Note: Descriptions are shown in the official language in which they were submitted.
CA 02736240 2011-04-04
IN SITU FORMING BIPHASIC OSTEOCHONDRAL PLUG
TECHNICAL FIELD
[0001] The present disclosure relates to methods and devices for the treatment
of tissue
defects. More particularly, the present disclosure relates to biphasic
osteochondral plugs and
methods of forming and using the same in the treatment of osteochondral
defects.
BACKGROUND
[0002] Osteochondral defects are combination lesions of the bone and
cartilage.
Particularly, osteochondral defects affect the joints, such as the knee,
ankle, shoulder, and
elbow, and include lesions to both the articular cartilage and underlying
subchondral bone.
Treatment options include filling the defect with a bone or cartilage filler,
as well as allograft or
autograph transplantation of cells/tissue.
[0003] Current void filling devices include hydrogels, sponges, scaffolds, and
cements.
The limitation with these devices, however, is that they are single phase.
Single phase void
fillers cannot cater to the needs of a void having more than one tissue type.
Solid layered plugs
have also been utilized to fill voids; however, these plugs may leave gaps
between the tissue
and the implanted device.
[0004] It would be advantageous to provide a biphasic, or multiphasic, plug
tailored to
specific tissue types by customizing the physical properties of each phase to
accommodate the
biomechanical properties of each tissue type, as well as optimizing the
biochemical compatibility
of each phase to its respective tissue type to favor growth and repair of the
distinct tissues.
1
CA 02736240 2011-04-04
SUMMARY
[0005] The present osteochondral plugs include a first scaffold and a second
scaffold.
The first scaffold is a solid scaffold containing pendant reactive functional
groups. The second
scaffold includes a hydrogel capable of reacting with the pendant reactive
groups of the first
scaffold. The first scaffold may be porous. In embodiments, the first scaffold
may be a sponge,
or foam.
[0006] The second scaffold includes at least one hydrogel precursor. In
embodiments,
the at least one precursor is an electrophile or a nucleophile. In other
embodiments, the second
scaffold includes at least two precursors. The at least two precursors may
include an
electrophile and a nucleophile. In embodiments, the nucleophile is a natural
component. In
embodiments, the at least two precursors include a PEG star and collagen. In
other
embodiments, the at least two precursors include a PEG star and NHS. The term
PEG star is
meant to include a multibranched molecule which contains polyethylene glycol
segments.
[0007] The first and second scaffolds may be designed to interact with one
another to
form covalent bonds. In addition, the first and the second scaffolds may be
designed to interact
with the tissue to also form covalent bonds between the plug and the tissue.
[0008] Methods of filling an osteochondral defect with the osteochondral plugs
of the
present disclosure are also provided. In accordance with an embodiment of the
present
methods, an osseous scaffold may be placed within a tissue defect and a
hydrogel may be
injected into the tissue defect over the osseous scaffold. The osseous
scaffold may be loaded
into a delivery device prior to placement of the osseous scaffold in the
tissue defect. The
delivery device includes an outer shaft having an inner channel for housing
the osseous
scaffold. A plunger, optionally including a central bore, is adapted for
slidable engagement with
the inner channel for driving the osseous scaffold into the tissue defect. The
osseous scaffold
may then be ejected from the delivery device. The hydrogel may be injected
into the tissue
2
CA 02736240 2011-04-04
defect by introducing the hydrogel into the defect through the central bore of
the plunger of the
delivery device.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The accompanying drawings, which are incorporated in and constitute a
part of
this specification, illustrate embodiments of the disclosure and, together
with a general
description of the disclosure given above, and the detailed description of the
embodiment(s)
given below, serve to explain the principles of the disclosure, wherein:
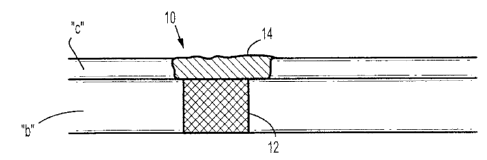
[0010] FIGURE 1 schematically shows an osteochondral plug placed within an
osteochondral defect in accordance with one embodiment of the present
disclosure;
[0011] FIGURE 2 schematically shows an osteochondral plug placed within an
osteochondral defect in accordance with another embodiment of the present
disclosure;
[0012] FIGURE 3 schematically shows an osteochondral plug placed within an
osteochondral defect in accordance with yet another embodiment of the present
disclosure;
[0013] FIGURE 4 schematically shows an osteochondral plug placed within an
osteochondral defect in accordance with yet another embodiment of the present
disclosure; and
[0014] FIGURES 5A-5D schematically illustrate a method of inserting an
osteochondral
plug into an osteochondral defect in accordance with one embodiment of the
present disclosure.
3
CA 02736240 2011-04-04
DETAILED DESCRIPTION
[0015] Osteochondral plugs in accordance with the present disclosure are at
least
biphasic and include a first, osseous phase and a second, chondral phase. Upon
implantation,
the first phase promotes bone repair by favoring the growth of osteogenic cell
types and
providing similar mechanical properties to that of bone. The second phase
promotes cartilage
repair by favoring the growth of chondrogenic cell types and providing similar
mechanical
properties to that of cartilage.
[0016] The first osseous phase may include a first scaffold, at least a
portion of which
includes one or more pendant reactive groups. By pendant, the one or more
reactive groups
may be positioned at or near a surface of the scaffold in a manner conducive
for interaction with
the tissue and/or the second chondral phase of the plug.
[0017] The second chondral phase may include a second scaffold, at least a
portion of
which includes one or more pendant complimentary reactive groups. The one or
more pendant
complimentary reactive groups of the second scaffold are capable of covalently
bonding with the
one or more pendant reactive groups of the first scaffold and/or the tissue to
form a multiphase
osteochondral plug.
[0018] The multiphase osteochondral plugs may be used in a variety of surgical
and
wound applications involving defects of two or more tissue types. As used
herein, a "tissue
defect" may include any breakdown of tissue from a normal, healthy state. This
breakdown may
be due to internal factors such as degenerative disease, or external factors
such as injury. Any
variation from the normal structure of a tissue may be a "tissue defect."
Thus, the
osteochondral plugs of the present disclosure may be used to fill voids as a
tissue filler, bone
filler, or filler for soft/hard tissue interfaces; to promote tissue growth as
a tissue scaffold; and/or
to deliver bioactive agents and/or cells to a tissue defect or lesion.
The Osseous Phase
4
CA 02736240 2011-04-04
[0019] The osseous phase of the osteochondral plug of the present disclosure
includes
a first scaffold or structure upon, or within, which the desired osteogenic
cells may grow in order
to regenerate the desired tissue. At least a portion of the first scaffold may
include one or more
pendant reactive functional groups suitable for interacting with the tissue
and/or the chondral
phase of the plugs described herein. The osseous phase may be in the form of a
rod, cylinder,
sponge, foam, gel, or any other desired configuration that provides both a
structure having the
necessary strength to support the defect, as well as a structure upon or
within which the
desirable cells may grow. The osseous phase may be provided as a composition
in liquid form
which hardens to form a solid scaffold. The composition may harden in vivo or
in vitro, prior to
or after, implantation in tissue.
