAAGGTGGAG
AGACTGTTTTCGATGGATCTAAAGCAATATGTCTATAAAATGCATTGATATAATAA
TTATCTGAGAAAATCCAGAATTGGCGTTGGATTATTTCAGCCAAATAGAAGTTTG
TACCATACTTGTTGATTCCTTCTAAGTTAAGGTGAAGTATCATTCATAAACAGTTT
TCCCCAAAGTACTACTCACCAAGTTTCCCTTTGTAGAATTAACAGTTCAAATATAT
GGCGCAGAAATTACTCTATGCCCAAAACCAAACGAGAAAGAAACAAAATACAGG
GGTTGCAGACTTTATTTTCGTGTTAGGGTGTGTTTTTTCATGTAATTAATCAAAAA
ATATTATGACAAAAACATTTATACATATTTTTACTCAACACTCTGGGTATCAGGGT
GGGTTGTGTTCGACAATCAATATGGAAAGGAAGTATTTTCCTTATTTTTTTAGTTA
ATATTTTCAGTTATACCAAACATACCTTGTGATATTATTTTTAAAAATGAAAAACTC
GTCAGAAAGAAAAAGCAAAAGCAACAAAAAAATTGCAAGTATTTTTTAAAAAAGA
AAAAAAAAACATATCTTGTTTGTCAGTATGGGAAGTTTGAGATAAGGACGAGTGA
GGGGTTAAAATTCAGTGGCCATTGATTTTGTAATGCCAAGAACCACAAAATCCAA
TGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCGTTAGATAGG
AAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTAACCAATTATT
TCAGCACCA TGG
SEC ID NO. 23: Bamboo GPT DNA sequence
ATGGCCTCCGCGGCCGTCTCCACCGTCGCCACCGCCGCCGACGGCGTCGCGA
AGCCGACGGAAAGCAGCCGGTAAGGTCGCAAAGCGTTTG GAAAAGTTTAAG
ACAACAATTTTCACACAGATGAGCATGCTTGCCATCAAGCATGGAGCAATAAAC
CTCGGCCAGGGCTTTCCGAATTTTGATGGCCCTGACTTTGTGAAAGAAGCTGCT
ATTCAAGCTATCAATGCTGGGAAGAATCAGTATGCAAGAGGATATGGTGTGCCT
GAACTGAACTCGGCTGTTGCTGAAAGGTTCCTGAAGGACAGTGGCTTGCAAGTC
GATCCCGAGAAGGAAGTTACTGTCACATCTGGGTGCACGGAAGCGATAGCTGC
AACGATATTGGGTCTTATCAACCCTGGCGATGAAGTGATCTTGTTTGCTCCATTC
TATGATTCATACGAGGCTACGCTGTCGATGGCTGGTGCCAATGTAAAAGCCATT
ACTCTCCGTCCTCCAGATTTTGCAGTCCCTCTTGAGGAGCTAAAGGCCACAGTC
TCTAAGAACACCAGAGCGATAATGATAAACACACCACACAATCCTACTGGGAAA
ATGTTTTCTAGGGAAGAACTTGAATTCATTGCTACTCTCTGCAAGAAAAATGATG
100
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TGTTGCTTTTTGCTGATGAGGTCTATGACAAGTTGGCATTTGAGGCCAGATCATAT
ATCAATGGCTTCTATTCCTGGCATGTATGAGAGACTGTGACTATGAACTCTCTG
G GGAAGACATTCTCTCTAACAG GATGGAAGATCGGTTGGGCAATACAC CACCA
CACCTGACATGG G GTGTAAGG CAG GCACACTCATTCCTCACATTTGC CACCTGC
ACACCAATGCAATCGGCGGCGGCGGCGGCTCTTAGAGCACCAGATAGCTACTA
TGGGG.AGCTGAAGAGGGATTACGGTGCAAAGAAAGCGATACTAGTCGACGGAC
TCAAGGCTGCAGGTTTTATTGTTTACCCTTCAAGTGGAACATACTTTGTCATGGT
