Note: Descriptions are shown in the official language in which they were submitted.
CA 02737594 2011-03-16
DESCRIPTION
HEAT-RESISTANT FUEL-ACTIVATING SUBSTANCE
TECHNICAL FIELD
[0001]
The present invention relates to a heat-resistant
fuel-activating substance suitable for use in combustion
devices such as boilers in which liquid fossil fuels such
as heavy oil and kerosene, gas fossil fuels such as LPG and
natural gas, and solid fossil fuels such as coal are used
as fuels, and enhancing a combustion-activating effect for
the combustion therein.
BACKGROUND ART
[0002]
Heretofore, various studies have been conducted for
the improvement of thermal efficiency at the time of
combustion in combustion devices such as boilers. For that
purpose, for example, like the invention described in
Patent Document 1, there were some proposals to improve
burners.
The inventors of the present invention have proposed
that combustion efficiency at the time of combustion is
improved by activating methane-based molecules in a thermal
decomposition region using electromagnetic waves from a
- 1 -
CA 02737594 2011-03-16
fuel-activating substance. That is, methane-based
molecules as a kind of active chemical species generated by
the thermal decomposition of the fuel during the combustion
have an absorption band that absorbs electromagnetic waves
with specific electromagnetic wavelengths, specifically
around 8 pm (a range approximately 3 to 20 pm) . Thus,
radiation of the electromagnetic waves in the wavelength
region to the methane-based molecules in the thermal
decomposition region causes stronger vibration of the
methane-based molecules as a kind of active chemical
species that are combustion precursors. Thereby, frequency
of collision between the methane-based molecules and oxygen
molecules in air is enhanced and combustion reactions are
accelerated, thus leading to a rise in flame temperature.
As a result, combustion efficiency comes closer to that of
complete combustion, thus realizing a reduction in the
amount of the fuel use. The present inventors have tried
to develop a heat-resistant fuel-activating substance that
exhibits a high spectral emissivity in such wavelengths.
j0003]
For that purpose, focusing on tourmaline having an
action of radiating electromagnetic waves, tests of
radiating electromagnetic waves from tourmaline to methane-
based molecules in a thermal decomposition region were
carried out. However, there was no significant effect that
- 2 -
CA 02737594 2011-03-16
enables an improvement in combustion efficiency at the time
of combustion.
Based on these findings, the present inventors
disclosed an invention described in Patent Document 2.
This invention is intended to obtain an energy saving
effect by disposing a far infrared ray generator, formed by
mixing tourmaline, iron powder and carbon, in a methane gas
passageway located before a portion where combustion occurs,
thereby activating the fuel.
Patent Literature
Patent Document 1: JP 11-1707 A
Patent Document 2: WO 2006/088084 A
SUMMARY OF INVENTION
[0004]
After the above prior art, focusing particularly on a
spectral emissivity, the present inventors have intensively
made an improvement of a fuel-activating substance and
found that a flame temperature rise of 100 to 150 C is
obtained by using a fuel-activating material in which a
spectral emissivity of electromagnetic waves in the above
wavelength region becomes 0.85 or more and radiating
electromagnetic waves in the relevant wavelength region to
methane-based molecules in the thermal decomposition region.
- 3 -
CA 02737594 2011-03-16
By the way, a conventional fuel-activating substance
is prepared by forming an activating material into a sheet
using an organic resin such as a urethane resin as a binder,
or by forming the activating material into a coating
material to be affixed by coating. Therefore, in case the
fuel-activating substance is affixed to a place at high
temperature of 100 C or more in a combustion device, the
binder was sometimes carbonized with a lapse of time,
resulting in decrease of a spectral emissivity of the
electromagnetic waves from the fuel-activating substance.
[0005]
Then, an object of the present invention is that an
improved fuel-activating material is used and also heat
resistance is imparted to a fuel-activating substance using
this fuel-activating material thereby making it possible to
affix even under temperature conditions where a
conventional fuel-activating substance could not be used,
and thus an energy saving effect in various combustion
devices is further enhanced.
[ 0006]
The heat-resistant fuel-activating substance
according to a first invention among the present invention
is formed by melt-mixing 50 to 150% by weight of a metallic
thermal spray material with 100% by weight of a fuel-
activating material having a spectral emissivity of 0.85 or
- 4 -
CA 02737594 2011-03-16
more for electromagnetic waves with wavelengths in a range
of 3 to 20 pm, thereby making the mixture capable of
thermal spraying.
Regarding "a spectral emissivity of 0.85 or more for
electromagnetic waves with wavelengths in a range of 3 to
20 pm" as stated herein, the relevant wavelength range is a
wavelength range of electromagnetic waves, that contributes
the most to activation of methane-based molecules in a
thermal decomposition region, and is a portion that is
referred to as so-called "far infrared rays." This
spectral emissivity is a numerical value assumed that an
emissivity in the relevant wavelength range of a blackbody
is 1, and has significance as a numerical value enough to
radiate far infrared rays contributing to activation of
methane-based molecules. On this point, the same shall
apply in the respective inventions described hereinafter.
[0007]
Herein, application of the heat-resistant fuel-
activating substance by thermal spraying enables
application even to the place having a complicated surface
shape.
That is, the heat-resistant fuel-activating substance
according to the first invention is applicable to the site
to be applied in the combustion device at a temperature
within a range from about 100 to 400 C. Herein, it is
- 5 -
CA 02737594 2011-03-16
possible to use, as the metallic thermal spray material,
the group of materials having comparatively low melting
temperature, for example, copper, aluminum and nickel. In
particular, materials having a grain size of 5 to 150 um
are desirable.