[0020] In embodiments, the osseous phase may include a porous scaffold. The
term
"porous" as used herein means that the scaffold or structure may possess
defined openings
and/or spaces which are present as a surface characteristic or a bulk material
property, partially
or completely penetrating the scaffold. Pores may be created using any method
within the
purview of those skilled in the art including, but not limited to, processes
such as sintering,
lyophilization, leaching of salt, and sugar or starch crystals, or the
addition of gas-forming
agents (i.e., sodium bicarbonate), or the addition of gas-filled microbubbles_
Porous scaffolds
may have an open-cell structure, where the pores may be connected to each
other, forming an
interconnected network. Conversely, the porous scaffolds may include pores
which are not
interconnected.
[0021] In some embodiments, the pores may be formed after implantation in
situ. The in
situ pore formation may be performed using any suitable method. Some non-
limiting examples
include the use of contact lithography, living radical photopolymer (LRPP)
systems, and salt
leaching. Those skilled in the art reading the present disclosure will
envision other pore
distribution patterns and configurations for the osseous phase.
CA 02736240 2011-04-04
[0022] In some embodiments, the first scaffold may be a foam or a sponge
containing
openings or pores over at least a portion of a surface thereof, upon and
within which desired
cells may grow. The foam or sponge may be formed using any suitable method
including, but
not limited to the lyophilization or freeze-drying of a composition. The foam
or sponge may be
cross-linked or non-cross-linked, and may include covalent or ionic bonds.
[0023] The first scaffold of the osseous phase may be fabricated from any
biodegradable or non-biodegradable polymer that can be used in surgical
procedures. The term
"biodegradable" as used herein is defined to include both bioabsorbable and
bioresorbable
materials. By biodegradable, it is meant that the material decomposes, or
loses structural
integrity under body conditions (e.g., enzymatic degradation or hydrolysis) or
is broken down
(physically or chemically) under physiologic conditions in the body such that
the degradation
products are excretable or absorbable by the body. It should be understood
that such materials
include natural, synthetic, bioabsorbable, and/or non-absorbable materials, as
well as
combinations thereof, for forming the osseous phase of the present disclosure.
[0024] Representative natural biodegradable polymers include: polysaccharides,
such
as alginate, dextran, chitin, chitosan, hyaluronic acid (HA), cellulose,
fucans,
glycosaminoglycans, and chemical derivatives thereof (substitutions and/or
additions of
chemical groups, for example, alkyl, alkylene, hydroxylations, oxidations, and
other
modifications routinely made by those skilled in the art); and poly(amino
acids) including
proteins, such as albumin, casein, zein, silk, collagen (I, II, and III),
elastin, fibrin, fibrinogen,
gelatin, and copolymers and blends thereof, alone or in combination with
synthetic
biodegradable polymers.
[0025] Synthetically modified natural polymers include cellulose derivatives,
such as
alkyl celluloses, hydroxyalkyl celluloses, cellulose ethers, cellulose esters,
nitrocelluloses, and
chitosan. Examples of suitable cellulose derivatives include methyl cellulose,
ethyl cellulose,
hydroxypropyl cellulose, hydroxypropyl methyl cellulose, hydroxybutyl methyl
cellulose,
6
CA 02736240 2011-04-04
cellulose acetate, cellulose propionate, cellulose acetate butyrate, cellulose
acetate phthalate,
carboxymethyl cellulose, cellulose triacetate, and cellulose sulfate sodium
salt. These may be
collectively referred to herein, in embodiments, as "celluloses."
[0026] Representative synthetic biodegradable polymers include polyhydroxy
acids
prepared from lactone monomers, such as glycolide, lactide, caprolactone, E-
caprolactone,
valerolactone, and 6-valerolactone, as well as carbonates (e.g., trimethylene
carbonate,
tetramethylene carbonate, and the like), dioxanones (e.g., 1,4-dioxanone and p-
dioxanone),
1,dioxepanones (e.g., 1,4-dioxepan-2-one and 1,5-dioxepan-2-one), and
combinations thereof.
Polymers formed therefrom include: polylactides; poly(lactic acid);
polyglycolides; poly(glycolic
acid); poly(trimethylene carbonate); poly(dioxanone); poly(hydroxybutyric
acid);
poly(hydroxyvaleric acid); poly(lactide-co-(E-caprolactone-)); poly(glycolide-
co-(E-caprolactone));
poly(lactic-co-glycolic acid); polycarbonates; poly(pseudo amino acids);
poly(amino acids);
poly(hydroxyalkanoate)s; polyalkylene oxalates; polyoxaesters; polyanhydrides;
polyortho
esters; and copolymers, block copolymers, homopolymers, blends, and
combinations thereof.
[0027] Other non-limiting examples of biodegradable materials from which the
osseous
phase may be made include: poly (phosphazine); aliphatic polyesters;
polyethylene glycols;
glycerols; copoly (ether-esters); polyalkylene oxalates; polyamides; poly
(iminocarbonates);
polyalkylene oxalates; polyoxaesters; polyphosphazenes; and copolymers, block
copolymers,
homopolymers, blends, and combinations thereof.
[0028] Rapidly bioerodible polymers, such as poly(lactide-co-glycolide)s,
polyanhydrides, and polyorthoesters, which have carboxylic groups exposed on
the external
surface as the surface of the polymer erodes, may also be used.
[0029] Some non-limiting examples of suitable nondegradable materials from
which the
osseous phase may be made include polyolefins, such as polyethylene and
polypropylene
including atactic, isotactic, syndiotactic, and blends thereof, polyethylene
glycols, polyethylene
oxides, ultra high molecular weight polyethylene, copolymers of polyethylene
and
7
CA 02736240 2011-04-04
polypropylene, as well as, polyisobutylene and ethylene-alphaolefins
copolymers, and
fluorinated polyolefins such as polytetrafluoroethylene; polyamides such as
nylon and
polycaprolactam; polyamines; polyimines; polyesters such as polyethylene
terephthalate and
polybutylene terephthalate; aliphatic polyesters; polytetrafluoroethylene;
polyethers; polyether-
esters such as polybutester; polytetramethylene ether glycol; 1,4-butanediol;
polyurethanes;
acrylic polymers and copolymers; modacrylics; vinyl halide polymers and
copolymers such as
polyvinyl chloride; polyvinyl alcohols; polyvinyl ethers such as polyvinyl
methyl ether;
polyvinylidene halides such as polyvinylidene fluoride and polyvinylidene
chloride;
polyacrylonitrile; polyvinyl ketones; polyvinyl aromatics such as polystyrene;
polyvinyl esters
such as polyvinyl acetate; copolymers of vinyl monomers with each other and
olefins such as
etheylene-methyl methacrylate copolymers, acrylonitrile-styrene copolymers,
ABS resins, and
ethylene-vinyl acetate copolymers; alkyd resins; polycarbonates;
polyoxymethylenes;
polyphosphazine; polyimides; epoxy resins; aramids, and combinations thereof.
[0030] The biodegradable materials may be crosslinked with a crosslinking
agent to
enhance the mechanical strength of the osseous phase. Crosslinking agents are
within the
purview of those skilled in the art, and include, for example, calcium salts
such as
hydroxyapatite; aldehyde crosslinking agents such as glutaraldehyde;
isocyanate crosslinking
agents such as hexamethylene diisocyanate; carbodiimide crosslinking agents
such as1-ethyl-
3-(3-dimethylaminopropyl) carbodiimide hydrochloride; polyepoxy crosslinking
agents such as
ethylene glycol diglycidyl ether; and transglutaminase.