CGATCACACCCCGTTTGGTTTCGACAATGATATTGAGTTCTGCGAGTATTTGATC
CGCGAAGTCGGTGTTGTCGCCATACCACCAAGCGTATTTTATCTCAACCCTGAG
GATGGGAAGAACTTGGTGAGGTTCACCTTCTGCAAGGATGATGATACGCTGAGA
GCCGCAGTTGAGAGGATGAAGACAAAGCTCAGGAAAAAATGA
SEQ ID NO: 24: Bamboo GPT amino acid sequence
MASAAVST ATAADGV'AKPTEKQPVCVAKRLEKFKTTIFTQ lSIMLAIKHGAINLGQG
FPNFDGPD VK AAIGAI AGKNGYA GYGVPEL. SAVA RFLKD G GVDP K V
TVTSGCTEAIAATILGLINPGDE\ILFAPFYDSYEATL MAGANVKAITLRPPDFAVPL
EE.LKATVSKNTR.AIMINTPHNPTGKMFSREELEFIATLCKKNDVLLFADEVYDKLAFE
AI HI A IPGMYERTVTI1 N LGKTF LTG KIGWAIAPPHLTWGVPGAH FLTFA
TCTPMGSAAAAALR.API SY GELKRDYGAKKAILVDGLK.AAGFIVYP SGTYF I"+ V
DHTPFGFDND EFCEY ,I EVGWAIPP aVFYLNPEDGKNLVPFTFCKDODTLPAAVE
RMKTKLRKK
SEQ ID NO: 25, 1305.1+rbcS3C promoter + catl i tron with rice GPT gene.
Cambial 305.1 with (3 end of) rbc 3C+rice GPT. Underlined ATG is start site,
parentheses are the catl intron and the underlined actagt is the spel cloning
site used
to splice in the rice gene.
AAAAAAGAAAAAAAAAACATATCTTGTTTGTCAGTATGGGAAGTTTGAGATAAGG
ACG AG TGAG GGG TTTAAAATTCAGTG GCCATTGATTTTGTAATGCCAAGAACCAC
AAAATCCAATGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCG
TTAGATAGGAAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTA
ACCAATTATTTCAGCACCA TGGTAGATCTGAGG(GTAAATTTCTAGTTTTTCTCCT
TCATTTTCTTGGTTAGGACCCTTTTCTCTTTTTATTTTTTTGAGCTTTGATCTTTCT
TTAAACTGATCTATTTTTTAATTGATTGGTTATGGTGTAAATATTACATAGCTTTAA
CTGATAATCTGATTACTTTATTTCGTGTGTCTATGATGATGATGATAGTTACAG)A
ACCGACGAACTAGTATGAATCTGGCCGGCTTTCTCGCCACGCCCGCGACCGCG
ACCGCGACGCGGCATGAGATGCCGTTAAATCCCTCCTCCTCCGCCTCCTTCCTC
CTCTCCTCGCTCCGCCGCTCGCTCGTCGCGTCGCTCCGGAAGGCCTCGCCGG
CGGCGGCCGCGGCGCTCTCCCCCATGGCCTCCGCGTCCACCGTCGCCGCCGA
GAACGGCGCCGCCAAGGCGGCGGCGGAGAAGCAGCAGCAGCAGCCTGTGCA
101
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
GGTTGCAAAGCGGTTGGAAAAAGTTTAAAGACGACCATTT TCACACAGATGAGTAT
GCTTGCCATCAAGCATGGAGCAATAArCCTTGGCCAGGGTTTTCCGAATTTCGA
TGGCCCTGACTTTGTAAAAGAGGCTGCTATTCAAGT ATCAATGCTGGGAAG
TCA:GTACGCAAGAGGATATGGTGTGCCTGAACTGAACTCAGCTATTGCTGAAAG
ATTCCTGAAGGACAGCGGACTGCAAGTCGATCCGGAGAAGGAAGTTACTGTCA
