Note: Descriptions are shown in the official language in which they were submitted.
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
METERED DROP PUSH BUTTON DISPENSER SYSTEM
FIELD OF THE INVENTION
The present invention is directed to a dispensing and delivery system
including a continuously sealing one way valve assembly for dispensing a
sterile flowable
substance, which can include preservatives or be preservative free, while
preventing a
backflow of contaminants into the source of the flowable substance. The
dispensing and
delivery system includes, for example, a valve assembly enclosed by a pressure
displaceable
flexible member or elastomeric member for effecting the passage of the
flowable substance to
a controllable outlet, while preventing any backflow to the source of the
flowable substance
after dispensing individual portions or doses of the flowable substance.
BACKGROUND INFORMATION
In the past, to maintain the flowable substance free of contaminants,
preservatives have been mixed in with the flowable substance in the reservoir
from which it
is to be dispensed. The use of preservatives tends to be detrimental to users
and often limits
the effectiveness of the flowable substance, particularly when the flowable
substance is a
pharmaceutical such as an eye care solution, an intranasal drug, cosmetic
treatment or skin
treatment product. This group of prescription and nonprescription medications
are often
formulated with preservatives in multi-dose formats. The flowable substance
may also be a
food stuff, a beverage, a nutraceutical or cosmeceutical product.
Another consideration is the ability of the valve assembly to deliver a
selected
amount of the flowable substance to the outlet without causing any damage to
the user, such
as when applying an eye care solution directly into the eye.
In the past, flexible membranes have been used to control the flow of the
flowable substance to the valve assembly outlet while preventing any backflow
to the source
of the flowable substance. However such valves, such as the valve described in
U.S. Patent
1
SUBSTITUTE SHEET (RULE 26)
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
No. RE34,243, which is incorporated by reference herein in its entirety,
describe the use of
O-rings in conjunction with a uniformly thick flexible membrane to effect a
seal. Other valve
assemblies also used cylindrical parts which required, for example, sliding
the pretensioned
flexible membrane over the straight sided core during assembly, preventing
automated high
speed assembly. Still other valve assemblies require squeezing a reservoir of
flowable
substance in order to dispense the flowable substance. Such squeezing is often
difficult for
the very young or very old and for physically challenged or disabled
individuals. Therefore,
an effectively designed, easy to operate valve assembly which was able to be
manufactured,
for example via high speed automated production, and limited the costs of
manufacture by
reducing component parts and allowing the use of high speed automated
production, was not
provided in the past.
SUMMARY OF THE INVENTION
According to an exemplary embodiment of the present invention, a dispensing
and delivery system conveys a flowable substance from a closed source, such as
a collapsible
reservoir, while preventing any backflow of oxygen or other contaminants from
the ambient
atmosphere through the valve assembly and into the source of the flowable
substance after a
portion of the substance has been dispensed.
The collapsible reservoir can be, for example, a bellows type reservoir, a
collapsible tube, an internal bag or other type of suitable reservoir designed
to dispense
practically all of its contents. According to an exemplary embodiment of the
present
invention, the dispensing delivery system has a normally closed controllable
outlet orifice for
dispensing a controlled amount of the flowable substance out of the valve
assembly. The
reservoir is in sealed contact with the valve assembly so that its contents do
not receive any
contaminants when the flowable substance is dispensed.
2
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Dispensation of the flowable substance is effected by applying pressure to a
reservoir directly or through a pump so that its contents flow to and through
the valve
assembly. The contents may be, for example, a pharmaceutical, such as an eye
care solution
or other substance which is to be kept free of contaminants during dispensing.
According to
an exemplary embodiment of the present invention, a multiple number of
dispensed amounts
can be provided while keeping the undispensed flowable substance preservative-
free. Other
flowable substances which are preservative-free can be food stuffs, juices or
beverages,
cosmetics, or other flowable substances intended to be maintained free of
preservatives and
contaminants, notwithstanding multiple uses of the dispenser delivery system.
The flowable
substance reservoir is protected by a housing so that pressure is not
accidentally applied.
The valve assembly includes, for example, an axially extending structure open
to the dispenser or reservoir of the flowable substance. The valve assembly
can be formed of
an axially extending inner core open to the reservoir and formed of a rigid
plastic component.
The interior of the core can have a passageway for receiving the flowable
substance from the
reservoir. At least one port extending from the passageway can be provided and
affords an
opening for conveying the flow substance out of the inner core. The inner core
can be
designed with a substantially tapered or substantially conical shape.
An axially extending flexible membrane tightly encloses the inner core and
covers the outlet end of the port through the inner core. The flexible
membrane moves
outwardly from the inner core when the flowable substance is pressurized and
passes through
the port and flows toward the outlet end of the flexible membrane. The
flexible membrane is
structured such that it is, for example, thicker at the end closest to the
valve opening, e.g. the
flexible membrane is not uniformly thick along its length. This thickness
allows the valve to
seal at the thicker end first. Alternatively, even if the membrane was of
uniform thickness,
the elasticity of the membrane can be varied so that the portion of the
membrane closest to
3
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
the valve opening is less elastic, resulting in the portion of the membrane
closest to the valve
opening closing first.