[0031] At least a portion of the first scaffold may include one or more
pendant reactive
functional groups suitable for interacting with the tissue and/or the chondral
phase of the plugs
described herein. The term "reactive functional group" as used herein refers
to electrophilic or
nucleophilic groups capable of reacting with each other to form a bond.
Electrophilic functional
groups include, for example, N-hydroxysuccinimides ("NHS"), sulfosuccinimides,
carbonyldiimidazole, sulfonyl chloride, aryl halides, sulfosuccinimidyl
esters, N-
8
CA 02736240 2011-04-04
hydroxysuccinimidyl esters, succinimidyl esters such as succinimidyl
succinates and/or
succinimidyl propionates, isocyanates, thiocyanates, carbodiimides,
benzotriazole carbonates,
epoxides, aldehydes, maleimides, imidoesters, combinations thereof, and the
like. In certain
embodiments, the electrophilic functional group is a succinimidyl ester.
[0032] Suitable nucleophilic groups which may be present on at least a portion
of the
first scaffold surface include, but are not limited to, -NH2, -SH, -OH, -PH2, -
CO-NH-NH2 and
combinations thereof,
[0033] It is contemplated by the present disclosure that the functional groups
may be the
same or different at each occurrence. Thus, the first scaffold may have two
different
electrophilic groups, or two different nucleophilic groups.
[0034] The reactive groups may be positioned on or near the surface of the
first scaffold
using any suitable manner. For example, the first scaffold may be formed from
materials which
naturally position reactive groups toward the outer surface of the scaffold.
In other examples,
the first scaffold may be surface-modified to covalently attach the reactive
groups. In still other
examples, the first scaffolds may be coated with an additional layer of
material which includes
the pendant reactive groups necessary to interact with the tissue and/or the
second phase of
the plugs described herein.
[0035] In some embodiments, the coating process includes surface treatment of
the
osseous phase in order to promote adhesion of the coating to the surface of
the osseous phase.
The surface of the osseous phase can be treated using plasma, physical or
chemical vapor
deposition, pulsed laser ablation deposition, surface modification, or any
other means within the
purview of those skilled in the art to activate the surface of the osseous
phase with the amine-
functionalized coating. In other embodiments, treatment may include the use of
a primer such
as a cross-linkable compound. In yet other embodiments, one or more deposition
treatments
could be used alone or in conjunction with the primer to achieve the desired
association of
amine-functionalized coating with the osseous phase.
9
CA 02736240 2011-04-04
[0036] In embodiments, the osseous phase includes a first scaffold made from
bone
cement. For example, the first scaffold may be made from a poly(methyl
methacrylate). In
certain embodiments, the first scaffold may be made from a two-component
material which
includes an aminated poly(methyl methacrylate) powder which can be mixed with
a liquid methyl
methacrylate monomer. An accelerator, such as for example, N,N-dimethyl-p-
toluidine, N,N-
dimethylaniline, N,N-bis(2-hydroxylethyl)-p-toluidine, and organic copper(II)
salts, may be used
to initiate free radical polymerization of the monomer. The two-component
material may be
molded to fit the tissue defect and allowed to harden to match the mechanical
properties of the
bone. In some embodiments, the two-part material may be hardened in the tissue
defect. In
other embodiments, the two-part material may be hardened prior to
implantation.
The Chondral Phase
[0037] The chondral phase of the osteochondral plug of the present disclosure
includes
a second scaffold or structure upon, or within, which desired cartilage cells
may grow in order to
regenerate the desired tissue. The second scaffold being capable of reacting
with the one or
more pendant reactive groups of the first scaffold to form a bond. In
embodiments, the second
scaffold may be porous.
[0038] The second scaffold may include at least hydrogel precursor suitable
for forming
a hydrogel material. At least one of the hydrogel precursors of the chondral
phase may be
capable of reacting with the pendant reactive groups of the osseous phase. The
hydrogel
precursor may be, e.g., a monomer or a macromer. The hydrogel precursor may be
a solid or a
liquid. One type of precursor may have a reactive functional group that is an
electrophile or a
nucleophile. Electrophiles react with nucleophiles to form covalent bonds.
Covalent crosslinks
or bonds refer to chemical groups formed by reaction of functional groups on
different materials
that serve to covalently bind the different materials to each other. In
certain embodiments, a
first set of electrophilic functional groups on a first precursor may react
with a second set of
CA 02736240 2011-04-04
nucleophilic functional groups on a second precursor. When the precursors are
mixed in an
environment that permits reaction (e.g., as relating to pH or solvent), the
functional groups react
with each other to form covalent bonds. The precursors become crosslinked when
at least
some of the precursors can react with more than one other precursor. For
instance, a precursor
with two or more functional groups of a first type may be reacted with a
crosslinking precursor
that has two or more functional groups of a second type capable of reacting
with the first type of
functional groups.
[0039] The hydrogel may be formed from single or multiple precursors. For
example,
where the hydrogel is formed from multiple precursors, for example two
precursors, the
precursors may be referred to as a first and a second hydrogel precursor. The
terms "first
hydrogel precursor" and "second hydrogel precursor" each are meant to include
any of a
polymer, functional polymer, macromolecule, small molecule, or crosslinker
that can take part in
a reaction to form a network of crosslinked molecules, e.g., a hydrogel.
[0040] The term "reactive functional group" as used herein refers to
electrophilic or
nucleophilic groups capable of reacting with each other to form a bond.
Electrophilic functional
groups include, for example, N-hydroxysuccinimides ("NHS"), sulfosuccinimides,
carbonyldiimidazole, sulfonyl chloride, aryl halides, sulfosuccinimidyl
esters, N-
hydroxysuccinimidyl esters, succinimidyl esters such as succinimidyl
succinates and/or
succinimidyl propionates, isocyanates, thiocyanates, carbodiimides,
benzotriazole carbonates,
epoxides, aldehydes, maleimides, imidoesters, combinations thereof, and the
like. In
embodiments, the electrophilic functional group is a succinimidyl ester.
[0041] As noted above, the present disclosure provides hydrogels which include
an
electrophilic precursor, sometimes referred to herein as an electrophilic
crosslinker, and a
nucleophilic component. In embodiments, the nucleophilic component is a
natural component,
which may be cross-linked by the electrophilic crosslinker to form a hydrogel.
In embodiments,
the hydrogel may be biodegradable.
11
CA 02736240 2011-04-04
[0042] The hydrogel may be formed prior to implantation or may be formed in
situ at the
time of implantation. The components for forming hydrogels on or in tissues
may include, for
example, in situ forming materials. When formed in situ, the hydrogels may
conform to the
surface geometry of the osseous phase, mechanically interlocking with the
osseous phase. The
in situ forming material may include a single precursor or multiple precursors
that form "in situ,"
meaning formation occurs at a tissue in a living animal or human body. In
general, this may be
accomplished by having a precursor that can be activated at the time of
application to create, in
embodiments, a hydrogel. Activation can be through a variety of methods
including, but not
limited to, environmental changes such as pH, ionicity, temperature,
ultraviolet light (UV), etc.
In other embodiments, the components for forming a hydrogel may be contacted
outside the
body and then introduced into a patient as an implant such as a (pre-formed)
tissue scaffold.