CAT CTG GATE CACAGAAG CTATAG CTG C AACAATTTTAGG TCTAATTAATCCAG G
CGATGAAGTGATATTGTTTGCTCCATTCTATGATTCATATGAGGCTACCCTGTCA
ATGGCTGGTGCCAACGTAAAAGCCATTACTCTCCGTCCTCCAGATTTTTCAGTC
CCTCTTGAAGAGCTAAAGGCTGCAGTCTCGAAGAACACCAGAGCTATTATGATA
AACACCCCGCACAATCCTACTGGGAAAATGTTTACAAGGGAAGAACTTGAGTTT
ATTGCCACTCTCTGCAAGGAAAATGATGTGCTGCTTTTTGCTGATGAGGTCTAC
GACAAGTTAGCTTTTGAG GCAGATCATATATCAATGGCTTCTATTCCTG GCATGT
ATGAGAGGACCGTGACCATGAACTCTCTTGGGAAGACATTCTCTCTTACAG GAT
GGAAGATCGGTTGGGCAATCGCACCGCCACACCTGACATGGGGTGTAAGGCAG
GCACACTCATTCCTCACGTTTGCGACCTGCACACCAATGCAAGCAGCTGCAGCT
GCAGCTCTGAGAGCACCAGATAGCTACTATGAGGAACTGAGGAGGGATTATGG
AGCTAAGAAGGCATTGCTAGTCAACG GACTCAAGGATGCAGGTTTCATTGTCTA
TCCTTCAAGTGGAACATACTTCGTCATGGTCGACCACACCCCATTTGGTTTCGA
CAATGATATTGAGTTCTGCGAGTATTTGATTCGCGAAGTCGGTGTTGTCGCCATA
CCACCTAGTGTATTTTATCTCAACCCTGAGGATGGGAAGAACTTGGTGAGGTTC
ACCTTTTGCAAGGATGATGAGACGCTGAGAGCCGCGGTTGAGAGGATGAAGAC
AAAGCTCAGGAAAAAATGA
SEC ID NO: 26HORDEUM GPT SEQUENCE IN VECTOR
Cambial 30. with (3 end of) rbc C+hordeum ID14. Underlined AIG is start site,
parentheses are the tl intron and the underlined actagt is the sp l cloning
site used
to splice in the horde urn gene.
AAAAAAGAAAAAAAAAACATATCTTGTTTGTCAGTATGGGAAGTTTGAGATAAGG
ACGAGTGAGGGGTTAAAATTCAGTGGCCATTGATTTTGTAATGCCAAGAACCAC
AAAATCCAATGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCG
TTAGATAGGAAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTA
ACCAATTATTTCAGCACCA TGGTAGATCTGAGG(GTAAATTTCTAGTTTTTCTCCT
TCATTTTCTTGGTTAGGACCCTTTTCTCTTTTTATTTTTTTGAGCTTTGATCTTTCT
TTAAACTGATCTATTTTTTAATTGATTGGTTATGGTGTAAATATTACATAGCTTTAA
CTGATAATCTGATTACTTTATTTCGTGTGTCTATGATGATGATGATAGTTACAG)A
ACCGACGAACTAGTATGGCATCCGCCCCCGCCTCCGCCTCCGCGGCCCTCTCC
ACCGCCGCCCCCGCCGACAACGGGGCCGCCAAGCCCACGGAGCAGCGGCCG
GTACAGGTGGCTAAGCGATTGGAGAAGTTCAAAACAACAATTTTCACACAGATG
AGCATGCTCGCAGTGAAGCATGGAGCAATAAACCTTGGACAGGGGTTTCCCAAT
TTTGATGGCCCTGACTTTGTCAAAGATGCTGCTATTGAGGCTATCAAAGCTGGA
AAGAATCAGTATGCAAGAGGATATGGTGTGCCTGAATTGAACTCAGCTGTTGCT