In exemplary embodiments, the flexible membrane and, as described above,
the inner core, are of a substantially tapered or substantially conical shape,
allowing for the
rapid assembly and natural resting of the flexible membrane over the inner
core.
A valve cover located laterally outwardly from the flexible membrane ends at
the controllable outlet orifice. The pressurized flowable substance travels
between the
radially outwardly extended flexible membrane and the outer surface of the
inner core and
flows to the controllable outlet orifice. The outlet orifice provides for
controlled amounts of
the flowable substance to be dispensed. An over cap covers the exterior of the
valve cover to
protect the valve assembly during storage. A collar can join the valve
assembly to the
reservoir and afford a sealed arrangement preventing any flow of contaminants
into the
reservoir. The collar and the neck area of the reservoir are designed with
locking features
that permit the override of the collar during assembly but subsequently
prevent the
unscrewing and disassembly of the collar and the opening of and likely
contamination of the
system.
In exemplary embodiments, metered dispensing is achieved through the
placement of a check valve and chamber between the reservoir and valve
assembly or
through the use of a check valve and chamber alone. Such a configuration may
be push
button actuated, which also allows for significantly easier dispensing in
terms of the force
required to be exerted by the user to dispense flowable substance.
The various features of novelty which characterize the present invention are
pointed out with particularity in the claims annexed to and forming a part of
this disclosure.
For a better understanding of the present invention, its operation, advantages
and specific
objects attained by its use, reference should be had to the accompanying
drawings and
4
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
descriptive matter in which preferred embodiments of the invention are
illustrated and
described.
BRIEF DESCRIPTION OF THE DRAWINGS:
Fig. 1 is an axially extending view of a dispensing and delivery system
according to an exemplary embodiment of the present invention.
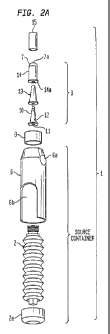
Fig. 2A is an exploded view of a dispensing and delivery system such as that
shown in Fig 1 according to an exemplary embodiment of the present invention.
Fig. 2B is an exploded view of a dispensing and delivery system such as that
shown in Fig 1 according to an exemplary embodiment of the present invention
which
includes a pump for dispensing flowable substance.
Fig. 3 is an exploded view of the soft cover and its controllable outlet
orifice
according to an exemplary embodiment of the present invention wherein the
controllable
outlet orifice is a cross slit.
Fig. 4A is an enlarged axially extending partial view of the continuously
sealing one way valve assembly with a flat topped soft cover according to an
exemplary
embodiment of the present invention.
Fig. 4B is an enlarged axially extending partial view of the continuously
sealing one way valve assembly with a rounded soft cover according to an
exemplary
embodiment of the present invention wherein the continuously sealing one way
valve
assembly is in the rest position.
Fig. 4C is an enlarged axially extending partial view of the continuously
sealing one way valve assembly with a rounded soft cover according to an
exemplary
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
embodiment of the present invention wherein the continuously sealing one way
valve
assembly is in the dispensing position.
Fig. 4D is an enlarged axially extending partial view of the continuously
sealing one way valve assembly where the opening in the soft cover contains a
portion of the
flexible membrane and inner core of the valve assembly according to an
exemplary
embodiment of the present invention.
Fig. 5 is an enlarged partial axially extending view of the continuously
sealing
one way valve assembly shown in Figs. 4B and 4C according to an exemplary
embodiment of
the present invention.
Fig. 6A is an axially extending partial view of the continuously sealing one
way valve assembly with one port and an outlet port according to an exemplary
embodiment
of the present invention.
Fig. 6B is an enlarged axially extending partial view of the continuously
sealing one way valve assembly with one port and an outlet port according to
an exemplary
embodiment of the present invention.
Fig. 7 is an axially extending view of a metered push button dispenser system
according to an exemplary embodiment of the present invention.
DETAILED DESCRIPTION OF THE INVENTION
As shown in Figs. 1, 2A and 2B, dispensing and delivery system 1 according
to exemplary embodiments of the present invention is comprised of a bellows
reservoir or
source 2 located within a housing 6. The housing 6 holds reservoir 2 of
flowable substance,
preferably a sterile or pure flowable substance, a valve assembly 3 (shown in
detail in Figs.
2A, 2B and 4A-D) for conveying the flowable substance from the reservoir 2 to
an outlet
when pressure is applied to the reservoir 2 or to an actuator 2a connected to
the reservoir 2.