When the content of the metallic thermal spray
material is less than 50% by weight in addition to 100% by
weight of the activating material, adhesion to the site to
be applied becomes worse. In contrast, when the content is
more than 150% by weight, the spectral emissivity decreases
with the decrease of the proportion of the fuel-activating
material. Therefore, the content is suitably from 50 to
150% by weight.
[0008]
Such a metallic thermal spraying material is mixed
with a predetermined fuel-activating material and the
obtained mixture is filled in a commercially available
thermal spraying apparatus, and then the mixture is
thermally sprayed onto a predetermined site to be applied
of a burner. A specific place to be thermally sprayed
includes a flange portion to which a burner is mounted a
combustion device, or the place behind the site where
combustion flame occurs inside a combustion device that
accommodates the burner. It becomes possible to form a
heat-resistant fuel-activating substance as a metal coating
- 6 -
CA 02737594 2011-03-16
layer containing a fuel-activating material as a component
on the relevant place with a desired thickness. Moreover,
thermal spraying enables application even onto the place
having a surface shape with complicated unevenness where it
is difficult to affix with a sheet-like material.
The heat-resistant fuel-activating substance
according to a second invention among the present invention
is formed by melting 50 to 150% by weight of a metallic
material having a melting point of 420 C or lower with 100%
by weight of a fuel-activating material having a spectral
emissivity of 0.85 or more for electromagnetic waves with
wavelengths in a range of 3 to 20 pm to be formed into a
sheet.
[0009]
That is, the heat-resistant fuel-activating substance
according to the present second invention is applicable to
the site to be applied at a temperature within a range from
about 100 to 300 C. Herein, it is possible to use, as a
metallic material, metals having a comparatively low
melting point, such as lead and zinc.
When the content of the metallic material is less
than 50% by weight in addition to 100% by weight of the
total amount of the fuel-activating material, it becomes
impossible to be formed into a sheet. In contrast, when
the content is more than 150% by weight, the spectral
- 7 -
CA 02737594 2011-03-16
emissivity decreases with the decrease of the proportion of
the fuel-activating material. Therefore, the content is
suitably from 50 to 150% by weight.
Such formation into a sheet enables affixing to a
predetermined site to be applied in the vicinity of a
burner in a combustion device, for example, a flange
portion to which a burner mounted, or the place behind the
site where combustion flame occurs inside a combustion
device that accommodates the burner.
[0010]
The heat-resistant fuel-activating substance
according to the third invention among the present
invention is formed by mixing 75 to 150% by weight of an
inorganic resin having a heat-resistant temperature
exceeding 300 C with 100% by weight of a fuel-activating
material having a spectral emissivity of 0.85 or more for
electromagnetic waves with wavelengths in a range of 3 to
20 pm.
That is, the heat-resistant fuel-activating substance
according to the present third invention is applicable to
the site to be applied at a temperature within a range from
about 100 to 300 C. Herein, the inorganic resin having a
heat-resistant temperature exceeding 300 C does not refer
to a resin that is composed only of an organic resin, but
refers to a resin in which an inorganic material is
- 8 -
CA 02737594 2011-03-16
partially or entirely used as the component. It is
possible to use, for example, a silicone resin, a
fluororesin, a water glass and the like, or a material
having heat resistance, such as a mixture that is
optionally used after mixing among these examples.
[0011]
When the content of the inorganic resin is less than
75% by weight in addition to 100% by weight of the total
amount of the fuel-activating material, it becomes
impossible to be formed into a sheet. In contrast, when
the content is more than 150% by weight, the spectral
emissivity decreases with the decrease of the proportion of
the fuel-activating material. Therefore, the content is
suitably from 75 to 150% by weight. The fuel-activating
material may contain 0.5 to 1.5% by weight of silicon in
100% by weight of the activating material.
The heat-resistant fuel-activating substance
according to the third invention can be formed into a sheet,
and can also be thermally sprayed onto the site to be
applied in a molten state, or sprayed or coated onto the
site to be applied in a mixed state. Formation into a
sheet enables application as a sheet to a predetermined
site to be applied in the vicinity of a burner in a
combustion device, for example, a flange portion to which a
burner is mounted, or the place behind the site where
- 9 -
CA 02737594 2011-03-16
combustion flame occurs inside a combustion device that
accommodates the burner. It is also possible to conduct
thermal spraying after melt-mixing, and to conduct thermal
spraying onto the position to form, on the relevant
position, a heat-resistant fuel-activating substance that
is an inorganic substance coating layer containing the
fuel-activating material as a component with a desired
thickness.
[00121
It is preferable that the fuel-activating materials
in the first invention to the third invention are formed by
blending tourmaline, iron powder and carbon in proportions
within a range of 30 to 44% by weight, 55 to 69% by weight,
and 0.5 to 1.5% by weight, respectively.
Herein, it has already been confirmed by the test of
the present applicant that, when the proportion of at least
one of the respective components deviates from the range of
the above blending ratio, the spectral emissivity of the
heat-resistant fuel-activating substance is less than 0.85.
The heat-resistant fuel-activating substance may
contain 1.5% by weight or less of silicon in 100% by weight
of the activating material. The significance of inclusion
of this silicon lies in that, in case the content of carbon
had to be decreased, silicon supplements lack of carbon,
thus enabling the heat-resistant fuel-activating substance
- 10 -
CA 02737594 2011-03-16
to exhibit the spectral emissivity of 0.85 or more.
[0013]
Each of the heat-resistant fuel-activating substances
shown above can be used not only in a once-through boiler,
a flame-tube smoke-tube boiler and a water-tube boiler
(including an industrial boiler and a power station boiler
that are equipped with two or more burners), but also in
burning appliances equipped with a combustion device that
uses combustion flame as a heat source, and a combustion
chamber, such as a kiln, a dryer, and a hot and chilled
water generator.