GAGAGATTTCTCAAGGACAGTGGATTGCACATCGATCCTGATAAGGAAGTTACT
102
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
GTTACATCTGGGTG+ACAGGAAGCAATAGCTGCAACGATATTGGGTCTGATCAAC
CCTGGGGATGAAGTCATACTGTTTGCTCCATTCTATGATTCTTATGAGGCTAAC
TGTCCATGGCTGGTGCGAATGTCAAAGCCATTACACTCCGCCCTCCGGACTTTG
CAGTCCCTCTTGAAGAGCTAAAGGCTGCAGTCTCGAAGAATACCAGAGCAATAA
TGATTAATACACCTCACAACCCTACCGGGAAAATGTTCACAAGGGAGGAACTTG
AGTTCATTGCTGATCTCTGCAAGGAAATGACGTGTTGCTCTTTGCCGATGAGG
TCTACGACAAGCTGGCGTTTGAGGCGGATCACATATCAATGGCTTCTATTCCTG
GCATGTATGAGAGGACCGTCACTATGAACTCCCTGGGGAAGACGTTCTCCTTGA
CCGGATGGAAGATCGGCTGGGCGATAGCACCACCGCACCTGACATGGGGCGT
AAGGCAGGCACACTCCTTCCTCACATTCGCCACCTCCACGCCGATGCAATCAGC
AGCGGCGGCGGCCCTGAGAGCACCGGACAGCTACTTTGAGGAGCTGAAGAGG
GACTACGGCGCAAAGAAAGCGCTGCTGGTGGACGGGCTCAAGGCGGCGGGCT
TCATCGTCTACCCTTCGAGCGGAACCTACTTCATCATGGTCGACCACACCCCGT
TCGGGTTCGACAACGACGTCGAGTTCTGCGAGTACTTGATCCGCGAGGTCGGC
GTCGTGGCCATCCCGCCAAGCGTGTTCTACCTGAACCCGGAGGACGGGAAGAA
C CTGGTGAGGTTCACCTTCTGCAAGG ACGACG ACACG CTAAG GGCGGCG GTG
GACAGGATGAAGG CCAAGCTCAGGAAAGAAATGATTGAGGG GCG CA CG TG TGA
SEC !D NO, 27 Cambia 1201 + Ara ido sis GPT sequence (35S promoter from
CaMV in italics)
CATGGAGTCAAAGATTCAAATAGAGGACCTAACAGAACTCGCCGTAAAGACTGG
CGAACAGTTCATACAGAGTCTCTTACGACTCAATGACAAGAAGAAAATCTTCGTC
AACATGGTGGAGCACGACACACTTGTCTACTCCAAAAATATCAAAGATACAGTCT
CAGAAGACCAAAGGGCAATTGAGACTTTTCAACAAAGGGTAATATCCGGAAACC
TCCTCGGA TTCCA TTGCCCAGC TA TCTGTCACTTTA TTG TGAAGA TAG TGGAAAA
GGAAGGTGGCTCCTACAAATGCCATCATTGCGATAAAGGAAAGGCCATCGTTGA
AGA TGCCTCTGCCGACAGTGGTCCCAAAGATGGACCCCCACCCACGAGGAGCA
TCGTGGAAAAAGAAGACGTTCCAACCACGTCTTCAAAGCAAGTGGATTGATGTG
ATATCTCCACTGACGTAAGGGATGACGCACAATCCCACTATCCTTCGCAAGACC
CTTCCTCTATATAAGGAAGTTCATTTCATTTGGAGAGAACACGGGGGACTCTTGA
CCATGTACCTGGACATAAATGGTGTGATGATCAAACAGTTTAGCTTCAAAGCCTC
TCTTCTCCCATTCTCTTCTAATTTCCGACAAAGCTCCGCCAAAATCCATCGTCCT
ATCGGAGCCACCATGACCACAGTTTCGACTCAGAACGAGTCTACTCAAAAACCC
GTCCAGGTGGCGAAGAGATTAGAGAAGTTCAAGACTACTATTTTCACTCAAATG
AGCATATTGGCAGTTAAACATGGAGCGATCAATTTAGGCCAAGGCTTTCCCAATT
TCGACGGTCCTGATTTTGTTAAAGAAGCTGCGATCCAAGCTATTAAAGATGGTAA