[0043] In some embodiments, as discussed further below, the hydrogel itself
may
include a natural component such as collagen, gelatin, hyaluronic acid,
combinations thereof,
and the like. In certain embodiments the natural component may be released at
the site of
implantation as the hydrogel degrades. The term "natural component" as used
herein includes
polymers, compositions of matter, materials, combinations thereof, and the
like, which can be
found in nature or derived from compositions/organisms found in nature.
Natural components
also may include compositions which are found in nature but can be synthesized
by man, for
example, using methods to create natural/synthetic/biologic recombinant
materials, as well as
methods capable of producing proteins with the same sequences as those found
in nature,
and/or methods capable of producing materials with the same structure and
components as
natural materials, such as synthetic hyaluronic acid, which is commercially
available, for
example, from Sigma Aldrich.
[0044] The hydrogel precursors, e.g., the electrophilic hydrogel precursors,
may have
biologically inert and water soluble cores. When the core is a polymeric
region that is water
soluble, suitable polymers that may be used include: polyethers, for example,
polyalkylene
12
CA 02736240 2011-04-04
oxides such as polyethylene glycol(" PEG"), polyethylene oxide ("PEO"),
polyethylene oxide-co-
polypropylene oxide ("PPO"), co-polyethylene oxide block or random copolymers,
and polyvinyl
alcohol ("PVA"); poly(vinyl pyrrolidinone) ("PVP"); poly(amino acids);
polysaccharides such as
dextran, chitosan, alginates, chitin, carboxymethylcellulose, oxidized
cellulose,
hydroxyethylcellulose, and hydroxymethylcellulose; hyaluronic acid (HA); and
poly(amino acids)
including proteins such as albumin, collagen (I, II, and III), elastin,
fibrin, fibrinogen, casein, and
gelatin. Other suitable hydrogels may include components such as methacrylic
acid,
acrylamides, methyl methacrylate, hydroxyethyl methacrylate, combinations
thereof, and the
like. In embodiments, combinations and components of the foregoing polymers
may be utilized.
[0045] The polyethers, and more particularly poly(oxyalkylenes) or
polyethylene glycol)
or polyethylene glycol, may be utilized in some embodiments. When the core is
small in
molecular nature, any of a variety of hydrophilic functionalities can be used
to make the first and
second hydrogel precursors water soluble. For example, functional groups like
hydroxyl, amine,
sulfonate and carboxylate, which are water soluble, may be used to make the
precursor water
soluble. For example, the n-hydroxysuccinimide ("NHS") ester of subaric acid
is insoluble in
water, but by adding a sulfonate group to the succinimide ring, the NHS ester
of subaric acid
may be made water soluble, without affecting its reactivity towards amine
groups. In
embodiments, the precursor having electrophilic functional groups may be a PEG
ester.
[0046] As noted above, each of the first and second hydrogel precursors may be
multifunctional, meaning that it may include two or more electrophilic or
nucleophilic functional
groups, such that, for example, a nucleophilic functional group on the first
hydrogel precursor
may react with an electrophilic functional group on the second hydrogel
precursor to form a
covalent bond. At least one of the first or second hydrogel precursors
includes more than two
functional groups, so that, as a result of electrophilic-nucleophilic
reactions, the precursors
combine to form cross-linked polymeric products.
13
CA 02736240 2011-04-04
[0047] A macromolecule having the electrophilic functional group may be multi-
armed.
For example, the macromolecule may be a multi-armed PEG having four, six,
eight, or more
arms extending from a core. The core may be the same or different from the
macromolecule
forming the arms. For example, the core may be PEG and the multiple arms may
also be PEG.
In embodiments, the core may be a natural polymer.
[0048] The molecular weight (MW) of the electrophilic crosslinker may be from
about
2,000 to about 100,000; in embodiments from about 10,000 to about 40,000.
Multi-arm
precursors may have a molecular weight that varies depending on the number of
arms. For
example, an arm having a 1000 MW of PEG has enough CH2CH2O groups to total at
least 1000
MW. The combined molecular weight of an individual arm may be from about 250
to about
5,000; in embodiments from about 1,000 to about 3,000; in embodiments from
about 1,250 to
about 2,500. In embodiments, the electrophilic crosslinker may be a multi-arm
PEG
functionalized with multiple NHS groups having, for example, four, six or
eight arms and a
molecular weight from about 5,000 to about 25,000. Other examples of suitable
precursors are
described in U.S. Patent Nos. 6,152,943; 6,165,201; 6,179,862; 6,514,534;
6,566,406;
6,605,294; 6,673,093; 6,703,047; 6,818,018; 7,009,034; and 7,347,850, the
entire disclosures of
each of which are incorporated herein by reference.
[0049] The electrophilic precursor may be a cross-linker that provides an
electrophilic
functional group capable of bonding with nucleophiles on another component, in
embodiments a
natural component. The natural component may be endogenous to the patient to
which the
electrophilic crosslinker is applied, or may be exogenously applied.
[0050] In embodiments, one of the precursors may be a natural component
possessing
nucleophilic groups. Nucleophilic groups which may be present include, for
example, -NH2,
-SH, -OH, -PH2, and -CO-NH-NH2. Any monomer, macromer, polymer, or core
described
above as suitable for use in forming the electrophilic precursor may be
functionalized with
14
CA 02736240 2011-04-04
nucleophilic groups to form a nucleophilic precursor. In other embodiments, a
natural
component possessing nucleophilic groups may be utilized as the nucleophilic
precursor.
[0051] The natural component may be, for example, collagen, gelatin, blood
(including
serum, which may be whole serum or extracts therefrom), hyaluronic acid,
proteins, albumin,
other serum proteins, serum concentrates, platelet rich plasma (prp),
chondroitin sulfate,
combinations thereof, and the like. Additional suitable natural components
which may be utilized
or added to another natural component, sometimes referred to herein as a
bioactive agent,
include, for example, stem cells, DNA, RNA, enzymes, growth factors, peptides,
polypeptides,
antibodies, other nitrogenous natural molecules, combinations thereof, and the
like. Other
natural components may include derivatives of the foregoing, for example
modified hyaluronic
acid, dextran, other polysaccharide, polymers and/or polypeptides, including
aminated
polysaccharides which may be naturally derived, synthetic, or biologically
derived. For example,
in embodiments hyaluronic acid may be modified to make it nucleophilic.
[0052] In embodiments, any of the above natural components may be
synthetically
prepared, e.g., synthetic hyaluronic acid, utilizing methods within the
purview of those skilled in
the art. Similarly, in embodiments the natural component could be a natural or
synthetic long
chain aminated polymer. The natural component may also be modified, i.e.,
aminated to create
a nucleophilic polymer.
[0053] The natural component may provide cellular building blocks or cellular
nutrients
to the tissue that it contacts in situ. For example, serum contains proteins,
glucose, clotting
factors, mineral ions, and hormones which may be useful in the formation or
regeneration of
tissue.
[0054] In embodiments, the natural component includes whole serum. In
embodiments,
the natural component is autologous, i.e., collagen, serum, blood, and the
like, from the body
where the hydrogel is (or is to be) formed. In this manner, the person or
animal in which the
hydrogel is to be used may provide the natural component for use in formation
of the hydrogel.
CA 02736240 2011-04-04
In such embodiments, the resulting hydrogel is semi-autologous, including a
synthetic
electrophilic precursor and an autologous/endogenous nucleophilic precursor.