The "combustion chamber" as used herein refers to a
portion where a fuel blown from a burner quickly undergoes
ignition and combustion, and the generated combustible gas
undergoes combustion by satisfactory mixing and contacting
with air.
In addition, the "burner" as used herein refers to a
liquid fuel burner, a gas fuel burner and a solid fuel
burner, and is specifically as follows.
[0014]
The liquid fuel burner atomizes a fuel oil thereby
increasing the surface area and accelerates vaporization
thereby enabling satisfactory contact with air, thus
completing a combustion reaction, and specifically refers
to a pressure spraying-type burner, a steam (air) spraying-
- 11 -
CA 02737594 2011-03-16
type burner, a low-pressure air atomizing-type burner, a
rotary burner, a gun type burner and the like.
The gas fuel burner often utilizes a diffusion
combustion system, and specifically refers to a center-type
burner, a ring-type burner, a multispud burner and the like.
The solid fuel burner specifically refers to a burner
of a pulverized coal burner combustion system.
[0015]
With the constitution of the present invention shown
above, it becomes possible to affix a heat-resistant fuel-
activating substance onto the place at comparatively high
temperature, such as inside of a combustion device, thus
making it possible for the electromagnetic waves radiated
from this heat-resistant fuel-activating substance to more
directly act on combustion flame. As a result, vibration
of methane-based molecules as a kind of active chemical
species generated by thermal decomposition of a fuel is
activated and the combustion is accelerated, thus leading
to a rise in flame temperature and stable combustion flame.
As a result, it becomes possible to further decrease the
amount of the fuel use.
BRIEF DESCRIPTION OF DRAWINGS
[0016]
Fig.l schematically shows a measuring device used to
- 12 -
CA 02737594 2011-03-16
examine a relationship between the spectral emissivity and
the flame temperature in a heat-resistant fuel-activating
substance according to the present invention.
Fig.2 schematically shows a flame-tube smoke-tube
boiler affixed with a heat-resistant fuel-activating
substance as a first embodiment of the present invention.
Fig.3 enlarges a burner portion in Fig.2.
Fig.4 schematically shows a once-through boiler
affixed with a heat-resistant fuel-activating substance as
a second embodiment of the present invention.
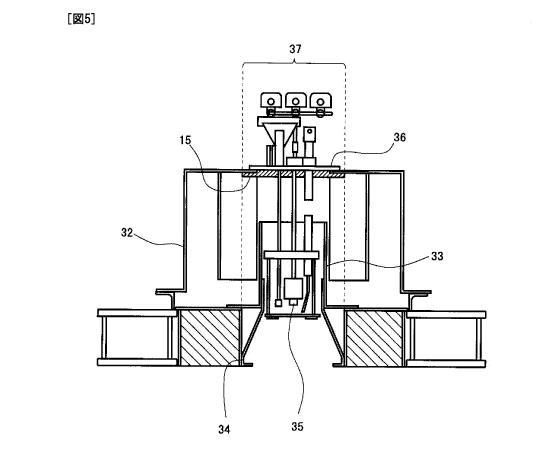
Fig.5 enlarges a burner portion in Fig.4.
Fig.6 schematically shows a water-tube boiler affixed
with a heat-resistant fuel-activating substance as a third
embodiment of the present invention.
Fig.7 enlarges a burner portion in Fig.6.
DESCRIPTION OF EMBODIMENTS
[0017]
(1) Verification of Blending ratio of Fuel Activating
Material
The following materials were used as a fuel-
activating material.
Tourmaline: Schorl tourmaline, 42 mesh (Adam Kozan
Chuo Kenkyusho Co., Ltd.).
Iron powder: RS-200A (POWDER TECH).
- 13 -
CA 02737594 2011-03-16
Carbon: activated carbon powder (C-AW; 12.011, SHOWA
CHEMICAL INDUSTRY CO., LTD.).
The above materials mixed in each blending ratio
shown in Table 1 described below was used as the fuel-
activating material and an inorganic silicone resin
(ES-1002T, Shin-Etsu Chemical Co., Ltd.) as a binder was
added thereto. The obtained mixture was kneaded and was
thereafter coated on a 2-mm thick aluminized steel sheet so
that a thickness of the obtained coating film became 0.6 mm
to obtain samples. The obtained samples were subjected to
the measurement of the spectral emissivity.
[0018]
The spectral emissivity was measured using a Fourier
transform infrared spectrophotometer of Shimadzu
(IRPrestiga-21 (P/N206-72010), Shimadzu Corporation).
Specifically, first, the spectral emissivity was read as
1.0 by a blackbody furnace (at 300 C) and a measuring
sample coated with a pseudo-blackbody coating material
(spectral emissivity: 0.94) was then placed in a sample
furnace. The spectral emissivity was set to 0.94 at a
temperature in the sample furnace. Thereafter, each sample
was placed in the sample furnace under this condition and
the spectral emissivity was measured. The results were
also shown in Table 1 below.