AAACCAGTATGCTCGTGGATACGGCATTCCTCAGCTCAACTCTGCTATAGCTGC
GCGGTTTCGTGAAGATACGGGTCTTGTTGTTGATCCTGAGAAAGAAGTTACTGT
TACATCTGGTTGCACAGAAGCCATAGCTGCAGCTATGTTGGGTTTAATAAACCCT
GGTGATGAAGTCATTCTCTTTGCACCGTTTTATGATTCCTATGAAGCAACACTCT
CTATGGCTGGTGCTAAAGTAAAAGGAATCACTTTACGTCCACCGGACTTCTCCA
TCCCTTTGGAAGAGCTTAAAGCTGCGGTAACTAACAAGACTCGAGCCATCCTTA
103
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TGAACACTCCGCACAACCCGACCGGGAAGATGTTCACTAGGGAGGAGCTTGAA
ACCTT GCATCTCTCTGCATTGAAAACGATGTGCTTTGTTCTCGGATGAAGTAT
ACGATAAGCTTGCGTTTGA,AATG GATCCATTTCTATAGCTTCTCTTCCCGGTA.T
GTATGAAAGAACTGTGACCATGAATTCCCTGGGAAAGGACTTTCTCTTTAACCGGA
TGGAAGATCGGCTGGGCGATTGCGCCGCCTCATCTGACTTGGGGAGTTCGACA
AGCACACTCTTACCTCACATTCG CCACATC,ACACCAGCACAATGCG CAG CCGT
TGCAGCTCTCAAGGCACCAGAGTCTTACTTCAAAGAGCTGAAAAGAGATTACAA
TGTGAAAAAGGAGACTCTG GTTAAGG GTTTGAAGGAAGTCG GATTTACAGTGTT
CCCATCGAGCGGGACTTACTTTGTGGTTGCTGATCACACTCCATTTGGAATGGA
GAACGATGTTGCTTTCTGTGAGTATCTTATTGAAGAAGTTGGGGTCGTTGCGAT
CCCAACGAGCGTCTTTTATCTGAATCCAGAAGAAGGGAAGAATTTGGTTAGGTT
TGCGTTCTGTAAAGACGAAGAGACGTTGCGTGGTGCAATTGAGAGGATGAAGC
AGAAGCTTAAGAGAAAAGTCTGA
SEQ ID NO 28 Cambia p1305.1 with (Tend of) rbcS3C+Arabidopsis GPT.
Underlined ATG is start site, parentheses are the cat! Ãntron and the
underlined
actagt is the spel cloning site used to splice in the Arabidep is gene.
AAAA.AAGAAA.AAAAAACATATCTTGTTTGTCAGTATGGGAAGTTTGAGATAAGG
AC GAGTG AG G G G TTAAAATTC AGTG G C CATT+ATTTTG TAATGCCAAGAAC CAC
AAAATCCAATGGTTACCATTCCTGTAAGATGAGGTTTGCTAACTCTTTTTGTCCG
TTAGATAGGAAGCCTTATCACTATATATACAAGGCGTCCTAATAACCTCTTAGTA
ACCAATTATTTCAGCACCA TGGTAGATCTGAGG(GTAAATTTCTAGTTTTTCTCCT
TCATTTTCTTGGTTAGGACCCTTTTCTCTTTTTATTTTTTTGAGCTTTGATCTTTCT
TTAAACTGATCTATTTTTTAATTGATTGGTTATGGTGTAAATATTACATAGCTTTAA
CTGATAATCTGATTACTTTATTTCGTGTGTCTATGATGATGATGATAGTTACAG)A
ACCGACGAA CTAGTATGTACCTGGACATAAATGGTGTGATGATCAAACAGTTTA
GCTTCAAAGCCTCTCTTCTCCCATTCTCTTCTAATTTCCGACAAAGCTCCGCCAA
AATCCATCGTCCTATCGGAGCCACCATGACCACAGTTTCGACTCAGAACGAGTC
TACTCAAAAACCCGTCCAGGTGGCGAAGAGATTAGAGAAGTTCAAGACTACTAT