6
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
An over cap 15 covers the valve assembly 3 to prevent contamination from
entering the valve
assembly 3 during storage. The housing 6 has surfaces 6a for holding the
assembly. A
collar 8 connects the valve assembly 3 to the reservoir 2 affording a sealed
connection so that
ambient contaminants cannot pass into the reservoir 2.
Referring again to Figs. 1, 2A and 2B, the bellows reservoir 2 is sufficiently
large to allow for multiple doses to be dispensed from the reservoir and
collapses when
pressure is applied to the reservoir. Other suitable reservoirs may be used,
such as a
collapsible tube or an internal bag in a reservoir that permit multi-dose
dispensation of the
flowable substance. The valve assembly 3 and collar 8 preferably prevents air
or other
contamination from entering the reservoir following the dispensing procedure.
Referring yet again to Figs. 1, 2A and 2B, the bellows reservoir or source 2
is
laterally enclosed, for example, by an axially extending housing 6 to prevent
the accidental
application of pressure to the reservoir. A slot 6b extending axially in the
housing 6 permits a
user to gain access to an actuator 2a of the reservoir as the flowable
substance is pressed out.
The housing 6 has surfaces 6a for holding the housing when the flowable
substance is being
dispensed.
Referring now to Figs. 2A and 2B, the valve assembly 3 has valve cover 14
which encircles the flexible membrane 13. The valve assembly 3 is comprised of
an inner
core 10, an axially extending blind passageway 11, ports 12, a flexible
membrane 13, a valve
cover 14 with a flange 14a, and a soft cover 7 with a controllable outlet
orifice 7a (all of
which are described in greater detail below in connection with the
descriptions of Figs. 4A-
D). While the flexible membrane 13 is hollow so as to accommodate the inner
core 10, it is
understood that when assembled with the device, it is filled with the inner
core 10 such that
no gap remains when the valve assembly is at rest.
7
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
The end of the valve cover 14 adjacent the reservoir 2 has a radially
outwardly
extending flange 14a bearing against the flange at the end of the flexible
membrane effecting
the seal for the valve assembly at the opening from the reservoir 2. The
opening or neck area
of reservoir 2 seals against flange 14a, for example, by way of a screw thread
which mates
with the collar 8. Alternatively, or in addition, the collar 8 and the opening
or neck area of
the reservoir 2 are designed with locking features that permit the override of
the collar 8
during assembly but subsequently prevent the unscrewing and disassembly of the
collar 8 and
the opening of the system. This prevents any unintended contamination by the
consumer and
also eliminates the possibility of refilling the system.
Referring now especially to Fig. 2B, in an embodiment suitable for pumping
flowable substance, a pump assembly 16 is joined to a valve assembly 3a and to
a reservoir 2
and bottle 6b. The collar 8 surrounds the connection between the pump assembly
16 and
valve assembly 3a. The pump assembly 16 is connected to the bottle 6 by screw
threads.
The opening or neck area of bottle 6 seals against pump assembly 16, for
example, by way of
a screw thread which mates with the pump assembly 16 sealing flange 2c of
reservoir 2
between the bottle 6 and the pump assembly 16. Alternatively, or in addition,
the collar 8 and
the opening or neck area of the reservoir 2 are designed with locking features
that permit the
override of the pump assembly 16 during assembly but subsequently prevent the
unscrewing
and disassembly of the pump assembly 16 and the opening of the system. This
prevents any
unintended contamination by the consumer and also eliminates the possibility
of refilling the
system.
The pump assembly 16 is thus connected to a valve assembly 3a having an
actuator 17, an inner core 10, an axially extending blind passageway 11, ports
12, a flexible
membrane 13, a valve cover 14 with a flange 14a, and a soft cover 7 with a
controllable outlet
orifice 7a (further described below in connection with the descriptions of
Figs. 4A-D).
8
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Optionally, the actuator 17 may be connected to or include an atomizer. In
operation the
actuator 17 serves to transfer force via a check valve of the pump assembly 16
to draw
flowable substance from the reservoir 2, thus providing the force necessary to
dispense
flowable substance. For example, conventional pumps may be utilized in this
manner.
Furthermore, the reservoir 2 can be disposed within a bottle 6 whose open end
is sealed by a plug 2c. Plug 2c serves to protect the reservoir 2 from damage,
rupture or
inadvertent application of force on the reservoir 2.
Referring now to Fig. 3, the controllable outlet orifice 7a is a cross-slit
enabling substantially dripless dispensing of the flowable substance. The
cross-slit causes the
controllable outlet orifice 7a to self close on itself after pressure is
released.
The controllable outlet orifice 7a can be formed as desired to provide a spray
or a stream of the flowable substance. Alternatively, by selectively
dimensioning the
controllable outlet orifice 7a, a drop-like amount of the flowable substance
can be dispensed,
for example if an eye care solution is being dispensed. If a greater amount of
the flowable
substance is to be dispensed, the controllable outlet orifice 7a can be formed
for dispensing a
larger quantity of the flowable substance, for example, a eye or nasal
solution and/or gel.