[0019]
- 14 -
CA 02737594 2011-03-16
Table 1
Sample Tourmaline Iron powder Carbon Total Binder Spectral
No. g % g - g g g emissivity
1 150 22.5% 508 76.0% 10 1.5% 668 668 100% 0.77
2 201 30.1% 458 68.6% 9 1.3% 668 668 100% 0.92
3 240 35.9% 420 62.9% 8 1.2% 668 668 100% 0.94
4 293 43.9% 368 55.1% 7 1.0% 668 668 100% 0.89
320 47.9% 344.5 51.6% 3.5 0.5% 668 668 100% 0.72
6 308 46.1% 350 52.4% 10 1.5% 668 668 100% 0.78
7 291.5 43.6% 367.5 55.0% 9 1.3% 668 668 100% 0.91
3 240 35.9% 420 62.9% 8 1.2% 668 668 100% 0.94
8 203 30.4% 460 68.9% 5 0.7% 668 668 100% 0.87
9 184 27.5% 480.5 71.9% 3.5 0.5% 668 668 100% 0.70
243 36.4% 424 63.5% 1 0.1% 668 668 100% 0.75
11 242.5 36.3% 422 63.2% 3.5 0.5% 668 668 100% 0.90
3 240 35.9% 420 62.9% 8 1.2% 668 668 100% 0.94
12 239 35.8% 419 62.7% 10 1.5% 668 668 100% 0.89
13 236 35.3% 417 62.4% 15 2.2% 668 668 100% 0.74
*Percentages are % by weight based on the total.
As shown in the above results, the spectral
emissivity of Sample No.3, in which the amount of
tourmaline in the fuel-activating material was 240 g (35.9%
by weight), the amount of iron powder was 420 g (62.9% by
weight) and the amount of carbon was 8 g (1.2% by weight),
was 0.94, which was considered to be the best mode. Using
this sample as a center value, when the blending ratio of
tourmaline was 30% by weight or more and 44% by weight or
less (from Samples No.2 and No.4), the blending ratio of
iron powder was 55% by weight or more and 69% by weight or
less (from Samples No.7 and No.8) and the blending ratio of
carbon was 0.5% by weight or more and 1.5% by weight or
less (from Samples No.11 and No.12), the spectral
emissivity was found to become 0.85 or more.
[0020]
- 15 -
CA 02737594 2011-03-16
(2) Heat-Resistant Fuel-Activating Substance formed by
Metal Spraying
Next, an appropriate weight ratio of a binder for
metal spraying was examined using the fuel-activating
material of Sample No.3, which was considered as the best
mode by the results of (1) described above.
Metallizing 29029 as a binder (Eutectic of Japan
Ltd.) containing nickel and aluminum as main components in
the weight ratio shown in Table 2 below was melt-mixed with
100% by weight of the fuel-activating material of Sample
No.3 described above, and then the obtained melt mixture
was thermally sprayed onto a 2-mm thick aluminized steel
sheet so that a thickness of the obtained coating film
became 0.6 mm, using Tero-Dizing System 2000 (Eutectic of
Japan Ltd.). With respect to the heat-resistant fuel-
activating substance formed by this thermal spraying, the
spectral emissivity was measured in the same manner as in
(1) described above and also adhesion to the thermal
sprayed site was examined. The results were as shown in
Table 2 below.
[0021]
Table 2
Sample Tourmaline Iron powder Carbon Total Binder Spectral
No. 9 g g % g g % emissivity
14 240 35.9% 420 62.9% 8 1.2% 668 300 45%
-
15 240 35.9% 420 62.9% 8 1.2% 668 334 50% 0.91
16 240 35.9% 420 62.9% 8 1.2% 668 668 100% 0.94
17 240 35.9% 420 62.9% 8 1.2% 668 1000 150% 0.90
18 240 35.9% 420 62.9% 8 1.2% 668 1150 172% 0.72
*Percentages are % by weight based on the total.
- 16 -
CA 02737594 2011-03-16
As shown in the above results, the spectral
emissivity of Sample No.16 in which the weight ratio of the
binder compared to 100% by weight of the fuel-activating
material is 100% by weight is the highest value of 0.94 and,
using this sample as a center value, the spectral
emissivity of Sample No.15, in which the weight ratio of
the binder is 50% by weight, and that of Sample No.17 in
which the weight ratio of the binder is 150% by weight were
0.85 or more. To the contrary, in Sample No.18 in which
the weight ratio of the binder is more than 150%, the
spectral emissivity was less than 0.85. In Sample No.14 in
which the weight ratio of the binder is less than 50% by
weight, when the sample was rubbed by hands after thermal
spraying onto the steel sheet, the spray coating film was
easily peeled off. As a result, it has been found that the
sample showed poor adhesion performance as the heat-
resistant fuel-activating substance and was not suited for
practical use.
[0022]
As described above, in the case of forming a heat-
resistant fuel-activating substance by mixing with the
binder for metal spraying, an appropriate weight ratio of
the binder compared to 100% by weight of the fuel-
activating material is 50% by weight or more and 150% by
- 17 -
CA 02737594 2011-03-16
weight or less.
(3) Heat-Resistant Fuel-Activating Substance formed as
Metal Sheet
Next, an appropriate weight ratio of a binder for
forming into a metal sheet was examined using the fuel-
activating material of Sample No.3, which was considered as
the best mode by the results of (1) described above.
Lead as a binder in the weight ratio shown in Table 3
below was blended with 100% by weight of the fuel-
activating material of Sample No.3 described above, and
then the obtained mixture was melted at 350 C and formed
into a 1-mm thick sheet. The spectral emissivity of the
sheet was measured in the same manner as in (1) described
above and also formability as the sheet was examined. The
results were as shown in Table 3 below.
[0023]
Table 3
Sample Tourmaline Iron powder Carbon Total Binder Spectral
No. g % g % g % g % emissivity
19 240 35.9% 420 62.9% 8 1.2% 668 300 45%
-
20 240 35.90 420 62.9% 8 1.2% 668 334 50% 0.90
21 240 35.9% 420 62.9% 8 1.2% 668 668 100 % 0.94
22 240 35.9% 420 62.9% 8 1.2% 668 1000 150% 0.88
23 240 35.9% 420
62.9% 8 1.2% 668 1150 172% 0.70
*Percentages are % by weight based on the total.