TTTCACTCAAATGAG CATATTG G CAGTTAAACATGGAG C GATCAATTTAG G CCAA
GGCTTTCCCAATTTCGACGGTCCTGATTTTGTTAAAGAAGCTGCGATCCAAGCTA
TTAAAGATGGTAAAAACCAGTATGCTCGTGGATACGGCATTCCTCAGCTCAACT
CTGCTATAGCTGCGCGGTTTCGTGAAGATACGGGTCTTGTTGTTGATCCTGAGA
AAGAAGTTACTGTTACATCTGGTTGCACAGAAGCCATAGCTGCAGCTATGTTGG
GTTTAATAAACCCTGGTGATGAAGTCATTCTCTTTGCACCGTTTTATGATTCCTAT
GAAGCAACACTCTCTATGGCTGGTGCTAAAGTAAAAGGAATCACTTTACGTCCA
CCGGACTTCTCCATCCCTTTGGAAGAGCTTAAAGCTGCGGTAACTAACAAGACT
CGAGCCATCCTTATGAACACTCCGCACAACCCGACCGGGAAGATGTTCACTAG
GGAGGAGCTTGAAACCATTGCATCTCTCTGCATTGAAAACGATGTGCTTGTGTT
CTCGGATGAAGTATACGATAAGCTTGCGTTTGAAATGGATCACATTTCTATAGCT
TCTCTTCCCGGTATGTATGAAAGAACTGTGACCATGAATTCCCTGGGAAAGACTT
TCTCTTTAACCGGATGGAAGATCGGCTGGGCGATTGCGCCGCCTCATCTGACTT
104
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
GG GGAGTTCGACAAGCACACTCTTAC CTCACATTCG CCACATCAAACCAGCAC
AATGGGCAGCCGTTGCAGCTCTCRAGGCACCAGAGTCTTACTTCAAAGAGCTGA
AAAGAGATTACAATGTGAAAAGGAGACTCTGGTTAAGGGTTTGAAGGAAGTCG
GATTTACAGTGTTCCCATCGAGCGGGACTTACTTTGTGGTTGCTGATCACACTC
CATTTGGAATGGAGICGATGTTGCTTTCTGTGAGTATCTTATTGAAGGTTGG
GGTCGTTGCGATCCCAACGAGCGTCTTTTATCTGAATCCAGAAGAAGGGAAGAA
TTTG GTTAGGTTTGCGTTCTGTAAAGACGAAGAGACGTTGCGTGGTG CAATTGA
GAGGATGAAGCAGAAGCTTAAGAGAAAAGTCTGA
SECS ID NCB: 29 Ar bi psis GPT coding sequence (mature protein, no targeting
sequence)
GTGGCGAAGAGATTAGAGAAGTTCAAGACTACTATTTTCACTCAAATGAGCATAT
TGGCAGTTAAACATGGAGCGATCAATTTAGGCCAAGGCTTTCCCAATTTCGACG
GTCCTGATTTTGTTAAAGAAGCTG CGATCCAAGCTATTAAAGATGGTAAAAACCA
GTATGCTCGTGGATACGGCATTC CTCAGCTCAACTCTGCTATAGCTGCGCGGTT
TC G T GAAG ATACG G GTCTTGTTG TTGATC CTGAGAAAGAAG TTA CTG TTAC AT CT
G G TTG CACAG AAG C CATAG CTG CAG C TATGTTG G GTTTAATAAACC CT G GTGAT
GAAGTCATTCTCTTTGCACCGTTTTATGATTCCTATGAAGCAACACTCTCTATGG
CTGGTGCTAAAGTAAAAGGAATCACTTTACGTCCACCGGACTTCTCCATCCCTTT
GGAAGAGCTTAAAGCTGCGGTAACTAACAAGACTCGAGCCATCCTTATGAACAC
TCCGCACAACCCGACCGGGAAGATGTTCACTAGGGAGGAGCTTGAAACCATTG
CATCTCTCTGCATTGAAAACGATGTGCTTGTGTTCTCGGATGAAGTATACGATAA
GCTTGCGTTTGAAATGGATCACATTTCTATAGCTTCTCTTCCCGGTATGTATGAA