Referring now to Figs. 4A-D, the valve assembly 3 preferably has an inner
core 10, an axially extending blind passageway 11, ports 12, a flexible
membrane 13, a valve
cover 14 with a flange 14a, and a soft cover 7 with a controllable outlet
orifice 7a. An over
cap 15 is placed over the valve assembly 3 when it is not in use, protecting
it from contact
with ambient contaminants.
In the valve assembly 3, an axially extending inner core 10 bears against the
opening of the reservoir 2 so that flow from the reservoir enters into an
axially extending
blind passageway 11 in the inner core. The passageway 11 extends for a major
portion of the
axial length of the inner core. At approximately half the length of the
passageway 11, the
9
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
inner core has a pair of ports 12 extending transversely of the passageway
axis from the
surface of the passageway to the outer surface of the inner core 10. The inner
core 10 is
formed of, for example, a rigid plastic material and terminates inwardly of
the outlet end of
the valve assembly. Furthermore, in exemplary embodiments, upon assembly and
filling of
the assembly no air is present inside the passageway 11 and the ports 12. It
should be noted
that additional ports 12 may be located through the inner core 10.
Furthermore, in exemplary embodiments the inner core 10 and the flexible
membrane 13 are constructed such that they fit tightly together, for example
having very
close tolerances which allow for an air-tight seal to be formed between the
flexible membrane
13 and the inner core 10. In further exemplary embodiments the molding process
for the
flexible membrane 13 and the inner core 10, as well as other components
described above as
sealing against one another is an asymmetric molding process which creates a
surface
substantially free of defects or seam lines at the areas of contact where
sealing occurs.
Accordingly, in an exemplary embodiment, very close tolerances between the
parts, for
example the inner core 10 and flexible membrane 13 and the other parts, are
used to provide
an optimal seal and operation of the valve assembly.
A flexible membrane 13, such as an elastomeric member, is fitted tightly over
the outer surface of the inner core and extends from the opening in the
reservoir 2 to the
opposite end of the inner core 10. As can be noted in Figs. 4A-D, the
thickness of the
membrane is preferably variable along its axial length. In the region of the
outlet end of the
inner core has, for example, an axially extending continuous uninterrupted end
considerably
thicker than the remainder of the flexible membrane 13. That is, the band is
not separated in
the axial direction by axially extending cuts. The thicker end ensures that
after the valve has
dispensed fluid, as further described below, the valve closes at the end
closest to the opening
7a first, therefore preventing any backflow. This is effected by the heavy
wall thickness
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
which provides for greater tension. As a result, the flexible membrane 13
exhibits non-
uniform tension.
In a further example, in yet other embodiments, the thickness of the membrane
may be variable along its axial length and the region surrounding the outlet
end of the inner
core has, for example, an axially extending continuous uninterrupted annular
band
considerably thicker than the remainder of the flexible membrane 13.
Furthermore, in certain
embodiments, the band is not separated in the axial direction by axially
extending cuts.
Alternatively, the elasticity or durometer of the end of the flexible membrane
closest to the
valve opening may be varied, for example it may be reduced, such that the end
closest to the
valve opening seals first when pressure is relieved.
In a further embodiment, flexible membrane 13 and inner core 10 are
substantially tapered or substantially conical at the ends closest to the
controllable outlet
orifice 7a such that the inner core 10 nest into the flexible membrane 13 one
another when
being assembled by high speed automated production equipment.
At its end adjacent to the opening of the reservoir 2, the flexible membrane
13
has an outwardly extending flange bearing against a flange on the inner core
located at the
opening from the reservoir.
An axially extending valve cover 14 encircles the flexible membrane 13 and,
as shown in the rest position in Fig. 2a, is spaced radially outwardly from
the outer surface of
the flexible membrane. The end of the valve cover 14 adjacent the reservoir 2
has a radially
outwardly extending flange 14a bearing against the flange at the end of the
flexible
membrane effecting the seal for the valve assembly at the opening from the
reservoir 2.
The valve cover 14 is formed, for example, of an inner layer of an elastomeric
material extending axially from its flange 14a to and over the outlet end of
the valve
assembly 3. Elastomeric material forms a soft cover 7 over the outlet end of
the valve cover
11
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
14 which is particularly advantageous when the valve assembly is used for
dispensing an eye
care solution. Such a soft cover 7 prevents, for example, any likelihood of
harm to the
delicate outer surfaces of the eye or surrounding tissue. The soft cover 7 has
a controllable
outlet orifice 7a for dispensing the flowable substance. The outlet orifice is
closed in the rest
position of the continuously sealing one way valve assembly and open in the
dispensing
position.
Referring yet again to Figs. 4A-D and to Fig. 5, various embodiments of the
valve assembly 3 are depicted having variations in the structure of the soft
cover 7 as
described below.