As shown in the above results, the spectral
emissivity of Sample No.21 in which the weight ratio of the
binder compared to 100% by weight of the fuel-activating
material is 100% by weight is the highest value of 0.94 and,
- 18 -
CA 02737594 2011-03-16
using this sample as a center value, the spectral
emissivity of Sample No.20 in which the weight ratio of the
binder is 50% by weight, and that of Sample No.22 in which
the weight ratio of the binder is 150% by weight were 0.85
or more. To the contrary, in Sample No.23 in which the
weight ratio of the binder is more than 150%, the spectral
emissivity was less than 0.85. In Sample No.19 in which
the weight ratio of the binder is less than 50% by weight,
it was impossible to form into a sheet. As a result, it
has been found that the sample was not suited for practical
use as a heat-resistant fuel-activating substance.
[00241
As described above, in the case of forming a heat-
resistant fuel-activating substance by mixing with a metal
binder and forming the mixture into a sheet, an appropriate
weight ratio of the binder compared to 100% by weight of
the fuel-activating material is 50% by weight or more and
150% by weight or less.
(4) Heat-Resistant Fuel-Activating Substance formed as
Inorganic Resin Sheet
Next, in the case of forming into a sheet using the
fuel-activating material of Sample No.3, which was
considered as the best mode by the results of (1) described
above, and using an inorganic resin as a binder, a suitable
weight ratio of the binder was examined. The inorganic
- 19 -
CA 02737594 2011-03-16
silicone resin used also in (1) described above as an
inorganic resin in the weight ratio shown in Table 3 below
was blended with 100% by weight of the fuel-activating
material of (1) described above, and then the obtained
mixture was kneaded and formed into a 1-mm thick sheet.
The spectral emissivity of the sheet was measured in the
same manner as in (1) described above and also formability
as the sheet was examined. The results were as shown in
Table 4 below.
[0025]
Table 4
Sample Tourmaline Iron powder Carbon Total Binder Spectral
No. g % g % g % g g % emissivity
24 240 35.9% 420 62.9% 8 1.2% 668 470 70% -
25 240 35.9% 420 62.9% 8 1.2% 668 500 75% 0.91
26 240 35.9% 420 62.9% 8 1.2% 668 688 100% 0.94
27 240 35.9% 420 62.9% 8 1.2% 668 1000 150% 0.90
28 240 35.9% 420 62.9% 8 1.2% 668 1150 172% 0.71
*Percentages are % by weight based on the total.
As shown in the above results, the spectral
emissivity of Sample No.26 in which the weight ratio of the
binder compared to 100% by weight of the fuel-activating
material is 100% by weight is the highest value of 0.94 and,
using this sample as a center value, the spectral
emissivity of Sample No.25 in which the weight ratio of the
binder is 75% by weight, and that of Sample No.27 in which
the weight ratio of the binder is 150% by weight were 0.85
or more. To the contrary, in Sample No.28 in which the
weight ratio of the binder is more than 150%, the spectral
- 20 -
CA 02737594 2011-03-16
emissivity was less than 0.85. In Sample No.24 in which
the weight ratio of the binder is less than 75% by weight,
it was impossible to form into a sheet. As a result, it
has been found that the sample was not suited for practical
use as a heat-resistant fuel-activating substance.
[0026]
As described above, in the case of forming a heat-
resistant fuel-activating substance by mixing with an
inorganic resin binder and forming the mixture into a sheet,
an appropriate weight ratio of the binder compared to 100%
by weight of the fuel-activating material is 75% by weight
or more and 150% by weight or less.
(5) Heat-Resistant Fuel-Activating Substance formed As
Inorganic Resin Melt Thermal Spraying Sheet
Next, in the case of forming into a sheet by melting
and thermal spraying using the fuel-activating material as
Sample No.3, which was considered as the best mode by the
results of (1) described above, and using an inorganic
resin as a binder, a suitable weight ratio of the binder
was examined. The inorganic silicone resin used also in
(1) described above as an inorganic resin in the weight
ratio shown in Table 3 below was blended with 100% by
weight of the fuel-activating material of (1) described
above, and then the obtained mixture was melted and
thermally sprayed onto a 2-mm thick aluminized steel sheet
- 21 -
CA 02737594 2011-03-16
so that the film thickness became 1 mm. The spectral
emissivity of the sheet was measured in the same manner as
in (1) described above and also adhesion as the sheet was
examined. The results were as shown in Table 5 below.
[0027]
Table 5
Sample Tourmaline Iron powder Carbon Total Binder Spectral
No. g g % g g % emissivity
29 240 35.9% 420 62.9% 8 1.2% 668 470 70% -
30 240 35.9% 420 62.9% 8 1.2% 668 500 75% 0.89
31 240 35.9% 420 62.9% 8 1.2% 668 668 100% 0.94
32 240 35.9% 420 62.9% 8 1.2% 668 1000 150% 0.87
33 240 35.9% 420 62.9% 8 1.2% 668 1150 172% 0.72
*Percentages are % by weight based on the total.
As shown in the above results, the spectral
emissivity of Sample No.31 in which the weight ratio of the
binder compared to 100% by weight of the fuel-activating
material is 100% by weight is the highest value of 0.94 and,
using this sample as a center value, the spectral
emissivity of Sample No.30 in which the weight ratio of the
binder is 75% by weight, and that of Sample No.32 in which
the weight ratio of the binder is 150% by weight were 0.85
or more. To the contrary, in Sample No.33 in which the
weight ratio of the binder is more than 150%, the spectral
emissivity was less than 0.85. In Sample No.29 in which
the weight ratio of the binder is less than 75% by weight,
when the sample was rubbed by hands after thermal spraying
onto a steel sheet, the spray coating film was easily
peeled off. As a result, it has been found that the sample
- 22 -
CA 02737594 2011-03-16
showed poor adhesion performance as the heat-resistant
fuel-activating substance and was not suited for practical
use.