AGAACTGTGACCATGAATTCCCTGGGAAAGACTTTCTCTTTAACCGGATGGAAG
ATCGGCTGGGCGATTGCGCCGCCTCATCTGACTTGGGGAGTTCGACAAGCACA
CTCTTACCTCACATTCGCCACATCAACACCAGCACAATGGGCAGCCGTTGCAGC
TCTCAAGGCACCAGAGTCTTACTTCAAAGAGCTGAAAAGAGATTACAATGTGAAA
AAGGAGACTCTGGTTAAGGGTTTGAAGGAAGTCGGATTTACAGTGTTCCCATCG
AGCGGGACTTACTTTGTGGTTGCTGATCACACTCCATTTGGAATGGAGAACGAT
GTTGCTTTCTGTGAGTATCTTATTGAAGAAGTTGGGGTCGTTGCGATCCCAACG
AGCGTCTTTTATCTGAATCCAGAAGAAGGGAAGAATTTGGTTAGGTTTGCGTTCT
GTAAAGACGAAGAGACGTTGCGTGGTGCAATTGAGAGGATGAAGCAGAAGCTT
AAGAGAAAAGTCTGA
SEC: ID NO: 30 Arabidpsis GPT amino acid sequence (mature protein, no
targeting
sequence)
VA RLE F TTIFTG ILAV HGAiNLGQGFP Ft3G DF EAAiQAI DGKNGYAR
GYGIPGLN AIAARFREDTGL '' DPE TVT GCTEAIAAAMLGLI PGCEVILFAP
FYDS' EATLSM G :I ITLRPPCFSI LEELKAAVTN TRAILMNTPHNPTG MFT
REELETIASLCIENDVLVFSDEV'YDKLA MDHICIA LPG 'IYER +''TMNSLGKTFSL
105
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
TGIl KIG 'AIAPPHLTWGVRQAHSYLTFATSTPAQW'AAVAALKAPESYFKELKRDYN
VÃ ETLVV GL.KE FT "FP TYFV ADHTPF DVAFCEYL_IEE G VAIPT
VFYLNPEEGKNLVRFAFCKDEETLRGAIERMKQKLKRKV
c
SEQ ID N : 31 Grape GPT amino acid sequence (mature protein, no targeting
sequence)
VAKRLEKFKTTIFTQMSMLAIKHGAINLGC GFPNF PEFVKE IQAIKD NQY R
Y VPDLN AVAI RFKKI T+GLVV'I PEI EVTVTSG TEAI TMLGL.INP DEVILFA
PFYDSYEATLSMAGAQIKSITLRPPL FAVPMDELKSAISKNTRAILINTPHNPTGKMFT
REELNVIASLCIENDVLVFTDEVYDK.LAFEMDHISMA LP MYERTVTMNSL KTFS
LTGWKIGWTVAPPHLT dGVRQAHSFLTFATCTIPICIQW+ ATALI APDSYYEEL.I R
YSAKKAILVEGLKAVGFRVYP aS TYFWVDHTPF LKDDIAF EYLI EV VVAIPT
SVF' L.HPEI KNLVI FTFCKDEGTLR VERMKEKLKPKQ
SECS ID NO: 32 Rice GPT amino acid sequence (mature protein, no targeting
sequence)
VAKRLEKFKTTIFTQMSMLAII HGAINL FPNFD PI FVKEAAIQAINAB`KNQYAR
+GYVPELNSAIAERFLIDSLQVDPEKEVTVT PG EVILFAPF
YDSYEATLSMAGANVKAITLRPPI FSVPLEELKAAVSKNTRAIMINTPHNPTGKMFT
REELEFIATLCKENDV LLFADEVY'DKLAFEADHISMASIP MÃYERTVTMNSL KTFSL
TGWKIGWAIAPPHLTWGV'R AHSFLTFATCTPMQAA AALRAPDSYYEELR.RDY
GAKKALLVNGLKI AGFIVYPSSGTYFVMVDHTPFGFDNDIEF .EYLIREVGVVAIPPS