[0055] In embodiments, a multifunctional nucleophilic polymer, such as a
natural
component having multiple amine groups, may be used as a first hydrogel
precursor and a
multifunctional electrophilic polymer, such as a multi-arm PEG functionalized
with multiple NHS
groups, may be used as a second hydrogel precursor. In embodiments, the
precursors may be
in solution(s), which may be combined to permit formation of the hydrogel. Any
solutions
utilized as part of the in situ forming material system should not contain
harmful or toxic
solvents. In embodiments, the precursor(s) may be substantially soluble in a
solvent such as
water to allow application in a physiologically-compatible solution, such as
buffered isotonic
saline.
[0056] In embodiments, a hydrogel may be formed from collagen, or a
combination of
collagen and/or gelatin, as the natural component, with a multi-functional PEG
utilized as a
crosslinker. In embodiments, the collagen and/or gelatin may be placed in
solution, utilizing a
suitable solvent. To this solution, hyaluronic acid may be added along with a
high pH buffer.
Such a buffer may have a pH from about 8 to about 12, in embodiments from
about 8.2 to about
9. Examples of such buffers include, but are not limited to, borate buffers,
and the like.
[0057] In a second solution, an electrophilic crosslinker, in embodiments a
multi-arm
PEG functionalized with electrophilic groups such as n-hydroxysuccinimide, may
be prepared in
a buffer such as Hanks Balanced Salt Solution, Dulbecco's Modified Eagle's
Medium,
Phosphate Buffered Saline, water, phosphate buffer, combinations thereof, and
the like. The
electrophilic crosslinker, in embodiments a multi-arm PEG functionalized with
n-
hydroxysuccinimide groups, may be present in a solution including the above
buffer at a
concentration from about 0.02 grams/ml to about 0.5 grams/ml, in embodiments
from about 0.05
grams/ml to about 0.3 grams/ml.
16
CA 02736240 2011-04-04
[0058] The two components may be combined, in some embodiments upon
introduction
in situ, wherein the electrophilic groups on the multi-arm PEG crosslink the
amine nucleophilic
components of the collagen and/or gelatin. The ratio of natural component to
electrophilic
component (i.e., collagen:PEG) may be from about 0.1:1 to about 100:1, in
embodiments from
about 1:1 to about 10:1.
[0059] The nucleophilic components, in embodiments the natural components,
e.g.,
collagen, gelatin, and/or hyaluronic acid, may together be present at a
concentration of at least
about 1.5 percent by weight of the hydrogel, in embodiments from about 1.5
percent by weight
to about 20 percent by weight of the hydrogel, in other embodiments from about
2 percent by
weight to about 10 percent by weight of the hydrogel. In certain embodiments,
collagen may be
present from about 0.5 percent to about 7 percent by weight of the hydrogel,
in further
embodiments, from about 1 percent to about 4 percent by weight of the
hydrogel. In another
embodiment, gelatin may be present from about 1 percent to about 15 percent by
weight of the
hydrogel, in further embodiments, from about 2 percent to about 15 percent by
weight of the
hydrogel. In yet another embodiment, hyaluronic acid and collagen combined as
the natural
component(s) may be present from about 0.5 percent to about 8 percent by
weight of the
hydrogel, in further embodiments, from about 1 percent to about 5 percent by
weight of the
hydrogel. It is also envisioned that the hyaluronic acid may not be present as
a "structural"
component, but as more of a bioactive agent. For example, hyaluronic acid may
be present in
solution/gel in concentrations as low as 0.001 percent by weight of the
solution/gel and have
biologic activity.
[0060] The electrophilic crosslinker may be present in amounts of from about
0.5
percent by weight to about 20 percent by weight of the hydrogel, in
embodiments from about 1.5
percent by weight to about 15 percent by weight of the hydrogel.
[0061] Hydrogel materials may be formed either through covalent, ionic or
hydrophobic
bonds. Physical (non-covalent) crosslinks may result from complexation,
hydrogen bonding,
17
CA 02736240 2011-04-04
desolvation, Van der Waals interactions, ionic bonding, combinations thereof,
and the like, and
may be initiated by mixing two precursors that are physically separated until
combined in situ, or
as a consequence of a prevalent condition in the physiological environment,
including:
temperature, pH, ionic strength, combinations thereof, and the like. Chemical
(covalent)
crosslinking may be accomplished by any of a number of mechanisms, including:
free radical
polymerization, condensation polymerization, anionic or cationic
polymerization, step growth
polymerization, electrophile-nucleophile reactions, combinations thereof, and
the like.
[0062] In some embodiments, hydrogel systems may include biocompatible multi-
precursor systems that spontaneously crosslink when the precursors are mixed,
but wherein the
two or more precursors are individually stable for the duration of the
deposition process. In
other embodiments, in situ forming materials may include a single precursor
that crosslinks with
endogenous materials and/or tissues.
[0063] The crosslinking density of the resulting biocompatible crosslinked
polymer may
be controlled by the overall molecular weight of the crosslinker and natural
component and the
number of functional groups available per molecule. A lower molecular weight
between
crosslinks, such as 600 daltons (Da), will give much higher crosslinking
density as compared to
a higher molecular weight, such as 10,000 Da. Elastic gels may be obtained
with higher
molecular weight natural components with molecular weights of more than 3000
Da.
[0064] The crosslinking density may also be controlled by the overall percent
solids of
the crosslinker and natural component solutions. Increasing the percent solids
increases the
probability that an electrophilic group will combine with a nucleophilic group
prior to inactivation
by hydrolysis. Yet another method to control crosslink density is by adjusting
the stoichiometry
of nucleophilic groups to electrophilic groups. A one to one ratio may lead to
the highest
crosslink density, however, other ratios of reactive functional groups (e.g.,
electrophile:nucleophile) are envisioned to suit a desired formulation.
18
CA 02736240 2011-04-04
[0065] The hydrogel thus produced may be bioabsorbable, so that it does not
have to be
retrieved from the body. Absorbable materials are absorbed by biological
tissues and disappear
in vivo at the end of a given period, which can vary, for example, from one
day to several
months, depending on the chemical nature of the material. Absorbable materials
include both
natural and synthetic biodegradable polymers, as well as bioerodible polymers.
[0066] In embodiments, one or more precursors having biodegradable linkages
present
in between functional groups may be included to make the hydrogel
biodegradable or
absorbable. In some embodiments, these linkages may be, for example, esters,
which may be
hydrolytically degraded in physiological solution. The use of such linkages is
in contrast to
protein linkages that may be degraded by proteolytic action. A biodegradable
linkage optionally
also may form part of a water soluble core of one or more of the precursors.
Alternatively, or in
addition, functional groups of precursors may be chosen such that the product
of the reaction
between them results in a biodegradable linkage. For each approach,
biodegradable linkages
may be chosen such that the resulting biodegradable biocompatible crosslinked
polymer
degrades or is absorbed in a desired period of time. Generally, biodegradable
linkages may be
selected that degrade the hydrogel under physiological conditions into non-
toxic or low toxicity
products.