[0028]
As described above, in the case of forming a heat-
resistant fuel-activating substance by subjecting an
inorganic resin binder to melting and thermal spraying and
forming the melt into a sheet, an appropriate weight ratio
of the binder compared to 100% by weight of the fuel-
activating material is 75% by weight or more and 150% by
weight or less.
(6) Addition of Silicon
In the case of further adding silicon (silicon powder
(Si.14, SHOWA CHEMICAL INDUSTRY CO., LTD.)) to Sample No.11
in which the content of carbon was the lower limit of 0.5%
by weight in (1) described above, samples were made under
the same conditions as in (1) described above and then
subjected to the measurement of the spectral emissivity.
The results were as shown in Table 6 below.
[0029]
Table 6 Iron Sample Tourmaline powder Carbon Silicon Total Binder Spectral
No. emissivity
9 % % g % g g g %
11 242.5 36.3% 422 63.2% 3.5 0.5% 0 0.0% 668 668 100% 0.90
34 242.5 36.1% 422 62.9% 3.5 0.5% 3.3 0.5% 671.3 668 100% 0.92
35 242.5 35.9% 422 62.5% 3.5 0.5% 6.7 1.0% 679.7 668 99% 0.94
36 242.5 35.8% 422 62.2% 3.5 0.5% 10 1.5% 678 668 99% 0.91
37 242.5 35.7% 422 62.1% 3.5 0.5% 12 1.8% 680 668 98% 0.87
*Percentages are % by weight based on the total.
- 23 -
CA 02737594 2011-03-16
As shown in the above results, the spectral
emissivity of Sample No.11 in which silicon was not added
was 0.90, whereas the spectral emissivity was increased to
0.92 in Sample No.34 in which 0.5% by weight of silicon was
added. Furthermore, the spectral emissivity was 0.94 in
Sample No.35 in which 1.0% by weight of silicon was added
and the spectral emissivity was 0.91 in Sample No.36 in
which 1.5% by weight of silicon was added. In both samples,
the spectral emissivity was increased as compared with the
case where silicon was not added. However, the spectral
emissivity was rather decreased to 0.87 in Sample No.37 in
which the additive percentage of silicon was more than 1.5%
by weight (1.8% by weight).
[0030]
As described above, when the additive percentage of
silicon is 1.5% by weight or less, the significance of
supplementing the spectral emissivity was recognized in
case the content of carbon is comparatively low.
(7) Continuous Use of Heat-Resistant Fuel-Activating
Substance
Next, an influence of continuous use on the spectral
emissivity under a high-temperature environment was
examined.
A test piece obtained by coating an aluminum sheet
- 24 -
CA 02737594 2011-03-16
measuring 100 mm x 200 mm x 2 mm in thickness with the
heat-resistant fuel-activating substance of Sample No.31 in
Table 5 described above was placed on a horizontal steel
plate supported by a prop, and then heated by a gas ring to
a temperature of 280 to 300 C for 7 hours per day from
under the steel plate. After completion of heating, the
test piece was subjected to the measurement of the spectral
emissivity in the same manner as in (1) described above.
This operation was continued for 20 hours with respect to
the same test piece.
[0031]
As a result, a change with time of the spectral
emissivity of the test piece was as shown in Table 7 below.
[0032]
Table 7
Elapsed days Spectral
emissivity
1 0.95
2 0.96
3 0.88
4 0.87
0.87
6 0.86
7 0.86
8 0.86
9 0.86
0.86
0.86
0.86
As described above, the spectral emissivity was kept
at 0.85 or more over the entire test period.
Over the entire test period, blister, peeling or
- 25 -
CA 02737594 2011-03-16
cracking did not occur in the aluminum sheet coated with
the heat-resistant fuel-activating substance.
After the measurement of the spectral emissivity, a
peeling test was conducted in a state where the temperature
was returned to room temperature. Using a cutter, a
lattice-shaped cut reaching an aluminum layer was formed on
a surface of a heat-resistant fuel-activating substance at
an interval of 5 mm, followed by adhering an adhesive
cellophane tape thereonto. The tape was peeled off
immediately was observed whether the peeled heat-resistant
fuel-activating substance adheres onto the tape or not. As
a result, over the entire test period, neither peeling of
the heat-resistant fuel-activating substance nor any burr
was observed at all.
[0033]
Furthermore, an impact resistance test was conducted
with respect to tight adhesion. The same aluminum sheet
coated with the heat-resistant fuel-activating substance
was placed on a floor and a steel ball of 1 kg was dropped
thereon three times from a height of 1 m, and then it was
observed whether peeling occurs or not. As a result, any
peeling of the heat-resistant fuel-activating substance was
not observed over the entire test period.
As shown in each observation described above, tight
adhesion of the heat-resistant fuel-activating substance
- 26 -
CA 02737594 2011-03-16
onto a material to be coated is extremely satisfactory.
It is additionally noted herein that the observation
results with respect to a change of the spectral emissivity
and tight adhesion with time were observed in common not
only in mode of use of spraying of the inorganic material
of (1) described above, but also in all of other modes of
use.
[0034]
(8) Relationship between Spectral Emissivity and Flame
Temperature
With respect to the presence or absence of affixing
of the heat-resistant fuel-activating material, and those
having different spectral emissivities among heat-resistant
fuel-activating substances, various tests were conducted
and a change in flame temperature was examined.