Referring now especially to Fig. 4A, a valve assembly having a flat topped
soft cover 7 is provided. The soft cover 7 has a flattened top, which allows
for less flowable
substance to adhere to the controllable outlet orifice 7a because the
flattened top results in a
shorter controllable outlet orifice 7a. The soft cover 7 has a controllable
outlet orifice 7a
which can be formed as desired to provide a spray or a stream of the flowable
substance.
Furthermore, the controllable outlet orifice 7a can be a cross-slit as shown
in Fig. 3.
Alternatively, by selectively dimensioning the controllable outlet orifice 7a,
a drop-like
amount of the flowable substance can be dispensed, for example if an eye care
solution or
other solution typically delivered in droplet form, is being dispensed. If a
greater amount of
the flowable substance is to be dispensed, the controllable outlet orifice 7a
can be formed for
dispensing a larger quantity of the flowable substance, for example by having
a larger
diameter opening.
Referring now especially to Figs. 4B-C, a valve assembly having a rounded
soft cover 7 is provided. The soft cover 7 has a rounded top useful for
dispensing flowable
substance into the outer surfaces of the eye and surrounding tissue or other
sensitive body
areas. Because the rounded tip lacks sharp edges, damage to the eye or other
sensitive tissues
12
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
is avoided or reduced if incidental contact occurs during administration of
the flowable
substance. The soft cover 7 has a controllable outlet orifice 7a which can be
formed as
desired to provide a spray or a stream of the flowable substance. Furthermore,
the
controllable outlet orifice 7a can be a cross-slit as shown in Fig. 3.
Alternatively, by
selectively dimensioning the controllable outlet orifice 7a, a drop-like
amount of the flowable
substance can be dispensed, for example if an eye care solution or other
solution typically
delivered in droplet form, is being dispensed. If a greater amount of the
flowable substance is
to be dispensed, the controllable outlet orifice 7a can be formed for
dispensing a larger
quantity of the flowable substance, for example by having a larger diameter
opening.
Referring now especially to Fig. 4D, a valve assembly having a flat cover 7
which has an enlarged version of controllable outlet orifice 7a is provided.
The enlarged
version of controllable outlet orifice 7a is able to accommodate the inner
core 10 and flexible
membrane 13 and is suitable for dispensing viscous flowable substances such as
lotions,
creams and emollients, but may also be used for any flowable substance. The
enlarged
version of controllable outlet orifice 7a allows flowable substance to be
dispensed without
having to move through two openings - namely the opening at the end of the
flexible
elastomer 13 and the controllable outlet orifice 7a, since these are now
flush.
Referring now to Fig. 5 the gap formed between inner core 10 and the flexible
membrane 13 by the pressurized fluid flowing out of ports 12 can more easily
be seen. The
controllable outlet orifice 7a in soft cover 7 can also be seen and may for
example be a
substantially uniform circular bore thought the material of soft cover 7 or
may be suitably
dimensioned as described in the preceding paragraphs.
Referring now to Figs. 6A-B, in another embodiment, flowable substance
flows through a single port 12 in inner core 10 and expands the flexible
membrane 13,
swirling around the exterior of inner core 10, and exiting via an outlet port
12a as shown in
13
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Figs. 6A and 6B. This results in the need for less cracking pressure to
dispense flowable
substance and is particularly advantageous for use with, though not limited
to, flowable
substances having higher viscosities such as lotions, creams and emollients.
It should be
noted that additional ports 12 may be located through the inner core 10.
In exemplary operation, when the flowable substance is to be dispensed, the
over cap 15 is removed and pressure is applied to the actuator 2a of the
reservoir 2 so that an
amount of the flowable substance passes out of the reservoir into the
passageway 11 in the
inner core 10. The substance flows through the ports 12 and expands the
flexible membrane
13 radially outwardly and flows toward the outlet end of the flexible membrane
where it exits
from the flexible membrane radially inwardly into the controllable outlet
orifice 7a in the
cover and is dispensed.
When the flowable substance is being dispensed and exits the outlet end of the
flexible membrane, it flows radially inward to the controllable outlet orifice
7a which then
opens allowing the substance to flow out of the valve assembly. When the
flowable
substance is dispensed and pressure on the source is withdrawn the
controllable outlet orifice
7a closes blocking any backflow into the valve assembly. An over cap 15 is
placed over the
valve assembly 3 when it is not in use, protecting it from contact with
ambient contaminants.
In another embodiment, as depicted in Figs. 6A and 6B for example, flowable
substance flows through a single port 12 in inner core 10 and expands the
flexible membrane
13, swirling around the exterior of inner core 10, and exiting via an outlet
port 12a as shown
in Figs. 6A and 6B. This results in the need for less cracking pressure to
dispense flowable
substance and is particularly advantageous for use with, though not limited
to, flowable
substances having higher viscosities such as creams and emollients.