[0067] Biodegradable gels utilized in the present disclosure may degrade due
to
hydrolysis or enzymatic degradation of the biodegradable region, whether part
of the natural
component or introduced into a synthetic electrophilic crosslinker. The
degradation of gels
containing synthetic peptide sequences will depend on the specific enzyme and
its
concentration. In some cases, a specific enzyme may be added during the
crosslinking reaction
to accelerate the degradation process. In the absence of any degradable
enzymes, the
crosslinked polymer may degrade solely by hydrolysis of the biodegradable
segment. In
embodiments in which polyglycolate is used as the biodegradable segment, the
crosslinked
polymer may degrade in from about 1 day to about 30 days depending on the
crosslinking
19
CA 02736240 2011-04-04
density of the network. Similarly, in embodiments in which a polycaprolactone
based
crosslinked network is used, degradation may occur over a period of time from
about 1 month to
about 8 months. The degradation time generally varies according to the type of
degradable
segment used, in the following order:
polyglycolate<polylactate<polytrimethylene
carbonate<polycaprolactone. Thus, it is possible to construct a hydrogel with
a desired
degradation profile, from a few days to months, using a proper degradable
segment.
[0068] Where utilized, the hydrophobicity generated by biodegradable blocks
such as
oligohydroxy acid blocks or the hydrophobicity of PPO blocks in PLURONICTM or
TETRONICTM
polymers utilized to form the electrophilic crosslinker may be helpful in
dissolving small organic
drug molecules. Other properties which will be affected by incorporation of
biodegradable or
hydrophobic blocks include: water absorption, mechanical properties and
thermosensitivity.
[0069] Certain properties of the hydrogel material can be useful, including
adhesion to a
variety of tissues, desirable setting times to enable a surgeon to accurately
and conveniently
place the hydrogel materials, high water content for biocompatibility,
mechanical strength for
use in implants, and/or toughness to resist destruction after placement.
Synthetic materials that
are readily sterilized and avoid the dangers of disease transmission involved
in the use of
natural materials may thus be used. Indeed, certain in situ polymerizable
hydrogels made using
synthetic precursors are within the purview of those skilled in the art, e.g.,
as used in
commercially available products such as FOCALSEAL (Genzyme, Inc.), COSEAL
(Angiotech
Pharmaceuticals), and DURASEAL (Confluent Surgical, Inc). Other known
hydrogels include,
for example, those disclosed in U.S. Patent Nos. 6,656,200; 5,874,500;
5,543,441; 5,514,379;
5,410,016; 5,162,430; 5,324,775; 5,752,974; and 5,550,187.
[0070] As noted above, in embodiments a branched multi-arm PEG, sometimes
referred
to herein as a PEG star, may be included to form a hydrogel of the present
disclosure. A PEG
star may be functionalized so that its arms include pendant reactive
biofunctional groups for
biological signaling and/or molecular binding, such as amino acids, peptides,
antibodies,
CA 02736240 2011-04-04
enzymes, drugs, affinity binders, thiols, combinations thereof, or other
moieties such as
bioactive agents in its cores, its arms, or at the ends of its arms. The
biofunctional groups may
also be incorporated into the backbone of the PEG, or attached to a reactive
group contained
within the PEG backbone. The binding can be covalent or non-covalent,
including electrostatic,
thiol mediated, peptide mediated, or using known reactive chemistries, for
example, biotin with
avidin.
[0071] Amino acids incorporated into a PEG star may be natural or synthetic,
and can
be used singly or as part of a peptide. Sequences may be utilized for cellular
adhesion, cell
differentiation, combinations thereof, and the like, and may be useful for
binding other biological
molecules such as growth factors, drugs, cytokines, DNA, antibodies, enzymes,
combinations
thereof, and the like. Such amino acids may be released upon enzymatic
degradation of the
PEG star.
[0072] These PEG stars may also include functional groups as described above
to
permit their incorporation into a hydrogel. The PEG star may be utilized as
the electrophilic
crosslinker or, in embodiments, be utilized as a separate component in
addition to the
electrophilic crosslinker described above. In embodiments, the PEG stars may
include
electrophilic groups that bind to nucleophilic groups. As noted above, the
nucleophilic groups
may be part of a natural component utilized to form a hydrogel of the present
disclosure.
[0073] In some embodiments a biofunctional group may be included in a PEG star
by
way of a degradable linkage, including an ester linkages formed by the
reaction of PEG
carboxylic acids or activated PEG carboxylic acids with alcohol groups on a
biofunctional group.
In this case, the ester groups may hydrolyze under physiological conditions to
release the
biofunctional group.
21
CA 02736240 2011-04-04
Optional Bioactive Agents
[0074] Bioactive agents may be added to the osteochondral plug of the present
disclosure to provide specific biological or therapeutic properties thereto.
Any product which
may enhance tissue repair, limit the risk of sepsis, and modulate the
mechanical properties of
the osteochondral plug, or specific phase portion thereof, may be added during
the preparation
of the device or may be coated on the device. In embodiments, agents which may
be added to
the osteochondral plug include: fucans for antiseptic properties; chitosan and
glutaraldehyde
crosslinked collagen for their degradation time; and growth factors, peptides,
proteins, drugs,
and DNA for their tissue properties.
[0075] Moreover, the osteochondral plug may also be used for delivery of one
or more
bioactive agents. The bioactive agents may be incorporated into one or both of
the phases of
the osteochondral plug during formation of the device, such as by free
suspension, liposomal
delivery, microspheres, microbubbles, etc., or by coating a surface of the
plug, or portion
thereof, such as by polymer coating, dry coating, freeze drying, applying to a
mesh surface,
ionically, covalently, or affinity binding to functionalize the degradable
components of the plug.
Thus, in some embodiments, at least one bioactive agent may be combined with a
phase of the
osteochondral plug, i.e., the osseous phase and/or chondral phase, during
formation to provide
release of the bioactive agent during degradation of the plug. As the plug
degrades or
hydrolyzes in situ, the bioactive agents are released. In other embodiments,
bioactive agents
may be coated onto a surface or a portion of a surface of the osseous phase or
chondral phase
of the plug for quick release of the bioactive agent.
[0076] A bioactive agent as used herein is used in the broadest sense and
includes any
substance or mixture of substances that have clinical use. Consequently,
bioactive agents may
or may not have pharmacological activity per se, e.g., a dye. Alternatively a
bioactive agent
could be any agent that provides a therapeutic or prophylactic effect; a
compound that affects or
participates in tissue growth, cell growth, and/or cell differentiation; an
anti-adhesive compound;
22
CA 02736240 2011-04-04
a compound that may be able to invoke a biological action such as an immune
response; or
could play any other role in one or more biological processes. A variety of
bioactive agents may
be incorporated into the plug.
[0077] Examples of classes of bioactive agents, which may be utilized in
accordance
with the present disclosure include, for example, anti-adhesives,
antimicrobials, analgesics,
antipyretics, anesthetics, antiepileptics, antihistamines, anti-
inflammatories, cardiovascular
drugs, diagnostic agents, sympathomimetics, cholinomimetics, antimuscarinics,
antispasmodics,
hormones, growth factors, muscle relaxants, adrenergic neuron blockers,
antineoplastics,
immunogenic agents, immunosuppressants, gastrointestinal drugs, diuretics,
steroids, lipids,
lipopolysaccharides, polysaccharides, platelet activating drugs, clotting
factors and enzymes. It
is also intended that combinations of bioactive agents may be used.