Specifically, a measuring device 10 as shown in Fig.l was
used. That is, a burner 13 made of a stainless steel tube
having an inner diameter of 8.0 mm was connected to a
burner connection portion 12 equipped with an air hole 11,
and also a fuel pipe 14 protrudes from behind the burner
connection portion 12 to halfway of the burner cylinder 13.
A heat-resistant fuel-activating substance 15 formed into a
sheet using the inorganic resin of (4) described above as a
binder was affixed on the portion that was an outer side
face of this burner cylinder 13 and was also behind a tip
- 27 -
CA 02737594 2011-03-16
of the fuel pipe 14.
[0035]
This measuring apparatus 10 was disposed at room
temperature under an atmospheric pressure and a test was
conducted. A flow rate of fuel (city gas (13A, 88% of
methane)) from the fuel pipe 14 was adjusted to 73 cm/sec
and a flow rate of air from the air hole 11 was adjusted to
27 cm/sec. Flame 16 occurring in the burner cylinder 12 as
a result of mixing them was videotaped by a high-speed
video camera (HPV-l, Shimadzu Corporation) and the obtained
video images were analyzed by a dichroic temperature
measurement/camera system (Thermera, Nobby Tech. Ltd.)
thereby measuring a flame temperature. The results are
shown in Table 8 below.
[0036]
Table 8
Affixing of heat- Spectral Flame
Test No. resistant fuel-
emissivity temperature (K)
activating substance
1 Not affixed - 2158
2 Affixed 0.70 2163
3 Affixed 0.75 2163
4 Affixed 0.80 2172
Affixed 0.85 2246
6 Affixed 0.87 2246
7 Affixed 0.90 2258
8 Affixed 0.92 2258
9 Affixed 0.94 2258
As described above, there was a tendency that the
flame temperature rose by affixing of the heat-resistant
fuel-activating substance, and also the flame temperature
- 28 -
CA 02737594 2011-03-16
rose as the spectral emissivity of the affixed heat-
resistant fuel-activating substance became higher. It has
also been found that flame temperature rise of 100 K was
particularly observed in the test No.1 in which the heat-
resistant fuel-activating substance was not affixed, and in
the tests Nos. 7 to 9 in which the spectral emissivity was
0.90 or more.
As is also apparent from the test of the heat-
resistant fuel-activating substance other than (4)
described above, the flame temperature depended on the
spectral emissivity.
Embodiments
[0037]
(1) Test Results in Boiler
The above heat-resistant fuel-activating substance
was affixed in a specific boiler and the energy saving
efficiency was verified. Herein, the "energy saving
efficiency" was defined as follows.
First, a coefficient obtained by dividing the amount
of fuel (unit: liter in the case of liquid fuel, m3 in the
case of gas fuel) used during the test by the amount of
water (unit: m3) used to obtain steam before affixing of
the heat-resistant fuel-activating substance was defined as
a "fuel use coefficient before affixing" (Eb).
- 29 -
CA 02737594 2011-03-16
On the other hand, a coefficient obtained by dividing
the amount of fuel used during the test by the amount of
water used to obtain steam after affixing of the heat-
resistant fuel-activating substance is similarly defined as
a "fuel use coefficient after affixing" (Ea).
[0038]
Then, an energy saving ratio (rj) is defined by the
following equation:
rl = (Eb - Ea) /Eb x 100.
That is, a ratio (%) of a decrease in amount before
and after affixing of the heat-resistant fuel-activating
substance of the amount of fuel required to convert 1 cubic
meter of water into steam to the amount of fuel required
before affixing was the energy saving ratio (rj).
This was verified by various kinds of boilers below.
(1-1) First Embodiment
As the first embodiment, verification was conducted
using a flame-tube smoke-tube boiler as a specific boiler.
The fuel used in this flame-tube smoke-tube boiler (KMS-16A,
IHI PACKAGED BOILER CO., LTD.) was A-heavy oil, the burner
used was a gun type burner, the boiler capacity was 8,000
kg/h, and the control method was a proportional control
method. Fig.2 is a schematic view of the flame-tube smoke-
tube boiler 20, and Fig.3 enlarges a gun type burner
portion thereof. A combustion device 22 was attached to
- 30 -
CA 02737594 2011-03-16
one end (left end in Fig.2) of a combustion chamber 28 in a
boiler body 21, and a combustion cone 23 enabled a cone
maximum diameter portion 24 having the maximum outer
diameter to open toward inside the boiler body 21
(rightward in Fig.2, upward in Fig.3), and emitted flame
from the tip of gun type burner 25 located in almost the
shaft center to a center direction of a combustion chamber
28. A flange 26 that fixed the gun type burner 25 was
provided at the rear end of the combustion device 22. Each
kind of heat-resistant fuel-activating substances 15 in
Table 9 below was affixed onto the inner side face of the
flange 26, whose area 27 was 100% of a projected area of
the cone maximum diameter portion 24 to the flange 26 (cf.
Fig.3), and the fuel use coefficient before and after
affixing was calculated and then the energy saving ratio
was calculated therefrom. The results were shown in Table
9 below. Regarding the spectral emissivity in the heat-
resistant fuel-activating substance, the weight ratio of
each binder was appropriately adjusted so as to become each
numerical value shown in the table below.
[0039]
Table 9
Method of affixing Spectral Fuel use coefficient Energy
heat-resistant fuel- Before After saving rate
activating substance emissivity affixing affixing (o)
Metal spraying 0.90 72.46 68.86 4.97
Metal sheet 0.88 72.40 68.89 4.85
Inorganic resin sheet 0.94 72.30 68.46 5.31
Inorganic resin 0.92 72.35 68.62 5.16
- 31 -
CA 02737594 2011-03-16
thermal spray
As described above, even in each of the affixing
methods, if the spectral emissivity was 0.85 or more, a
decrease of at least 4.85% or more of the fuel use
coefficient before affixing was observed. In particular,
even if the heat-resistant fuel-activating substance was
different, there was a tendency that the energy saving rate
also increased with the increase of the spectral emissivity
of the heat-resistant fuel-activating substance. This is
assumed that the flame temperature may increase with the
increase of the spectral emissivity (cf. item (8) in "BEST
MODE FOR CARRYING OUT THE INVENTION").