By releasing the pressure on the actuator 2a of the reservoir, the dispensing
operation is terminated and the flexible membrane 13 returns inwardly into
contact with the
14
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
outer surface of the inner core 10. The inward movement of the flexible
membrane starts at
its outlet end because of its increased thickness and affords gradual contact
with the outer
surface of the inner core, returning any flowable substance through the ports
back into the
reservoir whereby contaminants cannot enter the reservoir. Dispensing
individual portions of
the flowable substance can be continued until the reservoir is almost
completely emptied. As
a result of the structure and operation of the valve assembly, the valve
assembly according to
an exemplary embodiment of the present invention provides uniform pressure on
the valve
components via the pressurization of the flowable substance.
In still another exemplary embodiment, for example a spray pump such as that
depicted in Fig 2B, an actuator 17 serves to transfer force to the pump
assembly 16 when it is
depressed. This in turn compress the reservoir 2, thus providing the force
necessary to open
the valve assembly and in certain embodiments described above, controllable
outlet port 7a,
to dispense flowable substance.
Referring now to Fig. 7, in accordance with yet other embodiments of the
present invention, a metered drop push button dispenser system which prevents
contamination of the reservoir 2 and which allows for a metered volume of
flowable
substance to be dispensed is provided. Such a device can be achieved by the
use of a button
17 optionally having rounded front tabs, check valve 18, chamber 19 optionally
having
angled cam flanges on each side, spring 20, piston 21, which may be hollow and
optionally
have cam flanges, and tip 22 all of which may be contained in at least one
housing 23 located
in between the reservoir 2 and the outlet of the device (for example, the
previously described
outlet orifice 7a). The check valve 18 prevents flowable substance from the
valve assembly 3
and other components downstream of the reservoir 2 from reversing back into
the reservoir 2.
The reservoir 2 may be any of the above mentioned reservoirs or may also be a
tube, bellows,
pouch or other similar reservoir, for example a pouch.
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Referring again to Fig. 7, flowable substance enters the chamber 19 through a
rear port and through the check valve 18. As the button 17 is depressed it
pivots down and its
rounded front tabs contact the angled cam flange on each side of the chamber
19 forcing it
forward against the spring. This forces a measured amount of flowable
substance through the
hollow piston 21 and out of the tip 22 through the valve assembly 3 and out of
the device
outlet (for example, the outlet orifice 7a).
In addition to metered dispensing, the button 17 eliminates the need for user
applied pressure on the reservoir itself in order to dispense flowable
substance. Elimination
of mechanical pressure on the reservoir itself is especially useful in
dispensing the contents of
partially empty reservoirs which would otherwise require increasing mechanical
pressure on
the reservoir itself.
Adjustment of the piston 21 and chamber 19 can provide for the dispensing of
various volumes of flowable substance. Adjustment of the spring force and the
angle of the
cam flanges of the piston 21 provide variation of the force required to
depress the button 17.
Furthermore, adjustment of the spring force and the angle of the cam flanges
of the piston
provide variation of the force required to depress the button 17.
The check valve 18 prevents the flowable substance from reversing back into
the reservior 2. As previously described, the reservoir 2 can be, for example,
a collapsible
container such as a tube or bellows. A dynamic seal is maintained internally
between the
piston 21 and chamber 19 which prevents leakage and entry of solid, gas or
liquid
contaminants, including, for example, bacteria.
Referring yet again to Fig. 7, as described above, such metered drop push
button dispenser can be combined with the valve assembly 3 in one embodiment,
or
alternatively be provided without the valve assembly 3.
16
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Elastomers suitable to form the soft cover 7, the flexible membrane 13 and the
valve cover 14 in exemplary embodiments of the present invention include
thermoplastic
elastomers such as Dynaflex manufactured by GLS Corp., C-Flex manufactured by
CPT Inc.,
or Santoprene manufactured by Advanced Elastomer Systems, Inc. The elastomers,
and the
materials comprising any of the other components of the device may have
integrated,
impregnated, otherwise placed within them anti-microbial ingredients such as
silver ions
contained within a ceramic carrier, such as those supplied by AgION, or
sustained-release
ionic silver compounds, such as those supplied by Westlake Plastic
Technologies which are
known to be used in the making of anti-microbial plastics. Furthermore, other
anti-microbial
suitable for compounding with or coating plastics may be used. Furthermore,
the soft cover 7
or the flexible membrane 13 or both could, for example, be positively charged
to repel
residual flowable substance, coated in for example, Teflon type-plastics, have
increased
surface tension or be anti-wetting, or any combination of the above so as to
repel flowable
substance.