[0078] Other bioactive agents, which may be included as a bioactive agent
include:
local anesthetics; non-steroidal antifertility agents; parasympathomimetic
agents;
psychotherapeutic agents; tranquilizers; decongestants; sedative hypnotics;
steroids;
sulfonamides; sympathomimetic agents; vaccines; vitamins; antimalarials; anti-
migraine agents;
anti-parkinson agents such as L-dopa; anti-spasmodics; anticholinergic agents
(e.g.,
oxybutynin); antitussives; bronchodilators; cardiovascular agents, such as
coronary vasodilators
and nitroglycerin; alkaloids; analgesics; narcotics such as codeine,
dihydrocodeinone,
meperidine, morphine and the like; non-narcotics, such as salicylates,
aspirin, acetaminophen,
d-propoxyphene and the like; opioid receptor antagonists, such as naltrexone
and naloxone;
anti-cancer agents; anti-convulsants; anti-emetics; antihistamines; anti-
inflammatory agents,
such as hormonal agents, hydrocortisone, prednisolone, prednisone, non-
hormonal agents,
allopurinol, indomethacin, phenylbutazone and the like; prostaglandins and
cytotoxic drugs;
chemotherapeutics; estrogens; antibacterials; antibiotics; anti-fungals; anti-
virals;
anticoagulants; anticonvulsants; antidepressants; antihistamines; and
immunological agents.
23
CA 02736240 2011-04-04
[0079] Other examples of suitable bioactive agents, which may be included in
the
osteochondral plug include, for example, viruses and cells; peptides,
polypeptides and proteins,
as well as analogs, muteins, and active fragments thereof; immunoglobulins;
antibodies;
cytokines (e.g., lymphokines, monokines, chemokines); blood clotting factors;
hemopoietic
factors; interleukins (IL-2, IL-3, IL-4, IL-6); interferons ((3-IFN, a-IFN and
y-IFN); erythropoietin;
nucleases; tumor necrosis factor; colony stimulating factors (e.g., GCSF, GM-
CSF, MCSF);
insulin; anti-tumor agents and tumor suppressors; blood proteins such as
fibrin, thrombin,
fibrinogen, synthetic thrombin, synthetic fibrin, synthetic fibrinogen;
gonadotropins (e.g., FSH,
LH, CG, etc.); hormones and hormone analogs (e.g., growth hormone); vaccines
(e.g., tumoral,
bacterial and viral antigens); somatostatin; antigens; blood coagulation
factors; growth factors
(e.g., nerve growth factor, insulin-like growth factor); bone morphogenic
proteins; TGF-B;
protein inhibitors; protein antagonists; protein agonists; nucleic acids, such
as antisense
molecules, DNA, RNA, RNAi; oligonucleotides; polynucleotides; and ribozymes.
In some
embodiments, peptides or antibodies may be used to bind growth factors to the
plug.
[0080] It may be desirable to include bioactive agents which promote wound
healing
and/or tissue growth, including colony stimulating factors, blood proteins,
fibrin, thrombin,
fibrinogen, hormones and hormone analogs, blood coagulation factors, growth
factors, bone
morphogenic proteins, TGF-[3, IGF, combinations thereof, and the like. In
embodiments, the
scaffold of either or both the osseous and chondral phases may deliver and/or
release biological
factors/molecules and/or cells at the site of implantation. Thus, it may
assist in native tissue
regrowth by providing the surrounding tissue with needed nutrients and
bioactive agents.
[0081] As noted above, in embodiments that include a multi-arm PEG or PEG
star, the
bioactive agent may be incorporated into the core of the PEG, the arms of the
PEG, or
combinations thereof. In embodiments, the bioactive agent may be attached to a
reactive group
in the PEG chain. The bioactive agent may be bound covalently, non-covalently,
i.e.,
24
CA 02736240 2011-04-04
electrostatically, through a thiol-mediated or peptide-mediated bond, or using
biotin-avidin
chemistries and the like.
[0082] In embodiments, the bioactive agent may be encapsulated by the
hydrogel. For
example, the hydrogel may form polymer microspheres around the bioactive
agent. As the
hydrogel hydrolyzes in situ, the bioactive components and any added bioactive
agents are
released. This may provide nutrients from the natural components, as well as
bioactive agents,
to the surrounding tissue, thereby promoting growth and/or regeneration of
tissue.
Combining the Osseous and Chondral Phases
[0083] Various combinations of osseous and chondral phases may be used to
fabricate
the osteochondral plug according to the present disclosure. For example, any
of the osseous
phase materials and configuration as described above may be combined with any
of the
chondral phase hydrogels also described above, dependent upon the type of
defect to be
treated and the properties desired from the osteochondral plug.
[0084] The osseous phase may be a solid or cement-like scaffold which can
become
solid after a set cure time. Alternatively, the osseous phase may be a
hydrogel that may be
formed prior to implantation or in situ. The osseous phase should mimic the
biomechanical
properties of bone. In embodiments, the osseous phase is adapted to recruit
and/or deliver
endogenous growth factors, proteins, and/or cells. In embodiments, the
material of the osseous
phase includes reactive groups which may crosslink with the chondral phase.
[0085] The chondral phase may be a single or multi-component hydrogel
containing
water soluble biopolymers as at least one component. The precursor(s) of the
hydrogel may be
dissolved to form a solution prior to use, with the solution being delivered
to the osteochondral
defect. As used herein, a solution may be homogeneous, heterogeneous, phase
separated, or
the like. In other embodiments, the precursor(s) may be in an emulsion. Where
two solutions
are employed, each solution may contain one precursor of the hydrogel forming
material which
CA 02736240 2011-04-04
forms upon contact. The solutions may be separately stored and mixed when
delivered to
tissue.
[0086] In a single component system, the precursor, i.e., the electrophile,
reacts with
natural components of the tissue environment to produce a crosslinked
polymeric network. In a
multi-component system, the precursors react with each other to form a
hydrogel. In
embodiments, the precursors may be nucleophilic/electrophilic reactive
components, such as
succinimide and primary amines. In both the single and multi-component
hydrogel systems, the
hydrogel may crosslink with the osseous phase. In embodiments, the biopolymer
component of
the hydrogel may promote cell attachment and proliferation. In some
embodiments, the
hydrogel may contain proteins, peptides, and/or growth factors for promoting
chondrogenesis.
[0087] Formulations may be prepared that are suited to make precursor
crosslinking
reactions occur in situ. In general, this may be accomplished by having a
precursor that can be
activated at the time of application to a tissue to form a crosslinked
hydrogel. Activation can be
made before, during, or after application of the precursor to the tissue,
provided that the
precursor is allowed to conform to the tissue's shape before crosslinking and
associated
gelation is otherwise too far advanced. Activation includes, for instance,
mixing precursors with
functional groups that react with each other. Thus, in situ polymerization
includes activation of
chemical moieties to form covalent bonds to create an insoluble material,
e.g., a hydrogel, at a
location where the material is to be placed on, within, or both on and within,
a patient. In situ
polymerizable polymers may be prepared from precursor(s) that can be reacted
such that they
form a polymer within the patient. Thus precursor(s) with electrophilic
functional groups can be
mixed or otherwise activated in the presence of precursors with nucleophilic
functional groups.
[0088] In other embodiments, where electrophilic precursors are used, such
precursors
may react with free amines in tissue, thereby serving as a means for attaching
the hydrogel to
tissue.
26
CA 02736240 2011-04-04
[0089] The crosslinking reaction leading to gelation can occur, in some
embodiments
within a time from about 1 second to about 5 minutes, in embodiments from
about 3 seconds to
about 1 minute; persons of ordinary skill in these arts will immediately
appreciate that all ranges
and values within these explicitly stated ranges are contemplated. For
example, in
embodiments, the in situ gelation time of hydrogels according to the present
disclosure is less
than about 20 seconds, and in some embodiments, less than about 10 seconds,
and in yet
other embodiments less than about 5 seconds.