(1-2) Second Embodiment
As the second embodiment, verification was conducted
using a once-through boiler as a specific boiler. The fuel
used in this once-through boiler (STE2001GLM, Nippon
Thermoener Co., Ltd.) was LPG, the burner used was a gun
type burner, the boiler capacity was 1,667 kg/h, and the
control method was a 3-position control method. Fig.4 is a
schematic view of the once-through boiler 30, and Fig.5
enlarges a gun type burner portion thereof. A combustion
device 32 was attached to one end (upper end in Fig.4) of a
combustion chamber 38 in a boiler body 31, and a combustion
cone 33 enabled a cone maximum diameter portion 34 having
the maximum outer diameter to open toward inside the boiler
- 32 -
CA 02737594 2011-03-16
body 31 (downward in Fig.4 and Fig.5), and emitted flame
from the tip of gun type burner 35 located in almost the
shaft center to a center direction of a combustion chamber
38. A flange 36 that fixed the gun type burner 35 was
provided at the rear end of the combustion device 32. Each
kind of heat-resistant fuel-activating substances 15 in
Table 10 below was affixed onto the inner side face of the
flange 36, whose area 37 was 100% of a projected area of
the cone maximum diameter portion to the flange 36, and the
fuel use coefficient before and after affixing was
calculated and then the energy saving ratio was calculated
therefrom. The results were shown in Table 10 below. The
heat-resistant fuel-activating substances used herein were
respectively the same as those used in the first embodiment.
[0040]
Table 10
Method of affixing Spectral Fuel use coefficient Energy
heat-resistant fuel- emissivity After saving rate
activating substance ssivity affixing affixing (%)
Metal spraying 0.90 27.14 25.80 4.94
Metal sheet 0.88 27.12 25.83 4.76
Inorganic resin sheet 0.94 27.10 25.60 5.54
Inorganic resin 0.92 27.15 25.71 5.30
thermal spray
As described above, even in each of the affixing
methods, if the spectral emissivity was 0.85 or more, a
decrease of at least 4.76% or more of the fuel use
coefficient before affixing was observed. In particular,
even if the heat-resistant fuel-activating substance was
- 33 -
CA 02737594 2011-03-16
different, similar to the first embodiment described above,
there was a tendency that the energy saving rate also
increased with the increase of the spectral emissivity of
the heat-resistant fuel-activating substance.
(1-3) Third Embodiment
As the third embodiment, verification was conducted
using a water-tube boiler as a specific boiler. The fuel
used in this water-tube boiler (SCM-160, IHI Corporation)
was C-heavy oil, the burner used was a gun type burner, the
boiler capacity was 16,000 kg/h, and the control method was
a proportional control method. Fig.6 is a schematic view
of the water-tube boiler 40, and Fig.7 enlarges a gun type
burner portion thereof. A combustion device 42 was
attached to one end (lower end in Fig.6) of a combustion
chamber 48 in a boiler body 41, and a combustion cone 43
enabled a cone maximum diameter portion 44 having the
maximum outer diameter to open toward inside the boiler
body 41 (upward in Fig.6 and Fig.7), and emitted flame from
the tip of gun type burner 45 located in almost the shaft
center to a center direction of a combustion chamber 48. A
flange 46 that fixed the gun type burner 45 was provided at
the rear end of the combustion device 42. Each kind of
heat-resistant fuel-activating substances 15 in Table 11
below was affixed onto the inner side face of the flange 46,
whose area 47 was 100% of a projected area of the cone
- 34 -
CA 02737594 2011-03-16
maximum diameter portion 44 to the flange 46, and the fuel
use coefficient before and after affixing was calculated
and then the energy saving ratio was calculated therefrom.
The results were shown in Table 11 below. The heat-
resistant fuel-activating substances used herein were
respectively the same as those used in the first embodiment.
[0041]
Table 11
Method of affixing Fuel use coefficient Energy
heat-resistant fuel- Spectral emissivity Before After saving rate
activating substance affixing affixing (%)
Metal spraying 0.90 70.50 68.31 3.11
Metal sheet 0.88 70.52 68.35 3.08
Inorganic resin
0.94 70.38 67.89 3.54
sheet
Inorganic resin 0.92 70.42 68.05 3.37
thermal spray
As described above, even in each of the affixing
methods, if the spectral emissivity was 0.85 or more, a
decrease of at least 3% or more of the fuel use coefficient
before affixing was observed. In particular, even if the
heat-resistant fuel-activating substance was different,
similar to the first and second embodiments described above,
there was a tendency that the energy saving rate also
increased with the increase of the spectral emissivity of
the heat-resistant fuel-activating substance.
(2) Others
It is additionally noted herein that almost the same
effects were obtained even in the case of using boilers
- 35 -
CA 02737594 2011-03-16
other than the above respective general-purpose boilers,
industrial boilers and using, in addition to the above
fuels, biofuel, propane gas and the like as fuels used in
the boilers, regardless of the kind.
INDUSTRIAL APPLICABILITY
[0042]
The present invention can be utilized not only in a
once-through boiler, a flame-tube smoke-tube boiler and a
water-tube boiler (including an industrial boiler and a
power station boiler that are equipped with two or more
burners), but also in burning appliances equipped with a
combustion device, such as a kiln and a dryer.
- 36 -