VFYLNPEDGKNLVRFTFCKDDETLRAAVERMKTKLRKK
SEQ ID NO. 33 Soybean GPT amino acid sequence (-I mature protein, no targeting
sequence)
AKRLEKFGTTIFTQMSLLAIKHGAIN LGGGFPNFDGPEFVKEAAI AIRDGKNQYARG
YGVPI LNIAIAERFKKDTGLWDPEKEITVTSGCTEAIAATMIGLI NPGDEVI MFAPFY
DSYErTLMAGAKVKITLR'PDFAVPLEELKSTISKNTRAILINTPHNPTKMTIE
SMASLPGM FERTVTLNSLGI TFSLTG
`KI G AIAPPHLSGVRQAHAFLTFAT.AH PFsCAAAAALRAPDSYYV E LKRDYMAK
RAILIEGLKAVGFKVFPSSGTYFVVVDHTPFGLEN DVAFCEYLVKEVGWAIPTSVFY
LNPEEG NLVRFTFCKDE ET I RSAVERM KAKLRKVD
106
CA 02735646 2011-02-28
WO 2010/025466 PCT/US2009/055557
SEQ ID NO: 34 Barley GPT amino acid sequence (mature protein, no targeting
sequence)
VAKRLEKFKTTIFTQMSMLAVKHGAINLGQGFPNFD PD V DAA AIKA .NQY
RGYGVPE N SAVAERFLKDSG L I PDKEVTVTS CTEA ATIL LIN PG
FYDSYEATLSMAGANV AIT ,RPP. FAVPLEELKAAV KNTRAI INTPHNPT KI FT
REELEFIADLGKENDVLLFADEVYDKLAFEADHISMA IP MYERT TMN L+ KTF L
TG KIGWAIAPPHLT `GVRQAHSFLTFATSTPMQSAAAAALRAPDSYFEELKRDYG
AKKALL.VD LIB FIVYPS GTY IMVD TPF FDNDVEFGE `LIREVGWAIPP '
FYLNPEDGKNLVRFTFCKDDDTLRAAVDRMKAKLRKK
SEQ ID NO: 35 Zebra fish GPT amino acid sequence (mature protein, no targeting
sequence)
VAKI~ LEI FKTTIFTQMSI LAIKHGAINLGQGFPNFDGPDFVKEAAIQAII DGI NQ' A
YGVPDLNIAISERYK DTGLAVDPEKEIT' T G TEAIAATVLGLINPGDE 'I 'FAP
FYI S' EATLSMAGAI VI GITLRPPI FAL.PIEELKSTI KNTI ILLNTP NPTGKMFTP
EELNTIASLCIENDVLVFSDEVYDKLAFDMEHISIASLPGMFERTVTMNSLGKTFSLT
G KIG AIAPPHLT GVRQAHAFLTFATSNPMQWAAA ALRAPD Y TELKRDY
AKR.SILVEGL.KAVGFKVFPSSGTYFVVVDHTPFGHENDIAFCEYLVKEVGVVAIPTSV
FYLNPEEGKNLVRFTFGKDEGTLR VDRMKEKLRK
SEQ ID NO: 36 Bamboo GPT amino acid sequence (mature protein, no targeting
sequence)
VAKRLEKFKTTIFTQMS1I LAIKHGAINLG+ GFPNFDGPDFVKEAAIQAINAGKNQYAR
GYGVPELNSAV,+ EI FL DSGLQVDPEKEVVTVTSGGTEAIAATILGLINPGDEVILFAP
FYDSYEr TLSMAG:ANVI ITL.RPI DFAVPLEELKATVSI NTRAIMINTPHNPTGI MFS
REELEFIATL KKNDVLLF.AD VYDKLAF ADHISMASIPGMYERTVTMNSLGKTFSL
TGWKIGi AIAPPHLT GVRQAHSFLTFATCTPMQSAAAAALRAPDSYYGELKRDY
GAKKAILVDGLKAAGFIVYPS GTYFVMVDHTPFGF N IEFGE 'LIREVGVVAIPPS
VFYLNPEDGKNLVRFTFGKI DDTLRAA' ERM TKLI KK
107