[0090] The osteochondral plug of the present disclosure promotes tissue repair
in an
osteochondral defect by filling the void of the lesion with a tissue specific
scaffold which
promotes its respective tissue regeneration. The osteochondral defect also
promotes
integration with a tissue void by form fitting the defect.
[0091] Embodiments of the present disclosure will now be described, by way of
example
only, with reference to the accompanying drawings.
[0092] Referring to FIGURE 1, osteochondral plug 10 includes osseous phase 12
and
chondral phase 14. Osseous phase 12 is a solid scaffold formed from a
poly(lactic-co-glycolic
acid) sponge modified to includes bone growth factors which are released into
bone "b." The
osseous phase 12 also contains free amines for bonding with the chondral phase
14. It is
envisioned that portions of the osseus phase may also bond with bone "b" to
enhance tissue
integration. The chondral phase 14 is a hydrogel formed from PEG star and
collagen
precursors. The chondral phase 14 includes cartilage growth factors which are
released into
cartilage "c." In embodiments, the chondral phase 14 may be formed in situ and
thus added to
the tissue void containing the osseous phase 12 as a liquid. The liquid gels
in situ thus filling
any gaps that may form between the bone "b" and the osseous layer 12 of the
osteochondral
plug 10.
[0093] Turning now to FIGURE 2, an osteochondral plug 20 includes osseous
phase 22
and chondral phase 24. The osseous phase 22 is a soft, self-curing porous bone
cement
27
CA 02736240 2011-04-04
modified with free amines for covalently bonding with the bone "b" in which it
is placed as well
as for bonding with the chondral phase 24. Chondral phase 24 is a hydrogel
formed from PEG
star and collagen precursors. In embodiments, the bone cement is formed and
added to the
bone "b" as a slurry for curing in situ. The chondral phase 24 may be added to
the tissue void in
liquid form as described in FIGURE 1 above, or may be pre-formed and placed
within cartilage
"c. 11
[0094] FIGURE 3 illustrates an osteochondral plug 30 including an osseous
phase 32
and a chondral phase 34. Both the osseous phase 32 and the chondral phase 34
are formed
from a hydrogel containing NHS functionalized PEG star and collagen
precursors, respectfully.
The PEG star architecture of the osseous phase 32 and the chondral phase 34,
as well as the
concentration of the PEG star, are different thus altering the mechanical
properties and gelation
kinetics of each phase. As described above, variations of the molecular weight
and chemistry of
the biopolymer can also control the mechanical properties and gelation
kinetics of a hydrogel.
Additionally, these parameters can control the pore volume, release kinetics
for biological
materials (e.g., growth factors, DNA, etc.), and cellular response (e.g.,
migration). The hydrogel
of the osseous phase 32 is in the form of a stiff gel and includes bone growth
factors which are
released into bone "b." The hydrogel of the osseous phase 32 may be introduced
as a liquid
into bone "b" and allowed to soldify before the introduction of the hydrogel
of the chondral phase
34. The hydrogel of the chondral phase 34 may also be introduced in liquid
form and may
include cartilage growth factors which are released into cartilage "c."
[0095] FIGURE 4 illustrates an ostechondral plug 40 including a solid osseous
phase 42
and a solid chondral phase 34. The osseous phase 42 is a solid scaffold formed
from a
poly(lactic-co-glycolic acid) sponge modified to includes bone growth factors
which are released
into bone "b." The osseous phase 42 also contains free amines for covalently
bonding with the
bone "b" in which it is placed as well as for bonding with the chondral phase
44. The chondral
phase 44 is a solid scaffold such as a collagen sponge with dry PEG star
precursors disposed
28
CA 02736240 2011-04-04
therein and thereon. Bioactive agents may also be added to the collagen
sponge, such as
cartilage growth factors. Upon placement of the solid chondral phase 44 within
cartilage "c," the
scaffold hydrates from contact with bodily fluids, such as blood, and the PEG
star precursors
react to form a gel. The gel can seal any gaps within the tissue void and can
covalently bond
the scaffold to the cartilage "c" as well as the osseous phase 42 of plug 40.
[0096] While biphasic embodiments are shown above, the. osteochondral plug of
the
present disclosure may have more than two phases, each being formed from any
of the variety
of materials as described above, and including any of the bioactive agents as
also described
above.
[0097] A method for implanting an osteochondral plug of the present disclosure
is
illustrated in FIGURES 5A-5D. Implantation of an osteochondral plug may be by
way of open or
minimally invasive surgery. After an osteochondral defect has been identified
and cleaned, a
delivery device 100, loaded with at least the osseous phase 112 of the
osteochondral plug, may
be placed over the defect "d" as illustrated in FIGURE 5A. The delivery device
includes an
outer shaft 102 including an inner channel, or lumen, 104 housing the osseous
phase 112 of the
osteochondral plug. An inner shaft, or plunger, 106 is slidably engaged within
the inner channel
104 of the outer shaft 102 for driving material disposed therein into defect
"d." As illustrated in
FIGURE 5B, surface guides 108 are deployed to stabilize the device 100 against
the tissue as
well as to align the device with the defect "d." The osseous phase 112 is
ejected from the outer
shaft 102 of the delivery device 100 by advancing the plunger 106 in the
direction of the defect
"d." As described above, the osseous phase 112 may be in a solid or viscous
form. Turning
now to FIGURE 5C, the osseous phase 112 fills the defect "d" up to the
subchondral bone
surface "b." After placement of the osseous phase 112, the plunger 106 is
drawn back up into
the inner channel 106 of the outer shaft 102 of the delivery device 100, at a
level that may be
substantially aligned with the cartilage surface "c." As illustrated next in
FIGURE 5D, the
plunger 106 may include a central bore 107 through which the material of the
chondral phase
29
CA 02736240 2011-04-04
114 may be passed to fill the remainder of the defect, i.e., cartilage "c."
Thus, a hydrogel
composition (chondral phase) may be introduced into the defect "d" through
central bore 107 of
plunger 106. The osteochondral plug is then allowed to cure and the delivery
device 100 may
be removed. Alternatively, plunger 106 may be removed from outer shaft 102
after placement
of osseous phase 112 and the precursor(s), which may be placed into solution
prior to use, may
be delivered to the defect via a syringe (not shown). One may use a syringe
for delivery of a
single precursor, i.e., an electrophilic crosslinker, or a dual syringe or
similar device to apply
more than one precursor solutions, such as those described in U.S. Patent Nos.
4,874,368;
4,631,055; 4,735,616; 4,359,049; 4,978,336; 5,116,315; 4,902,281; 4,932,942;
6,179,862;
6,673,093; and 6,152,943.
[0098] While several embodiments of the disclosure have been described, it is
not
intended that the disclosure be limited thereto, as it is intended that the
disclosure be as broad
in scope as the art will allow and that the specification be read likewise.
Therefore, the above
description should not be construed as limiting, but merely as
exemplifications of embodiments
of the present disclosure. Various modifications and variations of the
osteochondral plug, the
desired properties of the osseous and chondral phases, as well as methods of
forming the
.osseous and chondral phases of the device and attaching the components
together, will be
apparent to those skilled in the art from the foregoing detailed description.
Such modifications
and variations are intended to come within the scope and spirit of the claims
appended hereto.