In further embodiments, one or more of the button 17, check valve 18,
chamber 19, spring 20, piston 21, tip 22 and housing 23 may be formed from
hydrophobic or
antimicrobial material or be coated with a hydrophobic or anti-microbial
coating. For
example, components of the device may have integrated, impregnated, otherwise
placed
within them anti-microbial ingredients such as silver ions contained within a
ceramic carrier,
such as those supplied by AgION, or sustained-release ionic silver compounds,
such as those
supplied by Westlake Plastic Technologies which are known to be used in the
making of anti-
microbial plastics. Furthermore, other anti-microbial suitable for compounding
with or
coating plastics may be used. Still furhter, components of the device may for
example, be
positively charged to repel residual flowable substance, coated in for
example, Teflon type-
17
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
plastics, have increased surface tension or be anti-wetting, or any
combination of the above
so as to repel flowable substance.
In yet other embodiments, including those described above, the durometer of
the elastomers can be varied in relation to the viscosity of the flowable
substance. For
example, assemblies containing substances with comparatively higher
viscosities would
utilize softer, i.e. lower durometer elastomers, in order to reduce the
cracking force needed to
dispense flowable substance, whereas lower viscosity flowable substances would
utilized
harder, i.e. higher durometer elastomers to maintain a strong seal. Likewise,
flowable
substances containing lubricants would also utilize harder, i.e. higher
durometer elastomers to
maintain a strong seal.
As described above, the parts of the dispending and delivery device, including
the valve assembly may be manufactured to close tolerances such that they form
airtight seals
and are close fitting ensuring optimal seals and operation of the device.
A variety of pharmaceuticals, cosmetics, food stuffs and other flowable
materials can be dispensed where it is important to maintain them free of
contaminants from
the ambient atmosphere. The flowable characteristics of the material being
dispensed
determines or at least may affect the type and dimension of the valve
assembly.
According to exemplary embodiments of the present invention, the material
forming the controllable outlet orifice 7a does not absorb the flowable
substance. As a result,
any substance entering the outlet orifice 7 is ejected from the dispenser and
does not return
into the space between the inner core and the flexible membrane, thereby
maintaining the
purity or sterility of the product remaining in the reservoir.
It should be understood that the various embodiments of the valve assembly
described above can each be used in the various embodiments of the
continuously sealing one
way valve assembly device.
18
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
As mentioned, the flowable substance may be a pharmaceutical cosmeceutical,
or nutraceautical, an eye care solution, other ophthalmological product,
otorhinolarygology
product, dermatological product, gynecological product, or product for
treating or preventing
anorectal, dermatological or pulmonary disorders or any formulation
administered to the
body through the mucus membranes; a food stuff, such as dairy products,
beverages or juices;
a cosmetic, such as a skin care solution or toiletries; and liquid vitamins,
all of which are
intended to be maintained free of contaminants from the ambient atmosphere and
of
preservatives during storage within the reservoir 2.
According to exemplary embodiments of the present invention, many existing
commercial products that contain preservatives can be reformulated into
preservative free
versions and provided for multiple dose dispensing with the valve assembly and
delivery
system of the present invention. For example, conventional creams, emollients,
eye drops,
nasal sprays, cosmetic creams that currently require preservatives, notably
parabens and
benzalkonium chloride that have proved to be deleterious to tissue, may be
reformulated in a
preservative free form and are amenable to storage and dispensing from
reservoirs using the
continuously sealing one way valve assembly and delivery system of the present
invention.
This can be accomplished by, for example, formulating the product according to
its original
formulation, but without the preservative, or by readjusting the formulation
of the product,
for example by changing the excipients or the amount of the excipients or
both. Thus, these
preservative free products are amenable to storage and dispensing from
reservoirs using the
continuously sealing one way valve assembly and delivery system of the present
invention
because they are preservative free formulations.
The following examples provide embodiments describing categories of
medical products which are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
19
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Preservative-free storage and delivery of formulations also can be
accomplished by
providing, for example, multi-dose metered, high barrier and for preservative-
free systems as
described in U.S. Patent No. RE 34,243, incorporated by reference above and
U.S. Patent
Nos. 5,092,855; 5,305,783; 5,279,447; 5,305,786; and 5,353,961 all of which
are hereby
incorporated by reference in their entirety.
Examples
Example 1
In an exemplary embodiment, preservative free ophthalmological products are
amenable to storage and dispensing from reservoirs using the continuously
sealing one way
valve assembly and delivery system of the present invention. For example, eye
drops, and
preferably those eye drops involved in chronic care, for example, dry eye,
glaucoma, allergies
and NSAIDs, and also those eye drops intended for acute care, for example
during ocular
surgery, are amenable to storage and dispensing from reservoirs using the
continuously
sealing one way valve assembly and delivery system of the present invention.
As a further
example, those eye drops used to relieve eye fatigue, those eye drops used to
relieve dry eye,
those eye drops used relieve dry eye due to computer use, television use, or
fatigue due to
prolonged awake periods are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
Examples of dry eye products can include dry eye products comprising
methycellulose, hyaluronic acid, polyethelene glycol 400 0.4%, propylene
glycol 0.3%,
glycerin, and mineral oils. Examples of glaucoma products include glaucoma
products
comprising timolol 0.25%/0.50%, brimonidine tartrate 0.1%, bimatoprost 0.03%
and
travaprost 0.004%. Examples of allergy products include allergy products
comprising
olopatadine HCL 0.1 % and predisalone acetate 1 %. Examples of NSAID products
include
NSAID products comprising ketorolac 0.5% and diclofenac 0.1%.
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
Example 2
In an exemplary embodiment, preservative-free otorhinolarygological
products are amenable to storage and dispensing from reservoirs using the
continuously
sealing one way valve assembly and delivery system of the present invention.
For example,
nasalia medicines, and preferably nasal sprays, external ear creams, ear
drops, steroid ear
drops, antibiotic ear drops, nose drops, and nose drops comprising
phenylephrine 0.25% and
pseudoephedrine 30mg, are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
Example 3
In an exemplary embodiment, preservative free dermatological products are
amenable to storage and dispensing from reservoirs using the continuously
sealing one way
valve assembly and delivery system of the present invention. For example,
preservative free
skin preparations; scalp preparations; corticosteroid creams, lotions and
ointments; topical
antibiotics and topical anti-fungal agents are amenable to storage and
dispensing from
reservoirs using the continuously sealing one way valve assembly and delivery
system of the
present invention. Thus, these preservative free dermatological products are
amenable to
storage and dispensing from reservoirs using the continuously sealing one way
valve
assembly and delivery system of the present invention because they are
preservative free
formulations.
Example 4
In an exemplary embodiment, preservative free products for the treatment or
prevention of dermatologic disorders are amenable to storage and dispensing
from reservoirs
using the continuously sealing one way valve assembly and delivery system of
the present
invention. For example, preservative free skin preparations; scalp
preparations;
corticosteroid creams, lotions and ointments; topical antibiotics; topical
anti-fungal agents;
21
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
therapeutic skin creams including anti-bacterial, anti-fungal/parasitic,
allergic and non-
specific dermatitis creams and emollients and all cosmetic dermatologic
compounds used for
dermatologic disorders are amendable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
Example 5
In an exemplary embodiment, products for the treatment or prevention of
anorectal disorders are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
For example, preservative free creams, topical anaesthetics, lubricating
jellies and jelly or
other preparations for hemorrhoid treatment, prevention or management, are
amenable to
storage and dispensing from reservoirs using the continuously sealing one way
valve
assembly and delivery system of the present invention.
Example 6
In an exemplary embodiment, preservative free products for the treatment or
prevention of pulmonary disorders are amenable to storage and dispensing from
reservoirs
using the continuously sealing one way valve assembly and delivery system of
the present
invention. For example preservative free formulations of products for chronic
obstruction
disorder, for example, aerosol nebulizers using B-adrenergic, anticholinergic,
corticosteroid
and theophyline derivatives requiring multi-dose application are amenable to
storage and
dispensing from reservoirs using the continuously sealing one way valve
assembly and
delivery system of the present invention.
Example 7
In an exemplary embodiment, preservative free gynecological products are
amenable to storage and dispensing from reservoirs using the continuously
sealing one way
valve assembly and delivery system of the present invention. For example,
vulvovaginal
22
CA 02736180 2011-03-04
WO 2009/033053 PCT/US2008/075443
treatment medicines, such as medicines for contact irritant or allergic
vulvitis, chemical
irritation, bacterial vaginosis, Candidal vaginitis therapy including all
azoles and nystatins,
butoconazole, butoconalzole 2%, clotrimazole, clotrimazole 1%, metronidazole
and
trichomonas treatments are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly and delivery system of the present
invention.
Example 8
In an exemplary embodiment, preservative free lens care products are
amenable to storage and dispensing from reservoirs using the continuously
sealing one way
valve assembly described herein. For example, contact lens rinsing, cleaning
disinfecting and
storage solutions, or a multi-purpose solution encompassing contact lens
rinsing, cleaning
disinfecting and storage are amenable to storage and dispensing from
reservoirs using the
continuously sealing one way valve assembly and delivery system of the present
invention.
Example 9
In an exemplary embodiment, preservative free eye wash products (e.g.
irrigation solutions) are amenable to storage and dispensing from reservoirs
using the
continuously sealing one way valve assembly described herein. For example, eye
wash
products used to clear the eye of environmental contamination are amenable to
storage and
dispensing from reservoirs using the continuously sealing one way valve
assembly described
herein. As a further example, eye wash products used to clear the eye of
environmental
contamination such as pollen or dirt are amenable to storage and dispensing
from reservoirs
using the continuously sealing one way valve assembly and delivery system of
the present
invention.
Although the system is designed for use with various preservative free
formulations it may also be used with formulations which are not preservative
free.
23